Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
TETRAHYDROCARBOLINE COMPOUNDS AS ANTICANCER AGENTS
FIELD OF THE INVENTION
The present invention relates to new tetrahydrocarboline compounds, their
pharmaceutically acceptable salts, and prodrugs thereof; compositions of the
new
compounds, either alone or in combination with at least one additional
therapeutic agent,
with a pharmaceutically acceptable carrier; and uses of the new compounds,
either alone
or in combination with at least one additional therapeutic agent, in the
prophylaxis or
treatment of proliferative diseases.
BACKGROUND OF THE INVENTION
Kinesins are motor proteins that use adenosine triphosphate (ATP) to bind to
microtubules and generate mechanical force. Kinesins are characterized by a
motor
domain having about 350 amino acid residues. The crystal structures of several
kinesin
motor domains have been resolved.
Currently, about one hundred kinesin-related proteins (KRP) have been
identified.
Kinesins are involved in a variety of cell biological processes including
transport of
organelles and vesicles, and maintenance of the endoplasmatic reticulum.
Several KRPs
interact with the microtubules of the mitotic spindle or with the chromosomes
directly,
and appear to play a pivotal role during the mitotic stages of the cell cycle.
These mitotic
KRPs are of particular interest for the development of cancer therapeutics.
Kinesin spindle protein (KSP) (also known as EgS, HsEgS, KNSL1, or KIFII) is
one of several kinesin-like motor proteins that are localized to the mitotic
spindle and
known to be required for formation and/or function of the bipolar mitotic
spindle.
In 1995, the depletion of KSP using an antibody directed against the C-
terminus
of KSP was shown to arrest HeLa cells in mitosis with monoastral microtubule
arrays
(Blangy et al., Cell 83:1159-1169, 1995). Mutations in bimC and cut? genes,
which are
considered to be homologues of KSP, cause failure in centrosome separation in
Aspergillus Nidulans (Enos, A.P., and N.R. Morris, Cell 60:1019-1027, 1990)
and
Schizosaccharomyces Pombe (Hagan, L, and M. Yanagida, Natu~°e 347:563-
566, 1990).
Treatment of cells with either ATRA (all traps-retinoic acid), which reduces
KSP expression on protein level, or depletion of KSP using antisense
oligonucleotides
revealed a significant growth inhibition in DAN-G pancreatic carcinoma cells
indicating
that KSP might be involved in the antiproliferative action of all traps-
retinoic acid
-1-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
(Kaiser, A., et al., J. Biol. Chew. 274, 18925-18931, 1999). Interestingly,
the Xenopus
Laevis Aurora-related protein kinase pEg2 was shown to associate and
phosphorylate
XlEgS (Giet, R., et al., J. Biol. ClZena. 274:15005-15013, 1999). Potential
substrates of
Aurora-related kinases are of particular interest for cancer drug development.
For
example, Aurora l and 2 kinases are overexpressed on protein and RNA level and
the
genes are amplified in colon cancer patients.
The first cell permeable small molecule inhibitor for KSP, "monastrol," was
shown to arrest cells with monopolar spindles without affecting microtubule
polymerization as do conventional chemotherapeutics such as taxanes and vinca
alkaloids
(Mayer, T.U., et al., Science 286:971-974, 1999). Monastrol was identified as
an
inhibitor in phenotype-based screens and it was suggested that this compound
may serve
as a lead for the development of anticancer drugs. The inhibition was
determined not to
be competitive in respect to adenosine triphosphate and to be rapidly
reversible (DeBous,
S., et al., Biochemistry 42:338-349, 2003; Kapoor, T.M., et al., .I. Cell
Biol. 150:975-988,
2000).
Recently, other KSP kinesin inhibitors have been described. WO 02/057244 and
WO 02/056880 describe phenothiazine compounds and triphenylmethane compounds,
respectively, for treating proliferative diseases. WO 02/078639 describes
cyano-substituted dihydropyrimidine compounds for treating proliferative
diseases.
U.S. Patent No. 6,472,521 describes oligonucleotides and oligonucleotide
derivatives for
inhibiting human KSP expression.
WO 01/98278, WO 01130768, and WO 03/039460 describe quinazolinone
compounds that are useful in treating cellular proliferative diseases
associated with
KSP activity. The compounds described in these references are
2-(2-aminomethyl)quinazolinone derivatives. The quinazolinone compounds
described
in WO 01/98278 and WO 01/30768 have 2-aminomethyl substituents that are either
amine, amide, or sulfonamide substituents. The quinazolinone compounds
described in
WO 03/039460 have the amino group of the 2-aminomethyl substituent
incorporated into
a 5-12 membered nitrogen-containing heterocycle.
WO 03/050064 describes thienopyrimidinone compounds that are useful for
treating cellular proliferative disease, for treating disorders associated
with KSP activity,
and for inhibiting KSP.
_2_
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
WO 03/103575 describes heterocyclic-fused pyrimidinone derivatives that are
inhibitors of the mitotic KSP and that are useful in the treatment of cellular
proliferative
diseases.
Tetrahydrocarbolines have been used as topoisomerase II inhibitors, protein
tyrosine phosphatases (PTPases) inhibitors, and phosphodiesterase inhibitors.
U.S. Patent
No. 5,606,060 describes azatoxin and derivatives as antitumor drugs that are
topoisomerase II inhibitors. WO 03/033496 describes beta-carboline derivatives
as
anticancer drugs that are protein tyrosine phosphatases inhibitors. U.S.
Patent
No. 6,069,150 describes beta-carboline derivatives bearing at least a free or
esterified
carboxylic group on the piperidine ring that have antimetastatic properties.
WO 01/87038 describes beta-carboline derivatives for the treatment of diseases
and
conditions related to phosphodiesterase inhibitors (PDE) such as male erectile
dysfunction.
SUMMARY OF THE INVENTION
In one aspect of the present invention, new tetrahydrocarboline compounds,
their
pharmaceutically acceptable salts, and prodrugs thereof are provided. The
tetrahydrocarboline compounds, pharmaceutically acceptable salts, and prodrugs
are
KSP inhibitors and are useful in the treating cellular proliferation diseases.
-3-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
In one embodiment, the tetrahydrocarboline compounds have the formula (I):
Rio
(I)
or a stereoisomer, tautomer, pharmaceutically acceptable salt, ester, or
prodrug
thereof, wherein,
Rl is selected from the group consisting of
(1) hydrogen, and
(2) substituted or unsubstituted C1-C6 alkyl;
Ra is selected from the group consisting of
(1) substituted or unsubstituted CS-Cg
cycloalkyl,
(2) substituted or unsubstituted aryl,
(3) substituted or unsubstituted heteroaryl,
(4) substituted or unsubstituted heterocyclyl,
and
(5) CONR2aR2b,
wherein R2a
and R2b are selected
from the group
consisting of
(a) hydrogen
(b) substituted or unsubstituted C3-C8
cycloalkyl,
(c) substituted or unsubstituted aryl,
(d) substituted or unsubstituted heteroaryl,
and
(e) substituted or unsubstituted heterocyclyl;
Rg is selected
from the group
consisting of
( 1 ) hydrogen, and
(2) substituted or unsubstituted alkyl;
-4-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Rq. is selected from the group consisting of
(1) substituted or unsubstituted alkyl, wherein substituted alkyl is not a
halomethyl,
(2) substituted or unsubstituted cycloalkyl, and
(3) substituted or unsubstituted heterocyclyl;
RS is selected from the group consisting of
(1) hydrogen,
(2) CO2Rsa~ ~d
(3) CONRsbRs~,
wherein Rsa, Rsb, and Rs~ are selected from the group consisting of
(a) substituted or unsubstituted alkyl,
(b) substituted or unsubstituted aryl,
(c) substituted or unsubstituted heteroaryl, and
(d) substituted or unsubstituted heterocyclyl;
R6 is hydrogen;
R7 is hydrogen or hydroxy;
R8 is hydrogen, or
R7 and R8 taken together with the carbon atom to which R7 and R8 are attached
form a carbonyl;
. Rlp is selected from the group consisting of
(1) substituted or unsubstituted alkyl,
(2) substituted or unsubstituted aryl,
(3) substituted or unsubstituted heteroaryl,
(4) substituted or unsubstituted heterocyclyl,
(5) halogen,
(6) ORloa~
(7) C02Rloa~
(8) NRloaRlla~
(9) CONRIOaRna~
(10) SOaNRIOaRIIa~
-5-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
wherein Rloa and Rila are selected from the group consisting of
(a) hydrogen,
(b) substituted or unsubstituted alkyl, and
(c) substituted or unsubstituted aryl; and
R9, Rl l, and R12 are selected from the group consisting of
(1) hydrogen,
(2) substituted or unsubstituted
alkyl,
(3) substituted or unsubstituted
aryl,
(4) halogen, and
(5) OR9~,
wherein R9a is
selected from
the group consisting
of
(a) hydrogen,
(b) substituted or unsubstituted alkyl, and
(c) substituted or unsubstituted aryl,
with the proviso that when Rl is hydrogen, RZ is phenyl, and Rlo is bromo, R4
is
not methyl,
with the proviso that when Rl is hydrogen, R2 is nitrophenyl or chloro
nitrophenyl, and Rlo is methoxy, R4 is not adamantyl,
with the proviso that when Rl is hydrogen, R2 is phenyl, R4 is methyl, and Rlo
is
hydroxy, RS is not C02CH3, and
with the proviso that when R4 is methyl or hydroxymethyl, Rz is not phenyl,
4-hydroxyphenyl, 3-methoxy-4-hydroxyphenyl, or 3,5-dimethoxy-4-hydroxyphenyl.
In another aspect, the present invention provides methods for treating
proliferative
diseases in a human or animal subject in need of such treatment comprising
administering
to said subject an amount of a compound of formula (I) effective to reduce or
prevent
cellular proliferation in the subject.
In another aspect, the present invention provides methods for treating
proliferative
diseases in a human or animal subject in need of such treatment, comprising
administering to said subject an amount of a compound of formula (I) effective
to reduce
-6-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
or prevent cellular proliferation in the subject in combination with at least
one additional
agent for the treatment of cancer.
In other aspects, the present invention provides therapeutic compositions,
comprising at least one compound of formula (I) in combination with one or
more
additional agents for the treatment of cancer, as are commonly employed in
cancer
therapy.
The compounds of the invention are useful in the treatment of cancers,
including,
for example, lung and bronchus; prostate; breast; pancreas; colon and rectum;
thyroid;
stomach; liver and intrahepatic bile duct; kidney and renal pelvis; urinary
bladder; uterine
corpus; uterine cervix; ovary; multiple myeloma; esophagus; acute myelogenous
leukemia; chronic myelogenous leukemia; lymphocytic leukemia; myeloid
leukemia;
brain; oral cavity and pharynx; larynx; small intestine; non-hodgkin lymphoma;
melanoma; and villous colon adenoma.
The invention further provides compositions, kits, methods of use, and methods
of
manufacture as described in the detailed description of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing aspects and many of the attendant advantages of this invention
will
become more readily appreciated as the same become better understood by
reference to
the following detailed description, when taken in conjunction with the
accompanying
drawings, wherein:
FIGURE 1 is a synthetic scheme illustrating the preparation of representative
substituted tryptamine compounds useful in synthesizing the
tetrahydrocarboline
compounds of the invention;
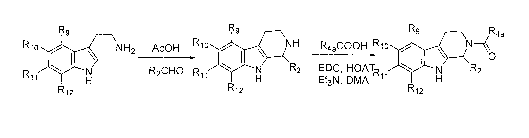
FIGURE 2 is a synthetic scheme illustrating the preparation of representative
N-acyl substituted tetrahydrocarboline compounds of the invention;
FIGURE 3 is a synthetic scheme illustrating the preparation of representative
N-aminosubstituted alkylcaxbonyl-containing tetrahydrocarboline compounds of
the
invention;
FIGURE 4 is a synthetic scheme illustrating the preparation of representative
aryl
substituted tetrahydrocarboline compounds of the invention;
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
FIGURE 5 is a synthetic scheme illustrating the preparation of representative
3-hydroxyphenyl substituted tetrahydrocarboline compounds of the invention;
FIGURE 6 is a synthetic scheme illustrating the preparation of representative
tetrahydrocarboline-4-one and tetrahydrocarboline-4-of compounds of the
invention;
FIGURE 7 is a synthetic scheme illustrating the preparation of representative
carboxy and amide substituted tetrahydrocarboline compounds of the invention;
and
FIGURE ~ is a synthetic scheme illustrating the preparation of representative
carbamate, sulfonamide, and amide substituted tetrahydrocarboline compounds of
the
invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
In one aspect of the present invention, new tetrahydrocarboline compounds,
their
pharmaceutically acceptable salts, and prodrugs thereof are provided. The
tetrahydrocarboline compounds, pharmaceutically acceptable salts, and prodrugs
are I~SP
inhibitors and are useful in the treating cellular proliferation diseases.
In one embodiment, the tetrahydrocarboline compounds of the invention have the
formula (I):
R~ c
R~
(I)
or a stereoisomer, tautomer, pharmaceutically acceptable salt, ester, or
prodrug
thereof, wherein,
Rl is selected from the group consisting of
(1) hydrogen, and
(2) substituted or unsubstituted Cl-C6 alkyl;
_g_
R1z
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
R2 is selected from the group consisting of
(1) substituted or unsubstituted Cs-C8
cycloalkyl,
(2) ~ substituted or unsubstituted aryl,
(3) substituted or unsubstituted heteroaryl,
(4) substituted or unsubstituted heterocyclyl,
and
(5) CONR2aR2b,
wherein R2a and
R2b are selected
from the group
consisting of
(a) hydrogen
(b) substituted or unsubstituted C3-Cg
cycloalkyl,
(c) substituted or unsubstituted aryl,
(d) substituted or unsubstituted heteroaryl,
and
(e) substituted or unsubstituted heterocyclyl;
R3 is selected
from the group
consisting of
(1) hydrogen, and
(2) substituted or unsubstituted alkyl;
R4 is selected
from the group
consisting of
(1) substituted or unsubstituted alkyl, wherein substituted alkyl is not a
halomethyl,
(2) substituted or unsubstituted cycloalkyl, and
(3) substituted or unsubstituted heterocyclyl;
RS is selected from the group consisting of
(1) hydrogen,
(2) COzRsa~ ~d
(3) CONRsbRs~,
wherein Rsa, Rsb, and Rs~ are selected from the group consisting of
(a) substituted or unsubstituted alkyl,
(b) substituted or unsubstituted aryl,
(c) substituted or unsubstituted heteroaryl, and
(d) substituted or unsubstituted heterocyclyl;
-9-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
R6 is hydrogen;
R7 is hydrogen or hydroxy;
R$ is hydrogen, or
R7 and R8 taken together with the carbon atom to which R7 and R$ are attached
form a carbonyl;
Rlp is selected from the group consisting of
(1) substituted or unsubstituted alkyl,
(2) substituted or unsubstituted aryl,
(3) substituted or unsubstituted heteroaryl,
(4) substituted or unsubstituted heterocyclyl,
(5) halogen,
(6) ORloa,
(7) C02R~oa,
(8) NR~oaRua~
(9) CONRIOaRua,
(10) S02NR1o~Rlla,
wherein Rloa and Rlia are selected from the group consisting of
(a) hydrogen,
(b) substituted or unsubstituted alkyl, and
(c) substituted or unsubstituted
aryl; and
Rg, Rl l, and R12 are selected from the group
consisting of
(1) hydrogen,
(2) substituted or unsubstituted
alkyl,
(3) substituted or unsubstituted
aryl,
(4) halogen, and
(5) ORga,
wherein Rga is
selected from
the group consisting
of
(a) hydrogen,
(b) substituted or unsubstituted alkyl, and
(c) substituted or unsubstituted aryl,
-10-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
with the proviso that when Rl is hydrogen, Ra is phenyl, and Rlo is bromo, R4
is
not methyl,
with the proviso that when Rl is hydrogen; Ra is nitrophenyl or chloro
nitrophenyl, and Rlo is methoxy, R4 is not adamantyl,
S with the proviso that when Rl is hydrogen, R2 is phenyl, R4 is methyl, and
Rlo is
hydroxy, RS is not C02CH3, and
with the proviso that when R4 is methyl or hydroxymethyl, R2 is not phenyl,
4-hydroxyphenyl, 3-methoxy-4-hydroxyphenyl, or 3,5-dimethoxy-4-hydroxyphenyl.
In another embodiment, the tetrahydrocarboline compounds of the invention have
formula (I) above, or a stereoisomer, tautomer, pharmaceutically acceptable
salt, ester, or
prodrug thereof, wherein,
Rl is selected from the group consisting of
(1) hydrogen, and
(2) substituted or unsubstituted C1-Cg alkyl;
R2 is selected from the group consisting of
(1) substituted or unsubstituted aryl, and
(2) substituted or unsubstituted heteroaryl;
R3 is selected from the group consisting of
( 1 ) hydrogen, and
(2) substituted or unsubstituted alkyl;
R4 is a nitrogen-containing substituent;
RS is selected from the group consisting of
( 1 ) hydrogen,
(2) C02Rsa~ ~d
(3) CONRSbRs~,
wherein Rsa, Rsb, and Rs~ are selected from the group consisting of
(a) substituted or unsubstituted allcyl,
(b) substituted or unsubstituted aryl,
(c) substituted or unsubstituted heteroaryl, and
-11-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
(d) substituted or unsubstituted heterocyclyl;
R6 is hydrogen;
R7 is hydrogen or hydroxy;
R8 is hydrogen, or
R7 and R8 taken together with the carbon atom to which R7 and R$ are attached
form a carbonyl;
Rlo is selected
from the group
consisting of
(1) substituted or unsubstituted alkyl,
(2) substituted or unsubstituted aryl,
(3) substituted or unsubstituted heteroaryl,
(4) substituted or unsubstituted heterocyclyl,
(5) halogen,
(6) ORloa~
(7) C02R~oa,
(8) NR~oaRna~
(9) CONR~oaRua~
(10) S02NR1oaRla,
wherein Rloa and
Rua are selected
from the group
consisting of
(a) hydrogen,
(b) substituted or unsubstituted alkyl, and
(c) substituted or unsubstituted aryl, and
R9, Rl 1, and R12
are selected from
the group consisting
of
( 1 ) hydrogen,
(2) substituted or unsubstituted
alkyl,
(3) substituted or unsubstituted
aryl,
(4) halogen, and
(5) OR9a,
-12-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
wherein R9a is selected from the group consisting of
(a) hydrogen,
(b) substituted or unsubstituted alkyl, and
(c) substituted or unsubstituted aryl.
The following embodiments relate to the tetrahydrocarboline compounds having
formula (I) described above.
In one embodiment, Rl is hydrogen.
In one embodiment, R2 is phenyl or substituted phenyl. The phenyl group may be
substituted with one or more substitiuents including, for example, alkyl,
alkoxy, amino,
carboxy, halogen, hydroxy, thioalkyl, and alkylsulfonate groups. In one
embodiment,
R2 is 3-hydroxyphenyl.
In one embodiment, R3 is hydrogen.
In one embodiment, R2 is a phenyl or substituted phenyl, and R3 is hydrogen.
In one embodiment, Rq, is a nitrogen-containing substituent, for example, an
alkyl
group substituted with a nitrogen-containing group. Representative nitrogen-
containing
groups include amino groups (e.g., primary, secondary, and tertiary amino
groups) and
cyclic amino groups (e.g., azetidinyl, pyrrolidinyl, piperidinyl, piperizinyl,
and
morpholinyl); amide groups (e.g., -C(=O)-NR2 , where R is hydrogen or alkyl;
and
-NH-C(=O)-R, where R is alkyl or aryl, such as methyl, trifluoromethyl, and
phenyl);
carbamate groups (e.g., methyl, ethyl, isopropyl, or t-butyl caxbamate
groups); and
sulfonamide groups (e.g., -NHS02R, where R is alkyl or aryl, such as methyl
and
phenyl). In one embodiment, R4 is aminoalkyl, for example, aminomethyl, 2-
aminoethyl,
3-aminopropyl.
In one embodiment, R5, R6, R7, and Rg are hydrogen.
In one embodiment, R9, Rl l, and R12 are hydrogen.
In another embodiment, Rlo is selected from alkyl (e.g., methyl, ethyl,
isopropyl),
alkoxy (e.g., methoxy, trifluoromethoxy), aryl (e.g., phenyl, methoxyphenyl,
tolyl) halo
(e.g., fluoro, chloro, bromo), and hydroxy.
-13-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
For the compounds of formulas (I), representative substituted alkyl groups
include
arylalkyl, heteroarylalkyl, heterocyclyalkyl, aminoalkyl, alkylaminoalkyl,
dialkyaminoalkyl, and sulfonamidoalkyl groups. Representative substituted aryl
groups
include sulfonamidoaryl groups. Representative substituted heteroaryl groups
include
alkylheteroaryl groups.
The synthesis of representative tetrahydrocarboline compounds are described in
Examples 2-11. Representative tetrahydrocarboline compounds that were prepared
are
shown in Table 1 in Example 12.
In other aspects, the present invention provides methods for manufacture of
tetrahydrocarboline compounds. Methods of making representative compounds of
the
invention are described in Examples 2-11. It is further contemplated that, in
addition to
the compounds of formula (I), intermediates, and their corresponding methods
of
syntheses are included within the scope of the invention. Representative
compounds of
the invention are illustrated in Table 1 in Example 12.
In other aspects, the present invention provides compositions that include the
KSP inhibitors described herein, and methods that utilize the KSP inhibitors
described
herein.
In one aspect, the present invention provides pharmaceutical compositions
comprising at least one tetrahydrocarboline compound (e.g., a compound of
formula (I))
together with a pharmaceutically acceptable carrier suitable for
administration to a human
or animal subject, either alone or together with other anticancer agents.
A number of suitable anticancer agents to be used as combination therapeutics
are
contemplated for use in the compositions and methods of the present invention.
Suitable
anticancer agents to be used in combination with the compounds of the
invention include
agents that induce apoptosis; polynucleotides (e.g., ribozymes); polypeptides
(e.g., enzymes); drugs; biological mimetics; alkaloids; alkylating agents;
antitumor
antibiotics; antimetabolites; hormones; platinum compounds; monoclonal
antibodies
conjugated with anticancer drugs, toxins, and/or radionuclides; biological
response
modifiers (e.g., interferons [e.g., IFN-a] and interleukins [e.g., IL-2]);
adoptive
immunotherapy agents; hematopoietic growth factors; agents that induce tumor
cell
differentiation (e.g., all-trans-retinoic acid); gene therapy reagents;
antisense therapy
reagents and nucleotides; tumor vaccines; inhibitors of angiogenesis, and the
like.
Numerous other examples of chemotherapeutic compounds and anticancer therapies
-14-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
suitable for coadministration with the tetrahydrocarboline compounds of the
invention are
known to those skilled in the art.
In certain embodiments, anticancer agents to be used in combination with
tetrahydrocarboline compounds of the invention comprise agents that induce or
stimulate
apoptosis. Agents that induce apoptosis include, but are not limited to,
radiation; kinase
inhibitors (e.g., Epidermal Growth Factor Receptor [EGFR] kinase inhibitor,
Vascular
Endothelial Growth Factor Receptor [VEGFR] kinase inhibitor, Fibroblast Growth
Factor
Receptor [FGFR] kinase inhibitor, Platelet-derived Growth Factor Receptor
[PGFR]
I kinase inhibitor, and Bcr-Abl kinase inhibitors such as STI-571 [Gleevec or
Glivec]);
antisense molecules; antibodies [e.g., Herceptin and Rituxan]; anti-estrogens
[e.g., raloxifene and tamoxifen]; anti-androgens [e.g., flutamide,
bicalutamide,
finasteride, amino-glutethamide, ketoconazole, and corticosteroids];
cyclooxygenase
2 (COX-2) inhibitors [e.g., Celecoxib, meloxicam, NS-398, and non-steroidal
anti-inflammatory drugs (NSAIDs)]; and cancer chemotherapeutic drugs [e.g.,
irinotecan
(Camptosar), CPT-11, fludarabine (Fludara), dacarbazine (DTIC), dexamethasone,
mitoxantrone, Mylotarg, VP-16, cisplatinum, 5-FU, Doxrubicin, TAXOTERE or
TAXOL]; cellular signaling molecules; ceramides and cytokines; and
staurosparine; and
the like.
In other aspects, the invention provides methods for using the compounds
described herein. For example, the compounds described herein can be used in
the
treatment of cancer. The compounds described herein can also be used in the
manufacture of a medicament for the treatment of cancer.
In one embodiment, the present invention provides methods of treating human or
aiumal subjects suffering from a cellular proliferative disease, such as
cancer. The
present invention provides methods of treating a human or animal subject in
need of such
treatment, comprising administering to the subject a therapeutically effective
amount of
an tetrahydrocarboline compound (e.g., a compound of formula (I)), either
alone or in
combination with other anticancer agents.
In another embodiment, the present invention provides methods for treating a
cellular proliferative disease in a human or animal subject in need of such
treatment
comprising, administering to said subject an amount of an tetrahydrocarboline
compound
(e.g., a compound of formula (I)) effective to reduce or prevent cellular
proliferation or
tumor growth in the subj ect.
-15-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
In another embodiment, the present invention provides methods for treating a
cellular proliferative disease in a human or animal subject in need of such
treatment
comprising administering to said subject an amount of an tetrahydrocarboline
compound
(e.g., a compound of formula (I)) effective to reduce or prevent cellular
proliferation in
the subject in combination with at least one additional agent for the
treatment of cancer.
The present invention provides compounds that are inhibitors of KSP. The
inhibitors are useful in pharmaceutical compositions for human or veterinary
use where
inhibition of KSP is indicated, e.g., in the treatment of cellular
proliferative diseases such
as tumor and/or cancerous cell growth mediated by KSP. In particular, the
compounds
are useful in the treatment of human or animal (e.g., marine) cancers,
including, for
example, lung and bronchus; prostate; breast; pancreas; colon and rectum;
thyroid;
stomach; liver and intrahepatic bile duct; kidney and renal pelvis; urinary
bladder; uterine
corpus; uterine cervix; ovary; multiple myeloma; esophagus; acute myelogenous
leukemia; chronic myelogenous leukemia; lymphocytic leukemia; myeloid
leukemia;
brain; oral cavity and pharynx; larynx; small intestine; non-hodgkin lymphoma;
melanoma; and villous colon adenoma.
In another embodiment, the invention provides methods of treating an
KSP mediated disorder. In one method, an effective amount of an
tetrahydrocarboline
compound is administered to a patient (e.g., a human or animal subject) in
need thereof to
mediate (or modulate) KSP activity.
A representative assay for determining KSP inhibitory activity is described in
Example 13.
In some embodiments of the method of inhibiting KSP using a
tetrahydrocarboline compound of the invention, the ICSO value of the compound
is less
than or equal to 1mM with respect to KSP. In other such embodiments, the ICSo
value is
less than or equal to 100 ~,M, is less than or equal to 25 ~.M, is less than
or equal to
10 ~M, is less than or equal to 1 ~M, is less than or equal to 0.1 ~M, is less
than or equal
to 0.050 ~,M, or is less than or equal to 0.010 ~,M.
In some embodiments of the method of inhibiting KSP using a
tetrahydrocarboline compound of the invention, the ICSO value of the compound
is
between 1 nM to 10 nM. In other such embodiments, the ICSO value is between 10
nM to
50 nM, between 50 nM to 100 nM, between 100 nM to 1 ~,M, between 1 ~,M to 25
~,M,
or is between 25 ~M to 100 ~M.
-16-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
The following definitions are provided to better understand the invention.
"Alkyl" refers to alkyl groups that do not contain heteroatoms. Thus the
phrase
includes straight chain alkyl groups such as methyl, ethyl, propyl, butyl,
pentyl, hexyl,
heptyl, octyl, nonyl, decyl, undecyl, dodecyl and the like. The phrase also
includes
branched chain isomers of straight chain alkyl groups, including but not
limited to, the
following which are provided by way of example: -CH(CH3)2, -CH(CH3)(CH2CH3),
-CH(CH2CH3)2, -C(CH3)3, -C(CH2CH3)3, -CHZCH(CH3)2, -CH2CH(CH3)(CH2CH3),
-CH2CH(CH2CH3)2, -CH2C(CH3)3, -CHZC(CHZCH3)3, -CH(CH3)-CH(CH3)(CH2CH3),
-CH2CH2CH(CH3)2, -CH2CH2CH(CH3)(CH2CH3), -CHZCH2CH(CH2CH3)2,
-CHZCH2C(CH3)3, -CH2CH2C(CH2CH3)3, -CH(CH3)CH2_CH(CH3)2,
-CH(CH3)CH(CH3)CH(CH3)2, -CH(CH2CH3)CH(CH3)CH(CH3)(CH2CH3), and others.
Thus the phrase "alkyl groups" includes primary alkyl groups, secondary alkyl
groups,
and tertiary alkyl groups. Preferred alkyl groups include straight and
branched chain
alkyl groups having 1 to 12 carbon atoms.
"Alkylene" refers to the same residues as noted above for "alkyl," but having
two
points of attachment. Exemplary alkylene groups include ethylene (-CH2CH2-),
propylene (-CH2CH2CH2-), dimethylpropylene (-CH2C(CH3)2CH2-), and
cyclohexylpropylene (-CH2CH2CH(C6H13)-).
"Alkenyl" refers to straight chain, branched, or cyclic radicals having one or
more
carbon-carbon double bonds and from 2 to about 20 carbon atoms. Preferred
alkenyl
groups include straight chain and branched alkenyl groups and cyclic alkenyl
groups
having 2 to 12 carbon atoms.
"Alkynyl" refers to straight chain, branched, or cyclic radicals having one or
more
carbon-carbon triple bonds and from 2 to about 20 carbon atoms. Preferred
alkynyl
groups include straight chain and branched alkynyl groups having 2 to 12
carbon atoms.
Alkyl, alkenyl, and alkynyl groups may be substituted. "Substituted alkyl"
refers
to an alkyl group as defined above in which one or more bonds to a carbons) or
hydrogen(s) are replaced by a bond to non-hydrogen and non-carbon atoms such
as, but
not limited to, a halogen atom such as F, Cl, Br, and I; an oxygen atom in
groups such as
hydroxyl groups, alkoxy groups, aryloxy groups, and ester groups; a sulfur
atom in
groups such as thiol groups, alkyl and aryl sulfide groups, sulfone groups,
sulfonyl
groups, and sulfoxide groups; a nitrogen atom in groups such as amines,
amides,
alkylamines, dialkylamines, arylamines, alkylarylamines, diarylamines, N-
oxides, imides,
-17-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
and enamines; a silicon atom in groups such as in trialkylsilyl groups,
dialkylarylsilyl
groups, alkyldiarylsilyl groups, and triarylsilyl groups; and other
heteroatoms in various
other groups. Substituted alkyl groups also include groups in which one or
more bonds to
a carbons) or hydrogen(s) atom is replaced by a higher-order bond (e.g., a
double- or
triple-bond) to a heteroatom such as oxygen in oxo, carbonyl, carboxyl, and
ester groups;
nitrogen in groups such as imines, oximes, hydrazones, and nitriles.
Substituted alkyl
groups further include alkyl groups in which one or more bonds to a carbons)
or
hydrogen(s) atoms is replaced by a bond to an aryl, heteroaryl, heterocyclyl,
or cycloalkyl
group. Preferred substituted alkyl groups include, among others, alkyl groups
in which
one or more bonds to a carbon or hydrogen atom is/are replaced by one or more
bonds to
fluoro, chloro, or bromo group. Another preferred substituted alkyl group is
the
trifluoromethyl group and other alkyl groups that contain the trifluoromethyl
group.
Other preferred substituted alkyl groups include those in which one or more
bonds to a
carbon or hydrogen atom is replaced by a bond to an oxygen atom such that the
substituted alkyl group contains a hydroxyl, alkoxy, or aryloxy group. Other
preferred
substituted alkyl groups include alkyl groups that have an amine, or a
substituted or
unsubstituted alkylamine, dialkylamine, arylamine, (alkyl)(aryl)amine,
diarylamine,
heterocyclylamine, diheterocyclylamine, (alkyl)(heterocyclyl)amine, or
(aryl)(heterocyclyl)amine group. Still other preferred substituted alkyl
groups include
those in which one or more bonds to a caxbon(s) or hydrogen(s) atoms is
replaced by a
bond to an aryl, heteroaryl, heterocyclyl, or cycloalkyl group. Examples of
substituted
alkyl are: -(CHZ)3NH2, -(CHz)3NH(CH3), -(CH2)3NH(CH3)a, -CH2C(=CH2)CH2NHz,
_CH2C(=O)CHZNH2, -CH2S(=O)ZCH3, -CH20CH2NH2, -C02H. Examples of
substituents of substituted alkyl axe: -CH3, -C2H5, -CHaOH, -OH, -OCH3, -
OCZHS,
-OCF3, -OC(=O)CH3, -OC(=O)NH2,-OC(=O)N(CH3)Z,-CN, N02, -C(=O)CH3, -COZH,
-C02CH3, -CONH2, NH2,-N(CH3)2, NHS02CH3, NHCOCH3, NHC(=O)OCH3,
-NHSO-2CH3, -SOZCH3, -SOZNHZ, Halo.
"Substituted alkenyl" has the same meaning with respect to alkenyl groups that
substituted alkyl groups had with respect to unsubstituted alkyl groups. A
substituted
alkenyl group includes alkenyl groups in which a non-carbon or non-hydrogen
atom is
bonded to a carbon double bonded to another carbon and those in which one of
the
non-carbon or non-hydrogen atoms is bonded to a carbon not involved in a
double bond
to another carbon.
-18-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
"Substituted alkynyl" has the same meaning with respect to alkynyl groups that
substituted alkyl groups had with respect to unsubstituted alkyl groups. A
substituted
alkynyl group includes alkynyl groups in which a non-carbon or non-hydrogen
atom is
bonded to a carbon triple bonded, to another carbon and those in which a non-
carbon or
non-hydrogen atom is bonded to a carbon not involved in a triple bond to
another carbon.
"Alkoxy" refers to RO- wherein R is alkyl. Representative examples of alkoxy
groups include methoxy, ethoxy, t-butoxy, trifluoromethoxy, and the like.
"Halogen" or "halo" refers to chloro, bromo, fluoro, and iodo groups. The term
"haloalkyl" refers to an alkyl radical substituted with one or more halogen
atoms. The
term "haloalkoxy" refers to an alkoxy radical substituted with one or more
halogen atoms.
"Amino" refers herein to the group NH2. The term "alkylamino" refers herein to
the group NRR' where R is alkyl and R' is hydrogen or alkyl. The term
"arylamino"
refers herein to the group NRR' where R is aryl and R' is hydrogen, alkyl, or
aryl. The
term "aralkylamino" refers herein to the group NRR' where R is aralkyl and R'
is
hydrogen, alkyl, aryl, or aralkyl.
"Alkoxyalkyl" refers to the group -alkl-O-alk2 where alkl is alkyl or alkenyl,
and
alk2 is alkyl or alkenyl. The term "aryloxyalkyl" refers to the group -alkyl O-
aryl. The
term "aralkoxyalkyl" refers to the group -alkylenyl-O-aralkyl.
"Alkoxyalkylamino" refers herein to the group NR-( alkoxyalkyl), where R is
typically hydrogen, aralkyl, or alkyl.
"Aminocarbonyl" refers herein to the group -C(O)-NH2. "Substituted
aminocarbonyl" refers herein to the group -C(O)-NRR' where R is alkyl and R'
is
hydrogen or alkyl. The term "arylaminocarbonyl" refers herein to the group -
C(O)-NRR'
where R is aryl and R' is hydrogen, alkyl or aryl. "Aralkylaminocarbonyl"
refers herein
to the group -C(O)-NRR' where R is aralkyl and R' is hydrogen, alkyl, aryl, or
aralkyl.
"Aminosulfonyl" refers herein to the group -S(O)2-NH2. "Substituted
aminosulfonyl" refers herein to the group -S(O)2-NRR' where R is alkyl and R'
is
hydrogen or alkyl. The term "aralkylaminosulfonlyaryl" refers herein to the
group
-aryl-S(O)2 NH-aralkyl.
"Carbonyl" refers to the divalent group -C(O)-.
"Carbonyloxy" refers generally to the group -C(O)-O. Such groups include
esters, -C(O)-O-R, where R is alkyl, cycloalkyl, aryl, or aralkyl. The term
"carbonyloxycycloalkyl" refers generally herein to both a
"carbonyloxycarbocycloalkyl"
-19-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
and a "carbonyloxyheterocycloalkyl," i.e., where R is a carbocycloalkyl or
heterocycloalkyl, respectively. The term "arylcarbonyloxy" refers herein to
the group
-C(O)-O-aryl, where aryl is a mono- or polycyclic, carbocycloaryl or
heterocycloaryl.
The term "aralkylcarbonyloxy" refers herein to the group -C(O)-O-aralkyl.
"Sulfonyl" refers herein to the group -SOZ-. "Alkylsulfonyl" refers to a
substituted sulfonyl of the structure -S02R- in which R is alkyl.
Alkylsulfonyl groups
employed in compounds of the present invention are typically alkylsulfonyl
groups
having from 1 to 6 carbon atoms in its backbone structure. Thus, typical
alkylsulfonyl
groups employed in compounds of the present invention include, for example,
methylsulfonyl (i.e., where R is methyl), ethylsulfonyl (i.e., where R is
ethyl),
propylsulfonyl (i.e., where R is propyl), and the like. The term
"arylsulfonyl" refers
herein to the group -S02-aryl. The term "aralkylsulfonyl" refers herein to the
group
-S02-aralkyl. The term "sulfonamido" refers herein to -S02NH2.
"Caxbonylamino" refers to the divalent group -NH-C(O)- in which the hydrogen
atom of the amide nitrogen of the carbonylamino group can be replaced alkyl,
aryl, or
axalkyl group. Such groups include moieties such as caxbamate esters (-NH-C(O)-
O-R)
and amides NH-C(O)-R, where R is a straight or branched chain alkyl,
cycloalkyl, or
aryl or aralkyl. The term "alkylcaxbonylamino" refers to alkylcarbonylamino
where R is
alkyl having from 1 to about 6 carbon atoms in its backbone structure. The
term
"arylcarbonylamino" refers to group NH-C(O)-R where R is an aryl. Similarly,
the term
"aralkylcarbonylamino " refers to carbonylamino where R is aralkyl.
"Guanidino" or "guanidyl" refers to moieties derived from guanidine,
H2N-C(--NH)-NH2. Such moieties include those bonded at the nitrogen atom
carrying the
formal double bond (the "2"-position of the guanidine, e.g.,
diaminomethyleneamino,
(HZN)2C--NH-)) and those bonded at either of the nitrogen atoms carrying a
formal single
bond (the "1-" and/or "3"-positions of the guandine, e.g., H2N-C(=NH)-NH-)).
The
hydrogen atoms at any of the nitrogens can be replaced with a suitable
substituent, such
as alkyl, aryl, or axalkyl.
"Amidino" refers to the moieties R C(--N)-NR'- (the radical being at the
"Nl" nitrogen) and R(NR')C--N- (the radical being at the "N2" nitrogen), where
R and R'
can be hydrogen, alkyl, aryl, or aralkyl.
"Cycloalkyl" refers to a mono- or polycyclic, heterocyclic or carbocyclic
alkyl
substituent. Representative cycloalkyl groups include cyclopropyl, cyclobutyl,
_20_
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl and such rings
substituted with
straight and branched chain alkyl groups as defined above. Typical cycloalkyl
substituents have from 3 to ~ backbone (i.e., ring) atoms in which each
backbone atom is
either carbon or a heteroatom. The term "heterocycloalkyl" refers herein to
cycloalkyl
substituents that have from 1 to 5, and more typically from 1 to 4 heteroatoms
in the ring
structure. Suitable heteroatoms employed in compounds of the present invention
are
nitrogen, oxygen, and sulfur. Representative heterocycloalkyl moieties
include, for
example, morpholino, piperazinyl, piperadinyl, and the like. Carbocycloalkyl
groups are
cycloalkyl groups in which all ring atoms are carbon. When used in connection
with
cycloalkyl substituents, the teen "polycyclic" refers herein to fused and non-
fused alkyl
cyclic structures.
"Substituted heterocycle," "heterocyclic group," "heterocycle," or
"heterocyclyl,"
as used herein refers to any 3- or 4-membered ring containing a heteroatom
selected from
nitrogen, oxygen, and sulfur or a 5- or 6-membered ring containing from one to
three
heteroatoms selected from the group consisting of nitrogen, oxygen, or sulfur;
wherein
the 5-membered ring has 0-2 double bonds and the 6-membered ring has 0-3
double
bonds; wherein the nitrogen and sulfur atom maybe optionally oxidized; wherein
the
nitrogen and sulfur heteroatoms maybe optionally quarternized; and including
any
bicyclic group in which any of the above heterocyclic rings is fused to a
benzene ring or
another 5- or 6-membered heterocyclic ring independently defined above. The
term
"heterocycle" thus includes rings in which nitrogen is the heteroatom as well
as partially
and fully-saturated rings. Preferred heterocycles include, for example:
diazapinyl, pyrryl,
pyrrolinyl, pyrrolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, imidazoyl,
imidazolinyl,
imidazolidinyl, pyridyl, piperidinyl, pyrazinyl, piperazinyl, N-methyl piper-
azinyl,
azetidinyl, N-methylazetidinyl, pyrimidinyl, pyridazinyl, oxazolyl,
oxazolidinyl,
isoxazolyl, isoazolidinyl, morpholinyl, thiazolyl, thiazolidinyl,
isothiazolyl,
isothiazolidinyl, indolyl, quinolinyl, isoquinolinyl, benzimidazolyl,
benzothiazolyl,
benzoxazolyl, furyl, thienyl, triazolyl, and benzothienyl.
Heterocyclic moieties can be unsubstituted or monosubstituted or disubstituted
with various substituents independently selected from hydroxy, halo, oxo
(C=O),
alkylimino (RN=, wherein R is alkyl or alkoxy group), amino, alkylamino,
dialkylamino,
acylaminoalkyl, alkoxy, thioallcoxy, polyalkoxy, alkyl, cycloalkyl or
ha.loalkyl.
-21-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
The heterocyclic groups may be attached at various positions as will be
apparent
to those having skill in the organic and medicinal chemistry arts in
conjunction with the
disclosure herein.
0 0
~N \N N ~N ~N~ ~N~
O N C ~ ~.~ ~.N,
O ~ O o
~N
~N~
~NH
O
wN~ O wN~ O
~N O ~N~ O~N O ~N NHZ
O~NH
O H~ \NV ~
wN N II O N// O WN
O ~ O
O ~ ~ S
OH R
~N R,N~R
O R~Nw
/Nw IIO
where R is H or a heterocyclic substituent, as described herein.
Representative heterocyclics include, for example, imidazolyl, pyridyl,
piperazinyl, azetidinyl, thiazolyl, furanyl, triazolyl benzimidazolyl,
benzothiazolyl,
benzoxazolyl, quinolinyl, isoquinolinyl, quinazolinyl, quinoxalinyl,
phthalazinyl, indolyl,
naphthpyridinyl, indazolyl, and quinolizinyl.
"Aryl" refers to optionally substituted monocyclic and polycyclic aromatic
groups
having from 3 to 14 backbone carbon or hetero atoms, and includes both
carbocyclic aryl
groups and heterocyclic aryl groups. Carbocyclic aryl groups are aryl groups
in which all
ring atoms in the aromatic ring are carbon. The term "heteroaryl" refers
herein to aryl
groups having from 1 to 4 heteroatoms as ring atoms in an aromatic ring with
the
remainder of the ring atoms being carbon atoms. When used in connection with
aryl
substituents, the term "polycyclic aryl" refers herein to fused and non-fused
cyclic
structures in which at least one cyclic structure is aromatic, such as, for
example,
benzodioxozolo (which has a heterocyclic structure fused to a phenyl group,
i.e., <°~ , na hth l, and the like. Exemplary aryl moieties em loyed as
substituents
p Y p
-22-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
in compounds of the present invention include phenyl, pyridyl, pyrimidinyl,
thiazolyl,
indolyl, imidazolyl, oxadiazolyl, tetrazolyl, pyrazinyl, triazolyl,
thiophenyl, fitranyl,
quinolinyl, purinyl, naphthyl, benzothiazolyl, benzopyridyl, and
benzimidazolyl, and the
like.
"Aralkyl" or "arylalkyl" refers to an alkyl group substituted with an aryl
group.
Typically, aralkyl groups employed in compounds of the present invention have
from 1 to
6 carbon atoms incorporated within the alkyl portion of the aralkyl group.
Suitable
aralkyl groups employed in compounds of the present invention include, for
example,
benzyl, picolyl, and the like.
Representative heteroaryl groups include, for example, those shown below.
These
heteroaryl groups can be further substituted and may be attached at various
positions as
will be apparent to those having skill in the organic and medicinal chemistry
arts in
conjunction with the disclosure herein.
-23-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
\N~ \ _N N wN ~ ~N ~
N H N~/
J ~ 0 0
F F \N~ \N_N ~N
w
~N ~ F ~ N ~N F~N~N HN
,NJ F
O F
N
N
N~
\N- ~ y \N'~NH~ \N- ~ \N_N
'N ~ 'N'N
O
~! N \
O \N N N
N
H~NN / N~ N I /
S N
\ -N
wN~NH N vN
O _ ~
I /
Representative heteroaryls include, for example, imidazolyl, pyridyl,
thiazolyl,
triazolyl benzimidazolyl, benzothiazolyl, and benzoxazolyl.
"Biaryl" refers to a group or substituent to which two aryl groups, which are
not
condensed to each other, are bound. Exemplary biaryl compounds include, for
example,
phenylbenzene, diphenyldiazene, 4-methylthio-1-phenylbenzene, phenoxybenzene,
(2-phenylethynyl)benzene, diphenyl ketone, (4-phenylbuta-1,3-diynyl)benzene,
phenylbenzylamine, (phenylmethoxy)benzene, and the like. Preferred optionally
substituted biaryl groups include: 2-(phenylamino)-N-[4-(2-phenylethynyl)-
phenyl]acetamide, 1,4-diphenylbenzene, N-[4-(2-phenylethynyl)phenyl]-2-[benzyl-
amino]-acetamide, 2-amino-N-[4-(2-phenylethynyl)phenyl]propanamide, 2-amino-N-
[4-
(2-phenyl-ethynyl)phenyl]acetamide, 2-(cyclopropylamino)-N-[4-(2-
phenylethynyl)-
phenyl]-acetamide, 2-(ethylamino)-N-[4-(2-phenylethynyl)phenyl] acetamide,
2-[(2-methyl-propyl)amino]-N-[4-(2-phenylethynyl)phenyl]acetamide, 5-phenyl-2H-
benzo-[d]1,3-dioxolene, 2-chloro-1-methoxy-4-phenylbenzene, 2-
[(imidazolylmethyl)-
amino]-N-[4-(2-phenylethynyl)phenyl]acetamide, 4-phenyl-1-phenoxybenzene,
N-(2-amino-ethyl)-[4-(2-phenylethynyl)phenyl]carboxamide, 2-~ [(4-
-24-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
fluorophenyl)methyl]-amino}-N-[4-(2-phenylethynyl)phenyl]acetamide, 2- f [(4-
methylphenyl)methyl]amino}-N-[4-(2-phenyl-ethynyl)phenyl]acetamide, 4-phenyl-1-
(trifluoromethyl)benzene, 1-butyl-4-phenyl-benzene, 2-(cyclohexylamino)-N-[4-
(2-
phenylethynyl)phenyl] acetamide, 2-(ethyl-methyl-amino)-N-[4-(2-
phenylethynyl)phenyl]acetamide, 2-(butylamino)-N-[4-(2-phenyl-ethynyl)-
phenyl]acetamide, N-[4-(2-phenylethynyl)phenyl]-2-(4-pyridylamino)-acetamide,
N-[4-
(2-phenylethynyl)phenyl]-2-(quinuclidin-3-ylamino)acetamide, N-[4-(2-phenyl-
ethynyl)phenyl]pyrrolidin-2-ylcarboxamide, 2-amino-3-methyl-N-[4-(2-phenyl-
ethynyl)-
phenyl]butanamide, 4-(4-phenylbuta-1,3-diynyl)phenylamine, 2-(dimethyl-amino)-
N-[4-
(4-phenylbuta-1,3-diynyl)phenyl]acetamide, 2-(ethylamino)-N-[4-(4-phenylbuta-
1,3-
diynyl)-phenyl]acetamide, 4-ethyl-1-phenylbenzene, 1-[4-(2-phenyl-ethynyl)-
phenyl]ethan-1-one, N-(1-carbamoyl-2-hydroxypropyl)[4-(4-phenylbuta-1,3-
diynyl)-
phenyl]-carbox-amide, N-[4-(2-phenylethynyl)phenyl]propanamide, 4-methoxy-
phenyl
phenyl ketone, phenyl-N-benzamide, (tent-butoxy)-N-[(4-phenylphenyl)-methyl]-
carboxamide, 2-(3-phenyl-phenoxy)ethanehydroxamic acid, 3-phenylphenyl
propanoate,
1-(4-ethoxyphenyl)-4-methoxybenzene, and [4-(2-phenylethynyl)phenyl]pyrrole.
"Heteroarylaryl" refers to a biaryl group where one of the aryl groups is a
heteroaryl group. Exemplary heteroarylaryl groups include, for example,
2-phenylpyridine, phenylpyrrole, 3-(2-phenylethynyl)pyridine, phenylpyrazole,
5-(2-phenyl-ethynyl)-1,3-dihydropyrimidine-2,4-dione, 4-phenyl-1,2,3-
thiadiazole,
2-(2phenyl-ethynyl)pyrazine, 2-phenylthiophene, phenylimidazole, 3-(2-
piperazinyl-
phenyl)-furan, 3-(2,4-dichlorophenyl)-4-methylpyrrole, and the like. Preferred
optionally
substituted heteroarylaryl groups include: 5-(2-phenylethynyl)pyrimidine-2-
ylamine,
1-methoxy-4-(2-thienyl)benzene, 1-methoxy-3-(2-thienyl)benzene, 5-methyl-2-
phenyl-
pyridine, 5-methyl-3-phenylisoxazole, 2-[3-(trifluoromethyl)phenyl]fixran, 3-
fluoro-5-
(2-furyl)-2-methoxy-1-prop-2-enylbenzene, (hydroxyimino)(5-phenyl(2-thienyl))-
methane, 5-[(4-methylpiperazinyl)methyl]-2-phenylthiophene, 2-(4-ethylphenyl)-
thio-
phene, 4-methyl-thio-1-(2-thienyl)benzene, 2-(3-nitrophenyl)thiophene, (tert-
butoxy)-N-
[(5-phenyl-(3-pyridyl))methyl]carboxamide, hydroxy-N-[(5-phenyl(3-
pyridyl))methyl]-
amide, 2-(phenyl-methylthio)pyridine, and benzylimidazole.
"Heteroarylheteroaryl" refers to a biaryl group where both of the aryl groups
is a
heteroaryl group. Exemplary heteroarylheteroaryl groups include, for example,
3-pyridylimidazole, 2-imidazolylpyrazine, and the like. Preferred optionally
substituted
-25-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
heteroarylheteroaryl groups include: 2-(4-piperazinyl-3-pyridyl)furan, diethyl-
(3-pyrazin-
2-yl(4-pyridyl))amine, and dimethyl f 2-[2-(5-methylpyrazin-2-yl)ethynyl](4-
pyridyl)~ amine.
"Optionally substituted" or "substituted" refers to the replacement of
hydrogen
with a monovalent or divalent radical. Suitable substitution groups include,
for example,
hydroxyl, vitro, amino, imino, cyano, halo, thio, sulfonyl, thioamido,
amidino, imidino,
oxo, oxamidino, methoxamidino, imidino, guanidino, sulfonamido, carboxyl,
formyl,
alkyl, haloalkyl, alkyamino, haloalkylamino, alkoxy, haloalkoxy, alkoxy-alkyl,
alkylcarbonyl, aminocarbonyl, arylcarbonyl, aralkylcarbonyl,
heteroarylcarbonyl,
heteroaralkyl-carbonyl, alkylthio, aminoalkyl, cyanoalkyl, aryl and the like.
The substitution group can itself be substituted. The group substituted onto
the
substitution group can be carboxyl, halo, vitro, amino, cyano, hydroxyl,
alkyl, alkoxy,
aminocarbonyl, -SR, thioamido, -S03H, -S02R, or cycloalkyl, where R is
typically
hydrogen, hydroxyl or alkyl.
When the substituted substituent includes a straight chain group, the
substitution
can occur either within the chain (e.g., 2-hydroxypropyl, 2-aminobutyl, and
the like) or at
the chain terminus (e.g., 2-hydroxyethyl, 3-cyanopropyl, and the like).
Substituted
substituents can be straight chain, branched or cyclic arrangements of
covalently bonded
carbon or heteroatoms.
"Carboxy-protecting group" refers to a carbonyl group which has been
esterified
with one of the commonly used carboxylic acid protecting ester groups employed
to
block or protect the carboxylic acid function while reactions involving other
functional
sites of the compound are carried out. In addition, a carboxy protecting group
can be
attached to a solid support whereby the compound remains connected to the
solid support
as the carboxylate until cleaved by hydrolytic methods to release the
corresponding free
acid. Representative carboxy-protecting groups include, for example, alkyl
esters,
secondary amides and the like.
Certain of the compounds of the invention comprise asymmetrically substituted
carbon atoms. Such asymmetrically substituted carbon atoms can result in the
compounds of the invention comprising mixtures of stereoisomers at a
particular
asymmetrically substituted carbon atom or a single stereoisomer. As a result,
racemic
mixtures, mixtures of diastereomers, as well as single diastereomers of the
compounds of
the invention are included in the present invention. The terms "S" and "R"
configuration,
-26-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
as used herein, are as defined by the ILTPAC 1974 "RECOMMENDATIONS FOR SECTION
E,
FUNDAMENTAL STEREOCHEMISTRY," Pure Appl. Chem. 45:13-30, 1976. The terms a and
~i are employed for ring positions of cyclic compounds. The a-side of the
reference plane
is that side on which the preferred substituent lies at the lower numbered
position. Those
substituents lying on the opposite side of the reference plane are assigned (3
descriptor. It
should be noted that this usage differs from that for cyclic stereoparents, in
which "a"
means "below the plane" and denotes absolute configuration. The terms a and [3
configuration, as used herein, are as defined by the "Chemical Abstracts Index
Guide,"
Appendix IV, paragraph 203, 1987.
As used herein, the term "pharmaceutically acceptable salts" refers to the
nontoxic
acid or alkaline earth metal salts of the tetrahydrocarboline compounds of the
invention.
These salts can be prepared in situ during the final isolation and
purification of the
tetrahydrocarboline compounds, or by separately reacting the base or acid
functions with
a suitable organic or inorganic acid or base, respectively. Representative
salts include,
but are not limited to, the following: acetate, adipate, alginate, citrate,
aspartate,
benzoate, benzenesulfonate, bisulfate, butyrate, camphorate, camphorsulfonate,
digluconate, cyclopentanepropionate, dodecylsulfate, ethanesulfonate,
glucoheptanoate,
glycerophosphate, hemi-sulfate, heptanoate, hexanoate, fumarate,
hydrochloride,
hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, lactate, maleate,
methanesulfonate, nicotinate, 2-napth-alenesulfonate, oxalate, pamoate,
pectinate,
persulfate, 3-phenylproionate, picrate, pivalate, propionate, succinate,
sulfate, tartrate,
thiocyanate, p-toluenesulfonate, and undecanoate. Also, the basic nitrogen-
containing
groups can be quaternized with such agents as alkyl halides, such as methyl,
ethyl,
propyl, and butyl chloride, bromides, and iodides; dialkyl sulfates like
dimethyl, diethyl,
dibutyl, and diamyl sulfates, long chain halides such as decyl, lauryl,
myristyl, and stearyl
chlorides, bromides and iodides, aralkyl halides like benzyl and phenethyl
bromides, and
others. Water or oil-soluble or dispersible products are thereby obtained.
Examples of acids that may be employed to form pharmaceutically acceptable
acid addition salts include such inorganic acids as hydrochloric acid,
sulfuric acid and
phosphoric acid and such organic acids as oxalic acid, malefic acid,
methanesulfonic acid,
succinic acid and citric acid. Basic addition salts can be prepared isz situ
during the final
isolation and purification of the tetrahydrocarboline compounds, or separately
by reacting
carboxylic acid moieties with a suitable base such as the hydroxide, carbonate
or
-27-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
bicarbonate of a pharmaceutically acceptable metal cation or with ammonia, or
an
organic primary, secondary or tertiary amine. Pharmaceutically acceptable
salts include,
but are not limited to, cations based on the alkali and alkaline earth metals,
such as
sodium, lithium, potassium, calcium, magnesium, aluminum salts and the like,
as well as
nontoxic ammonium, quaternary ammonium, and amine cations, including, but not
limited to ammonium, tetramethylammonium, tetraethylammonium, methylamine,
dimethylamine, trimethylamine, triethylamine, ethylamine, and the like. Other
representative organic amines useful for the formation of base addition salts
include
diethylamine, ethylenediamine, ethanolamine, diethanolamine, piperazine, and
the like.
The term "pharmaceutically acceptable prodrugs" as used herein refers to those
prodrugs of the compounds of the present invention which are, within the scope
of sound
medical judgment, suitable for use in contact with the tissues of humans and
lower
animals without undue toxicity, irritation, allergic response, and the like,
commensurate
with a reasonable benefit/risk ratio, and effective for their intended use, as
well as the
zwitterionic forms, where possible, of the compounds of the invention. The
term
"prodrug" refers to compounds that are rapidly transformed ivy vivo to yield
the parent
compound of the above formula, for example by hydrolysis in blood. A thorough
discussion is provided in Higuchi, T., and V. Stella, "Pro-drugs as Novel
Delivery
Systems," A.C.S. SynPosium Series 14, and in "Bioreversible Carriers in Drug
Design,"
in Edward B. Roche (ed.), AnaeYicah Pha~~fnaceutical Association, Pergamon
Press, 1987,
both of which are incorporated herein by reference.
The term "KSP mediated disorder" refers to a disorder that can be beneficially
treated by the inhibition of KSP.
The term "cellular proliferative diseases" refers to diseases including, for
example, cancer, tumor, hyperplasia, restenosis, cardiac hypertrophy, immune
disorder
and inflammation.
The term "cancer" refers to cancer diseases that can be beneficially treated
by the
inhibition of KSP, including, for example, lung and bronchus; prostate;
breast; pancreas;
colon and rectum; thyroid; stomach; liver and intrahepatic bile duct; kidney
and renal
pelvis; urinary bladder; uterine corpus; uterine cervix; ovary; multiple
myeloma;
esophagus; acute myelogenous leukemia; chronic myelognous leukemia;
lymphocytic
leukemia; myeloid leukemia; brain; oral cavity and pharynx; larynx; small
intestine;
non-hodgkin lymphoma; melanoma; and villous colon adenoma.
_28_
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
The compounds of the invention are useful in vitro or in vivo in inhibiting
the
growth of cancer cells. The compounds may be used alone or in compositions
together
with a pharmaceutically acceptable carrier or excipient. Suitable
pharmaceutically
acceptable carriers or excipients include, for example, processing agents and
drug
delivery modifiers and enhancers, such as, for example, calcium phosphate,
magnesium
stearate, talc, monosaccharides, disaccharides, starch, gelatin, cellulose,
methyl cellulose,
sodium carboxymethyl cellulose, dextrose, hydroxypropyl-[3-cyclodextrin,
polyvinyl-pyrrolidinone, low melting waxes, ion exchange resins, and the like,
as well as
combinations of any two or more thereof. Other suitable pharmaceutically
acceptable
excipients axe described in "Remington's Pharmaceutical Sciences," Mack Pub.
Co., New
Jersey, 1991, incorporated herein by reference.
Effective amounts of the compounds of the invention generally include any
amount sufficient to detectably inhibit KSP activity by any of the assays
described herein,
by other KSP activity assays known to those having ordinary skill in the art,
or by
detecting an inhibition or alleviation of symptoms of cancer.
The amount of active ingredient that may be combined with the carrier
materials
to produce a single dosage form will vary depending upon the host treated and
the
particular mode of administration. It will be understood, however, that the
specific dose
level for any particular patient will depend upon a variety of factors
including the activity
of the specific compound employed, the age, body weight, general health, sex,
diet, time
of administration, route of administration, rate of excretion, drug
combination, and the
severity of the particular disease undergoing therapy. The therapeutically
effective
amount for a given situation can be readily determined by routine
experimentation and is
within the skill and judgment of the ordinary clinician.
For purposes of the present invention, a therapeutically effective dose will
generally be a total daily dose administered to a host in single or divided
doses may be in
amounts, for example, of from 0.001 to 1000 mg/kg body weight daily and more
preferred from 1.0 to 30 mg/kg body weight daily. Dosage unit compositions may
contain such amounts of submultiples thereof to make up the daily dose.
The compounds of the present invention may be administered orally,
parenterally,
sublingually, by aerosolization or inhalation spray, rectally, or topically in
dosage unit
formulations containing conventional nontoxic pharmaceutically acceptable
carriers,
adjuvants, and vehicles as desired. Topical administration may also involve
the use of
-29-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
transdermal administration such as transdermal patches or ionophoresis
devices. The
term parenteral as used herein includes subcutaneous injections, intravenous,
intramuscular, intrasternal injection, or infusion techniques.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions may be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation may
also be a
sterile injectable solution or suspension in a nontoxic parenterally
acceptable diluent or
solvent, for example, as a solution in 1,3-propanediol. Among the acceptable
vehicles
and solvents that may be employed are water, Ringer's solution, and isotonic
sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent
or suspending medium. For this purpose any bland fixed oil may be employed
including
synthetic mono- or di-glycerides. In addition, fatty acids such as oleic acid
find use in the
preparation of inj ectables.
Suppositories for rectal administration of the drug can be prepared by mixing
the
drug with a suitable nonirritating excipient such as cocoa butter and
polyethylene glycols,
which are solid at ordinary temperatures but liquid at the rectal temperature
and will
therefore melt in the rectum and release the drug.
Solid dosage forms for oral administration may include capsules, tablets,
pills,
powders, and granules. In such solid dosage forms, the active compound may be
admixed with at least one inert diluent such as sucrose lactose or starch.
Such dosage
forms may also comprise, as is normal practice, additional substances other
than inert
diluents, e.g., lubricating agents such as magnesium stearate. In the case of
capsules,
tablets, and pills, the dosage forms may also comprise buffering agents.
Tablets and pills
can additionally be prepared with enteric coatings.
Liquid dosage forms for oral administration may include pharmaceutically
acceptable emulsions, solutions, suspensions, syrups, and elixirs containing
inert diluents
commonly used in the art, such as water. Such compositions may also comprise
adjuvants, such as wetting agents, emulsifying and suspending agents,
cyclodextrins, and
sweetening, flavoring, and perfuming agents.
The compounds of the present invention can also be administered in the form of
liposomes. As is known in the art, liposomes are generally derived from
phospholipids or
other lipid substances. Liposomes are formed by mono- or mufti-lamellar
hydrated liquid
crystals that are dispersed in an aqueous medium. Any non-toxic,
physiologically
-30-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
acceptable and metabolizable lipid capable of forming liposomes can be used.
The
present compositions in liposome form can contain, in addition to a compound
of the
present invention, stabilizers, preservatives, excipients, and the like. The
preferred lipids
are the phospholipids and phosphatidyl cholines (lecithins), both natural and
synthetic.
Methods to form liposomes are known in the art. See, for example, Prescott
(ed.),
"Methods in Cell Biology," Volume XIV, Academic Press, New York, 1976, p. 33
et seq.
While the compounds of the invention can be administered as the sole active
pharmaceutical agent, they can also be used in combination with one or more
other agents
used in the treatment of cancer. Representative agents useful in combination
with the
compounds of the invention for the treatment of cancer include, for example,
irinotecan,
topotecan, gemcitabine, gleevec, herceptin, 5-fluorouracil, leucovorin,
carboplatin,
cisplatin, taxanes, tezacitabine, cyclophosphamide, vinca alkaloids, imatinib,
anthracyclines, rituximab, trastuzumab, topoisomerase I inhibitors, as well as
other cancer
chemotherapeutic agents.
The above compounds to be employed in combination with the compounds of the
invention will be used in therapeutic amounts as indicated in the Physicians'
Desk
Reference (PDR) 47th Edition (1993), which is incorporated herein by
reference, or such
therapeutically useful amounts as would be known to one of ordinary skill in
the art.
The compounds of the invention and the other anticancer agents can be
administered at the recommended maximum clinical dosage or at lower doses.
Dosage
levels of the active compounds in the compositions of the invention may be
varied so as
to obtain a desired therapeutic response depending on the route of
administration, severity
of the disease and the response of the patient. The combination can be
administered as
separate compositions or as a single dosage form containing both agents. When
administered as a combination, the therapeutic agents can be formulated as
separate
compositions, which are given at the same time or different times, or the
therapeutic
agents, can be given as a single composition.
Antiestrogens, such as tamoxifen, inhibit breast cancer growth through
induction
of cell cycle arrest, that requires the action of the cell cycle inhibitor
p27Kip. Recently, it
has been shown that activation of the Ras-Raf MAP Kinase pathway alters the
phosphorylation status of p27Kip such that its inhibitory activity in
arresting the cell
cycle is attenuated, thereby contributing to antiestrogen resistance (Donovan,
et al,
J. Biol. Chem. 276:40888, 2001). As reported by Donovan et al., inhibition of
MAPK
-31-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
signaling through treatment with MEK inhibitor changed the phosphorylation
status of
p27 in hormone refactory breast cancer cell lines and in so doing restored
hormone
sensitivity. Accordingly, in one aspect, the compounds of formula (I) may be
used in the
treatment of hormone dependent cancers, such as breast and prostate cancers,
to reverse
hormone resistance commonly seen in these cancers with conventional anticancer
agents.
In hematological cancers, such as chronic myelogenous leukemia (CML),
chromosomal translocation is responsible for the constitutively activated BCR-
ABL
tyrosine kinase. The afflicted patients are responsive to gleevec, a small
molecule
tyrosine kinase inhibitor, as a result of inhibition of Abl kinase activity.
However, many
patients with advanced stage disease respond to gleevec initially, but then
relapse later
due to resistance-conferring mutations in the Abl kinase domain. hz
vita°o studies have
demonstrated that BCR-Avl employs the Raf kinase pathway to elicit its
effects. In
addition, inhibiting more than one kinase in the same pathway provides
additional
protection against resistance-conferring mutations. Accordingly, in another
aspect of the
invention, the compounds of formula (I) are used in combination with at least
one
additional agent, such as gleevec, in the treatment of hematological cancers,
such as
chronic myelogenous leukemia (CML), to reverse or prevent resistance to the at
least one
additional agent.
In another aspect of the invention, kits that include one or more compounds of
the
invention are provided. Representative kits include a tetrahydxocarboline
compound of
the invention (e.g., a compound iof formula (I)) and a package insert or other
labeling
including directions for treating a cellular proliferative disease by
administering an KSP
inhibitory amount of the compound.
The present invention will be understood more readily by reference to the
following examples, which are provided by way of illustration and are not
intended to be
limiting of the present invention.
EXAMPLES
Referring to the examples that follow, compounds of the present invention were
synthesized using the methods described herein, or other methods, which are
well known
3 0 in the art.
The compounds andlor intermediates were characterized by high performance
liquid chromatography (HPLC) using a Waters Millenium chromatography system
with a
2690 Separation Module (Milford, MA). The analytical columns were Alltima C-1
~
-32-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
reversed phase, 4.6 x 250 mm from Alltech (Deerfield, IL). A gradient elution
was used,
typically starting with 5% acetonitrile/95% water and progressing to 100%
acetonitrile
over a period of 40 minutes. All solvents contained 0.1 % trifluoroacetic acid
(TFA).
Compounds were detected by ultraviolet light (UV) absorption at either 220 or
254 nm.
HPLC solvents were from Burdick and Jackson (Muskegan, MI), or Fisher
Scientific
(Pittsburgh, PA). In some instances, purity was assessed by thin layer
chromatography
(TLC) using glass or plastic backed silica gel plates, such as, for example,
Baker-Flex
Silica Gel 1B2-F flexible sheets. TLC results were readily detected visually
under
ultraviolet light, or by employing well known iodine vapor and other various
staining
techniques.
Mass spectrometric analysis was performed on one of two LCMS instruments: a
Waters System (Alliance HT HPLC and a Micromass ZQ mass spectrometer; Column:
Eclipse XDB-C18, 2.1 x 50 mm; solvent system: 5-95% (or 35-95%, or 65-95% or
95-95%) acetonitrile in water with 0.05%TFA; flow rate 0.8 mL/min; molecular
weight
range 500-1500; cone Voltage 20 V; column temperature 40°C) or a
Hewlett Packard
System (Series 1100 HPLC; Column: Eclipse XDB-C18, 2.1 x 50 mm; solvent
system:
1-95% acetonitrile in water with 0.05%TFA; flow rate 0.4 mL/min; molecular
weight
range 150-850; cone Voltage 50 V; column temperature 30°C). All masses
were reported
as those of the protonated parent ions.
GCMS analysis is performed on a Hewlett Packard instrument (HP6890 Series
gas chromatograph with a Mass Selective Detector 5973; injector volume: 1 ~L;
initial
column temperature: 50°C; final column temperature: 250°C; ramp
time: 20 minutes;
gas flow rate: 1 mL/min; column: 5% phenyl methyl siloxane, Model
No. HP 190915-443, dimensions: 30.0 m x 25 m x 0.25 m).
Nuclear magnetic resonance (NMR) analysis was performed on some of the
compounds with a Varian 300 MHz NMR (Palo Alto, CA). The spectral reference
was
either TMS or the known chemical shift of the solvent. Some compound samples
were
run at elevated temperatures (e.g., 75°C) to promote increased sample
solubility.
The purity of some of the invention compounds is assessed by elemental
analysis
(Desert Analytics, Tucson, AZ)
Melting points are determined on a Laboratory Devices Mel-Temp apparatus
(Holliston, MA).
-33-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Preparative separations were carried out using a Flash 40 chromatography
system
and IMP-Sil, 60A (Biotage, Charlottesville, VA), or by flash column
chromatography
using silica gel (230-400 mesh) packing material, or by HPLC using a C-18
reversed
phase column. Typical solvents employed for the Flash 40 Biotage system and
flash
column chromatography were dichloromethane, methanol, ethyl acetate, hexane,
acetone,
aqueous hydroxyamine, and triethyl amine. Typical solvents employed for the
reverse
phase HPLC were varying concentrations of acetonitrile and water with
0.1 % trifluoroacetic acid.
The following are abbreviations used in the examples:
AcOH: Acetic acid
aq: Aqueous
ATP: Adenosine triphosphate
9-BBN 9-Borabicyclo[3.3.1]nonane
Boc: tert-Butoxycarbonyl
Celite Filter agent
DAP or Dap: Diaminopropionate
DCM: Dichloromethane
DEAD: Diethyl azodicarboxylate
DIEA: Diisopropylethylamine
DMA N,N-Dimethylacetamide
DMAP 4-Dimethylaminopyridine
DME: 1,2-Dimethoxyethane
DMF: N,N-Dimethylformamide
DMSO: Dimethyl sulfoxide
DPPA: biphenyl phosphoryl azide
Et3N: Triethylamine
EDC: N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide
EDCI: 1-(3-Dimethylaminopropyl)3-ethylcarbodiimide
EtOAc: Ethyl acetate
EtOH: Ethanol
Fmoc: 9-Fluorenylmethoxycarbonyl
GC Gas Chromatography
Gly-OH: Glycine
-34-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
HATU: O-(7-Azabenzotriaazol-1-yl)-N,N,N'N'-tetramethyluronium
hexafluorophosphate
HBTU: 2-(1H-Benzotriazol-1-yl)-1,1,3,3-tetramethyluronium
hexafluorophosphate
Hex: Hexane
HOAT 1-Hydroxy-7-azabenzotriazole
HOBT: 1-Hydroxybenzotriazole
HPLC: High performance liquid chromatography
NIS N-Iodosuccinimide
ICSO value: The concentration of an inhibitor that causes
a 50% reduction
in a measured activity.
iPrOH: Isopropanol
LC/MS: Liquid chromatography/mass spectrometry
LRMS: Low resolution mass spectrometry
MeOH: Methanol
NaOMe: Sodium methoxide
nm: Nanometer
NMP: N-Methylpyrrolidone
PPA Polyphosphoric acid
PPh3: Triphenyl phosphine
PTFE Polytetrafluoroethylene
PyBOP Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium
RP-HPLC: Reversed-phase high-performance liquid chromatography
RT: Room temperature
sat: Saturated
TEA: Triethylamine
TFA: Trifluoroacetic acid
THF: Tetrahydrofuran
Thr: Threonine
TLC: Thin layer chromatography
Trt-Br: Triphenylmethyl bromide
Nomenclature for the Example compounds (Examples 2-11 and
Table 1 in
Example 12) was
provided using
ACD Name version
5.07 software
(November 14,
2001)
-35-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
or ACD Name Batch version 5.04 (May 28, 2002) available from Advanced
Chemistry
Development, Inc. Some of the compounds and starting materials were named
using
standard IUPAC nomenclature.
It should be understood that the organic compounds according to the invention
may exhibit the phenomenon of tautomerism. As the chemical structures within
this
specification can only represent one of the possible tautomeric forms, it
should be
understood that the invention encompasses any tautomeric form of the drawn
structure.
It is understood that the invention is not limited to the embodiments set
forth
herein for illustration, but embraces all such forms thereof as come within
the scope of
the above disclosure.
Example 1
The Preparation of a Representative Substituted Tryptomine
In this example, the preparation of a representative substituted tryptomine
useful
in synthesizing the tetrahydrocarboline compounds of the invention is
described. The
preparation is illustrated schematically in FIGURE 1 and described below with
reference
to FIGURE 1.
Synthesis of phthalimide lb. To a stirred solution of 4-aminobutyraldehyde
diethyl acetal la (89.3 mmol) in CH3CN (100 mL) was added N-
carbethoxyphthalimide
(93.8 mmol). Once the reaction was complete, the acetonitrile was removed
under
reduced pressure and the aqueous phase extracted with EtOAc (x3). The organic
phase
were combined, then washed with water (x4), saturated brine (x3), then dried
(Na2SO4),
filtered, and evaporated under reduced pressure to give the title compound lb,
which
crystallized on standing.
~nthesis of indole ld. A mixture of the hydrochloride salt of the hydrazine
(1.0 mmol) and lc (1.0 mmol) and lb in EtOH (6 mL) and water (1.2 mL) was
heated at
85°C. Once the reaction was complete, the EtOH was removed by
evaporation under
reduced pressure and the aqueous phase treated with sat. aqueous NaHC03 (5 mL)
and
then extracted with EtOAc (x3). The organic phase were combined, then washed
with
-36-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
H20 (x3), saturated brine (x3), dried (Na2S04), filtered, and evaporated under
reduced
pressure to give the crude indole ld.
Synthesis of le. To a stirred solution of crude indole ld (1.0 mmol) in EtOH
(4.0 mL) was added anhydrous hydrazine (5.0 mmol). Once the reaction was
complete,
the mixture was diluted with toluene/EtOH and filtered. The filtrate was
evaporated
under reduced pressure, and then diluted with EtOH and evaporated under
reduced
pressure to azeotrope the excess hydrazine. This procedure was repeated 3
times to yield
the crude le which was used directly as shown in Example 2.
Example 2
~ The Preparations of Representative Sulfonamide and
N-Acyl Substituted Tetrahydrocarboline Compounds
In this example, the preparations of representative sulfonamide and N-acyl
tetrahydrocarboline compounds of the invention are described. The preparations
are
illustrated schematically in FIGURE 2 and described below with reference to
FIGURE 2.
Synthesis of 2b. 2a (1 equiv) was suspended in acetic acid (~0.5 M) and the
aldehyde (1.2 equiv) was added. The reaction mixture was stirred at 80
°C for 4 h. The
reaction mixture was then cooled down to room temperature. The excess acetic
acid was
removed by adding ether to the reaction mixture and decanting. The residue was
then
partitioned between EtOAc and saturated aqueous NaHC03, the aqueous phase was
extracted orie additional time with EtOAc, the organic extracts were collected
and dried
(Na2S04). Evaporation of the solvent under reduced pressure afforded the
desired
tetrahydrocarboline 2b, wluch was used in the next step without further
purification.
General procedure for N-sulfonatin t~ydrocarbolines: synthesis of 2d. To a
stirred solution of tetrahydro-(3-carboline 2b (0.76 mmol) in CH2C12 (6 mL)
was added
DIPEA (2.3 mmol) followed by the appropriate sulfonyl chloride (1.5 mmol).
Once the
reaction was complete, the mixture was diluted with CH2C12 and then washed
with
saturated aqueous NaHC03 (x2), H20 (x2), saturated brine (x2), then dried
(Na2S04),
filtered, and evaporated under reduced pressure to give the desired 2d.
-3 7-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Example 3
The Preparation of Representative N- Aminosubstituted Alkylcarbon~tainin~
Tetrahydrocarboline Compounds
In this example, the preparation of representative N-aminosubstituted
alkylcarbonyl-containing tetrahydrocarboline compounds of the invention is
described.
The preparation is illustrated schematically in FIGURE 3 and described below
with
reference to FIGURE 3.
Synthesis of 3b. 3a (1 equiv) was dissolved in DMA (0.1 M) and
N-Boc-CHR4~(CH2)n-COZH (4 equiv) was added. Et3N (4 equiv) was then added,
followed by EDC (2 equiv) and HOAT (2 equiv). The reaction mixture was stirred
at
room temperature overnight, then poured into water. The precipitate 3b was
dried and
used in the next step without further purification.
Synthesis of 3c. 3b was dissolved in excess 4N HCl in dioxane, and stirred for
min. The solvent was evaporated under reduced pressure and the residue was
purified
15 by reverse phase preparatory HPLC, to afford 3c.
-3 8-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Example 4
The Preparation of Representative Aryl Substituted Tetrahydrocarboline
Com,_pound
In this example, the preparation of representative aryl substituted
tetrahydrocarboline compounds of the invention is described. The preparation
is
illustrated schematically in FIGURE 4 and described below with reference to
FIGURE 4.
Synthesis of 4b. To a stirred solution of bromo-tetrahydro-[3-carboline 4a
(0.1 mmol) in DME (0.5 ml) and H20 (0.17 mL) was added the appropriate boronic
acid
(0.12 mmol) followed by 2M aqueous Na2C03 (0.15 mL). The mixture was degassed
by
bubbling argon through the mixture for 1 minute, and then added Pd(dppf)Cl2
(0.01 mmol). The mixture was heated at 90°C under argon. Once the
reaction was
complete, the mixture was allowed to cool and then diluted with EtOAc and H20.
The
organics were separated and the aqueous phases extracted with EtOAc (x3). The
organic
phase were combined then washed with H20 (x2), saturated brine (x2), then
dried
(Na2S04), filtered, and evaporated under reduced pressure to give product 4b.
Example 5
The Preparation of Representative 3-Hydroxyphenyl Substituted
Tetrahydrocarboline Compounds
In this example, the preparations of representative 3-hydroxyphenyl
substituted
tetrahydrocarboline compounds of the invention are described. The preparations
axe
illustrated schematically in FIGURE 5 and described below with reference to
FIGURE 5.
Sa was synthesized as described in Example 1. 5b was synthesized using similar
procedure described in Example 2 for 2b.
Synthesis of Sc. Sb (1 equiv) was dissolved in MeOH, and solid I~2C03 (3
equiv)
was added. The reaction mixture was stirred at room temperature for 1 h, then
filtered
and evaporated. Purification by reverse phase preparatory HPLC afforded Sc.
Synthesis of Sd and 5~. Sb (1 equiv) was dissolved in DMA (0.1 M) and
BocNH(CH2)2C02H (3 equiv) was added. Et3N (4 equiv) was then added, followed
by
EDC (2 equiv) and HOAT (2 equiv). The reaction mixture was stirred at room
-39-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
temperature overnight, then poured into water. A precipitate formed, which was
filtered
and dried to yield Sd. Removal of the Boc group using the procedure described
above for
3c, and purification by reverse phase preparatory HPLC afforded 5g.
Example 6
The Preparation of Representative Tetrahydrocarboline-4-one
and Tetrahydrocarboline-4-of Compounds
In this example, the preparations of representative tetrahydrocarboline-4-one
and
tetrahydrocarboline-4-of compounds of the invention are described. The
preparations are
illustrated schematically in FIGURE 6 and described below with reference to
FIGURE 6.
6a was synthesized using similar procedure as described in Example 2.
Synthesis of 6b. To a mixture of 6a (1 equiv) and triethyl amine (2 equiv) in
dichloromethane was added a solution of Boc20 (2 equiv) in dichloromethane at
0°C.
The mixture was allowed to stir at room temperature overnight and diluted with
dichloromethane. The orgaiuc phase was washed with water, dried over NaZS04.
The
solvent was removed and purified to give 6b.
Synthesis of 6c and 6d. Substituted 1,2,3,4-tetrahydro-beta-carboline-2-
carboxylic acid tert-butyl ester 6b (1 equiv) and DDQ (2.5 equiv) were mixed
together
until a uniform color was observed. The mixture of powders was cooled to -
7S°C. The
solvent mixture (THF/H20, 9:1) was added slowly to the mixture of powders. The
slurry
was stirred and allowed to warm to room temperature. The solution was then
poured into
aqueous NaOH (1N) and extracted with EtOAc. The combined organic layers were
washed with H20, dried and this dried mixture of 6c and 6d were separated by
flash
chromatography.
Synthesis of 6e. To a solution of 6c in DCM was added 10% TFA at
0°C. The
mixture was stirred at room temperature for 4 h and then concentrated to give
6e as a
TFA salt.
-40-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Synthesis of 6~ To a solution of 6e (1 equiv) in THF was added PyBOP
(1.2 equiv), carboxylic acid (1.2 equiv) and Et3N (2.2 equiv). After being
stirred
overnight, the solvent was removed to give a residue, which was purified to
give 6~
Synthesis of 6~. To a solution of 6d in DCM was added 10% TFA at
0°C. The
mixture was stirred at room temperature for 4 h and then concentrated to give
6g as TFA
salt.
Synthesis of 6h. To a solution of 6g (1 equiv) in THF was added PyBOP
(1. 2 equiv), acid (1.2 equiv) and Et3N (2.2 equiv). After being stirred
overnight, solvent
was removed to give a residue, which was purified to give 6h.
Example 7
The Preparation of Representative Tetrahydrocarboline Compounds
In this example, the preparations of a variety of representative
tetrahydrocarboline
compounds listed in Table 1 are described.
General procedure for the p~aration of 1,2,3,4-tetrahydro-beta-carboline. A
mixture of substituted tryptamine (1 equiv) and 3-hydroxybenzaldehyde (1.05
equiv) in
acetic acid was heated at 90°C overnight. After cooling to room
temperature, the solid
was filtered and washed twice with acetic acid, and dried to give substituted
1-(3-hydroxyphenyl)-1,2,3,4-tetrahydro-beta-carboline.
R9
R~~
R~,
The following compounds were prepared according to the above procedure.
-41-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
H3C
3-(6-methyl-1,2,3,4-tetrahydrobeta-carbolinyl)phenol
CI
H
3-(6-chloro-1,2, 3,4-tetrahydrobeta-carbolinyl)phenol
3-(6-methoxy-1,2,3,4-tetralrydrobeta-carbolinyl)phenol
General procedure for the preparation of 2-acyl-1-(3-hydroxyphen~)-6-
substituted-1,2,3,4-tetrahydro-beta-carboline. To a solution of 6-substituted-
1-(3-
hydroxyphenyl)-1,2,3,4-tetrahydro-beta- carboline (1 equiv) in THF was added
Et3N
(2.2 equiv) and acyl chloride (1.1 equiv). After one hour, the product was
purified on
silica gel to give 2-acyl-1-(3-hydroxyphenyl)-6-substituted-1,2,3,4-tetrahydro-
beta-
carboline as shown below.
Rio
R4a
3-(2-acetyl-6-methyl-2,3,4,9-tetrah~dro-1H-beta-carbolin-1-yl)phenol 1. To a
solution of 6-methyl-1-(3-hydroxyphenyl)-1,2,3,4-tetrahydro-beta-carboline (1
equiv) in
-42-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
THF was added Et3N (2.2 equiv) and acetic anhydride (1.2 equiv). After one
hour, the
solution was purified by prep-HPLC to give 3-(2-acetyl-6-methyl-2,3,4,9-
tetrahydro-1H-
beta-carbolin-1-yl)phenol 1.
~CH3
H
The following compounds were prepared according to the above procedure.
~CH3
O
OH
3-(6-methyl-2-propionyl-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 2
CH3
3-(2-acetyl-6-methoxy-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 3
-43-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
CI
~CH3
3-(6-chloro-2-propionyl-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 4
CI
~CH3
H
3-(2-acetyl-6-chloro-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 5
CI CH3
"CH3
H
3-(6-chloro-2-isobutyryl-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 6
CI
~CH3
H
3-(2-butyryl-6-chloro-2,3,4,9-tetrahydxo-1H-beta-carbolin-1-yl)phenol 7
-44-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
CI
v
O
OH
3-[6-chloro-2-(cyclohexylcarbonyl)-2,3,4,9-tetrahydro-1H-beta-carbolin-1-
yl]phenol 8
CI
O /
3-[6-chloro-2-(phenylacetyl)-2, 3,4,9-tetrahydro-1 H-beta-carbolin-1-yl]phenol
9
~CH3
H
3-(6-methoxy-2-propionyl-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 10
3-[6-meth~(morlaholin-4- l~tyl)-2,3,4,9-tetrahydro-1H-beta-carbolin-1-
yl]phenol 16. To a solution of 6-methyl-1-(3-hydroxyphenyl)-1,2,3,4-tetrahydro-
beta-
carboline (1 equiv) in THF was added Et3N (2.2 equiv) and alpha-bromoacetyl
bromide
(1.2 equiv). The solution was stirred at room temperature for 30 min to give
crude
2-bromo-1-[ 1-(3-hydroxyphenyl)-6-methyl-( 1,2, 3,4-tetrahydro-beta-carbolin-2-
yl)] ethan-
1-one. To the solution was added morpholine (2 equiv). The solution was
stirred
overnight and purified to give 3-[6-methyl-2-(morpholin-4-ylacetyl)-2,3,4,9-
tetrahydro-
1 H-beta-carbolin-1-yl]phenol 16.
-45-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
H
L / N I N N
H
\I
OH
The following compounds were prepared according to the above procedure.
OH
3-[6-methyl-2-(piperidin-1-ylacetyl)-2,3,4,9-tetrahydro-1H-beta-carbolin-1-
yl]phenol 17
H /
,~ N \ I
H
3-{6-methyl-2-[N-(2-phenylethyl)glycyl]-2,3,4,9-tetrahydro-1H-beta-carbolin-1-
yl}phenol 18
3~ I \ I
/ N N~N~N
H O H
/I
\ OH
3-{6-methyl-2-[N-(2-morpholin-4-ylethyl)glycyl]-2,3,4,9-tetrahydro-1H-beta-
carbolin-1-
yl}phenol 19
-46-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
3-f 2-[N-(tert-butoxycarbon~) ~l~y_]!-6-methyl-2,3,4,9-tetrahydro-1H-beta-
carbolin-1-
~~phenol 11. To a solution of 6-methyl-1-(3-hydroxyphenyl)-1,2,3,4-tetrahydro-
beta-
carboline (1 equiv) in THF was added Et3N (2.2 equiv) and Boc-Gly-OSu (1.2
equiv).
After 2 h, the solvent was removed to give a residue, which was purified via
prep-HPLC
to give 3-{2-[N-(tert-butoxycarbonyl)-glycyl]-6-methyl-2,3,4,9-tetrahydro-1H-
beta-
carbolin-1-yl~phenol 11.
O
~N~O'tBu
O H
OH
3 -(2-~lycyl-6-methyl-2,3 ,4,9-tetrahydro-1 H-beta-carbolin-1-yl)phenol 14.
(tert-Butoxy)-N-~2-[1-(3-hydroxyphenyl)-6-methyl-(1,2,3,4-tetrahydro-beta-
carbolin-2-
yl)]-2-oxoethyl~carboxamide 11 was treated with HCl in dioxane (4 N, 10 equiv)
for
30 min, and the solvent was removed to give 3-(2-glycyl-6-methyl-2,3,4,9-
tetrahydro-1H-
beta-carbolin-1-yl)phenol 14 as its HCl salt.
H3C
~NH2
O
OH
-47-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
~N
]~ ''O
OH
General procedure for coupling of beta-carboline with carboxylic acid: 3-f2-fN-
~tert-butoxycarbon~)-beta-alan~]-6-methyl-2,3,4,9-tetrahydro-1H-beta-carbolin-
1-
yl)phenol 12. To a solution of 6-methyl-1-(3-hydroxyphenyl)-1,2,3,4-
tetrahydrobeta-
carboline (1 equiv) in THF was added PyBOP (1. 2 equiv), Boc-beta-Ala-OH (1.2
equiv),
and Et3N (2.2 equiv). After being stirred overnight, solvent was removed to
give a
residue, which was purified to give 3- f 2-[N-(tert-butoxycarbonyl)-beta-
alanyl]-6-methyl-
2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl]phenol 12.
H3C
H
~N~O
~O '' ~O'~tgu
OH
The following compound was prepared according to the above procedure.
/CH3
~N(~CH3
-4~-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
3-[2-(N,N-diethyl-beta-alanyl)-6-methyl-2,3,4,9-tetrahydro-1H-beta-carbolin-1-
yl]phenol 20
H3C
/ I N NJ
N
H / O
I
OH
3-[6-methyl-2-(3-piperidin-1-ylpropanoyl)-2,3,4,9-tetrahydro-1H-beta-carbolin-
1-
yl]phenol 21
3C
O
I / N I N N~O~tBu
H ~~H
/I
OH
(tert-butoxy)-N-{4-[1-(3-hydroxyphenyl)-6-methyl(1,2,3,4-tetrahydrobeta-
carbolin-2-yl)]-4-oxobutyl J carboxamide
CI
H
~N~O
~O'~ tBu
3-(2-N-tert-butoxycarbonyl-beta-alanyl-6-chloro-2,3,4,9-tetrahydro-1 H-beta-
carbolin-1-yl)phenol
-49-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
3-(2-beta-alanyl-6-methyl-2,3,4,9-tetrahydro-1 H-beta-carbolin-1-)phenol 15.
(tert-Butoxy)-N- f 3-[1-(3-hydroxyphenyl)-6-methyl-(1,2,3,4-tetrahydro-beta-
carbolin-2-
yl)]-3-oxopropyl]carboxamide was treated wvith HCl in dioxane (4 N, 10 equiv)
for
30 min, and the solvent was removed to give 3-(2-beta-alanyl-6-methyl-2,3,4,9-
tetrahydro-1H-beta-carbolin-1-yl)phenol 15 as its HCl salt.
~ NH2
3-(2-beta-alanyl-6-chloro-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 23.
(tent-Butoxy)-N- f 3-[1-(3-hydroxyphenyl)-6-chloro-(1,2,3,4-tetrahydro-beta-
carbolin-2-
yl)]-3-oxopropyl}carboxamide was treated with HCl in dioxane (4 N, 10 equiv)
for
30 min, and the solvent was removed to give a residue which was purified to
yield
3-(2-beta-alanyl-6-chloro-2,3,4,9-tetrahydro-1H-beta-carbolin-1-yl)phenol 23.
CI
-~NH2
-50-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Example 8
The Preparation of a Representative Tetrahydrocarboline Compound: Compound 98
In this example, the preparation of a representative tetrahydrocarboline
compound, Compound 98 in Table 1, is described. The preparation is illustrated
schematically in FIGURE 7 and described below with reference to FIGURE 7.
Synthesis of TBS protected Phenol 8b. To a stirred solution of phenol 8a
(1.2 mmol) in DMF (2.0 mL) was added imidazole (3.67 mmol) followed by
tent-butyldimethylsilyl chloride (1.52 mmol). Once the reaction was complete,
the
mixture was partitioned between EtOAc and saturated NH4Cl. The organic phase
was
separated, then washed with saturated NH4C1 (x3), water (x2), saturated brine
(x3), then
dried (Na2SO4), filtered, and evaporated under reduced pressure to give
compound 8b,
which was used directly in the next step.
Synthesis of amine 8c. Boc-amine 8b was treated with 10% TFA/ CH2C12. Once
the reaction was complete, the solvent was removed by evaporation under
reduced
pressure and the residue partitioned between EtOAc and sat. aqueous NaHCO3.
The
organic phase was separated, then washed with sat. aqueous NaHC03 (x3), H20
(x3),
saturated brine (x3), then dried (Na2S04), filtered, and evaporated under
reduced pressure
to give the free amine 8c.
Synthesis of 98. To a stirred solution of TBS protected tetrahydro-(3-
carboline 8c
(0.0165 mmol) in CH2C12 (0.3 mL) was added DIPEA (0.033 mmol) followed by
methyl
chloroformate (0.02 mmol). Once the reaction was complete, the mixture was
concentrated in vacuo. The residue was then dissolved in THF (0.3 mL) and
treated with
1M tetra-butyl ammonium fluoride (0.033 mmol). Once the reaction was complete,
the
mixture was diluted with EtOAc and then washed with saturated aqueous NaHC03
(x2),
H20 (x2), saturated brine (x2), then dried (Na2S04), filtered, and evaporated
under
reduced pressure. The residue was purified by reverse phase prep HPLC to give
Compound 98.
-51-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Compounds 99 and 100 were synthesized using procedures similar to the
procedure for the synthesis of Compound 98.
Example 9
The Preparation of a Representative Tetrahydrocarboline Compound: Compound 101
In this example, the preparation of a representative tetrahydrocarboline
compound, Compound 101 in Table 1, is described. The preparation is
illustrated
schematically in FIGURE 7 and described below with reference to FIGURE 7.
To a stirred solution of TBS protected tetrahydro-(3-carboline 8c from Example
8
(0.0165 mmol) in CH2C12. (0.3 mL) was added DIPEA (0.033 mmol) followed by the
appropriate sulfonyl chloride (e.g., methyl sulfonyl chloride) (0.02 mmol).
Once the
reaction was complete, the mixture was concentrated ifz vacuo. The residue was
then
dissolved in THF (0.3 mL) and treated with 1M tetra-butyl ammonium fluoride
(0.033 rnmol). Once the reaction was complete, the mixture was diluted with
EtOAc and
then washed with saturated aqueous NaHC03 (x2), H2O (x2), saturated brine
(x2), then
dried (Na2S04), filtered, and evaporated under reduced pressure. The residue
was
purified by reverse phase prep HPLC to give Compound 101.
Compounds 102 and 103 were synthesized using procedures similar to the
procedure for the synthesis of Compound 101.
Exam lp a 10
The Preparation of a Representative Tetrahydrocarboline Compound: Compound 104
In this example, the preparation of a representative tetrahydrocarboline
compound, Compound 104 in Table 1, is described. The preparation is
illustrated
schematically in FIGURE 7 and described below with reference to FIGURE 7.
To a stirred solution of TBS protected tetrahydro-[3-carboline 8c from Example
8
(0.0165 mmol) in CH2Cl2 (0.3 mL) was added DIPEA (0.033 mmol) followed by the
appropriate acid chloride (e.g., benzoyl chloride) (0.02 mmol). Once the
reaction was
complete, the mixture was concentrated in vacuo. The residue was then
dissolved in THF
(0.3 mL) and treated with 1M tetra-butyl ammonium fluoride (0.033 mmol). Once
the
-52-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
reaction was complete, the mixture was diluted with EtOAc and then washed with
saturated aqueous NaHCO3 (x2), H20 (x2), saturated brine (x2), then dried
(Na2S04),
filtered, and evaporated under reduced pressure. The residue was purified by
reverse
phase prep HPLC to give Compound 104.
Compounds 105 and 106 were synthesized using procedures similar to the
procedure for the synthesis of Compound 104.
Exam lp a 11
General Procedure for 2-(3-Aminopropano~, -2,3,4,9
tetrahydro-1H-beta-carboline-3-carboxamides
In this example, the preparation of representative tetrahydrocarboline
compounds,
Compounds 110-112,138, and 139 in Table l, is described. The preparation is
illustrated
schematically in Figure 8 and described below with reference to Figure 8.
The ethyl ester of lla (1 equiv) was dissolved in DMA (0.1 M) and
BocNH(CH2)ZCOZH (3 equiv) was added. Et3N (4 equiv) was then added, followed
by
EDC (2 equiv) and HOAT (2 equiv). The reaction mixture was stirred at room
temperature overnight, then poured into water. A precipitate formed, which was
filtered
and dried to yield llb.
Ethyl ester llb was then hydrolyzed using NaOH in EtOH and the resulting
carboxylic acid llc was coupled with vaxious amines following a procedure
identical to
the one described for the synthesis of llb. Removal of the Boc using the same
deprotection condition as in the synthesis of 3c, and purification by reverse
phase
preparatory HPLC afforded the final compounds lld. Compounds 110-112, 138, and
139 were synthesized in this manner.
Example 12
Representative Tetrahydrocarboline Compounds
Representative tetrahydrocarboline compound compounds of the invention are
shown in Table 1. In Table 1, MH+ refers to the molecular ion observed by mass
spectrometry.
-53-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Table 1. Representative Tetrahydrocarboline Compounds.
Com Structure Name MH+
ound
3-(2-acetyl-6-methyl-2,3,4,9-tetrahydro-
/ \
1 H 1 H-beta-carbolin-1-yl)phenol321.4
OH
N
O
H3C I ~ / N
u~
Hs
3-(6-methyl-2-propionyl-2,3,4,9-
/ ~
2 OH t etrahydro-1 H-beta-carbolin-1-yl)phenol335.4
H
N
O
H3C I / / N
a
~
CH3
HO 3-(2-acetyl-6-methoxy-2,3,4,9-
3 ~ ~ t etrahydro-1 H-beta-carbolin-1-yl)phenol337.3
H
N
CH3
/
H3C,0 ~
N
O
3-(6-chloro-2-propionyl-2,3,4,9-
4 pH t etrahydro-1 H-beta-carbolin-1-yl)phenol355.8
H
N
O
N
CI CH
3
3-(2-acetyl-6-ch to ro-2,
3, 4, 9-tetrahyd ro-
OH 1 H-beta-carbolin-1-yl)phenol341.8
H
~ N
O
N
CI CH3
3 -(6-chloro-2-isobutyryl-2,3,4,9-
~ ~
6 H etrahydro-1 H-beta-carbolin-1-yl)phenol369.8
OH t
N
O
N
CI
CH3
H3C
3 -(2-butyryl-6-chloro-2,3,4,9-tetrahydro-
~ ~
7 H H-beta-carbolin-1-yl)phenol369.8
OH 1
N
O
N
CI
CH3
-54-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com ound Structure Name MH+
3-[6-chloro-2-(cyclohexylcarbonyl)-
8 H /_~ ~H 2,3,4,9-tetrahydro-1 H-beta-carbolin- 409.9
1-yl]phenol
I i / N
ci
3-[6-chloro-2-(phenylacetyl)-2,3,4,9-
9 H ~ ~ OH tetrahydro-1 H-beta-carbolin-1-yl]phenol 417.9
N
O
N
CI
3-(6-methoxy-2-propionyl-2,3,4,9-
H ~ ~ OH tetrahydro-1 H-beta-carbolin-1-yl)phenol 351.4
N
O
H3C,0 I ~ / N
CH3
c cH 3-f2-[N-(tert-butoxycarbonyl)-glycyl]-
11 ~ / N I N~N~OxCH 6-methyl-2,3,4,9-tetrahydro-1 H-beta- 436.5
carbolin-1-yl}phenol
I
OH
3-{2-[N-(tert-butoxycarbonyl)-beta-
12 I / N I N N o alanyl]-6-methyl-2,3,4,9-tetrahydro-1 H- 450.5
o~ o cH beta-carbolin-1-yl}phenol
/ ~ 3
I OH CFCfHs
CI ~ 3-(6-chloro-2-glycyl-2,3,4,9-tetrahydro-
13 ~ ~ I 1 H-beta-carbolin-1-yl)phenol 356.8
N~NH2
O
OH
H3C ~ 3-(2-glycyl-6-methyl-2,3,4,9-tetrahydro-
14 ~ ~ I 1H-beta-carbolin-1-yl)phenol 336.4
N~NHz
O
OH
H3C ~ 3-(2-beta-alanyl-6-methyl-2,3,4,9-
~ ~ ~ N NH etrahydro-1 H-beta-carbolin-1-yl)phenol 350.4
N
H O
OH
-55-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com ound Structure Name MH+
3C ~ 3-[6-methyl-2-(morpholin-4-ylacetyl)-
16 ~ ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin- 406.4
1-yl]phenol
OH
H3C ~ 3-(6-methyl-2-(piperidin-1-ylacetyl)-
17 ~ ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin- 404.5
N~N~ 1-yl]phenol
OH
H3c ~ ~ 3-{6-methyl-2-[N-(2-phenylethyl)glycyl]-
18 ~ ~ ~ N ~ I 2,3,4,9-tetrahydro-1H-beta-carbolin- 440.5
1-yl}phenol
I
OH
H3c ~ ~0 3-{6-methyl-2-[N-(2-morpholin-4-
19 I i ( N ~NJ ylethyl)glycyl]-2,3,4,9-tetrahydro-1H- 449.5
beta-carbolin-1-yl}phenol
I
OH
H3c ~ ~cH3 3-[2-(N,N-diethyl-beta-alanyl)-6-methyl-
20 ~ ~ I N NvCH3 2,3,4,9-tetrahydro-1H-beta-carbolin- 406.5
1-yl]phenol
OH
H3c ~ 3-[6-methyl-2-(3-piperidin-1-
21 ~ ~ I N N~ Ipropanoyl)-2,3,4,9-tetrahydro-1 H-beta- 418.5
carbolin-1-yl]phenol
0
off
H3C ~ 3-[2-(4-aminobutanoyl)-6-methyl-
22 ~ ~ ~ N 2,3,4,9-tetrahydro-1 H-beta-carbolin- 364.4
~NHz 1-yl]phenol
OH
CI ~ 3-(2-beta-alanyl-6-chloro-2,3,4,9-
23 ~ , ~ N NH etrahydro-1 H-beta-carbolin-1-yl)phenol 370.8
N ~ z
H O
OH
-56-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com ound Structure Name MH+
CI ~ 3-[6-chloro-2-(N,N-dimethyl-beta
24 ~ ~ I N NH3 alanyl)-2,3,4,9-tetrahydro-1 H-beta- 398.9
CHs carbolin-1-yl]phenol
OH
CI ~ 3-{6-chloro-2-[4-
25 I ~ ~ N .cH (dimethylamino)butanoyl]-2,3,4,9- 412.9
tetrahydro-1 H-beta-carbolin-1-yl}phenol
~ I off
CI ~ 3-[2-(4-aminobutanoyl)-6-chloro-2,3,4,9-
26 ~ ~ I tetrahydro-1 H-beta-carbolin-1-yl]phenol 384.8
N~NH2
O
OH
o cI'" tert-butyl4-[6-chloro-1-(3
27 ~ ~ N I N~N~O~CH3 hydroxyphenyl)-1,3,4,9-tetrahydro-2H- 484.9
" ~ IoI " beta-carbolin-2-yl]-4-oxobutylcarbamate
~ I o"
CHs 3-{6-methyl-2-[(1-methylpiperidin-3-
2g HsC ~ N yl)carbonyl]-2,3,4,9-tetrahydro-1H-beta- 404.5
carbolin-1-yl}phenol
N
H O
OH
HsC ~ .CHs 3-{6-methyl-2-[(1-methylpiperidin-4-
29 ~ ~ I N N yl)carbonyl]-2,3,4,9-tetrahydro-1 H-beta- 404.5
N carbolin-1-yl}phenol
H O
OH
CHs 3-{6-chloro-2-[(1-methylpiperidin-3-
30 CI ~ N yl)carbonyl]-2,3,4,9-tetrahydro-1 H-beta- 424.9
carbolin-1-yl}phenol
N
H O
OH
CI ~ N.CHs 3-{6-chloro-2-[(1-methylpiperidin-4-
31 ~ ~ I yl)carbonyl]-2,3,4,9-tetrahydro-1H-beta- 424.9
N N
H carbolin-1-yl}phenol
O
off
-57-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com ound Structure Name MH+
v
0 3-(2-beta-alanyl-6-chloro-2,3,4,9-
32 0~ I ~ ~ N~ tetrahydro-1 H-beta-carbolin-1-yl)phenyl 412.1
~ NHa acetate
H
O
~O
H3C
0 3-{2-[N-(tert-butoxycarbonyl)-beta-
v
33 c~ I ~ ~ N~ ~ cH3 alanyl]-6-chloro-2,3,4,9-tetrahydro-1 H- 512.2
N O--~CH3 beta-carbolin-1-yl}phenyl acetate
H ~ ~ H CHa
O
-O
H3C
0 3-{2-[N-(tert-butoxycarbonyl)-beta-
34 c~ I ~ ~ \N~ ~ oH3 alanyl]-6-chloro-2,3,4,9-tetrahydro-1 H- 470.2
N O--~CH3 beta-carbolin-1-yl}phenol
H ~ ~ H CHa
HO
cH3 3-{2-[N-(tert-butoxycarbonyl)-beta-
35 Br ~ ~ I ~gC+CH3 alanyl]-6-bromo-2,3,4,9-tetrahydro-1 H- 515.4
o beta-carbolin-1-yl}phenol
I
OH
Br ~ 3-(2-beta-alanyl-6-bromo-2,3,4,9-
36 ~ , ~ N NH tetrahydro-1 H-beta-carbolin-1-yl)phenol 415.2
N ~ z
H O
OH
HO ~ 2-beta-alanyl-1-(3-hydroxyphenyl)-
37 ~ , ~ N NH 2~3,4,9-tetrahydro-1H-beta-carbolin-6-of 352.4
N ~ z
H O
OH
NHz N-[3-(2-beta-alanyl-6-methyl-2,3,4,9-
38 tetrahydro-1 H-beta-carbolin- 445.5
H3c I ~ ~ N o 1-yl)phenyl]-2,2,2-trifluoroacetamide
F
H ~ ~ N~ F
O/~F
-$ g-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
NHZ 3-[1-(3-aminophenyl)-6-methyl-1,3,4,9-
39 ~ tetrahydro-2H-beta-carbolin-2-yl]-349.5
N 3-oxopropylamine
H3C
~
\ O
i
H \ ~ NHz
/ 3-(2-beta-alanyl-6-phenyl-2,
3,4, 9-
40 ~ I tetrahydro-1H-beta-carbolin-1-yl)phenol412.3
w
I / N I N\ ~/NHZ
H
I
OH
cH3 3-[2-beta-alanyl-6-(4-methoxyphenyl)-
41 ~ 2,3,4,9-tetrahydro-1 H-beta-carbolin-442.4
~ 1-yl]phenol
N I N~NHZ
II
H
O
OH
CI ~ 5-(2-beta-alanyl-6-chloro-2,3,4,9-
42 ~ ~ I tetrahydro-1 H-beta-carbolin-386.3
N' ~ /NHZ 1_yl)benzene-1,3-diol
J
[ v
O
HO ~ OH
/ 3-[2-beta-alanyl-6-(3-methylphenyl)-
43 ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin-426.4
HC v
I N\~/NH 1-YI]phenol
,N, z
H O
/I
OH
/ 3-[2-beta-alanyl-6-(2-methylphenyl)-
44 ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin-426.4
( N 1-YI]phenol
I
NH
cH
/
H ~ z
I
OH
3-[2-beta-alanyl-6-(3-methoxyphenyl)-
45 H3c.o w ~ ~ 2,3,4,9-tetrahydro-1 H-beta-carbolin-442.4
~ N I N~NHz 1-yl]phenol
II
H
O
OH
-59-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com ound Structure Name MH+
CI ~ CH 3-[6-chloro-2-(N,N-dimethyl-beta
46 ~ ~ I N N, 3 alanyl)-9-methyl-2,3,4,9-tetrahydro-1 H- 412.2
CH3 beta-carbolin-1-yl]phenol
CH / O
OH
F ~ 3-(2-beta-alanyl-6-fluoro-2,3,4,9-
47 ~ ~ ~ N NH etrahydro-1H-beta-carbolin-1-yl)phenol 354
N
H O
OH
CI ~ 3-[6-chloro-1-(3-methoxyphenyl)-
48 ~ ~ I N NH 1,3,4,9-tetrahydro-2H-beta-carbolin- 384
2-yl]-3-oxopropan-1-amine
I
CH3
H3c ~ 3-[1-(3-methoxyphenyl)-6-methyl-
49 I ~ I N NH 1,3,4,9-tetrahydro-2H-beta-carbolin- 364
2-yl]-3-oxopropan-1-amine
I
0
CH3
Br ~ 3-(6-bromo-1-phenyl-1,3,4,9-tetrahydro-
50 ~ ~ I 2H-beta-carbolin-2-yl)-3-oxopropan- 398.2
N~NH~ 1-amine
OO
CI ~ 3-(6-chloro-1-phenyl-1,3,4,9-tetrahydro-
51 ~ ~ I 2H-beta-carbolin-2-yl)-3-oxopropan- 354.3
N~NHZ 1-amine
]] ~O
Ho ~ o cH 2-[N-(tert-butoxycarbonyl)-glycyl]-3-
52 I i N I N N~O~CH3 (hydroxyphenyl)-2,3,4,9-tetrahydro-1 H- 438.49
H ~H 3 beta-carbolin-6-of
I
OH
o ~ 3-{6-bromo-2-[N-(tert-butoxycarbonyl)-
53 I ~ N I N N~O~~H3 glycyl]- 2,3,4,9-tetrahydro-1 H-beta- 500.11
H ~H 3 carbolin-1-yl}phenol
I
OH
-60-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
Br ~ 3-(6-bromo-2-glycyl-2,3,4,9-tetrahydro-
54 ~ ~ I 1 H-beta-carbolin-1-yl)phenol400.06
N~NHZ
O
OH
H3C ~ 3-(6-methyl-1-phenyl-1,3,4,9-tetrahydro-
55 ~ ~ ~ N NH 2H-beta-carbolin-2-yl)-3-oxopropan-334.2
1-amine
O
CI ~ 3-[6-chloro-1-(3-methylphenyl)-1,3,4,9-
56 ~ ~ ~ N NH etrahydro-2H-beta-carbolin-2-yl]-370
3-oxopropan-1-amine
O
CH3
CI , 3-[6-chloro-1-(2-methylphenyl)-1,3,4,9-
57 ~ ~ ~ N NH tetrahydro-2H-beta-carbolin-2-yl]-368
N ~ z 3-oxopropan-1-amine
H3~ ~ O
CI ~ 3-[6-chloro-1-(3-fluorophenyl)-1,3,4,9-
58 ~ ~ N tetrahydro-2H-beta-carbolin-2-yl]-372
~ 3-oxopropan-1-amine
NH
O
F
CI , 3-(2-beta-alanyl-6-chloro-2,3,4,9-
69 ~ ~ ~ tetrahydro-1 H-beta-carbolin-379
w
N
NH
H 1-yl)benzonitrile
~
2
N
CI ~ 3-(6-chloro-1-thien-2-yl-1,3,4,9-
60 ~ ~ N tetrahydro-2H-beta-carbolin-2-yl)-360
~ 3-oxopropan-1-amine
NH
O
/
S
H3C ~ 3-[1-(3-fluorophenyl)-6-methyl-1,3,4,9-
61 ~ ~ ~ N NH tetrahydro-2H-beta-carbolin-2-yl]-352
3-oxopropan-1-amine
O
F
-61-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure _Name MH+
ound
Br ~ 3-[6-bromo-1-(3-fluorophenyl)-1,3,4,9-
62 ~ ~ ~ N NH tetrahydro-2H-beta-carbolin- 2-yl]-418
3-oxopropan-1-amine
O
F
CI ~ 3-(6-chloro-1-cyclohexyl-1,3,4,9-
63 ~ ~ ~ N NH tetrahydro-2H-beta-carbolin-2-yl)-360
3-oxopropan-1-amine
O
cl 3-{6-chloro-1-[4-(methylthio)phenyl]-
64 ~ ~ 1,3,4,9-tetrahydro-2H-beta-carbolin-401
N~NHZ 2-yl}-3-oxopropan-1-amine
H I'
O
i
H30.S
cl 3-[6-ch loro-1-(5-m ethyl
isoxazol-3-yl )-
65 ~ 1,3,4,9-tetrahydro-2H-beta-carbolin-360
\
N~ NHZ 2-yl]-3-oxopropan-1-am
N i ne
H
O
N'
O
CH3
methyl 3-(2-beta-alanyl-6-ch
loro-2, 3,4, 9-
66 ~ ~ ~ N NH tetrahydro-1 H-beta-carbolin-412
N ~ 2 1-yl)benzoate
H
O CH
s
O
O
3-(6-chloro-2-L-phenylalanyl-2,3,4,9-
H N tetrahydro-1 H-beta-carbolin-1-yl)phenol446.3
2
N
CI
O
~ ~
H ~ ~ OH
3-(2-beta-alanyl-6-chloro-2,3,4,9-
68 ~ ~ ~ N NH tetrahydro-1 H-beta-carbolin-398
N ~ 2 1-yl)benzoic acid
H
OH
O
-62-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
cl ~ 3-[2-(N-acetyl-beta-alanyl)-6-chloro-
69 I ~ ~ N N CH 2,3,4,9-tetrahydro-1 H-beta-carbolin-412.1
1-yl]phenol
I
HO
3-[6-bromo-1-(2-naphthyl)-1,
3,4, 9-
70 I ~ I N NH tetrahydro-2H-beta-carbolin-2-yl]-449
N z
H ~ 3-oxopropan-1-amine
~I
Br ~ 3-[6-bromo-1-(4-fluorophenyl)-1,3,4,9-
71 I ~ I N NH tetrahydro-2H-beta-carbolin-2-yl]-417
N
H ~ 3-oxopropan-1-amine
I
F
Br ~ 3-[6-bromo-1-(4-chlorophenyl)-1,3,4,9-
72 I ~ ~ N NH tetrahydro-2H-beta-carbolin-2-yl]-434
N ~ 2 3-oxopropan-1-amine
H
CI
Br ~ 3-[6-bromo-1-(3-chlorophenyl)-1,3,4,9-
73 ~ ~ I tetrahydro-2H-beta-carbolin-2-yl]-434
NH
N
2 3-oxopropan-1-amine
~
OO
CI
CI ~ 3-(2-beta-alanyl-6-chloro-1-methyl-
74 ~ ~ ~ 2,3,4,9-tetrahydro-1 H-beta-carbolin-384.4
NH
~H~ 1-yl)phenol
2
8
OH
CI ~ -[2-(3-aminopropanoyl)-6-chloro-
75 ~ ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin-370.3
NH
N\~
Z 1_yl]pyridin-2-amine
/
OO
N NHz
-63-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
CI ~ 3-[6-chloro-1-(3,4-dichlorophenyl)-
76 ~ , I N NH 1,3,4,9-tetrahydro-2H-beta-carbolin-423
N 2
a l
3
i
1
~ -y
H ]-
-oxopropan-
-am
ne
CI
CI
3-{6-bromo-1-[4-(methylsulfonyl)phenyl]-
77 I ~ N ~ N~NHZ 1,3,4,9-tetrahydro-2H-beta-carbolin-477
~
H 2-jrl~-3-oxopropan-1-amine
0
I
H3C-S=O
O
CH3 3-(2-beta-alanyl-6-isopropyl-2,3,4,9-
78 H C ~ tetrahydro-1 H-beta-carbolin-1-yl)phenol378.2
s ~
~
~ N
N II NHa
H
O
OH
CH3 3-[1-(3-fluorophenyl)-6-isopropyl-
79 H c ~ 1,3,4,9-tetrahydro-2H-beta-carbolin-380.2
~
(
N NH 2-yl]-3-oxopropan-1-amine
i
N ~ a
H
0
F
3-(2-beta-alanyl-7,8-dimethyl-2,3,4,9-
80 I N NH tetrahydro-1 H-beta-carbolin-1-yl)phenol364.2
I ~
2
H C
N
3
CH3 H
OH
H3C ~ 3-(2-beta-alanyl-6,8-dimethyl-2,3,4,9-
81 ~ ~ ~ N NH tetrahydro-1 H-beta-carbolin-1-yl)phenol364.2
N a
CH3 H
OH
3-(2-beta-alanyl-8-methyl-2,
\ 3,4, 9-
82 ~ tetrahydro-1 H-beta-carbolin-1-yl)phenol350.2
I ~
N N~NHZ
CH3 H , OO
OH
-64-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
ci ~ -(2-beta-alanyl-6-chloro-2,3,4,9-
83 I ~ I N NH tetrahydro-1 H-beta-carbolin-398
1-yl)benzoic acid
HO O
CI \ 3-[1-(1,3-benzodioxol-5-yl)-6-chloro-
84 I ~ I N NH 1,3,4,9-tetrahydro-2H-beta-carbolin-398
N ~ 2 2-yl]-3-oxopropan-1-amine
H
O
/I
O
~-O
3-(2-beta-alanyl-7-fluoro-2,3,4,9-
85 ~ tetrahydro-1 H-beta-carbolin-1-yl)phenol354.3
~ N
F
, N
NH
H
O
OH
-(2-beta-alanyl-6-chloro-2,3,4,9-
86 \ I I N NH tetrahydro-1 H-beta-carbolin-1-yl)-388.3
N ~ 2 2-fluorophenol
H
I
\ F
OH
CI \ 3-[6-chloro-1-(4-chloro-3-fluorophenyl)-
87 I , I N NH 1,3,4,9-tetrahydro-2H-beta-carbolin-406
2-yl]-3-oxopropan-1-amine
I
\ F
CI
CI \ 3-{6-chloro-1-[3-fluoro-5-
88 I ~ (trifluoromethyl)phenyl]-1,3,4,9-440
I N NH
N tetrahydro-2H-beta-carbolin-2-yl}-
z
H ~
i ~ 3-oxopropan-1-amine
F \
F
F
F
CI ~ 3-[6-chloro-1-(3,5-dimethylphenyl)-
89 ~ ~ I 1,3,4,9-tetrahydro-2H-beta-carbolin-382
i
NH
N
~
H 2-yl]-3-oxopropan-1-amine
\
/
z
OO
H3C \ CH3
-65-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
5-(2-beta-alanyl-6-chloro-2,3,4,9-
I N NH tetrahydro-1 H-beta-carbolin-1-yl)-400
2-methoxyphenol
OH
H3C.0
cH3 c~~ tert-butyl1-{[6-chloro-1-(3-
~ 3
91 hydroxyphenyl)-1,3,4,9-tetrahydro-484.2
CH3
2H-beta-carbolin-2-
cl I ~ \ N yl]carbonyl}propylcarbamate
i
H / \ OH
CH3 3-[2-(2-aminobutanoyl)-6-chloro-2,3,4,9-
92 NH tetrahydro-1 H-beta-carbolin-1-yl]phenol384.3
2
~N
CI
~ ~
I o
H ~ ~ OH
_ cn~~a~ tert-butyl (1 R)-2-[6-chloro-1-(3-
H
c
H
93 \ / hydroxyphenyl)-1,3,4,9-tetrahydro-532.4
~
~(
3
cH3 2H-beta-carbolin-2-yl]-2-oxo-1-
~
cl I ~ \ phenylethylcarbamate
N o
H ~ \ OH
3-{2-[(2R)-2-amino-2-phenylethanoyl]-6-
94 \ / chloro-2,3,4,9-tetrahydro-1431.92
H-beta-
~NHz carbolin-1-yl~phenol
CI ~ \ N
I O
H / \ OH
3-(6-chloro-2-D-phenylalanyl-2,3,4,9-
95 tetrahydro-1 H-beta-carbolin-1-yl)phenol446.3
HzN,
CI
N
~ \
O
H I \ OH
O 2-beta-alanyl-6-methyl-1-phenyl-1,2,
3, 9-
96 H3C ~ H tetrahydro-4.H-beta-carbolin-4-one348.2
/ I N II N.H
N
' O
H
~I
-66-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
O-H 2-beta-alanyl-6-methyl-1-phenyl-2,3,4,9-
7 H3c ~ H tetrahydro-1 H-beta-carbolin-4.-of350.2
~ I N II N_H
N
H O
I
methyl 3-[6-chloro-1-(3-hydroxyphenyl)-
98 I i N I N~N~O.CH 1,3,4,9-tetrahydro-2H-beta-carbolin-428.1
j
~ - I
I '
H 2-yl]-3-oxopropylcarbamate
o
I
HO
ethyl 3-[6-chloro-1-(3-hydroxyphenyl)-
N I N N O~CH3 1,3,4,9-tetrahydro-2H-beta-carbolin-442.1
~ 0
H 2-yl]-3-oxopropylcarbamate
I
HO \
i sopropyl 3-[6-chloro-1-(3-
100 I ~ N I N N O CH3 hydroxyphenyl)-1,3,4,9-tetrahydro-456.2
~ o ~
H ~ 2H-beta-carbolin-2-yl]-3-
3
I oxopropylcarbamate
HO
N-{3-[6-chloro-1-(3-hydroxyphenyl)-
I
I N
O
101 ~ 1,3,4,9-tetrahydro-2H-beta-carbolin-448.1
N-
-CH
0 3
2-yl]-3-oxopropyl}methanesulfonamide
OH
o _ N-{3-[6-chloro-1-(3-hydroxyphenyl)-
102 I ~ N I N'~'N-S ~ ~ 1,3,4,9-tetrahydro-2H-beta-carbolin-510.1
[
ff -
H ~ 2-yl]-3-oxopropyl}benzenesulfonamide
o
I
OH
o _ N-{3-[6-chloro-1-(3-hydroxyphenyl)-
103 I ~ N I N\~/N-S ~ ~ F ,3,4,9-tetrahydro-2H-beta-carbolin-546.1
1
[
~ - O
H , -yl]-3-oxopropyl}-2,4-
O
F 2
I d ifluorobenzenesulfonamide
OH
N-{3-[6-chloro-1-(3-hydroxyphenyl)-
104 I ~ 3 474
I N 4 2
N ~ I 1 9-tetrahydro-2H-beta-carbolin-
N , .
~ ,
,
H 0 2 -yl]-3-oxopropyl}benzamide
I
OH
-67-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
\ N-f3-[6-chloro-1-(3-hydroxyphenyl)-
C'
F
105 ~ 1,3,4,9-tetrahydro-2H-beta-carbolin-466.1
I
F
/ N N
2-yl]-3-oxopropyl)-2,
2, 2-
I trifluoroacetamide
OH
F N-f3-[6-chloro-1-(3-hydroxyphenyl)-
106 I N beta-carbolin- 522
N 2H 2
~ I 3
~ 4
9
t
h
d
1
t
o N ro- .
~ -
~o ,
,
-
ra
y
,
e
" ~ 0 0 2-yl]-3-oxopropyl)-2-(4-
I fluorophenoxy)acetamide
off
3-[2-(3-aminopropanoyl)-6-ethyl-2,3,4,9-
H3C
107 ~ ~ ~ N tetrahydro-1 H-beta-carbolin-1-yl]phenol363.2
NH
,
~
~
N
H
O
OH
F F o 3-[2-(3-aminopropanoyl)-6-
~
~
108 I (trifluoromethoxy)-2,3,4,9-tetrahydro-419.2
(
F / H N'~/NHZ 1H-beta-carbolin-1-yl]phenol
I
OH
c1 ~ 3-[6-chloro-2-(piperidin-4-ylacetyl)-
109 ~ ~ ~ N 2,3,4,9-tetrahydro-1 H-beta-carbolin-423.2
1-yl]phenol
~
NH
I
~
OH
o c" ~", 2-(3-aminopropanoyl)-N-[2-
110 N~N~CH3 (dimethylamino)ethyl]-1-(3-478.6
"3c
~
~ hydroxyphenyl)-N,6-dimethyl-2,3,4,9-
I
N NHZ
tetrahydro-1 H-beta-carboline-
~ off 3-carboxamide
O NHZ 2-(3-aminopropanoyl)-N-(3-
111 H l)-1-(3-h 449
c drox 55
~ hen
l)-
amino
ro
3 y .
w N yp
y
p
py
" NH 6-methyl-2,3,4,9-tetrahydro-1
H-beta-
carboline-3-carboxamide
i ~
OH
0 2-(3-aminopropanoyl)-1-
112 H3c ~ N;C"a (3-hydroxyphenyl)-N,N,6-trimethyl-420.51
O~H 2,3,4,9-tetrahydro-1 H-beta-carboline-
N
Z
~ 3-carboxamide
H
OH
-68-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
Br ~ 3-{6-bromo-2-[(methylamino)acetyl]-
113 ~ ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin-416.2
,N~NH 1-yl}phenol
O CH3
OH
Br ~ 3-[6-bromo-2-(pyrrolidin-1-ylacetyl)-
114 ~ 2,3,4,9-tetrahydro-1 H-beta-carbolin-456.3
( N
~ 1-yl]phenol
i V~
OH
Br ~ 3-(2-{[(2-aminoethyl)amino]acetyl}-6-
115 ~ bromo-2,3,4,9-tetrahydro-1445.2
~ N H-beta-
~ carbolin-1-yl)phenol
H ~H~NHz
OH
Br ~ 3-(6-bromo-2-{[(2-
116 ~ ~ ~ N o methoxyethyl)(methyl)amino]acetyl}-414.3
~ ~ 2
3
4
9
t
h
d
1 H
b
t
b
li
~~ ,
cH3 ,
,
-
etra
y
ro-
-
e
a-car
o
n-
H 1-yl)phenol
OH
Br ~ 3-[6-bromo-2-(piperazin-1-ylacetyl)-
117 ~ 2,3,4,9-tetrahydro-1 H-beta-carbolin-471.2
~ N
~ 1-yl]phenol
~
~
H
OH
Br ~ 3-{6-bromo-2-[(4-methylpiperazin-1-
118 I ~ ( N yl)acetyl]-2,3,4,9-tetrahydro-1485.3
H-beta-
carbolin-1-yl)phenol
CH3
\ OH
\ 3-{2-[(4-acetylpiperazin-1-yl)acetyl]-6-
Br
119 ~ bromo-2,3,4,9-tetrahydro-1513.3
( H-beta-
o carbolin-1-yl}phenol
i
OH
N-{2-[6-ethyl-1-(3-fluorophenyl)-1,
H3C 3,4, 9-
120 ~ ~ ~ N~ .CH tetrahydro-2H-beta-carbolin-2-yl]-379.2
3
II C 2-oxoethyl}-N,N-dimethylamine
H3
-69-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com Structure Name MH+
ound
3-[6-ethyl-1-(3-fluorophenyl)-1,3,4,
H3C 9-
121 I / I N NH t etrahydro-2H-beta-carbolin-2-yl]-365.2
3-oxopropan-1-amine
/I
F
2-[6-ethyl-1-(3-fluorophenyl)-1,3,4,9-
H3C
~
122 t etrahydro-2H-beta-carbolin-2-yl]-351.2
I I
N
/ H 2-oxoethanamine
~NH2
/ O
I
C
F
~ N-{3-[6-ethyl-1-(3-hydroxyphenyl)-
H ~
F
123 ~ ,3,4,9-tetrahydro-2H-beta-carbolin-459.2
~
F 1
/ N N
~F
2 -yl]-3-oxopropyl}-2,2,2-
t rifluoroacetamide
OH
F N-{3-[6-ethyl-1-(3-fluorophenyl)-1,3,4,9-
124 3 I / I N N F t etrahydro-2H-beta-carbolin-2-yl]-461.2
~F
3 -oxopropyl}-2,2,2-trifluoroacetamide
/
F
o N-{2-[6-ethyl-1-(3-fluorophenyl)-1,3,4,9-
125 3 ( / I N ~F t etrahydro-2H-beta-carbolin-2-yl]-447.2
2 th
H 2
H 2
t
ifl
l
2
t
id
F F -oxoe
' y
' ,
,
r
uoroace
}-
-
am
e
O
F
CI ~ 3 -(2-acetyl-6-chloro-2,3,4,9-tetrahydro-
126 1 H-beta-carbolin-1-yl)-4-fluorophenol359.6
~
~ N
,
CH
N 3
O
OH
CI ~ 3 -{6-chloro-2-[(dimethylamino)acetyl]-
127 I / I N .CH 2 ,3,4,9-tetrahydro-1H-beta-carbolin-402.1
3
1 -yl}-2-fluorophenol
~
CH
/ 3
HO
CI ~ 3 -(6-chloro-2-[(dimethylamino)acetyl]-
128 I , ( N .CH 2 ,3,4,9-tetrahydro-1H-beta-carbolin-402.1
3 1 l
4
fl
h
l
~ -y
}-
-
uorop
eno
CH
/ 3
OH
-70-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Gom ound Structure Name MH+
ci cn~rai tert-butyl (1 R)-1-{[6-chloro-1-(3-
129 / ~ H c H3~cH, hydroxyphenyl)-1,3,4,9-tetrahydro-2H- 484.2
~ cH3 beta-carbolin-
0 2-yl]carbonyl}propyicarbamate
0
~ I off
ci cnirai 3-{2-[(2S)-2-amino-3-methylbutanoyl]-6-
130 / ~ chloro-2,3,4,9-tetrahydro-1 H-beta- 398.2
N I N~CH3 carbolin-1-yl}phenol
TH
O CHs
OH
ci cnirai 3-{2-[(2R)-2-amino-3-methylbutanoyl]-6-
131 / ~ chloro-2,3,4,9-tetrahydro-1 H-beta- 398.2
I N~CH3 carbolin-1-yl}phenol
~O TCH3
OH
ci cnirai 3-{2-[(2R)-2-aminobutanoyl]-6-chloro-
132 / ~ 2,3,4,9-tetrahydro-1 H-beta-carbolin- 384.2
_. I N NHS cH 1-yl}phenol
H
O
OH
3-(6-bromo-2-{[(2-
133 I ~ N I N N~o, methoxyethyl)amino]acetyl}-2,3,4,9- 460.0
H ~H CHs tetrahydro-1 H-beta-carbolin-1-yl)phenol
I
OH
Br ~ 3-[2-(azetidin-1-ylacetyl)-6-bromo-
134 ~ ~ I 2,3,4,9-tetrahydro-1 H-beta-carbolin- 442.0
1-yl]phenol
i
OH
-[6-ch loro-1-(3-hydroxyphenyl)-1, 3,4, 9-
135 \ ~ o etrahydro-2H-beta-carbolin-2-yl]- 398.1
I ~~ -oxobutanamide
N N II NH2
H
O
I OH
H3~ ~ 3-(6-ethyl-1-phenyl-1,3,4,9-tetrahydro-
136 ~ ~ ~ N NH 2H-beta-carbolin-2-yl)-3-oxopropan- 347.2
N ~ ~ 1-amine
H
-71 _
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
Com oundStructure Name MH+
F ~ 3-(2-acetyl-6-fluoro-2,3,4,9-tetrahydro-
137 ~ 1 H-beta-carbolin-1-yl)phenol325.1
~ N
~
CH
N ~ s
H
O
OH
o NH2 N-(2-aminoethyl)-2-(3-aminopropanoyl)-
138 H 3 435.52
~ 4
9-
1-(3-hydroxyphenyl)-6-methyl-2
3 ,
~ ,
,
I ~ I ~NH tetrahydro-1H-beta-carboline-3-
carboxamide
I
OH
cH3 2-(3-aminopropanoyl)-N-[2-
139 0 rN'cH, (dimethylamino)ethyl]-1-(3-463.58
N hydroxyphenyl)-6-methyl-2,3,4,9-
N~NHZ tetrahydro-1 H-beta-carboline-
I
I
H ~ I 3-carboxamide
o
OH
Using the procedure described in Example 13, the above compounds were shown
to have an KSP inhibitory activity at an ICsp of less than about 50 p,M.
Certain of the
compounds have an ICsp of less than about 1 p.M, and certain others of the
compounds
have an ICsp of less than about 100 nM.
Example 13
Assay for Determining KSP Activity
In tlus example, a representative in vitro assay for determining KSP activity
is
described.
Purified microtubules from bovine brain were purchased from Cytoskeleton Inc.
The motor domain of human KSP (EgS, KNSL1) was cloned and purified to a purity
of
greater than 95%. Biomol Green was purchased from Affinity Research Products
Ltd.
Microtubules and the KSP motor protein were diluted in assay buffer (20 mM
Tris-HCI, pH 7.5, 1 mM MgCl2, 10 mM DTT and 0.25 mg/ml BSA) to a concentration
of
35 ug/ml for microtubules and 45 nM for KSP. The microtubule/KSP mixture was
then
pre-incubated at 37°C for 10 min to promote the binding of KSP to
microtubules.
ATP was also diluted to a concentration of 300 uM in the same assay buffer. To
each
well of the testing plate (384 well plate) containing 1.25 uL of compounds in
DMSO or
DMSO only, 25 uL of ATP solution. To start the reaction, 25 uL of
microtubule/KSP
-72-
CA 02554150 2006-07-19
WO 2005/070930 PCT/US2005/002270
solution was added to the ATP/compound mixture. The plates were incubated at
room
temperature for 1 hr. At the end of incubation period, 65 uL of Biomol Green
was added
to each well. The plates were incubated for 5-10 min and then the absorbance
at 630 nm
was determined. Biomol Green reagent is a malachite green based dye that
detects the
release of inorganic phosphate. Developed color signal was read using a Victor
II
reader. The concentration of each compound for 50% inhibition (ICSO) was
calculated by
nonlinear regression using either XLFit for Excel or Prism data analysis
software by
GraphPad Software Inc.
While the preferred embodiment of the invention has been illustrated and
described it will be appreciated .that various changes can be made therein
without
departing from the spirit and scope of the invention.
-73-