Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02538962 2006-03-17
1
TITLE OF THE INVENTION
[0001] Process for recovering value metal species from laterite-type
feedstock.
FIELD OF THE INVENTION
[0002] The present invention relates to a process for recovering value
metal
species from laterite-type feedstock. More specifically, the present invention
relates
to a process for recovering metal species such as nickel, cobalt, iron,
aluminum
and/or magnesium from laterite-type feedstock.
BACKGROUND OF THE INVENTION
[0003] Laterite minerals consist of residual weathering products of rocks
such as basalts, granites and shales. These metamorphic materials are found on
all continents in tropical or semi-tropical zones. They can have variable
compositions where iron, aluminum, magnesium and/or silica predominate. In
several instances, significant amounts of nickel and cobalt are associated
with the
dominant constituents.
[0004] Serpentine minerals can also be found associated with laterites
ores.
Serpentine is a magnesium-rich silicate mineral that is found as a major
constituent in many metamorphic and weathered igneous rocks. Some serpentinic
minerals contain significant amounts of nickel and cobalt.
[0005] A large number of processes have been reported in order to gain
access to the nickel/cobalt values, in laterite ores or other materials. These
processes can be grouped in four broad categories, namely:
a) Pressure leaching or atmospheric pressure leaching;
CA 02538962 2006-03-17
2
b) Acidic or basic leaching;
C) Reductive or oxidative leaching; and
d) Open or closed circuit leaching.
[0006] In many instances, two or more of these categories are combined,
such as in the well-known and commercially used processes of Pressure Acid
Leaching (PAL) or Ammonia Leaching (the Caron Process).
[0007] In PCT application No. WO 02/08477 published January 31, 2002,
Lalancette discloses a closed-circuit method for recovering nickel and cobalt
from
laterite ores, which essentially comprises the steps of grinding the ore,
treating the
ore with gaseous hydrochloric acid (gaseous HCI), wherein the remainder of
gaseous HCI is scrubbed with water into a concentrated HCI solution, curing
the
ores in the concentrated HCI solution, followed by filtration of the resulting
lixiviate
and selective recovery of nickel and cobalt with known techniques. Gaseous HCI
is
thereafter recycled by roasting or pyrohydrolysis at a minimum of 450 C and
returned to the treating stage.
[0008] However, high temperature roasting, which allows recycling of the
leaching agent or part of it, often fails to provide a significant commercial
advantage regarding capital or operation costs and remains more difficult to
implement.
[0009] In PCT application No. WO 2005/093107, published October 6,
2005, Moyes et al. disclose a process for recovering a target metal from an
oxidized metalliferous material comprising the steps of: leaching the oxidized
metalliferous material with an acidic aqueous halide solution to leach the
target
metal into solution, the leaching solution being generated by adding sulfuric
acid to
a solution comprising a metal halide; passing the solution from the leaching
stage
to a target metal recovery stage in which the target metal is recovered from
the
CA 02538962 2012-09-21
3
solution whilst the metal halide is retained in solution; and returning the
solution with the
metal halide therein from the target metal recovery stage to the leaching
stage. The
process preferably comprises two leaching stages, wherein the solid residue
from the
first leaching stage is directed to the second leaching stage, and the liquid
residue of
the second leaching stage is at least partially redirected to the first
leaching stage. The
process also possibly includes a separate hydrohalous acid generation stage in
which
sulfuric acid is added to a solution comprising the metal halide, thereby
forming an
acidic leaching solution that is then fed to the second leaching stage and
mixed with the
first leached solids.
[0010] However, this two-step leaching process seems unduly complicated
since
it requires recycling part of the respective residues of each leaching step
into the other.
It also seems to leave the residual solid material loaded with halide solution
that has to
be removed before discarding, which adds a substantial operational
complication.
[0011] Thus remains a need for a simple and economical process for the
recovery of value metal species from laterite-type feedstock.
SUMMARY OF THE INVENTION
[0012] The present invention generally relates to an essentially open-
circuit
process for recovering value metal species from a laterite-type feedstock.
[0013] More specifically, in accordance with the present invention, there
is
provided an essentially open-circuit process for recovering value metal
species from a
laterite-type feedstock, the process comprising the sequential or unsequential
steps of:
a) separating the laterite-type feedstock into a first and a second fraction;
b) reacting an acid with a chloride salt in a first compartment, thereby
generating
gaseous HCI;
C) chlorinating the first fraction with the gaseous HCI in a second
compartment,
thereby producing a chlorinated fraction, wherein excess HCI is recovered and
CA 02538962 2012-09-21
4
dissolved in water, thereby producing a concentrated HCI solution;
d) combining the chlorinated fraction and the second fraction into a mixture;
e) leaching the mixture with the concentrated HCI solution in a third
compartment,
thereby producing a reaction mass;
f) submitting the reaction mass to a separation of phases, thereby separating
an
insoluble residue from a head solution; and
g) selectively recovering value metal species from the head solution.
[0014] Other objects, advantages and features of the present invention
will
become more apparent upon reading of the following non-restrictive description
of
specific embodiments thereof, given by way of example only with reference to
the
accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] In the appended drawings:
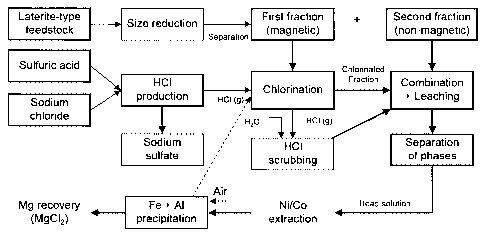
[0016] Figure 1 is a block diagram illustrating the broad aspects of the
process of
the present invention.
[0017] Figure 2 is a block diagram illustrating the various steps of an
embodiment
of the process of the present invention.
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0018] In general terms, the present invention relates to an essentially
open-circuit process for recovering value metal species from a laterite-type
feedstock.
[0019] As used herein, the expression "laterite-type feedstock" refers to
nickel-containing sedimentary oxide materials that are relatively rich in iron
oxide,
magnesium oxide and/or aluminum oxide, and/or nickel-containing magnesium-rich
CA 02538962 2012-09-21
silicates, which possibly further contain trace amounts of cobalt and/or
chromium. Non-
limiting examples of such laterite-type feedstock include laterites, laterite
ores, lateritic
materials, serpentine, serpentine ores, serpentinic materials and talc.
[0020] As used herein, the expression "value metal species" refers to any
valuable metal species that can be found in the laterite-type feedstock, non-
limiting
examples of which include nickel, cobalt, iron, aluminum, magnesium and
chromium.
[0021] As used herein, the expression "essentially open-circuit process"
means
that the process is mainly unidirectional, in a forward direction, that the
hydrochloric
acid, when combined to give an essentially stable chloride, is not recycled
alter the
leaching step and that a by-product is recovered and used for itself outside
the process,
without being recycled to a step that precedes its formation in the overall
process. It is
to be understood that an "essentially open-circuit" process as used herein
does not
preclude a product or reagent used in the process to be redirected to another
step of
the overall process.
[0022] As used herein when referring to a metal with which HCI may be
combined during the process of the present invention, an "essentially stable
chloride"
refers to a metal chloride that would not be hydrolyzed under oxidant
conditions at a
temperature less than 400 C, a non-limiting example of which is magnesium
chloride.
As used herein, the contrary of this expression would be an "essentially
unstable
chloride", non-limiting examples of which are iron or aluminum chlorides.
[0023] Referring now to Figure 1, gaseous HCI is generated by reacting an
acid
with a chloride salt in a first compartment, white the laterite-type feedstock
is separated
into a first and a second fraction. These steps are in no particular order and
can be
performed simultaneously and independently.
[0024] The hydrochloric acid generation can involve different couples of
acid/chloride salts, non-limiting examples of which are sulfuric acid/sodium
chloride,
sulfuric acid/calcium chloride, sulfuric acid/potassium chloride, nitric
acid/sodium
chloride, nitric acid/potassium chloride or phosphoric acid/sodium chloride
(although this
CA 02538962 2012-09-21
6
last example is not preferred because of the rather low reactivity of
phosphoric acid.
[0025] The first fraction of feedstock is then chlorinated with gaseous
HCI (which
constitutes a first, dry digestion) in a second compartment, and the resulting
chlorinated
fraction is combined with the second fraction of feedstock. Unreacted gaseous
HCI is
dissolved in water, thereby forming a concentrated HCI solution, which is used
for
leaching the combined fractions (which constitutes a second, wet digestion),
in a third
compartment. In operation, both the combination of the "chlorinated" and
"second"
fractions and leaching may occur simultaneously. The reaction mass resulting
from the
leaching is then submitted to a separation of phases, which produces an
insoluble
residue (solid) and a head solution (liquid). This head solution is thereafter
subjected to
further conventional steps allowing selectively recovering value metal
species, including
in particular nickel, cobalt, iron, aluminum and magnesium.
[0026] Referring now to Figure 2, which illustrates a more particular
embodiment
of the present invention, gaseous hydrochloric acid is generated by reaction
of sulfuric
acid with sodium chloride, which are both very cheap reagents, according to
the
following equation:
H2SO4+ 2 NaCI ---+ Na2SO4 + 2 HCI(g)
[0027] Hydrochloric acid is advantageous because of its fast dissolving
capability,
as compared to sulfuric or phosphoric acid for example. Since hydrochloric
acid is about
five times more expensive than sulfuric acid on an acidic-proton basis, in
situ generation
of hydrochloric acid from sulfuric acid provides a substantial economy. In
addition, such
reaction further generates a sellable product, namely sodium sulfate (in form
of a salt
cake), used in large amounts in pulp and paper production, glass making and
other
industries. This by-product may therefore substantially amortize the cost of
the reagents
required to produce the hydrochloric acid.
[0028] The amount of gaseous HCI needed is determined experimentally and
depends on the feedstock composition. For example, the more iron or magnesium
is
present in the feedstock, the higher amount of HCI will generally be needed.
CA 02538962 2012-09-21
7
[0029] The acid-generation reaction from sulfuric acid and sodium
chloride is
performed at a temperature between 300 and 400 C and therefore requires an
input of
energy. However, from that point, the highly reactive hot gaseous HCI, by the
reaction
with the first fraction, liberates a substantial amount of energy so that the
following
chlorination and leaching steps can be operated without further energy input,
which is
another significantly cost-effective aspect of the process of the present
invention at an
industrial scale.
[0030] According to a particular embodiment of the present invention (as
seen in
Figure 2), the laterite-type feedstock is separated into a magnetic and a non-
magnetic fractions. In such a case, the "first fraction" used for chlorination
is the
magnetic fraction, which tends to be more acidic (since it is richer in iron
and silica) and
therefore less reactive in the presence of another acid. The process of the
present
invention thus takes advantage of the high reactivity of the hot gaseous HCI
produced
to operate a reaction that would otherwise have been difficult. The leaching
is then done
on the combination of the magnetic, chlorinated fraction and the nonmagnetic
fraction
(which tends to be more basic since it is richer in alumina and magnesia).
These
chlorination and leaching reactions are advantageously performed at
temperatures
ranging from about 90 to about 120 C, preferably about 100 C. The dry
chlorination
takes approximately 15 to 30 minutes and the wet leaching step preferably for
a
duration of from 4 to 9 hours.
[0031] In another embodiment of the present invention, which occurs
mainly in a
case where a magnetic separation would not give substantial magnetic and non-
magnetic fractions, the laterite-type feedstock is simply separated into two
fractions of
approximately equal weight before treatment by HCI.
[0032] The chlorination and leaching steps are usually performed at
atmospheric
pressure, thereby limiting operation costs. These reactions are usually made
in vats or
stirred reactors.
[0033] Following the leaching step, the reaction mass, comprised of solid
particles and a liquid lixiviate, is filtered or centrifuged (separation of
phases in Figure 2)
CA 02538962 2012-09-21
8
and the insoluble residue discarded, optionally after rinsing. The
nickel/cobalt chlorides
are then recovered from the resulting head solution (to which the rinsing
solution is
optionally added) by conventional techniques, such as, contacting with
selective ion
exchange resins, solvent extraction, electrowinning or sulfide precipitation.
[0034] The first soluble residue after Ni/Co removal is then usually
deprived of
iron and aluminum by simple pH adjustment in the range of 3-3.5 after
aeration. The
resulting second soluble residue is then adjusted to a pH ranging between 6
and 7, and
reduced in volume so as to separate magnesium chloride, which may be
crystallized as
a solid and/or commercialized as such.
[0035] However, in the case of a relatively high-iron (for example, with
an iron
content of more than 8% of the feedstock dried at 100 C) and low-magnesium
feedstock, the first soluble residue, still rich in iron (in the form of an
essentially unstable
chloride), can optionally be hydrolyzed and oxidized in the presence of air at
a
temperature ranging between about 200 and 400 C, preferably between about 200
and
350 C, to liberate a substantial amount of gaseous HCI, which can then be used
as a
chlorination agent, thereby partly replacing the NaCl/H2SO4 mixture. It is to
be
understood that such additional step does not necessitate an energy input
higher than
that required forHCI production by NaCl/H2SO4.
[0036] Such hydrolysis converts the essentially unstable iron and
aluminium
chlorides into insoluble oxides, without affecting the magnesium chloride in
the resulting
second soluble residue. A simple rinsing may be used to recover it, followed
or not by
crystallization.
[0037] As stated above, the process according to the present invention
essentially operates in "open circuit", wherein the essentially stable
magnesium chloride
is usually recovered as a useful product rather than submitted to a high
energy-
consuming, high-temperature roasting so as to recycle the hydrochloric acid.
Only when
a relatively high-iron feedstock allows it, HCI may advantageously be recycled
by a
hydrolysis at a temperature between about 200 and 400 C. In either case, the
process
according to the present invention may generally lead to surprisingly low
operational
CA 02538962 2012-09-21
9
costs.
[0038] An additional step of size reduction may be added to the process
of the
present invention. Indeed, it has been noted that submitting the laterite-type
feedstock
to grinding before or after the separation step accelerates the reaction and
improves the
yield of metal recovery. Such grinding preferably takes place before the
separation step,
particularly in a case of magnetic separation, which is facilitated by smaller
particles.
[0039] The granulometry of the feedstock resulting from such grinding may
range
between minus 35 and minus 200 mesh, but preferably the whole feedstock would
pass
through a 120 mesh screen (i.e. granulometry of 100% minus 120 mesh).
[0040] The process of the present invention can be advantageously applied
to
laterite-type feedstocks comprising (as a result of an analysis on an ore
dried at 100 C):
4 to 50% iron, 0.1 to 10% aluminum, <1% to 15% magnesium and 0.2 to 5% nickel.
[0041] When applied to laterites with a nickel content ranging from 0.24%
to
3.1%, the process of the present invention allows a recovery of nickel in the
range of
about 95 to 99 %, while the magnesium recovery was observed in the range of
about 98
percent.
[0042] The useful products at the end of the process are mainly in the
form of
chlorides of nickel, cobalt, iron and magnesium and sulfate of sodium.
[0043] The present invention is illustrated in further details by the
following non-
limiting examples.
EXAMPLE 1
[0044] Five different samples of laterites originating from Brazil were
treated in
accordance with the process of the present invention, as further explained
below.
The chemical analysis of the dry (analyzed after drying at 100 C) samples was
as
CA 02538962 2012-09-21
follows:
Table 1: Chemical composition of samples
Sample Fe Co Ni Mn Cr Al Ca Mg LOI
cyo cyo cyo
1 7.70
0.07 3.09 <0.05 0.34 3.25 0.55 8.61 16.5
2 26.8 0.04 0.58 0.80 2.71 0.71 0.084 0.71
7.06
3 22.6 0.04 1.08 0.40 1.10 2.86 0.39 4.83
11.5
4 16.4 0.06 1.14 0.27 1.12 2.21 0.12 3.07
7.62
5 8.52 0.04 0.50 0.11 0.89 0.34 0.097 1.19
3.19
[0045] Each of these samples were reduced to minus 120 mesh and submitted
to
magnetic separation. In the case of samples 1 and 2, there was not a very
significant
magnetic fraction, as can be seen from Table 2.
Table 2: Magnetic fractionation of samples
Sample Magnetic fraction Non magnetic fraction
1 1.7% 98.3%
2 7% 93%
3 46% 54%
4 60% 40%
5 48% 52%
[0046] In the case of samples 1 and 2, the material to be treated was
split into
two fractions of approximately equal weight, whereas with the last three, the
first fraction
treated with the dry gaseous HCI was the magnetic fraction.
[0047] Gaseous HCI was generated by reaction of sodium chloride with
sulfuric
acid in a silica lined reactor at 350 C, so as to produce the required amount
of HCI
(which is determined experimentally).
[0048] The dry, gaseous HCI was first contacted with the first fraction
in a Vycor
tube kept at 120 C, at atmospheric pressure. The non-reacted HCI was taken up
with
CA 02538962 2012-09-21
11
water and the reaction completed by leaching of the combined first and second
fractions
in a stirred reactor at a temperature ranging from 90 C to 120 C, still at
atmospheric
pressure.
[0049] The reason why the Vycor tube and reactor have to be maintained at
the
desired temperatures in this case stems from the fact that the quantifies of
feedstock
and reagents are relatively small, and that there is an important loss of
energy at such
laboratory scale. At an industrial scale however, the introduction of energy
would
essentially be required for the generation of gaseous HCI only.
[0050] The residual mass was then filtered and the insoluble residue
discarded
after rinsing. The nickel/cobalt in solution was then collected over an ion
exchange
resin. Iron was then precipitated along with minor components such as
chromium,
aluminium and manganese, and the residual solution contained the magnesium as
chloride. The conditions of reaction and extraction results are reported in
Table 3 below.
Table 3: Conditions and Results of extraction on 25 g samples
extraction results
Sample Weight Leaching Temperature % Ni % Co % Mg
HCI (g) duration ( C)
(hrs)
1 12.5 9 100 94 86 99
2 8.75 8 110 100 100 96
3 14.0 7 120 100 100 99
4 13.5 7 100 100 100 97
5.75 5 90 100 73 98
EXAMPLE 2
[0051] A dry sample of overburden from the laterite deposit of Pinares in
Cuba
was showing the following elemental composition (analyzed after drying at 100
C):
CA 02538962 2012-09-21
12
Sample Fe Co Ni Mn Cr Al Mg LOI
A % % % % % % %
laterite,
36.4% 0.03% 0.24% 0.48% 1.60% 7.52% 0.77% 13.9%
Pinares
[0052] A 25 gram sample of this material was submitted to a magnetic
separation
(wet separation of a 20% solid slurry previously reduced to minus 120 mesh,
With a
5000 gauss permanent magnet). The resulting dried magnetic and non-magnetic
fractions represented respectively 55% and 45% of the starting sample. The
chlorination
and leaching were done following the procedure described in Examples 1 to 5,
the
amount of HCI used being 31.5 g and the duration of the whole digestion being
of 6.5
hours at 95 C. The workup of the solution gave a recovery of 96% of the nickel
and of
91% of the cobalt in the starting sample.
[0053] It is to be noted that iron generally predominates in the feedstock
sample
and therefore constitutes the biggest consumer of HCI drying the whole
digestion. An
oxydative hydrolysis of the ferrous chloride was performed on the solution
after removal
of the nickel and cobalt, which allowed regenerating 91% of the HCI used
initially for
lixiviation.
EXAMPLE 3
[0054] A sample of serpentinic tailings from the chrysotile mining at Bell
Mine
(Black Lake, Quebec, Canada) was showing the following elemental composition
(analyzed after drying at 100 C):
Sample MgO 5i02 A1203 Fe Ni Cr LOI
% % % % %
serpentinic
tailings, 38.25% 38.6% 1.28% 5.2% 0.23% 0.09% 12.5%
Black Lake
CA 02538962 2012-09-21
=
13
[0055] A 100 g sample of this ore was dried at 100 C and reduced to
minus 14
mesh in a hammer mill. The resulting mass was taken up in water to give a 10%
solid
pulp and the suspended chrysotile fiber was decanted. The residual weight of
fiber free
ore was 81.1g after drying.
[0056] A 25 g sample of the dried fiber free ore was reduced to minus
70 mesh in
a hammer mill and separated into magnetic (41%) and non-magnetic (59%)
fractions.
[0057] The chlorination and leaching were done as described in
Example 1, the
amount of HCI used being 14.9 g and the duration of whole digestion 7.5 hours
at 95 C.
[0058] The processing of the solution indicated a recovery of 85% of
the nickel
and 76% of the magnesium in the starting dried fiber free ore. It is to be
noted that an
extraction yield is always to be read in relation to the amount of the metal
species to be
recovered in the feedstock. Therefore a 85% recovery of nickel where nickel is
present
in an amount of 0.23% in the serpentinic tailing is a very good yield.
[0060] The scope of the claims should not be limited by the preferred
embodiments set forth in the examples, but should be given the broadest
interpretation
consistent with the description as a whole.