Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
DIFFERENTIAL COVERING AND COATING METHODS
FIELD OF INVENTION
The present invention relates generally to methods of covering
and/or coating medical devices and more particularly to methods of
manipulating the compliance of the cover to modify fluid mechanics
on the interior of the medical device and the friction points on the
exterior thereof.
BACKGROUND OF THE INVENTION
Stents are devices that are inserted into a vessel or passage to
keep the lumen open and prevent closure due to a stricture, external
compression, or internal obstruction. In particular, stents are
commonly used to keep blood vessels open in the coronary arteries
and they are frequently inserted into the ureters to maintain drainage
from the kidneys, the bile duct for pancreatic cancer or
cholangiocarcinoma or the esophagus for strictures or cancer.
Vascular as well as not vascular stenting has evolved significantly;
unfortunately there remain significant limitations with respect to the
technology for producing scents suitable to various portions of a
patient's anatomy.
Historically, in order to provide a stent with varying
characteristics, the stent had to be manufactured from multiple
materials, at least one for each characteristic desired. As a result,
many of these stents are woven from two or more metals having
differing shape-memories for example. Unfortunately, braided stents
are vulnerable to premature obsolescence. Moreover, providing
multiple material types in a single stent may lead to inconsistent
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-2-
characteristics along the surface area of the stent. This is particularly
undesirable when the stent is to be placed in vascular or nonvascular
lumens that have been occluded for one reason or another. The stent
needs to be stiffer in some regions while more flexible in others.
Additionally, medical device companies have identified the
need to cover stents at least partially to prevent the epithelialization
of the scaffolding. Most covered stents however have an
elastomeric cover that is subject to bunching particularly about
stenotic tissue. This can lead to additional tissue granulation.
Additionally, the stents are dip coated which can lead to uneven
coating as well as inconsistency in stent performance from batch to
batch.
Additionally the ends of the stent tend to be exposed in order
to encourage granulation tissue formation, which helps to anchor the
stent in place. With metal stents, the direct metal to tissue contact
accelerates tissue granulation and galvanic current generation is also
an undesirable byproduct. Such direct current can have indirect
effects on tissue granulation and direct effects on fluid flow dynamics.
Moreover, since many medical device companies have chosen
to use poorly adapted cardiovascular stents for Pulmonary, GI and
Peripheral Vascular indications, many of the anatomical differences in
the lumens are not accounted for in scent design. For example, the
pulsation of the cardiovascular lumen and the concomitant radial
force requirements of a cardiovascular stent differ substantially from
that of a tightly constricted lumen such as the trachea during
repeated coughing. When a stent developed for the former is
indicated for the latter, the stent tends to fail under the extreme
conditions and lose its elasticity and therefore its ability of ensure
airway patency. Non-vascular lumens also tend to have ciliated
epithelia so as to facilitate clearance of fluids and particulates. As a
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-3-
general principal, coated stents were not specifically designed for
ciliated lumen in that the external coating damages the cilia and
prevents the body's natural clearing function. Moreover, the coating
itself is usually made of a predominately hydrophilic polymer, which
can lead to mucous formation and/or fluid stagnation. Stagnation of
fluids or material passing through the lumen can lead to additional
complications such as in stent restenosis or bacterial infections.
Therefore, there remains an existing need for a therapeutic
stent that can have varying characteristics along its surface area
while being stamped, not braded, from a single base material.
Moreover, there is a need for such a therapeutic stent where the
relative hardness, softness, flexibility, stiffness and radial force can be
modified as a function of geometric considerations rather than
material considerations. In particular, there is a need for a stent that
is divided into zones so as to allow the stent to have predetermined
characteristics in one zone and could conceivably have drastically
different characteristics in an adjacent zone so as to allow for stents
that can be tailored to anatomical lumens in general and the
particular lumen topography of a specific patient in particular. An
additional need exists for a method of differentially modifying the
location, compliance, and density of the cover to achieve desired
behavior. In particular, there is a need for a covered stent that is
preferably covered internally such that the outer scaffolding surface
of the stent is raised from the outer surface of the coating. To this end,
cilia function is only partially limited and mucociliary clearance is not
significantly affected. A need also remains for a coating that itself
has anti-adherent properties or is complexed with an anti-adherent
such that bacteria, fungi or other microbials cannot colonize the
cover in particular and the stent generally. There also remains a need
for a cover for the proximal and distal ends of the stent that prevent
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-4-
epithelialization and granulation tissue formation while achieving the
benefits of traditional uncovered stents.
SUMMARY OF EXEMPLARY EMBODIMENTS
It is a principal purpose of the present invention to provide a
stent, in accordance with an exemplary embodiment of the present
invention, which combines many of the excellent characteristics of
both silicone and metal stents while eliminating the undesirable ones.
In particular, it is an objective of a preferred embodiment in
accordance with the present invention to provide a stent that is
easily installed, yet in alternative embodiments, removable.
Moreover the scent in accordance with this embodiment of the
present invention would not cause material infections and may be
capable of reducing infection. Therefore, a principal objective of a
preferred embodiment in accordance with the present invention is to
provide a prosthesis that is suitable for both permanent and
temporary use while being easy to insert, reposition and remove.
A principal objective of a preferred embodiment of the present
invention is to provide a stent that may be stamped from preferably a
single material that is capable of maintaining its axial working length
when radially compressed. To this end, the stent does not have a
seam that could aggravate luminal tissue. In particular, a stent in
accordance with the present invention is formed using a tool that
molds the stents outer contour as well as its interstices.
It is yet another objective of an exemplary embodiment of the
present invention to provide a stent that can be indicated for the
treatment of benign and malignant disease and improve the way
clinicians treat malignant obstructions.
Still another objective of the present invention is to provide a
stent and method for installing the stent that is economical and
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-5-
suitable for routine purposes. Moreover, the stent will have minimal
migration, cause minimal tissue granulation, will not foreshorten after
deployment and mucociliary clearance will not be problematic.
Yet another objective of an exemplary embodiment in
accordance with the present invention is to provide a prosthesis that
will have superior internal to external diameter ratio, superior radial
force with dynamic expansion, while being suitable for use in pediatric
and adult patients with malignant and benign disease.
A principal objective of an exemplary stent in accordance with
the present invention is to provide a family of stents where the
relative hardness/softness of regions of the stent can differ from other
regions of the stent to provide additional patient comfort and
resistance to radial forces.
An additional objective in accordance with an exemplary
embodiment is to provide a family of stents with novel interstice
configurations that facilitate flexibility, durability and/or proper
installation.
Still another objective of a preferred embodiment of the
present invention is to provide a self-expanding stent having the
above benefits that also defines a plurality of apertures at the termini
of the stent or along the scaffolding there between for, inter alia,
removal of the scent. In the furtherance of this and other objective,
suture may be treaded through one or more of these apertures to
facilitate purse string like removal of the device.
An additional objective in accordance with a preferred
embodiment of the present invention is to provide a prosthesis that
minimizes cilia destruction at the site of implantation. In the
furtherance of this and other objectives, the preferred prosthesis is
coated internally with a polyurethane such that the surfaces of the
struts that come into contact with the lumen of the patient are
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-6-
elevated above the surface of the coating such that the cilia can
move to allow for free fluid action of ciliated epithelium.
Still another objective in accordance with the present
invention is to provide a cover and method for applying the cover to
a scent. The cover may be applied such that the cover is at various
levels of compliance with respect to the stent struts. To this end, it
provides an opportunity to manipulate flow mechanics for the inner
diameter of the stent as well as the friction points of the outer
diameter of the stent.
Yet another objective in accordance with the present
invention is to provide covering about the distal end, the proximal
end or combination so as to retain the benefits of an uncovered stent
while retaining the ability to remove the stent. Moreover, the end
only and full stent covering may be provided in addition to the
coating to eliminate galvanic current.
Further objectives, features and advantages of the invention
will be apparent from the following detailed description taken in
conjunction with the accompanying drawings.
BRIEF DESCRIPTION OF THE FIGURES
FIG. 1 shows a perspective view of the cross-section of select
struts of an exemplary scent with a covering applied to the internal
diameter so as to cause the cover to conform to the stent struts.
FIG. 2 shows an alternative perspective view of the select stent
strut cross-section of FIG. 1 where the cover is not compliant with the
scent struts.
FIG. 3 shows an enlarged perspective view of a stent viewed
from one end through the lumen thereof showing the compliant cover
of FIG. 1.
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
FIG. 4 shows an alternative end-to-end perspective view of the
stent where the cover is super-compliant in certain regions of the
stent such that the cover extends through the interstices toward the
outer surface of the stent.
FIG. 5 shows an enlarged perspective view of the struts where
the cover does not conform to the geometry of the struts.
DETAILED DESCRIPTION OF AN EMBODIMENT
A preferred embodiment of the stent, in accordance with the
present invention, provides a stent that prevents epithelialization of
the scent and is not subject to premature elongation and
foreshortening but is capable of engaging the desired implantation
location. The stent also retains its axial length while undergoing radial ,
compression.
The stent is preferably formed from a composite material
selected from the group consisting essentially of Ni, C, Co, Cu, Cr, H,
Fe, Nb, O, Ti and combinations thereof. The composite material is
generally formed into a compressed tube from which the stent is
etched and is formed on a suitable shaping device to give the stent
the desired external geometry. Both the synthetic collar techniques
and in vitro valuation techniques show the remarkable ability of stents
in accordance with the present invention to convert acting force
into deformation work absorbed by the angled structure, which
prevents excessive scaffolding stress and premature material fatigue
and accelerated obsolescence.
Though one skilled in the stent engineering art, once apprised of
the present application, would be able to manufacture a scent
consistent with the present invention by other methods, a preferred
method of manufacturing such stents follows. As stated above a
composite material is selected and a blank is formed there from. The
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
_g_
blank is preferably laser etched and the etch work is generally verified
for accuracy using visual recording microscopy. Dimensional
measurements are taken to ensure strut thickness, segment angles,
zone placement, etc. Moreover, the stent is preferably formed on a
shaping tool that has substantially the desired contour of the external
stent dimensions.
In the event the stent is to be shaped to the dimensions of a
particular lumen, optical photography and/or optical videography of
the target lumen may be conducted prior to scent formation. The
geometry of corresponding zones and connector regions of the stent
then can be etched and formed in accordance with the requirements
of that target lumen. For example, if the stent were designed for the
trachea, which has a substantially D shaped lumen and additionally
the middle zones needed to be softer than the end zones, the stent
could be designed to those specifications. Stent angles may be
modified to provide different characteristics to different zones of the
stent. In particular, if the topography of the trachea of a particular
patient is captured optically and the appropriate dimension
provided, a patient specific prosthesis could be engineered. These
techniques can be adapted to other non-vascular lumen but is very
well suited for vascular applications where patient specific
topography is a function of a variety of factors such as genetics,
lifestyle, etc.
It should be pointed out that unlike the use of differing shape
memory materials to change regions of a stent, stents in accordance
with the present invention can take on an infinite number of
characteristic combinations as zones and segments within a zone can
be modified by changing angles, segment lengths and segment
thicknesses during the etching and forming stages of stent
engineering or during post formation processing and polishing steps.
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-7-
Moreover, by modifying the geometry of the connectors between
zones, additional functionality may be achieved.
Exemplary stents in accordance with the present invention are
shown in FIGS. 1-3 showing the preferred interstice geometry. Not
shown are a wide variety of interstice geometries that are also
acceptable alternatives to the preferred, namely, U, V, W, Z, S and X
geometries to name a few.
The stent also is formed of memory metal and preferably has
unique geometrical interstices that are laser etched therein.
However, other conventional ways of forming interstices in unitary
stents, though not equivalent are contemplated, may be employed
and would be within the skill set of one in the art.
It cannot be overemphasized, however, that this does not _
mean the knowledge that changes in the geometry of interstices
affect stent functionality is currently known in the art. To the contrary,
the present inventors discovered the interrelation between interstice
geometry, width, length and relative resistance to torsional stress and
radial force. In fact, it can be said that the stent has circumferential
bands extending perpendicularly with respect to the luminal device's
longitudinal axis. These bands are referred to generally as zones. A
connector connects these bands to one another; the connector is an
additional means for adjusting stent functionality. In particular, the
connector defines a substantially U shaped member, but could define
other geometries such as U, V, W, Z, S and X to name a few. Also a
plurality of eyelets that allow a physician to purse string the stent with
suture to facilitate removability. The eyelets are preferably between
about 200~,m and 300~,m, however, the eyelets may be smaller or
larger to accommodate the need of the target site. The preferred
eyelet size is about 350~,m as the preferred suture type is 4-0. The
,a
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-10-
medical appliance may be pre-threaded with suture or the user may
provide the suture after implantation.
An exemplary stent in accordance with the present invention
with relatively great torsionality and radial flexibility would be rated
soft. An exemplary soft rated stent comprises distance between U
shaped connectors of about 4.5 ~,m in the compressed state (i.e.,
contracted in the 3mm tube subject to laser etching). Moreover, the
length of the crossing member is preferably about 1.0 ~,m. The lengths
of the leg members are preferably about 1.5 ~,m long. Additionally the
leg members may further comprise feet that attached to the
remainder of the stent scaffolding. The feet can be adjusted from a
standard length of about 0.25 ~m to further adjust the characteristics
of the stent. There is additionally a substantially rectangular member
incorporated in the U shaped connector with similar capacity for
variability. The variability factors and results of modifying the
dimensions of the substantially rectangular members are similar to
those evinced by leg length dimensional modifications.
By way of example, but not to be construed in any way as
limiting, the softness index or relative flexibility can be increase by
increasing the various lengths discussed above. For example, by
increasing the length of the legs and crossing members of the U
shaped connector, flexibility increases. However, with respect to the
distance between U shaped members and distance between
interstices in a preferred stent embodiment, there is an inverse
correlation between length and softness. This relative
softness/hardness indexing as a corollary of interstice dimensions is a
novel aspect of preferred embodiment of the present invention. As a
practical rule of thumb, longer leg lengths coupled with acute angles
provide for greater flexibility. Conversely, shorter leg lengths and
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-11-
more obtuse angles provide more rigidity. By way of non-limiting
example, a U shaped connector with short legs deviating from the
crossing member at angles greater than 90°, will be extremely rigid
and resistant to torsional strain as compared to a U shaped connector
with longer legs diverging from the crossing member at angles less
than 90°.
In addition to the length and spacing differences, the interstices
themselves may define various shapes that by their very nature afford
novel functionality to the stent. The changes of functionality,
however, are more a function of the dimensional differences of the
various shapes rather than a function of the shapes themselves.
Therefore, it is important to keep in mind that the dimensional
differences discussed in the previous paragraph are determinative of
the functionality accorded the stent by the varying interstice
geometries. It is for this reason that one of ordinary skill in the art, after
being apprised of the present invention, would be able to conceive
of a number of interstice geometries to satisfy certain functionality
criteria by keeping certain dimensional parameters constant.
FIGS. 1-3 also show the coating provided in select embodiments
in accordance with the present invention. The coating preferably
comprises a stable polymeric material such as polyurethane that can
be deposited on a stent to form a thin film. The film preferably forms
layers when annealed to the scent such that the hydrophobic moieties
within the polymer are predominately oriented outward and the
hydrophilic moieties are predominately oriented inward. It should be
noted that depending on the characteristics desired by the user, the
relative hydroaffinity may be altered. For example, in the event the
implant was placed with the intention of collecting mucous in the
respiratory system, the coating would more suitably have a
predominately hydrophilic outer surface. Moreover, by manipulating
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-12-
the hydroaffinity of the coating, the physiochemical parameters such
as surface-free energy, charge density provide a substantial barrier to
biofilm formation in general and ligand-binding events mediated by
microbial adhesions and extracellular polymers. However, additional
anti-adherents know in the art may be applied to provide lubricity as
well as an additional barrier for microbials. For example, a preferred
coating in accordance with the present invention would be
hydrophilic and hydroscope to ensure the surface would always be
wet which prevents mucostasis as well as microbial adherence.
Regardless of desired coating surface characteristics, preferred
scents in accordance with the present invention are covered from the
interior of the stent lumen such that the scent scaffolding. The cover
may be strategically applied to either form a strut compliant
membrane, a non-compliant membrane within the internal diameter
of the stent or incrementally in between. One of the principal
functions of the variable covering method is to enhance friction
points on the exterior of the stent and/or control flow dynamics
through the interior lumen of the stent.
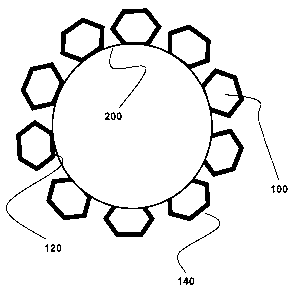
Making specific reference to FIGS. 1 and 3, the stent struts 100
are shown with the interior lumen surface 120 facing upward. When
covering the stent struts 100 from the interior surface 140, a compliant
cover forms angles between the struts 100 that can cause fluid
retention. If this is a desirable characteristic based on the target
lumen of the scent, such covering can be achieved by using a
compliant heating mechanism when coupling the cover 200 to the
struts. Alternatively, as shown in FIGS. 2 and 5, if a relatively smooth
interior diameter is desired, the cover 200 would be applied to the
struts 100 with a non-compliant device so as to prevent the cover 200
from conforming to the contours of the stent struts 100.
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-13-
With respect to FIG. 4, there may be instances wflere the cover
200 needs to be super compliant such that the cover 200, though
applied to the inner surface 120 of the stent, the cover extends
through the interstices to the outer surface 140. By modifying the
cover compliance between these extremes, it is possible to optimize
the degree of friction and flow based on desired design metrics.
The scent is preferably coated in a multi-step process, which
comprises providing a stem and initially spraying the stent with a
polymeric material to coat the struts. Though the steps may be
reversed it is preferable to follow the spraying step with the interior
coating step. In particular, the scent is placed into a hollow mold to
retain the stent shape as the internal diameter of the stent is coated
with the polymeric material to form a non-porous cover 200. An
alternative cover could be porous for breathability or selective
leaching. The cover 100 can be provided in sheets or additional spray
applications, however, the preferred embodiment is thin sheets.
Sheets are generally preferred to facilitate the proper orientation of
the polymer side chains to ensure that the desired moiety (e.g.,
hydrophilic and/or hydrophobic) is facing the lumen. Once the layer
of polymer is introduced into the inner diameter of the stent, an
application device such as a balloon or other device in which
temperature can be regulated is implanted to sandwich the layer of
polymer between the stent inner diameter and the balloon. The
balloon is expanded and heated to a temperature of about between
200° and 400° F to anneal the polymer to the stent. Preferred
polymers
such as various designer polyurethanes (e.g.. Cronoflex~
manufactured by Cardiotech International) are suitable for such
applications but other polymers are acceptable. Degree of
conformity may depend on the compliance of the balloon as well as
the presence or absence of a collar about the external surface of the
CA 02546809 2006-05-19
WO 2005/110282 PCT/US2004/039162
-14-
stent. The collar may have ribs complementary to the stent interstices
or alternatively recessed wells to facilitate the extent of super
compliance of the cover.
Additionally, the same methods may be employed to cover and
coat portions of the stent rather than the complete stent. In
particular, smaller pieces of covering material may be applied to the
distal and proximal ends of the stent to prevent excessive granulation
material. Such stents would be valuable in cases such as
photodynamic therapy and lung transplants where bare metal scents
are generally employed.
The present invention may be embodied in other specific forms
without departing from its spirit or essential characteristics. The
described embodiments are to be considered in all respects only as
illustrative, and not restrictive. The scope of the invention is,
therefore, indicated by the appended claims, rather than by the
foregoing description. All changes, which come within the meaning
and range of equivalency of the claims, are to be embraced within
their scope.