Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
CYTOTOXIC 1NDENO AND ISOINDOLOISOQUINOLONES
CROSS REFERENCE TO RELATED APPLICATIONS
This patent application claims the benefit of U.S. provisional patent
application serial Ilo. 60/469,718 filed May 12, 2003, the disclosure of which
is
incorporated herein by reference.
FIELD OF THE INVENTION
This invention relates to indeno and isoindoloisoquinolones, and uses
of indeno and isoindoloisoquinolones as cytotoxic agents.
BACKGROUND OF THE INVENTION
The cytotoxicity profile of the topoisomerase I (top 1) inhibitors
camptothecin (3) and indenoisoquinoline 2 (see FIG. 3) has been described by
Kohlhagen et al. in Mol. PhaYmacol. 54:50-58 (1998); Pommier et al. in
Biochem.
Bioplays. Acta, 1400:83-105 (1998); and Pommier in ~piniou ira Oncologic,
EyadocYihe ~ Metabolic Iravestigatioyaal DYUgs, 1:168-169 (1999), the
disclosures of
which are incorporated herein by reference. The synthesis of
indenoisoquinoline 2 is
accomplished by treatment of the cis-substituted isoquinolone 1 (FIG. 3) with
thionyl
chloride, as described by Cushman & Cheng in J. Org. Chem. 43:3781-83 (1978),
the
disclosure of which is incorporated herein by reference. Both camptothecin (3)
and
indenoisoquinoline 2 were also shown to be inhibitors of DNA religation
reactions
occuring after DNA cleavage by the enzyne, i.e., topl-catalyzed single strand
brealcage. Both camptothecin (3) and indenoisoquinoline 2 might be better
classified
as a topl poisons than a topl suppressors because camptothecin (3) and
indenoisoquinoline 2 showed the ability to stabilize the cleavable complexes.
However, the DNA single-strand breaks induced by indenoisoquinoline 2 were
more
stable than those induced by camptothecin (3). Furthermore, the cleavage site
specificity of 2 was different from that of camptothecin (3).
Although several camptothecin (3) derivatives such as irinotecan and
topotecan are clinically useful anticancer agents, they are unstable due to
opening of
the lactone ring present on each of these compounds; thus, subsequent rapid
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-2-
reversibility of the cleavage complexes is observed after drug removal.
Consequently, there is a present need for additional therapeutic agents that
inhibit
topl lilce the camptothecins, but that induce novel I~NA cleavage patterns,
have
modified toxicity profiles and extended durations of action, and display
different
antitumor spectra relative to the camptothecins themselves. A number of
analogs of
the indenoisoquinoline 2 have been synthesized, as described by Strumberg et
al. in .I.
Med. ClZerya. 42:446-457 (1999); Cushman et al. in.J. Med. Chem. 43:368-3698
(2000); and Jayaraman et al. in J. Med. Chem. 45:242-249 (2002).
SUMMARY OF THE INVENTION
A compound of formula I is described:
Y
11 ~ ~e
\ / RB
N,R~
wherein
Q is oxygen or sulfur;
X is hydrogen and Y is CHRZR3, NHR2, NHORZ, or NHNR2R; or X
and Y are taken together to form =CR2R3; NR2; NOR2; or NNR2R3;
Rl, R2, and R3 are each independently selected from the group
consisting of hydrogen and a radical -(CH2)mZ, where m is an integer from 0-6
and Z
is selected from the group consisting of halogen, hydroxy, fornyl, C1-C6
alkanoyloxy,
optionally substituted benzoyloxy, C1-C6 alkyl, C1-C6 alkoxy, C3-C8
cycloalkyl, C3-C$
cycloalkoxy, Cz-C6 alkenyl, Ca-C6 all~ynyl, C1-C6 haloalkyl, C1-C6 haloalkoxy,
C3-C8
halocycloalkyl, C3-C8 halocycloallcoxy, amino, C1-C6 alkylamino, (C1-C6
allcyl)(C1-C6
alleyl)amino, allcylcarbonylamino, N-(C1-C6 alkyl)allcylcarbonylamino,
aminoalkyl,
C1-C6 allcylaminoalkyl, (C1-C6 alkyl)(C1-C6 allcyl)aminoallcyl,
allcylcarbonylaminoalkyl, N-(C1-C6 alkyl)alkylcarbonylaminoallcyl, cyano,
vitro, C1-
C6 all~ylsulfonyl, optionally substituted phenyl, optionally substituted
phenoxy, and
optionally substituted heteroaryl; or Z is selected from the group consisting
of -N3,
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-3-
-COZR4, -CONRSR6, -P(O)(OR4)Z, -P(O)(NR4R5)Z, and -P(O)(NR4R5)(OR4), where
R4, R5, and R6 are each independently selected in each occurrence from the
group
consisting of hydrogen, C1-C6 alkyl, C3-C8 cycloallcyl, C1-C6 haloalkyl,
optionally
substituted phenyl, and optionally substituted phenyl-C1-C6 alkyl; or
when X and Y are taken together to form =NNR2R3, R2 and R3 are
talcen together with the attached nitrogen to form an optionally substituted
heterocycle;
RA represents 1-4 substituents each independently selected from the
group consisting of hydrogen and a radical -(CHZ)",~Z', where m' is an integer
from
0-6 and Z' is selected from the group consisting of halogen, hydroxy, C1-C6
alkanoyloxy, optionally substituted benzoyloxy, C1-C6 alkyl, C1-C6 alkoxy, C3-
C8
cycloalkyl, C3-C8 cycloalkoxy, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 haloalkyl,
C1-C6
haloalkoxy, C3-C8 halocycloalkyl, C3-C8 halocycloalkoxy, amino, C1-C6
alkylamino,
(C1-C6 allcyl)(C1-C6 alkyl)amino, alkylcarbonylamino, N-(Ci-C6
alkyl)alkylcarbonylamino, aminoallcyl, C1-C6 alkylaminoalkyl, (C1-C6 alkyl)(C1-
C6
alkyl)aminoalkyl, alkylcarbonylaminoalkyl, N-(C1-C6
alkyl)alkylcarbonylaminoalkyl,
cyano, nitro, C1-C6 alkylsulfonyl, optionally substituted phenyl, optionally
substituted
phenoxy, and optionally substituted heteroaryl; or Z' is selected from the
group
consisting of-N3, -CO2R4~, -CONRSR6~, -P(O)(OR4~)2, -P(O)(NR4R5~)2, and
-P(O)(NR4R5~)(OR4~), where R4~, RS~, and R6~ are each independently selected
in each
occurrence from the group consisting of hydrogen, C1-C6 alkyl, C3-C$
cycloalkyl, C1-
C6 haloallcyl, optionally substituted phenyl, and optionally substituted
phenyl-C1-C6
alkyl; or
RA represents 2-4 substituents where 2 of said substituents are adjacent
substituents and are taken together with the attached carbons to form an
optionally
substituted carbocycle or an optionally substituted heterocycle, and the
remaining 2
substituents are each independently selected from the group consisting of
hydrogen
and a radical -(CH2)",~Z', where m' is an integer from 0-6 and Z' is selected
from the
group consisting of halogen, hydroxy, Cl-C6 alkanoyloxy, optionally
substituted
benzoyloxy, C1-C6 allcyl, C1-C6 allcoxy, C3-C$ cycloalkyl, C3-C8 cycloalkoxy,
CZ-Cs
alleenyl, CZ-C6 allcynyl, C1-C6 haloallcyl, C1-C6 haloall~oxy, C3-C8
halocycloalkyl,
C3-C8 halocycloalkoxy, amino, C1-C6 alkylamino, (Cl-C6 alkyl)(Cl-C6
alkyl)amino,
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-4-
alkylcarbonylamino, N-(C1-C6 alkyl)alkylcarbonylamino, aminoalkyl, C1-C6
alkylaminoalkyl, (C1-C6 alkyl)(C1-C6 alkyl)aminoalhyl,
alkylcarbonylaminoalkyl, N-
(C1-C6 alkyl)alkylcarbonylaminoalkyl, cyano, nitro, C1-C6 alkylsulfonyl,
optionally
substituted phenyl, optionally substituted phenoxy, and optionally substituted
heteroaryl; or Z' is selected from the group consisting of -N3, -C~zR4~, -
CONRS R6~,
-P(~)(~R4~)z, -P(O)(NR4R5)z, and -P(O)(NR4R5)(OR4), where R4~, RS~, and R6~
are
each independently selected in each occurrence from the group consisting of
hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6 haloalkyl, optionally
substituted
phenyl, and optionally substituted phenyl-C1-C6 alkyl; and
RB represents 1-4 substituents each independently selected from the
group consisting of hydrogen and a radical -(CHz)m--Z", where m" is an integer
from
0-6 and Z" is selected from the group consisting of halogen, hydroxy, Ci-C6
alkanoyloxy, optionally substituted benzoyloxy, C1-C6 alkyl, C1-C6 alkoxy, C3-
C8
cycloalkyl, C3-C8 cycloalkoxy, Cz-C6 alkenyl, Cz-C6 alkynyl, Cl-C6 haloalkyl,
C1-C6
haloalkoxy, C3-C$ halocycloalkyl, C3-C$ halocycloalkoxy, amino, C1-C6
alkylamino,
(C1-C6 alkyl)(C1-C6 alkyl)amino, alkylcarbonylamino, N-(C1-C6
alkyl)alkylcarbonylamino, aminoalkyl, C1-C6 alkylaminoalkyl, (C1-C6 alkyl)(C1-
C6
allcyl)aminoallcyl, alkylcarbonylaminoalkyl, N-(C1-C6
alkyl)alkylcarbonylaminoalkyl,
cyano, nitro, Ci-C6 alkylsulfonyl, optionally substituted phenyl, optionally
substituted
phenoxy, and optionally substituted heteroaryl; or Z" is selected from the
group
4" S" 6" 4" 4" 5"
consisting of -N3, -C02R , -CONK R , -P(O)(OR )z, -P(O)(NR R )z, and
-P(O)(NR4~~R5~~)(OR4~~), where R4~~, RS~~, and R~~~ are each independently
selected in
each occurrence from the group consisting of hydrogen, C1-C6 alkyl, C3-C8
cycloalkyl, C1-C6 haloalkyl, optionally substituted phenyl, and optionally
substituted
phenyl-C1-C6 allcyl; or
RB represents 2-4 substituents where 2 of said substituents are adjacent
substituents and are taken together with the attached carbons to form an
optionally
substituted carbocycle or an optionally substituted heterocycle, and the
remaining 2
substituents are each independently selected from the group consisting of
hydrogen
and a radical -(CHz)r"'~Z", where m" is an integer from 0-6 and Z" is selected
from the
group consisting of halogen, hydroxy, C1-C6 alkanoyloxy, optionally
substituted
benzoyloxy, C1-C6 alkyl, Cl-C6 all~oxy, C3-C8 cycloallcyl, C3-C$ cycloalkoxy,
Cz-C6
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-5-
allcenyl, Ca-C6 alkynyl, C1-C6 haloalkyl, C1-C6 haloalkoxy, C3-C8
halocycloalkyl,
C3-C8 halocycloall~oxy, amino, C1-C6 alkylamino, (C1-C6 alkyl)(C1-C6
alkyl)amino,
alkylcarbonylamino, N-(C1-C6 alkyl)alkylcarbonylamino, aminoalkyl, C1-C6
alkylaminoalkyl, (C1-C6 allcyl)(C1-C6 alkyl)aminoalkyl,
alkylcarbonylaminoalkyl, N-
(C1-C6 alkyl)alkylcarbonylaminoalkyl, cyano, nitro, C1-C6 alkylsulfonyl,
optionally
substituted phenyl, optionally substituted phenoxy, and optionally substituted
heteroaryl; or Z" is selected from the group consisting of -N3, -C~2R4~~, -
C~NRS R6 ,
-P(C)(OR4,-)z, -P(O)(NR4"RS-~)a, and -P(O)(NR4"RS")(~R4"), where R4", RS", and
R6" aT,e
each independently selected in each occurrence from the group consisting of
hydrogen, C1-C6 alkyl, C3-C$ cycloalkyl, Cl-C6 haloalkyl, optionally
substituted
phenyl, and optionally substituted phenyl-C1-C6 alkyl is described.
In one illustrative embodiment of the compounds of formula I, Q is
oxygen; and RA is 2,3-bis(Cl-C6 alkoxy). In another illustrative embodiment of
the
compounds of formula I, Q is oxygen; and Ri is Cl-C6 alkyl, aminoalkyl, or C1-
C6
haloalkyl. In one illustrative embodiment of the compounds of formula I, RB
represents 2-4 substituents where 2 of the substituents are adjacent
substituents and
are taken together with the attached carbons to form an heterocycle selected
from
dioxolane and dioxane.
In another illustrative embodiment of the compounds of formula I, Q is
oxygen, RA is 2,3-bis(C1-C6 alkoxy), RB is 8,9-alkylenedioxy, X and Y are
taken
together to form =CRZR3, where RZ is hydrogen and R3 is as described above,
and Rl
is as described above. hl another illustrative embodiment of the compounds of
formula I, Q is oxygen, RA is 2,3-bis(C1-C~ alkoxy), RB is 8,9-alkylenedioxy,
X and Y
are taken together to form =CRZR3, where R2 is hydrogen and R3 is as described
above, and Rt is hydrogen, Cl-C6 alkyl, C3-C8 cycloalkyl, Cl-C6 haloalkyl, C3-
C8
halocycloalkyl, amino-C1-C6 alkyl, C1-CG alkylamino-C1-C6 allcyl, or (C1-C6
allcyl)(Cl-C6 allcyl)amino-Cl-C6 alkyl.
In another illustrative embodiment of the compounds of formula I, X
and Y are talcen together to form =CRZR3, where R2 and R3 are as described
above. In
one aspect, the carbon-carbon double bond formed thereby is an E-double bond.
In
another aspect, the carbon-carbon double bond formed thereby is an Z-double
bond.
In another illustrative embodiment of the compounds of formula I, X and Y are
taken
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-6-
together to form =CR2R3; and RZ is C1-C6 haloalkyl or aminoalkyl. In one
aspect, Ri
is hydrogen.
In another illustrative embodiment of the compounds of formula I, Z is
selected from hydroxy, amino, Ci-C6 alkylamino, and vitro. In another
illustrative
embodiment of the compounds of formula I, Z' is selected from Ci-C6 alkoxy and
vitro. In another illustrative embodiment of the compounds of formula I, Z" is
selected from C1-Cg alkoxy and vitro.
In another illustrative embodiment of the compounds of formula I, RB
represents 2-4 substituents where 2 of said substituents are adjacent
substituents and
are taken together with the attached carbons to form an optionally substituted
heterocycle. In another illustrative embodiment of the compounds of formula I,
RB
represents 2-4 substituents where 2 of said substituents are adjacent
substituents and
are taken together with the attached carbons to form an optionally substituted
heterocycle; and Z" is selected from C1-C6 alkoxy and vitro.
A compound of formula II is described:
Rc
II
wherein
Q is oxygen or sulfur;
Rl, R2, and R3 are each independently selected from the group
consisting of hydrogen and a radical -(CH2)mZ, where m is an integer from 0-6
and Z
is selected from the group consisting of halogen, hydroxy, formyl, C1-C6
alkanoyloxy,
optionally substituted benzoyloxy, C1-C6 alkyl, C1-C6 alkoxy, C3-C8
cycloalkyl, C3-C8
cycloallcoxy, C2-C6 alkenyl, CZ-C6 all~ynyl, Ci-C6 haloalkyl, C1-C6
haloalkoxy, C3-C8
halocycloalkyl, C3-C8 halocycloalkoxy, amino, C1-C6 alkylamino, (C1-C6
alkyl)(C1-C6
allcyl)amino, alkylcarbonylamino, N-(Cl-C6 alkyl)alkylcarbonylamino,
aminoalkyl,
C1-C6 all~ylaminoalkyl, (C1-C6 allcyl)(C1-C6 alkyl)aminoalkyl,
allcylcarbonylaminoalkyl, N-(Cl-C6 alkyl)alkylcarbonylaminoallcyl, cyano,
vitro, C1-
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
C6 all~ylsulfonyl, optionally substituted phenyl, optionally substituted
phenoxy, and
optionally substituted heteroaryl; or Z is selected from the group consisting
of -N3,
-COzR4, -CONRSR6, -P(O)(OR~)a~ -P(O)(NR4R5)z, and -P(O)(NR4R5)(OR ), where
R4, R5, and R6 are each independently selected in each occurrence from the
group
consisting of hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, Cl-C6 haloalkyl,
optionally
substituted phenyl, and optionally substituted phenyl-C1-C6 alkyl; or
Rl is selected from the group consisting of hydrogen and a radical
-(CHz)mZ, where m is an integer from 0-6 and Z is selected from the group
consisting
of halogen, hydroxy, formyl, C1-C6 alkanoyloxy, optionally substituted
benzoyloxy,
C1-C6 all~yl, C1-C6 alkoxy, C3-C8 cycloalkyl, C3-C8 cycloallcoxy, Cz-C6
alkenyl, Cz-C6
alkynyl, Cl-C6 haloalkyl, Ci-C6 haloalkoxy, C3-C8 halocycloalkyl, C3-C8
halocycloalkoxy, amino, Cl-C6 alkylamino, (C1-C6 alkyl)(G1-C6 alkyl)amino,
alkylcarbonylamino, N-(C1-C6 alkyl)allcylcarbonylamino, aminoalkyl, C1-C6
alkylaminoalkyl, (C1-C6 all~yl)(C1-C6 alkyl)aminoalkyl,
alkylcarbonylaminoalkyl, N-
(C1-C6 alkyl)alkylcarbonylaminoalkyl, cyano, nitro, C1-C6 alkylsulfonyl,
optionally
substituted phenyl, optionally substituted phenoxy, and optionally substituted
heteroaryl; or Z is selected from the group consisting of -N3, -COZR4, -
CONRSR6,
-P(O)(OR4)z, -P(O)(NR4R5)z, and -P(O)(NR4R5)(ORø), where R4, R5, and R6 are
each
independently selected in each occurrence from the group consisting of
hydrogen, C1-
C6 alkyl, C3-C8 cycloallcyl, Cl-C6 haloalkyl, optionally substituted phenyl,
and
optionally substituted phenyl-C1-C6 alkyl; and Rz and R3 are taken together
with the
attached carbon to form an optionally substituted carbocycle or heterocycle;
RA represents 1-4 substituents each independently selected from the
group consisting of hydrogen and a radical -(CHz)",~Z', where m' is an integer
from
0-6 and Z' is selected from the group consisting of halogen, hydroxy, Cl-C6
allcanoyloxy, optionally substituted benzoyloxy, C1-C6 alkyl, C1-C6 alkoxy, C3-
C8
cycloallcyl, C3-C8 cycloalkoxy, Cz-Cs allcenyl, Cz-C6 alkynyl, C1-C6
haloalkyl, C1-C6
haloalkoxy, C3-C8 halocycloallcyl, C3-C$ halocycloalkoxy, amino, C1-C6
alkylamino,
(C1-C~ alkyl)(C1-CG alkyl)amino, alkylcarbonylamino, N-(C1-C6
alkyl)alkylcarbonylamino, aminoalkyl, C1-C6 all~ylaminoalkyl, (C1-C6 alkyl)(C1-
C6
alkyl)aminoalkyl, alkylcarbonylaminoalkyl, N-(C1-C6
alkyl)alkylcarbonylaminoalkyl,
cyano, nitro, C1-C6 alkylsulfonyl, optionally substituted phenyl, optionally
substituted
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
_g_
phenoxy, and optionally substituted heteroaryl; or Z' is selected from the
group
Con5lStlng Of -N3, -C~2R4I, -C~NRS R6 I, -~(~)(~R~~)2~ -~(~)(~4 RS )2r and
-~(~)~4~5')(~R4')9 where R4~, RS~, and R6~ are each independently selected in
each
occurrlle'nlc~e from the group consisting of hydrogen, C1-C6 alkyl, C3-C8
cycloalkyl, Ci-
C6 haloalkyl, optionally substituted phenyl, and optionally substituted phenyl-
Cl-C6
alkyl; or
RA represents 2-4 substituents where 2 of said substituents are adjacent
substituents and are taken together with the attached carbons to form an
optionally
substituted caxbocycle or an optionally substituted heterocycle, and the
remaining 2
substituents are each independently selected from the group consisting of
hydrogen
and a radical -(CH2)",~Z', where m' is an integer from 0-6 and Z' is selected
from the
group consisting of halogen, hydroxy, C1-C6 alkanoyloxy, optionally
substituted
benzoyloxy, CI-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, C3-C8 cycloalkoxy, CZ-
C6
alkenyl, C2-C6 alkynyl, Ci-C6 haloalkyl, C1-C6 haloalkoxy, C3-C$
halocycloalkyl,
C3-C$ halocycloalkoxy, amino, C1-C6 alkylamino, (Ci-C6 alkyl)(Cl-C6
alkyl)amino,
allcylcarbonylamino, N-(Cl-C6 alkyl)allcylcarbonylamino, aminoalkyl, C1-C6
alkylaminoalkyl, (Ci-C6 alkyl)(C1-C6 alkyl)aminoalkyl,
alkylcarbonylaminoalkyl, N-
(C1-C6 alkyl)allcylcarbonylaminoalkyl, cyano, nitro, C1-C6 alkylsulfonyl,
optionally
substituted phenyl, optionally substituted phenoxy, and optionally substituted
heteroaryl; or Z' is selected from the group consisting of -N3, -COzR4~, -
CONRS Rs~,
-p(~)(~R4~)a, -P(~)(NR4IR5~)Z, and -P(O)(NR4R5)(OR4~), where R4~, RS~, and R6~
are
each independently selected in each occurrence from the group consisting of
hydrogen, CI-C6 alkyl, C3-C$ cycloallcyl, C1-C6 haloalkyl, optionally
substituted
phenyl, and optionally substituted phenyl-C1-C6 alkyl;
RB is selected from the group consisting of hydrogen and a radical
-(CH2)",~~Z", where m" is an integer from 0-6 and Z" is selected from the
group
consisting of halogen, hydroxy, Ci-C6 alkanoyloxy, optionally substituted
benzoyloxy, C1-Cg alkyl, C1-C6 alkoxy, C3-C8 cycloallcyl, C~-C8 cycloalk~xy,
CZ-C6
allcenyl, Ca-C6 alkynyl, C1-C6 haloalkyl, C1-C6 haloalkoxy, C3-C8
halocycloalkyl,
C3-C8 halocycloalkoxy, amino, C1-C6 alkylamino, (C1-C6 alkyl)(Cl-C6
all~yl)amino,
allcylcarbonylamino, N-(C1-C6 alkyl)alkylcarbonylamino, aminoalkyl, C1-C6
allcylaminoalkyl, (C1-C6 alkyl)(Ci-C6 alkyl)aminoalkyl,
alkylcarbonylaminoalkyl, N-
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-9-
(C1-C6 all~yl)alkylcarbonylaminoalkyl, cyano, nitro, C1-C6 alkylsulfonyl,
optionally
substituted phenyl, optionally substituted phenoxy, and optionally substituted
heteroaryl; or Z" is selected from the group consisting of -N3, -C02R~~~, -
CONRS~~R6 ,
., .. 4~~ 5'~ 4~, 4~~ 5~~ 6,~
-P(O)(OR4 )2, -P(O)(NR4"RS )2, and -P(O)(NR R )(OR ), where R , R , and R are
each independently selected in each occurrence from the group consisting of
hydrogen, C1-C6 alkyl, C3-C$ cycloallcyl, C1-C6 haloalkyl, optionally
substituted
phenyl, and optionally substituted phenyl-C1-C6 all~yl; and
Re represents 1-4 substituents each independently selected from the
group consisting of hydrogen and a radical -(CHa)",~~~Z"', where m"' is an
integer from
0-6 and Z"' is selected from the group consisting of halogen, hydroxy, C1-C6
alkanoyloxy, optionally substituted benzoyloxy, C1-C6 alkyl, Cl-C6 alkoxy, C3-
C8
cycloalkyl, C3-C8 cycloalkoxy, C2-C6 allcenyl, C2-C6 alkynyl, C1-C6 haloalkyl,
C1-C6
haloalkoxy, C3-C8 halocycloalkyl, C3-C8 halocycloalkoxy, amino, C1-C6
alkylamino,
(Cl-C6 alkyl)(C1-C6 alkyl)amino, alkylcarbonylamino, N-(C1-C6
allcyl)alkylcarbonylamino, aminoalkyl, CI-C6 alkylaminoalkyl, (C1-C6 alkyl)(C1-
C6
allcyl)aminoalkyl, alkylcarbonylaminoalkyl, N-(C1-C6
alkyl)alkylcarbonylaminoalkyl,
cyano, nitro, C1-C6 alkylsulfonyl, optionally substituted phenyl, optionally
substituted
phenoxy, and optionally substituted heteroaryl; or Z"' is selected from the
group
consisting of -N3, -CO2R4~~~, -CONRSrarR6nr~ -p(O)(OR4"~)z, -P(O)(NR4"RS"~)a,
and
4~~~ 5"~ 4~~~ 4~~~ 5~~~ 6~~~
-P(O)(NR R )(OR ), where R , R , and R are each independently selected in
each occurrence from the group consisting of hydrogen, C1-C6 alkyl, C3-C8
cycloalkyl, C1-C6 haloallcyl, optionally substituted phenyl, and optionally
substituted
phenyl-C1-C6 alkyl; or
R~ represents 2-4 substituents where 2 of said substituents are adjacent
substituents and are taken together with the attached carbons to form an
optionally
substituted carbocycle or an optionally substituted heterocycle, and the
remaining 2
substituents are each independently selected from the group consisting of
hydrogen
and a radical -(CHZ)m---Z"', where m"' is an integer from 0-6 and Z"' is
selected from
the group consisting of halogen, hydroxy, C1-C6 alkanoyloxy, optionally
substituted
benzoyloxy, C1-C6 alkyl, Cl-C6 alkoxy, C3-C$ cycloalkyl, C3-C8 cycloallcoxy,
CZ-C6
allcenyl, Ca-C6 alkynyl, C1-Cs haloalkyl, C1-C6 haloalkoxy, C3-C8
halocycloalkyl,
C3-C8 halocycloalkoxy, amino, C1-C6 alkylamino, (C1-C6 alkyl)(Cl-C6
alkyl)amino,
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-10-
alkylcarbonylamino, N-(C1-C6 alkyl)alkylcarbonylamino, aminoalkyl, C1-C6
alkylaminoalkyl, (C1-C6 alkyl)(C1-C6 alkyl)aminoalkyl,
alkylcarbonylaminoalkyl, N-
(C1-C6 allcyl)alkylcarbonylaminoalkyl, cyano, ntro, Ci-C6 alkylsulfonyl,
optionally
substituted phenyl, optionally substituted phenoxy, and optionally substituted
heteroaryl; or Z"' is selected from the group consisting of -N3, -C02R4~~~, -
C~NRS~~R6"',
-p(~)(~R4~~~)2~ -P(~)(NR4~~~5~'~2~ and -P(~)(NR4 RS",)(~R4re,)~ where Rq'"~~
RS",, and R6",
are each independently selected in each occurrence from the group consisting
of
hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, C1-C6 haloalkyl, optionally
substituted
phenyl, and optionally substituted phenyl-Cl-C6 alkyl is described.
In one illustrative embodiment of the compounds of formula II, at least
one of Rl, R2, R3, RA, RB, or Ro is not hydrogen. In another illustrative
embodiment
of the compounds of formula II, RA is 2,3-bis(Cl-C6 alkoxy). In another
illustrative
embodiment of the compounds of formula II, Q is oxygen, RA is 2,3-bis(C1-Cs
alkoxy), and RB, Ro, Rl, RZ, and R3 are each hydrogen.
In another illustrative embodiment of the compounds of formula II, Z'
is selected from the group consisting of hydroxy and nitro. In another
illustrative
embodiment of the compounds of formula II, RA represents 2-4 substituents
where 2
of said substituents are adjacent substituents and are taken together with the
attached
carbons to form an optionally substituted carbocycle or an optionally
substituted
heterocycle, and the remaining 2 substituents are each independently selected
from
the group consisting of hydrogen and a radical -(CH2)r"~Z', where Z' is
selected from
the group consisting of hydroxy and nitro.
In another illustrative embodiment of the compounds of formula II,
wherein Z" is nitro. In another illustrative embodiment of the compounds of
formula
II, Z"' is nitro. In another illustrative embodiment of the compounds of
formula II, R~
represents 2-4 substituents where 2 of said substituents are adjacent
substituents and
are taken together with the attached carbons to form an optionally substituted
caxbocycle or an optionally substituted heterocycle, and the remaining 2
substituents
are each independently selected from the group consisting of hydrogen and a
radical
-(CH2)",~~~Z"'; and Z"' is nitro.
A pharmaceutical composition including one or more compounds of
formula I and/or one or more compounds of formula II, and one or more
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-11-
pharmaceutically acceptable carriers, excipients, and/or diluents therefor is
also
described.
A method for treating a mammal in need of relief from a disease state,
including but not limited to cancer, is also described. The method comprises
the step
of administering to the mammal an effective amount of one or more compounds of
formula I and/or one or more compounds of formula II; or an effective amount
of a
pharmaceutical composition including one or more compounds of formula I and/or
one or more compounds of formula II.
BRIEF DESCRIPTION OF THE DRAWINGS
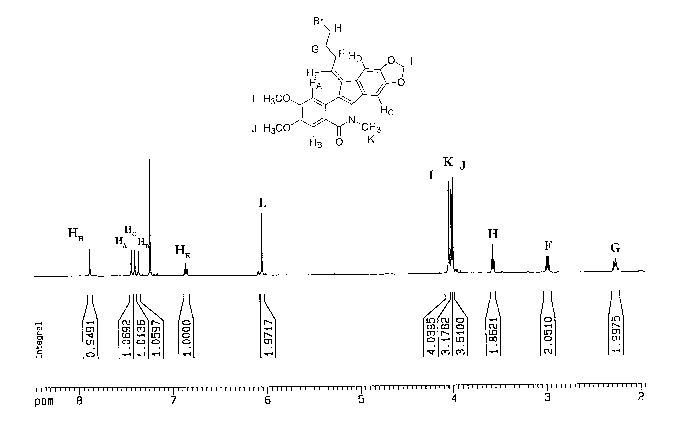
FIG. 1 shows a 1H NMR spectrum of indenoisoquinolone 19.
FIG. 2 shows a model of the binding of isoindoloisoquinolone 13 in a
ternary complex comprising DNA, topl, and the inhibitor. The diagram is
programmed for wall-eyed viewing.
FIG. 3 shows various indenoisoquinolones, isoindoloisoquinolones,
and derivatives thereof.
FIG. 4 shows an illustrative model of the orientation of compound 7
relative to DNA in a ternary complex containing topl, DNA, and compound 7.
DETAILED DESCRIPTION OF THE INVENTION
In one embodiment, indenoisoquinolones of formula I are described
herein:
a
RB
I
wherein R1, RA, RB, Q, X, and Y are as defined above.
In an embodiment of the compounds of formula I, haloall~yl,
aminoall~yl, haloallcenyl, and/or aminoalkenyl side chains may be attached to
C-11, as
described herein. Halo refers to fluoro, chloro, bromo, and iodo. In another
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-12-
embodiment of the compounds of formula I, heteroaryl alkyl and/or heteroaryl
alkenyl
side chains may be attached to C-11, as described herein. Heteroaryl refers to
any
cyclic aromatic stuucture comprising carbon and including at least one
heteroatom,
such as nitrogen, oxygen, phosphorus, or sulfur. Illustrative heteroaryls
include, but
are not limited to, furanyl, thiofuranyl, pyrrolyl, pyrazolyl, imidazolyl,
triazolyl,
tetrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxathiazolyl,
pyridinyl,
pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl, and the like.
It is appreciated that when X and Y are taken together to form =CR~'R3;
NR2; =NOR2; or =NNRZR3, either the E double bond, the Z double bond, or any
mixture of E and Z double bond isomers may be formed depending upon the
reaction
conditions chosen. For example, double bond formation using the McMurry
coupling
may tend to form more of, substantially more of, or exclusively the E double
bond
isomer. Other double bond forming reactions, such Wittig olefmations, Peterson
olefmations, condensation reactions, dehydration reactions, and the like, may
preferentially form other double bond isomers or mixtures thereof.
In another embodiment, isoindoloisoquinolone compounds are
described herein. In one aspect, benzo-fused isoindoloisoquinolone compounds
are
described herein. W another aspect, isoindoloisoquinolone compounds of formula
II
are described herein:
4
R1 - 3
Rc
s1 \ \
vRB
R/A~' ~ R~2 R3
Q
II
wherein Rl, R2, R3, RA, RB, R~, and Q are as defined above.
It is appreciated that compounds of formulae I and II may derive their
activity in modifying the growth or proliferation cells, such as cancer cells,
from their
ability to intercalate into DNA. In particular, DNA unwinding studies
indicated that
N 3-aminoalkyl derivatives of indenoisoquinolines, such as compound 7, can
intercalate. Without being bound by theory, it is suggested that the cationic
side chain
on the indenoisoquinoline nitrogen of compound 7 may project into the major
groove
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-13-
of DNA, as shown in FIG. 4. When the DNA is in a complex with a protein such
as
topl, side chains on the indenoisoquinoline nitrogen of compounds such as
compound
7 may project into the major groove of DNA towards the Asn352 residue of the
protein. Regarding compounds of formula I and without being bound by theory,
it is
suggested that side chains attached at C-11, such as alkyl, alkylidenyl,
aminoalkyl,
aminoalkylidenyl, hydroxylaminoalkyl, hydroxylaminoalkylidenyl,
hydrazinoalkyl,
and hydrazinoalkylidenyl side chains, may proj ect into the minor groove of
DNA.
When the DNA is in a complex with a protein such as top 1, side chains may
proj ect
into the minor groove of DNA toward the Arg364 and Asp533 residues of the
enzyme.
In another embodiment, pharmaceutical compositions and formulations
are contemplated herein. Such pharmaceutical compositions and formulations
comprise an effective amount of one or more indenoisoquinolone or
isoindoloisoquinolone compounds of formulae I and II for treating a patient
having
cancer. As used herein, an effective amount of the indenoisoquinolone or
isoindoloisoquinolone compound is defined as the amount of the compound which,
upon administration to a patient, inhibits growth of cancer cells, kills
malignant cells,
reduces the volume or size of the tumors, or eliminates the tumor entirely in
the
treated patient.
The effective amount to be administered to a patient is typically based
on body surface area, patient weight, and/or patient condition. The
interrelationship
of dosages for animals and humans (based on milligrams per meter squared of
body
surface) is described by Freireich, E.J., et al., Cayace~ Chemother. Rep.
1966, 50 (4),
219. Body surface area may be approximately determined from patient height and
weight (see e.g., Scientific Tables, Geigy Pharmaceuticals, Ardley, New York,
pages
537-538 (1970)). An effective amount of the indenoisoquinolone or
isoindoloisoquinolne compounds described herein includes any amount useful for
inhibiting the growth of (or killing) cancer cells in a patient. Typically,
such effective
amounts range from about 5 mg/lcg to about 500 mg/kg, more preferably from
about 5
mglkg to about 250 mg/kg, and most preferably about 5 to about 150 mg/kg. It
is
appreciated that effective doses may also vary dependent on a selected route
of
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-14-
administration, excipient usage, and/or the possibility of co-usage with other
therapeutic treatments including other anti-tumor agents, and/or radiation
therapy.
The pharmaceutical formulation may be administered via the
parenteral route, including subcutaneously, intraperitoneally,
intramuscularly, and
intravenously. Examples of parenteral dosage forms include aqueous solutions
of the
active agent, in isotonic saline, 5% glucose, or other well-known
pharmaceutically
acceptable liquid carrier. In one preferred aspect of the present embodiment,
the
indenoisoquinolone or isoindoloisoquinolone compound is dissolved in a saline
solution containing 5% dimethyl sulfoxide and 10% Cremphor EL (Sigma Chemical
Company). Additional solubilizing agents such as cyclodextrins, which can form
specific, more soluble complexes with the indenoisoquinolone or
isoindoloisoquinolone compounds described herein, or other solubilizing agents
well-
known to those familiar with the art, can be utilized as pharmaceutical
excipients for
delivery of the indenoisoquinolone or isoindoloisoquinolone compounds.
The present compound can also be formulated into dosage forms for
other routes of administration utilizing well-known methods. The
pharmaceutical
compositions can be formulated, for example, in dosage forms for oral
administration
in a capsule, a gel seal, or a tablet. Capsules may comprise any well-known
pharmaceutically acceptable material such as gelatin or cellulose derivatives.
Tablets
may be formulated in accordance with conventional procedure by compressing
mixtures of the active indenoisoquinoline or isoindoloisoquinoline and solid
carriers,
and lubricants well-lcnown to those familiar with the art. Examples of solid
carriers
include starch, sugar, and bentonite. The compounds of the present invention
can also
be administered in a form of a hard shell tablet or capsule containing, for
example,
lactose or mannitol as a binder and conventional fillers and tableting agents.
The following examples are intended to illustrate various embodiments
of the invention, and are not intended to in any way limit the scope of the
invention as
set forth in this specification and claims. Unless otherwise indicated,
melting points
were determined in capillary tubes and are uncorrected; Infrared spectra were
obtained using CHC13 as the solvent; 1H NMI~ spectra were determined at 300
MHz
using CDC13 as solvent and TMS as internal standard; microanalyses were
performed
at the Purdue University Microanalysis Laboratory; reactions were monitored by
thin-
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-15-
layer chromatography (TLC) and visualized with short wavelength UV light; and
compounds were purified by silica gel flash chromatography.
EXAMPLES
Example 1. General synthesis of compounds of formula II.
Syntheses of the benzoisoindoloisoquinolone 8 and the analog
compound 13, which lacks the two methoxyl groups of 8, are outlined in Scheme
1.
Scheme 1
X
R
O
R
N ~ NH3 N ~ O
Ms0 ~ / ~ / 11 R = OCH3, X = Br
12 R=H,X=CI
OMs HN
9 10
N- /
R ~ ~ \ /
R ~ , Nr
0
8 R = OCH3
13 R=H
Treatment of a THF solution of the dimesylate 9 with liquid ammonia
afforded the 2,3-dihydro-1H pyrrolo[3,4-b]quinoline (10), which was reacted in
situ
with bromide 11 to afford the desired compound 8. Similarly, reaction of 10
with the
chloride 12 yielded the corresponding unsubstituted derivative 13. Additional
synthetic details and/or reaction conditions are generally described in Corey
et al. in J.
Oyg. Cl2em. 40:2140-41 (1975); Claus & Steinitz in.Iustus Liebigs Ann. Chem.
282:107-30 (1894); Parnck & Ragunathan in J. Chem. Soc. Perkin Trans. 1 211-16
(1993); Sloan & Loch in.l. Org. Chem. 48:635-640 (1983), and Slemon et al. in
Can.
J. Chem. 59:3055-60 (1981), the disclosures of which axe incorporated herein
by
reference. For example, the preparation of dimesylate 9 is described in Claus
~
Steinitz, the preparation of bromide 11 is described in Slemon et al., and the
preparation of chloride 12 is described in Sloan ~ Loch.
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-16-
Example 2. General synthesis of compounds of formula I.
As portrayed in Scheme 2, the 11-indenoisoquinolones 18-21 were
prepared using a Ii~IcI~urry reaction of the ketone 2 with the haloaldehydes
14-17 (see
Cushman ~z Cheng in .I ~~~. Claern. 43:3781-3783 (1978), the disclosure of
which is
incorporated herein by reference).
Scheme 2
0
O - 1 ~~. V THF, Zn dust
H~o
H3C0 ~ w TiC14~2THF
14 n=3,X=CI
H3C0 CH3 15 n = 3, X = Br
O, 16 n=4,X=Br
2 17 n=5,X=Br
18 n=3,X=CI
19 n=3,X=Br
20 n=4,X=Br
21 n=5,X=Br
In each case, the production of a single double bond isomer was
observed. The stereochemistry of the double bond was determined by obtaining
nuclear Overhauser effect (NOE) difference spectra of compound 19. The
interpretation of the NOE difference spectra is based on the assignments of
the signals
in the 1H NMR spectrtnn of 19 (FIG. 1). The HB, HE, L, F, G, and H protons
were
assigned to the resonances at 7.89, 6.86, 6.08, 3.00, 2.28, and 3.61 ppm,
respectively
(see FIG. 1). The resonance at 7.89 ppm was assigned to HB because this proton
is
adjacent to the amide carbonyl, which would presumably deshield HB and cause
it to
shift farthest downfield with respect to the other aromatic protons. The
resonance at
6.68 ppm was assigned to the vinylic proton HE because this is the only
triplet that
might integrate for one proton. Furthermore, the resonance at 6.08 ppm was
assigned
to the L protons because they are the only protons that might appear as a two-
proton
singlet. The assignment of protons at F, G, and H was accomplished by
evaluating
their chemical shifts. The protons at H are adjacent to a bromide and are
faxthest
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-17-
downfield at 3.60 pprn. The allylic protons at F are further upfield at 3.00
ppm, and
finally the protons at G are farthest upfield at 2.28 ppm.
The remaining aromatic resonances were assigned using hT~E
difference spectrometry. Irradiation of the L protons (6.08 ppm) resulted in
enhancement of the resonances at 7.39 and 7.36 ppm, corresponding to HD and
HC.
Because the resonance at 7.89 ppm was assigned to HB, the resonance at 7.44
ppm
should correspond to HA by the process of elimination. Irradiation of HE (6.86
ppm)
resulted in a strong enhancement of the resonance at 7.44 ppm corresponding to
HA
instead of HD. Therefore, the double bond at C-11 of indenoisoquinoline 4
supports
the assigned E stereochemistry.
The assignments of the remainder of the resonances in the NMR
spectrum of 19 (FIG. 1) were accomplished in the same manner and supported the
assignments made above. Specifically, irradiation of the F protons (3.00 ppm)
caused
an enhancement of the resonance at 7.36 ppm. Therefore, the resonance at 7.36
ppm
corresponds to HD. Through the process of elimination, the resonance at 7.39
ppm
must belong to H~. Irradiation of H~ (7.39 ppm) resulted in enhancement of the
resonance at 4.040 ppm, resulting in assignment of this resonance to the
protons of the
methyl amine I~. Finally, irradiation of HB (7.89 ppm) resulted in enhancement
of the
resonance at 4.023 ppm. Therefore, the resonance at 4.023 ppm corresponds to
the
protons at J, and by process of elimination, the resonance at 4.044 belongs to
the
protons at I.
Though as exemplified herein, the McMurry coupling reaction affords
only the double bond having the E-double bond configuration, it is appreciated
that
the Z-double bond configuration or various mixtures of E and Z double bond
isomers
may be obtained using other conventional double bond forming reactions.
The 4-iodobutenyl compound 24 was derived from the bromide 19
using the Finl~elstein reaction (Scheme 3).
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-18-
Scheme 3
19
Nal, MeZCO
24
As with the haloalkene derivatives, the McMurry reaction of the lead
compound 2 with Boc-protected /3-alaninal (25) afforded the desired 3-
aminopropenyl
compound 26 after acidic work-up (Scheme 4).
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-19
Scheme 4
0 1
\ / o
H3CO ~ w
I
H3G0 I / N~CH3
O
2
H
~uN\~SH
IOI - f IO
1) Zn dust, TiC14~2THF
2) HCI
26
Alaninal 25 may be prepared as described by Blaney et al, in
Tetrahedroya 58:1719-37 (2002), the disclosure of which is incorporated herein
by
reference.
5 Example 3. 8,9-Dimethoxy-12H 5,11a-diaza-dibenzo[b,h.]fluoren-11-one (8).
A solution of 9 (described in Claus & Steinitz, "Alkyl Derivatives of
(3-Quinaldic Acid," Justus Liebigs Ayzya. Che~z., 2~2, 107-130 (1894), the
disclosure of
which is incorporated herein by reference) (200 mg, 0.58 mmol) in anhydrous
THF
(20 mL) was degassed by bubbling argon through the solution for 30 min. Liquid
10 NH3 was added via cold finger for 5 min at approximately 1 drop/Ssec. The
cold
finger was removed and the reaction mixture was allowed to warm to room
temperature under argon. The reaction mixture was stirred at room temperature
for 12
h, at which point argon was bubbled through the solution for 1.5 h to remove
excess
NH3. Anhydrous THF (10 mL) and NEt3 (3 mL) were added and the reaction mixture
15 was stirred at room temperature for 30 min. Bromide 11 (described in Slemon
et al.,
"Synthesis of Phthalideisoquinolines from 3-Halopyridines and 3,4-
Dihydroisoquinolinium Salts," Can. J. Chem., 59, 3055-3060 (1981), the
disclosure of
which is incorporated herein by reference) was added and the reaction mixture
was
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-20-
stirred at room temperature for 24 h. The solvent was removed in vacuo and
replaced
with 10% NaOAc/AcOH (30 mL). The reaction mixture was stirred at room
temperature for 24 h, at which point the solvent was removed in vacuo. The
resulting
solid was dissolved in water (100 mL) and extracted with CHC13 (3 x 100 mL).
The
organic layers were pooled, washed with saturated aqueous NaHCO3 (1 x 100 mL),
brine, dried (MgS04), filtered, and concentrated in vacuo to provide a brown
solid.
Purification (silica gel, CHC13) provided 8 (106 mg, 53%) as a yellow solid:
1H NMR
(300 MHz, DMSO-d6) b 8.61 (s, 1 H), 8.12 (m, 2 H), 7.84 (m, 1 H), 7.71 (s, 1
H),
7.82 (m, 1 H), 7.61 (s, 1 H), 7.50 (s, 1 H), 5.32 (s, 2 H), 3.94 (s, 3 H),
3.91 (s, 3 H);
ESIMS fnlz (rel intensity) 345.2 (100, MH+). Anal. Calcd for
C21H16N203~0.5H20:
C, 71.38; H, 4.85; N, 7.93. Found: C, 71.07; H, 4.72; N, 7.85.
Example 4. 12H 5,lla-Diaza-dibenzo[b,h]fluoren-11-one (13).
A solution of 9 (200 mg, 0.58 mmol) in anhydrous THF (20 mL) was
degassed by bubbling argon through the solution for 30 min. Liquid NH3 was
added
via cold finger for 5 min at approximately 1 drop/5sec. The cold finger was
removed
and the reaction mixture was allowed to warm to room temperature under argon.
The
reaction mixture was stirred at room temperature for 12 h, at which point
argon was
bubbled through the solution for 1.5 h to remove excess NH3, affording a
solution of
intermediate 10. Anhydrous THF (10 mL) and NEt~ (3 mL) were added and the
reaction mixture was stirred at room temperature for 30 min. Chloride 12
(Sloan &
Loch, "Effect of Nucleophilicity and Leaving Group Ability on the SN2
Reactions of
Amines with (Acyloxy)alkyl a-Halides," J. Org. Chern., 48, 635-640 (1983), the
disclosure of which is incorporated herein by reference) (195 mg, 1.16 mmol)
was
added and the reaction mixture stirred at room temperature for 24 h. The
solvent was
removed in vacuo and replaced with 10% NaOAc/AcOH (30 mL). The reaction
mixture was stirred at room temperature for 24 h, at which point the solvent
was
removed in vacuo. The resulting solid was dissolved in water (100 mL) and
extracted
with CHC13 (3 x 100 mL). The organic layers were pooled, washed with saturated
aqueous NaHC03 (1 x 100 mL), brine, dried (MgS04), filtered, and concentrated
in
vacuo to provide a brown solid. Purification (silica gel, CHC13) provided 13
(90 mg,
55%) as a yellow solid: IR (film) 3062, 2952, 2839, 1714, 1619, 1566, 1456,
1438,
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-21-
1256, 1067 cni 1; 1H NMR (300 MHz, CDCl3) b 8.56 (d, J= 7.56 Hz, 1 H), 8.34
(s, 1
H), 8.23 (d, J= 8.64. Hz, 1 H), 7.91 (d, J= 8.19 Hz, 1 H), 7.90-7.71 (m, 3 H),
7.68 (s,
1 H), 7.66-7.56 (m, 2 H), 5.38 (d, J=1.07 Hz, 2 H); ESIMS rfalz (rel
intensity) 285.2
(100, MH+). Anal. Calcd for C19H1zNzO'0.25HzO: C, 79.01; H, 5.46; N, 9.70.
Found: C, 79.30; H, 5.36; N, 9.66.
Example 5. 11-(4'-Chlorobutylidene)-5,6-dihydro-2,3-dimethoxy-6-methyl-8,9-
methylenedioxy-5-oxo-11FI indeno[1,2-c]isoquinoline (18).
TiCl4'2THF (508 mg, 1.52 mmol), Zn dust (199 mg, 3.04 mmol), and
dry THF (15 mL) were added to a flame-dried two-necked flask equipped with a
magnetic stir bar and reflux condenser. The suspension was heated at reflux
under
argon for 3 h, after which a solution of 4-chlorobutanal (14) (Li et al.,
"Synthesis of
the Tricyclic ABC Ring Subunit of Mazamine A," Tetrahedron, 54, 6661-6676
(1998), the disclosure of which is incorporated herein by reference) (108.1
mg, 1.01
mmol) and indenoisoquinoline 2 (185 mg, 0.51 mmol) in dry THF (15 mL) was
introduced by syringe. The reaction mixture was heated at reflux for 2.5 h,
after
which 4 N HCl (20 mL) was added. The solution was stirred at room temperature
for
1 h and then allowed to stand for 3 h. The resulting orange precipitate was
collected
by vacuum filtration. The solid was purified by flash chromatography (silica
gel, 5:1
CHC13/hexanes) to provide 18 (96.1 mg, 43%) as an orange solid: mp 196-201
°C;1R
(film) 2926, 1636, 1610, 1517, 1483, 1255, 1034 cm 1; 1H NMR (300 MHz, CDC13)
8
7.89 (s, 1 H), 7.78 (s, 1 H), 7.40 (s, 1 H), 7.37 (s, 1 H), 6.88 (t, J= 7.19
Hz, 1 H), 6.04
(s, 2 H), 4.05 (s, 3 H), 4.03 (s, 3 H), 4.02 (s, 3 H), 3.74 (t, J= 6.32 Hz, 2
H), 3.00 (dt,
J= 7.30 and 7.45 Hz, 2 H), 2.20 (qn, J= 6.90 Hz, 2 H); ESIMS m/z (rel
intensity)
440.7 (100, MH+), 442.6 (43, MH+). Anal. Calcd for Cz4HzzC1N05: C, 65.53; H,
5.04; N, 3.18. Found: C, 65.30; H, 4.96; N, 3.08.
Example 6. 11-(4'-Bromobutylidene)-5,6-dihydro-2,3-dimethoxy-6-methyl-8,9-
methylenedioxy-5-oxo-11H indeno[1,2-c]isoquinoline (19).
A 100 mL two-necked round-bottomed flaslc equipped with a magnetic
stirring bar, reflux condenser, septa, and argon line was charged with zinc
dust (537
mg, 8.21 rmnol) and was flame dried. THF (30 mL) and a 1 M solution of TiCl4
in
toluene (4.11 mL, 4.11 mmol) were added. The suspension was heated at reflux
for 5
h, at which point a suspension of 2 (500 mg, 1.37 mmol) and 4-bromobutanal
(15)
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
_22_
(Canan Loch ~z Chamberlin, "Enantioselective Preparation of [3-Alkyl-y-
butyrolactones from Functionalized I~etene Dithioacetals," ~: ~y~. Claefn.,
5~, 2725-
2737 (1993); Somekawa et al., "Intramolecular [2 + 2]Photocycloadditions of 1-
(e~-
Alkenyl)-2-pyridones Possessing an Ester Group on the ~lefinic Carbon Chain,"
.I.
~rg. Chena, 57, 5708-5712, (1992), the disclosures of which axe incorporated
herein
by reference) (413 mg, 2.74 mmol) in THF (30 mL) was added by pipette. The
reaction mixture was heated at reflux for 1 h and then quenched with 4 N HCl
(40
mL). The solution was stirred for 1 h and then cooled at 0 °C for 2 h.
The orange
precipitate was collected by vacuum filtration to provide an orange solid.
This was
purified by flash chromatography (silica gel, CHC13) to provide 19 (161.6 mg,
30%)
as an orange solid: mp 197-199 °C; IR (fllm) 2921, 1610, 1517, 1483,
1381, 1296,
1253, 1207, 1033 cm 1; 1H NMR (500 MHz, CDC13) b 7.88 (s, 1 H), 7.44 (s, 1 H),
7.40 (s, 1 H), 7.36 (s, 1 H), 6.83 (t, J= 7.11 Hz, 1 H), 6.05 (s, 2 H), 4.00
(s, 6 H), 3.97
(s, 3 H), 3.57 (t, J= 6.33 Hz, 2 H), 2.97 (q, J= 7.23 Hz, 2 H), 2.25 (qn, J=
6.80 Hz, 2
H); ESIMS m/z (rel intensity) 486.2 (97, MH+), 484.2 (100, MH+). Anal. Calcd
for
Ca4HaaBrN05: C, 59.52; H, 4.58; N, 2.89. Found: C, 59.56; H, 4.56; N, 2.88.
Example 7. 11-(5'-Bromopentylidene)-5,6-dihydro-2,3-dimethoxy-6-methyl-8,9-
methylenedioxy-5-oxo-11H indeno[1,2-c]isoquinoline (20).
A 100 mL two-necked round-bottomed flask equipped with a reflux
condenser and magnetic stir bax was charged with zinc dust (537 mg, 8.21 mmol)
and
flame dried. THF (30 mL) and 1 M TiCl4 in toluene (4.11 mL, 4.11 mmol) were
added to the round-bottomed flaslc and the mixture was heated at reflux for 6
h. THF
(30 mL), 5-bromopentanal (16) (452 mg, 2.74 mmol) and 2 (S00 mg 1.37 mmol)
were
added to the reaction mixture, which was heated at reflux for 2 h. The
reaction
mixture was cooled to room temperature, 4 N HCl (40 mL) was added, and this
mixture was stirred for 30 min and cooled in a-20 °C freezer overnight.
The
resulting yellow precipitate was collected by vacuum filtration and purified
by flash
chromatography to provide 20 (133.7 mg, 33%) as an orange solid: mp 196-197
°C;
IR (film) 2932, 1632, 1612, 1517, 1483, 1254, 1032 cm 1; 1H NMR (500 MHz,
CDCl3) ~ 7.89 (s, 1 H), 7.46 (s, 1 H), 7.41 (s, 1 H), 7.32 (s, 1 H), 6.86 (t,
J= 6.83 Hz,
1 H), 6.05 (s, 2 H), 4.05 (s, 3 H), 4.03 (s, 3 H), 4.01 (s, 3 H), 3.49 (t, J=
6.47 Hz, 2
H), 2.86 (q, J= 7.15 Hz, 2 H), 2.06 (m, 2 H), 1.88 (m, 2 H); ESIMS rnlz (rel
intensity)
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-23-
500.2 (95, MHO), 498.2 (100, MH+). Anal. Calcd for C2sHZ4BrN~5: C, 60.25; H,
4.85; N, 2.81. Found: C, 60.57; Ii, 4.90; N, 2.83.
Example 8. 11-(6'-Bromohexylidene)-5,6-dihydro-2,3-dimethoxy-6-methyl-8,9-
methylenedioxy-5-oxo-llfl indeno[1,2-c]isoquinoline (21).
A 100 mL two-necked round-bottomed flask equipped with a reflux
condenser and magnetic stir bar was charged with zinc dust (537 mg, 8.21 mmol)
and
flame dried. THF (30 mL) and 1 M TiCl4 in toluene (4.11 mL, 4.11 mmol) were
added to the round-bottomed flask and the mixture was heated at reflux for 4
h.
Anhydrous THF (30 mL), 6-bromohexanal (17) (491 mg, 2.74 mmol) and 2 (500 mg
1.37 mmol) were added to the reaction mixture, which was heated at reflux for
2 h.
The reaction mixture was cooled to room temperature, 4 N HCl (40 mL) was
added,
and this mixture was stirred for 30 min and then cooled in a -20 °C
freezer overnight.
The resulting yellow precipitate was collected by vacuum filtration and
purified by
flash chromatography to provide 21 (118.0 mg, 17%) as an orange solid: mp 182-
185
°C; IR (film) 2929, 1636, 1610, 1516, 1482, 1296, 1255, 1033 cm 1; 1H
NMR (500
MHz, CDC13) 8 7.88 (s, 1 H), 7.46 (s, 1 H), 7.40 (s, 1 H), 7.32 (s, 1 H), 6.88
(t, J=
6.96 Hz, 1 H), 6.05 (s, 2 H), 4.06 (s, 3 H), 4.02 (s, 3 H), 4.00 (s, 3 H),
3.44 (t, J= 6.62
Hz, 2 H), 2.84 (q, J= 7.13 Hz, 2 H), 1.95 (qn, J= 7.04 Hz, 2 H), 1.74 (m, 2 H)
1.66
(rn, 2 H); ESIMS m/z (rel intensity) 514.2 (100, MH+), 512.2 (91, MH+). Anal.
Calcd
for C2~Hz6BrN05: C, 60.95; H, 5.11; N, 2.73. Found: C, 60.55; H, 5.08; N,
2.72.
Example 9. 11-(4'-Iodobutylidene)-5,6-dihydro-2,3-dimethoxy-6-methyl-8,9-
methylenedioxy-5-oxo-11H indeno[1,2-c]isoquinoline (24).
NaI (217 mg, 1.45 mmol) was added to a suspension of bromide 19 (70
mg, 0.15 mmol) in acetone (15 mL). The reaction mixture was heated at reflux
for 12
h, after which the resulting orange precipitate was collected by vacuum
filtration and
purified by flash chromatography (silica gel, CHC13) to provide 24 (73.0 mg,
95%) as
an orange solid: mp 173-174.5 °C; IR (I~Br) 2944, 1612, 1522, 1481,
1254, 1033 cm
i;1H NMR (500 MHz, CDC13) ~ 7.88 (s, 1 H), 7.43 (s, 1 H), 7.40 (s, 1 H), 7.36
(s, 1
H), 6.84 (t, J= 7.22 Hz, 1 H), 6.05 (s, 2 H), 4.08 (s, 3 H), 4.04 (s, 3 H),
4.00 (s, 3 H),
3.35 (t, .I= 6.69 Hz, 2 H), 2.95 (q, .T= 7.32 Hz, 2 H), 2.19 (m, 2 H); ESIMS
m/z (rel
intensity) 532.1 (100, MH+). Anal. Calcd for C24H22IN05: C, 54.25; H, 4.17; N,
2.64. Found: C, 54.64; H, 4.25; N, 2.60.
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-24-
Example 10. 11-(3'-Aminopropylidene)-5,6-dihydro-2,3-dimethoxy-6-methyl-8,9-
methylenedioxy-5-oxo-11I~ indeno[1,2-c]isoquinoline (26).
TiCl4-THF (1:2) complex (730 mg, 2.19 mmol) and zinc dust (284 mg,
4.37 mmol) were put in a three-necked round-bottomed flaslc. THF (30 mL) was
added. The resulting suspension was heated under reflux for 4 h. At this
point, a
mixture of aldehyde 25 (Blaney et al., "Fused and Bridged Bi- and Tri-Cyclic
Lactams via Sequential Metallo-Azomethine Ylide Cycloaddition-Lactamisation,"
Tetf°ahedrofz., 58, 1719-37 (2002), the disclosures of which are
incorporated herein by
reference) (189 mg, 1.09 mmol) and indenoisoquinoline 2 (266 mg, 0.73 mmol) in
THF (30 mL) was added via syringe. The reaction mixture was stirred under
reflux
for an additional 4 h. Then 3 N HCl (10 mL) was added after cooling to room
temperature and the mixture was stirred at room temperature for 1 h, followed
by 0 °C
for 2 h, and finally at room temperature overnight. The mixture was cooled to
0 °C
and solid NaHC03 was added to neutralize HCI. The solvents were evaporated and
the residue was subjected to flash chromatography, eluting with CHC13-MeOH
(4:1)
to provide 26 as a yellow powder (121 mg, 41%): mp >180 °C (dec); 1H
NMR (300
MHz, DMSO-d6) 7.68 (s, 1 H), 7.62 (s, 1 H), 7.51 (s, 1 H), 7.48 (s, 1 H), 6.94
(t, J=
6.0 Hz, 1 H), 6.15 (s, 2 H), 4.01 (s, 3 H), 3.95 (s, 3 H), 3.87 (s, 3 H), 3.12-
3.18 (m, 4
H). ESIMS m/z (rel intensity) 407.0 (100, MH+). Anal. Calcd for
2O C23H22N205'0~9CHCl3: C, 55.86; H, 4.49; N, 5.45. Found: C, 55.86; H, 4.72;
N,
5.24.
Example 11. Topl-Mediated DNA Cleavage Reactions.
Human recombinant topl was purified from Baculovirus as described
by Pourquier et al. in J Biol. ClZem. 274:8516-23 (1999), the disclosure of
which is
incorporated herein by reference. The 161 by fragment from pBluescript SIB(-)
phagemid DNA (Stratagene, La Jolla, CA) was cleaved with the restriction
endonuclease Pvu II and Hind III (New England Biolabs, Beverly, MA) in
supplied
NE buffer 2 (10 ~,L reactions) for 1 h at 37 °C, and separated by
electrophoresis in a
1% agarose gel made in 1X TBE buffer. The 161 by fragment was eluted from the
gel slice (centrilutor by Amicon) and concentrated in a centricon 50
centrifugal
concentrator (Amicon, Beverly, MA). Approximately 200 ng of the fragment was
3'-
end labeled at the Hind III site by fill-in reaction with [alpha-3aP]-dGTP and
0.5 rnM
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-25-
dATP, dCTP, and dTTP, in React 2 buffer (50 mM Tris-HCl, pH 8.0, 100 mM MgCl2,
50 mM NaCl) with 0.5 units of DNA polylnerase I (I~lenow fragment).
Unincorporated 3ZP-dGTP was removed using mini Quick Spin DNA columns
(Ruche, W dianapolis,1N), and the eluate containing the 3'-end-labeled 161 by
fragment was collected. Aliquots (approximately 50,000 dpm/reaction) were
incubated with topl at 22 °C for 30 min in the presence of the tested
drug. Reactions
were terminated by adding SDS (0.5% final concentration). The samples (10 ~L)
were mixed with 30 ~,L of loading buffer (80% formamide, 10 mM sodium
hydroxide, 1 mM sodium EDTA, 0.1 % xylene cyanol, and 0.1 % bromophenol blue,
pH 8.0). Aliquots were separated in denaturing gels (16% polyacrylamide, 7 M
urea).
Gels were dried and visualized by using a Phosphoimager and ImageQuant
software
(Molecular Dynamics, Sunnyvale, CA).
Selected indenoisoquinolones described herein were examined for
antiproliferative activity against the human cancer cell lines in the National
Cancer
Institute screen, in which the activity of each compound was evaluated with
approximately 55 different cancer cell lines of diverse tumor origins. The
mean graph
midpoint (MGM) shown in Table 1 is based on a calculation of the average GI50
for
all of the approximately 55 cell lines tested, where GI50 values below and
above the
test range (10-$ to 10-4 molar) are taken as the minimum (10-8 molar) and
maximum
(10-4 molar) drug concentrations used in the screening test. The GI50 values
obtained
from selected cell lines for these indenoisoquinolones are also summarized in
Table 1.
The relative activities of selected indenoisoquinolines described herein were
obtained
from the topl-mediated DNA cleavage assay, and are also shown in Table 1.
Compounds 2 and 7 are also included the Table 1.
Example 12. Biological activity of compounds of formula I.
The halogenated 11-alkenyl side chain derivatives 18-21 and 24 were
all approximately in the same range of cytotoxicity as compound 2. However,
compounds 21 and 24 were slightly more cytotoxic than compounds 18-20. In
addition, the five-carbon bromide 20 and the six-carbon bromide 21 showed
higher
inhibitory activity against topl than the four-carbon allcenyl halides 19 and
24.
The most potent compound in the present series of new
indenoisoquinolines, both in terms of cytotoxicity and topl inhibitory
activity, was
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-26-
the compound 26. having a cytotoxicity mean graph midpoint (MGM) of 0.34 ~,M,
indicating a large increase in overall cytotoxicity in cancer cell cultures.
In addition,
the resulting compound 26 was more potent as a top 1 poison than compound 2.
V6Tithout being bound by theory, a hydrogen bonding interaction
between the hydroxyl group of the camptothecin analogue topotecan and the
carboxyl
group of Asp533 of the enzyme may contribute to the binding of the
camptothecin
ring system. Similarly, the 3'-aminopropenyl side chain in compound 26 may
project
into the minor groove, where it might possibly interact with Asp533 or Arg364,
or
with a stacked base residue of the DNA.
It is appreciated that cytotoxicity profile of compounds described
herein may be improved by altering solubility properties, facilitating
cellular uptake,
and/or the inclusion of components that take advantage of the electrostatic
attraction
of a positively charged ammonium cation to a negatively charged DNA
phosphodiester backbone prior to intercalation into the cleavage complex, as
illustrated by compounds 26 and 7.
Example 13. Biological activity of compounds of formula II.
Both compounds 8 (MGM 91.2 ~.M) and 13 (MGM 58.9 ~,M) were
generally less cytotoxic than compound 2 (MGM 20.0 ~M). The hybrid molecule 13
and the lead compound 2 displayed similar potencies as topl iWibitors. It was
observed that the pattern of topl-mediated DNA cleavage sites induced by 13
better
resembled that of compound 2 than that of camptothecin, though the only
difference
between camptothecin (3) and analogues 8 and 13 was the replacement of the
lactone
ring of camptothecin (3) by either the dimethoxybenzene ring in 8 or the
benzene ring
in 13.
Example 14. Computer model of the ternary complex consisting of DNA, top 1,
and
compounds of formula II.
To investigate the nature of the binding of compound 13 in the
published ternary complex, a model was constructed by overlapping the
structure of
the hybrid 13 with the structure of topotecan in the published ternary complex
(see
Staker et al. in PYOC. Natl. Acad. Sci. U.S.A. 99:15387-15392 (2002), the
disclosure of
which is incorporated herein by reference) and then deleting the camptothecin
structure (FIG. 2). It was reported that the synthetic double-stranded DNA in
the
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
crystalline ternary complex contained a phosphorothiolate at the cleavage
site.
Compound 13 can be modeled into the camptothecin binding site in the ternary
complex without any obvious steric constraints.
CA 02525099 2005-11-08
WO 2004/100891 PCT/US2004/014581
-2$-
U
O C~ ~ -~ ~ O
C~ C~
O U N ~ ~ ~ ~ ~ ~ M O ~ ~ ,.~
~ ~ v~
cd (~ ~ o~ M l~ M ~ ~ ~ N O '
O1 V7 M N M w
~'
~ ~
~
'r
O ~ U r
O W
O Ml'O ~ O O O ~ ~ ~ N .~ U z N
N
n n n n ~ ~ o
O - ' ~
O b
U
O 1
0_ 0 ~ ~ ~ ~ ~ M ~ O _ _,7,0 7-a
U O ~ n n M ~ ~ ~ N ~ M O ~ U
N N
O f~., O ~A
N p O O O O ~ ~ ~ ~ M O U
~
d ~ ~ ~ ~ ~ V ~ -N
~i ..i~ ~ 0 U
n n n n n O
...1 ;
N
a
O M ~ O M M .~', U ~ N
O 0 p 0 ' .O
O O ~ ~ ~ O ', N N "d
N" N N O
O U ~ O O
N N N
d o : W V ~ ..,~~.~,O
N O U ? ~ , N ~ ~ ,~ ~ _c~
~ U ~ 01 M ~ n M M N ~
U n ~ ~ '~
~','~ ~ 4-1 n
O ~ h M ~ ~ N ~ O ~~ ,+~.-'
n ~ z
U U ~ ~ ~ ~ ~ o ~ .~ o U
., ~ ~ o
M M
~i ".,~ ~ ; ~ ~ fl N M
E-1O 1 -1 ~ O
M d' M ~ ~ O ~ ~ i
~ M r 3
~ N ~ ~ ~ O 4a O
y ~ : O ~
N N O ~ ~~ ~ O
b
,M-~ ~ N N N N N l~
O
U