Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
ARTIFICIAL RECEPTORS INCLUDING GRADIENTS
Cross Reference to Related Applications
This application is being filed as a PCT International Patent application on
02 December 2004, in the name of Receptors LLC, a U.S. national corporation,
applicant for the designation of all countries except the US, and Robert E.
Carlsan, a
U.S. citizen, applicant for the designation of the US only, arid claims
priority to U.S.
Provisional Patent Application Serial Nos. 60/527,190, filed December 2, 2003,
and
60!622,086, filed October 25, 2004; 60/607,438, 60/607,458, 60/607,457, each
filed
September 3, 2004; 60/608,557, 60/608,654, each filed September 10, 2004;
60/609,160, filed September 11, 2004; and 60/612,666, filed September 23,
2004.
Field of the Invention
The present invention relates to gradients of artificial receptors or building
blocks, methods of making the gradients, and methods employing the gradients.
The gradient can include one or more building blocks. The gradient can include
change in any of a variety of characteristics of the artificial receptor ar
building
block.
Background
The preparation~of artificial receptors that bind ligands like proteins,
peptides, carbohydrates, microbes, pollutants, pharmaceuticals, and the like
with
high sensitivity and specificity is an active area of research. None of the
conventional approaches has been particularly successful; achieving only
modest
sensitivity and specificity mainly due to low binding affinity.
Antibodies, enzymes, and natural receptors generally have binding constants
in the 10$-1012 range, which results in both nanomolar sensitivity and
targeted
specificity. By contrast, conventional artificial receptors typically have
binding
constants of about 103 to 105, with the predictable result of millimolar
sensitivity and
limited specificity.
~ Several conventional approaches are being pursued in attempts to achieve
highly sensitive and specific artificial receptors. These;~approaches include,
for
example, affinity isolation, molecular imprinting, and rational and/or
combinatorial
design and synthesis of synthetic or semi-synthetic receptors.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Such rational or combinatorial approaches have been limited by the
relatively small number of receptors which are evaluated and/or by their
reliance on
a design strategy which focuses on only one building block, the homogeneous
design strategy. Common combinatorial approaches form microarrays that include
10,000 or 100,000 distinct spots on a standard microscope slide. However, such
conventional methods for combinatorial synthesis provide a single molecule per
spot. Employing a single building block in each spot provides only a single
possible
receptor per spot. Synthesis of thousands of building blocks would be required
to
make thousands of possible receptors.
Further, these conventional approaches are hampered by the currently lirriited
understanding of the principals which lead to efficient binding and the large
number
of possible structures for receptors, which makes such an approach
problematic.
There remains a need for methods for detecting ligands and for detecting
compounds that disrupt one or more binding interactions.
Summary
The present invention relates to gradients of artificial receptors or building
blocks, methods of making the gradients, and methods employing the gradients.
The gradient can include one or more building blocks. The gradient can include
change in any of a variety of characteristics of the artificial receptor or
building
block including change in the concentration of an artificial receptor or
building
block; change in the identity of an artificial receptor or building block;
change in the
topography of an artificial receptor or building block; change' in the mode of
binding
of an artificial receptor or building block to the support; change in the lawn
or lawn
modifier; change in charge, volume, lipophilicity, or hydrophilicity of the
artificial
receptor or building bloclc; or change in a molecular descriptors for the
artificial
receptor or building block.
In an embodiment, the present invention relates to a building block gradient.
The building block gradient can include a support. A portion of the support
can
include at least one building block. The building block being coupled to the
support
and the building block forming a gradient. In an embodiment, the building
block
gradient includes a surface. The surface includes a region including at least
one
2
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
building block. The building block being coupled to the support and the
building
block forming a gradient. The gradient can include a plurality of building
blocks.
In an embodiment, the present invention relates to a method of making a
building block gradient. The method can include forming a region on a solid
support. The region can include at least one building block, the building
block
forming a gradient. The method also includes coupling the building block to
the
solid support in the region. The method can include coupling a plurality of
building
blocks to the solid support in the region.
In an embodiment, the present invention relates to a method of using a
building block gradient. This method can include contacting a the building
block
gradient with a test ligand. and monitoring the gradient for binding of the
test
ligand. The test ligand can be a protein or proteome.
Brief Description of the Figures
Figure 1 schematically illustrates two dimensional representations of an
embodiment of a receptor according to the present invention that employs 4
different
building blocks to make a ligand binding site.
Figure 2 schematically illustrates two and three dimensional representations
of an embodiment of a molecular configuration of 4 building blocks, each
building
block including a recognition element, a framework, and a linker coupled to a
support (immobilization/anchor).
Figure 3 schematically illustrates an embodiment of the present methods and
artificial receptors employing shuffling and exchanging building blocks.
Figure 4 schematically illustrates a gradient extending in one direction along
a support, such as a slide or plate.
Figure 5 schematically illustrates gradients extending in two opposite
directions along a support, such as a slide or plate.
Figure 6 schematically illustrates multiple gradients extending in each of two
opposite directions along a support, such as a slide or plate.
Figure 7 schematically illustrates two gradients extending in different
directions on a support, such as a slide or plate.
Figure 8 schematically illustrates three gradients extending in different
directions on a support, such as a slide or plate.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Figure 9 schematically illustrates four gradients extending in different
directions on a support, such as a slide or plate.
Figure 10 also schematically illustrates four gradients extending in different
directions on a support, such as a slide or plate.
Figure 11 schematically illustrates a step gradient extending across a
support,
such as a slide or plate.
Figure 12 schematically illustrates a plate including both step gradient A and
a second gradient B.
Figure 13 schematically illustrates an embodiment of a method for making
and using a gradient for evaluating an analyte or mixture of analytes.
Figure 14 schematically illustrates a false color fluorescence image of a
labeled microarray according to an embodiment of the present invention.
Figure 15 schematically illustrates a two dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding
phycoerythrin.
Figure 16 schematically illustrates a three dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding
phycoerythrin.
Figure 17 schematically illustrates a two dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding a fluorescent
derivative of ovalbumin.
Figure 18 schematically illustrates a three dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding a fluorescent
derivative of ovalbumin.
Figure 19 schematically illustrates a two dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding a fluorescent
derivative of bovine serum albumin.
Figure 20 schematically illustrates a three dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding a fluorescent
derivative of bovine serum albumin.
Figure 21 schematically illustrates a two dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding an acetylated
horseradish peroxidase.
4
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Figure 22 schematically illustrates a three dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding an acetylated
horseradish peroxidase.
Figure 23 schematically illustrates a two dimensional plot of data obtained
for candidate artificial receptors contacted with andlor binding a TODD
derivative of
horseradish peroxidase.
Figure 24 schematically illustrates a three dimensional plot of data obtained
for candidate artificial receptors contacted with and/or binding a TODD
derivative of
horseradish peroxidase.
Figure 25 schematically illustrates a subset of the data illustrated in Figure
16.
Figure 26 schematically illustrates a subset of the data illustrated in Figure
16.
Figure 27 schematically illustrates a subset of the data illustrated in Figure
16.
Figure 28 schematically illustrates a correlation of binding data for
phycoerythrin against loge for the building blocks making up the artificial
receptor.
Figure 29 schematically illustrates a correlation of binding data for
phycoerythrin against loge for the building blocks making up the artificial
receptor.
Figure 30 schematically illustrates a two dimensional plot comparing data
obtained for candidate artificial receptors contacted with andlor binding
phycoerythrin to data obtained for candidate artificial receptors contacted
with
andlor binding a fluorescent derivative of bovine serum albumin.
Figures.3l, 32, and 33 schematically illustrate subsets of data from Figures
16, 20, and 18, respectively, and demonstrate that the array of artificial
receptors
according to the present invention yields receptors distinguished between
three
analytes, phycoerythrin, bovine serum albumin, and ovalbumin.
Figure 34 schematically illustrates a gray scale image of the fluorescence
signal from a scan of a control plate which was prepared by washing off the
building
blocks with organic solvent before incubation with the test ligand.
Figure 35 schematically illustrates a gray scale image of the fluorescence
signal from a scan of an experimental plate which was incubated with 1.0 pg/ml
Cholera Toxin B at 23 °C.
5
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Figure 36 schematically illustrates a gray scale image of the fluorescence
signal from a scan of an experimental plate which was incubated with 1.0
,ug/ml
Cholera Toxin B at 3 °C.
Figure 37 schematically illustrates a gray scale image of the fluorescence
signal from a scan of an experimental plate which was incubated With 1.0
,uglml
Cholera Toxin B at 43 °C.
Figures 38-40 schematically illustrate plots of the fluorescence signals
obtained from the candidate artificial receptors illustrated in Figures 35-37.
Figure 41 schematically illustrate plots of the fluorescence signals obtained
from the combinations of building blocks employed in the present studies, when
those building blocks are covalently linked to the support. Binding was
conducted
at 23 °C.
Figure 42 schematically illustrates the changes in fluorescence signal from
individual combinations of covalently immobilized building blocks at 4
°C, 23 °C, or
44 °C.
Figure 43 schematically illustrates a graph of the changes in fluorescence
si~,mal from individual combinations of building blocks at 4 °C, 23
°C, or 44 °C.
Figure 44 schematically illustrates the data presented in Figure 42 (lines
marked A) and the data presented in Figure 43 (lines marked B).
Figure 45 schematically illustrates a graph of the fluorescence signal at 44
°C
divided by the signal at 23 °C against the fluorescence signal obtained
from binding
at 23 °C for the artificial receptors with reversibly immobilized
receptors.
Figure 46 illustrates fluorescence signals produced by binding of cholera
toxin to a microarray of the present candidate artificial receptors followed
by
washing with buffer in an experiment reported in Example 4.
Figure 47 illustrates the fluorescence signals due to cholera toxin binding
that were detected upon competition with GM1 OS (0.34 ~.M) in an experiment
reported in Example 4.
Figure 48 illustrates the ratio of the amount bound in the absence of GM1 OS
to the amount bound in competition with GMl OS(0.34 ~.M) in an experiment
reported in Example 4.
Figure 49 illustrates fluorescence signals produced by binding of cholera
toxin to a microarray of the present candidate artificial receptors followed
by
6
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
washing with buffer in an experiment reported in Example 4 and for comparison
with competition experiments using 5.1 ,uM GM1 OS.
Figure 50 illustrates the fluorescence signals due to cholera toxin binding
that were detected upon competition with GM1 OS (5.1 ,uM) in an experiment
reported in Example 4.
Figure 51 illustrates the ratio of the amount bound in the absence of GMl OS
to the amount bound in competition with GM1 OS(5.1 ~,M) in an experiment
reported in Example 4.
Figure 52 illustrates the fluorescence signals produced by binding of cholera
toxin to the microarray of candidate artificial receptors alone and in
competition
with each of the three concentrations of GM1 in the experiment reported in
Example
5.
Figure 53 illustrates the ratio of the amount bound in the absence of GM1 OS
to the amount bound upon competition with GM1 for the low concentration of GM1
employed in Example 5.
Figure 54 illustrates the fluorescence signals produced by binding of cholera
toxin to the microarray of candidate artificial receptors without pretreatment
with
GM1 in the experiment reported in Example 6.
Figures 55-57 illustrate the fluorescence signals produced by binding of
cholera toxin to the microarray of candidate artificial receptors with
pretreatment
with GMl (100 ~ug/ml, 10 ~.g/ml, and 1 ,ug/ml GM1, respectively) in the
experiment
reported in Example 6.
Figure 58 illustrates the ratio of the amount bound in the presence of 1
,uglml
GM1 to the amount bound in the absence of GM1 in the experiment reported in
Example 6.
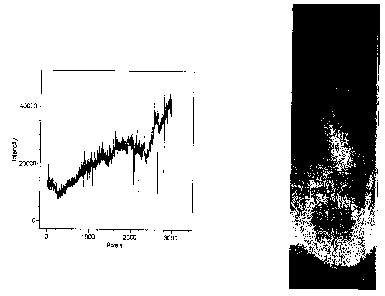
Figure 59 presents an image obtained from a run of phycoerythrin over a step
gradient of increasing concentrations of the building block TyrA3B3. Figure 60
shows increasing peaks of fluorescence for the 3rd, 4tn, Sth, and 6t" steps of
the
building block gradient.
Figure 61 presents an image obtained from a run of phycoerythrin over a step
gradient of increasing concentrations of the building block TyrA4B4. Figure 62
shows increasing peaks of fluorescence for the 5th and 6th steps of the
building block
gradient.
7
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Figure 63 presents an image obtained from a run of phycoerythrin over a step
gradient of increasing concentrations of the building block TyrA5B5. Figure 64
illustrates that phycoerythrin did not bind to any step of the gradient of
this building
block, no peaks of fluorescence were obtained.
Figure 65 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building block TyrA3B3. Figure 66
shows increasing peaks of fluorescence for the 2"a, 3ra, 4th, Stn, and 6th
steps of the
building block gradient. Figure 67 presents an image obtained from a run of
cholera toxin over a step gradient of increasing concentrations of the
building block
TyrA4B4. Figure 68 shows increasing peaks of fluorescence for at least the
2"a, 3rd,
4ch~ Sth~ and 6t1' steps of the building block gradient and possibly also for
the first
step.
Figure 69 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building block TyrA5B5. Figure 70
illustrates that cholera toxin did not bind to any step of the gradient of
this building
block, no peaks of fluorescence were obtained.
Figure 71 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building blocks TyrA3B3 and
TyrA4B4
(in a 1:1 molar ratio). Figure 72 shows increasing peaks of fluorescence for
the 3rd,
4t1', Sth, and 6th steps of the building block gradient.
Figure 73 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building blocks TyrA3B3 and
TyrA4B4
(in a 1:1 molar ratio). Figure 74 shows peaks of fluorescence for at least the
6t'', 5th,
4ch~ 3ra~ ~d 2na steps of the building block gradient.
Figure 75 presents an image obtained from a run of a mixture of cholera
toxin and phycoerythrin over a step gradient of increasing concentrations of
the
building blocks TyrA3B3 and TyrA4B4 (in a 1:1 molar ratio). Figure 76 shows
fluorescence intensities obtained for cholera toxin (top line) and
phycoerythrin
(bottom line).
Figure 77 presents an image obtained from a run of a mixture of cholera
toxin and phycoerythrin over a step gradient of increasing concentrations of
the
building blocks TyrA4B4 and TyrA4B6 (in a 1:1 molar ratio). Figure 78 shows
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
fluorescence intensities obtained for cholera toxin (top line) and
phycoerythrin
(bottom line).
Figure 79 presents a fluorescence image from a run of cholera toxin flowed
over a continuous gradient. of increasing concentrations of the building
blocks
TyrA3B3 and TyrA4B~ (in a 1:1 molar ratio). Figure 80 illustrates increasing
fluorescence, which indicates that the cholera toxin bound in increasing
amounts to
the higher concentration portions of the gradient.
Detailed Description
Definitions
As used herein, the term "peptide" refers to a compound including two or
more amino acid residues joined by amide bond(s).
As used herein, the terms "polypeptide" and "protein" refer to a peptide
including more than about 20 amino acid residues connected by peptide
linkages.
As used herein, the term "proteome" refers to the expression profile of the
proteins of an organism, tissue, organ, or cell. The proteome can be specific
to a
particular status (e.g., development, health, etc.) of the organism, tissue,
organ, or
cell.
Reversibly immobilizing building blocks on a support couples the building
blocks to the support through a mechanism that allows the building blocks to
be
uncoupled from the support without destroying or unacceptably degrading the
building block or the support. That is, immobilization can be reversed without
destroying or unacceptably degrading the building block or the support. In an
embodiment, immobilization can be reversed with only negligible or ineffective
levels of degradation of tla.e building block or the support. Reversible
immobilization can employ readily reversible covalent bonding or noncovalent
interactions. Suitable noncovalent interactions include interactions between
ions,
hydrogen bonding, van der Waals interactions, and the like. Readily reversible
covalent bonding refers to covalent bonds that can be formed and broken under
conditions that do not destroy or unacceptably degrade the building block or
the
support.
A combination of building blocks immobilized on, for example, a support
can be a candidate artificial receptor, a lead artificial receptor, or a
working artificial
9
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
receptor. That is, a heterogeneous building block spot on a slide ox a
plurality of
building blocks coated on a tube or well can be a candidate artificial
receptor, a lead
artificial receptor, or a working artificial receptor. A candidate artificial
receptor
can become a lead artificial receptor, which can become a working artificial
receptor.
As used herein the phrase "candidate artificial receptor" refers to an
immobilized combination of building blocks that can be tested to determine
whether
or not a particular test ligand binds to that combination. In an embodiment,
the
combination includes one or more reversibly immobilized building blocks. In an
embodiment, the candidate artificial receptor can be a heterogeneous building
block
spot on a slide or a plurality of building blocks coated on a tube or well.
As used herein the phrase "lead artificial receptor" refers to an immobilized
combination of building blocks that binds a test ligand at a predetermined
concentration of test ligand, for example at 10, 1, 0.1, or 0.01 ~,g/ml, or at
1, 0.1, or~
0.01 ng/ml. In an embodiment, the combination includes one or more reversibly
immobilized building blocks. In an embodiment, the lead artificial receptor
can be a
heterogeneous building block spot on a slide or a plurality of building blocks
coated
on a tube or well.
As used herein the phrase "working artificial receptor" refers to a
combination of building blocks that binds a test ligand with a selectivity
andlor
sensitivity effective for categorizing or identifying the test ligand. That
is, binding
to that combination of building blocks describes the test ligand as belonging
to a
category of test ligands or as being a particular test ligand. A working
artificial
receptor can, for example, bind the ligand at a concentration of, for example,
100,
10, 1, 0.1, 0.01, or 0.001 ng/ml. In an embodiment, the combination includes
one or
more reversibly immobilized building blocks. In an embodiment, the working
artificial receptor can be a heterogeneous building block spot on a slide or a
plurality
of building blocks coated on a tube, well, slide, or other support or on a
scaffold.
As used herein the phrase "working artificial receptor complex" refers to a
plurality of artificial receptors, each a combination of building blocks, that
binds a
test ligand with a pattern of selectivity andlor sensitivity effective for
categorizing or
identifying the test ligand. That is, binding to the several receptors of the
complex
describes the test ligand as belonging to a category of test ligands or as
being a
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
particular test ligand. The individual receptors in the complex can each bind
the
ligand at different concentrations or with different affinities. For example,
the
individual receptors in the complex each bind the ligand at concentrations of
100,
10, 1, 0.1, 0.01 or 0.001 ng/ml. In an embodiment, the combination includes
one or
more reversibly immobilized building blocks. In an embodiment, the working
artificial receptor complex can be a plurality of heterogeneous building block
spots
or regions on a slide; a plurality of wells, each coated with a different
combination
of building blocks; or a plurality of tubes, each coated with a different
combination
of building blocks.
As used herein, the phrase "significant number of candidate artificial
receptors" refers to sufficient candidate artificial receptors to provide an
opportunity
to find a working artificial receptor, working artificial receptor complex, or
lead
artificial receptor. As few as about 100 to about 200 candidate artificial
receptors
can be a significant number for finding working artificial receptor complexes
suitable for distinguishing two proteins (e.g., cholera toxin and
phycoerythrin). In
other embodiments, a significant number o~f candidate artificial receptors can
include
i
about 1,000 candidate artificial receptors, about 10,000 candidate artificial
receptors,
about 100,000 candidate artificial receptors, or more.
Although not limiting to the present invention, it is believed that the
significant number of candidate artificial receptors required to provide an
opportunity to find a working artificial receptor may be larger than the
significant
number required to find a working artificial receptor complex. Although not
limiting to the present invention, it is believed that the significant number
of
candidate artificial receptors required to provide an opportunity to find a
lead
artificial receptor may be larger than the significant number required to find
a
working artificial receptor. Although not limiting to the present invention,
it is
believed that the significant number of candidate artificial receptors
required to
provide an opportunity to fmd a working artificial receptor for a test ligand
with few
features may be more than for a test ligand with many features.
As used herein, the term "building block" refers to a molecular component of
an artificial receptor including portions that can be envisioned as or that
include one
or more linkers, one or more frameworks, and one or more recognition elements.
In
an embodiment, the building block includes a linker, a framework, and one or
more
11
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
recognition elements. In an embodiment, the linker includes a moiety suitable
for
reversibly immobilizing the building block, for example, on a support, surface
or
lawn. The building block interacts with the ligand.
As used herein, the term "linker" refers to a portion of or functional group
on
a building block that can be employed to or that does (e.g., reversibly)
couple the
building block to a support, for example, through covalent link, ionic
interaction,
electrostatic interaction, or hydrophobic interaction.
As used herein, the term "framework" refers to a portion of a building block
including the linker or to which the linker is coupled and to which one or
more
recognition elements are coupled.
As used herein, the term "recognition element" refers to a portion of a
building block coupled to the framework but not covalently coupled to the
support.
Although not limiting to the present invention, the recognition element can
provide
or form one or more groups, surfaces, or spaces for interacting with the
ligand.
As used herein, the phrase "plurality of building blocks" refers to two or
more building blocks of different structure in a mixture, in a kit, or on a
support or
scaffold. Each building block has a particular structure, and use of building
blocks
in the plural, or of a plurality of building blocks, refers to more than one
of these
particular structures. Building blocks or plurality of building blocks does
not refer
to a plurality of molecules each having the same structure.
As used herein, the phrase "combination of building blocks" refers to a
plurality of building blocks that together are in a spot, region, or a
candidate, lead, or
working artificial receptor. A combination of building blocks can be a subset
of a
set of building blocks. For example, a combination of building blocks can be
one of
the possible combinations of 2, 3, 4, 5, or 6 building blocks from a set of N
(e.g.,
N=10-200) building blocks.
As used herein, the phrases "homogenous immobilized building block" and
"homogenous immobilized building blocks" refer to a support or spot having
immobilized on or within it only a single building block.
As used herein, the phrase "activated building block" refers to a building
block activated to make it ready to form a covalent bond to a functional
group, for
example, on a support. A building block including a carboxyl group can be
converted to a building bloclc including an activated ester group, which is an
12
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
activated building block. An activated building block including an activated
ester
group can react, for example, with an amine to form a covalent bond.
As used herein, the term "naive" used with respect to one or more building
blocks refers to a building block that has not previously been determined or
known
to bind to a test ligand of interest. For example, the recognition elements)
on a
naive building block has not previously been determined or known to bind to a
test
ligand of interest. A building bloclc that is or includes a known ligand
(e.g., GM1)
for a particular protein (test ligand) of interest (e.g., cholera toxin) is
not naive with
respect to that protein (test ligand).
As used herein, the teen "irmnobilized" used with respect to building blocks
coupled to a support refers to building blocks being stably oriented on the
support so
that they do not migrate on the support or release from the support. Building
blocks
can be immobilized by covalent coupling, by ionic interactions, by
electrostatic
interactions, such as ion pairing, or by hydrophobic interactions, such as van
der
Waals interactions.
As used herein a "region" of a support, tube, well, or surface refers to a
contiguous portion of the support, tube, well, or surface. Building blocks
coupled to
a region can refer to building blocks in proximity to one another in that
region.
As used herein, a "bulky" group on a molecule is larger than a moiety
including 7 or 8 carbon atoms.
As used herein, a "small" group on a molecule is hydrogen, methyl, or
another group smaller than a moiety including 4 carbon atoms.
As used herein, the term "lawn" refers to a layer, spot, or region of
functional
groups on a support, for example, at a density sufficient to place coupled
building
blocks in proximity to one another. The fwctional groups can include groups
capable of forming covalent, ionic, electrostatic, or hydrophobic interactions
with
building blocks.
As used herein, the term "alkyl" refers to saturated aliphatic groups,
including straight-chain alkyl groups, branched-chain alkyl groups, cycloalkyl
(alicyclic) groups, alkyl substituted cycloalkyl groups, and cycloalkyl
substituted
alkyl groups. In certain embodiments, a straight chain or branched chain alkyl
has
30 or fewer carbon atoms in its baclcbone (e.g., C1-C12 for straight chain, Cl-
C6 for
13
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
branched chain). Likewise, cycloalkyls can have from 3-10 carbon atoms in
their
ring structure, for example; 5, 6 or 7 carbons in the ring structure.
The term "alkyl" as used herein refers to both "unsubstituted alkyls" and
"substituted alkyls", the latter of which refers to alkyl moieties having
substituents
replacing a hydrogen on one or more carbons of the hydrocarbon backbone. Such
substituents can include, for example, a halogen, a hydroxyl, a carbonyl (such
as a
carboxyl, an ester, a formyl, or a ketone), a thiocarbonyl (such as a
thioester, a
thioacetate, or a thioformate), an alkoxyl, a phosphoryl, a phosphonate, a
phosphinate, an amino, an amido, an amidine, an imine, a cyano, a nitro, a~i
azido, a
sulfhydryl, an alkylthio, a sulfate, a sulfonate, a sulfamoyl, a sulfonamido,
a
sulfonyl, a heterocyclyl, an aryl alkyl, or an aromatic or heteroaromatic
moiety. The
moieties substituted on the hydrocarbon chain can themselves be substituted,
if
appropriate. For example, the substituents of a substituted alkyl can include
substituted and unsubstituted forms of the groups listed above.
The phrase "aryl alkyl", as used herein, refers to an alkyl group substituted
with an aryl group (e.g., an aromatic or heteroaromatic group).
As used herein, the terms "alkenyl" and "alkynyl" refer to unsaturated
aliphatic groups analogous in length and optional substitution to the alkyls
groups
described above, but that contain at least~one double or triple bond
respectively.
The term "aryl" as used herein includes 5-, 6- and 7-membered single-ring
aromatic groups that may include from zero to four heteroatoms, for example,
benzene, pyrrole, furan, thiophene, imidazole, oxazole, thiazole, triazole,
pyrazole,
pyridine, pyrazine, pyridazine and pyrimidine, and the like. Those aryl groups
having heteroatoms in the ring structure may also be referred to as "aryl
heterocycles" or "heteroaromatics". The aromatic ring can be substituted at
one or
more ring positions with such substituents such as those described above for
alkyl
groups. The term "aryl" also includes polycyclic ring systems having two or
more
cyclic rings in which two or more carbons are common to two 'adjoining rings
(the
rings are "fused rings") wherein at least one of the rings is aromatic, e.g.,
the other
cyclic rings) can be cycloalkyls, cycloalkenyls, cycloalkynyls, aryls andlor
heterocyclyls.
As used herein, the terms "heterocycle" or "heterocyclic group" refer to 3- to
12-membered ring structures, e.g., 3- to 7-membered rings, whose ring
structures
14
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
include one to four heteroatoms. Heterocyclyl groups include, for example,
thiophene, thianthrene, furan, pyran, isobenzofuran, chromene, xanthene,
phenoxathiin, pyrrole, imidazole, pyrazole, isothiazole, isoxazole, pyridine,
pyrazine, pyrimidine, pyridazine, indolizine, isoindole, indole, indazole,
purine,
quinolizine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline,
quinazoline, cinnoline, pteridine, carbazole, carboline, phenanthridine,
acridine,
pyrimidine, phenanthroline, phenazine, phenarsazine, phenothiazine, furazan,
phenoxazine, pyrrolidine, oxolane, thiolane, oxazole, piperidine, piperazine,
morpholine, lactones, lactams such as azetidinones and pyrrolidinones,
sultams,
sultones, and the like. The heterocyclic ring can be substituted at one or
more
positions with such substituents.such as those described for alkyl groups.
As used herein, the term "heteroatom" as used herein means an atom of any
element other than carbon or hydrogen, such as nitrogen, oxygen, sulfur and
phosphorous.
Overview of the Artificial Receptor
Figure 1 schematically illustrates aai embodiment employing 4 distinct
building blocks in a spot on a microarray to make a ligand binding site. This
figure
illustrates a group of 4 building blocks at the corners of a square forming a
unit cell.
A group of four building blocks can be envisioned as the vertices on any
quadrilateral. Figure 1 illustrates that spots or regions of building blocks
can be
envisioned as multiple unit cells, in this illustration square unit cells.
Groups of unit
cells of four building blocks in the shape of other quadrilaterals can also be
formed
on a support.
Each immobilized building block molecule can provide one or more "arms"
extending from a "framework" and each can include groups that interact with a
ligand or with portions of another immobilized building block. Figure 2
illustrates
that combinations of four building bloclcs, each including a framework with
two
,arms (called "recognition elements"), provides a molecular configuration of
building
blocks that form a site for binding a ligand. Such a site formed by building
blocks
such as those exemplified below can bind a small molecule, such as a drug,
metabolite, pollutant, or the like, andlor can bind a larger ligand such as a
macromolecule or microbe.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
The present artificial receptors can include building blocks reversibly
immobilized on a support or surface. Reversing immobilization of the building
blocks can allow movement of building blocks to a different location on the
support
or surface, or exchange of building blocks onto and off of the surface. For
example,
the combinations of building blocks can bind a ligand when reversibly coupled
to or
immobilized on the support. Reversing the coupling or immobilization of the
building blocks provides opportunity for rearranging the building blocks,
which can
improve binding of the ligand. Further, the present invention can allow for
adding
additional or different building blocks, which can further improve binding of
a
ligand.
Figure 3 schematically illustrates an embodiment employing an initial
artificial receptor surface (A) with four different building blocks on the
surface,
which are represented by shaded shapes. This initial artificial receptor
surface (A)
undergoes (1) binding of a ligand to an artificial receptor and (2) shuffling
the
building blocks on the receptor surface to yield a lead artificial receptor
(B).
Shuffling refers to reversing the coupling or immobilization of the building
blocks
and allowing their rearrangement on the receptor surface. After forming a lead
artificial receptor, additional building blocks can be (3) exchanged onto
and/or off of
the receptor surface (C). Exchanging refers to building blocks leaving the
surface
and entering a solution contacting the surface and/or building blocks leaving
a
solution contacting the surface and becoming part of the artificial receptor.
The
additional building blocks can be selected for structural diversity (e.g.,
randomly) or
selected based on the structure of the building blocks in the lead artificial
receptor to
provide additional avenues for improving binding. The original and additional
building blocks can then be (4) shuffled and exchanged to provide higher
affinity
artificial receptors on the surface (D).
Gradients of Artificial Receptors and Building Blocks
The present invention includes artificial receptors, building blocks, or
combinations of building blocks configured as a gradient on a support. The
gradient
can be made up of change in the concentration (e.g., density) of an artificial
receptor
or building block. The gradient can be made up of change in the identity
(e.g.,
structure) of an artificial receptor or budding block. The gradient can be
made up of
16
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
change in the topography (e.g., size, shape, or flexibility) of an artificial
receptors or
building blocks. The gradient can be made up of change in the mode of binding
(e.g., irreversible or reversible) of an artificial receptor or building block
to the
support. The gradient can be made up of changes in the lawn or lawn modifier.
The
gradient can be any of a variety of types of gradients, such as step,
continuous, or the
like.
One or more of building blocks according to the present invention can be
immobilized on a support in regions. These regions can include one or more of
the
building blocks at different concentrations in different sub-regions. For
example,
one or more of the building blocks can be in different concentrations in bands
on or
across the region. For example, one or more of the building blocks can be in a
gradient from zero or low concentration at one side (e.g., edge or corner) of
the
region to higher concentration at the opposite side (e.g., edge or corner) of
the
region. These regions can include distinct building blocks or combinations of
building blocks in different sub-regions. For example, one or more of the
building
blocks can be in one sub-region but not another. For example, one or more of
the
building blocks can be at a first concentration in one sub-region and at a
another
(e.g., second) concentration in another sub-region. For example, one or more
building blocks can be in each sub-region. For example, one or more building
blocks can be in only a subset of sub-regions.
Figures 4 through 9 illustrate embodiments of supports or regions including
gradients of artificial receptors or building blocks. Figures 4 through 6
generally
xelate to the number and direction of gradients in a single dimension (i.e.,
along a
line) on a support. Figures 7 through 9 generally relate to the orientation of
gradients in two dimensions on a support. Of course, each gradient with an
orientation shown in Figures 7 through 9 can have the variation in number and
direction illustrated in Figures 4 through 6. Further, although the gradients
are
illustrated as generally straight lines, a gradient according to the present
invention
can vary along any of a variety of traj ectories. The change that occurs along
the
gradient can occur as a linear or other fixnction of distance traveled from
the
beginning of or from a point along the gradient. For example, concentration
can
change as a linear or exponential function of distance from the beginning of
the
gradient.
17
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Figure 4 schematically illustrates a gradient extending in one direction along
a support, such as a slide or plate. As described above, such a gradient can
be made
up of at least one of change in the concentration (e.g., density) of an
artificial
receptor or building block; change in the identity (e.g., structure) of an
artificial
receptor or building block; change in the topography (e.g., size, shape, or
flexibility)
of an artificial receptor or building block; change in the mode of binding
(e.g.,
irreversible or reversible) of an artificial receptor or building block to the
support;
and changes in the lawn or lawn modifier. The gradient can provide or be made
up
of changes in at least one of a variety of characteristics of the artificial
receptor or
building block, such as charge, volume, lipophilicity, hydrophilicity. The
gradient
can be described by or made up of changes in at least one of a variety of
molecular
descriptors for the artificial receptor or building block.
Figure 5 schematically illustrates gradients extending in two opposite
directions along a support, such as a slide or plate. Gradient A can be as
described
above with respect to Figure 4. Gradient B can be made up of any of the
changes in
characteristics described above with respect to Figure 4. Gradient B will
generally
not be selected to be the same gradient as Gradient A but in an opposite
direction,
which would just result in one gradient canceling the other and produce a
generally
uniform receptor surface. In an embodiment, gradient B can be selected to be
of a
different character than gradient A.
For example, if gradient A is made up of change in the concentration (e.g.,
density) of a first artificial receptor or building block, then gradient B can
be made
up of at least one of: change in the concentration (e.g., density) of a second
artificial
receptor or building block; change in the identity (e.g., structure) of an
artificial
receptor or building block; change in the topography (e.g., size, shape, or
flexibility)
of an artificial receptor or building block; change in the mode of binding
(e.g.,
irreversible or reversible) of an artificial receptor or building block to the
support;
and change in the lawn or lawn modifier.
For example, if gradient A is made up of change in the identity (e.g.,
structure) of a first artificial receptor or building block, then gradient B
can be made
up of at least one of: change in the concentration (e.g., density) of an
artificial
receptor or building block; change in the identity (e.g., structure) of a
second
artificial receptor or building block; change in the topography (e.g., size,
shape, or
18
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
flexibility) of an artificial receptor or building block; change in the mode
of binding
(e.g., irreversible or reversible) of an artificial receptor or building block
to the
support; and change in the lawn or lawn modifier.
For example, if gradient A is made up of change in the topography (e.g., size,
shape, or flexibility) of a first artificial receptor or building block, then
gradient B
can be made up of at least one of change in the concentration (e.g., density)
of an
artificial receptor or building block; change in the identity (e.g.,
structure) of an
artificial receptor or building block; change in the topography (e.g., size,
shape, or
flexibility) of a second artificial receptor or building block; change in the
mode of
binding (e.g., irreversible or reversible) of an artificial receptor or
building block to
the support; and change in the lawn or lawn modifier.
For example, if gradient A is made up of change in the mode of binding (e.g.,
irreversible or reversible) of a first artificial receptor or building block
to the
support, then gradient B can be made up of at least one of: change in the
concentration (e.g., density) of an artificial receptor or building block;
change in the
identity (e.g., structure) of an artificial receptor or building block; change
in the
topography (e.g., size, shape, or flexibility) of a second artificial receptor
or building
block; change in the mode of binding (e.g., irreversible or reversible) of a
second
artificial receptor or building block to the support; and change in the lawn
or lawn
modifier.
For example, if gradient A is made up of a first change in the lawn or lawn
modifier, then gradient B can be made up of at least one of: change in the
concentration (e.g., density) of an artificial receptor or building block;
change in the
identity (e.g., structure) of an artificial receptor or building block; change
in the
topography (e.g., size, shape, or flexibility) of a second artificial receptor
or building
block; change in the mode of binding (e.g., irreversible or reversible) of a
second
artificial receptor or building block to the support; and a second change in
the lawn
or lawn modifier.
For example, if gradient A is made up of a first change in charge of the
artificial receptor or building block, then gradient B can be made up of at
least one
of: a second change in charge of the artificial receptor or building block, a
change in
volume of the artificial receptor or building block, a change in lipophilicity
of the.
19
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
artificial receptor or building block, or a change in hydrophilicity of the
artificial
receptor or building block.
For example, if gradient A is made up of a first change in volume of the
artificial receptor or building block, then gradient B can be made up of at
least one
of: a change in charge of the artificial receptor or building block, a second
change in
volume of the artificial receptor or building block, a change in lipophilicity
of the
artificial receptor or building block, or a change in hydrophilicity of the
artificial
receptor or building block.
For example, if gradient A is made up of a first change in lipophilicity of
the
artificial receptor or building block, then gradient B can be made up of at
least one
of: a change in charge of the artificial receptor or 'building block, a change
in
volume of the artificial receptor or building block, a second change in
lipophilicity
of the artificial receptor or building block, or a change in hydrophilicity of
the
artificial receptor or building block.
For example, if gradient A is made up of a first change in hydrophilicity of
the artificial receptor or building block, then gradient B can be made up of
at least
one of a change in charge of the artificial receptor or building block, a
change in
volume of the artificial receptor or building block, a change in lipophilicity
of the
artificial receptor or building block, or a second change in hydrophilicity of
the
artificial receptor or building block.
For example, if gradient A is made up of change in a first molecular
descriptor for the artificial receptor or building block, then gradient B can
be made
up of change in a second molecular descriptor for the artificial receptor or
building
block.
Figure 6 schematically illustrates multiple gradients extending in each of two
opposite directions along a support, such as a slide or plate. Each of the
multiple
gradients can be as described above with respect to Figures 4 and 5. A first
gradient
illustrated in Fi ure 6 can relate to a second
g gradient in the ways described above for
the relationships of gradients A and B. For example, gradient AA can relate to
gradient A as gradient B in Figure 5 relates to gradient A in Figure S.
Gradient n
can relate to gradient A as gradient B in Figure 5 relates to gradient A.
Generally
gradient n will not be the same as gradient A, as this would just result in an
additive
increase in gradient A. In an embodiment, gradient B can be selected to be of
a
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
different character than gradient A. AA ... nn and A ... n as used in Figure 6
each
describe a plurality gradients.
Figure 7 schematically illustrates two gradients extending in different
directions on a support, such as a slide or plate. Each of gradients A and B
can be as
described above with respect to Figures 4 and 5. Typically gradient A will be
different in character than gradient B, as described above with respect to
Figure 5.
The examples of different characteristics for gradients A and B as described
above
can apply to gradients A and B in Figure 7, which are oriented in different
but not
necessarily opposite directions. Either or both of gradients A and B can be
made up
of multiple gradients as described above with respect to Figure 6. For
example,
gradient A in Figure 7 can be or include gradients A through n. For example,
gradient B in Figure 7 can be or include gradients AA through nn. The
orientation
of gradients of artificial receptors or building blocks shown in Figure 7 can
be
viewed as providing a support for a process analogous to 2-dimensional
electrophoresis.
Figure 8 schematically illustrates three gradients extending in different
directions on a support, such as a slide or plate. Each of gradients A, B and
C can be
as described above with respect to Figures 4 and 5. The examples of different
characteristics for gradients A and B as described above in the description of
embodiments of Figure 5 can apply to gradients A, B, and C in Figure 8, which
are
oriented in different but not necessarily opposite directions. Typically
gradient A
will be different in character than gradient B, gradient A will be different
in
character than gradient C, and gradient B will be different in character than
gradient
C, as described above for gradients A and B in Figure 5. One or more of
gradients
A, B or C can be made up of multiple gradients as described above with respect
to
Figure 6. For example, gradient A, B, or C in Figure 8 can be or include
gradients A
through n or gradients AA through nn. The orientation of gradients of
artificial
receptors or building bloclcs shown in Figure 8 can be viewed as providing a
support
for a process analogous to 2-dimensional electrophoresis but including
gradients of
three features providing characterization of analytes in three directions.
Figure 9 schematically illustrates four gradients extending in different
directions on a support, such as a slide or plate. Each of gradients A, B, C
and D can
be as described above with respect to Figures 4 and 5. The examples of
different
21
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
characteristics for gradients A and B as described above in the description of
embodiments of Figure 5 can apply to gradients A, B, C, and D in Figure 9,
which
are oriented in different but not necessarily opposite directions. Typically
gradient
A will be different in character than gradient B, gradient A will be different
in
character than gradient C, gradient A will be different in character than
gradient D,
gradient B will be different in character than gradient C, gradient B will be
different
in character than gradient D, and gradient C will be different in character
than
gradient D, as described above for gradients A and B in Figure 5. One or more
of
gradients A, B, C or D can be made up of multiple gradients as described above
with
respect to Figure 6. For example, gradient A, B, C, or D in Figure 9 can be or
include gradients A through n or gradients AA through nn. The orientation of
gradients of artificial receptors or building blocks shown in Figure 9 can be
viewed
as providing a support for a process analogous to 2-dimensional
electrophoresis but
including gradients of four features providing characterization of analytes in
four
directions.
Figure 10 also schematically illustrates four gradients extending in different
directions on a support, such as a slide or plate. Each of gradients A, B, C
and D can
be as described above with respect to Figure 9. In this embodiment, gradients
A and
B are oriented in opposite directions as are gradients C and D. In an
embodiment,
Figure 10 can schematically illustrate a rectangular plate with 1, 2, 3, 4, or
more
building blocks in a gradient from as many as each of the edges of the plate
to the
opposite edge.
In an embodiment, individual or combinations of building blocks according
to the present invention can be immobilized on a support in regions. Each
region
can include one or more building blocks each at a single concentration. The
concentration can differ from region to region. In an embodiment, a
concentration
of building block can increase as the regions become more distant from an edge
or
origin. The regions can be adjacent to one another or separated from one
another.
The area between separated building blocks can include, for example, lawn or
modified lawn. Such a gradient of building blocks can be envisioned as a
region
including one or more of the building bloclcs at different concentrations in
different
sub-regions. For example, one or more of the building blocks can be in
different
concentrations in bands on or across the region. For example, the one or more
22
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
building blocks can be at zero or low concentration at one edge of the region
and at a
higher concentration at the opposite edge of the region.
Figure 11 schematically illustrates a step gradient extending across a
support,
such as a slide or plate. Step gradient A includes steps a-i. The steps can be
contiguous or separated by lawn or modified lawn. Gradient A can be as
described
with respect to Figure 4 above. Step gradients can also be configured as
described
above for any one of Figures 5-10.
Figure 12 schematically illustrates a plate including both step gradient A and
a second gradient B. Step gradient A includes steps a-j. The steps can be
contiguous or separated by lama or modified lawn. Gradient B can be a step
gradient, a continuous gradient, or any other type of gradient. Gradients A
and B
can be independently as described with respect to Figure 4 above. Gradients A
and
B can also be independently configured as described above for any one of
Figures 5
10. In an embodiment, the plate of Figure 12 can include different building
blocks
or different concentrations of building blocks) in bands along, for example,
one
edge of a region. The second dimension of the region can be characterized by a
gradient of concentrations or identity of building blocks.
Methods of Making a Surface-Bound Molecular Gradient of Building Blocks
The present invention relates to a method of making a surface-bound
molecular gradient of building blocks. In an embodiment, this method includes
preparing a spot or region on a support, the spot or region including a
plurality of
building blocks immobilized on the support in a gradient. The method can
include
forming a plurality of spots or regions on a solid support, each spot or
region
including a plurality of immobilized building blocks, in which the density,
concentration, and number of building blocks varies across the support. In an
embodiment, the concentration of building blocks varies across a region on a
support
or between a plurality of spots or regions on a solid support.
In an embodiment, a gradient of building blocks is formed on a support
through the kinetics of the immobilization reactions described herein. The
kinetics
of immobilization can be influenced by a number of variables including, but
not
limited to: varying the concentration of the building blocks; varying the
concentration of reactive sites within or between spots or regions on the
surface,
23
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
support, or lawn; and varying the time of reaction. Additional factors, such
as
temperature, pH, solvation, and catalysis can also be used to influence the
immobilization conditions (e.g., rate of immobilization) and density of
immobilized
building block or lawn modifier.
In an embodiment, a method can be employed to produce a solid support
having on its surface a plurality of regions or spots, each region or spot
including a
different concentration or identity of building blocks and forming a gradient
across
the solid support. In an embodiment, the solid support is exposed at a
controlled
rate to the building blocks. In an embodiment, the solid support has a lawn of
reactive groups (e.g., amines) for the immobilization of building blocks.
In an embodiment, the solid support is placed non-horizontally (e.g.,
standing at an angle greater than 0°, typically between about
30° to 90°) in a
container so that the support stands on one end against a wall of the
container (e.g.,
beaker). The composition (e.g., solution) containing one or more building
blocks is
delivered into the container at a predetermined rate. For example, the
solution can
be introduced using a burette or pump. In an embodiment, the identity of the
building blocks in the composition being delivered changes with time. In an
embodiment, the concentration of building blocks in the composition being
delivered changes with time. When the solution reaches the top of the solid
support,
the flow of solution is stopped and the solid support is subsequently removed
from
contact with the solution. In an embodiment, the solid support is rinsed to
remove
any remaining free building blocks. In an embodiment, quenching or termination
agents are optionally applied to the solid support to limit further
immobilization.
In an embodiment, a portion of the solid support near the bottom of the
container has the longest time to react with the building block solution, and
therefore
has the highest concentration or density of immobilized building blocks. The
portion of the solid support near the top of the solid support will have the
least time
to react, and therefore has a lower concentration or density of immobilized
building
blocks. The time of solution exposure in combination with the rate of the
immobilization reaction determines the concentration or density of building
blocks
immobilized within regions or spots across the solid support.
In an embodiment, a constant flow of building block solution creates a linear
density gradient across the solid support. In an embodiment, the flow rate may
be
24
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
changed thereby altering the slope of the gradient. In an embodiment,
exponential
gradients are created by varying the flow rate exponentially in time, or by
simply
using, for example, an Erlenmeyer flask to contain the support in the
composition.
An Erlenmeyer flask is narrower towards the top, such that the solution
contacts the
solid support at times that decrease exponentially from bottom to top. In an
embodiment, turning the support, for example by either or 90° or
180°, with respect
to the first gradient and repeating the procedure creates a 2-dimensional or
multi-
directional gradient.
Any of a variety of known methods can be employed to produce a gradient
of building blocks across a region on a support, such as the surface of a
plate or
slide. For example, one or more building blocks can be eluted or diffused
across the
region to form a gradient. For example, one or more of the building blocks can
be
applied using a moving screen to form a gradient. For example, one or more of
the
building blocks can be poured into the center of the region and eluted or
diffused
across the region to form a gradient. In an embodiment, the solution of
building
blocks can be dispersed across a solid substrate by liquid or vapor phase
diffusion.
In an embodiment, building blocks can be distributed across a solid surface by
diffusion through a matrix.
In an embodiment, a surface-bound molecular gradient can be made utilizing
an energy-catalyzed (e.g., irradiation-catalyzed or initiated) chemistry to
immobilize
building blocks on the surface, support, or lawn. In an embodiment, the
surface can
be exposed to free building blocks (e.g., in solution). The surface can then
be
exposed to an energy source to catalyze the immobilization reaction. In an
embodiment, either the solution of free building blocks or surface comprises
an
energy-activated reactant (e.g., visible light, IJV iutiator).
In an embodiment, the energy can be applied non-uniformly to create a
gradient across the surface (e.g., high energy to low energy). The
differential energy
exposure can be achieved, for example, by source positioning relative to the
sample
or through the use of masks (either stationary or mobile). A variety of energy
sources can be used including, but not limited to: UV, IR, visible light,
corona
discharge, plasma treatment, radio frequency glow discharge, and ion
deposition
with increasing current. Various combinations of chemistries and energy
sources
are known.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, an initiator is associated with the support, surface, or
lawn. In an additional embodiment, an energy-activated reactant (e.g., LTV
initiator)
is present in a concentration gradient across the solid support. Where the
energy-
activated reactant is present in a concentration gradient, the solid support
is
uniformly exposed to the energy source. In an additional embodiment, the
energy-
activated reactant is present in a concentration gradient and the energy is
applied
non-uniformly to create either a 1D or 2D gradient.
Methods of Using Gradients of Artificial Receptors or Building Blocks
The present gradient of artificial receptor or building block can be contacted
with analyte or mixture of analytes by any of a variety of known methods. For
example, the analyte or mixture of analytes can be flowed across the support.
For
example, the analyte or mixture of analytes can be placed in contact with an
edge of
the support and diffusion can draw the analyte or mixture into, onto, and/or
across
the support and/or gradient. During or after contact with the analyte or
mixture of
analytes, one or more LE wins can be flowed or diffused into, onto, and/or
across
the support and/or gradient.
The present gradient of artificial receptor or building block can be employed
for separations of analytes such as proteins, nucleic acids, natural products,
or the
like in any of a variety of directions across a plate or slide. The present
gradient of
artificial receptor or building block can be employed for separating or
characterizing
mixtures of proteins, such as a proteome, e.g., a plasma proteome.
In an embodiment, the invention can include methods and/or gradients for
binding or detecting a protein, one or more of a plurality of proteins, or a
proteome.
Methods and systems for detection can include methods and systems for clinical
chemistry, environmental analysis, diagnostic assays, and for proteome
analysis.
For example, the gradient can be contacted with a sample including at
least~one
protein or one proteome. The building blocks making up the artificial
receptors can
be naive to the test ligand. Then, binding of one or more proteins to the
artificial
receptors can be detected. Next, the binding results can be interpreted to
provide
information about the sample, e.g., the proteome. In an embodiment, the
invention
includes a method for detecting a protein in a sample including contacting a
gradient
suitable for characterizing the protein with a sample suspected of containing
the
26
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
protein. The method can also include detecting or quantitating binding of the
protein to the gradient.
Figure 13 schematically illustrates an embodiment of a method for making
and using a gradient for evaluating an analyte or mixture of analytes. The
method
can include probing a receptor array with a sample known to contain the
analyte or
mixture of analytes of interest. The analyte or mixture thereof can be a
protein or
mixture of proteins. Probing can be followed by selecting receptors suitable
for
binding and analyte of interest or distinguishing among a mixture of analytes
of
interest. The building blocks from a selected receptors can be employed for
producing one or more gradients on a support. In an embodiment, a first
gradient on
the support can be a step ,gradient. In an embodiment, each step can be a
receptor
surface suitable for binding a particular analyte or analytes of interest. In
an
embodiment, a second gradient can be generally orthogonal to the first
gradient.
The second gradient can include variation on a characteristic of the building
blocks
such as described above with respect to Figures 4-9. The gradient on the
support
can then be probed to detect one or more of the analytes bound to the
gradient.
In an embodiment, each analyte or mixture of analytes (e.g., protein or
proteome) can provide a pattern of binding to the gradient. The pattern of
binding
can be characteristic of the analyte or mixture of analytes (e.g., protein or
proteome)
or a sample including the analyte or mixture of analytes (e.g., protein or
proteome).
The method can include storing a representation of the binding pattern as an
image
or a data structure. The representation of the binding pattern can be
evaluated either
by an operator or data processing system. The method can include such
evaluating.
A binding pattern from an unknown sample that matches the binding pattern for
a
particular protein then characterizes the unknown sample as containing that
analyte
or mixture of analytes (e.g., protein or proteome). A binding pattern from an
unknown sample that matches the binding pattern for a particular proteome then
characterizes the unknown sample as including or being that proteome or as
including or being the organism or cell having that proteome. Similarly, a
binding
pattern from an unknown sample can be evaluated against the patterns of a
plurality
of particular analyte or mixture of analytes (e.g., protein or proteome) and
the
sample can be characterized as containing one or more of the analytes (e.g.,
protein
or proteome). A plurality of binding patterns can be stored as a database.
27
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
An embodiment of the illustrated method can include creating a gradient.
This embodiment can also include compiling a database of the binding patterns
of
specific analyte or mixture of analytes (e.g., protein or proteome), for
example, by
probing the array with a plurality of individual analytes (e.g., proteins or
proteomes).
Contacting the array with an unidentified analyte or mixture of analytes
(e.g., protein
or proteome) can create a test binding pattern. The method can then compare
the
test binding pattern with the binding patterns of known analytes or mixtures
of
analytes (e.g., proteins or proteomes) in the database in order to
characterize or
classify the unidentified analyte, protein, proteome, or cell or organism. In
an
embodiment, the database and the gradient have already been constructed and
the
method involves probing the gradient with an unknown analyte or mixture of
analytes (e.g., protein or proteome) to create a test binding pattern and then
comparing this binding pattern with the binding patterns in the database in
order to
characterize or classify the unidentified analyte, protein, proteome, or cell
or
organism.
A proteome gradient can be contacted with samples from an organism, cell,
or tissue of interest. Proteins that bind to the proteome gradient can
characterize or
detect the organism, cell or tissue; can indicate a disorder caused by the
organism or
affecting the cell or tissue; can indicate successful therapy of a disorder
caused by
the organism or affecting the cell or tissue; characterize disease processes;
identify
therapeutic leads or strategies; or the like.
Molecular Descriptors
Any of a variety of molecular descriptors can be employed to describe or
characterize the change that makes up a gradient. Suitable molecular
descriptors
include constitutional descriptors, electrostatic descriptors, geometrical
descriptors,
physicochemical descriptors, topological descriptors, and the like. Such
descriptors
are known and can be calculated using commercially available software
packages.
Suitable constitutional descriptors include: formal charge, fraction of
rotatable bonds, molecular formula, molecular weight, the number of aromatic
bonds, the number of double bonds, the number of h-bond acceptors, the number
of
h-bond donors, the number of negative chargable groups, the number of negative-
charged groups, the number of positive chargable groups, the number of
positive-
2~
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
charged groups, the number of rigid bonds, the number of rings, the number of
rotatable bonds, the number of single bonds, the number of total atoms, the
number
of triple bonds, ratio donors to acceptor, the number of negative-charged
groups, the
number of positive chargable groups, the number of positive-charged groups,
functional groups. Such descriptors are known and can be calculated using
commercially available software packages.
Suitable electrostatic descriptors include: charge polarization, local dipole
index, maximum negative charge, maximum positive charge, maximum positive
hydrogen charge, polarity parameter, relative negative charge, relative
positive
charge, total absolute atomic charge, total negative charge, total positive
charge,
charge partial surface area descriptors. Such descriptors are known and can be
calculated using commercially available software packages.
Suitable charge partial surface area descriptors include: ACGD, charge on
acceptors atoms 1st type (CHAAl), charge on acceptors atoms 2nd type (CHAA2)
charge on acceptors atoms 3rd type (CHAA3),Charge on donatable hydrogens 1st
type (CHDH1), Charge on donatable hydrogens 2nd type (CHDH2), Charge on
donatable hydrogens 3rd type (CHDH3), CHGD, Difference in charged partial
surface area (DPSAl), Difference in total charge weighted surface area
(DPSA2),
Difference in atomic charge weithed surface area (DPSA3), Fractional charged
partial negative surface area 1st type (FNSA1), Fractional charged partial
negative
surface area 2nd type (FNSA2), Fractional charged partial negative surface
area 3rd
type (FNSA3), Fractional charged partial positive surface area 1st type
(FPSA1),
Fractional charged partial positive surface area 2nd type (FPSA2), Fractional
charged partial positive surface area 3rd type (FPSA3), HRNCG, HRNCS, HRPCG,
HRPCS, Hydrophobic SA - MPE,OE, Negative charged polar SA ? MPEOE, Partial
negative surface area 1st type (PNSA1), Partial negative surface area 2nd type
(PNSA2), Partial negative surface area 3rd type (PNSA3), Positive charged
polar SA
- MPEOE, Partial positive surface area 1st type (PPSA1), Partial positive
surface
area 2nd type (PPSA2) Partial positive surface area 3rd type (PPSA3), Relative
negative charge surface area (RNCS), Relative positive charge surface area
(RPCS),
Surface area on acceptor atoms 1st type (SAAAl), Surface area on acceptor
atoms
2nd type (SAAA2), Surface area on acceptor atoms 3rd type (SAAA3), Surface
area
on donor hydrogens 1st type (SADH1), Surface area on donor hydrogens 2nd type
29
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
(SADH2), Surface area on donor hydrogens 3rd type (SADH3), Surface weighted
charged area on acceptor atoms 1st type (SCAAl), Surface weighted charged area
on acceptor atoms 2nd type (SCAA2), Surface weighted charged area on acceptor
atoms 3rd type (SCAA3), Surface weighted charged area on donor hydrogens 1st
type (SCDH1), Surface weighted charged area on donor hydrogens 2nd type
(SCDH2), Surface weighted charged area on donor hydrogens 3rd type (SCDH3),
Surface weighted charged area on acceptor atoms 1st type (SCAAl), Surface
weighted charged area on acceptor atoms 2nd type (SCAA2), Surface weighted
charged area on acceptor atoms 3rd type (SCAA3), Surface weighted charged area
on donor hydrogens 1st type (SCDH1), Surface weighted charged area on donor
hydrogens 2nd type (SCDH2), Surface weighted charged area on donor hydrogens
3rd type (SCDH3), Surface weighted charged partial negative surface area 1 st
type
(WNSAl), Surface weighted charged partial negative surface area 2nd type
(WNSA2), Surface weighted charged partial negative surface area 3rd type
(WNSA3), Surface weighted charged partial positive surface area 1st
type(WPSAl),
Surface weighted charged partial positive surface area 2nd type (WPSA2),
Surface
weighted charged partial positive surface area 3rd type (WPSA3). Such
descriptors
are known and can be calculated using commercially available software
packages.
Suitable geometric descriptors include: 2D van der Waals surface area
(VSA), 2D van der Waals volume, Fraction of 2D-VSA chargable groups, Fraction
of 2D-VSA hydrophobic, Fraction of 2D-VSA polar, Topological Polar Surface
Area, 2D van der Waals chemical features surface area. Such descriptors are
known
and can be calculated using commercially available software packages.
Suitable 2D van der Waals chemical features surface area descriptors
include: 2D-VSA Hbond acceptor, 2D-VSA Hbond all, 2D-VSA Hbond donor, 2D-
VSA hydrophobic, 2D-VSA hydrophobic sat, 2D-VSA hydrophobic unsat, 2D-
VSA negative chargable groups, 2D-VSA other, 2D-VSA polar, 2D-VSA positive
chargable groups. Such descriptors are known and can be calculated using
commercially available software packages.
Suitable physicochemical descriptors include: AlogP98 value, AMR value,
Buffer solubility, Polarizability Miller, Polarizability MPEOE, SK BP, SK MP,
SKIogP value, SklogPvp, SKIogS value, SKIogS buffer, Solvation Free Energy,
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Vapor pressure, Water solubility, AlogP98 atomic types. Such descriptors are
known and can be calculated using commercially available software packages.
Suitable topological descriptors include: Autocorrelation descriptors
(AlogP98) (e.g., ATS Geary 110 AlogP98, ATS Moran 010 AlogP98, ATS
Moreau-Bruto 010 AlogP98, ATS Moreau-Bruto 010 AlogP98 average);
Autocorrelation descriptors (Mass) (e.g., ATS Geary 110 Mass, ATS Moran 010
Mass, ATS Moreau-Bruto 010 Mass, ATS Moreau-Bruto 010 Mass average);
Autocorrelation descriptors (Polarizability) (e.g., ATS Geary 110
Polarizability,
ATS Moran 010 Polarizability, ATS Moreau-Bruto 010 Polarizability, ATS
Moreau-Bruto 010 Polarizability average); Adjacency and distance matrix based
descriptors (e.g., 1st Zagreb,2-MTI, 2-MTI prime, 2nd Zagreb, Balaban index
JX,
Balaban index JY, Centralization distance matrix, Degree complexity,
Dispersion distance matrix, Eccentric adjacency index, Eccentric comlectivity
index, Edge connectivity index, Edge Gutman MTI, Edge Hyper Wiener index,
Edge MTI, Edge Wiener index, Graph diameter, Graph distance complexity, Graph
distance index, Graph Petitjean, Graph radius, Graph vertex complexity, Gutman
MTI,Harary index Hyper Wiener index, Mean distance deviation, Mean square
distance index, Odd-even index, Platt number, Pogliani index, Quadratic index,
Ramification index, Ring degree-distance index, Rouvray index, Superpendentic
index, Unipolarity_distance matrix, Variation distance matrix, Vertex degree-
distance index, Wiener index, Xu); Atom-type Electrotopological state indices,
E-
state; Atom-type AI topological indices (AI); Autocorrelation descriptors
(Charge)
(e.g., ATS Geary 110 Charge, ATS Moran 010 Charge, ATS Moreau-Bruto 010
Charge, ATS Moreau-Bruto 010 Charge average); Autoconelation descriptors
(Electronegativity) (e.g., ATS Geary 110 Electronegativity, ATS Moran 010
Electronegativity, ATS Moreau-Bruto 010 Electronegativity, ATS Moreau-Bruto
010 Electronegativity average); Autocorrelation descriptors (E-state) (e.g.,
ATS
Geary 110 E-state ATS Moran 010 E-state ATS Moreau-Bruto 010 E-state ATS
Moreau-Bruto 010 E-state average); Autocorrelation descriptors (VDW radius)
(e.g., ATS Geary 110 VDW radius ATS Moran 010 VDW radius ATS Moreau-
Bruto 010 VDW radius ATS Moreau-Bruto 010 VDW radius average); BCUT
descriptors (Charge) (e.g., BCUT highest eigenvalue 1~5 MPEOE charge BCUT
lowest eigenvalue 1~5 MPEOE charge); BCUT descriptors (Electronegativity)
(e.g.,
31
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
BCUT highest eigenvalue 1~5 Electronegativity, BCUT lowest eigenvalue 1~5
Electronegativity); BCUT descriptors (AlogP98) (e.g., BCUT highest eigenvalue
1~5 AlogP98"BCUT lowest eigenvalue 1~5 AlogP98); BOUT descriptors (Mass)
(e.g., BCUT highest eigenvalue 1~5 Mass BOUT lowest eigenvalue 1~5 Mass);
BOUT descriptors (Polarizability) (e.g., BCUT highest eigenvalue 1~5
Polarizability, BCUT lowest eigenvalue 1~5 Polarizability); BCUT descriptors
(VDW radius) (e.g., BCUT highest eigenvalue 1~5 VDW radius, BOUT lowest
eigenvalue 1~5 VDW radius); BCUT descriptors (E-state) (e.g., BCUT highest
eigenvalue 1~5 E-state, BOUT lowest eigenvalue 1~5 E-state); Delta
connectivity
indices (e.g., Delta Chi 0, Delta Chi 1, Delta Chi 2, Delta Chi 3 cluster,
Delta Chi 3
path, Delta Chi 4 cluster, Delta Chi 4 path, Delta Chi 4 path/cluster, Delta
Chi 5
path); Difference connectivity indices (e.g., Difference chi 0, Difference chi
1,
Difference chi 2, Difference chi 3, Difference chi 4, Difference chi 5);
Galvez
topological charge indices (e.g., Bound charge index 010, Charge index 010,
Charge transfer index algebraic, Global topological charge index, Valence
bound
charge index 010, Valence charge index 010); Atom-type Hydrogen
electrotopological state indices, HE-state; Information content related
descriptors
(e.g., BIC, CIC, I adj deg equ, I adj deg mag, I adj equ, I adj mag, I dist
equ,
I dist mag, I edge adj deg equ, I edge adj deg mag, I edge adj equ,
I edge adj mag, I edge dist equ, I edge dist mag, IAC total, IC, SIC); Kappa
indices (e.g., Kier alpha 1, Kier alpha 2, Kier alpha 3, Kier flexibility,
Kier shape 1,
Kier shape 2, Kier shape 3, Kier steric descriptor, Kier symmetry index); Kier
&
Hall molecular connectivity indices (e.g., Chi 0, Chi 1, Chi 2, Chi 3 cluster,
Chi 3
path, Chi 4 cluster, Chi 4 path, Chi 4 path/cluster, Chi 5 path, Total
structure
connectivity index); Kier & Hall valence connectivity indices (e.g., VChi 0,
VChi 1,
VChi 2, VChi 3 cluster, VChi 3 path, VChi 4 cluster, VChi 4 path, VChi 4
path/cluster, VChi S path); Molecular walk count (e.g., Molecular walk count 2
Molecular walk count 3 Molecular walk count 4 Molecular walk count 5 Path/walk
2 Path/walk 3 Path/walk 4 Path/wallc 5); Narumi topological indices (e.g.,
Narumi
ATI, Narumi GTI, Narumi HTI); Subgraph count indices (e.g., SC-0, SC-1, SC-10
path, SC-2, SC-3 cluster, SC-3 path, SC-4 cluster, SC-4 path, SC-4
path/cluster, SCr
5 path, SC-6 path, SC-7 path, SC-8 path, SC-9 path); Solvation molecular
connectivity indices (e.g., Solvation chi 0, Solvation chi 1, Solvation chi 2,
32
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Solvation chi 3 cluster, Solvation chi 3 path, Solvation chi 4 cluster,
Solvation chi 4
path, Solvation chi 4 path/cluster, Solvation chi 5 path); Valence shell count
(e.g.,
VS-0, VS-1, VS-2, VS-3, VS-4, VS-5). Such descriptors are known and can be
calculated using commercially available software packages.
Methods for Detecting
Contacting a gradient of an artificial receptor or building block with a test
ligand can identify or characterize the test ligand. Binding of the test
ligand to the
gradient can produce a detectable signal. The detectable signal can be
produced, for
example, through mechanisms and properties such as scattering, absorbing or
emitting light, producing or quenching fluorescence or luminescence, producing
or
quenching an electrical signal, and the like. Spectroscopic detection methods
include use of labels or enzymes to produce light for detection by optical
sensors or
optical sensor gradients. The light can be ultraviolet, visible, or infrared
light, wluch
can be produced and/or detected through fluorescence, fluorescence
polarization,
chemiluminescence, bioluminescence, or chemibioluminescence.
Systems and methods for detecting electrical conduction, and changes in
electrical conduction, include ellipsometry, surface plasmon resonance,
capacitance,
conductometry, surface acoustic wave, quartz crystal microbalance, Love-wave,
infrared evanescent wave, enzyme labels with electrochemical detection,
nanowire
field effect transistors, MOSFETS - metal oxide semiconductor field effect
transistors, CHEMFETS - organic membrane metal oxide semiconductor field
effect
transistors, ICP - intrinsically conducting polymers, FRET - fluorescence
resonance
energy transfer.
Apparatus that can detect binding to or signal from a gradient includes UV,
visible, or infrared spectrometer, fluorescence or luminescence spectrometer,
surface
plasmon resonance, surface acoustic wave or quartz crystal microbalance
detectors,
pH, voltammetry or amperometry meters, radioisotope detector, or the like.
The detectable signal can originate from, for example, a signaling moiety
incorporated into the gradient or a signaling moiety added to the gradient.
The
signal can also be intrinsic to the gradient or to the test ligand. The signal
can come
from, for example, the interaction of the test ligand with the gradient or the
interaction of the test ligand with a signaling moiety that has been
incorporated into
33
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
the gradient. In an embodiment, the present method can include selecting a
gradient
for which binding induces a change in the signal from the signaling moiety,
e.g., a
fluorescent moiety. Such a change can signal binding to the gradient.
The present gradient can be part of products used in: analyzing a genome
and/or proteome; pharmaceutical development; detect~rs for any of the test
ligands;
drug of abuse diagnostics or therapy; hazardous waste analysis or remediation;
toxic
agent alert or intervention; disease diagnostics or therapy; cancer
diagnostics or
therapy; food chain contamination analysis or remediation; and the like.
Test Li~ands
The test ligand can be any ligand for which binding to an array or surface can
be detected. The test ligand can be a pure compound, a mixture, or a "dirty"
mixture
containing a natural product or pollutant. Such dirty mixtures can be tissue
homogenate, biological fluid, soil sample, water sample, or the like.
Test ligands include prostate specific antigen, other cancer markers, insulin,
warfarin, other anti-coagulants, cocaine, other drugs-of abuse, markers for E.
coli,
markers for Salfnonella sp., markers for other food-borne toxins, food-borne
toxins,
markers for Smallpox virus, markers for anthrax, markers for other possible
toxic
biological agents, pharmaceuticals and medicines, pollutants and chemicals in
hazardous waste, toxic chemical agents, markers of disease, pharmaceuticals,
pollutants, biologically important cations (e.g., potassium or calcium ion),
polynucleotides, peptides, carbohydrates, enzymes, bacteria, viruses, mixtures
thereof, and the like. In certain embodiments, the test ligand can be at least
one of
small organic molecules, inorganic/organic complexes, metal ion, mixture of
proteins, protein, nucleic acid, mixture of nucleic acids, mixtures thereof,
and the
like.
Suitable test ligands include any compound or category of compounds
described elsewhere in this document as being a test ligand, including, for
example,
the microbes, proteins, cancer cells, drugs of abuse, and the like described
above.
Methods of Making an Artificial Receptor
The present invention relates to a method of making an artificial receptor or
immobilized combination of building blocks. In an embodiment, this method
34
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
includes preparing a spot or region on a support, the spot or region including
a
plurality of building blocks immobilized on the support. The method can
include
forming a plurality of regions on a solid support, each region including a
plurality of
building blocks, and immobilizing (e.g., reversibly) a plurality of building
blocks on
the solid support in each region.
The method can include mixing a plurality of building blocks and employing
the mixture in forming the region(s). Alternatively, the method can include
immobilizing (e.g., reversibly) individual building blocks on the support.
Coupling
building blocks to the support can employ covalent bonding or noncovalent
interactions. Suitable noncovalent interactions include interactions between
ions,
hydrogen bonding, van der Waals interactions, and the like. In an embodiment,
the
support can be fwctionalized with moieties that can engage in covalent bonding
or
noncovalent interactions. Forming regions can yield a gradient of
heterogeneous
combinations of building blocks. The method can apply or spot building blocks
onto a support in combinations of 2, 3, 4, or more building blocks.
In an embodiment, the present method can be employed to produce a solid
support having on its surface a plurality of regions, each region including a
plurality
of building blocks. For example, the method can include immobilizing on a
glass
slide in each of a plurality of regions a plurality of building blocks. Such a
region
can be referred to as including heterogeneous building blocks.
In an embodiment, the present method includes making a receptor surface.
Making a receptor surface can include forming a region on a solid support, the
region including a plurality of building blocks, and immobilizing (e.g.,
reversibly)
the plurality of building blocks to the solid support in the region. The
method can
include mixing a plurality of building blocks and employing the mixture in
forming
the region or regions. Alternatively, the method can include applying
individual
building bloclcs in a region on the support. Forming a region on a support can
be
accomplished, for example, by soaking a portion of the support with the
building
block solution. The resulting coating including building blocks can be
referred to as
including heterogeneous building blocks.
A region including a plurality of building blocks can be independent and
distinct from other regions including a plurality of building blocks. In an
embodiment, one or more regions including a plurality of building blocks can
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
overlap to produce a region including the combined pluralities of building
blocks.
In an embodiment, two or more regions including a single building block can
overlap to form one or more regions each including a plurality of building
blocks.
The overlapping regions can be envisioned, for example, as portions of overlap
in a
Ven diagram, or as portions of overlap in a pattern like a plaid or tweed.
In an embodiment, the method produces a surface with a density of building
blocks sufficient to provide interactions of more than one building block with
a
ligand. That is, the building blocks can be in proximity to one another.
Proximity
of different building blocks can be detected by determining different (e.g.,
greater)
binding of a test ligand to a spot or surface including a plurality of
building blocks
compared to a spot or surface including only one of the building blocks.
In an embodiment, the method includes forming an array including one or
more regions that function as controls for validating or evaluating binding to
gradients of the present invention. In an embodiment, the method includes
forming
one or more regions that function as controls for validating or evaluating
binding to
gradients of the present invention. Such a control region can include no
building
block, only a single building block, only functionalized lawn, or combinations
thereof.
The method cane immobilize (e.g., reversibly) building blocks on supports
using known methods for immobilizing compounds of the types employed as
building blocks. Coupling building blocks to the support can employ covalent
bonding or noncovalent interactions. Suitable noncovalent interactions include
interactions between ions, hydrogen bonding, van der Waals interactions, and
the
like. In an embodiment, the support can be functionalized with moieties that
can
engage in reversible covalent bonding, moieties that can engage in noncovalent
interactions, a mixture of these moieties, or the like.
In an embodiment, the support can be functionalized with moieties that can
engage in covalent bonding, e.g., reversible covalent bonding. The present
invention can employ any of a variety of the numerous known functional groups,
reagents, and reactions fox forming reversible covalent bonds. Suitable
reagents for
forming reversible covalent bonds include those described in Green, TW; Wuts,
PGM (1999), Protective Groups in Organic Synthesis Third Edition, Wiley-
Interscience, New York, 779 pp. For example, the support can include
functional
36
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
groups such as a carbonyl group, a carboxyl group, a silane group, boric acid
or
ester, an amine group (e.g., a primary, secondary, or tertiary amine, a
hydroxylamine, a hydrazine, or the like), a thiol group, an alcohol group
(e.g.,
primary, secondary, or tertiary alcohol), a diol group (e.g., a 1,2 diol or a
1,3 diol), a
phenol group, a catechol group, or the like. These functional groups can form
groups with reversible covalent bonds, such as ether (e.g., alkyl ether, silyl
ether,
thioether, or the like), ester (e.g., alkyl ester, phenol ester, cyclic ester,
thioester, or
the like), acetal (e.g., cyclic acetal), ketal (e.g., cyclic ketal), silyl
derivative (e.g.,
silyl ether), boronate (e.g., cyclic boronate), amide, hydrazide, imine,
carbamate, or
the like. Such a functional group can be referred to as a covalent bonding
moiety,
e.g., a first covalent bonding moiety.
A carbonyl group on the support and an amine group on a building block can
form an imine or Schiff's base. The same is true of an amine group on the
support
and a carbonyl group on a building block. A carbonyl group on the support and
an
alcohol group on a building block can form an acetal or lcetal. The same is
true of an
alcohol group on the support and a carbonyl group on a building block. A thiol
(e.g., a first thiol) on the support and a thiol (e.g., a second thiol) on the
building
block can form a disulfide.
A carboxyl group on the support and an alcohol group on a building block
can form an ester. The same is true of an alcohol group on the support and a
carboxyl group on a building block. Any of a variety of alcohols and
carboxylic
acids can form esters that provide covalent bonding that can be reversed in
the
context of the present invention. For example, reversible ester linkages can
be
formed from alcohols such as phenols with electron withdrawing groups on the
aryl
ring, other alcohols with electron withdrawing groups acting on the hydroxyl-
bearing carbon, other alcohols, or the like; and/or carboxyl groups such as
those with
electron withdrawing groups acting on the acyl carbon (e.g., nitrobenzylic
acid, R-
CF2-COOH, R-CClz-COOH, and the like), other carboxylic acids, or the like.
In an embodiment, the support, matrix, or lawn can be functionalized with
moieties that can engage in noncovalent interactions. For example, the support
can
include functional groups such as an ionic group, a group that can hydrogen
bond, or
a group that can engage in van der Waals or other hydrophobic interactions.
Such
37
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
functional groups can include cationic groups, anionic groups, lipophilic
groups,
amphiphilic groups, and the like.
In an embodiment, the support, matrix, or lawn includes a charged moiety
(e.g., a first charged moiety). Suitable charged moieties include positively
charged
moieties and negatively charged moieties. Suitable positively charged moieties
(e.g., at neutral pH in aqueous compositions) include amines, quaternary
ammonium
moieties, ferrocene, or the like. Suitable negatively charged moieties (e.g.,
at neutral
pH in aqueous compositions) include carboxylates, phenols substituted with
strongly
electron withdrawing groups (e.g., tetrachlorophenols), phosphates,
phosphonates,
phosphinates, sulphates, sulphonates, thiocarboxylates, hydroxamic acids, or
the
like.
In an embodiment, the support, matrix, or lawn includes groups that can
hydrogen bond (e.g., a first hydrogen bonding group), either as donors or
acceptors.
The support, matrix, or lavcnl can include a surface or region with groups
that can
hydrogen bond. For example, the support, matrix, or lawn can include a surface
or
region including one or more carboxyl groups, amine groups, hydroxyl groups,
carbonyl groups, or the like. Ionic groups can also participate in hydrogen
bonding.
In an embodiment, the support, matrix, or lawn includes a lipophilic moiety
(e.g., a first lipophilic moiety). Suitable lipophilic moieties include
branched or
straight chain C6_3s alkyl, Ca_z4 alkyl, Clz-z4 alkyl, Clz_la alkyl, or the
like; C6_36
alkenyl, Ca_z4 alkenyl, Clz_z4 alkenyl, Clz-is alkenyl, or the like, with, for
example, 1
to 4 double bonds; C6_36 alkynyl, Ca_z4 alkynyl, Clz-z4 alkynyl, Clz_la
alkynyl, or the
like, with, for example, 1 to 4 triple bonds; chains with 1-4 double or triple
bonds;
chains including aryl or substituted aryl moieties (e.g., phenyl or naphthyl
moieties
at the end or middle of a chain); polyaromatic hydrocarbon moieties;
cycloalkane or
substituted alkane moieties with numbers of carbons as described for chains;
combinations or mixtures thereof; or the like. The alkyl, alkenyl, or alkynyl
group
can include branching; within chain functionality like an ether group;
terminal
functionality like alcohol, amide, carboxylate or the like; or the like. A
lipophilic
moiety like a quaternary ammonium lipophilic moiety can also include a
positive
charge.
38
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Artificial Receptors
A candidate artificial receptor, a lead artificial receptor, or a working
artificial receptor includes combination of building blocks immobilized (e.g.,
reversibly) on, for example, a support. An individual artificial receptor can
be a
heterogeneous building block region on a slide or a plurality of building
blocks
coated on a slide, tube, or well. The building blocks can be immobilized
through
any of a variety of interactions, such as covalent, electrostatic, or
hydrophobic
interactions. For example, the building block and support or lawn can each
include
one or more functional groups or moieties that can form covalent,
electrostatic,
hydrogen bonding, van der Waals, or like interactions.
An gradient of candidate artificial receptors can be a commercial product
sold to parties interested in using the gradients as implements in developing
methods
for detecting or characterizing test ligands of interest. In an embodiment, a
useful
gradient of candidate artificial receptors includes at least one glass slide,
the at least
one glass slide including a region including a gradient.
One or more lead artificial receptors can be developed from a plurality of
candidate artificial receptors. In an embodiment, a lead artificial receptor
includes a
combination of building blocks and binds detectable quantities of test ligand
upon
exposure to, for example, several picomoles of test ligand at a concentration
of 1,
0.1, or 0.01 ~,g/ml, or at 1, 0.1, or 0.01 ng/ml test ligand; at a
concentration of 0.01
~,g/ml, or at l, 0.1, or 0.01 ng/ml test ligand; or a concentration of 1, 0.1,
or 0.01
ng/ml test ligand.
In an embodiment, a gradient according to the present invention includes a
combination of building blocks and binds categorizing or identifying
quantities of
test ligand upon exposure to, for example, several picomoles of test ligand at
a
concentration of 100, 10, 1, 0.1, 0.01, or 0.001 ng/ml test ligand; at a
concentration
of 10, 1, 0.1, 0.01, or 0.001 ng/ml test ligand; or a concentration of 1, 0.1,
0.01, or
0.001 ng/ml test ligand.
Artificial receptors, particularly candidate or lead artificial receptors, can
be
in the form of an array of artificial receptors. Each spot is a candidate
artificial
receptor and a combination of building blocks. The array can also be
constructed to
include lead artificial receptors. For example, the array of artificial
receptors can
include combinations of fewer building blocks and/or a subset of the building
39
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
blocks. In an embodiment, an array of candidate artificial receptors includes
building blocks of general Formula 2 (shown hereinbelow), with REl being B1,
B2,
B3, B3a, B4, B5, B6, B7, B8, or B9 (shown hereinbelow) and with REZ being Al,
A2, A3, A3a, A4, A5, A6, A7, A8, or A9 (shown hereinbelow). In an embodiment,
the framework is tyrosine.
In. an embodiment, the artificial receptor of the invention includes a
plurality
of building blocks coupled to a support. In an embodiment, the plurality of
building
blocks can include or be building blocks of Formula 2 (shown below). An
abbreviation for the building block including a linker, a tyrosine framework,
and
recognition elements AxBy is TyrAxB'y. In an embodiment, a candidate
artificial
receptor can include combinations of building blocks of formula TyrAlBl,
TyrA2B2, TyrA2B4, TyrA2B6, TyrA2B8, TyrA3B3, TyrA4B2, TyrA4B4,
TyrA4B6, TyrA4B8, TyrA5B5, TyrA6B2, TyrA6B4, TyrA6B6, TyrA6B8,
TyrA7B7, TyrA8B2, TyrA8B4, TyrA8B6, or TyrA8B8.
The present artificial receptors can employ any of a variety of supports to
which building blocks or other array materials can be coupled. For example,
the
support can be glass or plastic; a slide, a tube, or a well; an optical fiber;
or the like.
The present invention includes obtaining a result employing a gradient
according to the invention and communicating or forwarding that result. ,
Further,
the result obtained from the gradient can be processed are configured to
provide
patterns or conclusions. Communicating or forwarding such patterns or
conclusions
is also a part of the present invention. Forwarding or communicating can
include
forwarding or communicating between a first and second location, which can be
remote from one another. Forwarding and communicating can include sending
and/or receiving. In an embodiment, the present invention includes forwarding
to a
remote location a result obtained from a method employing a gradient, building
block, or artificial receptor according to the present invention. In an
embodiment,
the present invention includes transmitting data representing a result or
reading
obtained employing a gradient, building block, or artificial receptor
according to the
present invention.
Remote objects can be separated by, for example, being in two different
buildings, being one or more miles apart, being 10 or more~miles apart, or
being 100
or more miles apart. Communicating can include transmitting data or other
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
information, for example, as electrical signals over any of a variety of
transmission
systems. Forwarding can include any mechanism for translocating an item such
as
data or other information from one location to another. Forwarding can include
physically transporting or transmitting the item, data, or information.
Building Blocks
The present invention relates to building blocks for making or forming
candidate artificial receptors. Building blocks can be designed, made, and
selected
to provide a variety of structural characteristics among a small number of
compounds. A building block can provide one or more structural characteristics
such as positive charge, negative charge, acid, base, electron acceptor,
electron
donor, hydrogen bond donor, hydrogen bond acceptor, free electron pair, ~
electrons, charge polarization, hydrophilicity, hydrophobicity, and the like.
A
building block can be bulky or it can be small.
A building, block can be visualized as including several components, such as
one or more frameworks, one or more linkers, and/or one or more recognition
elements. The framework can be covalently coupled to each of the other
building
block components. The linker can be covalently coupled to the framework. The
linker can be coupled to a support through one or more of covalent,
electrostatic,
hydrogen bonding, van der Waals, or like interactions. The recognition element
can
be covalently coupled to the framework. In an embodiment, a building block
includes a framework, a linker, and a recognition element. In an embodiment, a
building block includes a framework, a linker, and two recognition elements.
A description of general and specific features and functions of a variety of
building blocks and their synthesis can be found in copending U.S. Patent
Application Nos. 10/244,727, filed September 16, 2002, 10/813,568, filed March
29,2004, and Application No. PCT/LTS03/05328, filed February 19, 2003, each
entitled "ARTIFICIAL RECEPTORS, BUILDING BLOCKS, AND METHODS";
U.S. Patent Application Nos. 10/812,850 and 10/813,612, and application No.
PCT/LTS2004/009649, each filed March 29, 2004 and each entitled "ARTIFICIAL
RECEPTORS INCLUDING REVERSIBLY IMMOBILIZED BUILDING
BLOCKS, THE BUILDING BLOCKS, AND METHODS"; and U.S. Provisional
Patent Application Nos. 60/499,965, filed September 3, 2003, and 60/526,699,
filed
41
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
December 2, 2003, each entitled BUILDING BLOCKS FOR ARTIFICIAL
RECEPTORS. These patent documents include, in particular, a detailed written
description of function, structure, and configuration of building blocks,
framework
moieties, recognition elements, synthesis of building blocks, specific
embodiments
of building blocks, specific embodiments of recogution elements, and sets of
building blocks.
Embodiments of Building Blocks
The building block can include one or more functional groups, structural
features, or moieties that form the recognition moiety. For example, the
building
block can include one or more carboxyl, amine, hydroxyl, phenol, carbonyl, and
thiol groups, which can be a recognition moiety. For example, the building
block
can include one or more moieties with positive charge, negative charge, acid,
base,
electron acceptor, electron donor, hydrogen bond donor, hydrogen bond
acceptor,
free electron pair, ~r electrons, charge polarization, hydrophilicity,
hydrophobicity,
and the like. The building block can include two, three, or four such
functional
groups, structural features, or moieties.
The building block can include one or more functional groups, structural
features, or moieties that form all or part of the linking moiety. For
example, the
building block can include one or more carboxyl, amine, hydroxyl, phenol,
carbonyl,
and thiol groups, which can be a linking moiety. For example, the building
block
can include one or more moieties with positive charge, negative charge, acid,
base,
electron acceptor, electron donor, hydrogen bond donor, hydrogen bond
acceptor,
free electron pair, ~ electrons, charge polarization, hydrophilicity,
hydrophobicity,
and the like. The linking moiety is configured for coupling (e.g., reversibly)
to the
support.
A building block can be or can include any of a variety of compounds or
substructures. For example, a building block can be or include an amino acid
(natural or synthetic), a dipeptide, a monosaccharide, a disaccharide, another
carbohydrate, a mixture or combination thereof, or the like; a catalytic
moiety such
as a coenzyme, a metal, a metal complex, or the like; a polymer of up to 2000
carbon atoms (e.g., up to 4~ carbon atoms), e.g., a polyether,
polyethyleneimine, a
polyacrylamide, or like polymer; an a hydroxy acid, a thioic acid; an enzyme
42
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
inhibitor (e.g., a protease inhibitor (such as pepstatin), a statin, or the
like), a
receptor antagonist (e.g., a benzodiazepine), a receptor agonist, a
pharmaceutical, a
peptide hormone; a natural product, a starting material, intermediate, or end
product
of a metabolic pathway (e.g., glycolysis, the citric acid cycle,
photosynthesis,
glucogenesis, mitochondrial electron transport, oxidative phosphorylation,
biosynthetic pathways, catabolic pathways, or the like); a mixture or
combination
thereof, or the like. A building block can be a naturally occurring or
synthetic
compound; can be racemic, optically active, or achiral; can include positional
isomers of any specifically described structure; or can include
conformationally
restricted functional groups.
In an embodiment, the building block is or includes a monosaccharide. Any
of a variety of naturally occurring or synthetic monosaccharides can be
employed as
a building block. Suitable monosaccharides include pyranoses and furanoses,
such
as glucose, fructose, ribulose, allose, altrose, mannose, gulose, idose,
galactose,
talose, ribose, arabinose, xylose, lyxose, or the like; erythrose, threose, or
the like;
inositol, or the like; amino sugars, such as rhammose, fucose, glucosamine,
galactosamine, or the like; aldonic and uronic acids, such as gluconic acid,
glucuronic acid, glucaric acid, or the like; glycosides including these
monosaccharides; disaccharides or oligosaccharides including these
monosaccharides, such as sucrose, raffinose, gentianose, cellobiose, maltose,
lactose, trehalose, gentiobiose, meliobiose, or the like; a mixture or
combination
thereof, or the like.
hi an embodiment, the building block is or includes a disaccharide. Any of a
variety of naturally occurring or synthetic disaccharides can be employed as a
building block. Suitable disaccharides include disaccharides or
oligosaccharides
including the monosaccharides listed above. Such disaccharides include
sucrose,
raffmose, gentianose, cellobiose, maltose, lactose, trehalose, gentiobiose,
meliobiose, or the like; a mixture or combination thereof, or the like.
In an embodiment, the building block is or includes a carbohydrate. Any of
a variety of naturally occurring or synthetic carbohydrates can be employed as
a
building block. Suitable carbohydrates include cellulose, chitin, starch,
glycogen,
hyaluronic acid, chondroitin sulfates, keratosulfate, heparin, glycoproteins,
or the
like; a mixture or combination thereof, or the like.
43
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, the building block is or includes a catalytic moiety. Any
of a variety of naturally occurnng or synthetic catalytic moieties can be
employed as
or can be a moiety on a building block. Suitable catalytic moieties include
coenzymes, metals, metal complexes, pronucleophiles, proelectrophiles,
proreducing
agents, prooxidizing agents, general acid catalysts, general base catalysts, a
mixture
or combination thereof, or the like.
In an embodiment, the building block is or includes a metal binding or
complexing moiety. Any of a variety of naturally occurring or synthetic metal
binding or complexing moieties can be employed as or can be a moiety on a
building
block. Suitable metal binding or complexing moieties include synthetic and
naturally occurring porphyrin (e.g., etioporphyrin, mesoporphyrin,
protoporphyrin
(e.g., heme or hematin), coproporphyrin, tetraphenylporphyrin,
octaethylporphyrin,
or the like), a cobamide coenzyme (e.g., coenzyme B12, a cobalamin such as
methyl-
cobalamin, or the like), selenocysteine, selenomethionine, ferritin; naturally
occurring or synthetic complexes of magnesium, zinc, copper, chromium, iron,
cobalt, aluminum (e.g., A13+), titanium (e.g., Ti4+) or the like; salt
thereof, a mixture
or combination thereof, or the like.
In an embodiment, the building block is or includes a coenzyme (which can
also be called a prosthetic group or cofactor). Any of a variety of naturally
occurring or synthetic coenzymes can be employed as or can be a moiety on a
building block. Suitable coenzymes include a nicotinamide coenzyme (e.g., NAD,
NADH, NADP, NADPH, and the like), a flavin compound (e.g., FAD, FADH2,
FMN, FMNH2), a lipoic acid (e.g., oxidized or reduced lipoic acid), a
glutathione
(e.g., oxidized or reduced glutathione), an ascorbic acid, a quinone (e.g.,
ubiquinone,
vitamins K, or the like), a porphyrin (e.g., etioporphyrin, mesoporphyrin,
protoporphyrin (e.g., heme or hematin), coproporphyrin, or the like), a
nucleoside
(e.g., adenine, guanine, cytosine, thymine, uracil), a nucleotide (e.g., AMP,
ADP,
ATP, GMP, GDP, GTP, CMP, CDP, CTP, TMP, TDP, TTP, UMP, UDP, UTP), a
glycerol phosphate, a biotin (e.g., biotin or carboxybiotin), a pyridoxal
(e.g.,
pyridoxal phosphate, pyridoxal, pyridoxamine, pyridoxamine phosphate, or
Schiff's
bases thereof), an oxoglutaric acid (e.g., 2-oxoglutarate), a coenzyme A, a
carnitine,
a folic acid (e.g., tetrahydrofolic acid, 5-formyltetrahydrofolic acid, 10-
formyltetrahydrofolic acid, 5,10-methenyltetrahydrofolic acid, 5,10-
44
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
methylenetetrahydrofolic acid, 5-hydroxymethyltetrahydrofolic acid, 5-
formiminotetrahydrofolic acid, or the like), an adenosylhomocysteine, a
cobamide
coenzyme (e.g., coenzyme Blz, a cobalamin such as methyl-cobalamin, or the
like),
adenosine 3',5'-bisphosphate, thiamin diphosphate, ferritin, salt thereof, a
mixture or
combination thereof, or the like.
In an embodiment, the building block is or includes a polymer of up to 2000
carbon atoms (e.g., up to 48 carbon atoms). Such a polymer can be naturally
occurring or synthetic. Suitable polymers include a polyether or like polymer,
such
as a PEG, a polyethyleneimine, polyacrylate (e.g., substituted polyacrylates),
salt
thereof, a mixture or combination thereof, or the like. Suitable PEGS include
PEG
1500 up to PEG 20,000, for example, PEG 1450, PEG 3350, PEG 4500, PEG 8000,
PEG 20,000, and the like.
In an embodiment, the present building block can be or include a lipophilic
moiety. Suitable lipophilic moieties include one or more branched or straight
chain
C6_3s alkyl, Cs_24 alkyl, Cla-z4 alkyl, Cla-is alkyl, or the like; C6_3s
alkenyl, Cg_24
alkenyl, Cla-as alkenyl, Cla-is alkenyl, or the like, with, for example, 1 to
4 double
bonds; C6_3~ alkynyl, Cs_24 alkynyl, Cla-a4 alkynyl, Cla-is alkynyl, or the
like, with,
for example, 1 to 4 triple bonds; chains with 1-4 double or triple bonds;
chains
including aryl or substituted aryl moieties (e.g., phenyl or naphthyl moieties
at the
end or middle of a chain); polyaromatic hydrocarbon moieties; cycloalkane or
substituted alkane moieties with numbers of carbons as described for chains;
combinations or mixtures thereof; or the like. The alkyl, alkenyl, or alkynyl
group
can include branching; within chain functionality like an ether group;
terminal
functionality like alcohol, amide, carboxylate or the like; or the like.
Suitable building blocks include carboxylic acids (e.g., mono and di-
carboxylates) with the carboxylate appended to a lipophilic moiety, such as
one or
more branched or straight chain C6_3s alkyl, Cs_24 alkyl, Cla-za alkyl, Cla-is
allcyl, or
the like; C~_3~ alkenyl, Cs_24 alkenyl, Cla-a4 alkenyl, Cla-is alkenyl, or the
like, with,
for example, 1 to 4 double bonds; C~_3~ alkynyl, Cs_a4 alkynyl, Cla-za
allcynyl, Cla-is
allcynyl, or the like, with, for example, 1 to 4 triple bonds; chains with 1-4
double or
triple bonds; chains including aryl or substituted aryl moieties (e.g., phenyl
or
naphthyl moieties at the end or middle of a chain); or the like. Such
carboxylic acids
include arachidonic acid, linoleic acid, linolenic acid, oleic acid, and the
like. Such
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
carboxylic acids can be immobilized on a support through covalent bonding or
electrostatic interaction between
Suitable building blocks include carboxylic acids (e.g., mono and di-
carboxylates) with the carboxylate appended to a an organic radical, such as
one or
more branched or straight chain CZ_8 alkyl, arylalkyl, alkenyl, alkynyl, or
the like.
These carboxylic acids can include substituted aryl moieties (e.g., phenyl or
naphthyl moieties). Such carboxylic acids include acetic acid, propionic acid,
butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid,
oxalic
acid, malonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid,
benzoic
acid, and the like. Such carboxylic acids can be immobilized on a support
through
covalent bonding or electrostatic interaction between the carboxyl(ate) and
the
support or lawn.
In an embodiment, the building block is or includes an amino acid. Suitable
amino acids include a natural or synthetic amino acid. Amino acids include
carboxyl and amine functional groups. In their side chains, amino acids can
also
include a moiety with one or more of positive charge, negative charge, acid,
base,
electron acceptor, electron donor, hydrogen bond donor, hydrogen bond
acceptor,
free electron pair, ~- electrons, charge polarization, hydrophilicity, or
hydrophobicity.
Suitable amino acids include those with a functional group on the side chain.
The
side chain functional group can include, for natural amino acids, an amine
(e.g.,
alkyl amine, heteroaryl amine), hydroxyl, phenol, carboxyl, thiol, thioether,
or
amidino group.
Any of the natural amino acids can be employed as a building block. The
natural amino acids include aliphatic amino acids (e.g., alanine, valine,
leucine, and
isoleucine), hydroXyamino acids (e.g., serine, threonine, and tyrosine),
dicarboxylic
acids (e.g., glutamic acid and aspartic acid), amides (e.g., glutamine and
asparagine),
amino acids with basic sidechains (e.g., lysine, hydroxylysine, histidine, and
arginine), aromatic amino acids (e.g., histidine, phenylalanine, tyrosine,
tryptophan,
and thyroxine), sulfur containing amino acids (e.g., cysteine, cystine, and
methionine), imino acids (e.g., proline and hydroxyproline). Natural amino
acids
suitable for use as building blocks include, for example, serine, threonine,
tyrosine,
aspartic acid, glutamic acid, asparagine, glutamine, cysteine, lysine,
arginine,
histidine.
46
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Synthetic amino acids can include the naturally occurring side chain
functional groups or synthetic side chain functional groups which modify or
extend
the natural amino acids with alkyl, substituted alkyl, cycloalkyl,
heterocyclic,
substituted heterocyclic, aryl alkyl, aryl, heteroaryl, heteroaryl alkyl,
and'like
moieties and with carboxyl, amine, hydroxyl, phenol, carbonyl, or thiol
functional
groups. Suitable synthetic amino acids include N-substituted glycine and
oligomers
of N-substituted glycines. Suitable synthetic amino acids include ~i-amino
acids and
homo or ~i analogs of natural amino acids.
In an embodiment, the building block is or includes a dipeptide. Any of the
400 dipeptides including the 20 natural amino acids in any order can be
employed as
building blocks. Suitable dipeptides include muramyl dipeptide or the like.
In an embodiment the building block can be or include a therapeutic or
pharmacologically active agent. Suitable therapeutic or pharmacologically
active
agents include a nitrate, nitric oxide, a nitric oxide promoter, nitric oxide
donors,
dipyridamole, or another vasodilator; HYTRIN~ or another antihypertensive
agent;
a glycoprotein IIb/IIIa inhibitor (abciximab) or another inhibitor of surface
glycoprotein receptors; aspirin, ticlopidine, clopidogrel or another
antiplatelet agent;
colchicine or another antimitotic, or another microtubule inhibitor; a
retinoid or
another antisecretory agent; cytochalasin or another actin inhibitor;
methotrexate or
another antimetabolite or antiproliferative agent; tamoxifen citrate, TAXOL~,
paclitaxel, or derivatives thereof, rapamycin, vinblastine, vincristine,
vinorelbine,
etoposide, tenopiside, dactinomycin (actinomycin D), daunorubicin,
doxorubicin,
idarubicin, an anthracycline, mitoxantrone, bleomycin, plicamycin
(mithramycin),
mitomycin, mechlorethamine, cyclophosphamide and its analogs, chlorambucil, an
ethylenimine, a methylmelamine, an alkyl sulfonate (e.g., busulfan), a
nitrosourea
(carmustine, etc.), streptozocin, methotrexate (used with many indications),
fluorouracil, floxuridine, cytarabine, mercaptopurine, thioguanine,
pentostatin, 2-
chlorodeoxyadenosine, cisplatin, carboplatin, procarbazine, hydroxyurea, or
other
anti-cancer chemotherapeutic agents; cyclosporin, tacrolimus (FK-506),
azathioprine, mycophenolate mofetil, mTOR inhibitors, or another
immunosuppressive agent; cortisol, cortisone, dexamethasone, dexamethasone
sodium phosphate, dexamethasone acetate, a dexamethasone derivative,
betamethasone, fludrocortisone, prednisone, prednisolone, 6U-
methylprednisolone,
47
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
triamcinolone (e.g., triamcinolone acetonide), or another steroidal agent;
trapidil (a
PDGF antagonist); dopamine, bromocriptine mesylate, pergolide mesylate, or
another dopamine agonist; captopril, enalapril or another angiotensin
converting
enzyme (ACE) inhibitor; angiotensin receptor blockers; ascorbic acid, alpha
tocopherol, deferoxamine, a 21-aminosteroid (lasaroid) or another free radical
scavenger, iron chelator or antioxidant; estrogen or another sex hormone; AZT
or
another antipolymerase; acyclovir, famciclovir, rimantadine hydrochloride,
ganciclovir sodium, Norvir, Crixivan, a methyl-1-adamantanemethylamine,
hydroxy-ethoxylnethylguanine, adamantanamine, 5-iodo-2'-deoxyuridine,
trifluorothymidine, adenine arabinoside, or another antiviral agent; 5-
aminolevulinic
acid, meta-tetrahydroxyphenylchlorin, hexadecafluorozinc phthalocyanine,
tetramethyl hematoporphyrin, rhodamine 123 or other photodynamic therapy
agents;
PROSCAR~, HYTRIN~ or other agents for treating benign prostatic hyperplasia
(BHP); mitotane, aminoglutethimide, breveldin, acetaminophen, etodalac,
tolmetin,
ketorolac, ibuprofen and derivatives, mefenamic acid, meclofenamic acid,
piroxicam, tenoxicam, phenylbutazone, oxyphenbutazome, nabumetone, auranofin,
aurothioglucose, gold sodium thiomalate, a mixture of any of these, or
derivatives of
any of these.
In an embodiment, the building block can be or can include an antibiotic.
Examples of antibiotics include penicillin, tetracycline, chloramphenicol,
minocycline, doxycycline, vancomycin, bacitracin, kanamycin, neomycin,
gentamycin, erythromycin and cephalosporins. Examples of cephalosporins
include
cephalothin, cephapirin, cefazolin, cephalexin, cephradine, cefadroxil,
cefamandole,
cefoxitin, cefaclor, cefuroxime, cefonicid, ceforanide, cefotaxime,
moxalactam,
ceftizoxime, ceftriaxone, and cefoperazone.
111 an embodiment, the building block can be or can include an enzyme
inhibitor. Suitable enzyme inhibitors include edrophonium chloride, N-
methylphysostigmine, neostigmine bromide, physostigmine sulfate, tacrine HCL,
tacrine, 1-hydroxy maleate, iodotubercidin, p-bromotetramisole, 10-(cx-
diethylaminopropionyl)-phenothiazine hydrochloride, calmidazolium chloride,
hemicholinium-3,3,5-dinitrocatecho-1, diacylglycerol kinase inhibitor I,
diacylglycerol lcinase inhibitor II, 3-phenylpropargylaminie, N-monomethyl-L-
arginine acetate, carbidopa, 3-hydroxybenzylhydrazine HCI, hydralazine HCI,
48
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
clorgyline HCI, deprenyl HCl L(-), deprenyl HCl D(+), hydroxylamine HCI,
iproniazid phosphate, 6-Me0-tetrahydro-9H-pyrido-indole, nialamide, pargyline
HCI, quinacrine HCI, semicarbazide HCI, tranylcypromine HCI, N,N-
diethylaminoethyl-2,2-di-phenylvalerate hydrochloride, 3-isobutyl-1-
methylxanthne,
papaverine HCI, indomethacind, 2-cyclooctyl-2-hydroxyethylamine hydrochloride,
2,3-dichloro- a -methylbenzylamine (DCMB), 8,9-dichloro-2,3,4,5-tetrahydro-1H-
2-
benzazepine hydrochloride, p-aminoglutethimide, p-aminoglutethimide tartrate
R(+), p-aminoglutethimide tartrate S(-), 3-iodotyrosine, alpha-methyltyrosine
L(-),
alpha-methyltyrosine D(-), cetazolamide, dichlorphenamide, 6-hydroxy-2-
benzothiazolesulfonamide, allopurinol, and the like.
In an embodiment, the building block is or includes a signal element that
produces a detectable signal when a test ligand is bound to the receptor. In
an
embodiment, the signal element can produce an optical signal or a
electrochemical
signal. Suitable optical signals include chemiluminescence or fluorescence.
The
signal element can be a fluorescent moiety. The fluorescent molecule can be
one
that is quenched by binding to the artificial receptor. For example, the
signal
element can be a molecule that fluoresces only when binding occurs. Suitable
electrochemical signal elements include those that give rise to current or a
potential.
Suitable electrochemical signal elements include phenols and anilines, such as
those
with substitutents oriented ortho or para to one another, polynuclear aromatic
hydrocarbons, sulfide-disulfide, sulfide-sulfoxide-sulfone, polyenes,
polyeneynes,
and the like. Suitable electrochemical signal elements include quinones and
ferrocenes.
In an embodiment, the building block includes or is substituted with a moiety
providing a positive charge (e.g., at neutral pH in aqueous compositions).
Suitable
positively charged moieties include one or more groups such as amines,
quaternary
ammonium moieties, sulfonium, phosphonium, ferrocene, and the like. Suitable
amines include alkyl amines, alkyl diamines, heteroalkyl amines, aryl amines,
heteroaryl amines, aryl alkyl amines, pyridines, heterocyclic amines
(saturated or
unsaturated, the nitrogen in the ring or not), amidines, hydrazines, and the
like.
Alkyl amines generally have 1 to 12 carbons, preferably 1-8, rings can have 3-
12
carbons, preferably 3-8. Any of the amines can be employed as a quaternary
ammonium compound. Additional suitable quaternary ammonium moieties include
49
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
trimethyl alkyl quaternary ammonium moieties, dimethyl ethyl alkyl quaternary
ammonium moieties, dimethyl alkyl quaternary ammonium moieties, aryl alkyl
quaternary ammonium moieties, pyridinium quaternary ammonium moieties, and
the like.
In an embodiment, the building block includes or is substituted with a moiety
providing a negative charge (e.g., at neutral pH in aqueous compositions).
Suitable
negatively charged moieties include one or more groups such as carboxylates, j
phenols substituted with strongly electron withdrawing groups (e.g.,
substituted
tetrachlorophenols), phosphates, phosphonates, phosphinates, sulphates,
sulphonates, thiocarboxylates, and hydroxamic acids. Suitable carboxylates
include
alkyl carboxylates, aryl carboxylates, and aryl alkyl carboxylates. Suitable
phosphates include phosphate mono-, di-, and tri- esters, and phosphate mono-,
di-,
and tri- amides. Suitable phosphonates include phosphonate mono- and di-
esters,
and phosphonate mono- and di- amides (e.g., phosphonamides). Suitable
phosphinates include phosphinate esters and amides.
In an embodiment, the building block includes or is substituted with a moiety
providing a negative charge and a positive charge (at neutral pH in aqueous
compositions), such as sulfoxides, betaines, and amine oxides.
In an embodiment, the building block includes or is substituted with an
acidic moiety. Suitable acidic moieties include one or more groups such as
carboxylates, phosphates, sulphates, and phenols. Suitable acidic carboxylates
include thiocarboxylates. Suitable acidic phosphates include the phosphates
listed
hereinabove.
In an embodiment, the building block includes or is substituted with a basic
moiety. Suitable basic moieties include one or more groups such as amines.
Suitable basic amines include alkyl amines, aryl amines, aryl alkyl amines,
pyridines, heterocyclic amines (saturated or unsaturated, the nitrogen in the
ring or
not), amidines, and any additional amines listed hereinabove.
In an embodiment, the building block includes or is substituted with a
hydrogen bond donor. Suitable hydrogen bond donors include one or more groups
such as amines, amides, carboxyls, protonated phosphates, protonated
phosphonates,
protonated phosphinates, protonated sulphates, protonated sulphinates,
alcohols, and
thiols. Suitable amines include alkyl amines, aryl amines, aryl alkyl amines,
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
pyridines, heterocyclic amines (saturated or unsaturated, the nitrogen in the
ring or
not), amidines, ureas, and any other amines listed hereinabove. Suitable
protonated
carboxylates, protonated phosphates include those listed hereinabove. Suitable
alcohols include primary alcohols, secondary alcohols, tertiary alcohols, and
aromatic alcohols (e.g., phenols).
In an embodiment, the building block includes or is substituted with a
hydrogen bond acceptor or a moiety with one or more free electron pairs.
Suitable
groups can include one or more groups such as amines, amides, carboxylates,
carboxyl groups, phosphates, phosphonates, phosphinates, sulphates,
sulphonates,
alcohols, ethers, thiols, and thioethers. Suitable amines include alkyl
amines, aryl
amines, aryl alkyl amines, pyridines, heterocyclic amines (saturated or
unsaturated,
the nitrogen in the ring or not), amidines, ureas, and amines as listed
hereinabove.
Suitable carboxylates include those listed hereinabove. Suitable phosphates,
phosphonates and phosphinates include those listed hereinabove. Suitable
alcohols
include primary alcohols, secondary alcohols, tertiary alcohols, aromatic
alcohols,
and those listed hereiriabove. Suitable ethers include alkyl ethers, aryl
alkyl ethers.
In an embodiment, the building block includes or is substituted with a an .
uncharged polar or hydrophilic group. Suitable groups include one or more
groups
such as amides, alcohols, ethers, thiols, thioethers, esters, thio esters,
boranes,
borates, and metal complexes. Suitable alcohols include primary alcohols,
secondary alcohols, tertiary alcohols, aromatic alcohols, and those listed
hereinabove. Suitable ethers include those listed hereinabove.
In an embodiment, the building block includes or is substituted with an
uncharged hydrophobic group. Suitable groups include one or more groups such
as
alkyl (substituted and unsubstituted), alkene (conjugated and unconjugated),
alkyne
(conjugated and unconjugated), aromatic. Suitable alkyl groups include lower
alkyl,
substituted alkyl, cycloalkyl, aryl alkyl, and heteroaryl alkyl. Suitable
alkene groups
include lower allcene and aryl alkene. Suitable aromatic groups include
unsubstituted aryl, heteroaryl, substituted aryl, aryl alkyl, heteroaryl
alkyl, alkyl
substituted aryl, and polyaromatic hydrocarbons.
In an embodiment, the building block includes or is substituted with a spacer
(e.g., small) moiety, such as hydrogen, methyl, ethyl, and the like.
51
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Framework
The framework can be selected for functional groups that provide for
coupling to the recognition moiety and for coupling to or being the linking
moiety.
The framework can interact with the ligand as part of the artificial receptor.
In an
embodiment, the framework includes multiple reaction sites with orthogonal and
reliable functional groups and with controlled stereochemistry. Suitable
functional
groups with orthogonal and reliable chemistries include, for example,
carboxyl,
amine, hydroxyl, phenol, carbonyl, and thiol groups, which can be individually
protected, deprotected, and derivatized. In an embodiment, the framework has
two,
three, or four functional groups with orthogonal and reliable chemistries. In
an
embodiment, the framework has three functional groups. In such ail embodiment,
the three functional groups can be independently selected, for example, from
carboxyl, amine hydroxyl, phenol, carbonyl, or thiol group. The framework can
include alkyl, substituted alkyl, cycloalkyl, heterocyclic, substituted
heterocyclic,
aryl alkyl, aryl, heteroaryl, heteroaryl alkyl, and like moieties.
A general structure for a framework with three functional groups can be
represented by Formula la:
Fz
F1__R1__ F3
A general structure for a framework with four functional groups can be
represented
by Formula 1b:
Fz
Fi--R1-- F3
F4
In these general structures: R1 can be a 1-12, a 1-6, or a 1-4 carbon alkyl,
substituted
alkyl, cycloalkyl, heterocyclic, substituted heterocyclic, aryl alkyl, aryl,
heteroaryl,
heteroaryl allcyl, or like group; and Fl, Fz, F3, or F4 can independently be a
carboxyl,
amine, hydroxyl, phenol, carbonyl, or thiol group. Fl, Fz, F3, or F4 can
independently be a 1-12, a 1-6, a 1-4 carbon alkyl, substituted alkyl,
cycloalkyl,
heterocyclic, substituted heterocyclic, aryl alkyl, aryl, heteroaryl,
heteroaryl alkyl, or
52
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
inorganic group substituted with carboxyl, amine, hydroxyl, phenol, carbonyl,
or
thiol group. F3 and/or F4 can be absent.
A variety of compounds fit the formulas and text describing the framework
including amino acids, and naturally occurnng or synthetic compounds
including,
for example, oxygen and sulfur functional groups. The compounds can be
racemic,
optically active, or achiral. For example, the compounds can be natural or
synthetic
amino acids, a hydroxy acids, thioic acids, and the like.
Suitable molecules for use as a framework include a natural or synthetic
amino acid, particularly an amino acid with a functional group (e.g., third
functional
group) on its side chain. Amino acids include carboxyl and amine functional
groups. The side chain functional group can include, for natural amino acids,
an
amine (e.g., alkyl amine, heteroaryl amine), hydroxyl, phenol, carboxyl,
thiol,
thioether, or amidino group. Natural amino acids suitable for use as
frameworks
include, for example, serine, threonine, tyrosine, aspartic acid, glutamic
acid,
asparagine, glutamine, cysteine, lysine, arginine, histidine. Synthetic amino
acids
can include the naturally occurring side chain functional groups or synthetic
side
chain functional groups which modify or extend the natural amino acids with
alkyl,
substituted alkyl, cycloalkyl, heterocyclic, substituted heterocyclic, aryl
alkyl, aryl,
heteroaryl, heteroaryl alkyl, and like moieties as framework and with
carboxyl,
amine, hydroxyl, phenol, carbonyl, or thiol functional groups. Suitable
synthetic
amino acids include ~3-amino acids and homo or (3 analogs of natural amino
acids. In
an embodiment, the framework amino acid can be serine, threonine, or tyrosine;
e.g.,
serine or tyrosine, e.g., tyrosine.
Although not limiting to the present invention, a framework amino acid, such
as serine, threonine, or tyrosine, with a linker and two recognition elements
can be
visualized with one of the recognition elements in a pendant orientation and
the
other in an equatorial orientation, relative to the extended carbon chain of
the
framework.
All of the naturally occurring and many synthetic amino acids are
commercially available. Further, forms of these amino acids derivatized or
protected to be suitable for reactions for coupling to recognition elements)
andlor
linkers can be purchased or made by known methods (see, e.g., Green, TW; Wuts,
PGM (1999), Protective Groups in Organic Synthesis Third Edition, Wiley-
53
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Interscience, New York, 779 pp.; Bodanszky, M.; Bodanszky, A. (1994), The
Practice of Peptide Synthesis Second Edition, Springer-Verlag, New York, 217
pp.).
Embodiments of Frameworks
A framework can be or can include any of a variety of compounds or
substructures. For example, a framework can be or include an amino acid
(natural
or synthetic), a dipeptide, a monosaccharide, a disaccharide, another
carbohydrate, a
mixture or combination thereof, or the like; a catalytic moiety such as a
coenzyme, a
metal, a metal complex, or the like; a polymer of up to 2000 carbon atoms
(e.g., up
to 48 carbon atoms), e.g., a polyether, polyethyleneimine, a polyacrylamide,
or like
polymer; an a hydroxy acid, a thioic acid; an enzyme inhibitor (e.g., a
protease
inhibitor (such as pepstatin), a statin, or the like), a receptor antagonist
(e.g., a
benzodiazepine), a receptor agonist, a pharmaceutical, a peptide hormone; a
natural
product, a starting material, intermediate, or end product of a metabolic
pathway
(e.g., glycolysis, the citric acid cycle, photosynthesis, glucogenesis,
mitochondria)
electron transport, oxidative phosphorylation, biosynthetic pathways,
catabolic
pathways, or the like); a mixture or combination thereof, or the like. A
framework
can be a naturally occurring or synthetic compound; can be racemic, optically
active,
or achiral; can. include positional isomers of any specifically described
structure; or
can include conformationally restricted functional groups.
In an embodiment, the framework is or includes a monosaccharide. Any of a
variety of naturally occurring or synthetic monosaccharides can be employed as
a
framework. Suitable monosaccharides include pyranoses and furanoses, such as
glucose, fructose, ribulose, allose, altrose, mannose, gulose, idose,
galactose, talose,
ribose, arabinose, xylose, lyxose, or the like; erythrose, threose, or the
like; inositol,
or the like; amino sugars, such as rhammose, fucose, glucosamine,
galactosamine, or
the like; aldonic and uronic acids, such as gluconic acid, glucuronic acid,
glucaric
acid, or the like; glycosides including these monosaccharides; a mixture or
combination thereof, or the like.
In an embodiment, the framework is or includes a disaccharide. Any of a
variety of naturally occurring or synthetic disaccharides can be employed as a
framework. Suitable disaccharides include disaccharides or oligosaccharides
including the monosaccharides listed above. Such disaccharides include
sucrose,
54
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
raffmose, gentianose, cellobiose, maltose, lactose, trehalose, gentiobiose,
meliobiose, a mixture or combination thereof, or the like.
In an embodiment, the framework is or includes a carbohydrate. Any of a
variety of naturally occurring or synthetic carbohydrates can be employed as a
framework. Suitable carbohydrates include cellulose, chitin, starch, glycogen,
hyaluronic acid, chondroitin sulfates, keratosulfate, heparin, glycoproteins,
or the
like; a mixture or combination thereof, or the like.
In an embodiment, the framework is or includes a catalytic moiety. Any of a
variety of naturally occurring or synthetic catalytic moieties can be employed
as or
can be a moiety on a framework. Suitable catalytic moieties include coenzymes,
metals, metal complexes, pronucleophiles, proelectrophiles, proreducing
agents,
prooxidizing agents, general acid catalysts, general base catalysts, a mixture
or
combination thereof, or the like.
In an embodiment, the framework is or includes a metal binding or
complexing moiety. Any of a variety of naturally occurring or synthetic metal
binding or complexing moieties can be employed as or can be a moiety on a
framework. Suitable metal binding or complexing moieties include synthetic and
naturally occurring porphyrin (e.g., etioporphyrin, mesoporphyrin,
protoporphyrin
(e.g., heme or hematin), coproporphyrin, tetraphenylporphyrin,
octaethylporphyrin,
or the like), a cobamide coenzyme (e.g., coenzyme B12, a cobalamin such as
methyl-
cobalamin, or the like), selenocysteine, selenomethionine, ferritin; naturally
occurring or synthetic complexes of magnesium, zinc, copper, chromium, iron,
cobalt, aluminum (e.g., A13+), titanium (e.g., Ti4~) or the like; salt
thereof, a mixture
or combination thereof, or the like.
In an embodiment, the framework is or includes a coenzyme (which can also
be called a prosthetic group or cofactor). Any of a variety of naturally
occurring or
synthetic coenzymes can be employed as or can be a moiety on a framework.
Suitable coenzymes include a nicotinamide coenzyme (e.g., NAD, NADH, NADP,
NADPH, and the like), a flavin compound (e.g., FAD, FADH2, FMN, FMNFia), a
lipoic acid (e.g., oxidized or reduced lipoic acid), a glutathione (e.g.,
oxidized or
reduced glutathione), an ascorbic acid, a quinone (e.g., ubiquinone, vitamins
K, or
the like), a porphyrin (e.g., etioporphyrin, mesoporphyrin, protoporphyrin
(e.g.,
heme or hematin), coproporphyrin, or the like), a nucleoside (e.g., adenine,
guanine,
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
cytosine, thymine, uracil), a nucleotide (e.g., AMP, ADP, ATP, GMP, GDP, GTP,
CMP, CDP, CTP, TMP, TDP, TTP, UMP, UDP, UTP), a glycerol phosphate, a
biotin (e.g., biotin or carboxybiotin), a pyridoxal (e.g., pyridoxal
phosphate,
pyridoxal, pyridoxamine, pyridoxamine phosphate, or Schiff's bases thereof),
an
oxoglutaric acid (e.g., 2-oxoglutarate), a coenzyme A, a carnitine, a folic
acid (e.g.,
tetrahydrofolic acid, 5-fonnyltetrahydrofolic acid, 10-formyltetrahydrofolic
acid,
5,10-methenyltetrahydrofolic acid, 5,10-methylenetetrahydrofolic acid, 5-
hydroxymethyltetrahydrofolic acid, 5-formiminotetrahydrofolic acid, or the
like), an
adenosylhomocysteine, a cobamide coenzyme (e.g., coenzyme Blz, a cobalamin
such as methyl-cobalamin, or the like), adenosine 3',5'-bisphosphate, thiamin
diphosphate, ferntin, salt thereof, a mixture or combination thereof, or the
like.
In an embodiment, the framework is or includes a polymer of up to 2000
carbon atoms (e.g., up to 48 carbon atoms). Such a polymer can be naturally
occurring or synthetic. Such a polymer can be naturally occurring or
synthetic.
Suitable polymers include a polyether or like polymer, such as a PEG, a
polyethyleneimine, polyacrylate (e.g., substituted polyacrylates), salt
thereof, a
mixture or combination thereof, or the like. Suitable PEGS include PEG 1500 up
to
PEG 20,000, for example, PEG 1450, PEG 3350, PEG 4500, PEG 8000, PEG
20,000, and the like.
In an embodiment, the building block is or includes a dipeptide. Any of the
400 dipeptides including the 20 natural amino acids in any order can be
employed as
building blocks. Suitable dipeptides include muramyl dipeptide or the like.
In an embodiment the framework can be or include a therapeutic or
pharmacologically active agent. Suitable therapeutic or pharmacologically
active
agents include a nitrate, nitric oxide, a nitric oxide promoter, nitric oxide
donors,
dipyridamole, or another vasodilator; HYTRIN~ or another antihypertensive
agent;
a glycoprotein IIb/IIIa inhibitor (abciximab) or another inhibitor of surface
glycoprotein receptors; aspirin, ticlopidine, clopidogrel or another
antiplatelet agent;
colchicine or another antimitotic, or another microtubule inhibitor; a
retinoid or
another antisecretory agent; cytochalasin or another actin inhibitor;
methotrexate or
another antimetabolite or antiproliferative agent; tamoxifen citrate, TAXOL~,
paclitaxel, or derivatives thereof, rapamycin, vinblastine, vincristine,
vinorelbine,
etoposide, tenopiside, dactinomycin (actinomycin D), daunorubicin,
doxorubicin,
56
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
idarubicin, an anthracycline, mitoxantrone, bleomycin, plicamycin
(mithramycin),
mitomycin, mechlorethamine, cyclophosphamide and its analogs, chlorambucil, an
ethylenimine, a methylmelamine, an alkyl sulfonate (e.g., busulfan), a
nitrosourea
(carmustine, etc.), streptozocin, methotrexate (used with many indications),
fluorouracil, floxuridine, cytarabine, mercaptopurine, thioguanine,
pentostatin, 2-
chlorodeoxyadenosine, cisplatin, carboplatin, procarbazine, hydroxyurea, or
other
anti-cancer chemotherapeutic agents; cyclosporin, tacrolimus (FK-506),
azathioprine, mycophenolate mofetil, mTOR inhibitors, or another
immunosuppressive agent; cortisol, cortisone, dexamethasone, dexamethasone
sodium phosphate, dexamethasone acetate, a dexamethasone derivative,
betamethasone, fludrocortisone, prednisone, prednisolone, 6U-
methylprednisolone,
triamcinolone (e.g., triamcinolone acetonide), or another steroidal agent;
trapidil (a
PDGF antagonist); dopamine, bromocriptine mesylate, pergolide mesylate, or
another dopamine agonist; captopril, enalapril or another angiotensin
converting
enzyme (ACE) inhibitor; angiotensin receptor blockers; ascorbic acid, alpha
tocopherol, deferoxamine, a 21-aminosteroid (lasaroid) or another free radical
scavenger, iron chelator or antioxidant; estrogen or another sex hormone; AZT
or
another antipolymerase; acyclovir, famciclovir, rimantadine hydrochloride,
ganciclovir sodium, Norvir, Crixivan, a methyl-1-adamantanemethylamine,
hydroxy-ethoxymethylguanine, adamantanamine, 5-iodo-2'-deoxyuridine,
trifluorothymidine, adenine arabinoside, or another antiviral agent; 5-
aminolevulinic
acid, meta-tetrahydroxyphenylchlorin, hexadecafluorozinc phthalocyanine,
tetramethyl hematoporphyrin, rhodamine 123 or other photodynamic therapy
agents;
PROSCAR~, HYTRIN~ or other agents for treating benign prostatic hyperplasia
(BHP); mitotane, aminoglutethimide, breveldin, acetaminophen, etodalac,
tolmetin,
lcetorolac, ibuprofen and derivatives, mefenamic acid, meclofenamic acid,
piroxicam, tenoxicam, phenylbutazone, oxyphenbutazone, nabumetone, auranofin,
aurothioglucose, gold sodium thiomalate, a mixture of any of these, or
derivatives of
any of these.
' In an embodiment, the framework can be or can include an antibiotic.
Examples of antibiotics include penicillin, tetracycline, chloramphenicol,
minocycline, doxycycline, vancomycin, bacitracin, kanamycin, neomycin,
gentamycin, erythromycin and cephalosporins. Examples of cephalosporins
include
57
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
cephalothin, cephapirin, cefazolin, cephalexin, cephradine, cefadroxil,
cefamandole,
cefoxitin, cefaclor, cefuroxime, cefonicid, ceforanide, cefotaxime,
moxalactam,
ceftizoxime, ceftriaxone, and cefoperazone.
In. an embodiment, the framework can be or can include an enzyme inhibitor.
Suitable enzyme inhibitors include edrophonium chloride, N-
methylphysostigmine,
neostigmine bromide, physostigmine sulfate, tacrine HCL, tacrine, 1-hydroxy
maleate, iodotubercidin, p-bromotetr'amisole, 10-(a-diethylaminopropionyl)-
phenothiazine hydrochloride, cahnidazolium chloride, hemicholinium-3,3,5-
dinitrocatecho-1, diacylglycerol kinase inhibitor I, diacylglycerol kinase
inhibitor II,
~ 3-phenylpropargylaminie, N-monomethyl-L-arginine acetate, carbidopa, 3-
hydroxybenzylhydrazine HCI, hydralazine HCI, clorgyline HCI, deprenyl HCl L(-
),
deprenyl HCl D(+), hydroxylamine HCI, iproniazid phosphate, 6-Me0-tetrahydro-
9H-pyrido-indole, nialamide, pargyline HCl, quinacrine HCI, semicarbazide HCI,
tranylcypromine HCl, N,N-diethylaminoethyl-2,2-di-phenylvalerate
hydrochloride,
3-isobutyl-1-methylxanthne, papaverine HCI, indomethacind, 2-cyclooctyl-2-
hydroxyethylamine hydrochloride, 2,3-dichloro- a -methylbenzylamine (DCMB),
8,9-dichloro-2,3,4,5-tetrahydro-1H-2-benzazepine hydrochloride, p-
aminoglutethimide, p-aminoglutethimide tartrate R(+), p-aminoglutethimide
tartrate
S(-), 3-iodotyrosine, alpha-methyltyrosine L(-), alpha-methyltyrosine D(-),
cetazolamide, dichlorphenariiide, 6-hydroxy-2-benzothiazolesulfonamide,
allopurinol, and the like.
In an embodiment, the framework is or includes a signal element that
produces a detectable signal when a test ligand is bound to the receptor. 11i
an
embodiment, the signal element can produce an optical signal or a
electrochemical
signal. Suitable optical signals include chemiluminescence or fluorescence.
The
signal element can be a fluorescent moiety. The fluorescent molecule can be
one
that is quenched by binding to the artificial receptor. For example, the
signal
element can be a molecule that fluoresces only when binding occurs. Suitable
electrochemical signal elements include those that give rise to current or a
potential.
Suitable electrochemical signal elements include phenols and anilines, such as
those
with substitutents oriented ortho or para to one another, polynuclear aromatic
hydrocarbons, sulfide-disulfide, sulfide-sulfoxide-sulfone, polyenes,
polyeneynes,
5~
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
and the like. Suitable electrochemical signal elements include quinones and
ferrocenes.
Recognition Element
The recognition element can be selected to provide one or more structural
characteristics to the building block. The recognition element can interact
with the
ligand as part of the artificial receptor. For example, the recognition
element can
provide one or more structural characteristics such as positive charge,
negative
charge, acid, base, electron acceptor, electron donor, hydrogen bond donor,
,10 hydrogen bond acceptor, free electron pair, ~ electrons, charge
polarization,
hydrophilicity, hydrophobicity, and the like. A recognition element can be a
small
group or it can be bulky.
In an embodiment the recognition element can be a 1-12, a 1-6, or a 1-4
carbon alkyl, substituted alkyl, cycloalkyl, heterocyclic, substituted
heterocyclic,
aryl alkyl, aryl, heteroaryl, heteroaryl alkyl, or like group. The recognition
element
can be substituted with a group that includes or imparts positive charge,
negative
charge, acid, base, electron acceptor, electron donor, hydrogen bond donor,
hydrogen bond acceptor, free electron pair, ~ electrons, charge polarization,
hydrophilicity, hydrophobicity, and the like.
Embodiments of Recognition Elements
Recognition elements with a positive charge (e.g., at neutral pH in aqueous
compositions) include amines, quaternary ammonium moieties, sulfonium,
phosphonium, ferrocene, and the like. Suitable amines include alkyl amines,
alkyl
diamines, heteroalkyl amines, aryl amines, heteroaryl amines, aryl alkyl
amines,
pyridines, heterocyclic amines (saturated or unsaturated, the nitrogen in the
ring or
not), amidines, hydrazines, and the like. Alkyl amines generally have 1 to 12
carbons, e.g., 1-8, and rings can have 3-12 carbons, e.g., 3-8. Suitable alkyl
amines
include that of formula B9. Suitable heterocyclic or alkyl heterocyclic amines
include that of formula A9. Suitable pyridines include those of formulas AS
and B5.
Any of the amines can be employed as a quaternary ammonium compound.
Additional suitable quaternary ammonium moieties include trimethyl alkyl
quaternary ammonium moieties, dimethyl ethyl alkyl quaternary ammonium
59
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
moieties, dimethyl alkyl quaternary ammonium moieties, aryl alkyl quaternary
ammonium moieties, pyridinium quaternary ammonium moieties, and the like.
Recognition elements with a negative charge (e.g., at neutral pH in aqueous
compositions) include carboxylates, phenols substituted with strongly electron
withdrawing groups (e.g., substituted tetrachlorophenols), phosphates,
phosphonates, phosplunates, sulphates, sulphonates, thiocarboxylates, and
hydroxamic acids. Suitable carboxylates include alkyl carboxylates, aryl
carboxylates, and aryl alkyl carboxylates. Suitable phosphates include
phosphate
mono-, di-, and tri- esters, and phosphate mono-, di-, and tri- amides.
Suitable
phosphonates include phosphonate mono- and di- esters, and phosphonate mono-
and di- amides (e.g., phosphonamides). Suitable phosphinates include
phosphinate
esters and amides.
Recognition elements with a negative charge and a positive charge (at neutral
pH in aqueous compositions) include sulfoxides, betaines, and amine oxides.
Acidic recognition elements can include carboxylates, phosphates, sulphates,
and phenols. Suitable acidic carboxylates include thiocarboxylates. Suitable
acidic
phosphates include the phosphates listed hereinabove.
Basic recognition elements include amines. Suitable basic amines include
alkyl amines, aryl amines, aryl alkyl amines, pyridines, heterocyclic amines
(saturated or unsaturated, the nitrogen in the ring or not), amidines, and any
additional amines listed hereinabove. Suitable alkyl amines include that of
formula
B9. Suitable heterocyclic or alkyl heterocyclic amines include that of formula
A9.
Suitable pyridines include those of formulas AS and B5.
Recognition elements including a hydrogen bond donor include amines,
amides, carboxyls, protonated phosphates, protonated phosphonates, protonated
phosphinates, protonated sulphates, protonated sulphinates, alcohols, and
thiols.
Suitable amines include allcyl amines, aryl amines, aryl alkyl amines,
pyridines,
heterocyclic amines (saturated or unsaturated, the nitrogen in the ring or
not),
amidines, areas, and any other amines listed hereinabove. Suitable alkyl
amines
include that of formula B9. Suitable heterocyclic or allcyl heterocyclic
amines
include that of formula A9. Suitable pyridines include those of formulas AS
and B5.
Suitable protonated carboxylates, protonated phosphates include those listed
hereinabove. Suitable amides include those of formulas A8 and B8. Suitable
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
alcohols include primary alcohols, secondary alcohols, tertiary alcohols, and
aromatic alcohols (e.g., phenols). Suitable alcohols include those of formulas
A7 (a
primary alcohol) and B7 (a secondary alcohol).
Recognition elements including a hydrogen bond acceptor or one or more
free electron pairs include amines, amides, carboxylates, carboxyl groups,
phosphates, phosphonates, phosphinates, sulphates, sulphonates, alcohols,
ethers,
thiols, and thioethers. Suitable amines include alkyl amines, aryl amines,
aryl alkyl
amines, pyridines, heterocyclic amines (saturated or unsaturated, the nitrogen
in the
ring or not), amidines, ureas, and amines as listed hereinabove. Suitable
alkyl
amines include that of formula B9. Suitable heterocyclic or alkyl heterocyclic
amines include that of formula A9. Suitable pyridines include those of
formulas AS
and B5. Suitable carboxylates include those listed hereinabove. Suitable
amides
include those of formulas A8 and B8. Suitable phosphates, phosphonates and
phosphinates include those listed hereinabove. Suitable alcohols include
primary
alcohols, secondary alcohols, tertiary alcohols, aromatic alcohols, and those
listed
hereinabove. Suitable alcohols include those of formulas A7 (a primary
alcohol)
and B7 (a secondary alcohol). Suitable ethers include alkyl ethers, aryl alkyl
ethers.
Suitable alkyl ethers include that of formula A6. Suitable aryl alkyl ethers
include
that of formula AA.. Suitable thioethers include that of formula B6.
Recognition elements including uncharged polar or hydrophilic groups
include amides, alcohols, ethers, thiols, thioethers, esters, thin esters,
boranes,
borates, and metal complexes. Suitable amides include those of formulas A8 and
B8. Suitable alcohols include primary alcohols, secondary alcohols, tertiary
alcohols, aromatic alcohols, and those listed hereinabove. Suitable alcohols
include
those of formulas A7 (a primary alcohol) and B7 (a secondary alcohol).
Suitable
ethers include those listed hereinabove. Suitable ethers include that of
formula A6.
Suitable axyl alkyl ethers include that of formula A4.
Recognition elements including uncharged hydrophobic groups include alkyl
(substituted and unsubstituted), alkene (conjugated and unconjugated), alkyne
(conjugated and unconjugated), aromatic. Suitable alkyl groups include lower
alkyl,
substituted alkyl, cycloalkyl, aryl alkyl, and heteroaryl alkyl. Suitable
lower alkyl
groups include those of formulas Al, A3, A3a, and B1. Suitable aryl alkyl
groups
include those of formulas A3, A3a, A4, B3, B3a, and B4. Suitable alkyl
cycloalkyl
61
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
groups include that of formula B2. Suitable alkene groups include lower alkene
and
aryl alkene. Suitable aryl alkene groups include that of formula B4. Suitable
aromatic groups include unsubstituted aryl, heteroaryl, substituted aryl, aryl
alkyl,
heteroaryl alkyl, alkyl substituted aryl, and polyaromatic hydrocarbons.
Suitable
aryl alkyl groups include those of formulas A3, A3a and B4. Suitable alkyl
heteroaryl groups include those of formulas AS and B5.
Spacer (e.g., small) recognition elements include hydrogen, methyl, ethyl,
and the like. Bulky recognition elements include 7 or more carbon or hetero
atoms.
Formulas Al-A9 and Bl-B9 are:
CHZ CH3 Al
CH2CH(CH3)a A2
CH2CH2
A3
A3 a
25
CHZCH2 ~ ~ OCH3
A4
CH2CH2
AS
CH2CH2-O-CH3 A6
CH2CH2-OH A~
62
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
CH2CH2-NH-C(O)CH3 A8
CH2CH2 N
A9
CH3 B 1
CHZCH2
B2
C1
HzC
B3
/ /
t \ \ B3a
CH=CH
B4
CHZCHZ
BS
CH2-S-CH3 B6
CH2CH(OH)CH3 B7
CH2CHZC(O)-NH2 B8
CH2CH2CH2-N-(CH3)2 B9
These A and B recognition elements can be called derivatives of, according
to a standard reference: Al, ethylamine; A2, isobutylamine; A3,
phenethylamine;
A4, 4-methoxyphenethylamine; A5, 2-(2-aminoethyl)pyridine; A6, 2-
63
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
methoxyethylamine; A7, ethanolamine; A8, N-acetylethylenediamine; A9, 1-(2-
aminoethyl)pyrrolidine; B 1, acetic acid, B2, cyclopentylpropionic acid; B3, 3-
chlorophenylacetic acid; B4, cinnamic acid; B5, 3-pyridinepropionic acid; B6,
(methylthio)acetic acid; B7, 3-hydroxybutyric acid; B8, succinamic acid; and
B9, 4-
(dimethylamino)butyric acid.
In an embodiment, the recognition elements include one or more of the '
structures represented by formulas A1, A2, A3, A3a, A4, A5, A6, A7, A8, and/or
A9 (the A recognition elements) and/or B1, B2, B3, B3a, B4, B5, B6, B7, B8,
and/or
B9 (the B recognition elements). In an embodiment, each building block
includes an
A recognition element and a B recognition element. In an embodiment, a group
of
81 such building blocks includes each of the 81 unique combinations of an A
recognition element and a B recognition element. In an embodiment, the A
recognition elements are linked to a framework at a pendant position. In an
embodiment, the B recognition elements are linked to a framework at an
equatorial
position. In an embodiment, the A recognition elements are linked to a
framework
at a pendant position and the B recognition elements are linked to the
framework at
an equatorial position.
Although not limiting to the present invention, it is believed that the A and
B
recognition elements represent the assortment of functional groups and
geometric
configurations employed by polypeptide receptors. Although not limiting to the
present invention, it is believed that the A recognition elements represent
six
advantageous functional groups or configurations and that the addition of
functional
groups to several of the aryl groups increases the range of possible binding
interactions. Although not limiting to the present invention, it is believed
that the B
recognition elements represent six advantageous functional groups, but in
different
configurations than employed for the A recognition elements. Although not
limiting
to the present invention, it is further believed that this increases the range
of binding
interactions and further extends the range of functional groups and
configurations
that is explored by molecular configurations of the building blocks.
In an embodiment, the building bloclcs including the A and B recognition
elements can be visualized as occupying a binding space defined by
lipophilicity/hydrophilicity and volume. A volume can be calculated (using
known
methods) for each building block including the various A and B recognition
64
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
elements. A measure of lipophilicity/hydrophilicity (loge) can be calculated
(using
known methods) for each building block including the various A and B
recognition
elements. Negative values of loge show affinity for water over nonpolar
organic
solvent and indicate a hydrophilic nature. A plot of volume versus loge can
then
show the distribution of the building blocks through a binding space defined
by size
and lipophilicity/hydrophilicity.
Reagents that form many of the recognition elements are commercially
available. For example, reagents for forming recognition elements Al, A2, A3,
A3a, A4, A5, A6, A7, A8, A9 B1, B2, B3, B3a, B4, B5, B6, B7, B8, and B9 are
commercially available.
Additional Embodiments of Recognition Elements
A recognition element can be or can include any of a variety of compounds
or substructures. For example, a recognition element can be or include an
amino
acid (natural or synthetic), a dipeptide, a monosaccharide, a disaccharide,
another
carbohydrate, a mixture or combination thereof, or the like; a catalytic
moiety such
as a coenzyme, a metal, a metal complex, or the like; a polymer of up to 2000
carbon atoms (e.g., up to 48 carbon atoms), e.g., a polyether,
polyethyleneimine, a
polyacrylamide, or like polymer; an a hydroxy acid, a thioic acid; an enzyme
inhibitor (e.g., a protease inhibitor (such as pepstatin), a statin, or the
like), a
receptor antagonist (e.g., a benzodiazepine), a receptor agonist, a
pharmaceutical, a
peptide hormone; a natural product, a starting material, intermediate, or end
product
of a metabolic pathway (e.g., glycolysis, the citric acid cycle,
photosynthesis,
glucogenesis, mitochondria) electron transport, oxidative phosphorylation,
biosynthetic pathways, catabolic pathways, or the like); a mixture or
combination
thereof, or the like. A building block can be a naturally occurring or
synthetic
compound; can be racemic, optically active, or achiral; can include positions)
isomers of any specifically described structure; or can include
conformationally
restricted functional groups. .
In an embodiment, the recognition element is or includes a monosaccharide.
Any of a variety of naturally occurring or synthetic monosaccharides can be
employed as a recognition element. Suitable monosaccharides include pyranoses
and furanoses, such as glucose, fructose, ribulose, allose, altrose, mannose,
gulose,
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
idose, galactose, talose, ribose, arabinose, xylose, lyxose, or the like;
erythrose,
threose, or the like; inositol, or the like; amino sugaxs, such as rhammose,
fucose,
glucosamine, galactosamine, or the like; aldonic and uronic acids, such as
gluconic
acid, glucuronic acid, glucaric acid, or the like; glycosides including these
monosaccharides; a mixture or combination thereof, or the like.
In an embodiment, the recognition element is or includes a disaccharide.
Any of a variety of naturally occurring or synthetic disaccharides can be
employed
as a building block. Suitable disaccharides include disaccharides or
oligosaccharides including the monosaccharides listed above. Such
disaccharides
include sucrose, raffinose, gentianose, cellobiose, maltose, lactose,
trehalose,
gentiobiose, meliobiose, a mixture or combination thereof, or the like.
In an embodiment, the recognition element is or includes a carbohydrate.
Any of a variety of naturally occurring or synthetic carbohydrates can be
employed
as a recognition element. Suitable carbohydrates include cellulose, chitin,
starch,
glycogen, hyaluronic acid, chondroitin sulfates, keratosulfate, heparin,
glycoproteins, or the like; a mixture or combination thereof, or the like.
In an embodiment, the recognition element is or includes a catalytic moiety.
Any of a variety of naturally occurring or synthetic catalytic moieties can be
employed as or can be a moiety on a recognition element. Suitable catalytic
moieties include coenzymes, metals, metal complexes, pronucleophiles,
proelectrophiles, proreducing agents, prooxidizing agents, general acid
catalysts,
general base catalysts, a mixture or combination thereof, or the like.
In an embodiment, the recognition element is or includes a metal binding or
complexing moiety. Any of a variety of naturally occurring or synthetic metal
binding or complexing moieties can be employed as or can be a moiety on a
recognition element. Suitable metal binding or complexing moieties include
synthetic and naturally occurring porphyrin (e.g., etioporphyrin,
mesoporphyrin,
protoporphyrin (e.g., heme or hematin), coproporphyrin, tetraphenylporphyrin,
octaethylporphyrin, or the like), a cobamide coenzyme (e.g., coenzyme Bla, a
cobalamin such as methyl-cobalamin, or the like), selenocysteine,
selenomethionine,
ferritin; naturally occurring or synthetic complexes of magnesium, zinc,
copper,
chromium, iron, cobalt, aluminum (e.g., Al3+), titanium (e.g., Ti4+) or the
like; salt
thereof, a mixture or combination thereof, or the like.
66
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, the recognition element is or includes a coenzyme (which
can also be called a prosthetic group or cofactor). Any of a variety of
naturally
occurring or synthetic coenzymes can be employed as or can be a moiety on a
recognition element. Suitable coenzymes include a nicotinamide coenzyme (e.g.,
NAD, NADH, NADP, NADPH, and the like), a flavin compound (e.g., FAD,
FADHz, FMN, FMNHz), a lipoic acid (e.g., oxidized or reduced lipoic acid), a
glutathione (e.g., oxidized or reduced glutathione), an ascorbic acid, a
quinone (e.g.,
ubiquinone, vitamins K, or the like), a porphyrin (e.g., etioporphyrin,
mesoporphyrin, protoporphyrin (e.g., heme or hematin), coproporphyrin, or the
like), a nucleoside (e.g., adenine, guanine, cytosine, thymine, uracil), a
nucleotide
(e.g., AMP, ADP, ATP, GMP, GDP, GTP, CMP, CDP, CTP, TMP, TDP, TTP,
UMP, UDP, UTP), a glycerol phosphate, a biotin (e.g., biotin or
carboxybiotin), a
pyridoxal (e.g., pyridoxal phosphate, pyridoxal, pyridoxamine, pyridoxamine
phosphate, or Schiff's bases thereof), an oxoglutaric acid (e.g., 2-
oxoglutarate), a
coenzyme A, a carrutine, a folic acid (e.g., tetrahydrofolic acid, 5-
formyltetrahydrofolic acid, 10-formyltetrahydrofolic acid, 5,10-
methenyltetrahydrofolic acid, 5,10-methylenetetrahydrofolic acid, 5-
hydroxyrnethyltetrahydrofolic acid, 5-formiminotetrahydrofolic acid, or the
like), an
adenosylhomocysteine, a cobamide coenzyme (e.g., coenzyme Blz, a cobalamin
such as methyl-cobalamin, or the like), adenosine 3',5'-bisphosphate, thiamin
diphospliate, ferritin, salt thereof, a mixture or combination thereof, or the
like.
In an embodiment, the present recognition element can be or include a
lipophilic moiety. Suitable lipophilic moieties include one or more branched
or
straight chain C~_36 alkyl, C8_z4 alkyl, Clz-z4 alkyl, Clz-is alkyl, or the
like; C6_36
alkenyl, C8_z4 alkenyl, Clz-z4 alkenyl, Ciz_l8 alkenyl, or the like, with, for
example, 1
to 4 double bonds; C6_3s alkynyl, C$_z4 alkynyl, Clz-za alkynyl, Clz-is
alkynyl, or the
like, with, for example, 1 to 4 triple bonds; chains with 1-4 double or triple
bonds;
chains including aryl or substituted aryl moieties (e.g., phenyl or naphthyl
moieties
at the end or middle of a chain); polyaromatic hydrocarbon moieties;
cycloalkane or
substituted alkane moieties with numbers of carbons as described fox chains;
combinations or mixtures thereof; or the like. The alkyl, alkenyl, or alkynyl
group
can include branching; within chain functionality like an ether group;
terminal
functionality like alcohol, amide, carboxylate or the like; or the like.
67
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Suitable recognition elements include carboxylic acids (e.g., mono and di-
carboxylates) with the carboxylate appended to a lipophilic moiety, such as
one or
more branched or straight chain C6_3s alkyl, C8_z4 alkyl, Clz-z4 alkyl, Clz-is
alkyl, or
the like; C6_36 alkenyl, Cs_z4 alkenyl, CIZ-z4 alkenyl, Clz_ls alkenyl, or the
like, with,
for example, 1 to 4 double bonds; C6_36 alkynyl, C8_z4 alkynyl, Clz-za
alkynyl, Clz-is
alkynyl, or the like, with, for example, 1 to 4 triple bonds; chains with 1-4
double or
triple bonds; chains including aryl or substituted aryl moieties (e.g., phenyl
or
naphthyl moieties at the end or middle of a chain); or the like. Such
carboxylic acids
include arachidonic acid, linoleic acid, linolenic acid, oleic acid, and the
like. Such
carboxylic acids can be immobilized on a support through covalent bonding or
electrostatic interaction between
Suitable recognition elements include carboxylic acids (e.g., mono and di-
carboxylates) with the carboxylate appended to a an organic radical, such as
one or
more branched or straight chain Cz_8 alkyl, arylalkyl, alkenyl, alkynyl, or
the like.
These carboxylic acids can include substituted aryl moieties (e.g., phenyl or
naphthyl moieties). Such carboxylic acids include acetic acid, propionic acid,
butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid,
oxalic
acid, malonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid,
benzoic
acid, and the like. Such carboxylic acids can be immobilized on a support
through
covalent bonding or electrostatic interaction between the carboxyl(ate) and
the
support or lawn.
In an embodiment, the recognition element is or includes an amino acid.
Suitable amino acids include a natural or synthetic amino acid. Amino acids
include
carboxyl and amine functional groups. In their side chains, amino acids can
also
include a moiety with one or more of positive charge, negative charge, acid,
base,
electron acceptor, electron donor, hydrogen bond donor, hydrogen bond
acceptor,
free electron pair, ~- electrons, charge polarization, hydrophilicity, or
hydrophobicity.
Suitable amino acids include those with a functional group on the side chain.
The
side chain functional group can include, for natural amino acids, an amine
(e.g.,
alkyl amine, heteroaryl amine), hydroxyl, phenol, carboxyl, thiol, thioether,
or
amidino group.
Any of the natural amino acids can be employed as a recognition element.
The natural amino acids include aliphatic amino acids (e.g., alanine, valine,
leucine,
68
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
and isoleucine), hydroxyamino acids (e.g., serine, threonine, and tyrosine),
dicarboxylic acids (e.g., glutamic acid and aspartic acid), amides (e.g.,
glutamine
and asparagine), amino acids with basic sidechains (e.g., lysine,
hydroxylysine,
histidine, and arginine), aromatic amino acids (e.g., histidine,
phenylalanine,
tyrosine, tryptophan, and thyroxine), sulfur containing amino acids (e.g.,
cysteine,
cystine, and methionine), imino acids (e.g., proline and hydroxyproline).
Natural
amino acids suitable for use as recognition elements include, for example,
serine,
threonine, tyrosine, aspartic acid, glutamic acid, asparagine, glutamine,
cysteine,
lysine, arginine, histidine.
Synthetic amino acids can include the naturally occurring side chain
functional groups or synthetic side chain functional groups which modify or
extend
the natural amino acids with alkyl, substituted alkyl, cycloalkyl,
heterocyclic,
substituted heterocyclic, aryl alkyl, aryl, heteroaryl, heteroaryl alkyl, and
like
moieties as framework and with carboxyl, amine, hydroxyl, phenol, carbonyl, or
thiol functional groups. Preferred synthetic amino acids include ,Q-amino
acids and
homo or (3 analogs of natural amino acids.
In an embodiment, the building block is or includes a dipeptide. Any of the
400 dipeptides including the 20 natural amino acids in any order can be
employed as
building blocks. Suitable dipeptides include muramyl dipeptide or the like.
In an embodiment the recognition element can be or include a therapeutic or
pharmacologically active agent. Suitable therapeutic or pharmacologically
active
agents include a nitrate, nitric oxide, a nitric oxide promoter, nitric oxide
donors,
dipyridamole, or another vasodilator; HYTRIN~ or another antihypertensive
agent;
a glycoprotein IIb/IIIa inhibitor (abciximab) or another inhibitor of surface
glycoprotein receptors; aspirin, ticlopidine, clopidogrel or another
antiplatelet agent;
colchicine or another antimitotic, or another microtubule inhibitor; a
retinoid or
another antisecretory agent; cytochalasin or another actin inhibitor;
methotrexate or
another antimetabolite or antiproliferative agent; tamoxifen citrate, TAXOL~,
paclitaxel, or derivatives thereof, rapamycin, vinblastine, vincristine,
vinorelbine,
etoposide, tenopiside, dactinomycin (actinomycin D), daunorubicin,
doxorubicin,
idarubicin, an anthracycline, mitoxantrone, bleomycin, plicamycin
(mithramycin),
mitomycin, mechlorethamine, cyclophosphamide and its analogs, chlorambucil, an
ethylenimine, a methylmelamine, an alkyl sulfonate (e.g., busulfan), a
nitrosourea
69
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
(carmustine, etc.), streptozocin, methotrexate (used with many indications),
fluorouracil, floxuridine, cytarabine, mercaptopurine, thioguanine,
pentostatin, 2-
chlorodeoxyadenosine, cisplatin, carboplatin, procarbazine, hydroxyurea, or
other
anti-cancer chemotherapeutic agents; cyclosporin, tacrolimus (FK-506),
azathioprine, mycophenolate mofetil, mTOR inhibitors, or another
immunosuppressive agent; cortisol, cortisone, dexamethasone, dexamethasone
sodium phosphate, dexamethasone acetate, ~a dexamethasone derivative,
betamethasone, fludrocortisone, prednisone, prednisolone, 6U-
methylprednisolone,
triamcinolone (e.g., triamcinolone acetonide), or another steroidal agent;
trapidil (a
PDGF antagonist); dopamine, bromocriptine mesylate, pergolide mesylate, or
another dopamine agonist; captopril, enalapril or another angiotensin
converting
enzyme (ACE) inhibitor; angiotensin receptor blockers; ascorbic acid, alpha
tocopherol, deferoxamine, a 21-aminosteroid (lasaroid) or another free radical
scavenger, iron chelator or antioxidant; estrogen or another sex hormone; AZT
or
another antipolymerase; acyclovir, famciclovir, rimantadine hydrochloride,
ganciclovir sodium, Norvir, Crixivan, a-methyl-1-adamantanemethylamine,
hydroxy-ethoxymethylguanine, adamantanamine, 5-iodo-2'-deoxyuridine,
trifluorothymidine, adenine arabinoside, or another antiviral agent; 5-
aminolevulinic
acid, meta-tetrahydroxyphenylchlorin, hexadecafluorozinc phthalocyanine,
tetramethyl hematoporphyrin, rhodamine 123 or other photodynamic therapy
agents;
PROSCAR~, HYTRIN~ or other agents for treating benign prostatic hyperplasia
(BHP); mitotane, aminoglutethimide, breveldin, acetaminophen, etodalac,
tolmetin,
ketorolac, ibuprofen and derivatives, mefenamic acid, meclofenamic acid,
piroxicam, tenoxicam, phenylbutazone, oxyphenbutazone, nabumetone, auranofm,
aurothioglucose, gold sodium thiomalate, a mixture of any of these, or
derivatives of
any of these.
In an embodiment, the recognition element can be or can include an
antibiotic. Examples of antibiotics include penicillin, tetracycline,
chloramphenicol,
minocycline, doxycycline, vancomycin, bacitracin, kanamycin, neomycin,
gentamycin, erythromycin and cephalosporins. Examples of cephalosporins
include
cephalothin, cephapirin, cefazolin, cephalexin, cephradine, cefadroxil,
cefamandole,
cefoxitin, cefaclor, cefuroxime, cefonicid, ceforanide, cefotaxime,
moxalactam,
ceftizoxime, ceftriaxone, and cefoperazone.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, the recognition element can be or can include an enzyme
inhibitor. Suitable enzyme inhibitors include edrophonium chloride, N-
methylphysostigmine, neostigmine bromide, physostigmine sulfate, tacrine HCL,
tacrine, 1-hydroxy maleate, iodotubercidin, p-bromotetramisole, 10-(cx
diethylaminopropionyl)-phenothiazine hydrochloride, calinidazolium chloride,
hemicholinium-3,3,5-dinitrocatecho-l, diacylglycerol kinase inhibitor I,
diacylglycerol kinase inhibitor II, 3-phenylpropargylaminie, N-monomethyl-L-
arginine acetate, carbidopa, 3-hydroxybenzylhydrazine HCl, hydralazine HCI,
clorgyline HCI, deprenyl HCl L(-), deprenyl HCl D(+), hydroxylamine HCI,
iproniazid phosphate, 6-Me0-tetrahydro-9H-pyrido-indole, nialamide, pargyline
HCI, quinacrine HCI, semicarbazide HCI, tranylcypromine HCI, N,N-
diethylaminoethyl-2,2-di-phenylvalerate hydrochloride, 3-isobutyl-1-
methylxanthne,
papaverine HCI, indomethacind, 2-cyclooctyl-2-hydroxyethylamine hydrochloride,
2,3-dichloro- a -methylbenzylamine (DCMB), 8,9-dichloro-2,3,4,5-tetrahydro-1H-
2-
benzazepine hydrochloride, p-aminoglutethimide, p-aminoglutethimide tartrate
R(+), p-aminoglutethimide tartrate S(-), 3-iodotyrosine, alpha-methyltyrosine
L(-),
alpha-methyltyrosine D(-), cetazolamide, dichlorphenamide, 6-hydroxy-2-
benzothiazolesulfonamide, allopurinol, and the like.
In an embodiment, the recognition element is or includes a signal element
that produces a detectable signal when a test ligand is bound to the receptor.
In an
embodiment, the signal element can produce an optical signal or a
electrochemical
signal. Suitable optical signals include chemiluminescence or fluorescence.
The
signal element can be a fluorescent moiety. The fluorescent molecule can be
one
that is quenched by binding to~the artificial receptor. For example, the
signal
element can be a molecule that fluoresces only when binding occurs. Suitable
electrochemical signal elements include those that give rise to current or a
potential.
Suitable electrochemical signal elements include phenols and anilines, such as
those
with substitutents oriented ortho or para to one another, polynuclear aromatic
hydrocarbons, sulfide-disulfide, sulfide-sulfoxide-sulfone, polyenes,
polyeneynes,
and the like. Suitable electrochemical signal elements include quinones and
ferrocenes.
71
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Linkers
The linker is selected to provide a suitable coupling of the building block to
a
support. The framework can interact with the ligand as part of the artificial
receptor.
The linker can also provide bulk, distance from the support, hydrophobicity,
hydrophilicity, and like structural characteristics to the building block.
Coupling
building blocks to the support can employ covalent bonding or noncovalent
interactions. Suitable noncovalent interactions include interactions between
ions,
hydrogen bonding, van der Waals interactions, and the like. In an embodiment,
the
linker includes moieties that can engage in covalent bonding or noncovalent
interactions. In an embodiment, the linker includes moieties that can engage
in
covalent bonding. ' Suitable groups for forming covalent and reversible
covalent
bonds are described hereinabove.
Linkers for Reversibly Irmnobilizable Building Blocks
The linker can be selected to provide suitable reversible immobilization of ,
the building block on a support or lawn. In an embodiment, the linker forms a
covalent bond with a functional group on the framework. In an embodiment, the
linker also includes a functional group that can reversibly interact with the
support
or lawn, e.g., through reversible covalent bonding or noncovalent
interactions.
i
Tn an embodiment, the linker includes one or more moieties that can engage
in reversible covalent bonding. Suitable groups for reversible covalent
bonding
include those described hereinabove. An artificial receptor can include
building
blocks reversibly immobilized on the lawn or support through, for example,
imine,
acetal, ketal, disulfide, ester, or like linkages. Such functional groups can
engage in
reversible covalent bonding. Such a functional group can be referred to as a
covalent bonding moiety, e.g., a second covalent bonding moiety.
In an embodiment, the linker can be functionalized with moieties that can
engage in noncovalent interactions. For example, the linker can include
functional
groups such as an ionic group, a group that can hydrogen bond, or a group that
can
engage in van der Waals or other hydrophobic interactions. Such functional
groups
can include cationic groups, anionic groups, lipophilic groups, amphiphilic
groups,
and the like.
72
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, the present methods and compositions can employ a
linker including a charged moiety (e.g., a second charged moiety). Suitable
charged
moieties include positively charged moieties and negatively charged moieties.
Suitable positively charged moieties include amines, quaternary ammonium
moieties, sulfonium, phosphonium, ferrocene, and the like. Suitable negatively
charged moieties (e.g., at neutral pH in aqueous compositions) include
carboxylates,
phenols substituted with strongly electron withdrawing groups (e.g.,
tetrachlorophenols), phosphates, phosphonates, phosphinates, sulphates,
sulphonates, thiocarboxylates, and hydroxamic acids.
In an embodiment, the present methods and compositions can employ a
linker including a group that can hydrogen bond, either as donor or acceptor
(e.g., a
second hydrogen bonding group). For example, the linker can include one or
more
carboxyl groups, amine groups, hydroxyl groups, carbonyl groups, or the like.
Ionic
groups can also participate in hydrogen bonding.
In an embodiment, the present methods and compositions can employ a
linker including a lipophilic moiety (e.g., a second lipophilic moiety).
Suitable
lipophilic moieties include one or more branched or straight chain C6_36
alkyl, Cg_z4
alkyl, Clz-za alkyl, Clz-is alkyl, or the like; C~_3s alkenyl, C8_z4 alkenyl,
Clz-z4 alkenyl,
Ciz-is alkenyl, or the like, with, for example, 1 to 4 double bonds; C6-ss
alkynyl, Cg_z4
alkynyl, Clz-z4 alkynyl, Clz-is alkynyl, or the like, with, for example, 1 to
4 triple
bonds; chains with 1-4 double or triple bonds; chains including aryl or
substituted
aryl moieties (e.g., phenyl or naphthyl moieties at the end or middle of a
chain);
polyaromatic hydrocarbon moieties; cycloalkane or substituted alkane moieties
with
numbers of carbons as described for chains; combinations or mixtures thereof;
or the
like. The alkyl, alkenyl, or alkynyl group can include branching; within chain
functionality like an ether group; terminal functionality like alcohol, amide,
carboxylate or the like; or the like. In an embodiment the linker includes or
is a
lipid, such as a phospholipid. In an embodiment, the lipophilic moiety
includes or is
a 12-carbon aliphatic moiety.
In an embodiment, the linker includes a lipophilic moiety (e.g., a second
lipophilic moiety) and a covalent bonding moiety (e.g., a second covalent
bonding
moiety). In an embodiment, the linker includes a lipophilic moiety (e.g., a
second
lipophilic moiety) and a charged moiety (e.g., a second charged moiety).
73
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, the linker forms or can be visualized as forming a
covalent bond with an alcohol, phenol, thiol, amine, carbonyl, or like group
on the
framework. Between the bond to the framework and the group participating in or
formed by the reversible interaction with the support or lawn, the linker can
include
an alkyl, substituted alkyl, cycloalkyl, heterocyclic, substituted
heterocyclic, aryl
alkyl, aryl, heteroaryl, heteroaryl alkyl, ethoxy or propoxy oligomer, a
glycoside, or
like moiety.
For example, suitable linkers can include: the functional group participating
in or formed by the bond to the framework, the functional group or groups
participating in or formed by the reversible interaction with the support or
lawn, and
a linker backbone moiety. The linker backbone moiety can include about 4 to
about
48 carbon or heteroatoms, about 8 to about 14 carbon or heteroatoms, about 12
to
about 24 carbon or heteroatoms, about 16 to about 18 carbon or heteroatoms,
about
4 to about 12 carbon or heteroatoms, about 4 to about 8 carbon or heteroatoms,
or
the like. The linker backbone can include an alkyl, substituted alkyl,
cycloalkyl,
heterocyclic, substituted heterocyclic, aryl alkyl, aryl, heteroaryl,
heteroaryl alkyl,
ethoxy or propoxy oligomer, a glycoside, mixtures thereof, or like moiety.
In an embodiment, the linker includes a lipophilic moiety, the functional
group participating in or formed by the bond to the framework, and,
optionally, one
or more moieties for forming a reversible covalent bond, a hydrogen bond, or
an
ionic interaction. In such an embodiment, the lipophilic moiety can have about
4 to
about 48 carbons, about 8 to about 14 carbons, about 12 to about 24 carbons,
about
16 to about 18 carbons, or the like. In such an embodiment, the linker can
include
about 1 to about 8 reversible bond/interaction moieties or about 2 to about 4
reversible bond/interaction moieties. Suitable linkers have structures such as
(CHZ)"COOH, with n=12-24, n=17-24, or n=16-18.
Additional Embodiments of Linlcers
The linker can be selected to provide a suitable covalent coupling of the
building block to a support. The framework can interact with the ligand as
part of
the artificial receptor. The linker can also provide bulk, distance from the
support,
hydrophobicity, hydrophilicity, and like structural characteristics to the
building
block. In an embodiment, the linker forms a covalent bond with a functional
group
74
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
on the framework. In an embodiment, before attachment to the support the
linker
also includes a functional group that can be activated to react with or that
will react
with a functional group on the support. In an embodiment, once attached to the
support, the linker forms a covalent bond with the support and with the
framework.
In an embodiment, the linker forms or can be visualized as forming a
covalent bond with an alcohol, phenol, thiol, amine, carbonyl, or like group
on the
framework. The linker can include a carboxyl, alcohol, phenol, thiol, amine,
carbonyl, maleimide, or like group that can react with or be activated to
react with
the support. Between the bond to the framework and the group formed by the
attachment to the support, the linker can include an alkyl, substituted alkyl,
cycloalkyl, heterocyclic, substituted heterocyclic, aryl alkyl, aryl,
heteroaryl,
heteroaryl alkyl, ethoxy or propoxy oligomer, a glycoside, or like moiety.
The linker can include a good leaving group bonded to, for example, an alkyl
or aryl group. The leaving group being "good" enough to be displaced by the
alcohol, phenol, thiol, amine, carbonyl, or like group on the framework. Such
a
linker can include a moiety represented by the formula: R-X, in which X is a
leaving group such as halogen (e.g., -Cl, -Br or -I), tosylate, mesylate,
triflate, and R
is alkyl, substituted alkyl, cycloalkyl, heterocyclic, substituted
heterocyclic, aryl
alkyl, aryl, heteroaryl, heteroaryl alkyl, ethoxy or propoxy oligomer, a
glycoside, or
like moiety.
Suitable linker groups include those of formula: (CHZ)"COOH, with n=1-16,
n=2-8, n=2-6, or n=3. Reagents that form suitable linkers are commercially
available and include any of a variety of reagents with orthogonal
functionality.
Additional Embodiments of Building Blocks
In an embodiment, building blocks can be represented by Formula 2:
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
~z
Y
C=O
REI X N C R3 Z1 L
H i
R2
in which: RE1 is recognition element 1, RE2 is recognition element 2, and L is
a
linker. X is absent, C=O, CHz, NR, NR2, NH, NHCONH, SCONH, CH=N, or
OCHZNH. In certain embodiments, X is absent or C=O. Y is absent, NH, O, CH2,
or NRCO. In certain embodiments, Y is NH or O. In an embodiment, Y is NH. Z1
and Z2 can independently be CH2, O, NH, S, CO, NR, NRa, NHCONH, SCONH,
CH=N, or OCH2NH. In an embodiment, Zl and/or Z2 can independently be O. Z2 is
optional. R2 is H, CH3, or another group that confers chirality on the
building block
and has size similar to or smaller than a methyl group. R3 is CH2; CHZ-phenyl;
CHCH3; (CHZ)" with n=2-3; or cyclic alkyl with 3-8 carbons, e.g., 5-6 carbons,
phenyl, naphthyl. In certain embodiments, R3 is CH2 or CH2-phenyl.
REl is B1, B2, B3, B3a, B4, B5, B6, B7, B8, B9, A1, A2, A3, A3a, A4, A5,
A6, A7, A8, or A9. In certain embodiments, REl is B1, B2, B3, B3a, B4, B5, B6,
B7, B8, or B9. REZ is A1, A2, A3, A3a, A4, A5, A6, A7, A8, A9, B1, B2, B3,
B3a,
B4, B5, B6, B7, B8, or B9. In certain embodiments, REZ is A1, A2, A3, A3a, A4,
A5, A6, A7, A8, or A9. In an embodiment, RE1 can be B2, B3a, B4, B5, B6, B7,
or
B8. Tn an embodiment, REa can be A2, A3a, A4, A5, A6, A7, or A8.
hi an embodiment, L is the functional group participating in or formed by the
bond to the framework (such groups are described herein), the functional group
or
groups participating in or formed by the reversible interaction with the
support or
lawn (such groups are described herein), and a linker backbone moiety. In an
embodiment, the linker backbone moiety is about 4 to about 48 carbon or
heteroatom alkyl, substituted allcyl, cycloalkyl, heterocyclic, substituted
heterocyclic, aryl alkyl, aryl, heteroaryl, heteroaxyl alkyl, ethoxy or
propoxy
oligomer, a glycoside, or mixtures thereof; or about 8 to about 14 carbon or
76
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
heteroatoms, about 12 to about 24 carbon or heteroatoms, about 16 to about 18
carbon or heteroatoms, about 4 to about 12 carbon or heteroatoms, about 4 to
about
8 carbon or heteroatoms.
In an embodiment, the L is the functional group participating in or formed by
the bond to the framework (such groups are described herein) and a lipophilic
moiety (such groups are described herein) of about 4 to about 48 carbons,
about 8 to
about 14 carbons, about 12 to about 24 carbons, about 16 to about 18 carbons.
In an
embodiment, this L also includes about 1 to about 8 reversible
bond/interaction
moieties (such groups are described herein) or about 2 to about 4 reversible
bond/interaction moieties. In an embodiment, L is (CH2)"COOH, with n=12-24,
n=17-24, or n=16-18.
In an embodiment, L is (CH2)"COOH, with n=1-16, n=2-8, n=4-6, or n=3.
Building blocks including an A and/or a B recognition element, a linker, and
an amino acid framework can be made by methods illustrated in general Scheme
1.
Building Blocks Including a Tether
A building block can be visualized as including several components, such as
one or more frameworks, one or more linkers, one or more recognition elements,
and/or one or more tethers. The framework can be covalently coupled to each of
the
other building block components. The linker can be covalently coupled to the
framework. The linker can be coupled to a support through one or more of
covalent,
electrostatic, hydrogen bonding, van der Waals, or like interactions. The
recognition
element can be covalently coupled to the framework. The tether can be
covalently
coupled to the linker and to the framework.
In an embodiment, a building block includes a framework, a linker, a
recognition element, and a tether. In an embodiment, a building block includes
a
framework, a linker, a tether, and two recognition elements. The framework can
be
selected for functional groups that provide for coupling to the recognition
moiety
and for coupling to or being the tether and/or linking moieties.
In an embodiment, the present invention relates to a building block including
a tether moiety. The tether can include the framework. The tether moiety can
provide spacing or distance between the recognition element and the support or
scaffold to which the building block is immobilized. A tether moiety can have
any
of a variety of characteristics or properties including flexibility, rigidity
or stiffness,
77
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
ability to bond to another tether moiety, and the like. The tether moiety can
include
the linker. The framework moiety be envisioned as forming all or part of the
tether
moiety.
Suitable tether moieties can include a polyethylene glycol, a polyamide, a
linear polymer, a peptide, a polypeptide, an oligosaccharide, a
polysaccharide, a
semifunctionalized oligo- or polyglycine. In an embodiment, the tether is or
includes a polyner of up to 2000 carbon atoms (e.g., up to 48 carbon atoms).
Such a
polymer can be naturally occurring or synthetic. Suitable polymers include a
polyether or like polymer, such as a PEG, a polyethyleneimine, polyacrylate
(e.g.,
substituted polyacrylates), salt thereof, a mixture or combination thereof, or
the like.
Suitable PEGS include PEG 1500 up to PEG 20,000, for example, PEG 1450, PEG
3350, PEG 4500, PEG 8000, PEG 20,000, and the like.
Suitable tether moieties can include one or more branched or straight chain
C6-36 alkyl, C8_z4 alkyl, Clz-z4 alkyl, Clz_is alkyl, or the like; C6_36
alkenyl, C8_z4
alkenyl, Clz-z4 alkenyl, Clz-is alkenyl, or the like, with, for example, 1 to
4 double
bonds; C6_3~ alkynyl, C8_z4 alkynyl, Clz_z4 alkynyl, Clz_1$ alkynyl, or the
like, with,
for example, 1 to 4 triple bonds; chains with 1-4 double or triple bonds;
chains
including aryl or substituted aryl moieties (e.g., phenyl or naphthyl moieties
at the
end or middle of a chain); polyaromatic hydrocarbon moieties; cycloalkane or
substituted alkane moieties with numbers of carbons as described for chains;
combinations or mixtures thereof; or the like. The alkyl, alkenyl, or alkynyl
group
can include branching; within chain functionality like an ether group;
terminal
functionality like alcohol, amide, carboxylate or the like; or the like. In an
embodiment, the lipophilic moiety includes or is a 12-caxbon aliphatic moiety.
Rigid tether moieties can include conformationally restricted groups such as
imines, aromatics, and polyaromatics. Rigid tether moieties can include one or
more
branched or straight chain C~_3~ alkenyl, C8_z4 alkenyl, Clz-z4 alkenyl, Clz-
is alkenyl,
or the like, with, for example, 2 to 8 double bonds; C6_36 alkynyl, C8_z4
alkynyl, Clz-
z4 alkynyl, Clz_l8 alkynyl, or the like, with, for example, 1 to 8 triple
bonds; chains
with 3-8 double or triple bonds; chains including aryl or substituted aryl
moieties
(e.g., phenyl or naphthyl moieties at the end or middle of a chain);
polyaromatic
hydrocarbon moieties; and the like. The alkenyl or alkynyl group can include
branching; within chain functionality like an ether group; terminal
functionality like
78
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
alcohol, amide, carboxylate or the like; or the like. Rigid tether moieties
can include
a steroid moiety, such as cholesterol, a cornn or another porphyrin, a
polynuclear
aromatic moiety, a polar polymer fixed with metal ions, or the like.
In an embodiment, a rigid tether moiety can include more than one tether
moiety. For example, a rigid tether moiety can include a plurality of
hydrophobic
chains, such as those described in the paragraph above and in the paragraph
below.
The hydrophobic chains if held in sufficient proximity on the support or
scaffold
will, in a hydrophobic solvent, form a grouping sufficiently rigid to hold one
or
more sets of recognition elements in place. In another embodiment, a rigid
tether
moiety can include a plurality of otherwise flexible tether moieties
crosslinked to
one another. The crosslinking can include, for example, covalent bonding,
electrostatic interactions, hydrogen bonding, or hydrophobic interactions.
Groups
for forming such interactions are disclosed herein.
Flexible tether moieties can include one or more branched or straight chain
C~_3~ alkyl, Cs_24 alkyl, C12_24 alkyl, Cla-la alkyl, or the like; C6_36
alkenyl, Cg_24
alkenyl, C12_24 alkenyl, Cla-is alkenyl, or the like, with, for example, 1 to
2 double
bonds; C6_3s alkynyl, Cs_24 alkynyl, Clz-z4 alkynyl, Clz_ls alkynyl, or the
like, with,
for example, 1 to 2 triple bonds; chains with 1-2 double or triple bonds;
chains
including 1 to 2 aryl or substituted aryl moieties (e.g., phenyl or naphthyl
moieties at
the end or middle of a chain); cycloalkane or substituted alkane moieties with
numbers of carbons as described for chains; combinations or mixtures thereof;
or the
like. The alkyl, alkenyl, or alkynyl group can include branching; within chain
functionality like an ether group; terminal functionality like alcohol, amide,
carboxylate or the like; or the like. In an embodiment, the lipoplulic moiety
includes
or is a 12-carbon aliphatic moiety.
In an embodiment, the tether forms or can be visualized as forming a
covalent bond with an alcohol, phenol, thiol, amine, carbonyl, or like group
on the
frameworlc. Between the bond to the framework and the group participating in
or
formed by the interaction with the support or lawn, the linker can include an
alkyl,
substituted alkyl, cycloalkyl, heterocyclic, substituted heterocyclic, aryl
alkyl, aryl,
heteroaryl, heteroaryl alkyl, ethoxy or propoxy oligomer, a glycoside, or like
moiety.
Suitable tethers can include, for example: the functional group participating
in or formed by the bond to the framework, the functional group or groups
79
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
participating in or formed by the interaction with the support or lawn, and a
tether
backbone moiety. The tether backbone moiety can include about 8 to about 200
carbon or heteroatoms, about 12 to about 150 carbon or heteroatoms, about 16
to
about 100 carbon or heteroatoms, about 16 to about 50 carbon or heteroatoms,
or the
like. The tether backbone can include an alkyl, substituted alkyl, cycloalkyl,
heterocyclic, substituted heterocyclic, aryl alkyl, aryl, heteroaryl,
heteroaryl alkyl,
ethoxy or propoxy oligomer, a glycoside, mixtures thereof, or like moiety.
Suitable
tethers have structures such as (CH2)"COOH, with n=12-24, n=17-24, or n=16-18.
The tether can interact with the ligand as part of the artificial receptor.
The
tether can also provide bulk, distance from the support, hydrophobicity,
hydrophilicity, and like structural characteristics to the building block. In
an
embodiment, the tether forms a covalent bond with a functional group on the
framework. In an embodiment, the tether also includes a functional group that
can
couple to the tether or to the support or lawn, e.g., through covalent bonding
or
noncovalent interactions.
In an embodiment, the tether includes one or more moieties for forming a
reversible covalent bond, a hydrogen bond, or an ionic interaction, e.g., with
another
tether moiety. For example, the linker can include about 1 to about 20
reversible
bondlinteraction moieties or about 2 to about 10 reversible bondlinteraction
moieties.
In an embodiment, the tether includes one or more moieties that can engage
in reversible covalent bonding. Suitable groups for reversible covalent
bonding
include those described hereinabove. Such groups for reversible covalent bonds
can
be part of links between tether moieties. The tether-tether links can include,
for
example, imine, acetal, ketal, disulfide, ester, or like linkages. Such
functional
groups can engage in reversible covalent bonding. Such a functional group can
be
referred to as a covalent bonding moiety.
In an embodiment, the tether can be functionalized with moieties that can
engage in noncovalent interactions. For example, the tether can include
functional
groups such as an ionic group, a group that can hydrogen bond, or a group that
can
engage in van der Waals or other hydrophobic interactions. Such functional
groups
can include cationic groups, anionic groups, lipophilic groups, amphiphilic
groups,
and the like.
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
In an embodiment, the present methods and compositions can employ a
tether including a charged moiety. Suitable charged moieties include
positively
charged moieties and negatively charged moieties. Suitable positively charged
moieties include protonated amines, quaternary ammonium moieties, sulfonium,
sulfoxonium, phosphonium, ferrocene, and the like. Suitable negatively charged
moieties (e.g., at neutral pH in aqueous compositions) include carboxylates,
phenols
substituted with strongly electron withdrawing groups (e.g.,
tetrachlorophenols),
phosphates, phosphonates, phosphinates, sulphates, sulphonates,
thiocarboxylates,
and hydroxamic acids.
In an embodiment, the present methods and compositions can employ a
tether including a group that can hydrogen bond, either as donor or acceptor
(e.g., a
second hydrogen bonding group). For example, the tether can include one or
more
carboxyl groups, amine groups, hydroxyl groups, carbonyl groups, or the like.
Ionic
groups can also participate in hydrogen bonding.
In an embodiment, building blocks can be represented by Formula 3:
~2
Y
C=O
RE1 X NH ~ R Z2 T Z1-L
R2
in which: RE1 is recognition element 1, RE2 is recognition element 2, T is an
optional tether, and L is a linker. X is absent, C=O, CHZ, NR, NR2, NH,
NHCONH,
SCONH, CH=N, or OCH2NH. In certain embodiments, X is absent or C=O. Y is
absent, NH, O, CH2, or NRCO. In certain embodiments, Y is NH or O. In an
embodiment, Y is NH. Z1 and Z2 can independently be CH2, O, NH, S, CO, NR,
NR2, NHCONH, SCONH, CH=N, or OCHZNH. In an embodiment, Zl and/or Za
can independently be O. Z2 is optional. R2 is H, CH3, or another group that
confers
chirality on the building block and has size similar to or smaller than a
methyl
group. R3 is CHa; CH2-phenyl; CHCH3; (CHa)" with n=2-3; or cyclic alkyl with 3-
8
81
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
carbons, e.g., 5-6 carbons, phenyl, naphthyl. In certain embodiments, R3 is
CHZ or
CHZ-phenyl.
REl is B1, B2, B3, B3a, B4, BS, B6, B7, B8, B9, A1, A2, A3, A3a, A4, A5,
A6, A7, A8, or A9. In certain embodiments, RE1 is B1, B2, B3, B3a, B4, B5, B6,
B7, B8, or B9. RE2 is A1, A2, A3, A3a, A4, A5, A6, A7, A8, A9, B1, B2, B3,
B3a,
B4, B5, B6, B7, B8, or B9. In certain embodiments, RE2 is Al, A2, A3, A3a, A4,
A5, A6, A7, A8, or A9. In an embodiment, REl can be B2, B3a, B4, B5, B6, B7,
or
B8. In an embodiment, RE2 can be A2, A3a, A4, A5, A6, A7, or A8.
T can be any of the tether moieties described hereinabove.
In an embodiment, L is the functional group participating in or formed by the
bond to the framework (such groups are described herein), the functional group
or
groups participating in or formed by the reversible interaction with the
support or
lawn (such groups are described herein), and a linker backbone moiety. In an
embodiment, the linker backbone moiety is about 4 to about 48 carbon or
heteroatom alkyl, substituted alkyl, cycloalkyl, heterocyclic, substituted
heterocyclic, aryl alkyl, aryl, heteroaryl, heteroaryl alkyl, ethoxy or
propoxy
oligomer, a glycoside, or mixtures thereof; or about 8 to about 14 carbon or
heteroatoms, about 12 to about 24 carbon or heteroatoms, about 16 to about 18
carbon or heteroatoms, about 4 to about 12 carbon or heteroatoms, about 4 to
about
8 carbon or heteroatoms.
In an embodiment, the L is the functional group participating in or formed by
the bond to the framework (such groups are described herein) and a lipophilic
moiety (such groups are described herein) of about 4 to about 48 carbons,
about 8 to
about 14 carbons, about 12 to about 24 carbons, about 16 to about 18 carbons.
In an
embodiment, this L also includes about 1 to about 8 reversible
bond/interaction
moieties (such groups are described herein) or about 2 to about 4 reversible
bond/interaction moieties. In an embodiment, L is (CH2)"COOH, with n=12-24,
n=17-24, or n=16-18.
In an embodiment, L is (CHz)"COOH, with n=1-16, n=2-8, n=4-6, or n=3.
Building blocks including an A and/or a B recognition element, a linker, and
an amino acid framework can be made by methods illustrated in general Scheme
1.
The present invention may be better understood with reference to the
following examples. These examples are intended to be representative of
specific
82
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
embodiments of the invention, and are not intended as limiting the scope of
the
invention.
EXAMPLES
Example 1 - Synthesis of Building Blocks
Selected building blocks representative of the alkyl-aromatic-polar span of
the an embodiment of the building blocks were synthesized and demonstrated
effectiveness of these building blocks for making candidate artificial
receptors.
These building blocks were made on a framework that can be represented by
tyrosine and included numerous recognition element pairs. These recognition
element pairs include enough of the range from alkyl, to aromatic, to polar to
represent a significant degree of the interactions and functional groups of
the full set
of 81 such building blocks.
Synthesis
Building block synthesis employed a general procedure outlined in Scheme
1, which specifically illustrates synthesis of a building block on a tyrosine
framework with recognition element pair A4B4. This general procedure was
employed for synthesis of building blocks including TyrAlB1 [1-1], TyrA2B2,
TyrA2B4, TyrA2B6, TyrA2B8, TyrA4B2, TyrA4B4, TyrA4B6, TyrA4B8,
TyrA6B2, TyrA6B4, TyrA6B6, TyrA6B8, TyrA8B2, TyrA8B4, TyrA8B6,
TyrA8B8, and TyrA9B9, respectively.
83
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
H
NH(BOC~ NOT ISOLATED
R-NH2 / EDCC I H H
TYR-BOC > ~ N
H y~~~~H
O
OCH3
Br~ester / K2C03
Et00 O
I ~IH{BOC)
H H
N
4-X BOC Intermediate
H ~~~~~H
O
OCH3
TFA
Et00 O
dH2 NOT ISOLATED
H H
N
H~ y't~~H
O
OCH3
R-COCI / TEA
4-4 ESTE
R-COOH [4-4]
Et00
Scheme 1
Results
Synthesis of the desired building blocks proved to be generally
straightforward. These syntheses illustrate the relative simplicity of
preparing the
building blocks with 2 recognition elements having different structural
84
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
characteristics or structures (e.g. A4B2, A6B3, etc.) once the building blocks
with
corresponding recognition elements (e.g. A2B2, A4B4, etc) have been prepared
via
their X BOC intermediate.
The conversion of one of these building blocks to a building block with a
lipophilic linker can be accomplished by reacting the activated building block
with,
for example, dodecyl amine.
Example 2 - Preparation and Evaluation of Microarrays of Candidate Artificial
Receptors
Microarrays of candidate artificial receptors were made and evaluated for
binding several protein ligands. The results obtained demonstrate the 1) the
simplicity with which microarrays of candidate artificial receptors can be
prepared,
2) binding affinity and binding pattern reproducibility, 3) significantly
improved
binding for building block heterogeneous receptor enviromnents when compared
to
the respective homogeneous controls, and 4) ligand distinctive binding
patterns (e.g.,
working receptor complexes).
Materials and Methods
Building blocks were synthesized and activated as described in Example 1.
The building blocks employed in this example were TyrAlBl [1-1], TyrA2B2,
TyrA2B4, TyrA2B6, TyrA4B2, TyrA4B4, TyrA4B6, TyrA6B2, TyrA6B4, and
TyrA6B6. The abbreviation for the building block including a linker, a
tyrosine
framework, and recognition elements AxBy is TyrAxBy.
Microarrays for the evaluation of the 130 n=2 and n=3, and for evaluation of
the 273 n=2, n=3, and n=4, candidate receptor enviromnents were prepared as
follows by modifications of known methods. As used herein, "n" is the number
of
different building blocks employed in a receptor environment. Briefly: Amine
modified (amine "lawn"; SuperAmine Microarray plates) microarray plates were
purchased from Telechem Inc., Sunnyvale, CA (www.arrayit.com). These plates
were manufactured specifically for microarray preparation and had a nominal
amine
load of 2-4 amines per square nm according to the manufacturer. The CAM
microarrays were prepared using a pin microarray spotter instrument from
Telechem
Inc. (SpotBotTM Arrayer) typically with 200 um diameter spotting pins from
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Telechem Inc. (Stealth Micro Spotting Pins, SMP6) and 400-420 um spot spacing.
The 9 building blocks were activated in aqueous dimethylformamide (DMF)
solution as described above. For preparing the 384-well feed plate, the
activated
building block solutions were diluted 10-fold with a solution of
DMF/HZO/PEG400
(90/10/10, v/v/v; PEG400 is polyethylene glycol nominal 400 FW, Aldrich
Chemical Co., Milwaukee, WI). These stock solutions were aliquotted (10 ,u1
per
aliquot) into the wells of a 384-well microwell plate (Telechem Inc.). A
separate
series of controls were prepared by aliquotting 10 ,u1 of building block with
either 10'
~.1 or 20 ~,l of the activated [1-1] solution. The plate was covered with
aluminum
foil and placed on the bed of a rotary shaker for 15 minutes at 1,000 RPM.
This
master plate was stored covered with aluminum foil at -20°C when not in
use.
For preparing the 384-well SpotBotTM plate, a well-to-well transfer (e.g. A-1
to A-l, A-2 to A-2, etc.) from the feed plate to a second 384-well plate was
performed using a 4 ~,1 transfer pipette. This plate was stored tightly
covered with
aluminum foil at -20°C when not in use. The SpotBotTM was used to
prepare up to
13 microarray plates per run using the 4 ,u1 microwell plate. The SpotBotTM
was
programmed to spot from each microwell in quadruplicate. The wash station on
the
SpotBotTM used a wash solution of EtOH/H20 (20/80, v/v). This wash solution
was
also used to rinse the microarrays on completion of the SpotBotTM printing
run. The
plates were given a final rinse with deionized (DI) water, dried using a
stream of
compressed air, and stored at room temperature.
Certain of the microarrays were further modified by reacting the remaining
amines with succinic anhydride to form a carboxylate lawn in place of the
amine
lawn.
The following test ligands and labels were used in these experiments:
1) r-Phycoerythrin, a commercially available and intrinsically fluorescent
protein with a FW of 2,000,000.
2) Ovalbumin labeled with the AlexaTM fluorophore (Molecular Probes Inc.,
Eugene, OR).
3) BSA, bovine serum albumin, labeled with activated Rhodamine (Pierce
Chemical, Rockford, IL) using the known activated carboxyl protocol. BSA has a
FW of 68,000; the material used for this study had ca. 1.0 rhodamine per BSA.
4) Horseradish peroxidase (HRP) modified with extra amines and labeled as
86
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
the acetamide derivative or with a 2,3,7,8-tetrachlorodibenzodixoin derivative
were
available through known methods. Fluorescence detection of these HRP
conjugates
was based on the Alexa 647-tyramide kit available from Molecular Probes,
Eugene,
OR.
5) Cholera toxin labeled With the AlexaTM fluorophore (Molecular Probes
Inc., Eugene, OR).
Microarray incubation and analysis was conducted as follows: For test
ligand incubation with the microarrays, solutions (e.g. 500 ~,l) of the target
proteins
in PBS-T (PBS with 20 p,lJL of Tween-20) at typical concentrations of 10, 1.0
and
0.1 ~,g/ml were placed onto the surface of a microarray and allowed to react
for, e.g.,
30 minutes. The microarray was rinsed with PBS-T and DI water and dried using
a
stream of compressed air.
The incubated microarray was scanned using an Axon Model 4200A
Fluorescence Microarray Scanner (Axon Instruments, Una.on City, CA). The Axon
scanner and its associated software produce a false color 16-bit image of the
fluorescence intensity of the plate. This 16-bit data is integrated using the
Axon
software to give a Fluorescence Units value (range 0 - 65,536) for each spot
on the
microarray. This data is then exported into an Excel file (Microsoft) for
further
analysis including mean, standard deviation and coefficient of variation
calculations.
Results
The CARATM: Combinatorial Artificial Receptor ArrayTM concept has been
demonstrated using a microarray format. A CARA microarray based on N=9
building blocks was prepared and evaluated for binding to several protein and
substituted protein ligands. This microarray included 144 candidate receptors
(18
n=1 controls plus 6 blanks; 36 n=2 candidate receptors; 84 n=3 candidate
receptors).
This microarray demonstrated: 1) the simplicity of CARA microarray
preparation,
2) binding affinity and binding pattern reproducibility, 3) significantly
improved
binding for building block heterogeneous receptor environments when compared
to
the respective homogeneous controls, and 4) ligand distinctive binding
patterns.
Reading the Arrays
A typical false colorlgray scale image of a microarray that was incubated
87
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
with 2.0 pg/ml r-phycoerythrin is shown in Figure 14. This image illustrates
that the
processes of both preparing the microarray and probing it with a protein test
ligand
produced the expected range of binding as seen in the visual range of relative
fluorescence from dark to bright spots.
The starting point in analysis of the data was to take the integrated
fluorescence units data for the array of spots and normalize to the observed
value for
the [1-1] building block control. Subsequent analysis included mean, standard
deviation and coefficient of variation calculations. Additionally, control
values for
homogeneous building blocks were obtained from the building block plus [1-1]
data.
First Set of Experiments
The following protein ligands were evaluated for binding to the candidate
artificial receptors in the microarray. The resulting Fluorescence Units
versus
candidate receptor environment data is presented in both a 2D format where the
candidate receptors are placed along the X-axis and the Fluorescence Units are
shown on the Y-axis and a 3D format where the Candidate Receptors are placed
in
an X-Y format and the Fluorescence Units are shown on the Z-axis. A key for
the
composition of each spot was developed (not shown). A key for the building
blocks
in each of the 2D and 3D representations of the results was also developed
(not
shown). The data presented are for 1-2 p.g/ml protein concentrations.
Figures 15 and 16 illustrate binding data for r-phycoerythrin (intrinsic
fluorescence). Figures 17 and 18 illustrate binding data for ovalbumin
(commercially available with fluorescence label). Figures 19 and 20 illustrate
binding data for bovine serum albumin (labeled with rhodamine). Figures 21 and
22
illustrate binding data for HRP-NH-Ac (fluorescent tyramide read-out). Figures
23
and 24 illustrate binding data for HRP-NH-TCDD (fluorescent tyramide read-
out).
These results demonstrate not only the application of the CAR.A microarray
to candidate artificial receptor evaluation but also a few of the many read-
out
methods (e.g. intrinsic fluorescence, fluorescently labeled, ih situ
fluorescence
labeling) which can be utilized for high throughput candidate receptor
evaluation.
The evaluation of candidate receptors benefits from reproducibility. The
following results demonstrate that the present microarrays provided
reproducible
ligand binding.
88
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
The microarrays were printed with each combination of building blocks
spotted in quadruplicate. Visual inspection of a direct plot (Figure 25) of
the raw
fluorescence data (from the run illustrated in Figure 14) for one block of
binding
data obtained for r-phycoerythrin demonstrates that the candidate receptor
environment "spots" showed reproducible binding to the test ligand. Further
analysis of the r-phycoerythrin data (Figure 14) led to only 9 out of 768
spots (1.2%)
being deleted as outliers. Analysis of the r-phycoerythrin quadruplicate data
for the
entire array gives a mean standard deviation for each experimental
quadruplicate set
of 938 fluorescence units, with a mean coefficient of variation of 19.8%.
Although these values are acceptable, a more realistic comparison employed
the standard deviation and coefficient of variation of the more strongly
bound, more
fluorescent receptors. The overall mean standard deviation unrealistically
inflates
the coefficient of variation for the weakly bound, less fluorescent receptors.
The
coefficient of variation for the 19 receptors with greater than 10,000
Fluorescent
Units of bound target is 11.1 %, which is well within the range required to
produce
meaningful binding data.
One goal of the CARA approach is the facile preparation of a significant
number of candidate receptors through combinations of structurally simple
building
blocks. The following results establish that both the individual building
blocks and
combinations of building blocks have a significant, positive effect on test
ligand
binding.
The binding data illustrated in Figures 23-24 demonstrate that heterogeneous
combinations of building blocks (n=2, n=3) are dramatically superior candidate
receptors made from a single building block (n=1). For example, Figure 16
illustrates both the diversity of binding observed for n=2, n=3 candidate
receptors
with fluorescent units ranging from 0 to ca. 40,000. These data also
illustrate and
the ca. 10-fold improvement in binding affinity obtained upon going from the
homogeneous (n=1) to heterogeneous (n=2, n=3) receptor environments.
The effect of heterogeneous building blocks is most easily observed by
comparing selected n=3 receptor environments candidate receptors including 1
or 2
of those building blocks (their n=2 and n=1 subsets). Figures 26 and 27
illustrate
this comparison for two different n=3 receptor environments using the r-
phycoerythrin data. In these examples, it is clear that progression from the
89
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
homogeneous system (n=1) to the heterogeneous systems (n=2, n=3) produces
significantly enhanced binding.
Although van der Waals interactions are an important part of molecular
recognition, it is important to establish that the observed binding is not a
simple case
of hydrophobic/hydrophilic partitioning. That is, that the observed binding
was the
result of specific interactions between the individual building blocks and the
target
The simplest way to evaluate the effects of hydrophobicity and hydrophilicity
is to
compare building block loge value with observed binding. Loge is a known and
accepted measure of lipophilicity, which can be measured or calculated by
known
. methods for each of the building blocks. Figures 28 and 29 establish that
the
observed target binding, as measured by fluorescence units, is not directly
proportional to building block loge. The plots in Figures 28 and 29 illustrate
a rion-
linear relationship between binding (fluorescence units) and building block
loge.
One advantage of the present methods and arrays is that the ability to screen
large numbers of candidate receptor environments will lead to a combination of
useful target affinities and to significant target binding diversity. High
target affinity
is useful for specific target binding, isolation, etc. while binding diversity
can
provide multiplexed target detection systems. This example employed a
relatively
small number of building blocks to produce ca. 120 binding environments. The
following analysis of the present data clearly demonstrates that even a
relatively
small number of binding environments can produce diverse and useful artificial
receptors.
The target binding experiments performed for this study used protein
concentrations including 0.1 to 10 ~,g/ml. Considering the BSA data as
representative, it is clear that some of the receptor environments readily
bound 1.0
ug/ml BSA concentrations near the saturation values for fluorescence units
(see, e.g.,
Figure 20). Based on these data and the formula weight of 68,000 for BSA,
several
of the receptor environments readily bind BSA at ca. 15 picomole/ml or 15
nanomolar concentrations. Additional experiments using lower concentrations of
protein (data not shown) indicate that, even with a small selection of
candidate
receptor environments, femptomole/ml or picomolar detection limits have been
attained.
One goal of artificial receptor development is the specific recognition of a
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
particular target. Figure 30 compares the observed binding for r-phycoerythrin
and
BSA. Comparison of the overall binding pattern indicates some general
similarities.
However, comparison of specific features of binding for each receptor
environment
demonstrates that the two targets have distinctive recognition features as
indicated
by the (*) in Figure 30.
One goal of artificial receptor development is to develop receptors which can
be used for the multiplexed detection of specific targets. Comparison of the r-
phycoerythrin, BSA and ovalbumin data from tlus study (Figures 16, 18, and 20)
were used to select representative artificial receptors for each target.
Figures 31, 32
and 33 employ data obtained in the present example to illustrate
identification of
each of these three targets by their distinctive binding patterns.
Conclusions
The optimum receptor for a particular target requires molecular recognition
which is greater than the expected sum of the individual hydrophilic,
hydrophobic,
ionic, etc. interactions. Thus, the identification of an optimum (specific,
sensitive)
artificial receptor from the limited pool of candidate receptors explored in
this
prototype study, was not expected and not likely. Rather, the goal was to
demonstrate that all of the key components of the CARA: Combinatorial
Artificial
Receptor Array concept could be assembled to form a functional receptor
microarray. This goal has been successfully demonstrated.
This study has conclusively established that CAR.A microarrays can be
readily prepared and that target binding to the candidate receptor
environments can
be used to identify artificial receptors and test ligands. In addition, these
results
demonstrate that there is significant binding enhancement for the building
block
heterogeneous (n=2, n=3, or n=4) candidate receptors when compared to their
homogeneous (n=1) counterparts. When combined with the binding pattern
recognition results and the demonstrated importance of both the heterogeneous
receptor elements and heterogeneous building blocks, these results clearly
demonstrate the significance of the CARA Candidate Artificial Receptor -> Lead
Artificial Receptor -> Working Artificial Receptor strategy.
91
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Example 3 - Preparation and Evaluation of Microarrays of Candidate Artitxcial
Receptors Including Reversibly Immobilized Building Blocks
Microarrays of candidate artificial receptors including building blocks
immobilized through van der Waals interactions were made and evaluated for
binding of a protein ligand. The evaluation was conducted at several
temperatures,
above and below a phase transition temperature for the lawn (vide infra).
Materials and Methods
Building blocks 2-2, 2-4, 2-6, 4-2, 4-4, 4-6, 6-2, 6-4, 6-6 where prepared as
described in Example 1. The C12 amide was prepared using the previously
described carbodiimide activation of the carboxyl followed by addition of
dodecylamine. This produced a building block with a 12 carbon alkyl chain
linker
for reversible immobilization in the C18 lawn.
Amino lama microarray plates (Telechem) were modified to produce the C18
lawn by xeaction of stearoyl chloride (Aldrich Chemical Co.) in A)
dimethylformamide / PEG 400 solution (90:10, v/v, PEG 400 is polyethylene
glycol
average MW 400 (Aldrich Chemical Co.) or B) methylene chloride l TEA solution
(100 ml methylene chloride, 200 ~,1 triethylamirie) using the lawn
modification
procedures generally described in Example 2.
The C18 lawn plates where printed using the SpotBot standard procedure as
described in Example 2. The building blocks were in printing solutions
prepared by
solution of ca. 10 mg of each building block in 300 ~,1 of methylene chloride
and 100
~,1 methanol. To this stock was added 900 ~.1 of dimethylformamide and 100 ~,1
of
PEG 400. The 36 combinations of the 9 building blocks taken two at a time
(N9:n2,
36 combinations) where prepared in a 384-well microwell plate which was then
used
in the SpotBot to print the microarray in quadruplicate. A random selection of
the
print positions contained only print solution.
The selected microarray was incubated with a 1.0 ~,g/ml solution of the test
ligand, cholera toxin subunit B labeled with the AlexaTM fluorophore
(Molecular
Probes Inc., Eugene, OR), using the following variables: 1) the microarray was
washed with methylene chloride, ethanol and water to create a control plate;
and 2)
the microarray was incubated at 4 °C, 23 °C, or 44 °C.
After incubation, the plates)
92
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
were rinsed with water, dried and scanned (AXON 4100A). Data analysis was as
described in Example 2.
Results
A control array from which the building blocks had been removed by
washing with organic solvent did not bind cholera toxin (Figure 34). Figures
35-37
illustrate fluorescence signals from arrays printed identically, but incubated
with
cholera toxin at 4 °C, 23 °C, or 44 °C, respectively.
Spots of fluorescence can be
seen in each array, with very pronounced spots produced by incubation at 44
°C.
The fluorescence values for the spots in each of these three arrays are shown
in
Figures 38-40. Fluorescence signal generally increases with temperature, with
many
nearly equally large signals observed after incubation at 44 °C. Linear
increases
with temperature can reflect expected improvements in binding with
temperature.
Nonlinear increases reflect rearrangement of the building blocks on the
surface to
achieve improved binding, which occurred above the phase transition for the
lipid
surface (vide infi°a).
Figure 41 can be compared to Figure 39. The fluorescence signals plotted in
Figure 39 resulted from binding to reversibly immobilized building blocks on a
support at 23 °C. The fluorescence sig~ials plotted in Figure 41
resulted from
binding to covalently immobilized building blocks on a support at 23
°C. These
figures compare the same combinations of building blocks in the same relative
positions, but immobilized in two different ways.
The binding to covalently immobilized building blocks was also evaluated at
4 °C, 23 °C, or 44 °C. Figure 42 illustrates the changes
in fluorescence signal from
individual combinations of covalently immobilized building blocks at 4
°C, 23 °C, or
44 °C. Binding increased modestly with temperature. The mean increase
in
binding was 1.3-fold. A plot of the fluorescence signal for each of the
covalently
immobilized artificial receptors at 23 °C against its signal at 44
°C (not shown)
yields a linear correlation with a correlation coefficient of 0.75. This
linear
correlation indicates that the mean 1.3-fold increase in binding is a
thermodynamic
effect and not optimization of binding.
Figure 43 illustrates the changes in fluorescence signal from individual
combinations of reversibly immobilized building blocks at 4 °C, 23
°C, or 44 °C.
93
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
This graph illustrates that at least one combination of building blocks
(candidate
artificial receptor) exhibited a signal that remained constant as temperature
increased. At least one candidate artificial receptor exhibited an
approximately
linear increase in signal as temperature increased. Such a linear increase
indicates
normal temperature effects on binding. The candidate artificial receptor with
the
lowest binding signal at 4 °C became one of the best binders at 44
°C. This indicates
that rearrangement of the building blocks of this receptor above the phase
transition
for the lawn, which increases the building blocks' mobility, produced
increased
binding. Other receptors characterized by greater changes in binding between
23 °C
and 44 °C (compared to between 4 °C and 23 °C) also
underwent dynamic affinity
optimization.
Figure 44 illustrates the data presented in Figure 42 (lines marked A) and the
data presented in Figure 43 (lines marked B). The increases in binding
observed
with the reversibly immobilized building blocks are significantly greater than
the
increases observed with covalently bound building blocks. Binding to
reversibly
immobilized building blocks increased from 23 bC and 44 °C by a median
value of
6.1-fold and a mean value of 24-fold.
This confines that movement of the reversibly immobilized building blocks
within
the receptors increased binding (i.e., the receptor underwent dynamic affinity
optimization).
A plot of the fluorescence signal for each of the reversibly immobilized
artificial receptors at 23 °C against its signal at 44 °C (not
shown) yields no
correlation (correlation coefficient of 0.004). A plot of the fluorescence
signal for
each of the reversibly immobilized artificial receptors at 44 °C
against the signal for
the corresponding covalently immobilized receptor (not shown) also yields no
correlation (correlation coefficient 0.004). This lack of correlation provides
further
evidence that movement of the reversibly immobilized building blocks within
the
receptors increased binding.
Figure 45 illustrates a graph of the fluorescence signal at 44 °C
divided by
the signal at 23 °C against the fluorescence signal obtained from
binding at 23 °C for
the artificial receptors with reversibly immobilized receptors. This
comparison
indicates that the binding enhancement is independent of the initial affinity
of the
receptor for the test ligand.
94
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Table 1 identifies the reversibly immobilized building blocks making up
each of the artificial receptors, lists the fluorescence signal (binding
strength) at 44
°C and 23 °C, and the ratios of the observed binding at these
two temperatures.
These data illustrate that each artificial receptor reflects a unique
attribute for each
combination of building blocks relative to the role of each individual
building block.
TABLE 1
Building Signal at Signal at Ratio of
Blocks 44 C 23 C Signals,
Making Up 44 C /23
Receptor C
22 24 24136 4611 5.23
22 26 16660 43 387.44
22 42 17287 -167 -103.51
22 44 16726 275 60.82
22 46 25016 3903 6.41
22 62 13990 3068 4.56
22 64 15294 3062. 4.99
22 66 11980 3627 3.30
24 26 22688 1291 17.57
24 42 26808 -662 -40.50
24 44 23154 904 25.61
24 46 42197 2814 15.00
24 62 19374 2567 7.55
24 64 27599 262 105.34
24 66 16238 5334 3.04
26 42 22282 4974 4.48
26 44 26240 530 49.51
26 46 23144 4273 5.42
26 62 29022 4920 5.90 ,
26 64 23416 5551 4.22
26 66 , 19553 5353 3.65
42 44 29093 6555 4.44
42 46 18637 3039 6.13
42 62 22643 4853 4.67
42 64 20836 6343 3.28
42 66 14391 9220 1.56
44 46 25600 3266 7.84
44 62 15544 4771 3.26
44 64 25842 3073 8.41
44 66 22471 5142 4.37
46 62 32764 8522 3.84
46 64 21901 3343 6.55
46 66 23516 3742 6.28
62 64 24069 7149 3.37
62 66 15831 2424 6.53
64 66 21310 2746 7.76
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Conclusions
This experiment demonstrated that an array including reversibly immobilized
building blocks binds a protein substrate, like an array with covalently
immobilized
building blocks. The binding increased nonlinearly as temperature increased,
indicating that movement of the building blocks increased binding. Many of the
candidate artificial receptors demonstrated improved binding upon mobilization
of
the building blocks.
Examule 4 - The Oli~osaccharide Portion of GMl Competes
With Artificial Receptors for Binding to Cholera Toxin
Microarrays of candidate artificial receptors were made and evaluated for
binding of cholera toxin. The arrays were also evaluated for disrupting that
binding.
Disrupting of binding employed ~a compound that binds to cholera toxin, the
oligosaccharide moiety from GMl (GMl OS). The results obtained demonstrate
that a ligand of a protein specifically disrupted binding of the protein to
the
microarray.
Materials and Methods
Building blocks were synthesized and activated as described in Example 1.
The building blocks employed in this example were TyrAlB1 [1-1], TyrA2B2,
TyrA2B4, TyrA2B6, TyrA2B8, TyrA3B3, TyrA3B5, TyrA3B7, TyrA4B2,
TyrA4B4, TyrA4B6, TyrA4B8, TyrA5B3, TyrA5B5, TyrA5B7, TyrA6B2,
TyrA6B4, TyrA6B6, TyrA6B8, TyrA7B3, TyrA7B5, TyrA7B7, TyrA8B2,
TyrA8B4, TyrA8B6, and TyrA8B8. The abbreviation for the building block
including a linker, a tyrosine framework, and recognition elements AxBy is
TyrAxBy.
Microarrays for the evaluation of the 171 n=2 candidate receptor
environments were prepared as follows by modifications of known methods. An
"n=2" receptor environment includes two different building blocks. Briefly:
Amine
modified (amine "lawn"; SuperAmine Microarray plates) microarray plates were
purchased from Telechem Inc., Sunnyvale, CA. These plates were manufactured
specifically for microarray preparation and had a nominal amine load of 2-4
amines
per square nm according to the manufacturer. The microarrays were prepared
using
96
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
a pin microarray spotter instrument from Telechem Inc. (SpotBotTM Arrayer)
typically with 200 ~,m diameter spotting pins from Telechem Inc. (Stealth
Micro
Spotting Pins, SMP6) and 400-420 pin spot spacing.
The 19 building blocks were activated in aqueous dimethylformamide
(DMF) solution as described above. For preparing the 384-well feed plate, the
activated building block solutions were diluted 10-fold with a solution of
DMF/H20/PEG400 (90110/10, vlv/v; PEG400 is polyethylene glycol nominal 400
FW, Aldrich Chemical Co., Milwaukee, WI). These stock solutions were
aliquotted
(10 ~.1 per aliquot) into the wells of a 384-well microwell plate (Telechem
Inc.).
Control spots included the building block [1-1]. The plate was covered with
aluminum foil and placed on the bed of a rotary shaker for 15 minutes at 1,000
RPM. This master plate was stored covered with aluminum foil at -20 °C
when not
m use.
For preparing the 384-well SpotBotTM plate, a well-to-well transfer (e.g. A-1
to A-l, A-2 to A-2, etc.) from the feed plate to a second 384-well plate was
performed using a 4 ~,1 transfer pipette. This plate was stored tightly
covered with
aluminum foil at -20°C when not in use. The SpotBotTM was used to
prepare up to
13 microarray plates per run using the 4 ~.1 microwell plate. The SpotBotTM
was
programmed to spot from each microwell in quadruplicate. The wash station on
the
SpotBotTM used a wash solution of EtOH/H20 (20/80, v/v). This wash solution
was
adjusted to pH 4 with 1 M HCl and used to rinse the microarrays on completion
of
the SpotBotTM printing run. The plates were given a final rinse with deionized
(DI)
water, dried using a stream of compressed air, and stored at room temperature.
The
microarrays were further modified by reacting the remaining amines with acetic
aWydride to fonn an acetamide lawn in place of the amine lawn.
The test ligand employed in these experiments was cholera toxin labeled
with the AlexaTM fluorophore (Molecular Probes Inc., Eugene, OR). The
candidate
disruptor employed in these experiments was GM1 OS (GM1 oligosaccharide), a
known ligand for cholera toxin.
Microarray incubation and analysis was conducted as follows: For control
incubations with the microarrays, solutions (e.g. 500 ~,1) of the cholera
toxin in PBS-
T (PBS with 20 ~.l/L of Tween-20) at a concentrations of 1.7 pmol/ml (0.1
~glml)
was placed onto the surface of a microarray and allowed to react for 30
minutes. For
97
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
disruptor incubations with the microarrays, solutions (e.g. 500 ~.l) of the
cholera
toxin (1.7 pmol/ml, 0.1 ~.g/ml) and the desired concentration of GM1 OS in PBS-
T
(PBS with 20 ~.1/L of Tween-20) was placed onto the surface of a microarray
and
allowed to react for 30 minutes. GM1 OS was added at 0.34 and at 5.1 ~.M in
separate experiments. After either of these incubations, the microarray was
rinsed
with PBS-T and DI water and dried using a stream of compressed air.
The incubated microarray was scanned using an Axon Model 4200A
Fluorescence Microarray Scanner (Axon Instruments, Union City, CA). The Axon
scanner and its associated software produce a false color 16-bit image of the
fluorescence intensity of the plate. This 16-bit data is integrated using the
Axon
software to give a Fluorescence Units value (range 0 - 65,536) for each spot
on the
microarray. This data is then exported into an Excel file (Microsoft) for
further
analysis including mean, standard deviation and coefficient of variation
calculations.
Table 2 identifies the building blocks in each of the 171 receptor
environments.
TABLE 2
Building Building
Blocks Blocks
1 22 24 22 24 42
2 22 26 23 24 44
3 22 28 24 24 46
4 22 33 25 24 48
5 22 42 26 24 55
6 22 44 27 24 62
7 22 46 28 24 64
8 22 48 29 24 66
9 22 55 30 24 68
10 22 62 31 24 77
11 22 64 32 24 82
12 22 66 33 24 84
13 22 68 34 24 86
14 22 77 35 24 88
15 22 82 36 26 28
16 22 84 37 26 33
17 22 86 38 26 42
18 22 88 39 26 44
19 24 26 40 26 46
24 28 41 26 48
21 24 33 42 26 55
98
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Building Building
Blocks Blocks
43 26 62 89 42 77
44 26 64 90 42 82
45 26 66 91 42 84
46 26 68 92 42 86
47 26 77 93 42 88
48 26 82 94 44 46
49 26 84 95 44 48
50 26 86 96 44 55
51 26 88 97 44 62
52 28 33 98 44 64
53 28 42 99 44 66
54 28 44 10044 68
55 28 46 10144 77
56 28 48 10244 82
57 28 55 10344 84
58 28 62 ~ 10444 86
59 28 64 10544 88
60 28 66 10646 48
61 28 68 10746 55
62 28 77 10846 62
63 28 82 10946 64
64 28 84 11046 66
65 28 86 11146 68
66 28 88 11246 77
.
67 33 42 11346 82
68 33 44 11446 84
69 33 46 11546 86
70 33 48 11646 88
71 33 55 11748 55
72 33 62 11848 62
73 33 64 11948 64
74 33 66 12048 66
75 33 68 12148 68
76 33 77 12248 77
77 33 82 12348 82
78 33 84 12448 84
79 33 86 12548 86
80 33 88 12648 88
81 42 44 12755 62
82 42 46 12855 64
83 42 48 12955 66
84 42 55 13055 68
85 42 62 13155 77
86 42 64 13255 82
87 42 66 13355 84
88 42 68 13455 86
99
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Building Building
Blocks Blocks
135 55 88 154 66 84
136 62 64 155 66 86
137 62 66 156 66 88
138 62 68 157 68 77
139 62 77 158 68 82
140 62 82 159 68 84
141 62 84 160 68 86
142 62 86 161 68 88
143 62 88 162 77 82
144 64 66 163 77 84
145 64 68 164 77 86
146 64 77 165 77 88
147 64 82 166 82 84
148 64 84 167 82 86
149 64 86 168 82 88
150 64 88 169 84 86
151 66 68 170 84 88
152 66 77 171 86 88
153 66 82
laesults
Low Concentration of GMl OS
Figure 46 illustrates binding of cholera toxin to the microarray of candidate
artificial receptors followed by washing with buffer produced fluorescence
signals.
These fluorescence signals demonstrate that the cholera toxin bound strongly
to
certain receptor enviromnents, weakly to others, and undetectably to some.
Comparison to experiments including those reported in Example 2 indicates that
cholera toxin binding was reproducible from array to array and from month to
month.
Binding of cholera toxin was also conducted with competition from GMl OS
(0.34 ~.M). Figure 47 illustrates the fluorescence signals due to cholera
toxin
binding that were detected after this competition. Notably, many of the
signals
illustrated in Figure 47 are significantly smaller than the corresponding
signals
recorded in Figure 46. The small signals observed in Figure 47 represent less
cholera toxin bound to the array. GM1 OS significantly disrupted binding of
cholera
toxin to many of the receptor environments.
The disruption in cholera toxin binding caused by GM1 OS can be visualized
as the ratio of the amount bound in the absence of GM1 OS to the amount bound
in
100
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
competition with GM1 OS. This ratio is illustrated in Figure 48. The larger
the
ratio, the less cholera toxin remained bound to the artificial receptor after
competition with GMl OS. The ratio can be as large as about 30. The ratios are
independent of the quantity bound in the control.
High Concentration of GMl OS
Binding of cholera toxin to the microarray of candidate artificial receptors
followed by washing with buffer produced fluorescence signals illustrated in
Figure
49. As before, cholera toxin was reproducible and it bound strongly to certain
receptor enviromnents, weakly to others, and undetectably to some. Figure 50
illustrates the fluorescence signals detected due to cholera toxin binding
that were
detected upon competition with GM1 OS at 5.1 ~,M. Again, GM1 OS significantly
disrupted binding of cholera toxin to many of the receptor environments.
This disruption is presented as the ratio of the amount bound in the absence
of GM1 OS to the amount bound after contacting with GM1 OS in Figure 51. The
ratios range up to about 18 and are independent of the quantity bound in the
control.
Conclusions
This experiment demonstrated that binding of a test ligaald to an artificial
receptor of the present invention can be diminished (e.g., competed) by a
candidate
disruptor molecule. In this case the test ligand was the protein cholera toxin
and the
candidate disruptor was a compound known to bind to cholera toxin, GM1 OS. The
degree to which binding of the test ligand was disrupted was independent of
the
degree to which the test ligand bound to the artificial receptor.
Example 5 - GM1 Competes With Artificial Receptors for Binding to Cholera
Toxin
Microarrays of candidate artificial receptors were made and evaluated for
binding of cholera toxin. The arrays were also evaluated for disrupting that
binding.
Disrupting of binding employed a compound that binds to cholera toxin, the
liposaccharide GM1. The results obtained demonstrate that a ligand of a
protein
specifically disrupts binding of the protein to the microarray.
101
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Materials and Methods
Building blocks were synthesized and activated as described in Example 1.
The building blocks employed in this example were TyrAlBl [1-1], TyrA2B2,
TyrA2B4, TyrA2B6, TyrA4B2, TyrA4B4, TyrA4B6, TyrA6B2, TyrA6B4, and
TyrA6B6 in groups of 4 building blocks per artificial receptor. The
abbreviation for
the building block including a linker, a tyrosine framework, and recognition
elements AxBy is TyrAxBy.
Microarrays for the evaluation of the 126 n=4 candidate receptor
environments were prepared as described above for Example 4. The test ligand
employed in these experiments was cholera toxin labeled with the AlexaTM
fluorophore (Molecular Probes Inc., Eugene, OR). Cholera toxin was employed at
5.3 nM in both the control and the competition experiments. The candidate
disruptor employed in these experiments was GMl, a known ligand for cholera
toxin, which competed at concentrations of 0.042, 0.42, and 8.4 ,uM.
Microarray
incubation and analysis was conducted as described for Example 4.
Table 3 identifies the building blocks in each receptor environment.
TABLE 3
Building Building
Blocks Blocks
1 22 24 26 21 22 24 64
42 66
2 22 24 26 22 22 26 42
44 44
3 22 24 26 23 22 26 42
46 46
4 22 24 26 24 22 26 42
61 62
5 22 24 26 25 22 26 42
64 64
6 22 24 26 26 22 26 42
66 66
7 22 24 42 27 22 26 44
44 46
8 22 24 42 28 22 26 44
46 62
9 22 24 42 29 22 26 44
62 64
10 22 24 42 30 22 26 44
46 66
11 22 24 42 31 22 26 46
66 62
12 22 24 44 32 22 26 46
46 64
13 22 24 44 33 22 26 46
62 66
14 22 24 44 34 22 26 62
64 64
15 22 24 44 35 22 26 62
66 66
16 22 24 46 36 22 26 64
62 66
17 22 24 46 37 22 42 44
64 46
18 22 24 46 3 22 42 44
66 8 62
19 22 24 62 39 22 42 44
64 64
22 24 62 40 22 42 44
66 66
102
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Building Building
Blocks Blocks
41 22 42 46 85 24 44 62
62 64
42 22 42 46 86 24 44 62
64 66
43 22 42 46 87 24 44 64
66 66
44 22 42 62 88 24 46 62
64 64
45 22 42 62 89 24 46 62
66 66
46 22 42 64 90 24 46 64
66 66
47 22 44 46 91 24 62 64
62 66
48 22 44 46 92 26 42 44
64 46
49 22 44 46 93 26 42 44
66 62
50 22 44 62 94 26 42 44
64 64
51 22 44 62 95 26 42 44
66 66
52 22 44 64 96 26 42 46
66 62
53 22 46 62 97 26 42 46
64 64
54 22 46 62 98 26 42 46
66 66
55 22 46 64 99 26 42 62
66 64
56 22 62 64 100 26 42 62
66 66
57 24 26 42 101 26 42 64
44 66
58 24 26 42 102 26 44 46
46 62
59 24 26 42 103 26 44 46
62 64
60 24 26 42 104 26 44 46
64 66
61 24 26 42 105 26 44 62
66 64
62 24 26 44 106 26 44 62
46 66
63 24 26 44 107 26 44 64
62 66
64 24 26 44 108 26 46 62
64 64
65 24 26 44 109 26 46 62
66 66
66 24 26 46 110 26 46 64
62 66
67 24 26 46 111 26 62 64
64 66
68 24 26 46 112 42 44 46
66 62
69 24 26 62 113 42 44 46
64 64
70 24 26 62 114 42 44 46
66 66
71 24 26 64 115 42 44 62
66 64
72 24 42 44 116 42 44 62
46 66
73 24 42 44 117 42 44 64
62 66
74 24 42 44 118 42 46 62
64 64
75 24 42 44 119 42 46 62
66 66
76 24 42 46 120 42 46 64
62 66
77 24 42 46 121 42 62 64
64 66
78 24 42 46 122 44 46 62
66 64
79 24 42 62 123 44 46 62
64 66
80 24 42 62 124 44 46 64
66 66
81 24 42 64 125 44 62 64
66 66
82 24 44 46 126 46 62 64
62 66
83 24 44 46
64
84 24 44 46
66
103
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Results
Figure 52 illustrates the fluorescence signals produced by binding of cholera
toxin to the microarray of candidate artificial receptors alone and in
competition
with each of the three concentrations of GM1. The magnitude of the
fluorescence
signal decreases steadily with increasing concentration of GMl. The amount of
decrease is not quantitatively identical for all of the receptors, but each
receptor
experienced decreased binding of cholera toxin. These decreases indicate that
GMl
competed with the artificial receptor for binding to the cholera toxin.
The decreases show a pattern of relative competition for the binding site on
cholera toxin. This can be demonstrated through graphs of fluorescence signal
obtained at a particular concentration of GM1 against fluorescence signal in
the
absence of GM1 (not shown). Certain of the receptors appear at similar
relative
positions on these plots as concentration of GM1 increases.
The disruption in cholera toxin binding caused by GM1 can be visualized as
the ratio of the amount bound in the absence of GM1 OS to the amount bound
upon
competition with GM1. This ratio is illustrated in Figure 53. The larger the
ratio,
the more cholera toxin remained bound to the artificial receptor upon
competition
with GM1. The ratio can be as large as about 14. The ratios are independent of
the
quantity bound in the control.
20, Interestingly, in several instances minor changes in structure to the
artificial
receptor caused significant changes in the ratio. For example, the artificial
receptor
including building blocks 24, 26, 46, and 66 differs from that including 24,
42, 46,
and 66 by only substitution of a single building block. (xy indicates building
block
TyrAxBy.) The substitution of building block 42 for 26 increased binding in
the
presence of GM1 by about 14-fold.
By way of further example, the artificial receptor including building blocks
22, 24, 46, and 64 differs from that including 22, 46, 62, and 64 by only
substitution
of a single building block. The substitution of building block 24 for 62
increased
binding in the presence of GM1 by about 3-fold.
Even substitution of a single recognition element affected binding. The
artificial receptor including building blocks 22, 24, 42, and 44 differs from
that
including 22, 24, 42, and 46 by only substitution of a single recognition
element.
104
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
The substitution of building block 44 for 46 (a change of recognition element
B6 to
B4) increased binding in the presence of GMl by about 3-fold.
Conclusions
This experiment demonstrated that binding of a test ligand to an artificial
receptor of the present invention can be diminished (e.g., competed) by a
candidate
disruptor molecule. In this case the test ligand was the protein cholera toxin
and the
candidate disruptor was a compound known to bind to cholera toxin, GM1. Minor
changes in structure of the building blocks making up the artificial receptor
caused
significant changes in the competition.
Example 6 = GMl Employed as a Building Block
Alters Binding of Cholera Toxin to the Present Artificial Receptors
Microarrays of candidate artificial receptors were made, GMl was bound to
the arrays, and they were evaluated for binding of cholera toxin. The results
obtained demonstrate that adding GM1 as a building block in an array of
artificial
receptors can increase binding to certain of the receptors.
Materials and Methods
Building blocks were synthesized and activated as described in Example 1.
The building blocks employed in this example were those described in Example
4.
Microarrays for the evaluation of the 171 n=2 candidate receptor environments
were
prepared as described above for Example 4. The test ligand employed in these
experiments was cholera toxin labeled with the AlexaTM fluorophore (Molecular
Probes Inc., Eugene, OR). Cholera toxin was employed at 0.01 ug/ml ( 0.17 pM)
or
0.1 ug/ml (1.7 pM) in both the control and the competition experiments. GM1
was
employed as a test ligand for the artificial receptors and became a building
block for
receptors used to bind cholera toxin. The arrays were contacted with GM1 at
either
100 ,ug/ml, 10 ~,g/ml, or 1 ,ug/ml as described above for cholera toxin and
then rinsed
with deionized water. The arrays were then contacted with cholera toxin under
the
conditions described above. Microarray analysis was conducted as described for
Example 4. Table 2 identifies the building blocks in each receptor
environment.
105
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Results
Figure 54 illustrates the fluorescence signals produced by binding of cholera
toxin to the microarray of candidate artificial receptors without pretreatment
with
GM1. Binding of GM1 to the microarray of candidate artificial receptors
followed
by binding of cholera toxin produced fluorescence signals illustrated in
Figures 55,
56, and 57 (100 ,ug/ml, 10 ~,g/ml, and 1 ,ug/ml GM1, respectively).
The enhancement of cholera toxin binding caused by pretreatment with GM1
can be visualized as the ratio of the amount bound in the presence of GM1 to
the
amount bound in the absence of GM1. This ratio is illustrated in Figure 58 for
1
~,g/ml GM1. The larger the ratio, the more cholera toxin bound to the
artificial
receptor after pretreatment with GM1. The ratio can be as large as about 16.
In several instances minor changes in structure to the artificial receptor
caused significant changes in the ratio. For example, the artificial receptor
including
building blocks 46 and 48 differs from that including 46 and 88 by only
substitution
of a single recognition element on a single building block. (xy indicates
building
block TyrAxBy.) The substitution of building block 48 for 88 (a change of
recognition element A8 to A4) increased the ratio representing increased
binding the
presence of GM1 building block from about 0.5 to about 16. Similarly, the
artificial
receptor including building blocks 42 and 77 differs from that including 24
and 77
by only substitution of a single building block. The substitution of building
block
42 for 24 increased the ratio representing increased binding the presence of
GM1
building block from about 2 to about 14.
Interestingly, several building blocks that exhibited high levels of binding
of
cholera toxin (signals of 45,000 to 65,000 fluorescence units) and that
include the
building block 33 were not strongly affected by the presence of GM1 as a
building
block.
Conclusions
This experiment demonstrated that binding of GMl to an artificial receptor
of the present invention can significantly increase binding by cholera toxin.
Minor
changes in structure of the building blocks making up the artificial receptor
caused
significant changes in the degree to which GM1 enhanced binding of cholera
toxin.
106
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Discussion of Examples 4-6
We have previously demonstrated that an array of working artificial
receptors bind to a protein target in a manner which is complementary to the
specific
environment presented by each region of the proteins surface topology. Thus
the
pattern of binding of a protein target to an array of working artificial
receptors
describes the proteins surface topology; including surface structures which
participate in e.g., protein~small molecule, protein peptide, protein-protein,
protein~carbohydrate, protein~DNA, etc. interactions. It is thus possible to
use the
binding of a selected protein to a working artificial receptor array to
characterize
these protein~small molecule, protein~peptide, protein-protein,
protein~carbohydrate, protein~DNA, etc. interactions. Moreover, it is possible
to
utilize the protein to array interactions to define "leads" for the disruption
of these
interactions.
Cholera Toxin B sub-unit binds to GM1 on the cell surface. Studies to
identify competitors to this binding event have shown that competitors to the
cholera
toxin: GM1 binding interaction (binding site) can utilize both a sugar and an
alkyl/aromatic functionality (Pickens, et al., Chemistry and Biolo~y, vol. 9,
pp 215-
224 (2002)). We have previously demonstrated that fluorescently labeled
Cholera
Toxin B sub-unit binds to arrays of the present artificial receptors to give a
defined
binding pattern which reflects cholera toxin B's surface topology. For this
study, we
sought to demonstrate that the binding of the cholera toxin to at least some
members
of the array could be disrupted using cholera toxin's natural ligand, GMl.
The results presented in the figures clearly demonstrate that these goals have
been achieved. Specifically, competition between the GM1 OS pentasaccharide or
GM1 and an artificial receptor array for cholera binding clearly gave a
binding
pattern which was distinct from the cholera binding pattern control. Moreover,
these
results demonstrated the complementarily between several of the working
artificial
receptors which contained a naphthyl moiety when compared to working
artificial
receptors which only contained phenyl functionality. These results are in
keeping
with the active site competition studies in Pickens, et al. and indicate that
the
naphthyl and phenyl derivatives represent good mimicslprobes for the cholera
to
GM1 interaction. The specificity of these interactions was demonstrated by the
observation that the change of a single building block out of 4 in a
combination of 4
107
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
building blocks system changed a non-competitive to a significantly
competitive
environment. These results also indicated that selected working artificial
receptors
can be used to develop a high-throughput screen for the further evaluation of
the
cholera:GMl interaction.
Additionally, we sought to demonstrate that an affinity support/membrane
mimic could be prepared by pre-incubating an array of artificial receptors
with GMl
which would then bind/capture cholera toxin in a binding pattern which could
be
used to select a working artificial receptors) for, for example, the high-
throughput
screen of lead compounds wluch will disrupt the "cholera:membrane~GMl mimic".
The GMl pre-incubation studies clearly demonstrated that several of the
working
artificial receptors which were poor cholera binders significantly increased
their
cholera binding, presumably through an affinity interaction between the
cholera
toxin and both the immobilized GM1 pentasaccharide moiety and the working
artificial receptor building block environment.
Example 7 - Gradients of Building Blocks Bind and Distinguish
Cholera Toxin and Phycoerythrin
Gradients of the artificial receptors were made and protein was flowed along
the gradient. The gradients were evaluated for binding of cholera toxin and/or
phycoerythrin. The results obtained demonstrate that gradients of the
artificial
receptors bind and distinguish proteins, such as cholera toxin and
phycoerythrin.
Materials and Methods
Gradients of the present artificial receptors were constructed using known
methods for making gradients of molecules along a surface. Two types of
gradients
were prepared, step gradients and continuous gradients.
The step gradients were prepared by methods employed for coupling
building blocks to surfaces in regions or arrays. Briefly, a stock solution of
each
building block or mixture of building blocks was prepared at 15 mg/ml in
DMF/H20/PEG400 (90/10/10, v/v/v), some also included dimethylaminopyridine.
This stock solution was diluted 1/10, 1/20, 1/40, 1/80, 1/160, and 1/320 for
applying
to amine functionalized slides (ArrayIt SuperAmine). The diluted solutions of
building blocks were applied to the slides in stripes (steps) across the
shorter
108
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
dimension of the slide. After applying the building blocks to the slide, the
slide was
allowed to sit for 60 minutes at room temperature. The slides were then washed
with EtOHIH20 (20J80 v/v, adjusted to pH about 2 with 1N HCl) followed by
deionized water, dried using a stream of compressed air, and stored at room
temperature. The slides were further modified by reacting the remaining amines
with acetic anhydride to form an acetamide lawn in place of the amine lawn. Tn
the
experiments reported in this Example, the stripes (steps) of building blocks
on the
slide were separated by stripes of acetamide lawn. Tn other experiments, the
steps of
building blocks were contiguous.
The continuous gradients were prepared by knomn methods for preparing
surface-bound molecular gradients (Kramer et al., J. Amer. Chem. Soc. 126:5388-
5395 (2004) and references therein). Briefly, an amine functionalized slide
was
placed in a 150 ml beaker so that it was standing on one end against the wall
of the
beaker. A solution of building block as described above at the 1/80 or 1/120
dilution
was introduced into the beaker at a rate of 2 ml/min over a period of 60
minutes. In
this manner, the bottom of the slide was in contact with the building block
solution
for a longer time than the top of the slide. Thus, the bottom of the slide
included a
lugher density of coupled building blocks than the top.
Proteins (e.g., cholera toxin andlor phycoerythrin) were contacted with the
gradients by flowing protein solutions down the length of the slide in a small
trough
or flow-cartridge. The cholera toxin was labeled with the AlexaTM fluorophore
(Molecular Probes Inc., Eugene, OR). The flow-cartridge included an inlet for
protein solution at one end and an outlet at the other. A total of 2 ml of
protein
r
solution was run through the flow-cartridge having a volume of about 1.8 ml
over 5
or 120 minutes at room temperature followed by rinsing with 2 m1 of PBS-T and
with 10 ml of deionized water. Phycoerythrin was at 0.2 or 2 p,g/ml. Cholera
toxin
was at 0.01 or 0.1 ~,g/ml.
Results
Figures 59, 61, 63, 65, 67, 69, 71, 73, 75, 77, and 79 show fluorescence
images of gradient slides after protein has been flowed over them. In each of
these
Figures, the concentration of the building block increased in steps from the
top of
109
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
each slide to the bottom. The protein flowed in a direction shown as from top
to
bottom in the Figure, although the slide was horizontal during flow.
Figures 60, 62, 64, 66, 68, 70, 72, 74, 76, 78, and 80 show fluorescence
intensity plots corresponding to the images. In each of these Figures, the
concentration of the building block increased in steps from right to left. The
protein
flowed from left to right (unless described otherwise).
Figure 59 presents an image obtained from a run of phycoerythrin over a step
gradient of increasing concentrations of the building block TyrA3B3. The image
as
reproduced shows fluorescence obtained from binding of phycoerythrin to at
least
receptor surfaces including the three highest concentration (the 4th, 5th, and
6th) steps
of this building block. Figure 60 shows increasing peaks of fluorescence for
the 3ra,
4cn, 5th, and 6th steps of the building block gradient. Phycoerythrin bound to
receptors made up of building block TyrA3B3 and bound to a greater extent to
receptors including higher concentrations of this building block.
Figure 61 presents an image obtained from a run of phycoerythrin over a step
gradient of increasing concentrations of the building block TyrA4B4. The image
as
reproduced shows fluorescence obtained from binding of phycoerythrin to at
least
receptor surfaces including the highest concentration (the 6th) step of this
building
block. Figure 62 shows increasing peaks of fluorescence for the 5th and 6th
steps of
the building block gradient. Phycoerythrin bound to receptors made up of
building
block TyrA4B4 and bound to a greater extent to receptors including higher
concentrations of this building block.
Figure 63 presents an image obtained from a run of phycoerythrin over a step
gradient of increasing concentrations of the building block TyrA5B5. The image
as
reproduced shows that phycoerythrin did not bind to any step of the gradient
of this
building block. The plot in Figure 64 also illustrates that phycoerythrin did
not bind
to any step of the gradient of this building block.
Figure 65 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building block TyrA3B3. The image
as
reproduced shows fluorescence obtained from binding of cholera toxin to at
least
receptor surfaces including the four highest concentration (the 3ra, 4th, 5th,
and 6th)
steps of this building block. Figure 66 shows increasing peaks of fluorescence
for
the 2°a, 3ra, 4th, 5th, and 6th steps of the building block gradient.
Cholera toxin bound
110
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
to receptors made up of building block TyrA3B3 and bound to a greater extent
to
receptors including higher concentrations of this building block.
Figure 67 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building block TyrA4B4. The image
as
reproduced shows fluorescence obtained from binding of cholera toxin to at
least
receptor surfaces including the three highest concentration (the 4th, Stt',
and 6th) steps
of this building block. Figure 68 shows increasing peaks of fluorescence for
at least
the 2°d, 3rd, 4th, 5th, and 6th steps of the building block gradient
and possibly also for
the first step. Cholera toxin bound to receptors made up of building block
TyrA4B4
and bound to a greater extent to receptors including higher concentrations of
this
building block.
Figure 69 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building block TyrA5B5. The image
as
reproduced shows that cholera toxin did not bind to any step of the gradient
of this
building block, but may have bound to the edges of several of the steps. This
binding to the edges may be analogous to the "doughnut" effect sometimes
observed
in spots on microarrays. The plot in Figure 70 also illustrates that cholera
toxin did
not bind to any step of the gradient of this building block.
Figure 71 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building blocks TyrA3B3 and
TyrA4B4
(in a 1:1 molar ratio). The cholera toxin was flowed across the slide from the
lower
concentration steps to the higher concentration steps. The image as reproduced
shows fluorescence obtained from binding of cholera toxin to at least receptor
surfaces including the three highest concentrations (the 4t'', 5th, and 6th)
steps of these
building blocks. Figure 72 shows increasing peaks of fluorescence for the 3rd,
4th,
5th, and 6th steps of the building block gradient. Cholera toxin bound to
receptors
made up of building blocks TyrA3B3 and TyrA4B4 and bound to a greater extent
to
receptors including higher concentrations of these building blocks.
Figure 73 presents an image obtained from a run of cholera toxin over a step
gradient of increasing concentrations of the building blocks TyrA3B3 and
TyrA4B4
(in a 1:1 molar ratio). The cholera toxin was flowed across the slide from the
higher
concentration steps to the lower concentration steps. The image as reproduced
shows fluorescence obtained from binding of cholera toxin to at least receptor
111
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
surfaces including the three highest concentration (the 6th, 5th, and 4th)
steps of this
building block. Figure 74 shows peaks of fluorescence for at least the 6th,
Stn, 4th,
3rd, and 2"d steps of the building block gradient.
This plot obtained from flow of protein from high to low concentration steps
is different in appearance from those obtained with protein flow from low to
high
concentrations. The plot in Figure 74 indicates that the cholera toxin first
encountered and saturated the highest concentration step, then encountered and
saturated the next highest concentration (5th) step, subsequently encountered
and
saturated the next highest concentration (4th) step, and also bound to the 3rd
and 2"d
steps.
Evaluating binding of cholera toxin and phycoerythrin to arrays of candidate
artificial receptors identified receptors that preferentially bind cholera
toxin rather
than phycoerythrin (e.g., receptors including the building blocks TyrA4B4 and
TyrA4B6). Such testing also identified receptors that bind both cholera toxin
and
phycoerythrin (e.g., receptors including the building blocks TyrA3B3 and
TyrA4B4).
These receptors were employed in step gradients to demonstrate selective
binding of
one protein compared to another on gradients of artificial receptors.
Figure 75 presents an image obtained from a run of a mixture of cholera
toxin and phycoerythrin over a step gradient of increasing concentrations of
the
building blocks TyrA3B3 and TyrA4B4 (in a 1:1 molar ratio). The gradient
included
five steps of increasing concentrations of the building blocks. The mixture
included
cholera toxin at 0.1 ~.g/ml and phycoerythrin at 2 ~,g/ml. The mixture flowed
across
the slide from the lower concentration steps to the higher concentration
steps. The
image as reproduced shows fluorescence obtained from binding of these proteins
to
at least receptor surfaces including the four highest concentration (2"d, 3rd,
4tn, and
5th) steps of these building blocks.
. Figure 76 shows fluorescence intensities obtained for cholera toxin (top
line)
and phycoerythrin (bottom line). This plot shows increasing peaks of
fluorescence
for cholera toxin bound to at least the 2"d, 3rd, 4th, and Stn steps of the
building block
gradient. This plot shows increasing peaks of fluorescence for phycoerythrin
bound
to at least the 3rd, 4th, and 5th steps of the building block gradient.
Gradients made up of receptors known to bind both cholera toxin and
phycoerythrin, in fact, bound both of these proteins.
112
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
Figure 77 presents an image obtained from a run of a mixture of cholera
toxin and phycoerythrin over a step gradient of increasing concentrations of
the
building blocks TyrA4B4 and TyrA4B6 (in a 1:1 molar ratio). The gradient
included
five steps of increasing concentrations of the building blocks. The mixture
included
cholera toxin and phycoerythrin at concentrations as above. The mixture flowed
across the slide from the lower concentration steps to the higher
concentration steps.
The image as reproduced shows fluorescence obtained from binding of cholera
toxin
to at least receptor surfaces including the three highest concentration (the
3rd, 4tn,
and 5th) steps of these building blocks.
Figure 78 shows fluorescence intensities obtained for cholera toxin (top line)
and phycoerythrin (bottom line). This plot shows increasing peaks of
fluorescence
for cholera toxin bound to at least the 3rd, 4th, and 5th steps of the
building block
gradient. This plot shows that phycoerythrin, as expected, did not bind to any
steps
of this building block gradient.
Gradients' made up of receptors known to bind cholera toxin but not
phycoerythrin, in fact, bound cholera toxin but not phycoerythrin.
Figure 79 pr esents a fluorescence image from a run of cholera toxin flowed
over a continuous gradient of increasing concentrations of the building blocks
TyrA3B3 and TyrA4B4 (in a 1:1 molar ratio). The lower concentrations of
building
blocks are at the top of the image and the higher concentrations at the
bottom. The
present building blocks formed a continuous gradient on the derivatized slide.
The
cholera toxin bound in increasing amounts to the higher concentration portions
of
the gradient (Figure 80).
It should be noted that, as used in this specification and the appended
claims,
the singular forms "a," "an," and "the" include plural referents unless the
content
clearly dictates otherwise. Thus, for example, reference to a composition
containing
"a compound" includes a mixture of two or more compounds. It should also be
noted that the term "or" is generally employed in its sense including "and/or"
unless
the content clearly dictates otherwise.
It should also be noted that, as used in this specification and the appended
claims, the term "configured" describes a system, apparatus, or other
structure that is
constructed or configured to perform a particular task or adopt a particular
113
CA 02544614 2006-05-03
WO 2005/053835 PCT/US2004/040578
configuration. The term "configured" can be used interchangeably with other
similar phrases such as arranged and configured, constructed and arranged,
adapted,
adapted and configured, constructed, manufactured and arranged, and the like.
All publications and patent applications in this specification are indicative
of
the level of ordinary skill in the art to which this invention pertains.
The invention has been described with reference to various specific and
preferred embodiments and techniques. However, it should be understood that
many
variations and modifications may be made while remaining within the spirit and
scope of the invention.
114