Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
FUEL FOR HOMOGENEOUS CHARGE COMPRESSION IGNITION (HCCI)
SYSTEMS AND A PROCESS FOR PRODUCTION OF SAID FUEL
Field of the Invention
The invention relates to a fuel for Homogeneous Charge Compression Ignition
(HCCI)
systems and to a process for producing such a fuel.
Background to the Invention
The HCCI engine is a relatively new concept under development by several
institutions and companies. The principle of HCCI combustion is that a dilute,
premixed, homogenous mixture of fuel and air reacts and burns volumetrically
throughout the cylinder as it is compressed by the piston. Combustion
reactions start
when the mixture reaches a sufficiently high temperature to autoignite. These
reactions initiate at multiple locations simultaneously, proceed very quickly,
and there
is a complete absence of localized high-temperature regions or flame-fronts.
In essence, the HCCI combustion process seeks to combine the low nitrogen
oxides
(NOx)exhaust emissions associated with the gasoline engine, with the high
thermal
efficiency associated with the diesel or compression ignition (CI) engine. In
theory,
HCCI offers the potential for sootless combustion and very low emissions of
nitrogen
oxides (NOx), together with an energy efficiency that can exceed that of the
CI engine.
Successful implementation of HCCI combustion would therefore increase the
competitiveness of the internal combustion (IC) engine against emerging
technologies
such as fuel cells, thereby extending its lifespan.
Because HCCI is effectively an evolution of the IC engine, there are no
external
barriers to its implementation, and the gradual adoption of this technology
may see it
eventually being implemented in the majority of automotive IC engines, in one
form or
another. A 2001 ~ report by the US Department of Energy to the US Congress
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
2
speculated that, with successful R&D, passenger car HCCI engines might be
commercialised by 2010.
Thus a need exists for a fuel for HCCI systems and engines.
Summary of the Invention
According to one aspect of the invention, there is provided a HCCI fuel, which
fuel
includes at least n-paraffins and iso-paraffins, and which fuel has an
ignition delay of
less than 7 ms. The HCCI fuel may also be used as a fuel component.
Typically, the fuel contains hydrocarbon species having from 7 to 14 carbon
atoms.
The fuel may be substantially cyclo-paraffins free. Thus, the fuel may have
less than 5
mass%, typically less than 1 mass% cyclo-paraffins.
Moreover, it contains less than 1 wt % of aromatic and negligible levels of
sulphur.
In this specification, the ignition delay is measured using the ASTM Method
D6890 in
a constant volume combustion bomb, Ignition Quality Tester (IQTT"' )
The ignition delay of the fuel may be less than 5 ms.
The ignition delay of the fuel may be between 2 and 5 ms.
The weight % of the n-paraffins may exceed that of any other single component
in the
fuel.
The n-paraffins may be in excess of 25% by weight of the fuel
The n-paraffins may be in excess of 50% by weight of the fuel.
The n-paraffins may be in excess of 80% by weight of the fuel.
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
3
The n-paraffins may be in the order of 95% by weight of the fuel.
The n-paraffins may be Fischer-Tropsch (FT) reaction derived n-paraffins.
The iso-paraffins may be FT reaction derived iso-paraffins.
The fuel may include olefins.
The HCCI fuel may include oxygenates.
The HCCI fuel may be substantially sulphur free.
The HCCI fuel may be substantially oxygenate free.
The fuel may have an ASTM D86 distillation range from 90°C to
270°C.
The fuel may include a lubricity improver or other fuel additives to malee
meeting
product specifications possible.
The fuel may be used as blending component with conventional fuel.
The invention extends to a process for preparing a HCCI fuel or fuel
component,
which fuel or fuel component includes at least n-paraffins and iso-paraffins,
which
fuel has an ignition delay of less than 7 ms, said process including one or
more steps
selected from:
a) hydrotreating at least a Condensate fraction of a Fischer-Tropsch (FT)
synthesis reaction product, ora derivative thereof;
b) hydroconverting a Wax fraction of the FT synthesis product or a derivative
thereof;
c) fractionating in a single unit or in separate units, one or more of the
hydrotreated Condensate fractions of step a) and the hydroconverted fraction
of step b) to obtain the desired HCCI fuel or fuel component; and
d) optionally, blending two or more of said components from step c) in a
desired
ratio to obtain the desired HCCI fuel.
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
4
The hydroconversion may be by way of hydrocracking.
The properties of the fuel made according to the process may be as disclosed
above
and elsewhere in the specification.
The blending of step d) may be the blending of FT condensate derivative and
hydroconverted FT wax derivative from 1:99 to 99:1 by volume
The table below gives a typical composition of the two fractions.
Typical FT product after separation into two fractions (vol % distilled)
FT Condensate FT Wax
(< 270C fraction) (> 270C fraction)
C5-160C 44 3
160-270C 43 4
270-370C 13 25
370-500C 40
> 500C 28
The >160°C fraction, contains a considerable amount of hydrocarbon
material, which
boils higher than the normal naphtha range. The 160°C to 270°C
fraction may be
regarded as a light diesel fuel. This means that all material heavier than
270°C
needs to be converted into lighter materials by means of a catalytic process
often
referred to as hydroprocessing, for example, hydrocracking.
Catalysts for this step are typically of the bifunctional type; i.e. they
contain sites
active for cracking and for hydrogenation. Catalytic metals active for
hydrogenation
include group VIII noble metals, such as platinum or palladium, or a sulphided
Group
VIII base metals, e.g. nickel, cobalt, which may or may not include a
sulphided
Group VI metal, e.g. molybdenum. The support for the metals can be any
refractory
oxide, such as silica, alumina, titania, zirconia, vanadia and other Group
III, IV, V and
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
VI oxides, alone or in combination with other refractory oxides.
Alternatively, the
support can partly or totally consist of zeolite.
Specific Description and Examples
The following table summarises the origin and carbon number ranges for the
proposed
fuels usable in HCCI engines of this invention:
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
6
Class Typical (LTFT)Com Carbon
iti Number
range
F pos
d on
ee C,-C9 C,-Cy4 C~p-C14
stock
SR FT FT CondensateParaffins, olefinsX X X
and ox enates
HT SR FT CondensateMostly linear X X X
FT
araffins
HX FT FT Wax Mostl iso- araffinsX X X
GTL FT CondensateFully paraffinicX X X
and Wax
Definitions
~ SR FT Straight Run Fischer-Tropsch
~ HT SR FT Hydrotreated Straight Run Fischer-Tropsch
~ HX FT Hydrocracked Fischer-Tropsch
~ GTL Hydroconverted Product as expected from a Fischer-Tropsch
Gas-to-Liquid plant
The fuel might contain hydrocarbon species having from 7 to 14 carbon atoms
and
has been found to define unique characteristics with respect to vapour
pressure and
ignition delay . Moreover, the criteria also made consideration to the highly
paraffinic
nature of the fuel as well as the high linearity of the hydrocarbon species.
The C7 to C14 carbon number range has been found to exclude hydrocarbons like
pentane or hexane that have high vapour pressures. Adequate volatility is
important
to establish a homogeneous gaseous charge in the combustion chamber, with
enough cetane character (propensity to auto-ignite) to effect the homogeneous
ignition throughout the whole volume.
Furthermore, the C7 to C14 carbon number range has been found to exclude
hydrocarbons like n-hexadecane that conventionally has cetane number of 100.
The
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
7
cetane number of the HCCI fuel must not be too high and its ignition delay not
too
short to ensure controlled in-cylinder combustion.
The inventors believe that the abovementioned twelve options cover almost all
practical options for FT-based synthetic HCCI fuels.
The key quality requirements for these fuels are summarised in Table 1.
Table 1 Selected Quality Characteristics of Synthetic FT HCCI Fuels
Desired Ran Analytical
a Procedure
g
Distillation Ran 90-270C ASTM D86
a
Densit 0.65-0.78 k ASTM D1298
/I
Com osition h drocarbon GC-FID
I nition dela IQT 2-7 ms ASTM D6890-03
Cetane Number 25-75 ASTM D613-03a
Aromatics content <1.0% wt ASTM D5186-99
ASTMD6591-00
Sulfur content < 1 ppm wt ASTM D5453
Oxygen content <5000 ppm ~ GC-TCD
~
The ignition delay is a good indication of the elevated pressure, high
temperature
autoignition characteristics of the fuel and can be correlated to the
distillation range
and cetane number of the fuel, which in turn relate to its chemical
composition. The
conditions at which the ignition delay is determined in the IQTTM; at 22.4 bar
air
pressure and 565 °C, are comparable to the conditions that an HCCI fuel
could
experience in an HCCI engine, thus the ignition delay (ID) can be used as an
appropriate yardstick for HCCI fuel ignition quality. The implications are
that fuels with
a high propensity for autoignition under compression will have short ignition
delays
(~2-4 ms), while fuels with increased resistance against autoignition
(equivalent to
high octane spark ignition gasoline) will have longer ignition delays (~7-11
ms).
Since the resistance against autoignition is no different to a resistance
against
oxidation at the specific pressure and temperature conditions to which the
fuel is
exposed in an HCCI engine's combustion chamber, it follows that those sulphur
(S)
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
8
and nitrogen (N) heteroatoms present in crude oil derived HCCI fuel will act
as
oxidation inhibitors, leading to longer ignition delays and a lower propensity
towards
autoignition.
FT fuels are virtually sulphur free, with lower levels of nitrogen-containing
compounds,
and the absence of these naturally occurring anti-oxidants represent a benefit
when
FT fuels are applied in HCCI engines. This results in FT fuels outperforming
conventional fuels in terms of their propensity to autoignite under HCCI
conditions.
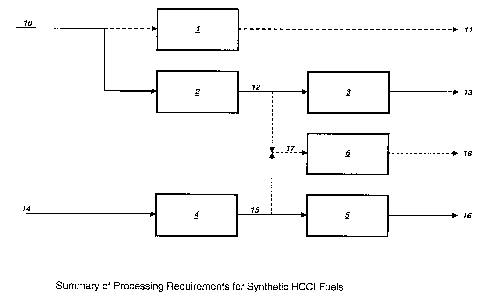
Process Scheme
A generic block diagram flow scheme is included as figure 1. The process
options for
all four classes of HCCI fuels are shown in a simple format. The following
table 2
summarises the basic processing for these fuels and feeds.
Table 2 Generic Requirement for FT Feedstock Processing
Process Ste Process Descri tion Reference
DistillationAtmos heric Distillation 1
H2 saturation of olefinic double
bonds.
HydrotreatmentH2 saturation of oxygen-containingUS 6
475
375
hydrocarbons with formation of ,
water ,
Other h droconversion reactions
Cracking of heavy molecules (mostly
paraffinic)
Hydrocracking~ H2 saturation of olefinic doubleEP 1129155
bonds.
H2 saturation of oxygen-containing
hydrocarbons with formation of
water
Other h droconversion reactions
(1 ) There are many references for this unit operation. For example, refer to
PA
Schweitzer, Handbook of Separation Techniques for Chemical Engineers (McGraw-
Hill, 1979) or RH Perry and CH Chilton, Chemical Engineers' Handbook (McGraw-
Hill,
5t" Edition, 1973)
The production of the synthetic HCCI fuel components can be achieved following
at
least four process configurations. The selection of one for a specific plant
is an
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
9
exercise in process synthesis that demands additional site and market specific
information.
A first group of HCCI fuels - named SR FT in this description - can be
produced by
fractionation of a light synthetic FT hydrocarbon stream 10 in Distillation
unit 1. The
operation of this fractionation unit to the required product specification
results in the
group of products 11.
A second group of HCCI fuels - named SR HT FT in this description - can be
obtained
1,0 from a light synthetic FT hydrocarbon stream 10 which is first
hydrogenated in
hydrogenation unit 2 to saturate the olefinic double bonds and remove the
oxygen
from the oxygenate species. Then the hydrogenated products can be fractionated
in
fractionation unit 3 to the required specification, obtaining the group of
products 13.
, A third group of HCCI fuels - named HX FT in this description - can be
obtained from
a heavy synthetic FT hydrocarbon stream 14 which is hydrocracked in
hydrocracking
unit 4 to result in lighter saturated hydrocarbon species. Then the
hydrocracked
products can be fractionated in fractionation unit 5 to the required
specification,
obtaining the group of products 16.
An alternative to produce a fourth group of HCCI fuels - named GTL (GTL = gas
to
liquid) in this description - can be produced by direct blending of the
hydrotreated and
hydrocracked products described above. This can be done in an optimised way by
using a common fractionator unit 6 to the required specification, obtaining
the group of
products 18.
It is also possible to blend the products 11 and 16, either by sharing a
common
fractionator or after fractionation to also obtain synthetic HCCI fuels.
In all of these process options there is co-production of non-HCCI hydrocarbon
stream, both lighter and heavier than the designed HCCI synthetic products.
The
former can be described as a light naphtha and the latter as a heavy diesel
stream.
These can be used in fuel and non-fuel applications.
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
All fuels in any of these four groups can be used as blends components for
final HCCI
fuels.
Emissions Performance of the Synthetic FT HCCI Fuels
5
There is wide acceptance to the fact that the synthetic FT fuels produce less
noxious
emissions than conventional fuel. This point has been brought into the public
domain
several times - for example refer to "Processing of Fischer-Tropsch Syncrude
and
Benefits of Integrating its Products with Conventional Fuels" presented at the
National
10 Petrochemical & Refiners Association Annual Meeting held in March 2000 in
San
Antonio, Texas - paper AM-00-51. This document makes reference to both FT
naphthas and FT diesels.
Typical Quality of Synthetic FT HCCI Fuels
Table 3 contains the typical quality of synthetic FT HCCI fuels produced as
described
and conforming to the selected requirements. Table 4 shows a comparison
between
HT SR FT fuel and crude derived fuel.
Table 3 Typical Quality of Synthetic FT HCCI Fuels
SR HT
FT SR
FT
Desired C~-C9C7-C14Cio-C14CrCs C7-C14Cio-
Range C14
Distillation 90-270 ~C 103- 103- 164- 90- 90- 165-
Range 183 251 251 160 254 254
Densit 0.65-0.78k /1 0.67 0.71 0.76 0.71 0.74 0.76
Com osition ' f
n- araffins wt 52.5 63.1 68.4 94.6 94.9 95.1
%
i- araffins ' wt 0.4 1.6 2.2 5.4 5.1 4.9
%
Olefins wt 38.5 26.5 20.5 0 0 0
%
Ox enates wt 8.6 8.8 8.9 0 0 0
%
Ignition delay2-7 ms 3.34 2.79 2.60 3.44 2.74 2.54
IQ'~'TM
Cetane Number30-70 60 75 83 58 77 86
Aromatics <1.0 % wt <0.1 <0.1 <0.1 <0.1 <0.1 <0.1
content %
Sul hur content< 1 m wt <1 <i <i <i <1 <i
Oxygen content<5000 ppm 700 2000 2150 <80 <80 <80
wt
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
11
HX GTL
Desired CrC9 C7-C14C C~-C9 C~-C~a
Range 4
Distillation90-270 ~C 80-16380-250135- 90-16390-250155-
Range 250 250
Densit 0.65-0.78k /I 0.68 0.72 0.74 0.69 0.72 0.75
Com osition
~
n- araffins wt 46.0 30.7 26.6 57.5 41.0 38.0
%
i- araffins wt 54.0 69.3 73.4 42.5 59.0 62.0
%
Olefins wt 0 0 0 0 0 0
%
Ox enates ' 4 wt 0 0 0 0 ~ 0
% 0
Ignition 2-7 ms 4.92 4.06 3.50 4.55 3.34 3.08
delay
IQTTM
Cetane Number30-70 41 49 57 44 60 66
Aromatics <1.0% wt <0.1 <0.1 <0.1 <0.1 <0.1 <0.1
content %
Sul hur content< 1 m wt <1 <i <i <i <1 <i
Oxygen content<5000 ppm <80 <80 <80 <80 <80 <80
wt
CA 02549927 2006-06-15
WO 2005/059063 PCT/ZA2004/000157
12
Table 4 Comparison between equivalent synthetic FT Fuel for HCCI Fuels
and Crude Derived Fuels
HT T Crude Fuels
SR Derived
F
Desired C~-C9C,a Ci4 CrC9 C~-C14 C1p-C~4
Range
Distillation90-270 C g0- 90- 165- 80-15980-257 151-257
Range 160 254 245
Densit 0.65-0.78k /I 0.71 0.74 0.76 0.73290.7715 0.7961
Com osition '
. ~
.
n- araffins wt 94.6 94.9 95.1 28.2 23.8 24.7
%
i- araffins wt 5.4 5.1 4.9 32.8 53.0 55.3
%
Olefins wt 0 0 0 0.4 0.4 0.5
%
Ox enates wt 0 0 0 0 0 0
%
Aromatics wt 0 0 0 10.3 14.2 18
%
Na hthenes wt 0 0 0 28.3 8.6 1.5
%
IgOni~ioM -7 ms 344 2.74 2.54 6.17 5.22 4.79
delay 2
Cetane Number30-70. 58 77 86 34.1 39.0 42
0
Sul hur content< 1 m wt <i <1 <i 50 50 .
50
10
Table 5 below presents an example of the quality characteristics of blends of
the C7-
C9 GTL HCCI fuel with an equivalent Petroleum fraction. The benefits of
including
synthetic FT fuel in conventional blends are quite evident.
Table 5 Quality of blends of the C7-C9 GTL HCCI fuel with an equivalent
Petroleum fraction
GTL tent
Fuel
Con
0% 25% 50% 75% 100%
Densit kg/I 0.733 0.722 0.711 0.700 0.690
Com osition
'
n- araffins wt 28.2 35.4 42.8 50.1 57.5
%
i- araffins wt 32.8 35.1 37.6 40.0 42.5
%
Olefins wt 0.4 0.3 0.2 0.1 0.0
%
Ox enates wt 0.0 0.0 0.0 0.0 0.0
%
Aromatics wt 10.3 7.8 5.2 2.6 0.0
%
Na hthenes wt 28.3 21.3 14.2 7.1 0.0
%
Total wt 100.0 99.9 100.0 99.9 100.0
%
I nition dela ms 6.17 5.75 5.22 4.75 4.55
IQTT"'
Cetane Number 34.1 36.0 39.1 41.9 44.0
Sul hur content m 50 38 25 13 <1