Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02554027 2006-07-19
WO 2005/075656 1 PCT/EP2004/000789
Biochip Electroporator and its use in multi-site, single-cell electroporation.
Field of the invention
This invention refers to a method and apparatus for the individual
electroporation
of any kind of cell adhering to a substrate at any stage of development.
State of the art
Electroporation is a widely used technique employed to introduce genetic
material
or molecules of biological interest into cells. Application of an electrical
signal to
the cell produces the opening of pores in the membrane, allowing the molecules
in
the extracellular solution to enter the cell.
io Several electroporation techniques have been established. They can be
divided in
two classes:
(i) techniques for the electroporation of a population of cells
(ii) techniques for the electroporation of single cells.
Electroporation of a population of cells.
is The electroporation of a population of cells in suspension, which is the
most used
and representative of the first class, is routinely performed after the cells
have
been removed from the culture substrate, by using enzymes such as trypsine.
Then, cells are suspended in electroporation medium and high voltages (up to
1000V and more) are applied between two large metal electrodes, typically
spaced
20 2 or 4 mm. The cells suspension is then transferred into a suitable
culture
chamber, where cells are free to settle down and adhere onto the bottom
substrate
where they are cultured.
This technique implies several drawbacks.
- The actual voltage drop across each cell can not be controlled. Many cells
close
25 to the electrodes die during the high voltage application, others are
electroporated
to different extent depending on their position within the electric field
between the
electrodes and on the local variations of the applied electrical field that is
not
uniform.
- The method can only be applied to a large population of cells.
30 - Cells must be detached from the adhesion substrate, thus cells are
stressed and
damaged in the process.
Electroporation of single cells.
CA 02554027 2006-07-19
WO 2005/075656 2 PCT/EP2004/000789
A technique for single cell electroporation has been developed by Rubinsky et
al.
and described in the US patent n. 6300108.
This technique employs a cell sized electroporation chamber integrated into a
silicon microchip. The cell, is first introduced in the chamber and placed
onto an
opening in a silicon substrate, then it is electroporated and made permeable
to .
genetic constructs.
The above technique has some drawbacks:
- The system set-up is complex, moreover, the integration of a large array of
"single cell electroporators" into a microchip has not been accomplished yet
and
- The cell membrane cannot adhere extensively to the substrate and therefore
growth and development are impossible for all cell types requiring adhesion
(which
is the case for most type of cells);
- The cell has to be placed into .a rnicrochamber; this is a difficult
procedure and far
From the above it is clear the need of an electroporation apparatus allowing
to
overcome the above said drawbacks.
Summary
The Applicant has now found an apparatus for individual electroporation of any
Therefore, it is an object of the present invention an apparatus for
electroporation
comprising a wave generator, a biochip containing an array of microelectrodes
and
a control system that permits to transfer the signal to a pre-selected single
It is a further object of the present invention the biochip comprising an
array of
microelectrodes comprised on a suitable insulating layer mounted on a solid
substrate; means to electrically connect said microelectrodes to a switching
system; a cell culture chamber where the cells can be grown and adhere in
CA 02554027 2006-07-19
WO 2005/075656 3 PCT/EP2004/000789
Further objects of the invention are the methods for cell electoporation using
said
apparatus, this methods further performing electroporation to at least a
single
adhering cell and electroporated cells obtained with the same.
These and other objects as well as features and advantages of the present
invention will be better understood from the detailed description set forth
below.
Brief description of the drawings:
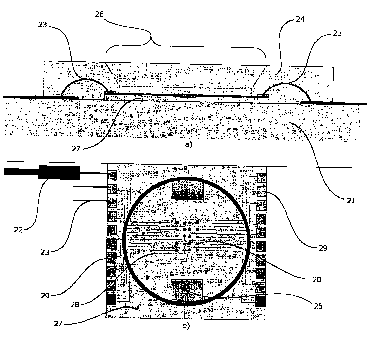
Fig. 1 shows a schematic representation of the apparatus
Fig. 2 shows a biochip according to the invention; top view (Fig. 2b) and
cross
section (Fig. 2a)
Fig. 3 shows a cross section of a conductive microelectrode according to the
invention
Fig. 4 shows top view and cross section of an array of MOSFET conductive
microelectrode according to the invention
Fig. 5 shows a schematic representation of an array of MOSFET conductive
microelectrode according to the invention
Fig. 6 shows a cross section of a capacitive microelectrode according to the
invention
Fig. 7 and Fig. 8 display two particular waveforms of the electroporation
driver
signal. Parameters value, such as time and amplitude are given in the
experiments
descriptions
Detailed description of the invention
The features of the invention will become apparent in the course of the
following
description and from the preferred embodiments which are given for
illustration
and have no limiting purposes.
The present invention allows to overcome the drawbacks of existing techniques
through an apparatus comprising an electrical waveform generator, a biochip
comprising an array of microelectrodes and a control system that permits to
transfer the signal to a pre-selected single microelectrode of the biochip.
Preferably the control system consists of a personal computer, equipped with a
software program, capable of designing various waveform signals within the
wave
generator and selecting the microelectrodes by the use of a switching system.
The apparatus according to the present invention is schematically represented
in
CA 02554027 2006-07-19
WO 2005/075656
PCT/EP2004/000789
4
Fig. 1 wherein a personal computer 10, a switching system 11, a signal
generator
12 and a biochip 13 are displayed.
The personal computer 10 is equipped with a software program allowing the
operator to fully control the electroporation process, programming the shape
and
the timing of the waveform of the signal to be delivered to the biochip.
The electrical waveform generator 12 used in the apparatus according to the
present invention is a common device, readily available on the market; the
switching system 11 can easily be designed and implemented by the man skilled
in the art.
The biochip 13, comprising an array of microelectrodes, a suitable insulating
layer, where said insulating layer is on a solid substrate, means to
electrically
connect said microelectrodes to a switching system, a cell culture chamber
where
the cells can be grown in contact with said array of microelectrodes and
adhere on
the surface formed by said insulating layer containing said array of
microelectrodes on a solid substrate, is described hereinafter in detail
according to
its preferred embodiments.
The lay-out of the biochip according to the present invention is displayed in
Fig. 2a
and 2b as one preferred embodiment and will be described with reference to
both
figures, wherein the reference numbers designate identical or corresponding
parts
throughout the two views.
In the Fig. 2a said biochip is displayed mounted on a dielectric support 21
=
mounting, in turn, a cell culture chamber 24 with an opening 26 placed in the
centre, the base surface of which is formed by an insulating layer, comprising
an
array of microelectrodes of a size comparable to the cell to be
electroporated, on a
solid substrate 27. Said array of microelectrodes 20 being integrated on an
insulating layer mounted on a solid substrate 27 on which is placed a cell
culture
chamber 24 provided with an opening 26, the bottom of said opening 26 is the
top
surface of the biochip 13 where the cells are grown adhering on and in contact
with said array of microelectrodes. Said array of microelectrodes are
electrically
connected via conductive traces 28 to conductive pads 29 electrically
connected,
in turn, to a couple of external parallel connectors 22 through wire bonding
23
covered by the outer portion of the cell culture chamber 24 encircling the
opening
CA 02554027 2006-07-19
WO 2005/075656 5 PCT/EP2004/000789
26.
The biochip 13 is electrically connected to the switching system 11, as
displayed in
Fig. 1, through appropriate means of connection such as a cable plugged in the
connectors 22
In a preferred embodiment of the present invention, the dielecrtric support 21
is
made of vetronite. Other equivalent materials such as glass or ceramic are not
excluded.
In a preferred embodiment of the present invention the solid substrate of the
biochip 13 consists of a semiconductor substrate, such as for example a
silicon
io substrate, of a suitable form, such as, for example, of square form,
having suitable
lateral dimension, preferably of about ten millimetres and covered with an
insulating layer preferably of Si02. Silicon is not the only possible
substrate for
such a device: for example, glass or other transparent substrates can be used
as
well. However, silicon has the advantage of well consolidated and repeatable
is manufacturing techniques derived from the microelectronics industry,
allowing
precise control of each device parameter.
In any case transparent substrates can be preferred, allowing the use of
inverted
microscopes for cell observation.
Two electrodes of large dimensions 25 (around 1 square mm), integrated in the
20 solid substrate covered with an insulating layer 27, act as ground
reference. In
alternative it is possible to have the said electrodes acting as cold terminal
for the
electroporation electrical current and a wire, made of Ag/AgCI, as ground
potential
reference.
In the array of microelectrodes 20 of a size comparable to the cell to be
25 electroporated and comprised in an insulating layer covering a solid
substrate,
= each microelectrode can be driven individually and separately from the
others
thanks to their individual connections to the switching system 11, thus
allowing
very precise and punctual control of the electroporation process.
Microelectrodes
employed can be of conductive or capacitive type.
30 Fig. 2b shows (not in scale) the layout of twenty microelectrodes 20,
each one with
an active area (that is the area delivering the signal to the cell) of twenty
jtm long
by twenty lam wide. This layout provides a relatively good balance between the
CA 02554027 2006-07-19
WO 2005/075656 6 PCT/EP2004/000789
number of stimulating sites, the microelectrodes, and the interconnections
between microelectrodes and of the biochip required for the external supplying
of
the microelectrodes. Higher number and density of microelectrodes can be
achieved, depending on the type of cell to be electroporated.
In order to favour cell adhesion on the biochip, the surface of said opening
26 of
the biochip cell culture chamber 24 is covered with adhesion molecules (for
example poly-L-lysine) before starting cell culture. Many different adhesion
molecules can be used for coating the substrate allowing good cell adhesion
with
a distance of a few tens of nanometers between cell membrane and
io semiconductor substrate covered with an insulating layer 27 comprised in
the
opening 26 of the culture chamber.
Microelectrodes may be of different shape and dimensions as long as their size
is
comparable to cell size, normally they are planar or, in general, designed to
allow
a good adhesion of the cell membrane on their surface (normally at least ten
per
cent of the total cell membrane has to be in contact with the electrode),
their
dimensions range from 1 gm to 50 m. Said microelectrodes are made of bio-
compatible conductive material capable to deliver the necessary
current/voltage
AC and/or DC signals to the cells.
Conductive microelectrodes are made of materials which can be chosen among
several bio-compatible metals and alloys and can be single layer or a
multilayer.
A preferred embodiment of a conductive microelectrode used in the biochip of
the
apparatus according to the present invention is depicted in Fig. 3. Said
microelectrodes are realised over a silicon substrate 31 covered with a Si02
insulating layer 32. Microelectrodes and their connecting traces 38 are made
by a
"sandwich" of two titanium nitride (TiN) layers 33 and an aluminium layer 34,
covered with a gold layer 37. This solution has several advantages, combining
the
high thermal budget and biocompatibility of TiN to low electrical resistance
of Al.
Microelectrodes are separated by S102 insulating layer 35. The part of the
chip not
to be directly exposed to the cells (comprising traces 38 connecting
microelectrodes) is covered by a silicon nitride (Si3N4) layer 36, which
leaves
exposed the active part of the microelectrodes only. Different materials (such
as
Si02 or organic polymers) can be used for this purpose.
CA 02554027 2006-07-19
WO 2005/075656 PCT/EP2004/000789
7
In another embodiment of the invention, microelectrodes of capacitive type are
realised introducing a thin insulating material to cover the active part of
the
microelectrode itself. With reference to Fig. 6, microelectrodes are realised
on an
insulating substrate 60 by using a conductive layer 61 (that can be made of
metal
or semiconductor or the above mentioned sandwich of TiN/Al/TiN, or other
equivalent materials and combination of materials) and a thin insulating layer
64
(typically thinner than 25nm) which manages to electroporate the cell via a
capacitive coupling. Microelectrodes are separated by insulating material 62
and
covered in non exposed areas by a passivation layer 63.
io According to a further embodiment of the microelectrodes of the biochip
according
to the present invention, said microelectrodes can be realised using Metal
Oxide
Semiconductor (MOS) technology in order to achieve a higher electrode density,
and each of them can be addressed in a way similar to that of semiconductor
memory cells.
With reference to Fig.4, an array of MOSFET (Metal Oxide Semiconductor Field
= Effect Transistor) is realised on the silicon substrate 40 with
conventional
microelectronic techniques, that is by implanting in a p-type substrate 40,
two n-
doped regions, 41 and 42, which constitute the drain and the source of the
transistor, respectively. The gate 43 of these MOSFETs is realised in n+ doped
polysilicon and is common to all devices in a row (word line). The drain 41 of
all
devices in a column are connected together by using a metal contact plug
= (normally realised in tungsten) and a metal line 44 (bit line). The metal
is
surrounded by isolation oxide 45. The source 42 of the transistor is connected
via
a metal (usually tungsten) plug 46 to a thin gold layer 47 which acts as the
active
microelectrode. Part of the chip not to be exposed can be covered with a
passivation layer 48.
Inverting p-type regions with n-type regions and n doping with p doping it is
possible to realise a pMOSFET instead of the nMOSFET described above with
analogous results in terms of integration.
With this technology, the minimum distance between two microelectrodes can be
as low as one micrometer thus allowing to reach and electroporate single cells
with high spatial resolution.
CA 02554027 2006-07-19
WO 2005/075656 8 PCT/EP2004/000789
In Fig. 5 a schematic of an array of microelectrodes as described above is
displayed. Microelectrodes 50 are connected to the sources 51 of MOSFETs,
whose drains 52 are connected to word line 53 and gates 54 to bit lines 55. If
the
gate of a MOSFET is kept at a voltage VG higher than the threshold voltage
VTH,
the drain is kept at VD, and the source is left open, the voltage of the
latter terminal
is VD-VTH. This mechanism can be used to control, the voltage of the
microelectrode 50 connected to the source 51 of a selected MOSFET by
controlling the voltages of the word line 53 and the bit line 55 which define
its
position in the array.
The electroporation apparatus according to the present invention does not
require
particular adhesion molecules and is independent from the procedures for cells
plating, positioning and culturing as well.
The apparatus according to the present invention allows the performing of
electroporation with the following advantages:
- electroporation on adhering cells
- electroporation onto a number of different single cells within the same
culture
- electroporation timing arbitrarily chosen for each target cell,
- electroporation degree (i.e. pores number, size and duration) controlled for
each
target cell, by controlling the voltage developing across the membrane via the
individual cell/microelectrode electrical coupling,
- electroporation in different sites of the same cell using different
microelectrodes,
in case of cells large enough to cover different microelectrodes.
Due to its features, the method according to the present invention is
extremely
useful for high throughput screening of molecules impermeable to the cell
membrane. Drugs, genetic constructs and proteins can be tested individually on
a
number of single cells on the same microchip. Timing of delivery and
combinations
of different molecules can be set for each target cell.
The method according to the present invention can be employed in basic
research
(for example for screening of genes function) as well as in the phase of
"discovery'
of new drugs. A substantial increase of the experimental yield and a decrease
of
the experimental costs are expected. Due to the good efficiency and control of
cell
CA 02554027 2006-07-19
WO 2005/075656 PCT/EP2004/000789
9
electroporation, it is not excluded that the technique will become useful even
in the
"production" phase of drugs from transfected cells and for gene therapy.
The method for electroporation achievable with the apparatus hereinbefore
describe substantially comprises the following steps:
- cultivate cells since the adhering stage is reached
- add in the culture medium at least one compound to be
electroporated in
at least one single cell of the said cells
- selected at least one single cell and at least one microelectrode on
which said selected single cell is adherent
- generate at least one electric signal suitable to electroporate said at
least one single cell with said at least one compound to be
electroporated and drive said electric signal to the said one
microelectrode on which said selected single cell is adherent.
Hereinafter four experiments performed with the method using the apparatus
according to the present invention are described in the following examples.
Example 1: Transfection of Cos-7 cells with oligonucleotides
The goal of the experiment was the transfection of individual target cells
with
double-stranded DNA oligonucleotides.
Oligonucleotides marked with a fluorescent label were used and their
penetration
into the target cell after electroporation was afterwards detected by the
presence
of intracellular fluorescence.
Cos-7 cells were mantained in culture using a conventional culture medium.
After
having been kept two days in culture, they were trypsinized, resuspended in
culture medium and plated on the biochip cell culture chamber according to the
present invention at a density of about 35,000 cells/cm2 of chip surface.
Before plating, the surface of the chip was washed carefully, rinsed, dried,
and
sterilised with UV light. The biochip was then coated with poly-L-lysine by
adsorption from a 20 pg/ml aqueous solution for 2 h and dried. Cells were
incubated at 37 C and 5% CO2.
Double-stranded DNA oligonucleotides of 87-113 pair bases and containing each
one a residue labelled with a fluorescent dye (either NED or FAM) were then
synthesised. The oligonucleotides were solubilized in water during the
synthesis to
CA 02554027 2006-07-19
WO 2005/075656 PCT/EP2004/000789
I 0
a final concentration of about 40 ng/pl. Prior electroporation the
oligonucleotides
solution was diluted 1:1 in HBS 2x. After removing the culture medium, about
60 pl
of the oligonucleotides solution were applied to the culture chamber just
before
electroporation.
Individual cells growing in contact with a single microelectrode were
identified by
microscopic observation. After selecting with the control system one of the
microelectrodes in contact with a single cell, a suitable electroporation
signal was
delivered and the same operation was repeated for each target cell.
A wave form generator driven by a personal computer, generates the electrical
io signal that is delivered to the biochip according to the present
invention. Said
signal is send through a 50n coaxial cable to the switching system that
permits to
transfer the signal to a pre-selected single electrode of the biochip
according to
the present invention. The external parallel connectors are connected to the
biochip through a printed circuit. The reference ground is made by connecting
the
previous switch ground to an Ag/AgCI electrode dipped into the electrolyte
solution
where the cultured cells lie.
The waveform of the electroporation signal is depicted in Fig. 7. Five trains
of 25
square pulses (1 ms duration, 10ps rise/fall time) repeated at a time interval
of 500
ms were found to be suitable for a successful transfection of Cos-7 cells.
After electroporation cells were washed with a standard physiological solution
and
observed with a fluorescence microscope. Successful transfection of each
target
cell was detected by the presence of cell fluorescence. Cell survival was
monitored for 24 h after transfection by observation of the cell morphology.
About 80% of target cells were successfully transfected and a good percentage
of
cells survival (50-80%) was achieved with pulses of 6-10 V amplitude and 1-10
ps
rise/fall time.
The degree of transfection, judging from the intensity of the intracellular
fluorescence, was directly related to the amplitude and inversely related to
the rise
time. Contrary, cell survival was decreasing with the voltage amplitude and
increasing with the rise time. The time interval between trains was critical:
for a
time interval larger than 500 ms, transfection did not occur. For a time
interval
smaller than 500 ms cell survival substantially decreased. As a best
compromise
CA 02554027 2011-02-11
11
in order to obtain the highest percentage of transfection and cell survival we
routinely used pulses of 9 V and 10 jis rise time.
Example 2 : Transfection of Cos-7 cells with a DNA plasm id vector.
In another experiment performed with the method according to the present
invention the goal was the transfection of individual target cells with a DNA
plasmid vector.
Individual Cos-7 target cells were transfected using the apparatus according
to
the present invention with a plasmid vector coding for the GFP (Green
Fluorescent Protein). The. synthesis of GFP in the cell cytoplasm was observed
with a fluorescence microscope and was indicating successful transfection.
Transfection with plasmid vectors is a technique routinely used in many
laboratories for the investigation of genes function.
The Cos-7 target cells were identical to the previous experiment.
A DNA plasmid vector of 5 kB containing the GFP coding gene was used. After
amplification and purification the DNA was solubilized to 50 g/m1 in water.
Prior
electroporation the DNA solution was diluted 1: 1 in HBS 2x. After removing
the
culture medium, about 60 pi of the DNA solution (25 jig/m1) were applied to
the
culture chamber just before electroporation.
Target cells were selected as in the previous experiment. The waveform of the
electroporation signal used was identical to the previous experiment except
that
the number of pulses per train was elevated to 50. After 48 h the transfected
target cell has undergone a replication cycle splitting in two cells
expressing the
GFP.
The typical cytoplasmic pattern of GFP expression is visible in both cells.
The
two cells, because of replication and of intrinsic cell motility, have
slightly moved
away from the microelectrode which was originally covered by the mother cell
alone.
Example 3 :-Electroporation of rat hippocampal neurons with fluoroscein.
In a third experiment, the electroporation with the apparatus according to the
invention of rat hippocampal neurons with fluoroscein was performed.
Neurons were dissociated from the hippocampi of Wistar rats at 18 d gestation
(Banker GA, Cowan VVM. (1977) Rat hippocampal neurons in dispersed cell
culture, Brain Res 126:397-442). They were preplated twice to get rid of glia
cells and suspended in DMEM with glutamax I (no. 61965026, Gibco,
Eggenstein, Germany) supplemented with 10% (vol) fetal bovine serum
(10106078, Gibco) and 1% (vol) penicillin (15140114, Gibco) (Brewer G.J.,
Torricelli, J.R., Evege, E.K. and Price, P.J. (1993) Optimized survival of
CA 02554027 2011-02-11
,
12
hippocampal neurons in B27-supplemented Neurobasal, a new serum-free
combination. J. Neurosci. Res., 35: 567-576); Vassanelli S., Fromherz P.,
(1998)
Transistors Records of Excitable Neurons from Rat Brain. APPLIED PHYSICS A,
Vol. 66; P. 459-463). The final concentration was 350,000 cells per
milliliter.
The surface of the chip was wiped carefully with a 1% solution of a liquid
dish
detergent, rinsed with milli-Q water (Millipore, Bedford, MA), dried, and
sterilized
with UV light. The chips were coated with poly-L-lysine (molecular weight
>300,000 ; Sigma, Heidelberg, Germany) by adsorption from a 20 ug/ml
aqueous solution for 1 h and dried. We applied 350 NI of the cell suspension
culture chamber. Leibovitz L-15 medium (100 ui) with glutamax 1(31415029,
Gibco) supplemented with 5% fetal bovine serum were added. The density of
cells was ¨100, 000 cm2. The chips were kept at 37 C and 10% CO2 for 2 h.
Then the medium was removed, and the cells were cultured in a serum-free
medium (Brewer G.J., Torricelli, J.R., Evege, E.K. and Price, P.J. (1993)
Optimized survival of hippocampal neurons in B27-supplemented Neurobasal,
a new serum-free combination. J. Neurosci. Res., 35: 567-576; Evans MS,
Collings MA and Brewer GJ. (1998) Electrophysiology of embryonic, adult and
aged rat hippocampal neurons in serum-free culture. J. Neurosci. Methods
79:37-46; Vassanelli S., Fromherz P. (1998) Transistors Records of Excitable
Neurons from Rat Brain. APPLIED PHYSICS A, Vol. 66; P. 459-463) using 450 ul
neurobasal medium (Gibco, 21103049) supplemented with 2% (vol) B27-
medium (17504036, Gibco) and 1 % (vol) glutamaxl (35050038 ? Gibco) for 4-7
d.
Electroporation was performed with neurons maintained for a minimum of 6 d
to a maximum of 12 d in culture.
Fluorescein was solubilized in standard physiological solution (10 uM) and
applied to the culture chamber just prior electroporation. After washing twice
with physiological solution cells were observed under a fluorescence
microscope.
Electroporation of single rat hippocampal neurons was obtained by application
to the microelectrode of trains of triangular voltages, each triangular
waveform
has amplitude equal to 4V and single triangular duration equal to lms with
rise
time equal to fall time (Fig 8).
It has to be noted that contrary to Cos-7 cells and numerous other cell lines,
neurons are excitable cells and unwanted stimulation of their electrical
activity
during electroporation has to be limited. We reduced unwanted stimulation (i)
by
diminishing the number of pulses per train (10 pulses versus 25 or 50 used
with Cos-7 cells), (ii) by increasing the interval between trains (5 s instead
of
500 ms) and (iii) by use of triangular voltages.
Example 4 :-Electrophysiological activity of cell during electroporation (Cos-
7
and Rat hippocampal neurons).
CA 02554027 2006-07-19
WO 2005/075656 13 PCT/EP2004/000789
A target cell was contacted with a patch-clamp electrode. After establishing a
whole-cell configuration the intracellular potential of the cell was
monitored.
Application of the appropriate electroporation signal to the corresponding
microelectrode induced an intracellular voltage transient up to 0 mV (the cell
electroporation pores and the consequent electrical communication with the
grounded external electrolyte, was slowly decreasing and the cell completely
recovering to the resting potential within 1-2 minutes. This was likely the
time
required for pores resealing. Pores formation was also demonstrated by
into the cell.
Electroporation performed with the apparatus according to the present
invention,
and suitable electroporation signals, could be repeated during the whole patch-
clamp recording session (typically 20-30 min) without any electrophysiological
sign
As expected, the amplitude of the intracellular voltage transient induced by
electroporation and the time of recovery to the resting potential were varying
depending on the electroporation signal applied.
The results herein described demonstrate that the apparatus and the method
different kinds of cells with a variety of compounds thus fulfilling the
purposes of
the present invention. Further implementation or adaptations as well as
embodiments readily apparent to those skilled in the art are to be considered
within the scope of the present invention.