Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02536050 2006-02-16
Boehringer Ingelheim International GmbH Case 1/1548
55216 Ingelheim foreign filing text
85155fft
New inhalative powder formulations containing the CGRP-antagonist
1-[N2-[3,5-d ibromo-N-[[4-(3,4-d i hyd ro-2(1 I-n-oxoqu inazol i n-3-yl)-1
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine
The invention relates to powdered preparations for pulmonary or nasal
inhalation,
containing the CGRP antagonist 1-[NZ-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11-~-
oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-
pyridinyl)-
piperazine (A) or a pharmaceutically acceptable salt thereof, processes for
preparing
~ o them and the use thereof for preparing a pharmaceutical composition for
the
treatment of headaches, migraine and cluster headache.
Background to the invention
The CGRP antagonist 1-[NZ-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11-oxoquinazolin-3-
15 yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine
(A) is known
from International Patent Application PCT/EP97/04862 (published as WO
98/11128)
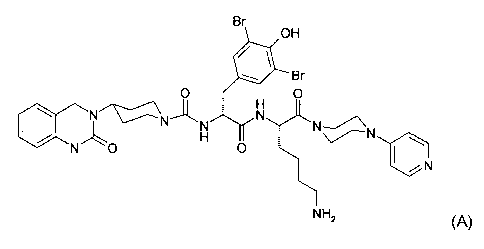
and has the following structure:
Br
OH
~Br
O O
~ H J~
N/~~N~N~ N~N-~N
N ~o H Iol ~ W
~N
NHZ (A)
Prior art
The active substance base (A) is a highly effective CGRP antagonist for the
acute
and prophylactic treatment of headaches, particularly migraine and cluster
headache,
which cannot be administered orally using conventional formulations, as the
substance has very limited oral bioavailability.
CA 02536050 2006-02-16
- 2 - Case 1/1548
foreign filing text
For treating sudden attacks of migraine it is essential that an active
substance is
systemically available as quickly as possible. The treatment should be
uncomplicated
for the patient to administer and no other conditions which could affect
bioavailability
(e.g. the food effect) should restrict the use of the medicament for the
patient.
Active substances which are intended to be systemically available are usually
administered by oral route. If this route is unsuitable or undesirable on
account of
particular properties of the active substance or particular demands made of
the
application, other possible ways of administering substances systemically are
known
1o in the art. For example, inhalation, by means of which active substances
may be
administered systemically as well as topically, has been under discussion for
some
time. For substances which prove critical on account of their decomposition in
solution or which have poor solubility per se, powder inhalation is an option.
The
absolute amount of the active substance which has to be administered per
application makes particular demands of the formulation. On the other hand,
the
physical stability (e.g. aerodynamic particle size, dispersibility,
physicochemical
properties) of the active substance has proved to be a critical requirement
for the
development and production of an inhalable powder.
2o With formulations of the powder inhalant type, inhalable powders, which are
packaged for example in suitable capsules (inhalettes), are delivered to the
lungs by
means of powder inhalers. Similarly, other systems in which the quantity of
powder
to be administered is pre-dosed (e.g. blisters) and multidose powder systems
are
also known. Alternatively, the medicament may also be inhaled by the use of
suitable
powdered inhalable aerosols which are suspended for example in HFA134a,
HFA227 or mixtures thereof as propellant gas.
In powder inhalation, the microparticles of a pure active substance are
administered
through the airways onto the surface of the lungs, e.g. in the alveoli, by the
inhalation
so process. These particles settle on the surtace and can only be absorbed
into the
body after the dissolving process by active and passive transporting
processes.
CA 02536050 2006-02-16
- 3 - Case 1 /1548
foreign filing text
Inhalation systems are known in the literature in which the active substance
is
present in the form of solid particles either as a micronised suspension in a
suitable
solvent system as carrier or in the form of a dry powder.
Usually, powder inhalants, e.g. in the form of capsules for inhalation, are
prepared on
the basis of the general teaching as described in DE-A-179 22 07.
A critical factor in multi-substance systems of this kind is the uniform
distribution of
the pharmaceutical composition in the powder mixture.
Another important aspect of powder inhalants is that when the active substance
is
1o administered by inhalation only particles of a certain aerodynamic size
reach the
target organ, the lungs. The average size of these lung-bound particles
(inhalable
fraction) is in the region of a few microns, typically between 0.1 and 10 Nm,
preferably less than 6 Nm. Such particles are usually produced by
micronisation (air-
jet grinding).
A formulation is only suitable for use as an inhalable powder containing the
active
substance if a micronised preparation and an excipient (carrier material) with
special
properties is used, the ratio of active substance to excipient is within a
defined range
and a specified amount of powder is available for the application. In
addition, special
2o climatic conditions must be adhered to during the manufacture of the
pharmaceutical
composition.
In the light of these fundamental technical requirements the problem is to
propose
solutions in accordance with the special properties of the active substance
base
1-[N2-[3, 5-d i bromo-N-[[4-(3,4-d i hyd ro-2( 1 I-~-oxoq ui nazol i n-3-yl)-1-
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) and
the
physiologically acceptable salts thereof by means of which the substances may
be
made sufficiently systemically bioavailable in the form of a powder inhalant.
ao Brief description of the invention
The invention consists in providing a novel, stable formulation for the CGRP
antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11-x-oxoquinazolin-3-yl)-1-
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine or a
physiologically
CA 02536050 2006-02-16
- 4 - Case 1 /1548
foreign filing text
acceptable salt thereof, by means of which it is possible to produce adequate
systemic blood levels for these substances which are not orally bioavailable.
Similarly, the invention also includes methods of preparing such formulations
and the
use thereof for preparing a pharmaceutical composition.
It has been found that the active substance base 1-[N2-[3,5-dibromo-N-[[4-(3,4-
dihydro-2(1I-~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-
4-(4-
pyridinyl)-piperazine (A) as well as the physiologically acceptable salts
thereof are
physically stable in the form of powder mixtures with excipients and may be
made
~o sufficiently bioavailable by pulmonary or nasal inhalation.
Detailed description of the invention
Surprisingly it has been found that the micronised active substance 1-[N2-[3,5
dibromo-N-[[4-(3,4-dihydro-2(11~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D
15 tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A), present in its amorphous
form, or a
physiologically acceptable salt thereof has proved to be physically stable in
formulations of powder mixtures with a physiologically acceptable, homogeneous
excipient. The powdered preparations described here enable the powder to be
dispersed during inhalation and the active substance is made available for
systemic
2o administration by being taken in this way.
It is known that the amorphous state of solids is thermodynamically unstable.
In
particular, this contributes to the fact that microparticles which have
amorphous parts
or are purely amorphous are metastable in their physicochemical properties.
25 Typically, amorphous or partially amorphous pharmaceutical active
substances and
excipients, such as sugars, for example, recrystallise spontaneously during
storage
under normal conditions. The process may be accelerated by an increase in the
relative humidity and possibly the temperature. In association with this
recrystallisation, the particles change their surface characteristics, their
particle
so morphology and their particle size.
Surprisingly it has been found that the amorphous active substance 1-[N2-[3,5-
dibromo-N-[[4-(3,4-dihydro-2(1 I~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-
D-
CA 02536050 2006-02-16
- 5 - Case 1 /1548
foreign filing text
tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) in the form of the amorphous
micronised
particles prepared therefrom (detected by X-ray crystallography / X-ray powder
diffractometry) may be used to prepare a stable inhalable powder. The
amorphous
form of the active substance is maintained throughout the shelf life of the
medicament.
According to the invention in addition to the active substance base the acid
addition
salts are used which are selected for example from among 1-[N2-[3,5-dibromo-N-
[[4-
(3,4-dihydro-2(11-~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-
lysyl]-4-(4-
to pyridinyl)-piperazine hydrochloride, sulphate, phosphate, hydrobromide,
carbonate,
methanesulphonate, p-toluenesulphonate, nitrate, citrate, malate, tartrate,
lactate,
succinate, gluconate, acetate, formate, propionate, capronate, oxalate,
maleate,
fumarate, mandelate and hydroxysuccinate, while the 1-[NZ-[3,5-dibromo-N-[[4-
(3,4-
dihydro-2(11-~-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-
4-(4-
15 pyridinyl)-piperazine hydrochloride, the sulphate and the hydrobromide are
particularly preferred and the 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11~-
oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-
pyridinyl)-
piperazine hydrochloride is most particularly preferred.
2o The formulations described here are designed so that the micronised active
substance 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11~-oxoquinazolin-3-yl)-1-
piperidi-
nyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) with a
particle size X5o
in the range from 1 Nm to 6 Nm, preferably from 1 Nm to 3.5 pm, and a
proportion of
particles (by volume) Q~s.a> < 5.8 Nm of at least 60%, is mixed with an inert
excipient
2s in a ratio of 1:9 to 5:1, in order to ensure a high active substance
content according
to the invention.
By the median value X5o is meant the particle size below which 50% of the
quantity of
particles fall. The Q~s.a~ value describes the percentage of particles which
are less
than 5.8 Nm in size.
so It has been found that in order to prepare doses in which the
dispersibility of the
inhalable powder is sufficiently guaranteed for each inhalation, the
micronised active
substance may be combined with a coarser excipient (e.g. lactose) in the above
proportions.
CA 02536050 2006-02-16
- 6 - Case 1/1548
foreign filing text
Powdered preparations of this kind are administered in amounts of from 25 mg
to
100 mg, preferably 50 mg, per application by inhalation. During use it is also
possible
to achieve therapeutic blood levels by multiple administration.
Thus, in a first aspect the present invention relates to an inhalable powder
for
administration by pulmonary or nasal inhalation, containing as active
substance the
CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1l~-oxoquinazolin-3-
yl)-1-
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) in
the form of the
active substance base or a physiologically acceptable salt thereof and an
inert,
1o homogeneous excipient, characterised in that
(a) the parameter X5o for the particle size of the active substance in the
range from 1 Nm to 6 pm, preferably from 1 pm to 3.5 Nm, and
15 (b) the characteristic value Q~s.a> of the active substance is at least
60°/a.
Normal carrier materials or flow adjuvants may be used as physiologically
acceptable
homogeneous excipients according to the invention. The normal carrier
materials
may be selected from among the monosaccharides (e.g. glucose or arabinose),
2o disaccharides (e.g. lactose, saccharose, maltose, trehalose), oligo- and
polysaccharides (e.g. dextrans, starch, cellulose derivatives), polyalcohols
(e.g.
mannitol, sorbitol, xylitol), salts (e.g. sodium chloride, calcium carbonate),
polylactides, polyglycolides and mixtures of these excipients. The flow
adjuvants may
for example be selected from a group consisting of magnesium stearate, calcium
25 stearate, stearic acid, stearyl alcohols, calcium behenate, calcium
arachinate,
hydrogenated vegetable oils such as for example hydrogenated castor oil or
hydrogenated cottonseed oil, fatty acid esters, sodium stearyl fumarate,
sodium
dodecyl sulphate, magnesium dodecyl sulphate and mixtures of these flow
adjuvants.
so The inhalable powders according to the invention may for example be
administered
using inhalers which meter a single dose from a reservoir by means of a
measuring
chamber (e.g. according to US 4570630A) or using other equipment (e.g.
according
to DE 36 25 685 A). Preferably, however, the inhalable powders according to
the
CA 02536050 2006-02-16
- 7 - Case 1/1548
foreign filing text
invention are packed into capsules (to form so-called inhalettes), which are
used in
inhalers as described, for example, in WO 94/28958.
The inhalable powders according to the invention may be obtained by the method
s described below.
As the active substance base (A) as well as the salts thereof are hygroscopic,
particular ambient conditions must be maintained when weighing out these
substances.
After suitable micronisation of the active substance it is conditioned at a
specified
~o temperature and humidity and in this way an equilibrium is achieved between
the
water content of the active substance and the relative humidity of the
environment.
Then the conditioned active substance is suitably mixed with one or more
excipients
and the amount of the resulting powder mixture which is to be administered is
packaged in single doses under defined climatic conditions (temperature and
15 humidity) taking into account the water content of the active substance
obtained
according to these conditions (corrected weight). The mixture is packed into
inhalettes which are later used in inhalers suitable for this purpose. The
preparation
of the inhalable powders is then followed by the production of the powder-
filled
capsules, which have to be put into their final packaging (blister backs) in
suitable
2o manner.
In a second aspect the present invention relates to a process for preparing a
powder
inhalant according to the invention, characterised in that
25 (a) the active substance is micronised,
(b) the micronised active substance is conditioned,
(c) suitably mixed with one or more excipients according to the invention
3o and
(d) the amount of the resulting powder mixture which is to be administered
is packaged as single doses in inhalettes under defined climatic
CA 02536050 2006-02-16
- $ - Case 1 /1548
foreign filing text
conditions.
The powder mixture according to the invention may be inhaled, the powder
preferably being presented to the patient in the form of a pre-metered
pharmaceutical
preparation. One example of this is an inhalable capsule system. Also possible
are
systems in which the powder preparation is provided, for example, in the form
of
single doses backed into wells in blister packs.
The powdered preparations described here may be inhaled and thus delivered to
the
lungs using a suitable device.
The inhalable powder containing the active substance which may be produced
from
such preparations has a particle size which is characterised in that the
active
substance base 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1hn-oxoquinazolin-3-yl)-
1-
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) or
the
physiologically acceptable salts thereof is or are in the form of
microparticles of a size
suitable for penetration into the lungs. At the same time, it is found that
powder
mixtures such as may be obtained according to the description of this
formulation are
sufficiently easy to process, in terms of their cohesive properties, to
reproducibly form
a pharmaceutical composition. It is thus possible to design a powdered
preparation
2o for use in pulmonary (and optionally nasal) inhalation such that on the one
hand,
during the dispersion of the powder in the course of the inhalation process by
the
patient, the amorphous active substance has an aerodynamic particle size which
leads to settling of the active substance in the lungs after nasal or
pulmonary
inhalation, and on the other hand the powder (consisting of the micronised
active
substance and a carrier material) is designed to be suitable for processing by
machine. Thanks to this administration of the active substance by nasal or
pulmonary
inhalation in the lungs, which can be achieved using this technique, the
active
substance has sufFcient systemic bioavailability.
so It has also been found that, surprisingly, a micronised preparation of the
active
substance base 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(11-oxoquinazolin-3-yl)-
1-
piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A),
which may be
prepared for example by known methods such as air-jet grinding, is only
suitable if in
CA 02536050 2006-02-16
- 9 - Case 1/1548
foreign filing text
addition to the above-mentioned conditions relating to particle size it also
has special
properties with regard to the specific surface area of (A) in relation to the
surface
area of the inert excipient of the formulation. It is found that the
formulation is
particularly suitable if the quotient of the specific surface area of the
micronised
preparation of (A) to the specific surface area of the inert excipient, based
in each
case on the total quantity of powder available per application, is greater
than 0.05,
preferably greater than 0.1, particularly preferably greater than 0.5, most
particularly
preferably greater than 0.7, and in each case is less than 22, preferably less
than 15.
~o In addition to the use of air-jet-ground 1-[N2-[3,5-dibromo-N-[[4-(3,4-
dihydro-2(1H)-
oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-
pyridinyl)-
piperazine (A) it is also suitable to use a micronised preparation of (A)
which is
present in a particle size as stated above, produced by alternative methods.
Therefore, such formulations may also be prepared for example using micronised
15 preparations of the active substance (A) or a physiologically acceptable
salt thereof,
which are prepared by spray-drying, irrespective of whether this spray
micronised
preparation is obtained as a single-component system or in the form of spray
particles consisting of the active substance and one or more excipients.
2o Powdered preparations which consist of components that satisfy the above
requirements regarding the particle size of the active substance and the ratio
of the
specific surface area of the active substance to the excipient can be
processed by
known methods to form homogeneous powder mixtures and packed by known
methods into capsules or other systems for pre-dosing. However, such
25 manufacturing steps are only successful when the handling of the powder
takes
place under strict climate control. For the successful production the maximum
temperature difference and the band width within which the relative humidity
should
fluctuate during the particular manufacturing step are critical as the active
substance
according to the invention is strongly hygroscopic. Ideally, the temperature
should
3o differ by not more than ~5°C, preferably ~3°C, either side of
a freely selected mean
value and the humidity should fluctuate by not more than ~15%, preferably
~10%,
either side of the freely selected mean value. Adjusting the amount of active
substance weighed out (weight correction) should be carried out after
adjusting the
CA 02536050 2006-02-16
- 10 - Case 1/1548
foreign filing text
equilibrium between the relative humidity of the environment and the water
content of
the active substance, depending on the hygroscopicity of the active substance.
In a third aspect the present invention relates to the use of an inhalable
powder
according to the invention as a pharmaceutical composition, particularly for
preparing
a pharmaceutical composition for the treatment of headaches, migraine or
cluster
headache.
In a fourth aspect the present invention relates to the use of an inhalable
powder
1o according to the invention for preparing a capsule (inhalette).
Such a capsule (inhalette) is characterised by a content of 2 to 50 mg of
inhalable
powder according to the invention.
Experimental section
1 ) Methods of measurement
a) Determining the particle size by laser diffraction (Frauenhofer
diffraction):
2o Measuring method: In order to determine the particle size the powder is fed
into a laser diffraction spectrometer using a dispersing unit.
The median value X5o refers to the particle size below which
50% of the quantity of particles fall. The Q~S.s> value
describes the percentage of particles which are less than
5.8 Nm in size.
Measuring device: Laser diffraction spectrometer (HELOS), Messrs. Sympatec
Software: WINDOX Version 3.3/REL 1
Dispersing unit: RODOS / dispersing pressure: 3 bar
Focal length: 100 mm [measuring range: 0.9.....175 Nm]
so Evaluation method: HRLD (V 3.3 Rel. 1)
CA 02536050 2006-02-16
- 11 - Case 1 /1548
foreign filing text
b) Determining the Specific Surface Area:
Measuring method: The specific surface is determined by exposing the powder
sample to a nitrogen atmosphere at different pressures. Cooling
the sample causes the nitrogen molecules to be condensed on
the surtace of the particles. The quantity of condensed nitrogen
is determined by means of the drop in pressure in the system
and the specific surface area of the sample is calculated by
means of the surface nitrogen requirement and the weight of the
1o sample.
Measuring device: Tri Star Multi Point BET, Messrs. Micromeritics
Heating station: VacPrep 061, Messrs. Micromeritics
Heating: approx. 12 h / 40 °C
Analysis parameters
sample tube: 'h inch; with filler rod
analysis method: 10 point BET surface measurement
0.1 to 0.20 p/p0
2o absolute pressure tolerance: 5.0 mm Hg
relative pressure tolerance: 5.0%
evacuation rate: 50.0 mm Hg/second
evacuation threshold: 10.0 mm Hg
evacuation time: 0.1 h
free space: lower Dewar,
t: 0.5 h
retention time: 20 seconds
minimum equilibration 600 seconds
delay:
adsorptive: nitrogen
CA 02536050 2006-02-16
- 12 - Case 1 /1548
foreign filing text
2) Examples
s a) 50 g of (anhydrous) air-jet-ground active substance with a specific
surface
area of 20.2 mZ/g are conditioned at 25°C and 45% relative humidity for
8 hours and
mixed with 450 g Pharmatose~ 200M (manufactured by Danone), specific surface
area 0.96 mz/g (screened in layer by layer, Turbula mixer).
Under the same ambient conditions as for the preparation of the starting
materials
~o and the mixing of the individual components the mixture is transferred into
single
capsules. A filling of 20.12 mg of the powder mixture with the above
composition
corresponds to a micronised active substance content per capsule of 2 mg
(anhydrous).
15 b) 100 g (anhydrous) air-jet-ground active substance with a specific
surface area
of 20.2 m2/g are conditioned at 25°C and 45% relative humidity for 8
hours and
mixed with 200 g Pharmatose~ 325M (manufactured by DMV), specific surtace area
0.25 mZ/g (screened in layer by layer, Turbula mixer).
Under the same ambient conditions as for the preparation of the starting
materials
2o and the mixing of the individual components the mixture is transferred into
single
capsules. A filling of 48.96 mg of the powder mixture with the above
composition
corresponds to a micronised active substance content per capsule of 16 mg
(anhydrous).
2s c) 200 g (anhydrous) spray-dried active substance with a specific surface
area
of 7.8 m2/g are conditioned at 25°C and 30% relative humidity for 8
hours and mixed
with 200 g Lactochem~ Super Fine Powder (manufactured by Borculo), specific
surface area 0.75 m2/g (screened in layer by layer, Turbula mixer).
Under the same ambient conditions as for the preparation of the starting
materials
3o and the mixing of the individual components the mixture is transferred into
single
capsules. A filling of 51 mg of the powder mixture with the above composition
corresponds to a micronised active substance content per capsule of 25 mg
(anhydrous).
CA 02536050 2006-02-16
- 13 - Case 1/1548
foreign filing text
d) 400 g (anhydrous) spray-dried active substance with a specific surface area
of 1.4 m2/g are conditioned at 25°C and 30% relative humidity for 8
hours and mixed
with 100 g Pharmatose~ 200M (manufactured by Danone), specific surface area
0.96 m2/g (screened in layer by layer, Turbula mixer).
Under the same ambient conditions as for the preparation of the starting
materials
and the mixing of the individual components the mixture is transferred into
single
capsules. A filling of 51.6 mg of the powder mixture with the above
composition
corresponds to a micronised active substance content per capsule of 40 mg
~o (anhydrous).