Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 1 -
ELECTROLYSIS PROCESS AND CELL FOR USE IN SAME
Field of the Invention
The present invention relates generally to an electrolysis process for the
recovery of metals from an aqueous solution and to an improved cathode for use
in such
a process. The primary application of the invention disclosed herein is in
relation to the
recovery of copper, although the invention finds equal application with
electro-recovery
of other metals such as nickel, lead, zinc etc.
Background Art
Processes for leaching base metals from ores and concentrates with subsequent
recovery of the base metal in electrolytic cells are known in the field of the
hydrometallurgy. One example is disclosed in Australian Patent Application No.
42999/93 (669906). This process is multi-stage and produces a pregnant liquor
stream
following leaching of the mineral in a chloride medium. The pregnant liquor
stream is
electrolysed in an electrolysis cell to recover the metal from the solution
which deposits
on a cathode of the cell. Under high current densities, dendritic copper of
high purity is
2 0 produced on the cathode . In the past it has been necessary to regularly
remove the
cathodes to strip the plates of the metal deposits so as to maintain current
efficiency in
the cell.
Optimisation of the electrowinning operation is a function of the purity of
the
pregnant liquor stream , and general cell parameters such as current density,
stripping
2 5 cycle, cell configuration and the degree of agitation. Accordingly, an aim
of the present
invention is to improve the efficiency of the electrowinning operation. In
particular, an
aim is to provide an electrolysis process and cell configuration which is able
to better
control current density across the deposition surface of the cathode so as to
assist in
both formation and removal of the metal deposit.
Surnnnar~of the Invention
In a first aspect the present invention provides an electrolysis process for
the
recovery of metal from an aqueous solution wherein on electrolysing the
solution metal
3 5 is caused to deposit on a deposition surface of a cathode, the process
including the step
of
- inducing a non-uniform current density across the deposition surface so as
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 2 -
to form areas of high current density interspaced by areas of low current
density, the difference between the areas of high current density and low
current density being sufficient to cause metal deposition to be
concentrated on the areas of high current density so as to promote non-
uniform deposition of metal across the deposition surface.
In the context of the invention, the deposition surface may be of unitary
structure or alternatively may be formed from discrete elements which may be
spaced
apart or in direct contact with one another.
Providing a non-uniform current density across the deposition surface provides
a mechanism by which the deposition of metal on that surface can be
controlled. In
particular, it allows the metal deposition to be concentrated in certain areas
(i.e. the
areas of high current density) so as to promote non-uniform deposition across
the
surface. Non-uniform deposition of the metal is beneficial as it is easier to
remove from
the cathode which assists in the metal recovery process.
Preferably, the metal deposition is heavily concentrated on the areas of high
current density so that the metal deposition is effectively discontinuous
across the
deposition surface. Preferably, the concentration of metal deposition in
operation of the
cell is greater than 80% in the areas of high current density and more
preferably greater
than 95%.
2 0 Preferably, the areas of high current density and low current density
extend
along the surface in one direction and alternate across the surface in an
opposite
direction. With this arrangement, the metal deposits in a series of generally
linear bands
which is ideally suited for removal using a wiping action as will be described
in more
detail below.
2 5 Preferably, the electrolysis process induces a non-uniform current density
across the deposition surface by providing a cathode which in operation of the
cell,
creates a non-uniform electrical field having areas of strong electrical field
and weak
electrical field. With this arrangement, the areas of strong electrical field
induce the
areas of the high current density and the areas of weak electrical field
induce the areas
3 0 of low current density.
The non-uniform electrical field can be created through numerous mechanisms,
including the geometry of the surface, and by varying the electrical
resistance between
the cathode and anode along the deposition surface, or by a combination of
both these
mechanisms.
3 5 The geometry of the surface influences the electrical field and is related
to its
surface curvature. Electrical fields are always parallel to the surface so
that, sharp
edges, or peaks at the deposition surface induce areas of high electrical
field as
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 3 -
compared to areas of flat surface, or valleys. The electrical resistance can
be varied by
using different materials along the deposition surface (e.g. providing
sections with
insulating material) or by changing the current path length between the
cathode and the
anode.
In a preferred form, the non-uniform electrical field is induced at the
deposition
surface by the geometry of the surface and in particular by forming a series
of alternate
ridges and valleys across the surface. By virtue of this geometry, in
operation of the
cell, there is a higher electrical field along the ridges as compared to the
valleys. In
addition, the current path length at the ridges is shorter as compared to the
valleys
thereby creating a situation where there is less resistance at the ridges as
compared to
the valleys.
The variation in current density across the deposition suxface may be such
that
there is a sharp demarcation between the areas of high current density and low
current
density, or alternatively there may be a more gradual transition between the
axeas of
highest current density and lowest current density.
The applicant has found that inducing a gradual transition between the areas
of
highest and lowest current density still provides good deposition patterns so
as to
promote substantially discontinuous growth across the deposition surface. In
particular,
the applicant has found that using a cathode which includes a deposition
surface having
2 0 ridges and valleys which do not include a sharp transition between the
ridge and valleys
so that there is a more gradual change between the highest current density and
the
lowest current density provides excellent performance. This arrangement
induces
secondary effects which assist in concentration of the metal deposition at the
ridges as
described in more detail below and also provides for easier removal of the
metal as it
2 5 allows easier access to the entire deposition surface in contrast to a
sharp transition
between the ridge and, the valley may provide areas which are difficult to
access:
In a preferred form, where the cell is operative to remove copper from an
aqueous solution, the current density in the areas of high current density is
in the range
of 500 to 2,SOOA/m2 and more preferably 1,000.A/m2. Preferably, the areas of
low
3 o current density is in the range of 0 to 2,OSOA/m2 and more preferably 0 to
SOOA/m2.
Where there is a gradual transition between the areas of highest current
density
and lowest current density, the demarcation between an area of "high current
density"
and "low current density" is somewhat arbitrary. In this arrangement, the
transition
region may be regarded as an area of moderate current which in turn is located
between
3 5 areas of adj acent "high current density" and areas of "low current
density".
Preferably, the process further includes the step of removing deposited metal
from the deposition surface by passing an element over the surface.
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 4 -
Preferably, in the arrangement where the areas of high current density and low
current density extend along the surface in one direction and alternate across
the surface
in an opposite direction, the element is moved in the direction in which the
areas of high
and low current density extend.
Preferably, the deposited metal is removed by the element whilst maintaining
current flow in the aqueous solution. In this way, the process can be
substantially
continuous.
In yet a fixrrher aspect, the present invention relates to an electrolysis
cell for
the electrorecovery of metal from an aqueous solution, the cell including a
cathode
1 o which includes a deposition surface on which metal is deposited on
electrolysing of the
aqueous solution; wherein in operation of the cell, the deposition surface has
a non-
uniform electrical field so as to have areas of strong electrical field
interspaced by areas
of low electrical field, the difference between the areas of high electrical
field and low
electrical field being sufficient to cause metal deposition to be concentrated
on the areas
of high electrical field so as to promote non-uniform deposition of metal on
the surface.
Preferably, the areas of high electrical field and low electrical field extend
along the surface in one direction and alternate across the surface in an
opposite
direction. In a particularly preferred form, the deposition surface of the
cathode
includes an array of alternate ridges and valleys, with the ridges forming
areas of high
2 0 electrical field and the valleys forming the areas of low electricalfield.
Profiling the deposition surface to have an array of alternate ridges and
valleys
has significant benefit in promoting substantially discontinuous metal
deposition on the
cathode. Typically such profiling promotes metal deposition as a dendrite
growth on
each of the ridges. Advantageously, the resulting dendrites are easy to remove
(as
2 5 described below). Not only does the profile provide, in the initial
operation of the cell,
the appropriate non-uniform current density to concentrate metal deposition as
dendrites
on the ridges, but it also assists in maintaining discontinuous growth as the
process
continues. As will be appreciated, once metal deposits on the deposition
surface, the
deposited metal forms an extension of a deposition surface. An advantage of
having an
3 0 arrangement of ridges and valleys is that as the dendrites grow on the
ridges, they tend
to "shadow" the valleys which further inhibits metal deposition in the
valleys. In
addition, the aqueous solution tends to stagnate in the valleys which further
inhibits
deposition of metal in the valleys. In tests conducted by the applicant, using
a profile of
alternate ridges and valleys, more than 98.8% of metal was deposited on the
ridges of
3 5 the deposition surface.
Whilst the beneficial effects of including the ridges and valleys may be
achieved over a range of profiles, the applicants have found that a regular
profile where
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 5 -
the surfaces between the top of the ridge and the base of the valley is
substantially linear
and have an internal angle of approximately 60° between adjacent
surfaces provides
good results. Furthermore preferably the pitch distance between adjacent
ridges is in
the order of 10-40mm, and more preferably 15-25mm, and the depth between the
ridge
and the valley is in the order of ~-32mm and more preferably in the range of
12-20mm.
A deposition surface having these characteristics has been found to produce
substantially discontinuous metal deposits. A further advantage is that this
profile
enables the surface to be substantially cleaned without creating "hot spots"
of current
density which would lead to impure metal deposits. If the current density at a
site is too
1 o high, as the deposition progresses, it leads to concentration polarisation
(which takes
place around the growing deposit). When this phenomenon occurs, a relative
increase
in impurity inclusions in the depositing metal (e.g. in copper) can occur.
Thus it is
important to control the current density at the site. The advantage of the
profile
mentioned above is that the areas of high current density where metal deposits
still
takes up a substantial part of the total area of the cathode. (i.e. in the
vicinity of 25-35%
of the total area of the deposition surface). With this arrangement, the
current is able to
be maintained at a substantially constant rate regardless of whether the
surface is clean
of metal deposits or whether deposition has already occurred. As such, there
is no need
to ramp up the current on initiating the cell as the profile itself does not
tend to induce
2 o strong "hot spots" of current density which is likely to cause problems in
initial metal
deposition.
In a particularly preferred form, the cathode includes a sheet having at least
one major surface which forms the deposition surface of the cathode, the sheet
being
preformed so as to incorporate the alternate ridges and valleys. The sheet may
thus
2 5 define a corrugated profile. Preferably, this preforming operation is
achieved by folding
of the sheet but it could be made by any other appropriate process such as a
stamping,
milling, swaging, casting process or combinations thereof.
In a particularly preferred form, the sheet is formed from titanium or similar
oxidation resistant material. Whilst other oxidation resistant materials may
be used,
3 0 such as platinum, stainless steel, corrosion resistant metal alloys,
titanium is most
preferred because of its excellent oxidation resistance, its capacity to
resist forming a
metallurgical bond with metals such as copper, and because of its relative
availability.
A further advantage of using a corrugated profile is that it assists in
maintaining dimensional stability for the sheet. Such an arrangement can
assist in
3 5 overcoming the disadvantages of prior art arrangements where sheet
cathodes had a
tendency to flex and buckle. Further, when metal deposits on the sheet as a
dendritic or
crystalline growth, the dimensional stability of the sheet enables wiping
methods to be
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 6 -
used to easily remove the deposit from the sheet. The applicants have found
that
titanium sheets in the order of l.6mm thickness provide sufficient dimensional
stability
for this process.
Preferably, the sheet is adapted in use for attachment to a conductive header
bar. This header bar supports the cathode in use and supplies electrons to it.
In one form, the opposite major surfaces of the folded sheet are used as
deposition surfaces in operation of the cathode.
In an alternative form, the cathode is made from a composite structure and
fixrther includes a conducting element which extends along the sheet. The
conducting
element is in electrocommunication with the sheet so as in use to supply the
deposition
surface with electrons in the electrolysis process. One advantage of using a
conducting
element which extends along the sheet is that it minimises ohmic drop which
occurs
when the electrons are supplied solely from one edge of the sheet. A second
advantage
of using a conducting element is that is may be of sufficient size to provide
rigidity to
the sheet to further assist in maintaining dimensional stability of the
cathode. With the
composite arrangement it may thus be possible to use thinner sheet structures
for the
deposition surface(s).
In a preferred form of this latter arrangement, the cathode includes a second
sheet which is connected to the first sheet and which has a major surface
which forms a
2 0 second deposition surface of the cathode, the second sheet being preformed
so as to
incorporate the alternate ridges and valleys along that deposition surface.
Preferably,
the second sheet is connected to the first sheet of the cathode so as to form
a plurality of
pockets which extend in the direction of the alternate ridges and valleys. At
least some
of these pockets are operative to receive the conducting element of the
cathode.
2 5 In a preferred form, the wiping device is operative to pass over a
deposition
surface of the cathode so as to remove deposited material from the deposition
surface.
In a particularly preferred form, where the cathode includes the ridge and
valley profile,
the wiping device includes a plurality of proj ections which are operative to
locate
within respective valleys of the deposition surface. In a preferred form,
these
3 o projections are made from a ceramic material but can be made of any other
corrosive
resistant material.
In a preferred form, the projections are movable between a first and a second
position and are operative to pass over the surface in either of these
positions. In a first
position, the element is in contact or in close proximity to the deposition
surface so as to
3 5 remove substantially all of the deposition material from that surface. In
the second
position, preferably the element is spaced from the deposition surface and is
operative
to remove deposited material which extends a predetermined distance from the
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
deposition surface.
In yet a further aspect, the present invention relates to a cathode for use in
a
process or electrolysis cell as defined in any form above.
In yet a further aspect, the present invention relates to a wiping system for
use
in an electrolysis cell in any form as defined above.
In yet a further aspect, the present invention relates to a cathode for use in
an
electrolysis cell for the electrorecovery of metal from an aqueous solution,
the cathodes
including a deposition surface having a plurality of ridges which are
interspaced by a
plurality of valleys, the profile of the cathode being operative on operation
of the cell to
1 o cause metal deposition to be concentrated on the ridges so as to promote
non-uniform
deposition of metal on that surface.
Brief Description of the Drawings
Notwithstanding any forms which may fall within the scope of the present
invention, preferred forms of the invention will now be described, by way of
example
only, with reference to the accompanying drawings in which:
Figure 1 is a generalised flowchart for processing and recovery of copper;
Figure 2 is a sectional elevation of an electrolysis cell in accordance with
one
2 o embodiment of the invention with wiper sets of the cell in a closed
position;
Figure 3 is a sectional side view of the cell of Figure 2;
Figure 4 is a sectional elevation of the cell of Figure 2 with the wipers in
an
open position;
Figure 5 is a detailed view of the linkage assembly in the cell of Figure 2;
2 5 Figure 6 is a cut-away perspective view of the cell of Figure 2;
Figure 7 is a schematic view to an enlarged scale showing the wipers located
in
an open position at the top of the cathode plates;
Figure 8 is a detailed view to an enlarged scale of the wipers in a closed
position;
3 o Figure 9 is a front elevation of a cathode panel used in the cell of
Figure 2;
Figure 10 is an end view of the panel of Figure 9;
Figure 1 I is a schematic perspective view of a wiper engaging a cathode in
the
cell of Figure 2;
Figure I2 is a sectional view along section line XII-XII in Figurel l;
3 5 Figure 13 is a detailed view of the blade construction of the wipers used
in the
cell of Figure 2;
Figures 14 and 15 are variations of the blade instruction shown in Figurel3;
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
_ g _
Figure 16 is a schematic perspective view of an alternative cathode designed
for use in the cell of Figure 2; and
Figure 17 is a cross sectional view along section line XVII-XVII of the
cathode
of Figure 16.
Modes for Carr ''~ng out the Invention
In Figure 1, a schematic representation of a combined process 100 including
leach and electrorecovery 104 of metal is depicted. In a preferred form of
this process,
1 o ground copper sulfide 106 is fed to a multistage counter current leaching
process in
which the metals are solubilised through oxidation by a lixiviant. In a
preferred form,
the lixiviant includes a complex halide species which is formed in the anode
of the
subsequent electrolysis stage and is fed back into the leach stage as part of
the
electrolyte recycle 108.
Dissolved metals in desirable oxidation states are removed at various stages
from the leach process in the leachate. The leachate is passed through
filtration 110 to
remove unwanted solids such as sulfur and ferric oxide. The leachate is then
passed to
purification 112 to remove metals which may otherwise contaminate subsequent
electrolysis (such as silver and mercury). The contaminant metals may be
precipitated
2 0 as the metal oxide or carbonate form.
The purified leachate is then fed to the electrolysis stage 104 which may
include a plurality of electrolysis cell groups in series and/or in
parallel.In each group, a
different metal may be produced, with typically copper metal being
electrorecovered in
a first cell group and metals such as zinc, lead and nickel being recovered in
subsequent
2 5 or parallel cell groups. The electrolysis process is typically operated
such that a highly
oxidising lixiviant (such as a complex halide species) is produced at the
anode. The
spent electrolyte (anolyte) is then recycled to the leaching stage and
includes the highly
oxidising lixiviant which participates in further counter current leaching.
Thus, the
process can operate continuously.
3 o The present invention is concerned with optimising the electrorecovery of
metals and relates to significant design improvements in the electrolysis
process,
including improved cathode design and geometry.
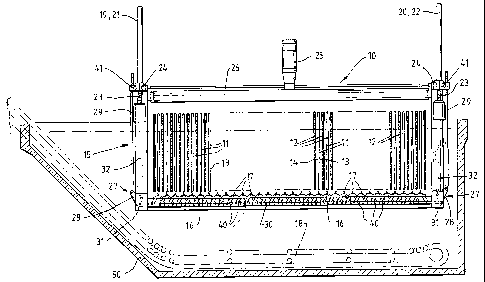
Referring now to Figures 2-5 the electrolysis cell 10 for use in the process
100
includes a series of cathode plates 11 which are disposed within the
electrolysis cell
3 5 tank 50 and interspaced by anodes 12. Electrolyte fed to the cell enables
current flow
between the anodes and the cathodes. The outer surfaces 13, 14 of the
respective
cathodes form a deposition surface for the cell on which the metal to be
recovered
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 9 -
deposits in operation of the cell 10. As will be described in more detail
below, the
cathode plates are formed from a generally corrugated profile having alternate
ridges
and valleys so as to-influence the mode of deposition of the metal on the
respective
deposition surfaces 13 and 14.
The cell 10 includes a wiper system 15 which includes a plurality of wiper
sets
16 operative to interfit between respective cathodes and anodes with the
wipers 17 of
respective wiper sets 16 being operative to move across the deposition
surfaces 13 and
14 of respective cathodes 11 so as to remove metal deposits from those
surfaces. The
wipers 17 are arranged to be wiped down the respective deposition surfaces 13
and 14
at predetermined intervals to cause the dislodged metal to drop to the bottom
of the cell
10 wherein it is transferred to a conveyor 18 for removal from the cell.
To achieve this wiping action, the wiping system 15 includes two principle
movements; the first being a vertical movement to allow the wiper sets 16 to
move
between the top and the bottom of the respective cathodes 11, the second being
to allow
the wipers 17 in each set 16 to move from an open position (as best
illustrated in Figure
7) to a closed position (as best illustrated in Figure 8).
The wiper sets 16 are mounted on a frame 32 which is secured at its upper end
to four supporting struts 19, 20, 21 and 22. Each of the struts include a
helical track 23
which cooperates with a worm gear 24 connected to the frame 32. In this way,
the
2 0 frame 32 moves relative to the struts. An electric motor 25 mounted on a
cross beam 26
is operative to drive the worm gears 24 so as to achieve the vertical movement
of the
wiper sets relative to the deposition surfaces 13 and 14. Under this action,
the wipers
are able to move between a lower position as disclosed in Figure 2 to an upper
position
as disclosed in Figure 4.
2 5 The frame 32 supports a linkage assembly 27 which in turn is connected to
the
wiper sets 16. The linkage assembly 27 includes a pair of link plates 28 at
each end of
the wiper sets 16 which are connected to respective link arms 29. A crank 30
is
pivotally connected to respective pairs of the link plates 28 through pivot
points 31.
Crank arms 40 extend from the crank 30 to the wiper sets 16 so as to support
each end
3 0 of the wiper sets. The link arms 29 are capable of vertical movement
through a second
actuator 41. In the illustrated form, the second actuator is in the form of
worm gears
which cooperate with helical tracks formed on the respective link arms. The
worm
gears rotate which impacts the rotation to the the link arms 29 to cause
vertical
displacement of those arms relative to the frame 18 which in turn drives the
crank 30 so
3 5 as to move the wipers between their open and closed positions. The second
activator
can be damped to prevent over-tightening and j amming of the wipers against
the
cathode. Damping can be provided by a spring-loaded coupling or by using a
pneumatic
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 10 -
cylinder in place of the worm gear.
As best illustrated in Figure 6 each line of cathodes in the cell 10 is formed
from a plurality of cathode plates 11 which are connected to a header bar 34
so that the
individual plates are suspended in the tank 50. The header bar 34 is
conductive and
connected to a power source so as to supply electrons to the cathode.
Typically the electrolyte is highly corrosive, resulting from typically a 5
molar
or greater concentration of alkali or alkaline-earth metal halides. To enable
the
components to be able to operate in this environment, the wiper system 15 is
made from
a corrosion resistant material which is preferably titanium. Other suitable
materials
include platinum, stainless steel, corrosion resistant metal alloys (such as
Hastalloy C
22), or even some plastics. Also, titanium is most preferred for the cathode
because of
its excellent corrosion resistance and its capacity to resist forming a
metallurgical bond
with metal such as copper, and because of its relative availability (hence
cost benefit).
Its resistance to forming a metallurgic bond improves the ability of the
plates to be
stripped using the wiper system described above.
Figures 9 and 10 illustrate the construction of the individual cathode plates
11.
In the illustrated form, the cathode plate 11 is formed from a titauium sheet
having a
thickness which is preferably about 1.6mm. Sheets of this thickness have been
found
by the applicant to give adequate rigidity to the cathode plate to prevent
buckling in use.
2 0 The titanium sheet is folded to form a generally corrugated profile so as
to provide on
each deposition surface 13, 14 alternate valleys and ridges 35, 36
respectively. These
corrugations run along the entire length of the cathode between its upper and
lower
edges 37, 38.
In the illustrated form, the distance between adjacent ridges 36 is 20mm,
2 5 whereas the depth between the top of the ridges 36 and the bottom of the
valleys 35 is
approximately l6mm. The wall surfaces 43 formed on the corrugated sheet are
generally linear and have an internal angle at the top at the ridges and
bottom of the
valleys of approximately 60°.
A primary purpose for incorporating the corrugations in the cathode is to
3 o influence the current density on the deposition surfaces 13, 14 under
operation of the
cell. In particular, the corrugations on the deposition surface cause a non-
uniform
electrical field across that surface in operation of the cell.
The corrugated deposition surface on the cathode creates bands of high current
density along the ridges of the cathode due to its corresponding high
electrical field at
3 5 those areas and relatively low current densities in the valleys. This
causes metal
deposition to be concentrated in the areas of high current density and
promotes non-
uniform deposition across the surface so that the vast majority of the
deposition
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 11 -
includes in the ridge regions 35 of the deposition surface, Creating
substantially
discontinuous deposition improves the ability to be able to remove recovered
metal
from the cathode using the wiping system 15.
The profile of the deposition surface with the valleys and ridges causes the
non-uniform electric field by two mechanisms. Firstly, in view of the geometry
of the
profile, the electrical field will be stronger at the ridges than the valleys
because of its
surface curvature. In general, the electric field Iines are always parallel to
the surface.
Therefore, at each ridge there will be a concentration of a field along those
points.
Secondly, the current flow path at the ridges is less than the current flow
path at the
1 o valleys. As a result, there is less resistance at the ridges than there is
at the valleys.
In addition, the use of the corrugated profile of the cathode allows better
control at the main sites of deposition (i.e. along the ridges). If the
current density at a
site is too high, as deposition progresses, it leads to concentration
polarisation (which
takes place around the growing deposit). When this phenomenon occurs, a
relative
increase in impurity inclusion in the depositing metal (e.g. in copper) can
occur. With
the corrugated profile, the main sites of deposition account for approximately
25-35%
of the total surface area of the cathode. As a function of the mass transfer,
ideally the
current at the deposition surface should be in the vicinity of 1,OOOAlm2 or
less. As the
dendrites grow on the surface, the actual area of deposition surface increases
as metal is
2 o deposited on the previously deposited metal. If the initial deposition
sites on the
cathode are too small, then there is a tendency that once. the dendrite is
removed from
the cathode the current density at that site becomes too high. Through trials
conducted
by the applicant, using the corrugated profile, it is found that the current
density at the
deposition sites both on initial operation of the cell and after dendritic
growth has
2 5 occurred, it is able to be maintained in the vicinity of 1,OOOA/m2 so as
to provide high
quality metal deposition. As such, there is no need to vary the current during
the
process.
A further advantage of using the corrugated profile on the cathode is that it
improves the rigidity of the cathode plate, as the corrugated profile is
inherently stiffer
3 o than a flat plate along the direction of the ridges and valleys. In
addition, the corrugated
profile is ideally suited to be cleaned using the wiping blade system as is
described in
more detail below.
With reference to Figures 11 to 15, the wipers 17 include fingers 39 which are
mounted between a pair of rails 42. In the illustrated form, each of the
individual
3 5 fingers are formed from a ceramic material with the rails being made of
titanium. Each
of the fingers is spaced along the rail 42 so that the wipers 17 conform
generally to the
shape of the corrugated cathode plate 11, with the individual forgers locating
within the
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 12 -
valleys 35 of the deposition surface and over the associated ridges 36.
As best illustrated in Figure 12, the wiping system 15 is designed so that
when
the wiper sets 16 are in their closed position, the wipers 17 are angled to
the cathode
plate 11 so that the individual fingers 39 are in a trailing position relative
to the line of
movement of the wiper 17 down the cathode plate 11. This arrangement is
preferred as
it inhibits jamming of the fingers in the valleys as may occur if the fingers
39 were in a
leading position relative to the direction of movement of the wipers down the
cathode
plate.
As described above, in view of the configuration of the cathode plates 11, the
metal recovered from the electrolysis cell is concentrated on the ridges of
the respective
deposition surfaces of the cell. As such, when the wiper 17 is moved across
the
deposition surface the dislodged material from the ridges tends to move into
the
adjacent valleys of the deposition surface. This causes an accumulation of the
metal
within the valleys which tends to envelop the fingers 34 thereby protecting
the ceramic
fingers 39 from wear. In addition, there is a build up in frictional force as
the mass of
material is moved down the deposition surface thereby aiding removal of
material as the
material is dragged from the surface under this frictional force. It is not
necessary that
the fingers 39 are in direct contact with the deposition surface to ensure
adequate
cleaning of that surface.
2 o Another advantage of the design of the wiping system 15 is that it enables
different stages of cleaning of the cathodes. In particular, as described
above, the
wipers 17 can be operated to remove the bulk of the deposited material on the
deposition surfaces by dragging across those surfaces when in their closed
position.
The wipers can also be moved across the deposition when in their open
position. This
2 5 is used not to fully clean the deposition surface but rather to ensure
that there is no
extended dendritic growth on part of the deposition surface which could
otherwise grow
to an extent that it contacts the anode and thereby causes short circuiting of
the
electrolysis cell. Also, this allows for more consistent growth across the
ridges of the
cathode, which aids in control of the current density along the deposition
surface.
3 0 Figures 14 and 15 illustrate some variations in the design of the wipers
17. As
in the arrangement of Figure 13, each of the wipers 17 include ceramic fingers
39.
However, rather than using the rail arrangement 42 as disclosed in Figure 13,
the fingers
39 are interconnected by an internal connecting bar 44. In the embodiment of
Figure 14
the bar 44 is formed as a square section, whereas in Figure 15 the connecting
bar is
3 5 made up of two cylindrical bars 45.
Refernng now to Figures 16 and 17, an alternative cathode construction is
depicted. In this embodiment the cathode is funned as a composite structure
wherein
CA 02502650 2005-04-18
WO 2004/035868 PCT/AU2003/001393
- 13 -
the outer deposition surfaces 13, 14 are defined by separate sheets which are
fastened
together along their respective lateral edges 60, 61, and which may optionally
be
fastened together at intermittent regions 62.
In this embodiment, a plurality of conducting bars 63 form part of the
construction and extend downwardly from the header bar 34, the conducting bars
typically also being formed from titanium (or a titanium coated copper bar to
further
improve conductively). Typically the conducting bars extend for the full
length the
plates 13,14 through each of the passages defined between a plate and are
fastened
thereto. Such an arrangement provides enhanced distribution of electrons
through the
assembly, thereby minimising ohmic drop which can occur when the electrons are
supplied solely to one edge of the sheet. In addition, it has been found that
the
composite arrangement, including the arrangement of the conducting bars in the
passages, enhances the dimensional stability of the sheet so that thin plate
structures
(e.g. as small as lmm) or alternatively wide plate structures can be employed
for the
cathode. Otherwise, the principles of operation of the cathode of Figures 16
and I7 are
described above.
Whilst the invention has been described with reference to a number of
preferred embodiments, it should be appreciated that the invention can be
embodied in
many other forms.