Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
A phakic intraocular lens with improved fluid circulation properties
Field of the invention
The present invention relates to the field of implants for ophthalmic surgery.
More specifically, the present invention is concerned with implantable phakic
intraocular lenses, PIOLs, which are suitable as correction lenses together
with the
intact natural crystalline lens, or optionally an implanted crystalline Lens
substitute. The
inventive lenses are provided with one or more penetrating channels to allow
for
IO improved fluid transport in the eye.
Background of the invention
PIOLs are increasingly conceivable as an alternative to correct for optical
deficiencies besides spectacles and conventional contact lenses. In a general
sense,
I5 PIOLs can be considered for implantation, either in the anterior (front)
chamber of the
eye between the cornea and the iris, or in the posterior (rear) chamber
located between
the iris and the natural crystalline lens.
PIOLs positioned in the anterior chamber have been considered as desirable in
several earlier embodiments for the reason that this chamber is considerably
larger than
20 the posterior chamber and thereby admitting a less complicated surgical
process.
However, these types of lenses show series of drawbacks essentially related
with an
irritation action from the support means (haptics) on the sensitive eye
strictures. For
example, the support means can, when positioned in the corner between cornea
and iris,
disturb the aqueous outflow and consequently generate an increase in the
intraocular
25 pressure, a condition, which at worst may induce glaucoma. The haptic may
press on
the iris and disturb the blood circulation causing the pupil to aquire an oval
shape. The
PIOL optic in the vicinity of the cornea may contact the cornea intermittent
and cause
damage to the endothelium. The present invention is concerned with PIOLs to be
implanted in the posterior chamber.
30 Tt is a general complication when designing PIOLs to be implanted in the
posterior chamber between the iris and the natural crystalline lens that the
available
space is small. Consequently, the PIOLs cannot be bulky, as frequently is
required when
CONFIRMATION COPY
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
2
a high power optical coi~ection is considered. Application of diffractive
optics may
reduce the profile of the lens, malting it thinner.
In particular, consideration must be taken to avoid or restrict any contacts
with
the intact natural crystalline lens, in order to prevent it from damages,
which may lead
to local opacifications, or at worst case cataract formation.
Considerations must also be taken to that contact with posterior iris could
result
in abrasive intraocular damages with resulting pigment dispersion, and that
the pupil
must not be blocked. Blocl~ing of the pupil prevents the flow of aqueous
humor, which
may lead to raised intraocular pressure and reduced circulation of nutrients
and
metabolites to and from the natural crystalline Iens.
Various types of PIOLs are known. They could be grouped according to their
design, one-piece or multiple piece PIOLs. A one-piece PIOL is one where both
optic
and haptic portions are made from one material. The haptic portions are used
for
attachment purposes. Two general designs for the haptics are a "plate-type"
and a "C-
haptic" type, both of which have a variety of shapes.
The preferred positioning of the PIOL is free-floating as opposed to sulcus-
fixated. The PIOL will either rest on the zonula or be pushed forwards by the
aqueous
humor flowing from the ciliary body in anterior direction. The iris restricts
the
movement of the PIOL in the anterior direction. Since the PIOL is pushed
forward, a
distance is created between the PIOL and the crystalline lens. Thereby, the
aqueous
flow can reach the posterior surface of the PIOL, bring nutrients to the
anterior surface
of the crystalline lens, and remove products from the metabolic processes.
Since the shape of the anterior crystalline lens varies from person to person,
it is
not possible to avoid contact points or line contacts at all times between the
PIOL and
the crystalline lens. There is a risk that a line contact around the optic of
the PIOL will
create a sealed chamber between the central PIOL and the crystalline lens.
This is a
highly undesirable situation, since it will prevent nutrients to reach the
central part of
the PIOL and prevent derivatives of the crystalline lens metabolism from being
removed. It can lead to a serious distLUbance of the crystalline lens
metabolism and the
osmotic balance, resulting in reduced transmission of light through the
crystalline lens
and opacifications. The sealed chamber will also interfere with the
accommodation.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
3
When the crystalline lens accommodates, the volume of the liquid between the
PIOL and the crystalline lens decreases. If this is not possible due to that
the PIOL is in
contact with the crystalline lens, thereby creating a sealed chamber, the
accommodation
will be hindered. A force will be exerted on the crystalline lens, leading to
a temporary
deformation of the anterior surface of the lens. A force of the same magnitude
will be
exerted on the~PIOL, which will be pressed forward. As the force on the optic
and/or
optic/haptic transition zone increases, the seal thus created will improve in
strength. The
liquid will eventually be squeezed out of the chamber due to increased
pressure, the
volume of the chamber will decrease, and the crystalline lens can accommodate.
In
practice, there will be a mix of the two mechanisms described. The PIOL will
be
pressed forward, and the accommodation of the crystalline lens will to some
extent be
hindered. The forward movement of the PIOL can cause the anterior chamber
angle to
close and the risk for an increased intraocular pressure, IOP, and associated
closed-
angle glaucoma will increase.
If the eye changes its geometry from accommodated to relaxed state, the
opposite will happen. The volume of the chamber between the PIOL and the
crystalline
lens will increase. If the chamber is sealed, the PIOL is sucked to the
surface of the
crystalline lens. The crystalline lens will deform again, and the return of
the crystalline
lens geometry to the relaxed state will be hindered. These periodic movements
of the
crystalline lens in the direction of the optical axis are also important to
facilitate for the
PIOL to adjust its position, i.e. center itself.
The risk for this undesired contact with the crystalline lens increases if the
PIOL
does not fit properly in the space between the iris and the crystalline lens.
A resulting
effect is that the iris is rubbing against the implant with a force. Depending
on the
surface characteristics of the implant, its biocompatibility and adhesion to
the iris, this
can cause pigment dispersion, which may lead to pigmentary glaucoma. During
accommodation, the crystalline lens moves forward and increases thereby the
pressure
in the anterior chamber. This will cause the iris to bow posteriorly and press
against the
PIOL and the crystalline lens. As a result, pigment dispersion will clog the
trabecular
meshwork.
If the PIOL does not respect the space between the iris and the crystalline
lens,
the PTOL pushes the iris forwards and results in a larger contact zone between
the iris
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
4
and the implant. Such a situation increases the risk for papillary block,
where no
aqueous fluid will be able to move between the posterior chamber and the
anterior
chamber via the pupil. If the papillary block persists, it may develop into
papillary
block glaucoma.
These mentioned effects could be avoided by preventing the formation of the
undesired seal between the PIOL and the crystalline lens. By creating a
channel between
the fluid in the space that is central and posterior of the PIOL, and the
anterior of the
PIOL, fluid exchange at the spaces between the PIOL and the crystalline lens,
and
between the posterior and the anterior chamber, is secured.
These channels or holes can in theory be placed anywhere in the central part
of
the optic. See e.g. US 5,450,425, which describes a corrective intraocular
lens including
a central, axially aligned opening that enhances liquid circulation in the
eye. However,
the hole scatters light, which can lead to undesired reflection images on the
retina,
which are experienced as glare by the end user. Moreover, the hole provides
aqueous
fluid from the anterior chamber, which fluid has a Iower concentration of
glucose than
the corresponding aqueous fluid in the posterior chamber.
Summary of the Invention
It is an object of the present invention to provide a PIOL that is suitable
for
implantation between the iris and the native Lens in an eye, and not is prone
to form a
sealed chamber between its posterior and the anterior of the crystalline lens.
It is another object of the present invention to provide a PIOL that allows
for a
suitable circulation of fluids between its posterior and the anterior of the
crystalline
lens.
It is one object of the present invention to provide a PIOL that allows for a
suitable supply of nutrient-rich aqueous fluid to the space between the
posterior of the
PIOL and the anterior of the crystalline Iens.
It is also an object of the present invention to provide a PIOL that prevents
or
decreases undesired glare phenomena experienced by its end user.
It is a further object of the present invention to provide a PIOL that
prevents or
decreases undesired reflection images on the retina.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
For these and other objects that will be evident from the following
disclosure,
the present invention provides a PIOL for implantation between the iris and
the natural
lens in an eye, wherein the PIOL is allowing fluid circulation between its
posterior and
the anterior of said natural lens after implantation, comprising a central
optic part, a
5 peripheral haptic part, and at least one penetrating channel with an
anterior orifice and a
posterior orifice, characterized in that the channel is arranged at the border
of, or
outside, the central optic part.
The invention is based on the insight that the presence of such a penetrating
channel outside the optic part has the advantages that formation of a sealed
chamber
between the PIOL and the crystalline lens and an accompanying decrease in
fluid
circulation are avoided, while undesired glare phenomena are prevented or
decreased.
According to one aspect of the invention, the axis of symmetry of the channel
intersects the optical axis of the PIOL at a point posterior to the central
optic part. This
configuration further improves fluid circulation in the vicinity of the PIOL.
According to an aspect of the invention, the channel is tapered towards the
posterior orifice. This configuration has the advantage that light scattering
by the
channel is decreased, thereby avoiding or decreasing undesirable reflection
images on
the retina. Moreover, this setup allows for facilitated use of blunt
instruments during
insertion of the PIOL, which further decreases the risk for injuries during
surgery.
Optionally, the channel has a surface that diffuses refracted or reflected
light in its
anterior orifice region.
According to one aspect, the PIOL according to the invention further comprises
an optic/haptic transition zone arranged between the optie part and the haptic
part,
which provides a smooth transition between the optic and the haptic part,
thereby
avoiding potential stress.
According to an aspect, the PIOL according to the invention further comprises
at
least one recess on its anterior side, wherein said recess is arranged outside
the central
optic part and is connected to the anterior orifice of the channel. This
arrangement
prevents the iris from blocking the orifice(s), and thereby further prevents
the PIOL
from sticking to the iris
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
6
According to a preferred aspect of the invention, the area of said orifices)
is in
the range of from 0.005 to 0.4 mm2, such as from O.OI25 to 0.05 mm2. This area
is
estimated to allow for a suitable flow of aqueous fluids.
According to a first aspect of the invention, the area of each orifice is
larger than
0.0003 mm2. This allows for transport of cells through the channels. According
to a
second aspect of the invention, the area of each orifice is less than 1 ~m2.
This prevents
transport of cells through the channels.
According to a preferred aspect of the invention, the PIOL is made of a
viscoelastic and oxygen-permeable material.
According to another aspect, the present invention provides a method of
preventing glaucoma associated with implantation of a PIOL between the iris
and the
native lens in an eye, comprising the step of implanting a PIOL according to
the
invention. Optionally, said glaucoma is selected from pi~nentary glaucoma,
papillary
block glaucoma, and closed-angle glaucoma.
I5
Brief description of the drawings
Fig 1 is a side and a front plan view of one embodiment of the PIOL according
to present invention.
Fig 2 is a side sectional view of an eye with a human crystalline Iens in an
accommodated and in a not accommodated state and a PIOL according to the
invention.
Fig 3 is a side sectional view of a PTOL according to the invention and the
aqueous flow anterior and posterior of the PIOL.
Fig 4 is a front view of an alternative embodiment of the PIOL according to
the
invention, where the positions of the channel orifices are rotated slightly
with respect to
the symmetry axis of the lens.
Fig 5 is a front view of an alternative embodiment of the PIOL according to
the
invention with a plurality of small channels at the periphery of and
peripheral to the
central optic pant of the PIOL.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
7
Detailed description of the invention
In the most general terms, the present invention pertains to a PIOL, i.e. an
intraocular correction lens for implantation in the posterior chamber of the
eye between
the iris and the intact natural lens (phallic posterior chamber intraocular
lenses, PPC-
IOLs). The correction lens comprises a centrally located optical part, capable
of
providing an optical correction, and a peripherally located supporting
element, or haptic
part, capable of maintaining said optical part in the central location. Viewed
from above
at use, the correction lens will generally have a total length of from about 9
to about 13
mm and a width of from about 6 to about 8 mm. These values are confined by,
and
determined individually from, the size of the posterior chamber of the
individual
patient.
The terms "natural Lens" and "crystalline Lens" are used synonymously
throughout this application to denote the natural accomodative lens in the
eye. These
terms are also intended to encompass any replacement intraocular lenses, IOLs,
if
present. Removal of the natural lens and implantation of such IOLs are
standard
procedures in cataract surgery.
The natural lens typically varies in diameter between about 9 and 10.5 mm,
depending on the individual patient and his/her age. The diameter of the
natural lens can
be estimated as a part of the pre-surgical considerations and a suitable
correction lens
with a suitably extended curvature can thereby readily be selected. The
curvature of the
correction lens shall preferably be such that it sufficiently covers the
natural lens,
thereby providing for that no local pressure points are built up that can form
stress
concentration points or zones on the natural lens which may impair its natural
metabolism and form local opacifications, which in worst case result in
cataract
formation and the subsequent need of surgical intervention.
The support elements preferably comprise an inner part neighboring the central
optical part and an outer, peripheral part, which is designed to at least
partially be in
contact with the ciliary sulcus and the zonulas. According to an embodiment,
the
peripheral part is essentially flawlessly connected to the inner part of the
support
elements. Preferably, the peripheral part of the support means follows a curve
that
converges towards a plane perpendicular to the optical axis. This ensures that
the
support means are directed from the zonulas attached to the natural lens and
that the
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
corrective lens advantageously adapts to be accommodated in the free space
confined by
the posterior chamber of the eye between the iris and the natural lens.
According to a preferred embodiment of the present invention, the PIOLs shall
be freely floating in the aqueous humor of the posterior chamber and not have
any
permanent engagement with ciliary sulcus constituting its inner periphery. A
free
floating PIOL is consequently not kept in a constant position by the ciliary
sulcus, but
will to a certain degree follow the eye movements, i.e. those of the natural
lens during
accommodation and the dilations of the pupil, while being surrounded by the
aqueous
humor flowing through the zonulas in anterior direction. For this reason, the
PIOLs
IO according to the present invention will preferably have a maximum diameter
(including
optic part and support means, i.e. haptic part) less than the average diameter
of the
ciliary sulcus. Suitably, the overall length of the PIOL (maximum diameter)
should be
about 1 mm shorter than the ciliary sulcus, or largex, to avoid excessive
decentration of
the PIOL from the optical axis. The overall PIOL length according to the
invention is
15 generally a compromise to obtain a floating effect while retaining a
centering effect
from the sulcus. Therefore, preferred PIOLs according to the invention will be
centered
by a combined controlled interaction with the .iris and the ciliary sulcus. It
is to be
understood that the sulcus in practice is not circular, but rather elliptical
and irregular,
so a frequent touching contact between the PIOL and the sulcus will in reality
be
20 attained, which contributes to the mentioned centering effect. Should the
PIOL not be
sufficiently centered by the iris movements or the forces of the aqueous fluid
between
the PIOL and the natural lens, excessive decentration will prevented by the
sulcus. For
this reason and since the sulcus diameter has a tendency to shrink with
increasing age of
the patient, it cannot always be avoided that the overall length (maximum
diameter) of
25 the PIOL at least at some points exceeds the sulcus diameter. For PIOLs
having a large
diameter (above about 10.5 mm), the probability of sulcus contact increases
considerably and thereby the risk of.sulcus engagement that may lead to a
compression
of the PIOL and its axial displacement.
Favorable PIOL designs according to the invention can be found in US patent
30 application 20010051826.
In a preferred embodiment, the optical part of the PIOL is essentially
circular
and can be designed to correct various optical defects, including myopia and
hyperopia.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
For example, the inventive PIOLs can be designed to correct astigmatism by
designing
their anterior surface toroidal or superimposing a cylindrical surface on the
anterior side
of the PIOL. As another example, the inventive PIOLs can con:ect presbyopia by
applying a bi- or multifocal surface of the anterior side of the PIOL. The
optically
skilled person can readily apply a number of alternative anterior surfaces to
provide a
desired optical correction.
The size of optical part (the optical diameter) generally varies between about
4
to about 7 mm, depending on the patient and the desired optical correction.
The chamber between the central part of the PIOL and the anterior surface of
the
crystalline lens shall always be in contact with aqueous fluid that has a high
concentration of glucose and low concentration of lactic acid. A channel in
the center of
the PIOL, as proposed in US 5,480,428, will provide contact with the aqueous
fluid in
the anterior chamber, but this fluid has a low concentration of glucose. The
distance to
the source, the eilliary body, may in certain subjects be longer, especially
if there is a
pupillary block and the aqueous fluid flows into the anterior chamber through
iridotomies. In contrast, the ehannel(s) according to the present invention
provides
direct contact with the nutrient-rich aqueous fluid of the posterior chamber
of the eye.
At elder age, the pupil size will decrease. At 60 years the pupil size at
night is
4.1 mm, while in bright light conditions the pupil size will be 3.1 mm. At
night or
during sleep, the pupil is for all ages small. The ixis will rest on the
anterior side of the
crystalline Iens or, when a PIOL is implanted, on the optic of the PIOL. To
avoid light
scattering by the ehannel(s), it is preferable to position the anterior
entrance of the
channels outside the optic. The anterior entrance should therefore be
positioned outside,
or at the outer border of, the optic zone, and preferably in the optic/haptic
transition
zone, such that it is in contact with the posterior chamber of the eye. The
posterior
entrance should be in the optic zone or in the optic/haptic transition zone,
if present,
such that it is in contact with the central chamber between the PIOL and the
crystalline
lens.
The geometry of the channels in the PIOL according to the present invention is
important. Generally, the channels) should be as small as possible, so that
the
disturbance of the optical function of the PIOL is nunimal.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
According to one aspect of the invention, the channels should be large enough
to
allow passage of cells. Macrophages are the largest cells in the eye, with a
typical
dimension of 20 ~,m, corresponding to an area of approximately 0.0003 mm2. It
has
been estimated that the aqueous flow is 2.5 ml/min. Aqueous fluid can flow
with this
5 rate from the posterior to the anterior chamber if the pupil size is 4 mm
and the gap
between the iris and the crystalline lens is from .I to 2 q:m. This gap
corresponds to a
surface of from 0.0125 to 0.0250 mm2. The cross-section of the channels should
be
equal to this surface. Tf two peripheral channels are applied, their diameter
should be in
the range of from 0.09 to O.I25 mm.
10 According to another aspect of the invention, the channels should be small
enough to prevent passage of cells. The diameter of the channels should be
from 0.5 qm
to 1 N,m, corresponding to an area of approximately 1 ~,m2. Closer to 0.5 pzn
is
desirable, since the effect on the optical behavior due to diffraction will be
minimal. If
the channels have a diameter of 0.5 ~.m, there should be a minimal of 128 000
channels
to comply with the required total flow surface. The channels should preferably
have a
total cross-section of 0.0125 to 0.4 mm2. In this manner, a gateway has been
constructed
from. the sulcus to the antexior chamber, independent of papillary block. The
PIOL
constructed in this way can be used in the treatment of papillary block
glaucoma.
The diameter of the channels is preferably relatively small, because some
resistance to the aqueous flow is desirable. During accommodation, aqueous
fluid will
be forced in between the implant's haptic and the crystalline lens, providing
the
crystalline lens with oxygen and nutrition. In this perspective, the
crystalline lens is
functioning as an aqueous pump.
Channels with a diameter exceeding 0.5 ~,m should be tapered or otherwise
dimensioned to avoid directing reflected light to the fovea or focus it on
other parts of
the retina, which light is otherwise perceived.by the patient as glare. The
tapering of the
channels has also the advantage that the channels can be used by the surgeon
as
positioning holes by using a blunt instrument smaller than the anterior
entrance of the
channel but larger than the posterior orifice diameter of the channel. The
surface of the
channel orifices may be modified so as to diffuse the refracted and reflected
light. Such
surface modifications include a rugged, grinded or sand blasted appearance.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
11
The channels may be positioned near the syn~netry axis in the long direction
of
the implant. Tf the line connecting the center of the channels is rotated
slightly with
respect to the symmetry axis, the orientation of the channel orifices can
function as a
reference to the surgeon for the anterior side of the PIOL. The surgeon will
be able to
tell by the orientation of the position holes if the PIOL is implanted in the
right
upside/downside orientation.
To further improve the function of the communication channels, they could be
connected to one or more recesses, or indentations, on the anterior side of
the PIOL. The
recesses are arranged outside the central optic part and are connected to the
anterior
orifice of the channels. The function of the recesses is to prevent blocking
of the
channels by the iris, thus facilitating the flow of aqueous fluid into and out
of the space
between the implant and the crystalline lens. In the initial situation without
the implant,
the aqueous fluid in the anterior and posterior chamber flows in the direction
of the
sulcus when the crystalline lens accommodates. When this flow is restricted,
for
example by the implant, pressure builds up in the anterior chamber, pressing
the iris
against the implant and the zonulas, causing papillary block and pigment
dispersion,
resulting in glaucoma. When posterior phakic lenses are implanted in the
posterior
chamber, iridotomies can be applied to compensate for the pupilary block. The
iris will
also in this case, due to the flow through the iridotomies, be pressed against
the surface.
The recesses in the PIOL will prevent the blocking of the pupil by the PIOL.
Preferably, the peripherally located indentation has a generally concave shape
extending towards the inner part of the support means and the optical axis.
The
preferred depth of the indentations is from 0.5 to 1.25 mril. The indentations
thereby
form free spaces, which will both contribute to fluid circulation around the
PIOL and to
that the contact between the PIOL and the sulcus is restricted by these
resilient
peripheral members in a manner that the floating effect of the PIOL can be
maintained,
while the benefit of the contributory PIOL centering effect from the sulcus
contact is
retained.
The material of the PIOL should be highly flexible and transparent. It is
preferred that the material also has viscoelastic properties. This implies
that the PIOL is
stiff to sudden changes and is flexible for long-term geometric changes. It
should
preferably be permeable to oxygen, since oxygen reaches the lens from the
cornea by
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
12
means of diffusion and is essential for the metabolism. The lenses according
to the
present invention can be made from conventional biocompatible optically clear
materials of a suitable refractive index by suitable molding technologies.
Depending on
the material, the lenses can be molded in one singular piece (silicones or
poly(methyl)methacrylate (PMMA)) or be machined by precision milling and lathe
cutting (PMMA or hydrogels). The lenses can be made from stiff materials like
PMMA
and similar acrylates. Alternatively, the lenses can be made of a material
that is foldable
or compressible like polysiloxanes, hydrogels such as polyHEMA, soft acrylates
and the
similar. A particularly suitable polysiloxane material is described in US
Patent
No.5,306,297 and a particularly suitable hydrogel is described in US Patent
No.5,717,049. The skilled person can readily conceive alternatives to these
materials for
the inventive correction lenses.
A suitable material for the PIOL according to the invention is a material that
posses both oxygen permeability and viscoelastic properties. Examples of such
materials are co-polymers of siloxane and acrylic hydrogels, often used in
daily-wear
contact lenses.
The corrective Lenses will be described in more detail below according to
specific embodiments that serve to illustrate non-limiting examples of the
present
invention.
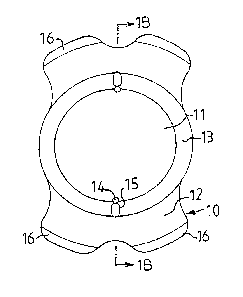
Referring to Figure 1, there is shown a phakic intraocular lens 10 according
to
the present invention. The PIOL 10 includes an optic portion 11, haptic parts
12 and an
optic/haptic transition zone 13. The optic portion 11 has a concave posterior
surface 11 a
and an anterior surface 11b. The posterior surface 11a shall in use be
arranged on the
side corresponding to the anterior surface of the natural crystalline lens 20
(Fig. 2).
The peripherally extending recesses 15 are connected to the communication
channels 14. The recesses 15 are placed outside the optical zone 11,
preferrably within
the optic/haptic transition zone 13.
Referring to Fig 2, the opening in the middle of the iris 21 is the pupil 24.
The
chamber behind the iris is the posterior chamber 26, and the chamber in front
of the iris
is the anterior chamber 25. The PIOL 10 should be freely floating in the space
between
the crystalline lens 20 and the iris 21. This implies that the overall length
of the PIOL
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
13
should be shorter than the diameter of the sulcus 23. To avoid excessive
decentration, the overall length of the PIOL 10 should not be less than 1 mm
shorter
than the sulcus diameter. The sulcus 23 is however not perfectly circular.
Therefore, the
outer periphery of the implant should not be circular, rather be straight or
having
5 protrusions, footplates 12 (Fig 1), in the direction of the sulcus 23. It is
not necessary
that the lens optic portion 11 (Fig 1) is circular; it could also be oval,
square, or any
other shape as desired.
The intraocular lens 10 can be any type of PIOL, one-piece or multiple pieces
IOL. The diameter of the optic portion 11 is limited within the space
available. It should
10 be large enough to avoid edge glare, but not larger, in order to minimize
disturbance of
the aqueous flow. The zonular free diameter is 6.86 mm. At this point the
posterior
radius of the PIOL 10 should increase considerable to avoid intrusion of the
zonulas 22.
The optic diameter should preferably not be longer than 6.5 mm. Outside a 7 mm
radius, the PIOL 10 should have a thin profile in order to reduce the
stiffness of the
implant. An average pupil diameter is 5.1 mm at 15 Lumen. This corresponds
with 4.5
mm real pupil size. The minimum optic diameter is therefore preferably set to
4.5 mm.
The zonula free diameter shows variation between eyes. The design of the PIOL
10
should be robust to this. A solution is to make the design flexible. This
implies a
material with a lower modulus of elasticity or thinner haptics. The best
option is to have
the haptics thin and flexible at a diameter equal to the zonula free diameter
or above.
Referring to Fig 1 and 2, the PIOL 10 has two communication channels 14 for
the aqueous flow, positioned at the periphery of or outside the optic portion
11. To
avoid blocking of the communication channels 14 by the iris 21, the anterior
entrance of
the communication channels 14 are positioned outside the optical portion 11.
The
posterior entrances of the communication channels 14 should be in the optic
portion 11,
or within the optic/haptic transition zone 13 (fig 1), and in contact with the
central
chamber between the PIOL 10 and the crystalline Iens 20 (fig 2).
The crystalline lens 20 is shown in Fig 2. When the crystalline lens 20
accommodates, the anterior radius of the central part of the crystalline lens
20
decreases, and the anterior surface moves relatively forwards with respect to
the
peripher y.
CA 02563348 2006-06-12
WO 2005/058204 PCT/EP2004/014250
14
Fig 3 illustrates an example of an intraocular lens 10 in accordance with the
present invention. The anterior openings 14b of the communication channels 14
are here
placed just outside the optic portion 11 of the crystalline lens 10. The
posterior openings
14a are in the optic portion 11. These channels 14 will guarantee proper
circulation of
liquid in the eye, from the sulcus 23 (Fig 2) into the central area posterior
to the
intraocular Iens 10.
The communication channels 14 could be of any desired shape, straight or
tapered. Communication channels 14 that are tapered towards the posterior
orifice 14a
have the capability to avoid scattering of incident light, which is perceived
by the
patient as glare. The tapering of the communication channels 14 also has the
advantage
that they can be used by the surgeon as positioning holes by using a blunt
instrument
that is smaller than the anterior entrance 14b of the communication channel 14
but
larger than the posterior entrance 14a.
The communication channels 14 may be positioned near the symmetry axis in
the Iong direction of the PIOL 10, as can be seen in the cross-sectional views
of Fig 1
and 4. If the line connecting the center of the communication channels 14 is
rotated
slightly with respect to the symmetry axis, the orientation of the
communication
channels 14 can function as a reference to the surgeon for the anterior side
of the PIOL
10. The surgeon will be able to tell by the orientation of the communication
channels 14
if the PIOL 10 is implanted in the right upside/downside orientation.
Fig 5 illustrates an example of a PIOL 10 according to the invention with a
large
number of small communication channels 14.