Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
POLYVINYL CHLORIDE GLOVE HAVING IMPROVED CHEMICAL
RESISTANCE
BACKGROUND
In recent years, there has been an increasing emphasis in the medical
community on developing gloves that offer various degrees and types of
protection. Medical practitioners are frequently exposed to solvents such as
isopropyl alcohol and other chemicals that may puncture the glove and
compromise the barrier afforded by the glove. Gloves formed from
thermoplastic resins, such as polyvinyl chloride (1'VC), have a history of
poor
permeation resistance to some chemicals relative to gloves formed from a
coagulated rubber latex, such as natural rubber or nitrile rubber. As such,
there
is a recognized need for a PVC glove with improved resistance to chemical
p ermeation.
SUMMARY OF THE INVENTION
The present invention generally relates to a glove having improved
chemical permeation resistance. The glove includes a substrate body formed
from polyvinyl chloride, and a barrier layer overlying at least a portion of
the
substrate body, where the barrier layer is formed from an acrylic polymer
having
a glass transition temperature of from about -30°C to about
30°C. The baxrier
layer may be present in any suitable amount, and in some instances, may be
present in an amount of from about 3 mass % to about ~ mass % of the glove.
In other instances, the barrier layer may be present in an amount of from
about 4
mass % to about 6 mass % of the glove. The barrier layer may be a skin-
contacting layer. In some instances, the barrier layer may be visually
distinct
from the substrate body. The glove may also include a donning layer overlying
at
least a portion of the barrier layer, where the donning layer is a skin-
contacting
layer.
The present invention further relates to a glove having improved chemical
permeation resistance including a substrate body formed polyvinyl chloride, a
barrier layer overlying at least a portion of the substrate body, where the
barrier
layer is formed from an acrylic polymer, and a donning layer overlying at
least a
portion of the barrier layer, where the donning layer is formed from a
1
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
polyurethane. In some instances, the acrylic polymer may have a glass
transition
temperature of from about -30°C to about 30°C. In other
instances, the acrylic
polymer may have a glass transition temperature of from about -20°C to
about
20°C. In yet other instances, the acrylic polymer may have a glass
transition
temperature of from about -10°C to about 10°C. The glove is
generally resistant
to 70% isopropyl alcohol for at least 90 minutes using ASTM F739-99a. In
some instances, the glove may be resistant to 70% isopropyl alcohol for at
least
100 minutes using ASTM F739-99a. In other instances, the glove may be
resistant to 70% isopropyl alcohol for at least 110 minutes using ASTM F739-
99a. In yet other instances, the glove may be resistant to 70% isopropyl
alcohol
for at least 120 minutes using ASTM F739-99a.
The present invention also relates to a method of forming a glove having
improved chemical permeation resistance. The method includes preparing a
substrate body from a polyvinyl chloride plastisol, and forming a barrier
layer
over at least a portion of the substrate body, where the barrier layer is
formed
from a barrier layer composition comprising an acrylic emulsion. The method
may include forming a donning layer over at least a portion of the barrier
layer.
The method may further include rendering the barrier layer visually distinct
from
the substrate body by, for example, adding a colorant to the barrier layer
2o composition.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a perspective view of a glove that may be formed according to
the present invention;
FIG. 2A is an exemplary cross-sectional illustration of the article of FIG. 1
taken along a line 2-2, the article including a substrate body and a barrier
layer;
and
FIG. 2B is an exemplary cross-sectional illustration of the article of FIG. 1
taken along a line 2-2, the article including a substrate body, a barrier
layer, and a
donning layer.
DESCRIPTION
The present invention generally relates to an article having improved
resistance to chemical permeation, and a method of forming such an article.
The
2
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
article of the present invention features improved resistance to chemical
permeation through use of a barrier layer formed from an acrylic emulsion. In
particular, the article of the present invention features improved permeation
resistance to isopropyl alcohol as measured by ASTM F739-99a entitled
"Standard Test Method for Resistance of Protective Clothing Materials to
Permeation by Liquids or Gases Under Conditions of Continuous Contact".
The glove is generally resistant to 70% isopropyl alcohol for at least 80
minutes
using ASTM F739-99a. In some instances, the glove may be resistant to 70%
isopropyl alcohol for at least 85 minutes using ASTM F739-99a. In other
instances, the glove may be resistant to 70% isopropyl alcohol for at least 90
minutes using ASTM F739-99a. In other instances, the glove may be resistant to
70% isopropyl alcohol for at least 100 minutes using ASTM F739-99a. In yet
other instances, the glove may be resistant to 70% isopropyl alcohol for at
least
110 minutes using ASTM F739-99a. In yet other instances, the glove may be
resistant to 70% isopropyl alcohol for at least 120 minutes using ASTM F739-
99a.
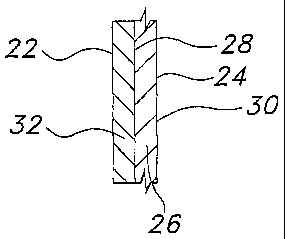
An article made according to the present invention, for example, a glove
20, generally includes an inside surface 22 and an outside surface 24 (FIG.
1). As
used herein, the "inside surface" refers to the surface of the article that
contacts
the body of the wearer. As used herein, the "outside surface" refers to the
surface of the article that is distal from the body of the wearer. The glove
includes a substrate body 26 having a first surface 28 and a second surface 30
(FIG.'s 2A-2B). As used herein, "first surface" refers to the surface of the
substrate body proximal to the body of the wearer. As used herein, "second
surface" refers to the surface of the substzate body distal to the body of the
wearer.
The article of the present invention may include a single layer or multiple
layers as desired. In a single layer glove including only the substrate body,
the
first surface may form the inside surface of the glove. However, in a multi-
layer
glove having additional layers proximal to the body of the wearer, the
additional
layer or layers may each form a portion of the inside surface, or the entire
inside
surface, as desired. Likewise, in a single layer glove including only the
substrate
body, the second surface may form the outside surface of the glove. However,
in a multi-layer glove having additional layers distal from the body of the
wearer,
3
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
the additional layer or layers may each form a portion of the outside surface,
or
the entire outside surface, as desired.
For example, as depicted in FIG. 2A, the article may include a barrier layer
32 overlying at least a portion of the first surface 28 of the substrate
body26. In
such an article, the barrier layer 32 forms at least a portion of the inside
surface
22 of the glove 20. .As depicted in FIG. 2B, the article may also include
other
layers, such as a donning layer 34 that overlies at least a portion of the
barrier
layer 32. In such an article, the donning layer 34 forms at least a portion of
the
inside surface 22 of the glove 20.
The substrate body 26 (FIG.'s 2A 2B) is generally formed from a
polymeric material, and in some instances, may be formed from a thermoplastic
polymer resin. In one such embodiment, the substrate body may be formed
from a polyvinyl chloride (PVC) resin. While articles formed from a PVC resin
are described in detail herein, it should be understood that any other
suitable
polymeric material or combination of polymeric materials may be used with the
present invention.
The barrier layer 32 (FIG.'s 2A 2B) may be formed from any suitable
polymer that provides increased chemical resistance, and in some embodiments,
may be formed from an acrylic polymer. While any suitable acrylic polymer may
2o be used as desired, it has been discovered that use of an acrylic polymer
that has
a glass transition temperature of from about -30°C to about 30°C
provides a
barrier layer that is durable even after donning or manipulation of the
article. In
some embodiments, an acrylic polymer having a glass transition temperature of
from about -20°C to about 20°C may be used to form the barrier
layer of the
present invention. In other embodiments, an acrylic polymer having a glass
transition temperature of from about -10°C to about 10°C may be
used to form
the barrier layer of the present invention. In one such embodiment, an acry3ic
polymer having a glass transition temperature of about -3 °C may be~
used to form
the barrier layer of the present invention.
3o The acrylic polymer may be provided in any suitable manner, and in some
instances, may be provided as an acrylic emulsion. In some instances, it may
be
desirable to select an acrylic emulsion that readily forms a film without use
of
crosslinking agents, curatives, or the like. In some such instances, the
acrylic
emulsion may form a film at room temperature. This provides a significant
4
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
process advantage over other polymer coatings that require curing to form a
durable coating.
One such acrylic emulsion that may be suitable for use with the present
invention is commercially available from Noveon, Inc. (Cleveland, Ohio) under
the trade name HYCAR~ 2679 Emulsion ("HYCAR° 2679"). HYCAR~ 2679
has a glass transition temperature of about -3 °C and is believed to
contain about
50 mass % TSC, less than 50 mass % water, and a small amount of formaldehyde.
Another acrylic emulsion that may be suitable for use with the present
invention
is commercially available from Noveon, Inc. (Cleveland, Ohio) under the trade
1o name HYCAR° 2671 Emulsion ("HYCAR° 2671"). HYCAR~ 2671 is
believed to contain about 53 mass % TSC, less than 47 mass % water, and a
small amount of formaldehyde. Yet another acrylic emulsion that may be
suitable for use with the present invention is commercially available from
Noveon, Inc. (Cleveland, Ohio) under the trade name HYCAR~ 26349
Emulsion ("HYCAR~ 26349"). HYCAR~ 26349 has a glass transition
temperature of about 15°C and is believed to contain about 49 mass %
TSCy less
than about 51 mass % water, and a small amount of formaldehyde.
In some embodiments, the barner layer may be visually distinct from the
substrate body. For instance, the barrier layer may include a colorant that
2o enables the wearer to recognize the existence of multiple layers in the
glove.
Alternatively, the substrate body may include a colorant to create
visually.distinct
layers. In one embodiment, the substrate body and the barrier layer may each
include a colorant, so that the inside of the glove is predominantly one
color,
while the outside of the glove is another color.
Any suitable colorant may be used to create a visual distinction between
layers as desired. One such colorant that may be suitable for use with the
present
invention is commercially available from Sun Chemical Corporation (Ameba,
Ohio) under the trade name FLEXIVERSE° Phthalo Blue Dispersion
("FLEXIVERSE° "). FLEXIVERSE~ is a resin based aqueous dispersion that
3o is believed to contain an acrylic resin, phthalocyanine blue, and water.
Additionally, the glove of the present invention may include a donning
layer 34 (FIG. 2B). The donning layer may be formed from any polymeric
material that facilitates donning of the article, and in some instances, may
include
a polyurethane. One such polyurethane that may be suitable for use with the
5
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
present invention is available from Soluol Chemical Co., Inc. (West Warwick,
Rhode Is land) under the trade name S OLUCOTE ~ 117-179. S OLUCOTE
117-179 is provided as a waterborne polyurethane dispersion having from about
10-20 mass % total solids content (TSC).
In other embodiments, the donning layer may be formed from a blend of
an acrylic polymer and a polyurethane. One such blend that may be suitable for
use with the present invention is available from Jatrac, Inc. (Kyoto, Japan)
under
the trade name SMOOTHER Anti-Stick Agent ("SMOOTHER").
SMOOTHER is believed to contain about 5 mass % polyurethane latex, 3 mass
% polyacrylic latex, 2 mass % polyvinyl chloride latex, 3 mass % mica, and
water.
While exemplary donning layer materials are set forth herein, it should be
understood that any suitable donning layer material may be used as desired.
Furthermore, various lubricating materials may be added to the donning layer
composition as desired or needed to enhance donning. Some such materials may
include a flattening agent, a lubricant, for example, a wax or a silicone, or
particulate matter, for example, silica.
The glove of the present invention may be formed using a variety of
processes, for example, dipping, spraying, tumbling, drying, and curing. An
exemplary dipping process for forming a glove is described herein, though
other
2o processes may be employed to form various gloves having different
characteristics. Furthermore, it should be understood that a batch, semi
batch,
or a continuous process may be used with the present invention.
A glove is formed on a hand-shaped mold, termed a "former". The
former may be made from any suitable material, such as glass, metal,
porcelain,
or the like. The surface of the former defines at least a portion of the
surface of
the glove to be manufactured.
In general, the glove is for~.ned by dipping the former into a series of
compositions as needed to attain the desired glove characteristics. The glove
may be allowed to solidify between layers. Any combination of layers may be
3o used, and although specific layers are described herein, it should be
understood
that other layers and combinations of layers may be used as desired. Thus, in
one embodiment, the glove may include a substrate body 26 and a barrier layer
32 (FIG. 2A). In another embodiment, the glove may include a substrate body
26, a barrier layer 32, and a donning layer 34 (FIG. 2B).
6
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
In one embodiment, the substrate body may be formed from a plastisol
using a dipping process. As used herein, a "plastisol" refers to a dispersion
of
fine resin particles in a plasticizer. The plastisol is formed by mixing the
resin
particles into the plasticizer with sufficient shear to form a stable system.
Any
suitable resin may be used as desired, and in some instances, the resin
includes
polyvinyl chloride (PVC). While articles formed from PVC are described in
detail herein, it should be understood that any other suitable thermoplastic
material or combination of thermoplastic materials may be used with the
present
invention. Thus, for example, the resin may include a styrene-ethylene-
butylene-
1 o styrene block copolymer, a nitrile butadiene polymer, or any other polymer
capable of forming a film without use of a coagulant. Furthermore, while
exemplary process conditions are described herein, it should be understood
that
such conditions depend on the desired thickness of the article, the viscosity
of
the composition, the time required to gel the article, and so forth.
The former may first be heated to a temperature of about 100°F
(38°C) to
about 200°F (93°C), for example, 150°F (66°C). The
former is then dipped into
a plastisol containing a suitable thei~noplastic resin, for instance, PVC, and
a
plasticizer. The composition may be maintained at any suitable temperature,
and
in some instances, is maintained at a temperature of from about 75°F
(24°C) to
2o about 175°F (79°C), for example, 105°F (40°C).
The formers are then removed from the composition to drain. The time
permitted to drain ("drain time") determines the mass of the glove, its
thickness,
and so forth, based on the temperature of the former and the viscosity of the
plastisol. The formers are then advanced to a fusion oven where the substrate
body fuses on the former. In one instance, the fusion oven may be maintained
at about 300°F (149°C) to about 500°F (260°C), for
example, 450°F (232°C),
and the former may be in the oven for about 3 to about 8 minutes, for example,
6 minutes.
The fused PVC substrate body on the former is then cooled to a
3o temperature of about 100°F (38°C) to about 200°F
(93°C), for example, 150°F
(66°G~, by exposing the formers to one or more cooling fans, as
appropriate.
The former is then dipped into a composition to form the barrier layer.
As stated herein, the barner layer may be formed from any suitable material,
and
in some instances, may be formed from an acrylic emulsion. One such acrylic
7
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
emulsion that may be suitable is HYCAR~ 2679, described in detail above.
Where desired, the composition may include other additives. In one
embodiment, the composition may include a colorant to render the barrier layer
visually distinct from the substrate body. Where other layers are present, the
barner layer may also be visuallydistinct from such other layers as desired.
The barrier layer is then dried in an oven maintained at a temperature of
about 350°F to about 450°F, for example, 392°F
(200°C) for about 60 seconds
to about 120 seconds, for example, 90 seconds, and cooled to a temperature of
about 100°F (38°C) to about 200°F (93°C), for
example, 150°F (66°C), by
exposing the formers to one or more cooling fans, as appropriate.
The barrier layer may be present in the finished article any suitable amount,
and in some embodiments, the barrier layer may be present in an amount of
from about 3 mass % to about 8 mass % of the article. In other embodiments,
the barrier layer may be present in an amount of from about 4 mass % to about
6 mass % of the article. In yet another embodiment, the barrier layer may be
present in an amount of about 5.7 mass % of the article.
~Uhere no separate donning layer is desired, various lubricating materials
may be added to the barrier layer composition as desired or needed to enhance
donning. Some such materials may include a flattening agent, a lubricant, for
2o example, a wax or a silicone, or particulate matter, for example, silica.
As used
herein, the term "silicone" generally refers to a broad family of synthetic
polymers that have a repeating silicon-oxygen backbone, including, but not
limited to, polydimethylsiloxane and polysiloxanes having hydrogen bonding
functional groups selected from the group consisting of amino, carboxyl,
hydroxyl, ether, polyether, aldehyde, ketone, amide, ester, and thiol groups.
In
some embodiments, polydimethylsiloxane and/or modified polysiloxanes may be
used as the silicone component in accordance with the present invention. For
instance, some suitable modified polysiloxanes that can be used in the present
invention include, but are not limited to, phenyl modified polysiloxanes,
vinyl-
3o modified polysiloxanes, methyl modified polysiloxanes, fluoro-modified
polysiloxanes, allyl-modified polysiloxanes, alkoxy modified polysiloxanes,
amino-modified polysiloxanes, and combinations thereof.
In some embodiments, the barrier layer may include a silicone emulsion.
One such silicone emulsion that may be used is DC 365, a pre-emulsified
silicone
8
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
(35% TSC) that is commercially available from Dow Corning Corporation
(Midland, Michigan). DC 365 is believed to contain 40-70 mass % water, 30-60
mass % methyl-modified polydimethylsiloxane, 1-5 mass % propylene glycol, 1-5
mass % polyethylene glycol sorbitan monolaurate, and 1-5 mass % octylphenoxy
polyethoxy ethanol. Another silicone emulsion that may be used with the
present invention is SM 2140, commercially available from GE Silicones
(~Xjaterford, New York). SM 2140 is a pre-emulsified silicone (50% TSC) that
is
believed to contain 30-60 mass % water, 30-60 mass % amino-modified
polydimethylsiloxane, 1-5% ethoxylated nonyl phenol, 1-5 mass % trimethyl-4-
nonyloxypolyethyleneoxy ethanol, and minor percentages of acetaldehyde,
formaldehyde, and 1,4 dioxane. Another silicone emulsion that may be suitable
for use with the present invention is SM 2169 available from GE Silicones
(~X~aterford, New York). SM 2169 is a pre-emulsified silicone that is believed
to
contain 30-60 mass % water, 60 to 80 mass % polydimethylsiloxane, 1-5 mass
polyoxyethylene lauryl ether, and a small amount of formaldehyde. Yet another
silicone that may be useful with the present invention is commercially
available
from GE Silicones (~Xjaterford, New York) under the trade name AF-60. AF-60
is believed to contain polydimethylsiloxane, acetylaldehyde, and small
percentages of emulsifiers. If desired, these pre-emulsified silicones may be
diluted with water or other solvents prior to use.
In another embodiment, the barrier layer composition may contain a
quaternary ammonium compound, such as that commercially available from
Goldschmidt Chemical Corporation of Dublin, Ohio under the trade name
VERISOFT~ BTMS. VERISOFT~ BTMS is believed to contain behnyl
trimethyl sulfate and cetyl alcohol. Thus for example, in one embodiment, the
lubricant layer may include a quaternary ammonium compound such as
VERISOFT~ BTMS and a silicone emulsion such as SM 2169. In other
embodiments, such a barrier layer composition may include, for example, a
cationic surfactant (e.g., cetyl pyridinium chloride), an anionic surfactant
(e.g.,
sodium lauryl sulfate), a nonionic surfactant, or the like.
Where desired, the former may be dipped into a composition to form a
donning layer to facilitate donning of the glove. One such donning layer
composition that may be suitable for use with the present invention may
include
SMOOTHER Anti-Stick Agent, described in detail above. The donning layer
9
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
composition may be maintained at about 100°F (38°C) to about
200°F (93°C),
for example, 150°F (66°C). The donning layer on the former may
then be dried
in an oven, for example, for about 2-3 minutes at a temperature of about
200°F
(93°C) to about 400°F (204°C), for example, 300°F
(149°C).
The donning layer may be present in the finished article any suitable
amount, and in some embodiments, the donning layer may be present in an
amount of from about 0.1 mass % to about 2 mass % of the article. In other
embodiments, the donning layer may be present in an amount of from about 0.3
mass % to about 1 mass % of the article. In yet another embodiment, the
donning layer may be present in an amount of about 0.6 mass % of the article.
Alternatively, the barrier layer may be dusted with a powder to facilitate
donning. One such dusting powder that may be suitable for use with the present
invention is USP grade starch.
The former is then sent to a bead rolling station, where the cuff is rolled
slightly and permitted to solidify. The former may then be transferred to a
stripping station where the glove is removed from the former. The stripping
station may involve automatic or manual removal of the glove from the former.
For example, in one embodiment, the glove is manually removed and turned
inside out as it is stripped from the former. By inverting the glove in this
manner, the donning layer formed on the exposed surface of the substrate body
on the former becomes the interior of the glove.
The resulting glove features improved barrier characteristics when
exposed to isopropyl alcohol. These discoveries are evidenced by the following
example, which is not intended to be limiting in any manner.
EXAMPLE
Improved chemical permeation of the glove of the present invention was
demonstrated. Various competitive polyvinyl chloride gloves (Samples A-D)
were compared with a glove formed according to the present invention (Sample
E).
The experimental gloves (Sample E) were prepared by first heating the
glove formers to about 65°C. The formers were then dipped into a
polyvinyl
chloride plastisol maintained at about 45°C. The plastisol was then
fused in an
oven at 200°C for about 5 minutes. The formers were then cooled to a
CA 02535907 2006-02-15
WO 2005/023031 PCT/US2004/019858
temperature of about 100°C and dipped into a barrier layer composition
including about 10 mass % HYCAR~ 2679. The barrier layer was then dried at
about 200°C. The formers were again cooled to a temperature of about
100°C
and dipped into a donning layer composition including about 1 mass
SMOOTHER. The donning layer was then dried at about 200°C. The
gloves
were then cooled and removed from the formers.
The gloves were tested for chemical permeation resistance according to
ASTM F739-99a entitled "Standard Test Method for Resistance of Protective
Clothing Materials to Permeation by Liquids or Gases Under Conditions of
Continuous Contact" using 70% isopropyl alcohol as the challenge chemical.
Three repetitions were performed. The results of the analysis are presented
b slow.
Sample Mass/unit areaSample thicknessBreakthrough
/m~2 mm detection time
min
A 154.0 0.130 G7
B 131.8 0.118 47
C 143.4 0.128 77
D 150.8 0.129 77
E 127.4 0.113 130
The results indicate that the glove formed according to the present
invention (Sample E) offers a significant improvement in chemical permeation
resistance when compared with several competitive products (Samples A-D).
Thus, although the glove of the present invention (Sample E) has a lower
mass/unit area and a lower thickness, it provides a greater barrier to
isopropyl
alcohol.
The invention may be embodied in other specific forms without departing
from the scope and spirit of the inventive characteristics thereof. The
present
embodiments therefore axe to be considered in all respects as illustrative and
not
restrictive, the scope of the invention being indicated by the appended claims
rather than by the foregoing description, and all changes which come within
the
meaning and range of equivalency of the claims are therefore intended to be
embraced therein.
11