Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2004/058196 CA 02510317 2005-06-23PCT/US2003/040534
POLYORGANOS I LOXANE DENTAL IMPRESSION MATERIAL
[0001] The present invention is generally directed toward polyorganosiloxane
dental impression materials. More
particularly, the invention is directed toward such a material having improved
physical properties, including
improved wetting and tear strengths. Specifically, the present invention
employs a silicone glycol surfactant.
BACKGROUND OF THE INVENTION
[0002] This invention is directed to improvements in room temperature
polymerizable polyorganosiloxanes having
good dimensional stability upon curing or hardening. More particularly, this
invention is directed to improvements
in compositions that are generally of the type comprising two components, one
component comprising
organopolysiloxanes having vinyl groups, capable of undergoing addition
reactions with organopolysiloxanes
having silicone-bonded hydrogen atoms. The second component comprises a
catalyst capable of promoting the
addition of hydrogen atoms bonded to silicone atoms across the vinyl groups.
[0003] A major field for the use of certain of these room temperature curable
polyorganosiloxane compositions is
dentistry. Such materials are typically employed as impression materials for
securing an analog representation of
oral hard and soft tissue to support subsequent elaboration of crowns,
bridges, dentures, an other oral prostheses. For
dental use, extraordinary fidelity of structural reproduction is required in
order to ensure good fidelity of oral
prosthetic fit and the like. In this regard, changes in the dimensions of the
impression material during curing are to
be avoided. Moreover, the surface of the reproductions or oral prosthetics and
the like must be exceptionally free
from irregularities, blemishes, pits, and other imperfections. This is so
because castings and prostheses derived from
such impressions must have good surface qualities and be free from pits and
irregularities in order to have proper fit,
to achieve good adhesion, and to avoid irritation of sensitive mouth
structures. These polyorganosiloxanes will also
be useful in other fields where detailed reproductions are important such as
in the science of metrology, laboratory
processing of SEM and even jewelry fabrication and the like.
1
WO 2004/058196 CA 02510317 2005-06-23PCT/US2003/040534
[0004] In employing polyorganosiloxanes as dental impression materials, a
number of difficulties have arisen.
First of all, tear strength tends to be low. It is necessary, in effectively
taking an impression, to be able to easily
remove the impression, from the dentition without tearing, particularly at
thin marginal areas, to preserve fine detail.
In the past, fillers of various types have been added to improve tear
strength. Such additions may result in some
improvement, on the order of about 10%, but such improvements have proved
inadequate.
[0005] Paradiso in WO 93/17654 describes improving tear strength by
incorporating multi-functional, including
quadri-functional, polysiloxane components into the impression material, to
add increased cross-linking to the
resulting cured impression material matrix, particularly along the length of
the linear vinyl end-stopped polysiloxane
principal component. The Paradiso composition comprises SiOH groups capped off
with Me<sub>3</sub> Si units that form
pendants from the molecule. These pendants provide only mechanical or physical
interlinking between the linear
polysiloxane chains. This solution is deficient, being non-chemical and low in
cross-linking density.
[0006] Voigt et al in EP 0 522 341 Al describes very short processing times of
35-45 seconds for forming
dentition bite registration devices, utilizing a "QM" resin as a means of
speeding and increasing cross-linking. These
resins comprise as Q, the quadri-functional SiO<sub>4</sub>/2 and as M, building
blocks such as monofunctional units
R<sub>3</sub> SiO<sub>1</sub>/2 wherein R is vinyl, methyl, ethyl or phenyl, or similar in
or bi-functional units. Voigt notes that
an elastomer with small elastic deformation having a higher tenacity and
hardness results. However, such material
lacks flexibility, having a low strain value, and is unsuitable for impression
taking. The increased cross-linking rate
of the QM resin also results in very limited processing times that are
unsatisfactory.
[0007] The other major, well-known difficulties with polyorganosiloxane
impression materials are caused by its
inherent hydrophobic character. Such characteristics make reproduction of hard
and soft oral tissue difficult since
the oral cavity environment is wet and often contaminated with saliva or
blood. The hydrophobicity of the
impression material can result in loss of surface detail often at critical
surfaces of the dentition.
[0008] A number of improvements of polyorganosiloxane impression materials
focus upon adding a surfactant
component to the dental impression material in order to reduce the hydrophobic
nature of the polysiloxanes and
make the composition more hydrophilic. Thus, Bryan et al in U.S. Pat. No.
4,657,959 describes adding an
ethoxylated nonionic surface active agent containing siloxane or
perfluoroalkyl solubilizing groups to achieve a
three minute water contact angle below about 65°. While surfactants
including hydrocarbyl groups, for
2
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
rendering the surfactant soluble or dispersible in silicone prepolyrner, are
mentioned, including ethyleneoxy groups,
the results achieved appeared to be less than optimal.
[0009] In sum, polyorganosiloxane impression materials still need improvement
in tear strength and wettability in
order to provide improved use of these compositions for taking impressions of
oral hard and soft tissues such that
adequate working time, tear strength and wettability are provided.
SUMMARY OF THE INVENTION
[0010] The new polyvinylsiloxane impression materials are useful in low and
high viscosity impression materials
to record hard and soft tissues in the mouth. The new impression material is a
two component, platinum-catalyzed,
vinylpolysiloxane material. The two component polymerizable organosiloxane
composition, one component
including a catalyst for polymerization, for making a dental impression,
comprises:
(a) a QM resin, containing vinyl groups;
(b) a linear vinyl terminated polydimethylsiloxane fluid, forming with said QM
resin a dispersion having a
vinyl content of about 0.16 to 0.24 m-mole/g;
(c) an organohydrogen polysiloxane for cross-linking said vinyl groups;
(d) an organoplatinum catalyst complex for accelerating polymerization of said
components;
(e) an emulsifying plasticizer for said catalyst complex;
(f) a retarder component in sufficient amount for temporarily delaying the
onset of said polymerization;
(g) a filler; and
(h) a surfactant that imparts wettability to said composition, wherein said
composition surface contact
angle with water is less than 50° after three minutes.
[0011] There is also provided according to the invention, a polyorganosiloxane
impression material employing a
silicone glycol surfactant that achieves a water contact angle of less than
about 10 degrees at 30 seconds. The
preferred surfactant is PEG-8 methicone, such as is available from BASF as
Masil SF 19. According to one
embodiment of the invention, a contact angle of 2 degrees was achieved at 30
seconds, as will be demonstrated
hereinbelow.
[0012] Preferably, the dispersion of (a) and (b) has a viscosity of about
5,000-60,000 cps. The dispersion of (a) and
(b) may comprise a plurality of dispersion components having desired
viscosities and QM resin contents. Preferably
3
CA 02510317 2011-08-16
64053-516
the QM resin-containing dispersions comprise a first dispersion component
having a viscosity of about 5,000-7,000
cps; and a second dispersion component having a viscosity of about 45,000-
60,000 cps, said QM resin comprising
about 20-25 weight % of each dispersion.
10013] A preferred QM resin comprises a polyorganosiloxane comprising units of
SiO4/2 and units of RIR22Si01/2
wherein
RI is unsaturated, preferably vinyl and
2
R is alkyl, aryl, etc., such as methyl, ethyl, phenyl, etc. More preferably,
the QM resin comprises
the formula: Si0414
[0014] The retarder component of the composition is a low molecular weight,
vinyl functional fluid that is a linear
or cyclic polysiloxane in an amount of at least about 0.030 weight percent of
said composition. Preferably, the
retarder component comprises: a fluid 1,3-divinyl, dimethyldisiloxane, in an
amount of about 0.030 to 0.10 weight
percent of said composition.
[00151 The composition includes an emulsifying plasticizer that imparts
desired handling and flow properties to
the catalyst complex, to match those of the second component, wherein a
suitable composition for taking a dental
impression may conveniently be formed. Preferably, the plasticizer comprises
an alkylphthalate at about 0.5 to 2.0%
by weight of said catalyst component and is, most preferably, octyl benzyl
phthalate.
[00161 The filler component of the invention comprises about 15 to about 45
weight percent of said composition
and preferably includes a filler mixture of about 20 to about 40 weight
percent.
[00171 A key component of the composition of the invention is the surfactant
for imparting wettability, preferably
comprising an HLB of about 8-11 and a pH of about 6-8. A most preferred
surfactant is a nonionic surfactant,
nonylphenoxy poly (ethyleneoxy) ethanol having an HLB of about 10.8.
[0018] After polymerization, the compositions of the invention include a tear
strength of 270-300 PSI (1.86-2.06
MPa) and a contact angle with water of less than 50° at three minutes.
[0019] According to another embodiment of the invention, a base paste and
catalyst paste are prepared, wherein a
silicone glycol surfactant is present in both pastes or only in one. According
to the invention, a contact angle of less
than 10 degrees has been achieved in under 15 seconds.
4
CA 02510317 2012-03-27
64053-516
[0019a] Specific aspects of the invention include:
a two component polymerizable organosiloxane composition for making
a dental impression comprising, (i) one component being a catalyst paste
comprising
a catalyst for polymerization, and (ii) another component being a base paste,
the two
component polymerizable organosiloxane composition comprising:
(a) a QM resin, containing vinyl groups;
(b) a linear vinyl terminated polydimethylsiloxane fluid, forming with
said QM resin a dispersion having a vinyl content of about 0.16 to 0.24 m-
mole/g;
(c) an organohydrogen polysiloxane for cross-linking said vinyl groups;
(d) an organoplatinum catalyst complex for accelerating polymerization
of said components;
(e) an emulsifying plasticizer for said catalyst complex;
(f) a retarder component in sufficient amount for temporarily delaying
the onset of said polymerization;
(g) a filler; and
(h) an nonionic surfactant having an HLB of 8-11 that imparts
wettability to the composition, wherein said composition surface contact angle
with
water is less than 10 degrees at 15 seconds; and
use of a nonionic surfactant comprising an HLB of 8-11 in only the base
paste or only the catalyst paste of a polymerizable organosiloxane composition
for
making a dental impression, which comprises a polyvinylsiloxane,
for imparting wettability such that the material has a surface contact
angle with water of less than 10 degrees at 15 seconds.
4a
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
BRIEF DESCRIPTION OF THE DRAWINGS
[0020] FIG. 1 is a graph showing Wetting Contact Angle, in degrees, as a
function of Time, in minutes.
[0021] FIG. 2 is a graph showing Impression Material Viscosity as a function
of Time, in minutes.
[0022] FIG. 3 is a graph showing percent elongation and tear strength, in psi.
[0023] FIG. 4 is a graph showing Wetting Contact Angle, in degrees, as a
function of Time, in seconds.
[0024] FIG. 5 is a graph showing Wetting Contact Angle, in degrees, as a
function of Time, in seconds.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0025] An exemplary polymerizable polysiloxane compositions of the instant
invention comprises, in general: an
organopolysiloxane having at least about two vinyl groups per molecule,
further including, dispersed therein, a
quadri-functional vinyl polysiloxane resin; an organohydrogen-polysiloxane
having at least about two hydrogen
atoms bonded to at least two silicone atoms per molecule; a catalyst for
accelerating the addition of the silicone
atoms bonded to the hydrogen atoms to the polysiloxane vinyl groups, including
an emulsifying plasticizer; a filler;
a low molecular weight retarder composition for delaying onset of
polymerization; and an emulsifying surfactant
that imparts wettability to said impression material.
[0026] The composition of the invention is divided into two components. A
first component, which is conveniently
referred to as a "Base Paste", contains the vinylorganopolysiloxanes
dispersion, the organo-hydrogen-polysiloxane,
a portion of the filler and the surfactant. The second component of this two-
part composition is referred to as a
"Catalyst Paste" and comprises a second portion of the vinyl polysiloxanes,
together with the catalyst for
accelerating the addition reaction, the emulsifying plasticizer, a scavenging
agent for hydrogen released during
polymerization and usually, additional quantities of fillers and pigments.
[0027] A wide variety of organopolysiloxanes having at least about two vinyl
groups per molecule are known for
inclusion in the dental polysiloxane compositions of the invention to form the
dispersion including a quadri-
functional vinyl polysiloxane. Each of these materials may be included in
greater or lesser degree in accordance with
the practice of the instant invention. Preferred for use herein are linear
vinyl terminated polydivinytsiloxanes
preferably a divinyl polydimethylsiloxane. Such polymers are sold having
varying average molecular weights with
concomitant variations in viscosity. It is preferred that these materials be
selected to have a viscosity appropriate for
the conditions to be experienced by the resulting silicone material.
5
CA 02510317 2011-08-16
64053-516
[0028] The dispersions of interest have a viscosity range of 5,000-60,000 cps.
In practice, it is convenient to
employ a blend of the dispersing polymers having differing viscosities and
physical properties to provide
compositions having a desired thixotropicity and viscosity.
[0029] The dispersions of interest are preferably formed in two viscosity
ranges: (1) a first dispersion having a
viscosity of about 5000-7000 cps; and (2) a second dispersion having a
viscosity of about 45,000-65,000 cps. While
it is convenient to provide polysiloxane oligomers for this purpose having
methyl substituents, other substituents
may also be included in the compositions in accordance with this invention.
Thus, alkyl, aryl, halogen, and other
substituents may be included in greater or lesser degree as part of the vinyl
polysiloxanes which are useful. Those of
ordinary skill in the art will be able to determine which polysiloxane
materials are preferred for any particular utility
from the foregoing considerations.
[00301 The quadri-functional polysiloxanes, designated and known in the art as
QM resins, provide improved tear
strength to the polymerized impression composition, by increasing its
resulting polymerized crosslink density. As is
known, the QM resin is made up of: quadri-functional SiO4/2 units; and M
units, such as R1R22Si01/2 wherein RI is
unsaturated, preferably vinyl and R2 is alkyl, aryl or the like, such as
methyl, ethyl or phenyl. In a preferred
composition RI is vinyl and both R2 are methyl. A most preferred composition
is represented by the formula: SiO4R4.
100311 The QM resin provides a vinyl concentration in the dispersions with the
vinyl-terminated
polydivinylsiloxanes of at least about 0.16 m-mole/g. Preferably the vinyl
concentration is 0.16-0.24 m-mole/g. The
amount of QM resin is preferably about 20-25% by weight of the dispersion.
Such dispersions are sold by Miles,
Inc. of Pittsburgh, Pa. Other QM resin formulations may be used, including
those that are "neat" or dispersed in
carriers other than the preferred fluid polydivinylsiloxane.
[0032] A key element of the invention is a retarder component that delays
onset of polymerization of the QM
resin/dispersion such that sufficient working times to employ the composition
are provided. It functions, as it is
consumed, to offset what would otherwise be a too rapid polymerization. The
preferred retarder fluid in the
preferred impression material of interest is 1,3 divinyldimethyldisiloxane at
a sufficient concentration level to
perform its retarding functions, which is in at least about 0.03 weight
percent of the composition, preferably within a
range of about 0.03 to 0.10 weight percent. This preferred amount is in
contrast with the lower amounts of 0.0015-
0.020 weight percent typically employed in PVS systems to stabilize
compositions. Other suitable retarders are any
6
WO 2004/058196 CA 02510317 2005-06-23 PCT/US2003/040534
low molecular weight, vinyl functional material that would be initially
consumed in the polymerization, to delay
hardening suitably and as desired, including linear and cyclic polysiloxanes.
[0033] The organohydrogen-polysiloxanes useful in the practice of the present
inventions are well-known to those
of ordinary skill in the art. It is required only that polysiloxanes having
hydrogen atoms directly bonded to silicone
atoms be employed, and that they have suitable viscosities and other physical
properties. Substituents in the
molecules such as alkyl (especially methyl), aryl, halogen, and others may be
employed as well. It is necessary only
that such substituents not interfere with the platinum-catalyzed addition
reaction. It is preferred that molecules be
employed having at least two silicone-bonded hydrogen atoms per molecule.
Polyrnethylhydrogensiloxane is
preferred, having viscosity range of about 35-45 cps.
[0034] The catalysts which are useful for catalyzing the reaction of the
silicone atoms (bonded to hydrogen atoms)
to the vinyl groups of the vinyl polysiloxane molecules are preferably based
upon platinum. In this regard, it is
preferred to employ a platinum compound such as chloroplatinic acid,
preferably in admixture or complex with one
or more vinyl materials, especially vinyl polysiloxanes. While such materials
have been found to be preferred, other
catalysts are also useful. Thus, platinum metal together with other noble
metals including palladium, rhodium, and
the like and their respective complexes and salts are also useful. In view of
the toxicological acceptability of
platinum, however, it is greatly to be preferred for dental use.
[0035] The compositions of the present invention also include a filler,
preferably a mixture of hydrophobic fillers.
A wide variety of inorganic, hydrophobic fillers may be employed such as
silicas, aluminas, magnesias, titanias,
inorganic salts, metallic oxides and glasses. It is preferred, however, that
forms of silicone be employed, In
accordance with the present invention, it has been found to be preferable to
employ mixtures of silicones, including
those derived form: crystalline silicone dioxide, such as pulverized quartz (4-
6µ); amorphous silicone dioxides,
such as a diatomaceous earth (4-7µ); and silanated fumed silica, such as
Cab-o-Sil TS-530 (160-240 m<sup>2</sup> /g),
manufactured by Cabot Corporation. The sizes and surface areas of the
foregoing materials are controlled to control
the viscosity and thixotropicity of the resulting compositions. Some or all of
the foregoing hydrophobic fillers may
be superficially treated with one or more silanating or "keying" agents, as
known to those of ordinary skill in the art.
Such silanating may be accomplished through use of known halogenated silanes
or silazides. The fillers are present,
preferably, in amounts of from about 15 to about 45 weight percent of the
composition, forming an impression
composition that is polymer rich and, thus, having improved flow properties.
The fillers, more preferably, are about
7
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
35-40 weight percent of the composition. A preferred filler mixture includes
14-24 weight percent crystalline
silicone dioxide, 3-6 weight percent amorphous silicone dioxide and 4-8 weight
percent silanated fumed silicone
dioxide. A most preferred filler is about 19% cristobalite at about 4-6µ
particle diameter, about 4% diatomaceous
earth at about 4-7µ particle diameter and about 6% silanated fumed silica
at about 160-240 m<sup>2</sup> /g.
[0036] A chemical system may be employed to diminish the presence or degree of
hydrogen outgassing which
may be typically generated as a result of the vinyl polymerization. The
composition thus may comprise a fmely
divided platinum metal that scavenges for and takes up such hydrogen. The Pt
metal may be deposited upon a
substantially insoluble salt having a surface area of between about 0.1 and
40m<sup>2</sup> /g. Suitable salts are barium
sulphate, barium carbonate and calcium carbonate of suitable particle sizes.
Other substrates include diatomaceous
earth, activated alumna, activated carbon and others. The inorganic salts are
especially preferred to lend improved
stability to the resulting materials incorporating them. Dispersed upon the
salts is about 0.2 to 2 parts per million of
platinum metal, based upon the weight of the catalyst component. It has been
found that employment of the
platinum metal dispersed upon inorganic salt particles substantially
eliminates or diminishes hydrogen outgas sing
during curing of dental silicones.
[0037] An important improvement of the invention is inclusion in the
composition of the PEG-8 Methicone
surfactant that imparts wettability to said composition, as indicated by a
surface contact angle with water at three
minutes of less than 50.degree, or more preferably, less than about 10 degrees
at 30 seconds.. An unexpected result
of the selection of surfactant provides a major clinical advantage in that the
wetting contact angle of less than 10
degree is achieved in less than about 30 seconds, decreasing and remaining
below 10 degrees throughout the
working time of the composition, in contrast with prior art polyvinylsiloxanes
and surfactant formulations that
require more time to wet out. This higher wetting rate of the composition of
the invention is particularly
advantageous during the impression taking process and is shown in the
Drawings.
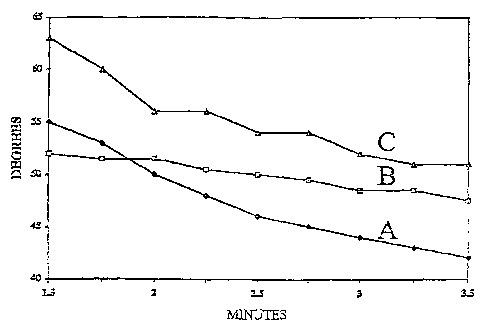
[0038] Referring to FIG. 1, the Wetting Contact Angle, in degrees, as a
function of Time, in minutes, is shown for
the polyvinyl siloxane composition of the invention, in comparison with prior
art compositions. Curve A is the
composition of the invention showing a wetting contact angle of about
50° at two minutes after mixing of the
base and catalyst components. FIG. 1 demonstrates that good weftability is
achieved early and improves at a fast rate
over the about 3.5 minutes of useful working life of the impression taking
material. Curves B and C are,
respectively, polyether and conventional polyvinyl siloxane impression
materials of the prior art. FIG. 2 shows
8
= 64053-516 CA 02510317 2011-08-16
Impression Material Viscosity as a function of Time for composition of the
invention, Curve A, and the two prior art
compositions B and C noted above. It shows the progression of the
polymerization process from mixing and, in
combination with FIG. 1, demonstrates that the improved wettability of the
composition of the invention occurs
during the critical working time for the impression material, an important
advantages over other known systems.
[0039] According to another embodiment of the invention, the surfactant is
placed in only one of the base paste or
the catalyst paste, preferably the base paste. Homogeneity of the mixture is
preferably controlled, such as by using
a preselected number of stators in the mixer. It has been unexpectedly found
that improved stability and wettability
of the formulation is acheived. Problems of the surfactant in the presence of
moisture and the catalyst causing
decomposition of the platinum catalyst are avoided. More unexpectedly, the
wettability of the material was greatly
improved, achieving a 10 degree or better contact angle in 15 seconds, or even
better, as will be below
demonstrated.
[0040] One surfactant of the invention may be of cationic, anionic, amphoteric
or nonionic type. A key criteria for
selection is that the Hydrophobic Liphophilic Balance (HLB) value (described
by Gower, "Handbook of Industrial
Surfactants", 1993) must be in the range of 8-11. As is well-known, the higher
the HLB the more hydrophobic is the
substance. In addition, the pH of the surfactant must be in the 6-8 range to
prevent side reactions that may be
detrimental the polymerization of the impression. A preferred surfactant is
nonionic, having an HLB value of 10.8
comprising nonylphenoxypoly(ethyleneoxy) ethanol, sold by Rhone-Poulenc of
Cranbury, N.J. as Igepal CO-530. In
comparison it is noted above with respect to Bryan et al, in U.S. Pat: No.
'959 that Igepal CO-630, having an HLB of
13.0, differing in structure from CO-530 wherein the number of repeating units
in CO-630 is 9 and those of CO-530
is 6, is not effective, demonstrating the criticality of the HLB limitation.
[0041] A preferred surfactant is PEG-8 Methicone available from BASF.
[0042] The composition of the invention may include plasticizers that
beneficially alter the handling and flow
properties of the impression material, particularly the catalyst component. A
preferred emulsifying plasticizer is
octyl benzyl phthalate. Other phthalates are useful.
[0043] The composition of the invention may include various pigments to
achieve a preferred color. Such
pigments are well known and include titanium dioxide as well as many others.
[0044] The two component compositions prepared in accordance with the instant
invention are employed in the
same way that conventional impression materials have been employed. Thus,
appropriately equal portions of base
*Trade¨mark 9
CA 02510317 2011-08-16
64053-516
paste and catalyst paste are mixed together thoroughly and applied to the oral
dentition or other region for a period
of time sufficient for the polymerizations or hardening of the composition.
Once the composition has been
substantially hardened, it is removed from the mouth or other surface and,
used for the elaboration of casts and the
like from which representations of the casting surface are subsequently
prepared.
[0045] As will be appreciated by those of ordinary skill in the art, it is
important that dental silicone materials be
capable of being stored for reasonably long periods of time and at reasonable
storage temperature in order to
maximize their commercial utility. Accordingly, it is necessary that such
materials not suffer from decreased
physical properties or substantial changes in working time or hardening time
upon such storage. In this regard,
accelerated storage tests employing high ambient temperatures are now capable
of determining the shelf stability of
such materials.
[0046] Certain embodiments of the present invention are described below.
Numerous other compositions and
formulations may be prepared within the scope of the invention. The following
examples are not to be construed as
limiting and are offered by way of illustration.
Example 1
[0047] The two component composition of the invention is formulated in a Base
Paste and Catalyst Paste
components. Mixing of each component's ingredients is done in a double
planetary mixer having a mixing pot
heated with circulating water at 45° C.-50° C. and under 65 mm
mercury vacuum.
BASE PASTE COMPONENT
[0048] In making the Base Paste, the mixing pot is first charged with all
organohydrogen polysiloxane and
incrementally thereafter, with QM dispersion and filler component, with mixing
continuing until a uniform mixture
is achieved. The finished Base Paste is discharged into a storage container.
CATALYST PASTE COMPONENT
[0049] The Catalyst Paste component is formulated and mixed under conditions
and in equipment as described
above. The platinum catalyst, 1,3 divinyldimethyldisiloxane, QM resin
dispersions, fillers and pigments are added
10
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
incrementally to the mixing pot and mixing carried out until a uniformly mixed
mass is achieved. The compounded
Catalyst Paste is then discharged into a storage container
[0050] The composition of each component is indicated in the table below,
wherein amounts are in weight percent
of the component.
BASE CATALYST
Organohydrogen Polysiloxane
9.00 0.00
(5000-7000 cps) QM resin dispersion
19.62 23.95
(45000-60000 cps) QM resin dispersion
34.59 42.89
Cristobalite 19.01 19.06
Diatomaceious earth 6.53 6.41
Cab-O-Sil TS-530 6.53 6.00
Pigments Predispersed in Divinyl Polysiloxane
0.65 0.25
Titanium Oxide Pigment 0.07 0.07
Surfactant (Igepal CO-530)
4.00 0.00
Plasticizer 0.00 0.50
Platinum Catalyst 0.00 0.64
1,3-Divinyldimethyidisiloxane
0.00 0.07
Finely divided Platinum metal
0.00 0.16
on Calcium Carbonate
100.00 100.00
Example 2
[0051] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
9.00 0.00
(5000-7000 cps) QM resin dispersion
20.18 31.71
(45000-60000 cps) QM resin dispersion
35.61 35.23
Cristobalite 19.74 20.67
Diatomaceious earth 4.30 4.28
Cab-O-Sil TS-530 6.45 6.42
11
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
Pigments Predispersed in Divinyl Polysiloxane
0.65 0.25
Titanium Oxide Pigment 0.07 0.07
Surfactant (Igepal CO-530)
4.00 0.00
Plasticizer 0.00 0.50
Platinum Catalyst 0.00 0.64
1,3-Divinyldimethyidisiloxane
0.00 0.07
Finely divided Platinum metal
0.00 0.16
on Calcium Carbonate
100.00 100.00
Example 3
[0052] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
10.00 0.00
(5000-7000 cps) QM resin dispersion
14.73 26.91
(45000-60000 cps) QM resin dispersion
43.80 43.80
Cristobalite 17.00 17.40
Diatomaceious earth 5.00 5.00
Cab-O-Sil TS-530 5.00 5.00
Pigments Predispersed in Divinyl Polysiloxane
0.40 0.50
Titanium Oxide Pigment 0.07 0.07
Surfactant (Igepal CO-530)
4.00 0.00
Plasticizer 0.00 0.50
Platinum Catalyst 0.00 0.65
1,3-Divinyldimethyidisiloxane
0.00 0.07
Finely divided Platinum metal
0.00 0.01
on Calcium Carbonate
100.00 100.00
12
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
Example 4
[0053] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
10.00 0.00
(5000-7000 cps) QM resin dispersion
19.40 32.37
(45000-60000 cps) QM resin dispersion
36.03 36.03
Cristobalite 20.00 20.00
Diatomaceious earth 5.00 5.00
Cab-O-Sil TS-530 5.00 5.00
Pigments Predispersed in Divinyl Polysiloxane
1.50 0.00
Titanium Oxide Pigment 0.07 0.07
Surfactant (Igepal CO-530)
3.00 0.00
Plasticizer 0.00 0.50
Platinum Catalyst 0.00 1.00
1,3-Divinyldimethyidisiloxane
0.00 0.03
Finely divided Platinum metal
0.00 0.00
on Calcium Carbonate
100.00 100.00
Example 5
[0054] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
11.00 0.00
(5000-7000 cps) QM resin dispersion
14.36 28.44
(45000-60900 cps) QM resin dispersion
43.07 42.64
Cristobalite 17.00 17.19
Diatomaceious earth 5.00 4.95
Cab-O-Sil TS-530 5.00 4.95
13
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
Pigments Predispersed in Divinyl Polysiloxane
1.50 0.00
Titanium Oxide Pigment 0.07 0.07
Surfactant (Igepal CO-530)
3.00 0.00
Plasticizer 0.00 0.49
Platinum Catalyst 0.00 1.13
1,3-Divinyldimethyidisiloxane
0.00 0.06
Finely divided Platinum metal
0.00 0.09
on Calcium Carbonate
100.00 100.00
Example 6
[0055] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
9.52 0.00
(5000-7000 cps) QM resin dispersion
11.19 27.91
(45000-60000 cps) QM resin dispersion
38.07 38.21
Cristobalite 22.84 21.21
Diatomaceious earth 5.71 5.73
Cab-O-Sil TS-530 5.71 5.73
Pigments Predispersed in Divinyl Polysiloxane
1.58 0.00
Titanium Oxide Pigment 0.13 0.13
Surfactant (Igepal CO-530)
4.76 0.00
Plasticizer 0.48 0.48
Platinum Catalyst 0.00 0.48
1,3-Divinyldimethyidisiloxane
0.00 0.05
Finely divided Platinum metal
0.00 0.08
on Calcium Carbonate
100.00 100.00
14
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
Example 7
[0056] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
9.52 0.00
(5000-7000 cps) QM resin dispersion
11.19 27.91
(45000-60000 cps) QM resin dispersion
38.07 38.21
Cristobalite 22.84 21.21
Diatomaceious earth 5.71 5.73
Cab-O-Sil TS-530 5.71 5.73
Pigments Predispersed in Divinyl Polysiloxane
1.58 0.00
Titanium Oxide Pigment 0.13 0.13
Surfactant (Igepal CO-530)
4.76 0.00
Plasticizer 0.48 0.48
Platinum Catalyst 0.00 0.48
1,3-Divinyldimethyidisiloxane
0.00 0.05
Finely divided Platinum metal
0.00 0.08
on Calcium Carbonate
100.00 100.00
Example 8
[0057] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
9.52 0.00
(5000-7000 cps) QM resin dispersion
11.19 27.91
(45000-60000 cps) QM resin dispersion
38.07 38.21
Cristobalite 22.84 21.21
Diatomaceious earth 5.71 5.73
Cab-O-Sil TS-530 5.71 5.73
Pigments Predispersed in Divinyl Polysiloxane
1.58 0.00
15
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
Titanium Oxide Pigment 0.13 0.13
Surfactant (Igepal CO-530)
4.76 0.00
Plasticizer 0.48 0.48
Platinum Catalyst 0.00 0.48
1,3-Divinyldimethyidisiloxane
0.00 0.05
Finely divided Platinum metal
0.00 0.08
on Calcium Carbonate
100.00 100.00
Example 9
[0058] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
9.52 0.00
(5000-7000 cps) QM resin dispersion
11.19 27.91
(45000-60000 cps) QM resin dispersion
38.07 38.21
Cristobalite 22.84 21.21
Diatomaceious earth 5.71 5.73
Cab-O-Sil TS-530 5.71 5.73
Pigments Predispersed in Divinyl Polysiloxane
1.58 0.00
Titanium Oxide Pigment 0.13 0.13
Surfactant (Igepal CO-530)
4.76 0.00
Plasticizer 0.48 0.48
Platinum Catalyst 0.00 0.48
1,3-Divinyldimethyidisiloxane
0.00 0.05
Finely divided Platinum metal
0.00 0.08
on Calcium Carbonate
100.00 100.00
16
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
Example 10
[0059] A two component composition of the invention is made by first making a
Base Paste and then a Catalyst
Paste as described in Example 1, having the composition indicated in the table
below.
BASE CATALYST
Organohydrogen Polysiloxane
9.52 0.00
(5000-7000 cps) QM resin dispersion
11.19 27.91
(45000-60000 cps) QM resin dispersion
38.07 38.21
Cristobalite 22.84 21.21
Diatomaceious earth 5.71 5.73
Cab-O-Sil TS-530 5.71 5.73
Pigments Predispersed in Divinyl Polysiloxane
1.58 0.00
Titanium Oxide Pigment 0.13 0.13
Surfactant (Igepal CO-530)
4.76 0.00
Plasticizer 0.48 0.48
Platinum Catalyst 0.00 0.48
1,3-Divinyldimethyidisiloxane
0.00 0.05
Finely divided Platinum metal
0.00 0.08
on Calcium Carbonate
100.00 100.00
Example 11
[0060] A representative sample of each of the above described Examples, of 10
grams, is mixed in equal parts and
the properties of the mixture and resulting polymerized composition tested.
The table below reports the results said
measurements. The first five properties reported are tested in accord with ADA
Specification 19: Non-Aqueous
Elastomer Impression Materials (1976, as amended in 19a of 1982).
[0061] The following procedure was used to provide tensile tear strength,
percent elongation, and modulus of
elasticity of the Examples.
[0062] Equal parts of the base and catalyst components are mixed and the
samples or specimen is placed in a
specimen mold having an I-shaped cavity that is 1.5 mm thick, 20 rnm×11
mm, with top arms of 8 mm depth
and center I portion 5 mm wide. The filled mold is clamped between two
stainless steel plates and the assembly is
17
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
placed in a 32° C. water bath. At six minutes from start of mix, the
assembly is removed from the bath. The
mold is unclamped, the specimen is removed from the mold and any flash is
removed from the specimen. At 10
minutes from start of mix the specimen is clamped into the specimen test grips
of an instron Model 1123 in the
extension mode. The Instron is attached to a Microcon II micropressor that has
been programmed to calculate the
tear strength [psi],% elongation, and modulus of elasticity. At 11 minutes,
the specimen is stressed by the Instron at
a rate of 10 mm/min. until the specimen reaches peak failure. (The maximum
load is set to 5 kg.) This is repeated for
five specimens and then statistically evaluated results are reported, as shown
in the Table.
[0063] Wetting contact angles are measured for each Example as follows. One
gram (1 g) of base and one gram (1
g) of catalyst paste are mixed together until uniform (.about.30 seconds). A
one-half gram (0.5 g) of mixed paste is
placed between two sheets of polyethylene (Dentsilk) and pressed flat using a
glass plate, about 2-3 mm thick. The
specimen is allowed to stand undisturbed until set (.about.15 minutes). The
polyethylene sheets are removed, being
careful not to touch the surface of the specimen, and the specimen placed on
the table of a gynometer, a well known
device for measuring contact angles. The eyepiece recticle is adjusted to the
horizontal and vertical planes of the
specimen surface and stop watch is started as a drop of water is dropped onto
the specimen surface. At 1.5 minutes
to 3.5 minutes, the inside contact angle, in degrees, of the water/specimen
interface is measured using the gynometer
scale, recorded for the specimen and reported below.
TABLE
PROPERTIES OF EXAMPLES
Examples
Property 1 2 3 4 5 6 7 8 9 10
Work Time (min)
3 3 3 2 3 4.25
2.50
3.33
3.18
2.50
Set Time (min)
6 6 6 4 6 9 5 7 7 5.75
% Deformation
0.5
0.25
0.45
0.3
18
CA 02510317 2005-06-23
WO 2004/058196 PCT/US2003/040534
1.9
4.25
1.75
2.25
23 1.65
% Strain 2.75
3.15
3.25
2.75
3.5
NA NA NA NA NA
Consistency (mm)
33 34 36 32 38 33 29 32 31 30
Contact Angle° with
30 35 38 37 42 28 52 56 42 31
water at 3 min.
Tear Strength PSI
277
277
295
289
216
NA NA NA NA NA
[0064] Examples 1-3 are preferred compositions. Example 1 is suitable for
dispensing from a tube and hand
mixing. Example 2 is most preferred for cartridge dispensing and static-
mixing. Example 3 describes a composition
of the invention that is suitable for forming a lower viscosity composition
suitable for either tube or cartridge
dispensing.
[0065] The composition of Example 4, having a high viscosity, exhibited severe
gassing, having a higher hydride
concentration and no degassing component. Example 5, having a low viscosity,
demonstrated good syringe
consistency but had a high percent deformation and percent strain while tear
strength was lower. This composition
had a high hydride, low surfactant, low retarder and low catalyst
concentration. Compositions of Examples 6, 8 and
9 did not polymerize properly. The composition of Example 6 had too low
retarder and catalyst. The surfactant was
also too high an HLB and too acid. The composition of Example 7 lacked wetting
capability having a surface
contact angle exceeding desirable limits. Examples 8 and 9 both were too low
in retarder and catalyst concentrations
The composition of Example 10 exceeded desired percent deformation.
19
CA 02510317 2011-08-16
=
64053-516
EXAMPLES USING PEG-8 METHICONE SURFACTANT
[0066] As is otherwise conventional, the impression materials according to the
present invention may be
formulated in a number of viscosities or the like. It is common in the dental
industry to formulate impression
materials having monophase, heavy, rigid, low viscosity, extra low viscosity
and the like. The preferred impression
materials, as discussed above, are two-component addition curing polyvinyl
siloxane in nature. The materials may
also contain silanated, fumed, amorphous and/or crystalline silicas, pigments,
flavorants, plasticizers and/or other
surfactants. The present material is emplyed in a conventional manner for
dental impression materials, taking
advantage of its unexpected and improved characteristics as discussed herein.
[0067] Examples of useful impression materials with which the present
invention may be employed include the
AQUAS IL line of impression materials available from DENTSPLY International
Inc. of York, PA, with the addition
of the PEG-8 Methicone surfactant, such as the BASF MASIL SF 19 surfactant.
[0068] The following are examples of compositions prepared according to the
present invention.
MONOPHASE REGULAR & FAST SET
' COMPONENT CHEMICAL NAME ' Eclipse Mono kS Eclipse Mono
FS
1:1 Cartridge 1:1 Cartridge
Baysilone Crosslinlcing Polymethylhydrogensiloxane 4.25 4.25
Agent =
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 58.51 58.59
Polymer Blend
Baysilone Polymer Divinyl Polydimethylsiloxane 3.508 3.748
Masil SF 19 Polyoxyalkylene Modified 2.00 2.00
Polyclimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.023 0.028
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.30 0.005
Cristobalite Silicon Dioxide, Crystalline 11.00 11.00
Silica PF-5 Silicon Dioxide, Amorphous 7.00 7.00
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 7.00 7.00
Baylith T Sodium AlumiknoSilicate 5.00 5.00
Degass Concentrate Platinum 0.005 0.005
Calcium Carbonate 0.095 0.095
Silicon Dioxide, Crystalline 0.400 0.400
TiO2 #3328 Titanium Dioxide 0.70 0.70
Irgalite Red C2B Calcium Sale Of Beta Oxynapthoic 0.04 0.04
Acid
RD&C Blue #1 FD&C Blue #1 0.04 0.04
100.00 100.00
*Trade-mark
20
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
MONOPHASE REGULAR SET
COMPONENT CHEMICAL NAME
Base 1:1 Cartridge Catalyst _
Baysilone Crosslinldng Polymethylhydrogensiloxane
8.50 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion
55.73 61.89
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane
2.81 4.205
Masil 0 SF 19 Polyoxyalkyene Modified
2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA
0.10 0.10
Catalyst Fluid Organoplatinum Complex
0.0 0.045
Retarder Fluid 1,3 Divinyltetramethyldisiloxane
0.0 0.06
Cristobalite Silicon Dioxide, Crystalline
11.0 11.0
Silica PF-5 Silicon Dioxide, Amorphous
7.0 7.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide
7.0 7.0
Baylith T Sodium AlumilmoSilicate
5.0 5.0
Degass Concentrate Platinum
0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide
0.70 0.70
Irgalite Red C2B Calcium Sale Of Beta Oxynapthoic
0.08 0.00
Acid
RD&C Blue #1 FD&C Blue #1
0.08 0.00
100.00 100.00
=
21
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
=
MONOPHASE FAST SET
COMPONENT CHEMICAL NAME 1:1 Cartridge
Base Catalyst
Baysilone Crosslinldng Polymethylhydrogensiloxane 8.50 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 55.73 61.44
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 2.81 4.695
Masil SF 19 Polyoxyalkyene Modified 2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.055
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.01
Cristobalite Silicon Dioxide, Crystalline 11.0 11.0
Silica PF-5 Silicon Dioxide, Amorphous 7.0 7.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 7.0 7.0
Baylith T Sodium AlumilmoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide 0.70 0.70
Irgalite Red C2B Calcium Sale Of Beta Oxynapthoic 0.08 0.00
Acid
RD&C Blue #1 FD&C Blue #1 0.08 0.00
100.00 100.00
,
22
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
HEAVY REGULAR & FAST SET
COMPONENT CHEMICAL NAME 1:1 Cartridge
Base Catalyst
Baysilone Crosslinking Polymethylhydrogensiloxane 4.00 4.00
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 54.04
53.82
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 8.61 8.85
Masil SF 19 Polyoxyalkyene Modified 2.00 2.00
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.023
0.028
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.030
0.0005
Cristobalite Silicon Dioxide, Crystalline 11.00
11.00
Silica PF-5 Silicon Dioxide, Amorphous 8.00 8.00
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 6.00 6.00
Baylith T Sodium AlumiknoSilicate 5.00 5.00
Degass Concentrate Platinum 0.005 0.0005
Calcium Carbonate 0.095 0.095
Silicon Dioxide, Crystalline 0.400 0.400
TiO2 #3328 Titanium Dioxide 0.20 0.20
Irgalite Red C2B Calcium Sale Of Beta Oxynapthoic 0.25 0.25
Acid
RD&C Blue #1 FD&C Blue #1 0.25 0.25
100.00 100.00
=
=
23
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
HEAVY REGULAR SET
COMPONENT CHEMICAL NAME 1:1 Cartridge
Base Catalyst
Baysilone Crosslinlcing Polymethylhydrogensiloxane 8.00 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 51.20 56.89
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 7.50 9.71
Masil SF 19 Polyoxyalkyene Modified 2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.045
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.06
Cristobalite Silicon Dioxide, Crystalline 11.0 11.0
Silica PF-5 Silicon Dioxide, Amorphous 8.0 8.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 6.0 6.0
Baylith T Sodium AlumiknoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide 0.20 0.20
Irgalite Red C2B Calcium Sale Of Beta Oxynapthoic 0.50 0.00
Acid
RD&C Blue #1 FD&C Blue #1 0.50 0.00
100.00 100.00
24
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
HEAVY FAST SET
COMPONENT CHEMICAL NAME 1:1 Cartridge
Base Catalyst
Baysilone Crosslinking Polymethylhydrogensiloxane 8.00 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 51.20
56.44
Polymer Blend
_
Baysilone Polymer Divinyl Poydimethylsiloxane 7.50
10.20
Masil 0 SF 19 Polyoxyallcyene Modified 2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
_
Catalyst Fluid Organoplatinum Complex 0.0
0.055
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.01
Cristobalite Silicon Dioxide, Crystalline 11.0 11.0
Silica PF-5 Silicon Dioxide, Amorphous 8.0 8.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 6.0 6.0
Baylith T Sodium AlumilmoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide 0.20 0.20
Irgalite Red C2B Calcium Sale Of Beta Oxynapthoic 0.50 0.00
Acid
RD&C Blue #1 FD&C Blue #1 0.50 0.00
100.00 100.00
25
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
RIGID REGULAR AND FAST SET
COMPONENT CHEMICAL NAME 1:1 Cartridge 1:1 Cartridge
Baysilone Crosslinking Polymethylhydrogensiloxane 3.75 3.75
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 31.88 31.90
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 19.15 19.15
_
Masil @ SF 19 Polyoxyalkyene Modified 2.00 2.00
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.023 0.028
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.030 0.005
Cristobalite Silicon Dioxide, Crystalline 13.00 13.00
Silica PF-5 Silicon Dioxide, Amorphous 17.00 17.00
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 7.00 7.00
Baylith T Sodium AlumiknoSilicate 5.00 5.00
Degass Concentrate Platinum 0.005 0.0005
Calcium Carbonate 0.095 0.095
Silicon Dioxide, Crystalline 0.400 0.400
TiO2 #3328 Titanium Dioxide 0.20 0.20
Cosmetic Green Pigment Chromium oxide 0.38 0.38
100.00 100.00
RIGID REGULAR SET
' COMPONENT , ' CHEMICAL NAME ' 1:1 Cartridge '
Base Catalyst
Baysilone Crosslinldng Polymethylhydrogensiloxane 7.50 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 29.15 34.60
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 18.30 20.00
Masil @ SF 19 Polyoxyalkyene Modified 2.00 2.00
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 _ 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.045
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.06
Cristobalite Silicon Dioxide, Crystalline 13.00 _ 13.00
Silica PF-5 Silicon Dioxide, Amorphous 17.00 17.00
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 7.0 7.0
Baylith T Sodium AlumiknoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide 0.20 0.20
Cosmetic Green Pigment Chromium oxide 0.75 0.00
100.00 100.00
26
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
RIGID FAST SET
COMPONENT CHEMICAL NAME
Base 1:1 Cartridge Catalyst .
Baysilone Crosslinking Polymethylhydrogensiloxane
7.50 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion
29.15 34.64
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane
18.30 20.00
Masil @ SF 19 Polyoxyallcyene
Modified 2.00 2.00
Polydimethylsiloxane
Artificial Mint flavor NA
0.10 0.10
Catalyst Fluid Organoplatinum Complex
0.0 0.055
Retarder Fluid 1,3 Divinyltetramethyldisiloxane
0.0 0.01
Cristobalite Silicon Dioxide, Crystalline
13.00 13.00
Silica PF-5 Silicon Dioxide, Amorphous
17.00 17.00
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide
7.0 7.0
Baylith T Sodium AlumiknoSilicate
5.0 5.0
Degass Concentrate Platinum
0.00 0.01
Calcium Carbonate 0.00
0.19
Silicon Dioxide, Crystalline 0.00
0.80
TiO2 #3328 Titanium Dioxide
0.20 0.20
Cosmetic Green Pigment Chromium oxide
0.75 0.00
100.00 100.00
LOW VISCOSITY
COMPONENT CHEMICAL NAME
, ' 1:1 Cartridge
Baysilone Crosslinlcing Polymethylhydrogensiloxane
4.00 4.00
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion
52.99 52.45
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane
3.56 4.11
Masil @ SF 19 Polyoxyallcyene
Modified 2.00 2.00
Polydimethylsiloxane
Artificial Mint flavor NA
.010 0.10
Catalyst Fluid Organoplatinum Complex
0.025 0.030
Retarder Fluid 1,3 Divinyltetramethyldisiloxane
0.030 0.005
Cristobalite Silicon Dioxide, Crystalline
_ 26.50 26.50
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide
_ 4.00 4.00
Baylith T Sodium AlumiknoSilicate
5.00 5.00 .
Degass Concentrate Platinum
0.005 0.005
Calcium Carbonate 0.095
0.095
Silicon Dioxide, Crystalline 0.400
0.400
FD & C Blue #1 FD & C Blue #1
_ 0.05 0.05 .
Dayglo Saturn Yellow Aminotriazine Formaldehyde
0.25 0.25
Pigment Sulphonamide
_
Dayglo Horizon Blue Aminotriazine Formaldehyde
1.00 1.00
Pigment Sulphonamide
_
100.00 100.00
27
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
LOW VISCOSITY
COMPONENT CHEMICAL NAME 1:1 Cartridge
Base Catalyst
Baysilone Crosslinking Polymethylhydrogensiloxane 8.00 0
Agent
_
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 47.40
58.58
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 2.9 4.21
Masil 0 SF 19 Polyoxyalkyene Modified 2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.05
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.06
Cristobalite Silicon Dioxide, Crystalline 28.0 25.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 4.0 4.0
Baylith T Sodium AlumiknoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
FD & C Blue #1 FD & C Blue #1 0.10 0.00
Dayglo Saturn Yellow Aminotriazine Formaldehyde 0.50 0.00
Pigment Sulphonamide
Dayglo Horizon Blue Aminotriazine Formaldehyde 2.0 0.00
Pigment Sulphonamide
100.00 100.00
28 .
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
LOW VISCOSITY
COMPONENT CHEMICAL NAME 1:1 Cartridge
Base Catalyst _
Baysilone Crosslinking Polymethylhydrogensiloxane 8.00 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 47.40 58.14
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 2.9 4.70
Masil SF 19 Polyoxyalkyene Modified 2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.06
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.01
Cristobalite Silicon Dioxide, Crystalline 28.0 25.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 4.0 4.0
Baylith T Sodium AlumiknoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
FD & C Blue #1 FD & C Blue #1 0.10 0.00
Dayglo Saturn Yellow Aminotriazine Formaldehyde 0.50 0.00
Pigment Sulphonamide _
Dayglo Horizon Blue Aminotriazine Formaldehyde 2.0 0.00
Pigment Sulphonamide
100.00 100.00
EXTRA LOW VISCOSITY
COMPONENT CHEMICAL NAME 1:1 Cartridge 1:1
Cartridge
Baysilone Crosslinking Polymethylhydrogensiloxane 4.50 4.50
Agent _
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 61.12 61.15
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 2.86 2.86
Masil SF 19 Polyoxyalkyene Modified 2.00 2.00
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.020 0.020
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.030 0.005
Cristobalite Silicon Dioxide, Crystalline 18.00 18.00
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 4.00 4.00
Baylith T Sodium AlumilcnoSilicate 5.00 5.00
Degass Concentrate Platinum 0.005 0.005
Calcium Carbonate 0.095 0.095
Silicon Dioxide, Crystalline 0.400 0.400
TiO2 #3328 Titanium Dioxide 0.10 0.10
Suntan Iron oxide pigment Iron oxide blend 0.03 0.03
Dayglo Arc Yellow Anubitruazube Formaldehyde 1.75 1.75
pigment _ Sulphonamide
100.00 100.00
29
L
CA 02510317 2005-06-23
WO 2004/058196
PCT/US2003/040534
EXTRA LOW VISCOSITY
COMPONENT CHEMICAL NAME 1:1 Cartridge 1;1
Cartridge
Baysilone Crosslinldng Polymethylhydrogensiloxane 9.00 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 56.24 65.99
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 2.0 3.715
Masil SF 19 Polyoxyalkyene Modified 2.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.035
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.06
Cristobalite Silicon Dioxide, Crystalline 18.0 18.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 4.0 4.0
Baylith T Sodium AlumiknoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide 0.10 0.10
Suntan Iron oxide pigment Iron oxide blend 0.06 0.00
Dayglo Arc Yellow Anubitruazube Formaldehyde 3.5 0.00
pigment Sulphonamide
100.00 100.00
EXTRA LOW VISCOSITY
COMPONENT CHEMICAL NAME 1:1. Cartridge , ;' 1:1
Cartridge
Baysilone Crosslinking Polymethylhydrogensiloxane 9.00 0
Agent
Vinylsilicone Resin Siloxane Vinyl Z-Resin Dispersion 56.24 66.04
Polymer Blend
Baysilone Polymer Divinyl Poydimethylsiloxane 2.0 3.715
Masil SF 19 Polyoxyalkyene Modified 0.00 2.0
Polydimethylsiloxane
Artificial Mint flavor NA 0.10 0.10
Catalyst Fluid Organoplatinum Complex 0.0 0.035
Retarder Fluid 1,3 Divinyltetramethyldisiloxane 0.0 0.01
Cristobalite Silicon Dioxide, Crystalline 18.0 18.0
Cab-O-Sil TS-530 Silinated Fumed Silicon Dioxide 4.0 4.0
Baylith T Sodium AlumiknoSilicate 5.0 5.0
Degass Concentrate Platinum 0.00 0.01
Calcium Carbonate 0.00 0.19
Silicon Dioxide, Crystalline 0.00 0.80
TiO2 #3328 Titanium Dioxide 0.10 0.10
Suntan Iron oxide pigment Iron oxide blend 0.06 0.00
Dayglo Arc Yellow Anubitruazube Formaldehyde 3.5 0.00
pigment Sulphonamide 100.00 100.00
30
CA 02510317 2011-08-16 _
64053-516
10069] The following table (and FIG. 3) shows the physical characteristics of
the extra low viscosity (A), low viscosity
(B), monophase (C) and rigid (D) forms of the invention employing MASIL SF-19.
As can be seen in the table and in
FIG. 3, the wetability and tear strengths are greatly improved over what has
been heretofore accomplished in the art.
In the table, XLV is extra low viscosity, LV is low viscosity, and RS is
regular set, as such terms are conventionally
used in the art.
PVS COMPETITIVE TESTING COMPANY: LDC
BRAND: ECLIPSE SUPERWET IM REGULAR SET
VISCOSITY /TYPE
BATCH it's
XLV RS LV RS MONO RS RIGID RS
PROPERTY TESTED
ADA 19 WORK TIME N/A N/A N/A N/A
BENCH WORK TIME 3'00" 4'00" 3'20" 3'05"
BENCH SET TIME 5'45" 7'35" 6'30" 6'00"
BASE CONSISTENCY (mm) 44 39 34 33
CATALYST CONSISTENCY (mm) _ 43 40 34 33
MIX CONSISTENCY (min) 42 39 33 33
% RECOVERY @ 5' MRT 99.7 99.1 98.9 98.5
% STRAIN ISO @5' MRT 3.4 3.0 2.7 1.2
SHORE A HARDNESS 60 62 63 75
DETAIL REPRODUCTION 20 micron 20 micron 20 micron 20 micron
% DIMENSIONAL CHANGE
IMMEDIATE -0.39 -0.31 -0.30 -0.33
24 HOUR -0.45 -0.34 -0.42 -0.42
1 WEEK -0.44 -0.42 -0.46 -0.47
WATER CONTACT ANGLE @ 0" 46 deg. 47 deg. 36 deg.
35 deg.
WATER CONTACT ANGLE @ 15" 9 deg. 11 deg. 2 deg. 3 deg.
WATER CONTACT ANGLE @ 30" 7 deg. 6 deg. 2 deg. 2 deg.
15 min. DELAYED POUR GASSING Passes Passes Passes Passes
CAPATIBILITY WITH GYPSOM 20 micron 20 micron 20 micron 20 micron
TEAR STRENGTH (psi) 346 318 361 272
% ELONGATION 65 55 66 26
MODULUS OF ELASTICITY 701 834 748 1179
[0070] There is also demonstrated below, the embodiment of the invention
wherein all of the surfactant is
incorporated into the base paste before it is combinded with the catalyst
paste. It has been unexpectedly found, that
when the surfactant according to the invention is incorporated into the base
paste only, improved contact angles
were achieved. These contact angles arc even superior to those of the present
invention where the surfactant is
incorporated into both the base and the catalyst pastes, which themselves show
an improvement over the prior art.
This was unexpected from the prior art which does show impression materials
with a surfactant in the base paste
31
WO 2004/058196 CA 02510317 2005-06-23PCT/US2003/040534
only. Even though such materials exist, none show the improvement of the
present surfactant, and none disclosed
further improvements in contact angle data as compared to when the surfactant
is incorporated into both pastes.
[0071] For exemplary purposes, a number of materials according to this
embodiment of the invention were
prepared. These included examples of extra low viscosity (XLV), low viscosity
(LV), Monophase, and heavy
viscosity and rigid, as such terms are conventionally used in the dental arts.
RS is "regular set" as such term is
conventionally used in the dental arts.
32
.9e
99)
kr)
o
.9e
o
99)
o
o
el
ci)
Aquasil Ultra Rigid RS ( 1:1 Cartridge)
E=1
c.) COMPOSITION
(% BY WEIGHT)
pi.
_
COMPONENT CHEMICAL NAME ..: - .
CAS NO. Rigid RS
,
(1:1) Cartridge
.
. . Base Catalyst '
co ' Baysilone Crosslinking Agent
Polyinethylhydrogensiloxane 68037-59-2
8.50 - 0
C \I
1 Vinylsilicone Resin Polymer Blend Siloxane Vinyl Q-
Resin Dispersion 68083-19-2 26.05
36.50 '
ko
0
1 Baysilone Polymer Divinyl
Polydimethylsiloxane 68083-19-2 18.30
20.00
Lo
0= Masil @ SF 19 Polyoxyalkyene
modified polydimethylsiloxane 68937-54-2 4.00
0.00 ,
0
C \I Oil of Peppermint NA
80006-90-4 0.20
0.20
r-
H 1 Catalyst Fluid Organoplatinwn
Complex . 68748-92-2 0.0
0.045
co
0 Retarder Fluid 1,3
Divinyltetramethyldisiloxane 2627-95-4 0.0
0.06
H
Lo
cn
C \I Cristobalite Silicon Dioxide,
Crystalline 14464-46-1 13.00
13.00 c,-)
0
Silica PF-5 Silicon Dioxide, Amorphous
7631-86-9 17.00 17.00
4
U Cab-O-Sil TS-530 Silanated Fumed
Silicon Dioxide 68909-20-6 7.0
7.0
UOP T- POWDER Sodium AluminoSilicate
1318-02-1 5.0_ 5.0
Degass Concentrate Platinum
471-344 0.00 0.01
Calcium Carbonate 7440-06-4
0.00 0.19
Silicon Dioxide, Crystalline 14464-46-1
0.00 0.80
TiO2 #3328 Titanium Dioxide
13463-67-7 0.20 0.20
Cosmetic Green Pigment Chromium oxide
1308-38-9 0.75 0.00
,--i T9TOTAL' '
' ' 100.00
100.000
_
kr)
o
.9e
o
o
el
0
.9e
99)
kr)
o
.9e
o
99)
o
o
el
ci)
E=1
Aquasil Ultra Heavy
RS ( 1:1 Cartridge)
c.)
pi.
COMPOSITION (% BY WEIGHT)
.
COMPONENT
CHEMICAL NAME.i.õ ,..., ....: .= = CAS NO.
Heavy RS
- ,. = .. = .. . ==
.
. . (1:1) Cartridge
. ==_ . -... = =
= .. ' .
Base Catalyst
C \ I
, Baysilone Crosslinking A . cut
Polymethylhydrogensiloxane
68037-59-2
8.50
0
ko
0 Vin lsilicone Resin Pot mer
Blend Siloxane
Viliy1Q-Resin Dispersion
68083-19-2
48.60 58.79
1
Lo Baysilone Polymer
Divinyl
Polydimethylsiloxane
68083-19-2
7.50
9.71
0
0
Masil SF 19
Polyoxyalkyene modified polydimethylsiloxane
68937-54-
2 4.00
0.0
C \ I
N Oil of Peppermint
NA
80006-90-4
0.20 0.20
. --i-
H
VI
co Catalyst Fluid
0.0 0,045
Orgattoplatinum Complex
68748-92-2
0
_
H
Lo Retarder Fluid
1,3
Divinyltetramethyldisiloxane
2627-95-4
0.0
0.06
C \ I
0 Cristobalite
14464-46-1
11.0 11.0
Silicon Dioxide, Crystalline
4
8.0
Silica PP-5
Silicon Dioxide, Amorphous7631-86-9
8.0
c.)
Cab-O-Sit TS-530
Silanated Fumed Silicon Dioxide
68909-20-
6 6.0
6.0
UOP T- POWDER
Sodium AluminoSiiicate
1318-
02-1 5.0
5.0
Degass Concentrate
Platinum
471-34-
1 0.00
0.01
Calcium Carbonate
7440-06-4
0.00 %.,
0.19
Silicon Dioxide, Crystalline
14464-46-1
0.00
0.80
TiO2 113328
Titanium Dioxide
13463-67-
7 0.20
0.20
c: Cosmetic Green Pigment
Chromium oxide
1308-38-9
0.50 0.00
,--1
o Dayglo Saturn Yellow Pigment
Aminotriazine
Formaldehyde Sulphonamide
39277-28-6
0.50 0.00
kr)
o. .. :..
._ .-
.9e TOTAL
. . ..
*
100.00 100.00
!
. .... = .
_:.. _ ... ____ _ ______ . ..__ ...
= . .. . . .
o
. .
o
el
0
=
.9e
99) -
kr)
o
.9e
o
99)
o
o
el
v)
Aquasil Ultra Moimpliase RS ( 1:1 Cartridge)
E=1
COMPOSITION (% BY WEIGHT)
c.)
pi.
COMPONENT CHEMICAL NAME : .. . .
CAS NO. . (eWirtid rn gphase RS ,
.. .= =
_ Bas 1:1) Ca e
.. .
. - Catalyst
_
Baysilone Crosslinking Agent Polymethylhydrogensiloxane
68037-59-2 8.50 0
co
C\ I Vinylsilicone Resin Polymer Blend
58.00 69.65
1 Siloxane Vinyl Q-Resin
Dispersion 68083-19-2
ko
0 Baysilone Polymer Divinyl
Polyditnethylsiloxane 68083-19-2 3.26
4.106
Lc)1
Masil 0 SF 19 Polyoxyalkyene modified
polyditnethylsiloxane 68937-54-2 4.00 0.0
0
;
0
Oil of Peppermint NA
80006-90-4 0.20 0.20
C \ I
.
N Catalyst Fluid Organoplatinurn Complex
68748-92-2 0.0 0.044
H
co Retarder Fluid 1,3
Divinyltetramethyldisiloxane 2627-95-4 0.0
0.06
0
H
Ln
Lo Cristobalite Silicon Dioxide,
Crystalline _ 14464-46-1 10.83
9.87
CO
C \ I
0 Silica PF-5 Silicon Dioxide,
Amorphous _ 7631-86-9 5.0
5.0
4
5.0
Cab-O-Sil TS-530 Silanated Fumed Silicon Dioxide
68909-20-6 _ 5.0
o
UOP T- POWDER Sodium AluminoSilicate
_ 1318-02-1 _ 5.0 5.0
._
Degass Concentrate Platinum
471-34-1 0.00 0.01
Calcium Carbonate 7440-06-4 0.00
0.19
Silicon Dioxide, Crystalline 14464-46-1 0.00
'= 0.80
TiO2 113328 Titanium Dioxide
13463-67-7 0.07 0.07
Irgalite Red C2B Calcium Salt Of Beta Oxynapthoic Acid
65997-06-0 _ 0.10 0.00
FD&C Blue 111 FD&C Blue #11
2650-18-2 0.04 0.00
c:
.-
oc, 1 TOTAL-100.00
= ' = " 100.000
kr),
,
o
.9e
o
o
el
0
.9e
99)
kr)
o
.9e
o
99)
o
o
el
ci)
E=1
Aquasil Ultra
LV RS ( 1:1 Cartridge)
c.)
pi.
COMPOSITION (% BY WEIGHT)
COMPONENT
CUEMICAL NAME .... : .
. .. CAS NO.
LV RS
õ. .,... . ,
.. . ...% . = .
(1:1) Cartridge
. .
.. =
Base Catalyst
co
C\I
, Baysilone Crosslinking Agent
Polymethylhydrogensiloxane
, 68037-59-2
8.00
0
ko
0 Vinylsilicone Resin Polymer
Blend Siloxane
Vinyl Q-Resin Dispersion
_ 68083-19-2
43.50
60.48
1
Lo Baysilone Polymer
4.21
Divinyl Polydimethylsiloxane , 68083-19-2
2.9
0
0
Masi! SF 19
_ Polyoxyalkyene modified polydimethylsiloxane
68937-
54-2 6.00
0.00
C\I
N Oil of Peppermint
NA
80006-90-4
0.20 0.20
H
co Catalyst Fluid
Organoplatinum Complex
68748-92-2
0.0
0.05
0
H
Lo Retarder Fluid
1,3
Divinyltetramethyldisiloxane
2627-95-4
0.0
0.06 ,
C\I
0 Cristobalite
Silicon
Dioxide, Crystalline
14464-46-1
28.0
25.0 VD
Cn
4 Cab-O-Sil TS-530
Silanated
Fumed Silicon Dioxide
4.0
4.0
68909-20-6
o
UOP T- POWDER
Sodium AluminoSilicate
1318-
02-1 5.0
5.0
Degass Concentrate
Platinum
471-
34-1 0.00
0.01
Calcium Carbonate
7440-06-4
0.00
0.19
Silicon Dioxide, Crystalline
14464-46-1
0.00 ..,
0.80
FD&C Blue ill
FD&C Blue III
2650-
18-2 0.10
0.00
Dayglo Saturn Yellow Pigment
Aminotriazine Formaldehyde Sulphonamide
39277-
28-6 0.50
0.00
Dayglo Horizon Blue Pigment
Atninotriazine Formaldehyde Sulphonamide
2.0
0.00 1
39277-28-6
c:
,-1
oc, TOTAL
= .
= .
100.00 100.000
kr)
o
.
.9e
o
o
el
0
CA 02510317 2011-08-16
64053-516
[0072] Contact angle data was collected at one second intervals for fifteen
seconds after the base paste and the
catalyst paste were mixed. For purposes of comparison, the same contact angle
data was determined for a number of
commercially available commercially available impression materials. These
included AQUASIL XLV, LV, RIGID
and Monophase impression materials, available from LD Caulk Division of
DENTSPLY International. The contact
angle data for the inventive and commercially available products is shown in
graph form in Fig. 4.
[0073] As shown in Fig. 4, the XLV, LV, Rigid, Monophase and Heavy
formulations had a contact angle of below
80 degrees or even below 20 degrees, after 1 second, whereas the AQUASIL
materials had contact angles of greater9'
than 100 degrees. At 15 seconds, the inventive materials all showed a contact
angle of less than 10 degrees, whereas
the commercially available products shdwed a contact angle of greater than 60
degrees.
[0074] A similar set of data were determined for the inventive materials and
graphed with data from the
commercially available IMPREGNUM impression material from ESPE. At two
seconds, the contact angle of the
IMIPREGNUM materials were all above 50 degrees, with no or little improvement
out to as long as 15 seconds. The
inventive materials showed a contact angle of 10 or close to 10 at 2 seconds,
and better than 10 degrees at 15
seconds. This data is reported in Fig. 5.
[0075] Test data was obtained as follows.
Tear Strength
[0076] 1. Materials are mixed according to the manufactures directions and
loaded into molds to prepare
specimens (see the attached figure) for measuring the tear strength in the
tensile mode. Ten specimens
for each material are made.
[0077] 2. All specimens are cured in a 37 degree oven for 15 minutes before
removing from the mold in
preparation for testing.
[0078] 3. All specimens are pulled in the tensile mode, within one hour, on a
Instron universal testing machine.
The specimens are pulled at a rate of 100mmimin until the specimens tears at
the internal right angle of
the specimen.
*Trade¨mark
37
WO 2004/058196 CA 02510317 2005-06-23PCT/US2003/040534
[0079] 4. The Force required to tear the specimen is calculated using Instrons
series IX software. The average
value for each test is reported.
Contact angle
[0080] 1. Materials are mixed according to the manufactures directions.
[0081] 2. Material is dispensed and placed in a 5 x 10 mm specimen ring
between two mylar sheets and pressed
flat using a glass plates.
[0082] 3. The specimen is allowed to cure undisturbed for 15 minutes.
[0083] 4. The mylar sheets are removed and the specimen removed from the ring,
being careful not to touch the
test surface of the specimen.
[0084] 5. The specimen is transferred to the surface of the testing platform
on a VCA 2500xe video contact
angle instrument.
[0085] 6. The instrument has been set up to measure the contact angle of a
drop of water, in the static mode, at 1
sec intervals for 35 seconds from initial water contact.
[0086] 7. The determination of the contact angle from 1-30 seconds is recorded
and graphed.
Viscosity build-up
[0087] 1. TA Instruments AR-1000 rheometer equipped with temperature
controlling peltier plate and a 4 cm 2
deg cone geometry was used for collecting viscosity build-up data.
[0088] 2. Using Advantage software and navigator script the instrument was set
to collect viscosity data in the
shear mode. A shear stress of 1000 Pa's was used. The temperature was ramped
from 28 ¨35 degrees
Celsius to simulate mixing and insertion into the oral environment.
[0089] 3. The test materials were mixed and immediately placed under the 4 cm
2 deg cone geometry on the
surface of the test stage. The test script was begun, the geometry was gapped
to 54 microns, a 5 sec
pre-shear of 1500 Pa's was applied, the viscosity data was then collected at
1000 Pa's for 5 minutes
as the temperature ramped from 28-35 deg C.
[0090] 4. Viscosity change versus Global Time was analyzed and transferred to
Excel for graphical
representation. 38
WO 2004/058196 CA 02510317 2005-06-23PCT/US2003/040534
[0091] Other properties were tested according to standardized procedures, such
as ISO 4823 for Elastomeric
Impression materials. It was also observed that tear strength data for the
inventive materials was as good as or
improved over the prior art materials. Further, particularly with the
embodiment of the invention wherein the
surfactant is incorporated into the base paste only, improvements in product
stability were noted. All of these
improvements were determined together with the greatly improved contact
angles.
[0092] Based upon these results, the inventive materials have superior
characteristics and out performed
commercially available impression material products especially in the areas of
hydrophilicity and tear strength.
39