Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-1-
PHARMACEUTICAL PRODUCT TRACKING
FIELD OF THE INVENTION
The present invention broadly relates to a method and apparatus for the
protection of products and security
documents using machine readable tags disposed on or in a surface of the
product or security document.
CO-PENDING APPLICATIONS
The following applications have been filed by the Applicant simultaneously
with the present application:
HYN HYS
The disclosures of these co-pending applications are incorporated herein by
reference. The above
applications have been identified by their filing docket number, which will be
substituted with the
corresponding application number, once assigned.
CROSS-REFERENCES
Various methods, systems and apparatus relating to the present invention are
disclosed in the following US
co-pending applications and granted patents filed by the applicant or assignee
of the present invention. The
disclosures of all of these US co-pending applications and granted patents are
incorporated herein by cross-
reference.
6,795,215 10/884,881 PEC01NP 09/575,109 10/296,535 09/575,110 6,805,419
09/607,985 6,398,332 6,394,573 6,622,923 6,747,760 10/189,459 10/943,941
10/949,294 10/727,181 10/ 727,162 10/727,163 10/727,245 10/727,204 10/727,233
10/727,280 10/727,157 10/727,178 10/727,210 10/727,257 10/727,238 10/727,251
10/727,159 10/727,180 10/727,179 10/727,192 10/727,274 10/727,164 10/727,161
10/727,198 10/727,158 10/754,536 10/754,938 10/727,227 10/727,160 10/934,720
10/854,521 10/854,522 10/854,488 10/854,487 10/854,503 10/854, 5 04 10/854,509
10/854,510 10/854,496 10/854,497 10/854,495 10/854,498 10/854,511 10/854,512
10/854,525 10/854,526 10/854,516 10/854,508 10/854,507 10/854,515 10/854,506
10/854,505 10/854,493 10/854,494 10/854,489 10/854,490 10/854,492 10/854,491
10/854,528 10/854,523 10/854,527 10/854,524 10/854,520 10/854,514 10/854,519
PLT036US 10/854,499 10/854,501 10/854,500 10/854,502 10/854,518 10/854,517
10/934,628 10/728,804 10/728,952 10/728,806 10/728,834 10/729,790 10/728,884
10/728,970 10/728,784 10/728,783 10/728,925 10/728,842 10/728,803 10/728,780
10/728,779 10/773,189 10/773,204 10/773,198 10/773,199 10/773,190 10/773,201
10/773,191 10/773,183 10/773,195 10/773,196 10/773,186 10/773,200 10/773,185
10/773,192 10/773,197 10/773,203 10/773,187 10/773,202 10/773,188 10/773,194
10/773,193 10/773,184 6,746,105 6,623,101 6,406,129 6,505,916 6,457,809
6,550,895 6,457,812 6,428,133 09/575,141 10/407,212 10/815,625 10/815,624
10/815,628 09/517,539 6566,858 09/112,762 6,331,946 6,246,970 6,442,525
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-2-
09/517,384 09/505,951 6,374,354 09/517,608 09/505,147 6,757,832 6,334,190
6,745,3 31 09/517,541 10/203,559 10/203,540 10/203,564 10/636,263 10/63,6283
10/866,608 10/902,889 10/902,883 10/940,653 10/942,858 10/409,876 10/409,848
10/409,845 09/575,197 09/575,195 09/575,159 09/575,132 09/575,123 6,825,945
09/575,130 09/575,165 6,813,039 09/693,415 09/575,118 6,824,044 09/608,970
09/575,131 09/575,116 6,816,274 NPA019NUS 09/575,139 09/575,186 6,681,045
6,678,499 6,679,420 09/663,599 09/607,852 6,728,000 09/693,219 09/575,145
09/607,656 6,813,558 6,766,942 09/693,515 09/663,701 09/575,192 6,720,985
09/609,303 09/610,095 09/609,596 09/693,705 09/693,647 09/721,895 09/721,894
09/607,843 09/693,690 09/607,605 09/608,178 09/609,553 09/609,233 09/609,149
09/608,022 09/575,181 09/722,174 09/721,896 10/291,522 6,718,061 10/291,523
10/291,471 10/291,470 6,825,956 10/291,481 10/291,509 10/291,825 10/291,519
10/291,575 10/291,557 10/291,661 10/291,558 10/291,587 10/291,818 10/291,576
6,829,387 6,714,678 6,644,545 6,609,653 6,651,879 10/291,555 10/291,510
10/291,592 10/291,542 10/291,820 10/291,516 10/291,363 10/291,487 10/291,520
10/291,521 10/291,556 10/291,821 10/291,525 10/291,586 10/291,822 10/291,524
10/291,553 10/291,511 10/291,585 10/291,374 10/685,523 10/685,583 10/685,455
10/685, 5 84 10/757,600 10/804,034 10/793, 93 3 10/853,356 10/831,232 10/8 84,
882
10/943,875 10/943,938 10/943,874 10/943,872 10/944,044 10/943,942 10/944,043
10/949,293 10/943,877 10/965,913 10/954,170 NPA174US NPA175US NPA176US
NPA177US NPA178US NPA179US NPA181US NPA182US NPA183US NPA184US
NPA185US NPA186US NPA187US NPA188US 09/575,193 09/575,156 09/609,232
09/607,844 6,457,883 09/693,593 10/743,671 NPBOlOUS 09/928,055 09/927,684
09/928,108 09/927,685 09/927,809 09/575,183 6,789,194 09/575,150 6,789,191
10/900,129 10/900,127 10/913,328 10/913,350 NPKO10US NPKO11US 6,644,642
6,502,614 6,622,999 6,669,385 6,827,116 10/933,285 NPM016US 6,549,935
NPNO04US 09/575,187 6,727,996 6,591,884 6,439,706 6,760,119 09/575,198
09/722,148 09/722,146 6,826,547 6,290,349 6,428,155 6,785,016 6,831,682
6,741,871 09/722,171 09/721,858 09/722,142 10/171,987 10/202,021 10/291,724
10/291,512 10/291,554 10/659,027 10/659,026 10/831,242 10/884,885 10/884,883
10/901,154 10/932,044 NPPO51US NPPO52US NPPO53US 10/965,733 10/965,933
NPPO58US NPPO60US NPPO61US NPPO62US 10/659,027 09/693,301 09/575,174
6,822,639 6,474,888 6,627,870 6,724,374 6,788,982 09/722,141 6,788,293
09/722,147 6,737,591 09/722,172 09/693,514 6,792,165 09/722,088 6,795,593
10/291,823 6,768,821 10/291,366 10/291,503 6,797,895 10/274,817 10/782,894
10/782,895 10/778,056 10/778,058 10/778,060 10/778,059 10/778,063 10/778,062
10/778,061 10/778,057 10/846,895 10/917õ468 10/917,467 10/917,466 10/917,465
10/917,356 10/948,169 10/948,253 10/948,157 10/917,436 10/943,856 10/919,379
10/943,843 10/943,878 10/943,849 NPSO86US 09/575,154 09/575,129 6,830,196
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-3-
09/575,188 09/721,862 10/473,747 10/120,441 10/291,577 10/291,718 6,789,731
10/291,543 6,766,944 6,766,945 10/291,715 10/291,559 10/291,660 10/409,864
10/309,358 10/410,484 10/884,884 10/853,379 10/786,631 10/853,782 10/893,372
10/893,381 10/893,382 10/893,383 10/893,384 NPT046US NPT047US NPT048US
NPT049US NPT050US NPWOOIUS 10/492,152 NPWO03US NPWO04US 10/492,154
NPWO07US 10/683,151 10/683,040 NPWO12US 10/919,260 NPWO13US 10/919,261
10/778,090 09/575,189 09/575,162 09/575,172 09/575,170 09/575,171 09/575,161
10/291,716 10/291,547 10/291,538 6,786,397 10/291,827 10/291,548 10/291,714
10/291,544 10/291,541 10/291,584 10/291,579 10/291,824 10/291,713 10/291,545
10/291,546 10/917,355 10/913,340 10/940,668 NPX041US 6,593,166 10/428,823
10/849,931 10/815,621 10/815,612 10/815,630 10/815,637 10/815,638 10/815,640
10/815,642 10/815,643 10/815,644 10/815,618 10/815,639 10/815,635 10/815,647
10/815,634 10/815,632 10/815,631 10/815,648 10/815,614 10/815,645 10/815,646
10/815,617 10/815,620 10/815,615 10/815,613 10/815,633 10/815,619 10/815,616
10/815,614 10/815,636 10/815,649 10/815,609 10/815,627 10/815,626 10/815,610
10/815,611 10/815,623 10/815,622 10/815,629
Some application has been listed by docket numbers, these will be replace when
application number are
known.
BACKGROUND
PHARMACEUTICAL COUNTERFEITiNG
The pharmaceutical industry is large, and it has continued to grow steadily
with worldwide sales reaching
US$400 billion in 2002. Around 20% of gross sales revenues are spent on R&D.
The industry is also global,
and its security structure is largely the result of the need to protect
massive R&D investments. Many
Governments are large buyers of pharmaceuticals in publicly-fmanced health
care systems.
Pharmaceutical expenditure now represents about 15% of total health
expenditure in Organization for
Economic Co-operation and Development (OECD) nations. While trade and
manufacturing activities are
operating at an international level, national authorities take into account
the position of their country within a
global perspective when designing their national policies. There is also a
need to balance global industry
interests with concerns for public health and safety.
In this environment there is a need to improve security of the pharmaceutical
supply chain through:
= Global trade regulation through International Trade Agreements (ITAs),
= Protection of R&D expenditure through patent activity, and
= Concerns for public health and safety.
Global trade is regulated through ITAs and involves many public agencies
pursuing multiple goals that relate
to public health, industry and trade regulation, and security policies. The
World Trade Organization (WTO) is
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-4-
therefore just one of the many organizations providing agreements of specific
interest to the pharmaceutical
industry.
The following Agreements highlight some of the WTO's key trade requirements
now influencing the way the
pharmaceutical industry is organized.
Agreement on Rules of Origin: The rules of origin are the criteria needed to
determine the national origin of a
product, and they are necessary because goods may be subject to different
discriminatory measures depending
on their origin. Rules of origin are the criteria needed to determine:
= What imported products will receive most-favored nation treatment or
preferential treatment,
= When to implement measures and instruments of commercial policy such as anti-
dumping duties and
safeguard measures,
= Trade statistics,
= Labeling and ticketing requirements, and
= Procedures for government procurement.
Agreement on Import Licensing Procedures: The agreement on import licensing
procedures requires
governments to publish sufficient information for traders to know how and why
the licenses are granted. It
also describes how countries should notify the WTO when they introduce new
import licensing procedures, or
change existing procedures.
Preshipment Inspection Agreement: The obligations that apply to governments
which use preshipment
inspections include:
= Non-discrimination,
= Transparency,
= Protection of confidential business information,
= Avoiding unreasonable delay,
= The use of specific guidelines for conducting price verification, and
= Avoiding conflicts of interest by the inspection agencies.
Agreement on Trade-Related Aspects of Intellectual Property Rights, Including
Trade in Counterfeit Goods:
The agreement recognizes that widely varying standards in the protection and
enforcement of intellectual
property rights. The lack of a multilateral framework of principles, rules and
disciplines dealing with
international trade in counterfeit goods has been a growing source of tension
in international economic
relations.
The above WTO provisions are just a few of the wide range of statutory and
regulatory requirements now
governing the international and domestic trade behaviour of the pharmaceutical
industry. They also highlight
the impending regulatory and statutory pressures likely to mandate the use of
unique item identification.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-5-
An additional area that has always been of particular concern to the
International AntiCounterfeiting Coalition
(IACC) is the increasing availability of counterfeit products that have caused
and continue to present threats
to public health and safety. Given the heightened awareness of the past two
years, the IACC's concerns with
respect to public health and safety risk, have only increased. WHO estimates
that counterfeit drugs account
for ten percent of all pharmaceuticals, and of these 16% contain the wrong
ingredients, with 60% having no
active ingredients at all. That proportion of counterfeit drugs can rise to as
high as 60% in developing
countries.
In addition to using the pharmaceutical industry as a means to raise funds,
the potential exists for terrorists to
use the commission of the crime itself as a means of attack, for example by
shipping counterfeit product
containing deadly biotoxins. The United States Congress recognizes the
increasing role of organized crime
and terrorist activity in the theft of intellectual property through Trademark
misuse and drug counterfeiting,
and the threat that these pose for public health and safety. Proceeds are
often used to fund more violent
activities. The IACC have been tracking the influx of terrorist organizations
into criminal and counterfeiting
and there is now ample evidence to suggest that links exist.
It should also be noted here, that there is a significant overlap between
security concerns and issues raised by
the need to strengthen brand protection. It is simply not possible for the
pharmaceutical industry to
effectively address all of the security and brand protection concerns raised
without adopting an automated
unique item identification process to improve drug authentication, and to be
able to actively monitor the
physical flow of goods from its source to the customer.
In view of pressures for the pharmaceutical industry to address security and
brand protection concerns, it
becomes necessary to consider the adoption of new technologies. The two key
capabilities required to
improve overall efficiency and to protect the supply chain, are track and
trace and product authentication.
While there are some compelling arguments supporting the introduction of track
and trace, and product
authentication solutions, there are also complexities that need to be
addressed.
Two fundamental goals of the pharmaceutical industry are consumer care and
public safety. To achieve these
goals in the United States, the FDA and individual states regulate the
industry through laws and
administrative orders designed to protect the integrity of drugs throughout
the pharmaceutical supply chain.
Implicit in the laws is the administrative requirement for drug authentication
and the ability to do track and
trace.
Track and trace forms the foundation for improved patient safety by giving
manufacturers, distributors and
pharmacies a systemic method to detect and control counterfeiting, drug
diversions and mishandling.
The introduction of track and trace capabilities also introduces the concept
of a pedigree. Florida recently
gained national attention by introducing a legislative bill to establish a
pedigree for each drug sold in the
State. Although this bill has not yet become law, its intention is to verify
authenticity and reduce the risk of
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-6-
counterfeit items entering the supply chain. Specifically, the bill calls for
the following pedigree information
to accompany each drug through all steps of the supply chain:
= Drug name,
= Dosage,
= Container size,
= Number of containers,
= Drug lots or control numbers,
= Business name and address of all parties to each prior transaction, starting
with the
manufacturer, and
= The date of each previous transaction.
Other countries have also moved forward with pedigree regulations. Most
notably the Italian government,
with financial support from the European Union, began to enforce the track and
trace of pharmaceuticals with
the Bollini Law in 2000. This law requires the use of a special sticker
containing a serial number and a trace
of all parties within the supply chain. However, this has created great
difficulty for manufacturers and
distributors. As a result, the full implementation of the law will not take
place until June 2004 because of a
lack of technology to handle the task of recording and archiving the serial
numbers. An additional problem is
that the design specifications of the database structure needed to support
track and trace, have still not yet
been determined.
Although the physical form of goods changes throughout manufacturing and
distribution, a link still exists for
all raw materials and the work processes used to produce the finished goods.
This type of link demonstrates
inheritance of specific attributes. Each medicine used by the patient has a
specific lot number and expiration
date printed on the container. The drug is shipped on a identifiable truck, at
a particular temperature for a
specific duration. The effectiveness of the medicine ultimately depends on the
quality of the manufacturing
process and the environmental conditions of transport and storage. These are
all inherited attributes that form
the pedigree.
Organizing the large number of informational links for all pharmaceutical
product items in the supply chain
becomes complex. To simplify product data management, two additional concepts
are required. These are
data aggregation and data inheritance.
Data inheritance is the history of the parent data. It is the logical
equivalent of item aggregation or assembly.
By viewing data within a supply chain as a series of parent-child
relationships, track and trace becomes
possible. To reconstruct the history of an item, each change in form must
transfer from parent to child.
Data aggregation joins linked or like data together to reduce the number of
readings at critical points within
the supply chain, and thus making the capture of informational links needed
for large-scale drug
authentication, and track and trace, more feasible. If data aggregation were
not possible, the identifiers for
each product on the pallet would need to be read, resulting in a number of
additional reads, especially when
dealing with pallet level shipments.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-7-
To overcome the problems associated with the generation of a huge amount of
product data, there is
considerable interest in expanding or replacing the Universal Product Code
(UPC) now in use for barcodes. In
North America a product is typically identified by a 12-digit Universal
Product Code (UPC), and in Europe
and other regions by a 13-digit European Article Number (EAN) which are
machine-readable product codes
in the form of a printed 2D bar code. The Uniform Code Council (UCC) and EAN
defme and administer the
UPC and related codes as subsets of the 14-digit Global Trade Item Number
(GTIN).
The Auto-ID Center has defined a standard for mapping of the GTIN into the 96-
bit Electronic Product Code
(EPC) to help ensure compatibility between the EPC and current practices. The
MIT Auto-ID Center has
developed a standard for a 96-bit Electronic Product Code (EPC), coupled with
an Internet-based Object
Naming Service (ONS) and a Product Markup Language (PML). Once an EPC is
scanned, it is used to look
up, via the ONS, matching product information encoded in PML. The EPC consists
of an 8-bit header, a 28-
bit object class, and a 36-bit serial number. Although EPCs can be encoded in
many physical forms, and
carried over a range of interfaces, the Auto-ID Center strongly advocate the
benefits of using low cost passive
RFID tags to carry EPCs for individual item identification.
The appeal of an Auto-ID solution lies in the ability to use the EPC as a
pointer to look up information about a
drug that is contained in a remote database. The EPC acts as a persistent link
to verify if the item has been
legitimately obtained. This will act as a strong deterrent for fraud at many
points along the supply chain. If
and when a customer decides to return an item, or if there is a suspected
problem with the contents, then there
is a persistent link to information to validate product details. It prevents
illegal returns, protects customers in
the event of medical problems resulting from product use - or misuse - and it
makes it possible to track
customers and customized products that might be used by the wrong person and
resulting in medical
problems. Inventory control and reordering functions will be far more
reliable.
The item's EPC serves as a key into a distributed PML database which records
the characteristics of the item
and its evolving history as it proceeds through the pharmaceutical supply
chain. PML servers, located at each
node of the supply chain, and secure Internet based communication combine to
provide the primary handling
structure and means. Tracking of higher level units (e.g. pallet or shipping
company, dispatch/order number
and transport route) in the supply chain is implicit. Readers installed at all
transit entry and exit points can be
used to automatically track movement and update dispatch logs at all points in
the supply chain. Either the
Internet or dedicated computer networks can provide the communication links.
The hardware components for an Auto-ID solution are technologically feasible
with significant development
having taken place during the past several years. A number of vendors are
capable of producing key
infrastructure components to meet the specific requirements of the
pharmaceutical industry.
Besides the proposed applications in improving track and trace, and drug
authentication, Auto-ID
infrastructure also serves as the foundation for future applications of
importance to the health care industry.
For example, the Human Genome Project creates greater opportunities for
engineering drugs to treat small
groups of individuals that suffer from specific illnesses. These 'designer
drugs' will be manufactured in small
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-8-
lot sizes on a make to order basis. In this environment, logistics and
coordination takes on a new form as
thousands of biotechnology drugs flood the pharmaceutical supply chain.
Delivery of these new drugs to the
right group of people presents a challenge that the current logistical system
may not handle effectively.
However, this new capability does have drawbacks: The task of handling
streaming information for the
estimated 6 billion individual pharmaceutical items sold in the United States
last year alone, taxes the capacity
of the Internet or dedicated computer networks - even when the data
aggregation and inheritance concepts are
used. An additional complexity is that the Auto-ID approach would have to be
fine-tuned in terms of
information synchronisation among many different supply chain partners to
ensure a high level of reliability
for pedigree and drug authentication information. If a single supply chain
partner did not properly handle
information, pedigrees might show gaps that would raise counterfeit questions.
The Auto-ID approach also
assumes different entities within the pharmaceutical supply chain can achieve
a common level of cooperation
in supporting this information infrastructure.
A further difficulty to overcome is that the Auto-ID approach assumes that all
drug manufacturers, carriers,
wholesalers and pharmacies have the necessary hardware and computing ability
to read and process EPC
information. lt is therefore unrealistic to believe that this capability will
occur immediately.
Thus, it is likely that the pharmaceutical industry will continue to adopt an
evolutionary approach to the
standardisation of unique item identification technology throughout the
manufacturing and supply chains. It is
also likely that a range of technologies will need to be adopted and
integrated to introduce incremental
improvements in security and supply chain efficiency.
Currently two main types of technologies offering alternative methods of
unique product item identification,
such as EPCs, namely:
= 2D optical barcodes, and
= Radio Frequency Identification tags (RFID).
A 2D optical barcode consists of a composite image that can store about 2,000
bytes of data along two
dimensions. The Uniform Code Council and European Article Numbering (EAN)
International have
standardized a range of 2D barcodes, all with a significantly larger data
capacity than the existing EPC.
2D optical barcodes are now widely used in the global pharmaceutical industry.
In the United States, the Food
and Drug Administration (FDA) has mandated their use on all pharmaceutical
goods manufactured within its
jurisdiction to identify product lines. The main advantage driving their
acceptance is that they are inexpensive
to produce.
The main disadvantage of 2D optical barcodes is that they are often difficult
to read due to label damage and a
direct 'line-of-sight' is needed for scanning. In addition to this, 2-D
optical barcodes are unsightly and
therefore detrimental to the packaging of the product. This problem is
exacerbated in the case of
pharmaceuticals, which generally use small packaging, but require a relatively
large bar-code which can
therefore obscure a substantial part of the packaging.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-9-
An RFID tag is a technology that incorporates the use of electromagnetic or
electrostatic coupling to uniquely
identify an object. It consists of three parts: antenna, transceiver, and
transponder.
In the case of Pharmaceuticals RFID tags provide unique product item
identification encoded in the form of
an EPC. The pharmaceutical industry recognizes the many advantages of
introducing accurate and reliable
unique item identification technology. The expected advantages include:
= Dramatically eliminate inventory loss and write-offs due to 'shrinkage',
= Improve productivity in dispatch and receiving of goods,
= Significantly reduce the time required to identify the location of products
for recall if required,
= Provide an efficient basis for satisfying regulatory requirements,
= Increase assurance of shipment accuracy, and therefore reduce the number of
customer complaints,
and
= Provide a lot and expiration date tracking capability.
RFID tracking could be automated to help improve the integrity of the
pharmaceutical supply chain. By
identifying and tracking products in the supply chain, companies can maintain
a much tighter control over
legitimate shipments, and ensure that they are not hijacked or stolen. This
can prevent products from falling
into the hands of counterfeiters, who could dilute or alter the drugs, and
then distribute them to unsuspecting
pharmacies and customers. For this reason, many major pharmaceutical
companies, such as Johnson &
Johnson and Eli Lilly and Company, are now exploring the possibility of using
RFID tags on all drug
shipments.
There are also regulatory requirements driving the adoption of RFID
technologies. The FDA plays a lead role
in providing a forum and guidance for new technology adoption and is actively
encouraging the
implementation of ways to authenticate prescription drugs through the supply
chain to ensure compliance and
patient safety. Working with pharmaceutical companies and vendors of anti-
counterfeit security systems, the
FDA identifies technologies that are able to protect the industry against
various threats to customers and brand
protection. Although it does not specify which technologies are able to
respond to the identified threats, it
does specify the security features that need to be employed on the product
packaging and shipping materials.
The Healthcare Distribution Management Association (HDMA) has also recommended
that pharmaceutical
manufacturers and wholesalers use product identifiers on cases by 2005, and
that RFID tags at item level
should be deployed by 2007.
Recently, the FDA has published a proposed rule referred to as 'Bar Code Label
Requirements for Human
Drug Products and Blood FDA Proposed Rule (14 March, 2003). Bar Code Label
Requirements for Human
Drug Products and Blood, Federal Register (Vol. 68, No. 50) pp.12499-12534.
The proposed rule will require
pharmaceutical companies to identify each drug, and dosage using linear
barcodes. The need to include lot
number and expiration dates are still under consideration. Although the
benefits of using unique item
identification are now being considered, this is not likely for some time as
at present there are no suitable
solutions available.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-10-
However, in addition to the forecast benefits and regulatory pressures for
RFID use, there are also some
disadvantages that make RFID tags unsuitable for some pharmaceutical products.
First, RFID tags are costly to produce. The current cost of producing an RFID
is around 30-50 cents. While
this cost can be significantly reduced once high production volumes and wide
acceptance have been achieved,
it is unlikely that the cost will fall below 5 cents per RFID tag in the
foreseeable future. There are also some
additional costs associated with integrating the RFID tags into packaging and
labeling.
A further problem is that the presence of metals, liquids and other
electromagnetic frequency (EMF) signals
can interfere with RFID tag scanners, and thus seriously jeopardize the
reliability and integrity of the RFID
system. In the pharmaceutical industry, many radiopaque materials are used in
both the content and containers
of goods. To overcome this problem for RFID systems, it is necessary to split
individual boxes of goods so
that they can be conveyed past RFID tag readers, making dock-to-dock transfers
more time-consuming. These
restrictions will also apply at item level at the point-of-sale (POS). This
alone would make them unsuitable for
large-scale deployment.
A third disadvantage of RFID tags concerns customer privacy. If, for example,
a terminally ill cancer patient
collected their RFID tagged morphine sulphate prescription, then it might be
possible for a person to illegally
use a scanning device to detect the nature of the contents by reading an RFID
tag without the knowledge of
the owner. Knowing the contents, that person may then decide to steal the
goods. However, the risk can be
reduced by ensuring that once an item has gone to a customer, access to
information over the network is then
dynamically altered and secured to protect personal information or product
details. This could be done using
virtual customer records that comply with emerging standards for managing and
securing patient information
folders (PIFs) for the healthcare system.
Collectively, these disadvantages mean that it is unlikely that RFID tags will
ever become suitable for all
pharmaceutical or therapeutic items, and as such will only ever be able to be
deployed as an alternative
technology and adopted alongside some other form of product identification
system.
SURFACE CODING BACKGROUND
The Netpage surface coding consists of a dense planar tiling of tags. Each tag
encodes its own location in the
plane. Each tag also encodes, in conjunction with adjacent tags, an identifier
of the region containing the tag.
This region ID is unique among all regions. In the Netpage system the region
typically corresponds to the
entire extent of the tagged surface, such as one side of a sheet of paper.
The surface coding is designed so that an acquisition field of view large
enough to guarantee acquisition of an
entire tag is large enough to guarantee acquisition of the ID of the region
containing the tag: Acquisition of
the tag itself guarantees acquisition of the tag's two-dimensional position
within the region, as well as other
tag-specific data. The surface coding therefore allows a sensing device to
acquire a region ID and a tag
position during a purely local interaction with a coded surface, e.g. during a
"click" or tap on a coded surface
with a pen.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-11-
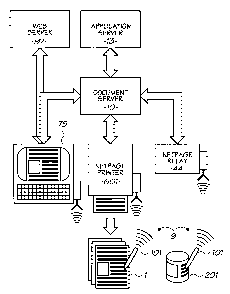
The use of netpage surface coding is described in more detail in the following
copending patent applications,
USSN 10/815,647 (docket number HYGOOlUS), entitled "Obtaining Product
Assistance" filed on 2 d April
2004; and USSN 10/815,609 (docket number HYTOOIUS), entitled " Laser Scanner
Device for Printed
Product Identification Cod" filed on 2 d April 2004.
CRYPTOGRAPHY BACKGROUND
Cryptography is used to protect sensitive information, both in storage and in
transit, and to authenticate parties
to a transaction. There are two classes of cryptography in widespread use:
secret-key cryptography and
public-key cryptography.
Secret-key cryptography, also referred to as symmetric cryptography, uses the
same key to encrypt and
decrypt a message. Two parties wishing to exchange messages must first arrange
to securely exchange the
secret key.
Public-key cryptography, also referred to as asymmetric cryptography, uses two
encryption keys. The two
keys are mathematically related in such a way that any message encrypted using
one key can only be
decrypted using the other key. One of these keys is then published, while the
other is kept private. They are
referred to as the public and private key respectively. The public key is used
to encrypt any message intended
for the holder of the private key. Once encrypted using the public key, a
message can only be decrypted using
the private key. Thus two parties can securely exchange messages without first
having to exchange a secret
key. To ensure that the private key is secure, it is normal for the holder of
the private key to generate the
public-private key pair.
Public-key cryptography can be used to create a digital signature. If the
holder of the private key creates a
known hash of a message and then encrypts the hash using the private key, then
anyone can verify that the
encrypted hash constitutes the "signature" of the holder of the private key
with respect to that particular
message, simply by decrypting the encrypted hash using the public key and
verifying the hash against the
message. If the signature is appended to the message, then the recipient of
the message can verify both that
the message is genuine and that it has not been altered in transit.
Secret-key can also be used to create a digital signature, but has the
disadvantage that signature verification
can also be performed by a party privy to the secret key.
To make public-key cryptography work, there has to be a way to distribute
public keys which prevents
impersonation. This is normally done using certificates and certificate
authorities. A certificate authority is a
trusted third party which authenticates the association between a public key
and a person's or other entity's
identity. The certificate authority verifies the identity by examining
identity documents etc., and then creates
and signs a digital certificate containing the identity details and public
key. Anyone who trusts the certificate
authority can use the public key in the certificate with a high degree of
certainty that it is genuine. They just
have to verify that the certificate has indeed been signed by the certificate
authority, whose public key is well-
known.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-12-
To achieve comparable security to secret-key cryptography, public-key
cryptography utilises key lengths an
order of magnitude larger, i.e. a few thousand bits compared with a few
hundred bits.
Schneier B. (Applied Cryptography, Second Edition, John Wiley & Sons 1996)
provides a detailed discussion
of cryptographic techniques.
SUMMARY OF THE INVENTION
In a first broad form the invention provides a transaction terminal for
performing transactions relating to a
pharmaceutical product, the pharmaceutical product being associated with
packaging having disposed thereon
or therein coded data including a number of coded data portions, each coded
data portion being indicative of
an identity of the pharmaceutical product, the transaction terminal including:
a radiation source for exposing
at least one coded data portion; a sensor for sensing the at least one exposed
coded data portion; and, a
processor for: determining, using the at least one sensed coded data portion,
a sensed identity; and,
performing the transaction using the sensed identity.
Optionally, the processor uses the identity to perform at least one of:
authentication of the pharmaceutical
product; approval of the transaction; determination of transaction details;
and, updating of tracking
information relating to the pharmaceutical product.
Optionally, the transaction terminal is at least one of: a cash register; a
vending machine; a supermarket
checkout; a handheld scanner attached to a computer system.
Optionally, the identity is formed at least in part from at least one of: a
serial number of the pharmaceutical
product; an identity of the pharmaceutical product; an EPC; an identity of the
packaging; and, an identity of a
region of the packaging.
Optionally, coded data portion is indicative of at least part of a signature,
the signature being a digital
signature of at least part of the identity, and wherein the processor:
determines, using the at least one sensed
coded data portion, at least one sensed signature part; causes authentication
of the pharmaceutical product
using the sensed identity and the at least one sensed signature part.
Optionally, the processor: generates indicating data at least partially
indicative of the sensed identity and the
at least one sensed signature part; and, transfers the indicating data to a
computer system, the computer
system being responsive to the indicating data to: determine, using the
indicating data, the sensed identity and
the at least one sensed signature part; and, authenticate the pharmaceutical
product using the sensed identity
and the at least one sensed signature part.
Optionally, the pharmaceutical product is authenticated by at least one of the
processor and a computer
system, which: determines, using the sensed identity, a key; determines, using
the sensed identity and the key,
a determined signature; and, compares the at least one sensed signature part
and the determined signature to
thereby authenticate the pharmaceutical product.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 13 -
Optionally, the entire signature is encoded within a plurality of coded data
portions, the processor determines,
from a plurality of sensed coded data portions, a sensed signature, and
wherein pharmaceutical product is
authenticated by at least one of the processor, and a computer system, which:
determines, using the sensed
identity, a key; determines, using the sensed signature and the key, a
determined identity; and, compares the
determined identity and the sensed identity to thereby authenticate the
pharmaceutical product.
Optionally, the coded data includes a plurality of layouts, each layout
defming the position of a plurality of
first symbols encoding the identity, and a plurality of second symbols
defining at least part of the signature.
Optionally, the transaction terminal includes a communication system for
communicating with a database, the
database storing data relating the pharmaceutical product, including at least
one of: authentication data,
including at least a key associated with a signature, the signature being a
digital signature of at least part of
the identity; tracking data, the tracking data being at least partially
indicative of tracking information
including at least one of: an owner of the pharmaceutical product; one or more
transactions performed using
the pharmaceutical product; a location of the pharmaceutical product; and, a
location of the sensing device.
product data, the product data being at least partially indicative of product
information including at least one
of: a product cost; a patient identifier; a user identifier; an owner
identifier; manufacture date; batch number;
product manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions;
disposal conditions; serial number; expiry date; effects; side-effects;
conditions for use; instructions for use;
links to further information; contra-indications; and, dosage.
Optionally, the transaction terminal includes a display for displaying at
least one of: results of authentication;
tracking information; and, product information.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-14-
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the transaction terminal includes a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity; and,
printing the packaging by printing the
coded data and the visible information.
Optionally, the transaction terminal further performs a method of allowing a
user to interact with the
pharmaceutical product, the method including, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data at least
partially indicative of the identity; determining, using the indicating data,
at least one action; and, performing
the action associated with the pharmaceutical product, the action including at
least one of: providing
information to a user; updating tracking information relating to the
pharmaceutical product; performing a
transaction relating to the pharmaceutical product; authenticating the
pharmaceutical product; and, receiving
feedback from the user.
Optionally, the transaction terminal further performs a method for
authenticating the pharmaceutical product,
each coded data portion being further indicative of a signature, the signature
being a digital signature of at
least part of the identity and being encoded within a plurality of coded data
portions, wherein the method
includes: in a sensing device: sensing at least one coded data portion; and,
generating, using the sensed coded
data portion, indicating data indicative of: the identity; and, the at least
one signature part; in a processor:
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-15-
determining, from the indicating data: a determined identity; and, at least
one determined signature part;
authenticating the pharmaceutical product using the determined identity and
the at least one determined
signature part.
Optionally, the transaction terminal further performs a method for
authenticating the pharmaceutical product,
each coded data portion being further indicative a signature, the signature
being a digital signature of at least
part of the identity, wherein the method includes, in a computer system:
receiving indicating data from a
sensing device, the sensing device being responsive to sensing of the coded
data to generate indicating data at
least partially indicative of: the identity of the pharmaceutical product;
and, the signature part; determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the transaction terminal further performs a method of determining
a possible duplication of
pharmaceutical product packaging, wherein the method includes, in a computer
system: receiving indicating
data from a sensing device, the sensing device being responsive to sensing of
the coded data to generate
indicating data indicative of the identity; determining, from the indicating
data, a determined identity;
accessing, using the determined identity, tracking data relating to the
pharmaceutical product, the tracking
data being at least partially indicative of the location of the pharmaceutical
product; and, determining, using
the tracking data, if the pharmaceutical product is a possible duplicate.
Optionally, the transaction terminal further performs a method of tracking the
pharmaceutical product, the
method including, in a computer system: receiving indicating data from a
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data
indicative of the identity of the
product item; and, updating, using the received indicating data, tracking data
at least partially indicative of
tracking information.
Optionally, the transaction terminal further performs a method of producing
pharmaceutical product
packaging, wherein the method includes, in a computer system: determining a
serial number associated with
the pharmaceutical product; generating, using the serial number, an identity;
generating, using the identity, a
signature; causing generation of coded data using the identity, the coded data
including a number of coded
data portions, each coded data portion encoding the identity; and, at least
part of the signature, the signature
being a digital signature of at least part of the identity; causing printing
of, on the pharmaceutical product
packaging: at least some coded data, and at least one o~ the identity; and,
the serial number.
Optionally, the transaction terminal further performs a method of dispensing
the pharmaceutical product, the
method including, in a computer system: receiving indicating data from a
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data and from a dispensing
database, at least one criterion for
dispensing the pharmaceutical product; and, causing the pharmaceutical product
to be dispensed if the at least
one criterion is satisfied.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-16-
Optionally, the transaction terminal further includes a sensing device for use
with the pharmaceutical product,
the sensing device including: a housing adapted to be held by a user in use; a
radiation source for exposing at
least one coded data portion; a sensor for sensing the at least one exposed
coded data portion; and, a processor
for determining, using the at least one sensed coded data portion, a sensed
identity. In a second broad form
the invention provides a printer for printing packaging associated with a
pharmaceutical product, the printer
being for: determining visible information to be provided on the packaging;
determining an identity associated
with the pharmaceutical product; generating coded data using the identity, the
coded data including a number
of coded data portions, each coded data portion being indicative of at least
the identity of the pharmaceutical
product; and, printing the packaging by printing the coded data and the
visible information.
Optionally, each coded data portion is indicative of at least part of a
signature, the signature being a digital
signature of at least part of the identity, and wherein the printer:
determines the signature; and, generates the
coded data using the signature.
Optionally, printer includes: a secure data store data; and, a processor which
generates the signature using
data stored in the data store.
Optionally, the printer encodes the entire signature within a plurality of
coded data portions.
Optionally, the printer: determines a layout, the layout being at least one
of: a coded data layout, the layout
being indicative of the position of each coded data portion on the packaging;
and, a document description, the
document description being indicative of the position of the visible
information on the packaging; and, prints,
using the layout, at least one of the coded data and the visible information.
Optionally, the printer includes a communication system for communicating with
a database, the database
storing data relating the pharmaceutical product, including at least one of:
authentication data, including at
least a key associated with a signature, the signature being a digital
signature of at least part of the identity;
tracking data, the tracking data being at least partially indicative of
tracking information including at least one
of: an owner of the pharmaceutical product; one or more transactions performed
using the pharmaceutical
product; a location of the pharmaceutical product; and, a location of the
sensing device; and, product data,
the product data being at least partially indicative of product information
including at least one of: a product
cost; a patient identifier; a user identifier; an owner identifier;
manufacture date; batch number; product
manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions; disposal
conditions; serial number; expiry date; effects; side-effects; conditions for
use; instructions for use; links to
further information; contra-indications; and, dosage.
Optionally, the printer, at least one of: updates at least some of the data
relating to the pharmaceutical product;
and, generates the coded data using at least some of the data relating to the
pharmaceutical product.
Optionally, the identity is formed at least in part from at least one of: a
serial number of the pharmaceutical
product; an identity of the pharmaceutical product; an EPC; an identity of the
packaging; and, an identity of a
region of the packaging.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-17-
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the printer: receives the packaging; scans the packaging to
determine information indicative of at
least one of: a source of the pharmaceutical product; a pharmaceutical product
type; the identity of the
pharmaceutical product; and, the serial number of the pharmaceutical product;
and, generates using the
determined information, at least part of the coded data.
Optionally, the coded data is indicative of a plurality of reference points
such that sensing of at least one
coded data portion, by a sensing device, allows for determination of at least
one of: the identity; at least one
signature part; a position of the sensing device relative to the packaging;
and, movement of the sensing device
relative to the packaging.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 18-
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the printer includes a transaction terminal for performing
transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the printer further performs a method of allowing a user to
interact with the pharmaceutical
product, the method including, in a computer system: receiving indicating data
from a sensing device, the
sensing device being responsive to sensing of the coded data to generate
indicating data at least partially
indicative of the identity; determining, using the indicating data, at least
one action; and, performing the
action associated with the pharmaceutical product, the action including at
least one of: providing information
to a user; updating tracking information relating to the pharmaceutical
product; performing a transaction
relating to the pharmaceutical product; authenticating the pharmaceutical
product; and, receiving feedback
from the user.
Optionally, the printer further performs a method for authenticating the
pharmaceutical product, each coded
data portion being further indicative of a signature, the signature being a
digital signature of at least part of the
identity and being encoded within a plurality of coded data portions, wherein
the method includes: in a
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the printer further performs a method for authenticating the
pharmaceutical product, each coded
data portion being further indicative of a signature, the signature being a
digital signature of at least part of the
identity, wherein the method includes, in a computer system: receiving
indicating data from a sensing device,
the sensing device being responsive to sensing of the coded data to generate
indicating data at least partially
indicative of: the identity of the pharmaceutical product; and, the signature
part; determining, from the
indicating data: a determined identity; and, at least one determined signature
part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the printer further performs a method of determining a possible
duplication of the packaging,
wherein the method includes, in a computer system: receiving indicating data
from a sensing device, the
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-19-
sensing device being responsive to sensing of the coded data to generate
indicating data indicative of the
identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the printer further performs a method of tracking the
pharmaceutical product, the method
including, in a computer system: receiving indicating data from a sensing
device, the sensing device being
responsive to sensing of the coded data to generate indicating data indicative
of the identity of the product
item; and, updating, using the received indicating data, tracking data at
least partially indicative of tracking
information.
Optionally, the printer further performs a method of producing the packaging,
wherein the method includes, in
a computer system: determining a serial number associated with a
pharmaceutical product; generating, using
the serial number, an identity; generating, using the identity, a signature;
causing generation of coded data
using the identity, the coded data including a number of coded data portions,
each coded data portion
encoding the identity; and, at least part of the signature, the signature
being a digital signature of at least part
of the identity; causing printing of, on the pharmaceutical product packaging:
at least some coded data, and at
least one of: the identity; and, the serial number.
Optionally, the printer further performs a method of dispensing the
pharmaceutical product, the method
including, in a computer system: receiving indicating data from a sensing
device, the sensing device being
responsive to sensing of the coded data to generate indicating data at least
partially indicative of the identity;
determining, using the indicating data and from a dispensing database, at
least one criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the printer further includes a sensing device for use with the
pharmaceutical product, the sensing
device including: a housing adapted to be held by a user in use; a radiation
source for exposing at least one
coded data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for
determining, using the at least one sensed coded data portion, a sensed
identity. In a third broad form the
invention provides a method of allowing a user to interact with a
pharmaceutical product, the pharmaceutical
product being associated with packaging having disposed thereon or therein
coded data, at least some of the
coded data being indicative of at least an identity, the method including, in
a computer system: receiving
indicating data from a sensing device, the sensing device being responsive to
sensing of the coded data to
generate indicating data at least partially indicative of the identity;
determining, using the indicating data, at
least one action; and, performing the action associated with the
pharmaceutical product, the action including
at least one o~ providing information to a user; updating tracking information
relating to the pharmaceutical
product; performing a transaction relating to the pharmaceutical product;
authenticating the pharmaceutical
product; and, receiving feedback from the user.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-20-
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging, the region being associated with a respective action.
Optionally, the method includes, in the computer system: determining, using
the indicating data, a
description; and, determining, using the description, the action.
Optionally, the coded data is indicative of a plurality of reference points,
the indicating data being at least
partially indicative of at least one of: a position of the sensing device
relative to the packaging; and,
movement of the sensing device relative to the packaging, and wherein the
method includes, in the computer
system: determining, using the indicating data, at least one of a determined
position and determined
movement; and, performing the action at least in part based on at least one of
the detennined position and
determined movement.
Optionally, the coded data includes a number of coded data portions, each
coded data portion being indicative
of at least part of a signature, the signature being a digital signature of at
least part of the identity, wherein, in
response to sensing of at least one coded data portion, the sensing device
generates indicating data at least
partially indicative of at least one signature part, and wherein the method
includes, in the computer system:
determining, from the indicating data, a determined identity and at least one
determined signature part; and,
authenticating the pharmaceutical product using the determined identity and
the at least one determined
signature part.
Optionally, the entire signature is encoded within a plurality of coded data
portions and wherein the method
includes, in the sensing device, sensing a number of coded data portions to
thereby determine the entire
signature.
Optionally, the coded data includes a plurality of layouts, each layout
defming the position of a plurality of
first symbols encoding the identity, and a plurality of second symbols
defining at least part of the signature.
Optionally, the information includes at least one of: a product cost; a
patient identifier; a user identifier; an
owner identifier; manufacture date; batch number; product manufacturer;
product distributor; product
supplier; issue country; ingredients; storage conditions; disposal conditions;
serial number; expiry date;
effects; side-effects; conditions for use; instructions for use; links to
further information; contra-indications;
and, dosage.
Optionally, the feedback includes at least one of: an indication of the
effects of the pharmaceutical product;
marketing feedback; questions relating to the pharmaceutical product; and,
answers to customer surveys.
Optionally, the tracking information is indicative of at least one of: the
current owner of the pharmaceutical
product; one or more transactions performed using the pharmaceutical product;
a location of the
pharmaceutical product; and, a location of the sensing device.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-21 -
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated l/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-22-
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity and being encoded within a plurality of coded data portions,
wherein the method includes: in the
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of: the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity, wherein the method includes, in a computer system: receiving
indicating data from the sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data at least
partially indicative o~ the identity of the pharmaceutical product; and, the
signature part; determining, from
the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for determining a possible duplication
of pharmaceutical product
packaging, wherein the method includes, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data indicative
of the identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the method is further used for tracking the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data indicative of the
identity of the product item; and,
updating, using the received indicating data, tracking data at least partially
indicative of tracking information.
Optionally, the method is further used for producing pharmaceutical product
packaging, wherein the method
includes, in a computer system: determining a serial number associated with a
pharmaceutical product;
generating, using the serial number, an identity; generating, using the
identity, a signature; causing generation
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-23-
of coded data using the identity, the coded data including a number of coded
data portions, each coded data
portion encoding the identity; and, at least part of the signature, the
signature being a digital signature of at
least part of the identity; causing printing of, on the pharmaceutical product
packaging: at least some coded
data, and at least one of: the identity; and, the serial number.
Optionally, the method is further used for dispensing the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data at least partially
indicative of the identity; determining,
using the indicating data and from a dispensing database, at least one
criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In another broad form
the invention provides a method
of allowing a user to interact with a pharmaceutical product, the
pharmaceutical product being associated with
packaging having disposed thereon or therein coded data, at least some of the
coded data being indicative of
at least an identity, the method including, in a sensing device: sensing at
least some of the coded data;
determining, using the sensed coded data, indicating data at least partially
indicative of the identity; and,
transferring the indicating data to a computer system, the computer system
being responsive to the indicating
data for: determining, using the indicating data, at least one action; and,
performing the action associated with
the pharmaceutical product, the action including at least one of providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user. In
a fifth broad form the invention provides a method for authenticating a
pharmaceutical product, the
pharmaceutical product being associated with packaging having disposed thereon
or therein coded data
including a number of coded data portions, each coded data portion being
indicative of an identity of the
pharmaceutical product and at least part of a signature, the signature being a
digital signature of at least part of
the identity and being encoded within a plurality of coded data portions,
wherein the method includes: in a
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of: the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method includes, in the processor: obtaining, using the
identity, a key from a secure data
store, the secure data store storing at least one secret key; generating,
using the determined identity and the
key, a generated signature; and, comparing the at least one determined
signature part and the generated
signature to thereby authenticate the pharmaceutical product.
Optionally, the secure data store forms an internal memory of the processor.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-24-
Optionally, the method includes, in the processor: in the sensing device:
sensing a plurality of coded data
portions; and, generating, using the sensed coded data portions, indicating
data indicative of the signature; in
a processor: determining, from the indicating data, a determined signature;
determining, using the identity, a
key; generating, using the determined signature and the key, a generated
identity; and, comparing the
determined identity and the generated identity to thereby authenticate the
pharmaceutical product.
Optionally, the method includes, in the processor: obtaining the key from a
local data store using the identity;
and, obtaining the key from a remote data store using the identity, if the key
cannot be obtained from the local
data store.
Optionally, the method includes in the at least one of the sensing device and
the processor: determining when
sufficient in a sensing device: sensing at least one coded data portion; and,
generating, using the sensed coded
data portion, indicating data indicative of the identity; and, the at least
one signature part; in a processor:
determining, from the indicating data: a determined identity; and, at least
one determined signature part;
authenticating the pharmaceutical product using the determined identity and
the at least one determined
signature part.
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging.
Optionally, the coded data includes a plurality of layouts, each layout
defining the position of a plurality of
first symbols encoding the identity, and a plurality of second symbols defming
at least part of the signature.
Optionally, the method includes, in the processor, communicating with a local
database, the database storing
data relating the pharmaceutical product, including at least one of:
authentication data, including at least a key
associated with a signature, the signature being a digital signature of at
least part of the identity; tracking data,
the tracking data being at least partially indicative of tracking information
including at least one of: an owner
of the pharmaceutical product; one or more transactions performed using the
pharmaceutical product; a
location of the pharmaceutical product; and, a location of the sensing device.
product data, the product data
being at least partially indicative of product information including at least
one of: a product cost; a patient
identifier; a user identifier; an owner identifier; manufacture date; batch
number; product manufacturer;
product distributor; product supplier; issue country; ingredients; storage
conditions; disposal conditions;
serial number; expiry date; effects; side-effects; conditions for use;
instructions for use; links to further
information; contra-indications; and, dosage.
Optionally, the method includes displaying at least one of: results of
authentication; tracking information; and,
product information.
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 25 -
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-26-
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for allowing a user to interact with a
pharmaceutical product, the
method including, in a computer system: receiving indicating data from the
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data, at least one action; and,
performing the action associated with
the pharmaceutical product, the action including at least one of: providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user.
Optionally, the method is further used for authenticating the pharmaceutical
product, wherein the method
includes, in a computer system: receiving indicating data from the sensing
device, the sensing device being
responsive to sensing of the coded data to generate indicating data at least
partially indicative of: the identity
of the pharmaceutical product; and, the signature part; determining, from the
indicating data: a determined
identity; and, at least one determined signature part; authenticating the
pharmaceutical product using the
determined identity and the at least one determined signature part.
Optionally, the method is further used for determining a possible duplication
of pharmaceutical product
packaging, wherein the method includes, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data indicative
of the identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the method is further used for tracking the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data indicative of the
identity of the product item; and,
updating, using the received indicating data, tracking data at least partially
indicative of tracking information.
Optionally, the method is further used for producing pharmaceutical product
packaging, wherein the method
includes, in a computer system: determining a serial number associated with a
pharmaceutical product;
generating, using the serial number, an identity; generating, using the
identity, a signature; causing generation
of coded data using the identity, the coded data including a number of coded
data portions, each coded data
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-27-
portion encoding the identity; and, at least part of the signature, the
signature being a digital signature of at
least part of the identity; causing printing of, on the pharmaceutical product
packaging: at least some coded
data, and at least one of: the identity; and, the serial number.
Optionally, the method is further used for dispensing the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data at least partially
indicative of the identity; determining,
using the indicating data and from a dispensing database, at least one
criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In a fourth broad form
the invention provides a method
for authenticating a pharmaceutical product, the pharmaceutical product being
associated with packaging
having disposed thereon or therein coded data including a number of coded data
portions, each coded data
portion being indicative of an identity of the pharmaceutical product and at
least part of a signature, the
signature being a digital signature of at least part of the identity, wherein
the method includes, in a computer
system: receiving indicating data from a sensing device, the sensing device
being responsive to sensing of the
coded data to generate indicating data at least partially indicative of: the
identity of the pharmaceutical
product; and, the signature part; determining, from the indicating data: a
determined identity; and, at least one
determined signature part; authenticating the pharmaceutical product using the
determined identity and the at
least one determined signature part.
Optionally, the method includes, in the computer system: determining, using
the determined identity, a key;
generating, using the determined identity and the key, a generated signature;
and, comparing the at least one
determined signature part and the generated signature to thereby authenticate
the pharmaceutical product.
Optionally, the entire signature is encoded within a plurality of coded data
portions, the sensing device being
responsive to sensing a plurality of coded data portions to generate
indicating data indicative of the entire
signature, and wherein the method includes, in the computer system:
determining, using the indicating data, a
determined signature; determining, using the determined identity, a key;
generate, using the determined
signature and the key, a generated identity; and, compare the determined
identity and the generated identity to
thereby authenticate the pharmaceutical product.
Optionally, the method includes, in the computer system: determining, using
the determined identity, tracking
data at least partially indicative of the location of the pharmaceutical
product; and, determining, using the
tracking data, if the pharmaceutical product is a possible duplicate.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-28-
Optionally, the method includes, in the computer system, and in response to a
successful authentication:
authorising a transaction relating to the pharmaceutical product; and,
updating tracking data relating to the
phartnaceutical product, the tracking data being at least partially indicative
of the location of the
pharmaceutical product.
Optionally, the method includes, in the computer system, communicating with a
database, the database storing
data relating the pharmaceutical product, including at least one o~
authentication data, including at least a key
associated with a signature, the signature being a digital signature of at
least part of the identity; tracking data,
the tracking data being at least partially indicative of tracking information
including at least one of: an owner
of the pharmaceutical product; one or more transactions performed using the
pharmaceutical product; a
location of the pharmaceutical product; and, a location of the sensing device;
and, product data, the product
data being at least partially indicative of product information including at
least one of: a product cost; a
patient identifier; a user identifier; an owner identifier; manufacture date;
batch number; product
manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions; disposal
conditions; serial number; expiry date; effects; side-effects; conditions for
use; instructions for use; links to
further information; contra-indications; and, dosage.
Optionally, the method includes, in the computer system, causing the display
of at least one of: results of
authentication; tracking information; and, product information.
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging.
Optionally, the coded data includes a plurality of layouts, each layout
defining the position of a plurality of
first symbols encoding the identity, and a plurality of second symbols
defining at least part of the signature.
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-29-
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for allowing a user to interact with a
pharmaceutical product, the
method including, in a computer system: receiving indicating data from the
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data, at least one action; and,
performing the action associated with
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-30-
the pharmaceutical product, the action including at least one of: providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user.
Optionally, the method is further used for authenticating the pharmaceutical
product, wherein the method
includes: in the sensing device: sensing at least one coded data portion; and,
generating, using the sensed
coded data portion, indicating data indicative of: the identity; and, the at
least one signature part; in a
processor: determining, from the indicating data: a determined identity; and,
at least one determined signature
part; authenticating the pharmaceutical product using the determined identity
and the at least one determined
signature part.
Optionally, the method is further used for determining a possible duplication
of pharmaceutical product
packaging, wherein the method includes, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data indicative
of the identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the method is further used for tracking the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data indicative of the
identity of the product item; and,
updating, using the received indicating data, tracking data at least partially
indicative of tracking information.
Optionally, the method is further used for producing pharmaceutical product
packaging, wherein the method
includes, in a computer system: determining a serial number associated with a
pharmaceutical product;
generating, using the serial number, an identity; generating, using the
identity, a signature; causing generation
of coded data using the identity, the coded data including a number of coded
data portions, each coded data
portion encoding the identity; and, at least part of the signature, the
signature being a digital signature of at
least part of the identity; causing printing of, on the pharmaceutical product
packaging: at least some coded
data, and at least one of: the identity; and, the serial number.
Optionally, the method is further used for dispensing the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data at least partially
indicative of the identity; determining,
using the indicating data and from a dispensing database, at least one
criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-31-
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In another broad form
the invention provides a method
for authenticating a pharmaceutical product, the pharmaceutical product being
associated with packaging
having disposed thereon or therein coded data including a number of coded data
portions, each coded data
portion being indicative of an identity of the pharmaceutical product and at
least part of a signature, the
signature being a digital signature of at least part of the identity, wherein
the method includes, in a sensing
device: sensing at least one coded data portion; generating, using the sensed
coded data portion, indicating
data at least partially indicative of: the identity; and, at least one
signature part; and, transferring the indicating
data to a computer system, the computer system being responsive to the
indicating data to: determine, from
the indicating data: a determined identity; and, at least one determined
signature part; authenticate the
pharmaceutical product using the determined identity and the at least one
determined signature part. In a sixth
broad form the invention provides a method of determining a possible
duplication of pharmaceutical product
packaging, the packaging having disposed thereon or therein coded data
including a number of coded data
portions, each coded data portion being indicative of at least an identity of
the pharmaceutical product, and
wherein the method includes, in a computer system: receiving indicating data
from a sensing device, the
sensing device being responsive to sensing of the coded data to generate
indicating data indicative of the
identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the tracking data is indicative of tracking information for each
of a number of existing
pharmaceutical products, and wherein the method includes, in the computer
system, determining if the
pharmaceutical product is a duplicate of one of the existing pharmaceutical
products.
Optionally, the method includes, in the computer system: determining, using
the indicating data, a current
location of the pharmaceutical product; comparing the current location to the
tracking information; and,
determining the pharmaceutical product to be a possible duplicate if the
current location is inconsistent with
the tracking information.
Optionally, the method includes, in the computer system, determining if the
current location is inconsistent
with the tracking information using predetermined rules.
Optionally, each coded data portion is indicative of at least part of a
signature, the signature being a digital
signature of at least part of the identity, wherein the indicating data is at
least partially indicative of at least
one signature part, and wherein the method includes, in a computer system:
determining, from the indicating
data, at least one determined signature part; and, authenticating the
pharmaceutical product using the
determined identity and the at least one determined signature part.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-32-
Optionally, the method includes, in the computer system, and in response to a
successful authentication:
authorising a transaction relating to the pharmaceutical product; and,
updating the tracking data relating to the
pharmaceutical product.
Optionally, the method includes, in the computer system, communicating with a
database, the database storing
data relating the pharmaceutical product, including at least one of:
authentication data, including at least a key
associated with a signature, the signature being a digital signature of at
least part of the identity; tracking data,
the tracking data being at least partially indicative of tracking information
including at least one of: an owner
of the pharmaceutical product; one or more transactions performed using the
pharmaceutical product; a
location of the pharmaceutical product; and, a location of the sensing device;
and, product data, the product
data being at least partially indicative of product information including at
least one of: a product cost; a
patient identifier; a user identifier; an owner identifier; manufacture date;
batch number; product
manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions; disposal
conditions; serial number; expiry date; effects; side-effects; conditions for
use; instructions for use; links to
further information; contra-indications; and, dosage.
Optionally, the method includes, in the computer system, causing the display
of at least one of: results of
authentication; tracking information; and, product information.
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging.
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 33 -
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for allowing a user to interact with a
pharmaceutical product, the
method including, in a computer system: receiving indicating data from the
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data, at least one action; and,
performing the action associated with
the pharmaceutical product, the action including at least one of: providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-34-
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity and being encoded within a plurality of coded data portions,
wherein the method includes: in the
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of: the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity, wherein the method includes, in a computer system: receiving
indicating data from the sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data at least
partially indicative of: the identity of the pharmaceutical product; and, the
signature part; determining, from
the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is fizrther used for tracking the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data indicative of the
identity of the product item; and,
updating, using the received indicating data, tracking data at least partially
indicative of tracking information.
Optionally, the method is further used for producing pharmaceutical product
packaging, wherein the method
includes, in a computer system: determining a serial number associated with a
pharmaceutical product;
generating, using the serial number, an identity; generating, using the
identity, a signature; causing generation
of coded data using the identity, the coded data including a number of coded
data portions, each coded data
portion encoding the identity; and, at least part of the signature, the
signature being a digital signature of at
least part of the identity; causing printing of, on the pharmaceutical product
packaging: at least some coded
data, and at least one of: the identity; and, the serial number.
Optionally, the method is further used for dispensing the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data at least partially
indicative of the identity; determining,
using the indicating data and from a dispensing database, at least one
criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In another broad form
the invention provides a method
of determining a possible duplication of pharmaceutical product packaging, the
packaging having disposed
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-35-
thereon or therein coded data including a number of coded data portions, each
coded data portion being
indicative of at least an identity of the pharmaceutical product, and wherein
the method includes, in a sensing
device: sensing at least some of the coded data; determining, using the sensed
coded data, indicating data at
least partially indicative of the identity; and, transferring the indicating
data to a computer system, the
computer system being responsive to the indicating data for: determining, from
the indicating data, a
determined identity; accessing, using the determined identity, tracking data
relating to the pharmaceutical
product, the tracking data being at least partially indicative of the location
of the pharmaceutical product; and,
determining, using the tracking data, if the pharmaceutical product is a
possible duplicate. In a seventh broad
form the invention provides a method of tracking a pharmaceutical product, the
pharmaceutical product being
associated with packaging having disposed thereon or therein coded data
including a number of coded data
portions, each coded data portion being indicative of an identity of the
pharmaceutical product, the method
including, in a computer system: receiving indicating data from a sensing
device, the sensing device being
responsive to sensing of the coded data to generate indicating data indicative
of the identity of the product
item; and, updating, using the received indicating data, tracking data at
least partially indicative of tracking
information.
Optionally, at least one of the tracking information and the indicating data
are indicative of at least one of: an
identity of the sensing device; an identity of a patient; an identity of a
current owner of the pharmaceutical
product; one or more transactions performed using the pharmaceutical product;
a location of the
pharmaceutical product; and, a location of the sensing device.
Optionally, each coded data portion is indicative of at least part of a
signature, the signature being a digital
signature of at least part of the identity, wherein the indicating data is at
least partially indicative of at least
one signature part, and wherein the method includes, in a computer system:
determining, from the indicating
data, at least one determined signature part; and, authenticating the
pharmaceutical product using the
determined identity and the at least one determined signature part.
Optionally, the method includes, in the computer system, and in response to a
successful authentication:
authorising a transaction relating to the pharmaceutical product; and,
updating the tracking data.
Optionally, the entire signature is encoded within a plurality of coded data
portions and wherein the system
includes the sensing device configured to sense a number of coded data
portions to thereby determine the
entire signature.
Optionally, the method includes, in the computer system, communicating with a
database, the database storing
data relating the pharmaceutical product, including at least one of:
authentication data, including at least a key
associated with a signature, the signature being a digital signature of at
least part of the identity; and, product
data, the product data being at least partially indicative of product
information including at least one of: a
product cost; a patient identifier; a user identifier; an owner identifier;
manufacture date; batch number;
product manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions;
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-36-
disposal conditions; serial number; expiry date; effects; side-effects;
conditions for use; instructions for use;
links to further information; contra-indications; and, dosage.
Optionally, the method includes, in the computer system, causing the display
of at least one of: results of
authentication; tracking information; and, product information.
Optionally, the coded data includes a plurality of layouts, each layout
defming the position of a plurality of
first symbols encoding the identity, and a plurality of second symbols
defining at least part of the signature.
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging.
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-37-
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for allowing a user to interact with a
pharmaceutical product, the
method including, in a computer system: receiving indicating data from the
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data, at least one action; and,
performing the action associated with
the pharmaceutical product, the action including at least one of: providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity and being encoded within a plurality of coded data portions,
wherein the method includes: in the
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of: the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-38-
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity, wherein the method includes, in a computer system: receiving
indicating data from the sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data at least
partially indicative of: the identity of the pharmaceutical product; and, the
signature part; determining, from
the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for determining a possible duplication
of pharmaceutical product
packaging, wherein the method includes, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data indicative
of the identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the method is further used for producing pharmaceutical product
packaging, wherein the method
includes, in a computer system: determining a serial number associated with a
pharmaceutical product;
generating, using the serial number, an identity; generating, using the
identity, a signature; causing generation
of coded data using the identity, the coded data including a number of coded
data portions, each coded data
portion encoding the identity; and, at least part of the signature, the
signature being a digital signature of at
least part of the identity; causing printing of, on the pharmaceutical product
packaging: at least some coded
data, and at least one of: the identity; and, the serial number.
Optionally, the method is further used for dispensing the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data at least partially
indicative of the identity; determining,
using the indicating data and from a dispensing database, at least one
criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In another broad form
the invention provides a method
of tracking a pharmaceutical product, the pharmaceutical product being
associated with packaging having
disposed thereon or therein coded data including a number of coded data
portions, each coded data portion
being indicative of an identity of the pharmaceutical product, the method
including, in a sensing device:
sensing at least some of the coded data; determining, using the sensed coded
data, indicating data at least
partially indicative of the identity; and, transferring the indicating data to
a computer system, the computer
system being responsive to the indicating data to update, using the received
indicating data, the tracking data.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-39-
In an eighth broad form the invention provides a method of producing
pharmaceutical product packaging,
wherein the method includes, in a computer system: determining a serial number
associated with a
pharmaceutical product; generating, using the serial number,. an identity;
generating, using the identity, a
signature; causing generation of coded data using the identity, the coded data
including a number of coded
data portions, each coded data portion encoding the identity; and, at least
part of the signature, the signature
being a digital signature of at least part of the identity; causing printing
of, on the pharmaceutical product
packaging: at least some coded data, and at least one of: the identity; and,
the serial number. 2. A method
according to claim 1, wherein the identity is formed at least in part from at
least one of: a serial number of the
pharmaceutical product; an identity of the pharmaceutical product; an EPC; an
identity of the packaging; and,
an identity of a region of the packaging.
Optionally, the method includes, encoding the entire signature within a
plurality of coded data portions.
Optionally, the method includes: determining visible information relating to
the pharmaceutical product;
determining a description, the description describing a layout of the visible
information; recording an
association between the identity and the layout; and, causing printing of the
packaging using the layout.
Optionally, the description is indicative of at least one action associated
with the pharmaceutical product, the
action including at least one of: providing information to a user; updating
tracking information relating to the
pharmaceutical product; performing a transaction relating to the
pharmaceutical product; authenticating the
pharmaceutical product; and, receiving feedback from the user.
Optionally, the method includes, in the computer system, communicating with a
database, the database storing
data relating the pharmaceutical product, including at least one of:
authentication data, including at least a key
associated with a signature, the signature being a digital signature of at
least part of the identity; tracking data,
the tracking data being at least partially indicative of tracking information
including at least one of: an owner
of the pharmaceutical product; one or more transactions performed using the
pharmaceutical product; a
location of the pharmaceutical product; and, a location of the sensing device;
and, product data, the product
data being at least partially indicative of product information including at
least one of: a product cost; a
patient identifier; a user identifier; an owner identifier; manufacture date;
batch number; product
manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions; disposal
conditions; serial number; expiry date; effects; side-effects; conditions for
use; instructions for use; links to
further information; contra-indications; and, dosage.
Optionally, the method includes, in the computer system: updating at least
some of the data relating to the
pharmaceutical product; and, generating the coded data using at least some of
the data relating to the
pharmaceutical product.
Optionally, the method includes, in the computer system, receiving from a
scanning system, in response to
scanning of the packaging, information indicative of at least one of: a source
of the pharmaceutical product; a
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-40-
pharmaceutical product type; the identity of the pharmaceutical product; and,
the serial number of the
pharmaceutical product; and,
Optionally, the coded data is indicative of a plurality of reference points
such that sensing of at least one
coded data portion, by a sensing device, allows for determination of at least
one of: the identity; at least one
signature part; a position of the sensing device relative to the packaging;
and, movement of the sensing device
relative to the packaging.
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging.
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-41 -
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for allowing a user to interact with a
pharmaceutical product, the
method including, in a computer system: receiving indicating data from the
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data, at least one action; and,
performing the action associated with
the pharmaceutical product, the action including at least one of: providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity and being encoded within a plurality of coded data portions,
wherein the method includes: in the
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of: the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-42-
the identity, wherein the method includes, in a computer system: receiving
indicating data from the sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data at least
partially indicative of: the identity of the pharmaceutical product; and, the
signature part; determining, from
the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for determining a possible duplication
of pharmaceutical product
packaging, wherein the method includes, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data indicative
of the identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the method is further used for tracking the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data indicative of the
identity of the product item; and,
updating, using the received indicating data, tracking data at least partially
indicative of tracking information.
Optionally, the method is further used for dispensing the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data at least partially
indicative of the identity; determining,
using the indicating data and from a dispensing database, at least one
criterion for dispensing the
pharmaceutical product; and, causing the pharmaceutical product to be
dispensed if the at least one criterion is
satisfied.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In a ninth broad form
the invention provides a method
of dispensing a pharmaceutical product, the pharmaceutical product being
associated with packaging having
disposed thereon or therein coded data, at least some of the coded data being
indicative of at least an identity
of the pharmaceutical product, the method including, in a computer system:
receiving indicating data from a
sensing device, the sensing device being responsive to sensing of the coded
data to generate indicating data at
least partially indicative of the identity; determining, using the indicating
data and from a dispensing database,
at least one criterion for dispensing the pharmaceutical product; and, causing
the pharmaceutical product to be
dispensed if the at least one criterion is satisfied.
Optionally, the at least one criterion is indicative of an intended recipient
of the pharmaceutical product, and
wherein the method includes, in the computer system: determining identity data
indicative of an identity of an
individual requesting the pharmaceutical product; comparing the identity data
to the at least one criterion; and,
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 43 -
determining the at least one criterion to be satisfied if the identity of the
individual is the same as the identity
of the intended recipient.
Optionally, the sensing device includes a data store for storing the identity
data, and wherein the method
includes determining the identity data from the data store.
Optionally, the at least one criterion is indicative of at least one of: an
intended time for dispensing the
pharmaceutical product; an intended date for dispensing the pharmaceutical
product; and, an intended location
for dispensing the pharmaceutical product.
Optionally, the coded data includes a number of coded data portions, each
coded data portion being indicative
of at least part of a signature, the signature being a digital signature of at
least part of the identity, wherein, in
response to sensing of at least one coded data portion, the sensing device
generates indicating data at least
partially indicative of at least one signature part, and wherein the method
includes, in the computer system:
determining, from the indicating data, a determined identity and at least one
determined signature part;
authenticating the pharmaceutical product using the determined identity and
the at least one determined
signature part; and, dispensing the pharmaceutical product in response to a
successful authentication.
Optionally, the entire signature is encoded within a plurality of coded data
portions and wherein the method
includes, in the sensing device, sensing a number of coded data portions to
thereby determine the entire
signature.
Optionally, the method includes, in the computer system, communicating with a
database, the database storing
data relating the pharmaceutical product, including at least one of:
authentication data, including at least a key
associated with a signature, the signature being a digital signature of at
least part of the identity; tracking data,
the tracking data being at least partially indicative of tracking information
including at least one of an owner
of the pharmaceutical product; one or more transactions performed using the
pharmaceutical product; a
location of the pharmaceutical product; and, a location of the sensing device;
and, product data, the product
data being at least partially indicative of product information including at
least one of: a product cost; a
patient identifier; a user identifier; an owner identifier; manufacture date;
batch number; product
manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions; disposal
conditions; serial number; expiry date; effects; side-effects; conditions for
use; instructions for use; links to
further information; contra-indications; and, dosage.
Optionally, the method includes, in the computer system, causing display of at
least one of: results of
authentication; tracking information; and, product information.
Optionally, the identity is formed at least in part from at least one of: a
coded data portion identity; an identity
of the pharmaceutical product; an EPC; an identity of the packaging; and, an
identity of a region of the
packaging.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-44-
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of: an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one of: Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one o~ a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 45 -
Optionally, the method includes the use of a transaction terminal for
performing transactions relating to the
pharmaceutical product, the transaction terminal including: a radiation source
for exposing at least one coded
data portion; a sensor for sensing the at least one exposed coded data
portion; and, a processor for:
determining, using the at least one sensed coded data portion, a sensed
identity; and, performing the
transaction using the sensed identity.
Optionally, the method includes the use of a printer for printing the
packaging, the printer being for:
determining visible information to be provided on the packaging; determining
an identity associated with the
pharmaceutical product; generating coded data using the identity, the coded
data including a number of coded
data portions, each coded data portion being indicative of at least the
identity of the pharmaceutical product;
and, printing the packaging by printing the coded data and the visible
information.
Optionally, the method is further used for allowing a user to interact with a
pharmaceutical product, the
method including, in a computer system: receiving indicating data from the
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data at
least partially indicative of the
identity; determining, using the indicating data, at least one action; and,
performing the action associated with
the pharmaceutical product, the action including at least one of: providing
information to a user; updating
tracking information relating to the pharmaceutical product; performing a
transaction relating to the
pharmaceutical product; authenticating the pharmaceutical product; and,
receiving feedback from the user.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
tha identity and being encoded within a plurality of coded data portions,
wherein the method includes: in the
sensing device: sensing at least one coded data portion; and, generating,
using the sensed coded data portion,
indicating data indicative of: the identity; and, the at least one signature
part; in a processor: determining,
from the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is further used for authenticating the pharmaceutical
product, each coded data portion
being further indicative of at least part of a signature, the signature being
a digital signature of at least part of
the identity, wherein the method includes, in a computer system: receiving
indicating data from the sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data at least
partially indicative of: the identity of the pharmaceutical product; and, the
signature part; determining, from
the indicating data: a determined identity; and, at least one determined
signature part; authenticating the
pharmaceutical product using the determined identity and the at least one
determined signature part.
Optionally, the method is fiarther used for determining a possible duplication
of pharmaceutical product
packaging, wherein the method includes, in a computer system: receiving
indicating data from a sensing
device, the sensing device being responsive to sensing of the coded data to
generate indicating data indicative
of the identity; determining, from the indicating data, a determined identity;
accessing, using the determined
identity, tracking data relating to the pharmaceutical product, the tracking
data being at least partially
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-46-
indicative of the location of the pharmaceutical product; and, determining,
using the tracking data, if the
pharmaceutical product is a possible duplicate.
Optionally, the method is further used for tracking the pharmaceutical
product, the method including, in a
computer system: receiving indicating data from the sensing device, the
sensing device being responsive to
sensing of the coded data to generate indicating data indicative of the
identity of the product item; and,
updating, using the received indicating data, tracking data at least partially
indicative of tracking information.
Optionally, the method is further used for producing pharmaceutical product
packaging, wherein the method
includes, in a computer system: determining a serial number associated with a
pharmaceutical product;
generating, using the serial number, an identity; generating, using the
identity, a signature; causing generation
of coded data using the identity, the coded data including a number of coded
data portions, each coded data
portion encoding the identity; and, at least part of the signature, the
signature being a digital signature of at
least part of the identity; causing printing of, on the pharmaceutical product
packaging: at least some coded
data, and at least one of: the identity; and, the serial number.
Optionally, the sensing device is for use with the pharmaceutical product, the
sensing device including: a
housing adapted to be held by a user in use; a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for determining, using the at
least one sensed coded data portion, a sensed identity. In another broad form
the invention provides a method
of dispensing a pharmaceutical product, the pharmaceutical product being
associated with packaging having
disposed thereon or therein coded data, at least some of the coded data being
indicative of at least an identity
of the pharmaceutical product, the method including, in a sensing device:
sensing at least some of the coded
data; determining, using the sensed coded data, indicating data at least
partially indicative of the identity; and,
transferring the indicating data to a computer system, the computer system
being responsive to the indicating
data for: determining, using the indicating data and from a dispensing
database, at least one criterion for
dispensing the pharmaceutical product; and, causing the pharmaceutical product
to be dispensed if the at least
one criterion is are satisfied.
In an tenth broad form the invention provides a sensing device for use with a
pharmaceutical product, the
pharmaceutical product being associated with packaging having disposed thereon
or therein coded data
including a number of coded data portions, each coded data portion being
indicative of an identity of the
pharmaceutical product, the sensing device including: a housing adapted to be
held by a user in use; a
radiation source for exposing at least one coded data portion; a sensor for
sensing the at least one exposed
coded data portion; and, a processor for determining, using the at least one
sensed coded data portion, a
sensed identity.
Optionally, the sensing device includes a communication system and wherein the
processor: generates
indicating data at least partially indicative of the sensed identity; and,
transfers the indicating data to a
computer system, the computer system being responsive to the indicating data
to perform an action including
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-47-
at least one of authentication of the pharmaceutical product; approval of the
transaction; determination of
transaction details; and, updating of tracking information relating to the
pharmaceutical product.
Optionally, the processor uses the sensed identity to perform at least one of:
authentication of the
pharmaceutical product; approval of the transaction; determination of
transaction details; and, updating of
tracking information relating to the pharmaceutical product.
Optionally, the identity is formed at least in part from at least one of: a
serial number of the pharmaceutical
product; an identity of the pharmaceutical product; an EPC; an identity of the
packaging; and, an identity of a
region of the packaging.
Optionally, coded data portion is indicative of at least part of a signature,
the signature being a digital
signature of at least part of the identity, and wherein the processor:
determines, using the at least one sensed
coded data portion, at least one sensed signature part; causes authentication
of the pharmaceutical product
using the sensed identity and the at least one sensed signature part.
Optionally, the pharmaceutical product is authenticated by at least one of the
processor and a computer
system, which: determines, using the sensed identity, a key; determines, using
the sensed identity and the key,
a determined signature; and, compares the at least one sensed signature part
and the determined signature to
thereby authenticate the pharmaceutical product.
Optionally, the entire signature is encoded within a plurality of coded data
portions, the processor determines,
from a plurality of sensed coded data portions, a sensed signature, and
wherein pharmaceutical product is
authenticated by at least one of the processor, and a computer system, which:
determines, using the sensed
identity, a key; determines, using the sensed signature and the key, a
determined identity; and, compares the
detennined identity and the sensed identity to thereby authenticate the
pharmaceutical product.
Optionally, the coded data includes a plurality of layouts, each layout
defining the position of a plurality of
first symbols encoding the identity, and a plurality of second symbols
defining at least part of the signature.
Optionally, packaging includes at least one of: a blister pack; a bottle; a
bottle lid; a label; a box; and, a
leaflet.
Optionally, the sensing device includes a communication system for
communicating with a database, the
database storing data relating the pharmaceutical product, including at least
one of: authentication data,
including at least a key associated with a signature, the signature being a
digital signature of at least part of
the identity; tracking data, the tracking data being at least partially
indicative of tracking information
including at least one of: an owner of the pharmaceutical product; one or more
transactions performed using
the pharmaceutical product; a location of the pharmaceutical product; and, a
location of the sensing device.
product data, the product data being at least partially indicative of product
information including at least one
of: a product cost; a patient identifier; a user identifier; an owner
identifier; manufacture date; batch number;
product manufacturer; product distributor; product supplier; issue country;
ingredients; storage conditions;
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 48 -
disposal conditions; serial number; expiry date; effects; side-effects;
conditions for use; instructions for use;
links to further information; contra-indications; and, dosage.
Optionally, the sensing device includes a display for displaying at least one
of: results of authentication;
tracking information; and, product information.
Optionally, the coded data is substantially invisible to an unaided human.
Optionally, the coded data is printed on the surface using at least one of an
invisible ink; and, an infrared-
absorptive ink.
Optionally, the coded data is provided substantially coincident with visible
human-readable information.
Optionally, the coded includes a number of coded data portions, and wherein at
least some of the coded data
portions encode at least one of: a location of the respective coded data
portion; a position of the respective
coded data portion on the surface; a size of the coded data portions; a
signature part; a size of a signature; an
identity of a signature part; units of indicated locations; and, at least part
of a data object, the entire data object
being encoded at least once by a plurality of coded data portions.
Optionally, the data object includes at least one o~ Multipurpose Internet
Mail Extensions (MIME) data; text
data; image data; audio data; video data; application data; contact data;
information; business card data; and,
directory data.
Optionally, the coded data includes at least one of: redundant data; data
allowing error correction; Reed-
Solomon data; and, Cyclic Redundancy Check (CRC) data.
Optionally, the digital signature includes at least one of: a random number
associated with the identity; a
keyed hash of at least the identity; a keyed hash of at least the identity
produced using a private key, and
verifiable using a corresponding public key; cipher-text produced by
encrypting at least the identity; cipher-
text produced by encrypting at least the identity and a random number; and,
cipher-text produced using a
private key, and verifiable using a corresponding public key.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout including n identical sub-
layouts rotated 1/n revolutions apart
about a centre of rotation, at least one sub-layout including rotation-
indicating data that distinguishes that sub-
layout from each other sub-layout.
Optionally, the coded data is arranged in accordance with at least one layout
having n-fold rotational
symmetry, where n is at least two, the layout encoding orientation-indicating
data comprising a sequence of
an integer multiple m of n symbols, where m is one or more, each encoded
symbol being distributed at n
locations about a centre of rotational symmetry of the layout such that
decoding the symbols at each of the n
orientations of the layout produces n representations of the orientation-
indicating data, each representation
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 49-
comprising a different cyclic shift of the orientation-indicating data and
being indicative of the degree of
rotation of the layout.
Optionally, is coupled to a transaction terminal for performing transactions
relating to the pharmaceutical
product, the transaction terminal including: a radiation source for exposing
at least one coded data portion; a
sensor for sensing the at least one exposed coded data portion; and, a
processor for: determining, using the at
least one sensed coded data portion, a sensed identity; and, performing the
transaction using the sensed
identity.
Optionally, is coupled to a printer for printing packaging associated with the
pharmaceutical product, the
printer being for: determining visible information to be provided on the
packaging; determining an identity
associated with the pharmaceutical product; generating coded data using the
identity, the coded data including
a number of coded data portions, each coded data portion being indicative of
at least the identity of the
pharmaceutical product; and, printing the packaging by printing the coded data
and the visible information.
Optionally, the sensing device is further used to perform a method of allowing
a user to interact with the
pharmaceutical product, the method including, in a computer system: receiving
indicating data from the
sensing device, the sensing device being responsive to sensing of the coded
data to generate indicating data at
least partially indicative of the identity; determining, using the indicating
data, at least one action; and,
performing the action associated with the pharmaceutical product, the action
including at least one of
providing information to a user; updating tracking information relating to the
pharmaceutical product;
performing a transaction relating to the pharmaceutical product;
authenticating the pharmaceutical product;
and, receiving feedback from the user.
Optionally, the sensing device is further used to perform a method for
authenticating the pharmaceutical
product, each coded data portion being further indicative of at least part of
a signature, the signature being a
digital signature of at least part of the identity and being encoded within a
plurality of coded data portions,
wherein the method includes: in the sensing device: sensing at least one coded
data portion; and, generating,
using the sensed coded data portion, indicating data indicative of the
identity; and, the at least one signature
part; in a processor: determining, from the indicating data: a determined
identity; and, at least one determined
signature part; authenticating the pharmaceutical product using the determined
identity and the at least one
determined signature part.
Optionally, the sensing device is further used to perform a method for
authenticating the pharmaceutical
product, each coded data portion being further indicative at least part of a
signature, the signature being a
digital signature of at least part of the identity, wherein the method
includes, in a computer system: receiving
indicating data from the sensing device, the sensing device being responsive
to sensing of the coded data to
generate indicating data at least partially indicative of: the identity of the
pharmaceutical product; and, the
signature part; determining, from the indicating data: a determined identity;
and, at least one determined
signature part; authenticating the pharmaceutical product using the determined
identity and the at least one
determined signature part.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-50-
Optionally, the sensing device is further used to perform a method of
determining a possible duplication of
pharmaceutical product packaging, wherein the method includes, in a computer
system: receiving indicating
data from the sensing device, the sensing device being responsive to sensing
of the coded data to generate
indicating data indicative of the identity; determining, from the indicating
data, a determined identity;
accessing, using the determined identity, tracking data relating to the
pharmaceutical product, the tracking
data being at least partially indicative of the location of the pharmaceutical
product; and, determining, using
the tracking data, if the pharmaceutical product is a possible duplicate.
Optionally, the sensing device is further used to perform a method of tracking
the pharmaceutical product, the
method including, in a computer system: receiving indicating data from a
sensing device, the sensing device
being responsive to sensing of the coded data to generate indicating data
indicative of the identity of the
product item; and, updating, using the received indicating data, tracking data
at least partially indicative of
tracking information.
Optionally, the sensing device is further used to perform a method of
producing pharmaceutical product
packaging, wherein the method includes, in a computer system: determining a
serial number associated with
the pharmaceutical product; generating, using the serial number, an identity;
generating, using the identity, a
signature; causing generation of coded data using the identity, the coded data
including a number of coded
data portions, each coded data portion encoding the identity; and, at least
part of the signature, the signature
being a digital signature of at least part of the identity; causing printing
of, on the pharmaceutical product
packaging: at least some coded data, and at least one o~ the identity; and,
the serial number.
Optionally, the sensing device is further used to perform a method of
dispensing the pharmaceutical product,
the method including, in a computer system: receiving indicating data from a
sensing device, the sensing
device being responsive to sensing of the coded data to generate indicating
data at least partially indicative of
the identity; determining, using the indicating data and from a dispensing
database, at least one criterion for
dispensing the pharmaceutical product; and, causing the pharmaceutical product
to be dispensed if the at least
one criterion is satisfied.
In an eleventh broad form the present invention provides a method of verifying
an object, wherein the method
includes, in a computer system: receiving a verification request, the request
being at least partially indicative
of: an identity of the object; at least one signature fragment, the signature
being a digital signature of at least
part of the identity; determining, using the verification request, a
determined identity; determining, using the
determined identity, and from a database, at least one criterion relating to
verification; and, comparing the
received verification request to the at least one criterion; and causing the
object to be verified if the at least
one criterion is satisfied.
Optionally the at least one criterion relates to a limit on at least one of: a
number of received verification
requests; a rate of received verification requests; and, timing of received
verification requests.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-51-
Optionally the limit is defmed in respect of at least one of the identity of
the object; the signature; the
signature fragment; a verification request source; and, the object.
Optionally the limit is proportional to a size of the signature fragment.
Optionally the method includes, in the computer system: determining, using the
verification request: a request
history indicative of a number of previously received verification requests;
and, a corresponding limit;
determining, using the verification request and the request history, a request
number; and, causing the object
to be verified if the request number does not exceed the corresponding limit.
Optionally the method includes, in the computer system, and in response to a
verification request, updating
the request history.
Optionally the request history is indicative of the timing of the received
verification request.
Optionally the request history is associated with: the identity of the object;
the signature; the signature
fragment; a verification request source; and, the object.
Optionally the method includes, in the computer system, verifying the object
by authenticating the object
using the identity of the object and the at least one signature fragment.
Optionally the verification request is at least partially indicative of an
identity of the signature fragment.
Optionally the object is associated with a surface having disposed thereon or
therein coded data including a
number of coded data portions, each coded data portion being indicative of at
least the identity and a signature
fragment, and wherein, in response to sensing of at least one coded data
portion, a sensing device generates
the verification request.
Optionally the verification request is at least partially indicative of an
identity of the signature fragment, the
fragment identity being based on at least one of: a number encoded within the
at least one sensed coded data
portion; and, a position of the at least one sensed coded data portion on the
surface.
Optionally the method includes, in the computer system, only comparing the
received verification request to
the at least one criterion after a failed verification.
Optionally the method includes, in a computer system: receiving a verification
request, the request being at
least partially indicative of: an identity of the object; a concatenation of a
signature fragment, the signature
fragment being a digital signature of at least part of the identity; and a
random signature; determining, using
the verification request, a determined identity; determining, using the
concatenation, the signature fragment;
and, verifying the object using the determined identity and the signature
fragment.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-52-
Optionally the method includes, in the computer system: determining, using the
determined identity, a key;
generating, using the determined identity and the key, a generated signature;
comparing the generated
signature to the concatenation to thereby identify and authenticate the
signature fragment.
In another broad form the present invention provides coded data for disposal
on or in a surface, the coded data
including a number of coded data portions, each coded data portion encoding:
an identity; and, a fragment of a
signature, the signature being a digital signature of at least part of the
identity; and a random signature.
In another broad form the present invention provides coded data for disposal
on or in a surface, the coded data
including a number of coded data portions, each coded data portion being at
least partially indicative of an
identity; at least fragment of a signature, the signature being a digital
signature of at least part of the identity;
and, a position of the coded data on the surface.
Optionally each coded data portion is at least partially indicative of a data
portion identity, the data portion
identity being unique for each coded data portion, the data portion identity
being indicative of the position.
Optionally the coded data is disposed on or in the surface using a layout, the
layout being indicative of, for
each data portion identity, the position of the corresponding coded data
portion.
Optionally the signature is generated using RSA encryption.
BRIEF DESCRIPTION OF THE DRAWINGS
An example of the present invention will now be described with reference to
the accompanying drawings, in
which: -
Figure 1 is an example of a document including Hyperlabel encoding;
Figure 2 is an example of a system for interacting with the Hyperlabel
document of Figure 1;
Figure 3 is a further example of system for interacting with the Hyperlabel
document of Figure 1;
Figure 4. is a first example of a tag structure;
Figure 5. is an example of a symbol unit cell for the tag structure of Figure
4;
Figure 6. is an example of an array of the symbol unit cells of Figure 5;
Figure 7. is an example of symbol bit ordering in the unit cells of Figure 5;
Figure 8. is an example of the tag structure of Figure 4 with every bit set;
Figure 9. is an example of tag types within a tag group for the tag structure
of Figure 4;
Figure 10. is an example of continuous tiling of the tag groups of Figure 9;
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-53-
Figure 11. is an example of the orientation-indicating cyclic position
codeword R for the tag group of Figure
4;
Figure 12. is an example of a local codeword A for the tag group of Figure 4;
Figure 13. is an example of distributed codewords B, C, D and E, for the tag
group of Figure 4;
Figure 14. is an example of a layout of complete tag group;
Figure 15. is an example of a code word for the tag group of Figure 4;
Figure 16. is an example of an alternative tag group for the tag structure of
Figure 4;
Figure 17. is a second example of a tag structure;
Figure 18. is a third example of a tag structure;
Figure 19 is an example of an item signature object model;
Figure 20 is an example of Hyperlabel tags applied to a pharmaceutical item;
Figure 21 is an example of a pharmaceutical distribution process;
Figure 22. is an example of Scanning at Retailer interactions;
Figure 23. is an example of Online Scanning interaction detail;
Figure 24. is an example of Offline Scanning interaction details;
Figure 25. is an example of netpage Pen Scanning interactions;
Figure 26. is an example of netpage Pen Scanning interaction details;
Figure 27. is an example of a Hyperlabel tag class diagram;
Figure 28. is an example of a item ID class diagram
Figure 29. is an example of a pharmaceutical ID class diagram
Figure 30. is an example of an Object Description, ownership and aggregation
class diagram;
Figure 31. is an example of an Object Scanning History class diagram;
Figure 32. is an example of scanner class disgram;
Figure 33. is an example of an object ID hot list diagram;
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-54-
Figure 34. is an example of a valid ID range class diagram;
Figure 35. is an example of Public Key List class diagram;
Figure 36. is an example of a Trusted Authenticator class diagram;
Figure 37. is an example of Tagging and Tracking Object Management;
Figure 38. is an example of a Hyperlabel supermarket checkout;
Figure 39. is an example of a cash register;
Figure 40. is an example of a handheld validity scanner.
DETAILED DESCRIPTION OF THE DRAWINGS
The Netpage surface coding consists of a dense planar tiling of tags. Each tag
encodes its own location in the
plane. Each tag also encodes, in conjunction with adjacent tags, an identifier
of the region containing the tag.
In the Netpage system, the region typically corresponds to the entire extent
of the tagged surface, such as one
side of a sheet of paper.
Hyperlabel is the adaptation of the Netpage tags for use in unique item
identification for a wide variety of
applications, including security document protection, object tracking,
pharmaceutical security, supermarket
automation, interactive product labels, web-browsing from printed surfaces,
paper based email, and many
others.
Using MemjetT"' digital printing technology (which is the subject of a number
of pending US patent
applications including USSN 10/407,212), Hyperlabel tags are printed over
substantially an entire surface,
such as a security document, bank note, or pharmaceutical packaging, using
infrared (IR) ink. By printing the
tags in infrared-absorptive ink on any substrate which is infrared-reflective,
the near-infrared wavelengths,
and hence the tags are invisible to the human eye but are easily sensed by a
solid-state image sensor with an
appropriate filter. This allows machine readable information to be encoded
over a large portion of the note or
other surface, with no visible effect on the original note text or graphics
thereon. A scanning laser or image
sensor can read the tags on any part of the surface to performs associated
actions, such as validating each
individual note or item.
An example of such a hyperlabel encoded document, is shown in Figure 1. In
this example, the hyperlabel
document consists of graphic data 2 printed using visible ink, and coded data
3 formed from hyperlabel tags 4.
The document includes an interactive element 6 defined by a zone 7 which
corresponds to the spatial extent of
a corresponding graphic 8. In use, the tags encode tag data including an ID.
By sensing at least one tag, and
determining and interpreting the encoded ID using an appropriate system, this
allows the associated actions to
be performed.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-55-
In one example, a tag map is used to define a layout of the tags on the
hyperlabel document based on the ID
encoded within the tag data. The ID can also be used to reference a document
description which describes the
individual elements of the hyperlabel document, and in particular describes
the type and spatial extent (zone)
of interactive elements, such as a button or text field. Thus, in this
example, the element 6 has a zone 7 which
corresponds to the spatial extent of a corresponding graphic 8. This allows a
computer system to interpret
interactions with the hyperlabel document.
In position indicating techniques, the ID encoded within the tag data of each
tag allows the exact position of
the tag on the hyperlabel document to be determined from the tag map. The
position can then be used to
determine whether the sensed tag is positioned in a zone of an interactive
element from the document
description.
In object indicating techniques, the ID encoded within the tag data allows the
presence of the tag in a region
of the document to be determined from the tag map (the relative position of
the tag within the region may also
be indicated). In this case, the document description can be used to determine
whether the region corresponds
to the zone of an interactive element.
An example of this process will now be described with reference to Figures 2
and 3 which show how a
sensing device in the form of a netpage or hyperlabel pen 101, which interacts
with the coded data on a
printed hyperlabel document 1, such as a security document, label, product
packaging or the like.
The hyperlabel pen 101 senses a tag using an area image sensor and detects tag
data. The hyperlabel pen 101
uses the sensed coded data to generate interaction data which is transmitted
via a short-range radio link 9 to a
relay 44, which may form part of a computer 75 or a printer 601. The relay
sends the interaction data, via a
network 19, to a document server 10, which uses the ID to access the document
description, and interpret the
interaction. In appropriate circumstances, the document server sends a
corresponding message to an
application server 13, which can then perform a corresponding action.
In an alternative embodiment, the PC, Web terminal, netpage printer or relay
device may communicate
directly with local or remote application software, including a local or
remote Web server. Relatedly, output is
not limited to being printed by the netpage printer. It can also be displayed
on the PC or Web terminal, and
further interaction can be screen-based rather than paper-based, or a mixture
of the two.
Typically hyperlabel pen users register with a registration server 11, which
associates the user with an
identifier stored in the respective hyperlabel pen. By providing the sensing
device identifier as part of the
interaction data, this allows users to be identified, allowing transactions or
the like to be performed.
Hyperlabel documents are generated by having an ID server generate an ID which
is transferred to the
document server 10. The document server 10 determines a document description
and then records an
association between the document description and the ID, to allow subsequent
retrieval of the document
description using the ID.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-56-
The ID is then used to generate the tag data, as will be described in more
detail below, before the document is
printed by the hyperlabel printer 601, using the page description and the tag
map.
Each tag is represented by a pattern which contains two kinds of elements. The
first kind of element is a
target. Targets allow a tag to be located in an image of a coded surface, and
allow the perspective distortion of
the tag to be inferred. The second kind of element is a macrodot. Each
macrodot encodes the value of a bit by
its presence or absence.
The pattern is represented on the coded surface in such a way as to allow it
to be acquired by an optical
imaging system, and in particular by an optical system with a narrowband
response in the near-infrared. The
pattern is typically printed onto the surface using a narrowband near-infrared
ink.
In the Hyperlabel system the region typically corresponds to the surface of an
entire product item, or to a
security document, and the region ID corresponds to the unique item ID. For
clarity in the following
discussion we refer to items and item IDs (or simply IDs), with the
understanding that the item ID
corresponds to the region ID.
The surface coding is designed so that an acquisition field of view large
enough to guarantee acquisition of an
entire tag is large enough to guarantee acquisition of the ID of the region
containing the tag. Acquisition of
the tag itself guarantees acquisition of the tag's two-dimensional position
within the region, as well as other
tag-specific data. The surface coding therefore allows a sensing device to
acquire a region ID and a tag
position during a purely local interaction with a coded surface, e.g. during a
"click" or tap on a coded surface
with a pen.
A wide range of different tag structures can be used, and some examples will
now be described.
FIRST EXAMPLE TAG STRUCTURE
Figure 4 shows the structure of a complete tag. Each of the four black circles
is a target. The tag, and the
overall pattern, has four-fold rotational symmetry at the physical level.
Each square region represents a symbol, and each symbol represents four bits
of information.
Figure 5 shows the structure of a symbol. lt contains four macrodots, each of
which represents the value of
one bit by its presence (one) or absence (zero).
The macrodot spacing is specified by the parameter s throughout this document.
It has a nominal value of
143 m, based on 9 dots printed at a pitch of 1600 dots per inch. However, it
is allowed to vary by 10%
according to the capabilities of the device used to produce the pattern.
Figure 6 shows an array of nine adjacent symbols. The macrodot spacing is
uniform both within and between
symbols.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-57-
Figure 7 shows the ordering of the bits within a symbol. Bit zero is the least
significant within a symbol; bit
three is the most significant. Note that this ordering is relative to the
orientation of the symbol. The orientation
of a particular symbol within the tag is indicated by the orientation of the
label of the symbol in the tag
diagrams. In general, the orientation of all symbols within a particular
segment of the tag have the same
orientation, consistent with the bottom of the symbol being closest to the
centre of the tag.
Only the macrodots are part of the representation of a symbol in the pattern.
The square outline of a symbol is
used in this document to more clearly elucidate the structure of a tag. Figure
8, by way of illustration, shows
the actual pattern of a tag with every bit set. Note that, in practice, every
bit of a tag can never be set.
A macrodot is nominally circular with a nominal diameter of (5/9)s. However,
it is allowed to vary in size by
10% according to the capabilities of the device used to produce the pattern.
A target is nominally circular with a nominal diameter of (17/9)s. However, it
is allowed to vary in size by
10% according to the capabilities of the device used to produce the pattern.
The tag pattern is allowed to vary in scale by up to 10% according to the
capabilities of the device used to
produce the pattern. Any deviation from the nominal scale is recorded in the
tag data to allow accurate
generation of position samples.
Each symbol shown in the tag structure in Figure 4 has a unique label. Each
label consists an alphabetic prefix
and a numeric suffix.
TAG GROUP
Tags are arranged into tag groups. Each tag group contains four tags arranged
in a square. Each tag therefore
has one of four possible tag types according to its location within the tag
group square. The tag types are
labelled 00, 10, 01 and 11, as shown in Figure 9.
Each tag in the tag group is rotated as shown in the figure, i.e. tag type 00
is rotated 0 degrees, tag type 10 is
rotated 90 degrees, tag type 11 is rotated 180 degrees, and tag type 01 is
rotated 270 degrees.
Figure 10 shows how tag groups are repeated in a continuous tiling of tags.
The tiling guarantees.the any set
of four adjacent tags contains one tag of each type.
ORIENTATION-INDICATING CYCLIC POSITION CODE
The tag contains a 24-ary (4, 1) cyclic position codeword which can be decoded
at any of the four possible
orientations of the tag to determine the actual orientation of the tag.
Symbols which are part of the cyclic
position codeword have a prefix of "R" and are numbered 0 to 3 in order of
increasing significance.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-58-
The cyclic position codeword is (0, 7, 9, E16). Note that it only uses four
distinct symbol values, even though a
four-bit symbol has sixteen possible values. During decoding, any unused
symbol value should, if detected, be
treated as an erasure. To maximise the probability of low-weight bit error
patterns causing erasures rather than
symbol errors, the symbol values are chosen to be as evenly spaced on the
hypercube as possible.
The minimum distance of the cyclic position code is 4, hence its error-
correcting capacity is one symbol in the
presence of up to one erasure, and no symbols in the presence of two or more
erasures.
The layout of the orientation-indicating cyclic position codeword is shown in
Figure 11.
LOCAL CODEWORD
The tag locally contains one complete codeword which is used to encode
information unique to the tag. The
codeword is of a punctured 24-ary (13, 7) Reed-Solomon code. The tag therefore
encodes up to 28 bits of
information unique to the tag.
The layout of the local codeword is shown in Figure 12.
DISTRIBUTED CODEWORDS
The tag also contains fragments of four codewords which are distributed across
the four adjacent tags in a tag
group and which are used to encode information common to a set of contiguous
tags. Each codeword is of a
24-ary (15, 11) Reed-Solomon code. Any four adjacent tags therefore together
encode up to 176 bits of
information common to a set of contiguous tags.
The layout of the four complete codewords, distributed across the four
adjacent tags in a tag group, is shown
in Figure 13. The order of the four tags in the tag group in Figure 13 is the
order of the four tags in Figure 9.
Figure 14 shows the layout of a complete tag goup.
REED-SOLOMON ENCODING
LOCAL CODEWORD
The local codeword is encoded using a punctured 24 -ary (13, 7) Reed-Solomon
code. The code encodes 28
data bits (i.e. seven symbols) and 24 redundancy bits (i.e. six symbols) in
each codeword. Its error-detecting
capacity is six symbols. Its error-correcting capacity is three symbols.
As shown in Figure 15, codeword coordinates are indexed in coefficient order,
and the data bit ordering
follows the codeword bit ordering.
The code is a 2 -ary (15, 7) Reed-Solomon code with two redundancy coordinates
removed. The removed
coordinates are the most significant redundancy coordinates.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-59-
The code has the following primitive polynominal:
(EQ 1)
P(x) = x4+x+ 1
The code has the following generator polynominal:
(EQ 2)
g(x) = (x+ (X)(x+ az)... (x + a8)
DISTRIBUTED CODEWORDS
The distributed codewords are encoded using a 24-ary (15, 11) Reed-Solomon
code. The code encodes 44 data
bits (i.e. eleven symbols) and 16 redundancy bits (i.e. four symbols) in each
codeword. Its error-detecting
capacity is four symbols. Its error-correcting capacity is two symbols.
Codeword coordinates are indexed in coefficient order, and the data bit
ordering follows the codeword bit
ordering.
The code has the same primitive polynominal as the local codeword code.
The code has the following generator polynominal:
(EQ 3)
g(x) = (x + (X) (x + a2 ) .. . (x + a4 )
TAG COORDINATE SPACE
The tag coordinate space has two orthogonal axes labelled x and y
respectively. When the positive x axis
points to the right then the positive y axis points down.
The surface coding does not specify the location of the tag coordinate space
origin on a particular tagged
surface, nor the orientation of the tag coordinate space with respect to the
surface. This information is
application-specific. For example, if the tagged surface is a sheet of paper,
then the application which prints
the tags onto the paper may record the actual offset and orientation, and
these can be used to normalise any
digital ink subsequently captured in conjunction with the surface.
The position encoded in a tag is defined in units of tags. By convention, the
position is taken to be the position
of the centre of the target closest to the origin.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-60-
TAG INFORMATION CONTENT
FIELD DEFINITIONS
Table I defines the information fields embedded in the surface coding. Table 2
defines how these fields map
to codewords.
Table 1. Field definitions
field width description
(bits)
per tag
coordinate 9 or 13 The unsigned x coordinate of the tag allows
aximum coordinate values of approximately 0.9m
and 14m respectively.
coordinate 9 or 13 The unsigned y coordinate of the tag allows
aximum coordinate values of approximately 0.9m
and 14m respectively
active area flag I ' l' indicates whether the area (the diameter of the
area ntered on the tag, is nominally 5 times the
diagonal size of the tag) immediately surrounding the
ag intersects an active area
data fragment flag 1 flag indicating whether a data fragment is present
(see next field).
' 1' indicates the presence of a data fragment.
If the data fragment is present then the width of the x
and y coordinate fields is 9. If it is absent then the
idth is 13.
data fragment 0 or 8 fragment of an embedded data stream.
per tag group
(i.e. per region)
encoding format 8 The format of the encoding.
0: the present encoding
Other values are reserved.
egion flags 8 Flags controlling the interpretation of region data.
0: region ID is an EPC
1: region has signature
: region has embedded data
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-61-
3: embedded data is signature
Other bits are reserved and must be zero.
ag size ID 8 The ID of the tag size.
0: the present tag size the nominal tag size is
1.7145mm, based on 1600dpi, 9 dots per
macrodot, and 12 macrodots per tag
Other values are reserved.
egion ID 96 The ID of the region containing the tags.
signature 36 The signature of the region.
igh-order coordinate 4 The width of the high-order part of the x and y
idth (w) coordinates of the tag.
'gh-order x coordinate 0 to 15 igh-order part of the x coordinate of the tag
expands the maximum coordinate values to
approximately 2.4km and 38km respectively
igh-order y coordinate 0 to 15 igh-order part of the y coordinate of the tag
expands
the maximum coordinate values to
approximately 2.4km and 38km respectively.
CRC 16 CRC of tag group data.
An active area is an area within which any captured input should be
immediately forwarded to the
corresponding hyperlabel server for interpretation. This also allows the
hyperlabel server to signal to the user
that the input has had an immediate effect. Since the server has access to
precise region definitions, any active
area indication in the surface coding can be imprecise so long as it is
inclusive.
The width of the high-order coordinate fields, if non-zero, reduces the width
of the signature field by a
corresponding number of bits. Full coordinates are computed by prepending each
high-order coordinate field
to its corresponding coordinate field.
Table 2. Mapping of fields to codewords
codeword codeword field width field
bits bits
12:0 coordinate 13 all
12:9 data fragment 3:0
5:13 coordinate 13 all
5:22 data fragment 7:4
6 active area flag 1 all
7 data fragment flag 1 all
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-62-
7:0 encoding format 8 all
15:8 egion flags 8 all
3:16 ag size ID 8 all
39:24 CRC 16 all
13:40 gh-order coordinate 3:0
idth (w)
C 35:0 signature 36 all
(35-w):(36-2w) gh-order x coordinate all
35:(36-w) gh-order y coordinate all
13:36 egion ID 8 7:0
3:0 egion ID 4 51:8
13:0 egion ID 14 95:52
EMBEDDED DATA
If the "region has embedded data" flag in the region flags is set then the
surface coding contains embedded
data. The data is encoded in multiple contiguous tags' data fragments, and is
replicated in the surface coding
as many times as it will fit.
The embedded data is encoded in such a way that a random and partial scan of
the surface coding containing
the embedded data can be sufficient to retrieve the entire data. The scanning
system reassembles the data from
retrieved fragments, and reports to the user when sufficient fragments have
been retrieved without error.
As shown in Table 3, a 200-bit data block encodes 160 bits of data. The block
data is encoded in the data
fragments of a contiguous group of 25 tags arranged in a 5x5 square. A tag
belongs to a block whose integer
coordinate is the tag's coordinate divided by 5. Within each block the data is
arranged into tags with
increasing x coordinate within increasing y coordinate.
A data fragment may be missing from a block where an active area map is
present. However, the missing data
fragment is likely to be recoverable from another copy of the block.
Data of arbitrary size is encoded into a superblock consisting of a contiguous
set of blocks arranged in a
rectangle. The size of the superblock is encoded in each block. A block
belongs to a superblock whose integer
coordinate is the block's coordinate divided by the superblock size. Within
each superblock the data is
arranged into blocks with increasing x coordinate within increasing y
coordinate.
The superblock is replicated in the surface coding as many times as it will
fit, including partially along the
edges of the surface coding.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-63-
The data encoded in the superblock may include more precise type information,
more precise size
information, and more extensive error detection and/or correction data.
Table 3. Embedded data block
field width description
data type 8 The type of the data in the superblock.
alues include:
0: type is controlled by region flags
1: MIME
Other values are TBA.
superblock width 8 The width of the superblock, in blocks.
superblock height 8 The height of the superblock, in blocks.
data 160 The block data.
CRC 16 CRC of the block data.
otal 200
It will be appreciated that any form of embedded data may be used, including
for example, text, image, audio,
video data, such as product information, application data, contact data,
business card data, and directory data.
REGION SIGNATURES
If the "region has signature" flag in the region flags is set then the
signature field contains a signature with a
maximum width of 36 bits. The signature is typically a random number
associated with the region ID in a
secure database. The signature is ideally generated using a truly random
process, such as a quantum process,
or by distilling randomness from random events.
In an online environment the signature can be validated, in conjunction with
the region ID, by querying a
server with access to the secure database.
If the "region has embedded data" and "embedded data is signature" flags in
the region flags are set then the
surface coding contains a 160-bit cryptographic signature of the region ID.
The signature is encoded in a one-
block superblock.
In an online environment any number of signature fragments can be used, in
conjunction with the region ID
and optionally the random signature, to validate the signature by querying a
server with knowledge of the full
signature or the corresponding private key.
In an offline (or online) environment the entire signature can be recovered by
reading multiple tags, and can
then be validated using the corresponding public signature key.
Signature verification is discussed in more detail below.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-64-
MIME DATA
If the embedded data type is "MIME" then the superblock contains Multipurpose
Internet Mail Extensions
(MIME) data according to RFC 2045 (Freed, N., and N. Borenstein, "Multipurpose
Internet Mail Extensions
(MIME) - Part One: Format of Internet Message Bodies", RFC 2045, November
1996), RFC 2046 (Freed, N.,
and N. Borenstein, "Multipurpose Internet Mail Extensions (MIME) - Part Two:
Media Types", RFC 2046,
November 1996 ) and related RFCs. The MIME data consists of a header followed
by a body. The header is
encoded as a variable-length text string preceded by an 8-bit string length.
The body is encoded as a variable-
length type-specific octet stream preceded by a 16-bit size in big-endian
format.
The basic top-level media types described in RFC 2046 include text, image,
audio, video and application.
RFC 2425 (Howes, T., M. Smith and F. Dawson, "A MIME Content-Type for
Directory Information", RFC
2045, September 1998) and RFC 2426 (Dawson, F., and T. Howes, "vCard MIME
Directory Profile", RFC
2046, September 1998) describe a text subtype for directory information
suitable, for example, for encoding
contact information which might appear on a business card.
ENCODING AND PRINTING CONSIDERATIONS
The Print Engine Controller (PEC) (which is the subject of a number of pending
US patent applications,
including: 09/575,108; 10/727,162; 09/575,110; 09/607,985; 6,398,332;
6,394,573; 6,622,923) supports the
encoding of two fixed (per-page) 24 -ary (15,7) Reed-Solomon codewords and
four variable (per-tag) 24-ary
(15,7) Reed-Solomon codewords, although other numbers of codewords can be used
for different schemes.
Furthermore, PEC supports the rendering of tags via a rectangular unit cell
whose layout is constant (per
page) but whose variable codeword data may vary from one unit cell to the
next. PEC does not allow unit
cells to overlap in the direction of page movement.
A unit cell compatible with PEC contains a single tag group consisting of four
tags. The tag group contains a
single A codeword unique to the tag group but replicated four times within the
tag group, and four unique B
codewords. These can be encoded using five of PEC's six supported variable
codewords. The tag group also
contains eight fixed C and D codewords. One of these can be encoded using the
remaining one of PEC's
variable codewords, two more can be encoded using PEC's two fixed codewords,
and the remaining five can
be encoded and pre-rendered into the Tag Format Structure (TFS) supplied to
PEC.
PEC imposes a limit of 32 unique bit addresses per TFS row. The contents of
the unit cell respect this limit.
PEC also imposes a limit of 384 on the width of the TFS. The contents of the
unit cell respect this limit.
Note that for a reasonable page size, the number of variable coordinate bits
in the A codeword is modest,
making encoding via a lookup table tractable. Encoding of the B codeword via a
lookup table may also be
possible. Note that since a Reed-Solomon code is systematic, only the
redundancy data needs to appear in the
lookup table.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 65 -
IMAGING AND DECODING CONSIDERATIONS
The minimum imaging field of view required to guarantee acquisition of an
entire tag has a diameter of 39.6s,
i.e.
(2 x (12+2))j2-s
allowing for arbitrary alignment between the surface coding and the field of
view. Given a macrodot spacing
ofl43 m, this gives a required field of view of 5.7mm.
Table 4 gives pitch ranges achievable for the present surface coding for
different sampling rates, assuming an
image sensor size of 128 pixels.
Table 4
Pitch ranges achievable for present surface coding for different sampling
rates, computed
using Optimize Hyperlabel Optics; dot pitch = I600dpi, macrodot pitch = 9
dots, viewing
distance = 30mm, nib-to-FOV separation = 1mm, image sensor size = 128 pixels
sainpling rate pitch range -40 to +49
5 27 to +36
3 lO to +18
For the surface coding above, the decoding sequence is as follows:
= locate targets of complete tag
= infer perspective transform from targets
= sample cyclic position code
= decode cyclic position code
= determine orientation from cyclic position code
= sample and decode local Reed-Solomon codeword
= determine tag x-y location
= infer 3D tag transform from oriented targets
= determine nib x-y location from tag x-y location and 3D transform
= determine active area status of nib location with reference to active area
map
= generate local feedback based on nib active area status
= determine tag type
= sample distributed Reed-Solomon codewords (modulo window alignment, with
reference to tag type)
= decode distributed Reed-Solomon codewords
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-66-
= verify tag group data CRC
= on decode error flag bad region ID sample
= determine encoding type, and reject unknown encoding
= determine region flags
= determine region ID
= encode region ID, nib x-y location, nib active area status in digital ink
= route digital ink based on region flags
Region ID decoding need not occur at the same rate as position decoding and
decoding of a codeword can be
avoided if the codeword is found to be identical to an already-known good
codeword.
If the high-order coordinate width is non-zero, then special care must be
taken on boundaries between tags
where the low-order x or y coordinate wraps, otherwise codeword errors may be
introduced. If wrapping is
detected from the low-order x or y coordinate (i.e. it contains all zero bits
or all one bits), then the
corresponding high-order coordinate can be adjusted before codeword decoding.
In the absence of genuine
symbol errors in the high-order coordinate, this will prevent the inadvertent
introduction of codeword errors.
ALTERNATIVE TAG ARRANGEMENTS
It will be appreciated that a range of different tag layouts and tag
structures can be utilised.
For example, the tag group shown in Figure 9 can be replaced with the tag
group shown in Figure 16, in
which the tags are not rotated relative to each other. Figure 17 shows an
arrangement that utilises a six-fold
rotational symmetry at the physical level, with each diamond shape
representing a respective symbol. Figure
18 shows a version of the tag in which the tag is expanded to increase its
data capacity by adding additional
bands of symbols about its circumference.
The use of these alternative tag structures, including associated encoding
considerations, is described shown
in more detail in the copending patent application numbers [we will need to
include a docket number here for
HYG, HYT cases], the contents of which is incorporated herein by cross
reference.
SECURITY DISCUSSION
As described above, authentication relies on verifying the correspondence
between data and a signature of that
data. The greater the difficulty in forging a signature, the greater the
trustworthiness of signature-based
authentication.
The item ID is unique and therefore provides a basis for a signature. If
online authentication access is
assumed, then the signature may simply be a random number associated with the
item ID in an authentication
database accessible to the trusted online authenticator. The random number may
be generated by any suitable
method, such as via a deterministic (pseudo-random) algorithm, or via a
stochastic physical process. A keyed
hash or encrypted hash may be preferable to a random number since it requires
no additional space in the
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-67-
authentication database. However, a random signature of the same length as a
keyed signature is more secure
than the keyed signature since it is not susceptible to key attacks.
Equivalently, a shorter random signature
confers the same security as a longer keyed signature.
In the limit case no signature is actually required, since the mere presence
of the item ID in the database
indicates authenticity. However, the use of a signature limits a forger to
forging items he has actually sighted.
To prevent forgery of a signature for an unsighted ID, the signature must be
large enough to make exhaustive
search via repeated accesses to the online authenticator intractable. If the
signature is generated using a key
rather than randomly, then its length must also be large enough to prevent the
forger from deducing the key
from known ID-signature pairs. Signatures of a few hundred bits are considered
secure, whether generated
using private or secret keys.
While it may be practical to include a reasonably secure random signature in a
tag (or local tag group),
particularly if the length of the ID is reduced to provide more space for the
signature, it may be impractical to
include a secure ID-derived signature in a tag. To support a secure ID-derived
signature, we can instead
distribute fragments of the signature across multiple tags. If each fragment
can be verified in isolation against
the ID, then the goal of supporting authentication without increasing the
sensing device field of view is
achieved. The security of the signature can still derive from the full length
of the signature rather than from
the length of a fragment, since a forger cannot predict which fragment a user
will randomly choose to verify.
A trusted authenticator can always perform fragment verification since they
have access to the key and/or the
full stored signature, so fragment verification is always possible when online
access to a trusted authenticator
is available.
Fragment verification requires that we prevent brute force attacks on
individual fragments, otherwise a forger
can determine the entire signature by attacking each fragment in turn. A brute
force attack can be prevented
by throttling the authenticator on a per-ID basis. However, if fragments are
short, then extreme throttling is
required. As an alternative to throttling the authenticator, the authenticator
can instead enforce a limit on the
number of verification requests it is willing to respond to for a given
fragment number. Even if the limit is
made quite small, it is unlikely that a normal user will exhaust it for a
given fragment, since there will be
many fragments available and the actual fragment chosen by the user can vary.
Even a limit of one can be
practical. More generally, the limit should be proportional to the size of the
fragment, i.e. the smaller the
fragment the smaller the limit. Thus the experience of the user would be
somewhat invariant of fragment size.
Both throttling and enforcing fragment verification limits imply serialisation
of requests to the authenticator.
Enforcing fragment verification limits further requires the authenticator to
maintain a per-fragment count of
satisfied verification requests.
A brute force attack can also be prevented by concatenating the fragment with
a random signature encoded in
the tag. While the random signature can be thought of as protecting the
fragment, the fragment can also be
thought of as simply increasing the length of the random signature and hence
increasing its security.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-68-
Fragment verification may be made more secure by requiring the verification of
a minimum number of
fragments simultaneously.
Fragment verification requires fragment identification. Fragments may be
explicitly numbered, or may more
economically be identified by the two-dimensional coordinate of their tag,
modulo the repetition of the
signature across a continuous tiling of tags.
The limited length of the ID itself introduces a further vulnerability.
Ideally it should be at least a few hundred
bits. In the Netpage surface coding scheme it is 96 bits or less. To overcome
this the ID may be padded. For
this to be effective the padding must be variable, i.e. it must vary from one
ID to the next. Ideally the padding
is simply a random number, and must then be stored in the authentication
database indexed by ID. If the
padding is deterministically generated from the ID then it is worthless.
Offline authentication of secret-key signatures requires the use of a trusted
offline authentication device. The
QA chip (which is the subject of a number of pending US patent applications,
including 09/112,763;
09/112,762; 09/112,737; 09/112,761; 09/113,223) provides the basis for such a
device, although of limited
capacity. The QA chip can be programmed to verify a signature using a secret
key securely held in its internal
memory. In this scenario, however, it is impractical to support per-ID
padding, and it is impractical even to
support more than a very few secret keys. Furthermore, a QA chip programmed in
this manner is susceptible
to a chosen-message attack. These constraints limit the applicability of a QA-
chip-based trusted offline
authentication device to niche applications.
In general, despite the claimed security of any particular trusted offline
authentication device, creators of
secure items are likely to be reluctant to entrust their secret signature keys
to such devices, and this is again
likely to limit the applicability of such devices to niche applications.
By contrast, offline authentication of public-key signatures (i.e. generated
using the corresponding private
keys) is highly practical. An offline authentication device utilising public
keys can trivially hold any number
of public keys, and may be designed to retrieve additional public keys on
demand, via a transient online
connection, when it encounters an ID for which it knows it has no
corresponding public signature key.
Untrusted offline authentication is likely to be attractive to most creators
of secure items, since they are able
to retain exclusive control of their private signature keys.
A disadvantage of offline authentication of a public-key signature is that the
entire signature must be acquired
from the coding, violating our desire to support authentication with a minimal
field of view. A corresponding
advantage of offline authentication of a public-key signature is that access
to the ID padding is no longer
required, since decryption of the signature using the public signature key
generates both the ID and its
padding, and the padding can then be ignored. A forger can not take advantage
of the fact that the padding is
ignored during offline authentication, since the padding is not ignored during
online authentication.
Acquisition of an entire distributed signature is not particularly onerous.
Any random or linear swipe of a
hand-held sensing device across a coded surface allows it to quickly acquire
all of the fragments of the
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-69-
signature. The sensing device can easily be programmed to signal the user when
it has acquired a full set of
fragments and has completed authentication. A scanning laser can also easily
acquire all of the fragments of
the signature. Both kinds of devices may be programmed to only perform
authentication when the tags
indicate the presence of a signature.
Note that a public-key signature may be authenticated online via any of its
fragments in the same way as any
signature, whether generated randomly or using a secret key. The trusted
online authenticator may generate
the signature on demand using the private key and ID padding, or may store the
signature explicitly in the
authentication database. The latter approach obviates the need to store the ID
padding.
Note also that signature-based authentication may be used in place of fragment-
based authentication even
when online access to a trusted authenticator is available.
Table 5 provides a summary of which signature schemes are workable in light of
the foregoing discussion.
Table 5 Summary of workable signature schemes
encoding acquisition signature online offline
in tags from tags generation authentication authentication
ocal full andom ok Impractical to
store per ID
information
secret key Signature too ndesirable to
short to be secure store secret keys
rivate key Signature too
short to be secure
istributed fragment(s) andom ok impractical
secret key ok impractical
rivate key ok impractical
full andom ok impractical
secret key ok impracticalc
rivate key ok ok
SECURITY SPECIFICATION
Figure 19 shows an example item signature object model.
An item has an ID (X) and other details (not shown). It optionally has a
secret signature (Z). It also optionally
has a public-key signature. The public-key signature records the signature (S)
explicitly, and/or records the
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-70-
padding (P) used in conjunction with the ID to generate the signature. The
public-key signature has an
associated public-private key pair (K, L). The key pair is associated with a
one or more ranges of item IDs.
Typically issuers of security documents and pharmaceuticals will utilise a
range of IDs to identify a range of
documents or the like. Following this, the issuer will then use these details
to generate respective IDs for each
item, or document to be marked.
Authentication of the product can then be performed online or offline by
sensing the tag data encoded within
the tag, and performing the authentication using a number of different
mechanisms depending on the
situation.
Examples of the processes involved will now be described for public and
private key encryption respectively.
AUTHENTICATION BASED ON PUBLIC-KEY SIGNATURE
Setup per ID range:
= generate public-private signature key pair (K, L)
= store key pair (K, L) indexed by ID range
Setup per ID:
= generate ID padding (P)
= retrieve private signature key (L) by ID (X)
= generate signature (S) by encrypting ID (X) and padding (P) using private
key (L):
S<-- EL(X,P)
= store signature (S) in database indexed by ID (X) (and/or store padding (P))
= encode ID (X) in all tag groups
= encode signature (S) across multiple tags in repeated fashion
Online fragment-based authentication (user):
= acquire ID (X) from tags
= acquire position (x, y)i and signature fragment (Ti) from tag
= generate fragment number (i) from position (x, y)i:
i E--F[(x,y)il
= look up trusted authenticator by ID (X)
= transmit ID (X), fragment (Si) and fragment number (i) to trusted
authenticator
Online fragment-based authentication (trusted authenticator):
= receive ID (X), fragment (Si) and fragment number (i) from user
= retrieve signature (S) from database by ID (X) (or re-generate signature)
= compare received fragment (Ti) with corresponding fragment of signature (Si)
= report authentication result to user
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-71-
Offline signature-based authentication (user):
= acquire ID from tags (X)
= acquire positions (x, y)i and signature fragments (Ti) from tag
= generate fragment numbers (i) from positions (x, y)i:
i <- FL(x,y)i )
s <-- s0lslI ...Isõ-,
. generate signature (S) from (n) fragments:
= retrieve public signature key (K) by ID (X)
= decrypt signature (S) using public key (K) to obtain ID (X') and padding
(P'):
X I P' <-- DK(S)
= compare acquired ID (X) with decrypted ID (X')
= report authentication result to user
AUTHENTICATION BASED ON SECRET-KEY SIGNATURE
Setup per ID:
= generate secret (Z)
= store secret (Z) in database indexed by ID (X)
= encode ID (X) and secret (Z) in all tag groups
Online secret-based authentication (user):
= acquire ID (X) from tags
= acquire secret (Z) from tags
= look up trusted authenticator by ID
= transmit ID (X) and secret (Z) to trusted authenticator
Online secret-based authentication (trusted authenticator):
= receive ID (X) and secret (Z) from user
= retrieve secret (Z) from database by ID (X)
= compared received secret (Z') with secret (Z)
= report authentication result to user
As discussed earlier, secret-based authentication may be used in conjunction
with fragment-based
authentication.
CRYPTOGRAPHIC ALGORITHMS
When the public-key signature is authenticated offline, the user's
authentication device typically does not
have access to the padding used when the signature was originally generated.
The signature verification step
must therefore decrypt the signature to allow the authentication device to
compare the ID in the signature with
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-72-
the ID acquired from the tags. This precludes the use of algorithms which
don't perforrn the signature
verification step by decrypting the signature, such as the standard Digital
Signature Algorithm U.S.
Department of Commerce/National Institute of Standards and Technology, Digital
Signature Standard (DSS),
FIPS 186-2, 27 January 2000.
RSA encryption is described in:
= Rivest, R.L., A.Shamir, and L.Adleman, "A Method for Obtaining Digital
Signatures and Public-Key
Cryptosystems", Conununications of the ACM, Vol.21, No.2, February 1978,
pp.120-126
= Rivest, R.L., A.Shamir, and L.M.Adleman, "Cryptographic communications
system and method",
U.S. Patent 4,405,829, issued 20 September 1983
= RSA Laboratories, PKCS #1 v2.0: RSA Encryption Standard, October 1, 1998
RSA provides a suitable public-key digital signature algorithm that decrypts
the signature. RSA provides the
basis for the ANSI X9.31 digital signature standard American National
Standards Institute, ANSI X9.31-
1998, Digital Signatures Using Reversible Public Key Cryptography for the
Financial Services Industry
(rDSA), September 8, 1998. If no padding is used, then any public-key
signature algorithm can be used.
In the hyperlabel surface coding scheme the ID is 96 bits long or less. It is
padded to 160 bits prior to being
signed.
The padding is ideally generated using a truly random process, such as a
quantum process [14,15], or by
distilling randomness from random events Schneier, B., Applied Cryptography,
Second Edition, John Wiley
& Sons 1996.
In the hyperlabel surface coding scheme the random signature, or secret, is 36
bits long or less. It is also
ideally generated using a truly random process.
SECURITY TAGGING AND TRACKING
Currency, checks and other monetary documents can be tagged in order to detect
currency counterfeiting and
counter money laundering activities. The Hyperlabel tagged currency can be
validated, and tracked through
the monetary system. Hyperlabel tagged products such as pharmaceuticals can be
tagged allowing items to be
validated and tracked through the distribution and retail system.
A number of examples of the concepts of Hyperlabel security tagging and
tracking referring specifically to
bank notes and pharmaceuticals, however Hyperlabel tagging can equally be used
to securely tag and track
other products, for example, traveller's checks, demand deposits, passports,
chemicals etc.
Hyperlabel tagging, with the Netpage system, provides a mechanism for securely
validating and tracking
objects.
Hyperlabel tags on the surface of an object uniquely identify the object. Each
Hyperlabel tag contains
information including the object's unique ID, and the tag's location on the
Hyperlabel tagged surface. A
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
- 73 -
Hyperlabel tag also contains a signature fragment which can be used to
authenticate the object. A scanning
laser or image sensor can read the tags on any part of the object to identify
the object, validate the object, and
allow tracking of the object.
PHARMACEUTICAL TAGGING
An example of the protection of pharmaceuticals will now be described with
reference to the specific
protection of currency, such as bank notes, although it will be appreciated
that the techniques may be applied
to any security document.
Hyperlabel tags can be printed over the entire surface of the pharmaceutical
packaging, or only on a smaller
area of the packaging. A Hyperlabel pharmaceutical tag contains the item's
product ID and a serial number, to
uniquely identify an individual item. The product ID identifies the item's
National Drug Code (NDC) number.
The NDC number is allocated and administered by the FDA (U.S. Food and Drug
Administration) for drugs
and drug-related items and identifies the product and manufacturer.
Alternatively the tag may contain another
product ID code, such as the European International Article Numbering (EAN)
code, or EPC etc.
In this example, each hexagonal Hyperlabel currency tag is around 2.5 mm
across, and incorporates a variety
of data in the form of printed dots of infrared ink. An example of a tag
included on pharmaceutical packaging
is shown in Figure 20.
The tag may also include:
= Alignment marks (these are the larger dots in the image above)
= A code indicating that the tag is a pharmaceutical tag, as opposed to
another commercial Hyperlabel
or Hyperlabel tag
= A horizontal position code, specifying where the tag is along the packaging
= A vertical position code, specifying where the tag is across the packaging
= A cryptographic signature
= Error detection and correction bits
Each tag is unique. That is, of all tags ever to be printed on any packaging
or other document, no two valid
tags will ever be the same. The tags are designed to be easily read with low
cost scanners that can be built into
a variety of validation devices.
The pharmaceutical ID can be read by a scanner and used to look up details of
the item's lot number and
expiry date. Alternatively the lot number and expiry date may be contained in
the pharmaceutical tag to allow
off-line retrieval of this information by any scanner. The pharmaceutical ID
may also be used to access details
such as dosage and administration information, drug interactions, precautions,
contraindications, product
warnings, recall information, place of manufacture etc.
Hyperlabel currency tags can be read by any Hyperlabel scanner. These scanners
can be incorporated into a
variety of devices to facilitate authentication and tracking, as will be
described in more detail below.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-74-
TRACKING
For the purpose of tracking and item validation the manufacturer, or other
central authority, maintains a
database which tracks the location and status of all items.
Each time a pharmaceutical item is scanned its location is recorded. This
location information can be
collected in a central database allowing analysis and identification of
abnormal product movements and
detection of counterfeit pharmaceuticals.
This allows the creation of highly accurate intelligence about criminal
activity and the real-time detection of
the location of stolen or counterfeit pharmaceuticals at many locations within
the supply chain or in
distribution. For example, in the case of sophisticated forgeries where
Hyperlabel dot patterns are exactly
duplicated, there will be multiple copies of exactly forged pharmaceutical
items (at a minimum, the original
and the forgery). If multiple identical pharmaceutical items appears in
different places at the same time, all
but one of the pharmaceutical items must be a forgery. All can then be treated
as suspect.
Thus, when a transaction is performed involving pharmaceutical items, the
general process is as follows:
= a transaction is agreed
= currency is provided relating to the transaction
= the pharmaceutical item is scanned using an appropriate sensing device
= the sensing device sense at least one tag and generates predetermined data
= the predetermined data is transferred to a central government database
In this regard, the following predetermined data is automatically sent from
the scanners to the central
government currency database:
= The unique identifier for the pharmaceutical item
= The nature of pharmaceutical item
= validity data
= The serial number of the scanner
= The time and date of the scan
= The physical location of the scanner at the time the scan was taken (for
fixed scanners this is
automatic, and for mobile scanners the physical location is determined using a
GPS tracker)
= The network location of the scanner
= The identity of the person making reportable transactions
Thus, Hyperlabel technology makes it possible to build databases containing
the history of all pharmaceutical
items produced, and it allows them to be tracked through to distribution and
use by the consumer. The data
collected can be used to build up flow maps based on the validation data
received, and its presence will
provide a powerful tool for law enforcement agencies to combat pharmaceutical
item counterfeiting.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-75-
There are also a large number of transactions involved - several hundred
million per day. These are within the
capability of conventional distributed transaction processing systems.
However, the Hyperlabel currency
system can be implemented at substantially lower cost by using new generation
database systems that perform
transactions in semiconductor memory, instead of disk drives. These
transactions can then be continually
streamed to disk as a background 'backup' task. Such systems are likely to be
sufficiently mature by the time
that a Hyperlabel based currency tracking system comes on-line that they will
be a viable choice.
As well as basic tracking and validation functions, the database system may
have the following additional
features:
= Indication of abnormal pharmaceutical item movement patterns within the
system
= The provision of pharmaceutical item demand forecasts
= Data mining features that could be used to detect and prosecute
counterfeiters
= Neural network based fraud detection
= Geographic trends identification
A central database maintains up-to-date information on valid object IDs, an
object ID hotlist (for all suspect
object IDs), and a list of public keys corresponding to object IDs. The
central server also maintains an object
scanning history to track an object's movements. Each time an object is
scanned, its timestamped location is
recorded. If known, the details of the object owner may also be recorded. This
information may be known
particularly in the case of large transactions. This object scanning history
data can be used to detect illegal
product movements, for example, the illegal import of a pharmaceutical. It can
also be used to detect
abnormal or suspicious product movements which may be indicative of product
counterfeiting.
If an object is known to be stolen it can be immediately added to an object ID
hotlist on the central server.
This hotlist is automatically distributed to (or becomes accessible to) all on-
line scanners, and will be
downloaded to all off-line scanners on their next update. In this way the
stolen status is automatically and
rapidly disseminated to a huge number of outlets. Similarly, if an object is
in any other way suspect it can be
added to the hotlist so that its status is flagged to the person scanning the
object.
An on-line scanner has instant access to the central server to allow checking
of each object ID at the time of
scanning. The object scanning history is also updated at the central server at
the time the object is scanned.
An off-line scanner stores object status data internally to allow validation
of a scanned object. The object
status data includes valid ID range lists, an object ID hotlist, a public key
list, and an object scanning history.
Each time an object is scanned the details are recorded in the object scanning
history. The object status data is
downloaded from the central server, and the object scanning history is
uploaded to the central server, each
time the scanner connects.
A mobile scanner's location can be provided to the application by the scanner,
if it is GPS-equipped.
Alternatively the scanner's location can be provided by the network through
which it communicates.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-76-
For example, if the hand-held scanner uses the mobile phone network, the
scanner's location can be provided
by the mobile phone network provider. There are a number of location
technologies available. One is Assisted
Global Positioning System (A-GPS). This requires a GPS-equipped handset, which
receives positioning
signals from GPS satellites. The phone network knows the approximate location
of the handset (in this case
the handset is also the scanner) from the nearest cell site. Based on this,
the network tells the handset which
GPS satellites to use in its position calculations. Another technology, which
does not require the device to be
GPS-equipped, is Uplink Time Difference of Arrival (U-TDOA). This determines
the location of a wireless
handset, using a form of triangulation, by comparing the time it takes a
wireless handset's signal to reach
several Location Measurement Units (LMUs) installed at the network's cell
sites. The handset location is then
calculated based on the differences in arrival times of the three (or more)
signals.
AUTHENTICATION
Each object ID has a signature. Limited space within the Hyperlabel tag
structure makes it impractical to
include a full cryptographic signature in a tag so signature fragments are
distributed across multiple tags. A
smaller random signature, or secret, can be included in a tag.
To avoid any vulnerability due to the limited length of the object ID, the
object ID is padded, ideally with a
random number. The padding is stored in an authentication database indexed by
object ID. The authentication
database may be managed by the manufacturer, or it may be managed by a third-
party trusted authenticator.
Each Hyperlabel tag contains a signature fragment and each fragment (or a
subset of fragments) can be
verified, in isolation, against the object ID. The security of the signature
still derives from the full length of
the signature rather than from the length of the fragment, since a forger
cannot predict which fragment a user
will randomly choose to verify.
Fragment verification requires fragment identification. Fragments may be
explicitly numbered, or may by
identified by the two-dimensional coordinate of their tag, modulo the
repetition of the signature across
continuous tiling of tags.
Note that a trusted authenticator can always perform fragment verification, so
fragment verification is always
possible when on-line access to a trusted authenticator is available.
ESTABLISHING AUTHENTICATION DATABASE
Prior to allocating a new range of IDs, some setup tasks are required to
establish the authentication database.
For each range of IDs a public-private signature key pair is generated and the
key pair is stored in the
authentication database, indexed by ID range.
For each object ID in the range the following setup is required:
= generate ID padding and store in authentication database, indexed by object
ID
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-77-
= retrieve private signature key by object ID
= generate signature by encrypting object ID and padding, using private key
= store signature in authentication database indexed by object ID, and/or
store the padding,
since the signature can be re-generated using the ID, padding and private key
= encode the signature across multiple tags in repeated fashion
This data is required for the Hyperlabel tags therefore the authentication
database must be established prior to,
or at the time of, printing of the Hyperiabels.
Security issues are discussed in more detail above.
Figure 21 summarises printing and distribution of pharmaceutical packaging
with Hyperlabel tags.
Pharmaceuticals are also logged in the database whenever they are scanned in
circulation, and also when they
are destroyed.
While the technology to print commercial Hyperlabel tags will be commercially
available, only the authorized
manufacturers will be able to print the codes corresponding to their products.
These codes can be protected
by 2048 bit RSA cryptography embedded within the integrated circuits (chips)
embedded in the MemjetTM
printers used to print Hyperlabel tags. This is a highly secure form of
asymmetric cryptography, using private
and public keys. The private keys relating to any particular currency would be
kept only by authorised
national security agencies.
OFF-LINE PUBLIC-KEY-BASED AUTHENTICATION
An off-line authentication device utilises public-key signatures. The
authentication device holds a number of
public keys. The device may, optionally, retrieve additional public keys on
demand, via a transient on-line
connection when it encounters an object ID for which it has no corresponding
public key signature.
For off-line authentication, the entire signature is needed. The
authentication device is swiped over the
Hyperlabel tagged surface and a number of tags are read. From this, the object
ID is acquired, as well as a
number of signature fragments and their positions. The signature is then
generated from these signature
fragments. The public key is looked up, from the scanning device using the
object ID. The signature is then
decrypted using the public key, to give an object ID and padding. If the
object ID obtained from the signature
matches the object ID in the Hyperlabel tag then the object is considered
authentic.
The off-line authentication method can also be used on-line, with the trusted
authenticator playing the role of
authenticator.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-78-
ON-LINE PUBLIC-KEY-BASED AUTHENTICATION
An on-line authentication device uses a trusted authenticator to verify the
authenticity of an object. For on-
line authentication a single tag can be all that is required to perform
authentication. The authentication device
scans the object and acquires one or more tags. From this, the object ID is
acquired, as well as at least one
signature fragment and its position. The fragment number is generated from the
fragment position. The
appropriate trusted authenticator is looked up by the object ID. The object
ID, signature fragment, and
fragment number are sent to the trusted authenticator.
The trusted authenticator receives the data and retrieves the signature from
the authentication database by
object ID. This signature is compared with the supplied fragment, and the
authentication result is reported to
the user.
ON-LINE SECRET-BASED AUTHENTICATION
Alternatively or additionally, if a random signature or secret is included in
each tag (or tag group), then this
can be verified with reference to a copy of the secret accessible to a trusted
authenticator. Database setup then
includes allocating a secret for each object, and storing it in the
authentication database, indexed by object ID.
The authentication device scans the object and acquires one or more tags. From
this, the object ID is acquired,
as well as the secret. The appropriate trusted authenticator is looked up by
the object ID. The object ID and
secret are sent to the trusted authenticator.
The trusted authenticator receives the data and retrieves the secret from the
authentication database by object
ID. This secret is compared with the supplied secret, and the authentication
result is reported to the user.
Secret-based authentication can be used in conjunction with on-line fragment-
based authentication is
discussed in more detail above.
PRODUCT SCANNING INTERACTIONS
Product Scanning at a retailer is illustrated in Figure 22. When a store
operator scans a Hyperlabel tagged
product the tag data is sent to the service terminal (A). The service terminal
sends the transaction data to the
store server (B). The store server sends this data, along with the retailer
details, to the manufacturer server
(C). The Hyperlabel server knows which manufacturer server to send the message
to from the object ID. On
receipt of the input, the manufacturer server authenticates the object, if the
manufacturer is the trusted
authenticator. Alternatively the manufacturer server passes the data on to the
authentication server to verify
the object ID and signature (D). The authentication server sends the
authentication result back to the
manufacturer server (E). The manufacturer server checks the status of the
object ID (against its valid ID lists
and hotlist), and sends the response to the store server (F), which in turn
send the result back the store service
terminal (G). The store server could also communicate with the relevant
authentication server directly.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-79-
The interaction detail for on-line product scanning at a retailer is shown in
Figure 23. The store operator scans
the Hyperlabel tagged product. The scanner sends the scanner ID and tag data
to the service terminal. The
service terminal sends this data along with the terminal ID and scanner
location to the store server. The store
server then sends the request on to the manufacturer server, which performs
authentication (either itself or via
a third party authentication server) and determines the object status. The
response is then sent back to the
store server, and on to the operator service terminal.
The interaction detail for off-line product scanning at a retailer is shown in
Figure 24. The store operator
scans the Hyperlabel tagged product. The scanner sends the scanner ID and tag
data from multiple tags to the
service terminal. The service terminal sends this data, along with the
terminal ID and scanner location, to the
store server. The store server then performs off-line authentication, as
described in Section 3.4.2, and
determines the object status through its cached hotlist, valid object ID
lists, and public key list. The store
server records the scan details in its internal object scanning history. The
response is then sent back to the
operator service terminal.
An alternative for off-line product scanner occurs where the scanner is a hand-
held, stand-alone scanner. In
this case the cached authentication data is stored within the scanner itself,
and the scanner performs the
validation internally. The object scanning history is also cached within the
scanner. Periodically the scanner
connects to the central database, uploads it's object scanning history, and
downloads the latest public key list,
object ID hotlist and valid ID range list. This connection may be automatic
(and invisible to the user), or may
be initiated by the user, for example, when the scanner is placed in a docking
station/charger.
Product scanning with a Netpage pen is illustrated in Figure 25. When a user
scans a Hyperlabel tagged item
with their Netpage pen, the input is sent to the Netpage System, from the
user's Netpage pen, in the usual way
(A). To scan a product rather than interact with it, the pen can be placed in
a special mode. This is typically a
one-shot mode, and can be initiated by tapping on a <scan> button printed on a
Netpage. Alternatively, the
pen can have a user-operable button, which, when held down during a tap or
swipe, tells the pen to treat the
interaction as a product scan rather than a normal interaction. The tag data
is transmitted from the pen to the
user's Netpage base station. The Netpage base station may be the user's mobile
phone or PDA, or it may be
some other Netpage device, such as a PC. The input is relayed to the
Hyperlabel server (B) and then on to
manufacturer server (C) in the usual way. On receipt of the input, the
manufacturer server authenticates the
object if the manufacturer is the trusted authenticator. Alternatively the
manufacturer server passes the data on
to the authentication server to verify the object ID and signature (D). The
authentication server sends the
authentication result back to the manufacturer server (E). The manufacturer
server checks the status of the
object ID (against its valid ID lists and hotlist), and sends the response to
the Hyperlabel server (G). The
Hyperlabel server, as part of the Netpage system, can know the identity and
devices of the user. The
Hyperlabel server will relay the manufacturer server's response to the user's
phone (G) or Web browsing
device (H) as appropriate. If the user's Netpage pen has LEDs then the
Hyperlabel server can send a
command to the user's pen to light the appropriate LED(s) (I,J).
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-80-
The interaction detail for scanning with a Netpage pen is shown in Figure 26.
The Netpage pen clicks on the
Hyperlabel tagged product. The Netpage pen sends the pen id, the product's tag
data and the pen's location to
the Hyperlabel server. If the pen ID is not already associated with a scanner,
the Hyperlabel server may create
a new scanner record for the pen, or may use the pen ID as a scanner ID. The
Hyperlabel server sends the
scanner ID, tag data, and scanner location (if known) to the manufacturer
server, which performs
authentication (either itself or via a third party authentication server) and
determines the object status. The
response is then sent back to the Hyperlabel server, and on to the user's
default Web browsing device.
SECURITY TAGGING AND TRACKING OBJECT MODEL
The Security Tagging and Tracking object model revolves around Hyperlabel
tags, object IDs, and signatures.
Figure 37 illustrates the management and organisation of these objects.
As shown in Figure 27, a Hyperlabel tag comprises a tag type, object ID, two-
dimensional position and a
signature fragment. The tag type indicates whether this is a tag on a common
object, or whether the tag is on a
special type of object such as a currency note or a pharmaceutical product. A
signature fragment has an
optional fragment number which identifies the fragment's place within the full
signature.
As described above, a product's unique item ID may be seen as a special kind
of unique object ID. The
Electronic Product Code (EPC) is one emerging standard for an item ID. An item
ID typically consists of a
product ID and a serial number. The product ID identifies a class of product,
while the serial number
identifies a particular instance of that class, i.e. an individual product
item. The product ID in turn typically
consists of a manufacturer number and a product class number. The best-known
product ID is the EAN.UCC
Universal Product Code (UPC) and its variants. The Item ID class diagram is
shown in Figure 28.
Pharmaceuticals are identified by a pharmaceutical ID. Typically the
pharmaceutical ID will be an EPC. A
pharmaceutical ID consists of a product ID and a serial number. The product ID
in turn typically consists of a
manufacturer number and a product class number. The best known product ID for
pharmaceutical products is
the National Drug Code (NDC), allocated and administered by the US Food and
Drug Administration. The
Pharmaceutical ID class diagram is shown in Figure 29.
Object Description, ownership and aggregation class diagram is shown in Figure
30. This is described in more
detail above.
The Object Scanning History class diagram is shown in Figure 31. An object has
an object scanning history,
recording each time the scanner scans an object. Each object scanned event
comprises the scanner ID, the date
and time of the scan, and the object status at the time of the scan, and the
location of the scanner at the time
the object was scanned. The object status may be valid, stolen, counterfeit
suspected, etc. If known, the object
owner details may also be recorded.
A scanner has a unique scanner ID, a network address, owner information and a
status (e.g. on-line, off-line).
A scanner is either a mobile scanner, whose location may vary, or a fixed
scanner, whose location is known
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-81-
and constant. A scanner has a current location, comprising the location
details and a timestamp. A scanner
may be a Netpage pen, in which case it will be associated with a Netpage Pen
record. If a scanner in off-line,
it will keep an object scanning history, and will optionally store a public
key list, a valid ID range list and an
object ID hotlist. The scanner class diagram is shown in Figure 32.
The manufacturer, or other central authority, maintains a number of Object ID
Hot Lists, each with a unique
list ID, and the time the list was last updated. Each hot list comprises a
list of suspect object IDs, comprising
the object ID, date, time, status (suspected counterfeit, stolen, etc.) and
other information. The Object 1D Hot
List class diagram is shown in Figure 33.
The manufacturer, or other central authority, maintains a list of valid ID
ranges. Each valid object ID range
entry in the list comprises the start object ID and end object ID (the valid
ID range) and the time the entry was
updated. The Valid ID Range List class diagram is shown in Figure 34.
The manufacturer, or other central authority, maintains a public key list. The
public key list consists of a
number of entries identifying the public key for a range of Object IDs. Each
valid object ID range entry
comprises the update time for the entry, the start object ID for the range,
the end object ID for the range, and
the public key applicable to each object ID in the given range. The Public Key
List class diagram is shown in
Figure 35.
Object authentication may be performed by the manufacturer, or by a third-
party trusted authenticator. A
trusted authenticator has an authenticator ID, name and details. A trusted
authenticator holds a list of public-
private key pairs, each associated with one or more ID ranges. This is a list
of object ID ranges (identified by
the start and end ID) and the corresponding public/private signature key pair.
A trusted authenticator also
holds a list of secret signatures, and a list of public-key signatures. Each
public-key signature identifies the
actual signature and/or the padding used to generate the signature. Each
secret signature and public-key
signature is associated by object ID with a unique object. The Trusted
Authenticator class diagram is shown in
Figure 36.
SCANNERS
Hyperlabel scanners can be built into a variety of devices. Scanners may be
fixed or mobile. A fixed scanner
has a permanent, known location. A mobile scanner has no fixed location. A
scanner may be on-line, i.e. have
immediate access to the central database, or it may be off-line.
Scanners may be specific to a particular product application, such as a
currency counter, or may be a generic
Hyperlabel scanner. Hyperlabel scanners may be embedded in other multi-
function devices, for example, a
mobile phone or PDA.
Hyperlabel currency tags can be read using many types of scanner, including:
= Cash registers
= POS checkouts
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-82-
= Mobile phone with inbuilt scanner
= Hyperlabel pens
= Vending machines
The Hyperlabel technology used in these devices can be implemented in a wide
range of applications. As a
result, the development and deployment costs can be shared by the key
stakeholders. It will be realised that
these can therefore be implemented in a manner similar to that described above
with respect to security
documents.
Hyperlabel scanners built into a variety of products will include the
following features, currently under
development at Silverbrook Research.
= An infrared image sensor to read the Hyperlabel tags that uniquely identify
each pharmaceutical
item.
= A 32 bit RISC processor with 20 megabits of secure code space signed using
2048 bit RSA
cryptography.
= A highly secure processor with cryptographic and physical security features
for verifying the
cryptographic signature on Hyperlabel tags (under development at Silverbrook
Research).
= Infrared optics, including filters tuned to the Hyperlabel ink infrared
spectrum.
= A real-time clock to verify the time of each transaction reported.
= Software to decode the Hyperlabel tags, record the details of each scan, to
validate each note
scanned, and to facilitate automatic and secure communications with an online
database.
= Communications systems to create secure network connections to the central
currency verification
database.
Various of the Hyperlabel scanners described below are also planned to include
the following units:
= An inbuilt display and data entry mechanism to indicate to the operator
details of the pharmaceutical
item being dispensed, items that are suspected of being counterfeit, and the
identity of the person
requesting reportable cash transactions.
= A cache of the serial numbers of all known counterfeit pharmaceutical item.
= Other spectral filters tuned to the secure currency ink spectrum (which
differs from the commercially
available Hyperlabel ink).
= A GPS tracker to verify the location of the currency counter at the time of
use.
MOBILE PHONE WITH INBUILT SCANNER
A mobile phone with an inbuilt Hyperlabel infrared scanner to scan and
validate each item can be used in a
range of locations such as where medications are stored for distribution, or
dispensed. It is intended for wide
use and distribution among the hundreds of millions of mobile phone
subscribers. It can be used by consumers
to validate items, or to quickly fmd out additional information about a
prescription item. It can also be used
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-83-
for inventory management and validity checking applications, such as for
policing trademark infringement,
and stocktaking.
HYPERLABEL SUPERMARKET CHECKOUT SCANNER
One of the other major applications of Hyperlabel is in consumer packaged
goods, where it has the potential
of being the next generation bar code and allowing automatic tracking of
individual items. The application of
Hyperlabel laser scanners makes it possible to automatically scan products at
supermarket checkouts. These
checkouts will be able to read pharmaceutical Hyperlabels to validate each
item at the point of sale. An
example of a hyperlabel is shown in Figure 38, and is described in more detail
in copending application
number [cross ref any application describing Hyperlabel checkout], the
contents of which is incorporated
herein by cross reference.
CASH REGISTERS
Cash registers can have an add-on or built-in currency scanner for a small
additional cost per unit. The
pharmaceutical item is scanned as it is processed for sale. An example of a
cash register is shown in Figure
39.
HYPERLABEL PEN
A Hyperlabel Pen can be used as a miniature low cost image sensor for consumer
and small business use. It
uses an infrared image sensor, and to image a Hyperlabel tag whenever it is
clicked against a surface. These
pens are also intended for high volume consumer use, with intended
distribution exceeding 100 million units.
While its primary application is a wide range of 'interactive paper' and
computer peripheral uses, it also
allows consumers to validate pharmaceuticals and other goods by clicking on
the Hyperlabel.
When read by a Hyperlabel Pen, the Hyperlabel allows the pen to track its own
nib movement relative to the
label. The pen uses the position and orientation of each tag in its 5mm field
of view to determine a much more
precise position than just the position encoded in a tag. The pen transmits
its interaction data to a Hyperlabel
Server for interpretation. The interaction data consists of movement data (or
'digital ink'), defined relative to
the product label identified by its EPC, thus enabling consumers to use a
product label to interact directly with
the manufacturer's Web site. The Hyperlabel network will be managed by
dedicated Hyperlabel servers, and
any pharmaceutical scans from Hyperlabel Pens will be routed through these
servers to the Pharmaceutical
server.
An example of a handheld validity scanner is shown in Figures 2 and 25, and is
described in more detail in
copending application number [cross ref any application describing validity
scanner], the contents of which
is incorporated herein by cross reference.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-84-
HANDHELD VALIDITY SCANNER
Handheld Hyperlabel validity scanners may also be used where currency counters
are not required or suitable.
These devices are expected to be significantly more common than currency
counters, as they have multiple
uses, and will be much cheaper.
The validity scanner has multiple uses, including pharmaceutical security,
brand-name security, stocktaking,
forensic investigations, and policing. As it is not a dedicated currency
device. It does not communicate
directly with the government currency server as otherwise, large numbers of
non-currency related messages
would need to be routed through that server. Instead, it communicates directly
with commercial Hyperlabel
servers, and any currency related validation requests are passed on to the
government server. To reduce the
transaction load on the government server, note related information can be
cached at the Hyperlabel server,
much as they are cached in the currency counters.
The link to the database would typically be relayed over a radio link to allow
local mobility. The radio link
can be WiFi, GPRS, 3G mobile, Bluetooth, or other IP link, as appropriate.
Internet transactions are secured
using encrypted packets.
An example of a hand held scanner is shown in Figure 40, with an alternative
example being shown in Figure
22, and being described in more detail in copending application number [cross
ref any application describing
validity scanner], the contents of which is incorporated herein by cross
reference.
SECURITY FEATURES
Hyperlabel currency security features include:
= Notes can be tracked whenever they are scanned - at banks, supermarket
checkouts, vending
machines, cash registers, and low cost home scanners.
= The unique range of currency tag numbers can be printed only by the
government printing agency.
= Currency IR ink with unique spectral properties, can be made available only
to government printing
agencies.
= Note serial number printed in tag must match printed serial number.
= Tags are printed all over both sides of the note.
= Tags vary across the note - a forger must match the thousands of tags
printed on any note.
= Additional proprietary security features not disclosed in this document.
= The ability to determine both the validity and the value of currency.
ADVANTAGES OF HYPERLABEL
Unlike 2D optical barcodes that are often difficult to read due to label
damage and a direct 'line-of-sight'
requirement needed for scanning, optically readable, but invisible, infrared
Hyperlabel tags, are printed all
over, or on a large section of a product label. Hyperlabel tags support line-
of-sight omnidirectional reading. In
practice, the Hyperlabel reader is designed to scan the scanning field from at
least two substantially
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-85-
orthogonal directions. This helps the reader to avoid occlusions which may
occur if a hand is holding an item.
Hyperlabel tags also incorporate Reed-Solomon error correction methods to
improve reliability.
A further advantage of Hyperlabels over barcodes is that they are unobtrusive
to the customer as they do not
use visible label space, and tag information is not restricted to only one
section of a label.
Hyperlabel tags are therefore easy to locate, easy to read, and enable
accurate automatic scanning. Automatic
checkouts minimize the possibility of collusion between the operator and the
customer or the shipping
agent(s). That is, it significantly reduces shrinkage due to the operator or
shipping agent deliberately not
scanning selected items. It also helps prevent substitution-based fraud.
Hyperlabels are less promiscuous than RFID tags since they require line-of-
sight for reading. This means that
it will be difficult for customers to have their product scanned for
information without their knowledge.
Hyperlabels provide customers with the means to protect their privacy in much
the same way as they can now
when carrying pharmaceutical goods.
HYPERLABELS AS INTERACTIVE WEB PAGES
A distinctive and unique feature of Hyperlabel technology is that Hyperlabels
provide the opportunity to
design packaging labels as interactive 'Web pages' - and thus make it possible
for a whole new range of
product-linked customer services to be introduced by the pharmaceutical
industry.
In a few years from now when digital pen use becomes widespread, product
graphics can be added to labels to
indicate interactive areas and prompting customers to write or click using a
Hyperlabel Pen. A digital
Hyperlabel Pen can identify the x-y position on a label, and enable a link to
be established between the
information on the label, and a Web page on a server. The Hyperlabel Pen
connects the customer to an
Internet-based Hyperlabel Server through a companion device such as a mobile
phone or computer.
Using a Hyperlabel Pen to interact with the label, customers can be offered
additional information on drug
use, risks and advice on potential interactions between drugs. It could also
provide an opportunity for
customers to register for participation in new drug trials, to enter
promotions, to participate in Web chat
sessions, or to receive 'free' samples. Web pages can be customised based on
customer profiles, local area
health data, or by using a range of product supply chain data such as
geographic location.
Hyperlabels therefore make it possible for the pharmaceutical industry to
extend the use of product labels and
packaging to increase brand strength, and to establish closer links with
customers. Thus, with Hyperlabels, the
customer can become an integral part of the product supply chain, and supply
chain data can be integrated
with customer relationship management (CRM) or healthcare databases to improve
the overall efficiency and
level of service offered to customers.
The following sections highlight how Hyperlabel technology can be implemented
to improve overall brand
strength and increase security.
BRAND PROTECTION
One of the most important challenges now confronting the pharmaceutical
industry is brand protection. A
strong brand protection strategy is crucial for the pharmaceutical industry to
protect profits and R&D
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-86-
investment, as well as customers. The illegal activities now used by criminals
to erode brand value, and that
are of most concern are:
= Parallel trade and illegal imports,
= Product substitution and counterfeiting, and
= Product tampering.
PARALLEL TRADE AND ILLEGAL IMPORTS
Parallel importation of pharmaceuticals exists where there is a significant
price difference for the same
product in different markets.
Pharmaceutical manufacturers are firmly against parallel importation as
parallel traders reduce their profits,
and manufacturers claim that they will have less money to spend on R&D.
Manufacturers also often express
concern that parallel imports do not meet international standards on safety,
quality and efficacy, and that they
give rise to additional opportunities for counterfeiting. The distribution by
unregulated drug outlets of expired,
contaminated, subpotent, superpotent and counterfeit drugs is also a
significant potential danger to customers.
Unregulated dispensers may provide patients with incorrect or contraindicated
medications, incorrect
strengths, or medications without adequate directions for use. State agencies
governing the local
pharmaceutical industry may not have implemented the appropriate standards and
safeguards to protect the
public against such occurrences.
Another concern is that the current outlook for international parallel 'trade
is one of controversy among
governments. For example, prices for the drug Amoxil (amoxicillin) vary
considerably around the world - the
cheapest US$8 in Pakistan, and the most expensive US$60 in Germany. It is
therefore now common for drugs
like Amoxil to be illegally imported across international borders to avoid
high prices. The U.S. Food and
Drug Administration (FDA) estimates that approximately two million parcels
containing FDA-regulated
products for personal use enter the United States annually through
international mail facilities. Other sources
estimate that nearly 70 pharmacies in Canada (40 in Manitoba) shipped almost
US$500 million dollars worth
of prescriptions into the United States in 2002. In the United States this
year, the pharmaceutical industry's
trade group - Pharmaceutical Research and Manufacturers of America (PhRMA) -
has spent US$8.5 million
lobbying against a bill to allow the import of Canadian drugs. Much is
therefore expected from the World
Trade Organization (WTO). The outcome is likely to be further lobbying by the
pharmaceutical industry to
the WTO for regulations mandating the introduction of individual item
identification and verification
capabilities to overcome differences between national governments.
Hyperlabels can be used to reduce the possibility of parallel trade and
illegal imports by using EPC linked
data to determine the origin and supply chain details for each item.
PRODUCT SUBSTITUTION AND COUNTERFEITING
Counterfeit products may include products with the wrong ingredients, without
active ingredients, with
insufficient active ingredients, or with fake packaging. This type of illegal
behavior can lead to compromises
in patient safety, and economic loss for established drug manufacturers.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-87-
Although counterfeiting laws and regulations have been in place for the past
20 years, evidence of
counterfeiting is increasing. The FDA estimates that up to 40% of
pharmaceuticals shipped from countries
such as Argentina, Colombia, and Mexico may be counterfeit. There is no
unified world authority to
promulgate investigations, nor a world tribunal for enforcement. Consequently,
pharmaceutical firms
themselves must augment legal approaches with alternative ones to protect
industry, stakeholders, and
customers.
Hyperlabels can assist manufacturers by making it easy to recognize non-
authentic product and product
tampering. A specific 'fmgerprint' is created for each vial, drum, shipping
container, and label. Unique
identifiers for every product item coming out of the plant shipping dock can
be used to collect data such as the
site of manufacture, the date of packaging, storage location and time,
distribution path, repackaging details,
testing and possibly other information. Products might also include multiple
layers of tamper evidence.
Therefore, a Hyperlabel item identification system can be used to assist the
pharmaceutical industry to
implement an effective item level tracking and tracing system.
PRODUCT TAMPERING
Today, the pharmaceutical industry is in danger from the threat of
pharmaceutical product tampering. Some of
today's biopharmaceutical products cost in excess of $2000 - $3000 per gram to
produce, and some criminals
(and even pharmacists) are tampering for profit. Since pharmaceuticals are
critical to the social, economic and
political stability of many nations, they are also vulnerable targets for
terrorism. In each case, the lack of
ability to determine the actual country of origin is of utmost concern.
To address these fears, a range of protection methods are now being
implemented. These may include seals
that need to be removed, features that must be broken, or permanent measures
such as color, taste, and
fragrance that are part of the product itself. Other tools include codes whose
color spectrum is only visible
under UV light or special-coded label words/symbols visible only with special
viewing devices.
25. The pharmaceutical industry is also acting to secure the supply chain
using new technology to protect all
stakeholders, patients and manufacturers alike. This also means that Brand
protection needs to be applied at
several levels of packaging or containment because every level offers a
possible introduction point for
tampered product, and it could involve the use of several different methods.
The levels for tamper evidence
start with the largest unit (the warehouse building) and need to be applied at
each package configuration level,
down to the item label and contents. Hyperlabels, when used in parallel with
other product brand protection
methods, can offer the potential to improve brand protection at each of these
points in the supply chain.
ADDING THE CUSTOMER TO THE PHARMACEUTICAL SUPPLY CHAIN
One of the key advantages that Hyperlabel can offer the pharmaceutical
industry, is the ability to extend the
use of labeling to make them Web-interactive so that customers can become an
integral part of the supply
chain.
There are many ways for Web-interactive Hyperlabels to bring benefits to the
pharmaceutical industry. Some
of these are to:
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-88-
= Enable customers to authenticate a product themselves,
= Protect brand strength through improved market segmentation and
customization,
= Become more customer-centric by introducing new customer-led marketing
models,
= Refine the marketing mix by introducing Web-based direct to customer (DTC)
marketing campaigns,
and,
= Differentiate products by using extended label marketing models.
CUSTOMER AUTHENTICATION OF PRODUCTS
While the changing of anti-counterfeit approaches helps manufacturers and
regulatory agencies differentiate
genuine product from false product, the rapid changes can be confusing to the
public. This creates a situation
where customers find it difficult to check the authenticity of a product
themselves. Using a Hyperlabel Pen,
customers can validate an item for themselves, as well as access additional
product information and customer-
centric services.
MARKET SEGMENTATION AND CUSTOMIZATION
Pharmaceutical markets can be divided into three broad product segments:
= Prescription-only medicines (comprise about 80% of the market by value, and
50% by volume),
= Generic branded medicines, and
= Over the counter medicines (OTCs), which may be purchased without
prescription and may also
be branded or generic.
Each of these market segments requires different strategic approaches.
Suppliers of branded prescription
drugs need to protect R&D efforts. Generic companies focus on supply chain
logistics and manufacturing
costs. OTCs are rarely prescribed, and they require direct-to-customer (DTC)
marketing methods. In each
case, Hyperlabel Web-interactive labeling makes it possible for the
pharmaceutical industry to establish a
direct marketing relationship with customers in each segment, and at the same
time it provides an opportunity
to improve market segmentation.
CUSTOMER-LED MARKETING
Most pharmaceutical companies have been product-led rather than customer-led.
This has probably been a
consequence of the unpredictability of the R&D process because it has not been
easy to develop a product to
meet specific customer needs. Web-interactive hyperlinks provide customers
with an 'opt in' method of
participating in new customer-led marketing activities. For example, they may
make suggestions about what
Web character stickers they want to appear on the packaging to make a child
'feel better'.
WEB DIRECT TO CUSTOMER (DTC) MARKETING
Another emerging trend has evolved around the growing importance of direct-to-
customer (DTC) advertising.
As a medium, DTC TV advertising was one of factors responsible for increasing
sales in the US
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-89-
pharmaceutical market during the 1990s. Although the EU does not yet allow DTC
advertising of
pharmaceuticals, customers can access product information on the Web. In the
year 2000, health was one of
the top 2 reasons for people to conduct Web searches. With many customers now
using the Internet as a
productive source of health-related information, more pharmaceutical suppliers
are using the Web as a DTC
advertising medium.
EXTENDED LABEL MARKETING MODELS
With 70% of purchasing choices made at the point of sale, marketers can build
brand recognition and drive
sales with packaging that makes an immediate impact on customers. Because of
this, pharmaceutical
companies now spend more on packaging than they do on advertising. As markets
have matured and
competitive differentiation has narrowed, packaging has become a very
important component of marketing
strategy.
A product's package is often its most distinctive marketing effort, and it
performs a number of essential
functions: The package provides a means of communicating with the customer.
However, as regulations
require more information to be placed on labels, while there is also a trend
for packaging to 'shrink', the use
of the space available becomes more important. Barcodes take up some of this
space. By using invisible Web-
interactive Hyperlabels, the pharmaceutical industry can conceive a range of
extended label marketing
models. For example, they can include hyperlinks to a Web page to request SMS
reminders to take medicines,
enter competitions, reorder goods, or to access support services.
Thus, there are many ways for Web-interactive Hyperlabels to bring benefits to
the manufacturer, and to
improve market acceptance of a pharmaceutical product.
HYPERLABEL BENEFITS ANALYSIS MATRIX
From the conclusions drawn in the preceding sections it is evident that
Hyperlabels present a unique
opportunity for the pharmaceutical industry to introduce a long term, low
cost, unique item identification
solution that has significant advantages over alternative technologies.
A summary of the comparative advantages of Hyperlabels over 2D optical
barcodes, and RFID tags is
provided in Table 12. The range of benefits these advantages can deliver to
the pharmaceutical industry
include:
= Meet current and anticipated statutory requirements,
= Protect expensive R&D investment,
= Limit business and customer risk through criminal and terrorist activity,
= Reduce parallel trade and illegal imports,
= Reduce substitution and counterfeiting,
= Prevent product tampering,
= Establish Web links between the industry and customers (via Hyperlabels),
and
= Improve pharmaceutical supply chain logistics and efficiency.
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-90-
It is also clear that unique product identification, and track and trace
capabilities are the foundation stones for
achieving the above goals.
Table 12
Nleets ctu7ent FDA standards for food.
Ldru-and costnetic labeline. Meets WHO FD-1 track and trace "ciide.lines Por
rieterrin- and detcctin~
countcrleit dru~'S.
f'rovidcs a ne%\ revenue strearn for X X
existin packagin~~ substratcs.
{
1
Provides customer-centered 'opt-in' X X
VJeb interactivitv 1'Or speciiic product
a items and special oltzrs.
~ Yrovides a solution that is acceptable to X
privac.y advocatesconcerned abont the
ability to read tags without the
custonier knowledge.
owcost to produce. ~ X
Omnidirectional reading. X
... . . . . ' .:.1
CA 02567253 2006-11-15
WO 2005/111922 PCT/AU2005/000070
-91 - IZEQUIIZENIENI-S
Unobtrusive to customer. X
~ _ ...:._ .. _
~Individual product iteni idcntific3tion.
'
Introduce new customer-centric X X
niarketinL niodels based on Vveb-label
interactivitN.
-x
C.an bz used ith radiopaque iriaterials. ~ X