Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
A PROCESS FOR THE MANUFACTURE OF SULPHUR-CONTAINING
AMMONIUM PHOSPHATE FERTILIZERS
FIELD OF THE INVENTION
The invention relates to a process for the
manufacture of sulphur-containing ammonium phosphate
fertilizers.
The invention further relates to sulphur-containing
fertilizers of the ammonium phosphate type, such as
sulphur-containing diammonium phosphate (S-DAP), sulphur-
containing mono-ammonium phosphate (S-MAP) or ammonium
phosphate based sulphur-containing nitrogen-phosphorous-
potassium compounds (S-NPK).
The invention further relates to the use of these
sulphur-containing fertilizers, especially to grow
agricultural products on sulphur-deficient soil.
The invention also relates to the agricultural
products grown on soil, especially on sulphur-deficient
soil which has been treated with the sulphur-containing
fertilizers of the present invention.
BACKGROUND OF THE INVENTION
In the past a tremendous amount of work has been
devoted to the manufacture of sulphur-containing
fertilizers. The growing worldwide demand for sulphur-
containing fertilizers stems from the discovery that low
crop yields in certain cases may be related to
deficiencies in sulphur in the soil. An example of a
species with high sulphur requirements is Canola. Canola
is an important cash crop in Alberta, Canada, and has
high sulphur requirements at any growth stage. A shortage
of sulphur can cause serious reductions in crop yield.
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
2 -
Manufacturing processes for sulphur-containing
fertilizers of the ammonium phosphate type often involve
the use or incorporation of sulphates, see e.g.
US 4,377,406, or US 4,762,546. A disadvantage of
sulphates is that they are very mobile in the soil and
leachable. Elemental sulphur is not leached out of the
soil, as are sulphates. It is therefore more advantageous
to have the sulphur present as elemental sulphur.
Furthermore, elemental sulphur offers some additional
benefits in fertilizers: elemental sulphur acts as a
fungicide against certain micro organisms, as a pesticide
against certain soil and plant pests, it assists the
decomposition of plant residues and it improves
phosphorus and nitrogen utilization and reduces the pH of
alkaline and calcareous soils.
Thus, it is advantageous to incorporate sulphur as
elemental sulphur in the sulphur-containing fertilizers.
Processes for the manufacture of sulphur-containing
fertilizers, wherein elemental sulphur is used, are known
in the art. Most of the methods involve the incorporation
of molten sulphur into the fertilizer.
In US 5,653,782, a process for the manufacture of
sulphur-containing fertilizers has been described,
wherein a substrate containing fertilizer particles is
heated to a temperature above the melting point of
sulphur and admixed with sulphur. According to
US 5,653,782, the sulphur is melted by the heat provided
by the preheated fertilizer particles, thereby producing
a homogeneous coating on the fertilizer particles.
US 3,333,939, describes the coating of ammonium
phosphate granules with molten sulphur. The granules are
coated in a separate coating unit into which the sulphur
is fed, by contacting the granules with molten sulphur or
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
3 -
with a solution of ammonium polysulphide. Subsequently,
the coated granules are dried. Alternatively,
US 3,333,939 teaches a process for preparing sulphur-
containing fertilizer particles in which the sulphur is
interspersed throughout the particles. In this process
ammonia and phosphoric acid are allowed to react to form
ammonium phosphate. The ammonium phosphate formed is fed
into a granulator in which it is mixed with urea and dry
sulphur. The granules obtained are dried in a dryer. The
disadvantage of the first process of US 3,333,939 is that
the coating prevents a uniform distribution of ammonium
sulphate and sulphur into the soil. The second process
has the disadvantage that it requires solid sulphur
handling. The handling and grinding of solid sulphur is
highly hazardous due to the dust and risks of explosions.
As mentioned in a review by H.P. Rothbaum et al (New
Zealand Journal of Science, 1980, vol. 23, 377),
explosion hazards are always due to sulphur dust which is
inflammable. Therefore, a more complex process design is
necessary to ensure the safety of the process.
US 5,571,303 discloses a process for the manufacture
of fertilizers in which first ammonia, water and
phosphoric acid are reacted to form ammonium phosphate.
Subsequently, the ammonium phosphate/water mixture is
mixed with molten sulphur. The mixture thus obtained is
kept at temperatures of 120-150 C until granulation. A
disadvantage of this process is that due to the
preforming of ammonium phosphate either much water is
needed to keep the salt dissolved or, when relatively
small amounts of water are used, solid ammonium phosphate
is formed. The homogeneous distribution of sulphur
throughout the granule may be hampered by the existence
of solid ammonium phosphate.
CA 02506093 2011-04-14
-4-
Since problems with the manufacture of fertilizers of the sulphur-
containing ammonium-phosphate type, continue to be in existence, there is a
need
for a manufacturing process for such fertilizers which would diminish or even
prevent the problems experienced in the art.
It has now been found that a process for manufacturing sulphur-containing
fertilizers, wherein sulphur is introduced as a liquid phase comprising
elemental
sulphur, offers advantages over the manufacturing processes known in the art,
with regard to safety aspects as well as with respect to process control.
The process according to the invention enables the manufacture of
fertilizers with an even distribution of sulphur throughout the fertilizer
product,
thereby enhancing conversion in the soil to plant usable form, namely
sulphates.
The fertilizer is thus enabled to deliver sulphates to the intended crop in a
more
reliable and consistent manner.
SUMMARY OF THE INVENTION
The invention therefore provides a process for the manufacture of sulphur-
containing fertilizers comprising the steps of.
(a) mixing ammonia, phosphoric acid and water in a reactor unit to obtain an
ammonium phosphate mixture;
(b) introducing the mixture obtained in step (a) into a granulator unit to
obtain
granules, wherein a liquid phase comprising elemental sulphur is brought into
contact with ammonia, phosphoric acid and water in the reactor unit in step
(a) or
is introduced in the granulator unit in step (b).
More especially, the process comprises the steps of:
(a) bringing a liquid phase comprising elemental sulphur into contact with
ammonia, phosphoric acid and water in a reactor unit to obtain an ammonium
phosphate mixture, wherein the elemental sulphur is introduced into the
reactor
unit substantially at the same time as the other reactants; and
(b) introducing the mixture obtained in step (a) into a granulator unit to
obtain
granules.
BRIEF DESCRIPTION OF THE DRAWING
Fig. 1 depicts a typical process scheme of the process according to the
invention for the manufacture of sulphur-containing ammonium phosphate
fertilizers.
DOCSMTL: 3023028\1
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 5 -
DETAILED DESCRIPTION OF THE INVENTION
In step (a) of the process according to the
invention, ammonia, phosphoric acid and water are
contacted in a reactor unit to obtain an ammonium
phosphate mixture. The phosphoric acid is typically
manufactured by reacting sulphuric acid with phosphate or
is commercially available phosphoric acid. Suitable
phosphoric acids are for example orthophosphoric acid, or
pyrophosphoric acid or mixtures thereof. To avoid the
introduction of excess process water, the ammonia is
preferably introduced as a concentrated aqueous solution
or as anhydrous gaseous ammonia. The advantage of having
a mixture with as little water as possible is that any
additional water introduced into a fertilizer process
must be handled in the process and eliminated at a later
stage. Therefore, any additional water introduced into
fertilizer manufacturing processes leads to a more
complex process. Preferably, the water content in the
ammonium phosphate mixture is kept as low as possible,
preferably between approximately 10 and 20% based on the
total weight of the mixture, more preferably between 12
and 15% based on the total weight of the mixture.
The amounts of ammonia and phosphoric acid are
adjusted to achieve the desired products. For the
production of S-MAP, the molar ratio of ammonia and
phosphoric acid is typically kept between values of about
0.5-1.0, for the production of S-DAP the molar ratio of
ammonia and phosphoric acid is typically kept between
values of about 1.2-2.0 and for the production of S-NPK
the molar ratio of ammonia and phosphoric acid is
typically kept between values of about 0.7-1.7. Preferred
values for the ammonia:phosphoric acid molar ratios are
about 0.6-0.8 for the production S-MAP, 1.3-1.8 for the
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 6 -
production S-DAP and about 1.0-1.5 for S-NPK. More
preferred values for the ammonia:phosphoric acid molar
ratios are about 0.7 for the production S-MAP, about 1.5
for the production S-DAP and about 1.3 for NPK.
Typically, the mixing takes place at atmospheric
pressure and at temperatures between about 100 C and
about 130 C. Preferably, water or sulphuric acid is
added to the reactor unit to control the temperature of
the mixture. Typically, water is added when a temperature
reduction is needed, sulphuric acid is added to when a
temperature increase is needed.
In one embodiment of the process according to the
invention, a liquid phase comprising elemental sulphur is
brought into contact with ammonia, phosphoric acid and
water in the reactor unit in step (a). In a preferred
embodiment, ammonia, phosphoric acid, water and a sulphur
slurry comprising a sulphur dispersion are mixed in a
reactor unit to obtain an ammonium phosphate mixture;
this mixture is then introduced into a granulator unit to
obtain granules. In another embodiment of the process
according to the invention, ammonia, phosphoric acid and
water are mixed in a reactor unit to obtain an ammonium
phosphate mixture; this mixture is then introduced into a
granulator unit to obtain granules, while a liquid phase
comprising elemental sulphur is also introduced in the
granulator unit in step (b).
In a preferred process according to the invention,
elemental sulphur is introduced into the reactor unit in
step (a) substantially at the same time as the other
reactants. It has been found that the crushing strength
of the granules can be improved if the sulphur is added
into the reactor unit in step (a).
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
7 -
In a preferred process, the elemental sulphur is
introduced as a slurry of water and sulphur particles.
Typically, the sulphur particles are dispersed or
suspended in the slurry. Preferably, the particles have a
size ranging from between about 0.5 to about 150 microns,
preferably between about 1.0 and about 100 microns. To
avoid the removal of excess water at a later stage in the
process, the water content in the sulphur slurry is
typically kept as low as possible, preferably between
approximately 10 and 40% based on the total weight of the
mixture, more preferably between 15 and 30% based on the
total weight of the slurry. In the case where the sulphur
particles are suspended in the slurry, the sulphur slurry
is preferably stirred or mixed in a suitable apparatus to
homogenise the slurry prior to introducing it into the
manufacturing process. In a preferred embodiment, the
sulphur slurry contains sulphur particles which are
dispersed in the water. This type of slurry, henceforth
referred to as dispersed or emulsified sulphur slurry,
comprises dispersed sulphur particles in water,
preferably dispersed micron-sized sulphur particles in
water. The sulphur particles are suitably kept in
dispersion through the addition of a suitable emulsifier.
Suitable emulsifiers are known in the art and are not
critical to the invention. An advantage of using
dispersed sulphur particles is that the precipitation of
sulphur particles is kept to a minimum and the sulphur is
distributed more homogeneously throughout the water.
Thus, the need for stirring or mixing prior to
introducing the sulphur slurry into the reactor unit is
reduced. Typically, the slurry is introduced by pumping
the slurry from a sulphur slurry reservoir unit into the
reactor unit.
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
8 -
In yet another preferred process according to the
invention, the elemental sulphur is introduced into the
reactor unit in step (a) as molten sulphur. Molten
sulphur can be obtained from solid sulphur, by melting in
a suitable melting apparatus, for instance a tube melter.
The use of molten sulphur is advantageous when
sulphur is obtained in the molten state from an
industrial process. Processes for the removal of unwanted
sulphur components from natural gas usually produce
sulphur in the molten state and the use of this molten
sulphur directly in the fertilizer manufacturing process
according to the invention avoids the need for additional
steps, such as drying and grinding of the sulphur, to
obtain a sulphur slurry. An additional advantage of using
molten sulphur is that no additional water is introduced
into the fertilizer manufacturing process. When adding
elemental sulphur in the molten state, the temperature of
the sulphur-containing mixture is preferably kept above
the melting point of sulphur, preferably between
temperatures of 115 C and 121 C.
In an especially preferred process according to the
invention, biologically produced elemental sulphur is
used. Reference herein to biologically produced elemental
sulphur is to sulphur obtained from a process wherein
sulphur-containing components, such as sulphides or H2S,
are converted to elemental sulphur via biological
conversion. Biological conversion can suitably be
effected using sulphide-oxidising bacteria. Suitable
sulphide-oxidising bacteria can be selected for instance
from the known autotropic aerobic cultures of the genera
Thiobacillus and Thiomicrospira. An example of a suitable
biological conversion process to obtain the biologically
produced elemental sulphur suitable for the process
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
9 -
according to the invention is the process for the removal
of sulphur compounds from gases wherein the gas is washed
with an aqueous washing liquid and the washing liquid is
subjected to sulphide-oxidising bacteria, as described in
WO 92/10270. Biologically produced elemental sulphur has
a hydrophilic nature, making it especially suitable for
agricultural use as fertilizer due to the relative ease
with which the biologically produced sulphur is taken up
by the soil. An additional advantage of biologically
produced elemental sulphur is that fouling or blocking of
equipment is substantially reduced or even eliminated due
to the hydrophilic nature.
The reactor unit used in step (a) is any device
wherein the ammonia, phosphoric acid and water are
reacted to obtain an ammonium phosphate mixture, for
example a pipe cross reactor or a preneutralizer unit. A
preneutralizer unit comprises a tank reactor equipped
with mixing equipment and appropriate inlet and outlet
devices. In a preferred embodiment, a liquid phase
comprising elemental sulphur is introduced in step (a)
and a preneutralizer unit is used. In the preneutralizer
unit, the starting components are mixed using a stirring
device and ammonia is typically introduced as gaseous
ammonia. The advantage of using a preneutralizer unit
when elemental sulphur is introduced in step (a) is that
a larger amount of sulphur can be used without
experiencing operating problems such as clogging, most
likely due to a more effective mixing. Another advantage
of using a preneutralizer unit in step (a) is that the
resulting sulphur-containing granules obtained after
step (b) are stronger, reflected in their higher crush
strength, even at higher amounts of sulphur in the
granule. In a pipe cross reactor, the liquid phase
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 10 -
comprising elemental sulphur, water and phosphoric acid
are simultaneously fed in a pipe reactor through which
the reactants are passed.
After step (a), a mixture comprising ammonium
phosphate, water and optionally elemental sulphur is
obtained. In step (b) of the process according to the
invention, this mixture is introduced into a granulator
unit to obtain granules.
In a preferred embodiment, ammonia, phosphoric acid
and water are mixed in a reactor unit to obtain an
ammonium phosphate mixture; this mixture is then
introduced into a granulator unit to obtain granules,
while a slurry comprising elemental sulphur is also
introduced in the granulator unit in step (b).
In a preferred process, the elemental sulphur is
introduced into the granulator unit as a slurry of water
and sulphur particles, including suspended sulphur or
dispersed sulphur, as hereinbefore described.
In an especially preferred process according to the
invention, the elemental sulphur is introduced into the
granulator unit as a slurry of water and biologically
produced elemental sulphur particles, including suspended
sulphur or dispersed sulphur as hereinbefore described.
Reference herein to a granulator is to a device for
forming granules or pellets of fertilizer product.
Commonly used granulators are described in Perry's
Chemical Engineers' Handbook, chapter 20 (1997).
Preferred granulators are drum granulators or pan
granulators. Typically, the mixture is pumped and
distributed on a rolling bed of material in a drum
granulator. In the granulator, granules are formed.
Reference herein to granules is to discrete particles
comprising ammonium phosphate and elemental sulphur.
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 11 -
Optionally, ammonia can be introduced in the granulator
to complete the ammoniation of the ammonium phosphate
mixture. Optionally, water and steam can also be fed to
the granulator to control the temperature of the
granulation process as needed.
Optionally, additional ammonia and/or recycled
fertilizer particles may be added to the granulator unit.
Recycled fertilizer particles add granulation and
nucleating agents. They are obtained from the final
fertilizer product. Suitably they have small particle
sizes (so-called off-spec fines). The recycle of fines is
also described in Us 3,333,939.
Other ingredients may be added during the
manufacturing process to tailor the fertilizer products
to their intended end-use. Examples include plant micro
nutrients such as boron, potassium, sodium, zinc,
manganese, iron, copper, molybdenum, cobalt, calcium,
magnesium and combinations thereof. These nutrients may
be supplied in elemental form or in the form of salts,
for examples as sulphates, nitrates or halides. In this
way, granules enriched in plant nutrients are obtained.
The amount of plant micronutrients depends on the type of
fertilizer needed and is typically in the range of
between 0.1 to 5%, based on the total weight of the
granules.
The sulphur-containing ammonium phosphate granules
obtained after the granulation step are optionally dried
in a drying unit. In a preferred embodiment, the granules
are air-dried in the drying unit, thereby avoiding the
need for additional drying equipment. Alternatively,
drying units wherein heat transfer for drying is
accomplished by direct contact between the wet solid and
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 12 -
hot gases are used, thereby enabling a faster drying
step. Typically, the drying unit is a rotary dryer.
In a preferred process according to the invention,
the granules are sorted on their size in a sorting unit
to achieve a more uniform size distribution. Typically,
oversized granules are crushed and returned to the
sorting unit while undersized granules are returned to
the granulator as so-called off-spec fines. A preferred
size range for the granules is between about 1.5 and
5.0 mm, more preferably between about 2 and 4 mm,
expressed as the average diameter of the granules. The
use of granules which fall within this range is more
likely to enable a more even distribution of the
fertilizer ingredients in the soil after applying the
granules to the soil.
It will be appreciated that the process parameters in
the reactor unit and in the granulator unit have to be
adjusted depending on the desired products.
After a typical manufacturing process according to
the invention, sulphur-containing monoammoniumphosphate,
sulphur-containing diammoniumphosphate or sulphur-
containing NPK (nitrogen-phosphorous-potassium)
fertilizer granules, optionally enriched in plant
nutrients, are obtained. The sulphur in the sulphur-
containing fertilizer granules according to the invention
may be incorporated into the fertilizer granules, or the
sulphur may be distributed on the granules or the sulphur
may be both incorporated into the granules and be
distributed on the granules. The content of elemental
sulphur in these fertilizer granules is typically up to
25%, based on the total weight of the fertilizer,
preferably between 2 and 18%, more preferably between 5
and 15%. An elemental sulphur content higher than 25%
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 13 -
will generally lead to a less even distribution of
sulphur on and throughout the granules, due to clustering
of elemental sulphur. In addition, the crush strength of
granules decreases with an increasing elemental sulphur
content. The most homogeneous distribution of sulphur on
and throughout the granules is achieved when the content
of elemental sulphur is between 5 and 15%, based on the
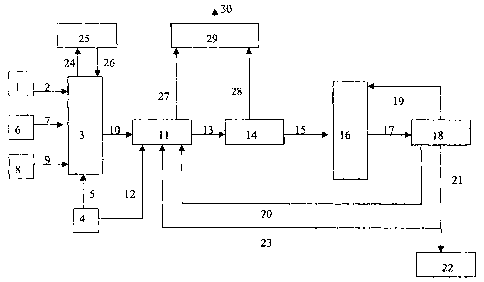
total fertilizer granule.
The invention will now be illustrated by means of
schematic figure 1.
Figure 1 depicts a typical process scheme of the
process according to the invention, wherein the elemental
sulphur is introduced in step (a).
Phosphoric acid is led from tank (1) via line (2) to
a reactor (3). Gaseous ammonia is led from tank (4) via
line (5) to reactor (3). Water is led from tank (6) via
line (7) to reactor (3). Sulphur is led from tank (8) via
line (9) to reactor (3).
In reactor (3), the anhydrous ammonia and phosphoric
acid are reacted to form a sulphur-containing ammonium-
phosphate mixture. This mixture is pumped via line (10)
to a drum granulator (11), where it is introduced on top
of a rolling bed of fertilizer material. Gaseous ammonia
is led from tank (4) via line (12) into the drum
granulator to increase the mole ratio to approximately
1.8 or 1.0 when producing S-DAP or S-MAP respectively.
In granulator (11), moist sulphur-containing
ammonium-phosphate granules are formed. The moist
granules are led via line (13) to a rotary dryer 14). In
the rotary dryer (14), the granules are dried. The dried
granules are led via line (15) to a sizing unit (16).
In the sizing unit, granules that are too large or
too small, relative to a pre-determined granules size,
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 14 -
are removed from the granules stream. The oversized
granules are led via line (17) to a crusher (18) where
they are crushed. The crushed granules are returned via
line (19) to the sizing unit. The undersized granules are
recycled via line (20) to the granulator. The granules
with a size range of between 2.0 and 4.0 mm are led via
line (21) to a cooler (22) where they are cooled. A
portion of granules with a size range of between 2.0 and
4.0 mm is recycled via line (23) to the drum granulator
to help control the granulation process.
Ammonia and water vapours escaping from reactor (3)
are led via line (24) to a wet scrubber unit (25), where
they are scrubbed with phosphoric acid. The scrubber
liquid containing ammonium phosphate is led back via
line (26) to reactor (3).
The air and dust collected from the drum granulator,
dryer discharge elevator and drum granulator surroundings
are led via lines (27) and (28) to a suitable
commercially available wet scrubber (29) where they are
treated and then vented via line (30) to the atmosphere.
The invention will now be illustrated by means of the
following non-limiting examples.
EXAMPLE 1 (comparative)
DAP granules without added sulphur were prepared
using the process according to schematic figure 1, but
without added sulphur from tank (8). A preneutralizer
reactor was used as reactor (3). The reaction mixture in
the preneutralizer reactor was maintained at 115 C, with
a NH3:H3P04 mole ratio of 1.42. Chemical analysis of the
resulting granules indicated 19.0% N, 50.5% P205 and 0.9%
sulphate sulphur (expressed as weight percentages based
on the total weight). The average crush strength of the
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 15 -
granules, the minimum force required to crush an
individual granule, was 4.7 kg/granule.
EXAMPLE 2 (according to the invention)
DAP granules with added sulphur were prepared using
the process according to schematic figure 1. The reactor
used was a preneutralizer reactor. The reaction mixture
in the preneutralizer reactor was maintained at 117 C,
with a NH3:H3P04 ratio of 1.44. Chemical analysis of the
resulting granules indicated 15.7%N, 41.8% P205, 0.6%
sulphate sulphur and 17.6% elemental sulphur (expressed
as weight percentages based on the total weight).
Scanning electron microscopy (SEM) analysis was performed
to evaluate if the added sulphur was evenly dispersed in
the fertilizer granules. SEM analysis of the granules and
of split granules indicated that the sulphur was
distributed both on the surface of the granules and
throughout the granules. The average crush strength of
the granules was 4.3 kg/granule.
EXAMPLE 3 (comparative)
MAP granules without added sulphur were prepared
using the process according to schematic figure 1, but
without added sulphur from tank (8). A pipe cross reactor
was used as reactor (3). The reaction mixture in the pipe
cross reactor was maintained between 120 and 126 C, with
a NH3:H3P04 mole ratio of 0.67. Chemical analysis of the
resulting granules indicated 11.3% N, 56.0% P205 and
1.0% sulphate sulphur (expressed as weight percentages
based on the total weight). The average crush strength of
the granules was 4.8 kg/granule.
EXAMPLE 4 (according to the invention)
MAP granules with added sulphur were prepared using
the process according to schematic figure 1. The reactor
used was a pipe cross reactor. Sulphur was added as
CA 02506093 2005-05-12
WO 2004/043878 PCT/EP2003/050821
- 16 -
emulsified sulphur. The emulsified sulphur was agitated
in a container and then transferred directly from the
container to the sulphur feed tank (8). The reaction
mixture in the pipe cross reactor was maintained at about
122 C, with a NH3:H3P04 ratio of 0.69. Chemical analysis
of the resulting granules indicated 10.3% N, 50.3% P205,
0.7% sulphate sulphur and 11.0% elemental sulphur
(expressed as weight percentages based on the total
weight). Scanning electron microscopy (SEM) analysis was
performed to evaluate if the added sulphur was evenly
dispersed in the fertilizer granules. SEM analysis of the
granules and of split granules indicated that the sulphur
was distributed both on the surface of the granules and
throughout the granules. The average crush strength of
the granules was 4.2 kg/granule