Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02562655 2006-10-05
METHOD AND APPARATUS FOR NON-INVASIVE RINSE DIAGNOSIS
AND MONITORING OF PERIODONTAL DISEASES
SCOPE OF THE INVENTION
The present invention relates to a quick, easy-to-use, non-invasive diagnostic
test for
diagnosing and/or monitoring the severity of periodontal diseases in human
patients.
BACKGROUND OF THE INVENTION
Screening for periodontal diseases has heretofore required regular visits to
the dentist in
order for a regular examination to be carried out. Periodontal diseases are
inflammatory
conditions that result in loss of the bone, gingiva and ligament that support
the teeth. The
destruction of the tooth supporting tissues (periodontium) occurs as a result
of collateral damage
caused by enzymes released by specialized white blood cells called neutrophils
as they attempt to
contain the bacterial infection. Periodontal diseases are one of the most
prevalent diseases
occurring in man, with between 70 and 90% of the population experiencing this
disease during
their lifetime.
Typically, diagnosis of the severity of periodontal diseases are determined by
periodic
professional dental examination of the amount of lost bone, ligament and
gingival tissues. This
examination requires the insertion of a thin metal probe under the gum tissues
surrounding the
teeth. The depth to which to probe extends is noted indicating the degree of
"detachment" and
loss of the supporting tissues around the teeth. A key measure is the degree
of bleeding that
occurs following probe insertion which indicates the degree of inflammation
and ongoing
disease. This bleeding provides a crude quantitative measure of disease level.
Many studies have
shown that the degree of bleeding is the most accurate predictor of future
periodontal tissue loss
around a given tooth.
CA 02562655 2006-10-05
2
There are also biochemical tests that have been developed to identify enzymes
that are
released by cells of the periodontium and the immune system into the oral
cavity. However these
tests require specialized equipment and training to carry out.
Since periodontal diseases usually do not cause pain, patients will often not
be aware that
there is any active disease occurring in their mouths. Some may notice
occasional bleeding when
they brush their teeth but most choose to ignore this as the bleeding is often
transient in nature.
Earlier studies have correlated the presence of neutrophils entering into the
mouth through the
gingival crevice surrounding the teeth in the crevicular fluid as a possible
measure of oral
inflammation. Currently, microscopes are sold to dentists to enable them to
visually count or
quantify neutrophils taken from samples around teeth.
SUMMARY OF THE INVENTION
A significant problem in society is rapidly and inexpensively identifying
geriatric and
institutionalized patients in need of dental care. This is a significant
healthcare issue as recent
studies have identified significant links between periodontal diseases and
1)cardiovascular
diseases including heart attacks and strokes, 2) diabetes and 3) aspiration
pneumonia. Being able
to identify periodontal diseases in institutionalized elderly patients using a
rapid non-invasive
test that can be administered by non-specialized staff has the potential to
have a significant
impact on this population and the healthcare system.
The inventor has proposed an earlier method of quantifying oral mucosal
neutrophils to
detect a likelihood of periodontal diseases. In particular, neutrophils are
collected with an oral
saline rinse, stained with acridine orange and counted using fluorescence
microscopy. In the
inventor's earlier studies comparing a control group of healthy patients, and
a group of
individuals with moderate and severe chronic periodontal diseases, oral
neutrophil counts were
compared together with the degree of periodontal disease and oral
inflammation. The inventor
has appreciated that oral neutrophil counts may be used to provide an
excellent measure of not
CA 02562655 2006-10-05
3
only identifying patients with periodontal disease in need of treatment, but
also as a means of
monitoring the elimination of infection and the effectiveness of any remedial
treatments.
It is clear based on the available knowledge relating to neutrophil kinetics
in the human
organism, that access to a non-invasive, simple, quick colourimetric, test
capable of being self
administered by outpatients and by non-skilled personnel will readily benefit
patients by helping
identify those in need of treatment. The inventor's conclusions have led to an
investigation into
the development of a rapid test based on a colourimetic assay employing a
neutrophil-specific
enzymatic reaction to assess their presence of periodontal diseases.
Accordingly, an object of the invention is to provide an assay test for
assessing the
presence of periodontal disease which is simple enough to enable non-
specialized health care
workers or even patients to self-administer the test, eliminating the need for
dentist visits and/or
time-consuming microscopic counting of neutrophils.
A further object is to provide a simplified kit for the identification of
periodontal diseases
which uses a colourimetric change reaction in the rinse solution, such as a
colour change, a
colour intensity change, or other suitable visual indicator. The visual
indicator may then be
manually or electronically compared to a predetermined standard colour or
colour chart relating
the solution colour to the number of neutrophils present in the sample and the
level of oral
inflammation present in the patient's mouth.
In another aspect the applicant has proposed a rapid non-invasive diagnostic
test to
quantify neutrophils and/or oral inflammation in the mouth.
In one possible embodiment, a 15 to 60 second and more preferably a 30 second
mouth
rinse sample is obtained. A diagnostic reagent is added to the sample which is
colour reactive
with neutrophils. A resulting colour change is observed preferably in as
little as 5 to 10 seconds,
and more preferably in about 60 seconds which indicates a number of white
blood cells present
CA 02562655 2006-10-05
4
in the rinse based on a standardized scale. The detected oral neutrophil
levels may be then used
to correlate with the severity of the periodontal disease/oral inflammation
present in the patient.
In a more preferable assay, the patient may first pre-rinse with a cleansing
solution such
as water or an alcohol or antiseptic based solution, to effectively sterilize
the oral cavity prior to
obtaining the mouth rinse sample.
In an alternate embodiment, the present invention employs an assay test using
a modified
mouth rinse sample collection procedure. The test involves collecting a mouth
rinse sample from
a patient. A diagnostic reagent that specifically reacts with white blood cell
specific enzymes
and results in a colour change n the rinse soluton is then delivered to the
rinse sample
immediately after sample collection. Suitable diagnostic reagents contain a
diammonium salt that
are reactive with neutrophil enzymes which create a visually discernable
colour change in the
solution after a selected period of time. Most preferably, the reagents are
selected to produce a
colour change in between about 3 and 120 seconds, and most preferably about 60
seconds. At 60
seconds a detergent is added to stop and fix the colour reaction. The
intensity of the colour
reaction may be measured by reflectance or absorbance, or due to the large
colour range by eye
when comparing the sample to a standardized colour chart/swatch that
correlates with
predetermined neutrophil concentrations and/or periodontal disease levels.
The correlation of the colour changes and/or changing colour intensity are
compared to
standards that relate observed colour intensity levels to neutrophils numbers
present in samples
with varying oral/periodontal disease levels. The colour chart correlations
may be supplied on
printed labels, card and or instruction materials as part of an assay kit
supplied to dentists, or
patients/health care workers to facilitate the self-administration of the
test, and enhance
understanding of the test results.
Accordingly, in one aspect the present invention resides in an in vitro method
for
identifying the presence of periodontal diseases in a human patient's mouth
comprising the steps
of:
CA 02562655 2006-10-05
obtaining an oral rinse sample from said patient's mouth;
adding a colourimetric reagent to the rinse sample;
admixing the oral rinse sample and the colourimetric reagent to form a mixed
solution;
and
comparing a colour intensity or shade of the mixed solution with at least one
predetermined colour standard representative of selected number of human
neutrophils in the
mixed solution.
In another aspect, the present invention resides in an assay test for the in
vitro
identification of the presence of periodontal diseases in a human patient,
said assay test
including;
a volume of sampling solution for use in obtaining an oral rinse sample;
a receptacle for obtaining a volume of said oral rinse sample expectorated by
said patent;
a colourimetric reagent for addition to the rinse sample, the diagnostic
reagent comprises
a lysic agent selected to lyse neutrophils in said oral rinse sample, a buffer
for regulating the pH
of the lysed solution and a neutrophil reactant salt; and
at least one predetermined colour standards representative of selected numbers
of human
neutrophils in the solution.
In yet a further aspect, the present invention resides in a method for
identifying the
presence of periodontal diseases in a human patient's mouth comprising the
steps of (a)
obtaining an oral rinse sample from said patient's mouth by swishing a
measured quantity of a
saline/bicarbonate buffer mouthwash around said mouth for a timed interval of
about 30 second;
(b) adding a measured quantity of a diagnostic colourimetric reagent to the
rinse sample; (c)
agitating and/or inverting the solution at least once to mix; (d) comparing
the colour and/or
CA 02562655 2006-10-05
6
intensity of the colour of the rinse sample with a chart of predetermined
colour standards that
correlate with a predetermined number of human neutrophils in the sample.
BRIEF DESCRIPTION OF THE DRAWINGS
Reference may now be had to the following detailed description, taken together
with the
accompanying drawings in which:
Figure 1 shows schematically an oral rinse sample container having affixed
thereto a
colour intensity chart correlating the average neutrophil levels in patients
with healthy mouths
and those with moderate and severe periodontal disease;
Figure 2 shows a comparison of resultant sample colours utilizing an ABTS
diammonium
salt as a neutrophil reactive reagent with predetermined colour charts in
accordance with the
preferred embodiment of the invention;
Figure 3 shows graphically luminosity histograms of control samples showing
the
luminosity of differing neutrophil levels;
Figure 4 shows a sample predetermined colour scale which is representative of
neutrophil
levels in the patient, used to provide an indication of a likelihood of
periodontal disease/oral
infections in a patient;
Figure 5 illustrates the colour/neutrophil concentration relationship using an
ABTS
diammonium salt as a reagent;
Figure 6 illustrates graphically mucosal neutrophil concentrations in clinical
subjects over
time (from day to day);
CA 02562655 2006-10-05
7
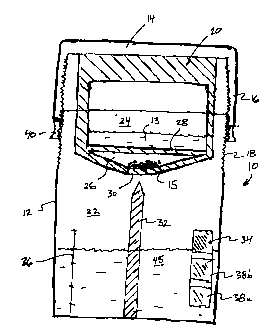
Figure 7 illustrates schematically an assay kit for use in the home assay of
neutrophil
levels in the diagnosis of periodontal disease, in accordance with a preferred
embodiment of the
invention.
Figure 8 illustrates the assay kit of Figure 8 when activated in use; and
Figure 9 illustrates graphically the luminosity of the neutrophil levels
between control
patients and those exhibiting periodontal disease.
DETAILED DESCRIPTION OF THE INVENTION
The present invention resides in a method of quantifying neutrophil levels in
the oral
cavity of a patient as an accurate indicator of the existence and/or severity
of a periodontal
disease in a patient's mouth, and consequently of the need for dental
treatment.
In a first embodiment, the present invention provides a method and rinse for
use in
determining neutrophil concentrations in a patient's mouth as a means of
identifying the
presence of periodontal disease. Preferably, the patientfirst pre-rinses his
or her mouth with a
cleansing solution to clear debris and any excess neutrophils which may be
residually present.
Suitable cleansing solutions would include tap water, distilled or purified
water, as well as
alcohol and/or antiseptic based mouthwashes. Most preferably, the patient pre-
rinses with
between about 3 and 20 ml, and preferably about 5 ml of a sterile saline
mouthwash (0.9%)
solution for 10 to 30 seconds to effectively clear debris.
Within 10 minutes, and most preferably substantially immediately following pre-
rinsing,
the patient undertakes a second oral rinse to obtain an assay sample. The
second oral rinse
involves rinsing between about 3 and 20 ml., and preferably about 5 ml of a
sterile saline
mouthwash (0.9%) for 30 seconds.
CA 02562655 2006-10-05
8
Following the collection of the assay sample, a colourimetric indicator
solution is added
to the rinse after collection in a container/mixing tube. Suitable indicator
solutions include
diamonium salts in preferred concentrations of between about 200 mg to 500
mg/100m1 together
with a lysic agent such as 0.5 to 5% anionic detergent selected to lyse
neutrophils in the assay
sample. Optionally, hydrogen peroxide 100 to 500 ul/100ml and/or a buffer may
also be
provided for regulating the pH of the lysed solution. Suitable buffers would
include citrate based
buffers. The sample mixture is agitated and/or inverted at least once, and
preferably twice to
thoroughly mix whereby the reaction between the salt and the neutrophils
produces a resultant
change in colour and/or intensity. Sodium dodecyl sulphate can be added after
reaction
(approximately 60 seconds) to allow for stabilization of the solution colour.
The intensity of the
resultant sample colour is thereafter compared to the provided colour scale
34, as for example, is
shown in Figure 1.
The colourimetric indicator most preferably contains: a) an anionic detergent
selected to
lyse the cells and expose the characteristic human neutrophil enzyme, such as
those sold as
Triton X-100T"' or NP-40T""); b) a buffer to correct the pH of the lysed cell
solution (ie. Sodium
citrate); and c) a neutrophil reactant salt which acts as a reagent that
reacts with
myeloperoxidase, an enzyme found in neutrophils, to form a green-blue colour
(2,2'-Azino-
bis(3-ethylbenzo-thiazoline-6-sulfonic acid).
(ABTS) salt is most preferred as it serves as a substrate for myeloperoxidase,
and one of
the reaction's products is chromogenic, producing a light blue-green colour
which is visible to
the human eye. Other possible examples of salts could, however, also be used
including, without
restriction: 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)diammonium
salt; 2,7-
diaminofluorene, 3,3',5,5'-tetramethylbenzidine and its dihydrochloride salt;
5-aminosalicylic
acid, o-phenylenediamine and its dihydrochloride salt; 5-amino-2,3-dihydro-1,4-
phthalazinedione, 3-amino-9-ethylcarbazole, 4-chloro-l-naphthol, 3,3'-
diaminobenzidine, o-
dianisidine and its dihydrochloride salt, guaiacol and pyrogallol.
CA 02562655 2006-10-05
9
The present invention satisfies the need to provide a simple test for oral
neutrophils to
provide an indication of periodontal disease, and which can be used outside as
well as inside a
hospital/clinical setting. The test can also be administered by the patient
him/herself, by patient,
family members, non-specialist primary care physicians, dental office
hygienist assistants, and/or
private duty nurses caring for patients in the home.
Furthermore, this test can be used to easily monitor and/or map any subsequent
changes
of a patient's oral health status, to enable more rapid and frequent screening
and diagnosis.
The assay test employs an indicator having a novel composition in solid or
solution form. The kit
includes a container 12 (Figure 7) which may optionally include two
compartments which are
used to maintain one or more components of the solution and/or reagent
discrete and apart prior
to use. As will be described, in one preferred construction, ABTS is
maintained in a solid form
separately from the remaining components of the indication which may be solid,
but more
preferably which are housed in liquid form (ie. detergent, citrate buffer, and
hydrogen peroxide).
Immediately prior to use the powder is mixed with the liquid solution to form
an active
colourmetric indicator.
a) Determination Of Colour Scale (independent of a patient's age, sex and
systemic health
status)
To produce the colour scale 34, the inventor has first developed a set
protocol. This
protocol set rules for several parameters, including the conditions for taking
the photographs of
the oral rinse samples. Lighting conditions, background, aperture size and
exposure had to be
standardized in order to obtain consistent results. Furthermore, the
quantities of neutrophils for
the samples were, after experimentation, set to 1, 3, 6 and 12 million per
sample. These were
selected to correspond to a realistic range of neutrophil quantities in
patients with various levels
of periodontal disease severity (see Figure 1).
After the establishment of this protocol, neutrophils were isolated from human
blood and
used to obtain multiple samples and photographed using digital photography.
CA 02562655 2006-10-05
The digital photographs were then cropped as for example shown and analyzed
using
image analysis software. The resultant luminosity histograms were compared for
the different
samples to further establish the consistency of the method. As can be seen
from Figure 2,
successive samples with the same approximate number of neutrophils have the
same dominant
color. In other words, Figure 2 shows peaks, from left to right, which
symbolize samples with
decreasing concentrations of neutrophils. The lighter blue colors have higher
luminosity values,
and the peaks at 255 represent white - total absence of neutrophils.
From the test criteria, the inventor has proposed the production of a single
color scale
shown in Figure 3.
b) Quantitative Correlation
To obtain a quantitative relationship between the color of the oral rinse and
the quantity
of neutrophils, a calibration curve was constructed, with the reflectance
percentage as a function
of the quantity of neutrophils. To accomplish the above, the color range of
neutrophil dilutions
was compared to the reflectance values.
The equation of the standard curve as shown in Figure 4 is calculated by
equation 4.1 as
follows:
(4.1) y = -0.0815Ln(x) + 0.6869
In equation (4.1), "y" stands for the percentage reflectance of the solution
and the "x"
stands for the quantity of neutrophils in the sample. The symbol "Ln(x)" as
used in the equation
represents the natural logarithm of "x" - logarithm with base e. Although the
relationship
between the concentration of neutrophils and the reflectance is an inverse
one, the most accurate
representation was found to be logarithmic. This was so due to the fact that
in a real experimental
situation, the reflectance would never be 100%, but rather would approach the
100% value
CA 02562655 2006-10-05
11
asymptotically. The reflectance can be read in a spectrometer to obtain
numeric values. This
would facilitate research but would have minimal benefit in a purely clinical
setting.
c) Establishment Of A Reproducibility of day to day measurements in a given
patient
Using the sample collection method described earlier, the measurement
methodology
established in Example 1, and the ABTS solution, the day to day
reproducibility of an
individuals oral mucosal neutrophil levels was established. In the control
population, the
mouthwash samples were obtained at exactly the same time of day on five
separate days over a
time period spanning four weeks. Each collection was evenly spaced one week
after the
previous collection. Figure 5 below shows the measured reflectance results for
each test day for
the control population.
The individual lines in the study (Figure 5) show a high degree of
reproducibility in
mucosal neutrophil concentration for each individual.
d) Clinical Example
To provide an example of the in depth luminosity analysis, the previously
established
methodology was employed to analyze the oral neutrophil levels of 4 patients.
After
photographing their oral rinses, the data was analyzed to produce Figure 9.
As can be seen from Figure 9, participants 1 and 2 (PT 1 and PT 2) have fewer
than 1
million neutrophils in their rinses, indicating a healthy oral cavity. This
was subsequently
confirmed by a dental examination. The oral rinse supplied by Participant 3
has fewer than 3
million neutrophils in the sample rinse. This neutrophil activity level also
suggested a healthy
individual, and minor gingivitis was confirmed in the patient by a dental
examination. On the
other hand, the oral rinse provided by participant 4 revealed elevated levels
of neutrophil of a
somewhere in the range between 6 and 12 million in each sample rinse. These
levels suggested
that the patient has periodontal disease or oral infection and should be
referred to a dental
CA 02562655 2006-10-05
12
practitioner for further treatment. Subsequent dental examination confirmed
active periodontal
infections in participant 4, resulting in loose teeth and alveolar bone loss.
Although digital analysis of the luminosity of the sample was undertaken for
the clinical
example study, it is to be appreciated that this is not, in fact, essential.
In a simplified test, it is
possible to simply compare the rinse colour or colour intensity to provide
standardized color
swatch with the unaided eye, to arrive at a diagnosis in a similar manner.
Overall, the studies support the claim that a patient's oral neutrophil levels
tend to
maintain at a stable level, unless the state of the patient's oral health
changes. Furthermore, the
studies show that a calibration curve can be constructed, quantitatively tying
color indicators,
such as colour shade and/or colour intensity, to a patient's oral mucosal
neutrophil concentration.
e) Assay Kit For Home Use
Reference may be had to Figures 7 and 8 which illustrate schematically a
single use in-
home assay kit 10 for use in the diagnosis and/or monitoringof periodontal
diseases.
The single assay test kit 10 is used with a single serving colourimetric
indicator solution
is prepared which includes as a diamonium salt, 2,2'-Azino-bis (3-ethylbenzo-
thiazoline-6-
sulfonic acid) (ABTS) diammonium salt; in a concentration of 3.2 to 3.6 mg/ml,
a detergent (1%
Triton X-100), 30% hydrogen peroxide (200 ul/100 ml) and sodium citrate buffer
(380 mM) in a
recipe as follows:
Single Serving Recipe
188.7 L-10% Triton (1.88%)
377.3 L-1 M Citrate Buffer (377 mM)
L H2O2
431.4 L H2O
3.6 mg-ABTS powder
.... as a liquid component 13
CA 02562655 2006-10-05
13
3.6 mg-ABTS powder
.... as a solid component 15
The assay kit 10 includes a transparent and most preferably clear plastic
container 12 and
a cap member 14. As shown best in Figure 2, the cap member 14 is provided with
an internally
threaded peripheral flange 16 which is configured for threaded engagement with
an externally
threaded upper edge portion 18 of the container 12. As shown best in Figure 7,
a two-part
chamber 20 is mounted to the underside of the cap member 14. The chamber 20 is
sized and
positioned for partial insertion into the container interior 22 as the cap
member 14 is secured in
place. The chamber 20 is used to house the colourmetric indicator solution
used in assaying
neutrophil concentrations in two-part form. The chamber 20 includes an
uppermost liquid cavity
24 which houses the liquid component 13 and a lowermost solid reagent cavity
26 which houses
the solid component 15. As will be described, prior to the use of the assay
kit 10, the solid cavity
26 is maintained separate and discrete from the liquid cavity 24 by a
detachable seal member 28
which delineates the bottom of the liquid cavity 24 from the top of the solid
cavity 26. The
bottom of the solid cavity 26 is furthermore initially sealed by a detachable
septum 30.
Figure 7 shows best the container 12 as including a bayonet member 32. The
bayonet
member 32 is secured to the bottom of the container 12 and projects axially
upwardly therefrom
so as to be selectively engageable with both the septum 30 and seal member 28
as the cap
member 14 is tightened downwardly onto the container 12.
Optionally, a colour scale 34 and graduation markings 36 may be provided along
the
outside of the container 12. The colour scale 34 is provided with a number of
pre-printed colour
slides 38a,38b,38c which represent resulting colours produced by the reaction
of predetermined
threshold members of neutrophils with a colourmetric indicator solution formed
from mixing the
dry solid component 15 contained in the solid cavity 26 with the liquid
component 13 contained
in the liquid cavity 24. As shown best in Figure 7, when initially purchased,
the container 12 of
the assay kit 10 is precharged with a preselected volume and preferably
approximately 5 ml of a
sterile saline mouthwash solution (0.9% salt). The two-part chamber 20 of the
cap member 14 is
furthermore preloaded with the indicator solution in two-part form and
including 3.2-3.6glml of
CA 02562655 2006-10-05
14
ABTS powder as the solid reactive component 15. The liquid cavity 24 is
similarly preloaded
with a liquid solution of Triton X- 100 as a detergent, hydrogen peroxide and
sodium citrate
buffer as the liquid component 13. A sodium dodecyl sulphate powder/tablet
(between 1 and 50
mg but preferably 25mg) is provided as an optional stop reagent to be added
after 60 seconds of
colour reaction to stabilize the colour change if the rinse is to be
preserved/colour reaction needs
to be saved for any reason. At the time of initial purchase of the assay kit
10, the cap member 14
is secured to the container 12 so that only the lowermost peripheral threaded
edge of the flange
16 engages the uppermost portion of the externally threaded edge 18 of the
container.
Optionally, a detachable locking ring 40 (Figure 7) may be provided to prevent
unintentional
tightening of the cap member 14 onto the container.
In use of the assay kit 10, the patient initially pre-rinses with either tap
water, or more
preferably, distilled water for a period of about 30 to 60 seconds. Following
a predetermined
period of time which preferably is selected at between about 5 seconds and 5
minutes, the user
activates the assay kit 10 by first fully detaching the cap member 14 so as to
break and remove
the locking ring 40. With the cap member 14 removed, the user then rinses with
the sterile saline
mouthwash solution 45 for a period of approximately 10 to 30 seconds.
Following rinsing, the
solution is expectorated back into the container 12 as an oral rinse solution
45' ensuring that a
desired volume of fluid is achieved according to the graduated marking 36. The
cap member 14
is then immediately repositioned onto the container 12 and fully tightened by
the engagement of
the flange 16 with the threaded edge 18 to the position shown in Figure 8. As
the cap member
14 is moved further onto the container 12, the bayonet member 32 is moved
initially into bearing
contact with the septum 30. Initially, engagement with the bayonet member 32
results in the
detachment of the septum 30 allowing the solid component 15 to move under
gravity into the
oral rinse solution 45'. Continued tightening of the cap member 14 results in
the bayonet
member 32 next engaging and detaching the seal member 28. The detachment of
the seal
member 28 enables liquid component 13 to flow downwardly first into and
through the solid
cavity 26 and outwardly through the bottom therefrom into the container
interior 12 where it
admixes with the solution 45'. The flow of the liquid component 13' into the
solid cavity 26
CA 02562655 2006-10-05
advantageously flushes any residual solid component 15 from the chamber 20
into the container
interior 22.
The container 12 is thereafter shaken, agitated or inverted to allow full
mixing of the
colourmetric reagents and reaction with any neutrophils from the patient's
mouth which are
present in the oral rinse solution 45'. Following reaction with the reagent
for a period of time
preferably selected at between 5 and 60 seconds, the resulting solution will
change colour having
regard to the neutrophil concentration. The user thereafter may visually
compare the colour of
the admixed solution with the appropriate colour panel 38a,38b,38c of the
colour scale 34 to
provide a visual indication of the likely presence and/or severity of
periodontal disease.
Although Figures 7 and 8 illustrate an assay kit 10 having a two-part chamber
containing
cap member 14, the invention is not so limited. It is to be appreciated that
assay kits could
equally be provided in which the colourmetric indicators are provided in
separate containers for
subsequent decantation into the container 12. Optionally, the kit 10 may be
provided without a
predetermined volume of sterile saline mouthwash solution or with separate
containers
containing both volumes of pre-rinse solutions and sampling solutions.
The colourimetric method and assay kit of the present invention shows
outstanding
sensitivity, precision and accuracy relative to standard methods used to
diagnose periodontal
diseases. Furthermore, the assay kit may be self-administered and is more
rapid than current
microscope based methods used for counting oral neutrophils.
Although the detailed description describes and illustrates preferred
embodiments, the
invention is not so limited. Many modifications and variations will now occur
to persons skilled
in the art. For a definition of the invention reference may be had to the
appended claims.