Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
METHODS AND COMPOSITIONS FOR THE TREATMENT OF
CONDITIONS RELATED TO GASTRIC ACID SECRETION
By Inventors
Jie Shen, Devin F. Welty and Diane D. Tang-Liu
BACKGROUND OF THE INVENTION
Description of the Related Art
to Benzimidazole derivatives intended for inhibiting gastric acid secretion
are disclosed in U.S. Pat. Nos. 4,045,563; 4,255,431; 4,628,098; 4,686,230;
4,758,579; 4,965,269; 5,021,433; 5,430,042 and 5,708,017. Generally
speaking, the benzimidazole-type inhibitors of gastric acid secretion are
believed to work by undergoing a rearrangement to form a thiophilic species
15 which then covalently binds to gastric H,K-ATPase, the enzyme involved in
the
final step of proton production in the parietal cells, and thereby inhibits
the
enzyme. Compounds which inhibit the gastric.H,K-ATPase enzyme are
generally known in the field as "proton pump inhibitors" (PPI).
Some of the benzimidazole compounds capable of inhibiting the gastric
2o H,K-ATPase enzyme have found substantial use as drugs in human medicine
and are known under such names as LANSOPRAZOLE (U.S. Pat. No.
4,628,098), OMEPRAZOLE (U.S. Pat. Nos. 4,255,431 and 5,693,818),
ESOMEPRAZOLE (U.S. Pat No. 6,369,085) PANTOPRAZOLE (U.S. Pat. No.
4,758,579), and RABEPRAZOLE (U.S. Pat. No. 5,045,552). Some of the
25 diseases treated by proton pump inhibitors and specifically by the five
above-
mentioned drugs include peptic ulcer, heartburn, reflux esophagitis, erosive
esophagitis, non-ulcer dyspepsia, infection by Helicobacter pylori, alrynitis
and
asthma.
Whereas the proton pump inhibitor type drugs represent a substantial
30 advance in the field of human and veterinary medicine, they are not totally
without shortcomings or disadvantages. For example, it is believed that the
short systemic half-life of the drug limits the degree of gastric acid
suppression
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
currently achieved. Furthermore, it appears that the short plasma half life of
the
drug may contribute to significant gastric pH fluctuations that occur several
times a day in patients undergoing PPI therapy. Additionally, PPIs are acid-
labile, and in most cases it is necessary to enterically coat the drug in
order to
prevent the acidic milieu of the stomach from destroying the drug before the
drug is absorbed into systemic circulation. Thus, any contribution that might
improve the acid stability or plasma half life of the presently used proton
pump
inhibitors will be a significant improvement in the art.
As further pertinent background to the present invention, applicants note
the concept of prodrugs which is well known in the art. Generally speaking,
prodrugs are derivatives of per se drugs, which after administration undergo
conversion to the physiologically active species. The conversion may be
spontaneous, such as hydrolysis in the physiological environment, or may be
enzyme catalyzed. From among the voluminous scientific literature devoted to
prodrugs in general, the foregoing examples are cited: Design of Prodrugs
(Bundgaard H. ed.) 1985 Elsevier Science Publishers B. V. (Biomedical
Division), Chapter 1; Design of Prodrugs: Bioreversible derivatives for
various
functional groups and chemical entities (Hans Bundgaard); Bundgaard et al.
Int.
J. of Pharmaceutics 22 (1984) 45-56 (Elsevier); Bundgaard et al. Int. J. of
2o Pharmaceutics 29 (1986) 19-28 (Elsevier); Bundgaard et al. J. Med. Chem. 32
(1989) 2503-2507 Chem. Abstracts 93, 137935y (Bundgaard et al.); Chem.
Abstracts 95, 138493f (Bundgaard et al.); Chem. Abstracts 95, 138592n
(Bundgaard et al.); Chem. Abstracts 110, 57664p (Alminger et al.); Chem.
Abstracts 115, 64029s (Buur et al.); Chem. Abstracts 115, 189582y (Hansen et
al.); Chem. Abstracts 117, 14347q (Bundgaard et al.); Chem. Abstracts 117,
55790x (Jensen et al.); and Chem. Abstracts 123, 17593b (Thomsen et al.).
A publication by Silz., et al. (Journal of Medicinal Chemistry, 1991, vol.
34, pp 1049-1062), describes N-acyloxyalkyl, N-alkoxycarbonyl, N-
(aminoethyl), and N-allcoxyalkyl derivatives of benzimidazole sulfoxide as
prodrugs of proton-pump inhibitors. According to this article these prodrugs
exhibited improved chemical stability in the solid state and in aqueous
solutions, but had similar activity or less activity than the corresponding
parent
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
compounds having a free imidazole N-H group. This publication provides no
data nor suggestion regarding the duration of the inhibitory activity of these
prodrugs.
United States Patent No. 6,093,734 and PCT Publication WO 00109498
(published on February 24, 2000) describe prodrugs of proton pump inhibitors
which include a substituted arylsulfonyl moiety attached to one of the
benzimidazole nitrogens of proton pump inhibitors having the structure
identical with or related to proton pump inhibitor drugs known by the names
LANSOPRAZOLE, OMEPRAZOLE, PANTOPRAZOLE and
1o RABEPRAZOLE.
PCT Publication WO 02/30920 describes benzimidazole compounds
which are said to have gastric acid secretion inhibitory and anti H. pylori
effects. PCT Publication WO 02/00166 describes compounds that are said to be
nitric oxide (NO) releasing derivatives of proton pump inhibitors of the
benzimidazole structure.
Copending U.S. Patent Application No. 10/620,252, filed July 15, 2003
discloses prodrugs of the proton pump inhibitor type drugs having an
arylsulfonyl group with an acidic functional group attached, which provided
improved solubility in physiological fluids and improved cell penetration.
BRIEF DESCRIPTION OF THE FIGURES
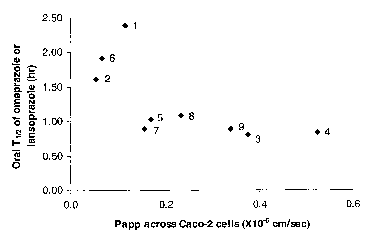
Figure 1 is a plot of the systemic half life (Tli2) of proton pump inhibitors
omeprazole and lansoprazole, following oral administration of their
corresponding prodrugs in dog, as a function of membrane permeability of the
prodrugs, measured as the permeability coefficient (Papp) across Caco-2 cells
in
the apical to basolateral direction.
3o Figure 2 depicts compound 1 transport in the basolateral to apical
direction
across Caco-2 cells in the presence and absence of 50 ,uM of MK-571 and 250
~M of reduced glutathione.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Figure 3 depicts the mean omeprazole concentration in blood following oral
administration of 16 mg/kg compound 6, with and without co-administration of
MK-571 (n = 9).~
DETAILED DESCRIPTION OF THE INVENTION
A method comprising orally administering to a mammal a proton pump
inhibitor and a compound which modulates the activity of an efflux transporter
l0 protein in the gastrointestinal tract epithelium; or prodrugs or
pharmaceutically
acceptable salts thereof; is disclosed herein; said method being effective for
the
prevention or treatment of a disease or condition related to gastric acid
secretion.
While not intending to limit the scope of the invention in any way, one
method comprises orally administering to a mammal a proton pump inhibitor, or
a pharmaceutically acceptable salt or prodrug thereof; and a compound which
modulates the activity of MRP2, or a pharmaceutically acceptable salt or
prodrug thereof.
~ A composition comprising a proton pump inhibitor and a compound
which modulates the activity of an efflux transporter protein in the
gastrointestinal tract epithelium; or prodrugs or pharmaceutically acceptable
salts thereof; is also disclosed herein.
While not intending to limit the scope of the invention in any way, one
composition comprises a proton pump inhibitor, or a pharmaceutically
acceptable salt or prodrug thereof; and a modulator of MRP2 activity or a
pharmaceutically acceptable salt or prodrug thereof.
The use of a proton pump inhibitor and a compound which modulates
the activity of an efflux transporter protein in the gastrointestinal tract
epithelium; or prodrugs or pharmaceutically acceptable salts thereof; in the
manufacture of a medicament for the prevention or treatment of a condition or
disease related to gastric acid secretion afflicting a mammal, is disclosed
herein.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
While not intending to limit the scope of the invention in any way, one
embodiment comprises use of a compound which modulates the activity of
MRP2, or a salt or a prodrug thereof; in combination with a proton pump
inhibitor, or a salt or a prodrug thereof; in the manufacture of a medicament
for
the prevention or treatment of a condition or disease related to gastric acid
secretion afflicting a mammal, is disclosed herein.
While not intending to limit the scope of the invention in any way, or be
bound
in any way by theory, it is believed that the methods and composition
disclosed
herein confer significant advantages to the use and formulation of proton pump
to inhibitors and their prodrugs for the treatment and prevention of diseases
and
conditions related to gastric acid secretion.
While not intending to limit the scope of the invention in any way, we
have surprisingly discovered that a multidrug resistance-associated protein
family member, MRP2, which is expressed at the luminal membrane of the
intestinal epithelium, is likely to be one of the transporter proteins
responsible
for intestinal efflux of proton pump inhibitors and their prodrugs, thus
prolonging the systemic half life of these compounds. In other words, it is
believed that two-way transport occurs for these compounds, the first
transport
is absorptive, i.e. from the gut toward the bloodstream, and the second
transport
2o is effluxive, i.e. from the gut lining back into the lumen of the
gastrointestinal
tract. Thus, while not intending to be bound in any way by theory, the
effluxive
action slows the absorption of the compounds, and the apparent systemic half
live of the proton pump inhibitors is increased. We have surprisingly
discovered that compounds believed to modulate the activity efflux transporter
proteins of the gastrointestinal tract epithelium, including modulators of
MPR2,
are capable of altering the net flux of prodrugs of proton pump inhibitors and
proton pump inhibitors from the gut into the bloodstream and thus altering the
pharmacokinetic profile of proton pump inhibitors. Thus, while not intending
to
be bound in any way by theory, compounds which modulate the activity efflux
transporter proteins in the gastrointestinal tract epithelium are believed to
be
capable of helping to tune the pharmacokinetics of prodrugs of proton pump
inhibitors and proton pump inhibitors themselves, thus improving sustained-
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
release, bioavailability, or peak proton pump inhibitor concentration,
according
to the particular need. Furthermore, while not intending to be bound in any
way
by theory, this discovery should enable separate control of the
pharmacokinetic
and physicochemical properties of these compounds, thus improving flexibility
in formulating therapeutic dosage forms.
The term "prodrug" has the meaning previously described herein, and in
relation to this disclosure refers to a prodrug of a proton pump inhibitor.
The
term should be construed broadly, such that if functional groups are present
on
the prodrug that are capable of forming salts, a salt of such a compound is
also
considered to be a "prodrug". The term "proton pump inhibitor" also has the
meaning previously described herein.
"A compound a compound which modulates the activity of an efflux
transporter protein in the gastrointestinal tract epithelium" is any compound
which affects the activity of efflux transporter proteins. This includes any
compound which stimulates activity or inhibits activity, regardless of the
manner in which this is accomplished. Although the compound may selectively
affect the activity of an efflux transporter protein, nonselective compounds
may
also be used.
"A compound which modulates the activity of MRP2" is any compound,
0 salt, or prodrug which affects the activity of MRP2, whether it stimulates
activity or inhibits activity, regardless of the manner in which this is
accomplished. Although the compound may selectively affect the activity of
MRP2, nonselective compounds may also be used.
In one embodiment, an MRP modulator is used. Known inhibitors of
MRP proteins include MK-571, sildenafil (Viagra"), leukotriene C4,
gemfibrozil, probenecid, and verapamil. Compounds such as glutathione,
which stimulate MRP activity may also be used. Pharmaceutically acceptable
salts of these compounds may also be used, and for the purposes herein, the
name of any compound applies to both the neutral form and any
pharmaceutically acceptable salt.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
CI
MK-571
SH
O O O
H
N
HO H ~ OH
NH2 O
glutathione
Both proton pump inhibitors and prodrugs may be used in the
compositions and methods disclosed herein. While not intending to limit the
scope of the invention in any way, commercially available proton pump
inhibitors (PPI) include lansoprazole, esomeprazole, omeprazole, pantoprazole,
to and rabeprazole. Although a prodrug may be prepared from any proton pump
inhibitor, it may be desirable to use a prodrug of a commercially available
proton pump inhibitor. In situations where the prodrug is derived from one of
the commercially available PPIs circumstances related to the individual to
which the prodrug is administered are often relevant to the compositions and
15 methods practiced as disclosed herein. For example, if the person to which
the
prodrug is being administered is known to respond well to omeprazole, then one
may consider using a prodrug of omeprazole as disclosed herein. In another
situation, a person may have a history of being effectively treated by
lansoprazole, in which case one may consider using a prodrug of lansoprazole
2o as disclosed herein. The specific compounds disclosed herein are given
merely
to provide guidance and direction to one practicing the invention, and are not
intended to limit the overall scope of the invention in any way.
In one embodiment the proton pump inhibitor is lansoprazole. In
another embodiment the proton pump inhibitor is omeprazole. In another
25 embodiment the proton pump inhibitor is esomeprazole. In another
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
embodiment the proton pump inhibitor is pantoprazole. In another embodiment
the proton pump inhibitor is rabeprazole. Other embodiments comprise a
prodrug of omeprazole. Other embodiments comprise a prodrug of
pantoprazole. Other embodiments comprise a prodrug of rabeprazole. Other
embodiments comprise a prodrug of lansoprazole. Other embodiments
comprise a prodrug of esomeprazole.
Certain compounds have been shown to be useful as prodrugs in relation
to the embodiments disclosed herein. In certain embodiments, the prodrug
comprises a sulfonyl moiety. A "sulfonyl" moiety is defined herein as a moiety
comprising an SO2 group, where a sulfur atom is directly covalently bonded to
two oxygen atoms. In other embodiments, the prodrug comprises a
phenylsulfonyl moiety. The term "phenylsulfonyl" moiety should be broadly
interpreted to mean any moiety where the sulfur of the S02 group is directly
covalently bonded to a carbon that is part of a phenyl ring. The term "phenyl
ring" should be broadly understood to mean any ring comprising six carbon
atoms having three conjugated double bonds. Thus, a phenylsulfonyl moiety
could be monosubstituted, meaning that the sulfonyl group is the only group
directly attached to the phenyl ring, or the phenylsulfonyl moiety could have
from 1 to 5 additional substituents which are not a hydrogen atom, and are
directly attached to a carbon of the phenyl ring. In certain embodiments, the
prodrug comprises both a phenylsulfonyl moiety and a carboxylic acid or a
pharmaceutically acceptable salt thereof.
Prodrugs may also comprise
N O
- ~~ N-
I
o. -s=o
R1
RS
Rz
R3
or a pharmaceutically acceptable salt thereof
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
wherein
A is H, OCH3, or OCHF~;
B is CH3 or OCH3;
D is OCH3, OCH2CF3, or O(CH~)30CH3;
E is H or CH3;
Rl, R2, R3, and RS are independently H, CH3, CO~H, CH2C02H, (CH~)aCOaH,
CH(CH3)2, OCH2C(CH3)~COZH, OCHZC02CH3, OCHZC02H, OCHZCOZNH~,
OCHaCONH2(CH2)SCO~CH3, or OCH3.
In another embodiment related to the one just described, Rl, R2, R3, and
to RS are independently H, CH3, COZH, CH2C02H, (CH2)2C02H, OCH~C02CH3,
OCHZC02H, OCH2CONH2(CH2)SCO2CH3, Or OCH3.
In certain embodiments, the prodrug has a structure comprising
or a pharmaceutically acceptable salt thereof.
Other prodrugs comprise
or a pharmaceutically acceptable salt thereof.
Other prodrugs comprise
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
or a pharmaceutically acceptable salt thereof.
or a pharmaceutically acceptable salt thereof.
In other embodiments, the prodrug has a structure comprising
In other embodiments, the prodrug has a structure comprising
l0 In other embodiments, the prodrug has a structure comprising
Other prodrugs comprise
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
HsCHO-
In embodiments comprising a prodrug, the apical to basolateral
membrane permeability of the prodrug may vary. The term "apical to
basolateral membrane permeability" used in relation to this disclosure refers
to
the value obtained by carrying out the procedure described in Example 1
herein.
In one embodiment the apical to basolateral membrane permeability of the
prodrug is less than 1 x 10-~ cm/sec. In another embodiment the apical to
basolateral membrane permeability of the prodrug is less than 5 x 10-7 cm/sec.
In another embodiment the apical to basolateral membrane permeability of the
prodrug is less than 1 x 10-7 cm/sec. In another embodiment the apical to
basolateral membrane permeability of the prodrug is less than 5 x 10-s cm/sec.
In certain embodiments, the apical to basolateral membrane permeability
of a prodrug as it relates to that of the parent proton pump inhibitor is
relevant.
In one embodiment the apical to basolateral membrane permeability of the
proton pump inhibitor is more than twice the apical to basolateral membrane
permeability of the prodrug. In another embodiment, the apical to basolateral
membrane permeability of the proton pump inhibitor is more than 10 times the
apical to basolateral membrane permeability of the prodrug. In another
embodiment the apical to basolateral membrane permeability of the proton
pump inhibitor is more than 100 times the apical to basolateral membrane
permeability of the prodrug. In another embodiment the apical to basolateral
membrane permeability of the proton pump inhibitor is more than 150 times the
apical to basolateral membrane permeability of the prodrug.
The prodrugs of the present invention can be prepared by the methods
described in the following U.S. Patent documents, all of which are expressly
incorporated by reference herein: U.S. Pat. No. 6,093,734; U.S. Pat. App. No.
09/783,807, filed February 14, 2001; and U.S. Patent Application No.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
10/620,252, filed July 15, 2003; U.S. Pat. App. 10/487,340, filed July 15,
2003.
However, these methods are only given to provide guidance, and are not meant
to limit the scope of the invention in any way. One of ordinary skill in the
art
will recognize that there are many ways in which the prodrugs of the present
invention can be prepared without departing from the spirit and scope of the
present invention.
Certain embodiments disclosed herein relate to prodrugs comprising an
acidic group. An "acidic functional group" as used herein refers to an oxygen
containing functional group which has a pKa below 10. Thus, while not
l0 intending to limit the scope of the claims in any way an acidic functional
group
may include an organic acid such as a carboxylic acid, a phosphonic acid, or a
sulfonic acid.
Acidic functional groups can be in one of two forms, the acid form or
the salt form, depending upon whether the particular group has undergone an
acid-base reaction. The two forms of these functional groups may also be
known by other names. The term "acidic functional group" should be broadly
understood to incorporate either the acid or the salt form of the functional
group.
A "pharmaceutically acceptable salt" is any salt that retains the activity
of the parent compound and does not impart any deleterious or untoward effect
on the subject to which it is administered and in the context in which it is
administered as compared to the parent compound.
Pharmaceutically acceptable salts of acidic functional groups may be
derived from organic or inorganic bases. The salt may be a mono or polyvalent
ion. Of particular interest are the inorganic ions, lithium, sodium,
potassium,
calcium, and magnesium. Organic salts may be made with amines, particularly
ammonium salts such as mono-, di- and trialkyl amines or ethanol amines. Salts
may also be formed with caffeine, tromethamine and similar molecules.
Hydrochloric acid or some other pharmaceutically acceptable acid may form a
salt with a compound that includes a basic group, such as an amine or a
pyridine
ring.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
A disease or conc~tion related to gastric acid secretion is any disease
where gastric acid is a cause or a contributing factor, or contributes to a
symptom of the diseases, or where inhibition of gastric acid secretion may be
helpful in treating or preventing the disease. While not intending to limit
the
scope of the invention in any way, some examples of such diseases or
conditions are peptic ulcer, heartburn, reflux esophagitis, erosive
esophagitis,
non-ulcer dyspepsia, infection by Helicobacter pylori, alrynitis, and other
conditions.
In certain embodiments disclosed herein, the prodrug is not enterically
to coated. The term "enterically coated" means the prodrug or the dosage form
comprising the prodrug is coated by a coating which protects the prodrug from
the acids present in the stomach, but which coating disintegrates in the
higher
pH environment of the intestines. In many dosage forms, small particles of the
prodrug are coated with the enteric coating. In other dosage forms, an entire
capsule, tablet, or other solid dosage form is coated with the enteric
coating.
While not intending to be bound in any way by theory, it is believed that the
prodrugs disclosed herein are sufficiently stable in the presence of the
acidic
milieu of the stomach that enteric coating of the prodrug is generally not
necessary.
Those skilled in the art will readily understand that for oral
administration the compounds of the invention are admixed with
pharmaceutically acceptable excipients which per se are well known in the art.
Specifically, a drug to be administered systemically, it may be confected as a
powder, pill, tablet or the like, or as a syrup or elixir suitable for oral
administration. Description of the substances normally used to prepare
tablets,
powders, pills, syrups and elixirs can be found in several books and treatise
well
known in the art, for example in Remington's Pharmaceutical Science, Edition
17, Mack Publishing Company, Easton, Pa.
Prodrugs of the present invention can be combined with certain amounts
3o of the proton pump inhibitors to which they are related to provide a drug-
prodrug combination, and the combination administered for inhibition of
gastric
acid secretion. Thus, certain embodiments relate to a mixture of the prodrug
and
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
the proton pump inhibitor. Other embodiments relate to the administration of
both the prodrug and the proton pump inhibitor. While not intending to limit
the scope of these embodiments, it is believed that the proton pump inhibitor
(drug) initially inhibits gastric acid secretion of the patient, and as the
effective
concentration of the proton pump inhibitor (drug) is decreased by metabolism,
the prodrug is used to maintain a sustained presence of a therapeutically
effective systemic concentration of the proton pump inhibitor. In certain
embodiments the ratio of the molar concentration of the prodrug to the molar
concentration of the proton pump inhibitor is from 1 to 1000. In other
l0 situations, two prodrugs of a proton pump inhibitor are administered to a
person
to a similar end.
The following examples provide guidance and direction in making and
using the invention, and to demonstrate the advantages of the present
invention.
However, except in the case of Example 1, they are not to be interpreted as
15 limiting the scope of the invention in any way. In the case of Example 1,
it
should only be interpreted as limiting in relation to those claims where
apical to
basolateral membrane permeability is used as a limitation.
Test Compounds
Membrane permeability and oral bioavailability tests were carried out
for the compounds shown in Table 1 below. The generic structure, I, is shown
as a combination of a proton pump inhibitor (X) and a sulfonyl-bearing moiety
which is attached to the proton pump inhibitor to form the prodrug according
to
the formula below. The identity of each group represented by Rl-RS is shown in
the table.
R1 R2
O _
Rs
O
Rs Ra
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
The different possibilities for X are shown below.
N O N O
HaCO ~ N- ~ ~ ~/ N-
N CH ~ N
HaC OCHa H3C OCHZCFa
OME LNZ
//
/% N
~5 I
N
~(CHZ)a
) O
H3C
PNT RAB
Table 1
Com ound X R R ~ R R
1 OME H H OCHZCOZH H H
2 OME CH3 H OCHZCOZH H CH3
3 OME H H OCHZC(CH3)zCOZHH H
4 OME CHs H OCHZC(CH3)ZCOzHH CH3
OME H H CHZCOZH H H
6 OME H COZH H H H
7 LNZ H COZH H H H
8 LNZ H COzH OCH3 H H
9 LNZ H H CHZCOZH H H
LNZ H H OCHZCOZH H H
11 LNZ H H OCHzC(CH3)zCOZHH H
12 LNZ H CHZCOZH CHZCOzH H H
13 LNZ H C02H H H CH3
14 LNZ H COzH H H OCH3
LNZ CH(CH3)zH CHZCOZH H H
16 LNZ H OCHZCOZH COzH H H
17 LNZ CH(CH3)ZH OCHZCOZH H CH3
18 LNZ H H COZH H H
19 LNZ H (CHz)ZCOZHCH3 H H
OME H H OCHZCOZCH3 H H
21 OME H H OCHZCOZNHz H H
22 OME H COZH COZH H H
23 OME H COzH OCHZCOzH H H
24 OME H OCHZCOzH OCHzCOzH H H
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
25 OME OCH3 H COZH H H
26 OME H COzH H H
27 OME H COZH H H CH3
2$ pj~' H H OCHZCOzH H H
29 PNT H COZH H H CH3
30 RAB H COZH H H H
31 RAB H COZH H H CH3
32 RAB CHs H OCHZCOZH H CH3
33 RAB H H COZH H H
34 LNZ CH3 H OCHZCOZH H CH3
35 LNZ H OCHZCOZH OCHzCO2H H H
36 LNZ H H COZH H H
37 LNZ CH3 H COZH H H
38 LNZ H (CHZ)ZCOzHOCH3 H H
39 OME CH3 H OCHZCONHZ(CHz)5 CH3
H
COZCH3
40 OME H H OCHZCONHZ(CHZ)5H H
COZCH3
41 OME H H (CHZ)zCOZH H H
42 OME H (CHz)zCO2HOCH3 H H
Compounds were prepared according to procedures described the U.S_
Pat. App. No. 101620,252, filed July 15, 2003 and U.S. Pat. App. No.
101487,340, filed July 15, 2003 incorporated by reference herein.
Omeprazole and lansoprazole were purchased from Sigma (St. Louis,
MO).
Example 1
to Determination of membrane permeability in all examples described
herein was accomplished by the following procedure. This procedure is also
used to determine whether a given prodrug falls within the scope of those
claims given herein which relate to membrane permeability.
Materials/Methods
Test System: Cultured Caco-2 cells and MDR1-MDCK cells
Seeding Density: 2 x 105 cells/cm' in Costar 12 well TranswellT""
plates
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Culture Age: 17-21 days post seeding for Caco-2 cells, 2-3 days
post seeding for MDR1-MDCK cells
Source: American Type Culture Collection, Manassas,
VA (Caco-2)
Dr. Piet Borst at the Netherlands Cancer Institute
(Amsterdam, Netherlands) (MDR1-MDCK)
Growth Media: Dulbecco's Modified Eagle Media (DMEM)
(Gibco BRL) supplemented with 10% fetal bovine
serum and 0.1 % nonessential amino acids
Dosing Formulation: 10 ~,M proton pump inhibitor or prodrug in
DMEM. Make on the day of dosing.
Assay: LC-MS/MS
Bi-directional transport experiment:
Caco-2 and MDR1-MDCK cells were seeded on Costars l2mm
diameter, 0.4 ~.m pore size transwell filters, and were cultured at
37°C, 5% C02
in a humidified tissue culture chamber.
DMEM was equilibrated as a transport buffer in 37°C water bath an
hour before experiment. The cells were then equilibrated in transport buffer
for
1 hr at 37°C.
Dosing solution (10 ~M) was prepared by adding a 20 ~,L aliquot of a 10
mM stock solution of the prodrug to 20 mL of transport buffer.
Test Conditions:
Transport across Caco-2 or MDR1-MDCK cell monolayer was
measured at 37°C, in the apical to basolateral direction (n=3).
Transport buffer was removed from both apical and basolateral
compartment of filters. Dosing solution (0.2 mL) was added to the apical
compartment of the cell layers on transwell filters, and 0.8 ml fresh pre-
warmed
transport buffer was added to basolateral compartment. Timing was started for
transport, and at 5, 20, and 60 min after transport started, sample fluid (400
~,L)
was collected from the basolateral compartment. Fresh transport buffer (400
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
~.L) was added back to the basolateral compartment, and the fluid was
thoroughly mixed.
Transport samples, dosing solution, and standards(100 p,L) each were
mixed with 100 ~.1 of a 500 ng/ml internal standard (Lansoprazole-D) for LC-
MS/MS analysis, and part of each sample (100~,L) was vortexed and transferred
into glass LC-MS/MS vials for analysis.
Data Analysis
The apparent permeability coefficient (Papp, cm/sec), otherwise known
to herein as the membrane permeability, is determined from the following
relationship:
Papp = J/(ACo)
where J (pmol/min) is the transport rate, meaning the rate of prodrug movement
through the cell layer, A (cm2) is the filter surface area, and Co (p,M) is
the
initial dosing concentration.
The transport rate J, is calculated as the slope of the linear regression fit
for the
transport amount over time data using Microsoft Excel" 97 SR-2 (Microsoft
Corp. Redmond, WA),
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Reference Standard:
Lucifer yellow (LY) was used as a paracellular permeability reference
standard to determine integrity of cell layers used in the experiments. LY
transport in the apical to basolateral direction was carried out in the same
manner as described above. Fluorescence level in basoL ateral fluid sampled at
5, 20, and 60 min post dose was determined using Fluostar Galaxy (BMG
Labtechnologies, Durham, NC) at excitation/emission wavelengths of 485/520
nm. A standard curve covering the range from 0.002 to 0.5 mg/mL is
constructed to quantify the amount of LY in the transport sample to calculate
i0 permeability coefficient (Papp). Papp values below 1 x 10-6 cm/sec were
considered acceptable and were used to normalize Papp values for test articles
across experiments by multiplying the Papp values for tL-~e test articles by
the
factor x according to the following equation,
x = (1 x 10-6)/(S)
where S is the value of Papp obtained for LY.
Example 2
Oral bioavailability of omeprazole, lansoprazole~ pantoprazole,
2o rabeprazole, and test compounds was determined in rats (Sprague-Dawley) and
dogs (beagle) by administering an oral solution to the animal and collecting
serial blood samples through 24 hr post dose. Blood concentrations of the
compounds omeprazole, lansoprazole, pantoprazole, rabeprazole, and test
compounds were quantified using an achiral liquid chromatography tandem
mass spectrometry method (LC-MS/MS). Systemic pharinacokinetic parameters
were determined for omeprazole or lansoprazole using non-compartmental
analysis in Watson°' version 6.3, available from InnaPhase Corporation,
Philadelphia, PA. Results of the oral pharmacolcinetic stZtdies are presented
in
Tables 2A-2D below.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Table 2A. Systemic Omeprazole Half life in Rats
Compound Dosing Equivalent Systemic
Administered Route omeprazole omeprazole
dose (mg/k half life
) (hr)
Omeprazole Oral 10 0.31
1 Oral 10 1.7
Omeprazole Intravenous1 0.15
1 Intravenous1 0.18
Table 2A shows the systemic half life of omeprazole in rats after oral
and intravenous administration of omeprazole and compound 1. Surprisingly,
these results show that the systemic half life of omeprazole after intravenous
administration of omeprazole is nearly identical to that after intravenous
administration of the prodrug (compound 1). The prodrug was not detected in
the bloodstream 5 minutes after it was administered intravenously. These
unexpected results demonstrate that in the case of compound l, systemic
conversion of the prodrug to omeprazole does not take an appreciable amount of
time compared to the amount of time omeprazole is present systemically. By
contrast, slowed absorption of the prodrug from the gastrointestinal tract
into
the blood unexpectedly prolongs the systemic half-life of omeprazole to a
significant extent relative to both the intravenous and oral administration of
omeprazole. Table 2B shows a similar effect in dogs. Thus, these results show
that oral administration of a prodrug will increase the systemic half-life of
a
proton pump inhibitor. While not intending to limit the scope of the
invention,
results that will be discussed later, and which are presented in Table 2D,
indicate that a relationship may exist between the membrane permeability of
the
prodrug and the systemic half-life of the proton pump inhibitor.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Table 2B. Systemic Omeprazole Half life in Dogs
Compound Dosing Equivalent Systemic
Administered Route omeprazole omeprazole
dose (mg/kg) half life
(hr)
Omeprazole Oral 10 0.70
1 Oral 10 2.4
Omeprazole Intravenous1 0.60
1 Intravenous1 1.0
Table 2C summarizes the systemic half-lives of the prodrugs and the
PPIs for compounds 1-42 in dogs and rats. While not intending to be limited or
bound in any way by theory, these results demonstrate that slow absorption of
the prodrug from the gastrointestinal tract can contribute to an increase in
the
systemic half-life of the proton pump inhibitor. For many of the prodrugs in
the
table, the systemic half life of the prodrug (i.e. the intact prodrug
molecule) is
either very short relative to the systemic half-life of the proton pump
inhibitor,
or is so short that the intact prodrug cannot be detected in the blood, and
thus
the half-life cannot be detected (NC). By contrast, however, for many of these
same prodrugs, the measured systemic half life of the proton pump inhibitor is
significantly increased relative to the orally administered prodrug. Since the
hydrolysis of the prodrugs in the blood does not contribute significantly to
the
increased systemic half-life of the proton pump inhibitors, it follows that
the
absorption of the prodrug from the gastrointestinal tract is slowed
sufficiently to
prolong the systemic half-life of the proton pump inhibitor. Thus, while not
intending to be bound or limited in any way by theory, in the case of these
particular prodrugs, it is the absorption step rather than the hydrolysis step
that
is the rate-limiting step of the pharmacolcinetic process. In other words, the
gastrointestinal tract, rather than the bloodstream, acts as the depot for the
prodrug.
Table 2C. Systemic Half-Life of Prodrugs and PPIs in Dogs and Rats
Compound Do Rat
Tliz ProdrugTliz PPI Tliz ProdrugTliz PPI
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Omeprazole 0.696 (0.116) 0.308
1 NC 2.08 (1.19)NC 2.4
2 0.113 1.61
(n=1)
3 0.311 0.813 NC 1.76(0.93)
4 1.26 0.837 0.342 0.708 (0.479)
0.269 1.03 NC 1.7
6 0.303 1.91 NC 1.93 (0.39)
20 NC 2.70 (0.62)
21 NC 0.855 (0.143)1.51 (1.44)0.523 (0.338)
22 NC 3.89
23 NC 1.22 NC 2.72 (1.35)
24 1.37 NC 0.384
25 NC 1.03
26 1.19 0.881
27 0.117 1.10 NC 2.17 (0.53)
(n=1)
39 NC 1.50 (1.18)
40 NC 2.69 (0.76)
41 NC 0.761 (0.497)
42 0.521 1.47 (0.29)
Lansoprazole 0.573 (0.150) 0.510 (0.168)
7 0.206 0.893 NC 1.93 (1.41)
8 NC 1.08 NC 1.80 (1.20)
9 NC 0.894 NC 0.341 (0.151)
NC 0.989 (0.307)
11 NC 0.873 (0.288)NC 0.933 (1.009)
12 NC 0.931
13 0.122 1.77 NC 2.35 (1.22)
14 0.118 1.39 0.536 (0.217)
NC 0.923
16 NC 1.00 NC 1.86 (0.74)
17 1.49 1.13
18 0.0899 0.909
19 1.84 0.484
34 NC 1.11 (0.71)
35 NC 1.84 (0.87)
36 NC 0.389 (0.085)
37 NC 2.19 (0.80)
38 1.04 (0.35)1.43 (0.42)
Panto razole 0.743 0.696 (0.116)
28 NC 2.61 NC 1.45 (0.73)
29 ~ NC ~ 0.958 ~ NC 1 Ol (0
30)
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Rabe razole 0.369
30 1.12 0.491
31 0.843 0.855
32 0.526 1.52
33 0.746 0.894
Values in parenthesis indicate the standard deviation, when obtained.
NC: plasma concentration of prodrug was too low to calculate half-life, or
undetected.
The results in Table 2D demonstrate that apical to basolateral membrane
permeability correlates with the systemic half-life of a PPI after oral
administration of a PPI or a prodrug. They also demonstrate that apical to
basolateral membrane permeability is a good predictive test for how much a
given prodrug will increase the systemic half life of a PPI because the data
1o shows that decreasing the membrane permeability of a prodrug increases the
systemic half-life of the PPI. It should be noted that there is some scatter
in the
data, which is believed to be due to the relatively large random error in
determining the systemic half-life. However, Figure 1 is a plot that
graphically
demonstrates that despite the scatter, as a general trend, systemic half-life
of a
15 PPI resulting from oral administration of its prodrug increases with
decreasing
membrane permeability of the prodrug.
Table 2D. Membrane permeability of proton pump
inhibitors and their prodrugs, and their systemic half-life
2o in do s after their oral administration.
Compound Parent PPI Permeabilitytut (hours)
(x 10 cm/sec)
Ome razole - 13 0.70
1 Ome razole 0.12 2.4
2 Ome razole 0.054 1.6
3 Ome razole 0.38 0.81
4 Omeprazole 0.52 0.84
Ome razole 0.17 1.0
6 Ome razole 0.067 1.9
Lanso razole- 15 0.57
7 Lanso razole0.16 0.89
8 Lanso razole0.23 1.1
9 Lanso razole0.34 0.89
Example 3
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
To more completely understand the membrane permeability results
presented in the previous examples, transport across Caco-2 or MDRl-MDCK
cell layers in both the apical to basolateral (A to B) and basolateral to
apical (B
to A) directions was measured (n = 3 - 4) for compounds 1 and 6 using the
methods described in Example 1. Briefly, dosing solution containing the test
compounds at 10 ,uM was applied to either the apical or the basolateral side
of
the cell layer, while the receiver compartment was bathed in DMEM low
glucose medium (free of FBS) as the transport buffer. The cells were incubated
to at either 37°C or 4°C. At 5, 20, and 60 min post dose,
aliquots were sampled
from the receiver compartment. Each time after sampling, same volume of
fresh transport buffer was immediately added back to the receiver compartment
and mixed well with the remaining fluid.
Papp values in the A to B and B to A directions in Caco-2 and MDRl-
MDCI~ cells for the three different test compounds are listed in Table 3. In
Caco-2 cells, all compounds demonstrated preferential transport in the B to A
as
compared to the A to B direction at 37°C, corresponding to efflux into
intestinal
lumen in vivo. In other words, while not intending to be bound in any way by
theory, these results suggest that following absorption, the cells lining the
2o gastrointestinal tract are able to transport the compounds back into the
gastrointestinal tract lumen in the opposite direction of absorption.
When transport was measured for compound 1 in the P-gp over-
expressing cell line MDR1-MDCK cells, transport in the efflux direction was
not greater. While not intending to be bound in any way by theory, this result
suggests P-gp was not involved in the efflux of the prodrugs in Caco-2 cells.
Thus, while not intending to be bound in any way by theory, as shown in
Example 4, it appears that the activity of MRP2 and other transporter proteins
are responsible for the preferred transport of the compounds in the efflux
direction, and consequently, contributes to the reduced absorption rate of the
prodrugs and the increased systemic half life of the PPIs observed when
prodrugs are administered orally.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Caco-2 MDR1-MDCK
cells cells
Ratio Ratio
CompoundPapp Papp Papp Papp Papp Papp
AB BA AB BA
(cm/sec (cm/sec BA / (cm/sec (cm/secBA /
x10'5) x10'S) Papp x10'5) x10'5) Papp
AB AB
OME
1,26 1.63 1.29 2.60 1.71 1.52
1 0.0165 0.0415 2.52 2.39 1.61 0.67
6 0.0172 0.144 8.37 - - -
Table 3. Permeability coefficient estimation for omeprazole, compound 1, and
compound 6 across Caco-2 and MDR1-MI~CK cells.
Example 4
Modulating reagents for the multidrug resistant-associated MRP protein
were tested in vitro to determine their effect upon transport of compound 1 in
Caco-1 cells. In this experiment, dosing solution containing 10 ~.M of
to compound 1 was applied to the basolateral compartment of Caco-2 cell layers
(n=4) in the presence and absence of MK-571 (50 ~,M) and glutathione (250
~.M) in the apical compartment. MK-571 is a known specific inhibitor for the
MRP family [Walgren RA, Karnaky KJ Jr, Lindenmayer GE, and Walle T.
Efflux of dietary flavonoid quercetin 4'-beta-glucoside across human
intestinal
Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. J
Pharmacol Exp Ther 2000; 294:830-6] whereas reduced glutathione (GSH) has
been shown to stimulate MRP transport activity [Van Aubel RA, Koenderink
JB, Peters JG, Van Os CH, and Russel FG. Mechanisms and interaction of
vinblastine and reduced glutathione transport in membrane vesicles by the
2o rabbit multidrug resistance protein MRP2 expressed in insect cells. Mol
Pharmacol 1999; 56:714-9]. The cells were incubated at 37 °C. At 5, 20,
and 60
minutes postdose, samples were taken from the apical chamber.
As shown in Figure 2, in the presence of MK-571 in the apical
compartment, transport in the B to A direction was significantly reduced
compared to control (Papp = 5.21 ~ 0.50 x 10-6 cm/sec versus control PaPp =
8.17 ~ 0.38 x 10-6 cm/sec, p<0.001). On the other hand, presence of
glutathione increased B to A transport by over 60% (Papp = 13.0 ~ 1.7 x 10'6
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
cm/sec, p<0.002). While not intending to be bound in any way by theory, the
inhibition and enhancement of compound 1 B to A transport by MK-571 and
GSH respectively suggest that compound 1 is a substrate of MRP2 and is
transported by MRP2 in the basolateral to apical direction. While not
intending
to be limit the invention in any way, or be bound by theory, the in vivo
implication of this finding is that MRP2 or other transporter proteins
expressed
at the luminal membrane of intestinal epithelial cells may efflux compound 1
in
the export direction. Thus, while not intending to be bound by theory, as
compound 1 is continuously absorbed and effluxed in the GI tract, its
residence
l0 time is effectively prolonged and its absorption time window expanded.
Further, while not intending to be bound by theory, or limit the scope of the
invention in any way, these results suggest that MRP2 modulators or
modulators for other transporter proteins may be used to modify the rate the
prodrugs are effluxed, thus modifying the absorption time window, as well as
15 the maximum concentration and the plasma half-life of the PPI.
Example 5
In Vivo Pharmacolunetic Study on compound 6:
While not intending to limit the scope of the invention, or be bound in
any way by theory, the following in vivo study demonstrates how MRP2
modulators can be used to alter the systemic half life of a PPI after oral
administration of a prodrug. Male Sprague-Dawley rats (200-220 g) cannulated
in both jugular and femoral veins were purchased from Charles River
Laboratories (Wilmington, MA). Two treatment groups with nine animals per
group were dosed orally with 16 mg/kg compound 6 in a solution. One group
of animals were co-administered an oral dose of 10 mg/kg MIA-571 in a
solution. Blood samples were collected at 5, 10, 20, 4.0 minutes, 1, 2, 4, 6
and 8
hours post dosing, mixed with five volumes of acetonitrile, and stored frozen
at
below -70°C until bioanalysis.
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
Both in vitro transport and in vivo blood samples were analyzed by LC-
MS/MS for concentration of omeprazole and the prodrugs, with detection range
of 1-1000 ng/mL. Deuterated omeprazole served as the internal standard.
Figure 3 shows mean blood omeprazole concentration following dosing.
With MIA-571 co-administration, maximum blood concentration of omeprazole
was significantly increased, as would be expected if efflux of its prodrug is
inhibited in the GI lumen by an inhibitor of MRP2. Interestingly, systemic
half-
life of omeprazole was shortened significantly also. The numeric results of
this
experiment are compiled in Table 5. Thus while not intending to be bound in
l0 any way by theory, this result indicates that the efflux of a prodrug may
contribute to the prolonged oral half life of a parent proton pump inhibitor
following oral administration of the prodrugs by prolonging the GI residence
time of the prodrug. While not intending to be bound in any way by theory, or
limit the scope of the invention in any way, this result also demonstrates
MRPZ
modulators or modulators for other transporter proteins involved in efflux can
be used to alter the pharmacolcinetic profile of a proton pump inhibitor.
ALTCo_~ Cmax (ng/mL) tll2 (hr)
(n hr/mL)
Control 56.2 17.021.8 12.4 1.64 0.44
+MK-571 81.039.8 43.023.9* 1.170.41*
Table 5. Estimated pharmacokinetic parameters for omeprazole following oral
administration of its prodrug compound 6 with and without co-administration of
MK-571 (n = 9). "'~" denotes statistically significant difference (p<0.05).
While not intending to limit the scope of the invention in any way, the
results presented herein suggest that there are a number of ways that MRP2
modulators or modulators for other transporter proteins could be used to
modify
the pharmacolcinetics to improve the properties, depending upon the
circumstances. For example, adding a compound which stimulates MRP2
activity or activity of other transporter proteins involved in efflux could be
used
to increase the plasma half life of a proton pump inhibitor by oral
administration
3o with a prodrug. Thus, if a particular prodrug has desirable physicochemical
properties, but slower absorption is desired, a compound such as glutathione
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
could be administered either separately, or in a single composition with the
prodrug. A compound which stimulates MRP2 activity or the activity of other
transporter proteins involved in efflux may also be used to improve sustained
release of a proton pump inhibitor when it is administered orally, and not as
the
prodrug. Alternatively, a compound which inhibits MRP2 activity or activity of
other transporter proteins involved in efflux may be used to provide rapid
onset
of action and increase the bioavailability of the PPI when used in conjunction
with a prodrug that is absorbed more slowly than is desired. This may be
useful
in providing a faster acting dosage form.
While not intending to limit the scope of the invention in any way, a
non-obvious use of a compound such as MK-571, which inhibits MRP2
activity, would be to enhance sustained release of a proton pump inhibitor by
oral administration of a prodrug. For example, Figure 3 shows that the
concentration of the PPI in the blood is higher from about 0-2 hours when MK-
571 is administered with the compound 6 as compared to when compound 6 is
administered alone. While not intending to be bound by theory, it is believed
that the reduced efflux allows greater systemic absorption of the prodrug,
thus
producing a higher plasma concentration of the PPI. However, the inhibition of
the efflux activity need not occur when the prodrug is administered. For
example, in the case of compound 6, if MK-571 is administered about 2 hours
after the prodrug is administered, one may expect that the absorption would
increase, thus increasing the amount of PPI in the blood for as long as the
prodrug is available for absorption. Thus, the total bioavailability of the
prodrug might be increased, and the uneven control of stomach pH which is
associated with PPI use might be improved. Alternatively, a dosage form might
be formulated which allows delayed release of the MRP inhibitor. Thus, both
the inhibitors and stimulators of MRPZ or other transporter proteins involved
in
efflux are useful in improving the pharmacokinetic properties of PPIs and
their
prodrugs through a large variety of compositions and methods.
Example 6
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
The physicochemical properties of compound 1 were analyzed.
Compound 1 was found to be hygroscopic, in that 9~/o weight gain was observed
for the compound after 14 days of storage at 25 °C at 75% relative
humidity.
Table 3A. Solubility Profile of Compound
lat 25 °C in Buffered Aaueous Solutions
pH Buffer CompositionSolubility
(m mL)
1 0.1 M HCl 1.g
3 Citric Acid (0.10.4
M)/
NazHP04 (0.2
M)
5 Citric Acid (0.1>50
M)
/Na2HP04 (0.2
M)
7 sodium phosphate>50
(0.1 -
0.2 M)
9 sodium phosphate>50
(0.1 -
0.2 M)
The solubility profile of compound 1 in at various pH values is
1o presented in Table 3A. This data shows that the aqueous solubility of the
compound is significantly enhanced at around pH 5. While not intending to be
bound in any way by theory, it is believed that this improvement in solubility
is
due to the deprotonation of a sufficient quantity of the acid. While not
intending to be bound in any way by theory, this suggests that the prodrug
should be significantly easier to formulate, particularly in the case of
liquid
dosage forms, when the pH is around 5 or higher.
Table 3A. Stability Profile of Compound 1 at 25 °C in Buffered
Adueous Solutions
Half-life Degradation
Buffer Shelf life
pH Composition (tl~~) (t9o~o) Rate Constant
hours
hours (k) 1/hours
1 0.1 M HCl 3.6 0.5 0.194
3 Citric Acid 7g,p 11.9 0.009
(o.l
M)/ Na2HP04
(0.2
M)
5 Citric Acid 89.2 ~ 13.6 0.008
(0.1 M)
/Na2HP04 (0.2
M)
7 sodium phosphate286.8 43.6 0.002
(0.1 - 0.2
M)
7,4 sodium phosphate291.2 44.3 0.002
(0.1 - 0.2 .
M)
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
9 sodium phosphate23.0 3.5 0.030
(0.1 - 0.2
M)
sodium phosphate2,3 0.4 0.298
(0.1 - 0.2
M)
The aqueous stability data of compound 1 is presented in Table 3B.
These results show that, the half-life (tl,~), the shelf-life (t9o%a), and the
rate
constant for degradation (k) for compound 1 are significantly improved in the
5 pH range of 3-9. While not intending to be bound in any way by theory, these
results suggest that formulation of dosage forms in the pH range of from 3 to
9
should greatly improve the stability of the prodrugs, thus improving shelf-
life
and facilitating formulation. Further, these results suggest that dosage forms
having a pH from 6 to 8 will be particularly useful in certain situations.
to Additionally, these results demonstrate that the prodrugs are
significantly more stable in acidic and neutral aqueous solutions than the
proton
pump inhibitors. The stability of omeprazole and other proton pump inhibitors
have been reported (Kromer et al., "Differences in pH-Dependent Activation
Rates of Substituted Benzimidazoles and Biological in vitro Correlates",
Pharmacology 1998; 56:57-70; and Ekpe et al, "Effect of Various Salts on the
Stability of Lansoprazole, Omeprazole, and Pantoprazole as Determined by
High Performance Liquid Chromatograpy", Drug Development and Industrial
Pharmacy, 25(9), 1057-1065 (1999)), and while the stability is somewhat buffer
dependent, typical half lives for omeprazole are about 1 hour at pH 5 and
about
40 hours at pH 7, which is about 1-2 orders of magnitude shorter than the
prodrug half lives presented in Table 3A. This instability of the proton pump
inhibitors has generally necessitated their formulation in enterically-coated
dosage forms. Thus, while not intending to limit the scope of the invention in
any way, or to be bound in any way by theory, these results suggest that the
prodrugs disclosed herein have sufficient stability to allow the
gastrointestinal
tract to act as a depot for the prodrug, and also have sufficient stability
that the
use of enteric coatings is not necessary for effective formulation of a dosage
form.
3o Example 7
CA 02559127 2006-09-08
WO 2005/089758 PCT/US2005/007015
A capsule containing 40 mg of compound 6 is administered to a patient
suffering from heartburn. Two hours later, a capsule containing 40 mg of MK-
571 is administered to the same patient. This therapy is repeated daily, and
relief from heartburn is experienced for as long as the therapy continues.
Example 8
Compound 1 (60 mg) and MK-571 (40 mg) are dissolved by stirring into
l0 5 rnL of water at 50 °C. The solution is allowed to cool to room
temperature,
and the entire volume of solution is administered to a patient suffering from
heartburn. This procedure is repeated daily, and relief from heartburn is
experienced for as long as the therapy continues.
Example 9
A capsule containing compound 6 (60 mg) and glutathione (200 mg) is
administered to a patient having an ulcer. This therapy is repeated daily, and
relief from symptoms is experienced for as long as the therapy continues.