Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
SYNTHESIS OF ULTRAFINE RUTILE PHASE TITANIUM DIOXIDE PARTICLES
AT LOW TEMPERATURE
Field of the invention
The present invention relates to a low temperature process for the synthesis
of
ultrafine rutile phase titanium dioxide particles through vapor phase
hydrolysis of titanium
tetra chloride. In particular the present invention relates to a method for
the manufacture of
rutile grade titanium dioxide powder using ethanol as dopant to bring down the
rutile
formation temperature to as low as 150 - 400 C during calcination with time
duration of 1 to
4 hrs. The process includes a novel combination of operational steps to
economically produce
ultrafine titanium dioxide powders of rutile phases in a flexible
manufacturing process.
Background of the invention
Titanium dioxide (titania) is extensively used as pigments, catalysts,
inorganic
membranes, semi-conductors, optical coating reagent and as photocatalysts in
water
purification process. Titanium dioxide (Ti02) has two phases of crystalline
structure of
industrial importance, namely, anatase and rutile. Titanium dioxide with
anatase phase has
been used as a photocatalyst for photodecomposition of acetone, phenol or
trichloro ethylene,
oxidation such as nitrogen mono-oxide and hitrogen dioxide and conversion
system using
solar energy due to its high photo-activity. Titanium dioxide with rutile
phase has been
widely used as white pigment because of its good scattering effect that
protects the
ultraviolete light. It has also been used in optical coatings, beam splitter
and anti-reflection
coatings since it has a high dielectric constant and high refractive index,
good oil absorption
ability, tinting power and chemical stability even under strongly acidic or
basic conditions.
Titanium dioxide shows different electrical characteristics according to
oxygen partial
pressure since it has wide chemical stability and non-stoichiometric phase
region. Because of
this, it can also be used as a humidity sensor and as high-temperature oxygen
sensor and the
field of its use has become wide.
Titanium dioxide powders for pigment use generally have an average particle
size of
150 to 250 nanometer and is considered the principal white pigment of
commerce. It has an
exceptionally high refractive index, negligible color and is quite inert.
Titanium dioxide is
having a smaller average particle size, for instance in the 10 to 100
nanometer median
particle size range, is used commercially in cosmetics and personal care
products, plastics,
surface coating, self - cleaning surfaces, and photo voltaic applications.
This grade of
titanium dioxide is referred to as ultrafine or nano-sized titanium dioxide.
More than four
million tons of titanium dioxide are produced annually; there are several
processes for
making ultrafme titanium dioxide, some in commercial use and some in
development. Some
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
use anhydrous titanium dioxide, some in commercial use and some in
development. Some
use anhydrous titanium tetrachloride as a feed stock. Another process use a
titanyl sulfate
solution as the feed stock.
Generally, titanium dioxide powders are manufactured by a chloride process,
which is
a gas phase process, or by a sulfate process, which is a liquid phase process.
In the chloride process, which was commercialized by Du Pont of USA in 1956,
titanium tetrachloride, is used as a starting material and the reaction
temperature needs to be
higher than 1,000 C. This method also requires extra protection devices
because of the
corrosive C12 gas product at high temperature in the process, leading to
higher production
costs. Because titanium dioxide powders produced by the chloride process are
fine but rough,
additive equipment for providing external electrical fielfs or controlling
reactant mixing
ratios are required to control the particle shape and the particle size of
titanium dioxide
powders. High pure oxygen is required for oxidation of TiC14 and that leads to
high capital
and operating costs.
In the sulfate process, which was commercialized by Titan company of Norway in
1961, titanium sulfate (TiSO4) is conventionally hydrolyzed at temperatures
higher than
100 C, calcined at 800 - 1000 C and then pulverised to produce titanium
dioxide powders.
During the calcination and pulverization processes, impurities are introduced
causing the
quality of the final titanium dioxide powder to be low.
Funaki, Saeki, et al. in Kogyo Kagaku Zasshi, 59 (11), pp. 1291 (1956), teach
that
fine particles of anatase-type titanium dioxide can be produced by mixing
titanium
tetrachloride and water in the vapor phase at a temperature in the range from
200 C to 800 C
or fine particles of anatase-type titanium dioxide containing or not
containing a very small
amount of rutile-type particles can be produced by the reaction of titanium
tetrachloride and
water in the liquied phase and a much higher temperature treatment to obtain
rutile phase
titanium dioxide.
A method for preparing spherical particles of a metal oxide comprising
hydroysis of a
hydrolysable titanium (IV) compound in the form of a liquid aerosol by being
contacted with
water vapor in a dynamic flow is taught in US Patent 4,241,042. A method in
which a
precursor of a metal oxide in the form of a very fine droplet suspension of
the liquid is heated
and gasified by evaporation and thermal decomposition and then contacted and
reacted with
an oxygen containing gas in the vapor phase to give spherical fine particles
of a metal oxide
is taught in Japanese Patent Kokai 59-107904 and 59-107905.
2
CA 02551663 2009-01-23
Recently considerable interest has been directed toward the synthesis of
rutile grade
titania at low temperature. There have been some reports about new liquid
phase processes to
synthesis of rutile grade titanium dioxide powder using titanium
tetrachloride. Kim, Park et
al, (US Patent 6,001,326) show new liquid phase process in which Tio2
precipitates with pure
rutile phase having spherical shapes having 200-400 mm in diameter formed
between room
temperature and 65 C, by the homogeneous precipitation method simply by
heating and
stirring an aqueous TiOCI2 solution.
Tang et al., (Mater. Chem. Phys. 77 (2): pp. 314, (2003) disclose the
preparation of
nanosized rutile TiOZ powder by hydrolysis of Ti (OC4H9) 4 solution at 40-50
C. When the
solution is neutral and basic, the hydrolysis product is a precipitate and the
dried precipitate is
amorphous. The rutile phase Ti02 cannot be obtained even when the dried
precipitate is
calcined at 600 C. However, when the solution is acidic, the hydrolysis
product is a sol, in
order to obtain rutile Ti02 by drying the gel at 40-50 C. However, tight
control of reaction
conditions is required, since alkoxide is intensely hydrolyzed in air.
Furthermore, he high
price of the alkoxide limits its commercialization.
Yang et al., (Mater. Chem. Phys., 77 (2) : pp. 501, (2003) also reported that
titania
nanocrystals in the rutile form were prepared in liquid phase at room
temperature under
normal pressure. Li Y.Z. et al, also reported in Jour. Mater. Chem., 12 (5):
pp. 1387, (2002),
the preparation of nano-crystalline rutile Ti02 with average crystal sizes of
6.9 - 10.5 nm by
hydrolysis of TiC14 aqueous solution at lower temperatures. All the above
reported techniques
of synthesizing rutile phase titanium dioxide are based on liquid phase
processing.
In comparison with liquid phase routes, vapor phase hydrolysis of titanium
chloride to
synthesize anatase has been reported. For example; B. Xia et al. (Jour. Mater.
Sci., 34, pp.
3505, (1999), reported the preparation of anatase Ti02 nanopowders by vapor-
phase
hydrolysis of TiC14 below 600 C. As an independent preparation route, it
hasn't been paid
much attention.
Compared with liquid phase process, the vapor phase process carried out in an
aerosol
reactor offers many advantages including product purity, ease of collection,
energy efficiency
and avaoids treatment like filtration, washing, drying etc., involving large
liquid volumes.
However, the chloride process is carried out at high temperature and has
encountered several
problems such as control of product characteristics, corrosion of reactor
material of
construction and operational problems, mainly due to the high temperatures and
corrosive
gases involved. Therefore, there is a need for a process to produce ultrafine
titanium dioxide
3
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
at temperature much reduced from those encountered in the chloride process but
involving
only gas phse processing without involvement of liquids.
Objects of the invention
The main objective of the invention is to develop a low temperature process
for the
synthesis of ultrafine rutile particles through vapor phase hydrolysis of
TiC14.
Another object of the present invention is to develop a flexible low
temperature
process for the synthesis of anatase, rutile and mixtures thereof in the same
reactor system.
Summary of the Invention
The present invention provides a low temperature process for the synthesis of
ultrafine rutile phase titanium dioxide particles through vapor phase
hydrolysis of titanium
tetrachloride comprising the step of:
a) hydrolyzing a mixture of TiC14 and H20 and a dopant in vapour phase in an
aerosol
reactor;
b) collecting amorphous or anatase titanium dioxide powder formed as dry
powders;
c) calcining the dry powder to obtain rutile phase titanium dioxide.
In one embodiment of the invention, the amorphous particles of titanium
dioxide are
calcined at a temperature in the range of 150 to 400 C and for a period in the
range of 1 to 4
hrs to generate rutile particles.
In another embodiment of the invention, the dopant contains a carbon atom and
is
selected from the group consisting of an aliphatic alcohol, an aromatic
hydrocarbon, and any
mixture thereof.
In yet another embodiment of the invention, the dopant is ethanol.
In yet another embodiment of the invention, the molar concentration of the
dopant is 1
to 10 based on the water vapour.
In another embodiment of the invention, the reaction mixture contains from 0
to 10 %
ethanol on a molar basis based on TiC14.
In another embodiment of the invention, the flow rate of TiC14 is in the range
of 10
cm3/min to 200 cm3/min.
In yet another embodiment of the invention, the TiC14 vapor concentration
inside the
reactor is in the range of 7 x 10-4moUmin to 1 x 10-2 mol/min.
In yet another embodiment of the invention, the flow rate of water vapour is
in the
range of 240 to 1500 cm3/min, preferably from 500 to 1000 cm3/min.
In one embodiment of the invention, temperature at the exit of the aerosol
reactor is
maintained at less than 100 C for obtaining titanium dioxide particles having
anatase phase.
4
CA 02551663 2009-01-23
In another embodiment of the invention, the aerosol reactor is externally
heated in
order to avoid particle coating on the walls through thermophoresis.
In yet another embodiment of the invention, the aerosol reactor consists of 3 -
tube
concentric jet assembly wherein TiC14 is introduced into the innermost tube,
dopant is
introduced into the outermost tube and water vapor is introduced into the
middle tube.
In another embodiment of the invention, the 3-tube assembly comprises a
concentric
arrangement of three inconel tubes at the entrance of the aerosol reactor.
In another embodiment of the invention, the vapor phase TiC14 is introduced
into a
center tube of the three concentric inconel tubes.
In another embodiment of the invention, the vapor phase TiC14 is formed by
bubbling
an inert gas through TiC14 liquid.
In a further embodiment of the invention, the inert gas is selected from the
group
consisting of argon, nitrogen, krypton, helium and any mixture thereof.
In yet another embodiment of the invention, the molar ratio of water to
titanium tetra
chloride in the feed is in the range 10 to 15.
In another embodiment of the invention, the water vapor is formed by bubbling
air or
inert gases through water under superheated condition.
In another embodiment of the invention, the reactor wall temperature is from
200 to
450 C.
In yet another embodiment of the invention, the rutile titanium dioxide
particles
formed have an average diameter in the range of from 25 to 150 nanometers.
The present invention also provides a low temperature process for the
synthesis of
ultrafine rutile phase titanium dioxide particles through vapor phase
hydrolysis of titanium
tetrachloride comprising the step of:
a. vaporizing a titanium chloride liquid, water and a dopant comprising
ethanol
separately to generate a reaction mixture ;
b. hydrolyzing vapor phase TiC14 and H20 and dopant mixture in a continuous
aerosol
reactor under non-isothermal conditions at a temperature in the range of 80 to
135 C;
c. collecting amorphous and anatase phase titanium dioxide powder as dry
powder;
d. Calcining the titanium dioxide particles having amorphous phase at a
temperature
range of 150-400 C and for a period in the range of 1 to 4 hrs to obtain
titanium
dioxide particles with rutile phase.
5
CA 02551663 2009-01-23
According to another aspect of the present invention, there is provided a
process for
the low temperature synthesis of rutile phase titanium dioxide particles,
comprising the steps
of:
(a) vaporizing TiCl4, water and ethanol separately for generating a reaction
mixture;
(b) hydrolyzing the reaction mixture of TiC14 and water and ethanol in vapour
phase
in a continuous aerosol reactor at a temperature in the range of 80 to 135 C
such
that all reactants are in vapour form;
(c) collecting as synthesized titanium dioxide product as dry powder using a
filter
bag;
(d) calcining the dry powder at a temperature in the range of 150 to 400 C to
obtain
rutile phase titanium dioxide particles.
According to a further aspect of the present invention, there is provided a
vapour
phase process for the synthesis of rutile phase titanium dioxide particles
having a diameter of
25 to 150 nanometers, comprising the steps of:
(a) vaporizing a TiC14 liquid, water and ethanol separately for generating a
reactant;
(b) hydrolyzing TiCl4, water and ethanol in vapour phase in a continuous
aerosol
reactor at temperature in the range of 80 to 135 C such that all the reactants
are in vapour form;
(c) collecting as synthesized powder as dry product;
(d) calcining the as synthesized powder in the temperature range of 150 to 400
C
and time duration in the range of 1 to 4 hrs to obtain titanium dioxide
particles
with rutile phase.
Brief description of the Drawing
In the drawings accompanying this specification
5a
CA 02551663 2009-01-23
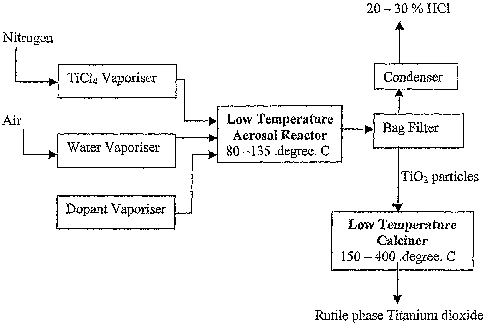
Figure 1 represents a flow sheet of the general aspect of the rutiel phase
titanium
dioxide synthesis using low temperature vapore phase process according to the
present
invention.
Figure 2 represents a layout of nozzle inlet assembly for mixing of reactants
of
reactants and dopant in the inlet part to the reactor.
Detailed description of the invention
The present invention relates to a gas phase based aerosol synthesis of rutile
phase
titanium dioxide particles at a much lower temperature so as to avoid the
several unit
operations for the treatment of large liquid volumes and non-requirement of a
need for high
purity oxygen as in the chloride process. The present invention has
successfully led high
purity oxygen as in the chloride process. The present invention has
successfully led to the
development of to new titanium dioxide powder manufacturing method. In this
method, it is
possible to prepare ultrafine titanium dioxide powders of rutile phase
continuously with
excellent control of particle characteristics such s particle shase, particle
size, and specific
crystallographic modifications. This invention also provides a low
temperature, low cost,
environmentally friendly flexible process for preparing titanium dioxide
powders. In addition,
it is easy to control the mixture ratio of rutile and anatase phase of the
titanium dioxide
crystallites.
The present invention relates to a process for the synthesis of titanium
dioxide
powders having rutile phase by TiCl4 hydrolysis in vapor phase followed by low
temperature
calcination. The process defined herein consists of three basis steps:
(1) Hydrolyzing a reactant mixture containing TiCl4 vapor, water vapor and
optionally a
dopant in a vapour phase reactor.
(2) Collecting titanium dioxide powder having amorphous phase formed inside
the vapor
phase reactor.
(3) Low temperature calcination of the collected powder.
The precise details of these steps are set forth below. Although specific
executions
and examples are discussed in this application, it is envisioned that the
present invention
encompasses the full range of obvious variants of those specifically disclosed
herein.
Hydrolyzing Step
The hydrolyzing reaction takes place in the aerosol reactor of ID 2.5 cm and
1.5m in
length, heated externally in a horizontal electrical furnace (Fig. 1). The
reactor comprises of a
metallic tube made of InconelTM in which the reactants (TiCl4, H20, and the
dopant) are
introduced as vapor. The aerosol reactor consists of three concentric Inconel
tubes as shown
6
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
in Fig. 2. the inner diameter of the central tube is 2mm and the spacing
between successive
tubes is lmm respectively. The mixture of TiC14 vapor and nitrogen is
introduced through the
concentric inconel tube (a), water vapor is introduced through the tube (b)
and dopant vapor
is introduced into the system through the concentric inconel tube (c).
The TiC14 reactant is introduced into the reactor in the vapor phase. In this
invention
TiC14 vapor can be generated by bubbling an inert gas through liquid TiC14,
the nitrogen gas/
TiC14 vapor is preferably directed through the concentric inconel tube (a) of
the reactor. The
TiC14 flow rates utilized in the process of the present invention are
generally from about 10
cm3/min to about 200 cm3/min. This flow rate (together with the liquid TiC14
temperature)
essentially define the concentration of TiC14, which is present inside the
reactor. The TiC14
vapor concentration ranges inside the reactor, which are useful in the present
invention, are
from about 7 x 10-4mo1/min to about 1 x 10-2 mol/min. Heating the TiCi4 liquid
through
which the nitrogen gas is bubbled controls the actual concentration of TiC14
vapor in the
nitrogen gas. The higher the temperature utilized, the greater the TiC14 vapor
concentration
achieved. In this regard, it is preferred that the TiC14 through which the
nitrogen is bubbled
has a temperature of from about 20 C to about 100 C.
The other required reactant utilized in the process of the invention is water
vapor.
Water vapor is generated by bubbling air through water and directing that gas
(air with water
vapor) into the reactor through the concentric inconel tube (b). This
procedure allows for
precise control of water vapour flow rate and concentration in the reactor.
The air (containing
water vapour) flow rate is generally from about 240 to about 1500 cm3/min,
preferably from
about 500 to about 1000 cm3/min. The reaction mixture, which is utilized in
the present
invention also, includes a dopant material, in vapor phase, it positively
affect the physical
attributes of the titanium dioxide formed. The TiC14 reactant, water vapor and
the dopant may
be mixed in the reactor. It is preferred that the dopant vapor be introduced
through the
concentric inconel tube (c). Aliphatic alcohols, aromatic hydrocarbons and
mixtures thereof
can be used as dopants out of which ethanol is used for this present
invention. In selecting the
amount of dopant to the used in the process, it is generally advisable to use
dopant molar
concentration in the range one to ten percent of the concentration of water
vapor.
Reaction
In chemical terms, the reactions being carried out in the present invention.
are as
follows:
TiCl4 + 4 H20 P. Ti (OH) 4+ 4 HCL
Ti (OH) ¾ oo Ti02 + 2H20
7
CA 02551663 2009-01-23
The size range of particles formed due to the above reactions can be
controlled by
reaction temperature and molar ratio of H20/ TiC14 in the reactor.
Separation of titanium dioxide particle from gas phase
The Ti02 particles formed are either amorphosuse or anatase and this powder is
collected on a bag filter, made of TeflonTM which is aided by vacuum pump. The
filter bag is
maintained at temperatures in the range 130 to 140 C to avoid condensation.
Calcination
The titanium dioxide powder having amorphous phase resulting from gas phase
hydrolysis of titanium chloride without dopant is calcined at a temperature in
the range of
300 - 600 C and for a length of time in the range of 1 -4 hours to obtain
rutile phase or
mixtures there of with anatase phase. In the presence of a vapor phase dopant
such as ethanol
during the gas phase hydrolysis, the rutile formation temperature is reduced
to as much as
150 - 400 C compared to other usual calcination treatments, and the
calcination duration is
also sufficiently shortened to limit the excessive particle growth through
sintering. In
comparison in the absence of dopant during the hydrolysis step, calcination
temperature can
range between 800 C to 1100 C in the gas phase hydrolysis for anatase to
rutile
transformation. In the presence of dopant, anatase to rutile transformation
takes place during
the vapor phase hydrolysis, calcinations temperature range can be reduced
between 500 to
700 C.
The following illustrative examples are not intended to restrict the scope of
the
present invention. The following examples also illustrate the unique advantage
of using
dopants during the hydrolysis in the process of this invention.
Example 1 illustrates the vapor phase hydrolysis of TiCi4 and water without
any dopant to
synthesize titania nanopowders having the rutile phase.
Example 2 illustrates the vapor phase hydrolysis of TiCl4 and water with
dopant as ethanol to
synthesize titania nanopowders having the rutile phase.
Example 1
Dry nitrogen (99.9%) is bubbled through a gas bottle containing titanium
tetrachloride
(commercial grade) maintained at a temperature of 90 C and is directed through
the central
tube of the aerosol reactor. Concentration of TiC14 in the gas stream is
determined by
recording the weight of TiC14 before and after each experiment. A constant N2
flow rate of
500 cm3/min through the TiCi4 bubbler is used. The corresponding molar flow
rate of TiCl4 is
1.7 x 10-3mo1/min. Air is bubbled through a gas washed bottle containing water
(temperature
= 90 C) and is directed through the second tube of the nozzle distributor.
Mass flow
8
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
controllers (1259 B, MKS) precisely control all flows into reactor. The TiC14
vapor and the
water vapor are mixed rapidly around the nozzle and form Ti02 aerosol at near
atmospheric
pressure. Titanium dioxide particles produced by gas phase hydrolysis of TiC14
in the. aerosol
reactor are collected in a bag filter made of Teflon. The titanium dioxide
powder is obtained
directly as dry powders for characterization. The exhoust gas is completely
absorbed by a set
of bubblers. Portions of the powders produced were heat treated in a
conventional muffle
furnace. Powder was calcined at 800 C for 3 hrs. Rotameter is used for
measuring the flow
rate of air.
Ti02 is synthesized (without the use of dopants), in this example, the
following range
of reaction conditions is utilized.
Inlet gas stream temperature = 70 - 80 C
Exit gas stream temperature = 130 - 150 C
Air flow rate = 1000.00 cm3/min. (STP)
TiC14 molar flow rate = 1.7 x 10-3mo1/min.
H20 / TiC14 molar ration = 15
Phase composition of collected particles was determined by X-ray diffraction
(XRD)
in, a Philips Holland Exper-Pro diffractometer operating at 40kV, 20mA, using
CuK ,.
Radiation. Weight fractions of the rutile and anatase phases in samples are
calculated from
the relative intensities of strongest peaks corresponding to anatase (20 =
25.6 for the (101)
reflection of anatase) and rutile (26 = 27.5 for the (110) reflection of
rutile) peaks as
described by Spurr and Myers, Quantitative Analysis of Anatase - Rutile
Mixture with an X-
ray Diffractometer, Analytical Chem., 29: 760 (1957). Specific surface area of
the powders is
measured using a BET Nitrogen adsorption apparatus (Gemini 2375 V4.02).
Scanning
Electron Microscopy SEM-JIOL: 1.5 kV) is used for morphological analysis of
the powders.
Titanium dioxide powders synthesized at different molar ratios of TiC14 and
water
vapor in the reactor are given in Table 1 below. Table 2 shows the specific
surface areas of
the powders produced, as well as the rutile and anatase contents of those
powders. Powders
produced at the various molar ratios are designated as H1, H2, H3 and H4.
Table 1:
Aerosol Synthesis Condition of Ti02 powder (Without gas phase dopant)
Powder Temperature, C Molar flow rate of TiC14 H20/TiC14 molar ratio
H1 135 0.0026 14
H2 135 0.0015 20
H3 135 0.0007 33
H4 135 0.0013 49
9
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
Table 2:
Characteristics of Titanium Dioxide powder
Powder BET Surface Avg. particle (nm*) Rutile content, Anatase content,
No. Area m2l wt% wt%
H1 19 81 >99.9 <0.1
H2 22 69 87.0 13.0
H3 30 51 76.0 24.0
H4 33 46 21.0 79.0
= Based on BET surface area
Example 2
Using the reactor and the analytical methods of Example 1, doped titanium
dioxide
was prepared as follows. The dopant ethanol kept at room temperature (28 C) is
introduced
into the reactor through the third concentric tube. TiC14 vapor, water vapor
and ethanol are
mixed rapidly around the nozzle and form Ti02 aerosol at near atmospheric
pressure. Ethanol
molar concentration iri the range one to ten percent of the concentration of
water vapor.
Portions of the powders ,produced were heat treated in a conventional furnace.
Powder was
calcined at 500 C for 3 hrs.
Titanium dioxide powders are synthesized at different molar ratios of
H20/TiC14 in
the reactor is given in Table 3 below. Table 4 show the specific surface areas
of the powders
produced as well as the rutile and anatase contents of these powders. Powders
produced at the
various molar ratios are designated as EHl, EH2 EH3 and EH4.
Table 3:
Aerosol Synthesis Condition of Titanium Dioxide powder (with gas phase dopant)
Powder Temperature, C H20/TiC14 molar ratio H20/TiC14 molar ratio
EHl 137 14 7.0
EH2 137 20 6.0
EH3 1137 33 3.5
EH4 137 49 3.0
~Table 4:
Characteristics of Titanium Dioxide powder
Powder BET Surface Avg. particle size,nm* Rutile Anatase
No. Area ( m2/g) conten content,
t, wt% wt%
EH1 43.5 35 >99.9 <0.1
EH2 39.6 39 87.0 13.0
EH3 36.0 43 76.0 24.0
EH4 33.0 47 51.0 49.0
= Based on Bet surface area
CA 02551663 2009-01-23
Table 5:
Comparison of rutile transition temperature observed with and without the
dopant
Gas H20/ H20/ Titanium Commencement Completion of
stream TiC14 Ethanol Dioxide of phase phase
temp, C molar ratio Molar ratio particles Transformation transformation
Obtained on to rutile on to rutile,
From vapor temperature. C temperature.
Phase OC*
hydrolysis
80 12 - Amorphous 300 600
80 12 7.4 Amorphous 150 400
137 15 - Anatase 800 1100
137 15 7.0 Anatase 500 700
= Calcined duration: 3 hrs.
Table 5 illustrates the unique advantage of using dopant such as ethanol
during the
vapor phase hydrolysis step to achieve substantial reduction of the
calcinations temperature
required for obtaining titanium dioxide particles with rutile phase.
1. Nano and sub micron size titanium dioxide particles having the rutile
phase, anatase
phase and mixtures there of could be synthesized at temperatures less than 400
C
through vapor phase reaction with TiC14 as the precursor.
2. The other reactant involved in the process is water and ethanol, which are
of low cost
and are environmentally green chemicals.
3. The process is less energy consuming that the other available process and
involves
negligible maintenance.
Prior art processes such as the chloride process developed for rutile
manufacture (by
Dupont) involves oxidation of titanium tetra chloride at a temperature of 1000
- 1200 C. The
high purity oxygen is obtained through cryogenic air separation and the
reaction is highly
exothermic leading to release of large amount of energy (-130.98KJ/mol at 1100
C) which is
removed from the reactor through heat exchangers containing cooling water. The
high energy
consumption and wastage in this process is due to
1. The energy for cryogenic separation of air into high purity oxygen
2. Pre-heating of TiC14 and oxygen to 1200 C.
3. Wastage of exothermic heat of reaction.
The process of the present invention does not require pure oxygen and the
maximum
reaction temperature in the aerosol reactor can be controlled to about 150 C.
Thus reduced
energy consumption is due to the absence of energy requirement of cryogenic
oxygen
separation and negligible preheat temperature of only 150 C. Additionally,
there is no need
11
CA 02551663 2006-06-27
WO 2005/063629 PCT/IN2003/000429
for heat exchangers since the TiC14 hydrolysis reaction has a much lower
exothermic heat of
reaction (-20 kJ /mol at 150 C).
The significance of the role played by ethanol to reduce the temperature of
conversion
of amorphous precursor to rutile is very clearly indicated in the XRD of the
amorphous
precursors. Specifically the XRD of the amorphous precursor synthesized with
ethanol as
dopant contains rutile fingerprints having shallow and broad peaks typical of
non-
crystallinity. However these features are absent in the XRD of amorphous
precursors
generated without ethanol. Without wishing to be bound by any theory, it is
believed that the
use of organic dopant influences the nucleation process of titanium dioxide
powder by
generating unique solid structures capable of being converted to rutile phase
under mild
calcination temperatures.
20
30
12