Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
DEVICE FOR CHANGING THE SHAPE OF THE MITRAI~ ANNUhUS
CROSS-REFERENCE TO REhATED APPhICATION
[001] This application claims priority to and the benefit
of U.S. Provisional Patent Application 60/530,352 filed
December 16, 2003 titled Device to Change the Shape of the
Mitral Valve Annulus, U.S. Provisional Patent Application
60/547,741 filed February 25, 2004 titled Methods and
Apparatus for Treatment of Mitral Insufficiency, and U.S.
Provisional Patent Application 60/624,224 filed November 2,
2004 titled Device for Changing the Shape of the Mitral
Annulus, the entire content of which is expressly incorporated
herein by reference.
FIELD OF THE INVENTION
[002] This invention relates to devices and methods for
heart valve repair and, more particularly, to endovascular
devices and methods for improving mitral valve function using
devices inserted into the coronary sinus.
BACKGROUND
[003] Heart valve regurgitation, or leakage from the
outflow to the inflow side of a heart valve, is a common
occurrence in patients with heart failure and a source of
morbidity and mortality in these patients. Usually,
regurgitation will occur in the mitral valve, located between
the left atrium and the left ventricle, or in the tricuspid
valve, located between the right atrium and right ventricle.
Mitral regurgitation in patients with heart failure is caused
by changes in the geometric configurations of the left
ventricle, papillary muscles and mitral annulus. Similarly,
tricuspid regurgitation is caused by changes in the geometric
-1-
CONFIRMi4Ti~N ~0~'Y
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
configurations of the right ventricle, papillary muscles, and
tricuspid annulus. These geometric alterations result in
mitral and tricuspid leaflet tethering and incomplete
coaptation in systole.
[004] Mitral valve repair is the procedure of choice to
correct mitral regurgitation of all etiologies. With the use
of current surgical techniques, between 40% and 60% of
regurgitant mitral valves can be repaired depending on the
surgeon's experience and the anatomic conditions. The
advantages of mitral valve repair over mitral valve
replacement are well documented. These advantages include
better preservation of cardiac function and reduced risk of
anticoagulant-related hemorrhage, thromboembolism and
endocarditis.
[005] In current practice, mitral valve surgery requires
an extremely invasive approach that includes a chest wall
incision, cardiopulmonary bypass, cardiac and pulmonary
arrest, and an incision on the heart itself to gain access to
the mitral valve. Such a procedure is associated with high
morbidity and mortality. Due to the risks associated with this
procedure, many of the sickest patients are denied the
potential benefits of surgical correction of mitral
regurgitation. In addition, patients with moderate,
symptomatic mitral regurgitation are denied early intervention
and undergo surgical correction only after the development of
cardiac dysfunction.
[006.] More particularly, current surgical practice for
mitral valve repair generally requires that the posterior
mitral valve annulus be reduced in radius by surgically
opening the left atrium and then fixing sutures, or sutures in
combination with a support ring, to the internal surface of
the annulus. This structure is used to pull the annulus back
-2-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
into a smaller radius, thereby reducing mitral regurgitation
by improving leaflet coaptation.
[007] This method of mitral valve repair, generally termed
"annuloplasty," effectively reduces mitral regurgitation in
heart failure patients. This, in turn, reduces symptoms of
heart failure, improves quality of life and increases
longevity. Unfortunately, however, the invasive nature of
mitral valve surgery and the attendant risks render most heart
failure patients poor surgical candidates. Thus, a less
invasive means to increase leaflet coaptation and thereby
reduce mitral regurgitation in heart failure patients would
make this therapy available to a much greater percentage of
patients.
[008] Several recent developments in minimally invasive
techniques for repairing the mitral valve without surgery have
been introduced. Some of these techniques involve introducing
systems for remodeling the mitral annulus through the coronary
sinus.
[009] The coronary sinus is a blood vessel commencing at
the coronary ostium in the right atrium and passing through
the atrioventricular groove in close proximity to the
posterior, lateral and medial aspects of the mitral annulus.
Because of its position adjacent to the mitral annulus, the
coronary sinus provides an ideal conduit for positioning an
endovascular prosthesis to act on the mitral annulus and
therefore reshape it.
[0010] One example of a minimally invasive technique for
mitral valve repair can be found in U.S. Patent Publication
No. 2003/0083,538 to Adams et al. ("the '538 publication").
The '538 publication describes a balloon expandable device
insertable into the coronary sinus to reshape the mitral valve
annulus, the device taking the form of a frame structure
-3-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
having an elongated base and integral columnar structures
extending therefrom. The columnar structures form the force
applier to apply force to discrete portions of the wall of the
coronary sinus.
[0011] Another device is described in U.S. Patent No.
6,656,221 issued to Taylor et al. ("the '221 patent"). The
'835 publication describes a substantially straight rigid
elongated body including relatively flexible portions to help
better distribute the stress exerted on the walls of the
coronary sinus.
[0012] U.S. Patent Publication 2002/0183838 to Liddicoat et
al. ("the '838 publication) describes multiple devices for
minimally invasive mitral valve repair. In one embodiment,
the '838 publication describes a device including an internal
member having a plurality of slots and an external member
having a plurality of slots. When the slots on the internal
member are aligned with the slots on the external member, the
device is flexible so as to follow the natural curvature of
the coronary sinus. When the slots on both members are
oriented away from each other, the device is straight and
rigid and able to apply an anteriorly-directed force to the
mitral valve annulus.
[0013] In another embodiment, the '838 publication
describes an elongated body having a "w" shape. When the body
is positioned in the coronary sinus, the center of the "w" is
directed towards the anterior mitral annulus and inverts the
natural curvature of the coronary sinus.
[0014] Another example of a minimally invasive technique
for mitral valve repair can be found in U.S. Patent No. 6,
402, 781 issued to Langberg et al. ("the '781 patent"). The
'781 patent describes a two-dimensional prosthesis deployed
into the coronary sinus via a delivery catheter. The tissue
-4-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
contacting surface of the prosthesis is provided with ridges,
teeth or piercing structures that exert tension and enhance
friction to engage to discrete portions of the wall of the
coronary sinus. Moreover, the device provides an open loop
through the coronary sinus and the entire coronary venous
system with control lines that extend outside of the patient.
[0015] Another device is described in U.S. Patent No.
6,790,231 to Liddicoat et al. ("the '231 patent"). The '231
patent describes a two-dimensional elongated body having a
guide wire that controls a spine of the elongated body to form
an arc. The elongate body has discrete barbs along its spine
to apply frictional force to discrete portions of the wall of
the coronary sinus.
[0016] U.S. Patent No. 6,676,702 to Mathis ("the '702
patent") describes a two-dimensional mitral valve therapy
device that forms an arc inside the coronary sinus to exert
force on the mitral annulus. A guide wire extending from the
device changes the shape of the device and the device applies
pressure on discrete portions of the coronary sinus.
[0017] Despite recent attempts at minimally invasive repair
of the mitral annulus using devices residing in the coronary
sinus, there is a need for such endovascular correction
devices that do not require an external member, such as a
wire, to alter the shape of the device, yet still provide
enough force to reshape the mitral annulus. Further, there is
a need for devices, including those that use an external
member, that are less traumatic to the sinus, both during and
after their insertion into the coronary sinus, and are also
more reliable over long periods of time. Finally, there is a
need for better control over~the shape in which the mitral
annulus is deformed by such endovascular correction devices.
-5-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
SUI~1ARY
[0018] The invention described herein provides a more
reliable and a safer way to treat a dilated mitral annulus.
Devices in accordance with principles of the present invention
may comprise one or more components suitable for deployment in
the coronary sinus and adjoining coronary veins. The devices
may be configured to bend in-situ to apply a compressive load
to the mitral valve annulus with or without a length change,
or may include multiple components that are drawn or
contracted towards one another to remodel the mitral valve
annulus . Any of a number of types of anchors may be used to
engage the surrounding vein and tissue, including anchors
comprising ultraviolet (UV) curable materials, hydrogels,
hydrophilic materials, or biologically anchored components.
Remodeling of the mitral valve annulus may be accomplished
during initial deployment of the device, or by biological
actuation during subsequent in-dwelling of the device.
[0019] One embodiment of the invention comprises an
elongate body having a proximal, central and distal stmt
section, wherein a backbone fixes the stmt sections relative
to one another and wherein the central stmt section has a
plurality of rings connected to the backbone. The elongate
body has two states: a first state wherein the elongate body
has a shape that is adaptable to the shape of the coronary
sinus and a second state wherein the elongate body pushes on
the coronary sinus to reduce dilatation. Further, the
elongate body has a greater axial length in the first state
than in the second state.
[0020] When the body is deployed, the proximal and distal
stmt sections are expanded to act as anchors in the coronary
sinus. Expansion of the central stmt section foreshortens
the elongate body, drawing the proximal and distal stmt
-6-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
sections toward the central stmt section, and cinching the
mitral valve and closing the gap between mitral valve
leaflets. When the gap between the mitral valve leaflets is
closed, the effects of mitral valve regurgitation are
drastically reduced or eliminated.
[0021] In another embodiment, the device comprises proximal
and distal transitional sections in addition to the proximal,
central and distal stmt sections. The transitional sections
allow the body to have. enough flexibility to conform to the
curvature of the coronary sinus.
[0022] Yet another embodiment comprises a proximal stmt
module and a distal stmt module, wherein each stmt module
has an anchor section, a central section and a backbone. When
both stmt modules are inserted into the coronary sinus, the
central sections of the two modules may overlap, effectively
providing for one continuous stmt. Additionally, based on
the degree of rigidity desired, the backbones of the ste ms
may be misaligned to provide for increased flexibility.
[0023] Yet another embodiment comprises a tubular elongate
body having such dimensions so as to be insertable into the
coronary sinus. The body has two states: a first state
wherein the body has a linear shape adaptable to the shape of
the coronary sinus and a second state, to which the body is
transferable from the first state, wherein the device has a
nonlinear shape.
[0024] In yet another embodiment, the invention comprises a
proximal stmt section, a central stmt section, and a distal
stmt section, where a diameter of the elongate body varies
from the proximal stmt section to the distal stmt section.
The body expands into a three-dimensional shape that conforms
to the anatomy of the coronary sinus, thereby applying more
uniform stress to the walls of the inner radius of the
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
coronary sinus. The device achieves remodeling of the mitral
annulus through foreshortening, which reduces the overall
length of the coronary sinus and as a result, reduces the
circumference of the mu ral annulus.
[0025] In accordance with the invention, in one embodiment,
the elongate body is a mufti-filament woven structure, where
an angle of weave in the woven structure determines the degree
of expansion force and foreshortening of the coronary sinus.
The woven structure is made of metal with memory effect, such
as Nitinol, Elgiloy, or spring steel.
[0026] Also in accordance with this aspect of the
invention, in one embodiment a rigid inner elongated body is
placed.inside of the elongate body. In one example, the rigid
inner elongate body is placed along the central stmt section
of the elongate body and fitted into the central stmt section
of the elongate body. The inner elongate body is made from
rigid metal, such as stainless steel. Moreover, the elongate
body may be self expandable or balloon expandable.
[0027] In yet another embodiment, the invention comprises a
proximal and distal anchor, and a bridge between the proximal
and distal anchors. The bridge has an elongated state, having
first axial length, and a shortened state, having a second
axial length, wherein the second axial length is shorter than
the first axial length. A resorbable thread may be woven into
the bridge to hold the bridge in the elongated state and to
delay the transfer of the bridge to the shortened state. In
an additional embodiment, there may be one or more central
anchors between the proximal and distal anchors with a bridge
connecting adjacent anchors.
[0028] In another embodiment of the present invention, the
device comprises proximal and distal anchor elements, wherein
the proximal anchor element comprises a deployable flange.
_g_
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
The proximal and distal anchor elements are delivered into the
coronary sinus in a contracted state, and then are deployed
preferably within the coronary sinus so that the flange of the
proximal anchor element engages the coronary sinus ostium. A
cinch mechanism, for example, comprising a plurality of wires
and eyelets, is provided to reduce the distance between
proximal and distal anchor elements, thereby reducing the
circumference of the mitral valve annulus.
[0029] To reduce trauma to the intima of the coronary sinus
during actuation of the cinch mechanism, the distal anchor
element preferably is chemically or mechanically bonded to the
intima of the coronary sinus prior to actuation of the cinch
mechanism. The distal anchor element may comprise a UV-
curable material that causes the distal anchor element to bond
with the intima of the coronary sinus when a UV source is
provided. Alternatively, the distal anchor element may
comprise a hydrogel or hydrophilic foam that causes the distal
anchor element to chemically bond with the intima of the
coronary sinus, which in effect may reduce trauma to the
intima of the vessel wall during actuation of the cinch
mechanism.
[0030] In another embodiment of the present invention, a
proximal balloon catheter is used in conjunction with a distal
balloon catheter to treat mitral insufficiency. The balloons
of the proximal and distal catheters may be deployed spaced
apart a selected distance, preferably substantially within the
coronary sinus, and then manipulated so that they remodel the
curvature of the coronary sinus. This remodeling in turn
applies a compressive force upon the mitral valve to remodel
the mitral valve annulus. With the compressive force applied,
a substance, such as a biological hardening agent, may be
introduced into a cavity formed between the two balloons to
-9-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
cause a hardened mass to form in the cavity. When the
balloons of the proximal and distal catheters subsequently are
removed, the mass ensures that the coronary sinus is retained
in the remodeled shape.
[0031] In yet a further embodiment of the present
invention, a stmt is provided having proximal and distal
sections coupled to one another by a central section, so that
expansion and/or curvature of the central section causes the
proximal and distal sections to be drawn together. In this
embodiment, the central section includes one or more
biodegradable structures, such as biodegradable sutures, that
retain the central section in its contracted state until the
vessel endothelium has overgrown a portion of the proximal and
distal sections. This provides biological anchoring of the
proximal and distal sections of the stmt within at least a
portion of the coronary sinus.
[0032] After the proximal and distal sections have become
endothelialized, the biodegradable structure degrades,
releasing the central section and enabling it to expand and/or
assume a desired curvature. The expansion and/or curvature of
the central section causes the stmt to reduce the radius of
curvature of the coronary sinus, thereby causing remodeling of
the mitral valve annulus.
[0033] In another embodiment, a device for the treatment of
mitral annulus dilatation includes a cylindrical proximal
stmt module having an anchor section and a central section
and a cylindrical distal stmt module having an anchor section
and a central section, wherein the proximal and distal stmt
modules have two states, a first state wherein the proximal
and distal st m t modules have a shape that is adaptable to the
shape of the coronary sinus, and a second state wherein the
elongate body pushes on the coronary sinus to reduce
-10-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
dilatation, wherein each stmt module has a backbone, and each
backbone fixes the anchor section relative to the central
section on each module along one side of the module, and
wherein, when the proximal and distal stmt modules are in the
second state, the central section of the proximal stent
overlaps the central section of the distal st m t.
[0034] In this embodiment, the device may be inserted into
a coronary sinus, and the anchor sections of the proximal
stmt module and the distal stmt module anchor each module,
respectively, to the coronary sinus when the modules are in
the second state. The proximal and distal stmt modules may
be made from stainless steel.
(0035] In this embodiment, the stmt modules may be
inserted into the coronary sinus, and the backbone of the
proximal st m t section may be separated from the backbone of
the distal stmt section.
[0036] For example, the backbone of the proximal stmt
section may be angularly separated from the backbone of the
distal stmt section by between about 60° - 180°.
[0037] In this embodiment, the proximal and distal stmt
sections may be transferable from the first state to the
second state by a balloon. The proximal and distal stent
modules may have a greater axial length in the first state
than in the second state.
[0038] In another embodiment, a device for the treatment of
mitral annulus dilatation includes a tubular elongate body
having such dimensions as to be insertable into a coronary
sinus, wherein the elongate body has two states, a first state
wherein the elongate body has a linear shape that is adaptable
to the shape of the coronary sinus, and a second state, to
which the elongate body is transferable from the first state,
wherein the device has a nonlinear shape.
-11-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
[0039] In another embodiment, the tubular elongate body in
the second state has a substantially w-shaped configuration.
The elongate body may be transferable from a first state to a
second state by a balloon. The elongate body may also include
at least two spines. In another embodiment, the tubular
elongate body further includes a plurality of interconnecting
members extending between the at least two spines.
[0040] In another embodiment, a device for treatment of
mitral annulus dilation includes an outer elongate body having
such dimensions as to be insertable into a coronary sinus, the
outer elongate body comprising a proximal stmt section, a
central stmt section, and a distal stmt section, wherein a
diameter of the outer elongate body varies from the proximal
stmt section to the distal stmt section, the outer elongate
body having two states, a first state wherein the outer
elongate body is adaptable to be inserted into the coronary
sinus, and a second state wherein the outer elongate body
expands inside the coronary sinus to provide foreshortening of
the coronary sinus; and a rigid inner elongate body being
placed inside of the outer elongate body when the outer
elongate body is in the second state.
[0041] In another embodiment, a method of treating mitral
annulus dilation includes providing an elongate body for
treatment of mitral annulus dilation, the elongate body
comprising a curved configuration to conform to an anatomy of
a coronary sinus, the elongate body having a proximal stmt
section, a central stmt section, and a distal stmt section,
wherein a diameter of the elongate body varies from the
proximal stmt section to the distal stmt section; inserting
the elongate body into the coronary sinus; expanding the
elongate body into a three-dimensional shape to make
substantial contact with walls of the coronary sinus; and
-12-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
foreshortening the elongate body.
[0042] In another embodiment, the method includes inserting
a rigid inner elongate body inside the expanded elongate body
using a balloon; and expanding the inner elongate body to make
a substantial contact with the outer elongate body.
[0043] In another embodiment, an apparatus for treating
mitral annulus dilatation includes (a) a proximal anchor
element; (b) a distal anchor element adapted to be at least
partially bonded to an intima of a patient's vessel; and (c)
means for drawing the distal anchor element towards the
proximal anchor element.
[0044] In another embodiment, the proximal anchor element
further comprises a flange configured to abut a coronary
ostium.
[0045] In another embodiment, the proximal anchor element
comprises a self-deploying stmt.
[0046] In another embodiment, the distal anchor element
comprises a self-deploying stmt configured to engage an
intima of a patient's vessel in an expanded state.
[0047] In another embodiment, the distal anchor element
further comprises an expandable foam member having proximal
and distal ends and a bore extending therebetween, wherein the
foam member is configured to engage an intima of a patient's
vessel in an expanded state.
[0048] In another embodiment, the foam member comprises a
hydrophilic foam.
[0049] In another embodiment, the distal anchor element
further comprises a light-reactive binding agent.
[0050] In another embodiment, a catheter having proximal
and distal ends, a lumen extending therebetween, and at least
one port disposed at the distal end, wherein the catheter is
configured to transmit light from the proximal end to the port
-13-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
via the lumen.
[0051] In another embodiment, at least one radiopaque
marker band disposed on the distal end of the catheter.
[0052] In another embodiment, the distal anchor element
further comprises a hydrogel.
[0053] In another embodiment, a method for treating mitral
annulus dilatation includes (a) providing apparatus comprising
a proximal anchor element and a distal anchor element in
contracted states, (b) deploying the distal anchor element at
a first location in a patient's vessel; (c) deploying the
proximal anchor element at a second location in a patient's
vessel; (d) bonding at least a portion of the distal anchor
element to an intima of the patient's vessel; and (e) drawing
the distal anchor towards the proximal anchor element to apply
a compressive force upon the mitral annulus.
[0054] In another embodiment, the distal anchor element is
chemically bonded to an intima of a patient's coronary sinus.
[0055] In another embodiment, the method further includes
(a) providing a light-reactive binding agent disposed on at
least a portion of the distal anchor element; (b) providing a
light source; and (c) exposing the light-reactive binding
agent to the light source to cause at least a portion of the
distal anchor element to polymerize.
[0056] In another embodiment, the method further includes
(a) providing a hydrogel disposed on at least a portion of the
distal anchor element; and (b) causing the hydrogel to harden.
[0057] In another embodiment, the method further includes
(a) providing a hydrophilic foam member; and (b) causing the
hydrophilic foam member to engage an intima of the patient's
coronary sinus and or great cardiac vein.
[0058] In another embodiment, a method for treating mitral
annulus dilatation includes (a) providing a first balloon
-14-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
catheter having proximal and distal ends, a lumen extending
therebetween, and a balloon disposed at the distal end; (b)
providing a second balloon catheter having proximal and distal
ends, a lumen extending therebetween, and a balloon disposed
at the distal end; (c) deploying the balloon of the first
catheter at a first location in a patient's coronary sinus;
(d) deploying the balloon of the second catheter at a second
location in a patient's vessel, the second location being
proximal to the first location; (e) drawing the balloon of the
first catheter towards the balloon of the second catheter to
apply a compressive force upon the mitral annulus; (f) forming
a coherent mass in a cavity formed between the balloon of the
first catheter and the balloon of the second catheter; (g)
contracting the balloon of the first catheter and the balloon
of the second catheter; and (h) removing the first catheter
and the second catheter.
[0059] In another embodiment, forming a coherent mass
comprises, injecting a substance into the cavity.
[0060] In another embodiment, injecting the substance into
the cavity comprises injecting the substance into the cavity
via an annulus formed between an outer surface of the first
catheter and an interior surface of the second catheter.
[0061] In another embodiment, drawing the balloon of the
first catheter towards the balloon of the second catheter
further comprises causing a plurality of ribs or bumps
disposed about the balloon of the first catheter to engage a
portion of a vessel wall.
[0062] In another embodiment, at least an exterior surface
of the first catheter is coated with a non-stick adherent.
[0063] In another embodiment, an apparatus for treating
mitral annulus dilatation includes (a) a stmt having proximal
and distal sections, wherein the proximal and distal sections
-15-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
have a radially contracted state suitable for insertion into a
vessel and radially expanded state in which they are
substantially flush with a vessel wall; and (b) a central
section disposed between the proximal and distal sections,
wherein the central section has a elongated state suitable for
insertion into a vessel and a foreshortened state having a
curvature configured to apply a compressive force to and a
foreshortening force on the mitral valve annulus.
[0064] In another embodiment, one or more biodegradable
structures are disposed on the central section in the
contracted state.
[0065] In another embodiment, the proximal section is
configured to become biologically anchored to a vessel before
the one or more biodegradable structures degrade.
[0066] In another embodiment, the distal section is
configured to become biologically anchored to a vessel before
the one or more biodegradable structures degrade.
[0067] In another embodiment, the central section comprises
a shape memory material.
[0068] In another embodiment, an apparatus for treating
mitral annulus dilatation includes a st m t having proximal and
distal sections, wherein the proximal and distal sections have
a radially contracted state suitable for insertion into a
vessel and radially expanded state in which they have a
diameter greater than the diameter of the vessel wall; and a
central section disposed between the proximal and distal
sections, wherein the central section has an elongated long
state suitable for insertion into a vessel and a foreshortened
state having a curvature configured to apply a compressive
force upon the mitral annulus and a foreshortening force on
the mitral valve annulus.
-16-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
BRIEF DESCRIPTION
OF THE DRAWINGS
[0069] In th e drawings, like reference characters generally
refer to the sa me parts throughout the different views. Also,
the drawings are
not necessarily
to scale, emphasis
generally
being placed upon illustrating the principles of the
invention.
[0070] FIG. 1 is a three-dimensional view of the mitral
valve, coronary sinus and adjacent aortic valve.
[0071] FIG. 2 is a side view of an embodiment of an
elongate body of the present invention including a central
stmt section w ith a backbone and a severed region.
[0072] FIG. 3 is a perspective schematic view of the body
of FIG. 2 in an expanded state.
[0073] FIG. 4 is a cross-sectional view of a mitral valve
and a coronary sinus into which an embodiment of a body of
the
present inventi on and a first balloon have been inserted.
[0074] FIG. 5 is a cross-sectional view of a mitral valve
and a coronary sinus in which proximal and distal sections
of
an embodiment of a body of the present invention have been
expanded and wherein
a balloon has
been inserted
into a
central section of the body.
[0075] FIG. 6 is a side view of an embodiment of an
elongate body of the present invention including a proximal
and a distal tr ansitional section.
[0076] FIG. 7 is a side view of a distal stmt module of
an
embodiment of he present invention.
t
[0077] FIG. 8 is a side view of a proximal stmt module
of
an embodiment
of the present
invention.
[0078] FIG. 9 is a side view of a distal and proximal stmt
module as they may be oriented when inserted into a coronary
sinus.
[0079] FIG. 10 is a flat view of a camel stmt of the
-17-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
present
invention.
[0080] FIG. 11 is a top view of a camel stent embodiment
of
the present vention.
in
[0081] FIG. 12 is a side view of a camel stmt embodiment
of the invention.
present
[0082] FIG. 13 is a three-dimensional view of an exemplary
embodiment an elongate body of the present invention.
of
[0083] FIG. 14 is another three-dimensional view of the
elongate body of FIG. 13 depicted from a different angle.
[0084] FIGS . 15A-15S are side views of further alternative
devices present invention.
of the
[0085] FIG. 16 is a perspective view of an alternate device
of the invention.
present
[0086] FIG. 17 schematically depicts a first state of the
elongate body of FIG. 13.
[0087] FIG. 18 schematically depicts a second state of the
elongate body of FIG. 13.
[0088] FIG. 19 schematically depicts a second state of an
alternate
embodiment
of the
present
invention
having
an outer
elongate body and an inner elongate body positioned inside
the
coronary sinus .
[0089] FIG. 20 is a side view of an embodiment of an
elongate body of the present invention including a proximal
anchor, a distal
anchor
and
a
bridge
having
resorbable
thread
connecting proximal and distal anchors.
the
[0090] FIG. 21 is a detail of the bridge of FIG. 20.
[0091] FIG. 22 is a side view of an embodiment of an
elongate body of the present invention including a proximal
anchor, a dis tal anchor and a central anchor with a bridge
having ble thread connecting the anchors together.
resorba
[0092] FIG. 23 is a side view of an embodiment of an
elongate body of the present invention including a proximal
-18-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
anchor, a distal anchor and two central anchors with a bridge
having resorbable thread connecting the anchors together.
[0093] FIGS. 24A-24D describe a further embodiment of the
present invention.
[0094] FIGS. 25A-25C illustrate exemplary embodiments of
the anchor elements of FIGS. 24A-24D.
[0095] FIGS. 26A-26B illustrate deployment and actuation of
the device of FIGS. 24A-24D.
[0096] FIGS. 27A-27L illustrate alternative embodiments of
the present invention.
[0097] FIGS. 28A-28C illustrate alternative embodiments of
the present invention.
DETAILED DESCRIPTION
[0098] Referring to FIG. 1, a coronary sinus 20 extends
from a right atrium 22 and a coronary ostium 24 and wraps
around a mitral valve 26. The term coronary sinus is used
herein as a generic term to describe a portion of the vena
return system that is situated adjacent to the mitral valve 26
along the atrioventricular groove. The term coronary sinus 20
used herein generally includes the coronary sinus, the great
cardiac vein and the anterior intraventricular vein. A mitral
annulus 28 is a portion of tissue surrounding a mitral valve
orifice to which several leaflets attach. The mitral valve 26
has two leaflets, an anterior leaflet 29 and a posterior
leaflet 31 having three scallops P1, P2 and P3.
[0099] The problem of mitral regurgitation often results
when a posterior aspect of the mitral annulus 28 dilates and
displaces one or more of the posterior leaflet scallops P1, P2
or P3 away from the anterior leaflet 29. To reduce or
eliminate mitral regurgitation, therefore, it is desirable to
move the posterior aspect of the mitral annulus 28 in an
-19-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
anterior direction. For instance, in the specific case of
ischemic mitral regurgitation, the posterior section of the
mitral valve may dilate symmetrically or asymmetrically. In
the case of symmetric dilatation, the dilation is usually more
pronounced in the P2 scallop of the posterior section, while
in the case of asymmetric dilatation, the dilation is usually
more pronounced in the P3 scallop of the posterior section.
Consequently, it is desirable to move the area of the mitral
annulus 28 adjacent to the area of dilatation of the mitral
valve 26 while leaving the remaining section of the mitral
annulus unaltered. The catheter-based devices of the present
invention can be inserted within the coronary sinus 20 to the
proper location so as to perform the desired reshaping
procedure on the mitral annulus 28.
[00100] The following embodiment comprises an elongate body
10, as shown, for example, in FIG. 2. The elongate body 10 is
manufactured by programming a desired pattern into a computer
and cutting the pattern into a tube of stainless steel. The
tube may be, however, cut by any other appropriate means.
FIG. 2 is a "flat pattern" view showing the elongate body 10
cut along its axial length and laid flat.
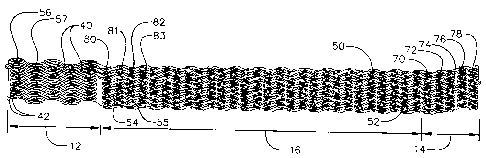
[00101] As shown in FIG. 2, the elongate body 10 has a
proximal stmt section 12, a distal stmt section 14, and a
central stmt section 16. As used herein, "distal" means the
direction of the device as it is being inserted into a
patient's body or a point of reference closer to the leading
end of the device as it is inserted into a patient's body.
Similarly, as used herein "proximal" means the direction of
the device as it is being removed from a patient's body or a
point of reference closer to a trailing end of a device as it
is inserted into a patient's body.
[00102] The distal and proximal stmt sections 14, 12 are
-20-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
used to anchor the body 10 into the distal and proximal ends,
respectively, of the coronary sinus 20. The proximal end of
the coronary sinus is located at or near the coronary sinus
ostium 24. The central stmt section 16 is attached between a
distal end of the proximal stmt section 12 and a proximal end
of the distal stmt section 14 and serves to "foreshorten" the
coronary sinus 20. The reduction in length of a stmt section
when it is expanded is referred to as foreshortening.
(00103] The elongate body 10 has two states, a compressed
state (not shown) and an expanded state, as shown in FIG 3.
In the compressed state, the elongate body 10 has a diameter
that is less than the diameter of the coronary sinus 20 and
the elongate body is generally flexible enough to conform to
the shape of the coronary sinus. In this state, the elongate
body 10 has a substantially uniform diameter of between about
1.5 to 4 mm. In the expanded state, the elongate body 10 has
a diameter that is about equal to or greater than a diameter
of a non-expanded coronary sinus 20. Specifically, in the
expanded state the diameter of the distal stmt section 14 is
between about 3 to 6 mm, the diameter of the proximal stmt
section 12 is between about 10 to 15 mm, and the diameter of
the central stmt section 16 is between about 6 to 10 mm.
[00104] Referring to FIGS. 2 and 3, one embodiment of the
device comprises a tubular elongate body 10 made of stainless
steel in a mesh configuration. The mesh configuration
includes a series of connected stainless steel loops, for
example, 56, 57. In the depicted embodiment, the loops have a
zigzag shape including alternating peaks 42.
[00105] In the depicted embodiment, the proximal stmt
section 12 includes five loops. When a first loop 56 loop is
connected to an adjacent loop 57 at at least two peaks 42, a
four-sided opening 40 is formed. In an exemplary embodiment,
-21-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
the four-sided openings 40 of the proximal stmt section have
a compressed length of about 2 to 10 mm and a height of
essentially 0 to 1 mm.
[00106] As shown in FIG. 2, the distal stmt section 14
includes five loops. A first loop 70 and an adjacent second
loop 72 are connected at each peak 42 to form a ring of four-
sided openings 40. The second loop 72 is partially connected
to a third loop 74 at four peaks 42 and the third loop is
partially connected to a fourth loop 76 at four peaks. The
fourth loop 76 is partially connected to a fifth loop 78 at
two peaks. The number of loops and the number of peaks by
which each loop is connected to an adjacent loop is not
critical and numerous permutations are possible. However, the
distal stmt 14 should be flexible enough to make the body 10
steerable through the coronary sinus 20. In an exemplary
embodiment, the four-sided openings 40 of the distal stmt
section 14 have a compressed length of about 2 to 10 mm and a
height of essentially 0 to 1 mm.
[00107] As further shown in FIG. 2, the central stmt
section 16 separates the proximal stent section 12 and the
distal stmt section 14. The connections between the stmt
sections 12, 14 and 16 are flexible joints to allow the stent
to conform to the local curvature of the coronary sinus 20.
For example, in the depicted embodiment, the central stmt
section 16 is partially connected to the proximal stmt
section 12 at three peaks 42 and it is also connected to the
distal stmt section 14 at three peaks.
[00108] The central stmt section 16 includes twenty-eight
loops. In this section, a first loop 80 is joined to a second
loop 81 at every peak to form a first ring 54. Further, a
third loop 82 is joined to a fourth loop 83 to form a second
ring 55. The adjacent first and second rings 54, 55 are
-22-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
partially connected to each other at three peaks 42. The
central stmt section 16 of the depicted embodiment includes
fourteen rings each partially connected to an adjacent ring at
three peaks. The structure of the rings allows the axis of
the central stmt section 16 to conform to the curvature of
the coronary sinus 20. The region of the central stmt
section 16 that forms continuous four-sided openings 40, i.e.
where the peaks 42 of adjacent rings are connected to each
other, is a backbone 50. The region of the central stmt
section 16 where the rings are not connected to each other is
a severed region 52. In an exemplary embodiment, the four-
sided openings 40 of the central stmt section 16 have a
compressed length of about 2 to 10 .mm and a height of
essentially 0 to 1 mm. Again, the number of loops and the
number of peaks by which each loop is connected to an adjacent
loop is not critical and numerous permutations are possible.
[00109 The device of the first embodiment is deployed as
follows. As shown in FIG. 4, the elongate body 10, in the
compressed state, is mounted onto a first balloon 58, which
acts as a delivery catheter. The first balloon 58 has a
length generally corresponding to the length of the distal
st m t section 14 and is inserted so that it is enveloped by
the distal stmt section. The elongate body 10 and the first
balloon 58 are inserted into the coronary sinus 20 from the
coronary sinus ostium 24, e.g., until the central stent
section 16 is generally aligned with the P2 scallop. Once the
elongate body 10 and the first balloon 58 are positioned in
the coronary sinus, the first balloon is expanded by
introducing, for example, a saline solution through the
delivery catheter and into the balloon. Alternately, any
biocompatible solution may be used to inflate the balloon.
The force of the expansion of the first balloon 58 expands the
-23-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
distal stmt section 14 so that its circumference is forced
against the circumference of the coronary sinus 20 and anchors
it into the wall of the coronary sinus. Once the distal stmt
section 14 is anchored, the first balloon 58 is deflated and
removed.
[00110] A second balloon (not shown) having a length
generally corresponding to the length of the proximal stmt
section 12 is then inserted into the elongate body 10 so that
it is enveloped by the proximal stmt section. The second
balloon is then expanded as above using a saline solution to
fill the balloon. The expansion force of the second balloon
expands the proximal stm t section 12 so that its
circumference is forced against the coronary sinus 20 and
anchors it to the wall of the coronary sinus. The second
balloon is then deflated and removed. In one embodiment, the
proximal stmt section 12 is sized such that expansion of the
proximal stmt section makes it into a funnel shape adjacent
to the right atrium 22. The funnel shape conforms to the
coronary sinus ostium 24 to help secure the proximal stent
section 12 in place.
[00111] Although the described method of deployment and
expansion of the stmt sections involves expanding the distal
section prior to expanding the proximal section, it will be
appreciated that the proximal section may be expanded prior to
the distal section. In addition, the same balloon or
different balloons, or balloons shorter or longer than the
proximal and distal stmt sections may be used as desired.
[00112] Once both the proximal and distal stmt sections 12,
14 have been expanded and anchored to the coronary sinus 20, a
third balloon 62 is inserted into the elongate body 10 so that
it is enveloped by the central stmt section 16 as shown in
FIG. 5. The third balloon 62 has a length generally
-24-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
corresponding to the length of the central stm t section 16.
The central stent section 16 is then expanded by filling the
third balloon 62 with a saline solution. The severed regions
52 of the central stmt section 16 allow the body 10 the
flexibility to generally conform to the shape of the coronary
sinus 20 as the body expands.
(00113] In an alternate embodiment, a shorter balloon may be
used to expand the central stmt section 16 in sections to
achieve the desired diameters along the central stmt section.
By expanding the central stmt section 16 in sections, the
amount of foreshortening of the coronary sinus 20 can be more
accurately adjusted.
[00114] When the central stmt section 16 expands, the
length of the four-sided openings 40 is reduced as the height
of the four-sided openings is increased. The body 10 is
designed such that when it is expanded, it has a curved shape
that generally follows the anatomical curvature of the
coronary sinus 20. Additionally, as a result of the reduction
in the length of the four-sided openings 40, the length of the
entire central stmt section 16 is foreshortened. The
foreshortening of the central stmt section 16 pulls the
distal stmt section 14 and the proximal stent section 12
toward each other. As a result, the distance between the
proximal and distal stmt sections 12, 14 is reduced. Since
the proximal and distal stmt sections 12, 14 are anchored to
the walls of the coronary sinus 20, the length of the coronary
sinus is thereby also reduced. The reduction in length of the
coronary sinus 20 cinches the coronary sinus more tightly
around the P1, P2 and P3 scallops of the mitral valve 26 and
pushes one or more of the scallops, closer to the anterior
leaflet 29 of the mitral valve. This allows a gap between the
anterior leaflet 29 and the P1, P2 and P3 scallops of the
-25-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
posterior leaflet 31 to close. When the gap between the
mitral valve leaflets is closed, the effects of mitral valve
regurgitation are drastically reduced or eliminated.
[00115] A second embodiment of the elongate body is shown in
FIG. 6. In this embodiment, an elongate body 110 has a mesh
configuration similar to that described with respect to the
previous embodiment. In addition to a distal stmt section
114, a proximal stmt section 112, and a central stmt section
116, the second embodiment also includes a distal transitional
section 120 and a proximal transitional section 118. The
distal and proximal stmt sections 114, 112 are used to anchor
the body 110 into the distal and proximal ends, respectively,
of the coronary sinus 20. The distal and proximal
transitional sections 120, 118, located between the central
stmt section 116 and the distal and proximal stmt sections
114, 112, respectively, provide a flexible transition zone for
improved load distribution. In addition, the transitional
sections 112 and 120 may experience significant foreshortening
during expansion providing the additional benefit of coronary
sinus contraction.
[00116] The second embodiment is similar to the first
embodiment in that it has two states, a compressed state and
an expanded state. Further, the structure of the proximal and
distal stmt sections 112, 114 are identical to those of the
first embodiment. The purpose of these flexible stmt
sections 112 and 114 is to provide a large conforming contact
area between the stmt and the outer wall of the coronary
sinus 20 which better distributes the force exerted on the
body 110 by the vessel wall. The central st m t section 116
includes eighteen loops to form seventeen rings of four-sided
openings 40. Since each ring of the central stmt section 116
of the second embodiment is connected to the ring adjacent to
-26-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
it at each peak 42, the rings form a continuous mesh
configuration.
[00117] The proximal transitional section 118 of the second
embodiment is connected to the distal end of the proximal
stmt section 112 and the proximal end of the central stmt
section 116. The proximal transitional section 118 includes
two loops. As shown in FIG. 6, a first loop 170 is connected
to a most distal loop 171 of the proximal st m t section 112 at
three peaks 42 and a second loop 172 is connected to a most
proximal loop 173 of the central stmt section 116 at three
peaks. The first loop 170 is also connected to the second
loop 172 at three peaks 42 along the same axis as it is
connected to the proximal and central stmt sections 112, 116,
thus forming a backbone 50 and a severed region 52 for
flexibility similar to the central st mt section 116 of the
first embodiment. It will be appreciated that a fewer number
or greater number of loops may be used in the proximal
transitional section 118, or no loops, wherein the proximal
stmt section 112 is connected to the central stm t section
116.
[00118 As also shown in FIG. 6, the distal transitional
section 120 is located between a distal end of the central
stmt section 116 and a proximal end of the distal stent
section 114. Specifically, a most proximal loop 174 in the
distal transitional section 120 is partially connected to a
distal-most loop 179 in the central stmt section 116 at three
peaks and a distal-most loop 181 in the distal transitional
section 120 is partially connected to a proximal-most loop 180
in the distal stmt section at three peaks. The distal
transitional region 120 includes ten loops. The first loop
174 in the distal transitional section 120 is joined to a
second loop 175 at every peak to form a first ring 154.
-27-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
Further, a third loop 176 is joined to a fourth loop 177 to
form a second ring 155. The adjacent rings 154 and 155 are
partially connected to each other at three peaks 42. The
distal transitional section 120 of the present embodiment
includes five such rings each connected to an adjacent ring at
three peaks. The region that forms continuous four-sided
openings 40 is a backbone 50 and the region where the rings
are not connected is a severed region 52. It will be
appreciated that a fewer number or greater number of loops may
be used in the distal transitional section 120, or no loops,
wherein the distal stent section 114 is connected to the
central stmt section 116.
[00119] The proximal and distal stmt sections 112 and 114
of the second embodiment are deployed as described above with
respect to the first embodiment. The elongate body 110 is
positioned in the coronary sinus 20 so that the central stmt
section 116 is generally aligned with the P2 scallop of the
posterior leaflet 31 of the mitral valve 26. In an alternate
embodiment, the distal stmt section 114 may be of increased
flexibility to allow for placement in the proximal region of
the great cardiac vein (not shown). In addition, the same
balloon or different balloons, or balloons shorter or longer
than the proximal and distal st m t sections may be used as
desired.
[00120] Once both the proximal and distal stmt sections
112, 114 are balloon expanded and anchored to the coronary
sinus 20, a third balloon (not shown) having a length
generally corresponding to the combined lengths of the central
stmt section 116, the proximal transitional st m t section 118
and the distal transitional stmt section 120 is inserted into
the elongate body 110 so that it is enveloped by all three
stent sections 116, 118 and 120. These three sections 116,
-28-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
118, 120 are then expanded using the third balloon. As the
central stmt section 116 is expanded, its rigidity
straightens a central section of the coronary sinus. As the
coronary sinus 20 straightens, the P1, P2 and/or P3 scallops,
of the mitral valve 26 are moved anteriorly, thereby closing
the gap between the scallops and the anterior leaflet 29 of
the mitral valve 26. Additionally, expanding the central
stmt section 116 and the proximal and distal transitional
sections 118, 120 foreshortens the elongate body 110, reducing
the distance between the proximal and distal stmt sections
112, 114 and cinching the coronary sinus 20 more tightly
around the P1, P2 and P3 scallops. The severed region 52 of
the transitional sections 118, 120 allows the elongate body
110 the flexibility to generally conform to the curvature of
the coronary sinus 20 as the body expands.
[00121] Alternatively, a shorter balloon may be used to
expand the central stent section 116, proximal transitional
section 118 and distal transitional section 120 in steps to
achieve the desired diameters along the central stmt section
116. By expanding the central stmt section 116 in parts, the
amount of foreshortening and straightening of the coronary
sinus 20 can be better adjusted.
[00122] Inserting a stmt deep into the coronary sinus 20
toward the anterior intraventricular vein may sometimes be
difficult because of the curved shape of the distal region of
the coronary sinus. Therefore, the distal part of a device
insertable into the coronary sinus 20 needs to be flexible.
One possible way to achieve a more flexible stmt is to reduce
the wall thickness of a stmt and provide for a more flexible
design of the stmt. On the other hand, using two overlapping
stems allows for a flexible stmt in the curvy distal region
of the coronary sinus 20 and a stronger, more rigid part in
-29-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
the proximal region. More specifically, the area where two
stems overlap will have a higher radial strength and become
more rigid when it is expanded. This rigidity in turn will
provide a more effective straightening effect in the desired
area of the coronary sinus 20.
[00123] In that regard, a third embodiment of the present
invention, as shown in FIGs. 7 and 8, comprises a proximal
stmt module 200 (FIG. 8) and a distal stmt module 205 (FIG.
7). Both the proximal and distal st m t modules 200, 205 have
a compressed and expanded state, as described above with
respect to the previous embodiments.
[00124] In one embodiment, the distal stmt module 205 has
an anchor section 214, located at the distal end of the distal
stmt module, and a central section 217. The anchor section
214 includes three loops. A first loop 270 is connected to a
second loop 271 at four peaks 42 and the second loop is
connected to a third loop 272 at two peaks. Accordingly, the
distal stmt module will be more flexible in the distal
direction. The central stmt section 217 includes thirty-six
loops. As with respect to the first embodiment described
above, alternating pairs of loops are connected at each peak
to form rings of four-sided openings 40. Each ring is
connected to an adjacent ring at three peaks, where the
connected portion forms a backbone 250 and the unconnected
portion forms a severed region similar to the central stmt
section 16 of the first embodiment. FIGS. 7 and 8 both
include lines 220 in places of the modules 200 and 205 where
larger pieces of material will be removed by laser cutting.
These single lines 220 represent a cut to be made by the laser
that will allow the large pieces of material to be more easily
removed while leaving the remaining material undamaged.
[00125] As shown in FIG. 8, the proximal stmt module 200
-30-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
has an anchor section 212, located at the proximal end of the
proximal stent module 200, and a central section 215. The
anchor section 212 is a combination of the proximal stmt
section 112 and the proximal transitional section 118 as
described above with respect to the second embodiment. The
central section 215 includes twenty-four loops. Similarly to
the central section 217 of the distal stmt module 205,
alternating pairs of loops are connected at each peak to form
rings of four-sided openings 40. Each ring is connected to an
adjacent ring at three peaks 42, where the connected portion
forms a backbone 254 and the unconnected portion forms a
severed region.
[00126] The device of the third embodiment is deployed as
follows. The distal stmt module 205 in a compressed state is
mounted onto a first balloon (not shown), which acts as a
delivery catheter. The first balloon has a length generally
corresponding to the length of the anchor section 214 and is
inserted so that it is enveloped by the anchor section. The
distal st m t module 205 and the first balloon are inserted
into the coronary sinus 20 from the coronary sinus ostium 24
so that the central section 215 is generally aligned with,
e.g., the P2 scallop. Once the distal stmt module 205 and
the first balloon are positioned in the coronary sinus 20, the
first balloon is expanded by introducing a saline solution
through the delivery catheter and into the balloon. The
balloon expands the distal stmt module 205 so that the
module's circumference is forced against to the circumference
of the coronary sinus 20 and so that the module is anchored to
the wall of the coronary sinus. Once the distal stmt module
205 is anchored, the first balloon is deflated and removed.
[00127] A second balloon (not shown) is then mounted on the
proximal stmt module 200, the second balloon having a length
-31-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
corresponding to the length of the anchor section 212. The
proximal stmt module 200 and the second balloon are then
inserted into the coronary sinus so that the central section
215 of the proximal stmt module 200 overlaps the central
section 217 of the distal stmt module 205 by at least about 2
cm. Further, as shown in FIG. 9, upon insertion, the backbone
250 of the proximal stmt module 200 is angularly separated
from the backbone 254 of the distal stmt module 205 depending
on the anatomy of the patient and the desired rigidity of the
overlapping section. Although the backbones 250 and 254 may
be aligned, in alternate embodiments the backbones are
separated by about 60°-180°. The closer the backbones 250,
254 are together, the less rigid the overlapping section will
be. On the other hand, if the backbones 250 and 254 are
spaced 180° apart, the overlapping section will be as rigid as
possible and able to provide the most strength to straighten
the coronary sinus 20.
[00128] Once the proximal stmt module 200 is in place, the
second balloon 260 is expanded using a saline solution to fill
the balloon. The balloon expands the proximal stmt module
200 so that the module's circumference is forced against the
circumference of the coronary sinus 20 and so that the module
is anchored to the wall of the coronary sinus. Once the
proximal stmt module 200 is anchored, the second balloon is
deflated and removed. In addition, the same balloon or
different balloons, or balloons shorter or longer than the
proximal and distal stmt sections may be used as desired.
[00129] Once the proximal and distal stmt modules 200, 205
have been anchored in the coronary sinus, a third balloon (not
shown) is inserted. The third balloon has a length generally
corresponding to the entire length of the combined central
sections 215 and 217, i.e., the balloon extends the entire
-32-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
distance between the anchor sections 212 and 214. The third
balloon is then expanded using a saline solution, and such
expansion simultaneously expands the central sections 215 and
217 so that these sections have a circumferences of
approximately the circumference of the coronary sinus 20. The
proximal and distal stmt modules 200, 205 effectively become
one stmt as they expand due to the overlapping region of the
central stmt sections 215 and 217 becoming secured together
as a result of the proximal stmt module 200 expanding into
the distal stmt module 205. The expanded central sections
215, 217 serve to straighten the coronary sinus 20 and push
the posterior leaflet 31 of the mitral valve 26 anteriorly.
Further, expanding the central sections 215 and 217
foreshortens the "combined" stmt and cinches the coronary
sinus around the Pl, P2 and/or P3 scallops, of the posterior
leaflet 31.
[00130] A fourth embodiment of the invention comprises a
"camel" stmt 310. The camel stmt is an elongate tubular
member having two diametrically opposed spines 320 and 322.
FIG. 9 is a "flat pattern" view showing the camel stmt 310
cut along its axial length and laid flat. In this case, the
stmt 310 has been cut along one spine 322 of the two spines
320, 322 running the length of the stmt. In an exemplary
embodiment, the length of the stmt 310 is about 40 to 120 mm.
The stmt 310 includes two stainless steel loops 354 and 356,
each loop having a zigzag shape with alternating peaks 42.
One loop 354 is located at a proximal end 312 and one loop 356
is located at a distal end 314 of the stmt 310. Extending
between the loops 354 and 356 are the two spines 320 and 322
spaced 180° apart. In a proximal half of the stmt 310,
angularly extending about one quarter the length of the stent
from the first spine 320 to the second spine 322 are first and
-33-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
second interconnecting members 324, 326. At the location
where the first two interconnecting members 324, 326 meet the
second spine 322, a third and a fourth interconnecting member
328, 330 extend angularly about one quarter of the length of
the stmt 310 from the second spine 322 to the first spine
324. The third and fourth interconnecting members 328, 330
meet the first longitudinal member 320 at about the middle of
the camel stmt 310. The distal half of the stmt 310 is a
mirror image of the proximal half, the distal half having two
interconnecting members 332, 334 that extend from the first
spine 320 to the second spine 322 and two interconnecting
members 336, 338 extend from the second spine 322 to the first
spine 320.
[00131] On the proximal half of the st mt extending between
the first and second interconnecting members 324, 326 bisected
by the second spine 322 are four strands 311 of zigzag shaped
stainless steel having at least one peak 42. Similarly, there
are four strands 311 extending between the first and second
interconnecting members 324, 326 bisected by the first spine
320. Further, four strands 311 extend between the third and
fourth interconnecting members 328, 330 and are bisected by
the second spine 322 and four strands are bisected by the
first spine 320. The structure of the distal half of the
stmt 310 is a mirror image of the structure of the proximal
half of the stmt.
[00132] The camel stmt 310 has two states, a compressed
state and an expanded state. In the compressed state, the
camel stmt 310 has a diameter that is less that the diameter
of the coronary sinus 20 and the stmt is flexible enough to
be suitably located in the coronary sinus. In this state, the
camel stmt 310 has a substantially uniform diameter of about
1.5 to 4 mm. In the expanded state, as shown in FIGS. 11 and
-34-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
12 the camel stmt is generally "w" shaped and has a diameter
of about 4 to 12 mm.
[00133] The camel stmt 310 is deployed as follows. The
camel stmt is mounted on a balloon catheter (not shown). The
balloon has a length generally corresponding to the entire
length of the camel stmt 310. The camel stmt 310 and the
balloon are inserted into the coronary sinus 20 from the
coronary sinus ostium 24 so that the center of the stent is
generally aligned, e.g., with the P2 scallop. Once the stent
310 is positioned in the coronary sinus 20, the balloon is
expanded using a saline solution, as described above. The
expansion of the zigzag shaped strands 311 and the structure
of the spines 320, 322 and interconnecting members 324, 326,
328, 330, 332, 334, 336 and 338 causes the expanded stent 310
to have a substantially w-shaped structure.
[00134] The "w" shape of the camel stmt 310 in its expanded
state anchors the camel stmt inside the coronary sinus 20.
Further, since the center of the stmt 310 is adjacent to the
P2 scallop, it pushes the P2 scallop anteriorly, thereby
closing the gap between the anterior leaflet 29 and posterior
leaflet 31 of the coronary sinus 20. In other embodiments,
the design of the camel stmt 310 may be modified to have only
a single bend, two bends or more than three bends and/or may
have a nonuniform diameter. Additionally, the camel stmt 310
may be part of a stmt system having proximal and distal stmt
sections.
[00135] FIG. 13 shows yet another embodiment of the
invention comprising an elongate body 1300. In this
embodiment, the elongate body 1300 self expands into a three
dimensional shape that conforms to the anatomy of the coronary
sinus, thereby applying substantially uniform stress to the
walls of the coronary sinus 20. Such expansion of the
-35-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
elongate body 1300 achieves remodeling of the mitral annulus
through foreshortening, which reduces the overall length of
the coronary sinus 20 and, in turn, reduces the circumference
of the mitral annulus 28.
[00136] As illustrated in FIG. 1, the coronary sinus 20 is a
curved tubular structure that enwraps the posterior leaflet 31
of the mitral valve 26 with scallops P1, P2, and P3. The
coronary sinus 20, as shown, has a central portion Y located
in an x-y plane defining the annulus of the mitral valve 26.
A proximal portion of the coronary sinus 20 extends slightly
upwardly out of the x-y plane towards the coronary ostium 24
of the right atrium 22. A distal portion X of the coronary
sinus 20 extends downwardly behind the P1 scallop out of the
x-y plane into the great cardiac vein and anterior
interventricular vein.
[00137] The diameter of the coronary sinus 20 decreases from
the proximal end to the distal end of the coronary sinus 20.
The diameter of the central section of the coronary sinus 20
remains generally uniform throughout its length.
[00138] FIG. 13 illustrates a three-dimensional view of an
embodiment of the elongate body 1300 in its unstressed,
natural state. The elongate body 1300 is compressible to
permit insertion into the coronary sinus 20 percutaneously and
has the ability to self expand into a three-dimensional shape
to conform to the anatomy of the coronary sinus 20. The
elongate body 1300 has a proximal stmt section 1305, a
central stmt section 1310, and a distal st m t section 1315,
each of which conforms generally in size and shape to the part
of the coronary sinus 20 into which it will be inserted. In
one exemplary embodiment, in its unstressed state, the
diameter of the elongate body 1300 along its length is greater
than the diameter of the coronary sinus 20 along its length
-36-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
for reasons to be discussed below. The proximal and distal
stmt sections 1305 and 1315 are used to anchor the elongate
body 200 into the proximal and distal ends, respectively, of
the coronary sinus 20. The central st m t section 1310 is
attached between a distal end of the proximal stmt section
1305 and a proximal end of the distal stm t section 1315.
After the elongate body is deployed in the coronary sinus, the
central stmt section 1310 is located in the x-y plane shown
in FIG. 13 generally aligned, for example, with the P2 scallop
along the posterior leaflet 31 of the mitral valve 26 (FIG.
The proximal ste m section 1305 extends slightly upwardly out
of the x-y plane towards the coronary ostium 24. The distal
stmt section 1315 extends downwardly behind the P1 scallop
extending out of the x-y plane into the great cardiac vein.
(00139] FIG. 14 illustrates another three-dimensional view
of the embodiment of the elongate body 1300 depicted from a
different angle wherein the viewer is looking into the
proximal end of the elongate body. As shown in FIG. 14, to
better emulate the slight upward extension of the proximal
portion of the coronary sinus 20, the end of the proximal
stmt section 1305 slightly bends and faces upward. Moreover,
the slightly upward facing end of the proximal stmt section
1305 and the downward facing end of the distal stmt section
1315 of the elongate body 1300 flare out in a funnel shape to
securely anchor the elongate body to the wall of the coronary
sinus 20.
[00140] To match with the varying diameters of the coronary
sinus 20, the diameter of the elongate body 1300 decreases
from the proximal stmt section 1305 to the distal stmt
section 1315 and the diameter of the central stmt section
1310 remains generally uniform. In one embodiment, for the
elongate body 1300 having the initial total length of about
-37-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
155 mm, the proximal stmt section 1305 has the diameter of
about 22 mm, the central stmt section 1310 has the diameter
of about 6 mm, the distal stmt section 1315 has the diameter
of about 11 mm in its unstressed state. In another embodiment
of the elongate body 1300 also having the initial total length
of about 155 mm, the proximal stmt section 1305 has the
diameter of about 21 mm, the central stent section 1310 has
the diameter of about 8 mm and the distal stmt section 1315
has the diameter of about 19 mm in its unstressed state.
[00141] Furthermore, referring again to FIG. 13, to conform
with a radial arc of the coronary sinus along the x-y plane of
the P2 scallop, a radial arc 1320 of the central stmt section
1310 of the elongate body 1300 arches along the x-y plane in
the range of 90 to 150 degrees in its unstressed state.
[00142] Referring again to FIG. 13, the elongate body 1300
has a multi-filament woven structure made from shape metal
with .memory effect, such as, but not limited to, Nitinol,
Elgiloy, or spring steel. The self-expansion force and the
anchoring force of the elongate body 1300, which affects the
degree of foreshortening of the coronary sinus 20, is
controlled by various factors, such as the angle of the weave
(i.e., intersection of the strands), the thickness of the
material, and the spacing between the strands. For example,
depending on the angle of the weave, the degree of expansion
and anchoring forces may vary. And, depending on the degree
of expansion and anchoring forces exerted onto the wall of the
inside surface of the coronary sinus 20, which results in
reshaping of the wall, the diameter and the length of the
coronary sinus 20 will gradually change over a period of time.
For example, a smaller angle of weave (i.e., tight weaving)
generally exerts greater expansion force as the elongate body
1300 expands. Moreover, due to its spring-like configuration,
-38-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
when the elongate body 1300 is compressed along the
longitudinal axis of the elongate body 1300, the angle of the
weave also tightens or reduces, preferably close to 0 degrees.
However, when the elongate body 1300 is released or expanded
along the longitudinal axis of the elongate body 1300, the
angle of the weave expands, for example, in the range of 45 to
90 degrees radially along the longitudinal axis, to retain its
original shape. As the angle of the weave expands further in
the radial direction along the longitudinal axis of the
elongate body 1300, the expansion force weakens.
[00143] With regard to the thickness of the material,
thicker material exerts greater expansion force as the
elongate body 1300 transforms from its compressed state to the
expanded state. With regard to the spacing between the
strands, smaller spacing between the strands requires a
greater number of strands in the elongate body, resulting in
greater expansion force as the elongate body 1300 transforms
from its compressed state to the expanded state. At the same
time, it is important to select a material and control the
above-mentioned factors to ensure a smooth surface of the
elongate body 1300 that minimizes trauma to the coronary sinus
20.
[00144] As briefly mentioned above, the elongate body 1300
has two states, a compressed state and an expanded state, as
shown in FIGs. 17 and 18, respectively. Referring to FIG 17,
in the compressed state, the elongate body 1300 is enclosed
within a lumen 1505 of a sheath 1500 and is inserted into the
coronary sinus 20 via the sheath 1500, which acts as a
delivery catheter. The elongate body 1300, still enclosed
within the lumen 1505 is positioned in the coronary sinus 20
so that the central stmt section 1310 is generally aligned,
for example, with the P2 scallop. In the compressed state,
-39-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
the elongate body 1300 has a diameter that has been compressed
to fit into the lumen 1505 and is flexible enough to move with
the sheath 1500 along the curvatures of the coronary sinus 20.
In this state, the elongate body 1300 has a uniform diameter
that ranges from about 1.5 to 4 mm as it is enclosed within
the lumen 1505.
[00145] Referring to FIG. 18, the sheath is pulled from the
elongate body 1300 to expose the elongate body 1300 to the
walls of the coronary sinus 20 and to allow it to expand into
a three-dimensional shape that conforms to the anatomy of the
coronary sinus 20. As the elongate body 1300 expands, the
strands of the weave of the three-dimensional shape make
contact with the circumference of the coronary sinus 20 and
the entire length of the elongate body 1300 anchors tightly
onto the wall of the inside surface of the coronary sinus 20.
In addition to the anchoring provided by the woven structure
of the elongate body 20, the funnel-shaped flare ends and
slight bend of the proximal and distal stmt sections 1305,
1315 provide further anchoring of the elongate body 1300. In
one embodiment, the flare end of the proximal stent section
1305 expands against the circumference of the coronary sinus
ostium 24 and the flare end of the distal stmt section 1315
expands against the circumference at the distal end of the
coronary sinus 20.
[00146] As discussed above, the elongate body 1300 is
designed so that when it is expanded, it has a curved shape
that follows the anatomical curvature of the coronary sinus 20
and makes substantial contact with the walls along the inside
of the arcuate path of the coronary sinus 20. The expansion
force of the elongate body 1300, which has been determined by
various factors such as the angle of the weave, continues to
push the walls of the coronary sinus 20 radially outward and
-40-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
pull the ends of the elongate body 1300 toward the central
section 1310 of the elongate body 1300. Over a period of
time, e.g. several weeks, the diameter elongate body continues
to expand. As the elongate body 1300 expands, radially, it
gradually grows through the wall of the coronary sinus 20 and
attaches to scar tissue created by the elongate body's
penetration of the wall of the coronary sinus (FIG. 16).
Radial expansion of the elongate body 1300 through the wall of
the coronary sinus 20 foreshortens the coronary sinus and also
reduces the radius of curvature of the coronary sinus. Such
changes in the coronary sinus 20 cinches the coronary sinus
more tightly around the P1, P2 and P3 scallops of the mitral
valve 26 and pushes one or more of the scallops, closer to the
anterior leaflet 28 of the mitral valve. This allows a gap
between the anterior leaflet 29 and the P1, P2 and P3 scallops
of the posterior leaflet 31 to close and achieve remodeling of
the mitral annulus 28 over the span of several weeks. When
the gap between the mitral valve leaflets is closed, the
effects of mitral valve regurgitation are drastically reduced
or eliminated. The elongate body 1300 may be coated with
antithrombogenic material to prevent thrombosis and occlusion
of the coronary sinus, which may occur in the remodeling of
the coronary sinus.
[00147] FIGS. 15A to 15S in general show various additional
embodiments of the present invention.
[00148] Referring now to FIGS. 15A-15C, a further
alternative embodiment of the present invention is described,
in which the device comprises a tapered stmt having proximal
and distal sections that are joined by a central section
capable of assuming a predetermined curvature. In FIG. 15A,
elongate body 1300 includes a wire mesh stmt having proximal
stmt section 1305, distal stmt section 1315 and central
-41-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
stmt section 1310, and is designed to conform to the taper of
the coronary sinus. In FIG. 15A, the elongate body 1300 is
shown in its elongated and radially crimped state. Elongate
body 1300 is shown in its fully radially expanded and axially
foreshortened state in FIG. 15C. Further in accordance with
the principles of the present invention, elongate body 1300
includes one or more biodegradable structures 858, such as
sutures, disposed on central stmt section 1310 to retain that
section in the contracted shape for a predetermined period
after placement of the device in a patient's coronary sinus.
Examples of biodegradable structures are described in more
detail below.
[00149] Elongate body 1300 also includes at least one
proximal retaining element 853 that retains proximal stmt
section 1305 in a contracted state, and further includes at
least one distal retaining element 855 that retains distal
stmt section 1315 in a contracted state. Proximal and distal
retaining elements 853 and 855 may comprise one or more
sutures disposed about proximal and distal sections 1305 and
1315, respectively. Proximal and distal retaining elements
853 and 855 may be coupled to distal ends of strands 863 and
865, respectively. A physician may actuate strands 863 and
865, e.g., by retracting proximal ends of the strands, to
deploy proximal and distal sections 1305 and 1315,
respectively, as shown in FIG. 15B.
[00150] Proximal and distal sections 1305 and 1315 may
comprise a shape-memory alloy, such as Nitinol, that self
expands to a predetermined shape when retaining elements 853
and 855 are removed.
[00151] In another embodiment of the present invention as
shown in FIGS. 15D - 15F, the central stmt section 1310 of
the elongate body 1300 delivered in a restraining catheter has
-42-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
a restraining thread 867 extending outside of the vasculature
and the patient to be retracted by the physician at the
desired time. Retraction of the restraining thread 867 will
allow the central section 1310 of the elongate body 1300 to
expand radially.
[00152] Additionally, as shown in FIGS. 15G - 15I, a single
restraining thread 869 may cover the entire elongate body
1300. The thread may be wrapped around the elongate body 1300
in such a way that, when it is retracted by the physician, it
unravels from the proximal end 1305 to the distal end 1315 of
the elongate body 1300. Alternatively, as shown in FIGS. 15J
- 15L, the single restraining thread 869 may be wrapped around
the elongate body 1300 in such a way that, when it is
retracted by the physician, it unravels from the distal end
854 to the proximal end 152 of the elongate body 1300. Such
restraint, as described by at least the last two embodiments,
makes a restraining catheter unnecessary. Alternatively,
retaining elements 853 and 855 may be omitted, and proximal
and distal sections 1305 and 1315 may self-expand to the
predetermined shape upon retraction of a constraining sheath.
[00153] In yet another embodiment of the present invention,
as shown in FIGS. 15M - 15P, a restraining catheter 881 is
placed over the elongate body 1300 before the device is
inserted into a patient. Additionally, a biodegradable
restraining thread 858 is placed around the central stmt
section 1310 of the elongate body 1300. When the restraining
catheter 881 is removed, the proximal and distal stmt
sections 1305, 1315 of the elongate body 1300 expand
immediately, while the central stmt section 1310 will expand
over time as the restraining thread 858 is absorbed by the
body. Alternatively, as shown in FIGS. 15Q - 155, only a
restraining catheter 881 is placed over the elongate body
-43-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
1300. Thus, as the restraining catheter is retracted, the
elongate body 1300 expands immediately from the distal end
1315 to the proximal end 1305.
[00154] In one exemplary embodiment, all three sections
1305, 1310, 1315 of the stmt are integrally formed from a
single shape memory alloy tube, e.g., by laser cutting. The
sections. 1305, 1310, 1315 are then processed, using known
techniques, to form a self-expanding unit. In another
embodiment, the device may be braided from Nitinol, stainless
steel or other metal alloy threads and cut to the appropriate
length. Such braiding permits the creation of three
dimensional shapes, allowing the device to more closely
15. conform to the shape of the coronary sinus.
[00155] Unlike some of the preceding embodiments, which rely
upon drawing proximal and distal elements together at the time
of deploying the device, this embodiment of the present
invention permits proximal and distal sections 1305 and 1315
to become biologically anchored in the venous vasculature
before those sections are drawn together by expansion and/or
curvature of central stmt section 1310 to remodel the mitral
valve annulus.
[00156] The elongate body 1300 may be deployed as follows.
Elongate body 1300 is loaded into a delivery sheath and
positioned within the patient's coronary sinus. The delivery
sheath then is retracted proximally to expose distal stmt
section 1315, as shown in FIG. 15B. Distal stent section 1315
may be deployed when the proximal end of strand 865, which is
coupled to retaining element 855, is actuated by a physician.
Alternatively, retaining element 855 may be omitted and distal
stmt section 1315 may self-expand upon retraction of the
delivery sheath. Upon deployment using either technique,
-44-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
distal stmt section 1315 radially expands to engage the
intima of the coronary sinus.
[00157] The delivery sheath is then further proximally
retracted to expose proximal stmt section 1305 as shown in
FIG. 15B. Proximal stmt section 1305 may be deployed when
strand 863, which is coupled to retaining element 853, is
actuated by a physician. Alternatively, retaining element 853
may be omitted and proximal stmt section 1305 may self-expand
upon further retraction of the delivery sheath. Upon
deployment using either technique, proximal st mt section 1305
radially expands to engage the intima of the coronary sinus.
[00158] At the time of deployment of proximal and distal
sections 1305 and 1315, central stmt section 1310 is retained
in a contracted state by biodegradable structures 858,
illustratively biodegradable sutures, e.g., a poly-glycol
lactide strand or VICREL suture, offered by Ethicon, Inc., New
Brunswick, NJ, USA.
[00159] Over the course of several weeks to months, proximal
and distal sections 1305 and 1315 of the stmt will
endothelialize, i.e., the vessel endothelium will form a layer
that extends through the apertures in the proximal and distal
sections of elongate body 1300 and causes those sections to
become biologically anchored to the vessel wall. This
phenomenon may be further enhanced by the use of a copper
layer on the proximal and distal stmt sections, as this
element is known to cause an aggressive inflammatory reaction.
Conversely, to reduce thrombosis on the central stmt section
1310 of the stent 850, the central section and associated
structures may be coated with an anticoagulant material. As a
further alternative, the central section of the st m t may be
coated with a taxol derivative or other elutable drug.
-45-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
[00160] Over the course of several weeks to months, or after
the proximal and distal sections have become anchored in the
vessel, biodegradable structures 858 that retain central stmt
section 1310 in the contracted state will biodegrade.
Eventually, the self-expanding force of the central section
will cause the biodegradable structures to break, and release
central stmt section 1310. Because central stmt section
1310 is designed to assume a predetermined curvature as it
expands radially, it causes the proximal and distal sections
1305 and 1315 of elongate body 1300 to curve accordingly,
resulting in the fully deployed shape depicted in FIG. 15C.
The forces created by expansion and curvature of central stmt
section 1310 thereby compressively loads, and thus remodels,
the mitral valve annulus.
[00161] In an alternative embodiment, as shown in FIG. 16,
the elongate body 1300 is "oversized." In other words, the
elongate body 1300 is manufactured deliberately to be larger
than the natural size of the coronary sinus, even in the
coronary sinus' most expanded state. Thus, as the elongate
body 1300 expands, it slowly passes through the wall of the
coronary sinus, causing the coronary sinus to form tissue and
grow around the device. Since the device "outgrows" the
coronary sinus, additional foreshortening may be achieved and
the mitral valve annulus will be able to be more remodeled
than with an ordinary sized device.
[00162] Biodegradable sutures may be designed to rupture
simultaneously, or alternatively, at selected intervals over a
prolonged period of several months or more. In this manner,
progressive remodeling of the mitral valve annulus may be
accomplished over a gradual period, without additional
interventional procedures. In addition, because the
collateral drainage paths exist for blood entering the
-46-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
coronary sinus, it is possible for the device to accomplish
its objective even if it results in gradual total occlusion of
the coronary sinus.
[00163] Another embodiment of the present invention, as
shown in FIG. 19, comprises an outer elongate body 1700 and a
rigid inner elongate body 1705 placed inside of the outer
elongate body 1700 and eventually tightly fitted onto the wall
of the inside surface of the outer elongate body 1700. The
outer elongate body 1700 is flexible such that it can evenly
distribute the expansion forces along the wall of the coronary
sinus 20 during the foreshortening of the coronary sinus 20.
For example, elongate body 1300 described in FIG. 13 may be
used. The rigid inner elongate body 1705, which is placed
inside of the outer elongate body 1700 and has the length in
the range of 30 mm to 80 mm in its unstressed state, provides
higher radial strength and rigidity to further straighten the
coronary sinus 20 and to exert greater force onto the mitral
annulus 28, in addition to the foreshortening provided by the
outer elongate body 1700 (shown by the arrows 1730 in FIG.
19). To provide sufficient rigidity with an effective
straightening effect, the inner elongate body 1705 is made of
a rigid metal, such as stainless steel. In one configuration,
the inner elongate body 1705 is a tubular structure made of
stainless steel in a mesh configuration. The mesh
configuration includes a series of connected stainless steel
loops, each loop having a zigzag shape with peaks. For
example, the elongate body 10 described in FIG. 2 may be used.
[00164] The two elongate bodies 1700, 1705 are deployed with
separate delivery means. First, the outer elongate body 1700,
which may be self-expandable, as described with respect to the
elongate body 1300 of FIGS. 13 and 14, or balloon-expandable,
is deployed and placed into the coronary sinus 20 as shown in
-47-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
FIG. 19. The expansion of the outer elongate body 1700
results in foreshortening of the coronary sinus 20, which in
turn results in reshaping of the mitral annulus 28.
[00165] Next, the inner elongate body 1705, which may be
self-expandable or balloon-expandable, is deployed and placed
inside of the inner surface of the outer elongate body 1700.
In one configuration, the inner elongate body 1705 is deployed
with a balloon. In this configuration, the inner elongate
body 1705 is mounted onto a balloon (not shown), which acts as
a delivery catheter. Once the inner elongate body 1705 and
the balloon are appropriately positioned inside of the outer
elongate body 1700, the balloon is expanded by introducing,
for example, a saline solution through the delivery catheter
and into the balloon. Alternately, any biocompatible solution
may be used to inflate the balloon. Once the inner elongate
body 1705 is expanded to make substantial contact with the
outer elongate body 1700 and is tightly fitted along the walls
of the inside surface of the outer elongate body 1700, the
balloon is deflated and removed. Depending on the location of
the regurgitation jet in the mitral valve, the rigid inner
elongate body 1705 can be placed anywhere along the wall of
the coronary sinus 20 that aligns with the posterior section
of the mitral annulus 28 to further increase the effect of the
inward displacement of the mitral annulus 28 (as shown by the
arrows of FIG. 19). Typically, the inner elongate body 1705
is placed within the central stmt section of the outer
elongate body 1700 to straighten the central section of the
coronary sinus 20, which is generally aligned with the P2
scallop.
[00166] Resorbable materials have been used in connection
with valve repair devices as a means to provide a "delayed
release" mechanism allowing a device to effect a change to a
-48-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
valve over time. Examples of embodiments that include
resorbable material may be found in U.S. Patent Application
10/141,348 to Solem, et al., 10/329,720 to Solem, et al., and
10/500,188 to Solem, et al., which are incorporated herein by
reference.
[00167] As shown in FIG. 20, a new embodiment of the present
invention includes an elongate body 410 having resorbable
thread sutured through the openings of a bridge 416. The
elongate body further includes a proximal anchor 412 and a
distal anchor 414 connected by the bridge 416 with the
resorbable material..
[00168] Resorbable materials are those that, when implanted
into a human body, are resorbed by the body by means of
enzymatic degradation and also by active absorption by blood
cells and tissue cells of the human body. Examples of such
resorbable materials are PDS (Polydioxanon), Pronova (Poly
hexafluoropropylen-VDF), Maxon (Polyglyconat), Dexon
(polyglycolic acid) and Vicryl (Polyglactin). As explained in
more detail below, a resorbable material may be used in
combination with a shape memory material, such as nitinol,
Elgiloy or spring steel to allow the superelastic material to
return to a predetermined shape over a period of time.
[00169] In one embodiment as shown in FIG. 20, the proximal
and distal anchors 412, 414 are both generally cylindrical and
are both made from tubes of shape memory material, for
example, nitinol. However, the anchors 412 and 414 may also
be made from any other suitable material, such as stainless
steel. Both anchors 412, 414 have a mesh configuration
comprising loops 54 of zigzag shaped shape memory material
having alternating peaks 42. The loops 54 are connected at
each peak 42 to form rings 56 of four-sided openings 40.
Other configurations may also be used as known in the art.
-49-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
Additionally, other types of anchors known in the art may also
be used.
[00170] The proximal and distal anchors 412, 414 each have a
compressed state and an expanded state. In the compressed
state, the anchors 412, 414 have a diameter that is less than
the diameter of the coronary sinus 20. In this state, the
anchors 412 and 414 have a substantially uniform diameter of
between about 1.5 to 4 mm. In the expanded state, the anchors
412, 414 have a diameter that is about equal to or greater
than a diameter of the section of a non-expanded coronary
sinus 20 to which each anchor will be aligned. Since the
coronary sinus 20 has a greater diameter at its proximal end
than at its distal end, in the expanded state the diameter of
the proximal anchor 412 is between about 10 - 15 mm and. the
diameter of the distal anchor is between about 3 - 6 mm.
[00171] In one embodiment, the bridge 416 is connected
between the proximal anchor 412 and distal anchor 414 by links
418, 419. More specifically as shown in FIG. 20, a proximal
link 418 connects the proximal stmt section 412 to a proximal
end of the bridge 416 and a distal link 419 connects the
distal stmt section 414 to a distal end of the bridge 416.
The links 418 and 419 have a base 421 and arms 422 that extend
from the base and which are connected to two peaks 42 on each
anchor 412, 414. Further, the links 418 and 419 contain a
hole 428, as shown in FIG. 21, which serves as a means through
which to pass the end of the resorbable thread and secure it
to the bridge 416.
[00172] The bridge 416 in one embodiment is made from a
shape memory material and is flexible to allow the body 410 to
conform to the shape of the coronary sinus 20. The bridge 416
comprises X-shaped elements 424 wherein each X-shaped element
is connected to an adjacent X-shaped element at the
-50-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
extremities of the "X," allowing a space 425 to be created
between adjacent X-shaped elements, as shown in FIG. 23. The
X-shaped elements 424 further have rounded edges that
minimizes the chances that a sharp edge of the bridge 416 will
puncture or cut a part of the coronary sinus 20 as the device
is inserted. The bridge 416 has two states: an elongated
state in which the bridge 416 has a first length, and a
shortened state in which the bridge has a second length, the
second length being shorter than the first length. In the
present embodiment, resorbable thread 420 is woven into the
spaces 425 between adjacent X-shaped elements 424 to hold the
bridge 416 in its elongated state. The thread 420 acts as a
temporary spacer. When the resorbable thread 420 is dissolved
over time by means of resorption, the bridge assumes its
shortened state.
[00173] The present embodiment is deployed as follows. An
introduction sheath (not shown) made of synthetic material is
used to gain access to the venous system. A guide wire (not
shown) is then advanced through the introduction sheath and
via the venous system to the coronary sinus 20. The guide
wire and/or introduction sheath is provided with radiopaque
distance markers which can be identified using X-rays which
allows the position of the body 410 in the coronary sinus 20
to be monitored.
[00174] The elongate body 410 is mounted onto a stmt
insertion device (not shown) so that the self-expanding
anchors 412 and 414 are held in the compressed state.
Thereafter, the stmt insertion device with the elongate body
410 mounted thereon is pushed through the introduction sheath
and the venous system to the coronary sinus 20 riding on the
guide wire. After the body 410 is positioned in the coronary
sinus 20 so that the center of the body is generally aligned
-51-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ~ ECV-5780/53998/OSB/E303
with the center of the P2 scallop, the stmt insertion device
is removed. When the stmt insertion device is removed, the
self-expandable anchors 412 and 414 are released so that they
expand and contact the inner wall of the coronary sinus 20 and
provide temporary fixation of the elongate body 410 to the
coronary sinus. Alternatively, the anchor may be expanded by
balloons or other means known in the art . In one embodiment,
the device can be rotated so that the bridge contacts the wall
of the coronary sinus that is closest to the mitral valve 26.
The guide wire and the introduction sheath are then removed.
[00175] After the body 410 is inserted into the coronary
sinus 20, the wall of coronary sinus will grow around the mesh
configuration of the anchors 412 and 414. Simultaneously, the
resorbable thread 420 will be resorbed by the surrounding
blood and tissue in the coronary sinus 20. After a period of
a few weeks, the anchors 412 and 414 will be secured into the
wall of the coronary sinus 20. During that time period, the
resorbable thread 420 will be resorbed to such a degree that
eventually it can no longer hold the bridge 416 in its
elongated state. As the resorbable thread 420 is resorbed,
the bridge 416 retracts from its elongated state to its
shortened state. This shortening of the bridge 416 draws the
proximal anchor 412 and the distal anchor 414 together,
cinching the coronary sinus 20 and/or reducing its
circumference. This cinching and/or reduction of the
circumference of the coronary sinus 20 closes the gap created
by dilatation of the posterior leaflet 31 of the mitral valve.
[00176] The body 410 may be positioned in the coronary sinus
20 by catheter technique or by any other adequate technique.
The body 410 may be heparin-coated so as to avoid thrombosis
in the coronary sinus 20, thus reducing the need for aspirin,
ticlopedine or anticoagulant therapy. At least part of the
-52-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
body 410 may contain or be covered with any therapeutic agents
such as Tacrolimus, Rappamycin or Taxiferol to prohibit
excessive reaction with surrounding tissue. Further, at least
parts of the body 410 may contain or be covered with Vascular
Endothelial Growth Factor (VEGF) to ensure smooth coverage
with endothelial cells.
[00177] In some cases of ischemic mitral regurgitation, the
dilatation of the mitral annulus may be asymmetric with, for
example, one region of the mitral annulus being more dilated
than another. Thus, it may be advantageous to be able to
control the degree of cinching along a particular segment of
the mitral annulus.
[00178] As shown in FIG. 22, an alternate embodiment of the
present invention similar to the delayed release device
described above comprises an elongate body 510 including a
proximal anchor 512, a distal anchor 514 and a central anchor
516. A first bridge 518 connects the proximal anchor 512 to
the central anchor 516, and a second bridge 520 connects the
distal anchor 514 to the central anchor.
[00179] The structure of the elongate body 510 is
substantially similar to the structure of the elongate body
410 described above. More specifically, each anchor 512, 514,
516 is generally cylindrical and has a compressed state and an
expanded state. Further, each bridge 518, 520 has an
elongated and a shortened state and comprises X-shaped
elements with resorbable thread woven into spaces created
between adjacent X-shaped elements. Also, each bridge 518,
520 is connected to its respective anchors 512, 514, 516 by a
link as described above.
[00180] The amount of foreshortening of the bridge 518 may
be variable depending on, for example, the size of the X-
shaped elements, the size of the openings between adjacent X-
-53-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
shaped elements, the type of material used to manufacture the
bridge, and the diameter of the material threaded into the
bridge.
[00181] The present embodiment is deployed as follows. An
introduction sheath (not shown) made of synthetic material is
used to gain access to the venous system. A guide wire (not
shown) is then advanced through the introduction sheath and
via the venous system to the coronary sinus 20. The guide
wire and/or introduction sheath is provided with X-ray
distance markers so that the position of the body 510 in the
coronary sinus 20 may be monitored.
[00182] The elongate body 510 is mounted onto a stent
insertion device (not shown) so that the self-expanding
anchors 512, 514 and 516 are held in the compressed state.
Thereafter, the stmt insertion device with the elongate body
510 mounted thereon is pushed through the introduction sheath
and the venous system to the coronary sinus 20 riding on the
guide wire. After the body 510 is positioned in the coronary
sinus 20 so that the central anchor 516 is generally aligned
with the center of the P2 scallop, the stmt insertion device
is removed. When the stmt insertion device is removed, the
self-expandable anchors 512, 514 and 516 are released so that
they expand and contact the inner wall of the coronary sinus
20 and provide temporary fixation of the elongate body 510 to
the coronary sinus. In one embodiment, the device may be
rotated so that the bridges contact the wall of the coronary
sinus that is closest to the mitral valve 26. The guide wire
and the introduction sheath are then removed.
[00183] After the body 510 is inserted into the coronary
sinus 20, the wall of coronary sinus will grow around the mesh
configuration of the anchors 512, 514 and 516.
Simultaneously, the resorbable thread (not shown in detail)
-54-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
will be resorbed by the surrounding blood and tissue in the
coronary sinus 20. After a period of a few weeks, the anchors
512, 514 and 516 will be more permanently secured into the
wall of the coronary sinus 20. During that time period, the
resorbable thread will be resorbed to such a degree that
eventually it will not hold the bridges 518, 520 in their
elongated state any longer. As the resorbable thread is
resorbed, the bridges 518, 520 retract from their elongated
state to their shortened state. This shortening of the
bridges 518, 520 draws the proximal and distal anchors 512,
514 toward each other, cinching the coronary sinus 20 and
reducing its circumference. The reduction of the
circumference of the coronary sinus 20 closes the gap created
by dilatation of the posterior leaflet 31 of the mitral valve.
[00184] Having the central anchor 520 between the proximal
and distal anchors 512, 514 may allow for a different amount
of foreshortening between each pair of adjacent anchors,
depending on the length of the bridges 518, 520. Thus, the
elongate body 510 may be more specifically tailored to reshape
the mitral annulus according to a patient's needs. For
example, the bridge between the proximal anchor 512 and
central anchor 516 may shorten more than the bridge between
the distal anchor 514 and the central anchor or vice versa.
Further, having an additional anchor serves to improve the
distribution of forces that act on the proximal and distal
stems as well as improving the distribution of the forces
that the bridges exert on the inner wall of the coronary
sinus.
[00185] The delayed release device described above is not
limited to three anchors. FIG. 23 shows an embodiment 610 of
the present invention wherein four anchors 612, 614, 616, 618
and three bridges 620, 622, 624 are used, but it will be
-55-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
apparent to one skilled in the art that any number of anchors
may be used and that the length of the bridges between each
anchor may vary.
[00186] In addition to the embodiments described in detail
above, those skilled in the art will appreciate other
embodiments for connecting a proximal anchor, a distal anchor
and at least one central anchor. Some of those embodiments
may include a thread of shape memory material held in an
elongated state by a sheath of resorbable material, scissors-
shaped memory material held in an elongated state by a sheath
of resorbable material or by resorbable material in tension, a
coil of shape-memory material wrapped around a tube of
resorbable material, ribbons of resorbable material wrapped
around a tube of shape memory material. See, for example, the
embodiment in Serial No. 10/500,188.
[00187] Referring now to FIGS. 24A-24D, another embodiment
of the present invention is described. Apparatus 758 includes
proximal anchor element 762 that is joined to distal anchor
element 764 via wire 766 and cinch mechanism 767. Proximal
and distal anchor elements 762 and 764 also include
substantially tubular members that self-expand to engage the
intima of the vessel in which they are deployed. In
accordance with principles of the present invention, distal
anchor element 764 includes a means for bonding the distal
anchor element to at least a portion of an intima of coronary
sinus C. Preferred configurations for proximal and distal
anchor elements 762 and 764, as well as preferred means for
bonding distal anchor element 764 to the intima of the
coronary sinus, are described in detail with respect to FIGS.
25A-25C.
[00188] As shown in FIG. 25A, proximal anchor element 762
includes self-deploying stmt 785 having proximal and distal
-56-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
ends, deployable flange 769 disposed at the proximal end, and
cinch mechanism 767 coupled to stmt 785. Stent 785 and
deployable flange 769 of proximal anchor element 762 are
initially constrained within delivery sheath 760, as shown in
FIG. 24A, and are composed of a shape memory material, e.g.,
Nitinol, so that stmt 785 and flange 769 self-deploy to the
predetermined shapes shown in FIG. 25A upon retraction of
delivery sheath 760.
[00189] Flange 769 may include a substantially circular
shape-memory member, as illustrated in FIG. 25A, a plurality
of wire members, e.g., manufactured using Nitinol, that self
deploy upon removal of sheath 764 and abut ostium O, or other
suitable shape.
[00190] As shown in FIG. 25B, distal anchor element 764
preferably includes wire mesh stmt 787 manufactured using a
shape memory material, e.g., Nitinol. Wire 766 is coupled to
distal anchor element 764 and is used in combination with
cinch mechanism 767 of proximal anchor element 762 to remodel
the coronary sinus, as described hereinbelow. Stems 785 and
787 are illustratively described as comprising wire mesh, but
one of skill in the art will appreciate that other types of
anchor elements including self-expanding slotted tubular
stems also may be employed.
[00191] Distal anchor element 764, as depicted in FIG. 25B,
in one exemplary embodiment is at least partially coated with
a bonding material 791. Bonding material 791 may have light-
reactive binding agents that undergo polymerization when
exposed to radiation, for example, ultraviolet (UV) radiation.
When bonding material 791 has such UV-curable agents, the
agents may include acrylates, and more specifically, acrylates
with UV or free radical polymerization or, for example,
polymethylmethacrylate.
-57-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
[00192] Apparatus 758 may further comprise catheter 770
having proximal and distal ends, a lumen extending
therebetween, and at least one port 771 disposed at the distal
end of the catheter, as shown in FIG. 24A. A light source,
for example, including UV light, may be coupled to the
proximal end of catheter 770 so that the light is transmitted
throughout the lumen of catheter 770 and exits via port 771.
Catheter 770 further includes radiopaque marker bands 772 and
774 to aid in the positioning of port 771 under fluoroscopy,
which in turn ensures the proper positioning of the UV light.
[00193] Alternatively, bonding material 791 may include a
synthetic molding material, such as a starch-based poly
ethylene glycol hydrogel, that is heat hardenable or
hydrophilic. In an exemplary embodiment, a starch-based poly
ethylene glycol hydrogel is used that swells when exposed to
an aqueous solution. Hydrogels also may be selected to
harden, for example, upon exposure to body temperature or
blood pH. Hydrogels suitable for use with the present
invention may be obtained, for example, from Gel Med, Inc.,
Bedford, Mass.
[00194] Referring to FIG. 25C, alternative distal anchor
element 794 may be used in lieu of distal anchor element 764
of FIG. 25B. Distal anchor element 794 includes foam member
796 having proximal and distal ends and bore 797 extending
therebetween. Foam member 796 is depicted in a deployed state
in FIG. 25C, but is capable of being contracted within
delivery sheath 760 of FIG. 24A. Foam member 796 is made from
a hydrophilic foam, i.e., a foam material that has a tendency
to absorb water and swell into engagement with the vessel
intima.
[00195] Referring back to FIG. 24A, preferred method steps
for using the proximal and distal anchor elements of FIGS.
-58-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
25A-25C are described. Apparatus 758 is navigated through the
patient's vasculature with proximal and distal anchor elements
762 and 764 in a contracted state and into coronary sinus C,
as shown in FIG. 24A. The distal end of sheath 760 is
disposed, under fluoroscopic guidance, at a suitable position
within the coronary sinus, great cardiac vein, or adjacent
vein. Push tube 768 then is held stationary while delivery
sheath 760 is retracted proximally so that distal anchor
element 764 deploys from within sheath 760, thereby permitting
distal anchor element 764 to self-expand into engagement with
the vessel wall, as shown in FIG. 24B.
[00196] In accordance with principles of the present
invention, after distal anchor element 764 self-deploys, an
outer surface of distal anchor element 764 will become at
least partially chemically or mechanically bonded to an intima
of coronary sinus C. When bonding material 791 of FIG. 25B
comprises a light-reactive binding agent, the light-reactive
binding agents will at least partially contact the vessel wall
when distal anchor element 764 self-deploys. At this time,
light 773, for example, UV light, may be emitted from port 771
of catheter 770 to cause light-reactive agents 791 to
polymerize, and thereby form bond B with the intima of
coronary sinus C, as shown in FIG. 25B. Catheter 770 then may
be removed upon satisfactory bonding of distal anchor element
764.
[00197] Alternatively, when bonding material 791 of FIG. 25B
comprises a hydrogel, the exposure of the hydrogel to flow in
the vessel will cause at least a portion of distal anchor
element 764 to chemically bond with the intima of coronary
sinus C. In yet another alternative embodiment, when
alternative distal anchor element 794 of FIG. 25C is used,
foam member 796 will cause distal anchor element 794 to
-59-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
chemically or mechanically bond with the intima of coronary
sinus C when exposed to flow in the vessel due to the
hydrophilic properties of foam member 796.
[00198] Using any of the techniques described above, it is
possible to chemically bond distal anchor element 764, or
distal anchor element 794, to at least a portion of the intima
of coronary sinus C. As will be described in detail
hereinbelow, this is advantageous because shear stress to the
vessel will be reduced when actuating wire 766 and cinch
mechanism 767.
[00199] Referring now to FIG. 24C, in a next method step,
delivery sheath 760 is retracted proximally, under
fluoroscopic guidance, until proximal anchor element 762 is
situated extending from the coronary sinus. Push tube 768 is
held stationary while sheath 760 is further retracted, thus
releasing proximal anchor element 762. Once released from
delivery sheath 760, proximal anchor element 762 self-expands
into engagement with the wall of the coronary sinus C, and
flange 769 abuts against coronary ostium O, as shown in FIG.
24C.
[00200] Delivery sheath 760 (and/or push tube 768) then may
be positioned against flange 769 of proximal anchor element
762, and wire 766 retracted in the proximal direction to draw
distal anchor element 764 towards proximal anchor element 762,
as shown in FIG. 24D. As will of course be understood, distal
anchor element 764 is drawn towards proximal anchor element
762 under fluoroscopic, ultrasound or other types of guidance,
so that the degree of remodeling of the mitral valve annulus
may be assessed.
[00201] As wire 766 is drawn proximally, cinch mechanism 767
prevents distal slipping of the wire. For example, wire 766
may include a series of grooves along its length that are
-60-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
successively captured in a V-shaped groove, a pall and ratchet
mechanism, or other well-known mechanism that permits one-way
motion. Upon completion of the procedure, delivery sheath 760
and push tube 768 are removed from the patient's vessel.
[00202] Referring now to FIGS. 26A-26D, a method for using
apparatus 758 of FIGS. 6 and 7 to close a central gap 782 of
mitral valve 780 is described. In FIG. 26A, proximal and
distal anchor elements 762 and 764 are deployed in coronary
sinus C, preferably so that flange 769 of proximal anchor
element 762 abuts coronary ostium O. Distal anchor element
764 is disposed at such a distance apart from proximal anchor
element 762 that the two anchor elements apply a compressive
force upon mitral valve 780 when wire 766 and cinch 767 are
actuated.
[00203] In FIG. 26B, cinch 767 is actuated from the proximal
end to reduce the distance between proximal and distal anchor
elements 762 and 764, e.g., as described hereinabove with
respect to FIG 24D. When wire 766 and cinch mechanism 767 are
actuated, distal anchor element 764 is pulled in a proximal
direction, while proximal anchor element 762 may be urged in a
distal direction using delivery sheath 760 and/or push tube
768, as shown in FIG. 24D.
[00204] When proximal anchor element 762 comprises flange
769, proximal anchor element 762 is urged in the distal
direction until flange 769 abuts coronary ostium O. The
reduction in distance between proximal and distal anchor
elements 762 and 764 reduces the circumference of mitral valve
annulus 781 and thereby reduces gap 782. Flange 769 provides
a secure anchor point that prevents further distally-directed
movement of proximal anchor element 762, and reduces shear
stresses applied to the proximal portion of the coronary
sinus. Moreover, because distal anchor element 764 is bonded
-61-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
to the intima of coronary sinus C using any of the techniques
described above, shear stress to the intima of coronary sinus
C will be reduced when actuating wire 766 and cinch mechanism
767.
[00205] Referring now to FIGS. 27A-27L, alternative
apparatus and methods suitable for treating mitral
insufficiency are described. In FIG. 27A, distal balloon
catheter 804 having proximal and distal ends, lumen 815
extending therebetween, and balloon 805 disposed at the distal
end is positioned within coronary sinus C with balloon 805 in
a contracted state. Distal catheter 804 may be positioned
using a conventional guidewire (not shown), according to
techniques that are known in the art. Distal catheter 804
further comprises an inflation lumen (not shown) extending
between the proximal and distal ends that is in fluid
communication with an opening of balloon 805, so that balloon
805 may be inflated via the inflation lumen, as shown in FIG.
27B.
[00206] Balloon 805 preferably includes a plurality of ribs
or bumps 806 disposed about its circumference that are
configured to engage the intima of a vessel wall and resist
movement of balloon 805, when inflated, relative to the
vessel.
[00207] After balloon 805 of distal catheter 804 is deployed
in coronary sinus C, proximal balloon catheter 802 having
proximal and distal ends, lumen 816 extending therebetween,
and balloon 803 disposed at the distal end then may be
advanced distally over distal catheter 804.
(00208] Lumen 816 of proximal catheter 802 comprises an
inner diameter that is larger than an outer diameter of distal
catheter 804, so that annulus 807 is defined as the space
-62-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
between an interior surface of proximal catheter 802 and an
outer surface of distal catheter 804.
[00209] Proximal catheter 802 is provided with balloon 803
in a contracted state, and may be under fluoroscopy at a
location whereby proximal section 819 of balloon 803 remains
proximal of coronary ostium O, as shown in FIG. 27B. At this
time, balloon 803 is inflated via an inflation lumen (not
shown) of proximal catheter 802 to deploy balloon 803.
[00210] In the deployed state, balloon 803 of proximal
catheter 802 comprises flange 809 disposed about proximal
section 819 of balloon 803, as shown in FIG. 27C. In the
deployed state, flange 809 is configured to abut against the
wall of coronary ostium O, while a distal section of balloon
803 is configured to be substantially flush with the intima of
coronary sinus C, as shown in FIG. 27C. An interior portion
of coronary sinus C that is formed between deployed balloons
803 and 805 defines cavity 827.
[00211] Referring to FIG. 27D, balloon 805 of distal
catheter 804 then may be retracted proximally and/or balloon
803 of proximal catheter 802 may be urged distally so that the
distance between balloons 803 and 805 is reduced. Balloon 805
is disposed at such a distance apart from balloon 803 that the
two balloons will apply a compressive force upon mitral valve
820 when the distance between balloons is reduced.
[00212] Ribs 806 of balloon 805 may engage the intima of
coronary sinus C when balloon 805 is retracted, so that
balloon 805 does not move with respect to coronary sinus C.
Proximal retraction of balloon 805 causes coronary sinus C to
shorten and remodel the curvature of the mitral valve annulus,
as shown in FIG. 27D. The reduction in distance between
balloons 803 and 805 applies a compressive force upon mitral
-63-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
valve 820 that reduces the circumference of mitral valve
annulus 121 and thereby closes gap 822.
[00213] Referring now to FIG. 27E, with gap 822 reduced or
closed as described hereinabove with respect to FIG. 27D,
substance 811 then may be introduced into cavity 827 via
annulus 807. Substance 811 may be a biological or synthetic
biocompatible material that is injected in a fluid state, and
which hardens to a rigid or semi-rigid state.
[00214] For example, substance 811 may comprise a biological
hardening agent, such as fibrin, that induces blood captured
in cavity 827 to form a coherent mass, or it may comprise a
tissue material, such as collagen, that expands to fill the
cavity. If fibrin is employed, it may be obtained from
commercially available sources, or it may be separated out of
a sample of the patient's blood prior to the procedure, and
then injected into cavity 827 via annulus 807 to cause
thrombosis. On the other hand, collagen-based products, such
as are available from Collatec, Inc.,. Plainsboro, N.J., may be
used to trigger thrombosis of the volume of blood in cavity
827.
[00215] Alternatively, substance 811 may comprise a
synthetic molding material, such as a starch-based poly
ethylene glycol hydrogel or a polymer, such as poly-capro
lactone, that is heat hardenable or hydrophilic. In a
preferred embodiment, a starch-based poly ethylene glycol
hydrogel is used that swells when exposed to an aqueous
solution. Hydrogels suitable for use with the present
invention are described hereinabove with respect to FIG. 25B.
Hydrogels or polymers also may be selected to harden, for
example, upon exposure to body temperature or blood pH.
[00216] The injection of substance 811 between balloons 803
and 805 and into cavity 827 forms coherent mass 812, as shown
-64-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
in FIG. 27F. It is expected that, depending upon the type of
hardening agent or molding material used, solidification of
the content of cavity 827 may take about ten minutes or less.
[00217] After solidification of mass 812 has occurred,
balloons 803 and 805 may be deflated. To facilitate removal
of distal catheter 804 and balloon 805 from solidified mass
812, the exterior surface of distal catheter 804 and balloon
805 may be coated with a suitable non-stick coating, for
example, Teflon, a registered trademark of the E.I. duPont de
Nemours Company, Wilmington, Del. (polytetrafluorethylene), or
other suitable biocompatible material, such as Oparylene,
available from Paratech~, Inc., Aliso Viejo, Calif. Proximal
catheter 802 and/or balloon 803 also may be coated with such a
non-stick coating to facilitate removal from within the
patient's vessel.
[00218] Upon removal of proximal and distal catheters 802
and 804, solidified mass 812 maintains mitral valve 820 in the
remodeled shape with gap 822 closed. The removal of distal
catheter 804 from within solidified mass 812 may form bore 828
within the mass, as shown in FIG. 27F, which allows blood flow
to be maintained within coronary sinus C. Because blood
oxygenating the myocardium can drain directly into the left
ventricle via the Thebesian veins, it is also permissible for
the coronary sinus to be completely occluded with little or no
adverse effect.
[00219] In an alternate embodiment of the present invention
as shown in FIGS. 27G and 27H, the catheter 802 reaches all
the way to the distal balloon 805. The distal balloon 805
with the catheter 802 is inserted into the great cardiac vein
beyond where the vein turns away from the mitral valve plane
at about 90 degrees. When a substance 811 is introduced into
the device, the substance may also enter side branches 813
-65-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
creating small arms there. These arms will aid in axially
fixing the device once the substance is cured as described
below. After the device has foreshortened as described above
by moving the balloons 803, 805 towards each other and
temporarily fixing their positions relative to each other, the
lumen 816 of catheter 802 is filled with a substance 811 that
when cured, for example by an ultraviolet light or by adding a
proper chemical, becomes a hardened mass. Using this
technique, a three-dimensional mass 812 having a small central
bore 828 is created. This mass 812 is smaller in diameter than
the coronary sinus C and the great cardiac vein, permitting
close to normal blood flow in the vessel. Due to its three-
dimensional shape and rigid configuration, the mass 812 is
restricted to almost no axial movement. Thus, the shape of
the coronary sinus C, the great cardiac vein and the mitral
valve held temporarily by means of the two balloons 803, 805
may be held permanently by the mass 812.
[00220] In another embodiment as shown in FIGS. 27I and 27J,
a film sack 880 is attached to the distal end of the proximal
balloon 803. The diameter of the film sack is approximately
equal to the diameter of the coronary sinus C and tapers down
to approximately the diameter of the distal catheter 804 near
the distal balloon 805 as shown in FIG. 27J. The film sack
880 is removably attached to the distal balloon 805 and may be
manufactured from any thin plastic biocompatible material. A
curable substance 811 is then introduced via the annulus 807
and cured by ultraviolet light or by the addition of a
chemical as described above. When cured, the substance 811
forms a hardened mass that retains its shape and forces the
affected vessels to also retain that shape. Once the
substance 811 has hardened, the catheter 804, balloons 803,
805 and film sack 880 are removed.
-66-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
[00221] In yet another embodiment, as shown in FIGS. 27K and
27L, the film sack 880 extends to outside the patient's body
rather than being attached to the proximal balloon 803. Once
the substance 811 is introduced, it can then be cured so as to
form a hardened mass that extends all the way to the ostium O.
This allows the cured substance to encompass a greater amount
of the mitral valve annulus and ensures better closure of the
gap created by mitral valve dilatation. The excess substance
811 that is not cured remains fluid and may be removed when
the catheter 804, balloons 803, 805 and film sack 880 are
removed.
(00222] Dilatation of the heart ventricles may lead to heart
failure, which affects both the electrical and mechanical
properties of the heart. Specifically, dilatation may cause
distortion of the synchronization between the heart ventricles
and atria. To correct this distortion, a pacemaker to
stimulate contraction of the heart may be implanted into the
heart, either through the chest wall or percutaneously through
the venous system. Stent-type mechanisms are known that are
connected to the tip of a pacing lead to securely anchor the
pacing lead into a target vessel, such as those described in
U.S. Patents 5,071,407 (Termin, et al.), 5,224,491 (Mehra),
5,496,275 (Sirhan, et al.), 5,531,779 (Dahl, et al.) and
6,161,029 (Spreigl, et al.).
[00223] FIGS. 28A-28C illustrate another embodiment of the
present invention. A pacing lead 901 such as described above
may be attached to any of the previously described mitral
valve annulus reshaping devices, for example elongate body 10
(FIG. 28A), elongate body 1300 (FIG. 28B) or elongate body 110
(FIG. 28C), to combine the function of the pacing lead with
the function of the annulus reshaping device. Such a
combination would allow for simultaneous treatment of
-67-
CA 02548541 2006-06-02
WO 2005/058206 PCT/EP2004/014464
1 ECV-5780/53998/OSB/E303
arrhythmia and mitral regurgitation and would eliminate the
need for a separate procedure to treat both conditions.
Additionally, potential interference of the annulus reshaping
device with the pacing lead would be avoided. As shown in
FIGS. 28A-C, two pacing activity leads are used with each
depicted elongate body which allows for effect at two
locations. However, the number of pacing leads used is not
critical and more or fewer than two leads may be used.
[00224] While the foregoing describes the preferred
embodiments of the invention, various alternatives,
modifications and equivalents may be used. For instance,
although the described embodiments have generally been
directed to placement in the coronary sinus for treatment of
the mitral valve, the embodiments may also be placed in, for
example, the anterior right ventricular cardiac vein to treat
the tricuspid valve. Additionally, the order in which the
stmt sections of the various embodiments are expanded may be
varied. Moreover, it will obvious that certain other
modifications may be practiced within the scope of the
appended claims.
30
-68-