Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
JTJICE PURIFICATION SYSTEM
This International Patent Cooperation Treaty patent application claims the
benefit
of United States Provisional Patent Application No. 60/403,594, hereby
incorporated by
S reference herein.
I. TECHNICAL FIELD
The present invention relates to a process system for the production of sugar
along
with other products from sucrose containing juice obtained from plant
material, such as,
sugar cane, sugar beets, or sweet sorghum. The invention further relates to
apparatus and
methods to produce sucrose containing juice having a reduced amount of
dissolved
material. The invention further relates to the conversion of conventional
sugar process
systems to produce or to utilize such sucrose containing juices that have a
reduce amount
of dissolved materials.
II. BACKGROUND
Sucrose, C1zH22~11~ a disaccharide, is a condensation molecule that links one
glucose monosaccharide and one fructose monosaccharide. Sucrose occurs
naturally in
many fruits and vegetables of the plant kingdom, such as sugarcane, sugar
beets, sweet
sorghum, sugar palms, or sugar maples. The amount of sucrose produced by
plants can be
dependent on the genetic strain, soil or fertilization factors, weather
conditions during
growth, incidence of plant disease, degree of maturity or the treatment
between harvesting
and processing, among many factors.
Sucrose may be concentrated in certain portions of the plant, for example, the
stalks of the sugarcane plant or the sugar beet root. The entire plant, or a
portion of the
plant in which the sucrose is concentrated, may be harvested and the plant
juices may be
removed or extracted to obtain a juice containing a certain concentration of
sucrose.
Typically, the removal or extraction of juices from plant material involves
milling,
diffusion, pressing, or a combination thereof. Milling is one of the
conventional methods
for extracting juice from sugar cane stalks. The sugar cane stalks may be cut
up into
pieces having the desired size and then passed through rollers to squeeze out
the juices.
1
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Tlus process may be repeated several times down a series of mills to ensure
that
substantially all the sugar cane juice is removed.
Diffusion is considered to be the conventional method for extracting juice
from
the root of the sugar beet. Sugar beets may be sliced into thin strips called
"cossettes"
that may then be introduced into one end of a diffuser while a diffusion
liquid, such as
warm water, enters the other. When such counter current processing is used
about 98
percent of the sucrose from the cossette or sugar beet material can be
removed. The
resulting sucrose containing liquid is often called "diffusion juice." The
cossettes or beet
slices from the diffuser can still be very wet and the juice, which can be 88-
92% water,
associated with them can still hold some sucrose. The cossettes or beet slices
may,
therefore, be pressed in a screw press, or other type of press, to squeeze as
much juice out
of them as possible. This juice often referred to as "pulp press water" can
have a pH value
of about 5 and in some cases is returned to the diffuser. The resulting pulp
may contain
about 75% moisture. The addition to the press feed of cationic charged
pressing aids can
lower the pulp moisture content by about 1.5 to 2%. Sucrose from sugarcane
stalks can
also be removed by diffusion. One difftision process for sugarcane involves a
moving
bed of finely prepared sugarcane pieces passed through the diffuser allowing
the sucrose
to be leached out of the sugarcane.
The diffusion process, the milling process, other processes that remove juice
from
plant material, or bring plant juice into aqueous solution, result in a juice
containing
sucrose, non-sucrose substances, and water. The nature and amount of the non-
sucrose
substances in the juice obtained by these processes can vary and may include
all manner
of plant derived substances and non-plant derived substances, including but
not limited
to: insoluble material, such as, plant fiber or soil particles; and soluble
materials, such as,
fertilizer, sucrose, saccharides other than sucrose, organic and inorganic non-
sugars,
organic acids, dissolved gases, proteins, inorganic acids, organic acids,
phosphates, metal
ions (for example, iron, aluminum, or magnesium ions), pectins, colored
materials,
saponins, waxes, fats, or gums, their associated or linked moieties, or
derivatives thereof.
These non-sucrose substances are often highly colorized, thermally unstable,
or
otherwise interfere with certain processing steps or adversely impact the
quality or
quantity of the sugar product resulting from the purification process. It has
been estimated
2
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
that on average one pound of non-sucrose substances reduces the quantity of
sugar
product resulting from the purification process by one and one-half pounds. It
may be
desirable to have all or a portion of these non-sucrose substances separated
from or
removed from the juice resulting from the diffusion, milling, or other methods
used to
remove juice from the plant material. A good diffusion operation can eliminate
25-30%
of entering impurities. Returned pulp or carbonation press water can reduce
this level to
17-20%, however it is still economical due to: heat recovery, make up water
saved,
wastewater pollution reduced, sugar recovered.
Conventional process systems utilize the remaining plant material, or the
juices)
resulting from the diffusion, milling, or other methods used to remove juice
from the
plant material, such as those described by United States Patent Nos.
6,051,075; 5,928,42;
5,480,490, each hereby incorporated by reference, or such as those described
by "Sugar
Technology, Beet and Cane Sugar Manufacture" by P. W. van der Poel et al.
(1998);
"Beet-Sugar Technology" edited by R.A. McGinnis, Third Edition (1982); or Cane
Sugar
Handbook: A Manual for Cane Sugar Manufacturers and Their Chemists by James C.
P.
Chen, Chung Chi Chou, 12th Edition (1993), each hereby incorporated by
reference
herein, to generate various types of: process juices; solids prepared from the
remaining
plant material or separated from such process juices during their
clarification, purification
or refining; sugar or sucrose containing juices; sugar or sucrose crystallized
from such
sugar or sucrose containing juices; mother liquors of such crystallization of
sugar or
sucrose, along with the various combinations, permutations, by products, or
derivative
products thereof, each having a level of impurities consistent with the
process steps
described herein or any portion thereof, or actually utilized in their
production, or
consistent with conventional standards for a type or kind of product
including, but not
limited to: animal feeds containing plant material from which juice has been
removed
such as exhausted beet cossettes, pulp, bagasse, or other solids or juices
separated from
process juices; power generated using plant material from which juice has been
removed
as a fuel to boil water to generate high pressure steam to drive turbines) in
order to make
electricity, or to generate low pressure steam for the process system, or to
generate low
grade heat; syrup ranging from pure sucrose solutions such as those sold to
industrial
users to treated syrups incorporating flavors and colors, or those
incorporating some
invert sugar to prevent crystallization of sucrose, for example, golden syrup;
molasses
obtained by removal of all or any part of the crystallizable sucrose or sugar,
or products
3
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
derived from molasses, one example being treacle; alcohol distilled from
molasses;
blanco directo or plantation sugars generated by sulfitation using sulfur
dioxide (S02) as
a bleaching agent; juggeri or gur generated by boiling sucrose or sugar
containing juices
until essentially dry; juice sugar from melting refined white sugar or from
syrups) which
may be further decolorized; single-crystallization cane sugars often referred
to as
"unrefined sugar" in the United Kingdom or other parts of Europe, or referred
to as
"evaporated cane juice" in the North American natural foods industry to
describe a free-
flowing, single-crystallization cane sugar that is produced with a minimal
degree of
processing; milled cane; demerara; muscovado; rapedura; panels; turbina; raw
sugar
which can be 94-98 percent sucrose, the balance being molasses, ash, and other
trace
elements; refined sugars such as extra fine granulated having a quality based
upon
"bottlers" quality specified by the National Soft Drink Association being
water white and
at least 99.9 percent sucrose; specialty white sugars, such, as, caster sugar,
icing sugar,
sugar cubes, or preserving sugar; brown sugars that can be manufactured by
spraying and
blending white refined sugar with molasses which can be light or dark brown
sugar
depending on the characteristics of the molasses; or powdered sugar made in
various
degrees of fineness by pulverizing granulated sugar in a powder mill and which
may
further contain corn starch or other chemicals to prevent caking. This list is
not meant to
be limiting with respect to the products generated from conventional sugar
process
systems, but rather, it is meant to provide a few examples of the enormous
variety of
sugar process system products that are generated.
As can be understood, conventional process systems, in part, comprise steps
that
increasingly clarify, purify, or refine juices) resulting from the diffusion,
milling, or
other methods used to remove juice from the plant material. Typically, a
portion of the
insoluble or suspended material in sucrose containing juice derived from plant
material
can be removed using one or more mechanical processes such as screening. The
resulting
screened juice, when derived from sugar beets, for example, may contain about
82%-85%
by weight water, about 13-15% by weight sucrose, about 2.0-3.0% by weight
dissolved
non-sucrose substances or impurities, and some amount of remaining insoluble
materials.
Typically, the resulting sucrose containing juice or juices, which can have a
volume of 1000-2500 gallons per minute, may be treated by the gradual addition
of base
to increase the pH of the juice. In certain conventional process systems, the
pH of the
4
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
juice may be raised from between about 5.5 pH to about 6.5pH up to between
about 11.5
pH to about11.8pH to enable certain non-sucrose substances contained in such
juices to
reach their respective iso-electric points. This step is often referred to as
"preliming".
However, the subsequent use of this term is not meant to limit the step of
adding base to
sucrose containing juice or juices solely to those process systems that refer
to tlus
addition of base as "preliming". Rather it should be understood that in the
various
conventional juice process systems it may be desirable to first utilize base
to raise pH of
juice prior to a subsequent process step, such as a filtration step, as
described by United
States Patent Nos. 4,432,806, 5,759,283, or the like; an ion exchange step as
described in
British Patent No. 1,043,102, or United States Patent Nos. 3, 618, 589,
3,785,863,
4,140,541, or 4,331,483, 5,466,294, or the like; a chromatography step as
described by
United States Patent Nos. 5,466,294, 4,312,678, 2,985,589, 4,182,633,
4,412,866, or
5,102,553, or the like; or an ultrafilitration step as described by United
States Patent No.
4,432,806, or the like; phase separation as described by United States Patent
No.
6,051,075, or the like; process systems that add active materials to the final
carbonation
vessel as described by United States Patent No. 4,045,242, that may be an
alternative to
the conventional juice process steps of main liming and carbonation, each
reference
hereby incorporated by reference herein.
The use of the term "base" involves the use materials that are capable of
increasing the pH of a juice including, but not limited to the use of lime or
the underflow
from processes that utilize lime. The use of the term "lime" typically
involves the
specific use of quick lime or calcium oxides formed by heating calcium
(generally in the
form of limestone) in oxygen to form calcium oxide. Milk of lime is preferred
in many
juice process systems, and consists of a suspension of calcium hydroxide
(Ca(OH)2) in
accordance with the following reaction:
CaO+H2 O ~ Ca(OH)2 +15.5 Cal.
The term "iso-electric point" involves the pH at which dissolved or colloidal
materials, such as proteins, within the juice have a zero electrical
potential. When such
dissolved or colloidal materials reach their designated iso-electric points,
they may form a
plurality of solid particles, flocculate, or flocs.
5
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Flocculation may be further enhanced by the addition of calcium carbonate
materials to juice, which functionally form a core or substrate with which the
solid
particles or flocculates associate. This process increases the size, weight or
density of the
particles, thereby facilitating the filtration or settling of such solid
particles or materials
and their removal from the juice.
The resulting mixture of juice, residual lime, excess calcium carbonate, solid
particles, flocculants, or flocs, may then be subjected to subsequent process
steps as
described above. Specifically, with regard to the process system for the
clarification,
purification, or refining of juices generated by the prior processing of sugar
beets, the
mixture may first be subjected to a cold main liming step to stabilize the
solids formed in
the preliming step. The cold main liming step may involve the addition of
about another
0.3-0.7% lime by weight of prelimed juice (or more depending on the quality of
the
prelimed juice) undertaken at a temperature of between about 30 degrees
Centigrade to
about 40 degrees Centigrade.
The cold main limed juice may then be hot main limed to further degrade invert
sugar and other components that are not stable to this step. Hot main liming
may involve
the further addition of lime to cause the pH of the limed juice to increase to
a level of
between about 12 pH to about12.5 pH. This results in a portion of the soluble
non-
sucrose materials that were not affected by preceding addition of base or lime
to
decompose. In particular, hot main liming of the limed juice may achieve
thennostability
by partial decomposition of invert sugar, amino acids, amides, and other
dissolved non-
sucrose materials.
After cold or hot main liming, the main limed juice can be subjected to a
first
carbonation step in which carbon dioxide gas can be combined with the main
limed juice.
The carbon dioxide gas reacts with residual lime in the main limed juice to
produce
calcium carbonate in the form of precipitate. Not only may residual lime be
removed by
this procedure (typically about 95% by weight of the residual lime), but also
the surface-
active calcium carbonate precipitate may trap substantial amounts of remaining
dissolved
non-sucrose substances. Furthermore, the calcium carbonate precipitate may
function as a
6
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
filter aid in the physical removal of solid materials from the main limed and
carbonated
juice.
The clarified juice product obtained from the first carbonation step may then
be
subjected to additional liming steps, heating steps, carbonation steps,
filtering steps,
membrane ultrafiltration steps, chromatography separation steps, or ion
exchange steps as
above described, or combinations, permutations, or derivations thereof, to
further clarify
or purify the juice obtained from the first carbonation step resulting in a
process juice
often referred to as "thin juice".
Tlus further clarified juice or "thin juice" may be thickened by evaporation
of a
portion of the water content to yield a product conventionally referred to as
"syrup".
Evaporation of a portion of the water content may be performed in a mufti-
stage
evaporator. This technique is used because it is an efficient way of using
steam and it can
also create another, lower grade, steam which can be used to drive the
subsequent
crystallization process, if desired.
The thickened clarified juice or "syrup" can be placed into a container, which
may
typically hold 60 tons or more. In the container, even more water is boiled
off until
conditions are right for sucrose or sugar crystals to grow. Because it may be
difficult to
get the sucrose or sugar crystals to grow well, some seed crystals of sucrose
or sugar are
added to initiate crystal formation. Once the crystals have grown the
resulting mixture of
crystals and remaining juice can be separated. Conventionally, centrifuges are
used to
separate the two. The separated sucrose or sugar crystals are then dried to a
desired
moisture content before being packed, stored, transported, or further refined,
or the like.
For example, raw sugar may be refined only after shipment to the country where
it will be
used.
There is a competitive global commercial market for the products derived from
sucrose containing plant materials and juices. The market for products
produced from
sucrose containing plant material has sufficient size that even a slight
reduction in the cost
of a single process system step can yield a substantial and desired monetary
savings. As
such, there is great incentive to perform research in sugar or juice process
systems by the
sugar industry to yield process system savings, by independent researchers and
by
7
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
distributors who may be paid for novel process system chemicals and equipment,
and in
some cases have a further incentive by additional payments based upon a
percentage of
the savings within the process when improvements are made.
However, even though process systems for the purification of sucrose
containing
juices from certain plant materials have been established and improved upon
for at least
1000 years, and specifically with regard to sugar beets, there have been
commercial
process systems for more than 100 years, and even though there is great
incentive to
generate improvements within sugar or juice process systems, significant
problems with
regard to the processing of juices obtained from plant material remain.
A significant problem with conventional sugar processing systems can be the
expense of obtaining and using base, such as calcium oxide, to raise the pH of
the sucrose
containing liquids or juices) obtained from plant materials. As discussed
above, calcium
oxide or calcium hydroxide may be added to juice to raise the pH allowing
certain
dissolved materials to come out of solution as solids, flocculent, or flocs.
Calcium oxide
is typically obtained through calcination of limestone a process in which the
limestone is
heated in a kiln in the presence of oxygen until carbon dioxide is released
resulting in
calcium oxide.
As shown by Figure 5, calcination can be expensive because it requires the
purchase of the kiln (40), limestone (41), and fuel (42), such as gas, oil,
coal, coke, or the
like, that can be combusted to raise the temperature of the kiln sufficiently
to release
carbon dioxide (43) from the limestone (41). Ancillary equipment to transport
the
limestone and the fuel to the kiln and to remove the resulting calcium oxide
from the kiln
must also be provided along with equipment to scrub certain kiln gases and
particles from
the kiln air exhausted during calcination of the limestone. Naturally, labor
must be
provided to operate and maintain the equipment, as well as, monitor the
quality of the
calcined limestone generated and also to monitor the clean up of gases and
particulates
released during operation of the kiln.
Additionally, the calcium oxide generated by calcination must be converted to
calcium hydroxide for use in typical juice process systems. Again this
involves the
purchase of equipment to reduce the calcium oxide to suitably sized particles
and to mix
8
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
these particles with water to generate calcium hydroxide. Again, labor must be
provided
to operate and maintain this equipment.
Finally, the investment in equipment and labor associated with the use of
calcium
oxides incrementally increases as the amount used increases. This may involve
the
incremental expenditure for the additional labor to mix additional amounts of
calcium
hydroxide with juice, or it may involve an incremental expenditure to use
equipment
having greater loading capacity or having greater power, or the like.
Another significant and related problem with the production of and use of base
in
conventional process systems can be disposal of excess base or the products
formed when
the base reacts with organic acids or inorganic acids dissolved in the juice.
For example,
when the process system uses one or more carbonation steps in clarifying or
purifying
juice, the amount of calcium carbonate or other salts formed, often referred
to as "spent
lime", will be proportionate to the amount of lime added to the juice. Simply
put, the
greater the amount of lime added to the juice, generally the greater the
amount of
precipitates formed during the carbonation step The "carbonation lime" may be
allowed
to settle to the bottom of the carbonation vessel forming what is sometimes
referred to as
a "lime mud". The lime mud can be separated by a rotary vacuum filter or plate
and
frame press. The product formed is then called "lime cake". The lime cake or
lime mud
may largely be calcium carbonate precipitate but may also contain sugars,
other organic
or inorganic matter, or water. These separated precipitates are almost always
handled
separately from other process system wastes and may, for example, be slurried
with water
and pumped to settling ponds or areas surrounded by levees or transported to
land fills.
Alternately, the carbonation lime, lime mud, or lime cake can be recalcined.
However, the cost of a recalcining kiln and the peripheral equipment to
recalcine spent
lime can be substantially more expensive than a kiln for calcining limestone.
Furthermore, the quality of recalcined "carbonation lime" can be different
than calcined
limestone. The purity of calcined limestone compared to recalcined carbonation
lime
may be, as but one example, 92% compared with 77%. As such, the amount of
recalcined
lime required to neutralize the same amount of hydronium ion in juice may be
correspondingly higher. Also, the carbon dioxide content of spent lime can be
much
higher than limestone. As such, not only can recalcined lime be expensive to
generate, it
9
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
can also require the use of substantially larger gas conduit and equipment to
transfer the
generated COZ from recalcining spent lime, larger conveying equipment to move
the
recalcined lime, larger carbonation tanks, or the like.
Also, whether spent lime is disposed of in ponds, landfills, or by recycling,
the
greater the amount of lime utilized in a particular process system, generally
the greater
the expense of disposing the spent lime.
Another significant problem with conventional sugar processing systems may be
an incremental decrease in process system throughput corresponding with an
incremental
increase in the amount of lime used in processing juice(s). One aspect of this
problem
may be that there is a limit to the amount of or rate at which lime can be
produced or
provided to juice process steps. As discussed above, limestone must be
calcined to
produce calcium oxide prior to its use as a base in juice process systems. The
amount of
lime produced may be limited in by availability of limestone, kiln capacity,
fuel
availability, or the like. The rate at which lime can be made available to the
juice process
system may vary based on the size, kind, or amount of the lime generation
equipment,
available labor, or the like. Another aspect of this problem can be that the
amount of lime
used in the process system may proportionately reduce volume available for
juice in the
process system. Increased use of base, such as lime, may also require the use
of larger
containment areas, conduits, or the like to maintain throughput of the same
volume of
juice.
Another significant problem with conventional sugar processing systems may be
excess acids within plant material generated prior to extraction of the plant
juice. Organic
acids act as a buffering system in the acid-base equilibrium of the plant
cell, in order to
maintain the required pH value in the plant tissue. The origin of these acids
can be
divided into two groups, the first, are acids taken up by the plant from the
soil in the
course of the growing cycle, and the second, are acids formed by biochemical
or
microbial processes. When the uptake of acids from the soil is insufficient,
plants may
synthesize organic acids, primarily oxalic acid, citric acid and malic acid,
to maintain a
healthy pH value of the plant cell juice. As such, juice extracted from the
plant tissue will
contain a certain amount of various organic acids.
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
In addition to this naturally occurring amount of organic acids within the
plant
tissue, acids may be formed during storage primarily by microbial processes.
Badly
deteriorating plant material may generate large amounts of organic acids,
primarily lactic,
acetic acid, as well as citric acid. The total acid content within the plant
tissue can
increase threefold, or more, under certain circumstances.
Moreover, carbon dioxide (C02) can be generated in the plant tissues due to
breakdown of the natural alkalinity in the juice. In this process, bicarbonate
ion and
carbonate ion are converted to carbon dioxide. The resulting carbon dioxide to
the extent
it remains in solution generates carbonic acid that provides a source of
hydronium ion.
Organic acids contained within the plant cell juice, in whole or in part,
remain within the
juice obtained from the plant material. As such, to raise the pH of the juice,
these organic
and inorganic acids must be neutralized with base. The higher the
concentration of
organic acids or inorganic acids within the juice, the greater the amount of
base that may
be necessary to raise the pH of the juice to a desired value.
Another significant problem with conventional sugar processing systems may be
that plant materials or juices) treated with antimicrobial chemicals can have
higher acid
content then untreated plant materials or juices. For example, sulfur dioxide
(SOS) or
ammonium bisulfate (NH4HSO3) can be added continuously or intermittently to
help
control microbial growth or infection. The amount of SOa added depends on the
severity
of the microbial growth or infection. Lactic acid an'd nitrite levels can be
monitored or
tracked to determine severity of growth or infection. Up to about 1000 ppm of
SOa can
be used to shock or treat an infected system. Up to 400-500 ppm can be fed
continuously
to control an infection. The SOa or NH4HS03 addition used for antimicrobial
protection
can lower the pH and alkalinity of juice(s). The alkalinity reduction may
occur due to
conversion of naturally occuaTing bicarbonate ions to COa and carbonic acid.
Another significant problem with conventional sugar processing systems may be
the formation of scale in containment vessels, such as, evaporators or sugar
crystallization
equipment. The calcium salt of oxalic acid often foams the main component of
scale.
Oxalate has low solubility in solution and that solubility can be reduced as
the amount of
calcium in solution increases. Even after juice purification .to "thin" or
"thick" juices
there can be sufficient calcium in solution to force oxalate out of solution.
The process of
11
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
removing scale from the surfaces of equipment can be expensive, including, but
not
limited to, costs due to production slowdowns and efficiency losses, or the
reduction in
the effective life of equipment.
Another significant problem with convention sugar processing systems may be
the
lack of recognition that juice extraction equipment or processes used to
obtain juice from
plant material can alter or reduce the pH of the extracted juice. With respect
to diffusors
used to extract juice from sugar beet material, there may have been a failure
to recognize
that the pH value of sugar beet juice can be altered or reduced during the
diffusion
process. Another aspect of this problem may be that there may be a lack of
recognition
that different apparatus or different methods used to diffuse juice from sugar
beet material
alters or reduces the pH of the juice obtained differentially. To the extent
that
improvements in diffusion technology have generally resulted in increasingly
lower pH
values of the juice obtained, these apparatuses and methods teach away from
the solutions
provided by the invention.
Another significant problem with conventional sugar processing systems may be
that organic substances, dissolved gases, or other materials soluble in juice
(such as COZ
or S02 as examples) extracted, removed, or diffused from sugar beets or added
to the
extracted or diffusion juice may not to the extent possible be allowed to move
toward
equilibrium or to equilibrate with atmospheric partial pressures or a selected
mixture of
partial pressures of gases prior to pre-liming steps in conventional sugar
process
purification. As such, dissolved materials that could have been transferred
from the
extracted, removed, or diffusion juices) to the atmosphere or other selected
mixture of
gases, thereby reducing partial pressures or concentration of those dissolved
materials in
the diffusion juice, remain to directly contribute to or indirectly contribute
by conjunction
with other pH adjusting processes in the juice(s), that result in lowering of
pH of the
diffusion juices) prior to or at the time of performing pre-liming, initial
liming, lime
addition step(s). Lower pH can result in the use of additional lime, as
described above, to
achieve the desired pH of the juice.
One aspect of this problem with respect to conventional diffusion of sugar
beet
cossettes (or other conventional methods of removing or extracting juice or
materials)
from plant material(s)) may be that convention diffusion equipment (or other
convention
12
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
equipment used to remove or extract juice or other materials from plant
material(s)) does
not provide, or provides an inadequate, surface interface between the
diffusion juices) or
liquids containing extracted or removed plant materials) and the atmosphere,
or other
selected or desired mixture of gases, to allow materials dissolved in the
diffusion juice or
other liquids that contain extracted or removed plant materials to move toward
an
equilibrium that would substantially reduce the concentration of such
materials in the
juice or other liquids containing extracted or removed plant materials.
Another aspect of this problem may be that conventional sugar beet-diffusion
methods or equipment (or other convention equipment used to remove or extract
juice or
other materials from plant material(s)) does not provide sufficient re-
circulation of
atmospheric partial pressures, or other selected partial pressures of gases,
within the
equipment to maintain a difference in partial pressures between the
concentration of
dissolved material in the juice or other liquid containing extracted or
removed plant
material that can potentially be equilibrated with the partial pressures of
gases presented
at the gas-liquid interface to be effective in achieving the desired,
potential, or possible
reduction of pH reducing materials in the diffusion juice or other liquid
containing
extracted or removed plant material. As such, partial equilibrium or complete
equilibrium
between the partial pressures of gases presented at the liquid interface and
the partial
pressures of gases in solution prevents or slows the further reduction in
concentration of
pH reducing materials, compounds, or gases in the diffusion juice.
A third aspect of this problem may be that conventional sugar beet-diffusion
methods or equipment (or other convention equipment or methods used to remove
or
extract juice or other materials from plant material(s)) may be that the
diffusion juices)
are not mixed sufficiently to allow the entire volume, or a sufficient volume,
of the
diffusion juice or other liquid containing extracted or removed plant
materials that
contribute to the reduction in pH to move toward equilibrium with the
atmosphere or
other mixture of gases presented at the liquid-gas interface.
A fourth aspect of this problem may be that conventional sugar beet-diffusion
methods or equipment (or other convention equipment or methods used to remove
or
extract juice or other materials from plant material(s)) do not heat the
diffusion juices) or
other liquids containing extracted or removed plant material(s), to a
temperature that
13
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
sufficiently reduces the solubility of the diffusion juice or other liquids
containing
extracted or removed plant materials) to allow concentrations of pH reducing
materials
to move toward, or equilibrate with, the concentration in the partial
pressures of gases
presented at the liquid-gas interface, or move the point of equilibrium such
that the
concentration of pH reducing materials can be reduced to the desired,
potential, or
possible concentration, or move toward or equilibrate with the partial
pressure of gases
presented to the gas-liquid interface at the rate desired, or at the potential
or possible
equilibration rate that may be desired or achieved.
Another significant problem with conventional sugar processing systems may be
that extracted or diffusion juices) are allowed to move toward equilibrium, or
equilibrate
with, atmospheric partial pressures or other mixture of gases having a higher
concentration of pH reducing materials that may be presented to the surface of
the juice
as it cools. As diffusion juice or other liquids containing extracted or
removed plant
material cool the solubility of atmospheric gases or other mixture of gases
can increase.
As such, the concentration of gases or other materials that can be dissolved
into the juice
(including but not limited to pH reducing materials) may increase as the
diffusion juice
cools. As but a single example, solubility of atmospheric COa increases as
diffusion juice
cools from a range of between about 55°C to about 70°C during
diffusion steps to a range
of temperature between about 20°C to 30°C prior to the pre-
liming or liming steps.
Exposure to atmospheric partial pressures of C02, or any mixture of gases
having
sufficient partial pressure of CO2 to allow transfer of C02 to the juice as it
cools, increases
the concentration of COZ in the diffusion juice relative to that amount
present at higher
temperatures. The increased concentration of COZ in the diffusion juice may
reduce the
pH of the juice. As such, the increased concentration of C02 or other gases in
the
diffusion juice may require addition of greater amounts of lime during
subsequent lime
addition, pre-liming or other liming steps to achieve a desired or necessary
pH.
Another significant problem with conventional sugar processing systems may be
that the partial pressures of gases presented at the gas-liquid interface of
diffusion juice or
other liquids containing removed or extracted plant materials to be effective
in
establishing a concentration gradient sufficient to volatilize, move, remove
or otherwise
transfer the necessary or desired portion of materials dissolved in the
diffusion juice or
other liquid containing removed or extracted plant materials to substantially
increase the
14
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
pH of the diffusion juice or reduce the concentration of pH reducing materials
in the
diffusion juice.
The present invention provides a juice process system involving both
apparatuses
and methods that address each of the above-mentioned problems.
III. DISCLOSURE OF INVENTION
Accordingly, a broad object of the invention can be to provide a juice process
system to generate products from sucrose containing liquids or juices obtained
from plant
material. One aspect of this broad object can be to provide an alternative to
conventional
juice or sugar process system(s). As such, the invention can provide an entire
process
system, including both apparatus and methods, to generate products from
sucrose
containing liquids or juice. A second aspect of this broad object can be to
provide juice
process system methods compatible with conventional juice or sugar process
system
methods. As to this object, the invention provides method steps and apparatus
that can
be further added to, replace, or modify conventional methods and apparatus
used to
process sucrose containing liquids or juice(s).
A second broad object of the invention can to reduce the cost of generating
products from sucrose containing liquids or juices. One aspect of this object
of the
invention can be to increase juice process throughput that may be, in whole or
in part,
limited by availability of base, such as a reduced availability of limestone
or the a lack of
capacity to convert limestone to calcium oxide, or the like. Another aspect of
this object
can be to provide a cost savings by reducing the amount of base, such as lime,
that has to
be used to process sucrose containing liquids or juice into products. A third
aspect of this
object of the invention can be to reduce the amount of waste generated, such
as a
reduction in the amount of spent lime.
A third broad object of the invention can be to provide a sucrose containing
liquid
product or juice product resulting from use of the invention. One aspect of
this object can
be to provide a sucrose containing liquid or juice product having a reduced
amount or
reduced concentration of dissolved material, such as aqueous acids, volatile
organic
compounds, dissolved gases (e.g. COa or SOS), ammonia, or the like. A second
aspect of
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
this object can be to provide a sucrose containing liquid or juice product
that has a higher
pH value after treatment in accordance with the invention. A third aspect of
this object
can be to provide a sucrose containing liquid or juice product that has a
higher pH value
after treatment in accordance with the invention without the use of any base.
A fourth
aspect of the invention can be to provide a sucrose containing liquid or juice
product that
has a higher pH even when an amount of base, such as lime, or the underflow
from
conventional processing of juice, or the like, has been added prior to
treatment in
accordance with the invention. A fifth aspect of this object can be to provide
a sucrose
containing liquid product or juice product that has a reduced capacity to
generate
hydronium ion. A sixth aspect of this object of the invention can be to
provide a sucrose
containing liquid or juice product that requires less base to raise the pH to
a desired value,
iso-electric focus dissolved material(s), perform preliming or main liming
steps in
conventional process systems, degrade invert sugars, or otherwise generate
products from
sucrose containing liquids or juices.
Another fourth broad object of the invention can be to provide methods and
apparatus that reduce the amount or concentration of dissolved material in
juice obtained
from plant material by conventional juice extraction procedures such as
pressing, milling ,
or diffusion. One aspect of this object can be to provide a method of reducing
the amount
or concentration of dissolved material without the addition of base,
necessitating tlae
addition of base, or prior to the addition of base. A second aspect of this
object can be to
provide a method that can be used prior to, in conjunction with, or after, the
addition of
base to sucrose containing liquids or juices to reduce the amount or
concentration of
dissolved material in such juice. A third aspect of this object can be to
provide a method
that assists in reducing the amount or concentration of dissolved materials in
sucrose
containing liquid or juice. A fourth aspect of this object can be to provide a
method of
reducing dissolved material in sucrose containing liquids or juices compatible
with
conventional juice clarification or purification methods, including but not
limited to,
preliming, main liming, ion exchange, or filtering, as above described.
A fifth broad object of the invention can be to provide various apparatus and
methods to increase the area of interface between the sucrose containing
liquid or juice
and a desired partial pressures of gases.
16
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
A sixth broad object of the invention can be to provide various apparatus that
inject, introduce, or otherwise mix desired partial pressures of gases with
juice obtained
from plant material. One aspect of this object can be to provide apparatus to
introduce a
mixture of gases into juice to provide a mixed stream of juice comprising the
juice and
the desired partial pressures of gases.
A seventh broad object of the invention can be to provide various apparatus to
separate or remove mixtures of gases having come to partial or complete
equilibrium with
the dissolved material, or partial pressures of gases contained by, or
dissolved within, the
juice.
An eighth broad obj ect of the invention can be to assess, monitor, generate,
or
maintain liquids containing materials) extracted or removed plant material at
a
temperature or temperatures, ~or temperatures adjusted to (either manually or
automatically) in response to or with respect to: an elapse of time; a
concentration of any
particular materials) or components) contained therein; a specific processes)
or steps)
to purify or otherwise process such liquids; method of extracting, removing,
or diffusing
such materials from such plant material; or any manner of preparation or
storage of such
liquid to establish a range or specific values) of solubility to materials to
control the
concentration of materials that reduce or potentially reduce pH of such
liquids.
A ninth broad object of the invention can be to provide apparatus and methods
of
treating diffusion juice or liquids containing materials extracted or removed
from plant
material to prevent, minimize, or control the partial pressures of gases that
are presented
at the liquid-gas interface prior to the initial addition of lime or
subsequent additions of
lime.
A tenth broad object of the invention can be to provide apparatus and methods
that
allow the desired or necessary volume of juice to interact with the liquid-gas
interface to
allow the desired. or necessary transfer of materials from the diffusion juice
to
atmospheric partial pressures or selected partial pressure of gases.
Naturally, further objects of the invention are disclosed throughout other
areas of
the specification and drawings.
17
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
IV. BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a particular embodiment of the invention to reduce amount of
substances in juice obtained from plant material that includes a juice
transfer means
having a mixture of gases being delivered to the juice to generate a mixed
stream of juice
with the mixture of gases, which can further include a gas distribution
element, such as
channels or grooves within the juice transfer means, or the impellor of a
pump.
Figure 2 shows a particular embodiment of the invention to produce a juice
containing a reduced amount of substance.
Figure 3 shows second particular embodiment of the invention to produce a
juice
containing a reduced amount substances
Figure 4 shows third particular embodiment of the invention to produce a juice
containing a reduced amount of substances.
Figure 5 shows a particular embodiment of the invention to produce a juice
containing a reduces amount of substances which further includes the use of
liming and
carbonation to further clarify or purify such juice prior to reduction of
water content to
produce syrup, or prior to crystallization of sugar.
Figure 6 shows a particular embodiment of the invention to produce a juice
containing a reduced amount of substances which further includes the use of
ion
exchange to further clarify or purify the juice prior to reduction of water
content to
produce syrup, or prior to crystallization of sugar.
Figure 7 shows a particular embodiment of the invention to produce a juice
containing a reduced amount of substances which further includes filtration
steps such as
ultrafilration to further clarify or purify the juice prior to reduction of
water content to
produce syrup, or prior to crystallization of sugar.
18
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Figure 8 shows that as the temperature of a juice, diffusion juice, or other
juice
process liquid increases the solubility of certain substances, materials or
components
contained within such juice, diffusion juice, or other juice process liquid
can decrease.
Figure 9 shows several particular embodiments of the invention to process
sugar
beet cossettes in a manner that reduces certain substances in pulp juice or
diffusion juice
liquids prior to pre-liming steps.
Figure 10 shows another particular embodiment of the invention to process
sugar
beet cossettes in a manner that reduces certain substances in juice, or juice
process liquids
prior to conventional pre-liming steps.
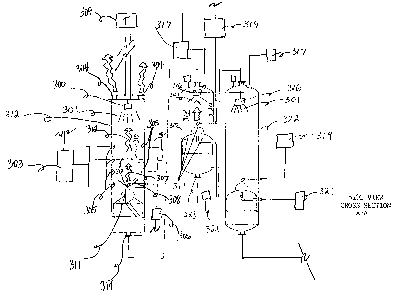
Figure 11 shows a particular embodiment of the invention.
Figure 12 shows a top view of a particular embodiment of the invention
indicating
cross section A-A.
Figure 12 shows side view cross section A-A of the particular embodiment of
the
invention shown in Figure 13.
V. MODES) FOR CARRYING OUT THE INVENTION
Generally, the invention involves a juice process system to purify juice
without
addition of base or with reduced addition of base prior to evaporation of
excess water
content or fractional crystallization,of sucrose. Specifically, the invention
provides juice
having reduced dissolved material, reduced dissolved gases, higher pH, or
lower acidity
for use in juice process systems.
As discussed above juice can be obtained from plant material such as sugar
beets,
sugar cane, sweet sorghum, or the like. Naturally, there may be large
commercial
maxkets or niche markets for products that necessitate obtaining juice from
other types of
plant material and it should be understood that the invention is not limited
to juice
removed, extracted, or obtained from any particular type of plant, or any
portion of the
plant or plant material harvested. Moreover, the term juice can be broadly
understood to
19
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
be any sucrose containing juice or liquid at, or from, any step in any process
system prior
to sugar crystallization. As such, sucrose containing liquids obtained from
plant material
by milling or pressing steps, or the juice resulting from the steps of
diffusing the plant
material, as but two examples, are juice. As further described above, the term
juice
includes liquid containing sucrose, non-sucrose substances, and water that can
occur in
various proportions depending on the nature of the plant material and the
steps used to
remove juice from the plant material. It may be desirable to remove all or a
portion of the
dissolved materials because they are highly colorized, thermally unstable, or
otherwise
interfere with certain processing steps or adversely impact the quality or
quantity of the
sugar product resulting from the purification process. The sucrose containing
liquids
resulting from these various clarification or purification steps axe also
included in the
term juice.
Particular embodiments of the invention involve the removal of at least a
portion
of the dissolved materials, volatile materials, dissolved gases, aqueous
acids, or the like,
such as carbon dioxide or sulfur dioxide that can form aqueous acids that
generate
hydronium ion in solution, change the concentration of hydronium ion in the
juice, or
lower the pH of the juice.
For example, when juice contains sufficient rations, hydroxide ion OH- can act
as
a anion, which enables carbon dioxide CO2 to dissolve into the juice as
carbonate ions
(C03)-2, or as bicarbonate ions HC03-. The dissociation of HC03 provides a
very weak
acid. However, when juice contains an insufficient number of rations to allow
dissolved
C02 to form carbonate or bicarbonate ions, an equilibrium results between
carbon dioxide
and carbonic acid H2C03. Carbonic acid can act as a strong acid in the pH
range that
juice is obtained. The consequent production of hydronium ion increases the
existing
concentration in the juice resulting in values of pH that can be lower.
Similarly, sulfur dioxide (S02) or ammonium bisulfite (NH4HS03) can be
introduced into the juice to control, reduce, or eliminate microbiologic
activity, sucrose
hydrolysis, formation of invert sugars, or loss of sucrose, or to adjust pH
lower. Again,
when juice contains sufficient rations, such as calcium, sulphites, such as
calcium sulfite
can result. However, when juice contains an insufficient number of rations to
allow
dissolved sulfur dioxide (S02) to form sulphites, an equilibrium results
between sulfur
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
dioxide (S02), sulfurous acid (HaS03), and sulfuric acid (H2S04). Sulfuric
acid and
sulfurous acid can act as strong acids. The consequent production of hydronium
ion
increases the existing concentration in the juice resulting in values of pH
that can be
lower.
Additionally, other aqueous acids can be generated by the plant during normal
growth and other acids are generated by microbial activity including, but not
limited to,
phosphoric acid, hydrochloric acid, sulfuric acid, citric acid, oxalic acid,
succinic acid,
fumaric acid, lactic acid, glycolic acid, pyrrolidone-carboxylic acid, formic
acid, acetic
acid, butyric acid, malefic acid, lactic acid, or the like.
Moreover, other dissolved materials, such as ammonia NH3, can be generated by
the breakdown of amino acids or by the conversion of materials added to the
juice such as
ammonium bisulfate.
Now refernng primarily to Figure 1, an embodiment of the invention can
comprise exposing juice (1) obtained from plant material (2) to a mixture of
gases (3) in a
manner that generates an increased interface surface area (4) between the
juice (1) and the
mixture of gases (3). By generation of the increased interface surface area
(4) between
the juice (1) and the mixture of gases ( 3), the transfer rate of various
types of dissolved
materials (5) from the juice (1) to the mixture of gases (3) can be increased
as the
concentration of each component of the dissolved material (5) moves toward
equilibrium
with the concentration of that component in the mixture of gases (3). The
mixture of
gases (or stripping gas) can be selected to provide the desired partial
pressures necessary
to allow transfer of the undesired dissolved material (5) from the juice (1)
to the mixture
of gases (3). The mixture of gases (3) can be refreshed, or the partial
pressures of the
gases adjusted, continuously or periodically, at the increased interface
surface area (4)
with the juice (1) to prevent equilibrium between the mixture of gases (3) and
the
dissolved material (5) from occurring, thereby maintaining transfer of
dissolved material
5) from the juice to the mixture of gases (3).
When the invention is utilized dissolved materials or volatile materials, such
as,
volatile inorganic compounds, volatile organic compounds, or dissolved gases
(e.g.
carbon dioxide, sulfur dioxide, or ammonia) can be removed from the juice. The
juice
21
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
product resulting from use of the invention can have reduced dissolved
material, reduced
dissolved gases, reduced capacity to generate hydronium ion, or a decreased
concentration of hydronium ion, lower acidity, or a higher pH as compared to
the same
juice without application of the invention. As but one example, the
concentration of
carbon dioxide in the juice can be reduced substantially when atmospheric
partial
pressures are used to strip the juice. The pH of the juice product resulting
from the
process can have a pH value that is higher by 0.05 pH, 0.1 pH, 0.2 pH, 0.3 pH,
0.4 pH,
0.5 pH, 0.6 pH, 0.7 pH, 0.8 pH, 0.9 pH, 1.0 pH, 1.1 pH, 1.2, pHl.3, pHl.4,
pHl.S, pHl.6,
pHl.7, pHl.B, pHl.9, 2.0 pH, however, any upward adjustment of the pH value
from the
initial pH value of the untreated juice can result in a substantial monetary
savings and can
be important commercially. The actual amount of upward adjustment of the pH
value
from the initial pH value generally depends upon the kind and quality of juice
treated by
the invention, the extent of the increased interface surface area generated
throughout the
volume of juice, the duration of time the mixture of gases is responsive to
the increased
interface surface area generated, and the partial pressures provided in the
mixture of
gases. As such, the upward adjustment of the pH value can vary with respect to
the
embodiment of the invention utilized. For example, varying the volume or
amount of
juice treated per unit time, but otherwise using the same embodiment of the
invention, can
yield a different increment in change of the pH value.
The invention can further comprise the step of reducing the amount of base
added
per unit weight or unit volume of the juice treated with the invention to
achieve a
necessary or desired pH, concentration of hydronium ion, or acidity as
compared to
untreated juice or conventional process treated juice. The amount of base
added after
reducing dissolved material in the juice by treatment with the invention can
be
substantially less to establish a desired pH value, such as, between about
11.0 to about
12.0, or between 11.5 to about 12.5, or the range of pH used to "prelime",
"main lime",
"intermediate lime, or to establish a pH value corresponding to the iso-
electric point of
any particular non-sucrose substance in the juice, or required to adjust the
acidity or
alkalinity of the juice to a desired concentration. With respect to lime
usage, for
example, a reduction of up to about 30% can be achieved by using the various
embodiments of the invention as compared to untreated juices or conventional
process
treated juices.
22
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Now referring primarily to Figure 2, embodiments of the invention can comprise
a
mixture of gases (3) that can comprise atmospheric gases , or air; atmospheric
gases or air
that have been passed through one or more filters to reduce, or to
substantially eliminate,
non-biological particulate or biological particles (such as bacteria, viruses,
pollen,
microscopic flora or fauna, or other pathogens); atmospheric gases or air that
have been
passed through chemical scrubbers or otherwise processed to generate a desired
concentration or range of concentrations of partial pressures of gases;
purified gases; or
combinations or permutations thereof.
Particular embodiments of the invention can further include a gas filter (6)
responsive to the flow of the mixture of gases (3). The gas filter (6) can be
located
before, or can be located after, a gas flow generator (7) made fluidicly
responsive to the
mixture of gases (3). The gas filter (6) responsive to the flow of the mixture
of gases ~s~
can comprise a Hepa filter, or a Ulpa filter, or other type of macro-
particulate or micro-
particulate filter. Additional prefilters may also be used to capture
particles in the
mixture of gases prior to entering the gas flow generator (7), or may be used
after the gas
flow generator but prior to the gas filter (6).
An unfiltered mixture of gases (3) can be drawn into 'a first stage prefilter
(8) then
through the second stage prenlter (9) and then through the gas flow generator
(7). The
prefiltered mixture of gases can then flow through the gas filter (6) (Hepa
filter, or Ulpa
filter, or other type filter). The resulting filtered mixture of gases (up to
99.99% of all
particles as small as about 0.3 microns removed from the mixture of gases (3)
when a
Hepa filter is used, and up to 99.99% of all particles as small as about 0.12
microns
removed from the mixture of gases ( 3) when a Ulpa filter is used) can then be
made to
generate or be responsive to the increased interface surface area (4) between
the juice (1)
and the mixture of gases (3). As to other embodiments of the invention, the
mixture of
gases (3) or the juice (1) can be exposed to short wavelength ultraviolet
radiation source
(10) in order to reduce the number of pathogen particles or bacterial
particles. The
invention can further comprise temperature control means (11) for establishing
a desired
temperature of the mixture of gases (3) prior to making them responsive with
the juice (1)
or the increased interface surface area (4). The temperature control means
(11) can be
made responsive to a temperature sensor (12) that can detect the temperature
of the
mixture of gases (3) or the juice (1) and can signal or cause the temperature
control means
23
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
(11) to adjust the temperature of the mixture of gases (3) or the juice (1),
or both, to a
desired temperature.
With respect to certain embodiments of the invention, the~mixture of gases
(3),
whether filter or unfiltered, can be used to form or assist in the formation
of the increased
interface surface area (4). For example, juice (1) can be transferred to a gas
injector (13)
by gravity feed or transferred under pressure generated by a pump (14) or
other liquid
transfer element. The gas injector (13) can have an inlet port (15) through
which juice (1)
enters the gas injector (13), an outlet port (16) from which juice (1) exits
the gas injector
(13), and at least one injection port (17) through which the mixture of gases
(3) can be
delivered into at least a portion of the volume of juice ( 1) contained within
or passed
through the gas injector (13).
When the gas injector (13) has a configuration for batch processing of juice
(the
gas injector is periodically filled and emptied) the inlet port (15) and the
outlet port (16)
can, with respect to certain embodiments of the invention, be the same port.
When the
gas injector (13) has a configuration for pulsatile flow processing (the flow
of the juice
1) can be periodically diminished or interrupted to increase residence time of
the juice (1)
in or responsive to the gas injector (13)), or continuous flow processing (a
stream of juice
(1) flows continuously through the gas injector ( 13) although the rate or
volume of juice
(1) flowing through the gas injector (13) may be adjusted) the inlet port (15)
and the
outlet port (16) can be discrete.
As to each embodiment of the invention, the mixture of gases (3) can be
injected
into the juice (1) with a sufficient volume, at a sufficient pressure, or with
a pattern of
distribution (e.g. diffused or as small bubbles) to generate the desired
increased interface
surface area (4) between the juice (1) and the mixture of gases (3). The
increased
interface surface area (4) can provide the interface at which at least a
portion of the
dissolved material (5) in the juice can transfer from the juice (1) to the
mixture of gases
(3).
The gas injector (13) whether configured to operate as a batch, pulsatile,
intermittent, or continuous embodiment of the invention, can further agitate,
move, stir or
otherwise provide mixing means (18) to further distribute the mixture of gases
(3) into the
24
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
juice (1) to further generate the increased interface surface area (4). Where
the
configuration of the gas injector (13) generates a stream of juice (1),
whether continuous,
pulsatile, or discontinuous, injecting the mixture of gases (3) into the juice
(1) can
generate a mixed stream of juice (19). The mixture of gases in the mixed
stream of juice
(19) may be further distributed in the mixed stream of juice (19) by further
extensions,
channels, or the like coupled to the interior surface of the gas injector
(13). The
extensions or channels can be oriented to create a desired perturbation of the
stream of
juice within the gas injector (13). The invention can further provide a
injection pressure
adjustment means (20) to which the gas flow generator (7) can be responsive to
increase
or decrease the pressure or volume of the mixture of gases (3) injected,
mixed, or sparged
into the juice (1). In some embodiments of the invention, the injection
pressure
adjustment means (20) can individually or in combination comprise a variably
adjustable
restriction means located between the gas flow generator (7) and the injection
port (17).
The invention, with respect to certain embodiments, can generate total
dissolved
gases within the juice greater than the initial concentration in the juice.
This can be up to
about 10 times the concentration that would be obtained by saturating the
juice at
atmospheric pressure. The pressure of the mixture of gases (3) injected into
the juice (1)
can be between the initial pressure exerted by the juice ( 1) to about a
pressure of about
20 bars.
Multiple gas injectors (13) can be used in series or in parallel, and each gas
injector can have multiple gas injection ports (17) at substantially the same
location or
different locations in a series or in parallel. Each injection port (17 ) may
be separately or
variably controlled with respect to the volume and pressure of the mixture of
gases (3)
injected in the juice (1). The variably adjustable injection ports (17) can be
made
responsive to the volume of juice (1), the residence time of the juice in the
gas injector
(13), the concentration or amount of dissolved materials (5) in the juice (1),
or the
concentration of dissolved gases in the juice (1), or the like.
With respect to other embodiments of the invention, the mixture of gases (3)
can
be injected into the juice (1) prior to the pump (14), whereby the pump (14)
can act to
distribute the mixture of gases (3) with the stream of juice (1) to generate
the mixed
stream (19 ) and increased interface surface area (4). As to certain types of
pumps, the
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
mixed stream (19) can comprise at least 35% mixture of gases with
substantially 100%
saturation of the stream of juice (1) with bubbles of the mixture of gases
(3). As but one
example, a Shanley Pump, can be used to generate the mixed stream (19).
Shanley Pump,
hereby incorporated by reference herein. A plurality of pumps (14) can be run
in series or
parallel as required to process a certain volume of juice (1) within the
desired duration of
time.
With respect to other embodiments of the invention, a stream of juice (1) can
be
further configured to provide a venturi effect, or otherwise develop a reduced
pressure
responsive to the stream of juice (1) to draw the mixture of gases (3) into
the stream of
juice (1), whether pulstile, continuous, or intermittent.
With respect to certain embodiments of the invention, only a portion of the
stream
of juice (1) may be exposed to the mixture of gases (3). For example, if the
juice (1)
contains a low amount of dissolved material (5), then the stream of juice (1)
can be split
and only a portion of the juice (1) exposed to the mixture of gases (3). The
streams of
juice (1) can then later be recombined in the proportions desired.
Now referring primarily to Figure 3, with respect to other embodiments of the
invention, juice (1) can be sprayed through a juice distribution element (21),
such as a
nozzle. The juice distribution element (21) can create a spray of very fine
juice droplets
(22) or particles. As such, spraying generates an increased interface surface
area (4). The
juice can be sprayed in an aeration containment element (23) and the mixture
of gases (3),
whether or not filtered or scrubbed as described above, can be exposed to the
sprayed
juice droplets. Juice can be discharged into the top region of the aeration
containment
element (23) (e.g. via a spray nozzle) and then exposed to the mixture of
gases (3) passed
through the aeration containment element (23). The mixture of gases (3) can be
passed
through the aeration containment element (23) counter current to the direction
of the of
the juice droplets (22) to increase the efficiency of transfer of dissolved
material (5) in the
juice (1) to the mixture of gases (3). The aeration containment element (23)
can be, for
example, a 150 gallon tank but it can be appreciated that the size and shape
of this tank
can vary depending upon the quantity of the juice that is being processed.
26
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
In certain embodiments of the invention the aeration containment element (23)
can further contain a juice distribution surface (24). Juice (1) can be
distributed to the
juice distribution surface (24) to provide a further increased interface
surface area (4).
Again, juice can be discharged into the top region of the aeration containment
element
(23) spread over the juice distribution surface (24) and can be exposed to the
mixture of
gases (3) passed through the aeration containment element (23). Again, the
mixture of
gases (3) can be passed through the aeration containment element (23) counter
current to
the general direction that the juice (1) flows on the juice distribution
surface (24) to
increase the efficiency of transfer of dissolved material (5) in the juice (1)
to the mixture
of gases ( 3).
As to each of these embodiments of the invention utilizing the aeration
containment element (23), the juice (1) can be collected and cycled through
the aeration
containment element (23) as many times as may be desired.
Now referring primarily to Figure 4, in other embodiments of the invention,
juice
(1) can be transferred to a juice containment element (25), and the mixture
of. gases (3)
can be introduced into the juice (1) by sparging the juice (26). The pressure
and volume
of the mixture of gases (3) can be adjusted relative to the volume of juice
(1) and the size
of the juice containment element (25). The juice containment element can
further be
combined with the aeration containment element (23) described above.
A general discussion of gas absorption provided by Chemical Engineer's
Handbook, Perry, ed., McGraw-Hill Book Company, pg. 66~ et seq. (1950) is
hereby
incorporated by reference to the extent necessary for an understanding of the
general
principals of gas absorption.
It can be appreciated that a variety of conventional conduits, valves, or
other
devices, for example, pressure gauges, can be provided to generate relevant
information
concerning the transfer of the juice (1) to the gas injector (13), aeration
containment
element (23), or juice containment element (25), the amount and pressure of
the mixture
of gases (3) injected, sprayed, or sparged, the amount of dissolved material
(5) in the juice
( 1 ), or the like.
27
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Again referring primarily to Figure 2, the invention can further include a gas
separator (27) to release the mixture of gases (3) which contain dissolved
material (5)
transferred from the juice (1). In certain embodiments of the invention, where
an aeration
containment element (23) is utilized, as described above, the gas separator
(27) can
comprise an aperture in the aeration containment element allowing the mixture
of gases
passed through the aeration containment element to be discharged to
atmosphere. In
those embodiments of the invention where the gas injector (13) comprises a
sparger (26),
the gas separator (27) can be an aperture allowing the mixture of gases (3)
containing
dissolved material to be discharged to atmosphere. In those embodiments of the
invention where the gas injector (13) introduces the mixture of gases (3) into
a stream of
juice (1) to generate a mixed stream of juice (19), whether continuous,
pulsatile, or
intermittent, transferred in a conduit closed from atmosphere, the gas
separator (27) can
comprise a portion of the conduit that further provides an interior volume
fluidicly
coupled to atmosphere. Specifically, the gas separator (27) fluidicly coupled
to
atmosphere can comprise a portion of the conduit configured to, or having
restriction
means to, adjust the time that the mixed stream (19) is responsive to
atmosphere.
Specifically, one configuration of the gas separator (27) can be an increase
in the
internal volume of the conduit to spread the mixed stream (19) over the
interior surface of
the conduit to increase the residence time that, or to increase the surface
area when, the
juice is fluidicly coupled to atmosphere, or both. In certain embodiments of
the gas
separator (27), the juice can be spread over a surface area sufficiently large
to allow the
mixture of gases (3) within the juice (1) to substantially equilibrate with
atmospheric
partial pressures prior to transfer of the juice from the gas separator (27).
The interior
surfaces of the gas separator (27) can be further configured to provide
extensions,
corrugates, grooves, or the like, to fiuther mix or agitate the juice (1)
within the gas
separator (27) to increase the rate at which the mixture of gases (3) can be
transferred
from the juice (1) to atmosphere.
~ A gas flow of the mixture of gases (28) transferred from the juice (1) to
atmosphere can be generated by coupling a source of reduced pressure (29) to
the gas
separator (27). Reduced pressure involves generating partial pressures of
gases at the
increased surface area (4) of the juice (1) that are lower than the partial
pressures of the
dissolved materials (5) transferred to the mixture of gases (3). As can be
understood, the
28
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
source of reduced pressure (29) can be atmosphere when the partial pressures
of the
mixture of gases containing dissolved materials (5) removed from the juice
exceeds
atmospheric pressure. With respect to some embodiments of the invention, as
described
above, a source of reduced pressure (29) can be generated by increasing the
interior
volume of the conduit in which the mixed stream (19) flows. The source of
reduced
pressure (29) can also be generated by a vacuum pump, a venturi, or other
device
fluidicly coupled to the gas separator (27). The partial pressure of gases
generated at the
increased surface area (4) of the juice can then be adjusted as desired (e.g.
below
atmospheric pressure) to increase the transfer rate of the mixture of gases
(3) containing
dissolved material (5) from the mixed stream (19) of juice.
As to some embodiments, the gas separator can further include a relief valve
(30)
or further include a signal generator (31), coupled to the source of reduced
pressure (29)
that can be responsive to accumulation of, or partial pressures of, gases
within the gas
separator (27), or responsive to a reduction in dissolved materials in the
juice (total
dissolved material, certain dissolved materials, concentration of dissolved
materials, or
concentration of certain dissolved materials), a reduction in acidity of the
juice, alkalinity
of the juice, an increase in pH of the juice, or other measure, that indicates
sufficient
dissolved material has been transferred from the juice (1).
The invention can further include storage or conveyance of the mixture of
gases
(32) containing dissolved materials removed from the juice that avoids
discharging all or
a portion to atmosphere. In certain embodiments of the invention the mixture
of gases
containing dissolved materials from the juice (e.g. containing carbon dioxide)
can be
utilized for carbonation steps as described above, as but one example.
The invention can also include the addition of antifoaming agents (33) to the
juice
(1). Juice contains a large amount of material that can be surface active or
that can alter
the surface tension of water. As such, air inclusion within the juice, or
dissolved gases
transferred from the juice to atmosphere, can result in foam. There are many
kinds of
antifoaming agents that can be used to reduce the amount of foam. Including,
but not
limited to, fatty acids, oils, or the like. To accomplish injection of the
mixture of gases
(3) into juice (1) or to transfer the mixture of gases (3 ) containing at
least some dissolved
material (5), as described above, can further require the step of adding an
amount of
29
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
antifoaming agent simultaneous with, or at about the time the juice is exposed
to or
injected with, the desired mixture of gases (3).
Once the desired amount of dissolved material, volatile material, dissolved
gases,
aqueous acids, or the like, have been transferred from the juice (1) the
resulting juice
product can be transported to existing sugar process facilities for further
clarification or
purification. Alternately, the various embodiments of the invention can be
incorporated
into sugar process facilities to produce juice having reduced dissolved
material in situ.
Now referring to Figure 5, with respect to sugar process systems which utilize
base, such as calcium oxide or calcium hydroxide, to raise pH for the purpose
of initially
reaching the iso-electric point of the various materials dissolved in the
juice (1), or as part
of conventional method of preliming juice (33) either separate from or in
conjunction
with further steps such as cold liming (34), main liming (35) or intermediate
liming (36)
again separate from or in conjunction with a first carbonation step (37) or
second
carbonation step (38) that can result in a precipitate of calcium carbonate
(39) to trap at
least a portion of the non-sucrose substances from the juice (1) so that the
resulting
clarified or purified juice can be filtered (44) prior to evaporation (45) of
the desired
amount of water, the method and apparatus involving the invention can be
utilized to
produce a juice product having reduced dissolved material or reduced dissolved
gases
consistent for introduction into one, or more, or all of these conventional
steps, or
conventional steps modified to the extent to benefit from the characteristics
of the juice
treated in accordance with the invention.
As can be appreciated the invention can be used to reduce dissolved materials
within the juice prior to any addition of base. Because the invention can
substantially
increase the pH or reduce the acidity of the juice, the amount of base used in
conventional
preliming or main liming steps can be reduced. Alternately, in those process
systems in
which the underflow in the process system, such as spent lime, is used to
neutralize some
portion of the acid in the juice, or used to reduce foaming, the under flow
can be
introduced either before or after utilizing the invention
Specifically, a method of purifying juice utilizing the invention can comprise
obtaining juice (1) from plant material (2) where the juice as above described
contains
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
sucrose, non-sucrose substances, and water. Utilizing the invention in the
various
embodiments shown or described to either raise the pH or lower acidity of the
juice prior
to preliming (33) the juice. Cold main liming (34) the juice (1) or hot main
liming (35),
or both, can be utilized in conjunction with carbonating (37)(38). Where
calcium oxide
or calcium hydroxide has been employed as the base (46) in the preliming (33)
or main
liming (34)(35) steps, a carbonation step (37) precipitating calcium carbonate
(39) can
result in trapping at least a portion of the non-sucrose substances in the
juice (1). These
precipitates (39) allow removal of the trapped non-sucrose substances by
separation of the
juice (1) from the precipitates (39). In some embodiments of the invention, an
intermediate liming (36) step in conjunction with an additional carbonation
(38) step can
be performed. Again precipitating calcium carbonate (39) can allow removal of
trapped
non-sucrose substances. Removing calcium carbonate precipitates (39) can yield
a juice
(1) that after by removing water content (45) to the desired amount can yield
desired
syrups (46). Alternately, crystallizing (47) the sucrose content within the
juice can yield
sugar products (48).
Now referring primarily to Figure 6, with respect to sugar process systems
that
utilize ion exchange (49) to replace conventional calcium carbonate
purification steps in
the sugar process system as described above, it can be understood from United
States
Patent Nos. 3,785,863; 4,331,483; or 4,140,541, each hereby incorporated by
reference,
that base, such as lime can be used to pretreat juice so that it may more
readily be filtered
prior to ion exchange steps (49), to regenerate ion exchange material to
generate the
calcium form so that the polar load of the juice is exchanged for calcium, or
to reduce
acidity of the juice after ion exchange processes.
In these types of processes, the invention can be used to reduce the amount of
dissolved materials, or dissolved gases, or reduce acidity of the juice prior
to or in
conjunction with pretreatment of the juice, or to reduce the polar load of the
juice prior to
ion exchange, or to reduce the acidity of the juice after the ion exchange
steps. Each of
these can be accomplished by processing the juice in accordance with the
invention.
Now refernng primarily to Figure 7, with respect to sugar process systems that
utilize filtration or ultrafiltration to replace conventional calcium
carbonate purification
steps in the sugar process system as described above, it can be understood
from United
31
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
States Patent No. 4,432,806, hereby incorporated by reference, that base, such
as lime can
be used to pretreat juice so that it may more readily be filtered (50).
In these types of processes, the invention can be used to reduce the amount of
dissolved materials, or dissolved gases, or reduce acidity of the juice prior
to or in
conjunction with pretreatment of the juice with base to allow non-sucrose
substances to
reach their isoelectric points and aggregate, or to otherwise generate solid
particulates that ~
can be filtered from the remaining liquid portion of the juice. Each of these
can be
accomplished by processing the juice in accordance with the invention.
Now referring primarily to Figure 8, the invention can include apparatus for
processing or methods of processing liquids containing sucrose, or diffusion
juice(s),
which take advantage of the lower solubility of pH reducing materials in such
liquids. As
sucrose containing liquids are heated the solubility of certain materials
including gases,
such as C02 and S02 decreases. As such, the transfer of these materials from
such
liquids can be initiated or increased at the interface between such liquid and
a mixture of
partial pressures of gases, even when the material could not be transferred,
or could not
be further transferred to such partial pressure of gases at a lower liquid
temperature.
Now referring primarily to Figure 9, a particular embodiment of the invention
is
shown in which sugar beet cossettes (51) are introduced into a mixer (52)
typically with a
conveyor belt or other conveyance means, or alternately, sugar beet cossettes
(51) can be
introduced directly into a cossette diffuser (53) using a pump (54). In
particular
embodiments of the invention in which sugar beet cossettes (51) are introduced
into the
mixer (52), the sugar beet cossettes (51) may within the mixer (52) be exposed
to a
portion, or all, of the diffusion juice or effluent (55) from the cossette
diffuser (53) before
being transferred by a pump (54) to the cossette diffuser (53).
In the diffuser (53) the sugar beet cosettes are treated with heated water
(59)
(typically between 50°C and 80°C), sometimes in a counter
current fashion, to remove or
transfer sugar beet juice (which can contain a variety of other soluble and
non-soluble
substances and materials as described above) from the sugar beet cossettes
(51) to the
heated water (59). The heated water (59) now containing sugar beet juice
diffused from
the sugar beet cossettes (51) (sometimes referred to as "diffusion juice") is
collected and
32
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
transferred by pump (60) to the mixer (52) in a single or in multiple effluent
streams
(55)(58).
Importantly, while diffuser technology has been used for decades, it has not
been
lcnown until the instant invention that the diffuser (53) itself can prevent
or reduce
transfer of certain substances or pH reducing materials from the diffuser
liquids resulting
in diffusion juice which can contain amounts of certain substances or
materials which can
be reduced in accordance with the invention. Embodiments of the invention take
advantage of the increased temperatures used during diffusion of sugar beet
cossettes and
which reduces solubility of certain substances contained in diffuser liquids,
pulp liquids,
diffusion juice, or the like, to remove, reduce or transfer certain substances
or materials
such as alcohols, aldehydes, ketones, esters, nitrites, sulfides, pyrazines,
carbon dioxide,
carbonic acid, sulfur dioxide, phosphoric acid, hydrochloric acid, sulfuric
acid, sulfurous
acid, citric acid, oxalic acid, succinic acid, fumaric acid, lactic acid,
glycolic acid,
pyrrolidone-carboxylic acid, formic acid, acetic acid, butyric acid, malefic
acid, propanoic
acid, 3-methylbutanoic acid, butanoic acid, pentanoic acid, 5-methylhexanoic,
hexanoic,
heptanoic, or lactic acid.
The monitoring, assessment, and manipulation of the diffusion juice, pulp
juice, or other
diffusion liquids with the configurations of the invention (such as those
shown by Figures
1 to 7 and 11, 12, 13 and described herein) to take advantage of higher
temperatures
(lower solubility of pH reducing material) of the diffusion liquids or
diffusion juice can
occur at the diffuser (53) itself or at various locations as shown in Figure 9
(200)(201)(202) to treat pulp press liquids or to treat diffusion juice
between the diffuser
(53) and the pre-Timer (57). Heaters (T)(203)(204) can be added in line to
maintain juice
or diffusion juice to establish or maintain temperature of the juice between
about 60°C
and about 80°C. Various embodiments of the invention with heaters can
establish or
maintain juice at about 60°C, about 61°C, about 62°C,
about 63°C, about 64°C, about
65°C, about 66°C, about 67°C, about 68°C, about
69°C, about 70°C, about 71°C, about
72°C, about 73°C, about 74°C, about 75°C, about
76°C, about 77°C, about 78°C, about
79°C, about 80°C, or other temperature as desired.
As such, there are numerous embodiments of the invention which manifest a
variety of configurations for use with diffusers and diffusion technology of
sugar beet
33
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
cossettes, certain of these configurations are shown by shown by Figure 9
(showing
exemplary locations (200)(201)(202) at which the various configurations of the
invention
such as those shown by Figures 10 to 13 and described below can be positioned)
which
provide a sufficient number of illustrative examples to make and use the
various
embodiments of the invention. These illustrative examples are not to be
considered
limiting with respect to the wide variety of alternate embodiments not shown.
Certain embodiments of the invention comprise a controlled exchange rate of
atmospheric partial pressures or maintains a partial pressure of gases (62)
within the
diffuser that provides for additional transfer of substances or pH reducing
materials from
heated diffusion juices (205) within the diffuser (53). In some embodiments of
the
invention, the diffuser (53) can be modified to include additional fluidic
coupling with the
atmosphere to allow increased exchange of atmospheric partial pressures at the
surface of
the heated diffusion juice. In other embodiments of the invention a gas flow
generator
(64) can be installed where the configuration of the diffuser cannot be
modified to
increase exchange of atmospheric pressures within the diffuser (53). Increased
ventilation (63) may be balanced with the established gas flow within the
diffuser (53).
Assessment element(s)(65) which monitors the transfer of certain substances
from the
diffuser liquids to the gas flow can provide information about the exchange
rate of
substances between the diffuser liquids or atmospheric partial pressures (or
other selected
mixtures of gases or partial pressures of gases) at the diffusion juice
interface can be
controlled.
Now referring primarily to Figure 10, other embodiments of the invention
comprises heated diffusion juice (66) transferred by means of a pump (60) or
other liquid
conveyance to a containment element (67) that increases the surface area of
the heated
diffusion juice (66) to provide a greater reduction in concentration of pH
reducing
materials (or the desired level of pH reducing materials, such as C02 or S02)
or to
generate a more rapid transfer of the pH reducing materials from the heated
diffusion
juice. The increased surface area (or the desired surface area which could
also be
adjustably variable) of the heated diffusion juice (66) can be obtained in
various ways as
described above by injection of desired partial pressures of gases to strip
the diffusion
juice, sprayed into a containment element, or delivered over a increased area
substrate.
34
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Certain embodiments of the invention can include container (67) having a
substantially open top and can further provide a substantially open bottom
(which for
convenience can have a reduced size opening to transfer treated diffusion
juice to the'
mixer, a settling tank (68), or pump (56)(54), or other transfer means. The
heated juice
(66) from the diffuser can be introduced near the top of the container (67)
such that the
heated juice (66) has a substantially increased surface area with~respect to
atmospheric
partial pressures within the container. As shown in Figure 10, one embodiment
of the
invention introduces the heated juice near the top of the container (67) such
that the
heated juice spreads over the interior walls and can have sufficient force to
spiral down at
least a portion of the height of the interior surface to increase the
residence time in the
container (67).
As to some embodiments of the invention, the manner of introduction of the
heated juice into the container (67) can be the means of increasing the
surface area of the
heated juice (66) while the container (67) serves only to contain and collect
the treated
diffusion juice. In these embodiments of the invention the configuration of
the fluid
stream of heated juice can be modified to create additional surface area
fluidicly coupled
to atmospheric or desired partial pressures of gases by agitation, pulsation,
division into
multiple streams, spraying, droplet formation, or otherwise.
Alternate embodiments of the invention can utilize the configuration of the
container (67) to optimize the increase in surface area of the heated
diffusion juice (66).
For example, the container can have a circular or conical configuration or
even a variably
adjustable configuration that controllably increases or decreases the surface
area of the
heated juice introduced onto the container (67) surface and the residence time
on the
surfaces of the container. As to some embodiments of the invention the
container can be
an increase in the diameter of the conduit (69) transferring the juice
providing fluidic
coupling with atmospheric partial pressures or the desired partial pressures
of gases can
be injected into the conduit to strip the heated diffusion juice of pH
reducing materials or
undesirable strippable components.
In certain embodiments of the invention, the partial pressures of gases to
which
the surface area of the heated juice is exposed can be controlled by
evacuation or desired
exchange of selected mixtures of gases to maintain a continuously lower
concentration of
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
desired partial pressures of gases to increase transfer of the desired gases
or materials
from the heated diffusion juice, including gas stripping as described above.
Now referring primarily to Figure 11, a further embodiment of the invention
can
comprise a pump (70) or other liquid transfer element that achieves adequate
process
liquid pressure (between about 20 pounds per square inch and about 25 pounds
per square
inch) at the an injection port of an gas injector (71). As described above,
the process
liquid may be heated to between about 50°C to about SO°C to
decrease solubility of gases
in the process liquid, such as C02, SOZ, volatile organic compounds, or
volatile inorganic
compounds, or otherwise described above, in the process liquid. After air or
other partial
pressures of gases as desired, are injected into the process liquid at the
injection port (71),
the process liquid can be transferred to a gas-liquid separator (72) which in
some
embodiments of the invention can be a centrifugal gas-liquid separator that
can achieve
forces of about four times gravity. The gas-liquid separator (72) allows
partial pressures
of gases injected into the process liquid to transfer dissolved gases,
volatile organic
compounds, or volatile inorganic compounds to atmosphere to lower the
concentration of
these materials in the process liquid. In some embodiments of the invention,
the gas-
liquid separator can be a container that contains the process liquid in a
manner that
increases the atmosphere-process liquid interface allowing the transfer of
materials from
the process liquid to the atmosphere in a shorter duration of time. When a
centrifugal
gas-liquid separator is used, centrifugal forces applied to the process liquid
can spread the
process liquid over the inside surface of a cylindrical container (although
other
configurations can be used as well) with forces in some cylindrical
embodiments of the
invention of about four times gravity. Spreading the process liquid over the
inside
surface of the cylindrical container of the centrifugal gas-liquid separator
increases the
area of the atmosphere (or other partial pressures of gases)-process liquid
interface by
maintaining a column of gases at the center of the cylindrical to which gases
in the
process liquid can be transferred to. A gas relief system (73) allows partial
pressures of
gases transferred from the process liquid to atmosphere. In some embodiments
of the
invention, the process liquid from the gas-process liquid separator (72) can
enter the pre-
liming step of convention sugar process systems, or enter other processing
steps as
described above.
36
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
As to other embodiments of the invention, pump (74) or other process liquid
transfer element transfers process liquid to a liquid dispersion element (76),
such as a
nozzle, to distribute the process liquid in a manner that increases the
atmosphere (or other
partial pressure of gases)-process liquid surface area. In some embodiments
the liquid
dispersion element (76) can create droplets or a spray. A gas distribution
manifold (77)
or other gas distribution element moves air or other partial pressure of gases
through the
dispersion of process liquid to further allow gas partitioning between
dissolved gases,
volatile organic compounds, or volatile acids, or the like, in the process
liquid and the
partial pressure gases introduced by the gas distribution manifold. In some
cases, the
flow of such partial pressure of gases introduced by the gas distribution
manifold (77) can
be comter current to the direction of the dispersed process liquid from the
liquid
dispersion element (76) to make the process of gas partitioning or gas
stripping more
efficient. A foam dispersion element (78) can be further included to knock
down foam
generated by the liquid during the gas partitioning or gas stripping process.
A mesh or
screen having apertures of a suitable size can be used. The liquid dispersion
element
(76), the gas distribution manifold (77) and the foam dispersion element (78)
can be
located inside a containment element (79) or gas partition column. Gas flow
volume to
the gas distribution manifold (77) can be established with a gas transfer
element (80).
The gas flow volume can be regulated in amount based upon analysis of the
conditions
within the containment element (79) or the chemical conditions within the
process liquid,
separately or in combination. In some embodiments of the invention the process
liquid
can enter the pre-liming step of convention sugar process systems, or enter
other
processing steps as described above.
Certain embodiments of the invention can fixrther include a vacuum chamber
(84)
into which process liquid can be transferred. The pressure within the vacuum
chamber
(84) can be adjusted or regulated to transfer the desired amount of volatile
materials from
the volume of process liquid passed through the vacuum chamber (84) (or
achieve the
desired pH). The vacuum within the chamber can be generated by a vacuum pump,
or
with respect to some embodiments of the invention movement of liquid through
an
eductor system (88) (89)(90). The amount of process liquid entering the vacuum
chamber
(84) can also be regulated by a liquid control valve (81) and can be dispersed
through a
second liquid dispersion element (82) to increase the process liquid-gas
interface area.
The process liquid can then be transferred from the vacuum chamber (84) to the
pre-
37
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
liming step of convention sugar process systems, or enter other processing
steps as
described above.
The invention can further comprise a vent system (91) from various components
(72)(79)(84)(90) to transfer overflow process liquid or process liquid foam to
a vent
collection container (93) into which an anti-foam agent can be added through
an anti-
foam agent dispersion element (92). The process liquid collected in the vent
collection
container (93) can then be transferred from the vacuum chamber (84) to the pre-
liming
step of convention sugar process systems, or enter other processing steps as
described
above.
Now referring primarily to Figures 12 and 13, an embodiment of the invention
can
comprise an a juice treatment system which provides juice dispersal element
(300) which
can as a non-limiting example be a BEX PSQ full square spray nozzle or a BEX
PSWSQ
wide angle full square spray nozzle (300). See for example
Juice (301), whether or not heated as described above, can be dispersed into a
gas (302),
or a mixture of gases, or partial pressure of gases (such as atmospheric
gases, or
atmospheric gases supplemented or stripped to the desired partial pressures)
having gas
characteristics that allow transfer of at least one substance from said juice
to said gas. An
adjustable gas flow generator (303) maintains a flow of said gas (302)
sufficient to
maintain said gas characteristics (gas partial pressures, gas volume, gas
residence time,
gas velocity, or the like) which allow transfer of said at least one substance
from said
juice to gas (302). A gas discharge element(s)(304) allow gas containing
substances
transferred from the juice to discharge to the atmosphere or be carried to a
desired
location or be discharged to a desired process or into a desired process step.
Gas flow
(302) can be established by a single gas discharge location or by multiple gas
discharge
locations (305). In certain embodiments of the invention gas is first directed
to a gas
distribution element (310), such as the gas distribution ring shown in Figure
13 (providing
numerous apertures (313) in the ring). The gas distribution element serves to
generate
desired gas flow characteristics within the containment vessel (312), whether
counter
current or otherwise.
For example, juice (301), diffusion juice, pulp juice, diffuser liquids, or
juice
process liquids dispersed at about 60 to about 110 cubic foot per minute
(about 500 to 133
38
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
gallons per minute) dispersed into flow of gas generated at about 450 to 850
cubic foot
per minute can result in a transfer from the juice to the gas flow of certain
substances
such as alcohol, an aldehyde, a ketone, an ester, a nitrite, a sulfide, a
pyrazine, carbon
dioxide, carbonic acid, sulfur dioxide, phosphoric acid, hydrochloric acid,
sulfuric acid,
sulfurous acid, citric acid, oxalic acid, succinic acid, fumaric acid, lactic
acid, glycolic
acid, pyrrolidone-carboxylic acid, formic acid, acetic acid, butyric acid,
malefic acid,
propanoic acid, 3-methylbutanoic acid, butanoic acid, pentanoic acid, 5-
methylhexanoic,
hexanoic, heptanoic, and lactic acid, or the like. With respect to embodiments
of the
invention such as those configurations shown in Figures 12 and 13 a gas flow
(302) in
cubic feet of about four times the amount of juice dispersed (301) has been
used to reduce
the amount of a variety of substance in diffusion juice obtained from sugar
beets. See
Examples 1 through 3. Similarly, the juices obtained from milled sugar cane
can be
treated similarly with similar results. Depending upon the amount of juice
dispersed and
the gas flow generated the configuration can be sized accordingly or multiple
components
comprising the invention can be used in series or in parallel to treat juice
generated by a
typical sugar beet process facility (typically between 1000 to 5000 gallons of
diffusion
juice per minute).
Certain embodiments of the invention fixrther include a supplemental gas flow
generator (306) to generate supplemental gas flow (307) which can comprise
oxygen,
ozone, air stripped of certain partial pressures of gases, an oxidant capable
of converting
primary alcohols to corresponding aldehydes or carboxylic acids. Alternately,
embodiments of the invention further include supplemental oxidants (308) which
cam be
dispersed into the dispersed juice through nozzle (311).
As discussed above, a heater (309) can establish or maintain juice at a
substantially constant temperature selected within the range of 60°C
and 80°C as said
juice (301) disperses into said gas (302) having gas characteristics which
allow transfer of
said at least one substance from said juice to said gas. As to different
embodiments of the
invention, juice can have a temperature as said juice (301) disperses into
said gas (302)
having gas characteristics which allow transfer of said at least one substance
from said
juice (301) to the gas selected from the group consisting of about
60°C, about 61°C, about
62°C, about 63°C, about 64°C, about 65°C, about
66°C, about 67°C, about 68°C, about
39
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
69°C, about 70°C, about 71°C, about 72°C, about
73°C, about 74°C, about 75°C, about
76°C, about 77°C, about 78°C, about 79°C, and
about 80°C.
Some embodiments of the invention can further include baffles (311) to
increase the area
of interface between dispersed juice (301) and the gas (302) having gas
characteristics
which allow transfer of at least one substance from said juice to said gas.
Treatment of juice as described above can occur in a first containment element
(312) as shown in Figure 13 after which treated juice is transferred from an
exit port (314)
to the prelimer (57) or other process step or can be transferred to a second
containment
element (315). In those embodiments, in which the treated juice is transferred
to a second
containment element, juice (301) can again be dispersed by at least one second
dispersal
element (316). A pressure reduction generator (317) can reduce pressure within
said
second containment element (316) to reduce gas partial pressures which allows
transfer of
at least one substance from said juice (301) to lowered partial pressures of
gas (318).
The pressure reduction generator establishes and maintains a reduced pressure
(318) within the second containment element (315) sufficient to boil dispersed
juice
(102). The reduced pressure (318) within the second containment element (315)
can be
varied or adjusted (automatically or manually) based upon the temperature of,
composition of dispersed juice (301). A stripping gas flow generator (319) can
introduce
a flow of stripping gas (320) into the second containment element (315) to
transfer
volatilized substances to atmosphere. The stripping gas (320) can comprise
air,
atmospheric gases, nitrogen, oxygen, other desired gas.
Certain embodiments of the invention can ftzrther include a supplemental gas
reduction generator (321) to assist gas reduction generator (317) in
establishing or
maintaining boiling of dispersed juice (301) in the second containment element
(315).
Similar to the first containment element (312) baffles (311) can be included
in the
second containment element (315) to increase the area of interface between the
juice
(301) and the reduced partial pressures of gas (318).
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Some embodiments of the invention can comprise a third containment element
(322) in which a reduced pressure can be established and maintained as
described above.
Juice (301) can be transferred from the second containment element (315)
through exit
port (323) and dispersed into the third containment element through a juice
disbursement
element similar to that in the first and second containment elements.
Alternately, juice
(301) exiting the second containment element (315) can be transferred directly
to the
preliming steps or filtration steps or other steps or processes as desired.
These examples of specific embodiments of the invention are specifically
intended
to be illustrative of the broader generic concept of utilizing the lowered
solubility of
heated juice to certain materials, gases, volatile compounds, acids, or the
like to
affirmatively monitor, assess, or control the concentration of these materials
through one
of or a combination of controlling the partial pressures of gases presented to
the surface
of heated diffusion juices or increasing the surface area of the heated juice
exposed to a
desired partial pressure of gases prior to pre-liming steps. The advantages of
the
invention are to be understood even in the context of small additions of base
such as lime
to control foaming of juices) during processing prior to the pre-liming step.
EXAMPLE 1
Juice was obtained by conventional tower diffusion of sugar beet cossettes. A
control group and an experimental group each consisting of six substantially
identical 500
mL aliquots of the diffusion juice were generated. Each aliquot within the
control group
and the experimental group was analyzed to ascertain the pH value. As to each
aliquot of
the diffusion juice in the control group the pH value was about 6.3. Each
aliquot within
the control group without any further treatment was titrated to an 11.2 pH
endpoint with a
solution of 50% wt./vol. caustic soda. Each aliquot within the experimental
group was
treated in accordance with the invention after which the pH of each aliquot
was
ascertained and each experimental aliquot titrated in substantially identical
fashion to the
control group to an 11.2 pH endpoint with a solution of 50% wt./vol. caustic
soda.
The results are set out in Table 1 below. As can be understood from the
table each aliquot of juice prior to any treatment had a pH of about 6.3. The
experimental
group after treatment in accordance with the invention had increased pH values
without
41
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
the addition of any base, and required a reduced amount of caustic soda to
achieve the
11.2 pH endpoint as compared to the control group.
42
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Table 1
Untreated Juice mL Treated xnL % reduction
pH Caustic Juice Caustic Caustic
Soda pH Soda Soda
6.3 1.8 6.5 1.5 1 6.6
6.3 1.8 6.6 1.4 22.2
6.3 1.8 6.6 1.4 22.2
6.3 1.9 6.6 1.6 1 5.8
6.3 1.9 6.5 1.5 2 1.0
6.3 1.9 6.5 1.6 1 5.8
The reduction in the amount of caustic soda to reach the 11.2 pH endpoint for
the
aliquots of juice in the experimental group treated in accordance with the
invention as
compared to the aliquots of juice in the untreated control group was between
about 15.8%
and about 22.2%.
EXAMPLE 2.
Juice was obtained by conventional tower diffusion of sugar beet cossettes. A
control group and an experimental group each consisting of five substantially
identical
500 mL aliquots of the diffusion juice were generated. Each aliquot within the
control
group and the experimental group was analyzed to ascertain the pH value. As to
each
aliquot of the diffusion juice in the control group the pH value was about
6.1. Each
aliquot within the control group without any further treatment was titrated to
an 11.2 pH
endpoint with a solution of 30 brixs mills of lime. Each aliquot within the
experimental
group was treated in accordance with the invention after which the pH of each
aliquot
was ascertained and each experimental aliquot titrated in substantially
identical fashion to
the control group to an 11.2 pH endpoint with a solution of 30 brixs milk of
lime.
The results are set out in Table 2 below. As can be understood from the table
each
aliquot of juice prior to any treatment had a pH of about 6.1. The
experimental group
after treatment in accordance with the invention had increased pH values
without the
43
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
addition of any base, and required a reduced amount of milk of lime to achieve
the 11.2
pH endpoint as compared to the control group.
Table 2
Untreated Juice mL Milk Treated mL Milk % reduction
pH of Juice of Milk of Lime
Lime pH Lime
6.1 4.6 6.5 3.3 28.3
6.1 4.4 6.6 3.2 27.3
6.1 4.7 6.6 3.5 25.5
6.1 4.4 6.6 3.3 25.0
6.1 4.5 6.6 3.3 26.7
The reduction in the amount of milk of lime to reach the 11.2 pH endpoint for
the
aliquots of juice in the experimental group treated in accordance with the
invention as
compared to the aliquots of juice in the untreated control group was between
about 25.0%
and about 2~.3%.
Also, the data set out in Table 1 and Table 2 provides a comparison of two
different types of diffusion apparatus and diffusion methods. Importantly, the
data shows
that different diffusers or different diffusion methods can generate diffusion
juice having
significantly different pH values even though pH values attributed to each
type of
diffusion technology can be substantially internally consistent. See for
example the initial
pH value of the untreated diffusion juice in Table 1 which shows a pH value of
6.3 as
compared to the untreated diffusion juice in Table 2 which a pH value of 6.1.
EXAMPLE 3.
Diffusion juice was obtained by conventional tower diffusion of sugar beet
cossettes and treated in accordance with the invention using the embodiment
shown by
Figures 12 and 13 having location between the mixer and the pre-limer.
Diffusion juice
dispersed at a rate of about 100 cubic foot per minute into a flow of
atmospheric gases
generated at a rate of about 400 cubic foot per minute (counter current path
of 72 inches x
44
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
72 inches with counter current path height of about 144 inches) generated
transfer a
variety of substances from the dispersed juice as identified by gas
chromatograph/mass
spectra analysis shown in Tables 1 and 2 below:
1 Acetic
2 Propanoic
3 3-Methylhutanoic
4 8utanoic
4-Methylpentanoic
6 Pentanoic
7 5-Methylhexauoic
8 Hexanoic
9 I-Ieptanoic
SM.BSC # ~
S11~IBSC # 2
s
1 Z_ 3 ~l 9
I
s~~z
5
TABLE 1. ,
Table 1 shows gas chromatography analysis of samples SMBSC 1 and SMBSC 2
(condensates obtained from gas flow after counter current exchange with juice
as
described herein) with the chromatographs of those samples compared with a gas
chromatograph of a sample of a standard mixture of organic acids listed as 1-9
above. As
can be understood, treatment of juice in accordance with the invention removed
varying
amounts of each organic acid included in the standard mixture.
45
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Abmndanca
2t300000
2nooooo
2200000
2000000
'I 800000
1800000
X400000
120000
~oooaoc
sooooc
~ooooc
aOOOOc
20000(
Tirne--~
TrC:~ RMRRC:S A
TABLE 2. ,
Table 2 shows gas chromatography/ mass spectrometry analysis of sample
SMSBCS D (condensates obtained from gas flow after counter current exchange
with
juice as described herein without use of reduced pressure with a juice
temperature of
between 60°C and 70°C with the chromatograph of this sample
showing various volatile
compounds rising above a base line having a curvature predominated by a
variety of
alcohols.
Each of these compounds has been identified with GCMS and the kekule
structures are set out below in Table 3:
46
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
Aldebydes
iT
OOH ~OH ~OH ~leal~o(s
O
~~-~(~~ Ketones
O
~O~
O O esters
IOI IIO
N~ N~ Nitrites
Sulfides
,O N i0 i N Substituted 1'yrazines
N~ ~ Nw
TABLE 3.
While there may be different types of diffusion apparatus and different
diffusion
methods, there is a lack of recognition within the ordinary skill in the art
that pH can be
altered or reduced during diffusion of sugar beet material or other types of
plant material,
or a lack of recognition that different diffusion apparatus or different
methods yield juice
or liquids having different pH values, or a lack of recognition that newer
types of
diffusers typically result in diffused juice that has lower pH values. To the
extent that
diffusion technology generates diffusion juice having different pH values
using the same
diffusion technology or different diffusion technology, or that improvements
to diffusion
technology have altered or reduced the pH value of diffusion juice, it can be
understood
that these conventional approaches to extracting juice from plant material
teach away
from the teaching of the invention.
As can be easily understood from the foregoing, the basic concepts of the
present
invention may be embodied in a variety of ways. It involves both analysis
techniques as
well as devices to accomplish the appropriate analysis. In this application,
the analysis
47
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
techniques are disclosed as part of the results shown to be achieved by the
various devices
described and as steps that are inherent to utilization. They are simply the
natural result
of utilizing the devices as intended and described. In addition, while some
devices are
disclosed, it should be understood that these not only accomplish certain
methods but also
can be varied in a number of ways. Importantly, as to all of the foregoing,
all of these
facets should be understood to be encompassed by this disclosure.
The discussion included in this application is intended to serve as a basic
description. The reader should be aware that the specific discussion may not
explicitly
describe all embodiments possible; many alternatives are implicit. It also may
not fully
explain the generic nature of the invention and may not explicitly show how
each feature
or element can actually be representative of a broader function or of a great
variety of
alternative or equivalent elements. Again, these are implicitly included in
this disclosure.
Where the invention is described in device-oriented terminology, each element
of the
device implicitly performs a function. Apparatus claims may not only be
included for the
device described, but also method or process claims may be included to address
the
functions the invention and each element performs. Neither the description nor
the
terminology is intended to limit the scope of the claims herein included.
It should also be understood that a variety of changes may be made without
departing from the essence of the invention. Such changes are also implicitly
included in
the description. They still fall within the scope of this invention. A broad
disclosure
encompassing both the explicit embodiments) shown, the great variety of
implicit
alternative embodiments, and the broad methods or processes and the like are
encompassed by this disclosure and may be relied for support of the claims of
this
application. It~should be understood that any such language changes and broad
claiming
is herein accomplished. This full patent application is designed to support a
patent
covering numerous aspects of the invention both independently and as an
overall system.
Further, each of the various elements of the invention and claims may also be
achieved in a variety of manners. This disclosure should be understood to
encompass
each such variation, be it a variation of an embodiment of any apparatus
embodiment, a
method or process embodiment, or even merely a variation of any element of
these.
Particularly, it should be understood that as the disclosure relates to
elements of the
4~
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
invention, the words for each element may be expressed by equivalent apparatus
terms or
method terms -- even if only the function or result is the same. Such
equivalent, broader,
or even more generic terms should be considered to be encompassed in the
description of
each element or action. Such terms can be substituted where desired to make
explicit the
implicitly broad coverage to which this invention is entitled. As but one
example, it
should be understood that all actions may be expressed as a means for taking
that action
or as an element which causes that action. Similarly, each physical element
disclosed
should be understood to encompass a disclosure of the action which that
physical element
facilitates. Regarding this last aspect, as but one example, the disclosure of
a "injector"
should be understood to encompass disclosure of the act of "injecting" --
whether
explicitly discussed or not -- and, conversely, were there effectively
disclosure of the act
of "injecting", such a disclosure should be understood to encompass disclosure
of a
"injector" and even a "means for injecting." Such changes and alternative
terms are to be
understood to be explicitly included in the description.
Any patents, publications, or other references mentioned in this application
for
patent are hereby incorporated by reference. In addition, as to each term used
it should be
understood that unless its utilization in this application is inconsistent
with such
interpretation, common dictionary definitions should be understood as
incorporated for
each term and all definitions, alternative terms, and synonyms such as
contained in the
Random House Webster's Unabridged Dictionary, second edition are hereby
incorporated
by reference. However, as to each of the above, to the extent that such
information or
statements incorporated by reference might be considered inconsistent with the
patenting
of this/these inventions) such statements are expressly not to be considered
as made by
the applicants.
Thus, the applicants) should be understood to claim at least: i) each of the
juice
process systems as herein disclosed and described, ii) the related methods
disclosed and
described, iii) similar, equivalent, and even implicit variations of each of
these devices
and methods, iv) those alternative designs which accomplish each of the
functions shown
as are disclosed and described, v) those alternative designs and methods which
accomplish each of the functions shown as are implicit to accomplish that
which is
disclosed and described, vi) each feature, component, and step shown as
separate and
independent inventions, vii) the applications enhanced by the various systems
or
49
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
components disclosed, viii) the resulting products produced by such systems or
components, ix) methods and apparatuses substantially as described
hereinbefore and
with reference to any of the accompanying examples, x) the various
combinations and
permutations of each of the previous elements disclosed, xi) processes
performed with the
aid of or on a computer as described throughout the above discussion, xii) a
programmable apparatus as described throughout the above discussion, xiii) a
computer
readable memory encoded with data to direct a computer comprising means or
elements
which function as described throughout the above discussion, xiv) a computer
configured
as herein disclosed and described, xv) individual or combined subroutines and
programs
as herein disclosed and described, xvi) the related methods disclosed and
described, xvii)
similar, equivalent, and even implicit variations of each of these systems and
methods,
xviii) those alternative designs which accomplish each of the functions shown
as are
disclosed and described, xix) those alternative designs and methods which
accomplish
each of the functions shown as are implicit to accomplish that which is
disclosed and
described, xx) each feature, component, and step shown as separate and
independent
inventions, xxi) the various combinations and permutations of each of the
above, and
xxii) each potentially dependent claim or concept as a dependency on each and
every one
of the independent claims or concepts presented.
It should be understood that for practical reasons and so as to avoid adding
potentially hundreds of claims, the applicant may eventually present claims
with initial
dependencies only. Support should be understood to exist to the degree
required under
new matter laws -- including but not limited to European Patent Convention
Article
123(2) and United States Patent Law 35 U.S.C ~132 or other such laws-- to
permit the
addition of any of the various dependencies or other elements presented under
one
independent claim or concept as dependencies or elements under any other
independent
claim or concept.
Further, if or when used, the use of the transitional phrase "comprising" is
used to
maintain the "open-end" claims herein, according to traditional claim
interpretation.
Thus, unless the context requires otherwise, it should be understood that the
term
"comprise" or variations such as "comprises" or "comprising", are intended to
imply the
inclusion of a stated element or step or group of elements or steps but not
the exclusion of
any other element or step or group of elements or steps. Such terms should be
interpreted
CA 02537038 2005-09-27
WO 2004/015144 PCT/US2003/026209
in their most expansive form so as to afford the applicant the broadest
coverage legally
permissible.
The claims set forth in this specification are hereby incorporated by
reference as
part of this description of the invention, and the applicant expressly
reserves the right to
use all of or a portion of such incorporated content of such claims as
additional
description to support any of or all of the claims or any element or component
thereof,
and the applicant further expressly reserves the right to move any portion of
or all of the
incorporated content of such claims or any element or component thereof from
the
description into the claims or vice-versa as necessary to define the matter
for which
protection is sought by this application or by any subsequent continuation,
division, or
continuation-in-part application thereof, or to obtain any benefit of,
reduction in fees
pursuant to, or to comply with the patent Laws, rules, or regulations of any
country or
treaty, and such content incorporated by reference shall survive daring the
entire
pendency of this application including any subsequent continuation, division,
or
continuation-in-part application thereof or any reissue or extension thereon.
51