Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
PORTABLE GAS OPERATING INHALER
Field of the Invention
This invention relates to the field of inhalers
used to administer a drug to a patient through the
patient's lungs and, more particularly, to an improved
gas inhaler.
BACKGROUND OF THE INVENTION
Definitions
As used herein, "Heliox" is defined as a gas
mixture of helium and oxygen whose physical properties
are summarized in Table 1 depending on the
concentration of Helium.
Table 1: Physical properties of Heliox at 273 K,
1 atmosphere.
Percentage of 0 20 40 60 80 100
Helium
Density (g/L) 1.429 1.179 0.929 0.679 0.429 0.179
Viscosity (uP) 204 201.2 198.4 195.6 192.8 190
Kinematic 14.3 17.1 21.4 28.8 44.9 106.1
Viscosity
(um2-s 1)
As used herein, "ambient air" is defined as that
air which normally exists around us which is either
inhaled and exhaled from the environment, or, pumped
into a mechanical hand held device from the environment
and then inhaled.
As used herein, "aerosolization" is primarily
defined as the generation and then breakup of a liquid
sheet into primary and satellite droplets, generally 1
micron to 20 microns in size, although the physical
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 2 -
form of particles in an aerosol as used herein may be
liquid drops or solid dry powder particles.
As used herein, "fluidization" is defined as the
deagglomeration of a compact mass of drug in micronized
dry powder form manufactured with a preferred particle
size range of 1 micron to 5 microns into a cloud, with
the objective being the generation of particles in the
preferred 1-10 micron range, and more preferably in the
1-3 micron range.
As used herein, "heterodisperse aerosol" or
"heterodisperse particle cloud" shall be defined as a
deliverable form of a liquid drug formulation or dry
powder drug formulation, such that there are particles
of many different sizes.
, As used herein, "monodisperse aerosol" or
'"monodisperse particle cloud" shall be defined as a
deliverable form of a liquid drug formulation or dry
powder drug formulation, such that the particles are
all the same, or very near the same size.
As used herein, "alveoli" are air sacs deep in the
lung at the terminal end of the smallest and last
branch of bronchioles, where gas exchange takes place
between the airspace in the lungs and arterial blood.
Small particulate drug matter can enter the alveolar
spaces, depending on their size and deposition
characteristics. After entering the alveoli, the drug
matter becomes engulfed by alveolar macrophages, which
exist around each alveolus under its surfactant layer
and enter the acinus by way of the terminal bronchiolar
lumen. Drug particles may be absorbed from the lung
primarily by alveolar macrophages.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 3 -
As used herein, "fine particle dose" shall mean
particles that are preferably about 5~,m or less,
generally 3~,m or less, and more preferably 2~m or less.
As used herein, "respirable fraction" (RF) is a
dose fraction of aerosolized drug particles small
enough in diameter to escape the filtration machinery
of the airways and be deposited in the lungs.
As used herein, the terms "dry powder formulation"
and "liquid formulation" are pharmacologically active
drug by itself, or with any of the following including
but not limited to propellants, carriers, excipients,
surfactants, anti-microbial, flavoring, and other
additions to the formulation that enhance production,
shelf life stability, generation of particles, delivery
to the desired site in the lungs, and absorption,
macrophage or other pr~cessed base transfer from the
air space into the tissue and blood, or taste.
General Medical Background
Delivery of therapeutic drugs via the lungs for
respiratory and non-respiratory systemic diseases, is
increasingly being recognized as a viable if not
superior alternative to administration of drugs
orally/nasally, rectally, transdermally, by intravenous
needle injection, intra-muscular needle injection, or
gas jet driven non-needle injection through the skin
and into the muscle.
Around 1 million patients in the US receive
intravenous morphine for the relief of chronic and
terminal pain. Morphine actually acts more rapidly
with respect to pain management when inhaled than when
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 4 -
injected. In addition, there is a major effort to move
away from CFC or other vapor pressure based propellant
driven inhalers toward alternative technology, due to
environmental issues.
All but oral and rectal modes of administration,
ideally require a liquid form of drug. Hard
particulate drug forms are being explored for gas jet
driven needle-less injection through the skin for
deposit into the muscle for extended or timed release
of the drug substance.
In each of these non-pulmonary methods of drug
administration, far higher doses of drug substance than
that required for actual therapeutic effectiveness on
the target system must be administered to assure that
the required therapeutic amount of drug substance is
actually delivered to the target system or site. This
represents a risk factor to the patient, in that there
is a therapeutic variable regarding the amount of dose
delivered to the target system or site. The exception
is where that target is very local to the site of
administration (i.e., mouth, colon, patch of skin, area
of muscle, etc) .
In addition, many new drugs being developed by
companies in the biotechnology field based on peptides
and proteins, exist as dry powder in their optimum
and/or most stable form, and so these drugs cannot be
injected using a needle or needle-less method, or
administered transdermally. Genetically produced
peptide and protein based drugs are also very sensitive
to being altered by in-vivo environmental factors such
as enzymes and acids.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 5 -
If such sensitive drug molecules in dry powder
form are delivered orally, they are subjected to the
enzymes and acids in the digestive tract. This can
reduce the quantity of these sensitive therapeutic
molecules available for absorption into the blood in
their original therapeutic structure, increasing the
need to initially deliver a higher oral dose. Rectal
drug administration is neither pleasant, socially
acceptable, or commercially viable except in extreme
cases where no other choice exists.
The intravenous needle method of administering
therapeutic drugs in liquid form in the arm or
femorally, results in the dilution and loss of
administered drug potency as the blood passes through
the venous system back to the heart, then to the lungs,
and finally into the~arterial circulation for delivery. '
Intra-muscular needle injection adds a pathway
where part of the administered dose can be lost. The
same is true for a gas driven jet needle-less
injection, where the drug substance must go through the
skin, into the muscle, (usually and primarily) into the
venous blood system, and then into the arterial system.
Hence, it is necessary to inject more drugs,
regardless of the method, than is really needed to
achieve the desired therapeutic effect on, for example,
a specific organ system or organ based receptor target
fed by arterial blood. However, by introducing a drug
substance into the arterial blood stream at its source,
the lungs, a bolus of drug delivered to the target is
less diluted, and, therefore, less drug needs to be
deposited in-vivo at the site or entry point of
administration (the alveoli).
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 6 -
Delivery of drugs via the lungs is the optimal
approach to treat diseases in the lung. In addition,
drugs delivered via the lungs for other than
respiratory diseases, go rapidly and directly into the
arterial blood, then to the heart, and then to the
other critical organs such as brain, liver and kidneys,
and receptor sites residing thereon. This reduces the
effect of dilution on the administered therapeutic dose
in the bloodstream. Furthermore, there is minimal
enzymatic or acid activity in the lungs compared to the
stomach that can impact the therapeutic molecular
integrity of sensitive drug molecules such as
genetically engineered peptides and proteins.
Pulmonary drug delivery can, depending on the drug and
disease:
a) improve the efficacy of.a drug;
b) improve the bioavailability of a drug, which
is particularly important for biological compounds
such as peptides and proteins;
c) improve targeting to an organ or receptor
site thus reducing unwanted side effects (which is an
important consideration with, for example, anticancer
agents); and
d) mimic the biopattern of a disease, or
circadian rhythm, e.g., as in the case of sustained-
release anti-hypertensives designed to peak coinciding
with the early morning blood pressure surge.
Commercially, a new method of pulmonary drug
delivery for an existing drug, can extend its
therapeutic indications, lower cost, and facilitate a
more rapid time to market. Since drugs administered by
the pulmonary route do not require sterility, a sterile
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
device or sterile environment, they are ideal for the
delivery of drugs in difficult environments.
U.S. Patent No. 6,125,844 discloses an apparatus
for portable gas-assisted dispensing of medication not
using a fluorocarbon propellant. The apparatus
comprises a pressurized supply of gas containing
therapeutic gas or mixture of therapeutic gases, and
one or more drugs mixed therein, connected to a
pressure regulator, wherein the pressure regulator is
connected to a gas release switch which is connected to
a breath activator. The breath activator .is connected
to an aspiration chamber, whereby in use when a patient
inhales from the aspiration chamber, the inhalation
causes the breath activator to engage with the gas
release switch to release the therapeutic gas/drug
mixture into the aspiration chamber, wherein the
therapeutic gas and drug in the aspiration chamber are
simultaneously delivered to a patient during
inhalation. Alternatively, medication can be stored in
a separate drug reservoir adjacent the pressurized
supply of therapeutic gas, which medication is drawn
into the aspiration chamber by a venturi assembly.
Variables that affect inhaler generated
particulate drugs being delivered to the right location
rroutinely mentioned in the medical literature include:
a) those that are breathing related including
the volume of inspiration, inspiration flow rate
(velocity), breath holding period after inspiration of
a dose, the total lung volume at the time the bolus of
medication is administered, and the expiration flow
rate;
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
_ g _
b) those that are particulate related including
aerosol particle size, shape, density of the liquid or
powder drug particles, and size distribution in the dry
powder or liquid aerosol cloud produced; and
c) the medical status of the patient, and in
particular, the status of the respiratory system of the
patient.
The objective with any method and technology
involving inhalers, is: a) to generate particles of
the optimum size range for deep lung delivery, and b)
to get any administered particles past the larger
airways where they will be lost to turbulence and
impaction and into the middle (for treating respiratory
diseases) and deep (for delivering drugs to the target
area where they can enter the arterial blood) lung.
Unlike intravenously administered drugs, drugs
administered via the lungs are not subject to prior
first pass hepatic metabolism. They are also less
subject to reacting with or being affected by fewer
receptors prior to reaching their intended target
either in the lungs or systemically, resulting in a
reduced amount of drug being needed, if the particle
size and delivery to the target location in the lungs
are optimized. However, because any systemic drug
administered by the lung does go straight to the heart
first, the cardiac side effects of excipients and drugs
administered by this method are an issue. As an
example of the rapid effects drugs administered via the
lungs can have systemically, administration of the pain
killer morphine via the lungs is faster acting than
morphine administered intravenously.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 9 -
Recognition of the ability to deliver systemic
therapeutic drugs by inhalation due to the
physiological properties of the lung and circulatory
system, has led to a large number of different
therapeutic drugs being developed and evaluated for
administration by inhalation to treat even non
respiratory diseases.
A key problem is in the maximizing the number of
these smallest particles that are delivered to the
terminal branches of the bronchioles and the alveoli.
Small particles, preferably lam-3~,m in size, are
optimal for this purpose. Generally, only about 10-200
of the amount of particulate drug dispensed by
conventional inhalers is delivered in this range.
Large molecule drugs, such as peptides and-
proteins which are now possible due to genetic'
engineering, do not pass easily through the airway
surface because it is lined with a ciliated mucus-
covered cell layer of some depth, making it highly
impermeable. The alveoli however, have a thin single
cellular layer enabling absorption into the
bloodstream. The alveoli are the door to the arterial
blood and are at the base of the lungs.
So, to reach the alveoli, a particulate drug must
be administered in small size particles, and the
inhalation must be moderated, slow, and deep. Large
particles will impact in the oropharyngeal area or
settle in the upper bronchi. If the particles are too
small and/or ultra light, they will be exhaled (the
latter is especially true if air is the tidal front of
gas entraining the ultra light particles).
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 10 -
The larger passages through which the air and drug
particles travel generates turbulence, which also
results in the impaction and loss of drug particles. A
desired goal is to increase the laminar flow of the gas
stream in the larger air passages, so that particles
reach the smaller passages where laminar flow is
naturally induced. If there are any constrictions in
the bronchi or bronchioles, resulting, for example,
from asthma, the turbulence and rate of impaction of
drug particles can also increase at those points of
constriction.
Any variability in the dose deposited in the
lungs, and where it is deposited in the lungs, could
have a major effect on treatment because of the narrow
15... therapeutic range of many drugs, and the potency of
~. such drugs. One well known such example is insulin.
Aerosol particles are deposited in the airways by
gravitational sedimentation, inertial impaction, and
diffusion. All three mechanisms act simultaneously.
However, the first two are the principle methods that
apply to the deposition of large particles. Diffusion,
is the primary factor of deposition of smaller
particles in peripheral regions of the lung.
The optimum size particles of drug for delivery to
the alveoli are in the range generally of 1-3 microns,
and usually particles less than 2 microns reach the
alveoli.
The diameter of therapeutically usable particles
is generally between 0.5 and 5 microns. Particles 1-5
microns are deposited in the larger airways while
particles generally below 3 microns in diameter reach
the terminal bronchioles and alveoli and are optimal
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 11 -
for transference into the arterial blood. The depth of
penetration of a particle into the bronchial tree is
inversely proportional to the size of the particle,
down to 1 Vim. Particles smaller than 1 Vim, however, are
so light that a large proportion does not deposit in
the lungs.
The small airways are the optimal sites for the
inhalative treatment of obstructive pulmonary diseases.
Diffusion is a process that applies to particles
smaller than about 3 microns. The maximum collection
of particles by the deep lung is by the process of
sedimentation.
Some of the sub-micron particles of a drug may be
exhaled because their sedimentation may not be high
enough in air -- which is normally the ambient
entrainment gas and environment in the lungs.
Prior art, whether metered dose inhalers (MDI)or
dry powder inhalers (DPI), use air as the exclusive or
primary means of conveying fluidized powder or
aerosolized liquid drug into the lungs. In the case of
MDIs, it is assumed that the propellant evaporates as
intended or constitutes a very small fraction of the
total gas inhaled at full tidal volume with the drug
dose and air.
Heliox has been administered to a patient in a
hospital setting prior to the administration of a dry
powder or liquid aerosol drug. Heliox has also been
used to administer a liquid drug using a nebulizer,
which is a different type of device for pulmonary drug
administration lasting 10-60 minutes. That is distinct
from "puffs" received through an inhaler.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 12 -
Additionally, in both cases, the systems in which
Heliox were used were designed for the physical
properties of air and not Heliox, and so were not
optimized for Heliox.
Prior art and medical publications pertaining to
inhalers, address other factors but do not focus on the
specific gas involved in the transport of particles
into the lung. In the case of DPIs, the gas is always
assumed to be, or stated specifically to be, air. In
the case of MDIs, the "gas" is always assumed to be a
liquid propellant having a vapor pressure, CFC in most
cases, and is only a negligible, fraction of the inhaled
volume, the balance being air.
MDI is a metered dose inhaler consisting of a
propellant generating a vapor pressure and a drug in
suspension or solution form, where, when the device is
activated, the vapor pressure of said propellant pushes
a predetermined amount of liquid drug through a nozzle
generating an aerosol for inhalation. MDIs contain
suspensions or solutions of a drug, a propellant, and a
surfactant that acts as a lubricant to stop particles
from aggregating and to reduce clogging of the aerosol
nozzle. MDIs rely on the use of propellants that have
a high vapor pressure. The higher the vapor pressure,
the faster a liquid containing a drug can be pushed out
of a nozzle, and thus a thinner liquid sheet is formed,
and smaller particles are produced. Vapor pressure is
therefore directly related to the velocity generated
and the fraction of fine or desirable small particles
generated.
Pressurized aerosols historically used
chlorofluorocarbon (or CFC) propellants generating a
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 13 -
pressure of approximately.400 kPa or higher. The
aerosol cloud therefore emerges from the canister at a
high speed. Furthermore, the drug crystals are
initially enclosed within large propellant droplets
whose mass median diameter may exceed 30 Vim. Large
particles traveling at high velocities are very
susceptible to oropharyngeal deposition by inertial
impaction. While the propellant evaporates and the
particles slow down when the device is held away from
the mouth, or when an MDI spacer is used, on average,
only about 200 of the original or nominal dose actually
enters the lungs.
In an MDI, the generation of an aerosol occurs in
what can only be described as an explosive manner since
the propellant containing the therapeutic solution or
suspension disintegrates as it passes through the
aerosol nozzle at very high velocity. As the
propellant flash rapidly evaporates, the liquid
particles decrease rapidly in diameter to the state of
a "dried solute" .
The velocity of the discharged particles entrains
the evaporating particles as they exit the device and
move into the airstream. This velocity is much higher
than an inhalation velocity by a user. The result can
be impaction of particles in the oropharyngeal area. A
spacer, which is discussed later, is a solution to this
problem, i.e., reducing the velocity of the "cloud" of
particles prior to inhalation. Another technique is to
use the "open-mouth" method that implies activating the
device a few cms away from an open mouth.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 14 -
MDIs containing a suspension require that they be
shaken before use. MDIs containing a solution need not
be. This presents a problem to patients using more
than one type of drug, i.e., one in suspension and one
in solution, as the patient may shake the wrong MDI, or
not shake the MDI that needs to be shaken before use.
The latter one would result in an incorrect dose of the
drug being delivered and inhaled. .This is an advantage
to the use of DPIs, as there is no "to shake or not to
shake" decision. MDIs containing propellant and a
suspension or solution, also present a challenge
concerning stability over a temperature range.
A problem with both MDIs and DPIs is that there is
often poor coordination between the patient pressing
the actuator and the timing of the inhalation. One
solution is to use a spacer' between the device and
patient, that will also allow for the heavier particles
to settle before the patient inhales.
Another problem with MDIs is that they are based
on propellants that rely on vaporization to generate
pressure, and a drop in temperature occurs when
vaporization occurs. The vaporized propellant can hit
the back of a user's throat before it has completely
evaporated if no spacer is used. This can lead to
reflex gagging which interrupts the continuous and deep
inhalation required for optimum delivery of the drug.
In addition, water moisture in the mouth will condense
rapidly in the cold vapor, causing the small liquid
medication droplets to coagulate and drop out, reducing
the percentage of drug actually deliverable past the
oropharyngeal area.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 15 -
DPI is a dry powder inhaler consisting of a drug
in micronized dry powder form provided in a compact
shape and contained in a unit dose container or
reservoir, which is fluidized by the flow of a gas and
inhaled by the patient.
Micronized dry powder formulations are very
soluble and quickly dissolve in the fluid layer on the
surface of the deep lung before passing through the
thin single cellular layer of the alveoli. They are
then deposited in the alveolar region and can be
absorbed into the bloodstream without using what are
commonly referred to as penetration enhancers. Dry
powder aerosols can carry approximately five times more
drug in a single breath than metered dose inhaler (MDI)
systems and many more times than; liquid or nebulizer
systems. -
Micronized dry powder drugs used in inhalers are
usually produced with an original particle range of 1-
10 microns. An individual dose as loaded can take from
5 mg to 20 mg of dry powder drug. A lower total amount
of dry powdered drug is possible with purer drugs, or
with drugs that do not require or are packageable
without excipients. Examples of excipient carriers
used in dry powder drug formulations include lactose,
trehalose, or crystalline or non-crystalline mannitol.
Trehalose and mannitol, which are spray dried sugars,
are better dispersal agents than lactose.
Thus, the "drug substance" in a DPI consists of
the pure drug, plus a sugar if an excipient is used,
compared to the multitude of constituents contained in
a MDI. This multitude of constituents in a MDI
increases the work involved in production of the
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 16 -
product and its packaging, can effect formulation
stability, can cause aerosolization problems by
clogging the nozzle, and may require either the shaking
or non-shaking of the MDI Inhaler before use.
In DPI devices, providing compressed gas or
propeller/impeller assisted fluidization, basing the
fluidization on the patient's inhalation produces a
major variability in dosing and particle size
formation. The velocity, ramp up rate, and continuous
event of this inhalation are variables that can effect
the fluidization of the powdered drug and the effective
delivery of the optimum size particles to the deep
lung. The higher the rate of gas velocity, the finer
the particle size created during fluidization, but the
greater the possibility for impaction of:,particles in
the oropharyngeal area during inhalation; where the gas
velocity which fluidizes the dry powder drug is derived
from the " suction°' or negative pressure of a strong
inhalation.
Devices that rely on the force of the patients
inhalation, also operate based on the "suction" or
pulling effect of said gas flow, i.e. a negative
pressure, to pull apart and fluidize the drug powder.
This is less effective than a highly focused directed
stream of high pressure gas, which is consistently
delivered at the same pressure.
Some DPIs use compressed air generated by a
pumping mechanism, which the patient utilizes, whereby
the pressure is released for fluidization of the powder
drug when the system is actuated. The pressure, and
therefore velocity, of a gas that can be generated by a
hand pump or an inhaler device, is far less than that
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 17 -
available from a compressed gas cartridge. The
uniformity of fluidization of the dry powder would
therefore lae less using a manual hand pump, with the
possibility therefore of generating larger percentages
of larger size particles, which result in the variable
and inconsistent loss of drug in the oropharyngeal and,
upper bronchi.
The higher the velocity of the gas hitting the dry
powder, the greater the amount of powder dislodged and
the turbulence induced, which can create a cloud of
particles for inhalation. In the case of dry powder
inhalers, the ramp speed to the velocity required to
deaggregate'or deagglomerate the dry powder into fine
particles, is as important a factor as velocity in
determining effectiveness.
Systems using dry powder drug in capsules;.~r_equire
the patient to load the capsules individually, whether
the system is capable of being loaded with one dose at
a time, or several doses for multi dose use over time.
In some of these devices, the capsule is crushed to
thereby release the powder contained therein.
A DPI entrains the fluidized drug powder and sends
it through a narrow gap, increasing the velocity of the
gas and powder to improve deagglomeration by turbulence
and reduce the number of large particles by impaction
or settling out. Often, a baffle is also included in
the system to trap larger particles.
One problem in using compressed gas vs. a hand
pump to generate compressed air DPI (or a liquid MDI
driven by CFC vapor pressure) is that the compressed
gas pressure will decrease with usage. In the case of
the hand pump driven DPI, the gas pressure is
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 18 -
consistent during each dose fluidization procedure. In
the case of gas driven MDIs, the pressure available for
aerosolization decreases over time near the end of the
capacity, unless the MDI has a cut off which does not
allow dose administration below a certain minimal
pressure required to achieve sufficient aerosolization.
A spacer is a plastic or metal tubelike device
that is placed between the inhaler device and the
patient, and into which the inhaler device delivers the
particulate cloud generated by dry powder fluidization
or liquid aerosolization. A spacer can be open-ended,
allowing a slowing down of the gas, or closed-ended
(holding chamber) to reduce the loss of dose inhaled
due to poor hand-breath coordination. The spacer slows
down the gas mass and particles leaving the inhaler,
traps larger particles by impaction and settling, and.:
wprovides a better control of inhalation rate and
timing, delivery of the desired size range of
particles, and reduced oropharyngeal loss of particles
due to impaction, versus inhaling directly from the
inhaler device. It also reduces the gagging effect
from inhaling a cold gas like Freon. Spacers have been
incorporated into the routine use of MDIs.
Inhalation flow velocity in inhalation driven
inhaler determines the quality of the aerosol cloud, as
the greater velocity fluidizing the dry powder drug,
the finer the particles produced. However, the
inhalation of particles at a fast rate, leads to
impaction of a large percentage of particles on the
back of the throat.
Heliox, which is commercially available in a
combination of 700 or 80o helium in oxygen, has been
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 19 -
used for over 70 years in respiratory therapy. Heliox
is administered in some hospitals and emergency rooms
in large gas cylinders. The most popular types are the
"K" cylinder that stands 51 inches in height, 9 inches
in diameter and weighs 130 1b when fully filled.
Heliox is supplied at 2,200 psig and requires a two-
stage pressure regulator to reduce the pressure for
administering to patients. However, due to its
bulkiness and requirement of sophisticated pressure and
flow regulators, it is used only in research and
hospital facilities.
Gas flow within the tracheobronchial tree is
complex and depends on many factors. For a given
pressure gradient, the volumetric flow rate of a gas is
inversely proportional to the square root of its
density. In accordance with the subject invention, it
has been found that substituting helium for nitrogen in
inhaled gas mixtures results in increased gas flow
rates because the density of helium is much lower than
that of nitrogen.
Resistance to the flow of gas within the
tracheobronchial tree results from connective
acceleration and friction. Connective acceleration is
the increase in the linear velocity of fluid molecules
in a system of flow in which the cross-sectional area
is decreasing. Frictional resistance may be either
turbulent or laminar depending on the nature of the
flow. Since resistance associated with these factors
is density-dependent, breathing a less dense gas should
decrease flow resistance and, consequently, reduce
respiratory work. An obstruction in the upper airway
causes a resistance to flow that is primarily
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 20 -
convective and turbulent and therefore susceptible to
modulation through a change in -gas density. For
respiratory treatment, it is desirable to create a flow
of minimum pressure drop or flow-resistance.
Gas flow in airways may be laminar, turbulent, or
a combination of the two. Turbulence is predicted by a
high Reynolds number, which is a unitless quantity
proportional to the product of gas velocity, airway
diameter, and gas density divided by viscosity. The
Reynolds number is also expressed as the ratio of
kinetic to viscous forces. The decreased density of
helium, when substituted for nitrogen, lowers the
Reynolds number and may convert turbulent flow to
laminar in various parts of the airway. Turbulence is
highly dependent on the surface roughness, so that a
flow in a rough cavity might be turbulent even if the
Reynolds number predicts a laminar flow. Even in the
absence of turbulent flow, the decreased density of
helium improves flow and decreases work of breathing
along broncho-constricted airways.
The efficacy of Heliox in respiratory therapy
occurs because it is a low-density gas. The rate of
diffusion of a gas through a narrow orifice is
inversely proportional to the square root of its
density (Graham's Zaw). When an area of stenosis
occurs in the airway, there is resistance to flow at
the site of the stenosis. The resistance varies
directly with gas density. Downstream from the
stenosis, airflow becomes turbulent. By substituting
helium for nitrogen in inspired air, resistance at
stenotic areas is reduced and turbulence downstream
from the stenosis is either reduced or eliminated.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 21 -
In the tracheobronchial tree, a laminar flow
normally exists in airways that are generally less than
2 mm in diameter. Turbulent flow has been observed in
the upper respiratory tract, the glottis, and the
central airways. This upper portion of the airway,
especially the throat, and the main bronchioles, are
considered to be the region where the turbulent
intensity is sensitive to the gas density.
Since airway resistance in turbulent flow is
directly related to the density of the gas, Heliox,
with its lower density than nitrogen or oxygen, results
in lower airway resistance. Heliox further lowers
airway resistance by reducing the Reynolds number, such
that some areas of turbulent flow are converted to
laminar flow. The Mgher flow rate of Heliox has the
ability to stay laminar at velocities under which air
would be turbulent.
Heliox does not. need to be laminar to provide
higher flow rates and its benefits persist under
turbulent conditions. Some have the misconception
that, due to its lower density, helium is less viscous
than air, so it flows faster. Actually, the absolute
viscosity of helium is slightly higher than that of
air, and its kinematic viscosity (absolute viscosity
divided by density) is about seven times that of air.
Thus, from the fluid-dynamical standpoint, helium is
more viscous than air.
The linear relationship between helium
concentration and resistance to flow is predictable on
the basis of fluid mechanics. Helium has two major
effects in reducing resistance in an obstructed airway.
First, helium reduces the probability of turbulence.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 22 -
Flow of air in the upper airway is turbulent, except at
rest, because of the rough walls of the airway and the
relatively short lengths of the airway segments
compared to their diameters.
The probability of turbulent flow is predicted by
the Reynolds number:
Re ='°YD ( 1 )
Where
D - Diameter of the mouth, airway or throat
(cm)
V - Gas velocity (cm/sec)
p - Density of the gas (g/cc)
- Viscosity (g/cm/sec)
Second, gas flow through an orifice requires an
increase in pressure to maintain the flow:
II C'a 2 ~Pa - Pb ) ( 2
where Pa-Pb is the pressure difference caused by
the orifice (dynes/cm2) , and Co, is the discharge
coefficient, which depends on the sharpness of the edge
of the orifice.
Uo - Velocity through the orifice
(3 - Ratio of orifice diameter to pipe
diameter
Pa - Pressure at upstream before orifice
Pb - Pressure at downstream after orifice.
p - Density
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 23 -
In summary, Heliox is more beneficial because of
its lower density. Compared to air, it flows at a
higher flow rate for fixed pressure gradient, or needs
a lower pressure gradient or work of breathing (or
patient inhalation effort) for a given flow rate. This
is valid even in turbulent conditions.
There is medical literature where Heliox has been
provided to a patient prior to dosing with an Inhaler
based on a CFC based propellant. There is also a study
where a small volume of Heliox (40-70m1) was delivered
as bolus but with a shallow breath during pulmonary
administration of a particulate to see if the entrained
particles would diffuse deeper into the lungs by
themselves within the Heliox gas.
There is also literature where Heliox was used
with a nebulizer to deliver a drug~in liquid form. Most
of the time, the velocity of the nebulizer gas flow was
based on that used for air. In other cases where the
gas flow velocity was altered, the aerosol nozzle used
was designed for air and not Heliox or pure helium, so
that the particle size distribution was not adapted to
the change of gas.
Two factors that can influence the delivery of an
optimally fluidized dry powder drug formulation are
static electricity and humidity. It is desirable to
avoid imparting a static electricity charge to the fine
particles, especially those 1 micron or less in size.
The static charge will form an attractive force on the
particles, causing them to clump together, rendering
them of a collective size that is unsuitable for deep
lung delivery. This type of particle cohesion is
highly undesirable because a few particles that are
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 24 -
attracted together can double or triple the terminal
settling velocity. This is a key reason why
conventional inhalers using inhaled air, propeller
driven air, or compressed air pumps, have more than 500
of the drug lost in the mouth and throat, before they
can enter the lung.
Moisture in the fluidization gas can also result
in the clumping of particles. This is a disadvantage
of using inhaled air, air from the surrounding
environment driven through a propeller, or air
compressed using a hand pump that is part of an
inhaler. If an inhaler is used in a humid geographical
location or during humid seasonal conditions, the
humidity can affect the deliverable dose of drug
particles in the size range required for::penetration
into the deep lung, thereby affecting the dose.
In addition, if moisture comes in contact with the
powder before it is fluidized, the moisture can
accumulate on the outer layer of the powder, forming
lumps before fluidization occurs.
The subject invention system can be light enough
to be portable, and small enough for a child up to an
adult to hold and use.
It is an object of the subject invention to
provide an inhaler that can deliver appropriate sized
particles to the lungs efficiently using a propellant
with sufficient pressure to fluidize or aerosolize a
drug to be used by a patient.
SUMMARY OF THE INVENTION
The present invention describes in detail an
inhaler for medical purposes where the main carrier gas
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 25 -
is Heliox or helium. One embodiment of the invention
is an, inhaler for introducing a drug into a user, said
inhaler comprising:
a first chamber adapted for containing first
a compressed gas at a first pressure;
a second chamber in selective communication
with said first chamber, said second chamber adapted
for containing a second compressed gas at a second
pressure lower than the first pressure, said first and
second chambers cooperating so as to yield said second
pressure of said compressed gas within said second
chamber;
a means to administer two different volumes
of gas in successive applications from the second
chamber;
a storagesection coupled to said second
chamber, said storage section adapted for containing a
drug and operating such that a portion of said second
compressed gas can fluidize and aerosolize said drug to
thereby produce a drug cloud; and
a mouthpiece coupled to said storage section,
said mouthpiece adapted for receiving said drug cloud
and convey said drug cloud to a user.
The inhaler is comprised of three mostly
independent parts: a high-pressure canister, a drug
delivery holder; and a spacer. The three parts can be
separable from each other or affixed in a non-separable
way. The canister can have a resealable, refilling
opening and the drug holder can be removable and have a
resealable refilling means. The high pressure canister
holds pressurized Heliox or helium and delivers two
constant volumes of gas at a fixed pressure
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 26 -
independently of the inside pressure of the canister.
One volume of gas can go directly to the spacer to
purge it from ambient air, while the second, smaller,
volume of gas will.interact with the drug. The drug
drum holds several doses of drug in liquid or powder
form that will be nebulized or liquefied using the
second volume of gas from the canister. Finally a
spacer is used to hold and mix the two volumes of gas
from the canister and opens up to the patient.
Alternatively, only one volume of gas can be released
by the canister to purge and nebulize the drug in one
process.
These aspects, as well as others, will become
apparent upon reading the following disclosure and
.corresponding drawings. The drawings will cover only.
r~some embodiments of the invention to explain its
overall functionality. There~is wide room for design
changes on the technical aspect of the gas delivery for
instance. No figure is drawn to scale.
In order to position a helium/Heliox canister on
the market, it is necessary to produce a product of
similar weight and dimensions as current MDIs. The
weight when full is estimated at 50 grams. Helium
itself is a light gas and will contribute only slightly
to the overall mass of the canister. Indeed, 300 ml of
pure helium weights 50 mg, so 100 doses of 300 ml would
only weight 5 grams.
It is hence preferable to minimize the weight of
the canister. Its sizing, however, depends on the
inside pressure of the gas, but pressure will limit the
total amount of gas in the device, or the total number
of doses available. We will base our calculations on
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
27 _
average canister dimensions of 80 mm height by 40 mm
diameter, containing roughly 100 cc of gas. Assuming
doses, or 3 liters of gas, the canister will need to
be pressurized at 500 psig. The device would then
5 weight 50 grams using steel (stainless or carbon). If
we want to deliver 50 doses (comparable to existing
MDIs), the canister should then be pressurized at 3,200
psig and will weight 320 g (steel). See Table 2 for
details.
Table 2: Design of proposed canister.
Height 80 mm, diameter 40 mm (dimensions based on
existing MDIs).
Number of doses 1 10 50
Inside pressure (prig) 50 500 3,200
Volume of gas (liters) 0.34 3.3 15
Thickness (mm) 0.05 0.5 3.2a
Weight Full (grams) 5 50 320
aThe overall dimensions of the canister are changed
due to the high thickness.
An optimization of the dimensions of the canister can
be easily done so to have an acceptable overall weight,
inside pressure and number of doses available. For
instance a 25 cc container at 1,600 psig can also
deliver 10 doses, while weighting 30 g.
A helium canister can not compare to existing MDIs
as described herein. For a similar number of doses, it
will be too heavy and pressurized at dangerous levels.
This is due to the fact that the canister needs a much
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
t - 28 -
higher amount of gas per dose to fully use helium
properties.
The solution is to design the canister for a very
limited number of uses. Synchronizing the number of
available doses in the canister with the number of drug
packages in the drum (plus a residual volume necessary
to delivering the gas) ensures that patients will never
operate their devices without the necessary drug. A
cylinder containing the necessary amount of
helium/Heliox for 10 doses would weight roughly 40
grams full (37 grams material, less than 1 gram for the
gas), which is comparable to existing devices. Finally
it would market itself along with existing DPIs in
terms of number of available doses, but with a much
better efficiency due to the use of Heliox/helium for
better drug delivery and the absence of patient hand-
breathing synchronization.
In order to help leverage the cost of the inhaler
over longer period of uses, the canister can be
refillable. In this case, the user would also have a
bigger, high-pressurized helium/Heliox cylinder at home
and would refill his small inhaler canister with a
simple process after a certain number, 10 for instance,
of uses. This idea is novel to inhalers and would
allow the patient to use their inhalers for months at a
time without a refill from health care providers. In
this case, the drug drum could be allowed to contain a
much higher number of doses. Refilling the cartridge
could be done with no or very little modification to
the current proposed cartridge and gas delivery
designs.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 29 -
The canister's maximum pressure is 500 psig. An
E-cylinder is typically filled up to 2,200 psig for a
total content of 623 liters for 1000 helium or 708
liters for pure oxygen. Using an E-cylinder to refill
the canister with a basic regulator set up for a
delivery of 500 psig would allow refilling the canister
with 1,600 doses or 480 liters based on the content for
the helium E-cylinder.
Practically, the in-home Heliox tank would have a
standard regulator set up for a delivery of 500 psig.
The easiest way to refill the canister is to have a
separate value on the canister for refilling purposes
only. The valve could be similar to a standard one-way
refilling valve as used on footballs for instance and
located on top or on:the side of the canister to avoid
any interference with the metering chamber inside the
canister. For aesthetic and safety reasons, it is
preferable that no extension protrudes from the
cylinder. The valve would only open if the proper stem
from the in-home refilling tank is inserted and, due to
the regulator of the home cylinder, would refill the
portable cylinder to exactly 500 prig, or 10 doses.
The operation would only require the user to push the
cylinder on the valve stem and would last a few
seconds. A pressure gage on the home cylinder would
let the user know when the inside pressure falls below
500 psig, the pressure when the cylinder would be
considered having reached the end of its usable life.
Alternatively a counter device would let the user know
how many refills are available in the home cylinder.
The whole refilling system would only require the
regulator on top of a standard medical cylinder along
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 30 -
with the specific valve stem and the pressure gage or
dose counter. This clearly limits the overall cost of
the device. Renting the home cylinder to the user
would further reduce the costs by reusing the device
and refilling it in specialized facilities in a similar
fashion to existing Oxygen cylinders.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a side view of an inhaler, diffuser, and
spacer in accordance with the invention;
Fig. 2 side view of a piston-chamber assembly to
deliver the two volumes of gas;
Fig. 3 is a an alternative to Fig. 2 to deliver
the two volumes of gas using two gas orifices, one
being a calibrated orifice;
Fig. 4 is side view of,a drug drum assembly;
Fig. 4A is a sectional~view of A-A of the drum of
Figure 4 used to hold a drug in accordance with the
invention;
Fig. 5 is an alternative to Fig. 4.
Fig. 6 is a side view showing the engagement of
the drum and an equalization chamber;
Fig. 7 is an enlarged side view of a tube
containing a liquid drug;
Fig. 8 is an enlarged side view of an alternative
embodiment of a tube containing a liquid drug;
Fig. 9 is a side view of a tube adapted to be
coupled to a fixed nozzle;
Fig. 10 is a side view of an alternative coupling
of a tube with a fixed nozzle;
Fig. 11 is a side view of a spacer in accordance
with the invention;
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 31 -
Fig. 12 is a side view of another embodiment of an
inhaler and diffuser in accordance with the invention.
DETALLED DESCRIPTION OF THE PREFERRED EMBODIMENTS)
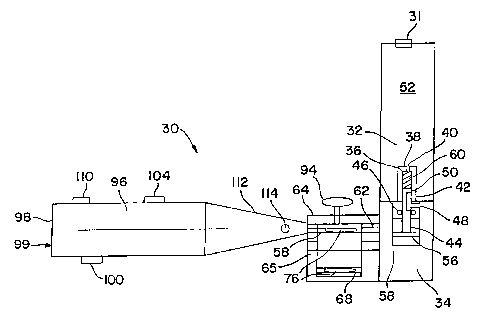
Referring to Fig. 1, there is shown an inhaler 30
in accordance with the invention. Inhaler 30 comprises
a high pressure chamber 32 coupled to an equalization
chamber 34. High pressure chamber 32 is a small, cold
rolled, low carbon steel container containing gas 52
compressed to a pressure between about 30 psig and
about 1600 prig, preferably between 100 psig and about
500 psig. Gas 52 is a gas preferably containing from
Oo to 1000 of helium, the balance if needed being
oxygen. Other compressed gases could also be used. It
is preferred that the gas that is~:.used be a dry gas.
The high pressure storage allows H.eliox to be stored in
a container preferably 10 cc to 100 cc in volume but
still provide sufficient gas for a;large number of
inhalations. For example, 100 cc of Heliox at 200
atmosphere will expend 200 times in volume to a volume
of 20 liters when the gas is released to atmospheric
pressure.
To provide Heliox at a constant pressure, the
storage pressure in chamber 32 should be significantly
higher than the regulating pressure. When the supply
pressure of the compressed Heliox falls below the
pressure required to fluidize the powder (or aerosolize
a liquid) to the uniform standard established, then the
inhaler should become inoperative, and a cut off
mechanism is thus desirable. The chamber could have a
resealable, refilling opening 31 to which a user can
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 32 -
couple the canister to a larger high-pressurized Heliox
tank.
High pressure chamber 32 includes a housing 36
defining a third chamber 38. Housing 36 includes an
opening 40 on a top portion thereof and a gas passage
42 on a side. Third chamber 38 communicates with both
high pressure chamber 32 and equalization chamber 34.
Equalization chamber 34 is needed to produce a
consistent volume of gas throughout the lifecycle of
the high-pressure canister 32 independent of its inside
pressure. This is achieved with the help of a simple
regulator via the diaphragm plate 56. Gas will flow
from the high chamber 32 to the equalization chamber 34
until equalization chamber 34 has reached its nominal
pressure, constant value much smaller than the high-
pressure at which the gas is stored in the canister 32.
Equalization chamber 34 includes a housing 58
having a gasket 46 disposed therein. Gasket 46
includes a gas passage 48 on a side thereof for
allowing gas disposed in third chamber 38 to
communicate with second chamber 34.
A piston 44 is slidably mounted within gasket 46
and within housing 36. Piston 44 includes a
communication opening 50. Piston 44 is pushed
downwards with a spring 60 located inside chamber 38 to
allow gas communication between chambers 32 and 34.
When the canister 32 is separated from the inhaler, the
spring 60 is pushing the piston 44 sealing the canister
by closing the opening 42. When the canister is
inserted in the inhaler, the tip of the piston 44 will
rest on the diaphragm 56, and pushing the piston 44 up
inside chamber 38 just so that high-pressure gas
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 33 -
passage 42 is communicating with the communication
opening 50. Communication opening 50 is designed to
selectively allow gas 52 stored in high pressure
chamber 32 to communicate with gas 54 stored in
equalization chamber 34. A pressure plate 56 is
also disposed within housing 58. One side of pressure
plate 56 is coupled to piston 44.
Through the use of high pressure chamber 32 and
equalization chamber 34, inhaler 30 produces a desired
gas pressure without requiring an external pump or
inhalation pressure from a patient. When the pressure
inside the equalization chamber 34 is too low to allow
inhaler 30 to be used, it is desirable that the high-
pressure Heliox 52 from high pressure chamber 32 will
fill into equalization chamber 34. Spring 60 anal
pressure plate 56 are designed so as to facilitate this
operation. As stated above, piston 44 has a
communication opening 50 that selectively allows high
pressure chamber 32 to communicate with equalization
a chamber 34 through gas passages 42 and 48 when passages
42, 48 are aligned with communication opening 50.
Gas 52 applies pressure against a small area
defined by the top of piston 44. The net force from
gas 52 pressing on piston 44 is the pressure of the gas
multiplied by the surface area of the top of piston 44.
This net force applied by the high-pressure side of
high pressure chamber 32 on piston 44 works with the
biasing force of spring 60 and against the force
applied by gas 54 on pressure plate 56.
The spring constant of spring 60 and the surface
area of pressure plate 56 are chosen so that when
equalization chamber 34 has received sufficient
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 34 -
pressure to utilize inhaler 30, the force applied by
gas 54 on pressure plate 56 will exceed that of the
force produced by gas 52 on piston 44 on the high-
pressure side of the device and the force of the spring
60. At such a time, the force applied by gas 54 will
cause piston 44 to move upward within housings 58 and
36. As piston 44 moves upwardly, communication opening
50 will move away from gas passage 48 effectively
stopping any additional high-pressure Heliox 52 from
entering equalization chamber 34. The spring 60 will
push the piston 44 downwards to allow gas passage from
chamber 32 to chamber 34 no matter what the pressure is
inside chamber 32. Since the surface of the pressure
plate 56 is quite important, the two main forces
..balancing the piston are the spring force and the
.,..:pressure force from the equalization chamber 34. The
spring constant of spring 60 and the area of pressure
plate 56 are thus selected for a specific pressure
rating so that a patient will always receive the same
volume of gas and dosage for their applications,
independent of the pressure change in high-pressure
chamber 32.
Once gas 54 is dispensed (i.e., inhaler 30 has
been actuated and the medication in inhaler 30 is
delivered to the patient), the pressure exerted by gas
54 on pressure plate 56 is lower and the high-pressure
Heliox 52 along with the spring 60 will force piston 44
downwardly thereby repeating the cycle described above
until equalization chamber 34 once again has a desired
pressure of gas therein.
An alternative to this delivery system can be done
using a mechanical actuation by the user. As the high-
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 35 -
pressure chamber 32 is depressed, the piston 44 will
allow the gas to escape from the high-pressure gas
passage 42 and be stored into a secondary chamber. The
amount of gas released into equilibrium chamber 34 is
then defined by the volume of this secondary chamber.
The gas is released from this chamber into the
equilibration chamber 34 when the high-pressure
cylinder 32 is returned to its original position. In
this configuration the housing 58 could be used as the
secondary chamber.
The high-pressure Heliox 52 can be stored at, for
example, 1,600 psig. Equalization chamber 34
effectively decompresses this gas so that it has a
pressure of, for example, 32 to 200 psig. Using 22 ml
of the.,200 prig Heliox, the gas will expand to 300 ml
at a pressure of one atmosphere. This is a sufficient
amount of gas for drug delivery in one inhalation.
It should be noted that the equalization chamber
34 can be part of the separable high-pressure canister
32. In other words, the design of the delivery of a
constant volume of gas can be an internal mechanism
inherent to a high-pressure canister that the user can
buy independently of the rest of the inhaler, or it can
be part of the inhaler itself.
Equalization chamber 34 contains a fixed volume of
pressurized gas 54. This gas will be released in two
widely different volumes to the rest of the inhaler.
The first volume released is around 270 ml of gas; the
second is roughly 1/l0th that value, or 30 ml. It is
proposed here that the creation of the two volumes
occurs in separate activation, either triggered
manually by the user (i.e. pressing a trigger twice or
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 36 -
at two positions) or sequentially inside the device.
The drawings will cover 2 different embodiments of the
invention: mainly either delivering two volumes of gas
using a two-chamber piston (Fig. 2), or with the use of
two gas orifices, one being a calibrated orifice (Fig.
3) .
Option 1:
The two-volume delivery can be done first by
having two-chambers as seen in Figure 2. The novelty
aspect lies in the presence of two internal chambers
and a unique piston shape, selectively isolating the
chambers. Option 1 also allows for the delivery of the
two-volumes of gas to be an inside component of the
high-pressure canister 32. The two-volume delivery can
be inherent to the canister design where the two
chambers are an internal mechanism of the canister,
along with the equalization chamber. It is for that
reason that the valve assembly has been designed to
closely resembles existing MDI canister design. If the
two-volume delivery is thought of belonging to the
inhaler instead, the piston shape can be changed to
ease its manufacturing.
The shape of the piston is adapted to deliver
first with a low push activation a high volume of gas
(i.e. 270 ml) that can be used to purge the spacer.
Using a higher push activation will deliver a much
smaller volume of gas (i.e. 30 ml) to nebulize the
drug. Releasing the stem valve allows the two chambers
to communicate with the equalization chamber refilling
them for the next use.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 37 -
Equalization chamber 201 includes an internal
housing 203 defining two different size chambers 204
and 205. Housing 203 includes an opening 208 on top
for communication with the equalization chamber. First
chamber 204 communicates with second chamber 205 via
opening 209, and the outside via the opening 210 in the
main piston 202. Second chamber 205,communicates with
both equalization chamber 201 and the first chamber 204
via the openings 208 and 209 in housing 203.
A piston 202 is slidably mounted within the gasket
203. Piston 202 includes a communication opening 210
comprising of a hollow passage terminated at the bottom
of the piston. The communication opening 210 is
designed to selectively allow gas stored in chamber 204
to communicate with-;the outside of the canister. The
unique shape of thevpiston 202 allows for the two-
process operation.
At rest, piston 202 is pushed downwards by spring
206 located inside the housing 203, isolating the
chambers with an isolation ring. Communication
openings 208 and 209 are opened allowing filling of the
second and third chamber 204 and 205 from the
equalization chamber 201. When the user wants the
delivery of the first volume of gas, piston 202 is
sliding upward relative to the chamber 201. The
opening 210 is now communicating with chamber 204,
releasing the first initial large volume of gas. In
this position, piston 202 pushes against the isolation
ring surrounding opening 209, closing opening 209 and
isolating chamber 204 from chamber 205. All the gas in
chamber 204 will leave until equilibrium is reached
with the outside of the canister.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 38 -
To release the second volume of gas, the piston
202 is moved further up. Gas can now flow from chamber
204 to chamber 205 via opening 209, and to the outside
via opening 210. The O-ring 207 affixed to the piston
202 will now isolate chamber 205 from the main high-
pressure gas in the canister 201 by sealing opening
208. Since opening 208 is smaller in diameter than the
piston, it will also limit the maximum upward movement
of the piston 202 and the overall amount of gas
delivered in one dose. Since chamber 205 is much
smaller than chamber 204, it will deliver a smaller
volume of gas to be used for the drug delivery.
Option 2:
The other option is to control the delivery of the
two volumes using calibrated orifices. In this case,
the design of the high-pressure canister is similar to
existing ones, the novelty lying in the design of the
inhaler main chamber.
The main process is located inside the inhaler,
communicating with the equalization chamber 34 via a
piston 302, as seen in Figure 3. In this case, the
canister 32 along with the equalization 34 can be a
detachable item of the inhaler; it will now be referred
in general term as the canister 301. The delivery of
the two volumes is inherent of the inhaler body.
Piston 302 is pushed open by a user-activated
valve 311. The valve 311 is encased inside a casket
312 which has two gas passages 315A and 315B connecting
the high-pressure gas from the canister 301 to the rest
of the inhaler. 315B is a calibrated orifice that will
allow a very small known flow rate to the inhaler. For
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 39 -
instance a .004" diameter orifice will allow a volume
in one-half second of 21 ml for pure helium at 200 psig
while 315A is a larger orifice, sealed by the secondary
piston 313. Piston 313 is pushed against the casket
312 by the large diameter of the valve 311.
When the valve 311 is first pushed up, it pushes
up piston 302, releasing high-pressure gas from the
canister 301 to the outside via openings 309 and 310.
The hollow diameter of piston 311 is now at the same
level as the secondary piston 313. Aided by the spring
314, the piston 313 slides towards the valve 311
opening channel 315A. High-pressure gas flows via both
orifices 315A and 315B producing the large amount of
gas needed for the purge bolus. When the second volume
of gas is desired, the valve 311 is..pus~ed farther up.
Due to the expansion in the valve~.diameter; the piston
313 is pushed back against 312 sealing orifice 315A.
High-pressure gas can only flow via the calibrated
orifice 315B alone creating the small amount of gas
needed to mix with and nebulize the drug. Valve 311 is
then pushed back down, releasing piston 302, and
sealing the canister 301.
Equalization chamber 34, after the process
described above, now has a desired pressure of gas 54
within it. Upon actuation of inhaler 30, gas 54 will
be utilized by being injected into a gas passage 62 of
a medical storage or drum section 64. Referring now
also to Figs. 1 and 4-6, drum section 64 includes a
housing 65 that contains a rotating drum 66 and
includes gas passage 62. An elastic material 67 is
disposed between drum section 66 and housing 65 so that
drum 66 can rotate freely within housing 65 but still
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 40 -
retain drugs stored therein. Drum 66 is made of
plastic or coated plastic to decrease or eliminate
static electricity, which can lead to agglomeration of
particles of an injected drug. Drum 66 includes a
plurality of tubes 68, 70 that are substantially
cylindrical and extend longitudinally therethrough.
Drum 66 further includes a substantially cylindrical
bore 72 also extending longitudinally therethrough.
Tubes 68 contain a powdered drug formulation to be
administered to a patient, whereas tubes 70 are empty
and hollow to allow communication of gas 54 from
equalization chamber 34 to a spacer 96 so that spacer
96 can be rapidly filled with several hundred ml of
Heliox prior to injection of the fluidized dry powder
drug formulation into spacer 96.
Fig. 1 shows an embodiment where drum 66 includes
tubes 68 and so an attached spacer is not pre-purged.
This is especially useful when the spacer is small in
size. Fig. 4 shows an embodiment of drum 66 that
includes tubes 70.
Fig. 4A shows a cross sectional view along line A-
A of Fig. 4. As shown in Fig. 4A, tubes 70 are empty
and have a diameter that is larger than the diameter of
tubes 68. Tubes 68 contain a powdered medication 76.
The diameter of each tube 68 is dependent on the volume
and weight of dry powder of a specific drug to be
delivered. Both tubes 68 and 70 are packed within
rotating drum 66 so as to maximize the amount of doses
available per rotating drum. For each drug filled tube
68, there is a corresponding hollow tube 70. A
preferred arrangement is for the drug filled tubes and
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 41 -
hollow empty tubes to be arranged in pairs vertical to
each other. A multitude of such tube pairs can exist.
Micronized dry powder drugs can be made in
particle ranges from preferably slightly smaller than 1
micron to 5 microns. A fluidized particle range of
less than 1 micron to 3 microns is most beneficial for
optimal drug delivery, to the deep lung for systemic
diseases. However, particle sizes should be optimized
for both the delivery system, i.e. the inhaler design,
and the targeted location in the lung. Therapeutic
drugs to treat the upper or middle lung for respiratory
diseases, can be up to 5 microns in size in their final
fluidized and delivered form.
Referring to Fig. 4, when rotating drum 66 is to
be used, drum 66 is placed upon spindle 78 so that
spindle 78 is inserted into bore 72 and drum 66 i~s
coaxial with spindle 78: As indicated by arrows 82,
rotating drum 66 is selectively placeable upon and
removable from spindle 78. A plurality of ducts 80 are
disposed between gas passage 62 and both tubes 68 and
70 so as to provide a gaseous communication between
these elements.
Two ducts 80 may be used to feed Heliox from
equalization chamber 34 to spacer 96 with gas flow
25' occurring through ducts 80 first to tubes 70 and then
to corresponding tubes 68. After the allocated number
.of tubes per one concentric ring has been emptied, the
ducts are moved to a different concentric ring and
corresponding tubes. This can be accomplished through
a simple appendage on drum 66 engaging a switch, or, a
contact switch operated by low level current.
Alternatively, one duct 80 may provide gas to both
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 42 -
tubes 68, 70 which is on an assembly that moves, and
which changes position moving from one concentric ring
of tubes to the other.
For example, this can be done by a set of gears
(not shown). The multidose barrel will rotate after
(or before) each use using a gear drive activated by an
external trigger. Duct 80 will be moved gradually to
an inner concentric tube (if the tubes are arranged in
spiral form or to the next concentric filled tube if
all the tubes are concentric). Duct 80 can be also
dropped suddenly after completing most of one rotation.
This is accomplished by removing the last tooth of a
gear so that duct 80 can be forced into an inner track
with the help of a spring (not shown.
. In another embodiment of drum 66, illustrated in
:r~Fig. 5, all of the tubes 68 in drum 66 contain dry
powder drug and there are no corresponding hollow tubes
70. Instead, Heliox used to prefill spacer 96 is
channeled through a spindle 78 on which drum 66 is
mounted and rotates. This allows for a doubling of the
number of tubes in drum 66 that contain the dry powder
drug formulation. In both embodiments, shown in Figs.
4 and 5, tubes 68 shown in dark indicate tubes that
still have medication 86a and 86b within them. Tubes
68 that are open indicate tubes that no longer have
medication to be dispensed within them.
In this embodiment, only one duct 80 at a time
need be coupled to a corresponding tube 68. This is
because spindle 78 is used to provide communication of
gas 54 with spacer 96. Additional ducts 80 could be
used for a different concentric ring of tubes 68. The
embodiment of Fig. 5 would increase the number of tubes
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 43 -
containing drug powder or liquid in rotating drum 66,
raising the mufti-unit dose capacity of a single
disposable plastic barrel.
If the embodiment of Fig. 5 were used, ducts 80
would act as a source of propellant and fluidizing
energy for the drug in the tubes. Each duct is
activated by a mechanical means when the ducts are to
be utilized. Alternatively, a single Heliox source
needle can change position to access each circular row
O
of drug bearing tubes in succession.
As shown in Figs. 1, 4 and 5, a clear sealed
plastic overlay 86 is disposed on the front 86a and
back 86b of drum 66 covering .all tubes 68. Plastic
overlay 86 contains and protects the dry powdered drug
76 from moisture, provides an anti-microbial barrier,
and keeps tubes 68 clean and moisture free for pre-dose
generation of Heliox gas injection into the spacer 96.
Plastic overlay 86 will have a surface strength
marginally less then the pressure of gas 54. When gas
,54 is injected into drug filled tube 68, plastic
overlay 86a bursts inward into tube 68. A buildup of
pressure from Heliox 54 then occurs in tube 68, and
explosively blows plastic overlay 86b existing on the
spacer side of tube 68 thereby fluidizing powder 76
into the environment of the spacer.
The engagement of rotating drum 66 with ducts 80
is illustrated with reference to Fig. 6. Rotating drum
66 includes a receptacle 88 for hermetically receiving
duct 80 therein. Rotating drum 66 can be designed so
that plastic overlay 86a covers the entire front of
rotating drum 66 and receptacle 88 is affixed over
plastic overlay 86a. Alternatively, receptacle 88 may
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 44 -
have a plastic membrane similar to plastic overlay 86
built into it. Duct 80 includes beveled portions 90
made of a strong but pliable material so that when
ducts 80 are inserted into receptacle 88, a small
amount of physical pressure is required to maintain a
tight friction based seal between receptacle 88 and
duct 80 during injection of the Heliox gas. Receptacle
88 further has a trapezoidal shaped appendage 92
designed to accept duct 80 on a hermetic fitting basis
at the pressure required for operation and drug powder
fluidization.
Alternative embodiments of rotating drum 66 are
shown in Figs. 7 and 8 where tubes 68 may contain,
instead of powdered drug 76, a liquid drug 69 disposed
therein. In the embodiment shown in Fig. 7, a
micropore aerosol nozzle 71 is disposed in tube 68 at
an end opposite duct 80. As with the prior
embodiments, plastic overlay 86 keeps liquid drug 69
within tube 68 until it is desired that liquid drug 69
be administered. In this embodiment, a space 85 is
provided between aerosol nozzle 71 and plastic overlay
86B. Space 85 could be 0.25 inches or larger. Aerosol
nozzle 71 is a hard structure with micropores. Plastic
overlay 86B will expand and stretch before it ruptures
so it should not lay on top of the aerosol nozzle 71.
Space 85 will also prevent plastic overlay 86 from
sticking over aerosol holes in aerosol nozzle 71 when
plastic overlay 86 ruptures.
Referring to Figs. 9 and 10, another embodiment of
tube 68 is shown. In this embodiment, again tube 68 is
divided into a liquid container part 68a and gas
conveyer part 68b. Liquid drug 69 is contained within
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 45 -
liquid container part 68a through the use of plastic
overlays 86a,b. Tube 68 further includes a friction
seat 124 that is designed to selectively mate with a
fixed nozzle 126 of spacer 96. In Fig. 10, spindle 78
is shown as the conduit for compressed gas 54. It
should be clear though that tubes 70 could also be used
in a drum 66 that has a friction seat 124 which mates
with a friction nozzle 126.
In the embodiments shown, where a fixed nozzle is
implemented, when drum 66 rotates to align a new drug
to be administered, drum 66 is moved forward toward
spacer 96 and press fitted therein. This forward
performed movement may be accomplished mechanically or
manually by the patient.
In all of the embodiments discussed above, when
gas 54 is applied to::.tube 68; the application causes an
explosion of plastic overlay 86a blowing into chamber
68. This explosion, combined with the combination of
gas pressure plus drug 69, 76 inside tube 68 bursts the
plastic overlay 86b covering the other side of tube 68.
This bursting of plastic overlay 86 provides a large
explosive and subsequent turbulent effect to fluidize
powder 76 or aerosolize liquid drug 69. The drug-
Heliox combination is then introduced to a spacer 96
(Fig. 1), which has an environment of about 270 ml of
Heliox gas. An advantage of this method, is that the
ducts do not have to penetrate a pre-scored vapor
barrier which might clog the duct but instead the ducts
provide a gas to blow out the plastic overlays based on
a predetermined plastic film strength and: gas pressure.
Disposable multidose drum 66 is designed so that
it can only be inserted onto spindle 78 on which it
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 46 -
rotates in the correct manner. That is, a position
where the front of the drum 66 is inserted correctly
juxtaposed to where the Heliox sources) ducts are
located.
Referring now to Fig. 1, the operation of inhaler
30 will now be explained. An activating trigger 94 is
disposed on drum section 64. Trigger 94 can have
several stops like a multi-action pistol trigger, or
may have an activating button plunger with the same
multi-action inducing activities. When trigger 94 is
depressed, drum 66 is rotated about spindle 78 to a
correct position for the next dose. An inhalation port
door 98 coupled to a mouth piece 99 of spacer 96
closes, thereby inhibiting a user from inhaling gas
disposed within spacer 96. Alternatively, inhalation
port door 98 could be first,:left open so that the air
in spacer 96 is more efficiently purged out (as
discussed immediately below). Thereafter, inhalation
port door 98 is closed as above. Yet another
embodiment includes using a combination pressure/vacuum
port (not shown) which opens to let air out during the
purging phase and closes during the drug delivery
phase.
If an embodiment shown in the drawings is used, a
quantity of 230-270m1 of Heliox gas is injected into
spacer 96 from equalization chamber 34 to gas passage
62, through tubes 70 or spindle 78, through another gas
passage 63 (not shown in figures) in housing 65 and
finally through a compressed gas input port of spacer
96. The air in spacer 96 is pushed out or purged
through a pressure port 100 so as to assure as close to
a 100% Heliox is present in the ambient spacer
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 47 -
environment as possible. The Heliox gas provides both
a spacer environment for settling of the heavier
undesirable particles, and provides a large bolus front
wave of gas in which the desired fine particle fraction
will be entrained during inhalation. This will provide
a sufficient amount of volume of Heliox gas to have the
desired effect on delivery of particles in to the deep
lung.
In the embodiment shown in Fig. 1, the spacer 96
is not necessary pre-purged. Therefore, all the volume
of Heliox in the equalization chamber is used to
fluidize and deliver the drug only. If pre-purge is
desired, the first volume of Heliox delivered by any
option presented in figures 2 and 3 is first sent
through the tubes 70 or 78 to purge the inside of
spacer 96 then drum 66 rotates, allowing the second
volume of Heliox to nebulize the drug. A double action
trigger can be used to activate the. process in sequence
as presented in figures 2 and 3.
When the process of filling spacer 96 with gas 54
ends, inhaler 30, based on a sequence automated or.
manually activated by trigger 94, shoots 30 to 70 ml of
gas 54 obtained via the options presented in figures 2
and 3 into tube 68 containing the drug formulation.
The gas fluidizes powder drug 76 (or aerosolizes drug
69), driving the drug through drug input port 68 into
spacer 96, and causes turbulence which helps to further
fluidize and deagglomerate the drug. In fact, one port
could be used to deliver both the compressed gas alone,
and a combination of the compressed gas and drug.
Alternatively, Heliox gas 54 used to aerosolize
drug 76 or 69 may be provided in two pulses, of, for
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 48 -
example, 60o and then 400 of the total intended volume.
This procedure assures all powder 76 from tube 68 is
injected into spacer 96, and further adds turbulence to
spacer 96 so,that the particles are kept separated.
At a pre-determined period of time thereafter,
e.g. 0.5 to 5 seconds, a mechanical timer opens
inhalation port 98 so that the patient can inhale cloud
of particles. A combination of a spring, gear and wire
(not shown) attached to trigger 94 can be used to make
inhalation port door 98 close when trigger 94 is
depressed. Depressing trigger 94 also activates the
Heliox purge at the same time. When the patient
releases trigger 94, the spring, gear and wire open
inhalation port door 98 and start the drug delivery. A
vacuum/pressure valve 104 will close automatically
until inhalation door 98 opens and will equalize the
pressure inside spacer 96 until the patient has
finished inhaling.
As the patient inhales cloud of particles, a
vacuum begins to form in spacer 96. At a certain
pressure, vacuum/pressure valve 104 opens allowing
ambient air into spacer 96. By opening vacuum/pressure
valve 104, the patient may continue a steady deep
inhalation of room air following the Heliox bolus, slug
or gas front entraining particle cloud.
Vacuum/pressure valve 104 also ensures that larger
particles that may settle on the bottom of spacer 96 do
not get inhaled by a user. Vacuum/pressure valve 104
can be opened/closed automatically. For example, it
can be made of a piece of flexible metal strip. At
atmospheric pressure, the strip will line up perfectly
with the wall of spacer 96. When the Heliox builds up
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 49 -
excess pressure during purging, vacuum pressure valve
104 will coil upward, keeping the Heliox in. When
inhalation port door 98 is opened by the trigger wire,
the pressure will drop to normal. During the drug
delivery, the patient will breathe in much larger
volume of gases (500 ml to 1.5 liters) than the 230-270
ml. During the inhalation, a vacuum will form as the
Heliox with the drug will be inhaled. Now the metal
strip will coil inwards to allow air to rush in.
This structure prevents excess pressure or
velocity of the Heliox/drug combination. Moreover,
since the whole process occurs within a few seconds, it
is not necessary to make the system leak proof or
strong enough to sustain, any particular high pressure.
... 15 Spacer 96 provides the following benefits:'..
::r..- a) slows down the velocity of the Heliox gas
w plus drug formulation injected~into the spacer;
b) allows sufficient turbulence to keep the
small desirable particles suspended and separated;
c) allows the heavier particles unsuitable for
pulmonary drug delivery to settle out or be trapped in
the spacer; and
d) provides a bolus, slug or initial front of
gas plus drug formulation that is 100% Heliox,
followed, thereafter, by air as part of the same
continuous breath.
Spacer 96 may be further provided with a scented
receptacle 110 disposed on an upper and outer part of
the spacer near to where a patient's nose would be.
Receptacle 110 may be a scented strip containing
essence of vanilla, mint, or another scent, and is
placed near the nose to make the use of the inhaler
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 50 -
pleasant for children and older adults who dominate the
usage population.
Spacer 96 should be constructed of a plastic, or
provided with an inner coating, that eliminates the
generation of static electricity. This is because
static electricity imparted to the drug particles
injected into the spacer could result in clumping and
adversely affect the dose delivered to the patient.
It is desirable for spacer 96 to incorporate in
its inhalation port door 98 an apparatus to prevent the
accidental exhalation by the patient into spacer 96
prior to inhalation, so as to avoid mixing of exhaled
gases with the Heliox and suspended drug cloud of
particles, and to avoid agglomeration of the particles
due to exhaled moisture. It is also desirable for
::~:nhalation port door 98 to be closed upon introduction .:.
of the Heliox ambient atmosphere and Heliox plus drug
formulation into spacer 96, so that only Heliox is in
spacer 96 and a minimal amount of that Heliox is lost
external to spacer 96.
Spacer 96 can be made of rough materials on its
surface. The rough surface serves two different
purposes. It can slow down the Heliox-powder mixture
to a laminar flow by inducing additional boundary layer
drag force. The rough surface can also provide a trap
to hold the large particles.
After the injection of the Heliox gas and Heliox
plus drug formulation into the spacer, it is further
desirable to reduce the Heliox velocity immediately
prior to transit from turbulent flow to laminar flow.
An impact plate (ball or other object) and a diffuser
can accomplish this reduction in velocity. Referring
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 51 -
again to Fig. 1, a diffuser 112 is disposed between
spacer 96 and drum section 64."
Diffuser 112 includes an impact ball 114 at a
portion of diffuser 112 that is proximate to gas
passage. Impact ball 114 is used to reduce the initial
high velocity of highly turbulent gas and drug that
enters diffuser 112. When gas and drug is injected
into diffuser 112, a high-energy flow may concentrate
in the center of the unit. Impact ball 114 helps avoid
this channeling effect. Diffuser 112 is shaped as an
expansion cone to slow down the gas-powder mixture.
The size of diffuser 112 is dependent on the desired
gas-powder mixture velocity hitting the back of the
throat. The velocity should be low enough so that the
flow is:vlaminar.~ At an inhalation flow rate of, 60
Z/min (often the necessary flow rate required b,y some
DPIs to release the drug), the Reynolds number is 670
for pure helium compared to 5,400 for air. Using a
certain mix of helium and air changes the Reynolds
number accordingly, as shown in Table 2. Even if~the
Reynolds number is low enough so that it falls into a
laminar category, the flow might still be turbulent due
to the roughness of the surface for instance. Spacer
96 can be combined with diffuser 112 so that the large
particles can drop out of particle cloud in spacer 96.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 52 -
Table 2: Effect of concentration of helium on flow
regime.
Percentage
of
Helium
(balance
oxygen)
o Helium 0 20 40 60 80 100
Diameter of 1.8
throat (cm)
Inhalation rate 60
(L/min)
Velocity (m/s) 3.9
Gas density 1.43 1.18 0.93 0.68 0.43 0.18
(g/cc)
Viscosity (~,P) 204 201 198 196 193 190
Reynolds number 4950 4140 3310 2460 1670 666
Flow Regime* T T T Trans Trans L
* T= Turbulent, Trans=Transitional, L=Laminar
The function of impact ball 114 can be
incorporated into spacer 96. Spacer 96 could include
an impact plate disposed at an end of spacer 96
proximate to inhalation port door 98. In this design,
the injected Heliox stream and drug particles would hit
impact plate causing impaction and turbulence, and
resulting in a reduction in the velocity of particle
cloud. The impact plate could be tilted~so that the
injected Heliox and drug particles would reflect off
impact plate, thereby resulting in the accelerated
settling of heavier particles and the formation of a
cloud of desired particles containing a desired fine
particle fraction.
Diffuser 112 and spacer 96 can also include a
flow-straightening device. For example, diffuser 112
and spacer 96 can be sub-divided into parallel
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 53 -
channels. The channels will adsorb all the energy from
the random motion of a turbulent flow.
Referring to Fig. 11, there is shown a flow
straightening device that can be used in spacer 96.
Spacer 96 further includes a plurality of shelves 122
disposed proximate to inhalation port door 98. Shelves
122 function so that gas 54 passes over or just above
and below shelves 122, thereby helping to induce a
straightened flow of the Heliox and entrained particles
into the patient.
It should be made clear that spacer 96 and
diffuser 112 are merely additional options that could
be used with inhaler 30. A patient may use spacer 96
only, diffuser 112 only, or neither appendage when
using inhaler 30. If spacer ~96 is not used, mouthpiece
99 should be placed on the end of diffuser 112. If
diffuser 112 is also not used, then mouthpiece 99
should be placed on an end of gas passage 62. It
should also be'clear that when mouthpiece 99 is not
disposed on spacer 96, it is not necessary to further
include inhalation port door 98.
Referring to Fig. 12, there is shown another
embodiment of the invention. Similar elements contain
the same reference numerals described above and their
description is omitted for the sake of brevity. The
inhaler comprises, a venturi section 142 coupled to
chamber 34. Venturi section 142 includes a liquid drug
reservoir 144 having a store of liquid drug 146
contained therein. A gas passage 148 selectively
provides communication between equalization chamber 34
and a venturi 150. The inlet of venturi 150
communicates with gas passage 148. The outlet of
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 54 -
venturi 150 communicates with diffuser 112. The throat
of venturi 150 communicates with a liquid metering tube
152 coupled to liquid drug reservoir 144.
As would be understood by one with ordinary skill
in the art, when gas 52 passes through venturi 150,
since the throat of venturi 150 is constricted, a
decrease in pressure of gas 54 is experienced at the
throat of venturi 150. This apparent vacuum sucks a
quantity of liquid drug 146 out of liquid drug
reservoir 144. This quantity of liquid drug 146 is
aerosolized by gas 54 and injected into diffuser 112.
Clearly, diffuser 112 is not necessary as the gas/drug
combination could go directly to spacer 96 or to the
patient.
Another embodiment of the invention would use
ultrasonic nebulization. Ultrasonic nebulization is
more efficient in delivering~properly sized particles
and reducing dead (unused) volume of medication. Its
main disadvantage comes from an increase in temperature
over long~use. This is avoided in the present
invention since the nebulization will only occur via
short puffs. Although not as extensively used as the
Venturi principle, it is in the core of new inhaled
drug delivery systems such as the AeroDose (AeroGen
Inc, Sunnyvale, CA, patent USD474536), Premaire Metered
Solution Inhaler (Sheffield Pharmaceuticals), or the
Vibrating Membrane Nebulizer (Pari GmbH, Germany).
Ultrasonic nebulization uses the excitation of a
piezoelectric crystal vibrated at high frequency to
create waves in the liquefied drug solution placed
directly above the crystal. The oscillation waves then
disrupt the surface and create a geyser-like behavior
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 55 -
at the surface, nebulizing the drug that is then
carried by the Heliox gas passing above the surface on
its way to the spacer. The practical means is not
specifically addressed here, only the concept of adding
ultrasonic nebulization to the helium/Heliox inhaler is
presented. Ultrasonic nebulization can easily be
adapted to the present invention.
In all of the above arrangements, the spacer is
designed to accommodate the total volume of Heliox gas,
to be injected into it both as a bolus of gas and with
the drug dose. The spacer is designed so that the
Heliox gas displaces the ambient air that is in the
spacer prior to the introduction of Heliox and then
replaces it with Heliox gas and drug formulation.
Sufficient gas pressure is needed to optimally
fluidize the powder (or aerosolize:::the liquid drug) in
a manner that particles of the desired size range and
grouping are generated. Therefore,.it is desirable to
have a cut off pressure valve which, when the pressure
in equalization chamber 34 is insufficient to provide
sufficient Heliox volume to fill spacer 96 and a
pressure wave to optimally fluidize or aerosolize the
drug, the inhaler will cease to function. Such a out
off switch could be comprised of a pin hook that is
effective to disengage trigger 94. Pin hook could be
coupled to a spring that could be in turn coupled to a
diaphragm. Thus, when the pressure in equalization
chamber 34 is high enough, the diaphragm would be
pushed towards equalization chamber 34 thereby
elongating the spring. This elongation of the spring
clears the hook from trigger 94 and allows trigger 94
to operate. When there is insufficient pressure in
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 56 -
equalization chamber 34, the hook engages trigger 94
and thereby precludes trigger 94 from operating.
Another embodiment includes employing a pre-calibrated
Heliox gas cylinder that will provide more than enough
Heliox for all the medication inside drum 66.
Furthermore, a pressure activated flag or signal, could
be implemented to tell the user that a cartridge needs
to be replaced.
Since it is critical that patients have access to
medication when needed, a counting method is desired
concerning the number of doses remaining. A counting
method can be placed above or by each drug tube in drum
66,with an indicator for indicating the number of doses
left. Each application of trigger 94 will rotate drum
66 once and when the medication is empty,. the indicator
on drum 66 would indicate that there is no...medication
left in the device.
As the inhaler in accordance with the invention
can deliver different drugs by use of different
multidose drug drum, a clear label with black letters
stating the drug and potency and a color band coding
system can be affixed to the outside of each drum. This
clear label material will not obstruct the contents of
the tubes within the barrel containing the drug
formulation. These features also provide an added
safety measure by allowing a visual verification of
remaining doses, in addition to one provided by a
simple automatic counting mechanism which is a part of
the device which tells the user how many doses have
been used, or how many doses are left. The "zeroing"
of said device can be done manually, or, be
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 57 -
automatically done by a feature of the barrel such as
an appendage.
One advantage of this system, is that drug
containing tubes in a single disposable multi unit dose
barrel can contain the same drug, or, a sequence of
drugs to be taken over the course of a day. For
example tubes 1, 2, 3, 4 may contain a sequential
medication group and tubes 5, 6, 7, 8 a repeat of the
same medication group with each dose, for example,
within tubes 1-4 to be inhaled every 6 hours.
Examples of classes of drugs being investigated
and formulated for pulmonary administration, which may ,
be administered with the invention, include, but are
not limited to, those for chronic obstructive lung
. 15 diseases such as the classes of agents commonly:
referred to as anticholinergic agents, beta-adrenergic
agents, corticosteroids, antiproteinases, and
mucolytics, and include such specific drugs.
Other therapeutic pharmaceuticals for respiratory
disease use in dry powder and/or liquid form with which
the invention could also be used include, but are not
limited to, benzamil, phenamil, isoproterenol,
metaproterenol, Beta 2 agonists in general,
Proctaterol, Salbutamol, Fenoterol, ipratropium,
fulutropium, oxitropium, beclomethasone dipropionate,
fluticasone propionate, salmeterol xinafoate,
albuterol, terbutaline sulphate, budesonide,
beclomethasone di propionate monohydrate, surfactants
such as colfosceril palmitate, cetyl alcohol and
tyloxapol, P2Y2 agonist (rapid stimulates mucus and is
potentially for use in CORD and OF), aerosolized
dextran (for OF), and mannitol powders (for bronchial
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 58 -
provocative challenge). An example of another
therapeutic drug that could be delivered by the
invention is Pentamidine for AIDS related therapy.
Examples of proteins and peptide hormone drugs
that may be administered with the invention, which may
or not be glycosolated, include somatostatin, oxytocin,
desmopressin, LHRH, nafarelin, leuprolide, ACTH analog,
secretin, glucagon, calcitonin, GHRH, growth hormone,
insulin, parathyroid, estradiol and follicle
stimulating hormone and prostaglandin El.
In addition, genes, oligonucleotides, anti
coagulants such as heparin and tPA, anti-infective to
treat localized and systemic bacterial or fungal
infections, enzymes, enzyme inhibitors, vaccines,
anesthetics, pain Millers, and agents that can turn
.:..:certain types of receptors on, off, or enhance their
response are possible therapeutic drugs or action
inducing substances which may be delivered via the
invention.
Ergotamine for the treatment of migraine headaches
and nicotine to substitute for and eventually eliminate
cravings for tobacco, are also therapeutic formulations
that may be administered lay the invention, along with
insulin.
Furthermore, controlled release drugs such as
those that are liposome based and which are designed
for pulmonary drug delivery to treat respiratory and
systemic diseases over a period of time due to the
chronic nature of the illness or the mode in which the
illness responds to medication, or the mode in which
the medication operates, may be administered by the
invention.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 59 -
Existing DPIs use the patient's inhalation alone,
the patient's inhalation assisted by a propeller, or
compressed air generated by a hand pump in a DPI, to
fluidize the dry powder drug formulation. One DPI also
uses compressed air in a plastic pillow that contains
the dry powder drug formulation as an aid to
fluidization.
The present invention offers several advantages
over these approaches to fluidizing a dry powder drug
formulation. First, the volume of gas (Heliox) and its
pressure are independent of the operator's inhalation
velocity, ability to generate a given,level of
inhalation velocity (if, as in some devices, a minimum
threshold is required for release of the powder for
fluidization), or physical. motion. No batteries need
to be checked and replaced periodically as is required
for the propeller driven system. Compressed air does
not have to be pumped prior to each dosing.
DPIs that rely on inhalation power, a propeller,
or hand pumped compressed air, all use air that is from
the environment where the user is present. If the air
is humid, it can cause clumping of the micronized dry
powder drug formulation, resulting in larger particles
that may not reach the upper lung, let alone the deep
lung. A factory produced compressed Heliox source can
be produced as a desiccated dry gas, eliminating this
problem in humid climates. This in turn, would cause
variability in the fluidization, deagglomeration, and
post clumping of dry powder drug formulations, which in
turn effects the fine particle fraction available for
pulmonary administration and effective therapy.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 60 -
A factory-produced source of pressurized Heliox
also provides the advantage of a high velocity gas
stream, which provides the advantage of a more forceful
impact on and fluidization of a dry powder drug
formulation, compared to the force generated by inhaled
air, battery powered propeller assisted air, or hand
pumped. compressed air. The result is that the powder
can be fluidized and deagglomerated more completely,
with the result being a more consistent and effective
use of a unit dose of the formulation, and potentially
a reduction in the nominal drug powder formulation that
must be loaded into the inhaler as more is consistently
deliverable.
The use of tubes, instead of blisters and
reservoirs of powder as in the prior art, allows the
effect of the gas pressure to be magnified in terms of
velocity generated and impact of the gas on the
particles.
The use of a mufti-unit dose disposable drum
with pre-loaded tubes of drug formulation prepared
under factory controlled conditions, is an improvement
over the prior art, which uses a barrel permanently
open on one end, into which the user must insert
capsules, and after use, remove the capsules. First,
there is the factor of user variability in loading the
capsules in the prior art, and of loading the right
capsules if a user takes more than one medication that
may be administrable by the system. Second, powder can
remain in the capsule and tube due to physical
obstruction of airflow by the debris after the capsule
is punctured. Third, because the broken capsules have
to be removed by the user as the barrel is fixed and
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 61 -
not merely used and disposed, there is also residual
powder in the tube. This residual powder can alter the
dose delivered to the patient when another capsule is
put into that tube and used, or, worse, if another
medication is administered, the two powders might mix
with an unknown variable, or perhaps undesired effect
on the patient system.
Another advantage of the compressed low molecular
weight Heliox gas is that it can also be used for
delivering liquid drug formulations. Heliox is a much
better liquid aerosolizing/atomizing agent because of
its high velocity of release and it does not have the
same cooling characteristics of liquid CFCs.
In the present invention, the multidose insert
containing multiple sealed unit doses of liquid drug,
or a reservoir multidose liquid drug source, is stored
separately from the compressed gas. In contrast, in
MDIs, the propellant and drug formulation are stored
together, along with many other additional additive
ingredients. In the case of liquid drug suspension
formulations, the MDI must be shaken before each use to
try to achieve a uniform consistent dosing.
Additionally, temperature changes can make the drug
compound, which is packaged with the propellant, come
out of solution.
The uniquely designed spacer reduces the high
velocity of the Heliox gas and the dry powder particles
or aerosolized droplets of liquid drug that have been
generated, resulting in the desired settling within the
spacer of the larger particles which are neither
desirable nor effective for pulmonary drug delivery.
Existing spacers are filled with ambient air prior to
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 62 -
the entrance from the MDI or DPI of air plus entrained
drug powder or liquid drug droplets.
In the present invention, the spacer is pre-filled
with Heliox gas prior to the mixture of Heliox gas and
entrained drug powder or liquid droplets entering the
spacer. This provides a unique gas environment for a)
a differential settling of heavier particles than air,
and b) a large volume bolus of Heliox plus a desired
fine particle fraction which is then inhaled by the
patient, followed on a continuous inhalation basis with
air, with the Heliox and particles being the inhaled
tidal gas front. The spacer also can have laminar flow
shelves, which help induce the laminar flow of Heliox
plus entrained particles from the "cloud" of fluidized
powder or aerosolized liquid drug formulation upon
inhalation by the patient.:s.The spacer reduces the
velocity of the gas stream to an acceptable cloud of
particles, the undesirable particles settle out, and
the resulting cloud of remaining particles that are of
the desired particle size range can be inhaled. The
laminar flow shelves aid in the introduction of a
laminar flow out of the spacer of the helium gas and
entrained particles.
Then, upon inhalation, it is highly desirable to
keep the particles of the desired size range from
settling. With viscous drag greater than the
gravitational settling velocity, the fine solid
particles can be suspended indefinitely without
settling. On the other hand, additional viscous drag
will cause an excess pressure drop. It is therefore,
desirable to control the viscosity.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 63 -
The ability of this invention to generate initial
high turbulent flow and provide rapid flow deceleration
is important to the performance of the inhaler for
powdered drug delivery.
A high pressure chamber and an equalization
chamber are provided so that Heliox gas can be stored
efficiently under a high pressure and also be used as a
propellant to fluidize or aerosolize a drug at a lower
pressure. Using a mechanical way to systemically
provide two widely different volumes of gas allows to
create first a bolus of gas then a second volume of gas
to fluidize, nebulize the drug, independently of a
variable user activation. By providing a disposable
chamber for storing medication, a user does not have to
manually insert and remove drugs and there is no
concern that the tubes carrying the a drugs will become
soiled from prior administered medication. By
injecting a hermetically sealed spacer with some Heliox
prior to injecting the same spacer with a Heliox/drug
combination, the heavier particles in the Heliox/drug
combination can be settled quicker than in air. Also,
a large volume bolus of Heliox plus a desired fine
particle fraction can be inhaled by the patient,
followed by inhalation of air, with the Heliox and
particles being the inhaled tidal gas front. The
drug/Heliox combination in the spacer is also less
susceptible to external factors such as humidity in the
ambient air as the spacer is hermetically sealed.
Finally, by using Heliox as a propellant, a drug
fluidized or aerosolized by this propellant has a
better chance of navigating the airways and reaching
desired portions of the lung.
CA 02549174 2006-06-02
WO 2005/060480 PCT/US2004/038477
- 64 -
The main costs of the inhaler are the drug
and the manufacturing/parts. The cost of Heliox, while
being an expensive gas by itself, is less than the
other costs. There is then an incentive for the
patient to be able to use the inhaler for a much longer
period than the limited number of doses available in
the canister. Providing the user with an in-home mean
to refill his canister allows him to continue using his
inhaler for longer periods of time without going to the
pharmacy or doctor. The higher cost of the inhaler
would then be paid for by the longer use.
While preferred embodiments of the invention have
been disclosed, various modes of carrying out the
principles disclosed herein are contemplated as being
within the scope of the following claims.. Therefore,
it is understood that the scope of the invention is not
to be limited except as otherwise set forth in the
claims.