Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
PROTEOSOME-BASED FOR VACCINATING AGAINST INFECTIOUS DISEASES
TECHNICAL FIELD
This disclosure relates generally to nasally-delivered vaccines for
protecting against infectious disease or toxic agents and, in particular, to
Proteosome-
based compositions and methods of use thereof for treating or preventing
various
diseases associated with microbial pathogens or toxic agents, such as Yersinia
pestis,
Variola virus, ricin toxin and botulinum toxin.
BACKGROUND
An efficient immune response depends on the communication between
the innate immune system (the first line of defense against invading
pathogens) and the
adaptive (specific) immune response (see generally, Klein et al., Immunology
(2nd),
Blackwell Science Inc., Boston, 1997). T lymphocytes are important for
coordinating
the adaptive immune response by controlling the release of effector molecules.
For
example, T helper (Th) 1 cells produce interleukin-2 (IL-2), tumor necrosis
factor alpha
(TNF-a), and interferon gamma (IFN-y), which are important for the development
of
cell-mediated immunity (Mosmann et al., J. Immunol. 136: 2348, 1986; Street
and
Mosmann, FASEB J. 5: 171, 1991). In contrast, Th2 cells produce IL-4, IL-13,
IL-5,
IL-9, IL-6 and IL-10, which are important for the stimulation of IgE
production,
rnucosal mastocytosis, and eosinophilia (Mosmann et al.; Street and Mosmann).
While
a shift toward a Thl (type 1 response) or Th2 (type 2 response) phenotype may
be
important fox the defense against pathogens, a shift in one direction or
another can also
be associated with the induction of autoimmune disease (ThI) or inflammatory
disease
(Th2).
In this regard, vaccines have been developed and used to induce an
adaptive immune response. Vaccines typically include an attenuated microbe or
a
microbial antigen (i. e., a microbe component such as a protein or nucleic
acid) to
activate a specific immune response. However, the ability of an antigen to
induce a
protective immune response in a host sometimes must be enhanced by formulating
the
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
antigen with an immunostimulant or an adjuvant. For over 100 years, aluminum-
based
mineral salts (generically known as alum) have been the only adjuvants
approved for
human use. Historically, alum has been an effective adjuvant by significantly
enhancing responses to adsorbed proteins when injected intramuscularly.
However, in
recent years, alum adjuvants has come under scrutiny due to reports (1) that
alum is a
weak adjuvant for the new generation of vaccine antigens, which are based on
highly
purified recombinant proteins; (2) that alum can reportedly induce IgE
antibodies and
has been associated with allergic reactions in some vaccine recipients; and
(3) that alum
can induce persistent inflammatory responses which are manifested as an
adverse
reaction at injection sites. These adverse reactions have been found to be
associated
with the tendency of alum-adsorbed vaccines to polarize antigen-specific
immune
responses towards a type 2 phenotype (i. e., inflammatory response).
Furthermore,
alum-based vaccines have only been approved for use in humans by the
injectable
route.
Other known adjuvants include Freund's adjuvant (complete or
incomplete), lipopolysaccharide (LPS, also referred to as endotoxin), and
pertussis
adjuvant (a saline suspension of killed Bordatella pertussis organisms).
Freund's
adjuvant consists of a mixture of mycobacteria in an oillwater emulsion.
However, due
to frequent toxic physiological and immunological reactions to this material,
Freund's
adjuvant cannot be used in humans. LPS stimulates the immune system by
triggering
an innate immune response, but LPS is too toxic to be a viable adjuvant.
Molecules that
are structurally related to endotoxin, such as monophosphoryl lipid A ("MPL"
or
detoxified LPS), are being tested as adjuvants in clinical trials. Other
potential
adjuvants being investigated include saponins (see U.S. Patent Nos. 6,080,725;
6,262,029; and 5,977,081); amphipathic aldehydes (see U.S. Patent No.
6,649,172);
proteosomes (see U.S. Patent Nos. 5,726,292 and 5,985,284; U.S. Patent
Application
Nos. 2001/0053368 and 2003/0044425); and cyclic aminoalkyl glucosaminide
phosphates (see U.S. Patent No. 6,525,028).
Hence, there is a recognized need in the art for identifying and
developing superior immunostimulatory compounds that can be co-administered
with
2
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
antigens to promote more robust immune responses to protect against microbial
infections or toxic agents. The present invention meets such needs, and
further
provides other related advantages.
BRIEF SLJI~IMARI' ~F TFIE INVEIVTI~N
The present disclosure provides adjuvant formulations for antigens, in
particular Proteosome-based adjuvants for formulation with various antigens
for use in
a variety of therapeutic settings, such as in treating or preventing, for
example,
infectious disease or the action of toxic agents.
In one aspect, the present invention provides a method for eliciting an
immune response to an antigen, comprising administering to a subject in need
thereof a
therapeutically effective amount of an immunogenic composition, wherein said
immunogenic composition comprises Proteosomes, liposaccharide and an antigen.
In
another aspect, the present invention provides a method for treating or
preventing a
microbial infection, comprising administering to a subject in need thereof a
therapeutically effective amount of an immunogenic composition, wherein said
immunogenic composition comprises Proteosomes, liposaccharide and an antigen.
In
certain embodiments, the antigen of the immunogenic compositions comprises one
or
more from Bacillus anthracis, Chlamydia trachornatis, Staphylococcus aureus,
Clostridium perfi~ingens, or Yer~sinia pesos. In certain other embodiments,
the antigen
of the immunogenic compositions comprises one or more from Poxvirus,
Alphavirus,
Flavivirus, Arenavirus, Bunyavirus, Filovirus, or Picornavirus. In yet other
embodiments, the antigen of the immunogenic compositions comprises one or more
toxins. In still other embodiments, the antigen is recombinant. In yet other
embodiments, the immunogenic composition comprises a plurality of different
antigens.
In a related embodiment, the plurality of antigens can comprise viral,
bacterial,
parasitic, toxin, or a combination thereof.
In certain embodiments, the immunogenic composition is administered
by a route selected from mucosal, enteral, parenteral, transdermal,
transmucosal, nasal,
or inhalation, or a combination thereof. In a related embodiment, the
immunogenic
3
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
composition elicits a protective immune response. In other embodiments, the
antigen
comprises a Protective Antigen from Bacillus anthracis, filamentous
hemagglutinin
(FIIA) of Boretella p~rtussis, an F1 antigen or a V antigen from Yersinia
pesos, an Fl-
V antigen fusion protein antigen, or a combination thereof. In still other
embodiments,
the antigen comprises one or more antigens from a Variola virus or from a
Vaccinia
virus. . In yet other embodiments, the antigen comprises one or more of
botulinum
toxin, ricin toxin, Staphylococcus enterotoxin B, or fragment or variant
thereof.
In still other embodiments, any of the aforementioned immunogenic
compositions have a liposaccharide final content by weight as a percentage of
Proteosome protein ranges from about 1% to about 500% in the immunogenic
composition. In other embodiments, the Proteosomes and liposaccharide are
obtained
from the same bacteria or from different bacteria, such as Gram-negative
bacteria. In
some embodiments, the liposaccharide is from Gram-negative bacteria selected
from
Shigella species, Chlamydia species, Yersinia species, Pseudomonas species,
Plesiomonas species, Escherichia species, Salmonella species, or a combination
thereof. In other embodiments, the Proteosomes are from Neisseria species. In
certain
embodiments, the immunogenic compositions of this disclosure have Proteosomes
are
from Neisseria meningitides and the liposaccharide is from Shigella flexneri.
In other
embodiments, any of the aforementioned immunogenic compositions have a ratio
of
Proteosomes to antigen that ranges from about 5:1 to about 1:500, or the ratio
is at least
1:2~ 1:5, or 1:10. In still other embodiments, any of the aforementioned
immunogenic
compositions further comprise a pharmaceutically acceptable carrier, excipient
or
diluent.
In another aspect, the instant disclosure provides a method for eliciting
an immune response to an antigen, comprising administering to a subject in
need
thereof a therapeutically effective amount of an immunogenic composition,
wherein
said immunogenic composition comprises an adjuvant and an antigen, wherein
said
adjuvant comprises Proteosomes and liposaccharide and said adjuvant to antigen
ratio
ranges from about 1:5 to about 1:500 or is at least 1:5, such that an immune
response to
said antigen is elicited. In a related aspect, there is provided a method for
treating or
4
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
preventing a microbial infection, comprising administering to a subject in
need thereof
a therapeutically effective amount of an immunogenic composition, wherein said
immunogenic composition comprises an adjuvant and an antigen, wherein said
adjuvant
comprises Proteosomes and liposaccharide and said adjuvant to antigen ratio
ranges
from about 1:5 to about 1:500 or is at least 1:5, such that a microbial
infection is treated
or prevented. In certain embodiments, provided is any of the aforementioned
immunogenic compositions wherein the antigen is at least one of a bacterial,
viral, or
toxin antigen.
These and other aspects of the present invention will become evident
upon reference to the following detailed description and attached drawings. In
addition,
various references are set forth herein which describe in more detail certain
procedures
or compositions, and are therefore incorporated by reference in their
entirety.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows serum IgG titers from mice immunized twice intranasally
with 50, 20 or S~.g of Fl-V with or without Protollin (2.5, 1 or 0.25 ~,g), or
injected
intramuscularly with 20wg F1-V adsorbed onto alum (Alhydrogel~). Half the mice
were euthanized on day 35 post-primary immunization, and the other half on day
55.
Titers are expressed as the geometric mean of specific antibody concentrations
(~g/ml
for serum IgG; ng/ml for lung IgA and IgG) and 95% confidence limits are
shown.
Figure 2 shows lung IgG titers from mice immunized as described for
Figure 1.
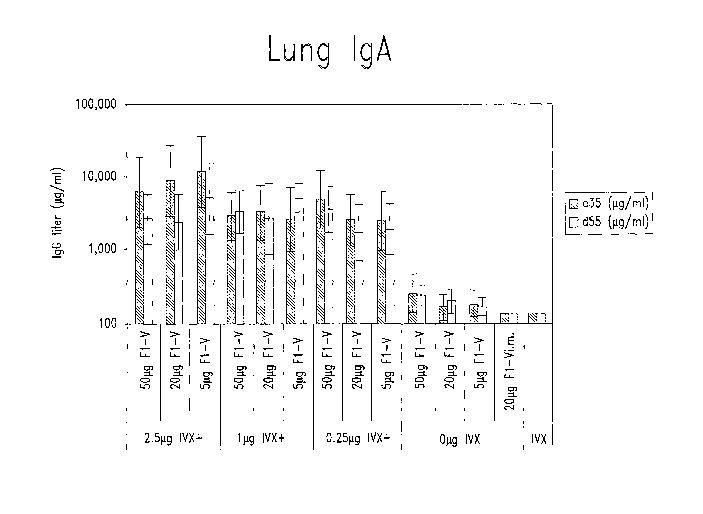
Figure 3 shows lung IgA titers from mice immunized as described for
Figure 1.
Figures 4A and 4B show the survival of mice against challenge with
lethal doses of aersolized Yersi~cia pestis. Mice immunized twice with 20 ~g
Fl-V
intranasally with (0.25, l, or 2.5 ~,g) or without Protollin, or
intramuscularly adsorbed
onto Alhydrogel~, were challenged by whole body exposure to 170 LDSO of Y.
pestis
(A) 35 days or (B) 55 days post-primary immunization. In all studies, all
control mice
5
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
(that received Protollin only) died when challenged with Y. pestis, confirming
the
inoculum used was lethal.
Figures SA and SB show the survival of mice against challenge with
lethal doses of aersolized Yersirzia pesos. Mice immunized twice with 50 ~g F1-
V
intranasally with or without 1 ~g of Protollin, or intramuscularly adsorbed
onto
Alhydrogel~, were challenged by whole body exposure to (A) 255 LDSO of Y.
pesos
55 days post primary immunization; (B) shows the survival of mice challenged
on day
35 and day 55 (different groups of mice) post-primary immunization with 170
lethal
doses of aersolized Y. pestis. Mice were immunized twice with 5 ~g F1-V
intranasally
with (0.25, l, or 2.5 fig) or without Protollin. All the control mice (those
that received
Protollin only) died when challenged with Y. pestis, confirming the inoculum
used was
lethal.
Figure 6 shows the amounts of IFN-y, TNF-a and IL-5 released from
splenocytes stimulated in vitro with antigen F1-V. Splenocytes were harvested
from
mice immunized intranasally with 50 ~.g of F1-V with or without 1 ~g
Protollin, or
injected intramuscularly with 20 ~,g of F1-V adsorbed onto Alhydrogel~, on day
35
after immunization.
Figures 7A and 7B show serum IgG and lung IgA levels, respectively, in
mice immunized nasally (on days 0 and 14) with 5 ~,g or 25 ~g of recombinant
Protective Antigen (rPA) from Bacillus authracis admixed with (1 fig) or
without
Protollin.
Figures 8A and 8B show results of anthrax neutralization assays using
serum and lung lavage fluid from mice immunized with rPA admixed with
Protollin.
Figure 9 shows serum IgG in mice immunized (on days l, 21 and 31)
with 15 ~,g of recombinant filamentous hemagglutinin (rFHA) from Bordetella
pertusis
admixed with liposome (intranasally, i.n.), Protollin (i.n.), or Alhydrogel~
(intraperitoneally, i.p.). As controls, mice were immunized i.p. with
QuadracelTM
(positive) and i.n. with excipient PBS alone (negative). QuadracelTM (Aventis
Pasteur
Ltd.) is a vaccine containing, among other antigens, B~rdetella pertusis FHA.
Titers
were determined by measuring the level of anti-rFHA in serum harvested seven
days
6
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
after the last immunization (day 38) (less the background titer level found,
if any, in
serum from mice on day 1).
Figure 10 shows salivary IgA in mice immunized (on days 1, 21 and 31)
with 15 ~g of recombinant filamentous hemagglutinin (rFHA) from Bordetella
per~tusis
formulated in the same compositions as described for Figure 9.
Figure 11 shows anti-rFHA serum IgG subclasses (IgG2a and IgGl)
found in pooled sera from mice immunized as described for Figure 9.
Figure 12 shows the level of Bor~detella pertusis found. in the lungs of
immunized mice as described for Figure 9 when challenged with aerosolized B.
pertusis
eleven days after the final immunization (day 42). Lung counts were measured
on days
1, 3, 7, and 14 post-aerosol challenge.
DETAILED DESCRIPTION OF THE INVENTION
Prior to setting forth the invention, it may be helpful to an understanding
thereof to set forth definitions of certain terms that will be used
hereinafter.
"Proteosome or Projuvant" as used herein refers to preparations of outer
membrane proteins (OMPs, also known as porins) from Gram-negative bacteria,
such
as Neisseria species (see, e.g., Lowell et al., J. Exp. Med. 167:658,1988;
Lowell et al.,
Science 240:800, 1988; Lynch et al., Biophys. J. 45:104, 1984; Lowell, in "New
Generation Vaccines" 2nd ed., Marcel Dekker, Inc., New York, Basil, Hong Kong,
page
193, 1997; U.S. Patent No. 5,726,292; U.S. Patent No. 4,707,543), which are
useful as. a
carrier or an adjuvant for immunogens, such as bacterial or viral antigens.
Proteosomes
are hydrophobic and safe for human use, and comparable in size to certain
viruses.
Proteosomes have the interesting ability to auto-assemble into vesicle or
vesicle-like
OMP clusters of 20-800 run, and to noncovalently incorporate, coordinate,
associate
(e.g., electrostatically or hydrophobically), or otherwise cooperate with
protein antigens
(Ags), particularly antigens that have a hydrophobic moiety. Any preparation
method
that results in the outer membrane protein component in vesicular or vesicle-
like form,
including mufti-molecular membranous structures or molten globular-like OMP
compositions of one or more OMPs, is included within the definition of
Proteosome.
7
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
Proteosomes may be prepared, for example, as described in the art (see, e.g.,
U.S.
Patent Nos. 5,726,292 or 5,985,284).
"Liposaccharide" as used herein refers to native (isolated or prepared
synthetically with a native structure) or modified lipopolysaccharide or
lipooligosaccharide (collectively, also referred to as "LPS") derived from
Gram-
negative bacteria, such as Shigella flexneri or Plesiomonas shigelloides, or
other Gram-
negative bacteria (including Alcaligenes, Bacteroides, Bordetella, Borrellia,
Brucella,
Campylobacter, Chlamydia, Citrobacter, Edwardsiella, Ehrlicha, Enterobacter,
Escherichia, Francisella, Fusobacterium, Cpardnerella, Hemophillus,
Helicobacter,
Klebsiella, Legionella, Leptospira (including Leptospira interrogans),
Moraxella,
Morganella, Neiserria, Pasteurella, Proteus, Providencia, other Plesiomonas,
Porphyrornonas (including Porphyromonas gingivalis), Prevotella, Pseudomonas,
Rickettsia, Salrnonella, Serratia, other Shigella, Spirillum, heillonella,
llibrio, or
Yersinia species). The liposaccharide may be in a detoxified form (i.e.,
having the
Lipid A core removed) or may be in a form that has not been detoxified. In the
instant
disclosure, liposaccharide need not be and preferably are not detoxified. The
liposacchaxide may be prepared, for example, as described in U.S. Patent
Application
Publication No. 2003/0044425.
"Proteosome:LPS or Protollin or IVX or IVX-908" as used herein refers
to preparations of projuvant admixed as described herein with at least one
kind of
liposaccharide to provide an OMP-LPS composition (which can function as an
immunostimulatory composition). Thus, the OMP-LPS adjuvant can be comprised of
two of the basic components of Protollin, which include ( 1 ) an outer
membrane protein
preparation of Proteosomes (i.e., Projuvant) prepared from Gram-negative
bacteria,
such as Neisseria meningitides, and (2) a preparation of one or more
liposaccharides.
Protollin should also be understood to optionally include lipids, glycolipids,
glycoproteins, small molecules, or the like, and combinations thereof. The
Protollin
may be prepared, for example, as described in U.S. Patent Application
Publication No.
2003/0044425.
8
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
Projuvant is generally used in conjunction with antigens that possess a ',
(naturally-occurring or modified) hydrophobic moiety (also referred to as a
"foot" or
"anchor"). Protollin (with exogenously added LPS), which although can be used
with
an antigen that do not contain a hydrophobic foot domain and which are largely
hydrophilic in nature, or can be associated with a antigen containing a
hydrophobic
foot, or a combination thereof.
"Toxin or Toxic Agent" as used herein refers to is a noxious or
poisonous substance that is antigenic and is formed or elaborated as an
integral part of a
cell or tissue (e.g., endotoxin), as an extracellular product (e.g.,
exotoxin), or as a
combination of the two, during the metabolism or growth of certain
microorganisms or
some higher plant and animal species. For use in the immunogenic compositions
of the
instant disclosure, it should be understood that a toxin or toxic agent will
be in the form
of a "toxoid" so that the immunogenic compositions axe not toxic or poisonous.
A
"toxoid" form is a toxin that has been treated so as to destroy the toxic
property of the
agent while still retaining antigenicity (i.e., capable of eliciting anti-
toxin or a
neutralizing immune response). The "treatment" of a toxin to generate a toxoid
includes chemical treatment (e. g. , formaldehyde), mutations, fragments, and
combinations thereof. Exemplary toxins include ricin~toxin, botulinum toxin,
alfatoxin,
episolntoxin, Staphylococcus enterotoxin B, tetrodotoxin, snae venom toxin,
diptheria
toxin, cholera toxin, saxitoxin, trichothecene mycotoxins, etc.
"Immuno$enic composition" as used herein refers to any one or more
compounds or agents capable of priming, potentiating, activating, stimulating,
augmenting, boosting, amplifying, or enhancing an adaptive (specific) immune
response, which may be cellular (T cell) or humoral (B cell), or a combination
thereof.
Preferably, the adaptive immune response will be protective, neutralizing, or
both. A
representative example of an immunogen is a microbial antigen (such as one or
more
bacterial, viral, or parasite proteins of interest) or a non-microbial antigen
(e.g., antigens
obtained from other sources, such as from plants (ricin), from dinoflagellates
(saxitoxin), and the like).
9
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
"Specific or Acquired or Adaptive immune res onse" as used herein
refers to a resistance mediated by a mammalian immune system resulting from
previous
exposure to an infectious agent or antigen. For example, specific immunity can
be a
result of a naturally acquired (patent or latent) infection or from an
intentional
vaccination. In addition, specific immunity may be passively and transitorily
acquired
from the transfer of antibodies from another naturally (e.g., maternally
inherited), or
from transfer of antibodies or immune cells by intentional inoculation (e.g.,
antibody
mediated immunotherapy).
In the present description, any concentration range, percentage range,
ratio range or other integer range is to be understood to include the value of
any integer
within the recited range and, when appropriate, fractions thereof (such as one
tenth and
one hundredth of an integer), unless otherwise indicated. As used herein,
"about" or
"comprising essentially of mean ~ 15%. The use of the alternative (e.g., "or")
should
be understood to mean one, both, or any combination thereof of the
alternatives. As
used herein, the use of an indefinite article, such as "a" or "an", should be
understood to
refer to the singular and the plural of a noun or noun phrase. In addition, it
should be
understood that the individual compositions, formulations, or compounds, or
groups of
compositions, formulations, or compounds, derived from the various components
or
combinations of the composition or sequences, structures, and substituents
described
herein, axe disclosed by the present application to the same extent as if each
composition or compound or group of compositions or compounds was set forth
°
individually. Thus, selection of particular sequences, structures, or
substituents is
within the scope of the present invention.
As set forth above and described herein, the present invention provides
compositions and methods for using and making immunogenic compositions to
treat or
prevent a variety of infectious diseases or to treat or prevent pathology
associated with
toxic agents. In particular, provided are immunogenic compositions comprising
an
adjuvant (such as Proteosomes or Proteosomes with liposaccharide) and an
antigen
(such as microbial antigens or toxins) formulated with, for example, limited
amounts of
adjuvant and excess amounts of antigen. The instant invention, therefore,
relates
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
generally to the surprising discovery that Proteosome-based adjuvants have
such robust
immunostimulatory activity that more antigen can be formulated with less
adjuvant to
provide new and useful vaccine formulations. Discussed in more detail below
are
immunogenic compositions comprising Proteosome:LPS or Proteosomes formulated
with one or more microbial antigens. In certain aspects, the compositions of
the instant
description are suitable for therapeutic uses such as treating or preventing a
microbial
infection by inducing a specific immune response, or eliciting an immune
response
capable of providing protective immunity (against infection) or neutralizing
immunity
(against toxic agents).
1 O PROTEOSOME-BASED VACCINE ADJUVANTS - ("PROJUVANT" AND "PROTOLLIN"~
The invention relates to immunogenic compositions that contain one or
more antigen capable of eliciting an immune response. As set forth above, many
antigens are poorly immunogenic unless formulated with an adjuvant. The only
adjuvant licensed for use in humans is alum, but this adjuvant has limitations
as .
described above. Despite the multiplicity of efforts to identify alternative
adjuvants,
there remains a need for effective compositions to immunize individuals in
need
thereof, particularly against infectious diseases and toxic agents.
The Proteosome-based adjuvant of the instant disclosure can be used in
vaccine formulations, which may include a variety of antigen sources. For
example,
live attenuated microbes, killed microbes, split antigens, subunit antigens,
toxic agent
antigens, and combinations thereof. To maximize the effectiveness of a subunit
vaccines, the antigens should be combined with a potent immunostimulant or
adjuvant.
Exemplary adjuvants include alum (aluminum hydroxide, REHYDRAGEL~),
aluminum phosphate, Proteosome adjuvant (see, e.g., U.S. Patent Nos. 5,726,292
and
5,985,284, and U.S. Patent Application Publication Nos. 2001/0053368 and
2003/0044425), virosomes, liposomes with and without Lipid A, Detox
(Ribi/Corixa),
MF59, or other oil and water emulsions type adjuvants, such as nanoemulsions
(see,
e.g., U.S. Patent No. 5,716,637) or submicron emulsions (see, e.g., U.S.
Patent No.
11
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
5,961,970), and Freund's complete and incomplete adjuvant. A particularly
preferred
adjuvant is a Proteosome or Protollin.
Proteosomes are comprised of outer membrane proteins (OMP) from
Neisseria species typically, but can be derived from other Gram-negative
bacteria (see,
e.g., Lowell et al., .I. ~xla. Med 167:658, 1988; Lowell et al., Scieizce
240:800, 1988;
Lynch et al., Biophys. .I. 45:104, 1984; U.S. Patent No. 5,726,292; U.S.
Patent No.
4,707,543). Proteosomes have the interesting ability to auto-assemble into
vesicle or
vesicle-like OMP clusters of 20-800 nm, and to noncovalently incorporate,
coordinate,
associate, or otherwise cooperate with protein antigens (Ag), particularly
antigens that
have a hydrophobic moiety. Proteosomes are hydrophobic and safe for human use,
and
comparable in size to certain viruses. By way of background, and not wishing
to be
bound by theory, mixing of Proteosomes with a protein (e.g., antigen) provides
a
composition comprising non-covalent association or coordination between the
antigen
and Proteosomes, which association or coordination forms when solubilizing
detergent
is selectively removed or reduced, for example, by dialysis. Proteosomes may
be
prepared as described in the art or as described in U..S. Patent Application
Nos.
2001/0053368 and 2003/0044425.
Any preparation method that results in the outer membrane protein
component in vesicular or vesicle-like form, including molten globular-like
OMP"
compositions of one or more OMP, is included within the definition of
"Proteosome."
In one embodiment, the Proteosomes are from Neisseria species, and nriore
preferably
from Neisseria meningitides. In certain embodiments, Proteosomes are not a
carrier but
are an adjuvant. In certain other embodiments, Proteosomes may be an adjuvant
and an
antigen delivery composition. In a preferred embodiment, an immunogenic
composition comprises one or more antigens (e.g., bacterial, viral, parasitic,
fungal,
toxin antigens, and fragments or variants thereof) as described herein and an
adjuvant,
wherein the adjuvant comprises Projuvant or Protollin. As described herein,
the
antigens can be from a recombinant source or comprise, for example, a
(detergent) split
antigen.
12
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
In certain embodiments, the invention provides an immunogenic
composition that further comprises an immunostimulant, such as a
liposaccharide. That
is, the adjuvant may be prepared to include an additional immunostimulant. For
example, the projuvant may be mixed with a liposaccharide to provide am OMP-
LPS
adjuvant. Thus, the OMP-LPS (Protollin) adjuvant can be comprised of two basic
components. The first component is an outer membrane protein preparation of
Proteosomes (i.e., Projuvant) prepared from CJram-negative bacteria, such as
Neisser~ia
meningitidis. The second component is a preparation of liposaccharide. The
liposaccharide may be prepared as described in U.S. Patent Application Nos.
2001/0053368 and 2003/0044425. It is also contemplated that the second
component
may include lipids, glycolipids, glycoproteins, small molecules or the like,
and
combinations thereof.
As described herein, the two components of an OMP-LPS adjuvant may
be formulated at specific initial ratios to optimize interaction between the
components
resulting in stable association and formulation of the components for use in
the
preparation of an immunogenic composition of the invention. The process
generally
involves the mixing of components in a selected detergent solution (e.g.,
Empigen~ BB,
Triton~ X-100, or Mega-10) and then effecting complexing of the OMP and LPS
components while reducing the amount of detergent to a predetermined,
preferred
concentration, by dialysis or, preferably, by diafiltration/ultrafiltration
methodologies.
Mixing, co-precipitation, or lyophilization of the two components may also be
used to
effect an adequate and stable association or formulation. In a preferred
embodiment, an
immunogenic composition comprises one or more antigens as described herein and
an
adjuvant, wherein the adjuvant comprises a Projuvant (i. e., Proteosome) and
liposaccharide.
In the preferred embodiment, the final liposaccharide content by weight
as a percentage of the total Proteosome protein can be in a range from about 1
% to
about 500%, more preferably in range from about 10% to about 200%, or in a
range
from about 30% to about 150%. Another embodiment of the instant invention
includes
an adjuvant wherein the Proteosomes are prepared from Neisseria meningitides
and the
13
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
liposaccharide is prepared from Shigella flexneri or Plesiomonas shigelloides,
and the
final liposaccharide content is between 50% to 150% of the total Proteosome
protein by
weight. In another embodiment, Proteosomes are prepared with endogenous
lipooligosaccharide (LOS) content ranging from about 0.5°/~ up to about
5°/~ of total
OMP. Another embodiment of the instant invention provides Proteosomes with
endogenous liposaccharide in a range from about 12% to about 25%, and in a
preferred
embodiment between about 15% and about 20°/~ of total OMP. The instant
invention
also provides a composition containing liposaccharide derived from any Gram-
negative
bacterial species, which may be from the same Gram-negative bacterial species
that is
the source of Proteosomes or is a different bacterial species.
In certain embodiments, the Proteosome or Protollin to antigen ratio in
the mixture is greater than l :l, greater than 2:1, greater than 3:1 or
greater than 4:1.
The ratio can be as high as 8:1 or higher. In other embodiments, the ratio of
Proteosomes to antigen of the immunogenic composition ranges from about 1:1 to
about 1:500, preferably the ratio is at least 1:5, at least 1:10, at least
1:20, at least 1:50,
or at least 1:100. An unexpected result of the instant invention is that
Protollin:antigen
ratios could be reduced dramatically (from 1:2 to 1:200) with no significant
effect on
the ability of an antigen to elicit an immune response.
ANTIGENS
The invention relates to immunogenic compositions that contain one or
more antigens, which can be used to elicit an immune response, such as a
protective
immune response. The invention further relates to methods for treating and
preventing
microbial infections by administering to a subject one or more antigens or
fragments or
variant thereof, fusion protein, multivalent immunogen, or a mixture of such
immunogens at a dose sufficient to elicit an immune response (cellular or
humoral)
specific for the antigen (which may be a protective immune response), as
described
herein. The antigens are preferably from clinically relevant microorganisms,
such as
bacteria, viruses (e.g., Influenza, Measles, Coronavirus), parasites (e.g.,
Trypansome,
Plasmodium, Leishmania, pathogenic bacteria) fungi (e.g., Aspergillus,
Candida,
14
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
Coccidioides, Cryptococcus), and the like. For example, the antigen may be
from
bacteria, such as the causative agent of anthrax (Bacillus anthracis), plague
(Yersinia
pestis), stomach cancer (Flelic~bacter pylori), Q fever (Coxiella burnetii),
whooping
cough (B~rdetella pertussis, e.g , FHA), botulism (Cl~stridiuna b~tulinurn,
types A,
B,C, D, and E), gas gangrene (Cl~stridiurrZ p~rfringens), tularemia
(Francisella
tularensis), meliodiosis (Pseudornonas pseudomallei), brucellosis (Brucella
suis, B.
abortus, B. canis, B. tnelitensis), sexually transmitted diseases (Chlamydia
tf~ach~matis
or Neisseria gonorrhea), toxin producing organisms, such as Clostridium
perfringens
(epsilon toxin, one of twelve protein toxins produced by these gram positive,
anaerobic
spore-forming bacteria).
There are five strains of C. perfringens (A through E), each producing a
unique set of toxins; epsilon toxin is produced by the B and D types. Epsilon
toxin is
known as a performing polypeptide that causes potassium and fluid leakage from
cells.
In addition to epsilon toxin the A through E types may produce one or more of
Alpha,
beta, epsilon or iota toxin. The epsilon toxin can be disseminated by
ingestion of
contaminated food or water, or by inhalation of aerosolized toxin. Another
exemplary
toxin of concern is Staphylococcal enterotoxin B (SEB) produced by
Staphylococcus
aureus. SEB is known to cause illness if inhaled and maybe the cause of food
poisoning. The immune system of SEB intoxicated individuals is compromised and
although not frequently associated with death, there is significant and wide
spread
incapacitating illness, such as high fever, chills, headache, muscle aches,
inflammation
of the lining of the eyelids, nausea, vomiting and diarrhea. There is no
vaccine or anti-
SEB currently available.
In certain embodiments, isolated LPS may be an antigen, for example,
LPS isolated from P. gingivalis, which may be formulated with Proteosomes for
use in
stimulating an immune response to P. gingivalis for treating or preventing gum
disease,
periodontal disease, tooth decay, and the like.
Other representative examples include antigens from certain viruses,
such as Norwalk virus, small pox virus, West Nile virus, SARS virus,
respiratory
syncytial virus, and the like. Examples of Filoviruses include lVlarburg virus
and Ebola
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
virus; examples of Flaviviruses include Dengue virus, Yellow Fever virus,
Japanese
encephalitis virus, Russian spring-summer encephalitis virus, St. Louis
encephalitis
virus; examples of Arenaviruses include Junin (Argentine hemorrhagic fever)
virus;
Lassa fever virus, lymphocytic choriomeningitis virus, Machupo (Bolivian
hemorrhagic
fever) virus; examples of Bunyaviruses include Crimean-Congo hemorrhagic fever
virus, Hantaan (Korean hemorrhagic fever) virus, Rift Valley fever virus;
examples of
Picornavirus includes Hepatitis A virus, polio virus; examples of
orthomyxovirus
includes influenza virus A, B, C (e.g., HA antigen), Thogoto virus; examples
of
alphaviruses include Chikungunya virus, Eastern encephalitis virus, Venezuelan
encephalitis virus, Western encephalitis virus. Exemplary fungi include
Candida
albicans, Aspergillus spp., Coccidioides immites, Histoplasma capsulatum,
Norcardia
farcinica; and exemplary parasites include the causative agents of
trypanosomiasis,
leishmania, pneumonic plague, and lyme disease (Borrellia burgdorferi). In
addition,
antigens of interest may be toxic agents (e.g., ricin, botulinum).
As described herein, the antigens can be prepared recombinantly,
synthetically, isolated from a biological source, recombinantly or chemically
modified,
and any combination thereof. Microbial antigens or fragments thereof can be
prepared
from a variety of biological sources, such as tissues of an infected subject
or cultured
cell lines. Primary isolation may be from, for example, peripheral blood cells
or from
respiratory secretions. Preferably, the isolated microbes are amplified on
primary cell
cultures or on established cell lines known in the art. In certain
embodiments, the
antigens or fragments thereof are isolated from intact microbial particles. As
used
herein, the term "isolated" or "derived from" means that the material is
removed from
its original or natural environment. For example, a naturally occurring
nucleic acid
molecule or polypeptide present in a living animal or cell, or virus is not
isolated, but
the same nucleic acid molecule or polypeptide is isolated when separated from
some or
all of the co-existing materials in the natural system. For example, nucleic
acid
molecules could be contained in a vector and/or such nucleic acids could be
part of a
composition and still be isolated in that such a vector or composition is not
part of the
original nucleic acid molecule's natural environment. In other embodiments,
peptides
16
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
or polypeptides, such as antigens or variants and fragments thereof, may be
either
partially purified or purified to homogeneity.
The present invention further provides methods for producing synthetic
antigens, including fusion proteins. The immunogenic polypeptide components
may be
synthesized by standard chemical methods, including synthesis by automated
procedure. In general, immunogenic polypeptides or peptides are synthesized
based on
the standard solid-phase Fmoc protection strategy with HATLT as the coupling
agent.
The immunogenic peptide can be cleaved from the solid-phase resin with
trifluoroacetic
acid containing appropriate scavengers, which also deprotects side chain
functional
groups. Crude immunogenic peptide may be further purified using preparative
reverse
phase chromatography. Other purification methods, such as partition
chromatography,
gel filtration, gel electrophoresis, or ion-exchange chromatography may be
used. Other
synthesis techniques known in the art may be employed to produce similar
immunogenic peptides, such as the tBoc protection strategy, use of different
coupling
reagents, and the like. In addition, any naturally or non-naturally occurring
amino acid
or derivative thereof may be used, including D- or L-amino acids and
combinations
thereof.
As described herein, the microbial antigens or fragments thereof of the
invention may be recombinant, wherein a desired antigen is expressed from a
polynucleotide that is operably linked to an expression control sequence
(e.g.,
promoter) in a nucleic acid expression construct. For example, host cells
(such as
baculovirus and mammalian cell lines) containing the immunogen-encoding
nucleic
acid expression constructs can be cultured to produce recombinant protein
immunogens, or fragments thereof. The antigens may be further fused or
conjugated to
an amino acid sequence, which sequence may be a hydrophobic anchor or foot to
facilitate or otherwise enhance non-covalent association with Projuvant or
Protollin.
The antigen polypeptides may comprise any portion of such polypeptides that
have at
least one epitope capable of eliciting a protective immune response (cellular
or
humoral) against microbial infection. Immunogenic polypeptides of the instant
invention may also be arranged or combined in a linear form, and each
immunogen may
17
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
or may not be reiterated, wherein the reiteration may occur once or multiple
times. In
addition, a plurality of different immunogenic polypeptides (e.g., protein
variants, or
fragments thereof) can be selected and mixed or combined into a cocktail
composition
to provide a multivalent vaccine for use in eliciting a protective immune
response.
To provide more detail about certain exemplary antigens for use in the
immunogenic compositions of the instant disclosure, the following descriptions
is
provided.
A. PLAGUE
Plague, also known as the Black Death, is a disease with a long history.
The plague pandemic of the Middle Ages killed up to 30 million people in
Europe.
Natural outbreaks still occur in endemic areas throughout the world, but
because of the
availability of antibiotic treatments, the disease causes little morbidity and
death is a
rare outcome. The fear surrounding plague, however, has been recently
heightened
with the possibility that it could be used as a biological warfare agent.
Bubonic plague are spread to humans through bites from fleas infected
with Yersinia pestis, are relatively easy to diagnose and, therefore treat
effectively with
anti-bacterials. The most fatal form of plague, pneumonic plague, is
transmitted
person-to-person by the respiratory route, or as the result of spread of
bubonic or
septicemic plague, by an infected and untreated person. Treatment of pneumonic
plague after exposure is more problematic because of its rapid course - unless
antibacterial treatment begins within 24 hours of showing symptoms of
infection, most
people who inhale Y. pestis will die. With the growing concern over the
possible
intentional transmission of plague, high priority has been given to the
development of a
safe vaccine capable of protecting against the most virulent pneumonic form of
plague.
In some embodiments, the present invention provides a method for
eliciting an immune response against plague, comprising administering to a
subject in
need thereof a therapeutically effective amount of an immunogenic composition,
wherein said immunogenic composition comprises Proteosomes, liposacchaxide and
one or more plague antigens, or variants or fragments thereof. In other
embodiments,
18
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
the present invention provides a method for treating or preventing a plague
infection,
comprising administering to a subject in need thereof a therapeutically
effective amount
of an immunogenic composition, wherein said immunogenic composition comprises
Proteosomes, liposaccharide and one or more plague antigens or variants or
fragments
thereof, wherein the plague infection is treated or prevented. In certain
embodiments,
the plague antigen used in the immunogenic composition can comprise an F1
antigen or
a V antigen from Ye~si~cia pestis, or an F1-V antigen fusion protein antigen,
or a
combination thereof.
B. SMALL POX
In one embodiment, Protollin formulations comprising viral antigens that
evoke a protective immune response against infection by, for example, variola
virus
causing smallpox disease in man is contemplated herein. Variola virus is the
most
notorious member of the virus family termed the Poxviridae and has
historically had a
significant and dramatic effect on human civilization. The Poxviridae is a
large family
of DNA viruses, which replicate in the cytoplasm of infected host cells.
Infection with
Variola virus kills 20-30% of those infected, and is highly contagious. Even
though
twenty years of prophylactic immunizations with cowpox and vaccinia virus
vaccines
have essentially eradicated occurrence of smallpox disease in man by the late
1970s
there remains concern that smallpox virus could reemerge as a significant and
deadly
disease either from a naturally occurring virus population or as a consequence
of
intentional introduction into an un-expecting population as a biological
warfare agent.
Virus particles purified from infected host cells are composed of as
many as 30 or more discreet polypeptide bands when resolved by polyacrylamide
gel
electrophoresis. The genomic sequence of the Copenhagen strain of vaccinia
virus
contains about 185 unique, nonoverlapping open reading frames (ORFs) of more
than
65 amino acids. By convention, the naming of the orthopoxvirus ORFs consists
of
using the IiindII restriction endonuclease fragment letter followed by the ORF
number
(from left to right) and L or R depending on the direction of the ORF. The
complete
and nearly identical sequences of the India and Bangladesh strains of variola
virus also
19
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
contain about 187 ORFs of which 150 have more than 90% sequence identity to
those
of vaccinia virus. One or more of he polypeptides encoded by vaccinia virus
ORFs
with 90% sequence identity to variola virus encoded polypeptides is assumed to
mediate the immune response responsible for protection following vaccinia
virus
immunization. By way of example but not in limitation, a number of ORFs are
known
to encode extracellular virus-specific polypeptides that may be involved in
eliciting a
protective immune response. They include, the non-glycosylated 41.8 kilodalton
protein encoded by the F 13L ORF; the N-glycosylated 19.5 kilodalton protein
encoded
by the A34R ORF; the 25.1 kilodalton protein encoded by the A36R ORF; the N-
and
O-glycosylated hemagglutinin protein encoded by the A56R ORF; and the
glycosylated
35.1 kilodalton protein encoded by the BSR ORF. The immune response to one or
more of these proteins is expected to elicit a humoral or cell mediated immune
response
which may protect from variola virus infection or reduce the pathological
effects of
infection. An infected host immune response preferentially involving a
balanced or fme
tuned TH1/Th2 cytokine profile is expected to be effective in preventing or
treating
variola virus infection and occurrence of smallpox disease.
Four members of the Orthopoxvirus cause human infection, they are
monkeypox, cowpox, variola and vaccinia viruses. Cowpox and vaccinia virus
have
been widely used to vaccinate humans against smallpox infection because they
produced less serious complications compared to infection by variola virus. To
a large
extent, smallpox has been eradicated from being a serious health threat, and
continued
wide spread vaccination of the general population has been halted. However,
reemergence of a natural virus or the rapid and wide deployment of a smallpox
virus as
a biological threat agent, and the possible unwanted side effects resulting
from
vaccination with live virus creates the need for the development of
alternative vaccines
against small pox.
To date, there is no non-living sub-unit vaccine against smallpox virus
(i.e., variola virus) infection. Current vaccine formulations require the use
of attenuated
live virus to evoke a protective immune response, often with unwanted side
effects. In
certain embodiments, the present invention provides a method for eliciting an
immune
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
response against small pox, comprising administering to a subject in need
thereof a
therapeutically effective amount of an immunogenic composition, wherein said
immunogenic composition comprises Proteosomes, liposaccharide and one or more
small pox antigens or a small pox viral particle extract. In other
embodiments, the
present invention provides a method for treating or preventing a small pox
infection,
comprising administering to a subject in need thereof a therapeutically
effective amount
of an immunogenic composition, wherein said immunogenic composition comprises
Proteosomes, liposacchaxide and one or more small pox antigens or a small pox
viral
particle extract, wherein the small pox infection is treated or prevented.
C. RICIN
In yet another aspect of the current invention, Proteosome-based vaccine
compositions capable evoking an immune response to non-infectious, toxic or
poisonous agents are contemplated herein. Such non-infectious agents include,
for
. example, ricin toxin produced by the bean plant Ricinus communis, which is
poisonous
to humans, animals and insects. Ricin is a cytotoxin produced by castor beans,
poisoning by inhalation or ingestion is caused by ricin. Ricin poisoning can,
depending
on dose, begin within a few hours of ingestion, causing abdominal pain,
vomiting,
diarrhea. Ricin poisoning, if unchecked, develops within several days into
severe
dehydration and a decrease in blood pressure resulting in death within 3-5
days after
ingestion (or inhalation). Ricin is a potent toxin that is fairly easily
produced.and can
cause poisoning (toxicity) by inhalation as a small particle or following
ingestion.
Ricin is also quite stable. Symptoms of ricin poisoning include weakness,
fever, cough
and pulmonary edema followed by severe respiratory distress and death from
hypoxemia. In rodents, the histopathology of aerosol exposure is characterized
by
necrotizing airway lesions causing tracheitis, bronchitis and interstitial
pneumonia with
perivascular and aleveolar edema. In rodent, ricin is more toxic by inhalation
than by
other routes of exposure. Symptoms of ricin poisoning in a significant number
of
geographically localized individuals may suggest exposure to aerosolized
ricin.
21
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
Cytotoxicity is mediated by the inhibition of protein synthesis through a
mechanism that specifically and irreversibly inactivates the eukaryotic
ribosome.
Accordingly, ricin is also known a ribosome-inactivating protein (RIP). The
RIPS such
as ricin are generally made up of two disulfide bonded polypeptide chains. ~ne
polypeptide chain mediates binding to cells and uptake into the cytoplasm
while the
other chain functions to inhibit protein synthesis. In certain embodiments,
the present
invention provides a method for eliciting an immune response against ricin
toxin,
comprising administering to a subject in need thereof a therapeutically
effective amount
of an immunogenic composition, wherein said immunogenic composition comprises
Proteosomes, liposaccharide and one or more ricin toxin antigens or fragments
or
variant thereof, wherein the ricin toxin antigen elicits a neutralizing
antibody response.
Thus, proteosome vaccine formulations comprising ricin immunogenic
polypeptides
that modulate the host Thl/Th2 immune response, and inactivates or attenuates
toxin
activity, are contemplated herein.
C. BOTULINUM
Botulism is caused by intoxication with any of the seven distinct
neurotoxins produced by the bacillus, Clostridium botulinum. The botulinum
toxins axe
proteins with molecular mass of approximately 150,000 KDa, which bind to the
presynaptic membrane of neurons at peripheral cholinergic synapses to prevent
release
of acetylcholine and block neurotransmission. The blockade is most evident
clinically
in the cholinergic autonomic nervous system and at the neuromuscular junction.
Symptoms of inhalation botulism may begin as early as 24-36 hours following
exposure
or as late as several days. Initial signs and symptoms include ptosis,
generalized
weakness, lassitude, and dizziness. Development of respiratory failure may be
abrupt.
In certain embodiments, the present invention provides a method for
eliciting an immune response against botulinum toxin, comprising administering
to a
subject in need thereof a therapeutically effective amount of an immunogenic
composition, wherein said immunogenic composition comprises Proteosomes,
22
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
liposaccharide and one or more botulinum toxin antigens or fragments or
variant
thereof, wherein the botulinum toxin antigen elicits a neutralizing antibody
response.
IMMUNOCiENIC COMPOSITIONS AND METHODS OF USE
The invention also relates to immunogenic compositions that contain one
or more antigens, which can be used to elicit an immune response, such as a
protective
immune response. The invention further relates to methods for treating and
preventing
microbial infections or effects of toxic agents by administering to a subject
one or more
antigens or fragments thereof, fusion protein, multivalent immunogen, or a
mixture of
such antigens at a dose sufficient to elicit an immune response (cellular or
humoral)
specific for the antigens) (which may be a protective immune response), as
described
herein. Antigens and variants thereof, or a cocktail of such immunogens are
preferably
part of a composition comprising an adjuvant, such as Projuvant or Protollin,
when used
in the methods of the present invention. In one embodiment, the immunogenic
compositions of the instant invention may further comprise one or more
additional
microbial antigens, such as viral antigens, bacterial antigens, parasitic
antigens, or a
combination thereof. For example, an immunogenic composition for plague or
anthrax
may also include antigens for rubella and mumps antigens.
The immunogenic compositions may further include a pharmaceutically
acceptable vehicle, carrier, diluent, or excipient, in addition to one or more
antigen or
fragment thereof and, optionally, other components. For example,
pharmaceutically
acceptable carriers or other components suitable for use with an immunogenic
composition of this invention include a thickening agent, a buffering agent, a
solvent, a
humectant, a preservative, a chelating agent, an additional adjuvant, and the
like, and
combinations thereof.
In addition, the pharmaceutical composition of the instant invention may
further include a diluent such as water or phosphate buffered saline (PBS).
Preferably,
diluent is PBS with a final phosphate concentration range from about 0.1 mM to
about
1 M, more preferably from about 0.5 mM to about 500 mM, even more preferably
from
about 1 mM to about 50 mM, and most preferably from about 2.5 mM to about 10
mM;
23
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
and the final salt concentration ranges from about 100 mM to about 200 mM and
most
preferably from about 125 mM to about 175 mM. Preferably, the final PBS
concentration is about 5 mM phosphate and about 150 mM salt (such as NaCI). In
certain embodiments, any of the aforementioned immunogenic compositions
comprising a cocktail of antigens and an adjuvant (such as Projuvant or
Protollin) of the
instant invention are preferably sterile.
The compositions can be sterilized either by preparing them under an
aseptic environment or they can be terminally sterilized using methods
available in the
. art. Many pharmaceuticals are manufactured to be sterile and this criterion
is defined
by the USP XXII <1211>. Sterilization in this embodiment may be accomplished
by a
number of means accepted in the industry and listed in the USP XXII <1211>,
including gas sterilization, ionizing radiation or filtration. Sterilization
may be
maintained by what is termed aseptic processing, defined also in USP XXII
<1211>.
Acceptable gases used for gas sterilization include ethylene oxide. Acceptable
radiation
types used for ionizing radiation methods include gamma, for instance from a
cobalt 60
source and electron beam. A typical dose of gamma radiation is 2.5 MRad. When
appropriate, filtration may be accomplished using a filter with suitable pore
size, for
example 0.22 ~,m and of a suitable material, for instance Teflon~. The term
"USP"
refers to U.S. Pharmacopeia (see www.usp.org; Rockville, MD). Due to the fact
that
Proteosomes or OMP-LPS result in particles small enough that the immunogenic
compositions of the invention can be filtered through a 0.8 ~, filter, a 0.45
~. filter, or a
0.2 ~, filter. Thus, in preferred embodiments the immunogenic compositions of
this
invention are sterilized by filtration. This is highly advantageous as it is
desirable to
eliminate any complications by virtue of the presence of such contaminants.
The present invention also pertains to methods for treating or preventing
a microbial infection, comprising administering to a subject in need thereof
an
immunogenic composition comprising an adjuvant and one or more antigens,
wherein
the adjuvant comprises either Proteosomes or ~MP-LPS. In another embodiment,
the
immunogenic compositions of this invention may be used to elicit an immune
response
(cellular or humoral or both, which may favor a Type 1 or Type 2 cellular
response). A
24
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
subject suitable for treatment or for eliciting an immune response with an
antigen
formulation may be identified by well-established indicators of risk for
developing a
disease or well-established hallmarks of an existing disease. As used herein,
it should
be understood that the terms "treat" and "ameliorate" refer to the therapeutic
administration of a desired composition or compound, in an amount or for a
time
sufficient to treat, inhibit, attenuate, ameliorate, reduce, prevent or alter
at least one
aspect or marker of a disease, in a statistically significant manner.
Infections that may
be treated, ameliorated or prevented with an antigen of the subject invention
include
those caused by or due to any of the microbes described herein, whether the
infection is
primary, secondary, opportunistic, or the like. Other exemplary antigens are
the toxins
or variants thereof described herein.
As described herein, the antigens can be prepared recombinantly,
synthetically, isolated from a biological source, recombinantly or chemically
modified,
and any combination thereof. Microbial antigens or fragments thereof can be
prepared
from a variety of biological sources, such as tissues of an infected subject
or cultured
cell lines. Primary isolation'may be from, for example, peripheral blood cells
or from
respiratory secretions. Preferably, the isolated microbes are amplified on
primary cell
cultures or on established cell lines known in the art. In certain
embodiments, the
antigens or fragments thereof are isolated from intact microbial particles. As
used
herein, the term "isolated" or "derived from" means that the material is
removed from
its original or natural environment. For example, a naturally occurring
nucleic acid
molecule or polypeptide present in a living animal or cell, or virus is not
isolated, but
the same nucleic acid molecule or polypeptide is isolated when separated from
some or
all of the co-existing materials in the natural system. For example, nucleic
acid
molecules could be contained in a vector or such nucleic acids could be part
of a
composition and still be isolated in that such a vector or composition is not
part of the
original nucleic acid molecule's natural environment. In other embodiments,
peptides
or polypeptides, such as antigens or variants and fragments thereof, may be
either
partially purified or purified to homogeneity.
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
The present invention further provides methods for producing synthetic
antigens, including fusion proteins. The immunogenic polypepfide components
may be
synthesized by standard chemical methods, including synthesis by automated
procedure. In general, immunogenic polypeptides or peptides are synthesized
based on
the standard solid-phase Fmoc protection strategy with I3ATIJ as the coupling
agent.
The immunogenic peptide can be cleaved from the solid-phase resin with
trifluoroacetic
acid containing appropriate scavengers, which also deprotects side chain
functional
groups. Crude immunogenic peptide may be further purified using preparative
reverse
phase chromatography. Other purification methods, such as partition
chromatography,
gel filtration, gel electrophoresis, or ion-exchange chromatography may be
used. Other
synthesis techniques known in the art may be employed to produce similar
immunogenic peptides, such as the tBoc protection strategy, use of different
coupling
reagents, and the like. In addition, any naturally or non-naturally occurring
amino acid
or derivative thereof may be used, including D- or L-amino acids and
combinations
thereof.
As described herein, the microbial antigens or fragments thereof of the
invention may be recombinant, wherein a desired antigen is expressed from a
polynucleotide that is operably linked to an expression control sequence
(e.g.,
promoter) in a nucleic acid expression construct. For example, host cells
(such as .
baculovirus and mammalian cell lines) containing the immunogen-encoding
nucleic
acid expression constructs can be cultured to produce recombinant protein
immunogens, or fragments thereof. The antigens may be further fused or
conjugated to
an additional amino acid sequence, which may be a hydrophobic anchor or foot
to
facilitate or otherwise enhance non-covalent association of the antigen fusion
with
Projuvant or Protollin. The antigen polypeptides may comprise any portion of
such
polypeptides that have at least one epitope capable of eliciting a protective
immune
response (cellular or humoral) against a microbial infection. Immunogenic
polypeptides of the instant invention may also be arranged or combined in a
linear
form, and each immunogen may or may not be reiterated, wherein the reiteration
may
occur once or multiple times. In addition, ~a plurality of different
immunogenic
26
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
polypeptides (e.g., protein variants, or fragments thereof) can be selected
and mixed or
combinedinto a cocktail composition to provide a multivalent vaccine for use
in
eliciting a protective immune response.
Methods for preparing the immunostimulatory compositions,
immunomodulatory compositions and immunogenic compositions of the instant
disclosure are described herein and are known in art (see, e.g., LJ.S. Patent
Application
Publications Nos. 2001/0053368 and 2003/0044425). The antigens) and adjuvant
are
formulated at specific initial ratios to optimize interaction (or cooperation)
between the
components resulting in non-covalent association (or non-specific
juxtaposition) of a
significant portion of the two components with each other. For example, a
mixture of at
least one antigen with a Proteosome (projuvant) or Protollin is prepared in
the presence
of detergent, and reducing or removal of the detergent from the mixture by
diafiltration/ultrafiltration leads to association (or coordination) of the
antigens with the
adjuvant. In certain embodiments, the Protollin (or Proteosome) to antigen
ratio in the
mixture ranges from about 1:1 to about 5:1. The ratio can be as high as 8:l or
higher.
In certain other embodiments, the Protollin (or Proteosome) to antigen ratio
in the
mixture ranges from about 1:1 to about 1:500, or in a range of about 1:2 to
about 1:200,
or in a range of about 1:2 to about 1:100, or in a range of about 1:5 to about
1:50, or in a
range of about 1:2 to about 1:20, or at least 1:5, 1:10, 1:20 or 1:100. The
c~etergent-
based solutions of the two components may contain the same detergent or
different
detergents, and more than one detergent may be present in the mixture
subjected to
ultrafiltration/diafiltration. Suitable detergents include Triton~, Empigen~
BB, and
Mega-10. Other detergents can also be used (e.g., octoglucoside). The
detergents serve
to solubilize the components used to prepare the composition. The use of a
mixture of
detergents may be particularly advantageous. This mixture is, of course,
removed or
the concentration is reduced by diafiltration/ultrafiltration prior to final
formulation.
The immunogenic compositions that contain one or more antigens and a
Proteosome-based adjuvant of the invention may be in any form that allows for
the
composition to be administered to a subject, such as a human or animal. For
example,
immunogenic compositions of the present invention may be prepared and
administered
27
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
as a liquid solution or prepared as a solid form (e.g., lyophilized), which
may be
administered in solid form, or resuspended in a solution in conjunction with
administration. The immunogenic polypeptide compositions are formulated to
allow
the active ingredients contained therein to be bioavailable upon
administration of the
composition to a subject or patient or bioavailable via slow release.
Compositions that
will be administered to a subject or patient take the form of one or more
dosage units,
where for example, a drop may be a single dosage unit, and a container of one
or more
compounds of the invention in aerosol form may hold a plurality of dosage
units. In
certain preferred embodiments, any of the aforementioned pharmaceutical
compositions
comprising an immunogen or cocktail of immunogens (i.e., anitgens) of the
invention
are in a container, preferably in a sterile container. The design of a
particular protocol
for administration, including dosage levels and timing of dosing are
determined by
optimizing such procedures using routine methods well known to those having
ordinary
skill in the art.
In one embodiment, the immunogenic composition is administered
nasally. Other typical routes of administration include enteral, parenteral,
transdermal/transmucosal, nasal, and inhalation: The term "enteral", as used
herein, is a
route of administration in which the immunogenic composition is absorbed
through the
gastrointestinal tract or oral mucosa, including oral, rectal, and sublingual.
The term
"parenteral", as used herein, describes administration routes that bypass the
gastrointestinal tract, including intraarterial, intradermal, intramuscular,
intranasal,
intraocular, intraperitoneal, intravenous, subcutaneous, submucosal, and
intravaginal
injection or infusion techniques. The term "transdermal/transmucosal", as used
herein,
is a route of administration in which the immunogenic composition is
administered
through or by way of the skin, including topical. The terms "nasal" and
"inhalation"
encompass techniques' of administration in which an immunogenic composition is
introduced into the pulmonary tree, including intrapulmonary or
transpulmonary.
Preferably, the compositions of the present invention are administered
nasally.
For example, as described herein, intranasally administered F1-V
combined with Frotollin elicited comparable serum anti-Fl-V IgG titers to
those
28
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
induced by intramuscular injection of Fl-V formulated with Alhydrogel~.
However,
unlike Alhydrogel~/F1-V, Protollin/F1-V also elicited specific IgA and IgG
responses
as detected in lung lavage fluids. hnmunized mice were subsequently challenged
with
aerosolized h pesos. Protollin/F1-V immunized mice were ~0% protected against
high
dose (255 LDso) challenge as compared to 60°/~ in the Alhydrogel/F1-V
group, while
both vaccines were 90-100% protective against challenge with a lower dose (170
LDSO)
of the organism.
All of the U.S. patents, U.S. patent application publications, U.S. patent
applications, foreign patents, foreign patent applications and non-patent
publications
referred to in this specification and/or listed in the Application Data Sheet,
are
incorporated herein by reference, in their entirety. The invention having been
described, the following examples are intended to illustrate, and not limit,
the invention.
29
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
EXAMPLES
EXAMPLE 1
ELISA ASSAYS TO EVALUATE SERUM AND MUCOSAL IMMUNE RESPONSE
ELISAs were used to determine total and antigen-specific IgG, IgA and
IgM titers in biosamples obtained from animals immunized with test
immunostimulatory or immunogenic formulations according to the instant
application,
and controls. Samples include serum, nasal and lung mucosal washes in mice and
rabbits. A standard ELISA protocol was used to determine linearity,
specificity,
sensitivity and reproducibility. Briefly, serial dilutions of the test samples
(serum and
lavage fluids) were added to the wells of ELISA plates coated with purified
antigen, or
derivatives thereof. Antigen=specific antibodies that adhere to the
immobilized antigen
were detected with animal and antibody subtype specific horse radish
peroxidase (HRP)
conjugated antibodies. Following HRP antibody interaction and washing, the
amount
of bound HRP antibody was detected following incubation with a HRP substrate
(TMB)
and measuring absorbance at 490 nm. Antibody concentrations in the test
samples were
calculated from standard curves, run in parallel, using purified standard
antibodies for
IgA, IgM, IgG, IgGl and IgG2a. Where appropriate, specific antibody levels in
mucosal wash fluid samples are standardized and normalized by expressing the
specific
antibodies detected in comparison to the total amount of IgA or IgG in the
sample as
measured in a separate assay. This technique has resulted in superior
reproducibility
and consistency in assays performed on nasal wash fluids collected in human
clinical
trials. ELISA data is expressed as geometric means at 95% confidence levels
with
statistical analysis using log-transformed data.
EXAMPLE 2
ANALYSIS OF MUC~SAL ANTIBODY PRODUCTION
Lung and nasal washes in mice and rabbits were collected to analyze the
immune response to immunostimulatory or immunogenic formulations according to
the
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
instant application. In mice, nasal washes and lung lavage were performed by
cannulating the trachea and 1 ml PBS supplemented with 0.1% bovine serum
albumin
and protease inhibitors (General IJse Protease Inhibitor Cocktail9 Sigma
Chemicals
containing 0.2 mM AEBSR, 1 ~,glmL aprotinin, 3.25 ~.M bestatin, 10 ~,M
Leupeptin)
were pumped upwards through the trachea. Fluid emerging from the nostrils was
collected, vortexed and then centrifuged to remove tissue and cell debris. The
supernatants were stored at -70°C until assayed. A cannula reinserted
into the trachea
and keeping the cannula directed toward the lung allowed the collection of
lung fluids.
Lungs were lavaged twice with 1.0 mL protease supplemented PBS, the fluid was
collected and vortexed, and the cell debris removed by centrifugation. Lung
and nasal
washes were stored at -70°C until being assayed. Rabbit mucosal fluids
were similarly
collected, adjusting the volumes as appropriate. In certain experiments, after
collecting
mucosal samples, cervical and mediastinal lymph nodes were surgically removed
and
mononuclear cells isolated and cultured for ELISPOT antibody, cytokine and CMI
assays as described herein.
EXAMPLE 3
IMMUNIZATION WITH PROTOLLIN FORMULATED WITH PLAGUE ANTIGEN F1-V
Examined is the ability of Proteosome:LPS (Protollin) compositions
formulated with, for example, plague antigen (F1-V) to evoke an immune
response
capable of protecting against a lethal challenge with Yersinia pestis. The F1-
V immune
response was assessed by immunizing groups of twenty 6-8 week old female Swiss-
Webster mice (Charles River, St-Constant, Quebec) on days 0 and 21. For
immunization, freshly thawed aliquots of Protollin and F1-V solutions were
mixed <16
hrs prior to immunization. For intranasal (i.n.) administration, mice were
first lightly
anesthetized by isoflurane inhalation, then 25 ~l of vaccine or appropriate
control
samples (Protollin or F1-V alone) was applied to the nares (12.5 ~1 per
nostril) of each
mouse. In parallel experiments, mice were immunized intramuscular (i.m.) with
25 ~1
of F1-V adsorbed to SOOmg of Alhydrogel~ injected into hind limbs. Control
i.m.
injections were also performed. Thirty-five and 55 days thereafter, 10 mice
from each
31
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
group were euthanized by asphyxiation with C02 and exsanguination. Serum was
obtained and stored at -80°C until assay. Nasal wash and lung lavage
samples were
obtained and stored at -80°C until assay. Spleens were processed for i~
vit~~o
restimulation and assessment of released cytokines. The remaining 10 mice from
each
group were challenged on day 35 or 55 by inhalation of 170-250 LD50 of
aerosolized Y
pestis (Colorado 92 strain) to assess protection. Mice were monitored for 28
days after
challenge for determination of morbidity and mortality.
Antibodies present in serum and lung lavage fluid samples obtained from
mice immunized intranasally with two doses of F1-V antigen formulated with
Protollin
were compared with those from mice immunized i.n. with Fl-V alone or with mice
immunized intramuscularly with Alhydrogel~-adsorbed F1-V. The results are
shown in
Figures 1 and 2. Surprisingly, at all concentrations tested, all combinations
of Protollin
and F1-V were highly immunogenic and elicited F1-V specific serum IgG titers
of
between 1 mglml and 9 mg/ml (Figures 1 and 2). There were no significant
differences
in the specific IgG titers elicited by any combination of F1-V and Protollin
concentrations or those elicited by intramuscular injection of 20~,g of Fl-V
adsorbed
onto Alhydrogel (P > 0.05). All specific serum IgG titers elicited by F1-V
formulated
vaccines were significantly higher than those elicited by i.n. administration
of non-
adjuvanted Fl-V controls (P < 0.001), and no F1-V specific antibodies were
detected in
serum from control mice. An unexpected result of these experiments is that
Protollin:antigen ratios could be reduced dramatically (from 1:2 to 1:200)
with no
significant effect on the ability of an antigen to elicit an immune response.
Prior to this
disclosure, one would have expected that reducing the amount of adjuvant would
have
resulted in a reduction in the immune response.
Lung lavage samples were assayed by ELISA to determine the level of
specific anti-Fl-V, anti-F1 and anti-V antibodies present (Figure 3). The
results
confirm that immunization by mucosal (e.g., intranasal) routes is an efficient
means of
eliciting mucosal antibodies as all groups of mice immunized i.n. with Fl-V
antigen
plus Protollin responded with high titers of F1-V specific lung IgA. In these
experiments, the IgA responses were highest in lung lavage samples from mice
32
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
immunized with the highest dose of Protollin; ANOVA analysis indicated, again
unexpected, that there were no significant differences in the IgA titers
elicited by any
combination of F1-V with Protollin. Non-adjuvanted F1-V, administered i.n.,
elicited
IgA levels that were barely detectable; no secretory IgA was detected in
samples from
mice injected i.m. with Alhydrogel~-adsorbed F1-V. Assays of lung lavage
samples
against the F1 and V portions of the F1-V antigen indicated that the immune
response
was primarily directed against the V component of the F 1-V fusion protein
(Table 1 ).
However, lung lavage samples also contained significant titers of F1-V
specific IgG,
even though the titers represented only a small percentage of the serum titers
(range
0.11% - 0.56%; median 0.175%).
To determine if serum antibodies recognizing both the F1 and V
components were elicited, sera from all mice immunized with the 20 ~,g dose of
F1-V
antigen were analyzed separately against Fl and V. In all instances, and at
both
sampling times, serum IgG and lung lavage fluid IgA antibodies specific for
both the F1.
and V portions of the F1-V antigen were detected (Table 1). There were rio
significant
differences in the serum anti-F 1 and anti-V antibody ratios, irrespective of
the
formulation or route of delivery of the F1-V antigen evaluated in these
experiments.
Table 1. Ratios of anti-Fl to anti-V Antibodies in Serum and Lung Lavage
fluids
of T~~firP Tmmnni~Prl with several Formulations of Plague Antigen Fl-V
F1-V+ F1-V + ~ F1-V +
Fl-V+ leg
2.S~.g 0,25~g F1-V i.n.
protollin Alhydrogel
i.m.
Protollin protollin
Serum IgG 0,31 0.30 0.34 0.31 0.65
d35
Serum IgG p.25 0.26 0.33 0.58 0.29
d55
Lung IgA 0.42 0.39 0.33 N/A N/A
d35
Lung IgA 0.49 0.29 0.32 N/A N/A
d55
Lung IgG 0.40 0.53 0.44 N/A 1.02
d35
Lung IgG 0.48 0.48 0.46 N/A 1.03
d55
33
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
EXAMPLE 4
CHALLENGE OF IMMUNIZED MICE WITH AEROSOLIZED Y. PESTIS
These experiments were designed to assess the level of immune
protection elicited by intranasal immunization with Fl-V formulated with
Protollin.
Mice were challenged by whole-body exposure to live aerosolized Y. pesos, and
the
level of protection from challenge was compared with that elicited by
injection of F1-V
adsorbed onto Alhydrogel9 as well as control mice administered Fl-V alone or
Protollin
alone, intranasally. In these experiments, only the results for mice immunized
with the
20 ~,g doses of F1-V are shown in Figures 4 and 5. On day 35 and at a
challenge dose
of 170 LD50 Y. pesos, mice immunized intranasally with 5, 20 or 50 ~,g Fl-V
plus 1 or
2.5 ~.g Protollin were 100% protected against death, as were the mice injected
with F1-
V adsorbed onto Alhydrogel. Mice immunized i.n. with 5, 20 or 50 ~,g F1-V and
0.25 ~.g Protollin were 90%, 100% and 90% protected, respectively, while mice
immunized i.n. with the same doses of F1-V without Protollin were only 30%,
40% and
40% protected, respectively. None of the control mice, which received
Protollin alone,
survived longer than 4 days post challenge. Survival for all mouse groups
immunized
with formulated F1-V was highly significant compared to survival in control
mice or
mice immunized with unformulated F1-V (P <_ 0.05 or better by Fisher's Exact
Probability Test). Survival following challenge on day 55 was very similar to
the day
35 data. All mice immunized with 2.5 ~g of Protollin formulated with F1-V were
completely protected against challenge, as were mice immunized by injection of
F1-V
adsorbed onto Alhydrogel~. Mice immunized with 1 ~g of Protollin formulated
with
50 ~,g or 20 ~.g of F1-V were also 100% protected, while all other
combinations of
Protollin and F1-V elicited 90% protection. In all mice immunized with
formulated F1-
V, the observed protection was highly significant (P <_ 0.01 or better)
compared to mice
immunized with non-adjuvanted F1-V (10-30% protection) or the control group of
mice
in which there were no survivors. Mice immunized i.n. with 50 ~g of Fl-V with
or
without l~.g of Protollin, or injected with 20 ~g of F1-V adsorbed onto
Alhydrogel~,
were challenged on day 55 by whole body exposure to 255 LD50 aerosolized live
Y
pestis. 80%, 20% and 60% respectively of the mice survived the high dose
lethal
34
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
challenge while control mice given Protollin alone all died. Immunization with
formulated Fl-V induced significant protection against death compared to
control mice
(P < 0.001 for nasal F1-V plus Protollin; P <_ 0.01 for injected F1-V). Nasal
inununization with F1-V plus Protollin was also significantly better at
protecting mice
against death than immunization with Fl-V alone (P < 0.05), but this was not
the case
for injected Fl-V when the induced protection was not significantly better
than that
induced by i.n. Fl-V without Protollin (P = 0.095).
EXAMPLE 5
DETERMINATION OF CYTOKINE PROFILE AFTER
1 O IMMUNIZATION WITH PROTOLLIN:PALGUE ANTIGEN
To compaxe the phenotype (type 1 or type 2) of the adaptive immune
response elicited by i.n. adminstered Protollin or injected Alhydrogel~
adjuvanted F1-V
vaccine, splenocytes from selected groups of immunized mice were re-stimulated
in
vitro with F1-V and the amount of IFN-'y, TNF-a and IL-5 cytokines released
into
culture supernatants were measured (Figure 6). Splenocytes from mice immunized
i.n.
with Fl-V (50 fig) mixed with Protollin (1 fig) responded to in vitro re-
stimulation by
secreting high levels of both IFN-y and TNF-a, and a very low amount of IL-5
was also
detected. In contrast, splenocytes from mice immunized by injection of Fl-V
(20 ~,g)
adsorbed onto Alhydrogel responded by secreting comparatively lower amounts of
IFN-
y and TNF-a, although a significant amount of IL-5 was detected. Thus, the
cytokine
profile elicited by i.n, administration of Protollin formulated (adjuvanted)
with F1-V
antigen was consistent with eliciting a type 1 immune response, whereas the
cytokine
profile induced by i.m. injection of F1-V antigen formulated with Alhydrogel~
is more
consistent with a type 2 response.
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
EXAMPLE 6
PROTOLLIN:ANTHRAX IMMUNOGENIC FORMULATIONS EVOKE
A NEUTRALIZING IMMUNE RESPONSE
Protollin formulations with a recombinant Protective Antigen (rPA) of
Bacillus anthracis were evaluated for the ability to induce an immune response
capable
eliciting a statistically significant reduction in PA-mediated macrophage
killing, using a
cell culture assay system. Mice immunized i.n. with 5 ~,g or 25 ~,g rPA (List
Laboratories) admixed with 1 ~.g of Protollin (on days 0, 14) showed specific
anti-rPA
serum IgG and lung IgA levels significantly higher than those of mice that
were i.n.
immunized with 5 ~g or 25 ~g of rPA alone (p<0.05) (Figure 7), indicating that
adjuvanticity of low levels of Protollin is also effective with rPA antigen.
Mucosal IgA
levels in the Protollin alone and rPA alone control groups were below the
detection
level of this assay.
The ability of specific anti-rPA antibodies to neutralize PA-mediated
macrophage killing was evaluated. An anthrax PA neutralization assay was also
performed as described using serum and lung lavage fluid samples from these
animals.
For this assay, 2 x 105 RAW264.7 cells were plated out in sterile 96 well
plates and
incubated at 37°C for 24 h in 5% C02. Serial dilutions of serum or lung
lavage fluid
samples (from PA immunized animals) were incubated with a PA solution for 1 h
at
37°C, which was then added to the wells containing cells. Thereafter, a
solution of LF
was added to the wells and the plates incubated at 37°C in 5% COZ for 4
h. A solution
of MTT (to measure cell viability) was then added to each well and the plates
incubated
for a further 4h at 37°C in 5% C02. The reaction was stopped by
addition of 20% SDS
in 50% DMF pH 4.3 and the plates read at 570 nm with a reference at 690 nm.
This
assay showed that the Proteosome-adjuvanted i.n. vaccine of rPA with Protollin
elicited
comparable levels of antibodies that neutralized rPA activity as did the i.m,
alum-
adjuvanted vaccine (Figure 8). However, a notable distinction between samples
is the
presence of neutralizing IgA antibodies present in lung lavage samples frorr~
mice
immunized with the Protollin formulation rPA.
36
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
EXAMPLE 7
PREPARATION OF ADDITIONAL ANTHRAX VACCINE FORMULATIONS
Nasal Protollin anthrax vaccines were made by admixing the anthrax PA
antigens with soluble pre-formed Proteosome plus LPS (i.e., Protollin) prior
to
immunization. Both rPA and rPA-anchor antigens are evaluated with several
versions
of Protollin to determine the formulations) having the optimal Protollin to
immunogenic antigen ratio. Control formulations include, for example,
Protollin alone
or mixed with one or more control antigens (as appropriate), such as
recombinant
streptococcal protein that contains or lacks a hydrophobic anchor sequence.
Accordingly, formulations of Protollin containing LPS from different sources,
various
Proteosome:LPS ratios, and various Protollin:rPA antigen ratios are evaluated.
rPA-
anchor are formulated with Proteosomes with very low levels of LPS (<2% by
weight).
In certain embodiments, the Proteosome adjuvant preparations do not have
exogenous
LPS added. Previosuly, Proteosomes themselves have been used extensively in
pre-
clinical toxicity studies as well as in close to 500 persons in Phase 1 and
Phase 2 safety,
immunogenicity and experimental challenge trials of a Proteosome nasal
influenza
vaccine.
Process development of the preferred version of LPS (in terms of LPS
bacterial source) and the preferred formulation of Protollin (in terms of LPS
source and
Proteosome:LPS ratio) are performed based on the results of the immunogenicity
studies designed to identify the components of Protollin in terms of LPS
source and
Proteosome:LPS ratio. Fermentation of the preferred bacterial source for LPS
is
followed by LPS purification and analysis. The selected purified LPS is then
mixed
with Proteosome OMP particles at the selected ratio to form a preferred
Protollin. The
extent of association of the LPS with the OMPs is verified in specially
developed "free-
vs.-bound" assays using capillary electrophoresis, LPS "spiking" studies and
other
analyses, as necessary. Characterization of Protollin typically includes
analyses of LPS
content using KDO, NMR, and silver stain PAGE, for Proteosome OMP content
using
LC-MS, I2P-HPLC, SDS-Page (C. Blue ~c Western Blot w/IVIAb 8c PAbs), N-
terminal
Sequencing, Amino Acid Analysis, total protein by Lowry or BCA, and MALDI-
37
CA 02524485 2005-11-02
WO 2004/098636 PCT/US2004/014236
TOFMS where appropriate, and for residual LPS, Nucleic Acids, and detergents
using
KDO, nucleic acid and HPLC assays, respectively.
EXAMPLE ~
CELL MEDIATED IMMUNITY ASSAY TO EVALUATE
IMMUNIZATION AGAINST ANTHRAX
Cellular immune responses induced following immunization with the
Proteosome-based PA vaccines is studied using a variety of methods. For
example,
T cell-derived cytokines are assessed on PA-re-stimulated purified or enriched
T cells
isolated from mouse spleen and/or mediastinal lymph nodes. Type 1 (e.g., IFN-
y) and
type 2 (e.g., IL-4 and IL-5) cytokines are determined by one or more methods
including
ELISA, ELISPOT and intracellular cytokines by flow-cytometry. Proliferation of
PA
re-stimulated PBMC T cells from PBMC axe used to evaluate CMI responses in
rabbits
due to lack of reagents specific for rabbit cytokines. T lymphocyte
proliferation assays
are used to measure the effect of immunization on clonal expansion and
presence of
memory lymphocytes in various animal models. Following animal sacrifice,
mediastinal and cervical lymph nodes are removed surgically using standard
techniques, the lymphocytes isolated and cultured and PA added to the isolated
cells.
Proliferation is measured by uptake of H3-thymidine. Cells from animals
immunized
with sham vaccine are used as negative controls. Assay results are used to
determine
the effect of immunization on T cell differentiation in the lymph nodes
involved in
mucosal immunity, and correlated with efficacy of immunization as determined
in
anthrax challenge studies.
From the foregoing it will be appreciated that, although specific
embodiments of the invention have been described herein for purposes of
illustration,
various modifications may be made without deviating from the spirit and scope
of the
invention. Accordingly, the invention is not limited except as by the appended
claims.
38