Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
1
GAS-FILLED MICROVESICLE ASSEMBLY FOR CONTRAST IMAGING
The present invention relates to an assembly comprising as a first
component a gas-filled microvesicle and as a second component a structural
entity which is capable to associate to the outer surface of the microvesicle,
thereby modifying the physico-chemical properties thereof. Optionally, said
second component may comprise a targeting ligand, a bioactive agent, a
diagnostic agent or any combination thereof. The invention further relates to
formulations comprising said assembly, to the use of said formulations, to a
method for preparing said assembly and formulations and to a diagnostic kit
comprising said assembly. The assembly of the invention can be used as an
active component in diagnostically and/or therapeutically active formulations,
in
particular for enhancing the imaging in the field of ultrasound contrast
imaging,
including targeted ultrasound imaging and/or ultrasound-mediated drug delivery
and other imaging techniques such as molecular resonance imaging (MRI) or
nuclear imaging.
Background of the invention
Rapid development of ultrasound contrast agents in the recent years has
generated a number of different formulations, which are useful in ultrasound
imaging of organs and tissue of human or animal body. These agents are
designed to be used primarily as intravenous or intra-arterial injectables in
conjunction with the use of medical echographic equipment which employs for
example, B-mode image formation (based on the spatial distribution of
backscatter tissue properties) or Doppler signal processing (based on
Continuous Wave or pulsed Doppler processing of ultrasonic echoes to
determine blood or liquid flow parameters).
A class of injectable formulations useful as ultrasound contrast agents
includes suspensions of gas bubbles having a diameter of few microns dispersed
in an aqueous medium.
Use of suspensions of gas bubbles in carrier liquid, as efficient ultrasound
reflectors is well known in the art. The development of microbubble
suspensions
as echopharmaceuticals for enhancement of ultrasound imaging followed early
observations that rapid intravenous injections of aqueous solutions can cause
dissolved gases to come out of solution by forming bubbles. Due to their
substantial difference in acoustic impedance relative to blood, these
intravascular gas bubbles were found to be excellent reflectors of ultrasound.
The injection of suspensions of gas bubbles in a carrier liquid into the blood
stream of a living organism strongly reinforces ultrasonic echography imaging,
CONFIRMATION COPY
CA 02547024 2011-12-22
2
thus enhandng the visualisation of internal organs. Since imaging of organs
and
deep seated tissues can be crudeì in establishing medical diagnosis, a lot of
effort has been devoted to the development of stable suspensions of highly
concentrated gas bubbles which at the same time would be simple to prepare
and administer, would contain a minimum of inactive spedes and would be
capable of long storage and simple administration.
The simple dispersion of free gas bubbles in the aqueous medium is
however of limited practical interest, since these bubbles are in general not
stable enough to be useful as ultrasound contrast agents.
Interest has accordingly been shown in methods of stabilising gas bubbles
for echography and other ultrasonic studies, for example using emulsifiers,
oils,
thickeners or sugars, or by entrapping or encapsulating the gas or a precursor
thereof in a variety of systems. These stabilized gas bubbles are generally
referred to in the art as .microvesidee, and may be divided into two main
categories.
A first category of stabilized bubbles or microvesicles is generally referred
to
in the art as "microbubbles" and includes aqueous suspensions In which the
bubbles of gas are bounded at the gas/liquid interface by a very thin envelope
(film) involving a stabilizing amphiphilic material disposed at the gas to
liquid
interface:- Microbubbles suspensions are typically prepared by contacting
powdered amphiphilic materials, e.g. freeze-dried preformed liposomes or
freeze-dried or spray-dried phospholipid solutions, with air or other gas and
then with an aqueous carrier, while agitating to generate a microbubbie
suspension which can then be administered, preferably shortly after its
preparation.
Examples of aqueous suspension of gas microbubbles and preparation
thereof are disclosed, for Instance, in US 5,271,928, US 5,445,82.3,
US 5,413,774, US 5,556,610, 5,597,549, US 5,827,504 and WO 04/069284,
A second category of microvesicies is generally referred to in the art as
"mideballoonsw or "microcapsuies" and indudes suspensions in which the
bubbles of gas are surrounded by a solid material envelope of a lipid or of
natural or synthetic polymers. Examples of microballoons and of the
preparation
thereof are disclosed, for instance, In US 5,711,933 and US 6,333,021.
Microvesides bearing an overall net charges are also known (see for
instance International patent application WO 97/29783,
CA 02547024 2011-12-22
3
the outer envelope of these microvesides contains ionic compounds
which are capable to confer the desired overall charge to the final
microveside.
Further to these formulations of gas-filled microvesides, interest has more
recently been shown also towards modified formulations of gas-filled
mkrovesides, either for improving the diagnostic effect and/or for therapeutic
purposes.
For instance, the mkrovesides can be assodated (e.g. by inclusion in its
boundary envelope) with spedflc components (known as 'targeting ilgands")
which are capable to link to a determined target within a patient's body, e.g.
to
a specific pathogenic site. These formulations are generally known in the art
as
"targeted microvesides". Examples of targeted microvesides, of targeting
ligands and of the preparation thereof are disclosed for instance in
International
patent application WO 98/18051.
Another example of modified formulations are those where a therapeutic
agent is associated with the microvesicie. When the formulation comprising the
microveside reaches the pathogenic site, the drug can be advantageously
released, e.g. by applying a controlled acoustic energy capable of disrupting
the
veside, thus locally releasing the therapeutic agent. This technique is
generally
known in the field as "ultrasound-mediated drug release". Examples of
microvesicies' formulations comprising a therapeutic agent are disclosed for
Instance In International patent application WO 94/28873.
Further developments In the field have brought to the preparation of
assemblies wherein the microveslde is assodated with a second component,
bearing a desired therapeutic agent or targeting compound.
For instance, WO 99/39738, discloses an assembly comprising a gas-fllied
microvesIde and a liquid-filled liposome assodated therewith, where the
liposome comprises a therapeutically active substance therein. The liposome is
assodated to the microveside by simple admixture with microvesides or
through a link between a conjugated pair, each of the microveside and liposome
being provided with a component bearing one of the two the respective
complementary moieties of said pair (e.g. biotin and avidin or streptavidin).
WO 03/015831 discloses a formulation comprising gas-filled microveskles
("microspheres" in the application) assodated to liposomes, referred to as
microsphere-liposome composites. The liposomes of the composite may indude
a drug and/or a targeting moiety. The microvesides and liposomes forming the
composite are made from a same starting material; the composite is obtained
preparing an aqueous solution comprising a mblure of lipids, introdudrog saki
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
4
solution in a sealed vial comprising the desired gas and finally agitating the
solution. The so obtained composite is thus a simple mixture of microvesicles
and liposomes of the same chemical nature. In particular, no specific chemical
or physical interaction between microvesicles and liposomes is disclosed in
said
document.
Furthermore, International patent application WO 99/53963 discloses a
combined preparation which comprises a first composition comprising gas-filled
microvesicles dispersed in an aqueous medium and stabilized by a material and
a second composition which is an oil-in-water emulsion comprising a material
which stabilize the emulsion. The surface materials stabilizing the
microvesicles
and the dispersed oil phase have affinity for each other. In one embodiment,
said affinity is obtained by using surface materials with opposite charges, so
that they interact and bind electrostatically to each other. Alternatively,
the
association of the respective surface materials may comprises compounds
capable of interaction through chemical or biological binding. The oil of the
emulsion is a substance which is capable of generating a gas or vapor pressure
in vivo and is referred to as the "diffusable component". The association of
droplets of said emulsified substance with the microvesicle is capable of
determining a controllable growth of the dispersed gas phase in the
microvesicle, through inward diffusion thereto of molecules of gas or vapour
from said substance.
Summary of the invention
The Applicant has now found a novel assembly, for use inpharmaceutically
active formulations, comprising a gas-filled microvesicle which is associated
to a
second component through a substantially electrostatic interaction, said
second
component optionally comprising a targeting ligand, a bioactive agent, a
diagnostic agent or any combination thereof.
An aspect of the present invention relates to an assembly comprising a
gas-filled microvesicle bearing a first overall net charge and a component
associated to said microvesicle wherein said component bears a second overall
net charge opposite in sign to said first net charge, said associated
component
comprising a biocompatible surface active agent and having a diameter of 100
nm or lower.
According to a preferred embodiment, said associated component comprises
a targeting ligand, a bioactive agent, a diagnostic agent or any combination
thereof.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
Preferably, said surface active agent is an emulsifying agent, a dispersing
agent or any combination thereof, particularly preferred being an amphiphilic
material.
In the following of this specification, the second component of the assembly
5 will be referred to as Microvesicle's Associated Component ("MAC").
According to an embodiment of the invention, said ultrasound contrast
agent is in the form of a suspension of a plurality of said assemblies
dispersed in
a pharmaceutically acceptable aqueous carrier.
According to an alternative embodiment of the invention said ultrasound
contrast agent is in the form of a freeze-dried composition.
Another aspect of the invention relates to a method for preparing an
assembly as above described, which comprises admixing a preparation
comprising gas-filled microvesicles or a precursor thereof with a preparation
comprising said second component or a precursor thereof.
For the purposes of the present application the term "precursor of a gas-
filled microvesicles" includes within its meaning any intermediate substance,
composition, formulation or structure which is capable of forming a suspension
of gas-filled microvesicles including, for instance, freeze-dried formulations
capable of being reconstituted with an aqueous carrier to form said
microvesicle
suspension, or microemulsions capable to undergo a freeze-drying process to
obtain a freeze-dried product which can then be reconstituted with an aqueous
carrier to form said suspension.
Similarly, the term "precursor of the second component", includes any
intermediate substance, composition, formulation or structure which is capable
of forming said second component, including, for instance, freeze-dried
compositions reconstitutable into an aqueous suspension comprising said MAC.
According to an embodiment of the present invention, the assembly of the
invention can be obtained by:
1) preparing a first aqueous suspension comprising a gas-filled
microvesicle;
2) preparing a second aqueous suspension comprising a component to be
associated with said gas-filled microvesicle;
3) admixing said two suspensions, to obtain an aqueous suspension
comprising said assembly.
Optionally a washing step can be included, after the preparation of either
the first and/or the second suspension. An optional washing step of the final
suspension can also be performed. The term "washing step" includes within its
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
6
meaning any method or process directed to separate and/or at least partially
remove the excess of non-associated materials, components, particles and the
like from a suspension of a desired compound (e.g. microvesicle, MAC or
assembly). Suitable separation methods include, for instance, decantation,
centrifugation, ultrafiltration or microfiltration.
According to an alternative embodiment, the assembly of the invention can
be obtained by:
1) preparing a first aqueous suspension comprising a gas-filled
microvesicle;
2) freeze-drying said suspension, to obtain a first lyophilized product;
3) preparing a second suspension comprising a component to be associated
with said gas-filled microvesicle;
4) freeze-drying said suspension, to obtain a second lyophilized product;
5) reconstituting said first and said second lyophilized product with a
physiologically acceptable aqueous carrier in the presence of a gas, to obtain
an
aqueous suspension comprising the assembly.
Optionally a washing step can be included, after the preparation of either
the first and/or the second suspension. An optional washing step of the final
suspension can also be performed.
According to a preferred embodiment, the last step 5) of the preparation
process comprises the steps of a) reconstituting the second lyophilized
product
with a physiologically acceptable aqueous carrier to obtain a suspension
comprising the component to be associated to the gas-filled microvesicle and
b)
reconstituting the first lyophilized product with said suspension in the
presence
of a gas.
According to a further preferred embodiment, said assembly is obtained as
a freeze-dried composition by:
1) preparing an aqueous emulsion comprising a water immiscible organic
solvent, a phospholipid and a lyoprotecting agent;
2) preparing an aqueous suspension comprising a component to be
associated with a gas-filled microvesicle;
3) admixing said aqueous suspension with said aqueous emulsion; and
4) freeze drying the mixture to remove the water and the organic solvent,
to obtain a lyophilized product comprising said assembly.
The obtained lyophilized product can be reconstituted into an aqueous
suspension comprising an assembly of the invention by agitating said
lyophilized
product in the presence of a gas and of an aqueous carrier.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
7
A further aspect of the invention relates to a method for ultrasound
diagnostic imaging comprising administering a contrast-enhancing amount of an
aqueous suspension of an assembly as above defined, optionally comprising a
targeting ligand.
A further aspect of the invention relates to a therapeutic method comprising
administering a therapeutically-effective amount of an aqueous suspension of
an
assembly as above defined comprising a bioactive agent.
A still further aspect of the invention relates to a pharmaceutical kit which
contains the components of said assembly in any of the following forms: a) as
two separate suspensions of microvesicles and MACS; b) as separate freeze-
dried preparations of the two components, optionally together with an aqueous
carrier for reconstitution; or c) as a freeze-dried preparation of the
assembly,
together with an aqueous carrier for reconstitution.
An advantage of an assembly according to the invention is that the
electrostatic interaction between the microvesicle and the MAC can be obtained
by using conventional components typically employed for forming the envelope
of the microvesicles, without the need of introducing additional components or
moieties in said envelope, which may otherwise impair the stability of the
microvesicles.
The obtained assembly can advantageously modify or modulate the
behavior of gas-filled microvesicles once administered in the body of a
patient
(such as, for instance, the rate of clearance from bloodstream circulation).
For
instance, assemblies comprising positively charged microvesicles and
negatively
charged MACs can be used to administer a preparation of positively charged
microvesicle which will however show a behavior similar to negatively charged
microvesicles once inside the body. Alternatively, assemblies comprising
negatively charged microvesicles and positively charged MACS can be used to
administer a preparation of negatively charged microvesicles which will
however
show a behavior similar to positively charged microvesicles once inside the
body. In addition it is possible to associate a desired targeting compound or
a
pharmaceutically active agent to the microvesicle without impairing its
stability
(in particular the stability of the boundary layer surrounding the gas), as
said
targeting compound or pharmaceutically active agent are in fact associated to
the second component of the assembly, which stability is substantially
unaffected by the presence of said compound or agent
A further advantage of the present invention is the extreme flexibility in the
preparation of different assemblies for different purposes. As a matter of
fact,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
8
a single basic preparation of charged microvesicles can be associated to
different preparations of MACs of opposite charge, if necessary more than one
at
the same time, depending on the specific diagnostic/therapeutic needs. For
instance, it is possible to associate to the microvesicles' preparation a
first
preparation of MAC bearing a targeting ligand (e.g. for binding the assembly
to
a specific pathogenic site) and a second preparation of MAC including a
bioactive
agent (which can be released at the specific pathogenic site once the assembly
has been linked thereto).
In addition, the Applicant has observed that an assembly of the invention
may show an increased pressure resistance with respect to the sole
microvesicle.
Figures
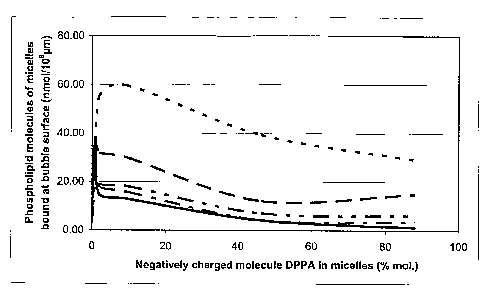
Figure 1. is a graph showing the composition of different assemblies formed by
microvesicles and MACs comprising the same materials but in different amounts.
Figures 2 and 3 show the in vivo behaviour of charged microvesicles and of
corresponding assemblies with MACs of opposite charge with respect to the
microvesicle .
Detailed Description of the Invention
An assembly according to the invention typically comprises a first
component (also identified as the 'carrier" component) in the form of a gas-
filled microvesicle bearing an overall net charge and a second component
associated with said carrier component (MAC) having a diameter of less than
100 nm, which bears an overall net charge of opposite sign with respect to the
first component and which comprises at least one surface active agent, in
particular an emulsifying agent and/or a dispersing agent, more preferably an
amphiphilic compound.
Preferably, the MAC contains a desired targeting ligand, a bioactive agent,
a diagnostic agent or any combination thereof.
The microvesicle's associated component (MAC) is preferably in the form of
a stable supermolecular structure formed by the association of a plurality of
molecules of one or more surface active agent. Preferably, said supermolecular
structure comprises at least one surface active agent bearing a net charge,
more preferably a ionic surface active agent. Said stable supermolecular
structure can for instance be determined by a hydrophobic interaction between
the hydrophobic portions of said molecules. According to a particularly
preferred
embodiment, the MAC is in the form of a micelle. Alternatively, said MAC can
be
CA 02547024 2006-05-24
WO 2005/063305
PCT/IB2004/004230
9
formed by a single molecule of a polymeric ionic surfactant, optionally
functionalized to include a suitable targeting, bioactive and/or diagnostic
moiety
The assembly of the invention is useful for preparing a pharmaceutically
active formulation for use in diagnostic and/or therapeutic methods.
The term "pharmaceutically active formulation" includes within its meaning
any formulation, or precursor thereof, including diagnostically, bioactive
and/or
therapeutically active formulations, capable of exerting a pharmaceutical
effect
(e.g. a diagnostic, bioactive and/or therapeutic effect) when administered in
an
effective amount to a patient in need thereof. Similarly, the term
"pharmaceutical active" when referred to a compound, an agent or kit includes
within its meaning diagnostic, bioactive and/or therapeutic compounds, agents
or kits.
The term "targeting ligand" includes within its meaning any compound,
moiety or residue having, or being capable to promote, a targeting activity of
the assembly of the invention towards any biological or pathological site
within a
living body. Targets to which targeting ligand may be associated include
tissues
such as, for instance, myocardial tissue (including myocardial cells and
cardiomyocites), membranous tissues (including endothelium and epithelium),
laminae, connective tissue (including interstitial tissue) or tumors; blood
clots;
and receptors such as, for instance, cell-surface receptors for peptide
hormones,
neurotransmitters, antigens, complement fragments, and immunoglobulins and
cytoplasmic receptors for steroid hormones.
The term "bioactive agent" includes within its meaning any substance,
composition or particle which may be used in any therapeutic application, such
as in methods for the treatment of a disease in a patient, as well as any
substance which is capable of exerting or responsible to exert a biological
effect
in vitro and/or in vivo. Examples of bioactive agents are drugs,
pharmaceuticals,
proteins, natural or synthetic peptides, including oligopeptides and
polypeptides,
vitamins, steroids and genetic material, including nucleosides, nucleotides
and
polynucleotides. A therapeutic method or treatment of a patient typically
includes the use of a bioactive agent.
The term "diagnostic agent" includes within its meaning any compound,
composition or particle which may be used in connection with diagnostic
methods, including imaging of an internal region of a patient and/or
diagnosing
the presence or absence of a disease in a patient. Exemplary diagnostic agents
include, for example, contrast agents for use in connection with magnetic
resonance imaging, X-ray imaging, in particular computed tomography, optical
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
imaging, nuclear imaging or molecular imaging of a patient including, for
example, magnetite nanoparticles.
"Bioconnpatible" or "physiologically acceptable" refers to any compound,
material or formulation which can be administered, in a selected amount, to a
5 patient without negatively affecting or substantially modifying its
organism's
healthy or normal functioning (e.g. without determining any status of
unacceptable toxicity, causing any extreme or uncontrollable allergenic
response
or determining any abnormal pathological condition or disease status).
The term "surface active agent" refers to any compound which is capable of
10 stabilizing mixtures of otherwise generally immiscible materials, such
as
mixtures of two immiscible liquids (e.g. water and oil), mixtures of liquids
with
gases (e.g. gas microbubbles in water) or mixtures of liquids with insoluble
particles (e.g. metal nanoparticles in water). These compounds are also
generally referred to in the art as "emulsifying agents" or "dispersing
agents".
Preferably said compound is an "arnphiphilic compound", i.e. a compound
having a molecule with a hydrophilic polar head (e.g. a polar or ionic group)
and
a hydrophobic organic tail (e.g. a hydrocarbon chain). Examples of surface
active agent, in particular of emulsifying and/or dispersing agents, are: (C2-
C10)
organic acids, organic fatty acids comprising a (C12-C24), Preferably a (C14-
C22),
aliphatic chain, the pharmaceutically acceptable (alkali) salts thereof and
the
respective esters with polyoxyethylene, such as palmitic acid, stearic acid,
arachidonic acid, oleic acid, sodium dodecanoate, sodium oxalate or sodium
tartrate or polyoxyethylene fatty acid stearate; polyionic (alkali) salts,
such as
sodium citrate, sodium polyacrylate, sodium phosphate; organic amines,
amides, quaternary amine (halide) salts, preferably containing a (C8-C22)
hydrocarbon chain, including polyoxyethylated derivative thereof, such as
ethanolamine, triethanolamine, alkylamines, alkanolamides, trimethylalkylamine
chloride, polyoxyethylated alkylannines, polyoxyethylated alkanolamides;
aminoacids; phospholipids, such as fatty acids di-esters of
phosphatidylcholine,
ethylphosphatidylcholine, phosphatidylglycerol, phosphatidic acid,
phosphatidylethanolannine, phosphatidylserine or of sphingomyelin; esters of
mono- or oligo-saccharides with (C12-C24), preferably a (C14-C22), organic
fatty
acids, such as sorbitan laurate; polymeric surfactants, i.e. block copolymers
including hydrophobic and hydrophilic portions, such as
ethyleneoxide/propyleneoxide block copolymers; organic sulfonates such as
alkali (e.g. sodium) (C12-C24)alkyl, preferably (C14-C22)alkyl, sulfonates;
perfluoroorganic acids, such as perfluorooctanoic acid; and mixtures thereof.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
11
Because of its nanometric dimensions, the MAC will also be referred to as the
nanocomponent of the assembly, as opposed to microvesicles having
micrometric dimensions. Microvesicles typically have dimensions of at least
0.5
pm, preferably of 0.8 pm and up to e.g. 20 pm, more preferably from about 1 to
8 pm; the respective mean diameter in number of microvesicles (DN), measured
e.g. by means of a Coulter Counter, is preferably of at least 0.8 pm, more
preferably of at least 1 pm (up to about e.g. 8 pm) and much more preferably
from about 1 pm to about 5 pm.
In general, depending on the respective method of preparation,
microvesicles and MACs are obtained as a population of particles having a more
or less narrowly distribution of dimensions. Thus, for comparing different
populations of microvesicles or MACS, mean values of said distribution are
generally used. As known by those skilled in the art, the dimensions of
micro/nano particles and their respective size distribution can be
characterized
by a number of parameters, the most frequently used being the mean diameter
in number DN, the median diameter in number DN50/ the mean diameter in
volume Dv and the median diameter in volume Dvso. While diameters in number
provide an indication of the mean number dimension of the particles, the
diameter in volume provides information on how the total volume of the
particles is distributed among the whole population. As the presence of very
few
large volume particles in a population of otherwise small volume particles may
cause the corresponding Dv value to be shifted towards high values, it is
sometimes more convenient to use the Dv50 value for evaluating the
distribution
of a particles' population. Dvso is a calculated value indicating that half of
the
total of particles' internal volume is present in particles having a diameter
lower
than DV50; this allows to reduce the effects of accidentally formed large
volume
particles in the evaluation of the size distribution. Clearly, mono-sized
particles
show identical DN, DNS , Dv and Dv50 values. On the other side, an increasing
broadening of particles' distribution will result in a larger difference
between
these various values with a corresponding variation of the respective ratio
thereof (e.g. increase of Dv/DN ratio). For example, particles populations
containing primarily small particles (e.g. particles with a diameter around 2
pm)
with nevertheless a small percentage of large particles (for instance
particles
with a diameter above 8 pm) show higher Dv or DV50 values as compared to the
DN value, with correspondingly higher Dv/DN or Dv50/DN ratios.
The electrostatic interaction between the two components of the assembly
is basically obtained by using a first molecular compound (comprised in the
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
12
microvesicle's envelope) bearing a first net charge and a second molecular
compound (comprised in the structure of the MAC) bearing a second net charge,
which is opposite in sign to the first one. The microvesicles having a first
overall
net charge and the MAC having a second overall net charge, opposite in sign to
the first one, are then associated to each other through an electrostatic
interaction to obtain an assembly according to the invention.
The gas-filled microvesicle forming the first component of an assembly
according to the present invention can be any microvesicle known in the art
bearing an overall net charge. Preferred examples of microvesicles are
microbubbles and microballoons (or microcapsules).
Microbubbles
A first example of suitable gas-filled microvesicle will be referred to
hereinafter as "gas-filled microbubble".
Gas-filled nnicrobubbles useful for preparing an assembly according to the
present invention are generally bubbles of gas dispersed in an aqueous
suspension which are stabilized by a (very thin) envelope comprising an
amphiphilic (film-forming) compound, disposed at the gas to liquid interface.
Said stabilizing envelope, sometimes referred to as an "evanescent envelope"
in
the art, has in general a thickness of less than 5 nm, typically of about 2-3
nm,
thus often amounting to a substantially mononnolecular layer. At least a
portion
of the amphiphilic material comprised in the envelope is composed of charged
molecules, so to confer the desired overall net charge to the microbubble's
envelope.
The amphiphilic compound included in the microvesicles' envelope can be a
synthetic or naturally-occurring biocompatible compound and may include, for
example a film forming lipid, in particular a phospholipid. Examples of
amphiphilic compounds include, for instance phospholipids; lysolipids; fatty
acids, such as palmitic acid, stearic acid, arachidonic acid or oleic acid;
lipids
bearing polymers, such as chitin, hyaluronic acid, polyvinylpyrrolidone or
polyethylene glycol (PEG), also referred as "pegylated lipids"; lipids bearing
sulfonated mono- di-, oligo- or polysaccharides; cholesterol, cholesterol
sulfate
or cholesterol hemisuccinate; tocopherol hemisuccinate; lipids with ether or
ester-linked fatty acids; polymerized lipids; diacetyl phosphate; dicetyl
phosphate; stearylamine; ceramides; polyoxyethylene fatty acid esters (such as
polyoxyethylene fatty acid stearates), polyoxyethylene fatty alcohols,
polyoxyethylene fatty alcohol ethers, polyoxyethylated sorbitan fatty acid
esters, glycerol polyethylene glycol ricinoleate, ethoxylated soybean sterols,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
13
ethoxylated castor oil or ethylene oxide (BO) and propylene oxide (PO) block
copolymers; sterol aliphatic acid esters including, cholesterol butyrate,
cholesterol iso-butyrate, cholesterol palmitate, cholesterol stea rate,
lanosterol
acetate, ergosterol palmitate, or phytosterol n-butyrate; sterol esters of
sugar
acids including cholesterol glucuronides, lanosterol glucoronides, 7-
dehydrocholesterol glucoronide, ergosterol glucoronide, cholesterol gluconate,
lanosterol gluconate, or ergosterol gluconate; esters of sugar acids and
alcohols
including lauryl glucoronide, stearoyl glucoronide, myristoyl glucoronide,
lauryl
gluconate, myristoyl gluconate, or stearoyl gluconate; esters of sugars with
aliphatic acids including sucrose laurate, fructose laurate, sucrose
palmitate,
sucrose stearate, glucuronic acid, gluconic acid or polyuronic acid; saponins
including sarsasapogenin, smilagenin, hederagenin, oleanolic acid, or
digitoxigenin; glycerol or glycerol esters including glycerol tripalmitate,
glycerol
distearate, glycerol tristearate, glycerol dimyristate, glycerol trimyristate,
glycerol dilaurate, glycerol trilaurate, glycerol dipalmitate,; long chain
alcohols
including n-decyl alcohol, lauryl alcohol, myristyl alcohol, cetyl alcohol, or
n-
octadecyl alcohol; 6-(5-cholesten-313-yloxy)-1-thio- p -D-galactopyranoside;
digalactosyldiglyceride; 6-(5-cholesten-3 p -yloxy)hexy1-6-amino-6-deoxy-1-
thio- 13 -D-galactopyranoside; 6-(5-cholesten-3 13 -yloxy)hexy1-6-amino-6-
deoxy1-1-thio- 13 -D-mannopyranoside; 12-(((7'-diethylaminocoumarin-3-
yl)carbonyOrnethylamino)octadecanoic acid; N-[12-(((7'-diethylaminocoumarin-
3-yl)carbonyl)rnethylamino)octadecanoy1]-2-aminopalmitic acid; N-succinyl-
dioleylphosphatidylethanolamine; 1,2-dioleyl-sn-glycerol; 1,2-dipalmitoyl-sn-3-
succinylglycerol; 1,3-dipalmitoy1-2-succinylglycerol; 1-hexadecy1-2-
palmitoylglycerophosphoethanolamine or palmitoylhomocysteine;
alkylarnmonium salts comprising at least one (C10-C20), preferably (C14-C18),
alkyl chain, such as, for instance, stearylammonium chloride,
hexadecylammonium chloride, dimethyldioctadecylamrnonium bromide (DDAB),
hexadecyltrimethylammonium bromide (CTAB); tertiary or quaternary
ammonium salts comprising one or preferably two (Co-Cm), preferably (C14-
C18), acyl chain linked to the N-atom through a (C3-C6) alkylene bridge, such
as,
for instance, 1,2-distearoy1-3-trimethylarnmonium-propane (DSTAP), 1,2-
dipalmitoy1-3-trirnethylammonium-propane (DPTAP), 1,2-oleoy1-3-
trimethylammonium-propane (DOTAP), 1,2-distearoy1-3-dimethylammonium-
3 5 propane (DSDAP): and mixtures or combinations thereof.
Depending on the combination of components and on the manufacturing
process of the microbubbles, the above listed exemplary compounds may be
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
14
employed as main compound for forming the microvesicle's envelope or as
simple additives, thus being present only in minor amounts. .
According to a preferred embodiment, at least one of the compounds
forming the microvesicles' envelope is a phospholipid, optionally in admixture
with any of the other above cited film-forming materials. According to the
present description, the term phospholipid is intended to encompass any
amphiphilic phospholipid compound, the molecules of which are capable of
forming a stabilizing film of material (typically in the form of a mono-
molecular
layer) at the gas-water boundary interface in the final microbubbles
suspension.
Accordingly, these materials are also referred to in the art as "film-forming
phospholipids".
Amphiphilic phospholipid compounds typically contain at least one
phosphate group and at least one, preferably two, lipophilic long-chain
hydrocarbon group.
Examples of suitable phospholipids include esters of glycerol with one or
preferably two (equal or different) residues of fatty acids and with
phosphoric
acid, wherein the phosphoric acid residue is in turn bound to a hydrophilic
group, such as choline (phosphatidylcholines - PC), serine
(phosphatidylserines -
PS), glycerol (phosphatidylglycerols - PG), ethanolamine
(phosphatidylethanolamines - PE), inositol (phosphatidylinositol), and the
like
groups. Esters of phospholipids with only one residue of fatty acid are
generally
referred to in the art as the "Iyso" forms of the phospholipid. Fatty acids
residues present in the phospholipids are in general long chain aliphatic
acids,
typically containing from 12 to 24 carbon atoms, preferably from 14 to 22; the
aliphatic chain may contain one or more unsaturations or is preferably
completely saturated. Examples of suitable fatty acids included in the
phospholipids are, for instance, lauric acid, myristic acid, palmitic acid,
stearic
acid, arachidic acid, behenic acid, oleic acid, linoleic acid, and linolenic
acid.
Preferably, saturated fatty acids such as myristic acid, palmitic acid,
stearic acid
and arachidic acid are employed.
Further examples of phospholipid are phosphatidic acids, i.e. the diesters of
glycerol-phosphoric acid with fatty acids; sphingolipids such as
sphingomyelins,
i.e. those phosphatidylcholine analogs where the residue of glycerol diester
with
fatty acids is replaced by a ceramide chain; cardiolipins, i.e. the esters of
1,3-
diphosphatidylglycerol with a fatty acid; glycolipids such as gangliosides GM1
(or
GM2) or cerebrosides; glucolipids; sulfatides and glycosphingolipids.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
As used herein, the term phospholipids include either naturally occurring,
semisynthetic or synthetically prepared products that can be employed either
singularly or as mixtures.
Examples of naturally occurring phospholipids are natural lecithins
5 (phosphatidylcholine (PC) derivatives) such as, typically, soya bean or
egg yolk
lecithins.
Examples of semisynthetic phospholipids are the partially or fully
hydrogenated derivatives of the naturally occurring lecithins. Preferred
phospholipids are fatty acids di-esters of phosphatidylcholine,
10 ethylphosphatidylcholine, phosphatidylglycerol, phosphatidic acid,
phosphatidylethanolamine, phosphatidylserine or of sphingomyelin.
Examples of preferred phospholipids are, for instance, dilauroyl-
phosphatidylcholine (DLPC), dimyristoyl-phosphatidylcholine (DMPC),
dipalmitoyl-phosphatidylcholine (DPPC), diarachidoyl-phosphatidylcholine
15 (DAPC), distearoyl-phosphatidylcholine (DSPC), dioleoyl-
phosphatidylcholine
(DOPC), 1,2 Distearoyl-sn-glycero-3-Ethylphosphocholine (Ethyl-DSPC),
dipentadecanoyl-phosphatidylcholine (DPDPC), 1-myristoy1-2-palmitoyl-
phosphatidylcholine (MPPC), 1-palrnitoy1-2-myristoyl-phosphatidylcholine
(PMPC), 1-palmitoy1-2-stearoyl-phosphatidylcholine (PSPC), 1-stearoy1-2-
palmitoyl-phosphatidylcholine (SPPC), ), 1-palmitoy1-2-
oleylphosphatidylcholine
(POPC), 1-oley1-2-palmitoyl-phosphatidylcholine (OPPC), dilauroyl-
phosphatidylglycerol (DLPG) and its alkali metal salts,
diarachidoylphosphatidyl-
glycerol (DAPG) and its alkali metal salts, dimyristoylphosphatidylglycerol
(DMPG) and its alkali metal salts, dipalmitoylphosphatidylglycerol (DPPG) and
its
alkali metal salts, distearoylphosphatidylglycerol (DSPG) and its alkali metal
salts, dioleoyl-phosphatidylglycerol (DOPG) and its alkali metal salts,
dimyristoyl
phosphatidic acid (DMPA) and its alkali metal salts, dipalmitoyl phosphatidic
acid
(DPPA) and its alkali metal salts, distearoyl phosphatidic acid (DSPA),
diarachidoylphosphatidic acid (DAPA) and its alkali metal salts, dimyristoyl-
phosphatidylethanolamine (DMPE), dipalmitoylphosphatidylethanolamine
(DPPE), distearoyl phosphatidyl-ethanolamine (DSPE), dioleylphosphatidyl-
ethanolamine (DOPE), diarachidoylphosphatidylethanolamine (DAPE),
dilinoleylphosphatidylethanolamine (DLPE), dimyristoyl phosphatidylserine
(DMPS), diarachidoyl phosphatidylserine (DAPS), dipalmitoyl phosphatidylserine
(DPPS), distearoylphosphatidylserine (DSPS), dioleoylphosphatidylserine
(DOPS), dipalmitoyl sphingomyelin (DPSP), and distearoylsphingomyelin
(DSSP).
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
16
The term phospholipid further includes modified phospholipid, e.g.
phospholipids where the hydrophilic group is in turn bound to another
hydrophilic group. Examples of modified phospholipids are
phosphatidylethanola mines modified with polyethylenglycol (PEG), i.e.
phosphatidylethanolarnines where the hydrophilic ethanolamine moiety is linked
to a PEG molecule of variable molecular weight e.g. from 300 to 5000 daltons),
, such as DPPE-PEG or DSPE-PEG, i.e. DPPE (or DSPE) having a PEG polymer
attached thereto. For example, DPPE-PEG2000 refers to DPPE having attached
thereto a PEG polymer having a mean average molecular weight of about 2000.
Particularly preferred phospholipids are DAPC, DSPC, DPPA, DSPA, DMPS,
DPPS, DSPS and Ethyl-DSPC. Most preferred are DAPC or DSPC.
Mixtures of phospholipids can also be used, such as, for instance, mixtures
of DPPC, DSPC and/or DAPC with DSPS, DPPS, DSPA, DPPA, DSPG, DPPG, Ethyl-
DSPC and/or Ethyl-DPPC,
In some embodiments the phospholipid is the main component of the
stabilizing envelope of microbubbles, amounting to at least 50% (w/w) of the
total amount of components forming the envelope of the gas filled
microbubbles. In some preferred embodiments, substantially the totality of the
envelope (i.e. at least 90% and up to 100% by weight) can be formed of
phospholipids.
The phospholipids can conveniently be used in admixture with any of the
above listed amphiphilic compounds. Thus, for instance, lipids such as
cholesterol, ergosterol, phytosterol, sitosterol, lanosterol, tocopherol,
propyl
gallate or ascorbyl palmitate, fatty acids such as myristic acid, palmitic
acid,
stearic acid, arachidic acid and derivatives thereof or butylated
hydroxytoluene
and/or other non-phospholipid compounds can optionally be added to one or
more of the foregoing phospholipids in proportions ranging from zero to 50% by
weight, preferably up to 25%. Particularly preferred is palrnitic acid.
In order to confer the desired overall net charge to the microbubble, the
envelope shall comprise at least one component bearing an overall net charge,
in particular a charged amphiphilic material, preferably a lipid or a
phospholipid.
Examples of phospholipids bearing an overall negative charge are
derivatives, in particular fatty acid di-esters, of phosphatidylserine, such
as
DMPS, DPPS, DSPS; of phosphatidic acid, such as DMPA, DPPA, DSPA; of
phosphatidylglycerol such as DMPG, DPPG and DSPG. Also modified
phospholipids, in particular PEG-modified phosphatidylethanolarnines, such as
DMPE-PEG2000, DMPE-PEG3000, DMPE-PEG4000, DPPE-PEG5000, DPPE-
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
17
PEG2000, DPPE-PEG3000, DPPE-PEG4000, DPPE-PEG5000, DSPE-PEG2000,
DSPE-PEG3000, DSPE-PEG4000, DSPE-PEG5000, DAPE-PEG2000, DAPE-
PEG3000, DAPE-PEG4000 or DAPE-PEG5000 can be used as negatively charged
molecules. Also the lyso- form of the above cited phospholipids, such as
lysophosphatidylserine derivatives (e.g. lyso-DMPS, -DPPS or -DSPS),
lysophosphatidic acid derivatives (e.g. lyso-DMPA, -DPPA or -DSPA) and
lysophosphatidylglycerol derivatives (e.g. lyso-DMPG, -DPPG or -DSPG), can
advantageously be used as negatively charged compound. Examples of
negatively charged lipids are bile acid salts such as cholic acid salts,
deoxycholic
acid salts or glycocholic acid salts; and (C12-C24), preferably (C14-C22)
fatty acid
salts such as, for instance, palmitic acid salt, stearic acid salt, 1,2-
dipalmitoyl-
sn-3-succinylglycerol salt or 1,3-dipalmitoy1-2-succinylglycerol salt.
Preferably, the negatively charged compound is selected among DPPA,
DPPS, DSPG, DSPE-PEG2000, DSPE-PEG5000 or mixtures thereof.
The negatively charged component is typically associated with a
corresponding positive counter-ion, which can be mono- (e.g. an alkali metal
or
ammonium), di- (e.g. an earth-alkali metal) or tri-valent (e.g. aluminium).
Preferably the counter-ion is selected among alkali metal cations, such as Li,
Na, or K+, more preferably Na.
Examples of phospholipids bearing an overall positive charge are derivatives
of ethylphosphatidylcholine, in particular esters of ethyl phosphatidylcholine
with
fatty acids, such as 1,2-Distearoyl-sn-glycero-3-Ethylphosphocholine (Ethyl-
DSPC or DSEPC), 1,2-Dipalmitoyl-sn-glycero-3-Ethylphosphocholine (Ethyl-DPPC
or DPEPC). The negative counterion is preferably an halogen ion, in particular
chlorine or bromine. Examples of positively charged lipids are alkylammonium
salts with a halogen counter ion (e.g. chlorine or bromine) comprising at
least
one (C10-C20), preferably (C14-C18), alkyl chain, such as, for instance
stearylammoniunn chloride, hexadecylammonium chloride,
dimethyldioctadecylammonium bromide (DDAB), hexadecyltrimethylannmoniurn
bromide (CTAB). Further examples of positively charged lipids are tertiary or
quaternary ammonium salts with a halogen counter ion (e.g. chlorine or
bromine) comprising one or preferably two (C10-C20), preferably (C14-C18),
acyl
chain linked to the N-atom through a (C3-C6) alkylene bridge, such as, for
instance, 1,2-distearoy1-3-trirnethylammonium-propane (DSTAP), 1,2-
dipalmitoy1-3-trimethylammonium-propane (DPTAP), 1,2-oleoy1-3-
trimethylammoniurn-propane (DOTAP), 1,2-distearoy1-3-dimethylammoniunn-
propane (DSDAP).
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
18
DSEPC, DPEPC and/or DSTAP are preferably employed as positively charged
compounds in the microvesicle's envelope.
The positively charged component is typically associated with a
corresponding negative counter-ion, which can be mono- (e.g. halogen), di-
(e.g. sulphate) or tri-valent (e.g. phosphate). Preferably the counter-ion is
selected among halogen ions, such as F (fluorine), C1 (chlorine) or Br
(bromine).
In order to allow an effective electrostatic interaction with the MAC, the
total amount of charged compounds in the microvesicle's envelope should be of
at least 1% by mole with respect to the total amount of material forming said
envelope, preferably of at least 5% and much more preferably of at least 10%.
In some preferred combinations of microvesicles and MACS, it has been
observed that an amount of at least 20%, preferably of at least 40%, of
charged
compounds in the microvesicles' envelope allows binding relatively higher
amounts of MACS to said microvesicles. Although in some embodiments the
totality of the envelope of the microvesicle can be formed by charged
compounds, it has been observed that it may be advantageous to add at least
minimum amounts of neutral compounds to the formulation forming said
envelope. Preferably, the total amount of charged component can thus be equal
to or lower than about 95% by mole with respect to the total amount of
components forming the envelope of the nnicrovesicle, more preferably equal to
or lower than 90%, down to particularly preferred amounts equal to or lower
than 80%.
Mixtures of neutral and charged phospholipids and/or charged lipids can be
satisfactorily employed to form the microvesicles of an assembly of the
present
invention. Preferably, mixtures of two or more lipids or phospholipids, at
least
one with a neutral charge and at least one with an overall net charge, are
employed. More preferably, mixtures of two or more lipids or phospholipids, at
least one with neutral and at least one with positive charge are employed, to
obtain microvesicles with an overall positive charge. The amount of charged
lipid
or phospholipid may vary from about 95% to about 1% by mole, with respect to
the total amount of lipid and phospholipid, preferably from 80% to 20% by
mole.
Preferred mixtures of neutral phospholipids and charged lipids or
phospholipids are, for instance, DPPG/DSPC , DSTAP/DAPC, DPPS/DSPC,
DPPS/DAPC, DSPA/DAPC, DSPA/DSPC and DSPG/DSPC.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
19
Other excipients or additives may be present either in the dry formulation or
may be added together with the aqueous carrier used for the reconstitution,
without necessarily being involved (or only partially involved) in the
formation of
the stabilizing envelope of the microvesicle. These include pH regulators,
osmolality adjusters, viscosity enhancers, emulsifiers, bulking agents, etc.
and
may be used in conventional amounts. For instance compounds like
polyoxypropylene glycol and polyoxyethylene glycol as well as copolymers
thereof can be used. Examples of viscosity enhancers or stabilizers are
compounds selected from linear and cross-linked poly- and oligo-saccharides,
sugars, hydrophilic polymers like polyethylene glycol.
As the preparation of gas-filled microvesicles may involve a freeze drying or
spray drying step, it may be advantageous to include in the formulation one or
more agents with cryoprotective and/or lyoprotective effect and/or one or more
bulking agents, for example an amino-acid such as glycine; a carbohydrate,
e.g.
a sugar such as sucrose, mannitol, maltose, trehalose, glucose, lactose or a
cyclodextrin, or a polysaccharide such as dextran; or a polyglycol such as
polyethylene glycol.
The microbubbles usable in an assembly according to the invention can be
produced according to any known method in the art. Typically, the
manufacturing method involves the preparation of a dried powdered material
comprising an amphiphilic material as above indicated, preferably by
lyophilization (freeze drying) of an aqueous or organic suspension comprising
said material.
For instance, as described in WO 91/15244 film-forming amphiphilic
compounds can be first converted into a lamellar form by any liposome forming
method. For instance, an aqueous solution comprising the film forming lipids
and optionally other additives (e.g. viscosity enhancers, non-film forming
surfactants, electrolytes etc.) can be submitted to high-speed mechanical
homogenisation or to sonication under acoustical or ultrasonic frequencies,
and
then freeze dried to form a free flowable powder which is then stored in the
presence of a gas. Optional washing steps, as disclosed for instance in US
5,597,549, can be performed before freeze drying.
According to an alternative embodiment (described for instance in the
above cited US 5,597,549) a film forming compound and a hydrophilic stabiliser
(e.g. polyethylene glycol, polyvinyl pyrrolidone, polyvinyl alcohol, glycolic
acid,
malic acid or maltol) can be dissolved in an organic solvent (e.g. tertiary
CA 02547024 2011-12-22
butanol, 2-methy1-2-butanol or C2CI4F2) and the solution can be freeze-dried
to
form a dry powder.
Alternatively, as disclosed In the above dted WO 04/069284, a phospholipid
(selected among those cited above and induding at least one of the above-
5 identified charged phospholipids) and a lyoprotecting agent (such as
those
previously listed, in particular carbohydrates, sugar alcohols, polyglycols
and
mixtures thereof) can be dispersed in an emulsion of water with a water
immisdble organk solvent (e.g. branched or linear alkanes, aikenes, cydo-
alkanes, aromatic hydrocarbons, alkyl ethers, ketones, halogenated
10 hydrocarbons, perfluorinated hydrocarbons or mixtures thereof). The so
obtained emulsion, which contains microdroplets of solvent surrounded and
stabilized by the phospholipid material (and optionally by other amphiphilic
film-
forming compounds), is then lyophilized according to conventional tedmiques to
obtain a lyophilized material, which is stored (e.g. In a vial In the presence
of a
15 suitable gas) and which can be reconstituted with an aqueous carrier to
finally
give a gas-filled microbubbles suspension.
A further process for preparing gas-filled microbubbies comprises
generating a gas microbubbie dispersion by submitting an aqueous medium
comprising a phospholipid (and optionally other amphiphilic film-forming
20 compounds and/or additives) to a controlled high agitation energy (e.g.
by
means of a rotor stator mixer) in the presence of a desired gas and subjecting
the obtained dispersion to iyophilisation to yield a dried recormtitutable
product.
An example of this process is given, for instance, In WO 97/29782.
Spray drying techniques (as disclosed for instance In US 5,605,673) can
also be used to obtain the dried powder containing the microvesides of the
assembly of the invention.
The dried or lyophilised product obtained with any of the above techniques
will generally be in the form of a powder or a cake, and can be stored (e.g.
In a
vial) In contact with the desired gas. The product is readily reconstitutable
in a
suitable aqueous liquid carrier, which is physiologically acceptable, sterile
and
injectable, to form the gas-filled miamesides. Suitable liquid carriers are
water,
aqueous solutions such as saline (which may advantageously be balanced so
that the final product for injection is not hypotonic), or solutions of one or
more
tonidty adjusting substances such as salts or sugars, sugar akohols, glycols
or
other non-ionic polyol materials (eg. glucose, sucrose, sorbitol, mannitol,
glycerol, polyethylene glycols, propylene glycols and the like).
CA 02547024 2011-12-22
21
Microbe
'loons
Other gas-filled microvesides suitable for an assembly according to the
invention are referred to in the art as "microballoone. In general, these gas-
filled mIcrovesides have a material envelope, the thickness of which Is
greater
than the thidmess of mlcrobubbles' stabilizing film-envelope. Depending from
the material forming said envelope (which can be e.g. polymeric,
proteinaceous,
of a water insoluble Oki or of any combination thereof), said thickness Is in
general of at least 50 nm, typically of at least 1.00 nm, up to few hundred
nanometers (e.g. 300 nm).
Microballoons also generally differ from rnicrobubbles in terms of acoustic
response to ultrasonication. While the ultrasonic behavior of microbubbles is
in
fact closer to the behavior of "free gas bubbles, microballoons (probably
because of a higher stiffness of the envelope) are in general less responsive
(In
terms of intensity of the reflected echo signal) when irradiated at low levels
of
acoustic pressure energy (e.g. at a mechanical Index of about 0.1).
Examples of microballoons which are useful for preparing an assembly
according to the invention are preferably microballoons having a polymeric
envelope, preferably comprising a biodegradable polymer, or an envelope based
on biodegradable water-Insoluble lipids, such as, for instance those described
in
US 5,711,933 and US 6,333,021.
Microbe'loons having a proteinaceous envelope, i.e. made of natural
proteins (albumin, haemoglobin) such as those described in US-A-4,276,885 or
EP-A-0 324 938, can also be employed
Polymers forming the envelope of the injectable microballoons are
preferably hydrophilic, biodegradable physiologically compatible polymers.
Examples of such polymers, which may be natural or synthetic, are
substantially
insoluble polysaccharides (e.g. dlitosan or chitin), polycyanoacrylates,
polylactides and polyglycolides and their copolymers, copolymers of lactides
and
lactones such as y-caprolactone or 8-valerolactone, copolymers of
ethyleneoxide
and lactides, polyethylenelmines, polypeptides, and proteins such as gelatin,
collagen, globulins or albumins. Other suitable polymers mentioned in the
above
dted US 5,711,933 include poly-(ortho)esters, polylactic and polyglycolic add
and their copolymers (e.g. DEXON6, Davis & Gedc, Montreal, Canada); poly(DL-
lactide-co-y-caprolactone), poly(DL-lactide-co-45-valerolactone), poly(DL-
lactIde-
co-y-butyrolactone), polyalkyicyanoacrylates; polyamides, polyhydroxybutyrate;
polydioxanone; poly-eraminoketones; polyphosphazenes; and polyanhydrldes.
Polyamlno-adds sud) as polyglot:an* and polyaspartic adds can also be used,
CA 02547024 2011-12-22
22
as well as their derivatives, such as partial esters with lower alcohols or
glycols.
Copolymers with other amino adds such as methiontne, leudne, vallne, proline,
glycine, adenine, etc. can also be used. Derivatives of polygiutamic and
poiyaspartic add with controlled biodegradability (such as those described In
W087/03891, US 4,888,398 or EP 1309354
can also be used. These polymers (and copolymers with other amino-adds)
have formulae of the following type: -(NH-CHA-00)õ, -(NH-CHX-CO)y-
where X designates the side chain of an amino add residue (e.g. methyl,
Isopropyl, Isobutyl, or benzyl); A is a group of formula -(CH2), COOR1 R2 -
OCOR,
-(CH2) COO-CHR1COOR, -(CH2), CO(NH-CHX-CO)m NH-CH(COOH)-(CH2), COOH,
or the respective anhydrides thereof, wherein R1 and R2 represent H or lower
alkyls, and R represents alkyl or aryl; or R and R1 are connected together by
a
subsdtuted or unsubstituteci linking member to provide 5- or 6- membered
rings; n, m and p are lower integers, not exceeding 5; and w and y are
integers
selected for having molecular weights not below 5000.
Non-biodegradable polymers (e.g. for making microballoons to be used in the
digestive tract) can be selected from most water-insoluble, physiologically
acceptable, bioresistant polymers induding polyolefins (polystyrene), acrylic
resins (polyaaylates, polyacrylonitrile), polyesters (polycarbonate),
polyurethanes, polyurea and their copolymers. ABS (acryl-butadiene-styrene) is
a preferred copolymer.
Biodegradable water-Insoluble lipids useful for forming a mIcroballoon for an
assembly according to the invention comprise, for Instance, solid water
insoluble
mono-, di- or VI-glycerides, fatty adds, fatty acid esters, sterols such as
cholesterol, waxes and mixtures thereof. Mono-, dl- and tri- glycerides
include
mainly the mono-, di- and tri-laurin compounds as well as the corresponding -
myristin, -palmitin, -stearin, -arachIciln and -behenln derivatives. Mono-, di-
and
tri- myristin, -palmitin -stearin and mixed triglycerides such as
dipalmitoylmonooley1 glyceride are particularly useful; tripaimitin and
distearin
are preferred. Fatty acids include solid (at room temperature, about 18-25 C)
fatty adds (preferably saturated) having 12 carbon atoms or more, including,
for Instance, lauric, arachldic, behenic, pafmltìc, stearic, sebadc, myristic,
cerotinlc, melissic and erudc acids and the fatty add esters thereof.
Preferably,
the fatty adds and their esters are used in admixture with other glycerides.
The sterols are preferably used in admixture with the other glycerides
and or fatty adds and are selected from cholesterol, phytostevol, lanosterol,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
23
ergosterol, etc. and esters of the sterols with the above mentioned fatty
acids;
however, cholesterol is preferred.
Preferred biodegradable lipids are triglycerides such as tripalmitin,
tristearin or mixtures of the above mentioned triglycerides.
Optionally, up to 75% by weight of a biodegradable polymer, such as
those listed previously, can be admixed together with the biodegradable water
insoluble lipid forming the envelope of the microballoon.
Advantageously, ionic polymers (i.e. polymers bearing ionic moieties in their
structure), preferably biodegradable ionic polymers, can also be used to form
the stabilizing envelope of the nnicroballoons, thus conferring the desired
overall
net charge thereto. Ionic polymers can be used as main components of the
stabilizing envelope or they can be admixed in various amounts (e.g. from 2 to
80% by weight) with non ionic polymers. Suitable ionic polymers are, for
instance, polymers comprising a quaternized nitrogen atom, such as quaternized
amines or polymers comprising an carboxylic, sulfate, sulfonate or phosphonate
moieities. Examples of suitable ionic polymers include, without limitation,
Polyethylenimine, poly(diallyldimethylamnnonium chloride), poly{bis(2-
chloroethypether-alt-1,3-bis[3-(dimethylamino)propyl]urea} quaternized
(Polyquaternium -2), poly(4-vinylpyridinium tribrornide),
hydroxyethylcellulose
ethoxylate quaternized (Polyquaterniunn -4, poly(p-xylene
tetrahydrothiophenium chloride), poly(L-lysine), chitin, diethyleneaminoethyl
dextran, poly(acrylic acid), poly(methacrylic acid), poly(styrene-a/t-maleic
acid),
poly(amino acids), alginic acid, poly(uridylic acid) , hyaluronic acid, i.e.
poly(B-
glucuronic acid-a/t-B-N-acetylclucosamide), poly(galacturonic acid),
poly(vinyl
acetate-co-crotonic acid), DNA, poly(3,3',4,4'-benzophenonetetracarboxylic
dianhydride-co-4,4'-oxydianiline), poly(isoprene-graft- maleic acid monomethyl
ether), copolymer of glutammic acid with alkyl glutammate, heparin,
poly(styrene sulfonate), sulfonated poly(isophthalic acid), poly(vinyl
sulfonate,
potassium salt), poly(vinyl sulfate, potassium salt), chondroitin sulfate A,
dextran sulfate, fucoidan, polyphosphoric acid, sodium polyphosphate, sodium
polyvinylphosphonate, poly-L-lisine hydrobromide, chitosan, chitosan sulfate,
sodium alginate, alginic acid and ligninsulfonate.
Conventional additives can also be incorporated into the envelope of the
microballoons, to modify physical properties thereof, such as dispersibility,
elasticity and water permeability. In particular, effective amounts of
amphiphilic
materials can be added to the emulsion prepared for the manufacturing of said
microballoons, in order to increase the stability thereof. Said materials can
CA 02547024 2011-12-22
24
advantageously be selected among those amphiphilic compounds, such as lipids,
phospholipids and modified phospholipids, listed in the foregoing of this
specification.
The added amphiphilic material can advantageously be a compound bearing
an overall net charge. Preferred charged lipids, phospholipids and modified
phospholipids are those previously listed.
In order to allow an effective electrostatic Interaction with the MAC, the
total amount of charged additive In the envelope of the microballoon should be
of at least 1% by mole with respect to the total amount of material forming
said
envelope. The total amount of charged component is however preferably lower
than about 70% by mole with respect to the total amount of the material
forming the envelope of the microballoon. Preferably the amount of charged
compound is from about 2% to 40%.
Other excipients or additives, in particular used for the preparation of
microbatioons, can be Incorporated into the envelope such as redispersing
agents or viscosity enhancers.
Biodegradable polymer containing microballoons can be prepared, for
instance, according to the process disdosed in US 5,711,933
which comprises (a) emulsifying a hydrophobic
organic phase into a water phase so as to obtain droplets of said hydrophobic
phase as an oll-in-water emulsion In said water phase; (b) adding to said
emulsion a solution of at least one polymer In a volatile solvent insoluble in
the
water phase, so that said polymer forms a layer around said droplets; (c)
evaporating said volatile solvent so that the polymer deposits by interfacial
precipitation around the droplets which then form beads with a core of said
hydrophobic phase encapsulated by a membrane of said polymer, said beads
being In suspension in said water phase; (d) removing said encapsulated
hydrophobic phase by evaporation by subjecting said suspension to reduced
pressure; and (e) replacing said evaporated hydrophobic phase with a suitable
gas.
Biodegradable lipid containing microballoons can be prepared, for instance,
according to the process disclosed in US 6,333,021 (herein incorporated by
reference), by dispersing a mixture of one or more of the solid constituents
of
the microcapsuie envelope dissolved in an organk solvent In a water carrier
phase, so as to produce an oil-in-water emulsion. The emulsion water phase
may contain an effective amount of amphiphilk materials which are used to
stabilise the emulsion.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
A certain amount of redispersing agent and/or of a cryoprotecting or
lyoprotecting agent, such as those previously indicated, is then added to the
emulsion of tiny droplets of the organic solution in the water phase, prior to
freezing at a temperature below -30 C. Any convenient redispersing agent may
5 be used; redispersing agents selected from sugars, albumin, gelatine,
polyvinyl
pyrolidone (PVP), polyvinyl alcohol (PVA), polyethylene glycol (PEG) and
ethyleneoxide-propyleneoxide block copolymer (e.g. Pluronic , or Synperonie)
or mixtures thereof are preferred. The redispersing agents which are added to
prevent particle agglomeration are particularly useful when the microcapsules
10 are in the form of non-coalescent, dry and instantly dispersible
powders. The
frozen emulsion is then subjected to reduced pressure to effect
lyophilisation,
i.e. the removal by sublimation of the organic solvent from the droplets and
of
the water of the carrier phase, and the freeze-dried product is then contacted
with the desired gas.
15 Biocompatible Gas
Any biocompatible gas, gas precursor or mixture thereof may be employed
to fill the above nnicrovesicles, the gas being selected depending on the
chosen
modality.
The gas may comprise, for example, air; nitrogen; oxygen; carbon dioxide;
20 hydrogen; nitrous oxide; a noble or inert gas such as helium, argon,
xenon or
krypton; a radioactive gas such as Xe133 or Kr81; a hyperpolarized noble gas
such as hyperpolarized helium, hyperpolarized xenon or hyperpolarized neon; a
low molecular weight hydrocarbon (e.g. containing up to 7 carbon atoms), for
example an alkane such as methane, ethane, propane, butane, isobutane,
25 pentane or isopentane, a cycloalkane such as cyclobutane or
cyclopentane, an
alkene such as propene, butene or isobutene, or an alkyne such as acetylene;
an ether; a ketone; an ester; halogenated gases, preferably fluorinated gases,
such as or halogenated, fluorinated or prefluorinated low molecular weight
hydrocarbons (e.g. containing up to 7 carbon atoms); or a mixture of any of
the
foregoing. Where a halogenated hydrocarbon is used, preferably at least some,
more preferably all, of the halogen atoms in said compound are fluorine atoms.
Fluorinated gases are preferred, in particular perfluorinated gases,
especially in the field of ultrasound imaging. Fluorinated gases include
materials
which contain at least one fluorine atom such as, for instance fluorinated
hydrocarbons (organic compounds containing one or more carbon atoms and
fluorine); sulfur hexafluoride; fluorinated, preferably perfluorinated,
ketones
such as perfluoroacetone; and fluorinated, preferably perfluorinated, ethers
CA 02547024 2011-12-22
26
such as perfluorodiethyl ether. Preferred compounds are perfluorinated gases,
such as SF6 or perfluorocarbons (perfluorinated hydrocarbons), i.e.
hydrocarbons where all the hydrogen atoms are replaced by fluorine atoms,
which are known to form particularly stable miatobubble suspensions, as
disclosed, for Instance, in EP 0554 213.
The term perfluorocarbon Includes saturated, unsaturated, and cydic
perfluorocarbons. Examples of blocompatible, physiologically acceptable
perfluorocarbons are: perfluoroalicanes, such as perfluoromethane,
perfluoroethane, perfluoropropanes, perfluorobutanes (e.g. perfluoro-n-butane,
optionally in admbcture with other isomers such as perfluoro-lsobutane),
perfluoropentanes, perfluorohexanes or perfluoroheptanes; perfluoroalkenes,
such as perfluoropropene, perfluorobutenes (e.g. perfluorobut-2ene) or
perfluorobutadiene; perfluoroalkynes (e.g. perfluorobut-2-yne); and
perfluorocydoalkanes (e.g. perfluorocydobutane, perfluoromethylcydobutane,
perfluorodimethylcydobutanes, perfluorotrimethylcydobutanes,
perfluorocydopentene, perfluoromethylcydopentane,
perfluorodimethylcydopentanes, perfluorocydohexane,
perfluoromethyicydohexane and perfluorocydoheptane). Preferred saturated
perfluorocarbons have the formula C.,F..2, where n Is from 1 to /2, preferably
from 2 to 10, most preferably from 3 to 8 and even more preferably from 3 to
6.
Suitable perfluorocarbons lndude, for example, CF4, C.2F6, C.3F8, ;Fe, C4F20,
C.3F22, C6F22, C6F24, C7F24, C.7F26, C8F28, and C.8F20.
Particularly preferred gases are SF6 or perfluorocarbons selected from CF4,
C2F6, C3F6, C4F8, C4F20 or mbctures thereof; SF6, C3F8 or C.4F10 are
particularly
preferred.
It may also be advantageous to use a mbcture of any of the above gases in
any ratio. For instance, the mixture may comprise a conventional gas, such as
nitrogen, air or carbon dioxide and a gas forming a stable microbubbie
suspension, such as sulfur hexafluorlde or a perfluorocarbon as indicated
above.
Examples of suitable gas mixtures can be found, for Instance, in WO 94/09829,
which is herein incorporated by reference. The following combinations are
particularly preferred: a mixture of gases (A) and (B) In which the gas (B) is
a
fluorinated gas, preferably selected from SF6, CF4, C2F9, C3F6, C3F8, C4F6,
C4F8,
Via, Ws, C5F22 or mixtures thereof, and (A) Is selected from air, oxygen,
nitrogen, carbon dioxide Or mixtures thereof. The amount of gas (B) can
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
27
represent from about 0.5% to about 95% v/v of the total mixture, preferably
from about 5% to 80%.
In certain circumstances it may be desirable to include a precursor to a
gaseous substance (i.e. a material that is capable of being converted to a gas
in
vivo). Preferably the gaseous precursor and the gas derived therefrom are
physiologically acceptable. The gaseous precursor may be pH-activated, photo-
activated, temperature activated, etc. For example, certain perfluorocarbons
may be used as temperature activated gaseous precursors. These
perfluorocarbons, such as perfluoropentane or perfluorohexane, have a
liquid/gas phase transition temperature above room temperature (or the
temperature at which the agents are produced and/or stored) but below body
temperature; thus, they undergo a liquid/gas phase transition and are
converted to a gas within the human body.
For ultrasonic echography, the biocompatible gas or gas mixture is
preferably selected from air, nitrogen, carbon dioxide, helium, krypton,
xenon,
argon, methane, halogenated hydrocarbons (including fluorinated gases such as
perfluorocarbons and sulfur hexafluoride) or mixtures thereof. Advantageously,
perfluorocarbons (in particular C4F10 or C3F8) or SF6 can be used, optionally
in
admixture with air or nitrogen.
For the use of the assembly in MRI the microvesicles will preferably contain
a hyperpolarized noble gas such as hyperpolarized neon, hyperpolarized helium,
hyperpolarized xenon, or mixtures thereof, optionally in admixture with air,
CO2, oxygen, nitrogen, helium, xenon, or any of the halogenated hydrocarbons
as defined above.
For use in scintigraphy, the microvesicle of an assembly according to the
invention will preferably contain radioactive gases such as Xe133 or Kr81 or
mixtures thereof, optionally in admixture with air, CO2, oxygen, nitrogen,
helium, kripton or any of the halogenated hydrocarbons as defined above.
Microvesicle's associated component (MAC)
The second component of the assembly associated to the microvesicle
(MAC) can be any structural entity comprising a biocompatible surface active
agent bearing an overall net charge. In particular, said structural entity is
preferably a supermolecular structure formed by the association of a plurality
of,
preferably amphiphilic, molecules. In some embodiments, said charged
compound is admixed with other surface active agents and/or additives which
are neutral. The MAC may further comprise a desired targeting ligand,
bioactive
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
28
agent and/or diagnostic agent, depending on the specific application of the
assembly. Biocompatible surface active materials suitable for preparing a MAC
for an assembly according to the invention can be selected among those
compound previously listed, such as (C2-C10) organic acids, organic fatty
acids
comprising a (C12-C24), preferably a (C14-C22), aliphatic chain, the
pharmaceutically acceptable (alkali) salts thereof and the respective esters
with
polyoxyethylene, such as palmitic acid, stearic acid, arachidonic acid, oleic
acid,
sodium dodecanoate, sodium oxalate or sodium tartrate or polyoxyethylene
fatty acid stearate; polyionic (alkali) salts, such as sodium citrate, sodium
polyacrylate, sodium phosphate; organic amines, amides, quaternary amine
(halide) salts, preferably containing a (C8-C22) hydrocarbon chain, including
polyoxyethylated derivative thereof, such as ethanolamine, triethanolamine,
alkylamines, alkanolamides, trimethylalkylamine chloride, polyoxyethylated
alkylamines, polyoxyethylated alkanolamides; aminoacids; phospholipids, such
as fatty acids di-esters of phosphatidylcholine, ethylphosphatidylcholine,
phosphatidylglycerol, phosphatidic acid, phosphatidylethanolamine,
phosphatidylserine or of sphingomyelin; esters of mono- or oligo-saccharides
with (C12-C24), preferably a (C14-C22), organic fatty acids, such as sorbitan
laurate; polymeric surfactants, i.e. block copolymers including hydrophobic
and
hydrophilic portions, such as ethyleneoxide/propyleneoxide block copolymers;
organic sulfonates such as alkali (e.g. sodium) (C12-C24) alkyl, preferably
(C14-C22)alkyl, sulfonate; perfluoroorganic acids, such as perfluorooctanoic
acid;
and mixtures thereof. Preferred compounds are those neutral or charged
amphiphilic materials previously listed among the suitable components of
microbubbles, including lipids, phospholipids and modified phospholipids. A
preferred MAC is in the form of a micelle.
The preparation of the MAC can be obtained according to conventional
techniques, e.g. by dispersing the relevant components forming the MAC in an
aqueous carrier and optionally washing the obtained suspension in order to
remove the excess material.
Said second component is a nanocomponent, i.e. its relative dimension is of
about 100 nm or lower, preferably of about 80 nm or lower and more preferably
of about 50 nm or lower. The dimensions of the MAC, in particular its mean
diameter in number, can be determined according to conventional techniques,
such as, for instance, photon correlation spectroscopy. For instance, a
ZetaSizer
3000 Has (Malvern Instruments Gmbh) can be used. Particularly when the MAC
incorporates a desired targeting ligand, bioactive agent and/or diagnostic
agent
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
29
within its structure, its dimensions are preferably of at least 0.1 nm, more
preferably of at least 1. nm.
Preferably, the MAC has a mean dimension which is at least 10 times less or
smaller than the mean dimension of the microvesicles to which the MAC is
associated, more preferably at least 50 times less or smaller. Said mean
dimension is in general not lower than 1000 times, preferably not lower than
500 times.
As it can be appreciated, because of the relatively smaller dimensions of the
MAC with respect to the gas-filled microvesicle, it is possible to associate a
relatively large amount of MACS to the microvesicles, thus increasing the
effectiveness of the assembly in terms of a higher number of binding targeting
moieties and/or of the amount of releasable therapeutic or diagnostic agent
incorporated therein. In addition, said relatively small dimensions of the MAC
allow to obtain assemblies with dimensions comparable to the dimensions of the
microvesicles. It is in fact preferred that the mean diameter in number of an
assembly according to the invention is not higher than about 30% the mean
diameter of the microvesicle measured before the assembling, more preferably
not higher than 20% and much more preferably not higher than 10%.
In some embodiments of the invention, the charged material may form the
substantial totality of the MAC, i.e. 90% by mole or more. In some other
embodiments, it is preferable that the charged molecules forming the structure
of said MAC do not represent the totality of the compounds forming said
structure, thus being admixed with a certain amount of neutral compounds. Said
charged molecules may thus represent less than about 90% by mole of the total
amount of the material forming said MAC. On the other hand, the Applicant has
observed that the amount of charged molecules in the MAC should preferably be
of at least 0.5% by mole with respect to the total amount of material forming
said envelope, in order to allow an effective interaction with the charged
microvesicle. Preferably, said amount is of at least 1%, more preferably of at
least 2% by mole. In some preferred embodiments of the invention, the amount
of charged molecules forming the structure of the MAC is preferably of about
50% or lower, more preferably of about 20% or lower.
Micelles
As previously mentioned, a preferred component to be associated with a
microvesicle in an assembly of the invention is a micelle. The term "micelle"
as
used herein includes both micelles and mixed micelles, where the term mixed
micelles refers to a micellar structure formed by a mixture of two or more
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
different compounds, at least one of which is an amphiphilic compound capable
of forming a micellar structure. The term mixed micelles thus includes within
its
meaning also micelles formed by at least one compound, preferably an
amphiphilic compound, which is in general unable to form a micellar structure
5 when dispersed as such in an aqueous carrier, but which is capable of
forming
said structure when used in combination with suitable amounts of a micelle-
forming amphiphilic compound. Examples of mixed micelles are micelles formed
by unmodified phospholipids (which are in general not capable of forming
micelles when dispersed as the sole material in an aqueous carrier) and by a
10 micelle-forming compound (e.g. PEG-modified phospholipid or a fatty acid
salt).
As know in the art, micelles are formed by amphiphilic molecules dispersed in
water when the concentration of these molecules exceeds a predetermined
value known as CMC (critical micellar concentration). At concentrations below
the CMC, the molecules are in general dispersed in the aqueous solution as
15 single molecules. Above the CMC, the amphiphilic molecules tend to
organize in
supermolecular structures, in equilibrium with the free molecules in the
solution,
said structures being characterized by the fact that the hydrophobic (lipid)
tail of
the molecule is disposed towards the inner portion of the structure while the
hydrophilic (polar or ionic) headgroup of the molecule is disposed on the
outer
20 portion of the structure. The CMC of an amphiphilic molecule can be
determined
experimentally using techniques standard in the art. For example, the CMC of a
surfactant can be determined by plotting a property as a function of the
concentration of the surfactant. The property usually varies linearly with the
increase of surfactant concentration up to the CMC, and after this
concentration,
25 the curve (or the property) becomes non-linear. Suitable properties
which can
be used for the determination of the CMC include refractive index, light
scattering, surface tension, electric conductivity, osmotic pressure and the
like.
For the purpose of the invention, preferred micelle-forming materials are
those
having a relatively low CMC, e.g. of about 10 mM or lower.
30 Micelles have typically a dimension comprised from about 0.1 nm to about
100 nm, preferably from about 1 nm to about 50 nm. The mean diameter in
number (DN) is of about 50 nm or less, preferably of about 20 nm or less and
much more preferably of 10 nm or less, down to e.g. 1 nm, preferably about 2
nm.
A review of micelles, micellar systems and methods of preparation thereof
can be found, for instance, in the reference book: "Surfactants and Polymers
in
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
31
Drug Delivery", by M. Malmsten, Ch. 2, pp. 19-50, Marcel Dekker Inc. Ed.,
2002).
Suitable materials useful for forming micelles to be associated with
microvesicles in an assembly of the invention can be selected among the lipids
and phospholipids material previously listed.
Examples of micelle-forming compounds are PEG-modified phospholipids,
including in particular PEG-modified phosphatidylethanolamines such as DMPE-
PEG2000, DMPE-PEG3000, DMPE-PEG4000, DPPE-PEG5000, DPPE-PEG2000,
DPPE-PEG3000, DPPE-PEG4000, DPPE-PEG5000, DSPE-PEG2000, DSPE-
PEG3000, DSPE-PEG4000, DSPE-PEG5000, DAPE-PEG2000, DAPE-PEG3000,
DAPE-PEG4000 or DAPE-PEG5000; alkylammonium salts comprising at least one
(C10-C20), preferably (C14-C18), alkyl chain, such as, for instance
stearylarnmonium chloride, hexadecylammonium chloride,
dimethyldioctadecylammonium bromide (DDAB), hexadecyltrimethylammonium
bromide (CTAB); tertiary or quaternary ammonium salts comprising one or
preferably two (C10-C20), preferably (C14-C18), acyl chain linked to the N-
atom
through a (C3-C6) alkylene bridge, such as 1,2-distearoy1-3-
trimethylammonium-propane (DSTAP), 1,2-dipalmitoy1-3-trimethylammonium-
propane (DPTAP), 1,2-dioleoy1-3-trimethylammonium-propane (DOTAP), 1,2-
distearoy1-3-dimethylammonium-propane (DSDAP); fatty acid salts, preferably
alkali, in particular sodium salts, such as sodium palmitate, sodium stearate,
sodium oleate, sodium linoleate, sodium dodecanoate, 1,2-dipalmitoyl-sn-3-
succinylglycerate sodium salt or 1,3-dipalmitoy1-2-succinylglycerol sodium
salt.
Polymers including hydrophobic and hydrophilic portions therein (also
known as "polymeric surfactants") can also be used to prepare micellar
suspensions. Examples of suitable polymeric surfactants include, without
limitation, polyethyleneoxides (PEO), such as (C8-C16)n-alkyl PEO monoether,
(C8-C10)n-alkyl phenyl PEO, tetramethylbutylphenyl PEO, PEO polysorbates,
these PEO being sold under commercial names of Brij , Lubrol , Triton ,
Nonidet or Tweee; block copolymers such as ethyleneoxide/propyleneoxide
block copolymers (e.g. Pluronic or Synperonic ), having preferably a MW of
from about 3000 to 20000 daltons, preferably of from 5000 to 15000 daltons;
sugar derivatives such as (C6-C10)alky1-0-D-glucopyranoside, (C8-C12)alkyl-3-D-
maltoside; (C8-C16)alkyldimethylammoniumpropane-sulfonate; and bile acids
and derivatives therof, such as sodium cholate or sodium deoxycholate.
Additional lipids which can be used for preparing a micelle to be included in
an assembly of the invention include, for instance, unmodified phospholipids,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
32
such as the previously mentioned fatty acids di-esters of phosphatidylcholine,
ethylphosphatidylcholine, phosphatidylglycerol, phosphatidic acid,
phosphatidylethanolamine, phosphatidylserine or sphingomyelin. As these
unmodified phospholipids are in general unable to form micellar structures
when
dispersed in an aqueous carrier (as these compounds tends rather to associate
as liposomes when dispersed in an aqueous solution), said unmodified
phospholipids are preferably used in admixture with any of the previously
mentioned micelle-forming compounds. In particular, their amount shall
preferably be less than about 80%, more preferably of about 70% or less of the
total weight of the mixture of compounds forming the micellar structure.
According to a preferred embodiment the micellar component is formed from a
mixture comprising from about 30% to 70%, preferably form about 40% to
60% by weight of unmodified phospholipids. The remainder of the mixture can
be any of the above listed micelle-forming surfactants.
The desired overall net charge is conferred to the micelle by any of the
previously listed negatively or positively charged compounds, in particular
lipids
or phospholipids, including modified phospholipids.
Thus, examples of phospholipids suitable for conferring an overall negative
charge to the micelle are phosphatidylserine derivatives, such as DMPS, DPPS,
DSPS; phosphatidic acid derivatives, such as DMPA, DPPA, DSPA;
phosphatidylglycerol derivatives such as DMPG, DPPG and DSPG. Also modified
phospholipids, in particular PEG-modified phosphatidylethanolamines, can
advantageously be employed, such as, for instance DMPE-PEG750, -PEG1000, -
PEG2000, -PEG3000 or -PEG5000; DPPE-PEG750, -PEG1000, -PEG2000,
PEG3000 or PEG5000; DSPE-PEG750, -PEG1000, -PEG2000, PEG3000 or
PEG5000; DAPE-PEG750, -PEG1000, -PEG2000, PEG3000 or PEG5000; and the
respective lyso- form of the above cited phospholipids, such as
lysophosphatidylserine derivatives, lysophosphatidic acid derivatives (e.g.
lyso-
DMPA, -DPPA or -DSPA) and lysophosphatidylglycerol derivatives (e.g. lyso-
DMPG, -DPPG or -DSPG). Examples of negatively charged lipids are bile acid
salts such as cholic acid salts, deoxycholic acid salts or glycocholic acid
salts;
and fatty acid salts such as palmitic acid salt, stearic acid salt, 1,2-
dipalmitoyl-
sn-3-succinylglycerol salt or 1,3-dipalmitoy1-2-succinylglycerol salt.
Preferably, the negatively charged compound is selected among DPPA,
DPPS, DSPG, DSPE-PEG2000, DSPE-PEG5000 or mixtures thereof.
The negatively charged component is typically associated with a
corresponding positive counter-ion, which can be mono- (e.g. an alkali metal),
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
33
di- (e.g. an earth-alkali metal) or tri-valent (e.g. aluminium). Preferably
the
counter-ion is selected among alkali metal cations, such as Li, Na, or K+,
more
preferably Na.
Examples of phospholipids suitable for conferring an overall positive charge
to the micelle are esters of phosphatidylcholines, such as 1,2-Distearoyl-sn-
glycero-3-Ethylphosphocholine (Ethyl-DSPC), 1,2-Dipalmitoyl-sn-glycero-3-
Ethylphosphocholine (Ethyl-DPPC). The negative counterion is preferably an
halogen ion, in particular chlorine or bromine. Examples of positively charged
lipids are alkylammonium salts, comprising at least one (C10-C20), preferably
(C14-C18), alkyl chain, or tertiary or quaternary ammonium comprising one or
preferably two (C10-C20), Preferably (C14-C18), acyl chain linked to the N-
atom
through a (C3-C6) alkylene bridge, such as those previously listed.
Ethyl-DPPC, Ethyl-DSPC, DSTAP or mixtures thereof are preferably
employed as positively charged compounds.
The positively charged component is typically associated with a
corresponding positive counter-ion, which can be mono- (e.g. halogen), di-
(e.g.
sulphate) or tri-valent (e.g. phosphate). Preferably the counter-ion is
selected
among halogen ions, such as F (fluorine), C1 (chlorine) or Br (bromine).
Furthermore, ionic polymers such as those previously listed among the
microballoons-forming materials can advantageously be used to form a micelle
having an overall (negative or positive) net charge.
As above, the charged molecules can, in some embodiments,
advantageously be admixed with a neutral amphiphilic compound, such as those
previously listed (including neutral phospholipids), to form the desired
micellar
structure. Preferred neutral compounds to be admixed with the above listed
charged compounds are polymeric surfactants, such as ethyleneoxide-
propylenoxide block copolymers, e.g. Pluronic F68, Pluronic F108, Pluronic F-
127
(Sigma Aldrich, Missouri, USA); Polyoxyethylated alkyl ether such as Brij 78
(Sigma Aldrich, Missouri, USA); Polyoxyethylene fatty acid ester such as Myrj
53 or Myrj 59 (Sigma Aldrich, Missouri, USA); Polyoxyethylenesorbitan fatty
acid ester such as Tween 60 (Sigma Aldrich, Missouri, USA); Polyethylene
glycol tert-octylphenyl ether such as Triton X-100 (Sigma Aldrich, Missouri,
USA); sodium dodecyl sulfate (SDS). According to one embodiment of the
invention, the micelles are formed by mixtures of a charged amphiphilic
compound with a neutral phospholipid and one or more of the above listed
neutral compounds.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
34
In some preferred embodiments of the invention, the amount of charged
surfactant can form the substantial totality of the micelle (i.e. at least
80%,
preferably at least 90% and more preferably about 100% of the total weight of
micelle forming material). In some other preferred embodiments, in particular
when at least one compound forming the micelle is an unmodified phospholipid,
the total amount of charged surfactant forming the micelle is preferably from
about 1% to 80%, more preferably from about 2% to about 50%.
Micelles can be prepared as known in the art by dispersing the above
compounds in an aqueous liquid carrier and optionally agitating the mixture.
Examples of suitable liquid carriers are water, saline solution (sodium
chloride
0.9%), Phosphate buffered saline (10 mM, pH 7.4) , HEPES buffer (20 mM, pH
7.4), Glucose 5% w/w in water. For instance, the above compounds can be
dispersed in a concentration of from about 1 to 100 mg/ml in an aqueous liquid
and dissolved by means of agitation or sonication.
The micelles can then be stored as an aqueous dispersion (e.g. in the
aqueous carrier used for their preparation) before being admixed with a
suspension containing microvesicles or (as explained in detail in the
following of
the specification) to an aqueous-organic emulsion from which microvesicles are
prepared. Alternatively, the micelle suspension can be freeze-dried according
to
conventional techniques, to eliminate the liquid and store the final dry
product
for the subsequent uses.
Liposomes
Another supermolecular structure which can be associated as a MAC to a
microvesicle in an assembly according to the invention is a liposome, in
particular small unilamellar vesicle (SUV) liposomes.
The term liposome includes substantially spherical aggregations of
amphiphilic compounds, including lipid compounds, typically in the form of one
or more concentric layers. Typically they are formed in aqueous suspensions
and contain at least one bilayer of an amphiphilic compound. The hydrophilic
heads of the amphiphilic compounds forming the external layer of the bilayer
are directed towards the exterior of the spherical structure, while the
hydrophilic
heads of the amphiphilic compounds forming the internal layer of the bilayer
are
directed towards the interior of said spherical structure. The interior of the
spherical structure of the liposonnes is in general filled with the same
liquid of
the aqueous suspension, optionally containing additional compounds which are
not present (or are present to a lesser extent) in the outer aqueous
suspension.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
Preferred materials for preparing liposomes are phospholipids, such as those
previously listed, optionally in admixture with any of -the other previously
listed
amphiphilic compounds.
SUV liposomes can be formed according to conventional techniques, e.g. by
5 suitably processing MLV (Multilamellar large vesicles) suspensions, for
instance
by ultrasonication, extrusion or microfluidisation. MLV can be obtained, for
instance, by dissolving phospholipids in an organic solvent, evaporating the
organic solvent under vacuum to obtain a phospholipid film and finally
hydrating
the film at a temperature above phospholipid transition temperature. The
10 obtained MLV may thus be exposed to ultrasonic radiations to obtain the
desired
SUV liposomes. Alternatively, the MLV can be extruded through a plurality of
membranes (e.g. of polycarbonate) with decreasing pore size (e.g. 1.0, 0.8,
0.6,
0.4, and 0.2 pm) and then through an extruder with smaller pore dimensions
(for example LIPEX Biomembranes , Canada,) to obtain the final SUV. As a
15 further alternative preparation process of SUV, MLV can be homogenised
under
high pressure in a microfluidizer (e.g. from Microfluidics Corporation), to
reduce
the liposome size to approximately 100 nm or less, depending on the amount of
recirculation of the liposomes in the microfluidizer. These and other
preparation
methods of SUV are disclosed, for instance, in the reference book "Liposomes,
a
20 practical approach", edited by Roger R.C. New, Oxford University Press,
1989.
Dimensions of SUV liposomes are typically from about 25 nm to about 100
nm, preferably from about 30 nm to about 100 nm. The mean diameter in
number can vary from about 30 nm to about 60 nm, preferably from about 30
to about 50 nm.
25 A review of liposomes and their preparation methods is also given in the
above cited reference book "Surfactants and Polymers in Drug Delivery", by M.
Malmsten, Ch. 4, pp. 87-131, Marcel Dekker Inc. Ed., 2002).
Other structures which can be associated as a MAC to a microvesicle in
an assembly of the invention include colloidal nanoparticles, e.g. colloidal
gold
30 nanoparticles. These nanoparticles are typically obtained by adding a
suitable
dispersing agent to an aqueous solution comprising substantially insoluble
solid
nanoparticles, thus forming an aqueous suspension of colloidal nanoparticles
(i.e. solid nanoparticles coated with the dispersing agent). For instance,
colloidal
gold nanoparticles can be obtained by dispersing gold nanoparticles (with a
35 diameter of from about 2 to 50 nm) with sodium citrate in an aqueous
solution
(see e.g. Grabar, "Preparation and Characterization of Au colloid monolayers",
Analytical. Chemistry, vol. 67, p. 735,1995). Colloidal gold nano-particles
CA 02547024 2011-12-22
36
associated with gas-ftlied microvesides can be used to increase penetration
depth In a selected tissue when said microvesicies are caused to disintegrate
(e.g. induced by controlled high energy ultrasound irradiation). Thus, an
assembly comprising colloidal gold nanopartides can be for instance associated
with a further MAC comprising a bloactive agent, in order to increase the
penetration depth of said bioactive agent into the selected tissue, thus
enhancing the effectiveness of the therapeutic treatment.
Further MACs can be formed by solid polymeric nanoparticies. These solid
polymeric nanopartides can be formed by any of the polymeric materials
previously listed In connection with the preparation of gas-fllied
microballoons,
thus induding biodegradable physiologically acceptable polymers, such as
substantially water insoluble polysaccharides (e.g. chitosan or chitin),
polycyanoacrylates, polylactides and polyglycolides and their copolymers,
copolymers of iactides and lactones such as rcaprolactone or 5-valerolactone,
copolymers of ethyieneoxide and lactides, polyethyleneimines, poiypeptides,
and
proteins such as gelatin, collagen, globulins or albumins. Other suitable
polymers are those mentioned in the above dted US 5,711,933 and previously
listed. Non-biodegradable polymers in particular water-insoluble,
physiologically
acceptable and bioresistant polymers, can also be used, preferably in
admixture
with any of the above biodegradable polymer. Said polymer can be, for
instance, a polyoleffn, such as polystyrene, an acrylic resin such as
poiyacrylates
of polyeaylonitrIle, a polyester, such as potycarbonate, polyurethane,
polyurea
and their copolymers. ABS (acryl-butadiene-styrene) is a preferred copolymer.
Targeting Ugands and Bloactive/Diagnostic agents
The MAC, in particular in the form of micelles, of an assembly according to
the invention, may advantageously indude within Its structure compounds
having a targeting, diagnostic and/or biological activity.
The targeting ligand induded in the MAC may be synthetic, semi-
synthetic, or naturally-occurring. Materials or substances which may serve as
targeting ligands indude, for example, but are not limited to proteins,
induding
antibodies, antibody fragments, receptor molecules, receptor binding
molecules,
glycoproteins and Iectins; peptides, including oligopeptIdes and polypeptides;
peptIdomimetics; sac:charides, including mono and polysacchartdes; vitamins;
steroids, steroid analogs, hormones, cofactors, bloactive agents and genetic
material, including nucleosides, nucleotides and polynudeotides.
Examples of suitable targets and targeting Wands are disclosed, for
instance, in US patent no. 6,139,819,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
37
The targeting ligand can be a compound per se which is admixed with the
other components of the MAC composition to be included in the final structure
of
the MAC or can be a compound which is bound to an amphiphilic molecule
employed for the formation of the MAC.
In one preferred embodiment, the targeting ligand can be bound to an
amphiphilic molecule of the MAC through a covalent bond. In such a case, the
specific reactive moiety that needs to be present on the amphiphilic molecule
will depend on the particular targeting ligand to be coupled thereto. As an
example, if the targeting ligand can be linked to the amphiphilic molecule
through an amino group, suitable reactive moieties for the amphiphilic
molecule
may be isothiocyanate groups (that will form a thiourea bond), reactive esters
(to form an amide bond), aldehyde groups (for the formation of an imine bond
to be reduced to an alkylamine bond), etc.; if the targeting ligand can be
linked
to the amphiphilic molecule through a thiol group, suitable complementary
reactive moieties for the amphiphilic molecule include haloacetyl derivatives
or
maleimides (to form a thioether bond); and if the targeting ligand can be
linked
to the amphiphilic molecule through a carboxylic group, suitable reactive
moieties for the amphiphilic molecule might be amines and hydrazides (to form
amide or alkylamide bonds). In order to covalently bind a desired targeting
ligand, at least part of the amphiphilic compound forming the MAC shall thus
contain a suitable reactive moiety and the targeting ligand containing the
complementary functionality will be linked thereto according to known
techniques, e.g. by adding it to an aqueous dispersion comprising the
amphiphilic components of the MAC. The amphiphilic compound can be
combined with the desired targeting ligand before preparing the MAC, and the
so obtained combination can be used in the preparation process of the MAC.
Alternatively, the targeting ligand can be linked to the respective
amphiphilic
compound during the preparation process of the MAC or can be directly linked
to
the amphiphilic compound already in a micellar structure.
According to an alternative embodiment, the targeting ligand may also
be suitably associated to the MAC via physical and/or electrostatic
interaction.
As an example, a functional moiety having a high affinity and selectivity for
a
complementary moiety can be introduced into the amphiphilic molecule, while
the complementary moiety will be linked to the targeting ligand. For instance,
an avidin (or streptavidin) moiety (having high affinity for biotin) can be
covalently linked to a phospholipid while the complementary biotin moiety can
be incorporated into a suitable targeting ligand, e.g. a peptide or an
antibody.
CA 02547024 2011-12-22
38
The biotin-labelled targeting ligand will thus be associated to the avidin-
labelled
phospholipid of the MAC by means of the avidin-blotin coupling system.
Alternatively, both the phospholipid and the targeting ligand can be provided
with a biotin moiety and subsequently coupled to each other by means of avidin
(which is a bifunctional component capable of bridging the two biotin
moieties).
Examples of blotin/avIdin coupling of phospholipids and peptides are also
disclosed in the above cited US 6,139,819. Alternatively, van der Waal's
interactions, electrostatic interactions and other association processes may
associate or bind the targeting ligand to the amphlphilic molecules.
According to an alternative embodiment, the targeting ligand can be a
compound whkh is admixed with the components forming the MAC, to be
eventually incorporated the MAC structure, such as, for instance, a
lipopeptide
as disclosed e.g. In International patent Applications WO 98/18501 or
99/55383,
Alternatively, a MAC can first be manufactured, which comprises a
compound having a suitable moiety capable of interacting with a corresponding
complementary moiety of a targeting ligand; thereafter, the desired targeting
ligand is added to the MAC suspension, to bind to the corresponding
complementary moiety on the MAC. As an additional alternative, an assembly
can be prepared, which comprises a MAC including a compound having a
suitable moiety capable of interacting with a corresponding complementary
moiety of a targeting ligand; thereafter, the desired targeting ligand Is
added to
the assembly suspension, to bind to the corresponding moiety on the MAC.
Examples of suitable specific targets to which the assembly can be
directed are, for instance, fibrin and the GPIIbIlla binding receptor on
activated
platelets. Fibrin and platelets are in fact generally present in *thrombi",
i.e.
coagula which may form in the blood stream and cause a vascular obstruction.
Suitable binding peptides are disclosed, for instance, in the above dted US
6,139,819. Further binding peptkies specific for fibrin-targeting are
disdosed,
for instance, in International patent application WO 02/055544,
Other examples of important targets indude receptors In vulnerable
plaques and tumor specific receptors, such as kinase domain region (KDR) and
VEGF (vascular endothelial growth factor)/KDR complex. Binding peptides
suitable for KDR or VEGF/KDR complex are disclosed, for Instance, in
International Patent application WO 03/74005 and WO 03/084574,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
39
Bioactive agents include any compound or material capable of being used
in the treatment (including diagnosis, prevention, alleviation, pain relief or
cure)
of any pathological status in a patient (including malady, affliction, disease
lesion or injury). Examples of bioactive agents are those previously listed.
Among these, drugs or pharmaceuticals are preferred, in particular those drugs
consisting of an organic molecule (typically a synthetic molecule) which is
substantially hydrophobic or which contain a relevant portion thereof which is
substantially hydrophobic. These molecules may in fact be incorporated
relatively easily in the structure of a MAC, in particular of a micelle,
because of
their affinity with the lipophilic (or hydrophobic) portion of the amphiphilic
material forming the MAC. For instance, the organic molecule can be dispersed
in the aqueous carrier containing the amphiphilic material forming the MAC, in
particular the micelle, where it will be incorporated by affinity into the
hydrophobic portion of the MAC. Alternatively, also hydrophilic drugs or
organic
molecules can be incorporated into the MAC, in particular when this latter is
in
the form of a liposome. In this case, said hydrophilic compound will
preferably
be contained in the internal aqueous portion of the liposome.
Examples of drugs which can be incorporated into or associated to the
MAC's structure are, for instance those mentioned in the above cited
WO 99/53963, thus including antineoplastic agents such as vincristine,
vinblastine, vindesine, busulfan, chlorambucil, spiroplatin, cisplatin,
carboplatin,
methotrexate, adriamycin, mitomycin, bleomycin, cytosine arabinoside,
arabinosyl adenine, mercaptopurine, mitotane, procarbazine, dactinonnycin
(antinomycin D), daunorubicin, doxorubicin hydrochloride, taxol, plicamycin,
aminoglutethimide, estramustine, flutamide, leuprolide, megestrol acetate,
tamoxifen, testolactone, trilostane, amsacrine (m-AMSA), asparaginase
(Lasparaginase), etoposide, interferon a-2a and 2b, blood products such as
hematoporphyrins or derivatives of the foregoing; biological response
modifiers
such as muramylpeptides; antifungal agents such as ketoconazole, nystatin,
griseofulvin, flucytosine, miconazole or amphotericin B; hormones or hormone
analogues such as growth hormone, melanocyte stimulating hormone, estradiol,
beclomethasone dipropionate, betamethasone, cortisone acetate,
dexamethasone, flunisolide, hydrocortisone, methylprednisolone,
parannethasone acetate, prednisolone, prednisone, triamcinolone or
fludrocortisone acetate; vitamins such as cyanocobalamin or retinoids; enzymes
such as alkaline phosphatase or manganese superoxide dismutase; antiallergic
agents such as amelexanox; anticoagulation agents such as warfarin,
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
phenprocoumon or heparin; antithrombotic agents; circulatory drugs such as
propranolol; metabolic potentiators such as glutathione; antituberculars such
as
p-aminosalicylic acid, isoniazid, capreomycin sulfate, cyclosexine,
ethambutol,
ethionamide, pyrazinamide, rifannpin or streptomycin sulphate; antivirals such
5 as acyclovir, amantadine, azidothymidine, ribavirin or vidarabine; blood
vessel
dilating agents such as diltiazern, nifedipine, verapamil, erythritol
tetranitrate,
isosorbide dinitrate, nitroglycerin or pentaerythritol tetranitrate;
antibiotics such
as dapsone, chloramphenicol, neomycin, cefaclor, cefadroxil, cephalexin,
cephradine, erythromycin, clindamycin, lincomycin, amoxicillin, ampicillin,
10 bacampicillin, carbenicillin, dicloxacillin, cyclacillin, picloxacillin,
hetacillin,
methicillin, nafcillin, penicillin or tetracycline; antiinflammatories such as
diflunisal, ibuprofen, indomethacin, meclefenannate, nnefenamic acid,
naproxen,
phenylbutazone, piroxicann, tolnnetin, aspirin or salicylates; antiprotozoans
such
as chloroquine, metronidazole, quinine or meglumine antimonate;
15 antirheumatics such as penicillamine; narcotics such as paregoric;
opiates such
as codeine, morphine or opium; cardiac glycosides such as deslaneside,
digitoxin, digoxin, digitalin or digitalis; neuromuscular blockers such as
atracurium mesylate, gallamine triethiodide, hexafluorenium bromide,
metocurine iodide, pancuronium bromide, succinylcholine chloride, tubocurarine
20 chloride or vecuronium bromide; sedatives such as amobarbital,
amobarbital
sodium, apropbarbital, butabarbital sodium, chloral hydrate, ethchlorvynol,
ethinamate, flurazepam hydrochloride, glutethimide, methotrimeprazine
hydrochloride, methyprylon, midazolam hydrochloride, paraldehyde,
pentobarbital, secobarbital sodium, talbutal, temazepam or triazolam; local
25 anaesthetics such as bupivacaine, chloroprocaine, etidocaine, lidocaine,
mepivacaine, procaine or tetracaine; general anaesthetics such as droperidol,
etomidate, fentanyl citrate with droperidol, ketamine hydrochloride,
methohexital sodium or thiopental and pharmaceutically acceptable salts (e.g.
acid addition salts such as the hydrochloride or hydrobromide or base salts
such
30 as sodium, calcium or magnesium salts) or derivatives (e.g. acetates)
thereof;
and radiochemicals, e.g. comprising alpha-, beta-, or gamma-emitters such as,
for instance 177Lu, 90Y. or 1311. Of particular importance are antithrombotic
agents
such as heparin and agents with heparin-like activity such as antithrombin
III,
dalteparin and enoxaparin; blood platelet aggregation inhibitors such as
35 ticlopidine, aspirin, dipyridamole, iloprost and abcixirnab; and
thrombolytic
enzymes such as streptokinase and plasminogen activator. Other examples of
bioactive agent include genetic material such as nucleic acids, RNA, and DNA
of
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
41
natural or synthetic origin, including recombinant RNA and DNA. As mentioned
in the above patent, DNA encoding certain proteins may be used in the
treatment of many different types of diseases. For example, tumour necrosis
factor or interleukin-2 may be provided to treat advanced cancers; thymidine
kinase may be provided to treat ovarian cancer or brain tumors; interleukin-2
may be provided to treat neuroblastoma, malignant melanoma or kidney
cancer; and interleukin-4 may be provided to treat cancer.
Diagnostic agents which may be incorporated into or associated to the
MAC in an assembly of the invention are any compound, composition or particle
which may allow imaging enhancement in connection with diagnostic
techniques, including, magnetic resonance imaging, X-ray, in particular
computed tomography, optical imaging, nuclear imaging or molecular imaging.
Examples of suitable diagnostic agents are, for instance, magnetite
nanoparticles, iodinated compounds, such as Iomeprol , or paramagnetic ion
complexes, such as hydrophobic gadolinium complexes. For instance, magnetite
nanoparticles can be admixed with a negatively charged amphiphilic
material(and optionally a neutral one), such as those previously mentioned, in
order to stabilize said particles and keep them dispersed in an aqueous
solution.
US 5,545,395, herein incorporated by reference, gives some examples of
preparation of said stabilized magnetite particles, e.g. by using a mixture of
DPPA and Pluronic for stabilizing said particles. Alternatively, gadolinium
complexes can be admixed with suitable micelle-forming compounds, for
instance as disclosed in European Patent EP 804 251 (herein incorporated by
reference), to form a gadolinium containing MAC.
The assembly
In order to evaluate the relative compositions of the assemblies of the
invention, the Applicant has found it useful to refer to the amounts of
charged
compounds in the microvesicles and in the MAC (expressed as "equivalents of
charge") and to the potential of the suspensions of microvesicles and
assemblies.
The term "equivalent of charge" (EC), indicates the number of charges per
mole of said compound. Thus, one mole of a mono-ionic compound contains one
EC, one mole of a di-ionic compound contains two EC and so on.
The '.-potential (zeta-potential), also called electrokinetic potential, is
the
electric potential at the surface of a colloidal particle relative to the
potential in
the bulk medium at a long distance. It can be measured according to
conventional micro-electrophoresis analytical methods, e.g. via the
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
42
determination of the velocity of the particles in a driving electric field by
Laser-
Doppler-Anemometry. For example, the ZetaSizer 3000 Has (Malvern
Instrument GmbH) can be advantageously used. In the practice, the potential
of the initial suspension of microvesicles is first determined, which can have
a
positive or negative value, depending whether the microvesicles contain
positively or negatively charged compounds, respectively. Then, the -potential
is measured on the final suspension containing the assembly (i.e. after the
necessary washing steps for removing possibly unbound MACs). In general, the
addition of MACs of opposite sign with respect to the microvesicles determines
a
more or less pronounced decrease in absolute value of the C-potential of the
suspension. In particular, suspensions comprising positively charged
microvesicles will show a decrease of the .-potential upon addition of a
suspension of negatively charged MACs, while suspensions comprising
negatively charged microvesicles will show a relative increase of the '-
potential
(i.e. a decrease in absolute value) upon addition of a suspension of
positively
charged MACs. As observed by the Applicant, preferred assemblies are those
suspensions showing a substantial decrease in absolute value with respect to
the '-potential of the initial microvesicles suspension, i.e. a decrease of at
least
50%, preferably of at least 75% and more preferably of at least 90% of said
initial value. Particularly preferred assemblies' suspensions are those
showing a
substantially neutral -potential (i.e. 0 10 mV, corresponding to an absolute
decrease of about 100% with respect to the initial potential of the
microvesicles
suspension) or a potential opposite in sign with respect to the potential of
the initial microvesicles' suspension. As observed by the Applicant, when the
C-
potential of the assembly suspension remains equal in sign with an absolute
decrease of less than 50% with respect to the -potential of the initial
microvesicles suspensions, this may be an indication that an insufficient
number
of MACs are associated to the microvesicles.
According to a preferred embodiment, the amount of charged MACs in the
assembly is such as to confer a substantially neutral -potential to said
assembly
or a i-potential which is opposite in sign with respect to the -potential of
the
microvesicle. As observed by the Applicant, to obtain said neutral or opposite
in
sign potential of the assembly it is however not necessary that the assembly
contains an excess of equivalents of charge from the MAC. As a matter of fact,
it
has been observed that assemblies composed of positive microvesicles and
negative MACs and having a ratio between EC in the MAC and equivalents of
opposite charge in the microvesicle of about 1:5 (i.e. an excess of about 5
times
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
43
of positive charges on the microvesicles) may nevertheless show a
substantially
neutral or negative (-potential. Although not wishing to be bound to any
particular theory, it may be supposed that the (negative) charges comprised in
the MACs are disposed on the outer surface of the assembly; if the number of
MACs associated to the microvesicle is sufficiently high, the excess of
(positive)
opposite charges on the microvesicle may result, at least partially, screened
by
said MACs. Thus, as the (-potential measured on a particle is strongly
influenced
by the charges present on the outer boundary of said particle, even an
assembly
having an excess of (positive) equivalents of charge deriving from a
microvesicle
may show a negative (-potential, if the amount of (negatively) charged MACs is
sufficient to partially screen the (positive) charges of the microvesicle. All
the
above is of course also applicable to assemblies formed by negatively charged
microvesicles and positively charged MACs.
In general, the ratio between the EC on the microvesicles and the EC of
charge on the MAC in the final suspension of the assembly can vary from about
10:1 to about 1:10. According to a preferred embodiment, the microvesicle/MAC
EC ratio in the formed assembly is preferably of about 3:1 or less, more
preferably of about 2:1 or less and much more preferably of about 3:2 or less.
Depending from the amount of charged compounds forming the microvesicles
and the MACs, said ratio can of course be lower, for instance of about 1:1 and
down to e.g. about 1:4 or less.
In view of the relatively small dimensions of the MAC, the dimensions
(mean diameter in number) of the assembly are typically of about 10 pm or
lower and in general of about 1 pm or more. Preferred dimension of an
assembly according to the invention are from about 1 pm to about 8 pm, more
preferably from about 2 pm to about 5 pm.
According to a further embodiment of the invention, multi-layer assemblies
can be formed by associating a gas-filled microvesicle to a plurality of
layers of
components, having an alternate charge. Thus, for instance, it is possible to
associate to a negatively charged microvesicle a first layer of components
(e.g.
micelles) having a positive charge; then a second layer of components (e.g.
again micelles or liposomes) having a negative charge can be associated to
this
assembly, and so on. Whilst the association of the first layer of components
to
the microvesicles will cause a reduction in absolute value of the (-potential
(with respect to the one measured on a suspension of the sole microvesicles),
the further association of a second layer of components (having an opposite
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
44
charge with respect to the first component) will cause the C-potential to
change
again towards values closer to those of the suspension of sole microvesicles.
According to a first method of preparation, the assembly can be obtained by
admixing an aqueous suspension comprising the microvesicles (obtained
according to any of the above cited manufacturing methods) with an aqueous
suspension comprising the second component of the assembly (obtained
according to any of the above cited manufacturing methods).
Optionally, the so obtained mixture can be subjected to one or more
washing steps, in order to remove the excess of non-associated components.
The washing can be performed with any conventional washing technique, by
using suitable washing solutions, such as distilled water, phosphate buffered
saline, Tris/glycerol buffer, saline or 5% glucose solution. The phase of the
washed mixture comprising the assembly of the invention (in general the
supernatant phase) is thus separated and collected; optionally, the recovered
assembly-containing suspension is finally diluted before use, e.g. with any of
the
above cited physiologically acceptable carrier.
Upon formation, the suspension comprising the assembly of the invention
can be stored for a subsequent administration or can be directly administered.
If
desired, the liquid carrier of the suspension can be eliminated (e.g. by
freeze-
drying) to obtain a dry powder of the assembly which can be stored (preferably
in the presence of a gas suitable for forming the gas-filled microvesicles
upon
reconstitution) for relatively long periods of time before reconstitution.
Alternatively, the two components of the assembly can be stored as
separate compositions in dried form (e.g. freeze dried) and reconstituted as a
suspension before administration. For the storage, the dried components are
preferably kept in an atmosphere of the gas which will form the microvesicles
upon reconstitution with water. The reconstitution with an aqueous liquid
carrier
may take place separately on the two dried compositions comprising the
respective components of the assembly, thus obtaining two separate
suspensions which are subsequently admixed to obtain the desired assembly
suspension. Alternatively, the two dried compositions may be admixed together
and then reconstituted as a single suspension with an aqueous liquid carrier.
In
this latter case, the mixed components of the assembly are stored in the
presence of the gas which will form the microvesicles upon reconstitution with
the aqueous liquid carrier. According to a preferred embodiment, the dried MAC
composition is first reconstituted with a physiologically acceptable aqueous
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
carrier and the obtained suspension is then used for reconstituting the dried
microvesicle composition, to finally obtain a suspension of the assembly.
Any of the above preparation methods can also be used for preparing a
multi-layer assembly as described above, by first admixing the charged gas
5 filled microvesicles with a first component having an opposite charge and
then
by admixing the formed assembly with a second component having the same
charge as the microvesicles.
For the preparation of the assembly from two separate preparations of
microvesicles and MACs, it may be advantageous to add an excess amount of
10 MACs with respect to the relative amount of MACs which is desired in the
final
assembly, in particular because a certain amount of said MACs can be removed
during the optional washing steps of the assemblies' suspension. In general,
it is
preferred that the amount of EC in the composition employed for the
preparation of the MAC is at least substantially equal to the EC in the
15 composition employed for the preparation of the microvesicles (i.e. EC
ratio of
about 1:1). Preferably said EC ratio is of about 2:1 or higher, more
preferably of
at least about 3:1 or higher, up to e.g. 30:1.
According to a preferred embodiment, an aqueous suspension of a MAC (in
particular of micelles as above defined) is added to an aqueous/organic
20 emulsion comprising a phospholipid and a lyoprotecting agent, prepared
according to the method disclosed in the above cited WO 04/069284. In this
case, the charged MACs will associate with the opposite charged layer of
amphiphilic material surrounding the microdroplets of the emulsion. The MAC is
generally added in an amount such that ratio between the equivalents of charge
25 in the MAC and the EC in the microvesicles of the suspension is of at
least about
1:2 or higher, preferably 2:3 or higher and much more preferably of at least
1:1
or higher, up to e.g. 10:1. Similarly, an aqueous suspension of MAC can be
added to a gas microbubble dispersion which has been obtained by submitting
an aqueous medium comprising a phospholipid (and optionally other amphiphilic
30 film-forming compounds and/or additives) to a controlled high agitation
energy
in the presence of a desired gas, as previously mentioned.
Freeze drying of the mixture provides the desired assembly as a lyophilized
powder, which can be stored in contact with the desired gas and subsequently
reconstituted as a physiological suspension by addition of an aqueous carrier.
35 The gas in contact with the stored freeze-dried products (assembly,
microvesicles and/or MACs) can be present in the storage container at a
substantial atmospheric pressure (i.e. about 1020 mbar +/- 5%) or at a
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
46
pressure lower than the atmospheric one (e.g. 900 mbar or lower) as disclosed
in European patent application EP 1228770.
Injectable compositions after reconstitution of the lyophilised contrast agent
should be, as far as possible, isotonic with blood. Hence, before injection,
small
amounts of isotonic agents may also be added to the suspensions comprising
the assembly of the invention. The isotonic agents are physiological solutions
commonly used in medicine such as, for example, aqueous saline solution
(0.9% NaCI), 2.6% glycerol solution or 5% dextrose solution. The
reconstitution
of the aqueous suspensions is generally obtained by simple dissolution of the
gas-stored dried film forming surfactant and gentle agitation.
The volume and concentrations of the reconstitution liquid may desirably be
balanced to make the resulting ready-to-use formulations substantially
isotonic.
Hence the volume and concentration of reconstitution fluid chosen will be
dependent on the type and amount of stabilizer (and other bulking agents)
present in the freeze-dried product.
As it will be appreciated by those skilled in the art, the assembly according
to the invention allows an extreme flexibility in the preparation of different
assemblies for different purposes. As a matter of fact, the structure of the
basic
carrier component employed for the ultrasound diagnostic/therapeutic methods
(i.e. the microvesicle) does not need to be subjected to any particular
modifications, thus avoiding possible drawbacks in terms of stability of said
component. Such component only needs to have an overall net charge on its
envelope, which result can be easily obtained by using conventional materials
normally used for forming said envelope. As a matter of fact, the
electrostatic
interaction between the microvesicle and the MAC allows an effective
association
between the two components, without the need of modifying the structure of
the microvesicle. On the other side, the second component of the assembly, the
stability of which is much less sensitive to possible modifications of its
composition, can be easily adapted to the specific purpose requested to the
assembly, by associating the desired targeting ligand and/or bioactive
compound to it. Furthermore, due to the relatively small dimensions of the MAC
with respect to the microvesicle, it is possible to associate a relatively
large
number of MACS to each microvesicle, thus increasing the efficiency of the
system.
In addition, the microvesicles of the assembly can be easily associated with
more than one type of different nano-components, thus resulting in a
"multipurpose" assembly. In particular, one single preparation of charged
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
47
microvesicles (e.g. positively charged) can be used as a carrier to be
associated
with any desired type of MAC bearing an opposite charge (e.g. negative).
Alternatively, a multipurpose assembly can also be obtained by preparing a
multilayer assembly as previously described, where the different components of
opposite charge are disposed as alternate layers around the microvesicle. The
different MAC's associated to the microvesicle can differ in their chemical
composition or supermolecular structure (e.g. micelles vs. liposomes), as well
as
in the targeting ligand, diagnostic agent and/or bioactive agent contained
therein; advantageously, a multipurpose assembly will contain a combination of
any of these. For instance, the microvesicle component can be combined with a
first nano-component (e.g. in micellar form), comprising in its structure at
least
one targeting ligand (capable to link to a specific receptor associated to a
pathologic status or disease), and with a second nano-component (e.g. either
in
micellar form or as a liposome), comprising either a second targeting ligand
or a
bioactive compound (e.g. a therapeutic compound for treating said pathologic
status or disease). When an assembly comprising a combination of a "targeting
ligand bearing component" and of a "bioactive compound bearing component"
are employed, particularly when a "multilayer" assembly is prepared, the
component bearing the targeting ligand is preferably separately associated as
last component to the gas-filled microvesicle, in order to allow an effective
targeting activity of the assembly. An example of a multipurpose assembly is,
for instance, an assembly comprising a gas filled microvesicle, a first
component
in micellar form, which comprises a targeting ligand binding to a tumor
specific
receptor, and a second component comprising a radiochemical (bound to a
micelle-forming compound or incorporated into a liposome) for the therapeutic
treatment of the tumor.
An assembly of the invention can thus be used for a variety of diagnostic
and/or therapeutic methods.
For instance, an assembly comprising a MAC with a suitable targeting ligand
can be used to target a specific organ or tissue, which can then be
selectively
imaged according to conventional ultrasound imaging techniques, because of
the enhanced imaging determined by the gas-filled microvesicles bound to said
organ or tissue. If a diagnostic agent (e.g. for MRI) is further included in
the
assembly, use of combined diagnostic techniques is possible. Furthermore, if a
bioactive agent is included in the assembly (e.g. included in a liposome), it
is
possible to provoke an ultrasound-mediated release of said bioactive agent at
a
selected target (e.g. where a targeting ligand binds) by applying a controlled
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
48
acoustic power capable of destroying the gas-filled microvesicles, as
disclosed
for instance in WO 99/39738, herein incorporated by reference.
Of course, an assembly of the invention may also contain, together with
components bearing a targeting ligand or a pharmaceutical active agent, also
components which are free of said compounds, which are employed, for
instance, for balancing the overall charge of the assembly.
As previously mentioned, it has also been observed that the association of a
component, in particular of a plurality of micelles, to a gas-filled
microvesicle to
form an assembly according to the invention, results in an increased
resistance
of said microvesicles towards pressure. For instance, it has been observed
that
microvesicles showing a Pcso (i.e. a critical pressure at which more than 50%
of
the microvesicle population is destroyed) of about 500 mm Hg, may increase
said value of Pc50 to at least 600 mm Hg and up to about 800 mm Hg, when
associated to different types of micelles to form an assembly of the
invention.
Kit
Another aspect of the invention relates to diagnostic kits comprising the
assembly of the invention or its respective separate components, optionally
further comprising the aqueous liquid carrier.
According to a first embodiment, said kit is a two component kit comprising
the assembly of the invention together with an aqueous liquid carrier. Said
two
component kit can include two separate containers or a dual-chamber container.
In the former case the first container is preferably a conventional septum-
sealed vial, wherein the vial containing the assembly as a lyophilized residue
(obtained according to any of the above illustrated methods) in contact with
the
desired gas is sealed with a septum through which the carrier liquid may be
injected for reconstituting the suspension of the gas-filled
nnicrovesicles/MACs
assemblies. The carrier liquid is contained into a second container which
preferably takes the form of a syringe. The syringe is preferably re-filled
with
the reconstituted suspension and used subsequently to administer the contrast
agent by injection. Instead of the formed assembly, the first container can
alternatively contain mixture of separately freeze-dried MAC and microvesicles
compositions, which will form the desired assembly upon reconstitution with
the
aqueous carrier. Although in general hand shaking of the container provides
the
desired energy for reconstituting the suspension, means for directing or
permitting application of sufficient energy towards the container can be
provided
(e.g. a Vortex mixer), in order to assure suitable reconstitution of the
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
49
assemblies' suspension. The dual-chamber container is preferably a dual-
chamber syringe, where the components are kept separated e.g. by means of a
removable septum, and once the lyophilisate has been reconstituted by gentle
shaking, the container can be used directly for injecting the contrast agent.
As
before, means for directing or permitting application of sufficient energy
towards
of the container can be provided.
It can be appreciated by one ordinary skilled in the art that other two-
chamber reconstitution systems capable of combining the dried powder with the
aqueous solution in a sterile manner are also within the scope of the present
invention. In such systems, it is particularly advantageous if the aqueous
phase
can be interposed between the water-insoluble gas and the environment, to
increase shelf life of the product.
According to another embodiment, a kit according to the invention is an at
least two component kit comprising a MAC composition, a microvesicle
composition and, optionally, an aqueous carrier.
These are preferably presented as at least two separate containers, the first
one containing the lyophilized microvesicle composition (e.g. in contact with
a
desired gas) and the second one containing the desired lyophilized MAC
composition (optionally in contact with a desired gas or under vacuum). A
third
optional container, containing the aqueous carrier for reconstitution can
advantageously be included in the kit. If desired, additional containers
containing further lyophilized MAC compositions can be included in the kit.
For
administration, the MAC suspension is first reconstituted in the aqueous
carrier
and the obtained suspension is then used for reconstituting the microvesicle
composition, thus forming the desired assembly suspension.
No specific containers vial or connection systems are required; the present
invention may use conventional containers, vials and adapters. The only
requirement is a good seal between the stopper and the container. The quality
of the seal, therefore, becomes a matter of primary concern; any degradation
of
seal integrity could allow undesirables substances to enter the vial. In
addition
to assuring sterility, vacuum retention is essential for products stoppered at
ambient or reduced pressures to assure safe and proper reconstitution. The
material of the stopper forming the gas-seal of the container is preferably an
elastomeric compound or multicomponent formulation based on an elastomer,
such as poly(isobutylene) or butyl rubber. Conveniently a butyl rubber stopper
from Daiko Seiko ltd. can be used.
CA 02547024 2011-12-22
EXAMPLES
The following materials are employed in the examples:
PBS Phosphate buffered saline: 10 mM sodium phosphate, NaCI
0.9% w/w, pH = 7.4
Tris buffer Tris buffered saline:10 mM Tris (hydroxy
methyl)aminomethane, NaCi 0.9%, pH = 7.4
HEPES buffer 4-(2-hydroxyethyl)-1-piperazineethanesulfonic add (20 mM)
and NaC1 (150 mM), pH = 7.4
Tris glycerol buffer TrIs (hydroxymethyl) aminomethan 1g/I and 0.3 M glycerol,
pH = 7.2
DI018 marker 3,3'-dloctadecyloxacarbocyanine (Molecular Probes Inc.,
U.S.A.)
Gd-DTPA-(SE)2 DIstearoyi ester of gadolinium-diethylenetriaminepentacetic
add complex (prepared according to G. W. Kabalka et al.,
Magnetic Resonance in Medidne 8 (1988), 89-95)
DSPG Distearoylphosphatidylglycerol sodium salt (Genzyme)
IUPAC: 1,2 -DIstearoyl-sn-glycero-3-[phospho-rac-(1-
glycerol)]
DAPC Diarachidoylphosphatidylcholine (Avant! polar Lipids)
IUPAC: 1,2-Diarachidoyi-sn-glycero-3- phosphodioline
DSTAP 1,2 -Distearoy1-3-trimethylammonium-propane chloride
(Avantl Polar Lipids)
DSPC Distearoylphosphatidylcholine (Genzyme)
IUPAC: 1,2-Distearoyl-sn-glycero-3- phosphocholine
DPPG Dipaimitoylphosphatidyiglycerol sodium salt (Genzyme)
IUPAC: 1,2 -DipalmItoyl-sn-glycero-3- rphospho-rac-(1-
glycerol)]
DPPA Dipaimitoyl phosphatidlc add sodium salt (Genzyme)
IUPAC: 1,2 -01palmItoyl-sn-glycero-3- phosphate
DPPC DIpalmItoylphosphatidylchollne (Genzyme)
IUPAC: 1,2 -DIpalmItoyl-sn-glycero-3- phosphodrolIne
DSEPC Distearoyiethylphosphatklylcholine (Avanti Polar Lipids)
IUPAC: 1,2-DIstearoyl-sn-glycero-3-EthylphosphocholIne
NaDOC sodium deoxycholate (Fluka)
OSPE-PEG2000 Distearoylphosphatidylethanolamine modified with PEG2000,
sodium salt (Nelctar Therapeutics)
Ethyl-SPC3 Soy ethyl phosphocholine: 4:1 (w/w) mbcture of Ethyl-DSPC
and Ethyl-DPPC
DPPE-cap-blotIn 1,2 dipalmItoyl-sn-glycero-3-phosphoethanolamlne-N-(cap
blotinyi) sodium salt (Avanti Polar Lipids)
PEG4000 Polyethyleneglycol, MW = 4000 (Fluke)
Plunbnr 68 Ethylenemdde/propylenemdde block copolymer (Fluke)
CA 02547024 2011-12-22
51
C4Eta Perfluorobutane
Dknensions and concentration of microvesides are determined by using
Coulter counter Multisizer (aperture: 30pm).
4-potentials of miavvesides suspensions are determined by using a Malvern
ZetasIzar 3000Nsa In Naa 1mM.
Dimensions of micelle preparations are determined using a Malvern
/ft
Zetasizer 3000Hsa.
Example &
Preparation of positively charged microballoons
Trlpalmidn (60mg) is dissolved In cydohexane (0.6 ml) at 40 C. This
organic phase is kept at 40 C until emulsification. 40 mg of Ethyl-SPC3
(cationic
phospholipid) are dispersed in 30 mi of distilled water at 65 C for 15 min and
then the dispersion is allowed to cool to 40 C.
The organic phase is emulsified in the aqueous phase using a Polytrone
homogeniser PT3000 (10000 rpm, 1 min). The emulsion is then diluted with Smi
of PVA (200 mg, Mw: 9000 from Aldrich) In distilled water, then cooled to 5 C,
frozen at -45 C for 10 min and then lyophilized (0.2 mbar, 24 h).
The lyophilisate is redispersed In disdlled water (20 ml) In the presence of
air, mIcroballoons are washed twice by centrifugation (600 g for 10 min) with
phosphate buffered saline and the final suspension of microballoons (20 ml).
The size characterization for this preparationgave the following results: Dvso
as
2.54 pm; Dm = 1.57 pm.
11/MMR1.1111.
Preparation of fiqoresgent inariceP positively charged microbailoons
Example 1 is repeated by adding 5% by weight (with respect to the total
weight of tripaimItIn) of lipophMc fluorescent probe D1018 in the organic
phase
for fiuorescently maridng the midoballoon. The size characterization for this
preparation gave the following results: Dvso = 2.38 pm; DN = 1.45 pm.
ExamPles 3a-2.2
Preparation of DSTAP-contalningpositiyely chaiged microbubbies
15 mg of a mixture of DAPC and cationic lipid OSTAP (see relative ratio In
table 1) and 985 mg PEG4000 are dissolved In tert-butanol (10 ml) at 50 C. The
solu'don Ls sampled In 10 ml vlals (SO mg of dry matter per vial) then freeze
dried in a Christ Emi1io's:4i 2-12DS freeze dryer (-30 C, 0.56 mbar for 24h).
After
CA 02547024 2011-12-22
52
additional drying (25 C, 0.1. mbar for 5 hours), the vials are stoppered with
an
eiastomeric stopper and sealed with an aluminium 111p off.
The obtained lyophilisates are exposed to the desired gas (5050 viv of
Clio/N2) and then redispersed In 5 nil of the PBS buffer solution thus
obtaining
a suspension of positively charged microbubbles. Size characterization of
suspended microbubbles is reported in table 1.
Table 1: DSTAP-containing microbubbies
Example DAPC/DSTAP Dv DN
molar ratio ,
2a 99:1 8.57 3.11
2b 955 8.64 1.73
2c 90:10 8.80 1.77
2d 8020 9.22 1.82
2e 5050 8.51 1.94
EXIMEd131.287.2E
Preparation of negatively charged rnicrobobbles
The preparation of examples 2a-2e Is repeated by repladng the
DAPC/DSTAP mixture with the same total amount (15 rag) of a DPPG/DSPC
rnbcttrre in different relative amounts, as indlatted in Table 2. Size
characterization of suspended microbubbles is reported lu table 2.
Table 2: 01)PG-containing microbubbles
Example DEPC/DPPG Dv
:War *lc
3a 75:25 6.05 1.97 =
3b 50;50 12.97 1.97
3o2575 S.09 1.88 'µ
-
1114MIS11.4
a3133311ftti01 ilitabIlly4233Verintalrbibb*
DSTAP (200 mg) is dispersed In 100 ml of water containhig 5.4% (w/w) of a
mixture of propyien 0/yea and glycerol (3:10 w/w) at 80 C for 5 'shut= and
then cooled to room temperature.
The dispersion is transferred In a reactor under C4Fio atmosphere and
nornogenalsed at 20000 rpm (PolytroTAIPT3000) for 10 min, keeping ttie rotor
stator mixing shaft such that the openings are slightly above the surftias of
the
CA 02547024 2011-12-22
53
liquid. The obtained microbubbies are washed twice by centrifugation with
water, then redispersed In a dextran 7.5% solution.
The suspension is sampled In 10m1 vial (2m1 per vial). The vials are cooled
to -45 C and lyophilized for 24 hours, then stoppered, sealed and kept at room
temperature. Size diaracterlzation of microbubbles re-suspended In distilled
water was as follows: Dv= 4.04 ; D14= 1.75.
Example 5a-54
Preparation of negatively charged micelles
50 mg of Gd-D1PA-(SE)2 (containing traces of radioactive iS3Gd) and 10 mg
of NaDOC are dispersed In 5% aqueous glucose (1.0 ml) using a 3mm sonication
probe attached to a Branson 250 sonlfier (output: 30% for 10 min), to obtain
an
aqueous suspension of anionic micelles. The same preparation Is repeated by
dispersing different amounts of different compounds in the same volume of
aqueous glucose solution, as indicated In the following table 3.
Table 3: Negatively charged micelles
Example Gd-DTPA- NaDOC DPPE-Cap- DPPA Pluronic F68
(SE), (rng) (mg) Biotin (mg) img) (mg)
Sa 50 10
Sb 50 1.0 3.8
5c 16 16
5d 2,5 16 16
Example 6a-61
algetalkm of_PPPA-contaiging negatlyely chergej micelles
Various amounts of anionic phospholipld DPPA and neutral phospholipid
DPPC (as indicated in table 4) together with 16mg Pluronic F68 are dispersed
in
10 mi of PBS, using a 3mm sonicstion probe (Branson 250 soniffer, output 30%
for 10 min). A small amount of DPPC-H3(approximately 2.5 pCI for 10mi of final
suspension) is added to the micelle preparation as radioactive marker.
After sonlcation, the solutkin Is filtered through 0.2 pm filters (Millipore).
After cooling to room temperature, micelle size is measured using Malvern
Zetzsize73000HSA and spedfic radioactivity is determined using 50 pl of
solution dllubsd In 10mt of LSC coddltil Hlonic Fluert6.1(Padcard Blosdence)
and
counted In Tricarr) 2200A liquid sdntillatIon analyzer (Packard Biosderice).
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
54
Table 4: DPPA containing negative micelles
Example DPPC DPPA DPPA/DPPC Pluronic F68 1 Amount of
(mg) (mg) molar ratio (mg) 1 DPPA (% w/w)
6a 17.62 0 16.0
17.30 0.16 1:99 16.0 0.48
6c 17.26 0.32 2:98 16.0 0.95
6d 15.86 1.61 10:90 16.0 4.81
6e 8.80 I 8.00 50:50 16.0 24.39
6f 0.88 15.27 95:5 16.0 47.5
Example 7a-7b
Preparation of negatively charged micelles containing DSPE-PEG
20 mg of DSPE-PEG 2000 are dissolved in 1 ml of chloroform/ethanol (1/1, v/v)
at 60 C in a round bottom flask and the solvent mixture is evaporated under
vacuum, leaving a thin film on the inner wall of the flask. This film is
further
dried overnight in a vacuum chamber. The lipid film is then hydrated with 10m1
Hepes buffer at 60 C for 30 min. The solution is then filtered on 0.2 pm
filters
and allowed to cool to room temperature prior to characterization. The
filtered
solution is diluted in water (dilution ratio 1:3) and analyzed by Malvern
Zetasizer 3000HSA for size distribution. The results of two different
preparations
according to the above procedure are summarized in Table 5.
Table 5
Example Dv (nm) Dn (nm)
7a 15 9.9
7b 10.4 4.4
Example 8
Preparation of positively charged micelles containing Ethyl-SPC3
16 mg of Ethyl-SPC3 and 16 mg of Pluronic F68 are dispersed in 5%
aqueous glucose (10 ml) using a 3mm sonication probe attached to a Branson
250 sonifier (output: 30% for 10 min), to obtain an aqueous suspension of
cationic micelles.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
Examples 9a-9e
Preparation of DSTAP-containing positively charged micelles
The preparation of examples 6a-6f is repeated by replacing negatively
5 charged DPPA with positively charged DSTAP. Relative amounts of lipids
and
phospholipids of the different preparations are reported in table 6.
Table 6: DSTAP-containing positively charged micelles
Example DPPC DSTAP DPPC/DSTAP Pluronic
(mg) (mg) Molar ratio F68 (mg)
9a 17.62 0 16.0
9b 17.44 0.17 99:1 16.0
9c 17.26 0.34 98:2 16.0
9d 15.86 1.69 90:10 16.0
9e 8.81 8.43 50:50 16.0
10 Examples 10a-10b
Preparation of assemblies with cationic microballoons and anionic micelles
The microballoons suspension of example 1 (1 ml) is admixed with different
volume amounts (indicated in table 7) of the micelle preparation of Example 5a
or of Example 5b, respectively. After 1 hour, the suspension is washed twice
15 with PBS by centrifugation (600g for 5min) and redispersed in PBS (1.2
m1). The
amount of bound micelles (expressed as the percentage of the radioactivity
measured on the assembly suspension with respect to the radioactivity
measured on the initial micelle preparation) is determined by measuring the
Gd153 radioactivity (gamma count) of the suspension, by using a Cobra II
20 Autogarnma instrument (Packard Bioscience). The results are given in
table 7.
Table 7
pl micelle /ml DV50 DN % of bound
microballoons (gm_ _(pm), micelles
Example 1 - 2.38 l 1.45
-
2.5
_ _ 100
Example 10a
10 _2.83 1.70 90.9__
(assembly with non-
25 281 171
91.4
biotinated micelles of ex. 5a) . . --
100 f 2.37 1.581
31.1
____________________________________ 2.5 2.63 1.66 100
Example 10b
10_ 2.84 1.70 96.8
(assembly with biotinated
-
micelles from ex. 5b) 25 2.84 1.73
88.1
100 j 2.43 1.62
30.1
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
56
As inferable from the above table, while the relative amount of bound
micelles (i.e. the percentage of bound micelles with respect to the total
amount
of added micelles) decreases by increasing the total amount of micelles (i.e.
the
volume of micelle suspension) added to the microvesicle suspension, the
absolute amount of bound micelles (given by the product of the first and last
column in table 7) is nevertheless increasing.
Substantially similar results are obtained by preparing an assembly with the
microballoons of example 1 and the micelle preparations of examples 5c or 5d,
respectively.
Example 11
Determination of binding activity of the assemblies of example 10a-10b
To test the binding activity of the assemblies of examples 10a and 10b (10
pl and 100 pl of each micelle suspension preparations), a neutravidin coated
surface is prepared as follows:
Carbonate Buffer (pH 9.5 - 300p1) and NeutrAvidinTM (Pierce - 1 mg/ml - 50p1)
are added to each well of a twelve wells plate (NuncTm). After incubation
(overnight - 4 C), the well is washed twice with PBS containing Tween 20 0.1%
and twice with PBS. Bovine serum albumin (2% in PBS - 350p1) is added and
after incubation (25 C - 1h), the well is washed twice with PBS containing
Tween 20 0.1% and twice with PBS.
An amount of 2.108 assemblies prepared according to examples 10a and 10b
are added to each well, then the well is filled with PBS, sealed and the plate
is
turned. After inverse incubation (2h - 25 C), the well is washed twice with
PBS
and the surface is observed through an optical microscope with a 40x
magnification lens. Both the assemblies from example 10b, containing
biotinated micelles, show affinity for the neutravidin coated surface, the 100
pl/ml preparation providing a higher coverage of the surface with respect to
the
10 pl/ml preparation. Corresponding non-biotinated preparations of example
10a show instead no binding activity on the neutravidin coated surface.
Substantially similar results are obtained by comparing the binding activity
of assemblies comprising microballoons of example 1 and non-biotinated
micelles of example 5c with corresponding assemblies comprising microballoons
of example 1 and biotinated micelles of example 5d.
CA 02547024 2006-05-24
WO 2005/063305 PCT/1B2004/004230
57
Example 12a-12b
Preparation of assemblies with cationic microbubbles and anionic micelles
The microbubbles suspension of example 2d (1 ml) is admixed with different
volume amounts (indicated in the following table 8) of the micelle preparation
of
Examples 5a or 5b, respectively. Suspensions are gently stirred for 1 hour
then
washed twice by centrifugation (180g for 5min) with Tris glycerol buffer.
Infranatant is discarded and the residue is dispersed in Tris glycerol buffer
(1
ml). Size, concentration and -potential of the obtained assemblies are
reported
in table 8.
Table 8
- - ¨ - - - ¨
micelle/microbubble DV50 DN
susp. (pl/ml) (Pm) (pm)
potential
(mV)
- - -
Example 2d _ 1 4.74 1 1.82
57.1
_
10 6.92 1 2.75 37.6
Example 12a0
2.81 -19.0
(with non-biotinated _8.79
micelles from example 5a)7 100 .81
¨ , 2 29 -38.0
300 = 6.12 1 1.91 1 -50.4
10 6.43 2.53 1 30.1
Example 12b r-
30 7.31 2.37 -28.2
(with biotinated micelles
100 _________ 7.22 1.94 -42.4
from example 5b) -
300 6.11 1 1.87 -44.4
In both cases, increasing volumes of micelle suspension determine a
reduction of the -potential of the obtained respective assembly suspension.
Substantially similar results are obtained by using the microbubbles
suspensions of examples 2c or 2e, in place of the nnicrobubble suspension of
example 2d, or by replacing the micelle preparations of examples 5a and 5b
with those of examples Sc and 5d, respectively.
Example 13
Determination of binding activity of the assemblies of examples 12a-12b
To test the binding activity of the assembly of example 12a-12b, a
neutravidin coated surface is prepared as described in example 11 and tested
with different amounts (300, 100, 30 and 10 pl) of the preparations of
examples
12a and 12b.
A marked binding activity is observed at the optical microscope for the 100
pl/ml and 300 pl/ml preparations of example 12b. A lower binding is observed
for the 30 pl/ml preparation while the 10 pl/ml mixture shows poor binding.
All
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
58
the assemblies of example 12a (not containing biotinated micelles) show no
binding activity.
Example 14a-14b
Preparation of assemblies with cationic microbubbles and anionic micelles
The lyophilized content of a vial obtained according to example 4 is
exposed to C4F10 and redispersed in 2 ml of distilled water. The suspension is
washed twice by centrifugation (180g for 10min) with PBS and redispersed in
2m1 of PBS.
50 pl of a micelles preparation prepared according to example 5a or 5b,
respectively, are added, the mixture is stirred overnight with a rotating
stirrer
under C4F10 atmosphere, then washed twice with PBS by centrifugation (180g
for 10 min) and finally redispersed in 2m1 of PBS.
Table 9 provides the characterization of the assemblies of examples 14a and
14b.
Table 9
¨ ¨ - - - - ¨ - -
DV50 DN Conc.
Micelles
(pm) (pm)
(part./m1) Yield
ok
- - - - _
Example 14a Microbubbles 4.57
2.75 5.50E+08
Assemblies 4.81 1 2.72 1 4.04E+08 91.9
_ _
Example 14b Microbubbles 1_ 4..39 2.65 3.48E+08 1
Assemblies 4.45 2.44 3.45E+08
83.8
_ _ _ _
As inferable from the above table, the substantial totality of the micelles is
associated to microbubbles in the formed assemblies, said assemblies having
substantially the same mean diameter as the initial microbubbles.
Example 15
Preparation of assemblies with anionic microbubbles and cationic micelles
A microbubbles suspension prepared according to example 3b (1 ml) is
admixed with different volume amounts (indicated in the following table 10) of
the micelles preparation of Example 8. The suspension is gently stirred for 1
hour, then washed twice by centrifugation (180g for 5nnin) with Tris glycerol
buffer. Infranatant is discarded and the resulting assemblies are dispersed in
Tris glycerol Buffer (1 ml). Table 10 shows some characteristics of the
assemblies.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
59
Table 10
_ ¨ --
pl micelles suspension Dv DN -potential
per ml of microbubbles (pm) (pm) (mV)
--suspension
-
0 7.87 1.86 -60.2
11.43 4.98 ______ -44.9
100 9.16 2.48 +17.1
3009.20 2.12 +49.4
It can be observed that with an amount of micelles capable of determining a
reversal in sign of the initial (-potential of the microbubbles suspension,
the
mean dimensions of the assembly become closer to the dimensions of the initial
5 microbubbles.
Example 16
Determination of the amount of bound micelles as a function of the amount
of charged compounds in assembly preparations comprising cationic
10 microbubbles and anionic micelles
Different assembly suspensions are prepared by admixing 300 pl of a
micelle solution prepared according to examples 6a-6f to 1 ml of a microbubble
suspension in PBS prepared according to examples 2a-2e in a 5 ml glass tube,
for a total of 30 assembly preparations. The mixed suspensions are gently
stirred for 30 min and then washed twice by centrifugation (180g for 10mn) to
remove unbound material. The amount of the lipid molecules in micelles bound
to the microbubbles is evaluated by dosing radioactively labelled molecule
DPPC-3H incorporated within the micelles.
Figure 1 shows the results of the measurements, where lines A to E
represent the amounts of micelles bound to the microvesicles as a function of
the amount of charged compounds in said micelles, for assemblies comprising
the respective microvesicles prepared according to examples 2a to 2e.
From said figure, it can be noticed that substantially no bound micelles are
observed for assembly preparations including micelles of example 6a (no
charged surfactant). Furthermore, the amount of micelles bound to the
microvesicles increases with the increase of the amount of charged compounds
included in the nnicrovesicle. Finally, for this particular combination of
micelles/microvesicles assemblies, it can be observed that higher amounts of
micelles bind to the microvesicles when the relative amount of charged
compound in the micelle is from about 1% to 5% (w/w) of the total weight.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
Example 17
Determination of the amount of bound micelles as a function of the amount
of charged compounds in assembly preparations comprising anionic
5 microbubbles and cationic micelles
Different assembly suspensions are prepared by admixing 300 pl of each of
the micelle solutions prepared according to examples 9a-9e to 1 ml of each of
the microbubble suspensions prepared according to examples 3a-3c in a 5 ml
glass, respectively, for a total of 15 assembly preparations. The mixed
10 suspensions are gently stirred for 30 min and then washed twice by
centrifugation (180g for 10nnn) to remove unbound material. The amount of the
lipid molecules in micelles bound to the microbubbles is evaluated by dosing
radioactively labeled molecule DPPC-3H incorporated within the micelles.
Similar results are observed as for the assembly preparations of example
15 16, i.e. that by increasing the amount of charged compounds included in
the
microvesicle it is possible to increase the amount of micelles bound to the
microvesicles and that, in particular for assemblies where the microvesicles
contain a lower amount of charged compounds, a higher amount of micelles is
bound to the microvesicles when the relative amount of charged component in
20 the micelle is from about 1% to 5% (w/w).
Example 18
Determination of the amount of bound micelles as a function of the amount
of micelles added to microbubbles suspensions including different amounts of
charged compounds
25 Different amounts (50, 100, 250 and 500 pl) of micelle preparations
prepared according to example 7a or 7b are combined with 1 ml of the
nnicrobubble preparations prepared according to examples 2b, 2d and 2e for a
total of 12 assembly preparations (in particular 2b and 2d are combined with
7a,
while 2d is combined with 7b). The mixtures are gently stirred for 30 min, and
30 washed twice by centrifugation (180g/10min) in water to remove the
unbound
material.
The resulting suspensions are characterized by Coulter Counter for the
measurement of size distribution and by Malvern Zetasizer for potential. A
portion of the samples is freeze-dried at 0.2 mbar for 24 hours and the
35 lyophilisate analyzed by HPLC to determine the amount of DSPE-PEG in the
assemblies (pg PE-PEG/ml bubbles). The results are summarized in the following
table 11, illustrating the initial amount of DSPE-PEG included in the mixture
for
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
61
forming the assembly, the final amount of DSPE-PEG in the assembly
(corresponding to the amount of bound micelles), the ratio (expressed as
equivalent of charges) between positive and negative charges in the final
assembly and the respective potential of the final suspension.
Table 11: Cationic microbubbles and anionic micelles
Initial
mixture Final mixture
DSPE-PEG DSPE-PEGEC ratio potential
(nmoles) (nmoles) (mV)
Examples 35.87 1.35 0.18 35.8
2d and 7a 71.75 2.34 0.43 15.6
179.37 2.28 0.46 10.9
358.75 3.26 0.59 -13.3
Examples 35.87 3.30 0.14 26.2
2d and 7a 71.75 3.69 0.18 20.8
179.37 3.36 0.21 12.4
358.75 4.77 0.22 -10.0
Examples 35.87 3.81 0.07 39.3
2e and 7b 71.75 5.24 0.10 31.5
179.37 8.04 0.15 18.3
358.75 9.79 0.20 9.6
From the above table, it can be observed that, in general, the higher the
amount of charged compounds in the microvesicle, the higher the amount of
bound micelles in the final assembly. In addition, with respect to a same
microbubble preparation, the higher the amount of bound DSPE-PEG, the higher
the EC ratio and the lower the respective potential value.
Example 19
Assembly of cationic microvesicles with anionic micelles and comparative
mixture of anionic microvesicles and anionic micelles
20mg of DSPE-PEG 2000 are weighted and dissolved in
chloroform/Methanol (1/1, v/v) at 60 C in a round bottom flask and the solvent
mixture is evaporated under vacuum, deposing a thin film on the inner wall of
flask. This film is further dried overnight in a vacuum chamber.
The lipid film is hydrated with 10 ml 5% glucose at 60 C during 30 min, the
solution is filtered on 0.2pm filters and then cooled down to room temperature
prior to the characterization.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
62
The preparation is repeated twice.
Microbubbles are prepared as described in example 2e (positively charged)
and 3b (negatively charged), by using a 50/50 (w/w) mixture of DAPC and
DSTAP or a 50/50 (w/w) mixture of DSPC and DPPG. Vials are exposed to
C4F10/N2 50/50 (v/v) prior to reconstitution.
2.5 ml of micelles solution are diluted with 2.5 ml of 5% glucose. The
lyophilized microbubbles are reconstituted using the diluted solution of
micelles,
vortexed for 2 minutes then mixed gently for 30 minutes.
The obtained suspension is washed twice with glucose 5% (by
centrifugation, 180g/10 min) and the supernatant is redispersed in 2.5 ml of
5%
glucose. -potential of each suspension is measured by using a Malvern
Zetasizer 3000Hsa (50p1/10m1 NaCI 1mM). The amount DSPE-PEG2000 in each
suspension is determined using HPLC. Results are given in the following table
12.
Table 12: Mixture of anionic micelles with anionic or cationic microbubbles
Anionic micelle -potential DSPE-PEG2000
suspension with: (mV) (pg/nril)
Anionic -41.3 2.7 0.8
microbubbles
Cationic -18.3 2.5 129.0
microbubbles
From the above table, it can be observed that substantially no binding of
anionic micelles on anionic microbubbles is obtained, i.e. only negligible
amounts of DSPE-PEG are found in the final mixture, while the c-potential
remains substantially negative.
Example 20
Cationic microballoons - colloidal Gold Assembly
A suspension of cationic microballoons prepared according to example 1
is admixed with a colloidal suspension of gold particles stabilized with
sodium
citrate (Polysciences - 60 nm) in various ratios (expressed as number of gold
particles/number of microballoons, see table 13). After 2 hours, the .floating
particles are separated and redispersed in distilled water. Table 13 shows
that a
neutral value of c--potential is achieved at a ratio of about 200 gold
particles per
microballoon.
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
63
Table 13
Gold Cationic
colloid/Microballoons microballoons
Number ratio
0 +35.0
50 +23.8
100 +19.3
200 -0.7
800 -2.8
2000 -19.0
Example 21 - Cationic microbubbles-magnetites assembly
Magnetites coated with DPPA/Pluronic F108 (FE/DPPA/Pluronic F108 ratio
3/15/1.5 in mg/ml) were prepared according to US patent 5,545,395. The
solution was diluted 100 times with Tris(1g/1)/Glycerol(0.3M) buffer
(pH:7.05).
Cationic microbubbles (prepared according to example 2d, except that the
employed gas is SF6 instead of the C4F10/N2 mixture) are redispersed under SF6
atmosphere with 5m1 of magnetites solution. After 2min of vortexing, the
suspension is mixed gently for 1 hour. Then the floating particles are washed
twice with Tris/Glycerol buffer by centrifugation (180g/10min). Size and
concentration are measured by Coulter counter Multisizer. 4-potential is
determined with Malvern Zetasizer 3000Hsa (dilution: 50p1/10m1 water).
Magnetites binding was measured using relaxation time (T2) determination
(Bruecker: Minispec MQ20) and compared with a control carried out on a same
preparation of microbubbles without magnetite particles. The results are given
in table 14.
Table 14
Control suspension Suspension with
(without magnetite magnetite particles
particles)
4-potential (mV) +40.4 0.8 -30.8 4.1
T2 (ms) 1800 300 29.3 0.2
As shown in the above table, further to the reduction of the 4-potential with
respect to the control suspension a substantial reduction of the T2 is
observed,
confirming a substantial binding of magnetite-containing micelles to the
microbubbles.
CA 02547024 2011-12-22
64
Dcomole 22 - Effect of opposite charaed micelles on the s rface ot
charaed mtrobubblent In In vhro administratiork
Positively charged microbubbles are prepared as described in example 2d
using a 80/20 (w/w) mbdure of DAPC and DSTAP. Negatively charged
microbubbles are prepared as described in example 3b using a 50/50 (w/w)
mixture of DSPC and DPPG. Vials are exposed to C4Flo/N2 50/50 (v/v) prior to
reconstitution with Tris/glycerol buffer (5m1).
Micelles are prepared according to example 6f (negatively charged) and
example 8 (positively charged). Thereafter the following suspensions of
microbubbles or of assemblies are prepared:
Suspension A : 600p1 of Tris/Glyc.erol buffer are admixed with 2m1 of
mkrobubbles (example 2d - positively charged) and mixed gently for 30 min.
Suspension 13 : 600p1 of micelles according example 6g (negatively charged)
are admbced with 2m1 of microbubbles (example 2d - positively charged) and
mixed gently for 30 min.
Suspension C 600p1 of Iris/Glycerol buffer are admixed with 2m1 of
microbubbles (example 3b - negatively charged) and mixed gently for 30 min.
Suspension D : 600p1 of micelles according example 8 (positively charged)
are admixed with 2m1 of microbubbies (example 3b - negatively charged) and
mixed gently for 30 min.
A11 suspensions are washed twice with Tris/glycerol buffer (by centrifugation
180g/10 min) and the supernatants are redispersed in 2m1 of buffer. Sizes and
concentrations are determined using a Coulter counter. 4-potential of each
suspension is measured with a Malvern Zetasla; 3000Pisa (50p1/10m1 Nab
' 1mM) and are illustrated in the following table 15.
Table 15
4-potential
Suspension (mV)
A +48.6 9.3
-51.9*1.3
-61.5*7.2
+37.2 9.8
The suspensions were Injected In a rabbit ear vein at a dose of 5E+06
microbubbles per kg body weight. Two-dimensional echography was performed
in Coherent Contrast Imaging (ca) using an Acusonequola 512 equipped with
a 4C1-S transducer In intermittent imaging (two frames/s) and a high
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
mechanical index (MI). Images of the kidney were recorded on a video recorder
during 3 minutes and the sequence was analyzed to determine the mean pixel
intensity as a function of time in a region of interest (ROI) selected in the
cortex
(Figures 2 and 3).
5 As seen on the figures, the addition of micelles of opposite charge on
microbubbles changes dramatically the in vivo behaviour of microbubbles. Thus
positively charged bubbles are hardly detectable in the cortex of the kidney
(suspension A). However after incubation with negatively charged micelles, the
same microbubbles show a strong signal in the ROI (suspension B). Similarly
Exemple 23 ¨ Assembly of Cationic microbubbles with anionic micelles
2 ml of microbubble suspension (prepared according to example 2a
dispersed in PBS) are mixed with different amounts of Fungizone solutions
(micellar suspension of Amphotericin B with sodium deoxycholate in PBS -
Bristol Myers Squibb) as illustrated in the following table 16. Suspensions
are
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
66
Table 16 - Assemblies with drug
pl of micelles j -potential Amphotericin
suspension (mV)
per ml of (pg/ml)
microbubbles
suspension
Ex. 2a 49.1 3.0
= - - _____ - _________
+Fungizone 10 50.8 4.3 42.8
30 28.9 6.9 122.0
100 -24.7 0.3 367.9
Example 24: Assembly with double laver of micelles
Ex. 24a: Preparation of negatively charged bubbles
DPPC/DPPS-containing microbubbles are prepared using the method similar
to the one described in Example 3 of US patent no. 5,830,435. Briefly,
multilamellar liposomes (MLVs) are obtained by dispersing 59.2 mg of DPPC and
40.8 mg of DPPS in 100m1 of distilled water containing 1g of propylene glycol.
The liposomes are incubated at 70 C for 30 min under agitation. The mean
diameter of the liposomes is of about 1.4pm for DN and 2.7pm for Dv.
The liposome suspension is introduced in a gas tight glass reactor equipped
a high speed mechanical emulsifier (Megatron MT3000, Kinematica,
Switzerland). A gas bag containing C4F10 is connected to the mixing chamber of
the emulsifier. After homogenisation (1.0,000 rpm, 1 min), a milky suspension
of
microbubbles is obtained. The infranatant (about 90 ml containing mostly
liposomes) is removed by decantation. The supernatant (containing the
microbubbles) is recovered and resuspended in distilled water to a total
volume
of 100 ml. The decantation step is repeated and the final bubble suspension is
resuspended in 10% maltose. Aliquots of the suspension are collected in 10 ml
glass vials (1 ml of suspension per vial) and the samples are frozen at -45 C
and lyophilized.
After lyophilisation, the vials are closed with rubber stoppers, evacuated
and filled with a gas mixture containing a 1:1 (v/v) mixture of C4Fio and air.
Microbubbles are generated by injecting 2 ml distilled water intothe vials
through the stopper and hand shaking.
Ex. 246: Preparation of cationic and anionic micelles
Ex. 24b1
Cationic micelles are prepared with 3.73mg/m1 of DSPE-PTE020 (a multi-
arm PEG-phospholipid, NOF Corporation, Japan) and 1.27mg of cationic
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
67
phospholipid DPEPC (Dipalmitoyl Glycero-3-Ethylphosphocholine, Avanti Polar
Lipids, Inc. USA).
Ex. 2462
Anionic and functionalized micelles are prepared with 4.1 mg/ml of DSPE-
PEG2000 and 0.9 mg/ml of a GPIIbIIIa binding lipopeptide (DPPE-PEG2000-Lys-
Gln-Ala-Gly-Asp-Val, prepared according to example 3 of US 6,139,819).
Both positively and negatively charged micelles are prepared in 5% glucose
solution.
Ex. 24c: Preparation of assembly with neaatively charged bubbles and
multi-MACs layers having opposite electrically charges
50p1 and 500p1 of cationic micelles prepared according to Ex. 24b1 are
respectively added to two preparations containing about 1x109 negatively
charged microbubbles prepared according to Ex. 24a. The mixture is gently
stirred for 30 min and then washed twice by centrifugation (1071000 rpm), with
resuspensions in a solution of glucose 5%. Size and zeta potential of the
obtained assembly as determined are reported in table 17 below (rows
"Assembly 1"). The results show that after coating the negatively charged
microbubbles with a layer of cationic micelles, the measured zeta potential of
the assembly suspension becomes positive.
100 pl and 250 pl of anionic micelles suspension (prepared according to Ex.
24b2) are then respectively added to the assembly containing 50p1 of cationic
micelles and to the assembly containing 500p1 of cationic micelles. The two
mixtures are gently stirred for 30 min and washed twice by centrifugation
(107100Orpnn) with resuspensions in a solution of glucose 5%. The diameters
and zeta potential values of the obtained double layer assembly are given in
the
table 17 below (rows "Assembly 2"); the presence of the second layer of
negative micelles determines corresponding negative values of zeta potential.
Table 17
pl micelles Dv DN
added to 1x109 (pm) (pm) potential
microbubbles (mV)
Microbubbles 0 2.4 1.3 -63
Assembly 1 50 2.5 1.4 +5
Assembly 1 500 2.2 1.3 +8
Assembly 2 100 2.0 1.3 -30
Assembly 2 250 2.2 1.3 -38
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
Similar assemblies comprising a plurality of alternately charged layers can
also be manufactured with others types of MACs, such as liposomes and
nanoparticles. For instance, negatively charged microbubbles can be coated
with
cationic and drug containing liposomes and then with a second layer of anionic
micelles bearing targeting moieties.
Example 25: prenaration of assemblies from emulsion
50 ml of distilled water containing DAPC and DSTAP (80:20, 2mg/m1) are
heated at 70 C for 30 minutes then cooled at room temperature. 4 ml of
perfluorohexane is emulsified in this aqueous phase using a high speed
homogenizer (Polytron, 10,000 rpm, 1 minute). The resulting emulsion shows a
median diameter in volume (Dvso) of 5.0 pm and a mean diameter in number
(DN) of 2.7 pm as determined with a Malvern Mastersizer. The emulsion was
washed by centrifugation and re-suspended in water. Different amounts of
anionic micelles prepared according to example 24b2 are respectively added to
three aliquots of the above cationic emulsion, with respective concentrations
of
135 pl, 270 pl and 540p1 per ml of emulsion. After incubation (30 min at room
temperature under gentle stirring) and removal of the excess of micelles by
centrifugation, the micelle-coated emulsion was redispersed in a 20% (w/w)
aqueous PEG4000 solution. The emulsion-micelles assembly was distributed in
vials (2 ml/vial) then frozen and lyophilized in vials. Air in the vials of
lyophilisates was evacuated and replaced by C4F10. After reconstitution with 2
ml
of a 5% glucose solution, a milky microbubble-micelles assembly suspension is
obtained. were performed. Results of Coulter counter and zeta potential
analyses are gathered in table 18 below.
Table 18
Micelles/bubbles
(Pi/m0 Bubbles/ml DN (pm) Dv (pm) DV50 (pm) Z-pot.
5.88E+07 1.35 4.65 4.22 46.9
135 8.27E+08 1.23 3.70 2.35 20.3
270 8.61E+08 1.39 4.12 3.35 -4.3
540 1.04E+09 1.31 3.85 3.34 -6.3
Preparations with increasing amounts of anionic micelles, result in
increasing amounts of microbubble. Furthermore, surface charge properties can
CA 02547024 2006-05-24
WO 2005/063305
PCT/1B2004/004230
69
be also modulated (zeta potential values varying from positive to negative) as
desired.
Example 26: preparation of assemblies from gas emulsion
Negatively charged microbubbles are obtained according to Example 24a using
C4F10 as gas phase and DPPS as phospholipid (2 mg/ml) to stabilized
microbubbles. After bubble generation by high speed mechanical emulsification
(Megatron MT3000, Kinematica, Switzerland) of the DPPS liposome
suspension, the microbubbles are washed by diafiltration for 30 minutes using
a
1 pm polycarbonate membrane (Nuclepore ), to remove the excess of
phosphospholipids in the bubble suspension. Cationic micelles containing DPEPC
and DSPE-PEG2000 conjugated to rat anti-mouse monoclonal IgG1 against P-
selectin (70:30 in molar ratio, 5 mg/m1) are added to the bubble suspension
(50
pl of micelles for 1 ml of microbubbles about 5 x 109 bubbles/ml ). The
mixture
is gently stirred for 30 min at room temperature and centrifuged. The
assemblies (supernatant) are resuspended in a 10% maltose solution, frozen
and lyophilised (2rri1/vial). After freeze-drying, lyophilisates are gassed
with
C4F10 and reconstituted with 2 ml distilled water. The Coulter analysis showed
that more than 90% of bubble-micelles assemblies were still intact after
lyophilisation. These microbubbles showed a DN of 1.3 pm and Dv of 2.9 pm.
Flow cytometry measurements confirmed the presence of the biochemically
active IgG1 antibody at the surface of the assemblies.