Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02543003 2006-04-19
WO 2005/044803 PCT/EP2004/011244
BENGAMIDE DERIVATIVES, METHOD FOR THE PRODUCTION
THEREOF AND USE THEREOF FOR THE TREATMENT OF CANCER
Cancer is a disease of humans and animals which is for the most part fatal
and which is caused by the uncontrolled growth of endogenous cells.
Cancer is the term for the formation of malignant tumors (malignomas) and
of neoplasms (tumors or carcinomas) or for the malignant degeneration
and disturbed maturation of white blood cells (leukemia, blood cancer).
Cancer cells or tumor cells arise as the result of the transformation of
endogenous cells. The malignancy of the cancer cell is expressed in the
autonomy of its growth, that is in the ability of the cell to grow in an
uninhibited manner and without being fitted into the structure of the organs
and also to grow in an infiltrating manner, thereby destroying tissue. The
formation of disseminations (metastases) at a distance from the tumor,
after tumor cells have been spread by way of the blood or the lymph, is a
sure sign of malignancy. Cancer is one of the most frequent causes of
death in humans and there is therefore a great need for methods and
means for curing or treating malignant degenerations.
Aside from the, if possible radical, surgical removal of the tumor, the
possibilities for treating malignant tumors include radiological therapy using
X-rays, a-rays, ~-rays and y-rays, immunotherapy and chemotherapy. At
present, immunotherapy can only be used to a limited extent. The chemo-
therapy of tumors is understood as meaning the administration of cell
poisons (cytostatic agents) for treating tumors and tumor cells which
remain, usually following local surgical treatment or irradiation. These
substances interfere specifically in certain processes in cell division, which
means that tissues containing a high proportion of dividing cells, such as
rapidly growing tumor tissues, react more sensitively. The cytostatic agents
which are used are alkylating compounds, such as cyclophosphamide,
antimetabolites, such as methotrexate, alkaloids, such as vincristine, and
antibiotics, such as daunomycin or adriamycin. However, due to massive
side-effects, all these agents suffer from severe disadvantages, such that
the death of the affected patient is only delayed and not averted.
Furthermore, the degenerate (cancer) cells develop resistances to the
agents which are used; while the medicaments which are being used at the
time then no longer have any cytostatic effect, they are still toxic as a
CA 02543003 2006-04-19
2
consequence of the side-effects. In addition, it has been found that the
efficacy achieved by using cytostatic agents in combination or in sequence
exceeds that achieved by using a single cytostatic agent (monotherapy)
and, as a result, it is possible that the substantial side-effects are not
additive in connection with polychemotherapy. For all these reasons, novel
chemotherapeutic agents are urgently required and are therefore being
sought world-wide.
The first examples of bengamides were bengamides A and B, which are
dodecanoyl-substituted on the caprolactam ring and which were isolated
from the sea sponge Jaspis cf. Coriacea (family Coppatiidae, order
Choristida B Astrophorida) (Adamczewski et al., J. Org. Chem. 1986, 51,
4497-4498) and reported to be biotoxic to eukaryotic cells, nematodes and
bacteria.
Bengamide E
H
0
N O OH OH
N
H
OMe OH
and its N-methylated derivative bengamide F are examples of bengamide
derivatives which have been demonstrated to possess antitumor activity.
Bengamide E inhibits cell proliferation by stepping cell division at the G1/S
restriction point and in the G2/M phase of the cell cycle. Bengamide B
derivatives inhibit the proliferation of MDA-MB-435 breast cancer cells
(Kinder et al., J. Med. Chem. 2001, 44, 3692-3699).
A feature shared in common by the known bengamide derivatives is that
they have been isolated from sea sponges of the genus Jaspis sp. or
Pachastrissa sp. (Thale et al., J. Org. Chem. 2001, 66, 1733-1741).
It has now been found that the microorganism strain Myxococcus virescens
ST200611 (DSM 15898) is able to form novel bengamide derivatives which
inhibit cell proliferation at low concentrations and are consequently suitable
to be used for the treatment andlor prophylaxis of cancer diseases.
CA 02543003 2006-04-19
3
The present invention relates to a compound of the formula (I),
R~
I O
O OR3 OR3
R N /
2
OMe OR3
(I)
wherein
R~ is H or (C~-C6)-alkyl,
R2 is H or OH, and
R3 is H or -C(=O)-(C~-Cg)-alkyl,
or to a physiologically tolerated salt of a compound of the formula (I).
Independently of each other, R~ is preferably H or methyl and R3 is
preferably H.
The invention preferably relates to a compound of the formula (I) in which
R~ is H or methyl,
R2 is H or OH, and
R3 is H.
(C~-C6)-Alkyl is a straight-chain or branched alkyl group having 6 carbon
atoms, for example methyl (Me), ethyl, n-propyl, iso-propyl, tent-butyl or
n-hexyl, preferably methyl.
In addition, the invention relates to a compound of the formula (I) which is
characterized by a compound of the formula (II)
H
I 0
O OH OH
N
H
OMe OH (ll)
a compound of the formula (III)
CA 02543003 2006-04-19
4
Me
I o
O OH OH
H
OMe OH (lll),
and a compound of the formula (IV)
H
O
N O OH OH
HO
H
OMe OH (lV).
The present invention furthermore relates to all obvious chemical
equivalents of the compounds of the formula (I) according to the invention.
These equivalents are compounds which exhibit only a slight chemical
difference, and have the same pharmacological effect, or which are
converted into the compounds according to the invention under mild
conditions. Said equivalents also include, for example, salts, reduction
products, oxidation products, esters, ethers, acetals or amides of the
compounds of the formula (I) as well as equivalents which the skilled
person can prepare using standard methods and, in addition to this, all the
optical antipodes and diastereomers and all the stereoisomeric forms.
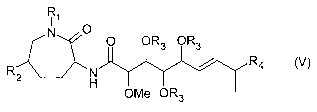
The invention also relates to a process for preparing a compound of the
formula (V)
R~
I O
N O OR3 OR3
Rz N / R4
H OMe OR3
(V),
wherein
R~ is H or (C~-C6)-alkyl,
R2 is H or OH,
CA 02543003 2006-04-19
R3 is H or -C(=O)-(C~-Cg)-alkyl, and
R4 is methyl or ethyl,
or to a physiologically tolerated salt of a compound of the formula (~,
5 which comprises
1. the strain Myxococcus virescens ST200611 (DSM 15898), or one of
its variants and/or mutants, being fermented under suitable
conditions in a culture medium until one or more of the compounds
of the formula (~ accrue(s) in the culture medium,
2. a compound of the formula (~ being isolated from the culture
medium, and
3. the compound of the formula (~ being derivatized, where
appropriate, andlor, where appropriate, being converted into a
physiologically tolerated salt.
The invention preferably relates to a process for preparing a compound of
the formula (~ where Rq. is ethyl. The product of such a process
corresponds to a compound of the formula (I) as described above.
The invention particularly preferably relates to a process for preparing a
compound of the formula (~ where, independently of each other, R~ is H
or methyl, R3 is H and R4 is ethyl.
In addition, the invention relates to a process for preparing a compound of
the formula (II), a compound of the formula (III) and a compound of the
formula (11~ as well as the bengamide derivatives E and F.
Unless otherwise indicated, the chiral centers in the compounds of the
formula (I) and (~ can be present in the R configuration or in the S
configuration. The invention relates both to the optically pure compounds
and to stereoisomeric mixtures, such as enantiomeric mixtures and
diastereomeric mixtures.
Physiologically tolerated salts of compounds of the formula (I) and (~ are
understood as being both their organic salts and their inorganic salts, as
are described in Remington's Pharmaceutical Sciences (17th edition, page
1418 (1985)). Because of their physical and chemical stability and their
solubility, sodium, potassium, calcium and ammonium salts are preferred,
inter alia, for acid groups; salts of hydrochloric acid, sulfuric acid or
CA 02543003 2006-04-19
6
phosphoric acid, or of carboxylic acids or sulfonic acids, such as acetic
acid, citric acid, benzoic acid, malefic acid, fumaric acid, tartaric acid and
p-toluenesulfonic acid, are preferred, inter alia, for basic groups.
The culture medium is a nutrient solution or a solid medium containing at
least one customary carbon source and nitrogen source as well as the
customary inorganic salts. If hydroxylysine is added to the culture medium,
the strain Myxococcus virescens ST200611 (DSM 15898) produces a
compound of the formula (~ in which R2 is OH as a result of digesting
hydroxylysine.
One part of the subject matter of the present invention is therefore a
process for preparing a compound of the formula (~ as described above
where R2 is OH and where the culture medium in step 1 contains hydroxy-
lysine.
The process according to the invention can be used for fermenting on a
laboratory scale (milliliter to liter scale) and for fermenting on an
industrial
scale (cubic meter scale).
Suitable carbon sources for the fermentation are assimilable carbohydrates
and sugar alcohols, such as glucose, lactose, sucrose or D-mannitol, as
well as carbohydrate-containing natural products, such as malt extract or
yeast extract. Examples of nitrogen-containing nutrients are amino acids;
peptides and proteins and also their breakdown products, for example
casein, peptones or tryptones; meat extracts; yeast extracts; gluten; ground
seeds, for example from corn, wheat, beans, soya or the cotton plant;
distillation residues from producing alcohol; meat meals; yeast extracts;
ammonium salts; nitrates. Preference is given to the nitrogen source being
one or more peptides) which has/have been obtained synthetically or
biosynthetically. Examples of inorganic salts are chlorides, carbonates,
sulfates or phosphates of the alkali metals, the alkaline earth metals, iron,
zinc, cobalt and manganese. Examples of trace elements are cobalt and
manganese.
Conditions which are suitable for forming the bengamides according to the
invention are as follows: the bengamides according to the invention are
preferably formed in a culture medium which contains from 0.05 to 5%,
preferably from 0.1 to 2.5%, yeast extract; from 0.2 to 5.0%, preferably
CA 02543003 2006-04-19
7
from 0.1 to 2%, casitone; from 0.02 to 1.0%, preferably from 0.05 to 0.5%,
CaCl2 x 2 H20; from 0.02 to 1.5%, preferably from 0.05 to 0.7%, MgS04 x
7 H20 and from 0.00001 % to 0.001 % cyanocobalamin. The percentage
values which are given are in each case based on the weight of the total
nutrient solution.
The microorganism is cultured aerobically, that is, for example, submerged
while being shaken or stirred in shaking flasks or fermenters, or on solid
medium, where appropriate while air or oxygen is being passed in. The
microorganism can be cultured in a temperature range of from about 18 to
35°C, preferably at from about 20 to 32°C, in particular at from
27 to 30°C.
The pH range should be between 4 and 10, preferably between 6.5 and
7.5. The microorganism is generally cultured under these conditions for a
period of from 2 to 10 days, preferably of from 72 to 168 hours. The micro-
organism is advantageously cultured in several steps, i.e. one or more
preliminary cultures are initially prepared in a liquid nutrient medium, with
these preliminary cultures then being inoculated into the actual production
medium, i.e. the main culture, for example in a ratio by volume of from 1:10
to 1:100. The preliminary culture is obtained, for example, by inoculating
the strain, in the form of vegetative cells or fruiting bodies, into a
nutrient
solution and allowing it to grow for from about 20 to 120 hours, preferably
for from 48 to 96 hours. Vegetative cells and/or fruiting bodies can be
obtained, for example, by allowing the strain to grow for from about 1 to
15 days, preferably for from 4 to 10 days, on a solid or liquid nutrient
substrate, for example yeast agar.
A bengamide derivative of the formula (V) is isolated or purified from the
culture medium using known methods and taking account of the chemical,
physical and biological properties of the natural substances. HPLC was
used to test the concentrations of the respective bengamide derivatives in
the culture medium or in the individual isolation steps, with the quantity of
the substance formed expediently being compared with a calibration
solution.
For the isolation, the culture broth or the culture together with the solid
medium is lyophilized, after which the bengamide derivatives are extracted
from the lyophilizate using an organic solvent, for example methanol or
2-propanol. The organic solvent phase contains the natural substances
CA 02543003 2006-04-19
8
according to the invention; it is concentrated, where appropriate, in vacuo
and subjected to further purification.
The further purification of one or more compounds according to the
invention is effected by chromatography on suitable materials, preferably,
for example, on molecular sieves, on silica gel, on aluminum oxide, on ion
exchangers or on adsorber resins or on reversed phases (RPs). This
chromatography is used to separate the bengamide derivatives. The
bengamide derivatives are chromatographed using buffered aqueous
solutions or mixtures of aqueous and organic solutions.
Mixtures of aqueous or organic solutions are understood as being all water-
miscible organic solvents, preferably methanol, 2-propanol or acetonitrile,
at a concentration of from 5 to 95% solvent, preferably from 5 to 40%
solvent, or else all buffered aqueous solutions which are miscible with
organic solvents. The buffers which are to be used are the same as
specified above.
The bengamide derivatives are separated, on the basis of their differing
polarities, by means of reversed phase chromatography, for example on
MCI~ (adsorber resin from Mitsubishi, Japan) or Amberlite XAD~
(TOSOHAAS), or on other hydrophobic materials, for example on RP-8 or
RP-18 phases. In addition, the separation can be effected by means of
normal-phase chromatography, for example on silica gel, aluminum oxide
and the like.
The bengamide derivatives are chromatographed using buffered, basic or
acidified aqueous solutions or mixtures of aqueous solutions with alcohols
or other water-miscible organic solvents. Preference is given to using
acetonitrile and methanol as organic solvent.
Buffered, basic or acidified aqueous solutions are understood as being, for
example, water, phosphate buffer, ammonium acetate and citrate buffer at
a concentration of up to 0.5 M, as well as formic acid, acetic acid, trifluoro-
acetic acid, ammonia and triethylamine, or all commercially available acids
and bases known to the skilled person, preferably at a concentration of up
to 1 %. In the case of buffered aqueous solutions, particular preference is
given to 0.1 % ammonium acetate.
CA 02543003 2006-04-19
9
The chromatography was carried out using a gradient which began with
100% water and ended with 100% solvent; the chromatography was
preferably run with a linear gradient of from 5 to 95% acetonitrile.
Alternatively, it is also possible to carry out a gel chromatography or
chromatography on hydrophobic phases. The gel chromatography is
carried out on polyacrylamide gels or copolymer gels, such as Biogel-P 2~
(Biorad) or Fractogel TSK HW 40~ (Merck, Germany). The sequence of
the abovementioned chromatographic steps can be reversed.
Insofar as bengamides are present as diastereomers, they can be
separated using known methods, for example by means of separation
using a chiral column.
The derivatization of the OH groups in the side chain of the compounds of
the formulae (I) and/or (~ (R3 is in each case H) to give an acyl group (R4
is in each case -C(=O)-(C~-Cg)-alkyl) is effected using methods which are
known per se (J. March, Advanced Organic Chemistry, John Wiley & Sons,
4th edition, 1992), for example by means of reaction with an acid
anhydride. For example, Adamczeski et al., J. Am. Chem. Soc. 1989, 111,
647-654 describe the reaction with acetic anhydride to give a compound of
the formula (I) and/or (~ in which R3 is -C(=O)-CH3.
The alkylation of the NH group in the caprolactam ring of a compound of
formula (I) or (~ (R~ is in each case H) is likewise effected using methods
which are known per se (J. March, Advanced Organic Chemistry, John
Wiley & Sons, 4th Edition, 1992), for example by reaction with Me2C03 or
Me2S04, to prepare the corresponding N-methylated derivatives, or by
reaction with (C~-Cg)-alkyl bromide in the presence of a base.
An isolate of the microorganism strain Myxococcus virescens ST200611
was deposited in the Deutschen Sammlung von Mikroorganismen and
Zellkulturen [German Collection of Microorganisms and Cell Cultures]
GmbH (DSMZ), Mascheroder Weg 1 B, 38124 Brunswick, Germany, in
accordance with the rules of the Budapest treaty, on 11.09.2003 under the
following number: DSM 15898.
CA 02543003 2006-04-19
The vegetative cells of the strain DSM 15898 have the rod form which is
characteristic for Myxococcus virescens. On solid nutrient substrates,
Myxococcus virescens ST200611 (DSM 15898) farms orange-yellow
fruiting bodies which contain round myxospores.
5
Instead of the strain Myxococcus virescens ST200611 (DSM 15898), it is
also possible to use its mutants andlor variants which synthesize one or
more of the compounds according to the invention.
10 A mutant is a microorganism in which one or more genes in the genome
has/have been modified, with the gene, or the genes, which is/are
responsible for the ability of the organism to produce the compound
according to the invention remaining functional and heritable.
Such mutants can be produced, in a manner known per se, using physical
means, for example irradiation, as with ultraviolet rays or X-rays, or
chemical mutagens, such as ethyl methanesulfonate (EMS); 2-hydroxy-
4-methoxybenzophenone (MOB) or N-methyl-N'-nitro-N-nitrosoguanidine
(MNNG), or as described by Brock et al. in "Biology of Microorganisms",
Prentice Hall, pages 238-247 (1984).
A variant is a phenotype of the microorganism. Microorganisms have the
ability to adapt to their environment and therefore exhibit highly developed
physiological flexibility. All the cells of the microorganism are involved in
the
phenotypic adaptation, with the nature of the change not being genetically
conditioned and being reversible under altered conditions (H. Stolp,
Microbial ecology: organism, habitats, activities. Cambridge University
Press, Cambridge, GB, page 180, 1988).
Screening for mutants and/or variants which synthesize one or more of the
compounds according to the invention takes place in accordance with the
following scheme:
- lyophilizing the fermentation medium;
- extracting the lyophilizate with an organic solvent
- extracting the compound from the culture filtrate using solid phases
- analyzing by means of HPLC or TLC or by testing the biological
activity.
CA 02543003 2006-04-19
11
The fermentation conditions which have been described apply for Myxo-
coccus virescens ST200611 (DSM 15898) and for mutants and/or variants
thereof.
The present invention also relates to the use of the microorganism
Myxococcus virescens ST200611 (DSM 15898), or of a mutant and/or
variant, for preparing a compound of the formula (V), in particular a
compound of the formula (IV), or a physiologically tolerated salt thereof, as
described above.
A test which is based on determining the intracellular concentration of ATP
is employed for detecting the inhibition of cell proliferation. It is possible
to
use known tumor cell lines such as Hep-G2 and Co1o205. In this test, the
ATP content of metabolically active cells serves, in a luciferase reaction, as
a measure of the number of living cells.
The compounds of the formula (II)-(VI) were used in the test in a single
dose of 0.3 - 40 p.M and a dose dependency given as a TC50 value, with
(IIA) and (IIB) in each case denoting a diastereomer of the compound of
the formula (II):
Table 1: Activity
of the bengamides
in a cell proliferation
test,
expressed as
TC50 value in
pM
Compound Hep-G2 Colo 205
(II) ~ 16 27
(IIA) 17 33
(IIB) 6 10
(III) 9 15
(IV) >40 42
Bengamide E 36 46
Bengamide F 27 33
The invention therefore also relates to the use of the compound of the
formula (I) or of a physiologically tolerated salt thereof as a pharmaceutical
in human or animal medicine, in particular for the treatment and/or
CA 02543003 2006-04-19
12
prophylaxis of cancer diseases. The invention preferably relates to the use
of a compound of the formula (I), or of a physiologically tolerated salt, for
treating breast cancer, intestinal cancer, stomach cancer, liver cancer,
brain tumors, ovarial tumors, esophageal cancer, renal cancer and muscle
cell carcinoma, in particular carcinoma of the head and neck muscles.
In addition, the present invention relates to a pharmaceutical having a
content of at least one compound of the formula (I) or of a physiologically
tolerated salt thereof, with it being possible for the compound or the
compounds of the formula (I) to be administered as such or, preferably, to
be present in a mixture with one or more of the customary, pharma-
cologically suitable carrier substances or auxiliary substances.
The compounds according to the invention are stable in the solid state and
in solutions in a pH range of between 2 and 9, in particular 5 and 7, and, as
a consequence, can be incorporated into customary galenic preparations.
While the pharmaceuticals according to the invention can be administered
orally or parenterally, a rectal use is also possible in principle. Examples
of
suitable solid or liquid galenic preparation forms are granules, powders,
tablets, sugar-coated tablets, (micro)capsules, suppositories, syrups,
emulsions, suspensions, aerosols, drops or injectable solutions in ampoule
form, as well as preparations giving a protracted release of active
compound, in connection with whose preparation use is customarily made
of pharmacologically suitable carrier substances or auxiliary substances,
such as disintegrants, binders, coating agents, swelling agents, glidants,
lubricants, flavoring substances, sweeteners or solubilizers, for example
magnesium carbonate, titanium dioxide, lactose, mannitol and other
sugars, talc, milk protein, gelatin, starch, vitamins, cellulose and its
derivatives, animal or vegetable oils, polyethylene glycols and solvents,
such as sterile water, alcohols, glycerol and polyhydric alcohols.
Where appropriate, the dosage units for oral administration can be micro-
encapsulated in order to delay release or to extend it over a relatively long
period, for example by means of coating or embedding the active
compound in particle form in suitable polymers, waxes or the like.
Preference is given to producing and administering the pharmaceutical
preparations in dosage units, with each unit containing, as the active
CA 02543003 2006-04-19
13
constituent, a defined dose of one or more compounds of the bengamide
derivatives of the formula (I). In the case of solid dosage units such as
tablets, capsules and suppositories, this dose can be up to about 500 mg,
preferably, however, from about 0.1 to 200 mg, and, in the case of injection
solutions in ampoule form, up to about 200 mg, preferably, however, from
about 0.5 to 100 mg, per day.
The daily dose which is to be administered depends on the bodyweight,
age, sex and condition of the mammalian subject. However, higher or lower
daily doses may also possibly be appropriate. The daily dose can be
administered both by means of once-only administration in the form of a
single dosage unit, or else in several smaller dosage units, and by means
of the multiple administration of subdivided doses at defined intervals.
The pharmaceuticals according to the invention are produced by bringing
one or more of the compounds of the formula (I) according to the invention
into a suitable form for administration, optionally together with one or more
of the customary carrier substances or auxiliary substances.
The following examples are intended to explain the invention in more detail
without limiting its scope in any way.
Unless otherwise indicated, percentage values refer to the weight and
mixing ratios in the case of liquids refer to the volume.
Example 1: Storing M.yxococ~us viresesns ST200611 (DSM 15898) at
-135°C
An agar plate (1 % fresh baker's yeast, 1 % CaCl2 x 2 H20, 20 mM HEPES,
0.00005°l° cyanocobalamin, 1.5°l° agar, pH 7.2) is
inoculated with the strain
Myxococcus virescens ST200611 (DSM 15898) and incubated at 30°C
far
approx. 7 days. The cells in this surface culture are scratched off the agar
surface using a sterile spatula, resuspended in 0.5 ml of casitone medium
(1 % casitone (Difco), 0.15°!o MgSOq. x 7 H20, pH 7.0) and stored at
-135°C.
CA 02543003 2006-04-19
14
Example 2: Preparing a preliminary culture of Myxococcus virescens
ST200611 (DSM 15898) in an Erlenmeyer flask
100 ml of nutrient solution (1 % fresh baker's yeast, 1 % CaCl2 x 2 H20,
20 mM HEPES, 0.00005% cyanocobalamin, pH 7.2) in a sterile 300 ml
Erlenmeyer flask are inoculated with the strain Myxococcus virescens
ST200611 (DSM 15898) and the culture is incubated for 4 days at
30°C
and 180 rpm on a rotating shaker. 5 ml of this preliminary culture are then
used for preparing the main cultures.
Example 3: Preparing a liquid main culture of Myxococcus virescens
ST200611 (DSM 15898)
A sterile 300 ml Erlenmeyer flask containing 100 ml of the following nutrient
solution (0.5% yeast extract, 0.5% casitone, 0.1 % CaCl2 x 2 H20, 0.2%
MgS04 X 7 H20, 0.00005% cyanocobalamin, pH 7.4) is inoculated with
5 ml of a preliminary culture (see example 2), or a culture which has grown
on a fresh agar plate (1 % fresh baker's yeast, 1 % CaCl2 x 2 H20, 20 mM
HEPES, 0.00005% cyanocobalamin, pH 7.2, plus 1.5% agar), and
incubated at 30°C and 180 rpm on a shaker. The maximum production of
the bengamides according to the invention is reached after 72-96 hours. A
72-96 hour-old submerged culture (inoculation quantity approx. 5-10%)
from the same nutrient solution as described in example 2 is sufficient for
inoculating from 10 to 200 I fermenters.
Example 4: Preparing a liquid main culture of Myxococcus virescens
ST200611 (DSM 15898) while feeding in hydroxylysine for producing the
bengamide derivative (I~
A sterile 300 ml Erlenmeyer flask containing 100 ml of the following nutrient
solution (0.5% yeast extract, 0.5% casitone, 0.1 % CaCl2 x 2 H20, 0.2%
MgS04 x 7 H20, 0.00005% cyanocobalamin and also 1 mM hydroxylysine
pH 7.4) is inoculated with 5 ml of a preliminary culture from example 2 or a
culture which has grown on a fresh agar plate (1 % fresh baker's yeast, 1
CaCl2 x 2 H20, 20 mM HEPES, 0.00005% cyanocobalamin, pH 7.2, plus
1.5% agar) and incubated at 30°C and 180 rpm on a shaker. The maximum
production of the bengamide derivative (I~ is reached after 72-96 hours.
HPLC-MS was used for the analysis. A 72-96 hour-old submerged culture
CA 02543003 2006-04-19
(inoculation quantity, approx. 5-10%) from the same nutrient solution as
described in example 2 is sufficient for inoculating 10 to 200 I fermenters.
Example 5: Preparing bengamide derivatives in a fermenter
5
The 10 I and 30 I fermenters were operated under the following conditions:
Inoculum: approx. 5% approx. 9%
Fermenter: 30 I 10 I
10 Nutrient medium: see example 2 see example 2
Incubation temperature:30C 30C
Stirrer speed: 112 rpm 150 rpm
Aeration: 8 I/min 4 Ilmin
pH regulation: from pH 7.8 to pH from pH 8.1 to
7.5 pH 7.5
15 p02 regulation: none none
The pH was always regulated using 10% KOH or, respectively, 10%
H2S04. Foam formation can be suppressed by repeatedly adding Clerol
FBA 265 (Cognis Deutschland GmbH). Maximum production is reached
after approx. 72 to 96 hours.
Example 6: Isolating bengamide derivatives (II) and (III), as well as
bengamides E and F, from the shaken cultures of Myxococcus virescens
ST200611 (DSM 15898)
After the Myxococcus ~irescen~ ST200611 (DSM 15898) fermentation had
come to an end, the culture broth from example 3 (30 I culture broth),
together with the biomass, was lyophilized and the lyophilizate was
extracted with methanol (2 x 5 I). The methanol extract was reduced to 1.2 I
under vacuum and then loaded onto a prepared column which was packed
with approx. 1.5 liters of CHP-20P material (MCI~ gel, 75-150 p, Mitsubishi
Chemical Corporation). The column was eluted with 95% methanol. The
column flowthrough (120 ml/min) was collected and reduced down to a
volume of 1.5 I in vacuo.
CA 02543003 2006-04-19
16
Example 7: Preseparating bengamide derivatives (II) and (III), and also
bengamides E and F, by means of RP-18 chromatography
1.5 I of the solution obtained as described in example 6 were loaded onto a
Phenomenex Lunar 10 ~, C18 (2) column (size: 50 mm X 250 mm)
possessing a Lunar 10 p C18 (2) precolumn (dimension: 21.2 mm X
60 mm) and eluted (0.1 % ammonium acetate, pH 4.6, adjusted with acetic
acid) over 60 min using a gradient of from 5% to 95% acetonitrile in water.
The flow rate was 150 ml/min and the fraction size was 200 ml.
Bengamides were present in fractions 5-9, 10-11 and 12-14.
Example 8: Purifying bengamide derivatives (II) and (III) and also
bengamides E and F
The individual fractions from example 7 were lyophilized and purified once
more by means of HPLC on a Phenomenex Luna~ 10 ~m C18 (2) column
(dimension: 21 mm X 250 mm) possessing an XTerra~ Prep MS C18
10 ~m (Waters, dimension: 19 X 10 mm) precolumn. The column was
eluted using a gradient of from 5% to 40% acetonitrile in water over 40 min
(in the added presence of 0.1 % ammonium acetate, pH 8.8, adjusted with
triethylamine). The column flowthrough (50 ml/min) was collected in
fractions (7.5 ml fractions in each case). Fractions 5-9 from example 7
contained the compound of the formula (III) and, after chromatography and
lyophilization, yielded 86 mg of bengamide E (purity > 95%). After chroma-
tography, fractions 10-11 from example 7 yielded 145 mg of bengamide (II)
(purity > 95%, 70/30 diastereomeric mixture) and 5 mg of bengamide F
(purity > 95%). 35 mg of bengamide (III) (purity > 95%) were obtained as a
diastereomeric mixture, in a 75:25 ratio, from fractions 12-14 from
example 7. The diastereomers are in each case the corresponding C-16
epimers.
Example 9: Isolating the hydroxybengamide (I~ from the shaken cultures
of Myxococcus virescens ST200611 (DSM 15898)
After the Myxococcus virescens ST200611 (DSM 15898) fermentation had
come to an end, the culture broth from example 5 (10 I of culture broth),
together with the biomass, was lyophilized and the lyophilizate was
extracted with methanol (2 X 3 I). The methanol extract was reduced under
CA 02543003 2006-04-19
17
vacuum to 400 ml and then loaded onto a prepared column which had
been packed with 0.6 liter of CHP-20P (MCI~ gel, 75-150 ~, Mitsubishi
Chemical Corporation) material. The column was eluted with 5% to 95%
methanol in water. The column tlowthrough (100 ml/min) was collected in
fractions over 60 min (0.5 min per fraction). The fractions containing the
desired derivative (fractions 45-109) were pooled and reduced in vacuo
down to a volume of 700 ml.
Example 10: Prepurifying the hydroxybengamide (I~ by means of RP-18
chromatography
The solution from example 9 was then loaded onto a Phenomenex Luna~
10 ~ C18 (2) column (size: 50 mm X 250 mm) possessing a Phenomenex
Luna~ 10 ~ C18 (2) precolumn (dimension: 21.2 mm X 60 mm) and eluted
(0.1 % ammonium acetate, pH 8.8, adjusted with triethylamine) using a
gradient of from 5% to 40% acetonitrile in water over 60 min. The flow rate
was 150 ml/min and the fraction size was 225 ml. Fraction 22 contained the
desired bengamide derivative.
Example 11: Purifying the hydroxybengamide (I~
Fraction 22 from example 10 was lyophilized and purified once again by
means of HPLC on a Phenomenex Luna~ 10 ~m C18 (2) column
(dimension: 21 mm x 250 mm) possessing a Waters XTerra~ Prep MS C18
10 ~.m precolumn (dimension: 19 x 10 mm). The column was eluted with a
gradient of from 5% to 95% acetonitrile in water over 60 min (in the added
presence of 0.1 % ammonium acetate, pH 4.6, adjusted with acetic acid).
The column flowthrough (50 ml/min) was collected in fractions (7.5 ml
fractions in each case). The bengamide-containing fractions (fractions 26-
28) were combined, desalted and freeze-dried. In connection with this,
7 mg of bengamide (I~ were obtained as a diastereomeric mixture in a
ratio of 75:25.
Example 12: Characterizing the compound of the formula (II)
Empirical formula: C~gH32N20g
Molecular weight: 372.47
Diastereomeric mixture: 75:25
CA 02543003 2006-04-19
18
H
1 O
0 OH OH
2 7 8 10 12 1 j 16 18
11 ~ 13 v 15 ~ 17 \
6 OMe OH
(II}
Table 2: NMR-chemical
shifts of bengamide
(II), diastereomeric
mixture, c = 3
mg/ml in DMSO,
300 K.
Position ~ H ~ 3C
1 - 173.99
2 7.91 -
3 3.19/3.06 40.56
4 1.74/1.20 28.75
1.87/1.64 27.55
6 1.87/1.36 30.72
7 4.39 51.27
8 7.78 -
9 - 169.61
3.69 81.60
10-OMe 3.25 57.32
11 ~ 3.58 70.72
11-O H 4.46 -
12 3.33 72.80
12-O H 4. 36 -
13 3.97 72.46
13-O H 4. 56 -
14 5.37 129.05
5.48 136.57
16 1.99 37.41
16-Me 0.93 19.90
17 1.26 29.15
19
18 0.81 11.58
CA 02543003 2006-04-19
Example 13: Characterizing the compound of the formula (III)
Empirical formula: C~gH34N206
Molecular weight: 386.49
Diastereomeric mixture: 75/25
Table 3: NMR-chemical
shifts of bengamide
(III), diastereomeric
mixture, c = 3
mg/ml in DMSO,
300 K.
Position ~ H
1 - 171.95
2 2.91 35.28
3 3.61 /3.21 49.20
4 1.71/1.31 26.13
5 1.82/1.67 27.11
6 1.84/1.32 30.80
7 4.55 51.14
8 7.84 -
g - 169.54
10 3.69 81.55
10-OMe 3.25 57.28
11 3.57 70.69
11-OH 4.45 -
12 3.33 72.77
12-O H 4.37 -
13 3.97 72.47
13-OH 4.56 -
14 5.37 129.04
15 5.48 136.58
16 1.99 37.41
16-Me 0.93 1 g.gg
17 1.26 29.15
18 0.81 11.58
CA 02543003 2006-04-19
21
Example 14: Characterizing the compound of the formula (I~
Empirical formula: C~gH32N207
Molecular weight: 388.46
Diastereomeric mixture: 75/25
Table 4: NMR-chemical
shifts of bengamide
(I~, diastereomeric
mixture, c = 3.1
mg/ml in DMSO,
300 K.
Position ~ H
1 - 173.45
2 7.55 -
3 3.35/3.02 45.05
4 3.74 63.48
4-OH 4.60 -
1.81 /1.75 34.28
6 1.68/1.64 24.25
7 4.32 51.18
8 7.77 -
- 169.63
3.70 81.59
10-OMe 3.26 57.30
11 3.58 70.73
11-O H 4.47 -
12 3.34 72.82
12-OH 4.38 -
13 3.97 72.47
13-O H 4.56 -
14 5.37 129.06
5.48 136.57
16 1.99 37.41
16-Me 0.93 19.90
17 1.26 29.15
18 0.81 11.58
CA 02543003 2006-04-19
22
Example 15: Characterizing bengamide E
Empirical formula: C~7H3oN206
Molecular weight: 358.44
Table 5: NMR-chemical
shifts of bengamide
E, c = 3 mg/ml
in
DMSO, 300 K.
Position ~ H
1 - 174.01
2 7.91 -
3 3.19/3.06 40.56
4 1.74/1.20 28.75
1.87/1.64 27.56
6 1.87/1.36 30.72
7 4.39 51.27
8 7.78 -
- 169.60
3.69 81.61
10-OMe 3.25 57.30
11 3.56 70.74
11-OH 4.49 -
12 3.33 I 72.78
12-OH 4.38 -
13 3.96 72.38
13-OH 4.57 -
14 5.38 127.68
5.58 137.85
16 2.24 30.08
17 0.95 22.27
18 0.95 22.17
CA 02543003 2006-04-19
23
Example 16: Characterizing bengamide F
Empirical formula: C~gH32N206
Molecular weight: 372.47
Table 6: NMR-chemical
shifts of bengamide
F,
c = 3 mg/ml in
DMSO, 300 K.
Position ~ H
1 -
2 2.91
3 3.62/3.21
4 1.69/1.33
5 1.84/1.69
6 1.84/1.33
7 4.56
8 7.84
9 _
3.70
10-OMe 3.25
11 3.57
12 3.33
13 3.97
14 5.38
5.59
16 2.25
17 0.95
18 0.95
Example 17: Separating the diastereomers of compound (II)
The diastereomeric mixture of the compound of formula (II) from example 8
10 was seperated on a chiral column (ADIH, Daicel, 20 X 200 mm, 0.5 ml flow,
mobile phase: acetonitrile:methanol 4:1 + 0.1 % NHq.Ac). The optical purity
CA 02543003 2006-04-19
24
was checked on an analytical AD/H column (Daicel) (4.6 X 250 mm, 30°C,
mobile phase: acetonitrile:methanol 4:1 + 0.1 % NH4Ac, 0.75 ml flow, Rt
peak1: 9.9 min, Rt peak2: 10.9 min).
Table 7:
NMR-chemical
shifts
of the
diastereomers
of bengamide
(II),
c = 3 mg/ml
in DMSO,
300 K.
1 H (A) 1 H (B) 13C (A) 13C (B)
1 - - 173.99 173.99
2 7.91 7.91 - -
3 3.19/3.06 3.19/3.06 40.56 40.56
4 1.74/1.20 1.74/1.20 28.75 28.75
1.87/1.64 1.87/1.64 27.55 27.55
6 1.87/1.36 1.87/1.36 30.72 30.72
7 4.39 4.39 51.27 51.27
8 7.78 7.78 - _
9 - - 169.61 169.61
3.69 3.69 81.60 81.60
10-OMe 3.25 3.25 57.32 57.32
11 3.58 3.58 70.72 70.77
11-OH 4.46 4.47 - -
12 3.33 3.33 72.80 72.85
12-OH 4.36 ' 4.36 ' - ' - '
13 3.97 3.97 72.46 72.37
13-O H 4. 56 4. 56 - -
14 5.37 5.38 129.05 129.01
5.48 5.49 136.57 136.44
16 1.99 1.99 37.41 37.28
16-Me 0.93 0.92 19.90 19.86
17 1.26 1.26 29.15 29.06
18 0.81 0.82 11.58 11.48
CA 02543003 2006-04-19
Example 18: Cell proliferation measurements performed on various tumor
cell lines
The tumor cell lines Hep-G2 (ATCC No. HB-8065) and COLD 205 (ATCC
5 No. CCL-222) were used for determining the cell proliferation. The cell
lines
were sown in cell culture medium at the rate of 1000 cells/well [Hep-G2]
and, respectively, 3500 cells/well [Co1o205] and incubated for 4 hours at
37°C and 5% C02.
10 Medium for Hep-G2: Dulbecco's modified Eagle's medium / Ham's F12 mix
(Gibco); NEAA (10°I°; nonessential amino acids, Gibco), sodium
pyruvate
(1 %, Gibco), L-glutamine (1 %, Gibco), fetal calf serum (5%; PAA)].
Medium for COLD 205: RPMI 1640 (Gibco), L-glutamine (1%, Gibco),
15 HEPES (1 %, Gibco), fetal calf serum (10%, PAA).
After 4 hours, compounds (II), (III) and (I~ and bengamides E and F,
dissolved in DMSO/cell culture medium, were added at various dilutions
and the mixtures were incubated for 72 hours at 37°C and 5% C02. The
20 intracellular content of ATP was determined using the test reagent
CeIITiterGlo (Promega).
The results of the cell proliferation tests are reported in table 1.