Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
1
NICOTINAMIDE DERIVATIVES USEFUL AS PDE4 INHIBITORS
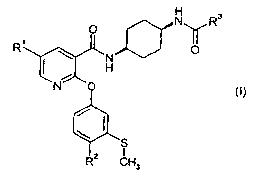
This invention relates to nicotinamide derivatives of general formula (I):
N R3
O
R~ ~ N O
H
N O
(I)
'S
R2 CH3
s
in which R~, R2 and R3 have the meanings indicated below, and to processes for
the preparation of, intermediates used in the preparation of, compositions
containing and the uses of such derivatives.
~o
The 3',5'-cyclic nucleotide phosphodiesterases (PDEs) comprise a large class
of
enzymes divided into at least eleven different families which are
structurally,
biochemically and pharmacologically distinct from one another. The enzymes
within each family are commonly referred to as isoenzymes, or isozymes.. A
total
is of more than fifteen gene products is included within this class, and
further
diversity results from differential splicing and post-translational processing
of
those gene products. The present invention is primarily concerned with the
four
gene products of the fourth family of PDEs, i.e., PDE4A, PDE4B, PDE4C, and
PDE4D. These enzymes are collectively referred to as being isoforms or
2o subtypes of the PDE4 isozyme family.
The PDE4s are characterized by selective, high affinity hydrolytic degradation
of
the second messenger cyclic nucleotide, adenosine 3',5'-cyclic monophosphate
(cAMP), and by sensitivity to inhibition by rolipram. A number of selective
2s inhibitors of the PDE4s have been discovered in recent years, and
beneficial
pharmacological effects resulting from that inhibition have been shown in a
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
2
variety of disease models (see, e.g., Torphy et al., Environ. Health Perspect.
,1994, 102 Suppl. 10, p. 79-84 ; Duplantier et al., J. Med. Chem., 1996, 39,
p.
120-125 ; Schneider ef al., Pharmacol. Biochem. Behav., 1995, 50, p. 211-217 ;
Banner and Page, Br. J. Pharmacol., 1995, 114, p. 93-98 ; Barnette et al., J.
s Pharmacol. Exp. Ther., 1995, 273, p. 674-679 ; Wright et al., Can. J.
Physiol.
Pharmacol., 1997, 75, p. 1001-1008 ; Manabe et al., Eur. J. Pharmacol., 1997,
332, p. 97-107 and Uleita et al., J. Med. Chem., 1999, 42, p. 1088-1099).
Accordingly, there continues to be considerable interest in the art with
regard to
the discovery of further selective inhibitors of PDE4s.
Io
Successful results have already been obtained in the art with the discovery
and
development of selective PDE4 inhibitors. In vivo, PDE4 inhibitors reduce the
influx of eosinophils to the lungs of allergen-challenged animals while also
reducing the bronchoconstriction and elevated bronchial responsiveness
1s occurring after allergen challenge. PDE4 inhibitors also suppress the
activity of
immune cells (including CD4+ T-lymphocytes, monocytes, mast cells, and
basophils), reduce pulmonary edema, inhibit excitatory nonadrenergic
noncholinergic neurotransmission (eNANC), potentiate inhibitory nonadrenergic
noncholinergic neurotransmission (iNANC), reduce airway smooth muscle
2o mitogenesis, and induce bronchodilation. PDE4 inhibitors also suppress the
activity of a number of inflammatory cells associated with the pathophysiology
of
COPD, including monocytes/macrophages, CD4+ T-lymphocytes, eosinopl~ils and
neutrophils. PDE4 inhibitors also reduce vascular smooth muscle mitogenesis
and potentially interfere with the ability of airway epithelial cells to
generate pro-
2s inflammatory mediators. Through the release of neutral proteases and acid
hydrolases from their granules, and the generation of reactive oxygen species,
neutrophils contribute to the tissue destruction associated with chronic
inflammation, and are further implicated in the pathology of conditions such
as
emphysema. Therefore, PDE4 inhibitors are particularly useful for the
treatment
30 of a great number of inflammatory, respiratory and allergic diseases,
disorders or
conditions and for wounds and some of them are in clinical development mainly
for treatment of asthma, COPD, bronchitis and emphysema.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
3
The effects of PDE4 inhibitors on various inflammatory cell responses can be
used as a basis for profiling and selecting inhibitors for further study.
These
effects include elevation of cAMP and inhibition of superoxide production,
degranulation, chemotaxis, and tumor necrosis factor alpha (TNFa) release in
eosinophils, neutrophils and monocytes.
Some nicotinamide derivatives having a PDE4 inhibitory activity have already
been synthetized. For example, the patent application WO 98/45268 discloses
nicotinamide derivatives having activity as selective inhibitors of PDE4D
isozyme.
Io
The patent applications WO 01/57036 and WO 03/068235 also disclose
nicotinamide derivatives which are PDE4 inhibitors useful in the treatment of
various inflammatory allergic and respiratory diseases and conditions.
Is However, there is still a huge need for additional PDE4 inhibitors that are
good
drug candidates. In particular, preferred compounds should bind potently to
the
PDE4 enzyme whilst showing little affinity for other receptors and enzymes.
They
should also possess favourable pharmacokinetic and metabolic activities, be
non-
toxic and demonstrate few side effects. Furthermore, it is also desirable that
the
2o ideal drug candidate will exist in a physical form that is stable and
easily
formulated.
The present invention therefore provides new nicotinamide derivatives of
formula
N R3
O
R~ ~ N O
H
N O
(I)
~S
R2 CH3
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
4
and pharmaceutically acceptable salts, pro-drugs, solvates and polymorphs
thereof wherein:
R~ and R2 are each independently selected from the group consisting of
s hydrogen, halo and (C~-C3)alkyl;
and wherein R3 is a 9- or 10-membered bicyclic heteroaryl containing from 1 to
4
nitrogen atoms wherein said bicyclic heteroaryl is optionally substituted by
one or
two groups selected from OH, halo, (C~-C4)alkyl, (C~-C4)alkoxy, hydroxy(C~
to C4)alkyl and hydroxy(C2-C4)alkoxy.
Preferably R~ is H, F, CI or methyl, more preferably R~ is F.
Preferably R2 is H or F, more preferably R2 is H.
Preferably R3 is a 9 or 10 membered bicyclic heteroaryl containing from 1 to 3
nitrogen atoms wherein said bicyclic heterocyclic ring system is optionally
substituted by one or two groups selected from OH, halo, (C~-C4)alkyl, (C~-
C4)alkoxy and hydroxy(C~-C4)alkyl.
More preferably R3 is a C-linked 9 or 10 membered bicyclic heteroaryl
containing
from 1 to 3 nitrogen atoms wherein said bicyclic heteroaryl is optionally
substituted by one or two groups selected from OH, F, CI, (C~-C4)alkyl, (C~-
C4)alkoxy and hydroxy(C~-C4)alkyl.
2s
Yet more preferably R3 is a C-linked 9 or 10 membered bicyclic heteroaryl
containing from 1 to 3 nitrogen atoms wherein said bicyclic heteroaryl is
optionally
substituted by one or two groups selected from OH, (C~-C3)alkyl, (C~-C3)alkoxy
and hydroxy(C~-C3)alkyl.
Particularly preferred R3 groups are selected from the group consisting of:
indole,
isoindole, indolizine, indazole, benzoimidazole, imidazopyridine,
pyrrolopyridazine, pyrrolopyridine, benzotriazole, pyrazolopyridine,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
s
imidazopyridine, quinoline, isoquinoline, cinnoline, quinoxaline, quinazoline,
phthalazine, and naphthyridine.
Highly preferred R3 groups are selected from the group consisting of:
indazole,
benzoimidazole, benzotriazole, imidazo[1,2-a]pyridine, pyrrolo[1,2-
b]pyridazine
s and quinoline.
Preferred optional substitutent groups for the bicyclic ring system of R3 are
selected from OH, methyl, ethyl, propyl, hydroxymethyl and hydroxyethyl.
io According to a further aspect the present invention provides compounds of
formula (I) wherein R~ is H, F, CI or methyl; R2 is H or F; and R3 is a C-
linked 9 or
membered bicyclic heteroaryl containing from 1 to 3 nitrogen atoms wherein
said bicyclic heteroaryl is optionally substituted by one or more groups
selected
from OH, halo, (C~-C4)alkyl, (C~-C4)alkoxy and hydroxy(C~-C4)alkyl.
is
According to a preferred aspect the present invention provides compounds of
formula (I) wherein R' is F; R2 is F; and wherein the ring system of R3 is an
optionally substituted bicyclic heteroaryl selected from the group consisting
of:
indazole, benzoimidazole, benzotriazole, imidazo[1,2-a]pyridine, pyrrolo-[1,2-
2o b]pyridazine and quinoline.
Preferred compounds according to the present invention are selected from the
group consisting of:
Syn-Pyrazolo[1,5-a]pyridine-2-carboxylic acid (4-{[5-fluoro-2-(3-
methylsulfanyl-
2s phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
Syn-1-Isopropyl-1 H-benzoimidazole-4-carboxylic acid (4-{[5-fluoro-2-(3-
methylsulfanyl-phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
Syn-Imidazo[1,2-a]pyridine-2-carboxylicacid (4-~[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
3o Syn-1 H-Indazole-3-carboxylic acid (4-f[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-
pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
Syn-2-Methyl-3H-benzoimidazole-4-carboxylic acid (4-{[5-fluoro-2-(3-
methylsulfanyl-phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
6
Syn-Imidazo[1,2-a]pyridine-8-carboxylic acid (4-{[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
Syn-3H-Benzotriazole-4-carboxylic acid (4-{[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-pyridine-3-carbonyl]-am i no}-cyclohexyl )-amide;
s Syn-Quinoline-8-carboxylic acid (4-{[5-fluoro-2-(3-methylsulfanyl-phenoxy)-
pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
Syn-3-Hydroxy-quinoline-8-carboxylic acid (4-{[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide; and
Syn-1-(2-Hydroxy-ethyl)-1 H-indazole-3-carboxylic acid (4-{[5-fluoro-2-(3-
to methylsulfanyl-phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide
and pharmaceutically acceptable salts, pro-drugs, solvates and polymorphs
thereof.
More preferred compounds are selected from the group consisting of:
Is Syn-2-Methyl-3H-benzoimidazole-4-carboxylic acid (4-f[5-fluoro-2-(3-
methylsulfanyl-phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide;
Syn-Imidazo[1,2-a]pyridine-8-carboxylic acid (4-{[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl )-amide;
Syn-Quinoline-8-carboxylic acid (4-f[5-fluoro-2-(3-methylsulfanyl-phenoxy)-
2o pyridine-3-carbonyl]-amino}-cyclohexyl)-amide; and
Syn-3-Hydroxy-quinoline-8-carboxylic acid (4-{[5-fluoro-2-(3-methylsulfanyl-
phenoxy)-pyridine-3-carbonyl]-amino}-cyclohexyl)-amide
and pharmaceutically acceptable salts, pro-drugs, solvates and polymorphs
thereof.
2s
The present invention additionally provides compounds of formula (I) wherein
R~,
R2, and R3 are as previously defined and wherein the optional substituent
groups
of R3 additionally comprise hydroxymethoxy.
3o It has been found that these nicotinamide derivatives are inhibitors of
PDE4
isoenzymes, particularly useful for the treatment of inflammatory, respiratory
and
allergic diseases and conditions or for wounds.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
7
In the here above general formula (I), halo denotes a halogen atom selected
from
the group consisting of fluoro, chloro, bromo and iodo in particular fluoro or
chloro.
s (C~-C3)alkyl or (C~-C4)alkyl radicals denote a straight-chain or branched
group
containing respectively 1 to 3 and 1 to 4 carbon atoms. This also applies if
they
carry substituents or occur as substituents of other radicals, for example in
(C~-
C4)alkoxy radicals and hydroxy(C~-C4)alkyl radicals. Examples of suitable (C~-
C3)alkyl and (C~-C4)alkyl radicals are methyl, ethyl, n-propyl, iso-propyl, n-
butyl,
to iso-butyl, sec-butyl and tart-butyl. Examples of suitable (C~-C4)alkoxy
radicals are
methoxy, ethoxy, n-propyloxy, iso-propyloxy, n-butyloxy, iso-butyloxy, sec-
butyloxy
and tent-butyloxy. Hydroxy(C~-C4)alkyl and hydroxy(C2-C4)alkoxy radicals may
contain more than one hydroxy group (-OH). According to a preferred
embodiment of said invention, such radicals contain one hydroxy substituent.
Is Examples of suitable hydroxy(C~-C4)alkyl radicals are hydroxymethyl, 1-
hydroxyethyl or 2-hydroxyethyl.
In the hereabove general formula (I), "9- or 10-membered bicyclic heteroaryl"
means a radical of a bicyclic aromatic system having 9 or 10 ring members,
which contains 1, 2, 3 or 4 nitrogen (N) atoms) depending in number and
quality
20 of the total number of ring members. Examples of additional, optional
heteroatoms are oxygen (O) and sulphur (S). If several heteroatoms are
contained, these can be identical or different. Heteroaryl radicals can also
be
unsubstituted, monosubstituted or polysubstituted, as indicated in the
definition of
R3 hereabove for general formula (I) according to the present invention.
2s Preferably bicyclic heteroaryl is a bicyclic aromatic radical which
contains 1, 2 or
3 nitrogen (N). Examples of suitable bicyclic heteroaryl radicals are the
radicals
derivated from indole, isoindole, indolizine, indazole, purine, napthyridine,
phthalazine, quinoline, quinazoline, quinoxaline, cinnoline, isoquinoline,
benzoimidazole, imidazo[1,2-a]pyridine, benzotriazole, pyrazolo[1,5-a]pyridine
3o and pyrazolopyrimidine. Particularly preferred are the bicyclic
heterocyclic
radicals selected from indole, isoindole, indolizine, indazole,
benzoimidazole,
imidazopyridine, pyrrolopyridazine, pyrrolopyridine, benzotriazole,
pyrazolopyridine, imidazopyridine, quinoline, isoquinoline, cinnoline,
quinoxaline,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
8
quinazoline, phthalazine, and naphthyridine. Nitrogen bicyclic heteroaryl
radicals
can also be present as N-oxides or as quaternary salts.
In the general formula (I) according to the present invention, when a radical
is
s mono- or poly-substituted, said substituent(s) can be located at any desired
position(s). Also, when a radical is polysubstituted, said substituents can be
identical or different, unless otherwise stated.
The nicotinamide derivatives of the formula (I) can be prepared using
io conventional procedures such as by the following illustrative methods in
which
R~, R2 and R3 are as previously defined for the nicotinamide derivatives of
the
formula (I) unless otherwise stated. '
The compounds of formula (I) may be prepared by the methods disclosed
Is hereunder, and exemplified in the Examples and Preparations. Other methods
may be used in accordance with the skilled person's knowledge.
Unless otherwise provided herein:
PyBOPO means Benzotriazol-1-yloxytris(pyrrolidino)phosphonium
2o hexafluorophosphate;
PyBrOP~ means bromo-tris-pyrrolidino-phosphonium hexafluoro-
phosphate;
CDI means N,N'-carbonyldiimidazole;
WSCDI means 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
2s hydrochloride;
Mukaiyama's reagent means 2-chloro-1-methylpyridinium iodide;
HATU means O-(7-Azabenzotriazol-1-yl)-N,N,N'N'-tetramethyluronium
hexafluorophosphate;
HBTU means O-Benzotriazol-1-yl-N,N,N'N'-tetramethyluronium
so hexafluorophosphate;
DCC means N,N'-dicyclohexylcarbodiimide;
CDI means N,N'-carbonyldiimidazole;
HOAT means 1-hydroxy-7-azabenzotriazole;
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
9
HOBT means 1-hydroxybenzotriazole hydrate;
Hianig's base means N-ethyldiisopropylamine;
Et3N means triethylamine;
NMM means N-methylmorpholine;
s NMP means 1-methyl-2-pyrrolidinone;
DMAP means 4-dimethylaminopyridine;
NMO means 4-methylmorpholine N-oxide;
KHMDS means potassium bis(tr-imethylsilyl)amide;
NaHMDS means sodium bis(trimethylsilyl)amide;
Io DIAD means diisopropyl azodicarboxylate;
DEAD means diethyl azodicarboxylate;
DIBAL means diisobutylammonium hydride;
Dess-Martin periodinane means 1,1,1-triacetoxy-1,1-dihydro-1,2-
benziodoxol-3(1 H)-one;
is TBDMS-CI means tert butyldimethylchlorosilane;
TMS-CI means chlorotrimethylsilane;
Boc means tent-butoxycarbonyl;
CBz means benzyloxycarbonyl;
MeOH means methanol, EtOH means ethanol, and EtOAc means ethyl
2o acetate;
THF means tetrahydrofuran; DMSO means dimethyl sulphoxide; DCM
means dichloromethane; DMF means N,N-dimethylformamideAcOH
means acetic acid; TFA means trifluoroacetic acid; RT means room
temperature; 3° means tertiary; eq means equivalents; Me means methyl;
2s Et means ethyl; Bn means benzyl; other abbreviations are used in
accordance with standard synthetic chemistry practice.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
Route A
O
R~ H
OH N~PG R
N%\LG +
H2N
(II) (II!) (IV)
H
O N~PG
R~
~N
H
(b) N O (c)
-,~
V
( ) R~ CH3
O NHS N R3
R~ _
\ R
I ~ H (d~
N O
/ I
(VI) S ( S
2
R CH3 CH3
Nicotinic acids or acid derivatives of formula (II) are either available
commercially
or may be obtained by analogy with the methods of Haylor et. al. (EP 0634413
examples 9 and 10, pages 12-13), or Marzi et. al. (European Journal of Qrg.
to Chem. 2001 (7), 1371-1376). The protected amines of formula (III) are
either
available commercially or may be prepared by analogy with the method of Oku et
al (WO 99/54284, for example, at page 80, preparation 77(1 )).
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
11
In the scheme above, R~, R2 and R3 are as previously defined, PG is a suitable
amine protecting group, typically Boc, CBS or Bn, and preferably Boc, and LG
is a
suitable leaving group, typically halo, and preferably CI.
s Step~a)-Acid-amine coupling,,
This acid/amine coupling may be undertaken by using either:
(i) an acyl chloride derivative of acid or acid derivative (II) + amine (III),
with an
excess of acid acceptor in a suitable solvent; or
(ii) the acid or acid derivative (I1) with a conventional coupling agent +
amine (III),
to optionally in the presence of a catalyst, with an excess of acid acceptor
in a
suitable solvent.
Typically the conditions are as follows:
(i) acid chloride of acid (II) (generated in-situ), an excess of amine (lll),
Is optionally with an excess of 3° amine such as Et3N, Hunig's base
or NMM,
in DCM or THF, without heating for 1 to 24 hrs; or
(ii) acid (II), WSCDI IDCC/CDI optionally in the presences of HOBT or
HOAT, an excess of amine (Ill), with an excess of NMM, Et3N, Hianig's
2o base in THF, DCM or EtOAc, at RT for 4 to 48 hrs; or, acid (II), PYBOP~I
PyBrOP~/Mukaiyama's reagent, an excess of amine (III), with an excess of
NMM, Et3N, Hiinig's base in THF, DCM or EtOAc, at RT for 4 to 24 hrs.
The preferred conditions are: either treatment of (II) with oxalyl chloride
and
2s catalytic DMF in DCM at RT for 3 hours followed by the addition of Hunig's
base
or Et3N and the amine and stirring at RT for 18 hours; treatment of (II) with
CDI in
DMF at RT for 1 hour followed by the addition of the amine and stirring at RT
for
72 hours.
3o Step (b)-Ether formation
Substitution of the leaving group, LG, wherein said leaving group is for
example a
halogen and is preferably chlorine, of the compound (iV) with a substituted
phenol to give compounds of formula (V).
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
12
Compounds of general formula (V) can be prepared from compounds of general
formula (IV) via treatment with an optionally substituted, 3-methylsulphanyl-
phenol in the presence of a suitable base, in a suitable solvent. Alkali metal
salts
s are used as the base (e.g. Cs2C03, K2COs, NaOH) and MeCN, dioxan, toluene or
NMP are suitable solvents for use. The reaction is carried out at elevated
temperature.
Preferred conditions are: reaction of compound (IV), wherein the LG is
chlorine,
io with an excess of optionally substituted, 3-methylsulphanyl-phenol in the
presence of caesium carbonate in dioxan or MeCN at about 100°C,
optionally at
reflux temperatures, for from about 24 to about 72 hours.
Step (c)-Removal of protectincLgroup
is Deprotection of the N protecting group (PG), from compounds of general
formula
(V) to provide compounds of general formula (VI) is undertaken using standard
methodology, as described in "Protective Groups in Organic Synthesis" by T.W.
Greene and P. Wutz.
2o For example when PG is Boc, the preferred conditions are: treatment of
compound (V) with a strong acid (e.g. TFA, HCI), in a suitable solvent such as
for
example dioxan or DCM at room temperature. Preferred conditions herein for
removal of a Boc group are treatment with hydrochloric acid (preferably 4M
HCI)
in dioxan at RT for about 5 hrs. Exemplified herein as preparation 18.
30
Step (d)-Reaction of de-protected amino Group with R3COOH
Compounds of the general formula (I) may be prepared by reaction of amines of
general formula (VI) via treatment with a suitable acid of formula R3COOH
according to the general methods described previously for step (a).
The preferred conditions are: treatment of a solution of amine (VI) and acid
R3COOH in NMP or DMF, with WSCDI, HOBT and NMM or Hunig's base, at RT
for from about 18 to about 72 hours.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
13
The transformation (VI) to (I) is exemplified by Examples 1 to 9.
Route B
s Compounds of general formula (I) may alternatively be prepared by the
following
route.
O
NH2 R3~H N R3
PG~
PG
(d) ~N (c)
H
(III) (VII)
(II) Rs
N R3 R
H2N ~ (a)
IV Lh
(VIII) (I~)
to
N R3
O
R~
(b) ~ \
--~ H
i
N O
\ ~ (I)
~S
R2 CH3
The compound of formula (VII) may be prepared from the amine (III) by reaction
is with R3COOH according to the methods described previously in step (d),
Route
A. Preferred conditions provide stirring a solution of amine (III) in DCM with
Hianig's base, HOBT, WSCDI and acid R3COOH at RT for about 48 hours.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
14
The de-protected amine compound of general formula (VIII) may be prepared
from the protected amine compound of general formula (V11) via removal of the
protecting group PG, preferably a Boc group, by analogy to the methods
s described previously in step (c), Route A. Preferred conditions provide
compounds of general formula (Vlll) via treatment of a solution of (VII) in
DCM at
0°C with bubbled hydrogen chloride gas for about 2 hours followed by
stirring for
about 90 minutes at RT.
to The amide compounds of genera! formula {IX) may be prepared by reaction of
the amine of general formula (VIII) with the acid (II) according to the
methods
described previously in steps (a) and (d), Route A. Preferred conditions
provide
amide {IX) via treatment of a solution of amine (VIII) and the appropriate
nicotinic
acid (II) in DCM with Hunig's base, HOBT and WSCDI with stirring for about 18
is hours at RT.
Compounds of formula (I) may be prepared by substitution of the leaving group,
LG, of the compounds of formula (fX) by an optionally substituted, 3-
methylsulphanyl-phenol group as described previously in step (b), Route A.
2o Preferred conditions provide compounds of general formula (I) via treatment
of
compounds of general formula (IX) and optionally substituted, 3-
methylsulphanyl-
phenol in MeCN and DMF in the presence of caesium carbonate ~t reflux
temperatures from about 18 to about 36 hours.
2s The compounds of formula (I) may also be prepared by the process outlined
in
Route C.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
is
Route C
O O
R~ \ ~Ra~k R~ Rack
I O (b) I \ O~ (e) ...
N LG ~ NCO
(X)
(XI )
S
R2 CH3
O O N R
R~ (a) R~
\ ,oH I \
H
N O (VIII) N O
\I ~I
S \ S
R2 CH3 R2 CH3
(XII) (I)
s
wherein Rack represents a C~-C4 alkyl group or Bn, preferably a C~-C3 alkyl
group
and more preferably Et.
Compounds of formula (X) are either available commercially or may be obtained
io from the compounds of formula (II), using standard esterification
conditions. The
protected amines of formula (III) are either available commercially or may be
prepared by analogy with the method of Oku et. al. (WO 99/54284) as described
hereinbefore.
is Compounds of formula (XI) may be prepared by reaction of the ester (X) with
optionally substituted, 3-methylsulphanyl-phenol, as described previously in
step
(b), Route A. Suitable optional catalysts for use in this reaction include
Cul.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
16
Preferred conditions for use herein are treatment with caesium carbonate in
dioxan at about 100°C for about 48 hours. Exemplified herein by
preparation 15.
Stec (e)-Ester hydrolysis
s Hydrolysis of the ester (XI) may be achieved in the presence of acid or
base, in a
suitable solvent, optionally at elevated temperature to afford the acid (XII).
Typically, the ester (XI) is treated with a suitable base such as an alkali
metal
hydroxide (eg LiOH, NaOH) or a carbonate base (eg K2C03, Cs2C03) in aqueous
solvent (MeOH, EtOH, dioxan, THF) at RT, to give the acid (XII). Preferred
to conditions herein provide for treatment of ester (XI) in THF with a 1 M
aqueous
solution of LiOH at RT for about 2 hours. Exemplified herein by preparation
16.
Alternatively compounds of formula (XII) may be prepared from compounds of
formula (II) by reaction with optionally substituted 3-methylsulphanyl-phenol,
as
Is described previously in step (b), Route A.
Reaction of the acid of formula (Xll) with the amine of formula (VIII) as
described
previously in Route A, step (a) and Route B, step (a) provides the compounds
of
formula (I). Preferred conditions herein for formation of compounds of formula
(I)
2o from the corresponding acid of formula (XII) are treatment of acid (XII) in
DCM
and DMF with oxalyl chloride for about 2 hours at RT (to form the acid
chloride),
followed by treatment with a solution of the amine (VIII) and Et3N in DCM at
RT
for about 48 hours.
2s Compounds of formula (V) as described in Route A, may alternatively be
prepared by reaction of acid (Xll) with the protected amine (III), according
to the
methods described for step (a), of Route A. This is exemplified herein by
preparation 17.
so Further Routes
Certain R3 groups may undergo further functional group interconversions (FGIs)
and transformations, such as aikylation of a hydroxy substituent group, using
a
suitable alkylbromide, in the presence of a suitable alkali metal base (such
as
K2C03), optionally in the presence of a catalyst (eg KI) in a suitable solvent
such
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
17
as acetonitrile and/or N,N-dimethylformamide at elevated temperature, or
demethylation of a methoxy group by treatment with lithium iodide in pyridine
or
collidine, or by treatment with BBr3 in dichloromethane.
s As detailed hereinbefore for certain compounds of the description, a
suitable
protecting group strategy may be employed. For example, a hydroxyl group may
be protected using a tetrahydropyran group, and deprotection may be achieved
by treatment with a solution of acetic acid:wateraetrahydrofuran (4:1:2 by
volume)
at RT for up to 18 hrs. Further, a benzyloxy group may be used and deprotected
to to give the corresponding hydroxyl compound, for example by using a
reduction
(e.g. with palladium black in acid).
For example, reaction of amine (VI) with a carboxylic acid of the formula,
QR3COOH, wherein Q is an alcohol protecting group (eg THP or phenyl,
is preferably THP), to provide a protected amide can be carried out as
described in
step (c) of Scheme A. Preferred conditions for such reaction are: treatment of
a
solution of amine (VI) in NMP with the carboxylic acid, QR3COOH, HOST,
WSCDI and Hunig's base at RT for about 72 hours.
2o Removal of protecting group, Q, from the protected amide can be achieved by
a
standard method specific for that protecting group, as described in
"Protective
Groups in Organic Synthesis" by T.W. Greene and P. Wutz. Preferred conditions
for such deprotection herein, when Q = THP, are: treatment of (VII) with a
AcOH:water (4:1 by volume) mixture at about 60°C for about 17
hours.
2s Protection/deprotection strategies are exemplified in Preparation 19 and in
Example 10 herein.
All of the above reactions and the preparations of novel starting materials
using in
the preceding methods are conventional and appropriate reagents and reaction
3o conditions for their performance or preparation as well as procedures for
isolating
the desired products will be well-known to those skilled in the art with
reference to
literature precedents and the examples and preparations hereto.
For some of the steps of the here above described process of preparation of
the
3s nicotinamide derivatives of formula (I), it can be necessary to protect the
potential
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
18
reactive functions that are not wished to react. In such a case, any
compatible
protecting radical can be used. In particular methods such as those described
by
T.W. GREENE (Protective Groups in Organic Synthesis, A. Wiley-Interscience
Publication, 1981 ) or by McOMIE (Protective Groups in Organic Chemistry,
s Plenum Press, 1973), can be used.
Also, the nicotinamide derivatives of formula (I) as well as intermediate for
the
preparation thereof can be purified according to various well-4cnown methods,
such as for example crystallization or chromatography.
to
Thus according to a further embodiment the present invention provides a
process
for the preparation of a nicotinamide derivative of the formula (I) as
described in
claim 1 comprising:
(i) reaction of amines of general formula (VI) via treatment with a suitable
is acid of formula R3COOH; or
(ii) substitution of the leaving group, LG, of the compounds of formula (IX)
by an optionally substituted, 3-methylsulphanyl-phenol group; or
(iii) reaction of the acid of formula (XII) with the amine of formula (VIII)
2o wherein formulae (VI), (IX) and (XII) are as defined hereinbefore.
The present invention additionally provides compounds of the general formulae
(VI), (IX) and (XII) as defined hereinbefore.
2s According to a yet further embodiment the present invention provides
processes
for the preparation of compounds of general formulae (VI), (IX) and (XII)
wherein
said processes are as illustrated by sfieps (a), (b) and (c) Route A, steps
(c) and
(a) Route B and steps (b) and (e) Route C herein.
3o The nicotinamide derivatives of formula (I) may also be optionally
transformed in
pharmaceutically acceptable salts. In particular, these pharmaceutically
acceptable salts of the nicotinamide derivatives of the formula (I) include
the acid
addition and the base salts (including disalts) thereof.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
19
Suitable acid addition salts are formed from acids which form non-toxic salts.
Examples include the acetate, aspartate, benzoate, besylate,
bicarbonate/carbonate, bisulphate, camsylate, citrate, edisylate, esylate,
fumarate, gluceptate, gluconate, glucuronate, hibenzate,
hydrochloride/chloride,
s hydrobromide/bromide, hydroiodide/iodie, hydrogen phosphate, isethionate, D-
and L-lactate, malate, maleate, malonate, mesylate, methylsulphate, 2-
napsylate,
nicotinate, nitrate, orotate, palmoate, phosphate, saccharate, stearate,
succinate
sulphate, D- and L-tartrate, 1-hydroxy-2-naphtoate, 3-hydroxy-2-naphthoate and
tosylate saltes.
to
Suitable base salts are formed from bases which form non-toxic salts. Examples
include the aluminium, arginine, benzathine, calcium, choline, diethylamine,
diolarnine, glycine, lysine, magnesium, meglumine, olamine, potassium, sodium,
tromethamine and zinc salts.
Is
For a review on suitable salts, see Stahl and Wermuth, Handbook of
Pharmaceutical Salts: Properties, Selection and Use, Wiley-VCH, Weinheim,
Germany (2002).
2o A pharmaceutically acceptable salt of a nicotinamide derivative of the
formula (I)
may be readily prepared by mixing together solutions of the nicotinamide
derivative of formula (I) and the desired acid or base, as appropriate. 'f'he
salt
may precipitate from solution and be collected by filtration or may be
recovered
by evaporation of the solvent.
2s
Pharmaceutically acceptable solvates in accordance with the invention include
hydrates and solvates wherein the solvent of crystallization may be
isotopically
substituted, e.g. D20, d6-acetone, d6-DMSO.
3o Also within the scope of the invention are clathrates, drug-host inclusion
complexes wherein, in contrast to the aforementioned solvates, the drug and
host
are are present in non-stoichiometric amounts. For a review of such complexes,
see J Pharm Sci, 64 (8), 1269-1288 by Haleblian (August 1975).
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
Hereinafter all references to nicotinamide derivatives of formula (I) include
references to salts thereof and to solvates and clathrates of compounds of
formula (I) and salts thereof.
s The invention includes all polymorphs of the nicotinamide derivatives of
formula
Also within the scope of the invention are so-called "prodrugs" of the
nicotinamide
derivatives of formula (I). Thus certain derivatives of nicotinamide
derivatives of
Io formula (I) which have little or no pharmacological activity themselves
can, when
metabolised upon administration into or onto the body, give rise to
nicotinamide
derivatives of formula (i) having the desired activity. Such derivatives are
referred
to as "prodrugs".
is Prodrugs in accordance with the invention can, for example, be produced by
replacing appropriate functionalities present in the nicotinamide derivatives
of
formula (1) with certain moieties known to those skilled in the art as "pro-
moieties"
as described, for example, in "Design of Prodrugs" by H Bundgaard (Elsevier,
1985).
Finally, certain nicotinamide derivatives of formula (I) may themselves act as
prodrugs of other nicotinamide derivatives of formula (I).
Nicotinamide derivatives of formula (I) containing one or more asymmetric
carbon
2s atoms can exist as two or more optical isomers. Where a nicotinamide
derivative
of formula (l) contains an alkenyl or alkenylene group, geometric cis/trans
(or Z/E)
isomers are possible, and where the nicotinamide derivative contains, for
example, a keto or oxime group, tautomeric isomerism ('tautomerism') may
occur.
It follows that a single nicotinamide derivative may exhibit more than one
type of
3o isomerism.
Included within the scope of the present invention are all optical isomers,
geometric isomers and tautomeric forms of the nicotinamide derivatives of
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
21
formula (I), including compounds exhibiting more than one type of isomerism,
and
mixtures of one or more thereof.
Cis/trans isomers may be separated by conventional techniques well known to
s those skilled in the art, for example, fractional crystallisation and
chromatography.
Conventional techniques for the preparation/isolation of individual
stereoisomers
include the conversion of a suitable optically pure precursor, resolution of
the
racemate (or the racemate of a salt or derivative) using, for example, chiral
to HPLC, or fractional crystallisation of diastereoisomeric salts formed by
reaction of
the racemate with a suitable optically active acid or base, for example,
tartaric
acid.
The present invention also includes all pharmaceutically acceptable isotopic
is variations of a nicotinamide derivative of formula (I). An isotopic
variation is
defined as one in which at least one atom is replaced by an atom having the
same atomic number, but an atomic mass difFerent from the atomic mass usually
found in nature.
2o Examples of isotopes suitable for inclusion in the nicotinamide derivatives
of the
invention include isotopes of hydrogen, such as 2H and 3H, carbon, such as ~3C
and ~4C, nitrogen, such as ESN, oxygen, such as X70 and ~s0, phosphorus, such
as 32P, sulphur, such as 35S, fluorine, such as ~$F, and chlorine, such as
36CI.
2s Substitution of the nicotinamide derivative of formula (I) isotopes such as
deuterium, i.e. 2H, may afford certain therapeutic advantages resulting from
greater metabolic stability, for example, increased in vivo half-life or
reduced
dosage requirements, and hence may be preferred in some circumstances.
3o Certain isotopic variations of the nicotinamide derivatives of formula (I),
for
example, those incorporating a radioactive isotope, are useful in drug and/or
substrate tissue distribution studies. The radioactive isotopes tritium, i.e.
3H, and
carbon-14, i.e. ~4C, are particularly useful for this purpose in view of their
ease of
incorporation and ready means of detection.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
22
Isotopic variations of the nicotinamide derivatives of formula (1) can
generally be
prepared by conventional techniques known to those skilled in the art or by
processes analogous to those described in the accompanying Examples and
s Preparations using appropriate isotopic variations of suitable reagents.
According to a further aspect, the present invention concerns mixtures of
nicotinamide derivatives of the formula (I), as well as mixtures with or of
their
pharmaceutically acceptable salts, solvates, polymorphs, isomeric forms andlor
to isotope forms.
According to the present invention, all the here above mentioned forms of the
nicotinamide derivatives of formula (I) except the pharmaceutically acceptable
salts (i.e. said solvates, polymorphs, isomeric forms and isotope forms), are
Is defined as "derived forms" of the nicotinamide derivatives of formula (I)
in what
fol lows.
The nicotinamide derivatives of formula (I), their pharmaceutically acceptable
salts and/or derived forms, are valuable pharmaceutical active compounds,
which
2o are suitable for the therapy and prophylaxis of numerous disorders in which
the
PDE4 enzymes are involved, in particular the inflammatory disorders, allergic
disorders, respiratory diseases and wounds.
The nicotinamide derivatives of formula (I) and their pharmaceutically
acceptable
25 salts and derived forms as mentioned above can be administered according to
the invention to animals, preferably to mammals, and in particular to humans,
as
pharmaceuticals for therapy or prophyVaxis. They can be administered per se,
in
mixtures with one another or in combination with other drugs, or in the form
of
pharmaceutical preparations which permit enteral (gastric) or parenteral (non-
3o gastric) administration and which as active constituent contain an
efficacious
dose of at least one nicotinamide derivative of the formula (I), its
pharmaceutically
acceptable salts and/or derived forms, in addition to customary
pharmaceutically
innocuous excipients and/or additives. The term "excipient" is used herein to
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
23
describe any ingredient other than the compound of the invention. The choice
of
excipient will to a large extent depend on the particular mode of
administration.
The nicotinamide derivatives of formula (I), their pharmaceutically acceptable
s salts and/or derived forms may be freeze-dried, spray-dried, or
evaporatively
dried to provide a solid plug, powder, or film of crystalline or amorphous
material.
Microwave or radio frequency drying may be used for this purpose.
ORAL ADMINISTRATION
to The nicotinamide derivatives of formula (I) their pharmaceutically
acceptable salts
and/or derived forms of the invention may be administered orally. Oral
administration may involve swallowing, so that the compound enters the
gastrointestinal tract, or buccal or sublingual administration may be employed
by
which the compound enters the blood stream directly from the mouth.
is
Formulations suitable for oral administration include solid formulations such
as
tablets, capsules containing particulates, liquids, or powders, lozenges
(including
liquid-filled), chews, multi- and nano-particulates, gels, films (including
muco-
adhesive), ovules, sprays and liquid formulations.
Liquid formulations include suspensions, solutions, syrups and elixirs. Such
formulations may be employed as fillers in soft or hard capsules and typically
comprise a carrier, for example, water, ethanol, propylene glycol,
methylcellulose,
or a suitable oil, and one or more emulsifying agents and/or suspending
agents.
2s Liquid formulations may also be prepared by the reconstitution of a solid,
for
example, from a sachet.
The nicotinamide derivatives of formula (I), their pharmaceutically acceptable
salts and/or derived forms of the invention may also be used in fast-
dissolving,
3o fast-disintegrating dosage forms such as those described in Expert Opinion
in
Therapeutic Patents, 11 (6), 981-986 by Liang and Chen (2001 ).
The composition of a typical tablet in accordance with the invention may
comprise:
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
24
Ingredient % w/w
Nicotinamide derivative of formula 10.00*
(1 ) ~
Microcrystalline cellulose 64.12
Lactose 21.38
Croscarmellose sodium 3.00
Magnesium stearate 1.50
* Quantity adjusted in accordance with drug activity.
s A typical tablet may be prepared using standard processes known to a
formulation chemist, for example, by direct compression, granulation (dry,
wet, or
melt), melt congealing, or extrusion. The tablet formulation may comprise one
or
more layers and may be coated or uncoated.
Io Examples of excipients suitable for oral administration include carriers,
for
example, cellulose, calcium carbonate, dibasic calcium phosphate, mannitol and
sodium citrate, granulation binders, for example, polyvinylpyrrolidine,
hydroxypropylcellulose, hydroxypropylmethylcellulose and gelatin,
disintegrants,
for example, sodium starch glycolate and silicates, lubricating agents, for
is example, magnesium stearate and stearic acid, wetting agents, for example,
sodium lauryl sulphate, preservatives, anti-oxidants, flavours and colourants.
Solid formulations for oral administration may be formulated to be immediate
and/or modified release. Modified release formulations include delayed-,
2o sustained-, pulsed-, controlled dual-, targeted and programmed release.
Details
of suitable modified release technologies such as high energy dispersions,
osmotic and coated particles are to be found in Verma et al, Pharmaceutical
Technology On-line, 25(2), 1-14 (2001). Other modified release formulations
are
described in US Patent No. 6,106,864.
as
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
PARENTERAL ADMINISTRATION
The nicotinamide derivatives of formula (I), their pharmaceutically acceptable
salts and/or derived forms of the invention may also be administered directly
into
the blood stream, into muscle, or into an internal organ. Suitable means for
s parenteral administration include intravenous, intraarterial,
intraperitoneal,
intrathecal, intraventricular, intraurethral, intrasternal, intracranial,
intramuscular
and subcutaneous. Suitable devices for parenteral administration include
needle
(including microneedle) injectors, needle-free injectors and infusion
techniques.
io Parenteral formulations are typically aqueous solutions which may contain
excipients such as salts, carbohydrates and buffering agents (preferably to a
pH
of from 3 to 9), but, for some applications, they may be more suitably
formulated
as a sterile non-aqueous solution or as a dried form to be used in conjunction
with a suitable vehicle such as sterile, pyrogen-free water.
is
The preparation of parenteral formulations under sterile conditions, for
example,
by lyophilisation, may readily be accomplished using standard pharmaceutical
techniques well known to those skilled in the art.
2o The solubility of nicotinamide derivatives of formula (I) used in the
preparation of
parenteral solutions may be increased by suitable processing, for example, the
use of high energy spray-dried dispersions (see WO 01!47495) and/or by~the use
of appropriate formulation techniques, such as the use of solubility-enhancing
agents.
Formulations for parenteral administration may be formulated to be immediate
and/or modified release. Modified release formulations include delayed-,
sustained-, pulsed-, controlled dual-, targeted and programmed release.
so TOPICAL ADMINISTRATION
The nicotinamide derivatives of the invention may also be administered
topically
to the skin or mucosa, either dermally or transdermally. Typical formulations
for
this purpose include gels, hydrogels, lotions, solutions, creams, ointments,
dusting powders, dressings, foams, films, skin patches, wafers, implants,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
26
sponges, fibres, bandages and microemulsions. Liposomes may also be used.
Typical carriers include alcohol, water, mineral oil, liquid petrolatum, white
petrolatum, glycerin and propylene glycol. Penetration enhancers may be
incorporated - see, for example, J Pharm Sci, 88 (10), 955-958 by Finnin and
s Morgan (October ~ 999).
Other means of topical administration include delivery by iontophoresis,
electroporation, phonophoresis, sonophoresis and needle-free or microneedle
infection.
to
Formulations for topical administration may be formulated to be immediate
andlor
modified release. Modified release formulations include delayed-, sustained-,
pulsed-, controlled dual-, targeted and programmed release. Thus nicotinamide
derivatives of formula (I) may be formulated in a more solid form for
Is administration as an implanted depot providing long-term release of the
active
compound.
INHALED/INTRANASAL ADMINISTRATION
The nicotinamide derivatives of formula (I) can also be administered
intranasally
20 or by inhalation, typically in the form of a dry powder (either alone, as a
mixture,
for example, in a dry blend with lactose in anhydrous or monohydrate form,
preferably monohydrate, mannitol, dextran, glucose, maltose, sorbitof,
xylitof,
fructose, sucrose or trehalose, or as a mixed component particle, for example,
mixed with phospholipids) from a dry powder inhaler or as an aerosol spray
from
2s a pressurised container, pump, spray, atomiser (preferably an atomiser
using
electrohydrodynamics to produce a fine mist), or nebuliser, with or without
the
use of a suitable propellant, such as dichlorofluoromethane.
The pressurised container, pump, spray, atomiser, or nebuliser contains a
3o solution or suspension of the active compound comprising, for example,
ethanol
(optionally, aqueous ethanol) or a suitable alternative agent for dispersing,
solubilising, or extending release of the active, the propellants) as solvent
and an
optional surfactant, such as sorbitan trioleate or an oligolactic acid.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
27
Prior to use in a dry powder or suspension formulation, the drug product is
micronised to a size suitable for delivery by inhalation (typically less than
5
microns). This may be achieved by any appropriate comminuting method, such
as spiral jet milling, fluid bed jet milling, supercritical fluid processing
to form
s nanoparticles, high pressure homogenisation, or spray drying.
A suitable solution formulation for use in an atomiser using
electrohydrodynamics
to produce a fine mist may contain from 1Ng to 20mg of the nicotinamide
derivative of formula (I) per actuation and the actuation volume may vary from
1,u1
to to 100,u1. A typical formulation may comprise a nicotinamide derivative of
formula
(I), propylene glycol, sterile water, ethanol and sodium chloride. Alternative
solvents which may be used instead of propylene glycol include glycerol and
polyethylene glycol.
is Capsules, blisters and cartridges (made, for example, from gelatin or HPMC)
for
use in an inhaler or insufflator may be formulated to contain a powder mix of
the
nicotinamide derivative of formula (I), a suitable powder base such as lactose
or
starch and a performance modifier such as I-leucine, mannitol, or magnesium
stearate.
In the case of dry powder inhalers and aerosols, the dosage unit is determined
by
means of a valve which delivers a metered amount. Units in accordance with the
invention are typically arranged to administer a metered dose or "puff'
containing
from 1 Ng to 4000 ,ug of the nicotinamide derivative of formula (I). The
overall
2s daily dose will typically be in the range 1 ,ug to 20 mg which may be
administered
in a single dose or, more usually, as divided doses throughout the day.
Formulations for inhaled/intranasal administration may be formulated to be
immediate and/or modified release. Modified release formulations include
3o delayed-, sustained-, pulsed-, controlled dual-, targeted and programmed
release. Sustained or controlled release can be obtained by using for example
poly(D,L-lactic-co-glycolic acid).
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
2$
Flavouring agents, such as methol and levomethol and/or sweeteners such as
saccharing or saccharin sodium can be added to the formulation.
RECTAUINTRAVAGINAL ADMINISTRATION
s The nicotinamide derivatives of formula (I) may be administered rectally or
vaginally, for example, in the form of a suppository, pessary, or enema. Cocoa
butter is a traditional suppository base, but various alternatives may be used
as
appropriate.
io Formulations for rectal/vaginal administration may be formulated to be
immediate
and/or modified release. Modified release formulations include delayed-,
sustained-, pulsed-, controlled dual-, targeted and programmed release.
OCULAR/ANDIAL ADMINISTRATION
is The nicotinamide derivatives of formula (I) may also be administered
directly to
the eye or ear, typically in the form of drops of a micronised suspension or
solution in isotonic, pH-adjusted, sterile saline. Other formulations suitable
for
ocular and andial administration include ointments, biodegradable (e.g.
absorbable gel sponges, coNagen) and non-biodegradable (e.g. silicone)
2o implants, wafers, lenses and particulate or vesicular systems, such as
niosomes
or liposomes. A polymer such as crossed-linked polyacrylic acid,
polyvinylalcohol,
hyaluronic acid, a celiulosic polymer, for example,
hydroxypropylmethylcellulose,
hydroxyethylcellulose, or methyl cellulose, or a heteropolysaccharide polymer,
for
example, gelan gum, may be incorporated together with a preservative, such as
2s benzalkonium chloride. Such formulations may also be delivered by
iontophoresis.
Formulations for ocular/andial administration may be formulated to be
immediate
and/or modified release. Modified release formulations include delayed-,
3o sustained-, pulsed-, controlled dual-, targeted, or programmed release.
ENABLING TECHNOLOGIES
The nicotinamide derivatives of formula (I) may be combined with soluble
macromolecular entities such as cyclodextrin or polyethylene glycol-containing
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
29
polymers to improve their solubility, dissolution rate, taste-masking,
bioavailability
and/or stability.
Drug-cyclodextrin complexes, for example, are found to be generally useful for
s most dosage forms and administration routes. Both inclusion and non-
inclusion
complexes may be used. As an alternative to direct complexation with the drug,
the cyclodextrin may be used as an auxiliary additive, i.e. as a carrier,
diluent, or
solubiliser. Most commonly used for these purposes are alpha-, beta- and
gamma-cyclodextrins, examples of which may be found in International Patent
to Applications Nos. WO 91111172, WO 94/02518 and WO 98/55148.
DOSAGE
For administration to human patients, the total daily dose of the nicotinamide
derivatives of formula (I) is typically in the range 0.001 mg/kg to 100 mglkg
Is depending, of course, on the mode of administration. The total daily dose
may be
administered in single or divided doses, The physician will readily be able to
determine doses for subjects depending on age, weight, health state and sex or
the patient as well as the severity of the disease.
2o According to another embodiment of the present invention, the nicotinamide
derivatives of the formula (I), their pharmaceutically acceptable salts and/or
their
derived forms, can also be used as a combination with one or more additional
therapeutic agents to be co-administered to a patient to obtain some
particularly
desired therapeutic end result. The second and more additional therapeutic
2s agents may also be a nicotinamide derivatives of the formula (I), their
pharmaceutically acceptable salts and/or their derived forms, or one or more
PDE4 inhibitors known in the art. More typically, the second and more
therapeutic
agents will be selected from a different class of therapeutic agents.
3o As used herein, the terms "co-administration", "co-administered" and "in
combination with", referring to the nicotinamide derivatives of formula (I)
and one
or more other therapeutic agents, is intended to mean, and does refer to and
include the following
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
~ simultaneous administration of such combination of nicotinamide
derivatives) and therapeutic agents) to a patient in need of treatment,
when such components are formulated together into a single dosage form
which releases said components at substantially the same time to said
s patient,
~ substantially simultaneous administration of such combination of
nicotinamide derivatives) and therapeutic agents) to a patient in need of
treatment, when such components are formulated apart from each other
into separate dosage forms which are taken at substantially the same time
to by said patient, whereupon said components are released at substantially
the same time to said patient,
~ sequential administration of such combination of nicotinamide derivatives)
and therapeutic agents) to a patient in need of treatment, when such
components are formulated apart from each other into separate dosage
is forms which are taken at consecutive times by said patient with a
significant time interval between each administration, whereupon said
components are released at substantially different times to said patient;
and
~ sequential administration of such combination of nicotinamide derivatives)
2o and therapeutic agents) to a patient in need of treatment, when such
components are formulated together into a single dosage form which
releases said components in a controlled manner whereupon they are
concurrently, consecutively, and/or overlappingly administered at the same
and/or different times by said patient.
Suitable examples of other therapeutic agents which may be used in combination
with the nicotinamide derivatives of the formula (I), their pharmaceutically
acceptable salts and/or their derived forms include, but are by no mean
limited to:
(a) 5-Lipoxygenase (5-LO) inhibitors or 5-lipoxygenase activating protein
(FLAP) antagonists,
(b) Leukotriene antagonists (LTRAs) including antagonists of LTB4, LTC4,
LTD4, and LTE4,
(c) Histaminic receptor antagonists including H1, H3 and H4 antagonists,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
31
(d) a1- and a2-adrenoceptor agonist vasoconstrictor sympathomimetic agents
for decongestant use,
(e) Muscarinic M3 receptor antagonists or anticholinergic agents,
(f) a2-adrenoceptor agonists,
s (g) Theophylline,
(h) Sodium cromoglycate,
(i) COX-1 inhibitors (NSAIDs) and COX-2 selective inhibitors,
(j) Oral or inhaled Glucocorticosteroids,
(k) Monoclonal antibodies active against endogenous inflammatory entities,
to (I) Anti-tumor necrosis factor (anti-TNF-a) agents,
(m) Adhesion molecule inhibitors including VLA-4 antagonists,
(n) Kinin-B1 - and B2 -receptor antagonists,
(o) Immunosuppressive agents,
(p) Inhibitors of matrix metalloproteases (MMPs),
is (q) Tachykinin NK1, NK2 and NK3 receptor antagonists,
(r) Elastase inhibitors,
(s) Adenosine A2a receptor agonists,
(t) Inhibitors of urokinase,
(u) Compounds that act on dopamine receptors, e.g. D2 agonists,
20 (v) Modulators of the NFkb pathway, e.g. IKK inhibitors,
(w) Agents that can be classed as mucolytics or anti-tussive,
(x) antibiotics, and
(y) p38 MAP kinase inhibitors
2s According to the present invention, combination of the nicotinamide
derivatives of
formula (I) with
~ muscarinic M3 receptor agonists or anticholinergic agents including in
particular ipratropium salts, namely bromide, tiotropium salts, namely
bromide, oxitropium salts, namely bromide, peren~epine, and telenzepine,
30 ~ /32-adrenoceptor agonists including albutarol, salbutamol, formoterol and
salmeterol,
~ p38 MAP kinase inhibitors,
~ H3 antagonists,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
32
~ glucocorticosteroids, in particular inhaled glucocorticosteroids with
reduced
systemic side effects, including prednisone, prednisolone, flunisolide,
triamcinolone acetonide, beclomethasone dipropionate, budesonide,
fluticasone propionate, and mometasone furoate,
s ~ or adenosine A2a receptor agonists,
are preferred.
It is to be appreciated that all references herein to treatment include
curative,
palliative and prophylactic treatment. The description which follows concerns
the
io therapeutic applications to which the nicotinamide derivatives of formula
(I) may
be put.
The nicotinamide derivatives of formula (I) inhibit the PDE4 isozyme and
thereby
have a wide range of therapeutic applications, as described further below,
is because of the essential role, which the PDE4 family of isozymes plays in
the
physiology of all mammals. The enzymatic role performed by the PDE4 isozymes
is the intracellular hydrolysis of adenosine 3',5'-monophosphate (CAMP) within
pro-inflammatory leuleocytes. cAMP, in turn, is responsible for mediating the
effects of numerous hormones in~ the body, and as a consequence, PDE4
2o inhibition plays a significant role in a variety of physiological
processes. There is
extensive literature in the art describing the effects of PDE inhibitors on
various
inflammatory cell responses, which in addition to CAMP increase, include
inhibition of superoxide production, degranulation, chemotaxis and tumor
necrosis factor (TNF) release in eosinophils, neutrophils and monocytes.
2s
Therefore, a further aspect of the present invention relates to the use of the
nicotinamide derivatives of formula (I), their pharmaceutically acceptable
salts
and/or derived forms, in the treatment of diseases, disorders, and conditions
in
which the PDE4 isozymes are involved. More specifically, the present invention
3o also concerns the use of the nicotinamide derivatives of formula (I), their
pharmaceutically acceptable salts and/or derived forms, in the treatment of
diseases, disorders, and conditions selected from the group consisting of
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
33
~ asthma of whatever type, etiology, or pathogenesis, in particular asthma
that is a member selected from the group consisting of atopic asthma, non-
atopic asthma, allergic asthma, atopic bronchial IgE-mediated asthma,
bronchial asthma, essential asthma, true asthma, intrinsic asthma caused
s by pathophysiologic disturbances, extrinsic asthma caused by
environmental factors, essential asthma of unknown or inapparent cause,
non-atopic asthma, bronchitic asthma, emphysematous asthma, exercise-
induced asthma, allergen induced asthma, cold air induced asthma,
occupational asthma, infective asthma caused by bacterial, fungal,
io protozoal, or viral infection, non-allergic asthma, incipient asthma and
wheezy infant syndrome,
~ chronic or acute bronchoconstriction, chronic bronchitis, small airways
obstruction, and emphysema,
~ obstructive or inflammatory airways diseases of whatever type, etiology, or
is pathogenesis, in particular an obstructive or inflammatory airways disease
that is a member selected from the group consisting of chronic eosinophilic
pneumonia, chronic obstructive pulmonary disease (COPD), COPD that
includes chronic bronchitis, pulmonary emphysema or dyspnea associated
therewith, COPD that is characterized by irreversible, progressive airways
20 obstruction, adult respiratory distress syndrome CARDS) and exacerbation
of airways hyper-reactivity consequent to other drug therapy
~ pneumoconiosis of whatever type, etiology, or pathogenesis, in particular
pneumoconiosis that is a member selected from the group consisting of
aluminosis or bauxite workers' disease, anthracosis or miners' asthma,
2s asbestosis or steam-fitters' asthma, chalicosis or flint disease, ptilosis
caused by inhaling the dust from ostrich feathers, siderosis caused by the
inhalation of iron particles, silicosis or grinders' disease, byssinosis or
cotton-dust asthma and talc pneumoconiosis;
~ bronchitis of whatever type, etiology, or pathogenesis, in particular
3o bronchitis that is a member selected from the group consisting of acute
bronchitis, acute laryngotracheal bronchitis, arachidic bronchitis, catarrhal
bronchitis, croupus bronchitis, dry bronchitis, infectious asthmatic
bronchitis, productive bronchitis, staphylococcus or streptococcal
bronchitis and vesicular bronchitis,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
34
~ bronchiectasis of whatever type, etiology, or pathogenesis, in particular
bronchiectasis that is a member selected from the group consisting of
cylindric bronchiectasis, sacculated bronchiectasis, fusiform
bronchiectasis, capillary bronchiectasis, cystic bronchiectasis, dry
s bronchiectasis and follicular bronchiectasis,
~ seasonal allergic rhinitis or perennial allergic rhinitis or sinusitis of
whatever type, etiology, or pathogenesis, in particular sinusitis that is a
member selected from the group consisting of purulent or nonpurulent
sinusitis, acute or chronic sinusitis and ethmoid, frontal, maxillary, or
to sphenoid sinusitis,
~ rheumatoid arthritis of whatever type, etiology, or pathogenesis, in
particular rheumatoid arthritis that is a member selected from the group
consisting of acute arthritis, acute gouty arthritis, chronic inflammatory
arthritis, degenerative arthritis, infectious arthritis, Lyme arthritis,
is proliferative arthritis, psoriatic arthritis and vertebral arthritis,
~ gout, and fever and pain associated with inflammation,
~ an eosinophil-related disorder of whatever type, etiology, or pathogenesis,
in particular an eosinophil-related disorder that is a member selected from
the group consisting of eosinophilia, pulmonary infiltration eosinophilia,
2o Loffler's syndrome, chronic eosinophilic pneumonia, tropical pulmonary
eosinophilia, bronchopneumonic aspergillosis, aspergilloma, granulomas
containing eosinophils, allergic granulomatous angiitis or Churg-Strauss
syndrome, polyarteritis nodosa (PAN) and systemic necrotizing vasculitis,
~ atopic dermatitis, allergic dermatitis, contact dermatitis, or allergic or
atopic
2s eczema,
~ urticaria of whatever type, etiology, or pathogenesis, in particular
urticaria
that is a member selected from the group consisting of immune-mediated
urticaria, complement-mediated urticaria, urticariogenic material-induced
urticaria, physical agent-induced urticaria, stress-induced urticaria,
3o idiopathic urticaria, acute urticaria, chronic urticaria, angioedema,
cholinergic urticaria, cold urticaria in the autosomal dominant form or in the
acquired form, contact urticaria, giant urticaria and papular urticaria,
~ conjunctivitis of whatever type, etiology, or pathogenesis, in particular
conjunctivitis that is a member selected from the group consisting of actinic
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
conjunctivitis, acute catarrhal conjunctivitis, acute contagious
conjunctivitis,
allergic conjunctivitis, atopic conjunctivitis, chronic catarrhal
conjunctivitis,
purulent conjunctivitis and vernal conjunctivitis,
~ uveitis of whatever type, etiology, or pathogenesis, in particular uveitis
that
s is a member selected from the group consisting of inflammation of all or
part of the uvea, anterior uveitis, iritis, cyclitis, iridocyclitis,
granulomatous
uveitis, nongranufomatous uveitis, phacoantigenic uveitis, posterior uveitis,
choroiditis; and chorioretinitis,
~ psoriasis;
io ~ multiple sclerosis of whatever type, etiology, or pathogenesis, in
particular
multiple sclerosis that is a member selected from the group consisting of
primary progressive multiple sclerosis and relapsing remitting multiple
sclerosis,
~ autoimmune/inflammatory diseases of whatever type, etiology, or
is pathogenesis, in particular an autoimmunelinflammatory disease that is a
member selected from the group consisting of autoimmune hematological
disorders, hemolytic anemia, aplastic anemia, pure red cell anemia,
idiopathic thrombocytopenic purpura, systemic lupus erythematosus,
polychondritis, scleroderma, Wegner's granulomatosis, dermatomyositis,
2o chronic active hepatitis, myasthenia gravis, Stevens-Johnson syndrome,
idiopathic sprue, autoimmune inflammatory bowel diseases, ulcerative
colitis, endocrin opthamopathy, Grave's disease, sarcoidosis, alveolitis,
chronic hypersensitivity pneumonitis, primary biliary cirrhosis, juveniie
diabetes or diabetes mellitus type I, keratoeonjunctivitis sicca, epidemic
2s keratoconjunctivitis, diffuse interstitial pulmonary fibrosis or
interstitial lung
fibrosis, idiopathic pulmonary fibrosis, cystic fibrosis, glomerulonephritis
with and without nephrotic syndrome, acute glomerulonephritis, idiopathic
nephrotic syndrome, minimal change nephropathy,
inflammatory/hyperproliferative skin diseases, benign familial pemphigus,
3o pemphigus erythematosus, pemphigus foliaceus, and pemphigus vulgaris,
~ prevention of allogeneic graft rejection following organ transplantation,
~ inflammatory bowel disease t;IBD) of whatever type, etiology, or
pathogenesis, in particular inflammatory bowel disease that is a member
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
36
selected from the group consisting of collagenous colitis, colitis polyposa,
transmuraf colitis, ulcerative colitis and Crohn's disease (CD),
~ septic shock of whatever type, etiology, or pathogenesis, in particular
septic shock that is a member selected from the group consisting of renal
s failure, acute renal failure, cachexia, malarial cachexia, hypophysial
cachexia, uremic cachexia, cardiac cachexia, cachexia suprarenalis or
Addison's disease, cancerous cachexia and cachexia as a consequence of
infection by the human immunodeficiency virus (HIV),
~ liver injury,
to ~ pulmonary hypertension of whatever type, etiology or pathogenesis
including primary pulmonary hypertension / essential hypertension,
pulmonary hypertension secondary to congestive heart failure, pulmonary
hypertension secondary to chronic obstructive pulmonary disease,
pulmonary venous hypertension, pulmonary arterial hypertension and
Is hypoxia-induced pulmonary hypertension,
~ bone loss diseases, primary osteoporosis and secondary osteoporosis,
~ central nervous system disorders of whatever type, etiology, or
pathogenesis, in particular a central nervous system disorder that is a
member selected from the group consisting of depression, Alzheimers
2o disease, Parkinson's disease, learning and memory impairment, tardive
dyskinesia, drug dependence, arteriosclerotic dementia and dementias
that accompany Huntington's chorea, Wilson's disease, paralysis agitans,
and thalamic atrophies,
~ infection, especially infection by viruses wherein such viruses increase the
2s production of TNF-a in their host, or wherein such viruses are sensitive to
upregulation of TNF-a in their host so that their replication or other vital
activities are adversely impacted, including a virus which is a member
selected from the group consisting of HIV-1, HIV-2, and HIV-3,
cytomegalovirus (CMV), influenza, adenoviruses and Herpes viruses
3o including Herpes zoster and Herpes simplex,
~ yeast and fungus infections wherein said yeast and fungi are sensitive to
upregulation by TNF-a or elicit TNF-a production in their host, e.g., fungal
meningitis, particularly when administered in conjunction with other drugs
of choice for the treatment of systemic yeast and fungus infections,
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
37
including but are not limited to, polymixins, e.g. Polymycin B, imidazoles,
e.g. clotrimazole, econazole, miconazole, and ketoconazole, triazoles, e.g.
fluconazole and itranazole as well as amphotericins, e.g. Amphotericin B
and liposomal Amphotericin B,
s ~ ischemia-reperfusion injury, ischemic heart disease, autoimmune diabetes,
retinal autoimmunity, chronic lymphocytic leukemia, HIV infections, lupus
erythematosus, kidney and ureter disease, urogenital and gastrointestinal
disorders and prostate diseases,
~ reduction of scar formation in the human or animal body, such as scar
to formation in the healing of acute wounds, and
~ psoriasis, other dermatological and cosmetic uses, including antiphlogistic,
skin-softening, skin elasticity and moisture-increasing activities.
According to one aspect the present invention relates in particular to the
Is treatment of a respiratory disease, such as adult respiratory distress
syndrome
CARDS), bronchitis, chronic obstructive pulmonary disease (COPD), cystic
fibrosis, asthma, emphysema, bronchiectasis, sinusitis and rhinitis.
According to another aspect the present invention relates in particular to the
2o treatment of gastrointestinal (GI) disorders, in particular inflammatory
bowel
diseases (IBD) such as Crohn's disease, ileitis, collagenous colitis, colitis
pofyposa, transmural colitis and ulcerative colitis.
According to a further aspect the present invention relates also to the
reduction of
2s scars formation.
A still further aspect of the present invention also relates to the use of the
nicotinamide derivatives of formula (I), their pharmaceutically acceptable
salts
and/or derived forms, for the manufacture of a drug having a PDE4 inhibitory
3o activity. In particular, the present inventions concerns the use of the
nicotinamide
derivatives of formula (I), their pharmaceutically acceptable salts and/or
derived
forms, for the manufacture of a drug for the treatment of inflammatory,
respiratory, allergic and scar-forming diseases, disorders, and conditions,
and
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
38
more precisely for the treatment of diseases, disorders, and conditions that
are
listed above.
As a consequence, the present invention provides a particularly interesting
s method of treatment of a mammal, including a human being, with a PDE4
inhibitor including treating said mammal with an effective amount of a
nicotinamide derivative of formula (1), its pharmaceutically acceptable salts
and/or
derived forms. More precisely, the present invention provides a particularly
interesting method of treatment of a mammal, including a human being, to treat
to an inflammatory, respiratory, allergic and scar-forming disease, disorder
or
condition, including treating said mammal with an effective amount of a
nicotinamide derivative of formula (i), its pharmaceutically acceptable salts
and/or
derived forms.
is The following examples illustrate the preparation of the nicotinamide
derivatives
of the formula (I):
Where Preparations or Examples are described as being effected by a method
"similar to" another method this means that minor differences in the practical
2o method may exist, such as for example use of recrystallisation rather than
column
chromatography in the purification stage or use of alternative solvents in
separation phase. However such minor differences are considered to be within
the common general knowledge and experimental experience of the skilled
chemist when approaching such reactions.
Preparation 1
2-Chloro-5-fluoro nicotinic acid
O
OH
i~
N- -CI
Ethyl-2-chloro-5-fluoro-nicotinoate (50.4g, 0.247mo1) (may be prepared
according
3o to the method of J. Med. Chem., 1993, 36(18), 2676-88, page 2684, column 2,
3~d
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
39
example, ethyl-2-chloro-5-fluoropyridine-3-carboxylate) was dissolved in
tetrahydrofuran, THF, (350mL) and a 2M aqueous solution of lithium hydroxide
(247mL, 0.495mo1) added. The reaction mixture was stirred at room temperature
for 3 days. The pH of the solution was reduced to pH1 by addition of 6M
s hydrochloric acid and then extracted with dichloromethane (x3). The combined
extracts were dried over magnesium sulphate and the solvent concentrated in
vacuo to give a solid which was triturated with diethyl ether and then dried
to give
the title compound as a white solid, 40.56g.
~ HNMR (DMSO-D6, 400MHz): 8.20 (s, 1 H), 8.62 (s, 1 H)
io MS ES+ m/z 174 [MH]+
Preparation 2
Syn-tent-Butyl 4-aminocyclohexylcarbamate
H CHs
N~O~CH3
IOI ~CH3
HZN
Is 5% Palladium on charcoal (5g) was mixed with toluene (10mL) and was added
to
syn-(4-azido-cyclohexyl)-carbamic acid terf butyl ester (170g, 0.71 mol)
(prepared
according to the method of WO 99/54284, pg 80, prep 77(1), cis-4-(N-tert-
Butoxycarbonylamino)cyclohexyl azide) in methanol (400mL). The mixture was
hydrogenated (80 atmospheres) at room temperature for 18 hours and then
2o filtered. The solvent was evaporated in-vacuo and the residue was
triturated with
ethyl acetate (50mL) and then with hexane (200mL). The solid obtained was
isolated by filtration, dissolved in ethyl acetate (600mL) and filtered
through
Celite~. The filtrate was concentrated in-vacuo to give a slush that was
diluted
with hexane (300mL). The solid obtained was isolated by filtration and was
2s washed with ethyl acetate in hexane (20:80). The mother liquors were
combined
and evaporated in-vaeuo. The residue was purified by chromatography on silica
gel using ethyl acetate and then methanol as eluant. The material obtained was
crystallised from ethyl acetate and hexane and combined with the first crop to
give the title compound as a white solid, 76.Og.
so Mpt 88-90°C
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
~HNMR (CDC13, 400MHz): 1.41 (s, 9H), 1.52-1.77(m, 8H), 1.82(m, 1 H), 1.97(m,
1 H), 2.61 (m, 1 H), 3.62(m, 1 H), 4.59(m, 1 H),
MS ES+ m/z 215 [MH]'~
Preparation 3
1-f2-(Tetrahydro-pyran-2-ylox~r -eth~]-1H-indazole-3-carboxylic acid ethyl
ester
'CH3
/O
O
/N
N
O O
Indazole-3-ethyl-carboxylate (prepared according to the method of Synthesis,
1984, {11 ), 982-983, page 983 product 6ca) (1.90g, 10.Ommol), 2-(2-
Io bromoethoxy)tetrahydropyran (2.25g, 10.8mmol), potassium carbonate (1.438,
10.4mmol) and lithium iodide (67mg, 0.50mmo1) was dissolved in 1-methyl-2-
pyrrolidinone (20mL) and the reaction mixture stirred at 80°C for 17
hours. The
reaction mixture was partitioned between ethyl acetate (250mL) and water
(250mL) and the organic layer washed with water (3x200mL), dried over
Is magnesium sulphate and concentrated in vacuo. The residue was purified by
column chromatography on silica gel eluting with pentane:ethyl acetate 10:1 to
5:1 to 3:1 to 2:1 to 1:1 to yield the title product, 1.88g.
~HNMR (DMSQ-D6, 400MHz): 1.20-1.53(m, 6H), 1.35)t, 3H), 3.30(m, 2H),
3.80(m, 1 H), 4.00(m, 1 H), 4.37(m, 2H), 4.48(m, 1 H), 4.70(m, 2H), 7.32(t,
2H),
20 7.80(d, 1 H), 8.05(d, 1 H),
MS ES+ m/z 341 [MNa]k
Preparation 4
1-f2- Tetra~dro-pyran-2-yloxy)-ethyll-1 H-indazole-3-carboxylic acid
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
41
A solution of sodium hydroxide (413mg, 10.33mmol) in water (3.75mL) was
added dropwise to a solution of the ester of preparation 3 (1.838, 5.74mmol)
in
ethanol (14.7mL) and the reaction mixture stirred at room temperature for 48
s hours. The reaction mixture was adjusted to pH 3 by treatment with 2M
hydrochloric acid. The solution was partitioned between ethyl acetate (75mL)
and water (75mL) and the aqueous layer extracted with ethyl acetate (3x60mL).
The organic layers were combined, dried over magnesium sulphate and
concentrated in vacuo to yield the title product as a white solid, 1.44g.
to ~HNMR (DMSO-D6, 400MHz): 1.20-1.55(m, 6H), 3.30(m, 2H), 3.80(m, 1H),
4.00(m, 1 H), 4.48(m, 1 H), 4.68(m, 2H), 7.28(m, 1 H), 7.46(m, 1 H), 7.80(d, 1
H),
8.08(d, 1 H), 12.90(s, 1 H)
MS ES- m/z 289 [M-H]'
is Preparation 5
2-Amino-1 ~3-ethoxy-2 3-dioxopropyl)pyridinium bromide
NH2 Br O
~ N+ O~CH3
/ O
Ethyl bromopyruvate (51.9g, 266mmol) was added dropwise to a solution of 2-
2o aminopyridine (25g, 266mmo1) in ethylene glycol dimethyl ether (270mL), and
the
reaction then stirred at room temperature for 1 hour. The resulting
precipitate
was filtered off, the solid washed with ether and dried to afford the title
compound
as a pale yellow solid, 71.9g.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
42
~ HNMR (CDC13, 300MHz): 1.35 (t, 3H), 4.35 (q, 2H), 4.70 (d, 1 H), 5.15 (d, 1
H),
7.10-7.20 (m, 2H), 8.10 (m, 1 H), 8.25 (d, 1 H).
Preparation 6
s Ethyl imidazof1 2-alpyridine-2-carbo~late hydrobromide
CH3
~N O-J
BrH
O
A suspension of the compound from preparation 5 (71.9g, 249mmol) in ethanol
(750mL) was heated at reflux for 3 hours, and then allowed to cool. The
mixture
to was concentrated in vacuo, the residue triturated with ether, filtered and
dried to
afford the title compound as a solid, 64.17g.
~HNMR (CD30D, 300MHz): 1.45 (t, 3H), 4.50 (q, 2H), 7.55 (m, 1 H), 7.95 (m, 1
H),
8.10 (dd, 1 H), 8.80 (s, 1 H), 8.85 (d, 1 H).
is Preparation 7
Imidazof1,2-alpyridine-2-carboxylic acid hydrobromide
N OH
BrH
O
A solution of the ester from preparation 6 (S.Og, 18.4mmol) in 10% aqueous
2o hydrobromic acid (90mL) was heated under reflux for 6 hours. The cooled
mixture was concentrated in vacu~ and the residue triturated with dioxan. The
resulting solid was filtered off, washing with hexane and the filtrate
concentrated
in vacuo. The residue was again triturated with dioxan, the solid filtered and
dried
to afford additional compound, 3.83g in total.
2s ~HNMR (CD30D, 300MHz): 7.57 (m, 1 H), 7.96 (d, 1 H), 8.06 (m, 1 H), 8.78
(s, 1 H),
8.84 (d, 1 H).
MS ES+ m/z 163 [MH]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
43
Preparation 8
Methyl imidazof 1,2-alpyridine-8-carboxylate
N~ N
,O
HsC v
O
A mixture of methyl 2-aminonicotinate (prepared according to the method of WO
s 89/01488 at page 33, prep 17) (1g, 6.56mmol), and chloroacetaldehyde
(1.05mL,
6.56mmol) in ethanol (5mL) was heated under reflux for 18 hours. The cooled
mixture was diluted with water (10mL), 0.88 ammonia (1 mL) added and the
solution concentrated in vacuo. The residue was dissolved in methanol and the
dark solution treated with charcoal, the mixture filtered and the filtrate
to concentrated in vacuo. The residue was purified by column chromatography on
silica gel using dichloromethane:methano1:0.88 ammonia (97:2.5:0.5) as eluant,
and the product triturated with ether, to afford the title compound, 768mg.
'HNMR (CDCI3, 400MHz): 4.02 (s, 3H), 6.83 (s, 1 H), 7.63 (s, 1 H), 7.79 (s, 1
H),
8.00 (d, 1 H), 8.31 (d, 1 H).
is MS TSP+ m/z 177.2 [MH+]
Preparation 9
Imidazof1,2-alpyridine-8-carboxylic acid
N~ N
HO
I
O
2o Lithium hydroxide solution (2.5mL, 1 M in water) was added to a solution of
the
ester from preparation 8 (400mg, 2.27mmol) in methanol (5mL) and the solution
stirred at room temperature for 90 minutes. The solution was concentrated in
vacuo to remove the methanol, the aqueous solution acidified using 2M
hydrochloric acid, and the mixture concentrated in vacuo to give the title
2s compound as a yellow solid.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
44
~ HNMR (DMSO-D6, 400MHz): 7.60 (dd, 1 H), 8.10 (s, 1 H), 8.41 (d, 1 H), 8.55
(s,
1 H), 9.18 (d, 1 H)
MS TSP+ m/z 163 [MH]+
Preparation 10
Ethyl 2-amino-3-isoprowlamino-benzoate
O O~CH3
NH2
NH
H C- 'CH
3 3
2-lodopropane (2.OmL, 20mmol) was added to a solution of ethyl 2,3-
to diaminobenzoate (prepared according to the method of WO 97/10219, page 81,
Example 51 (1 )) (3g, 16.67mmol) in N,N-dimethylformamide (20mL), and the
solution stirred at 50°C for 3 hours. The mixture was concentrated
under reduced
pressure and the residue partitioned between ethyl acetate (200mL) and water
(50mL), and the layers separated. The organic layer was washed with water
is (5x50mL), dried over magnesium sulphate and concentrated in vacuo. The
crude
product was purified by column chromatography on silica gel using ethyl
acetate:pentane (5:95 to 90:10) to afford the title compound as a yellow o.il,
1.4g.
~HNMR (CDCI3, 400MHz): 1.20 (d, 6H), 1.38 (t, 3H), 3.56 (m, 1H), 4.31 (q, 2H),
5.60 (brs, 2H), 6.84 (m, 1 H), 6.80 (d, 1 H), 7.42 (d, 1 H).
2o MS ES+ mlz 223 [MH]+
Preparation 11
1-Isopropyl-1 H-benzoimidazole-4-carboxylic acid
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
H3
H3C
A solution of the amine from preparation 10 (1.4g, 6.31mmol) in formic acid
(15mL) was stirred at 60°C for 45 minutes. 2M Hydrochloric acid (20mL)
and
additional formic acid (l5mL) were added and the reaction heated under reflux
for
s 12 hours. The cooled mixture was concentrated in vacuo and the residue
triturated initially with ethyl acetate and the solid filtered and dried. This
solid was
then triturated with hot ethyl acetate and the solid filtered and dried at
60°C to
give the title compound as a pale pink solid, 1.16g.
~HNMR (DMSO-D6, 400MHz): 1.61 (d, 6H), 5.10 (m, 1 H), 7.72 (m, 1 H), 8.13 (d,
to 1 H), 8.39 (d, 1 H), 9.75 (s, 1 H).
MS TSP+ m/z 205 [MH]+
Preparation 12
2-Amino-3-nitro-benzoic acid
N02
NH2
/ OH
I
2-Chloro-3-nitrobenzoic acid (20.6g, 0.24mo1) was dissolved in ammonium
hydroxide solution (120mL) and the reaction mixture stirred at 120°C in
a seated
vessel for 7 hours. The reaction mixture was diluted with water (250mL) and
acidified to pH 2 with hydrochloric acid. The precipitate formed was filtered
off
2o and dried in vacuo to yield the title product, 15.Og (80%).
~HNMR (CD30D, 400MHz): 6.65(dd, 1 H), 8.24(dd, 1 H), 8.32(dd, 1 H).
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
46
Preparation 13
2,3-Diamino-benzoic acid
NH2
NH2
/ OH
I
O
The nitro compound of preparation 12 (6.Og, 33.Ommol) was dissolved in
s methanol (150mL) and the solution treated with 10% Pd/C (300mg) and stirred
for
18 hours under 60psi of hydrogen. The reaction mixture was filtered to remove
the catalyst and the filtrate concentrated in vacuo. The residue was taken up
in
methanol (150mL) and treated with charcoal (S.Og) and the mixture stirred at
room temperature for 1 hour. The mixture was filtered and the filtrate
to concentrated in vacuo to yield the title product, 3.Og.
~HNMR (DMSO-D6, 400MHz): 6.29(m, 1 H), 6.64(m, 1 H), 7.03(m, 1 H).
Preparation 14
1 H-Benzotriazole-4-carboxylic acid
HO O
\ N,
N
N
is H
A solution of the diamine of preparation 13 (300mg, 1.97mmol) in acetic acid
(1 mL) and water (2mL) was treated dropwise with a solution of sodium nitrite
(151 mg, 2.20mmol) in water (2mL) and the reaction mixture stirred at room
temperature for 18 hours. The brown solid formed was filtered off, washed with
2o water and dried in vacuo to yield the title product, 220mg.
~HNMR (CD3OD, 400MHz): 7.53(dd, 1 H), 8.16(dd, 1 H), 8.24(m, 1 H).
MS ES+ m/z 164 [MH]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
47
Preparation 15
5-Fluoro-2-(3-methylsulfanyl-phenoxy)-nicotinic acid ethyl ester
O
F
O~CH3
NCO
S
I
CH3
A solution of ethyl-2-chloro-5-fluoro-nicotinoate (29g, 0.143mo1) (prepared
s according to the method of J. Med. Chem., 1993, 36(18), 2676-88, at page
2684,
column 2, 3~d compound, Ethyl 2-chloro-5-fluoropyridine-3-carboxylic acid) and
3-
methylsulphanyl-phenol (20g, 0.143mo1) (prepared according to the method of
WO 98/45268, page 68, preparation 61 ) in dioxane (300mL) was treated with
caesium carbonate (46.5g, 0.143mo1) at room temperature. The reaction mixture
to was heated to 100°C and stirred for 48 hours. The reaction mixture
was
concentrated in vacuo and the residue taken up in water (600mL) and extracted
with ethyl acetate (3 x 250mL). The organic layers were combined, washed with
brine (200mL), dried over magnesium sulphate and concentrated in vacuo. The
residue was purified by column chromatography on silica gel eluting with
is dichloromethaneaoluene (99.75:0.25 to 99.5:0.5) to yield the title product
as a
yellow oil , 27.1 g.
~HNMR(CDCI3, 400MHz): 1.37(t, 3H), 2.23(s, 3H), 4.40(q, 2H), 6.84(m, 1 H),
7.01 (m, 1 H) 7.08(m, 1 H), 7.26(m, 1 H), 7.98(m, 1 H), 8.13(m, 1 H).
MS APCI+ m/z 308 [MH]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
48
Preparation 16
5-Fluoro-2-(3-methylsulfanyl-phenoxy)-nicotinic acid
o
F
~OH
N O
S
I
CH3
The ester of preparation 15 (27.1g, 88.2mmol) was dissolved in tetrahydrofuran
s (300mL) and the solution treated with a 1 M aqueous solution of lithium
hydroxide
(220mL, 220mmol). The reaction mixture was stirred at room temperature for 2
hours. The reaction mixture was concentrated in vacuo to remove the
tetrahydrofuran and the aqueous was cooled to 0°C before being
acidified to pH
1 with hydrochloric acid. The resulting pink precipitate was removed by
filtration
to and washed with iced water. The solid was dissolved in dichloromethane
(800mL) and washed with acidified brine solution (200mL). The organic layer
was separated, dried over magnesium sulphate and concentrated in vacuo. The
residue was triturated with toluene to yield the title product as a white
solid,
22.13g.
is ~ HNMR(CD30D, 400MHz): 2.43(s, 3H), 6.83(m, 1 H), 7.01 (m, 1 H), 7.06(m, 1
H),
7.25(m, 1 H), 8.03(m, 2H).
MS APCI+ mlz 280 [MH]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
49
Preparation 17
Syn-(4-f (5-Fluoro-2-(3-methylsulfan~l-phenoxy)-wridine-3-carbonyll-amino~
cyclohexyl)-carbamic acid tert but I ester
H
O N O CH3
CHs
\ N O CH3
H
N"O
/I
S
I
CH3
s The acid of preparation 16 (5g, 17.9mmol) and N,N-dimethylformamide (5
drops)
were dissolved in dichloromethane (100mL) and the solution cooled to
0°C. This
was treated dropwise with oxalyl chloride (3.1 mL, 35.8mmol) over 15 minutes
and
then stirred at room temperature for 2 hours. The reaction mixture was
concentrated in vacuo and the residue taken up in dichloromethane (100mL).
to The solution was cooled to 0°C and treated with triethylamine
(7.5mL, 54mmol)
and the amine of preparation 2 (4.2g, 19.6mmol). The reaction mixture was
allowed to warm to room temperature and was stirred at room temperature for 48
hours. The reaction mixture was diluted with dichloromethane (100mL) and
washed with water (70mL), 10% citric acid solution (2 x 70mL), saturated
sodium
Is hydrogencarbonate solution (2 x 70mL) and water (70mL). The organic layer
was
dried over magnesium sulphate and concentrated in vacuo to yield the title
product, 8.Og.
~HNMR(CDCI3, 400MHz): 1.40(s, 9H), 1.53(m, 2H), 1.68(m, 2H), 1.77(m, 4H),
2.46(s, 3H), 3.60(m, 1 H), 4.18(m, 1 H), 4.37(m, 1 H), 6.88(m, 1 H), 7.02(m, 1
H),
20 7.17(m, 1 H), 7.37(m, 1 H), 7.93(m, 1 H), 8.06(m, 1 H), 8.36(m, 1 H).
MS ES+ m/z 476 [MH]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
Preparation 18
Syn-N-(4-Amino-cyclohexyl)-5-fluoro-2-i3-methylsulfanyl-phenoxy)-nicotinamide
h~rdrochloride
NH2
O
N
I H
N' _O .HC1
/ I
S
I
CH3
s The protected amine of preparation 17 (B.Og, 16.8mmol) was dissolved in
dioxan
(50mL) and the solution treated with a 4M solution of hydrochloric acid in
dioxan
(25mL). The reaction mixture was stirred at room temperature for 5 hours
before
being concentrated in yacuo and azeotroped with ethyl acetate and
dichloromethane to yield the title product, 5.Og.
to ~HNMR(CD30D, 400MHz): 1.67(m, 2H), 1.80-2.01(m, 6H), 2.45(s, 3H), 3.24(m,
1 H), 4.14(m, 1 H), 6.92(m, 1 H), 7.09(m, 1 H), 7.17(m, 1 H), 7.35(t, 1 H),
8.08(m,
2H).
MS ES+ m/z 376 [MH]+
Preparation 19
Syn-1-[2-(Tetrah~dro-pyran-2-foxy)-ethyll-1 H-indazole-3-carboxylic acid (4-
~f5-
fluoro-2-(3-meth Is~yl-phenoxY~~yridine-3-carbonyll-amino)-cyclohexyl)-
amide
i
~N~\/O O
~ /
O N
F \ N O
~~ "
S~c~3
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
51
The carboxylic acid of preparation 4 (145mg, 0.50mmol) was dissolved in 1-
methyl-2-pyrrolidinone (3mL) and the solution treated with 1-
hydroxybenzotriazole hydrate (74.3mg, 0.55mmol) and 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (115mg, 0.60mmol).
s The mixture was stirred for 10 minutes and was then treated with amine of
preparation 18 (200mg, 0.48mmol) and N-ethyldiisopropylamine (155mg,
1.20mmol) and stirred at room temperature for 72 hours. The reaction mixture
was partitioned between ethyl acetate (75mL) and water (75mL) and the organic
layer washed with water (2 x 75mL) and 5% sodium carbonate solution (75mL),
to dried over magnesium sulphate and concentrated in vacuo. The residue was
triturated with ether:pentane 1:1 to yield the title product as a white solid,
218mg.
~HNMR(DMSO-D6, 400MHz): 1.20-1.55(m, 6H), 1.73(m, 8H), 2.40(s, 3H), 3.23(m,
1 H), 3.32(m, ~1 H), 3.80(m, 1 H), 3.94(m, 1 H), 4.00(m, 2H), 4.48(m, 1 H),
4.66(m,
2H), 6.95(m, 1 H), 7.07(m, 2H), 7.23(m, 1 H), 7.33(m, 1 H), 7.44(m, 1 H),
7.50(m,
is 1 H), 7.75(m, 1 H), 8.00(m, 1 H), 8.12(m, 1 H), 8.25(s, 1 H), 8.29(m, 1 H).
MS ES+ m/z 670 [MNa]+
Example 1
Syn-Pyrazolo[1 5-a]pyridine-2-carboxylic acid (4-f(5-fluoro-2-(3-
methylsulfanyl-
2o phenoxy)-pyridine-3-carbon~rll-amino-cyclohexyl )-amide
i
H N
N ~N/
°
y N °
H
..iCHs
Pyrazolo[1,5-a]pyridine-2-carboxylic acid (J. Het. Chem., 18(6), 1981, 1149-
1152,
at page 1152) (81.8mg, 0.50mmol) was dissolved in 1-methyl-2-pyrrolidinone
(3mL) and the solution treated with 1-hydroxybenzotriazole hydrate (74.3mg,
2s 0.55mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
52
(115mg, 0.60mmol) and the mixture stirred at room temperature for 5 minutes.
The mixture was then treated with the amine of preparation 18 (200mg,
0.48mmol) and N-ethyldiisopropylamine (155mg, 1.20mmol) and the reaction
mixture stirred at room temperature for 48 hours. The reaction mixture was
s partitioned between ethyl acetate (75mL) and water (75mL) and the organic
layer
washed with water (3x50mL) and 0.880 ammonia in water (100mL), dried over
magnesium sulphate and concentrated in vacuo. The residue was triturated with
ether to yield the title product as a white solid, 223mg.
~HNMR(DMSO-D6, 400MHz): 1.70(m, 8H), 2.41 (s, 3H), 3.87(m, 1 H), 3.98(m, 1 H),
l0 6.94(m, 2H), 7.00(m, 1 H), 7.07(m, 2H), 7.38(m, 2H), 7.65(d, 1 H), 7.74(m,
1 H),
8.00(m, 1 H), 8.22(m, 2H), 8.62(m, 1 H).
MS ES+ miz 542 [MNa]+
Microanalysis: Observed - C = 62.39%, H = 5.06%, N = 13.35%
C2~H~sFN503S Calculated - C = 62.41 %, H = 5.04%, N = 13.48%
is
The following compounds, of the general formula below, were prepared by a
method similar to that described for example 1, using the appropriate
carboxylic
acid, R3COOH.
N R3
O
F O
\ ,H
N O
I
\ S/CHa
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
53
No. R Data
2 H3C' CH 'HNMR(DMSO-D6, 400MHz): 1.55(d, 6H),
Y 3
' 1.73(m, 8H), 2.38(s, 3H), 3.95(m,
1 H), 4.03(m,
N~ 1 H), 4.82(m, 1 H), 6.92(m, 1 H),
I 7.05(m, 2H),
/ N 7.27(t, 1 H), 7.36(m, 1 H), 8.20(m,
2H), 8.40(m,
2H), 9.93(d, 1 H).
MS ES+ m/z 584 [MNa]+
Microanalysis: Observed - C = 63.31
%, H =
5.84%, N = 12.41
CsoHs2FNsOsS Calculated - C = 64.15%,
H =
5.74%, N = 12.47%
3 ~ ~HNMR(DMSO-D6, 400MHz): 1.70(m, 8H),
J 2.41 (s, 3H), 3.90(m, 1 H), 3.99(m,
~ 1 H), 6.95(m,
N 2H), 7.06(m, 2H), 7.32(m, 2H), 7.56(d,
N 1 H),
8.00(m, 1 H), 8.23(m, 1 H), 8.27(m,
1 H), 8.35(s,
1 H), 8.55(d, 1 H).
MS ES+ m/z 542 [MNa]+
Microanalysis: Observed - C = 62.29%,
H =
5.19%, N = 13.28%
C2~H2sFN50sS Calculated - C = 62.41
%, H =
5.04%, N = 13.48%
4 N 'HNMR(DMSO-D6, 400MHz): 1.70(m, 8H),
N~ / ~ 2.42(s, 3H), 3.95(m, 2H), 6.95(d,
1 H), 7.07(m,
2H), 7.22(t, 1 H), 7.33(m, 1 H),
7.40(m, 1 H),
7.63(m, 2H), 8.01 (m, 1 H), 8.14(d,
1 H), 8.25(m,
2H), 13.50(s, 1 H).
MS ES+ m/z 542 [MNa]+
Microanalysis: Observed - C = 62.41
%, H =
5.05%, N = 13.44%
C2~H26FN503S Calculated - C = 62.41
%, H =
5.04%, N = 13.48%
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
54
H3C HNMR(CDC13, 400MHz): 1.83(m,4H), 1.95(m,
N
4H), 2.40(s, 3H), 2.51 4.22(m,
(s, 3H), 1 H),
HN / 4.31 (m, 1 H), 6.92(d, 1 H), 7.11
1 H), 7.03(m, (m,
1 H), 7.25(m, 2H), 7.29(m,7.58(m,
1 H), 1 H),
8.02(d, 1 H), 8.06(m, 1 H).
H), 8.36(m, 1
Microanalysis: Observed 60.69%,
- C = H = ~~
5.45%, N = 13.07%
C28H28FN5O3S Calculated 60.97%,
- C = H = ~
5.48I, N = 12.70%
~ Example 2 - The carboxylic acid of preparation 11 was used. The crude
product was purified by column chromatography on silica gel eluting with
dichloromethane:methanol 100:1 to 50:1 to 25:1 to 15:1.
s
~ Example 3 - The carboxylic acid of preparation 7 was used. The crude
product was purified by column chromatography on silica gel eluting with
dichloromethane:methanol 100:1 to 50:1 to 25:1 to 15:1.
to ~ Example 4 - 1 H-indazole-3-carboxylic acid, which is available from Fluka
was used. The crude product was purified by column chromatography on
silica gel eluting with dichloromethane:methanol 100:1 to 50:1 to 25:1 to
15:1 and then with pentane:ethyl acetate 10:1 to 3:1 to 2:1 to 1:1 fo 1:2 to
1:3.
is
~ Example 5 - 2-methyl-3H-benzoimidazole-4-carboxylic acid (J. Med.
Chem., 43, 2000, 4084-4097, at page 4090, column 2, 6'" compound) was
the carboxylic acid used. The organics were washed with 10% citric acid
before a basic wash.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
Example 6
Syn-Imidazof1,2-alpyridine-8-carboxylic acid- (4-ff5-fluoro-2-(3-methylsulfan~
phenoxy)-pyridine-3-carbonyl-amino-~clohexyl)-amide
N~ N
H
O ~ \
F
\N O
l
N O
S
I
CH3
s The amine of preparation 18 (250mg, 0.60mmol) was dissolved in N,N-
dimethylformamide (7mL) and the solution treated with 1-hydroxybenzotriazole
hydrate (84mg, 0.60mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (124mg, 0.69mmol), 4-methylmorpholine (205~,L, 1.88mmol) and
the carboxylic acid of preparation 9 (90mg, 0.55mmol). The reaction mixture
was
Io stirred at room temperature for 18 hours. The mixture was diluted with
ethyl
acetate:water 3:1 (28mL) and the aqueous extracted with ethyl acetate (x2).
The
organics were washed with 10% citric acid solution (7mL), saturated sodium
hydrogen carbonate solution (7mL) and brine (7mL). The organic layers were
dried over magnesium sulphate and concentrated in vacuo. The residue was
is purified by column chromatography on silica gel eluting with pentane:ethyl
acetate 70:30 to 20:80 to yield the title product, 80mg.
~HNMR(CDCI3, 400MHz): 1.91 (m, 8H), 2.43(s, 3H), 4.23(m, 1 H), 4.32(m, 1 H),
6.94(m, 2H), 7.06(m, 1 H), 7.14(m, 1 H), 7.31 (m, 1 H), 7.37(m, 1 H), 7.60(m,
1 H),
8.01 (m, 1 H), 8.06(m, 1 H), 8.17(x, 1 H), 8.24(d, 1 H), 8.36(m, 1 H),
10.53(m, 1 H).
2o MS ES+ m/z 520 [MH]+
Microanalysis: Observed - C = 60.71 °!°, H = 5.02%, N =
13.06%
C2~H26FN503S Calculated - C = 60.73%, H = 5.21 %, N = 13.11
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
56
Example 7
Syn-3H-Benzotriazole-4-carboxylic acid (4-~f5-fluoro-2-(3-methVlsulfan ~LI
phenoxy)-pyridine-3-carbonLrl]-amino-cyclohexyl)-amide
N=N
HN
H
O N \
F
~N O
H
N O
\ I
S
I
CH3
s The title compound was prepared by a method similar to that described for
example 6 using the carboxylic acid of preparation 14.
~HNMR(CD30D, 400MHz): 1.93(m, 8H), 2.38(s, 3H), 4.14(m, 1 H), 4.22(m, 1 H),
6.93(m, 1 H), 7.06(m, 2H), 7.28(m, 1 H), 7.61 (m, 1 H), 8.01 (m, 1 H), 8.04(m,
2H),
8.12(m, 1 H).
io MS ES+ m/z 521 [MH]+
Microanalysis: Observed - G = 59.28%, H = 4.95%, N = 15.75%
G2sHzsENsOsS Calculated - C = 59.17%, H = 4.93%, N = 15.92%
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
57
Example 8
Syn-Quinofine-8-carboxylic acid (4-(f5-fluoro-2-(3-methylsulfanyl-phenoxy)
pyridine-3-carbonyll-amino cl~ohexyl -amide
N
O
F O NJ
~N
H
N O
/
S
CH3
s The amine of preparation 18 (150mg, 0.36mmol), 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (91 mg, 0.47mmol), 1-hydroxybenzotriazole
hydrate (72mg, 0.47mmol), N-ethyldiisopropylamine (0.25mL, 1.40mmol) and 8-
quinolinecarboxylic acid (69mg, 0.40mmol) were dissolved in dichloromethane
(10mL) and the reaction mixture stirred at room temperature for 18 hours. The
io reaction mixture was diluted with 10% citric acid solution and the layers
separated by filtration through a phase separation tube. The organic layer was
concentrated and the residue purified by column chromatography on silica gel
eluting with dichloromethane:methanol 99:1 to 98:2 to yield the title product
as a
white solid, 51 mg.
is ~HNMR(DMSO-D6, 400MHz): 1.64-1.86(m, 8H), 2.36(s, 3H), 3.93(m, 1H), 4.14(m,
1 H), 7.09(m, 3H), 7.27(t, 1 H), 7.58(m, 1 H), 7.75(m, 1 H), 8.01 (m, 1 H),
8.20(m,
2H), 8.42(d, 1 H), 8.56(m, 2H), 8.82(m, 1 H), 11.35(m, 1 H).
MS ES+ mlz 553 [MNa]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
5~
Example 9
Syn-3-Hydroxy-auinoline-8-carboxylic acid (4-f)'5-fluoro-2-(3-methYlsulfanyl
phenoxy)-pyrid i ne-3-carbonyl]-amino)-cyclohexyl )-amide
H
N /
O
F O
~N OH
H
N O
S
CH3
s The title product was prepared by a method similar to that described for
example
8 using 3-hydroxy-quinoline-8-carboxylic acid as the starting acid (prepared
according to the method of Chem. Pharm. Bull., 17(11 ), 1969, 2293-2298, at
page 2297, compound (?CIV)).
'HNMR(DMSO-D6, 400MHz): 1.64-1.82(m, 8H), 2.36(s, 3H), 3.92(m, 1 H),
to 4.12(m, 1 H), 6.93(m, 1 H), 7.02(m, 2H), 7.25(t, 1 H), 7.60(t, 1 H),
7.67(m, 1 H),
8.00(d, 2H), 8.22(m, 1 H), 8.33(m, 1 H), 8.46(m, 1 H), 8.64(m, 1 H), 10.59(s,
1 H),
11.14(m, 1 H)
MS ES+ m/z 569 [MNa]+
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
s9
Example 10
Syn-1-(2-Hydroxy-ethyl)-1H-indazole-3-carboxylic acid (4-fj5-fluoro-2-(3-
methylsulfanyl-phenoxY~pyridine-3-carbonLrl]'-amino-cyclohexyl)-amide
H N
N 'N/ ~OH
F O
CH3
s The product of preparation 19 (200mg, 0.31 mmol) was dissolved in acetic
acid
(4mL), tetrahydrofuran (2mL) and water (1 mL) and the reaction mixture stirred
at
60°C for 17 hours. The reaction mixture was allowed to cool and was
then
poured into a 15% aqueous solution of potassium carbonate and extracted with
ethyl acetate (1x75mL, 3x40mL). The organic layers were combined, dried over
to magnesium sulphate and concentrated in vacuo. The residue was purified by
column chromatography on silica gel eluting with dichloromethane:ethyl acetate
5:1 to 3:1 to 1:1 to 1:2. The crude product was triturated with ether to yield
the
title product as a white solid, 105mg (60%).
~HNMR(DMSO-D6, 400MHz): 1.70(m, 8H), 2.42(s, 3H), 3.82(m, 2H), 3.92(m, 1 H),
is 4.00(m, 1 H), 4.50(t, 2H), 4.90(t, 1 H), 6.95(d, 1 H), 7.09(m, 2H), 7.24(t,
1 H), 7.33(t,
1 H), 7.42(m, 1 H), 7.54(d, 1 H), 7.73(m, 1 H), 8.05(m, 1 H), 8.13(m, 1 H),
8.25(s,
1 H), 8.33(d, 1 H).
MS ES+ m/z 586 [MNa]+
Microanalysis: Observed - C = 61.73%, H = 5.46%, N = 12.23%
2o C29H3oFN504S Calculated - C = 61.80%, H = 5.36%, N = 12.42%
IN VITRO ACTIVITY OF THE NICOTINAMIDE DERIVATIVES
The PDE4 inhibitory activity of the nicotinamide derivatives of the formula (1
) is
determined by the ability of compounds to inhibit the hydrolysis of cAMP to
AMP
2s by PDE4 (see also reference 1 ). Tritium labelled cAMP is incubated with
PDE4.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
Following incubation, the radiolabelled AMP produced is able to bind yttrium
silicate SPA beads. These SPA beads subsequently produce light that can be
quantified by scintillation counting. The addition of a PDE4 inhibitor
prevents the
formation of AMP from CAMP and counts are diminished. The ICSO of a PDE4
s inhibitor can be defined as the concentration of a compound that leads to a
50%
reduction in counts compared to the PDE4 only (no inhibitor) control wells.
The anti-inflammatory properties of the nicotinamide derivatives of the
formula (1 )
are demonstrated by their ability to inhibit TNFa release from human
peripheral
io blood mononuclear cells (see also reference 2). Venous blood is collected
from
healthy volunteers and the mononuclear cells purified by centrifugation
through
Histopaque (Ficoll) cushions. TNFa production from these cells is stimulated
by
addition of lipopolysaccharide. After 18 hours incubation in the presence of
LPS,
the cell supernatant is removed and the concentration of TNFa in the
supernatant
is determined by ELISA. Addition of PDE4 inhibitors reduces the amount of TNFa
produced. An ICSO is determined which is equal to the concentration of
compound
that gives 50% inhibition of TNFa production as compared to the LPS stimulated
control wells.
2o All the examples were tested in the assay described above and found to have
an
IC5o (TNFa screen) of less than 10 nM. And for most of the tested compounds,
they were found to have an IC5o (TNFa screen) of even less than 1 nM.
For illustrating purpose, the following table indicates the exact IC5o (TNFa
screen)
2s of some representative examples of the present invention:
Example N ICSO (nM) Example N ICSO (nM)
1 0.1 6 0.02
2 1.0 7 0.4
3 0.1 8 0.25
4 2.0 9 0.15
5 0.04 10 0.3
References.
CA 02533681 2006-O1-24
WO 2005/010001 PCT/IB2004/002365
61
1. Thompson JW, Teraski WL, Epstein PM, Strada SJ., "Assay of
nucleotidephosphodiesterase and resolution of multiple molecular forms of the
isoenzyme", Advances in cyclic nucleotides research, edited by Brooker G,
Greengard P, Robinson GA. Raven Press, New York 1979, 10, p. 69-92.
s 2. Yoshimura T, Kurita C, Nagao T, Usami E, Nakao T, Watanabe S, Kobayashi
J, Yamazaki F, Tanaka H, Nagai H., "Effects of CAMP-phosphodiesterase
isozyme inhibitor on cytokine production by lipopolysaccharide-stimulated
human
peripheral blood mononuclear cells", Gen. Pharmacol., 1997, 29(4), p. 63
to