Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02519650 2005-09-15
INCORPORATING RAPID COOLING IN TISSUE FUSION HEATING
PROCESSES
=
BACKGROUND
Technical Field
The present disclosure relates to electrosurgical instruments
used for open and endoscopic surgical procedures for sealing or fusing tissue.
More particularly, the present disclosure relates to a bipolar forceps for
sealing
vessels, vascular tissues and soft tissues having an electrode sealing
assembly which is designed to limit and/or reduce by rapid cooling thermal
spread to adjacent tissue structures.
Related Prior Art
Electrosurgical forceps utilize both mechanical clamping action
and electrical energy to effect hemostasis by heating the tissue and blood
vessels to coagulate and/or cauterize vessels or tissue. However, certain
surgical procedures may require sealing blood vessels or vascular tissue
rather
than just simply effecting hemostasis. "Vessel sealing' or "Tissue Fusion" is
defined as the process of liquefying the collagen, elastin and ground
substances in the tissue so that it reforms into a fused mass with
significantly
-
1
CA 02519650 2005-09-15
reduced demarcation between the opposing tissue structures. In contrast, the
term "cauterization" is defined as the use of heat to destroy tissue (also
called
"diathermy" or "electrodiathermy") and the term "coagulation" is defined as a
process of desiccating tissue wherein the tissue cells are ruptured and dried.
Coagulation of small vessels is usually sufficient to permanently close them.
Larger vessels or tissue need to be "sealed" to assure permanent closure.
Numerous electrosurgical instruments have been proposed in the
past for various open and endoscopic surgical procedures. However, most of
these instruments cauterize or coagulate tissue and are normally not designed
to provide uniformly reproducible pressure on the blood vessel or tissue
which,
if used for sealing purposes, would result in an ineffective or non-uniform
seal.
For example, U.S. Patent No. 2,176,479 to Willis, U.S. Patent Nos. 4,005,714
and 4,031,898 to Hiltebrandt, U.S. Patent Nos. 5,827,274, 5,290,287 and
5,312,433 to Boebel et al., U.S. Patent Nos. 4,370,980, 4,552,143, 5,026,370
and 5,116,332 to Lottick, U.S. Patent No. 5,443,463 to Stern et al., U.S.
Patent No. 5,484,436 to Eggers et al. and U.S. Patent No. 5,951,549 to
Richardson et al., all relate to electrosurgical instruments for coagulating,
cauterizing, and cutting vessels or tissue.
Many of these instruments include blade members or shearing
members which simply cut tissue in a mechanical and/or electromechanical
manner and are relatively ineffective for vessel sealing purposes. Other
instruments generally rely on clamping pressure alone to procure proper
sealing thickness and are often not designed to take into account gap
tolerances and/or parallelism and flatness requirements which are parameters
which, if properly controlled, can assure a consistent and effective tissue
seal.
For example, it is known that it is difficult to adequately control thickness
of the
resulting sealed tissue by controlling clamping pressure alone for either of
two
reasons: 1) if too much force is applied, there is a possibility that the two
poles
will touch and energy will not be transferred through the tissue resulting in
an
2
CA 02519650 2013-05-27
ineffective seal; or 2) if too low a force is applied, a thicker less reliable
seal is
created.
Commonly-owned U.S. Patent Publication US 2004/0082952 by
Dycus, et al. entitled "VESSEL SEALER AND DIVIDER", U.S. Patent
Publication US 2003/0014053 by Tetzlaff, et al. entitled "VESSEL
SEALING INSTRUMENT" and U.S. Patent Publication US 2004/0162557
by Tetzlaff et al. entitled "VESSEL SEALING INSTRUMENT" teach
that to effectively seal tissue or vessels, especially large vessels, two
predominant mechanical parameters must be accurately controlled: 1) the
pressure applied to the vessel; and 2) the gap distance between the conductive
tissue contacting surfaces (electrodes). As can be appreciated, both of these
parameters are affected by the thickness of the vessel or tissue being sealed.
Accurate application of pressure is important for several reasons: to reduce
the tissue impedance to a low enough value that allows enough electrosurgical
energy through the tissue; to overcome the forces of expansion during tissue
heating; and to contribute to the end tissue thickness which is an indication
of a
good seal.
It has been found that using electrosurgical instruments to seal
tissue may result in some degree of so-called "thermal spread" across adjacent
tissue structures. "Thermal spread" refers generally to the heat transfer
traveling along the periphery of the electrically conductive surfaces. This
can
also be termed 'collateral damage" to adjacent tissue. As can be appreciated,
reducing the thermal spread during an electrical procedure reduces the
likelihood of unintentional or undesirable collateral damage to surrounding
tissue structures which are adjacent to an intended treatment site. Reducing
the collateral damage to surrounding tissue or maintaining the viability of
surrounding tissue after the sealing process is known to promote tissue
healing
and decrease overall healing time by stimulating / improving healing response.
Controlling tissue cooling may also reduce adhesion or buildup of tissue on
the
3
CA 02519650 2013-05-27
electrodes and also assist during the formation of the tissue seal, e.g.,
cross-
linking or other chemical bonding, during the reformation or renaturation of
collagen.
Instruments which include dielectric coatings disposed on the
outer surfaces are known and are used to prevent tissue "blanching" at points
normal to the sealing site. In other words, these coatings are primarily
designed to reduce accidental burning of tissue as a result of incidental
contact
with the outer surfaces of the end effectors. So far as is known, these
coatings
are not designed or intended to reduce collateral tissue damage or thermal
spread to adjacent tissue (tissue lying along the tissue plane).
Commonly-owned U.S. Patent Publication US 2005/0021025
entitled "ELECTROSURGICAL INSTRUMENT WHICH REDUCES
COLLATERAL DAMAGE TO ADJACENT TISSUE" by Buysse et al.
relates to an instrument which is configured to control or regulate the
electrical
field around the electrically conductive sealing surfaces to reduce stray
current
concentrations which can result in thermal spread to adjacent tissue
structures.
Thus, a need exists to develop an electrosurgical instrument
which includes an electrode sealing assembly which can seal vessels and
tissue consistently and effectively and reduce the undesirable effects of
thermal spread across or to adjacent tissue structures by utilizing a
thermally
conductive, electrically non-conductive material.
In addition, in tissue fusion applications that utilize energy to treat
tissue, the need exists to maximize and enhance tissue strength at the tissue
fusion site and minimize detrimental tissue effects to adjacent or surrounding
tissue structures.
4
CA 02519650 2005-09-15
SUMMARY
It is an object of the present disclosure to provide an electrode
sealing assembly designed for use with an electrosurgical instrument for
sealing tissue which rapidly cools during or after tissue fusion heating
processes.
The present disclosure generally relates to an electrode sealing
assembly for use with an electrosurgical instrument for sealing tissue. The
electrode sealing assembly includes first and second jaw members which are
movable from a first position in spaced relation relative to one another to at
least one second position for grasping tissue therebetween. The jaw members
include electrically conductive sealing plates disposed in opposing relation
to
one another. At least one jaw member includes a thermoelectric cooling plate
having a first surface in direct contact with an outer surface of the sealing
plate.
The thermoelectric cooling plate include first and second electrical
connections
disposed on opposite sides of the thermoelectric cooling plate. The first
connection is configured to selectively transmit a first electrical potential
and
the second connection is configured to selectively transmit a second
electrical
potential such that heat generated by the sealing plates is transferred away
from the tissue via the thermoelectric cooling plate.
The heat sink may be configured to be coupled to an ultimate
heat sink for transferring heat from the jaw member(s). The heat sink may
include a coolant line disposed therethrough. The coolant line may be
configured to receive a coolant to transfer heat from the thermoelectric
cooling
plate. In one embodiment, the coolant is a thermally conductive, non-
electrically conductive fluid which may be one of the group consisting of air,
nitrogen, carbon dioxide, and 3MTm Fluorinerrm Electronic Liquid FC-7
(available from 3M Company, St. Paul, Minnesota).
In one particularly useful embodiment, the present disclosure
relates to an electrode sealing assembly designed for use with an
CA 02519650 2005-09-15
electrosurgical instrument for sealing tissue. The electrode sealing assembly
includes first and second electrode jaw members which are movable from a
first position in spaced relation relative to one another to at least one
second
position for grasping tissue therebetween. The jaw members include sealing
plates disposed in opposing relation relative to one another. Each jaw member
includes a cooling line disposed therethrough which is configured to convey a
cooling liquid therethrough to absorb heat from the sealing plates during or
after sealing.
The cooling line may be configured to be coupled to a second or
an ultimate heat sink for transferring heat from the jaw member(s). In
addition,
the coolant line may be configured to receive a coolant to transfer heat from
the jaw member(s). In one embodiment, the coolant is a thermally conductive,
non-electrically conductive fluid.
In another particularly useful embodiment, the present disclosure
relates to an electrode sealing assembly designed for use with an
electrosurgical instrument for sealing tissue, which includes: first and
second
jaw members being movable from a first position in spaced relation relative to
one another to at least one second position for grasping tissue therebetween.
Each of the jaw members includes: an insulating housing having at least one
electromechanical interface; and an electrically conductive sealing plate
having
at least one corresponding electromechanical interface which mates with the
electromechanical interface of the insulating housing. The insulating housing
has a coolant duct disposed therethrough which is configured to transport a
coolant to the insulating housing to dissipate heat away from surrounding
tissue.
In another embodiment, the coolant duct is configured to
transport the coolant through one or more nozzle(s) disposed on an upper
surface of the insulating housing. The nozzle(s) are configured to discharge
the coolant to an environment proximate the electrode sealing assembly. In
6
CA 02519650 2005-09-15
another embodiment, the coolant duct is configured to transport the coolant
through the insulating housing to an ultimate heat sink.
BRIEF DESCRIPTION OF THE DRAWINGS
Various embodiments of the subject instrument are described
herein with reference to the drawings wherein:
FIG. 1A is a perspective view of an endoscopic bipolar forceps
which is configured to support an electrode sealing assembly according to the
present disclosure;
FIG. 1B is a perspective view of an open bipolar forceps which is
configured to support the electrode sealing assembly according to the present
disclosure;
FIG. 2A is an enlarged, perspective view of the electrode sealing
assembly according to the present invention;
FIG. 2B is an enlarged, perspective view of the embodiment
shown in FIG. 2A with parts separated;
FIG. 3 is an enlarged, perspective view of an alternate, simplified
embodiment of the electrode sealing assembly with parts separated according
to the present disclosure;
FIG. 4 is an enlarged, perspective view of an alternate
embodiment of the electrode sealing assembly showing an active cooling
system designed to reduce thermal spread during activation;
FIG. 5A is an enlarged view of a seal utilizing a conventional
vessel sealing instrument with a conventional electrode sealing assembly;
7
CA 02519650 2005-09-15
FIG. 5B is an enlarged view of a seal utilizing a vessel sealing
instrument having the electrode sealing assembly according the present
disclosure;
FIG. 6 is a schematic, end view of an alternate electrode sealing
assembly which may be utilized to reduce thermal spread during activation;
FIG. 7 is a schematic, end view of another alternate electrode
sealing assembly which may be utilized to reduce thermal spread during
activation;
FIG. 8A shows a perspective view of a sealed tissue area of an
end-to-end anastomosis utilizing a straight electrode sealing assembly
according to the present disclosure;
FIG. 8B shows a perspective view of a sealed tissue area of an
end-to-end anastomosis utilizing a curved electrode sealing assembly
according to the present disclosure;
FIG. 9A shows an end view of the jaw members of an electrode
sealing assembly which are configured to support an alternate embodiment of
an electrode cooling assembly according to the present disclosure;
FIG. 9B shows a perspective view of the jaw members according
to FIG. 9A;
FIG. 9C shows a top perspective view of the jaw members of an
electrode sealing assembly which are configured to support still another
embodiment of an electrode cooling assembly according to the present
disclosure;
8
CA 02519650 2005-09-15
FIG. 9D shows a bottom perspective view of the jaw members
according to FIG. 9C.
FIG. 10A shows an end view of jaw members of an electrode
sealing assembly which are configured to support yet another alternate
embodiment of an electrode cooling assembly according to the present
disclosure;
FIG. 10B shows a perspective view of the jaw members
according to FIG. 10A;
FIG. 11 shows a perspective view of the jaw members of an
electrode sealing assembly which are configured to support yet another
alternate embodiment of an electrode cooling assembly according to the
present disclosure;
FIG. 12 is an enlarged, perspective view of yet another alternate
embodiment of the electrode sealing assembly of FIG. 4 showing an active
cooling system designed to reduce thermal spread during activation;
FIG. 13A is a cross-sectional end view of an embodiment of a
cooling line for an electrode cooling assembly;
FIG. 13B is a cross-sectional end view of an alternate
embodiment of a cooling line for an electrode cooling assembly;
FIG. 14A is a perspective view of the endoscopic bipolar forceps
of FIG. 1A which is configured to support the cooling lines of FIG. 4, FIG.
10A,
FIG. 10B, FIG. 11, and FIG. 12; and
9
CA 02519650 2013-05-27
FIG. 14B is a perspective view of the open bipolar forceps of FIG.
1B which is configured to support the cooling lines of FIG. 4, FIG. 10A, FIG.
10B, FIG. 11, and FIG. 12.
DETAILED DESCRIPTION
It has been found that by providing a thermally conductive and
electrically non-conductive material adjacent to the electrically conductive
sealing surfaces, surgeons can more readily and more easily produce a
consistent, high quality seal and effectively reduce thermal spread across or
to
adjacent tissue. For the purposes herein the term "thermal spread" refers
generally to the heat transfer (heat conduction, heat convection or electrical
current dissipation) dissipating along the periphery of the electrically
conductive
or electrically active surfaces to adjacent tissue. This can also be
termed
"collateral damage" to adjacent tissue.
It is envisioned that the configuration of the thermally conductive
material which surrounds the perimeter of the electrically conductive surface
will effectively absorb heat during electrosurgical activation (or thermally
dissipate the heat during electrosurgical activation) and generally restrict
heat
travel to areas between the opposing electrically conductive surfaces. In
other
words, the material acts like a so called "heat sink". As mentioned above, the
thermally conductive material is also electrically non-conductive which also
restricts current concentrations to between the two opposing surfaces.
It is important to note that this is different from dielectrically
coating the outer surfaces of the instrument to prevent tissue "blanching" at
points normal to the sealing site. These coatings are not designed or intended
CA 02519650 2005-09-15
to reduce collateral tissue damage or thermal spread to adjacent tissue
(tissue
lying along the tissue sealing plane).
It is contemplated that by providing a thermally conductive
material adjacent to the electrically conductive surface, the thermally
conductive path is altered thereby influencing the thermal spread/collateral
damage to adjacent tissue structures. In addition, the thermally conductive,
electrically non-conductive material also isolates the two electrically
opposing
poles (i.e., electrodes) from one another thereby reducing the possibility
that
tissue or tissue fluids can create an unintended bridge or path for current
travel
to adjacent tissue. The thermally
conductive material and electrically
conductive sealing surface may be dimensioned such that the current is
concentrated at the intended sealing site between the opposing electrically
conductive surfaces as explained in more detail below.
It is contemplated that by providing additional cooling of the
electrosurgical jaw members of the bipolar forceps such as by solid state
cooling via thermoelectric coolers (TEC) based on the Peltier effect, the
thermal spread/collateral damage to adjacent tissue structures may also be
further reduced. It is further contemplated that additional cooling may be
provided to the electrosurgical jaw members via a cooling duct passing
internally through the jaw members.
Referring now to FIGS. 1A and 18, two bipolar forceps 10 and 10'
are shown; a first forceps 10 for use with endoscopic surgical procedures and
a
second forceps 10' for use with open surgical procedures. For the purposes
herein, either an endoscopic instrument or an open instrument may be utilized
for supporting the electrode sealing assembly according to the present
disclosure. Obviously, different electrical and mechanical connections and
considerations apply to each particular type of instrument, however, the novel
aspects with respect to the electrode sealing assembly and its operating
characteristics remain generally consistent with respect to both the open or
11
CA 02519650 2005-09-15
endoscopic designs of FIGS. 1A and 1B. Forceps 10 and 10' are shown by
way of example and other electrosurgical forceps are also envisioned which
may support the electrode sealing assembly of the present disclosure. In the
drawings and in the description which follows, the term "proximal", as is
traditional, will refer to the end of the forceps 10, 10' which is closer to
the user,
while the term "distal" will refer to the end which is further from the user.
FIG. 1A shows one example of an endoscopic vessel sealing
instrument 10 which is configured to support an electrode sealing assembly
100. More particularly, forceps 10 generally includes a housing 20, a handle
assembly 30, a rotating assembly 80, a trigger assembly 70 and the end
effector assembly 100 which mutually cooperate to grasp, seal and, if
warranted, divide tissue. The forceps 10 includes a shaft 12 which has a
distal
end 14 dimensioned to mechanically engage the end effector assembly 100
and a proximal end 16 which mechanically engages the housing 20 proximate
the rotating assembly 80.
Forceps 10 also includes a plug 300 which connects the forceps
to a source of electrosurgical energy, e.g., an electrosurgical generator (not
shown) via an electrical cable 310. Handle assembly 30 includes a fixed handle
50 and a movable handle 40. Handle 40 moves relative to fixed handle 50 to
actuate the end effector assembly 100 and enable a user to grasp and
manipulate tissue 400 (See FIG. 6). More particularly, the end effector
assembly 100 includes a pair of opposing jaw members 110 and 120 which
move in response to movement of the handle 40 from an open position wherein
the jaw members 110 and 120 are disposed in spaced relation relative to one
another, to a clamping or closed position wherein the jaw members 110 and
120 cooperate to grasp tissue therebetween.
The housing 20 encloses a drive assembly (not shown) which
cooperates with the movable handle 40 to impart movement of the jaw
members 110 and 120 from the open position to the clamping or closed
12
CA 02519650 2013-05-27
position. The handle assembly 30 can generally be characterized as a four-bar
mechanical linkage which provides a unique mechanical advantage when
sealing tissue between the jaw members 110 and 120. For example, once the
desired position for the sealing site is determined and the jaw members 110
and 120 are properly positioned, handle 40 may be compressed fully to lock
the jaw members 110 and 120 in a closed position against the tissue. The
details relating to the inter-cooperative relationships of the inner-working
components of forceps 10 are disclosed in commonly-owned U.S. Patent
Publication US 2003/0199869 and U.S. Patent Publication US 2004/0254573.
When the jaw members 110 and 120 are fully compressed about the tissue the
forceps 10 is now ready for selective application of electrosurgical energy.
Experimental results suggest that the magnitude of pressure
exerted on the tissue by the electrically conductive sealing surfaces 112, 122
of
the jaw members 110 and 120, respectively, is important in assuring a proper
surgical seal. Pressures within a working range of about 3 kg/cm2 to about 16
kg/cm2 and, preferably, within a working range of about 6 kg/cm2 to about 13
kg/cm2 have been shown to be effective for sealing various tissue types. Most
preferably, the pressures are within a working range of about 4.5 kg/cm2 to
about 8.5 kg/cm2 to optimize sealing.
An open forceps 10' for use in connection with traditional open
surgical procedures and is shown by way of example in FIG. 1B. Open forceps
10' includes a pair of elongated shaft portions 12a', 12b' each having a
proximal end 16a and 16b', respectively, and a distal end 14a' and 14b',
respectively. The forceps 10' includes jaw assembly 100' which attaches to the
distal ends 14a' and 14b' of shafts 12a' and 12b', respectively. Jaw assembly
100' includes an upper jaw member 110' and a lower jaw member 120' which
are movable relative to one another to grasp tissue therebetween.
13
CA 02519650 2013-05-27
Each shaft 12a' and 12b' may include a handle 17a' and 17b'
disposed at the proximal end 16a' and 16b' thereof which each define a finger
hole 18a' and 18b', respectively, therethrough for receiving a finger of the
user.
As can be appreciated, finger holes 1Ba' and 18b' facilitate movement of the
shafts 12a' and 12b' relative to one another which, in turn, pivot the jaw
members 110' and 120' from the open position wherein the jaw members 110'
and 120' are disposed in spaced relation relative to one another for
manipulating tissue to a clamping or closed position wherein the jaw members
110' and 120' cooperate to grasp tissue therebetween.
A ratchet 30' is included for selectively locking the jaw members
110' and 120' relative to one another at various positions during pivoting.
Preferably, each position associated with the cooperating ratchet interfaces
30'
holds a specific, i.e., constant, strain energy in the shaft members 12a' and
12b' which, in turn, transmits a specific closing force to the jaw members
110'
and 120'. It is envisioned that the ratchet 30' may include graduations or
other
visual markings which enable the user to easily and quickly ascertain and
control the amount of closure force desired between the jaw members 110' and
120'. One of the shafts, e.g., 12b', includes a proximal shaft connector
/flange
19' which is designed to connect the forceps 10' to a source of RF energy (not
shown) via an electrosurgical cable 310 and plug 300. The details relating to
the inner-working electrical connections and various components of forceps 10'
are disclosed in commonly owned U.S. Patent Publication US 2003/0229344.
As mentioned above, two mechanical factors play an important
role in determining the resulting thickness of the sealed tissue and
effectiveness of the seal, i.e., the pressure applied between opposing jaw
members 110' and 120' and the gap between the opposing jaw members 110'
and 120' during the sealing process. Applying the correct force is also
important for other reasons: to reduce the impedance of the tissue to a low
enough value that allows enough current through the tissue; and to overcome
14
CA 02519650 2005-09-15
the forces of expansion during the heating of the tissue in addition to
contributing towards creating the required seal thickness necessary for a good
seal.
For the purposes herein, electrode assemblies 100 and 100'
include the same general configuration and are designed to reduce thermal
spread to adjacent tissue. However, certain modifications may have to be
made to each electrode sealing assembly 100 (or 100') to fit the electrode
sealing assembly 100 (or 100') to a specific support structure for an open or
endoscopic instrument. By controlling the intensity, frequency and duration of
the RF energy applied to the tissue, the user can selectively seal the tissue
as
needed for a particular purpose. As can be appreciated, different tissue types
and the physical characteristics associated with each tissue type may require
different electrical sealing parameters.
=
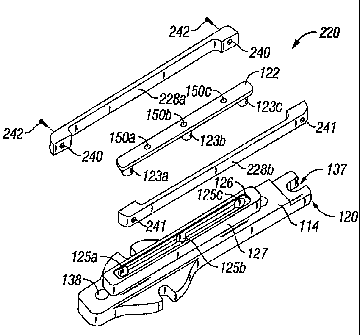
FIGS. 2A and 2B show enlarged views of the lower jaw 120 of the
electrode sealing assembly 100 (or 100') according to the present disclosure.
As can be appreciated a second jaw 110 with similar components as described
below is positioned in opposition to jaw member 120. Only the elements of jaw
member 120 are described herein, however, jaw member 110 also includes
identical or similar elements which are designed to accomplish similar
purposes
such that bipolar electrosurgical energy can be conducted through tissue held
between the two jaw members 110 and 120 to effect a seal.
More particularly, lower jaw member 120 includes an insulated
outer housing 114 which supports a thermally conductive, electrically non-
conductive material 128 and electrically conductive sealing surface or sealing
plate 122. As best seen in FIG. 2B, insulating housing 114 includes a support
surface 115 which houses an electrode support step 127. Support step 127
includes a series of electro-mechanical interfaces 125a, 125b and 125c which
matingly engage a set of corresponding interfaces 123a, 123b and 123c which
depend from sealing plate 122. The outer periphery of the support step 127 is
CA 02519650 2005-09-15
also preferably dimensioned to matingly engage the thermally conductive
material 128 as will be explained in more detail below.
Each electromechanical interface, e.g., 125a, is electrically
connected to an electrical potential by way of wire 160 which extends to the
generator (not shown). It is envisioned that other electrical configurations
are
plausible as is known in the art and the above is shown by way of example. For
example, electrically conductive tubes or plates may be utilized within the
jaw
members 110 and 120 to supply current to the sealing plate 122.
Support surface 115 also includes a series of notches 137, 121a,
121b and screw holes 138 which secure the insulating housing 114 to the
electrode sealing assembly 100. For example, and as best shown in FIG. 2A,
the support surface 115 includes a pair of flanges 139a and 139b which project
laterally from the distal end of the support surface 115 and which are each
dimensioned to receive the head of a screw 135a and 135b, respectively. In
turn, the screws 135a and 135b secure the support surface to the electrode
sealing assembly 100. A proximal notch 137 mates with another screw (not
shown) to position the end of the support surface 115 on the electrode sealing
assembly 100. Other apertures, e.g., 138, may also be utilized to align and/or
secure the support surface 115 on the electrode sealing assembly 100 during
the manufacturing process.
Thermally conductive material 128 is may be made from two
laterally-opposing segments 128a and 128b which mate to encompass the
sealing plate 122 and the support step 127 as best seen in FIG. 2A. A series
of set screws or pegs 142 secure the two thermally conductive segments 128a
and 128b about the sealing plate 122 and about the support step 127 once
assembled. As mentioned above, the thermally conductive material 128 is
designed to effectively absorb or thermally dissipate the heat during
electrosurgical activation and generally restrict heat travel to areas between
the
16
CA 02519650 2005-09-15
opposing sealing plates 122. In other words, the material acts like a "heat
sink"
to limit thermal damage to surrounding tissue.
As mentioned above, the thermally conductive material 128 is
also electrically non-conductive which also restricts current concentrations
to
between the two opposing sealing plates 122. The thermally conductive
material 128 may be made from a material having a high thermal conductivity
value or "k" value and minimum electrical conductively, e.g., anodized
aluminum. Alternatively, the thermally conductive material 128 may also be
made from or combined with a semi-resilient or elastomeric material so as not
to inflict mechanical damage to the tissue during compression. Mechanical
damage may also be diminished by minimizing the overall tissue contact area
of the thermally conductive material 128 (See, e.g., FIG. 3). Alternatively, a
spring loaded system (not shown) designed to apply pressures below critical
tissue pressure limits may be employed to reduce mechanical damage of the
tissue when under compression.
Other compression-reducing systems are also envisioned to
avoid over-compression of tissue adjacent the sealing plates 122 and between
the opposing thermally conductive materials 128, e.g., rubber-like inserts,
foam
or the like. Other examples of
thermally conductive and electrically non-
conductive materials which can be utilized to minimize thermal damage to
surrounding tissue include, but are not limited to: thermally conductive
plastic
materials which dissipate heat along a preferred isothermal profile to the
surrounding environment resulting in a lower maximum temperature and
reduced formation of hot spots. Examples of such materials are commonly
sold under the trademark CoolPoly by Cool Polymers, Inc., of Rhode Island
and composite materials such as AL02.
As mentioned above, the thermally conductive material 128
includes two segments 128a and 128b which mate about the sealing plate 122
and the support step 127. More particularly, each segment 128a and 128b
17
CA 02519650 2005-09-15
includes a tissue contacting surface 143a and 143b with a recessed portion
129a and 129b, respectively, along an inner peripheral edge of the tissue
contacting surface 143a and 143b such that, once the two segments 128a and
128b are assembled they form a slot 141 for seating the sealing plate .122
therein. The sealing plate 122 is typically seated to lie generally flush with
or
below the tissue contacting surfaces 143a, 143b of the thermally conductive
segments 128a and 128b. It is also envisioned that the thickness (or height
relative to the insulating housing 114) of the thermally conductive material
128
proximate the recessed portions 129a, 129b is about equal to the height of the
step 127 plus the thickness of the sealing plate 122 such that, once
assembled, the sealing plate 122 and the thermally conductive material 128 lie
substantially flush or below within the sealing plane.
The thermally conductive segments 128a and 128b may also
include a series of fin-like extensions 145a, 145b, 145c and 146a, 146b, 146c,
respectively, which extend laterally therefrom. It is envisioned that the fin-
like
extensions 145a, 145b, 145c and 146a, 146b, 146c further absorb or dissipate
heat emanating from the sealing plates 122 during or after activation. The
fins
145a, 145b, 145c and 146a, 146b, 146c may also be shaped and dimensioned
to facilitate manufacturing and assembly, i.e., the fins 145a, 145b, 145c and
146a, 146b, 146c may be shaped to include slots 132 therein which allow
passage of one or more screws 135a, 135b which attach the insulating housing
114 to the underlying electrode sealing assembly 100.
As mentioned above, the sealing plate 122 is electromechanically
connected to the underlying insulating housing 114 by virtue of a series of
electro-mechanical interfaces 123a, 123b and 123c which project outwardly
therefrom to mate with a series of corresponding electromechanical interfaces
125a, 125b and 125c. It is envisioned that the electromechanical interfacing
elements 123a, 123b, 123c and 125a, 125b, 125c maintain electrical continuity
from the insulating housing 114 to the sealing plate 122. As mentioned above,
once assembled and interfaced with the insulating housing 114, the thermally
18
CA 02519650 2005-09-15
conductive material 128 encapsulates and further secures the sealing plate 122
atop the insulating housing 114.
A series of stop members 150a, 150b and 150c may be disposed
on the tissue contacting surfaces or the inner-facing surfaces of the
electrically
conductive sealing plates 122 (and/or the opposite sealing plate 112 (See FIG.
1A) on jaw member 110) to facilitate gripping and manipulation of tissue and
to
define a gap distance between opposing jaw members 110 and 120 (or 110'
and 120') during sealing. In order to achieve a desired spacing between the
electrically conductive plates 112, 122 of the respective jaw members 110,
120,
(i.e., gap distance) and apply the required force to properly seal tissue, at
least
one jaw member 110 or 120 includes at least one stop member or stop
members, e.g., 150a, 150b and 150c, which limit the movement of the two
opposing jaw members 110 and 120 relative to one another. The stop
members, e.g., 150a, extends from the sealing plate or tissue contacting
surface 122 a predetermined distance according to the specific material
properties of the stop member 150a (e.g., compressive strength, thermal
expansion, etc.) to yield a consistent and accurate gap distance during
sealing.
The gap distance between opposing sealing surfaces 112, 122 (and the sealing
surface (not shown) of jaw member 110) during sealing preferably ranges from
about 0.001 inches to about 0.006 inches and, preferably, between about
0.002 inches and about 0.003 inches. For larger tissue structures such as
bowel, lung or intestine the gap distance ranges from about 0.001 inches to
about 0.012 inches and preferably from about 0.005 inches to about 0.007
inches.
Stop members 150a-150c are typically made from an insulative
material, e.g., parylene, nylon and/or ceramic. The stop members 150a-150c
can be disposed on one or both of the jaw members 110 and 120 and may be
dimensioned in a variety of different shapes and sizes, e.g., longitudinal,
circular, ridge-like, etc.
19
CA 02519650 2013-05-27
The non-conductive stop members 150a-150c are molded onto
the sealing plates 112 and 122 (e.g., overmolding, injection molding, etc.),
stamped onto the sealing plates 112 and 122, deposited (e.g., plasma
deposition) onto the sealing plates 112 and 122 and/or thermally sprayed onto
the surface of the sealing plates 112 and 122 (e.g., a ceramic material may be
thermally sprayed) to form the stop members 150a-150c. Many different
configurations for the stop members 150a-150c are discussed in detail in
commonly-assigned, co-pending U.S. Patent Publication US 2004/0122423
entitled "VESSEL SEALER AND DIVIDER WITH NON-CONDUCTIVE STOP
MEMBERS" by Dycus et al.
It is also envisioned that the thermally conductive material 128
may be dimensioned thicker than the height of step 127 and the thickness of
the sealing plate 122 such that the thermally conductive material 128 acts
like a
stop member for maintaining a gap distance between the sealing plates 122
during activation.
In addition to keeping the pressure within a working range (i.e.,
about 3 kg/cm2 to about 16 kg/cm2) and the gap distance within a specified
range (i.e., about 0.001 inches to about 0.012 inches for large tissue
structures) the electrical power should be kept within the range of about 1 W
to
about 350 W, about 1 Vrms to about 400 Vrms and about 0 Amps to about 5.5
Amps.
Thermal spread on each side of the sealing plates 122 is ideally
kept to less than about 2mm and preferably to less than about 0.5mm to
promote tissue healing. However, when sealing larger or well-vascularized
tissue structures, thermal spread is acceptable to about 5mm. It is envisioned
that maintaining the viability of tissue surrounding or adjacent the sealing
site or
fused tissue area will promote healing.
CA 02519650 2005-09-15
FIGS. 3 and 4 show alternate embodiments of lower jaw
members 220 and 320 of the electrode sealing assembly 100 which may be
utilized to reduce thermal spread to adjacent tissue during activation. More
particularly, FIG. 3 shows a lower jaw member 220 which includes the same
insulating housing 114 and sealing plate 122 configuration of FIGS. 2A and 2B.
The thermally conductive material 228 is modified to have a reduced width
which, as mentioned above, reduces the overall tissue contacting surface of
the thermally conductive material 128. It is envisioned that mechanical
damage may be diminished or at least maintained below critical tissue pressure
limits by minimizing the overall tissue contact area of the thermally
conductive
material 128. Much in the same fashion as described above with respect to
FIGS. 2A and 2B, the thermally conductive material 228 is secured about the
sealing plate 122 and the step 127 by a series of screws 242 which mate into
apertures 240 and 241 in segments 228a and 228b. As can be appreciated,
the overall required width of the thermally conductive material 228 may be
dependent upon type of tissue being sealed or the thickness of the tissue
being
sealed. Step 127 may include a reliefed portion 126 disposed therein which
seats or aligns the sealing plate 122 during assembly.
FIG. 4 shows yet another possible configuration of the lower jaw
member 320 of the electrode sealing assembly 100 (or 100') designed to
reduce thermal spread to adjacent tissue. In this embodiment, a thermally
conductive material is not utilized as the heat absorbing material or heat
sink,
but, rather, an active cooling system 340 surrounds the sealing plate 122 to
reduce heat dissipation to surrounding tissue. More particularly, insulating
housing 314 includes a series of ducts or tubes 355, 355a and 355b disposed
therethrough. The coolant ducts 355a, 355b are configured to transport a
coolant 370 to the insulating housing 314 to dissipate heat away from
surrounding tissue adjacent the sealing plates 122 to actively cool the tissue
during activation which reduces thermal spread.
21
CA 02519650 2005-09-15
The coolant ducts 355, 355a, 355b supply active cooling liquid
(preferably, non-electrically conductive cooling liquid) or gas (e.g., air)
370
through at least one of a series of nozzles or ports 350a and 350b disposed on
an upper surface 330 of the insulating housing 314. The nozzles or ports
350a and 350b are located immediately adjacent the sealing plate 122 and
extend longitudinally on opposite sides thereof, i.e., ports 350a extend along
one side of the sealing plate 122 and ports 350b extend along the opposite
side of the sealing plate 122. The nozzles or ports 350a and 350b are
configured to discharge the coolant 370 to an environment proximate the
electrode sealing assembly 100 (or 100').
As can be appreciated, the sealing system 340 supplies coolant
(liquid or gas (e.g., air)) 370 to the tissue areas adjacent the sealing
plates 122
to actively cool the tissue during activation which reduces thermal spread.
With respect to this particular embodiment and compared to the embodiments
of FIGS. 2A-3, the insulating housing 314 encapsulates the sealing plate 122
by virtue of a mechanical connection or manufacturing process, e.g. stamp
molding or injection molding.
FIGS. 5A and 5B show a side-by-side comparison of the resulting
tissue seals 420 and 420' utilizing a prior vessel sealing instrument (See
FIG.
5A) and a vessel sealing instrument designed to reduce thermal spread to
adjacent tissue 400 according to the present disclosure (See FIG. 5B). More
particularly and with respect to FIG. 5A, there is some notable thermal damage
430 to adjacent tissue 400 proximate the tissue seal 420. FIG. 58 shows the
resulting seal 420' utilizing one of the various electrode assemblies 100 (or
100') described herein. A more uniform and narrower seal 420' is evident with
a significant reduction of thermal damage 430' to adjacent tissue 400. It is
envisioned that reducing thermal damage to adjacent tissue 400 can improve
healing especially in sensitive tissue areas, e.g., small and large
intestines. As
mentioned above, the thermal spread is preferably kept to about 2mm with
22
CA 02519650 2005-09-15
sensitive large tissues and vessels and about 5mm with non-sensitive tissues
and vessels.
FIG. 6 shows an alternative electrode sealing assembly 500
which is also designed to reduce thermal spread to adjacent tissue. More
particularly, electrode sealing assembly 500 includes upper and lower jaws 510
and 520, respectively, which each include a thermally conductive, electrically
insulative material 530a and 530b, e.g., a so-called "cool polymer" material,
disposed on (or within) the respective tissue sealing plates, 512 and 522. The
cool polymers 530a, 530b may be centrally disposed within each sealing plate
512 and 522, respectively. It is envisioned that the cool polymers 530a and
530b will act as heat sinks (i.e., absorb heat) during activation which will
limit
the thermal spread to adjacent tissue 400. Examples of cool polymers include
thermally conductive plastic materials which dissipate heat in a more
isothermal
profile to the surrounding environment resulting in a lower maximum
temperature and reduced formation of hot spots such as materials commonly
sold under the trademark CoolPoly by Cool Polymers, Inc., of Rhode Island.
Alternatively, certain known ceramic materials may also be used to reduce
tissue effects.
FIG. 7 shows yet another electrode sealing assembly 600 which
is also designed to reduce thermal spread to adjacent tissue 400. More
particularly, electrode sealing assembly 600 includes upper and lower jaw
members 610 and 620, respectively which are designed to engage tissue 400
therebetween. Each of the jaw members 610 and 620 includes a recessed
portion 630 and 640, respectively which is dimensioned to allow bulging
portions 450a and 450b of the tissue 400 to bulge into each respective jaw
member 610 and 620 when the tissue 400 is under compression. It is
envisioned that the moisture in the less-compressed tissue bulges 450a and
450b essentially acts as a heat sink to absorb heat during activation and
reduce thermal spread to surrounding tissue.
23
CA 02519650 2013-05-27
It is envisioned that the jaw members 110 and 120 may be curved
in order to reach specific anatomical structures and promote more consistent
seals for certain procedures. For example, it is contemplated that
dimensioning the jaw members 110 and 120 at an angle of about 45 degrees
to about 70 degrees is preferred for accessing and sealing specific anatomical
structures relevant to prostatectomies and cystectomies, e.g., the dorsal vein
complex and the lateral pedicles. Other angles may be preferred for different
surgical procedures.
For example and as best shown in FIGS. 8A and 8B, it may be
preferable to use a curved jaw member (not shown) for an end-to-end
anastomosis of bowel tissues. FIG. 8A shows the resulting seal 420 of an
end-to-end anastomosis of two bowel segments 400a and 400b utilizing a
straight pair of jaw members. FIG. 8B shows a resulting seal 420' of an end-to-
end anastomosis of two bowel segments 400a' and 400b' utilizing a curved pair
of jaw members. As can be appreciated the resulting seal 420' from the curved
pair of jaw members tends to more closely conform to the general contours of
the two tissue segments 400a' and 400b. which is envisioned will promote
tissue healing around the anastomosis site.
It is also envisioned that the jaw members 110 and 120 may be
tapered which is advantageous for two reasons: 1) the taper will apply
constant
pressure for a constant tissue thickness at parallel; 2) the thicker proximal
portion of each jaw member 110 and 120 will resist bending due to the reaction
force of the tissue 400.
It is also envisioned that the above forceps 10 (or 10') may be
utilized in connection with a closed-loop RF control system which optimizes
sealing based upon pre-surgical conditions or changes in physical or
electrical
conditions during sealing. One example of a closed-loop, control system is
described in commonly-owned U.S. Patent Publication US 2004/0015163
entitled "METHOD AND SYSTEM FOR CONTROLLING
24
CA 02519650 2013-05-27
OUTPUT OF RF MEDICAL GENERATOR" and commonly-owned U.S. Patent
Publication US 2005/0004564 entitled "METHOD AND SYSTEM FOR
PROGRAMMING AND CONTROLLING AN ELECTROSURGICAL
GENERATOR SYSTEM". In general, the closed-loop control system
includes a user interface for allowing a user to select at least one pre-
surgical
parameter, such as the type of surgical instrument operatively connected to
the
generator, the type of tissue and/or a desired surgical effect. A sensor
module
is also included for continually sensing at least one of electrical and
physical
properties proximate the surgical site and generating at least one signal
relating
thereto.
The closed loop control system also includes a control module for
continually receiving or monitoring surgical parameters and each of the
signals
from the sensor module and processing each of the signals in accordance with
a desired surgical effect using a microprocessor, computer algorithm and/or a
look-up table. The control module generates at least one corresponding
control signal relating to each signal from the sensor module(s), and relays
the
control signal to the electrosurgical generator for controlling the generator.
The closed loop system may be employed in a feedback circuit or part of a
surgical method for optimizing a surgical seal. The method includes the steps
of: applying a series of electrical pulses to the surgical site; continually
sensing
electrical and physical properties proximate the surgical site; and varying
pulse
parameters of the individual pulses of the series of pulses in accordance with
the continually-sensed properties. Alternatively, the signal may be
continuous.
It is also contemplated that the sealing surfaces 122 of the jaw
members 110 and 120 can be made from or coated with non-stick materials to
reduce tissue adhesion. Alternatively, the jaw members 110 and 120 may be
surface treated, roughened, to reduce sticking, e.g., bead blasting, stamping.
When utilized on the sealing surfaces 122, these materials provide an optimal
surface energy for eliminating sticking due in part to surface texture and
CA 02519650 2005-09-15
susceptibility to surface breakdown due to electrical effects and corrosion in
the
presence of biologic tissues. It is envisioned that these materials exhibit
superior non-stick qualities over stainless steel and should be utilized on
the
forceps 10 (or 10') in areas where the exposure to pressure and RE energy can
create localized "hot spots" more susceptible to tissue adhesion. As can be
appreciated, reducing the amount that the tissue "sticks" during sealing
improves the overall efficacy of the instrument. Controlling tissue cooling
may
also reduce adhesion or buildup of tissue on the electrodes and also assist
during the formation of the tissue seal, e.g., cross-linking or other chemical
bonding, during the reformation or renaturation of collagen.
The non-stick materials may be manufactured from one (or a
combination of one or more) of the following "non-stick" materials: nickel-
chrome, chromium nitride, MedCoat 2000, Inconel 600, tin-nickel or various
nitride coatings which include, but are not limited to, TiN, ZrN, TiAIN and
CrN.
For example, high nickel chrome alloys, Ni200, Ni201 (-100% Ni) may be
made into electrodes or sealing surfaces by metal injection molding, stamping,
machining or any like process. Also and as mentioned above, the sealing
surfaces 122 may also be "coated" with one or more of the above materials to
achieve the same result, i.e., a "non-stick surface".
It is further envisioned that thermal spread may be reduced by
altering the physical dimensions of the insulating housing 114. For example,
in
some cases it may be preferable to manufacture the insulating housing 114
from a variety of materials (either alone or in combination) which include:
nylons and syndiotactic polystyrenes such as QUESTRA manufactured by
DOW Chemical; Polybutylene Terephthalate (PBT); Polycarbonate (PC);
Acrylonitrile Butadiene Styrene (ABS); Polyphthalamide (PPA); Polymide,
Polyethylene Terephthalate (PET); Polyamide-imide (PAI); Acrylic (PMMA);
Polystyrene (PS and HIPS); Polyether Sulfone (PES); Aliphatic Polyketone;
Acetal (POM) Copolymer; Polyurethane (PU and TPU); Nylon with
Polyphenylene-oxide dispersion; and Acrylonitrile Styrene Acrylate.
26
CA 02519650 2005-09-15
It is also contemplated that only one of the two jaw members 110
and 120 may include one of the aforedescribed mechanisms or configurations
for reducing thermal spread. For example and with reference to FIGS. 2A, 2B
and 3, it is contemplated that only the lower jaw member 120, 220 may include
the thermally conductive material 128, 228 disposed between the insulating
housing 114 and the sealing plate 122. With reference to FIG. 4, only the
lower jaw member 320 may include the active cooling system 340. With
reference to FIG. 6, only the top jaw member 510 may be configured to house
a cool polymer 530a for reducing thermal spread to adjacent tissue 400.
Likewise and with reference to FIG. 7, only the upper jaw member 610 may
include a recessed area 630 for receiving bulging tissue 450a. It is further
contemplated that the above configurations may be used in combination to
reduce thermal spread to adjacent tissue. For example, a cool polymer 530a
may be used in combination with the thermally conductive material 128 of FIG.
2A or used in replace of the thermally conductive material 128 of FIG. 2A
depending upon a particular purpose.
It is envisioned that the forceps 10 or 10' may be designed such
that it is fully or partially disposable depending upon a particular purpose
or to
achieve a particular result. For example, electrode sealing assembly 100 may
be selectively and releasably engageable with the distal end 14 of the shaft
12
and/or the proximal end 16 of shaft 12 may be selectively and releasably
engageable with the housing 20 and the handle assembly 30. In either of
these two instances, the forceps 10 would be considered "partially disposable"
or "reposable", i.e., a new or different electrode sealing assembly 100 (or
electrode sealing assembly 100 and shaft 12) selectively replaces the old jaw
assembly 110 as needed.
Another embodiment of an electrode cooling system for an
electrode assembly 700 according to the present disclosure is illustrated in
FIG. 9A. More particularly, FIG. 9A shows an end view of a distal end of
lower electrode jaw member 720 and a distal end of upper electrode jaw
27
CA 02519650 2005-09-15
member 710 of electrode assembly 700 adapted for use as a bipolar forceps
10. The upper electrode jaw member 710 includes upper electrically insulating
portions 711a, 711b joined at edges 713a, 713b to contact electrically
conductive seal plates 712a, 712b. The lower electrode jaw member 720
includes lower electrically insulating portions 721a, 721b joined at edges
723a,
723b to contact electrically conductive seal plates 722a, 722b. A knife
blade
702 is shown disposed within a knife slot 704 formed by inward lateral side
edges 706a and 706b of the electrically conductive seal plates 712a and 712b
and by inward lateral side edges 708a and 708b of the electrically conductive
seal plates 722a and 722b. The jaw members 710 and 720 have a generally
U-shaped cross-section with a generally flat central portion 710a, 710b, 720a,
720b, in the electrically conductive seal plates 712a, 712b, and 722a, 722b,
respectively.
During the tissue sealing process, heat Q is generated on inner
surface 727a, 727b in the generally flat central portion 710a, 710b of
electrically conductive seal plates 712a and 712b. Similarly, heat Q' is
generated on inner surface 729a, 729b in the generally flat central portion
720a, 720b of electrically conductive seal plates 722a and 722b..
At least one of the jaw members 710 and 720 includes a
thermoelectric plate such that heat generated by at least one of the jaw
members is transferred away from the tissue via the thermoelectric plate.
More particularly, a first surface 730 of an upper thermoelectric (TEC) plate
718 and an outer surface 714a, 714b of the upper electrically conductive seal
plates 712a, 712b in the generally flat central portion 710a, 710b have a
thermally conductive, electrically insulating material 780 disposed
therebetween. Correspondingly, a first surface 740 of a lower thermoelectric
(TEC) plate 728 and an outer surface 724a, 724b of the lower electrically
conductive seal plates 722a, 722b in the generally flat central portion 720a,
720b have a thermally conductive, electrically insulating material 782
disposed
there between
28
CA 02519650 2005-09-15
The heat Q generated on inner surface 727a, 727b of upper jaw
member 710 is transferred through the upper electrically conductive seal
plates 712a, 712b and through the thermally conductive, electrically
insulating
material 780 to the first surface 730 of the upper TEC plate 718 where the
heat Q is transferred to the TEC plate 718.
Similarly, the heat Q generated on inner surface 729a, 729b of
upper jaw member 720 is transferred through the lower electrically conductive
seal plates 722a, 722b and through the thermally conductive, electrically
insulating material 782 to the first surface 740 of the lower TEC plate 728
where the heat Q is transferred to the TEC plate 728.
It is contemplated that in most cases of electrosurgery, both of
the jaw members 710 and 720 would include their respective TEC plates 718
and 728 for cooling purposes. Furthermore, those skilled in the art will
recognize that TEC plates 718 and 728 may be alternatively referred to as
solid state heat pumps or Peltier coolers.
As shown in FIG. 9B, electrical lead 734a is connected to a
proximal end 749 of upper TEC plate 718, while electrical lead 734b is
connected to a distal end 750 of upper TEC plate 718. Similarly, electrical
lead
736a is connected to a proximal end 751 of lower TEC plate 728, while
electrical lead 736b is connected to a distal end 752 of lower TEC plate 728.
The leads 734a, 734b, 736a, 736b are routed through a conduit or cable 754 to
a direct current (DC) power supply 756. As noted previously, during the tissue
sealing process, heat 0 is generated on inner surface 727a, 727b in the
generally flat central portion 710a, 710b of upper seal plates 712a, 712b.
Similarly, heat Q' is generated on inner surface 729a, 729b in the generally
flat
central portion 720a, 720b of lower seal plate 722a, 722b.
The TEC plates 718 and 728 provide the capability of directing
this heat Q away from the inner surfaces 727a, 727b and 729a, 729b
29
CA 02519650 2005-09-15
depending upon direction of current flow through the electrical leads. In most
cases of electrosurgery, the TEC plates would be used for cooling rather than
heating. To achieve cooling, direction of current is controlled by the power
supply 756 and current is directed through the TEC plates 718 and 728 such
that the heat Q from the seal plates 712a, 712b, 722a, 722b is directed away
from the tissue and towards the opposite end of the TEC plates 718 and 728.
As can be appreciated, the heat Q generated during tissue sealing by the
electrodes 710 and 720 is transferred away from the tissue and is not
transmitted to surrounding tissue, thus reducing collateral damage to tissue.
The thermally conductive, electrically insulating materials 780, 782 may be
made of a cool polymer as described previously which prevents electrical
continuity between the DC power supply 756 and an AC power supply from
the previously discussed source of electrosurgical energy e.g., an
electrosurgical generator (not shown) via plug 300 and electrical cable 310
(see FIGS. 1A and 1B).
FIGS. 9C and 9D show one particularly useful embodiment
according to the present disclosure wherein TEC plate 718 is utilized to
dissipate heat from the jaw members 710 and 720 during tissue treatment.
More particularly, and with specific reference to jaw member 710, the jaw
member 710 includes upper electrically insulating portions 711a and 711b
joined at edges 713a, 713b to contact an electrically conductive seal plate
712.
TEC plate 718 is disposed within jaw member 710 on the opposite side 714' of
tissue engaging surface 714 of the electrically conductive sealing plate 712.
A
thermally conductive, electrically insulating material 784 is disposed between
the TEC sealing plate 718 and sealing plate 712 on outer surfaces 714a and
714b of the sealing plate 712. The plate 718 includes first and second sides
760 and 760', respectively. Side 760 abuts the opposite end 714' of sealing
plate 712. A series of electrical leads 765a, 765b, and 765c are connected to
the second side 760' while a series of electrical leads 766a, 766b, and 766c
are connected to the first side 760.
CA 02519650 2005-09-15
It is envisioned that a first electrical potential 758 may be
selectively transmitted through leads 765a, 765h and 765c and a second
electrical potential 759 may be selectively transmitted through leads 766a,
766b, and 766c such that different electrical potentials are created on
opposite
sides of the plate 718. As can be appreciated, heat Q in this instance may be
directed proximally for absorption by a second heat sink, e.g., cool polymer,
a
fluid through one or more ducts 854 disposed in contact with TEC plate 718, or
another TEC plate.
Jaw member 720 is configured in much the same manner and
includes similar elements for directing heat Q proximately. More particularly,
and with specific reference to jaw member 720, the jaw member 720 includes
lower electrically insulating portions 721a and 721b joined at edges 723a,
723b
to contact an electrically conductive seal plate 722. TEC plate 728 is
disposed
within jaw member 720 on the opposite side 724' of tissue engaging surface
724 of the electrically conductive sealing plate 722. A thermally conductive,
electrically insulating material 786 is disposed between the sealing plate 722
and the TEC plate 728 on outer surfaces 724a and 724b of the sealing plate
722. The plate 728 includes first and second sides 762 and 762', respectively.
Side 762 abuts the opposite end 724' of sealing plate 722. A series of
electrical leads 767a, 767b, and 767c are connected to the first side 762
while
a series of electrical leads 769a, 769b and 769c are connected to the second
side 762'.
The thermally conductive, electrically insulating materials 784,
786 may be made of a cool polymer as described previously which prevents
electrical continuity between the DC power supply 756 and an AC power
supply from the previously discussed source of electrosurgical energy.
It is envisioned that first electrical potential 758 may be selectively
transmitted through leads 767a, 767b and 767c and second electrical potential
759 may be selectively transmitted through leads 769a, 769b, and 796c such
31
CA 02519650 2005-09-15
that different electrical potentials are created on opposite sides of the
plate
728. As can be appreciated, heat Q' in this instance may be directed
proximally for absorption by a second heat sink, e.g., cool polymer, a fluid
through one or more ducts 856 disposed in contact with TEC plate 728,. or
another TEC plate. As can be appreciated, the two jaw members 710, 720
cooperate to remove excess heat from the tissue to reduce collateral tissue
effects during sealing.
FIG. 10A shows a proximal end of the electrode assembly 700
configured in one particularly useful embodiment for forced convection cooling
of the upper electrode jaw members 710 and lower electrode jaw members
120. FIG. 10A is in all respects identical to FIG. 9A except that electrode
assembly 700 is configured for forced convection cooling of the upper seal
plates 712a, 712b and lower seal plates 722a, 722b. More particularly, a heat
sink 818 is disposed in direct contact with a second surface 732 of
thermoelectric cooling plate 718. A coolant or cooling line 850 is disposed
through or embedded within heat sink 818. The coolant line 850 has a coolant
supply end 850a and a coolant return end 850b projecting from a proximal end
of the heat sink 818.
Similarly, a heat sink 828 is disposed in direct contact with a
second surface 742 of thermoelectric cooling plate 728. A coolant or cooling
line 852 is disposed through or embedded within heat sink 828. The coolant
line 852 has a coolant supply end 852a and a coolant return end 852b
projecting from a proximal end of the heat sink 828.
FIG. 10B shows a front perspective view of the electrode
assembly 700 of FIG. 10A as configured for forced convection cooling of the
upper seal plates 712a, 712b and lower seal plates 722a, 722b . More
particularly, the heat sink 818 is disposed in direct contact with the second
surface 732 of thermoelectric cooling plate 718. The coolant line 850 is
disposed through or embedded within heat sink 818. The coolant line 850 has
32
CA 02519650 2005-09-15
coolant supply end 850a and coolant return end 850b projecting from a
proximal end 838 of the heat sink 818. The coolant line 850 may form a U-
bend 850c proximate to a distal end 842 of heat sink 818.
Similarly, heat sink 828 is disposed in direct contact with the
second surface 742 of thermoelectric cooling plate 728. The coolant line 852
is disposed through or embedded within heat sink 828. The coolant line 852
has a coolant supply end (not shown) and a coolant return end (not shown)
projecting from a proximal end 840 of the heat sink 828. The coolant line 852
may form a U-bend 852c proximate to a distal end 844 of heat sink 828 in an
analogous manner as shown with respect to U-bend 850c of coolant line 850 in
heat sink 818.
In the foregoing embodiment, it is particularly suitable for the
coolant lines 850 and 852 to contain an active cooling fluid (e.g., a
thermally
conductive, non-electrically conductive cooling liquid or a gas, e.g., air).
In
particular, the cooling fluid may include a liquid coolant such as water or a
non-
conductive fluid such as a medicinal or biocompatible fluid. However, a gas
such as, but not limited to, air, nitrogen or carbon dioxide (preferably at
ambient or above ambient pressure conditions) may be applied under forced
flow conditions. Alternatively, coolant lines 850 and 852 may also be filled
with
a stagnant substance such as a below ambient temperature gas (including air,
nitrogen or carbon dioxide), or a liquid or solid or frozen substance such as
water ice or dry ice (solid carbon dioxide).
Coolant applied to coolant supply lines 850 and 852 removes the
heat Q generated during the tissue sealing process. As discussed in more
detail below with respect to FIGS. 14A and 14B, the heat sinks 818 and 828
may be configured to be coupled to an ultimate heat sink for transferring heat
from the jaw members 710 and 720. More particularly, via the coolant supply
ends 850a, 852a, the coolant or cooling lines 850 and 852 may be configured
to receive the coolant to transfer the heat from the respective thermoelectric
33
CA 02519650 2005-09-15
cooling plates 718 and 728. Furthermore, via the coolant return ends 850b,
852b, the coolant or cooling lines 850 and 852 may be configured to be
coupled to an ultimate heat sink via the forceps 10.
FIG. 11 shows yet another embodiment of an electrode cooling
system for an electrode assembly 900 according to the present disclosure.
More particularly, FIG. 11 shows a proximal end 938 of an upper electrode jaw
member 910 and a proximal end 940 of a lower electrode jaw member 920 of
electrode assembly 900 adapted to bipolar forceps 10. A knife blade 902 is
shown disposed within a knife slot 904 formed by the inward lateral side edges
906a and 906b of the upper jaw member 910 and by the inward lateral side
edges 908a and 908b of the lower jaw member 920. The jaw members 910
and 920 have a generally U-shaped cross-section.
At least one of the jaw members 910 and 920 includes a cooling
line disposed therethrough or embedded therein. More particularly, a coolant
or cooling line 950 may be disposed or embedded within upper electrode jaw
member 910. The coolant line 950 has a coolant supply end 950a and a
coolant return end 950b projecting from a proximal end 938 of the upper jaw
member 910. The coolant line 950 may form a U-bend 850c proximate to a
distal end 942 of upper jaw member 910.
Similarly, a coolant or cooling line 952 may be disposed or
embedded within lower electrode jaw member 920. The coolant line 952 has
a coolant supply end 952a and a coolant return end 952b projecting from a
proximal end 940 of the lower jaw member 920. The coolant line 952 may
form a U-bend 952c proximate to a distal end 944 of lower jaw member 920.
The coolant lines 950 and 952 may be configured to receive a
coolant to transfer heat from jaw members 910 and/or 920. In a similar
manner to the previous embodiment described above, it is particularly suitable
for the coolant received by the coolant lines 950 and 952 to be an active
34
CA 02519650 2005-09-15
cooling fluid (preferably, a non-electrically conductive cooling liquid or a
gas,
e.g., air).
Coolant applied to coolant supply lines 950 and 952 removes the
heat Q generated during the tissue sealing process. As discussed in more
detail below with respect to FIGS. 14A and 14B, the coolant supply ends 950a,
952a and coolant return ends 950b, 952b may be coupled to an ultimate heat
sink via the forceps 10.
FIG. 12 is an enlarged, perspective view of still another
embodiment of the electrode sealing assembly of FIG. 4. More particularly,
FIG. 12 shows yet another possible configuration of the lower jaw member 320
of the electrode sealing assembly 100 (or 100') designed to reduce thermal
spread to adjacent tissue. This embodiment is in all respects identical to the
embodiment disclosed by FIG. 4 except that open active cooling system 340
with a common supply line 355, which branches out into coolant lines 355a and
355b to supply coolant 370 through the series of nozzles or ports 350a and
350b located on an upper surface 330 of the insulating housing 314, is
replaced by closed active coolant system 1140 which includes a U-shaped
continuous coolant loop 1180 having a coolant supply end 1180a and a coolant
return end 1180b. The coolant supply loop 1180 is disposed through or
embedded within the insulating housing 314 surrounding the sealing plate 122.
The coolant loop 1180 is configured to receive the coolant 370, which is,
typically, a non-electrically conductive cooling liquid or gas (e.g., air)
such as
previously described. The active coolant 370 is caused to flow through the
coolant loop 1180 to reduce heat dissipation to surrounding tissue which is
generated by the tissue sealing process in sealing plate 122. As is the case
of
the embodiment of FIG. 4, a thermally conductive material is not utilized as
the
heat absorbing material or heat sink, but, rather, the active cooling system
1140 surrounds the sealing plate 122. As is discussed in more detail later
with
respect to FIGS. 14A and 14B, the coolant loop 1180 transports the coolant to
an ultimate heat sink for dissipating heat away from surrounding tissue.
CA 02519650 2005-09-15
With respect to this particular embodiment and compared to the
embodiments of FIGS. 2A, 2B, 3 and 4, again, the insulating housing 314
encapsulates the sealing plate 122 by virtue of a mechanical connection or
manufacturing process, e.g. stamp molding or injection molding.
FIG. 13A is a cross-sectional end view of one embodiment of
cooling loop 1180 for the electrode cooling assemblies of FIG. 12. More
particularly, the ends 1180a and 1180b of the cooling loop 1180 are joined
together in a common cooling line 1150. The common cooling line 1150
includes typically an inner tubular shaped conduit which can function as
either
supply line 1180a or return line 1180b, and an outer concentrically arranged
tubular shaped conduit which can function conversely as either return line
1180b or supply line 1180a, respectively.
FIG. 13B is a cross-sectional end view of an alternate
embodiment of a cooling line for the electrode assemblies of FIG. 12. More
particularly, in a similar manner to the embodiment of FIG. 13A, the ends
1180a and 1180b of the cooling loop 1180 are again joined in a common
cooling line designated as 1190. However, the common cooling line 1190
includes a generally tubular configuration which is segmented into two inner
flow channels 1192a and 1192b via a partition 1194. The inner flow channel
1192a can function as either supply line 1180a or return line 1180b, while
conversely, the inner flow channel 1192b can function as either return line
1180b or supply line 1180a, respectively.
Those skilled in the art will recognize that the coolant loops 850
and 852, and 950 and 952 (see FIGS. 10A, 106 and 11) may be configured in
an analogous manner as common cooling lines 1150 and 1190.
FIG. 14A is a perspective view of the endoscopic bipolar forceps
of FIG. 1A which is configured to support the common cooling lines 1150 and
1190 (see FIG. 12, FIG. 13A and FIG. 13B). More particularly, the forceps 10
36
CA 02519650 2005-09-15
includes the shaft 12 which has a distal end 14 dimensioned to mechanically
engage the end effector assembly 100 and a proximal end 16 which
mechanically engages the housing 20 proximate the rotating assembly 80.
The cooling line 1150, or 1190 extends from the upper and lower jaws, erg.,
jaw members 710, 720, 910, 920 through the shaft 12 and through the housing
20 at a port 1210 proximate the shaft 12 from which the cooling line 1150, or
1190 emerges at a port 1220 in the housing 20 proximate the electrosurgical
cable 310. Alternatively, the cooling line 1150, or 1190, may be configured to
bypass the housing 20 and only emerges from the shaft 12 at port 1210.
Typically, in either embodiment, the cooling line 1150 or 1190 is coiled
around
the electrosurgical cable 310 to a convenient point at which it is directed to
an
ultimate heat sink 1250. The cable 754 which provides DC power to the TEC
plates 718 and 728 as previously described extends from the TEC plates 718
and 728 through the shaft 12 and through the housing 20 from which cable 754
emerges at port 1220 (or a separate port) to connect to the DC power supply
756. It is contemplated that the forceps 10 described with respect to FIGS.
14A
and as follows in FIG. 14B may be utilized with any of the aforementioned end
effector assemblies and jaw members described herein.
More particularly, FIG. 14B is a perspective view of the open
bipolar forceps of FIG. 1B which is configured to support the cooling line of
FIG. 10, FIG. 11B and FIG. 11C. As disclosed previously with respect to FIG.
1B, open forceps 10' includes a pair of elongated shaft portions 12a', 12b'
each
having a proximal end 16a' and 16b', respectively, and a distal end 14a' and
14b', respectively. The forceps 10' includes jaw assembly 100' which attaches
to the distal ends 14a' and 14b' of shafts 12a' and 12b', respectively. Jaw
assembly 100' includes an upper jaw member 710' or 910' and a lower jaw
member 720' or 920' which are movable relative to one another to grasp tissue
therebetween. Those skilled in the art will recognize that upper jaw members
710' and 910' are substantially identical to upper jaw member 710 and 910,
respectively, except for being configured to adapt to the open forceps 10'.
Similarly, those skilled in the art will recognize that lower jaw members 720'
and
37
CA 02519650 2013-05-27
920' are substantially identical to upper jaw member 720 and 920,
respectively,
except for being configured to adapt to the open forceps 10'.
Each shaft 12a' and 12b' includes a handle 17a' and 17b'
disposed at the proximal end 16a' and 16b' thereof which each define a finger
hole 18a' and 18b', respectively, therethrough for receiving a finger of the
user.
As can be appreciated, finger holes 18a' and 18b' facilitate movement of the
shafts 12a' and 12b' relative to one another which, in turn, pivot the jaw
members 110' and 120' from the open position wherein the jaw members 110'
and 120' are disposed in spaced relation relative to one another for
manipulating tissue to a clamping or closed position wherein the jaw members
110' and 120' cooperate to grasp tissue therebetween.
One of the shafts, e.g., 12b', includes a proximal shaft connector
/flange 19' which is designed to connect the forceps 10' to a source of RF
energy (not shown) via an electrosurgical cable 310 and plug 300. Although
the details relating to the inner-working electrical connections and various
components of forceps 10' are disclosed in commonly-owned U.S.
Patent Publication US 2003/0229344, it is disclosed herein that
cooling line 1150 or 1190 and electrical cable
754 extends from the upper and lower jaw members 110' and 120' through the
shaft 12b' to the proximal shaft/connector flange 19' which interfaces with
electrosurgical cable 310. The cooling line 1150 or 1190 emerges from the
flange 19' at a port 1230 proximate the power cord 310. Typically, the cooling
line 1150 or 1190 is coiled around the electrosurgical cable 310 to a
convenient
point at which it is directed to the ultimate heat sink 1250. The electrical
cable
754 emerges at the port 1230 from which it extends to connect to DC power
supply 756.
From the foregoing and with reference to the various figure
drawings, those skilled in the art will appreciate that certain modifications
can
also be made to the present disclosure without departing from the scope of the
36
CA 02519650 2005-09-15
=
same. For example, although it is preferable that jaw members 110 and 120
meet in parallel opposition, and, therefore, meet on the same plane, in some
cases it may be preferable to slightly bias the jaw members 110 and 120 to
meet each other at the distal end such that additional closure force on .the
handles is required to deflect the electrodes in the same plane. It is
envisioned
that this could improve seal quality and/or consistency. Alternatively, the
jaws
members 110 and 120 may be configured to close in a heel-based manner or
in an independently floating (with respect to parallel) fashion.
It is envisioned that while the jaw members 710, 710', 910, 910'
and 720, 720', 920, 920' are configured for dissipating heat generated by
electrosurgical RF power, the cooling members disclosed herein (i.e.,
thermoelectric plates 718 and 728, corresponding heat sinks 818 and 828 and
the cooling lines 850, 852, 950, 952; and the cooling loops 340, 1150 and 1190
for cooling the insulating housing 314) may be adapted as well to other
heating
modalities. Such other heating modalities include, but are not limited to,
ultrasonic, capacitive or thermoelectric heating power sources.
While various embodiments of the disclosure have been
described, it is not intended that the disclosure be limited thereto, as it is
intended that the disclosure be as broad in scope as the art will allow and
that
the specification be read likewise. Therefore, the above descriptions should
not
be construed as limiting, but merely as exemplifications of preferred
embodiments. Those skilled in the art will envision other modifications within
the scope and spirit of the claims appended hereto.
39