Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02509581 2005-06-10
DESCRIPTION
HIGH-STRENGTH MARTENSITIC STAINLESS STEEL WITH
EXCELLENT RESISTANCES TO CARBON DIOXIDE GAS
CORROSION AND SULFIDE STRESS CORROSION CRACKING
FIELD OF THE INVENTION
The present invention relates to a steel material suitable for its use in
severe
corrosion environment containing corrosive materials such as carbon dioxide
gas,
hydrogen sulfide, chlorine ions and the like. Specifically, the present
invention
relates to a steel material for a seamless steel tube and a seam welded steel
tube
such as an electric resistance welding steel tube, a laser welding steel tube,
a spiral
welding tube or the like, which is used in applications for petroleum or
natural gas
production facilities, facilities for eliminating carbon dioxide gas, or for
geothermal
power generation, or for a tank for liquid containing carbon dioxide gas,
especially to
a steel material for oil well tubes fox oil wells or gas wells.
BACKGROUND ART
From the viewpoint of exhaustion of petroleum resources, which is expected
in the near future, development of an oil well under severe environment that
is an oil
well in a deeper layer, of a sour gas field or the like, has often been
performed. Thus,
high strength and excellent corrosion resistance and sulfide stress corrosion
cracking resistance are required for oil well steel tubes, which are used in
such uses.
As a steel material. for oil well tubes or the like, carbon steel or a low-
alloy
steel has been generally used. However, as the environment of the well becomes
severe, steel which contains increased amount of alloying elements, has been
used.
For example, as oil wells for steel material which contain a large amount of
carbon
1
CA 02509581 2005-06-10
dioxide gas, 13 Cr series martensitic stainless steel such as typical SUS 420
and the
like have been used.
However, although the SUS 420 steel has excellent corrosion resistance to
carbon dioxide gas, it has poor corrosion resistance to hydrogen sulfide.
Thus, the
SUS 420 steel is liable to generate sulfide stress-corrosion cracking (SSCC)
under
the environment containing carbon dioxide gas and hydrogen sulfide
simultaneously.
Therefore various steel materials in place of the SUS 420 steel have been
proposed.
Japanese Patent No. 2861024, Japanese Patent Application Publication No.
05-287455, and Japanese Patent Application Publication No. 07-62499 disclose
steel
having improved corrosion resistance by reducing carbon content of the SUS
420.
However, such a low carbon-content steel described in these publications may
not
have the enough strength required for use in a deep well, that is proof stress
of 860
MPa or more.
Alternatively, Japanese Patent Appilcation Publication No. 2000-192196
discloses steel of a maxtensitic single phase structure containing Co~ 0.5 - 7
% and
Mo: 3.1 - 7 % having high strength and excellent sulfide stress-corrosion
cracking
resistance. The invention described in the publication is a steel containing
Co in
the above-mentioned range to suppress the generation of retained austenite
during
cooling so that the structure is made to be a martensitic single phase.
However,
since Co is an expensive element, it is desirable not to use.
SITMMARY OF THE INVENTION
The present invention was made in consideration of the above-mentioned
circumstances. The object of the present invention is to provide a martensitic
stainless steel having sufficient strength to use for oil well tubes for a
deep well, that
is high strength of a proof stress of 860 MPa or more, and excellent carbon
dioxide
2
CA 02509581 2005-06-10
gas corrosion resistance and sulfide stress-corrosion cracking resistance
whereby it
can be used even under the environment containing carbon dioxide gas, hydrogen
sulfide or chlorine ions or two or more of them. The symbols of the respective
elements in the following expression show the content (mass %) of each
element.
Accordingly, the gist of the present invention is high strength maxtensitic
stainless steels described in the following (a) and (b).
(a) Ahigh strength martensitic stainless steel excellent in carbon dioxide gas
corrosion resistance and sulfide stress-corrosion cracking resistance and
having
0.2 % proof stress of 860 MPa or more, characterized by including, by mass %,
C:
0.005 - 0.04 %, Si: 0.5 % or less, Mn: 0.1 - 3.0 %, P: 0.04 % or less, S: 0.01
% or less,
Cr: 10 - 15 %, Ni: 4.0 - 8 %, Mo: 2.8 - 5.0 %, Al: 0.001- 0.10 % and N: 0.07 %
or less,
and the balance Fe and impurities, and also characterized by satisfying the
expression (I) given below wherein the microstructure mainly comprises
tempered
martensite, carbide precipitated during tempering, and intermetallic compounds
I5 such as Laves phase, a phase and the like finely precipitated during
tempering.
Mo ~ 2.3 - 0.89 Si + 32. 2 C ... (1)
wherein the symbols of the respective elements in the expression (1) show the
content (mass %) of each element.
Further, the gist of the present invention is martensitic stainless steels
containing at least one of alloying elements selected from at least one group
consisting of the following a first group, a second group and a third group,
in
addition to the components described in the above mentioned (a). In this steel
said
expression (1) is also satisfied and the microstructure is the same as
mentioned
above.
First group .... Ti: 0.005 - 0.25 %, V: 0.005 - 0.25 %, Nb: 0.005 - 0.25 %,
and Zr:
0.005 - 0.25 %.
3
CA 02509581 2005-06-10
Second group ... Cu: 0.05 - 1
Third group ... Ca: 0.0002 - 0.005 %, Mg: 0.0002 - 0.005 %, La: 0.0002 - 0.005
%,
and Ce: 0.0002 - 0.005 %.
(b) A high strength martensitic stainless steel excellent in carbon dioxide
gas
corrosion resistance and sulfide stress-corrosion cracking resistance and
having
0.2 % proof stress of 860 MPa or more, characterized by including compositions
defined in any one of (a) and characterized in that steel, which satisfies the
above
mentioned expression (1), is subjected to tempering in which (20 + log t)(T +
273)
satisfies 13500 - 17700 when, after quenching the steel at a quenching
temperature
of 880 °C - 1000 °C, a range of a tempering temperature is set
to 450 °C - 620 °C, a
tempering temperature is set to T (°C) and tempering time is set to t
(hour), whereby
the microstructure of said steel mainly comprises tempered martensite, carbide
precipitated during tempering, and intermetallic compounds such as a Laves
phase,
a a phase and the like finely precipitated during tempering.
BRIEF DESCRIPTION OF THE DRAWINGS
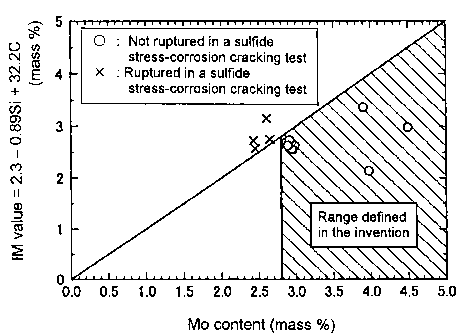
FIG. 1 is a view showing relationships between Mo contents of various types
of steels tested in examples and the right side in the expression (1), that is
"2.3 -
0.89 Si + 32. 2 C" (IM value).
FIG. 2 is a view for explaining tempering conditions defined in the present
invention, which shows relationships between 0.2 % proof stress obtained by
changing values of (20 + log t)(T + 273) while changing tempering temperatures
in
400 - 650 °C after quenching steel at 920 °C, and the (20 + log
t)(T + 273).
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The reasons for restrictions of contents of various elements defined in the
4
CA 02509581 2005-06-10
present inventors will be described hereinbelow. "%" of the respective
contents
means mass %.
C: 0.005-0.04
Although C (carbon) is an effective alloying element to enhance strength of
steel, from a viewpoint of corrosion resistance small C content is preferable.
However, if the content of C is less than 0.005%, proof stress does not reach
860 Mpa
or more. Thus, the lower limit of the C content was set to 0.005 %. On the
other
hand, if the C content exceeds 0.04 %, the hardness of the tempered steel
becomes
hard excessively, the steel has high sulfide stress-corrosion cracking
sensibility.
Accordingly, the C content was set to 0.005 - 0.04 %.
Si: 0.5 % or less
Si (Silicon) is an alloying element necessary as a deoxidizer. An amount of
Si retained in the steel may be a Ievel of impurities. However, to obtain a
large
deoxidation effect it is preferred that the Si content is set to 0.01 % or
more. On the
other hand, if the Si content exceeds 0.5 %, the toughness of the steel is
decreased
and the workability of the steel is also decreased. Accordingly, the Si
content was
set to 0.5 % or less.
Mn~0.1-3.0%
Mn (Manganese) is an effective alloying element to enhance the hot
workability. To obtain this effect Mn content of 0.1 % or more is needed. On
the
other hand, if the Mn content exceeds 3.0 %, the effect is saturated resulting
in an
increase in cost. Accordingly, the Mn content was set to 0.1- 3.0 %.
P: 0.04 % or less
P (Phosphorus) is an impurity element contained in the steel and the P
content is better as low as possible. Particularly, if the P content exceeds
0.04 %,
the sulfide stress-corrosion cracking resistance is remarkably decreased.
5
CA 02509581 2005-06-10
Accordingly, the P content was set to 0.04 % or less.
S: 0.01 % or less
S (Sulfur) is an impurity element contained in the steel and the S content is
better as low as possible. Particularly, if the S content exceeds 0.01 %, the
hot
workability, corrosion resistance and toughness are remarkably decreased.
Accordingly, the S content was set to 0.01 % or less.
Cr- 10 - 15
Cr (Chromium) is an effective alloying element to enhance the carbon
dioxide gas corrosion resistance. To obtain this effect Cr content of 10 % or
more is
needed. On the other hand, if the Cr content exceeds 15 %, it becomes
dif6.cult to
make the microstructure of tempered steel a martensite phase mainly
Accordingly,
the Cx content was set to 10 - 15 %.
Ni: 4.0 - 8
Ni (Nickel) is an alloying element, which is necessary for making the
microstructure of tempered steel a martensite phase mainly. However, if the Ni
content is 4.0 % or Less, a number of ferrite phases were precipitated in the
microstructure of tempered steel and the microstructure of tempered steel does
not
become a martensite phase mainly. On the other hand, if the Ni content exceeds
8 %, the microstructure of tempered steel becomes an austenite phase mainly.
Accordingly, the Ni content was set to 4.0 - 8 %. More preferably the Ni
content
was set to 4 - 7 %.
Mo: 2.8 - 5.0
Mo (Molybdenum) is an effective alloying element to enhance the sulfide
stress-corrosion cracking resistance for a high strength material. To obtain
this
effect Mo content of 2.8 % or more is needed. However, if the Mo content
exceeds
5.0 %, this effect is saturated, resulting in an increase in cost.
Accordingly, the Mo
6
CA 02509581 2005-06-10
content was set to 2.8 - 5.0 %.
Al: 0.001- 0.10
A1 (.Aluminum) is an alloying element, which is used as a deoxidizer in a
melting process. To obtain this effect A1 content of 0.001 % or more is
needed.
However, if the A1 content exceeds 0.10 %, many inclusions are formed in the
steel so
that the corrosion resistance is lost. Accordingly, the Al content was set to
0.001 -
0.10 %.
N: 0.07 % or less
N (Nitrogen) is an impurity element contained in the steel and the N content.
is better as low as possible. Particularly, if the N content exceeds 0.07 %,
many
inclusions are formed so that the corrosion resistance is lost. Accordingly,
the N
content was set to 0.07 % or less.
One of martensitic stainless steels according to the present invention
consists the above-mentioned chemical composition as well as the balance Fe
and
indispensable impurities. Another martensitic stainless steel according to the
present invention further contains, in addition to the above-mentioned
components,
at least one alloying element selected from at least one group consisting of a
first
group, a second group and a third group shown as follows. The components
(elements) of the respective groups will be described below.
First group (Ti, V, Nb, Zr: 0.005 - 0.25 % respectively)
Since Ti, V, Nb and Zr have effect to fix C so as to reduce variations of
strength, one or more selected from these elements may be optionally
contained.
However, if any one of the elements is less than 0.005%, the above-mentioned
effect
cannot be obtained. On the other hand, if any one of the elements exceeds 0.25
%,
the microstructure of the steel cannot become a martensite phase mainly so
that
highly strengthening of the steel with a proof stress of 860 MPa or more
cannot be
7
CA 02509581 2005-06-10
attained. Accordingly, the respective contents in selectively containing these
elements were set to 0.005 - 0.25 %.
Second group (Cu: 0.05 - 1 %)
Cu is an effective element to make the microstructure of tempered steel a
martensite phase mainly like Ni. To obtain the effect by the addition of Cu
the Cu
content may be 0.05 % or more. However, if the Cu content exceeds 1 %, the hot
workability of the steel is lowered. Accordingly, when Cu is contained in the
steel
the Cu content was set to 0.05 - 1 %.
Third group (Ca, Mg, La, Ce: 0.0002 - 0.005 % respectively)
Since Ca, Mg, La and Ce are effective elements to enhance the hot
workability of the steel, one or more selected from these elements may be
optionally
contained. However, if any one of the elements is less than 0.0002 %, the
above-mentioned effect cannot be obtained. On the other hand, if any one of
the
elements exceeds 0.005 %, coarse oxide is formed in the steel whereby the
corrosion
resistance of the steel is decreased. Accordingly, the respective contents in
selectively containing these elements wexe set to 0.0002 - 0.005 %.
Particularly, it
is preferred to contain Ca and/or La in the steel.
The steel according to the present invention should have the
above-mentioned chemical composition and satisfy the following expression (1).
This is because, if the steel satisfies the expression (1), strength of the
steel can be
enhanced to proof stress of 860 MPa or more without deteriorating sulfide
stress-corrosion cracking resistance.
Mo a 2.3 - 0.89 Si + 32. 2 C .. . ( 1)
wherein the symbols of the respective elements in the expression (1) show the
content (mass %) of each element.
FIG. 1 is a view showing relationships between Mo contents of various types
8
CA 02509581 2005-06-10
of steels tested in examples, which will be described later, and the right
side in the
expression (1), that is "2.3 - 0.89 Si + 32. 2 C" (IM value). Specifically,
the results
shown in FIG. 1 are based on steels of the present invention and comparative
steels
(test Nos. 18 - 21). The mark "o" shows an example that did not generate
rupture
in a sul.fi.de stress-corrosion cracking test, and the mark "X" shows an
example that
generated rupture therein. Even if the Mo content exceeds 2.8 %, if the Mo
content
does not satisfy the expression (1), the steel has a poor sulfide stress-
corrosion
cracking resistance.
When Mo content is out of a range (that is less than 2.8 %) defined in the
present invention, the 0.2 % proof stress of the steel is less than 860 MPa.
Further,
even if Mo content is in a range (that is 2.8 - 5 %) defined in the present
invention, if
the Mo content does not satisfy the above-mentioned expression (1), the 0.2 %
proof
stress of the steel is less than 860 MPa.
However, if steel satisfies the above-mentioned expression (1), the 0.2
proof stress of the steel reaches 860 MPa or more and the steel can endure the
use of
an oil well steel material due to its sufficient strength. Accordingly, the
steel
according to the present invention should be in a range of said chemical
composition
and satisfy the above-mentioned expression (1).
Further, the present inventors have checked the influences of
microstructure. As a result the present inventors have found that if the
microstructure is a structure mainly comprising tempered martensite, carbide
precipitated during tempering, and intermetallic compounds such as Laves
phase, a
phase and the like finely precipitated during tempering, the strength of the
steel can
be enhanced without deteriorating sulfide stress-corrosion cxacking
resistance.
It is noted that "mainly comprising tempered martensite" means that a 70
vol % or more of the microstructure of the steel is a tempered martensitic
structure,
9
CA 02509581 2005-06-10
and a retained austenitic structure and/or a ferritic structure other than a
tempered
martensitic structure may be present.
Further, the "intermetallic compounds such as Laves phase, ~ phase and the
like" may contain intermetallic compounds such as a phase ands phase other
than
Laves phase such as Fe2Mo and the like and 6 phase.
The microstructure of the steel according to the present invention contains
carbide precipitated during tempering. Although carbide is an effective
microstructure to ensure the strength of the steel, high strength of proof
stress of
860 MPa or more cannot be realized by only carbide contained in the steel.
Accordingly, in the present invention precipitation of carbide as well as fine
precipitation of intermetallic compounds such as the above-mentioned Laves
phase,
a phase and the like are needed.
Heat treatment for the steel of the present invention is typical
quenching-tempering. Zb precipitate fine intermetallic compounds during
tempering it is necessary to sufficiently dissolve the intermetallic compounds
during
quenching. The quenching temperature is preferably 880 - 1000 °C.
Further, conditions in which intermetallic compounds such as a fine Laves
phase, a phase and the like are precipitated and 0.2 % proof stress of 860 MPa
or
more can be obtained resides in a case where when a temperature range for
tempering is 450 - 620 °C, as well as the tempering temperature is set
to T(°C) and
the tempering time is set to t (hour), (20 + log t)(T + 273) can satisfy 13500
- 17700.
FIG. 2 is a view for explaining tempering conditions defined in the present
invention. FIG. 2 shows relationships between 0.2 % proof stress obtained by
changing values of (20 + log t)(T + 273) while changing tempering temperatures
in
400 - 650 °C after quenching steel at 920 °C, and the (20 + log
t)(T + 273).
As shown in FTG. 2,when (20 + log t)(T + 273) is in a range of 13500 - 17700,
CA 02509581 2005-06-10
0.2 % proof stress reaches 860 MPa or more.
When tempering is performed at a condition that (20 + log t)(T + 273)
exceeds 17700, dislocation density is reduced ox imtermetallic compounds are
dissolved in microstructure of the steel, whereby high strengthening of 0.2 %
proof
stress of 860 MPa or more cannot be attained. On the other hand, when the
steel is
tempered at a condition of less than 13500, intermetallic compounds and
carbide are
not precipitated. Accordingly, 0.2 % proof stress of 860 MPa or more cannot be
attained.
From the above-mentioned principal, the steel of the present invention
should have the above-mentioned chemical compositions and satisfy the
expression
(1) and the microstructure of the steel should be mainly comprising tempered
martensite, carbide precipitated during tempering, and intermetallic compounds
such as a Laves phase, a phase and the like finely precipitated during
tempering.
(Examples)
Steels having chemical compositions shown in Tables 1 (1) and 1 (2) were
melted and cast, and the obtained cast ingots were forged and hot rolled to
prepaxe
steel plates each having a thickness of 15 mm, a width of 120 mm and a length
of
1,000 mm. These steel plates were subjected to quenching (water cooling at 920
°C)
and tempering (air cooling after soaking at 550 °C for 30 min. ((20 +
log t)(T + 273)
16212], and the obtained steel plates were provided in various tests as
testing steel
plates.
11
CA 02509581 2005-06-10
'" o,r N N m m a m cn r -. o_ cc c:r m
~ o '
c~.~N ~W (noo cn_ c7N a d! cn N cV
: 0 0 0 ) 0 0 0 0 0 0
0
o o - o - 0 0
O O CD o0 ~Dm m.0 001f~Q)cD r c7o dJ'd'M
' u? cocc c r c~
~
~ cnc~ u~ m -~ cor cncm n m N N N n N N
, N N N M N N Pt N N N N N
~
CC
U
o.
r
I I I I I I I I I
Lj I I I t o I I
0
m
j I i I i o I I I I I I I I I I I i
o ~,
k
"' a>
z'
I I I I I o I I I I I I I I I I I
0
a>
W ~ ~. i
U I I I I I I I I I I I I I I $ o I
0 0
0
I I I I o I I I I I I I I I I I I o
0 0~o a ~ a~ oo m O
I I I I I I I O
o o o o 0 0 o o
0 0 o m ~ro ~ro 0 0 0 0
ca w ~ro o ca o cc ~ ~ ~r cc
a ' 0 0 0 0 0 0 0 0 0 0 0 o I I I I cd
0
0 0 0 0 0 0 0 0 0 0
~n m
cd
z
~,
101f~O N O ~ CD If~cD N N N N d' G)ID r
. y nn u~ ccm n ca cccc cDca ca ~ r m o cn
O O O O O O cOO O O O
z O O O O O O 0 0 0 0 0 0 0 0 0 0 0
o 0 0 0 0 0
0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0
~
.~0 0 0 00 ~ w o o mn~n ~ ~ ~r o N o m r c
N N d
O M M N M N M M N N M M m M N M O c?
O O O O O O
O O O O O O O O O O O O O O O O O
O O O O ~ O O O
O
V
C1j O ~
.H
O p rnm m m ~ ~ 0 '
m ~ ~ o rn m o a O~ o o ~
o o 0 , ~
N N ~ M m N N N N N N N N N N N N
~
y n o r a~r r.N ~ ~n ' w n u~o ooa~ oof.,'
.'~..a'
0oa~ cn m oo N ao 0oao o~~n co 000~ ~ m ooy,~ O
cDcO t~ r r ~1't cDCD cDcD cD IC~cD cDcD tD~ U
~
-.
V O
0000 N -~CO .-nO O t .-~.~ O a9pp ~ Co O
O O O O~ ~-~-n -aO O O O O O
~ N N N ri O er N N N ~ N N N N .-iN
.~...m--mr ..-n .-n.r.r ~ .r .w
d
O
~
n
~
Wit_
'
O O Oo N o0 i17i d; ,
:
U o 0 0 0 0 - c o 0
O O ~ O O O O O)00 00O O O N O C) O Gi
-.~ -r ..rO ~
.-n.-n.-n.~ O O O - Oy.
O O O O O O O O O O O O O O O O O
.w
~j
o O O O O o o O O O o 0 0 o O O O .
U
IfJIC~CD ~ W ~ M N U7 yf7r d' If7N ~'
~ D ~ H N r 'r .-r.-n.H.--r.~~
~
.--n.H .1 O . O . . O r O O O O O O O C~
O O O O O O O
O O O O O O O O O O O O O O O O O ~ ~ l!)
'~
~a
o
~
0
m cn ~ ~r-~ coN M d~ w w .r m r r ca ~ ~ v ~1
F
~ <r oo ~;~r aoo cod; r ~ ~ v;w c~~ w 0 ~ m
0 0 0 0 0 0 .: o .-.0 0 0 0 0 0 0 0 ,
~
r r a0 V'00 r r 10tiJ00~ O o0l 1C~N r
'-' a>
~ r~i
.-r.r .-nO ~ .~~ .-n.-i~ N N .-r'r ..-rO ""i~ In
O
O O O O O O O O O O O O O O O O
~ ca co w oo m r c mn w d' mnd~ c~
i -n-i
U o 0 N M O .r.~ ~ .H .-~.~ .-n.-n.- . . o s-~
0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
as .~
N
m
V~
IfW
D
t
GO
Q~
O
N
~
d'
ID
cD
r
uoyua~m z z z
auk
~o
sjaa~s
12
CA 02509581 2005-06-10
Q) I!7~ H'm 1n .-i
N tfJ N t- m O
b 0 0 0 0 0
~ 0 -.r. 0
* ~ ~
> .
O n,a m ca o ~ o ~ p
N ~ .,N N ~ m w
N N m N N N N N
N
U I I I
I I I I I
I I I I I I I
I i i I I I I I
U I I I I I I I I
O
N I I I I I I I I
H
O
m c.N m oo N aop
o rn cacc o rn a~ 00
0 0 0 0 0 0 0 ~n
O Ci O O O O O O ""i
~
O O O O O O O
O O O O O O O O
.N
r~
s z
a
0 0 o a o 0 0
p
a a o 0 0 0 0 0
a0m 0000 N I(J~' -1
~ er w n chm m .-
o a o 0 0 0 0 0
..ia ~ 0 0 0 0 0 0 0 0
-n~ ~ S~
p d cc m n ..,m ~ caI
d'er cecm n a~ o <rO
N N N N * O N N
.
.~ y p
~' - ~ a~~~ m m ~ o
, Z , .~ p
~
d c CD O CD c0cD Cn c0
a
U o 00 0 0 0 00 ~ o
s..mn o c-~ m ao o n W
C7 0 ~
~ ~ ~ ~ ; r.,~'
~+.
O
a
~rm m m m m m m O
0 0 0 0 0 0 0 0
U o 0 0
0 0 0 0 0
N O .~.-aQ~~ ~ N
H H H N Q ~ H H
O O O O O O a O
O O O O O O a O ~ f.
0
N .~ 1(7I(J1fJ1f~et'M
P.i O O O O o O 4 O
O o O
C O o O O O
H I
O O O O O O O O
~ S~
O ~
p
p
,
N ,--~O N cD ~ U7 O
(/~ O O O O O O O O
U o 0 0 0 0 0 0 0 ~t-~
o o > t
t~
~
t:
0 c 0 0 0 0 o c
p
~d
~np o~0O O .r N eJ ~i'if7
N N N N N N
E
S jaa~s z~~~~a~o~ z z z
a~,
13
CA 02509581 2005-06-10
First, round bar test pieces each having a diameter of 6.35 mm and a length
of the parallel portion of 25. 4 mm were taken from the respective testing
steel plates
and subjected to tensile tests at normal temperatures. The obtained 0.2 %
proof
stresses are shown in Table 2.
Then, test pieces each having a thickness of 3 mm, a width of 20 mm and a
length of 50 mm were taken from the respective testing steel plates and these
testing
pieces were polished with a No. 600 emery paper and degreased and dried. Then
the obtained testing pieces were immersed into 25% NaCl water solution
saturated
with 0.973 MPa COz gas and 0.0014 MPa HaS gas (temperature: 165 °C) for
720
hours.
After the immersion weight reductions of the test pieces by corrosion ((mass
before testing) - (mass after testing)] were measured and the presence and
absence
of local corrosion on surfaces of the testing pieces were confirmed by a
visual test.
As a result the corrosion rate of the steel according to the present invention
is 0.5
mm/year or less, and no local corrosion on its surface could be found.
Subsequently, examples in which 0.2 % proof stxesses were 860 MPa or more
in the tensile tests were subjected to fixed load tests by use of a spring
type (proof
ring type) testing machine in accordance with TM0177-96 Method A of NACE.
Specifically, round bar test pieces each having a diameter of 6.3 mm and a
length of
the parallel portion of 25. 4 mm were taken from the respective testing steel
plates
and subjected to 0.2 % proof stress-85 % (test stress) fixed load tests at a
test
temperature of 25 °C, for 720 hours by use of 0.003 MPa HzS gas (COz
bal.) saturated
25% NaCl water solution (pH 4.0). As a result all test pieces were not
ruptured.
The Microstructures of the test pieces were observed by an optical
microscope and an extraction replica. These results are also shown in Table 2.
14
CA 02509581 2005-06-10
Table 2
Test 2 % Poof Carbon dioxideSSC
0
No. . gas corrosionCorrosion Microstructure
stress (MPa)
test test
1 951 0 o M+IM+C
2 944 0 o M+IM+C
3 1,007 0 o M+IM+C
4 1,027 0 o M + IM + C
1 020 0 o M + IM + C
6 910 0 o M+IM+C
7 8$2 0 o M+F+IM+C
.., 8 944 0 o M+IM+C
9 965 0 o M + I_M_+ C
0 10 972 0 o M+IM+C
11 958 0 o M+IM+C
12 951 0 o M+IM+C
13 965 0 o M+IM+C
14 958 0 o M+IM+C
972 0 o M+IM+C
16 882 0 o M+IM+C
17 979 0 o M+IM+C
18 841 o x M + C
19 843 o x M + C
858 -. X M + C
21 840 o x M + C
22 829 o x M + C
23 832 o x M + C
U 24 849 o x M + C
841 x x M + C
Note 1) In carbon dioxide gas corrosion test a steel, whose corrosion rate is
0.5 mm/y or
less, and which did not generate local corrosion, is shown by "o" and
otherwise
"X"
5 Note 2) In SSC test, a steel, which did not generate rupture, is shown by
"o" and a steel,
which generated rupture, is shown by "X".
Note 3) In microstructure, tempered martensite is shown by "M", ferrite is
shown by "F",
intermetallic compounds are shown by "IM" and carbide is shown by "C".
10 As shown in Table 2, examples Nos. 1 to 17 of the present invention each
have 0.2 % proof stress of 860 MPa or more and excellent carbon dioxide gas
corrosion resistance and sulfide stress-corrosion cracking resistance. On the
other
hand, comparative examples Nos. 22 to 25, which have Cr and/or Mo contents out
of
range defined in the present invention, and comparative examples Nos. 18 to
21,
CA 02509581 2005-06-10
which have the content ranges of the respective components are in the range
defined
in the present invention but the expression (1) previously described was not
satisfied,
were not su~cient in carbon dioxide gas resistance and/or stress cracking
resistance.
INDUSTRIAL APPLICABILITY
The martensitic stainless steel according to the present invention can have
high strength of 0.2 % proof stress of 860 MPa or more and excellent carbon
dioxide
gas corrosion resistance and sulfide stress-corrosion cracking resistance by
limiting
the steel composition of specified elements and defining Mo content in the
steel by
relationships with IM values as well as by forming microstructure of the steel
with
tempered martensite mainly, carbide precipitated during tempering, and
intermetallic compounds such as a Laves phase, a 6 phase and the like. As a
result
the martensitic stainless steels of the pxesent invention can be applied to
practical
steels, which can be widely used in oil well tubes and the like under
environment
including carbon dioxide gas, hydrogen sulfide, chlorine ions or two or more
of them,
in wide fields.
16