Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02502706 2005-04-15
Description
Process for Recovering Argon by Low-Temperature Air Separation
The invention relates to a process for recovering argon by low-temperature
separation of air in a rectifying system, which has three rectifying sections
that are
arranged in series, whereby the first and the second as well as the second and
third
rectifying sections in each case are connected to one another on the gas and
liquid sides,
and whereby the second rectifying section has two subsections, which are not
connected
to one another on the gas and liquid sides and are flushed in a parallel
manner, whereby a
fluid that contains oxygen and argon is introduced into the first of two
subsections and a
stream that contains oxygen and argon is removed in the second of the two
subsections.
The boiling point of argon is located between the boiling points of oxygen and
nitrogen. In the standard low-temperature separation of air by two-stage
rectification, the
argon accumulates in a middle area of the low-pressure column. For argon
recovery, a
gaseous fraction, which essentially consists of oxygen and argon, is usually
removed
from this area. This fraction that is concentrated with about 10% argon is fed
to the so-
called crude argon column into which a separation of oxygen and argon by
rectification is
performed. At the head of the crude argon column, argon can be drawn off, and
in the
bottom thereof, a liquid that essentially contains oxygen is collected that
then is returned
into the low-pressure column.
In practice, argon purities of over 95% are frequently called for. In the
known
process, however, a stream that only contains approximately I O% argon is fed
to the
CA 02502706 2005-04-15
7
crude argon column. To concentrate the latter to the desired high argon
purities and to be
able to draw off the desired amount of product at the head of the crude argon
column,
considerable amounts of vapor must be introduced into the crude argon column
and
rectified in the latter. The cross-section of the crude argon column must be
selected in a
correspondingly large size, by which considerable investment costs arise.
In particular from the field of hydrocarbon recovery, it is already known to
use
so-called partition columns to separate ternary mixtures. In the case of a
partition
column, a portion of the column is divided into two subsections by a wall that
is arranged
in the lengthwise direction of the column. Above and below the partition, the
two
subsections are connected on the side of the flow in each case. In a
corresponding
approach, a three-component mixture that is introduced into the subsection on
one side of
the partition can be separated into three fractions in a single column. The
lowest-boiling
component can be recovered at the head of the partition column, the middle-
boiling
component can be recovered on the side of the partition that is opposite to
the feed, and
the highest-boiling component can be recovered from the bottom. In comparison
to a
column without a partition, higher concentrations of the middle-boiling
component can
be achieved with the partition column in the lateral draw-off.
In low-temperature air separation, partition columns have hardly been used to
date
because of their difficult adjustment. In EP 0 638 778 B1, a process for low-
temperature
separation of air in a partition column is described. The low-pressure column
is divided
by a partition in a middle area. On one side of the partition, bottom liquid
is fed from the
pressure column, while on the other side of the partition, the argon-
containing fluid is
drawn off. For better adjustment of the process, a waste fluid is removed on
the side of
CA 02502706 2005-04-15
3
the partition, in which the bottom liquid is introduced. The process
parameters are
selected such that the recovered argon-containing fluid has an argon
concentration of at
least 70%.
In the case of a product requirement in the range of 70% argon concentration,
the
number of theoretical plates in the crude argon column can be reduced with the
process
described in EP 0 638 778 B1, and thus overall height is saved. If, however,
high argon
concentrations of, for example, more than 95% are called for, the advantages
of a
concentration of the fluid that is drawn off from the low-pressure column and
fed to the
crude argon column to values of above 70% argon are always smaller. The reason
for
this is that to achieve higher argon concentrations, the majority of the
theoretical plates in
the crude argon column are necessary to remove the last remnants of oxygen
from the
argon. That is to say, in the case of high purity requirements, the starting
concentration
of the fluid that is introduced into the crude argon column plays a lesser
role.
The object of this invention is therefore to demonstrate an improved process
for
recovering argon by low-temperature air separation.
This object is achieved according to the invention by a process of the above-
mentioned type, whereby the argon concentration in the stream that is removed
in the
second subsection is between 15% and SO%, preferably between 15% and 40%,
especially preferably between 20% and 35%.
The invention is based on the knowledge that in the case of a specified amount
and purity of the argon product, an increase in the starting concentration of
argon in the
stream that is introduced into the crude argon column entails a reduction of
the amount of
CA 02502706 2005-04-15
4
vapor that is to be moved. This is positive in as much as the cross-section of
the crude
argon column is correspondingly reduced, and costs can be saved.
Such an increase in the argon concentration in the lateral draw-off of the air
separation column, however, is associated with a complicated design of the air
separation
column and a more expensive adjustment. It can also be noted that the
advantages of the
argon concentration in the lateral draw-off from the air separation column are
always
smaller in the case of high product requirements, since, as described above,
in this case
the number of theoretical plates in the crude argon column essentially depends
on the
final concentration to be achieved and not on the starting concentration.
Studies have now shown that the minimum amount of vapor that must be fed to
the crude argon column for proper operation first decreases with increasing
argon
concentration, but remains the same starting from an argon concentration of
50%. That is
to say, an additional argon concentration in the lateral draw-off to values of
over 50%
does not bring any further reduction of the amount of vapor to be introduced
into the
crude argon column and thus entails no possibility of an additional cross-
section
reduction of the crude argon column. There remains only the advantage of a
higher argon
concentration in the mixture that is fed to the crude argon column. Since,
however, in the
case of high requirements of argon purity, the number of theoretical plates in
the crude
argon column essentially depends on the starting concentration, a further
increase in the
argon concentration in the stream that is removed from the air separation
column is no
longer useful. Within the scope of this invention, this case was examined in
more detail,
and it was found that an argon concentration of between 15% and SO% in the
stream that
is removed in the second subsection is especially advantageous.
CA 02502706 2005-04-15
S
In practice, it has turned out that an approach in which a stream with an
argon
concentration of between 15% and 40%, preferably between 20% and 35%, is
removed
from the second subsection brings special advantages.
The invention is especially advantageous when using a partition column. In
this
case, the rectifying system has at least one air separation column, which has
three
rectifying sections that are arranged in series, whereby in each case adjacent
rectifying
sections can be connected to one another on the gas and liquid sides. The
middle
rectifying section has a partition that divides the rectifying section into
two subsections.
Inside the second rectifying section, a gas and liquid exchange between the
two
subsections is prevented by the partition. Both subsections, however, are
connected on
the flow side to the rectifying sections located above and below.
Instead of a partition column, the division into two subsections that are
flushed in
a parallel manner can also be carried out by two columns that are arranged
parallel to one
another. Liquid is drawn off at an intermediate point for a first air
separation column and
fed to a second column. Gas is drawn out from the first air separation column
at a second
intermediate point and introduced into the second column. Gas and liquid,
produced at
the head of the second column, from the bottom of the second column are
returned to the
first air separation column, preferably at the two intermediate points. The
two
subsections that are separated on the flow side are not produced in this
embodiment by a
partition but rather by two columns that are connected in parallel.
The stream that is removed from the second subsection, either from the air
separation column or from the second column, depending on the design, is
preferably
directed into a crude argon column. The bottom liquid that essentially
contains oxygen,
CA 02502706 2005-04-15
6
accumulating there, is preferably returned to the second subsection, i.e., in
the subsection
in which the argon-containing fraction is also removed.
The invention is preferably suitable for a rectifying system, which has a
pressure
column and a low-pressure column, whereby the partition in the low-pressure
column is
arranged and whereby a fluid, concentrated with oxygen, from the pressure
column,
preferably bottom liquid, is introduced into the first subsection.
The advantages of the process according to the invention are shown in
particular
if argon with a high purity of more than 95%, preferably more than 98%, and/or
argon
with an oxygen content of less than 100 ppm, preferably less than 10 ppm, is
to be
recovered in the crude argon column. The invention is then especially
advantageous if
more than 100 theoretical plates, preferably between 150 and 200 theoretical
plates, are
used in the crude argon column. In these cases, the overall height of the
crude argon
column is determined in any case by the number of theoretical plates necessary
for the
high final purity. The diameter of the crude argon column can be significantly
reduced,
however, compared to the conventional process without a partition column.
In the air separation column, packings are preferably used for rectification.
In this
connection, it is advantageous if the packings are arranged in several areas
that lie on top
of one another, so-called beds, whereby the liquid to be rectified and/or the
gas to be
rectified are collected between two beds each and redistributed onto the next
packing bed.
If, instead of packings, other internals or devices are used for rectification
in the air
separation column, it has also proven of value to provide collectors and/or
distributors at
specific intervals in the air separation column to be able to counteract poor
distribution in
the column.
CA 02502706 2005-04-15
7
The partition between the two compartmented areas in the air separation column
preferably ends in each case on the upper end or the lower end of a packing
bed, or when
other column internals are used on the upper or lower end of the corresponding
area,
which is divided from the adjacent area by a collector/distributor. Since, in
any case,
collectors/distributors are arranged at these points of impact of two column
areas, no
additional collectors/distributors have to be provided when using the
partition. Only the
collector/distributor arranged directly above the partition has to be
modified, such that it
distributes the liquid in the desired way to the two subsections that are
divided from one
another by the partition. The equivalent holds true if instead of a partition
column, a
second column that is arranged parallel to the first air separation column is
used.
It has proven especially advantageous to divide the air separation column, in
particular the low-pressure column of a double column system, into four areas,
or when
using packings, into four packing beds, and to provide the partition at the
level of the
second and the third area.
In the first and second subsection, metabolic elements that produce the same
pressure drop for the rising gas are preferably used.
The invention as well as additional details of the invention are explained in
more
detail below based on the embodiments that are depicted in the drawings. In
this
connection:
Figure 1 shows a device for implementing the process according to the
invention,
Figure 2 shows another embodiment according to the invention,
Figure 3 shows the specific amount of vapor to be fed to the crude argon
CA 02502706 2005-04-15
8
column based on the argon concentration thereof, and
Figure 4 shows the argon yield based on the argon concentration in the
vapor fed to the crude argon column.
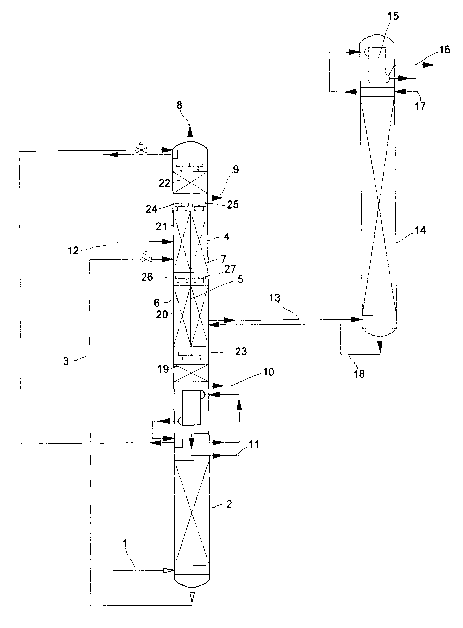
In Figure 1, the rectification portion of a low-temperature air separation
unit with
argon recovery is depicted. After corresponding purification and cooling,
charging air 1
is introduced into pressure column 2. The oxygen-concentrated liquid that
collects in the
bottom of pressure column 2 is conveyed via line 3 into low-pressure column 4.
Low-pressure column 4 is implemented as a partition column. As rectification
elements, packings, which are arranged in several beds 19, 20, 21, 22 that are
located on
top of one another and which in each case have a height of about 6 m, are
provided in
low-pressure column 4. Between two beds each, collectors/distributors 23, 24,
25, 26, 27
are provided for collecting and distributing the liquid that flows downward in
low-
pressure column 4.
In a middle area of low-pressure column 4, a partition S is arranged, such
that
low-pressure column 4 is divided into two subsections 6, 7. Partition 5
extends in this
case over the entire length of the two middle packing beds 20 and 21. A gas
and liquid
exchange between the two separate subsections 6, 7 is not possible in this
area.
Beds 19 and 22 below and above separate subsections 6, 7 extend, however, over
the entire cross-section of low-pressure column 4, such that the gas or liquid
streams that
separately flow out or flow down into two subsections 6, 7 are merged again.
In compartmented subsection 6, low-pressure column 4 is fed bottom liquid from
pressure column 2 via line 3. Moreover, turbine air can be introduced into low-
pressure
column 4 via line 12. At the head of low-pressure column 4, gaseous product
nitrogen
CA 02502706 2005-04-15
9
can be recovered via line 8. Also, above compartmented subsections 6, 7, a
draw-off 9
for impure nitrogen is provided. Gaseous or liquid product oxygen can be
removed from
the bottom of low-pressure column 4 via lines 10 and 11.
In two subsections 6 and 7, packings with identical specific surfaces are
installed.
The vapor that rises in low-pressure column 4 thus experiences the same
pressure loss in
both subsections 6, 7. The liquid that flows out is distributed in the two
subsections 6, 7
by means of distributors 24, 25. The same amount of liquid is preferably
released to both
subsections 6, 7. To optimize the way the process is performed, however, it
may very
well be useful to provide different liquid throughputs in subsections 6 and 7.
The
distribution of the rising vapor to the two subsections 6, 7 is advantageously
adjusted
automatically based on the amounts of liquid flowing toward it and the
pressure losses in
packing beds 20, 21.
A stream 13 that essentially contains argon and oxygen with an argon
concentration of 35% is drawn off from subsection 7 and introduced into a
crude argon
column 14 that is provided with packings. In crude argon column 14, the oxygen-
argon
mixture is rectified. At the head of crude argon column 14, the argon that is
produced in
a top condenser 15 is condensed and partially recovered as product 16 with a
residual
oxygen content of less than 10 ppm, and released to portion 17 as reflux
liquid in turn to
crude argon column 14. In the bottom of crude argon column 14, liquid oxygen,
which is
fed via line 18 into divided subsection 7 of low-pressure column 4, collects.
In low-pressure column 4, the feeding of bottom liquid 3 from pressure column
2
and turbine air 12 from argon draw-off 13 are separated by partition S. In
this way,
CA 02502706 2005-04-15
considerably higher argon concentrations can be adjusted in argon draw-off 13
than in
columns without partitions.
In Figure 2, an embodiment of the invention is depicted, in which instead of
partition 5, a parallel side column 30 is provided. The same elements are
provided in
both figures with the same reference numbers.
In this case, low-pressure column 4 is designed without a partition. The
liquid
that flows down from rectification section 22 is distributed, on the one hand,
by means of
distributor 24 to beds 20, 21, which form the first subsection. The second
subsection is
produced by side column 30. A portion of the liquid that flows down from
packing bed
22 is drawn off via line 31 from low-pressure column 4 and fed to side column
30 at the
head. Gas that is produced at the head of side column 30 is returned via line
32 above
packing bed 21 into low-pressure column 4. In a corresponding way, liquid from
side
column 30 is directed via line 33 into low-pressure column 4 or gas from low-
pressure
column 4 is directed by means of line 34 into side column 30.
The procedures in the embodiments according to Figure 1 and Figure 2 are
identical, whereby only in Figure 2, rectifying sections 20, 21 of low-
pressure column 4
represent first subsection 6, and side column 30 represents second subsection
7. In a
corresponding way, streams 3, 12 are introduced into low-pressure column 4,
while
argon-containing stream 13 of side column 30 is removed.
Within the scope of this invention, the specific amount of vapor to be fed to
crude
argon column 14, i.e., the amount of vapor relative to the amount of argon
product, was
determined by simulations based on the argon concentration of the vapor. The
determined dependence is shown in Figure 3. In this case, the process started
from an
CA 02502706 2005-04-15
11
argon product purity of 98.5% and a constant argon yield, i.e., a constant
ratio of argon
product to the amount of argon in the charging air.
The solid curve shows the theoretical minimum amount of vapor in the case of a
theoretically infinite number of plates. The dotted curve indicates the trace
of the
conditions calculated for a theoretical plate number of 50. Both curves have
essentially
the same course. It can be gathered from the curve for the finite plate
number, however,
that in this case, compared to the theoretical curve, about 30 to 40% larger
amounts of
vapor have to be used.
Both curves show that first with increasing argon concentration, increasingly
less
vapor in crude argon column 14 has to be converted to obtain argon of the
desired purity
and amount. The curves, however, in each case approach a lower boundary value
at
about 50% argon concentration. In the case of higher argon concentrations, no
drop or
only a slight further drop of the amount of vapor to be fed is to be expected.
Because of the dropping amount of vapor with the increasing argon
concentration
in the feed stream to crude argon column 14, the latter can be made
correspondingly
smaller in its diameter. The reduction of the amount of vapor, however, can be
observed
only up to an argon concentration of about 50%. With an increase of the
concentration to
over SO%, however, no further reduction of the amount of vapor can be achieved
under
the existing conditions, such that also no further reduction in size of the
crude argon
column cross-section can be achieved. The cost of adjustment associated with
the
increase in concentration in the low-pressure column increases significantly,
however.
Also, the number of theoretical plates in crude argon column 14 cannot be
significantly lowered by an increase of the argon concentration in vapor 13
that is to be
CA 02502706 2005-04-15
12
fed in the case of a desired product purity of 98.5%, since at high product
purities, the
number of plates is determined by the final concentration to be achieved and
not by the
starting concentration.
Low-pressure column 4 is operated, according to the invention, such that in
lateral
draw-off 13, an argon concentration of 45% is achieved. At this concentration,
the
amount of vapor that is introduced into crude argon column 14 can be
minimized, and the
diameter of crude argon column 14 corresponding to the amount of vapor can be
reduced.
Figure 4 shows the argon yield based on the argon concentration of the vapor
that
is fed into the argon column. The solid curve represents the calculated values
for a short
partition, the dotted curve for a long partition. The plate number in the low-
pressure
column was kept constant in this case.
On the solid curve, it is shown that the argon yield remains essentially
constant in
a range of between 10 and 25% argon concentration in the feed vapor. The curve
breaks
off at 25%, since no higher argon concentrations can be achieved with the
partition length
used. In the case of a longer partition, upon which the calculation of the
dotted curve was
based, an essentially constant argon yield can be observed even in the area of
higher
argon concentrations above 30% up to 90%. An increase in the argon
concentration
consequently does not have a negative effect on the yield.