Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
BIOCHIP DEVICES FOR ION TRANSPORT MEASUREMENT, METHODS
OF MANUFACTURE, AND METHODS OF USE
TECHNICAL FIELD
The present invention relates generally to the field of ion transport
detection
("patch clamp") systems and methods, particularly those that relate to the use
of
biochip technologies.
This application claims priority to United States patent application number
60/474,508, filed May 31, 2003, hereby incorporated by reference in its
entirety.
This application incorporates by reference the following applications: United
States patent application number 10/760,866, filed January 20, 2004; United
States
patent application number 10/428,565, filed May 2, 2003; United States patent
application number 60/380,007, filed May 4, 2002; United States patent
application
number 10/642,014, filed August 16, 2003; United States patent application
number
10/351,019, filed January 23, 2003; United States patent application number
60/351,849 filed January 24, 2002; United States patent application number
10/104,300, filed March 22, 2002; United States patent application number
60/311,327 filed August 10, 2001; and United States patent application number
60/278,308 filed March 24, 2001.
BACKGROUND
Ion transports are channels, transporters, pore forming proteins, or other
entities that are located within cellular membranes and regulate the flow of
ions
across the membrane. Ion transports participate in diverse processes, such as
generating and timing of action potentials, synaptic transmission, secretion
of
hormones, contraction of muscles etc. Ion transports are popular candidates
for drug
discovery, and many known drugs exert their effects via modulation of ion
transport
functions or properties. For example, antiepileptic compounds such as
phenytoin and
lamotrigine which block voltage
dependent sodium ion transports in the brain, anti-hypertension drugs such as
nifedipine and diltiazem which block voltage dependent calcium ion transports
in
smooth muscle cells, and stimulators of insulin release such as glibenclamide
and
tolbutamine which block an ATP regulated potassium ion transport in the
pancreas.
1
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
One popular method of measuring an ion transport function or property is the
patch-clamp method, which was first reported by Neher, Sakmann and Steinback
(Pflueger Arch. 375:219-278 (1978)). This first report of the patch clamp
method
relied on pressing a glass pipette containing acetylcholine (Ach) against the
surface of
a muscle cell membrane, where discrete jumps in electrical current were
attributable
to the opening and closing of Ach-activated ion transports.
The method was refined by fire polishing the glass pipettes and applying
gentle suction to the interior of the pipette when contact was made with the
surface of
the cell. Seals of very high resistance (between about 1 and about 100 giga
ohms)
could be obtained. This advancement allowed the patch clamp method to be
suitable
over voltage ranges which ion transport studies can routinely be made.
A variety of patch clamp methods have been developed, such as whole cell,
vesicle, outside-out and inside-out patches (Liem et al., Neurosurgery 36:382-
392
(1995)). Additional methods include whole cell patch clamp recordings,
pressure
patch clamp methods, cell free ion transport recording, perfusion patch
pipettes,
concentration patch clamp methods, perforated patch clamp methods, loose patch
voltage clamp methods, patch clamp recording and patch clamp methods in tissue
samples such as muscle or brain (Boulton et al, Patch-Clamp Applications and
Protocols, Neuromethods V. 26 (1995), Humana Press, New Jersey).
These and later methods relied upon interrogating one sample at a time using
large laboratory apparatuses that require a high degree of operator skill and
time.
Attempts have been made to automate patch clamp methods, but these have met
with
little success. Alternatives to patch clamp methods have been developed using
fluorescent probes, such as cumarin-lipids (cu-lipids) and oxonol fluorescent
dyes
(Tsien et al., U.S. Patent No. 6,107,066, issued August 2000). These methods
rely
upon change in polarity of membranes and the resulting motion of oxonol
molecules
across the membrane. This motion allows for the detection of changes in
fluorescence
resonance energy transfer (FRET) between cu-lipids and oxonol molecules.
Unfortunately, these methods do not measure ion transport directly but measure
the
change of indirect parameters as a result of ionic flux. For example, the
characteristics of the lipid used in the cu-lipid can alter the biological and
physical
characteristics of the membrane, such as fluidity and polarizability.
Thus, what is needed is a simple device and method to measure ion transport
directly. Preferably, these devices would utilize patch clamp detection
methods
2
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
because these types of methods represent a gold standard in this field of
study. The
present invention provides these devices and methods particularly miniaturized
devices and automated methods for the screening of chemicals or other moieties
for
their ability to modulate ion transport functions or properties.
BRIEF SUMMARY OF THE INVENTION
The present invention recognizes that the determination of one or more ion
transport functions or properties using direct detection methods, such as
patch-clamp,
whole cell recording, or single channel recording, are preferable to methods
that
utilize indirect detection methods, such as fluorescence-based detection
systems.
The present invention provides biochips for ion transport measurement, ion
transport measuring devices that comprise biochips, and methods of using ion
transport measuring devices and biochips that allow for the direct analysis of
ion
transport functions or properties. The present invention provides biochips,
devices,
apparatuses, and methods that allow for automated detection of ion transport
functions
or properties. The present invention also provides methods of making biochips
and
devices for ion transport measurement that reduce the cost and increase the
efficiency
of manufacture, as well as improve the performance of the biochips and
devices.
These biochips and devices are particularly appropriate for automating the
detection
of ion transport functions or properties, particularly for screening purposes.
A first aspect of the present invention is a biochip device for ion transport
measurement. A biochip device comprises an upper chamber piece that comprises
one
or more upper chambers and a biochip that comprises at least one ion transport
measuring means. In one preferred embodiment of this aspect of the present
invention, a biochip device is part of an apparatus that also comprises at
least one
conduit that that can be positioned to engage the one or more upper chambers,
where
the conduit comprises an electrode or can provide an electrolyte bridge to an
electrode.
A second aspect of the present invention is a biochip device having one or
more flow-through lower chambers. The device comprises an upper chamber piece
that comprises one or more upper chambers, a biochip that comprises at least
one ion
transport measuring means, and at least one lower chamber base piece that
comprises
3
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
one or more lower chambers and at least two conduits that connect with at
least one of
the one or more lower chambers.
A third aspect of the invention is biochip-based ion transport measurement
devices that are adapted for microscope stages. The devices comprise an upper
chamber piece that comprises one or more upper chambers, a biochip that
comprises
at least one ion transport measuring means, and at least one lower chamber
base piece,
in which the bottom surface of the lower chamber base piece is transparent.
Preferably, the device also includes a baseplate adapted to a microscope stage
into
which a lower chamber base piece can fit.
A fourth aspect of the invention is methods of making an upper chamber piece
for a biochip device for ion transport measurement. In one preferred
embodiment of
this aspect of the present invention, an upper chamber piece can be molded as
two
pieces, an upper well portion piece and a well hole portion piece. Preferably,
a well
hole portion piece comprises at least one groove into which at least one
electrode can
be inserted. After insertion of the electrode, the upper well portion piece
and the well
hole portion piece are attached to form an upper chamber piece. In another
embodiment of this aspect, an upper chamber piece can be molded as a single
piece,
where an electrode, such as a wire electrode, can be positioned in a mold and
then the
upper chamber piece can be molded around it. In yet another preferred
embodiment of
this aspect, an upper chamber piece can be molded as a single piece without an
electrode.
A fifth aspect of the invention is methods for making chips comprising ion
transport measuring holes. An ion transport measuring hole can be fabricated
by laser
drilling one or more counterbores, and then laser drilling a through-hole
through the
one or more counterbores.
A sixth aspect of the invention is an ion transport measuring device that
comprises an inverted chip comprising ion transport measuring holes. A chip
used in
inverted orientation can comprise one or more ion transport measuring holes
that are
fabricated by laser drilling of one or more coimterbores and a through-hole
through
the one or more counterbores.
A seventh aspect of the invention is methods of treating ion transport
measuring chips to enhance their sealing properties. In one aspect of the
present
invention, the chip or substrate comprising an ion transport measuring means
is
modified to become more electronegative, more smooth, or more electronegative
and
4
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
more smooth. In some aspects of the present invention, the chip or substrate
comprising the ion transport measuring means is modified chemically, such as
with
acids, bases, or a combination thereof. Treatment of chips of the present
invention
with chemical solution can be performed using treatment racks that fit into
vessels
that hold the chemical solutions and can hold multiple glass chips while
allowing
access of the chemical solutions to the chip surfaces.
An eighth aspect of the invention is a method to measure surface energy on a
surface, such as the surface of a chemically-treated ion transport measurement
biochip. The surface energy measurement can be used to evaluate the
hydrophilicity
of a biochip biochip of the present invention that has been chemically treated
to
improve its electrical sealing properties, such as, for example, at chip that
has been
treated with base. The method can also be used for any surface
characterization
purpose where a measurement of surface energy or hydrophilicity is desired.
A ninth aspect of the invention is the substrates, biochips, devices,
apparatuses, and/or cartridges comprising ion transport measuring means with
enhanced electric seal properties. In preferred embodiments, at least a
portion of at
least one chip that comprises at least one ion transport measuring means has
been
modified to become more electronegative. In preferred embodiments, at least a
portion of at least one chip that comprises at least one ion transport
measuring means
has been treated with at least one base, at least one acid, or both.
A tenth aspect of the present invention is a method for storing the
substrates,
biochips, cartridges, apparatuses, and/or devices comprising ion transport
measuring
means with enhanced electrical seal properties.
An eleventh aspect of the present invention is a method for shipping the
substrates, biochips, cartridges, apparatuses, and/or devices comprising ion
transport
measuring means with enhanced electrical seal properties.
A twelfth aspect of the invention is methods for assembling devices and
cartridges of the present invention. The methods include attaching an upper
chamber
piece to a biochip that comprises at least one ion transport measuring means
using a
UV adhesive. Preferably, the chip has been chemically treated to enhance its
electrical
sealing properties. During UV activation of the adhesive, at least a portion
of the
biochip is masked to prevent UV irradiation of ion transport measuring means
on the
chip.
5
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A thirteenth aspect of the present invention is a method of producing biochips
comprising ion transport measuring means by fabricating the biochips as
detachable
units of a large sheet. Ion transport measuring holes can be made by wet
etching and
laser drilling appropriate substrates, and the sheet can be scored with a
laser such that
portions of the sheet having a desired number of ion transport measuring holes
can be
separated along the score lines. In some embodiments, upper chamber pieces are
attached to the substrate sheet after the fabrication of holes and before
separation of
sections of the sheet. In this case, the detachable units that are separated
to produce
devices comprise cartridges having upper chambers attached to an ion transport
measuring chip.
A fourteenth aspect of the invention is a method of producing high density ion
transport measuring chips. The ion transport measuring chips preferably have
more
than 16 ion transport measuring holes, and wells can be fabricated in a chip
using wet
etching, followed by laser drilling of ion transport measuring holes through
the
bottoms of the wells.
A fifteenth aspect of the invention is a biochip device for ion transport
measurement comprising fluidic channel upper and lower chambers. The fluidic
channels have apertures that are aligned with ion transport measuring holes on
the
chip. The fluidic channels can be connected to sources for generating or
promoting
fluid flow, such as pumps, pressure sources, and valves. The fluidic channels
preferably provide electrolyte bridges to one or more electrodes that can be
used in
ion transport measurement.
A sixteenth aspect of the present invention is methods of preparing cells for
ion transport measurement. The methods include the use of filters that can
allow the
passage of single cells through their pores and monitoring of cell health
parameters
important for electrophysiological measurements.
A seventeenth aspect of the present invention is a logic and program
that uses a pressure control profile to direct an ion transport measurement
apparatus to
achieve and maintain a high-resistance electrical seal. The logic can follow
decision
pathways based on information from electrical measurements made by ion
transport
measuring electrodes in a feedbacl~ system.
6
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
BRIEF DESCRIPTION OF THE DRAWINGS
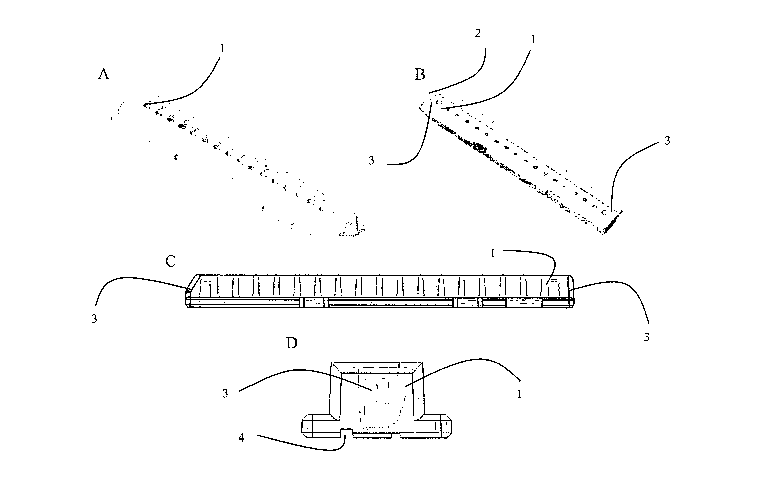
Figure 1 depicts four views of one example of an upper chamber piece of the
present
invention: A) top view; B) bottom view; C) side-on cross-sectional view; and
D) end-
on cross-sectional view.
Figure 2 depicts a cross-sectional view of a single ion transport measuring
unit of one
example of an ion transport measuring device of the present invention. Figure
is not
necessarily to scale.
Figure 3 provides photographs of a lower chamber piece of the present
invention that
is adapted to fit a microscope stage and has flow-through lower chambers. (A)
view
of a plastic lower chamber base piece with connectors for inflow and outflow
tubes,
B) a zoomed-in view of the lower chamber base piece showing inflow and outflow
tubes C) the lower chamber piece installed in a base plate.
Figure 4 provides photographs of one design of a base plate for adapting a
biochip
device to a microscope stage. (A) Top view and (B) bottom view of a base plate
cut
from aluminum stock. The holes (401) are threaded except for the four holes
closest
to the corners of the square-cut carve-out. The four unthreaded holes (402)
are sized
to accept a press-in 1 mm socket connector.
Figure 5 depicts one device of the present invention having a lower chamber
base
piece fitted to a baseplate (54) by means of a clamp (53) which also attaches
the upper
chamber piece (51) to the lower chamber base piece (not visible). The clamp
also
comprises wire electrodes (55) that extend into upper wells. Electrode
connectors (52)
have wires extending into the fluidics of each lower chamber below.
Figure 6 depicts a lower chamber piece of the present invention in the form of
a
gasket having multiple holes (601) that form the walls of lower chambers in an
assembled device. In this design, the holes are formed by O-ring structures
(602).
Figure 7 provides photographs of a clamp part (A) upside down and (B) viewed
from
the top fitted over a cartridge.
7
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 8 provides photographs of a cartridge device of the present invention
(black
item) shoran in relation to the rest of the parts of a device adapted for a
microscope
(A) and after assembly into a baseplate (B).
Figure 9 depicts an upper chamber piece of the present invention that is made
from
an upper well portion piece (91) and a well-hole portion piece (92). (A) the
upper well
portion piece (91) is shown above the well-hole portion piece (92). (B) the
upper well
portion piece (91) is shown fitted on the well-hole portion piece to form
wells (93),
with the groove (94) where an electrode can be inserted visible along the back
of the
wells (93).
Figure 10 is a graph that illustrates that a decreasing hole depth (x-axis)
and widening
the exit hole (as for "K-configuration" chips) decreases Re (y-axis). On the
left side
("K-configuration" chips): black circles, chips having 2.5 micron diameter
holes with
6 micron entrance holes; black squares, chips having 2 micron diameter holes
with 5
micron entrance holes; black double triangles, chips having 1.8 micron
diameter holes
with 4 and 6 micron entrance holes; and X's, chips having 1.5 micron diameter
holes
with 6 micron entrance holes. On the right side ("S-configuration chips) black
triangles, chips having 2.5 micron diameter holes with 10 micron entrance
holes;
black squares, chips having 2 micron diameter holes with 9 micron entrance
holes;
open triangles, chips having 1.8 micron diameter holes with 7 micron entrance
holes;
and black diamonds, chips having 1.5 micron diameter holes with 8 micron
entrance
holes.
Figure 11 is a graph illustrating that thinner chips (for example "K-
configuration"
chips of the present invention) have a lower Ra ("improved Ra") than those
with
greater hole depth. Ra also decreases as hole diameter increases, however at a
cost of
lower Rm. Increased Rm ("improved Rm") is found with increased hole depth.
Figure 12 gives depictions of a laser drilled chip (123) having a first
counterbore
(126) and a second counterbore (127) and a through-hole (128). In A) the
direction of
laser drilling of the counterbores (126 and 127) and through-hole (128) is
shown by
the arrow. In B), the chip is used in inverted orientation with a cell (129)
sealed to the
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
hole (128) that connects the upper chamber (121) with the lower chamber (125)
having walls formed by a gasket (124). Figure is not necessarily to scale.
Figure 13 depicts treatment fixtures for chemically treating chips and
devices. (A)
shows a single layer treatment fixture that can fit into a glass j ar
containing acid, base,
or other chemical solutions. (B) shows the stacked fixture.
Figure 14 shows one design of a shipping fixture for cartridges of the present
invention. In A), a blister pack having a plastic frame (141) and openings
(142) for
sealing cartridges (143) is viewed from the bottom. In B), the blister pack is
viewed
from the top side of the sealed-in cartridge (143).
Figure 15 depicts a glass chip (151) with multiple ion transport holes (152)
that can
be attached to a multichamber upper chamber piece to form a multiunit sheet
(154).
The multiunit sheet (154) comprising upper chambers and a chip (151) has mark
lines
or perforations in the chip (153) where the sheet can be separated into
sections.
Cartridges with a smaller number of units (155) can be separated from the
larger
multiunit sheet (154). Not to scale.
Figure 16 depicts one example of a high density array chip (161) of the
present
invention. The wells (162) of the chip can be made by wet etching followed by
laser
drilling through holes through the bottoms of the wells (162).
Figure 17 shows an example of a high density array having upper chambers (171)
that can be formed by a well plate (172) attached to the chip (173). Wells
(174) in the
chip (173) having laser drilled through-holes can be oriented in inverted
orientation
(top alternative) or standard orientation (bottom alternative).
Figure 18 depicts the general format for pressure bonding, in which a chip
(183)
comprising a hole (182) is attached to an upper chamber piece (181) using a
gasket
(184) to form a seal between the upper chamber piece (181) and chip (183) when
pressure (arrow) is applied. In this highly schematized depiction, a lower
chamber
piece (185) is also attached to the chip (183) using a second gasket (186) to
form a
seal between the lower chamber piece (185) and chip (183) when pressure
(arrow) is
applied.
9
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 19 depicts a schematic view of one design a planar patch clamping chip
(193)
having an upper fluid channel (191) for extracellular solution (ES) and a
lower fluidic
channel (195) for intracellular solutions (IS1, IS2). The upper and lower
channels are
interfaced at a point where the recording aperture (192) of the planar
electrode
resides. Separate fluidic pumps (P) drive the flow of fluids through the two
(upper and
lower) fluidic channels. Recording (196) and reference electrodes (197)
external to
the fluidic patch clamp chip are connected via an electrolyte solution bridge
to the
upper (191) and lower (195) fluidic channels. A pressure source such as a pump
with
pressure controller that can generate both positive and negative pressures is
linked to
the lower fluidic channels. A multi-way valve (194) is used to connect the
lower
fluidic channel (195) to different solution reservoirs (IS1, IS2, etc), and a
multi-way
valve (198) is used to connect the upper fluidic channel (191) to cell
reservoirs,
compound plate (CP), wash buffers and other solutions. (Not to scale).
Figure 20 provides graphs of the success rate of a test of patch clamp seals
using
cartridges of the present invention having chemically treated chips. A) gives
the
success duration of seals on 52 chips. B) plots the accumulative success rate
of cells
on 53 chips (achieved gigaseals and gave Ra<lSMOhm and Rm>200MOhm
throughout 15 min recording period).
Figure 21 provides graphs of results of tests performed on 52 clops. A) gives
Re
values of the chips. B) gives break-in pressures during the quality control
test.
Figure 22 provides graphs of Rm (membrane resistance) and Ra (access
resistance) at
the beginning and at end of tests using devices of the present invention. A)
shows Rm
after break-in (wide diagonals slanting upward) and at the end of the test
(narrow
diagonals slanting downward). B) shows Ra after break-in (wide diagonals
slanting
upward) and at the end of the test (narrow diagonals slanting downward).
Figure 23 provides typical patch clamp recordings immediately after break-in
using a
device of the present invention. A) uncorrected whole-cell recording, B)
corrected
whole cell recording, C) plot of corrected and uncorrected recording taken
during the
interval denoted by the arrowheads in A) and B).
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 24 provides typical patch clamp recordings fifteen minutes after break-
in
using a device of the present invention. A) uncorrected whole cell recording,
B)
corrected whole cell recording, C) plot of corrected and uncorrected recording
taken
during the interval denoted by the arrowheads in A) and B).
Figure 25 plots the Rm and Ra values for patch clamps of the experiment shown
in
Figures 23 and 24 beginning at break-in and continuing over a 15-minute
period.
Figure 26 is a flowchart of an overview of the pressure control profile
program.
Figure 27 is a flowchart of part 1 of Procedure Landing of the pressure
control profile
program.
Figure 28 shows a flowchart of part 2 of Procedure Landing of the pressure
control
profile program.
Figure 29 shows a flowchart of part 3 of Procedure Landing of the pressure
control
profile program.
Figure 30 shows a flowchart of part 1 of Procedure FormSeal of the pressure
control
profile program.
Figure 31 shows a flowchart of part 2 of Procedure FormSeal of the pressure
control
profile program.
Figure 32 shows a flowchart of part 3 of Procedure FormSeal of the pressure
control
profile program.
Figure 33 shows a flowchart of part 4 of Procedure FormSeal of the pressure
control
profile program.
11
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 34 shows a flowchart of part 5 of Procedure FormSeal of the pressure
control
profile program.
Figure 35 shows a flowchart of part 1 of Procedure Breakln of the pressure
control
profile program.
Figure 36 shows a flowchart of part 2 of Procedure Breakln of the pressure
control
profile program.
Figure 37 shows a flowchart of part 3 of Procedure BreakIn of the pressure
control
profile program.
Figure 38 shows a flowchart of part 4 of Procedure Breakln of the pressure
control
profile program.
12
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
DETAILED DESCRIPTION OF THE INVENTION
Definitions
Unless defined otherwise, all technical and scientific terms used herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which this invention belongs. Generally, the nomenclature used herein and the
manufacture or laboratory procedures described below are well known and
commonly
employed in the art. Conventional methods are used for these procedures, such
as
those provided in the art and various general references. Terms of orientation
such as
"up" and "down", "top" and "bottom", "upper" or "lower" and the like refer to
orientation of parts during use of a device. Where a term is provided in the
singular,
the inventors also contemplate the plural of that term. Where there are
discrepancies
in terms and definitions used in references that are incorporated by
reference, the
terms used in this application shall have the definitions given herein. As
employed
throughout the disclosure, the following terms, unless otherwise indicated,
shall be
understood to have the following meanings:
"Ion transport measurement" is the process of detecting and measuring the
movement of charge and/or conducting ions across a membrane (such as a
biological
membrane), or from the inside to the outside of a particle or vice versa. In
most
applications, particles will be cells, organelles, vesicles, biological
membrane
fragments, artificial membranes, bilayers or micelles. In general, ion
transport
measurement involves achieving a high resistance electrical seal of a membrane
or
particle with a surface that has an aperture, and positioning electrodes on
either side
of the membrane or particle to measure the current and/or voltage across the
portion
of the membrane sealed over the aperture, or "clamping" voltage across the
membrane and measuring current applied to an electrode to maintain that
voltage.
However, ion transport measurement does not require that a particle or
membrane be
sealed to an aperture if other means can provide electrode contact on both
sides of a
membrane. For example, a particle can be impaled with a needle electrode and a
second electrode can be provided in contact with the solution outside the
particle to
complete a circuit for ion transport measurement. Several techniques
collectively
known as "patch clamping" can be included as "ion transport measurement".
13
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
An "ion transport measuring means" refers to a structure that can be used to
measure at least one ion transport function, property, or a change in ion
channel
function, property in response to various chemical, biochemical or electrical
stimuli.
Typically, an ion transport measuring means is a structure with an opening
that a
particle can seal against, but this need not be the case. For example, needles
as well as
holes, apertures, capillaries, and other detection structures of the present
invention can
be used as ion transport measuring means. An ion transport measuring means is
preferably positioned on or within a biochip or a chamber. Where an ion
transport
measuring means refers to a hole or aperture, the use of the terms "ion
transport
measuring means" "hole" or "aperture" are also meant to encompass the
perimeter of
the hole or aperture that is in fact a part of the chip or substrate (or
coating) surface
(or surface of another structure, for example, a channel) and can also include
the
surfaces that surround the interior space of the hole that is also the chip or
substrate
(or coating) material or material of another structure that comprises the hole
or
aperture.
A "hole" is an aperture that extends through a chip. Descriptions of holes
found herein are also meant to encompass the perimeter of the hole that is in
fact a
part of the chip or substrate (or coating) surface, and can also include the
surfaces that
surround the interior space of the hole that is also the chip or substrate (or
coating)
material. Thus, in the present invention, where particles are described as
being
positioned on, at, near, against, or in a hole, or adhering or fixed to a
hole, it is
intended to mean that a particle contacts the entire perimeter of a hole, such
that at
least a portion of the surface of the particle lies across the opening of the
hole, or in
some cases, descends to some degree into the opening of the whole, contacting
the
surfaces that surround the interior space of the hole.
A "patch clamp detection structure" refers to a structure that is on or within
a
biochip or a chamber that is capable of measuring at least one ion transport
function
or property via patch clamp methods.
A "chip" is a solid substrate on which one or more processes such as physical,
chemical, biochemical, biological or biophysical processes can be carried out.
Such
processes can be assays, including biochemical, cellular, and chemical assays;
ion
transport or ion channel function or activity determinations, separations,
including
separations mediated by electrical, magnetic, physical, and chemical
(including
biochemical) forces or interactions; chemical reactions, enzymatic reactions,
and
14
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
binding interactions, including captures. The micro structures or micro-scale
structures such as for example, channels and wells, electrode elements, or
electromagnetic elements, may be incorporated into or fabricated on the
substrate for
facilitating physical, biophysical, biological, biochemical, chemical
reactions or
processes on the chip. The chip may be thin in one dimension and may have
various
shapes in other dimensions, for example, a rectangle, a circle, an ellipse, or
other
irregular shapes. The size of the major surface of chips of the present
invention can
vary considerably, for example, from about 1 mm2 to about 0.25 m2. Preferably,
the
size of the chips is from about 4 mm2 to about 25 cm2 with a characteristic
dimension
from about 1 mm to about 5 cm. The chip surfaces may be flat, or not flat. The
chips
with non-flat surfaces may include wells fabricated on the surfaces.
A "biochip" is a chip that is useful for a biochemical, biological or
biophysical
process. In this regard, a biochip is preferably biocompatible, in that it
does not
negatively affect cells or cell membranes.
A "chamber" is a structure that comprises or engages a chip and that is
capable
of containing a fluid sample. The chamber may have various dimensions and its
volume may vary between 0.001 microliter and 50 milliliter. In devices of the
present
invention, an "upper chamber" is a chamber that is above a biochip, such as a
biochip
that comprises one or more ion transport measuring means. In the devices of
the
present invention, a chip that comprises one or more ion transport measuring
means
can separate one or more upper chambers from one or more lower chambers.
During
use of a device, an upper chamber can contain measuring solutions and
particles or
membranes. An upper chamber can optionally comprise one or more electrodes. In
devices of the present invention, a "lower chamber" is a chamber that is below
a
biochip. During use of a device, a lower chamber can contain measuring
solutions and
particles or membranes. A lower chamber can optionally comprise one or more
electrodes.
A lower chamber "has access to" or "accesses" an upper chamber via (or
through) a hole in a chip when the chip separates or is between the upper and
lower
chambers and a hole in the chip provides fluid communication between the
referenced
lower chamber and the referenced upper chamber. An upper chamber "has access
to"
or "accesses" a lower chamber via (or through) a hole in a chip when the chip
separates or is between the upper and lower chambers and a hole in the chip
provides
fluid communication between the referenced upper chamber and the referenced
lower
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
chamber. Similarly an upper chamber can be "connected to" a lower chamber (or
vice
versa) via a hole in a chip when the hole in the chip provides fluid
communication
between the referenced upper chamber and the referenced lower chamber.
A "lower chamber piece" is a part of a device for ion transport measurement
that forms at least a portion of one or more lower chambers of the device. A
lower
chamber piece preferably comprises at least a portion of one or more walls of
one or
more lower chambers, and can optionally comprise at least a portion of a
bottom
surface of one or more lower chambers, and can optionally comprise one or more
conduits that lead to one or more lower chambers, or one or more electrodes.
A "lower chamber base piece" or "base piece" is a part of a device for ion
transport measurement that forms the bottom surface of one or more lower
chambers
of the device. A lower chamber base piece can also optionally comprise one or
more
walls of one or more lower chambers, one or more conduits that lead to one or
more
lower chambers, or one or more electrodes.
As used herein, a "platform" is a surface on which a device of the present
invention can be positioned. A platform can comprises the bottom surface of
one or
more lower chambers of a device.
An "upper chamber piece" is a part of a device for ion transport measurement
that forms at least a portion of one or more upper chambers of the device. An
upper
chamber piece can comprise one or more walls of one or more upper chambers,
and
can optionally comprise one or more conduits that lead to an upper chamber,
and one
or more electrodes.
An "upper chamber portion piece" is a part of a device for ion transport
measurement that forms a portion of one or more upper chambers of the device.
An
upper chamber portion piece can comprise at least a portion of one or more
walls of
one or more upper chambers, and can optionally comprise one or more conduits
that
lead to an upper chamber, or one or more electrodes.
A "well" is a depression in a substrate or other structure. For example, in
devices of the present invention, upper chambers can be wells formed in an
upper
chamber piece. The upper opening of a well can be of any shape and can be of
an
irregular conformation. The walls of a well can extend upward from the lower
surface
of a well at any angle or in any way. The walls can be of any shape and can be
of an
irregular conformation, that is, they may extend upward in a sigmoidal or
otherwise
curved or mufti-angled fashion.
16
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A "well hole" is a hole in the bottom of a well. A well hole can be a well-
within-a well, having its own well shape with an opening at the bottom.
A "well hole piece" is a part of a device for ion transport measurement that
comprises one or more well holes of the wells of the device.
When wells or chambers (including fluidic channel chambers) are "in register
with" ion transport measuring means of a chip, there is a one-to-one
correspondence
of each of the referenced wells or chambers to each of the referenced ion
transport
measuring means, and an ion transport measuring means is positioned so that it
is
exposed to the interior of the well or chamber it is in register with, such
that ion
transport measurement can be performed using the chamber as a compartment for
measuring current or voltage through or across the ion transport measuring
means.
A "port" is an opening in a wall or housing of a chamber through which a fluid
sample or solution can enter or exit the chamber. A port can be of any
dimensions,
but preferably is of a shape and size that allows a sample or solution to be
dispensed
into a chamber by means of a pipette, syringe, or conduit, or other means of
dispensing a sample.
A "conduit" is a means for fluid to be transported into or out of a device,
apparatus, or system for ion transport measurement of the present invention or
from
one area to another area of a device, apparatus, or system of the present
invention. In
some aspects, a conduit can engage a port in the housing or wall of a chamber.
In
some aspects, a part of a device, such as, for example, an upper chamber piece
or a
lower chamber piece can comprise conduits in the form of tunnels that pass
through
the upper chamber piece and connect, for example, one area or compartment with
another area or compartment. A conduit can be drilled or molded into a chip,
chamber, housing, or chamber piece, or a conduit can comprise any material
that
permits the passage of a fluid through it, and can be attached to any part of
a device.
In one preferred aspect of the present invention, a conduit extends through at
least a
portion of a device, such as a wall of a chamber, or an upper chamber piece or
lower
chamber piece, and connects the interior space of a chamber with the outside
of a
chamber, where it can optionally connect to another conduit, such as tubing.
Some
preferred conduits can be tubing, such as, for example, rubber, teflon, or
tygon tubing.
A conduit can be of any dimensions, but preferably ranges from 10 microns to 5
millimeters in internal diameter.
17
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A "device for ion transport measurement" or an "ion transport measuring
device" is a device that comprises at least one chip that comprises one or
more ion
transport measuring means, at least a portion of at least one upper chamber,
and,
preferably, at least a portion of at least one lower chamber. A device for ion
transport
measurement preferably comprises one or more electrodes, and can optionally
comprise conduits, particle positioning means, or application-specific
integrated
circuits (ASICs).
A "cartridge for ion transport measurement" comprises an upper chamber
piece and at least one biochip comprising one or more ion transport measuring
means
attached to the upper chamber piece, such that the one or more ion transport
measuring means are in register with the upper chambers of the upper chamber
piece.
An "ion transport measuring unit" is a portion of a device that comprises at
least a portion of a chip having a single ion transport measuring means and a
single
upper chamber, where the ion transport measuring means is in register with the
upper
chamber. An ion transport measuring unit can further comprise at least a
portion of a
lower chamber that is in register with the ion transport measuring means an
upper
chamber.
A "measuring solution" is an aqueous solution containing electrolytes, with
pH, osmolarity, and other physical-chemical traits that are compatible with
conducting function of the ion transports to be measured.
An "intracellular solution" is a measuring solution used in the upper or lower
chamber that is compatible with the electrolyte composition and physical-
chemical
traits of the intracellular content of a living cell.
An "extracellular solution" is a measuring solution used in the upper or lower
chamber that is compatible with the electrolyte composition and physical-
chemical
traits of the extracellular content of a living cell.
To be "in electrical contact with" means one component is able to receive and
conduct electrical signals (for example, voltage, current, or change of
voltage or
current) from another component.
An "ion transport" can be any protein or non-protein moiety that modulates,
regulates or allows transfer of ions across a membrane, such as a biological
membrane
or an artificial membrane. Ion transport include but are not limited to ion
channels,
proteins allowing transport of ions by active transport, proteins allowing
transport of
ions by passive transport, toxins such as from insects, viral proteins or the
like. Viral
1S
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
proteins, such as the M2 protein of influenza virus can form an ion channel on
cell
surfaces.
A "particle" refers to an organic or inorganic particulate that is suspendable
in
a solution and can be manipulated by a particle positioning means. A particle
can
include a cell, such as a prokaryotic or eukaryotic cell, or can be a cell
fragment, such
as a vesicle or a microsome that can be made using methods known in the art. A
particle can also include artificial membrane preparations that can be made
using
methods known in the art. Preferred artificial membrane preparations are lipid
bilayers, but that need not be the case. A particle in the present invention
can also be
a lipid film, such as a black-lipid film (see, Houslay and Stanley, Dynamics
of
Biological Membranes, Influence on Synthesis, Structure and Function, John
Wiley &
Sons, New York (1982)). In the case of a lipid film, a lipid film can be
provided over
a hole, such as a hole or capillary of the present invention using methods
known in the
art (see, Houslay and Stanley, Dynamics of Biological Membranes, Influence on
Synthesis, Structure and Function, John Wiley & Sons, New York (1982)). A
particle
preferably includes or is suspected of including at least one ion transport or
an ion
transport of interest. Particles that do not include an ion transport or an
ion transport
of interest can be made to include such ion transport using methods known in
the art,
such as by fusion of particles or insertion of ion transports into such
particles such as
by detergents, detergent removal, detergent dilution, sonication or detergent
catalyzed
incorporation (see, Houslay and Stanley, Dynamics of Biological Membranes,
Influence on Synthesis, Structure and Function, John Wiley & Sans, New York
(1982)). A microparticle, such as a bead, such as a latex bead or magnetic
bead, can
be attached to a particle, such that the particle can be manipulated by a
particle
positioning means.
A "cell" refers to a viable or non-viable prokaryotic or eukaryotic cell. A
eukaryotic cell can be any eukaryotic cell from any source, such as obtained
from a
subject, human or non-human, fetal or non-fetal, child or adult, such as from
a tissue
or fluid, including blood, which are obtainable through appropriate sample
collection
methods, such as biopsy, blood collection or otherwise. Eukaryotic cells can
be
provided as is in a sample or can be cell lines that are cultivated in vitro.
Differences
in cell types also include cellular origin, distinct surface markers, sizes,
morphologies
and other physical and biological properties.
19
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A "cell fragment" refers to a portion of a cell, such as cell organelles,
including but not limited to nuclei, endoplasmic reticulum, mitochondria or
golgi
apparatus. Cell fragments can include vesicles, such as inside out or outside
out
vesicles or mixtures thereof. Preparations that include cell fragments can be
made
using methods known in the art.
A "population of cells" refers to a sample that includes more than one cell or
more than one type of cell. For example, a sample of blood from a subject is a
population of white cells and red cells. A population of cells can also
include a
sample including a plurality of substantially homogeneous cells, such as
obtained
through cell culture methods for a continuous cell lines.
A "population of cell fragments" refers to a sample that includes more than
one cell fragment or more than one type of cell fragments. For example, a
population
of cell fragments can include mitochondria, nuclei, microsomes and portions of
golgi
apparatus that can be formed upon cell lysis.
A "microparticle" is a structure of any shape and of any composition that is
manipulatable by desired physical force(s). The microparticles used in the
methods
could have a dimension from about 0.01 micron to about ten centimeters.
Preferably,
the microparticles used in the methods have a dimension from about 0.1 micron
to
about several hundred microns. Such particles or microparticles can be
comprised of
any suitable material, such as glass or ceramics, and/or one or more polymers,
such
as, for example, nylon, polytetrafluoroethylene (TEFLONTM~, polystyrene,
polyacrylamide, sepaharose, agarose, cellulose, cellulose derivatives, or
dextran,
and/or can comprise metals. Examples of microparticles include, but are not
limited
to, plastic particles, ceramic particles, carbon particles, polystyrene
microbeads, glass
beads, magnetic beads, hollow glass spheres, metal particles, particles of
complex
compositions, microfabricated free-standing microstructures, etc. The examples
of
microfabricated free-standing microstructures may include those described in
"Design
of asynchronous dielectric micromotors" by Hagedorn et al., in Journal of
Electrostatics, Volume: 33, Pages 159-185 (1994). Particles of complex
compositions
refer to the particles that comprise or consists of multiple compositional
elements, for
example, a metallic sphere covered with a thin layer of non-conducting polymer
film.
"A preparation of microparticles" is a composition that comprises
microparticles of one or more types and can optionally include at least one
other
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
compound, molecule, structure, solution, reagent, particle, or chemical
entity. For
example, a preparation of microparticles can be a suspension of microparticles
in a
buffer, and can optionally include specific binding members, enzymes, inert
particles,
surfactants, ligands, detergents, etc.
"Coupled" means bound. For example, a moiety can be coupled to a
microparticle by specific or nonspecific binding. As disclosed herein, the
binding can
be covalent or noncovalent, reversible or irreversible.
"Micro-scale structures" are structures integral to or attached on a chip,
wafer,
or chamber that have characteristic dimensions of scale for use in
microfluidic
applications ranging from about 0.1 micron to about 20 mm. Example of micro-
scale
structures that can be on chips of the present invention are wells, channels,
scaffolds,
electrodes, electromagnetic units, or microfabricated pumps or valves.
A "particle positioning means" refers to a means that is capable of
manipulating the position of a particle relative to the X-Y coordinates or X-Y-
Z
coordinates of a biochip. Positions in the X-Y coordinates are in a plane. The
Z
coordinate is perpendicular to the plane. In one aspect of the present
invention, the X-
Y coordinates are substantially perpendicular to gravity and the Z coordinate
is
substantially parallel to gravity. This need not be the case, however,
particularly if
the biochip need not be level for operation or if a gravity free or gravity
reduced
environment is present. Several particle positioning means are disclosed
herein, such
as but not limited to dielectric structures, dielectric focusing structures,
quadropole
electrode structures, electrorotation structures, traveling wave
dielectrophoresis
structures, concentric electrode structures, spiral electrode structures,
circular
electrode structures, square electrode structures, particle switch structures,
electromagnetic structures, DC electric field induced fluid motion structure,
acoustic
structures, negative pressure structures and the like.
A "dielectric focusing structure" refers to a structure that is on or within a
biochip or a
chamber that is capable of modulating the position of a particle in the X-Y or
X-Y-Z
coordinates of a biochip using dielectric forces or dielectrophoretic forces.
A "horizontal positioning means" refers to a particle positioning means that
can position a particle in the X-Y coordinates of a biochip or chamber wherein
the Z
coordinate is substantially defined by gravity.
21
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A "vertical positioning means" refers to a particle positioning means that can
position a particle in the Z coordinate of a biochip or chamber wherein the Z
coordinate is substantially defined by gravity.
A "quadropole electrode structure" refers to a structure that includes four
electrodes arranged around a locus such as a hole, capillary or needle on a
biochip and
is on or within a biochip or a chamber that is capable of modulating the
position of a
particle in the X-Y or X-Y-Z coordinates of a biochip using dielectrophoretic
forces
or dielectric forces generated by such quadropole electrode structures.
An "electrorotation structure" refers to a structure that is on or within a
biochip or a chamber that is capable of producing a rotating electric field in
the X-Y
or X-Y-Z coordinates that can rotate a particle. Preferred electrorotation
structures
include a plurality of electrodes that are energized using phase offsets, such
as 360/N
degrees, where N represents the number of electrodes in the electroroation
structure
(see generally United States Patent Application Number 09/643,362 entitled
"Apparatus and Method for High Throughput Electrorotation Analysis" filed
August
22, 2000, naming Jing Cheng et al. as inventors). A rotating electrode
structure can
also produce dielectrophoretic forces for positioning particles to certain
locations
under appropriate electric signal or excitation. For example, when N=4 and
electrorotation structure corresponds to a quadropole electrode structure.
A "traveling wave dielectrophoresis structure" refers to a structure that is
on or
within a biochip or a chamber that is capable of modulating the position of a
particle
in the X-Y or X-Y-Z coordinates of a biochip using traveling wave
dielectrophoretic
forces (see generally United States Patent Application Number 09/686,737 filed
October 10, 2000, to Xu, Wang, Cheng, Yang and Wu; and United States
Application
Number 09/678,263, entitled "Apparatus for Switching and Manipulating
Particles
and Methods of Use Thereof' filed on October 3, 2000 and naming as inventors
Xiaobo Wang, Weiping Yang, Junquan Xu, Jing Cheng, and Lei Wu).
A "concentric circular electrode structure" refers to a structure having
multiple
concentric circular electrodes that are on or within a biochip or a chamber
that is
capable of modulating the position of a particle in the X-Y or X-Y-Z
coordinates of a
biochip using dielectrophoretic forces.
A "spiral electrode structure" refers to a structure having multiple parallel
spiral electrode elements that is on or within a biochip or a chamber that is
capable of
22
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
modulating the position of a particle in the X-Y or X-Y-Z coordinates of a
biochip
using dielectric forces.
A "square spiral electrode structure" refers to a structure having multiple
parallel square spiral electrode elements that are on or within a biochip or a
chamber
that is capable of modulating the position of a particle in the X-Y or X-Y-Z
coordinates of a biochip using dielectrophoretic or traveling wave
dielectrophoretic
forces.
A "particle switch structure" refers to a structure that is on or within a
biochip
or a chamber that is capable of transporting particles and switching the
motion
direction of a particle or particles in the X-Y or X-Y-Z coordinates of a
biochip. The
particle switch structure can modulate the direction that a particle takes
based on the
physical properties of the particle or at the will of a programmer or operator
(see,
generally United States Application Number 09/678,263, entitled "Apparatus for
Switching and Manipulating Particles and Methods of Use Thereof ' filed on
October
3, 2000 and naming as inventors Xiaobo Wang, Weiping Yang, Junquan Xu, Jing
Cheng, and Lei Wu.
An "electromagnetic structure" refers to a structure that is on or within a
biochip or a chamber that is capable of modulating the position of a particle
in the X-
Y or X-Y-Z coordinates of a biochip using electromagnetic forces. See
generally
United States Patent Application Number 09/685,410 filed October 10, 2000, to
Wu,
Wang, Cheng, Yang, Zhou, Liu and Xu and WO 00/54882 published September 21,
2000 to Zhou, Liu, Chen, Chen, Wang, Liu, Tan and Xu.
A "DC electric field induced fluid motion structure" refers to a structure
that is
on or within a biochip or a chamber that is capable of modulating the position
of a
particle in the X-Y or X-Y-Z coordinates of a biochip using DC electric field
that
produces a fluidic motion.
An "electroosomosis structure" refers to a structure that is on or within a
biochip or a chamber that is capable of modulating the position of a particle
in the X-
Y or X-Y-Z coordinates of a biochip using electroosmotic forces. Preferably,
an
electroosmosis structure can modulate the positioning of a particle such as a
cell or
fragment thereof with an ion transport measuring means such that the
particle's seal
(or the particle's sealing resistance) with such ion transport measuring means
is
increased.
23
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
An "acoustic structure" refers to a structure that is on or within a biochip
or a
chamber that is capable of modulating the position of a particle in the X-Y or
X-Y-Z
coordinates of a biochip using acoustic forces. In one aspect of the present
invention,
the acoustic forces are transmitted directly or indirectly through an aqueous
solution
to modulate the positioning of a particle. Preferably, an acoustic structure
can
modulate the positioning of a particle such as a cell or fragment thereof with
an ion
transport measuring means such that the particle's seal with such ion
transport
measuring means is increased.
A "negative pressure structure" refers to a structure that is on or within a
biochip or a chamber that is capable of modulating the position of a particle
in the X-
Y or X-Y-Z coordinates of a biochip using negative pressure forces, such as
those
generated through the use of pumps or the like. Preferably, a negative
pressure
structure can modulate the positioning of a particle such as a cell or
fragment thereof
with an ion transport measuring means such that the particle's seal with such
ion
transport measuring means is increased.
"Dielectrophoresis" is the movement of polarized particles in electrical
fields
of nonuniform strength. There are generally two types of dielectrophoresis,
positive
dielectrophoresis and negative dielectrophoresis. In positive
dielectrophoresis,
particles are moved by dielectrophoretic forces toward the strong field
regions. In
negative dielectrophoresis, particles are moved by dielectrophoretic forces
towaxd
weak field regions. Whether moieties exhibit positive or negative
dielectrophoresis
depends on whether particles are more or less polarizable than the surrounding
medium.
A "dielectrophoretic force" is the force that acts on a polarizable particle
in an
AC electrical field of non-uniform strength. The dielectrophoretic force FDEP
acting
on a particle of radius r subjected to a non-uniform electrical field can be
given, under
the dipole approximation, by:
3~y 2
FDEP = 2~Em r ll. DEP vErms
where E""S is the RMS value of the field strength, the symbol O is the symbol
for
gradient-operation, En, is the dielectric permittivity of the medium, and
,~DEP is the
particle polarization factor, given by:
24
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
~p - ~m
xDEP = Re * * ,
~p -~ 2~~n
"Re" refers to the real part of the "complex number". The symbol sX = sx - j a-
x ~2~f'
is the complex permittivity (of the particle x~, and the medium x=m) and j = ~
.
The parameters ~p and ~-p are the effective permittivity and conductivity of
the
particle, respectively. These parameters may be frequency dependent. For
example, a
typical biological cell will have frequency dependent, effective conductivity
and
permittivity, at least, because of cytoplasm membrane polarization. Particles
such as
biological cells having different dielectric properties (as defined by
permittivity and
conductivity) will experience different dielectrophoretic forces. The
dielectrophoretic
force in the above equation refers to the simple dipole approximation results.
However, the dielectrophoretic force utilized in this application generally
refers to the
force generated by non-uniform electric fields and is not limited by the
dipole
simplification. The above equation for the dielectrophoretic force can also be
written
as
1 S FDEP = 27C~nlf'3xDEP VZ v p(x, Y~ Z)
where p(x,y,z) is the square-field distribution for a unit-voltage excitation
(Voltage V
= 1 V) on the electrodes, V is the applied voltage.
"Traveling-wave dielectrophoretic (TW-DEP) force" refers to the force that is
generated on particles or molecules due to a traveling-wave electric field. An
ideal
traveling-wave field is characterized by the distribution of the phase values
of AC
electric field components, being a linear function of the position of the
particle. In this
case the traveling wave dielectrophoretic force F''~-DEP on a particle of
radius s°
subj ected to a traveling wave electrical field E = E cos~2~( ft - z l ~, o
)~Cax (i. e., a x
direction field is traveling along the z-direction) is given, again, under the
dipole
approximation, by
2
_ 4~ Vin: 3 2
FTW-DEP - ~ ~ ~TW-DEPE ~az
0
where E is the magnitude of the field strength, gin, is the dielectric
permittivity of the
medium. ~~,-DEP 1S the particle polarization factor, given by
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
j' Ep - E~~~
7 TW-DEP -
EP -~ 2~~n
"Im" refers to the imaginary part of the "complex number". The symbol
Ex = Ex - J ~'x ~2~f' is the complex permittivity (of the particle x~, and the
medium
x=m). The parameters Ep and ~p are the effective permittivity and conductivity
of
the particle, respectively. These parameters may be frequency dependent.
A traveling wave electric field can be established by applying appropriate AC
signals to the microelectrodes appropriately arranged on a chip. For
generating a
traveling-wave-electric field, it is necessary to apply at least three types
of electrical
signals each having a different phase value. An example to produce a traveling
wave
electric field is to use four phase-quardrature signals (0, 90, 180 and 270
degrees) to
energize four linear, parallel electrodes patterned on the chip surfaces. Such
four
electrodes may be used to form a basic, repeating unit. Depending on the
applications, there may be more than two such units that are located next to
each
other. This will produce a traveling-electric field in the spaces above or
near the
electrodes. As long as electrode elements are arranged following certain
spatially
sequential orders, applying phase-sequenced signals will result in
establishing
traveling electrical fields in the region close to the electrodes.
"Electric field pattern" refers to the field distribution in space or in a
region of
interest. An electric field pattern is determined by many parameters,
including the
frequency of the field, the magnitude of the field, the magnitude distribution
of the
field, and the distribution of the phase values of the field components, the
geometry of
the electrode structures that produce the electric field, and the frequency
and/or
magnitude modulation of the field.
"Dielectric properties" of a particle are properties that determine, at least
in
part, the response of a particle to an electric field. The dielectric
properties of a
particle include the effective electric conductivity of a particle and the
effective
electric permittivity of a particle. For a particle of homogeneous
composition, for
example, a polystyrene bead, the effective conductivity and effective
permittivity are
independent of the frequency of the electric field at least for a wide
frequency range
(e.g. between 1 Hz to 100 MHz). Particles that have a homogeneous bulk
composition may have net surface charges. When such charged particles are
suspended in a medium, electrical double layers may form at the
particle/medium
26
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
interfaces. Externally applied electric field may interact with the electrical
double
layers, causing changes in the effective conductivity and effective
permittivity of the
particles. The interactions between the applied field and the electrical
double layers
are generally frequency dependent. Thus, the effective conductivity and
effective
permittivity of such particles may be frequency dependent. For moieties of
nonhomogeneous composition, for example, a cell, the effective conductivity
and
effective permittivity are values that take into account the effective
conductivities and
effective permittivities of both the membrane and internal portion of the
cell, and can
vary with the frequency of the electric field. In addition, the
dielectrophoretic force
experience by a particle in an electric field is dependent on its size;
therefore, the
overall size of particle is herein considered to be a dielectric property of a
particle.
Properties of a particle that contribute to its dielectric properties include
but are not
limited to the net charge on a particle; the composition of a particle
(including the
distribution of chemical groups or moieties on, within, or throughout a
particle); size
of a particle; surface configuration of a particle; surface charge of a
particle; and the
conformation of a particle. Particles can be of any appropriate shape, such as
geometric or non-geometric shapes. For example, particles can be spheres, non-
spherical, rough, smooth, have sharp edges, be square, oblong or the like.
"Magnetic forces" refer to the forces acting on a particle due to the
application
of a magnetic field. In general, particles have to be magnetic or paramagnetic
when
sufficient magnetic forces are needed to manipulate particles. For a typical
magnetic
particle made of super-paramagnetic material, when the particle is subjected
to a
magnetic field B , a magnetic dipole ,u is induced in the particle
_ ~.~r _ l B
~P~P ~n:~~
m
-YP~P -~~'m~Hm
where VP is the particle volume, ~P and Vin, are the volume susceptibility of
the
particle and its surrounding medium, ,un, is the magnetic permeability of
medium,
Hn, is the magnetic field strength. The magnetic force F",ag"etic acting on
the particle
is determined, under the dipole approximation, by the magnetic dipole moment
and
the magnetic field gradient:
Fnt~gnert~ = W.5 VP(.2'P -xrn~Hm ~ vBm'
27
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
where the symbols " ~ " and "~ " refer to dot-product and gradient operations,
respectively. Whether there is magnetic force acting on a particle depends on
the
difference in the volume susceptibility between the particle and its
surrounding
medium. Typically, particles are suspended in a liquid, non-magnetic medium
(the
volume susceptibility is close to zero) thus it is necessary to utilize
magnetic particles
(its volume susceptibility is much larger than zero). The particle velocity
vp~,rticle
under the balance between magnetic force and viscous drag is given by:
_ F'magnetic
vpnrticle - 6~,
m
where r is the particle radius and r~,n is the viscosity of the surrounding
medium.
As used herein, "manipulation" refers to moving or processing of the
particles,
which results in one-, two- or three-dimensional movement of the particle, in
a chip
format, whether within a single chip or between or among multiple chips. Non-
limiting examples of the manipulations include transportation, focusing,
enrichment,
concentration, aggregation, trapping, repulsion, levitation, separation,
isolation or
linear or other directed motion of the particles. For effective manipulation,
the
binding partner and the physical force used in the method should be
compatible. For
example, binding partner such as microparticles that can be bound with
particles,
having magnetic properties are preferably used with magnetic force. Similarly,
binding partners having certain dielectric properties, for example, plastic
particles,
polystyrene microbeads, are preferably used with dielectrophoretic force.
A "sample" is any sample from which particles are to be separated or
analyzed. A sample can be from any source, such as an organism, group of
organisms
from the same or different species, from the environment, such as from a body
of
water or from the soil, or from a food source or an industrial source. A
sample can be
an unprocessed or a processed sample. A sample can be a gas, a liquid, or a
semi-
solid, and can be a solution or a suspension. A sample can be an extract, for
example a
liquid extract of a soil or food sample, an extract of a throat or genital
swab, or an
extract of a fecal sample. Samples are can include cells or a population of
cells. The
population of cells can be a mixture of different cells or a population of the
same cell
or cell type, such as a clonal population of cells. Cells can be derived from
a
biological sample from a subject, such as a fluid, tissue or organ sample. In
the case
of tissues or organs, cells in tissues or organs can be isolated or separated
from the
28
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
structure of the tissue or organ using known methods, such as teasing,
rinsing,
washing, passing through a grating and treatment with proteases. Samples of
any
tissue or organ can be used, including mesodermalhy derived, endodermally
derived or
ectodermally derived cells. Particularly preferred types of cells are from the
heart and
blood. Cells include but are not limited to suspensions of cells, cultured
cell lines,
recombinant cells, infected cells, eukaryotic cells, prokaryotic cells,
infected with a
virus, having a phenotype inherited or acquired, cells having a pathological
status
including a specific pathological status or complexed with biological or non-
biological entities.
"Separation" is a process in which one or more components of a sample is
spatially separated from one or more other components of a sample or a process
to
spatially redistribute particles within a sample such as a mixture of
particles, such as a
mixture of cells. A separation can be performed such that one or more
particles is
translocated to one or more areas of a separation apparatus and at least some
of the
remaining components are translocated away from the area or areas where the
one or
more particles are translocated to and/or retained in, or in which one or more
particles
is retained in one or more areas and at least some or the remaining components
are
removed from the area or areas. Alternatively, one or more components of a
sample
can be translocated to and/or retained in one or more areas and one or more
particles
can be removed from the area or areas. It is also possible to cause one or
more
particles to be translocated to one or more areas and one or more moieties of
interest
or one or more components of a sample to be translocated to one or more other
areas.
Separations can be achieved through the use of physical, chemical, electrical,
or
magnetic forces. Examples of forces that can be used in separations include
but are
not limited to gravity, mass flow, dielectrophoretic forces, traveling-wave
dielectrophoretic forces, and electromagnetic forces.
"Capture" is a type of separation in which one or more particles is retained
in
one or more areas of a chip. In the methods of the present application, a
capture can
be performed when physical forces such as dielectrophoretic forces or
electromagnetic forces are acted on the particle and direct the particle to
one or more
areas of a chip.
An "assay" is a test performed on a sample or a component of a sample. An
assay can test for the presence of a component, the amount or concentration of
a
component, the composition of a component, the activity of a component, the
29
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
electrical properties of an ion transport protein, etc. Assays that can be
performed in
conjunction with the compositions and methods of the present invention
include, but
not limited to, biochemical assays, binding assays, cellular assays, genetic
assays, ion
transport assay, gene expression assays and protein expression assays.
A "binding assay" is an assay that tests for the presence or the concentration
of
an entity by detecting binding of the entity to a specific binding member, or
an assay
that tests the ability of an entity to bind another entity, or tests the
binding affinity of
one entity for another entity. An entity can be an organic or inorganic
molecule, a
molecular complex that comprises, organic, inorganic, or a combination of
organic
and inorganic compounds, an organelle, a virus, or a cell. Binding assays can
use
detectable labels or signal generating systems that give rise to detectable
signals in the
presence of the bound entity. Standard binding assays include those that rely
on
nucleic acid hybridization to detect specific nucleic acid sequences, those
that rely on
antibody binding to entities, and those that rely on ligands binding to
receptors.
A "biochemical assay" is an assay that tests for the composition of or the
presence, concentration, or activity of one or more components of a sample.
A "cellular assay" is an assay that tests for or with a cellular process, such
as,
but not limited to, a metabolic activity, a catabolic activity, an ion
transport function
or property, an intracellular signaling activity, a receptor-linked signaling
activity, a
transcriptional activity, a translational activity, or a secretory activity.
An "ion transport assay" is an assay useful for determining ion transport
functions or properties and testing for the abilities and properties of
chemical entities
to alter ion transport functions. Preferred ion transport assays include
electrophysiology-based methods which include, but are not limited to patch
clamp
recording, whole cell recording, perforated patch or whole cell recording,
vesicle
recording, outside out and inside out recording, single channel recording,
artificial
membrane channel recording, voltage gated ion transport recording, ligand
gated ion
transport recording, stretch activated (fluid flow or osmotic) ion transport
recording,
and recordings on energy requiring ion transporters (such as ATP), non energy
requiring transporters, and channels formed by toxins such a scorpion toxins,
viruses,
and the like. See, generally Neher and Sakman, Scientific American 266:44-51
(1992); Sakmann and Heher, Ann. Rev. Physiol. 46:455-472 (1984); Cahalan and
Neher, Methods in Enzymology 207:3-14 (1992); Levis and Rae, Methods in
Enzymology 207:14-66 (1992); Armstrong and Gilly, Methods in Enzymology
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
207:100-122 (1992); Heinmann and Conti, Methods in Enzymology 207:131-148
(1992); Bean, Methods in Enzymology 207:181-193 (1992); Leim et al.,
Neurosurgery 36:382-392 (1995); Lester, Ann. Rev. Physiol 53:477-496 (1991);
Hamill and McBride, Ann. Rev. Physiol 59:621-631 (1997); Bustamante and
Varranda, Brazilian Journal 31:333-354 (1998); Martinez-Pardon and Ferrus,
Current
Topics in Developmental Biol. 36:303-312 (1998); Herness, Physiology and
Behavior
69:17-27 (2000); Aston-Jones and Siggins,
www.acnp.org/GAlGN40100005/CH005.htm1 (February 8, 2001); U.S. Patent No.
6,117,291; U.S. Patent No. 6,107,066; U.S. Patent No. 5,840,041 and U.S.
Patent No.
5,661,035; Boulton et al., Patch-Clamp Applications and Protocols,
Neuromethods V.
26 (1995), Humana Press, New Jersey; Ashcroft, Ion Channels and Disease,
Cannelopathies, Academic Press, San Diego (2000); Sakmann and Neher, Single
Channel Recording, second edition, Plenuim Press, New York (1995) and Soria
and
Cena, Ion Channel Pharmacology, Oxford University Press, New York (1998), each
of which is incorporated by reference herein in their entirety.
An "electrical seal" refers to a high-resistance engagement between a particle
such as a cell or cell membrane and an ion transport measuring means, such as
a, hole,
capillary or needle of a chip or device of the present invention. Preferred
resistance of
such an electrical seal is between about 1 mega ohm and about 100 giga ohms,
but
that need not be the case. Generally, a large resistance results in decreased
noise in
the recording signals. For specific types of ion channels (with different
magnitude of
recording current) appropriate electric sealing in terms of mega ohms or giga
ohms
can be used.
An "acid" includes acid and acidic compounds and solutions that have a pH of
less than 7 under conditions of use.
A "base" includes base and basic compounds and solutions that have a pH of
greater than 7 under conditions of use.
"More electronegative" means having a higher density of negative charge. In
the methods of the present invention, a chip or ion transport measuring means
that is
more electronegative has a higher density of negative surface charge.
An "electrolyte bridge" is a liquid (such as a solution) or a solid (such as
an
agar salt bridge) conductive connection with at least one component of the
electrolyte
bridge being an electrolyte so that the bridge can pass current with no or low
resistance.
31
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A "ligand gated ion transport" refers to ion transporters such as ligand gated
ion channels, including extracellular ligand gated ion channels and
intracellular ligand
gated ion channels, whose activity or function is activated or modulated by
the
binding of a ligand. The activity or function of ligand gated ion transports
can be
detected by measuring voltage or current in response to ligands or test
chemicals.
Examples include but are not limited to GABAA, strychnine-sensitive glycine,
nicotinic acetylcholine (Ach), ionotropic glutamate (iGlu), and 5-
hydroxytryptamine3
(5-HT3) receptors.
A "voltage gated ion transport" refers to ion transporters such as voltage
gated
ion channels whose activity or function is activated or modulated by voltage.
The
activity or function of voltage gated ion transports can be detected by
measuring
voltage or current in response to different commanding currents or voltages
respectively. Examples include but are not limited to voltage dependent Na+
channels.
"Perforated patch clamp" refers to the use of perforation agents such as but
not
limited to nystatin or amphotericin B to form pores or perforations in
membranes that
are preferably ion-conducting, which allows for the measurement of current,
including
whole cell current.
An "electrode" is a structure of highly electrically conductive material. A
highly conductive material is a material with conductivity greater than that
of
surrounding structures or materials. Suitable highly electrically conductive
materials
include metals, such as gold, chromium, platinum, aluminum, and the like, and
can
also include nonmetals, such as carbon, conductive liquids and conductive
polymers.
An electrode can be any shape, such as rectangular, circular, castellated,
etc.
Electrodes can also comprise doped semi-conductors, where a semi-conducting
material is mixed with small amounts of other "impurity" materials. For
example,
phosphorous-doped silicon may be used as conductive materials for forming
electrodes.
A "channel" is a structure with a lower surface and at least two walls that
extend upward from the lower surface of the channel, and in which the length
of two
opposite walls is greater than the distance between the two opposite walls. A
channel
therefore allows for flow of a fluid along its internal length. A channel can
be
covered (a "tunnel") or open.
32
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
"Continuous flow" means that fluid is pumped or injected into a chamber of
the present invention continuously during an assay or separation process, or
before or
after an assay or separation process. This allows for components of a sample
or
solution that are not selectively retained on a chip to be flushed out of the
chamber.
"Binding partner" refers to any substances that both bind to the moieties with
desired affinity or specificity and are manipulatable with the desired
physical force(s).
Non-limiting examples of the binding partners include cells, cellular
organelles,
viruses, particles, microparticles or an aggregate or complex thereof, or an
aggregate
or complex of molecules.
A "specific binding member" is one of two different molecules having an area
on the surface or in a cavity that specifically binds to and is thereby
defined as
complementary with a particular spatial and polar organization of the other
molecule.
A specific binding member can be a member of an immunological pair such as
antigen-antibody, can be biotin-avidin or biotin streptavidin, ligand-
receptor, nucleic
acid duplexes, IgG-protein A, DNA-DNA, DNA-RNA, RNA-RNA, and the like.
A "nucleic acid molecule" is a polynucleotide. A nucleic acid molecule can be
DNA, RNA, or a combination of both. A nucleic acid molecule can also include
sugars other than ribose and deoxyribose incorporated into the backbone, acid
thus can
be other than DNA or RNA. A nucleic acid can comprise nucleobases that axe
naturally occurring or that do not occur in nature, such as xanthine,
derivatives of
nucleobases, such as 2-aminoadenine, and the like. A nucleic acid molecule of
the
present invention can have linkages other than phosphodiester linkages. A
nucleic
acid molecule of the present invention can be a peptide nucleic acid molecule,
in
which nucleobases are linked to a peptide backbone. A nucleic acid molecule
can be
of any length, and can be single-stranded, double-stranded, or triple-
stranded, or any
combination thereof. The above described nucleic acid molecules can be made by
a
biological process or chemical synthesis or a combination thereof.
A "detectable label" is a compound or molecule that can be detected, or that
can generate readout, such as fluorescence, radioactivity, color,
chemiluminescence or
other readouts known in the art or later developed. Such labels can be, but
are not
limited to, photometric, colorimetric, radioactive or morphological such as
changes of
cell morphology that are detectable, such as by optical methods. The readouts
can be
based on fluorescence, such as by fluorescent labels, such as but not limited
to, Cy-3,
Cy-5, phycoerythrin, phycocyanin, allophycocyanin, FITC, rhodamine, or
33
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
lanthanides; and by fluorescent proteins such as, but not limited to, green
fluorescent
protein (GFP). The readout can be based on enzymatic activity, such as, but
not
limited to, the activity of beta-galactosidase, beta-lactamase, horseradish
peroxidase,
alkaline phosphatase, or luciferase. The readout can be based on radioisotopes
(such
as 33P, 3H , 14C, 3sS, izsh szp or 13y). A label optionally can be a base with
modified
mass, such as, for example, pyrimidines modified at the CS position or purines
modified at the N7 position. Mass modifying groups can be, for examples,
halogen,
ether or polyether, alkyl, ester or polyester, or of the general type XR,
wherein X is a
linking group and R is a mass-modifying group. One of skill in the art will
recognize
that there are numerous possibilities for mass-modifications useful in
modifying
nucleic acid molecules and oligonucleotides, including those described in
Oligonucleotides and Analogues: A Practical Approach, Eckstein, ed. (1991) and
in
PCT/LTS94/00193.
A "signal producing system" may have one or more components, at least one
component usually being a labeled binding member. The signal producing system
includes all of the reagents required to produce or enhance a measurable
signal
including signal producing means capable of interacting with a label to
produce a
signal. The signal producing system provides a signal detectable by external
means,
often by measurement of a change in the wavelength of light absorption or
emission.
A signal producing system can include a chromophoric substrate and enzyme,
where
chromophoric substrates are enzymatically converted to dyes, which absorb
light in
the ultraviolet or visible region, phosphors or fluorescers. However, a signal
producing system can also provide a detectable signal that can be based on
radioactivity or other detectable signals.
The signal producing system can include at least one catalyst, usually at
least
one enzyme, and can include at least one substrate, and may include two or
more
catalysts and a plurality of substrates, and may include a combination of
enzymes,
where the substrate of one enzyme is the product of the other enzyme. The
operation
of the signal producing system is to produce a product that provides a
detectable
signal at the predetermined site, related to the presence of label at the
predetermined
site.
In order to have a detectable signal, it may be desirable to provide means for
amplifying the signal produced by the presence of the label at the
predetermined site.
Therefore, it will usually be preferable for the label to be a catalyst or
luminescent
34
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
compound or radioisotope, most preferably a catalyst. Preferably, catalysts
are
enzymes and coenzymes that can produce a multiplicity of signal generating
molecules from a single label. An enzyme or coenzyme can be employed which
provides the desired amplification by producing a product, which absorbs
light, for
example, a dye, or emits light upon irradiation, for example, a fluorescer.
Alternatively, the catalytic reaction can lead to direct light emission, for
example,
chemiluminescence. A large number of enzymes and coenzymes for providing such
products are indicated in U.S. Pat. No. 4,275,149 and U.S. Pat. No. 4,318,980,
which
disclosures are incorporated herein by reference. A wide variety of non-
enzymatic
catalysts that may be employed are found in U.S. Pat. No. 4,160,645, issued
July 10,
1979, the appropriate portions of which are incorporated herein by reference.
The product of the enzyme reaction will usually be a dye or fluorescer. A
large
number of illustrative fluorescers are indicated in U.S. Pat. No. 4,275,149,
which is
incorporated herein by reference.
Other technical terms used herein have their ordinary meaning in the axt that
they are used, as exemplified by a variety of technical dictionaries.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Introduction
The present invention recognizes that using direct detection methods to
determine an ion transport function or property, such as patch-clamps, is
preferable to
using indirect detection methods, such as fluorescence-based detection
systems. The
present invention provides biochips and methods of use that allow for the
direct
detection of one or more ion transport fiinctions or properties using chips
and devices
that can allow for automated detection of one or more ion transport functions
or
properties. These biochips and methods of use thereof are particularly
appropriate for
automating the detection of ion transport functions or properties,
particularly for
screening purposes.
As a non-limiting introduction to the breath of the present invention, the
present invention includes several general and useful aspects, including:
1) a biochip device for ion transport measurement that comprises at least one
upper chamber piece and at least one biochip that comprises at least one ion
transport measuring means. The device can comprise one or more conduits
that provide an electrolyte bridge to at least one electrode.
2) a biochip ion transport measuring device having one or more flow-through
lower chambers.
3) a biochip devices adapted for a microscope stage.
4) methods of making an upper piece for a biochip device for ion transport
measurement .
5) methods for making chips comprising ion transport measurement holes
using laser drilling techniques.
6) devices that include an inverted chip for ion transport measurement.
7) methods of treating ion transport measuring chips to enhance their sealing
properties.
8) a method to measure surface energy, such as on the surface of a
chemically- treated ion transport measurement biochip.
9) substrates, biochips, cartridges, apparatuses, and/or devices comprising
ion
transport measuring means with enhanced electric seal properties.
36
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
10) methods for storing the substrates, biochips, cartridges, apparatuses,
and/or devices comprising ion transport measuring means with enhanced
electrical seal properties.
11) methods for shipping the substrates, biochips, cartridges, apparatuses,
and/or devices comprising ion transport measuring means with enhanced
electrical seal properties.
12) methods for assembling devices and cartridges of the present invention
using UV adhesives.
13) a method of producing ion transport measuring chips by fabricating them
as detachable units of a larger sheet.
14) a method of producing high density ion transport measuring chips.
15) a biochip device for ion transport measurement comprising fluidic channel
upper and lower chambers.
16) methods of preparing cells for ion transport measurement.
17) a software program logic that controls a pressure control profile to
direct
an ion transport measurement apparatus to achieve and maintain a high-
resistance electrical seal.
These aspects of the invention, as well as others described herein, can be
achieved by using the methods, articles of manufacture and compositions of
matter
described herein. To gain a full appreciation of the scope of the present
invention, it
will be further recognized that various aspects of the present invention can
be
combined to make desirable embodiments of the invention.
37
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
I. DEVICE FOR ION TRANSPORT MEASUREMENT
The present invention comprises devices for ion transport measurement and
components of ion transport measuring devices that reduce the costs of
manufacture
and use and are efficient and convenient to use. The devices of the present
invention
are also designed for maximum versatility, providing for different assay
formats
within the same basic design.
In some aspects, the present invention contemplates devices and apparatuses
that have parts that are manufactured separately and can be assembled to form
ion
transport measuring devices that have at least one, and preferably multiple,
ion
transport measuring units, each of which comprises an upper chamber and at
least a
portion of a biochip that comprises an ion transport measuring means that
during use
of the device can connect the upper chamber with a lower chamber. An ion
transport
measuring device of the present invention can further comprise at least a
portion of at
least one lower chamber that is connected to one or more upper chambers of the
device via an ion transport measuring means of the chip. These devices
comprising
ion channel measuring units can be assembled before the assay procedure, and
pieces
that make up the device can be reversibly or irreversibly attached to one
another.
In many preferred aspects of the present invention, a device or one or more
parts of a device can be removed from an apparatus and can be disposable after
a
single use (for example, a chip comprising ion transport measuring means; one
or
more upper chambers designed to contain cells), and can engage one or more
parts of
an ion transport measuring device or apparatus that can be permanent and
reusable
(for example, at least a portion of a lower chamber; one or more electrodes)
For
example, in some aspects of the present invention, devices comprising one or
more
upper chamber pieces and at least one biochip (called cartridges) are single-
use and
disposable, and lower chamber pieces, one or more electrodes, and platforms or
lower
base pieces are reusable. In these aspects, upper chamber pieces and biochips
can be
reversibly or irreversibly attached to one another during use of the device or
apparatus, and these attached upper chamber/biochip devices can be reversibly
attached to or contacted with lower chamber pieces, conduits, or electrodes.
In one embodiment, the present invention contemplates an ion transport
measuring device in the form of a cartridge that comprises an upper chamber
piece
that comprises at least one well that is open at its upper and lower ends, and
a biochip
that comprises at least one ion transport measuring means. The chip is
reversibly or
3~
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
irreversibly attached to the bottom of the upper chamber piece such that each
of the
one or more upper wells is in register with one of the one or more ion
transport
measiuing means, providing one or more independent upper chambers each in
contact
with a single ion transport measuring means. The chip can be in direct or
indirect
contact with the upper chamber piece.
In a cartridge in which an upper chamber piece is in indirect contact with an
attached chip, a spacer or gasket, for example, can be between the upper
chamber
piece and the chip. A chip can be in direct contact with an upper chamber
piece of a
cartridge if it is attached during molding of the cartridge, by heat sealing,
or by
adhesives, for example. Attachment of a chip to an upper chamber piece to make
a
cartridge can be performed by a machine, and can be automated.
A chip can also be intergral to an upper chamber piece in a cartridge or
device
of the present invention, where the chip forms or is part of the lower surface
of the
upper chamber piece that can comprise, for example, glass or one or more
plastics.
Preferably a biochip that is part of an ion transport measuring device of the
present invention comprises multiple holes used as ion transport measuring
means,
and an upper chamber piece comprises multiple upper chambers such that each of
the
upper chambers is in register with one of the ion transport measuring means of
the
chip. For example, preferred devices and apparatuses for ion transport
measurement
can have two or more, four or more, eight or more, or sixteen or more ion
transport
measuring units and comprise upper chamber pieces comprising a corresponding
number of upper chambers. For example, ion transport measuring devices can
have
sixteen, twenty-four, forty-eight, ninety-six or more ion transport measuring
units and
comprise upper chamber pieces comprising a corresponding number of upper
chambers.
The upper chambers or wells can be any shape or size. Typically, the upper
chambers will be in the form of wells which can be tapered or non-tapered. The
wells
of an upper chamber piece that can be part of an ion transport measuring
device
preferably can hold a volume of between about 0.5 microliters and about 5
milliliters
or more, more preferably between about 10 microliters and about 2 milliliters,
and
more preferably yet between about 25 microliters and about 1 milliliter. The
upper
diameter of a well can be from about 0.05 millimeter to about 20 millimeters
or more,
and is preferably between about 2 millimeters and about 10 millimeters or
more. The
depth, or height of a well can vary from about 0.01 to about 25 millimeters or
more,
39
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
and more preferably will be from about 2 millimeters to about 10 millimeters.
In
designs in which the upper well or wells are tapered, the well can be tapered
downward at an angle of from about 0.1 degree to about 89 degrees from
vertical, and
preferably from about 5 degrees to about 60 degrees from vertical. The well
can be
tapered at one or more ends, or throughout the circumference of the well.
An upper chamber piece can be made of any suitable material, (for example,
one or more plastics, one or more polymers, one or more ceramic, one or more
waxes,
silicon, or glass) but for ease of manufacturing is preferably made of a
moldable
plastic, such as, for example, polysulfone, polyallomer, polyethylene,
polyimide,
polypropylene, polystyrene, polycarbonate, cylco olefin polymer (such as, for
example, ZEONOR~), polyphenylene ether/PPO or modified polyphenylene oxide
(such as, for example, NORYL~), or composite polymers. In some aspects, base
resistant plastics such as polystyrene, cylco olefin polymers (such as, for
example,
ZEONOR~), polyphenylene ether/PPO or modified polyphenylene oxide (such as,
for example, NORYL~), can be preferred.
An upper chamber piece can optionally comprise one or more electrodes. An
upper chamber piece that comprises multiple upper chambers can comprise
multiple
electrodes, where each well contacts an independent electrode (such as, for
example,
independent recording electrodes). In an alternative design, an upper chamber
piece
can contain or contact at least a portion of a single electrode (which can be,
for
example, a reference electrode) that contacts all of the upper chambers of the
device.
In designs in which the upper chamber piece does not comprise one or more
electrodes, the upper chamber piece can optionally be used as part of an
apparatus for
ion transport measurement in which one or more electrodes can be introduced
into
one or more upper chambers (such as, for example, introduced via a conduit
that can
be connected to or can be inserted into one or more chambers). In an
alternative
configuration, conduits connected with or introduced into one or more upper
chambers can, during the use of the apparatus, be filled with a measuring
solution and
provide electrolyte bridges to one or more electrodes.
The chip can be reversibly or irreversibly attached the lower surface of an
upper chamber piece to form a cartridge by any feasible means that provides a
fluid-
impermeable seal between the chip and the upper chamber piece, such as by
adhesives
or by pressure mounting. The chip of the assembled cartridge can be in direct
or
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
indirect contact with an upper chamber piece. Preferably but optionally, the
chip is
irreversibly attached to the upper chamber piece, such as by one or more
adhesives, to
make a cartridge. Such cartridges can optionally single use and disposable.
Assembly
of a preferred cartridge of the present invention is provided in Example 1.
An upper chamber piece of the present invention can also have features that
aid in the manufacture of the piece or assembly of the cartridge. For example,
the
lower surface of the upper chamber piece can comprise one or more alignment
bumps
or registration edges on at least one end of the lower side of the piece that
allows a
chip to be positioned against the lower side of the upper chamber piece such
that the
ion transport measuring holes of the chip are in register with the wells.
Features that
facilitate manufacture of an upper chamber piece include one or more sink
holes that
prevent the piece from deforming through thermal contraction of the piece
during the
injection molding process, and one or more glue spillage grooves that allow
for
seepage of excess glue that may be used in attaching a chip to the upper
chamber
piece. Assembly of a cartridge can be done manually, or by a machine.
Preferably but
optionally, at least one of the steps in the assembly of a cartridge of the
present
invention by a machine is automated. For example, a machine may perform one or
more of the steps of picking up a chip from a rack or holder, picking up an
upper
chamber piece from a rack, platform, shelf, or holder, applying one or more
adhesives to an upper chamber piece or a chip, positioning a chip on the
bottom of an
upper chamber piece so that the ion transport measuring means of the chip are
in
register with the wells of the upper chamber piece, and allowing or promoting
attachment of the chip to the upper chamber piece (such as by treating with
LTV or
heat).
One design of an upper chamber piece is shown in Figure 1. Figure lA
depicts a top view of an upper chamber piece having sixteen wells (1) and
Figure 1B
depicts a bottom view of the upper chamber piece showing the lower openings of
the
sixteen wells (1), and also shows the openings of two sinkholes (3). (In an
assembled
cartridge or device comprising a chip, the chip preferably covers and thereby
seals
off, the sinkhole openings.) In this design, the wells (1) are tapered such
that the upper
diameters of the wells (1) (seen in Figure lA) are larger than the lower
diameters of
the wells (1) (seen in Figure 1B). In Figure 1C, the upper chamber piece is
shown
side-on in cross-section, showing the sixteen wells (1) as well as features
that increase
the efficiency of manufacture of a device, including an alignment bump (2) for
chip
41
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
positioning and sink holes (3) that prevent cave-in of the upper chamber piece
due to
contraction of the plastic as it cools after molding of the piece. Figure 1D
is an end-
on cross-sectional view of the piece showing a well (1) behind a sink hole
(3). In
Figure 1D a glue spillage groove (4) is also shown. A glue spillage groove can
allow
for seepage of an adhesive used to seal a chip to the lower chamber piece to
make a
cartridge.
A chip used in a device of the present invention is preferably a chip that
comprises ion transport measuring means in the form of holes. A chip used in a
device
of the present invention can comprise glass, silicon, silicon dioxide, quartz,
one or
more plastics, one or more waxes, or one or more polymers (for example,
polydimethylsiloxane (PDMS)), one or more ceramics, or a combination thereof.
Methods of fabricating such chips, including methods of fabricating ion
transport
measuring holes in chips, are disclosed in related applications, including
United States
patent application number 10/760,866 (pending), filed January 20, 2004; United
States patent application number 10/642,014, filed August 16, 2003; and United
States patent application number 10/104,300, filed March 22, 2002; each of
which is
incorporated by reference herein.
A chip used in a device of the present invention is preferably a "K-
configuration" chip, but this is not a requirement of the present invention.
As
described in a later section of this application and in the Examples, K-
configuration
chips have ion transport measuring holes that comprise a through-hole that is
laser
drilled through one or more counterbores. A chip used in a device of the
present
invention is preferably treated to have enhanced sealing properties. Methods
of
chemically treating ion transport measuring chips, for example with basic
solutions, to
enhance their ability to form electrical seals with particles such as cells
are disclosed
herein. A preferred device for ion transport measurement is a cartridge that
comprises
a K-configuration chip with enhanced electrical sealing properties that is
reversibly or
irreversibly attached to an upper chamber piece. Preferably, a chip assembled
into a
device of the present invention has one or more ion transport measuring holes
that is
able to seal to a cell or particle such that electrical access between the
chip and the
inside of the cell or particle (or between the chip and the inside of the cell
or particle)
has an access resistance that (Ra) is less than the seal resistance (R).
Preferably, the
access resistance of a whole-cell configuration seal that can be formed on the
hole of
a chip of a device of the present invention is less than 80 MOhm, more
preferably less
42
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
than about 30 MOhm, and more preferably yet, less than about 10 MOhm.
Preferably,
a chip of a device of the present invention can form a seal with a cell such
that the
seal has a resistance that is at least 200 MOhm, and more preferably, at least
500
MOhm, and more, preferably yet, about 1 GigaOhm or greater. Preferably, a chip
of a
device of the present invention comprises at least one ion transport measuring
means
in the form of a through-hole that has been laser-drilled through at least one
counterbore, in which at least the surface of the ion transport measuring
means has
been treated to enhance its electrical sealing properties, and the chip can
form a seal
between at least one ion transport measuring means and a cell such that the
resistance
(R) of the seal is at least ten times the access resistance of the seal. More
preferably, a
chip of a device of the present invention can form a seal with a cell such
that the seal
resistance is at least twenty times the Ra.
Preferably, a chip comprising laser-drilled ion transport measuring holes is
attached to an upper chamber piece in inverted orientation, as described in a
later
section of this application, such that the laser entrance hole of the ion
transport
measuring hole is exposed to the upper chambers, but this is not a requirement
of the
present invention. In the alternative, the chip can be attached to the upper
chamber in
"upside up" orientation.
A cartridge comprising an upper chamber piece and at least one biochip
comprising one or more ion transport measuring means can be assembled into a
device that comprises one or more lower chambers in which the one or more
lower
chambers access at least one upper chamber via a hole in the biochip. A
cartridge can
engage one or more parts that make up one or more lower chambers, where the
one or
more lower chambers are directly or indirectly attached to the underside of
the chip,
and at least one ion transport measuring hole in the chip connects the one or
more
lower chambers with one or more upper chambers of the device.
For example, a cartridge comprising an upper chamber piece and at least one
biochip comprising one or more ion transport measuring means can be assembled
with a lower chamber piece that comprises at least a portion of at least one
lower
chamber. The cartridge can be assembled with a lower chamber piece that
comprises
at least a portion of a single lower chamber, such as a dish, tray, or channel
that
provides a common lower chamber for ion transport measuring means that connect
to
separate upper chambers. In one embodiment, at least a portion of a lower
chamber
piece can be in the form of a gasket that seals around the bottom of the
biochip that
43
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
when sealed against a lower chamber base piece or platform provides an inner
space
as a lower chamber
Alternatively, the device can be assembled with a lower chamber piece that
comprises at least a portion of more than one lower chamber. In this case,
each
individual lower chamber preferably connects with a single upper chamber via
an ion
transport measuring hole in the biochip. The lower chamber piece can form the
walls
and lower surfaces of lower chambers, or the lower chamber piece can form at
least a
portion of the walls of a lower chamber and other parts can form the bottom
surface of
the lower chambers. In one embodiment, at least a portion of a lower chamber
piece
can be in the form of a gasket that seals around the bottom of the biochip and
having
openings such that when the gasket is sealed against a lower chamber base
piece or
platform the inner spaces of the gasket openings provide lower chambers.
A lower chamber piece can be irreversibly attached to a cartridge of the
present invention, such as by the use of adhesives, but preferably, a lower
chamber
piece is reversibly attached to a cartridge. Reversible attachment can be by
any
feasible means that provides a fluid-impermeable seal between the walls of the
lower
chamber or chambers and the lower surface of the chip, such as pressure
mounting,
and can use clamps, frames, screws, snaps, etc.
In one example of attachment of a lower chamber piece to a cartridge, a lower
chamber piece structure comprising a compressible material such as PDMS
contains
channels for fluid delivery and other channels for applying vacuum pressure
that can
maintain a strong seal between the biochip and the structure, where the vacuum
pressure provides the means of reversible attachment of the lower chamber
piece to
the biochip. Preferably, the applied vacuum pressure also scavenges any leaks
that
may occur or develop between lower chambers that would otherwise result in
electrical cross-talk between adjacent lower chambers.
Preferred embodiments encompass devices that comprise multiple ion
transport measuring units, comprising an upper chamber piece that comprises at
least
two upper chambers that are open at both their upper and lower ends and a chip
that
comprises at least two ion transport measuring means in the form of holes
through the
chip that are in register with the upper chambers. The upper chamber piece and
chip
can be reversibly or irreversibly attached to a lower chamber piece that
comprises at
least a portion of at least two lower chambers that are in register with the
ion transport
measuring means and upper chambers. Such preferred devices comprise multiple
ion
44
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
transport measuring units, where each unit comprises an upper chamber and a
lower
chamber, each in register with a hole in the biochip, in which the hole
connects the
upper with a lower chamber. The interaction between the chambers and the chip
are
such that at least one of the chambers of an ion transport measuring unit can
be
pneumatically sealed and can withstand pressures of at least plus or minus 100
mmHg, and preferably at least plus or minusl atmosphere of pressure.
In some preferred aspects of the present invention, a cartridge comprises an
upper chamber piece comprising multiple upper chambers irreversibly attached
to a
chip comprising multiple ion transport measuring holes that can be reversibly
engaged
with a lower chamber piece that comprises at least a portion of multiple lower
wells,
such that the upper wells and lower wells of the device are in register with
one
another and with the ion transport measuring holes of the chip.
Preferred devices and apparatuses for ion transport measurement can have two
or more, four or more, eight or more, or sixteen or more ion transport
measuring units.
For example, ion transport measuring devices can have sixteen, twenty-four,
forty
eight, or ninety-six or more ion transport measuring units.
Lower chamber pieces that comprise at least a portion of multiple lower
chambers of a multiple unit ion transport measuring apparatus can be provided
in a
variety of designs. Lower chamber pieces can comprise complete lower chamber
units, or can comprise all or a portion of the walls of the multiple chamber
units, such
that when the lower chamber piece is fixed to or pressed against the lower
side of a
biochip and attached to or pressed down on a platform or lower chamber base
piece,
the lower chamber piece forms the walls and the platform or lower chamber base
piece forms the bottoms of the lower chambers.
For example, a device for measuring ion transport function or activity can
comprise a multiple unit device that comprises an upper chamber piece having
multiple upper chambers in the form of wells that are open at both the top and
bottom,
and a chip attached to the upper chamber piece, where the chip comprises
multiple
holes for ion transport measurement that are spaced such that when the device
is
assembled each upper chamber is over a hole. A lower chamber piece can be held
or
fastened against the lower side of the chip of the device, where the lower
chamber
piece comprises multiple openings that fit over the biochip holes to form
lower
chambers.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
In a preferred embodiment, the lower chamber piece comprises at least one
compressible plastic or polymer on its upper surface that can form a fluid-
impermeable seal with the bottom of the biochip. The lower chamber piece can
also
comprise at least one compressible polymer as a gasket on its lower surface
that can
form a seal with a platform or a lower base piece. In this design, when the
device is
positioned on a lower base piece or platform so that the lower chamber piece
is
pressed against the lower base piece or platform, the lower base piece or
platform
forms the bottom of the lower chambers. Mechanical pressure can provide a seal
between the biochip and the lower chamber piece, and between the lower chamber
piece and the platform. Clamps can optionally be employed to hold the seal.
The
compressible plastic or polymer can comprise rubber, a plastic, or an
elastomer, such
as for example, polydimethylsiloxane (PDMS), silicon polyether urethane,
polyester
elastomer, polyether ester elastomer, olefinic elastomer, polyurethane
elastomer,
polyether block amide, or styrenic elastomer. Preferably, in cases where the
compressible plastic or polymer contacts cells, the compressible plastic or
polymer is
made of a biocompatible material, such as PDMS. Portions of the lower chamber
piece that do not form a gasket can be of any suitable material, including
plastics,
waxes, polymers, glass, metals, and ceramics. Portions of the lower chamber
piece
that contact measuring solutions preferably comprise materials that are not
affected by
electrical current (such as nonmetals).
For example, one preferred design of a device for ion transport measurement
comprises an upper chamber piece, a chip comprising ion transport measuring
holes, a
lower chamber piece, and a lower base piece in the form of a platform. The
chip has
been chemically treated, preferably with at least one base, to enhance its
sealing
properties. The lower chambers that are formed by a lower chamber piece that
comprises an aluminum frame having a PDMS gasket on its upper surface that
fits
over the lower surface of a chip. PDMS is also used to coat the inner surfaces
of the
holes that form the lower chambers, and is also used as a gasket on the bottom
of the
lower chamber piece. The lower chambers can be filled with a solution while
the
device is held in inverted orientation prior to positioning the device on the
platform.
During use of the device, mechanical pressure holds the lower chamber piece
against
the chip and against the platform.
The lower base piece can optionally comprise one or more electrodes. For
example, separate individual electrodes can be fabricated on or attached to
the
46
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
platform so that separate lower chambers of the device have independent
electrodes
that can be attached to independent circuits and used as patch clamp recording
electrodes. In an alternative design, the platform can comprise or be part of
a common
lower chamber with a reference electrode, or a common electrode that can be
used as
a reference electrode can contact all of the lower chambers of a device having
multiple lower chambers (optionally through separate electrode extensions that
meet a
common connector outside of the chambers).
The lower base piece can optionally comprise or engage one or more conduits
connected to tubing that can allow for the flow of fluids into and out of
individual
lower chambers. In preferred embodiments, a device of the present invention
comprises one or more flow-through lower chambers where each of the one or
more
lower chamber connects to at least one conduit for providing solutions to the
lower
chamber (the inflow conduit) and at least one additional conduit for removing
solutions from the lower chamber (the outflow conduit).
Figure 2 depicts a single ion transport measuring unit of a device in which a
gasket (24) forms the walls of the lower chamber (25). The upper well (21) is
part of
an upper chamber piece that is attached to a chip (23) having an ion transport
measuring means in the form of a hole (22). An inflow conduit (27) and outflow
conduit (28) connects to each lower chamber. W this type of design the lower
chambers can be filled with a measuring solution (such as an intracellular
solution)
after the gasket is positioned on a lower base piece. The conduits can also be
used for
the exchange of solutions during the use of the device. For example, solutions
containing test compounds, ionophores, inhibitors, drugs, different
concentrations or
combinations of ions or compounds, etc., can be delivered into and out of a
chamber
during ion transport measuring assays. At least some of the conduits or tubing
can
optionally comprise or lead to electrodes (such as, for example, recording
electrodes).
In the design depicted in Figure 2, a lower chamber electrode (26) is situated
on,
fabricated on, or attached to the lower chamber piece.
The present invention also includes methods of using an ion transport
measuring device of the present invention that comprises at least one upper
chamber
piece reversibly or irreversibly attached to a chip, wherein the clup
comprises at least
one ion transport measuring means in the form of a hole through the biochip,
wherein
the chip has been treated to have enhanced electrical sealing properties. The
device
further comprises at least one lower chamber, wherein at least one well of the
upper
47
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
chamber piece comprises, contacts, or is in electrical contact with at least
one
electrode, and the at least one lower chamber
In one preferred design, a lower chamber piece comprises conduits that engage
each lower chamber from one side (one per chamber), and conduits that engage
each
lower chamber from the opposite side. Conduits on one side of the lower
chamber
piece can be used for introducing solutions, such as "intracellular solutions"
that can
optionally comprise test compounds, into the chambers, and conduits on the
opposite
side of the lower chamber piece can be used for flushing solutions and/or air
bubbles
out of the lower chambers. At least one set of the conduits (such as, for
example, the
inflow conduits) can comprise wire electrodes that are independently connected
(with
respect to other ion transport measuring units) to a signal amplifier and used
for ion
transport activity recording.
Devices such as those described herein can be part of apparatuses that also
comprise patch clamp signal amplifiers and conduits, fluid dispensing means,
pumps,
electrodes, or other components. The apparatuses are preferably mechanized,
for
automated fluid dispensing or pumping, pressure generation for sealing of
particles,
and ion transport recording. The apparatuses can be part of a biochip system
for ion
transport measurement, in which software controls the automated functions.
The present invention also includes methods of using an ion transport
measuring device of the present invention to measure one or more ion transport
properties or activities of a cell or particle (such as, for example, a
membrane vesicle).
The methods include using a device that comprises at least one upper chamber
reversibly or irreversibly attached to a chip that comprises at least one ion
transport
measuring means in the form of a hole through the biochip, wherein the chip
has been
treated to have enhanced sealing properties. In the assembled device used in
the
methods of the present invention, the holes of the biochip access at least one
lower
chamber. In these methods, the device is reversibly or irreversibly attached
to a lower
chamber piece that forms all or a portion of a lower chamber. An upper chamber
piece
and chip can optionally additionally be reversibly or irreversibly attached to
a
platform or lower chamber base piece that can form at least the lower surface
of one
or more lower chambers. For example, a cartridge comprising an upper chamber
piece
and chip can be attached to at least one lower chamber piece that forms the
walls and
lower surfaces of one or more lower chambers, or a cartridge can be attached
to at
48
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
least one lower chamber piece that forms the walls of one or more lower
chambers
and at least one platform or lower chamber base piece that forms the lower
surfaces of
one or more lower chambers.
The device is assembled such that the one or more upper chambers are in
register with the one or more ion transport measuring holes of the chip, and
one or
more lower chambers access the one or more upper chambers via the one or more
holes of the chip. In preferred embodiments, each of the one or more upper
chambers
is in register with one of the ion transport measuring holes of the chip, and
each of the
lower chambers is aligned with one upper chamber that it accesses via an ion
transport
measuring hole.
During use of the device, the one or more upper chambers comprise, contact,
or are in electrical contact with at least one electrode. During use of the
device, the
one or more lower chambers comprise, contact, or are in electrical contact
with at
least one electrode. In one alternative, the one or more upper chambers
contact,
comprise, or are in electrical contact with a common reference electrode, and
the one
or more lower chambers contact, comprise, or are in electrical contact with a
individual reference electrodes. In another alternative, the one or more upper
chambers contact, comprise, or are in electrical contact with individual
reference
electrodes, and the one or more lower chambers contact, comprise, or are in
electrical
contact with a common reference electrode.
The method includes: filling at least one lower chamber of the device with a
measuring solution; adding at least one cell or particle to one or more of the
at least
upper chambers of the device, wherein the one or more upper chambers is
connected
to one of the at least one lower chambers that comprises measuring solution
via a hole
in the ion transport measuring chip; applying pressure to at least one lower
chamber,
at least one lower chamber, or to an upper chamber and a lower chamber that
are
connected via an ion transport measuring hole to create a high-resistance
electrical
seal between at least one cell or particle and at least one hole; and
measuring at least
one ion transport property or activity of the at least one cell or at least
one particle.
Preferably, one or more cells or one or more particles are in a suspension
when added to the upper chamber. Various measuring solutions and, optionally,
compounds can be provided in an upper chamber or a lower chamber.
In some preferred embodiments, the methods measure at least one ion
transport activity or property of a cell in the whole cell configuration, but
this is not a
49
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
requirement of the present invention, as the devices can be used in a variety
of
applications on particles such as, for example, vesicles, as well as cells.
The application of pressure can be manual or automated. If pressure is applied
manually (for example, by means of a syringe), preferably the user can make
use of a
pressure display system to monitor the applied pressure. Automated application
of
pressure can be through the use of a software program that is able to receive
feedback
from the device and direct and control the amount of pressure applied to one
or more
ion transport measuring units.
Various specific ion transport assay can be used for determining ion transport
function or properties. These include methods known in the art such as but not
limited to patch clamp recording, whole cell recording, perforated patch whole
cell
recording, vesicle recording, outside out or inside out recording, single
channel
recording, artificial membrane channel recording, voltage-gated ion transport
recording, ligand-gated ion transport recording, recording of energy requiring
ion
transports (such as ATP), non energy requiring transporters, toxins such a
scorpion
toxins, viruses, stretch-gated ion transports, and the like. See, generally
Neher and
Sakman, Scientific American 266:44-51 (1992); Sakman and Neher, Ann. Rev.
Physiol. 46:455-472 (1984); Cahalan and Neher, Methods in Enzymology 207:3-14
(1992); Levis and Rae, Methods in Enzymology 207:14-66 (1992); Armstrong and
Gilly, Methods in Enzymology 207:100-122 (1992); Heinmann and Conti, Methods
in
Enzymology 207:131-148 (1992); Bean, Methods in Enzymology 207:181-193
(1992); Leim et al., Neurosurgery 36:382-392 (1995); Lester, Ann. Rev. Physiol
53:477-496 (1991); Hamill and McBride, Ann. Rev. Physiol 59:621-631 (1997);
Bustamante and Varranda, Brazilian Journal 31:333-354 (1998); Martinez-Pardon
and
Ferrus, Current Topics in Developmental Biol. 36:303-312 (1998); Herness,
Physiology and Behavior 69:17-27 (2000); U.S. Patent No. 6,117,291; U.S.
Patent
No. 6,107,066; U.S. Patent No. 5,840,041 and U.S. Patent No. 5,661,035;
Boulton et
al., Patch-Clamp Applications and Protocols, Neuromethods V. 26 (1995), Humana
Press, New Jersey; Ashcroft, Ion Channels and Disease, Cannelopathies,
Academic
Press, San Diego (2000); Sakman and Neher, Single Channel Recording, second
edition, Plenuim Press, New York (1995) and Soria and Cena, Ion Channel
Pharmacology, Oxford University Press, New York (1998), each of which is
incorporated by reference herein in their entirety.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
II. AN ION CHANNEL MEASURMENT DEVICE HAVING FLOW-THROUGH
LOWER CHAMBERS
The present invention includes ion transport measurement devices and
apparatuses comprising flow-through lower chambers. As used herein, a "flow-
through chamber" is a chamber to which fluids can be added and from which
fluids
can be removed via continuous fluid flow. Thus, a flow-through chaanber will
preferably engage at least two conduits: at least one inflow conduit for
adding fluids
(such as solutions) and at least one outflow conduit for the removal of fluids
(such as
solutions). In the alternative, a flow-through chamber can be designed as a
channel
through which fluids can pass.
A flow-through lower chamber can be designed with two or more ports or
openings in the wall of the chamber, such that at least one inflow conduit and
at least
one outflow conduit engage one or more walls of the lower chamber at the
ports. In an
alternative, at least one inflow conduit and at least one outflow conduit can
engage
ports or openings at the bottom surface of a chamber. It is also possible to
have a
flow-through chamber in which at least one conduit engages the wall of the
chamber
and at least one conduit engages the bottom surface of the chamber.
Flow-through lower chambers have several advantages for ion transport
measuring devices. Because the exchange of lower chamber solutions can be
performed rapidly and continuously, without the need to empty the chamber of
liquid
when changing from a first solution to a second solution, a single patch clamp
(that is,
a cell or particle sealed with a high resistance electrical seal to an ion
transport
measuring hole) can be used for repeated tests, using, for example, different
solutions
that are delivered to the chamber in sequence. Adding and removing solutions
in a
flow-manner via conduits also facilitates automation of an ion transport
measurement
device, where the addition and removal of solutions can be through the
automated
control of pumps and valves. Addition or removal of solutions to one or more
lower
chambers can preferably but optionally be performed independently of the fluid
distribution to other chambers of a device, so that conditions of particular
patch
clamps can be changed without disrupting or changing the conditions of other
patch
clamps of the device.
In preferred embodiments, an ion transport measurement device comprises one
or more flow-through lower chambers, at least one chip comprising ion
transport
51
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
measuring holes, and at least one upper chamber. Preferably, a flow-through
chamber
is connected to two or more conduits that can provide fluid flow to and from a
lower
chamber. At least one of the at least two conduits can be used to provide
solutions to a
lower chamber, and at least one other of the at least two conduits can be used
to
remove solutions from a lower chamber.
Preferably, fluid flow is directed by one or more fluid pressure sources such
as
a pump or pumps. The conduits, or tubing or connectors leading to the
conduits, can
comprise valves that can be used to control the flow of solutions into or out
of a lower
chamber. In some preferred embodiments, control of the flow of solutions into
or out
of a chamber is automated, at least in part.
Lower chambers can be formed by one or more pieces of the device. At least a
portion of the upper surface of a lower chamber will be formed by a chip
comprising
an ion transport measuring hole. The walls and bottom surface of a lower
chamber
can be formed by one or more pieces of the device. For example, in some
embodiments at least a portion of the walls and the bottom surface of a lower
chamber
can be formed by a lower chamber piece. In other preferred embodiments, at
least a
portion of the walls of a lower chamber can be formed by a lower chamber piece
and
the bottom surface of a lower chamber can be formed by a lower chamber base
piece
or a platform.
In some embodiments, an ion transport measuring device with one or more
flow-through lower chambers can comprise a lower chamber piece that has inflow
and
outflow conduits that directly or indirectly connect to the walls or bottom
surfaces of
the one or more lower chambers. In some designs, the device can comprise a
platform
or a lower chamber base piece that comprises inflow and outflow conduits that
directly or indirectly connect to the bottom surface of one or more lower
chambers. In
an especially preferred embodiment of the present invention, a device for ion
transport measurement comprises a lower chamber base piece that forms the
bottom
of multiple lower chambers and comprises conduits that open to the lower
surfaces of
the lower chambers, such that each lower chamber is accessed by an inflow
conduit
and an outflow conduit. In this design, the device further comprises a lower
chamber
piece that forms at least a portion of the lower chamber walls, a chip
comprising ion
transport measuring holes that align with the lower chambers, and an upper
chamber
piece that comprises multiple upper wells that align with the ion transport
measuring
52
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
holes of the chip and the lower chambers formed by the lower chamber piece and
lower chamber base piece.
In preferred embodiments of ion transport measuring devices having one or
more flow-through lower chambers, the devices have multiple flow-through lower
chambers, each of which engages an inflow conduit and an outflow conduit, such
that
inflow and outflow conduits connected to different chambers are separate and
independent.
Components of an ion transport measuring device having one or more flow
through lower chambers, such as a lower chamber base piece, lower chamber
piece,
chip, and an upper chamber piece, can be reversibly or irreversibly attached
to one
another. In some preferred embodiments, an upper chamber piece and chip are
irreversibly attached (such as by adhesives) to one another as a cartridge,
and the
cartridge can be reversibly attached to a lower chamber piece and lower
chamber base
piece. A cartridge can be attached to a lower chamber piece by any feasible
means
that provides a fluid impermeable seal between the lower surface of the chip
of the
cartridge and the walls of the one or more lower chambers that are formed, at
least in
part, by a lower chamber piece. In designs in which the device comprises a
lower
chamber base piece, the lower chamber base piece can be attached to a lower
chamber
piece by any feasible means that provides a fluid impermeable seal between the
lower
chamber piece and the lower chamber base piece. The attachment of a lower
chamber
base piece to a lower chamber base can be irreversible, but is preferably
reversible.
For example, reversible attachment can be by pressure mounting, and can use
compressible materials as well as clamps, frames, screws, snaps, etc.
In preferred embodiments encompassing devices having more than one ion
transport measuring unit, when a multiunit device is assembled, the two or
more wells
of the upper chamber piece are in register with the two or more holes of the
biochip,
and the two or more lower chambers formed by a lower chamber piece and lower
chamber base piece are aligned with the holes with the biochip. The lower
chamber
base piece comprises at least two inflow conduits and at least two outflow
conduits,
such that each lower chamber is accessed by an inflow conduit and an outflow
conduit.
In some preferred embodiments, a cartridge, lower chamber piece that
comprises a compressible material and a lower chamber base piece are fastened
together using a clamp. In other preferred embodiments, a cartridge, lower
chamber
53
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
piece, and, optionally, a lower chamber base piece are attached using pressure
mounting and at least one gasket to form seals between the parts.
The present invention also includes a lower chamber base piece for use in a
device for ion transport measurement that can optionally be used independently
of a
larger automated apparatus and can be used to observe cells and particles
within the
device using an inverted microscope. In this embodiment, at least a portion of
the
lower chamber base piece that will form the bottom surface of the lower
chambers is
transparent. Preferably, the lower chamber base piece comprises at least two
conduits
that extend through the lower chamber base piece such that when the lower
chamber
base piece is assembled into a device of the present invention, the conduits
can be
used to transfer fluid from outside the device into lower chambers, and
transfer fluid
from inside lower chambers to the outside of the device. As part of a device
for ion
transport measurement, the base piece foims a bottom surface of lower
chambers. The
conduits that extend through the base piece allow for fluids such as solutions
to be
delivered in and out of lower chambers of ion transport measuring devices.
In this embodiment, two or more conduits go through the base piece, with
each conduit having one opening on one surface of the base piece, and the
other
opening on a different surface of the base piece. In preferred embodiments of
the
present invention, the conduits extend from a side of the base piece
essentially
horizontally toward the center, and then turn or curve upward to end in an
opening on
the top surface of the base piece which, in an assembled device, is the bottom
surface
of a lower chamber. The side opening can be the site where the conduit
connects with
tubing connected to solution reservoirs, pressure sources, and/or electrodes,
and the
top opening of the conduits is the site where the conduit opens into a lower
chamber.
In a preferred device of the present invention, each lower chamber of an ion
transport
measuring device is connected to two such conduits, and the conduits can
provide for
solutions to be delivered into and washed out of a lower chamber.
A lower chamber piece and lower chamber base piece can comprise one or
more plastics, one or more polymers, one or more ceramics, silicon or glass.
Preferably, the part or parts of a lower chamber base piece that will form the
bottom
of one or more lower chambers of an ion transport measuring device is
preferably
made of a transparent material that is impermeable to aqueous liquids so that
cells or
particles inside an ion transport measuring unit are visible using an inverted
microscope. Although not a requirement of the present invention, to simplify
54
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
manufacture of the base piece, the entire base piece (with the exception of
separate
attachments such as connectors, pins, screws, etc.) is preferably made of a
single
material by molding or machining. Glass and transparent polymers are preferred
materials, with transparent polymers such as polycarbonate and polystyrene
having
the advantage of easier manufacture.
Conduits can be molded into or drilled through the base piece, and can be
fitted with connectors. (Connectors can comprise glass, polymers, plastics,
ceramics,
or metals.) The connectors can be connected to tubing that can be used to
provide in-
flow and outflow of solutions to a lower chamber of an ion transport measuring
unit.
The conduits can also be used to deliver pressure to the lower chamber and to
an ion transport measuring hole of a chip exposed to the chamber. Pressure can
be
generated, for example, by a pump or a pressure source connected to the tubing
that
will be filled with an appropriate solution in at least the segment connecting
the lower
chamber. Preferably the pressure is regulatable and can be used for purging
air
bubbles and or other blocking micro-particles in the ion transport measuring
hole, cell
and particle positioning, sealing, and optionally, membrane rupture of an
attached cell
when carrying out ion transport measurement procedures.
In preferred embodiments, the conduits, or tubes leading to the conduits, can
also comprise electrodes. For example, a wire electrode can be threaded
through
tubing that is connected to a conduit of a base piece. The wire electrode can
optionally
extend through the conduit to the upper surface of the base piece (which will
be the
lower surface of a lower chamber of an ion transport measuring unit).
In the alternative, the base piece can comprise one or more electrodes on its
upper surface. Electrodes fabricated or attached to the upper surface of the
base piece
can be connected through leads to connectors on the outer edge of the base
piece, and
the connectors can be connected to a patch clamp amplifier.
In preferred aspects of the present invention, a lower chamber base piece is
designed to form the bottom of more than one lower chamber of an ion transport
measuring device. Preferably, a lower chamber base piece is designed to form
the
bottoms of all the lower chambers of an ion transport measuring device that
comprises
at least two ion transport measuring units, more preferably at least six ion
transport
measuring units, and more preferably yet, at least sixteen ion transport
measuring
units. In a preferred embodiment described in detail in Example 5, a lower
chamber
base piece forms the bottom of 16 lower chambers of a 16 unit device. In many
cases
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
(as illustrated in the example) multiple lower chambers will be arranged
linearly in a
row, but this is not a requirement of the present invention.
Thus, in preferred embodiments of the present invention, a flow-through lower
chamber base piece will comprise multiple conduits, two for each lower chamber
that
will occur in the ion transport measuring device: a first conduit for inflow
of solutions
(the "inflow conduit"), and a second conduit for outflow of solutions (the
"outflow
conduit"). A schematic cross-sectional view of a single ion transport
measuring unit
of one design of a device of the present invention having one or more flow-
through
lower chambers is shown in Figure 2. In this depiction, the lower chamber (25)
is
accessed by an inflow conduit (27) and an outflow conduit (28). In this
depiction the
lower chamber comprises an electrode (26) positioned on the upper surface of
the
lower chamber base piece. In an alternative design, one of each pair of
conduits that
leads to a single chamber of an ion transport measuring device can contain or
contact
an electrode.
The present invention also includes devices and apparatuses for ion transport
measurement that include a lower chamber base piece of the present invention.
In one
embodiment of the present invention, a device includes: a lower chamber base
piece
that comprises at least two conduits, where at least a portion of the lower
chamber
base piece is transparent; a chip comprising at least one ion transport
measuring hole;
and an upper chamber piece that comprises at least one chamber that attaches
to said
chip. Preferably, the device also includes a lower chamber piece in the form
of at least
one gasket that fits between the lower chamber base piece and the chip where
the one
or more gaskets comprise at least one opening, such that the one or more
gaskets form
the walls of the one or more lower chambers and seals the lower chamber base
piece
to the chip. The gasket or gaskets align with the lower surface of the chip
such that a
lower chamber formed by a gasket comprises a lower surface having the openings
of
two conduits, and an upper surface comprising a portion of a chip having a
single ion
transport measuring hole.
In preferred aspects of the present invention, a lower chamber base piece is
designed to fit a base plate that is adapted to fit the stage of a microscope,
such as an
inverted light microscope. The dimensions can be altered to fit a microscope
of
choice, such as, for example, an inverted light microscope sold by Leica,
Nikon,
Olympus, Zeiss, or other companies.
56
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 3A provides a photograph of a preferred design of a lower chamber
base piece having flow-through chambers for use in a sixteen unit device. In
Figure
3(A), connectors (302) for inflow conduits can be seen leading out from one
side of
the lower chamber base piece (301) and connectors (302) for outflow conduits
can be
seen leading out of the opposite side of the lower chamber base piece. Figure
3(B) is
a close-up photograph of the lower chamber piece showing the areas that
correspond
to what will be the transparent bottom surfaces (303) of the lower chambers
when the
device is fully assembled (black areas) with the conduit openings (304)
visible as
lighter areas within the black areas. A transparent gasket (305) lies over the
top of the
central portion of the lower chamber piece covering the areas that will be the
bottom
surfaces of the lower chambers (303). In this design, the gasket can be
aligned over
the lower chamber base piece by fitting a ridge that runs lengthwise down the
underside of the gasket into a groove the runs lengthwise down the length of
the upper
surface lower chamber base piece. The gasket depicted has two ridges running
along
either side of the gasket (on either side of the row of openings) and the
lower chamber
base piece has two corresponding grooves on either side of the surface having
conduit
openings (not visible in the photographs). When the gasket is placed on the
lower
chamber base piece such that the ridges of the gasket fit the grooves of the
lower
chamber base piece, the openings of the gasket align over the areas of the
surface of '
the lower chamber base piece that have conduit openings and will be the bottom
surfaces of the lower chambers.
The lower chamber base piece can also have "cuts" between the areas that will
correspond to the bottom surface of lower chambers (the cuts are perpendicular
to the
alignment grooves, not visible in the photographs). When the gasket is placed
on top
of the lower chamber base piece, the cuts in the lower chamber base piece axe
between lower chamber areas defined by the openings in the gasket. These cuts
can
reduce the possibility of solution seepage between lower chambers.
The three alignment dowels (306) seen in the foreground of Figure 3B at
lower left are used to align an upper chamber piece or cartridge over the
lower
chamber base piece, such that the ends of the lower chamber base piece fit
between
and abut the three pins. The two shorter pogo pins (307) are used to prevent a
clamp
placed on an assembly that includes a cartridge (comprising an upper chamber
piece
and attached chip) a gasket, 'and a lower chamber base piece from pressing
down on
the assembly prior to fastening of the clamp. Holding the clamp in standoff
position
57
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
by these pogo pins (307) prior to fastening prevents misaligned contact of the
cartridge with the gasket.
Also seen in Figure 3B, are inflow tubes (309) and outflow tubes (308)
attached to the connectors in this view. Female pin sockets (310) that connect
to the
lower chamber recording electrodes can also be seen. Electrical connectors
that are
attached to a signal amplifier can be inserted into these socket pins.
In Figure 3C, the lower chamber base piece is seated in a base plate (312)
adapted to a microscope stage. To the right of the base plate is a plexiglass
piece
(313) comprising ports (314) for the addition of lower chamber solutions and
screw-
down pinch valves (315) for the inflow tubing.
A baseplate can be made of any suitable material, such as glass, plastics,
polymers, ceramics, or metals. Metals, such as but not limited to stainless
steel, are
preferred, because metal materials have high mechanical strength needed during
pressure sealing of the lower chamber. A metal base plate can also, together
with a
grounded microscope stage, form an electrical noise shield around a lower
chamber
piece fitted to the base plate.
The base plate can be carved on the top side to catch any fluids that may leak
or spill and prevent the contamination of the microscope with the fluids.
Preferably,
the base plate is sealed around the lower chamber base piece, for example,
with
silicone glue, silicone grease, Vaseline, etc.
The base plate is preferably drilled and tapped so as to provide a mounting
point for the lower chamber base piece and for a clamp that can hold
additional
components of the ion transport measuring device together (for example,
gasket, chip,
upper chamber piece) to form the upper and lower chambers of ion transport
measuring units. The base plate is designed to hold an ion transport measuring
device
within a few millimeters of the level of the top of the microscope stage so as
to ensure
that the chip function may be monitored within the focal range of the
microscope.
Figure 4 illustrates the design of a base plate as adapted for a Nikon
Microscope.
Flow-through lower chamber designs described herein can be used in ion
transport measurement devices of the present invention'. In preferred
embodiments,
such devices comprise upper chamber pieces having multiple wells and chip
comprising multiple ion transport measuring holes. Upper chambers of such
devices
can comprise one or more electrodes. Such electrodes can be fabricated,
positioned, or
attached on a surface of an upper chamber, such as those described in a later
section
58
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
of this application on two-piece molding of upper chambers, can be inserted
into the
upper chambers of the assembled device from above (for example, wire
electrodes
inserted into the wells), or can be provided as within a tube or part of a
tube that can
be placed inside the upper chamber (such as a tube that delivers solutions or
cell
suspensions). Preferably, electrodes of upper chambers are connected as a
common
reference electrode, but this is not a requirement of the present invention.
It is also
possible for each upper well to have an individual (recording) electrode, and
to have
the electrodes of the lower chambers connected as a common reference
electrode.
In some preferred embodiments, the upper piece of a device of the present
invention comprises a common reference electrode that contacts all of the
wells. In
other preferred embodiments, an electrode is not within or attached to the
upper piece,
but during assembly of the device is inserted into an upper well through upper
opening of the well. In other preferred embodiments, an electrode can be
brought into
electrical contact with an upper chamber by way of a conduit that comprises an
electrode or can provide an electrolyte solution bridge to an electrode.
Electrodes that
are connected through electrolyte bridges can be recording electrodes, but in
most
preferred embodiments are reference electrodes.
Figure 5 depicts the design of a device of the present invention having an
upper chamber piece (51) and attached chip (not visible beneath the upper
chamber
piece) fixed on top of a gasket (not visible beneath the upper chamber piece)
and
lower chamber base piece (not visible beneath the upper chamber piece) by
means of
a clamp (53). The clamp (53) also fixes the device to a baseplate (54) adapted
to a
microscope. The plexiglass piece (52) holds female pin sockets (56) that
connect to
electrodes inserted into lower chamber piece conduits. The clamp has a wire
electrode
(55) that extends into upper chamber wells.
Figure 6 shows a gasket that can fit on top of a lower chamber base piece and
form the walls of lower chambers such that the openings (601) in the gasket
become
the lower chamber spaces.
Figure 7 provides three views of one design of a clamp that can be used in the
assembly of a device of the present invention. In Figure 7A, the clamp (71) is
shown
upside down to illustrate the cutout (72) that fits a cartridge. Thumb screws
(73) used
to attach the clamp to the base piece are alongside the clamp (71). In Figure
7B, the
top view of the clamp on the cartridge (74) reveals the presence of an array
of top
chamber electrodes (75) that reach into the cartridge wells.
59
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 8 provides photographs showing the parts of an ion transport
measuring device of the present invention including a baseplate (812), a
cartridge
(804) comprising an upper chamber piece with a chip attached at the bottom,
lower
chamber base piece (801), and clamp. In Figure 8A, the black upper chamber
piece
of the cartridge (804), transparent lower chamber base piece (801), inflow
tubing
(809) and outflow tubing (808) leading to the lower chamber base piece (801),
and
metallic clamp (802) can be seen. The transparent gasket (805) is lying over
the lower
chamber base piece (801) behind the upper chamber cartridge. In Figure 8B, the
device is assembled, with the clamp (802) screwed into a baseplate (812).
The present invention also encompasses compositions and devices that
incorporate novel elements of the compositions and devices described herein,
including: a transparent platform beneath the lower chambers, a baseplate
adapted for
microscope stage, one or more flow-through bottom chambers, reference or
recording
electrodes outside of upper or lower chambers and connected to chambers)
through
electrolyte bridges, and reference or recording electrodes introduced into
tubing
attached to upper or lower chambers. The present invention also encompasses
manufacture procedures and features for enhancing efficiency or accuracy of
manufacture of devices and devices disclosed herein and devices made using
such
methods, including tapering of upper chamber wells, geometry of holes drilled
into
chips, ion transport measuring holes comprising one or more counterbores in
chips,
treatment of chips to enhance electrical sealing of particles such as cells,
etc.
The present invention also includes methods of using an ion transport
measuring device of the present invention having one or more flow-through
lower
chambers to measure one or more ion transport properties or activities of a
cell or
particle (such as, for example, a membrane vesicle). The methods include using
a
device that comprises at least upper chamber reversibly or irreversibly
attached to a
chip that comprises at least one ion transport measuring means in the form of
a hole
through the biochip, wherein the chip has been treated to have enhanced
sealing
properties, and at least one flow-through lower chamber. In the assembled
devices
used in the methods of the present invention, the holes of the biochip access
the at
least one flow-through lower chamber. In these methods, an upper chamber piece
and
chip are reversibly or irreversibly attached to a lower chamber piece that
forms all or
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
a portion of a flow-through lower chamber. An upper chamber piece and chip are
optionally additionally reversibly attached to a lower chamber base piece that
can
form at least the lower surface of one or more lower chambers. Preferably, an
upper
chamber piece and chip are attached to at least one lower chamber piece that
forms
the walls of one or more lower chambers and at least one lower chamber base
piece
that forms the lower surfaces of one or more lower chambers and comprises
conduits
for the inflow and outflow of solutions.
The device is assembled such that the one or more upper chambers are in
register with the one or more ion transport measuring holes of the chip, and
one or
more lower chambers access the one or more upper chambers via the one or more
holes of the chip. In preferred embodiments, each of the one or more upper
chambers
is in register with one of the ion transport measuring holes of the chip, and
each of the
lower chambers is aligned with one upper chamber that it accesses via an ion
transport
measuring hole. Each of the lower chambers is connected to at least one inflow
conduit and at least one outflow conduit.
During use of the device, the one or more upper chambers comprise, contact,
or are in electrical contact with at least one electrode. During use of the
device, the
one or more lower chambers comprise, contact, or axe in electrical contact
with at
least one electrode. In one alternative, the one or more upper chambers
contact,
comprise, or are in electrical contact with a common reference electrode, and
the one
or more lower chambers contact, comprise, or axe in electrical contact with a
individual reference electrodes. In another alternative, the one or more upper
chambers contact, comprise, or are in electrical contact with individual
reference
electrodes, and the one or more lower chambers contact, comprise, or are in
electrical
contact with a common reference electrode.
The method includes: filling at least one flow-through lower chamber of the
device with a measuring solution; adding at least one cell or at least one
particle to
one or more of the at least one upper chamber of the device, wherein the one
or more
upper chambers is connected to one of the at least one lower chambers that
comprises
measuring solution via a hole in the ion transport measuring chip; applying
pressure
to at least one flow-through lower chamber, at least one upper chamber, or to
an
upper chamber and a lower chamber that are connected via an ion transport
measuring
hole to create a high-resistance electrical seal between at least one cell or
particle and
61
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
at least one hole of the biochip; and measuring at least one ion transport
property or
activity of the at least one cell or at least one particle.
Preferably, one or more cells or one or more particles are in a suspension
when added to the upper chamber. Various measuring solutions and, optionally,
compounds
In some preferred embodiments, the methods measure at least one ion
transport activity or property of a cell in the whole cell configuration, but
this is not a
requirement of the present invention, as the devices can be used in a variety
of
applications on particles such as, for example, vesicles, as well as cells.
The application of pressure can be manual or automated. If pressure is applied
manually (for example, by means of a syringe), preferably the user can make
use of a
pressure display system to monitor the applied pressure. Automated application
of
pressure can be through the use of a software program that is able to receive
feedback
from the device and direct and control the amount of pressure applied to one
or more
ion transport measuring units.
Various specific ion transport assay can be used for determining ion transport
function or properties. These include methods known in the art such as but not
limited to patch clamp recording, whole cell recording, perforated patch whole
cell
recording, vesicle recording, outside out or inside out recording, single
channel
recording, artificial membrane channel recording, voltage-gated ion transport
recording, ligand-gated ion transport recording, recording of energy requiring
ion
transports (such as ATP), non energy requiring transporters, toxins such a
scorpion
toxins, viruses, stretch-gated ion transports, and the like. See, generally
Neher and
Sakman, Scientific American 266:44-51 (1992); Sakman and Neher, Ann. Rev.
Physiol. 46:455-472 (1984); Cahalan and Neher, Methods in Enzyrnology 207:3-14
(1992); Levis and Rae, Methods in Enzymology 207:14-66 (1992); Armstrong and
Dilly, Methods in Enzymology 207:100-122 (1992); Heinmann and Conti, Methods
in
Enzymology 207:131-148 (1992); Bean, Methods in Enzymology 207:181-193
(1992); Leim et al., Neurosurgery 36:382-392 (1995); Lester, Ann. Rev. Physiol
53:477-496 (1991); Hamill and McBride, Ann. Rev. Physiol 59:621-631 (1997);
Bustamante and Varranda, Brazilian Journal 31:333-354 (1998); Martinez-Pardon
and
Ferrus, Current Topics in Developmental Biol. 36:303-312 (1998); Herness,
Physiology and Behavior 69:17-27 (2000); U.S. Patent No. 6,117,291; U.S.
Patent
No. 6,107,066; U.S. Patent No. 5,840,041 and U.S. Patent No. 5,661,035;
Boulton et
62
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
al., Patch-Clamp Applications and Protocols, Neuromethods V. 26 (1995), Humana
Press, New Jersey; Ashcroft, Ion Channels and Disease, Cannelopathies,
Academic
Press, San Diego (2000); Sakman and Neher, Single Channel Recording, second
edition, Plenuim Press, New York (1995) and Soria and Cena, Ion Channel
Pharmacology, Oxford University Press, New York (1998), each of which is
incorporated by reference herein in their entirety.
During the assay, while the cell or particle maintains a high-resistance seal
with the ion transport measuring hole, lower chamber solutions such as
intracellular
solutions can be exchanged using the inflow and outflow conduits. For example,
a
given patch-clamped cell can be assayed without drug, after addition of drug,
and
after washout of drug while maintaining a high-resistance seal. In another
example, a
cell or particle can be assayed for ion transport activity in the presence and
absence of
a particular ion by means of exchange of the lower chamber solution.
III. METHOD OF MAKING AN UPPER CHAMBER PIECE OF A DEVICE FOR
ION TRANSPORT MEASUREMENT
In ion transport measuring devices contemplated by the present invention, an
upper chamber is designed to contain the cells or particles on which ion
transport
measurements are to be performed. In these embodiments, an upper chamber of an
ion
transport measuring device can comprise or engage at least a portion of an
electrode
used to monitor ion transport activity. In the alternative, an upper chamber,
when
filled with an ion transport measuring solution, can be brought into
electrical contact
with at least a portion of an electrode. For example, an electrode (such as,
but not
limited to, a metal wire) can be inserted into the well so that electrical
current from
the electrode would be transmitted through the conductive measuring solution.
Alternatively, a tube that comprises a measuring solution (or otherwise
conductive
solution) that contains or contacts an electrode or a portion thereof can be
put in
contact with the upper chamber solution. In the latter case, the electrode (or
a portion
thereof) need not be within the upper chamber at all, as long as it is
electrically
connected to the inner part of the upper chamber conductive solution
(electrolyte
bridge).
Typically, an upper chamber electrode will be a reference electrode, although
this need not be the case. In cases in which upper chamber electrodes are
reference
63
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
electrodes, electrode extensions or electrolyte bridges that contact
individual upper
chambers can be connected with one another either outside or inside the upper
chamber piece.
In many of the devices of the present invention, an upper chamber piece
comprises at least one upper chamber in the form of a well. Preferably, an
upper
chamber piece comprises multiple upper chambers or wells that allow several
ion
transport assays to be performed simultaneously. The upper chamber piece can
optionally comprise one or more electrodes. The present invention provides
methods
of making upper chamber pieces that increase the efficiency and reduce the
cost of
making devices that measure ion transport activity of cells and particles.
Two-Piece Molding followed by Electrode Insertion
In one aspect of the present invention, an upper chamber piece that comprises
one or more wells is made in two pieces, an upper well portion piece and a
well hole
portion piece, and the well hole portion piece has a groove into which a wire
electrode
can fit. An upper well portion piece comprises the upper portion of one or
more wells.
The upper well portions are open at both ends. The well hole piece comprises
one or
more well holes that will form the bottom portion of the one or more wells. A
well
hole is, in effect, the lower portion of a well and can have different
dimensions
(height, diameter, and taper angle) than the upper well portion. The well
holes axe also
open at their upper and lower ends. The well holes have an upper diameter that
is
equal or smaller than the diameter of the lower opening of the upper well
portion.
When the upper well portion piece is attached on top of the well hole piece,
the upper
well portions are aligned over the well holes to form upper chambers (wells)
that have
well holes at their lower end.
After manufacturing the upper well portion piece and the well hole piece, a
wire electrode is inserted into the groove of the well hole piece, and then
the upper
well portion piece is attached, via, for example ultrasonic welding, to the
well hole
piece to form an upper chamber piece comprising one or more wells, each of
which is
in contact with a portion of a wire electrode.
An example of this manufacture (an upper well piece made by assembling an
upper well portion piece having upper portions of wells with an upper well
hole piece
having well holes) is depicted in Figure 9. In Figure 9A, the upper well
portion piece
(91) is shown suspended above the well-hole piece (92). The groove (94) into
which a
64
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
wire electrode can fit is seen along the backs of the wells (93) in the
assembled upper
well piece shown in Figure 9B.
The method includes: molding a well hole portion piece of an upper chamber
piece of an ion transport measuring device, wherein said well hole portion
piece
comprises: at least one well hole, and a groove that extends longitudinally
from one
end of the well hole portion piece toward the opposite end of the well hole
portion
piece, such that the groove contacts the one or more well holes; molding an
upper
well portion piece of an upper chamber piece that comprises at least one upper
well;
inserting a wire electrode into the groove of the well hole portion piece; and
attaching
the upper well portion piece to the well hole portion piece to form an upper
chamber
piece that comprises one or more wells, such that the wire electrode is
exposed to the
interior of said one or more wells.
In this embodiment, the upper piece is made from one or more plastics and
comprises wells that are open at their upper and lower ends, and each well
contacts or
contains a portion of a common electrode that can be used as a reference
electrode in
ion transport measuring assays. This method of manufacture is particularly
suited to
embodiments where the upper piece comprises multiple wells (at least two) that
will
contact a common electrode, and wells are arranged linearly in a row. However,
this
is not a requirement of the present invention, and the principle of two-piece
molding
and wire insertion can be adapted to the manufacture of device components in
which
multiple wells or chambers that will share a common electrode are arranged in
different geometries. In such embodiments, the path of the groove can be
designed
such that it contacts all of the wells or chambers that are intended to be in
contact with
the electrode. This includes embodiments where there are multiple rows of
wells or
chambers, arrangement of wells or chambers in concentric circles or spirals as
well as
other arrangements of wells or chambers.
It is also possible to adapt the methods of the present invention to designs
in
which one or more wells are to be contacted by one electrode and one or more
other
wells are to be contacted by a different electrode. It is also possible that
one well be
contacted with more that one electrode. In such cases, the well hole portion
piece will
comprise more than one continuous groove such that more than one wire
electrode
can be inserted into the lower well portion piece.
Injection molding or compression molding techniques as they are known in
the art can be used to make the well hole portion piece and the upper well
portion
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
piece. In the methods of the present invention, the upper well portion piece
comprises
an upper portion of at least one well or chamber and the well hole portion
piece
comprises a lower portion of at least one well or chamber, such that when the
upper
well portion piece is attached to the well hole portion piece, the two pieces
together
form at least one upper well or upper chamber. The well hole portion piece
comprises
at least one groove whose diameter corresponds to that of a wire electrode,
and the
groove contacts the well holes. Preferably, the well hole portion piece
comprises a
well hole whose upper diameter is equal to or smaller than the lower diameter
of the
upper portion of the well that is part of the upper well portion piece. Thus,
in
preferred embodiments, the well hole portion piece will have a top surface
around the
upper diameter of the well hole (see Figure 9), that, when looking down into a
well
after the entire top chamber piece is assembled, appears as a ledge around the
top of
the well hole. The groove can be in this top surface or ledge. In this way the
wire
electrode can be easily inserted into the groove, and its placement on this
"ledge"
ensures that it will be exposed to the interior of the well after attachment
of the upper
well portion piece.
The wire is easily inserted into the groove of the lower well portion piece,
as
the groove is totally accessible prior to attachment the upper and lower
portion pieces.
After insertion of the wire electrode, the upper well portion piece and well
hole portion piece are fused together to form a complete upper chamber piece.
Any
glues appropriate to the materials and applications of the devices can be used
for this
purpose. UV glues and other fast-curing glues are preferred for mass
production of the
upper chamber pieces, although slow-cure glues can also be used for mass
production
if a high capacity production process is used. Ultrasonic welding, pressuring,
heating,
or other bonding methods can also be used.
Upper Claafnber Pieces and Devices
The present invention includes upper chamber pieces that are made using the
methods of the present invention, and devices that comprise such pieces. Such
pieces
and devices can comprise wells or chambers that are open or closed at one or
both
ends, can comprise other components, such as, but not limited to, membranes,
microstructures, ports (optionally with attached conduits), fluidic channels,
particles
positioning means, specific binding members, polymers, etc., and are not
limited to
use as ion transport measuring devices. In fact, the same design and
manufacturing
66
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
principles can be used to fabricate pieces that comprise wells or chambers
that need
not function as "upper" pieces of devices or apparatuses. Two-piece molding,
wire
insertion, and attachment of two pieces can be used to make devices or
components of
devices that comprise wells or chambers regardless of whether the components,
chambers, or wells, can be considered "upper".
Plastics that can be used in the manufacture of upper and lower pieces
include,
but are not limited to polyallomer, polypropylene, polystyrene, polycarbonate,
cyclo
olefin polymers (e.g., ZeonorC~), polyimide, paralene, PDMS, polyphenylene
ether/PPO or modified polyphenylene oxide (e.g., Noryl~), etc. A very large
number
and variety of moldable plastics and their properties are known.
Electrodes can comprise conductive materials such as metals that can be
shaped into wires. Various metals, including aluminum, chromium, copper, gold,
nickel, palladium, platinum, silver, steel, and tin can be used as electrode
materials.
For electrodes used in ion channel measurement, wires made of silver or other
metal
halides are preferred, such as Ag/AgCI wires.
The design and dimensions of the upper and lower well pieces, as well as the
dimension of the upper wells and lower wells, can vary according to the
preferences
of the user and are not limiting to the present invention.
Preferred Embodimey~ts: Upper Chamber Pieces anal Devices
In preferred embodiments of the present invention, the upper chamber piece
comprises one or more upper wells that can function as the upper chambers of
ion
transport measuring units of ion transport measuring devices. Preferably, an
upper
chamber piece of the present invention comprises more than one upper well, and
more
preferably more than two upper wells. Even more preferably, an upper chamber
piece
comprises six or more upper wells, each of which can be a part of an ion
transport
measuring unit of an ion transport measuring device, where all of the six or
more
upper wells of the manufactured upper chamber piece contact a portion of a
common
wire electrode that extends along the upper chamber piece.
The wells of an upper chamber piece that can be part of an ion transport
measuring device preferably can hold a volume of between about 5 microliters
and
about S milliliters, more preferably between about 10 microliters and about 2
milliliters, and more preferably yet between about 25 microliters and about 1
milliliter. The depth, or height of a well can vary from about 0.01 to about
25
67
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
millimeters or more, and more preferably will be from about 2 milliliters to
about 10
milliliters or more in depth. In preferred embodiments of the present
invention in
which an upper well portion and a lower well portion together make up the
well, the
upper well portion is preferably from about 1 to about 25 milliliters in
depth, and the
S lower well is preferably from about 100 microns to about 10 milliliters in
depth.
A low cell or particle density is often preferred for attaining a high success
rate when using the ion channel measuring device described herein. W order to
reduce
the cell or particle density required for optimal cell or particle landing to
the recording
apertures, it is desirable to have an accurate means for delivering the cells
or particles
to the recording aperture. For a more accurate delivery of cells or particles
to the
recording aperture, the upper chamber well can have one or more tapered walls,
The
walls can be contoured such that the cells or particles, when delivered to the
upper
chamber well wall (such as by robotic dispenser), are directed to the
recording
aperture.
In these preferred embodiments, the shape of the well can vary, and can be
irregular or regular, and in many cases will be generally circular or oval at
its
circumference. In preferred embodiments, the diameter of a well at its upper
end will
generally be from about 2 millimeter to about 10 millimeters. In some
preferred
embodiments of the present invention such as those depicted in Figure 1 and
Figure
9, the upper circumferences of the wells of the upper chamber piece are
horseshoe-
shaped, and at least a portion of the sides of the wells are tapered. Figure
1D, for
example, shows that the wall of the well (1) corresponding to the rounded end
of the
horseshoe shape tapers toward the bottom of the well. In other preferred
embodiments, the walls along entire well can taper toward the bottom of the
upper
portion of the well. In some preferred embodiments of the present invention
the angle
of the taper of a portion of the walls of the well or the entire well walls
(the difference
from vertical) is from about one degree to about 80 degrees. More preferably,
the
angle of the taper of the well walls is between about 5 degrees and 60 degrees
from
vertical. The taper can extend down the full height of the well, or the well
can be
tapered for only a portion of its height. The upper well portion can
optionally be
tapered, or the well hole can optionally be tapered, or both the upper well
portion and
the lower well portion can be tapered. Where both are tapered, the tapering
need not
be to the same degree or extend around the well to the same extent.
68
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Molding of Single Upper Chamber Piece around Electrode
In another aspect of the present invention, an upper chamber piece with at
least one wire electrode can be manufactured as a single piece by molding an
upper
piece around a wire electrode. In this case, the mold has a means for
positioning the
wire electrode such that the upper chamber piece that includes the wells can
be
molded around it. The method includes: positioning an electrode in a mold; and
injection molding an upper chamber piece using the mold such that the
electrode
contacts one or more wells of the upper chamber piece. The electrode can be
positioned in any of a number of ways, for example it can extend through the
mold
and be held by apertures that it is threaded through on either end of the
mold.
The inj ection molded upper chamber piece can comprise one or more wells or
upper chambers, preferably two or more, more preferably six or more wells. The
wells
can be of any dimension of size, and can comprise a well hole within the well
as
described in the previous section.
Molding of Single Upper Piece without Electrode
In yet another aspect of the present invention, an upper chamber piece can be
manufactured without an electrode. In this case, an upper chamber piece with a
desirable number of wells is injection molded using a suitable plastic, such
as, but not
limited to, polyallomer, polypropylene, polystyrene, polycarbonate, polyimide,
paralene, PDMS, cyclo olefin polymers (for example, Zeonor~), or polyphenylene
ether/PPO or modified polyphenylene oxides (for example, Noryl~).
When the upper chamber piece is integrated into a device for ion transport
measurement, electrodes (for example, metal wires) can be inserted into the
wells.
Such electrodes are preferably reference electrodes and are preferably
connected
outside the chambers, but inserted electrodes can also be recording electrodes
connected separately to a power source/signal amplifier.
In a preferred embodiment of the present invention, an electrode connection
can be provided by a conduit that can be introduced into the upper chambers
during
use of the device. The conduit can comprise an electrode, or, when the conduit
is
filled with a conductive solution, can be in electrical contact with an
electrode. When
both the upper chamber and the conduit contain a conductive solution (such as
a
measuring solution), the upper chamber is in electrical contact with the
electrode
through the "electrolyte bridge" of solution provided by the conduit.
69
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Insert Molding of Glass Chip
In yet another embodiment, a pre-diced glass chip is insert-molded together
with an upper chamber piece to make a one-piece cartridge. In this process, a
glass
chip is inserted into a mold, and the upper chamber piece is molded around the
glass
chip such that it forms the bottoms of upper chambers of the upper chamber
piece.
Laser drilling of the recording apertures is done after the molding process,
and then
the cartridge is chemically treated to enhance its electrical sealing
properties. In this
embodiment, materials that can be treated with acid and base (such as, for
example,
polyphenylene ether/PPO or modified polyphenylene oxide (e.g., NORYL~) and
cylco olefin polymers (e.g.,ZEONOR~) are used for the construction of the
cartridge
other than the biochip.
Additional Features
In some preferred embodiments of the present invention, the upper chamber
pieces of the present invention or components of the upper chamber pieces of
the
present invention can have additional features that can aid in the manufacture
of upper
chamber pieces or of ion transport measuring devices. One such feature is an
alignment bump (also called a registration edge) (2) as seen on the chamber
piece
depicted in Figure 1B. One or more alignment bumps on the lower surface of an
upper chamber piece can be used during attachment of a chip that comprises ion
transport measuring means to the upper chamber piece. Attachment of the chip
and
the upper chamber piece must occur such that every ion transport measuring
hole in
the chip is aligned with a well hole. The alignment bump or registration edge
allows a
person or machine assembling the device to detect the location where the edge
of the
chip must be positioned.
Another useful feature for the manufacture of ion transport measuring devices
that can occur on upper chamber piece of the present invention is a glue
spillage
groove. This allows for overflow of glue that is used for the attachment of a
chip, such
as a chip that comprises ion transport measuring means. The glue spillage
groove (4)
is also shown as a notch in the bottom surface of the part shown in Figure 1D.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Yet another optional feature useful in the manufacturing process of an upper
chamber piece is the presence of sinkholes. Depicted in Figure 1C, these
sinkholes
(3) allow for appropriate expansion and contraction of the piece during
molding.
IV. METHODS OF MAKING A CHIP COMPRISING HOLES FOR ION
TRANSPORT MEASUREMENT
Fabrication of Ion TrarZSport MeasurirZg Holes ira a Chip
For optimal quality ion transport recording, ion transport measurement chips
comprising holes for ion transport measurement ideally should have a low hole
resistance (Re) across the chip. For chips having multiple holes, it is also
desirable to
have a high degree of uniformity of Re from recording site to recording site.
It is also
desirable to have ion transport measuring chips that can form seals of the ion
transport
measuring holes of the chip with a cell membrane such that the seal resistance
(R) is
high and the access resistance (Ra) is low.
Chip geometry determines hole resistance (Re) which in turn determines the
lowest achievable Ra. Figure 10 shows that chips of the present invention
having
shallower holes and reduced entrance hole diameters (known as "K configuration
chips" or "K chips"), have reduced Re relative to standard chips ("S
configuration
chips" or "S chips"). Figure 10 demonstrates that for S chips, the Re of seals
(y-axis)
decreases with increasing width of the exit hole (opening at the lower side of
the
chip), and increases with increasing hole depth (x-axis). For K chips, the
same
relationship holds, however the Re of seals of K chips is lower than those of
comparable S chips having holes with the same exit hole diameters (comparing
the K
configuration chips on the left side of the graph with the S configuration
chips on the
right side of the graph.) A wider tapering (greater angle from vertical) of
the hole also
decreases Re.
Figure 11 also shows that the Ra of a seal on a chip decreases with decreasing
depth of the hole in the chip and widening of the exit hole. Improved Ra,
however,
comes at the expense of reduced seal resistance (here, Rm).
The present invention includes methods of making chips that can form seals
with cells and cell membranes such that the seals have low access resistance
and high
seal resistance. The methods of the present invention seek to reduce hole
resistance
71
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
(Re) of ion transport measuring holes of chips by reducing hole depth. This is
achieved by laser drilling holes in thin substrates, such as glass, quartz,
silicon, silicon
dioxide, or polymer substrates.
A chip with shortened holes for ion transport measurement can be made by
laser drilling one or more counterbores into a glass chip, and then laser
drilling a
through-hole through the one or more counterbores. While a wide counterbore is
preferred for lower Re, increased width of the counterbore can weaken the
chip. It is
also difficult to control the drilling of the counterbore as the bottom of the
counterbore gets thinner and thinner. In addition, with increased (deeper)
drilling, the
peripheral areas of the counterbores tend to be deeper than the more central
portions
of the counterbore due to optical effects (this is sometimes called the wave
guide
effect). To avoid these problems, a second counterbore is laser drilled into
the bottom
of a first counterbore. This makes drilling to a greater depth easier control,
and has the
effect of reducing the thickness of the chip in the vicinity of the through-
hole. Thus,
preferred methods for synthesis of biochips for ion transport measurement
include
laser drilling at least one counterbore through a substrate, and then drilling
a through-
hole through the one or more counterbores. Preferably two counterbores are
laser
drilled into a substrate, such that a second counterbore is drilled through a
first
counterbore, that is, the counterbores are nested to form (along with a
through-hole) a
single hole structure. In some embodiments of the present invention, three,
four, or
more nested counterbores can be drilled into a substrate prior to drilling a
through-
hole through the counterbores.
Control of the depth of laser drilling can be done by using a separate laser
device that can measure the thickness of the glass. In preferred aspects of
this
embodiment of the present invention, a measuring laser is used to measure the
thickness of the substrate before or as it is being drilled, and the laser
used for drilling
can be regulated by the thickness of the remaining substrate at the bottom
surface of
the counterbore. Laser-based measuring devices have been used for the
determination
of glass thickness to an accuracy of 0.1 micron. Such a laser measurement
device is
available from the Keyence Company. A laser based measurement is made to
determine the exact thickness of the substrate. This measurement determines
the
number of pulses to be used by the drilling laser to drill a counterbore and
thereby
achieve uniformity of hole depth. To improve the consistency of through-hole
depth
72
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
and hole resistance, the invention contemplates the integration of a laser
unit with an
excimer laser drilling device, together with automated control software.
Thus, the present invention comprises methods of making chips comprising
holes for ion transport measurement that can form seals having a high seal
resistance
and low access resistance with cells and particles. The method includes:
providing a
substrate; laser drilling at least one counterbore in the substrate, and laser
drilling at
least one hole through the counterbore in the substrate. Preferably, laser
drilling is
done with sequential or simultaneous measurement of the glass thickness at the
site of
the pore.
In practice, a substrate made of glass, quartz, silicon, silicon dioxide,
polymers, or other substrates that preferably ranges in thickness from 5 to
1000
microns, and more preferably from 10 to 200 microns, is provided. A first
counterbore
is laser drilled, where the entrance of the counterbore has a diameter from
about 20 to
about 200 microns, preferably from about 40 to about 120 microns. The first
counterbore can be drilled to a depth of the thickness of the substrate minus
the
through-hole depth, with the depth depending on the thickness of the substrate
and the
number of counterbores that each ion transport measuring hole will have.
Subsequent
counterbores will have a smaller diameter than the first counterbore, and can
be of
lesser depth than the first counterbore. In general, after drilling of all of
the
counterbores that will be part of an ion transport measuring hole, the
remaining
thickness of the substrate that is to be drilled out to form the through-hole
(that is, the
depth of the through-hole) will range from about 0.5 to about 200 microns, and
preferably will range from about 2 to about 50 microns, more preferably from
about 5
to about 30 microns. The diameter of the through-hole can be from about 0.2 to
about
8 microns, and preferably will be from about 0.5 to about 5 microns, and even
more
preferably, from about 0.5 to about 3 microns.
Counterbores can be tapered. Preferably, a counterbore is tapered at an angle
ranging from about 1 degree to about 80 degrees from vertical, and more
preferably
from about 3 degrees to about 45 degrees from vertical. Ion transport
measuring holes
comprising multiple counterbores can have different taper angles for different
counterbores.
Through-holes can also be tapered. The angle of taper for a through-hole can
range from about 0 degree to about 75 degrees from vertical, and more
preferably,
where a through-hole is tapered, is from about 0 degree to about 45 degrees
from
73
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
vertical. In general an exit hole of a through-hole will have a narrower
diameter than
an entrance hole, although this is not a requirement of the present invention.
The present invention includes chips made using the methods of the present
invention having at least one counterbore and at least one through-hole
drilled through
the counterbore. Figure 12A depicts a chip of the present invention (123)
having a
laser drilled ion transport measuring means that comprises a first counterbore
(126), a
second counterbore (127), and a through-hole (128).
Preferably, the chips of the present invention that comprise through holes
laser
drilled through counterbores have electrical sealing properties such that when
appropriate pressure is applied to achieve a seal, a seal between the chip and
a cell or
particle has a seal resistance (R) that is greater than the resistance across
the hole
(Re). Preferably, the chips produced by the methods of the present invention
have ion
transport measuring holes that are able to seal to cells or cell membranes
such that
electrical access between said chip an the inside of said cell or particle, or
between
said chip and the outside of said cell or particle in the region of said hole
has an
access resistance (Ra) that is less than the seal resistance (R). Preferably,
the seal
between the ion transport measuring hole of a chip made by the methods of the
present invention and a cell or cell membrane has a seal resistance that is at
least 200
MOhm, more preferably at least 500 MOhm, and more preferably yet one gigaOhm
or
greater.
In preferred embodiments of chips of the present invention having at least one
ion transport measuring means comprising at least one laser drilled
counterbore and a
through-hole laser drilled through the one or more counterbores, the chip has
been
treated to enhance the electrical sealing properties of the chip. Preferably,
the chip has
been treated to make the surface of the chip at or near the ion transport
measuring
hole or holes more electronegative. For example, chips of the present
invention can be
chemically treated, such as by methods described herein, to become more
electronegative.
Preferably, a chip made by the methods of the present invention can produce a
seal with a cell or particle that has an access resistance that is less than
~0 MOhm,
more preferably less than about 30 MOhm, and more preferably yet, less than
about
10 MOhm. Preferably, a chip of the present invention comprising at least one
ion
transport measuring means in the form of a through-hole that has been laser-
drilled
74
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
through at least one counterbore can form a seal with a cell such that the
resistance of
the seal is at least ten times the access resistance. More preferably, a chip
of the
present invention can form a seal with a cell such that the seal resistance is
at least
twenty times the access resistance.
A chip produced by methods of the present invention can be used in any ion
transport measuring device, including but not limited to those described
herein.
Iftverted Chip
The present invention also includes methods of using chips comprising ion
transport measuring holes that are in inverted orientation for ion transport
measurement, that is, using chips in which the holes (or at least a portion of
the holes,
such as a portion of the holes made by at least one counterbore) have a
negative taper.
The method comprises: assembling a device for ion transport measurement
that comprises: at least one upper chamber, wherein the one or more upper
chambers
comprise or are in electrical contact with at least one electrode; at least
one chip that
comprises an ion transport measuring hole, wherein the one or more chips are
assembled in the device in inverted orientation; and at least one lower
chamber,
wherein the one or more lower chambers comprise or are in electrical contact
with at
least one electrode; connecting the electrodes with a power supply/signal
amplifier;
introducing at least one particle or at least one cell into at least one upper
chamber,
and measuring ion transport activity of at least one cell or at least one
particle.
By "inverted orientation" is meant that, for a chip in which ion transport
measuring holes are made by drilling, the chip is positioned such that the
side of the
chip having the laser entrance hole opening is exposed to a chamber that will
contain
cells or particles, instead of the side having the laser exit hole. This is
contrary to what
has previously been done in the art - the "upside- up" orientation in which
the cells or
particles seal against the side of the chip that has the laser exit hole.
Thus, sealing of a
cell or particles against the ion transport measuring hole occurs on the side
of the chip
opposite to the side that has smaller hole size (the "back side" of the chip).
The inverted chip orientation has several advantages. One advantage is that
the
chip does not require a laser polishing step, since the laser drilling
performs this
function as a "side-effect". A second advantage is that sealing occurs with
high
efficiency due to the geometry of the particle-chip interaction. Yet another
advantage
is that a stable low Ra can be produced using larger holes (for example, from
about 2
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
to about 5 microns in diameter), due to the position at which break-in occurs
during
whole cell recording.
When one or counterbores are used to reduced the through-hole depth, the
through-hole can be drilled from either the same direction as the
counterbores, or
from the opposite direction to the counterbores. In the former case, the chips
is
produced just like the "normal" chips are produced, they are simply assembled
up side
down. Figure 12B illustrates the use of a chip with laser drilled counterbores
(126,
127) and through-hole (128) used in inverted orientation. The single unit of
the ion
transport measuring device shown has an upper well (121) attached to a chip
(123)
comprising an ion transport measuring means in the form of a hole (122) that
connects
the upper chamber (121) with a lower chamber (125). In this case, a gasket
(124)
forms the walls of the lower chamber. A cell (129) is shown sealed to the
through-
hole (128) of the chip which is being used in inverted orientation.
The present invention includes devices and apparatuses having chips
comprising ion transport measuring holes that are in inverted orientation, as
well as
methods of using chips comprising ion transport measuring holes that are in
inverted
orientation for ion transport measurement.
Methods of Treating Chips Comprisi~rg lon Transport Measuring Means to Ehhance
tlae Electrical Seal of a Particle
The present invention also includes methods of modifying an ion transport
measuring means to enhance the electrical seal of a particle or membrane with
the ion
transport measuring means. Ion transport measuring means includes, as non-
limiting
examples, holes, apertures, capillaries, and needles. "Modifying an ion
transport
measuring means" means modifying at least a portion of the surface of a chip,
substrate, coating, channel, or other structure that comprises or surrounds
the ion
transport measuring means. The modification may refer to the surface
surrounding all
or a portion of the ion transport measuring means. For example, a biochip of
the
present invention that comprises an ion transport measuring means can be
modified
on one or both surfaces (e.g. upper and lower surfaces) that surround an ion
transport
measuring hole, and the modification may or may not extend through all or a
part of
the surface surrounding the portion of the hole that extends through the chip.
Similarly, for capillaries, pipettes, or for channels or tube structures that
comprises
ion transport measuring means (such as apertures), the inner surface, outer
surface, or
76
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
both, of the channel, tube, capillary, or pipette can be modified, and all or
a portion of
the surface that surrounds the inner aperture and extends through the
substrate (and
optionally, coating) material can also be modified. Methods of modifying an
ion
transport measuring means to enhance the electrical seal of a particle or
membrane
with the ion transport measuring means are also disclosed in United States
patent
application number 10/760,866 filed January 20, 2004, and United States patent
application number 10/642,014, filed August 16, 2003, both of which are herein
incorporated by reference in their entireties.
As used herein, "enhance the electrical seal", "enhance the electric seal",
"enhance the electric sealing" or "enhance the electrical sealing properties
(of a chip
or an ion transport measuring means)" means increase the resistance of an
electrical
seal that can be achieved using an ion transport measuring means, increase the
efficiency of obtaining a high resistance electrical seal (for example,
reducing the
time necessary to obtain one or more high resistance electrical seals), or
increasing the
probability of obtaining a high resistance electrical seal (for example, the
number of
high resistance seals obtained within a given time period).
The method comprises: providing an ion transport measuring means and
treating the ion transport measuring means to enhance the electrical sealing
properties
of the ion transport measuring means. Preferably, treating an ion transport
measuring
means to enhance the electrical sealing properties results in a change in
surface
properties of the ion transport measuring means. The change in surface
properties can
be a change in surface texture, a change in surface cleanness, a change in
surface
composition such as ion composition, a change in surface adhesion properties,
or a
change in surface electric charge on the surface of the ion transport
measuring means.
In some preferred aspects of the present invention, a substrate or structure
that
comprises an ion transport measuring means is subjected to chemical treatment
(for
example, treated in acid, and /or base for specified lengths of time under
specified
conditions). For example, treatment of a glass chip comprising a hole through
the chip
as an ion transport measuring means with acid and/or base solutions may result
in a
cleaner and smoother surface in terms of surface texture for the hole. In
addition,
treating a surface of a biochip or fluidic channel that comprises an ion
transport
measuring means (such as a hole or aperture) or treating the surface of a
pipette or
capillary with acid andlor base may alter the surface composition, and/or
modify
77
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
surface hydro~h~~a,_~ ~ or change surface charge density and/or surface charge
polarity.
Preferably, the altered surface properties improve or facilitate a high
resistance electric seal or high resistance electric sealing between the
surface
s modified ion transport measuring means and a membranes or particle. In
preferred
embodiments of the present invention in which the ion transport measuring
means are
holes through one or more biochips, one or more biochips having ion transport
measuring means with enhanced sealing properties (or, simply, a "biochip
having
enhanced sealing properties") preferably has a rate of at least 50% high
resistance
sealing, in which a seal of 1 Giga Ohm or greater occurs at 50% of the ion
transport
measuring means takes place in under 2 minutes after a cell lands on an ion
transport
measuring hole, and preferably within 10 seconds, and more preferably, in 2
seconds
or less. Preferably, for biochips with enhanced sealing properties, a 1 Giga
Ohm
resistance seal is maintained for at least 3 seconds.
In practice, in preferred aspects of the present invention the method
comprises
providing an ion transport measuring means and treating the ion transport
measuring
means with one or more of the following: heat, a laser, microwave radiation,
high
energy radiation, salts, reactive compounds, oxidizing agents (for example,
peroxide,
oxygen plasma), acids, or bases. Preferably, an ion transport measuring means
or a
structure (as nonlimiting examples, a structure can be a substrate, chip,
tube, or
channel, any of which can optionally comprise a coating) that comprises at
least one
ion transport measuring means is treated with one or more agents to alter the
surface
properties of the ion transport measuring means to make at least a portion of
the
surface of the ion transport measuring means smoother, cleaner, or more
electronegative.
An ion transport measuring means can be any ion transport measuring means,
including a pipette, hole, aperture, or capillary. An aperture can be any
aperture,
including an aperture in a channel, such as within the diameter of a channel
(for
example, a narrowing of a channel), in the wall of a channel, or where a
channel
forms a junction with another channel. (As used herein, "channel" also
includes
subchannels.) In some preferred aspects of the present invention, the ion
transport
measuring means is on a biochip, on a planar structure, but the ion transport
measuring means can also be on a non-planar structure.
78
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
The ion transport measuring means or surface surrounding the ion transport
measuring means modified to enhance electrical sealing can comprise any
suitable
material. Preferred materials include silica, glass, quartz, silicon, plastic
materials,
polydimethylsiloxane (PDMS), or oxygen plasma treated PDMS. In some preferred
aspects of the present invention, the ion transport measuring means comprises
SiOM
surface groups, where M can be hydrogen or a metal, such as, for example, Na,
K,
Mg, Ca, etc. In such cases, the surface density of said SiOM surface groups
(or
oxidized SiOM groups (Si0-)) is preferably more than about 1%, more preferably
more than about 10%, and yet more preferably more than about 30%. The SiOM
group can be on a surface, for example, that comprises glass, for example
quartz glass
or borosilicate glass, thermally oxidized Si02 on silicon, deposited SiOa,
deposited
glass, polydimethylsiloxane (PDMS), or oxygen plasma treated PDMS.
In preferred embodiments, the method comprises treating said ion transport
measuring means with acid, base, salt solutions, oxygen plasma, or peroxide,
by
treating with radiation, by heating (for example, baking or fire polishing) by
laser
polishing said ion transport measuring means, or by performing any
combinations
thereof.
An acid used for treating an ion transport measuring means can be any acid, as
nonlimiting examples, HCI, HZS04, NaHS04, HSO4, HN03, HF, H3P04, HBr,
HCOOH, or CH3COOH can be. The acid can be of a concentration about 0.1 M or
greater, and preferably is about 0.5 M or higher in concentration, and more
preferably
greater than about 1 M in concentration. Optimal concentrations for treating
an ion
transport measuring means to enhance its electrical sealing properties can be
determined empirically. The ion transport measuring means can be placed in a
solution of acid for any length of time, preferably for more than one minute,
and more
preferably for more than about five minutes. Acid treatment can be done under
any
non-frozen and non-boiling temperature, preferably at greater or equal than
room
temperature.
An ion transport measuring means can be treated with a base, such as a basic
solution, that can comprise, as nonlimiting examples, NaOH, KOH, Ba(OH)2,
LiOH,
CsOH,or Ca(OH)2. The basic solution can be of a concentration of about 0.01 M
or
greater, and preferably is greater than about 0.05 M, and more preferably
greater than
about 0.1 M in concentration. Optimal concentrations for treating an ion
transport
measuring means to enhance its electrical sealing properties can be determined
79
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
empirically (see examples). The ion transport measuring means can be placed in
a
solution of base for any length of time, preferably for more than one minute,
and more
preferably for more than about five minutes. Base treatment can be done under
any
non-frozen and non-boiling temperature, preferably at greater or equal than
room
temperature.
An ion transport measuring means can be treated with a salt, such as a metal
salt solution, that can comprise, as nonlimiting examples, NaCl, KCl, BaClz,
LiCI,
CsCI, NaZS04, NaN03, or CaCI, etc. The salt solution can be of a concentration
of
about 0.1 M or greater, and preferably is greater than about 0.5 M, and more
preferably greater than about 1 M in concentration. Optimal concentrations for
treating an ion transport measuring means to enhance its electrical sealing
properties
can be determined empirically (see examples). The ion transport measuring
means can
be placed in a solution of metal salt for any length of time, preferably for
more than
one minute, and more preferably for more than about five minutes. Salt
solution
treatment can be done under any non-frozen and non-boiling temperature,
preferably
at greater or equal than room temperature.
Where treatments such as baking, fire polishing, or laser polishing are
employed, they can be used to enhance the smoothness of a glass or silica
surface.
Where laser polishing of a chip or substrate is used to make the surface
surrounding
an ion transport measuring means more smooth, it can be performed on the front
side
of the chip, that is, the side of the chip or substrate that will be contacted
by a sample
comprising particles during the use of the ion transport measuring chip or
device.
Appropriate temperatures and times for baking, and conditions for fire and
laser polishing to achieve the desired smoothness for improved sealing
properties of
ion transport measuring means can be determined empirically.
In some aspects of the present invention, it can be preferred to rinse the ion
transport measuring means, such as in water (for example, deionized water) or
a
buffered solution after acid or base treatment, or treatment with an oxidizing
agent,
and, preferably but optionally, before using the ion transport measuring means
to
perform electrophysiological measurements on membranes, cells, or portions of
cells.
Where more than one type of treatment is performed on an ion transport
measuring
means, rinses can also be performed between treatments, for example, between
treatment with an oxidizing agent and an acid, or between treatment with an
acid and
a base. An ion transport measuring means can be rinsed in water or an aqueous
~0
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
solution that has a pH of between about 3.5 and about 10.5, and more
preferably
between about 5 and about 9. Nonlimiting examples of suitable aqueous
solutions for
rinsing ion transport measuring means can include salt solutions (where salt
solutions
can range in concentration from the micromolar range to SM or more),
biological
buffer solutions, cell media, or dilutions or combinations thereof. Rinsing
can be
performed for any length of time, for example from minutes to hours.
Some preferred methods of treating an ion transport measuring means to
enhance its electrical sealing properties include one or more treatments that
make the
surface more electronegative, such as treatment with a base, treatment with
electron
radiation, or treatment with plasma. Not intending to be limiting to any
mechanism,
base treatment can make a glass surface more electronegative. This phenomenon
can
be tested by measuring the degree of electro-osmosis of dyes in glass
capillaries that
have or have not been treated with base. In such tests, increasing the
electronegativity
of glass ion transport measuring means correlates with enhanced electrical
sealing by
the base-treated ion transport measuring means. Base treatment can optionally
be
combined with one or more other treatments, such as, for example, treatment
with
heat (such as by baking or fire polishing) or laser treatment, or treatment
with acid, or
both. Optionally, one or more rinses in water, a buffer, or a salt solution
can be
performed before or after any of the treatments.
For example, after manufacture of a glass chip that comprises one or more
holes as ion transport measuring means, the chip can be baked, and
subsequently
incubated in a base solution and then rinse in water or a dilution of PBS. In
another
example, after manufacture of a glass chip that comprises one or more holes as
ion
transport measuring means, the chip can optionally be baked, subsequently
incubated
in an acid solution, rinsed in water, incubated in a base solution, and
finally rinsed in
water or a dilution of PBS. In some preferred embodiments, the surfaces of a
chip
surrounding ion transport measuring means can be laser polished prior to
treating the
chip with acid and base.
To facilitate batch treatment of glass biochips, we have used the treatment
fixtures illustrated in Figure 13. Figure 13A shows a single layer treatment
fixture
that can fit into a glass jar containing acid, base, or other chemical
solutions. The rods
(131) facilitate handling and stacking of the treatment fixtures. Glass pins
can fit into
the holes (132) and chips can be stacked lengthwise on their edges between the
pins.
Sl
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Figure 13B shows the stacked treatment fixture. The fixture is made of acid
and base
resistant materials such as cyclo olefin polymers (for example, ZEONOR~),
polyphenylene ether/PPO or modified polyphenylene oxide (for example, NORYL~),
polytetrafluoroethylene, TEFLONTM, etc. Multiple layers of these racks can be
S stacked up to fit into one glass container, as shown in Figure 13B. This
design also
allows mechanisms of moving fluid to occur such as that brought about by a
rotary or
reciprocal shaker or a magnetic stir bar.
In an alternative design, chips are positioned flat on a treatment fixture,
and
are held in a tray by a door that can open and latch closed. This facilitates
manipulation of the chips, such as by a machine. For example, after treatment
of the
chips, a machine that assembles cartridges can pick up a treated chip from the
treatment fixture in order to attach it to a cartridge.
In some aspects of the present invention, it can be preferable to store an ion
transport measuring means that has been treated to have enhanced sealing
capacity in
an environment having decreased carbon dioxide relative to the ambient
environment.
This can preserve the enhanced electrical sealing properties of the ion
transport
measuring means. Such an environment can be, for example, water, a salt
solution
(including a buffered salt solution), acetone, a vacuum, or in the presence of
one or
more drying agents or desicants (for example, silica gel, CaCl2 or NaOH) or
under
nitrogen or an inert gas. Where an ion transport measuring means or structure
comprising an ion transport measuring means is stored in water or an aqueous
solution, preferably the pH of the water or solution is greater than 4, more
preferably
greater than about 6, and more preferably yet greater than about 7. For
example, an
ion transport measuring means or a structure comprising an ion transport
measuring
means can be stored in a solution having a pH of approximately 8.
Glass chips that have been base treated and stored in water with neutral pH
levels can maintain their enhanced sealability for as long as 10 months or
longer. In
addition, patch clamp chips bonded to plastic cartridges via adhesives such as
UV-
acrylic or UV-epoxy glues can be stored in neutral pH water for months without
affecting the sealing properties.
We have also tested patch clamp biochips and cartridges that were stored in a
dry environment with dessicant for 30 days. The chips were re-hydrated and
tested
for sealing. In one experiment, we got 6/7 seals for the dry-stored chips.
Similarly, we
82
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
stored mounted chips in dry environment and were able to obtain seals after a
few
weeks of storage.
Dehydration can, however, reduce the sealability of chemically treated chips.
To improve the seal rate for dry-stored chips, NaOH, NaCl, CaCl2 and other
salt or
basic solutions can be used to rejuvenate the chips out of dry storage to
restore the
sealability.
The present invention also includes methods of shipping or transporting ion
transport measuring means modified by the methods of the present invention to
have
enhanced electric sealing properties and structures comprising ion transport
means
that have been modified using the methods of the present invention to have
enhance
electric sealing properties. Such ion transport measuring means and structures
comprising ion transport measuring means can be shipped or transported in
closed
containers that maintain the ion transport measuring means in conditions of
low C02
or air. For example, the ion transport measuring means can be submerged in
water,
acetone, alcohol, buffered solutions, salt solutions, or under nitrogen (N2)
or inert
gases (e.g., argon). Where the ion transport measuring means or structure
comprising
an ion transport measuring means is stored in water or an aqueous solution,
preferably
the pH of the water or solution is greater than 4, more preferably greater
than about 6,
and more preferably yet greater than about 7. For example, an ion transport
measuring
means or a structure comprising an ion transport measuring means can be
shipped in a
solution having a pH of approximately 8.
In one method of shipping a chip that has been treated to have enhanced
sealing properties, the ion transport measuring devices comprising base-
treated chips
are shipped such that the chips are loaded up side down. The package for
commercial
shipments is designed to hold cartridges up side down, although the up side up
configuration can also be used for shipping. To allow easy opening and
facilitate
automation in sequential loading of the devices onto apparatuses for use, a
blister
pack with film sealing is designed. As illustrated in the Figure 14, a blister
pack is
provided in the form of a molded plastic frame (141) having (142) for
positioning
cartridges. One of the slots comprises a cartridge (143), viewed from the
bottom in
Figure 14A and from the top in Figure 145. The blister pack has an opening on
both
top and bottom sides for film sealing. The sealing film or "lidstock" is a
thin foil with
83
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
temperature activated adhesive and an inert coating such as EVA (ethyl vinyl
acetate)
polymer. For wet (water) storage, the blister pack is first sealed from top
(the opening
side, flipped over, and the cartridges are loaded up side up. A preservative
solution
such as water is then injected into each well and the rest of the open space
in each
chamber of the package. .Another lidstock film is then used to seal the
blister package
from the bottom. The blister package can be optionally sterilized with
radiation for
long shelf life.
Yet another aspect is related to the shipping of laser processed glass chips
as
finished goods between to production processes, particularly if the two
processes are
in different production locations. The current invention includes a shipping
fixture
allowing individual placement and securing of laser-processed glass chips for
shipment. The same fixture-chips assembly is then directly used for subsequent
chemical processing. To withstand strong acid and base treatment, the shipping
fixtures are molded with inert materials such as polyphenylene ether/or
modified
polyphenylene oxide (e.g., Noryl~), Teflon, and cylco olefin polymers (e.g.,
Zeonor~). A stack of these fixtures can be secured in one container for
chemical
treatments, or for shipping in aqueous solutions such as water. The liquid
shipping
provides buffering for vibrations during transportation, giving maximum
protection of
glass chips from being damaged.
The present invention also includes ion transport measuring means treated to
have enhanced electrical sealing properties, such as by methods disclosed
herein. The
ion transport measuring means can be any ion transport measuring means,
including
those disclosed herein. The present invention also includes chips, pipettes,
substrates,
and cartridges, including those disclosed herein, comprising ion transport
measuring
means treated using the methods of the present invention to have enhanced
electrical
sealing properties.
The present invention also includes methods of using ion transport measuring
means and structures comprising ion transport measuring means, such as
biochips, to
measure ion transport activity or functions of one or more particles, such as
cells. The
methods include: contacting a sample comprising at least one particle with an
ion
transport measuring means that has been modified to enhance the electrical
seal of a
particle or membrane with the ion transport measuring means, engaging at least
one
84
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
particle or at least one membrane on or at the modified ion transport
measuring
means, and measuring at least one ion transport function or property of the
particle or
membrane. The methods can be practices using the methods and devises disclosed
herein. Generally, the methods of the present invention provide the following
characteristics, but not all such characteristics are required such that some
characteristics can be removed and others optionally added: 1) the
introduction of
particles into a chamber that includes a biochip of the present invention, 2)
optionally
positioning particles at or near an ion transport detection structure, 3)
electronic
sealing of the particle with the ion transport detection structure, and 4)
performing ion
transport recording. Methods known in the art and disclosed herein can be
performed
to measure ion transport functions and properties using modified ion transport
measuring means of the present invention, such as surface-modified
capillaries,
pipette, and holes and apertures on biochips and channel structures.
V. METHODS FOR MEASURING THE SURFACE ENERGY OF THE SURFACE
OF A CHEMICALLY TREATED ION TRANPORT MEASURING BIOCHIP
Another aspect of the current invention originated from the need for an
inexpensive, fast, and sensitive method to measure surface energy on
solid/liquid
surface such as, for example, that of a chemically treated ion transport
measurement
biochip.
The method includes: dispensing a drop of defined volume of water or an
aqueous solution on a surface, measuring the time it takes for the drop to
evaporate;
and estimating the relative or absolute surface energy of the surface based on
the
evaporation time and the difference in evaporation time with respect to
control
samples.
The contact angle of a liquid drop on a solid surface is a measure of the
surface energy, assuming constant liquid/air surface energy. Very low
liquidlsolid
energy results in extremely small contact angles (close to 0 degrees). For
that reason,
contact angle measurements might not be a very sensitive method for low
surface
energy systems.
When a liquid drop with fixed volume is in contact with a solid surface, the
air/liquid surface of the drop will be inversely proportional to the
liquid/solid surface
energy. Lower liquid/solid surface energy will result in bigger spreading of
the drop.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
The evaporation of the drop will be proportional to the air/liquid surface
area at any
given moment. Thus the evaporation time will be proportional to the
liquid/solid
surface energy.
The method can be used to determine the hydrophilicity of any type of surface.
For example, the method can be used to determine the hydrophilicity of at
least a
portion of the surface of an ion transport measuring chip. In this case, a
drop of water
or aqueous solution is dispensed on the surface of a biochip comprising at
least one
ion transport measuring means, preferably a biochip that has been chemically
treated
to improve its electrical sealing properties. Controls can be performed
simultaneously
with the hydrophilicity test, or can be performed at another time. Preferably,
a range
of controls are performed on surfaces of known hydrophilicity to provide a
hydrophilicity scale. Evaporation of the drop is monitored, and the time
elapsed
between the time the drop contacts the chip and the time it has totally
evaporated is
measured. Preferably, the evaporation time of the test drop is compared with
the
evaporation times of the one or more controls, which can be expressed as a
scale. The
elapsed time is used as an index for hydrophilicity. This index can be used to
determine whether a chemically treated chip is within the optimal range for
achieving
high resistance electrical seals.
Evaporation can be monitored by diffraction, reflectance, or interference at
the
surface where the drop is deposited, or simply by visual observation.
Evaporation can
also be monitored by measuring the change in intensity or other physical or
chemical
properties of a dye or tracer agent that has been used to color or label the
solution.
The method is not limited to testing of biochips, but can be used to measure
the hydrophilicity of a surface used for any purpose. The invention uses the
evaporation time of a liquid drop on a solid surface as a measure of the
solid/liquid
surface energy. The method has very low cost (an accurate liquid dispenser is
the only
equipment needed). It is also very fast and accurate for low surface energy
systems.
Using the drop evaporation technique, we have demonstrated that the
evaporation time of a 0.25 microliter water drop is 2.5 times shorter for a
highly
hydrophilic glass surface (treated with base) compared to chemically untreated
glass.
86
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
VI. METHODS OF MANUFACTURING CHIPS FOR ION TRANSPORT
MEASUREMENT DEVICES
Yet another aspect of the present invention is a method of making a chip for
ion transport measurement devices by fabricating a chip that comprises
multiple rows
of ion transport measuring holes and subsequently breaking the chip into
discrete
segments that comprise a subset of the total number of ion transport measuring
holes.
In this method, a glass sheet is pre-processed with a laser to create patch
clamp recording apertures, and preferably treated chemically to improve
sealability as
described in this application. The glass sheet has also been pre-scored with a
laser to
produce mark lines by which sets of holes can be separated from one another.
Preferably, the mark lines are continuous slashes that go through the glass to
a depth
of about 30% or more of the thickness of the sheet.
In some preferred embodiments, an injection molded mufti-unit well plate is
bonded to the glass with adhesives so that each well of the plate is in
register with one
of the ion transport recording holes. Sections of the mufti-unit welled sheet
sheet
comprising a portion of the mufti-unit well plate and a portion of the glass
chip can be
separated later by two metal plates closing in from two sides of the scored
mark lines
against the glass sheet, followed by bending of the bonded mufti-well devices
along
with the metal plates and pulling of the segments away from each other. The
severed
sections can comprise one or more ion transport measuring units. Figure 15
shows a
glass chip (151) having ion transport measuring holes (152) and mark lines
(153)
created by a laser. The chip is attached to a multiwell plate that to form a
multiunit
sheet (154). Sections (155) that can comprise one or more ion transport
measuring
holes (152) can be detached from the sheet (154).
This approach allows for low cost, automated assembly of single well or low-
density arrays, such as 16-well planar patch clamp consumables. This method of
manufacture improves automation, and reduces individual unit assembly time.
VII. HIGH DENSITY ION TRANSPORT MEASUREMENT CHIPS
Another aspect of the present invention is a high density, high throughput
chip
for ion transport measurement. A high density chip for ion transport
measurement
87
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
comprises multiple ion transport measuring holes. The invention also
encompasses
methods of making high-density consumable patch clamp arrays for ultra high
throughput screening of ion transport function.
A high density chip for ion transport measurement comprises at least 24 ion
transport measuring holes, preferably at least 48 ion transport measuring
holes, and
more preferably, at least 96 ion transport measuring holes. A high density,
high
throughput chip for ion transport measurement of the present invention can
comprise
at least 384 ion transport measuring holes, or at least 1536 ion transport
measuring
holes.
A high density ion transport measuring chip can be made using a silicon,
glass, or silicon-on-insulator (SOI) wafer. The wafer is first wet-etched to
create wells
on the top surface, and then laser drilling is used to form the through-holes.
The
dimensions of the wafer and the wells can vary, however, in preferred
embodiments
in which a 1536 well array is fabricated, the thickness of the wafer can range
from
about 0.1 micron to 10 millimeters, preferably from about 0.5 micron to 2
millimeters,
depending on the substrate.
For wafers in the range of 1 millimeter thick, the etching tolerance should be
within 2% if the through-holes are approximately 30 microns in depth. This
applies to
silicon wafers etched with alkaline solutions such as KOH or glass wafers
etched with
buffered HF. With SOI wafers, a defined thickness of SiOa covers the Si
wafers, and
etching of the wells through the Si side with KOH will stop at the Si02
interface. This
way the thickness of the remaining material is consistent across the whole
wafer, and
even consistent among different batches of etched wafers. This permits laser
drilling
on these etched substrates to be more standardized, and reduces the time
needed for
laser measurement. In a preferred embodiment, the etched Si wells have a
volume of
approximately 2 microliters, assuming a footprint of approximately 2
millimeters x 2
millimeters for each well that extends as a prism or inverted pyramid shape
through
the Si substrate during anisotropic etching, leaving a distance of
approximately 1
millimeter between adjacent wells.
In one design, the bottom of the chip can be sealed against a single common
reservoir for measuring solution that is connected to a common reference
electrode,
while individual recording electrodes can be connected at the upper surface
directly or
via electrolyte bridges.
88
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Alternatively, a structure with 1536 or any preferred number of individual
isolated chambers can be sealed against the bottom of a 1536-well (or any
preferred
number of well) plate so that each chamber is in register with a well. In some
designs
of this embodiment, the top surface of the SOI wafer can be a common
electrode, with
the conductivity of Si material being adequate to provide electrical
connection;
however, additional metal coating on the top surface (applied before etching
as mask
layer) can increase conductivity of the upper surface. Wet etching that
creates the
wells removes this metal coating from the wells themselves. Chemical treatment
with
acid and/or base can optionally be performed on the chip for improved sealing.
Another way to make a high density chip is to use very thin wafers made of
glass, SiO2, quartz, Si, PDMS, plastics, polymers, or other materials, or a
thin sheet,
with thickness between about 1 micron and about 1 millimeter. Laser drilling
can be
performed on such sheets to create through-holes. A separate, "well plate"
with 1536
or any preferred number of wells, manufactured by molding, etching, micro-
machining or other processes, is then sealed against the holes via gluing or
by using
other bonding methods.
The laser drilling of the holes can be from the front or back side of the
chip.For high density ion transport measuring chips, either a "standard" or
inverted
drilling configuration can be used as described herein.
Figure 16 shows a high density array made on a Si, glass, or SOI wafer (161).
It is made with a wet etch process, which creates the wells (162) on the top
surface,
followed by laser drilling through the remaining of the material on the bottom
of each
of the wells. Figure 17 shows the high density array having upper chambers
(171) that
can be formed by a well plate (172) attached to the chip (173). Wells (174) in
the chip
(173) having laser drilled through-holes can be oriented in inverted (top
alternative) or
standard (bottom alternative) orientation.
89
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
VIII. METHODS FOR ASSEMBLING ION TRANSPORT MEASUREMENT
CARTRIDGES
Use ofAdlzesives
A preferred embodiment of the present invention is an ion transport
measurement device cartridge comprising one or more upper chamber pieces
bonded
via adhesive or other means to one or more ion transport measurement chips
that have
been treated to have enhanced electrical sealing properties in which the chip
or chips
contain at least one microfabricated ion transport measurement aperture
(hole),
optionally but preferably drilled by a laser. The one or more ion transport
measurement chips are optionally laser polished on the side of the small exit
hole, and
treated with a combination of acid and base treatment as described herein.
The present invention also includes a method of assembling ion transport
measurement cartridges by bonding the ion transport measurement chips) with an
upper chamber piece. In one embodiment, an ion transport measurement chip
containing one or more ion transport measuring apertures is bonded to an upper
chamber piece via a UV-activated adhesive, such that each well of the upper
chamber
piece is in register with a recording aperture on the ion transport
measurement chip,
and the smaller, exit holes from laser drilling of the ion transport measuring
holes are
exposed to the wells of the upper chamber piece.
To facilitate efficient assembly, a registration bump can preferably be molded
on the bottom of the upper chamber piece so that when the biochip is pressed
against
the bump and shoulder at the bottom of the upper chamber piece, the recording
apertures on the ion channel measurement chip are in register with the wells
of the
upper chamber piece. An example of an upper chamber piece having alignment
bumps (2) is shown in Figure 1B.
Preferred UV adhesive include, but are not limited to, LTV-epoxy, UV-acrylic,
UV-silicone, and UV-PDMS.
The UV dose required to completely cure the UV adhesive can at times
inactivate the treated surface of the chip. To avoid UV radiation to chip
surface areas
near the recording apertures where seals are to occur, a mask made of UV-
permeate
glass on which spots of size between 0.5 to 5 mm are provided by depositing a
thin
metal layer or paint (preferably a dark or black) layer.
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Pr~essuf-e Mouyzting
As an alternative to glue-based bonding, the upper chamber piece can be
designed to allow an O-ring type of gasket made with PDMS to be used as seal
cushion between the upper chamber piece and a biochip during a sandwich-type
pressure mounting procedure. Figure 18 depicts the general format for pressure
bonding, in which a chip (183) is attached to an upper chamber piece (181)
using a
gasket (184) to form a seal between the upper chamber piece (181) and chip
(183)
when pressure (arrow) is applied. In this highly schematized depiction, a
lower
chamber piece (185) is also attached to the chip (183) using a second gasket
(186) to
form a seal between the lower chamber piece (185) and chip (183) when pressure
(arrow) is applied. Mechanical pressure can be provided by a weight or clamp,
or by
any other means, including fasteners or holders.
IX. BIOCHIP DEVICE FOR ION TRANSPORT MEASUREMENT COMPRISING
FLUIDIC CHANNEL CHAMBERS
A further aspect of the present invention is a flow-through fluidic channel
ion
transport measuring device that can be part of a fully automated ion transport
measuring device and apparatus. This device comprises a planax chip that
comprises
ion transport measuring holes, and upper and lower chambers on either side of
the
chip that are fluidic channels. One or more fluidic channels is positioned
above the
chip and one or more fluid channels is positioned below the chip. Apertures
are
positioned in the fluidic channels such that an ion transport measuring hole
in the chip
has access to an upper fluidic channel (serving as an upper chamber) and a
lower
fluidic channel (serving as a lower chamber).
A chip of a fluidic channel ion transport measuring device can have multiple
ion transport measuring holes, and each of the holes can be in fluid
communication
with an upper fluidic channel and a lower fluidic channel. The upper fluidic
channel
or channels can be connected with one another, and more than one lower fluidic
channel can be independent; or the device can have two or more upper fluidic
channels that can be independent while the one or more lower fluidic channels
can be
connected with one another. In a yet another alternative, upper fluidic
channels that
service different ion transport measuring holes can be separate from one
another and
91
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
the lower fluidic channels that service different ion transport measuring
holes can also
be separate from one another.
Figure 19, depicts a schematic view of one possible design of a planar patch
clamping chip (193) having an upper fluid channel (191) for extracellular
solution
(ES) and a lower fluidic channel (195) for intracellular solutions (ISl, IS2).
The upper
and lower channels are interfaced at a point where the recording aperture
(192) of the
planar electrode resides. Separate fluidic pumps (P) drive the flow of fluids
through
the two (upper and lower) fluidic channels. Recording (196) and reference
electrodes
(197) external to the fluidic patch clamp chip are connected via an
electrolyte solution
bridge to the upper (191) and lower (195) fluidic channels. A pressure source
such as
a pump with pressure controller that can generate both positive and negative
pressures
is shown linked to the lower fluidic channels. A mufti-way valve (194) can be
used to
connect the lower fluidic channel (195) to different solution reservoirs (ISl,
IS2, etc),
and a mufti-way valve (198) can be used to connect the upper fluidic channel
(191)'~to
cell reservoirs, a compound plate (CP), wash buffers, or other solutions.
In some preferred aspects, the device can have a molded upper piece that
comprises one or more upper channels, and a molded lower piece that comprises
one
or more lower channels. The channels can be drilled through or molded into the
pieces, which preferably comprises at least one plastic. A chip comprising one
or
preferably, multiple ion transport measuring holes can be situated between the
upper
piece and the lower piece, such that an ion transport measuring hole through
the chip
connects an upper channel of the upper piece with a lower channel of the lower
piece.
In some preferred embodiments of these aspects, an upper conduit connects to
a well that is in register with a hole of the chip. In addition to being
accessed by the
conduit, the well can be open at the top, for the addition of, for example,
cell
suspensions or compounds. Preferably, these preferred embodiments, the chip
comprises multiple holes and the upper piece comprises multiple wells in
register with
the holes of the chip. Preferably, each well is accessed by a separated and
independent
channel. The lower piece can comprise one or more lower channels. Preferably,
in
these embodiments, the lower piece comprises at least one channel, and each of
the at
least one channel accesses two or more ion transport measuring holes in the
biochip.
The at least one lower channel can comprise or be in electrical contact with
an
electrode, such as, for example, a reference electrode. Upper chamber
electrodes can
92
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
be dunked into well from above, inserted into the upper channels, or otherwise
brought into electrical contact with the upper wells.
Designs comprising upper chamber fluidic channels, lower chamber fluidic
channels, or both upper and lower chamber fluidic channels have several
advantages.
The external electrodes can be of multiple use, but replaceable. This reduces
the cost
of the biochip. The flow-through fluidics of both the upper and lower chambers
minimizes the generation of air bubbles. Importantly, the closed fluidic
channels
allow for controlled delivery of low volume fluids without evaporation.
X. METHODS OF PREPARING CELLS FOR ION TR.ANPORT MEASUREMENT
In a further aspect of the present invention, methods for isolating attached
cells for planar patch clamp electrophysiology are provided. Conventional cell
isolation methods by non-enzymatic, trypsin, or reagent-based methods will not
produce cells that are in optimal condition for high throughput
electrophysiology.
Typically cells produced by available protocols are either over-digested and
tend to
function less than optimally in planar patch clamp studies, or under-digested
and
resulting in cell clumps with the cell suspension. In addition, the cells
isolated by
conventional methods tend to have large amounts of debris which are a major
source
of contamination at the recording aperture. The current protocols are
optimized for
better cell health, single cell suspension, less debris and good patch clamp
performance. The current protocols can be used to isolate cells for any
purpose,
particularly when cells in an optimal state of health and integrity are
desirable,
including purposes that are not related to electrophysiology studies.
This invention was developed to produce suspension CHO and HEK cells that
give high quality patch clamp recording when used with chips and devices of
the
present invention. Parameters such as cell health, seal rate, Rm (membrane
resistance), Ra (access resistance), stable whole cell access, and current
density, were
among the parameters optimized. The method includes: providing a population of
attached cells, releasing the attached cells using a divalent cation solution,
an enzyme-
containing solution, or a combination thereof; washing the cells with a
buffered cell-
compatible salt solution; and filtering the cells to produce suspension cells
that give
high quality patch clamp recordings using ion transport measuring chips.
93
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Enzyme free Cell Preparation
Enzyme-free dissociation is desirable when an ion transport expressed on a
cell surface can be digested by enzymatic methods, thereby causing a change in
ion
transport properties. Enzyme-free methods involve a dissociation buffer that
is either
Cap-chelator-based or non- Cap-chelator-based. The former is typically a
solution of
EDTA, while the latter can be calcium-free PBS. In such methods, attached
cells
grown on plates are first washed with calcium-free PBS, and then incubated
with the
dissociation buffer. In case of the calcium chelator-based dissociation, the
dissociated
cells must be washed at least once with a chelator-free solution before they
can be
used for ion transport measurement assays. The suspended cells are then passed
through a filter, such as a filter having a pore size of from about 15 to 30
microns (this
can vary depending on the type of cells and their average size).
Preparation of Cells using Efazynae
In some methods (see Example 6), trypsin is used to dissociate attached cells.
In such methods, the cells are typically rinsed with a solution devoid of
divalent
cations, and then briefly treated with trypsin. The trypsin digestion is
stopped with a
quench medium carefully designed to achieve the optimal divalent cation mix
and
concentration. In the methods provided herein, the suspended cells are then
passed
through a filter, such as a filter having a pore size of from about 15 to 30
microns (this
can vary depending on the type of cells and their average size).
Another enzyme-based method uses a preparation commercially available
from Innovative Cell Technologies (San Diego). Accumax is an enzyme mix
containing protease, collagenase, and DNAse. Example 6 provides a protocol for
CHO cells using Accumax and filtration.
Some preferred methods of the present invention use a combination of
enzyme-free dissociation buffer, Accumax reagent, and filtration to isolate
high
quality cells for patch clamping (see Example 6).
94
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
XI. PRESSURE CONTROL PROFILE PROTOCOL FOR ION TRANSPORT
MEASUREMENT
The present invention also provides a pressure protocol control program logic
that can be used by an apparatus for ion transport measurement to achieve a
high-
s resistance electrical seal between a cell or particle and an ion transport
measuring
means on a chip of the present invention in a fully automated fashion. In this
aspect,
the program interfaces with a machine that can receive input from an apparatus
and
direct the apparatus to perform certain functions.
Typically it has required months to years of experience on the part of an
experimenter to master the techniques required to achieve and maintain high
quality
seals during their experiments. It is an object of the invention to produce a
pressure
protocol for achieving and maintaining seal quality parameters for automated
patch
clamp systems. The present invention provides a logic that can direct
mechanical and
automated patch clamp sealing of particles and membranes.
The program logic includes: a protocol for providing feedback control of
pressure applied to an ion transport measuring means of an ion transport
measuring
apparatus, comprising: steps that direct the production of positive pressure;
steps that
direct the production of negative pressure; steps that direct the sensing of
pressure;
and steps that direct the application of negative pressure in response to
sensed
pressure in the form of multiple mufti-layer if then and loop logic, in which
the
positive and negative pressure produced is generated through tubing that is in
fluid
communication with an ion transport measuring means of an apparatus, and in
which
negative pressure is sensed through tubing that is in fluid communication with
an ion
transport measuring means of an apparatus. Preferably, these steps are
performed in a
defined order that depends on the feedback the apparatus receives. Thus, the
order of
steps of the protocol can vary according to a defined script depending on
whether a
seal between a particle and the ion transport measuring means is achieved
during the
operation of the program, and the properties of the seal achieved.
An apparatus for ion transport measurement that is controlled at least in part
by the pressure program preferably comprises: at least one ion transport
measurement
device comprising two or more ion transport units (each comprising at least a
portion
of a biochip that has an ion transport measuring means, at least a portion of
an upper
chamber, and at least a portion of a lower chamber, and is in electrical
contact with at
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
least one recording electrode and at least one reference electrode), tubing
that
connects to the device and is in fluid communication with the two or more ion
transport measuring means of an apparatus, and pumps or other means for
producing
pressure through the tubing. Preferably, the apparatus is fully automated, and
comprises means for delivering cells to upper chambers (such means can
comprise
tubing, syringe-type injection pumps, fluid transfer devices such as one or
more
automated fluid dispensors) and means for delivering solutions to lower
chambers
(such means can comprise tubing, syringe-type injection pumps).
Preferably, in addition to promoting and maintaining a high resistance seal,
the
pressure protocol program can also direct the rupture of a cell or membrane
delineated
particle that is sealed to an ion transport measuring means. Such rupture can
be by the
application of pressure after sealing, and can be used to achieve whole cell
access.
In operation, the program directs the apparatus to generate a positive
pressure
in the range of 50 torr to 2000 torn preferably between 500 and 1000 torr, to
purge
any blockage of the recording holes. Then the program directs the apparatus to
generate a positive holding pressure between 0.1 to 50 torn, preferably
between 1 to
torr to keep the recording aperture of an ion transport measuring chip clear
of
debris during the addition of cells to the upper chamber. After cell addition,
the
20 program directs the release of pressure and holds the pressure at null long
enough to
allow cells to approximate the aperture. The program then directs a negative
pressure
to be applied draw a cell onto (and partly into) the ion transport recording
aperture for
landing and the formation of a gigaohm seal. Additional pressure steps as
described
Example 7 may be required for achieving gigaohm seals if a seal does not occur
upon
cell landing.
To achieve whole-cell access, negative pressure is increased in progressive
steps until the electrical parameters indicate the achievement of whole-cell
access.
Alternatively, the program can direct the application of a negative pressure
to a
"sealed" cell that is insufficient to gain whole-cell access, and then use a
electric
"zap" method to disrupt the membrane patch within the aperture and thereby
achieve
whole-cell access. Upon achieving whole-cell access the pressure is either
released
immediately, or held for a few seconds then released, depending on the cell
quality.
Finally, during whole-cell access procedures, the seal quality could be
improved after
96
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
access is achieved, then held at optimal parameters by a more complex pressure
protocol.
The pressure protocol involves many branchpoints or "decisions" based upon
feedback from the seal parameters. It is easiest to describe the protocol as a
series of
steps in programming logic, or program. A pseudocode example of such logic is
provided as Example 7.
The program, also herein referred to as program logic, control logic or
programming logic, can be illustrated and described in different manners. The
procedures and processes described in this program herein are one possible
embodiment of the program. Decision branches, loops, and other components can
be
performed in substantially different methods to obtain the same or
substantially
similar results, such as the use of an "if then" loop in place of a "while"
loop. The
exemplary pseudocode and program description contained herein is not intended
to be
limiting, merely they are examples of one possible embodiment of encoding this
program. One skilled in the art will realize that the procedures and processes
of this
program can be accomplished in a number of programming and encoding methods,
on
devices such as personal computers, chipsets, mainframe computers, and other
electronic devices capable of performing and executing programmed code.
Additionally, the steps described herein may be executed and performed in
other step-
wise processes to achieve the same or substantially similar results.
The procedures and descriptions of this program are described and illustrated
across several pages. Some procedures are illustrated across several figures.
This is
not intended to limit the varied calculations and functions of these
procedures to sub-
routines separated from the rest of the procedure, instead it is a result of
space
limitations in the drawing of the figures. Certain aspects illustrated across
several
figures are intended to be connected seamlessly, and operate together as one
procedure or subroutine. Off page and on-page connectors are utilized to
illustrate
this continuity, and are not intended to confine the execution of certain code
to
specific areas of the illustrated figures. These illustrative connectors are
additionally
not intended to be additional steps in the execution of the program disclosed
herein.
The program disclosed herein can be run and executed on a variety of systems.
The program can be run on a device such as SealChipTM from Aviva Biosciences
97
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Corporation, the PatchXpressTM from Axon Instruments, or any other electronic
patch-clamp system, as described in this present application or known in the
art.
Additionally, the present invention can be executed in a computer-based
manner. The computer-based manner of the present invention includes computer
hardware and software. The computer-based program can run on a personal
computer
of the traditional type, including a motherboard. The motherboard contains a
central
processing unit (CPU), a basic inputloutput system (BIOS), one or more RAM
memory devices and ROM memory devices, mass storage interfaces which connect
to
magnetic or optical storage devices including hard disk storage and one or
more
floppy drives, and may include serial ports, parallel ports, and USB ports,
and
expansion slots. The computer is operatively connected by wires to a display
monitor, a printer, a keyboard, and a mouse, though a variety of connection
means
and input and output devices may be substituted without departing from the
invention.
Additionally, the present invention can be encoded on a chipset, or be encoded
on
computer-like components included in other devices.
A computer used in connection with the computer program may run an IBM-
compatible personal computer, running a variety of operating systems including
MS-
DOS~, Microsoft~ Windows, or Linux~. Alternatively, the computer program
may run on other computer environments, including mainframe systems such as
UNIX~ and VMS~, or the Apple~ personal computer environment, portable
computers such as palmtops, programmable controllers, or any other digital
signal
processors.
All of these elements and the manner in which they are connected are well-
known in the art. In addition, one skilled in the art will recognize that
these elements
need not be connected in a single unit such as personal computer or mainframe,
but
may be connected over a network or via telecommunications links. The computer
hardware described above may operate as a stand-alone system, or may be part
of a
local area network, or may comprise a series of terminals connected to a
central
system. Additionally, some or all aspects of the logic of the present
invention can be
encoded to run on a chipset or other electronic hardware. Additionally, the
entire
program may comprise a portion of a larger program wherein this section is
called as
part of the normal execution of the larger program, and all references to
stopping or
ending execution in this case refer to returning from this section of the
program to the
calling routine.
98
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
An overview of the program is disclosed in Figure 26. The program
comprises 4 separate procedures: Procedure Landing (2610), Procedure FormSeal
(2615), Procedure BreakIn (2620), and Procedure RaControl (2625). The program
starts (at step 2605) by being called from a separate controlling software or
as a result
of a user-initiated action. The program first runs the Procedure Landing
(2610) to
place a cell onto (and partly into) the ion transport recording aperture. When
Procedure Landing (2610) has ended, the program runs Procedure FormSeal (2615)
to form a gigaohm seal. Next the program calls Procedure BreakIn (2620) to
achieve
whole-cell access. The program then runs Procedure RaControl (2625). When
completed, the control logic continues to step 2630 and ends. After the
execution
stops, a separate program will handle the application of voltage clamp
protocols and
the acquisition of data pertaining to ion channel activity. An unillustrated
alternate
mode of execution for this program will skip directly to Procedure RaControl
(2625)
to handle cells that have already been accessed but whose access resistance
has
increased beyond RaIdeal. This provides an opportunity to improve the quality
of
recordings in the middle of an experiment. Once a procedure called or run by
the
program ends, the program returns to run or execute the next procedure
illustrated by
Figure 26. The individual procedures are described below.
With reference to Figures 27, 28, and 29, Procedure Landing is now described.
At step 2610, the program begins Procedure Landing. The start of Procedure
Landing
is identified by step 2705. All of the counters and variables used in the
program are
assigned and are reset (2710), then the variable KeyPress, which traps user
input
instructions, is set to null (2715). The program displays (2720), through a
screen or
other similar display device, the message "Attempting Landing" to indicate the
progress of the control logic. Next, the program runs a Washer (2725), a pump-
driven
fluid delivery system, to rinse fluidics channels, which purges any blockage
of the
recording holes and clears any particles that may be present in the chambers
before
they have an opportunity to block the recording hole. The program waits 5
seconds
(2730) while Washer is run, then the program stops the Washer (2735). The
program
then applies -300 tort of pressure (2740) to clear away any left-over bubbles,
waits 0.5
seconds (2745), then applies 0 tort of pressure (2750). The control logic then
waits 2
seconds (2755) for the measurements to stabilize. At step 2760, the program
checks
to see if the variable Repeat is equal to 1. If Repeat is not equal to 1, the
program
adds 1 to the value for Repeat (2765), and returns to step 2740. If at step
2760 the
99
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
value of Repeat is l, the control logic continues to step 2810 of Procedure
Landing (as
illustrated by off page connector 2770 pointing to its matching off page
connector
2805).
With reference to Figure 28, Procedure Landing continues. The program next
nulls the junction potential (2810), waits for a stable reading (2815), then
records the
average Re (2820), and saves the Re to logs in a file stored on the computer
(2825).
Next the program requests cells (2830)from a separate program or routine not
listed
here, and waits until 0.5 seconds before cells would be introduced to the
recording
chamber (2835). The program then applies + 10 tort of pressure (2840) to keep
the
holes cleared during cell delivery, and then waits until the pipette has
completed the
cell delivery and is removed after adding cells (2845). The program then
applies 0
tort (the units of tort and mmHg are interchangeable terms) of pressure
(2850), waits
3 seconds (2855) to enable the cells to settle closer to the recording
aperture. The
program then starts a timer for Elapsed (2860), then applies -50 tort of
pressure
(2865) to attract a cell to the aperture. The control program then resets the
Repeat
variable to 0 (2870), and continues to step 2910 of Procedure Landing (as
illustrated
by off page connector 2875 pointing to off page connector 2905).
With reference to Figure 29, Procedure Landing continues. The program then
checks at step 2910 to see whether the Seal is greater than 2 x Re for 0.5
seconds, or
whether Elapsed time is greater than or equal to 5 seconds. If Elapsed time is
greater
than or equal to 5 seconds, the program then adds 1 to the value of stored
variable
Repeat (2915), then checks whether Repeat is equal to 3 (2920). If Repeat is
not
equal to 3, the program continues to step 2925 and applies +50 tort of
pressure. The
program waits 1 second (2930), then applies -50 tort of pressure (2935), then
returns
to step 2910. If at step 2920, the program determines that Repeat is equal to
3, the
program continues to step 2940. The program aborts, records "failure to land"
in its
log, then ends the execution of the program (2945). At this point the chamber
should
be clean and prepared for removal.
If at step 2910 the program determines that Seal is greater than 2 x Re, the
program displays the message "Landing Detected" (2950), resets the value for
Elapsed (2955), and ends Procedure Landing at step 2960. As illustrated by the
program overview of Figure 26, once Procedure Landing is run, the program next
continues to step 2615 and runs Procedure FormSeal.
100
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Procedure FormSeal is illustrated by Figures 30, 31, 32, and 33. The program
calls Procedure FormSeal at step 2615. The start of Procedure FormSeal is
illustrated
by step 3005. The program resets KeyPress to null, and the timer to 0:00
(3010). As
used throughout this program, when the variable Timer or Elapsed is reset, it
immediately starts counting time in seconds. The program then displays the
message
"Attempting Seal" on an output device (3015). The program then applies a
negative
holding potential to the electrode immediately after landing by applying HP = -
80 mV
(3020). The program then applies -50 tort pressure (3025). At step 3030, the
program checks whether the seal between the cell and the recording aperture
presents
greater than or equal to 1 one gigaOhm (a "gigaseal") of resistance across the
recoding aperture. If the seal is greater than or equal to 1 gigaOhm, the
program
proceeds to step 3310 of Procedure FormSeal (as illustrated by off page
connector
3035 pointing to off page connector 3305). If at step 3030 the program
determines
that the seal is not greater than or equal to 1 gigaOhm, the program checks if
the seal
is increasing greater than 20 megaOhms per second (3040). If the seal is
increasing
greater than 20 megaOhms per second, the program continues to step 3045. If at
step
3040 the program determines that the seal is not increasing greater than 20
megaOhms per second, then the program continues to step 3050. At step 3045,
the
program checks whether the timer has reached 10 seconds. If it has not, the
program
returns to step 3030. If at step 3045 the program determines that the timer is
greater
than 10 seconds, the program continues to step 3050.
At step 3050 the program resets the timer to 0:00, and checks whether the
pressure is equal to -50 tort (3055). If pressure is -50 tort, the program
applies 0 tort
of pressure (3060), waits 2 seconds (3065), and returns to step 3030. If at
step 3055
the program determines that pressure is not equal to -50 torn, the program
continues
with Procedure FormSeal (as illustrated by off page connector 3070 pointing to
off
page connector 3105). This section of the program ensures that a landing
happens,
and tests whether simple pressure steps are capable of producing a gigaOhm
seal.
With reference to Figure 31, Procedure FormSeal continues by displaying the
status message "Ramping Pressure" (3110). The program then optimally assigns a
set
of values for variables to initially be used during the pressure ramp (3115).
Min is set
to 0 tort, Max is set to -50 tort, Duration is set to 20 seconds, Counter is
set to 0, and
Timer is set to 0:00. The program then executes a pressure ramp loop. Starting
with
step 3120, the program ramps the pressure from Min to Max over the Duration,
using
101
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
the assigned values for these variables. The program then checks to see if
seal is
greater than 1 gigaOhm, or if "whole-cell access" has been achieved (3125).
Whole-
cell test is where capacitance is greater than 3.5 pF. If either of the
conditions at step
3125 are true, the program continues with Procedure FormSeal at step 3310 (as
illustrated by off page connector 3130 pointing to off page connector 3305).
If at step 3125 both of the conditions are false, the program moves to step
3135, where it checks whether Timer is greater than 20 seconds. If Timer is
greater
than 20 seconds, the program modifies the set of values for the variables used
during
the pressure ramp (3140). Min is reduced by 20 tort, Max is decreased by 30
tort,
Duration is increased by 10 seconds, Counter is incremented by 1, and Timer is
set to
equal 0:00. The program checks whether Counter is greater than 4 (3145). If
Counter
is greater than 4, Procedure FormSeal continues to step 3210 (as illustrated
by off
page connector 3170 pointing to off page connector 3205). If Counter is less
than 4,
the program applies 0 tort of pressure (3150), waits 5 seconds (3155), then
returns to
the beginning of the pressure ramp loop that begins at step 3120.
If at step 3135 the program determines that Timer is not greater than 20
seconds, the program checks whether a user input key has been pressed (3160).
If a
key has been pressed, Procedure FormSeal continues with step 3205 (as
illustrated by
off page connector 3170 pointing to off page connector 3205). If at step 3160
a key
has not been pressed, the program returns to the beginning of the pressure
tamping
loop that begins at step 3120.
With reference to Figure 32, Procedure FormSeal continues. At step 3210, 0
tort of pressure is applied. The program then resets the value to null whether
a key
has been pressed by the user (3215). The program then displays "Not sealed-
Retry,
Skip, Abort?" (3220). The program waits for the user to input whether to retry
Procedure FormSeal, skip Procedure FormSeal, or abort the program altogether
(3225). The program checks for input by the user. If the user enters "Retry"
(3230),
the program returns to step 3110 of Procedure FormSeal (as illustrated by off
page
connector 3235 pointing to off page connector 3105) to rerun the pressure ramp
loop
from its start. If the user inputs "Skip" (3240), the Procedure FormSeal ends
(step
3245). Once Procedure FormSeal has run, as illustrated by the program overview
of
Figure 25, the program next continues to step 2620 and runs Procedure Breakln.
If
the user enters "Abort" (3250), the program stops executing and ends (3255).
If no
102
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
input has been received by step 3250, the program return to continue the input
loop
(as illustrated by connector 3260 pointing to connector 3265.
As illustrated by Figure 33, Procedure FormSeal continues with step 3310 and
displays the message "Sealed." The program applies 0 tort pressure (3315),
saves
Elapsed time as time to seal in the logs (3320). The program then resets the
values for
Min, Max, Counter, KeyPress, and duration to null (3325). The program monitors
the
stability of the seal (3330), and continues once the seal is stable. If
capacitance is not
greater than 3.5 pF ("whole-cell") (3335), Procedure FormSeal ends (3340), and
as
illustrated by the program overview of Figure 26, the program next continues
to step
2620 and runs Procedure BreakIn. If at step 3335 the program determines that
capacitance is greater than 3.5 pF, the program displays "Premature Access"
(3345),
then writes this feature to the logs (3350) and Procedure FormSeal ends
(3355). The
program next continues to step 2620 and runs Procedure Breakln.
With reference to Figures 34, 35, 36, and 37, Procedure BreakIn is now
described. The program runs Procedure BreakIn at step 2620. Procedure Breakln
starts, as illustrated by Figure 34, at step 3405. The program resets the
value for
KeyPress to null (3410), then applies holding potential that is appropriate
for the
assay (3415). The program displays "Attempting access" (3420), then verifies
whether whole-cell access has already been achieved (3425). If whole-cell has
been
achieved, Procedure Breakln continues to step 3610 (as illustrated by off page
connector 3430 pointing to off page connector 3605). If whole-cell has not
been
achieved at step 3425, the program nulls the chamber electrode capacitance
(3435).
The program then sets values for several variables (3440). Min is set to 0
tort, Max is
set to -300 tort, Delta is set to -20 tort, Duration is set to 1 second, and
Timer is set to
0:00. The program sets the value for Pressure to Min (3445), and then applies
force
equal to Pressure in the lower chamber (3450).
Procedure BreakIn continues at step 3510 as illustrated by Figure 35, and as
indicated by the illustrated off page connector 3455 pointing to 3505. The
program
checks whether Seal is less than 200 megaOhms (3510). If yes, the program
displays
the message "Cell Lost" (3580), then stops execution of the program (3585). If
at step
3510 the seal is not less than 200 megaOhms, the program checks if capacitance
is
greater than 3.5 pF (3515). If yes, Procedure Breakln continues to step 3610
(as
illustrated by off page connector 3520 pointing to off page connector 3605).
If
capacitance at step 3515 is not greater than 3.5 pF, the program checks
whether
103
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Pressure is greater than Max (3525). If yes, Procedure BreakIn continues to
step 3445
(as illustrated by off page connector 3530 pointing to off page connector
3460). If
Pressure at step 3525 is not greater than Max, the program checks whether
KeyPress
has a value (3535). If yes, Procedure BreakIn continues to step 3710 (as
illustrated by
off page connector 3540 pointing to off page connector 3705). If no KeyPress
value
is found at step 3535, the program checks whether Seal is decreasing by
greater than
200 megaOhms per second (3545). If yes, Procedure BreakIn continues to step
3445
(as illustrated by off page connector 3590 pointing to off page connector
3460). If at
step 3545 Seal is not decreasing by greater than 200 megaOhms per second, the
program checks whether Timer is greater than Duration (3550). If no, Procedure
BreakIn goes to step 3510 (as illustrated by connector 3555 pointing to
connector
3560). If at step 3550 Timer is greater than Duration, the program resets
Timer to
0:00 (3565), then the program increments Pressure by Delta (3570). The
Procedure
then returns to step 3510 (as illustrated by connector 3575 pointing to
connector
3560).
Procedure BreakIn continues as illustrated by Figure 36. The program checks
whether capacitance is greater than 3.5 pF for 1 second (3610). If no,
Procedure
BreakIn continues to step 3445 (as illustrated by off page connector 3615
pointing to
off page connector 3460) to restart the pressure steps. If at step 3610,
capacitance is
greater than 3.5 pF for 1 second, the program records Break-in pressure to the
log file
(3620), and applies 0 tort of pressure (3625). The program then resets Elapsed
to
0:00, then sets Elapsed to Global (3630). The whole cell access duration is
set to the
be a global variable. The program then displays the message "Whole-cell access
detected" (3635), writes the time of access to the log (3640) and then
Procedure
Breakln ends at step 3645. As illustrated by the program overview of Figure
26, the
program next continues to step 2625 and runs Procedure RaControl.
Procedure Breakln continues as illustrated by Figure 37. At step 3710, the
program resets the value for KeyPress to null. Next, the program displays the
message "Access not detected- Force access detect, Continue, Abort?" (3715) In
step
3717, the program waits for the user to input whether to force access detect,
continue
or abort. The program checks for input by the user. If the users enters "Force
access
detect" (3720), Procedure BreakIn goes to step 3610 (as illustrated by off
page
connector 3725 pointing to off page connector 3605). If the user enters
"Continue"
(3730), Procedure BreakIn goes to step 3510 (illustrated by off page connector
104
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
pointing 3735 pointing to off page connector 3505). If the user enters "Abort"
(3740), the program stops executing (3745). If no input has been received by
step
3740, the program returns to step 3705 and continues the input loop.
Procedure RaControl, as illustrated by Figures 38, 39, and 40, are now
described. The program runs Procedure RaControl from step 2625. Procedure
RaControl starts at step 3810. In step 3815, KeyPress is set to null. Next,
the
program displays the message "Adjusting seal quality" (3820). The program then
assigns RmInitial the value of Rm, and assigns Ralnitial the value of Ra
(3825). The
values for Cm, Rm, and Ra are recorded (3830). The program verifies if Ra is
less
than RaIdeal (3835). RaMax and RaIdeal are values that can be ascribed by the
user
beforehand. If yes, the procedure ends (3840). If Ra is not less than RaIdeal,
then the
program verifies if Ra is less than Ra Max and Ra is decreasing (3845). If
yes, the
program returns to step 3835. If the answer at 3845 is no, the program sets
Elapsed to
0 seconds (3850), then the program verifies if Ra is less than RaMax (3855).
If Ra is
less than RaMax, then Countdown is set to 20 seconds (3860), and Procedure
RaControl continues to step 3910 (as illustrated by off page connector 3865
pointing
to off page connector 3905). If at step 3855 Ra is not less than RaMax,
Procedure
RaControl continues to step 3910 (as illustrated by off page connector 3865
pointing
to off page connector 3905.
Procedure RaControl continues as illustrated by figure 39. At step 3910, the
program checks whether the user has inputted "Continue" or whether Ra is less
than
RaIdeal. If yes, the procedure ends (3915). If the answer at step 3910 is no,
the
program goes to step 3920.
At step 3920, the program verifies if Ra is increasing and Rm is greater than
300 megaOhms. If no, the program continues to step 3945. If at step 3920 Ra is
increasing and Rm is greater than 300 megaOhms, the program applies -50 torr
of
pressure (3925), waits 0.5 seconds (3930), applies 0 torr of pressure (3935),
then
waits 1.5 seconds (3940). The program then continues to step 3945. The program
verifies if Ra is increasing and Rm is greater than 500 megaOhms (3945). If
no, the
program continues to step 3970. If at step 3945 Ra is increasing and Rm is
greater
than 500 megaOhms, the program applies -80 torn pressure (3950), waits 0.5
seconds
(3955), applies 0 torr of pressure (3960), then waits 1.5 seconds (3965). The
program
then goes to step 3970.
105
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
At step 3970, the program checks if Rm is greater than 0.8 gigaOhm. If yes, it
applies -50 tort of pressure (3975). If no, it applies -10 tort pressure
(3980). From
both steps 3975 and 3980, Procedure RaControl continues to step 4006 (as
illustrated
by off page connector 3985 pointing to off page connector 4003.
Procedure RaControl continues as illustrated by Figure 40. The program
checks, at step 4006, if Ra is greater than RaIdeal, if Rm is greater than
(Rmlnitial -
25%), and if countdown is greater than 0. If no, the program continues to step
4084
(as illustrated by connector 4009 pointing to connector 4081). If at step 4006
the
answer is yes, then the program continues to step 4012 and waits 5 seconds.
Then the
program tests whether Ra is less than RaMax (4015). If yes, then the program
sets
Countdown to 20 seconds (4018), and will time down be seconds to zero and
continues to step 4021. If at step 4015 Ra is not less than RalVIax, the
program
continues to step 4021.
At step 4021, the program checks whether Ra is less than RaIdeal. If yes, the
1 S program continues to step 4084 (as illustrated by connector 4024 pointing
to
connector 4081). If at step 4021 Ra is not less than RaIdeal, the program
checks
whether Ra is decreasing (4027). If Ra is decreasing, the program continues to
step
4054. If at step 4027 Ra is not decreasing, the program checks if Rm is not
decreasing and Rm is greater than 1 gigaOhm (4030). If yes, -10 delta tort of
pressure
is applied (4033), and the program continues to step 4036. If at step 4030 the
value is
false, the program continues to step 4036. At step 4036, the program checks
whether
Rm is not decreasing and Rm is less than 1 gigaOhm. If yes, -5 delta tort of
pressure
is applied (4039) and the program continues to step 4042. If at step 4036 the
answer
is no, the program continues to step 4042. At step 4042 the program tests
whether
Rm is decreasing and Pressure is greater than -10 tort. If yes, +5 tort of
pressure is
applied (4045) and the program continues to step 4048. If at step 4042 the
answer is
no, the program continues to step 4048. At step 4048, the program checks
whether
Rm is less than (Rmlnitial - 25%). If yes, 0 tort of pressure is applied
(4051), and the
program continues to step 4054. If at step 4048 the answer is no, the program
continues to step 4054.
The program next checks whether Pressure is greater than BreakInPressure
(4054). If yes, 0 torn of pressure is applied (4057), and the program
continues to step
4060. If at step 4054 Pressure is not greater than BreakInPressure, the
program
continues to step 4060. The program checks whether Elapsed time is greater
than 120
106
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
seconds (4060). If yes, 0 torn of pressure is applied (4063), and Procedure
RaControl
ends (4066). If at step 4060 Elapsed is not greater than 120 seconds, the
program
checks whether Rm is less than 300 megaOhms (4069). If no, the program
continues
to step 4084, as illustrated by connector 4072 pointing to connector 4081. If
at step
4069 Rm is less than 300 megaOhms, pressure equal to (BreakInPressure less 10
tort)
is applied (4075). The program continues to step 4006, as illustrated by
connector
4078 pointing to connector 4099.
At step 4084 the program checks whether Ra is increasing. If yes, -60 tort
pressure is applied (4087) and the program continues to step 3815, as
illustrated by
off page connector 4090 pointing to off page connector 3805. If at step 4084
Ra is
not increasing, 0 tort of pressure is applied (4093), and the program returns
to the
beginning of the loop at step 3910, as illustrated by off page connector 4096
pointing
to off page connector 3905.
Once Procedure RaControl has ended, the program, in an unillustrated step,
records and outputs the data, preferably to a database. These data can be
recorded and
outputted by a variety of means, including electronic storage media (hard disk
or
floppy disk), electronic transfer via a network (such as TCP/IP or Bluetooth),
or
optical storage media. Additionally, in an unillustrated step, the program may
display
the results on an output device, such as a LCD display or computer monitor
screen. In
another unillustrated step, the program may optionally generate a printout of
the
results and other collected data via a printing device such as a laser
printer. The
results gathered by the program may, in an unillustrated step, be collated,
aggregated,
or compared to other previous results, or control results. Depending upon the
needs
and requirements of the user of this present invention, the program can be
configured
to use one or more of the above-referenced output methods. Having completed
these
steps, and having outputted the results and/or data, the program stops
execution
(2630).
107
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
EXAMPLES
Example 1. Device for ion transport measurement comprising upper chamber
piece and biochip.
An ion transport measuring device in the form of a cartridge known as the
SEALCHIPTM (Aviva Biosciences, San Diego, CA) comprising an upper chamber
piece and a chip comprising ion transport measuring holes was manufactured.
Upper chamber pieces with 16 wells having dimensions of 84.8mm(long)
xl4mm(wide) x7mm(high) were injection molded with polycarbonate or modified
polyphenylene oxide (NORYL~) material. The distance between centers of two
adjacent wells was 4.Smm. The well wall was slanted by 16 degrees on one side
and
23 degrees and contoured on the other side to allow guidance for cell
delivery. The
well holes had a diameter of 2 mm.
A biochip with 16 laser-drilled recording apertures had dimensions of 82 mm
(long) x 4.3 mm (wide) x 155 microns (thick). The distance between the first
hole and
a narrow edge is 7.25mm. The holes were laser drilled to have two counterbores
of
100 microns (diameter) x 100microns (deep) and 25 microns (diameter) x 35
microns
(deep), respectively. A final through-hole was drilled from the side of the
counterbores and had a 7 to 9 micron entrance hole and a 2.0 micron exit hole
with a
total through-hole depth of 20 microns. Chemical treatment with acid and base
was
done as described in Example 3.
The treated chip was attached to the upper chamber using UV epoxy glue.
Devices produced using this methods had anRe of ~2MOhm with standard ES
and IS solutions, and an average Ra of ~6.OMOhm using RBL cells with a
standaxd
pressure protocol described herein.
Example 2. A 52-chip bench mark study.
We have conducted a bench mark study using 52 single-hole biochips tested
using a CHO cell line expressing the I~vl.l potassium channel. The result
demonstrated a 75% success rate as determined by the following criteria: 1)
achievement of sealing of at least one gigaOhm (a "gigaseal") within five
minutes of
cell landing on a hole, and 2) maintenance of Ra of less than lSMOhm, and Rm
of
greater than 200MOhm throughout 15 minutes of whole cell access time.
108
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Chip fabf°icatioiz
Patch clamp chips were designed at Aviva Biosciences and fabricated using a
laser-based technology (without an on-line laser measurement device). The I~-
type
chips were made from 150 micron thick cover glass. The ion transport measuring
hole structures had 140 micron double counterbores and final through-holes of
16.5~2 micron depth. The apertures on the recording surface had a diameter of
1.8~0.5 microns. The recording surface was further smoothed (polished) by
laser.
Surface Treatnieht
Chips were received from FedEx overnight service and were inspected for
integrity and cleanness. About 5% of the chips were excluded from further
treatment
in this process. Selected chips were then treated according to Example 3.
Treated
chips were stored in ddH20 for 12 to 84 hours before the tests.
Bateh QC foy~ chips
Chips were acid and base treated in batches of 2025. Four to six pieces of
each batch were randomly picked for testing their patch clamp performance with
CHO-Kvl.l cells in terms of speed to seal and stability of the whole cell
access.
Batches with <75% success rate were excluded for the 50-chip tests.
Cell passage
CHO-Kvl.l cells (CHO cells expressing the I~vl.l ion channel) between
passage 47 and 54 were split daily at 1:10 or 1:15 for next-day experiments.
Complete
Iscove media (Gibco 21056-023) with 10% FCS, 1xP/S, lxNEAA, lxGln, lxHT with
O.Smg/ml Geneticin was present in media used to passage cells and not present
in
media used to grow cells for next-day experiments.
Cell p~eparatioh
Cells were isolated using the protocol for CHO cell preparation described in
Example 6. After isolation, cells were resuspended in PBS complete media and
109
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
passed through a 20 micron polyester filter into an ultra-low cluster plate
(Costar
3473). The cells were used for the study between 30 minutes and 3 hour 30
minutes
after the filtration.
Cell QC
Isolated cells were quality control tested with conventional pipette patch
clamp recordings for their speed to seal, break-in pressure, and Rm and Ra
stability.
Freshly pulled pipettes were typically used within 3 hrs. Only cell
preparations that
passed the pipette quality control test were used for the 50-cell tests. About
50% of
the preparations out of approximately 30 cell isolations passed and were used
for this
study.
Solutions
Intracellular solution was made according to the following formula : 8 mM
NaCI; 20
mM KCI; 1 mM MgCl2; 10 mM HEPES-Na; 110 mM K-Glt; 10 mM EGTA; 4 mM
ATP-Mg; pH 7.25 (1M KOH3); 285 mOsm.
Aliquoted at 10m1 per 15m1 corning centrifuge tube, and stored at
4°G
Extracellular solution (PBS complete) was DPBS (lx), with glucose, calcium and
magnesium (Gibco cat# 14287-080).
This solution contained:
0.9 mM CaCl2, 2.67 mM KCI, 1.47 mM KH2P04, 0.5 mM MgCl2, 138 mM NaCI,
8.1 mM Na2HP04, 5.6 mM Glucose, 0.33 mM Na-pyruvate, pH 7.2-7.3, 295 mOsm.
Chip Quality Coratf-ol (QC)
For each recording, the chip was assembled into a two-piece cartridge, and the
lower and upper chambers were filled with intracellular and extracellular
solutions,
respectively. The chip was further quality control tested by inspection under
the
microscope and seal-test resistance measurement. Chips that showed a dirty
surface,
visible cracks and/or had a seal test resistance greater than 2.1 MOhm were
excluded.
Experiment settings
Chips that passed quality control underwent electrode offset and the overall
recordings were done with 4KHz bass filter. Cell landing was monitored on
computer
screen.
110
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Criteria
A simple description of a positive result is: chips that achieved gigaseals
and
gave Ra<lSMOhm and Rm>200MOhm throughout 15 min recording period.
Results
A total of 58 chips were tested, 6 of which were excluded from final analysis.
Out of the 52 cells included, 39 (75%) passed the test criteria. 43 (83%)
achieved at
least 12 minutes of continuous high quality recordings (Ra<lSMOhm;
Rm>200MOhm); 47 (90%) achieved gigaseals.
Success rate
Success duration is plotted in Figure 20A. Accumulative success rate is
plotted in Figure 20B. Success rate was consistent throughout the tests, which
suggests that most of the critical experimental parameters were under control.
75% is
a representative success rate under the current controlled conditions.
Electroele Resistance (Re)
90% of the electrodes selected for the tests had Re between 1.3 to 2.0 MOhms
(Figure 21A). A total of 81 chips were mounted and tested. 23(28%) failed the
quality control test, among which 15(18.5%) were due to Re>2.1 MOhms. 5(6%)
chips were screened out because of their dirtiness of surface; 3(4%) had
blocked or
cracked holes. Chips were not screened at low Re values. The reason behind the
2.1
MOhm cut off is that historically chips with the current geometry (double
counterbore) showed lower than 75% success rate in achieving the test
criteria. Re is
more or less normally distributed except for a slightly higher peak at
~1.3MOhm.
Break-in Pressure
Break-in Pressure is an important parameter for cell condition. During the
tests, break-in pressures were tightly distributed between -100 to -130 torts
(Figure
21B). Our previous findings suggest that seals with more negative break-in
pressure
are likely to have higher and unstable Ra, while seals with lower break-in
pressure are
likely to have lower and unstable Rm.
111
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Membrane Resistance (Rm)
After break-in, Rm was mostly between 0.5 to 2MOhm (Figure 22A). Ending
Rm had a similar distribution, but more skewed to lower values. This is
consistent
with the deterioration of Rm over time. However, the amount of Rm
deterioration was
surprisingly small, which suggests that the seals were very stable during the
15
minutes test periods.
Access Resistaface (Ra)
Initial Ra had a normal distribution centered at 7MOhm (Figure 22B). 80% of
the seals had Ra starting from below 10 MOhm. In most cases, Ra increased
during
the 15 minutes with an ending value near 11 ~ 13MOhm. In order to minimize
disruption of the seals, great effort was not made trying to maintain minimal
possible
Ra. It is not known what the ending Ra would be and what percentage of seals
would
lose Rm if such efforts were made.
Typical Recof-dings
Figures 23-25 demonstrate sample data from one particular cell monitored
during the 52-cell test referred to above. Figure 23A demonstrates the whole-
cell
current record in response to a series of voltage steps from a holding
potential of -80
mV to various potentials between -60mV and +60mV. Figure 23B shows the
potassium current, extracted from the whole-cell current by P/4 leak
correction of the
same currents, compensated for leak and capacitance. Figure 23C illustrates
the
current-voltage relationship of the steady-state current averaged from data
recorded at
the time-points between the arrowhead indicators in Figure 23A and Figure 23B,
showing the voltage-dependence of the potassium current expressed in this cell
line.
The larger currents were the uncompensated currents (from Figure 23A) and the
smaller currents were compensated (from Figure 23B). The difference between
the
compensated and uncompensated currents represents the leak current, which was
negligible in relation to total whole-cell current.
Figure 24 shows data similar to those in Figure 23 but is recorded at the end
of a 15-minute recording period whereas data in was Figure 23 recorded at the
start
of the recording period, where the duration of the recording period is
relative to the
112
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
time at which whole-cell access was achieved. Figure 24A demonstrates the
whole-
cell current record in response to a series of voltage steps from a holding
potential of -
80 mV to various potentials between -60mV and +6pmV. Figure 24B shows the
potassium current, extracted from the whole-cell current by P/4 leak
correction of the
same currents, compensated for leak and capacitance. Figure 24C illustrates
the
current-voltage relationship of the steady-state current averaged from data
recorded at
the time-points between the arrowhead indicators in Figure 24A and Figure 24B,
showing the voltage-dependence of the potassium current expressed in this cell
line.
Once again, in Figure 24C, the leak current was still a small proportion of
the whole-
cell current.
Figure 25 shows the time-course of the measured seal quality parameters
during the same experiment that is represented in Figures 23 and 24. Over the
15
minute recording period, the membrane resistance (Rm) decreased (due to leak
current) slightly from 1.4 GOhms to 1.0 GOhms, and access resistance (Ra)
increased
from 8 MOhms to 13 MOhms. The non-uniform time-profile of the traces is
representative of the effect of the applied pressure control protocol used to
control Ra
during the experiment.
Example 3. Treatment of Ion Transport Measurement Chips to Enhance their
Electrical Sealing Properties
Detailed Procedure: (referenced to step numbers below). All incubation
processes
were carried out in self made Teflon or modified polyphenylene oxide (Noryl~)
fixtures assembled in a glass tank while shaking (80 rpm, with C24 Incubator
Shaker,
Edison, NJ, USA). Water was always as fresh as practical from a water
purification
system (NANOpure Infinity UV/LJF with Organic free cartridge). Nitric acid was
ACS grade (EM Sciences NX0407-2, 69-70 %). Sodium hydroxide was 10 N,
meeting AP13A requirements (VWR VWR3247-7). When necessary, chips were
inspected for QC before and after treatment.
The protocol used was:
3 hour shaking incubation in 6M nitric acid at 50 degrees C.
113
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
2. 6 x 2 minute rinses in DI water at room temperature.
3. 60 minute incubation in DI water (shaking)
4. 2 hour shaking incubation in SM NaOH at 33 degrees C
5. 6 x 2 minute rinses in DI water at room temperature.
6. 30 minute incubation in DI water (shaking) at 33 degrees C
7. Chips were stored in DI water at room temperature. A vial used for storage
was filled to the neck to minimize air space.
Chips treated according to this protocol demonstrated enhanced electrical
sealing when tested in ion transport detection devices.
Example 4. Achieving Seals with Inverted Chips
A biochip was fabricated from Bellco D263 or Corning 211 glass of thickness
of 155 micron. The 16 laser-drilled recording apertures on the chip had
dimensions
of 82 mm (long) x 4.3 mm (wide) x 155 microns (thick). The distance between
the
first hole and a narrow edge is 7.25mm. The apertures were laser drilled to
have one
counterbore of 100 microns (diameter) x 125microns (deep). A final through-
hole was
drilled from the side of the counterbores and had a ~10 micron entrance hole
and 4.5
micron exit hole with a total through-hole depth of 30 microns. After standard
chemical treatment as described in Example 3, the biochip was mounted to an
upper
chamber piece described in Example 1 in inverted configuration such that the
counterbore side faced the upper chamber piece (where RBL cells were added).
Recordings were done with a device adapted to Nikon microscope as described in
Example 5. Typical voltage clamp quality parameters such as Rm and Ra over
time
are shown in Figure 22.
Example 5. A biochip device adapted to a microscope and having flow-through
lower chambers.
A device for ion transport measurement known as the "Tester" device having
114
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
flow-through lower chambers was designed and constructed. The device has a
lower
chamber base piece that formed the bottom surfaces of the lower chambers and
comprises conduits for the inflow and outflow of solutions, and a gasket that
formed
the walls of the lower chambers. The device also comprises a cartridge that
provided
upper chambers and a chip comprising holes. The device was adapted for a
microscope, so that the bottom surfaces of the lower chambers are transparent,
and the
device was fitted to a baseplate adapted to a microscope stage. The following
description of the design and manufacture of the device makes reference to
Figures 3-
8.
In this design, a biochip cartridge that has a chemically-treated glass chip
sealed to an upper chamber piece can be assembled onto a microscope stage-
mounted
lower chamber base piece that allows simultaneous or sequential testing of all
recording apertures while simultaneously observing the experiment's
progression
microscopically.
The Tester device includes a metallic base plate, in this case made of
aluminum, shaped to insert onto a microscope stage, and sculpted to support
and align
a multi-well perfusion lower chamber base piece. The baseplate of the device
(as
shown in Figure 4) was shaped to take advantage of an existing mounting point
on
the Nikon microscopes by positioning the device into an aperture within the
microscope stage. It is round, with an edge intended to prevent it from
falling through
the hole on the stage. The depth of the device is intended to hold the
functional
portion of the biochips as well as the cells that are added to the biochip at
testing time
at a convenient focal point within the focal range of the microscopes, that
is, at
essentially the same level as the upper platform of the microscope stage.
To assemble the device, a gasket (as shown in Figure 6) was inserted over the
lower chamber base piece (301 in Figure 3A) seated in a baseplate, then the
cartridge,
was clamped onto the gasket by compression via a clamp assembly (shown in
Figures
7A and 7B) that bolted onto the base plate using four thumb-screws (73 in
Figure
7A). The lower chamber piece was made of plastic and contained an array of 16
conduits for inflow of intracellular solution, and another 16 conduits for
outflow of
same. The 32 conduits emerged on the top surface of the lower chamber base
piece in
alignment with the recording apertures of the biochip. The gasket was made of
PDMS and was situated between the lower chamber piece and the chip, and
contained
slits, or holes (601 in Figure 6), that aligned between the emerging holes of
the
115
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
perfusion conduits of the lower chamber piece and the recording apertures of
the chip,
such than intracellular "lower" chambers were formed within the array of slits
or
holes in the gasket. An electrode of silver-silver chloride was introduced
into each of
the 16 outflow conduits along one side of the base piece to function as
recording
electrodes.
With reference to Figure 8A, the device was made up of 1) a metallic base
plate
(812), specifically, but not exclusively, stainless steel, 2) a transparent
lower chamber
piece (801), sometimes referred to as an "inner chamber array", made from
polycarbonate (but could be any other convenient transparent substance) 3)
electrodes
(not visible in Figure) inserted into the outflow conduits of the lower
chamber piece,
made from wires of silver or any other conductor capable of being used as a
voltage
sensing and current-delivering electrode, and attached to a connector on the
outer side
of the lower chamber piece, 4) inert tubing connectors (not visible in Figure
8; 302 as
seen in Figure 3A) glued to the lower chamber base piece at the conduit
openings (or
any other means that may provide a connection for a fluid conveyance system)
in this
case made from glass, 5) a gasket (805) that provided a seal between the lower
chamber base piece and the biochip cartridge, where the gasket (in this case
made of
PDMS) simultaneously comprised the inner chambers, 6) a biochip cartridge
(804)
mounted onto the test apparatus over the gasket, and held in place by a
guidance
system, in this case alignment pins inserted into the plastic bottom chamber
array
body in such a way as to restrict movement of the biochip while simultaneously
guaranteeing alignment of the biochip's recording surface with the inner
chambers,.7)
a clamp (802) assembly intended to apply sufficient pressure onto the biochip
cartridge so as to generate a seal between the bottom of the chip and the
gasket, and 8)
an array of electrodes (not visible in Figure 8, 75 in Figure 7B)attached to
the clamp
shaped and oriented so as to enter into the top wells of the biochip
cartridge, all 16 at
a time, and where all electrodes were connected together so as to provide a
reference
electrode in the upper chambers of the cartridge.
Figure 5 shows the arrangement of parts installed in the baseplate (54)
schematically. The clamp (53) holds the cartridge (51) down on the gasket (not
visible) and lower chamber base piece (not visible). The clamp has attached
electrode
wires (55) that extend into the upper wells of the cartridge (51). This
depiction also
shows the lower chamber electrode array (52) of pin sockets (56) that connect
to
116
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
electrode wires that are threaded through conduits leading to lower chambers.
The pin
sockets (56) can be connected to the signal amplifier.
Figure 8B showed the assembled device, in which the clamp (802) is screwed
into the baseplate (812). The flow-through lower chamber base piece is not
visible
beneath the cartridge (804). Inflow tubing (809) is attached to one side of
the lower
chamber base piece and outflow tubing (808) is attached to the opposite side
of the
lower chamber base piece.
1) Metallic Base Plate:
This base plate serves multiple functions. First, the metallic body serves as
an
electrical noise shield for the bottom side of the test chamber. It completes
a type of
faraday cage that is contiguous with the grounded stage of the microscope.
Secondly,
the metal base was carved on the top side so as to catch any fluids that may
leak or
spill and prevent the contamination of the microscope with said fluids. To
this end,
the base plate was sealed, with silicone glue or with silicone grease (vacuum
grease)
or with any other such viscous immiscible substance (eg: Vaseline) to the
transparent
lower chamber piece described in 2) (below). Third, the base plate was shaped
to
optimize its use with a particular microscope. Specifically, in our case it
was
desirable for the base plate to be cut to fit onto the 107mm circular cutout
hole of a
Nikon microscope. Fourth, the base plate was drilled and tapped so as to
provide a
mounting point for the lower chamber piece and for the clamp of the Tester.
Its
design was such that held the recording aperture of the cartridge within a few
millimeters of the level of the top of the microscope stage so as to ensure
that the chip
function could be monitored within the focal range of the microscope. Figure 4
illustrates the design of the base plate as adapted for the Nikon Microscope.
2) Transparent Lower Chamber Base Piece (Inner Chamber Array):
This design of a lower chamber base piece, shown as (301) in Figure 3A may
also be
referred to as an inner chamber array, or an intracellular chamber array. For
the
convenience of being able to view under a microscope the progression of an
experiment, it was made of a transparent material. Polycarbonate was chosen
for its
ease of machining. Its shape was designed to support a cartridge over it, and
provide
tubing connections along the long edges of either side the cartridge, as well
as to
provide connections to electrodes placed inside one of each pair of conduits
(holes in
117
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
the base piece material that function as such) supplying each recording
aperture of the
chip. The conduits drilled into each side provided a connection between the
edge of
the lower chamber base piece and somewhere near the center, then another
conduit
was drilled perpendicularly from the top surface to connect to each conduit
coming
from the edge. The emerging conduits at the top surface were located so as to
provide
for an inflow and an outflow of solution to and from each of the lower
chambers. The
lower chamber base piece did not comprise chambers, but instead the lower
chambers
were created by openings within the gasket material. As seen in Figure 3B, the
inflow and outflow conduit openings (304) in the areas (303) of the upper
surface of
the base piece that corresponded to the bottom surfaces of the lower chambers
were
separated from one another so as to leave an undisturbed area of surface that
could be
seen through with a microscope so as to visualize the recording aperture
during
experimentation. To this end, the top surface that was in opposition to the
chip was
untouched with the exception of the emerging inflow and outflow conduit
openings
and as well the bottom surface of the lower chamber base piece was left
untouched so
as to not disrupt transparency of the part. Each conduit leading to the edges
of the
base piece had a means (such as tubing connectors) for interfacing it to
inflow tubing
and outflow tubing (309 and 308 in Figure 3B) (see also description of part 4)
that
provided for delivery of solutions, as well as for pneumatic pressure control.
Tubing
connectors (302) can be seen in Figure 3A. One of the conduits going to the
edge of
the part was left longer so as to house an electrode (wire) that is introduced
into the
lumen of the conduit. The added length also allowed for a second segment to be
glued onto the top surface so as to house the connectors for the electrodes.
The top
surface of this part was trimmed down around the periphery of area covered by
the
cartridge so as to provide an edge that functioned to hold the gasket in place
during
mounting and removal of the cartridge. Further, between each pair of inflow
and
outflow holes for each bottom well was a cut intended to prevent wetting of
the gasket
material to span from one bottom chamber to adjacent bottom chambers. This
lower
chamber base piece as a whole contained 6 pin holes 2mm in diameter to hold 6
pins
that functioned to keep the cartridge aligned during mounting. It also
contained a
further 4 holes to hold 4 spring-pins (307 of Figure 3B) that functioned to
provide an
electrical connection for an early version of the cartridge. The present
version of the
cartridge does not require these contacts, however they were kept in place so
as to
prevent contact with the gasket before the clamp part is pressed down during
the
118
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
mounting. Finally, two more holes were present so as to use two screws to hold
the
part onto the base plate.
3) Inner Chamber Electrodes:
Each lower chamber contained an electrode, which in this case is a silver wire
that was periodically chlorided. The wire was inserted into the lumen of the
longer
conduit of the base piece and bent upward into the electrode connector array
(315 in
Figure 3B). The segment of wire was sufficiently long that it remained exposed
within the lumen of the longer conduit after the inert tubing interface parts
were glued
into place, and the other end was soldered to a connector, in this case an
array of lmm
female pin-connector sockets inserted into holes in the part. The connector
pin sockets
(310) are seen in Figure 3B.
4) Inert Tubing interface:
Into each conduit of the base piece an inert tubing connector (in this case
made
from glass) was inserted that was fixed in place with epoxy glue. Epoxy was
chosen
only in so much as it is preferred for bonding glass to polycarbonate. The
tubing
segments were sufficiently long to butt against a countersunken segment of the
conduit drilled into the lower chamber piece and stick out of the part enough
to hold a
segment of silicone tubing that was press-fit onto the glass segment. This
junction
should withstand a pressure greater than two atmospheres positive pressure,
and
greater than 700 mmHg vacuum pressure. It was determined that 3 to 5 mm
insertion
into the silicone tubing was sufficient to accomplish this requirement.
5) Gasket:
For convenience the flexible gasket was molded from curing PDMS. The
gasket contained a raised edge on the bottom side that surrounded the chambers
as a
whole and was able to hug an edge present in the same periphery on the lower
chamber piece so as to hold the gasket in place. As depicted in Figure 6, the
gasket
had oblong holes (601) in it that aligned over the exit and entrance holes of
the lower
chamber piece for each chamber of the array. On the top surface of the gasket
was a
set of squared O-rings (602) that were part of the gasket but raised
sufficiently to form
a seal onto the cartridge when pressed against it with the clamp part.
119
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
5) Biochip
The fabrication of chips having holes for ion transport measurement has been
described herein . In this device, the chip was made of glass and has 16 laser
drilled
holes. The chip was laser polished on the top surface, and treated in acid and
base
prior to attaching the chip in inverted orientation to an upper chamber piece
with a
UV adhesive.
6) Clamp Assembly:
A clamp was made from an inflexible material so as to not allow bowing of
the cartridge during compression onto the gasket while mounted on the tester.
In this
case it was made of stainless steel for its inertness when wetted with
physiological
buffers. The clamp was shaped so as to fit snugly over the cartridge and was
drilled
so as to accommodate and be positioned by the guide-pins sticking out of the
lower
chamber piece. Four screws were finger-tightened to the base plate at each
corner of
the clamp assembly so as to press down the cartridge to seal it against the
gasket.
This part is shown in Figure 7A and 7B.
7) Upper Chamber Electrodes:
In early development it was expected that compression pins would contact the
bottom of the cartridge during testing to provide a connection to the
reference
electrodes built in to the cartridge. The present embodiment of the cartridge
does not
contain reference electrodes, therefore these electrodes were introduced into
the top
wells of the cartridge. To this end, periodically chlorided silver wires were
used as
electrodes. The electrodes were shaped to dip deep inside each well, and on
the
outside of the wells the wires were soldered to a wire running along the top
of the
clamp part (visible in Figure 7B). At each end of this wire was a 1mm female
pin
connector that was used to interface with the voltage clamp amplifier. The
upper
chamber electrode wires (55) are shown in Figure 5.
Method:
Before use the device should be clean and dry.
120
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
A SealChipTM cartridge was removed from its carrier, and rinsed with a jet of
deionized water of approximately 18 MOhms resistance. The product was them
dried
under a stream of pressurized dry air filtered through a 0.2 ~.m air filter to
remove
water from the recording apertures and their vicinity.
The clean cartridge was then placed with top-wells upward onto the pressure
contact pins of the tester such that movement of the cartridge was limited by
the six
alignment dowels of the bottom chamber piece. Prior to clamping the cartridge
to the
gasket and lower chamber base piece, the cartridge should be supported above
the
gasket but without yet touching the gasket. The clamp was them placed over the
cartridge such that the four mounting holes aligned with their threaded
counterparts
on the base plate. The four mounting screws were them used to press down the
clamp
uniformly thereby pressing the cartridge down onto the PDMS gasket with
sufficient
pressure to form a tight seal between the chip and the gasket and between the
gasket
and the lower chamber base piece. The recording aperture within each chamber
of the
cartridge should already be aligned with openings in the gasket that form the
lower
chambers.
The bottom chambers were then filled from one side with sufficient solution
(analogous to intracellular solution) to fill the bottom chambers and fill
enough of the
tubing on the other side such that capacitative distension of the tubing on
the filling
side would not introduce air into the recording chamber, and would not
introduce air
into the area of the tubing that contained the bottom-chamber electrode. (For
this
purpose, it is best to fill the chamber starting from the side that does not
contain the
electrode since higher pressures will be used for vacuum pressure than for
positive
pressure, thereby ensuring that the electrode will remain in full contact with
the
solution at all times.) Once the bottom chamber was filled and was free of
visible
bubbles, the tubing was sealed off by a clamp (a valve or any means that
ensures
electrical isolation between the bottom chambers of the array can also be
used).
Sufficient positive pressure was applied to the free end of the inner chamber
tubing so
as to cause solution to be forced into the counterbore and through the hole of
the
recording aperture of the chip.
Once solution was seen emerging into the top chamber, the pressure was
released,
and immediately the top chamber was filled with sufficient solution (analogous
to
extracellular solution) so as to completely immerse the top side of the chip
without
bubbles remaining on the chip surface, and to fill the top well sufficiently
to provide
121
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
good contact with the electrode in the top well. (It is also of benefit to
fill the top well
sufficiently to avoid a strong meniscus effect (60 to 70 microliters with the
present
version of the SealChipTM product) whenever it is intended to view under an
inverted
microscope the progression of the experiment (for upright microscopes it is
necessary
to fill with more solution, ~90 microliters, to allow good contact with a
coverslip that
must be placed over the well to enable a good view of the bottom of the
well).)
The assembled tester, now ready for testing, was placed on the microscope (and
connected to the voltage clamp amplifiers) as well as to the pressure control
devices) for testing.
After the termination of the experiment, the tester was disconnected and
removed
from its testing location. The extracellular medium was suctioned from each
well,
and each well was rinsed once with deionized water to removed any leftover
particulate (debris or cellular) material that may have been left over from
the
experiment. Both ends of the tubing of the bottom chambers were then opened
and
the solution was suctioned out of the bottom wells. Each well was well rinsed
with
clean deionized water, then dried completely with pressurized air. Finally the
screws
holding down the clamp were removed and the cartridge was disassembled from
the
tester. Any wetting at the gaskets was wicked away with a lint-free tissue.
(If any
liquid is pooled around the gasket, then the gasket should be removed, rinsed
then
dried, and the bottom chamber array should be likewise rinsed and dried,
ensuring
that the tubing is also rinsed and completely dried.)
Quality Control/Quality Assurance of SealChipTM product:
Internally to the company, the "tester unit" device described in this example
has
been used for QC/QA of the SealChipTM product before it is sent to a customer,
and
before it is used internally for further research. The success rate with a
product that
passes the QC has been as good as that with older testers that tested a single
chamber
at a time.
Quality Control/Quality Assurance of Cells:
Internally to the company, the tester unit device has been used to verify the
quality of the cells used for QCJQA using known good SealChipTM product.
Research and Development:
122
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
The tester unit has been used by our company for testing variations to the SOP
for the SealChipTM product. In the future it may be used for discovery and
screening
of compounds that require exchanging of solutions on the bottom well or where
compounds or particles must be delivered to the cytosolic chamber after a seal
is
formed with the cell membrane.
A great number of results have been achieved on the microscope adapted
device ("Tester Unit") since its development. The tester unit has been the
tool of
choice for performing quality control experiments on the SealChipTM product.
The
following gives examples of the quality of data obtained from it. (The seal
resistance
is designated Rm; G refers to GigaOhms and M refers to MegaOhms.)
Table 1. SeaIChipTM Data
Cell
Chip Hole T pe Re Rm(G Ra(M)Seal Note
Lot# ID G) Qlt
S2N22-40C RBL 3.4 0.5 5.7 +++
G 3.3 5 6.7 +++
I 3.3 2 2 +++
M 3.2 0.25 8.8 +++
O 3.2 0.5 6.5 +++
S2D18-114A 3.9 2.4 7.2 ++
C 3.9 2.2 18 +
G 3.7 4 10.8 ++
S2D20-28B 4.5 2.6 9.1 ++
C 4.2 1 13.3 -S
D 4.4 0.6 10.5 +
E 4.3 2.7 10 ++
F 4.3 1.6 10 ++
G 4.2 3.5 9.4 ++
H 4.3 3.3 8.8 ++
S2D20-8 A 4.1 1.7 12.2 ++
C 4.1 2.7 9.3 ++
G 4.2 1.7 8.4 ++
I 4.1 2 11 +
M 4.1 1.6 11.7 S Debris landed
before cell
O 4.1 2.6 7.6 ++
S2D20-50A 4.3 2.9 12.4 +
B 4.3 7 10.7 +++
C 4.1 1.1 10 +++
D 4.3 2.1 8.8 +++
E 4.2 4.5 8 ++
F 4.4 4.9 7,1 ++
G 4.3 1.5 10 ++
H 4.3 6.9 8.3 ++
I 4.2 6.2 8.3 +++
123
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
J 4.2 0.6 8.1 +++
K 4.3 0.9 9.8 ++
L 4.4 6.5 7.4 +++
N 4 6 7.7 +++
O 4 5.6 7.8 ++
P 4.1 6.5 12.8 +++
S2D219-21D 3.1 4.5 4.6 +++
E 3 1.5 11.6 +
F 3 1.5 5.6 ++
G 3 2.8 5.8 +++
H 3 3.1 4.8 +++
I 3 3.2 8 ++
J 3.1 3 5.7 ++
S2D18-191A 3.5 3.3 8.5 ++
C 3.5 2 13.9 ++
D 3.3 1.6 8.9 ++
E 3.6 2.5 9.2 +++
F 3.6 2 8 +++
G 3.5 0.4 7.7 +++
H 3.7 1.4 6 ++
S2D18-206A 3.3 4.1 7 +++
C 3.2 2.1 6.2 ++
D 3.3 4.6 6.7 ++
E 3.4 3.4 5.2 ++
F 3.1 0.7 5.8 +++
H 3.4 0.6 11 S
S2D20-6B 4.1 1.5 8.8 ++
C 4.3 0.5 8.9 ++
D 4.1 3.2 8.9 +++
E 4.1 3.3 6.8 +++
G 4.3 3.8 7.8 +++
H 4.3 3.2 10.4 +++
S2D20-133A 4.3 3.5 9.6 S
B 4.5 4.4 7.5 ++
C 4.4 5 11.4 ++
D 4.5 3.1 10.8 +
E 4.5 5.3 10 +++
F 4.4 5.1 8.8 ++
G 4.4 5.1 8.5 ++
H 4.3 1.1 10.5 +
S2D21-70A 4.2 2.1 22 + S ec near the
hole
B 4.2 2.7 8 +++
C 4.3 2.8 7.6 +++
D 4.3 1.3 12.3 ++
E 4 2.3 10.2 ++
F 4.2 0.5 7.2 +++
S2D20-130A 3.2 0.8 7.6 +
B 3 0.5 8.9 ++
E 3 1.3 11.1 ++
F 3.3 2 7.9 +++
G 3.3 0.5 11.9 S
-
H I I 3.1 2.1 7.8 ++
~
124
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
S2D20-194A 3.7 2.3 9.8 +++
C 3.6 3 7.9 ++
D 3.8 2.4 14 S
E 3.6 2.4 5.9 ++
F 3.9 2.1 12.1 ++
G 3.7 2.1 6.7 +++
H 3.8 0.9 8.3 ++
S2D18-81A 3 1.6 5.5 ++
C 3.1 2.1 6 ++
D 3.3 3.4 7.8 ++
E 3.3 2 6.6 ++
F 3.3 2.4 8.6 ++
G 3.4 2.9 8.6 +
H 3.3 2.8 5.7 +++
S2D20-171C 3.8 2.3 9.5 ++
E 3.8 2.7 8.3 ++
F 3.9 3.4 8.1 ++
G 3.7 3.3 6.2 +++
H 3.7 2.8 7.8 +++
I 3.7 2.8 12.7 +
J 3.8 3.3 5.9 +++
S2D16-26A 3.3 1.5 5.5 ++
C 3.5 1.9 7.5 +++
D 3.7 1.2 6.8 ++
E 3.5 1.7 7.5 +++
F 3.7 1.7 6.4 +++
H 3.7 1.7 8.8 ++
S2D19-20A 2.5 1.4 5.7 ++
C 2.5 1.8 4.5 +++
D 2.5 1.5 5.8 ++
E 2.5 1.1 5 ++
F 2.4 1.8 1.6 +++
G 2.7 1.4 4.8 ++
H 2.8 1.6 5 ++
S2D16-1B 3.2 1.2 10.3 S
C 3.1 1.6 6.5 +
D 3.1 0.6 17 S
E 2.9 2.3 6.1 ++
F 3.1 2.7 6.1 ++
G 3.1 2.7 7.8 +++
S3210-181A Cho-Her4.6 0.3 14 +++
B 4 0.5 11 +++
D 4 0.2 14 ++
E 4 1.3 17 ++
G 4 2.1 10 +++
H 4.1 0.6 12 S
S3214-60A 3.6 1.2 7 ++
B 2.9 1 7 +++
C 2.9 0.4 17 -S
D 2.9 1.3 11 +
G 3.1 1.7 10 +++
H 3 0.2 10 ++
125
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
031103-A1B RBL 3 1 4 ++
D 3.4 0.5 5.2 ++
F 3.1 1.1 4.1 +++
H 3 1.2 7 ++
N 3.2 0.4 4.4 ++
P 3.1 0.3 5.5 +
031103-A2A 3.8 0.6 4.1 ++
C 4.3 2.1 4.1 ++
I 4 2.3 8.1 ++
K 4.4 2.1 5.3 +++
M 4.8 2.3 7.8 ++
O 4.4 2.7 9.9 ++
030703-A1A 3.6 1.9 4.9 ++
C 3.7 2.3 3.6 +++
E 3.8 2.2 5.8 ++
G 3.7 1.8 5.2 ++
I 3.4 1.7 4.1 ++
M 3.5 2.1 5.4 ++
O 3.7 1.7 4.6 ++
031103-A3A 4.8 2.5 5.2 ++
C 4.6 1.4 5.4 ++
E 4.8 1 4.6 ++
I 4.9 0.3 6.1 ++
D 4.9 1.6 6.1 ++
F 4.9 0.7 8.1 ++
030603-A2B 4.3 1.2 4.2 +++
C 4.3 4 9.2 ++
F 4.3 2 8.2 ++
H 4.4 2.2 7 ++
G 4.6 2 7.7 +++
1 4.2 2.2 7.2 +++
J 4.3 1.2 5.8 ++
030603-A1A 4.4 1.8 6.4 +
B 4.4 1.2 8 ++
C 4.6 1.2 8 +
F 4.8 1.5 8.5 +
G 4.4 0.7 4.4 ++
H 4.3 1.4 5.9 +
I 4.2 1.1 8.6 ++
030603-A3B 4 1.7 6.9 +++
D 4 0.28 6.9 ++
F 4.2 0.35 4.4 ++
H 4.3 0.27 6.9 ++
L 4.4 0.25 7.2 ++
.
N 4.5 0.85 7.2 ++ L
Example 6. Cell preparation for ion transport measurement.
126
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
PART I. CHO wt. and CHO.I~v cells
1. Use cells @ 50%~70% confluency. (18 hrs after cells seeded 1:101:15)
2. Remove medium and wash x2 with X~-free PBS (extra wash might be
necessary if the Fnal cell suspension has too much small debris)
3. Treat for 2' 15" with 1:10 trypsin-EDTA, at this time the supernatant
might be a little turbid due to release of cells into the buffer.
4. Rock gently, aspirate to discard supernatant. Wait for 1'25".
5. Add 1 volume of X++-free DMEM complete with 10% FCS, NEAR, etc,
rock gently to loosen and detach cells, and spin down (do not try to blow
to remove the remaining cells sticking to the bottom)
6. Wash xl with PBS complete
7. Resuspend in PBS, triturate, and pass through 15~20~m filter into non-
stick plate.
Cells can be used after 10 minutes of recovery and should last for up to 4hr
PART II. Transiently transfected CHO cells
1. Remove medium and wash x2 with X~-free PBS
2. Treat for 1' with 5 ml 1:10 trypsin-EDTA (O.SmI 0.05% trysin 0.53mM
EDTA from GIBCO cat. No.25300-54 in 4.5 ml PBS)
3. Rock gently, aspirate to discard supernatant.
4. Add 0.5 ml fresh 1:1 trypsin-EDTA , Wait for 6 mins.
5. Add Sml of X~-free DMEM complete with 10% FCS, NEAR, etc, rock
gently to loosen and detach cells, leave cell at RT for 1 hour, and spin
down (do not try to blow to remove the remaining cells sticking to the
bottom)
6. Wash x2 with lml PBS complete
7. Resuspend in PBS, triturate, and pass through 15 to 20 micron filter into
non-stick plate.
Part III. CHO-Her cells.
1. Use cells at 50%~70% confluency in T-25 flasks (VWR, Cat. No. 29185-
302).
2. Remove medium and wash x2 with X~-free PBS (extra wash might be
necessary if the final cell suspension has too much small debris)
3. Treat for 1' with 2 ml trypsin-EDTA( O.SmI 0.05% trysin 0.53mM
EDTA from GIBCO cat. No.25300-54 in 1.5 ml PBS)
4. Rock gently, aspirate to discard supernatant. Wait for 2 mins.
5. Add Sml volume of X++-free DMEM complete with 10% FCS, NEAR,
etc, rock gently to loosen and detach cells, leave cell at RT for 30min,
and spin down (do not try to blow to remove the remaining cells sticking
to the bottom)
6. Wash x2 with lml PBS complete
7. Resuspend in PBS, triturate, and pass through 15~20micron polyester
filter into non-stick plate if cells still cluster together.
Part IV Protocol for isolation of CHO
127
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
1. Use cells at 70~ 80% confluences in T-25 flasks (24 hrs after seeding).
2. Remove medium and wash x2 with X~+-free PBS ( ( cell should not be leave
in X++-free PBS more than 10 mins, otherwise, the minimal digestion time will
be decreased)
3. Wash once with 1:4 AccuMax (available from Innovative Cell Technologies,
San Diego, CA) ( wait about 20 second, rocking to removed the loose
attached cell)
4. Treat at 37°C w 4 ml volume of l: 4 Accumax ( diluted with X~-free
PBS)
for minimal time ( cell dissociate from the flask and floated in the Accumax )
or 1.5 times minimal time.
CHO-KV
a. 1: 4 AccuMax 5' ( 1 ml AccuMax + 3 ml X~-free PBS) w/o rocking
b. 1: 4 AccuMax 8' ( 1 ml AccuMax + 3 ml X~-free PBS) w/o rocking
CHO-HERG
c. 1: 4 AccuMax 8' ( 1 ml AccuMax + 3 ml X~-free PBS) w/o rocking
d. 1: 4 AccuMax 12' ( 1 ml AccuMax + 3 ml X~-free PBS) w/o
rocking
5. Add Sml volume of Cap-free DMEM with 10% FBS, into the flasks, and
removed all cell suspension to a 15 ml centrifuge tube, spin down ~300g x
3min (do not try to blow to remove the remaining cells sticking to the
bottom).
6. Discard supernatant, add lml 1:4 ( PBSC : PBS), gently triturate to
resuspend
cell, centrifuge 2000rpm x lmin in an micro centrifuge tube.
7. Discard the supernatant, add 800,1 to lml 1:4 (PBSC * : PBS) , triturate,
and
pass through 1520 micron filter into non-stick plate.
30
Part V. Protocol for Isolation of HEIR
1. Use HEK-Na cells at 70~ 80% confluences in T-75 flasks (16 hrs after
seeding).
2. Remove medium and wash x 2 with X~-free PBS
3. Add 6 ml X++-free PBS, incubate at 37°C for 5 mins, aspirate
supernatant
4. Add 6 ml X++-free PBS, incubate at 37°C for 10 mins or until all
cells
dissociate from flask.
5. Add 2 ml Accumax directly into flask to finalize the Accumax concentration
to 1:4, incubate cell at 37°C for 4 mins
6. Add 6 ml volume of Cap-free DMEM with 10% FCS into the flasks to stop
the digestion
7. Put cell mixture into a 15 ml tube, and spin down 300g x 3min
8. Discard supernatant, gently suspend cell in 4 ml Cap free DMEM with 10%
FCS, incubate cell at 37°C incubator at least 30 mins or until use
it.
9. Carefully remove the supernatant, wash x1 with PBS with 100nM Cacl2, 1mM
Mgclz
10. Triturate, resuspend cell in PBS with 100nM Caclz, 1mM Mgcla, filter cell
mixture through 21 ~m filter into non-stick plate.
128
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Example 7. Program Logic and Pressure control profile
The following is a typical program logic for software pneumatic control. It
includes procedures for cell landing, form seal, break-in, and Ra control.
#start of program
Count=0
Turn off compensations
Procedure Landing:
Reset button-pressed
Label window "Attempting Landing"
Run washer # deliver clean ES to top chamber
Wait 5 seconds
Stop washer
Repeat twice:
Apply -300torr pressure # clear holes of any remaining debris after filling
Wait 0.5 seconds
Apply Otorr pressure
Wait 2 seconds
End repeat
Zero junction potential
Wait for stable reading
Record average Re value
Save Re to logs
Initiate cell addition
Wait until 0.5 seconds before cell delivery # before pipette touches ES
Apply +1 Otorr # before and during delivery
Wait for pipette removal # from ES chamber
Apply 0 torr
Wait 3 seconds
Apply -50torr
Wait until Seal > 2Re for 0.5sec or elapsed=15 seconds
If elapsed then
Count=count+1
If count >= 3 then abort test and write to log
Apply +50torr
Run proc Landing
Endif
Run FormSeal
End procedure
Reset elapsed
Procedure FormSeal
Reset button-pressed
Label window "Attempting Seal"
Apply-80mV HP #negative holding immediately after landing
Apply -50torr #this may not necessarily be the same as that used for landing
While Seal increasing >20MOhms/second
Wait until Seal >= 1 Gohm or elapsed=10 seconds
129
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Endwhile
Apply Otorr
Wait 2 seconds
While seal increasing >20MOhms/second and seal<1 GOhm,
Wait 1 second
Endwhile
#start tamping to attempt seal
Unless seal>1 GOhm, Apply ramp from Otorr to -50torr over 20 seconds
Unless seal>1 GOhm, Wait 5 seconds
Unless seal>1 GOhm, Apply Otorr
Unless seal>1 GOhm, wait 5 seconds
Unless seal>1 GOhm, Apply ramp from -30torr to -80torr over 30 seconds
Unless seal>1GOhm, Wait 5 seconds
Unless seal>1 GOhm, Apply Otorr
Unless seal>1 GOhm, wait 5 seconds
Unless seal>1 GOhm, Apply ramp from -50torr to -1 OOtorr over 40 seconds
Unless seal>1 GOhm, Wait 5 seconds
Unless seal>1GOhm, Apply Otorr
Unless seal>1GOhm, wait 5 seconds
Unless seal>1 GOhm, Apply ramp from Otorr to -200torr over 120 seconds
Unless seal>1 GOhm, Wait 5 seconds
Unless seal>1GOhm, Apply Otorr
Unless seal>1GOhm, wait 5 seconds
If not seal>1GOhm
Check button_pressed
If button-pressed = "continue" then abort test and write to log
Run FormSeal
Endif
#Seal detected, now check stability
Stop tamping and hold last pressure
Wait 1 second # let seal stabilize
If seal>1GOhm,
Apply Otorr
Record Seal value into Rseal, save to logs
Unless Seal<(Rseal-200MOhms) or Seal decreasing >200MOhms/second
Wait 5 seconds
End unless
If Seal<(Rseal-200MOhms) or Seal decreasing >200MOhms/second
Check button_pressed
If button-pressed = "continue", goto Procedure Breakln
Run FormSeal
Endif
#cell sealed
Endif
End Procedure
Procedure Breakln:
Reset button-pressed
Label window "Attempting break-in"
130
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Null chamber capacitance
Until capacitance > 3.5pF or Pressure>300torr or Seal<(Rseal-200MOhms) or
Seal decreasing >200MOhms/second
Wait 1 second
Apply -20 delta torr
End until
If capacitance > 3.5pF
Record break-in pressure value
Wait 0.5 seconds
Apply Otorr
Run procedure RaControl
Endif
If Pressure>300torr
Apply Otorr
Until capacitance > 3.5pF or Pressure>300torr or Seal<(Rseal-200MOhms)
or Seal decreasing >200MOhms/second
Wait 1 second
Apply -20 delta torr
Apply Zap
End until
If pressure>300torr then abort test and write to log
Endif
If capacitance > 3.5pF
Record break-in pressure value
Wait 0.5 seconds
Apply Otorr
Run procedure RaControl
Endif
If Seal<(Rseal-200MOhms) or Seal decreasing >200MOhms/second
Check button-pressed
If button-pressed = "continue", goto Procedure Breakln
Run FormSeal
Endif
End Procedure
Elapsed = 0
Procedure RaControl:
Reset button-pressed
Label window "Adjusting seal quality"
Record Cm, Rm, Ra to logs
Assign Rmlnitial = Rm, Ralnitial = Ra
If Ra < Raldeal then end #Raldeal does not need adjustment
If Ra < RaMax and Ra decreasing then end #no need for adjustment
If Ra < RaMax then countdown = 20 seconds else countdown = "true"
While countdown
Check button-pressed
131
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
If button_pressed = "continue" then end
If Ra increasing and Rm > 300MOhms
Apply -50torr
Wait 0.5seconds # max 2 seconds
Apply Otorr
Wait 1.5 seconds
Endif
If Ra increasing and Rm > 500MOhms
Apply -80torr
Wait 0.5seconds # max 2 seconds
Apply Otorr
Wait 1.5 seconds
Endif
If Rm>0.8GOhm then apply -50torr else apply -10torr
While Ra>Raldeal and Rm>(Rmlnitial-25%) and countdown
Unless Ra<Raldeal or Rm<(Rmlnitial-25%), wait 5 seconds
If Ra<RaMax then countdown=20 seconds
If Ra<Raldeal then Endwhile
If Ra not decreasing
If Rm not decreasing and Rm>1 GOhm then Apply -10 delta tort
If Rm not decreasing and Rm<1 GOhm then Apply -5 delta tort
If Rm decreasing and Pressure>10torr then Apply +5 delta tort
If Rm<(Rmlnitial-25%) then apply 0 tort
Endif
If pressure>BreakInPressure then apply Otorr
If elapsed > 120 seconds then apply Otorr and end
If Rm<300MOhms then apply (reaklnPressure-l0torr)
Endwhile
If -10torr>pressure>-50torr
Apply Otorr
If Ra increasing then apply -60torr
If Ra increasing then run RaControl Procedure
Endif
Endwhile
End Procedure
Example 8. Achieving High Resistance Seals in 52-Cell Test
An operator using a syringe based pressure system employed a pressure
control profile similar to that described in Example 7, except that it was
performed
manually rather than by computer automation. The 52-cell test described in
Example
2 was performed using a syringe controlled by had while the operator viewed a
pressure monitor.
132
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
The criteria for the test was the achievement of at least 75% success rate,
with
success defined as achieving a gigaohm seal to initiate a patch clamp, then
during the
patch clamp membrane maintaining resistance above 200 MOhms and maintaining
access resistance (or series resistance) below 15 MOhms for at least 15
minutes.
Table
2
Success Rate Data
No. of Pro onion
Chi
s
Total chi s tested 52 100%
Chi s achieved i aseals 47 90%
Chi s achieved >12' continuous43 83%
recordin s
Chi s achieved >15' continuous39 75%
recordin s
Table 2: 50-cell test that demonstrates the feasibility of the
pressure control protocol.
demonstrates the conclusion from this experiment, showing that the goals of
the 52-
cell test were met.
Figures 23-25 give a sample of the time-course of an experiment where
membrane resistance and access resistance values are kept within the
acceptable
20 parameters. At many locations in the recording there are deflections in the
access
resistance trace (Figure 25). These deflections represent locations where the
pressure
protocol was applied to maintain the seal quality parameters. The success rate
at
achieving gigaohm seals is demonstrated in Figure 20. This data is a graphical
representation of the data identified in Table 2, where 90% of the chips
produced a
25 gigaohm seal with CHO cells. Figure 22 shows a histogram of the parameters
achievable with this pressure control protocol. Data shown with wide diagonal
bars
represents initial values for Ra and Rm, and values with narrow diagonal bars
represent values for Ra and Rm after 15 minutes of continuous whole-cell
access
under voltage clamp conditions. These data demonstrated that overall, 75% of
the
30 cells achieved gigaohm seals, and then whole-cell access was attained with
acceptable
parameters that were well-controlled for at least 15 minutes.
133
CA 02527660 2005-11-29
WO 2005/007866 PCT/US2004/017134
Example 9. Single channel recording using a biochip comprising a hole for ion
transport measurement.
RBL cells were prepared for patch clamp recording by simple centrifugation.
The cells were then delivered onto an ion transport measurement device with a
single
recording aperture. The biochip device was assembled according to Example 2.
The
biochip had been treated with acid and base to improve sealability. The upper
chamber solution was PBS lacking calcium and magnesium. The lower chamber
solution was:
150 mM KCI, 10 mM HEPES-K, 1 mM EGTA-Na, 1mM ATP-Mg pH (KOH) 7.4,
the upper chamber solution was
8 mM NaCl, 20 mM KCI, 1 mM MgCla, 10 mM HEPES-Na, 125 mM K-Glu , 10
mM EGTA-K, 1 mM ATP-Mg pH (KOH) 7.2.
Seal formation was achieved as provided in the previous examples, but after
gigaseal formation, no break-in step was performed. Single-channel recordings
were
obtained from a cell-attached membrane patch on an RBL cell. An inward
rectifier
IRKl single channel was recorded in RBL cells. A low concentration of
extracellular
K+ which does not depolarize the cell and does not inactivate the channel was
used.
ATP was present in the internal solution, which prevents the rundown of the
channel
activity. The noise level of the recordings was reduced from 10 pA to 1 pA in
order to
observe single channel events, which have an amplitude of a few picoamps.
The devices and methods described herein can be combined to make
additional embodiments which are also encompassed in the present invention.
All headings are for the convenience of the reader and should not be used to
limit the meaning of the text that follows the heading, unless so specified.
All references cited herein, including patents, patent applications, and
publications axe incorporated by reference in their entireties.
134