Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 1 -
ELECTROCHEMICAL REDUCTION OF METAL OXIDES
The present invention relates to electrochemical
reduction of metal oxides.
The present invention relates particularly to
continuous and semi-continuous electrochemical reduction
of metal oxides in the form of powder to produce metal
having a low oxygen concentration, typically no more than
0.2% by weight.
The present invention was made during the course
of an on-going research project on electrochemical
reduction of metal oxides being carried out by the
applicant. The research project has'focussed on the
reduction of titania (Ti02).
During the course of the research project the
applicant carried out experimental work on the reduction
of titanea using electrolytic cells that included a pool
of molten CaCl2-based electrolyte, an anode formed from
graphite, and a range of cathodes.
The CaCl2-based electrolyte was a commercially
available source of CaClz, namely calcium chloride
dehydrate, that decomposed on heating and produced a very
small amount of CaO.
The applicant operated the electrolytic cells at
a potential above the decomposeteon potenteal of Ca0 and
below the decomposition potenteal of CaCl2.
The applicant found that at these potentials the
cell could electrochemically reduce titania to titanium
with low concentrations of oxygen, ie concentrations less
than 0.2 wt.%.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 2 -
The applicant operated the electrolytic cells on
a batch basis with titania in the form of pellets and
larger solid blocks in the early part of the work and
titania powder in the later part of the work. The
applicant also operated the electrolytic cells on a batch
basis with other metal oxides.
Whilst the research work established that it is
possible to electrochemically reduce titanic (and other
metal oxides) to metals having low concentrations of
oxygen in such electrolytic cells, the applicant has
realised that there are significant practical difficulties
operating the electrolytic cells commercially on a batch
basis.
In the course of considering the results of the
research work and possible commercialisation of the
technology, the applicant realised that it was possible
that commercial production could be achieved by operating
an electrolytic cell on a continuous or semi-continuous
basis with metal oxide powders and pellets being
transported through the cell in a controlled manner and
being discharged in a reduced form from the cell.
International application PCT/AU2003/001657,
which claims priority from Australian provisional
application 2002953282 lodged on 12 December 2002, in the
name of the applicant describes this invention in broad
terms as a process for electrochemically reducing a metal
oxide, such as titanic, in a solid state in an
electrolytic cell that includes a bath of molten
electrolyte, a cathode, and an anode, which process
includes the steps of: (a) applying a cell potential
across the anode and the cathode that is capable of
electrochemically reducing metal oxide supplied to the
molten electrolyte bath, (b) continuously or semi-
continuously feeding the metal oxide in powder and/or
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 3 -
pellet form into the molten electrolyte bath, (c)
transporting the powders and/or pellets along a path
within the molten electrolyte bath and reducing the metal
oxide as the metal oxide powders and/or pellets move along
the path, and (d) continuously or semi-continuously
removing reduced metal oxide powders and/or pellets from
the molten electrolyte bath.
The International application defines the term
"powder and/or pellet form" as meaning particles having a
particle size of 3.5 mm or less. The upper end of this
particle size range covers particles that are usually
described as pellets.
The term "powder" and "pellets" as used herein is
understood to mean particles that are less than 5 mm in a
major dimension.
The term "powder" and "pellets" as used herein is
not intended to limit the scope of patent protection to a
particular procedure for producing the particles.
The term "semi-continuously" is understood in the
International application and herein to mean that the
process includes: (a) periods during which metal oxide
powders and/or pellets are supplied to the cell and
periods during which there is no such supply of metal
oxide powders and/or pellets to the cell, and (b) periods
during which reduced material is removed from the cell and
periods during which there is no such removal of reduced
material from the cell.
The overall intention of the use of the terms
"continuously" and "semi-continuously" in the
International application and herein is to describe cell
operation other than on a batch basis.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 4 -
In this context, the term "batch" is understood
in the International application and herein to include
situations in which metal oxide is continuously supplied
to a cell and reduced metal builds up in the cell until
the end of a cell cycle, such as disclosed in
International application WO 01/62996 in the name of The
Secretary of State for Defence.
After making the initial invention described
above, the applicant carried out further research into the
possibility of commercial production based on operating an
electrolytic cell on a continuous or semi-continuous
basis.
The applicant realised that a commercial
production cell should include a cell cathode in the form
of a member, such as a plate, having an upper surface for
supporting metal oxides in pellet form, as described
herein, that is horizontally disposed or slightly inclined
and has a forward end and a rearward end and is immersed
in the electrolyte bath and is supported for movement,
preferably in forward and rearward directions, so as to
cause metal oxide pellets to move toward the forward end
of the cathode.
The applicant proposed that, with this
arrangement, in use, metal oxides in powder and/or pellet
form could be supplied onto the upper surface of the
cathode, preferably near the rearward end thereof, and
moved forward by the movement of the cathode and fall off
the upper surface at the forward end of the cathode and
ultimately be removed from the cell. With this
arrangement, the metal oxides would be reduced as the
metal oxides moved over the upper surface.
International application PCT/AU2004/000809,
which claims priority from Australian provisional
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 5 -
application 2003903150 lodged on 20 June 2003, in the name
of the applicant describes this so-called "shaker table"
cathode invention in broad terms.
The applicant has carried out further research
and development work on the "shaker table" invention and
has now designed a particular electrolytic cell in
accordance with the invention. The invention of the
particular electrolytic cell design is the subject of this
patent specification.
The particular electrolytic cell design of the
present invention is characterised by multiple anodes and
by support structures that separately support the "shaker
table" cathode and the anodes from above the cell, and
preferably with the anode support structure enabling
adjustment of the spacing of the anodes above the upper
surface of the "shaker table" cathode.
According to the present invention there is
provided an, electrolytic cell for electrochemically
reducing metal oxide powders and/or pellets (as described
herein), which electrolytic cell includes (a) a bath of a
molten electrolyte, (b) a cathode in the form of a member,
such as a plate, having an upper surface for supporting
metal oxide powders and/or pellets that is horizontally
disposed or slightly inclined and has a forward end and a
rearward end and is immersed in the electrolyte bath, (c)
a cathode support means for supporting the cathode from
above the electrolyte bath and for moving the cathode in
the cell so as to cause metal oxide powders and/or pellets
on the upper surface of the cathode to move toward the
forward end of the cathode while in contact with molten
electrolyte whereby electrochemical reduction of the metal
oxide can occur as the powders and/or pellets move toward
the forward end, (d) a plurality of anodes extending into
the electrolyte bath, (e) an anode support means for
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 6 -
supporting the anodes from above the electrolyte bath, (f)
a means for applying a potential across the anodes and the
cathode, (g) a means for supplying metal oxide powders
and/or pellets to the electrolyte bath so that the metal
oxide powders and/or pellets can deposit onto the upper
surface of the cathode, and (h) a means for removing at
least partially electrochemically reduced metal oxides
from the electrolyte bath.
Preferably the anodes are arranged in pairs
above the upper surface of the cathode.
Preferably there are a plurality of the pairs
along the length of the upper surface of the cathode.
Preferably each anode is in the form of a block
of a suitable anode material, such as graphite, mounted on
the end of a rod.
The term "rod" is used herein in a general sense
and includes any elongate member, such as a bar, that is
suitable as a support member for an anode block.
Preferably the anode support means includes a
fixed structure and a means for holding the anode rods to
the structure above the electrolyte bath.
Preferably the means for holding the anode rods
enables adjustment of the anode blocks vertically upwardly
or downwardly so that the spacing of the ends of the anode
blocks above the upper surface of the cathode can be
varied.
Preferably the cathode support means includes:
(a) a plurality of cathode support members,
such as rods, extending upwardly from the
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
cathode,
(b) a fixed structure,
(c) a movable structure supported by the fixed
structure and movable with respect to the
fixed structure, the movable structure
including a means for holding the cathode
support members so that the cathode is
immersed in the electrolyte bath, and
(d) a means coupled to the movable structure
for moving the movable structure to thereby
move the cathode in the cell so as to cause
metal oxide powders and/or pellets on the
upper surface of the cathode to move toward
the forward end of the cathode.
Preferably the fixed structure of the anode
support means is mounted to the fixed structure of the
cathode support means.
Preferably the means for holding the cathode
support members allows adjustment of the position of the
cathode vertically upwardly or downwardly within the
electrolyte bath.
Preferably the cathode support means is adapted
to move the cathode in the cell to cause metal oxide
powders and/or pellets on the upper surface of the cathode
member to move over the upper surface of the cathode in
forward and rearward directions.
Preferably the cathode is formed to cause metal
oxide powders and/or pellets to move on the upper surface
of the cathode toward the forward end of the cathode as a
packed mono-layer of powders and/or pellets.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
_ g _
For example, the cathode may be formed with an
upstanding lip at the forward end that causes powders
and/or pellets to build-up behind the lip. Alternatively,
or in addition, the upper surface of the cathode may be
formed with a series of transversely extending grooves
that promote close packing of the powders and/or pellets.
Preferably the means for applying an electrical
potential across the anode and the cathode includes an
electrical circuit in which a power source is connected to
a forward end of the cathode. The applicant has found
that this arrangement results in substantial reduction of
titania powders and/or pellets within a short distance
from the forward end of the cell.
Preferably the cathode support members extend
upwardly from the opposed sides of the cathode.
Preferably the means for applying a potential
across the anodes and the cathode includes (a) a power
source and (b) an electrical circuit that electrically
interconnects the power source, the anodes, and the
cathode.
Preferably the electrical circuit includes the
cathode support members.
Preferably the size and/or the positions of the
cathode support members is selected having regard to the
requirements of (a) supporting the cathode in a stable
manner within the electrolytic cell and (b) supplying a
pre-selected current distribution to the cathode.
Preferably the cell includes a means for treating
gases released from the cell.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
_ g _
The gas treatment means may include a means for
removing any one or more of carbon monoxide, carbon
dioxide, chlorine-containing gases, and phosgene from the
gases.
The gas treatment means may also include a means
for combusting carbon monoxide gas in the gases.
In a situation in which the metal oxide is
titanic it is preferred that the electrolyte be a CaCl2-
based electrolyte that includes Ca0 as one of the
constituents.
Preferably the particle size of the powders
and/or pellets is in the range of 1-4 mm.
Typically, the particle size of the pellets is in
the range of 1-3 mm.
According to the present invention there is also
provided a process for electrochemically reducing metal
oxide pellets, such as titanic pellets, in the above-
described electrolytic cell that includes the steps of:
(a) applying a cell potential across the anodes and the
cathode that is capable of electrochemically reducing
metal oxide supplied to the molten electrolyte bath, (b)
continuously or semi-continuously feeding metal oxide
powders and/or pellets into the molten electrolyte bath so
that the powders and/or pellets deposit on an upper
surface of the cathode, (c) causing metal oxide powders
and/or pellets to move over the upper surface of the
cathode toward the forward end of the cathode while in
contact with molten electrolyte whereby electrochemical
reduction of the metal oxide occurs as the powders and/or
pellets move toward the forward end, and (d) continuously
or semi-continuously removing at least partially
electrochemically reduced metal oxide powders and/or
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 10 -
pellets from the molten electrolyte bath.
Preferably step (b) includes feeding the metal
oxide powders and/or pellets into the molten electrolyte
bath so that the powders and/or pellets form a mono-layer
on an upper surface of the cathode.
In use, the metal oxide powders and/or pellets
may be deposited on the upper surface of the cathode in a
pile of powders and/or pellets and may be shaken out into
a mono-layer as the cathode moves the pellets towards the
forward end of the cathode.
Preferably step (c) includes causing metal oxide
powders and/or pellets to move on the upper surface of the
cathode toward the forward end of the cathode as a packed
mono-layer of powders and/or pellets.
The packed mono-layer may be produced by forming
the cathode appropriately. For example, the cathode may
be formed with an upstanding lip at the forward end that
causes powders and/or pellets to build-up behind the lip.
Alternatively, or in addition, the cathode may be formed
with a series of transversely extending grooves that
promote close packing of the powders and/or pellets.
Preferably step (c) includes selectively moving
the cathode so as to cause metal oxide powders and/or
pellets on the upper surface of the cathode to move toward
the forward end of the cathode.
There is a wide range of options for moving the
cathode to cause forward movement of powders and/or
pellets on the upper surface of the cathode. The applicant
has found that it is preferable to move the cathode in
forward and rearward directions. The applicant has found
that one option that can achieve controlled forward
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 11 -
movement of powders and/or pellets includes moving the
cathode in a repeated sequence that comprises a short
period of oscillating motion in the forward and rearward
directions and a short rest period. The applicant has
found that this sequence can cause powders and/or pellets
on the upper surface of the cathode to move over the upper
surface in a controlled series of short steps from the
rearward end to the forward end of the cell.
Moreover, the present invention is not confined
to operating a cell under constant operating conditions
and extends to situations in which the operating
parameters, such as the cathode movement, are varied
during the operating campaign of the cell.
Preferably step (c) includes moving the cathode
so as to cause powders and/or pellets across the width of
the cathode to move at the same rate so that the powders
and/or pellets have substantially the same residence time
within the bath.
Preferably the process electrochemically reduces
the metal oxide to metal having a concentration of oxygen
that is no more than 0.3~ by weight.
More preferably the concentration of oxygen is no
more than 0.2~ by weight.
The process may be a single or multiple stage
process involving one or more than one electrolytic cell.
In the ease of a multiple stage process involving
more than one electrolytic cell, the process may include
successively passing reduced and partially reduced metal
oxides from a first electrolytic cell through one or more
than one downstream electrolytic cell and continuing
reduction of the metal oxides in these cells.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 12 -
In a situation in which the cathode is in the
form of a plate, another option for a multiple stage
process includes successively passing reduced and
partially reduced metal oxides from one cathode plate to
another cathode plate or a succession of cathode plates
within one electrolytic cell.
Another option for a multiple stage process
includes recirculating reduced and partially reduced metal
oxides through the same electrolytic cell.
Preferably the process includes washing pellets
that are removed from the cell to separate electrolyte
that is carried from the cell with the powders and/or
pellets.
The process inevitably results in a loss of
electrolyte from the cell and, therefore make-up
electrolyte will be required for the cell.
The make-up electrolyte may be obtained by
recovering electrolyte that is washed from the powders
and/or pellets and recycling the electrolyte to the cell.
Alternatively, or in addition, the process may
include supplying fresh make-up electrolyte to the cell.
Preferably the process includes maintaining the
cell temperature below the vaporisation and/or
decomposition temperatures of the electrolyte.
Preferably the process includes applying a cell
potential above a decomposition potential of at least one
constituent of the electrolyte so that there are cations
of a metal other than that of the cathode metal oxide in
the electrolyte.
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 13 -
In a situation in which the metal oxide is
titania it is preferred that the electrolyte be a CaCl2-
based electrolyte that includes Ca0 as one of the
constituents.
In such a situation it is preferred that the
process includes maintaining the cell potential above the
decomposition potential for CaO.
The present invention is described further by
way of example with reference to the accompanying drawings
of which:
1 5 Figure 1 is a schematic diagram that illustrates
one embodiment of an electrochemical process and an
electrolytic cell in accordance with the present
invention;
Figure 2 is a perspective view of the
electrolytic cell shown in Figure 1, with the cathode
support rods removed to clarify the Figure;
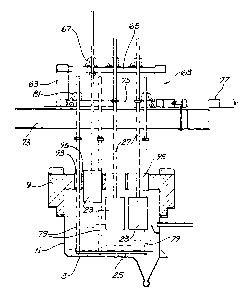
Figure 3 a vertical section through the
2 5 electrolytic cell shown in Figures 1 and 2;
Figure 4 is the vertical section shown in Figure
3 with the top cover and the anodes and the anode support
structure removed to illustrate the cathode and the
cathode support structure more clearly; and
Figure 5 is the vertical section shown in Figure
3 with the top cover and the cathode and the cathode
support structure removed to illustrate the anodes and the
3 5 anode support structure more clearly.
The following description is in the context of
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 14 -
electrochemically reducing titania pellets to titanium
metal having an oxygen concentration of less than 0.3
wt.°s. However, it is noted that the present invention is
not confined to this metal oxide and extends to other
metal oxides. It is also noted that the present invention
is not confined to electrochemical reduction of pellets
and also extends to electrochemical reduction of powders
as described herein.
The electrolytic cell 1 shown in the drawings is
an enclosed chamber, although not completely sealed, that
is rectangular in top plan and has a base wall 3, a pair
of opposed end walls 5, a pair of opposed side walls 7,
and a top cover 9.
The cell includes a series of inlets for titania
pellets in the top cover 9 near the left hand end of the
cell as viewed in Figures 1,3 4, and 5 and near the right
hand end of the cell as viewed in Figure 2. This end of
the cell is hereinafter referred to as "the rearward end"
of the cell. The inlets are identified by the numeral 11
in Figure 2.
The pellets are formed in a "green" state in a
pan pelletiser 51 and are then sintered in a sintering
furnace 53 and thereafter are stored in a storage bin 55.
Typically, the pellets have a size range of 1-4 mm.
Pellets from the storage bin 55 are supplied via a
vibratory feeder 57 to the cell inlets 11.
The cell further includes an outlet 13 for
titanium metal pellets in the base wall 3 near the right
hand end of the cell as viewed in Figures 1,3 4, and 5 and
near the right hand end of the cell as viewed in Figure 2.
This end of the cell is hereinafter referred to as "the
forward end" of the cell. The outlet 13 is in the form of
a sump defined by downwardly converging sides 15 and an
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 15 -
upwardly inclined auger 35 or other suitable means
arranged to receive titanium pellets from a lower end of
the sump and to transport the pellets away from the cell.
The cell contains a bath 21 of molten
electrolyte. The preferred electrolyte is CaClz with at
least some CaO.
The cell further includes a cathode 25 in the
form of a plate or other suitable member that is immersed
in the bath 21 and is positioned a short distance above
the base wall 3. The cathode plate 25 is supported in the
cell by a support structure described hereinafter so that
the upper surface of the cathode plate 25 is horizontal or
slightly inclined downwardly from the rearward end to the
forward end of the cell. The length and width dimensions
of the cathode plate 25 are selected to be as large as
possible to fit conveniently within the cell. The cathode
plate 25 is supported to move in the forward and rearward
directions in an oscillating motion as described
hereinafter.
The cathode support structure includes a fixed
support structure of vertical posts 71 and a pair of
horizontal cross members 73 mounted on the posts. In
addition, the support structure includes a carriage 75
that is arranged for forward and rearward horizontal
sliding movement on the cross members 73 and an hydraulic
actuator 77 that is mounted on the cross members 73 and is
coupled to the carriage 75 to move the carriage. The
support structure also includes 6 screw jacks 81 mounted
to the carriage 75 and 6 elongate cathode support members
79 that are connected at lower ends to the opposed sides
of the cathode plate 25 and are supported at upper ends by
the screw jacks 81. The support members 79 are arranged
in pairs on opposite sides of the cathode plate 25. Thus,
there are 3 support members 79 on each side of the cathode
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 16 -
plate 25. The screw jacks 81 hold the support members 79,
and thereby the cathode plate 25, for controlled movement
downwardly into or upwardly from the electrolyte bath 21
to enable height adjustment of the cathode plate within
the electrolyte bath 21. In addition, as will be apparent
from the above, sliding movement of the carriage 75 in
forward and rearward directions via operation of the
actuator 77 causes horizontal sliding movement of the
cathode plate 25 in the electrolyte bath 21. The top cover
9 of the cell includes openings 93 (see Figures 2 and 3)
for the support members 79 and the openings are
sufficiently large to accommodate such sliding movement of
the carriage 75 and therefore the support members 79.
The cell further includes 6 anodes generally
identified by the numeral 19 that extend into the bath 21.
The anodes 19 include graphite blocks 23 mounted to the
ends of rods or other suitable support members 27. The
anode blocks 19 include lengthwise extending slots 91 (see
Figure 2) to allow gas that evolves in the electrolyte
bath 21 to escape from the cell. The anodes 19 are
arranged in pairs and the size of the anode blocks 23 is
selected so that the anodes are positioned directly above
substantially the whole of the upper surface of the
cathode plate 25. The anodes 19 are supported by a
support structure described hereinafter so that the anode
blocks 23 can be progressively lowered into the bath 21 as
lower sections of the anode graphite are consumed by cell
reactions at the anodes. The top cover 9 of the cell
includes openings 95 (see Figures 2 and 3) for the support
members 27.
The anode support structure includes a fixed
support structure of vertical posts 63 and an assembly of
horizontal cross members 65 mounted on the posts. The
support structure also includes 6 screw jacks 67 mounted
to a pair of parallel cross members 65 and holding the 6
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 17 -
anodes 19. Specifically, the screw jacks 67 hold the
anode support members 27, and thereby the anode blocks 23,
for controlled movement downwardly into or upwardly from
the electrolyte bath 21.
The applicant has found that movement of the
cathode plate 25 in a repeated sequence that comprises a
short period of forward and backward, ie oscillating,
motion and a short rest period can cause pellets on the
upper surface of the cathode plate 25 to move over the
upper surface in a series of short steps from the rearward
end to the forward end of the cell.
Moreover, the applicant has found that the above-
described type of motion can cause pellets across the
width of the cathode plate 25 to move at a constant rate
so that the pellets have substantially the same residence
time within the bath 21.
More particularly, the cell is arranged so that,
in use, titania pellets supplied to the cell via the
inlets 11 fall downwardly onto the upper surface of the
cathode plate 25 near the rearward end of the cell and are
caused to move forwardly over the upper surface of the
cathode plate 25 and fall off the forward end of the
cathode plate 25 into the outlet 13. More particularly,
the cell is arranged so that, in use, the pellets move
forwardly over the upper surface of the cathode plate 25
as a closely packed mono-layer. In order to achieve close
packing of the pellets, the cathode plate 25 includes an
upstanding lip (not shown) at the forward end thereof that
causes pellets to build-up behind the lip along the length
of the cathode plate 25.
The applicant has found that it is preferable
that the titania pellets be substantially round since it
is possible to cause these pellets to move over the upper
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 18 -
surface of the cathode plate 25 in a more predictable
manner than is possible with more angular pellets.
In addition, the applicant has found that it is
undesirable that the pellets "stick" to the upper surface
of the cathode to an extent that inhibits forward movement
of the pellets and that the pellets "stick" together.
These considerations support the preference for round
pellets. It is relevant to note that oscillating movement
of the cathode plate 25 minimises sticking of pellets.
The applicant has also found that the size and
weight of the pellets should be selected so that the
pellets settle quite quickly onto the upper surface of the
cathode plate 25 and do not become suspended in the
electrolyte in the molten bath 21.
In overall terms, it is preferable to select the
smallest possible pellet size that can move over the
cathode plate 25 in an efficient manner, i.e. without
sticking to the cathode, in order to optimise mass
throughput of the cell.
The cell further includes a power source 31 for
applying a potential across the anode block 23 and the
cathode plate 25 and an electrical circuit (that includes
the above-described cathode support members 79)
electrically interconnects the power source 31, the anodes
23, and the cathode. The size and/or the positions of the
cathode support members 79 is selected to supply a
preselected current distribution to the cathode plate 25
to optimise electrochemical reduction of titania pellets
on the cathode plate 25. Depending on the circumstances,
there may be a range of current distributions required in
the operation of the cell.
In use of the cell, titania pellets are supplied
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 19 -
to the upper surface of the cathode plate 25 at the
rearward end of the cell so as to form a mono-layer of
pellets on the cathode plate 25 and the cathode is moved
as described above and causes the pellets to step forward
over the surface of the plate to the forward end of the
cell and ultimately fall from the forward end of the
cathode. The pellets are progressively electrochemically
reduced in the cell as the pellets are moved over the
surface of the cathode plate 25. The operating parameters
of the cathode plate 25 are selected so that the pellets
have sufficient residence time in the cell to achieve a
required level of reduction of the titanic pellets.
Typically, 2-4 mm titanic pellets require 4 hours
residence time to be reduced to titanium with a
concentration of 0.3 wt°s oxygen at a cell operating
voltage of 3 V.
The applicant has found that the above-described
arrangement results in substantial reduction of titanic
pellets within a short distance from the forward end of
the cell.
The applicant has found that there are a number
of factors that have an impact on the overall operation of
the cell. Some of these factors, namely pellet size and
shape and motion of the cathode plate 25, are discussed
above. ,Another relevant factor is the exposed surface
areas of the upper surface of the cathode plate 25 and the
anode block 23. On the basis of work to date, the
applicant believes that larger rather than smaller
cathodes 25 in relation to the exposed surface area of the
anode blocks 23 is preferable. In other words, the
applicant believes that a larger rather than a smaller
anodic current density is preferable.
In use of the cell, the anode blocks 23 are
progressively consumed by a reaction between carbon in the
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 20 -
anode block 23 and O-- anions generated at the cathode
plate 25, and the reaction occurs predominantly at the
lower edges of the anode blocks 23.
The distances between the upper surface of the
cathode plate 25 and the lower edges of the anode blocks
23 are maintained as required.
Preferably the distance between the upper surface
of the cathode plate 25 and the lower edges of the anode
block 23 is selected so that there is sufficient
resistance heating generated to maintain the bath 21 at a
required operating temperature.
Preferably the cell is operated at a potential
that is above the decomposition potential of. Depending
on the circumstances, the potential may be as high as 4-
5V. In accordance with the above-described mechanism,
operating above the decomposition potential of Ca0
facilitates deposition of Ca metal on the cathode plate 25
due to the presence of Ca++ cations and migration of O--
anions to the anode block 23 as a consequence of the
applied field and reaction of the O-- anions with carbon of
the anode block 23 to generate carbon monoxide and carbon
dioxide and release electrons. In addition, in accordance
with the above-described mechanism, the deposition of Ca
metal results in chemical reduction of titania via the
mechanism described above and generates O-- anions that
migrate to the anode block 23 as a consequence of the
applied field and further release of electrons. Operating
the cell below the decomposition potential of CaCl2
minimises evolution of chlorine gas, and is an advantage
on this basis.
As is indicated above, the operation of the cell
generates carbon monoxide and carbon dioxide and
potentially chlorine-containing gases at the anode blocks
CA 02540234 2006-03-24
WO 2005/031041 PCT/AU2004/001331
- 21 -
23 and it is important to remove these gases from the
cell. The cell further includes an off-gas outlet 41 in
the top cover 9 of the cell and a gas treatment unit 43
that treats the off-gases before releasing the treated
gases to atmosphere. The gas treatment includes removing
carbon dioxide and any chlorine gases and may also include
combusting carbon monoxide to generate heat for the
process.
Titanium pellets, together with electrolyte that
is retained in the pores of the titanium pellets, are
removed from the cell continuously or semi-continuously at
the outlet 13. The discharged material is transported via
the auger 35 to a water spray chamber 37 and quenched to a
temperature that is below the solidification temperature
of the electrolyte, whereby the electrolyte blocks direct
exposure of the metal and thereby restricts oxidation of
the metal. The discharged material is then washed to
separate the retained electrolyte from the metal powder.
The metal powder is thereafter processed as required to
produce end products.
The above-described cell and process are an
efficient and an effective means of continuously and semi-
continuously electrochemically reducing metal oxides in
the form of pellets to produce metal having a low oxygen
concentration
Specifically, the electrolytic cell shown in the
drawing is one example only of a large number of possible
cell configurations that are within the scope of the
present invention.