Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02557469 2008-09-22
FLUID DELIVERY DEVICE
FIELD OF THE INVENTION
The present invention relates to a fluid delivery device. In particular, the
invention
relates to a local anaesthetic fluid delivery device for slow delivery of
local anaesthetic
fluid to a patient while the device is attached to the patient.
BACKGROUND OF THE INVENTION
After a patient undergoes surgery, it is sometimes desirable to supply an
amount of
local anaesthetic to the patient at the site where the surgery was performed,
in order to treat
pain. A convenient way of administering such local anaesthetic is to allow the
patient to
carry an anaesthetic fluid delivery device which is arranged to allow release
of the local
anaesthetic fluid to the patient surgery site over a period of time.
One such local anaesthetic fluid delivery device is sold under the name "Pain
Buster" (RTM(V) made by I-Flow Corporation. The Pain Buster device is a 270 ml
capacity device with a spherical balloon designed to be placed in a cloth
pouch which can
be tied around the patient's waist. Disadvantages associated with this device
include that it
does not affix securely to the patient and can result in tangled tubing.
Because of its size,
it can introduce a relatively large volume of anaesthetic liquid to the
treatment site, but this
also results in it being cumbersome in size and weight.
A similar product, called "Pain Pump" made by Stryker Corporation includes a
spring-driven rigid pump that is also placed in a pouch in a similar fashion
to the Pain
Buster product. The Pain Pump is an even bigger device, being about 5 inches
in diameter
and 8 inches long, resulting in even more cumbersome size and weight
characteristics.
It is desired to provide a device which addresses or ameliorates one or more
disadvantages associated with prior art fluid delivery devices.
1
CA 02557469 2009-11-02
The reference to any prior art in this specification is not, and should not be
taken
as, an acknowledgment or any form of suggestion that that prior art forms part
of the
common general knowledge in Australia.
SUMMARY OF THE INVENTION
According to the present invention there is provided a local anaesthetic
delivery
device for supplying local anaesthetic to a patient, the device including:
anaesthetic supply means;
an outlet line which is supplied with local anaesthetic from said supply
means;
a housing within which the anaesthetic supply means is located;
means for adhering the housing to a patient; and
wherein the anaesthetic supply means includes a resilient reservoir in the
form of
a generally hollow cylindrical body when unexpanded, and having an inlet and
an outlet
at ends of the cylindrical body, the arrangement being such that a charge of
local
anaesthetic can be introduced into the reservoir through said inlet to
resiliently expand
the cylindrical body radially and said outlet line is connected to said outlet
of the
reservoir, so that the local anaesthetic is, in use, pressurised by the
radially expanded
resilient reservoir and the local anaesthetic is delivered to said outlet
line; and
wherein the outlet line includes a length of flow restricting line which
terminates
in a coupling which enables coupling to a catheter whereby the effective rate
of delivery
of local anaesthetic to the catheter is determined by the radial expansion of
the resilient
reservoir and the flow restricting line.
Preferably, the fluid delivery device is lightweight and the housing is mostly
formed of lightweight plastic.
Preferably, the housing is roughly dome shaped in cross-section on a side
facing
away from the patient when the housing is adhered to the patient. Preferably,
the
underside of the housing is relatively planar but of a flexible material to
accommodate
adhesion to non-planar areas of the patient's skin. Preferably, the housing
includes a
semi-cylindrical reinforcing member within an outer wall of the housing for
providing
increased
-2-
CA 02557469 2008-09-22
shape stability to the dome shape of the housing. Preferably, a raised area is
provided on
an outer portion of the domed area of the housing for affixing a label
thereto.
Preferably, the fluid delivery device has an inlet port at one end thereof and
the
inlet port is in fluid connection with the inflatable reservoir via a one-way
valve operative
such that fluid is only allowed to pass from the inlet port to the inflatable
fluid reservoir.
The inlet port may include a female luer fitting for engaging with a male luer
fitting of a
syringe, whereby the anaesthetic fluid can be injected in to the inflatable
fluid reservoir,
thereby inflating the reservoir with the anaesthetic fluid. Thus an amount of
local
anaesthetic fluid can be stored in the inflatable reservoir over a period of
time, for example
in the order of 24 hours, and the fluid delivery device will slowly deliver
the local
anaesthetic fluid to the patient site during that time.
Preferably, the fluid delivery device has a relatively large adhesive area on
its
underside, including wings extending to either side to increase the surface
area to which
the adhesive is applied.
Preferred features described above in relation to one embodiment may also be
applicable to any other described embodiments in any combination or singular
application
of such features.
Advantageously, fluid delivery devices according to embodiments of the present
invention are light and may be easily affixed to patients by means of an
adhesive underside
surface. The amount of local anaesthetic fluid held in the fluid reservoir is
generally less
than the amount held by prior art devices, thus reducing the weight carried by
the fluid
delivery device. Further, as the components of the device are all relatively
cheap to
purchase or manufacture, the device has a significantly lower manufacturing
cost than
prior art devices.
Further advantageously, the fluid reservoir, in the form of an inflatable
balloon or
bladder, is formed of a flexible polyisoprene material, rather than latex.
While latex would
normally be an ideal material for an inflatable bladder of the kind
contemplated, a number
3
CA 02557469 2008-09-22
of patients are allergic to latex and therefore the choice of an alternative
material, such as
polyisoprene, is desirable.
Advantageously, because of the secure attachment of the present fluid delivery
device to the patient's skin, the device can be better fixed in place, with
less chance of the
fluid delivery line becoming entangled or the fluid delivery device being
dislodged or
getting in the way when the patient moves. Advantageously, the dome form of
the device
helps to prevent or reduce external pressure being applied to the fluid
reservoir if, for
example, the patient should inadvertently contact the outside of the domed
outer wall of
the device.
The device of the invention can be made with inexpensive material so that it
is a
single use or disposable device.
Preferably further, the device is lightweight and compact so that it can be
discretely
worn under the clothing of a patient.
Preferably, the device has a housing which has a length in the range from 120
to
180mm and preferably about 160mm, a width in the range from 60 to 100mm and
preferably 80mm and a height in the range from 20 to 50mm and preferably 35mm.
Preferably further, the weight of the device (without local anaesthetic) is in
the range from
to 50gms and preferably about 35gms.
Further features of embodiments of the invention are hereinafter described, by
way
20 of example only, with reference to the drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a plan view of a fluid delivery device according to an embodiment
of
the invention;
4
CA 02557469 2008-09-22
Figure 2 is a longitudinal cross section through the device;
Figure 3 is a transverse cross-sectional view through the device;
Figure 4 is an enlarged view of part of the device;
Figure 5 is a cross-sectional view along the line 5-5;
Figure 6 shows the device with the fluid reservoir inflated;
Figure 7 shows an attachment for altering the delivery rate from the device.
and
Figure 8 is a schematic view showing deployment of the device on a patient.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
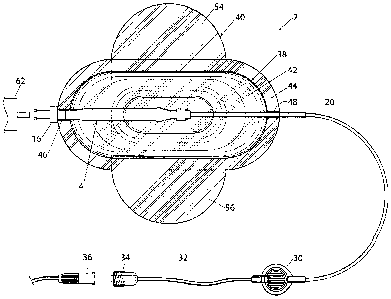
The device 2 illustrated in the drawings is for delivery of local anaesthetic
to a
patient 3 at a controlled rate. The device includes an inflatable reservoir 4
which as best
seen in Figures 4 and 5 includes a central cylindrical portion 8 and smaller
diameter end
portions 10 and 12. The end portion 12 is received within a threaded socket 14
of a female
luer connector 16. The luer connector may include a one-way valve (not shown)
which
permits fluid to pass from an inlet port 18 to the interior of the reservoir 4
but not in the
reverse direction. The other end portion 12 is connected to an outlet line 20.
In the
illustrated arrangement, the end of the reservoir 4 is a snug fit on the outer
diameter of the
line 20 and is retained in position by means of a retaining band 22.
The outlet line 20 is connected to a micro filter 30 of known type for
filtering out
any solid materials in the fluid delivered from the reservoir. The filter 30
is then connected
to a flow restricting line 32 which terminates in a male luer connector 34.
The male luer
connector 34 can be connected to a female luer connector 36 or to a cap (not
shown).
The reservoir 4 is located within a protective housing 38 which includes a
base wall
40 which is generally cruciform in shape and having rounded ends. The housing
can be
regarded as a lightweight flexible pouch which enables it to be directly
adhered to the skin
of the patient 3 whilst not causing significant discomfort. The housing 38
also includes a
top wall 42 which is generally complementary in shape to the base but is domed
so as to
accommodate the reservoir 4. The top wall 42 is formed with a peripheral
flange 44
5
CA 02557469 2008-09-22
enabling it to be glued or bonded to the base wall 40. As best seen in Figure
2, the top wall
42 is formed with an inlet formation 46 to permit the female luer connector to
pass
therethrough. The top wall 42 is formed with an outlet formation 48 which
permits the
outlet line 20 to pass therethrough. There is a relatively loose fit between
the outlet line 20
and the outlet formation 48. The luer connector 16 is glued to the formation
46 to hold it
in place. This holds the reservoir 4 in place within the housing 38. The
housing 38 also
includes a stiffening sleeve 50 which is generally semi-cylindrical and
located adjacent to
the top wall 42 as shown in Figures 2 and 3.
The cruciform shape of the base wall 40 defines two lateral wings 54 and 56,
the
ends of which are generally rounded, as shown in Figure 1. The underside
surface of the
wings 54 and 56 and the part of the base wall 40 therebetween are provided
with a layer 58
of adhesive. The adhesive layer 58 is covered by a removable backing strip 60.
In use of
the device, the backing strip 60 can be removed so that the adhesive layer 58
can be used
to adhere the housing 38 to the skin of a patient adjacent to the site 61
where the local
anaesthetic is to be delivered, as shown in Figure 8. Alternatively, the
housing 38 can be
adhered to a dressing which may be present near a surgical site rather than
directly to the
skin of the patient. The adhesive is preferably a hypoallergenic medical grade
adhesive
such as product code 467MP made by 3M. The surface area of the adhesive layer
is
preferably in the range from 80 to 150sq cm and most preferably about 115sq cm
in order
to provide secure adhesion to the patient's skin. The wings 54 and 56
preferably extend
from about 30 to 100mm and more preferably about 40mm laterally from the outer
periphery of the flange 44. This enables the flaps to be used to secure
delivery lines
beneath them and the patient's skin, as will be described below.
In the preferred form of the invention, the housing 38 is formed from clear
flexible
polyvinylchloride which is relatively soft to touch and is comfortable for the
patient. The
PVC materials can be glued together using a cyclohexanone adhesive.
Alternatively, the
PVC components can be ultrasonically welded. The domed shape of the top wall
42 and
the inlet and outlet formations 46 and 48 can be moulded by vacuum forming an
initially
flat sheet of PVC. The sheet of PVC which is used to form the top wall
preferably has an
initial thickness of say 0.6mm. The base wall 40 is preferably die cut from a
flat sheet of
PVC having a thickness of say 0.25mm. The stiffening sleeve 50 is preferably
formed
6
CA 02557469 2008-09-22
from a relatively stiff plastics material. It has been found that acrylic
sheet having a
thickness of 0.2mm is preferred. The stiffening sheet 50 is initially flat and
is held in the
hemi-cylindrical shape as shown in Figure 3 by elastic forces. Edges of the
stiffening
sleeve 50 butt against the upper side of the base wall 40. The stiffening
sleeve 50 helps to
maintain the domed shape of the top wall 42.
The reservoir 4 is preferably formed from flexible polyisoprene material and
may
be say 60mm long and having an outside diameter in the range from say 6.3 to
10.5mm.
The wall thickness is approximately 2mm. When the device is to be used, a
syringe 62,
preferably having a male luer connector on its end, can be coupled to the
female luer
connector 16. The syringe 62 is used to fill the reservoir 4 with local
anaesthetic and the
pressure of the anaesthetic inflates the reservoir to an inflated position as
shown in Figure
6. Normally the reservoir will hold about 120mis of local anaesthetic when
fully inflated.
Once the reservoir 4 has been inflated, the syringe 62 can be disconnected and
the resilient
pressure applied by the inflated reservoir 4 will force the local anaesthetic
through the
outlet line 20, through the filter 30 and into the flow restricting line 32 so
that the system is
flushed with anaesthetic in readiness for use. At this point, the cap 36 can
be placed in the
connector 34 so that the device remains primed with anaesthetic or
alternatively it can be
directly deployed at a site 61 on a patient.
The reservoir is preferably injection moulded from polyisoprene. It contains
no
latex and is therefore suitable for use with patients who may be allergic to
latex. The
reservoir 4 preferably complies with US FDA and International Pharmacopeia
requirements including requirements for ISO 10993-5. The fluid reservoir is
highly elastic
and behaves like a resilient bladder. Its initial volume is expanded from a
relaxed state of
about 7 to 10ml to an expanded state of 120m1. The elastic properties of the
reservoir 4
exert the pressure on the local anaesthetic and this property in conjunction
with the flow
restricting line 32 is a particularly simple and effective way of delivering a
relatively
constant supply of anaesthetic to the patient. After inflation of the
reservoir, the internal
pressure of the anaesthetic will be about 8.5 to 9 psi and this pressure will
remain
substantially constant as the anaesthetic is dispensed and the elastic
extension of the
reservoir becomes less. It has been found that there is little variation in
pressure until
7
CA 02557469 2008-09-22
about the last 5% of the anaesthetic remains in the reservoir after which
there will be a rise
in pressure and a brief increase in delivery rate. It will be appreciated that
the technique is
low cost and does not involve the use of any pump or motor or other form of
power input
to the device other than the initial energy which is expended when the syringe
62 is used to
inflate the reservoir 4.
If the flow restricting line 32 or other flow controlling device were not
present, the
rate of flow of local anaesthetic from the inflated reservoir 4 would be non-
linear and
generally proportional to the extent of expansion of the reservoir. The
provision of the
flow restricting line 32, however, ensures a generally uniform rate of
delivery of
anaesthetic to the patient, notwithstanding the variation in pressure applied
by the reservoir
4. This is because the flow restricting line 32 has a very fine bore and the
pressure drop
therein is substantial compared with pressure drops in other parts of the
device and hence
the flow rate is more or less constant. It is preferred that the flow
restricting line 32 is
formed of PVC microbore tubing having a nominal internal diameter of about 0.1
mm and
nominal outside diameter of 2 mm. It is preferred for flow restricting line 32
to be about
95 mm in length, which allows an anaesthetic fluid flow of about 5 ml per
hour. Generally
speaking, the flow rate is inversely proportional to the length of the flow
restricting line 32.
If a lower flow rate is required an additional flow restricting line 64 can be
provided, as
seen in Figure 7. In this arrangement the additional flow restricting line 64
has the same
length as the line 32 so that when connected in series the flow delivery rate
will be reduced
from about 5m1 per hour to about 2.5m1 per hour. The additional flow
restricting line 64
can be provided with a female luer coupling 65 at one end and a male luer
coupling 66 at
the other.
Figure 8 shows the device 2 deployed on the patient 3. In this drawing, the
reservoir 4 has been filled with local anaesthetic. The backing strip 60 has
been removed
so that the adhesive layer 58 can be applied directly to the skin of the
patient. It will also
be seen that part of the line 20 and/or flow restricting line 32 can be
located under one or
other of the wings 54 or 56 in order to prevent fouling of these components.
The male luer
connector 34 can then be connected to a female luer mounted on the end of a
catheter 68
which enters the skin of the patient beneath an adhesive bandage 70, as shown.
8
CA 02557469 2008-09-22
It will be appreciated by those skilled in the art that the device of the
invention is
simple and inexpensive to make which makes it suitable as a single use
product. It is also
convenient and comfortable for the patient 3 when compared to the bulky rigid
structures
of the prior art.
Preferably, the device has a housing which has a length in the range from 120
to
180mm and preferably about 160mm, a width in the range from 60 to 100mm and
preferably 80mm and a height in the range from 20 to 50mm and preferably 35mm.
Preferably further, the weight of the device (without local anaesthetic) is in
the range from
25 to 50gms and preferably about 35gms. Accordingly, the device is compact and
lightweight and so it can be discretely worn by a patient beneath the
patient's clothes.
Also, the wings 54 and 56 have sufficient lateral extent so that the outlet
line 20 and/or the
flow restricting lines 32 and 64 can be fastened between one or both wings 54,
56 and the
patient's skin adjacent to the site where the catheter 68 is located and this
minimises the
possibility of the lines being caught and torn away from the patient if these
lines were
external to the patient's clothing as they are in the prior art devices.
It will be appreciated by those skilled in the art that the compact,
lightweight device
of the invention having a pouch like housing with a flat base provided with
adhesive
material constitutes a significant advantage over prior art devices.
Whilst the primary use of the device of the invention is for delivery of local
anaesthetic, it is possible that the same or a similar device could be used
for delivering
local anaesthetic together with other medical agents or alternatively other
medical agents
not necessarily including local anaesthetic.
9