Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
New composition and process for the treatment of fibre material
Field of the invention
The present invention relates to a composition comprising a polymer, a
chelating
agent and an alkaline earth metal compound and to a process for the treatment
of
a fibre material, especially a cellulosic fibre material in the presence of a
polymer,
a chelating agent and an alkaline earth metal compound. The composition can be
used as a pretreatment in the bleaching with a peroxygen compound of chemical,
mechanical, chemi-mechanical and de-inked pulps and as a pretreatment in
deinking of recycled fibers and in alkaline peroxide bleaching of mechanical,
chemical, chemi-mechanical and de-inked pulps. The composition can also be
used in deinking of recycled fibers. The composition replaces partly or
totally
silicate as a stabilizer, especially in the treatment of mechanical and
deinked
pulps. The present invention also relates to a process for bleaching a
cellulosic
fibre material with a peroxide compound in an aqueous alkaline medium by using
said composition.
Description of the Related Art
It is well-known that chelating agents can be used as pretreatment for
removing
harmful metal ions, i.e. generally such transition metal ions as iron and
manganese before pulp is bleached with a peroxygen compound, such as
hydrogen peroxide, peracetic acid or Caro's acid. In alkaline peroxide
bleaching of
mechanical pulps, in bleaching of de-inked pulp (DIP) made from recovered
waste
paper and in the deinking of recovered waste paper, water glass (alkali
silicate)
and a chelating agent can be added.
Since the common chelating agents such as polyaminopolycarboxylates, e.g.
EDTA and DTPA and the corresponding methylenephosphonic acid derivatives of
the polyamines are non-biodegradable or show a low biodegradation, there is a
target to decrease the use of the common chelating agents as pretreatment
agents.
Alkaline silicate solutions normally called water glass have been used in
stabilizing
hydrogen peroxide solutions, which are used in alkaline peroxide bleaching of
mechanical pulps.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
2
Water glass is used alone or together with peroxide in de-inking of recovered
papers. Sometimes the de-inked pulp is also bleached with alkaline peroxide.
The use of water glass in alkaline peroxide bleaching of chemical pulps has
been
published, but the method cannot be utilized in full scale, since the silicate
can
cause very severe precipitation problems. Another disadvantage with water
glass
is that when the bleaching liquors are recycled and ultimately fed into the
recovery
boiler, where the so-called black liquor from the cooking process after
concentration is burned, the silicate will cause severe scaling and thus
decrease
the heat transfer in the recovery boiler, which in worst case can cause an
explosion of the recovery boiler.
If the silicates, e.g. in form of the water carry-over, will enter the paper
making
process, they will disturb the papermaking process, e.g. by precipitating on
hot
surface, causing holes in the paper reel etc.
It is known that hydrogen peroxide will decompose very rapidly in an alkaline
milieu in the presence of transition metal ions. The most abundant of these
ions in
pulps are iron and manganese. The copper ion is also very detrimental for
alkaline
hydrogen peroxide, but normally it can enter the process only via used process
waters.
It is also known that iron will start to precipitate already below pH 7, first
in colloidal
form. The formed iron hydroxides, oxyhydroxides etc are much more
catalytically
active than iron ions. Also manganese can, at least partly, be in precipitated
form,
but it has been shown that in the presence of hydrogen peroxide, manganese
should be in dissolved form.
The theory of the function of water glass varies, but one theory is that water
glass
will deactivate the catalytic surface of iron and other heavy metal ion
"precipitates".
In order to avoid the detrimental effect of manganese ions, a chelating agent
is
often introduced into the bleaching process or the pulp is pretreated with a
chelating agent. The most common chelating agents are EDTA and DTPA, which
belong to the group of polyaminopolycarboxylates. The corresponding phosphona-
tes, EDTMPA and DTPMPA can also be used, but they are much more expensive
than the polyaminopolycarboxylates. They have also the disadvantage that they
contain phosphorus, which is not a wanted component, when the environmental
regulations are becoming stricter and stricter.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
3
In the deinking of waste paper, water glass has also other functions, e.g.
water
glass improves ink detachment, it will disperse the ink and act as a buffer
keeping
the pH constant. Therefore a partly replacement of water glass would also be
advantageous and at the same time decrease the precipitation problems
connected with the use of water glass.
According to the above there is a need to partly or totally replace water
glass
(silicates) in alkaline peroxide bleaching processes and in pulping processes,
which use water glass, e.g. in alkaline peroxide bleaching of mechanical and
de-
inked pulps and in de-inking of recovered paper. There have been suggestions
to
use phosphonates, but they should be used in quite high dosages and the
phosphorus problem in the waste waters would still remain. Since the common
phosphonates are non-biodegradable, there has been much studies about they
adverse effect on mobilizing heavy metals, e.g. from sediments in waterways.
If
phosphonates would be used, the dosage of these substances should be
decreased.
A pretreatment method for bleaching pulp with hydrogen peroxide in alkaline
conditions in the presence of sodium silicate and adding 0.05-1% by weight
(based on dry pulp) of a copolymer of 3-allyloxy-2-hydroxypropanesulfonic acid
(AHPS) and (meth)acrylic acid in the pretreatment is described in the Japanese
patent publication JP 1266295 (published 24 October 1989).
According to the Japanese patent application JP 1148890 (published 12 June
1989) the same kind of polymer in an amount of 0.05-1 % by weight (based on
dry
pulp) has been used instead of e.g. DTPA in alkaline peroxide bleaching. In JP
1148890 the bleaching performance of a number of different AHPS-acrylic acid
copolymers are shown and compared e.g. with the performance of DTPA.
In the both JP patent applications the tested amounts are very big, since
normally
the chelating agents are used in an amount of 0.5 to 2 kg per ton pulp as 100%
sodium salt.
Finnish unpublished patent application FI-20040293 discloses a process for
bleaching a fibre material with an alkaline peroxide solution in the presence
of a
chelating agent and a copolymer of 3-allyloxy-2-hydroxypropanesulfonic acid
(AHPS) with (meth)acrylic acid, maleic acid or itaconic acid. This patent
application also discloses a composition comprising said copolymer and the
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
4
chelating agent for use as a stabilizer in alkaline peroxide bleaching for
replacing
partly or totally water glass.
Summary of the present invention
According to the present invention it has now surprisingly been found that by
using
a copolymer of AHPS and an unsaturated carboxylic acid, such as acrylic acid,
methacrylic acid, maleic acid or itaconic acid, together with a chelating
agent and
an alkaline earth metal compound, such as magnesium sulphate, either mixed
together or added separately, a very good bleaching performance can be
achieved
and a total replacement of water glass can be achieved, if necessary from the
pulping and paper making point of view. Surprisingly, the combination of the
copolymer, the chelating agent and the alkaline earth metal compound showed an
improved effect as compared to the effect of the combination of the copolymer
and
the chelating agent or the combination of the chelating agent and the alkaline
earth metal compound. Test results unexpectedly showed a clear synergistic
effect.
The combination of the three components, i.e. the copolymer, the chelating
agent
and the alkaline earth metal compound, can very effectively be used as a
stabilizer
in bleaching of a chemical, mechanical or de-inked pulp with a peroxygen
compound, such as hydrogen peroxide, peracetic acid or Caro's acid. The
present
invention makes it possible to partially or totally replace water glass in
bleaching
and deinking processes by using the combination of the three components.
The present invention provides a process for treatment of a fibre material
comprising the step of contacting the fibre material in an aqueous medium with
a
chelating agent, the above copolymer and an alkaline earth metal compound. The
copolymer, the chelating agent and the alkaline earth metal compound can be
added separately or preferably as a ready made mixture (composition).
The present invention also relates to a composition comprising the copolymer,
the
chelating agent and the alkaline earth metal compound.
The composition and process according to the invention can be used as a
pretreatment of all kind of pulps, chemical pulps, mechanical, chemi-
mechanical
pulps and deinked pulps, which are bleached with alkaline peroxide.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
The composition and process according to the invention can also be used in the
bleaching of all kind of pulps, chemical pulps, mechanical, chemi-mechanical
pulps and deinked pulps by using hydrogen peroxide as the bleaching agent.
The composition and process are also suitable in deinking of recycled pulps,
in
5 which water glass and hydrogen peroxide are commonly used.
The composition can also be used in sodium dithionite bleaching of mechanical
and de-inked pulps.
The alkaline peroxide bleaching process for mechanical, chemi-mechanical and
de-inked pulps according to the invention can be practiced as a single stage
of
bleaching or in a two-stage process, where the pre-bleached pulp is entering
the
second stage. Any consistency can be used, but it is most preferable to use
medium consistency in the first stage and high consistency in the second
stage.
If needed, the bleaching can be preceded by a pretreatment with a chelating
agent
or preceded by a pretreatment according to the invention in order to reduce
the
amount of transition metals entering the bleaching process.
In the de-inking process the composition of the present invention can be used
in
repulping or in a disperger or in a kneader which possibly is followed by a
soaking
tower whereto hydrogen peroxide can be fed. In the de-inking process the
composition of the present invention can also be used in a separate bleaching
stage or any process stage where hydrogen peroxide is present.
The composition, either as ready made mixture or as combination of the three
components, can be used as total or partial replacement in those processes,
where water glass are commonly used.
The theory how the three components will work together is not clear, since the
polymer itself cannot stabilize very well alkaline hydrogen peroxide solutions
and
also gives in general poor bleaching performance. Nor does the alkaline earth
metal compound stabilize alkaline hydrogen peroxide solutions very well
especially
in the presence of manganese. The chelating agents stabilize quite well the
above
mentioned alkaline peroxide, but cannot give a good bleaching result. The
common chelating agents mentioned above, will bind the soluble manganese ions
in the alkaline peroxide solutions, but since iron is then in solid form,
either
colloidal or in precipitated form, chelating agents cannot any more bind the
solid
compounds. The same is valid for the solid forms of manganese compounds. The
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
6
polymer somehow binds to the solid surfaces or inactivates the catalytic
effect of
the solid particles. Thus a combined effect will be obtained. The common
chelating
agents cannot, when used alone, give a good bleaching performance, i.e. for
chemical pulps, small viscosity loss and high brightness gain and a sufficient
amount of residual peroxide, and for mechanical pulps and deinked pulps high
brightness gain and a sufficient amount of residual peroxide, which indicates
that
peroxide has mainly been consumed for bleaching and not for decomposition
processes. Therefore there must be some synergetic effect between the three
components used according to the invention.
Detailed description of the invention
In a first aspect of the present invention there is provided a stabilizing
composition
comprising following components
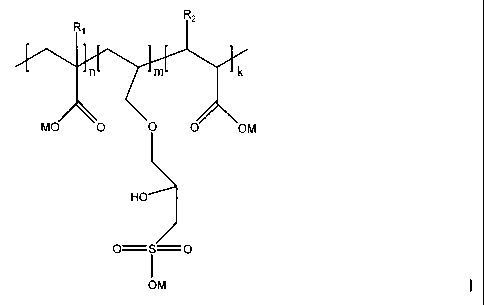
(A) a polymer having following formula
RZ
n m k
MO 0 0 0 OM
HO
0= S= O
I
OM
wherein
R, is a hydrogen atom or an alkyl group containing 1 to 12 carbon atoms,
R2 is -COOM or -CH2COOM,
M is a hydrogen atom, an alkali metal ion, an alkaline earth metal ion, an
ammonium ion or a mixture thereof,
n, m and k are molar ratios of corresponding monomers, wherein n is 0 to 0.95,
m
is 0.05 to 0.9, and k is 0 to 0.8, and (n+m+k) equals 1, and
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
7
the weight average molecular weight is between 500 and 20,000,000 g/mol,
(B) a chelating agent, and
(C) an alkaline earth metal compound.
In a second aspect of the present invention there is provided a process for
the
treatment of a fibre material comprising the step of contacting the fibre
material in
an aqueous medium with following components
(A) a polymer having following general formula
R2
n m k
"
MO O OM
HO
0=S O
I
OM I
wherein
R1 is a hydrogen atom or an alkyl group containing 1 to 12 carbon atoms,
R2 is -COOM or -CH2COOM,
M is a hydrogen atom, an alkali metal ion, an alkaline earth metal ion, an
ammonium ion or a mixture thereof,
n, m and k are molar ratios of corresponding monomers, wherein n is 0 to 0.95,
m
is 0.05 to 0.9, and k is 0 to 0.8, and (n+m+k) equals 1, and
the weight average molecular weight is between 500 and 20,000,000 g/mol,
(B) a chelating agent, and
(C) an alkaline earth metal compound.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
8
The composition of the present invention can be used as a stabilizer in the
bleaching of a fibre material in an aqueous medium or as a stabilizer in the
deinking of a recycled fibre material.
The above alkali metal ion is preferably sodium or potassium ion, and the
alkaline
earth metal ion is preferably magnesium ion.
A preferred comonomer with AHPS is acrylic acid (R,=H), methacrylic acid
(R,=CH3), maleic acid (R2=COOM) or itaconic acid (R2=CH2COOM). When k is 0
in formula I the preferred comonomer is acrylic acid or methacrylic acid, and
when
n is 0 the preferred comonomer is maleic acid or itaconic acid. When both k
and n
are not 0 the preferred comonomers with AHPS are (meth)acrylic acid and maleic
acid or itaconic acid.
The monomers are randomly distributed along the polymer chain of formula I,
and
preferably n is 0.4 to 0.9, m is 0.1 to 0.5, and k is 0 to 0.5.
If the system in pretreatment or in alkaline peroxide bleaching contains high
amount of calcium ions, as is the case, when so-called white water from
papermaking process is circulated to the pulping and/or bleaching operations,
it is
advantageous to use maleic acid or itaconic acid (k > 0) as one of the
comonomers in order to increase the calcium binding ability of the polymer. It
is
preferable in normal cases that the polymer only contains AHPS and a monomer
containing one carboxylic acid, such as acrylic acid, since a copolymer
comprising
multiple monomers is usually more difficult to produce.
The weight average molecular weight of the copolymer of formula I should be
between 500 and 20,000,000 g/mol, preferably between 1,000 and 1,000,000
g/mol and most preferably between 2,000 g/mol and 500,000 g/mol.
If the weight average molecular weight is lower than about 500 g/mol, the
efficiency of the polymer becomes too low. If the average molecular weight is
higher than 20,000,000 g/mol, handling and dosage become a problem due to
high viscosity of the polymer solution.
To increase the molecular weight of the copolymer and/or to enhance the
efficiency of the copolymer, a cross linker may be used in an amount of 0 to
20 %
by mole, preferably 0 to 10 % by mole, of the total monomer content. Suitable
cross linkers are, for example methylenebisacrylamide, ethylene glycol divinyl
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
9
ether, di(ethylene glycol) divinyl ether, tri(ethylene glycol) divinyl ether
and vinyl or
allyl terminated polymers, but are not limited to these.
To decrease molecular weight of the copolymer and/or to enhance the efficiency
of
the copolymer, a chain transfer agent may be used in an amount of 0 to 20 % by
mole, preferably 0 to 10 % by mole, of the total monomer content. Suitable
chain
transfer agents are, for example thiols (e.g. butylmercaptan) and alcohols
(e.g.
isopropanol), but are not limited to these.
The chelating agent (B) to be used together with the copolymer (A) of formula
I
may be a chelating having formula II, III or IV below.
A preferred chelating agent is a compound having following general formula
R7
I
Ra~ N R6
N N
p
R3 R5 I I
wherein
p is 0 or an integer of 1 to 10,
R3, R4, R5, R6 and R7 are independently a hydrogen atom or an alkyl chain
having
1 to 6 carbon atoms and containing one or more active chelating ligands, such
as
carboxylic, phosphonic or hydroxyl group(s) or a salt thereof.
The alkyl chain is preferably methylene -CH2- or ethylene -CH2CH2-.
In formula II R3, R4, R6 and R7 preferably represent the same group.
Examples of chelating agents according to the above formula II are
polyaminopolycarboxylic acids and polyaminopolymethylenephosphonic acids.
The polyaminopolycarboxylic acids can be produced by the conventional route
from the polyamine and formaldehyde and sodium cyanide or hydrocyanic acid.
The more suitable route for small scale production is to use a haloacetic
acid,
especially monochloroacetic acid as a reactant.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
Preferred polyaminopolycarboxylic acids are:
DTPA: p=1, R3 = R4 = R5 = R6 = R7 _-CH2COOH
TTHA: p=2, R3 = R4 = R5 = R6 = R7 _-CH2COOH
EDTA: p=0, R3= R4 = R5 = R6 =-CH2COOH
5 HEDTA: p=0, R3 = R4 = R5 =-CH2COOH, R5 =-CH2CH2OH
EDDS: p=0, R3 = R5 = H, R4 = R6 = -CH(COOH)CH2COOH
(ethylenediaminedisuccinic acid)
The polyaminopolymethylenephosphonic acids are made conventionally from the
corresponding polyamine, formaldehyde and phosphonic acid. With the higher
10 amines a full substitution with acetic acid groups or methylenphosphonic
acid
groups will become more and more difficult. These chelating agents will also
perform well in the composition but an incomplete substitution will make the
chelating agents more prone for decomposition by hydrogen peroxide.
Preferred polyaminopolymethylenephosphonic acids are:
DTPMPA: p=1, R3 = R4 = R5 = R6 = R7 _-CH2POO2H2
TTHMPA: p=2, R3 = R4 = R5 = R6 = R7 _-CH2POO2H2
EDTMPA: p=0, R3 = R4 = R5 = R6 =-CH2POO2H2
Another preferred chelating agent is a compound having following general
formula
. R
a\ R6
N-(CH2)q-N/
R 3 \
R5 III
wherein
q is an integer of 3 to 10,
R3, R4, R5 and R6 are independently a hydrogen atom or an alkyl chain having 1
to
6 carbon atoms and containing one or more active chelating ligands, such as
carboxylic, phosphonic or hydroxyl group(s) or a salt thereof.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
11
The alkyl chain is preferably methylene -CH2- or ethylene -CH2CH2-.
In formula III R3, R4 and R6 preferably represent the same group.
Examples of chelating agents according to the above formula III are the
commercially available hexamethylenediamine tetra(acetic acid) (q=6) and
tetramethylenediamine tetra(methylenephosphonic acid) (q=4) having following
formulae.
0
OH OH
HO HO ~j /
~ 0=P-OH
N ///~~~ ~~/OH O
N X N /J
II r N
HO O H \
O OH 0=POH
0 I HO/ O
hexamethylenediamine tetra(acetic acid) tetramethylenediamine
tetra(methylenephosphonic acid)
Yet another preferred chelating agent is a compound having following general
formula
I 03H2
P
R$ i Rjo
Rs IV
wherein
R8 is a hydrogen atom, an alkyl group containing 1 to 6 carbon atoms or an
alkyl
chain having 1 to 6 carbon atoms and containing a carboxylic, phosphonic or
hydroxyl group,
R9 is a hydrogen atom, hydroxyl group, phosphonic group, carboxylic group or
alkyl chain having 1 to 6 carbon atoms and containing one or two carboxylic
groups, and
Rio is a hydrogen atom, hydroxyl group, carboxylic group, alkyl group
containing 1
to 6 carbon atoms or alkyl chain having 1 to 6 carbon atoms and containing a
carboxylic group, or a salt thereof.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
12
The alkyl chain is preferably methylene -CH2- or ethylene -CH2CH2-.
An example of the non-nitrogen containing chelating agents according to the
above formula IV is 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP).
A further preferred chelating agent is a compound having following general
formula
COOR12 i ll COOR12
R1300C N COOR13
r O s
V
wherein Rll is
a hydrogen atom
an alkyl chain containing 1-30 carbon atoms,
an alkyl chain containing 1-30 carbon atoms and 1-10 carboxylic acid groups
attached to said chain, or alkali or alkaline earth metal salt thereof,
an alkyl chain containing 1-30 carbon atoms and 1-10 carboxylic acid esters
attached to said chain,
a (poly)ethoxylated hydrocarbon chain containing 1-20 ethoxyl groups, or
a carboxylic acid amide containing 1-30 carbon atoms, where N-RI, bond is an
amide bond,
R12 and R13 are: hydrogen, an alkali metal ion or an alkaline earth metal ion
or an
alkyl group containing 1-30 carbon atoms,
r is 0 or 1, and
sis0or1.
Preferred N-bis- or tris-[(1,2-dicarboxy-ethoxy)ethyl]amines of formula V are
following
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
13
A C
COOH
HOOC H
O NO~COOH COOH
HOOC COOH
O
B COOH H000 ~ N\ ~ O~-COOH
y C OOH HOOC COOH
HOOC COOH
HOOC~O N~O--<COOH
A = N-bis[(1,2-dikarboxy-ethoxy)-ethyl]-amine
B = N-bis[(1,2-dikarboxy-ethoxy)-ethyl]-aspartic (AES)
C = N-tris[(1,2-dikarboxy-ethoxy)-ethyl]-amine
A preferred N-bis-(1,2-dicarboxy-ethyl)amine of formula V is iminodisuccinic
acid
(ISA) having following formula
COOH
NH
HOOC
COOH
COOH
ISA
Though the formulas of the chelating agents are depicted above as acids, they
are
commercially normally sold as their alkali salts, mainly as their sodium salts
and
the formulas given above have to be understood as including both the free
acids
and their salts.
The alkaline earth metal compound (C) to be used together with the copolymer
(A)
of formula I and the chelating agent (B) is preferably a magnesium or a
calcium
compound or a mixture thereof, more preferably a magnesium compound.
Especially preferred are water-soluble magnesium or calcium salts, such as
magnesium or calcium chloride, sulphate or acetate or a mixture thereof, most
preferably magnesium sulphate. According to the present invention the alkaline
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
14
earth metal can also exist as a complex with the chelating agent, especially
an
Mg-chelating agent complex, such as Mg-DTPA complex. The form of the
alkaline earth metal in the stabilizer mixture has no effect here.
The polymer and the chelating agent can be added separately or as a
composition
mixture. The weight ratio of the polymer (calculated as solids) to the
chelating
agent (calculated as 100% chelating agent as sodium salt) is preferably from
1:4
to 4:1, more preferably from 1:3 to 3:1.
The alkaline earth metal compound can be added separately or as a composition
mixture with the polymer or the chelating agent or both.
The total amount of the polymer (as solids), the chelating agent (as 100%
sodium
salt) and the alkaline earth metal compound (as alkaline earth metal) added
separately or as a mixture, is preferably 0.05 - 10 kg per ton of dry fibre
material,
more preferably 0.1 - 5 kg per ton of dry fibre material, and most preferably
0.2 to
4 kg per ton of dry fibre material.
The amount of the polymer is preferably 0.05 - 5 kg per ton dry fibre
material,
more preferably 0.1 - 2 kg per ton dry fibre material calculated as solids.
The amount of the chelating agent is preferably 0.05 - 5 kg per ton dry fibre
material, more preferably 0.1 - 2 kg per ton dry fibre material calculated as
100%
sodium salt.
The amount of the alkaline earth metal compound is preferably 0.05 - 5 kg per
ton
dry fibre material, more preferably 0.1 - 2 kg per ton dry fibre material
calculated
as alkaline earth metal.
Preferably the three components (A), (B) and (C) are present in following
weight ratios 10 to 60 : 20 to 70 : 10 to 50, more preferably 15 to 55 : 25 to
65 : 15 : 45, most preferably 20 to 50 : 30 to 60 : 20 to 40 calculated as
active substance.
The fibre material is preferably a cellulosic fibre material, especially a
chemical,
mechanical, chemi-mechanical or deinked pulp. The cellulosic fibre material
can
also be any regenerated cellulose material, such as viscose, or flax or
cotton.
If a composition mixture is made according to the invention, the normal
content of
active materials in the mixture can be at least 10%, preferably at least 15%,
and
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
more preferably at least 20% by weight, but also more diluted solutions can be
used in the application process.
In one embodiment of the process of the present invention the treatment
comprises bleaching the fibre material with an alkaline peroxide solution in
the
5 presence of the chelating agent, the polymer and the alkaline earth metal
compound.
The bleaching of chemical pulp can be carried out at temperatures of from 50 C
to
150 C and at all practical consistencies. The residence time in the bleaching
can
vary within a wide range, from 30 to 240 minutes, preferably from 45 to 180
10 minutes and most preferably from 60 to 120 minutes. The residence time will
also
depend on the temperature used in the bleaching.
The stages can also be reinforced with oxygen, the abbreviation of stages
depicted in the professional literature as EOP, Eop, PO or OP.
The peroxide bleaching of mechanical pulps with the process according to the
15 invention can comprise all kind of mechanical pulps, e.g. stone groundwood
pulp
(SGW), refiner mechanical pulp (RMP), pressure groundwood (PGW), thermo-
mechanical pulp (TMP), but also chemically treated high-yield puips such as
chemithermomechanical pulp (CTMP). The invention is also useful in bleaching
of
deinked pulps. Deinked pulp can be made using mixed office waste (MOW),
newsprint (ONP), magazines (OMG) etc. as raw material and the composition of
the present invention can be used in any process stage where peroxide is used.
The invention can also be practiced in refiner bleaching of mechanical pulps
and in
alkaline peroxide mechanical pulp (APMP), in which wood chips are impregnated
with alkaline peroxide solution before refining. In these applications the
invention is
very advantageous, since the biggest obstacle to use hydrogen peroxide in
these
applications has been that water glass cannot be used, since the sodium
silicate
will e.g. fasten to the refiner plates and thus making the process
unpractical.
The residence time in the bleaching can vary within a wide range, from 30 to
240
minutes, preferably from 45 to 180 minutes and most preferably from 60 to 120
minutes. The residence time will also depend on the temperature used in the
bleaching.
The composition according to the invention can be used as a mixture or the
ingredients can be added separately.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
16
The bleaching of mechanical pulps can be carried out at a temperature of from
30 C to 90 C, preferably at a temperature of from 50 C to 90 C. The bleaching
can be carried out at a consistency of choice, but it is most preferably to
carry out
the bleaching at a high consistency, i.e. about 30% or higher. Bleaching can
also
be carried in two stages with a dewatering stage between the stages. The
stages
can be carried out at a consistency of choice, but it is most preferably to
use
medium consistency in the first stage and a high consistency in the second
stage.
This makes it possible to remove the detrimental substances efficiently.
The bleaching stage can be preceded by a chelating agent stage or a
pretreatment according to the invention, discussed in more detail below, and
dewatering and thus improve the bleaching performance. In the chelating agent
stage any of the above defined chelating agents can be used.
The ratio between the alkali and hydrogen peroxide can vary in a wide range,
depending on raw materials and degree of bleaching. Also alternative alkali
sources, like sodium carbonate, can be utilized. The use of sodium carbonate
is
especially preferably to use, at least as a partial replacement of sodium
hydroxide,
when wastepaper is deinked with the total replacement of water glass using the
composition according to the invention. The necessary buffer capacity can be
ensured in this way.
In another embodiment of the process of the present invention the treatment
comprises pretreating the fibre material in the aqueous medium comprising the
chelating agent, the polymer and the alkaline earth metal compound.
The pretreatment according to the invention can be utilized for all kind of
chemical
and mechanical pulps.
The pretreatment can be followed by a bleaching with a peroxygen compound
optionally in the presence of the chelating agent, the polymer and the
alkaline
earth metal compound. The peroxygen compound can be hydrogen peroxide,
peracetic acid or Caro's acid.
The pretreatment of chemical pulps can also precede such stages, in which
another peroxygen chemical than hydrogen peroxide is used, e.g. a peracetic
acid,
Caro's acid etc. stage. If the stage is followed by an alkaline stage
comprising the
use of hydrogen peroxide, the treatment can also carried out after the above
mentioned peroxygen stage. Depending on the raw material and the process the
treatment can also be carried out only after the mentioned peroxygen stage.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
17
The consistency of this pretreatment is preferably around 10% in order to
ensure
an efficient metal removal. The pH is preferably from 3 to 7, more preferably
from
4 to 6.5 and most preferably from 4.5 to 6. The pretreatment can be carried at
any
temperature, but it is preferably carried at the same temperature as the
bleaching
stage, but however below 100 C.
In yet another embodiment of the process of the present invention the
treatment
comprises de-inking recycled fibre material in the aqueous medium containing
the
chelating agent, the polymer and the alkaline earth metal compound.
In the de-inking process the polymer composition according to the invention
can
be used in repulping of wastepaper or in a disperger or in a kneader which
possibly is followed by a soaking tower whereto hydrogen peroxide can be fed.
In
the de-inking process the polymer composition of the present invention can
also
be used in a separate bleaching stage or any process stage where hydrogen
peroxide is present.
The pH in the alkaline bleaching, including the de-inking in the presence of
hydrogen peroxide, is from 7 to 13, preferably from 7 to 12, and more
preferably
from7to11.
The present invention is illustrated by following examples, which will not
limit the
scope of the invention.
In this specification the percentages are % by weight unless otherwise
specified.
In the tables below the amounts of chemicals given as kg refer to kg per ton
dry
pulp.
Example 1
Polymerization of AHPS and acrylic acid
Preparation of poly(acrylic acid-co-3-allyloxy-2-hydroxypropanesulfonic acid,
sodium salt) aqueous solution; a 65:35 (mol) polymer.
A four-necked glass reactor of 0.25 liters, equipped with a heating/cooling
jacket,
an overhead stirrer, a thermometer, a reflux condenser, a gas inlet and 2
reagent
pumps, was charged with 3-allyloxy-2-hydroxypropanesulfonic acid, sodium salt
40% aqueous solution (95.5g). The solution was degassed with nitrogen and
temperature raised to 85 C. While the solution was stirred, there were pumped
at
constant rate acrylic acid 50% aqueous solution (46.8g) within 3 hours, and
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
18
sodium persulfate 1.3% aqueous solution (47.6g) within 3 hours and 30 minutes.
The addition of the reagent solutions was started simultaneously. After
addition of
the sodium persulfate solution the reaction mixture was stirred for additional
1 hour
and 30 minutes, while maintaining the temperature at 85 C. The reactor was
cooled, and slightly yellow and viscous aqueous copolymer solution was
obtained.
A sample of the solution was treated with excess of concentrated hydrochloric
acid
solution to convert the corresponding sodium salts to free acids. Residual 3-
allyloxy-2-hydroxypropanesulfonic acid content of the thus obtained solution
was
determined by gas chromatography, and was approximated to be 2.0 % by weight.
This refers to 90% conversion of the 3-allyloxy-2-hydroxypropanesulfonic acid,
sodium salt monomer.
A sample of the first copolymer solution was neutralized with sodium hydroxide
to
pH about 10. The molecular weight of the thus obtained copolymer was
determined by gel permeation chromatography against poly(acrylic acid, sodium
salt) standards. Number and weight average molecular weights were
approximated to be 9,000 g/mol and 48,000 g/mol, respectively.
In order to make a preliminary test about the suitability of the stabilizers
for
alkaline peroxide solutions, stability tests were carried out, i.e. following
the
decomposition of hydrogen peroxide as a function of time. Since the results
very
nicely followed the first order kinetics, the results are given as half life
time figures.
If a very low half life time is obtained, e.g. under some tens of minutes, the
product
is not suitable for alkaline peroxide bleaching. If the half life time is more
than 100
minutes, the product may be suitable in alkaline peroxide bleaching without
sodium silicate, but the result does not guarantee a good bleaching
performance.
Since the transition metal ions, especially in wood abundantly present iron
and
manganese, will decompose alkaline hydrogen peroxide, the tests were carried
out in the presence of these ions.
Following Examples 2 to 7 relate to stability tests of alkaline peroxide
solutions
and Examples 8 and 9 relate to laboratory peroxide bleaching tests.
Example 2
A solution containing Fe and Mn (as sulphates) and a stabilizer composition
comprising one or more of following components: polymer (PAHPS-AA prepared
in Example 1), Mg sulphate, and DTPA was prepared and pH adjusted to 10. The
total amount of stabilizer in each test was 100 mg/I (calculated as active
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
19
substance). The temperature was raised to 50 C. The solution was stirred and
hydrogen peroxide added in a concentration of 3 g/I. The pH was readjusted to
10,
and the hydrogen peroxide concentration measured as a function of time
(determined by standard iodometric method). The half life time of the hydrogen
peroxide was calculated according to 1st order reaction rate. Following
stabilizer
compositions were tested:
Amount, mg/I (calculated as active substance)
DTPA 100
PAHPS-AA 100
PAHPS-AA + DTPA 50 + 50
MgSO4 100
DTPA + MgSO4 80 + 20
PAHPS-AA + DTPA + MgSO4 40 + 40 + 20
The results are shown in the table below.
Molar shares t,rz , min(t"orderreactionrate)
[Fe+Mn] , No DTPA+AHPS=AA+
Fe,ppm Mn, ppm Fe, mol-%Mn, mol-0 ~ol/I stabilizer DTPA AHPS PDTPA~+ Mg Mg
DTPA+
M
1. 4.0 0.0 100 0 72.2 892 45 61 8 880 260 1055
2. 3.0 1.0 75 25 72.2 16 57 8 224 35 254 1124
3. 2.5 1.5 62 38 72.2 9 69 9 227 19 285 861
4. 2.0 2.0 50 50 72.2 5 84 9 221 17 348 1043
5. 1.5 2.5 37 63 72.2 2 94 9 225 11 517 1267
6. 1.0 3.0 24 76 72.2 2 194 9 226 10 698 934
7. 0.0 4.0 0 100 72.2 48 5317 8 228 10 51 620
The results clearly show the synergistic effect of the combination of these
three
chemicals, i.e. PAHPS-AA, DTPA and Mg. Since iron and manganese are the
most abundant transition metals, and usually they both are present in
mechanical
pulps, it is very important to have good stabilizing performance in the
presence of
these both metals.
Example 3
In this example some additional stabilizer compositions were tested. The tests
were carried out in the same way as in Example 2. Following stabilizer
compositions were tested:
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
Amount, mg/I (calculated as active
substance)
ISA 100
AES 100
5 DTPMPA 100
ISA + PAHPS-AA + MgSO4 20 + 60 + 20
AES + MgSO4 80 + 20
PAHPS-AA + DTPA + DTPMPA + MgSO4 50 + 15 + 15 + 20
PAHPS-AA + AES + MgSO4 40 + 40 + 20
The results are shown in the table below.
Molar shares tii2 , min (1b' order reaction rate)
Fe,ppm Mn, ppm Fe, mol- Mn, mol- [Fe+Mn] No ISA AES DTPMPA
% % mol/I sta-
bilizer
1. 4.0 0.0 100 0 72.2 892 376 14001 376
2. 3.0 1.0 75 25 72.2 16 21 222 81
3. 2.5 1.5 62 38 72.2 9 9 274 64
4. 2.0 2.0 50 50 72.2 5 9 322 60
5. 1.5 2.5 37 63 72.2 2 9 386 71
6. 1.0 3.0 24 76 72.2 2 9 549 164
7. 0.0 4.0 0 100 72.2 48 592 242 1962
t1iZ , min (15t order reaction rate)
ISA+ AES+ PAHPS-AA+ PAHPS-AA+
PAHPS-AA+ Mg DTPA+ AES+
Mg DTPMPA+ Mg
Mg
1. 1089 587 2992 8455
2. 1027 1255 2594 1145
3. 745 513 2099 827
4. 671 199 1487 614
5. 601 31 876 338
6. 482 28 549 333
7. 36 29 260 264
The results show that very good results were obtained by the stabilizer
compositions of the present invention
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
21
Example 4
The effect of the composition of the stabilizer is shown in the table below.
The
tests were carried out in the same way as in Example 2.
Fe, ppm Mn; PAHPS- DTPA, Mg, % Stabilizer, t 1/2,
ppm AA, % % mg/I min
1 3 80 0 20 100 682
1 3 60 20 20 100 955
1 3 50 30 20 100 992
1 3 40 40 20 100 1443
1 3 30 50 20 100 1979
1 3 20 60 20 100 2294
1 3 10 70 20 100 734
1 3 0 80 20 100 607
As can be seen from the table, the combination of the polymer, the complexing
agent and the alkaline earth metal compound has better performance than the
combination of the polymer and the alkaline earth metal compound and the
combination of the complexing agent and the alkaline earth metal compound.
Example 5
In this example, the effect of the concentration of alkaline earth metal is
demonstrated. The tests were carried out in the same way as in Example 2.
Fe, ppm Mn, PAHPS- DTPA, Mg, % Stabilizer, t 1/2,
ppm AA, % % mg/I min
1 3 50 50 0 100 226
1 3 45 45 10 100 736
1 3 40 40 20 100 1443
1 3 35 35 30 100 1001
1 3 25 25 50 100 211
1 3 15 15 70 100 29
2 2 45 45 10 100 425
2 2 40 40 20 100 2376
2 2 35 35 30 100 1164
2 2 30 30 40 100 738
As can be seen from this example, there exists a certain optimal composition
that
gives the best stability.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
22
Example 6
In this example, the effect of the total concentration of the stabilizers is
demonstrated. The tests were carried out in the same way as in Example 2.
Fe, ppm Mn, PAHPS- DTPA, Mg, Stabilizer, t 1/2,
ppm AA, mg/I mg/I mg/I min
m /I
1 3 0 0 0 0 10
1 3 20 20 10 50 922
1 3 30 30 15 75 1514
1 3 40 40 20 100 2294
1 3 60 60 30 150 2993
Example 7
This example shows how the optimal PAHPS-AA: DTPA ratio changes when iron
and manganese concentration changes. The tests were carried out in the same
way as in Example 2.
Fe, ppm Mn, PAHPS- DTPA, Mg, % Stabilizer, t 1/2,
ppm AA, % % mg/I min
2 2 20 60 20 100 1307
2 2 40 40 20 100 2376
1 3 20 60 20 100 2294
1 3 40 40 20 100 1443
Example 8
An industrial TMP (spruce, picea abies) pulp was bleached in laboratory using
different stabilizers. The pulp contained 6 ppm Fe, 8 ppm Mn, 730 ppm Ca, and
<
2 ppm Cu. The PAHPS-AA used in this test was prepared in Example 1. DTPA
used in this test was of commercial grade containing the normal side products
of
the process. The reaction temperature was 70 C, reaction time 120 minutes,
consistency 12%. Chemical charges were: NaOH 35 kg/ton pulp, H202 45 kg/ton
pulp, stabilizer dosages are shown in table below (calculated as active
substance).
Initial pH in the bleaching was 10.4-10.2.
CA 02564015 2006-10-23
WO 2005/108673 PCT/F12005/000211
23
PAHPS-AA, kg 1.3 0.8 0.6 0.4 0 0
Na5DTPA, kg 1.3 0.8 0.6 0.4 0 0
Mg, kg 0.63 0.42 0.28 0.19 0 0
Stabilzer, as 100%, kg 3.13 2.10 1.41 0.94 0 0
Waterglass, kg/ton pulp 0 0 0 0 0 25
Residual H202 17.5 18.7 15.6 16 2 17.3
Brightness 77.8 78.6 77.7 77.7 75 77.6
According to the Yes Yes Yes Yes No No
invention?
The results show, that silicate can be efficiently replaced by the stabilizer
according to this invention.
Example 9
In this example, the effect of pH in bleaching is demonstrated. The pulp used
in
this test is the same as in example 8. The detailed reaction conditions and
chemical dosages are presented in the table below.
no 107 108 102 103 104 105 106 113 114 115 116
test P P P P P P P P P P P
T,C 70 70 70 70 70 70 70 70 70 70 70
t,min 120 120 120 120 120 120 120 120 120 120 120
Cs,% 12 12 12 12 12 12 12 12 12 12 12
Initial pH 10.3 10.4 10.5 10.3 10.1 10 10.1 10.4 10.3 10.1 10
Final pH 9.7 9.7 9.4 9 8.5 8.5 8.5 9.8 9.7 9.5 9
H202, kg 45 45 45 45 45 45 45 45 45 45 45
NaOH, kg 38 38 35 30 25 25 25 38 35 30 25
Waterglass, kg 0 0 0 0 0 0 0 25 25 25 25
DTPA, kg 2.5 1.25 2.5 2.5 2.5 1.75 1 0 0 0 0
PAHPS-AA, kg 2.5 1.25 2.5 2.5 2.5 1.75 1 0 0 0 0
MgSO4, kg/t 3.1 1.6 3.1 3.1 3.1 2.2 1.3 0 0 0 0
Residual H202,kg 17.4 16.8 21.8 27.2 31.5 30.2 27.8 16.2 17.3 28 32
Residual NaOH,kg 3.4 4 2.4 1.2 0.6 0.7 0.7 8.6 7.1 4.8 2.3
Brightness,% ISO 77 77.3 77.8 77.2 76.7 76.7 76.3 77.5 77.6 77.4 77.1
According to the invention? Yes Yes Yes Yes Yes Yes Yes No No No No
pH is a very significant factor in bleaching. Higher alkalinity leads to lower
stability
of peroxide. On the other hand higher alkalinity improves bleaching
performance
by increasing perhydroxyl anion concentration. This example shows that the
stabilizer according to this invention gives bleaching result equal to sodium
silicate
even with high alkali charge.