Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02516147 2005-08-17
TITLE: PROCESS FOR PREPARING CERTAIN HYDROHALIDE METAL
COMPLEX COMPOUNDS HAVING A SPECIFIC COARSE STRUCTURE
BACKGROUND
The present invention relates to a process for
preparing a granular hydrohalide salt of a particular
metal complex compound which is composed of a divalent
metal cation as central ion and of an amino
dicarboxylic acid ion and, where appropriate, water as
ligand, where the hydrohalide salt is obtained with a
specific particle size distribution.
Complexes of a divalent metal cation as central ion and
of an amino dicarboxylic acid ligand, and the
hydrohalides thereof and various processes for
preparing them are known. Complexes of a divalent metal
cation as central ion and of an amino dicarboxylic acid
ligand, for example magnesium L-aspartate can be
handled substantially without problems. In contrast
thereto, the hydrohalides thereof, especially magnesium
L-aspartate hydrochloride, are usually very
hygroscopic, so that they can be prepared only at
extremely low humidity. Magnesium L-aspartate
hydrochloride liquefies even at a humidity of more than
50%, making its further processing extremely difficult
or even impossible. Further processing to tablets or
granules is accordingly possible only in air-
conditioned zones with controlled low humidity. Because
of the extreme hygroscopicity, it has been possible to
date to granulate in particular magnesium L-aspartate
hydrochloride only using organic solvents, which is
undesired for environmental reasons.
In order to solve the problem of the hygroscopicity of
the hydrohalides described above, attempts have been
made to prepare a granular product with a reduced total
1
CA 02516147 2008-12-17
surface area. All attempts made to date to prepare a
granular product by spray drying have, however,
provided an inadequate, usually very finely powdered
material which, because of the large surface area or
the high proportion of very finely powdered material,
rapidly assumes a honey-like consistency, making
further processing impossible. For example, a very
finely powdered material is obtained under the spray-
drying conditions (air inlet temperature: about 180 C;
air outlet temperature: about 120 C) indicated in
DE 32 38 118 Al (cf. Examples 1 to 5 of
DE 32 38 118 Al).
SUI4MARY
The present invention is thus based on the technical
object of providing hydrohalides of complex compounds
which are composed of a divalent metal cation as
central ion and of an amino dicarboxylic acid ion and,
where appropriate, water as ligand, and which are
intended to have good flow and dissolving properties
and reduced hygroscopicity.
This object is achieved by providing the embodiments
characterized in the claims.
According to an aspect of the present invention is a
process for preparing a granular hydrohalide of a
complex compound which is composed of a divalent metal
cation as central ion and of an amino dicarboxylic acid
ion and, where appropriate, water as ligand is provided
and comprises the steps:
(a) preparation of an aqueous solution of the
hydrohalide of the complex compound,
(b) spray drying of the aqueous solution obtained in
step (a) at an air inlet temperature of from 300
to 350 C and at an air outlet temperature of from
100 to 140 C and with a spraying pressure of from 3
to 5 bar.
2
CA 02516147 2008-12-17
According to another aspect of the present invention is a
process for preparing a granular hydrohalide of a complex
compound which is composed of a divalent metal cation as
central ion and of an amino dicarboxylic acid ion and,
where appropriate, water as ligand comprising the steps:
(a) preparation of an aqueous solution of the
hydrohalide of the complex compound, by reacting an amino
dicarboxylic acid with a hydroxide, oxide and/or
carbonate of the metal (M) in aqueous solution or
suspension with a halide of the metal (M) and/or
hydrohalic acid; (b) spray drying of the aqueous
solution obtained in step (a) at a air inlet temperature
of from 300 to 350 C and at an air outlet temperature of
from 100 to 140 C and with a spraying pressure of from 3
to 5 bar, and wherein the freshly sprayed particles after
step (b) are passed through a fluidized bed to reduce the
residual moisture, and are subjected where appropriate
subsequently to a sieving step.
2a
CA 02516147 2005-08-17
BRIEF DESCRIPTION OF THE DRAWINGS
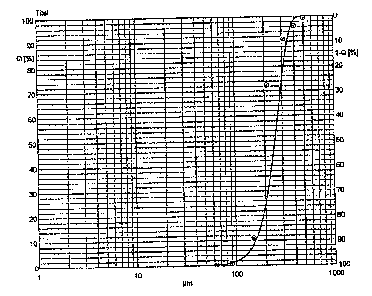
Fig. 1 is a graph showing the particle size
distribution of finely powdered magnesium L-aspartate
hydrochloride prepared by a standard process disclosed
in DE 32 38 118 Al.
Fig. 2 is a graph showing the particle size
distribution of granular magnesium L-aspartate
hydrochloride prepared by the process according to the
invention.
Fig. 3 a graphical representation of the measurements
shown in Table 5.
DETAILED DESCRIPTION
The hydrohalides of the complex compounds prepared
according to the invention can be prepared in various
ways. For example, they can be prepared by mixing
equimolar amounts of a metal salt of an amino
dicarboxylic acid (with a divalent metal such as, for
example, magnesium) and an appropriate metal halide in
aqueous solution. However, it is preferred to prepare
the hydrohalides of the complex compounds by reacting
an amino dicarboxylic acid with a hydroxide, oxide
and/or carbonate of the metal (M) in aqueous solution
and further reacting the resulting aqueous solution or
suspension with a halide of the metal (M) and/or
hydrohalic acid. The latter preparation process can
start from more favourable and easily available
starting compounds, making the overall process more
economic.
It is particularly preferred to mix an aqueous solution
or suspension of 2 mol of the particular amino
dicarboxylic acid with an aqueous solution or
suspension of 1 mol of the appropriate metal oxide,
hydroxide and/or carbonate and with an aqueous solution
3
CA 02516147 2005-08-17
or suspension of 1 mol of the appropriate metal halicte,
and to stir until a clear solution is obtained. Instead
of the metal halide it is also possible to use an
equimolar amount of a hydrohalic acid and of a metal
oxide, hydroxide and/or carbonate. The mixing
preferably takes place in a temperature range from
about 20 to about 90 C, or in the case of an exothermic
reaction at slightly elevated temperature until a clear
solution is obtained, which can be purified by
filtration. It may be advantageous in some cases
firstly to mix the solution or suspension of the amino
dicarboxylic acid with the metal oxide, hydroxide or
carbonate as solid, solution or suspension and only
then, when a clear solution has been obtained, to add
the solution of the metal halide.
The hydrohalide of the complex compound is preferably a
compound characterized by formula (I) below:
Hal 0
11
0 ~ (CHz)~
(H20) m ~, MZ; CH
-0. +
C
11 NH3
0
Formula (I)
in which M2+ is a divalent metal cation, Hal" is a
halide ion such as fluoride, chloride, bromide or
iodide, n is 1 or 2 and m is 0 to 10, preferably 0, 1,
2 or 3.
The amino dicarboxylic acids which can be used are
subject to no particular restrictions as long as they
are able to form a chelate with a divalent metal cation
such as, for example, magnesium, calcium or iron. A
skilled person is able to find a large number of
4
CA 02516147 2005-08-17
suitable substituted or unsubstitutea amino
dicarboxylic acids able to form a stable complex with a
divalent metal cation. It is particularly preferred to
use L-glutamic acid or L-aspartic acid as amino-
dicarboxylic acid in the process according to the
irivention.
The metal (M) which is present in the hydrohalide of
the complex compound and which represents the central
cation of the complex can in principle be any divalent
metal cation. The metal (M) is preferably an alkaline
earth metal, in particular magnesium, calcium or
strontium, or a heavy metal, in particular zinc, iron,
manganese, cobalt, copper or cadmium. In the complex
obtained according to the invention, the divalent metal
cation is complexed as central ion by the bidentate
amino dicarboxylic acid ligand to form a chelate
complex. Depending on the central ion and amino
dicarboxylic acid ligand, varying amounts of water may
be bound, normally up to about 10 molecules per metal
cation. The hydrohalide results through protonation of
the amino group of the amino dicarboxylic acid ligand,
the counter ion which is bound being a halide ion such
as fluoride, chloride, bromide or iodide.
In a preferred embodiment of the present invention, M
in the above formula (I) is magnesium, calcium or iron,
n is 1 or 2, Hal is chlorine and m is 0, 1, 2 or 3. The
compound of the formula (I) is in a particularly
preferred embodiment an alkaline earth metal
L-aspartate hydrohalide or an alkaline earth metal
L-glutamate hydrohalide, in particular magnesium
L-aspartate hydrochloride or magnesium L-glutamate
hydrochloride, or a hydrate thereof. The use of
magnesium L-aspartate hydrochloride in particular in
the process according to the invention results in an
excellently processable product having a specifically
5
CA 02516147 2005-08-17
coarse structure and a narrow parcicie sl:6e
distribution.
In the process according to the invention, it is
essential for achieving the advantageous coarse
structure of the hydrohalide of the complex compound
that the air inlet temperature and the air outlet
temperature in the spray-drying step (b) are controlled
in a targeted manner in combination with a suitable
spraying pressure in order to obtain granules with a
specifically narrow particle size distribution, good
flow and dissolving properties and a reduced
hygroscopicity. It has surprisingly been found that
with an air inlet temperature in a range from 300 to
350 C and with an air outlet temperature in a range
from 100 to 140 C it is possible to obtain a stable
granular product with excellent further processability
when the aqueous solution is sprayed or atomized with a
spraying pressure in a range from about 3 to about
5 bar. The exact temperature within these ranges
depends on the hydrohalide to be subjected to the spray
drying. However, a skilled person is capable of
accurate setting within the above ranges. It is
furthermore surprising in this connection that, despite
the use of a comparatively high air inlet temperature,
a coarse product is obtained.
The aqueous solution in step (a) can be sprayed or
atomized into the top or bottom of a spray-drying
tower. The gas used for drying, preferably air, can be
passed cocurrently or countercurrently to the sprayed
aqueous solution. However, it is preferred for the
sprayed aqueous solution, which is atomized to fine
droplets, to be sprayed into the top of a spray-drying
tower, and for the gas used for drying to be passed
cocurrently thereto, i.e. from the top to the bottom.
Subsequent to the air outlet it is possible to provide
6
CA 02516147 2005-08-17
a cyclone and/or a filter in order to separaLe a Line
powdery material.
The spraying pressure in step (b) of the process
according to the invention is in a range from about
3 bar to about 5 bar. The spraying pressure corresponds
to the liquid inlet pressure of the nozzles. The
atomization of the solution prepared in step (a)
normally takes place through a single fluid nozzle or
hollow cone nozzle which generates a hollow cone of
liquid at the outlet from the nozzle, resulting in
uniform droplets with a narrow droplet size
distribution.
In a preferred embodiment of the process according to
the invention, the freshly sprayed particles pass
immediately after the spray-drying step through a
fluidized bed in order for example to reduce the
residual moisture. When the freshly sprayed particles
impinge on the fluidized bed particles there is
formation of a granular product according to the
invention with excellent properties, which is
subsequently discharged from the spray tower,
preferably over a weir. A further possibility is to
provide a subsequent sieving step. It is particularly
preferred to provide a spray tower with integrated
fluidized bed. The air inlet temperature to the
fluidized bed is preferably in a range from 110 to
130 C, with the temperature of the product in the
fluidized bed preferably being adjusted to about 100 to
125 C. The height of the fluidized bed is not in
principle subject to special restrictions but is
preferably set at from 15 cm to 30 cm. The setting of
the holdup time of the product in the spray drier is
within the routine judgement of a skilled person and
can be determined in particular by adjusting the height
of the wheel and the material throughput. The material
7
CA 02516147 2005-08-17
throughput in the spray-drying step is in a range Lrum
50 kg to 200 kg per hour, a preferred throughput being
from 70 kg to 130 kg per hour. The volume of the spray
drier is subject to no particular restrictions.
However, it is preferably in a range from about 5 to
20 m3, with a volume of about 8 m3 being used most often
for economic reasons.
A product with a very favourable apparent volume can be
achieved in the process according to the invention for
preparing the granular hydrohalide of the complex
compound of relevance here. The apparent volume [volume
of the uncompressed product (ml)/100 g of the product]
is preferably in a range from 150 to 180 ml/100 g, with
a particularly preferred apparent volume being about
170 ml/100 g.
The concentration of the aqueous solution in step (a)
is subject in principle to no particular restrictions.
However, it is preferred to adjust the concentration of
the aqueous solution in step (a) to from 0.5 to
3 mol/1, preferably 1 mol/l to 2 mol/l, particularly
preferably to about 1.3 mol/l to 1.5 mol/l.
The present invention further relates to the granules
of a hydrohalide of a complex compound which is
composed of a divalent metal cation as central ion and
of an amino dicarboxylic acid ion and, where
appropriate, water as ligand, where 5 10% of the
particles have a particle size of < 50 m and 5 10% of
the particles have a particle size. of > 400 m,
obtained by the process according to the invention
described above.
It is particularly preferred for _ 70%, even more
preferred ? 80%, of the particles, with preference
? 85% of the particles, of the granules to have a
8
CA 02516147 2005-08-17
particle size in a range from about 100 m to about
315 m. It is further particularly preferred for _ 50%
of the particles, with preference >_ 55% of the
particles, of the granules to have a particle size in a
range from about 140 m to about 250 m. As stated
above, the apparent volume [volume of the uncompressed
product (ml)/100 g of the product] of such granules is
preferably in a range from 150 to 180 ml/100 g, with a
particularly preferred apparent volume being about
170 ml/100 g.
The present invention additionally relates to the use
of the granules, prepared by the process according to
the invention, of a hydrohalide of a magnesium-
containing complex compound in magnesium therapy and as
addition to animal feed. The granules obtained
according to the invention of such hydrohalides of the
magnesium complex compounds of relevance here are
valuable pharmaceuticals and additions to animal feed.
Thus, for example, magnesium L-aspartate hydrochloride
is employed for targeted magnesium therapy and also as
addition to animal feed and also as mineral supplement
for productive livestock. The compounds can be employed
in solid granular form or in aqueous solution.
The following examples are indicated in order to
explain the invention in more detail without
restricting it thereby.
Examples
Example 1
Preparation of magnesium L-aspartate hydrochloride
836 kg of L-aspartic acid are added with stirring to
1753 1 of demineralized water. 130 kg of magnesium
oxide as powder are added to the resulting dispersion,
and the mixture is heated to 60 C with stirring. Then,
9
CA 02516147 2005-08-17
while stirring, 628 g oz magnesium CnlULlUC 11=ly-1.2-U112-
are added, and the mixture is heated with stirring at
60 C for 2 h. The solution is subsequently filtered at
60 C and spray dried with an air inlet temperature of
about 320 C and an air outlet temperature of about
1200C with a spraying pressure of about 4 bar in a Niro
spray drier.
The final product obtained is a magnesium L-aspartate
hydrochloride having the following particle size
distribution: < 100 m (6.19%), 100-140 m (19.48%),
140-250 m (57.84%), 250-315 m (10.81%), 315-400 m
(4.78%) and 400-500 m (0.90%) . The apparent volume of
this final product was 170m1/100 g.
Example 2
Preparation of magnesium L-glutamate hydrochloride
936 1 of demineralized water are heated to 60 C, after
which 484 kg of L-glutamic acid are added with
stirring. 67 kg of magnesium oxide are added as powder
in portions to the resulting dispersion while stirring
continuously. The temperature rises, and the solution
becomes clear after about 1 h. A solution of 480 kg of
magnesium bromide hexahydrate and 384 1 of water (35%
strength solution) is added to this solution. The
concentration of the complex is adjusted to 30% by
using water. The solution is filtered and then spray
dried under the conditions described in Example 1.
This results in magnesium L-glutamate hydrochloride as
a white powder in 100% yield.
Example 3
Preparation of calcium L-aspartate hydrochloride
1010 1 of demineralized water are heated to 60 C, after
CA 02516147 2005-08-17
which 509 kg of L-aspartic acid are aaaea Wi111C
stirring. 142 kg of calcium hydroxide are added in the
form of a powder in portions to the resulting
dispersion while stirring continuously. The temperature
rises further and the solution becomes clear after
about 1 h. A solution of 281 kg of calcium chloride
dihydrate in 325 1 of water (35% strength solution) is
added to this solution. The concentration based on the
complex is adjusted to 30% with water. This is followed
by filtration and spray drying in the manner described
iri Example 1.
This results in calcium L-aspartate hydrochloride in
the form of a white powder in 100% yield.
Example 4
Preparation of zinc L-aspartate hydrochloride
1025 1 of demineralized water are heated to 60 C, after
which 464 kg of L-aspartic acid are added while
stirring. 142 kg of zinc oxide in the form of a powder
are added in portions to the resulting dispersion with
continuous stirring. The temperature rises somewhat,
but the solution does not become clear. The temperature
is therefore raised to about 90 C, after which a clear
solution is obtained. The concentration is adjusted to
30% by adding water.
A solution of 238 kg of zinc chloride in 441 1 of water
(35% strength solution) is added to the resulting
solution. The concentration based on the complex is
adjusted to 30% with water. This is followed by
filtration and spray drying in the manner described in
Example 1. This results in zinc L-aspartate
hydrochloride in the form of a white powder in 100%
yield.
11
CA 02516147 2008-12-17
Example 5
Preparation of magnesium L-aspartate hydrochloride
541 kg of L-aspartic acid are dispersed in 1016 1 of
demineralized water by stirring with heating to 60 C.
592 kg of a 25% by weight hydrochloric acid and then
164 kg of magnesium oxide as powder are added to this
dispersion and stirred. After a clear solution has been
obtained, it is filtered and spray dried in the manner
described in Example 1, resulting. in magnesium
L-aspartate hydrochloride in the form of a white powder
in 100% yield.
Example 6 (particle size distribution)
The particle size distribution of granular magnesium
L-aspartate hydrochloride prepared by the process
according to the invention was compared with a finely
powdered magnesium L-aspartate hydrochloride prepared
by a standard process (disclosed in DE 32 38 118 A1).
The comparative sieve analyses were performed using a
Hosokawa Alpine air jet sieve, and the results are
shown in Tables 1 and 2 below.12
CA 02516147 2005-08-17
~ O O O
b 00 00 N
y~ r1 r-I ~--I
u O O O
Ow O 00 co CN
0 ~ U1 I- O
a a M m in
Ln ,r LO
4J O f"1 %D
z O N N
V tIl N ~
0
H
_ O M a' w
O Cl1
b ~ N ~
ro
41
ri O O O O
r-i
ro
H
a w 00 N
1 O ~
r-I oM O Ln
..~ ~..{
^O o d~ N C9
a ui 0; w
ow 0 rn
fA co to N
tfl M lfl =
Lfl N r-1 ~
0
O O
r{
40 N M O LO
U kO arn
v
~4
0 O ~-I N M
z
CA 02516147 2005-08-17
The minimum particle size (dmin) was 0.7 m, tne maximum
particle size (dmax)was 260.0 m and the average
particle size (d50) was 54.9 m. Figure 1 shows the
graphical representation of the particle size
distribution of finely powdered magnesium L-aspartate
hydrochloride prepared by a standard process disclosed
iri DE 32 38 118 Al.
14
CA 02516147 2005-08-17
0 0 0 0 0 0
O O N N N N 0
1J ri r~ r~ ri ~-1 r~
O O 0 0 0 O
CO 00 N N N N O
a 0
rl ri ri r~ ri r-1 ~ . .
41
0
43
H N m O O O O
a X r-I ri r1 N N N N
4J
m O Q1 ~0 L1l GO ~ al d~
O 0~ Ol 4 M
=
d
$4
ZLn V w 14 1-4 O
a~
41 N
O m o rn~ ui %D rn-:r
43 O o; rn cf+ r~ ~, a
Ln Ln
a
.r.,
b
0
~4 ~Jl O 0 0 0 O 0 0 0
U H
rt
W
O
a a, - 00 N O l0 N a0 W
I i O
0 ~- oW O Ol 0~ oD N O~ (7 O
q o cv co O ct+ a0 N N
~ a o 0 O O r N O O 01
fa r~ I- m 01 41
H
N lp N C~ d' d~ O 00
~
o~ 0 O ~ ~ ~ tfl M O
I0000.O.cØ
N 0 0 N t!1 O O
lfl r-I rl O O
~ u k0 O% rl N M d' Lfl
~
0 0 r=1 N Md' Ltl l0 Il
z
L,
CA 02516147 2005-08-17
The minimum particle size (amin) was 4ts. u m, UiuC
maximum particle size (d,,,,,.)was 448.7 m and the average
particle size (d50) was 250.8 m. Figure 2 shows the
graphical representation of the particle size
distribution of granular magnesium L-aspartate
hydrochloride prepared by the process according to the
invention.
Example 7 (flow time and flowability)
The flowability of granular magnesium L-aspartate
hydrochloride prepared by the process according to the
invention was compared with a finely powdered magnesium
L-aspartate hydrochloride prepared by a standard
process (disclosed in DE 32 38 118 A1). The test
arrangement consisted of a conical flow funnel with a
height of 22.5 cm, an upper internal diameter of 7.3 cm
and a lower internal diameter of 8.2 mm. The test was
carried out by the 2.9.16 Method, Ph. Eur., 4th edition,
main volume 2002, as follows. 100 g portions of
material were put into the funnel. Opening of the
funnel orifice was followed by assessment of the
flowability, and the time for the whole sample to flow
out of the funnel was determined. Two samples were
measured in each case. The results are shown in Table 3
below.
Table 3 (flowability)
Preparation Standard Standard According According
process to the to the
invention invention
Flowability uneven, uneven, free free
not free- not free- flowing flowing
flowing flowing
Flow time Cannot be cannot be 10 sec 10 sec
determined determined
16
CA 02516147 2005-08-17
Example 8 (dissolving properties)
The dissolving properties of granular magnesium
L-aspartate hydrochloride prepared by the process
according to the invention were compared with a finely
powdered magnesium L-aspartate hydrochloride prepared
by a standard process (disclosed in DE 32 38 118 A1).
The test arrangement consisted of a glass beaker with
stirring bar which was adjusted to 20 revolutions per
min. 250 ml of water were introduced and then 10 g of
test material were added with the stirrer motor
running. The time until dissolution was complete was
measured. The results are shown in Table 4 below.
Table 4 (dissolving properties)
Preparation Standard Standard According According
process to the to the
invention invention
Dissolving 7 min 7 min 3 min 2 min
time
Example 9 (water uptake capacity or hygroscopicity)
The hygroscopicity of granular magnesium L-aspartate
hydrochloride prepared by the process according to the
invention was compared with a finely powdered magnesium
L-aspartate hydrochloride prepared by a standard
process (disclosed in DE 32 38 118 A1). The test
arrangement consisted of a desiccator which was
adjusted to a relative humidity of 75% with a saturated
sodium chloride solution. In each case a defined amount
of sample was weighed out and the sample dish was
placed in the desiccator. The percentage increase in
weight of the weighed amount was determined after 2, 4,
5 and 20 hours. The results are shown in Table S below,
from which it is unambiguously evident that the
hygroscopicity of the granules according to the
17
CA 02516147 2005-08-17
invention is distinctly reducect comparea wirn a 1111e1y
powdered product according to the prior art. Figure 3
shows a graphical representation of the measurements
shown in Table 5.
Table 5 (hygroscopicity)
Testing Standard Standard According According
time to the to the
invention invention
2 h 0.35 0.30 0.18 0.16
4 h 0.63 0.55 0.33 0.30
5 h 0.72 0.71 0.43 0.40
20 h 3.06 2.70 1.74 1.63
18