Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 1 -
INJECTION MOULDED CONTAINERS
The present invention relates to a process for preparing
a container by inj ection moulding a composition compris-
ing a water soluble polymer, such as polyvinyl alcohol)
(PVOH) .
Clothes washing compositions may be delivered to a
clothes washing machine by a delivery tray from which the
composition is fed into the washing drum, or they may be
placed directly into the washing drum. The washing com-
positions may be in powder, liquid or block form. Liquid
compositions have the disadvantage that they may be
spilt. The same applies to powder compositions. Powder
compositions have the additional disadvantage that they
may produce dust which can be inhaled. These problems
are overcome or lessened when blocks of washing composi-
tion are used. These are normally individually wrapped.
On unwrapping a block, for use, it is still possible that
some dust may be produced. Additionally it is an incon-
venience for the consumer to have to unwrap the block.
Furthermore it is almost impossible for the user to avoid
some contact between the block and his or her skin, so
leading to a requirement for the user to wash their hands
after starting the washing machine. In fact, all of the
methods described involve a risk of contact between the
composition and the skin, and it is desirable in all
cases for the user to wash their hands after starting the
washing machine. Tn this context it should be borne in
mind that many compositions contain enzymes to assist the
cleaning action. Even though the user may tolerate en-
zyme residues which may be left in clothes after washing,
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 2 -
they may still not tolerate contact between the concen-
trated washing composition containing the enzymes, and
the skin.
Similar considerations apply in relation to other areas
including fabric care, surface care and dishwashing.
Thus, in relation in particular to dishwashing composi-
tions, there are also problems of spillage, dust genera-
tion, skin contact and inconvenience.
It is known to package chemical compositions which may be
of a hazardous or irritant nature in water-soluble or wa
ter-dispersible materials such as films. The package can
simply be added to water in order to dissolve or disperse
the contents of the package into the water.
For example, WO 89/12587 discloses a package which com-
prises an envelope of a water-soluble or water-
dispersible material which comprises a flexible wall and
a water-soluble or water-dispersible heat seal. The
package may contain an organic liquid comprising, for ex-
ample, a pesticide, fungicide, insecticide or herbicide.
CA-A-1,112,534 discloses a packet made of a water-soluble
material in film form enclosing within it a paste-form,
automatic dishwasher-compatible detergent composition.
The water-soluble material may be, for example,
polyvinyl alcohol), polyethylene oxide or methyl cellu-
lose.
It is also known to form water-soluble containers by
thermoforming a water-soluble material. For example, WO
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 3 -
92/17382 discloses a package containing an agrochemical
such as a pesticide comprising a first sheet of non-
planar water-soluble or water-dispersible material and a
second sheet of water-soluble or water-dispersible mate-
s rial superposed on the first sheet and sealed to it by a
continuous closed water-soluble or water-dispersible seal
along a continuous region of the superposed sheets.
The above methods of packaging have, however, a number of
disadvantages.
The first disadvantage is that they do not have a par-
ticularly attractive appearance. In fields such as con-
tainers used in the domestic environment, an attractive
appearance for an article is extremely desirable. Liq-
uids contained in envelopes of water-soluble film can
have a limp, unattractive appearance.
The second disadvantage is that it is difficult to form
two or more separate compartments in the packaging so
that two incompatible components are both enclosed but
separated from each other. Although an arrangement has
been described to separate incompatible materials in
flexible pouches in WO 93/08095, the method proposed is
complex and is not currently achievable in large-scale
manufacturing. It cannot, therefore, be used for produc-
ing large numbers of containers.
The third disadvantage is that there is only limited con-
trol of the release profile of the compositions held in
the containers. For example, when a composition is held
between two planar water-soluble films or in a thermo-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 4 -
formed package, the composition is simply released at the
time when the films dissolve or disperse in water. While
it may be possible to control to a certain extent the
timing of the start of release of the contents, there can
be no control over the rate of release of the contents
since the entire film dissolves or disperses at about the
same time. Furthermore it can be difficult to provide an
extended time before the contents of the package are re-
leased. An additional problem also arises with thermo-
formed packages. If the thermoforming is not carefully
controlled there may be inadvertent thinning of the film
material at the points where the material is drawn down
into the mould when it is thermoformed. This could re-
lease the contents of the package early. Additionally,
in all of the above packages, it is not possible to re-
lease different compositions at different times or at
different rates since, as discussed above, it is not pos-
sible to incorporate more than one composition in each
water-soluble container.
The fourth disadvantage is that the containers cannot be
produced at a particularly fast rate. When the contain-
ers are produced by heat-sealing planar films or by ther-
moforming, the containers have to be immediately filled
and sealed. All of these procedures have to be carried
out in succession. This means that it is not possible to
obtain a quick throughput for mass-market goods such as
household products. For example, standard thermoforming
machines can only produce around 400 to 800 containers
per minute.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 5 -
The present invention seeks to provide water-soluble con-
tainers which overcome some or all of the above disadvan-
tages.
The present invention provides a process for the manufac-
ture of a single or mufti-compartment,. rigid, water-
soluble container, containing a detergent composition,
wherein the container is at least partially formed of in-
jection moulded water soluble polymer; the process com-
prising the steps of forming the container, filling with
the detergent composition and sealing, wherein the con-
tainer is allowed to contact / brought into contact with
a plasticiser after sealing.
Preferably the plasticiser is gaseous or in vapour form.
Generally the plasticiser is water. Thus the present in-
vention provides a process for the manufacture of a sin-
gle or mufti-compartment, rigid, water-soluble container,
containing a detergent composition, wherein the container
is at least partially formed of injection moulded water
soluble polymer; the process comprising the steps of
forming the container, keeping the container under sub-
stantially anhydrous conditions, filling with the deter-
251 gent composition and sealing, wherein the container is
allowed to contact / brought into contact with a plasti-
ciser after sealing.
Surprisingly we have found that when the process of the
present invention is used a rigid water soluble container
is produced. More specifically by keeping the injection
moulded container in substantially anhydrous conditions
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 6 -
after formation and before filling with the detergent
composition the rigidity of the container is preserved.
In this way we have found that in the preferred sealing
process (see later) wherein a top sealing film is applied
(usually in a pressure contact process) the container ex-
hibits sufficient rigidity for effective joining/sealing
of the film to occur.
Additionally we have also found that during the preferred
filling operation (see later) the high rigidity of the
container is especially beneficial.
The filling operation is usually performed using a dis-
pensing apparatus. The dispensing apparatus commonly
comprises a nozzle which directs the composition to be
filled into the container. For multi-compartment con-
tainers, especially where each compartment is filled with
a separate composition, it is vital that each compartment
be arranged relative to its appropriate dispensing means
with high predictability to avoid incorrect dispense.
This would otherwise lead to detrimental interaction of
compartment compositions (if more than one composition is
dispensed into one compartment) or wastage of compartment
compositions (if a composition is dispensed outside the
container). The method in accordance with the invention
allows this high level of predictability to be achieved.
Therefore filling issues caused by incorrect dispense are
minimised.
The anhydrous retention conditions may be effected using
common environmental controlled means. As an example, if
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
-
the containers are to be stored before filling the stor-
age conditions need to be controlled so that the humidity
level is low. This can be achieved by the use of de-
humidifiers controlling the atmosphere of the area where
the containers are stored. Alternatively a number of
containers may be stored in a sealed enclosure (such as a
water-tight bag/box, e.g. a metal/plastic vessel) from
which the bulk of the available moisture is withdrawn.
The latter method is particularly suitable, where the
containers need to be transported from the site of forma-
tion to the site of filling.
Most preferably the containers are kept under substan-
tially anhydrous conditions until filling.
The containers produced in accordance with the invention
need not be kept under anhydrous conditions (once filled
with the detergent composition and sealed). After time
the containers will begin to absorb moisture either from
the air or from the composition held within (if the com-
position is an aqueous liquid composition containing free
water). It is believed that the water plasticises the
water-soluble polymer, which was dried during the injec-
tion moulding process. It is believed that water (from
the air or from an aqueous composition held within the
container) lowers the rigidity of the container.
The reduction in rigidity has several benefits. Firstly,
although high rigidity (as described above) is beneficial
during the manufacturing (filling and/or sealing) proc-
ess, consumers generally find overly rigid containers un-
appealing. Following moisture uptake the container de-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
_ g _
velops a desirable tactile quality highly regarded by
consumers.
Additionally the reduction is rigidity (after processing)
improves container stability in storage/transport. The
low rigidity containers display a much greater ability to
withstand impact during storage and transport. Thus un-
desirable rupture of containers is vastly reduced.
The containers have the usual advantages associated with
injection moulded containers. The containers have an at-
tractive, uniform appearance which does not vary between
different containers. Also, a wide variety of different
shapes and designs are available. Furthermore, rigid
containers can easily have various elements incorporated
which are considered to be pleasing to the eye.
Moreover, it is possible to control the release profile
of the contents of the container. Since the container is
rigid, it is possible to adapt the width of all of the
walls of the container to control both the start of re-
lease of the composition as well as the rate of release .
For example, one or more walls may be made thin in order
to have an early release of the composition. Alterna-
tively all the walls may be thick in order to ensure that
there is a delayed release of the composition. The rate
of release of the composition may also be controlled by
ensuring that only part of the container has thin walls
which are dissolved or dispersed before the remainder of
the container.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 9 -
Preferably the water soluble polymer is PVOH or a deriva-
tive thereof.
Other water-soluble polymers may be used either as an al-
ternative or in addition to PVOH. Preferred examples in-
clude poly(vinylpyrollidone), poly(acrylic acid),
poly(maleic acid), a cellulose derivative (such as an
ether or hydroxypropyl methyl cellulose); and
poly(glycolide), poly(glycolic acid), poly(lactides),
poly (lactic acid) or a copolymer thereof
It will be appreciated that the container may be made in
part by any other water-soluble polymer. Therefore, a
further feature of the invention is a rigid, water-
soluble container made of at least two injection moulded
polymers, a "first" water-soluble polymer, preferably se-
lected from polyvinyl alcohol); a cellulose derivative
(such as an ether or hydroxypropyl methyl cellulose); and
poly(glycolide), poly(glycolic acid), poly(lactides),
poly (lactic acid) or a copolymer thereof; and a "second"
water-soluble polymer which polymer when dissolved in wa-
ter is active in detergency.
Thus in an alternative release control mechanism differ-
ent walls or parts of walls of the container may be pre-
pared from different water-soluble polymers which have
different dissolution characteristics. For example, a
first compartment may be fully enclosed by a polymer
which dissolves at a higher or lower temperature than the
polymer enclosing a second compartment. Thus different
components can be released at different times. If the
container holds a solid or gelled composition, it is not
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 10 -
even necessary for the container to fully enclose the
composition. A part may be left exposed, so that it im-
mediately begins to dissolve when added to water.
It is possible for suitable additives such as plasticiz-
ers (other than water) and lubricants to be included.
Plasticizers are generally used in an amount of up to
20wt%, for example from 8 to 20wto, lubricants are gener-
ally used in an amount of 0.5 to 5wt% and the polymer is
generally therefore used in an amount of 75 to 84.5wt%,
based on the total amount of the moulding composition.
Surprisingly we have found that if a plasticiser having a
melting higher than room temperature is used then the ri-
gidity of the containers formed is extremely high. This
has the advantage that the containers maintain their
shape after injection moulding. This in particular sur
prising as water-soluble polymers, especially PVOH, have
the non-desired characteristic to easily deform after the
shaping process.
Thus preferred plasticisers comprise a carbohydrate.
Carbohydrates are usually represented by the generalised
formula CX(H20)y. The term herein also includes materials
which are similar in nature like gluconic acids or amino
sugars which cannot be fully represented by this formula.
Other carbohydrate derivatives like sugar alcohols such
as sorbitol, glucitol, mannitol, galactitol, dulcitol,
xylitol, erythritol, isomaltutose and isomalt fall within
this term.
Most preferred carbohydrates include the more thermally
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 11 -
stable carbohydrates such as sorbitol, glucitol, manni-
tol, galactitol, dulcitol, xylitol, erythritol,
isomaltutose and isomalt.
We have also found that when the containers are formed in
the substantial absence of a plasticiser which is liquid
at room temperature then the rigidity of the containers
after shaping is extremely high.
The container is generally cold water (20°C) soluble, but
may be insoluble in cold water at 20°C and only become
soluble in warm water or hot water having a temperature
of, for example, 30°C, 40°C, 50°C or even 60°C.
For certain applications or uses, containers soluble in
aqueous environments at temperatures as low as 5°C are
also desirable.
In order to ensure that the polymer is capable of being
injection moulded, it is usual to incorporate components
such as plasticizers (as discussed above) and mould re
lease agents in an amount of up to, for example, l5wt% of
the composition. Solids such as talc, stearic acid, mag
nesium stearate, silicon dioxide, zinc stearate, and col
loidal silica may be used as mould release agents.
Poly(vinylpyrollidone) may be moulded at temperatures of
from 120-180°C, depending upon the formulation selected
and the melt flow index required.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 12 -
Poly(acrylic acid) may be moulded at temperatures of from
180-220°C, for example, depending upon the formulation
selected and the melt flow index required.
Poly(maleic acid) may be moulded at temperatures of, from
180-220°C for example, depending upon the formulation se-
lected and the melt flow index required.
PVOH may be moulded at temperatures of, for example, from
180-220°C, depending upon the formulation selected and
the melt flow index required. The PVOH preferably used
to form the container of the present invention may be
partially / fully alcoholised or hydrolysed. For example
it may be from 40-99%, preferably 70-92%, more preferably
about 88%, alcoholised or hydrolysed polyvinylacetate.
Preferably the container is a container enclosing a wash-
ing composition.
All of the polymer compositions may also include other
components such as colouring agents and components which
modify their properties.
Injection moulding techniques are well known to the
skilled person and are well described in the literature
(see, for example a good summary is provided in "The
Wiley Encyclopedia of Packaging Technology" Wiley Inter
science 1986). Special techniques, described below, are
preferred features of the invention for producing con
tamers having more than one type of polymer.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 13 -
Simultaneous injection moulding
1) two or more polymers are molten mixed and injected
into a mould;
2) two or more polymers are injected into a mould through
more than one gate, each gate allowing simultaneous
injection of a single polymer or molten mix into the
mould;
3) simultaneously injection moulding two or more compart-
ments and then joining the compartments together.
Sequential injection moulding
1) multi-component injection moulding;
2) sandwich injection moulding;
3) sequentially injection moulding two or more;
4) compartments and then joining the compartments to-
gether.
Multi-component injection moulding covers two distinct
processes
A) injection moulding a polymer or molten polymer
mix into a mould, removing the solid polymer and
inserting it into a second mould and injection
moulding a second polymer or polymer mix into
the second mould;
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 14 -
B) injection moulding a polymer or molten polymer
mix into a part of a mould, injection moulding a
second polymer or molten polymer mix into a fur-
ther part of the mould.
Steps A) and B) may be repeated more than once and may be
combined. It will be appreciated by the skilled person
that the first injection moulded polymer must survive the
pressure and temperature conditions of the second, or
subsequent, injection moulding.
For step B) the first polymer or molten mix may be pre-
vented from entering parts of the mould by any physical
means, such as, gates, gravity, positive or negative
pressure.
Sandwich injection moulding (or sometimes called skin-
core injection moulding) comprises injection moulding a
polymer or molten polymer mix into a mould until it is
partially filled and then injecting a second polymer or
molten polymer mix into the same mould through the same
gate to form the core. An additional step of sealing the
core may be performed.
It will be appreciated that any combination of simultane-
ous and sequential injection moulding may be used.
The closure part may itself be injection moulded or blow
moulded. Preferably, however, it is a plastics film se-
cured over the receptacle part. The film may, for exam-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 15 -
ple, comprise PVOH or a cellulose ether such as HPMC or
another water-soluble polymer.
Preferably for closing the container the receptacle part
and the closure are sealed together, for example by heat
sealing. A suitable heat sealing temperature is 120 to
195°C, more preferably 140 to 150°C. The sealing pres-
sure depends on the heat sealing machine used. A suit-
able sealing pressure is from 250 to 800 kPa (35 to 120
p.s.i.). Examples of sealing pressures are 400 to 800
kPa (60 to 120 p.s.i.), especially 276 to 552 kPa (40 to
80 p.s.i.), more especially 345 to 483 kPa (50 to 70
p.s.i.). Suitable sealing dwell times are at least 0.4
seconds, for example 0.4 to 2.5 seconds.
Other methods of sealing the receptacle part to the clo-
sure together may be used. These include infra-red, ra-
dio frequency, ultrasonic, laser, solvent, vibration,
electromagnetic, hot gas, hot plate, insert bonding,
fraction sealing or spin welding. An adhesive such as
water or an aqueous solution of PVOH may also be used.
The adhesive can be applied to the sheets by spraying,
transfer coating, roller coating or otherwise coating, or
the sheets can be passed through a mist of the adhesive.
The container walls have thicknesses such that the con-
tainers are rigid (as described above). For example, the
outside walls and any inside walls which have been injec-
tion moulded independently have a thickness of greater
than 100~m, for example greater than 150~,m or greater
than 200~.m, 300~.m, or 500~.m, 750~.m or lmm. Generally the
walls are moulded so as to be as thin as possible to re-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 16 -
duce container material consumption in the process and to
make the dissolution time of the container as short as
possible. Usually the wall thickness at more than 60% of
the wall area is less than 800~.m, more preferably less
than 600~.m and most preferably less than 400~.m.
Preferably, the closure part is of a thinner material
than the receptacle part. If different compartments hav-
ing different dissolution times are required, different
wall thicknesses can be used. A thickness difference of
from 100~.m to 500~,m, preferably from 250~.m to 350m, would
give a suitable difference in release times.
Preferably, the closure part dissolves in water (at least
to the extent of allowing the washing composition in the
receptacle part to be dissolved by the water; and pref-
erably completely) at 40°C in less than 5 minutes, pref-
erably in less than 2 minutes.
The receptacle part and the closure part could be of the
same thickness or different thicknesses. The closure
part may, for example, be of higher solubility than the
receptacle part, in order to dissolve more quickly.
Preferably, the washing container is generally cuboid in
its external shape, with the top wall being formed by the
closure part, and with the side walls and base wall being
formed by the receptacle part.
Preferably, a washing container of the invention is manu-
factured by forming an array of receptacle parts, each
receptacle part being joined to adjacent receptacle
parts, and being separable from them by a snap or tear
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 17 -
action. The array is preferably one which has columns
and rows of the receptacle parts. The receptacle parts
may be separated by frangible webs of the water-soluble
polymer such as PVOH or a cellulose ether.
Alternatively, the receptacle parts may be manufactured
with the aforementioned flanges, such that they are sepa-
rated from each other by a line of weakness. For example
the material may be thinner, and so able to be broken or
torn readily. The thinness may be a result of the mould
ing process or, preferably, of a later scoring step.
In the manufacturing method, the array, formed by inj ec-
tion moulding, is fed to a filling zone, and all the re-
ceptacle parts are charged with the washing composition.
A sheet of a water-soluble polymer such as PVOH or a cel-
lulose ether may then be secured over the top of the ar-
ray, to form the closure parts for all the receptacle
parts of the array. The array may then be split up into
the individual washing capsules, prior to packaging, or
it may be left as an array, for packaging, to be split by
the user.
The container, capsule or receptacle part defines two or
more compartments, which may contain different products
useful in a washing process . In such a situation a di-
viding wall or walls of the compartments preferably ter-
minate at the top of the container, i.e. in the same
plane as the top edges of the side walls, so that when
the receptacle part is closed by the closure part the
contents of the compartments cannot mix.. The container
may be provided with an upstand, preferably spaced from
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 18 -
the side walls thereof, and preferably of generally cy-
lindrical shape. If wished, the remaining volume of the
container can be divided into two or more parts by means
of~ walls extending between the upstand and the side
walls.
The container may be formed with an opening, for example
a depression, formed in the side wall or the base wall,
and preferably being open in the outward direction. That
is to say, it preferably does not form part of the main
volume defined by the container. Preferably the opening
is adapted to receive, in a press-fit manner, a solid
block (for example a tablet) of a composition, for exam-
ple a material useful in a washing process.
Preferably, the closure part is of a transparent or
translucent material, so that the contents of the washing
capsule can be seen.
Preferably, the container is of a transparent or translu-
cent material, so that the contents of the washing cap-
sule can be seen.
The washing composition within the container, or within a
compartment thereof, need not be uniform. For example
during manufacture it could be fed first with a settable
agent, for example a gel, useful in a washing process,
and then with a different material. The first material
could dissolve slowly in the washing process so as to de-
liver its charge over a long period within the washing
process. This might be useful, for example, to provide
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 19 -
immediate, delayed or sustained delivery of a softening
agent in a clothes washing container.
The container may, for example, be in at least two parts
(a body part and a cap part) which fit tightly, and pref-
erably sealingly and inseparably, together to form a com-
partment in which is stored the ingredient to be
achieved. In one example, the container or capsule may
have three parts - a body such as a receptacle part, a
first cap, and then a second cap to fit over the closed
end of either the body or the first cap, so as to result
in a capsule with two separate compartments. Where there
are three such parts (or more; four parts - a body and
three caps - make three compartments, and so on), then
naturally the ingredients in each compartment may be the
same or they may be different.
In all embodiments of the present invention one compart-
ment may contain, for example, a liquid or solid compo-
nent (such as a powder, granules or a compressed or
gelled tablet) and another may contain a different liquid
or solid component (such as a powder, granules or a com-
pressed or gelled tablet). Alternatively, more than one
component may be present in one or more compartments.
For example a compartment may contain a solid component,
for example in the form of a ball or pill (such as a pow-
der, granules or a compressed or gelled tablet), and a
liquid component.
Desirably the composition has a mass of at least 10g or
15g, for example, from 10g or 15g to 100g, especially
from 10g to 15g to 40g. For example, a dishwashing com-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 20 -
position may weigh from 10g or 15g to 20g, a water-
softening composition may weigh from 25g to 35g, and a
laundry composition may weigh from 10g to 40g, 20g to 40g
or 30g to 40g.
In general the maximum dimension of the container is 5cm.
For example, a cuboid container may have a length of 1 to
5cm, especially 3.5 to 4.5cm, a width of 1.5 to 3.5cm,
especially 2 to 3cm, and a height of l to 2cm, especially
1.25 to 1.75cm.
The composition may comprise a powder, gel, paste or low
water liquid foundation.
The composition contained by the container may be, for
example, any which is suitable for the designated appli-
cation, for example a clothes washing or dishwashing ap-
plication. It may be a powder or a liquid but if a liq-
uid, may be a low water formulation, preferably having a
maximum water content of 5wto, in order to maintain the
integrity of the walls of the capsule or a higher water
formulation containing, for example, at least 8wt% water.
It will be appreciated that higher water contents may be
present where the water is chemically or physically
bound. The composition may be formulated having regard
to the fact that the user will not come into contact with
the composition, whether by inhalation or by skin con
tact. For example, the composition may include an en
zyme, without concern about physical contact between the
composition containing the enzyme, and the user.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 21 -
If the container contains an aqueous liquid having a
relatively high free water content, it may be necessary
to take steps to ensure the liquid does not attack the
water-soluble polymer if it is soluble in cold water
(20°C) , or water at a temperature of up to, say, 35°C.
Steps may be taken to treat the inside surfaces of the
container, for example by coating it with agents such as
PVC (poly (vinylidene chloride)) or PTFE (polytetra-
fluoroethylene), or to adapt the composition to ensure
that it does not dissolve the polymer. For example, it
has been found that ensuring the composition has a high
ionic strength or contains an agent which minimises water
loss through the walls of the container will prevent the
composition from dissolving the polymer from the inside.
This is described in more detail in EP-A-518,689 and WO
97/27743.
The composition held within the container depends, of
course, on the intended use of the composition. It may,
for example, contain surface active agents such as an
anionic, non-ionic, cationic, amphoteric or zwitterionic
surface active agent or mixture thereof.
Examples of anionic surfactants are straight-chained or
branched alkyl sulfates and alkyl polyalkoxylated sul-
fates, also known as alkyl ether sulfates. Such surfac-
tants may be produced by the sulfation of higher C8-C2o
fatty alcohols.
Examples of primary alkyl sulfate surfactants are those
of formula:
ROS03-M+
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 22 -
wherein R is a linear C$-C~o hydrocarbyl group and M is a
water-solubilising ration. Preferably R is Cio-C16 alkyl,
for example C12-C14, and M is alkali metal such as lith-
ium, sodium or potassium.
Examples of secondary alkyl sulfate surfactants are those
which have the sulfate moiety on a "backbone" of the
molecule, for example those of formula:
CH3 ( CHZ ) n ( CHOS03-M+) ( CH2 ) mCH3
wherein m and n are independently 2 or more, the sum of
m+n typically being 6 to 20, for example 9 to 15, and M
is a water-solubilising ration such as lithium, sodium or
potassium.
Especially preferred secondary alkyl sulfates are the
(2,3) alkyl sulfate surfactants of formulae:
CH3 (CHI) X (CHOS03-M+) CH3 and
CH3 (CH2) x (CHOS03-M+) CH2CH3
for the 2-sulfate and 3-sulfate, respectively. In these
formulae x is at least 4, for example 6 to 20, preferably
10 to 16. M is ration, such as an alkali metal, for ex
ample lithium, sodium or potassium.
Examples of alkoxylated alkyl sulfates are ethoxylated
alkyl sulfates of the formula:
3 0 RO ( CZH40 ) nSp3-M+
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 23 -
wherein R is a C$-Coo alkyl group, preferably Clo-C18 such
as a C12-Cls, n is at least 1, for example from 1 to 20,
preferably 1 to 15, especially 1 to 6, and M is a salt-
forming ration such as lithium, sodium, potassium, ammo-
nium, alkylammonium or alkanolammonium. These compounds
can provide especially desirable fabric cleaning perform-
ance benefits when used in combination with alkyl sul-
fates.
The alkyl sulfates and alkyl ether sulfates will gener-
ally be used in the form of mixtures comprising varying
alkyl chain lengths and, if present, varying degrees of
alkoxylation.
Other anionic surfactants which may be employed are salts
of fatty acids, for example C$-C1$ fatty acids, especially
the sodium or potassium salts, and alkyl, for example CS-
C18, benzene sulfonates .
Examples of nonionic surfactants are fatty acid alkoxy-
lates, such as .fatty acid ethoxylates, especially those
of formula:
R (C~H40) nOH
wherein R is a straight or branched Ca-C16 alkyl group,
preferably a C9-CAS, for example Clo-C14, alkyl group and n
is at least 1, for example from 1 to 16, preferably 2 to
12, more preferably 3 to 10.
The alkoxylated fatty alcohol nonionic surfactant will
frequently have a hydrophilic-lipophilic balance (HLB)
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 24 -
which ranges from 3 to 17, more preferably from 6 to 15,
most preferably from 10 to 15.
Examples of fatty alcohol ethoxylates are those made from
alcohols of 12 to 15 carbon atoms and which contain about
7 moles of ethylene oxide. Such materials are commer-
cially marketed under the trademarks Neodol 25-7 and Neo-
dol 23-6.5 by Shell Chemical Company. Other useful Neo-
doll include Neodol 1-5, an ethoxylated fatty alcohol av-
eraging 11 carbon atoms in its alkyl chain with about 5
moles of ethylene oxide; Neodol 23-9, an ethoxylated pri-
mary C12-Csa alcohol having about 9 moles of ethylene ox
ide; and Neodol 91-10, an ethoxylated C9-C11 primary alco
' hol having about 10 moles of ethylene oxide.
Alcohol ethoxylates of this type have also been marketed
by Shell Chemical Company under the Dobanol trademark.
Dobanol 91-5 is an ethoxylated C9-C1l fatty alcohol with
an average of 5 moles ethylene oxide and Dobanol 25-7 is
an ethoxylated C12-Css fatty alcohol with an average of 7
moles of ethylene oxide per mole of fatty alcohol.
Other examples of suitable ethoxylated alcohol nonionic
surfactants include Tergitol 15-S-7 and Tergitol 15-S-9,
both of which are linear secondary alcohol ethoxylates
available from Union Carbide Corporation. Tergitol 15-5-
7 is a mixed ethoxylated product of a C11-Cls linear sec-
ondary alkanol with 7 moles of ethylene oxide and Tergi-
tol 15-S-9 is the same but with 9 moles of ethylene ox-
ide.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 25 -
Other suitable alcohol ethoxylated nonionic surfactants
are Neodol 45-11, which is a similar ethylene oxide con-
densation products of a fatty alcohol having 14-15 carbon
atoms and the number of ethylene oxide groups per mole
being about 11. Such products are also available from
Shell Chemical Company.
Further nonionic surfactants are, for example, Clo-C18 al-
kyl polyglycosides, such s C1~-C16 alkyl polyglycosides,
especially the polyglucosides. These are especially use-
ful when high foaming compositions are desired. Further
surfactants are polyhydroxy fatty acid amides, such as
Clo-C18 N- (3-methoxypropyl) glycamides and ethylene oxide-
propylene oxide block polymers of the Pluronic type.
Examples of cationic surfactants are those of the quater-
nary ammonium type.
The total content of surfactants in the composition is
desirably 60 to 95wt%, especially 75 to 90wto. Desirably
an anionic surfactant is present in an amount of 50 to
75wt%, the nonionic surfactant is present in an amount of
5 to 50wt%, and/or the cationic surfactant is present in
an amount of from 0 to 20wt%. The amounts are based on
the total solids content of the composition, i.e. exclud-
ing any solvent which may be present.
The compositions, particularly when used as laundry wash-
ing or dishwashing compositions, may also independently
comprise enzymes, such as protease, lipase, amylase, cel-
lulase and peroxidase enzymes. Such enzymes are commer-
cially available and sold, for example, under the regis-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 26 -
tered trade marks Esperase, Alcalase and Savinase by No-
vozymes. Desirably the enzymes are independently present
in the compositions in an amount of from 0.5 to 3wt%, es-
pecially 1 to 2wt%, when added as commercial preparations
they are not pure and this represents an equivalent
amount of 0.005 to 0.5wt% of pure enzyme.
The compositions may, if desired, independently comprise
a thickening agent or gelling agent. Suitable thickeners
are polyacrylate polymers such as those sold under the
trade mark CARBOPOL, or the trade mark ACUSOL by Rohm and
Haas Company. Other suitable thickeners are xanthan
gums. The thickener, if present, is generally present in
an amount of from 0.2 to 4wta, especially 0.5 to 2wt%.
Compositions used in laundry washing / dishwashing inde-
pendently usually comprise a detergency builder. The
builders counteract the effects of calcium, or other ion,
water hardness. Examples of such materials are citrate,
succinate, malonate, carboxymethyl succinate, carboxy-
late, polycarboxylate and polyacetyl carboxylate salts,
for example with alkali metal or alkaline earth metal ca-
tions, or the corresponding free acids. Specific exam-
ples are sodium, potassium and lithium salts of oxydisuc-
cinic acid, mellitic acid, benzene polycarboxylic acids,
C10-C22 fatty acids and citric acid. Other examples are
organic phosphonate type sequestering agents such as
those sold by Monsanto under the trade mark bequest and
alkylhydroxy phosphonates. Citrate salts and C12-Cls
fatty acid soaps are preferred. Further builders are;
phosphates such as sodium, potassium or ammonium salts of
mono-, di- or tri-poly or oligo-phosphates; zeolites;
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 27 -
silicates, amorphous or structured, such as sodium, po-
tassium or ammonium salts.
Other suitable builders are polymers and .copolymers known
to have builder properties. For example, such materials
include appropriate polyacrylic acid, polymaleic acid,
and polyacrylic/polymaleic and copolymers and their
salts, such as those sold by BASF under the trade mark
Sokalan.
The builder is desirably present in an amount of up to
90wto, preferably 15 to 90wt%, more preferable 15 to
75wto, relative to the total weight of the composition.
Further details of suitable components are given in, for
example, EP-A-694,059, EP-A-518,720 and WO 99/06522.
The compositions can also optionally comprise one or more
additional ingredients. These include conventional de-
tergent composition components such as further surfac-
tam s, bleaches, bleach enhancing agents, builders, suds
boosters or suds suppressors, anti-tarnish and anti-
corrosion agents, organic solvents, co-solvents, phase
stabilisers, emulsifying agents, preservatives, soil sus-
pending agents, soil release agents, germicides, pH ad-
justing agents or buffers, non-builder alkalinity
sources, chelating agents, clays such as smectite clays,
enzyme stabilizers, anti-limescale agents, colourants,
dyes, hydrotropes, dye transfer inhibiting agents,
brighteners, and perfumes. If used, such optional ingre-
diem s will generally constitute no more than l0wt%, for
example from 1 to 6wt%, the total weight of the composi-
tions.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 28 -
Compositions which comprise an enzyme may optionally con-
tain materials which maintain the stability of the en-
zyme. Such enzyme stabilizers include, for example,
polyols such as propylene glycol, boric acid and borax.
Combinations of these enzyme stabilizers may also be em-
ployed. If utilised, the enzyme stabilizers generally
constitute from 0.1 to lwto of the compositions.
The compositions may optionally comprise materials which
serve as phase stabilizers and/or co-solvents. Examples
are Cl-C3 alcohols such as methanol, ethanol and pro-
panol. C1-C3 alkanolamines such as mono-, di- and tri-
ethanolamines can also be used, by themselves or in com-
bination with the alcohols. The phase stabilizers and/or
co-solvents can, for example, constitute 0 to lwto, pref-
erably 0.1 to 0.5wt%, of the composition.
The compositions.may optionally comprise components which
adjust or maintain the pH of the compositions at optimum
levels. The pH may be from, for example, 1 to 13, such
as 8 to 11 depending on the nature of the composition.
For example a dishwashing composition desirably has a pH
of 8 to 11, a laundry composition desirable has a pH of 7
to 9, and a water-softening composition desirably has a
pH of 7 to 9. Examples of pH adjusting agents are NaOH
and citric acid.
The above examples may be used for dish or fabric wash-
ing. In particular dish washing formulations are pre-
ferred which are adapted to be used in automatic dish
washing machines. Due to their specific requirements
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 29 -
specialised formulation is required and these are illus-
trated below
Amounts of the ingredients can vary within wide ranges,
however preferred automatic dishwashing detergent compo-
sitions herein (which typically have a 1o aqueous solu-
tion pH of above 8, more preferably from 9.5 to 12, most
preferably from 9.5 to 10.5) are those wherein there is
present: from 5% to 900, preferably from 5% to 75%, of
builder; from 0 .1 o to 40%, preferably from 0 . 5% to 30%,
of bleaching agent; from 0.1% to 15%, preferably from
0.20 to 10%, of the surfactant system; from 0.0001% to
1%, preferably from 0.0010 to 0.05%, of a metal-
containing bleach catalyst; and from 0.1% to 400, pref-
erably from O.lo to 20% of a water-soluble silicate.
Such fully-formulated embodiments typically further. com-
prise from 0.1% to 150 of~a polymeric dispersant, from
0.01% to 100 of a chelant, and from 0.00001% to 100 of a
detersive enzyme, though further additional or adjunct
ingredients may be present. Detergent compositions
herein in granular form typically limit water content,
for example to less than 7% free water, for better stor-
age stability.
Non-ionic surfactants useful in ADW (Automatic Dish Wash-
ing) compositions of the present invention desirably in-
clude surfactants) at levels of from 2% to 60% of the
composition. In general, bleach-stable surfactants are
preferred. Non-ionic surfactants generally are well
known, being described in more detail in Kirk Othmer's
Encyclopedia of Chemical Technology, 3rd Ed., Vol. 22,
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 30 -
pp. 360-379, "Surfactants and Detersive Systems", incor-
porated by reference herein.
Preferably the ADW composition comprises at least one
non-ionic surfactant. One class of non-Tonics are eth-
oxylated non-ionic surfactants prepared by the reaction
of a monohydroxy alkanol or alkylphenol with 6 to 20 car-
bon atoms with preferably at least 12 moles particularly
preferred at least 16 moles, and still more preferred at
least 20 moles of ethylene oxide per mole of alcohol or
alkylphenol.
Particularly preferred non-ionic surfactants are the non-
ionic from a linear chain fatty alcohol with 16-20 carbon
15~ atoms and at least 12 moles particularly preferred at
least 16 and still more preferred at least 20 moles of
ethylene oxide per mole of alcohol.
According to one preferred embodiment the non-ionic sur-
factant additionally comprise propylene oxide units in
the molecule. Preferably this PO units constitute up to
25% by weight, preferably up to 20% by weight and still
more preferably up to 15% by weight of the overall mo-
lecular weight of the non-ionic surfactant. Particularly
preferred surfactants are ethoxylated mono-hydroxy alka-
nols or alkylphenols, which additionally comprises poly-
oxyethylene-polyoxypropylene block copolymer units. The
alcohol or alkylphenol portion of such surfactants con-
stitutes more than 30%, preferably more than 50%, more
preferably more than 70o by weight of the overall molecu-
lar weight of the non-ionic surfactant.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 31 -
Another class of non-ionic surfactants includes reverse
block copolymers of polyoxyethylene and polyoxypropylene
and block copolymers of polyoxyethylene and polyoxypro-
pylene initiated with trimethylolpropane.
.Another preferred non-ionic surfactant can be described
by the formula:
R10 [CH~CH (CH3) O] X [CH2CH20] Y [CH2CH (OH) R2]
wherein Rl represents a linear or branched chain ali-
phatic hydrocarbon group with 4-18 carbon atoms or mix-
tures thereof, R2 represents a linear or branched chain
aliphatic hydrocarbon rest with 2-26 carbon atoms or mix-
tures thereof, x is a value between 0.5 and 1.5 and y i~
a value of at least 15.
Another group of preferred nonionic surfactants are the
end-capped polyoxyalkylated-non-Tonics of formula:
R10 [CHZCH (R3) O] X [CH2] kCH (OH) [CH2] ~OR2
wherein R1 and R2 represent linear or branched chain,
saturated or unsaturated, aliphatic or aromatic hydrocar-
bon groups with 1-30 carbon atoms, R3 represents a hydro-
gen atom or a methyl, ethyl, n-propyl, iso-propyl, n-
butyl, 2-butyl or 2-methyl-2-butyl group , x is a value
between 1 and 30 and, k and j are values between 1 and
12, preferably between 1 and 5. When the value of x is
>2 each R3 in the formula above can be different. R1 and
R2 are preferably linear or branched chain, saturated or
unsaturated, aliphatic or aromatic hydrocarbon groups
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 32 -
with 6-22 carbon atoms, where groups with 8 to 18 carbon
atoms are particularly preferred. For the group R3 H,
methyl or ethyl are particularly preferred. Particularly
preferred values for x are comprised between 1 and 20,
preferably between 6 and 15.
As described above, in case x>2, each R3 in the formula
can be different. For instance, when x=3, the group R3
could be chosen to build ethylene oxide (R3=H) or propyl-
ene oxide (R3=methyl) units which can be used in every
single order for instance (PO) (E0) (E0) , (E0) (PO) (E0) ,
(E0) (E0) (PO) , (E0) (E0) (E0) , (PO) (E0) (PO) , (PO) (PO) (E0)
and (PO) (PO) (PO) . The value 3 for x is only an example
and bigger values can be chosen whereby a higher number
of variations of (E0) or (PO) units would arise.
Particularly preferred end-capped polyoxyalkylated alco-
hols of the above formula are those where k=1 and j=1
originating molecules of simplified formula:
R10 [ CH2 CH ( R3 ) O ] XCH2 CH ( OH ) CH20R2
The use of mixtures of different non-ionic surfactants is
particularly preferred in ADW formulations for example
mixtures of alkoxylated alcohols and hydroxy group con-
taining alkoxylated alcohols.
The composition, such as a washing composition within the
container, capsule or receptacle part, or within a com-
partment thereof if there is more than one compartment,
need not be uniform. For example during manufacture it
could be fed first with a settable agent, for example a
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 33 -
gel, useful in a washing process, and then with a differ-
ent material. The first material could dissolve slowly
in the washing process so as to deliver its charge over a
long period within the washing process. This might be
useful, for example, to provide delayed or sustained de-
livery of a softening agent in a clothes washing capsule.
The invention will now further explained with reference
to the following non-limiting Figures and Examples.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
The foregoing summary, as well as the following detailed
description of preferred embodiments of the invention,
will be better understood when read in conjunction with
the appended drawings. For the purpose of illustrating
the invention, there are shown in the drawings embodi
ments which are presently preferred. It should be under
stood, however, that the invention is not limited to the
precise arrangements shown. In the drawings:
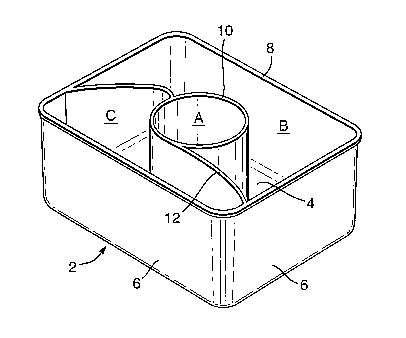
FIG. 1 is a view from below, of a receptacle part;
FIG. 2 is a perspective view, generally from above, of
the receptacle part of Fig. 1;
FIG. 3a and FIG 3b are side views along the longitudinal
and equatorial axes of the receptacle part as shown in
FIG.1 and FIG. 2.
FIGS. 1 to 3 show a receptacle part 2. The receptacle
part 2 has a base wall 4. The base wall 4 is substan-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 34 -
tially flat with a slight depression towards the main
chamber of the receptacle part 2. The receptacle part 2
has four upright side walls 6, and has no top wall.
Thus, each receptacle part 2 is upwardly open. Around
its opening, at the top of the side walls 6, is an out-
wardly-directed flange 8, which extends around the entire
opening. The flange 8 lies in one plane. When filled a
cover film (not shown) may be laid over the receptacle
part 2 and heat sealed against the flange 8, so that each
receptacle part 2 has, over it, a closure part (not
shown) .
Within the main chamber defined by the base wall 4 and
side walls 6 of each receptacle part 2, there is present
a generally cylindrical upstand 10, in a central posi-
tion. The cylindrical upstand 10 is open at its upper
end, and its upper end is in the same plane as the flange
6.
Adjacent the cylindrical upstand 10 there is a wave-
shaped upstand 12. The wave upstand 12 extends from the
base wall 4 of the receptacle part to the side walls 6 on
the longitudinal axis, up to the height of the side walls
6.
The cylindrical upstand 10 and the wave upstand 12 divide
the main chamber of the receptacle part into three sepa-
rate compartments. There is a first compartment (A)
within the volume defined by the cylindrical upstand 10.
There are two further compartments created by the divid-
ing effect of the wave upstand 12; a second compartment
(B) on the cylindrical upstand side 10 of the wave up-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 35 -
stand, being above the first compartment and a third com-
partment (C) on the opposite side of the wave upstand 12.
For clarity these three compartments are shown with the
letter references (A), (B) and (C) in FIG 2. The cover
film is preferably sealed against the wave upstand 12,
thus dividing the receptacle into separate compartments
as described above.
CONTAINER MANUFACTURING PROCESS
This process describes a method of manufacturing a con-
tainer as illustrated in the accompanying FIGS. 1-3. In
the filling stage it is filled with the compositions as
set out in the Composition Examples.
The Moulding Stage
Containers according to the invention were made by the
injection moulding method. The injection cavities were
in a two-impression (cap/body) composite water-cooled
stainless-steel mould. The PVOH had a material melt flow
index of 10-20 grams/10 min (DIN 53735). Injection tem-
peratures were 175°C, 180°C, 180°C and 185°C in
the feed,
zone 2 and 3, and Nozzle areas. The first stage injec-
tion pressure was 400 psi, and the hold stage pressure
was 270 psi. The pressure well time was 3 seconds in the
first stage and 5 seconds in the hold stage. Tool tem-
peratures were between ambient and 40°C.
The moulding pressures were just sufficient to fill the
cavities on the first pressure stage and then sufficient
packing pressure to hold on the second stage. Mould open
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 36 -
and close rates were as fast as possible.
The moulds were such that an array of container recepta-
cle parts were moulded simultaneously. In this case up
to 64 receptacle parts could be moulded in an array.
Where an array was formed separation of the receptacle
parts was necessary (see later).
Usually each receptacle weighed 2.0 to 2.5g. The resin
used for moulding comprised 85% PVOH, 11% Sorbitol and 4%
processing aids.
The Filling Stage
In the preferred embodiment of the invention where the
container is a multi-chamber product as illustrated in
the accompanying FIGS. a distinct detergent composition
was added to each of the compartments. This allowed the
formation of a multi-function detergent container which
could be used to achieve each of the required functions
in, for example, an automatic dishwasher. Indeed the
following example is in the field of automatic dishwash-
ing.
For compartment (A) generally a solidified melt formula-
tion was added. This formulation typically comprised a
surfactant which could be liquefied at elevated tempera-
ture, such as 60°C, (see Composition Examples). This
formulation was added at elevated temperature in the mol-
ten state using a hot-melt nozzle dispensing device. Af-
ter addition of the rinse aid the receptacle parts were
placed in a cooling system to allow the rinse aid to so-
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 37 -
lidify. The amount of this formulation added was usually
0.4 g.
For compartment (B) generally a powder formulation was
added. This formulation was added using a powder nozzle
dispensing device. For this stage of addition, vibration
of the receptacle part to aid settling of the powder was
employed. Also a compression station was used to aid
levelling. The amount of this formulation added was usu
ally 0.4 g.
For compartment (C) generally a gel formulation was
added. This formulation typically comprised a plurality
of materials suspended in a thickened gel formulation.
The formulation could be liquefied at elevated tempera-
ture, such as 60°C, (see Composition Examples). This
formulation was added at elevated temperature in the mol-
ten state using a hot-melt nozzle dispensing device. The
amount of this formulation added was usually 6.4 g.
The Sealing Stage
The filled receptacle parts were passed by a wetting sta-
tion, comprising a "wet plate" made of sand blasted alu-
minium.
A sealing top film (PVOH, 80-100~.m) was then applied to
the wet receptacle parts.
The film was pressed onto the receptacle parts using a
flat sealing surface which has been modified to follow
the seal contour along the flange of the receptacle part
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 38 -
and the wave upstand. The sealing surface operated at
150°C and pressed for 1.5 seconds.
The Separation Stage
A laser or other IR source was arranged to focus on the
area of the join between individual receptacle parts in
the array. The laser was applied to cut the joins thus
freeing up the individual containers.
Further Steps
Once sealed the containers have to be perforated on the
powder compartment top film. to allow vapours to exhaust
(e.g. those vapours arising from decomposition of the
bleach components of the powder (where present) during
storage). This was done with a plate with needles.
The containers were then removed from the trays to be
packed.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 39 -
COMPOSITION EXAMPLES
Composition Example 1: Phosphorus Containing Composition
Raw Material COMPARTMENT COMPARTMENT COMPARTMENT
A B C
Rinse Aid Powder Gel
(0.4 g) (10.4g) (6.4 g)
Sodium tripolyphosphate 43
Sodium carbonate 16
Sodium percarbonate 22
Phosphate speckles 4
Benzotriazol 0.4
HEDP 4 Na (88,5%) 0.3
Protease 1.5
Amylase 1
TAED 6
1,2-Propylenediglycol 1
Dye 0.02
Perfume 0.1
Sulphonated Polymer 5
~Sulphonated Polymer 5 i
Surfactant 24
Polyglykol g
1,2-Propylendiglycol 1
Dye 0.03
Antifoam 0.25
TAED 3
Sodium tripolyphoshate 57.5
Polyglycol 6000 0.3
Surfactant 100
100 100 100
1 Granules which contain approx. 10% active enzyme.
2 AMPS co-polymer.
3 Non-ionic low foaming surfactant.
4 Mixed poly alkoxylate.
5 Silicone oil.
6 Non-ionic surfactant (m.pt. approx. 50°C)
The receptacle part weighed 2.5g and comprised 85% of low
molecular weight PVOH (degree of hydrolysis 85-88%), 11%
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 40 -
sorbitol and 4o processing aids. The receptacle lid com-
prised a PVOH foil (90~,m thick) .
The contents of Compartment A (the non-ionic surfactant
(6)) were heated at 55°C until molten. The contents were
added into Compartment A and chilled to solid. The pow-
der was filled into Compartment B. The gel was heated to
65°C and stirred for 20 minutes. The gel was filled into
Compartment C and allowed to chill.
The receptacles were then sealed with PVOH film.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 41 -
Composition, Example 2: Phosphorus-Reduced Composition
Raw Material COMPARTMENT COMPARTMENT COMPARTMENT
A B C
Ria.se Aid Powder Gel
(0.4 g) (10.4g) (6.4 g)
Sodium Citrate 26
Sodium tripolyphosphate 19
Sodium carbonate 16
Sodium percarbonate 22
Phosphate speckles 4
Benzotriazol 0.4
HEDP 4 Na (88,5%) 0.35
Protease 1 1.5
Amylases 1
TAED 6
1,2-Propylenediglycol 1
Dye 0.02
Perfume 0.1
Sulphonated Polymer 2 5
Sulphonated Polymer 2 5
Surf actant3 24
Polyglykol4 - 9
1,2-Propylendiglycol 1
Dye 0.03
Antifoam 5 0.25
TAED 3
Sodium tripolyphoshate 57.5
Polyglycol 6000 0.3
Surfactant 6 100
100 100 100
1 Granules which contain approx. 10% active enzyme.
2 AMPS co-polymer.
3 Non-ionic low foaming surfactant.
4 Mixed poly alkoxylate.
5 Silicone oil.
6 Non-ionic surfactant (m.pt. approx. 50°C)
The preparation was as for Example 1.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 42 -
Composition. Example 3: Phosphorus Free Composition
Raw Material COMPARTMENT COMPARTMENT COMPARTMENT
A B C
Rinse Aid Powder Gel
(0.4 g) (10.4g) (6.4 g)
Tri-sodium citrate 51.5
Sodium carbonate 15
Sodium percarbonate 20
Benzotriazol 0.4
HEDP 4 Na (88,5%) ' 0.3
Protease 1.5
Amylase 1
1,2-Propylenediglycol 1
Dye 0.02
Perfume 0.1
Acrylate homo polymer
Sulphonated Polymer
5
Surfactant 24
Polyglykol
1,2-Propylendiglycol 1
Dye
0.03
Antifoam 0.25
TAED 8
Tri-sodium citrate 51.75
Polyglycol 35000 1
Surfactant 100
100 100 100
1 Granules which contain approx. 10% active enzyme.
2 AMPS co-polymer.
3 Non-ionic low foaming surfactant.
4 Mixed poly alkoxylate.
5 Silicone oil.
6 Non-ionic surfactant (m.pt. approx. 50°C)
The preparation was as for Example 1.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 43 -
Composition Example 4: Phosphorus Containing Composition
with a bleach activator composition in the centred com-
partment
Raw Material COMPARTMENT COMPARTMENT COMPARTMENT
A B C
Melt Powder Gel
(1.5 g) (9.2g) (6.4 g)
Sodium tripolyphosphate 52
!Sodium carbonate 15
'Sodium percarbonate 20
Phosphate speckles 4
Benzotriazol 0.4
HEDP 4 Na (88,50) 0.3
Protease 1 1.5
Amylases 1
1,2-Propylenediglycol 1
Dye 0.02
Perfume 0.1
Sulphonated Polymer ~ 5
Sulphonated Polymer 2 5
Surf actant3 24
Polyglykol 4 9
1,2-Propylendiglycol 1
Dye 0.03
Antifoam 5 0.25
Sodium tripolyphoshate 61
Polyglycol 6000 0.3
Gelatine 3
Glycerine 47
TAED 50
100 100 100
1 Granules which contain approx. 10% active enzyme.
2 AMPS co-polymer.
3 Non-ionic low foaming surfactant
4 Mixed poly alkoxylate grade.
5 Silicone oil
The preparation was as for Example 1.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 44 -
Composition Example 5: Phosphate Coatair~,ing Composition
Including PAP
Raw Material COMPARTMENT COMPARTMENT COMPARTMENT
A B C
Powder Gel
(1.5 g) (9.2 g) (6.4 g)
Sodium tripolyphosphate 42.5
Sodium carbonate 16
Tri-sodium citrate 22
Phosphate speckles 4
Benzotriazol 0.4
HEDP 4 Na ( 8 8 , 5 0 0 . 3
)
Protease 1.5
Amylase 1
TAED 6
1,2-Propylenediglycol 1
Dye 0.02
Perfume 0.1
Sulphonated Polymer 5
Sulphonated Polymer 5
Surfactant 24
Polyglykol 9
1,2-Propylendiglycol 1
Dye 0.03
Antifoam 0.25
TAED 3
Sodium tripolyphoshate 57.5
Polyglycol 6000 0.3
PAP 100
100 100 100
1 Granules which contain approx. 10% active enzyme.
2 AMPS co-polymer.
3 Non-ionic low foaming surfactant.
4 Mixed poly alkoxylate grade.
5 Silicone oil
6 PAP granules of particle size > 250~m.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 45 -
In the container used in this Example the cylindrical
part is raised to the height of the side wall. The PAP
is added as a powder. The remainder of the preparation
process is as for Example 1.
Rigidity Examples
Rigidity Example 1
A receptacle part as illustrated in the accompanying Fig-
ures was made in an injection moulding process. The re-
ceptacle part had the overall dimensions of 40mm
(length), 28mm (width) and l8mm (height). The thickness
of the receptacle was 300~,m with a rim thickness of
700~,m.
The composition of the receptacle part was the same as
that of the composition Examples.
The rigidity of the receptacle part was measured using a
measuring device available from Imatec in accordance with
the DIN 878 method. Using this apparatus a force of 0.8N
was applied to the receptacle part (across its width)
with a needle having a width of 2.4mm.
Measurements were taken immediately after production of
the receptacle part and after storage in moist conditions
(after 15 minutes and 30 minutes at 30°C and 70% humid-
ity. The rigidity data, shown in terms of the deforma-
tion extent of the receptacle part is shown in the fol-
lowing table.
CA 02549764 2006-06-15
WO 2005/058700 PCT/GB2004/005262
- 46 -
The following table also contains values for the brittle-
ness of the receptacle part, in terms of the force re-
quired to break the container, measured using the same
equipment.
Receptacle Deformation Brittleness
(mm)
(N)
Minimum
Average
Maximum
Immediately After 0.54 0.89 1.14 <80
Production
min. at 30C / 1.10 1.33 1.69 -
70o humidity
15 min. at 30C / 1.38 1.69 2.76 >100
70% humidity
The deformation ability of the receptacle party increases
significantly after plasticisation by water in the humid
10 atmosphere. This has the beneficial effect that as the
receptacle part has a low deformation ability (high ri-
gidity) after production it can be filled and sealed ef-
fectively without any incorrect filling / poor sealing.
After plasticisation the container has a high deformation
15 ability (low rigidity) which is pleasing to a consumer on
a tactile level.
The container also has a much lower brittleness after
plasticisation. This allows the container to be trans-
ported and handled without causing rupture of the con-
tainer.