Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
METHOD FOR RECOVERING GOLD
FIELD OF THE INVENTION
The invention relates to a method for recovering gold from an intermediate
product or residue containing sulphur and iron generated in the leaching of a
sulphidic raw material. The recovery of gold and the other valuable metals in
the raw material takes place in a chloride environment. The gold contained in
the intermediate product or residue is leached with divalent copper and
chlorine in a copper (II) chloride - sodium chloride solution in conditions
io where the oxidation-reduction potential is in the range of 650-750 mV and
the pH between 1 and 1.6. The acid generated during the feed of chlorine is
neutralized with a suitable alkali. Neutralization avoids the costs of
dissolving
the iron.
BACKGROUND OF THE INVENTION
Several methods are known in the prior art, which are used to leach gold
from material containing sulphur and iron in connection with a chloride-based
copper recovery process.
US patent 4,551,213 describes a method in which gold can be leached from
sulphur-containing material, in particular the residue from hydrometallurgical
processes. The preferred starting material for the method is the residue from
the CLEAR process. The CLEAR process is a hydrometallurgical copper
recovery process, which occurs in a chloride environment and at elevated
pressure. The gold-containing residue is elutriated into water and the
chloride concentration of the resulting suspension is adjusted so that it is
12
- 38 weight percent. The oxidation-reduction potential is adjusted to the
range of 650 - 750 mV and the pH to below 0. Copper (II) chloride or iron
(III) chloride are added to the suspension to oxidize the gold contained in
the
3o raw material, so that it dissolves.
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
2
EP patent 646185 relates to the recovery of copper from sulphidic
concentrates with chloride leaching in atmospheric conditions. The gold from
the leach residue is dissolved into electrolyte, which includes two or more
halides, such as sodium chloride and sodium bromide. The purpose is to
store oxidising energy in the bromine complex on the copper electrolysis
anode, and thereby leach the gold from the residue.
There are certain drawbacks to the above-mentioned methods. In the
method of US patent 4,551,213 the leaching conditions are very severe. The
lo patent mentions that sulphur is not dissolved in the patent conditions, but
the
mention is not universally applicable, since the dissolving tendencies of
elemental sulphur and the iron compounds mentioned in the patent depend
on the manner in which the sulphur and the compounds in question are
generated. In the tests we carried out it was found that when leaching
residues formed in atmospheric conditions are treated in conditions in
accordance with the said patent, the dissolution of sulphur and iron is
considerable. Since, according to the publication, the sulphur and iron do not
dissolve, there is no mention either of how to recover them from the solution.
The gold leaching method used in EP patent 646185 using a bromine
complex is not advantageous from an environmental point of view because
harmful bromine emissions may be generated in the concentrate leaching
stage.
WO patent application 03/091463 describes a method for leaching gold from
leaching residue or intermediate product containing iron and sulphur,
generated in the atmospheric chloride leaching of copper sulphide
concentrate. The publication states that gold may be leached from an iron-
and sulphur-containing material into an aqueous solution of copper (II)
chloride and sodium chloride with copper and oxygen in conditions where the
oxidation-reduction potential is below 650 mV and the pH value of the
solution in the range 1 - 3. In these conditions iron does not dissolve and
the
sulphur remains undissolved to a large extent. Thus the costs that arise
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
3
when iron and sulphur are removed from the solution are avoided. The
recovery of gold from the solution is made by one of the methods of the prior
art such as electrolysis or active carbon. The method in question is in itself
quite good, but in practice however it is somewhat slow.
PURPOSE OF THE INVENTION
Now a method has been developed for the recovery of gold from an
intermediate product or residue that contains sulphur and iron, generated in
the leaching of a sulphidic raw material. Raw material leaching is carried out
io using a concentrated aqueous solution of alkali chloride and copper (II)
chloride in atmospheric conditions. When oxygen or oxygen-containing gas
is fed into the sulphidic concentrate leaching stages, iron is oxidised and
precipitated as oxide or hydroxide and the valuable metals, with the
exception of gold, dissolve. The leaching of gold from the remaining residue
is carried out with an alkali chloride - copper (II) chloride aqueous solution
and chlorine in atmospheric conditions. The oxidation-reduction potential of
the leaching stage is raised to the range of 650-750 mV by means of
chlorine. A high oxidation-reduction potential enables the elemental sulphur
in the residue to dissolve and as a consequence, acid is formed in the stage,
which is neutralised by some suitable alkali. The simultaneous neutralisation
of the acid keeps the pH at a value of 1.0 - 1.6, whereby the dissolution of
iron is prevented. The dissolved gold is recovered by some method known
as such in the prior art.
The essential features of the invention will be made apparent in the attached
claims.
SUMMARY OF THE INVENTION
A gold-bearing intermediate product or residue is leached into sodium
chloride solution containing copper(II) chloride forming a suspension and the
oxidation-reduction potential required for gold leaching is obtained
particularly by means of divalent copper and chlorine gas. If the feed to gold
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
4
leaching still contains undissolved copper- or other sulphide, oxygen-
containing gas can be fed to the start of the leaching stage in order to
dissolve it. The oxidation-reduction potential is measured with Pt- and
Ag/AgCl electrodes and the potential is kept at a value of 650-750 mV. The
amount of divalent copper, Cu2+, in the solution is preferably 20 - 80 g/I and
the amount of sodium chloride in the region of 200 - 330 g/l. Gold dissolves
as a chloro complex in accordance with the following reaction:
Au + 3 Cu2+ + 6 CI" 4 AuC14 + 3 Cu+ + 2 CI" (1)
lo
Leaching occurs in atmospheric conditions at a temperature, which is
between room temperature and the boiling point of the suspension,
preferably, however, between 80 C and the boiling point of the suspension.
Thus, tests have now revealed that raising the redox potential of the reacting
slurry with chlorine gas accelerates the dissolution of gold. There is however
a drawback to this acceleration. Raising the redox potential increases the
dissolution of the elemental sulphur (S ) in the material to be leached, which
probably occurs in accordance with the following reaction (2):
S + 3 C12 + 4 H20 4 H2SO4 + 6 HCI (2)
Reaction (2) shows that a lot of acid is generated (8 mol H+/ mol S ). The
acid generated in the solution must however be neutralised, since at pH
values under 1 the iron in the solids begins to dissolve. The dissolution of
iron causes process costs, as dissolved iron is circulated and consumes
reagents. The preferred pH region to keep iron in the residue is between 1.0
and 1.6.
Some suitable alkali from the group NaOH, KOH, CaO, CaCO3 or MgO is
used to neutralise the acid. If the process is combined with a chloride-based
hydrometallurgical method of producing copper, in which basic copper (II)
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
chloride is generated in the precipitation of divalent copper from leaching,
the
use of the copper (II) chloride is the best option. When basic copper (II)
chloride dissolves, the copper (II) chloride generated can be used for raw
material leaching. The neutralization of hydrochloric acid and copper (II)
5 oxychloride leaching occur according to the following reaction:
3 Cu(OH)2.CuC12 + 6 HCI 4 4 CuC12 + 6 H20 (3)
The sulphuric acid generated can be neutralised for example with lime:
H2SO4 + CaCO3 4 CaSO4 2H2O + CO2 (4)
The recovery of gold from the solution takes place by some method known
as such in the prior art, for instance active carbon, electrolysis or chemical
precipitation.
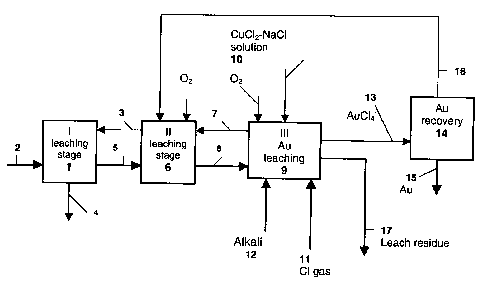
LIST OF DRAWINGS
The method of the invention is further described in the flow chart of Fig 1,
where the recovery of gold is combined with the hydrometallurgical recovery
of copper.
DETAILED DESCRIPTION OF THE DRAWINGS
According to Fig. 1, a sulphidic raw material such as copper sulphide
concentrate 2 is fed to the first leaching stage 1, and solution 3 from a
later
process stage, which is an aqueous solution of copper (II) chloride and
sodium chloride, is also circulated to this stage. The thicker arrows denote
solids and the thinner arrows the flow of solution. The stage always includes
one or more reactors and thickening. The copper and other valuable metals
of the concentrate mainly dissolve into the process solution and the resulting
solution 4 includes copper chloride, in which about 70 g/I of copper is mainly
monovalent. The further treatment of the copper chloride solution is not
presented in more detail here.
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
6
The leaching of the solids 5 from the first leaching stage is continued in the
second leaching stage 6 with solution 7, which is taken from a later process
stage. Air is fed into the reactors at this stage in order to enhance leaching
of
the valuable metals and to precipitate the iron. Thickening is done at the end
of this stage.
The solution 3 from the second stage is routed to the first leaching stage 1
to
leach the concentrate. The leaching of the solids 8 from the second leaching
io stage is continued in the third stage 9 in order to leach the rest of the
copper
and the gold. In the third leaching stage i.e. the gold leaching stage, the
residue is leached with copper (II) chloride - sodium chloride solution 10, in
which the Cu2+-content is 20 - 80 g/I and the sodium chloride content 200 -
330 g/l. If the residue entering this leaching stage still contains
undissolved
sulphide, oxygen, preferably in the form of air, can also be routed to the
first
reactor at the beginning of the stage. Copper and other sulphides should be
leached out of the residue before the gold dissolves. In order to raise the
redox potential to the range of 650-750 mV, chlorine gas 11 is also fed into
the reactor. Because of the high potential, sulphur starts to dissolve and as
a
2o result acid is formed in the stage. So that the pH of the stage does not
fall
below 1.0, some alkali 12 is fed into it such as NaOH, KOH, CaO, CaCO3 or
MgO. If the process is combined with a chloride-based hydrometallurgical
method to produce copper, in which basic copper (II) chloride is generated in
the precipitation of divalent copper from leaching, the basic copper (II)
chloride is used.
The gold-chloro complex solution 13 obtained from the leaching stage is
routed either as it is or filtered to gold recovery, which in this case occurs
in a
carbon column 14 by means of active carbon. The gold product 15 is
obtained from the column. The solution exiting column 14 is a gold-free
solution 16, which is circulated to the second stage of leaching 6 and sodium
chloride solution is routed there as required to achieve a suitable copper
(II)
CA 02564338 2006-10-25
WO 2005/106052 PCT/F12005/000194
7
chloride content for leaching. The residue of the gold recovery stage, after
normal after-treatment such as filtration and washing (not shown in detail in
the diagram), becomes the final leach residue 17, which contains nearly all
the sulphur and iron of the concentrate. The residue filtrate and rinse water
are returned for example to the concentrate leaching process.
The flow chart in Fig. 1 presents a gold leaching method in connection with
copper-bearing raw material leaching, but the method of the invention is not
limited to the copper-bearing raw material leaching process shown in the
lo chart. The crux of our method is that the leaching of gold-bearing material
is
performed with divalent copper and chlorine in conditions where the redox
potential of the solution is raised to a value of 650-750 mV, and the acid
formed during the dissolution of sulphur is neutralized so that the pH is
minimum 1, preferably at least 1.0 - 1.6.