Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-1-
85846pct
Glucopyranosyloxy-substituted aromatic compounds, medicaments containing
such compounds, their use and process for their manufacture
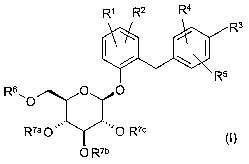
The present invention relates to glucopyranosyloxy-substituted aromatic groups
of
general formula I
R~ R2 Ra
R3
/ \
R5
R6~0 O O
Rya O ~~,, I~~~ O Roc
O Rib
(I),
wherein the groups R' to R6 and R'a, R'b and R'° are as defined
hereinafter, including
the tautomers, the stereoisomers, the mixtures thereof and the salts thereof.
The
invention also relates to pharmaceutical compositions containing a compound of
formula I according to the invention as well as the use of a compound
according to
the invention for preparing a pharmaceutical composition for the treatment of
metabolic disorders. Processes for preparing a pharmaceutical composition and
a
compound according to the invention are also the subject of this invention.
In the literature, compounds which have an inhibitory effect on the sodium-
dependent
glucose cotransporter SGLT2 are proposed for the treatment of diseases,
particularly
2o diabetes.
Glucopyranosyloxy- substituted aromatic groups and the preparation thereof and
their
possible activity as SGLT2 inhibitors are known from published International
applications WO 01/68660, WO 01/74834, WO 02/28872, WO 02/44192, WO
25 02164606, WO 03/11880 as well as WO 03/80635.
Aim of the invention
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-2-
The aim of the present invention is to find new glucopyranosyloxy-substituted
aromatic groups, particularly those which are active with regard to the sodium-
dependent glucose cotransporter SGLT, particularly SGLT2. A further aim of the
present invention is to discover glucopyranosyloxy-substituted aromatic groups
which
have an enhanced inhibitory effect on the sodium-dependent glucose
cotransporter
SGLT2 in vitro and/or in vivo compared with known, structurally similar
compounds
and/or have better pharmacological or pharmacokinetic properties.
A further aim of the present invention is to provide new pharmaceutical
compositions
1o which are suitable for the prevention and/or treatment of metabolic
disorders,
particularly diabetes.
The invention also sets out to provide a process for preparing the compounds
according to the invention.
Other aims of the present invention will become apparent to the skilled man
directly
from the foregoing and following remarks.
Object of the invention
2o In a first aspect the present invention relates to
glucopyranosyloxy-substituted aromatic groups of general formula I
R' R2 Ra
R3
i w
R5
R6~0 O O
Rya O ~~'~ I~~~ O Roc
O Rib (I),
wherein
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-3-
R' denotes C2_s-alkynyl, tetrahydrofuran-3-yloxy, tetrahydropyran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuranyl-C~_3-alkyloxy or tetrahydropyranyl-
C1_3-alkyloxy, or,
if R3
is selected from the group consisting of C2_s-alkynyl, tetrahydrofuran-3-
yloxy,
tetrahydropyran-3-yloxy, tetrahydropyran-4-yloxy, tetrahydrofuranyl-
C~_3-alkyloxy and tetrahydropyranyl-C~_3-alkyloxy,
then R' may additionally also represent hydrogen, fluorine, chlorine, bromine,
iodine, C»-alkyl, a methyl group substituted by 1 to 3 fluorine atoms, an
ethyl
group substituted by 1 to 5 fluorine atoms, C»-alkoxy, a methoxy group
substituted by 1 to 3 fluorine atoms, an ethoxy group substituted by 1 to 5
fluorine atoms, a C~~-alkyl group substituted by a hydroxy or C~_3-alkoxy
group, a CZ~-alkoxy group substituted by a hydroxy or C~_3-alkoxy group, C2~-
alkenyl, C3~-cycloalkyl, C3~-cycloalkyl-C~_3-alkyl, C3_s-cycloalkoxy, C3_s-
cycloalkyl-C~_3-alkoxy, hydroxy, amino or cyano, and
R2 denotes hydrogen, fluorine, chlorine, methyl, methyl or methoxy substituted
2o by 1 to 3 fluorine atoms, and
R3 denotes C2~-alkynyl, tetrahydrofuran-3-yloxy, tetrahydropyran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuranyl-C~_3-alkyloxy or tetrahydropyranyl-
C~_3-alkyloxy, or,
if R' is selected from the group consisting of
C2_s-alkynyl, tetrahydrofuran-3-yloxy, tetrahydropyran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuranyl-C~_3-alkyloxy and
tetrahydropyranyl-C~_3-alkyloxy,
then R3 may additionally also represent hydrogen, fluorine, chlorine, bromine,
iodine, C~_s-alkyl, CZ_s-alkenyl, C3_s-cycloalkyl, C3~-cycloalkylidenemethyl,
C~_s-alkoxy, C3_s-cycloalkyl-oxy, C3_s-cycloalkyl-C~_3-alkoxy, aryl, aryl-C~_3-
alkyl, heteroaryl, heteroaryl-C~_3-alkyl, aryloxy, aryl-C~_3-alkyl-oxy, a
methyl or
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-4-
methoxy group substituted by 1 to 3 fluorine atoms, a C2~-alkyl or C2_4-alkoxy
group substituted by 1 to 5 fluorine atoms, a C~~-alkyl group substituted by a
cyano group, a C»-alkyl group substituted by a hydroxy or C~_3-alkyloxy
group, cyano, carboxy, C~_3-alkoxycarbonyl, aminocarbonyl, (C1-s-
alkylamino)carbonyl, di-(C~_3-alkyl)aminocarbonyl, pyrrolidin-1-ylcarbonyl,
piperidin-1-ylcarbonyl, morpholin-4-ylcarbonyl, piperazin-1-yl-carbonyl,
4-(C~_3-alkyl)-piperazin-1-ylcarbonyl, nitro, amino, C~_3-alkylamino, di-(C~_3-
alkyl)amino, (C~~-alkyl)carbonylamino, C»-alkylsulphonylamino,
arylsulphonylamino, aryl-C~_3-alkylsulphonylamino, C~.~-alkylsulphanyl,
C~~-alkylsulphinyl, C»-alkylsulphonyl, arylsulphenyl, arylsulphinyl or
arylsulphonyl,
R4 and R5, which may be identical or different, represent hydrogen, fluorine,
chlorine,
bromine, C~_3-alkyl, C~_3-alkoxy, methyl or methoxy substituted by 1 to 3
~5 fluorine atoms, and
Rs ~ Rya
Rib, R'° independently of one another have a meaning selected from
among
hydrogen, (C~_~$-alkyl)carbonyl, (C~_~$-alkyl)oxycarbonyl, arylcarbonyl and
2o aryl-(C~_3-alkyl)-carbonyl,
while the aryl groups mentioned in the definition of the above groups are
meant to
indicate phenyl or naphthyl groups which may be mono- or disubstituted
25 independently of one another by Rh, while the substituents may be identical
or
different and R,, denotes a fluorine, chlorine, bromine, iodine, C~_3-alkyl,
difluoromethyl, trifluoromethyl, C~_3-alkoxy, difluoromethoxy,
trifluoromethoxy or
cyano,
so the heteroaryl groups mentioned in the definition of the above-mentioned
groups are
meant to indicate a pyrrolyl, furanyl, thienyl, imidazolyl, pyridyl, indolyl,
benzofuranyl,
benzothiophenyl, quinolinyl or isoquinolinyl group,
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-5-
or a pyrrolyl, furanyl, thienyl, imidazolyl or pyridyl group, wherein one or
two methyne
groups are replaced by nitrogen atoms,
or an indolyl, benzofuranyl, benzothiophenyl, quinolinyl or isoquinolinyl
group, wherein
one to three methyne groups are replaced by nitrogen atoms,
while the above-mentioned heteroaryl groups may be mono- or disubstituted by
Rh, while the substituents may be identical or different and Rh is as
hereinbefore defined,
while, unless otherwise stated, the above-mentioned alkyl groups may be
straight-
chain or branched,
the tautomers, the stereoisomers, the mixtures thereof and the salts thereof.
The compounds of general formula I according to the invention and the
physiologically acceptable salts thereof have valuable pharmacological
properties,
particularly an inhibitory effect on the sodium-dependent glucose
cotransporter SGLT,
particularly SGLT2. Moreover compounds according to the invention may have an
2o inhibitory effect on the sodium-dependent glucose cotransporter SGLT1.
Compared
with a possible inhibitory effect on SGLT1 the compounds according to the
invention
preferably inhibit SGLT2 selectively.
The present invention also relates to the physiologically acceptable salts of
the
compounds according to the invention with inorganic or organic acids.
Therefore, the invention also relates to the use of the compounds according to
the
invention, including the physiologically acceptable salts, as pharmaceutical
compositions.
3o This invention also relates to pharmaceutical compositions, containing at
least one
compound according to the invention or a physiologically acceptable salt
according to
the invention, optionally together with one or more inert carriers and/or
diluents.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-6-
A further subject of this invention is the use of at least one compound
according to the
invention or a physiologically acceptable salt of such a compound for
preparing a
pharmaceutical composition which is suitable for the treatment or prevention
of
diseases or conditions which can be influenced by inhibiting the sodium-
dependent
glucose cotransporter SGLT, particularly SGLT2.
This invention also relates to the use of at least one compound according to
the
invention or one of the physiologically acceptable salts thereof for preparing
a
pharmaceutical composition which is suitable for the treatment of metabolic
disorders.
This invention also relates to the use of at least one compound according to
the
invention or one of the physiologically acceptable salts thereof for preparing
a
pharmaceutical composition for inhibiting the sodium-dependent glucose
cotransporter SGLT, particularly SGLT2.
The invention further relates to a process for preparing a pharmaceutical
composition
according to the invention, characterised in that a compound according to the
invention is incorporated in one or more inert carriers and/or diluents by a
non-
chemical method.
The present invention also relates to a process for preparing the compounds of
general formula I according to the invention, characterised in that
a) in order to prepare compounds of general formula I wherein R6, R'a, R'b and
R'° are as hereinbefore defined, but do not represent hydrogen,
a compound of general formula
R6~ O Z~
O
Rya O ~~'~ I~~~ O Roc
O Rib
(II),
3o wherein
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-7-
R6 and R'a, R'b, R'' are as hereinbefore defined, but do not represent
hydrogen, and
Z' denotes a leaving group, is reacted with a compound of general formula
Ri R2 Ra
R3
/ \
R5
OH
(III),
wherein
R' to R5 have the meanings given hereinbefore, or
1o b) in order to prepare compounds of general formula I wherein R6, R'a, R'b
and
R'' represent hydrogen,
a compound of general formula I wherein R6 and R'a, R'b, R'° are as
hereinbefore
defined, but do not represent hydrogen, is hydrolysed, and
after step b) has been carried out, if desired a compound of general formula I
thus
obtained wherein Rs denotes a hydrogen atom is converted by acylation into a
corresponding acyl compound of general formula I, and/or
2o if necessary a protecting group used during the reactions described above
is cleaved
again and/or
if desired a compound of general formula I thus obtained is separated into its
stereoisomers and/or
a compound of general formula I thus obtained is converted into the salts
thereof,
particularly for pharmaceutical use into the physiologically acceptable salts
thereof.
Detailed description of the invention
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
_g_
Unless otherwise stated the groups, residues and substituents, particularly R'
to R6
and R'a, R'b, R'°, are defined as above and hereinafter.
If residues, substituents or groups occur several times in a compound, they
may
have the same or different meanings.
The term aryl used above and hereinafter, for example in the groups R3, R6,
R'a , R'b,
R'° and R'd preferably denotes phenyl. According to the general
definition and unless
otherwise stated, the aryl group, particularly the phenyl group, may be mono-
or
disubstituted by identical or different groups Rh.
The term heteroaryl used above and hereinafter, for example in the groups R3
preferably denotes pyridinyl, pyrimidinyl, pyridazinyl, pyrazinyl, triazinyl,
imidazolyl,
pyrazolyl, triazolyl, tetrazolyl, oxazolyl, oxadiazolyl, thiazolyl or
thiadiazolyl. According
to the general definition and unless otherwise stated, the heteroaryl group
may be
mono- or disubstituted by identical or different groups Rh.
Compounds according to the invention, in a first embodiment of this invention,
may be
described by general formula I, wherein
2o R' denotes CZ_6-alkynyl, tetrahydrofuran-3-yloxy, tetrahydropyran-3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuranyl-C~_3-alkyloxy or tetrahydropyranyl-
C~_3-alkyloxy and
the other groups R2 to R6 as well as R'a, R'b, R'° are as hereinbefore
defined,
including the tautomers, the stereoisomers, the mixtures thereof and the salts
thereof.
Preferred meanings of the group R~ according to this embodiment are ethynyl, 2-
propyn-1-yl, 2-butyn-1-yl, tetrahydrofuran-3-yloxy, tetrahydropyran-3-yloxy,
3o tetrahydropyran-4-yloxy, tetrahydrofuranylmethyloxy and
tetrahydropyranylmethyloxy.
Most particularly preferred meanings are ethynyl, tetrahydrofuran-3-yloxy and
tetrahydropyran-4-yloxy, particularly ethynyl.
Preferred meanings of the group R3 according to this embodiment are
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
_g_
hydrogen, fluorine, chlorine, methyl, ethyl, isopropyl, tert.-butyl, 2-cyano-2-
propyl,
difluoromethyl, trifluoromethyl, cyclopropyl, cyclobutyl, cyclopentyl,
methoxy, ethoxy,
isopropoxy, difluoromethoxy, trifluoromethoxy, 1,1,2,2-tetrafluoroethoxy,
cylopropyloxy, cyclobutyloxy, cyclopentyloxy, methylsulphanyl, 2-methyl-1-
propen-1-
y1, cyclopropylidenemethyl, ethynyl, tetrahydrofuran-3-yloxy, tetrahydropyran-
3-yloxy,
tetrahydropyran-4-yloxy, tetrahydrofuranylmethyloxy,
tetrahydropyranylmethyloxy,
phenyl, fluorophenyl, pyridinyl, pyrimidinyl, pyridazinyl, pyrazinyl,
imidazolyl, pyrazolyl,
triazolyl, tetrazolyl, oxazolyl, oxadiazolyl, thiazolyl or thiadiazolyl.
Particularly preferred
meanings are ethynyl, tetrahydrofuran-3-yloxy, methyl, ethyl, methoxy, ethoxy,
1o difluoromethoxy, trifluoromethoxy, particularly ethynyl, tetrahydrofuran-3-
yloxy and
methoxy.
Preferred meanings of the group R4 according to this embodiment are hydrogen
and
fluorine, particularly hydrogen.
Compounds according to the invention in a second embodiment of this invention
may
be described by general formula I, wherein
R' denotes hydrogen, fluorine, chlorine, bromine, iodine, C»-alkyl, methyl
2o substituted by 1 to 3 fluorine atoms, ethyl substituted by 1 to 5 fluorine
atoms,
C~~-alkoxy, methoxy substituted by 1 to 3 fluorine atoms, ethoxy substituted
by 1 to 5 fluorine atoms, C~~-alkyl substituted by a hydroxy or C~_3-alkoxy
group, C2~-alkoxy substituted by a hydroxy or C~_3-alkoxy group, Cz~-alkenyl,
C3_6-cycloalkyl, C3_s-cycloalkyl-C~_3-alkyl, C3_6-cycloalkoxy, C3_6-cycloalkyl-
C~_3-
alkoxy, hydroxy, amino or cyano, and
may also represent C2~-alkynyl, tetrahydrofuran-3-yloxy, tetrahydropyran-3-
yloxy, tetrahydropyran-4-yloxy, tetrahydrofuranyl-C~_3-alkyloxy or
tetrahydropyranyl-C~_3-alkyloxy, and
R3 is selected from a group consisting of C2_6-alkynyl, tetrahydrofuran-3-
yloxy,
tetrahydropyran-3-yloxy, tetrahydropyran-4-yloxy, tetrahydrofuranyl-
C~_3-alkyloxy and tetrahydropyranyl-C~_3-alkyloxy, and
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-10-
the other groups, particularly R2 and R4 to Rs as well as R'a, R'b, R'°
have the
meanings given hereinbefore,
including the tautomers, the stereoisomers, the mixtures thereof and the salts
thereof.
According to this embodiment preferred meanings of the group R' are hydrogen,
fluorine, chlorine, methyl, difluoromethyl, trifluoromethyl, methoxy,
difluoromethoxy,
trifluoromethoxy or cyano, particularly preferably hydrogen, fluorine, methyl
or cyano,
most particularly preferably hydrogen.
According to this embodiment preferred meanings of the group R3 are ethynyl
and
tetrahydrofuran-3-yloxy.
According to this second embodiment preferred meanings of the group R4 are
hydrogen and fluorine, particularly hydrogen.
The following remarks refer to the compounds of formula I, particularly the
first and
second embodiments mentioned above.
2o Preferred compounds according to the present invention, particularly
according to the
first and second embodiments, can be represented by the following formulae
(la), (1b),
(lc) and (Id), particularly (la), (1b) and (lc):
R2 Ra
R3
la
Rs
~O
R~
O Rib
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-11-
R' Ra
R3
Rz \
/ \ Ib
R5
R6~0 O O
Rya O ''~~ I~~~ O Roc
O R'b
Rz Ra
\ R~ R3
/ \
Rs Ic
R6~0 O O
'.,
R7a~''~~ '~ R7c
O R'b
Rz Ra
R3
\ ~ Id
R' / \
R5
R6~0 O O
Rya O ''~~ ~~~~ O Roc
O R'b
According to an alternative to the embodiments described above, other
preferred
compounds are those wherein the phenyl group which carries the substituents R3
has
at least one other substituent Ra and/or R5 which is not hydrogen. According
to this
alternative, particularly preferred compounds are those which have a
substituent Ra
which is fluorine.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-12-
The phenyl group which carries the substituent R3 is preferably at most
monofluorinated.
Preferred meanings of the group R5 are hydrogen and fluorine, particularly
hydrogen.
Preferred meanings of the group R2 according to the invention are hydrogen,
fluorine
and methyl, particularly hydrogen and methyl.
The group R6 according to the invention preferably denotes hydrogen, (C~_$-
alkyl)oxycarbonyl or C~_$-alkylcarbonyl, particularly hydrogen or (C~~-
alkyl)oxycarbonyl, particularly preferably hydrogen, methoxycarbonyl or
ethoxycarbonyl, most particularly preferably hydrogen or methoxycarbonyl.
The substituents R'a, R'b, R'° independently of one another preferably
represent
hydrogen, (C1_$-alkyl)oxycarbonyl, (C~_~$-alkyl)carbonyl, benzoyl,
particularly hydrogen
or (C~_6-alkyl)oxycarbonyl, (C~_$-alkyl)carbonyl, particularly preferably
hydrogen,
methoxycarbonyl, ethoxycarbonyl, methylcarbonyl or ethylcarbonyl. Most
particularly
preferably R'a, R'b and R'° represent hydrogen.
2o The compounds of formula I wherein R6, R'a, R'b and R'' have a meaning
according
to the invention other than hydrogen, for example C~_8-alkylcarbonyl, are
preferably
suitable as intermediate products in the synthesis of compounds of formula I
wherein
R'a, R'b and R'° represent hydrogen.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-13-
Particularly preferred compounds of general formula I are selected from among
(a) 1-(f3-D-glucopyranosyloxy)-2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-
benzene,
(b) 1-((3-D-glucopyranosyloxy)-2-(4-ethynylbenzyl)-benzene,
and the derivatives thereof, wherein R6 has a meaning according to the
invention
other than hydrogen, and in particular R6 denotes ethoxycarbonyl or
methoxycarbonyl,
including the stereoisomers and the mixtures thereof.
Some terms used above and hereinafter to describe the compounds according to
the
invention will now be defined more closely.
~5 The term halogen denotes an atom selected from the group consisting of F,
CI, Br
and I, particularly F, CI and Br.
The term C~_~-alkyl, wherein n may have a value of 1 to 18, denotes a
saturated,
branched or unbranched hydrocarbon group with 1 to n C atoms. Examples of such
2o groups include methyl, ethyl, n-propyl, iso-propyl, butyl, iso-butyl, sec-
butyl, tert-butyl,
n-pentyl, iso-pentyl, neo-pentyl, tert-pentyl, n-hexyl, iso-hexyl, etc.
The term C2_n-alkynyl, wherein n has a value of 3 to 6, denotes a branched or
unbranched hydrocarbon group with 2 to n C atoms and a C---C double bond.
25 Examples of such groups include ethynyl, 1-propynyl, 2-propynyl, iso-
propynyl, 1-
butynyl, 2-butynyl, 3-butynyl, 2-methyl-1-propynyl, 1-pentynyl, 2-pentynyl, 3-
pentynyl,
4-pentynyl, 3-methyl-2-butynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-
hexynyl
etc.
so The term C~_~-alkoxy denotes a C~_~-alkyl-O group, wherein C~_~-alkyl is as
hereinbefore defined. Examples of such groups include methoxy, ethoxy, n-
propoxy,
iso-propoxy, n-butoxy, iso-butoxy, sec-butoxy, tert-butoxy, n-pentoxy, iso-
pentoxy,
neo-pentoxy, tert-pentoxy, n-hexoxy, iso-hexoxy etc.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-14-
The term C~_~-alkylcarbonyl denotes a C~_"-alkyl-C(=O) group, wherein C~_~
alkyl is as
hereinbefore defined. Examples of such groups include methylcarbonyl,
ethylcarbonyl, n-propylcarbonyl, iso-propylcarbonyl, n-butylcarbonyl, iso-
butylcarbonyl, sec-butylcarbonyl, tert-butylcarbonyl, n-pentylcarbonyl, iso-
s pentylcarbonyl, neo-pentylcarbonyl, tert-pentylcarbonyl, n-hexylcarbonyl,
iso-
hexylcarbonyl, etc.
The term C3_~-cycloalkyl denotes a saturated mono-, bi-, tri- or
spirocarbocyclic group
with 3 to n C atoms. Examples of such groups include cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, cyclododecyl,
bicyclo[3.2.1.]octyl, spiro[4.5]decyl, norpinyl, norbonyl, norcaryl,
adamantyl, etc.
Preferably the term C3_~-cycloalkyl denotes saturated monocyclic groups.
The term C3_~-cycloalkylcarbonyl denotes a C3_~-cycloalkyl-C(=O) group wherein
~5 C3_~-cycloalkyl is as hereinbefore defined.
The style used above and hereinafter, in which a bond of a substituent in a
phenyl
group is shown towards the centre of the phenyl ring, denotes, unless
otherwise
stated, that this substituent may be bound to any free position of the phenyl
ring
2o bearing an H atom.
The compounds according to the invention may be obtained using methods of
synthesis known in principle. Preferably the compounds are obtained by the
following methods according to the invention which are described in more
detail
25 hereinafter.
a) In order to prepare compounds of general formula I wherein R6, R'a, R'b,
R'°
are as hereinbefore defined, but do not denote a hydrogen atom:
3o reacting a compound of general formula
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-15-
R6~ O Z~
O
Rya O ~~,, I~~~ O Roc
O Rib
(1l),
wherein
R6 and R'a, R'b, R'c are as hereinbefore defined, but do not represent
hydrogen, and
Z' denotes a leaving group such as for example a halogen atom, e.g. a
fluorine,
chlorine or bromine atom, or an acyloxy group, e.g. an acetyloxy or
trichloroacetimidoyloxy group, with a compound of general formula
R~ R2 Ra
R3
/ \
R5
OH (III),
wherein
R' to R5 have the meanings specified.
The reaction is conveniently carried out in a solvent, such as for example
methylene
chloride, chloroform, acetonitrile, toluene, tetrahydrofuran, dioxane,
dimethylformamide, dimethylsulphoxide or N-methylpyrrolidinone, optionally in
the
presence of a base, such as for example potassium carbonate, caesium
carbonate,
sodium hydride or potassium-tert.-butoxide, or a silver compound such as
silver (I)
oxide, silver (I) carbonate or silver (I) trifluoroacetate or a catalyst such
as for example
boron trifluoride etherate at temperatures between -60°C and
120°C. The reaction
may also be carried out for example in a phase transfer system such as sodium
hydroxide solution/methylene chloride/benzyl-triethylammonium bromide, while
other
protective groups such as the trimethylsilyl group on an ethynyl group may be
cleaved
at the same time.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-16-
b) In order to prepare compounds of general formula I wherein R6, R'a, R'b and
R'° denote hydrogen:
reacting a compound of general formula I wherein
R6, R'a, R'b and R'° are as hereinbefore defined, but do not represent
hydrogen, with
water or a lower alcohol such as methanol or ethanol.
The reaction is conveniently carried out in water, a lower alcohol such as
methanol or
ethanol or an aqueous solvent mixture such as methanol/tetrahydrofuran, in the
presence of a base, such as for example lithium hydroxide, sodium hydroxide,
potassium carbonate or sodium methoxide at temperatures between -20°C
and 60°C.
In this reaction other protective groups such as the trimethylsilyl group on
an ethynyl
group may be cleaved at the same time.
If according to the invention a compound of general formula I is obtained
wherein R6
denotes a hydrogen atom, this may be converted by acylation, for example by
acylation in the presence of a base such as pyridine, collidine, triethylamine
or N-
ethyl-diisopropylamine, into a compound wherein R6 denotes a (C~_~8-
alkyl)carbonyl
group, a (C~_~8-alkyl)oxycarbonyl group, an arylcarbonyl group or an aryl-
(C~_3-alkyl)-
2o carbonyl group. Suitable acylating agents may be, in particular, the
corresponding
activated acyl derivatives such as acid chlorides or anhydrides.
In the reactions described hereinbefore, any reactive groups present such as
ethynyl,
hydroxy, amino, alkylamino or imino groups may be protected during the
reaction by
conventional protecting groups which are cleaved again after the reaction.
For example, a protecting group for an ethynyl group may be the trimethylsilyl
group.
For example, a protecting group for a hydroxy group may be a trimethylsilyl,
acetyl,
3o trityl, benzyl or tetrahydropyranyl group.
Protecting groups for an amino, alkylamino or imino group may be, for example,
a
formyl, acetyl, trifluoroacetyl, ethoxycarbonyl, tert.butoxycarbonyl,
benzyloxycarbonyl,
benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-17-
Any protecting group used is optionally subsequently cleaved for example by
hydrolysis in an aqueous solvent, e.g. in water, isopropanol/water, acetic
acid/water,
tetrahydrofuran/water or dioxane/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an alkali
metal base such as lithium hydroxide, sodium hydroxide or potassium hydroxide
or
aprotically, e.g. in the presence of iodotrimethylsilane, at temperatures
between 0 and
120°C, preferably at temperatures between 10 and 100°C.
A trimethylsilyl group is cleaved for example in water, an aqueous solvent
mixture or a
lower alcohol such as methanol or ethanol in the presence of a base such as
lithium
hydroxide, sodium hydroxide, potassium carbonate or sodium methoxide.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is advantageously
~5 cleaved, for example, hydrogenolytically, e.g. with hydrogen in the
presence of a
catalyst such as palladium/charcoal in a suitable solvent such as methanol,
ethanol,
ethyl acetate or glacial acetic acid, optionally with the addition of an acid
such as
hydrochloric acid at temperatures between 0 and 100°C, but preferably
at ambient
temperatures between 20 and 60°C, and at a hydrogen pressure of 1 to 7
bar, but
2o preferably 3 to 5 bar. A 2,4-dimethoxybenzyl group, however, is preferably
cleaved in
trifluoroacetic acid in the presence of anisole.
A tert.butyl or tert.butyloxycarbonyl group is preferably cleaved by treating
with an
acid such as trifluoroacetic acid or hydrochloric acid or by treating with
25 iodotrimethylsilane optionally using a solvent such as methylene chloride,
dioxane,
methanol or diethylether.
A trifluoroacetyl group is preferably cleaved by treating with an acid such as
hydrochloric acid, optionally in the presence of a solvent such as acetic acid
at
3o temperatures between 50 and 120°C or by treating with sodium
hydroxide solution
optionally in the presence of a solvent such as tetrahydrofuran or methanol at
temperatures between 0 and 50°C.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-18-
Moreover, the compounds of general formula I obtained may be resolved into
their
enantiomers and/or diastereomers, as mentioned hereinbefore. Thus, for
example,
cisltrans mixtures may be resolved into their cis and traps isomers, and
compounds
with at least one optically active carbon atom may be separated into their
enantiomers.
Thus, for example, the cisltrans mixtures may be resolved by chromatography
into the
cis and traps isomers thereof, the compounds of general formula I obtained
which
occur as racemates may be separated by methods known per se (cf. Allinger N.
L.
and Eliel E. L. in "Topics in Stereochemistry", Vol. 6, Wiley Interscience,
1971) into
their optical antipodes and compounds of general formula I with at least 2
asymmetric
carbon atoms may be resolved into their diastereomers on the basis of their
physical-chemical differences using methods known per se, e.g. by
chromatography
and/or fractional crystallisation, and, if these compounds are obtained in
racemic
15 form, they may subsequently be resolved into the enantiomers as mentioned
above.
The enantiomers are preferably separated by column separation on chiral phases
or
by recrystallisation from an optically active solvent or by reacting with an
optically
active substance which forms salts or derivatives such as e.g. esters or
amides with
2o the racemic compound, particularly acids and the activated derivatives or
alcohols
thereof, and separating the diastereomeric mixture of salts or derivatives
thus
obtained, e.g. on the basis of their differences in solubility, whilst the
free antipodes
may be released from the pure diastereomeric salts or derivatives by the
action of
suitable agents. Optically active acids in common use are e.g. the D- and L-
forms of
25 tartaric acid or dibenzoyltartaric acid, di-o-tolyltartaric acid, malic
acid, mandelic acid,
camphorsulphonic acid, glutamic acid, aspartic acid or quinic acid. An
optically active
alcohol may be for example (+) or (-)-menthol and an optically active acyl
group in
amides, for example, may be a (+)-or (-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I may be converted into the salts
thereof,
particularly for pharmaceutical use into the physiologically acceptable salts
with
inorganic or organic acids. Acids which may be used for this purpose include
for
example hydrochloric acid, hydrobromic acid, sulphuric acid, methanesulphonic
acid,
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-19-
phosphoric acid, fumaric acid, succinic acid, lactic acid, citric acid,
tartaric acid or
malefic acid.
Moreover, the compounds obtained may be converted into mixtures, for example
1:1
or 1:2 mixtures with amino acids, particularly with alpha-amino acids such as
proline
or phenylalanine, which may have particularly favourable properties such as a
high
crystallinity.
The compounds of general formulae II to V used as starting materials are
partly
known from the literature or may be obtained by methods known from the
literature
(see Examples I to VI), optionally with the additional inclusion of protecting
groups.
The compounds according to the invention may advantageously also be obtained
by
the methods described in the following examples, which may also be combined
with
~5 methods known to the skilled man from the literature, for example,
particularly the
methods described in WO 01/68660, WO 01/74834, WO 02/28872, WO 02/44192,
WO 02/64606, WO 03/11880 and WO 03/80635.
As already mentioned, the compounds of general formula I according to the
invention
2o and the physiologically acceptable salts thereof have valuable
pharmacological
properties, particularly an inhibitory effect on the sodium-dependent glucose
cotransporter SGLT, preferably SGLT2.
The biological properties of the new compounds may be investigated as follows:
The ability of the substances to inhibit the SGLT-2 activity may be
demonstrated in a
test set-up in which a CHO-K1 cell line (ATCC No. CCL 61) or alternatively an
HEK293 cell line (ATCC No. CRL-1573), which is stably transfected with an
expression vector pZeoSV (Invitrogen, EMBL accession number L36849) , which
so contains the cDNA for the coding sequence of the human sodium glucose
cotransporter 2 (Genbank Acc. No.NM 003041) (CHO-hSGLT2 or HEK-hSGLT2).
These cell lines transport '4C-labelled alpha-methyl-glucopyranoside ('4C-AMG,
Amersham) into the interior of the cell in sodium-dependent manner.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-20-
The SGLT-2 assay is carried out as follows:
CHO-hSGLT2 cells are cultivated in Ham's F12 Medium (BioWhittaker) with 10%
foetal calf serum and 250 Ng/ml zeocin (Invitrogen), and HEK293-hSGLT2 cells
are
cultivated in DMEM medium with 10% foetal calf serum and 250 Ng/ml zeocin
(Invitrogen). The cells are detached from the culture flasks by washing twice
with
PBS and subsequently treating with trypsin/EDTA. After the addition of cell
culture
medium the cells are centrifuged, resuspended in culture medium and counted in
a
Casy cell counter. Then 40,000 cells per well are seeded into a white, 96-well
plate
coated with poly-D-lysine and incubated overnight at 37°C, 5% C02. The
cells are
washed twice with 250 NI of assay buffer (Hanks Balanced Salt Solution, 137 mM
NaCI, 5.4 mM KCI, 2.8 mM CaCl2, 1.2 mM MgS04 and 10 mM HEPES (pH7.4), 50
Ng/ml of gentamycin). 250 NI of assay buffer and 5 NI of test compound are
then
added to each well and the plate is incubated for a further 15 minutes in the
incubator.
5 NI of 10% DMSO are used as the negative control. The reaction is started by
~5 adding 5 NI of '4C-AMG (0.05 pCi) to each well. After 2 hours' incubation
at 37°C, 5%
C02, the cells are washed again with 250 NI of PBS (20°C) and then
lysed by the
addition of 25 NI of 0.1 N NaOH (5 min. at 37°C). 200 NI of
MicroScint20 (Packard) are
added to each well and incubation is continued for a further 20 min at
37°C. After this
incubation the radioactivity of the '4C-AMG absorbed is measured in a Topcount
20 (Packard) using a'4C scintillation program.
To determine the selectivity with respect to human SGLT1 an analogous test is
set up
in which the cDNA for hSGLT1 (Genbank Acc. No. NM000343) instead of hSGLT2
cDNA is expressed in CHO-K1 or HEK293 cells.
The compounds of general formula I according to the invention may for example
have EC50 values below 1000 nM, particularly below 50 nM.
In view of their ability to inhibit the SGLT activity, the compounds of
general formula I
3o according to the invention and the corresponding pharmaceutically
acceptable salts
thereof are theoretically suitable for the treatment and/or preventative
treatment of all
those conditions or diseases which may be affected by the inhibition of the
SGLT
activity, particularly the SGLT-2 activity. Therefore, compounds according to
the
invention are particularly suitable for the prevention or treatment of
diseases,
CA 02548353 2006-06-05
WO 2005/063785 PCT/EP2004/014319
-21 -
particularly metabolic disorders, or conditions such as type 1 and type 2
diabetes
mellitus, complications of diabetes (such as e.g. retinopathy, nephropathy or
neuropathies, diabetic foot, ulcers, macroangiopathies), metabolic acidosis or
ketosis,
reactive hypoglycaemia, hyperinsulinaemia, glucose metabolic disorder, insulin
resistance, metabolic syndrome, dyslipidaemias of different origins,
atherosclerosis
and related diseases, obesity, high blood pressure, chronic heart failure,
oedema and
hyperuricaemia. These substances are also suitable for preventing beta-cell
degeneration such as e.g. apoptosis or necrosis of pancreatic beta cells. The
substances are also suitable for improving or restoring the functionality of
pancreatic
cells, and also of increasing the number and size of pancreatic beta cells.
The
compounds according to the invention may also be used as diuretics or
antihypertensives and are suitable for the prevention and treatment of acute
renal
failure.
15 In particular, the compounds according to the invention, including the
physiologically
acceptable salts thereof, are suitable for the prevention or treatment of
diabetes,
particularly type 1 and type 2 diabetes mellitus, and/or diabetic
complications.
The dosage required to achieve the corresponding activity for treatment or
prevention
2o usually depends on the compound which is to be administered, the patient,
the nature
and gravity of the illness or condition and the method and frequency of
administration
and is for the patient's doctor to decide. Expediently, the dosage may be from
1 to
100 mg, preferably 1 to 30 mg, by intravenous route, and 1 to 1000 mg,
preferably 1
to 100 mg, by oral route, in each case administered 1 to 4 times a day. For
this
25 purpose, the compounds of formula I prepared according to the invention may
be
formulated, optionally together with other active substances, together with
one or
more inert conventional carriers and/or diluents, e.g. with corn starch,
lactose,
glucose, microcrystalline cellulose, magnesium stearate, polyvinylpyrrolidone,
citric
acid, tartaric acid, water, water/ethanol, water/glycerol, water/sorbitol,
so water/polyethylene glycol, propylene glycol, cetylstearyl alcohol,
carboxymethylcellulose or fatty substances such as hard fat or suitable
mixtures
thereof, to produce conventional galenic preparations such as plain or coated
tablets,
capsules, powders, suspensions or suppositories.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
- 22 -
The compounds according to the invention may also be used in conjunction with
other
active substances, particularly for the treatment and/or prevention of the
diseases and
conditions mentioned above. Other active substances which are suitable for
such
combinations include for example those which potentiate the therapeutic effect
of an
SGLT antagonist according to the invention with respect to one of the
indications
mentioned and/or which allow the dosage of an SGLT antagonist according to the
invention to be reduced. Therapeutic agents which are suitable for such a
combination include, for example, antidiabetic agents such as metformin,
sulphonylureas (e.g. glibenclamid, tolbutamide, glimepiride), nateglinide,
repaglinide,
thiazolidinediones (e.g. rosiglitazone, pioglitazone), PPAR-gamma-agonists
(e.g. GI
262570) and antagonists, PPAR-gamma/alpha modulators (e.g. KRP 297), alpha-
glucosidase inhibitors (e.g. acarbose, voglibose), DPPIV inhibitors (e.g.
LAF237, MK-
431), alpha2-antagonists, insulin and insulin analogues, GLP-1 and GLP-1
analogues (e.g. exendin-4) or amylin. The list also includes inhibitors of
protein
~5 tyrosinephosphatase 1, substances that affect deregulated glucose
production in the
liver, such as e.g. inhibitors of glucose-6-phosphatase, or fructose-1,6-
bisphosphatase, glycogen phosphorylase, glucagon receptor antagonists and
inhibitors of phosphoenol pyruvate carboxykinase, glycogen synthase kinase or
pyruvate dehydrokinase, lipid lowering agents such as for example HMG-CoA-
2o reductase inhibitors (e.g. simvastatin, atorvastatin), fibrates (e.g.
bezafibrat,
fenofibrat), nicotinic acid and the derivatives thereof, PPAR-alpha agonists,
PPAR-
delta agonists, ACAT inhibitors (e.g. avasimibe) or cholesterol absorption
inhibitors
such as, for example, ezetimibe, bile acid-binding substances such as, for
example,
cholestyramine, inhibitors of ileac bile acid transport, HDL-raising compounds
such as
25 CETP inhibitors or ABC1 regulators or active substances for treating
obesity, such as
sibutramin or tetrahydrolipostatin, dexfenfluramine, axokine, antagonists of
the
cannabinoid1 receptor, MCH-1 receptor antagonists, MC4 receptor agonists, NPYS
or
NPY2 antagonists or f33-agonists such as SB-418790 or AD-9677 and agonists of
the
5HT2c receptor.
Moreover, combinations with drugs for influencing high blood pressure, chronic
heart
failure or atherosclerosis such as e.g. A-II antagonists or ACE inhibitors,
ECE
inhibitors, diuretics, f3-blockers, Ca-antagonists, centrally acting
antihypertensives,
antagonists of the alpha-2-adrenergic receptor, inhibitors of neutral
endopeptidase,
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-23-
thrombocyte aggregation inhibitors and others or combinations thereof are
suitable.
Examples of angiotensin II receptor antagonists are candesartan cilexetil,
potassium
losartan, eprosartan mesylate, valsartan, telmisartan, irbesartan, EXP-3174, L-
158809, EXP-3312, olmesartan, medoxomil, tasosartan, KT-3-671, GA-0113, RU-
64276, EMD-90423, BR-9701, etc.. Angiotensin II receptor antagonists are
preferably
used for the treatment or prevention of high blood pressure and complications
of
diabetes, often combined with a diuretic such as hydrochlorothiazide.
A combination with uric acid synthesis inhibitors or uricosurics is suitable
for the
treatment or prevention of gout.
A combination with GABA-receptor antagonists, Na-channel blockers, topiramat,
protein-kinase C inhibitors, advanced glycation end product inhibitors or
aldose
reductase inhibitors may be used for the treatment or prevention of
complications of
15 diabetes.
The dosage for the combination partners mentioned above is usefully 1/5 of the
lowest dose normally recommended up to 1/1 of the normally recommended dose.
2o Therefore, in another aspect, this invention relates to the use of a
compound
according to the invention or a physiologically acceptable salt of such a
compound
combined with at least one of the active substances described above as a
combination partner, for preparing a pharmaceutical composition which is
suitable for
the treatment or prevention of diseases or conditions which can be affected by
25 inhibiting the sodium-dependent glucose cotransporter SGLT. These are
preferably
metabolic diseases, particularly one of the diseases or conditions listed
above, most
particularly diabetes or diabetic complications.
The use of the compound according to the invention, or a physiologically
acceptable
so salt thereof, in combination with another active substance may take place
simultaneously or at staggered times, but particularly within a short space of
time. If
they are administered simultaneously, the two active substances are given to
the
patient together; while if they are used at staggered times the two active
substances
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-24-
are given to the patient within a period of less than or equal to 12 hours,
but
particularly less than or equal to 6 hours.
Consequently, in another aspect, this invention relates to a pharmaceutical
composition which comprises a compound according to the invention or a
physiologically acceptable salt of such a compound and at least one of the
active
substances described above as combination partners, optionally together with
one or
more inert carriers and/or diluents.
Thus, for example, a pharmaceutical composition according to the invention
comprises a combination of a compound of formula I according to the invention
or a
physiologically acceptable salt of such a compound and at least one
angiotensin II
receptor antagonist optionally together with one or more inert carriers and/or
diluents.
15 The compound according to the invention, or a physiologically acceptable
salt thereof,
and the additional active substance to be combined therewith may both be
present
together in one formulation, for example a tablet or capsule, or separately in
two
identical or different formulations, for example as a so-called kit-of-parts.
2o In the foregoing and following text, H atoms of hydroxyl groups are not
explicitly
shown in every case in structural formulae. The Examples that follow are
intended to
illustrate the present invention without restricting it:
Preparation of the starting compounds:
Example I
O
O
Br
4-((R)-Tetrahydrofuran-3-yloxy)-bromobenzene
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-25-
Prepared by stirring 10 g of 4-bromophenol with 21 g of ((S)-tetrahydrofuran-3-
yl) p-
toluenesulphonate in the presence of 11.98 g of potassium carbonate in 100 ml
of
dimethylformamide at 60°C for 32 hours and subsequently purifying by
chromatographic purification.
Yield: 13.7 g (97% of theory)
Rf value: 0.80 (aluminium oxide; cyclohexane/ethyl acetate = 2:1 )
The following compound is prepared analogously to Example I:
(1) 4-((S)-tetrahydrofuran-3-yloxy)-bromobenzene
Mass spectrum: m/z = 242/244 [M+]
Example II
\ O
O
O O
~5 (2-Benzyloxy-phenyl)-[4-((R)-tetrahydrofuran-3-yloxy)phenyl]-methanol
5.17 ml of a 1.6 M butyllithium solution in hexane are added dropwise to a
solution of
2.0 g of 4-((R)-tetrahydrofuran-3-yloxy)-bromobenzene in 10 ml of
tetrahydrofuran at
-78°C and stirred for another hour at -78°C. Then 1.75 g of 2-
benzyloxy-
2o benzaldehyde dissolved in 5 ml of tetrahydrofuran are added dropwise and
the
mixture is stirred for 2 hours at -78°C. After heating to ambient
temperature it is stirred
for 1 hour. After aqueous working up and extraction with ethyl acetate the
organic
phase is dried and evaporated down. The residue is purified by chromatography
through a silica gel column with cyclohexane/ethyl acetate (8:2 to 1:1 ).
Yield: 2.6 g (84% of theory)
Rf value: 0.25 (silica gel, cyclohexane/ethyl acetate = 3:1 )
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-26-
The following compounds are prepared analogously to Example II:
(1 ) (2-benzyloxy-phenyl)-[4-((S)-tetrahydrofuran-3-yloxy)phenyl]-methanol
Mass spectrum (ESI+): m/z = 394 [M+NH4]+
(2) (2-benzyloxy-4-fluoro-phenyl)-[4-((R)-tetrahydrofuran-3-yloxy)phenyl]-
methanol
Mass spectrum (ESI+): m/z = 417 (M+Na]+
(3) (2-benzyloxy-6-methoxy-phenyl)-[4-((R)-tetrahydrofuran-3-yloxy)phenyl]-
methanol
Mass spectrum (ESI+): m/z = 424 [M+NH4]+
Example III
~ O
O
O
~5 2-[4-((R)-Tetrahydrofuran-3-yloxy)benzyl]-phenol
Prepared from 1.97 g of the compound of Example II by catalytic hydrogenation
in
methanol in the presence of 0.4 g palladium on activated charcoal (10% Pd) at
ambient temperature.
2o Rf value: 0.52 (silica gel, cyclohexane/ethyl acetate = 2:1 )
Mass spectrum (ESI-): m/z = 269 [M-H]-
The following compounds are prepared analogously to Example III:
25 (1) 2-[4-((S)-tetrahydrofuran-3-yloxy)benzyl]-phenol
Mass spectrum (ESI+): m/z = 271 [M+H]+
(2) 2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-4-fluoro-phenol
Mass spectrum (ESI-): m/z = 287 [M-H]-
(3) 2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-6-methoxy-phenol
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-27-
Mass spectrum (ESI+): m/z = 301 (M+H]+
Example IV
~ O
O
O v
O O O
O ,,,, .,,~ O
O O
O
1-(2,3,4,6-Tetra-O-acetyl-f3-D-glucopyranosyloxy)-2-[4-((R)-tetrahyd rofu ran-
3-
yloxy)benzyl]-benzene
500 mg of 2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-phenol, 820 mg of 2,3,4,6-
tetra-
O-acetyl-alpha-glucopyranosylbromide, 2 ml of 1 M sodium hydroxide solution
and 5
ml of chloroform are stirred for 16 hours at ambient temperature. Another 400
mg of
2,3,4,6-tetra-O-acetyl-alpha-glucopyranosylbromide, 1 ml of 1 M sodium
hydroxide
solution and 5 ml of methylene chloride are added and the mixture is stirred
for 2.5
days. The organic phase is separated off, washed with water, dried and
evaporated
down. The crude product is purified by chromatography through a silica gel
column
15 with a cyclohexane/ethyl acetate gradient (7:3 to 1:1 ).
Yield: 440 mg (40% of theory)
Rf value: 0.10 (silica gel; cyclohexane/ethyl acetate = 2:1 )
Mass spectrum (ESI+): m/z = 618 [M+NH4]+
2o The following compounds are obtained analogously to Example IV:
(1) 1-(2,3,4,6-Tetra-O-acetyl-f3-D-glucopyranosyloxy)-2-(4-ethynylbenzyl)-
benzene
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
_28_
/ ~ ~ \
\ /
O
O O O
0,,,. .,,~0
O O
O
reacting with the compound of Example VI in the presence of benzyl-
triethylammonium-bromide
Rf value: 0.30 (silica gel; cyclohexane/ethyl acetate = 2:1 )
Mass spectrum (ESI+): m/z = 556 [M+NH4]+
(2) 1-(2,3,4,6-Tetra-O-acetyl-(3-D-glucopyranosyloxy)-2-[4-((S)-
tetrahydrofuran-3-
yloxy)benzyl]-benzene
Rf value: 0.50 (silica gel; cyclohexane/ethyl acetate = 1:1 )
1o Mass spectrum (ESI+): m/z = 618 [M+NH4]+
(3) 1-(2,3,4,6-Tetra-O-acetyl-(3-D-glucopyranosyloxy)-2-[4-((R)-
tetrahydrofuran-3-
yloxy)benzyl]-4-fluoro-benzene
Mass spectrum (ESI+): m/z = 636 [M+NH4]+
(4) 1-(2,3,4,6-Tetra-O-acetyl-f3-D-glucopyranosyloxy)-2-[4-((R)-
tetrahydrofuran-3-
yloxy)benzyl]-6-methoxy-benzene
Mass spectrum (ESI+): m/z = 648 [M+NH4]+
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-29-
Example V
/ \ Br
\ ~ ~ /
O
2-(4-Bromobenzyl)-phenol
Prepared by reacting sodium phenoxide (from 4.0 g phenol and 1.7 g 60% sodium
hydride in paraffin oil) with 10.27 g of 4-bromobenzyl chloride in toluene at
reflux
temperature and purifying the reaction mixture by chromatography through a
silica gel
column with cyclohexane/ethyl acetate (8:2 to 1:1 ).
Yield: 1.8 g (16% of theory)
Rf value: 0.40 (silica gel; cyclohexane/ethyl acetate = 4:1 )
Mass spectrum (ESI-): m/z = 261/263 [M-H]-
~5 Example VI
S i\
\ /
O
2-[4-(2-Trimethylsilyl-ethynyl)-benzyl]-phenol
Prepared by reacting 1.6 g 2-(4-bromobenzyl)-phenol with 1.03 ml
trimethylsilyl-
acetylene in the presence of 86 mg of bis(triphenylphosphine)-palladium(II)-
chloride
2o and 23 mg of copper (I) iodide in 5 ml of triethylamine at 100°C in
the microwave
oven and purifying the reaction mixture by chromatography through a silica gel
column with cyclohexane/ethyl acetate (9:1 to 7:3)
Rf value: 0.62 (silica gel; cyclohexane/ethyl acetate = 4:1 )
Mass spectrum (ESI+): m/z = 281 [M+H]+
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-30-
Preparation of the final compounds:
Example 1
\ / O
O
/ \
O O
O
0~~~ ~~~~0
O
1-(f3-D-Glucopyranosyloxy)-2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-benzene
A solution of 400 mg of 1-(2,3,4,6-tetra-O-acetyl-(3-D-glucopyranosyloxy)-2-[4-
((R)-
tetrahydrofuran-3-yloxy)benzyl]-benzene in a mixture of 2.5 ml of methanol and
5 ml
of tetrahydrofuran is cooled in the ice bath and combined with 3.02 ml of a 1
M
aqueous lithium hydroxide solution and stirred for 1 hour. The reaction
mixture is
combined with 5 ml of water and extracted with ethyl acetate. The organic
phase is
separated off, washed with saturated saline solution, dried and evaporated
down.
Yield: 190 mg (65% of theory)
~5 Rf value: 0.23 (silica gel; methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 433 [M+H]+
The following compounds are obtained analogously to Example 1:
20 (1) 1-((3-D-glucopyranosyloxy)-2-(4-ethynylbenzyl)-benzene
\ /
/ \
O O
O
O ~~,, ~~~ O
O
Rf value: 0.55 (silica gel, methylene chloride/methanol = 6:1 )
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-31 -
Mass spectrum (ESI+): m/z = 388 [M+NH4]
(2) 1-((3-D-glucopyranosyloxy)-2-[4-((S)-tetrahydrofuran-3-yloxy)benzyl]-
benzene
\ / O'.,
~O
O O
O
0~~~ ~~~~0
O
melting point: 134-135 °C
(3) 1-((3-D-glucopyranosyloxy)-2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-4-
fluoro-
benzene
F
\ / O
O
O O
O
0,,,. ~~''O
O
1 o melting point: 145-147 °C
(4) 1-((3-D-glucopyranosyloxy)-2-[4-((R)-tetrahydrofuran-3-yloxy)benzyl]-6-
methoxy-
benzene
\ ~ O
O
O
O O
O
'~~~0
O
Mass spectrum (ESI+): m/z = 480 [M+NH4]
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-32-
Example 2
O
O O
\O~O
0,,,. ~~~'O
O
1-(6-O-Methoxycarbonyl-f3-D-glucopyranosyloxy)-2-(4-ethynylbenzyl)-benzene
100 mg of 1-(f3-D-glucopyranosyloxy)-2-(4-ethynylbenzyl)-benzene in 0.5 ml of
2,4,6-
collidine are combined with 0.026 ml of methyl chloroformate in the ice bath
and then
stirred for 16 hours at ambient temperature. 5 ml of 0.1 N hydrochloric acid
are added
to the reaction mixture which is then extracted with 10 ml of ethyl acetate.
The organic
1o phase is separated off, washed with saturated saline solution and
evaporated down.
The residue is stirred with 8 ml of diethyl ether/petroleum ether (1:1), the
solid is
suction filtered and dried at 40°C.
Yield: 73.5 mg (63% of theory)
Mass spectrum (ESI+): m/z = 429 [M+H]+
The following compounds are obtained analogously to Example 2:
(1) 1-(6-O-methoxycarbonyl-f3-D-glucopyranosyloxy)-2-[4-((R)-tetrahydrofuran-3-
yloxy)benzyl]-benzene
/ O
O
O
\O- _O O O
0~~~ I~~~O
Mass spectrum (ESI+): m/z = 508 [M+NH4]+
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-33-
(2) 1-(6-O-methoxycarbonyl-f3-D-glucopyranosyloxy)-2-[4-((S)-tetrahydrofuran-3-
yloxy)benzyl]-benzene
\ / O''
O
/ \
O
~ O O
\O- _O
0~~~ ,~~~0
O
melting point: 149-150 °C
The following compounds are also prepared analogously to the above-mentioned
Examples and other methods known from the literature:
\ \ / O\
(10) ~ / \
O O
O
O ,,,. ~''' O
O
(11) O
O O
O
0,,,. ~~''O
O
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-34-
\ / O F
(12)
\ F
O O
O
0,,,. ~~''O
O
( 3) ~ \ / ~ w
/ \
O O
O
0~~~~ ~~~0
O
F \ / O
O
(14) ~ / \
O O
O
0,,,. ~~~'O
O
O
(15) .O
O O
O
0,,,. ~~~'O
O
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-35-
O
(16) .O
O O
O
0,,,. ~~~'O
O
\ / O
(1 ) ~ / \ ~ O
O O F
O
0,,,. ~~''O
O
(18) ~ \ /
/ \
O O F
O
0,,,. ~~~'O
O
\ /
(19)
/ \
F
O O
O
0,,,. ~~''O
O
CA 02548353 2006-06-05
WO 2005/063785 PCT/EP2004/014319
-36-
\ // O
(20) I / \ I
0 0
0
o ,,,. '''' o
0
(21) I \ /
/ \
0 0
0
o,,,. '~''o
0
\ / o -..
'o
22 I / \ I
( )
O O
O
0,,,. '~''O
O
N
\ /
(23) I / \ I ,O
O O
O
0,,,. ''''O
O
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-37-
O
(24)
O \ /
/ \
O O
O
0,,,. ~~~'O
O
\ /
~O
2 ~/ \(
( 5)
O O
O
0,,,. ~~''O
O
(26) ~ \ /
/ \
O
O O
~O~O
0,,,. ~~''O
O
\ \ / O~CF3
(27) ~ / \
O
O O
O
0,,,. ~~~'O
O
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-38-
\ \ /
(28) ~ /
v
O O
O
O ~~,, ~ O
O
\ /
O
(29) I / \
O O F
O
0,,,. ~~~'O
O
O
(30)
O \ /
/
O
O O
O
0,,,. ~~''O
O
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-39-
Example A
Tablets containing 100 mg of active substance
Composition:
1 tablet contains:
active substance 100.0 mg
lactose 80.0 mg
corn starch 34.0 mg
1o polyvinylpyrrolidone 4.0 mg
magnesium stearate 2.0 mg
220.0 mg
Method of Preparation:
The active substance, lactose and starch are mixed together and uniformly
moistened
with an aqueous solution of the polyvinylpyrrolidone. After the moist
composition has
been screened (2.0 mm mesh size) and dried in a rack-type drier at 50°C
it is
screened again (1.5 mm mesh size) and the lubricant is added. The finished
mixture is
2o compressed to form tablets.
Weight of tablet: 220 mg
Diameter: 10 mm, biplanar, facetted on both sides and notched on one side.
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-40-
Example B
Tablets containing 150 mg of active substance
Composition:
1 tablet contains:
active substance 150.0 mg
powdered lactose 89.0 mg
corn starch 40.0 mg
colloidal silica 10.0 mg
polyvinylpyrrolidone 10.0 mg
magnesium stearate 1.0 mg
300.0 mg
Preparation:
The active substance mixed with lactose, corn starch and silica is moistened
with a
20% aqueous polyvinylpyrrolidone solution and passed through a screen with a
mesh
size of 1.5 mm. The granules, dried at 45°C, are passed through the
same screen
again and mixed with the specified amount of magnesium stearate. Tablets are
2o pressed from the mixture.
Weight of tablet: 300 mg
die: 10 mm, flat
Example C
Hard gelatine capsules containing 150 mg of active substance
Composition:
1 capsule contains:
3o active substance 150.0 mg
corn starch (dried) approx. 180.0
mg
lactose (powdered) approx. 87.0 mg
magnesium stearate 3.0 mg
approx. 420.0 mg
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-41 -
Preparation:
The active substance is mixed with the excipients, passed through a screen
with a
s mesh size of 0.75 mm and homogeneously mixed using a suitable apparatus. The
finished mixture is packed into size 1 hard gelatine capsules.
Capsule filling: approx. 320 mg
Capsule shell: size 1 hard gelatine capsule.
Example D
Suppositories containinct 150 mg of active substance
Composition:
15 1 suppository contains:
active substance 150.0 mg
polyethyleneglycol 1500 550.0 mg
polyethyleneglycol 6000 460.0 mg
polyoxyethylene sorbitan monostearate 840.0 mg
20 2,000.0 mg
Preparation:
After the suppository mass has been melted the active substance is
homogeneously
25 distributed therein and the melt is poured into chilled moulds.
Example E
Ampoules containingi 10 mq active substance
Composition:
active substance 10.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 2.0 ml
WO 2005/063785 CA 02548353 2006-06-05 PCT/EP2004/014319
-42-
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI, made
isotonic with common salt, filtered sterile and transferred into 2 ml
ampoules.
Example F
Ampoules containing 50 mg of active substance
Composition:
active substance 50.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water ad 10.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI, made
2o isotonic with common salt, filtered sterile and transferred into 10 ml
ampoules.