Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02511753 1993-07-09
PHOSPHOLIPID DERIVATIVES
The invention relates to new phospholiped derivatives,
pharmaceutical compositions containing them, and their use
in the treatment of tumours, and protozoal and fungal
diseases.
This patent application is divided from and comprises the
disclosure of Canadian Patent Application 2,100,228, filed
July 9, 1993. Consequently, the language "the invention"
and the like as used herein, and the description and
examples given, are not restricted to the subject matter
claimed in this divided application, but may include
subject matter not specifically claimed.
Published European Patent Application 108,565 describes
compounds of the general formula
O R2
11 +
R1(O)n P OCH2CH2N -R3
I O R4
and their pharmaceutically-acceptable salts, in which R' is
an aliphatic hydrocarbon radical having 8-30 carbon atoms
and the radicals R2, R3 and R4 are identical or different
and are hydrogen or lower alkyl radicals, or in which the
group NR2R3R4 is a cyclic ammonium group, and n has the
value 0 or 1. Antitumor and antifungal activity are
indicated for these compounds.
The present invention relates to alkyl or alkene phosphates
in which the choline radical is part of a heterocyclic
ring, to a process for the preparation of those compounds,
to pharmaceutical compositions containing the compounds as
1
CA 02511753 1993-07-09
active ingredients, and to processes for the preparation of
those drugs.
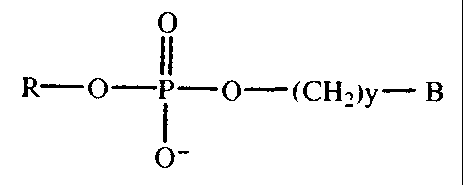
In a broad aspect the compounds have the general formula
0
R-X-A- I-0-(cH,2)y- .B
o-
wherein:
B is the ring system
(CH2)m\ R,
-CH\(CEiz)n / R2
or B is a tropanio or quinuclidinio ring system, which is
linked via a carbon-carbon bond and optionally is
substituted by one or more methyl groups;
R is a straight-chain or branched alkyl radical having 10
to 24 carbon atoms, which also can contain one or more
double or triple bonds;
R' and R2, independently of one another, are hydrogen, or
each is a straight-chain or branched saturated alkyl
radical having 1 to 6 carbon atoms, a straight-chain or
branched unsaturated alkyl radical having 2 to 6 carbon
atoms, or a cyclic saturated or unsaturated alkyl radical
having 3 to 6 carbon atoms, which also can contain a Cl, OH
or NH2 group, and where the straight-chain or branched alkyl
radical R' and R2 also can be bonded together to form a
ring;
A is a single bond, or one of the groups having the
formulae
2
CA 02511753 2006-11-01
-CH2-CH2-CH2-O- (II) -CH2-CH2-O- (III)
-CH2-CH-O- (IV) -S-(CH2)8-O- (V)
IH3'
-CH CH-CH2-O-
I ~ (VI)
H2C j
\CH2
wherein the groups (II) to (VI) are arranged such that
the oxygen atom is bonded to the phosphorus atom of
the compound (I);=
X is an oxygen or sulphur atom or NH if A is a single
bond, or is an oxygen or sulphur atom if A is one of the
groups (II) to (VI);
y is 0, 1, 2 or 3; and
m and n are independently 0 or an integer, with the
proviso that m + n = 2 to 8;
and pharmaceutically-acceptable salts thereof.
In a specific embodiment, the present invention provides
compounds of the general formula
0
11
R O P O (CH,)y B
O-
wherein:
B is a quinuclidinio ring system linked via a carbon atom
of the quinuclidinio ring and in which the nitrogen atom
optionally is substituted by a methyl group, or a tropanio
ring system linked via a carbon atom of the tropanio ring
3
CA 02511753 2006-11-01
and in which the nitrogen atom optionally is substituted by
one or two methyl groups;
R is a straight-chain or branched-chain alkyl radical
having 10 to 24 carbon atoms, which also can contain one or
more double or triple bonds; and
y is 0, 1, 2 or 3;
or a pharmaceutically-acceptable salt thereof.
Preferably, y is zero, and R is an octadecyl or hexadecyl
group. Also B advantageously is a 1-methylquinuclidinio-3-
yl or 1,1-dimethyltropanio-4-yl ring.
The present invention also provides a pharmaceutical
composition comprising, as an active ingredient, at least
one compound according to the invention, or a
pharmaceutically-acceptable salt thereof, and a
pharmaceutically-acceptable carrier therefor. The
pharmaceutical composition also may include
pharmaceutically-acceptable excipients, adjuncts, fillers
and diluents. The amount of active ingredient in the
4
CA 02511753 1993-07-09
pharmaceutical dosage unit the pharmaceutical composition
preferably is between 50 mg and 250 mg.
The present invention also provides for treating a tumor,
autoimmune disease or skin disease, and for combating
protozoal and fungal diseases, using effective amounts of
the compounds. They are particularly useful for treating
leishmaniasis, multiple sclerosis and psoriasis.
In addition, the invention provides for treating bone
marrow damage due to treatment with cytostatic agents and
other myelotoxic active ingredients which comprises
administering, to a host having bone marrow damage due to
treatment with cytostatic agents or other myelotoxic active
ingredients, an effective amount of a compound of the
invention.
The invention also provides for treatment of a viral
disease by administering to a host having such a disease an
effective amount of a compound of the invention.
Surprisingly, the compounds according to the invention have
better antitumor activity than the open-chain derivatives
described in E.P. 108,565.
The invention further provides processes for the
preparation and processes for the purification of the novel
compounds.
CA 02511753 1993-07-09
The first step of process A consists in reacting phosphorus
oxychloride with long-chain alcohol in halogenated
hydrocarbons, saturated cyclic ethers, acyclic ethers,
saturated hydrocarbons having 5 to 10 C atoms or liquid
aromatic hydrocarbons which can also be substituted by
halogen (especially chlorine), or in mixtures of the above-
mentioned solvents, or without a solvent, optionally in the
presence of a basic substance conventionally used for this
purpose.
Examples of possible halogenated hydrocarbons are
hydrocarbons having 1 to 6 C atoms, one or more or all of
the hydrogen atoms being replaced with chlorine atoms.
Methylene chloride, chloroform, ethylene chloride,
chlorobenzene and dichlorobenzene, for example, can be
used. In the case of halogen-substituted aromatic
hydrocarbons, these are preferably substituted by one or
two halogen atoms.
Examples of saturated cyclic ethers which can be used are
ethers with a ring size of 5-6 which consist of carbon
atoms and one or 2 oxygen atoms, examples of.such ethers
being tetrahydrofuran and dioxane.
The acyclic ethers consist of 2 to 8 carbon atoms and are
liquid, possible examples being diethyl ether, diisobutyl
ether, methyl tert-butyl ether and diisopropyl ether.
Possible saturated hydrocarbons are unbranched and branched
hydrocarbons which consist of 5 to 10 carbon atoms and are
liquid, possible examples being pentane, hexane, heptane
and cyclohexane.
Examples of possible aromatic hydrocarbons are benzene and
alkyl-substituted benzenes, the alkyl substituents
consisting of 1 to 5 carbon atoms.
6
CA 02511753 1993-07-09
Possible basic substances both for the reaction of the
phosphorus oxychloride with the long-chairi alcohol and for
the subsequent conversion to the phosphoric acid diester
are amines, for example aliphatic amines of the formula
NR1R2R3, R1, R2 and R3 being identical or different and,
being hydrogen or C1-C6- alkyl, or else aromatic amines such
as pyridine, picoline and quinoline. The basic substance
required for the conversion to the phosphoric acid diester
can be added simultaneously with or else before the amino
alcohol or ammonium alcohol salt.
A solvent is necessary in every case for this reaction,
i.e. if the first reaction step is carried out without a
particular solvent, one must now be added. The molar ratio
of phosphorus oxychloride to the long-chain alcohol is for
example between 1.5:1 and 0.8:1.
The amino alcohol or the ammonium alcohol salt is for
example used in excess, based on the long-chain alcohol
(about 1.1 - 1.5 molar excess).
If the reaction of the phosphorus oxychloride with the
long-chain alcohol is carried out in the presence of a
basic substance, the amount of the basic substance is for
example 1 to 3 mol, based on 1 mol of POC13. The amount of
basic substance used for the subsequent conversion to the
phosphoric acid diester is for example 1 to 5 mol, based on
1 mol.
The temperature of the reaction of phosphorus.oxychloride
with the long-chain alcohol is between -30 C and +30 C,
preferably between -15 C and +5 C and especially between
-10 C and -5 C.
The duration of this reaction is for example 0.5 - 5 hours,
preferably 1 - 3 hours and especially 1.5 - 2 hours. If it
7
CA 02511753 1993-07-09
is carried out in the presence of a basic substance, the
reaction generally proceeds rapidly (about 30 minutes).
The amino alcohol or the ammonium alcohol salt is then
added in portions or all at once. Possible ammonium
alcohol salts are those with mineral acids (for example
sulphuric acid, hydrochloric acid) and also those with
organic acids, for example acetic acid, paratoluene-
sulphonic acid and the like. This reaction step takes
place in an inert solvent. Possible solvents here are the
same ones as those used for the reaction of the phosphorus
oxychloride with the long- chain alcohol, in the case where
this reaction is carried out in a solvent.
The basic substance is then added dropwise, either
dissolved in one of the indicated solvents or without a
solvent. The following are preferably used here as
solvents for the basic substance: halogenated hydrocarbons,
saturated cyclic ethers, acyclic ethers, saturated
hydrocarbons having 5 to 10 carbon atoms, liquid aromatic
hydrocarbons or mixtures of the above-mentioned solvents.
These are the same solvents as those which can be used for
the reaction of the phosphorus oxychloride with the long-
chain alcohol.
The addition of the basic substance raises the temperature.
Care is taken to ensure that the temperature is kept in a
range of between 0 C and 40 C, preferably between 10 C and
30 C and especially between 15 C and 20 C.
The reaction mixture is then stirred at 5 C to 30 C,
preferably 15 C to 25 C (for example for 1 hour to 40
hours, preferably 3 hours to 15 hours).
The reaction mixture is hydrolyzed by the addition of
water, during which the temperature should be kept at
8
CA 02511753 1993-07-09
between 10 C and 30 C, preferably between 15 C and 30 C and
especially between 15 C and 20 C. --
The above-mentioned hydrolyzing liquids can also contain
basic substances, such basic substances possibly being
alkali metal and alkaline earth metal carbonates and
bicarbonates.
To complete the hydrolysis, stirring is then continued for
a further 0.5 hour to 4 hours, preferably 1 to 3 hours and
especially 1.5 to 2.5 hours, at 10 C to 30 C, preferably at
C to 25 C and especially at 18 C to 22 C.
The reaction solution is then washed with a mixture of
15 water and alcohols (preferably saturated aliphatic alcohols
having 1 to 4 carbon atoms) which can optionally also
contain a basic substance. The mixing ratio water:alcohol
can be for example between 5 and 0.5, preferably 1 - 3
(v/v).
Examples of possible basic substances for the washing
liquid are alkali metal and alkaline earth metal carbonates
and bicarbonates, as well as ammonia (for example aqueous
ammonia). A 3% solution of sodium carbonate in water is
particularly preferred.
The reaction solution can then optionally be washed with an
acid solution. The acid washing is advantageous for
removing basic components of the reaction solution which
have not yet reacted, especially when methylene chloride is
used as the solvent.
The washing solution consists of a mixture of water and
alcohols. Mixtures of saturated aliphatic alcohols having
1 to 4 carbon atoms are preferred, it optionally being
possible for an acid substance to be present as well. The
9
CA 02511753 1993-07-09
mixing ratio water:alcohol can be for example between 5 and
0.5, preferably 1 - 3 (v/v).
Examples of possible acid substances for the washing liquid
are mineral acids and organic acids, for example
hydrochloric acid, sulphuric acid, tartaric acid or citric
acid. A 10% solution of hydrochloric acid in water is
particularly preferred.
This is followed by a further washing with a mixture of
water and alcohols. Mixtures of saturated aliphatic
alcohols having l to 4 carbon atoms are preferred, it
optionally being possible for a basic substance to be
present as well. The mixing ratio water:alcohol can be for
example between 5 and 0.5, preferably 1 - 3.
The washed phases are then combined and dried in
conventional manner, after which the solvent is removed
(preferably under reduced pressure, for example at 5 to
100 mbar), optionally after the addition of 150 - 1000 ml,
preferably 300 - 700 ml and especially 450 - 550 ml of an
aliphatic alcohol (based on 1 molar part by weight of dry
product). Preferred alcohols are saturated aliphatic
alcohols with a chain length of 1 to 5 carbon atoms,
particularly preferred alcohols being n-butanol and
isopropanol. The purpose of this alcohol treatment is the
complete removal of residual water and the avoidance of
foaming.
Further purification of the.product can be effected for
example by dissolving the crude product in hot ethanol,
filtering off the residue and treating the filtrate with a
TM
mixed bed ion exchanger such as, for example, Amberlite MB3
in ethanolic solution. Any commercially available acid and
basic ion exchangers can be used, simultaneously or
successively, instead of a mixed bed ion exchanger.
CA 02511753 1993-07-09
The solution is then recrystallized from ketones such as,
for example, acetone or methyl ethyl ketone; digestion with
the above solvents is sufficient in some cases. It may be
convenient to purify the products by column chromatography
or flash chromatography on silica gel using mixtures of
chloroform, methylene chloride, methanol and 25% ammonia
solution, for example, as the eluent.
Process variant B consists in the subsequent alkylation of
products which are obtainable by process A using_amino
alcohols. Examples of alkylating agents which can be used
are methyl p-toluenesulphonate or dimethyl sulphate.
Possible solvents are those which have been mentioned
above.
Alkali metal carbonates are examples of basic substances
used. The reaction is carried out at elevated temperature,
for example at the boiling point of the solvents.
The following examples comprise some which are included
herein for illustrative and comparative purposes, and as
such may enhance an understanding of the invention,
although not all of the compounds prepared are specifically
claimed herein.
30
11
CA 02511753 1993-07-09
Examples:
Example 1: Name (IUPAC nomenclature)
4-(((Octadecyloxy)hydroxyphosphenyl)oxy)-
1,1-dimethylpiperidinium hydroxide internal
salt
Abbreviated name:
Octadecyl 1,1-dimethylpiperidinio-4-yl
phosphate
C25H52NO4P (461.66) - ZH2O
Preparation variant A:
10.3 ml (0.11 mol) of phosphorus oxychloride are placed in
100 ml of chloroform and cooled to 5 - 10 C. A solution of
27.0 g (0.10 mol) of 1-octadecanol in 100 ml of chloroform
and 35 ml of pyridine is added dropwise over 30 min, with
stirring. After subsequent stirring for 30 min at 5 - 10 C,
39.1 g (0.13 mol) of 4-hydroxy-l,1-dimethylpiperidinium
tosylate are added in a single portion. After the addition
of 40 ml of pyridine and 30 ml of DMF, the mixture is stirred
for 24 h at room temperature. It is then hydrolyzed with 15
ml of water and subsequently stirred for 30 min and the
organic phase is washed with 200 ml each of water/methanol
(1: 1) , 3% Na2CO3/methanol (1:1) and finally water/methanol
(1:1). The organic phase is concentrated, the residue is
dissolved in 300 ml of hot ethanol and the solution is
filtered after cooling. The filtrate is stirred with 80 g of
Amberlite* MB3 ion exchanger, the mixture is filtered and the
filtrate is concentrated. The residue is recrystallized from
300 ml of methyl ethyl ketone, filtered off with suction and
dried under vacuum over P205
* Trade-Mark
12
CA 02511753 1993-07-09
Yield: 4.71 g (10%)
Elemental analysis:
C H N
calc.: 65.26% 11.63% 2.620
found: 64.38% 11.61% -.2.730
65.04% 11.80% 2.78%
Thin layer chromatogram:
(chloroform/methanol/1 M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.17
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.12 -
Melting point: 270 - 271 C (decomposition)
Preparation variant B:
20.1 ml (0.22 mol) of phosphorus oxychloride are placed in
100 ml of methylene chloride and cooled to 5 - 10 C and a
solution of 54.1 g (0.20 mol) of octadecanol in 400 ml of
methylene chloride and 70.5 ml of pyridine is added over
min, with stirring. After subsequent stirring for one
hour, 29.9 g (0.26 mol) of 4-hydroxy-l-methylpiperidine in
80 ml of pyridine are added dropwise. After stirring for
3 h at ~0 C, the -mixture is hydrolyzed with 30 ml of water
30 while being cooled with ice and is subsequently stirred for
one hour. The organic phase is washed with 200 ml each of
water/methanol (1:1), 3 percent hydrochloric acid/ methanol
(1:1) and water/methanol (1:1). The organic phase is dried
over Na2SO4 and concentrated until turbidity appears, and
1 1 of methyl ethyl ketone is added. The crystals are
recrystallized from 1 1 of methyl ethyl ketone, filtered
off with suction and dried under vacuum over P205.
13
CA 02511753 1993-07-09
Yield: 54.1 g(600) of octadecyl 1-methylpiperidinio-
4-yl phosphate
98.1 g (0.22 mol) of octadecyl 1-methylpiperidinio-4-yl
phosphate are suspended in 500 ml of absolute ethanol and
heated to boiling. Under reflux, a total of 71.8 g (0.39
mol) of methyl p-toluenesulphonate and 26.5 g (0.19 mol) of
potassium carbonate are added alternately in eight portions
over 2 h. When the addition is complete, the mixture is
refluxed for a further hour. After cooling, it is
filtered, the filtrate is concentrated to half and 150 g of
TM
moist Amberlite MB3 ion exchanger are added to the
solution. After stirring for two hours, the mixture is
filtered with suction over kieselguhr/activated charcoal
and the filtrate is concentrated and crystallized with
acetone. The crystal cake is recrystallized from methyl
ethyl ketone and dried under vacuum over P205.
Yield: 46.1 g(46%) of octadecyl 1,1-dimethylpiperidinio-
4-yl phosphate
Elemental analysis:
C H N
calc.: 65.26% 11.63% 2.62%
found: 65.18% 11.62% 2.68%
65.070 11.710 2.700
Melting point: 271 - 272 C (decomposition)
Example 2: Hexadecyl piperidinio-4-yl phosphate
C21H44N04P (405.558)
14
CA 02511753 1993-07-09
7.1 ml (77 mmol) of phosphorus oxychloride are dissolved in
50 ml of dry tetrahydrofuran and, after cooling to
- 10 C, a solution of 17 g (70 mmol) of hexadecanol and
48 ml of triethylamine in 150 ml of tetrahydrofuran is
5 added dropwise, with stirring. When the addition is
complete, the mixture is subsequently stirred for 30 min in
an ice bath and then left to warm up to room temperature.
10.1 g (100 mmol) of 4-piperidinol are dissolved in 100 ml
of tetrahydrofuran and mixed with 17 ml of triethylamine
and the mixture is added dropwise to the reaction solution,
with stirring, so that the temperature does not exceed
40 C. When the addition is complete, the mixture is
refluxed for one hour. While still hot, the solution is
separated from the triethylammonium chloride by filtration
and, after cooling, is poured into an ice/2 M hydrochloric
acid mixture, with stirring. The product-obtained on
cooling in a refrigerator is taken up in methylene
chloride, dried over MgSO4, concentrated and chromato-
graphed on silica gel with methylene chloride/methanol/ 25%
ammonia (70:30:5). The product fractions are combined and
concentrated. After recrystallization from methanol, the
product is dried under vacuum over P205.
Yield: 10.0 g (35%)
Elemental analysis:
C H N
calc.: 62.19% 10.94% 3.45%
found: 65.15% 11.14% 3.54%
62.41% 11.19% 3.34%
Thin layer chromatogram:
(chloroform/methanol/25o ammonia 70:20:10)
Rf = 0.42
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.33
CA 02511753 1993-07-09
Example 3: Hexadecyl 1,1-dimethylpiperidinio-4-yl
phosphate -
C25H52NO4P ( 4 61. 64 )- H2O
5.7 g (14 mmol) of hexadecyl piperidinio-4-yl phosphate are
dissolved in 100 ml of methanol and mixed with 11.6 g
(84 mmol) of potassium carbonate. 4.0 ml (42 mmol) of
dimethyl sulphate are added dropwise over 30 min, with
thorough stirring. The mixture is subsequently,stirred for
4 h at 40 C, cooled, filtered and concentrated. The
residue is digested with acetone and, after filtration with
suction, is dissolved in 100 ml of 96% ethanol. 15 g of
Amberlite MB3 ion exchanger are added and the mixture is
stirred for 3 h. After filtration, the filtrate is
concentrated and recrystallized twice from methyl ethyl
ketone. The crystals are dried under vacuum over P205.
Yield: 3.70 g (61%)
Elemental analysis:
C H N
ca1c.: 61.17% 11.16% 3.10%
found: 60.83% 11.14% 2.99%
60.920. 11.26% 3.00%
Thin layer chromatogram:
(chloroform/methanol/25% ammonia 70:20:10)
Rf = 0.28
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.13
Melting point: 230 C (decomposition)
16
CA 02511753 1993-07-09
Example 4: Erucyl 1,1-dimethylpiperidinio-4-yl phosphate
C29HS8NO4P (515.765) - H20
10.3 ml (0.11 mol) of phosphorus oxychloride are placed in
50 ml of chloroform, and a solution of 32.5 g (0.10 mol) of
erucyl alcohol and 32 ml of pyridine in 100 ml of
chloroform is-added dropwise at 5 - 10 C_ After subsequent
stirring for half an hour, 39.1 g (0.13 mol) of 4-hydroxy-
1,1-dimethylpiperidinium tosylate are added in a single
portion. After the dropwise addition of 40 ml of pyridine,
the mixture is left to warm up to room temperature and
stirred for 3 h. It is then hydrolyzed with 15 ml of
water, subsequently stirred for half an hour and washed
with 100 ml each of water/methanol (1:1), 3% sodium
carbonate solution/methanol (1:1), 3% citric acid/methanol
(1:1) and water/methanol (1:1). The residue obtained after
concentration of the organic phase is digested with acetone
and then dissolved in 150 ml of 96% ethanol. This solution
is stirred for 3 h with 20 g of Amberlite* MB3 ion exchanger
and filtered over kieselguhr to give a clear solution.
This is concentrated and chromatographed on silica gel with
chloroform/ methanol/25% ammonia 70:40:10. The product
fractions are combined and concentrated to dryness under
vacuum.
~ Trade-Mark
17
CA 02511753 1993-07-09
Yield: 4.4 g (9%)
Elemental analysis:
C H N
caic.: 65.26% 11.63% 2.62%
found: 64.380 11.610 2.73%
65.04% 11.80% 2.78%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:20:10)
Rf = 0.30
Example 5: Hexadecyl 1,1-dimethylpiperidinio-3-yl
phosphate
C23H48NO4P (433.616) = H20
10.3 ml (0.11 mol) of phosphorus oxychioride are placed in
50 ml of chloroform and cooled to 0 - 10 C. 24.2 g
(0.10 mol) of n-hexadecanol are dissolved in 100 ml of
chloroform, 32 ml of pyridine are added and the mixture is
added dropwise to the phosphorus oxychloride solution over
one hour, with ice cooling. After subsequent stirring for
half an hour, 39.2 g (0.13 mol) of 3-hydroxy-1,1-dimethyl-
piperidinium tosylate are added in a single portion and
40 ml of pyridine are added dropwise over 15 min at room
temperature. After stirring for 16 h at room temperature,
the mixture is hydrolyzed with 15ml of water, stirred for
half an hour and washed with 100 ml each of water/ methanol
(1:1), 3% sodium carbonate solution/ methanol (1:1),
3% citric acid/methanol (1:1) and water/ methanol (1:1).
The organic phase is dried over sodium sulphate and
concentrated. The residue is dissolved in 150-ml of 96%
ethanol, the solution is filtered and the filtrate is
stirred with Amberlite*MB3 ion exchanger. After the ion
* Trade-Mark 18
CA 02511753 1993-07-09
exchanger has been filtered off, the filtrate is
concentrated and the residue is crystallized with acetone,
filtered off with suction and dried under vacuum over P205.
Yield: 13.5 g (31%)
Elemental analysis:
C H N
calc.: 61.170 11.16% 3.10%
found: 60.780 11.410 2.87%
60.85% 11.31% 2.86%
Thin layer chromatogram:
(chloroform/methanol/1 M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.37
30
19
CA 02511753 1993-07-09
Example 6: Octadecyl 1,1-dimethylpiperidinio-3-yl
phosphate
C2SH52NO4P (461.670) = zH20
This is prepared analogously to Example 5 from 10.3 ml
(0.11 mol) of phosphorus oxychioride, 27.0 g (0.10 mol) of
octadecanol, 32 + 40 ml of pyridine and 39.2 g (0.13 mol)
of 3-hydroxy-1,1-dimethylpiperidinium tosylate.
Yield: 18.7 g (40%)
Elemental analysis:
C H N
calc.: 63.80% 11.35% 2.98%
found: 63.38% 11.72% 2.63%
63.61% 11.98% 2.61%
Thin layer chromatogram:
(chloroform/methanol/1 M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.35
35
CA 02511753 1993-07-09
Example 7: Hexadecyl (1,1-dimethylpiperidinio-2-yl)
methyl phosphate
C24H50N04P (447.643) = zH20
This is prepared analogously to Example 5 from 10.3 ml
(0.11 mol) of phosphorus oxychloride, 24.2 g (0.10 mol) of
hexadecanol, 32 + 40 ml of pyridine and 41.0 g (0.13 mol)
of 2-hydroxymethyl-l,1-dimethylpiperidinium tosylate.
Yield: 22.9 g (51%)
Elemental analysis:
C H N
calc.: 63.13% 11.26% 3.07%
found: 63.69% 11.73% 3.04%
63.75% 11.71% 3.04%
Thin layer chromatogram:
(chloroform/methanol/1 M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.47
35
21
CA 02511753 1993-07-09
Example 8: Octadecyl (1,1-dimethylpiperidinio-2-yl)
methyl phosphate
C26H54NO4P (475.697) - 2H2O
This is prepared analogously to Example 5 from 10.3 ml
(0.11 mol) of phosphorus oxychloride, 27.0 g(0.10 mol) of
octadecanol, 32 + 40 ml of pyridine and 41.0 g (0.13 mol)
of 2-hydroxymethyl-1,1-dimethylpiperidinium tosylate.
Yield: 23.9 g (50%)
Elemental analysis:
C H N
calc.: 64.43% 11.44% 2.89%
found: 64.50% 11.61% 2.67%
64.11% 11.49% 2.77%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.47
35
22
CA 02511753 1993-07-09
Example 9: Hexadecyl (1,1-dimethylpiperidinio-3-yl)
methyl phosphate
C24H50NO4P (447.643) = 1H2O
This is prepared analogously to Example 5 from 10.3 ml
(0.11 mol) of phosphorus oxychloride, 24.2 g (0.10 mol) of
hexadecano1,.32 + 40 ml of pyridine and 41.0 g (0.13 mol)
of 3-hydroxymethyl-l,1-dimethylpiperidinium tosylate.
Yield: 17.2 g (39%)
Elemental analysis:
C H N
calc.: 61.91% 11.26% 3.01%
found: 62.32% 12.21% 2.86%
61.79% 11.96% 2.98%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.29
35
23
CA 02511753 1993-07-09
Example 10: Octadecyl (1,1-dimethylpiperidinio-3-yl)
methyl phosphate
C26H54NO4P (475.697) = H2O
This is prepared analogously to Example 5 from 10.3 ml
(0.11 mol) of phosphorus oxychloride, 27.0 g (0.10 mol) of
octadecanol, 32 + 40 ml of pyridine and 41.0 g (0.13 mol)
of 3-hydroxymethyl-1,1-dimethylpiperidinium tosylate.
Yield: 16.7 g (35%)
Elemental analysis:
C H N
calc.: 63.25% 11.43% 2.84%
found: 62.98% 12.210 2.760
63.67% 12.47% 2.80%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.30
35
24
CA 02511753 1993-07-09
Example 11: Tetradecyl 1,1-dimethylhexahydroazepinio-
4-yl phosphate
C22H46N04P (419.54) = HZO -
This is prepared analogously to Example 5 from 9.6 g
(45 mmol) of tetradecanol, 4.6 ml (50 mmol) of phosphorus
oxychloride, 10_+ 20 ml of pyridine and 21.3 g (67.5 mmol)
of hydroxy-l,l-dimethylhexahydroazepinium tosylate. It is
purified by flash chromatography on silica gel with
methylene chloride/methanol/ 25% ammonia 70:40:10.
Yield: 2.70 g (15%)
Elemental analysis:
C H N
calc.: 60.40% 11.05% 3.20%
found: 60.470 11.29% 3.63%
60.78% 11.52% 3.68%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.30
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.08
35
CA 02511753 1993-07-09
Example 12: Hexadecyl 1,1-dimethylhexahydroazepinio-
4-yl phosphate
C24H48NO4P (445.62)
This is prepared analogously to Example 5 from 10.8 g
(45 mmol) of hexadecanol, 4.6 ml (50 mmol) of phosphorus
oxychloride, 10 + 20 ml of pyridine and 21.3 g (67.5 mmol)
of 4-hydroxy-l,1-dimethylhexahydroazepinium tosylate. It
is purified by flash chromatography on silica gel with
methylene chloride/methanol/ 25% ammonia 70:30:10.
Yield: 5.0 g (25%)
Elemental analysis:
C H N
calc.: 64.69% 10.86% 3.14%
found: 63.90% 11.54% 3.22%
64.08% 11.59% 3.24%
Thin layer chromatogram:
(chloroform/methanol/25% ammonia 80:25:5)
Rf = 0.10
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.10
Melting point: >250 C (decomposition)
26
CA 02511753 1993-07-09
Example 13: Octadecyl 1,1-dimethylhexahydroazep'inio-
4-yl phosphate - -
C26H54NO4P (475.695) - 2H20
This is prepared analogously to Example 5 from 12.1 g
(45 mmol) of octadecanol, 4.6 ml (50 mmol) of phosphorus
oxychloride, 10 + 20 ml of pyridine and 21.3 g (67.5 mmol)
of 4-hydroxy-l,l-dimethylhexahydroazepinium tosylate. It
is purified by flash chromatography on silica gel with
methylene chloride/methanol/ 25% ammonia 70:30:10.
Yield: 5.5 g (26%)
Elemental analysis:
C H N
calc.: 64.43% 11.44% 2.89%
found: 64.54% 11.64% 2.82%
64.66% 11.58% 2.64%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.22
Melting point: >250 C (decomposition)
27
CA 02511753 1993-07-09
Example 14: Cis-A 9-octadecenyl 1,1-dimethylhexahydro
azepinio-4-yl phosphate
C26H52NO4P (473.679) - H2O
This is prepared analogously to Example 5 from 12.1 g
(45 mmol) of cis-O9-octadecenol, 4.6 ml (50 mmol) of
phosphorus oxychloride, 10 + 20 ml of pyridine and 21.3 g
(67.5 mmol) of 4-hydroxy-l,1-dimethylhexahydroazepinium
tosylate. It is purified by flash chromatography on silica
gel with methylene chloride/ methanol/25% ammonia 70:30:10.
Yield: 4.5 g (21%)
Elemental analysis:
C H N
calc.: 63.51% 11.070 2.85%
found: 64.05% 11.21% 3.10%
63.80% 11.06% 3.06%
Thin layer chromatogram:
(chloroform/methanol/25% ammonia 70:40:10)
Rf = 0.28
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.10
35
28
<
CA 02511753 1993-07-09
Example 15: Eicosyl 1,1-dimethylhexahydroazepinio-4-yl
phosphate
C28H58NO4P (503.754) = H2O
This is prepared analogously to Example 5 from 13.4 g
(45 mmol) of eicosanol, 4.6 ml (50 mmol) of phosphorus
oxychloride, 10 + 20 ml of pyridine and 21.3 g (67.5 mmol)
of 4-hydroxy-l,l-dimethylhexahydroazepinium tosylate. It
is purified by flash chromatography on silica gel with
methylene chloride/methanol/ 25% ammonia 70:30:10.
Yield: 5.7 g (25%)
Elemental analysis:
C H N
calc.: 64.46% 11.59% 2.68%
found: 63.51% 11.48% 2.95%
64.00% 11.79% 2.91%
Thin layer chromatogram:
(chloroform/methanol/25% ammonia 70:40:10)
Rf = 0.12
30
29
CA 02511753 1993-07-09
Example 16: Erucyl 1,1-dimethylhexahydroazepinio-4-yl
phosphate - -
C30H6oN04P (529.789) = H2O
This is prepared analogously to Example 5 from 16.2 g
(50 mmol) of erucyl alcohol, 5.1 ml (55 mmol) of phosphorus
oxychloride, 18 + 30 ml of pyridine and 20.5 g (65 mmol) of
4-hydroxy-1,1-dimethylhexahydroazepinium tosylate. It is=
purified by flash chromatography on silica gel with
methylene chloride/methanol/ 25% ammonia 70:30:10.
Yield: 4_1 g (15%)
Elemental analysis:
C H N
calc.: 65.78% 11.41% 2.56%
found: 65.76% 12.01% 2.97%
65.82% 11.63% 2.96%
Thin layer chromatogram:
(chloroform/methanol/l M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.30
CA 02511753 1993-07-09
Example 17: Octadecyl 1,1-dimethylpyrrolidinio-3-yl
phosphate
C24HS0N04P (447.643) - ZH2O
This is prepared analogously to Example 5 from 3.25 g
(12 mmol) of octadecanol, 1.21 ml (13 mmol) of phosphorus
oxychloride, 3.7 + 4.8 ml of pyridine and 4.31 g(15 mmol)
of hydroxy-1,1-dimethylpyrrolidinium tosylate. The crude
product is purified by dissolution in 96% ethanol and
treatment with Amberlite*MB3 ion exchanger.
Yield: 1.31 g (25%)
Elemental analysis:
C H N
calc.: 63.13% 11.26% 3.07%
found: 62.990 11.28a 2.80%
62.74% 11.27% 2.89%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.25
* Trade-Mark
31
CA 02511753 1993-07-09
Example 18: Hexadecyl 2-(1,1-dimethylpyrrolidinio-
2-yl)ethyl phosphate
C24H50NO4P (447.643) - H20
This is prepared analogously to Example 5 from 9.21 g
(38 mmol) of hexadecanol, 3.9 ml (42 mmol) of phosphorus
oxychloride, 13 + 16 ml of pyridine and 15.8 g (50 mmol) of
2-(2-hydroxyethyl)-1,1-dimethylpyrrolidinium tosylate. It
is purified by dissolution in 96% ethanol and treatment
with Amberlite*MB3 ion exchanger.
Yield: 6.0 g (35%)
Elemental analysis:
C H N
calc.: 61.91% 11.26% 3.01%
found: 61.82% 11.69% 3.21%
61.93% 11.86% 3.28%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.38
* Trade-Mark
32
CA 02511753 1993-07-09
Example 19: Octadecyl 2-(1,1-dimethylpyrrolidinio-
2-yl)ethyl phosphate
C26H54NO4P (475.697) - ZH2O
This is prepared analogously to Example 5 from 10.3 g
(38 mmol) of octadecanol, 3.9 ml (42 mmol) of phosphorus
oxychloride, 13 + 16 ml of pyridine and 15.8 g (50 mmol) of
2-(2-hydroxyethyl)-1,1-dimethylpyrrolidinium tosylate. It
is purified by dissolution in 96% ethanol and treatment
with Amberlite*MB3 ion exchanger.
Yield: 7.8 g (43%)
Elemental analysis:
C H N
caic.: 64.43% 11.440 2.89%
found: 64.69% 11.77% 2.64%
64.84% 11.880 2.690
Thin layer chromatogram:
(chloroform/methanol/1 M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.35
* Trade-Mark
33
CA 02511753 1993-07-09
Example 20: Hexadecyl (1,1-dimethylpyrrolidinio-2-yl)
methyl phosphate
C23H48NO4P (433.616) = 2H2O
This is prepared analogously to Example 5 from 9.21 g
(38 mmol) of hexadecanol, 3.9 ml (42 mmol) of phosphorus
oxychloride, 13 + 16 ml of pyridine and 15.1 g(50 mmol) of
2-hydroxymethyl-1,1-dimethylpyrrolidinium tosylate. It is
purified by dissolution in 96% ethanol and treatment with
Amberlite*MB3 ion exchan_ger_
Yield: 8.3 g (51%)
Elemental analysis:
C H N
ca1c.: 62.410 11_16% 3_160
found: 62.09% 11.48% 3.01%
62.25% 11.66% 3.09%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.33
* Trade-Mark
34
CA 02511753 1993-07-09
Example 21: Octadecyl (1,1-dimethylpyrrolidinio-2-yl)
methyl phosphate
C25H52NO4P ( 4 61. 67 )- ZH20
This is prepared analogously to Example 5 from 10.3 g
(38 mmol) of octadecanol, 3.9 ml (42 mmol) of phosphorus
oxychloride, 13 + 16 ml of pyridine and 15.1 g (50 inmol) of
2-hydroxymethyl-1,1-dimethylpyrrolidinium tosylate. It is
purified by dissolution in 96% ethanol and treatment with
Amberlite MB3 ion exchanger.
Yield: 9.0 g (520)
Elemental analysis:
C H N
caic.: 63.80% 11.35% 2.98%
found: 63.13% 11.57% 2.84%
63.55% 11.66% 2.82%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.35
* Trade-Mark
CA 02511753 1993-07-09
Example 22: Hexadecyl 1-methylquinuclidinio-
3-yl phosphate
C24H48NO4P (445.64) = 1.5 H20
2.7 ml (30 mmol) of phosphorus oxychloride are dissolved in
25 ml of chloroform and cooled to 5 - 10 C and a solution
of 6.4 g (26 mmol) of hexadecanol and 10 ml of pyridine in
50 ml of chloroform is added dropwise over one hour. After
subsequent stirring for half an hour at room temperature, a
solution of 4.5 g (35 mmol) of 3-hydroxyquinuclidine and
5 ml of pyridine in 10 ml of chloroform is added. After
stirring for 5 h at room temperature, the mixture is
hydrolyzed with 15 ml of water and subsequently stirred for
half an hour. It is then washed twice with 100 ml of
water/methanol (1:1) and the organic phase is dried over
magnesium sulphate and concentrated to dryness. The
residue is chromatographed on silica gel with methylene
chloride/methanol 80:25 and then methylene chloride/
methanol/25% ammonia 80:25:5. The product fractions are
purified, evaporated to dryness and crystallized with
acetone. The crystals are dried under vacuum over P205.
Yield: 4.95 g (44%) of hexadecyl quinuclidinio-3-yl
phosphate
36
CA 02511753 1993-07-09
4.95 g (11.5 mmol) of hexadecyl quinuclidinio-3-yl
phosphate are dissolved in 30 ml of inethariol, 13.7 g
(69 mmol) of potassium carbonate and 8.5 ml of water are
added and a solution of 3.3 ml (35 mmol) of dimethyl
sulphate in 5 ml of methanol is added dropwise, with
thorough stirring. After stirring for 14 h at room
temperature, the inorganic salts are filtered off, the
filtrate is concentrated to dryness and the residue is
taken up in methylene chloride. After filtration, the
filtrate is chromatographed on silica gel with methylene
chloride/methanol/25% ammonia 70:30:5. The product
fractions are combined, evaporated to dryness and stirred
with acetone until crystallization occurs. The crystals
are dried under vacuum over P205.
Yield: 2.7 g (49%)
Elemental analysis:
C H N
calc.: 60.99% 10.88% 2.96%
found: 61.38% 11.04% 3.29%
61.46% 11.22% 3.25%
Thin layer chromatogram:
(chloroform/methanol/25% ammonia 70:40:10)
Rf = 0.44
35 -
37
CA 02511753 1993-07-09
=Example 23: Octadecyl 1-methylquinuclidinio-
3-yl phosphate
C26H52NO4P (473.68) = 2H2O
This is prepared analogously to Example 5 from 18.3 g
(67.5=mmol) of octadecanol, 7.0 ml (75 mmol) of phosphorus
oxychloride, 18 + 20 ml of pyridine and 28.3 g(90 mmol) of
3-hydroxy-l-methylquinuclidinium tosylate_ It is purified
by dissolution in 96% ethanol and treatment with Amberlite*
MB3 ion exchanger.
Yield: 18.4 g (57%)
Elemental analysis:
C H N
calc.: 61.27% 11.07% 2.75%
found: 61.27% 10.91% 2.45%
61.95% 11.23% 2.51%
Thin layer chromatogram:
(chloroform/methanol/1 M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.37
(1-butanol/glacial acetic acid/water 40:10:10)
Rf = 0.13
*
Trade-Mark
38
CA 02511753 1993-07-09
Example 24: Hexadecyl 1,1-dimethyltropanio-4-yl phosphate
C25H50N04P (459.654) - H20
This is prepared analogously to Example 5 from 12.1 g
(50 mmol) of hexadecanol, 5.1 ml (55 mmol) of phosphorus
oxychloride, 17 + 40 ml of pyridine and 21.3 g (65 mmol) of
4-hydroxy-l,l-dimethyltropanium tosylate. It is purified
*
by dissolution in 96% ethanol, treatment with Amberlite MB3
ion exchanger and recrystallization from acetone._
Yield: 11.3 g (49%)
Elemental analysis:
C H N
calc.: 62.86% 10.97% 2.93%
found: 62.45g 11.52% 2.82%
62.58% 11.52% 2.75%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.28
* Trade-Mark
39
CA 02511753 1993-07-09
Example 25: Octadecyl 1,1-dimethyltropanio-4-yl phosphate
C27H54NO4P (487.708)
This is prepared-analogously to Example 5 from 13.5 g
(50 mmol) of octadecanol, 5.1 ml (55 mmol) of phosphorus
oxychloride, 17 + 20 ml of pyridine and 21.3 g (65 mmol) of
4-hydroxy-1,l-dimethyltropanium tosylate. It is purified
by dissolution in 96% ethanol and treatment with Amberlite*
MB3 ion exchanger_
Yield: 10.7 g (44%)
Elemental analysis:
C H N
calc.: 66.49% 11.16% 2.87%
found: 65.720 11.48% 2.64%
66.27% 11.78% 2.65%
Thin layer chromatogram:
(chloroform/methanol/i M sodium acetate in 25% ammonia
70:40:10)
Rf = 0.22
* Trade-Mark