Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
GEOSYNTHETIC COMPOSITE FOR BOREHOLE STRENGTHENING
Field of the Invention
This invention is related to borehole lining treatment technology. More
particularly it
is related to chemical formulations for creating geosynthetic composites in-
situ for
strengthening and reinforcing. Still more particularly it is related to
chemical formulations for
creating geosynthetic composites in-situ that are soluble in one or more non-
aqueous or invert
emulsion drilling fluids, or drilling fluids made with diesel and mineral
oils, and blends of any
of 'said drilling fluids with C7 to C20 olefins, esters, and paraffinic oils.
Additionally the
properties of the formulations can be varied by simply altering the
concentration of
crosslinking monomer and/or solvent.
Background of the Invention
Boreholes created into the earth for extraction of mineral deposits such as
oil and natural
gas pass through numerous and varied geologic formations. These geologic
formations have
varied chemical compositions, permeabilities, porosities, pore fluids,
internal (pore) pressures,
and material properties. Important material properties that significantly
impact well construction
operations include compressive strength, tensile strength, fracture initiation
pressure, fracture
propagation pressure, porosity, Young's (elastic) modulus, Poisson ratio and
bulk modulus.
Wide contrasts in formation pressures, formation material properties, and
formation
fluid types often require isolation and treatment of certain geologic
formations. Isolation and
treatment may be required to treat a weak formation, to increase near-wellbore
fracture
initiation pressure, to consolidate weak zones, to cure lost circulation, to
reduce formation
permeability, to seal off flow zones, to isolate high/low pressure zones, to
shut off undesirable
water or gas supply, to address damage to the tubing caused by collapse in
formation and
subsequent damage to pipes, or as a shut off plug for wells which are to be
shut off
permanently or temporarily, or as a so-called "kick-off' plug to prepare a
site for the drilling
of a new well from the remaining upper section of a former well. Also, in the
drilling of
multiple branched wells there is often a need to reinforce and seal the
transition zones of the
surrounding formation. These transition zones are subject to large mechanical
stresses. In
addition, there may be weak formations in wells that have been drilled such
that they deviate
substantially from a vertical position, or where part of the well is
horizontal.
1
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
Weak formations may result where, for example, the fracture initiation
pressure of one
formation may be lower than the internal pore pressure of another formation.
The increased
pressure in a borehole created by penetrating one formation may cause a lower
strength
formation to fracture. Similarly, the fluid pressure gradient in a borehole
required to contain
formation pore pressure during drilling may exceed the fracture pressure of
another, weaker
formation exposed in a borehole.
Attempts may be made to isolate specific formations and reinforce them with
steel
casing, or with cement or other treatments known in the art. Where steel
casings are cemented
in a borehole to isolate geologic formations having significantly different
properties, each of
these casing strings is costly and results in a reduction in the diameter of
the borehole in
subsequent sections as the borehole is deepened. It is desirable, therefore,
to minimize the
number of casing strings required to reach the desired depth.
It is known in the art to use cement to line boreholes, however a disadvantage
of cement
is that the curing step may require up to 24 hours, which is a
disproportionately long period of
time to wait, especially when the production site is a very costly offshore
operation. A further
disadvantage of cement is that in view of its particle based structure the
material exhibits
relatively poor penetration capabilities in formations, which may result in
reduced sealing effect.
There are references in the literature using resin based cementing materials
for geothermal
wellbores. In "New, Novel Well-Cementing Polymer Concrete Composite", American
Concrete
Institute (ACI), Special Publication 69: "Application of Polymer Concrete",
1981, part 69-5, pp.
73-92, Zelding, A. N., et al describe a system based on resin with initiator
and inhibitor, where
complete curing of the resin depends on the presence of water. The main
component of the resin
is organic siloxanes.
WO 94/12445 discloses an alternative material to cement and water slurries,
developed
for the completion of primary and secondary cementing of deep, hot oil wells
with static
background temperature in the range of 120-200 C (248-392 F). The binder is
based on diallyl
phthalate resin with the setting/curing time controlled by the addition of a
temperature sensitive
peroxide initiator and a suitable inhibitor.
Various sealants are known in the art for lining or strengthening boreholes.
Where
sealants are employed, a resin or monomer must be selected for each well that
is compatible with
the drilling/completion fluid used. Epoxy resins provide the best
comprehensive strength, tensile
2
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
strength and adhesion properties. However, epoxy resins and/or their curing
agents have poor
compatibility and poor performance with olefins, esters, and paraffinic
hydrocarbon fluid.
WO 97/15746 discloses a composition for sealing different types of zones in
oil wells,
which includes a monomer, initiator, inhibitor and optional filler as well as
other additives.
U.S. 4,556,109 discloses a system based on curing according to a condensation
mechanism, rather than free radical polymerization.
Acrylate or methacrylate resins/monomers are available that are soluble in
olefin, ester,
and paraffinic hydrocarbon fluids. However, alone, these monomers and resins
fail to provide the
type of material properties required for geosynthetic composite linings. They
typically have poor
tensile strength, poor fracture toughness, and low compressive strength.
Blends of acrylate
monomers containing prepolymers have improved tensile strength, compressive
strength, and
fracture toughness. However, the prepolymers used in these blends are often
insoluble in the
hydrocarbon fluids previously discussed.
There is a distinct need in the art for chemical formulations which can
provide in-situ
geosynthetic composites which are completely soluble in non-aqueous drilling
fluids, invert
emulsion drilling fluids, drilling fluids made with diesel and mineral oils,
and combinations
thereof, and blends of any of these with olefins, esters, and paraffinic oils.
There is also a need
for in-situ geosynthetic composites which provide good radial penetration and
which set faster.
It would constitute a distinct advance in the art if a formulation were
available for forming
a composite in-situ that contained a blend of components that could be adapted
to provide
optimum material properties depending on the properties and conditions
encountered in the
formation by simply altering the type and concentration of crosslinking
monomer and/or solvent
and by varying the amount of each component. It would be additionally
advantageous if the
formulation exhibited improved compatibility with any combination of non-
aqueous drilling
fluids, invert emulsion drilling fluids, or drilling fluids made with diesel
and mineral oils, and any
blend of any of said drilling fluids with one or more esters, olefins, and
paraffin oils, or
combinations thereof.
Summary of the Invention
The present invention is a composition useful for creating geosynthetic
composites in-
situ, said composition including a melamine-formaldehyde resin, or blend of
suitable
melamine-formaldehyde resins; optionally blended with a polyol and/or a
poly(hydroxy)ether,
3
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
or a combination thereof and a non-aqueous drilling fluid. In some
embodiments, the dilling
fluid is an invert emulsion drilling fluids containing C7 to C20 olefins,
esters, paraffinic oils
and blends thereof, or diesel and mineral oils and blends thereof with C7 to
C20 olefins, esters,
and paraffinic oils. The composition may also include additives to initiate
and control time of
polymerization selected from: an acidic or acid-generating additive to
initiate polymerization.
Optionally a reaction retarder or polymerization inhibitor to delay the onset
of polymerization
until completion of injection into the formation has been completed. The
composition may
also contain other additives selected from, for example, one or more of a)
coupling agents; b)
suspending agents; c) dyes; d) weighting agents; e) lost circulation
materials; and f) other
additives known in the art, or any combination thereof. The formulation,
including amounts of
each component, the type of acrylate/methacrylate monomer, where applicable,
and the
chemical composition and material properties of the thermoplastic elastomer,
where
applicable, are varied to provide the required material properties for the
geosynthetic
composite formed in-situ by chemical treatment.
Brief Description of the Drawings
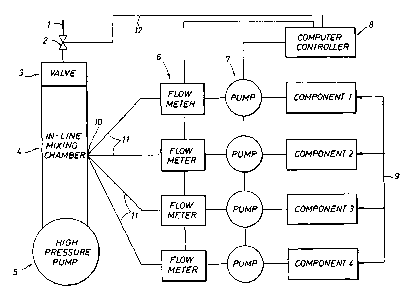
Figure 1 is a schematic drawing of the in-line apparatus for mixing and
pumping the
chemical treatment formulations in the present invention.
Figure 2 is a schematic drawing showing an alternative position for component
lines.
Detailed Description of the Invention
The present invention provides a chemical formulation for creating
geosynthetic rock-
plastic composites in-situ. The formulation is compatible, or miscible with,
and completely
soluble in non-aqueous drilling fluids and invert emulsion drilling fluids
containing C7 to C20
olefins, esters, paraffinic oils and blends thereof, as well as diesel and
mineral oils and blends
thereof with C7 to C20 olefins, esters, and paraffinic oils. The formulation
hardens in the pores
of the formation, bonds formation particles together, and forms a rock-plastic
composite. The
treatment is suitable for application to increase near-wellbore fracture
initiation pressure, in
depleted zones, over-pressured zones, flow zones, lost circulation zones and
other applications
that will be apparent to those skilled in the art.
The melamine-formaldehyde component in the formulation is a liquid melamine-
formaldehyde resin that is soluble in any hydrocarbon blend selected from one
or more of a
4
rCA 02567092 2006-11-18
~
r *4 1Lw P '.6 1 ;~4 SAX 713 241 6617 S,lELL(LEG ZP
fa v 'f.s . Y +7
S'/JyjP 1 . . 1iCriak tf.d d.Y`".[.r=i:.~,v:rs,t}v 'i,t
E 5;9
. ~ TH246 1-PCT
Y 4 i
Y
` r
non-aqueous drilling fluid, an invert emu]ision drilling fluid, diesel oil,
mineral oil, and any
blend of these with a C7 to C~ olefin, estz ors, and paraffin oils, and
combiuatious thereof.
Suitable melariune$armaldehyde resins nice amino crosslinkers designed for
thermosetting
surface coatings. Suitable resins should be capable of functioning tb
crosslinic the molecules
of the primary film former in a coating to' form a three-dimensional
lihermoset polymer
networks which involves the reaction of the functional groups on tbe' amino
with
complimentaryY reactive ,groups on. the primary film former. Suitable resins
are solvent soluble
and contain water extendable melamine. Suitable resins exhibit a versatile
catalyzed cure
response in a range between about 38 C to over 316 C (100 F to over 600 F),
more often
Y
between about 6 C to over 177 C (15O'F and 350 F) and provide good film
flexibility and
toughness. Resins are selected for the fo::mulation based on a combtiation of
performance and
cure parameters depending on the prape~:ties of the formation to be treated.
The melamine-formaldehyde resin may be used alone, may lie a blend of suitable
melamine-formaldehyde resins, or either may be blended will a polyol or blend
of polyols.
Suitable polyols must be soluble in the raelamine-formaldehyde resin or blend
of m.elaroin.e-
formaldehyde resins, and also soluble in the hydrocarbon phase of
drilling/completion fluids.
Suitable polyols include, for example, b iit are not limited to ethylene
glycol, propylene glycols
glycerol, diethylene glycol, triethylenetyco1, polyethylene glycol,
polypropylene glycol, and
polyethylene-propylene glycol. A suital:~le amount ofpolyol is from 0 to 50%
by volume,
more preferably 5 to 35% by volume, aI~Ld most preferably 10 to 30k by volume
of the
formulation. Suitable polyols for use in the present invention are seld
commercially under the
traderiame Voranol VoracflvTM P'alyols y Dow Chemical Company.
The melamine4ormaldehyde reE~in or blend of melange-fotznaldehyde resins may
also
optionally be blended with a poly (hycir xy) ether. It is also within the
scope of the invention
to blend the melee-formaldehyde re~tim.s or blends of resins with a blend of a
polyol and a
poly (hydroxy) ether. Suitable poly (liydroxy) ether material must be soluble
in the melamine-
. ;
formaldehyde,resin or blend of melamii,te-formaldehyde resins, and also in the
hydrocarbon
r
phase of drillinglcompletion fluids. Suitable poly (hydroxy) ethers; include
those that are high
Y
molecular weight with preferably a predominantly linear structure, ;which
typically provides a
TR246lFF.RSTYY 5
,+ M ~ Y
,e'+a1 ved at the EPO on Ap 03, 2006 21 2725, Pa
} AMENDED SHEET
CA 02567092 2012-07-18
63293-4091
combination of toughness and flexibility, and characterized by ether linkages
and pendant
hydroxyl groups that promote wetting and bonding to polar substrates and
fillers.
Suitable poly (hydroxy) ethers include substantially linear polymers having
the general
formula: -[D-O-E-O)n
wherein D is the radical residuum of a dihydric phenol, E is a hydroxyl
containing radical
residuum of an epoxide and n represents the degree of polymerization and is at
least 30. (See
U.S. 4,355,122)
These poly (hydroxy) ethers can be prepared by admixing from about 0.985 to
about
1.015 moles of an epihalohydrin with one mole of a dihydric phenol together
with from about
0.6 to 1.5 moles of an alkali metal hydroxide, such as, sodium hydroxide or
potassium
hydroxide, generally in a solution at a temperature of about 10 C to about. 50
C, until at least
about 60 mole percent of the epihalohydrin has been consumed.
The dihydric phenol contributing the phenol radical residuum, D, can be a
dihydric
mononuclear phenol, a dihydric polynuclear phenol, or mixtures thereof.
Preferred dihydric
poly nuclear phenols include his (hydroxyphenyl) alkanes, di (hydroxyphenyl)
sulfones, di
(hydroxyphenyl) ethers, and the like.
The epoxide contributing the hydroxyl containing radical residuum, E, can be
monoepoxide or diepoxide. By "epoxide" is meant a compound containing an
oxirane group,
i.e., oxygen bonded to two vicinal aliphatic carbon atoms. Suitable epoxides
include
monoepoxides, diepoxides, saturated epoxides and the like, and mixtures
thereof;
Blends of suitable poly (hydroxy) ethers may also be employed. A suitable
amount of
poly (hydroxy) ether is in the range of from 0 to 50% by volume, more
preferably 5 to 35% by
volume, and most preferably 10 to 30% by volume of the formulation. Suitable
poly
(hydroxy) ethers are available commercially under the tradename PAPHENO
Phenoxy Resins
from INCHEM Corp. They are available as solids, in solutions, waterborne
dispersions, resin
blends and micronized powders.
(Also see U.S. 6,034,160, to InChem.)
A solvent may be employed to dilute the blend of the selected formulation,
improve
wetting of formation surfaces, and improve tolerance to water contamination.
The solvent
should be miscible with water and hydrocarbons and may be selected from any
convenient
6
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
type, which would be apparent to those skilled in the art. Suitable solvents
include, but are not
limited to low molecular weight anhydrous alcohols such as methanol, ethanol,
propanol;
ethers and polyethers, such as tetrahydrofuran, dioxane, ethylene glycol
monoalkyl ethers,
polyethylene glycol monoalkylethers or glycol ether esters; ether alcohols
such as 2-
butoxyethanol, or mixtures thereof. Preferred solvents include ethylene glycol
monobutyl
ether, propylene glycol methyl ether acetate, and other solvents effective for
dissolving the
thermoplastic elastomer, or mixtures thereof. A suitable concentration of
solvent can range
from 0 to 50 wt percent, more preferably 1 to 35 wt percent, and most
preferably 5 to 25 wt
percent.
A catalyst or initiator is useful in the application of the present invention.
The use of
catalysts and initiators is known in the art and the invention is not intended
to be limited to any
particular type. An acidic catalyst or acid-producing catalyst is suitable for
condensation
polymerization of melamine-formaldehyde resins. Suitable catalysts may
include, for example,
but not be limited to strong acid catalysts such as mineral acids including,
for example,
hydrochloric acid, sulfuric acid, phosphoric acid, and nitric acid; strong
organic acids
including sulfonic or para-toluene sulfonic acid, benzene sulfonic acid,
xylene sulfonic acid,
dinonylnapthalene disulfonic acid, dinonylnapthalene sulfonic acid, and
dodecylbenzene
sulfonic acid; weak organic acids, including, but not limited to formic,
boric, phosphorous,
oxalic, and acid salts of hexamethylenetetramine, acetic acid, fumaric acid,
and formic acid;
esters of weak organic acids, including but not limited to butyl acetate,
isopropyl acetate, and
methyl formate; latent acid catalysts, such as ammonium chloride, alkyl acid
phosphates, and
phenyl acid phosphates; and acid-producing catalysts such as acid esters or
blocked acid
catalysts, including but not limited to amine salts of dinonlynapthalene
disulfonic acid,
dinonylnapthalene sulfonic acid, and dodecylbenzene sulfonic acid, or mixtures
thereof
Suitable free radical initiating catalysts or catalyst systems may include,
for example, but not
be limited to azo compounds, alkyl or acyl peroxides or hydroperoxides,
ketoperoxides,
peroxy esters, peroxy carbonates, and peroxy ketals, or mixtures thereof. Such
compounds
vary with respect to activation temperature and half-life or, in other words,
the temperature at
which their reaction is initiated and becomes extensive. Examples of suitable
alkyl peroxides,
dialkyl peroxides, hydroperoxides, acyl peroxides, peroxy esters and peroxy
ketals include, but
are not limited to benzoyl peroxide, dibenzoyl peroxide, diacetyl peroxide, di-
t-butyl peroxide,
7
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
cumyl peroxide, dicumyl peroxide, dilauryl peroxide, t-butyl hydroperoxide,
methyl ketone
peroxide, acetylacetone peroxide, methylethyl ketone peroxide, dibutylperoxyl
cyclohexane, di
(2,4-dichlorobenzoyl) peroxide, diisobutyl peroxide, t-butyl perbenzoate, and
t-butyl
peracetate, or mixtures thereof. The catalyst may be employed in total amounts
from about
0.001 to about 20 weight percent based upon the weight of the polymerizable
monomer.
Blocked catalysts may be used at elevated temperature to delay the
polymerization
reaction. Blocked catalysts are amine salts of aromatic sulfonic acids.
Examples include the
amine salts of dinonylnapthalene disulfonic acid, dinonylnapthalene sulfonic
acid,
dodecylbenzene sulfonic acid, and para-toluene sulfonic acid, and mixtures
thereof. These
blocked catalysts do not become effective catalysts until converted to their
acid form by
temperature. For example, para-toluene sulfonic acid can cure resins at room
temperature.
The amine salts of para-toluene sulfonic acid will not cure resins below 65 C
to 90 C.
Other additives can be incorporated into the formulation including, but not
limited to
coupling agents, suspending agents, dyes, weighting agents, and lost
circulation materials.
Numerous coupling agents are known in the art and the invention is not
intended to be
limited to particular agents. Preferred coupling agents include silane
coupling agents. A
suitable silane coupling agent may be selected from among
vinyltrimethoxysilane,
vinyltriethoxysilane, vinyltris ((3-methoxyethoxy) silane,
vinylmethyldimethoxysilane,
vinylmethyldiethoxysilane, 8-glycidoxypropyltrimethoxysilane, 6-
glycidoxypropylmethyldimethoxysilane, 8-methacryloxypropyltrimethoxysilane, 6-
methacryloxypropylmethyldimethoxysilane, acryloxypropyltrimethoxysilane,
acryloxypropylmethyldimethoxysilane, N-(3-(aminoethyl)-8-
aminopropyltrimethoxysilane, N-
(3-(aminoethyl)-6-aminopropyltriethoxysilane, N-(3-(aminoethyl)-8-
aminopropylmethyldimethoxysilane, and N-(3-(aminoethyl)-6-
aminopropylmethyldiethoxysilane, and mixtures thereof. Also suitable are
bifunctional sulfur-
containing organo silanes such as, for example, bis- (3-triethoxy-silylpropyl)
tetrasulfide, bis-
(3-trimethoxy-silyl-propyl) tetrasulfide, and bis- (3-trimethoxy-silylpropyl)
tetrasulfide grafted
silica, available from DeGussa AG. A suitable concentration for a coupling
agent is in the
range of 0 to 10 wt percent.
s
4- CA 02567092 2006-11-18
.Q4/3I46 X3:4 FAX 713 241 6617 SHJLL/LEG~IP 1O12
elftf Y a~1L . t >. t' Y j l41.ISI' 1 A
~. r tit _. 1 .1'A E~ rx1^ k"d""}=ic
t fr t k1f
Printed: 1 91Q4/2Op6' . pEa,o; :O5756398
L. , Ta2461-PCT I
I
1
i
i
i
i, 1 f 1
Suspending agents known iii the sort can be added to the formulation to
support solids.
The invention is not intended to be limited to any particular agents, however
stitable
= 1
suspending agents include, for example, is rganophihc clays, amine txeated
clays, oil soluble
polymers, quaternary amrnoniurn compolimds, polyamide resins, polycarboxylic
acids, and
soaps.
I
The formulation may also contain other common treatment f'uid ingredients such
as
fluid loss control additives, dyes, anti-fouuning agents when necessa y, and
the like, employed
in typical quantities, known to those skil ted in the art. Of course, the
addition of such other
additives should be avoided if it will detrimentally affect the basic desired
properties of the
treatment fluid:
r
weighting agents or density matcrials may be added to the f+kmuiation.
Suitable
materials inclu 1e, for example, galena, hematite, magnetite, iron oxides,
ilmenite, barite,
1
siderite, celestite, dolomite, calcite, man ;anese oxides, magnesium oxide,
zinc oxide,
zirconium oxides, spinels and the like. The quantity of such material added,
if any, depends
upon the desired density of the chemical treatment composition. Typically,
weight material is
1
added to result' in a drilling fluid density of up to about 1.1 kg/liter (9
pounds per gallon). The
weighted material is preferably added ups to 23 kg (5 pounds) per barrel and
most preferably
1
up to 225 kg (500) pounds per barrel of:resia blend.
Lost circulation materials may al so be incorporated into the formulation.
These
materials are generally categorized as tiers, flakes, granules, and mixtures.
Specific
1
examples inc1ide, but are not limited to ground mica, mica flakes, silica
slag, diatomaceous
earth, hydrated borate, graded sand, diatomaceous earth, gilsonite, found
coal, charcoal,
cellophane flakes or strips, cellulose fiber, expanded perlite, shredded paper
or paper pulp, and
.
the like, walnut or other nut hulls groum,l to different sizes, cottons ed
hulls or cottonseed
bo11s, sugar cane fibers or bagess, flax, straw, ground hemp, ground fir bar%,
ground redwood
1
bark and fibers, and grape extraction re~.idue, crystalline silicas, amorphous
silicas, clays,
1 ,
calcium carboI nate, i
and barite. Suitable amounts of additional solid agents for use in
i
combination with the copolymer(s) and/or iouomer(s) would be apparent to those
skilled in the
t
art
1
1
1 I
I
1 I
TH24 SIFF . RST 9
e2 ved at the EPO on Apr 03, 2006 21 2725. Pa AMENDED SHEET 1
t O3JO4I2QO6``
CA 02567092 2012-07-18
63293-4091
The formulation and the chemical composition and material properties of the
thermoplastic elastomer may be varied to provide required material properties
for the
geosynthetic composite formed in-situ.
The method and apparatus for utilizing the formulation of the present
invention is
described in more detail U.S. Patent No. 7,343,974. Advantages of certain
embodiments
include reducing the number of casing strings required to create a borehole of
a specified
depth, eliminating borehole diameter reductions necessitated by isolating
geologic
formations of significantly different properties with steel casings cemented
into the earth
at required intervals, and, ideally, allowing the creation of a single
diameter or
'monobore' wellbore lined with a single casing, or no more than two casing
strings after
reaching the required depth. U.S. Patent No. 7,696,133 is also related to this
case.
The first step in the method for use of the formulation of the present
invention is to
determine or estimate the material properties of the exposed geological
formation. Certain
properties are assessed in order to select the proper treatment. These
properties include, for
example, fracture initiation pressure, tensile strength, Young's modulus and
Poisson Ratio,
temperature, porosity and permeability. Methods of obtaining this data are
known to those
skilled in the art and the invention is not intended to be limited to any
particular methods of
performing tests to determine these properties.
Once properties of the formation are determined, data may be obtained and
analyzed to
determine the required changes in material properties of the exposed
geological formation that
would be desirable to eliminate the need for setting an additional casing
string. Given the
changes desired the appropriate chemical formulation of the treatment may be
selected, the
necessary minimum radial penetration distance of the chemical treatment from
the wellbore
may be determined, and volume of chemical treatment may be determined
The treatment process creates a cylindrical shell of a geosynthetic composite
extending
radially from the wellbore. Elastic modulus, tensile strength, compressive
strength, fracture
strength, fracture toughness and permeability of this geosynthetic composite
are different from
the surrounding formation. These properties allow the chemically treated
region of the
formation to withstand higher stresses (pressure) in the wellbore than the
untreated formation
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
without fracturing. The fracture pressure of the chemically treated region is
higher than the
untreated formation. The permeability of the chemically treated region is
typically lower that
the untreated formation.
The increased fracture pressure is a function of the material properties of
the
geosynthetic composite and thickness of the treatment. The change in fracture
initiation
pressure can be estimated by equations, known in the art of mechanics,
specifically rock
mechanics, for calculating the fracture strength of formations. The
impermeability of the
geosynthetic composite is important in the change of fracture initiation
pressure. The
impermeable, treated portion of the formation acts as an elastic layer re-
distributing the
pressure applied to the wellbore to the surrounding formation, allowing higher
stresses
(pressures) to be applied before fracturing or failure of the wellbore occurs.
Three modes of
failure are possible with the impermeable, geosynthetic composite layer
created by treatment
of the formation: (a) fracture of the geosynthetic composite, (b) fracture of
the untreated
formation beyond the thickness of the geosyntetic composite, or (c) plastic
failure of the
geosynthetic composite. With some modes of failure, the impermeability of the
layer is of
critical importance and the thickness of the layer is of little importance
except for the thickness
required to obtain complete impermeability. In other modes of failure,
thickness of the layer is
of critical importance. The increased fracture pressure may be a function of
the material
properties of the geosynthetic composite and thickness of the treatment. The
change in
fracture initiation pressure can be estimated by equations, known in the art
of mechanics,
specifically rock mechanics, for calculating the fracture strength of
formations. The
impermeability of the geosynthetic composite is important in the change of
fracture initiation
pressure. The impermeable, treated portion of the formation acts as an elastic
layer re-
distributing the pressure applied to the wellbore to the surrounding
formation, allowing higher
stresses (pressures) to be applied before fracturing or failure of the
wellbore occurs. Three
modes of failure are possible with the impermeable, geosynthetic composite
layer created by
treatment of the formation: (a) fracture of the geosynthetic composite, (b)
fracture of the
untreated formation beyond the thickness of the geosyntetic composite, or (c)
plastic failure of
the geosynthetic composite. With some modes of failure, the impermeability of
the layer is of
critical importance and the thickness of the layer is of little importance
except for the thickness
required to obtain complete impermeability. In other modes of failure,
thickness of the layer is
11
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
of critical importance. Those skilled in the art of geomechanics will be able
to use strength of
materials principles to calculate the most likely mode of failure and required
thickness of a
layer to obtain a specific increase in fracture initiation pressure.
The volume of treatment is determined from radial penetration distance from
the
wellbore, length of interval, wellbore diameter and formation porosity. The
volume of the
cylindrical shell is calculated and multiplied by the porosity of the
formation to estimate the
volume of pore space to be filled with the chemical treatment. An excess
volume may be
added to compensate for the uncertainty in the estimation. The volume of the
wellbore over
the treated interval must be filled with chemical treatment before or during
injection. This
volume is also added to the formation treating volume and any excess to yield
the required
treating volume.
Permeability of the geologic formation to be treated is important. The
formation must
have sufficient permeability to allow the chemical treatment to flow into
pores, displace pore
fluids and bond particles of the formation together more strongly. It is
desirable to treat
formations having matrix permeabilities between 1 millidarcy and 50,000
millidarcy.
Penetration rate typically increases with permeability at a constant injection
pressure. The
pressure required to inject the chemical treatment typically decreases with
increasing
permeability at a constant flow rate. Formations having permeability less than
about 0.1
millidarcy are difficult to treat. Very low viscosity treating fluids, high
injection pressures and
long treatment times are required for low permeability formations. A practical
solution for
low permeability formations is to use a ductile, high strength material that
requires a small
radial penetration distance from the wellbore to achieve the desired increase
in fracture
pressure.
The amount of time required to perform the treatment is necessary for the
formulation
of the chemical treatment. The polymerization reaction should occur after
injection of the
treatment volume into the formation has been completed. However, the
polymerization
reaction should not occur too long after injection has been completed because
dilution or flow-
back or cross-flow of fluids within the wellbore is possible. Treatment time
is estimated from
the sum of time required to pump the chemical treatment into the wellbore
adjacent to the
geologic formation to be treated, the time required to inject the treatment
into the formation,
the time to retrieve or withdraw any treating tools or pipes from the treated
interval plus a
12
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
safety margin. Time required to pump the chemical treatment into the wellbore
adjacent to the
formation to be treated is calculated using the volume of the chemical
treatement, the volume
of the workstring, and the pump rate. The time required to inject chemical
treatment volume
into the formation can be calculated using the volume of the chemical to be
injected and the
filtration rate.
The pseudo-steady-state filtration rate can be determined from a filtration or
fluid loss
test using a formation core or a synthetic core having filtration properties
representative of the
geologic formation to be treated. This time to inject the chemical treatment
must be less than
the time to cure the formulation used. Generally, the borehole is filled over
the volume to be
treated with the formulation, and then optionally pressurized to the extent
desired to force the
formulation into the formation at the rate predicted by the filtration rate,
plus a margin for
errors and inconsistencies. Thus the desired volume of the formulation is
forced into the
formation over the interval to be treated.
The chemical treatment may be selected based upon a number of considerations,
an
important one being compatibility with wellbore fluids (i.e. drilling fluids,
completion fluids,
formation fluids.) The chemical treatment should be compatible with or
miscible with
wellbore fluids and, most preferably the chemical treatment should be
completely soluble in
wellbore fluids. In addition, any catalyst or initiator should be compatible
with wellbore
fluids. For example, in a fluid containing a high amount of acid soluble or
acid neutralizing
components, one should not use an acid catalyzed chemical treatment fluid. In
addition, the
selected chemical treatment should be capable of penetrating drilling fluid
filter cake or near-
wellbore formation damage, commonly referred to as skin.
Additional important factors to consider when selecting the chemical treatment
are
strength and material properties of the chemical treatment, viscosity of the
chemical treatment,
initiation or catalyzation type of chemical treatment, wellbore temperature,
required placement
time of chemical treatment into geological formation, and permeability,
porosity, and lithology
of the geological formation.
In practice, the thickness of the treated formation (geosynthetic composite)
is greater
than the minimum thickness required to achieve the desired increase in
formation fracture
initiation pressure. This is done because of the inhomogeneity of the
formation and any
variability in the injection process. As a result, the geological formation is
treated to
13
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
improve/increase material property values to the required levels by injecting
a chemical
treatment into the formation to create a geosynthetic composite extending 0.05
to 5 meters,
preferably 0.1 to 3 meters, most preferably 0.25 to 2 meters radially from the
borehole. The
chemical treatment type and the depth of penetration are interrelated and
should be determined
based upon the geologic formation type; the permeability and porosity of the
untreated
formation; the presence of natural or induced fractures, fissures, faults or
vugs; and the
required material properties of the geosynthetic composite to be formed in-
situ by the
chemical treatment. Less radial penetration may by required in strong
formations or when
using high strength chemical treatments. Deeper radial penetration may be
required for weak
or unconsolidated formations and/or lower strength chemical treatments.
The first step in the method of utilizing the formulation may be to determine
or
estimate the material properties of the exposed geological formations. These
properties may,
for example, include the type of geological formation, the permeability and
porosity of the
untreated formation, and presence of natural or induced fractures, fissures,
faults, or vugs. In
some embodiments of the present invention the next step may be to determine
the required
changes in material properties of the exposed geologic formation necessary to
eliminate the
need for setting a casing string before drilling operations can continue.
With respect to the changes in material properties needed to continue drilling
and
eliminate the need for setting a casing string, the formulation may be
selected taking into
consideration compatibility with wellbore fluids, (i.e. drilling fluids,
completion fluids,
formation fluids), strength and material properties of chemical treatment,
viscosity of chemical
treatment solution, initiation or catalyzation type of chemical treatment,
wellbore temperature,
required placement time of chemical treatment in geological formation, and
permeability,
porosity and lithology of the formation.
The chemical treatment should be at least compatible with wellbore fluids, and
preferably completely soluble in wellbore fluids. The catalyst or initiator
should also be
compatible with wellbore fluids. For example, it would not be optimum to use
an acid
catalyzed chemical treatment in a fluid containing a high amount of acid
soluble or acid
neutralizing components. In addition, the chemical treatment should be capable
of penetrating
drilling fluid filter cake or near-wellbore formation damage. Less radial
penetration maybe
required in strong formations or with high strength chemical treatments, and
deeper radial
14
CA 02567092 2006-11-17
WO 2005/121500 PCT/US2005/019329
penetration may be required for weak or unconsolidated formations and/or lower
strength
chemical treatments.
The formation is treated to improve/increase material property values to the
required
levels by selecting an appropriate treatment formulation and injecting said
treatment into the
formation to create a geosynthetic composite extending 0.05 to 5 in, more
preferably 0.1 to 3
in, and most preferably 0.25 to 2 in radially from the borehole wall.
The chemical treatment formulation of the present invention may be applied to
the
wellbore through the drill string (BHA), by an open-ended treatment if a large
LCM (lost
circulation material) is used, by a spot-and-hesitation squeeze, or by a
bullhead-and-hesitation
squeeze (particularly in a severe loss zone). Preferably the composite will
exhibit radial
penetration away from the wellbore of 0.25 to 2 in. The monomer/resin
formulation hardens
in the pores of the formation and bonds formation particles together to form a
rock-plastic
composite.
After treatment the material properties of the geosynthetic composite are
improved
over the untreated geological formation. The fracture initiation pressure is
increased, tensile
strength increased, Young's modulus and Poisson Ratio are favorably altered to
improve
formation ductility, fracture toughness, and compressive strength, and the
permeability is
reduced.
After a zone is treated it can be pressure tested and drilling can be resumed.
It may be
appropriate at this point to use a higher or lower mud weight, as will be
apparent to those
skilled in the art.
The following examples will serve to illustrate the invention disclosed
herein. The
examples are intended only as a means of illustration and should not be
construed as limiting
the scope of the invention in any way. Those skilled in the art will recognize
many variations
that may be made without departing from the spirit of the disclosed invention.
Examples 1 through 3 demonstrate the use in the field of the formulation
utilizing at
least one melamine-formaldehyde resin, or a blend of melamine-formaldehyde
resins,
optionally blended with a polyol and/or poly (hydroxy) ether, soluble in non-
aqueous drilling
fluids and invert emulsion drilling fluids containing C7 to C20 olefins,
esters, paraffinic oils
and blends thereof, and also soluble in diesel and mineral oils and blends
thereof with C7 to
C20 olefins, esters, and paraffmic oils; and optionally other additives
selected from one or
'- CA 02567092 2006--18
r I.3 2 4 ~. 6 G~. i 11 = ,.~õ~ TF
1! Iry! Y !' JI L!`:4 yJy
1 11 jy~ I i.l e ! fl, ~r71. F s' 9+ 'v: 1 {f J' 1_1Sj
= ! I~~t~d;S i '!I I hk, ?. !s'f 1Ilf r r.. ', tlr.W f{ 4
V
1
J II' _} '1~I7~4! 2~~6: ; I IES 1 + CXP1M : y ,+pr . / 'T+f' If'. Al tf ff,--.
1'a r,l
Aw.w yr ~~r. D ~ T
1 1 1.
H ' T461 PCT . .
i
1
i
more of coupling agents, suspending agents, dyes, weighting agents, host
circulation materials,
and other additives known in the art.
E.am
1
Multipl treatments were perform d in seven wells having uncased wellbore
intervals
r
between he depths of about 2,740 meter' (9,000 feet) and about 5,490 meters
(18,000 feet),
in a southern Texas gas field, Static geothermal temperatures between about
113 to 160 C
(235 to 320 F) were encountered in this dept range Multiple geologic
formations are
exposed within this depth interval in the<<e wells. A series of permeable
sandstone formations
separated by s1a1e and silt intervals generally describes the exposed
formations in the
wellbore. Hydrocarbons, primarily gas, from some of the exposed s i n.ds have
been produced
from other wells in the field. Productiorc has decreased the formation pore
pressure of these
. 1
formations and reduced their fracture initiation and propagation pressure Sher
exposed,
permeable, hydrocarbon-bearing sands have not been produced and 'are at their
original
formation pore pressure and correspond,;ng higher fracture initcatiori and
propagation
pressures. In some cases the drilling fluid weight necessary to balance the
pore pressure of
unproduced formations exceeds the fraeibure initiation and propagation
pressure of previously
produced formations. Normally, casings are run and cemented in the weilbore to
isolate
geologic formations with such differenc es in pressure and strenggth.'
Failure to isolate formations with these differences in pressure and
properties generally
leads to fracturing ofthe weaker formation causing lost circulation, 1f the
lost circulation
cannot be cured. or controlled, then unct;rntroled flow ofhydrocarbons (or
other fluids under
pressure) can occur between fonrnationor to the surface. Such uncontrolled
flow from a
wellbore is referred to in the art as a blowout. In well o.1 of this study,
the weaker
1
formation was fractured by the higher mud weight required to control the pore
pressure from a
previously unproduced formation. Depth of the last circulation zone was at
approximately
12,000 feet, which was 3,000 feet deeper than the shoe of the previous casing
string. Lost
circulation was severe and difficult to c~ontro1. A resinMbased lost
irculation treatment was
1
performed to.repar the fractured formation. The formulation used for this
treatment was;
Per finished i S liter (42 gallon) barrel (bbl) of fluid..
i
r
50.7 liters (13.4 gallons) Diesel oil invert drilling fluid (2.0 kW1iter 163
lb/gal)
63.8 liters (168 gallons) Resimen e 755 Melamine-Fonnaldehyde Resin
TH2461FF.RST 16
r
vcd ut th a EPO on . y . ,,,..
' Apr, 03, 2006 21:27:25.
~~_
PaAMENDED SHEET O3/O4/2OO
CA 02567092 2006-11-18
04/03/06 13:24 FAX 713 241 661E SHELL/LEA IP LJO14
': sM f n .
f e t feu at.. ,:. "tUi 't'"rui
': ' rnl~ ~ 1 4 00 D SAP MDR ~ Oy5756398
=tr... .. rf s,: Y r.,.nr .,Y _.;: r.x.7.:. :. d:hr.;M rEi1 s.:.
'Wrr..ir<<~AiS7Y,u t.
`* r TH2461-PCT , .
r Y Y
I 6.0 liters (42r gallons) Ethylene glycol monobutyl ether
145.6 kg (323.6 pounds) Hematite
Y
I ,4 kg (3.06 pounds) Para-toluene sulfonic acid catalyst (40% by weight in
isopropyl
alcohol) Y
r
a
4.5 kg (l0 pounds) Magma Fil:~er Regular (Large fiber lost circulation
material)
Y
2.3 kg (5 pounds) Magma Fil:per Fine (Small fiber lost circulation material)
The original drilling fluid weight was 2(]) kg/liter (l67 lb/gal). Addition
ofthe melamine-
, ,.
formaldehyde resin, ethylene glycol motYobutyl ether solvent and catalyst
reduced the
solution weight. Hematite (iron oxide) as added as a weighting agent to
increase the weight
of the final mixture back to 2.0 kg/liter (116.7 lb/gal). .
The treatment was performed as follows i
Pull end of drill string up to 21,740 meter; (9,000 feet) just inside the shoe
of the previous
24.5 cm (9-5/8 inch) casing string.
Pump 50 bbls of resin mixture (formulation above) down the drillpipe to a dept
where the
leading edge of the mixture is 10 bbls above the end of the drill string.
Note, No spacer fluids were required ahead of or behind the resin mixture to
separate the
r
treatment fluid; from the driflurig fluid in the wellbore. This is because the
resin is completely
soluble in the drilling fluid and was con )tined with the drilling fluid to
make the treatment
mixture. The mutual solvent was included in this first formulation to insure
complete
compatibility between the drilling fluid ~ nd resin mixture. However, the
solvent was omitted
from later jobsY due to complete coompatil:~rlity ofthe resin with the dulling
fluid and the lack
of water in most formations being treate`;L ,
r
Close in the ananlar blowout pre'~enter to stop circulation of fluid out of
the well and
prepare to squeeze the resin treatment into the lost circulation zone.'
Squeeze the 50 bbls of resin. mixf:ure containing lost circulation material
into the loss
zone by pumping drilling fluid down thy: drill pipe to displace the resin
mixture out of the pipe
4 ,
Y
into the formation.
r
Once all the resin mixture has be en displaced from the drill pipe, pump an
additional
l0 bbls of fluff i down the drill pipe to displace the trailing edge of tie
resin mixture to a depth
r
below the end ofthe drill pipe but above; the top of the lost circulation
zone.
Y
Y .
TH24 S1FF . RST 17
r
Y ,
c.4 ved at the EPO on Apr 03, 2006 21 :27:25. Pa AMENDED SHEET `03/04/2000
CA 02567092 2006-11-18
_O 4/O6 13:24 FAI 713 241 661 SHELL/LEG xP EJ
`{ r f a N n I Y Y l .I i 7 M rP." sYJ'4 f kfr Y? ~ 015
'y1 /()r{p`')~ Y~rh.~ ~ f'y {:yr1 ;ak
bECPAAti y'~j'nfT`G d: +!r//~~0V ~6 r~F il~ VI
53' ,. a r Vy
b I TH2461-PCT ; ` .,
1 ~
I
r
I
Pump ai additional 10 bbls of dis placement down the annulus to complete
, y
displacement ofthe wailing edge of he resin mixture to just above he top of
the lest
circulation zone.
1 '
I .
.
Allow the well to remain shut-in or $ hours prior to resuming drilling
operations.
This treatment did not completely cure the lost circulation. However it did
seal off
exposed highRpressure zones that were f li:swing gas into the wellborer
Therefore, the risk of
loss of well control was accomplished by reducing the losses and selling offhe
flow zones.
No other treatment ofthis troublesome interval was required.
Example 2
l
. In well o. 2 of this study, an exposed lower pressure (depleted) formation
was
fractured by a high drilling fluid weight required to control an exposed high-
pressure zone.
This caused lost circulation and prevented further drilling operations to
deepen the welibore.
A resin based lost circulation treatment was performed, to repair the
fractured formation. The
fonnulatiou, used for this treatment was:
Per finished 159 liter (42 gallon.) barrel (bbl) of fluid:
50.9 liters (13.4 gallons) Diesel oil :,evert drilliri g fluid (2.02
kg/liter,I6,S lb/gal)
63.8 liters (16.8 gallons) Rexene 755 Melannine-Formaldehyde Resin
16,0 liters (4.2'gallons) Ethylene lycol monobutyl ether
147.6 kg (328,3 pounds) Hematite
3.4 kg (7.65 pounds) Formic acid catalyst (8S%)
2.3 kg (5 pounds) Magma Fiber Regular (Large fiber lost circulation material)
r
2.3 kg (5 pounds) Magma Fiber Fine (Small fiber lost circulation material)
The original drilling fluid weight was 2.02 kg/liter (16,5 1b/a1). Addition of
the
melamine-formaldehyde resin, ethylene glycol monobutyl ether solvent and
catalyst reduced the
solution weights Hematite (iron oxide) i~vas added as a weighting agent to
increase the weight of
the final mixture back to 2.02 loiter (1 I,$ lb/gal).
Treatment was peformed using the following procedure:
.
Pull end of drill string u to 90 meters (300 feet above the lost circulation
zone,
. Pump 35 bbls of resin. mixture (frmu1atioa above) down the drilipipe to a
depth where
the leading edge of the mixture is 10 bb:,s above the end of the drill string,
I
TH2 61F F . RST 1.8
I
e1, 5 Ived at the EPO on App 03, 2000 21 2725. Pa AMENDED SHEET
~a
. . 0310412006
CA 02567092 2006-11-18
94IU3j3S 13:25 FAX 7j3 241 8617 SHELL/LEG-IFs ala iar. rrrr J,I ' i 1 sE nsy
'<s t t
t - S~ r 14A~ Printed: 19/o412pp ], itii`~~,
,D
f
TH2461 PCT j `
r k
k
C
Note: No spacer fluids were required ahead of or behind, the zesin mixture to
separate
the treatment fluid from the drilling fluid in the wellbore.
Close in the annular blowout prey enter to stop circulation of fluid out ofthe
well and
prepare to squeeze the resin treatment into the lost circulation zone.
,
Squeeze the 35 bbls of resin mixtire containing lost circulation material into
the loss
zone by pumpi 'g drilling g fluid down the drill pipe to displace the resin
mixture out of the pipe
into the formation.
Displace the trailing edge of the resin mixture to a point 30 meters (100
feet) above
the top of the lost circulation zone.
r
Allow the well to remain shut-in 1 'or 8 hours prior to resuming drilling
operations.
After the treatment, the drilling fluid weight was increased tci 2.06 kg/liter
(17.2
lb/gal and thetellbore was deepened to its target total depth with no lost
circulation.
E)cample 3
Well No. 3 utilized resin treatme~ tts to strengthen a formation prior to
inducing lost
circulation. In this well., a resin treatment was pumped to cure a lost
circulation zone and seal
off a high-pressure flow zone at shallowLr depths. Sealing off the llbw zone
allowed a lower
drilling fluid weight to be used to drill dteper. The lower mud weight allowed
a weaker
formation at a deeper depth to be dri11ed.rthrough without fracturing he
formation and
inducing lost circulation.
r
After drilling throe the weaker formation at the deeper deal, the weak
formation
was treated with a resin nurture to strengthen it and increase its near-
wellbore fracture
initiation presre. The following mixture was used:
Per finished 159 liter (42 gallon) barrel t:bbl} of fluid
77.9 Liters (20.5 gallons) Diesel oil :invert drilling fluid (1.92 kliter or
16.0 lb/gal)
63.8 liters (16.8 gallons) Resinene 755 Melamine Fonnaldehye Resin
72.5 kg (161 pounds) Hematite
3.4 kg (7.65 pounds) Para-toluene sulfonic acid catalyst (4% by weight in
isopropyl
alcohol)
k
A spot; and~squeeze technique vas used for this treatment. The procedure
follows:
TH2461FF. RST ; 19
e 6 ved ~t tho EPO on Apr 03, 2005 21:27'25. Pa AMENDED SHEET 0310: /2006
CA 02567092 2006-11-18
_ . 310 3:25 FAQ 713 241 6817 SHELL/LEG IP
e
t i^ Sr t 4 a l i s e I i F ~y( ~si{~}l n ~I { ei wryly s i'. - die ~ ;7 4
}
I
Printed: 19/`J))1O4[/2aa t 1 } D .~iy V~1CPA ~r? If ?i 1 i j / ' 1
, i...! 1 k
[ff _ O575638
F.,.. ....... 1 . V =gf =i= i
1 ii:.. v i. , . v: iw ,'iõ . et Ae,.l'r,i(' . w .r 1 I 1 . t 1
`* ` TH2461-PCT '
.
.
e
Run drt 1string and tag total depth . Total depth of the wellbore w as
approximately 30
meters (loo feet) below the bottom of thy, weak interval to be ireatec1 The
weak formation
was approximately 45 meters (150 feet) thick,
Pull up drillstring 15 meters (50 ii~et ) above total depth.
Spot 3O~bbls of the resin mixture across and extending above; the top of the
weak
formation. The column length..ofthe re&.n mixture was approximate y 120 meters
(400 ft).
Excess volum.elwas spotted to allow a volume of the resin to be injected into
the matrix of the
formation.
Spotting the fluid is accomplished by pumping the resin mixture into the drib
string,
pumping a fluid (typically the drilling flhrrid) behind to displace the resin
mixture out the end of
the drill sting and out into the annulus between the wellbore and drill
string. The annulus is
1
open at the surface to allow fluid to be ci.rculated out of the wellbare as
the resin mixture is
placed.
After spotting the resin mixture Iii the welibore, the drill string was pulled
up to the
previous casing shoe.
The annular blowout preventer was closed.
Fluid was pumped down the drit. string to squeeze the resin into the weak
formation.
Radial penetration of the resin halo the weXlbore could be calculated from the
volume
of fluid squeezed into the formation, formation porosity, formation thickness
(height) and
1
wellbore diameter. Calculated radial pecietration distance for the ruin in
this treatment was
O.5 rn. (1,9 feet).
After squeezing resin into the fo:imstion, the well was shut in for 6 hours
prior to
cleaning set resin out of the wellbore and resuming drilling operations to
deepen the well.
The drilling fluid weight was increased to over 2.04 kgllitef(17 lb/gal)
during drilling
operations to total depth and no lost circulation occurred. The resiri
treatment effectively
strengthened the formation near the weliLbore,
1
The process described above for well No. 3 in this test study was repeated in
four
more wells with similar, successful resc.lts,
1
' r
1
.
r
r
1 ,
a
1 3
Tfl2461PF , RST , 20
....-.1 .i v 1
7 'ed at the EPO on Apr; 03, 2006 2127.25. Pa AMEN DED SH EET ,..
03/`04129.06
.1