Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
APPARATUS FOR IGNITING COMBUSTIBLE MEDIUMS
CROSS REFERENCE TO RELATED APPLICATION
This application claims priority from and incorporates herein U.S.
Provisional Application No. 60/509,813 filed October 10, 2003.
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
The present invention relates to ignition systems, particularly catalytic
ignition systems. More particularly, the present invention relates to
apparatuses
employing such ignition systems that can be used in remote environments to
ignite a combustible mixture, e.g., a hydrocarbon/oxygen gas containing
mixture.
DESCRIPTION OF PRIOR ART
Ignition systems for igniting a combustible mixture at a remote location are
used in a variety of applications. By way of example, such ignition systems
can
be used to ignite combustible mixtures issuing from flare stacks in
refineries,
chemical plants, etc. A prime example of the use of an ignition system in a
remote environment is their use in igniting burners disposed in earth
boreholes
drilled into a subterranean formation. Generally, the subterranean formation
is
one that contains a hydrocarbonaceous material e.g., coal, shale, tar sands,
oil,
etc. For example, it has been proposed to drill one or more boreholes into a
coal formation and then, by the generation of heat in the borehole, gasify the
coal in the formation to result in the in situ generation of synthesis gas.
United
1
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
States Patent Application Publications U.S. 200310173081 ('081 Publication)
and
US 2003/0141065, both of which are incorporated herein by reference, describe
methods and systems for the production of hydrocarbons, hydrogen and/or other
products from various hydrocarbon-containing formations. In the '081
Publication, there is described the in situ conversion of hydrocarbons to
produce
more valuable hydrocarbons, hydrogen and/or novel product streams from
underground oil containing formations. In the process proposed in the '081
Publication, one or more heat sources is installed into a subterranean,
hydrocarbon (oil) containing formation to heat the formation, one of the goals
being to raise the temperature in the formation above the pyrolyzation
temperature of the hydrocarbons in the formation. The '081 Publication
describes numerous embodiments and systems for supplying heat, preferably at
pyrolysis temperatures, to the oil containing formation to vaporize and/or
pyrolyze the oil and convert at least a portion of the oil to more valuable
and
more easily recoverable hydrocarbons, the produced more valuable
hydrocarbons being recovered from the subterranean formation.
While a number of electrical heating elements have been proposed to
heat subterranean formations, they all suffer from the inherent problems of
requiring hard wiring to the surface as well as being expensive and lacking
efficiency. To overcome some of these difficulties, it has been proposed to
combust a fuel, the combustion gases being used as the heat source. In this
regard, it has been proposed that the combustion may take place in the
formation in a well, and/or near or at the surface. For example, the
combustion
2
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
in the formation may be in the form of a fire-flood. An oxidizer may be pumped
into the formation. The oxidizer may be ignited to advance a fire front
towards
the production well.
Flameless combustors may be used to combust the fuel within the well.
Flameless combustors are demonstrated, for example, in U.S. Patents
5,255,742, 5,404,952, 5,862,858, 5,899,269, and 6,269,882, all of which are
incorporated herein by reference. Most of these flameless combustors operate
by preheating a fuel and combusting it to a temperature above an auto ignition
temperature of the mixture. The fuel and combustion air are then mixed in the
heating zone to combust. In the heating zone, the flameless combustor, and a
catalyst surface may be provided to lower the auto ignition temperature of the
fuel and air mixture.
It clearly would be desirable to have an ignition source or system which
could be' positioned in the wellbore, e.g. in a tubing having an ID as small
as 3"
positioned in the wellbore, and which, without the use of electrical igniters
or
heaters, and without any preheating of a fuel and oxygen containing mixture,
could ignite a fuel/oxygen gas containing mixture, the combustion gases from
the
fuel being used to heat an airstream being pumped into the tubing, the
combined
heated air and combustion gases then being used to heat the formation to the
desired temperature e.g. the vaporization andlor pyrolysis temperature of at
least
a portion of the hydrocarbons in the formation.
3
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
SUMMARY OF THE INVENTION
In one preferred embodiment, the present invention can include an
ignition system which can be positioned in a relatively small diameter tubing
e.g.
a 3'° I.D. tubing, which is disposable in an earth borehole and which
will ignite a
combustible fuel e.g. a mixture of a hydrocarbon and an oxidizing gas, e.g.,
air,
without the use of electrical heaters, preheating of gases, etc.
In another preferred embodiment, the present invention can employ a
composition of matter which, when exposed to a first gas/oxygen containing gas
(oxidizer) mixture, results in an exothermic reaction causing the temperature
of
the composition of the matter to be elevated above the auto ignition
temperature
of the first gas with consequent ignition of the first gas/oxidizer mixture to
produce a pilot flame. The resulting pilot flame can be propagated into (1 ) a
burner ignition zone to ignite a fuel mixture supplied to suitable burner(s),
or (2)
or near the opening in a flare stack from which is issuing a combustible
medium
to ignite the combustible mediums.
In another preferred embodiment of the present invention, there can be
employed a series of ignition systems as described above in conjunction with a
series of burners which can be spaced axially along the inside of a burner
tubing
positioned in an earth borehole so as to provide a multiplicity of heat
generating
sources along the length of the tubing. The hot combustion gases flowing
towards the bottom of the tubing disposed in the wellbore can then exit the
bottom of the tubing and flow up the annulus between the burner tubing and a
second concentrically disposed tubular, e.g., casing, surrounding the burner
4
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
tubing. Alternatively, the hot combustion gases can flow into the annulus
between the burner tubing and the formation in the case of an open, uncased
borehole. Additionally, air from the surface can be pumped down through the
burner tubing, the air being heated by the combustion gases and/or the flame
from the burners.
Yet another preferred aspect of the present invention can include an
igniter/burner module wherein an igniter system, as described above, is
mounted, on, in or in sufficiently close proximity to a burner that is
supplied with
a combustible fuel. The burner may take the form of one or more nozzles, jets
or
openings in a burner block or housing, the burner being supplied with a
combustible fuel mixture, the igniter system being positioned sufficiently
close to
at least one nozzle so that it will ignite the combustible fuel mixture
issuing from
the nozzle and subsequently all other nozzles.
Still a further preferred aspect of the present invention can include an
apparatus for igniting a combustible medium issuing from an opening in a flare
stack, the apparatus including an igniter assembly having a support, a
catalytic
material carried by the support, the catalytic material comprising a substance
which reacts with a hydrogen containing gas in the presence of an oxidizing
gas
to produce an exothermic reaction and preferably a temperature sufficient to
cause auto ignition of the hydrogen, a source of the hydrogen-containing gas
and a source of the oxidizing gas. There is a mount for positioning the
igniter
assembly adjacent the opening in the flare stack from which the combustible
medium is issuing.
s
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is an elevational, sectional view of one embodiment of an igniter
assembly used in the apparatus of the present invention.
Fig. 2 is an elevational, sectional view of another embodiment of the
igniter assembly used in the apparatus of the present invention.
Fig. 3 is an elevational, sectional view of another embodiment of the
igniter assembly used in the apparatus of the present invention.
Fig. 4 is an elevational view, partly in section of another embodiment of
the igniter assembly used in the apparatus of the present invention.
Fig. 5 is an elevational view of an igniter assembly in accordance with one
embodiment of the present invention used with a flare stack.
Fig. 6 is an elevational view of another embodiment of the igniter
assembly of the present invention used with a flare stack.
Fig. 7 is an elevational view of another embodiment of the igniter
assembly of the present invention used with a flare stack.
Fig. 8 is an elevational view, partly in section of a portion of an igniter
wand employing an igniter assembly in accordance with the present invention.
Fig. 9 is an elevational view showing the igniter wand as connected to the
source of a hydrogen-containing gas.
Fig. 10 is an elevational view, partly in section, showing a plurality of
igniter assembly/burner modules in a tubing in a earth borehole which is in a
subterranean formation containing hydrocarbonaceous materials.
6
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
Fig. 11 is an elevational view, partly in section, showing in greater detail
the igniter assembly/burner module shown in Fig. 10.
Fig. 12 is a cross-sectional view taken along the lines 12-12 of Fig. 11.
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
DESCRIPTION OF PREFERRED EMBODIMENTS
The present invention comprises a self-igniting system which includes a
chamber or support, a source of a first gas and an oxygen containing gas
(oxidizer) to the chamber or the support, and a composition of matter disposed
in
the chamber or supported on the support that reacts with the first
gas/oxidizer to
preferably cause auto ignition of the first gas and oxidizer mixture to
produce a
flame or at least increases the temperature of the composition of matter to a
point which could result in ignition of a combustible mixture e.g. a
hydrocarbon
such as methane, the system being positioned in tubing or other such earth
bore
hole tubulars, e.g., tubing, casing, etc. to ignite a combustible fuel mixture
in the
tubular as, for example, igniting a suitable burner disposed in the tubular,
the
burner being supplied with a combustible fuel mixture. Alternatively, the self
igniting system can be used to ignite a combustible mixture issuing from an
opening in a flare stack, the self-igniting system being supported
sufficiently
close to, or in the opening in the flare stack to effect ignition of the
issuing
combustible mixture.
The oxidizer is an oxidizing gas of the type that will support combustion.
Typically, the oxidizer will be an oxygen containing gas, e.g., air, 02, etc.
The
interaction or reaction between the first gas (hydrogen-containing gas) and
the
composition of matter produces an exothermic reaction, which can raise the
temperature of the composition of matter to above the auto ignition
temperature
of the first gas. At the present time, the invention contemplates that the
first gas
is hydrogen, the oxidizer is air and the composition of matter is a platinum
group
s
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
metal, e.g., palladium, in any form, which when contacted with the
hydrogen/oxidizer mixture results in the catalyzed reaction of hydrogen and
the
oxygen in the air. Typically, when the hydrogen/air mixture contacts the
catalytic
material, the temperature of the platinum/palladium, etc., is elevated well
above
the auto ignition temperature of hydrogen, i.e., greater than about
1,080°F. The
hydrogen/air mixture ignites and results in a flame that can be propagated to
a
burner ignition zone to ignite a combustible mixture issuing from a suitable
burner assembly into the burner igniter zone.
It is well known that the combination of hydrogen and oxygen to form
water is an exothermic process; however, hydrogen and oxygen will not react
automatically when mixed together because of the large activation energy
needed to initiate the reaction. It is also known that the mechanism of the
reaction is extremely complex and that one of the initiation steps is breaking
of
the bond between the two hydrogen atoms of the hydrogen molecule which
requires 432kJ/mole. This energy is typically initially provided by 'a spark
or
flame. After the reaction begins, the energy produced from it will provide the
necessary energy to continue breaking apart the hydrogen molecules. A catalyst
provides an alternative mechanism that has a lower activation energy that
allows
the reaction to proceed without the requirement of the initial addition of
energy
via a flame or spark. Hydrogen molecules will adsorb to the platinum or
palladium surface. The energy of the interaction between the hydrogen atoms
and the platinum or palladium surface contributes to the breaking of the bond
between the hydrogen atoms in the hydrogen molecule. The separate hydrogen
9
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
atoms are then free to react at the surface or leave the surface and
participate in
the water forming steps.
While the invention will be described with particular reference to the use
of hydrogen and an oxidizer (air) impinging on platinum, it is to be
understood
that it is not so limited. Thus, the invention contemplates the use of other
compositions of matter which will react with a gas in a fashion similar to
that
described above with respect to the reaction between hydrogen and platinum.
Additionally, any other metal, alloy, or composition of matter which will
react with
hydrogen in the presence of an oxidizer (air) to bring about the catalyzed
reaction of hydrogen and oxygen described above is also contemplated.
Further, although currently unknown to the inventor, the invention also
contemplates that other gases or mixtures thereof can be brought into contact
with other compositions of matter, also presently unknown to the inventor,
which
will result in a catalyzed auto ignition of such other gases in the presence
of a
suitable oxidizer such as air, oxygen or the like. The particular form of the
composition of matter, e.g., the platinum or palladium-containing material
will
vary depending upon the end-use, the specific design of the support or housing
for the catalyst and other factors. Thus, the platinum can be disposed or
carried
on an inert refractory-type carrier such as alumina, silica, titanic, etc.,
which can
be in the form of pellets, granules or formed bodies, e.g., a compacted mass
of
the platinum group metal/carrier mixture. The platinum group containing
material
can also take the form of a sponge. At the present time, applicant has found
that
platinum supported on a foam-like structure made of a material known as
to
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
Fecralloy and marketed by CRI Catalyst Company can be used to form an
excellent catalytic material for use in the present invention. The Fecralloy
which
is in the form of a hard foam pad or sponge normally contains 22 percent
chromium, 5.3 percent aluminum, a small amount of yithrium in addition to
iron.
The Fecralloy material can withstand temperatures in excess of
2000°F. The
Fecralloy material bearing the platinum metal deposited thereon can be formed
into numerous shapes including tubular bodies. It is desirable that, whatever
form the platinum group composition takes, it have some porosity so as to
provide the necessary surface area for the hydrogen/oxidizer gas mixture to
contact.
Referring now to Fig. 1, there is shown one embodiment of the igniter
assembly used in the apparatus of the present invention. The igniter assembly,
shown generally as 10, comprises a tubular housing 12 which is attached at one
end to an air intake, manifold 14 and at the opposite end to a flame
propagation
tube 16. Received in manifold 14 is a conduit 18 through which a hydrogen-
containing gas flows from a source not shown. Manifold 14 is provided with a
series of openings 20 which can be in generally surrounding relationship to
conduit 18. As seen, the end 22 of conduit 18 faces the throat portion of a
Venturi-type tube 24 attached to tubular housing 12. Venturi tube 24 also
forms
a retainer for a hollow cylindrical section 26 of a suitable catalytic
material as
described above. Tube 16 also serves as a retainer to hold section 26 in
position in tubular housing 12. In operation, a hydrogen-containing gas
passing
through conduit 18 under pressure passes into the throat of Venturi tube 24
and
11
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
then into the hollow core 27 of catalyst section 26. The flow of the hydrogen-
containing gas through conduit 18 toward the throat of tube 24 results in an
aspirating effect whereby air from an ambient source is drawn in through holes
20 of manifold 14. The air and hydrogen-containing gas mix upon entering tube
24, the combined mixture of hydrogen-containing gas and air (or other oxygen
containing gas) flowing through the hollow core 27 and into contact with
catalyst
section 26. The interaction of the hydrogen with the platinum in catalyst
section
26 and in the presence of oxygen results in an exothermic reaction which
causes
auto ignition of the hydrogen. At auto ignition, there is a burst of flame
resulting
in a flame front which propagates in both directions in housing 12, i.e., both
toward manifold 14 and out tube 16. This flame front propagates out tube 16 at
a high velocity and can ignite a combustible mixture that it contacts such as
a
combustible mixture from a burner or other suitable piece of equipment, e.g.,
a
flare stack, it being understood that the combustible mixture include an
oxygen-
containing gas from a suitable source. Generally speaking, the burning
velocity
of hydrogen in air is between about 2.7 to about 3.5 m/sec. Additionally, the
flame temperature is extremely hot, the flame temperature for a 19.6 percent
by
volume hydrogen and air mixture being 2321°K. In effect, this high
velocity, high
temperature flame front issuing from tube 16 behaves essentially like a spark
from an electrical discharge. Following this initial burst of flame, the
hydrogen
will continue to burn at the end 22 of conduit 18 but the hollow core 27 will
be
substantially free of flame. In this regard, the end 22 of conduit 18 is
positioned
a distance away from catalyst section 26 so as to ensure that following the
initial
12
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
burst of flame which propagates in both directions in housing 12, the issuing
hydrogen from conduit 18 will only burn at the tip 22 of conduit 18. This
protects
the catalyst section 26 from undue overheating which would result from a
direct
flame on catalyst section 26 if flame remained in the hollow core 27.
Referring now to Fig. 2, a modified tubular housing 26A~ is secured to a
tubular extension 30 in which is disposed a conduit 32 through which a
hydrogen-containing gas from a source (not shown) flows. Tubular extension 32
extends through a manifold block 34 which has an annulus 36 in surrounding
relationship to tubular extension 32 and which is in open communication with
an
L-shaped port 38 which in turn is in open communication with a conduit 40
through which an oxygen-containing gas, e.g., air, or other types of oxidizer
flows
under pressure from a source, e.g., a compressor (not shown). In the
embodiment shown in Fig. 2, the hydrogen issuing from the end 42 of tubular
extension 32 mixes with the air passing through conduit 40, port 38, annulus
36
and annulus 31 and passes into the throat of tube 24 resulting in an
exothermic
reaction and a burst of flame as described above with respect to the
embodiment
of Fig. 1. Once again, it is desirable to position the end 42 of conduit 32
sufficiently far away from the end of catalyst section 26a to ensure that once
the
initial burst of flame in the hollow core 27 has subsided, a small pilot flame
of
hydrogen will remain on the end 42 of conduit 32 if hydrogen flow is
continued.
Turning now to Fig. 3, there is shown yet another embodiment of the
igniter assembly for use in the apparatus of the present invention. A tubular
housing shown generally as 50 has an upset portion 52 which forms an annular
13
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
recess 54 in which is disposed a tubular catalyst section 56 as described
above
with respect to catalyst section 26. Tubular housing 50 also has an extended
flame front propagation tube 58.
Secured to tubular housing 50 is an annular flange 60 forming an annular
air intake 62 in surrounding relationship to an extension 64 of tubular
housing 50,
extension 64 being secured to upset portion 52. Extension 64 is also provided
with a series of openings or apertures 66 which communicate with air intake
62.
Generally concentrically mounted in extension 64 is a hydrogen feed tube 68
having a tapered tip 70 forming a nozzle. The end of extension 64 threadedly
receives a gland 72 which in turn threadedly receives a fitting 74. Fitting 74
is
threadedly engaged by a nut 76, a ferrule 78 being disposed between nut 76 and
fitting 74. It will be understood, in the well known manner, that when nut 76
is
tightened onto fitting 74, ferrule 78 will form a fluid-tight seal around
hydrogen
feed tube 68. In operation, hydrogen from a source not shown flows under
pressure through feed tube 68 and aspirates air through openings 66, the
mixture of the hydrogen-containing gas and the air flowing into the portion of
housing 52 forming recess 54 where it contacts catalyst section 56. As in the
cases described above, catalyst section 56 is of a generally hollow
cylindrical
configuration and is conveniently made of a material as described above with
respect to the embodiments discussed above. In any event, upon contact with
the catalyst section 56 there is an exothermic reaction which results in auto
ignition of the hydrogen with a sudden burst of flame which propagates a flame
front through flame propagation extension 58 as well as in the opposite
direction
14
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
towards the tip 70 of hydrogen feed tube 68. As described above, this flame
front propagation through tube 58 is much like a spark which is sufficient to
ignite
a combustible fuel in a burner or the like to which the end 59 of extension 58
is
operatively connected.
Referring now to Fig. 4, there is shown a slightly different modification of
the embodiment shows in Fig. 3 wherein the air or oxygen supply is from a
forced air source through a tube 80 into an annular plenum 82 formed by an
annular housing 84 which is attached to extension 64 of housing 58. The
operation of the embodiment shown in Fig. 4 is substantially the same as that
described above with respect to Fig. 3. It is to be noted that the use of
forced air
from a bottle source or other clean air source provides an advantage in that
contamination of the catalyst is minimized. In this regard, air from some
ambient
sources can contain soot and other particulates which; over time, can effect
the
efficiency of the catalyst.
Turning now to Fig. 5, there is shown one embodiment of the present
invention wherein the igniter assembly is used to ignite combustible materials
issuing from a flare stack such as would be found in a refinery, chemical
plant or
other industrial application wherein off-gases, flue gases or the like, are
flared so
as to not be released directly into the atmosphere. Referring then to Fig. 5,
a
flare stack 90 has an opening 92 through which waste gases containing
combustible gases would ordinarily escape to atmosphere if not burnt. Attached
to flare stack 90 via brackets 94 is an igniter assembly, shown generally as
96,
which is substantially the igniter assembly shown in Fig. 3, and includes a
flame
is
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
propagation tube 100 terminating in a nozzle or burner tip 102. Hydrogen is
supplied, under pressure, via hydrogen feed line 104 from a flow meter 106
connected to a line 108 which in turn is connected to a hydrogen source (not
shown). In the system shown in Fig. 5, it will be understood that a flame is
intermittently supplied to burner 102 as needed. In this regard, the system of
Fig. 5 can be automated by the use of solenoid control systems or other logic
control systems to provide hydrogen to the igniter assembly when ignition is
required.
Turning now to Fig. 6, there is shown another embodiment of the present
invention involving a flare stack. As in the case of the embodiment shown in
Fig.
5, the ignition assembly shown generally as 96 is attached to a flame
propagation. tube 112. It will be noted that with respect to the embodiments
shown in Figs. 5 and 6, air to be mixed with the hydrogen is by means of
aspiration of ambient air as shown in Fig. 3 rather than by forced air being
introduced as shown in Fig. 4. However, as seen hereafter, it will be
understood
that a system such as shown in Fig. 4 could be employed in either of the
embodiments of Figs. 5 or 6. Flame propagation tube 112 is attached to a pilot
burner 114. Pilot burner 114 is in turn attached to a tube 115 to which is
connected to an air aspirator 116 into which the end of a flow line 118
terminates
Methane, propane, or some light hydrocarbon gas from a source not shown
flows through flow line 118 into aspirator 116 where it mixes with air and
then
flows through tube 115 to pilot burner 114. In the embodiment shown in Fig. 6,
the methane or other light hydrocarbon gas flowing through line 118 and into
16
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
pilot burner 114 would be ignited by means of igniter assembly 96 and would
normally stay lit such that any combustible materials issuing from opening 92
and flare stack 90 would be ignited. However, it will be understood that pilot
burner 114 could be selectively lit when desired.
Fig. 7 shows yet another embodiment of the invention for use with a flare
stack. The embodiment shown in Fig. 7 difFers from that shown in Fig. 6 in
that
the igniter assembly, shown generally as 120 is substantially the same as
described with respect to the embodiment shown in Fig. 4, i.e., instead of
being
an air aspiration system, the embodiment shown in Fig. 7, like the embodiment
shown in Fig. 4, uses a forced air system wherein air via a line 124 is
supplied
through a flowline126 from a line 128 from a source such as an air compressor
or the like, not shown. Additionally, hydrogen supplied to igniter assembly
120 is
connected to a solenoid valve 130 forming part of an automatic flare pilot
ignition
system which typically incorporate thermocouples, optical flame monitors or
the
like which detect the absence of any flame, i.e., no pilot flame. In such
event
solenoid valve 130 would open such that hydrogen would be supplied to igniter
assembly 120 ultimately resulting, as described above, in the ignition of a
pilot
flame from pilot burner 114 via methane or the like being supplied via line
118.
Figs. 8 and 9 show a simplified hand-held wand employing an igniter
assembly of the present invention. The igniter assembly shown in Fig. 8 is of
a
self aspirating type substantially like that shown in Fig. 1. In this regard,
a wand
140 has disposed therein, a catalyst section carrier 142, catalyst section
144,
being in the form of a hollow cylinder with a central opening 146. A retainer
148
17
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
together with Venturi tube 150 hold catalyst section 144 in place. A hydrogen
feed line 152 running through wand 140 supplies hydrogen which aspirates air
through holes 154 in wand 140, holes 154 being located just upstream of tube
150. A hydrogen supply line 154 connected to a source of hydrogen such as a
tank 156 is connected by a coupling 158 to wand 140.
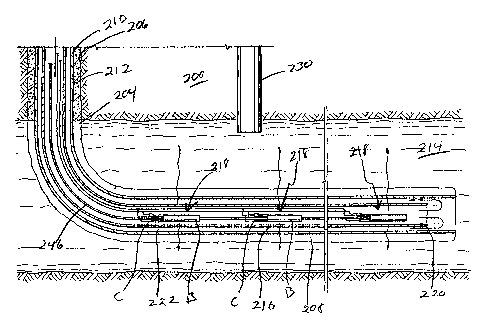
Turning now to Fig. 10, there is shown a typical subterranean usage of the
apparatus of the present invention. An earth formation 200 has drilled therein
a
borehole 204, borehole 204, as seen, having a vertical section 206 which
extends to the surface and a generally horizontal section 208 running
generally
parallel to the surface. Disposed in the borehole 204 is casing 210, casing
210
being cemented via cement 212 in the vertical section 206. As shown, the
horizontal section 208 of borehole 204 forms an annulus around casing 210 but
it will be recognized that it could be filled with substances which aid heat
conductivity or transfer, if desired. In any event, casing 210 extends into a
formation 214 containing hydrocarbonaceous materials such as coal, oil, heavy
oils, tar sands, shale oil or the like. Disposed generally concentrically in
casing
210 is tubing 216 which like casing 210 has a vertical section and a generally
horizontal section. Disposed in tubing 216 are a series of igniter
assembly/burner modules shown generally as 218 and described more fully
hereafter. As can be seen, modules 218 are staggered along the length of
tubing 216. The burners B of modules 218 are ignited by means of the ignition
assemblies C, air being forced down tubing 216 from a compressor or other
source of forced air. The air moving down tubing 216 is heated by combustion
is
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
gases and the flames issuing from the burner of modules 218. The mixture of
hot air and combustion gases exits the end 220 of tubing 216 and passes
upward in the annulus 222 between tubing 216 and casing 210. The heated air
combustion gases moving through the annulus 222 heats casing 210 and
ultimately the air or other material in borehole 204 surrounding casing 210
eventually heating the hydrocarbonaceous-containing formation 214. Depending
upon the temperature which is generated, hydrocarbons in the formation 214 are
vaporized and/or pyrolyzed to smaller more valuable and more easily
recoverable molecules which can then be recovered by a production well shown
generally as 230 which extends down into formation 214.
It will be apparent that a number of casing/tubing combinations such as
shown in Fig. 10 can be run side-by-side, stacked or virtually in as any other
array in formation 214 to achieve desired the degree of heating and/or
pyrolysis.
Further, any number of production wells can be positioned in formation 214 to
recover the vaporized/cracked hydrocarbons from the formation.
Referring now to Fig. 11 there is shown in greater detail the igniter
assembly/burner modules 218. Modules 218 comprise a burner B have a
housing forming a chamber 246A, chamber 246A being also defined by a front
wall 242 in which are positioned a series of holes 244 (see Fig. 12) and a
back
wall 243. Chamber 246A essentially forms an annular plenum inside housing
240. Extending through housing 240 is a main methane supply line 246 which
as seen in Fig. 10 extends through wall 242 and passes through each of the
igniter assembly/burner modules 218. A slip stream of methane is removed from
19
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
supply line 246 via a conduit 248 and introduced via a fitting assemblage 250
into an aspirator housing 280. Aspirator housing 280, as seen, is a tubular
member having a series of holes 282 and is attached to wall 243. Conduit 248
extends into aspirator housing 280 and terminates in a tapered tip or nozzle
284.
As methane flows through aspirator housing 280, air is drawn in from the
interior
of tubing 216 through holes 282 where it mixes with the methane issuing from
nozzle 284, the mixture being forced under the pressure of the methane into an
opening 286 in the back wall 243 of housing 240. In effect, in the embodiment
shown in Fig. 11, the same air that is ultimately used to heat the formation,
i.e.,
the air being pumped into tubing 216, forms the oxidizing gas and is aspirated
through holes 282 in aspirator/nozzle assembly 280. Also extending through
plenum 246A is an igniter assembly C of the forced air type substantially as
shown in Fig. 2. In this regard, a manifold 252 is connected to a main air
supply
line 254 by means of a conduit 256. Air supply line 254, as seen in Fig. 10,
runs
from the surface along the full length of the tubing to feed successive
igniter
assembly/burner modules 218. Manifold 252 is also connected via a conduit 258
to a hydrogen supply line 260 which as in the case of the methane line 246 and
air line 254 extends to the surface and feeds all of the igniter
assembly/burner
modules 218 staggered along the length of tubing 216. In the manner described
above, air and hydrogen are admixed and contact the catalyst segment such as
shown in Fig. 3 to emit a burst of flame at the front surface of wall 242
whereby
the methane/air mixture issuing from ports 244 is ignited.
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
It will be understood with respect to the embodiment shown in Figs. 10, 11
and 12, that once the burners B are lit, the flow of hydrogen and air to the
igniter
assembly C can be discontinued unless and until the burners extinguish and
need to be re-ignited.
In the preferred embodiment, the apparatus of the present invention
utilizes, as can be seen from the above, a tubular housing in which is
disposed
the catalytic material. Preferably the catalytic material is in the form of a
tube
disposed in the tubular housing as seen, for example, in Fig. 1. It will be
understood, however, that the catalytic material need not be in the form of a
tube
as noted above. Thus, the catalyst material could be in the form of pellets,
granules or the like, held in a perforate housing disposed in the tubular
housing.
In the preferred case when a pilot light is to be lit, the geometry of the
igniter
assembly is such that the tubular housing in which the catalytic material is
positioned has a flame front propagation tube attached to the tubular housing
at
one end. The flame front propagation tube can vary in length from about 2
inches up to about 15 feet depending upon the particular application. At the
other end of the tubular housing and when self air aspiration is used, a
Venturi-
type arrangement is preferably employed. In this regard and again as noted in
Fig. 1, there is a Venturi tube at the mouth of which the hydrogen-containing
gas
and the oxidizing gas mix and then flow at a higher velocity through the
tubular
housing containing the catalytic material. The presence of the Venturi tube,
as is
well known to those skilled in the art, increases the velocity of flow of the
hydrogen-containing gas and the oxidizer through the tubular housing and hence
21
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
through the bore through the cylinder of catalytic material. When there is
sufficient hydrogen and oxygen present in contact with the active ingredient
of
the catalytic material, auto ignition of the hydrogen occurs with a flame
front
which moves in both directions along the tubular member, i.e., toward the end
to
which the flame propagation tube is attached and toward the end through which
the Venturi tube is attached. The flame propagation, at high velocity, travels
through the flame propagation tube and essentially acts as a spark at the
source
of a combustible fuel which can be issuing from a pilot burner, a burner, a
flare
stack or the like. However, unless excessive hydrogen is being introduced into
the tubular housing containing the catalytic material, only a small flame of
hydrogen will remain lit at the outlet of the hydrogen feed tube. While in the
embodiments shown, and preferably, the tip of the hydrogen tube nozzle or the
like through which the hydrogen under pressure is flowing is displaced
somewhat
axially from the start of the catalyst section. However, it is to be
understood that
the tip of the hydrogen tube, nozzle, etc., could be positioned so as to be in
the
hollow core in the catalyst section. It is clearly preferably that the
hydrogen-
containing gas be introduced into the tubular housing in the form of a stream
as
opposed to simply being diffused into the tubular housing. It is believed that
by
introducing a stream of the hydrogen-containing gas into the tubular housing
containing the catalyst section, there results in a more uniform self ignition
of the
hydrogen in the catalyst section thereby providing better flame propagation
and
enhancing the "spark-like" igniting capability of the igniter assembly used in
the
present invention.
22
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
The ignition system of the present invention provides a virtually foolproof
method to ignite a combustible gas or other combustible mixture in a remote
environment such as in a tubular member disposed in an earth borehole or in
connection with a flare stack.
It is anticipated that the unique igniter assembly of the present invention
can be designed for multiple burners, e.g., 15 to 40, which can be spaced
along
a length of tubing disposed in a borehole. It is also anticipated that the
burner
output from each burner will be 50,000 to 125,000 BTU/hr. The igniter assembly
of the present invention will be capable of handling inlet combustion
temperatures from ambient to 500°F while withstanding downstream
combustion
air temperatures of up to 1,500°F. The igniter assembly of the present
invention
can be used in hot start-up conditions, as well as under conditions where
water
is present, i.e., wet conditions. As described above, the igniter
assembly/burner
module will fit inside a 3" I.D. burner tube. It is anticipated that the
service life of
the igniter assemblies will be 3-5 years with little to no maintenance.
The marked novelty of the igniter system of the present invention is best
demonstrated by the fact that under the parameters set out above, e.g., having
to dispose the burners in a 3" I.D. tube, in downhole conditions at elevated
temperatures, in the presence of moisture and with a repetitive and long
lifetime,
it accomplishes what electric igniters or any other heretofore known ignition
systems cannot accomplish. It overcomes the difficulty of having to protect
ignition wires in a hard wired system using an electric igniter from prolonged
exposure to a temperature of 1,500°F, a temperature that would almost
certainly
23
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
damage such electrical systems over time. It permits the use of multiple
burner
systems, e.g., 15 to 40 burner systems, each with an igniter assembly, and
such
a system would be virtually impossible using electrical igniters because the
small
I.D. of at least some of the burner tubes in which the igniter system will be
placed
would not permit the use of 15 to 40 ignition wires that would be required in
such
a multiple burner system. Furthermore, in any electric spark ignition system
it is
almost certain that delicate ceramics or the like would be used and such
materials are frangible, easily damaged and would require excessive
maintenance. Additionally, in an extended length of burner tubing, e.g., 5,000
ft.,
the voltage drop to the igniters would be excessive requiring repeaters, again
complicating the system and making it virtually impossible to fit it into a
small
diameter (3" I.D.).
As can be seen from the above, the igniter assembly used in the
apparatus of the present invention generally falls into two categories. In one
case, the combustion air required for auto ignition of the hydrogen-containing
gas is supplied to the igniter assembly from the surrounding ambient air by
introducing hydrogen into a Venturi at a sufficient velocity. In this case,
the
Venturi effect causes the required amount of oxidizer gas to be drawn into the
Venturi tube where it is mixed with the hydrogen-containing gas and then
introduced to the catalyst section.
The second basic type of igniter assembly is of the forced air type wherein
the oxidizer required for auto ignition of the hydrogen-containing gas
supplies to
the igniter assembly by an air compressor or any other controlled air source,
e.g.,
24
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
pressurized bottled air. In this regard, when forced air is employed, a flow
meter,
regulator, orifice plate or any other means of accurately controlling airflow
can
provide the desired specific amounts of combustion air into the igniter
assembly.
This type of igniter assembly is particularly suited for applications where
purity of
the ambient or surrounding outside air is in question.
Regardless of whether the igniter assembly is of the self aspirating or
forced air type, as described above, in all cases the hydrogen is introduced
into
the tubular housing which holds the catalyst under a pressure to ensure flow
of
the hydrogen-containing gas/oxidizer through the tubular housing and into
contact with the catalyst contained therein. Generally speaking, the hydrogen
pressure will range from about 0.1 to about 3 psi with flow rates from about
50 to
about 1400 cc/sec.
As can be seen from the above, the combination of the igniter assembly,
the burner, and the tubular member in which it is disposed and which can be
positioned downhole provides the basis for a method of vaporizing and/or
pyrolyzing hydrocarbons and subterranean formations such that they can be
recovered more easily from producing wells. It is also to be understood that
the
method can include simply heating the formation to make the oil less viscous
whereby it can be pumped more efficiently, i.e., without the necessity for
vaporizing and/or pyrolyzing any of the oil. The igniter assembly in
combination
with the flare stack provides an easy and efficient method of igniting
combustible
mixtures issuing from a flare stack and eliminates the need for spark
igniters,
continual pilot flames, etc.
2s
CA 02541169 2006-03-31
WO 2005/038347 PCT/US2004/033140
Modifications of the apparatus, compositions, procedures and conditions
disclosed herein that will still embody the concept of the improvements
described
should readily suggest themselves to those skilled in the art, and are
intended to
be encompassed within the spirit of the invention presently disclosed herein
as
well as the scope of the appended claims.
26