Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
POLYMERIC DRUG AGENTS FOR THE TREATMENT OF FIBROTIC
DISORDERS
FIELD OF THE INVEhITIOIV
[0001] The present invention relates generally to agents and methods for
treating aazd/or
preventing fibrosis. Idlore particularly, the present invention relates to
agents and methods for
treating fibrotic disorders through the release of drugs from within a
polymeric matrix.
BACKGROUND ~F THE II~VEIVTIOIV
[0002] Fibrosis, also known as scarring, is manifest in many clinical
diseases, conditions,
to and disorders (the terms have been used interchangeably herein). Hepatic
fibrosis, for
example, usually results from chronic liver disease, and is often complicated
by severe
distortion (cirrhosis). Cardiovascular fibrosis leads to the loss of
flexibility in the ventriculo-
arterial system, which is often accompanied by isolated systolic hypertension
and congestive
heart failure.
[0003] As well, surgical intervention leads to the formation of fibrotic
adhesions between
incision sites of organs, an undesirable side effect often requiring
corrective surgery
thereafter. By way of example, surgical intervention for correcting bowel
obstruction which
occurs in 60% of all laparotomy cases has a 5% to 30% mortality rate (Ellis,
1997). Pelvic
adhesions involving ovaries or the fallopian tubes following gynecological
operations result
2o in infertility for up to 45% of the patient population (Stangel et al.,
1984). Thus, there is a
significant need for methods of treating fibrosis.
[0004] Of the numerous extracellular matrix proteins that comprise a fibrotic
adhesion,
collagens are believed to contribute significantly to clinical fibrosis. In
addition to their
relative abundance at fibrotic adhesions, the proteolysis of collagen appears
to be a rate-
1
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
limiting step for extracellular matrix removal. Accordingly, a number of
approaches have
been attempted to retard and/or prevent the accumulation of extracellular
matrix proteins, and
in particular, collagen.
[000] In an attempt to minimise surgical trauma, for example, a wide range of
resorbable
s and nonresorbable barrier materials have been investigated (Sawhney, et al,
1994.; Edwards, et
al, 1997; Unman, et al, 1991). Despite the development in this field, the few
products
available for clinical use (principally barrier materials such as oxidised
regenerated cellulose
or hyaluronic acid) remain only marginally effective.
[0006] An alternative, molecular, approach has been to perturb the
functionally requisite
to three-dimensional structure of collagen, which structure contains a high
percentage of trayas-
hydroxylated proline and lysine residues. Indeed, hydroxylation is a
prerequisite for collagen
fiber formation, and proline analogues such as cis-4-hydroxy- (cHyp), fluoro-,
or bromo-
proline or 3,4-L-dehydroproline (DHP), that incorporate directly into nascent
chains of
procollagen, have been effective (Ditto and Prockop, 1974; Ditto and Prockop,
1977; Kao and
is Proclcop, 1976; Liotta et al., 1978). Administration of monomeric cHyp for
limited periods of
time has been shown to prevent collagen accumulation in animals in a variety
of experimental
models, including abdominal adhesions, cirrhosis, and pulmonary fibrosis
(Girl, 1990; Tan et
al, 1983).
[0007] Although short-term studies have demonstrated the efficacy of some anti-
fribrotic
2o dl-ugs, there are at least two limitations to the potential long-term use
of such drugs: (1)
systemic toxicity resulting from interference with general protein synthesis
(Eldridge, et al,
1988), and (2) rapid resorption, diffusion, and/or excretion of the drug.
Thus, there is a need
2
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
for treatments that may target anti-fibrotic agents to the fibrotic tissues
and/or increase
localized retention of the therapeutic agent.
SZJMMAR~ ~F THE IlV~EIVTI~N
[000] The foregoing needs are met, to an extent, by the present invention,
wherein in one
aspect a fibrotic tissue inhibiting agent is provided, comprising: a
drug/polyrner conjugate of
formula:
-[P-M]- n or Q-(P-L-D)k or (D-L)m-PP
L
to
D
wherein, P is a water-soluble polymer segment; M is a multifunctional moiety
joining water
soluble polymer segments P into a co-polymer backbone and providing attachment
for groups
-L-D to the backbone; L is a linlcer or a chemical bond; D is a fibrotic
tissue inhibiting
compound; Q is a multivalent coupler; n is an integer greater than 2; k is an
integer of 2 to
1000; PP is a polymer with one or more functional groups to attach the -L-D
groups; and, m
is an integer. D may be an anti-fibrotic agent, an anti-proliferative agent,
and/or a fibrotic
tissue inhibiting proline analogue: cis-4-hydroxy-L-proline (cHyp), cis-4-
hydroxy-D-proline,
cis-3-hydroxy-DL-proline, 3, 4-dehydro-L-proline(DHP), cis-4-fluoro-L-proline,
cis-4-chloro-
2o L-proline, cis-4-bromo-L-proline, L-azetidine-2-carboxylic acid (AZA), or L-
thiazolidine-4-
carboxylic acid (THP). Where D is an anti-fibrotic agent, D may be
pirfenidone, tranilast,
halofuginone, pentoxifylline, relaxin, estradiol, interleukin 10, pyridine-2,
or 4-dicaboxylic-
di(2-methoxyethyl) amide. Where D is an anti-proliferative agent, D may be 5-
flurouracil,
mitomycin-C, or paclitaxel. D may also be retinoic acid, a retinoic acid
analogue, a retinoic
acid antagonist, a plasminogen activator, a vastatin, or a non-steroidal anti-
inflammatory
compound. D may be released by enzymatic action or metabolic activity on
linker L. Polymer
3
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
segment P may be of average molecular weight 400-25,OOODa, derived from
compounds
having at least two functionalities for covalent attachment to M, selected
from the group
consisting of hydroxyl, amino, thiol, alkyl disulfide, aryl disulfide,
isothiocyanate,
thiocarbonylimidazole, thiocarbonylchloride, aldehyde, ketone, carboxylic
acid, carboxylic
acid ester, sulfonic acid, sulfonic acid ester, sulfonyl chloride, phosphoric
acid, alkyl
succinimidylcarbonate, aryl succinimidyl- carbonate, alkyl chlorocarbonate,
aryl
chlorocarbonate, alkyl succinimidylthiocarbonate, aryl
succinimidylthiocarbonate, allcyl
chlorothiocarbonate, aryl chlorothiocarbonate, halide, and thioester. W some
embodiments, P
may be a polyethylene glycol, polyvinyl alcohol, poly(2-hydroxyethyl
methacrylate),
1o poly(acrylic acid), poly(methacrylic acid), poly(maleic acid), polylysine,
or a mixture thereof.
In other embodiments, P may be a poly(carboxylic acid), poly(orthoester),
poly(anhydride),
pluronic polyol, poly(vinylpyrrolidone), poly(acrylate), polyamide,
polyphosphazine,
poly(amino acid),polypeptide, pseudo-poly (amino acid), linear or branched
polymer
containing PEG, copolymers of PEG, a dendrimer, or a PEG-dendrimer. PP may be
a
15 polyethylene glycol, polyvinyl alcohol, poly(2-hydroxyethyl methacrylate),
poly(acrylic acid),
poly(methacrylic acid), poly(maleic acid), polylysine, or a mixture thereof,
or a
poly(carboxylic acid), poly(orthoester), poly(anhydride), pluronic polyol,
poly(vinylpyrrolidone), poly(acrylate), polyamide, polyphosphazine, poly(amino
acid),polypeptide, pseudo-poly (amino acid), linear or branched polymer
containing PEG,
2o copolymers of PEG, a dendrimer, a PEG-dendrimer, collagen, hyaluronic acid,
fatty acid
lipid, polyhydroxyalkanoate, chondrotin sulfate, glycosaminoglycan, chitosan,
alginate,
starch, dextran, a carbohydrate-based polymer, cellulose, or cellulose
derivative. n may be an
integer from 3 to 100 inclusive, k may be an integer from 2 to 100 inclusive,
and m may be an
integer from 1 to 100 inclusive.
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
[0009] In another embodiment of the present invention, a method for treatment
of tissue
adhesions is provided, comprising: administering to a tissue in need of
treatment for
surgically-induced adhesions or fibrotic disease, an effective amount of a
fibrotic tissue
inhibiting agent, comprising: a dn~g/polyrner conjugate of formula:
-[P-M]- n or Q-(P-L-D)k or (D-L)m-PP
L
to D
wherein, P is a water-soluble polymer segment; M is a multifunctional moiety
joining water
soluble polymer segments P into a co-polymer backbone and providing attachment
for groups
-L-D to the backbone;L is a linker or a chemical bond; D is a fibrotic tissue
inhibiting
compound; Q is a multivalent coupler; n is an integer greater than 2; lc is an
integer of 2 to
1000; PP is a polymer with one or more functional groups to attach the -L-D
groups; and, m
is an integer. W some embodiments, m is an integer greater than 1 and in other
embodiments,
m is an integer of 1 to 1000. The agent may be administered topically by
inhalation, or by
injection, and may be encapsulated in an eroding polymer or entrapped in a
polymer matrix.
BRIEF DESCRIPTION OF THE DRAWINGS
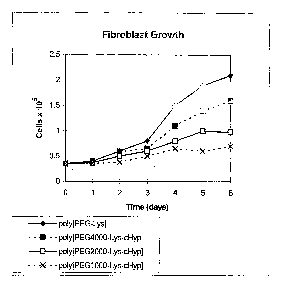
[0010] Figure 1 shows the growth of rat lung fibroblasts on uncoated plates.
All treatment
began at day 0 and at 2.0 mg/ml.
[0011] Figure 2 depicts the cytotoxicity of flee CHYP and Poly(PEG-Lys-cHyp)
constructs (2 synthesis batches) on RFL-6 rat hbroblast cells.
5
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
[0012] Figure 3 shows the effects of 0.1 mg/ml free proline analogs on RFL-6
cell growth
in regular or collagen-precoated wells (day 4). CHYP (0.76 mM), THP (0.75 mM),
DHP
(0.90 mM).
[001] Figure 4 shows the effect of four anti-fibrotic formulations on the
return of
adhesions after adhesiolysis and treatment. The percent of returning adhesions
(number of
adhesions present/number of adhesions at the initial observation).
[0014] Figure 5 depicts a comparison of the adhesion grade (as graded
according to Table
4) at the initial observation versus three weeks after adhesiolysis and
treatment.
DETAILED DESCRIPTION
to [0015] The compositions and methods provided herein can enhance delivery of
a fibrotic
tissue inhibiting (also referred to as "anti-fibrotic") drug and may do so by
extending the half
life of the drug, sustaining its release, releasing the drug by metabolic
activity and/or releasing
same at the site of inflammation. Such enhanced delivery of the fibrotic
tissue inhibiting drug
is accomplished by combining the drug with a polymer. Any anti-fibrotic agent
may be
incorporated into the compositions and methods of the invention for the
prevention and
treatment of fibrotic tissue and/or adhesions.
[0016] By way of example, fibrotic tissue inhibiting drugs can include, but
are not limited
to, proline analogues such as, for example, cis-4-hydroxy-L-proline, cis-4-
hydroxy-D-proline,
cis-3-hydroxy-DL-proline, 3,4-dehydro-L-proline, cis-4-fluoro-L-proline, cis-4-
chloro-L-
2o proline, cis-4-bromo-L-proline, L-azetidine-2-carboxylic acid (AZA), and L-
thiazolidine-4-
carboxylic acid (THP). Anti-fibrotic drugs including pirfenidone, tranilast,
halofuginone,
pentoxifylline~ relaxin, estradiol, interleukin 10, pyridine-2,4-dicarboxylic-
di(2-
methoxyethyl)amide may also be useful as drugs incorporated into the delivery
format
6
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
disclosed herein. In addition, anti-proliferative drugs, such as 5-
fluorouracil, mitomycin-C,
and paclitaxel may be employed. Drugs showing anti-fibrotic effects such as
retinoic acid,
retinoic acid analogues, retinoic acid antagonists, plasrninogen activator,
vastatins, and/or
non-steroidal anti-inflammatory drugs may also be used.
[001'x] In one embodiment of the invention, a fibrotic tissue inhibiting drug
can be
covalently linked to synthetic or naturally occun-ing polymers. Attaclnnent of
the drug to a
polymer confers a number of properties to the drug/polymer conjugate,
including, increased
acctunulation at the site of inflammation, which has been referred to as
"enhanced
permeability and retention" (Maeda, H, 2001); enhanced solubilization of
highly hydrophobic
to drugs (if the polymer itself is soluble); increased half life and/or
sustained delivery of the
drug. The macromolecular size of the drug/polymer conjugates can effect the
drug half life
and biodistribution.
[0018] A linker that attaches the drug to the polymer may contain one or more
chemical
bonds that may be cleaved by enzymes; alternatively, chemical bonds between
the drug and
linker may be used that can release the drug by local metabolic and/or
chemical activity such
as changes in pH, ionic, or redox conditions, or released by reaction with the
in situ
environment such as hydrolysis, reduction reactions, oxidative reactions, pH
shifts,
photolysis, or combinations thereof, thus releasing the active drug. The drug
from the
ch-ug/polymer conjugates can be released by a combination of enzymatic and non-
enzymatic
2o activity. In a preferred embodiment, the anti-fibrotic drug may be released
from the polymer
by a condition related to the formation of adhesions.
[0019] In some embodiments of the present invention, the drug/polymer
conjugates
comprise polymers of the type poly[D-L-M-P], in which M is a multifunctional
chemical
7
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
moiety that is used to join water soluble polymer segments P to form a regular
repeating
linear co-polymer backbone and additionally to provide the chemical
substituents for the
attachment of the fibrotic tissue inhibiting drug D via a linker L, and in
which n is the number
of repeats of D-L-M-P. The fibrotic tissue inhibiting drug D can be covalently
attached to
linker L or can be directly attached to M, with the polymer then being poly[D-
M-P] . The
water-soluble polymer segment P consists of a relatively short, water-soluble
polymeric
system (for example, average M.W. 400-25,000 Da) which contains at least two
homogenous
chemical functionalities (for example, including but not limited to, hydroxyl,
amino, thiol,
alkyl or aryl disulfide, isothiocyanate, thiocarbonylimidazole,
thiocarbonylchloride, aldehyde,
to lcetone, carboxylic acid, carboxylic acid ester, sulfonic acid, sulfonic
acid ester, sulfonyl
chloride, phosphoric acid, alkyl or aryl succinimidyl carbonate, alkyl or aryl
chlorocarbonate,
allcyl or aryl succinimidylthiocaxbonate, alkyl or aryl chlorothiocarbonate,
halide, or thioester)
that can be used for covalent attachment to the multifunctional chemical
moiety M.
[0020] P can be, but is not restricted to, polyethylene glycol), polyvinyl
alcohol), poly(2-
hydroxyethyl methacrylate), poly(acrylic acid), poly(methacrylic acid),
poly(maleic acid),
poly(lysine), and the like or a copolymer consisting of a mixture of these or
other polymeric
entities possibly substituted with organic functional groups. Similarly,
polymers of the type
Q-(P-L-D) k can be used for this invention, in which Q is designed to couple
to k number of
short water soluble polymer segments, P. For this polymer, the fibrotic tissue
inhibiting drug
2o D can be covalently attached to linker L or can be directly attached to P,
with the polymer
then being Q-(P-D) k.
8
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
P M -
L n - poly[D-L-M-P]
I
D
I~-I'~JI1 ~- - p~1~,[~-~~-~]
[0021] ~ther suitable polymers for covalent linkage of the fibrotic tissue
inhibiting drug
according to the invention include, but are not limited to, synthetic polymers
such as
poly(carboxylic acids), poly(ortho esters), poly(anhydrides), polyvinyl
alcohol), pluronic
polyols, poly(vinylpyrrolidone), poly(acrylates), poly(amides),
polyphosphazenes, poly(amino
acids), branched polypeptides, pseudo-poly(amino acids), polyethylene glycol),
branched
polymers containing PEG, co-polymers of PEG and other mentioned polymers, PEG-
to dendrimers, and other denrimers; natural polymers such as collagens,
hyaluronic acid, fatty
acids, lipids, polyhydroxyalkanoates, chondrotin sulfates, glycosaminoglycans,
chitosans,
alginates, starches, dextran, cellulose and its derivatives, and other
carbohydrate based
polymers.
[0022] The drug/polymer composite may be applied by injection or administered
topically. The drug/polymer composite can be encapsulated within an eroding
polymer, and
then applied by injection or administered topically. The drug/polyrner
composite can be
entrapped into a polymer matrix using synthetic or naturally occurring
polymers, and then
applied topically, by injection, or by implantation.
[002] The anti-fibrotic drug D or the linking group/drug D-L can be covalently
attached
2o to rnaazy of the above mentioned polymers by previously described methods,
forming a system
such as PP-(D-L)", whereby PP is one of the polymers listed above having a
molecular weight
9
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
between 1,000 and 1,000,000 Daltons and chemical groups that can be used to
attach n copies
of the linking group/drug conjugate D-L.
[0024] The polymer used for the covalent attachment of the fibrotic tissue
inhibiting drug
may also have a moiety that can be targeted to the tissue or cell types
associated with the
formation of adhesions or fibrotic diseases. In a preferred embodiment, the
polymers may be
of the type T-R-P-L-D, (T-R) q-poly[D-L-M-P] , (T-R) a poly[D-M-P] , (T-R) q-Q-
(P-L-D)k,
(T-R) q-Q-(P-D) k; poly[D-L-M]-poly[T-R-M] (with either random or sequential
repeats of the
pendant chains), and poly[D-M]-poly[T-R-M] (with either random or sequential
repeats of
the pendant chains) in which T is a targeting agent (such as an antibody,
receptor binder, or
to enzyme binder), R is a chemical group which links T to the polymer, and q
is the number of
repeats of T-R.
T-[M-P] n T
L - (T-R)q-poly[D-L-M-P]
D
T-[M-P] n T
DI - (T-R)q-poly[D-M-P]
P]n ~ P] n
T ~ = poly[D-L-M]-poly[T-R-M]
D
]n ~ P]n = poly[D-Ml-poly[T-R-Ml
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
[0025] In some embodiments, the fibrotic tissue inhibiting drug may be
encapsulated
using synthetic or naturally occurring polymers. The polymer composite may
surround the
core of drug, and can be in the form of particles such as microspheres that
can be used for
injection, implantation, or topical delivery. The en capsulatmg polymers) may
be degraded
by enzymes, by local metabolic activity such as changes in pH, ionic, or redox
conditions, or
the drug may be released by reaction with the in situ environment such as
hydrolysis,
reduction reactions, oxidative reactions, pH shifts, photolysis, or
combinations thereof. In a
preferred embodiment, the drug could be released from the polymer by a
condition related to
the formation of adhesions.
[0026] hi other embodiments, the fibrotic tissue inhibiting drug can be
entrapped into a
polymer matrix using synthetic or naturally occurring polymers. The drug may
be uniformly
distributed throughout the polymer. The fibrotic tissue inhibiting drug may be
crosslinlced
onto the polymer within the matrix (resulting in covalent attachment between
the drug and the
polymer), or held in place by non-covalent forces. The polymer/drug composite
may be in the
, form of particles (such as microspheres, applied by injection or
administered topically), gels
(used as an implant or applied topically), or films (used as an implant or
applied topically). In
particular, entrapping polymers) may be degraded by enzymes, released by local
metabolic
activity such as changes in pH, ionic, or redox conditions, or the drug may be
released by
reaction with the in situ environment such as hydrolysis, reduction reactions,
oxidative
2o reactions, pH shifts, photolysis, or combinations thereof. In a preferred
embodiment, the drug
would be released from the polymer by a condition related to the formation of
adhesions.
r~. Syntlxesis of polymers
[0027] A vaxiety of polymeric structures of the type poly[I~-IV!-P]" have been
synthesized
and tested for the controlled release of antifibrotic agents. These have
included alternating
11
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
polyethylene glycol (PEG) and lysine (PEG-lysine) co-polymers, using PEGS of
varying
subunt lengths (PEG1000, PEG2000, PEG4000, and PEG8000), as well as PEG2000 co-
polymerized with des-amino tyrosyl-tyrosine (PEG2000-TT).
[00~~] In one example, a poly[Poly(PEG-Lys-cHyp)] conjugate was synthesized
and
tested in vity~~ and in limited ifa viv~ experiments, and found to be
effective, where P is a
segment of polyethylene glycol) with and average molecular weight of 2000
Daltons (PEG-
2000), L-Lysine (Lys) is T~, and cis-4-hydroxy-L-proline (cHyp) is 1~. The
starting material
was Lys-CHYP dipeptides, and chain extension was accomplished by
copolymerizing with
bis(succinimidyl) polyethylene glycol)(BSC-PEG) to a,- and s-amines of the
cHyp-Lys
to dipeptide. For more efficient chain extension, CHYP construct using the
bis(succinimidyl)
polyethylene glycol)(BSC-PEG) is preferrably greater than 99.99% bi-
functional; mono-
functional contaminant of the PEG can act as a chain terminator. It was found
that some
commercial sources of bis(succinimidyl) polyethylene glycol)(BSC-PEG) MW=1000
had
more than 0.1 % mono-functional contaminant. Supplies of bis(succinimidyl)
polyethylene
glycol)(BSC-PEG) MW=2000 did not appear to pose such a problem. Longer PEG
building
bloclcs (MW = 4000 Da and 8000 Da) have more limited CHYP loading capacity,
and did not
demonstrate appreciably improved drug efficacy.
H H
O\ 'N N\ /O-(PEG-2000)
IO~I IO~I
HO ~N O
~COOH
poly [PEG-Lys-cHyp]
12
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
[0029] Proline analogs were linked through their imino group, forming a
peptide bond
with the lysine carboxylic group. The final constructs ranged between 20 to 35
kDa in
molecular weight, as determined by gel permeation chromatography on columns
calibrated
with PEGS of different length, and detected by refractive index. The molecular
weight,
however, can range from about 10 to about 200 lcDa. The amin~ acid content and
the ratio of
Lys to proline analog were determined by amino acid analysis. The co-polymers
typically
contained 8-12 pendent amino acids per construct, as estimated from the mean
conjugate
molecular weight and the known size of the PEG subunits. For example, Poly(PEG-
Lys-
cHyp) with PEG (molecular weight of 2000 "PEG2000") has a mean molecular
weight of
to 25,000 Da, and therefore has aaz average of 11 PEG units and 11 pendent
CHYPs. The
average polydispersity for the Poly(PEG-Lys-cHyp) is 1.6.
[0030] Ih vztro studies have shown that Poly(PEG-Lys-cHyp) formulations of
varying
PEG chain lengths (MW = 1000, 2000, and 4000 Da) can be effective in
inhibition of
collagen production. Figure 1 shows the inhibition of rat lung fibroblast
growth for the
various forms of Poly(PEG-Lys-cHyp). This design provides delivery of 4 times
more cHyp
for the PEG1000 polymer conjugate than the PEG4000 agent, and two times more
cHyp for
the PEG2000 polymer conjugate than the PEG4000 agent.
B. Stability of polymers
[0031] The stability of poly(PEG-[14C]-Lys) over a period of 48 h was
determined by
2o incubation in human plasma and physiological phosphate buffer at 37
°C, followed by GPC
analysis of the polymer molecular weight at predetermined time intervals.
There were no
noticeable changes in the molecular weight and the radioactivity per molecular
weight
(specific activity) over 48 h. HPLC analysis of Poly(PEG-Lys-cHyp) after
incubation in
13
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
human plasma, physiological phosphate buffer at 37 °C, trypsin,
thrombin, plasmin, or
collagenase showed no release of CHYP from the polymer.
C. Cell Gr~wth Inhibiti~n and Cytot~xicity Paz ~i~s~~
[003] Various proline analogs were tested alone, as peptidic prodrugs, and as
polyrner-
bound prodrug constructs on rat fibroblast RFL-6 cells grown as monolayers in
microtiter
plates. Test compounds were added in serial dilutions from the outset of the
culture.
Viability was assessed on day 4 using a tetrazolium viability indicator dye
(Dojindo).
[0033] A representative ICSa experiment is shown in Figure 2. In this test,
two different
synthesis batches of cis-hydroxyproline (CHYP) linked to PEG were tested,
along with free
to CHYP. As shown, the polymeric constructs (1 and 2) and the free amino acid
inhibit rat
fibroblast growth to a similar extent. The differences between the two batches
of Poly(PEG-
Lys-cHyp) were not significant. More importantly, the lack of difference
between the free
and conjugated CHYP activity indicates that the RFL-6 cells readily hydrolyze
the CHYP
from the polymer backbone. Similar experiments with other analogs of Pro are
summarized
in Table 1 below.
TABLE 1: Compiled ICso results for free proline
analogs, lysyl dipeptides, and PEG constructs
ICso mM
AA, mean
t SD n
Amino acid (AA) Free AA AA-L s AA-L s-PEG
cis-hydrox roline (CHYP) 8.8 ~ 5.1 7.0 (1) 5.6 ~ 1.5
(6) (11)
Azetidine carboxylic acid 1.7 ~ 1.8 2.5 ~ 0.7 7.0 t 1.4
(AZC) (4) (2) (2)
Dehydro roline (DHP) 0.7 ~ 0.3 1.9 (1) 5.4 ~ 3.7
(3) (2)
Thia roline (THP) 0.7 ~ 0.1 1.2 ( 1 8.4 ( 1 )
(3) )
[0034] The free amino acid CHYP is the least cytotoxic proline analog tested
in RFL-6
2o cultures. But its effects in the lysyl-dipeptide and PEG conjugate forms
are comparable to
those of the other proline analogs, indicating the ability of the cells to
easily cleave the
CHYP-Lys bond. This ability differs from the other analogs; AZC, DHP and THP
are
14
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
considerably more cytotoxic than CHYP as free drugs, as well as in their lysyl-
dipeptide
form, but their cytotoxicity as PEG constructs is reduced by factors of 4x, 8x
and 12x,
respectively, when compared to the free amino acid.
[003] Collagen synthesis by anchorage-dependent fibroblasts is required for
cell growth.
To differentiate between cell growth inhibition and cytotoxicity, the effect
of proline analogs
on RFL-6 cells grown on plain or collagen pre-coated wells was examined.
Cultures were
incubated in the presence or absence of proline analogs at concentrations
found to be
inhibitory (see Table 1). CHYP and DHP inhibited cell growth on plain wells,
but not on
collagen-coated wells, demonstrating the fact that pre-coating the wells with
collagen
to abrogated the effects of these proline analogs (Figure 3). THP was too
cytotoxic on both
surfaces at the tested concentration to observe any effect.
D. Poly(PEG-Lys-cHyp) Immunoassay
[0036] A polyclonal antiserum was generated in rabbits immunized with Poly(PEG-
Lys-
cHyp) coupled to keyhole limpet hemocyanin (KI,H). Pre-immune, immune, and
boosted
plasma samples were tested for the ability to bind solid-phase Poly(PEG-Lys-
cHyp) (1 q,g
Poly(PEG-Lys-cHyp)/well adsorbed onto Immulon microtiter plates). Specificity
was
determined by the ability of the antibody to bind immobilized conjugate
following pre-
incubation with competing haptens. Competing haptens included Poly(PEG-Lys-
cHyp) itself,
2o free CHYP, PEG-Lys (the actual copolymer backbone in Poly(PEG-Lys-cHyp)),
CHYP-Lys,
as well as structural analogs of hydroxyproline (dehydroproline, or DHP, and
azetidine
carboxylic acid, or ACA). From these competition-binding studies it was
determined that the
antiserum recognized only the intact Poly(PEG-Lys-cHyp) molecule (50%
inhibition at 2-5
~,g/ml). I~Tone of the other molecules competed effectively (50% inhibition at
>50 ~,g/ml).
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
E. Development of the Delivery Vehicle
[0037] Hyaluronic acid (HA) is a naturally occurnng bio-polymer that is a
component of
various medical products. In general, HA is a biocompatible material found to
retard
movement and proliferation of fibroblasts and related cell types. Iaz ~~iw~
studies of hyaluronic
acid gels and films that essentially form barriers between tissue planes have
been shown to
abrogate the formation of surgical adhesions.
[003] HA beads can be formed that contain anti-fibrotic agents, such as the
Poly(PEG-
Lys-cHyp) active agent. The HA beads can be manufactured by a number of
standard
teclmiiques, such as spray drying, or the use of non-aqueous solvents (such as
alcohols) to
to precipitate HA. The HA beads can be subsequently crosslinked using a number
of standard
chemical methods to enhance bead stability and regulate the bioresorption
rate. The final
form of the beads may be a free-flowing, freeze-dried powder.
[0039] A number of formation variables were tested to determine an optimal
release
characteristic of the Poly(PEG-Lys-cHyp) from the hyaluronic acid beads. Table
2 presents
the various formulations of HA/Poly(PEG-Lys-cHyp) beads and their release
kinetics:
TABLE 2: Release kinetics of various polymer formulations
Sample CrosslinkingPoly(PEG-Lys-Tliz** Tn**
. . H 7:4 BufferTissue.homo
cHyp) Content* mate
1 HA/Active None 30% 1.75 hr NA
2 HA/Active/LowX25 mM EDC 30% 47.8 hr 4.25 hr
3 HA/LowX 25 mM EDC 0 0 0
4 HA/Active/MedX50 mM EDC 30% > 72 hr 23.5 hr
5 HA/ MedX 50 mM EDC 0 0 0
6 HA/Active/Hi 75 mM EDC 30% > 72 hr 25.5 hr
hX
7 HAl MedX - 50 mM EDC 3.25 hr NA
Active
w weight nyamromc acid/weight Yoly(YYCi-Lys-cHyp) during bead formation
process.
*~° Defined as the tune at which 50% of the Poly(PEG-Lys-cHyp) is
released from the beads
j' For the first six formulations, the Poly(PEG-Lys-cHyp) was mixed with the
hyaluronic acid prior to bead
formation. For this last formulation, the beads were made first. Tlie beads
were then treated with a solution of
Poly(PEG-Lys-cHyp) (100 mg/ml), flien freeze-dried.
16
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
[0040] HA/Poly(PEG-Lys-cHyp) beads listed in Table 2 were subsequently tested
separately in three animals for each variable, observing after day 14. There
were no
noticeable adverse reactions from any of the materials. Therefore, the
HA/holy(PEG-Lys-
cHyp) beads were investigated in various ifz viv~ models.
F. Isa ~iv~ l~e~ult~
~lellae~i~ly~i~ l~~elel
[004.1] Injuries were made to the right abdominal wall of mature female
rabbits (removal
of the periternium and first muscle layer from an area of approx. 2 x 5 cm),
cecum adjacent to
the right abdominal wall (removing serosa), and abrasion of the right uterine
horn. The
bladder was protected during surgery in order to minimize unintentional
adhesions. The
injury to the side wall was electrocauterized; about 2 to 3 cc of blood was
withdraw from the
ear vein and placed on the wound. The animals were allowed to recover; a
second
laparotomy was done three weeks later, and the incidence, severity, and extent
of each
adhesion was scored for each animal (see Table 3 for adhesion grading system).
Animals
having no adhesions were removed from this study. The adhesions were lyzed,
and the
animals were treated and allowed to recover. Three weeks after adhesiolysis
and treatment, a
final open observation was done, whereby the incidence, severity, and extent
of each adhesion
were again scored and compared to the initial observation. For the control
group, 88% of all
the adhesions which were lyzed, reformed (Table 4).
1~
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
TABLE 3: Scale for Scoring Surgical Adhesions
Severity Fxt~~ t
Grade Descri tion Grade Descri tion
0 No adhesions 0 No adhesions
1 Filing, detaches easily 1 Focal; small, limited
area
2 Requires sharp dissection 2 Corers at least 1/2
of injbuy area
3 I ~asculari~ed and requires 3 Covers most of injury
sharp dissection area
[0042] In this series of studies, a viscous polymer fluid was used as a
carrier gel for the
sustained release polymer Poly(PEG-Lys-cHyp). The viscous polymer fluid
consisted of PEG
grafted to ~-hydroxy acids PLA and PGA, and phase-separated into a semi-solid
gel once
hydrated. The gel provided controlled release of Poly(PEG-Lys-cHyp). Four
formulations
were developed to test in the adhesiolysis model: A. Gel; B. Gel and 2 mg/ml
Poly(PEG-
Lys-cHyp); C. Gel and ~ mg/ml Poly(PEG-Lys-cHyp); and D. Gel and 2 mg/ml free
cHyp.
to All treatment tubes were coded to "blind" the surgeon.
[0043] Table 4 provides a summary of the data from this experiment. Each
treatment
group had six or more adhesions which were treated, with a mean incidence per
animal of
1.75 for the treated group (e.g., on average, there were approx. two adhesions
per animal).
More than 65% of the adhesions were Grade 2 or worse at the initial
observation
(adhesiolysis and treatment). The post-treatment observations, made three
weelcs later, are
presented on Table 4 (and graphically in FIGS. 4 and 5). The mean incidence
per animal of
adhesions after treatment for the combined groups A through D was less than 1,
which is a
statistically significant difference (p < 0.05) versus the mean incidence at
the initial
observation. The number of adhesions for groups B and C (the sustained release
2o formulations) were substantially less after treatment, which are
statistically significant
differences (p < 0.05), versus the number of adhesions at the initial
observation.
18
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
TABLE 4: Final Observations (3 weeks post adhesiolysis)
# of % #
# of Total Mean Returning~uiaxaals
rou # of IncidenceAdhes. OIncid.
Grade
3 Grade
2 Grade
1
Gel 1 2 2 5 1.25 83.33/~ 0.00
~cl ~ 1 0 1 2 0.50 28.57% 1.00
2 mg/ml Poly(PEG-Lys-
cHyp)
Gel & 0 1 0 1 0.25 14.29% 2.00
8 mg/ml Poly(PEG-Lys-
cHyp)
~~1 ~ 2 3 1 6 1.50 75.00/~ 0.00
2 mg/n~l CH~'1'
Controls 5 7 3 15 3.00 88.24% 0.00
_
Total of Treated4 ~ 6 ~ 4 ~ 14 0.875
Groups ~
[0044] The percentage of returning adhesions were also significantly less for
groups B
and C than the control or for groups A or D (see Fig. 9A). There is also a
statistically
significant difference between group C (8 mg/ml sustained release polymer) and
B (2 mg/ml
sustained release polymer), which suggests that the sustained release polymer
works in a
dose-dependent manner. Each adhesion was graded for severity and extent; the
combination
grade average for each group is presented in Fig. 9B, comparing the initial
observation grade
with the final observation grade. As with the percent of returning adhesions,
the extent and
to severity of adhesions was significantly reduced for groups B and C compared
to the control or
for groups A or D.
[0045] In a parallel study, the adhesiolysis and treatment model was again
used to
compare the current leading product for adhesion prevention, SEPR.AF1LMT""
(Genzyme),
with control (adhesiolysis, no treatment). SEPR.AFILMTM is a crosslinlced
hyaluronic acid
film. Table 8 provides a summary of the data from this experiment. Each
treatment group
had six or more adhesions which were treated (Table 5-A), with a mean
incidence of 1.2 for
the treated group and 2.25 for the control group. More than 65% of the
adhesions were Grade
19
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
2 or worse at the initial observation (adhesiolysis and treatment), which is
the same as for the
previous experiment.
[0046] The post-treatment observations, made three weeks later, are presented
in Table 5-
The mean incidence per animal of adhesions after treatment for the Seprafilm
group was
0.8, which is not statistically different (p < 0.05) versus the mean incidence
at the initial
observation. The mean incidence of adhesions after treatment for the control
group was 1.75,
which is not statistically different (p < 0.05) versus the mean incidence at
the initial
observation. The number of returning adhesions for the Seprafilm group was
66.7%, which is
not statistically different (p < 0.05) than the control group, which had a
reformation rate in
to ' this experiment of 77.8%. There was one animal in the Seprafilm group
which had no
adhesion reformation. In three of the remaining Seprafilm animals, the
severity of the
adhesions decreased by one grade.
TABLE 5-A: Adhesion Observation at Adhesiolysis and Treatment
#of #of #of
Grade Grade Grade Total Mean
3 2 1
Group AdhesionsAdhesionsAdhesions Incidence
Seprafilm 4 2 0 6 1.2
(n=5)
Control (n=4)1 6 2 9 2.25
20
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
TABLE 5-B: Adhesion Observation Three Weeks After Adhesiolysis and Treatment
# of # of # of % # # of Ave.
Grade Grade Grade TotalMean ReturningAnignalshTew Severity
3 2 1
Group Adhe~.Adhe~.Adhes. IneideneeAdite~. 0 Ineid.Adhe~.I~ev~
Ad.
Scprafilm 1 2 4 0.8 66.67!~ 1 2 2.5
(n=5) 1
Control 1 5 7 1.75 77.78% 0 3 2.3
(n=4) 1
[0047] A similar adhesiolysis study was performed using the HA/I'oly(PEG-Lys-
cHyp)
beads as described in Tables 4 and 5. In this study, formulations HA/MedX (no
active), and
HA/Active/LowX, HA/Active/MedX (see Table 2) were used. Bead material was
placed on
each wound to provide a complete and ever coverage (about 100 mg per wound).
Each bead
formulation adhered relatively strongly to tissue, and began to hydrate
shortly thereafter. The
beads formed a pasty film after about 15 minutes.
[0048] Observations were made three weeks after treatment. As previously
observed,
to there were no observable adverse effects from the material at the site of
delivery. Two
animals treated with HA/Active/MedX had residual polymer (most likely HA),
which was
encapsulated. There was slight inflammation at the site of the residual
polymer.
[0049] Table 6 provides a sununary of the data from this experiment. Each
treatment
group had six or more adhesions which were treated, with a mean incidence of
1.85 per
animal for the treated group and 2.1 for the control group. More than 65% of
the initial
adhesions were Grade 2 or worse at the initial observation (adhesiolysis and
treatment), which
is the same as for the previous experiment. The post-treatment observations,
made three
weeks later, confirmed that the incidence of returning adhesions after
adhesiolysis in the
control group was high (91.54%). The mean incidence of adhesions and percent
of returning
21
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
adhesions showed that each application group was effective. There was no
statistical
difference between HA/Active/MedX versus HA/Active/LowX with respect to the
mean
incidence or percent of returning adhesions (p < 0.05). However, the group
HA/MedX (no
Poly(PECa-Lys-cHyp) in the HA beads) did not appear to be as effective as the
two groups
containing Poly(PECa-Lys-eHyp).
°I'A~fLE 6: Adhesi~~a ~la~exvation Thr ee leeks After Acihe~i~ly~i~
ar~el 'g°reatment
# of # of # of % # #~ Ave.
of
Grade Grade Grade TotalMean ReturningAnimalsNew Severity
3 2 1
Group Adhes.Adhes.Adhes. IncidenceAdhes. 0 Incid.Adhes.New
Ad.
HA/MedX 1 2 7 0.76 21.2% 0 1 1
4
HA/Active/MedX0 1 2 0.22 6.67% 3 0 0
1
HA/Active/LowX1 1 4 0.43 12.5% 1 0 0
2
Control (n=4)3 5 15 2.6 91.54% 0 3 2.3
7
Small Bowel Model
[0050] A small bowel adhesion model was developed in rabbits to mimic at least
some of
to the clinical manifestation of small bowel obstruction in humans. A 4 to 5
cm section of
small bowel was isolated and the surface lightly abraded with gauze. A small
bowel loop was
formed at the midline of the abraded portion, and the loop of bowel was
secured by two
interrupted 5/0 prolene sutures, spaced about 0.5 cm apart. A branch of the
mesenteric artery
affecting the looped small bowel was tied off with 5/0 prolene suture, and the
area was
allowed to dry by exposing the loop to ambient conditions for 2 min. The
animals were
allowed to recover for 2 weeks before the extent and severity of the adhesions
was
investigated.
[0051] All animals treated (n=17) in this fashion formed adhesions between the
small
bowel loops, with 90% of the animals having a grade 3 adhesion (vascularized
adhesion
22
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
needing sharp dissection), and the remaining having grade 2 adhesions (non-
vascularized
adhesion needing sharp dissection). 30% of the animals showed signs of bowel
obstruction.
(0052] Table 7 provides a summary of the data from this experiment. The form
of
adhesion was separated into three categories: infra-loop adhesion, suture
adhesion, and
adhesion between small bowel and other organs. It should be noted that the
most severe
adhesions were the infra-loop adhesions, and Grade 3 adhesions in the control
group often
caused necrosis and obstruction. There were no Grade 3 adhesions for animals
treated with
V0426A. All treatment groups showed statiscally significant decreases in
incidence of
adhesions compared to the control group. However, the group V0425B (no
Poly(PEG-Lys-
1o cHyp) in the HA beads) was substantially less effective compared to the two
groups
containing Poly(PEG-Lys-cHyp). There was no statistical difference between
V0425A (50
rnM EDC crosslinked) versus V0426A (25 mM EDC crosslinked).
TABLE 7: Summary of Data from Small Bowel Adhesion Studies
# of of # of
Grade Grade Grade Total can
roup 3 2 1 ncidence
dhes. dhes. dhes.
Small Bowel
Loop
A/MedX 0 2 2 0.8
A/Active/MedX 0 0 2 2 0.4
A/Active/LowX 0 0 1 1 0.2
Control (n=17)15 5 0 20 1.18
Small Bowel
Suture
A/MedX 0 2 1 3 0.6
A/Active/MedX 0 1 0 1 0.2
A/Active/LowX 0 0 1 1 0.2
Control (n=4) 0 7 8 15 0.88
Small Bowel
to
~ther ~r ans
edX 1 0 2 3 0.5
A/Active/MedX 1 0 0 1 0.2
A/Active/LowX 0 1 0 1 0.2
HA/Ie~IedX 13 0 1 7 1
23
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
Hysterectomy Model
[0053] A rabbit hysterectomy model was developed to mimic some of the clinical
problems of adhesions to surrounding tissue after surgery in humans. This
model was created
by ligating the left or right pedicle with 5/0 prolene sutures in order to
isolate a 4 to 5 cm
portion of the uterus. Approximately 4 cm of the uterus was then surgically
removed and
hemostasis was achieved by electrocarterization. The excised surface was
treated and the
wound closed using standard procedures. The site was observed after 14 days.
[0054] Table 8 provides a summary of the data from this experiment. In this
study, the
material V0714A contained 30% Poly(PEG-Lys-cHyp), while material V0714B
contained
to none. All treatment groups showed statiscally significant decreases in
incidence of adhesions
compared to the control group. However, the group V0714B (no Poly(PEG-Lys-
cHyp) in the
HA beads) was less effective compared to V0714A that contained the active
agent Poly(PEG-
Lys-cHyp). Residual polymer (most likely HA) was found in 2 of the 5 animals
treated with
V0714A, and 6 out of 6 animals treated with V0714B. There was slight
inflammation noted
at the site of the residual polymer and an adhesion was formed from the
residual polymer to
the bladder in one case.
24
CA 02521407 2005-09-30
WO 2004/089311 PCT/US2004/009919
TABLE 8: Hysterectomy Model with HA Beads
# of # of # of
Grade Grade Grade Total Mean
3 2 1
Group Adhe~. Adhe~. Adhe~. Incidence
V0714A0 2 1 3 0.6
(n=6)
V0714B2 2 4 8 1.6
(n=6)
Control6 2 3 11 2.75
(n=~)
[0055] The same hysterectomy model was used to investigate delivery of the
Poly(PEG-
Lys-cHyp) agent without the use of a delivery gel. Dosing of Poly(PEG-Lys-
cHyp) was done
using 7-day Alzet miniosmotic pumps, which provide linear sustained release of
the agent
over 7 days. 20 mg of the Poly(PEG-Lys-cHyp) in 100 ~l was loaded into each
pump. After
the hysterectomy was performed and prior to closing, three pumps were loaded
into the
periotoneal space of each animal. Table 9 provides a summary of the data from
this
to experiment. This study shows that the active agent Poly(PEG-Lys-cHyp) alone
may be
sufficient to inhibit the formation of adhesions.
TABLE 9: Hysterectomy Model with Alzet Minipumps
# of # of # of
Grade Grade Grade Total Mean
3 2 1
Group Adhes. Adhes. Adhes. Incidence
VMT39 0 0 1 1 0.3
(n=3)
Control6 2 3 11 2.75
(n=4)
25