Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE I)E CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
1
TRICYCLIC COMPOUNDS PROTEIN KINASE INHIBITORS FOR ENHANCING THE
EFFICACY OF ANTI-NEOPLASTIC AGENTS AND RADIATION THERAPY
Field of The Invention
This invention relates to novel tricyclic compounds of formula I that inhibit
protein
kinases, preferably CHK-1. The invention further relates to pharmaceutical
compositions
containing such compounds, and to methods for the treatment of a condition
which can be treated
by the inhibition of protein kinases, preferably CHK-1, in a mammal by
administering effective
amounts of such compounds in conjunction with an anti-neoplastic agent, a
radiation therapy, or
as a single agent.
Background of The Invention
A eukaryotic cell cycle has a carefully regulated progression of phases:
initial gap (G1),
DNA synthesis (S), secondary gap (GA and mitosis (M). GI, S and G2 are known
as interphase.
In Gi, the cell, whose biosynthetic pathways were slowed during mitosis,
resumes a high rate of
RNA and proteinbiosynthesis. The S phase begins when DNA synthesis starts and
ends when
the DNA content of the nucleus has been replicated. The cell then enters G2
where again RNA
and protein biosynthesis occur. Following G2, the cell enters M phase that
begins with nuclear
division and ends with the complete division of the cytoplasm into two
daughter cells. This marks
the beginning of interphase for the new cells. Non-dividing cells exist at Go,
a time following
mitosis and before DNA synthesis.
Checkpoint enzymes, such as the serine/threonine protein kinase called
checkpoint
kinase 1(CHK-1 or p56CHK-1), are responsible for maintaining the order and
fidelity of events in
the cell cycle. CHK-1 transduces signals from the DNA damage sensory complex
to inhibit
activation of Cdc2-cyclin B complex which promotes mitotic entry (Science,
277, 1501-1505
(1997); Science, 277, 1497-1501 ((1997)). In eukaryotes, Cdc2 is known as Cdk1
(cyclin-
dependent kinase 1). CHK-1 regulates Cdc25, a dual specificity phosphatase
that activates Cdc2.
Thus, CHK-1 serves as the direct link between the G2 checkpoint and the
negative regulation of
Cdc2.
Healthy cells have both the G, and G2 checkpoints and their associated repair
processes
to ensure viability after treatment of DNA damage (chemotherapy and/or
radiation). Cancer cells,
however, rely exclusively on the Gz checkpoint and its associated repair
processes in order to
remain viable and to continue replication. Abrogation of the GZ checkpoint
would leave cancer
cells with no means to delay progression into mitosis following DNA damage.
Inactivation of
CHK-1 has been shown to abrogate G2 arrest induced by DNA damage inflicted by
either
anticancer agents or endogenous DNA damage. In addition, inactivation of CHK-1
results in
preferential killing of the resulting DNA damaged, checkpoint defective cells
(Cell, 91,_ 865-867
(1997); Science, 277, 1450-1451 (1997); Nature, 363, 368-371 (1993); Molec.
Biol. Cell, 5, 147-
160 (1994)). Therefore there is a need for small molecule inhibitors of CHK-1
to preferentially
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
2
abrogate the G2 checkpoint over G, and to effectively remove the only
checkpoint control found
in many types of cancers. When administered during the course of a DNA
damaging event, such
as chemotherapy employing anti-neoplastic agents, radiation therapy,
immunotherapies and
antiangiogenic therapies, a CHK-1 inhibitor can sensitize cancer cells thereby
triggering damage-
mediated apoptosis. Therefore there is a need for a combination therapy
involving CHK-1 inhibitor
in the course of a DNA damaging event.
Since protein kinases are ubiquitous and interrelated, selective modulation of
a single
kinase, such as CHK-1, or family of kinases may not result in an effective
therapeutic treatment.
There is therefore a need for small molecule inhibitors to influence one or
more targeted protein
kinases whose inhibition, taken as a whole, would produce the desired
therapeutic treatment.
Although kinase selectivity and its relation to generalized toxicity are
important, therapeutic
efficacy may rely on the inhibition of more than one protein kinase. Chemical
core structures that
can be suitably appended to interact selectively and potently with targeted
protein kinases
represent a valuable tool for drug discovery and scientific research.
Therefore there is a need for
such a core structure as an inhibitor of one, or more protein kinases. Whether
administered as a
single agent or as co-therapy, the protein kinase inhibitors, such as CHK-1
inihibitors, of the
present invention could prove beneficial in the treatment of a number of human
diseases, such as
cancer.
Certain CHK-1 inhibitors have been proposed for cancer therapy (see Sanchez,
Y. et.al.
(1997) Science 277: 1497-1501 and Flaggs, G. et. al. (1997) Current Biology
7:977-986; U.S.
Patent Nos. 6,413,755, 6,383,744, and 6,211,164; and International Publication
Nos. WO
01/16306, WO 01/21771, WO 00/16781, and WO 02/070494).
SUMMARY OF THE INVENTION
An object of the invention is to provide compounds that inhibit the activity
of one or more
protein kinases, such as CHK-1.
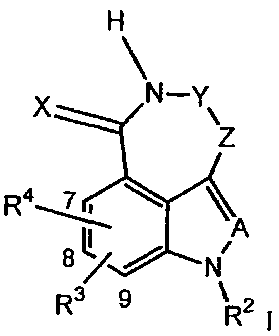
In a general aspect, the invention relates to a protein kinase inhibitor,
preferably CHK-1
inhibitor, tricyclic compounds of the Formula I:
H
~
X N-Y\
z
R4~
8LI NA
R3 9 R2 i
wherein:
X is =O or =S;
A is =CR'- or =N-;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
3
The group -Y-Z- has the formula -O-CH2- or -N=CH-;
R' is:
(a) (Cl-Ca)alkyl;
(b) -C(=O)-R5;
(c) -C(=O)-NRsR'; or
(d) R35, or R36, (C2-C8)alkenyl, or (C2-C8)alkynyl {wherein each of said (C2-
C8)alkenyl or
(C2-C8)alkynyl is unsubstituted or substituted with one to four substituents
independently selected
from the group consisting of F, Cl, OH, -NH2, R40, and R42};
R2is
(a) H, OH, or (CI-C$)alkyl;
(b) -C(=O)-R8;
(c) -(C=S)-R9 or -(C=S)-NR"R"; or
(d) R38 or.R3s;
R3 is
(a) (Cl-C$)alkyl;
(b) -C(=O)-R12;
(c) -C(=O)-NR'3R";
(d) -NR15-C(=O)-R16;
(e) -NR17-SOZR'$;
(f) -NR19-SOn-NR20R21 {wherein n is 1 or 2};
(g) -N R22-(C=S)-R23 or -N R22-(C=S)-N R23R24;
(h) R3s, (C2-C8)alkenyl, or (C2-C8)alkynyl {wherein each of said R3 (C2-
C8)alkenyl or
(C2-CB)alkynyl is unsubstituted or substituted with one to four substituents
independently selected
from the group consisting of -(C=O)-O-(CI-C8)alkyl, -O-(C=O)-(Cj-C$)alkyl, -
(C=O)-(Ci-C8)alkyl,
R41 R41 and R'};
(i) R37, -NH2, -NH((C2-C8)alkenyl), -NH((C2-CB)alkynyl), -N((Cl-C$)alkyl)((CZ-
C$)alkenyl), or
-N((CI-C8)alkyl)((C2-C$)alkynyl) {wherein each of said R26 (C2-C8)alkenyl or
(C2-C8)alkynyl is
unsubstituted or substituted with one to four substituents independently
selected from the group
consisting of R40 R41 and R42}; or
0) Rss;
R4 is selected from the group consisting of H, F, Br, Cl, and (CI-Ca)alkyl;
R5 is selected from the group consisting of H, (Cl-C8)alkyl, (CI-C8)alkyl-O-,
and R 36;
Each R6 and R' are independently selected from the group consisting of H, (Cl-
C8)aikyl,
and R3s;
R8 is selected from the group consisting of (CI-C$)alkyl, (C2-C$)alkenyl, (C2-
C8)alkynyl,
-NH2, R36, and R37;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
4
Each of R9, R10 and R11 are independently selected from the group consisting
of H,
(C1-C8)alkyl, and R 36;
R12 is selected from the group consisting of H, OH, (C1-C$)alkyl, (C1-C8)alkyl-
O-, and R36;
R13 is H or (C1-Ca)alkyl;
R14 is selected from the group consisting of H, (C1-C8)alkyl, -CHz-(C=O)-O-(C1-
Ca)alkyl,
and R3s;
R15 is H or (C1-Ca)alkyl;
R16 is selected from the group consisting of H, (C1-C8)alkyl, (C2-C8)alkenyl,
(C2-Ca)alkynyl,
-NH2, R36, and R37;
wherein each of said R15 and R16 (C2-C8)alkenyl or (C2-C8)alkynyl is
unsubstituted or
substituted with one to four substituents independently selected from the
group consisting of R40
R41, and R42;
R17 is selected from the group consisting of H, (C1-C8)alkyl, and R36;
R18 is (C1-C$)alkyl or R 36;
R19, R20, and R21 are independently selected from the group consisting of H,
(C1-Ca)alkyl,
and R36;
R22, R23 and R24 are independently selected from the group consisting of H,
(C1-C$)alkyi,
and R3s;
R25 is H or (C1-C8)alkyl;
R26 is selected from the group consisting of -C(=O)-O-C(CH3)3, (C1-C8)alkyl,
(C3-e'+1 )cycloalkyl, (C2-C10)heterocyclyl, (C6-C10)aryl, and (C1-
C10)heteroaryl;
or R25 and R26 may optionally be taken together with the nitrogen to which
they are
attached to form a 5 to 8-membered heteroaryl or heterocyclyl ring;
R 27 is selected from the group consisting of (C1-CS)alkyl, (C3-
C1o)cycloalkyl,
(C2-C10)heterocyclyl, (C6-C10)aryl, and (C1-C10)heteroaryl;
R28 is selected from the group consisting of (C1-C$)alkyl, (C3-C10)cycloalkyl,
(C2-C10)heterocyclyl, (C6-C10)aryl, and (C1-C10)heteroaryl;
R29 is H or (C1-C$)alkyl;
R30 is (C1-C$)alkyl, (C3-C10)cycloalkyl, (Cz-C10)heterocyclyl, (Cs-C1o)aryl,
or
(C1-C10)heteroaryl;
or R29 and R30 may optionally be taken together with the nitrogen to which
they are
attached to form a 5 to 8-membered heteroaryl or heterocyclyl ring;
R31 is H or (C1-C$)alkyl;
R32 is independently selected from the group consisting of (C1-C$)alkyl, (C3-
C10)cycloalkyl,
(CZ-C10)heterocyclyl, (C6-C10)aryl, and (C1-C10)heteroaryl;
or R31 and R32 may optionally be taken together with the nitrogen to which
they are
attached to form a 5 to 8-membered heteroaryl or heterocyclyl ring;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
R33 is (C1-C8)alkyl, (C3-C10)cycloalkyl, (C2-C10)heterocyclyl, (Cs-C10)aryl,
or
(C1-C10)heteroaryl;
R34 is (C1-Cs)alkyl, (C3-C10)cycloalkyl, (C2-C10)heterocyclyl, (Cs-C10)aryl,
or
(C1-C10)heteroaryl;
5 Each R35 is independently selected from the group consisting of H, F, Cl,
Br, I, CN, OH,
NO2i -NH2, -NH-C(=O)-O-C(CH3)3, and CF3;
Each R36 is independently selected from the group consisting of (C3-
C10)cycloalkyl,
(C2-C10)heterocyclyl, (Cs-C10)aryl, and (C1-C10)heteroaryl;
Each R37 is independently selected from the group consisting of:
(a) -NR2sRZS; and
(b) Rz'-0-;
R3B is RZS-SOn-; wherein n is 0,1, or 2 when -SO,,- is bonded to R28 via an
R28 carbon
atom, or wherein n is I or 2 when -SOn- is bonded to R2a via an R 28 ring
nitrogen atom;
R39 is R29R30N-SOn-; wherein n is 1 or 2;
wherein each of said (C1-C$)alkyl, wherever it occurs in any of said R1(a)-
(d), R2 (a)-(d),
R3(a)-Q), R4, R37, R38, or R39, is unsubstituted or substituted with one to
four substituents
independently selected from the group consisting of (C2-C$)alkenyl, R4o R41
and R42;
wherein each of said (C3-C10)cycloalkyl, (C2-C10)heterocyclyl, (Cs-C10)aryl,
or
(C1-C10)heteroaryl, wherever it occurs in said R36 R37 R38 or R39, is
independently unsubstituted
or substituted with one to four substituents independently selected from R40;
R40 is selected from the group consisting of (C1-C8)alkyl, R41, R42, and R43;
Each R41 is independently selected from the group consisting of F, Cl, Br, I,
CN, OH, NO2,
-NH2i -NH-C(=O)-O-C(CH3)3, COOH, -C(=0)(C1-C8)alkyl, -C(=O)-O-(C1-Cs)alkyl,
-NH-S02-(C1-C8)alkyl, -NH-S02-(C6-C10)aryl, and CF3;
Each R42 is independently selected from the group consisting of (C3-
C10)cycloalkyl,
(CZ-C10)heterocyclyl, (Cs-C10)aryl, and (C1-C10)heteroaryl;
Each R43 is independently selected from the group consisting of:
(a) -NR31R32.
,
(b) R33-O-; and
(c) R34-SOn-; wherein n is 0,1, or 2 when -SO,- is bonded to R34 via an R34
carbon atom,
or wherein n is 1 or 2 when -SO,- is bonded to R34 via an R34 ring nitrogen
atom;
wherein each of said (C1-Cs)alkyl, wherever it occurs in any of R40 is
independently
unsubstituted or substituted with one to four substituents independently
selected from the group
consisting of R44 and R 45;
wherein each of said (C3-C10)cycloalkyl, (C2-C10)heterocyclyl, (Cs-C10)aryl,
or
(C1-C10)heteroaryl, wherever it occurs in any of said R or R, is independently
unsubstituted or
42 43
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
6
substituted with one to four substituents independently selected from the
group consisting of R47
selected from the group consisting of P-C8)alkyl, R44, and R45;
Each R44 is independently selected from the group consisting of F, Cl, Br, I,
CN, OH, NOZ,
-NH2, -CF3, -C(=NH)-NH2, -C(=NH)-NH-OH, -C(=NH)-NH-O-(Cj-Ca)alkyl, -(C=O)-O-
(C1-C8)alkyl,
-O-(C=O)-(Cj-C8)alkyl, -(C=O)-(Cj-C$)alkyl, -(C=O)-NH2, -(C=O)-NH(Cj-C$)alkyl,
-(C=O)-N<[(Cj-Ca)alkyl]Z, -NH-(C=O)-(Cj-Ca)alkyi, R37, and R38;
Each R45 is independently selected from the group consisting of (C3-
CIo)cycloalkyl,
(C2-C10)heterocyclyl, (Cg-CIp)aryl, and (Cl-CIo)heteroaryl;
wherein each of said (Cj-C8)alkyl wherever it occurs in any of said R44 or R45
is
independently unsubstituted or substituted with one to four substituents
independently selected
from the group consisting of R46 and R47;
wherein each of said (C3-C10)cycloalkyl, (Ca-CIo)heterocyclyl, (C6-CIo)aryl,
or
(Cl-Clp)heteroaryl, wherever it occurs in I any of said R43 or R44 is
independently unsubstituted or
substituted with one to four substituents independently selected from the
group consisting of
(Cj-C8)alkyl, R46 and R47;
Each R46 is independently selected from the group consisting of F, Cl, Br, I,
CN, OH, NO2,
-C(=NH)-NH2, -C(=NH)-NH-OH, -C(=NH)-NH-O-(Cj-C$)alkyl, -(C=O)-O-(Cj-C8)alkyl,
-O-(C=O)-(Cj-C$)alkyl, -(C=O)-(Cj-C$)alkyl, -(C=O)-NH2, -(C=O)-NH(Cj-C$)alkyl,
-(C=O)-N<[(Cj-Ca)alkyl]2, -NH-(C=O)-(Cj-C$)alkyl, -C(=NH)-NH2, -C(=NH)-NH-OH,
-C(=NH)-NH-O-(Cj-C$)alkyl, -(C=O)-O-(Cj-C8)alkyl, -O-(C=O)-(Cj-C$)alkyl, -
(C=O)-(C1-C8)alkyl,
-(C=O)-NH2, -(C=O)-NH(Cj-C$)alkyl, -(C=O)-N>[(Cj-CB)alkyl]2, -NH-(C=O)-(Cj-
Ca)alkyl, R37, and
R3S; and
Each R47 is independently selected from the group consisting of (C3-
Clo)cycloalkyl;
(C2-Clo)heterocyclyl, (Cs-Clo)aryl, and (Cl-Clo)heteroaryl;
or a pharmaceutically acceptable salt thereof.
The invention is also directed to pharmaceutically acceptable prodrugs,
pharmaceutically
active metabolites, and pharmaceutically acceptable salts of the compounds of
Formula I.
Pharmaceutically acceptable salts of such active metabolites are also
provided. Advantageous
methods of making the compounds of the Formula I are also described.
The compounds of this invention include all stereoisomers (e.g., cis and trans
isomers) and
all optical isomers of compounds of the Formula I (e.g., R and S enantiomers),
as well as racemic,
diastereomeric and other mixtures of such isomers.
The compounds of the invention may also exist in different tautomeric forms.
This invention
relates to all tautomers of Formula I.
The compounds of this invention may contain olefin-like double bonds. When
such bonds
are present, the compounds of the invention exist as cis and trans
configurations and as mixtures
thereof.
CA 02512683 2008-05-27
50054-64
Fa
In one embodiment, the compound of formula I, is a
compourid of formula:
HN-N
0 I \. ~
N R-'
RP-_~
N
= H H
NH2
wherein:
R1 is H, Cl,
N
O-N
N, --- ~ \ N.
CH3 CH3 N CH3 or CH, ; and
Rp is
/ F / \ O
- `------ '- - ~------ or -
wherein "------" indicates points of attachment;
or a pharmaceutically acceptable salt thereof.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
7
In one embodiment, the invention relates to compounds of the Formula I wherein
R3 is
(CI-C8)aikyl substituted with one to four substituents independently selected
from the group
consisting of F, OH, -NHzi (Cj-C$)alkyl-NH-, (C3-Clo)cycloalkyl, (Cz-
CIo)heterocyclyl, (C6-Clo)aryl,
and (C6-CIo)heteroaryl.
In another embodiment, the invention relates to compounds of the Formula I
wherein R3
is selected from the group consisting of (C2-C8)alkenyl, (C2-Ca)alkynyl, (C3-
C6)cycloalkyl, (C2-
Clo)heterocyclyl, phenyl, and (Cl-CIo)heteroaryl; wherein each of said (C2-
C$)alkenyl or (C2-
C8)alkynyl is unsubstituted or substituted with one to three substituents
independently selected
from the group consisting of F, OH, -NH2, (Cj-C8)alkyl-NH-, [(Cl-Ca)alkyl]2>N-
, (C3-Clo)cycloalkyl,
(C2-Clo)heterocyclyl, (C6-Clo)aryl, and (Cl-Clo)heteroaryl; and wherein each
of said
(C3-C6)cycloalkyl, (C2-Clo)heterocyclyl, phenyl, or (Cl-Clo)heteroaryl is
unsubstituted or substituted
with one to four substituents independently selected from the group consisting
of (CI-C$)alkyl, F,
OH, -NH2, (Cj-C$)alkyl-NH-, [(Cl-C$)alkyl]2>N-, (C3-Clo)cycloalkyl, (C2-
C1o)heterocyclyl, (C6-
C1o)aryl, and (Cl-CIo)heteroaryl.
In a preferred embodiment, the invention relates to compounds of the Formula I
wherein
R3 is -C(=O)-NR13R14 {wherein R13 is H or (Cl-C$)alkyl}, wherein said R'3 (Cl-
C4)alkyl is
unsubstituted or substituted with one to four substituents independently
selected from the group
consisting of F, OH, -NH2, R41, and R42; wherein each of said R36 is
unsubstituted or substituted
with one or two substituents independently selected from the group consisting
of (C6-Clo)aryl,
(Cl-Clo)heteroaryl, (C2-C10)heterocyclyl, (Cj-C$)alkyl-NH-, and [(Cl-
C8)alkyl]Z>N-; and wherein
each of said (C6-Clo)aryl substituent is unsubstituted or substituted with one
to three substituents
independently selected from the group consisting of (Cl-C$)alkyl, F, Cl, -CF3i
and OH.
In another embodiment, the invention relates to compounds of the Formula I
wherein R15
is (CI-C8)alkyl unsubstituted or substituted with one to four substituents
independently selected
from the group consisting of OH, CN, -NH2, (Cj-C$)alkyl-NH-, [(Cl-C8)alkyl]Z>N-
,
[(Cl-C8)alkyl][(C3-Clo)cycloalkyl]>N-, (C3-Clo)cycloalkyl, (C2-
Clo)heterocyclyl, (C6-Clo)aryl, and
(CI-Clo)heteroaryl; wherein said (Cs-Clo)aryl substituent is unsubstituted or
substituted with one to
four substituents independently selected from the group consisting of (CI-
C8)alkyl, F, CI, Br, CN,
OH, and CF3; and wherein said (C2-Clo)heterocyclyl substituent is
unsubstituted or substituted
with one or two substituents independently selected from the group consisting
of (Cl-C$)alkyl,
-(C=O)-(C1-C$)alkyl, -(C=O)-O-(Cj-Ca)alkyl, -S-(Cl-C$)alkyl, F, Br, OH, and
CF3.
In another embodiment, the invention relates to compounds of the Formula I
wherein R3
is -NR15-C(=O)-R16; wherein R16 is (C2-C8)alkenyl unsubstituted or substituted
with one to four
substituents independently selected from the group consisting of (C3-
Clo)cycloalkyl,
(C2-CIo)heterocyclyl, (C6-Clo)aryl, and (Cl-CIo)heteroaryl; wherein said (C6-
Clo)aryl substituent is
unsubstituted or substituted with one to four substituents independently
selected from the group
consisting of (Cl-C$)alkyl, F, Cl, Br, CN, OH, and CF3i and wherein said (CZ-
Clo)heterocyclyl
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
8
substituent is unsubstituted or substituted with one or two substituents
independently selected
from the group consisting of (Cj-C8)alkyl, -(C=0)-(Cj-C$)alkyl, -(C=O)-O-(Cj-
Ca)alkyl,
-S-(CI-C8)alkyl, F, Br, OH, and CF3.
In a preferred embodiment, the invention relates to compounds of the Formula I
wherein
R3 is -NR15-C(=O)-R16; wherein R 16 is (CJ-Clo)heteroaryl unsubstituted or
substituted with one or
two substituents independently selected from the group consisting of (CI-
C8)alkyl, -(C=O)-
P-C$)alkyl, -S-(Cl-C8)alkyl, F, Cl, CN, OH, and CF3. More preferably the R16
(CI-CIo)heteroaryl
is pyridinyl.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein R3 is -NR15-C(=O)-R16; wherein R16 is (C3-Clo)cycloalkyl unsubstituted
or substituted with
one or two substituents independently selected from the group consisting of
(CI-C8)alkyl, F, CI,
CN, OH, NH2i CF3, (C2-Clo)heterocyclyl, (C6-C,o)aryl, and (CI-Clo)heteroaryl;
wherein said (C6-
CIo)aryl substituent is unsubstituted or substituted with one to four
substituents independently
selected from the group consisting of (CI-C8)alkyl, F, CI, Br, CN, OH, and
CF3; and wherein said
(C2-Clo)heterocyclyl substituent is unsubstituted or substituted with one or
two substituents
independently selected from the group consisting of (CI-C$)alkyl, -(C=O)-(Cl-
Cg)alkyl,
-(C=0)-O-(Cl-C$)alkyl, -S-(Cl-C8)alkyl, F, Br, OH, and CF3. More preferably
said R's
(C3-CIo)cycloalkyl is selected from the group consisting of cyclopropyl and
cyclohexyl. More
preferably said (C6-Clo)aryl substituents is unsubstituted.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein R3 is -NR15-C(=O)-R16; wherein R16 is (CZ-Clo)heterocyclyl
unsubstituted or substituted
with one to four substituents independently selected from the group consisting
of (Cl-C$)alkyl,
-(C=O)-(Cj-C$)alkyl, -(C=O)-O-(Cj-C$)alkyl, F, Cl, CN, OH, and CF3. More
preferably said R's
(C2-CIo)heterocyclyl is selected from the group consisting of piperazinyl,
piperidinyl, pyrrolidinyl,
pyrrolidinonyl, thiadiazolyl, tetrahydroisoquinolinyl, tetrahydronaphthalenyl,
and indanyl.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein R3 is -NR15-C(=O)-R16; wherein R's is phenyl unsubstituted or
substituted with one to
three substituents independently selected from the group consisting of (CI-
C$)alkyl, (Cl-Ca)alkyl-
O-, F, Cl, Br, CN, OH, and CF3.
In another embodiment, the invention relates to compounds of the Formula I
wherein R'
is (Cl-C8)alkyl substituted with one to two substituents independently
selected from the group
consisting of F, CI, -OH, -NH2, (Cl-C$)alkyl-NH-, [(C1-C8)alkyl]2>N-, and (Cj-
Ca)alkyl-O-; wherein
each of said (CI-Ca)alkyl substituent, wherever it occurs, is independently
unsubstituted or
substituted with one to three substituents independently selected from the
group consisting of -
NH2, (C1-C8)alkyl-NH-, [(Cj-Ca)alkyl]Z>N-, -O-(C=O)-(Cj-C$)alkyl, (C2-
Clo)heterocyclyl, (C6-CIo)aryl,
and (CI-Clo)heteroaryl.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
9
In another embodiment, the invention relates to compounds of the Formula I
wherein R'
is unsubstituted (Cl-Ca)alkyl; such as methyl or ethyl.
In another embodiment, the invention relates to compounds of the Formula I
wherein R'
is (C2-C8)alkenyl or (C2-C8)alkynyl; wherein each of said (C2-Ca)alkenyl or
(C2-C$)alkynyl is
unsubstituted or substituted with one to two substituents independently
selected from the group
consisting of -NH2, (Cl-Ca)alkyl-NH-, [(Cj-CB)alkyl]2>N-, (C2-
CIo)heterocyclyl, and
(CI-Clo)heteroaryl; wherein each of said (Cl-C8)alkyl substituent, wherever it
occurs, is
independently unsubstituted or substituted with one to three substituents
independently selected
from the group consisting of -NH2, (CI-C$)alkyl-NH-, [(CI-C$)alkyl]2>N-, -O-
(C=O)-(Cj-C8)alkyl,
(C2-CIo)heterocyclyl, (C6-Clo)aryl, and (CI-Clo)heteroaryl.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein R' is R36 selected from the group consisting of H, Cl, and Br.
In another embodiment, the invention relates to compounds of the Formula I
wherein R'
is selected from the group consisting of (C3-C6)cycloalkyl, (C2-
CIo)heterocyclyl, phenyl, and
(Cl-Cqp)heteroaryl; wherein each of said (C2-C10)heterocyclyl, phenyl, or (CI-
Clo)heteroaryl is
unsubstituted or substituted with one to three substituents independently
selected from the group
consisting of (Cl-C8)alkyl, F, Cl, -NH2, -OH, (Cl-C8)alkyl-NH-, and [(CI-
C8)alkyl]2>N-; wherein each
of said (Cl-C8)alkyl substituent, wherever it occurs, is unsubstituted or
substituted with one to
three substituents selected from -NH2, (Cl-Ca)alkyl-NH-, [(Cl-C8)alkyl]2>N-, -
O-(C=O)-(Cj-Ca)alkyl,
(C2-C10)heterocyclyl, (Cs-CIo)aryl, and (CI-Clo)heteroaryl. Within this
embodiment, preferably R' is
phenyl, tetrahydropyridinyl, piperidinyl or pyridinyl.
In another embodiment, the invention relates to compounds of the Formula I
wherein R'
is -C(=O)-R5, and R5 is (CI-Ca)alkyl-O- or (C2-CIo)heterocyclyl, such as
morpholinyl; wherein said
R5 (CZ-Clo)heterocyclyl is unsubstituted or substituted with (Cl-Ca)alkyl,
such as methyl or ethyl.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein R' is -C(=O)-NRsR7; wherein each of said R 6 and R7 are independently
H or (Cl-C$)alkyl;
and wherein each of said R6 and R7 (Cl-C8)alkyl are unsubstituted or
substituted with one to three
substituents independently selected from the group consisting of OH, -NH2, (Cl-
C8)alkyl-NH-,
[(Ci-C$)alkyl]z>N-, (C2-Clo)heterocyclyl, and (Cl-Clo)heteroaryl.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein R2 is H or (Cl-C8)alkyl unsubstituted or substituted with one to four
substituents
independently selected from the group consisting of OH, -NH2, (Cl-C8)alkyl-NH-
, [(CI-C8)alkyl]Z>N-
(CZ-C1o)heterocyclyl, and (Cl-Clo)heteroaryl.
In another embodiment, the invention relates to compounds of the Formula I
wherein A is
=N-.
In another embodiment, the invention relates to compounds of the Formula I
wherein R2
is -C(=O)-R8, wherein R8 is selected from the group consisting of (Cl-
C8)alkyl, (C2-C$)alkenyl, (C2-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
C8)alkynyl, -NH2, and R37 selected from the group consisting of (Cj-C$)alkyl-
NH-,
[(C1-Ca)alkyl]Z>N-, and (C1-C8)alkyl-O-; wherein each of said R8 and R37 (CI -
Ca)alkyl, wherever it
occurs, is independently unsubstituted or substituted with one to four
substituents independently
selected from R40 selected from the group consisting of F, OH, -NH2, (C3-
Clo)cycloalkyl,
5 (C2-CIo)heterocyclyl, (C6-Clo)aryl, (Cl-Clo)heteroaryl; (CI-C$)alkyl-NH- and
[(CI-C8)alkyl]z>N-;
wherein each of said R`'0 (C,-C8)alkyl, wherever it occurs, is independently
unsubstituted
or substituted with one to four substituents independently selected from R44
independently
selected from the group consisting of OH, -NH2, (Cl-C$)alkyl-NH-, [(Cj-
C8)alkyl]2>N-, and
(C3-C1o)cycloalkyl-N H-;
10 wherein each of said each of said R40 (C3-C10)cycloalkyl, (C2-
C10)heterocyclyl,
(Cs-C10)aryl, or (CI-C10)heteroaryl, wherever it occurs, is independently
unsubstituted or
substituted with one to four substituents independently selected from
R47selected from the group
consisting of (CI-C8)alkyl, OH, -NH2, (Cl-C8)alkyl-NH-, [(Cj-C$)alkyl]2>N-,
and (C3-C10)cycloalkyl-
NH-; and
wherein each of said R47 (C,-C8)alkyl, wherever it occurs, is independently
unsubstituted
or substituted with one to four substituents independently selected from the
group consisting of
OH, -NH2, (Cj-C8)alkyl-NH-, [(C1-C8)alkyl]2>N-, and (C3-C10)cycloalkyl-NH.
In another embodiment, the invention relates to compounds of the Formula I
wherein R 2
is -C(=O)-R 8, wherein Ra is selected from the group consisting of (C3-
C6)cycloalkyl, (C2-
C10)heterocyclyl, phenyl, or (Cl-CIo)heteroaryl; wherein each of said Ra (C3-
C6)cycloalkyl, (C2-
CIo)heterocyclyl, phenyl, or (CI-C10)heteroaryl is unsubstituted or
substituted with one to four
substituents independently selected from R40 selected from the group
consisting of (CI-Ca)alkyl, F,
OH, -NH2, (C1-C8)alkyl-NH-, [(Cj-Ca)alkyl]z>N-, (C3-Clo)cycloalkyl, (Ca-
Clo)heterocyclyl, (C6-
CI0)aryl, and (Cl-C10)heteroaryl; wherein each of said R40 (C,-Ca)alkyl,
wherever it occurs, is
independently unsubstituted or substituted with one to four substituents
independently selected
from R44 independently selected from the group consisting OH, -NH2, (Cj-
C8)alkyl-NH-,
[(C1-C8)alkyl]2>N-, and (C3-C1o)cycloalkyl-NH-; wherein each of said R40 (C3-
Clo)cycloalkyl,
(Cz-C10)heterocyclyl, (C6-C10)aryl, or (CI-CIo)heteroaryl is unsubstituted or
substituted with one to
four substituents independently selected from R47 selected from the group
consisting of (Cl-
C8)alkyl, OH, -NH2, (Cj-Ca)alkyl-NH-, [(Cj-C8)alkyl]z>N-, and (C3-
C1o)cycloalkyl-NH-; wherein each
of said R47 (CI-Ca)alkyl, wherever it occurs, is unsubstituted or substituted
with one to four
substituents independently selected from the group consisting of OH, -NH2, (Cj-
C8)alkyl-NH-,
[(Cj-C$)alkyl]2>N-, and (C3-C10)cycloalkyl-NH.
In another embodiment, the invention relates to compounds of the Formula I
wherein said
R3 is on any one of position 7, 8, or 9 of said compound of the formula I.
Preferably, the invention
relates to compounds of the Formula I wherein said R3 is on position 8 of said
compound of the
formula I.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
11
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein said R4 is on position 7 of said compound of the formula I. More
preferably, said R3 is on
position 8 of said compound of the formula I and said R4 is on position 7 of
said compound of the
formula I.
In another embodiment, the invention relates to compounds of the Formula I
wherein said
R4 is CI or Br on position 7 of said compound of the formula I.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein said R4 is H on position 7 of said compound of the formula I.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein X is O.
In another preferred embodiment, the invention relates to compounds of the
Formula I
wherein the group -Y-Z- has the formula -N=CH-.
Preferably the invention relates to compounds of the Formula I selected from
the group
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yi)-2-
phenyl-acetamide;
2-Cyclohexyl-N-(6-oxo-2-phenyl-5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-
acetamide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-4-phenyl-
butyramide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl)-3-
phenyl-
propionamide;
3-Fluoro-2-methyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
benzamide;
2-Fluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-
3-
trifluoromethyl-benzamide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-2-
trifluoromethyl-
benzamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
2-(3-Chlorophenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-
yl)acetamide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-4-thien-
2-
ylbutanamide;
1-Acetyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)piperidine-4-
carboxamide;
3-(2-Methylphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1H-[1,2]diazepino[4,5,6-
cd]indol-8-
yl)propanamide;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
12
(2S)-2-Amino-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-4-
phenyl-butyramide compound with trifluoro-acetic acid;
(2R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)ethanamide trifluoroacetate;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
2-Ethylsulfanyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
ce!]indol-8-yl)-
nicotinamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
N-[2-(3-Dimethylaminomethyl-phenyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,
5,6-cd]indol-
8-yl]-2-fluoro-3-trifl uoromethyl-benzam ide;
6-Oxo-2-phenyl-N-(2-phenylcyclopropyl)-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-
cd]indole-8-
carboxamide;
N-[1-(4-Fluorophenyl)ethyl]-6-oxo-2-phenyl-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indole-
8-carboxamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylaminomethyl-
phenyl)-6-oxo-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylaminomethyl-
phenyl)-6-oxo-
5,6-dihydro-1H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide (hydrochloric salt);
Acetic acid 3-{6-oxo-8-[((1R, 2R)-2-phenyl-cyclopropanecarbonyl)-amino]-5,6-
dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-2-yl}-benzyl ester;
(1 R, 2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-hydroxymethyl-phenyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(2R)-2-Amino-2-cyclohexyl-N-(.6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-acetamide (hydrochloric salt);
N-[1-(4-Hydroxyphenyl)ethyl]-6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indole-8-carboxamide;
2,3-Difluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-
benzamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4, 5,6-cd]indol-8-yl)-amide;
N-(4-Fluorobenzyl)-6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indole-8-
carboxamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-cyclobutylaminomethyl-
phenyl)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide(hydrochloric
salt);
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
13
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(3-pyrrolidin-1-
ylmethyl-phenyl)-
5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide(hydrochloric salt);
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl)-(1,2-
trans)-2-[6-
(trifluoromethyl)pyridin-3-yl]cyclopropanecarboxamide trifluoroacetate;
(2R)-2-Amino-N-(6-oxo-2-phenyl-5,6-dihydro-1H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-2-
phenyl-acetamide (hydrochloric salt);
(2R)-2-Amino-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-3-
phenyl-propionamide(hydrochloric salt);
(3E)-4-Phenyl-but-3-enoic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-amide;
2-Indan-2-yl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-
acetamide;
(1,2-trans)-2-(4-Fluoro-phenyl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1,2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-
dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide (hydrochloric salt);
(1,2-trans)-2-(3-Methoxy-phenyl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
2-Indan-2-yl-(6-oxo-5,6-dihydro-l-[1,2]diazepino[4,5,6-cd]indol-8-yl)-
acetamide;
(2R)-2-Hydroxy-N-(6-oxo-2-phenyl-5,6-dihydro-1H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
phenylethanamide;
(1,2-trans)-2-Pyridin-2-yl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-
dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide acetic acid;
(1,2-trans)-2-(1 H-Imidazol-4-yl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide acetic acid;
(2R)-Piperidine-2-carboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-amide (hydrochloric salt);
(2S)- 2-Amino-3-cyano-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-
yl)-propionamide acetic acid;
(2R)-2-amino-3-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)propanamide (hydrochloric salt);
(1 R,2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid methyl ester;
(2R)-3-(4-Hydroxyphenyl)-2-(methylamino)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)propanamide (hydrochloric salt);
(2R)-2-Amino-3-(4-fluorophenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)propanamide (hydrochloric salt);
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
14
(1 R,2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid methylamide;
(1 R,2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-hydroxy-ethyl)-amide;
(1 R,2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-dimethylamino-ethyl)-
amide;
(2R)-2-Amino-2-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)ethanamide (hydrochloric salt);
(1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-oxo-5,6-
dihydro-1 -
[1,2]diazepino[4,5,6]indol-8-yl)-amide;
(1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid . (6-oxo-2-phenyl-
5,6-
dihydro-l-[1,2]diazepino[4,5,6]indol-8-yl)-amide;
(1R,2R)- 2-Phenyl-cyclopropanecarboxylic acid (2-ethyl-6-oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1 R,2R)- 2-Phenyl-cyclopropanecarboxylic acid (2-chloro-6-oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1,2-trans)-2-(3-Bromo-phenyl)-cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-
1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1,2-trans)-2-(3-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-oxo-5,6-
dihydro-1 -
[1,2]diazepino[4,5,6]indol-8-yl)-amide;
2-(3,4-Dihydroisoquinolin-2(1 H)-yl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cc!]indol-8-yl)acetamide;
(1 R,2R)- 2-Phenyl-cyclopropanecarboxylic acid (2-bromo-6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(1,2,3,6-tetrahydro-
pyridin-4-yl)-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide (hydrochloric salt);
(1 R,2R)-N-(6-Oxo-2-pyridin-4-yI-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
phenylcyclopropanecarboxamide;
N-(6-Oxo-2-pyridin-4-yl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-
(1,2-trans)-2-
pyridin-3-ylcyclopropanecarboxamide;
N-(6-Oxo-2-pyridin-3-yi-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl)-
(1,2-trans)-2-
pyrid in-3-ylcyclopropanecarboxamide;
(1 R,2R)-N-(6-Oxo-2-pyridin-3-yl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
phenylcyclopropanecarboxamide;
(1R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylamino-prop-1-ynyl)-
6-oxo-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
(1 R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylamino-propyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl]-amide;
(1 R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylamino-propenyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
5 (1R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-methylamino-prop-l-ynyl)-
6-oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide (hydrochloric salt);
(1,2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid [2-(3-methylamino-prop-
l-ynyl)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-ccl]indol-8-yl]-amide (dihydrochloric
salt);
(2R)-2-Amino-2-cyclohexyl-N-[2-(3-methylamino-prop-l-ynyl)-6-oxo-5,6-dihydro-1
H-
10 [1,2]diazepino[4,5,6-cd]indol-8-yl]-acetamide (dihydrochloric salt);
(1,2-trans)-N-[1-(2-Hydroxyethyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl]-
2-phenylcyclopropanecarboxamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-dimethylaminomethyl-6-oxo-5,6-
dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl)-amide;
15 (1R,2R)-2-Phenyl-cyclopropanecarboxylic acid [1-(2-amino-ethyl)-6-oxo-5,6-
dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-morpholin-4-yl-prop-l-
ynyl)-6-oxo-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(3-pyrrolidin-1-yl-prop-
1-ynyl)-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide; and
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [1-(2-amino-ethyl)-2-chloro-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(1 H-imidazol-2-yl)-6-oxo-5,6-
dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-cyano-6-oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide; and
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(1 H-imidazol-2-yl)-6-oxo-5,6-
dihydro-
1 H-[1,2]diazepino[4,5,6-cc!]indol-8-yl]-amide; or
the pharmaceutically acceptable salts or solvates thereof.
Other preferred compounds are selected from the group consisting of:
3-Fluoro-2-methyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
benzamide;
2-Fluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-
3-
trifluoromethyl-benzam ide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-4-thien-2-
ylbutanamide;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
16
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylaminomethyl-
phenyl)-6-oxo-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
Acetic acid 3-{6-oxo-8-[((1R, 2R)-2-phenyl-cyclopropanecarbonyl)-amino]-5,6-
dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-2-yl}-benzyl ester;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-hydroxymethyl-phenyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl]-amide;
(2R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-acetamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo=5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-cyclobutylaminomethyl-
phenyl)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1R,2R)-2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(3-pyrrolidin-1-ylmethyl-
phenyl)-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
2-I ndan-2-yi-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[ 1,2]d iazepi no[4, 5, 6-
cd]indol-8-yl)-
acetamide;
(1,2-trans)-2-(4-Fluoro-phenyl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(2R)-2-Amino-3-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)propanamide;
(1 R,2 R)-6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-am ino]-5,6-d i hydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid methyl ester;
(1R,2R)-6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid methylamide;
(1 R,2R)-6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-hydroxy-ethyl)-amide;
(1 R,2R)-6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-hydroxy-ethyl)-amide;
(2R)-2-Amino-2-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[ 1,2]diazepino[4, 5,6-cd]indol-8-yl)ethanamide;
(1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-oxo-5,6-
dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-
dihydro-1 -[1,2]diazepino[4,5,6]indol-8-yl)-amide;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
17
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-ethyl-6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-ethyl-6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
2-(3,4-Dihydroisoquinolin-2(1H)-yI)-N-(6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-8-yl)acetamide;
2-(3,4-Dihydroisoquinolin-2(1 H)-yl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)acetamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(1,2,3,6-tetrahydro-
pyridin-4-yl)-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-N-(6-Oxo-2-pyridin-4-y1-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
phenylcyclopropanecarboxamide;
(1 R,2R)-N-(6-Oxo-2-pyridin-3-yl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
phenylcyclopropanecarboxamide;
(1R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylamino-prop-l-ynyl)-
6-oxo-
5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylamino-propenyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-methylamino-prop-1-ynyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(2R)-2-Amino-2-cyclohexyl-N-[2-(3-methylamino-prop-1-ynyl)-6-oxo-5,6-dihydro-1
H-
[1,2]diazepino[4, 5,6-cd]indol-8-yl]-acetamidede;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-hydroxymethyl-6-oxo-5,6-
dihydro-1 H-
[1,2-cd]diazepino[4,5,6]indol-8-yi)-amide;
(1R,2R)-2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(3-pyrrolidin-l-yl-prop-
1-ynyl)-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(1 H-imidazol-2-yl)-6-oxo-5,6-
dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-cyano-6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide; and
(2R)-2-Amino-2-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)ethanamide hydrochloride; or
the pharmaceutically acceptable salts or solvates thereof.
Other preferred compounds of formula I are selected from the group consisting
of:
2-Fluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-
3-
trifl uoromethyl-benzam ide;
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
18
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl)-4-thien-
2-
ylbutanamide;
(1R, 2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-hydroxymethyl-phenyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl]-amide;
(2R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-acetam ide(hydrochloride);
6-Oxo-2-phenyl-N-[(1 R)-1-phenylethyl]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indole-8-
carboxamide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(2R)-2-Hydroxy-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
phenylethanamide;
(1,2-trans)-2-Pyridin-2-yl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-
dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide (acetic acid salt);
(1,2-trans)-2-(1H-Imidazol-4-yl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-dihydro-
1H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide (acetic acid salt);
(2R)-Piperidine-2-carboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-amide (hydrochloric salt);
(2R)-2-Amino-3-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)propanamide hydrochloride;
(1 R,2R)-6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-dimethylamino-ethyl)-
amide;
Example 182: (1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-
oxo-2-
phenyl-5,6-dihydro-1-[1,2]diazepino[4,5,6]indol-8-yl)-amide;
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-ethyl-6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1 R,2R)-N-(6-Oxo-2-pyridin-3-yI-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-
8-yl)-2-
pheny[cyclopropanecarboxamide; and
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [1-(2-amino-ethyl)-2-chloro-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide; or
the pharmaceutically acceptable salts or solvates thereof.
In another embodiment, the invention also relates to compounds of the Formula
I that are
selective for CHK-1 over CHK-2 with selectivity ratio between about 5 folds
and about 5000 folds;
preferably between about 50 folds and about 1000 folds; and more preferably
between about 70
folds and about 830 folds. Within this embodiment, the more preferred
compounds are selected
from the group consisting of:
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
19
2-Fluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-
3-
trifluoromethyl-benzam ide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4, 5,6-cd]indol-8-yl)-2-
trifluoromethyl-
benzamide;
N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-4-thien-
2-
ylbutanamide;
N-[2-(3-Dimethylaminomethyl-phenyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-
8-yI]-2-fluoro-3-trifl uoromethyl-benzam ide;
(2R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-acetamide(hydrochloride);
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide;
(1 R,2R)-6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-dimethylamino-ethyl)-
amide;
(2R)-2-Amino-2-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)ethanamide hydrochloride;
(1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-oxo-2-phenyl-
5,6-
dihydro-l-[1,2]diazepino[4,5,6]indol-8-yl)-amide; and
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (2-ethyl-6-oxo-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide; or
the pharmaceutically acceptable salts or solvates thereof.
Another object of the invention is to provide a composition for the treatment
of neoplasms,
and for enhancing the antineoplastic effects of anti-neoplastic agents and
therapeutic radiation.
In an embodiment, the invention relates to a composition containing a compound
of the
Formula I, a pharmaceutically acceptable salt, solvate, or prodrug thereof and
an anti-neoplastic
agent as a combined preparation for the simultaneous, separate or sequential
use in treating a
neoplasm.
In another embodiment, the invention relates to a composition containing a
compound of
the Formula I, a pharmaceuticall,y acceptable salt, solvate, or prodrug
thereof and an anti-
neoplastic agent as a combined preparation for the simultaneous, separate or
sequential use in
treating a neoplasm wherein the anti-neoplastic agent is selected from the
group consisting of
alkylating agents, antibiotics and plant alkaloids, hormones and steroids,
synthetic agents having
anti-neoplastic activity, antimetabolites and biological molecules having anti-
neoplastic activity.
In another embodiment, the invention relates to a composition containing a
compound of
the Formula I, a pharmaceutically acceptable salt, solvate, or prodrug thereof
and an anti-
neoplastic agent as a combined preparation for the simultaneous, separate or
sequential use in
treating a neoplasm wherein the anti-neoplastic agent is selected from the
group consisting of
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Ara-c, VP-16, cis-platin, adriamycin, 2-chloro-2-deoxyadenosine, 9- (3-D-
arabinosyl-2-
fluoroadenine, carboplatin, gemcitabine, camptothecin, paclitaxel, BCNU, 5-
fluorouracil,
irinotecan, and doxorubicin.
Another object of the invention is to provide a method for the treatment of
neoplasms.
5 In an embodiment, the invention relates to a method for treating a neoplasm
which
comprises administering to a mammal in need thereof, an anti-neoplastic agent
in combination
with a compound of the Formula I, a pharmaceutically acceptable salt, solvate,
or prodrug thereof.
In another embodiment, the invention relates to a method for treating a
neoplasm which
comprises administering to a mammal in need thereof, an anti-neoplastic agent
in combination
10 with a compound of the Formula I, a pharmaceutically acceptable salt,
solvate, or prodrug thereof,
wherein the anti-neoplastic agent is selected from the group consisting of Ara-
c, VP-16, cis-platin,
adriamycin, 2-chloro-2-deoxyadenosine, 9-p-D-arabinosyl-2-fluoroadenine,
carboplatin,
gemcitabine, camptothecin, paclitaxel, BCNU, 5-fluorouracil, irinotecan, and
doxorubicin. In
another embodiment, more than one anti-neoplastic agents may be used in
combination with a
15 compound of the Formula I, the pharmaceutically acceptable salts, solvates,
or prodrugs thereof.
Another object of the invention is to provide methods for enhancing the anti-
neoplastic
effect of therapeutic radiation.
In an embodiment, the invention relates to a method for treating a neoplasm
which
comprises administering to a mammal in need thereof, therapeutic radiation
having an anti-
20 neoplastic effect in combination with a compound of the Formula I, a
pharmaceutically acceptable
salt, solvate, or prodrug thereof.
Another object of the invention is to provide methods for enhancing the
antineoplastic
effect of an anti-neoplastic agent.
In an embodiment, the invention relates to a method for enhancing the anti-
neoplastic
effect of an anti-neoplastic agent in a mammal which comprises administering
to a mammal in
need thereof, a compound of the Formula I, a pharmaceutically acceptable salt,
solvate, or
prodrug thereof, in combination with an antineoplastic agent. The
antineoplastic agents include
alkylating agents, antibiotics and plant alkaloids, hormones and steroids,
synthetic agents having
anti-neoplastic activity, antimetabolites and biological molecules having anti-
neoplastic activity.
Specific antineoplastic agents include Ara-c, VP-16, cis-platin, adriamycin, 2-
chloro-2-
deoxyadenosine, 9-(3-D-arabinosyl-2-fluoroadenine, carboplatin, gemcitabine,
camptothecin,
paclitaxel, BCNU, 5-fluorouracil, irinotecan, and doxorubicin.
In another embodiment, the invention relates to a method for enhancing the
anti-
neoplastic effect of therapeutic radiation in a mammal which comprises
administering to a
mammal in need thereof, a compound of the Formula I, a pharmaceutically
acceptable salt,
solvate, or prodrug thereof, in combination with therapeutic radiation having
an anti-neoplastic
effect.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
21
Another object of the invention is to provide a method for the treatment of a
condition
which can be treated by the inhibition of protein kinases. In one embodiment
of the invention, the
protein kinases are selected from the group consisting of Checkpoint kinase
1(CHK-1),
Checkpoint kinase 2 (CHK-2), Cyclin dependent kinase 1(CDK1), Serum and
glucocorticoid
regulated kinase (SGK), Adenosine 5' monophosphate (AMP)-activated protein
kinase (AMPK),
Lymphoid T cell tyrosine kinase (LCK), Mitogen activated protein kinase-2
(MAPK-2), Mitogen-
and stress-activated protein kinase 1(MSK1), Rho kinase (ROCK-II), P70 S6
kinase (p70S6K),
cAMP (adenosine 3;5' cyclic monophosphate)-dependent protein kinase (PKA),
Mitogen activated
protein kinase (MAPK), Mitogen activated protein kinase-I (MAPK-1), Protein
kinase C-related
kinase 2 (PRK2), 3'Phosphoinositide dependent kinase 1(PDK1), Fyn kinase
(FYN), Protein
kinase C (PKC), Protein Kinase C Beta 2(PKCPII), Protein Kinase C Gamma
(PKCy), Vascular
endothelial growth factor receptor 2 (VEGFR-2), Fibroblast growth factor
receptor (FGFR),
Phosphorylase kinase (PHK), Weel kinase (Weel), and Protein Kinase B (PKB).
Preferably, the
protein kinases are selected from the group consisting of Checkpoint kinase
1(CHK-1),
Checkpoint kinase 2 (CHK-2), Mitogen activated protein kinase (MAPK), Mitogen
activated protein
kinase-1 (MAPK-1), Mitogen activated protein kinase-2 (MAPK-2), Vascular
endothelial growth
factor receptor 2 (VEGFR-2), Fibroblast growth factor receptor (FGFR),
Phosphorylase kinase
(PHK), Protein Kinase B alpha (PKBa), and Weel kinase (Weel).
In an embodiment, the invention relates to a method for the treatment of a
condition which
can be treated by the inhibition of protein kinases in a mammal, including a
human, comprising
administering to a mammal in need thereof, a compound of the Formula I, a
pharmaceutically
acceptable salt, solvate, or prodrug thereof.
In another embodiment, said condition which can be treated by the inhibition
of protein
kinases is selected from the group consisting of connective tissue disorders,
inflammatory
disorders, immunology/allergy disorders, infectious diseases, respiratory
diseases, cardiovascular
diseases, eye diseases, metabolic diseases, central nervous system (CNS)
disorders, liver/kidney
diseases, reproductive health disorders, gastric disorders, skin disorders and
cancers.
Other aspects, advantages, and preferred features of the invention will become
apparent
from the detailed description below.
Detailed Description And Preferred Embodiments of The Invention
For purposes of the present invention, as disclosed and claimed herein, the
following
terms and abbreviations are defined as follows:
Unless otherwise indicated, the term "wherever it occurs" refers to any
occurrence of any
functional groups (such as R1, R2 or any substituents thereof), including any
occurrence of any
component of any functional groups referred to herein (e.g., the "(C1-C$)alkyl
component of (Cl-
C8)alkyl-O-).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
22
Unless otherwise indicated, the term "(Cj-C$)alkyl" as well as the (Cl-
C8)alkyl component
of other terms referred to herein (e.g., the "(Cj-Ca)alkyl component of (Cj-
Ca)alkyl-O-), may be
linear or branched (such as methyl, ethyl, n-propyl, isopropyl, n-butyl, iso-
butyl, secondary-butyl,
tertiary-butyl).
Unless otherwise indicated, the term "(C2-C$)alkenyl" means straight or
branched
hydrocarbon radical, substituent, moiety, or sub-moiety referred to herein
having 2 to 8 carbon
atoms having at least one double bond including, but not limited to ethenyl, 1-
propenyl, 2-propenyl
(allyl), iso-propenyl, 2-methyl-1-propenyl, 1-butenyl, or 2-butenyl.
Unless otherwise indicated, the term "(Cz-C8)alkynyl" is used herein to mean
straight or
branched hydrocarbon radical, substituent, moiety, or sub-moiety referred to
herein having 2 to 8
carbon atoms having one triple bond including, but not limited to, ethynyl (-
C=C-H), propynyl
(-CH2-C=C-H or -C=C-CH3), or butynyl (-CH2-CH2-C=C-H, or -CH2-C-C-CH3i or -C=C-
CH2CH3).
Unless otherwise indicated, the term "(C3-C1o)cycloalkyl" refers to a non-
aromatic,
saturated or partially saturated, monocyclic or fused, spiro or unfused
bicyclic or tricyclic
hydrocarbon radical, substituent, moiety, or sub-moiety referred to herein
containing a total of
from 3 to 10 carbon atoms, preferably 5-8 ring carbon atoms. Exemplary (C3-
Clo)cycloalkyls
include monocyclic rings having from 3-7, preferably 3-6, carbon atoms, such
as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and the like. Illustrative
examples of (C3-
C10)cycloalkyl are derived from, but not limited to, the following:
a , ^, ~ , _
0,0, o,,
and .
Unless otherwise indicated, the term "(C2-C1o)heterocyclyl" refers to a non-
aromatic,
saturated or partially saturated, monovalent, monocyclic or fused, spiro or
unfused bicyclic or
tricyclic radical, substituent, moiety, or sub-moiety referred to herein
containing a total of from 2 to
10 ring carbon atoms and 1 to 5 ring heteroatoms selected from nitrogen,
oxygen and sulfur.
Illustrative examples of (C2-CIo)heterocyclyl include azetidinyl, pyrrolidyl,
piperidyl, piperazinyl,
morpholinyl, chromenyl, tetrahydro-2H-1,4-thiazinyl, tetrahydrofuryl,
dihydrofuryl,
tetrahydropyranyl, dihydropyranyl, 1,3-dioxolanyl, 1,3-dioxanyl, 1,4-dioxanyl,
1,3-oxathiolanyl,
1,3-oxathianyl, 1,3-dithianyl, azabicyclo[3.'2.1]octyl,
azabicyclo[3.3.1]nonyl, azabicylo[4.3.0]nonyl,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
23
oxabicyclo[2.2.1]heptyl, 1,5,9-triazacyclododecyl, and the like. Additional
illustrative examples of
(C2-Clo)heterocyclyl are derived from, but not limited to, the following:
O H
NH 0
C N
O N) C
O N ~
~ H O H H H
NH
~ ~N ~ CN3 ~~\/ N
J N
S O H I H .
30~10 ~ _ O
C
N N
H H I H
/ I
O
-s
N
iH'\
H
O
O ~/O
~NH
and
unless otherwise indicated, the foregoing (C2-Clo)heterocyclyl can be
C-attached or N-attached where such is possible. For instance, piperidyl can
be piperid-1-yl
(N-attached) or piperid-2-yl (C-attached).
Unless otherwise indicated, the term "(C6-C1o)aryI" refers to an aromatic,
monovalent,
monocyclic or fused or unfused bicyclic or tricyclic radical, substituent,
moiety, or sub-moiety
referred to herein containing a total of from 6 to 10 ring carbon atoms.
Illustrative examples of
(C6-C,o)aryl are derived from, but not limited to, the following:
and \ I /
Unless otherwise indicated, the term "(Cl-Clp)heteroaryl" refers to an
aromatic,
monovalent monocyclic, fused or unfused bicyclic or tricyclic radical,
substituent, moiety, or sub-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
24
moiety referred to herein containing a total of from 1 to 10 ring carbon atoms
and I to 5 ring
heteroatoms selected from nitrogen, oxygen and sulfur. Illustrative examples
of (Cl-
CIo)heteroaryl include, but not limited to, thienyl, pyrrolyl, imidazolyl,
pyrazoiyi, furyl, isothiazolyl,
furazanyl, isoxazolyl, thiazolyl, pyridyl, pyrazinyl, pyrimidinyl,
pyridazinyl, triazinyl, benzo[b]thienyl,
naphtho[2,3-b]thianthrenyl, isobenzofuranyl, chromenyl, xanthenyl,
phenoxathienyl, indolizinyl,
isoindolyl, indolyi, indazolyl, purinyl, isoquinolyl, quinolyl, phthalazinyl,
naphthyridinyl, quinoxyalinyl,
quinzolinyl, benzothiazolyl, benzimidazolyl, tetrahydroquinolinyl, cinnolinyl,
pteridinyl, carbazolyl,
beta-carbolinyl, phenanthridinyl, acridinyl, perimidinyl, phenanthrolinyl,
phenazinyl, isothiazolyl,
phenothiazinyl, and phenoxazinyl. Further examples of (Cl-CIo)heteroaryl are
derived from, but
not limited to, the following:
N
H S3
r~
QQ
H N
~
~N N CN N~\N N
N \ ~N \N/ \N
H ~ O NI ~ N ' H
N':O~N N'::~~N / \ C I 1 11 N~/N N N
H H
/ \
/ I N N H
unless otherwise indicated, the foregoing (Cl-C10)heteroaryl can be
C-attached or N-attached where such is possible. For instance, pyridyl can be
pyrid-1-yl
(N-attached) or pyrid-3-yl (C-attached).
Unless otherwise indicated, the term "((C3-Clp)cycloalkyl)((Cl-C$)alkyl)>N"
refers to a
radical, substituent, moiety, or sub-moiety referred to herein having the
formula:
(C3-Cjo)cycloalkyl) \ N4
(CI-C8)alkyl)~ ; wherein the terms "(C3-Clo)cycloalkyl" and "(CI-C$)alkyl" are
as
defined above.
Unless otherwise indicated, the term "((Cs-Clo)aryl)((Cl-C$)alkyl)>N" refers
to a radical,
substituent, moiety, or sub-moiety referred to herein having the formula:
(Ca-Cjo)aryl )\ N4
(Cj-C8)alkyl) ; wherein the terms "(C6-C1o)aryl" and "(C1-C8)alkyl" are as
defined
above.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Unless otherwise indicated, the term "(C1-C1o)heteroaryl-NH" refers to a
radical,
substituent, moiety, or sub-moiety referred to herein having the formula:
(C1-CIo)heteroaryl)\
~N4
H ; wherein the term "(C1-C1o)heteroaryP" is as defined above and
wherein said (C,-CIo)heteroaryl is bonded to the -NH- via a ring (Cl-
Clo)heteroaryl carbon atom.
5 Unless otherwise indicated, the term "(CZ-CIo)heterocyclyl-NH '" refers to a
radical,
substituent, moiety, or sub-moiety referred to herein having the formula:
(C2-C1o)heterocyclyl) \
/N4
H ; wherein the term "(C2-C1o)heterocyclyP" is as defined above and
wherein said (C2-Clo)heterocyclyl is bonded to the -NH- via a ring (C2-
Clo)heterocyclyl carbon
atom.
10 The term "a pharmaceutically acceptable salt" refers to a salt that retains
the biological
effectiveness of the free acids and bases of the specified compound and that
is not biologically or
otherwise undesirable. A compound of the invention may possess a sufficiently
acidic, a
sufficiently basic, or both functional groups, and accordingly react with any
of a number of
inorganic or organic bases, and inorganic and organic acids, to form a
pharmaceutically
15 acceptable salt. Exemplary pharmaceutically acceptable salts include those
salts prepared by
reaction of the compounds of the present invention with a mineral or organic
acid or an inorganic
base, such as salts including sulfates, pyrosulfates, bisulfates, sulfites,
bisulfites, phosphates,
monohydrogenphosphates, dihydrogenphosphates, metaphosphates, pyrophosphates,
chlorides,
bromides, iodides, acetates, propionates, decanoates, caprylates, acrylates,
formates,
20 isobutyrates, caproates, heptanoates, propiolates, oxalates, malonates,
succinates, suberates,
sebacates, fumarates, maleates, butyne-1,4-dioates, hexyne-1,6-dioates,
benzoates,
chlorobenzoates, methylbenzoates, dinitrobenzoates, hydroxybenzoates,
methoxybenzoates,
phthalates, sulfonates, xylenesulfonates, phenylacetates, phenylpropionates,
phenylbutyrates,
citrates, lactates, y-hydroxybutyrates, glycollates, tartrates, methane-
sulfonates,
25 propanesulfonates, naphthalene-l-sulfonates, naphthalene-2-sulfonates, and
mandelates.
The term prodrug", as used herein, refers to a metabolic precursor of a
compound of
the Formula I (or a salt thereof) that is pharmaceutically acceptable. A
prodrug may be inactive
when administered to a subject but is converted in vivo to an active compound
of the Formula I.
The term "active metabolite", as used herein, refers to a metabolic product of
a compound of the
Formula I that is pharmaceutically acceptable and effective. Prodrugs and
active metabolites of
compounds of the Formula I may be determined using techniques known in the
art. Prodrugs and
active metabolites of a compound may be identified using routine techniques
known in the art.
See, e.g., Bertolini et al., J. Med. Chem., 40, 2011-2016 (1997); Shan, et
al., J. Pharm. Sci., 86
(7), 765-767; Bagshawe, Drug Dev. Res., 34, 220-230 (1995); Bodor, Advances in
Drug Res., 13,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
26
224-331 (1984); Bundgaard, Design of Prodrugs (Elsevier Press 1985); and
Larsen, Design and
Application of Prodrugs, Drug Design and Development (Krogsgaard-Larsen et
al., eds.,
Harwood Academic Publishers, 1991).
The CHK-1 inhibitor of the present invention may be administered in
combination with
other anti-neoplasm therapies including anti-neoplastic agents and radiation
therapy.
The term "in combination with" means that the compound of Formula I may be
administered shortly before, shortly after, concurrently, or any combination
of before, after, or
concurrently, with such other anti-neoplasm therapies. Thus, the compound and
the anti-
neoplastic agent may be administered simultaneously as either as a single
composition or as two
separate compositions or sequentially as two separate compositions. Likewise,
the compound
and radiation therapy may be administered simultaneously, separately or
sequentially. The
compound may be administered in combination with more than one anti-neoplasm
therapy. In a
preferred embodiment, the compound may be administered from 2 weeks to I day
before any
chemotherapy, or 2 weeks to 1 day before any radiation therapy. In another
preferred
embodiment, the CHK-1 inhibitor may be administered during anti-neoplastic
chemotherapies and
radiation therapies. If administered following such chemotherapy or radiation
therapy, the CHK-1
inhibitor may be given within I to 14 days following the primary treatments.
The CHK-1 inhibitor
may also be administered chronically or semi-chronically, over a period of
from about 2 weeks to
about 5 years. One skilled in the art will recognize that the amount of CHK-1
inhibitor to be
administered in accordance with the present invention in combination with
other antineoplastic
agents or therapies is that amount sufficient to enhance the anti-neoplasm
effects of anti-
neoplastic agents or radiation therapies or that amount sufficient to induce
apoptosis or cell death
along with the anti-neoplastic or radiation therapy and/or to maintain an
antiangiogenic effect.
Such amount may vary, among other factors, depending upon the size and the
type of neoplasia,
the concentration of the compound in the therapeutic formulation, the specific
anti-neoplasm
agents used, the timing of the administration of the CHK-1 inhibitors relative
to the other
therapies, and the age, size and condition of the patient.
The term "protein kinases", as used herein, refers to enzymes that catalyze
the
phosphorylation of hydroxy groups on tyrosine, serine and threonine residues
of proteins. The
consequences of this seemingly simple activity are staggering; cell growth,
differentiation and
proliferation, i.e., virtually all aspects of cell life in one way or another
depend on the protein
kinase activity. Furthermore, abnormal protein kinase activity has been
related to a host of
disorders, ranging from relatively non-life threatening diseases such as
psoriasis to extremely
virulent diseases such as glioblastoma (brain cancer). The protein kinases can
be conveniently
broken down into two major classes, the protein tyrosine kinases (PTKs) and
the serine-threonine
kinases (STKs). In addition, a third class of dual specificity kinases which
can phosphorylate both
tyrosine and serine-threonine residues is known. Examples of protein kinases
and their isoforms
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
27
contemplated within this invention include, but are not limited to, Checkpoint
kinase 1(CHK-1),
Checkpoint kinase 2 (CHK-2), Cyclin dependent kinase 1(CDK1), Serum and
glucocorticoid
regulated kinase (SGK), Adenosine 5=monophosphate (AMP)-activated protein
kinase (AMPK),
Lymphoid T cell tyrosine kinase (LCK), Mitogen activated protein kinase-2
(MAPK-2), Mitogen-
and stress-activated protein kinase 1(MSK1), Protein Kinase B (PKB), Protein
Kinase B alpha
(PKBa), Rho kinase (ROCK-II), P70 S6 kinase (p70S6K), cAMP (adenosine 3;5'
cyclic
monophosphate)-dependent protein kinase (PKA), Mitogen activated protein
kinase-1 (MAPK-1),
Protein kinase C-related kinase 2 (PRK2), 3'-Phosphoinositide dependent kinase
1(PDK1), Fyn
kinase (FYN), Protein kinase C (PKC), Protein Kinase C Beta 2(PKCRII), Protein
Kinase C
Gamma (PKCy), Vascular endothelial growth factor receptor 2 (VEGFR-2),
Fibroblast growth
factor receptor (FGFR), Phosphorylase kinase (PHK), Weel kinase (Weel), and
Protein Kinase B
(PKB).
Checkpoint kinase 2 (CHK-2) acts as a cell cycle checkpoint controller in
response to
DNA damage. CHK-2 is a downstream effector of ATM which phosphorylates p53
protein and
affects cell cycle progreesion from G, to the S phase. CHK-2 activation also
affects S phase
progression. In addition along with CHK-1, CHK-2 influences G2/M transition
and plays a role in
apoptosis if the damage cannot be repaired. CHK-2 could play a role in
sensitizing cancer cells to
DNA-damaging therapies. CHK-2 may also play a role as a tumor suppressor.
Bartek, J. et. al.
(2001) Nature Reviews, Molecular Cell biology 2:877-886.
Cyclin dependent kinase 1(CDK1) is also known as Cdc2 in yeast cells. The cell
cycle
directs specific events that control growth and proliferation of cells. The
cyclin B/Cdkl complex
promotes entry into mitosis. Cyclin BI overexpression has been found in 90% of
colorectal
carcinomas Since the cell cycle is dysregulated in human cancers, modulation
of CDK activity is a
possible therapy. Olomoucine, a CDK inhibitor, has been shown to inhibit
cellular proliferation in
human cancer cells. In lymphoma cells,olomoucine arrests the cell cycle in
both the G, and G2
phases by inhibiting cyclin E/CDK2 and cyclin B/CDK1. Buolamwini, J.K. (2000)
Current
Pharmaceutical Design 6:379-392; Fan, S. et. al. (1999) Chemotherapy 45:437-
445.
Serum and glucocorticoid regulated kinase (SGK) is rapidly and highly
regulated by
corticosteroids in A6 cells at the mRNA and protein levels. SGK is also
induced by aldosterone in
the kidney of adrenalectomized rats. SGK is activated by 3'-phosphoinositide
dependent kinase 1
(PDKI). SGK might play a critical role in aidosterone target cells and may be
physiologically
important in the early response to aldosterone. Aldosterone receptor
antagonists have recently
shown great promise in clinical trials for patients with heart failure. The
ability to mediate the
physiological responses to aidosterone may like-wise prove beneficial. See
Leslie, N. R. et. al.
(2001) Chemical Reviews 101(8):2365-2380; Funder, J. W. (1999) Molecular and
Cellular
Endocrinology 151(1-2):1-3; Verrey, F. et. al. (2000)
KidneyInternational57(4):1277-1282.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
28
Adenosine 5' monophosphate (AMP)-activated protein kinase (AMPK) isoform a2
(AMPK a2) is present in high concentrations in skeletal muscle, heart, and
liver while the a1
isoform is widely distributed. AMPK, probably the a2 isoform, phosphorylates
acetyl-CoA
carboxylase 0 isoform (ACCR) and inactivates it under conditions electrical
stimulation or
exercise. In rat skeletal muscle, malonyl-CoA is regulated by ACC(3is and
involved in the
regulatory mechanism of transferring long chain fatty acids into the
mitochondria where they are
oxidized. AMPK could therefore be linked to obesity and/or insulin resistance,
and modulation of
AMPK could be potentially beneficial in the treatment of these diseases. AMPK
inhibits enzymes
involved in glycogen and cholesterol synthesis. It is a possible regulatory
enzyme that in response
to adenosine 5'-triphosphate (ATP) depletion, reduces further ATP consumption
by initiating
cellular adjustments that are directed toward maintaining ATP levels. In
addition, AMPK has been
linked to transcription , regulation of creatinine kinase, apoptosis, and
glucose transport. See
Kemp, B. E. et. al. (1999) Trends in Biochemical Sciences 24(1):22-25;
Friedman, J. (2002)
Nature 415(6869):268-269; Ruderman, N. B. et. al. (1999) American Journal of
Physiology 276(1,
Pt.1):E1-E18.
Lymphoid T cell tyrosine kinase (LCK) is a cytosolic non-receptor tyrosine
kinase and a T-
lymphocyte member of the Src family. LCK has been implicated in early phase T-
cell receptor
activation by antigens and plays a critical role in T-cell mediated immune
responses. Upon
activation by autophosphorylation, LCK phosphorylates T-cell receptor i;-
chains which can then
recruit a second cytoplasmic protein-tyrosine kinase ZAP-70 to promote T-cell
activation.
Inhibitors could be used for the treatment of rheumatoid arthritis, diseases
related to immune
response and T-cell based Ieukemias and lymphomas. See Garcia-Echeverria, C.
(2001) Current
Medicinal Chemistry 8(13):1589-1604; Majolini, M. B. et. al. (1999) Leukemia &
Lymphoma
35(3/4):245-254.
Mitogen- and stress-activated protein kinase 1(MSK1) is activated on
stimulation of the
Ras-mitogen activated protein kinase (MAPK) pathway and also by the p38 stress
kinase
pathway. Both pathways are implicated in tumorigenesis. Stimulation of the Ras-
MAPK signal
transduction pathway by growth factors or phorbol esters results in
phosphorylation of histone H3.
MSK1 has been shown to mediate epidermal growth factor (EGF) or TPA (12-0-
tetradecanoylphorbol-13-acetate, a phorbol ester) induced phosphorylation of
H3. There is
evidence that persistent activation of Ras-MAPK pathway and MSK1 resulting in
elevated
phosphorylated H3 levels may contribute to aberrant gene expression observed
in oncogene-
transformed cells. Inhibition of MSKI suppressed the induction of c-fos (proto-
oncogene) and uPA
genes in parental and oncogene-transformed cells. Both c-fos and uPA are
involved in tumor
invasion and metastasis. See Strelkov, I. et. al. (2002) Cancer Research
62(1):75-78; Zhong, S.
et. al. (2001) Journal of Biological Chemistry 276(35):33213-33219; Nomura, M.
et. al. (2001)
Journal of Biological Chemistry 276(27);25558-25567.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
29
Rho kinase (ROCK-II) is also known as ROKa. By inhibiting ROCK-II, one could
potentially influence Rho GTPases which act as molecular controls that
regulate many essential
cellular processes, including actin dynamics, cell-cycle progression, and cell
adhesion. The in vitro
and in vivo biological effects of Y-27632, a specific inhibitor of ROCK, have
been described in the
literature and include lowering blood pressure in hypertensive rats,
inhibition of Rho-induced
formation of stress fibers and focal adhesions, and inhibition of tumor
growth. See Narumiya, S.
et. aI(2000) Methods in Enzymology 325 (Regulators and Effectors of Small
GTPases, Part D):
273-284 (and associated references listed therein); Bishop, et al. (2000)
Biochem. J. 348: 241-
255.
P70 S6 kinase (p70s6K) is found as two isoforms-one cytoplasmic and the other
in the
nucleus. They are similar except for N-terminus, and both are called p70s6K or
S6K1. A second
functional homologue S6K2 was also identified. P70s6K is a downstream target
of the lipid kinase
phosphoinositide 3-OH kinase (PI(3)K). P70s6K is implicated in cell cycle
control and neuronal cell
differentiation. P70S6K may also function in regulating cell motility which
could influence tumor
metastases, the immune response, and tissue repair. Along with PKB/Akt, p70S6K
is a crucial
effector in oncogenic protein-tyrosine kinase (PTK) signaling. P70S6K may be a
more potent kinase
for BAD than PKB/Akt (see above) in response to insulin growth factor 1(IGF-1)
stimulation.
P70s6K may therefore play an important antiapoptotic role. See Blume-Jensen,
P. et. al. (2001)
Nature 411(6835):355-365; Accili, D. (2001) Journal of Clinical Investigation
108(11):1575-1576;
Hidalgo, M. et al. (2000) Oncogene 19(56):6680-6686; Berven, L. et. al.,(2000)
Immunology and
Cell Biology 78(4):447-451.
cAMP (adenosine 3;5' cyclic monophosphate)-dependent protein kinase (PKA) is
involved in a wide range of physiological responses following interaction with
cAMP. cAMP is a
second messenger that regulates many different cellular activities such as
gene transcription, cell
growth and differentiation, ion channel conductivity, and release of
neurotransmitters. The
cAMP/PKA interaction acts as a major regulatory mechanism in mammals, and PKA
has been
shown phosphorylate a myriad of physiological substrates. PKA has two major
isoforms- PKAI
and PKAII. PKAI inhibitors have shown enhancing effects when used in
combination certain
cytotoxic cancer therapies. Antisense oligonucleotides targeting the PKAI
subunit Rla have shown
enhanced anti-tumor effects when combined with Taxol. Glucagon activates PKA
and PKA may
influence insulin response along with calmodulin-dependent protein kinase and
protein kinase C.
PKA is involved in regulating cardiac L-type calcium channels, and modulation
of the implicated
regulatory pathways may prove useful in the treatment'of heart disease. In
addition, dysfunctional
T-cells isolated from HIV patients have been restored by the addition of PKAI
antagonists. See
Skalhegg, B.S. et. al. (2000) Frontiers in Bioscience [Electronic Publication]
5:D678-D693;
Brandon, E. P. et. al. (1997) Current Opinion in Neurobiology 7(3):397-403;
Nesher, R. et
al. (2002) Diabetes 51(Suppl. 1): S68-S73; Shabb, J. B. (2001) Chemical
Reviews 101(8):2381-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
2411; Kamp, T.J. et. al. (2000) Circulation Research 87(12);1095-1102;
Tortora, G. et.al. (2002)
Clinical Cancer Research 8:303-304; Tortora, G. et.al. (2000) Clinical Cancer
Research 6:2506-
2512.
Mitogen activated protein kinase (MAPK) is also known as ERK. In
tumorigenesis, ras
5 oncogenes transmit extracellular growth signals. The MAPK pathway is an
important signaling
route between membrane-bound ras and the nucleus. A phosphorylation cascade
involving three
key kinases is involved. They are Raf, MEK (MAP kinase kinase) and MAPK/ERK.
Raf isoforms
phosphorylate and activate isoforms MEK1 and MEK2. MEK1 and 2 are dual
specificity kinases
that in turn phosphorylate and activate the,MAPK isoforms MAPK1/ERK1 and
MAPK2/ERK2. In
10 fibroblasts, MAPK1/ERK1 and MAPK2/ERK2 are both strongly activated by
growth factors and by
tumor-promoting phorbol esters. MAPK1/ERK1 and MAPK2/ERK2 are also involved
with glucose
regulation, neurotransmitter regulation, and secetagogue regulation (in
endocrine tissues). The
MAPK pathway has also been linked to the induction of cyclin Dl mRNA and thus
linked to G1
phase of cell cycle. See Webb, C.P. et. al. (2000) Cancer Research 60(2), 342-
349; Roovers, K.
15 et. al. (2000) BioEssays 22(9):818-826; Chen, Z. et. al. (2001) Chemical
Reviews 101(8):2449-
2476; Lee, J. C. et. al. (2000) Immunopharmacology 47(2-3):185-201, Sebolt-
Leopold J.S. (2000)
Oncogene 19:6594-6599; Cheng, F.Y. et. al. (2001) Journal of Biological
Chemistry
276(35):32552-32558; Cobb, M.H.et. al. (2000) Trends in Biochemical Sciences
25(1):7-9; Cobb,
M.H.et. al. (1995) Journal of Biological Chemistry 270(25):14843-14846; Deak,
M. et. al. (1998)
20 EMBO Journal 17(15):4426-4441; Davis, J.D. (1993) Journal of Biological
Chemistry
268(20):14553-14556.
cSrc (also known as p60 c-src) is cytosolic, non-receptor tyrosine kinase. c-
Src is
involved in the transduction of mitogenic signals from a number of polypeptide
growth factors
such as epidermal growth factor (EGF) and platelet-derived growth factor
(PDGF). c-Src is over
25 expressed in mammary cancers, pancreatic cancers, neuroblastomas, and
others. Mutant c-Src
has been identified in human colon cancer. c-Src phosphorylates a number of
proteins that are
involved in regulating cross-talk between the extracellular matrix and the
cytoplasmic actin
cytoskeleton. Modulation cSrc activity could have implications in diseases
relating to cell
proliferation, differentiation and death. See Bjorge, J.D. et. al. (2000)
Oncogene 19(49):5620-
30 5635; Halpern, M. S. et. al. (1996) Proc. Natl. Acad. Sci. U. S. A. 93(2),
824-7; Belsches, A.P. et.
al. (1997) Frontiers in Bioscience [Electronic Publication] 2:D501-D518; Zhan,
X. et. al (2001)
Chemical Reviews.101:2477-2496; Haskell, M.D. et. al. (2001) Chemical Reviews
101:2425-2440;
Protein kinase C-related kinase 2 (PRK2) is regulated by the G-protein Rho.
PRK2 is
found in regions of large actin turnover. Endogenous PRK2 kinase activity
increases with
keratinocyte differentiation and is associated with keratinocyte cell-cell
adhesion and Fyn kinase
activation. See Gross, C., et. al. (2001) FEBS Letters 496(2,3):101-104;
Calautti, E. et. al. (2002)
Journal of Cell Biology 156(1):137-148.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
31
3=Phosphoinositide dependent kinase 1(PDK1) phosphorylates and activates
members
of the AGC (cAMP-dependent, cGMP-dependent, and protein kinase C) kinase
family that are
activated downstream of phosphoinositide 3-kinase (P13K). P13K becomes active
through insulin
stimulation. PDKI activates a number of protein kinases and therefore can be
connected to the
regulation of a number of insulin specific events. PDK1 phosphorylation and
activation of PKC~ is
necessary for insulin-dependent GLUT4 translocation. Insulin-induced GLUT4
translocation is
physiologically related to the actin-based cytoskeleton. Disturbances in actin
filaments have been
linked to loss of insulin effect on glucose transport and decreased
translocation of GLUT4. See
Wick, K. L. et. al. (2001) Current Drug Targets: Immune, Endocrine and
Metabolic Disorders
1(3):209-221; Peterson, R. T. et. al. (1999) Current Biology 9(14):R521-R524;
Toker, A. et al.
(2000) Ce1l103(2):185-188; Leslie, N.R. (2001) Chem. Rev. 101: 2365-2380.
Fyn kinase (FYN) is a member of the Src family of tyrosine kinases. Fyn has
been
implicated in positive control of keratinocyte cell-cell adhesion. Adhesion
plays a crucial function in
establishment and maintenance of organized tissues. Fyn knockout and
transgenic mice
established that Fyn participates in T cell receptor (TCR) signaling.
Overexpression of the fyn(T)
transgene produces T cells with enhanced responsiveness to TCR signaling.
Conversely,
expression of an inactive kinase form is inhibitory. Fyn may be an appropriate
target for treatment
of autoimmune diseases. Fyn -/- mice are hypersensitive to alcohol which
suggests that Fyn might
be a target for the treatment of alcoholism. Alteration of Fyn levels may also
aid in the treatment of
skin disorders. Fyn has been implicated in the regulation of programmed cell
death, and Fyn-/-
mice exhibit reduced apoptosis. See also PRK2. See Calautti, E. et. al. (2002)
Journal of Cell
Biology 156(1):137-148; Resh, M. D. (1998) Journal of Biochemistry & Cell
Biology 30(11):1159-
1162.
Vascular endothelial growth factor receptor 2 (VEGFR-2) is also known as FLK-1
and as
KDR (kinase insert domain receptor). Other VEGF receptor tyrosine kinases
include VEGFR-
1(Flt-1) and VEGFR-3 (Flt-4). Angiogenesis or the development of new
vasculature is central to
the process by which solid tumors grow. The degree of vasculaturization has
been linked with
increased potential for metastasis. VEGFR-2, expressed only on endothelial
cells, binds the
potent angiogenic growth factor VEGF and mediates the subsequent signal
transduction.
Inhibition of VEGF-R2 activity has resulted in decreased angiogenesis and
tumor growth in in vivo
models, and inhibitors of VEGFR-1 are currently in clinical trials for the
treatment of cancer. See
Strawn et al.,(1996) Cancer Research 56:3540-3545; Millauer et al.,(1996)
Cancer Research
56:1615-1620; Sakamoto, K. M. (2001) IDrugs4(9):1061-1067; Ellis, L. M. et.al.
(2000) Oncologist
5(Suppl. 1):11-15; Mendel, D.B. et. al (2000) Anti-Cancer Drug Design 15:29-
41; Kumar, C.C.
et.al. (2001) Expert Opin. Emerging Drugs 6(2):303-315; Vajkoczy, P. et. al
(1999) Neoplasia
1(1):31-41.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
32
Fibroblast growth factor receptor (FGFR) binds the angiogenic growth factors
aFGF and
bFGF and mediates subsequent intracellular signal transduction. Growth factors
such as bFGF
may play a critical role in inducing angiogenesis in solid tumors that have
reached a certain size.
FGFR is expressed in a number of different cell types throughout the body and
may or may not
play important roles in normal physiological processes in adult humans.
Systemic administration
of a small-molecule inhibitor of FGFR has been reported to block bFGF-induced
angiogenesis in
mice. See Yoshiji et al., (1997) Cancer Research 57: 3924-3928; Mohammad et
al., (1998) EMBO
Journa117:5996-5904.
Phosphorylase kinase (PHK) activates glycogen phosphorylase. The primary
consequence of this activation is to release glucose 1-phosphate from
glycogen. Conversion to
glycogen is the major means by which glucose is stored in mammals.
Intracellular glycogen stores
are used to maintain blood-glucose homeostasis during fasting and are a source
of energy for
muscle contraction. In Vivo, PHK is phosphorylated by cAMP-dependent protein
kinase (PKA)
which increases the specific activity of PHK. Both Type 1 and 2 diabetics show
reduced glycogen
levels in liver and muscle cells. Glycogen levels are tightly regulated by
hormones and metabolic
signaling. Kinase inhibitors that could augment intracellular glycogen levels
may prove beneficial
in the treatment of diabetes. See Brushia, R.J. et.al. (1999) Frontiers in
Bioscience [Electronic
Publication] 4:D618-D641; Newgard, C.B. et. al. (2000) Diabetes 49:1967-1977;
Venien-Bryan, C.
et. al. (2002) Structure 10:33-41; Graves, D. et. al. (1999) Pharmacol. Ther.
82:(2-3)143-155;
Kilimann, M.W. (1997) Protein Dysfunction and Human Genetic Disease Chapter
4:57-75.
Weel kinase (Weel) along with Mik1 kinase has been shown to phosphorylate
Cdc2.
Phosphorylation of Cdc2 has been shown to prevent mitotic entry. Weel may play
an important
role the normal growth cycle of cells and may be implicated in cell-cycle
checkpoint control. Rhind,
N. et. al. (2001) Molecular and Cellular Biology 21(5):1499-1508.
Protein Kinase B (PKB) is also known as Akt. There are three very similar
isoforms known
as PKB q P. and y (or Akt 1, 2, and 3). Ultraviolet irradiation in the 290-
320nM range has been
associated with the harmful effects of sunlight. This irradiation causes
activation of PKB/Akt and
may be implicated in tumorigenesis. Over expressed PKB/Akt has been shown in
ovarian,
prostate, breast & pancreatic cancers. PKB/Akt is also involved in cell cycle
progression. PKB/Akt
promotes cell survival in a number of ways. It phosphorylates the proapoptotic
protein, BAD, so
that it is unable to bind and inactivate the antiapoptotic protein Bcl-xl.
PKB/Akt also serves to
inhibit apoptosis by inhibiting caspase 9 and forkhead transcription factor
and by activating IkB
kinase. See Barber, A.J. (2001) Journal of Biological Chemistry 276(35):32814-
32821; Medema,
R.H. et al. (2000) Nature 404:782-787; Muise-Helmericks, R.C. et. al (1998)
Journal of Biological
Chemistry 273(45): 29864-29872; Nomura, M. et. al. (2001) Journal of
Biological Chemistry
276(27): 2558-25567; Nicholson, K. M. et.al. (2002) Cellular Signaling 14(5):
381-395; Brazil,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
33
D.P. et. al. (2001) Trends in Biochemical Sciences 26(11): 657-664. Leslie,
N.R. (2001) Chem
Rev 101: 2365-2380.
Protein kinase C (PKC) classical isoforms are designated a,01, R2 and y, and
all are Ca2+
dependent. PKC isoforms are involved in signal transduction pathways linked to
a number of
physiological responses including membrane transport, cellular differentiation
and proliferation,
organization of cytoskeletal proteins and gene expression. Tumor promoting
phorbol esters
activate classical PKC isoforms and antisense oligonucleotides can block this
activation. PKC
isoforms are often over expressed in various cancers. PKC inhibitors have been
shown to reverse
p-glycoprotein-mediated multi-drug resistance and can increase intracellular
concentrations of
other anti-cancer agents. In myocytes, PKC isoforms have been implicated in
certain cardiac
pathologies. PKC-y is highly expressed in brain and spinal cord and is
primarily localized in
dendrites and neuron cell bodies. PKC-02 is involved in cell proliferation and
overexpression
increases sensitivity to cancer. PKCR inhibitors are a potential new therapy
for diabetic retinopathy
with clinical trials ongoing. See Magnelli, L. et. al. (1997) Journal of
Cancer Research and Clinical
Oncology 123(7):365-369; Clerk, A. et. al (2001) Circulation Research 89(10):
847-849; Carter, C.
(2000) Current Drug Targets 1 (2):163-183; Greenberg, S. et. al. (1998)
Alcohol 16(2);167-175;
Rosenzweig, T. et. al. (2002) Diabetes51(6):1921-1930; Deucher, A. et. al.
(2002) Journal of
Biological Chemistry 277(19):17032-17040; Frank, R.N. (2002) American Journal
of
Ophthalmology 133(5):693-698; Parekh, D. et. al. (2000) EMBO Journal 19(4):496-
503; Newton,
A.C. (2001) Chem. Rev.101:2353-2364.
The term "treating" or "treated", as used herein, refers to reversing,
alleviating, inhibiting the
progress of, or preventing the disorder or condition to which such term
applies, or one or more
symptoms of such disorder or condition. The term "treatment", as used herein,
refers to the act of
treating, as "treating" is defined immediately above.
"Connective tissue 'disorders" as used herein refers to disorders such as
degenerative
cartilage loss following traumatic joint injury, osteoarthritis, osteoporosis,
Paget's disease,
loosening of artificial joint implants, periodontal disease and gingivitis.
"Destruction of articular cartilage" as used herein refers to connective
tissue disorders
resulting in articular cartilage destruction, preferably joint injury,
reactive arthritis, acute
pyrophosphate arthritis (pseudogout), psoriatic arthritis, or juvenile
rheumatoid arthritis, more
preferably osteoarthritis.
"Inflammatory disorders" as used herein refers to disorders such as rheumatoid
arthritis,
ankylosing spondylitis, psoriatic arthritis, psoriasis, chondrocalcinosis,
gout, inflammatory bowel
disease, ulcerative colitis, Crohn's disease, fibromyalgia, and cachexia.
"Immunology/allergy disorders" as used herein refers to disorders such as
organ
transplant toxicity, allergic reactions, allergic contact hypersensitivity,
autoimmune disorders such
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
34
as those disorders associated with granulomatous inflammation/tissue
remodeling (such as
asthma), immunosuppression and sarcoid.
"Infectious diseases," including those mediated by viruses, bacteria, fungi or
mycobacterial infection, as used herein refers to disorders such as septic
arthritis, AIDS, fever;
Prion diseases, myasthenia gravis, Malaria, sepsis, hemodynamic shock and
septic shock.
"Respiratory diseases" as used herein refers to disorders such as chronic
obstructive
pulmonary disease (including emphysema), acute respiratory distress syndrome,
asthma,
hyperoxic alveolar injury and idiopathic pulmonary fibrosis and other fibrotic
lung diseases.
"Cardiovascular diseases" as used herein refers to disorders such as
atherosclerosis
including atherosclerotic plaque rupture; aortic aneurysm including abdominal
aortic aneurysm
and brain aortic aneurysm; congestive heart failure; myocardial and cerebral
infarction; stroke;
cerebral ischemia; coagulation and acute phase response; left ventricular
dilation; post ischemic
reperfusion injury; angiofibromas; hemangiomas; and restenosis.
"Eye diseases" as used herein refers to disorders such as aberrant
angiogenesis, ocular
angiogenesis, ocular inflammation, keratoconus, Sjogren's syndrome, myopia,
ocular tumors,
corneal graft rejection, corneal injury, neovascular glaucoma, corneal
ulceration, corneal scarring,
macular degeneration (including "Age Related Macular Degeneration (ARMD)
including both wet
and dry forms), proliferative vitreoretinopathy and retinopathy of
prematurity.
"Metabolic diseases" as used herein refers to disorders such as diabetes
(including non-
insulin dependent diabetes mellitus, diabetic retinopathy, insulin resistance,
diabetic ulceration).
"Central Nervous System" (CNS) disorders as used herein refers to disorders
such as
head trauma, spinal cord injury, Inflammatory diseases of the central nervous
system, neuro-
degenerative disorders (acute and chronic), Alzheimer's disease, demyelinating
diseases of the
nervous system, Huntington's disease, Parkinson's disease, peripheral
neuropathy, pain, cerebral
amyloid angiopathy, nootropic or cognition enhancement, amyotrophic lateral
sclerosis, multiple
sclerosis, migraine, depression and anorexia.
"Liver/Kidney diseases" as used herein refers to disorders such as nephrotic
syndromes
such as glomerulonephritis and glomerular disease of the kidney, proteinuria,
cirrhosis of the liver
and interstitial nephritis.
"Reproductive Health disorders" as used herein refers to disorders such as
endometriosis, contraception (male/female), dysmenorrhea, dysfunctional
uterine bleeding,
premature rupture of fetal membranes and abortifactant.
"Gastric disorders" as used herein refers to disorders such as colonic
anastomosis and
gastric ulcers.
"Skin disorders" as used herein refers to disorders such as skin aging,
pressure sores,
psoriasis, eczema, dermatitis, radiation damage, tissue ulceration, decubital
ulcers, epidermolysis
bullosa, abnormal wound healing (topical and oral formulations), burns and
scleritis.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
"Cancers" as used herein refers to disorders such as solid tumor cancer
including colon
cancer, breast cancer, lung cancer and prostrate cancer, tumor invasion, tumor
growth tumor
metastasis, cancers of the oral cavity and pharynx (lip, tongue, mouth,
pharynx), esophagus,
stomach, small intestine, large intestine, rectum, liver and biliary passages,
pancreas, larynx,
5 lung, bone, connective tissue, skin, cervix uteri, corpus endometrium,
ovary, testis, bladder,
kidney and other urinary tissues, eye brain and central nervous system,
thyroid and other
endocrine gland, Hodgkin's disease, non-Hodgkin's lymphomas, multiple myeloma
and
hematopoietic malignancies including leukemias and lymphomas including
lymphocytic,
granulocytic and monocytic.
10 The following reaction Schemes illustrate the preparation of the compounds
of the
present invention. Unless otherwise indicated each of A, the group -Y-Z-, X,
R', RZ, R3, and R4 in
the reaction Schemes and the discussion that follows are defined as above.
SCHEME 1
Lv
R4
IV\
R IV H
~ =
Lv
R ~~ NA
R III \R2
~ Vilsmeier-type reagent
Lv
CH2OH
Lv H
4 A
R 2(- VII 2 R4 ` \ ,
\~A
R ~/ N
R3 II '\R 2
== Lv
CHa-Lv2
R4 ~~ NA
R3 \R2
VI ~
H
N Y\Z
Lv
CHa-O-N-Pg
4 7 ~
R R
~A a~/
R N\R a R3 9 1 ~2
V
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
36
SCHEME 2
Lv
R4~ \ ~A
~/
R3 N\H
IV
Vilsmeier-type
reagent
Lv Lv H Lv H
CH2OH 4
R R ~ ~. ' R A
N ~ N s~ N\
R3 xi \Pg2 R3 IX \Pg2 R VIII H
Lv
CH2-Lv2
R4---
A
x N
R3 XII \Pgz
H H
\Y Y
Lv CHz-O-N-Pg X ~ ~
a R4 7,
R4~ N A R~ N A a ~ \
~ 3 9 R 9
R3 XIII \Pg2 R Pgz H
XIV Ia
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
37
SCHEME 3
H
N Y\
Z
R~4 \ ~ -H
R3 N
R
Id
H
N Y\Z
R -halogen
R 3 N
Ic
H
N Y~
x
R \ ~~
N
R3 R
lb
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
38
SCHEME 4
Lv
R CH3
~
R3 XVIII ~z
Lv
CH3
R~ 'CH
3
/No2
RXVII Lv
R Lv3
I` / b3
Rs; xvI z
Lv
R ~ Lv3
I~ / V3
R3~ x/ Hz
Lv
R 4 \ -H
R3/IVa H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
39
SCHEME 5
Lv
R Lv4
R 3/ x( 02
Lv
R\ Lv4
I'
3; H-COOCH3
R XIX
Lv
R ~C-R~
~H
R
IVb
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
SCHEME 6
Lv
4
R~
N
R8~ ~H
IVa
Lv H
0
R~1'~
N
R3~ H
Villa
H
~N-N
\\CH
R4~
R3 N
le
H
\N-N
\\CH
R~
N
R3
If \R 2
5
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
41
Scheme 1 refers to the preparation of compounds of the formula I. Referring to
Scheme
1, a compound of formula I, wherein the group -Y-Z- has the formula -N=CH- and
R2 is other than
hydrogen, can be prepared by reacting a compound of the formula II, wherein R2
is other than
hydrogen and wherein Lv is a leaving group, with hydrazine in a solvent.
Suitable Lv leaving
groups include methoxy, ethoxy, or benzyloxy, preferably methoxy. Suitable
solvents include
alcohols (such as ethanol), preferably methanol. The aforesaid reaction can be
conducted at a
temperature of about 25 C to about 90 C, preferably about 65 C. The aforesaid
reaction can be
conducted for a time period of about 5 minutes to about 24 hours, preferably
about 0.5 hour.
Compounds of formula II, wherein R2 is other than hydrogen and wherein Lv is
as
described above, can be prepared by reacting a compound of the formula III,
wherein R 2 is other
than hydrogen and wherein Lv is as described above, with a Vilsmeier-type
formylating reagent in
a solvent. Suitable Vilsmeier-type formylating reagents include POCI3 and DMF
or (CF3SO2)20
and DMF; preferably POCI3 and DMF. Suitable solvents include chloroform,
dioxane,
tetrahydrofuran, dimethylformamide, or methylene chloride; preferably
methylene chloride. The
aforesaid reaction can be conducted at a temperature of about 0 C to about 25
C, preferably
about 0 C during the reagent addition then allowing the reaction mixture to
warm to 23 C over
about 0.5 hour. The aforesaid reaction can be conducted for a time period of
about 5 minutes to
about 24 hours, preferably about 0.5 hour.
Compounds of formula III, wherein R2 is other than hydrogen and wherein Lv is
as
described above, can be prepared reacting a compound of formula IV, wherein Lv
is as described
above, with a compound of formula:
R2 -Lv'
wherein Lv' is a leaving group, such as halo, preferably bromo or chloro, in
the presence of a
suitable base in a polar solvent. Suitable bases include alkoxide bases (such
as sodium
methoxide, sodium ethoxide, or potassium tert-butoxide); hydride bases (such
as sodium
hydride); or carbonate bases (such as potassium carbonate or cesium
carbonate); preferably
potassium carbonate. Suitable polar solvents include tetrahydrofuran,
dimethylformamide,
dimethyl sulfoxide, or-alcohols (such as ethanol), preferably
dimethylformamide. The aforesaid
reaction can be conducted at a temperature of about 0 C to about 100 C,
preferably about 80
C. The aforesaid reaction can be conducted for a time period of about 0.5 hour
to about 72
hours, preferably about 24 hours.
A compound of formula I, wherein the group -Y-Z- is -O-CHZ- and wherein R 2 is
other than
hydrogen, can be prepared by reacting a compound of formula V, wherein R2 is
other than
hydrogen, wherein Pg is a protecting group, and Lv is as described above, with
a Pg deprotecting
agent. Suitable Pg includes phthaloyl, tert-butoxycarbonyl, benzyloxycarbonyl,
or ethoxycarbonyl;
preferably phthaloyl. Suitable Pg deprotecting agents include hydrazine,
trifluoroacetic acid,
hydrochloric acid, hydrogen chloride, hydrogen bromide in acetic acid, or
hydrogen gas and Pd
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
42
catalyst; preferably hydrazine. Acidic reactions can be neutralized after
deprotection with a
suitable base including tertiary amines (such as triethylamine or
diisopropylethylamine) or
carbonate bases (such as potassium carbonate); preferably triethylamine.
Suitable solvents
include dimethylformamide, methylene chloride, chloroform, or alcohol, (such
as methanol),
preferably methanol. The aforesaid reaction can be conducted at a temperature
of about 20 C to
about 130 C, preferably about 65 C. The aforesaid reaction can be conducted
for a time period
of about 0.5 hours to about 48 hours, preferably about 2 hours.
A compound of formula V, wherein R 2 is other than hydrogen, wherein Pg is a
protecting
group, and Lv is as described above, can be prepared by reacting a compound of
formula VI,
wherein R2 is other than hydrogen, Lv2 is a leaving group and Lv is as
described above, with a
compound of formula
Pg-N-OH
in the presence of a base in a solvent. Suitable Lv2 leaving groups include
halo, toluenesulfonyl,
methanesulfonyl, trifluoromethanesulfonyl, or Mitsunobu-type reaction adducts.
Suitable
compounds of formula Pg-N-OH include N-hydroxyphthalimide, tert-butyl N-
hydroxylcarbamate,
N-hydroxyurethane, or benzyl N-hydroxycarbamate. Suitable bases include sodium
hydride, 1,8-
diazabicyclo[5.4.0]undec-7-ene, pyridine, tertiary amines (such as
diisopropylethylamine or
triethylamine) or carbonate bases (such as sodium carbonate); preferably
sodium carbonate.
Suitable solvents include dimethylformamide, dimethylsulfoxide,
tetrahydrofuran, methylene
chloride, chloroform, or alcohol (such as methanol); preferably
dimethylsulfoxide. The aforesaid
reaction can be conducted at a temperature of about -25 C to about 80 C,
preferably about 23
C. The aforesaid reaction can be conducted for a time period of about 5
minutes to about 48
hours, preferably about 20 hours.
A compound of formula VI, wherein R2 is other than hydrogen and Lv2 and Lv are
as
described above, can be prepared by reacting a compound of formula VII,
wherein R 2 is other
than hydrogen and Lv is as described above, with an Lv2 producing agent, in a
solvent. Suitable
Lv2 producing agents include (C6H5)3P and CCI4; (C6H5)3P and PBr3; para-
CH3C6H4SO2CI;
CH3SO2CI; (CF3SO2)20; or Mitsunobu-type reagents (such as diethyl
azodicarboxylate and
(C6H5)3P); preferably (C6H5)3P and CCI4. Suitable solvents include methylene
chloride, chloroform,
tetrahydrofuran, carbon tetrachloride, benzene, or toluene; preferably
methylene chloride. The
aforesaid reaction can be conducted at a temperature of about -25 C to about
80 C, preferably
about 23 C. The aforesaid reaction can be conducted for a time period of
about 5 minutes to
about 24 hours, preferably about 20 hours.
A compound of formula VII, wherein R2 is other than hydrogen and Lv is as
described
above, can be prepared by reacting a compound of formula II, wherein R2 is
other than hydrogen
and Lv is as described above, with a reducing agent in a solvent. Suitable
reducing agents include
sodium borohydride, lithium borohydride, zinc borohydride, diborane, borane
complexes,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
43
triacetoxyborohydride, sodium cyanoborohydride, or lithium cyanoborohydride;
preferably sodium
borohydride. Suitable solvents include alcohol (such as methanol),
tetrahydrofuran, a mixture of
methanol and anhydrous HCI, or a mixture of methanol and acetic acid;
preferably methanol. The
aforesaid reaction can be conducted at a temperature of about 0 C to about 50
C, preferably
about 23 C. The aforesaid reaction can be conducted for a time period of about
5 minutes to
about 24 hours, preferably about 15 minutes.
Scheme 2 refers to the preparation of compounds of the formula Ia, which is a
compound
of formula I wherein R 2 is hydrogen. Referring to Scheme 2, a compound of
formula Ia, wherein
the group -Y-Z- has the formula -N=CH-, can be prepared by reacting a compound
of the formula
VIII, wherein Lv is a leaving group, as described above, with hydrazine in a
solvent. The reaction
condition is as described above in the description for Scheme 1 for the
preparation of a compound
of formula I from a compound of formula II.
Compounds of formula VIII, wherein Lv is as described above, can be prepared
by
reacting a compound of the formula IV, wherein Lv is as described above, with
a Vilsmeier
reagent in a solvent. The reaction condition is as described above in the
description for Scheme 1
for the preparation of compounds of formula II from a compound of formula Ill.
A compound of formula Ia, wherein the group -Y-Z- is -O-CH2-, can be prepared
by
reacting a compound of formula XIV, wherein pg2 is a protecting group, with a
pg2 deprotecting
agent in a solvent. Suitable pg2 protecting groups include tert-
butoxycarbonyl, benzyloxycarbonyl,
or 2-(trimethylsilyl)ethoxymethyl; preferably tert-butoxycarbonyl. Suitable
pg2 deprotecting agents
include trifluoroacetic acid, hydrochloric acid, hydrogen chloride, hydrogen
bromide in acetic acid,
hydrogen gas and Pd catalyst, or tetrabutylammonium fluoride; preferably
trifluoroacetic acid.
Suitable solvents include methylene chloride, chloroform, dioxane,
dimethylformamide, or alcohol
(such as methanol); preferably methylene chloride. The aforesaid reaction can
be conducted at a
temperature of about 20 C to about 80 C, preferably about 23 C. The
aforesaid reaction can be
conducted for a time period of about 15 minutes to about 48 hours, preferably
about 2 hours.
A compound 'of formula XIV, wherein pg2 is as described above, can be prepared
by
reacting a compound of formula XIII, wherein Pg is a protecting group as
described in the
compound of formula V of Scheme 1, and pg2 and Lv are as described above, with
a Pg
deprotecting agent. The reaction condition is as described above in the
description for Scheme I
for the preparation of a compound of formula I from a compound of formula V.
A compound of formula XIII, wherein PgZ, Lv and Lv2 are as described above,
can be
prepared by reacting a compound of formula XII, wherein pg2 , Lv and Lv2 are
as described above,
with a compound of formula
Pg-N-OH
CA 02512683 2008-05-27
50054-64
44
_ wherein Pg is as described above, in the presence of a base in a solvent,
The reaction condition
is as described above in the description for Scheme 1 for the preparation of a
compound of
formula V from a compound of formula VI.
A compound of formula XII, wherein Pg2, Lv2 and Lv are as described above, can
be
prepared by reacting a compound of formula XI, wherein pg2 and Lv are as
described above, with
an Lv2 producing agent, in a solvent. The reaction condition is as described
above in the
description for Scheme 1 for the preparation of a compound of formula VI from
a compound of
formula VII.
A compound of formula XI, wherein pg2 and Lv are as described above, can be
prepared
by reacting a compound of formula IX, wherein pg2 and Lv are as described
above, with a
reducing agent in a solvent The reaction condition is as described above in
the description for
Scheme 1 for the preparation of a compound of formula VII from a compound of
formula II.
A compound of formula IX, wherein pg2 and Lv are as described above, can be
prepared
by reacting a compound of formula VIII, wherein Lv is as described above, with
a pg2 protecting
agent in the presence of a suitable base in a solvent Suitable pg2 protecting
agents include
di-tert-butyl dicarbonate, benzyl chloroformate, or 2-
(trimethylsilyl)ethoxymethyl chloride;
preferably di-tert-butyl dicarbonate. Suitable bases include hydride bases
(such as sodium
hydride, lithium hydride, or potassium hydride); preferably sodium hydride.
Suitable solvents
include tetrahydrofuran or dimethylformamide; preferably tetrahydrofuran. The
aforesaid reaction
can be conducted at a temperature of about 0 C to about 60 C, preferably
about 23 C. The
aforesaid reaction can be conducted for a time period of about 15 minutes to
about 24 hours,
preferably about 1 hour.
Scheme 3 refers to the preparation of a compound of the formula lb, which is a
compound of the formula I wherein A is =CR'-. Referring to Scheme 3, a
compound of formula Ib,
can be prepared by reacting a compound of the formula Ic, wherein halogen is
selected from
bromo or iodo, with a coupling reagent of the formula:
R'-M
wherein M is a H or metal, in the presence of palladium and copper catalysts
in a solvent Suitable
metals include boron and tin, preferably boron. Suitable coupling reagents
include Stille coupling
reagent (see Chamoin, S., Houldsworth, S., Snieckus, V. Tetrahedron Lett.1998,
39, 4175-4178),
Suzuki coupling reagent (see Littke, A.F., Chaoyang, D., Fu, G.C. J.Am. Chem.
Soc. 2000, 122,
4020-4028), or Sonogashira coupling reagent (see Sonogashira, K., Tohda, Y.,
Hagihara, N.
Tetrahedron Lett. 1975, 16, 44467-4470); preferably Suzuki coupling reagent or
Stille
coupling reagent Suitable palladium and copper catalysts include Pd(C6H5)3P)4,
Pd(dba)2,
Pd(P(CsHS)3)CIZ and Cul. Suitable solvents include dimethylformamide or
tetrahydrofuran;
preferably dimethylformamide. The aforesaid reaction can be conducted at a
temperature of about
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
22 C to about 110 C, preferably about 90 C. The aforesaid reaction can be
conducted for a
time period of about 5 minutes to about 48 hours, preferably about 2 hours.
Some compounds of the formula lb, such as those wherein R' is a substituted
alkyne (for
example methylaminopropynyl) may need additional steps requiring the use of a
protecting group
5 (for example tert-butoxycarbonyl). These protection groups and their removal
processes are well
known in the art and can be found in Greene and Wuts, "Protecting Groups in
Organic Synthesis,"
(John Wiley & Sons, 2"d Ed). Furthermore, compounds of the Ib, such as those
wherein R1, R2 or
R3 is a substituted alkyl, alkynyl, aromatic or vinyl are subjected to
additional standard chemical
transformations (for example catalytic hydrogenation, OsO4/NMMO/NaIO4
oxidative cleavage,
10 mesylation/displacement, reductions and reductive amination). These
processes are also well
known in the art and can be found in Larock, R.C., "Comprehensive Organic
Transformations"
(Wiley-VCH, 2"d Ed.).
A compound of formula Ib, wherein A is =CR1- and R' is -(C=O)-O-(Cl-C6)alkyl,
can be
prepared by reacting a compound of the formula Ic, wherein halogen is selected
from bromo or
15 iodo, with carbon monoxide in the presence of a palladium catalyst, a base,
and a compound of
the formula H-O-(Cl-C6)alkyl (depending on the -(Cl-C6)alkyl part of the
desired R) in a solvent.
Suitable palladium catalysts include Pd(dppf)CI2. Suitable bases include
tertiary amine bases such
as triethylamine. Suitable compounds of the formula H-O-(Cl-C6)alkyl include
methanol, ethanol,
or propanol. Suitable solvents include dimethylformamide or tetrahydrofuran;
preferably
20 dimethylformamide. The aforesaid reaction can be conducted at a temperature
of about 22 C to
about 110 C, preferably about 85 C. The aforesaid reaction can be conducted
for a time period
of about 30 minutes to about 48 hours, preferably about 16 hours.
A compound of formula Ib, wherein A is =CR'- and R' is -(C=O)-NH-(CI-C6)alkyl
or
-(C=O)-NH-(Cl-C6)alkyl-OH or -(C=O)-NH-(CI-C6)alkyl-N(CH3)2 or -(C=O)-1-(4-N-
25 methylpiperazine) can be prepared by reacting a compound of the formula Ic,
wherein halogen is
selected from bromo or iodo, with carbon monoxide in the presence of a
palladium catalyst, a
base, and a compound of the formula HzN-(CI-C6)alkyl or H2N-(Cl-C6)alkyl-OH or
HZN-(Cl-
C6)alkyl-N(CH3)Z or N-methylpiperazine (depending on the -(Cl-Cs)alkyl part of
the desired R) in a
solvent. Suitable palladium catalysts include Pd(dppf)CIz. Suitable bases
include tertiary amine
30 bases such as triethylamine. Suitable compounds of the formulas H2N-(CI-
C6)alkyl or HzN-(Cl-
C6)alkyl-OH or H2N-(Cl-C6)alkyl-N(CH3)Z or N-methylpiperazine include 2-
aminoethanol, N,N-
dimethylethylenediamine, methylamine N-methylpiperazine Suitable solvents
include
dimethylformamide or toluene; preferably dimethylformamide. The aforesaid
reaction can be
conducted at a temperature of about 22 C to about 110 C, preferably about 85
C. The aforesaid
35 reaction can be conducted for a time period of about 30 minutes to about 48
hours, preferably
about 16 hours.
CA 02512683 2008-05-27
50054-64
46
A compound of formula Ic, wherein halogen is bromo or iodo, can be prepared by
reacting a compound of formula Id with a suitable halogenating agent in a
solvent. Suitable
halogenating agents include N-bromosuccinimide or N-iodosuccinimide;
preferably N-
bromosuccinimide. Suitable solvents include tetrahydrofuran or
dimethylformamide, preferably
dimethylformamide. The aforesaid reaction can be conducted at a temperature of
about 0 C to
about 75 C, preferably about 22 C. The aforesaid reaction can be conducted
for a time period of
about 5 minutes to about 24 hours, preferably about 1 hour.
A compound of formula Ic, wherein halogen is chloro, can be prepared by
reacting a
compound of formula Id with a suitable chlorinating agent in a solvent.
Suitable chlorinating
agents include N-chlorosuccinimide Suitable solvents include tetrahydrofuran
or
dimethylformamide; preferably dimethylformamide. The aforesaid reaction can be
conducted at a
temperature of about 0 C to about 75 C, preferably about 45 C. The aforesaid
reaction can be
conducted for a time period of about 5 minutes to about 24 hours, preferably
about 1 hour.
Scheme 4 refers to the preparation of a compound of the formula lVa, which is
a
compound of formula IV of Scheme 1, wherein A is =CH-. Referring to Scheme 4,
a compound of
formula IVa, wherein Lv is as described above, can be prepared by reacting a
compound of
formula XV, wherein Lv is as described above and each of Lv3 is a leaving
group, with a suitable
acid in a polar protic solvent (see Coe, J.W., Vetelino, M.G., Bradlee, M.J.
Tetrahedron Lett. 1996,
37, 6045-6047'). Suitable Lv3 leaving groups include methoxy or
ethoxy, preferably methoxy. Suitable acids include HCI, H.,S04i para
toluenasulfonic acid,
camphorsulfonic acid, or Lewis acids; preferably HCI. Suitable polar protic
solvents include
alcohols (such as methanol or ethanol), preferably methanol. The aforesaid
reaction can be
conducted at a temperature of about 0 C to about 100 C, preferably about 65
C. The aforesaid
reaction can be conducted for a time period of about 5 minutes to about 24
hours, preferably
about 1 hour.
A compound of formula XV, wherein Lv and Lv3 are as described above, can be
prepared
by reacting a compound of formula XVI, wherein Lv and Lv3 are as described
above, with a
reducing agent in a polar solvent. Suitable reducing agents include catalytic
transfer reagents
such as hydrazine hydrate, ammonium formate, ammonium chloride, cyclohexene,
or hydrogen
gas in the presence of catalysts [such as Pd on carbon (see Coe, J.W.,
Vetelino, M.G., Bradlee,
M.J. Tetrahedron Lett. 1996, 37, 6045-6047 ), Ru, Rh, Raney
nickel, or Pt]; HCI or acetic acid in the presence of In, Fe, Sn, or Zn;
HCUSnCIZ;SnC1z.2H20;
Bu3SnH/AIBN; or Fe3(CO)12; preferably SnC12.21-120 or hydrogen gas in the
presence of Pd on
carbon or Raney nickel. Suitable polar solvents include alcohols (such as
methanol or ethanol),
preferably methanol. The aforesaid reaction can be conducted at a temperature
of about 0 C to
about 100 C, preferably about 23 C. Where hydrogen gas is used the reaction
pressure can be
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
47
1 to 4 atm, preferably I atm. The aforesaid reaction can be conducted for a
time period of about
2 hours to about 48 hours, preferably about 24 hours.
A compound of formula XVI, wherein Lv and Lv3 are as described above, can be
prepared
by reacting a compound of formula XVII, wherein Lv is as described above, with
a suitable acid in
an anhydrous polar protic solvent of formula Lv3-H. Suitable acids include
HCI, H2SO4, or para
toluenesulfonic acid, preferably HCI. Alternatively, HCI can be generated in
situ by using an HCI
generating agent such as TMS-Cl or acetyl chloride, preferably TMS-Cl, in an
anhydrous polar
protic solvent such as methanol. Suitable anhydrous polar protic solvents of
formula Lv3-H include
anhydrous alcohols (such as methanol or ethanol), preferably anhydrous
methanol. The aforesaid
reaction can be conducted at a temperature of about 23 C to about 78 C,
preferably about 65
C. The aforesaid reaction can be conducted for a time period of about 30
minutes to about 48
hours, preferably about 24 hours.
A compound of formula XVII, wherein Lv is as described above, can be prepared
by
reacting a compound of formula XVIII, wherein Lv is as described above, with a
compound of
formula
H3C i v3
\N C-H
H3C~ LV3
wherein each of Lv3 is as described above, in a polar solvent. Suitable
compounds of formula
(CH3)2-N-CH-(Lv3)2 include dimethylformamide-dimethylacetal. Suitable polar
solvents include
dimethylformamide, toluene, or alcohol (such as ethanol), preferably
dimethylformamide. The
aforesaid reaction can be conducted at a temperature of about 22 C to about
150 C, preferably
about 110 C. The aforesaid reaction can be conducted for a time period of
about 15 minutes to
about 24 hours, preferably about 6 hours.
Compounds of formula (CH3)2-N-CH-(Lv3)2 are commercially available.
Compounds of the formula XVII, wherein Lv is as described above, are
commercially
available or alternatively can be made by methods well known to those skilled
in the art.
Scheme 5 refers to preparation of a-compound of the formula IVb, which is a
compound
of formula IV of Scheme 1, wherein A is =CR'-. Referring to Scheme 5, a
compound of formula IVb
can be prepared by reacting a compound of the formula XIX, wherein Lv is as
described above
and Lv4 is a leaving group, with a suitable substituted alkyne of formula
M'-C=C-Rl
wherein Ml is H or metal (such as Sn or B), preferably H or Sn, in the
presence of a metal catalyst
in a polar solvent. Suitable Lv4 leaving groups include halo preferably bromo
or iodo. Suitable
metal catalysts include palladium or copper catalysts (See Fagnola, M.C. et
al. Tetrahedron
CA 02512683 2008-12-15
50054-64
48
Lett.1997, 38, 2307-2310). Suitable polar solvents include
dimethylformamide, dioxane, dimethylsulfoxide, or mixtures thereof, preferably
a mixture of
dimethylformamide and dioxane. The aforesaid reaction can be conducted at a
temperature of
about 22 C to about 120 C, preferably about 90 C. The aforesaid reaction
can be conducted for
a time period of about 5 minutes to about 24 hours, preferably about 8 hours.
Compounds of formula M'-C=C-R' are commercially available or can be made by
methods well known to those skilled in the art.
A compound of formula XIX, wherein Lv and Lv4 are as described above, can be
prepared
by reacting a compound of formula XX, wherein Lv and Lv4 are as described
above, with a
reducing agent in the presence of (CH3CO)20 in a polar solvent. Suitable
reducing agents include
hydrogen gas in the presence of catalysts such as Pd/C (see Coe, J.W.,
Vetelino, M.G., Bradlee,
M.J. Tetrahedron Leif.1996, 37, 6045-6047), Rd, Raney nickel,
or Pt; acetic acid in the presence of In, Fe, or Zn; SnCl2; or Fe3(CO)12;
preferably hydrogen gas in
the presence of Pd/C; or acetic acid in the presence of Fe. Suitable polar
solvents include
dimethylformamide, methanol, ethanol, or acetic acid; preferably methanol or
acetic acid. The
aforesaid reaction can be conducted at a temperature of about 20 C to about
100 C, preferably
about 22 C. Where hydrogen gas is used the reaction pressure can be 1 to 4
atm, preferably I
atm. The aforesaid reaction can be conducted for a time period of about 2
hours to about 48
hours, preferably about 24 hours.
Compounds of the formula XX are commercially available or can be made by
methods
well known to those skilled in the art.
Scheme 6 refers to preparation of a compound of the formula If, which is a
compound of
formula I of Scheme 1, wherein the group -Y-Z- has the formula -N=CH-, A is =N-
, and R2 is other
than hydrogen. Referring to Scheme 6, a compound of formula If can be prepared
by reacting a
compound of the formula le, which is a compound of formula I of Scheme 1,
wherein the group
-Y-Z- has the formula -N=CH-, A is =N-, and R 2 is hydrogen, with a compound
of formula
RZ-Lv'
wherein Lv' is a leaving group, such as halo, preferably bromo or chioro, in
the presence of a
suitable base in a polar solvent. The reaction condition is as described above
in the description for
Scheme 1 for the preparation of compounds of formula III from a compound of
formula IV.
Compounds of formula le can be prepared by reacting a compound of the formula
Vllla,
which is a compound of formula VIII of Scheme 2, wherein A is =N- and Lv is as
described above,
with hydrazine in a solvent. The reaction condition is as described above in
the description for
Scheme 1 for the preparation of a compound of formula I from a compound of
formuia li.
Compounds of formula Villa can be prepared by reacting a compound of the
formula IVa,
which is a product of Scheme 4, with a nitrous acid.producing agent in the
presence of an acid in
a solvent. Suitable nitrous acid producing agents include NaNO2, KNO2, isoamyl
nitrite, or tert-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
49
butyl nitrite; preferably NaNOz. Suitable acids include acetic acid or aqueous
HCI; preferably
acetic acid. Suitable solvents include acetic acid, benzene,
dimethyiformamide, toluene, or
alcohols (such as methanol), preferably acetic acid. The aforesaid reaction
can be conducted at a
temperature of about 0 C to about 30 C, preferably about 0 C warming to 23
C. The aforesaid
reaction can be conducted for a time period of about 5 minutes to about 24
hours, preferably
about 1 hour.
Within the invention it is understood that a compound of Formula I may exhibit
the
phenomenon of tautomerism and that the formula drawings within this
specification represent only
one of the possible tautomeric forms. It is to be understood that the
invention encompasses any
tautomeric form which modulates and/or inhibits kinase activity and is not to
be limited merely to
any one tautomeric form utilized within the formula drawings.
Some of the inventive compounds may exist as single stereoisomers (i.e.,
essentially free
of other stereoisomers), racemates, and/or mixtures of enantiomers and/or
diastereomers. All
such single stereoisomers, racemates and mixtures thereof are intended to be
within the scope of
the present invention. Preferably, the inventive compounds that are optically
active are used in
optically pure form.
As generally understood by those skilled in the art, an optically pure
compound having
one chiral center (i.e., one asymmetric carbon atom) is one that consists
essentially of one of the
two possible enantiomers (i.e., is enantiomerically pure), and an optically
pure compound having
more than one chiral center is one that is both diastereomerically pure and
enantiomerically pure.
Preferably, the compounds of the present invention are used in a form that is
at least 90%
optically pure, that is, a form that contains at least 90% of a single isomer
(80% enantiomeric
excess ("e.e.") or diastereomeric excess ("d.e.")), more preferably at least
95% (90% e.e. or d.e.),
even more preferably at least 97.5% (95% e.e. or d.e.), and most preferably at
least 99% (98%
e.e. or d.e.).
Additionally, Formula I is intended to cover solvated as well as unsolvated
forms of the
identified structures. For example, Formula I include compounds of the
indicated structure in both
hydrated and non-hydrated forms. Other examples of solvates include the
structures in
combination with isopropanol, ethanol, methanol, DMSO, ethyl acetate, acetic
acid, or
ethanolamine.
If the inventive compound is a base, the desired pharmaceutically acceptable
salt may be
prepared by any suitable method available in the art, for example, treatment
of the free base with
an inorganic acid, such as hydrochloric acid, hydrobromic acid, sulfuric acid,
nitric acid,
phosphoric acid and the like, or with an organic acid, such as acetic acid,
maleic acid, succinic
acid, mandelic acid, fumaric acid, malonic acid, pyrovic acid, oxalic acid,
glycolic acid, salicylic
acid, a pyranosidyl acid, such as glucuronic acid or galacturonic acid, an
alpha-hydroxy acid, such
as citric acid or tartaric acid, an amino acid, such as aspartic acid or
glutamic acid, an aromatic
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
acid, such as benzoic acid or cinnamic acid, a sulfonic acid, such as p-
toluenesulfonic acid or
ethanesulfonic acid, or the like.
If the inventive compound is an acid, the desired pharmaceutically acceptable
salt may be
prepared by any suitable method, for example, treatment of the free acid with
an inorganic or
5 organic base, such as an amine (primary, secondary or tertiary), an alkali
metal hydroxide or
alkaline earth metal hydroxide, or the like. Illustrative examples of suitable
salts include organic
salts derived from amino acids, such as glycine and arginine, ammonia,
primary, secondary, and
tertiary amines, and cyclic amines, such as piperidine, morpholine and
piperazine, and inorganic
salts derived from sodium, calcium, potassium, magnesium, manganese, iron,
copper, zinc,
10 aluminum and lithium.
In the case of agents that are solids, it is understood by those skilled in
the art that the
inventive compounds and salts may exist in different crystal or polymorphic
forms, all of which are
intended to be within the scope of the present invention and specified
formulas.
The pharmaceutically acceptable salts of the compound of Formula I can also
exist as
15 various solvates, such as with water, methanol, ethanol, dimethylformamide,
ethyl acetate and the
like. Mixtures of such solvates can also be prepared. The source of such
solvate can be from the
solvent of crystallization, inherent in the solvent of preparation or
crystallization, or adventitious to
such solvent.
The compound of Formula I may be used in combination with conventional
antineoplasm
20 therapies to treat mammals, especially humans, with neoplasia. The
procedures for conventional
anti-neoplasm therapies, including chemotherapies using anti-neoplastic agents
and therapeutic
radiation, are readily available, and routinely practiced in the art, e.g.,
see Harrison's PRINCIPLES
OF INTERNAL MEDICINE 11th edition, McGraw-Hill Book Company.
Neoplasia is characterized by abnormal growth of cells which often results in
the invasion
25 of normal tissues, e. g., primary tumors or the spread to distant organs,
e. g., metastasis. The
treatment of any neoplasia by conventional non-surgical anti-neoplasm
therapies may be
enhanced by the present invention. Such neoplastic growth includes but not
limited to primary
tumors, primary tumors, that are incompletely removed by surgical techniques,
primary tumors
which have been adequately treated but which are at high risk to develop a
metastatic disease
30 subsequently, and an established metastatic disease.
Specifically, the CHK-1 inhibitor of Formula I above may enhance the anti-
neoplasm
effects of an anti-neoplastic agent. The wide variety of available anti-
neoplastic agents are
contemplated for combination therapy in accordance with present invention. In
a preferred
embodiment, anti-neoplastic agents that assert their cytotoxic effects by
activating programmed
35 cell death or apoptosis may be used in combination with the described CHK-1
inhibitor. The anti-
neoplastic agents contemplated in accordance with the present invention
include, but are not,
limited to alkylating agents, including busulfan, chlorambucil,
cyclophosphamide, iphosphamide,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
51
melphalan, nitrogen mustard, streptozocin, thiotepa, uracil nitrogen mustard,
triethylenemelamine, temozolomide, and
SARCnu; antibiotics and plant alkaloids including actinomycin-D, bleomycin,
cryptophycins,
daunorubicin, doxorubicin, idarubicin, irinotecan, L-asparaginase, mitomycin-
C, mitramycin,
navelbine, paclitaxel, docetaxel, topotecan, vinblastine, vincristine, VM-26,
and VP-16-213;
hormones and steroids including 5a-reductase inhibitor, aminoglutethimide,
anastrozole,
bicalutamide, chlorotrianisene, DES, dromostanolone, estramustine, ethinyl
estradiol, flutamide,
fluoxymesterone, goserelin, hydroxyprogesterone, letrozole, leuprolide,
medroxyprogesterone
acetate, megestrol acetate, methyl prednisolone, methyltestosterone, mitotane,
nilutamide,
prednisolone,
SERM3, tamoxifen, testolactone, testosterone, triamicnolone, and zoladex;
synthetics including
all-trans retinoic acid, BCNU (carmustine), CBDCA carboplatin (paraplatin),
CCNU (lomustine), cis-diaminedichloroplatinum (cisplatin), dacarbazine,
gliadel,
hexamethylmelamine, hydroxyurea, levamisole, mitoxantrone, o, p'-DDD
(lysodren, mitotane),
oxaliplatin, porfimer sodium, procarbazine, GleeVec; antimetabolites including
chlorodeoxyadenosine, cytosine arabinoside, 2'-deoxycoformycin, fludarabine
phosphate, 5-
fluorouracil, 5-FUDR, gemcitabine, camptothecin, 6-mercaptopurine,
methotrexate, MTA, and
thioguanine; and biologics including alpha interferon, BCG, G-CSF, GM-CSF,
interieukin-2,
herceptin; and the like.
In a preferred embodiment of the invention, the anti-neoplastic agent is
selected from the
group consisting of alkylating agents, antibiotics and plant alkaloids,
hormones and steroids,
synthetic agents having anti-neoplastic activity, antimetabolites and
biological molecules having
anti-neoplastic activity.
In a preferred embodiment of the invention the antineoplastic agent is
selected from the
group consisting of Ara-c, VP-16, cis-platin, adriamycin, 2-chloro-2-
deoxyadenosine, 9-R-D-
arabinosyl-2-fluoroadenine, carboplatin, gemcitabine, camptothecin,
paclitaxel, BCNU, 5-
fluorouracil, irinotecan, and doxorubicin; more preferably gemcitabine.
All the neoplastic conditions treatable with such anti-neoplastic agents may
be treated in
accordance with the present invention by using a combination of the compound
of Formula I with
one or more anti-neoplastic agents. The anti-neoplastic agents assert their
cytotoxicity or anti-
neoplasm effects in a variety of specific neoplastic conditions. For example,
Ara-c is normally
used for treatment of childhood-null acute lymphoid leukemia (ALL), thymic
ALL, B-cell ALL, acute
myeloid leukemia, acute granulocytic leukemia and its variants, non-Hodgkins
lymphoma,
myelomonocytoid leukemia, acute megakaryocytoid leukemia and Burkitt's
lymphoma, Adult-B-
ALL, acute myeloid leukemia, chronic lymphoid leukemia, chronic myeloid
leukemia, and T cell
leukemia. VP-16 is normally used for treatment of testicular carcinoma, small -
and large non-small
cell lung carcinoma, Hodgkin's lymphoma, non-Hodgkin's lymphoma,
choriocarcinoma, Ewing's
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
52
sarcoma, and acute granulocytic leukemia. Cis-platin can be employed for
treatment of testicular
carcinoma, germ cell tumors, ovarian carcinomas, prostate cancer, lung cancer,
sarcomas,
cervical cancer, endometrial cancer, gastric cancer, breast cancer, and cancer
of the head and
neck. 2-Chloro-2-deoxyadenosine and 9-R-D-arabinosyl-2-fluoroadenine can be
used to treat
chronic lymphoid leukemia, lymphomas and hairy cell leukemia. Doxorubicin can
be used to treat
acute granulocytic leukemia and its variants, ALL, breast cancer, bladder
cancer, ovarian cancer,
thyroid cancer, lung cancer, Hodgkin's lymphoma, non-Hodgkin's lymphoma,
sarcomas, gastric
carcinoma, prostate cancer, endometrial cancer, Wilm's tumor and
neuroblastoma.
Clinical effects of anti-neoplastic agents in all neoplastic conditions
treatable with
antineoplastic agents including the ones discussed above may be potentiated by
use of a
combination therapy with the identified CHK-1 inhibitor in accordance with the
present invention.
The CHK-1 inhibitor identified in the present invention may also enhance the
antineoplasm effects of radiation therapy. Usually, radiation can be used to
treat the site of a solid
tumor directly or administered by brachytherapy implants. The various types of
therapeutic
radiation which are contemplated for combination therapy in accordance with
the present
invention may be those used in the treatment of cancer which include, but are
not limited to X-
rays, gamma radiation, high energy electrons and High LET (Linear Energy
Transfer) radiation
such as protons, neutrons, and alpha particles. The ionizing radiation may be
employed by
techniques well known to those skilled in the art. For example, X-rays and
gamma rays are
applied by external and/or interstitial means from linear accelerators or
radioactive sources. High-
energy electrons may be produced by linear accelerators. High LET radiation is
also applied from
radioactive sources implanted interstitially.
The specific dosage amount of a compound of the Formula I, a pharmaceutically
acceptable salt, solvate, or prodrug thereof being administered to obtain
therapeutic or inhibitory
effects may be determined in a manner known in the art according to the
particular circumstances
surrounding the case, including, e.g., the specific agent being administered,
the route of
administration, the condition being treated, and the subject or host being
treated. An exemplary
total daily dose of a compound of the Formula I, which may be administered in
single or multiple
doses, contains a dosage level of from about 0.01 mg/kg body weight to about
50 mg/kg body
weight.
The compounds of the Formula I of the invention may be administered by any of
a variety
of suitable routes, such as orally, rectally, transdermally, subcutaneously,
intravenously,
intramuscularly, or intranasally. The compounds of the Formula I are
preferably formulated into
compositions suitable for the desired routes before being administered.
A pharmaceutical composition or preparation according to the invention
comprises an
effective amount of a compound of the Formula I, optionally one or more other
active agents, and
a pharmaceutically acceptable carrier, such as a diluent or excipient for the
agent; when the
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
53
carrier serves as a diluent, it may be a solid, semi-solid, or liquid material
acting as a vehicle,
excipient, or medium for the active ingredient(s). Compositions according to
the invention may be
made by admixing the active ingredient(s) with a carrier, or diluting it with
a carrier, or enclosing or
encapsulating it within a carrier, which may be in the form of a capsule,
sachet, paper container,
or the like. Exemplary ingredients, in addition to one or more compounds of
the Formula I and
any other active ingredients, include Avicel (microcrystalline cellulose),
starch, lactose, calcium
sulfate dihydrate, terra alba, sucrose, talc, gelatin, agar, pectin, acacia,
magnesium stearate,
stearic acid, peanut oil, olive oil, glyceryl monostearate, Tween 80
(polysorbate 80), 1,3-
butanediol, cocoa butter, beeswax, polyethylene glycol, propylene glycol,
sorbitan monostearate,
polysorbate 60, 2-octyidodecanol, benzyl alcohol, glycine, sorbic acid,
potassium sorbate,
disodium hydrogen phosphate, sodium chloride, and water.
The compositions may be prepared in any of a variety of forms suitable for the
desired
mode of administration. For example, pharmaceutical compositions may be
prepared in the form
of tablets, pills, powders, lozenges, sachets, cachets, elixirs, suspensions,
emulsions, solutions,
syrups, aerosols (as solids or in liquid media), ointments (e.g., containing
up to 10% by weight of a
compound of the Formula I), soft-gel and hard-gel capsules, suppositories,
sterile injectable
solutions, sterile packaged powders, and the like.
Similarly, the carrier or diluent may include time-delay or time-release
material known in
the art, such as glyceryl monostearate or glyceryl distearate alone or with a
wax, ethylcellulose,
hydroxypropylmethylcellulose, methylmethacrylate and the like.
A variety of pharmaceutical forms can be employed. Thus, if a solid carrier is
used, the
preparation can be tableted, placed in a hard gelatin capsule in powder or
pellet form or in the
form of a troche or lozenge. The amount of solid carrier may vary, but
generally will be from about
mg to about 1 g. If a liquid carrier is used, the preparation can be in the
form of syrup,
25 emulsion, soft gelatin capsule, sterile injectable solution or suspension
in an ampoule or vial or
non-aqueous liquid suspension.
To obtain a stable water-soluble dose form, a pharmaceutically acceptable salt
of an
inventive agent is dissolved in an aqueous solution of an organic or inorganic
acid, such as 0.3M
solution of succinic acid or citric acid. If a soluble salt form is not
available, the agent may be
dissolved in a suitable cosolvent or combinations of cosolvents. Examples of
suitable cosolvents
include, but are not limited to, alcohol, propylene glycol, polyethylene
glycol 300, polysorbate 80,
gylcerin and the like in concentrations ranging from 0-60% of the total
volume. A compound of
Formula I may be dissolved in DMSO and diluted with water. The composition may
also be in the
form of a solution of a salt form of the active ingredient in an appropriate
aqueous vehicle such as
water or isotonic saline or dextrose solution.
The compositions of the invention may be manufactured in manners generally
known for
preparing pharmaceutical compositions, e.g., using conventional techniques
such as mixing,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
54
dissolving, granulating, dragee-making, levigating, emulsifying,
encapsulating, entrapping or
lyophilizing. Pharmaceutical compositions may be formulated in a conventional
manner using one
or more physiologically acceptable carriers, which may be selected from
excipients and auxiliaries
that facilitate processing of the active compounds into preparations which can
be used
pharmaceutically.
Proper formulation is dependent upon the route of administration chosen. For
injection,
the agents of the invention may be formulated into aqueous solutions,
preferably in physiologically
compatible buffers such as Hanks's solution, Ringer's solution, or
physiological saline buffer. For
transmucosal administration, penetrants appropriate to the barrier to be
permeated are used in
the formulation. Such penetrants are generally known in the art.
For oral administration, the compounds can be formulated readily by combining
the active
compounds with pharmaceutically acceptable carriers known in the art. Such
carriers enable the
compounds of the invention to be formulated as tablets, pills, dragees,
capsules, liquids, gels,
syrups, slurries, suspensions and the like, for oral ingestion by a patient to
be treated.
Pharmaceutical preparations for oral use can be obtained using a solid
excipient in admixture with
the active ingredient (agent), optionally grinding the resulting mixture, and
processing the mixture
of granules after adding suitable auxiliaries, if desired, to obtain tablets
or dragee cores. Suitable
excipients include: fillers such as sugars, including lactose, sucrose,
mannitol, or sorbitol; and
cellulose preparations, for example, maize starch, wheat starch, rice starch,
potato starch, gelatin,
gum, hydroxypropylmethyl-cellulose, sodium carboxymethylcellulose, methyl
cellulose, or
polyvinylpyrrolidone (PVP). If desired, disintegrating agents may be added,
such as crosslinked
polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof such as sodium
alginate.
Dragee cores are provided with suitable coatings. For this purpose,
concentrated sugar
solutions may be used, which may optionally contain gum arabic, polyvinyl
pyrrolidone, Carbopol
gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions, and
suitable organic solvents
or solvent mixtures. Dyestuffs or pigments may be added to the tablets or
dragee coatings for
identification or to characterize different combinations of active agents.
Pharmaceutical preparations which can be used orally include push-fit capsules
made of
gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer,
such as glycerol or
sorbitol. The push-fit capsules can contain the active ingredients in
admixture with fillers such as
lactose, binders such as starches, and/or lubricarits such as talc or
magnesium stearate, and,
optionally, stabilizers. In soft capsules, the active agents may be dissolved
or suspended in
suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene
glycols. In addition,
stabilizers may be added. All formulations for oral administration should be
in dosages suitable
for such administration. For buccal administration, the compositions may take
the form of tablets
or lozenges formulated in conventional manner.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
For administration intranasally or by inhalation, the compounds for use
according to the
present invention are conveniently delivered in the form of an aerosol spray
presentation from
pressurized packs or a nebulizer, with the use of a suitable propellant, e.g.,
dichlorodifluoromethane, trichlorofluoromethane, dichlorotetrafluoroethane,
carbon dioxide or
5 other suitable gas. In the case of a pressurized aerosol the dosage unit may
be determined by
providing a valve to deliver a metered amount. Capsules and cartridges of
gelatin for use in an
inhaler or insufflator and the like may be formulated containing a powder mix
of the compound
and a suitable powder base such as lactose or starch.
The compounds may be formulated for parenteral administration by injection,
e.g., by
10 bolus injection or continuous infusion. Formulations for injection may be
presented in unit-dosage
form, e.g., in ampoules or in multi-dose containers, with an added
preservative. The compositions
may take such forms as suspensions, solutions or emulsions in oily or aqueous
vehicles, and may
contain formulatory agents such as suspending, stabilizing and/or dispersing
agents.
Pharmaceutical formulations for parenteral administration include aqueous
solutions of
15 the active compounds in water-soluble form. Additionally, suspensions of
the active agents may
be prepared as appropriate oily injection suspensions. Suitable lipophilic
solvents or vehicles
include fatty oils such as sesame oil, or synthetic fatty acid esters, such as
ethyl oleate or
triglycerides, or liposomes. Aqueous injection suspensions may contain
substances which
increase the viscosity of the suspension, such as sodium carboxymethyl
cellulose, sorbitol, or
20 dextran. Optionally, the suspension may also contain suitable stabilizers
or agents which increase
the solubility of the compounds to allow for the preparation of highly
concentrated solutions.
For administration to the eye, the active agent is delivered in a
pharmaceutically
acceptable ophthalmic vehicle such that the compound is maintained in contact
with the ocular
surface for a sufficient time period to allow the compound to penetrate the
corneal and internal
25 regions of the eye, including, for example, the anterior chamber, posterior
chamber, vitreous body,
aqueous humor, vitreous humor, cornea, iris/ciliary, lens, choroid/retina and
sclera. The
pharmaceutically acceptable ophthalmic vehicle may be an ointment, vegetable
oil, or an
encapsulating material. A compound of the invention may also be injected
directly into the
vitreous and aqueous humor.
30 Alternatively, the active ingredient may be in powder form for constitution
with a suitable
vehicle, e.g., sterile pyrogen-free water, before use. The compounds may also
be formulated in
rectal compositions such as suppositories or retention enemas, e.g.,
containing conventional
suppository bases such as cocoa butter or other glycerides.
The compounds may also be formulated as a depot preparation. Such long-acting
35 formulations may be administered by implantation (for example,
subcutaneously or
intramuscularly) or by intramuscular injection. Thus, for example, the
compounds may be
formulated with suitable polymeric or hydrophobic materials (for example, as
an emulsion in an
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
56
acceptable oil) or ion-exchange resins, or as sparingly soluble derivatives,
for example, as a
sparingly soluble salt.
A pharmaceutical carrier for hydrophobic compounds is a cosolvent system
comprising
benzyl alcohol, a nonpolar surfactant, a water-miscible organic polymer, and
an aqueous phase.
The cosolvent system may be a VPD co-solvent system. VPD is a solution of 3%
w/v benzyl
alcohol, 8% w/v of the nonpolar surfactant polysorbate 80, and 65% w/v
polyethylene glycol 300,
made up to volume in absolute ethanol. The VPD co-solvent system (VPD:5W)
contains VPD
diluted 1:1 with a 5% dextrose in water solution. This co-solvent system
dissolves hydrophobic
compounds well, and itself produces low toxicity upon systemic administration.
Naturally, the
proportions of a co-solvent system may be varied considerably without
destroying its solubility and
toxicity characteristics. Furthermore, the identity of the co-solvent
components may be varied: for
example, other low-toxicity nonpolar surfactants may be used instead of
polysorbate 80; the
fraction size of polyethylene glycol may be varied; other biocompatible
polymers may replace
polyethylene glycol, e.g. polyvinyl pyrrolidone; and other sugars or
polysaccharides may be
substituted for dextrose.
Alternatively, other delivery systems for hydrophobic pharmaceutical compounds
may be
employed. Liposomes and emulsions are known examples of delivery vehicles or
carriers for
hydrophobic drugs. Certain organic solvents such as dimethylsulfoxide also may
be employed,
although usually at the cost of greater toxicity. Additionally, the compounds
may be delivered
using a sustained-release system, such as semipermeable matrices of solid
hydrophobic
polymers containing the therapeutic agent. Various sustained-release materials
have been
established and are known by those skilled in the art. Sustained-release
capsules may,
depending on their chemical nature, release the compounds for a few weeks up
to over 100 days.
Depending on the chemical nature and the biological stability of the
therapeutic reagent, additional
strategies for protein stabilization may be employed.
The pharmaceutical compositions also may comprise suitable solid- or gel-phase
carriers
or excipients. Examples of such carriers or excipients include calcium
carbonate, calcium
phosphate, sugars, starches, cellulose derivatives, gelatin, and polymers such
as polyethylene
glycols.
Some of the compounds of the invention may be provided as salts with
pharmaceutically
compatible counter ions. Pharmaceutically compatible salts may be formed with
many acids,
including hydrochloric, sulfuric, acetic, lactic, tartaric, malic, succinic,
etc. Salts tend to be more
soluble in aqueous or other protonic solvents than are the corresponding free-
base forms.
The compounds of the Formula I, a pharmaceutically acceptable salt, solvate,
or
prodrug thereof are useful as anti-angiogenesis agents and as agents for
modulating and/or
inhibiting the activity of protein kinases, thus providing treatments for
cancer or other diseases
associated with cellular proliferation mediated by protein kinases.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
57
Therapeutically effective amounts of the agents of the invention may be used
to treat
diseases mediated by modulation or regulation of protein kinases. An
"effective amount" is
intended to mean that amount of an agent that, when administered to a mammal
in need of such
treatment, is sufficient to effect treatment for a disease mediated by the
activity of one or more
kinases. Thus, e.g., a therapeutically effective amount of a compound of the
Formula I, a
pharmaceutically acceptable salt, solvate, or prodrug thereof is a quantity
sufficient to modulate,
regulate, or inhibit the activity of one or more kinases such that a disease
condition which is
mediated by that activity is reduced or alleviated.
BIOCHEMICAL AND BIOLOGICAL EVALUATION
Enzyme Assays
CHK-1 Construct for Assay
As previously detailed in European Patent Application No. 1 096 014 A2 (filed
October 31,
2000), the C-terminally His-tagged kinase domain of human CHK-1 (KH289), amino
acid residues
1-289, can be expressed using the baculovirus/insect cell system. This
construct has been shown
to possess catalytic activity approximately 10-fold greater than full length
CHK-1. The Bac-to-Bac
system (Life Technologies) can be used to generate recombinant baculovirus for
the expression
of KH289 as per instructions. Recombinant viruses can be confirmed by PCR for
the presence of
CHK-1 cDNA insertion. Protein expression can be confirmed by SDS-PAGE or
Western blot with
CHK-1 polyclonal antibodies. Sf9 insect cells (Invitrogen, Carlsbad, CA, USA)
can be used for
initial amplification of recombinant virus stock. High titer stocks of
recombinant viruses can be
generated by 2 to 3 rounds of amplification using Sf21 insect cells. Hi-S
insect cells (Invitrogen,
Carlsbad, CA, USA) can be used for protein production. Both Sf9 and Hi-S cell
lines can be
adapted to grow in insect medium containing 1% Fetal Bovine Serum (Life
Technologies, Grand
Island, NY, USA). The viral stock was stored at 10 C and used for large-scale
protein production
within 2 months to avoid viral instability. For protein production, infected
Hi-S cells can be
harvested by centrifugation and stored at -80 C. From these cells, 6X-His
tagged KH289
(identified by SDS-PAGE) can be obtained after purification and can be flash-
frozen in liquid N2
and stored at -80 C. Maintaining salt concentration around 500 mM NaCI
including 5% glycerol
was found to be crucial for preventing aggregation of CHK-1 proteins during
purification and
storage.
CHK-1 Assay
As previously detailed in European Patent Application No. 1 096 014 A2 (filed
October 31,
2000), the enzymatic activity of a kinase can be measured by its ability to
catalyze the transfer of a
phosphate residue from a nucleoside triphosphate to an amino acid side chain
in a selected
protein target. The conversion of ATP to ADP generally accompanies the
catalytic reaction.
Herein, a synthetic substrate peptide, Syntide-2, having amino acid sequence
PLARTLSVAGLPGKK can be utilized. The production of ADP from ATP that
accompanies
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
58
phosphoryl transfer to the substrate can be coupled to oxidation of NADH using
phosphoenolpyruvate (PEP) through the actions of pyruvate kinase (PK) and
lactic
dehydrogenase (LDH). The oxidation of NADH can be monitored by following the
decrease of
absorbance at 340 nm (e340=6.22 cm-1 mM-1) using a HP8452 spectrophotometer.
Typical
reaction solutions contained: 4 mM PEP, 0.15 mM NADH, 28 units of LDH/mL, 16
units of PK/mL,
3 mM DTT, 0. 125 mM Syntide-2, 0.15 mM ATP and 25 mM MgC12 in 50 mM TRIS pH
7.5; 400
mM NaCI. Assays can be initiated with 10 nM of kinase domain of CHK-1, KH289.
Ki values can
be determined by measuring initial enzyme activity in the presence of varying
concentrations of
inhibitors. The data can be analyzed using Enzyme Kinetic and Kaleidagraph
software.
VEGF-R2 Construct for Assay
This construct determines the ability of a test compound to inhibit tyrosine
kinase activity.
A construct (VEGF-R2A50) of the cytosolic domain of (human) vascular
endothelial growth factor
receptor 2 (VEGF-R2) lacking the 50 central residues of the 68 residues of the
kinase insert
domain can be expressed in a baculovirus/insect cell system. Of the 1356
residues of full-length
VEGF-R2, VEGF-R2A50 contains residues 806-939 and 990-1171, and also one point
mutation
(E990V) within the kinase insert domain relative to wild-type VEGF-R2.
Autophosphorylation of
the purified construct can be performed by incubation of the enzyme at a
concentration of 4 M in
the presence of 3 mM ATP and 40 mM MgCI2 in 100 mM HEPES, pH 7.5, containing
5% glycerol
and 5 mM DTT, at 4 C for 2 hours. After autophosphorylation, this construct
has been shown to
possess catalytic activity essentially equivalent to the wild-type
autophosphorylated kinase domain
construct. See Parast et al. (1998) Biochemistry 37:16788-16801.
VEGF-R2 Assay
Coupled Spectrophotometric (FLVK-P) Assay
The production of ADP from ATP that accompanies phosphoryl transfer can be
coupled
to oxidation of NADH using phosphoenolpyruvate (PEP) and a system having
pyruvate kinase
(PK) and lactic dehydrogenase (LDH). The oxidation of NADH can be monitored by
following the
decrease of absorbahce at 340 nm (e340 = 6.22 cm"' mM"') using a Beckman DU
650
spectrophotometer. Assay conditions for phosphorylated VEGF-R2050 can be the
following: 1
mM PEP; 250 M NADH; 50 units of LDH/mL; 20 units of PK/mL; 5 mM DTT; 5.1 mM
poly(E4Y,);
1 mM ATP; and 25 mM MgCI2 in 200 mM HEPES, pH 7.5. Assay conditions for
unphosphorylated
VEGF-R2050 can be the following: 1 mM PEP; 250 M NADH; 50 units of LDH/mL; 20
units of
PK/mL; 5 mM DTT; 20 mM poly(E4Y,); 3 mM ATP; and 60 mM MgCIZ and 2 mM MnCIZ in
200 mM
HEPES, pH 7.5. Assays can be initiated with 5 to 40 nM of enzyme. Enzyme
percentage
inhibition values can be determined by measuring enzyme activity in the
presence of 0.05 M test
compound. The data can be analyzed using Enzyme Kinetic and Kaleidagraph
software.
FGFR
FGF-R1 Construct for Assay
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
59
The intracellular kinase domain of (human) FGF-R1 can be expressed using the
baculovirus vector expression system starting from the endogenous methionine
residue 456 to
glutamate 766, according to the residue numbering system of Mohammadi et al.
(1996) Mol. Cell.
Biol.16:977-989. In addition, the construct also has the following 3 amino
acid substitutions:
L457V, C488A, and C584S.
FGF-R Assay
The spectrophotometric assay can be carried out as described above for VEGF-
R2,
except for the following changes in concentration: FGF-R = 50 nM, ATP = 2 mM,
and poly(E4Y1)
= 15 mM. K; values can be determined by measuring enzyme activity in the
presence of varying
concentrations of test compounds.
PHK
Phosphorylase Kinase Construct for AssW.
The truncated catalytic subunit (gamma subunit) of phosphorylase kinase (amino
acids 1-
298) can be expressed in E.coli and isolated from inclusion bodies.
Phosphorylase kinase can
then be refolded and stored in glycerol at -20 C.
Phosphorylase Kinase Assax
In the assay, the purified catalytic subunit can be used to phosphorylate
phosphorylase b
using radiolabled ATP. Briefly, 1.5 mg/mI of phosphorylase b can be incubated
with 10 nM
phosphorylase kinase in 10 mM MgCI2, 50 mM Hepes pH 7.4, at 37 C. The
reaction can be
started with the addition of ATP to 100 uM and incubated for 15 min at 25 C
or 37 C. The
reaction can be terminated and proteins can be precipitated by the addition of
TCA to 10% final
concentration. The precipitated proteins can be isolated on a 96 well
Millipore MADP NOB filter
plate. The filter plate can be extensively washed with 20% TCA, and dried.
Scintilation fluid can
be then added to the plate and incorporated radiolabel can be counted on a
Wallac microbeta
counter. The % inhibition of phosphoryl transfer from ATP to phosphorylase b
in the presence of
10 M of compound can then be measured.
CHK-2 Assav
CHK-2 enzyme can be obtained from Upstate Group, Inc. and is an N-terminal,
GST-
tagged and C-terminal His-tagged fusion protein corresponding to amino acids 5-
543 of human
CHK-2 as confirmed by mass tryptic fingerprinting, expressed in E. coli; Mr-
87kDa. The assay
condition for CHK-2 can be as described above for CHK-1, except that the
enzyme CHK2
(0.059 M) can be utilized in place of KH289. Further, no NaCI can be added.
CDK-1 Assav
CDK-1/cyclin B, active complex can be obtained from Upstate Group, Inc. and is
a C-
terminal, His-tagged CDK-1 and an N-terminal GST-tagged-cyclin B as confirmed
by mass tryptic
fingerprinting and protein sequencing, produced individually in Sf21 cells and
then complexed in
vitro. The assay condition for CDK-1 can be as described above for CHK-1,
except that the
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
enzyme complex CDK-1/cyclin B(0.2 M) can be utilized in place of KH289, and
Histone-HI
(Upstate USA, Inc.) (0.059 M) can be utilized as a substrate in place of
Syntide-2. Further, no
NaCI can be added.
WEE-1 assay
5 Delfia (R) Assay Protocol for WEE-1
WEE-1 enzyme can be obtained from Upstate Group, Inc. and is an N-terminal,
GST-
tagged fusion protein to full length rat WEE-1, expressed in E. coli; Mr-
100kDa. This kinase
assay can be carried out on coated poly (Glu-Tyr) 4:1 (random copolymer) 96-
well filter plates
(NoAb Diagnostics). The assay volume can be 100 1 per well plus 2 l DMSO
(control) or 2 l of
10 compound in DMSO. Buffer A can be 10% glycerol, 20mM TRIS (pH7.5), 10mM
MgCIZ, 50mM
NaCI and 5mM DTT. The plates can be prepared by automation.
To an appropriate well can be added either 2 l of DMSO (control) or 2 l of
compound in
DMSO. To the positive control wells can be added 30 1 of 0.5M EDTA. To each
well can be added
50 1 ATP in Buffer A such that the ATP assay concentration can be 33 M. To
start the reaction,
15 501i1 Weel in Buffer A can be added to each well such that the Wee1 assay
concentration can be
0.1 ng/ l. The plate can be can be mixed by shaking and then allowed to remain
at room
temperature for 30 minutes. To stop the reaction, the plate can be washed once
with Delfia Wash
on an EL405 plate washer. To each well can be added 100 1 of EuPY in Delfia(R)
assay buffer
such that the EuPY assay concentration can be 0.0149 ng/ l. The plate can be
allowed to sit for 1
20 hours or overnight. The plate can be washed once again with Delfia(R) Wash
(EL405 plate
washer), and allowed to dry. To each well can be added 100 1 of Delfia (R)
Enhancement solution
and the plate can be allowed to sit for 10 minutes. The plate can be read on
Wallac's Victor
fluorescence reader (Europium Protocol). K; values can be determined by
measuring enzyme
activity in the presence of varying concentrations of test compounds.
25 SGK Assay
SGK (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 8mM MOPS pH7.0, 0.2mM EDTA, 30 M Crosstide, 10mM MgAcetate and [7-33P-
ATP]
(Specific activity approximately 500cpm/pmol, concentration as required) to
form a final reaction
volume of 25 1. Compounds can be tested at 1 M. The reaction can be initiated
by the addition of
30 Mg2+ [7_33P-ATP]. The ATP concentration can be 10 M. After incubation for
40 minutes at room
temperature, the reaction can be stopped by the addition of 5 l of a 3%
phosphoric acid solution.
10 1 of the reaction can then be spotted onto a P30 filtermat and washed three
times for 5
minutes in 50mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
Results represent an average of two experiments and enzymatic activity can be
expressed as a
35 percentage of that in control incubations without test compounds.
AMPK Assav
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
61
AMPK (rat) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with
50mM Hepes pH7.4, 1 mM DTT, 0.02% Brij35, 200 M AMP, 200 M AMARAASAAALARRR,
10mM MgAcetate and [r 33P-ATP] (Specific activity approximately 500cpm/pmol,
concentration as
required) to form a final reaction volume of 25111. Compounds can be tested at
1 M. The reaction
can be initiated by the addition of Mg2+ [y-33P-ATP]. The ATP concentration
can be 10 M. After
incubation for 40 minutes at room temperature, the reaction can be stopped by
the addition of 5 l
of a 3% phosphoric acid solution. 10 1 of the reaction can then be spotted
onto a P30 filtermat and
washed three times for 5 minutes in 75mM phosphoric acid and once in methanol
prior to drying
and scintillation counting. Results represent an average of two experiments
and enzymatic activity
can be expressed as a percentage of that in control incubations without test
compound.
LCK Assay
LCK (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 50mM Tris pH7.5, 0.1mM EGTA, 0.1mM NaVanadate, 2500M KVEKIGEGTYGVVYK
(CDC2
peptide), 10mM MgAcetate and [r33P-ATP] (Specific activity approximately
500cpm/pmol,
concentration as required) to form a final reaction volume of 25[tl. Compounds
can be tested at
1 M. The reaction can be initiated by the addition of Mg2+ [y33P-ATP]. The
ATP concentration can
be 10 M. After incubation for 40 minutes at room temperature, the reaction can
be stopped by the
addition of 5 l of a 3% phosphoric acid solution. 10 I of the reaction can
then be spotted onto a
P30 filtermat and washed three times for 5 minutes in 75mM phosphoric acid and
once in
methanol prior to drying and scintillation counting. Results represent an
average of two
experiments and enzymatic activity can be expressed as a percentage of that in
control
incubations without test compound.
MAPK2 Assay
MAPK2 (mouse) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be
incubated
with 25mM Tris pH 7.5, 0.02mM EGTA, 0.33mg/mI myelin basic protein, 10mM
MgAcetate and [y-
33P-ATP] (Specific activity approximately 500cpm/pmol, concentration as
required) to form a final
reaction volume of 25 l. Compounds can be tested at 1 M. The reaction can be
initiated by the
addition of Mga+ [y-33P-ATP]. The ATP concentration can be 10 M. After
incubation for 40 minutes
at room temperature, the reaction can be stopped by the addition of 5 l of a
3% phosphoric acid
solution. 10 1 of the reaction can then be spotted onto a P30 filtermat and
washed three times for
5 minutes in 75mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
Results represent an average of two experiments and enzymatic activity can be
expressed as a
percentage of that in control incubations without test compound.
MSK1 Assav
MSK1 (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 8mM MOPS pH7.0, 0.2mM EDTA, 30pM Crosstide, 10mM MgAcetate and [y-33P-
ATP]
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
62
(Specific activity approximately 500cpm/pmol, concentration as required) to
form a final reaction
volume of 25 l. Compounds can be tested at 1 M. The reaction can be initiated
by the addition of
Mg2+ [r 33P-ATP]. The ATP concentration can be 10 M. After incubation for 40
minutes at room
temperature, the reaction can be stopped by the addition of 5 l of a 3%
phosphoric acid solution.
10 I of the reaction can then be spotted onto a P30 filtermat and washed three
times for 5
minutes in 50mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
Results represent an average of two experiments and enzymatic activity can be
expressed as a
percentage of that in control incubations without test compound.
PKBa Assav
PKBa (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 8mM MOPS pH7.0, 0.2mM EDTA, 30 M Crosstide, 10mM MgAcetate and [y-33P-
ATP]
(Specific activity approximately 500cpm/pmol, concentration as required) to
form a final reaction
volume of 25 1. Compounds can be tested at 1 M. The reaction can be initiated
by the addition of
Mg2+ [7 33P-ATP]. The ATP concentration can be 10 M. After incubation for 40
minutes at room
temperature, the reaction can be stopped by the addition of 5 l of a 3%
phosphoric acid solution.
10 1 of the reaction can then be spotted onto a P30 filtermat and washed three
times for 5
minutes in 50mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
Results represent an average of two experiments and enzymatic activity can be
expressed as a
percentage of that in control incubations without test compound.
ROCKII Assay
ROCKII (rat) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 50mM Tris pH7.5, 0.1mM EGTA, 30^M KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK,
10mM MgAcetate and [Y33P-ATP] (Specific activity approximately 500cpm/pmol,
concentration
as required) to form a final reaction volume of 25 1. Compounds can be tested
at 1 M. The
reaction can be initiated by the addition of Mgz+ [7-33P-ATP]. The ATP
concentration can be 10 M.
After incubation for 40 minutes at room temperature, the reaction can be
stopped by the addition
of 5 l of a 3% phosphoric acid solution. 10 1 of the reaction can then be
spotted onto a P30
filtermat and washed three times for 5 minutes in 75mM phosphoric acid and
once in methanol
prior to drying and scintillation counting. Results represent an average of
two experiments and
enzymatic activity can be expressed as a percentage of that in control
incubations without test
compound.
p70 S6K Assay
p70S6K (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be
incubated
with 8mM MOPS pH7.0, 0.2mM EDTA, 100 M KKRNRTLTV, 10mM MgAcetate and [y-33P-
ATP]
(Specific activity approximately 500cpm/pmol, concentration as required) to
form a final reaction
volume of 250. Compounds can be tested at 1 M. The reaction can be initiated
by the addition of
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
63
Mg2+ [r 33P-ATP]. The ATP concentration can be 10 M. After incubation for 40
minutes at room
temperature, the reaction can be stopped by the addition of 5 l of a 3%
phosphoric acid solution.
1 of the reaction can then be spotted onto a P30 filtermat and washed three
times for 5
minutes in 75mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
5 Results represent an average of two experiments and enzymatic activity can
be expressed as a
percentage of that in control incubations without test compound.
PKA Assay
PKA (bovine) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 8mM MOPS pH7.0, 0.2mM EDTA, 30 M LRRASLG (Kemptide), 10mM MgAcetate and
[y-
10 33P-ATP] (Specific activity approximately 500cpm/pmol, concentration as
required) to form a final
reaction volume of 25 1. Compounds can be tested at 1 M. The reaction can be
initiated by the
addition of MgZ+ [y-33P-ATP]. The ATP concentration can be 10 M. After
incubation for 40 minutes
at room temperature, the reaction can be stopped by the addition of 5111 of a
3% phosphoric acid
solution. 10 1 of the reaction can then be spotted onto a P30 filtermat and
washed three,times for
5 minutes in 50mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
Results represent an average of two experiments and enzymatic activity can be
expressed as a
percentage of that in control incubations without test compound.
MAPK1 Assay
MAPKI (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be
incubated
with 25mM Tris pH7.5, 0.02mM EGTA, 1 mM synthetic peptide, 10mM MgAcetate and
[Y-33P-ATP]
(Specific activity approximately 500cpm/pmol, concentration as required) to
form a final reaction
volume of 25 1. Compounds can be tested at 1 M. The reaction can be initiated
by the addition of
Mg2+ [y-33P-ATP]. The ATP concentration can be 10 M. After incubation for 40
minutes at room
temperature, the reaction can be stopped by the addition of 5 l of a 3%
phosphoric acid solution.
10 1 of the reaction can then be spotted onto a P30 filtermat and washed three
times for 5
minutes in 75mM phosphoric acid and once in methanol prior to drying and
scintillation counting.
Results represent an average of two experiments and enzymatic activity can be
expressed as a
percentage of that in control incubations without test compound.
cSRC Assay
cSRC (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 8mM MOPS pH7.0, 0.2mM EDTA, 250 M KVEKIGEGTYGVVYK (CDC2 peptide), 10mM
MgAcetate and [Y-33P-ATP] (Specific activity approximately 500cpm/pmol,
concentration as
required) to form a final reaction volume of 251i1. Compounds can be tested at
1 M. The reaction
can be initiated by the addition of Mg2+ [/ 33P-ATP]. The ATP concentration
can be 10 M. After
incubation for 40 minutes at room temperature, the reaction can be stopped by
the addition of 5 l
of a 3% phosphoric acid solution, 10 I of the reaction can then be spotted
onto a P30 filtermat and
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
64
washed three times for 5 minutes in 75mM phosphoric acid and once in methanol
prior to drying
and scintillation counting. Results represent an average of two experiments
and enzymatic activity
can be expressed as a percentage of that in control incubations without test
compound.
PRK2 Assav
PRK2 (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 50mM Tris pH7.5, 0.1 mM EGTA, 0.1% R-mercaptoethanol, 30 M AKRRRLSSLRA,
10mM
MgAcetate and [y-33P-ATP] (Specific activity approximately 500cpm/pmol,
concentration as
required) to form a final reaction volume of 25 1. Compounds can be tested at
I M. The reaction
can be initiated by the addition of Mg2+ [y-33P-ATP]. The ATP concentration
can be 10 M. After
incubation for 40 minutes at room temperature, the reaction can be stopped by
the addition of 5 l
of a 3% phosphoric acid solution. 10 I of the reaction can then be spotted
onto a P30 filtermat and
washed three times for 5 minutes in 75mM phosphoric acid and once in methanol
prior to drying
and scintillation counting. Results represent an average of two experiments
and enzymatic activity
can be expressed as a percentage of that in control incubations without test
compound.
PDK1 Assay
PDK1 (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 50mM Tris pH7.5, 100 M KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC
(PDKtide), 0.1% R-mercaptoethanol, 10mM MgAcetate and [y-33P-ATP] (Specific
activity
approximately 500cpm/pmol, concentration as required) to form a final reaction
volume of 25 1.
Compounds can be tested at 1 M. The reaction can be initiated by the addition
of Mg2+ [y 33P-
ATP]. The ATP concentration can be 10 M. After incubation for 40 minutes at
room temperature,
the reaction can be stopped by the addition of 5 l of a 3% phosphoric acid
solution. 10 1 of the
reaction can then be spotted onto a P30 filtermat and washed three times for 5
minutes in 75mM
phosphoric acid and once in methanol prior to drying and scintillation
counting. Results represent
an average of two experiments and enzymatic activity can be expressed as a
percentage of that
in control incubations without test compound.
FYN Assav
FYN (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be incubated
with 50mM Tris pH7.5, 0.1 mM EGTA, 0.1 mM NaVanadate, 250 M KVEKIOEGTYGVVYK
(CDC2
peptide), 10mM MgAcetate and [r 33P-ATP] (Specific activity approximately
500cpm/pmol,
concentration as required) to form a final reaction volume of 25 1. Compounds
can be tested at
1 I.M. The reaction can be initiated by the addition of Mg2+ [y-33P-ATP]. The
ATP concentration can
be 10 M. After incubation for 40 minutes at room temperature, the reaction can
be stopped by the
addition of 5 l of a 3% phosphoric acid solution. 10 1 of the reaction can
then be spotted onto a
P30 filtermat and washed three times for 5 minutes in 75mM phosphoric acid and
once in
methanol prior to drying and scintillation counting. Results represent an
average of two
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
experiments and enzymatic activity can be expressed as a percentage of that in
control
incubations without test compound.
PKCRII (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be
incubated
with 20mM Hepes pH7.4, 0.03% Triton X-100, 0.1 mM CaC12, 0.1 mg/ml
phosphatidylserine,
5 10 g/ml diacy!g!ycerol, 0.1mg/ml histone HI, 10mM MgAcetate and [Y-33P-ATP]
(Specific activity
approximately 500cpm/pmol, concentration as required) to form a final reaction
volume of 25[L1.
Compounds can be tested at 1 M. The reaction can be initiated by the addition
of Mg2+ [,y-33P-
ATP]]. The ATP concentration can be 10 M. After incubation for 40 minutes at
room temperature,
the reaction can be stopped by the addition of 5 l of a 3% phosphoric acid
solution. 10 1 of the
10 reaction can then be spotted onto a P30 filtermat and washed three times
for 5 minutes in 75mM
phosphoric acid and once in methanol prior to drying and scintillation
counting. Results represent
an average of two experiments and enzymatic activity can be expressed as a
percentage of that
in control incubations without test compound.
PKCy Assav
15 PKCy (human) (Upstate Group, Inc., KINASEPROFILERTM) (5-10mU) can be
incubated
with 20mM Hepes pH7.4, 0.03% Triton X-100, 0.1 mM CaCIZ, 0.1 mg/mI
phosphatidy!serine,
10 g/ml diacylglycerol, 0.1mg/ml histone H1, 10mM MgAcetate and [-y-33P-ATP]
(Specific activity
approximately 500cpm/pmol, concentration as required) to form a final reaction
volume of 25 1.
Compounds can be tested at 1 M. The reaction can be initiated by the addition
of Mg2+[,y-33P-
20 ATP]. The ATP concentration can be 10 M. After incubation for 40 minutes at
room temperature,
the reaction can be stopped by the addition of 5yl of a 3% phosphoric acid
solution. 10 1 of the
reaction can then be spotted onto a P30 filtermat and washed three times for 5
minutes in 75mM
phosphoric acid and once in methanol prior to drying and scintillation
counting. Results represent
an average of two experiments and enzymatic activity can be expressed as a
percentage of that
25 in control incubations without test compound.
Whole Cell Checkpoint Abrogation Assay
Chk1 Mitotic Index ELISA Assav
To examine the in vitro effects of Chk1 inhibitory compounds, an ELISA assay
can be
designed to monitor the abrogation of DNA damage-induced checkpoint control.
The assay can
30 be based on the trapping and detection of mitotic cells following DNA
damage-induced arrest.
Phosphorylation of Histone H3 on serine 10 has been shown to correlate with
mitosis and
therefore can be required for chromosome condensation; consequently a mitosis
specific
phospho-epitope on Histone H3 can be used as a signal for checkpoint
abrogation.
CA-46 (lymphoma) cells can be treated with a DNA damaging agent, such as
35 camptothecin (Sigma), at 50nM for 8 hours to induce DNA damage. The control
compound or
Chkl inhibitor can be then added at increasing concentrations with Nocodazole
(Sigma) at
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
66
0.1 g/mI and plates can be incubated for 16 hours. Control cells, where only
Chkl inhibitors can
be added, can be prepared as well to assure that the inhibitors alone have no
effect on the cell
cycle. The cells can be then harvested, washed with PBS, and crude acid
extraction can be
performed. Pellets can be resuspended in 80 1 of Acid Extraction Buffer (10mM
Hepes pH 7.9,
1.5mM MgCl2, 10mM KCI, 0.5mM DTT, 1.5mM PMSF, 0.4N sulfuric acid), vortexed
briefly, and
incubated for 30 minutes on ice. Samples can be then centrifuged and 75 l of
the supernatant
can be transferred to a 96 well flat-bottom plate (VWR 3596). Next 15 1
Neutralizing Cocktail (#
of samples x(10 l 10N NaOH + 5 l 1 M Tris Base) can be added to each well, and
after mixing,
5 l of this can be transferred to another 96 well plate with 100 1 50mM Tris
base (pH 9.6) in each
well. Samples can be dried overnight. The wells can be then washed with 200 I
ELISA wash
buffer (PBS with 20mM Tris pH 7.5, 0.05% Tween 20) 5 times and blocked with
200 1 blocking
buffer (PBS with 20mM Tris pH 7.5, 0.05% Tween 20, 3.5% Dry milk, 1.5% BSA. pH
to 7.5 after
preparation) for 1 hour at room temperature. Following wash and block, anti-
phospho Histone H3
antibodies (Upstate USA, Inc., rabbit polyclonal) can be added at 0.5 g/ml in
block (100 i per
well) and incubated for 2 hours at room temperature. Wells can be washed again
to remove
unbound primary antibody and 100 1 alkaline phosphatase conjugated secondary
antibodies at
0.3mg/ml (Pierce, goat anti-rabbit IgG (HOURS+L)) in block can be added for 1
hour at room
temp. Wells can be washed 5 times to remove unbound secondary antibody, and
washed again 3
times with PBS alone to remove detergents. Then 100 1 alkaline phosphatase
substrate (Pierce
1-Step pNPP) can be added to wells. Plates can be protected from light and
incubated at room
temp for 1 hour. The OD can be read on Molecular Devices Vmax Kinetic
Microplate Reader at
405nm. The ratio of the OD (optical density) of a compound treated sample to
the Nocodazole
only treated sample (about 100% mitotic or abrogation) can be expressed in a
percentage, and
quantifies the percent abrogation of the checkpoint. The concentration at
which a compound
causes 50% abrogation of the checkpoint can be called the EC50. The raw OD
values can be
graphed in Excel, and an EC50 value can be generated using Kaleidograph
software. Strong signal
results from Nocodazole only treated cells, and equals 100% mitosis in this
assay. Camptothecin
+ Nocodazole treated control samples have low signal, signifying no mitosis
and therefore, no
checkpoint abrogation. When potent Chkl inhibitors are added to Camptothecin
treated cells with
Nocodazole, a high signal can be generated (generally in a dose dependent
manner), due to the
checkpoint abrogation activity caused by the combination treatment.
The examples above illustrate compounds according to Formula I and assays that
may
readily be performed to determine their activity levels against the various
kinase complexes. It will
be apparent that such assays or other suitable assays known in the art may be
used to select an
inhibitor having a desired level of activity against a selected target.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
67
The exemplary compounds described above may be formulated into pharmaceutical
compositions according to the following general examples.
Parenteral Composition
To prepare a parenteral pharmaceutical composition suitable for administration
by
injection, 100 mg of a water-soluble salt of a compound of Formula I or II may
be dissolved in
DMSO and then mixed with 10 mL of 0.9% sterile saline. The mixture may be
incorporated into a
dosage unit form suitable for administration by injection.
Oral Composition
To prepare a pharmaceutical composition for oral delivery, 100 mg of a
compound of
Formula I or II may be mixed with 750 mg of lactose. The mixture may be
incorporated into an
oral dosage unit for, such as a hard gelatin capsule, which may be suitable
for oral administration.
The starting materials used in the examples are commercially available and/or
can be
prepared by techniques known in the art. Freebases and salts of prepared
starting materials and
intermediates were used interchangeably and are indicated. Freebases were
prepared by addition
of a tertiary base to the salt followed by silica gel chromatograpy of the
resulting freebase if
necessary. Salts were prepared by adding an equivalent amount of the
appropriate acid to the
freebase in a slurry or solution.
The preparation of specific preferred compounds of the invention is described
in detail in
the following examples. The artisan will recognize that the chemical reactions
described may be
readily adapted to prepare a number of other kinase inhibitors of the
invention. For example, the
synthesis of non-exemplified compounds according to the invention may be
successfully
performed by modifications apparent to those skilled in the art, e.g., by
appropriately protecting
interfering groups, by changing to other suitable reagents known in the art,
or by making routine
modifications of reaction conditions. Alternatively, other reactions disclosed
herein or known in
the art will be recognized as having applicability for preparing other
compounds of the invention.
In the examples described below, unless otherwise indicated all temperatures
are set
forth in degrees Celsius and all parts and percentages are by weight. Reagents
were purchased
from commercial suppliers such as Aldrich Chemical Company or Lancaster
Synthesis Ltd. and
were used without further purification unless otherwise indicated.
Tetrahydrofuran (THF) distilled
from calcium hydride and N, N-dimethylformamide (DMF) were purchased from
Aldrich in Sure
seal bottles and used as received. All solvents were purified using standard
methods readily
known to those skilled in the art, unless otherwise indicated.
The reactions set forth below were done generally under a positive pressure of
argon or
with a drying tube, at ambient temperature (unless otherwise stated), in
anhydrous solvents, and
the reaction flasks were fitted with rubber septa for the introduction of
substrates and reagents via
syringe. Glassware was oven dried and/or heat dried. Analytical thin layer
chromatography (TLC)
was performed on glass-backed silica gel 60 F 254 plates Analtech (0.25 mm)
and eluted with the
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
68
appropriate solvent ratios (v/v), and are denoted where appropriate. The
reactions - were
assayed by TLC and terminated as judged by the consumption of starting
material.
Visualization of the TLC plates was done with an iodine chamber, UV, p-
anisaldehyde
spray reagent or phosphomolybdic acid reagent (Aldrich Chemical 20 wt% in
ethanol), ninhydrin
reagent, and activated with heat. Work-ups were typically done by doubling the
reaction volume
with the reaction solvent or extraction solvent and then washing with the
indicated aqueous
solutions using 25% by volume of the extraction volume unless otherwise
indicated. Product
solutions were dried over anhydrous Na2SO4 or MgSO4 prior to filtration and
evaporation of the
solvents under reduced pressure on a rotary evaporator and noted as solvents
removed in vacuo.
Flash column chromatography (Still et al., J. Org. Chem., 43, 2923 (1978)) was
done using Baker
grade flash silica gel (47-61 m) and a silica gel: crude material ratio of
about 20:1 to 50:1 unless
otherwise stated. Hydrogenation was done at the pressure indicated in the
examples or at
atmospheric pressure.
'H-NMR spectra were recorded on a Bruker instrument operating at 300 M Hz, 400
M Hz or 500
M Hz and13C-NMR spectra were recorded operating at 75 M Hz. NMR spectra were
obtained as
CDCI3 solutions (reported in ppm), using chloroform as the reference standard
(7.25 ppm and
77.00 ppm) or DMSO-D6 (2.50 ppm and 39.51 ppm) or or CD3OD (3.4 ppm and 4.8
ppm and 49.3
ppm), or internal tetramethylsilane (0.00 ppm) when appropriate. Other NMR
solvents were used
as needed. When peak multiplicities are reported, the following abbreviations
are used: s
(singlet), d (doublet), t (triplet), m (multiplet), br (broadened), dd
(doublet of doublets), dt (doublet
of triplets). Coupling constants, when given, are reported in Hertz ( Hz).
The starting materials used in the examples are commercially available and/or
can be
prepared by techniques known in the art. Freebases and salts of prepared
starting materials and
intermediates were used interchangeably and are indicated. Freebases were
prepared by addition
of a tertiary base to the salt followed by silica gel chromatograpy of the
resulting freebase if
necessary. Salts were prepared by adding an equivalent amount of the
appropriate acid to the
freebase in a slurry or solution.
The following, abbreviations may be used herein: Et20 (diethyl ether); DMF
(N,N-
dimethylformamide); DMSO (dimethylsulfoxide); MeOH (methanol); EtOH (ethanol);
EtOAc (ethyl
acetate); THF (tetrahydrofuran); Ac (acetyl); Me (methyl); Et (ethyl); and Ph
(phenyl).
EXAMPLES:
Example 1: 7-Pyridin-3-yl-1,5-dihydro-[1,2]diazepino[4,5,6-cd]indol-6-one
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
69
O~O O MeO O MeO 0
HN Na2CO3, MeOH H N NaNO2, H20 Br 310 \ a
~/ 87% 30% HBr (CH3CO2H)
NO~ NOz CuBr, 0-80 C, 81 % N02
1(a) 1(b)
OCH3 MeO O Me0
-O
1. ~ CH3 DMF Br I\ ~ POCI3. DMF Br I\ \
2. Ra/Ni, H2, DMF CH2CI2, 69% N
H H
rt, 46% 1(C)
1(d)
OH
B~ / I COZMe ~p / O HN-N
'OH N~ NH2NH2.H20, AcOH N I
Pd(OAc)2, Ph3P, Et3N I/ N MeOH, reflux, 23% N
DMF, 100 C, 30% H H
1(e)
Step 1. Preparation of 6-Amino-2-methyl-3-nitro-benzoic acid methyl ester 1(a)
5-Methyl-6-nitro-lH-benzo[d][1,3]oxazine-2,4-dione (8 g, 36.0 mmol,) prepared
from 2-Amino-6-
methyl-benzoic acid (Aldrich) as described by Abood, N.A., et al. (1997)
Bioorganic & Med. Chem.
Lett. 7: 2105-2108 and sodium carbonate (3.82, 36.0 mmol) were stirred in
methanol (180 mL, 0.1
M) at 0 C for 0.5 hours and then at ambient temperature for 3 hours. Strongly
acidic ion-
exchange resin (Dowex 50 WX4-200) was added until neutral pH and the solution
was filtered.
The solvent was removed under reduced pressure and silica gel chromatography
(60:40
hexane/ethyl acetate) afforded Intermediate 1(a) (6.59 g) in 87% yield.
'H-NMR (d6-DMSO): S 7.88 (d, 1 H, J= 9.1 Hz), 6.65 (d, 1 H, J= 9.2 Hz), 6.55
(s, 2H), 3.86 (s, 3H),
2.38 (s, 3H).
LCMS: (M-H+) 209.1
Step 2. Preparation of 6-Bromo-2-methyl-3-nitro-benzoic acid methyl ester 1(b)
Intermediate 1(a) (0.29 g, 1.38 mmol) was added to an ice-cold solution of HBr
(30% in acetic
acid, 1.6 mL) and water (3.0 mL). Sodium nitrite (0.103 g, 1.5 mmol) in water
(2.0 mL) was added
dropwise and the mixture stirred at 0 C for 0.5 hours. Excess nitric acid was
destroyed by the
addition of urea. The diazonium salt solution was added to a mixture of CuBr
(0.6 g, 4.18 mmol),
HBr (30% in acetic acid, 3.5 mL) and water (5.0 mL) at 35 C and the reaction
mixture was heated
at 80 C for 1.5 hours. The resulting precipitate was filtered and washed with
water to afford
Intermediate 1(b) (0.307 g) in 81 % yield.
1 H-NMR (d6-DMSO): 8 7.99 (d, 1 H, J = 8.8 Hz), 7.85 (d, 1 H, J = 8.8 Hz),
3.94 (s, 3H), 2.39 (s, 3H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Step 3. Preparation of 5-Bromo-lH-indole-4-carboxylic acid methyl ester 1(c)
Intermediate 1(b) (2.6 g, 9.5 mmol) was dissolved in anhydrous N,N-
dimethylformamide (0.5 M,
20 mL). N,N-Dimethylformamide dimethyl acetal (3.0 eq, 3.8 mL, 28.5 mmol) was
added under an
argon atmosphere at ambient temperature with stirring. The mixture was heated
at 130 C for 5
5 hours and cooled to ambient temperature. N,N-Dimethylformamide and the
unreacted N,N-
dimethylformamide dimethyl acetal was removed under reduced pressure (35 C,
c.a. 5 mm Hg).
Toluene (-50 mL) was added and the volatiles removed under vacuum. The crude
enamine was
dissolved in N,N-dimethylformamide (0.2 M, 50 mL) followed by the addition of
Ra/Ni (-300 mg).
The reaction mixture was stirred under hydrogen atmosphere for 7 hours,
filtered over celite and
10 concentrated. Silica gel chromatography (80:20 hexane/ethyl acetate)
afforded Intermediate 1(c)
(1.1 g) in 46% yield.
'H-NMR (d6-DMSO): S 11.54 (s, 1 H), 7.51 (d, 1 H, J= 1.8 Hz), 7.49 (dd, 1 H,
J= 8.5, 0.8 Hz), 7.33
(dd, 1 H, J= 8.7 Hz), 6.50-6.47 (m, 1 H), 3.92 (s, 3H).
LCMS: (M+H+) 252.1
Step 4. Preparation of 5-Bromo-3-formyl-lH-indole-4-carboxylic acid methyl
ester 1(d)
A premixed Vilsmeier reagent consisting of POCI3 (0.53 mL, 5.7 mmol) in N,N-
dimethylformamide
(1.2 mL, 15.6 mmol) was added dropwise at 0 C, to Intermediate 1(c) (0.66 g,
2.6 mmol) in
anhydrous CH2CI2 (13 mL, 0.2 M) with vigorous stirring. The mixture was
stirred for 0.5 hours at
ambient temperature, quenched with aqueous sodium acetate (2.0 M, 10 ml) and
neutralized with
solid Na2CO3. The mixture was partitioned between ethyl acetate (50 mL) and
H20 (10 mL). The
layers were separated and the aqueous layer was extracted with ethyl acetate
(1 X 20 mL). The
organic layers were combined, washed with brine, dried over Na2SO4 and
concentrated to give
Intermediate 1(d) (0.51 g) in 69% yield.
1 H-NMR (d6-DMSO): S 12.54 (s, 1 H), 9.80 (s, 1 H), 8.43 (s, 1 H), 7.59-7.48
(m, 2H), 3.91 (s, 3H).
LCMS: (M+H+) 306.0
Step 5. Preparation of 3-Formyl-5-pyridin-3-yl-IH-indole-4-carboxylic acid
methyl ester 1(e)
A solution of Intermediate 1(d) (0.05 g, 0.18 mmol), 3-boronic acid pyridine
(0.034 g, 0.27 mmol),
palladium (II) acetate (0.004 g, 0.0018 mmol), triphenylphosphine (0.009 g,
0.035 mmol) and
triethylamine (0.08 mL, 0.55 mmol) in N,N-dimethylformamide (0.7 mL, 0.25 M)
was heated at
100 C for 96 hours. The reaction mixture was cooled and filtered through
celite, providing after
column chromatography Intermediate 1(e) (0.015 g) in 30% yield.
LCMS: (M+H+) 281.1
Step 6. Preparation of Title Compound: 7-Pyridin-3-y1-1,5-dihydro-
[1,2]diazepino[4,5,6-
cd]indol-6-one
A solution of Intermediate 1(e) (0.015 g, 0.054 mmol), hydrazine (0.008 mL,
0.135 mmol) and
acetic acid (0.020 mL, 2%) in anhydrous methanol (1.0 mL, 0,05 M) was heated
at 80 C for 24
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
71
hours. The reaction mixture was cooled at ambient temperature and the title
compound (0.0035
g) was obtained after a preparative HPLC purification in 23% yield.
' H-NMR (d6-DMSO): 5 12.03 (s, 1 H), 9.97 (s, 1 H), 8.69 (s, 1 H), 8.65 (d, 1
H, J = 4.6 Hz), 8.09 (d,
1 H, J= 8.3 Hz), 7.77 (d, 1 H, J= 2.7 Hz), 7.76-7.68 (m, 1 H), 7.61 (d, 1 H,
J= 8.4 Hz), 7.54 (s, 1 H),
7.01 (d, 1 H, J= 8.4 Hz).
LCMS: (M+H+) 263.1
Example 2: 8-Amino-1.5-dihydro-[1,2]diazepino[4,5,6-cd]indol-6-one
(hydrochloric salt)
HO O MeO O OCH3
H2SO4, MeOH \ 1, ~CH3, DMF
02N NO reflux, 84% NO 2. Pd/C, H2, 55 p.s.i.
z 02N z
2(a) rt, 21 %
MeO 0 HN-N
WN 30 I~ \ 1. POCI3. DMF, CHzCIz HzN ~ H 2. NHzNHz.Hz0, AcOH HCI.H2N H
MeOH, reflux, 7%
2(b)
Step 1. Preparation of 2-Methyl-3,5-dinitro-benzoic acid methyl ester 2(a)
Concentrated sulfuric acid (0.5 mL) was added slowly at ambient temperature
with stirring to 2-
methyl-3,5-dinitro-benzoic acid (5.22 g, 23.06 mmol) in anhydrous methanol
(200 mL). After
refluxing overnight under an argon atmosphere, the reaction was determined to
be about 50%
complete. Toluene (100 mL) was used to azeotrope the H20 generated from the
reaction, and
fresh anhydrous methanol (300 mL) and H2SO4 (0.5 mL) were added and the
mixture was again
refluxed overnight under an argon atmosphere at which point the volatile
components were
reduced in vacuo. Ethyl acetate and 5% aqueous NaOH were added with stirring,
and the product
was extracted into the ethyl acetate. The ethyl acetate was then washed twice
each with 5%
aqueous NaOH and saturated aqueous NaHCO3, once with brine and dried with
Na2SO4 to give
Intermediate 2(a) (4.65-g, 19.37 mmol) as a white solid in 84% yield.
Step 2. Preparation of 6-Amino-1H-indole-4-carboxylic acid methyl ester 2(b)
Using a modification of the procedure described by Coe, J.W., et. al. (1996)
Tetrahedron Letters
37(34):6045-6048, Intermediate 2(a) (268 mg, 1.12 mmol) was dissolved in
anhydrous NN-
dimethylformamide (0.56 mL) and N,N-dimethylformamide dimethyl acetal (0.445
mL, 3.35 mmol)
was added under an Ar atmosphere with stirring. The mixture was heated at 120
C overnight at
which point the unreacted N,N-dimethylformamide dimethyl acetal was removed
under vacuum
(35-40 C, c.a. 5 mm Hg). To the resulting red enamine was added anhydrous N,N-
dimethylformamide (c.a. 10 mL) and 10% palladium on carbon (230 mg) and the
mixture was
hydrogenated at 55 p.s.i. for 5 hours. The Pd catalyst was filtered through
diatomaceous earth
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
72
and H20 was added to the filtrate. The aqueous component was then extracted
multiple times
with ethyl acetate and the combined extracts were dried with Na2SO4, filtered,
and the volatile
components were removed in vacuo to give the crude Intermediate 2(90 mg) as a
brown glass.
Purification was carried out by eluting through a silica plug with 20% ethyl
acetate and 20%
ethanol in hexane giving Intermediate 2(b) (45 mg, 0.24 mmol) as a brown solid
in 21 % yield.
Step 3. Preparation of Title Compound: 8-Amino-1,5-dihydro-
[1,2]diazepino[4,5,6-cd]indol-
6-one (hydrochloric salt)
With ice bath cooling under argon, to Intermediate 2(b) (45 mg, 0.24 mmol) in
anhydrous CH2CI2
(0.3 mL) and N,N-dimethylformamide (0.3 mL) was added dropwise a premixed
Vilsmeier reagent
(0.1 mL) consisting of POCI3 (0.47 mL) in N,N-dimethylformamide (0.77 mL).
After removing the
ice bath, the mixture was stirred for 0.5 hours at which point the reaction
was again cooled in an
ice bath and an additional Vilsmeier reagent (0.1 mL) was added. Following
removal of the ice
bath, the reaction was stirred 0.5 hours and then poured onto ice. Ethyl
acetate was added
followed by aqueous saturated NaHCO3. The product was then extracted into
ethyl acetate,
washed with brine, dried with Na2SO4, and filtered to give (by LCMS) di-N-
formylated 3-formyl-lH-
indole-4-carboxylic acid methyl ester (37 mg, 0.13 mmol) as a brown glass
which was then
dissolved in anhydrous methanol (2.2 mL). Acetic acid (0.022, 0.384 mmol) and
H2NNH2.H20
(0.038 mL, 0.78 mmol) were added, and the mixture was refluxed for 2 hours.
After removing the
volatile components in vacuo, the crude product was dissolved in H20 and
filtered, the water was
lyophilized and the resulting yellow glass (36 mg) was chromatographed on
silica gel eluting with
10% methanol in CH2CI2. Fractions judged pure were pooled, and the product in
methanol was
acidified with 1 M HCI. The volatile components were removed in vacuo using
acetonitrile to
azeotrope remaining water affording the title compound (4 mg, 0.016 mmol) as a
brown solid in
7% yield.
'H NMR (d6-DMSO): 8 11.99 (s, 1 H), 10.40 (s, 1 H), 7.70 (s, 1 H), 7.55 (s, 1
H), 7.52-7.41 (m, 2H).
HRMS (MALDI M+H+) Calcd for C,0H8N40: 201.0771. Found: 201.0776.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
73
Altenative Method for the Preparation of Intermediate 2(b) hydrochloride:
HO O OCH3
hl C02Me
CH3, DMF, 110 C OMe 1. Pd/C, H2, EtOAc
02N NO 2. Me3SiCl, MeOH, 70 C 02N I NOzMe 2. 4N HCI (Dioxane)
2
75% 2(c) MeOH, 92%
MeO 0
~
I ~N
~
HZN H
2(b) hydrochloride
Step 4. Preparation of 2-(2,2-Dimethoxy-ethyl)-3,5-dinitro-benzoic acid methyl
ester 2(c)
[Attn; 2-(2-Dimethylamino-vinyl)-3,5-dinitro-benzoic acid methyl ester
generated during (first step
of Step 4) enamine formation could lead to explosive decomposition!]
2-Methyl-3,5-dinitro-benzoic acid (100 g, 0.442 mol) was dissolved in
anhydrous N,N-
dimethy!formamide (1 M, 400 mL). N,N-Dimethylformamide dimethyl acetal (188
mL, 1.33 mol)
was added under an argon atmosphere over 10 min at ambient temperature with
stirring. The
mixture was heated at 110 C for 5 hours behind a shield, and cooled at
ambient temperature.
N,N-Dimethylformamide and the unreacted N,N-dimethy!formamide dimethyl acetal
were removed
under reduced pressure (35 C, c.a. 5 mm Hg). Toluene (-50 mL) was added and
the volatiles
removed under vacuum. 2-(2-Dimethy!amino-vinyl)-3,5-dinitro-benzoic acid
methyl ester, isolated
as a dark red solid, was mixed with anhydrous methanol (880 mL) and
chlorotrimethylsilane (140
mL, 1.10 mol) was added over 10 min. The solution was heated at reflux (oil
bath 67-70 C) under
argon for 20 hours, cooled to ambient temperature, and the volume of the
mixture was reduced
under vaccuum to approximately 100 mL. The precipitated solid was collected by
filtration and
washed with cold methanol (100 mL). The dark brown solid was dried under
vacuum, triturated
with acetone (100 mL), again collected by filtration and washed with diethyl
ether (150 mL) to
afford Intermediate 2(c) (79 g). The mother liquor from the first
precipitation and the various
triturations were combined and concentrated. Additional Intermediate 2(c) (21
g) was then
recrystallized from cyclohexane/ethyl acetate (9:1) providing a second batch.
Again the resulting
mother liquor was reduced in vacuo and a third batch of Intermediate 2(c) (4
g) was recrystallized
from acetone/H20 (6:4). The combined yield for all three batches of
Intermediate 2(c) (104 g) was
75%.
Step 5. Preparation of 6-Amino-1H-indole-4-carboxylic acid methyl ester
hydrochloride 2(b)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
74
Intermediate 2(c) (20 g, 63.6 mmol) was dissolved in anhydrous ethyl acetate
(350 mL) and 10%
palladium on carbon (7.4 g, 6.36 mmol) was added under argon. The mixture was
hydrogenated
at 1 atm until the reaction was judged complete by LCMS. The Pd catalyst was
removed by
filtering through diatomaceous earth, and the filtrate was reduced in vacuo.
The crude 3,5-
diamino-2-(2,2-dimethoxy-ethyl)-benzoic acid methyl ester was dissolved in
anhydrous methanol
(40 ml), and 4.0 M HCI in dioxane (160 mL) was added. The mixture was stirred
at ambient
temperature for one hour. The precipitated solid was collected by filtration
and washed with
CH2CI2 and diethyl ether and dried under vacuum to produce Intermediate 2(b)
(hydrochloride)
(11.85 g) as a gray solid. The filtrate was concentrated and more Intermediate
2(b) hydrochloride
(1.48 g) was precipitated. The combined yield for both batches of Intermediate
2(b) (13.33 g) was
92%
Example 3: N-(6-Oxo-5.6-dih rLdro-1H-11 2]diazepino[4 5 6-cdlindol-8-yl)-
acetamide
MeO O MeO 0 \' OCH3
\ (CH3C0)ZO, Et3N \ 1. / ~CH3 , DMF
H2N I~ NO2 4-DMAP, CH2CI2 AcHN I~ N02 2. Pd/C, H2, 55 p.s.i.
3(a)
26% 61%
Me0 0
Me0 0
I~ \ Zn (dust), CH3CO2H
I~ \
POCI3. DMF
AcHN ~ NbH 50-65 C, 94% AcHN N CH2CI2, 44%
3(b) 3(c)
MeO 0 HN-N
-0 O ~
NH2NH2.H20, AcOH I ~ \
AcHN ~ N MeOH, reflux, 51% AcHN ~ N
H H
3(d)
Step 1. Preparation of 5-Acetylamino-2-methyl-3-nitro-benzoic acid methyl
ester 3(a)
15 To 5-amino-2-methyl-3-nitro-benzoic acid methyl ester (428 mg, 2.04 mmol),
prepared as
described by Cannon et. al. (1984) J. Med. Chem. 27:386-389, in CH2CI2 (4 mL)
was added
triethylamine (1.71 mL, 12.2 mmol), acetic anhydride (0.77 mL, 8.14 mmol) and
4-
(dimethyiamino)pyridine (30mg, 0.25 mmol) with stirring at ambient
temperature. After stirring
overnight, LCMS indicated a mixture of mono and diacetylated products.
Saturated aqueous
20 NaHCO3 was added, and the mixture was again allowed to stir overnight.
Additional CH2CI2 was
added, and the layers were separated. The organic layer was reduced and ethyl
acetate was
added. The organic layer was washed with saturated aqueous NaHCO3i with H20,
with saturated
aqueous KHSO4, with brine, dried (Na2SO4), filtered and evaporated to an oil
which was then re-
dissolved in a minimal amount of ethyl acetate. Hexane was added and the
resulting precipitate
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
was isolated as tan solids (469 mg) which were then purified on silica gel,
eluting with 1:2 ethyl
acetate:hexane followed by 1:1 ethyl acetate:hexane, to afford Intermediate
3(a) (133 mg, 0.53
mmol) as a cream solid in 26% yield. (5-diacetylamino-2-methyl-3-nitro-benzoic
acid methyl ester
(248 mg, 0.84 mmol) was also isolated.)
5 ' H NMR (d6-DMSO): S 10.46 (br s, 1 H, exchanges), 8.34 (s, 1 H), 8.14 (s, 1
H), 3.89 (s, 3H), 2.43
(s, 3H), 2.08 (s, 3H).
LCMS: (M-H)" 251.3.
Step 2. Preparation of 6-Acetylamino-l-hydroxy-1H-indole-4-carboxylic acid
methyl ester
3(b)
10 To Intermediate 3(a) (117 mg, 0.46 mmol) in anhydrous N,N-dimethylformamide
(0.5 mL) was
added, under an Ar atmosphere with stirring, N,N-dimethylformamide dimethyl
acetal (0.185 mL,
1.39 mmol). The mixture was heated at 120 C for 5-6 hours at which point the
unreacted N,N-
dimethylformamide dimethyl acetal was removed under vacuum (35-40 C, c.a. 5
mm Hg). To the
resulting red enamine was added anhydrous N,N-dimethylformamide (c.a. 20 mL),
ethyl acetate
15 (10 mL) and 10% palladium on carbon (150 mg). The mixture was hydrogenated
at 55 p.s.i. for 4
hours at which point the N,N-dimethylformamide was removed in vacuo, methanol
was added,
and the Pd catalyst was removed by filtration. Again the volatile components
were removed in
vacuo. Following diethyl ether trituration, the triturate was evaporated to
afford crude Intermediate
3(b) (70 mg, 0.28 mmol) as tan solids in 61% yield which were carried forward
without further
20 purification.
LCMS: (M-H)" 247.3.
Step 3. Preparation of 6-Acetylamino-1H-indole-4-carboxylic acid methyl ester
3(c)
To Intermediate 3(b) (39 mg, 0.16 mmol) in acetic acid (1 mL) was added zinc
dust (206 mg, 3.15
mmol) with stirring. The mixture was heated at 50 C for 0.5 hours and then 65
C for 0.5 hours
25 during which time the mixture turns green. After cooling to room
temperature methanol is added
and the mixture is filtered though diatomaceous earth rinsing several times
with methanol.
Following evaporation the resulting tan solids are triturated with methanol
and the volatile
components of the triturate are remove in vacuo to afford crude Intermediate
3(c) (35 mg, 0.15
mmol) as tan solids in 94% crude yield which were then carried on without
further purification.
30 LCMS: (M-H)- 231.2.
Step 4. Preparation of 6-Acetylamino-3-formyl-IH-indole-4-carboxylic acid
methyl ester 3(d)
With ice bath cooling under argon, 0.2 mL of a premixed Vilsmeier reagent
consisting of POCI3
(0.47 mL) in N,N-dimethylformamide (0.77 mL) was added in two equal portions
to Intermediate
3(c) (35 mg, 0.15 mmol) in 1:1 CH2CI2: N,N-dimethylformamide (1 mL). After 0.5
hours the
35 reaction was quenched with water and extracted with ethyl acetate. The
aqueous layer was then
basified to about pH 8 with 5% aqueous NaOH and extracted again with ethyl
acetate. The
combined ethyl acetate extracts were dried with Na2SO4, filtered and
evaporated to give
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
76
Intermediate 3(d) (19 mg, 0.07 mmol) as yellow solids in 44% crude yield which
were then
carried on without further purification.
LCMS: (M-H)" 259.3.
Step 5. Preparation of Title Compound: N-(6-Oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-
cal]indol-8-yl)-acetamide
To Intermediate 3(d) (19 mg, 0.07 mmol) in anhydrous methanol (2.0 mL) was
added acetic acid
(0.006 mL, 0.1 mmol) and HZNNH2'H20 (11 mg, 0.22 mmol) and the mixture was
refluxed for
about 1 hour after which the volatile components were removed in vacuo and the
resulting yellow
glass was redissolved in methanol. Following recrystallization from
methanol/diethyl ether, the title
compound (9 mg, 0.04 mmol) was obtained as a yellow powder in 51 % yield.
' H NMR (d6-DMSO): S 11.60 (br s, 1 H, exchanges), 10.10 (s, IH, exchanges),
9.90 (s, IH,
exchanges), 8.00 (s, 1 H), 7.45 (br s, 2H), 7.30 (s, 1 H), 1.90 (s, 3H).
LCMS: (M+H+) 243.1, (M+Na+) 265.1.
Example 4: 2 2 2-Trifluoro-N-(6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5.6-
cd]indol-8-yl)-acetamide
HN-N
O \
O I ~ ~
F3C~N ~ N
H H
With stirring, trifluoroacetic anhydride (0.032 mL, 0.23 mmol) was added
dropwise to triethylamine
(0.088 mL, 0.63 mmol) and the title compound of Example 2 (freebase) (43 mg,
0.21 mmol) in
N,N-dimethylformamide (2 mL). After 2.5 hours, additional trifluoroacetic
anhydride (0.032 mL,
0.23 mmol) was added to drive the reaction to completion at which point
diethyl ether was added
to precipitate brown solids (25 mg), which were discarded. The triturate was
reduced in volume
and subjected to preparative HPLC (MetaChem Metasil AQ C18 reverse-phase 10 m,
120A,
250x21.2 mm column eluting with CH3CN/0.1%TFA in H20 at a flowrate of 20
mL/min using a
gradient of 5-95% CH3CN over 20 min) affording, after isolation, the title
compound (3.7 mg, 0.01
mmol) as a brown solid in 6% yield.
'H NM R(d6-DMSO): S 12.01 (s, IH), 11.41 (s, IH), 10.40 (s, IH), 8.15 (s, 1
H), 7.91 (s, 1 H), 7.78
(s, 1 H), 7.61 (s, 1 H)
LCMS: (M-H)" 295.2.
Example 5: 7-Amino-2-phenyl-1 5-dihydro-11 2ldiazepino[4 5 6-coindol-6-one
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
77
MeO 0 MeO 0 MeO 0
\ Br SnC12, H20 Br (CH3C02)20 01- Br
~/ MeOH, reflux, 98% CHaC12, rt, 81%
&NHAc
NO~ NH2 5(a) 5(b)
Me0 O _ Me0
HN03i H2SO4 02N \ Br Bu3Sn \/ 02N
0OC, 89% NHAc Pd(PPh3)4, toluene NHAc
5(c) 90 OC, 69%
5(d)
MeO 0 MeO 0
Cul, TMG, DMF O2N _ :::::0M H2N
\ -
Dioxane, 90 C, 86% I/ N
H H
5(e) 5(f)
HN-N
O ~
1. POCI3. DMF, CH2CI2 HzN
2. NH2NH2.H20, AcOH N
H
MeOH, reflux, 21 %
Step 1. Preparation of 3-Amino-2-bromo-benzoic acid methyl ester 5(a)
2-Bromo-3-nitro-benzoic acid methyl ester (12.9 g, 49.5 mmol) (prepared from 2-
amino-3-nitro-
benzoic acid as described by Webber E. S. et al., see patent application
number WO 01116136
A2) and SnCl2 (42 g, 223 mmol) were refluxed in methanol (225 mL, 0.2 M) and
H20 (5.3 g, 243
mmol) for 2 hours. After cooling at ambient temperature, diatomaceous earth
(20 g) and
dichloromethane (1 L) were added followed with 3N aqueous sodium hydroxide
(150 mL) with
vigorous stirring. The mixture was filtered and the organic phase was washed
with saturated
aqueous sodium chloride. The organic solution was dried over sodium sulfate,
filtered and all
volatiles were removed under reduced pressure to afford Intermediate 5(a)
(11.4 g) in 98% yield.
'H-NMR (ds-DMSO): S 7.12 (dd, 1 H, J = 8.1, 7.5 Hz), 6.93 (dd, 1 H, J = 8.1,
1.6 Hz), 6.80 (dd, 1 H,
J= 7.4, 1.6 Hz), 5.57 (s, 2H), 3.81 (s, 3H).
Step 2. Preparation of 3-Acetylamino-2-bromo-benzoic acid methyl ester 5(b)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
78
Intermediate 5(a) (2.21 g, 9.6 mmol) and acetic anhydride (1.82 mL, 19.2 mmol)
were stirred in
CH2CI2 (100 mL, 0.1 M) at 22 C for 24 hours. Volatiles were removed in vacuo
and silica gel
chromatography afforded Intermediate 5(b) (2.08 g) in 79% yield.
'H-NMR (ds-DMSO): S 9.58 (s, 1 H), 7.70 (dd, 1 H, J= 6.9, 2.7 Hz), 7.50-7.41
(m, 2H), 3.86 (s, 3H),
2.09 (s, 3H).
LCMS: (M+H+) 272.0, 274.0
Step 3. Preparation of 3-Acetylamino-2-bromo-6-nitro-benzoic acid methyl ester
5(c)
Intermediate 5(b) (1.0 g, 3.7 mmol) was nitrated in a manner analogous to step
1 of Example 2.
Intermediate 5(c) (1.0 g, 89%) was obtained after silica gel chromatography.
' H-NMR (d6-DMSO): S 9.83 (s, 1 H), 8.3 (d, 1 H, J= 9.0 Hz), 8.15 (d, 1 H, J=
9.0 Hz), 3.93 (s, 3H),
2.20 (s, 3H).
LCMS: (M+H+) 315.0, 317.0
Step 4. Preparation of 3-Acetylamino-6-nitro-2-phenylethynyl-benzoic acid
methyl ester
5(d)
Intermediate 5(c) (0.85 g, 2.7 mmol) was acetylated in a manner analogous to
step 3 of Example
6 to afford Intermediate 5(d) (0.4 g, 44%) after silica gel chromatography.
1H-NMR (d6-DMSO): S 9.93 (s, 1H), 8.32-8.25 (m, 2H), 7.65-7.59 (m, 2H), 7.53-
7.47 (m, 3H), 3.97
(s, 3H), 2.25 (s, 3H).
LCMS: (M-H) 337.1
Step S. Preparation of 5-Nitro-2-phenyl-1 H-indole-4-carboxylic acid methyl
ester 5(e)
Intermediate 5(d) (0.096 g, 0.28 mmol), copper iodide (0.076 g, 0.4 mmol),
N,N,N,N-
tetramethylguanidine (0.36 mL, 2.8 mmol) were stirred in a mixture of
dimethylformamide/dioxane
(1:4, 2 mL, 0.15 M) at 90 C for 2 hours. The reaction mixture was cooled to
ambient temperature
and poured into ethyl acetate (30 mL), The organic layer was washed
subsequently with a
saturated ammonium chloride solution (3 X 5 mL), H20 (2 X 5 mL), saturated
aqueous sodium
chloride solution (2 X 5 mL), dried over sodium sulfate, filtered and
volatiles removed in vacuo.
Silica gel chromatography afforded Intermediate 5(e) (0.073 g) in 86% yield.
1H-NMR (d6-DMSO): S 12.50 (s, IH), 7.98-7.88 (m, 3H), 7.69-7.40 (m, 4H), 7.13
(broad s, 1H),
3.97 (s, 3H).
LCMS: (M-H+) 295.1
Step 6. Preparation of 5-Amino-2-phenyl-lH-indole-4-carboxylic acid methyl
ester 5(f)
In an analogous manner to that of the preparation of Example 2 (step 5),
Intermediate 5(e) (0.072
g, 0.24 mmol) was hydrogenated to afford Intermediate 5(f) (0.06 g, 95%).
1 H-NMR (d6-DMSO): S 11.68 (s, 1 H), 7.86 (d, 2H, J= 7.4 Hz), 7.52-7.43 (m,
3H), 7.31 (dd, 1 H, J
7.4, 7.2 Hz), 7.20 (s, 1 H), 6.83 (d, 1 H, J= 8.5 Hz), 3.94 (s, 3H).
LCMS: (M+H+) 235.1
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
79
Step 7. Preparation of Title Compound: 7-Amino-2-phenyl-1,5-dihydro-
[1,2]diazepino[4,5,6-cd]indol-6-one
Carried out analogously to steps 4 and 5 of Example 3, Intermediate 5(f)
(0.055g, 0.21 mmol) was
formylated and cyclized to provide the title compound (0.012g, 21 %).
1H-NMR (d6-DMSO): 8 12.17 (s, 1 H), 8.32 (s, 1 H), 7.95-7.84 (m, 3H), 7.74 (d,
1 H, J = 1.8 Hz),
7.55-7.47 (m, 2H), 7.42 (d, 1 H, J = 8.6 Hz), 7.37 (dd, I H, J = 7.4, 7.3 Hz),
5.91 (s, 2H).
LCMS: (M+H+) 277.1
Example 6: N-(6-Oxo-2-Dhenyl-5,6-dihydro-lH-[1 2ldiazepinoj4 5 6-cdlindol-8-yl-
acetamide
1. Fe, CH3CO2H, 40 0 C
Me0 O MeO O 2. (CH3CO2)20, CH2CI2
Br HNO3, H2SO4 \ Br DMF, rt
I rt-40 OC, 65% I/ 3. Fe, (CH3CO2)20
O2N O2N NO2 CH3CO2H, 40 OC, 49%
6(a)
Me0 O Me0 O
Br Bu3Sn - o I\ ~ H2SO4
AcHN NHAc Pd(PPh3)4, toluene AcHN NHAc Dioxane, 90 OC, 98%
6(b) 90 OC, 90% 6( )
MeO O HN-N
O
1. POCI3. DMF, CH2CI2
AcHN N 2= NH2NH2.H2O, ACOH AcHN I/ N\ \/
H MeOH, reflux, 4% H
6(d)
Step 1. Preparation of 2-Bromo-3,5-dinitro-benzoic acid methyl ester 6(a)
Concentrated sulfuric acid (20 mL) was slowly added to 2-bromo-5-nitro-benzoic
acid methyl ester
(20.52 g, 78.91 mmol) with stirring. After a few minutes, fuming nitric acid
(20 mL) was added and
the mixture was capped and heated at 40 C for approximately 60 hours at which
point the flask
was cooled to ambient temperature, carefully opened, and the reaction was
poured onto ice water
and ethyl acetate. The product was extracted into ethyl acetate and washed
twice with H20, twice
with saturated aqueous NaHCO3, brine, dried (Na2SO4), and filtered to afford
Intermediate 6(a)
(15.64 g, 51.26 mmol) as a cream solid in 65% yield.
1H NMR (d6-DMSO): S 9.07 (s, 1 H), 8.73 (s, 1 H), 3.98 (s, 3H).
LCMS: (M-CO2CH3)" 245.1, 247.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Step 2. Preparation of 3,5-Bis-acetylamino-2-bromo-benzoic acid methyl ester
6(b)
To acetic acid (250 mL) at room temperature was added Intermediate 6(a) (15.49
g, 50.79 mmol).
The mixture was placed in an oil bath at 40 C for c.a. 10 min and stirred
vigorously under an Ar
atmosphere until the soution went clear. Iron powder (25.34 g, 453.72 mmol)
was added and the
5 mixture was heated at 40 C for c.a. 6 hours. The mixture was filtered
through diatomaceous
earth, rinsing with methanol. The combined filtrate and rinses were evaporated
to give orange
solids, which were determined to be a mixture of products (13.2 g) resulting
from incomplete
reduction of the nitro groups. This mixture (13.1 g) in CH2CI2 (48 mL) and N,N-
dimethylformamide
(5 mL) was then treated with acetic anhydride (36 mL, 382 mmol) and stirred
overnight under an
10 Ar atmosphere. The CH2CI2 was evaporated and the mixture was partitioned
between ethyl
acetate and saturated aqueous NaHCO3 The aqueous layer was extracted twice
with ethyl
acetate and the combined extracts were washed successively with 1:1
H20:saturated aqueous
NaHCO3 (multiple washings), 0.5 M aqueous HCI (X2), saturated aqueous NaHCO3
(X2) and
brine. The ethyl acetate solution was then dried (Na2SO4), filtered and
evaporated to give yellow
15 solids, which were then triturated with diethyl ether (c.a. 75 mL) to give
a mixture of acetylated
products (11.4 g) as a yellow solids. A portion of this mixture of acetylated
products (5.5 g) in
acetic acid (17.4 mL) was then treated with acetic anhydride (16.5 mL, 174
mmol) and iron
powder (9.74 g, 174 mmol) and heated at 40 C under an Ar atmosphere overnight.
Methanol was
then added and the reaction was stirred at room temperature for c.a. 1.5
hours. Then 9:1
20 CH2CI2:methanol was added, and the mixture was filtered through
diatomaceous earth, rinsing
with 9:1 CH2CI2: methanol. The combined filtrate and rinses were evaporated
and again methanol
was added and the mixture was stirred c.a. 0.5 hours after which the methanol
was evaporated.
Ethyl acetate was added followed by hexane to precipitate orange solids (6.9
g) which were
collected. Silica gel chromatography eluting with 1:1 acetone:hexane which
afforded, after
25 isolation, Intermediate 6(b) (4.1 g, 12.46 mmol) as a cream solid in 49%
yield.
Step 3. Preparation of 3,5-Bis-acetylamino-2-phenylethynyl-benzoic acid methyl
ester 6(c)
With stirring, argon was bubbled into anhydrous toluene (18 mL) containing
Intermediate 6(b)
(1.08 g, 3.29 mmol). Tributyl-phenylethynyl-stannane (1.73 mL, 4.94 mmol) and
tetrakis(triphenylphosphine) palladium(0) (310 mg, 0.28 mmol) were added
sequentially and more
30 Ar was bubbled into the reaction. After capping tightly, the mixture was
heated at 90 C overnight
under an argon atmosphere. After cooling to ambient temperature, H20, and
saturated aqueous
KHSO4 were added and the product was extracted into ethyl acetate and
isolated. Following silica
gel chromatography eluting with 2:3 acetone:hexane, fractions judged pure were
pooled.
Intermediate 6(c) (1.14 g, 3.25 mmol) was isolated, found to be contaminated
by approximately 5-
35 10% triphenylphosphine oxide, and carried on to the next step without
further purification.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
81
' H NMR (ds-DMSO): S 10.30 (s, 1 H), 9.50 (s, 1 H), 8.23 (s, 1 H), 8.08 s, 1
H), 7.63-7.51 (m, 2H,
contaminated by Ph3PO), 7.50-7.38 (m, 3H, contaminated by Ph3PO), 3.90 (s,
3H), 2.17 (s, 3H),
2.05 (s, 3H).
LCMS: (M+H+) 351.1, (M+Na+) 373.1, (M-H") 349.1.
Step 4. Preparation of 6-Acetylamino-2-phenyl-IH-indole-4-carboxylic acid
methyl ester
6(d)
To the impure Intermediate 6(c) (853 mg, 2.4 mmol) is added concentrated
sulfuric acid (15 mL).
After stirring 0.5 hours, the mixture is carefully poured onto methanol (30
mL) with vigorous
swirling. Ethyl acetate (c.a. 300 mL) and H20 (c.a.100 mL) are added. The
aqueous layer is
extracted three times with ethyl acetate, and the combined extracts are washed
with saturated
aqueous NaHCO3 until the evolution of CO2 ceases. The product in ethyl acetate
is then washed
with brine, dried (Na2SO4), filtered and volatile components evaporated to
afford crude
Intermediate 6(d) (790 mg, c.a. 2.4 mmol) as a yellow powder which was carried
on without
further purification.
'H NMR (d6-DMSO): S 11.75 (s, 1 H, exchanges), 10.07 (s, IH, exchanges), 8.33
(s, 1 H), 7.90-
7.80 (m, 3H), 7.66-7.23 (m, 4H, partially obscured), 3.93 (s, 3H), 2.07 (s,
3H).
LCMS: (M+H+) 309.1, (M+Na+) 331.1, (M-H)" 307.1.
Step 5. Preparation of Title Compound: N-(6-Oxo-2-phenyi-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indol-8-yl-acetamide
Crude Intermediate 6(d) (312 mg, c.a. 1 mmol) in N,N-dimethylformamide (2 mL)
and CH2CI2 (5
mL) was treated with Vilsmeier reagent (0.9 mL) in a manner similar to that
described for Example
3, step 4. After removal of the CH2CI2, adjusting the pH to c.a. 8 with 1 N
NaOH and removal of the
volatile components in vacuo, the yellow solids were triturated with ethyl
acetate and methanol.
The triturate was evaporated to afford the crude 6-acetylamino-3-formyl-2-
phenyl-1H-indole-4-
carboxylic acid methyl ester (405 mg) as a yellow solid contaminated with
salts from the aqueous
quench. In a procedure similar to that described for Example 3, step 5,
anhydrous methanol (15
mL), acetic acid (0.084 mL, 1.47 mmol) and H2NNH2'HZO (0.147 mL, 3.03 mmol)
were then added
and the mixture was refluxed for 2 hours. The volatile components were
evaporated and the
resulting solids were triturated with methanol to dissolve the product while
leaving behind most of
the insoluble solids. The triturate was evaporated, and this process was
repeated. The second
triturate was evaporated to give yellow solids (70 mg) enriched in product
which were then
subjected to silica gel chromatography eluting with hexane:ethyl
acetate:ethanol (4:1:1) to give the
title compound (12 mg, 0.04 mmol) after isolation as a yellow powder in
approximately 4% overall
yield.
'H NMR (d6-DMSO): S 12.10 (s, 1 H, exchanges), 10.33(s, 1 H, exchanges),
10.06(s, 1 H,
exchanges), 8.17 (s, 1 H), 7.70-7.43 (m, 7H), 2.05 (s, 3H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
82
LCMS: (M+H+) 319.1, (M+Na+) 341.1, (M-H)" 317.
Altenative Method for the Preparation of Intermediate 6(b).
MeO O MeO O
\ Br Fe, (CH3CO2)20 \ Br
02N I/ NOz CH3CO2H, 40 C, 56% AcHN I/ NHAc
(b)
To acetic acid (10 mL) at room temperature was added acetic anhydride (10.0
mL, 106 mmol),
iron powder (5.5 g, 98 mmol) and Intermediate 6(a) (3.0 g, 9.8 mmol). The
mixture was placed in
an oil bath at 43 C and stirred vigorously under argon for 48 hours. The
thick slurry goes from
orange to tan in color. Additional acetic acid (2 mL), acetic anhydride (2 mL,
21.2 mmol), and iron
powder (1.0 g, 17.9 mmol) were added, and the mixture was stirred at 43 C for
an additional 24
hours whereupon the mixture was poured into 10% methanol in CH2CI2 (300 mL)
and filtered
though diatomaceous earth. The filtrate was concentrated and ethyl acetate
(300 mL) and H20
(300 mL) were added. The product was extracted into ethyl acetate and washed
twice with H20,
twice with brine, dried (MgSO4), and filtered. Following recrystallization
from hot ethyl acetate (10
mL), collection by filtration and subsequent washing of the solids with CH2CI2
(5 mL) and diethyl
ether (30 mL), Intermediate 6(b) (1.8 g, 5.47 mmol) was obtained as a white
solid in 56% yield.
Example 7: 8-Amino-2-phenyl-1,5-dihydro-[1,2]diazepino[4,5,6-cd]indol-6-one
OMe O OMe
I \ ~ - 4N HCI (Dioxane), MeOH, 90 C I \ ~ -
AcHNO/ N \ / CIH.H2N N
H H
7(a)
HN-N
O \
1. POCI3. DMF, CH2CI2
\ ~ -
2. NH2NH2.H20, AcOH H2N N \~
MeOH, reflux, 30% H
Step 1. Preparation of 6-Amino-2-phenyl-IH-indole-4-carboxylic acid methyl
ester
(hydrochloric salt) salt 7(a)
Intermediate 6(d) of Example 6 (9.3 g, 30.2 mmol) and anhydrous 4M HCI in
dioxane (160 mL,
604 mmol) were heated in anhydrous methanol (160 mL) at reflux for 3 hours,
cooled to ambient
temperature and volatiles removed in vacuo. The resulting solid was triturated
with ethyl
acetate/CH2CI2 (1:1, 50 mL) and dried to afford Intermediate 7(a) (8.7 g) in
95% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
83
'H-NMR (d6-DMSO): 5 12.26 (s, 1 H), 10.10 (broad, 1 H), 7.93 (d, 2H, J= 7.7
Hz), 7.71 (d, 2H, J=
12.2 Hz), 7.52 (dd, 2H, J= 7.7, 7.6 Hz), 7.43-7.36 (m, 2H), 3.96 (s, 3H).
LCMS: (M+H+) 267.2.
Step 2. Preparation of Title Compound: 8-Amino-2-phenyl-1,5-dihydro-
[1,2]diazepino[4,5,6-
cd]indol-6-one
In two steps, analogous to steps 4 and 5 of Example 3, Intermediate 7(a) (8.7
g, 28.7 mmol) was
cyclized to afford the title compound (5.47 g, 69%).
'H-NMR (d6-DMSO): S 11.53 (s, 1 H), 10.15 (s, 1 H), 7.62-7.40 (m, 6H), 6.98
(d, 1 H, J= 1.8 Hz),
6.65 (d, 1 H, J= 1.8 Hz), 5.21 (s, 2H).
LCMS: (M+H+) 277.2.
Example 8: N-(6-Oxo-2-phenyl-5,6-dihydro-lH-[1.2]diazepino[4,5.6-cdlindol-8-
yl)-succinamic acid
HN-N
Z O HO~N N
O H
Succinic anhydride (3 eq, 0.022 g) was added to a solution of the title
compound of Example 7
(0.02 g, 0.072 mmol) in N,N-dimethylformamide (0.7 M, 1 mL) and methanol (2.5
M, 0.3 mL). The
reaction mixture was stirred at 22 C for 24 hours and concentrated under
reduced pressure. The
yellow solid was triturated with methanol (1.0 mL) and collected by
filtration. Following washes
with methanol (4 mL) and diethyl ether (5.0 mL), the title compound (21 mg)
was obtained in 77%
yield.
1H-NMR (d6-DMSO): S 12.03 (s, 1 H), 10.31 (s, 1 H), 10.09 (s, 1 H), 8.12 (s,
IH), 7.68-7.45 (m, 7H),
2.60-2.53 (m, 4H).
LCMS: (M+H+) 377.1.
Example 9: N-(6-Oxo-2-phenyl-5.6-dihydro-1 H-[1,2]diazepino[4.5.6-cdJindol-8-
yl)-
methanesulfonamide
HN-N
0 \
\
j~~N I / N
H H
Methanesulfonyl Chloride (1.5 eq, 0.003 g) was added to a solution of the
title compound of
Example 7 (0.005 g, 0.018 mmol) in CH2CI2 (0.045 M, 0.4 mL) and pyridine
(0.045 M, 0.4 mL).
The mixture was stirred at 22 C for 24 hours and concentrated under reduced
pressure. Silica gel
chromatography (triethylamine/methanol/ CH2CI2; 1:5:94) afforded the title
compound (1.7 mg) in
30% yield.
1 H-NMR (d6-DMSO): 5 12.15 (s, 1 H), 10.40 (s, 1 H), 9.71 (s, 1 H), 7.70-7.43
(m, 8H), 2.30 (m, 3H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
84
LCMS: (M+H+) 355.1.
Example 10: 1-(6-Oxo-2-phenyl-5 6-dihydro-1H-[1 2]diazepino[4 5 6-ccllindol-8-
yl)-pyrrolidine-2 5-
dione
HN-N
O ~
O \ -
N I / N ~ ~
H
O
Triethylamine (0.18 mmol, 0.025 mL) and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium hexafluorophosphate (0.04 mmol, 0.013 g) were added to a
solution of the
title compound of Example 8 (0.013 g, 0.036 mmol) in N,N-dimethylformamide
(0.05 M, 0.75 mL).
The reaction mixture was stirred at 22 C for 12 hours and concentrated under
reduced pressure.
The yellow solid was triturated with methanol (3.0 mL), collected by
filtration, and washed with
methanol (4.0 mL) and diethyl ether (5.0 mL) to afford the title compound (5.8
mg) in 47% yield.
'H-NMR (d6-DMSO): S 12.38 (s, 1 H), 10.44 (s, 1 H), 9.71 (s, 1 H), 7.74-7.42
(m, 8H), 2.79 (m, 3H).
LCMS: (M+H+) 359.1.
Example 11: 2-Methvl-cyclopropanecarboxylic acid(6-oxo-2-phenyl-5 6-dihydro-1H-
[1.2]diazepino[4.5,6-cdlindol-8-yl)-amide
HN-N 0 HN-N
0
H 0
~ \
H2N H HATU, Et3N, DMF, 36% ~N / H
H
Triethylamine (0.030 mL, 0.22 mmol), the title compound of Example 7 (15mg,
0.054 mmol) and
2-methyl-cyclopropanecarboxylic acid (6 mg, 0.062 mmol) were stirred in N,N-
dimethylformamide
(1.0 mL). O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (25 mg,
0.065 mmol) was added and the reaction was stirred overnight at which point
the volatile
components were removed under vacuum. The resulting mixture was purified on
silica gel, eluting
with 3:2 hexane:ethyl acetate. The purest fractions were combined and after
solvent removal, the
resulting solids were triturated with diethyl ether to give the title compound
(7 mg, 0.020 mmol) as
yellow powder in 36% yield.
' H-NMR (d6-DMSO): 5 12.03 (s, 1 H), 10.32 (s, 1 H), 10.27 (s, 1 H), 8.13 (s,
1 H), 7.67-7.48 (m, 7H),
1.54 (m, 1 H), 1.11(s, 1 H), 1.09 (d, 3H), 1.07 (m, 1 H), 1.03 (m, 1 H).
LCMS: (M+H+) 359.1, (M+Na+) 381.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Example 12: N-(6-Oxo-2-phen rLl-5 6-dihydro-lH-f1 2]diazepino[4 5 6-cdlindol-8-
,rl)-2-tetrazol-1-yl-
acetamide
HN-N
O
N1 O ~ ~ -
N.NA~N N
H H
Preparation of example 12 from the title compound of Example 7 (15 mg, 0.054
mmol), tetrazol-l-
5 yl-acetic acid (8 mg, 0.062 mmol), triethylamine (0.030 mL, 0.22 mmol), and
O-(7-
azabenzotriazol-l-yi)-N,N,N;M tetramethyluronium hexafluorophosphate (25 mg,
0.065 mmol) in
N,N-dimethylformamide (1.0 mL) was carried out analogously to Example 11.
Additional tetrazol-
1-yl-acetic acid acid (1.0 mg, 0.008 mmol) and O-(7-azabenzotriazol-1-yl)-
N,N,N;M
tetramethyluronium hexafluorophosphate (3.0 mg, 0.008 mmol) were added after
c.a. 18 hours to
10 drive the reaction to completion. Filtration, concentration and
recrystallization afforded the title
compound (12 mg, 0.031 mmol) as a yellow powder in 58% yield.
1H-NMR (d6-DMSO): 8 12.13 (s, 1 H), 10.71 (s, 1 H), 10.39 (s, 1 H), 9.44 (s, 1
H), 8.07 (m, 1 H),
7.68-7.47 (m, 7H), 5.05 (s, 2H).
LCMS: (M+H+) 387.2, (M+Na+) 409.1.
15 Example 13: 2-Cyclopentyl-N-(6-oxo-2-phenyl-5 6-dihydro-1H-[1 2]diazepino[4
5 6-cdlindol-8-yl)-
acetamide
HN-N
O
00
N I / N
H H
Preparation of example 13 from the title compound of Example 7 (15 mg, 0.054
mmol),
cyclopentyl-acetic acid (8 mg, 0.062 mmol), triethylamine (0.030 mL,
0.22,mmol), and O-(7-
20 azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (25
mg, 0.065 mmol) in
dimethylformamide (1.0 mL) was carried out analogously to Example 11. Silica
gel
chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by diethyl
ether trituration afforded the title compound (3 mg, 0.008 mmol) as a yellow
powder in 14% yield.
' H-NMR (d6-DMSO): S 12.03 (s, 1 H), 10.32 (s, 1 H), 9.99 (s, 1 H), 8.17 (s, 1
H), 7.67-7.49 (m, 7H),
25 2.33-2.26 (m, 3H), 1.78 (m, 2H), 1.65-1.53 (m, 4H), 1.22 (m, 2H).
LCMS: (M+H+) 387.2, (M+Na+) 409.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
86
Example 14: 2-Methyl-N-(6-oxo-2-phenyl-5.6-dihydro-1 H-[1 21diazepinof4 5 6-
cggindol-8-y,-
nicotinamide
HN-N
O
O ~ -
H
~ N
IN
H
Preparation of example 14 from the title compound of Example 7 (15 mg, 0.054
mmol), 2-methyl-
nicotinic acid (9 mg, 0.062 mmol), triethylamine (0.030 mL, 0.22 mmol), and O-
(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (25 mg,
0.065 mmol) in
N,N-dimethylformamide (1.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (ethyl acetate), also in an analogous manner, followed by
diethyl ether trituration
afforded the title compound (7 mg, 0.018 mmol) as a yellow powder in 33%
yield.
1H-NMR (ds-DMSO): S 12.13 (s, 1 H), 10.57 (s, 1 H), 10.36 (s, 1 H), 8.57 (m, 1
H), 8.25 (s, 1 H), 7.90
(m, 1H), 7.84 (m, 1H), 7.68 (m, 2H), 7.61-7.50 (m, 4H), 7.34 (m, 1H), 2.60 (s,
3H).
LCMS: (M+H+) 396.2, (M+Na+) 418.1.
Example 15: 4,4,4-Trifluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1H-[1,2)diazepino[4
5 6-cdjindol-8-yl)-
butyramide
HN-N
O ~
O ~
F3C^~N I / N
H H
Preparation of example 15 from the title compound of Example 7 (15 mg, 0.054
mmol), 4,4,4-
trifluorobutyric acid (9 mg, 0.062 mmol), triethylamine (0.030 mL, 0.22 mmol),
and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (25 mg,
0.065 mmol) in
N,N-dimethylformamide (1.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by diethyl
ether trituration afforded the title compound (7 mg, 0.017 mmol) as a yellow
powder in 32% yield.
~ H-NMR (d6-DMSO): 8 12.09 (s, 1 H), 10.34 (s, 1 H), 10.22 (s, 1 H), 8.14 (s,
1 H), 7.68-7.50 (m, 7H),
2.62 (m, 4H).
LCMS: (M+H+) 401.1, (M+Na+) 423Ø
Example 16: 4-Methyl-f1.2,31thiadiazole-5-carboxylic acid(6-oxo-2-phenyl-5.6-
dihydro-lH-
[1,21diazepino[4.5,6-cdlindol-8-yl)-amide
HN-N
O
O
N
_`ST H H
NN
%
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
87
Preparation of example 16 from the title compound of Example 7 (15 mg, 0.054
mmol), 4-
methyl-[1,2,3]thiadiazole-5-carboxylic acid (8 mg, 0.062 mmol), triethylamine
(0.030 mL, 0.22
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium
hexafluorophosphate (25
mg, 0.065 mmol) in N,N-dimethylformamide (1.0 mL) was carried out analogously
to Example 11.
Additional 4-methyl-[1,2,3]thiadiazole-5-carboxylic acid (1.0 mg, 0.006 mmol)
and O-(7-
azabenzotriazol-l-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (2.0 mg,
0.006 mmol)
were added after c.a. 18 hours to drive the reaction to completion. Silica gel
chromatography, also
in an analogous manner, followed by diethyl ether trituration afforded the
title compound (7 mg,
0.017 mmol) as a yellow powder in 32% yield.
'H-NMR (d6-DMSO): S 12.21 (s, 1 H), 10.85 (s, 1 H), 10.41 (s, 1 H), 8.18 (s, 1
H), 7.81 (m, 1 H), 7.69
(m, 2H), 7.61-7.51 (m, 4H), 2.85 (s, 3H).
LCMS: (M+H+) 403.1, (M+Na+) 425Ø
Example 17: N-(6-Oxo-2-phenyl-5 6-dihydro-1H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-2-phenyl-
propionamide
HN-N
O
/ O
~ ~ N N
H H
Preparation of example 17 from the title compound of Example 7 (15 mg, 0.054
mmol), 2-phenyl-
propionic acid (9 mg, 0.062 mmol), triethylamine (0.030 mL, 0.22 mmol), and O-
(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (25 mg,
0.065 mmol) in
N,N-dimethylformamide (1.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by diethyl
ether trituration afforded the title compound (7 mg, 0.017 mmol) as a yellow
powder in 32% yield.
' H-NMR (d6-DMSO): S 12.08 (s, 1 H), 10.33 (s, 1 H), 10.21 (s, 1 H), 8.16 (s,
1 H), 7.64 (m, 3H), 7.59
(m, 2H), 7.55 (m, 2H), 7.43 (m, 2H), 7.37 (m, 2H), 7.32 (m, 1 H), 3.31
(quart., 1 H), 1.45 (d, 3H).
LCMS: (M+H+) 409.1, (M+Na+) 431.1.
Example 18: N-(6-Oxo-2-phenyl-5 6-dihydro-lH-f1 2]diazepino[4 5 6-cdlindol-8-
yl)-2-phenoxy-
acetamide
HN-N
O \
O ~
~N ~ / N
H H
Preparation of example 18 from the title compound of Example 7 (15 mg, 0.054
mmol), phenoxy-
acetic acid (9 mg, 0.062 mmol), triethylamine (0.030 mL, 0.22 mmol), and O-(7-
azabenzotriazol-1-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
88
yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (25 mg, 0.065 mmol) in N,N-
dimethylformamide (1.0 mL) was carried out analogously to Example 11. Silica
gel
chromatography (1:1 ethyl acetate:hexane increasing to 100% ethyl acetate),
also in an
analogous manner, followed by diethyl ether trituration afforded the title
compound (4.5 mg, 0.011
mmol) as a yellow powder in 20% yield.
1 H-NMR (d6-DMSO): 8 12.13 (s, 1 H), 10.36 (s, 1 H), 10.26 (s, 1 H), 8.16 (s,
1 H), 7.73 (m, 1 H), 7.66
(m, 2H), 7.57 (m, 2H), 7.51 (m, 2H), 7.31 (m, 2H), 7.03 (m, 3H), 4.71 (s.,
2H).
LCMS: (M+H+) 411.0, (M+Na+) 433.1.
Example 19: Methyl-f(6-oxo-2-phenyl-5,6-dihydro-1 H-f 1,2]diazepino[4,5,6-
cdjindol-8-ylcarbamoyl)-
methyll-carbamic acid tert-butyl ester
HN-N
O \
O
~N N
H H
Preparation of example 19 from the title compound of Example 7 (hydrochloride)
(18 mg, 0.065
mmol), (tert-butoxycarbonyl-methyl-amino)-acetic acid (12 mg, 0.065 mmol),
triethylamine (0.012
mL, 0.085 mmol), and O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (27 mg, 0.072 mmol) in CH2CI2 (0.5 mL) and N,N-
dimethylformamide (0.5
mL) was carried out analogously to Example 11. Silica gel chromatography
(10:9:1
hexane:CH2CI2: methanol), also in an analogous manner, followed by diethyl
ether trituration
afforded the title compound (28 mg, 0.063 mmol) as a yellow powder in 96%
yield.
' H NMR (CDCI3): S 9.11 (br s, 1 H), 8.38 (br s, 2H), 7.62-7.46 (m, 7 H), 7.43
(s, IH), 4.02 (s, 2H),
3.03 (s, 3H), 1.50 (s, 9H).
LCMS: (M+H+) 448.1, (M+Na+) 470, (M-H") 446.1.
Example 20: 2-Methylamino-N-(6-oxo-2-phenyl-5 6-dihydro-lH-
[1,2]diazepinof4,5,6-cdlindol-8-yl)-
methy]-acetamide; compound with trifluoro-acetic acid
HN-N HN-N
O \
~ TFA, CH2CI2, 98% TFA O \
N~NOI/ N j~N I/ N
H H H H
The title compound of Example 19 (19 mg, 0.042 mmol) in CH2CIZ (0.65 mL) was
treated with
trifluoroacetic acid (0.45 mL) and allowed to stir for 0.5 hours. The volatile
components were
removed under vacuum, and diethyl ether was added and evaporated three times
to give the title
compound (19 mg, 0.041 mmol) as a yellow, orange powder in 98% yield.
'H NMR (d4-methanol): 8.15 (s, partially exchanged), 7.68-7.52 (m, 8H), 4.00
(s, 2H), 2.80 (s,
3H).
LCMS: (M+H+) 348.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
89
Example 21: N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepinof4,5.6-cd]indol-8-
Ll)-butyramide
HN-N
O ~
N I / N
H H
Preparation of example 21 from the title compound of Example 7 (hydrochloride)
(22 mg, 0.080
mmol), n-butyric acid (0.007 mL, 0.080 mmol), triethylamine (0.014 mL, 0.10
mmol), and O-(7-
azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (32 mg,
0.084 mmol) in
CH2CI2 (0.3 mL) and N,N-dimethylformamide (0.3 mL) was carried out analogously
to Example
11. Silica gel chromatography (10:9:1 hexane:CH2CI2:methanol followed by 9:5:1
CH2CI2:hexane:methanol), also in an analogous manner, afforded the title
compound (14 mg,
0.04 mmol) as a yellow powder in 50% yield.
'H NMR (d6-DMSO): S, 12.05 (s, 1 H, exchanges), 10.22 (s, IH, exchanges),
10.00 (s, 1 H,
exchanges), 8.15 (s, 1 H), 7.39-7.71 (m, 7H), 2.30 (m, 2H)l, 1.67 (m, 2H)2,
0.95 (m, 3H)'. '
Becomes triplet upon DCI addition, 2 Becomes quartet upon DCI addition.
LCMS: (M+H+) 347.1, (M+Na+) 369.1.
Example 22: N-(6-Oxo-2-phenyl-5,6-dihydro-lH-[1,2]diazepino[4,5,6-cdlindol-8-
yl)-nicotinamide
HN-N
O
O
o N N H H
N
Preparation of example 22 from the title compound of Example 7 (hydrochloride)
(21 mg, 0.076
mmol), nicotinic acid (7 mg, 0.076 mmol), triethylamine (0.014 mL, 0.10 mmol),
and O-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (32 mg,
0.084 mmol) in
CH2CI2 (0.3 mL) and N,N-dimethylformamide (0.3 mL) was carried out analogously
to Example
11. Silica gel chromatography (9:1 CH2CI2:methanol), also in an analogous
manner, afforded the
title compound (20 mg, 0.052 mmol) as a yellow powder in 69% yield.
'H NMR (d6-DMSO): S, 12.18 (s, 1 H, exchanges), 10.59 (s, 1 H, exchanges),
10.38 (s, IH,
exchanges), 9.17(s, 1 H), 8.80 (s, IH, partially obscurred, with fine
splitting), 8.40-8.26 (m, 2H),
7.91 (s, 1 H), 7.73-7.65 (m, 2H), 7.62-7.47 (m, 5H).
'H NMR (d6-DMSO/DCI): S 9.44 (s, 1 H), 9.10 (d, 1 H, J = 5.0 Hz), 9.00 (d, 1
H, J = 9.5), 8.32 (s,
1 H), 8.15 (dd, 1 H, J= 7.0, 7.5 Hz ), 7.92 (s, 1 H), 7.73 (s, 1 H,
overlapping), 7.68 (s, 1 H,
overlapping), 7.64-7.50 (m, 4H).
LCMS: (M+H+) 382.1, (M+Na+) 404.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Example 23: 2-Methyl-N-(6-oxo-2-phen yl-5,6-dihydro-1H-[1,2Liazepino[4,5,6-
cd]indol-8-yl)-
benzamide
HN-N
0
N ~ ~
0 c N I
H H
Preparation of example 23 from the title compound of Example 7 (hydrochloride)
(22 mg, 0.08
5 mmol), 2-methyl-benzoic acid (11 mg, 0.08 mmol), triethylamine (0.014 mL,
0.10 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (33
mg, 0.088 mmol)
in CH2CI2 (0.3 mL) and N,N-dimethylformamide (0.3 mL) was carried out
analogously to Example
11. Silica gel chromatography (8:5:2 CH2CI2:hexane:methanol), also in an
analogous manner,
afforded the title compound (23 mg, 0.058 mmol) as a yellow powder in 73%
yield.
10 'H NMR (d6-DMSO): S 12.11 (s, 1H, exchanges), 10.42 (s, IH, exchanges),
10.34 (s, 1H,
exchanges), 8.24 (s, 1 H), 7.88 (s, 1 H), 7.71-7.65 (m, 2H), 7.63-7.45 (m,
3H), 7.43-7.35 (m, 2H),
7.34-7.25 (m, 3H), 2.4 (s, 3H).
LCMS: (M+H+) 395.1, (M+Na+) 417Ø
Example 24: N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1.21diazepino[4.5,6-cdJindol-8-
yl)-benzamide
HN-N
0
0
e
N N 15 Preparation of example 24 from the title compound of Example 7
(hydrochloride) (23 mg, 0.082
mmol), benzoic acid (11 mg, 0.09 mmol), triethylamine (0.016 mL, 0.115 mmol),
and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (34 mg,
0.090 mmol) in
CH2CI2 (0.3 mL) and N,N-dimethylformamide (0.3 mL) was carried out analogously
to Example
20 11. Silica gel chromatography (10:9:1 hexane:CH2CIZ:methanol followed by
8:5:2
CH2CI2:hexane:methanol), also in an analogous manner, afforded the title
compound (20 mg,
0.053 mmol) as a yellow powder in 64% yield.
'H NMR (d6-DMSO): S 12.13 (s, 1 H), 10.41 (s, 1 H), 10.35 (s, 1 H), 8.32 (s, 1
H), 8.03 (d, 2H, J= 8
Hz), 7.94 (s, 1 H), 7.70 (s, 1 H, overlapping), 7.67 (s, 1 H, overlapping),
7.63-7.48 (m, 7H).
25 LCMS: (M+H+) 381.1, (M+Na+) 403.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
91
Example 25: N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepinof4,5.6-cd]indol-8-
yl)-2-phenyl-
acetamide
HN-N
O
O
N
H H
Preparation of example 25 from the title compound of Example 7 (hydrochloride)
(20 mg, 0.072
mmol), phenyl-acetic acid (11 mg, 0.08 mmol), triethylamine (0.014 mL, 0.10
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (30 mg,
0.080 mmol) in
CH2CI2 (0.3 mL) and N,N-dimethylformamide (0.3 mL) was carried out analogously
to Example
11. Silica gel chromatography (8:5:2 CH2CI2:hexane:methanol), also in an
analogous manner,
afforded the title compound (27 mg, 0.068 mmol) as a yellow powder in 95%
yield.
' H NMR (d6-DMSO): 8 12.08 (s, 1 H, exchanges), 10.31 (s, 2H, exchanges), 8.18
(s, IH), 7.72-
7.62 (m, 3H), 7.60-7.45 (m, 4H), 7.41-7.21 (m, 5H), 3.68 (s, 2H).
LCMS: (M+H+) 395.1, (M+Na+) 417.1.
Example 26: N-(6-Oxo-2-phenyl-5,6-dihydro-1H-[1,2)diazepino[4,5,6-cel]indol-8-
yl)-2-piperazin-l-
yi-acetamide Di-trifluoroacetic acid Salt
HN-N
0 \
H N O ~ -
~ N I / N
H H
2X TFA
To a suspension of the title compound of Example 27 in dichloromethane (0.3 M,
0.6 mL), was
added trifluoroacetic acid (0.3 M, 0.6 mL). The reaction mixture was stirred
at 22 C for 0.5 hours
and concentrated under reduced pressure. The solid was triturated with
dichloromethane (3.0
mL), collected by filtration, and washed with diethyl ether (5.0 mL) to afford
the title compound
(9.8 mg) in 95% yield.
'H-NMR (d6-DMSO): S 12.09 (s, 1 H), 10.36 (s, 1 H), 9.98 (s, 1 H), 8.53 (bs,
2H), 8.16 (d, 1 H, J
1.6 Hz), 7.66 (m, 2H), 7.69-7.48 (m, 7H), 3.32 (s, 2H), 3.20 (m, 4H), 2.82 (m,
4H).
HRMS: (M+H+) calcd for C22H23N602, 403.1882, found 403.1902.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
92
Example 27: 4_[(6-Oxo-2-phenyl-5 6-dihydro-1H-[1.2Ldiazepinol4,5.6-cdlindol-8-
ylcarbamoyl)-
methXl]-piperazine-l-carboxylic acid tert-butyl ester
HN-N
~ O
~N 0 H H
Preparation of example 27 from the title compound of Example 7 (0.025g, 0.09
mmol), 4-
carboxymethyl-piperazine-l-carboxylic acid tert-butyl ester (0.044 g, 0.18
mmol), triethylamine
(0.05 mL, 0.4 mmol), and O-(7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium
hexafluorophosphate (38 mg, 0.1 mmol) and N,N-dimethylformamide (0.05 M, 1.8
mL) was
carried out analogously to Example 11. Silica gel chromatography (95:5
CH2CI2/methanol), also in
an analogous manner, afforded the title compound (15 mg) as a yellow powder in
33% yield.
'H-NMR (d6-DMSO): S 12.07 (s, 1 H), 10.34 (s, 1 H), 9.92 (s, 1 H), 8.17 (s, 1
H), 7.69-7.64 (m, 3H),
7.61-7.54 (m, 2H), 7.52-7.47 (m, 2H), 3.40 (m, 4H), 3.29 (m buried, 4H), 3.18
(s, 2H).
HRMS: (M+H+) calcd for C29H31N604, 503.2407, found 503.2407.
The starting material 4-Carboxymethyl-piperazine-1-carboxylic acid tert-butyl
ester was prepared
as follow:
~\ N~Et (Boc)20, Et3N f~ OEt 5% KOH, THF 30 ~~OH
HN ~ BocN N~ BocN
CH2CI2, rt, 94% \-j 0-rt, 37% \-j
27(a) 27(b)
Step 1. Preparation of 4-Methoxycarbonylmethyl-piperazine-l-carboxylic acid
tert-butyl
ester 27(a)
Piperazin-1-yl-acetic acid ethyl ester (2.84 g, 15.7 mmol), triethylamine (7.6
mL, 55.0 mmol) and
di-tert-butyl dicarbonate (4.45 g, 20.4 mmol) were stirred in CH2CI2 (0.3 M,
55 mL) at 22 C for 24
hours. The volatiles were removed in vacuo and silica gel chromatography
(60:40 ethyl
acetate/hexanes), afforded Intermediate 27(a) (4.01 g) in 94% yield.
1H-NMR (d6-DMSO): S 4.08 (q, 2H, J = 7.1 Hz), 3.35-3.21 (m, 6H), 2.52-2.41 (m,
4H), 1.39 (s,
9H), 1.18 (t, 7.1 Hz).
13C-NMR (d6-DMSO): 8 170.2, 154.2, 79.1, 60.2, 58.6, 52.0, 28.4, 14.5.
Step 2. Preparation of 4-Carboxymethyl-piperazine-l-carboxylic acid tert-butyl
ester 27(b)
Intermediate 27(a) (3.6 g, 13.2 mmol) and a 5% aqueous KOH solution (90 mL,
80.0 mmol) were
stirred in tetrahydrofuran (30 mL, 0.44 M) at 22 C for 2 hours. The volatiles
were removed in
vacuo and treatment with strongly acidic Dowex-50TM (WX8-200), elution with
ammonium
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
93
hydroxide (1.0 N), and treatment with AmberliteTM CG-50 afforded Intermediate
27(b) (1.2 g) in
37% yield.
'H-NMR (d6-DMSO): S 3.35-3.31 (m, 4H), 3.21 (s, 2H), 2.52-2.41 (m, 4H), 1.39
(s, 9H).
Example 28: 2-Cyclohexyl-N-(6-oxo-2-phenyl-5 6-dihydro-lH-[1 2]diazepinof4,5,6-
cdlindol-8-yl)-
acetamide
HN-N WN: N
H H
Preparation of example 28 from the title compound of Example 7 (0.025g, 0.09
mmol),
Cyclohexyl-acetic acid (0.015g, 0.11 mmol), triethylamine (0.05 mL, 0.4 mmol),
O-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (38 mg,
0.1 mmol) and
N,N-dimethylformamide (0.05 M, 1.8 mL) was carried out analogously to Example
11. Silica gel
chromatography (95:5 CH2CI2/methanol) also in an analogous manner followed by
diethyl ether
trituration afforded the title compound (13 mg) as a yellow powder in 36%
yield.
'H-NMR (ds-DMSO): S 12.03 (s, 1 H), 10.32 (s, 1 H), 9.99 (s, 1 H), 8.17 (s, 1
H), 7.68-7.45 (m-, 7H),
2.20 (d, 2H, J= 7.0 Hz), 1.86-1.58 (m, 6H), 1.31-1.10 (m, 3H), 1.10-0.91 (m,
2H).
HRMS: (M+H+) calcd for C24H25N402, 401.1978, found 401.1987.
Example 29: 4-(6-Oxo-2-phenY-5 6-dihydro-1H-[1 2]diazepino[4,5,6-cdlindol-8-
vlcarbamoyl)-
piperidine-l-carboxylic acid tert-butyl ester
HN-N
O
O
N N
H H
BocN
Preparation of example 29 from the title compound of Example 7 (hydrochloride)
(22 mg, 0.07
mmol), piperidine-1,4-dicarboxylic acid mono-tert-butyl ester (19 mg, 0.084
mmol), triethylamine
(0.015 mL, 0.105 mmol), and O-(7-azabenzotriazol-l-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (32 mg, 0.084 mmol) in CH2CI2 (0.3 rnL) and N,N-
dimethylformamide (0.3
mL) was carried out analogously to Example 11. Silica gel chromatography
(8:5:2
CH2CI2:hexane:methanol), also in an analogous manner, afforded the title
compound (24 mg,
0.049 mmol) as a yellow powder in 70% yield.
'H NMR (d6-DMSO): 12.09 (s, IH), 10.38 (s, 1 H), 10.12 (s, 1 H), 8.17 (s, 1
H), 7.70-7.64 (m, 3H),
7.62-7.54 (m, 2H), 7.53-7.49 (m, 2H), 4.01 (br d, 2H, J= 12.06 Hz), 2.86-2.72
(m, 2H), 1.81 (br d,
2H, J= 13.00 Hz), 1.58-1.44 (m, 2H), 1.42 (s, 9H), 1.40 (m, 1 H, partially
obscured).
LCMS: (M-H)" 486.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
94
Example 30: 4-[(6-Oxo-2-phenyl-5 6-dihydro-lH-[1 2]diazepinof4 5 6-cdlindol-8-
ylcarbamoyl)-
methyl]-piperidine-l-carboxylic acid tert-butyl ester
HN-N
O
BocNI '\/\/\
` N N
H H
Preparation of example 30 from the title compound of Example 7 (hydrochloride)
(22 mg, 0.07
mmol), 4-carboxymethyl-piperidine-l-carboxylic acid tert-butyl ester (20 mg,
0.084 mmol),
triethylamine (0.015 mL, 0.105 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium hexafluorophosphate (32 mg, 0.084 mmol) in CH2CI2 (0.3 mL)
and N,N-
dimethylformamide (0.3 mL) was carried out analogously to Example 11. Silica
gel
chromatography (8:5:2 CHZCIZ:hexane:methanol), also in an analogous manner,
afforded the title
compound (35 mg, 0.070 mmol) as a yellow powder in 100% yield.
'H NMR (d6-DMSO): 12.08 (s, 1 H), 10.38 (s, 1 H), 10.09 (s, IH), 8.19 (s, 1
H), 7.70-7.46 (m, 7H),
3.93 (br d, 2H, J = 12.24 Hz), 2.81-2.66 (m, 2H), 2.27 (d, 2H, J = 7.16 Hz),
1.96 (br s, 1 H), 1.67
(br d, 2H, J= 13.94 Hz), 1.40 (s, 9H), 1.17-1.01 (m, 2H).
LCMS: (M+Na+) 524.2, (M-H)- 500.1.
Example 31: Piperidine-4-carboxylic acid (6-oxo-2-phenyl-5.6-dihydro-1H-
[1.21diazepinof4.5.6-
cd]indol-8-yl)-amide; compound with trifluoro-acetic acid
HN-N
O ~
O
N \ I / N
TFA HN H H
Preparation of example 31 from the title compound of Example 29 (20 mg, 0.041
mmol) and 45%
TFA in CH2CI2 (1 mL) was carried out analogously to Example 20. Isolation,
also in an analogous
manner, afforded the title compound (20 mg, 0.040 mmol) as a yellow powder in
97% yield.
'H NMR (d4-methanol): 8.03 (s, IH), 7.56-7.31 (m, 7H), 3.43-3.32 (m, 2H), 3.01-
2.90 (m, 2H),
2.61 (m, 1 H), 2.04 -1.78 (m, 4H).
LCMS: (M+H+) 388.1
Example 32: N-(6-Oxo-2-phenyl-5.6-dihydro-1 H-f 1.2]diazepino[4.5.6-cd]indol-8-
~rl)-2-pigeridin-4-yl-
acetamide: compound with trifluoro-acetic acid
HN-N
O
TFA HNI'\~ O \
` ,/III`N I / N ~ /
H H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
Preparation of example 32 from the title compound of Example 30 (31 mg, 0.062
mmol) and
45% TFA in CH2CI2 (1 mL) was carried out analogously to Example 20. Isolation,
also in an
analogous manner, afforded the title compound (29 mg, 0.056 mmol) as a yellow
powder in 91%
yield.
5 'H NMR (d4-methanol): S 8.20 (s, 1H), 7.70-7.50 (m, 7H), 3.50-3.40 (m, 2H,
partially obscurred),
3.10 (dd, 2H, J = 9.0, 9.2 Hz), 2.45 (d, 2H, J = 7.2 Hz), 2.25 (br m, 1 H),
2.0-2.11 (m, 2H), 1.68-
1.45 (m, 2H).
LCMS: (M+H+) 402.2
ExamQle 33: tert-butyl (1 S)-l-cyclohex rl-2-oxo-2-[(6-oxo-2-phenLl-5,6-
dihydro-1 H-
10 [1.2]diazepino[4,5,6-cd]indol-8-yl)amino]ethylcarbamate
HN-N
O 0rL&k1
BocHN Preparation of example 33 from the title compound of Example 7 (35 mg,
0.13 mmol), (2S)-[(tert-
butoxycarbonyl)amino](cyclohexyl)ethanoic acid (39 mg, 0.15 mmol),
triethylamine (0.073 mL,
15 0.52 mmol), and O-(7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium
hexafiuorophosphate
(58 mg, 0.15 mmol) in N,N-dimethylformamide (2.0 mL) was carried out
analogously to Example
11 except that after 24 hours additional (2S)-[(tert-
butoxycarbonyl)amino](cyclohexyl)ethanoic acid
(17 mg, 0.065 mmol) and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (25 mg, 0.065 mmol) were added to drive the reaction to
completion. Silica
20 gel chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by diethyl
ether trituration'afforded the title compound (45 mg, 0.087 mmol) as a yellow
powder in 67% yield.
'H-NMR (d6-DMSO): 8 12.08 (s, 1 H), 10.38 (s, 1 H), 10.13 (s, 1 H), 8.14 (s, 1
H), 7.71-7.44 (m, 6H),
6.90 (m, 1 H), 3.95 (m, 1 H), 1.78-1.44 (m, 5H), 1.43-1.24 (m, 10H, contains
singlet at 1.39), 1.23-
1.02 (m, 5H).
25 LCMS: (M+H)- 514.1.
Example 34: (2S)-2-amino-2-cyclohexyl-N-(6-oxo-2-phenyl-5.6-dihydro-1 H-
[1,2]diazepino(4,5,6-
cdlindol-8- r~i ethanamide trifluoroacetate
HN-N
O
O ~ -
N N
TFA NH2 H H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
96
Preparation of example 34 from the title compound of Example 33 (40 mg, 0.078
mmol), and 1:1
TFA/CH2CI2 (5 mL) was carried out analogously to Example 20. Isolation, also
in an analogous
manner, included a further trituration with methanol/diethyl ether and
afforded the title compound
(10 mg, 0.019 mmol) as an orange/yellow powder in 24% yield.
' H NMR (d6-DMSO): S 12.19 (s, 1 H), 10.60 (s, 1 H), 10.44 (s, 1 H), 8.25 (br
s, 3H), 8.11 (s, 1 H),
7.71-7.46 (m, 7H), 3.69 (m, 1 H), 1.93-1.52 (m, 6H), 1.29 -0.97 (m, 5H).
LCMS: (M+H+) 415.1.
Example 35: 3-Fluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2)diazepino[4,5,6-
cdJindol-8-yl)-
benzamide
HN-N
O
O
N N
H H
F
Preparation of example 35 from the title compound of Example 7 (0.025g, 0.09
mmol), 3-Fluoro-
benzoic acid (0.015g, 0.11 mmol), triethylamine (0.05 mL, 0.4 mmol), O-(7-
azabenzotriazol-1-yl)-
N,N,N;N'tetramethyluronium hexafluorophosphate (38 mg, 0.1 mmol) and N,N-
dimethylformamide (0.05 M, 1.8 mL) was carried out analogously to Example 11.
Silica gel
chromatography (95:5 CH2CI2/methanol), also in an analogous manner, afforded
the title
compound (15 mg) as a yellow powder in 42% yield.
' H-NMR (d6-DMSO): S 12.18 (s, 1 H), 10.47 (s, 1 H), 10.40 (s, 1 H), 8.31 (d,
1 H, J= 1.3 Hz), 7.92
(d, 1 H, J= 1.5 Hz), 7.90-7.81 (m, 2H), 7.72-7.41 (m, 8H).
HRMS: (M+H+) calcd for C23H16NAF, 399.1257, found 399.1257.
Example 36: N-(6-Oxo-2-phenyl-5,6-dihydro-1H-[1,21diazepino[4,5,6-cdJindol-8-
yl)-4-phenyl-
butyramide
HN-N
O cLJX}i-ci
Preparation of example 36 from the title compound of Example 7 (0.030g, 0.11
mmol), 4-Phenyl-
butyric acid (0.027g, 0.16 mmol), triethylamine (0.077 mL, 0.44 mmol), O-(7-
azabenzotriazol-1-yl)-
N,N,N;N=tetramethyluronium hexafluorophosphate (63 mg, 0.16 mmol) and N,N-
dimethylformamide (0.05 M, 2.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (95:5 CH2CI2/methanol) afforded the title compound (15 mg) as a
yellow powder
in 33% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
97
' H-NMR (ds-DMSO): S 12.06 (s, 1 H), 10.35 (s, 1 H), 10.06 (s, 1 H), 8.18 (d,
1 H, J= 1.5 Hz), 7.69-
7.46 (m, 7H), 7.33-7.16 (m, 5H), 2.64 (dd, 2H, J= 7.4, 7.0 Hz), 2.35 (m, 5H),
1.97-1.86 (m, 2H).
HRMS: (M+H+) calcd for C26H23N402, 423.1821, found 423.1826.
Anal. Calcd. for C26H22N402 = 0.2 H20: C, 73.29; H, 5.30; N, 13.15. Found: C,
73.33; H, 5.28; N,
13.23.
Example 37: 1-Methyl-piperidine-4-carboxylic acid (6-oxo-2-phenyl-5 6-dihydro
1H
11,21diazepinof4,5,6-cdlindol-8-yl)-amide: 2,2,2-trifluoro-acetic acid salt
HN-N
O ~
O ~ ~ -
N I / N
N H H
~ TFA salt
Preparation of example 37 from the title compound of Example 7 (0.030g, 0.11
mmol), 1-Methyl-
piperidine-4-carboxylic acid (0.023g, 0.16 mmol), triethylamine (0.077 mL,
0.44 mmol), O-(7-
azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (63 mg,
0.16 mmol) and
N,N-dimethylformamide (0.05 M, 2.0 mL) was carried out analogously to Example
11. HPLC
purification afforded the title compound (23 mg) as a yellow powder in 41 %
yield.
'H-NMR (d6-DMSO): S 12.10 (s, 1 H), 10.39 (s, 1 H), 10.23 (s, 1 H), 9.17 (bs,
1 H), 8.12 (d, 1 H, J=
1.5 Hz), 7.69-7.47 (m, 7H), 3.05-2.93 (m, 2H), 2.80 (d, 3H), 2.65-2.48 (buried
m, 1 H), 2.10-2.01
(m, 2H), 1.92-1.80 (m, 2H).
HRMS: (M+H+) calcd for CZ3H24N5O2, 402.1930, found 402.1937.
Example 38: N-(6-Oxo-2-phenyl-5 6-dihydro-1H-[1 21diazepino[4 5 6-cdlindol-8-
yl)-3-phenyl-
propionamide
HN-N
O ~
O ~
H I / N
H
Preparation of example 38 from the title compound of Example 7 (0.030g, 0.11
mmol), 3-Phenyl-
propionic acid (0.025g, 0.16 mmol), triethylamine (0.077 mL, 0.44 mmol), O-(7-
azabenzotriazol-1-
yl)-N,N,N;M tetramethyluronium hexafluorophosphate (63 mg, 0.16 mmol) and N,IV
dimethylformamide (0.05 M, 2.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (95:5 CH2CI2/methanol) afforded the title compound (15 mg) as a
yellow powder
in 34% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
98
'H-NMR (d6-DMSO): 5 12.09 (s, 1 H), 10.38 (s, 1 H), 10.36 (s, 1 H), 10.13 (s,
1 H), 8.15 (d, 1 H, J=
1.5 Hz), 7.68-7.46 (m, 11 H), 7.31 (d, 2H, J= 8.4 Hz), 7.24 (d, 1 H, J= 8.2
Hz), 3.65 (s, 2H), 3.45
(s, 2H).
HRMS: (M+H+) calcd for C25H2IN402, 409.1665, found 409.1683.
Example 39: 6-Oxo-2-phenyl-5,6-dihydro-lH-f1,21diazepino[4,5,6-cd]indole-8-
carboxylic acid
methyl ester
HO 0 HO 0 MeO 0
\ Br HNO3, H2SO4 Br H2SO4, MeOH Br
01. HO I/ <15 0C, 87% HO I NO reflux, 70% 30 MeO I NO
2 2
O O O
39(a) 39(b)
Me0 CO
SnCl2i H20
(C6H5CN)ZPdCl2, Cui MeO NO2 MeOH, reflux, 98%
t-Bu3P, DIPA,
Dioxane, rt, 53% 39(c)
Me0 O Me0 O
~O
I\ ~ - POCI3. DMF, CH2CI2 I\ ~ -
Me0 N 71% Me0 N
O H O H
39(d) 39(e)
HN-N
O \
NH2NH2.H2O, AcOH \ ~ -
MeOH, reflux, 72% Me0 I/ H
O
Step 1. Preparation of 4-bromo-5-nitroisophthalic acid 39(a)
To a 3-L 4-neck round-bottomed flask equipped with a mechanical stirrer,
thermometer, 250-mL
addition funnel and charged with concentrated H2SO4 (577 mL), was added 4-
bromoisophthalic
acid (75.0 g, 306 mmol). The white suspension was cooled to ice-bath
temperature and a nitrating
reagent (pre-prepared by the careful addition of H2SO4 (169 mL) to HNO3 (107
mL)) was slowly
added while maintaining an internal reaction temperature of less than 15 C.
When addition was
complete, the ice-bath was removed and the suspension stirred overnight at
room temperature.
After approximately 14 hours, the flask was placed in an ice-bath and crushed
ice was added to
quench the excess nitrating reagent while maintaining an internal temperature
less than 40 C.
The cream-colored suspension was filtered, and the solids were collected by
filtration and washed
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
99
with a small volume of ice-cold water. The solids were dissolved in methanol
and the volatile
components were evaporated. The resulting solids were dried at 60 C in a
vacuum oven
overnight to afford Intermediate 39(a) (79.6 g) in 87% yield.
'H NMR (d4-methanol): 5 8.47 (d, I H, J = 1.8 Hz), 8.39 (d, 1 H, J = 1.8 Hz).
LCMS: (M-H) 289.
Step 2. Preparation of Dimethyl 4-bromo-5-nitroisophthalate 39(b)
To a solution of Intermediate 39(a) (77.6 g, 267 mmol)' in methanol (500 mL)
was added
concentrated H2SO4 (10 mL). The reaction flask was heated in an oil bath at
reflux with stirring for
approximately 8 hours at which point LC/MS analysis indicated consumption of
starting material.
The mixture was allowed to cool to room temperature and white solids began to
crystallize in the
flask. After sitting overnight, the crystals were collected by filtration and
washed with water until
the pH of the filtrate was neutral. The crystals (51.9 g) were dried in an
Abderhalden drying
apparatus over refluxing acetone. The mother liquor was concentrated to
provide a second crop
(7.58 g) which was combined with the first to afford Intermediate 39(b) (59.4
g) in 70% yield.
'H NMR (d6-DMSO): 5 8.62 (s, 1 H), 8.45 (s, 1 H), 3.95 (s, 3H), 3.93 (s, 3H).
Step 3. Preparation of Dimethyl 5-nitro-4-(phenylethynyl)isophthalate 39(c)
A flask containing 1,4-dioxane (16 mL) was purged with nitrogen. Cul (0.12 g,
0.628 mmol) and
bis(benzonitrile)dichloropalladium(II) (0.361 g, 0.942 mmol) were added in
portions. Tri-tert-
butylphosphine (7.83 mL, 1.88 mmol) was added as a 0.24 M solution in 1,4-
dioxane via syringe.
The solution was allowed to stir for approximately 5 min at which point
diisopropylamine (2.65 mL,
18.9 mmol) was added via syringe. Intermediate 39(b) (5.00 g, 15.7 mmol) was
added in one solid
portion followed by phenylacetylene (2.08 mL, 18.9 mmol). A precipitate formed
immediately. The
reaction was capped and stirred at room temperature overnight. The mixture was
filtered through
diatomaceous earth which was subsequently washed with ethyl acetate to recover
any trapped
product. The combined filtrate and washings were concentrated and dissolved in
a minimal
amount of hot ethyl acetate. Hexane was added to recrystallize brown needles
which were
collected by filtration and further washed with hexane. The needles (4.5 g)
were dried in a vacuum
oven at 60 C overnight. The mother liquor was concentrated and subjected to
silica gel
chromatography (10-30% ethyl acetate/hexanes) to yield an additional 1.2 g of
a dark brown solid
which was combined with the first batch to afford Intermediate 39(c) (5.7 g)
in 53% yield.
'H NMR (CDCI3): S 8.73 (d, 1 H, J= 1.5 Hz), 8.65 (d, 1 H, J= 1.8 Hz), 7.61-
7.64 (m, 2H), 7.37-7.46
(m, 3H), 4.03 (s, 3H), 4.00 (s, 3H).
LCMS: (M+H) 340.
Step 4. Preparation of Dimethyl 2-phenyl-1H-indole-4,6-dicarboxylate 39(d)
To Intermediate 39(c) (6.37 g, 18.8 mmol) was added anhydrous methanol (120
mL). To the
resulting slurry was added Tin(II) chloride (35.6 g, 188 mmol), and the
reaction flask was refluxed
for 55 hours. Methanol was removed under reduced pressure and the resulting
residue was
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
100
suspended in a small volume of ethyl acetate. Dichloromethane was then added
such that the
resulting concentration was approximately 95% dichloromethane : 5% ethyl
acetate. This
suspension was then filtered through a short silica gel plug. The filtrate was
concentrated and
purified twice by flash silica gel chromatography (5-40% ethyl
acetate/dichloromethane) to obtain
3.1 g of Intermediate 39(d) in 53% yield.
'H NMR (ds-DMSO): S 12.35 (s, 1 H), 8.37 (s, 1 H), 8.28 (s, 1 H), 7.98-8.00
(m, 2H), 7.47-7.60 (m,
4H), 3.99 (s, 3H), 3.93 (s, 3H).
LCMS: (M+H) 310.
Step S. Preparation of Dimethyl 3-formyl-2-phenyl-IH-indole-4,6-dicarboxylate
39(e)
To a solution of Intermediate 39(d) (0.052 g, 0.17 mmol) in anhydrous N,N-
dimethylformamide (2
mL) at room temperature was added POCI3 (0.2 mL, 2.1 mmol). The resulting
violet solution was
stirred for 1 hour at room temperature. The mixture was poured into saturated
aqueous sodium
carbonate (15 mL) and extracted with ethyl acetate. The organic layer was
dried over sodium
sulfate, concentrated, and purified by silica gel chromatography (33% ethyl
acetate/hexanes) to
give Intermediate 39(e) (0.0404 g) in 71% yield.
'H NMR (d6-DMSO): S 13.00 (s, 1 H), 9.95 (s, 1 H), 8.21 (s, 1 H), 7.94 (s, 1
H), 7.85-7.75(m, 2H),
7.70-7.55 (m, 3H), 3.90 (s, 3H), 3.86 (s, 3H).
LCMS: (M+H) 338, (M-H) 336.
Step 6. Preparation of Title Compound: 6-Oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cdJindole-8-carboxylic acid methyl ester
To a solution of Intermediate 39(e) (1.39 g, 4.12 mmol) in anhydrous methanol
(70 mL) was
added anhydrous hydrazine (0.19 mL, 6.18 mmol). The mixture was refluxed
overnight. After
cooling, the yellow precipitate was collected by filtration and washed with
ice-cold methanol. After
drying under vacuum at 60 C overnight, the title compound (1.06 g) was
obtained as a bright
yellow solid in 72% yield.
'H-NMR (d6-DMSO): S 12.58 (s, 1 H), 10.54 (s, 1 H), 8.13 (d, 1 H, J = 1.3 Hz),
8.07 (d, 1 H, J = 1.3
Hz), 7.77-7.67(m, 2H), 7.65-7.54 (m, 3H), 7.52 (s, 1 H), 3.88 (s, 3H).
LCMS:, (M+H+) 320.3
Example 40: 3-Fluoro-2-methyl-N-(6-oxo-2-phenyl-5.6-dihydro-1 H-
[1,2]diazepinof4,5,6-cdlindol-8-
r~l -benzamide
HN-N
0
0 ~ ~ -
N
H
H
F
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
101
Preparation of example 40 from the title compound of Example 7 (0.030g, 0.11
mmol), 3-Fluoro-
2-methyl-benzoic acid (0.025g, 0.16 mmol), triethylamine (0.077 mL, 0.44
mmol), O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (63 mg,
0.16 mmol) and
N,N-dimethylformamide (0.05 M, 2.0 mL) was carried out analogously to Example
11. Silica gel
chromatography (95:5 CH2CI2/methanol) afforded the title compound (14 mg) as a
yellow powder
in 33% yield.
'H-NMR (d6-DMSO): S 12.15 (s, 1 H), 10.55 (s, 1 H), 10.39 (s, 1 H), 8.23 (d, 1
H, J= 1.5 Hz), 7.84
(d, 1 H, J= 1.4 Hz), 7.72-7.48 (m, 6H), 7.39-7.27 (m, 3H), 2.31 (s, 3H).
LCMS: (M-H+) 411Ø
Example 41: 2-Fluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4.5.6-
cdlindol-8-yl)-3-
trifluoromethyl-benzamide
HN-N
O
0 \ -
N
H H
F
F3
Preparation of example 41 from the title compound of Example 7 (0.1 g, 0.36
mmol), 2-Fluoro-3-
trifluoromethyl-benzoic acid (0.113 g, 0.54 mmol), triethylamine (0.2 mL, 1.45
mmol), O-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.207 g,
0.54 mmol)
and N,N-dimethylformamide (0.1 M, 3.6 mL) was carried out analogously to
Example 11. Silica gel
chromatography (95:5 CH2CI2/methanol) afforded the title compound (0.147 g) as
a yellow powder
in 87% yield.
' H-NMR (d6-DMSO): 6 12.20 (s, 1 H), 10.81 (s, 1 H), 10.43 (s, 1 H), 8.24 (d,
1 H, J= 1.5 Hz), 8.05-
7.93 (m, 2H), 7.79 (d, 1 H, J= 1.4 Hz), 7.72-7.67 (m, 2H), 7.62-7.49 (m, 5H).
HRMS: (M+H+) calcd for C24H15N402 F4, 467.1131, found 467.1119.
Anal. Calcd. for C24HI4N402F4 = 0.2 H20: C, 61.33; H, 3.09; N, 11.92; F,
16.17. Found: C, 61.17;
H, 3.09; N, 11.93; F, 16.65.
Example 42: N-(6-Oxo-2-phenyl-5,6-dihydro-1H-[1.2]diazepino[4,5.6-cd]indol-8-
yl)-2-
trifluoromethyl-benzamide
HN-N
0
0
N N H H
eCF3
Preparation of example 42 from the title compound of Example 7 (0.1 g, 0.36
mmol), 2-
Trifluoromethyl-benzoic acid (0.103g, 0.54 mmol), triethylamine (0.2 mL, 1.45
mmol), O-(7-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
102
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.207 g,
0.54 mmol)
and N,N-dimethylformamide (0.1 M, 3.6 mL) was carried out analogously to
Example 11. Silica gel
chromatography (95:5 CH2CI2/methanol) afforded the title compound (0.075 g) as
a yellow powder
in 46% yield.
'H-NMR (d6-DMSO): 5 12.14 (s, 1 H), 10.71 (s, 1 H), 10.40 (s, 1 H), 8.17 (d, 1
H, J= 1.4 Hz), 7.89-
7.66 (m, 7H), 7.62-7.49 (m, 4H).
HRMS: (M+H+) calcd for C24H16N402 F3, 449.1225, found 449.1223.
Example 43: N-(6-Oxo-2-phenyl-5.6-dihydro-lH-f 1 2]diazepinof4 5 6-cdlindol-8-
yl)-3-
trifluoromethyl-benzam ide
HN-N
O
O \ -
H N
H
F3
Preparation of example 43 from the title compound of Example 7 (0.03 g, 0.11
mmol), 3-
Trifluoromethyl-benzoic acid (0.031 g, 0.16 mmol), triethylamine (0.078 mL,
0.43 mmol), O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.062 g,
0.11 mmol)
and N,N-dimethylformamide (0.05 M, 2.0 mL) was carried out analogously to
Example 11. Silica
gel chromatography (95:5 CH2CI2/methanol) afforded the title compound (0.030
g) as a yellow
powder in 61 lo yield.
'H-NMR (d6-DMSO): S 12.20 (s, 1 H), 10.62 (s, 1 H), 10.42 (s, 1 H), 8.39-8.30
(m, 3H), 7.98 (d, 1 H,
J = 7.4 Hz), 7.91 (d, 1 H, J = 1.6 Hz), 7.80 (dd, 1 H, J = 7.7, 7.5 Hz), 7.69
(d, 2H, J = 7.2 Hz), 7.62-
7.48 (m, 4H).
HRMS: (M+H+) calcd for C24H16N402 F3, 449.1225, found 449.1229.
Example 44: 3-Chloro-N-(6-oxo-2=phenyl-5.6-dihydro-lH-[1.21diazepinof4.5 6-
cof]indol-8-yl)-
benzamide
HN-N
O
O
N N
H H
Preparation of example 44 from the title compound of Example 7 (0.03 g, 0.11
mmol), 3-
Chloromethyl-benzoic acid (0.025 g, 0.16 mmol), triethylamine (0.078 mL, 0.43
mmol), O-(7-
azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (0.062 g,
0.11 mmol)
and N,N-dimethylformamide (0.05 M, 2.0 mL) was carried out analogously to
Example 11. Silica
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
103
gel chromatography (95:5 CH2CI2/methanol) afforded the title compound (0.015
g) as a yellow
powder in 33% yield.
'H-NMR (d6-DMSO): S 12.18 (s, 1 H), 10.50 (s, 1 H), 10.41 (s, 1 H), 8.32 (d, 1
H, J= 1.5 Hz), 8.09
(s, 1 H), 7.98 (d, 1 H, J= 7.8 Hz), 7.91 (d, 1 H, J= 1.6 Hz), 7.72-7.65 (m,
3H), 7.62-7.47 (m, 5H).
HRMS: (M+H+) calcd for C23H16N4O20, 415.0962, found 415.0981.
Example 45:
(1 R.2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5 6-dihydro-1 H-
[1,2]diazepino[4,5,6-cdlindol-8-yl)-amide: and
(1S.2S)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-ghenyl-5 6-dihydro-1H-
[1,21diazepinof4,5.6-cdjindol-8-yl)-amide
HN-N HN-N
aN O / O \ \ I ,.~N H H H H H
Preparation of example 45 from the title compound of Example 7 (30 mg, 0.11
mmol), trans-2-
phenyl-l-cyclopropanecarboxylic acid (26 mg, 0.16 mmol), triethylamine (0.076
mL, 0.55 mmol),
and O-(7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate
(62 mg, 0.16
mmol) in N,N-dimethylformamide (1.0 mL) was carried out analogously to Example
11. Silica gel
chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by trituration
with ethyl acetate / diethyl ether afforded the title compound (6.5 mg, 0.015
mmol) (mixture of
trans diastereomers) as a yellow powder in 14% yield.
' H-NMR (d6-DMSO): S 12.09 (s, 1 H), 10.44 (s, 1 H), 10.37 (s, 1 H), 8.17 (s,
1 H), 7.71-7.44 (m, 7H),
7.35-7.25 (m, 2H), 7.24-7.10 (m, 3H), 2.39 (m, 1 H), 2.10 (m, 1 H), 1.52 (m, 1
H), 1.38 (m, 1 H).
LCMS: (M+H)- 419.1.
Example 46: 2-(3-Chlorophenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,21diazepino[4,5,6-cdlindol-
8-yl)acetamide
HN-N
/ 0
CI \ I N
WN
H H
Preparation of example 46 from the title compound of Example 7 (30 mg, 0.11
mmol), (3-
chlorophenyl)acetic acid (28 mg, 0.16 mmol), triethylamine (0.076 mL, 0.55
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (62 mg,
0.16 mmol) in
N,N-dimethylformamide (1.5 mL) was carried out analogously to Example 11.
Silica gel
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
104
chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by methanol
trituration afforded the title compound (6.5 mg, 0.015 mmol) as a yellow
powder in 14% yield.
1H-NMR (d6-DMSO): 5 12.10 (s, IH), 10.39 (s, IH), 10.17 (s, 1 H), 8.16 (s, 1
H), 7.70 -7.18 (m,
11 H), 3.69 (s, 1 H), 3.50 (s, 1 H).
LCMS: (M+H)" 427.1.
Example 47: N-(6-Oxo-2-phenyl-5.6-dihydro-1 H-[1,2]diazepinof4.5,6-cd]indol-8-
yl)-4-thien-2-
ylbutanamide
HN-N -
/ ~ ~
H
H
Preparation of example 47 from the title compound of Example 7 (30 mg, 0.11
mmol), 4-thien-2-
ylbutanoic acid (24 mg, 0.16 mmol), triethylamine (0.076 mL, 0.55 mmol), and O-
(7-
azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (62 mg,
0.16 mmol) in
N,N-dimethylformamide (1.5 mL) was carried out analogously to Example 11.
Silica gel
chromatography (3% methanol in CH2CI2 increasing to 6% methanol in CH2CI2),
also in an
analogous manner, followed by methanol trituration afforded the title compound
(6.5 mg, 0.015
mmol) as a yellow powder in 14% yield.
1H-NMR (d6-DMSO): 8 12.07 (s, IH), 10.38 (s, 1 H), 10.09 (s, IH), 8.18 (s, 1
H), 7.69-7.61 (m, 3H),
7.60-7.53 (m, 2H), 7.52-7.45 (m, 2H), 7.33 (m, 1 H), 6.96 (m, 1 H), 6.89 (m, 1
H), 2.86 (t, 2H, J
7.53 Hz), 2.40 (t, 2H, J = 7.35 Hz), 2.02 -1.89 (m, 2H).
LCMS: (M+H)" 427.1.
Example 48: 1-Acetyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-f 1.21diazepino[4.5,6-
cd]indol-8-
yl)piperidine-4-carboxamide
HN-N
0
N WN
"'Y N H H
O
Preparation of example 48 from the title compound of Example 7 (30 mg, 0.11
mmol), 1-
acetylpiperidine-4-carboxylic acid (28 mg, 0.16 mmol), triethylamine (0.076
mL, 0.55 mmol), and
O-(7-azabenzotriazol-l-yl)-N,N,N;N'-tetramethyluronium hexafluorophosphate (62
mg, 0.16
mmol) in N,N-dimethylformamide (1.5 mL) was carried out analogously to Example
11. Silica gel
chromatography (3% methanol in CH2CI2 increasing to 6% methanol in CH2CIZ),
also in an
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
105
analogous manner, followed by methanol trituration afforded the title compound
(12 mg, 0.028
mmol) as a yellow powder in 25% yield.
'H-NMR (d6-DMSO): S 12.07 (s, 1 H), 10.37 (s, 1 H), 10.10, s, 1 H), 8.17 (s, 1
H), 7.72-7.43 (m, 7H),
4.41 (d, 1 H, J = 15.07 Hz), 3.87 (d, 1 H, J = 14.13 Hz), 3.07 (t, 1 H, J =
12.81 Hz), 2.65-2.53 (m,
2H, partially obscured), 2.02 (s, 3H), 1.76-1.90 (m, 2H), 1.70-1.37 (m, 2H).
LCMS: (M+H+) 430.1, (M+Na+) 452.1.
Example 49: 6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepinof4,5,6-cdlindole-8-
carboxylic acid,
potassium salt
O HN-N
\ ~ -
+K"O I / N
H
O
To the title compound of Example 39 (3.0 g, 9.39 mmol) was added DMSO (20 mL).
To this semi-
suspension was added 2N KOH (19 mL, 37.8 mmol). The solution turned a deep red
color and
was stirred for approximately 1.5 hours at room temperature. The reaction was
purified by
preparative HPLC (5-60% CH3CN/H2O) over 60 minutes. Fractions containing the
product were
lyophilized to afford the title compound (0.8 g) as an orange solid in 25%
yield.
' H-NMR (d6-DMSO): S 12.21 (s, 1 H), 10.12 (s, 1 H), 8.16 (d, 1 H, J= 1.0 Hz),
8.01 (d, IH, J= 1.0
Hz), 7.73-7.65 (m, 2H), 7.62-7.46 (m, 3H), 7.44 (s, 1 H).
LCMS: (M+H+) 306.1
Example 50: 3-(2-Methylphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cdjindol-
8-yl)propanamide
HN-N
p \ ~ -
H
H
Preparation of example 50 from the title compound of Example 7 (30 mg, 0.11
mmol), 3-(2-
methylphenyl)propanoic acid (27 mg, 0.16 mmol), triethylamine (0.076 mL, 0.55
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (62 mg,
0.16 mmol) in
N,N-dimethylformamide (1.5 mL) was carried out analogously to Example 11.
Silica gel
chromatography (3% methanol in CH2CI2 increasing to 5% methanol in CH2CIA also
in an
analogous manner, followed by methanol trituration afforded the title compound
(6 mg, 0.014
mmol) as a yellow powder in 13% yield.
'H-NMR (d6-DMSO): S 12.08 (s, 1 H), 10.37 (s, 1 H), 10.10 (s, 1 H), 8.20 (s, 1
H), 7.72-7.42 (m, 7H),
7.26-7.05 (m, 4H), 2.96-2.87 (m, 2H), 2.64-2.55 (m, 2H, partially obscured),
2.32 (s, 3H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
106
LCMS: (M+H)" 421.1.
Example 51: N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-3-(1-trityl-1 H-
imidazol-4-yl)-propionamide
HN-N
O
O \ \ -
N~N I / H ~ ~
N H
/ I
\
Preparation of example 51 from the title compound of Example 7 (hydrochloride)
(31 mg, 0.10
mmol), 3-(1-Trityl-1H-imidazol-4-yl)-propionic acid (46 mg, 0.12 mmol),
triethylamine (0.021 mL,
0.15 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate
(46 mg, 0.12 mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was
carried out
analogously to Example 11. Silica gel chromatography (3% triethylamine in
5:4:1
hexane:CH2CI2:methanol), also in an analogous manner, afforded the title
compound (40 mg,
0.062 mmol) as a yellow powder in 62% yield.
'H NMR (CDCI3/d4-methanol): S 8.21 (s, 1H), 7.65-7.25 (m, 15H, partially
obscurred), 7.10-7.02
(m, 8H), 6.65 (s, 1H), 2.99-2.89 (m, 2H), 2.71-2.62 (m, 2H).
LCMS: (M+H+) 641.2.
Example 52: 3-(1 H-Imidazol-4-yl)-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-ccllindol-
8-,rl)-propionamide; compound with trifluoro-acetic acid
HN-N
O ~
O \ -
^~\/~ N ( / N
TFA HN~ N H H
~
Preparation of example 52 from the title compound of Example 51 (32 mg, 0.050
mmol) and 45%
TFA in CH2CI2 (1 mL) was carried out analogously to Example 20. (Deprotection
of trityl group
was effected using the same conditions as for tert-butoxycarbonyl
deprotection.) Isolation, also in
an analogous manner, additionally included recrystallization from
methanol/ethyl acetate and
diethyl ether to afford the title compound (16 mg, 0.031 mmol) as a yellow
powder in 62% yield.
'H NMR (d6-DMSO): 13.95 (br s, IH), 12.10 (s, 1 H), 10.40 (s, IH), 10.20 (s,
IH), 8.83 (s, 1 H),
8.13 (s, 1 H), 7.70-7.63 (m, 3H), 7.62-7.47 (m, 5H), 7.38 (s, 1 H), 3.02 -2.93
(m, 2H), 2.78-2.70 (m,
2H).
LCMS: (M+H+) 399.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
107
Example 53: f(S)-1-(6-Oxo-2-phenyl-5 6-dihydro-lH-[1 2jdiazepino~4 5 6-
cdlindol-8-ylcarbamoLrl)-
3-phenyi-propyll-carbamic acid tert-butyl ester
HN-N
NHBo~ H
Preparation of example 53 from the title compound of Example 7 (hydrochloride)
(31 mg, 0.10
mmol), (S)-2-tert-Butoxycarbonylamino-4-phenyl-butyric acid (34 mg, 0.12
mmol), triethylamine
(0.021 mL, 0.15 mmol), and O-(7-azabenzotriazol-l-yl)-
N,N,N;N=tetramethyluronium
hexafluorophosphate (46 mg, 0.12 mmol) in CH2CI2 (0.4 mL) and N,N-
dimethylformamide (0.4
mL) was carried out analogously to Example 11. Silica gel chromatography,
(5:4:1
hexane:CH2CI2:methanol), also in an analogous manner, afforded the title
compound (40 mg,
0.074 mmol) as a yellow powder in 74% yield.
' H NMR (d6-DMSO): 12.11 (s, 1 H), 10.40 (s, 1 H), 10.17 (s, 1 H), 8.40 (br s,
IH), 8.16 (s, 1 H),
7.71-7.45 (m, 6H), 7.35-7.15 (m, 6H), 4.06 (m, 1H), 2.77-2.53 (m, 2H,
partially obscured), 1.92
(m, 2H), 1.42 (s, 9 H).
LCMS: (M+H+) 538.1, (M+Na+) 560.2, (M-H)' 536Ø
Example 54: (2S)-2-Amino-N-(6-oxo-2-phenyl-5.6-dihydro-lH-[1,2]diazepino('4 5
6-cd]indol-8-yl)-4-
phenyl-butyramide: compound with trifluoro-acetic acid
HN-N
TFA NH2 H H
Preparation of example 54 from the title compound of Example 53 (30 mg, 0.056
mmol) and 45%
TFA in CH2CI2 (1 mL) was carried out analogously to Example 20. Isolation,
also in an analogous
manner, afforded the title compound (26 mg, 0.047 mmol) as a yellow powder in
84% yield.
'H NMR (d6-DMSO): 8 12.22 (s, 1 H), 10.71 (s, 1 H), 10.47 (s, 1 H), 8.38 (br
s, 3H), 8.13 (s, 1 H),
7.72-7.66 (m, 2H), 7.64-7.49 (m, 4H), 7.36-7.28 (m, 2H), 7.27-7.19 (m, 4H),
4.05 (br s, 1 H), 2.77-
2.65 (m, 2H), 2.21-2.09 (m, 2H).
LCMS: (M+H+) 438.2, (M+Na+) 460.1, (M-H)" 436.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
108
Example 55: N-Methyl-6-oxo-2-phenyl-5,6-dihydro-1H_[1 2ldiazepinof4 5 6-
cd]indole-8-
carboxamide
HN-N
O
N h
0 H
To a solution of the title compound of Example 49 (0.058 g, 0.17 mmol) in DMSO
(4 mL) was
added triethylamine (0.069 mL, 0.5 mmol), and O-(7-azabenzotriazol-1-yl)-
N,N,N;N'
tetramethyluronium hexafluorophosphate (0.084 g, 0.22 mmol). After
approximately 5 min, 2.0 M
methylamine in tetrahydrofuran (0.5 mL, 0.25 mmol) was added and the solution
was stirred for 2
hours. The mixture was purified using preparative HPLC (20-100% CH3CN/H20
containing 0.1%
HOAc). The pure fractions were combined and lyophilized to afford the title
compound (0.0036 g)
in 6.7% yield.
1 H-NMR (d6-DMSO): S 12.46 (s, 1 H), 10.45 (s, 1 H), 8.58 (s, 1 H), 8.20-
7.90(m, 2H), 7.85-7.35 (m,
6H), 2.79 (s, 3H).
LCMS: (M+H+) 319.1.
Example 56: N-[2-(1 H-Imidazol-4-yl)ethyl]-6-oxo-2-phenyl-5.6-dihydro-lH-
[1,2]diazepino[4.5,6-
cdlindole-8-carboxamide
HN-N
0 HN~N N
~-N O H
Preparation of example 56 from the title compound of Example 49 (0.0956 g,
0.28 mmol), 2-(1H-
imidazol-4-yl)ethylamine dihydrochloride (0.0618 g, 0.34 mmol), triethylamine
(0.2 mL, 1.4 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate
(0.129 g, 0.34
mmol) in DMSO (6 mL) was carried out analogously to Example 58. Preparative
HPLC (10-80%
CH3CN/H20), also in an analogous manner, afforded the title compound (0.0021
g) in 18% yield.
'H-NMR (d6-DMSO): S 12.48 (s, 1 H), 10.49 (s, 1 H), 8.77 (t, 1 H, J= 5.7 Hz),
8.08 (s, 1 H), 8.01 (s,
1 H), 7.75-7.66(m, 2H), 7.64-7.54 (m, 5H), 7.51 (s, 1 H), 6.85 (s, 1 H), 3.55-
3.45 (m, 2H), 2.84-2.73
(t, 2H, J = 7.2 Hz).
LCMS: (M+H+) 399.1
Anal. Calcd. for C22H1eN602 = 0.60 HCI = 0.75 H20: C, 60.91; H, 4.67; N,
19.37. Found: C, 60.74;
H, 4.74; N, 19.48.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
109
Example 57: 4-Dimethylamino-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepinof4,5,6-cd]indol-8-
yl)-butyramide (hydrochloric salt)
HN-N
O \
O ~
/~~N I / N
HCI H H
Preparation of example 57 from the title compound of Example 7 (hydrochloride)
(27 mg, 0.086
mmol), 4-dimethylamino-butyric acid(hydrochloride)(17 mg, 0.104 mmol),
triethylamine (0.036 mL,
0.258 mmol), and O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate
(40 mg, 0.104 mmol) in CH2CI2 (0.3 mL) and N,N-dimethylformamide (0.3 mL) was
carried out
analogously to Example 11. Silica gel chromatography (2% triethylamine in 9:1
CH2CI2: methanol)
of the freebase afforded, after acidification with HCI, the title compound (18
mg, 0.042 mmol) as a
yellow powder in 49% yield.
1 H NMR (d6-DMSO): S 12.10 (s, 1 H), 10.39 (s, 1 H), 10.21 (s, 1 H), 9.60 (br
s, 1 H, HCI), 8.13 (s,
1 H), 7.69-7.46 (m, 7H), 3.15-3.04 (m, 2H), 2.80 (s, 6H, with fine splitting),
2.45 (t, 2H, partially
obscurred), 2.02-1.90 (m, 2H).
LCMS: (M+H+) 390.2.
Example 58: 6-Oxo-2-phenyl-N-(2-pyridin-2-ylethyI)-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cdlindole-
8-carboxamide
O HN-N 0,NH2 HN-N
N H ~ ~ -
+KO I / \ N
H HATU, Et3N, DMSO, 20% H
N
I )Nf
To a solution of the title compound of Example 49 (0.0548 g, 0.16 mmol) in
DMSO (1.8 mL) was
added triethylamine (0.0268 mL, 0.192 mmol), and O-(7-azabenzotriazol-1-yl)-
N,N,N;N=
tetramethyluronium hexafluorophosphate (0.0669 g, 0.176 mmol). After
approximately 5 min, 2-
pyridin-2-ylethylamine (0.0215 g, 0.176 mmol) was added and the solution was
allowed to stir
overnight. The product was purified using preparative HPLC (5-35% CH3CN/H20).
The pure
fractions were combined and lyophilized to afford the title compound (0.013 g)
as a yellow powder
in 20% yield.
' H NMR (d6-DMSO): S 10.37 (s, 1 H), 8.74 (s, 1 H), 8.51 (d, 1 H, J = 3.0 Hz),
8.01 (d, 2H, J = 11
Hz), 7.49-7.71 (m, 7H), 7.20-7.29 (m, 2H), 3.62 (d, 1 H, J = 4.9 Hz), 3.02 (t,
1 H, J = 6.4 Hz).
LCMS: (M+H) 410.
Example 59: tert-Butyl (1R)-1-cyclohexyl-2-oxo-2-[(6-oxo-2-phenyl-5.6-dihvdro-
lH-
[1,21diazer)ino[4,5,6-cdlindol-8-yl)amino]ethylcarbamate
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
110
HN-N
O
WN:: N
NHBou H
Preparation of example 59 from the title compound of Example 7 (70 mg, 0.25
mmol), (2R)-[(tert-
butoxycarbonyl)amino](cyclohexyl)ethanoic acid (98 mg, 0.38 mmol),
triethylamine (0.139 mL, 1.0
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (144
mg, 0.38 mmol) in N,N-dimethylformamide (2.0 mL) was carried out analogously
to Example 11
except that after 24 hours additional (2R)-[(tert-
butoxycarbonyl)amino](cyclohexyl)ethanoic acid
(32 mg, 0.13 mmol) and O-(7-azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium
hexafluorophosphate (48 mg, 0.13 mmol) were added to drive the reaction to
completion. Silica
gel chromatography (1:1 ethyl acetate:hexane), also in an analogous manner,
followed by diethyl
ether trituration afforded the title compound (90 mg, 0.17 mmol) as a yellow
powder in 70% yield.
' H-NMR (d6-DMSO): S 12.09 (s, 1 H), 11.43 (s, 1H), 10.39 (s, 1 H), 10.14 (s,
1 H), 8.16 (s, 1 H),
7.70-7.64 (m, 3H), 7.61-7.54 (m, 2H), 7.53-7.46 (m, 2H), 3.96 (m, 1H), 1.79-
1.49 (m, 6H), 1.41-
1.29 (m, 14H, contains singlet at 1.38).
LCMS: (M+H)" 514.2.
Exampie 60: (2R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-ghenyl-5 6-dihydro-lH-[1
2]diazepinof4 5 6-
cd]indol-8-yl)ethanamide trifluoroacetate
HN-N
/ N
OlYiN::
TFA NH2 H H
Preparation of example 60 from the title compound of Example 59 (90 mg, 0.17
mmol), and 1:1
TFA/CH2CI2 (5 mL) was carried out analogously to Example 20. Isolation, also
in an analogous
manner, included a further trituration with methanol/diethyl ether and
afforded the title compound
(70 mg, 0.013 mmol) as an orange/yellow powder in 78% yield.
1 H-NMR (d6-DMSO): 5 12.22 (s, 1 H), 10.63 (s, 1 H), 10.47 (s, 1 H), 8.22 (br
s, 2H), 8.14 (s, 1 H),
7.72-7.66 (m, 3H), 7.64-7.49 (m, 4H), 3.72 (m, 1H), 1.91-1.59 (m, 6H), 1.29-
1.14 (m, 5H, partially
obscured by diethyl ether).
LCMS: (M+H+) 416.2, (M+Na+) 438.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
111
Example 61: (1R,2R)-2-Phenyl-cyclopropanecarboxvlic acid (6-oxo-2-phenyl-5,6-
dihydro-1H-
[1 2]diazepino[4,5,6-cdLndol-8-yl)-amide
HN-N
O
0
,.kN N
H H
Preparation of example 61 from the title compound of Example 7 (198 mg, 0.72
mmol), (1 R,2R)-
2-phenylcyclopropanecarboxylic acid (175 mg, 1.08 mmol; as described by A.
Thurkauf, et al., J.
Med. Chem. 43, 3923-3932, (2000)), triethylamine (0.401 mL, 2.88 mmol), and O-
(7-
azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (411 mg,
1.08 mmol) in
N,N-dimethylformamide (10.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (3% methanol in CH2CI2), also in an analogous manner, followed
by collection on
a frit and subsequent washing with diethyl ether afforded the title compound
(86 mg, 0.20 mmol)
as a yellow powder in 28% yield.
1H-NMR (d6-DMSO): S 12.09 (s, 1 H), 10.44 (s, 1 H), 10.37 (s, 1 H), 8.16 (d, 1
H, J= 1.6 Hz), 7.69-
7.63 (m, 3H), 7.60-7.46 (m, 4H), 7.34-7.17 (m, 5H), 2.44-2.35 (m, 1 H), 2.13-
2.05 (m, 1 H), 1.55-
1.46 (m, 1 H), 1.42-1.34 (m, 1 H).
LCMS: (M+H+) 421.1.
Alternative Method for the Preparation of Example 61
MeO O 1 MeO 0
POCI3. DMF
~ \ - HATU, Et3N, DMF, 86% / I p \ ~ - ~
H ~~ / I O \ .o=~N H CH2CI2, 98%
H2N
=~H
61(a)
HN-N
~
MeO 0 WN
/ OJ~NH2NH2, AoOH, MeOHO ~ I `P N N / MeOH, reflux, 88% J `N H H H H H
61(b)
Step 1. Preparation of 2-Phenyl-6-[((1R,2R)-2-phenyl-cyclopropanecarbonyl)-
amino]-1H-
indole-4-carboxylic acid methyl ester 61(a)
Preparation from Intermediate 7(a) (3.83 g, 12.64 mmol), (1 R,2R)-2-phenyl-
cyclopropanecarboxylic acid (2.27 mg, 13.97 mmol), triethylamine (3.52 mL,
25.28 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;Mtetramethyluronium hexafluorophosphate (5.31g,
13.97 mmol)
in CH2CI2 (6.5 mL) and N,N-dimethylformamide (6.5 mL) was carried out
analogously to Example
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
112
11. Extractive work-up from ethyl acetate and saturated aqueous NaHCO3
afforded the crude
product as an oil in N,N-dimethylformamide. Diethyl ether (c.a. 500 mL) and
CH2CI2 (80 mL) were
added and the mixture was capped and stirred vigorously overnight whereupon
the oil was
converted to greenish tan solids which were collected by filtration and washed
with diethyl ether,
methanol, and water. The filtrate was evaporated to an oil and water was added
to precipitate a
second batch of solids which was also collected by filtration and also washed
with methanol and
water. Both batches of precipitated and washed solids were combined to give
4.18 g of desired
product. In addition, the final fitrate was evaporated and subjected to silica
gel chromatography
eluting with 1:1 hexane:acetone to give a small amount of additional product
(0.275 mg).The
combined batches afforded Intermediate 61(a) (4.46 g, 10.86 mmol) as a tan
solid in 86% yield.
Step 2. Preparation of 3-formyl-2-phenyl-6-[((1 R,2R)-2-phenyl-
cyclopropanecarbonyl)-
amino]-1H-indole-4-carboxylic acid methyl ester 61(b)
Intermediate 61(a) (4.02 g, 9.79 mmol) was dissolved in CH2CI2 (80 mL) and
treated with
Vilsmeier reagent (2.94 mL) in a manner similar to that described in step 4 of
Example 3. After
c.a. 10 min stirring at ice bath temperature followed by 10 min at room
tempeature, the volume of
CH2CI2 was reduced under vacuum. With ice bath cooling and stirring, Na2CO3
(c.a. 1.2 g) in H20
(10 mL) was added as a paste to the reaction. Additional H20 (20 mL) was then
added to the still
cool reaction. Methanol (c.a. 100 mL) was added and the reaction was allowed
to warm to room
temperature, still stirring, whereup the slow evolution of gas was observed.
After 4 hours, the
volume of the mixture was reduced under vacuum, and CH2CI2 was added to adjust
the solvent
composition to approximately 4:1 CH2CIZ:methanol. Precipitated Na2CO3 was then
removed by
filtration, and the volume of filtrate was reduced under vacuum leaving mostly
methanol as the
solvent. Ethyl acetate was added and mixture was then dried (Na2SO4) and
filtered and
evaporated to give yellow solids. Silica gel chromatography (eluting with 4:1
hexane:acetone
increasing to 1:1 hexane:acetone) afforded Intermediate 61(b) (4.2 g, 9.64
mmol) as a yellow
powder in c.a. 98% yield.
Step 3. Preparation of Title Compound: (IR,2R)-2-Phenyl-cyclopropanecarboxylic
acid (6-
oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide
Intermediate 61(b) (4.1 g, 9.41 mmol) acetic acid (0.78 mL, 13.63 mmol) and
H2NNHZ ' H20 (1.37
mL, 28.23 mmol) in anhydrous methanol (100 mL) were refluxed in manner similar
to that
described for Example 3, step 5. The resulting precipitate was collected by
filtration and washed
with a minimum amount of methanol affording the title compound (3.5 g, 8.28
mmol) as a yellow
powder in 88% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
113
Example 62: (1 S,2S)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-phenyl-5,6-
dihydro-1 H-
[1.2]diazepino[4.5,6-cd]indol-8-yl)-amide
HN-N
O
~N
WN
H H
Preparation of example 62 from the title compound of Example 7 (79 mg, 0.28
mmol), (1S,2S)-2-
phenylcyclopropanecarboxylic acid (60 mg, 0.37 mmol; as described by A.
Thurkauf, et al., J.
Med. Chem. 43, 3923-3932, (2000)), triethylamine (0.160 mL, 1.14 mmol), and O-
(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (141 mg,
0.37 mmol) in
N,N-dimethylformamide (10.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (5% methanol in CH2CI2), also in an analogous manner, followed
by trituration
with methanol afforded the title compound (26 mg, 0.06 mmol) as a yellow
powder in 22% yield.
' H-NMR (d6-DMSO): S 12.10 (s, 1 H), 10.44 (s, 1 H), 10.37 (s, 1 H), 8.17 (s,
1 H), 7.72-7.43 (m, 7H),
7.35-7.26 (m, 2H), 7.24-7.17 (m, 3H), 2.40 (m, 1 H), 2.10 (m, 1 H), 1.52 (m, 1
H), 1.38 (m, 1 H).
LCMS: (M+H+) 421.3, (M+Na+) 423.2.
Example 63: 6-Oxo-2-phenyl-N-[2-(1 H-tetraazol-5-yl)ethyl]-5,6-dihydro-1 H-
[1,2]diazepinof4,5,6-
cdlindole-8-carboxamide
HN-N
O \
H I \ ~ -
N~N N
NNNH O H
Preparation of example 63 from the title compound of Example 49 (0.0989 g,
0.288 mmol), 2-(1H-
tetraazol-5-yl)ethylamine (0.072 g, 0.317 mmol), triethylamine (0.0883 mL,
0.634 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (0.121
g, 0.317
mmol) in DMSO (2 mL) was carried out analogously to Example 58. Preparative
HPLC (25-80%
CH3CN/H20), also in an analogous manner, afforded the title compound (0.002 g)
in 1.7% yield.
' H NMR (d6-DMSO): S 12.50 (s, 1 H), 10.49 (s, 1 H), 8.83 (t, 1 H, J = 4.9
Hz), 8.05 (s, 1 H), 7.98 (s,
1 H), 7.70 (d, 2H, J = 7.6 Hz), 7.51-7.62 (m, 4H), 3.64 (q, 2H, J = 6.1 Hz),
3.14 (t, 2H, J = 6.8 Hz).
HRMS calculated for CZOH17Na02 401.1474 (M+H), found 401.1476.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
114
Example 64: N-[2-(4-Morpholinyl)eth yl]-6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepinof4,5,6-
cd]indole-8-carboxamide
HN-N
O
N~iN N
OJ O H
Preparation of example 64 from the title compound of Example 49 (0.0991 g,
0.289 mmol), 2-
morpholin-4-ylethylamine (0.041 g, 0.317 mmol), triethylamine (0.0483 mL,
0.347 mmol), and 0-
(7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (0.121
g, 0.317
mmol) in DMSO (2 mL) was carried out analogously to Example 58. Preparative
HPLC (10-80%
CH3CN/H20), also in an analogous manner, afforded the title compound (0.018 g)
in 15% yield.
'H NMR (d6-DMSO): S 12.48 (s, 1 H), 10.49 (s, 1 H), 8.62 (t, 1 H, J= 5.3 Hz),
8.07 (s, 1 H), 8.00 (s,
1 H), 7.70 (d, 2H, J = 6.8 Hz), 7.51-7.62 (m, 4H), 3.57 (t, 4H, J = 4.3 Hz),
3.37-3.43 (m, 2H), 2.41
(br s, 6H).
HRMS calculated for C23H24N503 418.1879 (M+H), found 418.1858.
Example 65: 6-Oxo-N-[3-(2-oxo-l-pyrrolidinyl)propyl]-2-phenyl-5,6-dihydro-1 H-
[1.2]diazepino[4,5.6-cd]indole-8-carboxamide
HN-N
O
N
0 0 H
Preparation of example 65 from the title compound of Example 49 (0.109 g,
0.319 mmol), 1-(3-
aminopropyl)pyrrolidin-2-one (0.05 g, 0.351 mmol), triethylamine (0.0534 mL,
0.383 mmol), and
0-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(0.133 g, 0.351
mmol) in DMSO (2 mL) was carried out analogously to Example 58. Preparative
HPLC (10-80%
CH3CN/H2O), also in an analogous manner, afforded the title compound (0.035 g)
in 26% yield.
' H NMR (d6-DMSO): S 12.54 (s, IH), 10.51 (s, IH), 8.66 (s, IH), 8.08 (s, 1
H), 8.01 (s, 1 H), 7.70
(d, 2H, J = 7.2 Hz), 7.53-7.62 (m, 4H), 3.23-3.37 (m, 6H), 2.21 (t, 2H, J =
7.9 Hz), 1.89-1.96 (m,
2H), 1.72 (t, 2H, J= 6.8 Hz).
HRMS calculated for C24H24N503 430.1879 (M+H), found 430.1899.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
115
Example 66: 2-Ethylsulfanyl-N-(6-oxo-2-phenyl-5 6-dihydro-1H-[1 2]diazepino[4
5 6-cdlindol-8-
yI)-nicotinamide
HN-N
O \
~~S 0 \ -
N N I / N ~ ~
H H
Preparation of example 66 from the title compound of Example 7 (hydrochloride)
(30 mg, 0.096
mmol), 2-ethylsulfanyl-nicotinic acid (22 mg, 0.12 mmol), triethylamine (0.020
mL, 0.14 mmol),
and O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(46 mg, 0.12
mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was carried out
analogously to
Example 11. Silica gel chromatography (pre-rinsed silica with 3% triethylamine
in ethyl acetate,
eluted with 3% triethylamine in 19:6:1 ethyl acetate:hexane:methanol), also in
an analogous
manner, afforded the title compound (7 mg, 0.016 mmol) as a yellow powder in
17% yield.
' H NMR (d6-DMSO): S 12.15 (s, 1 H), 10.60 (s, 1 H), 10.39 (s, 1 H), 8.59 (s,
1 H, with fine splitting),
8.22 (s, 1 H), 7.95 (m,1 H), 7.82 (s, 1 H), 7.72-7.48 (m, 6H), 7.25 (m,1 H),
3.21-3.10 (m, 2H, partially
obscured), 1.35-1.23 (m, 3H).
LCMS: (M+H+) 442.3, (M+Na+) 464.1.
Example 67:
(1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5.6-dihydro-1 H41
2]diazepinof4 5 6-
cd]indol-8-yl)-amide; and
(1 S,2S)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H41
2]diazepino[4 5 6-
cd]indol-8-yl)-amide
HN-N HN-N
0 O
\ / I k
WN
I \ \ \ ,o N / N N H H VIIH H
Preparation of example 67 from the title compound of Example 2 (freebase) (21
mg, 0.105 mmol),
+ trans-2-phenyl-1-cyclopropanecarboxylic acid (20 mg, 0.126 mmol),
triethylamine (0.044 mL,
0.315 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N' tetramethyluronium
hexafluorophosphate
(48 mg, 0.126 mmol) in CH2CI2 (0.3 mL) and N,N-dimethyiformamide (0.3 mL) was
carried out
analogously to Example 11. Silica gel chromatography (5:4:1 hexane:CH2CI2:
methanol followed
by 9:1 CH2CI2:methanol), also in an analogous manner, afforded the title
compound (20 mg, 0.058
mmol) (mixture of trans diastereomers) as a yellow powder in 55% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
116
' H NMR (d6-DMSO): S 11.72 (s, 1 H), 10.38 (s, 1 H), 10.25 (s, 1 H), 8.13 (s,
1 H), 7.56 (s, 1 H,
overlapping), 7.55 (s, 1 H, overlapping), 7.46 (s, 1 H), 7.37-7.13 (m, 5H),
2.39 (m, 1 H), 2.10 (m,
1 H), 1.51 (m, 1 H), 1.39 (m, I H).
LCMS: (M+H+) 345.4, (M+Na+) 367.3.
Example 68: 8-(3-Chloro-benzvlamino)-2-phenyl-1 5-dihvdro-[1 2]diazepino[4 5 6-
cd]indol-6-one
HN-N
WN \ N
H H
To the title compound of Example 7 (hydrochloride) (40 mg, 0.13 mmol), and
triethylamine (0.053
mL, 0.38 mmol) in N,N-dimethylformamide (0.9 mL) was added 1-bromomethyl-3-
chloro-benzene
(0.17 mL, 0.13 mmol) dropwise with stirring. After stirring overnight, the
mixture was
chromatographed on silica eluting with 2:1 hexane:ethyl acetate to afford,
after isolation, the title
compound (36 mg, 0.090 mmol) as a yellow powder in 71 % yield.
'H NMR (d6-DMSO): S 11.59 (s, 1 H, exchanges), 10.22 (s, 1 H, exchanges), 7.60-
7.27 (m, IOH),
7.08 (s, 1 H), 6.60 (t, 1 H, J = 4.9 Hz, exchanges), 6.45 (s, 1 H), 4.36 (m,
2H).
LCMS: (M+H+) 401.3, 403.3.
Anal. Calcd. for C23HI7N40C1 ' 0.3 H20: C, 67.99; H, 4.37; N, 13.79.
Found: C, 67.98; H, 4.35; N, 13.58.
Example 69: 8-fBis-(3-chloro-benzyl)-aminol-2-phenyl-1 5-dihydro-f1
2]diazepinof4 5 6-cd]indol-6-
one
HN-N
CI
WN
~ N
H
The title compound (7 mg, 0.013 mmol) was isolated as a yellow powder in 10%
yield as a by-
product from the synthesis outlined in Example 68.
'H NMR (d4-methanol): S 7.51-7.31 (m, 6H), 7.25-7.10 (m, 9H), 6.64 (s, 1H),
4.64 (s, 4H).
LCMS: (M+H+) 525.3.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
117
Example 70: N-f2-(Dimethyiamino)ethyl]-6-oxo-2-phenyl-5 6-dihydro-lH-[1
2ldiazepino(4 5 6-
cd]indole-8-carboxamide trifluoroacetate
HN-N
O O
F3C-I-OH H \ ~ -
\N,,N N
O H
Preparation of example 70 from the title compound of Example 49 (0.08 g, 0.23
mmol), N,N-
dimethylethane-1,2-diamine (0.0247 g, 0.28 mmol), triethylamine (0.17 mL, 1.2
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.106 g,
0.28 mmol) in
DMSO (6 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid) also in an analogous manner
afforded the title
compound (0.0076 g) in 6.8% yield.
' H NMR (d6-DMSO): S 12.53 (s, IH), 10.54 (s, 1 H), 9.30 (s, IH), 8.84 (t, 1
H, J= 5.5 Hz), 8.12 (s,
1 H), 8.04 (s, 1 H), 7.75-7.67(m, 2H), 7.66-7.53 (m, 3H), 7.52 (s, 1 H), 3.62
(m, 2H), 3.39 (m, 2H),
2.86 (s, 6H).
LCMS: (M+H+) 376.1
HRMS: C21H21N502 = H: 376.1774. Found: 376.1785.
Example 71a: (S)-2-Dimethylamino-N-(6-oxo-2-phenyl-5 6-dihydro-1H-[1
2]diazepino[4 5 6-
cdlindol-8-yl)-3-phenyl-propionamide (hydrochloric salt)
HN-N
0 0
\ -
N N
H H
N,' H-CI
Preparation from the title compound of Example 7 (hydrochloride) (40 mg, 0.128
mmol), (S)-2-
Dimethylamino-3-phenyl-propionic acid (30 mg, 0.154 mmol), triethylamine
(0.071 mL, 0.512
mmol), and O-(7-azabenzotriazol-1-yl)-N,NN;M tetramethyluronium
hexafluorophosphate (59
mg, 0.154 mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was
carried out
analogously to Example 11. Silica gel chromatography (elute with 1.5%
triethylamine in CH2CIZ
followed by 1.5% triethylamine in 9:1 CH2CI2:methanol), also in an analogous
manner, afforded
the freebase of the title compound (46 mg, 0.10 mmol) in three separate
batches for a combined
80% yield. Two of the batches were carried on to Examples 71 b and 71c
respectively. To the third
batch (8 mg, 0.018 mmol) was added tetrahydrofuran and 1.2 equivalents of HCI
in dioxane (from
a 4M stock solution). After evaporation, trituration with diethyl ether and
1:1 CH2CI2:hexane gave
the title compound (6 mg, 0.012 mmol) as a yellow powder in 67% yield for the
salt formation.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
118
'H NMR (ds-DMSO): S 12.25 (s, IH, exchanges), 10.76 (br s, 2H, exchanges),
10.42 (s, 1 H,
exchanges), 7.95 (s, 1 H), 7.72-7.45 (m, 7H), 7.35-7.16 (m,5H), 4.28 (m, 1 H),
3.05 -2.90 (m, 6H).
LCMS (freebase): (M+H+) 452.3, (M+Na+) 474.3.
Example 71 b: (S)-2-Dimethvlamino-N-(6-oxo-2-phenyl-5 6-dihydro-lH-[1
2ldiazepino[4 5 6
cAindol-8-yl)-3-phenyl-propionamide
HN-N
O ~
O ~
Cr*' N I / N N,, H
H
One batch of freebase (S)-2-Dimethylamino-N-(6-oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-3-phenyl-propionamide (13 mg, 0.029 mmol)
from Example 71a
was triturated with 1:1 diethyl ether:hexane to give the purified title
compound (12 mg, 0.027
mmol) as a yellow powder in 93% yield for the trituration.
Example 71c: (S)-2-Dimethylamino-N-(6-oxo-2-phenyl-5 6-dihydro-1H-[1
2]diazepino[4 5 6-
cd]indol-8-vl)-3-phenvi-propionamiden compound with (2R 3R)-2 3-dihydroxy-
succinic acid
O O HN-N
HO-~'H O
HO \~~OH O N ~/ N
~
/ N~ H H
~
One batch of freebase (S)-2-Dimethylamino-N-(6-oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-3-phenyl-propionamide (22 mg, 0.049 mmol)
from Example 71a
was triturated with 1:1 diethyl ether:hexane giving purified material (20 mg,
0.044 mmol). A portion
(10 mg, 0.022 mmol) was then dissolved in a small amount of c.a. 1:1
tetrahydrofuran:methanol
and treated with L-tartaric acid (3.3 mg, 0.022 mmol). The volatile components
of the resulting
clear solution were removed under a stream of argon. To the resulting yellow
oil was added
diethyl ether plus one drop of methanol giving a fine yellow precipitate which
was isolated by
decanting most of the liquid and subsequent high vacuum romoval of the
remaining volatile
components to afford the title compound as a tartrate salt (13 mg, 0.022 mmol)
in quantitative
yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
119
Example 72: N-(2-{4-[(methylamino)methyl]phenyl}-6-oxo-5.6-dihydro-1H-
11.2]diazepinof4 5 6-
cd]indol-8-yl)acetamide trifluoroacetate
1. (CH3SO2)2O Me0 O
NBoc
Intermediate 73(c) 2,4,6-Collidine, CH2CI2 O ~ ~ -
of Example 73 II ~/ ~
J~N N
2. CH3NH2
3. Boc20, Et3N, CH2CI2 H H
64% 72(a)
1. POCI3. DMF, CH2CI2 WN
HN-N H 2. NH2NH2.HZ0, AcOH O - TFA
MeOH, reflux AN ~ ~
H H
3. TFA, CHZCI2, 4%
Step 1. Preparation of Methyl 6-(acetylamino)-2-(3-{[{[(1,1-
dimethylethyl)oxy]carbonyl}(methyl)amino] methyl}phenyl)-1H-indole-4-
carboxylate 72(a)
With stirring, to Intermediate 73(c) of Example 73 (54 mg, 0.16 mmol) in
CH2CI2 (2.5 mL) is added
2,4,6-trimethylpyridine (0.11 mL, 0.80 mmol) followed by methanesulfonic
anhydride (42 mg, 0.23
mmol). After 3.5 hours, additional methanesulfonic anhydride (7 mg, 0.04 mmol)
is added, and the
mixture is stirred for an additional 1 hour whereupon methylamine in
tetrahydrofuran (2.0 mL, 2.0
M) is added, and the reaction is stirred overnight at room temperature.
Following evaporation of
the volatile components, CH2CI2 (2.0 mL), triethylamine (0.064 mL, 0.46 mmol),
and di-tert-butyl
dicarbonate (50 mg, 0.23 mmol) are added and the reaction is stirred for 2.5
hours whereupon,
after removal of the volatile components, the crude product was purified by
silica gel
chromatography eluting with 1:1 ethyl acetate:hexane to afford Intermediate
72(a) (46 mg, 0.10
mmol) in 64% yield.
Step 2. Preparation of Title Compound: N-(2-{4-[(Methylamino)methyl]phenyl}-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cdJindol-8-yi)acetamide trifluoroacetate
In a manner similar to that described for Example 3, step 4, Intermediate
72(a) (45 mg, 0.10
mmole), in CH2CI2 (1.5 mL) was treated with a premixed Vilsmeier reagent
consisting of POCI3
(0.021 mL, 0.23 mmol) and N,N-dimethylformamide (0.05 mL, 0.65 mmol). After 2
h, 2 N sodium
acetate in water was added, and the crude 3-formylated product was isolated by
extractive work
up using ethyl acetate. After evaporation of the volatile components, methanol
(1.5 mL), HZNNHZ .
H20 (0.015 mL, 0.31 mmol) and acetic acid (0.010 mL, 0.17 mmol) were added,
and the mixture
was heated in a 70 C oil bath for 45 min. Purification was effected using
silica gel
chromatography eluting with 3:2 ethyl acetate:hexane. The protected
intermediate, 1,1-
dimethylethyl {4-[8-(acetylamino)-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-2-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
120
yl]phenyl}methyl(methyl)carbamate, was treated with 1:1 TFA/CH2CIZ (5 mL)
analogously to
Example 20. Isolation afforded the title compound (2 mg, 0.004 mmol) in 4%
yield.
LCMS: (M+H+) 362.3.
Example 73: N-f2-(3-Dimethylaminomethyl-pheny)-6-oxo-5,6-dihydro-1 H-
[1,2]diazepinof4,5,6-
cd]indol-8-yll-2-fluoro-3-trifluoromethyl-benzamide
,i
~~ OAc
MeO 0
73(a) OAc HZSO4, 0 C, 52%
Intermediate 6(b), 0
\ O
Example 6
(C6H5CN)2PdCI2, Cul A~ / ~
N NH
t-Bu3P, DIPEA, H
Dioxane, 70 C, 87% 73(b)
1. (CH3SO2)20 H
MeO O OH 2,4,6-Collidine, CH2CI2 0 N_N NMe
2
;; ~ I \ \ _ 2. (CH3)2NH AN H 3. 4N HCI (Dioxane), MeOH, 90 C H2N H
H 4. POCI3. DMF, CHZCIZ
73(c) 5. NH2NH2.H2O, AcOH 73(d)
MeOH, reflux, 30%
0
H HN-N
O
F 0 \
F3 N I / N
HATU, Et3N, DMF, 64% H
F -N
F3
Step 1. Preparation of 2-(3-Acetoxymethyl-phenylethynyl)-3,5-bis-acetylamino-
benzoic acid
methyl ester 73(b)
A mixture of (C6H5CN)2PdCI2 (0.8 g, 2 mmol), Cul (0.267 g, 1.4 mmol), and tri-
tert-butyl-
phosphane (1.05 mL, 4 mmol) in dioxane (35 mL, 2.0 M) was stirred at 22 C for
0.25 hours.
Diisopropylamine (17.1 mL, 122 mmol), Intermediate 6(b) of Example 6 (23 g,
69.9 mmol), acetic
acid 3-ethynyl-benzyl ester (17 g, 98.0 mmol) (see below for preparation) and
N,N-
dimethylformamide (35 mL, 2.0 M) were added sequentially. The reaction mixture
stirred at 70 C
for 16 hours. Ethyl acetate (300 mL) was added and the salts were filtered
through diatomaceous
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
121
earth. The solvent was removed under reduced pressure, and the resulting solid
was triturated
with ethyl acetate (25 mL), dichloromethane (50 mL) and diethyl ether (25 mL).
The precipitate
was collected by filtration and washed with 5% dichloromethane in diethyl
ether (100 mL) to
afford, after drying, Intermediate 73(b) (25.7 g) in 87% yield as a white
powder.
' H-N MR (d6-DMSO): S 10.35 (s, 1 H), 9.54 (s, 1 H), 8.21 (d, 1 H, J = 2.1
Hz), 8.08 (d, 1 H, J 2.1
Hz), 7.58-7.38 (m, 4H), 5.12 (s, 2H), 3.90 (s, 3H), 2.17 (s, 3H), 2.09 (s,
3H), 2.07 (s, 3H).
LCMS: (M-H+) 421.3
For step 1, compound 73(a) (acetic acid 3-ethynyl-benzyl ester) was prepared
as follows:
Step 1 a -1 c.
/ Pd(OAc)2, TMS = ~ I 1. KF, MeOH, rt
Br \ I ~O Ph3P, Et3N, 90 C 2. NaBH4, MeOH, 0 C
TMS
73(al)
(CH3CO2)20, DIPEA
OH 30- oAc
4-DMAP, CH2CI2, 63%
73(a2) 73(a)
Step 1a: Preparation of crude 3-trimethylsilanylethynvl-benzaldehyde 73(al)
3-Bromobenzaidehyde (30 g, 162 mmol), ethynyl-trimethyl-silane (30 mL, 211
mmol),
triphenylphosphine (2.13 g, 8 mmol), palladium (II) acetate (0.91 g, 4 mmol)
and triethylamine
(540 mL, 0.3 M) were heated at 90 C for 5 hours, after cooling to ambient
temperature, the
mixture was filtered. The filtrate was evaporated, and the residue was
subjected to silica gel
chromatography (hexane to 4:96 diethyl ether/hexane) to afford 3-
trimethylsilanylethynyl-
benzaldehyde 73(al).
Step 1 b: Preoaration of crude (3-eth ynyi-phenyl)-methanol 73(a2)
To 3-trimethylsilanylethynyl-benzaldehyde 73(al) from step 1a in methanol (540
mL, 0.3 M) was
added KF (18.8 g, 324 mmol), and the resulting mixture stirred at 22 C for 6
hours. After cooling
to 0 C, NaBH4 (6.13 g, 162 mmol) was carefully added over 0.5 hours. Aqueous
saturated NH4CI
was carefully added and the volatiles were removed in vacuo. Ethyl acetate
(200 mL) was added,
and the organic phase was washed with water and a saturated aqueous NaCI
solution, dried over
MgSO4 and filtered. The volatiles were removed in vacuo to afford crude (3-
ethynyl-phenyl)-
methanol 73(a2).
Step 1 c: Preparation of acetic acid 3-ethynyl-benzyl 73(a)
Crude (3-ethynyl-phenyl)-methanol 73(a2) from step 1 b, acetic anhydride (20
mL, 21 mmol),
diisopropylethylamine (85 mL, 486 mmol) and 4-dimethylamino-pyridine (0.3963.2
mmol) were
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
122
stirred in CH2CI2 (540 mL, 0.3 M) for 0.5 hours. Aqueous saturated NH4CI (100
mL) was carefully
added and the organic phase washed with brine, dried over MgSO4 and filtered.
The volatiles
were removed in vacuo to afford, after silica gel chromatography (hexanes to
12:88 diethyl
ether/hexanes), acetic acid 3-ethynyl-benzyl (a) (17.83 g) in 63% yield.
1 H-NMR (d6-DMSO): S 7.32-7.01 (m, 4H), 4.85 (s, 2H), 4.01 (s, 1H), 1.89 (s,
3H).
Step 2. Preparation of 6-Acetylamino-2-(3-hydroxymethyl-phenyl)-1H-indole-4-
carboxylic
acid methyl ester 73(c)
In a manner analogous to Step 4 of Example 6, Intermediate 73(b) (24 g, 56.8
mmol) was cyclized
to Intermediate 73(c) (10.1 g) in 52% yield.
'H-NMR (d6-DMSO): 8 11.89 (s, 1 H), 10.16 (s, 1 H), 8.35 (s, 1 H), 7.89 (bs,
2H), 7.51 (d, 1 H, J=
7.9 Hz), 7.49 (dd, 1 H, J= 7.7, 7.5 Hz), 7.33 (bs, 2H), 5.37 (dd, 1 H, J= 5.8,
5.7 Hz), 4.64 (d, 2H, J
= 5.65 Hz), 3.99 (s, 3H), 2.13 (s, 3H).
LCMS: (M+H+) 339.0
Step 3. Preparation of 8-Amino-2-(3-dimethylaminomethyl-phenyl)-1,5-dihydro-
[1,2]diazepino [4,5,6-cd]indol-6-one 73(d)
To a solution of Intermediate 73(c) (1 g, 3 mmol) in dichloromethane (0.1 M,
30 mL) was added
2,4,6-Collidine (1.56 mL, 12 mmol) followed by (CH3SO2)20 (0.62 g, 3.6 mmoL).
After stirring for 2
hours, dimethylamine (5.6 M solution in ethanol, 2.6 mL, 15 mmol) was added
and the reaction
mixture stirred for 24 hours at 22 C. The volatiles were removed in vacuo,
and the crude mixture
was dissolved in methanol (15 mL). Anhydrous 4M HCI in dioxane (15 mL, 60
mmol) was carefully
added, and the solution heated at 90 C for 2.5 hours. After cooling at 22 C,
the volatiles were
removed in vacuo and the crude 6-amino-2-(3-dimethylaminomethyl-phenyl)-1H-
indole-4-
carboxylic acid methyl ester was formylated and cyclized in a manner analogous
to steps 4 and 5
of Example 3. Silica gel chromatography (90:10 to 75:25 CH2CI2/ 2M ammonia in
isopropyl
alcohol) provided Intermediate 73(d) (0.3 g, 0.9 mmol) in 30% yield.
'H-NMR (d6-DMSO): 8 11.54 (s, 1 H), 10.15 (s, 1 H), 7.55-7.32 (m, 5H), 6.98
(d, 1 H, J= 1.3 Hz),
6.65 (d, 1 H, J= 1.4 Hz), 5.2 (bs, 2H), 3.49 (b, 2H), 2.20 (bs, 6H).
LCMS: (M+H+) 334.2
Step 4. Preparation of Title Compound: N-[2-(3-Dimethylaminomethyl-phenyl)-6-
oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-2-fluoro-3-trifluoromethyl-
benzamide
Preparation from Intermediate 73(d) (0.11 g, 0.4 mmol), 2-Fluoro-3-
trifluoromethyl-benzoic acid
(0.103 g, 0.6 mmol), triethylamine (0.183 mL, 1.6 mmol), O-(7-azabenzotriazol-
1-yl)-N,N,N;N=
tetramethyluronium hexafluorophosphate (0.188 g, 0.6 mmol) and N,N-
dimethylformamide (0.2 M,
1.7 mL) was carried out analogously to Example 11. Silica gel chromatography
(90:10 to 80:20
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
124
in DMSO (10 mL) was carried out analogously to Example 76. Preparative HPLC
(20-100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0145 g) in 3.5% yield.
'H NMR (ds-DMSO): S 12.46 (s, 1 H), 10.47 (s, 1 H), 8.88 (d, 1 H, J = 4.4 Hz),
8.10 (d, 1 H, J = 1.4
Hz), 8.02 (d, 1 H, J= 1.4 Hz), 7.75-7.66(m, 2H), 7.64-7.53 (m, 3H), 7.51 (s, 1
H), 7.32-7.25 (m,
2H), 7.23-7.10 (m, 3H), 3.08 (m, 1 H), 2.11 (m, 1 H), 1.41 (m, 1 H), 1.23 (m,
1 H).
LCMS: (M+H+) 421.1.
Example 76: N-(2-Hydroxy-2-phenylethyl)-6-oxo-2-phenyl-5 6-dihydro-lH-[1
2]diazepinol4 5 6-
cd]indole-8-carboxamide
HN-N
0 OH H \ ~ -
N N h
H
0
To a solution of the title compound of Example 49 (0.105 g, 0.306 mmol) in
DMSO (2 mL) was
added triethylamine (0.085 mL, 0.612 mmol), and O-(7-azabenzotriazol-1-yl)-
N,N,N;M
tetramethyluronium hexafluorophosphate (0.232 g, 0.612 mmol). After
approximately 5 min, 2-
amino-l-phenylethanol (0.084 g, 0.612 mmol) was added, and the mixture was
stirred overnight.
The mixture was subjected to preparative HPLC (20-100% CH3CN/H20 containing
0.1%
trifluoroacetic acid), and the purest fractions were combined and lyophilized
to afford the title
compound (0.018 g) as a yellow powder in 14% yield.
'H NMR (d6-DMSO): S 12.47 (s, 1 H), 10.45 (s, 1 H), 8.68 (t, 1 H, J= 4.5 Hz),
8.09 (s, 1 H), 8.01 (s,
1 H), 7.71 (d, 2H, J= 6.8 Hz), 7.51-7.62 (m, 4H), 7.24-7.41 (m, 5H), 4.80-4.84
(m, 1 H), 3.46-3.54
(m, 2H).
HRMS calculated for C25H21N403 425.1614 (M+H), found 425.1633.
Example 77: 6-Oxo-2-phenyl-N-[3-(trifluoromethyl)benzyll-5.6-dihydro-1 H-
[1.21diazepinof4.5.6-
cdlindole-8-carboxam ide
HN-N
O
H I \ ~ -
F3C N / N
H
o
Preparation of example 77 from the title compound of Example 49 (0.1 g, 0.291
mmol), 3-
(trifluoromethyl)benzylamine (0.102 g, 0.582 mmol), triethylamine (0.081 mL,
0.582 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.221
g, 0.582
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
123
CH2CI2/ 2M ammonia in isopropyl alcohol) afforded the title compound (0.11 g)
as a yellow
powder in 64% yield.
' H-NMR (d6-DMSO): 5 12.26 (s, 1 H), 10.84 (s, 1 H), 10.51 (s, 1 H), 9.62 (b,
1 H), 8.29 (d, 1 H, J
1.4 Hz), 8.08-7.94 (m, 2H), 7.85-7.53 (m, 7H), 4.39 (s, 2H), 2.73 (s, 6H).
LCMS: (M+H+) 524.2.
Example 74: Methyl 1-[3-(methylamino)propyl]-6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-8-carboxvlate trifluoroacetate
O' HN-N
/O N
O
O
HN F3C-I-OH
To a solution of the title compound from Example 39 (0.32 g, 1 mmol) in
anhydrous DMSO (10
mL) was added NaH (60% suspension in mineral oil) (0.088 g, 2.2 mmol). The
mixture was
allowed to stir for 5 min whereupon 1,2-dibromopropane (0.24 g, 1.2 mmol) was
added. The
mixture was allowed to stir overnight at room temperature at which point a 2M
solution of
methylamine in methanol (4 mL, 8 mmol) was added. The mixture was subjected to
preparative
HPLC (20-50% CH3CN/H20 containing 0.1% trifluoroacetic acid). The purest
fractions were
combined and lyophilized to afford the title compound (0.0209 g) in 4.1 %
yield.
'H NMR (d6-DMSO): S 10.61 (s, 1 H), 8.39 (d, 1 H, J = 1.0 Hz), 8.22 (d, 1 H, J
= 1.0 Hz), 7.70-
7.55(m, 5H), 7.07 (s, 1 H), 4.41 (t, 2H, J = 7.2 Hz), 3.92 (s, 3H), 2.80-2.60
(broad, 2H), 2.43 (s,
3H), 1.95-1.75 (m, 2H).
LCMS: (M+H+) 391.2
HRMS: C22H22N403 = H: 391.1770. Found: 391.1768.
Example 75: 6-Oxo-2-phenyl-N-((1,2-trans)-2-phenylcyclopropyl)-5,6-dihydro-1 H-
[1,21diazepino[4,5,6-cdJindole-8-carboxamide
HN-N
0 N N
H
O
Preparation of example 75 from the title compound of Example 49 (0.34 g, 1
mmol), (1,2-trans)-
2-phenylcyclopropylamine hydrochloride (0.2 g, 1.2 mmol), triethylamine (0.28
mL, 2 mmol), and
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(0.46 g, 1.2 mmol)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
125
mmol) in DMSO (2 mL) was carried out analogously to Example 76. Preparative
HPLC (20-100%
CH3CN/H20 containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.024 g) as a yellow powder in 18% yield.
'H NMR (d6-DMSO): S 12.49 (s, 1 H), 10.48 (s, 1 H), 9.33 (t, 1 H, J= 6.0 Hz),
8.14 (s, 1 H), 8.06 (s,
1 H), 7.52-7.72 (m, 10H), 4.58 (d, 3H, J= 5.7 Hz).
HRMS calculated for C25H18N402F3 463.1382 (M+H), found 463.1391.
Example 78: 6-Oxo-2-phenyl-N-(1-phenylethyl)-5,6-dihydro-lH-
[1.2]diazepinof4,5.6-cel]indole-8-
carboxamide
HN-N
O
/I H
\ N N h
O H
Preparation of example 78 from the title compound of Example 49 (0.103 g, 0.3
mmol), 1-
phenylethylamine (0.0727 g, 0.6 mmol), triethylamine (0.084 mL, 0.6 mmol), and
0-(7-
azabenzotriazol-1-yi)-N,N,N;N'tetramethyluronium hexafluorophosphate (0.228 g,
0.6 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H2O containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0081 g) as a yellow powder in 6.6% yield.
1 H NMR (d6-DMSO): S 12.44 (s, 1 H), 10.46 (s, 1 H), 9.03 (d, 1 H, J = 7.9
Hz), 8.14 (s, 1 H), 8.03 (s,
1 H), 7.71 (d, 2H, J= 7.2 Hz), 7.51-7.62 (m, 4H), 7.41 (d, 2H, J= 7.6 Hz),
7.32 (t, 2H, J= 7.6 Hz),
7.22 (t, 1 H, J= 7.2 Hz), 5.16-5.25 (m, 1 H), 1.51 (d, 3H, J= 6.8 Hz).
HRMS calculated for C25H21N402 409.1665 (M+H), found 409.1679.
Example 79: N-[1-(4-Fluoropheny,ethyl]-6-oxo-2-phenyl-5,6-dihydro-lH-
f1,2]diazepinof4,5.6-
cdlindole-8-carboxamide
HN-N
O
H I \ ~ -
N N h
H
O
Preparation of example 79 from the title compound of Example 49 (0.0979 g,
0.285 mmol), 1-(4-
fluorophenyl)ethylamine (0.0793 g, 0.57 mmol), triethylamine (0.079 mL, 0.57
mmol), and 0-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.217 g,
0.57 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0043 g) as a yellow powder in 3.5% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
126
' H NMR (ds-DMSO): 12.44 (s, 1 H), 10.47 (s, 1 H), 9.03 (d, 1 H, J = 7.9 Hz),
8.13 (s, 1 H), 8.02
(s, 1H), 7.71 (d, 2H, J 6.8 Hz), 7.51-7.62 (m, 4H), 7.42-7.47 (m, 2H), 7.12-
7.18 (m, 2H), 5.15-
5.24 (m, 1 H), 1.50 (d, 3H, J= 7.2 Hz).
HRMS calculated for C25H2ON402F 427.1570 (M+H), found 427.1584.
Example 80: N-(2,3-Dihydro-lH-inden-1-yl)-6-oxo-2-phenyl-5.6-dihydro-lH-f1
2ldiazepino[4 5 6-
cdlindole-8-carboxam ide
HN-N
O
Qr N N
O H
Preparation of example 80 from the title compourid of Example 49 (0.1 g, 0.291
mmol), 2,3-
dihydro-1H-inden-1-ylamine (0.0775 g, 0.582 mmol), triethylamine (0.081 mL,
0.582 mmol), and
O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(0.221 g, 0.582
mmol) in DMSO (2 mL) was carried out analogously to Example 76. Preparative
HPLC (20-100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0045 g ) as a yellow powder in 3.7% yield.
'H NMR (d6-DMSO): S 12.45 (s, 1 H), 10.45 (s, 1 H), 9.01 (d, 1 H, J= 8.3 Hz),
8.15 (s, 1 H), 8.09 (s,
1 H), 7.71 (d, 2H, J= 6.8 Hz), 7.51-7.62 (m, 4H), 7.18-7.28 (m, 4H), 5.56-5.64
(m, 1 H), 2.79-3.06
(m, 2H), 1.98-2.46 (m, 2H).
HRMS calculated for C25H2ON402F 427.1570 (M+H), found 427.1584.
Example 81: 6-Oxo-2-phenyl-N-(1,2,3,4-tetrahydronaphthalen-l-xl)-5,6-dihydro-1
H-
[1,21diazepino[4,5,6-cdlindole-8-carboxamide
HN-N
H I \ ~ -
N / N
O H
Preparation of example 81 from the title compound of Example 49 (0.103 g, 0.3
mmol), 1,2,3,4-
tetrahydronaphthalen-1-ylamine (0.0883 g, 0.6 mmol), triethylamine (0.084 mL,
0.6 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.228
g, 0.6 mmol)
in DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H2O containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0136 g) as a yellow powder in 10% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
127
' H NMR (d6-DMSO): S 12.44 (s, 1 H), 10.44 (s, 1 H), 9.01 (d, 1 H, J = 8.7
Hz), 8.15 (s, 1 H), 8.09
(s, 1 H), 7.71 (d, 2H, J = 6.8 Hz), 7.51-7.62 (m, 4H), 7.10-7.23 (m, 4H), 5.23-
5.30 (m, 1 H), 2.75-
2.81 (m, 2H), 1.96-2.01 (m, 2H), 1.74-1.90 (m, 2H).
HRMS calculated for C27H23N402 435.1821 (M+H), found 435.1810.
Example 82: N-f1-Methyl-1-(4-methylphenyl)ethyl]-6-oxo-2 phenyl-5 6-dihydro-lH-
[1.2]diazepinof4,5,6-cdlindole-8-carboxamide
HN-N
O
\ N / N
O H
Preparation of example 82 from the title compound of Example 49 (0.103 g, 0.3
mmol), 1-methyl-
1-(4-methylphenyl)ethylamine (0.0895 g, 0.6 mmol), triethylamine (0.084 mL,
0.6 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.228
g, 0.6 mmol)
in DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H2O containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0304 g) as a yellow powder in 23% yield.
'H NMR (d6-DMSO): S 12.41 (s, 1 H), 10.45 (s, 1 H), 8.60 (s, 1 H), 8.05 (s, 1
H), 7.94 (s, IH), 7.70
(d, 2H, J = 6.80 Hz), 7.51-7.62 (m, 4H), 7.26 (d, 2H, J = 8.3 Hz), 7.08 (d,
2H, J = 7.9 Hz), 2.25 (s,
3H), 1.67 (s, 6H).
HRMS calculated for C27H25N402 437.1978 (M+H), found 437.1987.
Example 83a: (1 R.2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-
dimethylaminomethyl-phenyl)-
6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide
/
O HN-N -N
0 \ -
,1.~'~ I / N \ ~
H H
Preparation of example 83a from Intermediate 73(d) of Example 73 (0.11 g, 0.33
mmol), (1 R,2R)-
2-phenyl-cyclopropanecarboxylic acid (0.08 g, 0.5 mmol), triethylamine (0.183
mL, 1.32 mmol), 0-
(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.188
g, 0.5 mmol)
and N,N-dimethylformamide (0.2 M, 1.7 mL) was carried out analogously to
Example 11. Silica gel
chromatography (90:10 to 80:20 CH2CI2/ 2M ammonia in isopropyl alcohol)
afforded the title
compound (0.12 g) as a yellow powder in 76% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
128
Example 83b: (1 R.2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-
dimethylaminomethvl-
phenyl)-6-oxo-5,6-dihydro-1 H-[1,21diazepino[4.5.6-cdJindol-8-yl]-amide HCI
Salt
HN-N -N HCI
O
O \ ~ -
N
H H
The title compound of Example 83a (0.075 g, 0.16 mmol) in dichloromethane (1.0
mL) was
treated with 4M HCI in dioxane (0.043 mL, 0.17 mmol). After concentrating to
dryness, the title
compound (0.08 g) was obtained in quantitative yield.
'H-NMR (d6-DMSO): 5 12.19 (s, 1 H), 10.48 (s, 1 H), 10.44 (s, 1 H), 10.02 (b,
1 H), 8.20 (d, 1 H, J=
1.5 Hz), 7.84 (s, 1 H), 7.76 (d, 1 H, J= 7.4 Hz), 7.71-7.59 (m, 4H), 7.34-7.17
(m, 5H), 4.38 (d, 1 H, J
= 5.0 Hz), 2.78 (d, 6H, J= 4.6 Hz), 2.45-2.37 (m, 1 H), 2.14-2.07 (m, 1 H),
1.56-1.47 (m, 1 H), 1.42-
1.35 (m, 1 H).
HRMS: (M+H+) calcd for C29H28N502, 478.2243, found 478.2261.
Example 84: N-(2-Hydroxymethyl-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-Lrl)-
acetamide
MeO O OH MeO O
I\ Br -j I\/ H TBDPSCI, imidazole
/ (C6H5CN)aPdC12, Cul / DMF, rt, 59%
AcHN NHAc AcHN NHAc
t-Bu3P, DIPEA,
Dioxane, 70 C, 9% 84(a)
MeO 0 MeO 0
TBDPS
Cul, TMG, DMF I(f....%,OTBDPS_1. POCI3. DMF, CH2CIz
AcHN NHAc Dioxane, 90 C, 57% AcHN H N 2. NH2NH2.H20, AcOH
MeOH, reflux, 69%
84(b) 84(c)
H H
O N-N N-N
x O
I~ OTBDPS TBAF, THF, 64% I\ \ OH
AcHN H AcHN N
H
84(d)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
129
Step 1. Preparation of 3,5-Bis-acetylamino-2-(3-hydroxy-prop-1-ynyl)-benzoic
acid methyl
ester 84(a)
Intermediate 6(b) (10 g, 30.4 mmol) of Example 6 and propargyl alcohol were
reacted in a manner
analogous to step 1 of Example 73. Intermediate 84(a) (0.85 g) was obtained in
9% yield.
'H-NM R(d6-DMSO): S 10.29 (s, 1 H), 9.11 (s, 1 H), 8.30 (s, 1 H), 8.00 (s, 1
H), 5.34 (t, 1 H, J= 5.84
Hz), 4.38 (d, 2H, J= 5.84 Hz), 3.83 (s, 3H), 2.13 (s, 3H), 2.05 (s, 3H).
Step 2. Preparation of 3,5-Bis-acetylamino-2-[3-(tert-butyl-diphenyl-
silanyloxy)-prop-l-
ynyl]-benzoic acid methyl ester 84(b)
Chloro-tert-butyl-diphenyl-silane (1.3 g, 2 rrimol), imidazole (0.54 g, 7.9
mmol) and Intermediate
84(a) (0.48 g, 1.6 mmol) were stirred in N,N-dimethylformamide (0.2 M, 15 mL)
for 0.5 hours at 22
C. Methanol was added, the volatiles removed in vacuo, and ethyl acetate (50
mL) was added.
The organic phase was washed with 1 N aqueous HCI, brine, dried over MgSO4i
filtered, and the
volatiles removed in vacuo. Silica gel chromatography (80:20 ethyl
acetate/hexanes) afforded
Intermediate 84(b) (0.5 g) in 59% yield.
' H-NMR (d6-DMSO): S 10.31 (s, 1 H), 9.18 (s, 1 H), 8.25 (d, 1 H, J = 1.8 Hz),
8.00 (d, 1 H, J = 1.8
Hz), 7.74-7.68 (m, 4H), 7.50-7.41 (m, 6H), 4.67 (s, 2H), 3.81 (s, 3H), 2.05
(s, 6H), 1.01 (s, 9H).
LCMS: (M-H+) 541.1
Step 3. Preparation of 6-Acetylamino-2-(te-t-butyl-diphenyl-silanyloxymethyl)-
1H-indole-4-
carboxylic acid methyl ester 84(c)
In a manner analogous to step 5 of Example 5, from Intermediate 84(b) (0.46 g,
0.84 mmol) was
reacted to provide Intermediate 84(c) (0.24 g) in 57% yield.
1H-NMR (d6-DMSO): 5 11.36 (s, 1 H), 10.04 (s, IH), 8.25 (s, IH), 7.78 (d, 1 H,
J= 1.5 Hz), 7.74-
7.66 (m, 4H), 7.50-7.41 (m, 6H), 6.72 (s, 1H), 4.85 (s, 2H), 3.87 (s, 3H),
2.06 (s, 3H), 1.03 (s, 9H).
LCMS: (M-H) 499.1
Step 4. Preparation of N-[2-(tert-Butyl-diphenyl-silanyloxymethyl)-6-oxo-5,6-
dihydro-lH-
[1,2]diazepino[4,5,6-cal]indol-8-yl]-acetamide 84(d)
In a manner analogous to steps 4 and 5 of Example 3, Intermediate 84(c) (0.2
g, 0.4 mmol) was
formylated and cyclized to provide Intermediate 84(d) (0.14 g) in 69% yield.
LCMS: (M-H) 509.1.
Step 5. Preparation of Title Compound: N-(2-Hydroxymethyl-6-oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cal]indol-8-yl)-acetamide
To a solution of Intermediate 84(d) (0.085 g, 0.17 mmol) in tetrahydrofuran
(0.1 M, 1.6 mL) was
added a IM solution of tetra-butyl ammonium fluoride in tetrahydrofuran (0.184
mL, 0.18 mmol).
The mixture was stirred at 22 C for 2 hours, and the yellow solid was
collected by filtration and
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
130
washed with methanol (5.0 mL) and diethyl ether (5.0 mL) to afford the title
compound (0.029 g)
in 64% yield.
'H-NMR (ds-DMSO): 8 11.74 (s, 1 H), 10.17 (s, 1 H), 10.02 (s, 1 H), 8.04 (d, 1
H, J = 1.3 Hz), 7,54
(s, 1 H), 7.53 (s, 1 H), 5.47 (dd, 1 H, J= 5.5, 5.5 Hz), 4.68 (d, 2H, J= 5.4
Hz).
LCMS: (M+H+) 273.1.
HRMS: (M+H+) calcd for C13H13N403, 273.1001, found 273.0988.
Example 85: Acetic acid 3-{6-oxo-8-[((1R. 2R)-2-phenyl-cyclopropanecarbony,-
aminol-5.6-
dihydro-1 H-[1,2]diazepino[4.5.6-cdJindol-2-Lrl}-benzyl ester
1. 4N HCI (Dioxane), MeOH, 90 C O OMe HO
avl~-OkN Intermediate 73(c) 2. HATU, Et3N, DMF
Example 73 O N
~~.
H H
17 85(a)
3. K2CO3, MeOH, 50%
OMe AcO
O
Ac2O, 4-DMAP cL.L..c?-c/ 2. NH2NH2, AcOH, MeOH
MeOH, reflux, 66%
85(b)
O HN-N Ac0
O \ \ -
.k N N
H H
Step 1. Preparation of 2-(3-Hydroxymethyl-phenyl)-6-[(1 R,2R)-(2-phenyl-
cyclopropanecarbonyl)-amino]-1 H-indole-4-carboxylic acid methyl ester 85(a)
Intermediate 73(c) from Example 73 (1.8 g, 5 mmol) was dissolved in methanol
(22 mL). 4M HCI
in dioxane (22 mL, 75 mmol) was carefully added, and the solution was heated
at 90 C for 1
hour. After cooling to 22 C, the volatiles were removed in vacuo giving 6-
amino-2-(3-
hydroxymethyl-phenyl)-1H-indole-4-carboxylic acid methyl ester which was then
combined with
(1 R,2R)-2-phenyl-cyclopropanecarboxylic acid (2 g, 12.5 mmol), triethylamine
(3.5 mL, 25 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(4.7 g, 12.5
mmol) in N,N-dimethylformamide (0.2 M, 25 mL) and stirred at 22 C for 12
hours. Volatiles were
removed in vacuo and the crude mixture dissolved in methanol (25 mL, 0.2 M)
was treated with
K2C03 (1.38 g, 10 mmol) for 1 hour at 22 C. Excess K2C03 was removed by
filtration, and acetic
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
131
acid (2 drops) was added to the filtrate. Following filtrate evaporation, the
residue was subjected
to silica gel chromatography (90:10 to 100:0 ethyl acetate/hexane) which
provided Intermediate
85(a) (1.1 g, 2.5 mmol) in 50% yield.
' H-NMR (d6-DMSO): 5 11.83 (s, IH), 10.41 (s, 1 H), 8.25 (s, 1 H), 7.90 (s,
IH), 7.84 (s, IH), 7.75
(d, 1 H, J= 7.9 Hz), 7.43 (dd, 1 H, J= 7.7, 7.5 Hz), 7.35-7.17 (m, 7H), 5.30
(dd, 1 H, J= 5.8, 5.6
Hz), 4.59 (d, 2H, J = 5.4 Hz), 2.45-2.36 (m, 1 H), 2.14-2.07 (m, 1 H), 1.58-
1.47 (m, 1 H), 1.42-1.34
(m, 1 H).
HRMS: (M+H+) calcd for C27H25N204, 441.1828, found 441.1814.
Step 2. Preparation of 2-(3-Acetoxymethyl-phenyl)-6-[(1 R,2R)-(2-phenyl-
cyclopropanecarbonyl)-amino]-1H-indole-4-carboxylic acid methyl ester 85(b)
To a suspension of Intermediate 85(a) (1.1 g, 2.3 mmol) in ethyl acetate (22
mL, 0.1 M) was
added 4-(dimethylamino)-pyridine (0.28 g, 2.3 mmol) and acetic anhydride (0.47
g, 4.6 mmol).
The mixture was stirred at 22 C for 1 h, and the volatiles were removed in
vacuo. Silica gel
chromatography (70:30 to 100:0 ethyl acetate/Hexanes) provided Intermediate
85(b) (1.1 g, 2.3
mmol) in quantitative yield.
'H-NMR (d6-DMSO): S 11.85 (s, 1 H), 10.43 (s, 1 H), 8.26 (s, 1 H), 7.92-7.81
(m, 3H), 7.49 (dd, 1 H,
J = 7.7, 7.6 Hz), 7.37-7.13 (m, 7H), 5.15 (s, 2H), 3.92 (s, 3H), 2.45-2.36 (m,
1 H), 2.14-2.07 (m,
1 H), 1.58-1.47 (m, 1 H), 1.42-1.34 (m, 1 H).
HRMS: (M+H+) calcd for C29H27N205, 483.1920, found 483.1945.
Step 3. Preparation of Title Compound: Acetic acid 3-{6-oxo-8-[((1 R, 2R)-2-
phenyl-
cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-2-
yl}-benzyl
ester
In a manner analogous to steps 4 and 5 of Example 3, Intermediate 85(b) (0.75
g, 1.56 mmol)
was formylated and cyclized. Purification by silica gel chromatography (40:60
to 100:0 ethyl
acetate/ Hexanes) afforded the title compound (0.505 g, 1.03 mmol) as a yellow
powder in 66%
yield.
' H-NM R(d6-DMSO): S 12.13 (s, 1 H), 10.44 (s, 1 H), 10.39 (s, 1 H), 8.16 (d,
1 H, J= 1.5 Hz), 7.68-
7.45 (m, 6H), 7.34-7.17 (m, 5H), 5.18 (s, 2H), 2.50-2.34 (m, 1 H), 2.14-2.07
(m, 4H), 1.58-1.47 (m,
1 H), 1.43-1.35 (m, 1 H).
HRMS: (M+H+) calcd for C29H25N404, 493.1876, found 493.1882.
Anal. Calcd. for C29H24N404 - 0.4 H20: C, 69.70; H, 5.00; N, 11.21. Found: C,
69.71; H, 5.03; N,
11.33.
Example 86: (1 R. 2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-hydroxymethyl-
phenyl)-6-oxo-
56-dihydro-1H-[1,2]diazegino14.5,6-cd]indol-8-yl]-amide
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
132
O HN-N HO
~
av O ,,~~N N
H H
The title compound of Example 85 (0.288 g, 0.58 mmol) and K2C03 (0.161 g, 1.17
mmol) were
stirred in methanol (0.2 M, 1.7 mL) and tetrahydrofuran (0.2 M, 1.7 mL) for
1.5 hours at 22 C.
After the solution was filtered and acidified with two drops of glacial acetic
acid, the volatiles were
removed in vacuo. Silica gel chromatography (40:60 to 0:100 hexane/ethyl
acetate) afforded the
title compound (0.26 g) as a yellow powder in 95% yield.
' H-NMR (d6-DMSO): S 12.11 (s, 1 H), 10.43 (s, 1 H), 10.36 (s, 1 H), 8.15 (d,
1 H, J= 1.0 Hz), 7.64
(d, 1 H, J= 1.0 Hz), 7.61 (s, 1 H), 7.55-7.17 (m, 9H), 5.35 (dd, 1 H, J= 5.8,
5.6 Hz), 4.60 (d, 2H, J=
5.6 Hz), 2.45-2.36 (m, 1 H), 2.14-2.07 (m, 1 H), 1.58-1.47 (m, 1 H), 1.42-1.34
(m, 1 H).
LCMS: (M-H+) 449.1.
Anal. Calcd. for C27H22N403 - 0.1 CH2CI2 - 0.1 C2H5O2CCH3: C, 70.61; H, 4.96;
N, 11.98. Found:
C, 70.01; H, 4.95; N, 11.95.
Example 87: N-(2,4-Difluorobenzyl)-6-oxo-2-phenyl-5.6-dihydro-1 H-
[1.2]diazepino[4.5.6-cellindole-
8-carboxamide
HN-N
O
F
H I \ ~ -
N / N
0 H
Preparation of example 87 from the title compound of Example 49 (0.102 g,
0.297 mmol), 2,4-
difluorobenzylamine (0.085 g, 0.594 mmol), triethylamine (0.083 mL, 0.594
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.226 g,
0.594 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0044 g) as a yellow powder in 3.4% yield.
' H NMR (d6-DMSO): S 12.48 (s, 1 H), 10.48 (s, 1 H), 9.20 (t, 1 H, J= 5.5 Hz),
8.13 (s, 1 H), 8.05 (s,
1 H), 7.71 (d, 2H, J = 7.2 Hz), 7.52-7.62 (m, 4H), 7.40-7.47 (m, 1 H), 7.16-
7.25 (m, 1 H), 7.03-7.10
(m, 1 H), 4.49 (d, 2H, J= 5.3 Hz).
HRMS calculated for C24H17N402F2 431.1320 (M+H), found 431.1324.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
133
Example 88: 4-(2-(6-Oxo-2-phenyl-5.6-dihydro-lH-f1 2ldiazepino[4 5 6-cd]indol-
8-ylcarbamoLrl)-
ethyll-piperidine-l-carboxylic acid tert-butyl ester
HN-N
O ~
p \
N I / N
BocN H H
Preparation of example 88 from the title compound of Example 7 (hydrochloride)
(44 mg, 0.141
mmol), 4-(2-carboxy-ethyl)-piperidine-l-carboxylic acid tert-butyl ester (43
mg, 0.169 mmol),
triethylamine (0.059 mL, 0.423 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium hexafluorophosphate (64 mg, 0.169 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethy[formamide (0.4 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 8:5:2 CH2CI2:hexane:methanol), also in an
analogous manner,
afforded the title compound (62 mg, 0.120 mmol) as a yellow powder in 85%
yield.
' H NMR (d6-DMSO): S 12.15 (s, 1 H), 10.37 (s, 1 H), 10.06 (s, 1 H), 8.20 (s,
1 H), 7.70-7.46 (m, 7H),
3.96-3.87 (m, 2H), 2.75-2.63 (m, 2H), 2.42-2.34 (m, 2H), 1.72-1.55 (m, 5H),
1.40 (s, 9H), 1.06-
0.98 (m, 2H).
LCMS: (M+H+) 416.3, (M+Na+) 538.3.
Example 89: (E)-N-(6-Oxo-2-phenyl-5.6-dihydro-lH-f1.2]diazepino[4 5 6-cdlindol-
8-yl)-3-phenyl-
acrylamide
HN-N
O ~
p \
I \ ~ HI /N
H
Preparation of example 89 from the title compound of Example 7 (hydrochloride)
(40 mg, 0.128
mmol), (E)-3-phenyl-acrylic acid (23 mg, 0.154 mmol), triethylamine (0.054 mL,
0.384 mmol), and
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (59
mg, 0.154
mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was carried out
analogously to
Example 11. Silica gel chromatography (eluted with 2:1 hexane:acetone), also
in an analogous
manner, after a final trituration with methanol afforded the title compound
(47 mg, 0.116 mmol) as
a yellow powder in 90% yield.
' H NMR (d6-DMSO): S 12.14 (s, 1 H), 10.43 (s, 1 H), 10.40 (s, 1 H), 8.38 (s,
1 H), 7.71-7.40 (m,
13H), 6.87 (d, 1 H, J= 16.2 Hz).
LCMS: (M+H+) 407.1, (M+Na+) 429.0, (M-H') 405.2.
Anal. Calcd. for C25HIBN4O2 , 2.7 H20: C, 65.98; H, 5.18; N, 12.31.
Found: C, 65.62; H, 4.63; N, 12.10.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
134
Example 90: (2E.4E)-Hexa-2,4-dienoic acid (6-oxo-2-phenyl-5.6-dihydro-1H-
[1,2]diazepino[4,5,6-
cdJindol-8-yl)-amide
HN-N
O
O \
~N I ~ N
H H
Preparation of example 90 from the title compound of Example 7 (hydrochloride)
(41 mg, 0.131
mmol), (2E,4E)-hexa-2,4-dienoic acid (18 mg, 0.157 mmol), triethylamine (0.055
mL, 0.393
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (60
mg, 0.157 mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was
carried out
analogously to Example 11. Silica gel chromatography (eluted with 2:1
hexane:acetone), also in
an analogous manner, after a final trituration with methanol afforded the
title compound (8 mg,
0.022 mmol) as a yellow powder in 16% yield.
' H NMR (d6-DMSO): S 12.13 (s, 1 H), 10.38 (s, 1 H), 10.21 (s, 1 H), 8.30 (s,
1 H), 7.71-7.48 (m, 7H),
7.15 (m, 1 H), 6.38-6.07 (m, 3H), 1.84 (d, 3H, J= 5.8 Hz).
LCMS: (M+H+) 371.1, (M+Nai') 393Ø
Example 91: (2R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-phenyl-5.6-dihydro-1 H-
f1,2]diazepinof4,5,6-
cd]indol-8-Lrl)-acetamide(hydrochloride)
HN-N HN-N
O O
4N HCI (Dioxane) I \
N N CHpCIp, 49% N ~ N
NHBou H NH2 H H-CIH
The title compound of Example 59 (210mg, 0.41 mmol) was treated with 4M HCI in
dioxane and
allowed to stir tightly capped as a slurry for about 4 hours after which the
volatile components
were evaporated and diethyl ether was added and evaporated several times. The
resulting solids
were dissolved in methanol, precipitated with diethyl ether, and collected to
afford the title
compound (161 mg, 0.36 mmol) as a yellow powder in 87% yield.
'H NMR (d6-DMSO): S 12.27 (s, 1 H), 10.83 (s, 1 H), 10.44 (s, 1 H), 8.36 (br
s, 2H), 8.13 (s, 1 H),
7.78-7.64 (m, 3H), 7.63-7.48 (m, 4H), 3.81 (brs, 1H), 1.91-1.58 (m, 6H), 1.31-
1.00 (m, 5H).
LCMS: (M+H+) 416.1,(M+Na+) 438.2.
Anal. Calcd. for C24H25N502 * 1.5 HCI ' 2.0 H20: C, 56.94; H, 6.07; N, 13.84.
Found: C, 57.20; H, 6.01; N, 13.57.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
135
Example 92: N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-co7indol-8-
rLl)-3-piperidin-4-
yl-propionamide; compound with trifluoro-acetic acid
HN-N
O ~
O 0 \
F3C~H N / N
HN H H
Preparation of example 92 from title compound of Example 88 (52 mg, 0.101
mmol) and 45%
TFA in CH2CI2 (1 mL) was carried out analogously to Example 20. Isolation,
also in an analogous
manner, afforded the title compound (50 mg, 0.084 mmol) as a yellow powder in
82% yield.
' H NMR (ds-DMSO): S 12.07 (s, 1 H), 10.38 (s, 1 H), 10.09 (s, 1 H), 8.45 (br
s, 1 H), 8.17 (s, 1 H),
7.70-7.44 (m, 7H), 3.31-3.20 (m, 2H, partially obscurred), 2.93-2.76 (m, 2H),
2.41-2.31 (m, 2H),
1.91-1.76 (m, 2H), 1.64-1.49 (m, 3H); 1.38-1.19 (m, 2H).
Anal. Calcd. for C24H25N502' 1.5 TFA ' 0.8 H20: C, 53.64; H, 4.75; N, 11.58.
Found: C, 53.59; H, 4.74; N, 11.55.
Example 93: 6-Oxo-2-phenyl-N-[(1R)-1-phenylethy]-5,6-dihydro-1H-
[1,2]diazepinoK5,6-
cdlindole-8-carboxamide
HN-N
O
H I \ -
N N
O H
Preparation of example 93 from the title compound of Example 49 (0.105 g,
0.306 mmol), (1R)-1-
phenylethylamine (0.0742 g, 0.612 mmol), triethylamine (0.085 mL, 0.612 mmol),
and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.233 g,
0.612 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0235 g) as a yellow powder in 19% yield.
' H NMR (d6-DMSO): S 12.44 (s, 1 H), 10.46 (s, 1 H), 9.03 (d, 1 H, J= 7.9 Hz),
8.14 (s, 1 H), 8.04 (s,
1 H), 7.70 (d, 2H, J= 6.8 Hz), 7.51-7.62 (m, 4H), 7.42 (d, 2H, J= 7.2 Hz),
7.30-7.36 (m, 2H), 7.20-
7.24 (m, 1 H), 5.16-5.25 (m, I H), 1.50 (d, 3H, J = 7.2 Hz).
HRMS calculated for C25H21N402 409.1665 (M+H), found 409.1666.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
136
Example 94: 6-Oxo-2-phenvl-N-[(1 S)-1-phenylethyl]-5,6-dihydro-1 H-f
1,2]diazepino[4,5,6-
cdJindole-8-carboxam ide
HN-N
O
H I \ ~ -
N N h
H
O
Preparation of example 94 from the title compound of Example 49 (0.0976 g,
0.284 mmol), (1 S)-
1-phenylethylamine (0.0688 g, 0.568 mmol), triethylamine (0.079 mL, 0.568
mmol), and O-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.216 g,
0.568 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0197 g) as a yellow powder in 17% yield.
'H NMR (d6-DMSO): S 12.44 (s, 1 H), 10.46 (s, 1 H), 9.04 (d, 1 H, J = 7.9 Hz),
8.14 (s, 1 H), 8.03 (s,
1 H), 7.70 (d, 2H, J= 8.3 Hz), 7.51-7.62 (m, 4H), 7.42 (d, 2H, J= 7.2 Hz),
7.30-7.36 (m, 2H), 7.20-
7.24 (m, 1 H), 5.16-5.25 (m, 1 H), 1.50 (d, 3H, J= 7.2 Hz).
HRMS calculated for C25H21N402 409.1665 (M+H), found 409.1666.
Example 95: N-[1-(4-Chlorophenyl)ethyl]-6-oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepinof4,5,6-
cd]indole-8-carboxamide
HN-N
O
= CI
/ I H I \ ~ -
\ N N h
H
O
Preparation of example 95 from the title compound of Example 49 (0.104 g,
0.303 mmol), 1-(4-
chlorophenyl)ethylamine (0.0943 g, 0.606 mmol), triethylamine (0.084 mL, 0.606
mmol), and 0-
(7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (0.23
g, 0.606 mmol)
in DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0025 g) as a yellow powder in 1.9% yield.
' H NMR (d6-DMSO): S 12.45 (s, 1 H), 10.47 (s, 1 H), 9.06 (d, 1 H, J= 7.9 Hz),
8.14 (s, 1 H), 8.03 (s,
1 H), 7.70 (d, 2H, J = 7.9 Hz), 7.51-7.62 (m, 4H), 7.38-7.44 (m, 4H), 5.13-
5.22 (m, 1 H), 1.49 (d,
3H, J = 7.2 Hz).
HRMS calculated for C25H2ON402C1443.1275 (M+H), found 443.1265.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
137
Example 96: N-f1-(4-Hydroxyphenyl)ethyll-6-oxo-2-phenyl-5,6-dihydro-1H-
[1,21diazepino[4 5 6-
cdjindole-8-carboxamide
HN-N
O
HO \/ I H I \ ~ -
N N
O H
Preparation of example 96 from the title compound of Example 49 (0.104 g,
0.303 mmol), 1-(4-
hydroxyphenyl)ethylamine (0.0831 g, 0.606 mmol), triethylamine (0.084 mL,
0.606 mmol), and 0-
(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.23
g, 0.606 mmol)
in DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0205 g) as a yellow powder in 16% yield.
'H NMR (d6-DMSO): S 12.42 (s, 1 H), 10.45 (s, 1 H), 9.21 (br s, 1 H), 8.91 (d,
1 H, J= 8.3 Hz), 8.11
(s, 1 H), 8.02 (s, 1 H), 7.70 (d, 2H, J= 6.8 Hz), 7.51-7.61 (m, 4H), 7.21 (d,
2H, J= 8.3 Hz), 6.71 (d,
2H, J= 8.3 Hz), 5.08-5.16 (m, 1 H), 1.46 (d, 3H, J= 7.2 Hz).
HRMS calculated for C25H21N403 425.1614 (M+H), found 425.1626.
Example 97: 2,3-Difluoro-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,21diazepino[4.5,6-cd]indol-8-yl)-
benzamide
HN-N
O N
O \ ~ -
H
I \ I /
H
F
Preparation of example 97 from the title compound of Example 7 (hydrochloride)
(40 mg, 0.128
mmol), 2,3-difluoro-benzoic acid (24 mg, 0.154 mmol), triethylamine (0.054 mL,
0.384 mmol), and
0-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (59
mg, 0.154
mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was carried out
analogously to
Example 11. Silica gel chromatography (eluted with 2:1 hexane:acetone
increasing to 1:1
hexane:acetone), also in an analogous manner, afforded the title compound (24
mg, 0.058 mmol)
as a yellow powder in 45% yield.
'H NMR (d6-DMSO): S 12.20 (s, 1 H), 10.72 (s, 1 H), 10.42 (s, 1 H), 8.24 (s, 1
H), 7.81 (s, 1 H), 7.72-
7.45 (m, 8H), 7.37 (m, 1 H).
LCMS: (M+H+) 417.0, (M+Na+) 439.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
139
(both eluted with 2:1 hexane:acetone increasing to 1:1 hexane:acetone)
afforded the title
compound (14 mg, 0.042 mmol) as a yellow powder in 40% yield.
' H NMR (d6-DMSO): S 11.79 (s, IH), 10.50 (s, 1 H), 10.25 (s, 1 H), 8.20 (s, 1
H), 7.78 (s, 1 H), 7.60
(s, 1 H), 7.49 (s, 1 H), 7.46-7.17 (m, 3H), 2.30 (s, 3H).
LCMS: (M+H+) 337.1, (M+Na+) 359.1.
Example 100: (1R,2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-5.6-dihydro-
lH-
j1,21diazepinof4,5.6-cdlindol-8-yl)-amide
MeO O
Intermediate 2(b) HATU, Et3N, DMF POCI3. DMF
~ ~ I \
Example 2 O \ ~.= / N CH2CI2, 66%
0 H H
~ `'=1~H
100(a)
MeO 0 HN-N
-O a O NH2NH2, AoOH, MeOH 01,;j1k,,WN
~`'=kH H MeOH, reflux, 57% H 100(b)
Step 1. Preparation of 6-[((1R,2R)-2-Phenyl-cyclopropanecarbonyl)-amino]-1H-
indole-4-
carboxylic acid methyl ester 100(a)
Preparation of intermediate 100(a) from Intermediate 2(b) of Example 2 (111
mg, 0.49 mmol),
(1 R,2R)-2-phenyl-cyclopropanecarboxylic acid (119 mg, 0.73 mmol),
triethylamine (0.273 mL,
1.96 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate
(278 mg, 0.73 mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was
carried out
analogously to Example 11. Extractive work-up from ethyl acetate and saturated
aqueous
NaHCO3 afforded crude Intermediate 100(a) (222 mg) as a tan solid which was
carried on without
purification.
Step 2. Preparation of 3-Formyl-6-[((1R,2R)-2-phenyl-cyclopropanecarbonyl)-
amino]-1H-
indole-4-carboxylic acid methyl ester 100(b)
Intermediate 100(a) (214 mg) was dissolved in CH2CI2 (3 mL) and N,N-
dimethylformamide (0.2
mL) and treated with Vilsmeier reagent (0.147 mL) in a manner similar to that
described for
Example 3, Step 4. Upon addition, an immediate precipitate formed causing a
thick slurry.
Additional CH2CI2 (5.0 mL) and N,IV dimethylformamide (0.2 mL) was added to
facilitate stirring.
Additional Vilsmeier reagent (0.147 mL) was also added. After c.a. 10 min,
hexane was added,
and the solids were allowed to settle. After decanting the supernatant,
additional hexane was
added and the trituration was repeated- discarding both triturates. To the
remaining solids,
methanol (8 mL) was added along with K2C03 (750 mg, 5.43 mmol) and H20 (4 mL),
and the
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
138
Example 98: 2,3-Dimethyl-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepinof4.5,6-cAindol-8-yl)-
benzamide
HN-N
O N
0 \ ~ -
I \ I /
H
H
Preparation of example 98 from the title compound of Example 7 (hydrochloride)
(42 mg, 0.134
mmol), 2,3-dimethyl-benzoic acid (24 mg, 0.161 mmol), triethylamine (0.056 mL,
0.402 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(61 mg, 0.161
mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was carried out
analogously to
Example 11. The mixture was stirred as a thick slurry and additional 2,3-
dimethyl-benzoic acid (12
mg, 0.08 mmol) and O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (30 mg, 0.08 mmol) were added after 48 hours to drive the
reaction to
completion. The mixture was filtered to collect the solids which were then
washed with methanol.
After drying the solids under high vacuum, the title compound (32 mg, 0.078
mmol) was obtained
as a yellow powder in 58% yield.
'H NMR (d6-DMSO): S 12.14 (s, 1 H), 10.47 (s, 1 H), 10.39 (s, 1 H), 8.25 (s, 1
H), 7.86 (m, 1 H), 7.72-
7.66 (m, 2H), 7.63-7.49 (m, 4H), 7.33-7.25 (m, 2H), 7.21 (m, 1 H), 2.31 (s,
3H), 2.29 (s, 3H).
LCMS: (M+H+) 409.1, (M+Na+) 431.1.
Example 99: 3-Fluoro-2-methyl-N-(6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cdlindol-8-yl)-
benzamide
HN-N
O
WN
H H
F
Preparation of example 99 from title compound of Example 2 (21 mg, 0.105
mmol), 3-fluoro-2-
methyl-benzoic acid (19 mg, 0.126 'mmol), triethylamine (0.044 mL, 0.315
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (48 mg,
0.126 mmol) in
CH2CI2 (0.2 mL) and N,N-dimethylformamide (0.2 mL) was carried out analogously
to Example
11. The mixture was stirred as a thick slurry and additional 3-fluoro-2-methyl-
benzoic acid (11 mg,
0.07 mmol) and O-(7-azabenzotriazol-l-yl)-NN,N;M tetramethyluronium
hexafluorophosphate
(27 mg, 0.07 mmol) were added after 24 hours to drive the reaction to
completion. Purification,
also in an analogous manner, except that it required two successive silica gel
chromatographies
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
140
mixture was allowed to stir. After c.a. 30 min, ethyl acetate was added and
the K2CO3/H20
aggregates were removed by decanting the product away in solution. The
solvents were then
reduced in volume, additional ethyl acetate was added, and the product was
subjected to
extractive work-up to afford Intemediate 100(b) (117 mg, 0.32 mmol) as a
brownish powder in
about a 66% combined, crude yield over steps I and 2.
Step 3. Preparation of Title Compound: (1R,2R)-2-Phenyl-cyclopropanecarboxylic
acid (6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cal]indol-8-yl)-amide
Intermediate 100(b) (105 mg), acetic acid (0.048 mL, 0.84 mmol) and H2NNH2 ,
H2O (0.084 mL,
1.74 mmol) in anhydrous methanol (4.4 mL) were refluxed in manner similar to
that described for
Example 3, Step 5. The crude product was purified on silica gel `eluting with
2:1 then 1:1
hexane:acetone to afford the title compound (61 mg, 0.17 mmol) in about a 57%
yield for the last
step.
'H NMR (d6-DMSO): S 11.74 (s, 1 H), 10.38 (s, 1 H), 10.23 (s, 1 H), 8.13 (s, 1
H), 7.60-7.53 (m, 2H),
7.46 (s, 1 H), 7.35-7.26 (m, 2H), 7.24-7.15 (m, 3H), 2.38 (m, 1 H), 2.08 (m, 1
H), 1.50 (m, 1 H), 1.36
(m, 1 H).
LCMS: (M+H+) 345.2, (M+Na+) 367.1.
Anal. Calcd. for C20H16N4O2 ' 0.6 H20 ' 0.1 methanol , 0.1 CHZCI2: C, 66.13;
H, 4.89; N, 15.27.
Found: C, 66.19; H, 5.03; N, 15.07.
Altenative Method for the Preparation of Example 100.
Preparation of example 100 from the title compound of Example 2 (3.0 g, 12.7
mmol), (1R,2R)-2-
phenylcyclopropanecarboxylic acid (2.36 g, 14.6 mmol) (prepared as described
by A. Thurkauf, et
al. (2000) J. Med. Chem. 43:3923-3932), triethylamine (8.8 mL, 63.4 mmol), and
O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (5.3 g,
14.5 mmol) in
N,N-dimethylformamide (30.0 mL, 0.4 M) was carried out analogously to Example
11. Silica gel
chromatography (5:50:45 methanol/ethyl acetate/CH2CI2), also in an analogous
manner, afforded
the title compound (3.67 g, 10.7 mmol) as a yellow powder in 84% yield.
Example 101: N-f2-Hydroxy-2-(3-hydroxyphenyl)ethyl]-6-oxo-2-phenyl-5 6-dihydro-
1H-
[1.2]diazepinof4,5.6-cdlindole-8-carboxamide
HN-N
0 OH H \ ~ -
HO I N / N
H
/ 0
Preparation of example 101 from the title compound of Example 49 (0.238 g,
0.693 mmol), 2-
hydroxy-2-(3-hydroxyphenyl)ethylamine hydrochloride (0.264 g, 1.39 mmol),
triethylamine (0.29
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
141
mL, 2.08 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (0.528 g, 1.39 mmol) in DMSO (2 mL) was carried out
analogously to
Example 76. Preparative HPLC (20-100% CH3CN/H20 containing 0.1%
trifluoroacetic acid), also
in an analogous manner, afforded the title compound (0.0414 g) as a yellow
powder in 14% yield.
1 H NMR (ds-DMSO): S 12.47 (s, 1 H), 10.45 (s, 1 H), 9.27 (br s, 1 H), 8.68
(t, 1 H, J= 5.7 Hz), 8.10
(s, 1 H), 8.02 (s, 1 H), 7.71 (d, 2H, J= 7.2 Hz), 7.51-7.62 (m, 4H), 7.08-7.13
(m, 1 H), 6.76-6.81 (m,
2H), 6.61-6.64 (m, 1 H), 4.70-4.74 (m, 1 H).
HRMS calculated for C25H21N404 441.1563 (M+H), found 441.1543.
Example 102: Methyl-6-oxo-2-phen rLl-1,3.5,6-tetrahydrol1.21oxazepinof6,5,4-
cdlindole-8-
carboxylate
MeO O O
NaH, (t-B0c)20 NaBH4, MeOH
Intermediate 39(e)
Do-
Example 39 THF, 67 lo Me0 I/ N 92%
O Boc
102(a)
0
Me0 O OH Me6ib HO-N ~/
::: Me0 Me0 Na2CO3, DMSO
O Boc O 55 l0
102(b) 102(c)
`
O
O HN-O
MeO 00 0
1. NH2NH2, MeOH, reflux ~ ~ -
Me0 Me0 I / N
N 2. TFA, CH202, 44% H
Boc
102(d)
Step I. Preparation of 1-tert-butyl-4,6-dimethyl-3-formyl-2-phenyl-lH-indole-
1,4,6-
tricarboxylate 102(a)
To a solution of Intermediate 39(e) of Example 39 (2.25 g, 6.7 mmol), and di-
tert-butyl dicarbonate
(11.68 g, 54 mmol) in 150 ml of tetrahydrofuran was added a 60% suspension of
NaH in mineral
oil (1.60 g, 40 mmol). The reaction mixture was stirred at room temperature
for 1 hour. Extractive
work-up from ethyl acetate and saturated aqueous NaHCO3 followed by silica gel
chromatography
afforded Intermediate 102(a) (1.97 g), in 67% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
142
'H NMR (ds-DMSO): S 9.56 (s, 1 H), 8.96 (s, 1 H), 8.08 (s, 1 H), 7.70-7.50 (m,
5H), 3.93 (s, 3H),
3.83 (s, 3H), 1.20 (s, 9H).
Step 2. Preparation of 1-(te-t-Butoxycarbonyl)-3-(hydroxymethyl)-6-
(methoxycarbonyl)-2-
phenyl-lH-indole-4-carboxylic acid 102(b)
Intermediate 102(a) (1.95 g) was dissolved in methanol (200 mL) and NaBH4
(1.70 g) was added
and stirred for 15 min. After removing solvent, silica gel chromatography
afforded Intermediate
102(b) (1.81 g) in 92% yield.
'H NMR (d6-DMSO): S 8.97 (d, 1 H, J= 1.5 Hz), 8.10 (d, 1 H, H=1.5 Hz), 7.35-
7.55 (m, 5H), 4.61 (t,
1 H, J= 5.1 Hz), 4.41 (d, 2H, J= 5.0 Hz), 3.92 (s, 3H), 3.87 (s, 3H), 1.16 (s,
9H).
Step 3. Preparation of 1-tert-Buty1-4,6-dimethyl-3-(chloromethyl)-2-phenyl-lH-
indole-1,4,6-
tricarboxylate 102(c)
To a solution of Intermediate 102(b) (1.68 g, 3.8 mmol) and CCI4 (3.50 g, 23
mmol) in 20 ml of
dichloromethane was added Ph3P (2.42 g, 9.2 mmol). The reaction mixture was
stirred at room
temperature for 24 hours. The reaction mixture was concentrated, and residue
was purified using
silica gel chromatography to afford Intermediate 102(c) (1.53 g) in 87% yield.
' H NMR (d6-DMSO): 5 8.97 (d, 1 H, J= 1.5 Hz), 8.10 (d, 1H, J= 1.5 Hz), 7.35-
7.55 (m, 5H), 4.61
(t, 1 H, J= 5.1 Hz), 4.41 (d, 2H, J= 5.0 Hz), 3.92 (s, 3H), 3.87 (s, 3H), 1.16
(s, 9H).
Step 4. Preparation of 1-tert-Butyl 4,6-dimethyl 3-{[(1,3-dioxo-1,3-dihydro-2H-
isoindol-2-
yl)oxy]methyl}-2-phenyl-1 H-indole-1,4,6-tricarboxylate 102(d)
A mixture of Intermediate 102(c) (0.23 g, 0.5 mmol), N-hydroxyphthalimide
(0.24 g, 1.5 mmol) and
Na2CO3 (0.32 g, 3 mmol) was stirred in anhydrous DMSO (10 mL) at room
temperature overnight.
Extractive work-up from ethyl acetate, followed by silica gel chromatography
afforded Intermediate
102(d) (0.16 g) in 55% yield.
'H NMR (d6-DMSO): S 9.02 (d, 1H, J = 1.1 Hz), 8.31 (d, IH, H=1.1 Hz), 7.75-
7.82 (m, 2H), 7.64-
7.71(m, 2H), 7.39 (t, 1 H, J = 7.6 Hz), 7,26 (t, 2H, J = 7.6), 7.18 (d, 2H, J=
7.8 Hz), 5.32 (s, 2H),
3.94 (s, 3H), 3.92 (s, 3H), 1.11 (s, 9H).
Step 5. Preparation of Title Compound Methyl-6-oxo-2-phenyl-1,3,5,6-
tetrahydro[1,2]oxazepi no[6,5,4-cdj indole-8-carboxylate
A mixture of Intermediate 102(d) (0.15 g, 0.26 mmol) and hydrazine (0.20 g,
6.3 mmol) in
methanol (15 mL) was refluxed for 2 hours. After solvent evaporation, the
residue was mixed with
CH2CI2 (15 ml) and trifluoroacetic acid (7.5 ml) and stirred for 2 hours.
After solvent removal, the
residue was subjected to reverse-phase preparative HPLC affording the title
compound (34.8 mg)
in 42% yield.
' H NMR (d6-DMSO): S 12.20 (s, 1 H), 11.23 (s, 1 H), 8.30 (d, 1 H, J= 1.2 Hz),
8.21 (d, 1 H, H=1.2
Hz), 7.40-7.75 (m, 5H), 5.44 (d, 1 H, J= 14.7 Hz), 5.22 (d, 1 H, J= 14.7 Hz),
3.92 (s, 3H).
LCMS (M++1): 323.0
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
143
Example 103: N-(4-Fluorobenzyl)-6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cd]indole-
8-carboxamide
HN-N
O
H I \ -
N N
O H
Preparation of example 103 from the title compound of Example 49 (0.108 g,
0.314 mmol), 4-
fluorobenzylamine (0.079 g, 0.628 mmol), triethylamine (0.088 mL, 0.628 mmol),
and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.239 g,
0.628 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0121 g) as a yellow powder in 9.3% yield.
'H NMR (d6-DMSO): S 12.47 (s, 1 H), 10.47 (s, 1 H), 9.23 (t, 1 H, J= 5.9 Hz),
8.13 (s, 1 H), 8.05 (s,
1H), 7.71 (d, 2H, J = 6.8 Hz), 7.51-7.63 (m, 4H), 7.35-7.40 (m, 2H), 7.12-7.18
(m, 2H), 4.46 (d,
2H, J = 5.7 Hz).
HRMS calculated for C24Hj8N402F 413.1414 (M+H), found 413.1394.
Example 104: 6-Oxo-2-phenyl-N-(2,3,5-trifluorobenzyl)-5,6-dihydro-1 H-
[1,2]diazepinof4,5,6-
cd]indole-8-carboxamide
HN-N
F O
H I \ ~ -
F \ N / N
H
F O
Preparation of example 104 from the title compound of Example 49 (0.101 g,
0.294 mmol), 2,3,5-
trifluorobenzylamine (0.0947 g, 0.588 mmol), triethylamine (0.082 mL, 0.588
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;Mtetramethyluronium hexafluorophosphate (0.224 g,
0.588 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.006 g) as a yellow powder in 4.5% yield.
' H NMR (d6-DMSO): S 12.50 (s, 1 H), 10.49 (s, 1 H), 9.27 (t, 1 H, J = 5.9
Hz), 8.13 (s, 1 H), 8.05 (s,
1 H), 7.71 (d, 2H, J = 6.8 Hz), 7.52-7.62 (m, 4H), 7.40-7.48 (m, 1 H), 7.05-
7.08 (m, 1 H), 4.56 (d,
2H, J = 4.9 Hz).
HRMS calculated for C24H16N402F3 449.1225 (M+H), found 449.1209.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
144
Example 105: N-f3,5-Bis(trifluoromethyl)benzyl]-6-oxo-2-phenyl-5 6-dihydro-1H-
[1,2]diazepinof4,5,6-cd]indole-8-carboxamide
HN-N
CF3 O \
H I \ ~ -
F3C N N
H
O
Preparation of example 105 from the title compound of Example 49 (0.101 g,
0.294 mmol), 3,5-
bis(trifluoromethyl)benzylamine (0.143 g, 0.588 mmol), triethylamine (0.082
mL, 0.588 mmol), and
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(0.224 g, 0.588
mmol) in DMSO (2 mL) was carried out analogously to Example 76. Preparative
HPLC (20-100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.004 g) as a yellow powder in 2.6% yield.
'H NMR (d6-DMSO): S 12.50 (s, 1 H), 10.50 (s, 1 H), 9.37 (t, IH, J= 5.9 Hz),
8.14 (s, 1 H), 8.05 (m,
3H), 8.00 (s, I H), 7.71 (d, 2H, J = 8.3 Hz), 7.52-7.62 (m, 4H), 4.67 (d, 2H,
J = 5.7 Hz).
HRMS calculated for C26H17N402F6 531.1256 (M+H), found 531.1272.
Example 106: N-[4-Fluoro-3-(trifluoromethyl)benzLrl]-6-oxo-2-phenyl-5.6-
dihydro-1 H-
j1.2]diazepino[4,5,6-cd]indole-8-carboxamide
HN-N
O
F
H I \ ~ -
F3C \ N / N
H
o
Preparation of example 106 from the title compound of Example 49 (0.105 g,
0.306 mmol), 4-
fluoro-3- (trifluoromethyl)benzylamine (0.118 g, 0.612 mmol), triethylamine
(0.085 mL, 0.612
mmol), and O-(7-azabenzotriazol-1-yl)=N,N,N;M tetramethyluronium
hexafluorophosphate (0.233
g, 0.612 mmol) in DMSO (2 mL) was carried out analogously to Example 76.
Preparative HPLC
(20-100% CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous
manner,
afforded the title compound (0.0205 g) as a yellow powder in 14% yield.
' H NM,R (d6-DMSO): S 12.49 (s, 1 H), 10.48 (s, 1 H), 9.30 (t, 1 H, J= 5.9
Hz), 8.13 (s, IH), 8.05 (m,
3H), 7.69-7.75 (m, 4H), 7.45-7.63 (m, 5H), 4.53 (d, 2H, J = 5.7 Hz).
HRMS. calculated for C25H17N402F4 481.1287 (M+H), found 481.1291.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
145
Example 107: N-f(1-Hydroxy-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-
yl)methyll-6-oxo-2-
phenyl-5,6-dihydro-1 H-[1,21diazepino[4.5,6-cd]indole-8-carboxamide
HN-N
O ~
\
N ~ / N ~
H O H
Preparation of example 107 from the title compound of Example 49 (0.104 g,
0.303 mmol), 1-
(aminomethyl)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-ol (0.124 g, 0.606
mmol),
triethylamine (0.084 mL, 0.606 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (0.23 g, 0.606 mmol) in DMSO (2 mL) was
carried out
analogously to Example 76. Preparative HPLC (20-100% CH3CN/H20 containing 0.1%
trifluoroacetic acid) also in an analogous manner afforded the title compound
(0.0145 g) as a
yellow powder in 9.7% yield.
' H NMR (d6-DMSO): 8 12.47 (s, 1 H), 10.48 (s, 1 H), 8.48-8.52 (m, 1 H), 8.10
(s, 1 H), 8.03 (s, 1 H),
7.70-7.73 (m, 2H), 7.52-7.62 (m, 4H), 7.25 (s, 1 H), 6.86 (s, 1 H), 3.66-3.73
(m, 2H), 2.55-2.63 (m,
2H), 2.22 (s, 3H), 2.14 (s, 3H), 1.81-1.98 (m, 4H).
HRMS calculated for C30H29N403 493.2240 (M+H), found 493.2252.
Example 108: N-[(1R)-1-(1-Naphthyl)ethyl]-6-oxo-2-phenyl-5.6-dihydro-lH-
[1,2]diazepino[4,5,6-
cd]indole-8-carboxamide
HN-N
O
H I \ \ _
N N h
I / - O H
Preparation of example 108 from the title compound of Example 49 (0.105 g,
0.306 mmol), 1-(1-
naphthyl)ethylamine (0.105 g, 0.612 mmol), triethylamine (0.085 mL, 0.612
mmol), and O-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.233 g,
0.612 mmol) in
DMSO (2 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0161 g) as a yellow powder in 12% yield.
'H NMR (ds-DMSO): S 12.44 (s, 1 H), 10.46 (s, 1 H), 9.14 (d, 1 H, J= 7.9 Hz),
8.17 (s, 1 H), 8.05 (s,
1 H), 7.87-7.90 (m, 4H), 7.69-7.72 (m, 2H), 7.46-7.63 (m, 7H), 5.34-5.140 (m,
1 H), 1.61 (d, 3H, J=
6.8 Hz).
HRMS calculated for C29H23N402 459.1821 (M+H), found 459.1795.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
146
Example 109: Diethyl 2-{[(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,2]diazepino[4,5.6-
cd]indol-8-
yl)carbonxllamino}malonate
HN-N
O
0 N / N
H
0 0 0
Preparation of example 109 from the title compound of Example 49 (0.106 g,
0.309 mmol), diethyl
2-aminomalonate hydrochloride (0.131 g, 0.618 mmol), triethylamine (0.129 mL,
0.926 mmol),
and O-(7-azabenzotriazol-l-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate
(0.235 g,
0.618 mmol) in DMSO (2 mL) was carried out analogously to Example 76.
Preparative HPLC (20-
100% CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous
manner, afforded
the title compound (0.0064 g) as a yellow powder in 4.5% yield.
'H NMR (d6-DMSO): S 12.53 (s, 1 H), 10.50 (s, 1 H), 9.55 (t, 1 H, J= 7.6 Hz),
8.14 (s, 1 H), 8.07 (s,
1 H), 7.71-7.73 (m, 2H), 7.52-7.62 (m, 4H), 5.29-5.32 (m, 2H), 4.15-4.25 (m,
4H), 1.23 (t, 6H, J=
7.2 Hz).
HRMS calculated for C24H23N406 463.1618 (M+H), found 463.1606.
Example 110: N-[(1R)-2-Hydroxy-1-phenylethyl]-6-oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indole-8-carboxamide
HN-N
0
H I \ ~ -
N N
H
O
HO
Preparation of example 110 from the title compound of Example 49 (0.17 g, 0.5
mmol), (2R)-2-
amino-2-phenylethanol (0.0822 g, 0.6 mmol), triethylamine (0.14 mL, 1 mmol),
and O-(7-
azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (0.23 g,
0.6 mmol) in
DMSO (8 mL) was carried out analogously to Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1 % trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.0351 g) in 17% yield.
'H NMR (d6-DMSO): S 12.45 (s, 1 H), 10.47 (s, 1 H), 8.92 (d, 1 H, J= 7.9 Hz),
8.17 (s, 1 H), 8.04 (s,
1 H), 7.75-7.65 (m, 2H), 7.65-7.50 (m, 4H), 7.45-7.37 (m, 2H), 7.37-7.27 (m,
2H), 7.27-7.15 (m,
1 H), 5.18-5.01 (m, 1 H), 3.83-3.60 (m, 2H).
LCMS: (M+H+) 425.1
Anal. Calcd. for C25H20N403 = 0.2 trifluoroacetic acid = 1.68 H20: C, 63.88;
H, 4.97; N, 11.73.
Found: C, 63.86; H, 4.97; N, 11.66.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
148
Step 2. Preparation of (1 R,2R)-Cyclobutyl-(3-{6-oxo-8-[(2-phenyl-
cyclopropanecarbonyl)-
amino]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cdjindol-2-yl}-benzyl)-carbamic
acid tert-butyl
ester 111(b)
In a manner analogous to steps 4 and 5 of Example 3, Intermediate 111(a) (0.13
g, 0.22 mmol)
was formylated and cyclized. After silica gel chromatography, Intermediate
111(b) (0.107 g, 0.18
mmol) was obtained as a yellow powder in 81 % yield.
'H-NMR (ds-DMSO): S 12.09 (s, 1 H), 10.43 (s, 1 H), 10.37 (s, 1 H), 8.16 (d, 1
H, J= 1.2 Hz), 7.63
(d, 1 H, J = 1.4 Hz), 7.55-7.42 (m, 4H), 7.35-7.16 (m, 6H), 4.53 (s, 2H), 2.45-
2.37 (m, 1 H), 2.14-
1.95 (m, 5H), 1.60-1.23 (m, 14H).
LCMS: (M-H) 602.2.
Step 3. Preparation of Title Compound: (IR,2R)-2-Phenyl-cyclopropanecarboxylic
acid [2-
(3-cyclobutylaminomethyl-phenyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl]-
amide (hydrochloric salt)
Preparation from Intermediate 111(b) (0.105 g, 0.17 mmol) and 4M HCI in
dioxane (1.7 mL) was
carried out analogously to Example 91. Isolation, also in an analogous manner,
included a further
trituration with CH2CI2/diethyl ether and afforded the title compound (0.09 g,
0.17 mmol) as an
orange/yellow powder in 96% yield.
1 H-NMR (d6-DMSO): S 12.20 (s, 1 H), 10.49 (s, 1 H), 10.43 (s, 1 H), 9.32 (b,
1 H), 8.18 (d, 1 H, J
1.4 Hz), 7.83 (s, 1 H), 7.75-7.58 (m, 5H), 7.35-7.15 (m, 5H), 4.13 (s, 2H),
3.80-3.50 (buried m, 1 H),
2.45-2.35 (m, 1 H), 2.28-2.08 (m, 5H), 1.88-1.75 (m, 2H), 1.55-1.47 (m, 1 H),
1.42-1.34 (m, 1 H).
LCMS: (M+H+) 504.2.
HRMS: (M+H+) calcd for C31H30N502i 504.2400, found 504.2378.
Example 112: f1R2Rl-2-Phenyl-cyclopropanecarboxylic acid f6-oxo-2-(3-
pyrrolidin-1-ylmethyl-
phenyl)-5.6-dihvdro-lH-f1.21diazepinof4 5 6-cd]indol-8-yll-amide (hydrochloric
salt)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
147
Example 111: (1 R,2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(3-
cyclobutylaminomethyl-
phenyl)-6-oxo-5.6-dihydro-1H-f1,2]diazepino[4,5,6-cdjindol-8-yl]-amide
(hydrochloric salt)
p
1. (CH3S02)20 0 OMe BocN
Intermediate 85(a) 2,4,6-Collidine, CH2CI2 10 asv'kOH I\ \
Example 85 2. ~NHa N
/ \ /
H
3. BocaO, Et3N, CH2CI2 111(a)
40%
p
HN-N BocN
0
1. POCI3. DMF, CH2CI2 0 \ \ _ ::,:::
.
MeOH, reflux, 81 % H H
111(b)
p
HN-N CIH.HN
0
0 \ \ -
,~~N N
H H
Step 1. Preparation of (IR,2R)- 2-{3-[(tert-Butoxycarbonyl-cyclobutyl-amino)-
methyl]-
phenyl)-6-[(2-phenyl-cyclopropanecarbonyl)-amino]-1H-indole-4-carboxylic acid
methyl
ester 111(a)
To a suspension of Intermediate 85(a) of Example 85 (0.260 g, 0.6 mmol) in
dichloromethane (6.0
mL, 0.1 M) was added 2,4,6-Collidine (0.312 mL, 2.4 mmol) followed by
(CH3SO2)20 (0.123 g, 0.7
mmol). After stirring for 1 hour, cyclobutylamine (0.252 mL, 3.0 mmol) was
added, and the
reaction mixture stirred for 24 hours at 22 C. The volatiles were removed in
vacuo and
dichloromethane (6.0 mL), triethylamine (3 mmol) and di-tert-butyl dicarbonate
(1.2 mmol) were
added. The mixture was stirred at 22 C for 12 hours and volatiles were
removed in vacuo. Silica
gel chromatography provided Intermediate 111(a) (0.14 g, 0.24 mmol) in 40%
yield.
'H-NMR (d6-DMSO): S 11.82 (s, 1 H), 10.42 (s, 1 H), 8.27 (s, 1 H), 7.89 (d, 1
H, J= 1.6 Hz), 7.72 (d,
1 H, J = 8.1 Hz), 7.67 (s, 1 H), 7.44 (dd, 1 H, J = 7.7, 7.6 Hz), 7.36-7.08
(m, 7H), 4.51 (s, 2H), 3.91
(s, 3H), 2.45-2.37 (m, 1 H), 2.14-1.95 (m, 5H), 1.62-1.22 (m, 14H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
149
OMe '-N
1. (CH3SO2)2O O
Intermediate 85(a) 2,4,6-Collidine, CH2CI2 / I I\ C
Example 85 ^ \ ~~.= N / N
2. I NH 0 H H
~/ 112(a)
1. POCI3. DMF, CH2CI2 O HN-N ~HCI
2. NHZNHZ.HaO, ACOH O \
I\ -
MeOH, reflux .JJ 'N / N ~ ~
H
3. 4N HCI (Dioxane)
CH2CI2, 9%
Step 1. Preparation of (1R,2R) 6-[(2-Phenyl-cyclopropanecarbonyl)-amino]-2-(3-
pyrrolidin-l-
ylmethyl-phenyl)-1H-indole-4-carboxylic acid methyl ester 112(a)
To a suspension of Intermediate 85(a) of Example 85 (0.260 g, 0.6 mmol) in
dichloromethane (6.0
mL, 0.1 M) was added 2,4,6-Collidine (0.312 mL, 2.4 mmol) followed by
(CH3SO2)20 (0.123 g, 0.7
mmol). After stirring for 1 hour, pyrrolidine (0.252 mL, 3.0 mmol) was added
and the reaction
mixture stirred for 24 hours at 22 C. Volatiles were removed in vacuo, and
the crude Intermediate
112(a) wa's carried on directly to the next step.
Step 2. Preparation of Title Compound: (IR,2R)-2-Phenyl-cyclopropanecarboxylic
acid [6-
oxo-2-(3-pyrrolidin-1-ylmethyl-phenyl)-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yi]-
amide (hydrochloric salt)
In a manner analogous to steps 4 and 5 of Example 3, Intermediate 112(a)
(0.42g, 0.85 mmol)
was formylated and cyclized. Silica gel chromatography (90:10:0 to 70:20:10
CH2CI2/2.0 M
ammonia in isopropyl alcohol/methyl alcohol) and conversion to the HCI salt
(4M HCI in dioxane)
afforded the title compound (0.04 g, 0.18 mmol) as a yellow-orange powder in
9% yield.
'H-NMR (d6-DMSO): 8 12.20 (s, 1 H), 10.55 (s, 1 H), 10.49 (s, 1 H), 10.43 (b,
1 H), 8.19 (d, 1 H, J=
1.3 Hz), 8.13 (s, 1 H), 7.76-7.62 (m, 5H), 7.34-7.16 (m, 5H), 4.45 (d, 2H, J=
5.5 Hz), 3.45-3.35 (m,
2H), 3.19-3.07 (m, 2H), 2.45-2.35 (m, 1 H), 2.14-1.85 (m, 5H), 1.55-1.47 (m, 1
H), 1.42-1.34 (m,
1 H).
LCMS: (M+H+) 504.2.
HRMS: (M+H+) calcd for C31H30N502i 504.2400, found 504.2404.
Example 113: N-(6-Oxo-2-phenyl-5,6-dihydro-1 H-[1.2]diazepino[4.5,6-cdJindol-8-
yiL-(1.2-trans)-2-
[6-(trifluoromethyl)pyridin-3-Ll]cyclopropanecarboxamide trifluoroacetate
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
150
F3C F3C
NaH, triethyl phosphite Me3SO+I-, NaH
N~ O N / OMe
THF, 0 C-RT, 42% DMSO, 53%
H 0
113(a)
F3C / I O LiOH=H2O F3C 0 HATU, Et3N, DMSO, 12%
N~ Me 1:1 THF:H20, 98% N H Title Compound
of Example 7
113(b) 113(c)
HN-N
O
F3C / O \
N'I N I/ N
CF3CO2H H
Step 1. Preparation of 3-(6-Trifluoromethyl-pyridin-3-yl)-acrylic acid methyl
ester 113(a)
To a solution of triethyl phosphite in anhydrous tetrahydrofuran (25 mL)
cooled to 0 C was added
a 60% suspension of sodium hydride in mineral oil (472 mg, 19.7 mmol) in small
portions. This
mixture was allowed to stir for 30 min at the same low temperature at which
point the cooling bath
was removed, and the mixture was allowed to warm to room temperature over 60
min. The
mixture was cooled again to 0 C and a solution of 6-trifluoromethyl-3-pyridine
carboxaldehyde in
anhydrous tetrahydrofuran (20 mL) was added dropwise. The reaction mixture was
allowed to
warm slowly to room temperature overnight. After 19 hours, the reaction
mixture was quenched
with water (100 mL) and extracted with ethyl acetate (3 x 50 mL). The combined
organic fractions
were dried over anhydrous magnesium sulfate and concentrated to a pale green
oil. Purification
was carried out using flash silica gel chromatography eluting with 5:95 then
1:9 ethyl
acetate:hexane. Pure fractions were combined and concentrated to afford
Intermediate 113(a)
(1.68 g, 6.85 mmol) as a white solid in 42% yield.
Step 2. Preparation of (1,2-frans)-2-(6-Trifluoromethyl-pyridin-3-yl)-
cyclopropanecarboxylic
acid 113(b)
Trimethylsulfoxonium iodide (574 mg, 2.61 mmol) was added to 60% NaH in
mineral oil (63 mg,
2.61 mmol), and the flask was purged with nitrogen. Methyl sulfoxide (10 mL)
was added slowly
over 20 minutes until evolution of hydrogen ceased. To this milky solution was
added Intermediate
113(a) (493 mg, 2.01 mmol) in methyl sulfoxide (15 mL) dropwise. The solution
was allowed to stir
at room temperature overnight. After 26 hours the excess NaH was carefully
quenched with water
(100 mL). Ethyl ether (100 mL) was added and the layers separated. The aqueous
layer was
extracted with fresh ethyl ether (3 x 50 mL). The combined ethereal layers
were washed with
brine, dried over anhydrous magnesium sulfate and concentrated in vacuo. Flash
silica gel
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
151
chromatography of the crude residue eluting with 1:9 then 1:4 ethyl
acetate:hexane gave two
pure fractions that upon combining and concentrating under reduced pressure
yielded
Intermediate 113(b) (275 mg, 1.06 mmol) as white feathers in 53% yield.
Step 3. Preparation of (1,2-trans)-2-[6-(Trifluoromethyi)pyridin-3-
yl]cyclopropanecarboxylic
acid 113(c)
To a solution of Intermediate 113(b) (275 mg, 1.06 mmol) in a 1:1 mixture of
tetrahydrofuran:water (4 mL) was added lithium hydroxide monohydrate (133 mg,
3.18 mmol).
The semi-suspension was allowed to stir at room temperature over 2 days. The
reaction mixture
was acidified with 2M aqueous hydrochloric acid (c.a. 2 mL). The reaction
mixture was then
concentrated and lyophilized to obtain Intermediate 113(c) as a white powder
containing lithium
chloride which was carried on directly without further purification.
Step 4. Preparation of Title Compound: N-(6-Oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-2-[6-(trifluoromethyl)pyridin-3-
yI]cyclopropanecarboxamide trifluoroacetate
To a solution of Intermediate 113(c) (85 mg, 0.368 mmol) in methyl sulfoxide
(2 mL) was added
triethylamine (0.056 mL, 0.405 mmol) and O-(7-azabenzotriazoi-1-yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (154 mg, 0.405 mmol). After c.a. 10
min, the title
compound of Example 7 (0.102 g, 0.368 mmol) was added. The reaction was capped
and stirred
overnight at room temperature. The mixture was subjected to preparative HPLC
(20-100%
acetonitrile/water containing 0.1% trifluoroacetic acid). The pure fractions
were combined and
lyophilized to afford the title compound (27 mg, 0.045 mmol) as an orange
powder in 12% yield.
'H-NMR (d6-DMSO): S 12.11 (b, 1 H), 10.50 (b, 1 H), 10.39 (b, 1 H), 8.71 (b, 1
H), 8.15 (b, 2H), 7.90-
7.80 (m, 2H), 7.70-7.45 (m, 6H), 2.65-2.55 (m, 1 H), 2.27-2.18 (m, 1 H), 1.67-
1.51 (m, 2H).
LCMS: (M+H+) 490.1.
Example 114: (2R)-2-Amino-N-(6-oxo-2-phenyl-5 6-dihydro-lH-[1 2]diazepino[4 5
6-cd]indol-8_yl)-
2-phenyl-acetamide (hydrochloric salt)
HN-N
O
\ I N N
K
H H
H-Cl NH2
Preparation of example 114 from the impure title compound of Example 117 (90
mg, 0.18 mmol)
and 4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, afforded the title compound (65 mg, 0.10 mmol) as a yellow
powder in 71%
yield over two steps (including Example 117).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
152
'H NMR (ds-DMSO): 5 12.26 (s, IH, exchanges), 11.08 (s, 1 H, exchanges), 10.42
(s, 1 H,
exchanges), 8.86 (br s, 3H, exchanges), 8.07 (s, 1 H), 7.72-7.63 (m, 5H), 7.62-
7.41 (m, 7H), 5.22
(m, 1 H).
LCMS: (M+H+) 410.1, (M+Na+) 432Ø
Anal. Calcd. for C24H19N502 ' 5.6 HCI ' 0.2 diethyl ether: C, 47.40; H, 4.27;
N, 11.14.
Found: C, 47.64; H, 4.21; N, 10.91.
Example 115: (2R)-2-Amino-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-f
1,2]diazepino[4,5,6-cd]indol-8-yl)-
3-phenxl-propionamide (hydrochloric salt)
HN-N
o
WN
N
NHz H H
H-Cl
Preparation of example 115 from the title compound of Example 118 (71 mg,
0.136 mmol) and
4M HCI in dioxane (20 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, afforded the title compound (66 mg, 0.12 mmol) as a yellow
powder in 87%
yield over two steps (from Example 118).
'H NMR (d6-DMSO): S 12.20 (s, IH, exchanges), 10.66 (s, 1 H, exchanges), 10.44
(s, 1 H,
exchanges), 8.39 (br s, 3H, exchanges), 8.05 (s, 1 H), 7.73-7.46 (m, 7H), 7.39-
7.22 (m, 5H), 4.19
(m, 1 H), 3.21-3.06 (m, 2H, partially obscured).
LCMS: (M+H+) 424.1.
Anal. Calcd. for C25H21N502 ' 2.8 HCI ' 0.1 diethyl ether' 0.2 dioxane: C,
57.15; H, 4.83; N, 12.72.
Found: C, 57.33; H, 5.01; N, 12.56.
Example 116: 1-Amino-cyclohexanecarboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-y)-amide (hydrochloric salt)
HN-N
O
O
H2N N N
H H-Cl
H
Preparation of example 116 from the title compound of Example 119 (311 mg,
0.062 mmol) and
4M HCI in dioxane (5 mL) was carried out analogously to Example 91. Isolation,
also in an
analogous manner, included a further trituration with CH2CI2/hexane and
afforded the title
compound (29 mg, 0.053 mmol) as an orange/yellow powder in 85% yield.
'H NMR (d6-DMSO): 8 12.29 (s, 1 H, exchanges), 10.44 (s, IH, exchanges), 10.28
(s, 1 H,
exchanges), 8.36 (br s, 3H, exchanges), 8.11 (s, 1 H), 7.81 (s, 1 H), 7.74-
7.46 (m, 6H), 2.37-2.14
(m, 2H), 1.96-1.35 (m, 8H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
153
LCMS: (M+H+) 402.2, (M+Na+) 424.1.
Anal. Calcd. for C23HZ3N5OZ * 3.1 HCI, 0.1 diethyl ether, 0.3 dioxane: C,
53.88; H, 5.42; N, 12.77.
Found: C, 53.86; H, 5.60; N, 12.70.
Example 117: f(R)-(6-Oxo-2-phenyl-5 6-dihydro-lH-[1 2ldiazepino[4 5 6-cdlindol-
8-ylcarbamoyl)-
phenyl-methyll-carbamic acid tert-butyl ester
HN-N
O
\ I N I / N
NHBoU H
Preparation of example 117 from the the title compound of Example 7
(hydrochloride) (44 mg,
0.141 mmol), (R)-tert-butoxycarbonylamino-phenyl-acetic acid (42 mg, 0.169
mmol), triethylamine
(0.059 mL, 0.423 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (64 mg, 0.169 mmol) in CH2CI2 (0.4 mL) and N,N-
dimethylformamide (014
mL) was carried out analogously to Example 11. Silica gel chromatography
(eluted with 1:1
hexane:acetone), also in an analogous manner, afforded the title compound (102
mg) as a yellow
powder contaminated with an unknown impurity. The compound was an intermediate
and carried
on without further purification to Example 91.
'H NMR (d6-DMSO): 5 12.12 (s, 1H), 10.41 (s, 2H), 8,12 (s, 1H), 7.69-7.48 (m,
10H), 7.42-7.27
(m, 3H), 5.36 (d, 1 H, J= 8.67 Hz), 1.41 (s, 9H).
LCMS: (M-H-) 508.1, (M+H+) 510.2, (M+Na+) 532.2.
Example 118: r(R)-1-(6-Oxo-2-phenyl-5.6-dihydro-1H-[1.2]diazepinof4 5 6-
cdlindol-8-
ylcarbamoyl)-2-phenyl-ethyll-carbamic acid tert-butyl ester
HN-N
O
O \ \ -
~ ~
0'-U = N I / N
HBoH
H
Preparation of example 118 from the title compound of Example 7
(hydrochloride) (43 mg, 0.138
mmol), (R)-tert-butoxycarbonylamino-3-phenyl-propionic acid (44 mg, 0.166
mmol), triethylamine
(0.058 mL, 0.414 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (63 mg, 0.166 mmol) in CH2CI2 (0.4 mL) and N,N-
dimethylformamide (0.4
mL) was carried out analogously to Example 11. Silica gel chromatography
(eluted with 1:1
hexane:acetone), also in an analogous manner, afforded the title compound (80
mg) as a yellow
powder contaminated with an unknown impurity. The compound was carried on
without further
purification to Example 115.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
154
' H NMR (ds-DMSO): S 12.12 (1 H), 10.41 (s, 1 H), 10.25 (s, 1 H), 8.18 (s, 1
H), 7.71-7.48 (m, 7H),
7.38-7.11 (m, 6H), 4.34 (br s, 1 H), 3.02 (m, 1 H), 2.87 (m, 1 H, partially
obscured by N,N-
dimethylformamide singlet), 1.34 (s, 9H).
LCMS: (M+H+) 524.2, (M+Na+) 546.2.
Example 119: f1-(6-Oxo-2-phenyl-5 6-dihydro-lH-[1 2ldiazegino[4 5 6-cdlindol-8-
ylcarbamoyl)-
cyclohexyll-carbamic acid tert-butyl ester
HN-N
O BocH
N N
WN
H H
Preparation of example 119 from the title compound of Example 7
(hydrochloride) (45 mg, 0.142
mmol), 1-tert-butoxycarbonylamino-cyclohexanecarboxylic acid (42 mg, 0.171
mmol),
triethylamine (0.059 mL, 0.426 mmol), and O-(7-azabenzotriazol-l-yi)-N,N,N;N=
tetramethyluronium hexafluorophosphate (65 mg, 0.171 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethylformamide (0.4 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 1:1 hexane:acetone), also in an analogous manner,
afforded the title
compound (38 mg, 0.076 mmol) as a yellow powder in 53% yield.
' H NMR (d6-DMSO): S 12.08 (s, 1 H), 10.37 (s, 1 H), 9.59 (s, 1 H), 8.14 (s, 1
H), 7.70-7.48 (m, 8H),
2.03-1.92 (m, 2H), 1.81-1.71 (m, 2H), 1.47 (m, 5H), 1.40-1.31 (m, 10H).
LCMS: (M+H+) 502.1, (M+Na+) 524.1.
Example 120: (3E)-4-Phenyl-but-3-enoic acid (6-oxo-2-phenyl-5 6-dihydro-1 H-
[1,2]diazepino[4.5,6-cdlindol-8-yl)-amide
HN-N
O ~
0-1/ O ~ N ~ N
H H
Preparation of example 120 from the title compound of Example 7
(hydrochloride) (44 mg, 0.141
mmol), (E)-4-phenyl-but-3-enoic acid (27 mg, 0.169 mmol), triethylamine (0.059
mL, 0.423 mmol),
and O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(64 mg, 0.169
mmol) in CHZCI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was carried out
analogously to
Example 11. When the reaction was judged complete, the mixture was filtered to
collect the
solids which were then washed with methanol. After drying under vacuum, the
title compound (41
mg, 0Ø095 mmol) was obtained as a yellow powder in 68% yield.
'H NMR (d6-DMSO): S 12.10, (s, 1 H, exchanges), 10.38 (s, 1 H, exchanges),
10.22 (s, IH,
exchanges), 8.21 (s, 1 H), 7.77-7.63 (m, 3H), 7.60-7.53 (m, 2H), 7.53-7.42 (m,
4H), 7.38-7.30 (m,
2H), 7.24 (m, 1 H), 6.57 (d, 1 H, J= 16.01 Hz), 6.45 (m, 1 H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
155
'H NMR (ds-DMSO/DCI): 5 8.20 (s, 1 H), 7.75 (s, 1 H), 7.71-7.65 (m, 2H), 7.60-
7.47 (m, 4H), 7.43-
7.37 (m, 2H), 7.38-7.29 (m, 2H), 7.22 (m, 1 H), 6.55 (d, 1 H, J = 16.23 Hz),
6.44 (m, 1 H), 3.33 (d,
2H, J = 9.09 Hz).
LCMS: (M+H+) 421.1, (M+Na+) 443.1.
Anal. Calcd. for C26H2oN402, 0.1 H20 ' 0.1 N,N-dimethylformamide: C, 73.53; H,
4.90; N, 13.37.
Found: C, 73.26; H, 4.50; N, 13.61.
Example 121: 2-Indan-2-yl-N-(6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cdlindol-8- rl)-
acetamide
HN-N O 00"~NaN ~
H H
Preparation of example 121 from the title compound of Example 7
(hydrochloride) (41 mg, 0.131
mmol), indan-2-yl-acetic acid (28 mg, 0.157 mmol), triethylamine (0.055 mL,
0.393 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (60
mg, 0.157 mmol)
in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was carried out
analogously to Example
11. When the reaction was judged complete, the mixture was filtered to collect
the solids, which
were then washed with methanol. After drying under vacuum, the title compound
(45 mg, 0.101
mmol) was obtained as a yellow powder in 77% yield.
'H NMR (d6-DMSO): S 12.08, (s, 1 H, exchanges), 10.37 (s, 1 H, exchanges),
10.11 (s, 1 H,
exchanges), 8.20 (s, 1 H), 7.70.-7.45 (m, 7H), 7.26-7.18 (m, 2H), 7.16-7.08
(m, 2H), 3.07 (dd, 2H,
J= 7.72, 15.83 Hz), 2.87 (m, 1 H), 2.65 (dd, 2H, J= 6.59, 15.26 Hz).
LCMS: (M+H+) 435.2, (M+Na+) 457.1.
Anal. Calcd. for C27H22N40200.5 H20: C, 73.12; H, 5.23; N, 12.63.
Found: C, 72.84; H, 4.99; N, 12.99.
Example 122: N-(6-Oxo-2-phenyl-5,6-dihydro-lH-[1,21diazepino[4,5.6-cd]indol-8-
Ll)-2-(toluene-4-
sulfonylamino)-benzamide
HN-N
O \
OO
0
~
~NH
N' ~ N
e H H
Preparation of example 122 from the title compound of Example 7 (100 mg, 0.362
mmol), 2-
(toluene-4-sulfonylamino)-benzoic acid (158 mg, 0.542 mmol), triethylamine
(0.201 mL, 1.446
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (206
mg, 0.542 mmol) in N,N-dimethylformamide (4.0 mL) was carried out analogously
to Example 11.
When the reaction was judged complete, N,N-dimethylformamide was evaporated
and methanol
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
156
was added. The mixture was filtered and the solids collected and washed with
methanol,
dichloromethane and diethyl ether. After drying under vacuum, the title
compound (121 mg, 0.220
mmol) was obtained as a yellow powder in 61 % yield.
'H NMR (d6-DMSO): S 12.19 (s, 1 H), 10.63 (s, 1 H), 10.45 (s, 1 H), 10.42 (s,
IH), 8.12 (s, 1 H),
7.85-7.78 (m, 2H), 7.74-7.67 (m, 2H), 7.64-7.56 (m, 4H), 7.56-7.42 (m, 4H),
7.29-7.20 (m, 3H),
2.27 (s, 3H).
LCMS: (M+H+) 550.1; (M-H)" 548.2.
HRMS: (M+H+) calcd for C30H24N504S, 550.1549, found 550.1551.
Anal. Calcd. for C30H23N5O4S ' 0.5 H20: C, 64.50; H, 4.33; N, 12.54.
Found: C, 64.51; H, 4.20; N, 12.71.
Example 123: 6-Oxo-2-Dhenyl-5 6-dihydro-lH-[1 2ldiazepinof4 5 6-cd]indole-8-
carboxylic acid
phenethyl-amide
HN-N
O
H
N I / N
H
O
Preparation of example 123 from the title compound of Example 49 (0.17 g, 0.5
mmol),
phenethylamine (73 mg, 0.6 mmol), triethylamine (100 mg, 1.0 mmol), and O-(7-
azabenzotriazol-
1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (0.23 g, 0.6 mmol) in
DMSO (8 mL) was
carried out analogously to the preparation of Example 76. Preparative HPLC (20-
100%
CH3CN/H20 containing 0.1% trifluoroacetic acid), also in an analogous manner,
afforded the title
compound (0.021 g) as a yellow powder in 9% yield.
'H NMR (d6-DMSO): S 12.49 (s, 1 H), 10.49 (s, 1 H), 8.77 (t, 1 H, H=5.5 Hz),
8.07 (s, 1 H), 8.00 (s,
1 H), 7.10-7.80 (m, 11 H), 3.48 (m, 2H), 2.86 (t, 2H, J= 7.5 Hz).
LCMS (M++1): 409.1
Example 124: (1.2-trans)-2-(4-Fluoro-phenyl)-cyclopropanecarboxylic acid (6-
oxo-2-phenyl-5.6-
dihydro-1 H-[1.2]diazepino[4,5.6-cdlindol-8-yl)-amide
HN-N
HN-N aN
H
ATU, EtgN, DMSO, 62% F~ ~ 10 - ~ I
H2NOI\ / N ~~ F O D H
H
H (a) H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
157
Preparation of example 124 was carried out analogously to the preparation of
Example 113
except that (1,2-trans)-2-(4'-fluorophenyl)-cyclopropanecarboxylic acid was
used instead of (1,2-
trans)-2-(6-trifluoromethyl-pyridin-3-yl)-cyclopropanecarboxylic acid in step
4. (1,2-trans)-2-(4'-
Fluorophenyl)-cyclopropanecarboxylic acid was prepared using procedures
similar to those
described in steps 1-3 of Example 113 except that 4-fluoro-benzaidehyde was
used instead of 6-
trifluoromethyl-3-pyridine carboxaldehyde. Final HPLC purification, also
analogous to Example
113, afforded the title compound (100 mg, 0.228 mmol) as a yellow powder in
62% yield.
1H-NMR (d6-DMSO): S 12.12 (b, 1 H), 10.46 (b, 1 H), 10.41 (b, 1 H), 8.18 (b, 1
H), 7.70-7.45 (m, 7H),
7.30-7.10 (m, 4H), 2.65-2.55 (m, 1 H), 2.27-2.18 (m, 1 H), 1.67-1.51 (m, 2H).
HRMS: C21H211\1502 = H: 439.1570. Found: 439.1584.
Example 125: (1.2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid (6-oxo-2-
phenyl-5.6-dihydro-
1 H-[1,2]diazepino[4.5.6-ccl]indol-8-yl)-amide (hydrochloric salt)
HN-N
O
HN-N
0 HATU, Et3N, DMSO, 100% / ~
~ \ -
10 CIH.N ~ ~ N I / N
H2N N (a) / H H
Preparation of example 125 was carried out analogously to the preparation of
Example 113
except that (1,2-trans)-2-pyridin-3'-yl-cyclopropanecarboxylic acid was used
instead of (1,2-trans)-
2-(6-trifluoromethyl-pyridin-3-yl)-cyclopropanecarboxylic acid in step 4. (1,2-
trans)-2-Pyridin-3'-yl-
cyclopropanecarboxylic acid was prepared using procedures similar to those
described in steps 1-
3 of Example 113 except that pyridine-3-carbaidehyde was used instead of 6-
trifluoromethyl-3-
pyridine carboxaldehyde. Final HPLC purification, also analogous to Example
113, afforded the
title compound (230 mg, 0.381 mmol) as an orange fluffy solid in quantitative
yield.
'H-NMR (d6-DMSO): S 12.15 (s, 1 H), 10.58 (s, 1 H), 10.38 (s, 1 H), 8.81 (s, 1
H), 8.15 (b, 2H), 7.82-
7.45 (m, 9H), 2.35-2.25 (m, 1 H), 1.70-1.51 (m, 1 H), 1.35-1.10 (m, 1 H), 0.90-
0.65 (m, 1 H).
LCMS: (M+H+) 422.1.
Example 126: (1.2-trans)-2-(3-Methoxy-phenyl)-cyclopropanecarboxylic acid (6-
oxo-2-phenyl-5.6-
dihydro-1 H-[1.21diazepino[4,5.6-cd]indol-8-yl)-amide
HN-N
O \
HN-N
0 HATU, Et3N, ~ ~ N DMSO, 74% / 0H/ ~
\ /
Me0 ~ H
H2N / N (a) / O
H ~I
Me0 H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
158
Preparation of example 126 was carried out analogously to the preparation of
Example 113
except that (1,2-trans)-2-(3'-methoxyphenyl)-cyclopropanecarboxylic acid was
used instead of
(1,2-trans)-2-(6-trifluoromethyl-pyridin-3-yl)-cyclopropanecarboxylic acid in
step 4. (1,2-trans)-2-
(3'-Methoxyphenyl)-cyclopropanecarboxylic acid was prepared using procedures
similar to those
described in steps 1-3 of Example 113 except that 3-methoxy-benzaidehyde was
used instead of
6-trifluoromethyl-3-pyridine carboxaldehyde. Final HPLC purification, also
analogous to Example
113, afforded the title compound (132 mg, 0.293 mmol) as a pale orange solid
in 74% yield.
1H-NMR (d6-DMSO): S 12.09 (s, 1 H), 10.43 (s, 1 H), 10.38 (s, 1 H), 8.15 (s, 1
H), 7.70-7.40 (m, 7H),
7.25-7.15 (m, 1 H), 6.77 (b, 3H), 3.74 (s, 3H), 2.40-2.30 (m, 1 H), 2.12-2.05
(m, 1H), 1.55-1.47 (m,
1 H), 1.42-1.33 (m, 1 H).
LCMS: (M+H+) 451.1.
Example 127: (R)-2-Amino-2-cyclohexyl-(6-oxo-5,6-dihydro-l-
[1,21diazepinof4,5,6-cd]indol-8-k)-
acetamide (hydrochloric salt)
HN-N I HN-N
RCO2H
(ne) i
Title Compound HATU ::,::
W
0-'-"iNWN of Example 2 Et3N, DMF 01t'N NHBot'
H H-Cl NH2 H H
127(a)
Step 1. Preparation of [(R)-Cyclohexyl-(6-oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-
8-ylcarbamoyl)-methyl]-carbamic acid tert-butyl ester 127(a)
Preparation of Intermediate 127(a) from the title compound of Example 2 (105
mg, 0.445 mmol),
(R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (172 mg, 0.668 mmol),
triethylamine (0.248
mL, 1.782 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium
hexafluorophosphate (254 mg, 0.668 mmol) in N,N-dimethylformamide (4.0 mL) was
carried out
analogously to Example 11. Silica gel chromatography (eluted with 1:1
hexane:ethyl acetate), also
in an analogous manner, afforded Intermediate 127(a) (110 mg, 0.250 mmol) as a
yellow powder
in 56% yield.
'H NMR (d6-DMSO): 6 11.71 (d, 1 H, J= 2.26 Hz), 10.23 (s, 1 H), 10.07 (s, 1
H), 8.10 (s, 1 H), 7.59
(s, 1 H), 7.55 (d, 1 H, J = 2.45 Hz), 7.46 (s, 1 H), 6.86 (d, 1 H, J = 8.85
Hz), 3.92 (dd, 1 H, J = 8.10,
7.91 Hz), 1.77-1.46 (m, 6H), 1.37 (s, 9H), 1.24-0.93 (m, 5H).
LCMS: (M+H+) 440.1, (M+Na+) 462.2; (M-H)" 438.2.
Step 2. Preparation of Title Compound: (R)-2-Amino-2-cyclohexyl-(6-oxo-5,6-
dihydro-l-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-acetamide (hydrochloric salt)
Preparation of the title compound from Intermediate 127(a) (66.1 mg, 0.150
mmol) and 4.0 M HCI
in dioxane (1.5 mL) was carried out analogously to Example 91. Isolation, also
in an analogous
manner, afforded the title compound (52.0 mg, 0.138 mmol) as a yellow powder
in 92% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
159
'H NMR (ds-DMSO): S 11.86 (s, 1 H), 10.69 (s, 1 H), 10.31 (s, 1 H), 8.29 (br
s, 3H), 8.08 (s, 1 H),
7.63 (s, 1 H), 7.49 (s, 1 H), 3.80-3.69 (m, 1 H), 1.93-1.55 (m, 6H), 1.29-0.98
(m, 5H).
LCMS: (M+H+) 340.3, (M+Na+) 362.3.
Example 128: 2-Indan-2-yl-(6-oxo-5.6-dihydro-l-[1 2]diazepinof4 5 6-cd]indol-8-
yl)-acetamide
HN-N
~ ~ /
0
WN
N 5 H H
Preparation of example 128 from the title.compound of Example 2 (freebase)
(88.7 mg, 0.443
mmol), indan-2-yl-acetic acid (117 mg, 0.665 mmol), triethylamine (0.247 mL,
1.774 mmol), and
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (253
mg, 0.665
mmol) in N,N-dimethylformamide (4.0 mL) was carried out analogously to Example
11. When the
reaction was judged complete, N,N-dimethylformamide was evaporated and
methanol was added.
The mixture was filtered to collect the solids, which were then washed with
methanol,
dichloromethane and diethyl ether. After drying under vacuum, the title
compound (115 mg, 0.321
mmol) was obtained as a yellow powder in 72% yield.
' H NMR (d6-DMSO): S 11.71 (s, IH), 10.23 (s, 1 H), 10.05 (s, IH), 8.15 (s, 1
H), 7.58(s, 1 H), 7.55
(d, 1 H, J = 2.26 Hz), 7.46 (s, 1 H), 7.26-7.16 (m, 2H), 7.16-7.06 (m, 2H),
3.06 (dd, 2H, J= 7.54,
5.54 Hz), 2.92-2.79 (m, 1 H), 2.65 (dd, 2H, J = 6.59, 6.78 Hz), 2.47 (d, 2H, J
= 9.80 Hz).
LCMS: (M+H+) 359.1, (M+Na+) 381.0; (M-H)- 357.2.
Example 129: (1,2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid (6-oxo-5 6-
dihydro-lH-
[1.2]diazepino[4.5.6-cdJindol-8-yl)-amide
HN-N
N
O W
H H
Preparation of example 129 from the title compound of Example 2 (freebase)
(200 mg, 1.00
mmol), (1,2-trans)-2-pyridin-3'-yl-cyclopropanecarboxylic acid (see Example
125 for preparation-
estimated purity c.a. 75%) (240 mg, c.a.1.10 mmol), triethylamine (0.550 mL,
3.96 mmol), and 0-
(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (570
mg, 1.50 mmol)
in N,N-dimethylformamide (8.0 mL) was carried out analogously to Example 11.
Silica gel
chromatography (eluted with 100:10:1 ethyl acetate: methanol: ammonium
hydroxide), also in an
analogous manner, afforded the title compound (38 mg, 0.110 mmol) as a yellow
powder in 11 %
yield.
'H NMR (d6-DMSO): S 11.73 (d, 1 H, J = 2.26 Hz), 10.41 (s, 1 H), 10.23 (s, 1
H), 8.50 (d, 1 H, J
1.88 Hz), 8.41 (dd, 1 H, J = 3.20, 1.51 Hz), 8.11 (d, 1 H, J= 1.51 Hz), 7.61-
7.48 (m, 3H), 7.45 (s,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
160
1 H), 7.32 (dd, 1 H, J = 5.09, 3.20 Hz), 2.45-2.36 (m, 1 H), 2.18-2.05 (m, 1
H), 1.58-1.49 (m, 1 H),
1.49-1.36 (m, 1 H).
LCMS: (M+H+) 346.1; (M-H)" 344.1.
HRMS: (M+H+) calcd for C19H16N502, 346.1304, found 346.1316.
Example 130: (1.2-trans)-2-(1'-Trityl-lH-imidazol-4'-yl)-
cyclopropanecarboxylic acid (6-oxo-2-
phenyl-5.6-d i hyd ro-1 H-[ 1.2]d iazep i no[4.5.6-cd] i n do l-8-yl)-am ide
HN-N HN-N HATU, Et3N, DMSO, 100% /-N ONN
WN
H2NO N (a) H
H
t---N O
Preparation of example 130 was carried out analogously to the preparation of
Example 113
except that (1,2-trans)-2-(1'-trityl-1H-imidazol-4'-yl)-cyclopropanecarboxylic
acid was used instead
of (1,2-trans)-2-(6-trifluoromethyl-pyridin-3-yl)-cyclopropanecarboxylic acid
in step 4. (1,2-trans)-2-
(1'-trityl-1H-imidazol-4'-yl)-cyclopropanecarboxylic acid was prepared using
procedures similar to
those described in steps 1-3 of Example 113 except that 1-trityl-1H-imidazole-
4-carbaldehyde was
used instead of 6-trifluoromethyl-3-pyridine carboxaldehyde. Final
purification consisted of
methanol trituration. The resulting solids were isolated by filtration, washed
with cold methanol,
and dried overnight under vacuum at room temperature to afford the title
compound (263 mg,
0.366 mmol) as a yellow powder in quantitative yield.
'H-NMR (d6-DMSO): 5 12.09 (s, 1 H), 10.43 (s, IH), 10.38 (s, 1 H), 8.13 (s, 1
H), 7.70-7.30 (m,
18H), 7.25 (s, 1 H), 7.15-7.05 (m, 6H), 6.87 (s, 1 H), 2.35-2.24 (m, 1 H),
2.20-2.13 (m, 1 H), 1.38-
1.23 (m, 2H).
LCMS: (M+H+) 653.3
Example 131: (6-Oxo-5, 6-dihydro-1-[12]diazepinof4.5.6-]indol-8-yl)-3-(pyridin-
2-vloxv)-
benzamide
HN-N
O
WN
N ~ O H
ration of example 131 from the title compound of Example 2 (25 mg, 0.125
mmol), 3-
Prepa
(pyridin-2-yloxy)-benzoic acid (88 mg, 0.348 mmol), N,N-diisopropylethylamine
(0.19 mL, 1.04
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (159
mg, 0.42 mmol) in N,N-dimethylformamide (3 mL) was carried out analogously to
Example 11.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
161
Silica gel chromatography (eluted with 2% methanol in CHZCI2), also in an
analogous manner,
afforded the title compound (20 mg, 0.05 mmol) as a yellow powder in 40%
yield.
'H NMR (d6-Acetone): S 7.92 (s, IH), 7.89 (s, 1 H), 7.55-7.52 (m, 4H), 7.39
(s, 1 H), 7.37 (s, IH),
7.34 (m, 1 H), 7.32-7.24 (m, 2H), 7.15 (m, 1 H), 6.95 (m, 1 H), 6.93 (m, 1 H),
6.77-6.73 (m, 5H), 6.88
(m, 1 H).
LCMS: (M+H+) 398.1.
Example 132: N-(6-Oxo-5.6-dihydro-lH-[1 2]diazegino[4 5 6-cdlindol-8-yl)-4-
thiophen-2-yl-
butvramide
HN-N
N / N
H H
Preparation of example 132 from the title compound of Example 2 (freebase)
(200 mg, 1.00
mmol), 4-thiophen-2-yl-butyric acid (187 mg, 1.10 mmol), triethylamine (0.550
mL, 3.96 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(570 mg, 1.50
mmol) in N,N-dimethylformamide (8.0 mL) was carried out analogously to Example
11. Silica gel
chromatography (eluted with ethyl acetate), also in an analogous manner,
afforded the title
compound (72 mg, 0.204 mmol) as a yellow powder in 20% yield.
'H NMR (d6-DMSO): S 11.70 (d, 1 H, J = 2.07 Hz), 10.22 (s, 1 H), 10.03 (s, 1
H), 8.13 (d, 1 H, J
1.51 Hz), 7.55 (d, 1 H, J = 1.51 Hz), 7.53 (d, 1 H, J = 2.45 Hz), 7.45 (s, 1
H), 7.31 (dd, 1 H, J = 4.33,
0.94 Hz), 6.94 (dd, 1 H, J= 3.39, 1.70 Hz), 6.86 (d, 1 H, J= 2.83 Hz), 3.84
(t, 2H, J= 7.54 Hz), 2.37
(t, 2H, J = 7.35 Hz), 1.93 (tt, 2H, J = 7.54, 7.35 Hz).
LCMS: (M+H+) 353.2, (M+Na+) 375.2; (M-H)- 351.2.
HRMS: (M+H+) calcd for C18H17N402S, 353.1072, found 353.1056.
Anal. Calcd. for C1$H16N402S: C, 61.35; H, 4.58; N, 15.90.
Found: C, 61.06; H, 4.52; N, 15.71.
Example 133: (2R)-2-Hydroxy-N-(6-oxo-2-phenyl-5 6-dihydro-1H-[1 2]diazepino[4
5 6-cdjindol-8-
yl)-2-phenylethanamide
HN-N
O
\ I N ~ / N
OH H H
Preparation of example 133 from the title compound of Example 7
(hydrochloride) (46 mg, 0.147
mmol), (2R)-hydroxy(phenyl)ethanoic acid (27 mg, 0.177 mmol), triethylamine
(0.061 mL, 0.44
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium
hexafluorophosphate (67
mg, 0.177 mmol) in CH2CI2 (0.4 mL) and N,N-dimethylformamide (0.4 mL) was
carried out
analogously to Example 11. Silica gel chromatography (eluted with 3:1:1
hexane:ethyl
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
162
acetate:ethanol), also in an analogous manner, followed by two successive
triturations (ethyl
acetate/diethyl ether then methanol/diethyl ether) afforded the title compound
(34 mg, 0.082
mmol) as a yellow powder in 56% yield.
'H NMR (d6-DMSO): S 12.12, (s, 1 H), 10.38 (s, 1 H), 10.16 (s, IH), 8.14 (s, 1
H), 7.81 (s, 1 H), 7.70-
7.64 (m, 2H), 7.61-7.45 (m, 6H), 7.42-7.26 (m, 3H), 6.35 (br s, 1H, partially
exchanged), 5.13 (s,
1 H).
LCMS: (M-H)" 409.2.
Example 134: (1,2-trans)-2-Pyridin-2-yl-cyclopropanecarboxylic acid (6-oxo-2-
phenyl-5,6-dihydro-
1H-[1,21diazepino[4,5,6-cd]indol-8-Lrl)-amide (acetic acid salt)
O
HN-N HO' ~CH3 HN-N
HATU, Et3N, DMSO, 69% CN O N OI / N
I/ ~~ (a) H H
H2NO N H / IN O
Preparation of example 134 was carried out analogously to the preparation of
Example 113
except that (1,2-trans)-2-pyridin-2'-yl-cyclopropanecarboxylic acid was used
instead of (1,2-trans)-
2-(6-trifluoromethyl-pyridin-3-yl)-cyclopropanecarboxylic acid in step 4. (1,2-
trans)-2-Pyridin-2'-yl-
cyclopropanecarboxylic acid was prepared using procedures similar to those
described in steps 1-
3 of Example 113 except that pyridine-2-carbaldehyde was used instead of 6-
trifluoromethyl-3-
pyridine carbaldehyde. Final HPLC purification, also in an analogous manner
but using 0.1%
acetic acid instead of 0.1% TFA, afforded the title compound (107 mg, 0.254
mmol) as a
greenish-yellow solid in 69% yield.
' H-NMR (d6-DMSO): S 12.09 (s, 1 H), 10.43 (s, 1 H), 10.38 (s, 1 H), 8.15 (s,
1 H), 7.70-7.40 (m, 7H),
7.25-7.15 (m, 1 H), 6.77 (b, 3H), 3.74 (s, 3H), 2.40-2.30 (m, 1 H), 2.12-2.05
(m, 1 H), 1.55-1.47 (m,
1 H), 1.42-1.33 (m, 1 H).
LCMS: (M+H+) 422.1.
Example 135: (1 2-trans)-2-(1H-Imidazol-4-yl)-cyclopropanecarboxylic acid (6-
oxo-2-phenyl-5,6-
dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide (acetic acid salt)
HN-N
a /-N O HN, ~%~~//~~ N N
~H H
O
2X HO"U"'CH3
Preparation of example 135 was carried out by suspending the title compound of
Example 130
(260 mg, 0.398 mmol) in anhydrous dichloromethane (5 mL) and adding anhydrous
trifluoroacetic
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
163
acid (5 mL) at room temperature. After 30 minutes, the mixture was
concentrated and subjected
to preparative HPLC, in a manner analogous to Example 134, to afford the title
compound (72 mg,
0.175 mmol) as a fluffy yellow solid in 44% yield.
' H-NMR (ds-DMSO): S 12.09 (s, 1 H), 10.43 (s, 1 H), 10.37 (s, 1 H), 8.15 (s,
1 H), 7.97 (b, 1 H), 7.70-
7.45 (m, 7H), 7.13 (b, 1 H), 2.40-2.30 (m, 1 H), 2.12-2.04 (m, 1 H), 1.45-1.33
(m, 2H).
LCMS: (M+H+) 422.1.
Example 136: (2R)-Piperidine-2-carboxylic acid (6-oxo-2-phenyl-5 6-dihydro 11-
I
f1,21diazepinof4,5,6-Cdlindol-8-yl)-amide (hydrochloric salt)
O HN-N O HN-N
O I\ 4N HCI (Dioxane) O
/ ~ ~ I \
N N CH ~~.k N N
H H 2CI2 96% H
NBoc c ON H H H-Cl
136(a)
Step 1. Preparation of (2R)-2-(6-Oxo-2-phenyl-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cdjindol-
8-ylcarbamoyl)-piperidine-l-carboxylic acid tert-butyl ester 136(a)
Preparation of Intermediate 136(a) from the title compound of Example 7 (0.11
g, 0.4 mmol),
(2R)-piperidine-1,2-dicarboxylic acid 1-tert-butyl ester (0.143 g, 0.6 mmol),
triethylamine (0.3 mL,
2 mmol),
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(0.23 g, 0.63
mmol) and N,N-dimethylformamide (0.1 M, 5 mL) was carried out analogously to
Example 11.
Silica gel chromatography afforded Intermediate 136(a) (0.21 g) as a yellow
powder in 88% yield.
1 H-NMR (d6-DMSO): 8 12.09 (s, 1 H), 10.40 (s, 1 H), 10.11 (s, 1 H), 8.10 (s,
1 H), 7.74-7.44 (m, 7H),
4.64-4.55 (m, 1 H), 3.83 (d, 1 H, J= 12.25 Hz), 3.35-3.20 (buried m, 1 H),
2.20-2.05 (m, 1 H), 1.80-
1.50 (m, 3H), 1.45-1.25 (bs, 11 H).
LCMS: (M+H+) 488.2.
Step 2. Preparation of Title Compound: (2R)-Piperidine-2-carboxylic acid (6-
oxo-2-phenyl-
5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide (hydrochloric salt)
Preparation of the title compound from Intermediate 136(a) (0.16 g, 0.33 mmol)
and 4M HCI in
dioxane (1.6 mL, 6.6 mmol) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, included a further trituration with CH2CI2/diethyl ether and
afforded the title
compound (0.137 g) as an orange/yellow powder in 96% yield.
' H-NMR (d6-DMSO): 8 12.31 (s, 1 H), 10.90 (s, 1 H), 10.45 (s, 1 H), 9.26 (b,
1 H), 8.80-8.75 (m, 1 H),
8.11 (s, 1 H), 7.76-7.46 (m, 7H), 3.95-3.81 (m, 1 H), 3.35-3.25 (m, 1 H), 3.10-
2.92 (m, 1 H), 2.36-
2.26 (m, 1 H), 1.76-1.51 (m, 5H).
LCMS: (M+H+) 388.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
164
Example 137: (2S)-Piperidine-2-carboxylic acid (6-oxo-2-phenyl-5,6-dihydro-1 H-
[1,2]diazepinoi4.5,6-cdJindol-8-yl)-amide (hydrochloric salt)
HN-N HN-N
O O
O 4N HCI (Dioxane) O I~ \
N N CHaCIZ, 95% N N
NBocH H NH H H-Cl H
137(a)
Step 1. Preparation of (2S)-2-(6-Oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cal]indol-
8-ylcarbamoyl)-piperidine-l-carboxylic acid tert-butyl ester 137(a)
Preparation of Intermediate 137(a) from the title compound of Example 7 (0.138
g, 0.5 mmol),
(2S)-Piperidine-1,2-dicarboxylic acid 1-tert-butyl ester (0.143 g, 0.6 mmol),
triethylamine (0.3 mL,
2 mmol), O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (0.23 g,
0.63 mmol) and N,N-dimethylformamide (0.1 M, 5 mL) was carried out analogously
to Example
11. Silica gel chromatography afforded Intermediate 137(a) (0.20 g) as a
yellow powder in 84%
yield.
'H-NMR (d6-DMSO): S 12.09 (s, 1 H), 10.40 (s, 1 H), 10.11 (s, 1 H), 8.10 (s, 1
H), 7.74-7.44 (m, 7H),
4.64-4.55 (m, 1 H), 3.83 (d, 1 H, J= 12.25 Hz), 3.35-3.20 (buried m, 1 H),
2.20-2.05 (m, 1 H), 1.80-
1.50 (m, 3H), 1.45-1.25 (bs, 11 H).
LCMS: (M+H+) 488.1.
Step 2. Preparation of Title Compound: (2S)-Piperidine-2-carboxylic acid (6-
oxo-2-phenyl-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cdJindol-8-yl)-amide (hydrochloric salt)
Preparation of the title compound from Intermediate 137(a) (0.16 g, 0.33 mmol)
and 4M HCI in
dioxane (1.6 mL, 6.6 mmol) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, included a further trituration with CH2CI2/diethyl ether and
afforded the title
compound (0.132 g) as an orange/yellow powder in 95% yield.
' H-NMR (d6-DMSO): 6 12.31 (s, 1 H), 10.90 (s, 1 H), 10.45 (s, 1 H), 9.26 (b,
1 H), 8.80-8.75 (m, 1 H),
8.11 (s, 1 H), 7.76-7.46 (m, 7H), 3.95-3.81 (m, 1 H), 3.35-3.25 (m, 1 H), 3.10-
2.92 (m, 1 H), 2.36-
2.26 (m, IH), 1.76-1.51 (m, 5H).
LCMS: (M+H+) 388.2.
Example 138: (2S,4R)-4-Hydroxy_pyrrolidine-2-carboxylic acid (6-oxo-2-phenyl-
5,6-dihydro-1 H-
[1,2]diazepino[4,5,6-cdJindol-8-yl)-amide (hydrochloric salt)
HN-N
O
HN-N a
De) O N HOu. N N HOu.. H
O :::::
oc H H C'NrH H H-CI
NB
138(a)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
165
Step 1. Preparation of (2S, 4R)-4-Hydroxy-2-(6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-8-ylcarbamoyl)-pyrrolidine-l-carboxylic acid
tert-butyl ester
138(a)
Preparation of Intermediate 138(a) from the title compound of Example 7 (0.138
g, 0.5 mmol),
(2S, 4R)-4-hydroxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester (0.144
g, 0.6 mmol),
triethylamine (0.3 mL, 2 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (0.23 g, 0.63 mmol) and N,N-dimethylformamide (0.1 M, 5
mL) was carried
out analogously to Example 11. Silica gel-chromatography afforded Intermediate
138(a) (0.127 g)
as a yellow powder in 52% yield.
'H-NMR (d6-DMSO): S 12.19 (s, 1 H), 10.46 (s, 1 H), 10.28 (s, 1 H), 8.29 (s,
0.8H, major rotamer),
8.29 (s, 0.2H, minor rotamer), 7.75-7.51 (m, 7H), 4.50-4.35 (m, 2H), 3.60-3.30
(m, 3H), 2.30-2.17
(m, 1 H), 2.10-1,.90 (m, 1 H), 1.48 (s, 2H, minor rotamer), 1.34 (s, 2H, major
rotamer).
LCMS: (M+H+) 490.1.
Step 2. Title Compound: (2S,4R)-4-Hydroxy-pyrrolidine-2-carboxylic acid (6-oxo-
2-phenyl-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yi)-amide (hydrochloric salt)
Preparation of the title compound from Intermediate 138(a) (0.105 g, 0.21
mmol) and 4M HCI in
dioxane (1 mL, 4.2 mmol) was carried out analogously to Example 91. Isolation,
also in an
analogous manner, included a further trituration with CH2CI2/diethyl ether and
afforded the title
compound (0.065 g) as an orange/yellow powder in 71 % yield.
1H-NMR (d6-DMSO): S 12.33 (s, 1 H), 10.93 (s, 1 H), 10.44 (s, 1 H), 10.10-9.95
(m, 1 H), 8.90-8.45
(m, 1 H), 8.08 (s, 1 H), 7.75-7.45 (m, 7H), 4.75-4.35 (bm, 3H), 3.45-3.30 (m,
1 H), 3.20-3.10 (m,
1 H), 2.50-2.35 (m, 1 H), 2.10-1.95 (m, 1 H).
LCMS: (M+H+) 390.1.
Example 139: (2S)- 2-Amino-3-cyano-N-(6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepinof4,5,6-
cdjindol-8-yl)-propionamide acetic acid salt
HN-N HN-N
a 0 4N HCI (Dioxane) 0 \
NCN N \/ CH2CI2, 49% % NC' )~N N
NHBoU H NH2 H AcO-H H
139(a)
Step 1. Preparation of (1S)- [2-Cyano-1-(6-oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cd]indol-8-ylcarbamoyl)-ethyl]-carbamic acid tert-butyl ester 139(a)
Preparation of Intermediate 139(a) from the title compound of Example 7 (0.138
g, 0.5 mmol),
(2S)-2-tert-butoxycarbonylamino-3-cyano-propionic acid (0.134 g, 0.6 mmol),
triethylamine (0.3
mL, 2 mmol), O-(7-azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium
hexafluorophosphate (0.23
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
166
g, 0.63 mmol) and N,N-dimethylformamide (0.1 M, 5 mL) was carried out
analogously to
Example 11. Silica gel chromatography afforded Intermediate 139(a) (0.094 g)
as a yellow powder
in 40% yield.
1H-NMR (d6-DMSO): S 12.15 (s, IH), 10.42 (s, 1 H), 10.37 (s, 1 H), 8.11 (s, 1
H), 7.75-7.48 (m, 8H),
4.55-4.40 (m, 1 H), 3.02 (dd, 1 H, J= 17.1, 4.9 Hz), 2.85 (dd, 1 H, J= 17.0,
9.0 Hz), 1.43 (s, 9H).
LCMS: (M+H+) 473.2.
Step 2. Preparation of Title Compound: (2S)- 2-Amino-3-cyano-N-(6-oxo-2-phenyl-
5,6-
dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-yl)-propionamide acetic acid salt
Preparation of the title compound from Intermediate 139(a) (0.084 g, 0.18
mmol) and 4M HCI in
dioxane (0.9 mL, 3.6 mmol) was carried out analogously to Example 91. A
preparative HPLC
afforded the title compound (0.038 g) as an orange/yellow powder in 49% yield.
'H-NMR (d6-DMSO): S 12.31 (s, 1 H), 10.50 (s, 1 H), 10.35 (bs, 1 H), 8.24 (s,
1 H), 7.80-7.55 (m,
7H), 3.84 (dd, 1 H, J= 7.0, 6.9 Hz), 2.99 (dd, 1 H, J= 16.8, 5.7 Hz), 2.85
(dd, 1 H, J= 16.6, 7.2 Hz),
2.73 (s, 6H).
LCMS: (M+H+) 373.1.
Example 140: (2S)-N-(6-Oxo-2-phenyl-5.6-dihydro-1H-f1.21diazepino[4 5 6-
cdlindol-8-yl)-2-
pyrrolidin-2-yl-acetamide (hydrochloric salt)
HN-N HN-N
O O
/' 4N HCI (Dioxane) ~f`( ~
`N %/\N N CH2CI2, 63% N N ~ N
Boc H H H H H-CI H
140(a)
Step 1. Preparation of (2S)-2-[(6-Oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-
8-ylcarbamoyl)-methyl]-pyrrolidine-l-carboxylic acid tert-butyl ester 140(a)
Preparation of Intermediate 140(a) from the title compound of Example 7 (0.138
g, 0.5 mmol),
(2S)-2-carboxymethyl-pyrrolidine-l-carboxylic acid tert-butyl ester (0.143 g,
0.6 mmol),
triethylamine (0.3 mL, 2 mmol), and O-(7-azabenzotriazol-1-yi)-N,N,N;M
tetramethyluronium
hexafluorophosphate (0.23 g, 0.63 mmol) and N,N-dimethylformamide (0.1 M, 5
mL) was carried
out analogously to Example 11. Silica gel chromatography afforded Intermediate
140(a) (0.155 g)
as a yellow powder in 64% yield.
1H-NMR (d6-DMSO): 8 12.09 (s, IH), 10.39 (s, 1 H), 10.17 and 10.10 (2s, 1 H,
minor and major
rotamer), 8.20 (s, 1 H), 7.75-7.45 (m, 7H), 4.10 (s, 1 H), 4.20-4.05 (m, 1H),
3.50-3.25 (m, 2H),
2.10-1.75 (m, 4H), 1.40 and 1.29 (2s, 9H, minor and major rotamer).
LCMS: (M-Boc+H+) 388.2.
Step 2. Preparation of Title Compound: (2S)-N-(6-Oxo-2-phenyl-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-2-pyrrolidin-2-yl-acetamide (hydrochloric
salt)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
167
Preparation of the title compound from Intermediate 140(a) (0.1 g, 0.20 mmol)
and 4M HCI in
dioxane (1 mL, 4.2 mmol) was carried out analogously to Example 91. Isolation,
also in an
analogous manner, included a further trituration with CH2CI2/diethyl ether and
afforded the title
compound (0.055 g) as an orange/yellow powder in 63% yield.
major rotamer; 1 H-NMR (d6-DMSO): S 12.23 (s, 1 H), 10.48 (s, 1 H), 10.41 (s,
1 H), 9.25-9.10 (m,
1 H), 8.95-8.80 (m, 1H), 8.11 (s, 1 H), 7.75-7.45 (m, 7H), 4.10 ( buried m, 1
H), 3.90-3.75 (m, IH),
3.25-3.15 (m, 2H), 2.95-2.85 (m, 2H), 2.25-1.55 (m, 5H).
minor rotamer; 1H-NMR (d6-DMSO): S 12.31 (s, 1 H), 10.92 (s, 1 H), 10.45 (s, 1
H), 9.85-9.75 (m,
1 H), 8.75-8.60 (m, 1 H), 3.35-3.25 (m, 2H).
LCMS: (M+H+) 388.2.
Example 141: (3R)-1.2.3,4-Tetrahydro-isoauinoline-3-carboxylic acid (6-oxo-5 6-
dihydro-1 H-
f1,21diazepino[4,5,6-cdJindol-8-yl)-amide (hydrochloric salt)
HN-N HN-N
O ~ O ~
O \ ~ O \ ~
~ 4N HCI (Dioxane) ~
OC~Bkoc N / N OCEH N /N
H H CH2CI2, 63% H H-Cl H
141(a)
Step 1. Preparation of (3R) 3-(6-Oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-
ylcarbamoyl)-3,4-dihydro-lH-isoquinoline-2-carboxylic acid tert-butyl ester
141(a)
Preparation of intermediate 141(a) from the title compound of Example 2 (0.1
g, 0.5 mmol), (3R)-
3,4-dihydro-lH-isoquinoline-2,3-dicarboxylic acid 2-tert-butyl ester (0.103 g,
0.6 mmol),
triethylamine (0.3 mL, 2 mmol), O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (0.23 g, 0.63 mmol) and N,N-dimethylformamide (0.1 M, 5
mL) was carried
out analogously to Example 11. Silica gel chromatography afforded Intermediate
141(a) (0.094 g)
as a yellow powder in 41 % yield.
1H-NMR (d6-DMSO): S 11.72 (bs, 1 H), 10.25 (s, 1 H), 10.14 (bs, 1 H), 8.09 and
7.96 (2s, 1 H, major
and minor rotamer), 7.57 (s, 2H), 7.47 (s, 1 H), 7.30-7.10 (m, 4H), 4.75-4.60
(m, 1 H), 4.48-4.34
(m, 1 H), 3.40-3.00 (m, 3H), 1.47 (s, 3H), 1.31(s, 6H).
LCMS: (M-H) 458.3.
Step 2. Preparation of Title Compound: (3R)-1,2,3,4-Tetrahydro-isoquinoline-3-
carboxylic
acid (6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide
(hydrochloric salt)
Preparation of the title compound from Intermediate 141(a) (0.094 g, 0.20
mmol) and 4M HCI in
dioxane (1 mL, 4.2 mmol) was carried out analogously to Example 91. Isolation,
also in an
analogous manner, included a further trituration with CH2CI2/diethyl ether and
afforded the title
compound (0.051 g) as an orange/yellow powder in 63% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
168
'H-NMR (d6-DMSO): 5 11.98 (s, 1 H), 11.08 (s, IH), 10.32 (s, IH), 9.93 (b, 1
H), 9.64 (b, 1 H),
8.09 (s, 1 H), 7.74 (s, 1 H), 7.64 (s, 1 H), 7.51 (s, 1 H), 7.29 (s, 5H), 4.50-
4.10 (buried m, 3H), 3.53
(dd, 1 H, J= 16.4, 4.0 Hz), 3.14 (dd, 1 H, J= 16.8, 12.06 Hz).
LCMS: (M+H+) 360.1.
Example 142: (2S, 4R)-4-Benzyloxy-pyrrolidine-2-carboxylic acid (6-oxo-5 6-
dihydro-1H-
f1,21diazepino[4,5,6-cdjindol-8-yl)-amide (hydrochloric salt)
HN-N HN-N
O ~
4N HCI (Dioxane) C I ~ ~
C
N / N
WN
CN H H CHpCIg, 92% H H-CI H
Boc e 142(a)
Step 1. Preparation of (2S, 4R) 4-Benzyloxy-2-(6-oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-
cd]indol-8-ylcarbamoyl)-pyrrolidine-l-carboxylic acid tert-butyl ester 142(a)
Preparation of intermediate 142(a) from the title compound of Example 2 (0.1
g, 0.5 mmol), (2S,
4R)-4-benzyloxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester (0.103 g,
0.6 mmol),
triethylamine (0.3 mL, 2 mmol), O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (0.23 g, 0.63 mmol) and N;N-dimethylformamide (0.1 M, 5
mL) was carried
out analogously to Example 11. Silica gel chromatography afforded Intermediate
142(a) (0.126 g)
as a yellow powder in 50% yield.
'H-NMR (d6-DMSO): S 11.76 (s, 1 H), 10.26 (s, 1 H), 10.18 (s, 1 H), 8.18 and
8.10 (s, 1 H,
rotamers), 7.60 (s, 1 H), 7.58 (s, 1 H), 7.48 (s, 1 H), 7.42-7.26 (m, 5H),
4.53 (s, 2H), 4.39-4.26 (m,
1 H), 4.22 (bs, 1 H), 3.54 (bs, 2H), 3.32 (s, 2H), 1.27 (s, 6H).
LCMS: (M-H) 502.2.
Step 2. Preparation of Title Compound: (2S, 4R)-4-Benzyloxy-pyrrolidine-2-
carboxylic acid
(6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cal]indol-8-yl)-amide
(hydrochloric salt)
Preparation of the title compound from Intermediate 142(a) (0.106 g, 0.21
mmol) and 4M HCI in
dioxane (1 mL, 4.2 mmol) was carried out analogously to Example 91. Isolation,
also in an
analogous manner, included a further trituration with CH2CI2/diethyl ether and
afforded the title
compound (0.085 g) as an orange/yellow powder in 92% yield.
1H-NMR (d6-DMSO): 6 11.96 (s, 1 H), 10.94 (s, IH), 10.33 (s, IH), 10.12 (b,
IH), 8.89 (b, IH),
8.06 (s, 1 H), 7.70 (s, 1 H), 7.64 (s, 1 H), 7.51 (s, 1 H), 7.45-7.28 (m, 7H),
4.40 (b, 2H), 3.47 (b, 2H),
2.80-2.65 (m, 1 H), 2.15-2.01 (m, 1 H).
LCMS: (M+H+) 404.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
169
Example 143: (2R)-2-Amino-3-(4-hydroxyphenyl)-N-(6-oxo-2-phenyl-5,6-dihydro-1
H-
[1,21diazepino[4,5.6-cdlindol-8-yl)propanamide hydrochloride
HN-N
O
0 ~ -
N ( / N ~ ~
HO H2 H H-CI H
Preparation of example 143 from title compound of Example 150 (64 mg, 0.119
mmol), and 4M
HCI in dioxane (5 mL) was carried out analogously to Example 91. Isolation,
also in an analogous
manner, included freebasing with triethylamine and subsequent silica gel
chromatography eluting
with 3:1:1 hexane:ethyl acetate:ethanol. With ice bath cooling, the purified
freebase in CH2CI2 (5
mL) was treated with 4M HCI in dioxane (0.1 mL). After removal of the volatile
components, the
title compound (32 mg, 0.067 mmol) was obtained as an orange/yellow powder in
57% yield.
'H NMR (d6-DMSO): S 12.25 (s, 1 H, exchanges), 10.71 (s, IH, exchanges), 10.45
(s, 1 H,
exchanges), 9.44 (br s, I H, exchanges), 8.45-8.31 (br m, 2H, exchanges), 8,08
(s, 1 H), 7.76-7.45
(m, 7H), 7.09 (d, 2H, J= 8 Hz), 6.72 9d, 2H, J= 8 Hz), 4.13 (m, 1 H), 3.19-
2.97 (m, 2H).
LCMS: (M+H+) 440.0, (M-H)' 438.2.
Example 144: (S)-1-Methyl-pyrrolidine-2-carboxylic acid (6-oxo-5. 6-dihydro-l-
[1,2]diazepino[4,5.6-lindol-8-yl)-amide
HN-N
0
N WN
eN H H
\
Preparation of example 144 from the title compound of Example 2 (190 mg, 0.95
mmol), N-
methylproline (129 mg, 1.47 mmol), triethylamine (0.19 mL, 1.43 mmol), and O-
(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (470 mg,
1.3 mmol) in
N,N-dimethylformamide (5 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 10 % methanol in CH2CIZ), also in an analogous
manner, afforded
title compound (100 mg, 0.32 mmol) as a yellow powder in 34% yield.
' H NMR (d6-DMSO): 11.83 (s, 1 H), 10.33 (s, 1 H), 9.96 (s, 1 H), 8.24 (s, 1
H), 7.73 (s, 1 H), 7.65 (s,
1 H), 7.55 (s, 1 H), 3.20 (m, 1 H), 3.03 (m, 1 H), 2.63 (m, 1 H), 2.23 (m, 1
H), 1.91-1.88 (m, 3H).
Anal. Calcd for C16H17N502= 0.2 H20: C, 61.02; H, 5.57; N, 22.24. Found: C,
60.83; H, 5.29; N,
22.23.
LCMS: (M+H+) 312.1.
Example 145: (2R)-5-Oxo-pyrrolidine-2-carboxylic acid (6-oxo-5,6-dihydro-l-
j1,2]diazepinof4,5,6]indol-8-yl)-amide
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
170
HN-N
O ~
I \ ~
O
N ~ N
~NH H H
O
Preparation of example 145 from the title compound of Example 2 (freebase)
(190 mg, 0.95
mmol), (R)-(+)-pyrrolidonecarboxylic acid (167 mg, 1.29 mmol), triethylamine
(0.25 mL, 1.77
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (538
mg, 1.42 mmol) in N,N-dimethylformamide (5 mL) was carried out analogously to
Example 11.
The mixture was concentrated and the residue was triturated with methanol. The
resulting solids
were then collected by filtration and washed with methanol to give the title
compound (220 mg,
0.57 mmol) as a yellow powder in 60% yield.
' H NMR (d6-DMSO): 11.79 (s, 1 H), 10.27 (s, 1 H), 10.20 (s, 1 H), 8.11 (s, 1
H), 7.91 (s, 1 H), 7.62 (s,
1 H), 7.59 (s, 1 H), 7.49 (s, 1 H), 4.21 (m, 1 H), 2.36 (m, 1 H), 2.24-2.16
(m, 2H), 2.03 (m, 1 H).
Anal. Calcd for C15H13N503= 1 H2O: C, 54.71; H, 4.59; N, 21.27. Found: C,
54.51; H, 4.68; N,
21.05.
LCMS: (M+H+) 312.2.
Example 146: N-(6-Oxo-5.6-dihydro-lH-[1 2]diazepino[4 5 6-cdlindol-8-Ll)-3-
piperidin-4-Lrl-
acrylamide acetic acid salt
H
O O I N-N
Title Compound BocNCa-CO2H
\
of Example 2 HATU, Et3N, DMF, 55% H H
BocN
146(a)
H
0 N-N
0
I \ \
4N HCI (Dioxane) AOH 0
CHZCI2, 31 % \ N
HN H H
Step I. Preparation of 4-[2-(6-Oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cdjindol-8-
ylcarbamoyl)-vinyl]-piperidine-l-carboxylic acid tert-butyl ester 146(a)
Preparation of intermediate 146(a) from the title compound of Example 2
(freebase) (49 mg,
0.245 mmol), 4-(2-carboxy-vinyl)-piperidine-l-carboxylic acid tert-butyl ester
(99 mg, 0.39 mmol),
triethylamine (0.069 mL, 0.49 mmol), and O-(7-azabenzotriazol-l-yl)-N,N,N;N=
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
171
tetramethyluronium hexafluorophosphate (148 mg, 0.39 mmol) in N,N-
dimethylformamide (3 mL)
was carried out analogously to Example 11. Silica gel chromatography (eluted
with 10 % methanol
in CH2CI2), also in an analogous manner, afforded Intermediate 146(a) (58 mg,
0.13 mmol) as a
yellow powder in 55% yield.
Step 2. Preparation of Title Compound: N-(6-Oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)-3-piperidin-4-yl-acrylamide acetic acid salt
Preparation of the title compound from Intermediate 146(a) (58 mg, 0.13 mmol)
in CH2CI2 (2 mL)
and 4M HCI in dioxane (2 mL) was carried out analogously to Example 91. After
concentration,
the residue was purified by preparative, HPLC (Peeke Scientific, HI-Q C18
reverse phase 5u,
100A, 250x21.2 mm column) eluting with CH3CN and 0.1 % acetic acid in water at
a flow rate of 20
mL/min using a gradient of 5-95% CH3CN over 40 min to give the title compound
(16 mg, 0.04
mmol) as a pale yellow powder in 31 % yield.
'H NM R(d6-DMSO): 11.55 (s, IH), 10.00 (s, 1 H), 9.91 (s, 1 H), 8.00 (s, 1 H),
7.32 (d, J = 8 Hz,
2H), 7.22 (s, 1 H), 6.50 (m, 1 H), 5.82 (m, 1 H), 2.77-2.74 (m, 2H), 2.03 (m,
1 H), 1.65-1.53 (m, 2H),
1.29-1.28 (m, 2H), 1.10-1.02 (m, 2H).
LCMS: (M+H+) 337.
Example 147: 8-tert-Butox rcarbonylamino-6-oxo-5.6-dihydro-1 H-
[1,2]diazepino[4.5,6-cdjindole-2-
carboxylic acid methyl ester
MeO 0 MeO 0
-O
Intermediate 2(b), Boc20, Et3N I~ POCI3. DMF
Example 2
CH3CN, 100% BocHN ~ N CH2CI2, 68% BocHN N
H H
147(a) 147(b)
~ HN-N O HN-N
NH2NH2.H20, AcOH I~ \ NBS, DMF
Br
MeOH, reflux, 100% BocHN ~ N -78 C to RT, 75% BocHN N
H H
147(c) 147(d)
HN-N
O \
Pd(dppflCla, MeOH ` I ~ ~ OMe
Et3N, CO, DMF, 35% BocHN ~ N
H
Step 1. Preparation of 6-tert-Butoxycarbonylamino-1H-indole-4-carboxylic acid
methyl
ester 147(a)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
172
Triethylamine (17.1 mL, 123 mmol) was added slowly to Intermediate 2(b) of
Example 2
(hydrochloride) (27.5 g, 121 mmol) cooled to 0 C in 400 ml anhydrous CH3CN.
After 0.5 hours,
di-tert-butyl dicarbonate (26.76 g, 123 mmol) in anhydrous CH3CN (50 mL) was
added, and the
mixture was allowed to stir at room temperature for 24 h at which point
volatile components were
removed in vacuo. Ethyl acetate (500 mL) and H20 (500 mL) were added, and the
aqueous layer
was extracted with ethyl acetate (4X120 mL). The combined ethyl acetate
extracts were washed
with H20 (2X170 mL) and brine (100 mL) and allowed to dry over Na2SO4.
Following filtration, the
volatile components were removed in vacuo to afford Intermediate 147(a) (35.1
g, 121 mmol) as a
brown solid in quantitative yield.
' H NMR (d6-DMSO): S 12.28 (s, 1 H), 9.43 (s, 1 H), 7.91 (br s, 1 H), 7.89 (s,
1 H), 7.42 (t, 1 H, J
2.73 Hz), 6.83 (t, 1 H, J= 2.17 Hz), 3.89 (s, 3H), 1.50 (s, 9H).
LCMS: (M+Na+) 313.1; (M-H)" 289.2.
Anal. Calcd. for C15H18N204: C, 62.06; H, 6.25; N, 9.65.
Found: C, 62.08; H, 6.30; N, 9.59.
Step 2. Preparation of 6-tert-Butoxycarbonylamino-3-formyl-lH-indole-4-
carboxylic acid
methyl ester 147(b)
A premixed Vilsmeier reagent consisting of phosphorus oxychloride (33.67 mL,
3624 mmol) in
N,N-dimethylformamide (81.40 mL) was added dropwise at 0 C to Intermediate
147(a) (33.87 g,
116 mmol) stirring in anhydrous CH2CI2 (584 mL). The mixture was stirred for 1
hour at room
temperature, quenched with aqueous 2.0 M sodium acetate (700 ml) at 0 C and
neutralized with
solid Na2CO3. A solid formed and the mixture was partitioned between ethyl
acetate (4000 mL)
and H20 (2000 mL). The layers were separated and the aqueous layer was
extracted with ethyl
acetate (4 X 500 mL). The organic layers were combined, washed with brine,
dried over Na2SO4,
filtered, and concentrated. Methanol (3500 mL) was added to the residue
followed by K2C03 (70
g). The mixture was stirred at room temperature for 16 hours at which point
volatile components
were removed in vacuo. Ethyl acetate (500 mL) and H20 (500 mL) were added. The
entire
mixture was then filtered and the insoluble solids were collected and dried
under vacuum to afford
Intermediate 147(b) (14.35 g, 45.1 mmol) as a white solid. The aqueous layer
was again extracted
with ethyl acetate (4X120 mL), and the ethyl acetate extracts were combined,
washed with H20
(2X170 mL), brine and allowed to dry over Na2SO4. Following filtration, the
volatile components
were removed in vacuo to afford additional Intermediate 147(b) (10.74 g, 33.8
mmol) as a white
solid. The combined yield for the two batches was 68%.
' H NMR (d6-DMSO): 5 12.28 (s, 1 H), 10.09 (s, 1 H), 9.60 (s, 1 H), 8.23 (d, 1
H, J= 3.01 Hz), 7.96
(d, 1 H, J = 1.32 Hz), 7.65 (d, 1 H, J = 1.88 Hz), 3.84 (s, 3H), 1.50 (s, 9H).
LCMS: (M+H+) 319, (M+Na+) 341.1; (M-H)" 317.1
Step 3. Preparation of (6-Oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-
yl)-carbamic
acid tert-butyl ester 147(c)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
173
Acetic acid (7.89 ml) was added to Intermediate 147(b) (25.1 g, 78.9 mmol) in
anhydrous
methanol (789 ml). The suspension was stirred at room temperature for 10
minutes and
H2NNH2=H20 (21.43 mL, 395 mmol) was added. The mixture was stirred at room
temperature for
another 10 minutes, heated at 70 C for 0.5 hours and cooled to room
temperature. The volatile
components were removed in vacuo and the residual oil was triturated with
methanol and toluene
to afford Intermediate 147(c) (23.6 g, 78.9 mmol) as a yellow powder in
quantitative yield.
' H NMR (ds-DMSO): 5 11.63 (s, 1 H), 10.19 (s, 1 H), 9.45 (s, 1 H), 7.76 (s,
1H), 7.62 (s, 1 H), 7.51
(d, 1 H, J = 2.07 Hz), 7.44 (s, I H), 1.49 (s, 9H).
LCMS: (M+H+) 301.1, (M+Na+) 323.1; (M-H)" 299.1.
Anal. Calcd. for C15H16N403 0.5 H2O: C, 59.10; H, 5.46; N, 18.38.
Found: C, 59.49; H, 5.43; N, 17.97.
Step 4. Preparation of (2-Bromo-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
carbamic acid tert-butyl ester 147(d)
Intermediate 147(c) (1.00 g, 3.33 mmol) was dissolved in anhydrous N,N-
dimethylformamide (15
ml) and cooled to -78 C. N-Bromosuccinimide (0.564 g, 3.17 mmol) in anhydrous
N,N-
dimethylformamide (3.5 ml) was added dropwise over 2 min. The reaction mixture
was allowed to
warm to room temperature and stirred for 1 hour. Additional N-bromosuccinimide
(0.337 g, 2.00
mmol) in N,IV dimethylformamide (c.a. 1.5 mL) was then added in portions over
a 0.5 hours period
to drive the reaction to completion. The reaction mixture was poured into H20
(70 mL), and a dark
solid precipitated. The solid was collected by filtration and dried under
vacuum to give
Intermediate 147(d) (0.95 g, 2.51 mmol) as dark solid in 75% yield.
1 H NMR (d6-DMSO): 6 12.54 (s, 1 H), 10.47 (s, 1 H), 9.57 (s, 1 H), 7.77 (s, 1
H), 7.71 (d, 1 H, J
1.70 Hz), 7.31 (s, 1 H), 1.53 (s, 9H).
LCMS: (M+H+) 379.0, 381.0, (M+Na+) 401.0, 403.0; (M-H)" 377.1, 379.1.
Anal. Calcd. for C15H15BrN4O3: C, 47.51; H, 3.99; N, 14.77.
Found: C, 47.42; H, 3.99; N, 14.51.
Step 5. Preparation of Title Compound: 8-tert-Butoxycarbonylamino-6-oxo-5,6-
dihydro-1H-
[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid methyl ester
Intermediate 147(d) (0.200 g, 0.529 mmol), triethylamine (0.147 mL, 1.06 mmol)
and anhydrous
methanol (2 mL) in anhydrous N,N-dimethylformamide (2 ml) were purged with Ar.
[1,1'-
Bis(diphenylphosphino)ferrocene]dichloropalladium (II) (86.4 mg, 0.106 mmol)
was added and CO
was bubbled into the reaction mixture for 5 minutes. The reaction was then
sealed and heated at
85 C for 16 hours. The mixture was filtered through a thin pack of
diatomaceous earth and the
filtrate reduced in vacuo. Silica gel chromatography (eluted with 1:1 ethyl
acetate: hexane)
afforded the title compound (66 mg, 0.184 mmol) as a yellow powder in 35%
yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
174
'H NMR (d6-DMSO): S 12.42 (s, 1 H), 10.80 (s, 1 H), 9.69 (s, 1 H), 8.14 (s, 1
H) 7.91 (s, 1 H), 7.76
(s, 1 H), 3.92 (s, 3H), 1.49 (s, 9H).
LCMS: (M+H+) 359.1, (M+Na+) 381.2; (M-H)" 357Ø
Example 148: 1-Amino-N-(6-oxo-2-phenyl-5,6-dihydro-1 H-[1,21diazepino[4.5,6-
cd]indol-8-yl)-2.3-
dihydro-1 H-indene-1-carboxamide hydrochloride
HN-N
6O \
O I \ ~ -
/ ~ N
- NH2 H H-Cl Preparation of example 148 from title compound of Example 149 (52
mg, 0.097 mmol), and 4M
HCI in dioxane (5 mL) was carried out analogously to Example 91. Isolation,
also in an analogous
manner, afforded the title compound (46 mg, 0.097 mmol) as a yellow powder in
quantitative yield.
'H NMR (d6-DMSO): S 12.42 (s, 1 H), 10.60 (s, 1 H), 10.20 (s, 1 H), 9.05-8.86
(m, 3H), 8.23 (s, 1 H),
7.90-7.73 (m, 3H), 7.72-7.57 (m, 6H), 7.55-7.41 (m 2H), 3.42 (m, 1 H), 3.31
(m, 1 H), 3.08 (m, 1 H),
2.55 (m, 1 H, partially obscured).
LCMS: (M+H+) 436.2, 419.2.
Example 149: 1,1-Dimethylethyl 1-{j(6-oxo-2-phenyl-5,6-dihydro-1 H-f
1,21diazepino[4,5,6-cdlindol-
8-Ll)amino]carbonyl}-2, 3-dihydro-1 H-inden-1-ylcarbamate
HN-N
O \
O ~ ~ -
/ \ N I / N ~ ~
NHBou H
Preparation of example 149 from the title compound of Example 7
(hydrochloride) (52 mg, 0.17
mmol), 1-({[(1,1-dimethylethyl)oxy]carbonyl}amino)-2,3-dihydro-lH-indene-1-
carboxylic acid (72
mg, 0.26 mmol), triethylamine (0.071 mL, 0.51 mmol), and O-(7-azabenzotriazol-
1-yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (99 mg, 0.26 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethylformamide (0.4 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 1:1 hexane:acetone), also in an analogous manner,
afforded the title
compound (63 mg, 0.12 mmol) as a yellow powder in 69% yield.
'H NMR (d6-DMSO): S 12.05 (s, 1 H), 10.36 (s, 1 H), 10.01 (m, IH), 8.09 (m, 1
H), 7.87-7.74 (m,
2H), 7.70-7.62 (m, 2H), 7.63-7.53 (m, 2H), 7.52-7.47 (m, 2H), 7.31-7.22 (m,
3H), 3.08-2.95 (m,
3H), 2.09 (m, 1 H), 1.40 (m, 9H).
LCMS: (M-H)" 534Ø
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
175
Examgle 150: 1.1-Dimethvlethyl (1R)-1-j(4-hydroxyphenyl)meth rl -2-oxo-2-f(6-
oxo-2-phenyl 5 6
dihvdro-1H-f1,21diazepino[4 5 6-cdlindol-8-yl)aminolethylcarbamate
HN-N
O
\ N N
HO I / NHBo~ H
Preparation of example 150 from the title compound of Example 7
(hydrochloride) (44 mg, 0.144
mmol), (2R)-2-({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-(4-
hydroxyphenyl)propanoic acid (46
mg, 0.173 mmol), triethylamine (0.060 mL, 0.43 mmol), and O-(7-azabenzotriazol-
1-yl)-N,N,N;N=
tetramethyluronium hexafluorophosphate (66 mg, 0.173 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethylformamide (0.4 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 1:1 hexane:acetone), also in an analogous manner,
afforded the title
compound (74 mg, 0.137 mmol) as a yellow powder in 95% yield.
'H NMR (d6-DMSO): S 12.11 (s, 1 H), 10.41 (s, 1 H), 10.20 (s, IH), 9.20 (s,
IH), 8.17 (s, 1 H), 7.71-
7.45 (m, 7H), 7.14 (d, 2H, J= 8.50 Hz), 6.68 (d, 2H, J= 8.40 Hz), 4.24 (m, 1
H), 2.94 (m, 1 H), 2.72
(m, 1 H), 1.35 (s, 9H).
LCMS: (M-H)" 538.1.
Example 151: 1.1-Dimethvlethyl (1R)-1-[(4-hydroxyphenyl)methyl]-2-oxo-2-[(6-
oxo-2-phenyl-5 6-
dihvdro-1 H-f 1,21diazepinof4,5,6-cdlindol-8-yl)aminolethyl(methyl)carbamate
HN-N
O N WN
\ ~ S
/NBocH H
HO
Preparation of example 151 from the title compound of Example 7
(hydrochloride) (40 mg, 0.128
mmol), (2R)-2-[{[(1,1-dimethylethyl)oxy]carbonyl}(methyl)amino]-3-(4-
hydroxyphenyl)propanoic
acid (57 mg, 0.192 mmol), triethylamine (0.054 mL, 0.384 mniol), and O-(7-
azabenzotriazol-1-yl)-
N,N,N;N=tetramethyluronium hexafluorophosphate (73 mg, 0.192 mmol) in CHZCIz
(0.4 mL) and
N,N-dimethylformamide (0.4 mL) was carried out analogously to Example 11.
Additional (2R)-2-
[{[(1,1-dimethylethyl)oxy]carbonyl}(methyl)amino]-3-(4-hydroxyphenyl)propanoic
acid (14 mg,
0.047 mmol) and O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate
(18 mg, 0.047 mmol) were added after 24 hours to drive the reaction to
completion. Silica gel
chromatography (eluted with 1:1 hexane:acetone), also in an analogous manner,
afforded the title
compound (55 mg, 0.099 mmol) as a yellow powder in 78% yield.
' H NMR (d6-DMSO) multiple : 5 12.10 (br s, IH), 10.38 (br s, IH), 9.20 (m,
IH), 8.13 (m, IH),
7.72-7.63 (m, 3H), 7.61-7.43 (m, 4H), 7.09-7.00 (m, 2H), 6.72-6.62 (m, 2H),
4.82-4.60 (m, 1 H),
3.19-3.08 (m, 1 H), 2.93-2.85 (m, 1 H), 2.80-2.62 (m, 3H), 1.37-1.21 (m, 9H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
176
LCMS: (M+H+) 554.2, (M+Na+) 576.2 (M-H)" 552Ø
Example 152: 1.1-Dimethylethyl (1R)-1-[(4-fluoropheny,methyll-2-oxo-2-[(6-oxo-
2-phenyl-5 6-
dihydro-1 H-[1,21diazepino[4.5,6-cdlindol-8-yl)aminolethylcarbamate
HN-N
Z O I N N
~H
F
Preparation of example 152 from the title compound of Example 7
(hydrochloride) (40 mg, 0.128
mmol), (2R)-2-({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-(4-
fluorophenyl)propanoic acid (54 mg,
0.192 mmol), triethylamine (0.054 mL, 0.384 mmol), and O-(7-azabenzotriazol-1-
yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (73 mg, 0.192 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethylformamide (0.4 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 1:1 hexane:acetone), also in an analogous manner,
afforded the title
compound (61 mg, 0.113 mmol) as a yellow powder in 88% yield.
' H NMR (d6-DMSO): S 12.12 (s, 1 H), 10.42 (s, 1 H), 10.25 (s, 1 H), 8.18 (s,
1 H), 7.71-7.46 (m, 7H),
7.42-7.34 (m, 2H), 7.21-7.09 (m, 3H), 4.41 (m, 1 H), 3.03 (m, 1 H), 2.85 (m 1
H), 1.34 (s, 9H).
LCMS: (M-H)- 540.2.
Example 153: 1,1-Dimethylethyl (1R)-1-(4-hydroxyphenyl)-2-oxo-2-[(6-oxo-2-
phenyl-5,6-dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)amino]ethylcarbamate
HN-N
~
_
HO / ONOI / ~ N ~ ~
~ (
NHBoo H
Preparation of example 153 from the title compound of Example 7
(hydrochloride) (42 mg, 0.134
mmol), (2R)-({[(1,1-dimethylethyl)oxy]carbonyl}amino)(4-hydroxyphenyl)ethanoic
acid (54 mg,
0.202 mmol), triethylamine (0.056 mL, 0.402 mmol), and O-(7-azabenzotriazol-l-
yl)-N,N,N;M
tetramethyluronium hexafluorophosphate (77 mg, 0.202 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethylformamide (0.4 mL) was carried out analogously to Example 11.
Additional (2R)-({[(1,1-
dimethylethyl)oxy]carbonyl}amino)(4-hydroxyphenyl)ethanoic acid (27 mg, 0.10
mmol) and O-(7-
azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (39 mg,
0.10 mmol)
were added after 24 hours to drive the reaction to completion. Silica gel
chromatography (eluted
with 1:1 hexane:acetone), also in an analogous manner, afforded the title
compound (48 mg,
0.091 mmol) as a yellow powder in 68% yield.
'H NMR (d6-DMSO): 5 12.11 (s, 1 H), 10.40 (s, IH), 10.30 (s, 1 H), 9.46 (s, 1
H), 8.12 (s, IH), 7.71-
7.48 (m, 7H), 7.33-7.27 (m, 2H), 6.80-6.69 (m, 2H), 5.22 (m, 1 H), 1.41 (s, 1
H).
LCMS: (M+H+) 526.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
177
Example 154: 1 1-Dimethylethyl (1 R)-1-(naphthalen-2-ylmethyl)-2-oxo-2-[(6-oxo-
2-phenyl-5.6-
dihydro-lH-[1 2ldiazepinof4 5.6-cd]indol-8-y,aminolethylcarbamate
HN-N
W
O Co-^-CkBo N
~ H
Preparation of example 154 from the title compound of Example 7
(hydrochloride) (40 mg, 0.128
mmol), (2R)-2-({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-naphthalen-2-
ylpropanoic acid (58 mg,
0.192 mmol), triethylamine (0.054 mL, 0.384 mmol), and O-(7-azabenzotriazol-1-
yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (73 mg, 0.192 mmol) in CH2CI2 (0.4 mL)
and N,N-
dimethylformamide (0.4 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 1:1 hexane:acetone), also in an analogous manner,
afforded the title
compound (69 mg, 0.120 mmol) as a yellow powder in 94% yield.
' H NMR (d6-DMSO): S 12.12 (s, 1 H), 10.41 (s, 1 H), 10.31 (s, 1 H), 8.18 (s,
1 H), 7.90-7.81 (m, 4H),
7.71-7.66 (m, 3H), 7.62-7.56 (m, 3H), 7.54-7.46 (m, 4H), 7.23 (d, 1 H, J = 8.1
Hz), 4.46 (m, 1 H),
3.23 (m, 1 H), 3.02 (m, 1 H), 1.30 (s, 9H).
LCMS: (M-H)- 572.2.
Example 155: 1 1-Dimethylethyl (1R)-1-f(4-hydroxyphenyl)methyl]-2-oxo-2-f(6-
oxo-5,6-dihvdro-1H-
[1 2ldiazepinof4 5 6-cd]indol-8-yl)aminolethylcarbamate
HN-N
o
WN
N H
HO \ / NHBou
Preparation of example 155 from the title compound of Example 2 (71 mg, 0.30
mmol), (2R)-2-
({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-(4-hydroxyphenyl)propanoic acid
(121 mg, 0.45
mmol), triethylamine (0.125 mL, 0.9 mmol), and O-(7-azabenzotriazol-l-yl)-
N,N,N;N'
tetramethyluronium hexafluorophosphate (171 mg, 0.45 mmol) in CH2CI2 (0.5 mL)
and N,N-
dimethylformamide (0.5 mL) was carried out analogously to Example 11.
Purification, also in an
analogous manner (eluted with 1:1 hexane:acetone) afforded the title compound
(100 mg, 0.22
mmol) as a yellow powder in 72% yield.
'H NMR (ds-DMSO): S 11.75 (s, 1 H), 10.26 (s, IH), 10,13 (s, IH), 9.19 (s, 1
H), 8.16 (s, 1 H), 7.61-
7.56 (m, 2H), 7.14 (d, 2H, J= 8.10 Hz), 7.04 (d, 1 H, J= 8.48 Hz), 6.66 (d,
2H, J= 8.10 Hz), 4.22
(m, 1 H), 3.08 (m, 1 H), 2.87 (m, 1 H), 1.34 (s, 9H).
LCMS: (M+H+) 464.2, (M+Na+) 486.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
178
Example 156: 1.1-Dimethylethyl (1R)-1-[(4-hydroxyphen~rl)methyl]-2-oxo-2-rj6-
oxo-5,6-dihydro-
1 H-[1,2]diazepinof4,5,6-cdlindol-8-yl)amino]ethyl(methyl)carbamate
HN-N
o AN WN
\
HO I / /NBocH H
Preparation of example 156 from the title compound of Example 2 (74 mg, 0.31
mmol), (2R)-2-
[{[(1,1-dimethylethyl)oxy]carbonyl}(methyl)amino]-3-(4-hydroxyphenyl)propanoic
acid (138 mg,
0.47 mmol), triethylamine (0.130 mL, 0.93 mmol), and O-(7-azabenzotriazol-l-
yl)-N,N,N;M
tetramethyluronium hexafluorophosphate (179 mg, 0.47 mmol) in CH2CI2 (0.5 mL)
and N,N-
dimethylformamide (0.5 mL) was carried out analogously to Example 11.
Purification, also in an
analogous manner (eluted with 1:1 hexane:acetone) afforded the title compound
(131 mg, 0.27
mmol) as a yellow powder in 88% yield.
'H NMR (ds-DMSO): S 11.77 (s, 1 H), 10.26 (s, 1 H), 9.21 (s, 1 H), 8.12 (m, 1
H), 7.65 (d, 1 H, J=
1.32 Hz), 7.58 (d, 1 H), J = 2.26 Hz), 7.48 (s, 1 H), 7.07 (d, 2H, J = 8.29
Hz), 6.73-6.64 (m, 2H),
4.75 (m, 1 H), 3.12 (m, 1 H), 2.86-2.65 (m, 4 H, partially obscurred by N,N-
dimethylformamide),
1.35-1.25 (m, 9H).
LCMS: (M+H+) 478.3, (M+Na+) 500.3.
Example 157: 1,1-Dimethylethyl (1R)-1-[(4-fluorophenyl)methkll-2-oxo-2-f(6-oxo-
5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-8-yl)amino]ethylcarbamate
HN-N
o
WN
\ N F I / NHBoU H
Preparation of example 157 from the title compound of Example 2 (80 mg, 0.34
mmol), (2R)-2-
({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-(4-fluorophenyl)propanoic acid
(145 mg, 0.51 mmol),
triethylamine (0.142 mL, 1.02 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (193 mg, 0.51 mmol) in CH2CI2 (0.5 mL)
and N,N-
dimethylformamide (0.5 mL) was carried out analogously to Example 11.
Purification, also in an
analogous manner (eluted with 1:1 hexane:acetone) afforded the title compound
(56 mg, 0.12
mmol) as a yellow powder in 35% yield.
'H NMR (ds-DMSO): S 11.73 (s, 1 H), 10.24 (s, 1 H), 10.16 (s, 1 H), 8.10 (s, 1
H), 7.55 (s, 1 H with
fine splitting), 7.45 (s, 1 H), (7.38-7.29 (m, 2H), 7.16-7.04 (m, 3H), 4.25
(m, 1 H), 2.97 (m, 1 H), 2.79
(m, 1 H), 1.30 (s, 9H).
LCMS: (M+H+) 466.2, (M+Na+) 488.3.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
179
Example 158: 1.1-Dimethylethyl (1 R)-1-(naphthalen-2-ylmethyl)-2-oxo-2-[(6-oxo-
5,6-dihydro-1 H-
(1,2]diazepinof4,5,6-cd]indol-8-yl)amino]ethylcarbamate
HN-N
W
~ N H
\ I / NHBo~
Preparation of example 158 from the title compound of Example 2 (76 mg, 0.32
mmol), (2R)-2-
({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-naphthalen-2-ylpropanoic acid (145
mg, 0.48 mmol),
triethylamine (0.134 mL, 0.96 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium hexafluorophosphate (182 mg, 0.48 mmol) in CH2CI2 (0.5 mL)
and N,N-
dimethylformamide (0.5 mL) was carried out analogously to Example 11.
Purification, also in an
analogous manner (eluted with 1:1 hexane:acetone) afforded the title compound
(67 mg, 0.13
mmol) as a yellow powder in 42% yield.
'H NMR (d6-DMSO): S 11.76 (br s, 1 H), 10.27 (s, 1 H), 10.27 (br s, 1 H), 8.15
(s, 1 H), 7.90-7.79 (m,
4H), 7.62-7.42 (m, 5H), 7.21 (d, 1 H, J = 7.73 Hz), 4.43 (m, 1 H), 3.18 (m, 1
H), 3.02 (m, 1 H), 1.29
(s, 9H).
LCMS: (M+H+) 498.2, (M+Na+) 520.2.
Example 159: 1.1-Dimethylethyl (1R)-2-oxo-2-[(6-oxo-5.6-dihydro-1H-
[1,2]diazepino[4,5,6-
cd]indol-8-yl)amino]-1-{[(phenylmethyl)oxy]methY}ethylcarbamate
HN-N
O ~
0 I \ \
I \ 0" Y `N / N
NHBou H
Preparation of example 159 from the title compound of Example 2 (97 mg, 0.41
mmol), (2R)-2-
({[(1,1-dimethylethyl)oxy]carbonyl}amino)-3-[(phenylmethyl)oxy]propanoic acid
(182 mg, 0.62
mmol), triethylamine (0.171 mL, 1.23 mmol), and O-(7-azabenzotriazol-1-yl)-
N,N,N;M
tetramethyluronium hexafluorophosphate (236 mg, 0.62 mmol) in CH2CI2 (0.5 mL)
and N,N-
dimethylformamide (0.5 mL) was carried out analogously to Example 11.
Purification, also in an
analogous manner (eluted with 1:1 hexane:acetone) afforded the title compound
(40 mg, 0.083
mmol) as a yellow powder in 20% yield.
'H NMR (d6-DMSO): S 11.78 (br s, 1 H), 10.29 (s, 1 H), 10.22 (s, 1 H), 8.14
(s, 1 H), 7.65 (s, 1 H),
7.61 (s, 1 H, with fine splitting), 7.75 (s, 1 H), 7.38-7.26 (m, 4H), 7.08 (d,
1 H, J = 6.02 Hz), 4.54 (s,
2H), 4.42 (m, 1 H), 3.74-3.63 (m, 2H), 1.42 (s, 9H).
LCMS: (M+H+) 478.3, (M+Na+) 500.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
180
Example 160: (1R,2R)- 6-Oxo-8-[(2-phenyl-ckclopropanecarbonyl)-amino]_5 6-
dihydro-lH-
j1,21diazepinor4,5.6-cdjindole-2-carboxylic acid methyl ester
O
HN-N
Title Compund I O \ \ .o,'\pH
4N HCI (Dioxane) OMe
of Example 147 CH2CI2, 99% CIH.HzN \ ~ N HATU, Et3N, DMF, 46%
H
160(a)
HN-N
O \
O \ \ OMe
..~~N I / N O
0 H H
Step 1. Preparation of 8-Amino-6-oxo-5,6-dihydro-1H-[1,2]diazepino[4,5,6-
cd]indole-2-
carboxylic acid methyl ester (hydrochloric salt) 160(a)
Preparation of intermediate 160(a) from the title compound of Example 147
(45.0 mg, 0.125
mmol) and 4.0 M HCI in dioxane (0.32 mL) was carried out analogously to
Example 91. Isolation,
also in an analogous manner, afforded Intermediate 160(a) (36.9 mg, 0.125
mmol) as a yellow
powder in 99% yield.
Step 2. Preparation of Title Compound: (1R,2R)- 6-Oxo-8-[(2-phenyl-
cyclopropanecarbonyl)-amino]-5,6-dihyd ro-1 H-[1,2]diazepino[4,5,6-cd]indole-2-
carboxylic
acid methyl ester
Preparation of the title compound from Intermediate 160(a) (36.9 mg, 0.125
mmol), (1 R,2R)-2-
phenyl-cyclopropanecarboxylic acid (22.0 mg, 0.136 mmol), triethylamine (0.175
mL, 1.26 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate
(72.0 mg,
0.189 mmol) in N,N-dimethylformamide (2.0 mL) was carried out analogously to
Example 11.
Silica gel chromatography (eluted with 1:1 ethyl acetate: hexane), also in an
analogous manner,
included a further trituration with methanol and ether and afforded the title
compound (23 mg,
0.0572 mmol) as a yellow powder in 46% yield.
'H NMR (ds-DMSO): S 12.53 (s, 1 H), 10.83 (s, 1 H), 10.58 (s, 1 H), 8.23 (d, 1
H, J= 1.51 Hz), 8.15
(s, 1 H), 7.76 (d, 1 H, J= 1.51 Hz), 7.37-7.25 (m, 2H), 7.25-7.14 (m, 3H),
3.93 (s, 3H), 2.45-2.35
(m, 1 H), 2.15-2.05 (m, 1 H), 1.58-1.47 (m, 1 H), 1.45-1.33 (m, 1 H).
LCMS: (M+H+) 403.3, (M+Na+) 425.1; (M-H)" 401Ø
HRMS: (M+H+) calcd for C22H19N404i 403.1406, found 403.1413.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
181
Example 161: (2-Methylcarbamoyl-6-oxo-5,6-dihydro-1H-[1 2]diazepino[4 5 6-
cdJindol-8-yI)-
carbamic acid tert-butyl ester
HN-N
WN Intermediate 147(d), Pd(dppf)C12, CH3NHz NHMe
Example 147 Et3N, CO, DMF, 29% BocHN 0
Preparation of example 161 was carried out in a manner analogous to step 5 of
Example 147,
except that methylamine hydrochloride was substituted for methanol. Thus CO
was bubbled
through a mixture of Intermediate 147(d) from Example 147 (200 mg, 0.529
mmol), triethylamine
(0.29 mL, 2.11 mmol), methylamine hydrochloride (71 mg, 1.06 mmol), and 1,1'-
bis(diphenylphosphino)ferrocenedichloropalladium (11) (86 mg, 0.106mmol).
Reaction conditions,
work up and silica gel chromatography (eluted with 46:4:25 dichloromethane:
methanol: ethyl
acetate) were also carried out in an analogous manner and afforded the title
compound (55 mg,
0.154 mmol) in 29% yield.
'H NM R(d6-DMSO): S 12.01 (s, IH), 10.55 (s, 1 H), 9.58 (s, IH), 8.33-8.25 (m,
1 H) 7.98 (s, 1 H),
7.80 (s, I H), 7.71 (d, I H, J = 1.51 Hz), 2.81 (d, 3H, J = 4.52 Hz), 1.48 (s,
9H).
LCMS: (M+H+) 358.3, (M+Na+) 380.1; (M-H") 356.1.
Example 162: f2-(2-Hydroxy-ethylcarbamoyl)-6-oxo-5,6-dihydro-1 H-[1
2ldiazepino[4 5 6-cdlindol-
8-yll-carbamic acid tert-butyl ester
HN-N
Z H
4:"-O
BocHN N H
Preparation of example 162 was carried out in a manner analogous to step 5 of
Example 147,
except that 2-aminoethanol was substituted for methanol, and chromatography
was not required.
Thus CO was bubbled through a mixture of Intermediate 147(d) from Example 147
(60 mg, 0.16
mmol), triethylamine (0.044 mL, 0.32 mmol), 2-aminoethanol (19 mg, 0.32 mmol),
and 1,1'-
bis(diphenylphosphino)ferrocenedichloropalladium (II) (26 mg, 0.032 mmol) in
N, N-
dimethylformamide (3.0 mL). After the reaction was complete, the mixture was
filtered through
diatomaceous earth. The filtrate was concentrated, and methanol was added. The
resulting solids
were collected by filtration and washed with methanol, dichloromethane and
diethyl ether. After
drying under vacuum, the title compound (24 mg, 0.062 mmol) was obtained as a
yellow powder
in 39% yield.
' H NMR (d6-DMSO): S 12.08 (s, 1 H), 10.56 (s, 1 H), 9.58 (s, 1 H), 8.30 (t, 1
H, J = 5.84 Hz) 8.06 (s,
1 H), 7.79 (s, 1 H), 7.74 (s, 1 H), 4.80 (br s, I H), 3.60-3.48 (m, 2H), 3.38
(m, 2H, partially obscured),
1.48 (s, 9H).
LCMS: (M+H+) 388.1, (M+Na+) 410.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
182
Example 163: (1 2-trans)-2-Piperidin-4-yl-cyclopropanecarboxylic acid (6-oxo-
5, 6-dihydro-lH-
11,21diazepinof4,5,6-cdjindol-8-yl)-amide (acetic acid salt)
~~ ~/-CO2Et NaH, Me3SO+I" C02Et 1 M LiOH, THF, H20
BocN_ }--~ BocN~
~/ DMSO, 67% 100%
163(a)
H
O N-N
CO2H Title Compound BocN O
BocN\~ of Example 2
N N
HATU, Et3N, DMF, 77% H
163(b) 163(c)
H
0 O N-N
\
AOH
4N HCI (Dioxane) HN O I\ ~
CH2CI2, 7% N N
H H
Step 1. Preparation of 4-((1,2-trans)-2-Ethoxycarbonyl-cyclopropyl)-piperidine-
l-carboxylic
acid tert-butyl ester 163(a)
To a mixture of NaH (303 mg, 7.57 mmol) and trimethylsulfoxonium iodide (1.67
g, 7.57 mmol)
was added dimethyl sulfoxide (10 mL). After stirring for 30 min, a solution of
4-(2-ethoxycarbonyl-
vinyl)-piperidine-l-carboxylic acid tert-butyl ester in dimethyl sufoxide (5
mL) was added drop-
wise. After stirring overnight, ethyl acetate and water were added to the
mixture. The aqueous
layer was extracted with ethyl acetate several times. The combined organic
layers were dried over
Na2SO4, filtered, and concentrated. The residue was purified by silica gel
chromatography eluting
with 10% ethyl acetate/hexane to give Intermediate 163(a)(1.16 g, 67%).
Step 2. Preparation of 4-((1,2-trans)-2-Carboxy-cyclopropyl)-piperidine-l-
carboxylic acid
tert-butyl ester 163(b)
To a mixture of Intermediate 163(a) (555 mg, 1.87 mmol) in 3:1 tetrahydrofuran-
H20 (12 mL) was
added aqueous 1 M LiOH (5.61 mL). The resulting mixture was stirred overnight.
The mixture was
then acidified with 1 M HCI to pH 1 and extracted with several times with
ethyl acetate. The
combined organic layers was then washed with brine and concentrated to give
Intermediate
163(b) (515 mg, 1.87 mmol) in quantitative yield which was carried on without
further purification.
Step 3. Preparation of 4-[(1,2-trans)-2-(6-Oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-
8-ylcarbamoyl)-cyclopropyl]-piperidine-l-carboxylic acid tert-butyl ester
163(c)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
183
Preparation of Intermediate 163(c) from the title compound of Example 2
(freebase) (374 mg,
1.87 mmol), Intermediate 163(b) (500 mg, 1.87 mmol), triethylamine (0.31 mL,
2.24 mmol), and
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (782
mg, 2.06
mmol) in N,N-dimethylformamide (10 mL) was carried out analogously to Example
11. Silica gel
chromatography (eluted with 40% acetone in hexane), also in an analogous
manner, afforded
Intermediate 163(c) as a yellow powder (649 mg, 1.44 mmol) in 77% yield.
Step 4. Preparation of Title Compound: (1,2-trans)-2-Piperidin-4-yi-
cyclopropanecarboxylic
acid (6-oxo-5, 6-dihydro-l-[1,2]diazepino[4,5,6]indol-8-yl)-amide (acetic acid
salt)
Preparation of the title compound from Intermediate 163(c) (603 mg, 1.34 mmol)
in CH2CI2 (10
mL) and 4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
After
concentration, the crude product was purified by preparative HPLC in a manner
analogous to
Example 146, Step 2, to give the title compound as a pale yellow powder (37
mg, 0.09 mmol) in
7% yield.
'H NMR (d6-DMSO): 11.71 (s, 1 H), 10.26 (s, 1 H), 10.23 (s, 1 H), 8.11 (s, 1
H), 7.55 (d, J= 8 Hz,
2H), 7.46 (s, IH), 3.05-3.03 (m, 2H), 2.51 (m, IH), 1.65-1.53 (m, 3H), 1.29-
1.28 (m, 2H), 1.10 (m,
1 H), 0.99 (m, 1 H), 0.96 (m, 1 H), 0.73 (m, 1 H).
LCMS: (M+H+) 352.
Example 164: (6-Oxo-2-vinvl-5 6-dihydro-1H-11 2ldiazepino[4 5 6-cdlindol-8-yl)-
carbamic acid tert-
bu I ester
HN-N
O ~
Intermediate 147(d), (n-Bu)3SnCH=CH2 ~ /
Example 147 ~ ~
Pd(PPh3)4, DMF, 85 C BocHN ~ H
99%
Under an argon atmosphere, Intermediate 147(d) of Example 147 (0.250 g, 0.661
mmol), tributyl-
vinyl-tin (0.420 g, 1.32 mmol) and tetrakis(triphenylphosphine)palladium(0)
(38 mg, 0.033 mmol)
in anhydrous N,N-dimethylformamide (5 mL) were heated at 85 C for 16 hours.
The reaction was
filtered through a thin pack of diatomaceous earth, and the filtrate
concentrated in vacuo. Silica
gel chromatography (eluted with 1:4 ethyl acetate: dichloromethane) afforded
the title compound
(0.18 g, 0.552 mmol) as a yellow powder in 84% yield.
'H NMR (d6-DMSO): 5 11.80 (s, 1 H), 10.32 (s, 1 H), 9.51 (s, 1 H), 7.72-7.62
(m, 3H), 7.05 (dd, 1 H,
J= 11.87, 6.59 Hz), 5.89 (d, 1 H, J= 17.33 Hz), 5.43 (d, 1 H, J= 11.11 Hz),
1.49 (s, 9H).
LCMS: (M+H+) 327.2, (M+Na+) 349.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
184
Example 165: [2-(2-Dimethylamino-ethylcarbamoyl)-6-oxo-5 6-dihydro-lH-[1
2]diazepino[4 5 6-
cd]indol-8-yl]-carbamic acid tert-butyl ester
HN-N ~
0 ~
~ HN~-
~ ~
BocHN ~ N O
H
Preparation of example 165 was carried out in a manner analogous to step 5 of
Example 147,
except that N,N-dimethylethylenediamine was substituted for methanol. Thus CO
was bubbled
through a mixture of Intermediate 147(d) from Example 147 (1.5 g, 3.97 mmol),
triethylamine (1.1
mL, 7.92 mmol), N,N-dimethylethylenediamine (0.7 g, 7.94 mmol) and 1,1'-
bis(diphenylphosphino)ferrocenedichloropalladium (II) (0.65 g, 0.796 mmol) in
N,N-
dimethylformamide (30.0 mL). Reaction conditions, work up and silica gel
chromatography (eluted
with 46:4:25 dichloromethane: methanol: ethyl acetate) were also carried out
in an analogous
manner and afforded the title compound (1.25 g, 3.02 mmol) in 76% yield.
'H NMR (ds-DMSO): S 10.55 (s, 1 H), 9.59 (s, 1 H), 8.25 (t, 1 H, J = 5.65,
4.71 Hz) 8.06 (s, 1 H),
7.80 (s, 1 H), 7.74 (s, 1 H), 3.45-3.37 (m, 2H, partially obscured), 2.42 (t,
2H, J = 6.59, 6.41 Hz),
2.20 (s, 6H), 1.49 (s, 9H).
LCMS: (M+H+) 415.3, (M+Na+) 437.1; (M-H)" 413.1.
Example 166: (2R,3R)-2-Phenyl-pyrrolidine-3-carboxylic acid (6-oxo-5.6-dihydro-
1H-
[1.21diazepino[4,5,6-cd]indol-8-yl)-amide (hydrochloric salt)
O 1 f O Title Compound
(Boc)2O, Et3N of Example 2
HN '~ ~~ OH CH2CI2, rt, 100% BocN =,J~OH HATU, Et3N, DMF, 68%
166(a)
H H
O N-N O N-N
e) 1 0 O
O ::::;an
JL ~~
BocN N N HN =A N N
H H H H-CI H
166(
b)
Step 1. Preparation of 2-Phenyl-pyrrolidine-1,3-dicarboxylic acid 1-tert-butyl
ester 166(a)
To a suspension of (2R,3R)-3-phenylpyrrolidine-2-carboxylic acid (100 mg,
0.556 mmol) in
dioxane (2 mL) and HZO (2 mL) was added triethylamine followed by di-tert-
butyl dicarbonate (127
mg, 0.583 mmol). The resulting mixture was stirred overnight. The mixture was
then partitioned
between ethyl acetate and 0.1 M HCI. The combined organic layers were dried
over anhydrous
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
185
Na2SO4, filtered, and concentrated to give Intermediate 166(a) (162 mg,
0.56mmol) in 100%
yield which was carried on without purification.
Step 2. Preparation of 3-(6-Oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-
ylcarbamoyl)-2-phenyl-pyrrolidine-l-carboxylic acid tert-butyl ester 166(b)
Preparation of intermediate 166(b) from the title compound of Example 2 (300
mg, 1.0 mmol),
Intermediate 166(a) (162 mg, 0.56 mmol), triethylamine (0.15 mL, 1.1 mmol),
and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (254 mg,
0.667 mmol) in
N,N-dimethylformamide (10 mL) was carried out analogously to Example 11.
Silica gel
chromatography (eluted with 40% acetone in hexane), also in an analogous
manner, afforded
Intermediate 166(b) as a yellow powder (180 mg, 0.38 mmol) in 68% yield.
Step 3. Preparation of Title Compound: (2R,3R)-2-Phenyl-pyrrolidine-3-
carboxylic acid (6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide (hydrochloric
salt)
Preparation of the title compound from Intermediate 166(b) (180 mg, 0.38 mmol)
in CH2CIZ (5 mL)
and 4M HCI in dioxane (5 mL) was carried out analogously to Example 91. The
solids were
collected by filtration and washed with diethyl ether to give the title
compound as a pale yellow
powder (154 mg, 0.37 mmol) in 99% yield.
'H NMR (d6-DMSO): 12.03 (s, 1 H), 10.72 (s, 1 H), 10.46 (s, 1 H), 10.20 (br s,
1 H), 9.24 (brs, 1 H),
8.17 (s, 1 H), 7.79 (d, J = 4.0 Hz, 1 H), 7.69 (d, J = 4.0 Hz, 1 H), 7.58-7.52
(m, 5H), 7.48 (m, 1 H),
4.43 (rn, 1 H), 3.77 (q, J= 8.0 Hz; 1 H), 3.75 (m, 1 H), 3.59 (m, 1 H), 2.66
(m, 1 H), 2.62 (m, 1 H).
LCMS: (M+H+) 374.2.
Example 167: (2R)-3-(4-Hydroxyphenyl)-2-(methylamino)-N-(6-oxo-2-phenyl-5.6-
dihydro-1 H-
[1.2~diazepino[4.5.6-cdjindol-8- rl propanamide hydrochloride
HN-N
O
~ _ H
WN
HOI/
NH H-CI H
Preparation of example 167 from the title compound of Example 151 (47 mg,
0.085 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, included freebasing with triethylamine and subsequent silica
gel
chromatography eluting with 3:1:1 hexane:ethyl acetate:ethanol. With ice bath
cooling, the purified
freebase in CH2CI2 (10 mL) was treated with 4M HCI in dioxane (0.1 mL). After
removal of the
volatile components, the title compound (20 mg, 0.041 mmol) was obtained as an
orange/yellow
powder in 48% yield.
'H NMR (ds-DMSO): 5 12.21 (s, 1 H), 10.70 (s, 1 H), 10.43 (s, 1 H), 9.44-9.06
(m, 1 H), 8.02 (s, 1 H),
7.72-7.64 (m, 2H), 7.61-7.54 (m, 3H), 7.53-7.47 (m, 2H) 7.05 (d, 2H, J = 8.0
Hz), 6.65 (d, 2H, J
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
186
8.0 Hz), 4.09 (m, 1 H), 3.40-3.31 (m, 2H), 3.16 (dd, 1 H, J = 6.07, 13.90 Hz),
3.07 (dd, 1 H, J
7.58, 13.89 Hz), 2.55 (s, 3H).
LCMS: (M+H+) 454.1, (M+Na+) 476.1.
Example 168: (2R)-2-Amino-3-(4-fluorophenyl)-N-(6-oxo-2=phenyl-5 6-dihydro-lH-
r1,21diazepino[4,5,6-cdlindol-8-,til)propanamide hydrochloride
HN-N
O N WN H
F NH2 H H-Cl
Preparation of example 168 from the title compound of Example 152 (49 mg,
0.091 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, included freebasing with triethylamine and subsequent silica
gel
chromatography eluting with 3:1:1 hexane:ethyl acetate:ethanol. With ice bath
cooling, the purified
freebase in CH2CI2 (10 mL) was treated with 4M HCI in dioxane (0.1 mL). After
removal of the
volatile components, the title compound (21 mg, 0.044 mmol) was obtained as an
orange/yellow
powder in 49% yield.
1 H NMR (d6-DMSO): 8 12.26 (s, 1 H), 10.83 (s, 1 H), 10.46 (s, 1 H), 8.48 (br
s, 3H), 8.08 (s, 1 H),
7.73-7.48 (m, 7H), 7.40-7.30 (m, 2H), 7.24-7.13 (m, 2H), 4.22 (m, 1 H), 3.24
(m, 1 H), 3.13 (m, 1 H).
LCMS: (M+H+) 442.1, (M+Na+) 464.1.
Example 169: (1 R,2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbony,-amino]-5 6-
dihydro-1H-
f1,2]diazepinof4,5,6-cellindole-2-carboxylic acid methylamide
O HN-N 0
4N HCI (Dioxane) NHMe
Intermediate 161(a), I \ \
Example 161 CH2CI2,99% CIH.H2N / N 0 HATU, Et3N, DMF, 41%
H
169(a)
O HN-N
O \ \ NHMe
N I / N O
H H
Step 1. Preparation of 8-Amino-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indole-2-
carboxylic acid methylamide (hydrochloric salt) 169(a)
Preparation of intermediate 169(a) from 161(a) of Example 161 (55.0 mg, 0.154
mmol) and 4.0 M
HCI in dioxane (0.77 mL) was carried out analogously to Example 91. Isolation,
also in an
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
187
analogous manner, afforded Intermediate 169(a) (45.0 mg, 0.154 mmol) as a
yellow powder in
99% yield.
'H NMR (d6-DMSO): 8 12.46 (s, 1 H), 10.71 (s, 1 H), 8.54 (m, 1 H), 8.05 (s, 1
H), 7.43 (s, 1 H), 7.34
(s, 1 H), 2.81 (s, 3H).
LCMS: (M+H+) 258.1; (M-H)" 256.1.
Step 2. Preparation of Title Compound: (1 R,2R)- 6-Oxo-8-[(2-phenyl-
cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indole-2-
carboxylic
acid methylamide
Preparation of the title compound from Intermediate 169(a) (45.0 mg, 0.154
mmol), (IR,2R)-2-
phenyl-cyclopropanecarboxylic acid (59.4 mg, 0.231 mmol), triethylamine (0.086
mL, 0.616
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (87.8
mg, 0.231 mmol) in N,N-dimethylformamide (2.0 mL) was carried out analogously
to Example 11.
When the reaction was judged complete, N,N-dimethylformamide was evaporated
and methanol
was added. The mixture was filtered to collect the solids, which were washed
with methanol and
diethyl ether. After drying under vacuum, the title compound (25.0 mg, 0.0623
mmol) was
obtained as a yellow powder in 41 % yield.
' H NMR (d6-DMSO): 5 12.13 (s, 1 H), 10.60 (s, 1 H), 10.51 (s, 1 H), 8.36 (d,
1 H, J= 4.52 Hz), 8.17
(s, 1 H), 8.01 (s, 1 H), 7.70 (s, 1 H), 7.37-7.25 (m, 2H), 7.25-7.14 (m, 3H),
2.83 (d, 3H, J= 4.52 Hz),
2.45-2.35 (m, 1 H), 2.14-2.04 (m, 1 H), 1.57-1.47 (m, 1 H), 1.45-1.33 (m, 1
H).
LCMS: (M+H+) 402.1, (M+Na+) 424.1.
HRMS: (M+H+) calcd for C22H20N503, 402.1566, found 402.1551.
Example 170: (1 R,2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbonLrl)-amino]-5 6-
dihydro-1 H-
L,2ldiazepinof4,5,6-cdlindole-2-carboxylic acid (2-hydroxy-ethyl)-amide
HN-N j
~OH
4N HCI (Dioxane) HN~OH Q
Intermediate 162 (a), I \
Example 162 CH2CI2, 99% CIH.HZN / N 0 HATU, Et3N, DMF, 17%
H
170(a)
HN-N
0 ~ OH
0 I ~ \ HN-~
N
17 H H
Step 1. Preparation of 8-Amino-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indole-2-
carboxylic acid (2-hydroxy-ethyl)-amide (hydrochloric salt) 170(a)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
188
Preparation of intermediate 170(a) from intermediate 162(a) of Example 162
(80.0 mg, 0.206
mmol) and 4.0 M HCI in dioxane (1.10 mL) was carried out analogously to
Example 91. Isolation,
also in an analogous manner, afforded Intermediate 170(a) (66.7 mg, 0.206
mmol) as a yellow
powder in 99% yield.
LCMS: (M+H+) 288.2.
Step 2. Preparation' of Title Compound: (1R,2R)- 6-Oxo-8-[(2-phenyl-
cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indole-2-
carboxylic
acid (2-hydroxy-ethyl)-amide
Preparation of the title compound from Intermediate 170(a) (66.7 mg, 0.206
mmol), (1 R,2R)-2-
phenyl-cyclopropanecarboxylic acid (46.0 mg, 0.284 mmol), triethylamine (0.143
mL, 1.03 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(147 mg,
0.387 mmol) in N,N-dimethylformamide (2.0 mL) was carried out analogously to
Example 11. The
reaction mixture was reduced in volume and subjected to preparative HPLC (H1-Q
C18 reverse-
phase 5 uM, 100A, 150x20 column eluting with CH3CN/0.1 % acetic acid in H20 at
a flowrate of 20
mL/min using a gradient of 20-60% CH3CN over 30 min) to afford the title
compound (15 mg,
0.0348 mmol) as a yellow powder in 17% yield.
'H NMR (d6-DMSO): S 12.19 (s, 1 H), 10.61 (s, 1 H), 10.52 (s, 1 H), 8.36 (t, 1
H, J= 5.65 Hz), 8.18
(d, 1 H, J= 1.70 Hz), 8.09 (s, 1 H), 7.70 (d, 1 H, J= 1.70 Hz), 7.35-7.26 (m,
2H), 7.25-7.16 (m, 3H),
4.82 (t, 1 H, J = 5.46 Hz), 3.54 (dd, 2H, J = 5.84, 5.65 Hz), 3.43-3.32 (m,
2H), 2.45-2.35 (m, 1 H),
2.14-2.04 (m, 1 H), 1.57-1.47 (m, 1 H), 1.44-1.33 (m, 1 H).
LCMS: (M+H+) 432.0, (M+Na+) 454Ø
HRMS: (M+H+) calcd for C23H22N504, 432.1672, found 432.1648.
Example 171: (1R.2R)- 6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-amino]-5 6-
dihydro-1H-
j1,21diazepino(4.5.6-cdlindole-2-carboxylic acid (2-dimethylamino-ethyl)-amide
HN-N 0
a O ~ Title Compound 4N HCI (Dioxane) \ \ HN N ~ 0
of Example 165 CH2CI2, 99% CIH.H2N I/ N 0 HATU, Et3N, DMF, 25%
H
171(a)
HN-N ~
0 \ HN~ ~
J]IN N
0 H H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
189
Step 1. Preparation of 8-Amino-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cdjindole-2-
carboxylic acid (2-dimethylamino-ethyl)-amide; dihydrochloride 171(a)
Preparation of intermediate 171(a) from the title compound of Example 165
(55.0 mg, 0.133
mmol) and 4.0 M HCI in dioxane (0.66 mL) was carried out analogously to
Example 91. Isolation,
also in an analogous manner, afforded Intermediate 171(a) (51.3 mg, 0.132
mmol) as a yellow
powder in 99% yield.
LCMS: (M+H+) 315.2.
Step 2. Preparation of Title Compound: (1R,2R)- 6-Oxo-8-[(2-phenyl-
cyclopropanecarbonyl)-amino]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indole-2-
carboxylic
acid (2-dimethylamino-ethyl)-amide
Preparation of the title compound from Intermediate 171(a) (51.3 mg, 0.132
mmol), (IR,2R)-2-
phenyl-cyclopropanecarboxylic acid (24.0 mg, 0.148 mmol), triethylamine (0.074
mL, 0.535
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate (76.0
mg, 0.200 mmol) in N,N-dimethylformamide (2.0 mL) was carried out analogously
to Example 11.
The reaction mixture was reduced in volume and subjected to preparative HPLC
(H1-Q C18
reverse-phase 5uM, 100A, 150x20 column eluting with CH3CN/0.1% acetic acid in
H20 at a
flowrate of 20 mL/min using a gradient of 20-60% CH3CN over 30 min) to afford
the title
compound (15 mg, 0.0328 mmol) as a yellow-green powder in 25% yield.
' H NMR (d6-DMSO): S 10.63 (s, IH), 10.53 (s, 1 H), 8.71 (br s, 1 H), 8.20 (d,
1 H, J= 1.13 Hz), 8.06
(s, 1 H), 7.70 (d, 1 H, J= 0.94 Hz), 7.37-7.26 (m, 2H), 7.26-7.15 (m, 3H),
3.70-3.60 (m, 2H), 3.22-
3.11 (m, 2H), 2.76 (s, 6H), 2.42-2.32 (m, 1 H), 2.16-2.04 (m, 1 H), 1.58-1.46
(m, 1 H), 1.46-1.33 (m,
1 H).
LCMS: (M+H+) 459.1, (M+Na+) 481.1.
HRMS: (M+H+) calcd for C25H27N603, 459.2145, found 459.2151.
Example 172: (1.2-trans)-2-(3-Morpholin-4-ylmethyl-phenyl)-
cyclopropanecarboxylic acid (6-oxo-
5.6-dihydro-1-[1,21diazepino[4.5,6]indol-8-y)-amide
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
190
~
N I ~02Et \ I~ 02Et Me3S0+1", NaH
NDM
SO, 35%
PdOAc)2, Et3N Q
P(Tol)3, DMF, 80 C 80% 172(a)
HN-N
O ~
~ I 1. LiOH, THF, H20
\ C02Et ON \ ~ O ~/ N
/~1` 2. HATU, Et3N, DMF, N H
r`v_ Jl Title Compound of H
172(b) Example 2
5%
Step 1. Preparation of 3-(3-Morpholin-4-ylmethyl-phenyl)-acrylic acid ethyl
ester 172(a)
To a solution of 4-(3-iodo-benzyl)-morpholine (3.44 g, 11.4 mmol) in N,N-
dimethylformamide (20
mL) was added triethylamine (1.7 mL, 12.5 mmol), ethyl acrylate (4.1 mL, 45.4
mmol), tri-o-
tolylphosphine (346 mg, 1.14 mmol) and palladium (II) acetate (127 mg, 0.57
mmol). The mixture
was heated at 80 C overnight. After concentration, the mixture was
chromatographed on silica
gel to afford Intermediate 172(a) as a colorless oil (2.37 g, 9.1 mmol) in 80%
yield.
Step 2. Preparation of (1,2-trans)-2-(3-Morpholin-4-ylmethyl-phenyl)-
cyclopropanecarboxylic acid ethyl ester 172(b)
Preparation of intermediate 172(b) from Intermediate 172(a) (472 mg, 1.81
mmol), NaH (94 mg,
2.35 mmol) and trimethylsulfoxonium iodide (517 mg, 2.35 mmol) was carried out
analogously to
step I of Example 181. After workup, the residue was purified by silica gel
chromatography,
eluting with 40% ethylacetate/hexane, to give Intermediate 172(b) as a
colorless oil (175 mg, 0.64
mmol) in 35 % yield.
Step 3. Preparation of Title compound (1,2-trans)-2-(3-Morpholin-4-ylmethyl-
phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
amide
To a solution of Intermediate 172(b) (175 mg, 0.64 mmol) in tetrahydrofuran (2
mL) was added 1
M aqueous LiOH (3.8 mL, 3.8 mmol). The resulting mixture was stirred overnight
whereupon
mixture was acidified to pH 2 and extracted with ethyl acetate. Concentration
of the organic layer
gave crude 2-(3-morpholin-4-ylmethyl-phenyl)-cyclopropanecarboxylic acid which
was combined
with the title compound from Example 2 (227 mg, 0.756 mmol), triethylamine
(0.32 mL, 2.27
mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium
hexafluorophosphate (345
mg, 0.907 mmol) in N,IV dimethylformamide (5 mL). After was stirring
overnight, the volatile
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
191
components were removed under vacuum, and the residue was purified by silica
gel
chromatography to give the title compound as yellow solid (15 mg, 0.03 mmol)
in 5% yield.
'H NMR (CD3OD): S 8.30 (s, 1H), 7.69 (s, 1H), 7.61 (s, 2H), 7.51-7.28 (m, 4H),
3.84 (s, 4H), 3.66
(s, 2H), 2.61 (s, 4H), 2.45 (m, 1 H), 2.21 (m, 1 H), 1.76 (m, 1 H), 1.53 (m, 1
H).
LCMS: (M+H+) 444.2
Example 173: (1,2-trans)-2-[3-(4-Methyl-piperazin-1-yl)-phenyl]-
cyclopropanecarboxylic acid (6-
oxo-5.6-dihydro-1 H-f 1,2]diazepino[4,5,6-cd]indol-8-yl)-amide
~
N H
,N ~
Intermediate 202(a), N~ CO2Et
Example 202 pdOAc)2, BINAP ,N J
Cs2CO3, toluene, 63% 173(a)
HN-N
O \
1. LiOH, THF, H20 / I O I~
2. HATU, Et3N, DMF, N ~ N ~ N
Title Compound of N~ H H
Example 2 ~
22%
Step 1. Preparation of 2-[3-(4-Methyl-piperazin-l-yl)-phenyl]-
cyclopropanecarboxylic acid
ethyl ester 173(a)
To a solution of Intermediate 202(a) of Example 202 (165 mg, 0.616 mmol) in
toluene (4 mL) was
added 2,2'-bis(diphenylphosphino)-1,1'-binaphthyi (34.5 mg, 0.055 mmol), 4-
methylpiperazine
(0.082 mL, 0.74 mmol), Cs2CO3 (281 mg, 0.862 mmol) and Pd(OAc)2 (8.3 mg, 0.037
mmol). The
mixture was refluxed overnight. The brown mixture was then filtered and the
filter cake was
washed with ethyl acetate. After concentrating the filtrate, the residue was
purified by silica gel
chromatography, eluting with 2-5% MeOH/CH2CI2, to afford Intermediate 173(a)
as a colorless oil
(111 mg, 0.39 mmol) in 63% yield.
Step 2. Preparation of Title compound: (1,2-trans)-2-[3-(4-Methyl-piperazin-1-
yl)-phenyl]-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
amide
In a manner similar to that described for step 3 of Example 172, Intermediate
173(a) was treated
with 1 M aqueous LiOH to give crude 2-[3-(4-methyl-piperazin-1-yl)-phenyl]-
cyclopropanecarboxylic acid. Crude 2-[3-(4-methyl-piperazin-1-yl)-phenyl]-
cyclopropanecarboxylic
acid was coupled to the title compound of Example 2 (71 mg, 0.3 mmol) also in
a manner similar
to that described for step 3 of Example 172. Extractive work-up from ethyl
acetate and saturated
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
192
aqueous NaHCO3 afforded the crude product, which was purified by silica gel
chromatography,
eluting with 3% MeOH\CHZCIz, to furnish the title compound (38 mg, 0.086 mmol)
in 22% yield.
' H NMR (d6-DMSO): S 11.70 (s, 1 H), 10.38 (s, 1 H), 10.23 (s, 1 H), 8.13 (s,
1 H), 7.59 (s, 1 H), 7.56
(s, 1 H), 7.47 (s, 1 H), 7.13 (m, 1 H), 6.79-6.74 (m, 2H), 6.58 (m, 1 H), 3.22-
3.12 (m, 4H), 2.51-2.47
(m, 4H), 2.33 (m, 1 H), 2.27 (s, 3H), 2.08 (m, 1 H), 1.46 (m, 1 H), 1.36 (m, 1
H).
LCMS: (M+H+) 443.2
Example 174: (1 2-trans)-2-(3-Morpholin-4-yi-phenyl)-cyclopropanecarboxylic
acid (6-oxo-5 6-
dihydro-1 H-[1.2]diazepinof4,5,6-cdiindol-8-yl)-amide
~NH
Intermediate 202(a), 0'-) NO CO Et
Example 202 ~
PdOAc)2, BINAP
Cs2CO3, toluene, 34% 174(a)
HN-N
O ~
1. LiOH, THF, H20 / I O I~
\
2. HATU, Et3N, DMF, N ~ N
Title Compound of I H H
Example 2
27%
Step 1. Preparation of 2-[3-Morpholin-4-yl)-phenyl]-cyclopropanecarboxylic
acid ethyl ester
174(a)
Preparation of intermediate 174(a) from Intermediate 202(a) of Example 202
(239 mg, 0.892
mmol), 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (50 mg, 0.081 mmol),
morpholine (0.12 mL,
1.34 mmol), Cs2CO3 (407 mg, 1.25 mmol) and Pd(OAc)2 (12 mg, 0.054 mmol) in
toluene (5 mL)
was carried analogously to Example 173. After concentration, the residue was
purified by silica gel
chromatography, eluting with 1-2 % MeOH/CH2CI2 to afford Intermediate 174(a)
as a colorless oil
(84 mg, 0.31 mmol) in 34% yield:
Step 2. Preparation of Title compound: (1,2-trans)-2-(3-Morpholin-4-yl-phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cdJindol-8-yl)-
amide
Preparation of the title compound was carried out analogously to step 3 of
Example 172 except
that Intermediate 174(a) was used instead of Intermediate 172(b). The title
compound was
obtained in 27% yield.
'H NMR (ds-DMSO): S 10.37 (d, 1 H, J= 2.26 Hz), 10.24 (s, 1 H), 8.24 (d, 1 H,
J= 1.88 Hz), 7.58 (d,
1 H, J = 1.51 Hz), 7.56 (d, 1 H, J= 3.30 Hz), 7.47 (s, 1 H), 7.15 (t, J = 8.0
Hz, 1 H), 6.79-6.77 (m,
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
193
2H), 6.61 (d, 1 H, J= 8.0 Hz), 3.74-3.72 (m, 4H), 2.23 (m, 1 H), 2.07 (m, 1
H), 1.46 (m, 1 H), 1.38
(m, 1 H).
LCMS: (M+H+) 430.2
Example 175: (2R)-2-Amino-2-(4-hydroxyphenLrl)-N-(6-oxo-2-phenyl-5 6-dihydro-1
H-
[1.21diazepinof4,5.6-cdlindol-8-yl)ethanamide hydrochloride
HN-N
O
HO
\ ION I\ / N
NH2 H H-CI H
Preparation of example 175 from the title compound of Example 153 (38 mg,
0.072 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, included freebasing with triethylamine and subsequent silica
gel
chromatography eluting with 3:1:1 hexane:ethyl acetate:ethanol. With ice bath
cooling, the purified
freebase in CH2CI2 (10 mL) was treated with 4M HCI in dioxane (0.1 mL). After
removal of the
volatile components, the title compound (20 mg, 0.043 mmol) was obtained as an
orange/yellow
powder in 60% yield.
'H NMR (d6-DMSO): S 12.23 (s, 1 H), 10.82 (s, IH), 10.45 (s, 1 H), 8.71 (br s,
3H), 8.10 (s, IH),
7.75-7.65 (m, 3H), 7.63-7.49 (m, 4H), 7.43 (d, 2H, J = 8.47 Hz), 6,86 (d, 2H,
J = 8.47 Hz), 5.02
(m, 1 H).
LCMS: (M+H+) 426.2.
Example 176: (2R)-2-Amino-3-naphthalen-2-yl-N-(6-oxo-2-phenyl-5 6-dihydro-1H-
[1,2]diazepinof4.5.6-cd]indol-8-yl)propanamide hydrochloride
HN-N
O - \ N ~ ~
WN
\ )/ NH~ H H-CI H
Preparation of example 176 from the title compound of Example 154 (59 mg,
0.103 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner afforded the title compound (52 mg, 0.102 mmol) as an
orange/yellow powder
in 99% yield.
'H NMR (d6-DMSO): S 1224 (s, 1 H), 10.82 (s, 1 H), 10.44 (s, 1 H), 8.43 (br s,
3H), 8.07 (s, 1 H),
7.93-7.81 (m, 5H), 7.71-7.45 (m, 9H), 4.32 (br s, 1 H), 3.44 (m, 1 H,
partially obscured), 3.28 (m,
1 H).
LCMS: (M+H+) 474.2, (M+Na+) 496.3.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
194
Example 177: (2R)-2-Amino-N-(6-oxo-5,6-dihydro-1 H-[1 2]diazepino[4 5 6-
cd]indol-8-yl)-3-
f(phenylmeth r~l oxy]propanamide hydrochloride
HN-N
O ~
O I \ ~
O~H / H
~ NH2 H-Cl
Preparation of example 177 from the title compound of Example 159 (35 mg,
0.072 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner afforded the title compound (30 mg, 0.072 mmol) as an
orange/yellow powder
in 100% yield.
' H NMR (d6-DMSO): S 11.89 (s, 1 H), 10.75 (s, 1 H), 10.32 (s, 1 H), 8.45 (br
s, 3H), 8.06 (s, 1 H),
7.63 (s, 2H), 7.50 (s, 1 H), 7.38-7.26 (m, 5H), 4.59 (dd, 2H, J= 12.25, 17.33
Hz), 4.24 (br s, 1 H),
3.89 (d, 2H, J = 4.15 Hz, partially obscured).
LCMS: (M+H+) 378.2, (M+Na+) 400.1.
Example 178: (2R)-2-Amino-3-(4-hydroxyphenyl)-N-(6-oxo-5 6-dihydro-1H-[1
2]diazepino[4 5 6-
cd]indol-8-yl)propanamide hydrochloride
HN-N
WN
0 \ N HO
I/ IVHZ H H-CI H
Preparation of example 178 from the title compound of Example 155 (90 mg,
0.194 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner afforded the title compound (56 mg, 0.140 mmol) as an
orange/yellow powder
in 72% yield.
'H NMR (d6-DMSO): S 11.89 (s, 1 H), 10.70 (s, 1 H), 10.30 (s, 1 H), 9.38 (br
s, 1 H), 8.34 (br s, 3H),
8.03 (s, 1 H), 7.62 (s, 1 H), 7.58 (s, 1 H), 7.49 (s, 1 H), 7.08 (d, 2H, J =
8.29 Hz), 6.70 (d, 2H, J
8.29 Hz), 4.11 (br s, 1 H), 2.93-3.15 (m, 2H).
LCMS:(M+Na+) 386.5, (M-H)" 362.4.
Example 179: (2R)-2-Amino-3-naphthalen-2-yl-N-(6-oxo-5 6-dihydro-1H41
2]diazepinoM5 6-
cd]indol-8-yl)propanamide hydrochloride
HN-N
0
O
I \
N / N
\ I/ NHg H H-Cl H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
195
Preparation of example 179 from the title compound of Example 158 (57 mg,
0.115 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner afforded the title compound (48 mg, 0.111 mmol) as an
orange/yellow powder
in 97% yield.
'H NMR (d6-DMSO): S 11.88 (s, 1 H), 10.82 (s, 1 H), 10.30 (s, 1 H), 8.43 (br
s, 3H), 8.04 (s, IH),
7.93-7.78 (m, 4H), 7.64-7.57 (m, 2H), 7.54-7.45 (m, 4H), 4.32 (br s, 1 H),
3.22-3.46 (m, 2H,
partially obscured).
LCMS: (M+H+) 398.5, (M+Na+) 420.4.
Example 180: 2-(1,1'-Biphenyl-4-yl)=N-(6-oxo-5 6-dihydro-1 H-[1 21diazepinof4
5 6-cd]indol-8-
yl)acetamide
3ION H H
Preparation of example 180 from the title compound of Example 2 (75 mg, 0.38
mmol), 1,1'-
biphenyl-4-ylacetic acid (93 mg, 0.44 mmol), triethylamine (0.16 mL, 1.15
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (167 mg,
0.44mmol) in
CH2CI2 (1.0 mL) and N,N-dimethylformamide (1.0 mL) was carried out analogously
to Example
11. Silica gel chromatography was performed twice eluting both times with 1:1
hexane:acetone
and the purest fractions were combined, evaporated, and the resulting solids
triturated with
methanol to afford the title compound (5 mg, 0.012mmol) as a yellow powder in
3% yield.
'H NMR (d6-DMSO): S 11.74 (s, 1 H), 10.35 (s, 1 H), 10.25 (s, 1 H), 8.14 (s, 1
H), 7.69-7.54 (m, 6H),
7.51-7.33 (m, 6H), 3.69 (s, 2H).
LCMS: (M+H+) 395.4, (M+Na+) 417.4.
Example 181: (1 2-trans)-2-(4-Hydroxy-phenyI)-cyclopropanecarboxylic acid (6-
oxo-5,6-dihydro-
I H-[1,21diazepino[4,5,6-cd]indol-8-yl)-amide
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
196
Me0 /02Et NaH, (CH3)3SO+I Me0 COZEt BBr3, CH2CI2, 25%
- DMSO, 72%
181(a)
CO2Et COzH
HO ~~ 1M LiOH, THF, H20
HO ~~
97%
181(b) 181(c)
H
0 N-N
Title Compound HO
of Example 2 O
N N
HATU, Et3N, DMF, 16% H H
Step 1. Preparation of (1,2-trans)-2-(4-Methoxy-phenyl)-cyclopropanecarboxylic
acid ethyl
ester 181(a)
To a mixture of NaH (200 mg, 5.0 mmol) and trimethylsulfoxonium iodide (1.1 g,
5.0 mmol) was
added DMSO (10 mL). After stirring for 30 min, a solution of 3-(4-methoxy-
phenyl)-acrylic acid
ethyl ester (400mg, 1.92 mmol) in DMSO (5 mL) was added drop-wise. After
stirring overnight, the
mixture was partitioned between ethyl acetate and water. The aqueous layer was
re-extracted
with ethyl acetate, and the combined organic layers were dried over Na2SO4 and
concentrated.
Purification by silica gel chromatography eluting with 10 % ethyl acetate in
hexane gave
Intermediate 181(a) (318 mg, 1.44 mmol) in 72% yield.
Step 2. Preparation of (1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic
acid ethyl
ester 181(b)
To a stirred solution of Intermediate 181(a) (318 mg, 1.45 mmol) in CH2CI2 (8
mL) at -78 C was
added 1 M BBr3 in CH2CI2 (1.7 mL). The resulting mixture was then warmed to 23
C and stirred
for 30 min. The mixture was quenched with saturated aqueous NaHCO3 and
extracted with
CH2CI2. The organic layer was dried over anhydrous Na2SO4, filtered,
concentrated, and
subjected to silica gel chromatography eluting with 15% ethyl acetate in
hexane to afford
Intermediate 181(b) as a colorless oil (75 mg, 0.364 mmol) in 25% yield.
Step 3. Preparation of (1,2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic
acid 181(c)
To a stirred solution of Intermediate 181(b) (75mg, 0.36 mmol) in
tetrahydrofuran (2.5 mL) was
added aqueous 1 M LiOH (2.5 mL). The resulting mixture was stirred at 23 C
for 12 hours. The
mixture was then acidified with 1 M HCI to pH 1 and extracted with ethyl
acetate. The organic layer
was then washed with brine and concentrated to give Intermediate 181(c) (63
mg, 0.35 mmol) in
97% yield which was carried on without further purification.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
197
Step 4. Preparation of Title Compound: (1,2-trans)-2-(4-Hydroxy-phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
amide
Preparation of the title compound from the title compound of Example 2 (66 mg,
0.278 mmol),
Intermediate 181(c) (62 mg, 0.348 mmol), triethylamine (0.073 mL, 0.52 mmol),
and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (159 mg,
0.42 mmol) in
N,N-dimethylformamide (2 mL) was carried out analogously to Example 11. Silica
gel
chromatography (eluted with 2% methanol in CH2CI2), also in an analogous
manner, afforded the
title compound (20 mg, 0.056 mmol) as a yellow powder in 16% yield.
'H NMR (d6-DMSO): 12.09 (s, 1 H), 10.38 (s, 1 H), 10.37 (s, 1 H), 9.26 (br s,
1 H), 8.16 (s, 1 H),
7.65-7.67 (m, 2H), 7.58 (t, J= 8.0 Hz, 1 H), 7.50 (s, 1 H), 7.00 (d, J= 8.0
Hz, 2H), 6.70 (d, J= 8.0
Hz, 2H), 2.33 (m, 1 H), 1.98 (m, 1 H), 1.43 (m, 1 H), 1.24 (m, 1 H).
LCMS: (M+H+) 361.3.
Example 182: (1.2-trans)-2-(4-Hydroxy-phenyl)-cyclopropanecarboxylic acid (6-
oxo-2-phenyl-5 6-
dihydro-1-f 1.2]diazepino[4,5,6]indol-8-yl)-amide
HN-N
O ~
\ -
HO / ON( / N ~ ~
\ I
H H
Preparation of example 182 from the title compound of Example 7 (35 mg, 0.127
mmol),
Intermediate 181(c) of Example 181 (66 mg, 0.372 mmol), triethylamine (0.062
mL, 0.45 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(156 mg, 0.41
mmol) in NN-dimethylformamide (3 mL) was carried out analogously to Example
11. Silica gel
chromatography (eluted with 2% methanol in CH2CIz), also in an analogous
manner, gave the title
compound (32 mg, 0.073 mmol) as a yellow powder in 20% yield.
' H NMR (d4- methanol): 8.04 (s, 1 H), 7.44 (s, 1 H), 7.35 (s, 1 H), 7.34 (s,
1 H), 6.91 (d, J = 9.0 Hz,
2H), 6.62 (d, J= 9.0 Hz, 2H), 2.31 (m, 1 H), 1.87 (m, 1 H), 1.43 (m, 1 H),
1.24 (m, 1 H).
LCMS: (M+H+) 437.4.
Example 183: (2-Ethyl-6-oxo-5,6-dihydro-lH-[1.2]diazegino[4,5.6-cdlindol-8-yl)-
carbamic acid tert-
bu I ester
HN-N
O ~
Title Compound Pd/C, H2, DMF, MeOH \
of Example 164 ~ ~
69% BocHN ~ N
H
Palladium (10% on activated carbon) (0.23 g, 0.198 mmol) was added to a
solution of the title
compound of Example 164 (0.65 g, 1.99 mmol) in 1:10 N,N-dimethylformamide:
methanol (11
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
198
mL). The reaction mixture was purged with H2 and stirred at room temperature
under H2 (1 atm.)
for 5 hours. The palladium was filtered and the volatile components were
removed in vacuo. The
resulting residue was dissolved in methanol and loaded onto a silica gel plug.
The plug was then
loaded onto a silica gel column and eluted with 1.2:1 dichloromethane: ethyl
acetate to afford the
title compound (0.45 g, 1.37 mmol) as a yellow solid in 69% yield.
'H NMR (d6-DMSO): 8 11.54 (s, 1 H), 10.05 (s, 1 H), 9.37 (s, 1 H), 7.63 (s, 1
H), 7.58 (s, 1 H), 7.44
(s, 1 H), 2.80 (dd, 2H, J= 7.35, 7.54 Hz), 1.48 (s, 9H), 1.23 (t, 3H, J= 7.54
Hz).
LCMS: (M+H+) 329.5, (M+Na+) 351.5; (M-H)" 327.4.
Example 184: 4-(8-tert-Butoxycarbonylamino-6-oxo-5,6-dihydro-1 H-
[1,2]diazepinof4,5,6-cdlindol-
2-yl)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester
/~ O HN-N
BocN ,~B(
O ~
Intermediate 147(d) V ~ &__OS1BOC
~ Example 147 BocHN N
Pd(dppf)CIa, 2 M Na2C03 H
DMF, 80 OC, 94%
Under an argon atmosphere, 2.0 M aqueous Na2CO3 (0.66 mL) was added to a
mixture of
Intermediate 147(d) of Example 147 (100 mg, 0.265 mmol), 4-(4,4,5,5-
tetramethyl-
[1,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-l-carboxylic acid tert-butyl
ester (122 mg, 0.395
mmol) and [1,1'- bis(diphenylphosphino)ferrocene]dichloropalladium (II) (10.8
mg, 0.013 mmol) in
anhydrous N,N-dimethylformamide (5 ml). The mixture was heated at 80 C for 16
hours. The
reaction was filtered through a thin pack of diatomaceous earth, and the
volatile components were
removed in vacuo. Silica gel chromatography (eluted with 1:1 ethyl acetate:
hexane) afforded the
title compound (120 mg, 0.249 mmol) as a yellow powder in 94% yield.
'H NMR (d6-DMSO): S 11.62 (s, 1 H), 10.22 (s, 1 H), 9.46 (s, 1 H), 7.69 (s, 1
H), 7.65 (d, 1 H, J
1.70 Hz), 7.49 (s, 1 H), 6.16 (s, 1 H), 4.06 (s, 2H), 3.56 (t, 2H, J = 5.65,
4.90 Hz), 3.31 (m, 2H,
partially obscured), 1.49 (s, 9H), 1.44 (s, 9H).
LCMS: (M+H+) 482.5, (M+Na+) 504.5; (M-H)- 480.5.
HRMS: (M+H+) calcd for C25H32N505i 482.2403, found 482.2417.
The 4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-1-
carboxylic acid tert
butyl ester starting material was prepared according to Eastwood, P.R. (2000)
Tetrahedron
Letters 41(19):3705-3708 from trifluoromethanesulfonic acid 1-tert-
butoxycarbonyl-1,2,3,6-
tetrahydropyridin-4-yl ester which in turn was prepared according to the
procedure outlined by
Barrow, J. C. et. al.(2000) J. Med. Chem. 43(14) 2703-2718.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
199
Example 185: 8-Amino-2-(1,2,3,6-tetrahydro-pyridin-4-Ll)-1,5-dihydro-
[1,2]diazepinof4.5,6-
cdlindol-6-one
HN-N
O \
\ ~
~ / N ~ NH H-Cl
H-Cl HZN H
Preparation of example 185 from the title compound of Example 184 (20 mg,
0.042 mmol) and
4.0 M HCI in dioxane (0.1 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, afforded the title compound (14.7 mg, 0.042 mmol) as a
yellow powder in
99% yield.
'H NMR (d6-DMSO): S 12.20 (br s, 1 H), 10.46 (s, 1 H), 9.14 (s, 3H), 7.57 (s,
1 H), 7.36 (s, 2H), 6.22
(s, 1 H), 3.83 (s, 2H), 3.36 (s, 2H), 2.74 (s, 2H).
LCMS: (M+Na+) 304.3; (M-H)" 280.2.
HRMS: (M+H+) calcd for C15H16N50, 282.1355, found 282.1349.
Example 186: (1R,2R)- 2-Phenyl-cyclopropanecarboxylic acid (2-ethyi-6-oxo-5,6-
dihydro-1H-
f 1,21diazepino[4,5,6-cd]indol-8-yl)-amide
/
HN-N 0
O \
OH
4N HCI (Dioxane)
Title Compound I \ ~
of Example 183 CH2CI2, 99% CIH.H2N / N HATU, Et3N, DMF, 59%
H
186(a)
HN-N
O
O \
,,`N I / N
H H
Step 1. Preparation of 8-Amino-2-ethyl-1,5-dihydro-[1,2]diazepino[4,5,6-
cd]indol-6-one
(hydrochloric salt) 186(a)
Preparation of intermediate 186(a) from the title compound of Example 183 (120
mg, 0.366 mmol)
and 4.0 M HCI in dioxane (0.92 mL) was carried out analogously to Example 91.
Isolation, also in
an analogous manner, afforded Intermediate 186(a) (95.5 mg, 0.366 mmol) as a
yellow powder in
99% yield.
LCMS: (M+H+) 229.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
200
Step 2. Preparation of Title Compound: (1 R,2R)- 2-Phenyl-
cyclopropanecarboxylic acid (2-
ethyl-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-amide
Preparation of the title compound from Intermediate 186(a) (95.5 mg, 0.366
mmol), (1 R,2R)-2-
phenyl-cyclopropanecarboxylic acid (65.0 mg, 0.401 mmol), triethylamine (0.510
mL, 3.66 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;Mtetramethyluronium hexafluorophosphate
(210 mg,
0.552 mmol) in N,N-dimethylformamide (4.0 mL) was carried out analogously to
Example 11. The
volatile components were removed in vacuo and the resulting residue was
dissolved in methanol
and loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted
with 1.2:1 dichloromethane: ethyl acetate to afford the title compound (80 mg,
0.215 mmol) as a
yellow solid in 59% yield.
'H NMR (d6-DMSO): S 11.63 (s, 1 H), 10.31 (s, 1 H), 10.09 (s, 1 H), 8.00 (d, 1
H, J= 1.51 Hz), 7.52
(d, 1 H, J= 1.51 Hz), 7.45 (s, 1 H), 7.34-7.24 (m, 2H), 7.24-7.13 (m, 3H),
2.81 (q, 2H, J= 7.54 Hz),
2.41-2.31 (m, 1 H), 2.11-2.00 (m, 1 H), 1.53-1.43 (m, 1 H), 1.40-1.29 (m, 1
H), 1.23 (t, 3H, J= 7.54
Hz).
LCMS: (M+H+) 373.1.
HRMS: (M+H+) calcd for C22H2jN402, 371.1665, found 373.1672.
Example 187: (2-Chloro-6-oxo-5.6-dihydro-1H-[1 2]diazepino[4 5 6-cdlindol-8-
yi)-carbamic acid
tert-butyl ester
HN-N
Intermediate 147(c), NCS, DMF
Example 147 00. CI
50 C, 90% BocHNO ~ N
H
N-Chlorosuccinimide (0.47 g, 3.52 mmol) was added to a solution of
Intermediate 147(c) of
Example 147 (1.00 g, 3.33 mmol) in anhydrous N,N-dimethylformamide (10 ml) and
anhydrous
chloroform (6.5 mL). The reaction was heated at 50 C for 3 hours at which
point the volatile
components were removed in vacuo. The resulting residue was dissolved in
methanol and loaded
onto a silica gel plug. The plug was then loaded onto a silica gel column and
eluted with 23:2:50
dichloromethane: methanol: ethyl acetate to afford the title compound (1 g,
2.99 mmol) as a
yellow solid in 90% yield.
'H NMR (d6-DMSO): S 12.52 (s, 1 H), 10.41 (s, 1 H), 9.51 (s, 1 H), 7.70 (s, 1
H), 7.66 (s, 1 H), 7.32
(s, 1 H), 1.47 (s, 9H).
LCMS: (M+H+) 335.1, (M+Na+) 357.0; (M-H)- 333Ø
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
201
Example 188: (1,2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid (2-ethyl-6-
oxo-5 6-dihydro-
1 H-[1,21diazepino[4, 5,6-cdJindol-8-yl)-amide
HN-N
N
i I O I \ ~
\ N / N
H H
Preparation of example 188 from Intermediate 186(a) of Example 186 (90 mg,
0.341 mmol),
crude (1,2-trans)-2-pyridin-3-yl-cyclopropanecarboxylic acid (see Example 125
for preparation-
estimated purity c.a. 75%) (79.5 mg, c.a.,0.341 mmol), triethylamine (0.237
mL, 1.73 mmol), and
O-(7-azabenzotriazol-l-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (194
mg, 0.510
mmol) in N,N-dimethylformamide (4.0 mL) was carried out analogously to Example
11. The
volatile components were removed in vacuo, and the resulting residue was
dissolved in methanol
and loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted
with 50:3 dichloromethane: methanol to afford the title compound (75 mg, 0.201
mmol) as a
yellow solid in 59% yield.
1 H NMR (d6-DMSO): S 11.65 (s, 1 H), 10.36 (s, 1 H), 10.11 (s, 1 H), 8.51 (d,
1 H, J= 1.88 Hz), 8.42
(dd, 1 H, J= 1.32, 1.32 Hz), 8.01 (d, 1 H, J= 1.70 Hz), 7.57 (dt, 1 H, J=
7.91, 1.88 Hz), 7.53 (d, 1 H,
J = 1.70 Hz), 7.47 (s, 1 H), 7.33 (dd, 1 H, J = 4.90, 4.71 Hz), 2.82 (q, 2H, J
= 7.54 Hz), 2.47-2.38
(m, 1 H), 2.17-2.08 (m, 1 H), 1.58-1.49 (m, 1 H), 1.49-1.39 (m, 1 H), 1.24 (t,
3H, J= 7.54 Hz).
LCMS: (M+H+) 374.2, (M+Na+) 396.0; (M-H)" 372Ø
HRMS: (M+H+) calcd for C21H2oN502, 374.1617, found 374.1620.
Example 189: (R)- 2-Amino-2-cyclohexyl-N-(2-ethyl-6-oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cdJindol-8-yl)-acetamide (hydrochloride salt)
01jOH O HN-N
4N HCI (Dioxane)
Intermediate 186(a) NHBoc \ \,
Example 186 HATU Et3N, DMF, 91% / 64%
N CH2CI2, 64/o
NHBoC' H
189(a)
HN-N
O
N
~ I \ \
H H
H-CI NH2
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
202
Step 1. Preparation of (R)-[Cyclohexyl-(2-ethyl-6-oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cd]indol-8-ylcarbamoyl)-methyl]-carbamic acid tert-butyl ester 189(a)
Preparation of intermediate 189(a) from Intermediate 186(a) of Example 186 (90
mg, 0.341
mmol), (R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (88 mg, 0.342
mmol), triethylamine
(0.237 mL, 1.73 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M
tetramethyluronium
hexafluorophosphate (194 mg, 0.510 mmol) in N,N-dimethylformamide (4.0 mL) was
carried out
analogously to Example 11. The volatile components were removed in vacuo, and
the resulting
residue vuas dissolved in methanol and loaded onto a silica gel plug. The plug
was then loaded
onto a silica gel column and eluted with 1.2:1 dichloromethane: ethyl acetate
to afford
Intermediate 189(a) (146 mg, 0.313 mmol) as a yellow solid in 91 % yield.
' H NMR (d6-DMSO): S 11.64 (s, 1 H), 10.12 (s, 1 H), 10.02 (s, 1 H), 7.99 (s,
1 H), 7.54 (s, 1 H), 7.47
(s, 1 H), 6.87 (d, 1 H, J= 7.72 Hz), 3.92 (t, 1 H, J= 8.48 Hz), 2.82 (q, 2H,
J= 7.54 Hz), 1.78-1.46
(m, 6H), 1.38 (s, 9H), 1.24 (t, 3H, J= 7.54'Hz), 1.19-0.94 (m, 5H).
LCMS: (M+H+) 468.2, (M+Na+) 490.2.
Step 2. Preparation of Title Compound: (R)- 2-Amino-2-cyclohexyl-N-(2-ethyl-6-
oxo-5,6-
dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-8-yl)-acetamide (hydrochloric salt)
Preparation of the title compound from Intermediate 189(a) (136 mg, 0.291
mmol) and 4.0 M HCI
in dioxane (1.5 mL) was carried out analogously to Example 91. Isolation, also
in an analogous
manner, afforded the title compound (75 mg, 0.186 mmol) as a yellow powder in
64% yield.
'H NMR (ds-DMSO): S 11.81 (s, 1 H), 10.66 (s, 1 H), 10.19 (s, 1 H), 8.42-8.20
(m, 3H), 7.98 (s, 1 H),
7.59 (s, 1 H), 7.50 (s, 1 H), 3.82-3.66 (m, 1 H), 2.84 (q, 2H, J = 7.54 Hz),
1.94-1.53 (m, 6H), 1.30-
0.98 (m, 8H).
LCMS: (M+H+) 368.1, (M+Na+) 390.2; (M-H)- 366.1.
HRMS: (M+H+) calcd for C20H26N502, 368.2087, found 368.2084.
Example 190: (1 R 2R)- 2-Phenyl-cyclopropanecarboxylic acid (2-chloro-6-oxo-
5.6-dihydro-1 H-
f 1 2]diazepino[4,5,6-cc!]indol-8-yl)-amide
o
HN-N
\
4N HCI (Dioxane) 0
Title Compound - ~ ~ ~ ci of Example 187 CH2CI2, 98% ~ N EDCI, Et3N, 4-DMAP
CIH.HzN H
DMF, 45%
190(a)
HN-N
O
O ~
CI
..~~~N I ~ N
H H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
203
Step 1. Preparation of 8-Amino-2-chloro-1,5-dihydro-[1,2]diazepino[4,5,6-
cd]indol-6-one
(hydrochloric salt) 190(a)
Preparation of intermediate 190(a) from the title compound of Example 187
(0.83 g, 2.48 mmol)
and 4.0 M HCI in dioxane (6.2 mL) was carried out analogously to Example 91.
Isolation, also in
an analogous manner, afforded Intermediate 190(a) (0.66 g, 2.44 mmol) as a
yellow powder in
98% yield.
'H NMR (d6-DMSO): 512.87 (s, 1 H), 10.57 (s, 1 H), 7.38 (s, 2H), 7.27 (s, 1
H).
LCMS: (M-H)" 233.1.
Step 2. Preparation of Title Compound: (IR,2R)- 2-Phenyl-
cyclopropanecarboxylic acid (2-
chloro-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cdjindol-8-yl)-amide
Intermediate 190(a) (120 mg, 0.443 mmol), (IR,2R)-2-phenyl-
cyclopropanecarboxylic acid (86.0
mg, 0.531 mmol), (3-dimethylamino-propyl)-ethyl-carbodiimide hydrochloride
(102 mg, 0.534
mmol), and 4-dimethylaminopyridine (65 mg, 0.533 mmol) were stirred in N,N-
dimethylformamide
(7.0 mL) at room temperature for 16 h at which point the volatile components
were removed in
vacuo. The resulting residue was dissolved in methanol and loaded onto a
silica gel plug. The
plug was then loaded onto a silica gel column and eluted with 1.2:1
dichloromethane: ethyl
acetate to afford the title compound (75 mg, 2.99 mmol) as a yellow solid in
45% yield.
' H NMR (ds-DMSO): S 12.65 (s, 1 H), 10.46 (s, 2H), 8.09 (s, 1 H), 7.63 (s, 1
H), 7.36 (s, 1 H), 7.34-
7.25 (m, 2H), 7.25-7.12 (m, 3H), 2.44-2.30 (m, 1 H), 2.13-2.01 (m, 1 H), 1.57-
1.44 (m, 1 H), 1.44-
1.31 (m, 1 H).
LCMS: (M-H)" 377.1.
HRMS: (M+H+) calcd for C20H16N402C1, 379.0962, found 379.0941.
Example 191: (1.2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid (2-chloro-
6-oxo-5.6-dihydro-
1 H-[1,21diazepino(4.5.6-cd]indol-8-yl)-amide
HN-N N
0 \ CI
U-~NaN
H H
Preparation of example 191 from Intermediate 190(a) of Example 190 (120 mg,
0.443 mmol),
crude 2-pyridin-3-yl-cyclopropanecarboxylic acid (estimated purity c.a. 75%)
(124 mg, c.a. 0.532
mmol), (3-dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (102 mg,
0.534 mmol), and 4-
dimethylaminopyridine (65 mg, 0.533 mmol) in N,N-dimethylformamide (7.0 mL)
was carried out
analogously to Example 190, Step 2. When the reaction was judged complete, the
volatile
components were removed in vacuo, and the resulting residue was dissolved in
methanol and
loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted with
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
204
50:3 dichloromethane: methanol to afford the title compound (80 mg, 0.210
mmol) as a yellow
solid in 48% yield.
' H NMR (d6-DMSO): S 12.65 (s, 1 H), 10.48 (s, 1 H), 10.46 (s, 1 H), 8.51 (s,
1 H), 8.42 (d, 1 H, J
3.20 Hz), 8.09 (d, 1 H, J= 1.13 Hz), 7.63 (d, 1 H, J= 1.32 Hz), 7.57 (d, 1 H,
J= 7.91 Hz), 7.40-7.28
(m, 2H), 2.47-2.37 (m, 1 H), 2.19-2.06 (m, 1 H), 1.60-1.50 (m, 1 H), 1.50-1.40
(m, 1 H).
,LCMS: (M+H+) 380.0, (M+Na+) 402.1; (M-H)- 378Ø
HRMS: (M+H+) calcd for C19H15N502CI, 380.0914, found 380.0922.
Example 192: N-(6-oxo-5.6-dihydro-lH-[1 2]diazepino[4 5 6-cdJindol-8-yl)-N'-
(phenylmethyl)urea
HN-N
I \ ~
NHz 0
~ O \
Title Compund N~N / N
of Example 2 DSC, EtN(iPr)2, DMF I/ H H H
25%
To the title compound of Example 2(45 mg, 0.19 mmol) and N,M-disuccinimidyl
carbonate (49
mg, 0.19 mmol) was added N,N-dimethylformamide (0.5mL) and triethylamine
(0.084 mL, 0.60
mmol). After 3 to 5 min, benzylamine (0.046 mL, 0.20 mmol) was added, and the
reaction was
stirred for about an hour. Methylene chloride:methanol (4:1) was added and the
resulting solids
were removed by trituration. The triturate was loaded onto a silica gel plug
and evaporated. The
plug was then loaded onto a silica gel column and eluted with hexane:acetone
(1:1) and the
purest fractions were combined. After solvent removal, the title compound (16
mg, 0.048 mmol)
was obtained as brown powder in 25% yield.
'H NMR (ds-DMSO): S 11.61 (s, 1 H, exchanges), 10.19 (s, 1 H, exchanges), 8.75
(s, 1 H,
exchanges), 7.93 (s, 1 H), 7.49 (m, 1 H), 7.40-7.30 (m, 7H), 6.55 (m, 1 H,
exchanges), 4.31 (d, 2H,
J = 5.84 Hz).
LCMS: (M+H+) 334.2, (M+Na+) 356.3
Example 193: (2R)-3-(4-Hydroxyphenyl)-2-(methylamino)-N-(6-oxo-5 6-dihydro-1H-
11,21diazepino[4,5,6-cd]indol-8-yl)propanamide hydrochloride
HN-N
O
N WN
HO NH H H-CI H
Preparation of example 193 from the title compound of Example 156 (121 mg,
0.25 mmol) and
4M HCI in dioxane (10 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, afforded the title compound (74 mg, 0.18 mmol) as an
orange/yellow powder
in 72% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
205
'H NMR (d6-DMSO): major component/conformer: S 11.89 (s, 1 H, exchanges),
10.73 (s, 1 H,
exchanges), 10.31 (s, 1 H, exchanges), 9.36 (br s, 2H, exchanges), 9.11 (br s,
1 H, exchanges),
7.99 (s, 1 H), 7.64 (s, 1 H), 7.56 (s, 1 H), 7.50 (s, 1 H), 7.06 (d, 2H, J =
8.48 Hz), 6.69 (d, 2H, J
8.48 Hz), 4.11 (m, 1 H), 3.18 (m, 1 H), 3.08 (m, 1 H), 2.55 (s, 3H, partially
obscurred).
LCMS: (M+H+) 378.0, (M+Na+) 400.1.
Example 194: (2R)-2-Amino-3-(4-fluorophenyl)-N-(6-oxo-5,6-dihydro-1
H41.21diazepinof4,5,6-
cd]indol-8-yl)propanamide hydrochloride
HN-N
O
WN
\ N F / NHZ H H-CI
Preparation of example 194 from the title compound of Example 157 (48 mg, 0.10
mmol) and 4M
HCI in dioxane (10 mL) was carried out analogously to Example 91. Isolation,
also in an
analogous manner afforded the title compound (34 mg, 0.08 mmol) as an
orange/yellow powder
in 80% yield.
' H NMR (d6-DMSO): S 11.88 (s, 1 H, exchanges), 10.72 (s, 1 H, exchanges),
10.32 (s, 1 H,
exchanges), 8.37 (br s, 4H, exchanges), 8.04 (s, 1 H), 7.64 (s, 1 H), 7.57 (s,
1 H), 7.50 (s, 1 H),
7.38-7.29 (m, 2H), 7.22-7.14 (m, 2H), 4.19 (m, 1 H), 3.26-3.03 (m, 2H).
LCMS: (M+H+) 366.0, (M+Na+) 388.1.
Example 195: N-(6-Oxo-5 6-dihydro-1H-f1,2]diazepino[4,5,6-cdlindol-8-yl)-2-(3H-
[1,2,31triazolo[4,5-b]pyridin-3-yloxx)acetamide
HN-N
WN
O N-O~N N=N H H
In a manner analogous to that of Example 19, to a stirred suspension of 8-
amino-1,5-dihydro-6H-
[1,2]diazepino[4,5,6-cd] indol-6-one hydrochloride in anhydrous N,N-
dimethylformamide (9 mL)
was added bromoacetic acid (168 mg. 1.2 mmol), O-(7-azabenzotriazol-l-yl)-
N,N,N;M
tetramethyluronium hexafluorophosphate (553 mg, 1.4 mmol) and triethylamine.
The reaction was
stirred at room temperature for 16 hours. The volatile components were
evaporated and water
was added to the residue. The precipitated orange solids were collected by
filtration and washed
with water and ethyl acetate. After drying, the title compound (158 mg) was
obtained as an
orange powder in 35% yield.
1 H NMR (DMSO -d6) d: 5.28 (1 H, s), 7.47 (1 H, s), 7.58 (3H, m), 8.09 (1 H,
d, J = 1.77 Hz), 8.63
(1 H, dd, J= 8.59 Hz), 8.83 (1 H, dd, J= 4.55 Hz), 10.27 (1 H, s), 10.52 (1 H,
s), 11.77 (1 H, s).
LCMS: (M+H+) 377.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
206
Example 196: 1 2-trans -2- 3-Metho - hen I-c clo ro anecarbo lic acid 6-oxo-5
6-dih dro-
1 H-f 1,21diazepino[4,5,6-cd]indol-8-yl)-amide
/ I Me3SO+i-, NaH / I
MeO \ ~ OZEt DMSO, 43% Me0 \ CO2Et
196(a)
HN-N
OH, THF, H20
W 1. Li
Me0 N H
O 2. HATU, Et3N, DMF,
H
Title Compound of
Example 2
9%
Step 1: Preparation of (1,2-trans)-2-(3-Methoxy-phenyl)-cyclopropanecarboxylic
acid ethyl
ester 196(a)
Preparation of intermediate 196(a) from NaH (1.04 g, 26 mmol) and
trimethylsulfoxonium iodide
(5.72 g, 26 mmol), 3-(methoxy-phenyl)-acrylic acid ethyl ester (4.12 g, 20
mmol) in DMSO (30 mL)
was carried out analogously to step 2 of Example 113 to afford -ntermediate
196(a) (1.89 g, 8.6
mmol) in 43% yield.
Step 2: Preparation of Title compound: (1,2-trans)-2-(3-Methoxy-phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-l-[1,2]diazepino[4,5,6-]indol-8-
yl)-amide
Preparation of the title compound was carried out analogously to step 3 of
Example 172 except
that Intermediate 196(a) was used instead of Intermediate 172(b). The title
compound was
obtained in 9% yield.
' H NMR (d6-DMSO): S 11.85 (d, IH, J= 2.26 Hz), 10.37 (s, 1 H), 10.23 (s, 1
H), 8.12 (s, 1 H), 7.57
(dd, 1 H, J= 3.20, 1.51 Hz), 7.46 (s, 2H), 7.20 (t, J= 8.0 Hz, 1 H), 6.77-6.74
(m, 3H), 3.75 (s, 3H),
2.36 (m, 1 H), 2.08 (m, 1 H), 1.48 (m, 1 H), 1.38 (m, 1 H).
LCMS: (M+H+) 375.1.
Example 197: 4-Acetylamino-N-(6-oxo-5 6-dihydro-lH-f1 2]diazepinof4 5 6-
cdJindol-B-rI)-
benzamide
HN-N
WN
/~ I \ H H
N /
H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
207
Preparation of example 197 from the title compound of Example 2 (124 mg, 0.525
mmol), 4-
acetylamino-benzoic acid (113 mg, 0.631 mmol), (3-dimethylamino-propyl)-ethyl-
carbodiimide
hydrochloride (120 mg, 0.628 mmol), and 4-dimethylaminopyridine (77 mg, 0.631
mmol) in N,N-
dimethylformamide (7.0 mL) was carried out analogously to Example 190, Step 2.
When the
reaction was judged complete, the volatile components were evaporated and
methanol was
added. The mixture was filtered to collect the solids, which were then washed
with methanol,
dichloromethane and diethyl ether. After drying, the title compound (32 mg,
0.0886 mmol) was
obtained as a yellow powder in 17% yield.
'H NMR (d6-DMSO): 8 11.79 (d, 1 H, J = 2.64 Hz), 10.25 (s, 1 H), 10.23 (s,
2H), 8.27 (d, 1 H, J
1.70 Hz), 7.96 (d, 2H, J = 8.67 Hz), 7.85 (d, 1 H, J = 1.70 Hz), 7.70 (d, 2H,
J = 8.67 Hz), 7.58 (d,
1 H, J = 2.64 Hz), 7.48 (s, I H), 2.09 (s, 3H).
LCMS: (M-H)- 360.2.
HRMS: (M+H+) calcd for C19H16N503, 362.1253, found 362.1280.
Example 198: (R)- 2-Amino-N-(2-chloro-6-oxo-5.6-dihydro-lH-
[1,2]diazepino[4.5,6-cdlindol-8-yl)-
2-cyclohexyl-acetamide (hydrochloric salt)
C\/ ./ 'O O HN-N
H ::;an1Intermediate 190(a), EDCEN, DP DMF, 46% NHBop H
198(a)
HN-N
O ~
~ CI
V~ ~ \
N
H-Cl NH2 H H
Step 1. Preparation of [(2-Chloro-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-
ylcarbamoyl)-cyclohexyl-methyl]-carbamic acid tert-butyl ester 198(a)
Preparation of intermediate 198(a) from Intermediate 190(a) of Example 190
(100 mg, 0.369
mmol), (R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (114 mg, 0.443
mmol), (3-
dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (85 mg, 0.445 mmol),
and 4-
dimethylaminopyridine (54 mg, 0.443 mmol) in N,N-dimethyiformamide (7.0 mL)
was carried out
analogously to Example 190, Step 2. When the reaction was judged complete, the
volatile
components were removed in vacuo, and the resulting residue was dissolved in
methanol and
loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted with
1.2:1 dichloromethane: ethyl acetate to afford Intermediate 198(a) (80 mg,
0.169 mmol) as a
yellow solid in 46% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
208
'H NMR (d6-DMSO): S 12.64 (s, 1 H), 10.47 (s, 1 H), 10.16 (s, 1 H), 8.06 (d, 1
H, J= 1.51 Hz), 7.66
(d, 1 H, J= 1.51 Hz), 7.36 (s, 1 H), 6.91 (d, 1 H, J= 9.04 Hz), 3.92 (dd, 1 H,
J= 8.29, 7.91 Hz), 1.78-
1.46 (m, 6H), 1.38 (s, 9H), 1.21-0.92 (m, 5H).
LCMS: (M-H)" 472.1.
Step 2. Preparation of Title Compound: (R)- 2-Amino-N-(2-chloro-6-oxo-5,6-
dihydro-lH-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-2-cyclohexyl-acetamide (hydrochloric salt)
Preparation of the title compound from Intermediate 198(a) (75 mg, 0.159 mmol)
and 4.0 M HCI in
dioxane (0.8 mL) was carried out analogously to Example 91. Isolation, also in
an analogous
manner, afforded the title compound (65 mg, 0.159 mmol) as a yellow powder in
99% yield.
'H NMR (d6-DMSO): S 12.81 (s, 1 H), 10.77 (s, 1 H), 10.53 (s, 1 H), 8.30 (br
s, 3H), 8.03 (d, 1 H, J=
1.70 Hz), 7.70 (d, 1 H, J= 1.70 Hz), 7.39 (s, 1 H), 3.73 (m, 1 H), 1.93-1.55
(m, 6H), 1.27-1.01 (m,
5H).
LCMS: (M+H+) 374.0; (M-H)" 372.2.
HRMS: (M+H+) calcd for C1$H21N502CI, 374.1384, found 374.1369.
Example 199: 2-(3.4-Dihydro-lH-isoauinolin-2-yI)-N-(6-oxo-5 6-dihydro-lH-[1
2ldiazepino[4 5 6-
cd]indol-8-yl)-acetamide
11~: BrN,,,CO2CaH5 CO,,,,,CO2C2H5 NaOH, MeOH
NH
CspC03, DMF 48 C, 98%
199(a)
HN-N
O ~
Title Compound
of Example 2
O ~
/ N~CO2H HATU, Et3N, DMF, 42% "/~N I/ N
H H
199(b)
Step 1. Preparation of (3,4-Dihydro-lH-isoquinolin-2-yl)-acetic acid ethyl
ester 199(a)
To a solution of 1,2,3,4-tetra-hydroisoquinoline (2.664 g, 20 mmol) and ethyl
bromoacetate (3.647
g, 22 mmol) in N,N-dimethylformamide (23 mL), was added Cs2CO3 (7.168 g, 22
mmol) under N2.
The mixture was stirred at room temperature for 4 hours. The solvent was
removed under
reduced pressure, and the remaining residue was subjected to silica gel
chromatography, eluting
with ethyl acetate/hexanes (33:66) to afford Intermediate 199(a) (3.39 g, 15.5
mmol) as yellow oil
in 77% yield.
'H-NMR (CDCI3): S 7.13-7.09 (m, 3H), 6.99 (t, 1H), 4.22 (q, 2H), 3.84 (s, 2H),
3.44 (s, 2H), 2.94
(s, 4H), 1.29 (t, 3H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
209
LCMS: (M+H+) 220.3.
Step 2. Preparation of (3,4-Dihydro-1H-isoquinolin-2-yl)-acetic acid 199(b)
To a suspension of Intermediate 199(a) (1.643 g, 7.5 mmol) in methanol (20 mL)
was added 2.5
N NaOH (7.8 mL). The reaction solution was heated at 48 C and stirred
overnight. With cooling,
the pH was adjusted to 8 by adding 1 M HCI. The volatile components were
removed under
vacuum, and the resulting mixture was suspended in methanol. After filtration
to remove the
insoluble solids, the filtrate was evaporated to give Intermediate 199(b) (1.4
g, 7.3 mmol) as white
foam in 98% yield.
1H-NMR (d6-DMSO): S 7.13-7.09 (m, 3H), 7.02 (t, IH), 3.76 (s, 2H), 3.27 (s,
2H), 2.88-2.82 (m,
4H).
LCMS: (M+H+) 192.2.
Step 3. Preparation of Title compound: 2-(3,4-Dihydro-lH-isoquinolin-2-yl)-N-
(6-oxo-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-acetamide
Preparation of the title compound from the title compound of Example 2 (185
mg, 0.79 mmol),
Intermediate 199(b) (150 mg, 0.79 mmol), triethylamine (0.274 mL, 1.98 mmol),
O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (330 mg,
0.879 mmol)
and N,N-dimethylformamide (3 mL) was carried out analogously to 11. Following
evaporation of
the volatile components, the resulting residue was triturated with a small
amount of N,N-
dimethylformamide and water to give the title compound (123.5 mg, 0.331 mmol)
as yellow solid
in 42% yield.
'H-NMR (d6-DMSO): S 11.73 (s, 1 H), 10.22 (s, 1 H), 9.97 (s, 1 H), 8.13 (s, 1
H), 7.62(s, 1 H), 7.55 (s,
1 H), 7.45 (s, 1 H), 7.12-7.04 (m, 4H), 3.72 (s, 2H), 2.88-2.80 (m, 6H).
LCMS: (M+H+) 374.4
Example 200: 2-(1.3-Dihydro-isoindol-2-yl)-N-(6-oxo-5.6-dihydro-lH-
[1.2]diazepinof4 5 6-coindol-
8-yl)-acetamide
O
Br\,COzCzHS C[~N CO2Et NaOH, MeOH
H 30- Cs2CO3, DMF 48 C, 54%
200(a)
HN-N
0 Title Compound O ~
\ C02H of Example 2 ~ 0 O I\ ~
HATU, EtgN, DMF, 67% N~ ~ N
N
H H
200(b)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
210
Step 1. Preparation of (1,3-Dihydro-isoindol-2-yl)-acetic acid ethyl ester
200(a)
Into a solution of isoindoline (1.788 g, 15 mmol) and ethyl bromoacetate
(2.756 g, 16.5 mmol) in
N,N-dimethylformamide (20 mL), was added Cs2CO3 (5.376 g, 16.5 mmol) under N2.
The mixture
was stirred at room temperature for 4 hours. The solvent was removed under
reduced pressure
and the resulting mixture was subjected to silica gel chromatography, eluting
with 33:66 ethyl
acetate:hexane to afford Intermediate 200(a) (1.14 g, 5.56 mmol) as a yellow
oil in 37% yield.
'H-NMR (CDCI3): 8 7.20 (s, 4H), 4.22 (q, 6H), 3.66 (s, 2H), 1.29 (t, 3H).
LCMS: (M+H+) 206.3.
Step 2. Preparation of 1,3-Dihydro-isoindol-2-yl)-acetic acid 200(b)
To a suspension of Intermediate 200(a) (1.14 g, 5.56 mmol) in methanol (20 mL)
was added 2.5
N NaOH (5.78 mL). The reaction solution was heated at 48 C and stirred
overnight. With cooling,
the pH was adjusted to 8 by adding IM HCI. The volatile components were
removed under
vacuum and the resulting mixture was suspended in methanol. After filtration
to remove the
insoluble solids, the filtrate was evaporated to give Intermediate 200(b) (531
mg, 3 mmol) as pale
solids in 54% yield.
LCMS: (M+H+) 178.2.
Step 3. Preparation of Title compound: 2-(1,3-Dihydro-isoindol-2-yl)-N-(6-oxo-
5,6-dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-acetamide
Preparation of the title compound from the title compound of Example 2 (118
mg, 0.5 mmol),
Intermediate 200(b) (88.5 mg, 0.5 mmol), triethylamine (0.274 mL, 1.98 mmol),
O-(7-
azabenzotriazol-1-yl)-N,N,N;N'tetramethyluronium hexafluorophosphate (209 mg,
0.55 mmol)
and N,N-dimethylformamide (5 mL) was carried out analogously to Example 11.
Following
evaporation of the volatile components, the resulting residue was triturated
with a small amount of
N,N-dimethylformamide and water to give the title compound (121 mg, 0.337
mmol) as a yellow
solid in 67% yield.
' H-NMR (d6-DMSO): 5 11.73 (s, 1 H), 10.21 (s, 1 H), 10.02 (s, 1 H), 8.13 (s,
1 H), 7.66(s, 1 H), 7.55
(s, 1 H), 7.46 (s, 1 H), 7.22 (d, 4H), 4.07 (s, 4H), 3.56 (s, 2H).
LCMS: (M+H+) 360.4
Example 201: l2-Morpholin-4-yl-6-oxo-5 6-dihydro-lH-[1 21diazepino[4 5 6-
cdlindol-8-yl)-carbamic
acid tert-butyl ester
HN-N HN-N
CI
~
O a Q ~ Et3N, n-BuOH ~Q ~ OH ~ H 120 C, 23% ~Q H H
Triethylamine (0.039 mL, 0.28 mmol), the title compound of Example 187 (46 mg,
0.14 mmol),
morpholine (96 mg, 1.1 mmol) and n-butanol (5 mL) were stirred in a flask with
a condenser at
120 C overnight. The mixture was allowed to stand at room temperature for two
more days. The
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
211
volatile components were removed under vacuum and the resulting mixture was
subjected to
silica gel chromatography, eluting with 95:5 dichloromethane:methanol to
afford the title
compound (12.5 mg, 0.032 mmol) as a yellow solid in 23% yield.
1 H-NMR (d6-DMSO): 3 9.53 (s, 1 H), 8.47 (s, 1 H), 7.80 (s, 1 H), 7.28 (s, 1
H), 6.06 (s, 2H), 3.74 (s,
8H), 1.48 (s, 9H).
LCMS: (M+H+) 386.4.
Example 202: (1,2-trans)-2-(3-Bromo-ghenyl)-cyclopropanecarboxylic acid (6-oxo-
5.6-dihydro-1H-
[1,2]diazepino[4.5,6-cd]indol-8-r~1 -amide
~ ~ Me3SO+l-, NaH ~ ~ /~
Br ~ ~ O2Et DMSO, 43% o Br' ~ W 'CO2Et
202(a)
HN-N
1. LiOH, THF, H20
2. HATU, Et3N, DMF, Br N N
Title Compound of H
Example 2
11%
Step 1. Preparation of (1,2-trans)-2-(3-Bromo-phenyl)-cyclopropanecarboxylic
acid ethyl
ester 202(a)
To a mixture of NaH (186 mg, 4.65 mmol) and trimethylsulfoxonium iodide (1.02
g, 4.65 mmol)
was added DMSO (5 mL). After stirring for 30 min, a solution of trans-3-(bromo-
phenyl)-acrylic
acid ethyl ester (933 mg, 3.57 mmol) in DMSO (2 mL) was added drop-wise. After
stirring
overnight, the mixture was partitioned between ethyl acetate and water. The
aqueous layer was
extracted with ethyl acetate. The combined organic layers were dried over
Na2SO4i filtered, and
concentrated. The residue was subjected to silica gel chromatography, eluting
with 10 % ethyl
acetate/hexane, to furnish Intermediate 202(a) as a colorless oil (408 mg,
1.52 mmol) in 43%
yield.
Step 2. Preparation of Title compound: (1,2-trans)-2-(3-Bromo-phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
amide
To a stirred solution of Intermediate 202(a) (505 mg, 2.25 mmol) in MeOH (10
mL) was added
aqueous 1 0M LiOH (10 mL). The mixture was stirred at 23 C for 12 hours,
acidified with 1 M HCI
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
212
to pH 1, and extracted with ethyl acetate. The combined organic layers was
then washed with
brine and concentrated to give crude (2,3-trans)-3-(3'-bromo-phenyl)-
cycloprapanecarboxylic acid
(632 mg), which was combined with the title compound of Example 2 (111 mg,
0.49 mmol),
triethylamine (0.273 mL, 1.96 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N=
tetramethyluronium hexafluorophosphate (278 mg, 0.73 mmol) in N,N-
dimethyiformamide (4 mL)
in a manner analogous to Example 11. Extractive work-up from ethyl acetate and
saturated
aqueous NaHCO3 follow by silica gel chromatography afforded the title compound
(100 mg, 0.24
mmol) as a yellow solid in 11 % yield.
1 H NMR (d6-DMSO): 11.85 (d, 1 H, J = 2.26 Hz), 10.50 (s, 1 H), 10.35 (s, 1
H), 8.23 (s, 1 H), 7.69 (s,
1 H), 7.67 (d, 1 H, J = 4.0 Hz), 7.58 (s, 1 H), 7.53-7.51 (m, 2H), 7.40-7.33
(m, 2H), 2.53 (m, 1 H),
2.23 (m, 1 H), 1.62 (m, 1 H), 1.54 (m, 1 H).
Example 203: (1,2-trans)-2-(3-Hydroxyphenyl)-cyclopropanecarboxvlic acid (6-
oxo-56-dihydro-
1 H-f 1,2]diazepinol4,5.6-cd]indol-8-Y)-amide
BBr3, CH2CI2
Intermediate 196(a), HO :I CO Et
Example 196 0 0 2
-78 C, 54%
203(a)
HN-N
1. LiOH, THF, H20 \ I O I~ \
Br N
2. HATU, Et3N, DMF, H
Title Compound of H
Example 2
5%
Step 1. Preparation of 2-(3-hydroxy-phenyl)-cyclopropanecarboxylic acid ethyl
ester 203(a)
To a stirred solution of Intermediate 196(a) of Example 196 (1.56 g, 7.12
mmol) in CH2CI2 at -78
C was added 1.OM BBr3 in CHZCI2 (8.56 ml, 8.56 mmol). The mixture was warmed
to room
temperature and stirred for 1 hour. Extractive work-up from ethyl acetate and
saturated aqueous
NaHCO3 followed by silica gel chromatography afforded Intermediate 203(a) as a
pale brown solid
(795 mg, 3.86 mmol) in 54% yield.
Step 2. Preparation of Title compound: (1,2-trans)-2-(3-Hydroxy-phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
coaindol-8-yl)-
amide
To a solution of Intermediate 203(a) (271 mg, 1.32 mmol) in tetrahydrofuran (2
mL) was added
aqueous 1 M LiOH (6 mL, 6 mmol). The mixture was stirred overnight. After
acidifying to pH 2, the
mixture was extracted with ethyl acetate. Concentration of the organic layer
gave crude 3-(3-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
213
hydroxy-phenyl)-cycloprapanecarboxylic acid (235 mg), where a portion (155 mg,
0.57 mmol)
was combined with the title compound of Example 2 (137 mg, 0.58 mmol),
triethylamine (0.24 mL,
1.72 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium
hexafluorophosphate
(331 mg, 0.87 mmol) in N,N-dimethylformamide (3 mL) in a manner analogous to
Example 11.
Extractive work-up from ethyl acetate and saturated aqueous NaHCO3 followed by
silica gel
chromatography afforded the title compound (10 mg, 0.028 mmol) as a tan solid
in 5% yield.
'H NMR (d6-DMSO): S 10.35 (s, 1 H), 10.27 (s, 1 H), 9.35 (s, 1 H), 8.12 (s, 1
H), 7.67 (dd, 1 H, J
3.20, 1.51 Hz), 7.55 (s, 1 H), 7.48(s, 1 H), 7.15 (t, J= 8.0 Hz, 1 H), 6.77-
6.74 (m, 3H), 2.32 (m, 1 H),
2.09 (m, 1 H), 1.45 (m, 1 H), 1.38 (m, 1 H).
LCMS: (M-H+) 359.1
Example 204 2-(3.4-Dihydroisoquinolin-2(1H)_ r~l -N-(6-oxo-2-phenyl-5.6-
dihydro-1H-
f 1.2]diazepino[4.5.6-cdJindol-8- rl acetamide
HN-N
O
O \ ~ -
N N
H H
In a manner analogous to that of Example 11, to a stirred solution of 8-amino-
1,5-dihydro-6H-
[1,2]diazepino[4,5,6-cd] indol-6-one in anhydrous N,N-dimethylformamide (6 mL)
was added 3,4-
dihydroisoquinolin-2(1H)-ylacetic acid (62 mg, 0.33 mmol) followed by
triethylamine (0.14 mL,
0.98 mmol) and O-(7-azabenzotriazol-1-yl)-N,N,N;Mtetramethyluronium
hexafluorophosphate
(372 mg, 0.98 mmol). The reaction was stirred at room temperature for 17
hours. After
concentrating, water was added and the precipitated solid was collected by
filtration. After
preparative HPLC, the title compound (50 mg) was obtained as a yellow powder
in 34% yield.
'H NMR (methanol-d4) d: 3.25 (2H, m), 3.72 (2H, bs), 4.27 (2H, s), 4.58 (2H,
s), 7.22 (1 H, d, J
7.58 Hz), 7.32 (3H, m), 7.52 (4H, m), 7.63 (3H, m), 8.13 (1 H, d, J= 1.52 Hz).
LCMS: (M+H+) 450.1.
Example 205: (1.2-trans)-2-Pyridin-3-yl-cyclogropanecarboxylic acid (2-chloro-
6-oxo-5,6-dihydro-
1 H-[1,2]diazepino(4.5.6-cdlindol-8-~rl)-amide (hydrochloric salt)
HN-N
HCI O
N
\ ~ O ~ ~ CI
N N
H H
The title compound of Example 191 (20 mg, 0.053 mmol) was converted to an HCI
salt in
anhydrous CH2CI2 (2mL) with 4.0 M HCI in dioxane (0.026 mL). The mixture was
stirred at room
temperature for 1 hour. The solid was collected by filtration and washed with
CH2CI2 and diethyl
ether. After drying, the title compound (20.4 mg, 0.049 mmol) was obtained as
a yellow powder in
93% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
214
'H NMR (ds-DMSO): & 12.70 (s, 1 H), 10.59 (s, 1 H), 10.46 (s, 1 H), 8.85 (s, 1
H), 8.72 (d, 1 H, J
5.84 Hz), 8.25 (d, 1 H, J= 8.29 Hz), 8.07 (d, 1 H, J = 1.70 Hz), 7.91 (dd, 1
H, J = 5.27, 5.27 Hz),
7.63 (d, 1 H, J= 1.70 Hz), 7.35 (s, 1 H), 2.75-2.59 (m, 1 H), 2.33-2.21 (m, 1
H), 1.67-1.56 (m, 2H).
Example 206: (1 R.2R - 2-Phenyl-cyclopropanecarboxylic acid (2-bromo-6-oxo-5 6-
dihydro-1 H-
[1.21diazepino[4,5.6-cellindol-8-kl)-amide
O HN-N 0
.o oH
4N HCI (Dioxane) ~ Q
Intermediate 147(d), 31- I \ r
Example 147 CH2CI2, 96% CIH.H2N ~ N EDCI, Et3N, 4-DMAP
H
206(a) DMF, 33%
HN-N
0~N Br
H H
Step 1. Preparation of 8-Amino-2-bromo-1,5-dihydro-[1,2]diazepino[4,5,6-
cd]indol-6-one
(hydrochloric salt) 206(a)
Preparation of intermediate 206(a) from Intermediate 147(d) of Example 147 (2
g, 5.29 mmol) and
4.0 M HCI in dioxane (26.4 mL) was carried out analogously to Example 91.
Isolation, also in an
analogous manner, afforded Intermediate 206(a) (1.59 g, 5.04 mmol) as a yellow
powder in 96%
yield.
'H NMR (d6-DMSO): S 13.13 (s, 1 H), 10.67 (s, 1 H), 7.52 (s, 1 H), 7.51 (s, 1
H), 7.35 (s, 1 H).
LCMS: (M+H+) 279.0, 281.1, (M+Na+) 401.0, 403.0; (M-H)- 277.0, 279Ø
Step 2. Preparation of Title Compound: (1 R,2R)- 2-Phenyl-
cyclopropanecarboxylic acid (2-
bromo-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yi)-amide
Preparation of the title compound from Intermediate 206(a) (120 mg, 0.380
mmol), (1 R,2R)-2-
phenyl-cyclopropanecarboxylic acid (74.0 mg, 0.457 mmol), (3-dimethylamino-
propyl)-ethyl-
carbodiimide hydrochloride (88 mg, 0.461 mmol), and 4-dimethylaminopyridine
(56 mg, 0.459
mmol) in N,N-dimethylformamide (15.0 mL) was carried out analogously to
Example 190, step 2.
When the reaction was judged complete, the volatile components were removed in
vacuo, and the
resulting residue was dissolved in methanol and loaded onto a silica gel plug.
The plug was then
loaded onto a silica gel column and eluted with 1.2:1 dichloromethane: ethyl
acetate to afford the
title compound (52.4 mg, 0.124 mmol) as a yellow solid in 33% yield.
'H NMR (d6-DMSO): S 12.59 (s, 1 H), 10.45 (s, 1 H), 10.44 (s, 1 H), 8.08 (d, 1
H, J= 1.51 Hz), 7.60
(d, 1 H, J= 1.70 Hz), 7.33-7.24 (m, 3H), 7.24-7.13 (m, 3H), 2.42-2.32 (m, 1
H), 2.11-2.00 (m, 1 H),
1.54-1.42 (m, 1 H), 1.42-1.30 (m, 1 H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
215
LCMS: (M+H+) 423.0, 425.0, (M+Na+) 445.0, 447.0; (M-H)" 421.0, 423Ø
HRMS: (M+H+) calcd for C2oH16N4O2Br, 423.0457, found 423.0471.
Example 207: N-Methyl-N-(2-methXlprop-2-enyl)-N'-L-oxo-5.6-dihydro-1 H-
[1.2]diazepino[4,5,6-
cd]indol-8-yl)urea
HN-N
WN
O 5 ~~ H H
Using a similar route as outlined in Example 192, the title compound of
Example 2 (25 mg, 0.11
mmol) and N,N'-disuccinimidyl carbonate (27 mg, 0.11 mmol) were stirred
together in N,N-
dimethylformamide (1 mL) while N,N-diisopropylethylamine (0.024 mL, 0.21 mmol)
was added.
After stirring three minutes N-2-dimethylprop-2-en-l-amine (0.046 mL, 0.21
mmol) was added
giving the crude product. Cation-exchange polystyrene scavenging resin
(Argonaut
TechnologiesTM, MP-TsOH) was added directly to the mixture. After stirring 2
hours the resin was
removed by filtration and washed twice with N,N-dimethylformamide (1 mL). The
combined N,N-
dimethylformamide solutions were then treated with anion-exchange polystyrene
scavenging resin
(Argonaut TechnologiesTM, MP-carbonate) and stirred for 2 hours. Again the
resin was removed
by filtration and washed twice with N,N-dimethylformamide (1 mL). The combined
N,N-
dimethylformamide solutions were then reduced under vacuum and subjected to
preparative
RPHPLC (Peeke Scientific HI-Q, C18 reverse-phase, 5 uM, 100A, 150x20 mm
column) eluting
with 0.1% acetic acid in CH3CN and 0.1% acetic acid in H20 at a flowrate of 20
mUmin using a
gradient of 30-70% 0.1% acetic acid in CH3CN over 30 min. Fractions judged
pure were pooled,
and the volatile components removed in vacuo. After a final trituation with
ethyl acetate, the title
compound (6 mg, 0.019 mmol) was obtained as brown powder in 18% yield.
' H NMR (CDCI3/methanol-d4): S 7.90 (s, 1 H), 7.42 (s, 1 H), 7.30 (s, 1 H),
7.28 (s, 1 H), 4.98 (s, 1 H),
4.90 (s, 1 H), 3.95 (s, 2H), 3.03 (s, 3H), 1.77 (s, 3H).
LCMS: (M+H+) 312.2, (M+Na+) 334.1.
Example 208: N-Methyl-N'-(6-oxo-5.6-dihydro-1H-[1,2]diazepino[4.5,6-cd]indol-8-
yl)-N-
(phenylmethyl)urea
HN-N
O
WN
H H
Preparation of example 208 from the title compound of Example 2 (48 mg, 0.20
mmol), N,M-
disuccinimidyl carbonate (52 mg, 0.20 mmol), triethylamine (0.084 mL, 0.60
mmol) and N-methyl-
1-phenylmethanamine (0.052 mL, 0.40 mmol) in N,N-dimethylformamide (0.5 mL)
was carried out
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
216
analogously to Example 192. Purification, also in an analogous manner,
afforded the title
compound (8 mg, 0.023 mmol) was obtained as brown powder in 12% yield.
1 H NMR (CDCI3/methanol-d4): S 7.88 (s, 1H), 7.46-7.23 (m, 8H), 4.64 (s, 2H,
obscured), 3.03 (s,
3H).
LCMS: (M+H+) 348.4, (M+Na+) 370.4.
Example 209: (1 2-trans)-2-(2'-Methoxy_phenyl)-cyclopropanecarboxylic acid (6-
oxo-5 6-dihydro-
1H-f1,2]diazepinof4.5.6-cd]indol-8- rLl)-amide
?me Me3SO+l", NaH ~ I
~ CO2Et DMSO, 43% \ C02Et
OMe
209(a)
HN-N
1. LiOH, THF, HZO O 2. HATU, Et3N, DMF, Title Compound of OMe H H
WN
Example 2
10%
Step 1: Preparation of (1,2-trans)-2-(2'-Methoxy-phenyl)-
cyclopropanecarboxylic acid ethyl
ester 209(a)
Preparation of intermediate 209(a) from NaH (1.04 g, 26 mmol) and
trimethylsulfoxonium iodide
(5.72 g, 26 mmol), 2-(methoxy-phenyl)-acrylic acid ethyl ester (4.12 g, 20
mmol) in
dimethylsulfoxide (30 mL) was carried out analogously to step 2 of Example
113. Intermediate
209(a) (1.89 g, 8.6 mmol) was obtained in 43% yield.
Step 2: Preparation of Title compound: (1,2-trans)-2-(2'-Methoxy-phenyl)-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
amide
Preparation of the title compound was carried out analogously to step 3 of
Example 172 except
that Intermediate 209(a) was used instead of Intermediate 172(b). The title
compound was
obtained in 10% yield.
'H NMR (d6-DMSO): S 11.74 (d, 1 H, J = 2.26 Hz), 10.35 (s, 1 H), 10.24 (s, 1
H), 8.14 (s, 1 H), 7.57
(dd, 1 H, J=3.20, 1.51 Hz), 7.46 (s, 2H), 7.20 (t, J = 8.0 Hz, 1 H), 6.77-6.74
(m, 3H), 3.80 (s, 3H),
2.36 (m, 1 H), 2.02 (m, 1 H), 1.44 (m, 1 H), 1.34 (m, 1 H).
LCMS: (M-H+) 373.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
217
Example 210: N-cyclohexyl-N-methyl-M-L-oxo-5 6-dihydro-1H-[1 2]diazepino[4 5 6-
cd]indol-8-
I urea
HN-N
O
WN
N N I H H
Preparation of example 210 from the title compound of Example 2 (25 mg, 0.11
mmol), N,N'-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and N-cyclohexyl-N-methylamine (0.028 mL, 0.021 mmol) in N,IV
dimethylformamide (1.0 mL)
was carried out analogously to Example 207. Purification, also in an analogous
manner, afforded
the title compound (7 mg, 0.021 mmol) as yellow powder in 19% yield.
'H NMR (CDCI3/methanol-d4): S 7.90 (s, 1 H), 7.41 (s, 1 H), 7.33 (s, 1 H),
7.27 (s, 1 H), 4.09 (m, 1 H),
2.92 (s, 3H), 1.91-1.80 (m, 2H), 1.79-1.65 (m, 3H), 1.56-1.37 (m, 4H), 1.14
(m, 1 H).
LCMS: (M+H+) 340.2, (M+Na+) 362.1.
Example 211: N-Cyclohexyl-N-ethyl-M-(6-oxo-5.6-dihydro-1 H-
[1.21diazepino[4,5,6-cdjindol-8-
l urea
HN-N
O
O I \
N~N ~ N
J H H
Preparation of example 211 from the title compound of Example 2 (25 mg, 0.11
mmol), N,M-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and N-cyclohexyl-N-methylamine (0.032 mL, 0.021 mmol) in N,N-dimethylformamide
(1.0 mL)
was carried out analogously to Example 207. Purification, also in an analogous
manner, afforded
the title compound (7 mg, 0.020 mmol) as yellow powder in 20% yield.
'H NMR (CDCI3/methanol-d4): 5 7.94 (s, IH), 7.40 (s, 1 H, obscured), 7.30 (s,
IH, partially
obscured), 7.26 (s, 1 H), 4.05 (m, 1 H, partially obscured), 3.36 (m, 2H,
partially obscured), 1.90-
1.74 (m, 4H), 1.71 (m, 1 H), 1.58-1.33 (m, 4H), 1.26 (t, 3H, J= 7.16 Hz), 1.14
(m, IH).
LCMS: (M+H+) 354.2, (M+Na+) 376.1.
Examgle 212: N.N-Diethyl-N'-(6-oxo-5,6-dihydro-1 H-[1.21diazepino[4.5.6-
cdJindol-8-y,urea
HN-N
O ~
O
/ I \ ~
NNN ~ N
J H H
Preparation of example 212 from the title compound of Example 2 (25 mg, 0.11
mmol), N,M-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
218
and N,N-diethylamine (0.022 mL, 0.021 mmol) in N,N-dimethylformamide (1.0 mL)
was carried
out analogously to Example 207. Purification, also in an analogous manner,
afforded the title
compound (5 mg, 0.016 mmol) as yellow powder in 15% yield.
'H NMR (CDCI3/methanol-d4): S 7.91 (s, 1 H), 7.41 (s, 1 H), 7.32 (s, 1 H),
7.27 (s, 1 H), 3,42 (q, 4H,
J = 7.16 Hz), 1.24 (t, 6H, J= 7.16 Hz).
LCMS: (M+H+) 300.2, (M+Na+) 322.1.
Example 213: N-(6-Oxo-S 6-dihydro-1H-f1 21diazepino[4.5,6-cdlindol-8-
yl)piperidine-1-
carboxamide
HN-N
0 \
O I ~ ~
^N ~ N
H H
Preparation of example 213 from the title compound of Example 2 (25 mg, 0.11
mmol), N,M-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and piperidine (0.021 mL, 0.021 mmol) in N,N-dimethylformamide (1.0 mL) was
carried out
analogously to Example 207. Purification, also in an analogous manner,
afforded the title
compound (7 mg, 0.022 mmol) as yellow powder in 20% yield.
'H NMR (CDCI3/methanol-d4): S 7.77 (s, 1 H), 7.65 (s, 1 H), 7.43 (s, 1 H,
partially obscured),, 7.32 (s,
1 H), 3.57-3.47 (m, 4H), 1.76-1.56 (m, 6H).
LCMS: (M+H+) 312.1, (M+Na+) 334.1.
Example 214: N-(6-Oxo-5 6-dihydro-lH-[1 2ldiazepinof4.5,6-cdlindol-8-yl)-4-
(phenylmethyl)piperidine-1-carboxamide
HN-N
0 \
O I ~ ~
0i,O
2
0 Preparation of example 214 from the title compound of Example 2 (25 mg, 0.11
mmol), N,N'-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,IV diisopropylethylamine (0.024
mL, 0.21 mmol)
and 4-(phenylmethyl)piperidine (0.037 mL, 0.021 mmol) in N,N-dimethylformamide
(1.0 mL) was
carried out analogously to Example 207. Purification, also in an analogous
manner, afforded the
title compound (7 mg, 0.017 mmol) as yellow powder in 16% yield.
'H NMR (CDCI3/methanol-d4): S 7.88 (s, 1H), 7.44-7.39 (m, 2H, partially
obscured), 7.34-7.12 (m,
6H), 4.16 (d, 2H, J = 13.75 Hz), 2.91-2.78 (m, 2H), 2.62-2.57 (m, 2H), 1.80-
1.69 (m, 3H), 1.36-
1.14 (m, 2H).
LCMS: (M+H+) 402.2, (M+Na+) 424.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
219
Example 215: N-Ethyl-M-(6-oxo-5,6-dihydro-1 H-[1,21diazepino[4,5,6-cdlindol-8-
yl)-N-
(phenylmethyl)urea
HN-N
O \
o
I ~
H N
H
Preparation of example 215 from the title compound of Example 2 (25 mg, 0.11
mmol), N,M-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and N-(phenylmethyl)ethanamine (0.032 mL, 0.021 mmol) in N,N-dimethylformamide
(1.0 mL)
was carried out analogously to Example 207. Purification, also in an analogous
manner, afforded
the title compound (7 mg, 0.019 mmol) as yellow powder in 18% yield.
'H NMR (CDCI3/methanol-d4): S 7.94 (s, 1H), 7.46-7.21 (m, 8H, partially
obscured), 4.63 (s, 2H),
3.51-3.34 (m, 2H, partially obscured), 1.28-1.19 (m, 3H).
LCMS: (M+H+) 362.1, (M+Na+) 384Ø
Example 216: N-Butyl-N-methyl-M-(6-oxo-5,6-dihydro-1 H-[1,21diazepino[4.5.6-
cd]indol-8-yl)urea
HN-N
O \
O I ~
/~/~NN N
I H H
Preparation of example 216 from the title compound of Example 2 (25 mg, 0.11
mmol), N,M-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and N-ethyl-N-propylamine (0.025 mL, 0.021 mmol) in N,N-dimethylformamide (1.0
mL) was
carried out analogously to Example 207. Purification, also in an analogous
manner, afforded the
title compound (4 mg, 0.013 mmol) as yellow powder in 12% yield.
'H NMR (CDCI3/methanol-d4): S 7.92 (s, 1 H), 7.41 (s, 1 H, partially
obscured), 7.29 (s, 1 H), 7.26 (s,
1 H), 3.04 (s, 3H), 1.66-1.55 (m, 2H), 1.45-1.21 (m, 4H), 0.97 (t, 3H, J =
7.35 Hz).
LCMS: (M+H+) 314.1, (M+Na+) 336.2.
Example 217: N-Methyl-M-(6-oxo-5 6-dihydro-1H-f 1 2]diazepino[4.5,6-cd]indol-8-
yl)-N-propylurea
HN-N
O \
O I ~ ~
\/~NN ~ N
( H H
Preparation of example 217 from the title compound of Example 2 (25 mg, 0.11
mmol), N,M-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and N-ethyl-N-propylamine (0.022 mL, 0.021 mmol) in N,N-dimethylformamide (1.0
mL) was
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
220
carried out analogously to Example 207. Purification, also in an analogous
manner, afforded the
title compound (4 mg, 0.013 mmol) as yellow powder in 12% yield.
1 H NMR (CDCI3/methanol-d4): S 7.87 (s, 1 H), 7.43 (s, 1 H), 7.36 (s, 1 H),
7.29 (s, 1 H), 3.05 (s, 3H),
1.72-1.58 (m, 2H), 1.37-1.21 (m, 2H), 0.96 (t, 3H, J= 7.25 Hz)
LCMS: (M+H+) 300.2, (M+Na+) 322.1.
Example 218: (1,2-trans)-2-[3-(2-Dimethylamino-ethoxy)-phenyl]-
cyclopropanecarboxylic acid (6-
oxo-5,6-dihydro-1 H-[1,21diazepinof4,5,6-cdJindol-8-Ll)-amide
i
Intermediate 203(a), H N ~ ) o
Example 203 ~ ~~O Et
PPh3, DIAD, CH2CI2
60% 218(a)
HN-N
O \
~ ~ ~
1. LiOH, THF, H20 /N~\ ~ ~ I~ N
O N H
2. HATU, Et3N, DMF, H
Title Compound of
Example 2
13%
Step 1. Preparation of (1,2-trans)-2-[3-(2-Dimethylamino-ethoxy)-phenyl]-
cyclopropanecarboxylic acid 218(a)
To a stirred solution of Intermediate 203(a) of Example 203 (125 mg, 0.607
mmol), 2-
dimethylethanol (81 mg, 0.91 mmol) and triphenylphosphine (239 mg, 0.91 mmol)
in CH2CI2 was
added diisopropyl azodicarboxylate (184 mg, 0.91 mmol). The mixture was
stirred at room
temperature for 4 hours, concentrated, and subjected to silica el
chromatography, eluting with 20-
30% ethyl acetate/hexane, to furnish Intermediate 218(a) as a colorless oil
(101 mg, 0.36 mmol)
in 60% yield.
Step 2. Preparation of Title compound: (1,2-trans)-2-[3-(2-Dimethylamino-
ethoxy)-phenyl]-
cyclopropanecarboxylic acid (6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
amide
Preparation of the title compound was carried out analogously to step 3 of
Example 172 except
that Intermediate 218(a) was used instead of Intermediate 172(b). The title
compound was
obtained in a 13% yield.
1H NMR (d6-DMSO): S 11.56 (d, 1 H, J = 2.26 Hz), 10.20 (s, 1 H), 10.06 (s, 1
H), 7.95 (d, 1 H, J
1.88 Hz), 7.38 (dd, 1 H, J= 3.20, 1.51 Hz), 7.29 (s, 1 H), 7.04 (t, 1 H, J=8.0
Hz), 6.62-6.58 (m, 3H),
6.36 (s, 1 H), 3.96-3.94 (m, 2H), 3.12-3.10 (m, 2H), 2.29 (m, 1 H), 1.91 (m, 1
H), 1.31 (m, 1 H), 1.20
(m, 1 H).
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
221
LCMS: (M+H+) 432.2.
Example 219: (R)- 2-Amino-2-cyclohexyl-N-[6-oxo-2-(1,2 3 6-tetrahydro-pyridin-
4-y,-5 6-dihydro-
1 H-f 1,21diazepino[4,5.6-cdlindol-8-yll-acetamide: dihydrochloride
~ HN-N
H
Intermediate 220(a), NHBoc O"JN:6 Example 220 NBoc
EDCI, Et3N, 4-DMAP N
DMF, 88% NHBot' H
219(a)
HN-N
4N HCI
(Dioxane) ~ NH H-CI
CH2CI2, 88% N \
WN
H-CI NH2 H H
Step 1. Preparation of (R)- 4-[8-(2-terLEButoxycarbonylamino-2-cyclohexyl-
acetylamino)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cal]indol-2-yl]-3,6-dihydro-2H-
pyridine-1-carboxylic
acid tert-butyl ester 219(a)
Preparation of intermediate 219(a) from Intermediate 220(a) of Example 220
(100 mg, 0.262
mmol), (R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (74.3 mg, 0.289
mmol), (3-
dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (55 mg, 0.288 mmol),
and 4-
dimethylaminopyridine (35.2 mg, 0.288 mmol) in N,N-dimethylformamide (6.0 mL)
was carried out
analogously to Example 190, step 2. When the reaction was judged complete, the
volatile
components were removed in vacuo, and the resulting residue was dissolved in
methanol and
loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted with
1:1 dichloromethane: ethyl acetate to afford Intermediate 219(a) (143 mg,
0.231 mmol) as a
yellow solid in 88% yield.
' H NMR (d6-DMSO): S 11.70 (s, 1 H), 10.27 (s, 1 H), 10.09 (s, 1 H), 8.06 (d,
1 H, J= 1.51 Hz), 7.59
(s, 1 H), 7.51 (s, 1 H), 0.88 (d, 1 H, J = 8.10 Hz), 6.17 (s, 1 H), 4.06 (br
s, 2H), 3.92 (dd, 1 H, J =
8.48, 7.72 Hz), 3.55 (t, 2H, J= 5.65, 5.27 Hz), 3.32 (m, 2H, obscured), 1.76-
1.46 (m, 6H), 1.43 (s,
9H), 1.37 (s, 9H), 1.19-0.94 (m, 5H).
LCMS: (M-H)- 619.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
222
Step 2. Preparation of Title Compound: (R)- 2-Amino-2-cyclohexyl-N-[6-oxo-2-
(1,2,3,6-
tetrahydro-pyridin-4-yl)-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-
acetamide;
dihydrochloride
Preparation of the title compound from Intermediate 219(a) (143 mg, 0.231
mmol) and 4.0 M HCI
in dioxane (2.3 mL) was carried out analogously to Example 91. Isolation, also
in an analogous
manner, afforded the title compound (100 mg, 0.203 mmol) as a red powder in
88% yield.
' H NMR (d6-DMSO): 5 12.18 (s, 1 H), 10.87 (s, 1 H), 10.39 (s, IH), 9.32 (br
s, 2H), 8.35 (br s, 3H),
8.07 (d, 1 H, J= 1.51 Hz), 7.70 (d, 1 H, J= 1.51 Hz), 7.55 (d, 1 H, J= 1.51
Hz), 6.19 (s, 1 H), 3.87-
3.74 (m, 3H), 3.41-3.26 (m, 2H), 2.81-2.69 (m, 2H), 1.90-1.55 (m, 6H), 1.28-
0.97 (m, 5H).
LCMS: (M+H+) 421.1, (M+Na+) 443.1; (M-H)- 419.1.
HRMS: (M+H+) calcd for C23H29N602, 421.2352, found 421.2338.
Example 220: (1 R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [6-oxo-2-(1,2,3,6-
tetrahydro-pyridin-
4-yI)-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-amide (hydrochloric
salt)
O HN-N
BocN0/B(
b O
I ~ ~
Intermediate 206(a), / OBoc
Example 206 N
Pd(dppf)Cla, 2 M Na2CO3 HZN H
DMF, 80 C, 82% 220(a)
O O HN-N
..,J~
~ H O ~ \ 4N HCI (Dioxane)
NBoc
EDCI, Et3N, 4-DMAP .==`,~N I~ N \ CHzCIg, 94%
DMF, 94% 0 H H
220(b)
HN-N
O \
O ~
',J~N ~ / N ~ NH.HCI
H H
Step 1. Preparation of 4-(8-Amino-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cal]indol-2-yl)-
3,6-dihydro-2H-pyridine-l-carboxylic acid tert-butyl ester 220(a)
In a manner analogous to that of Example 184, 2.0 M aqueous Na2CO3 (0.66 mL)
was added to a
mixture of Intermediate 206(a) of Example 206 (1 g, 3.17 mmol), 4-(4,4,5,5-
tetramethyl-
[1,2]dioxaborolan-2-yl)-3,6-dihydro-2H-pyridine-l-carboxylic acid tert-butyl
ester (1.18 g, 3.82
mmol) and [1,1'- bis(diphenylphosphino)ferrocene]dichloropalladium (II) (0.13
g, 0.159 mmol) in
anhydrous N,N-dimethy[formamide (50 ml), and the reaction was heated at 80 C
for 16 hours.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
223
When the reaction was judged complete, the volatile components were removed in
vacuo. Ethyl
acetate (50 mL), methanol (5 mL) and H20 (500 mL) were added, and the aqueous
layer was
extracted with ethyl acetate (3X20 mL). The combined ethyl acetate extracts
were washed with
H20 (2X50 mL) and brine (50 mL) and allowed to dry over Na2SO4. Following
filtration, the volatile
components were removed in vacuo and CH2CIZ was added to the residue. The
resulting solid
was collected by filtration and washed with CH2CI2 and diethyl ether to afford
Intermediate 220(a)
(1 g, 2.62 mmol) as a red solid in 82% yield.
'H NMR (ds-DMSO): S 11.15 (s, 1 H), 10.04 (s, 1 H), 7.42 (s, 1 H), 6.92 (d, 1
H, J= 1.88 Hz), 6.56
(d, 1 H, J= 1.88 Hz), 6.05 (br s, 1 H), 5.17 (s, 2H), 4.03 (s, 2H), 3.53 (t,
2H, J= 5.65, 5.27 Hz), 3.36
(m, 2H, partially obscured), 1.42 (s, 9H).
LCMS: (M+H+) 382.1, (M+Na+) 404.3; (M-H)- 380.1.
Step 2. Preparation of (1 R,2R)- 4-{6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-
amino]-5,6-
dihydro-1 H-[1,2]diazepino[4,5,6-cdJindol-2-yl}-3,6-dihydro-2H-pyridine-l-
carboxylic acid
tert-butyl ester 220(b)
Preparation of intermediate 220(b) from Intermediate 220(a) (100 mg, 0.262
mmol), (1 R,2R)-2-
phenyl-cyclopropanecarboxylic acid (47.0 mg, 0.290 mmol), (3-dimethylamino-
propyl)-ethyl-
carbodiimide, hydrochloride (55 mg, 0.288 mmol), and 4-dimethylaminopyridine
(35.2 mg, 0.288
mmol) in N,N-dimethylformamide (6.0 mL) was carried out analogously to Example
190, step 2.
When the reaction was judged complete, the volatile components were removed in
vacuo, and the
resulting residue was dissolved in methanol and loaded onto a silica gel plug.
The plug was then
loaded onto a silica gel column and eluted with 1:1 dichloromethane: ethyl
acetate to afford
Intermediate 220(b) (130 mg, 0.248 mmol) as a yellow solid in 94% yield.
' H NMR (d6-DMSO): S 11.71 (s, 1 H), 10.39 (s, 1 H), 10.26 (s, 1 H), 8.07 (d,
1 H, J= 1.70 Hz), 7.57
(d, 1 H, J = 1.70 Hz), 7.50 (s, 1 H), 7.33-7.25 (m, 2H), 7.23-7.15 (m, 3H),
6.16 (s, 1 H), 4.08-4.04
(m, 2H), 3.55 (t, 2H, J= 5.65, 5.27 Hz), 3.35 (m, 2H, obscured), 2.41-2.33 (m,
1 H), 2.11-2.02 (m,
1 H), 1.53-1.46 (m, 1 H), 1.43 (s, 9H), 1.40-1.30 (m, 1 H).
LCMS: (M-H)" 524.1.
Step 3. Preparation of Title Compound: (IR,2R)- 2-Phenyl-
cyclopropanecarboxylic acid [6-
oxo-2-(1,2,3,6-tetrahyd ro-pyrid i n-4-yl )-5,6-d i hyd ro-1 H-[1,2] d iaze pi
n o[4,5,6-cd]i n dol-8-y I]-
amide (hydrochloric salt)
Preparation of the title compound from Intermediate 220(b) (130 mg, 0.248
mmol) and 4.0 M HCI
in dioxane (1.24 mL) was carried out analogously to Example 91. Isolation,
also in an analogous
manner, afforded the title compound (107 mg, 0.232 mmol) as a red powder in
94% yield.
'H NMR (d6-DMSO): S 11.97 (s, IH), 10.47 (s, IH), 10.33 (s, IH), 9.31-9.10 (br
s, 2H), 8.11 (d,
1 H, J = 1.70 Hz), 7.61 (d, 2H, J = 1.70 Hz), 7.53 (s, 1 H), 7.34-7.24 (m,
2H), 7.24-7.12 (m, 3H),
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
224
6.17 (s, 1 H), 3.87-3.72 (m, 2H), 3.40-3.25 (m, 2H), 2.80-2.66 (m, 2H), 2.42-
2.31 (m, 1 H), 2.15-
2.04 (m, 1 H), 1.55-1.42 (m, 1 H), 1.42-1.29 (m, 1 H).
LCMS: (M+H+) 426.0, (M+Na+) 448.1; (M-H)- 424.1.
HRMS: (M+H+) calcd for C25H24N502, 426.1930, found 426.1924.
Examale 221: (1,2-trans)-2-Pyridin-3-vl-cyclopropanecarboxylic acid [6-oxo-2-
(1 2 3 6-tetrahydro-
pyridin-4-yl)-5,6-dihydro-1H-[1,2]diazepino[4 5 6-cdlindol-8-yll-amide=
dihydrochloride
N
01-~OH 0 O HN-N
CN \
Intermediate 220(a), O ~/ ~ ~ NBoc
N
Example 220 EDCI, Et3N, 4-DMAP N N
H H
DMF, 78% 221(a)
HN-N
HCI O
4N HCI (Dioxane) N\
~ ~ O ( \ H.HCI
CHgC12, 99% N ~ N ~
H H
Step 1. Preparation of (1,2-trans)-4-{6-Oxo-8-[(2-pyridin-3-yi-
cyclopropanecarbonyl)-amino]-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-2-yi}-3,6-dihydro-2H-pyridine-l-
carboxylic acid
tert-butyl ester 221(a)
Preparation of intermediate 221(a) from Intermediate 220(a) of Example 220
(100 mg, 0.262
mmol), crude 2-pyridin-3-yl-cyclopropanecarboxylic acid (estimated purity c.a.
75%) (67.3 mg, c.a.
0.289 mmol), (3-dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (55 mg,
0.288 mmol),
and 4-dimethylaminopyridine (35.2 mg, 0.288 mmol) in N,N-dimethylformamide
(10.0 mL) was
carried out analogously to Example 190, step 2. When the reaction was judged
complete, the
volatile components were removed in vacuo, and the resulting residue was
dissolved in methanol
and loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted
with 20:1 dichloromethane: methanol to afford Intermediate 221(a) (107.6 mg,
0.204 mmol) as a
yellow solid in 78% yield.
' H NM R(d6-DMSO): S 11.72 (s, 1 H), 10.42 (s, IH), 10.26 (s, IH), 8.50 (s, 1
H), 8.40 (d, 1 H, J
4.71 Hz), 8.07 (s, I H), 7.57 (s, 1 H), 7.55 (d, I H, J = 7.91 Hz), 7.50 (s, I
H), 7.32 (dd, 1 H, J = 4.71,
3.77 Hz), 6.16 (s, 1 H), 4.05 (br s, 2H), 3.55 (t, 2H, J = 4.52, 4.71 Hz),
3.35 (m, 2H, obscured),
2.46-2.37 (m, 1 H), 2.16-2.06 (m, 1 H), 1.57-1.48 (m, 1 H), 1.43 (s, 9H), 1.48-
1.38(m, 1 H).
LCMS: (M+H+) 527.2; (M-H)- 525Ø
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
225
Step 2. Preparation of Title Compound: (1,2-trans)-2-Pyridin-3-yl-
cyclopropanecarboxylic
acid [6-oxo-2-(1,2,3,6-tetrahydro-pyridin-4-yi)-5,6-dihydro-1 H-
[1,2]diazepino[4,5,6=cd]indol-
8-yI]-amide; dihydrochloride
Preparation of the title compound from Intermediate 221(a) (100 mg, 0.190
mmol) and 4.0 M HCI
in dioxane (0.95 mL) was carried out analogously to Example 91. Isolation,
also in an analogous
manner, afforded the title compound (95 mg, 0.190 mmol) as a red powder in 99%
yield.
' H NMR (ds-DMSO): 5 12.05 (s, 1 H), 10.66 (s, 1 H), 10.34 (s, 1 H), 9.29 (br
s, 2H), 8.89 (d, 1 H, J=
1.51 Hz), 8.74 (d, 1 H, J = 5.27 Hz), 8.32 (d, 1 H, J = 8.48 Hz), 8.10 (d, 1
H, J = 1.51 Hz), 7.94 (dd,
1 H, J = 6.03, 5.65 Hz), 7.65 (d, 1 H, J = 1.51 Hz), 7.53 (s, 1 H), 6.17 (s, 1
H), 3.85-3.74 (m, 2H),
3.38-3.25 (m, 2H), 2.80-2.70 (m, 2H), 2.70-2.59 (m, 1 H), 2.40-2.29 (m, 1 H),
1.69-1.55 (m, 2H).
LCMS: (M+H+) 427.1, (M+Na+) 449.1; (M-H)" 425Ø
HRMS: (M+H+) calcd for C24H23N602, 427.1882, found 427.1895.
Example 222: (1 R.2R)-2-Phenyl-cyclopropanecarboxylic acid (6-oxo-2-piperidin-
4-yI-5.6-dihydro-
1 H-[1,2]diazepino[4,5.6-cd]indol-8-yl)-amide (hydrochloric salt)
HN-N
O ~
Intermediate 220(a), Pd/C, H2, MeOH ~\ ~ NBoc
Example 220 H2N / N
DMF, 29% H
222(a)
0 O HN-N
..,,JLoH
~ 0 \ :::
10 HATU, EDMF H H
222(b)
HN-N
o ~
0 \
~ NH.HCI
..~'N I / N
H H
Step 1. Preparation of 4-(8-Amino-6-oxo-5,6-dihydro-lH-r1,21diazepinor4,5,6-
cdlindol-2-yl)-
piperidine-l-carboxylic acid terl-butyl ester 222(a)
In a manner analogous to that of Example 183, palladium (10% on activated
carbon) (0.186 g)
was added to a solution of Intermediate 220(a) of Example 220 (0.61 g, 1.60
mmol) in 9:1
methanol:N,N-dimethformamide (50 mL). The reaction mixture was purged with H2
and stirred at
room temperature under H2 (1 atm.) for 6.5 hours. The mixture was filtered,
and the filtrate
evaporated. The resulting residue was dissolved in methanol and loaded onto a
silica gel plug.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
226
The plug was then loaded onto a silica gel column and eluted with 50:3
dichloromethane:methanol to give Intermediate 222(a) (0.18 g, 0.470 mmol) as a
yellow solid in
29% yield.
'H NMR (d6-DMSO): S 11.03 (s, 1 H), 9.88 (s, 1 H), 7.43 (s, 1 H), 6.86 (s, 1
H), 6.54 (s, 1 H), 5.04 (br
s, 2H), 4.16-3.99 (m, 3H), 2.95-2.67 (m, 2H), 1.73-1.54 (m, 4H), 1.42 (s, 9H).
LCMS: (M+H+) 384.1, (M+Na+) 406.2; (M-H)" 382.1.
Step 2. Preparation of (1 R,2R)- 4-{6-Oxo-8-[(2-phenyl-cyclopropanecarbonyl)-
amino]-5,6-
dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-2-yl}-piperidine-l-carboxylic acid
tert-butyl ester
222(b)
Preparation of intermediate 222(b) from Intermediate 222(a) (60 mg, 0.157
mmol), (IR,2R)-2-
phenyl-cyclopropanecarboxylic acid (27.9 mg, 0.172 mmol), triethylamine (0.086
mL, 0.617
mmol), and O-(7-azabenzotriazol-1-yi)-N,N,N;M tetramethyluronium
hexafluorophosphate (71.5
mg, 0.188 mmol) in N,N-dimethylformamide (5.0 mL) was carried out analogously
to Example 11.
The volatile components were removed in vacuo, and the resulting residue was
dissolved in
methanol and loaded onto a silica gel plug. The plug was then loaded onto a
silica gel column and
eluted with 1:1 dichloromethane: ethyl acetate to afford Intermediate 222(b)
(104 mg) as a yellow
solid contaminated with NN-dimethylformamide which was carried on directly to
the next step.
' H NMR (ds-DMSO): S 11.57 (s, 1 H), 10.32 (s, 1 H), 10.12 (s, 1 H), 7.98 (s,
1 H), 7.55 (d, 1 H, J
1.32 Hz), 7.54 (s, 1 H), 7.33-7.25 (m, 2H), 7.22-7.15 (m, 3H), 4.19-4.00 (m,
3H), 2.92-2.77 (m,
2H), 2.41-2.30 (m, 1 H), 2.12-2.00 (m, IH), 1.80-1.56 (m, 4H), 1.53-1.45 (m, 1
H), 1.42 (s, 9H),
1.38-1.29 (m, 1 H).
LCMS: (M+H+) 528.2, (M+Na+) 550.1; (M-H)" 526.1.
Step 3. Preparation of Title Compound: (IR,2R)-2-Phenyl-cyclopropanecarboxylic
acid (6-
oxo-2-piperidin-4-y1-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cal]indol-8-yl)-
amide (hydrochloric
salt)
Preparation of the title compound from Intemediate 222(b) (100 mg) and 4.0 M
HCI in dioxane (1
mL) was carried out analogously to Example 91. Isolation, also in an analogous
manner, afforded
the title compound (65.3 mg, 0.141 mmol) as a yellow powder in a combined
yield of 90% for
steps 2 and 3.
'H NMR (d6-DMSO): S 11.82 (s, 1 H), 10.41 (s, 1 H), 10.18 (s, IH), 9.16-9.00
(m, IH), 8.93-8.73
(m, 1 H), 8.07 (s, 1 H), 7.58 (s, 2H), 7.34-7.23 (m, 2H), 7.23-7.09 (m, 3H),
3.46-3.26 (m, 3H), 3.10-
2.90 (m, 2H), 2.41-2.29 (m, IH), 2.15-2.03 (m, 1 H), 2.03-1.85 (m, 4H), 1.53-
1.40 (m, IH), 1.40-
1.27 (m, 1 H).
LCMS: (M+H+) 428.1, (M+Na+) 450.2; (M-H)" 426.2.
HRMS: (M+H+) calcd for C25H26N502, 428.2087, found 428.2086.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
227
Example 223: (R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-piperidin-4-yI-5.6-dihydro-1
H-
[1,21diazepino[4,5,6-cd]indol-8-yl)-acetamide: dihydrochloride
n O HN-N
~H
Interrmediate 222(a), NHBoc n NBoc
Example 222 HATU, Et3N, DMF NHBop H
223(a)
HN-N
O ~
4N HCI (Dioxane) C ~
( ~ NH H-Cl
CH2CI2, 82% OJLN..'C) ~ N
H-Cl NHZ H H
Step 1. Preparation of (R)- 4-[8-(2-tert-Butoxycarbonylamino-2-cyclohexyl-
acetylamino)-6-
oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-cd]indol-2-yl]-piperidine-l-carboxylic
acid tert-
butyl ester 223(a)
Preparation of intermediate 223(a) from Intermediate 222(a) of Example 222 (60
mg, 0.157
mmol), (R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (44.3 mg, 0.172
mmol), triethylamine
(0.086 mL, 0.617 mmol), and O-(7-azabenzotriazol-1-yl)-
N,N,N;N'tetramethyluronium
hexafluorophosphate (71.5 mg, 0.188 mmol) in N,N-dimethylformamide (8.0 mL)
was carried out
analogously to Example 11. The volatile components were removed in vacuo, and
the resulting
residue was dissolved in methanol and loaded onto a silica gel plug. The plug
was then loaded
onto a silica gel column and eluted with 1:1 dichloromethane: ethyl acetate to
afford Intermediate
223(a) (107 mg) as a yellow solid contaminated with N,N-dimethylformamide
which was carried
on directly to the next step.
' H NMR (d6-DMSO): S 11.57 (s, 1 H), 10.14 (s, 1 H), 10.03 (s, 1 H), 7.98 (s,
1 H), 7.94 (s, 1 H), 7.56
(s, 1 H), 7.54 (s, 1 H), 6.85 (d, 1 H, J = 8.48 Hz), 4.15-3.99 (m, 2H), 3.99-
3.85 (m, 1 H), 2.92-2.76
(m, 2H), 1.78-1.45 (m, 10H), 1.42 (s, 9H), 1.37 (s, 9H), 1.22-0.97 (m, 5H).
LCMS: (M+H+) 623.2, (M+Na+) 645.2; (M-H)' 621.2.
Step 2. Preparation of Title Compound: (R)-2-Amino-2-cyclohexyl-N-(6-oxo-2-
piperidin-4-yl-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl)-acetamide; dihydrochloride
Preparation of the title compound from Intermediate 223(a) (100 mg, 0.161
mmol) and 4.0 M HCI
in dioxane (1.6 mL) was carried out analogously to Example 91. Isolation, also
in an analogous
manner, afforded the title compound (63.2 mg, 0.128 mmol) as a yellow powder
in a combined
yield of 82% for steps 1 and 2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
228
'H NMR (d6-DMSO): 5 11.98 (s, 1 H), 10.82 (s, 1 H), 10.24 (s, 1 H), 9.21-9.06
(m, 1 H), 9.06-8.87
(m, 1 H), 8.35 (s, 3H), 8.04 (s, 1 H), 7.64 (d, 1 H, J= 1.70 Hz), 7.60(s, 1
H), 3.47-3.28 (m, 4H), 3.10-
3.29 (m, 2H), 2.10-1.86 (m, 4H), 1.86-1.52 (m, 6H), 1.28-0.94 (m, 5H).
LCMS: (M+H+) 423.2, (M+Na+) 445.1; (M-H)" 421.3.
HRMS: (M+H+) calcd for C23H31N60Z, 423.2508, found 423.2492.
Example 224: (1.2-trans)-2-Pyridin-3-yl-cyclopropanecarboxvlic acid (6-oxo-2
piperidin-4-yI-5 6-
dihydro-1 H-f 1.2]diazepinoi4.5,6-cdlindol-8yl)-amide: dihydrochloride
N p HN-N
O x
QN 0 Intermediate 222(a), H NBoc
Example 222 HATU, Et3N, DMF, 72% N N
H H
224(a)
HN-N
HCI ~ ~
4N HCI (Dioxane) N
~ ~ 0 ~ \ ~ NH.HCI
CH2CI2, 99% \ N ~ N
H H
Step 1. Preparation of 4-{6-Oxo-8-[(1,2-trans)-(2-pyridin-3-yl-
cyclopropanecarbonyl)-amino]-
5,6-dihydro-lH-[1,2]diazepino[4,5,6-cal]indol-2-yl}-piperidine-l-carboxylic
acid tert-butyl
ester 224(a)
Preparation of intermediate 224(a) from Intermediate 222(a) of Example 222 (60
mg, 0.157
mmol), (1,2-trans)-2-pyridin-3'-yl-cyclopropanecarboxylic acid (40.2 mg, 75%,
0.172 mmol),
triethylamine (0.086 mL, 0.617 mmol), and O-(7-azabenzotriazol-1-yl)-N,N,N;N'
tetramethyluronium hexafluorophosphate (71.5 mg, 0.188 mmol) in N,N-
dimethylformamide (8.0
mL) was carried out analogously to Example 11. The volatile components were
removed in vacuo,
and the resulting residue was dissolved in methanol and loaded onto a silica
gel plug. The plug
was then loaded onto a silica gel column and eluted with 25:1 dichloromethane:
methanol to
afford Intermediate 224(a) (59.2 mg, 0.112 mmol) as a yellow solid in 72%
yield.
'H NMR (d6-DMSO): S 11.59 (s, 1 H), 10.39 (s, 1 H), 10.14 (s, 1 H), 8.65 (d, 1
H, J= 2.07 Hz), 8.53
(dd, I H, J = 4.99, 1.41 Hz), 7.98 (d, 1 H, J = 1.70 Hz), 7.84 (d, I H, J =
7.91 Hz), 7.59-7.53 (m, 3H),
4.16-4.02 (m, 3H), 2.95-2.74 (m, 2H), 2.22-2.13 (m, 1 H), 1.78-1.45 (m, 7H),
1.42 (s, 9H).
LCMS: (M+H+) 529.1; (M-H)" 527.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
229
Step 2. Preparation of Title Compound: (1,2-trans)-(2-Pyridin-3-yl-
cyclopropanecarboxylic
acid (6-oxo-2-piperidin-4-y1-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cal]indol-8-
yl)-amide;
dihydrochloride
Preparation of the title compound from Intermediate 224(a) (50 mg, 0.095 mmol)
and 4.0 M HCI in
dioxane (0.5 mL) was carried out analogously to Example 91. Isolation, also in
an analogous
manner, afforded the title compound (47.4 mg, 0.095 mmol) as a yellow powder
in 99% yield.
'H NMR (d6-DMSO): 8 11.87 (s, 1 H), 10.59 (s, 1 H), 10.20 (s, 1 H), 9.18-9.00
(m, 2H), 8.89 (br s,
2H), 8.72 (d, 1 H, J= 5.09 Hz), 8.33 (d, 1 H, J= 8.10 Hz), 8.07 (s, 1 H), 7.96
(dd, 1 H, J= 8.10, 7.72
Hz), 7.60 (d, 2H, J= 5.09 Hz), 3.47-3.29 (m, 3H), 3.11-2.84 (m, 2H), 2.75-2.61
(m, 1 H), 2.40-2.29
(m, 1 H), 2.09-1.84 (m, 4H), 1.70-1.54 (m, 2H).
LCMS: (M+H+) 429.2, (M+Na+) 451.1; (M-H)" 427.1.
HRMS: (M+H+) calcd for C24H25N602, 429.2039, found 429.2021.
Examgle 225: (1R 2R)-N-(6-Oxo-2-pyridin-4-yl-5 6-dihydro-lH-[1 2]diazepino[4 5
6-cdlindol-8-yl)-
2-phenylcyclopropanecarboxamide
HO` _ O HN-N
\
B \ ~N
Intermediate 147(d), HO I\ \ N
Example 147 Pd(dppf)CI2, 2 M Na2CO3 BocHN / N
DMF, 80 oC, 82% H
225(a)
HN-N
O \
4N HCI (Dioxane) \ \ HATU, Et3N, DMF
N
CH2Cl2 CIH.HzN / H a ~ 225(b) )L..
H
O
25%
HN-N
O \
LX_GN
H H
Step 1. Preparation of tert-Butyl 6-oxo-2-pyridin-4-y1-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cd]indol-8-ylcarbamate 225(a)
A mixture of Intermediate 147(d) of Example 147 (0.50 g, 1.3 mmol), 4-pyridyl
boronic acid (0.25
g, 2.0 mmol), [1,1'- bis(diphenylphosphino)ferrocene]dichloropalladium (II)
(106 mg, 0.13 mmol)
and 3.0 M aqueous sodium carbonate (3.3 ml) in N,N-dimethylformamide (20 ml)
was stirred at
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
230
100 C for 4 hours. The volatile components were removed in vacuo and the
residue was
subjected to on silica gel chromatography, eluting with CHzCI2:methanol (95:5
increasing to
90:10). Intermediate 225(a) (0.41 g) was obtained in 82% yield.
'H NMR (ds-DMSO): S 12.22 (s, 1 H), 10.52 (s, 1 H), 9.60 (s, 1 H), 8.74 (s, 1
H), 8.72 (s, 1 H), 7.85
(d, 1 H, J= 1.7 Hz), 7.75 (d, 1 H, J= 1.7 Hz), 7.60-7.72 (m, 3H), 1.51 (s,
9H).
LCMS (M++1): 378.1
Step 2. Preparation of 8-Amino-2-pyridin-4-yl-1,5-dihydro-6H-
[1,2]diazepino[4,5,6-cd]indol-
6-one hydrochloride 225(b)
To a mixture of Intermediate 225(a) (0.38 g, 1.0 mmol) in CH2CI2 (30 ml), was
added 1 M HCI in
diethyl ether (20 ml). The mixture was then stirred at room temperature for 2
hours. The volatiles
were removed in vacuo to provide Intermediate 225(b) which was carried' on
directly to the next
step without further purification.
Step 3. Preparation of Title Compound: (1R,2R)-N-(6-Oxo-2-pyridin-4-y1-5,6-
dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl)-2-phenylcyclopropanecarboxamide
A solution of Intermediate 225(b) (ca. 0.5 mmol), (1R,2R)-2-phenyl-
cyclopropanecarboxylic acid
(113 mg, 0.7 mmol), triethylamine (0.4 ml) and O-(7-azabenzotriazol-1-yl)-
N,N,N;N=
tetramethyluronium hexafluorophosphate (0.23 g, 0.6 mmol) in 10 ml of N,N-
dimethylformamide
was stirred at room temperature for 3 hours. The mixture was subjected to
preparative HPLC to
afford the title compound (107 mg, 0.25 mmol) in 25% yield for steps 2 and 3.
'H NMR (d6-DMSO): $ 12.25 (s, 1 H), 10.49 (s, 1 H), 10.44 (s, 1 H), 8.67 (s, 1
H), 8.65 (s, 1 H), 8.16
(s, 1 H), 7.48-7.91 (m, 4H), 7.20-7.46 (m, 2H), 6.96-7.20 (m, 3H), 2.26-2.40
(m, 1 H), 1.94-2.13 (m,
1 H) 1.39-1.60 (m, 1 H), 1.22-1.39 (m, 1 H).
HRMS calculated for C25H19N502 422.1617 (M+H), found 422.1626.
Example 226: N-(6-Oxo-2-pyridin-4-LI-5,6-dihydro-lH-[1,2]diazepino[4,5.6-
cd]indol-8-yl)-(12
trans)-2-pyrid in-3-ylcxclopropanecarboxam ide
HN-N
O
CN O \ ~ N I / N X /N
H H
Preparation of example 226 from Intermediate 147(d) of Example 147 (200 mg,
0.529 mmol) was
carried out analogously to the preparation of Example 225 in three steps
except that (1,2-trans)-2-
pyridin-3-yl-cyclopropanecarboxylic acid was used instead of (1 R,2R)-2-phenyl-
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
231
cyclopropanecarboxylic acid in step 3. Isolation, also in an analogous manner,
afforded the title
compound (54 mg) as a yellow powder in 25% yield overall.
' H NMR (d6-DMSO): 5 12.26 (s, 1 H), 10.49 (s, 1 H), 10.47 (s, 1 H), 8.67 (s,
1 H), 8.65 (s, 1 H), 8.46
(s, 1 H), 8.30-8.43 (d, 1 H, J = 4.7 Hz), 8.16 (s, 1 H), 7.57-7.88 (m, 4H),
7.45-7.57 (m, 1 H), 7.19-
7.41 (m, 1 H), 2.32-2.42 (m, 1 H), 2.01-2.20 (m, 1 H), 1.46-1.65 (m, 1 H),
1.32-1.46 (m, 1 H).
HRMS calculated for C24H1$N602 423.1569 (M+H), found 423.1598.
Example 227: (2.9-Dichloro-6-oxo-5.6-dihydro-1H-L,21diazepino[4,5,6-cdjindol-8-
yl)-carbamic
acid tert-butyl ester
HN-N
O ~
Intermediate 147(c), NCS, DMF '
Example 147 0 Ci 00 60 C, 35% BocHN ,!! N
ci H
To a solution of Intermediate 147(c) of Example 147 (1.5 g, 5 mmol) in CHCI3
(10 mL) and N,N-
dimethylformamide (15 mL), was added N-chlorosuccinimide (701 mg, 5.25 mmol).
The mixture
was heated to 60 C and stirred for 3 hours. Chloroform, N,N-dimethylformamide
and water were
added. Following extractive work-up with chloroform, the organic layer was
dried over Na2SO4 and
filtered. Evaporation of the volatile components gave a deep brown residue to
which methanol
was added. Filtration and collection of the solids gave the title compound
(648 mg) as deep brown
solid whose purity by NMR and HPLC was estimated to be about 70%.
'H-NMR (d6-DMSO): S 13.15 (s, 1 H), 10.62 (s, IH), 8.90 (s, IH), 7.65 (s, IH),
7.39 (s, IH), 1.47
(s, 9H).
LCMS: (M+H+) 370.2.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
232
Example 228: (R)- 8-(2-Amino-2-cyclohexyl-acetylamino)-6-oxo-5,6-dihydro-1H-
j1 2]diazepino[4 5 6-cd]indole-2-carboxylic acid (2-dimethylamino-ethyl)-
amide; dihydrochloride
01- OHN-N ~H HN~ \
Intermediate 171(a), NHBoc
Example 171
EDCI, Et3N, 4-DMAP N N O
DMF, 24% NHBop H
228(a)
HN-N
4N HCI (Dioxane) 0 HN~N \ H-CI
CH2CI2, 99% ` 01~-NB H N
H-CI NH2 H
Step 1. Preparation of (R)-{Cyclohexyl-[2-(2-dimethylamino-ethylcarbamoyl)-6-
oxo-5,6-
dihydro-lH-[1,2]diazepino[4,5,6-cal]indol-8-ylcarbamoyl]-methyl}-carbamic acid
tert-butyl
ester 228(a)
Preparation of intermediate 228(a) from Intermediate 171(a) of Example 171
(200 mg, 0.571
mmol), (R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (147 mg, 0.571
mmol), (3-
dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (131 mg, 0.686 mmol),
and 4-
dimethylaminopyridine (84 mg, 0.688 mmol) in N,N-dimethylformamide (8.0 mL)
was carried out
analogously to Example 190, step 2. When the reaction was judged complete, the
volatile
components were removed in vacuo, and the resulting residue was dissolved in
methanol and
loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted with
40:3:0.3 dichloromethane: methanol: ammonium hydroxide to afford Intermediate
228(a) (77 mg,
0.139 mmol) as a yellow solid in 24% yield.
'H NMR (d6-DMSO): S 12.65 (s, 1 H), 10.66 (s, IH), 10.26 (s, 1 H), 9.83 (br s,
1 H), 8.98 (br s, 1 H),
8.16 (s, 2H), 7.76 (s, 1 H), 6.93 (d, 1 H, J = 9.23 Hz), 4.18-4.03 (m, 1 H),
4.01-3.85 (m, 1 H), 3.71-
3.56 (m, 2H), 2.83 (s, 6H), 1.78-1.45 (m, 6H), 1.37 (s, 9H), 1.21-0.93 (m,
5H). LCMS: (M+H+)
554.2, (M+Na+) 576.1; (M-H)- 552.2.
Step 2. Preparation of Title Compound: (R)- 8-(2-Amino-2-cyclohexyl-
acetylamino)-6-oxo-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indole-2-carboxylic acid (2-
dimethylamino-ethyl)-
amide; dihydrochloride
Preparation of the title compound from Intermediate 228(a) (75 mg, 0.136 mmol)
and 4.0 M HCI in
dioxane (1.36 mL) was carried out analogously to Example 91. Isolation, also
in an analogous
manner, afforded the title compound (71.3 mg, 0.136 mmol) as a yellow powder
in 99% yield.
' H NMR (d6-DMSO): S 12.95 (s, 1 H), 10.98 (s, 1 H), 10.70 (s, 1 H), 10.08 (br
s, 1 H), 9.16 (s, 1 H),
8.37 (s, 3H), 8.21 (s, 1 H), 8.13 (d, 1 H, J= 1.32 Hz), 7.79 (d, 1 H, J= 1.51
Hz), 3.85-3.74 (m, 1 H),
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
233
3.65 (d, 2H, J = 5.84 Hz), 3.30 (d, 2H, J = 6.41 Hz), 2.83 (d, 6H, J = 4.71
Hz), 1.93-1.52 (m, 6H),
1.29-0.97 (m, 5H).
LCMS: (M+H+) 454.2, (M+Na+) 476.1; (M-H)- 452.1.
HRMS: (M+H+) calcd for C23H32N703, 454.2567, found 454.2574.
Example 229: N-(Cyclohexylmethyl)-M-(6-oxo-5,6-dihydro-1H-[1,2]diazepino[4,5,6-
cdlindol-8-
I urea
HN-N
O ~
o I \ \
N N / N
~H H H
Preparation of example 229 from the/title compound of Example 2 (25 mg, 0.11
mmol), N,N'-
disuccinimidyl carbonate (27 mg, 0.11 mmol), N,N-diisopropylethylamine (0.024
mL, 0.21 mmol)
and 1-cyclohexylmethanamine (0.028 mL, 0.021 mmol) in N,N-dimethylformamide
(1.0 mL) was
carried out analogously to Example 207. Purification, also in an analogous
manner, afforded the
title compound (4 mg, 0.009 mmol) as yellow powder in 8% yield.
1 H NMR (CDCI3/methanol-d4): S 8.02 (s, 1 H), 7.42 (s, 1 H), 7.28 (s, 1 H),
7.18 (s, 1 H), 3.06 (d, 2H,
J= 5.84 Hz), 1.82-1.71 (m, 5H), 1.34-1.18 (m, 5H), 0.97 (m, 1 H).
LCMS: (M-H)" 338.1, (M+Na+) 362.1.
Example 230: (1 R, 2R)-2-Phenyl-cyclopropanecarboxylic acid (2-morpholin-4-
ylmethyl-6-oxo-5,6-
dihydro-1H-[1,2]diazepinof4,5.6-cdJindol-8-yl)-amide compound with acetic acid
HN-N
1. K3Fe(CN)6, K2Os02(OH)4 O ~
K2C03, THF, H20 A
\ \
Title compound (
of Example 164 2. Na104, THF, HZO, 22% BocHN / H
230(a)
O HN-N r-Q 1. 4N HCI (Dioxane)
Morpholine, NaBH(OAc)3 N___///) CH2CI2
HCI (Dioxane), THF, 71% BocHN I/ N 2. HATU, Et3N, DMF, 31%
H
2
30(b) ..~~
azl&
~ H
O HN-N ~0
/ I O I \ \ N_/
\ .,~N / N
0 H H
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
234
Step 1. Preparation of (2-Formyl-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-yl)-
carbamic acid tert-butyl ester 230(a)
To a solution of the title compound of Example 164 (48 mg, 0.146 mmol) in 1:1
tetrahydrofuran:H20 (4 mL) was added K2C03 (30 mg, 0.219 mmol), K20s02(OH)4 (7
mg) and
K3Fe(CN)6 (72 mg, 0.219 mmol). The mixture was stirred at room temperature for
2 hours
whereupon water and ethyl acetate were added. Following extractive work-up,
the organic layer
was then dried, filtered, and concentrated. The residue was dissolved in 1:1
tetrahydrofuran:H20
(2 mL) and sodium periodate (156 mg, 50.73 mmol) was added. The mixture was
stirred at room
temperature for 30 min whereupon water and ethyl acetate were added. Following
extractive
work-up, the organic layer was then dried, filtered, and concentrated. Silica
gel chromatography of
the residue, eluting with 30% ethyl acetate in hexane gave Intermediate 230(a)
as a pale yellow
solid (11 mg, 0.034 mmol) in 22% yield.
Step 2. Preparation of (2-Morpholin-4-ylmethyl-6-oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cal]indol-8-yl)-carbamic acid tert-butyl ester 230(b)
To a solution of morpholine (0.32 mL, 3.64 mmol) and powdered 4A molecular
sieves (1.0 g) in
tetrahydrofuran (5 mL) was added 4M HCI in dioxane (0.91 mL; 3.64 mmol). After
10 min, a
solution of Intermediate 230(a) (199 mg, 0.607 mmol) in tetrahydrofuran (2 mL)
was added
dropwise followed by NaBH(OAc)3 (254 mg, 1.2 mmol). The resulting suspension
was stirred at
room temperature for 24 hours. The mixture was quenched with saturated aqueous
sodium
bicarbonate and filtered through diatomaceous earth, which was then washed
with ethyl acetate.
More ethyl acetate was added to the filtrate, and the aqueous layer was
extracted. The combined
organic layers were dried over Na2SO4, filtered, and concentrated. The residue
was subjected to
silica gel chromatography eluting with 5 % MeOH/ethyl acetate to give
Intermediate 230(b) as a
pale yellow powder (173 mg, 0.43 mmol) in 71 % yield.
' H NMR (d6-DMSO): 11.71 (s, 1 H), 10; 21 (s, 1 H), 7.73 (s, 1 H), 7.62 (s,
2H), 3.74 (s, 2H), 3.63-
3.61 (m, 4H), 2.43-2.41 (m, 4H), 1.52 (s, 9H).
LCMS: (M+H+) 400.
Step 3. Preparation of Title compound: (IR, 2R)-2-Phenyl-
cyclopropanecarboxylic acid (2-
morpholin-4-ylmethyl-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cal]indol-8-
yl)-amide
compound with acetic acid
Preparation of the title compound from Intermediate 230(b) (173 mg, 0.433
mmol) in CH2CI2 (2
mL) and 4M HCI in dioxane (2 mL) was carried out analogously to the
preparation of Example 91.
After concentration, the residue was dissolved in N,N-dimethylformamide (5
mL). (1 R, 2R)-2-
Phenyl-cyclopropanecarboxylic acid (84 mg, 0.52 mmol), triethylamine (0.18 mL,
1.3 mmol), and
O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (247
mg, 0.65
mmol) were added. After 12 hours, the mixture was concentrated and subjected
to preparative
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
235
HPLC in a manner analogous to Example 146, Step 2. The title compound (59 mg,
0.13 mmol)
was obtained as a pale yellow powder in 31 % yield.
1 H NMR (ds-DMSO): 11.84 (s, 1 H), 10.42 (s, 1 H), 10.28 (s, 1 H), 8.10 (s, 1
H), 7.67 (s, 1 H), 7.64 (s,
1 H), 7.39-7.24 (m, 5H), 3.78 (s, 2H), 3.68-3.64 (m, 4H), 2.49-2.47 (m, 4H),
2.14 (m, 1 H), 1.56 (m,
1 H), 1.42 (m, 1 H).
LCMS: (M+H+) 444.1
Example 231: N-(6-Oxo-2-pyridin-3-y1-5,6-dihydro-1H-[1,21diazepino[4 5,6-
cdgindol-8-yl)_(1 2-
trans)-2-pyrid in-3-ylcyclopropanecarboxam ide
HN-N
N
\ I o N
aN
H H
Preparation of example 231 from Intermediate 147(d) of Example 147 was carried
out
analogously to the preparation of Example 225 in three steps except that 3-
pyridyl boronic acid
was used instead of 4-pyridyl boronic acid in step 1 and (1,2-trans)-2-pyridin-
3-yl-
cyclopropanecarboxylic acid was used instead of (1 R,2R)-2-phenyl-
cyclopropanecarboxylic acid in
step 3. Isolation, also in an analogous manner, afforded the title compound
(69 mg) as a yellow
powder in 33% yield overall.
' H NMR (d6-DMSO): $ 12.19 (s, 1 H), 10.30-10.50 (m, 2H), 8.81 (m, IH), 8.60
(m, 1 H), 8.46 (m,
1 H), 8.36 (m, 1 H), 8.13 (m, 1 H), 7.93-8.08 (m, 1 H), 7.37-7.69 (m, 4H),
7.27 (m, 1 H), 2.40 (m, 1 H),
2.08 (m, 1 H), 1.28-1.60 (m, 2H).
HRMS calculated for C24H18N602 423.1569 (M+H), found 423.1590.
Example 232: (1 R,2R)-N-(6-Oxo-2-pyridin-3-yl-5.6-dihydro-1 H-
[1,2]diazepino[4,5,6-cdlindol-8 -yl)-
2-phenylcycloproganecarboxamide
HN-N
.o
+1 ~ ~
N N
WN
H H
Preparation of example 232 from Intermediate 147(d) of Example 147 was carried
out
analogously to the preparation of Example 225 in three steps except that 3-
pyridyl boronic acid
was used instead of 4-pyridyl boronic acid in step 1. Isolation, also in an
analogous manner,
afforded the title compound (18 mg) as a yellow powder in 8% yield overall.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
236
'H NMR (ds-DMSO): S 12.18 (s, 1 H), 10.28-10.54 (m, 2H), 8.81 (m, 1 H), 8.60
(m, 1H), 8.13 (m,
1 H), 7.95-8.08 (m, 1 H), 7.57-7.70 (m, 1 H), 7.49-7.57 (m, 1 H), 7.38-7.48
(m, 1 H), 7.01-7.34 (m,
3H) 4.05 (m, 1 H), 2.32 (m, 1 H), 2.03 (m, 1 H), 1.46 (m, 1 H), 1.32 (m, 1 H).
HRMS calculated for C25H19N502 422.1617 (M+H), found 422.1598.
Example 233: (1 R 2R)- 2-Phenyl-cyclopropanecarboxylic acid [2-(3-
dimethylamino-prop-l-ynLl)-6-
oxo-5.6-dihydro-1 H-f 1.2]diazepinof4.5.6-cdJindol-8-yl]-amide
HN-N
O ~
~ \ - N
Intermediate 147(d), ~
Example 147 Pd(PPh3)2C12, Cul, TMG BOCHN H
DMF, 90 C, 43% 233(a)
O
HN-N azL-,J~01-
O H-CI ,o4N HCI (Dioxane) ~ ~ CH2CI2, 99% H-Cl H2N I~ N HATU, Et3N, DMF, 76%
H
233(b)
HN-N
O ~
i~ ~
0
~/` ,IN / N
H H
Step 1. Preparation of [2-(3-Dimethylamino-prop-l-ynyl)-6-oxo-5,6-dihydro-1H-
[1,2]diazepino[4,5,6-cd]indol-8-yl]-carbamic acid tert-butyl ester 233(a)
Under argon atmosphere, dimethyl-prop-2-ynyl-amine (198 mg, 2.38 mmol),
dichlorobis(triphenylphosphine)palladium(II) (27.6 mg, 0.039 mmol), copperl
iodide (7.8 mg, 0.041
mmol) and N,N,N,N-tetramethylguanidine (912 mg, 7.93 mmol) were added to a
solution of
Intermediate 147(d) from Example 147 (300 mg, 0.794 mmol) in N,N-
dimethylformamide (3 mL)
and dioxane (12 mL). The reaction was heated at 90 C for 4 hours at which
point the volatile
components were removed in vacuo. The resulting residue was dissolved in
methanol and loaded
onto a silica gel plug. The plug was then loaded onto a silica gel column and
eluted with 20:1:0.1
dichloromethane: methanol: ammonium hydroxide to afford Intermediate 233(a)
(130 mg, 0.341
mmol) as a yellow solid in 43% yield.
'H NMR (d6-DMSO): S 12.09 (s, 1 H), 10.45 (s, 1 H), 9.54 (s, 1 H), 7.71 (s, 1
H), 7.65 (d, 1 H, J
1.51 Hz), 7.40 (s, 1 H), 3.61 (s, 2H), 2.27 (s, 6H), 1.47 (s, 9H).
LCMS: (M+H+) 382.1, (M+Na+) 404.1; (M-H)" 380.1.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
237
Step 2. Preparation of 8-Amino-2-(3-dimethylamino-prop-1-ynyl)-1,5-dihydro-
[1,2]diazepino[4,5,6-cd]indol-6-one; dihydrochloride 233(b)
Preparation of intermediate 233(b) from Intermediate 233(a) (126 mg, 0.331
mmol) and 4M HCI in
dioxane (1.68 mL) was carried out analogously to Example 91. Isolation, also
in an analogous
manner, afforded Intermediate 233(b) (117 mg, 0.331 mmol) as a yellow powder
in 99% yield.
1 H NMR (d6-DMSO): S 12.56 (br s, 1 H), 10.68 (s, 1 H), 7.58 (s, 1 H), 7.36
(s, 1 H), 7.24 (s, 1 H), 4.46
(s, 2H), 2.88 (s, 6H).
LCMS: (M+Na+) 304.1; (M-H)"280.1.
Step 3. Preparation of Title Compound: (1R,2R)- 2-Phenyl-
cyclopropanecarboxylic acid [2-
(3-dimethylamino-prop-1-ynyl)-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-yl]-
amide
Preparation of the title compound from Intermediate 233(b) (110 mg, 0.312
mmol), (1 R,2R)-2-
phenyl-cyclopropanecarboxylic acid (55 mg, 0.339 mmol), triethylamine (0.189
mL, 1.36 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate
(155 mg,
0.408 mmol) in N,N-dimethylformamide (10.0 mL) was carried out analogously to
Example 11.
The volatile components were removed in vacuo, and the resulting residue was
dissolved in
methanol and loaded onto a silica gel plug. The plug was then loaded onto a
silica gel column and
eluted with 40:3:0.3 dichloromethane: methanol: ammonium hydroxide to afford
the title
compound (100 mg, 0.235 mmol) as a yellow-brown solid in 76% yield.
' H NMR (d6-DMSO): S 12.24 (s, 1 H), 10.53 (s, 1 H), 10.47 (s, 1 H), 8.10 (d,
1 H, J= 1.32 Hz), 7.62
(d, 1 H, J= 1.51 Hz), 7.45 (s, 1 H), 7.33-7.25 (m, 2H), 7.23-7.15 (m, 3H),
3.84 (s, 2H), 2.44 (s, 6H),
2.42-2.32 (m, 1 H), 2.12-2.02 (m, 1 H), 1.55-1.45 (m, 1 H), 1.42-1.32 (m, 1
H).
LCMS: (M+H+) 426.0, (M+Na+) 448.1; (M-H)' 424.1.
HRMS: (M+H+) calcd for C25H24N502i 426.1930, found 426.1911.
Example 234: (1 R. 2R)-2-Phenyl-cyclopropanecarboxylic acid [2-(4-methyl-
piperazine-1-carbonyl)-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5.6-cdlindol-8
yll-amide (acetic acid salt)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
238
O HN-N ~ H3
HN NCH3
Intermediate 147(d),
Example 147 Pd(PPh3)4, CO, DMF, 50% BocHN I~ N
H
234(a)
1. 4N HCI (Dioxane) HN-N NCH3
~~ O
CH2CI2 ~ O OH
2. HATU, EtgN, DMF, 15% ~~~~
N N
0 H H
O
J~OH
0 =
Step 1. Preparation of [2-(4-Methyl-piperazine-l-carbonyl)-6-oxo-5,6-dihydro-
1H-
[1,2]diazepino[4,5,6-cal]indol-8-yl]-carbamic acid tert-butyl ester; compound
with acetic
acid 234(a)
Preparation of intermediate 234(a) from Intermediate 147(d) of Example 147
(453 mg, 1.2 mmol),
N-methylpiperazine (0.66 mL, 6 mmol), and
tetrakis(triphenylphosphine)palladium(0) (105 mg,
0.09 mmol) in N,N-dimethyiformamide (7 mL) was carried out analogously to the
preparation of
Example 161 except that tetrakis(triphenylphosphine)palladium(0) was used as
the catalyst
instead of bis(diphenylphosphino)ferrocenedichloropalladium (II). After
concentration, the residue
was subjected to preparative HPLC (Peeke Scientific, HI-Q C18 reverse phase
5u, 100A,
250x21.2 mm column) eluting with CH3CN and 0.1% acetic acid in water at a flow
rate of 20
mL/min using a gradient of 5-95% CH3CN over 40 min to give Intermediate 234(a)
(254 mg, 0.6
mmol) as a pale green powder in 50% yield.
'H NMR (d6-DMSO): 12.06 (s, 1 H), 10.46 (s, 1 H), 9.52 (s, 1 H), 7.74 (s, 1
H), 7.64 (s, 1 H), 7.37 (s,
1 H), 3.49-3.48 (m, 4H), 2.29-2.28 (m, 4H), 2.20 (s, 3H), 1.42 (s, 9H).
LCMS: (M+H+) 426.1
Step 2. Preparation of Title compound: (1R, 2R)-2-Phenyl-
cyclopropanecarboxylic acid [2-
(4-methyl-piperazine-l-carbonyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl]-
amide (acetic acid salt)
Preparation of the title compound from Intermediate 234(a) (445 mg, 1.06 mmol)
in CH2CI2 (5 mL)
and 4M HCI in dioxane (5 mL) was carried out analogously to'Example 91. After
concentration,
the residue was dissolved in N,N-dimethylformamide (5 mL). (1 R, 2R)-2-Phenyl-
cyclopropanecarboxylic acid (162 mg, 1.0 mmol), triethylamine (0.42 mL, 3
mmol), and O-(7-
azabenzotriazol-1-yl)-N,N,N;M tetramethyluronium hexafluorophosphate (456 mg,
1.2 mmol) was
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
239
sequentially added. After 12 hours, the mixture was concentrated. The crude
product was
purified by preparative HPLC in a manner analogous to Example 146, Step 2, to
give the title
compound as a pale yellow powder (74 mg, 0.16 mmol) in 15% yield.
'H NMR (d6-DMSO): 12.25 (s, 1 H), 10.59(s, 1 H), 10.51 (s, 1 H), 8.18 (s, 1
H), 7.69 (s, 1 H), 7.49 (s,
IH), 7.34-7.19 (m, 5H), 3.63 (brs, 4H), 2.56 (s, 3H), 2.40-2.30 (m, 4H), 2.11
(m, 1 H), 1.56 (m,
1 H), 1.42 (m, 1 H).
LCMS: (M+H+) 444.1
Example 235: (1 R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [2-(3-
dimethylamino-propyl)-6-oxo-
5.6-dihydro-1 H-[1,21diazepino[4,5.6-cd]indol-8-yl]-amide
O HN-N
O ~
....kN I ~ N
0 H H
In a manner analogous to that of Example 183, 10% palladium on activated
carbon (20 mg) was
added to a solution of the title compound of Example 233 (20 mg, 0.047 mmol)
in methanol (3
mL). The reaction mixture was purged with H2 and stirred at room temperature
under H2 (1 atm.)
for 1 hour. The reaction mixture was loaded onto a silica gel plug. The plug
was then loaded onto
a silica gel column and eluted with 20:1:0.1 dichloromethane: methanol:
ammonium hydroxide to
afford the title compound (8 mg, 0.0186 mmol) as a yellow solid in 40% yield.
'H NMR (ds-DMSO): S 11.70 (s, 1 H), 10.34 (s, 1 H), 10.17 (s, 1 H), 8.04 (d, 1
H, J= 1.51 Hz), 7.52
(d, 1 H, J = 1.51 Hz), 7.48 (s, 1 H), 7.33-7.25 (m, 2H), 7.23-7.14 (m, 3H),
2.98-2.81 (m, 4H), 2.66
(br s, 6H), 2.41-2.31 (m, 1H), 2.11-2.02 (m, 1H), 2.01-1.87 (m, 2H), 1.53-1.43
(m, 1H), 1.41-1.31
(m, 1 H).
LCMS: (M+H+) 430.1, (M+Na+) 452.1; (M-H)" 428.1.
HRMS: (M+H+) calcd for C25H2$N502, 430.2243, found 430.2240.
Example 236: (1R,2R)- 2-Phenyl-cyclopropanecarboxylic acid [2-(3-dimethylamino-
propenyl)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yll-amide
HN-N
O NMe2
Example 233 H2, Lindlar`s Catalyst O I~
quinoline, MeOH, 66% ^'kN ~ N
H H
To a mixture of the title compound from Example 233 (45 mg, 0.106 mmol),
Lindlar catalyst (45
mg), and quinoline (4.1 mg, 0.032 mmol) was added anhydrous methanol (3 mL).
The reaction
mixture was purged with H2 and stirred at room temperature under H2 (1 atm)
for 2.5 hours. The
reaction mixture was loaded onto a silica gel plug. The plug was then loaded
onto a silica gel
column and eluted with 20:1:0.1 dichloromethane: methanol: ammonium hydroxide
to afford the
title compound (30 g, 0.070 mmol) as a yellow solid in 66% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
240
' H NMR (ds-DMSO): S 13.07 (s, 1 H), 10.41 (s, 1 H), 10.32 (s, 1 H), 8.11 (d,
1 H, J= 1.70 Hz), 7.57
(s, 1 H), 7.54 (d, 1 H, J = 1.70 Hz), 7.33-7.25 (m, 2H), 7.23-7.14 (m, 3H),
6.88 (d, 1 H, J = 12.25
Hz), 5.91 (dt, 1 H, J= 12.06, 6.22), 3.16 (d, 2H, J= 5.65 Hz), 2.42-2.33 (m, 1
H), 2.28 (s, 6H), 2.12-
2.03 (m, 1 H), 1.53-1.44 (m, 1 H), 1.41-1.31 (m, 1 H).
LCMS: (M+H+) 428.1, (M+Na+) 450.0; (M-H)" 426Ø
HRMS: (M+H+) calcd for C25H26N502, 428.2087, found 428.2082.
Example 237: (1R.2R)-2-Phenyl-cyclopropanecarboxylic acid j2-(3-methylamino-
prop-l-ynyl)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepinof4.5.6-cdJindol-8-yll-amide (hydrochloric
salt)
\NBoc HN-N
C
~ 0
\ \ -_
Title Compound 237(a) \ I ==,~N I~ N BocN-
of Example 206 Pd(PPh3)2CI2, Cul, TMG Q H H
DMF, 90 C, 30% 237(b)
HN-N
H-Cl
4N HCI (Dioxane) / 0 \
O \N HN
~i
CH2CIZ, 97% H H
Step 1. Preparation of (1 R,2R)-Methyl-(3-{6-oxo-8-[(2-phenyl-
cyclopropanecarbonyl)-
amino]-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-2-yl}-prop-2-ynyl)-
carbamic acid tert-
butyl ester 237(b)
Intermediate 237(a) was prepared by stirring methyl-prop-2-ynyl-amine (0.5 g,
7.23 mmol), di-tert-
butyl dicarbonate (1.74 g, 7.97 mmol) and 2,6-dimethyl-pyridine (0.088 g,
0.72mmol) in anhydrous
acetonitrile (10 mL) at room temperature for 16 hours. The solvent was
evaporated and the
residue was subjected to silica gel chromatography eluting with ethyl acetate
to afford
Intermediate 237(a) (1 g, 5.91 mmol) as colorless oil in 82% yield. In a
manner analogous to that
of Example 233, Step 1, under an argon atmosphere, intermediate 237(a) (66 mg,
0.39 mmol),
dichlorobis(triphenylphosphine)palladium(II) (4.6 mg, 0.0066 mmol), copperl
iodide (1.2 mg,
0.0063 mmol) and N,N,N,N-tetramethylguanidine (150 mg, 1.30 mmol) were added
to a solution
of the title compound from Example 206 (55 mg, 0.13 mmol) in N,N-
dimethylformamide (0.5 mL)
and dioxane (2 mL). The reaction was heated at 90 C for 3 hours at which
point the volatile
components were removed in vacuo. The resulting residue was dissolved in
methanol and loaded
onto a silica gel plug. The plug was then loaded onto a silica gel column and
eluted with 3:2
dichloromethane:ethyl acetate to afford Intermediate 237(b) (20 mg, 0.039
mmol) as a yellow solid
in 30% yield.
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
241
'H NMR (d6-DMSO): 5 12.22 (s, 1 H), 10.51 (s, 1 H), 10.46 (s, 1 H), 8.08 (d, 1
H, J= 1.51 Hz), 7.61
(d, 1 H, J= 1.70 Hz), 7.41 (s, 1 H), 7.33-7.25 (m, 2H), 7.22-7.15 (m, 3H),
4.37 (s, 2H), 2.90 (s, 3H),
2.41-2.33 (m, 1 H), 2.11-2.02 (m, 1 H), 1.54-1.45 (m, 1 H), 1.42 (s, 9H), 1.39-
1.32 (m, 1 H).
LCMS: (M-H)- 510Ø
Step 2. Preparation of Title Compound: (IR,2R)-2-Phenyl-cyclopropanecarboxylic
acid [2-
(3-methylamino-prop-1-ynyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-
cd]indol-8-yl]-amide
(hydrochloric salt)
Preparation of the title compound from Intermediate 237(b) (20 mg, 0.039 mmol)
and 4.0 M HCI in
dioxane (0.5 mL) was carried out analogously to Example 91. Isolation, also in
an analogous
manner afforded the title compound (17 mg, 0.038 mmol) as a yellow powder in
97% yield.
' H NMR (d6-DMSO): 5 12.37 (s, 1 H), 10.59 (s, 1 H), 10.55 (s, 1 H), 9.30 (br
s, 2H), 8.14 (s, 1 H),
7.65 (s, 1 H), 7.58 (s, 1 H), 7.36-7.24 (m, 2H), 7.24-7.12 (m, 3H), 4.27 (s,
2H), 2.66 (s, 3H), 2.41-
2.29 (m, 1 H, partial obscured), 2.17-2.03 (m, 1 H), 1.56-1.44 (m, 1 H), 1.43-
1.31 (m, 1 H).
LCMS: (M-H)- 410.1.
HRMS: (M+H+) calcd for C24H22N502, 412.1774, found 412.1768.
ExamQle 238: (12-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid !2-(3-
methylamino-prop-1-
ynyl)-6-oxo-5.6-dihvdro-1 H-[1.2]diazepino[4.5.6-cdlindol-8-yll-amiden
dihydrochloride
HN-N
~
i I 0 H N
~
Intermediate 206(a), 0 ~ \ Br
Example 206 EDC, 4 DMAP, DMF, 56% NO/ H
av-~ ~
H
238(a)
HN-N
Intermediate 237(a), N
Example 237 \ C I~ \ = 4N HCI (Dioxane)
zo, N N BocN-
Pd(PPh3)2CI2i Cul, TMG H H CH2CI2885%
DMF, 90 OC, 37% 238(b)
HN-N
H-Cl
H-Cl
CN)
O ~ /
N N HN-
H H
Step 1. Preparation of (1,2-trans)-2-Pyridin-3-yl-cyclopropanecarboxylic acid
(2-bromo-6-
20 oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cdJindol-8-yl)-amide 238(a)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
242
Preparation of intermediate 238(a) from Intermediate 206(a) of Example 206
(120 mg, 0.38
mmol), (1,2-trans)-2-pyridin-3-yl-cyclopropanecarboxylic acid (106 mg, c.a
0.487 mmol, purity c.a.
75%), (3-dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (88 mg, 0.461
mmol), and 4-
dimethylaminopyridine (56 mg, 0.459 mmol) in N,N-dimethylformamide (15.0 mL)
was carried out
analogously to the preparation of Example 190, step 2. When the reaction was
judged complete,
the volatile components were removed in vacuo, the resulting residue was
dissolved in methanol
and loaded onto a silica gel plug. The plug was then loaded onto a silica gel
column and eluted
with 50:3 dichloromethane:methanol to afford Intermediate 238(a) (90 mg, 0.212
mmol) as a
yellow solid in 56% yield.
' H NMR (d6-DMSO): S 12.60 (s, 1 H), 10.46 (s, 1 H), 10.45 (s, 1 H), 8.50 (d,
1 H, J= 1.88 Hz), 8.40
(dd, 1 H, J = 4.62, 1.41 Hz), 8.08 (d, 1 H, J = 1.70 Hz), 7.60 (d, I H, J =
1.51 Hz), 7.56 (dt, I H, J =
7.96, 1.86 Hz), 7.32 (dd, 1 H, J = 7.91, 4.90 Hz), 7.27 (s, IH), 2.45-2.37 (m,
1 H), 2.16-2.06 (m,
1 H), 1.57-1.49 (m, 1 H), 1.49-1.40 (m, 1 H).
LCMS: (M-H)" 422.0, 424Ø
Step 2. Preparation of Methyl-(3-(6-oxo-8-[(2-pyridin-3-yl-
cyclopropanecarbonyl)-amino]-
5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-2-yl}-prop-2-ynyl)-carbamic acid
tert-butyl
ester 238(b)
Under argon in a manner analogous to that of Example 233, step 1, Intermediate
237(a) of
Example 237 (54 mg, 0.32 mmol), dichlorobis(triphenylphosphine)palladium(II)
(3.7 mg, 0.0053
mmol), copperl iodide (1.0 mg, 0.0053 mmol) and N,N,N,N-tetramethylguanidine
(122 mg, 1.06
mmol) were added to a solution of Intermediate 238(a) (45 mg, 0.106 mmol) in
N,N-
dimethylformamide (1 mL) and dioxane (2 mL). The reaction was heated at 90 C
for 2 hours at
which point the volatile components were removed in vacuo. The resulting
residue was dissolved
in methanol and loaded onto a silica gel plug. The plug was then loaded onto a
silica gel column
and eluted with 20:1:0.1 dichloromethane: methanol: ammonium hydroxide to
afford Intermediate
238(b) (20 mg, 0.039 mmol) as a yellow solid in 37% yield.
'H NMR (d6-DMSO): S 12.23 (s, 1 H), 10.52 (s, 1 H), 10.50 (s, 1 H), 8.45 (br
s, 1 H), 8.08 (s, 1 H),
7.61 (s, 1 H), 7.56 (d, 1 H, J = 7.91 Hz), 7.41 (s, 1 H), 7.35 (br s, 1 H),
4.37 (s, 2H), 2.90 (s, 3H),
2.45-2.38 (m, 1 H, partial obscured), 2.16-2.07 (m, 1 H), 1.59-1.49 (m, 1 H),
1.50-1.44 (m, IH,
partial obscured), 1.42 (s, 9H),
LCMS: (M+H+) 513.2, (M+Na+) 535.1; (M-H)" 511.1.
Step 3. Preparation of Title Compound: (1,2-trans)-2-Pyridin-3-yl-
cyclopropanecarboxylic
acid [2-(3-methylamino-prop-1-ynyl)-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-
yI]-amide; dihydrochloride
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
243
Preparation of the title compound from Intermediate 238(b) (20 mg, 0.039 mmol)
and 4M HCI in
dioxane (0.5 mL) was carried out analogously to Example 91. Isolation, also in
an analogous
manner afforded the title compound (16 mg, 0.033 mmol) as a dark red solid in
85% yield.
' H NMR (d6-DMSO): S 12.46 (s, 1 H), 10.77 (s, 1 H), 10.59 (s, 1 H), 9.46 (br
s, 2H), 8.90 (s, 1 H),
8.76 (d, 1 H, J= 3.77 Hz), 8.34 (d, 1 H, J= 7.35 Hz), 8.11 (s, 1 H), 8.03-7.90
(m, 1 H), 7.69 (s, 1 H),
7.59 (s, IH), 4.25 (s, 2H), 2.65 (s, 3H), 2.49 (m, 1 H, obscured), 2.41-2.29
(m, 1 H, partial
obscured), 1.73-1.55 (m, 2H).
LCMS: (M-H)- 411.1.
HRMS: (M+H+) calcd for C23H21N602, 413:1726, found 413.1753.
Example 239: (2R)-2-Amino-2-ckclohexyl-N-[2-(3-methylamino-prop-1-ynyl)-6-oxo-
5,6-dihydro-lH-
[1.2]diazepino[4.5.6-cdjindol-8-yl]-acetamidede; dihydrochloride
0 HN-N
Intermediate 206(a), IVHBoc Br
OjNaN
Example 206 EDC, 4-DMAP, DMF, 30%
H
NHBoc
239(a)
HN-N
O \
Intermediate 237(a),
Example 237 ~ I \ _ 4N HCI (Dioxane)
_ N N BocN-
Pd(PPh3)2Ch, Cui, TMG - H H CHgCh, 11 %
NHBoc
DMF, 90 C 239(b)
HN-N
O \
H-Cl
N HN-
~N ~ I \
H H
H-Cl NH2
Step 1. Preparation of (R)-[(2-Bromo-6-oxo-5,6-dihydro-lH-[1,2]diazepino[4,5,6-
cd]indol-8-
ylcarbamoyl)-cyclohexyl-methyl]-carbamic acid tert-butyl ester 239(a) -
Preparation of intermediate 239(a) from Intermediate 206(a) of Example 206
(120 mg, 0.38
mmol), (R)-tert-butoxycarbonylamino-cyclohexyl-acetic acid (120 mg, 0.466
mmol), (3-
dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (87 mg, 0.455 mmol),
and 4-
dimethylaminopyridine (56 mg, 0.459 mmol) in N,N-dimethylformamide (7.0 mL)
was carried out
analogously to the preparation of Example 190, step 2. When the reaction was
judged complete,
the volatile components were removed in vacuo, and the resulting residue was
dissolved in
methanol and loaded onto a silica gel plug. The plug was then loaded onto a
silica gel column and
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
244
eluted with 1.2:1 dichloromethane: ethyl acetate to afford Intermediate 239(a)
(60 mg, 0.116
mmol) as a yellow solid in 30% yield.
'H NMR (d6-DMSO): S 12.59 (s, 1 H), 10.46 (s, 1 H), 10.14 (s, 1 H), 8.06 (s,
IH), 7.64 (s, 1 H), 7.27
(s, 1 H), 6.90 (d, 1 H, J= 8.85 Hz), 3.91 (t, 1 H, J= 8.29 Hz), 1.76-1.44 (m,
6H), 1.37 (s, 9H), 1.20-
0.93 (m, 5H).
LCMS: (M-H)" 518.0, 516Ø
Step 2. Preparation of (R)-{3-[8-(2-tert-Butoxycarbonylamino-2-cyclohexyl-
acetylamino)-6-
oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cdjindol-2-yl]-prop-2-ynyl}-methyl-
carbamic acid
tert-butyl ester 239(b)
Under an argon atmosphere in a manner analogous to that of Example 233 step 1,
Intermediate
237(a) of Example 237 (65 mg, 0.38 mmol),
dichlorobis(triphenylphosphine)palladium(II) (4.5 mg,
0.0064 mmol), copperl iodide (1.2 mg, 0.0063 mmol) and N,N,N,N-
tetramethylguanidine (146 mg,
1.27 mmol) were added to a solution of Intermediate 239(a) (66 mg, 0.127 mmol)
in N,N-
dimethylformamide (0.5 mL) and dioxane (2 mL). The reaction mixture was heated
at 90 C for 3
hours at which point the volatile components were removed in vacuo. The
resulting residue was
dissolved in methanol and loaded onto a silica gel plug. The plug was then
loaded onto a silica gel
column and eluted with 4:1 dichloromethane: ethyl acetate to afford
Intermediate 239(b) (10 mg)
as a yellow solid contaminated with an unknown impurity.
LCMS: (M+H+) 607.2, (M+Na+) 629.3; (M-H)- 605.2.
Step 3. Preparation of Title Compound: (2R)-2-Amino-2-cyclohexyl-N-[2-(3-
methylamino-
prop-l-ynyl)-6-oxo-5,6-dihydro-1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-
acetamidede;
dihydrochloride
Preparation of the title compound from Intermediate 239(b) (10 mg) and 4 M HCI
in dioxane (0.5
mL) was carried out analogously to Example 91. Isolation, also in an analogous
manner, included
a further trituration with CH2CI2 which removed the impurity carried along
from step 2. The title
compound (6 mg, 0.013 mmol) was obtained as a yellow powder in a combined
yield of 11% for
steps 2 and 3.
'H NMR (d6-DMSO): S 12.52 (s, 1 H), 10.90 (s, 1 H), 10.66 (s, 1 H), 9.37 (br
s, 2H), 8.33 (br s, 3H),
8.08 (s, 1 H), 7.73 (d, 1 H, J= 0.9 Hz), 7.61 (s, 1 H), 4.27 (s, 2H), 3.84-
3.74 (m, 1 H), 2.67 (s, 3H),
1.90-1.54 (m, 6H), 1.27-0.97 (m, 5H).
LCMS: (M+Na+) 429.2; (M-H)' 405.2.
HRMS: (M+H+) calcd for C22H27N602, 407.2195, found 407.2209.
Example 240: (1.2-trans)-N-[1-(2-Hydroxyethyl)-6-oxo-5.6-dihydro-lH-
[1,2]diazepino[4,5.6-
cd]indol-8-yl]-2-phenylcyclopropanecarboxamide
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
245
\ ~ O Me0 O
Intermediate 2(b)õ H p methyl bromoacetate
Example 2 HATU, Et3N, DMF, 74% o
N N K2C03, DMF, 80 C
H H
79%
240(a)
MeO 0 MeO 0
O I\ ~ NaBH4, THF 0 I\ ~ 1. POCI3. DMF, CH2CI2
N ~ N CH3CH2OH, 46% N N 2. NH2NHZ.H2O, AcOH
e H ~H
O ~ e MeOH, reflux, 43%
O O H
240(b) 240(c)
H
p N-N
o I \ ~
N N
OVH H
Step 1. Preparation of Methyl 6-{[(2-phenylcyclopropyl)carbonyl]amino}-1H-
indole-4-
carboxylate 240(a)
Preparation of intermediate 240(a) from Intermediate 2(b) of Example 2 (2.27
g, 10 mmol), (1,2-
trans)-2-phenyl-cyclopropanecarboxylic acid (1.79 g, 11 mmol), triethylamine
(3.0 g, 30 mmol),
and O-(7-azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate
(4.18 g, 11
mmol) in N,N-dimethylformamide (20 mL) was carried out analogously to Example
11.
Extractive work-up from ethyl acetate, followed by purification on silica gel
column eluting with
CH2CI2/ ethyl acetate afforded Intermediate 240(a) (2.47 g) in 74% yield.
' H NMR (d6-DMSO): fi 11.39 (s, IH), 10.39 (s, IH), 8.25 (s, 1 H), 7.88 (s,
IH), 7.47 (s, IH), 7.10-
7.40 (m, 5H), 6.86 (s, 1 H), 3.89 (s, 3H), 2.33-2.48 (m, 1 H), 2.04-2.21, (m,
1 H), 1.46-1.63 (m, 1 H),
1.29-1.45 (m, 1 H).
LCMS (M+H): 335.1.
Step 2. Preparation of Methyl 1-(2-methoxy-2-oxoethyl)-6-{[(2-
phenylcyclopropyl)carbonyl]amino}-1H-indole-4-carboxylate 240(b)
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
246
Intermediate 240(a) (2.40 g, 7.2 mmol), methyl bromoacetate (1.32 g, 8.6 mmol)
and K2C03 (2.0
g, 14.4 mmol) were mixed in N,N-dimethylformamide (25 mL) and stirred at 80 C
for 3 hours.
After filtration the filtrate was evaporated, and the residue was subjected to
silica gel
chromatography eluting with CH2CI2/ethyl acetate to afford Intermediate 240(b)
(2.31 g) in 79 %
yield.
' H NMR (d6-DMSO): S 10.50 (s, 1 H), 8.09 (s, 1 H), 7.95 (s, 1 H), 7.48 (m, 1
H), 7.10-7.38 (m, 5H),
6.90 (m, 1 H), 5.16 (s, 2H), 3.91 (s, 3H), 3.70 (s, 3H), 2.30-2.44 (m, 1 H),
2.03-2.21 (m, 1 H), 1.44-
1.58 (m, 1 H), 1.30-1.43 (m, 1 H).
LCMS (M+H): 407.1.
Step 3. Preparation of Methyl 1-(2-hydroxyethyl)-6-{[(2-
phenylcyclopropyl)carbonyl]amino}-
1H-indole-4-carboxylate 240(c)
To Intermediate 240(b) (2.0 g, 49 mmol) in 1:1 ethanol:tetrahydrofuran (80 mL)
was added NaBH4
(2.0 g, 53 mmol). The mixture was stirred at room temperature overnight.
Purification by silica gel
chromatography afforded Intermediate 240(c) (0.85 g) in 46% yield.
'H NMR (d6-DMSO): S 10.45 (s, 1 H), 8.27 (s, 1 H), 7.89 (d, 1 H, J = 1.7 Hz),
7.49 (d, 1 H, J= 1.5
Hz), 7.15-7.35 (m, 5H), 6.87 (d, 1 H, J= 3.0 Hz), 4.95 (t, 1 H. J= 5.2 Hz),
4.20 (t, 2H, J= 5.7 Hz),
3.91 (s, 3H), 3.60-3.80 (m, 2H), 2.33-2.46 (m, 1 H), 2.05-2.18 (m, 1 H), 1.43-
1.65 (m, 1 H), 1.30-
1.43 (m, 1 H).
LCMS (M+H): 379.1.
Step 4. Preparation of Title Compound: (1,2-trans)-N-[1-(2-Hydroxyethyl)-6-oxo-
5,6-dihydro-
1 H-[1,2]diazepino[4,5,6-cd]indol-8-yl]-2-phenylcyclopropanecarboxamide
With stirring, Intermediate 240(c) in N,N-dimethylformamide (7 ml) was added
to a mixture of
POCI3 (0.6 ml) and N,N-dimethylformamide (5 mL) at 0 C. After 1 hour, the
reaction was
quenched with water and extracted with ethyl acetate. The organic phase was
dried with Na2SO4
and filtered. After evaporation of the volatile components, the residue was
mixed with methanol
(50 ml) containing hydrazine (1 mL) and refluxed for 5 hours. After the
mixture was cooled to
ambient temperature, and the resulting yellow precipitate was collected by
filtration and washed
with methanol to afford the title compound (0.37 g) in 43% yield.
'H NMR (d6-DMSO): S 10.41 (s, 1 H), 010.25 (s, 1 H), 8.12 (d, 1 H, J = 1.6
Hz), 7.55 (s, 2H), 7.45
(s, 1 H), 7.10-7.33 (m, 5H), 4.12 (t, 2H, J = 5.1 Hz), 3.67 (t, 2H, J = 5.2
Hz), 2.29-2.40 (m, 1 H),
1.99-2.10 (m, 1 H), 1.41-1.52 (m, 1 H), 1.28-1.39 (m, 1 H).
HRMS calculated for C22H21N503 389.1614 (M+H), found 389.1627.
Example 241 =(1 R 2R -enyl-cyclopropanecarboxylic acid (2-hydroxymethyl-6-oxo-
5.6-
dihydro-1 H-f 1 2-cd]diazepino[4.5,6Jindol-8-yl)-amide
CA 02512683 2005-07-06
WO 2004/063198 PCT/IB2004/000026
247
HN-N
O ~
Intermediate 230(a), 1. NaBH4, MeOH ~ OH
Example 230 01- ~ ~
BocHN / N
H
241(a)
1. 4N HCI (Dioxane) HN-N
0 ~
CH2CI2 O I~ OH
\
2. HATU, Et3N, DMF, 10% ~ =`, N / N
H H
O
0'''J~OH
Step 1. Preparation of (2-Hydroxymethyl-6-oxo-5,6-dihydro-lH-
[1,2]diazepino[4,5,6-
cal]indol-8-yl)-carbamic acid tert-butyl ester 241(a)
A solution of intermediate 230(a) of Example 230 (26 mg, 0.079 mmol) in
methanol (2 mL) was
treated with NaBH4 (6 mg, 0.16 mmol) at 0 C for 15 min. The solution was
quenched with
saturated aqueous NH4CI and diluted with ethyl acetate. After extraction, the
organic phase was
washed with brine, dried (Na2SO4), filtered, and concentrated. Silica gel
chromatography of the
residue (3:1 ethyl acetate:hexane) gave intermediate 241(a) (13 mg) in 50%
yield.
Step 2. Preparation of Title compound: (IR, 2R)-2-Phenyl-
cyclopropanecarboxylic acid (2-
hydroxymethyl-6-oxo-5,6-dihydro-1 H-[1,2-cd]diazepino[4,5,6]indol-8-yl)-amide
To a suspension of Intermediate 241(a) (76 mg, 0.23 mmol) in CH2CI2 (2 mL) was
added 4M HCI
in dioxane (2 mL, 8 mmol). After stirring at room temperature for 2 hours, the
mixture was
concentrated and the residue was dissolved in N,N-dimethylformamide (2 mL).
(1R,2R)-2-Phenyl-
cyclopropanecarboxylic acid (33.6 mg, 0.21 mmol), triethylamine (0.1 mL, 0.69
mmol), and 0-(7-
azabenzotriazol-1-yl)-N,N,N;N=tetramethyluronium hexafluorophosphate (131 mg,
0.345 mmol)
were added. After stirring at room temperature for 2 hours, the mixture was
concentrated and the
residue was subjected to preparative HPLC in a manner analogous to Example
146, step 2. The
title compound was obtained as a pale yellow powder (9 mg, 0.024 mmol) in 10%
yield.
'H NMR (d6-DMSO): S 11.79 (s, 1 H), 10.36 (s, 1 H), 10.19 (s, 1 H), 8.05 (d, 1
H, J= 1.88 Hz), 7.57
(s, 1 H), 7.56 (s, 1 H), 7.32-7.19 (m, 5H), 5.70 (t, 1 H, J = 4.0 Hz), 4.70
(d, 2H, J = 5.4 Hz), 2.35 (m,
1 H), 2.07 (m, 1 H), 1.50 (m, 1 H), 1.36 (m, 1 H).
LCMS: (M+H+) 375.2
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.