Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
BIOACTIVE COATING COMPOSITIONS FOR MEDICAL DEVICES
TECHNICAL FIELD
In one aspect, the present invention relates to a method of treating
implantable
medical devices with coating compositions to provide for the controlled
release of
bioactive (e.g., pharmaceutical) agents from the surface of the devices under
physiological
conditions. In another aspect, the invention relates to the coating
compositions, per se. In
yet another aspect, the invention relates to devices or surfaces coated with
such
compositions. In yet another aspect, the present invention relates to the
local
administration of bioactive agents for the prevention and treatment of
diseases, such as
vascular and ocular diseases.
BACKGROUND OF THE INVENTION
Many surgical interventions require the placement of a medical device into the
body. One prevalent surgical intervention often requiring such a device is
percutaneous
transluminal coronary angioplasty ("PTCA"). Many individuals suffer from
circulatory
disease caused by a progressive blockage of the blood vessels, which often
leads to
hypertension, ischemic injury, stroke, or myocardial infarction. Percutaneous
transluminal
coronary angioplasty is a medical procedure performed to increase blood flow
through a
damaged artery and is now the predominant treatment for coronary vessel
stenosis. The
increasing use of this procedure is attributable to its relatively high
success rate and its
minimal invasiveness compared with coronary bypass surgery. A limitation
associated
with PTCA is the abrupt closure of the vessel which can occur soon after
angioplasty.
Insertion of small spring-like medical devices called stems into such damaged
vessels has
proved to be a better approach to keep the vessels open as compared to
systemic
pharmacologic therapy.
While often necessary and beneficial for treating a variety of medical
conditions,
metal or polymeric devices (e.g., stems, catheters...), after placement in the
body, can give
rise to numerous physiological complications. Some of these complications
include:
increased risk of infection; initiation of a foreign body response resulting
in inflammation
and fibrous encapsulation; and initiation of a detrimental wound healing
response resulting
in hyperplasia and restenosis. These problems have been particularly acute
with the
placement of stems in damaged arteries after angioplasty.
One promising approach is to provide the device with the ability to deliver
bioactive agents in the vicinity of the implant. By doing so, some of the
harmful effects
associated with the implantation of medical devices can be diminished. Thus,
for
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
2
example, antibiotics can be released from the surface of the device to
minimize the
possibility of infection, and antiproliferative drugs can be released to
inhibit hyperplasia.
Another benefit to the local release of bioactive agents is the avoidance of
toxic
concentrations of drugs encountered when given systemically at sufficiently
high doses to
achieve therapeutic concentrations at the site where they are needed.
Although the potential benefit from using such bioactive agent-releasing
medical
devices is great, development of such medical devices has been slow. Progress
has been
hampered by many challenges, including: 1 ) the requirement, in some
instances, for long
term (i.e., at least several weeks) release of bioactive agents; 2) the need
for a
biocompatible, non-inflammatory device surface; 3) the demand for significant
durability
(and particularly, resistance to delamination and cracking), particularly with
devices that
undergo flexion and/or expansion when being implanted or used in the body; 4)
concerns
regarding the ability of the device to be manufactured in an economically
viable and
reproducible manner; and 5) the requirement that the finished device can be
sterilized
using conventional methods.
Implantable medical devices capable of delivering medicinal agents from
hydrophobic polymer coatings have been described. See, for instance, U.S.
Patent No.
6,214,901; U.S. Patent No. 6,344,035; U.S. Publication No. 2002-0032434; U.S.
Publication No. 2002-0188037; U.S. Publication No. 2003-0031780; U.S.
Publication No.
2003-0232087; U.S. Publication No. 2003-0232122; PCT Publication No. WO
99/55396;
PCT Publication No. WO 03/105920; PCT Publication No. WO 03/105918; PCT
Publication No. WO 03/105919 which collectively disclose, inter alia, coating
compositions having a bioactive agent in combination with a polymer component
such as
polyalkyl(meth)acrylate or aromatic poly(meth)acrylate polymer and another
polymer
component such as polyethylene-co-vinyl acetate) for use in coating device
surfaces to
control and/or improve their ability to release bioactive agents in aqueous
systems.
SUMMARY OF THE INVENTION
The present invention provides a coating composition, and related methods for
preparing and using the coating composition to coat a surface with a bioactive
agent, for
instance to coat the surface of an implantable medical device in a manner that
permits the
surface to release the bioactive agent over time when implanted in vivo.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
The coating composition of this invention comprises one or more bioactive
agents
in combination with a plurality of polymers, including: (a) a first polymer
component
comprising a polymer selected from the group consisting of (i) ethylene
copolymers with
other alkylenes, (ii) polybutenes, (iii) aromatic group-containing copolymers,
(iv)
epichlorohydrin-containing polymers, (v) poly(alkylene-co-
alkyl(meth)acrylates), and (vi)
diolefin-derived, non-aromatic polymers and copolymers; and (b) a second
polymer
component comprising one or more polymers selected from the group consisting
of
poly(alkyl(meth)acrylates) and poly(aromatic (meth)acrylates), where "(meth)"
will be
understood by those skilled in the art to include such molecules in either the
acrylic and/or
methacrylic form (corresponding to the acrylates and/or methacrylates,
respectively).
Applicants have discovered a group of first polymers that when used in
combination with one or more second polymers can each meet or exceed the
variety of
criteria required of a composition of this invention, including in terms of
its formulation,
delivery, and/or coated characteristics.
In various embodiments, with regard to its formulation, a coating composition
of
this invention may be provided in the form of a true solution by the use of
one or more
solvents. Such solvents, in turn, are not only capable of dissolving the
polymers and
bioactive agent in solution, as compared to dispersion or emulsion, but they
are also
sufficiently volatile to permit the composition to be effectively applied to a
surface (e.g.,
as by spraying) and quickly removed (e.g., as by drying) to provide a stable
and desirable
coated composition. In turn, the coated composition is itself homogeneous,
with the first
and second polymers effectively serving as cosolvents for each other, and
bioactive agent
substantially equally sequestered within them both.
In some embodiments, the ability to form a true solution.using the claimed
polymer combinations is desirable when considering the inclusion of
potentially
significant amounts of bioactive agent with the polymer blend. In various
embodiments of
the present invention, the coating composition is not only in the form of a
true solution,
but one in which bioactive agent is present at saturated or supersaturated
levels. Without
intending to be bound by theory, it appears that it is by virtue of the
ability to achieve such
solutions, that release of the bioactive agent from the coated composition is
best
accomplished and facilitated. In turn, it appears that the release of
bioactive agent from
such a system is due, at least in part, to its inherent instability within the
coated
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
4
composition itself, coupled with its physical/chemical preference for
surrounding tissues
and fluids. In turn, those skilled in the art will appreciate the manner in
which the various
ingredients and amounts in a composition of this invention can be adjusted to
provide
desired release kinetics and for any particular bioactive agent, solvent and
polymer
combination.
With regard to its delivery, various embodiments including a composition of
this
invention meets or exceeds further criteria in its ability to be sterilized,
stored, and
delivered to a surface in a manner that preserves its desired characteristics,
yet using
conventional delivery means, such as spraying. In some embodiments, such
delivery
involves spraying the composition onto a device surface in a manner that
avoids or
minimizes phase separation of the polymer components.
Finally, and with regard to its coated characteristics, a composition of this
invention permits polymer ratios to be varied in a manner that provides not
only an
optimal combination of such attributes as biocompatibility, durability, and
bioactive agent
release kinetics, but also, in some embodiments, provides a coated composition
that is
homogeneous, and hence substantially optically clear upon microscopic
examination.
Even more surprisingly, in some embodiments, the compositions of this
invention will
provide these and other features, with or without optional pretreatment of a
metallic
surface. The ability to achieve or exceed any of these criteria, let alone
most if not all of
them, was not expected.
In turn, compositions of the present invention provide properties that are
comparable or better than those obtained with previous polymer blend
compositions.
This, in turn, provides a variety of new and further opportunities, including
with respect to
both the type and concentration of bioactive agents that can be coated, as
well as the
variety of medical devices, and surfaces, themselves. In turn, the present
invention also
provides a combination that includes a medical device coated with a
composition of this
invention, as well as a method of preparing and using such a combination.
BRIEF DESCRIPTION OF THE FIGURES
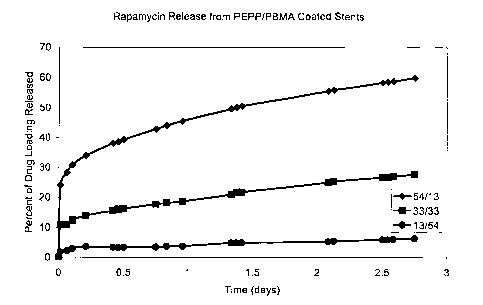
Figure 1 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 1.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
Figure 2 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 2.
Figure 3 depicts a graph illustrating the cumulative bioactive agent release
profiles for
5 coating compositions according to the present invention applied to stems, as
described in
Example 3.
Figure 4 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 4.
Figure S depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 5.
Figure 6 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 6.
Figure 7 depicts a graph illustrating the stresslstrain measurements of first
polymer
components used in coating compositions according to the present invention, as
described
in Example 8.
Figure 8 depicts a 100 micron wide and 10 micron deep Raman image taken by
measuring
the Raman intensity at 2900 cm' of a coating composition according to the
present
invention.
Figure 9 depicts a 100 micron wide and 10 micron deep Raman image taken by
measuring
the Raman intensity at 1630 cm 1 for the same region of stmt coating shown in
Figure 9.
Figure 10 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 10.
Figure 1 OA depicts a bar chart illustrating the durability profiles for
coating compositions
according to the present invention applied to stems, as described in Example
10.
Figure 11 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 11.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
6
Figure 12 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 12.
Figure 13 depicts a scanning electron microscope image a coated stmt including
a coating
composition according to the present invention after conventional crimping and
balloon
expansion procedures.
Figure 14 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 15.
Figure 15 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions according to the present invention applied to stems, as
described in
Example 15.
Figure 16 depicts a graph illustrating the cumulative bioactive agent release
profiles for
coating compositions and topcoats according to the present invention applied
to stems, as
described in Example 16.
Figure 17 shows a medical device as described in Example 17.
Figure 18 shows a medical device as described in Example 17.
Figure 19A shows a plot of data as described in Example 17.
Figure 19B shows the plot of Figure 19A with tilt and curvature correction.
Figure 20A shows a surface plot of a roughness test as described in Example
17.
Figure 20B shows a 3D representation of Figure 20A.
Figure 21A shows a surface plot of a roughness test as described in Example
17.
Figure 21 B shows a 3 D representation of Figure 21 A.
DETAILED DESCRIPTION
Without intending to be bound by theory, it appears that suitable first
polymers for
use in a composition of this invention provide an optimal combination of such
properties
as glass transition temperature (Tg) and diffusion constant for the particular
bioactive
agent of choice. Along with melting temperature (Tm), Tg is an important
parameter of a
given polymer (including copolymer), and particularly amorphous polymers, that
can be
used to characterize its properties over a wide temperature range. A polymer
is typically
brittle at temperatures below its Tg, and flexible at temperatures above. Both
Tm and Tg
can be affected by such things as polymer structure and backbone flexibility,
molecular
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
7
weight, attractive forces, and pressure. For random copolymers and compatible
polymer
blends, only a single Tg is observed, usually lying intermediate between the
Tg of the
corresponding pure homopolymers. Different Tg s are exhibited for incompatible
polymer
blends, and between the microdomains of block copolymers with mutually
incompatible
blocks. Tg can be measured by any suitable technique, e.g., dilatometry,
refractive index,
differential scanning calorimetry, dynamic mechanical measurement, and
dielectric
measurement.
Various second polymers (e.g., poly (n-butyl methacrylate)) of the present
composition generally provide a Tg in the range of room to body temperature
(e.g., from
about 20 °C to about 40 °C), and hence tend to be somewhat
stiffer polymers, in turn,
providing a slower diffusion constant for a number of bioactive agents.
Applicants have
discovered the manner in which certain new polymers can be used as a first
polymer
component, to essentially balance, or temper the desired properties of the
second polymer.
Such first polymers will generally provide a lower glass transition
temperature (e.g., below
room temperature, and in some embodiments in the range of about 0 °C or
less), together
with a relatively high diffusion constant for the bioactive agent. By
appropriately
combining the two polymers with bioactive agent, those skilled in the art,
given the
present description, will be able to vary both the selection and ratios of
first and second
polymers, in order to determine an optimal combination of physical and
mechanical
properties, including bioactive agent diffusion and release kinetics, as well
as durability
and tenacity of the coating itself upon a particular surface, that best fits
their particular
needs.
Hence embodiments of the first polymer of this invention will generally
provide an
optimal combination of glass transition temperature (e.g., at or lower than
that of the
second polymer), compatibility with the bioactive agent of choice, acceptable
solubility in
the solvents of choice, as well as commercial availability and cost.
The term "coating composition", as used herein, will refer to one or more
vehicles
(e.g., solutions, mixtures, emulsions, dispersions, blends, etc.) used to
effectively coat a
surface with bioactive agent, first polymer component and/or second polymer
component,
either individually or in any suitable combination.
The term "coated composition" will refer to the effective combination, upon
the
surface of a device, of bioactive agent, first polymer component and second
polymer
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
8
component, whether formed as the result of one or more coating vehicles or in
one or more
layers and/or steps.
Unless defined otherwise, the term "coating" will refer to the effective
combination of bioactive agent, first polymer component and second polymer
component,
independent of the device surface, and whether formed as the result of one or
more coating
vehicles or in one or more layers.
Unless otherwise indicated, the term "molecular weight" and all polymeric
molecular weights described herein are "weight average" molecular weights
("Mw"). As
used herein "weight average molecular weight" or MW, is an absolute method of
measuring
molecular weight and is particularly useful for measuring the molecular weight
of a
polymer preparation. The weight average molecular weight (MW) can be defined
by the
following formula:
~NiMa
L NiMi:
wherein N represents the number of moles of a polymer in the sample with a
mass of M,
and E; is the sum of all N;M; (species) in a preparation. The MW can be
measured using
common techniques, such as light scattering or ultracentrifugation. Discussion
of MW and
other terms used to define the molecular weight of polymer preparations can be
found in,
for example, Allcock, H. R. and Lampe, F. W., Contemporary Polymer Chemistry;
pg 271
( 1990).
As described and exemplified herein, a resultant composition can be coated
using a
plurality of individual steps or layers, including for instance, an initial
layer having only
bioactive agent (or bioactive agent with one or both of the polymer
components), over
which are coated one or more additional layers containing suitable
combinations of
bioactive agent, first polymer component and/or second polymer component, the
combined result of which is to provide a coated composition of the invention.
In turn, and
in various embodiments, the invention further provides a method of
reproducibly
controlling the release (e.g., elution) of a bioactive agent from the surface
of a medical
device implanted in vivo. Those skilled in the art will appreciate the manner
in which the
combined effect of these various layers can be used and optimized to achieve
various
effects in vivo. In addition, the surface to which the composition is applied
can itself be
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
pretreated in a manner sufficient to improve attachment of the composition to
the
underlying (e.g., metallic) surface. Examples of such pretreatments include
the use of
compositions such as Parylene TM coatings, as described herein. Additional
examples of
such pretreatments include silane coupling agents, photografted polymers,
epoxy primers,
polycarboxylate resins, and physical roughening of the surface. It is further
noted that the
pretreatment compositions may be used in combination with each other or may be
applied
in separate layers to form a pretreatment coating on the surface of the
medical device.
While not intending to be bound by theory, the release kinetics of the
bioactive
agent in vivo are thought to generally include both a short term ("burst")
release
component, within the order of minutes to hours after implantation, and a
longer term
release component, which can range from on the order of hours to days or even
months or
years of useful release.
Additionally, the ability to coat a device in the manner of the present
invention
provides greater latitude in the composition of various coating layers, e.g.,
permitting
more or less of the second polymer component (i.e., poly(alkyl (meth)acrylate)
and/or
poly(aromatic (meth)acrylate)) to be used in coating compositions used to form
different
layers (e.g., as a topcoat layer). This, in turn, provides the opportunity to
further control
release and elution of the bioactive agent from the overall coating.
The coating composition and method can be used to control the amount and rate
of
bioactive agent (e.g., drug) release from one or more surfaces of implantable
medical
devices. In various embodiments, the method employs a mixture of hydrophobic
polymers in combination with one or more bioactive agents, such as a
pharmaceutical
agent, such that the amount and rate of release of agents) from the medical
device can be
controlled, e.g., by adjusting the relative types and/or concentrations of
hydrophobic
polymers in the mixture. For a given combination of polymers, for instance,
this approach
permits the release rate to be adjusted and controlled by simply adjusting the
relative
concentrations of the polymers in the coating mixture. This provides an
additional means
to control rate of bioactive agent release besides the conventional approach
of varying the
concentration of bioactive agent in a coated composition.
Some embodiments of the invention include a method of coating a device
comprising the step of applying the composition to the device surface under
conditions of
controlled relative humidity (at a given temperature), for instance, under
conditions of
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
increased or decreased relative humidity as compared to ambient humidity.
Humidity can
be "controlled" in any suitable manner, including at the time of preparing
and/or using (as
by applying) the composition, for instance, by coating the surface in a
confined chamber
or area adapted to provide a relative humidity different than ambient
conditions, andlor by
5 adjusting the water content of the coating or coated composition itself.
Without intending
to be bound by theory, it appears that the elution rate of a bioactive agent
from a coating
composition generally increases as relative humidity increases.
In various embodiments, the coating composition of this invention includes a
mixture of two or more polymers having complementary physical characteristics,
and a
10 bioactive agent or agents applicable to the surface of an implantable
medical device. The
device can be of any suitable type or configuration, and in some embodiments,
is one that
undergoes flexion and/or expansion upon implantation or use, as in the manner
of a stmt
or catheter. The applied coating composition is cured (e.g., by solvent
evaporation) to
provide a tenacious and flexible bioactive-releasing composition on the
surface of the
medical device. Such coating compositions are particularly well suited for
devices that are
themselves sufficiently small, or have portions that are sufficiently small
(as in the struts
of an expandable stmt or the twists of an ocular coil), to permit the coated
composition to
form a contiguous, e.g., circumferential, coating, thereby further improving
the ability of
the coating to remain intact (e.g., avoid delamination).
The complementary polymers are selected such that a broad range of relative
polymer concentrations can be used without detrimentally affecting the
desirable physical
characteristics of the polymers. By use of the polymer combinations (including
mixtures
and blends) of the invention the bioactive release rate from a coated medical
device can be
manipulated by adjusting the relative concentrations of the polymers.
In additional embodiments, the present invention relates to a coating
composition
and related method for coating an implantable medical device which undergoes
flexion
andlor expansion upon implantation. However it is noted that the coating
composition
may also be utilized with medical devices that have minimal or do not undergo
flexion
andlor expansion. The structure and composition of the underlying device can
be of any
suitable, and medically acceptable, design and can be made of any suitable
material that is
compatible with the coating itself. The natural or pretreated surface of the
medical device
is provided with a coating containing one or more bioactive agents.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
11
A first polymer component of this invention provides an optimal combination of
similar properties, and particularly when used in admixture with the second
polymer
component. In some embodiments, a first polymer is a polymer selected from the
group
consisting of (i) ethylene copolymers with other alkylenes, (ii) polybutenes,
(iii) aromatic
group-containing copolymers, (iv) epichlorohydrin-containing polymers (v)
poly(alkylene-
co-alkyl(meth)acrylates), and (vi) diolefin-derived, non-aromatic polymers and
copolymers.
Examples of suitable first polymers are commercially available from sources
such
as Sigma-Aldrich.
A first polymer component may be selected from one or more ethylene copolymers
with other alkylenes. Various first polymers for use in this invention
comprise ethylene
copolymers with other alkylenes, which in turn, can include straight chain and
branched
alkylenes, as well as substituted or unsubstituted alkylenes. Examples include
copolymers
prepared from alkylenes that comprise from 3 to 8 branched or linear carbon
atoms,
inclusive, in various embodiments, alkylene groups that comprise from 3 to 4
branched or
linear carbon atoms, inclusive, and in some embodiments, the alkylene group
contains 3
carbon atoms (e.g., propylene). In some embodiments, the other alkylene is a
straight
chain alkylene (e.g., 1-alkylene).
Various copolymers of this type can comprise from about 20% to about 90%
(based on moles) of ethylene, and in some embodiments, from about 35% to about
80%
(mole) of ethylene. Such copolymers will have a molecular weight of between
about 30
kilodaltons to about 500 kilodaltons. Examples of such copolymers are selected
from the
group consisting of polyethylene-co-propylene), polyethylene-co-1-butene),
polyethylene-co-1-butene-co-1-hexene) and/or polyethylene-co-1-octene).
Examples of particular copolymers include polyethylene-co-propylene) random
copolymers in which the copolymer contains from about 35% to about 65% (mole)
of
ethylene; and in some embodiments, from about 55% to about 65% (mole)
ethylene, and
the molecular weight of the copolymer is from about 50 kilodaltons to about
250
kilodaltons, in some embodiments from about 100 kilodaltons to about 200
kilodaltons.
Copolymers of this type can optionally be provided in the form of random
terpolymers prepared by the polymerization of both ethylene and propylene with
optionally one or more additional dime monomers, such as those selected from
the group
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
12
consisting of ethylidene norborane, dicyclopentadiene and/or hexadiene.
Various
terpolymers of this type can include up to about S% (mole) of the third dime
monomer .
Other examples of suitable copolymers of this type are commercially available
from sources such as Sigma-Aldrich and include the following products. For
example,
suitable copolymers of this type and their related descriptions may be found
in the 2003-
2004 Aldrich Handbook of Fine Chemicals and Laboratory Equipment, the entire
contents
of which are incorporated by reference herein. Examples of such copolymers
include, but
are not limited to polyethylene-co-propylene), polyethylene-co-1-butene),
poly(ethylene-
co-1-butene-co-1-hexene), polyethylene-co-1-octene) and polyethylene-co-
propylene-
co-5-methylene-2-norborene).
Alternatively, a first polymer component may be selected from one or more
polybutenes. "Polybutenes" suitable for use in the present invention include
polymers
derived by homopolymerizing or randomly interpolymerizing isobutylene, 1-
butene and/or
2-butene. The polybutene can be a homopolymer of any of the isomers or it can
be a
copolymer or a terpolymer of any of the monomers in any ratio. In various
embodiments,
the polybutene contains at least about 90% (wt) of isobutylene or 1-butene,
and in some
embodiments, the polybutene contains at least about 90% (wt) of isobutylene.
The
polybutene may contain non-interfering amounts of other ingredients or
additives, for
instance it can contain up to 1000 ppm of an antioxidant (e.g., 2,6-di-tert-
butyl-
methylphenol).
In various embodiments, the polybutene has a molecular weight between about
100
kilodaltons and about 1,000 kilodaltons, in some embodiments, between about
150
kilodaltons and about 600 kilodaltons, and in some embodiments, between about
150
kilodaltons and about 250 kilodaltons. In other embodiments, the polybutene
has a
molecular weight between about 150 kilodaltons and about 1,000 kilodaltons,
optionally,
between about 200 kilodaltons and about 600 kilodaltons, and further
optionally, between
about 350 kilodaltons and about 500 kilodaltons. Polybutenes having a
molecular weight
greater than about 600 kilodaltons, including greater than 1,000 kilodaltons
are available
but are expected to be more difficult to work with. Other examples of suitable
copolymers
of this type are commercially available from sources such as Sigma-Aldrich.
Additional alternative first polymers include aromatic group-containing
copolymers, including random copolymers, block copolymers and graft
copolymers. In
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
13
various embodiments, the aromatic group is incorporated into the copolymer via
the
polymerization of styrene, and in some embodiments, the random copolymer is a
copolymer derived from copolymerization of styrene monomer and one or more
monomers selected from butadiene, isoprene, acrylonitrile, a C,-C4 alkyl
(meth)acrylate
(e.g., methyl methacrylate) and/or butene (e.g., isobutylene). Useful block
copolymers
include copolymer containing (a) blocks of polystyrene, (b) blocks of a
polyolefin selected
from polybutadiene, polyisoprene and/or polybutene (e.g., polyisobutylene),
and (c)
optionally a third monomer (e.g., ethylene) copolymerized in the polyolefin
block.
The aromatic group-containing copolymers may contain about 10% to about 50%
(wt) of polymerized aromatic monomer and the molecular weight of the copolymer
may
be from about 50 kilodaltons to about 500 kilodaltons. In some embodiments,
the
molecular weight of the copolymer may be from about 300 kilodaltons to about
S00
kilodaltons. In other embodiments, the molecular weight of the copolymer may
be from
about 100 kilodaltons to about 300 kilodaltons.
Other examples of suitable copolymers of this type are commercially available
from sources such as Sigma-Aldrich and include, but are not limited to,
polystyrene-co-
butadiene) (random), polystyrene-block-polybutadiene, polystyrene-block-
polybutadiene-
block-polystyrene, polystyrene-block-poly(ethylene-ran-butylene)-block-
polystyrene,
polystyrene-block-polyisoprene-block-polystyrene, polystyrene-block-
polyisobutylene-
block-polystyrene, polystyrene-co-acrylonitrile), polystyrene-co-butadiene-co-
acrylonitrile) and poly(styrene-co-butadiene-co-methyl methacrylate).
Additional alternative first polymers include epichlorohydrin homopolymers and
poly(epichlorohydrin-co-alkylene oxide) copolymers. In some embodiments, in
the case of
the copolymer, the copolymerized alkylene oxide is ethylene oxide. In various
embodiments, epichlorohydrin content of the epichlorohydrin-containing polymer
is from
about 30% to 100% (wt), and in some embodiments from about 50% to 100% (wt).
In
some embodiments, the epichlorohydrin-containing polymers have a Mw from about
100
kilodaltons to about 300 kilodaltons.
Other examples of suitable copolymers of this type are commercially available
from sources such as Sigma-Aldrich and include, but are not limited to,
polyepichlorohydrin and poly(epichlorohydrin-co-ethylene oxide).
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
14
As another example, a first polymer component may be selected from one or more
poly(alkylene-co-alkyl(meth)acrylates. Various poly(alkylene-co-
alkyl(meth)acrylates)
include those copolymers in which the alkyl groups are either linear or
branched, and
substituted or unsubstituted with non-interfering groups or atoms. In various
embodiments, such alkyl groups comprise from 1 to 8 carbon atoms, inclusive,
and in
some embodiments, from 1 to 4 carbon atoms, inclusive. In one example, the
alkyl group
is methyl.
In various embodiments, copolymers that include such alkyl groups comprising
from about 15 % to about 80% (wt) of alkyl acrylate. When the alkyl group is
methyl, the
polymer may contain from about 20% to about 40% methyl acrylate, and in some
embodiments from about 25 to about 30% methyl acrylate. When the alkyl group
is ethyl,
the polymer, in some embodiments, contains from about 15% to about 40% ethyl
acrylate,
and when the alkyl group is butyl, the polymer, in some embodiments, contains
from
about 20% to about 40% butyl acrylate.
The alkylene groups are selected from ethylene and/or propylene, and more in
various embodiments, the alkylene group is ethylene. In various embodiments,
the
(meth)acrylate comprises an acrylate (i.e., no methyl substitution on the
acrylate group).
Various copolymers provide a molecular weight (Mw) of about 50 kilodaltons to
about
500 kilodaltons, and in some embodiments, Mw is 50 kilodaltons to about 200
kilodaltons.
The glass transition temperature for these copolymers varies with ethylene
content,
alkyl length on the (meth)acrylate and whether the first copolymer is an
acrylate or
methacrylate. At higher ethylene content, the glass transition temperature
tends to be
lower, and closer to that of pure polyethylene (-120°C). A longer alkyl
chain also lowers
the glass transition temperature. A methyl acrylate homopolymer has a glass
transition
temperature of about 10°C while a butyl acrylate homopolymer has one of
-54°C.
Copolymers such as polyethylene-co-methyl acrylate), polyethylene-co-butyl
acrylate) and polyethylene-co-2-ethylhexyl acrylate) copolymers are available
commercially from sources such as Atofma Chemicals, Inc., Philadelphia, PA,
and can be
prepared using methods available to those skilled in the respective art.
Other examples of suitable polymers of this type are commercially available
from
sources such as Sigma-Aldrich and include, but are not limited to,
polyethylene-co-
methyl acrylate), polyethylene-co-ethyl acrylate), and polyethylene-co-butyl
acrylate).
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
First polymers may also include diolefin-derived, non-aromatic polymers and
copolymers, including those in which the diolefin monomer used to prepare the
polymer
or copolymer is selected from butadiene (CH2=CH-CH=CH2) and/or isoprene
(CHZ=CH-
C(CH3)=CHZ). A butadiene polymer can include one or more butadiene monomer
units
5 which can be selected from the monomeric unit structures (a), (b), or (c):
Hz Hz Hz
CSC=C/H ~HZC C
H \H ~H/
C
Hz
(a) (b) (c)
10 An isoprene polymer can include one or more isoprene monomer units which
can be
selected from the monomeric unit structures (d), (e), (f) or (g):
CH3
-_ ~CH3 ~HzC
H/ C\CH ~H~C C\CT
s H HC
-CHz
(d) (e)
~.3a_,- cna
c -c~u~
i
c;~ ~,
(g)
In some embodiments, the polymer is a homopolymer derived from diolefin
monomers or is a copolymer of diolefin monomer with non-aromatic mono-olefin
monomer, and optionally, the homopolymer or copolymer can be partially
hydrogenated.
Such polymers can be selected from the group consisting of polybutadienes
containing
polymerized cis-, traps- and/or 1,2- monomer units, and in some embodiments, a
mixture
of all three co-polymerized monomer units, and polyisoprenes containing
polymerized cis-
1,4- and/or traps-1,4- monomer units, polymerized 1,2-vinyl monomer units,
polymerized
3,4-vinyl monomer units and/or others as described in the Encyclopedia of
Chemical
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
16
Technology, Vol. 8, page 915 (1993), the entire contents of which is hereby
incorporated
by reference.
Alternatively, the first polymer is a copolymer, including graft copolymers,
and
random copolymers based on a non-aromatic mono-olefin co-monomer such as
acrylonitrile, an alkyl (meth)acrylate and/or isobutylene. In various
embodiments, when
the mono-olefin monomer is acrylonitrile, the interpolymerized acrylonitrile
is present~at
up to about 50% by weight; and when the mono-olefin monomer is isobutylene,
the
diolefin monomer is isoprene (e.g., to form what is commercially known as a
"butyl
rubber"). In some embodiments, the polymers and copolymers have a Mw between
about
50 kilodaltons and about 1,000 kilodaltons. In other embodiments, the polymers
and
copolymers have a Mw between about 100 kilodaltons and about 450 kilodaltons.
In yet
other embodiments the polymers and copolymers have a Mw between about 150
kilodaltons and about 1,000 kilodaltons, and optionally between about 200
kilodaltons and
about 600 kilodaltons.
Other examples of suitable first polymers of this type are commercially
available
from sources such as Sigma-Aldrich, and include, but are not limited to,
polybutadiene,
poly(butadiene-co-acrylonitrile), polybutadiene-block-polyisoprene,
polybutadiene-graft-
poly(methyl acrylate-co-acrylonitrile), polyisoprene, and partially
hydrogenated
polyisoprene.
A second polymer component of this invention provides an optimal combination
of
various structural/functional properties, including hydrophobicity,
durability, bioactive
agent release characteristics, biocompatibility, molecular weight, and
availability. In one
such an embodiment, the composition comprises at least one second polymer
component
selected from the group consisting of poly(alkyl (meth)acrylates) and
poly(aromatic
(meth)acrylates).
In various embodiments, the second polymer component is a
poly(alkyl)methacrylate, that is, an ester of a methacrylic acid. Examples of
suitable
poly(alkyl (meth)acrylates) include those with alkyl chain lengths from 2 to 8
carbons;
inclusive, and with molecular weights from 50 kilodaltons to 900 kilodaltons.
In various
embodiments the polymer mixture includes a poly(alkyl (meth)acrylate) with a
molecular
weight of from about 100 kilodaltons to about 1000 kilodaltons, in some
embodiments,
from about 150 kilodaltons to about 500 kilodaltons, and in some embodiments
from
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
17
about 200 kilodaltons to about 400 kilodaltons. An example of a particular
second
polymer is poly (n-butyl methacrylate). Examples of other polymers are poly(n-
butyl
methacrylate-co-methyl methacrylate, with a monomer ratio of 3:1, poly(n-butyl
methacrylate-co-isobutyl methacrylate, with a monomer ratio of 1: l and poly(t-
butyl
methacrylate). Such polymers are available commercially (e.g., from Sigma-
Aldrich,
Milwaukee, WI) with molecular weights ranging from about 150 kilodaltons to
about 350
kilodaltons, and with varying inherent viscosities, solubilities and forms
(e.g., as slabs,
granules, beads, crystals or powder).
Examples of suitable poly(aromatic (meth)acrylates) include poly(aryl
(meth)acrylates), poly(aralkyl (meth)acrylates), poly(alkaryl
(meth)acrylates),
poly(aryloxyalkyl (meth)acrylates), and poly (alkoxyaryl (meth)acrylates).
Such terms are
used to describe polymeric structures wherein at least one carbon chain and at
least one
aromatic ring are combined with (meth)acrylic groups, typically esters, to
provide a
composition of this invention. For instance, and more specifically, a
poly(aralkyl
(meth)acrylate) can be made from aromatic esters derived from alcohols also
containing
aromatic moieties, such as benzyl alcohol. Similarly, a poly(alkaryl
(meth)acrylate) can
be made from aromatic esters derived from aromatic alcohols such as p-anisole.
Suitable
poly(aromatic (meth)acrylates) include aryl groups having from 6 to 16 carbon
atoms and
with molecular weights from about 50 to about 900 kilodaltons. Examples of
suitable
poly(aryl (meth)acrylates) include poly(9-anthracenyl methacrylate),
poly(chlorophenyl
acrylate), poly(methacryloxy-2-hydroxybenzophenone),
poly(methacryloxybenzotriazole),
poly(naphthyl acrylate), poly(naphthylmethacrylate), poly-4-
nitrophenylacrylate,
poly(pentachloro(bromo, fluoro) acrylate) and methacrylate, poly(phenyl
acrylate) and
poly(phenyl methacrylate). Examples of suitable poly(aralkyl (meth)acrylates)
include
poly(benzyl acrylate), poly(benzyl methacrylate), poly(2-phenethyl acrylate),
poly(2-
phenethyl methacrylate) and poly(1-pyrenylmethyl methacrylate). Examples of
suitable
poly(alkaryl(meth)acrylates include poly(4-sec-butylphenyl methacrylate),
poly(3-
ethylphenyl acrylate), and poly(2-methyl-1-naphthyl methacrylate). Examples of
suitable
poly(aryloxyalkyl (meth)acrylates) include poly(phenoxyethyl acrylate),
poly(phenoxyethyl methacrylate), and poly(polyethylene glycol phenyl ether
acrylate) and
poly(polyethylene glycol phenyl ether methacrylate) with varying polyethylene
glycol
molecular weights. Examples of suitable poly(alkoxyaryl(meth)acrylates)
include poly(4-
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
18
methoxyphenyl methacrylate), poly(2-ethoxyphenyl acrylate) and poly(2-
methoxynaphthyl acrylate).
Acrylate or methacrylate monomers or polymers and/or their parent alcohols are
commercially available from Sigma-Aldrich (Milwaukee, WI) or from
Polysciences, Inc,
(Warrington, PA).
Optionally, the coating composition may include one or more additional
polymers
in combination with the first and second polymer components, the additional
polymers
being, for example, selected from the group consisting of (i) poly(alkylene-co-
alkyl(meth)acrylates, (ii) ethylene copolymers with other alkylenes, (iii)
polybutenes, (iv)
diolefin-derived, non-aromatic polymers and copolymers, (v) aromatic group-
containing
copolymers, (vi) epichlorohydrin-containing polymers, including each as
disclosed and
described above in the sections describing first polymers, and (vii) poly
(ethylene-co-vinyl
acetate). Generally, if one or more additional polymers are included, the one
or more
additional polymers are different from the first polymer component used in the
coating
composition. In some embodiments, the additional polymers may substitute up to
about
25% of the first polymer. In other embodiments, the additional polymers may
substitute
up to about 50% of the first polymer.
As discussed above, a suitable additional polymer that may be utilized in the
coating composition of the present invention includes polyethylene-co-vinyl
acetate)
(pEVA). Examples of suitable polymers of this type are available commercially
and
include polyethylene-co-vinyl acetate) having vinyl acetate concentrations of
from about
8% and about 90%, in some embodiments, from about 20 to about 40 weight
percent and
in some embodiments, from about 30 to about 34 weight percent. Such polymers
are
generally found in the form of beads, pellets, granules, etc. It has generally
been found
that pEVA co-polymers with lower percent vinyl acetate become increasingly
insoluble in
typical solvents.
In some embodiments, coating compositions for use in this invention includes
mixtures of first and second polymer components as described herein.
Optionally, both
first and second polymer components are purified for such use to a desired
extent and/or
provided in a form suitable for in vivo use. Moreover, biocompatible additives
may be
added, such as dyes and pigments (e.g., titanium dioxide, Solvent Red 24, iron
oxide, and
Ultramarine Blue); slip agents (e.g., amides such as oleyl palmitamide, N,N'-
ethylene
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
19
bisoleamide, erucamide, stearamide, and oleamide); antioxidants (e.g.
butylated
hydroxytoluene (BHT), vitamin E (tocopherol), BNXTM, dilauryl thiodipropionate
(DLTDP), IrganoxTM series, phenolic and hindered phenolic antioxidants,
organophosphites (e.g., trisnonylphenyl phosphite, IrgafosTM 168), lactones
(e.g.,
substituted benzofuranone), hydroxylamine, and MEHQ (monomethyl ether of
hydroquinone)); surfactants (e.g., anionic fatty acid surfactants (e.g.,
sodium lauryl
sulfate, sodium dodecylbenzenesulfonate, sodium stearate, and sodium
palmitate), cationic
fatty acid surfactants (e.g., quaternary ammonium salts and amine salts), and
nonionic
ethoxylated surfactants (e.g., ethoxylated p-octylphenol)); and teachable
materials (i.e.,
permeation enhancers) (e.g., hydrophilic polymers (e.g., poly(ethylene
glycol),
polyvinylpyrrolidone, and polyvinyl alcohol)) and hydrophilic small molecules
(e.g.,
sodium chloride, glucose)). In addition, any impurities may be removed by
conventional
methods available to those skilled in the art.
In various embodiments, the polymer mixture includes a first polymer component
comprising one or more polymers selected from the group consisting of (i)
ethylene
copolymers with other alkylenes, (ii) polybutenes, (iii) aromatic group-
containing
copolymers, (iv) epichlorohydrin-containing polymers, (v) poly(alkylene-co-
alkyl(meth)acrylates), and (vi) diolefin-derived, non-aromatic polymers and
copolymers,
and a second polymer component selected from the group consisting of poly
(alkyl(meth)acrylates) and poly (aromatic(meth)acrylates) and having a
molecular weight
of from about 150 kilodaltons to about 500 kilodaltons, and in some
embodiments from
about 200 kilodaltons to about 400 kilodaltons.
These mixtures of polymers have proven useful with absolute polymer
concentrations (i.e., the total combined concentrations of both polymers in
the coating
composition), of between about 0.1 and about 50 percent (by weight), and in
some
embodiments, between about 0.1 and about 35 percent (by weight). Various
polymer
mixtures contain at least about 10 percent by weight of either the first
polymer or the
second polymer.
In some embodiments, the polymer composition may comprise about 5% to about
95% of the first and/or second polymers based on the total weights of the
first and second
polymers. In a another group of embodiments, the composition may comprise
about 15%
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
to about 85% of the first and/or second polymers. In some embodiments, the
composition
may include about 25% to about 75% of the first and/or second polymers.
In various embodiments, the bioactive agent may comprise about 1% to about 75%
of the first polymer, second polymer, and bioactive agent mixture (i.e.,
excluding solvents
5 and other additives). In some embodiments, the bioactive agent may comprise
about 5%
to about 60% of such a mixture. In some embodiments, the bioactive agent may
comprise
about 25% to about 45% of such a mixture. The concentration of the bioactive
agent or
agents dissolved or suspended in the coating mixture can range from about 0.01
to about
90 percent, by weight, based on the weight of the final coating composition,
and in some
10 embodiments, from about 0.1 to about 50 percent by weight.
The term "bioactive agent" and "active agent", as used herein, will refer to a
wide
range of biologically active materials or drugs that can be incorporated into
a coating
composition of the present invention. In some embodiments of the present
invention, the
bioactive agents) to be incorporated do not chemically interact with the
coating
15 composition during fabrication or during the bioactive agent release
process. The
bioactive agents as described herein may also be included in one or more
additional layers
or coatings, such as, for example, a pretreatment coating and/or protective
coating. In
embodiments so provided, the bioactive agent in the coating composition may be
the same
as or different than the bioactive agent included in the pretreatment coating
and/or
20 protective coating. Further, such bioactive agents may sometimes be
referred to herein as
the "pretreatment coating bioactive agent" or the "protective coating
bioactive agent."
An amount of biologically active agent can be applied to the device to provide
a
therapeutically effective amount of the agent to a patient receiving the
coated device.
Particularly useful agents include those that affect cardiovascular function
or that can be
used to treat cardiovascular-related disorders. In an embodiment, the active
agent includes
estradiol. In an embodiment, the active agent includes rapamycin. In an
embodiment, the
active agent includes paclitaxel.
Active agents useful in the present invention can include many types of
therapeutics including thrombin inhibitors, antithrombogenic agents,
thrombolytic agents,
fibrinolytic agents, anticoagulants, anti-platelet agents, vasospasm
inhibitors, calcium
channel blockers, steroids, vasodilators, anti-hypertensive agents,
antimicrobial agents,
antibiotics, antibacterial agents, antiparasite and/or antiprotozoal solutes,
antiseptics,
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
21
antifungals, angiogenic agents, anti-angiogenic agents, inhibitors of surface
glycoprotein
receptors, antimitotics, microtubule inhibitors, antisecretory agents, actin
inhibitors,
remodeling inhibitors, antisense nucleotides, anti-metabolites, miotic agents,
antiproliferatives, anticancer chemotherapeutic agents, anti-neoplastic
agents,
antipolymerases, antivirals, anti-AIDS substances, anti-inflammatory steroids
or non-
steroidal anti-inflammatory agents, analgesics, antipyretics,
immunosuppressive agents,
immunomodulators, growth hormone antagonists, growth factors, radiotherapeutic
agents,
peptides, proteins, enzymes, extracellular matrix components, ACE inhibitors,
free radical
scavengers, chelators, anti-oxidants, photodynamic therapy agents, gene
therapy agents,
anesthetics, immunotoxins, neurotoxins, opioids, dopamine agonists, hypnotics,
antihistamines, tranquilizers, anticonvulsants, muscle relaxants and anti-
Parkinson
substances, antispasmodics and muscle contractants, anticholinergics,
ophthalmic agents,
antiglaucoma solutes, prostaglandin, antidepressants, antipsychotic
substances,
neurotransmitters, anti-emetics, imaging agents, specific targeting agents,
and cell
response modifiers.
More specifically, in embodiments the active agent can include heparin,
covalent
heparin, synthetic heparin salts, or another thrombin inhibitor; hirudin,
hirulog, argatroban,
D-phenylalanyl-L-poly-L-arginyl chloromethyl ketone, or another
antithrombogenic agent; urokinase, streptokinase, a tissue plasminogen
activator, or
another thrombolytic agent; a fibrinolytic agent; a vasospasm inhibitor; a
calcium channel
blocker, a nitrate, nitric oxide, a nitric oxide promoter, nitric oxide
donors, dipyridamole,
or another vasodilator; HYTRIN~ or other antihypertensive agents; a
glycoprotein IIb/IIIa
inhibitor (abciximab) or another inhibitor of surface glycoprotein receptors;
aspirin,
ticlopidine, clopidogrel or another antiplatelet agent; colchicine or another
antimitotic, or
another microtubule inhibitor; dimethyl sulfoxide (DMSO), a retinoid, or
another
antisecretory agent; cytochalasin or another actin inhibitor; cell cycle
inhibitors;
remodeling inhibitors; deoxyribonucleic acid, an antisense nucleotide, or
another agent for
molecular genetic intervention; methotrexate, or another antimetabolite or
antiproliferative
agent; tamoxifen citrate, TAXOL~, paclitaxel, or the derivatives thereof,
rapamycin (or
other rapalogs), vinblastine, vincristine, vinorelbine, etoposide, tenopiside,
dactinomycin
(actinomycin D), daunorubicin, doxorubicin, idarubicin, anthracyclines,
mitoxantrone,
bleomycin, plicamycin (mithramycin), mitomycin, mechlorethamine,
cyclophosphamide
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
22
and its analogs, chlorambucil, ethylenimines, methylmelamines, alkyl
sulfonates (e.g.,
busulfan), nitrosoureas (carmustine, etc.), streptozocin, methotrexate (used
with many
indications), fluorouracil, floxuridine, cytarabine, mercaptopurine,
thioguanine,
pentostatin, 2-chlorodeoxyadenosine, cisplatin, carboplatin, procarbazine,
hydroxyurea,
morpholino phosphorodiamidate oligomer or other anti-cancer chemotherapeutic
agents;
cyclosporin, tacrolimus (FK-506), pimecrolimus, azathioprine, mycophenolate
mofetil,
mTOR inhibitors, or another immunosuppressive agent; cortisol, cortisone,
dexamethasone, dexamethasone sodium phosphate, dexamethasone, acetate,
dexamethasone derivatives, betamethasone, fludrocortisone, prednisone,
prednisolone,
6U-methylprednisolone, triamcinolone (e.g., triamcinolone acetonide), or
another steroidal
agent; trapidil (a PDGF antagonist), angiopeptin (a growth hormone
antagonist),
angiogenin, a growth factor (such as vascular endothelial growth factor
(VEGF)), or an
anti-growth factor antibody (e.g., ranibizumab, which is sold under the
tradename
LUCENTIS~), or another growth factor antagonist or agonist; dopamine,
bromocriptine
mesylate, pergolide mesylate, or another dopamine agonist; 6°Co (5.3
year half life), l9alr
(73.8 days), 32P ( 14.3 days), ' ~ lIn (68 hours), 9°Y (64 hours), 99Tc
(6 hours), or another
radiotherapeutic agent; iodine-containing compounds, barium-containing
compounds,
gold, tantalum, platinum, tungsten or another heavy metal functioning as a
radiopaque
agent; a peptide, a protein, an extracellular matrix component, a cellular
component or
another biologic agent; captopril, enalapril or another angiotensin converting
enzyme
(ACE) inhibitor; angiotensin receptor blockers; enzyme inhibitors (including
growth factor
signal transduction kinase inhibitors); ascorbic acid, alpha tocopherol,
superoxide
dismutase, deferoxamine, a 21-aminosteroid (lasaroid) or another free radical
scavenger,
iron chelator or antioxidant; a ~4C-, 3H-, ~3H-, 32P- or 36S-radiolabelled
form or other
radiolabelled form of any of the foregoing; an estrogen (such as estradiol,
estriol, estrone,
and the like) or another sex hormone; AZT or other antipolymerases; acyclovir,
famciclovir, rimantadine hydrochloride, ganciclovir sodium, Norvir, Crixivan,
or other
antiviral agents; 5-aminolevulinic acid, meta-tetrahydroxyphenylchlorin,
hexadecafluorozinc phthalocyanine, tetramethyl hematoporphyrin, rhodamine 123
or other
photodynamic therapy agents; an IgG2 Kappa antibody against Pseudomonas
aeruginosa
exotoxin A and reactive with A431 epidermoid carcinoma cells, monoclonal
antibody
against the noradrenergic enzyme dopamine beta-hydroxylase conjugated to
saporin, or
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
23
other antibody targeted therapy agents; gene therapy agents; enalapril and
other prodrugs;
PROSCAR~, HYTRIN~ or other agents for treating benign prostatic hyperplasia
(BHP);
mitotane, aminoglutethimide, breveldin, acetaminophen, etodalac, tolmetin,
ketorolac,
ibuprofen and derivatives, mefenamic acid, meclofenamic acid, piroxicam,
tenoxicam,
phenylbutazone, oxyphenbutazone, nabumetone, auranofin, aurothioglucose, gold
sodium
thiomalate, a mixture of any of these, or derivatives of any of these.
Other biologically useful compounds that can also be included in the coating
material include, but are not limited to, hormones, (3-blockers, anti-angina)
agents, cardiac
inotropic agents, corticosteroids, analgesics, anti-inflammatory agents, anti-
arrhythmic
agents, immunosuppressants, anti-bacterial agents, anti-hypertensive agents,
antimalarials,
anti-neoplastic agents, anti-protozoa) agents, anti-thyroid agents, sedatives,
hypnotics and
neuroleptics, diuretics, anti-parkinsonian agents, gastro-intestinal agents,
anti-viral agents,
anti-diabetics, anti-epileptics, anti-fungal agents, histamine H-receptor
antagonists, lipid
regulating agents, muscle relaxants, nutritional agents such as vitamins and
minerals,
stimulants, nucleic acids, polypeptides, and vaccines.
Antibiotics are substances which inhibit the growth of or kill microorganisms.
Antibiotics can be produced synthetically or by microorganisms. Examples of
antibiotics
include penicillin, tetracycline, chloramphenicol, minocycline, doxycycline,
vancomycin,
bacitracin, kanamycin, neomycin, gentamycin, erythromycin, geldanamycin,
geldanamycin analogs, cephalosporins, or the like. Examples of cephalosporins
include
cephalothin, cephapirin, cefazolin, cephalexin, cephradine, cefadroxil,
cefamandole,
cefoxitin, cefaclor, cefuroxime, cefonicid, ceforanide, cefotaxime,
moxalactam,
ceftizoxime, ceftriaxone, and cefoperazone.
Antiseptics are recognized as substances that prevent or arrest the growth or
action
of microorganisms, generally in a nonspecific fashion, e.g., either by
inhibiting their
activity or destroying them. Examples of antiseptics include silver
sulfadiazine,
chlorhexidine, glutaraldehyde, peracetic acid, sodium hypochlorite, phenols,
phenolic
compounds, iodophor compounds, quaternary ammonium compounds, and chlorine
compounds.
Antiviral agents are substances capable of destroying or suppressing the
replication
of viruses. Examples of anti-viral agents include a-methyl-
ladamantanemethylamine,
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
24
hydroxy-ethoxymethylguanine, adamantanamine, 5-iodo-2'-deoxyuridine,
trifluorothymidine, interferon, and adenine arabinoside.
Enzyme inhibitors are substances that inhibit an enzymatic reaction. Examples
of
enzyme inhibitors include edrophonium chloride, N-methylphysostigmine,
neostigmine
bromide, physostigmine sulfate, tacrine HCL, tacrine, 1-hydroxy maleate,
iodotubercidin,
p-bromotetramisole, 10-(a-diethylaminopropionyl)-phenothiazine hydrochloride,
calmidazolium chloride, hemicholinium-3,3,5-dinitrocatechol, diacylglycerol
kinase
inhibitor I, diacylglycerol kinase inhibitor II, 3-phenylpropargylaminie, N-
monomethyl-L-
arginine acetate, carbidopa, 3-hydroxybenzylhydrazine HC1, hydralazine HC1,
clorgyline
HC 1, deprenyl HC 1 L(-), deprenyl HC 1 D(+), hydroxylamine HC 1, iproniazid
phosphate,
6-Me0-tetrahydro-9H-pyrido-indole, nialamide, pargyline HC1, quinacrine HCI,
semicarbazide HC1, tranylcypromine HC1, N,N-diethylaminoethyl-2,2-
diphenylvalerate
hydrochloride, 3-isobutyl-1-methylxanthne, papaverine HC1, indomethacind, 2-
cyclooctyl-
2-hydroxyethylamine hydrochloride, 2,3-dichloro- a - methylbenzylamine (DCMB),
8,9-
dichloro-2,3,4,5-tetrahydro-1H-2-benzazepine
hydrochloride, p-aminoglutethimide, p-aminoglutethimide tartrate R(+),
paminoglutethimide tartrate S(-), 3-iodotyrosine, alpha-methyltyrosine L(-),
alphamethyltyrosine D(-), cetazolamide, dichlorphenarnide, 6-hydroxy-2-
benzothiazolesulfonamide, and allopurinol.
Anti-pyretics are substances capable of relieving or reducing fever. Anti-
inflammatory agents are substances capable of counteracting or suppressing
inflammation.
Examples of such agents include aspirin (salicylic acid), indomethacin, sodium
indomethacin trihydrate, salicylamide, naproxen, colchicine, fenoprofen,
sulindac,
diflunisal, diclofenac, indoprofen and sodium salicylamide.
Local anesthetics are substances that have an anesthetic effect in a localized
region.
Examples of such anesthetics include procaine, lidocaine, tetracaine and
dibucaine.
Imaging agents are agents capable of imaging a desired site, e.g., tumor, in
vivo.
Examples of imaging agents include substances having a label that is
detectable in vivo,
e.g., antibodies attached to fluorescent labels. The term antibody includes
whole
antibodies or fragments thereof.
Cell response modifiers are chemotactic factors such as platelet-derived
growth
factor (PDGF). Other chemotactic factors include neutrophil-activating
protein, monocyte
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
chemoattractant protein, macrophage-inflammatory protein, SIS (small inducible
secreted), platelet factor, platelet basic protein, melanoma growth
stimulating activity,
epidermal growth factor, transforming growth factor alpha, fibroblast growth
factor,
platelet-derived endothelial cell growth factor, insulin-like growth factor,
nerve growth
5 factor, bone growth/cartilage-inducing factor (alpha and beta), and matrix
metalloproteinase inhibitors. Other cell response modifiers are the
interleukins,
interleukin receptors, interleukin inhibitors, interferons, including alpha,
beta, and gamma;
hematopoietic factors, including erythropoietin, granulocyte colony
stimulating factor,
macrophage colony stimulating factor and granulocyte-macrophage colony
stimulating
10 factor; tumor necrosis factors, including alpha and beta; transforming
growth factors
(beta), including beta-1, beta-2, beta-3, inhibin, activin, and DNA that
encodes for the
production of any of these proteins, antisense molecules, androgenic receptor
blockers and
statin agents.
In an embodiment, the active agent can be in a microparticle. In an
embodiment,
15 microparticles can be dispersed on the surface of the substrate.
The weight of the coating attributable to the active agent can be in any range
desired for a given active agent in a given application. In some embodiments,
weight of
the coating attributable to the active agent is in the range of about 1
microgram to about 10
milligrams of active agent per cm2 of the effective surface area of the
device. By
20 "effective" surface area it is meant the surface amenable to being coated
with the
composition itself. For a flat, nonporous, surface, for instance, this will
generally be the
macroscopic surface area itself, while for considerably more porous or
convoluted (e.g.,
corrugated, pleated, or fibrous) surfaces the effective surface area can be
significantly
greater than the corresponding macroscopic surface area. In an embodiment, the
weight of
25 the coating attributable to the active agent is between about 0.01 mg and
about 0.5 mg of
active agent per cm2 of the gross surface area of the device. In an
embodiment, the weight
of the coating attributable to the active agent is greater than about 0.01 mg.
In some embodiments, more than one active agent can be used in the coating.
Specifically, co-agents or co-drugs can be used. A co-agent or co-drug can act
differently
than the first agent or drug. The co-agent or co-drug can have an elution
profile that is
different than the first agent or drug.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
26
In some embodiments, the active agent can be hydrophilic. In an embodiment,
the
active agent can have a molecular weight of less than 1500 daltons and can
have a water
solubility of greater than l Omg/mL at 25 °C. In some embodiments, the
active agent can
be hydrophobic. In an embodiment, the active agent can have a water solubility
of less
than l Omg/mL at 25 °C.
Some embodiments of the invention include a stmt coated with a coating
compositon including a first polymer, a second polymer, and at least one
bioactive agent
selected from the group of steroids and antiproliferatives. In some
embodiments, the
invention includes a wound dressing coated with a coating composition
including a first
polymer, a second polymer, and at least one bioactive agent selected from the
group
consisting of anesthetics, such as procaine, lidocaine, tetracaine and/or
dibucaine.
A comprehensive listing of bioactive agents can be found in The Merck Index,
Thirteenth Edition, Merck & Co. (2001 ), the entire contents of which is
incorporated by
reference herein. Bioactive agents are commercially available from Sigma
Aldrich (e.g.,
vincristine sulfate). The concentration of the bioactive agent or agents
dissolved or
suspended in the coating mixture can range from about 0.01 to about 90
percent, by
weight, based on the weight of the final coated composition. Additives such as
inorganic
salts, BSA (bovine serum albumin), and inert organic compounds can be used to
alter the
profile of bioactive agent release, as known to those skilled in the art.
In some embodiments, in order to provide a coating of the present invention, a
coating composition is prepared to include one or more solvents, a combination
of
complementary polymers dissolved in the solvent, and the bioactive agent or
agents
dispersed in the polymer/solvent mixture. The solvent, in some embodiments, is
one in
which the polymers form a true solution. The pharmaceutical agent itself may
either be
soluble in the solvent or form a dispersion throughout the solvent. Suitable
solvents
include, but are not limited to, alcohols (e.g., methanol, butanol, propanol
and
isopropanol), alkanes (e.g., halogenated or unhalogenated alkanes such as
hexane,
cyclohexane, methylene chloride and chloroform), amides (e.g.,
dimethylformamide),
ethers (e.g., tetrahydrofuran (THF ), dioxolane, and dioxane), ketones (e.g.,
methyl ethyl
ketone), aromatic compounds (e.g., toluene and xylene), nitrites (e.g.,
acetonitrile) and
esters (e.g., ethyl acetate). In some embodiments, THF and chloroform have
been found
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
27
to be effective solvents due to their excellent solvency for a variety of
polymers and
bioactive agents of the present invention.
A coating composition of this invention can be used to coat the surface of a
variety
of devices, and is particularly useful for those devices that will come in
contact with
aqueous systems. Such devices are coated with a coating composition adapted to
release
bioactive agent in a prolonged and controlled manner, generally beginning with
the initial
contact between the device surface and its aqueous environment.
The coated composition provides a means to deliver bioactive agents from a
variety of biomaterial surfaces. Various biomaterials include those formed of
synthetic
polymers, including oligomers, homopolymers, and copolymers resulting from
either
addition or condensation polymerizations. Examples of suitable addition
polymers
include, but are not limited to, acrylics such as those polymerized from
methyl acrylate,
methyl methacrylate, hydroxyethyl methacrylate, hydroxyethyl acrylate, acrylic
acid,
methacrylic acid, glyceryl acrylate, glyceryl methacrylate, methacrylamide,
and
acrylamide; vinyls, such as those polymerized from ethylene, propylene,
styrene, vinyl
chloride, vinyl acetate, vinyl pyrrolidone, and vinylidene difluoride.
Examples of
condensation polymers include, but are not limited to, nylons such as
polycaprolactam,
poly(lauryl lactam), poly(hexamethylene adipamide), and poly(hexamethylene
dodecanediamide), and also polyurethanes, polycarbonates, polyamides,
polysulfones,
polyethylene terephthalate), poly(lactic acid), poly(glycolic acid),
poly(lactic acid-co-
glycolic acid), polydimethylsiloxanes, polyetheretherketone, poly(butylene
terephthalate),
poly(butylene terephthalate-co-polyethylene glycol terephthalate), esters with
phosphorus
containing linkages, non-peptide polyamino acid polymers, polyiminocarbonates,
amino
acid-derived polycarbonates and polyarylates, and copolymers of polyethylene
oxides with
amino acids or peptide sequences.
Certain natural materials are also suitable biomaterials, including human
tissue
such as bone, cartilage, skin and teeth; and other organic materials such as
wood,
cellulose, compressed carbon, and rubber. Other suitable biomaterials include
metals and
ceramics. The metals include, but are not limited to, titanium, stainless
steel, and cobalt
chromium. A second class of metals include the noble metals such as gold,
silver, copper,
and platinum. Alloys of metals, such as nitinol (e.g. MP35), may be suitable
for
biomaterials as well. The ceramics include, but are not limited to, silicon
nitride, silicon
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
28
carbide, zirconia, and alumina, as well as glass, silica, and sapphire. Yet
other suitable
biomaterials include combinations of ceramics and metals, as well as
biomaterials that are
fibrous or porous in nature.
Optionally, the surface of some biomaterials can be pretreated (e.g., with a
silane
and/or Parylene TM coating composition in one or more layers) in order to
alter the surface
properties of the biomaterial. For example, in various embodiments of the
present
invention a layer of silane may be applied to the surface of the biomaterial
followed by a
layer of ParleneTM. Parylene TM C is the polymeric form of the low-molecular-
weight
dimer of para-chloro-xylylene. Shane and/or Parylene TM C (a material supplied
by
Specialty Coating Systems (Indianapolis)) can be deposited as a continuous
coating on a
variety of medical device parts to provide an evenly distributed, transparent
layer. In one
embodiment, the deposition of Parylene TM is accomplished by a process termed
vapor
deposition polymerization, in which dimeric Parylene TM C is vaporized under
vacuum at
150°C, pyrolyzed at 680°C to form a reactive monomer, then
pumped into a chamber
containing the component to be coated at 25°C. At the low chamber
temperature, the
monomeric xylylene is deposited on the part, where it immediately polymerizes
via a free-
radical process. The polymer coating reaches molecular weights of
approximately 500
kilodaltons.
Deposition of the xylylene monomer takes place in only a moderate vacuum (0.1
torr) and is not line-of sight. That is, the monomer has the opportunity to
surround all
sides of the part to be coated, penetrating into crevices or tubes and coating
sharp points
and edges, creating what is called a "conformal" coating. With proper process
control, it is
possible to deposit a pinhole-free, insulating coating that will provide very
low moisture
permeability and high part protection to corrosive biological fluids.
Adherence is a function of the chemical nature of the surface to be coated. It
has
been reported, for instance, that tantalum and silicon surfaces can be
overcoated with
silicon dioxide, then with plasma-polymerized methane, and finally with
Parylene TM C to
achieve satisfactory adherence.
Most applications of Parylene TM C coating in the medical device industry are
for
protecting sensitive components from corrosive body fluids or for providing
lubricity to
surfaces. Typical anticorrosion applications include blood pressure sensors,
cardiac-assist
devices, prosthetic components, bone pins, electronic circuits, ultrasonic
transducers,
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
29
bone-growth stimulators, and brain probes. Applications to promote lubricity
include
mandrels, injection needles, cannulae, and catheters.
Also, as previously described above, the surface to which the composition is
applied can itself be pretreated in other manners sufficient to improve
attachment of the
composition to the underlying (e.g., metallic) surface. Additional examples of
such
pretreatments include photografted polymers, epoxy primers, polycarboxylate
resins, and
physical roughening of the surface. It is further noted that the pretreatment
compositions
and/or techniques may be used in combination with each other or may be applied
in
separate layers to form a pretreatment coating on the surface of the medical
device.
As described above, the surface of a medical device may be roughened to
increase
adhesion of the coating composition to the medical device and/or alter elution
profiles.
Without intending to be bound by theory, the roughening of the surface
provides for a
greater surface area between the coating composition and the surface of the
medical
device, which may increase adhesion. Further, in embodiments with relatively
aggressive
roughening and/or relatively thin coatings, the peaks and valleys of the
roughened surface
may transfer through the coating composition, thereby increasing the surface
area of the
coating. Such increased surface area may alter the bioactive agent release
profile in situ.
The surface of the medical device may be roughened by any suitable method. In
some embodiments, the surface of the medical device may be roughened by
projecting
silica particles at the surface. The extent of the roughening may be
characterized by peak
to valley distances. For example, the extent of roughening may be
characterized by the
distance between the average of the ten highest peaks and the ten lowest
valleys. In some
embodiments, the extent of roughening may range from about 2 pm to about 20
Vim.
Optionally, the extent of roughening may range from about 5 pm to about 15 pm.
In some
embodiments, the extent of roughening may range from about 6.5 ~m to about 12
Vim.
In some embodiments, a tie-in layer may be utilized to facilitate one or more
physical and/or covalent bonds between layers. For example, the pretreatment
layer may
include a mufti-interface system to facilitate adhesion and cohesion
interaction relative to
the different materials positioned at the interface of each layer. For
example, the
application of Parylene pretreatments to metal surfaces may be aided by a
first application
of a reactive organosilane reagent. A reactive organosilane reagent containing
an
unsaturated pendant group is capable of participating with the Parylene
radicals as they
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
deposit on the surface from the vapor phase. After cleaning of the metal
surface, an
organosilane reagent with an unsaturated pendant group may be applied to the
metal oxide
surface on a metal substrate. Without intending to be bound by theory, it
appears that the
silicon in the organosilane reagent couples covalently to the metal oxide,
linking the
5 organosilane group to the surface. The substrate may then be placed in a
Parylene reactor
and exposed to the vapor-phase Parylene process. During this process, the
unsaturated
pendant groups on the organosilane-treated surface can react with the Parylene
diradicals
depositing from the vapor phase. This forms a covalent link between the
Parylene and the
organosilane layer. The Parylene also forms covalent bonds to itself as it
deposits. Thus,
10 this process yields a layered surface in which the layers are covalently
bonded to each
other. This forms a very strong bond between the Parylene and the metal
surface,
resulting in high durability to mechanical challenges. Further, in some
embodiments, the
Parylene may physically bond with the bioactive agent delivery coating or may
include a
reactive acrylate group that can be reacted with the bioactive agent delivery
coating to
15 improve durability to mechanical challenges.
The coating composition of the present invention can be used in combination
with
a variety of devices, including those used on a temporary, transient, or
permanent basis
upon and/or within the body.
Compositions of this invention can be used to coat the surface of a variety of
20 implantable devices, for example: drug-delivering vascular stems (e.g.,
self expanding
stems typically made from nitinol, balloon-expanded stems typically prepared
from
stainless steel); other vascular devices (e.g., grafts, catheters, valves,
artificial hearts, heart
assist devices); implantable defibrillators; blood oxygenator devices (e.g.,
tubing,
membranes); surgical devices (e.g., sutures, staples, anastomosis devices,
vertebral disks,
25 bone pins, suture anchors, hemostatic barriers, clamps, screws, plates,
clips, vascular
implants, tissue adhesives and sealants, tissue scaffolds); membranes; cell
culture devices;
chromatographic support materials; biosensors; shunts for hydrocephalus; wound
management devices; endoscopic devices; infection control devices; orthopedic
devices
(e.g., for joint implants, fracture repairs); dental devices (e.g., dental
implants, fracture
30 repair devices), urological devices (e.g., penile, sphincter, urethral,
bladder and renal
devices, and catheters); colostomy bag attachment devices; ophthalmic devices
(e.g. ocular
coils); glaucoma drain shunts; synthetic prostheses (e.g., breast);
intraocular lenses;
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
31
respiratory, peripheral cardiovascular, spinal, neurological, dental,
earlnose/throat (e.g.,
ear drainage tubes); renal devices; and dialysis (e.g., tubing, membranes,
grafts).
Examples of useful devices include urinary catheters (e.g., surface-coated
with
antimicrobial agents such as vancomycin or norfloxacin), intravenous catheters
(e.g.,
treated with antithrombotic agents (e.g., heparin, hirudin, coumadin), small
diameter
grafts, vascular grafts, artificial lung catheters, atrial septal defect
closures, electro-
stimulation leads for cardiac rhythm management (e.g., pacer leads), glucose
sensors
(long-term and short-term), degradable coronary stems (e.g., degradable, non-
degradable,
peripheral), blood pressure and stmt graft catheters, birth control devices,
benign prostate
and prostate cancer implants, bone repair/augmentation devices, breast
implants, cartilage
repair devices, dental implants, implanted drug infusion tubes, intravitreal
drug delivery
devices, nerve regeneration conduits, oncological implants, electrostimulation
leads, pain
management implants, spinal/orthopedic repair devices, wound dressings,
embolic
protection filters, abdominal aortic aneurysm grafts, heart valves (e.g.,
mechanical,
polymeric, tissue, percutaneous, carbon, sewing cuff), valve annuloplasty
devices, mural
valve repair devices, vascular intervention devices, left ventricle assist
devices, neuro
aneurysm treatment coils, neurological catheters, left atrial appendage
filters, hemodialysis
devices, catheter cuff, anastomotic closures, vascular access catheters,
cardiac sensors,.
uterine bleeding patches, urological catheters/stents/implants, in vitro
diagnostics,
aneurysm exclusion devices, and neuropatches.
Examples of other suitable devices include, but are not limited to, vena cava
filters,
urinary dialators, endoscopic surgical tissue extractors, atherectomy
catheters, clot
extraction catheters, percutaneous transluminal angioplasty catheters, PTCA
catheters,
stylets (vascular and non-vascular), coronary guidewires, drug infusion
catheters,
esophageal stems, circulatory support systems, angiographic catheters,
transition sheaths
and dilators, coronary and peripheral guidewires, hemodialysis catheters,
neurovascular
balloon catheters, tympanostomy vent tubes, cerebro-spinal fluid shunts,
defibrillator
leads, percutaneous closure devices, drainage tubes, thoracic cavity suction
drainage
catheters, electrophysiology catheters, stroke therapy catheters, abscess
drainage catheters,
biliary drainage products, dialysis catheters, central venous access
catheters, and parental
feeding catheters.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
32
Examples of medical devices suitable for the present invention include, but
are not
limited to catheters, implantable vascular access ports, blood storage bags,
vascular stems,
blood.tubing, arterial catheters, vascular grafts, intraaortic balloon pumps,
cardiovascular
sutures, total artificial hearts and ventricular assist pumps, extracorporeal
devices such as
blood oxygenators, blood filters, hemodialysis units, hemoperfusion units,
plasmapheresis
units, hybrid artificial organs such as pancreas or liver and artificial
lungs, as well as filters
adapted for deployment in a blood vessel in order to trap emboli (also known
as "distal
protection devices").
The compositions are particularly useful for those devices that will come in
contact
with aqueous systems, such as bodily fluids. Such devices are coated with a
coating
composition adapted to release bioactive agent in a prolonged and controlled
manner,
generally beginning with the initial contact between the device surface and
its aqueous
environment. It is important to note that the local delivery of combinations
of bioactive
agents may be utilized to treat a wide variety of conditions utilizing any
number of
medical devices, or to enhance the function and/or life of the device.
Essentially, any type
of medical device may be coated in some fashion with one or more bioactive
agents that
enhances treatment over use of the individual use of the device or bioactive
agent.
In various embodiments, the coating composition can also be used to coat
stems,
e.g., either self expanding stents, which are typically prepared from nitinol,
or balloon-
expandable stems, which are typically prepared from stainless steel. Other
stmt materials,
such as cobalt chromium alloys, can be coated by the coating composition as
well.
Devices which are particularly suitable include vascular stems such as self
expanding stems and balloon expandable stems. Examples of self expanding stems
useful
in the present invention are illustrated in U.S. Pat. Nos. 4,655,771 and
4,954,126 issued to
Wallsten and 5,061,275 issued to Wallsten et al. Examples of suitable balloon-
expandable
stems are shown in U.S. Pat. No. 4,733,665 issued to Palmaz, U.S. Pat. No.
4,800,882
issued to Gianturco and U.S. Pat. No. 4,886,062 issued to Wiktor.
In other embodiments, the coating composition can also be used to coat
ophthalmic
devices, e.g. ocular coils. A therapeutic agent delivery device that is
particularly suitable
for delivery of a therapeutic agent to limited access regions, such as the
vitreous chamber
of the eye and inner ear is described in U.S. patent number 6,719,750 and U.S.
Patent
Application Publication No. 2005/0019371 A1.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
33
The resultant coating composition can be applied to the device in any suitable
fashion (e.g., the coating composition can be applied directly to the surface
of the medical
device, or alternatively, to the surface of a surface-modified medical device,
by dipping,
spraying, ultrasonic deposition, or using any other conventional technique).
The
suitability of the coating composition for use on a particular material, and
in turn, the
suitability of the coated composition can be evaluated by those skilled in the
art, given the
present description. In one such embodiment, for instance, the coating
comprises at least
two layers which are themselves different. For instance, a base layer may be
applied
having bioactive agents) alone, or together with or without one or more of the
polymer
components, after which one or more topcoat layers are coated, each with
either first
and/or second polymers as described herein, and with or without bioactive
agent. These
different layers, in turn, can cooperate in the resultant composite coating to
provide an
overall release profile having certain desired characteristics, and in some
embodiments, for
use with bioactive agents of high molecular weight. In some embodiments, the
composition is coated onto the device surface in one or more applications of a
single
composition that includes first and second polymers, together with bioactive
agent.
However, as previously suggested a pretreatment layer or layers may be first
applied to the
surface of the device, wherein subsequent coating with the composition may be
performed
onto the pretreatment layer(s). The method of applying the coating composition
to the
device is typically governed by the geometry of the device and other process
considerations. The coating is subsequently cured by evaporation of the
solvent. The
curing process can be performed at room or elevated temperature, and
optionally with the
assistance of vacuum and/or controlled humidity.
It is also noted that one or more additional layers may be applied to the
coating
layers) that include bioactive agent. Such layers) or topcoats can be utilized
to provide a
number of benefits, such as biocompatibility enhancement, delamination
protection,
durability enhancement, bioactive agent release control, to just mention a
few. In one
embodiment the topcoat may include one or more of the first, second, and/or
additional
polymers described herein without the inclusion of a bioactive agent. In
various
embodiments, the topcoat includes a second polymer that is a
poly(alkyl(meth)acrylate).
An example of one embodiment of a poly(alkyl(meth)acrylate) includes poly (n-
butyl
methacrylate). In another embodiment, the first or second polymers could
further include
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
34
functional groups (e.g. hydroxy, thiol, methylol, amino, and amine-reactive
functional
groups such as isocyanates, thioisocyanates, carboxylic acids, acyl halides,
epoxides,
aldehydes, alkyl halides, and sulfonate esters such as mesylate, tosylate, and
tresylate) that
could be utilized to bind the topcoat to the adjacent coating composition. In
another
embodiment of the present invention one or more of the pretreatment materials
(e.g.
Parylene TM) may be applied as a topcoat. Additionally, biocompatible topcoats
(e.g.
heparin, collagen, extracellular matrices, cell receptors...) may be applied
to the coating
composition of the present invention. Such biocompatible topcoats may be
adjoined to the
coating composition of the present invention by utilizing photochemical or
thermochemical techniques known in the art. Additionally, release layers may
be applied
to the coating composition of the present invention as a friction barrier
layer or a layer to
protect against delamination. Examples of biocompatible topcoats that may be
used
include those disclosed in U.S. Patent No. U.S. Patent No. 4,979,959 and
5,744,515.
Optionally, a hydrophilic topcoat may be provided. Such topcoats may provide
several advantages, including providing a relatively more lubricious surface
to aid in
medical device placement in situ, as well as to further increase
biocompatibility in some
applications. Examples of hydrophilic agents that may be suitable for a
topcoat in
accordance with the invention includes polyacrylamide(36%)co-methacrylic
acid(MA)-
(10%)co-methoxy PEGl000MA-(4%)co-BBA-APMA compounds such as those described
in example 4 of US Patent Application Publication No. 200210041899,
photoheparin such
as described in example 4 of US Patent No. 5,563,056, and a photoderivatized
coating as
described in Example 1 of US Patent No. 6,706,408, the contents of each of
which is
hereby incorporated by reference.
In some embodiments, the topcoat may be used to control the elution rate of a
bioactive agent from a medical device surface. For example, topcoats may be
described as
the weight of the topcoat relative to the weight of the underlying bioactive
agent
containing layer. For example, the topcoat may be about 1 percent to about 50
percent by
weight relative to the underlying layer. In some embodiments, the topcoat may
be about 2
percent to about 25 percent by weight relative to the underlying layer.
Optionally, in some
embodiments, the topcoat may be about 5 percent to about 12 percent by weight
relative to
the underlying layer.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
Applicants have found that providing a relatively thin topcoat compared to the
underlying layer may significantly reduce initial drug elution rates to
provide for longer
elution times. For example, providing a topcoat weighing about 5% of the
underlying
layer may reduce initial elution rates (e.g., less than 20 hours) by more than
about 50%.
In some embodiments, the topcoat layer comprises a polymer that is also
included
in the underlying layer (e.g., first, second, and/or additional polymers as
described above).
Such topcoats may provide for superior adhesion between the top coat and the
underlying
layer.
Further, in some embodiments, one or more bioactive agents may be provided in
a
10 topcoat (sometimes referred to herein as a topcoat bioactive agent). The
topcoat bioactive
agent may be the same as or distinguishable from the bioactive agent included
in an
underlying layer. Providing bioactive agent within the topcoat allows for the
bioactive
agent to be in contact with surrounding tissue in situ while providing a
longer release
profile compared to coating compositions provided without topcoats. Such
topcoats may
15 also be used to further control the elution rate of a bioactive agent from
a medical device
surface, such as by varying the amount of bioactive agent in the topcoat. The
degree to
which the bioactive agent containing topcoat affects elution will depend on
the specific
bioactive agent within the topcoat as well as the concentration of the
bioactive agent
within the topcoat.
20 Any suitable amount of a bioactive agent may be included in the topcoat.
For
example, the upper limit of the amount of bioactive agent in the topcoat may
be limited
only by the ability of the topcoat to hold additional bioactive agent. In some
embodiments, the bioactive agent may comprise about 1 to about 75 percent of
the
topcoat. Optionally, the bioactive agent may comprise about 5 to about SO
percent of the
25 topcoat. In yet other embodiments, the bioactive agent may comprise about
10 to about 40
percent of the topcoat.
The polymer composition for use in this invention is generally biocompatible,
e.g.,
such that it results in no significant induction of inflammation or irritation
when
implanted. In addition, the polymer combination is generally useful throughout
a broad
30 spectrum of both absolute concentrations and relative concentrations of the
polymers.
This means that the physical characteristics of the coating, such as tenacity,
durability,
flexibility and expandability, will typically be adequate over a broad range
of polymer
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
36
concentrations. In turn, the ability of the coating to control the release
rates of a variety of
bioactive agents can be manipulated by varying the absolute and relative
concentrations of
the polymers.
Additionally, the coatings of the present invention are generally hydrophobic
and
limit the intake of aqueous fluids. For example, many embodiments of the
present
invention are coating compositions including two or more hydrophobic polymers
wherein
the resulting coating shows <10% (wt) weight change when exposed to water, and
in some
embodiments <5% (wt) weight change when exposed to water.
A coating composition can be provided in any suitable form, e.g., in the form
of a
true solution, or fluid or paste-like emulsion, mixture, dispersion or blend.
In some
embodiments, polymer combinations of this invention are capable of being
provided in the
form of a true solution, and in turn, can be used to provide a coating that is
both optically
clear (upon microscopic examination), while also containing a significant
amount of
bioactive agent. In turn, the coated composition will generally result from
the removal of
solvents or other volatile components and/or other physical-chemical actions
(e.g., heating
or illuminating) affecting the coated composition in situ upon the surface.
A further example of a coating composition embodiment may include a
configuration of one or more bioactive agents within an inner matrix
structure, for
example, bioactive agents within or delivered from a degradable encapsulating
matrix or a
microparticle structure formed of semipermeable cells and/or degradable
polymers. One or
more inner matrices may be placed in one or more locations within the coating
composition and at one or more locations in relation to the substrate.
Examples of inner
matrices, for example degradable encapsulating matrices formed of
semipermeable cells
and/or degradable polymers, are disclosed and/or suggested in U.S. Publication
No.
20030129130, U.S. Patent Application Serial No. 60/570,334 filed May 12, 2004,
U.S.
Patent Application Serial No. 60/603,707, filed August 23, 2004, U.S.
Publication No.
20040203075, filed April 10, 2003, U.S. Publication No. 20044202774 filed on
April 10,
2003, and U.S. Patent Application Serial No. 10/723,505, filed November 26,
2003, the
entire contents of which are incorporated by reference herein.
The overall weight of the coating upon the surface may vary depending on the
application. However, in some embodiments, the weight of the coating
attributable to the
bioactive agent is in the range of about one microgram to about 10 milligram
(mg) of
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
37
bioactive agent per cmz of the effective surface area of the device. By
"effective" surface
area it is meant the surface amenable to being coated with the composition
itself. For a
flat, nonporous, surface, for instance, this will generally be the
macroscopic, surface area
itself, while for considerably more porous or convoluted (e.g., corrugated,
pleated, or
fibrous) surfaces the effective surface area can be significantly greater than
the
corresponding macroscopic surface area. In various embodiments, the weight of
the
coating attributable to the bioactive agent is between about 0.005 mg and
about 10 mg,
and in some embodiments between about 0.01 mg and about 1 mg of bioactive
agent per
cm2 of the gross surface area of the device. This quantity of bioactive agent
is generally
required to provide desired activity under physiological conditions.
In turn, in various embodiments, the final coating thickness of a coated
composition will typically be in the range of about 0.1 micrometers to about
100
micrometers, and in some embodiments, between about 0.5 micrometers and about
25
micrometers. This level of coating thickness is generally required to provide
an adequate
concentration of drug to provide adequate activity under physiological
conditions.
The invention will be further described with reference to the following non-
limiting Examples. It will be apparent to those skilled in the art that many
changes can be
made in the embodiments described without departing from the scope of the
present
invention. Thus the scope of the present invention should not be limited to
the
embodiments described in this application, but only by the embodiments
described by the
language of the claims and the equivalents of those embodiments. Unless
otherwise
indicated, all percentages are by weight.
EXAMPLES
TEST PROCEDURES
The potential suitability of particular coated compositions for in vivo use
can be
determined by a variety of screening methods, examples of each of which are
described
herein. Not all of these test procedures were used in connection with the
example
included in this application, but they are described here to enable consistent
comparison of
coatings in accordance with the invention.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
38
Sample Preparation Procedure
Stainless steel stems used in the following examples were manufactured by
Laserage Technology Corporation, Waukegan, IL. In some cases, the metal
surface of the
stems may be coated without any pretreatment beyond washing. In other cases, a
primer
may be applied to the stems by first cleaning the stems with aqueous base,
then pre-
treating with a silane followed by vapor deposition of ParyleneTM polymer. The
silane
used may be [3-(methacroyloxy)propyl] trimethoxysilane, available from Sigma-
Aldrich
Fine Chemicals as Product No. 44,015-9. The silane may be applied as
essentially a
monolayer by mixing the silane at a low concentration in 50/50 (vol)
isopropanol/water,
soaking the stems in the aqueous silane solution for a suitable length of time
to allow the
water to hydrolyze the silane and produce some cross-linking, washing off
residual silane,
then baking the silane-treated stmt at 100°C for conventional periods
of time. Following
the silane treatment, ParyleneTM C coating (available from Union Carbide
Corporation,
Danbury, CT) may be vapor-deposited at a thickness of about 1 mm. Prior to
coating, the
stems should be weighed on a microbalance to determine a tare weight.
Bioactive agent/polymer solutions may be prepared at a range of concentrations
in
an appropriate solvent (typically tetrahydrofuran or chloroform), in the
manner described
herein. In all cases the coating solutions are applied to respective stems by
spraying, and
the solvent is allowed to evaporate under ambient conditions. The coated stems
are then
re-weighed to determine the mass of coating and consequently the mass of
polymer and
bioactive agent.
Rapamycin Release Assay Procedure
The Rapamycin Release Assay Procedure, as described herein, was used to
determine the extent and rate of release of an exemplary bioactive agent,
rapamycin, under
in vitro elution conditions. Spray-coated stems prepared using the Sample
Preparation
Procedure were placed in sample baskets into 10 milliliters of SotaxTM
dissolution system
(elution media containing 2% (wt) surfactant/water solution, available from
Sotax
Corporation, Horsham, PA). Amount of bioactive agent elution was monitored by
UV
spectrometry over the course of several days. The elution media was held at
37°C. After
the elution measurements, the stems were removed, rinsed, dried, and weighed
to compare
measured bioactive agent elution to weighed mass loss.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
39
Dexamethasone Release Assay Procedure
The Dexamethasone Release Assay Procedure, as described herein, may be used to
determine the extent and rate of dexamethasone release under in vitro
conditions. Spray-
coated stems made using the Sample Preparation Procedure are placed in 10
milliliters of
pH 7 phosphate buffer solution ("PBS") contained in an amber vial. A magnetic
stirrer
bar is added to the vial, and the vial with its contents are placed into a
37°C water bath.
After a sample interval, the stmt is removed and placed into a new buffer
solution
contained in a new vial. Dexamethasone concentration in the buffer is measured
using
ultraviolet spectroscopy and the concentration converted to mass of bioactive
agent
released from the coating. After the experiment, the stmt is dried and weighed
to correlate
actual mass loss to the loss measured by the elution experiment.
Durability Test Procedure
The durability of the coated composition can be determined by the following
manner. To simulate use of the coated devices, the coated stems are placed
over sample
angioplasty balloons. The stmt is then crimped onto the balloon using a
laboratory test
crimper (available from Machine Solutions, Brooklyn, NY). The stmt and balloon
are
then placed in a phosphate buffer bath having a pH of 7.4 and temperature of
37°C. After
S minutes of soaking, the balloon is expanded using air at 5 atmospheres (3800
torr) of
pressure. The balloon is then deflated, and the stmt is removed.
The stmt is then examined by optical and scanning electron microscopy to
determine the amount of coating damage caused by cracking and/or delamination
and a
rating may be assigned. Coatings with extensive damage are considered
unacceptable for
a commercial medical device. The "Rating" is a qualitatitive scale used to
describe the
amount of damage to the coating from the stmt crimping and expansion procedure
based
on optical microscopy examination by an experienced coating engineer. A low
rating
indicates a large percentage of the coating cracked, smeared, and/or
delaminated from the
surface. For example, a coating with a rating of ten shows no damage while one
with a
rating of 1 will show a majority of the coating damaged to the point where
clinical
efficacy maybe diminished. Commercially attractive coatings typically have a
rating of
nine or higher.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
Stress-Strain Measurement Test Procedure
Polymer films can be prepared by hot pressing polymer beads at
100°C in a
constant film maker kit to a thickness of approximately 0.5 mm. The resulting
films are
cut into strips using a razor blade. A Q800 Dynamic Mechanical Analyzer
(available from
5 Texas Instruments, Dallas, TX) may be fitted with a film tension clamp. Each
sample is
equilibrated at 35°C for five minutes prior to straining the sample.
Then the sample is
loaded into the clamp such that the sample length is between 5 and 7 mm in
length. A
static force of O.O1N is applied to each sample throughout the measurements.
Simultaneously, a 0.5 N/min force is applied to the sample until the movable
clamp
10 reaches its maximum position. Films are elongated at constant stress and
the average
tensile modulus (i.e., the initial slope of the stress-strain curve, in MPa)
can be determined.
Example 1
Release of Rapamycin from Polyethylene-co-prop, l~ and Poly~butyl
methacrylate)
15 Three solutions were prepared for coating the stems. The solutions included
mixtures of polyethylene-co-propylene) ("PEPP", available from Sigma-Aldrich
Fine
Chemicals, Milwaukee, WI, as Product No. 18,962-6, contains 60% (mole)
ethylene,
having Mw of approximately 170 kilodaltons ), "PBMA" and "RAPA" ("PBMA",
available from Sigma-Aldrich Fine Chemicals as Product No. 18,152-8, having a
weight
20 average molecular weight (Mw) of about 337 kilodaltons), and rapamycin
("RAPA",
available from LC Laboratories, Woburn, MA) dissolved in THF to form a
homogeneous
solution. The stems were not given a primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
25 1 ) 16 mg/ml PEPP / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mg/ml PEPP / 10 mg/ml PBMA / 10 mg/ml RAPA
3) 4 mg/ml PEPP / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
30 stmt was monitored using the Rapamycin Release Assay Procedure.
Results, provided in Figure l, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of PEPP and PBMA in the polymer mixture as described herein.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
41
Example 2
Release of Rapamycin from Poly(e~ichlorol~drin~ and Poly(butvl methacrylate~
Three solutions were prepared for coating the stems. The solutions included
mixtures of poly(epichlorohydrin) ("PECH", available from Scientific Polymer
Products
as Catalog #127, CAS #24969-06-0, Mw approximately 700 kilodaltons),
poly(butyl
methacrylate) ("PBMA", available from Sigma-Aldrich Fine Chemicals as Product
No.
18,152-8, having a weight average molecular weight (Mw) of about 337
kilodaltons), and
rapamycin ("RAPA", available from LC Laboratories, Woburn, MA) dissolved in
tetrahydrofuran (THF) to form a homogeneous solution. The stems were not given
a
primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
1 ) 16 mg/ml PECH / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mg/ml PECH / 10 mg/ml PBMA 1 10 mg/ml RAPA
3) 4 mg/ml PECH / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Rapamycin Release Assay Procedure.
Results, provided in Figure 2, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of PECH and PBMA in the polymer mixture as described herein.
Example 3
Release of Rapamycin from Poly(isobutylene) and Po~~bu~l methac~late)
Three solutions were prepared for coating the stems. The solutions included
mixtures of poly(isobutylene) ("PIB", available from Scientific Polymer
Products as
Catalog #681, CAS #9003-27-4, Mw approx. 85 kilodaltons), ("PBMA", available
from
Sigma-Aldrich Fine Chemicals as Product No. 18,152-8, having a weight average
molecular weight (Mw) of about 337 kilodaltons), and rapamycin ("RAPA",
available
from LC Laboratories, Woburn, MA) dissolved in THF to form a homogeneous
solution.
The stems were not given a primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
42
1 ) 16 mg/ml PIB / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mg/ml PIB ! 10 mg/ml PBMA ! 10 mgiml RAPA
3) 4 mg/ml PIB / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Rapamycin Release Assay Procedure.
Results, provided in Figure 3, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of PIB and PBMA in the polymer mixture as described herein.
Example 4
Release of RapamYcin from Polystyrene-co-butadiene and Poly~butyl
methacrylate)
Three solutions were prepared for coating the stems. The solutions included
mixtures of polystyrene-co-butadiene) copolymer ("SBR", available from
Scientific
Polymer Products, Inc. Catalog #100, contains 23% (wt) styrene), poly(butyl
methacrylate) ("PBMA", available from Sigma-Aldrich Fine Chemicals as Product
No.
18,152-8, having a weight average molecular weight (Mw) of about 337
kilodaltons), and
rapamycin ("RAPA", available from LC Laboratories, Woburn, MA) dissolved in
THF to
form a homogeneous solution. The stems were not given a primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
1) 16 mg/ml SBR / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mg/ml SBR / 10 mg/ml PBMA / 10 mglml RAPA
3) 4 mg/ml SBR / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Rapamycin Release Assay Procedure.
Results, provided in Figure 4, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of SBR and PBMA in the polymer mixture as described herein.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
43
Example 5
Release of Rapamycin from Polyethylene-co-methyl acrylate)
and Poly(butyl methacrXlate)
Three solutions were prepared for coating the stems. All three solutions
included
mixtures of polyethylene-co-methyl acrylate) ("PEMA", available from Focus
Chemical
Corp. Portsmouth, NH, containing 28% (wt) methyl acrylate), poly(butyl
methacrylate)
("PBMA", available from Sigma-Aldrich Fine Chemicals as Product No. 18,152-8,
having
a weight average molecular weight (Mw) of about 337 kilodaltons), and
rapamycin
("RAPA", available from LC Laboratories, Woburn, MA) dissolved in
tetrahydrofuran
(THF) to form a homogeneous solution. The stems were not given a primer pre-
treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
1 ) 16 mg/ml PEMA / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mg/ml PEMA / 10 mg/ml PBMA / 10 mg/ml RAPA
3) 4 mg/ml PEMA / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Rapamycin Release Assay Procedure.
Results, provided in Figure 5, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of PEMA and PBMA in the polymer mixture as described herein.
The
lines in Figure 5 and similar figures are expressed in terms of percent by
weight of the first
and second polymers, respectively, in the coated compositions. This can be
compared to
the amounts provided above, which are stated in terms of "mg/ml" of the
respective
polymers in the coating compositions themselves, which are applied to the
stems. Hence
"54/13" corresponds to the coated compositions that results from the use of
the first
coating composition above, which upon removal of the solvent provides a coated
composition having 54% PEMA and 13% PBMA respectively, by weight.
Alternatively,
solutions such as the second solution above, e.g., which includes equal
amounts (by
weight) of the ingredients, will alternatively be referred to herein as
"33/33/33",
representing the weight ratio of ingredients to each other.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
44
Example 6
Release of Dexamethasone from Poly(ethXlene-co-methyl acrylate) and
Poly(butyl methacr~)
Three solutions were prepared for coating the stems. All three solutions
included
mixtures of polyethylene-co-methyl acrylate) ("PEMA"), poly(butyl
methacrylate)
"PBMA", and dexamethasone ("DEXA", available as Product No. 86,187-1 from
Sigma
Aldrich Fine Chemicals) dissolved in THF to form a homogeneous solution. The
stems
were not given a primer pre-treatment. The solutions were prepared to include
the
following ingredients at the stated weights per milliliter of THF:
1 ) 20 mg/ml PEMA / 0 mg/ml PBMA / 10 mg/ml DEXA
2) 10 mg/ml PEMA / 10 mg/ml PBMA / 10 mg/ml DEXA
3) 0 mg/ml PEMA / 20 mg/ml PBMA / 10 mg/ml DEXA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Dexamethasone Release Assay Procedure.
Results, provided in Figure 6, demonstrate the ability to control the elution
rate of
dexamethasone, a pharmaceutical agent, from a stmt surface by varying the
relative
concentrations of PEMA and PBMA in the polymer mixture.
Example 7
Surface Characterization of Coated Stents after Crimping and Expansion
Using the Sample Preparation Procedure, stems were sprayed with a coating of
second polymer/poly(butyl methacrylate)("PBMA")/rapamycin("RAPA"), mixed at a
weight ratio of 33/33/33 at 10 mg/ml each of THF. The first polymer was
poly(ethylene-
co-methyl acrylate) ("PEMA", available from Focus Chemical Corp. Portsmouth,
NH,
containing 28% (wt) methyl acrylate). The second polymer used was PBMA from
Sigma-Aldrich Fine Chemicals as Product No. 18,152-8, having a weight average
molecular weight (Mw) of about 337 kilodaltons. Stems were either used as
received (i.e.,
uncoated metal), were pre-treated with a silane/ParyleneTM primer using the
primer
procedure described in the Sample Preparation Procedure, were not pre-treated
with
primer but were given a subsequent PBMA topcoat using the spraying process
described
in the Sample Preparation Procedure, or were given both a silane/ParyleneTM
pre-treatment
primer and subsequent PBMA topcoat.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
After preparing the coated stems and allowing all solvents to dry at ambient
conditions, the stems were examined with an optical microscope under both
"bright field"
and "dark field" conditions. All coatings were optically transparent (i.e.,
clear, showing
no cloudiness). Raman microscopy taken of the coated stems of PEMA as first
polymer,
5 applied to bare metal stmt, indicated a high degree of homogeneity of mixing
of drug and
polymers.
The coated stems were crimped down on balloons and were expanded following
the Durability Test Procedure, which showed that, overall, all the coatings
remained intact
(i.e., the coating did not peel off or flake off, etc.), with only a few
localized sites where
10 coating delaminated from the metal stmt. When primer coatings were used,
essentially no
delamination was evident and cracks were all smaller than about 10 microns in
width.
Almost all stems had some degree of cracking of the coating around bends in
the struts, as
well as some mechanical damage caused by handling or balloon expansion. Adding
a
PBMA topcoat did not adversely affect the mechanical integrity of the coating
on the stmt
15 after crimping and expansion, as might be expected with an overall thicker
stmt coating.
Based on both the drug-eluting test results and mechanical test results,
coatings
containing bioactive agents incorporated into blends of PBMA with PEMA as the
first
polymer are viable candidates for commercial applications in drug-eluting
stems and are
expected to be particularly effective in minimizing the onset of restenosis
after stmt
20 implantation.
Example 8 and Comparative Example C 1
Stress-Strain Measurements for First and/or Additional Polymers
Tensile properties of various first polymers and additional polymers of this
25 invention were tested and average tensile modulus calculated using the
Stress-Strain
Measurement Test Procedure. The first and/or additional polymers evaluated
were
polyethylene-co-methyl acrylate) ("PEMA", same as used in Example 5),
poly(ethylene-
co-butyl acrylate) ("PEBA", containing 35% (wt) butyl acrylate, available from
Focus
Chemical Corp., Portsmouth, NH), polybutadiene ("PBD", available from
Scientific
30 Polymers Products, Inc., Ontario, NY, as Catalog # 688; CAS #31567-90-5; 7%
cis 1,4;
93% vinyl 1,2; Mw approx. 100 kilodaltons) and polyethylene-co-vinyl acetate)
("PEVA", available as Product No. 34,691-8 from Sigma-Aldrich Fine Chemicals).
PEVA was run as a comparative example.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
46
Stress-strain curves are shown in Figure 7. The calculated average tensile
modulus
for each of the tested polymers is shown in Table 1.
Table 1
Average Tensile
Example Polymer
Modulus, MPa (SD)
8a PEMA 5.54 (0.49)
8b PEBA 3.66 (0.67)
8c PBD 34.87 (4.83)
C 1 PEVA 2.17 (0.46
The data from Table 1 show that, when compared to PEVA, each of the first
polymers showed a higher average tensile modulus. The average tensile modulus
for the
PBD was significantly higher than that for any of the other polymers.
Example 9
Raman Microscopy
Raman measurements were made with a WITec CRM200 scanning confocal
Raman microscope. The Raman microscope can optically dissect a layer of
coating on a
stmt, looking into the coating and imaging the distribution of the coating
composition
ingredients within the thin coating. Since no Raman signal is obtained from
air and steel
materials, the air above the coating surface is black as is the steel
substrate upon which the
coating is deposited.
Figure 8 shows a 100 micron wide and 10 micron deep image (including a 10
micron bar in the lower left-hand corner for scale) taken by measuring the
Raman
intensity at 2900 cm ~ for a stmt with a 33/33/33 PEMA/PBMA/rapamycin coating.
Since
each of the composition ingredients, including first and second polymers as
well as
bioactive agent, contribute signal at this wavenumber, the image obtained is
one of the
entire coating. Figure 9 shows Raman intensity at 1630 cm 1 for the same
region of stmt
coating shown in Figure 8. When measuring the Raman intensity at 1630 cm-~,
only the
intensity of the bioactive agent signal is measured (the first and second
polymers do not
emit at this wavenumber), and so an image of the distribution of the bioactive
agent within
the coating is obtained (Figure 9).
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
47
Comparison of Figures 8 and 9 indicates that the bioactive agent is uniformly
distributed within the entire coating, since the intensity of the Raman signal
of the agent
varies only subtly from one region of the coating to another. Similar results
are seen with
other compositions of the present invention.
Example 10
Release of Rapamycin from Poly~butadiene) and Poly(but~,l methacr~ate)
Three solutions were prepared for coating the stems. The solutions included
mixtures of poly(1,2-butadiene) ("PBD", available from Scientific Polymers
Products,
Inc., Ontario, NY, as Catalog # 688; CAS #31567-90-5; 7% cis 1,4; 93% vinyl
1,2; Mw
approx. 100 kilodaltons), poly(butyl methacrylate) ("PBMA", available from
Sigma-
Aldrich Fine Chemicals as Product No. 18,152-8, having a weight average
molecular
weight (Mw) of about 337 kilodaltons), and rapamycin ("RAPA", available from
LC
Laboratories, Woburn, MA) dissolved in THF to form a homogeneous solution. The
scents were not given a primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
1) 16 mg/ml PBD / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mgiml PBD / 10 mg/ml PBMA / 10 mglml RAPA
3) 4 mg/ml PBD / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Rapamycin Release Assay Procedure.
Results, provided in Figure 10, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of PBD and PBMA in the polymer mixture as described herein. The
lines
in Figure 10 and similar figures are expressed in terms of percent by weight
of the first and
second polymers, respectively, in the coated compositions. This can be
compared to the
amounts provided above, which are stated in terms of "mg/ml" of the respective
polymers
in the coating compositions themselves, which are applied to the stems. Hence
"54/13"
corresponds to the coated compositions that results from the use of the first
coating
composition above, which upon removal of the solvent provides a coated
composition
having 54% PBD and 13% PBMA respectively, by weight. Alternatively, solutions
such
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
48
as the second solution above, e.g., which includes equal amounts (by weight)
of the
ingredients, will alternatively be referred to herein as "33/33/33",
representing the weight
ratio of ingredients to each other.
Additionally, the durability for PBD/PBMA coatings was also analyzed. Stems
were coated with PBD and PBMA in a procedure as described above but without
any
bioactive agent. The stems were then tested according to the method described
in the
Durability Test Procedure section. The results are displayed in Figure 10A.
The
PBD/PBMA coatings showed very little damage in the form of some small cracks
that did
not appear to reach the stmt surface. These coatings were applied to bare
metal stems
before ethylene oxide sterilization ("sterilization"), Parylene TM coated
stems before
sterilization, and Parylene TM coated stems after sterilization. These were
labeled in
Figure 10A "Bare Metal Pre-Sterile," "Parylene Pre-Sterile," and "Parylene
Post-Sterile,"
respectively. Parylene TM treatments and sterilization had little effect on
the exceptional
durability of the PBD/PBMA coatings.
Example 11
Release of Rapamycin from Poly(butadiene-co-acrylonitrile) and Poly~butyl
methacr~)
Three solutions were prepared for coating the stems. The solutions included
mixtures of
poly(butadiene-co-acrylonitrile) ("PBDA," available from Scientific Polymer
Products,
Inc., Catalog #533, contains 41% (wt) acrylonitrile), "PBMA" and "RAPA"
("PBMA" and
"RAPA" were obtained and used as described in Example 1) dissolved in THF to
form a
homogeneous solution. The stems were not given a primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
1) 16 mg/ml PBDA / 4 mg/ml PBMA / 10 mg/ml RAPA
2) 10 mg/ml PBDA / 10 mg/ml PBMA / 10 mg/ml RAPA
3) 4 mg/ml PBDA / 16 mg/ml PBMA / 10 mg/ml RAPA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Rapamycin Release Assay Procedure.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
49
Results, provided in Figure 11, demonstrate the ability to control the elution
rate of
rapamycin, a pharmaceutical agent, from a coated stmt surface by varying the
relative
concentrations of PBDA and PBMA in the polymer mixture as described herein.
Example 12
Release of Dexamethasone from Poly(butadiene~and Pol~(butyl methacr~ate)
Three solutions were prepared for coating the stems. All three solutions
included
mixtures of poly(1,2-butadiene) "PBD", poly(butyl methyl acrylate) ("PBMA"),
and
dexamethasone ("DEXA") dissolved in THF to form a homogeneous solution. The
stems
were not given a primer pre-treatment.
The solutions were prepared to include the following ingredients at the stated
weights per milliliter of THF:
1) 20 mg/ml PBD / 0 mg/ml PBMA l 10 mg/ml DEXA
2) 10 mg/ml PBD / 10 mg/ml PBMA / 10 mg/ml DEXA
3) 0 mglml PBD / 20 mg/ml PBMA / 10 mg/ml DEXA
Using the Sample Preparation Procedure, two stems were spray coated using each
solution. After solvent removal via ambient evaporation, the drug elution for
each coated
stmt was monitored using the Dexamethasone Release Assay Procedure.
Results, provided in Figure 12, demonstrate the ability to control the elution
rate of
dexamethasone, a pharmaceutical agent, from a stmt surface by varying the
relative
concentrations of PBD and PBMA in the polymer mixture.
Example 13
Surface Characterization of Coated Stents after Crimping and Expansion
Using the Sample Preparation Procedure, stems were sprayed with a coating of
second polymer/poly(butyl methacrylate)("PBMA")/rapamycin("RAPA"), mixed at a
weight ratio of 33/33/33 at 10 mg/ml each of THF. The first polymer was
polybutadiene
("PBD", available from Scientific Polymers Products, Inc., Ontario, NY, as
Catalog # 688;
CAS #31567-90-5; 7% cis 1,4; 93% vinyl 1,2; Mw approx. 100 kilodaltons), and a
polymer from the additional polymer class was polyethylene-co-methyl acrylate)
("PEMA", available from Focus Chemical Corp. Portsmouth, NH, containing 28%
(wt)
methyl acrylate). The second polymer used was PBMA from Sigma-Aldrich Fine
Chemicals as Product No. 18,152-8, having a weight average molecular weight
(Mw) of
about 337 kilodaltons. Stems were either used as received (i.e., uncoated
metal), were pre-
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
treated with a silane/ParyleneTM primer using the primer procedure described
in the
Sample Preparation Procedure, were not pre-treated with primer but were given
a
subsequent PBMA topcoat using the spraying process described in the Sample
Preparation
Procedure, or were given both a silane/ParyleneTM pre-treatment primer and
subsequent
5 PBMA topcoat.
After preparing the coated stems and allowing all solvents to dry at ambient
conditions, the stems were examined with an optical microscope under both
"bright field"
and "dark field" conditions. All coatings were optically transparent (i.e.,
clear, showing
no cloudiness). Raman microscopy taken of the coated stems of (PEMA as the
additional
10 polymer, applied to bare metal stmt) and (PBD as the first polymer, applied
to bare metal
stmt) indicated a high degree of homogeneity of mixing of drug and polymers.
The coated stems were crimped down on balloons and were expanded following
the Durability Test Procedure, which showed that, overall, all the coatings
remained intact
(i.e., the coating did not peel off or flake off, etc.), with only a few
localized sites where
15 coating delaminated from the metal stmt. When primer coatings were used,
essentially no
delamination was evident and cracks were all smaller than about 10 microns in
width.
Almost all stems had some degree of cracking of the coating around bends in
the struts, as
well as some mechanical damage caused by handling or balloon expansion. Adding
a
PBMA topcoat did not adversely affect the mechanical integrity of the coating
on the stmt
20 after crimping and expansion, as might be expected with an overall thicker
stmt coating.
Based on both the drug-eluting test results and mechanical test results,
coatings
containing bioactive agents incorporated into blends of PBMA with either PEMA
or PBD
as the other polymer are viable candidates for commercial applications in drug-
eluting
stems and are expected to be particularly effective in minimizing the onset of
restenosis
25 after stmt implantation.
Example 14
Scanning Electron Microscopy
Scanning Electron Microscopy can be used to observe coating quality and
30 uniformity on stems at any suitable point in their manufacture or use.
Crimped and
expanded stems were examined for coating failures in fine microscopic detail
using a
scanning electron microscope (SEM) at magnifications varying from ISOX to
5000X.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
51
Various coating defects tend to affect the manufacture and use of most polymer
coated stems in commercial use today, including the appearance of cracks or
tears within
the coating, smearing or displacement of the coating, as well as potentially
even
delamination of the coating in whole or in part. Such defects can occur upon
formation of
the coating itself, or more commonly, in the course of its further
fabrication, including
crimping the stmt upon an inflatable balloon, or in surgical use, which would
include
manipulating the stmt and expanding the balloon to position the stmt in vivo.
Figure 13 shows a scanning electron microscope image from a LEO Supra-35 VP
at 250X of a 33/33/33 PBD/PBMA/rapamycin coating on a stmt after conventional
crimping and balloon expansion procedures. The image shows that the coated
composition
maintains integrity after expansion, showing no evidence of delamination or
cracks.
When observed by SEM, many other compositions tended to show cracks,
however, typically of a type and number that are certainly on par with those
in commercial
use today, and would tend to be well within acceptable range, particularly
considering that
neither the coating compositions, or manner of applying particular
compositions, have yet
been optimized for any particular combination of surface, polymers, bioactive
agent. The
cracks were typically a few microns in width, with thin strands of polymer
stretching
between the edges of the crack. Overall, however, the coatings looked smooth,
uniform,
and in good condition.
Almost all the stems had some degree of cracking of the coating around bends
in
the struts, as well as some mechanical damage caused by handling or balloon
expansion.
Most surprisingly, polybutadiene-containing coatings exhibited less cracking
and in one
case no cracks, and when cracks occurred, they were typically smaller in size
in
comparison with the cracks found in PEMA or PEVA-containing coatings. For
comparison, cracks which opened up and delaminated from the metal stmt surface
were
found in coatings containing PEMA and PEVA in the absence of a Parylene TM
primer
coating. Polybutadiene-containing coatings without Parylene TM primer, as well
as
comparative PEMA (or comparative PEVA)-containing coatings with Parylene TM
primer,
showed cracks which tended to not result in delamination.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
52
Example 15
Release of Rapamycin from Poly(butadiene) and Poly~butyl methac~late)
provided with a Topcoat
Several solutions were prepared for coating non-sterile, non-deployed, self
expanding nitinol coronary stems having a primer layer. The solutions included
mixtures
of poly(1,2-butadiene) ("PBD", available from Scientific Polymers Products,
Inc., Ontario,
NY), poly(butyl methacrylate) ("PBMA", available from Sigma-Aldrich Fine
Chemicals),
and rapamycin ("RAPA", available from LC Laboratories, Woburn, MA) dissolved
to
form a homogeneous solution. In addition, a topcoat of PBMA was also prepared
and
applied to the coating composition on some of the stems, and the elution rate
profiles into
a 2% SLS buffer on a Sotax USP IV Apparatus were determined.
Results, provided in Figure 14, illustrates several elution rates of
rapamycin, a
pharmaceutical agent, from a coated stmt surface by varying the relative
concentrations of
rapamycin, PBD, and PBMA with and without utilizing a topcoat. Further, Figure
15
demonstrates the ability to control the elution rate of a bioactive agent by
varying the
amount of topcoat provided relative to the coating composition.
The lines in Figure 14 and Figure 15 are expressed in terms of percent by
weight of
the Rapamycin, PBD, and PBMA, respectively, in the coated compositions. Hence
"40/30/30" corresponds to the coated compositions that results from the use of
40%
Rapamycin, 30% PBD, and 30% PBMA, respectively, by weight. In Figure 15, the
weight
of the topcoat relative to the weight of the coating composition is shown. For
example,
6% topcoat corresponds to an amount of topcoat totaling 6% by weight of the
coating
composition weight.
Example 16
Release of Sirolimus from Polv(Butadiene) and Poly~butyl methacrylate) with
Poly~but,~l
methacrylate) and Sirolimus Topcoats
Stainless steel BX velocity stems manufactured by Cordis Corporation, Miami
Lakes, FL were used in the following examples. The stems were Parylene treated
and
weighted before coating.
Bioactive agent/polymer solutions were prepared at a range of concentrations
in an
appropriate solvent, in the manner described herein. The coating solutions
were applied to
respective stems by spraying procedures using an ultrasonic sprayer as
described in U.S.
Published Application 2004/0062875 (Chappa et al.); and in U.S. Application
Serial No.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
53
11/102,465, filed April 8, 2005 and entitled "Medical Devices and Methods for
Producing
Same." After spraying application of the bioactive agent/polymer solution, the
solvent was
allowed to evaporate. The coated stems were weighed to determine the mass of
coating
and consequently the mass of polymer and bioactive agent. The coating
thickness can be
measured using any suitable means, e.g. optical interferometry.
The Bioactive Agent Release Assay, as described herein, was used to determine
the extent and rate of drug release in vitro conditions. A Sotax dissolution
system (Sotax
Corporation, Horsham, PA) was utilized. The system used a 2wt%
surfactant/water
solution as elution media. The coated stems were placed in the sample baskets,
and the
drug elution monitored by UV spectrometry over the course of several days. The
elution
media was held at 37°C. After the elution measurement, the stems were
removed, rinsed,
dried, and weighed to compare measured drug elution to mass loss.
One basecoat solution was prepared for coating the stems. This solution
included
mixtures of "PBD" poly(butadiene), "PBMA" poly(butyl methacrylate), and
sirolimus
dissolved in tetrahydrofuran (THF). The basecoat solution contained 6 mg/ml
PBD, 6
mg/ml PBMA, and 6 mg/ml Sirolimus for a total "solids" concentration of 18
mg/ml.
Stems were coated with approximately 435 micrograms of total coating. The
basecoat
was allowed to dry before the topcoats were applied.
The following THF solutions were used for the topcoats:
1 ) 18 mg/ml PBMA and 2 mg/ml Sirolimus
2) 12 mg/ml PBMA and 8 mg/ml Sirolimus
3) 20 mg/ml PBMA
Average topcoat weights were 121 micrograms, and two stems were spray-coated
using each solution.
After the solvent was removed by evaporation, drug elution was tested via the
bioactive agent release assay described above. The results are provided in
Figure 16
where curve 1 is basecoat only, curve 2 is topcoat applied using coating
solution 1, curve 3
is topcoat applied using coating solution 2, and curve 4 is topcoat applied
using coating
solution 3. These curves demonstrated the ability to control the elution rate
of a bioactive
agent from a medical device surface by varying the amount of bioactive agent
in the
topcoat.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
54
Example 17
Pretreating the Surface of Medical Device by Roughening
Eye coils were roughened by blasting 50 ~m silica particles at the surface of
the
coils under high pressure and velocity. The roughness of the coil surfaces,
particularly the peak to valley distance, was measured with the VSI (Vertical
Scanning Interferometry) mode of an Optical Interferometer.
Roughness tests were taken over areas approximately 155 ~m x 120 pm on the top
of each turn in the coils, as shown in Figures 17 and 18. Three roughness
tests were taken
on one side of the coil, then the coil was rotated 180° and three more
tests were taken on
the other side of the coil.
The VSI mode of the optical interferometer was used to look at the surface
topography of uncoated eye coils over an area approximately 155 um x 120 Vim.
Three
separate areas were measured on each coil on two sides of each coil, to get an
average for
each. Each measurement comprised of a 30 ~m scan to acquire the raw data,
after which
Ra, Rt, and RZ roughness parameters were calculated. Ra, the roughness
average, is the
arithmetic mean of the absolute values of the surface departures from the mean
plane. Rt,
the maximum height (peak to valley distance), is the vertical distance between
the highest
and lowest points over the entire dataset (highest and lowest single pixels),
RZ, the
average maximum height (average peak to valley distance), is the average of
the
difference of the ten highest and ten lowest points in the dataset (10 highest
and 10 lowest
pixels at least 4.6 pm apart from each other laterally). The RZ value measures
the average
peak to valley distance from multiple locations to prevent a misrepresentation
of the data
caused by single data pixels that are random noise, or uncommon surface
features like
scratches or pits. As shown below in Figures 19A and B, tilt and curvature of
the surface
were removed in order to compare the relative surface finish of each coil.
Table 2 shows
the roughness statistics for coil 1, and Table 3 shows the roughness
statistics for coil 2.
Figure 20A shows a surface plot of test A-2 of coil 1, and Figure 20B shows a
3D
representation of Figure 20A. Figure 21 A shows a surface plot of test A-2 of
coil 2, and
Figure 21B shows a 3D representation of Figure 21A.
CA 02563253 2006-10-05
WO 2006/107336 PCT/US2005/035957
Table 1 - Coil #1 Roughness Statistics
Test PositionRa (nm) R m RZ m
A-1 625.03 8.51 6.78
A-2 756.07 9.11 7.84
A-3 686.93 13.92 10.75
B-1 795.54 9.24 8.29
B-2 782.50 15.27 11.78
B-3 778.56 10.46 8.95
Avg. +I- 737.44 t 67.2811.09 2.82 9.07 t 1.87
St.Dev.
Table 3 - Coil #2 Roughness Statistics
Test PositionRa (nm) R, m RZ m
A-1 790.22 10.88 8.34
A-2 626.39 10.01 7.35
A-3 1170.03 11.41 10.11
B-1 628.17 10.77 7.87
B-2 727.82 13.00 8.98
B-3 863.89 10.91 8.94
Avg. +I- 801.08 202.9611.17 t 1.01 8.60 t 0.97
St.Dev.
Other embodiments of this invention will be apparent to those skilled in the
art
upon consideration of this specification or from practice of the invention
disclosed herein.
Various omissions, modifications, and changes to the principles and
embodiments
described herein may be made by one skilled in the art without departing from
the true
scope and spirit of the invention which is indicated by the following claims.
All patents,
10 patent documents, and publications cited herein are hereby incorporated by
reference as if
individually incorporated.