Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
PLASMA ENHANCED CHEMICAL VAPOR DEPOSITION OF METAL OXIDE
Background of the Invention
The present invention relates to plasma enhanced chemical vapor deposition of
a
metal oxide onto a substrate, particularly a plastic substrate.
Metal oxide films are deposited onto glass substrates for a variety of
applications.
For example, in U.S. 5,830,530, Jones describes chemical vapor deposition
(CVD) coating
of semiconducting SnO2 onto a glass substrate at temperatures in the range of
250 C to
400 C at atmospheric or subatmospheric pressures. Similarly, McCurdy, in U.S.
6,238,738, describes a CVD method for laying down a tin or titanium oxide
coating on a
glass substrate at 630 C and at atmospheric pressure.
In U.S. 6,136,162, Shiozaki et al. describes a method for depositing a
transparent
electroconductive zinc oxide film onto the rear surface of a photoelectric
converter using
magnetron sputtering under high vacuum (2.2 mtorr).
In U.S. 6,540,884, Siddle et al. describes a process for producing an
electrically
conductive low emissivity coating on a glass substrate comprising 1)
depositing a
reflective metal layer onto the substrate, then 2) reactive sputter depositing
a metal oxide
layer over the reflective metal layer in the presence of an oxygen scavenger,
then 3) heat
treating the substrate to 400 C to 720 C. The metal oxide is described as
being an oxide
of tin, zinc, tungsten, nickel, molybdenum, manganese, zirconium, vanadium,
niobium,
tantalum, cerium, or titanium or mixtures thereof.
Woo, in U.S. 6,603,033, describes the preparation of organotitanium precursors
that can be used for metal-organic chemical vapor deposition (MOCVD). The thin
film of
titanium oxide was described as being formed on a glass substrate that was
heated to
375 C to 475 C. Conversely, Hitchman et al., in WO 00/47797, describes the
deposition
of thin films of rutile titanium dioxide onto a variety of substrates
including glass,
sapphire, steel, aluminum, and magnesium oxide, at temperatures as low as 268
C, but at
reduced pressures (1 torr).
As the art suggests, deposition of metal oxides onto temperature-resistant
substrates such as glass can be carried out at relatively high temperatures
without
-1-
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
degrading the glass. However, significantly lower temperatures would be
required to
deposit a metal oxide onto a plastic substrate. Moreover, for practical
reasons, it would
further be desirable to carry out such deposition at or near atmospheric
pressure. It would
therefore be advantageous to discover a method for depositing a metal oxide
onto a plastic
substrate at a teinperature below the glass transition temperature of the
substrate,
preferably at or near atmospheric pressure.
Summary of the Invention
The present invention addresses a need in the art by providing a method
comprising the steps of 1) carrying a metal-oxide precursor through a corona
discharge or
a dielectric barrier discharge in the presence of an oxidizing agent to
convert the precursor
to a metal oxide by plasma enhanced chemical vapor deposition (PECVD), and 2)
depositing the metal oxide onto a substrate.
Optionally, other precursors amenable to PECVD of organosiloxane and SiOx
coating may
be sequentially deposited or codeposited with metal oxides providing
multilayer and/or
composite compositions on the substrate.
Brief Description of Drawinjzs
Fig. 1 illustrates a corona discharge method of generating and depositing a
metal
oxide on a substrate.
Fig. 2 illustrates a dielectric barrier discharge device.
Detailed Description of the Invention
The present invention is a method for depositing a metal oxide onto a
substrate
using plasma enhanced chemical vapor deposition. In a first step a metal-
organic
precursor is carried through a corona discharge or a dielectric barrier
discharge in the
presence of an oxidizing agent and preferably a carrier gas. The discharge
converts the
precursor to a metal oxide, which is deposited on a substrate.
-2-
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
As used herein, the term "metal-oxide precursor" refers to a material capable
of
forming a metal oxide when subjected to plasma enhanced chemical vapor
deposition
(PECVD). Examples of suitable metal-oxide precursors include diethyl zinc,
dimethyl
zinc, zinc acetate, titanium tetrachloride, dimethyltin diacetate, zinc
acetylacetonate,
zirconium hexafluoroacetylacetonate, zinc carbamate, trimethyl indium,
triethyl indium,
cerium (IV) (2,2,6,6-tetramethyl-3,5-heptanedionate), and mixtures thereof.
Examples of
metal oxides include oxides of zinc, tin, titanium, indium, cerium, and
zirconium, and
mixtures thereof. An example of a particularly useful mixed oxide is indium-
tin-oxide
(ITO), which can be used as a transparent conductive oxide for electronic
applications.
The method of the present invention can be advantageously carried out using
well
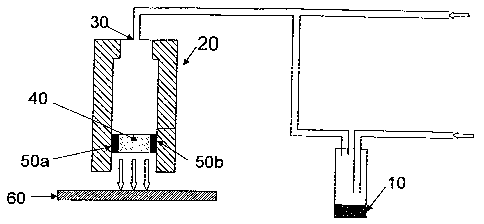
known corona discharge technology as illustrated in Fig. 1 a. Referring now to
Fig. 1 a, the
headspace from precursor (10), a carrier for the precursor, and the oxidizing
agent is
flowed into the jet (20) through a first gas intake (30) and corona discharge
(40) - which
breaks down gas between two electrodes 50(a) and 50(b) - to form the metal
oxide, which
is deposited on the substrate (60), preferably a plastic substrate that is
heated to impart
order thereto. If a plastic substrate is used, the plastic is advantageously
maintained at a
temperature near its Tg, preferably not exceeding 50 C higher than its Tg,
prior to and
during the deposition of the metal oxide. The method is preferably carried out
at or near
atmospheric pressure, typically in the range of 700 - 800 torr.
The carrier for the precursor is typically nitrogen, helium, or argon, with
nitrogen
being preferred; the oxidizing agent is an oxygen containing gas such as 02,
N20, air, 03,
C02, NO, or N204, with air being preferred. If the precursor is highly
reactive with the
oxidizing agent - for example, if the precursor is pyrophoric - it is
preferred to separate
the oxidizing agent from the precursor, as depicted in Fig. lb. According to
this scheme,
carrier and precursor are flowed through a second gas intake (70) situated
just above the
corona discharge (40) and the oxidizing agent is flowed through the first
intake (30).
Furthermore, a second carrier may be used to further dilute the concentration
of the
precursor prior to introduction into the jet (20). The oxidizing agent may not
need to be
affirmatively provided to the corona discharge or dielectric barrier discharge
region if it is
available to the region through the ambient air.
-3-
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
The corona discharge (40) is preferably maintained at a voltage in the range
of
about 2- 20 kV. The distance between the corona discharge (40) and the
substrate (60)
typically varies from about 1mm to 50 mm.
The precursor can be delivered to the jet by partially filling a container
with
precursor to leave a headspace and sweeping the headspace with the carrier
into the jet
(10). The container can be heated, if necessary, to generate the desirable
vapor pressure
for the precursor. Where the precursor is moisture- or air-sensitive or both,
it is preferable
to hold the precursor in a substantially moisture-free and oxygen-free
container.
Dielectric barrier discharge, also known as "silent" and "atmospheric-pressure-
glow" discharges, can also be used to carry out the process of the present
invention. Fig. 2
illustrates a schematic of a dielectric barrier discharge device (100), which
comprises two
metal electrodes (110 and 120) in which at least one is coated with a
dielectric layer (130)
superposed by a substrate (150). The gap between the electrodes (110 and 120)
typically
ranges from 1 to 100 mm and the applied voltage is on the order of 10-50 kV.
The plasma
(140) is generated through a series of micro-arcs that last for about 10-100
ns and that are
randomly distributed in space and time.
The concentration of the precursor in the total gas mixture (tlie precursor,
the
oxidizing agent, and the carrier gas) is preferably in the range of 10 ppm to
1% v/v. The
flow rate of the precursor is preferably in the range of 0.1-10 sccm and the
flow rate of the
oxidizing agent is preferably in the range of 10-100 scfin (2.7 x 105 to 2.7 x
106 sccm).
The thickness of the coating on the substrate is application dependent but is
typically in the
range; of 10 nm to 1 m.
The substrate is not limited but is preferably a plastic, examples of which
include
polycarbonates, polyuretlianes, thermoplastic polyurethanes,
poly(methylmethacrylates),
polypropylenes, low density polyethylenes, high density polyethylene,
etliylene-alpha-
olefm copolymers, styrene (co)polymers, styrene-acrylonitrile copolymers,
polyethylene
terephthalates, and polybutylene terephthalates. The method of the present
invention can
provide UV blocking coatings for plastic substrates at low temperature and at
or near
atmospheric pressure.
-4-
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
The following examples are for illustrative purposes only and not intended to
limit
the scope of the invention.
-5-
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
Example 1- Deposition of Tin Oxide on a Polycarbonate Substrate
Dimethyltin diacetate was placed in a closed precursor reservoir and heated to
62
C. Nitrogen gas was passed through the reservoir at 3000 sccm and combined
with a
stream of air passed at 15 scfin (420,000 sccm). The outcoming gas line of the
reservoir
was heated to 70 C. The total gas mixture was passed through a PLASMA-JET
corona
discharge (available from Corotec Corp., Farmington, CT., electrode spacing of
1 cm)
directed at a polycarbonate substrate. After 10 min., a clear monolithic
coating of tin
oxide was formed as evidenced by scanning electron microscopy and x-ray
photoelectron
spectroscopy (XPS). ,
Example 2 - Deposition of Titanium Oxide on a Polycarbonate Substrate
Titanium tetrachloride was placed in a closed precursor reservoir and cooled
to 0
C. Nitrogen gas was flowed through the reservoir at 600 scem and combined with
a
stream of dry (TOC grade) air passed at 20 scfin (570,000 sccm). The total gas
mixture
was passed through the plasma jet device directed at a polycarbonate
substrate. After 8
min., a clear monolithic coating of titanium oxide was formed as evidenced by
scanning
electron microscopy and XPS.
Example 3 - Deposition of Zinc Oxide on a Polycarbonate Substrate
Diethyl zinc was placed in a closed precursor reservoir. Nitrogen gas was
passed
through the reservoir at 150 sccm and combined with another stream of nitrogen
passed at
3500 sccm. This gas mixture was introduced into a stream of air plasma
generated by the
plasma jet device and directed onto the polycarbonate substrate. The flow rate
of the air
(TOC grade) was 20 scfm (570,000 sccm). After 10 min., a clear coating of zinc
oxide
was formed as evidenced by scanning electron microscopy and XPS.
Example 4- Deposition of a UV absorbing Zinc Oxide on a Polycarbonate
Substrate
Diethyl zinc was placed in a closed precursor reservoir. Nitrogen gas was
passed
through the reservoir at 100sccm and combined with another stream of nitrogen
passed at
3800 sccm. This gas mixture was introduced into a stream of air plasma
generated by the
plasma jet device and directed onto the polycarbonate substrate. The flow rate
of the air
(low humidity conditioned air) was 15 scfin (570,000sccm). The applied power
to the
-6-
CA 02562914 2006-10-17
WO 2005/113856 PCT/US2005/017747
electrodes was 720 W and the distance from jet to substrate was 20 mm. After
15 min, a
clear coating of zinc oxide about 0.6 m thick was formed on a polycarbonate
sheet as
evidenced by scanning electron microscopy and XPS. During deposition, the
polycarbonate sheet (Tg = 150 C) was heated to a temperature of 180 C to
induce
crystallinity in the coating, as evidenced by XRD analysis. Zinc oxide
coatings were in
tact after 1000 hours of QUV-B weathering tests according to ASTM G53-96.
Coatings
exhibited yellow Index < 5 and < 18% Delta Haze, 85% light transmission and a
UV
absorption cutoff of about 360 nm.
Example 5. Deposition of Zinc Oxide Using a Dielectric Barrier Discharge on a
Polycarbonate Substrate
Diethylzinc was placed in a closed reservoir. Nitrogen gas was passed through
the
reservoir at 150sccm and combined with another stream of nitrogen at 60scfin.
This gas
mixture was introduced downstream and mixed with air prior to exiting the
electrode into
the discharge zone, which contacts the polycarbonate substrate. The flow rate
of air was
11357sccm. The applied power to the electrodes was 1,000W and a distance from
electrode to substrate was about 4mm. After 10min, a clear coating of zinc
oxide was
formed on a polycarbonate film as evidenced by scanning electron microscopy
and XPS.
Example 6. Deposition of a SiOxCyHz or SiOx/Zinc Oxide Multilayer'Coating
An organosiloxane coating similar to VPP according to patent US 5,718,967, was
deposited onto a polycarbonate substrate. The precursor tetramethyldisiloxane
flowing at
6000sccm is mixed with N20 at a flowrate of 1 000sccm. This gas mixture was
introduced
into a stream of nitrogen plasma generated by the plasma jet device and
directed onto the
polycarbonate substrate. A balance gas of nitrogen is passed at a flowrate of
25scfin. The
applied power to the electrodes was 78W and the distance from jet to substrate
was 5 mm.
A Zinc Oxide coating was deposited on top of the organosiloxane coating
according to Example 4. Optionally, another organosiloxane layer was deposited
on top of
the Zinc Oxide layer.
-7-