Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 38
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 38
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
SARS VACCINES AND METHODS TO PRODUCE
HIGHLY POTENT ANTIBODIES
This application claims benefit of U.S. Serial No. Not Yet
Known, Filed May 31, 2005 and U.S. Serial No. 60/576,118, filed
June 2, 2004, which is incorporated in its entirety by reference
into this application.
Throughout this application, various publications are
referenced. Disclosures of these publications in their
entireties are hereby incorporated by reference into this
application in order to more fully describe the state of the art
to which this invention pertains.
BACKGROUND OF THE INVENTION
Severe acute respiratory syndrome (SARS), a newly emerging
infectious disease, is caused by a SARS-associated coronavirus
(SARS-CoV) (1-7), which may originate from some wild animals
(8). A global outbreak of SARS in 2002/2003 resulted in
thousands of cases and hundreds of deaths, seriously threatening
public health worldwide. In late 2003 and early 2004, new
infections caused by SARS-CoV strains different from those
predominant in 2002/2003 epidemic were reported in China (9).
Several isolated outbreaks that resulted from accidental
releases of the SARS-CoV isolates were reported in Taiwan,
Singapore, and China (http://www.who.int/csr/sars/en). These
indicate that SARS epidemics may recur at any time in the
future, either by animal-to-human transmission of the SARS-CoV
or by the virus escaping from laboratory samples. Therefore,
development of effective and safe vaccines is urgently needed
for protection of at-risk populations.
Currently, one candidate vaccine using inactivated SARS-CoV is
in a phase I clinical trial in China (9, 10) . Although the
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
inactivated SAI;S-CoV h=as been shown to be effective in
protecting animals from challenge by SARS-CoV, its efficacy in
humans is unclear. There has been a serious concern about its
safety since some antigens in the virions may elicit antibodies
that do not neutralize, but rather enhance virus infection (10).
Some viral proteins may induce harmful immune and inflammatory
responses, a potential cause of SARS pathogenesis and the
rationale for using immunosuppressants (e.g., steroids) for SARS
treatment, although there are apparent contradictions to this
regimen (11, 12). Most recently, it was reported that SARS-CoV
infection of ferrets caused mild liver inflammation and the
liver damage became much more serious if the ferrets were first
immunized with vaccinia virus-based SARS vaccines before virus
challenge (13 ) .
The S proteins of coronaviruses are responsible for virus
binding, fusion and entry, and are major inducers of
neutralizing antibodies (14-16). Besides, they play critical
roles in viral pathogenesis and virulence (17). The S protein
of SARS-CoV is also important for viral functions and
antigenicity (18, 19). It is a type I transmemberane glycoprotein
consisting of two domains, S1 and S2 (18) (Fig. 1). Sl is
responsible for virus binding to the receptor on the target
cell. It has been demonstrated that angiotensin-converting
enzyme 2 (ACE2) is a functional receptor for SARS-CoV (20-23) . A
fragment located in the middle region of Sl is the receptor-
binding domain (RBD)(24-26). S2 domain, which contains a
putative fusion peptide and two heptad repeat (HR1 and HR2)
regions =(Fig. 1), is responsible for fusion between viral and
target cell membranes. Like the anti-HIV peptides derived from
the HIV-1 gp4l HR2 region (27, 28), a peptide derived from the
HR2 region of SARS-CoV S protein was identified to be an
inhibitor of SARS-CoV infection (29) . HR1 and HR2 regions can
associate to form a six-helix bundle structure (29, 30),
resembling the fusion-active core structure of gp41 in HIV (31)
- 2 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
~E I .. ~ ~, õ~
and~"' r~f ~'~~e=~ other coronavirus, such as mouse
hepatitis coronavirus (MHV) (32, 33) These suggest that upon
binding of RBD on the viral S protein to ACE2 on the target
cell, S2 changes conformation by interaction between the HR1 and
HR2 regions to form fusogenic core and bring viral and target
cell membrane into close proximity, resulting in virus fusion
and entry (29). This indicates that the fragments containing the
functional domains on the S protein may be used as antigens for
inducing antibodies to block virus binding or fusion.
Several live attenuated and genetically engineered vaccines
encoding SARS-CoV S protein have been in preclinical studies.
Recently, Nabel and colleagues (34) reported that a DNA vaccine
candidate encoding the S protein induced T-cell and
neutralizing-antibody' responses (neutralizing antibody titers
range from 1:50 to 1:150), and protected mice from SARS-CoV
challenge as shown by reduced titers of SARS-CoV in the
respiratory tracts. They proved that the protection was mediated
by neutralizing antibodies but not a T-cell-dependent mechanism.
Most recently, Moss and co-workers (35) demonstrated that
intranasal or intramuscular inoculations of mice with highly
attenuated modified vaccinia virus vectors virus Ankara (MVA)
containing the gene encoding full-length SARS-CoV S protein
(MVA/S) produced S-specific antibodies with SARS-CoV
neutralizing activity (mean neutralizing titer is 1:284), and
protected mice from SARS-CoV infection after transfer of serum
from immunized mice. These data suggest that the S protein can
induce protective neutralizing antibodies, although the
neutralizing antibody titers are relatively low.
A recombinant fusion protein containing RBD linked to a human
IgG-Fc fragment (for facilitating RBD purification) as an
antigen (designated RBD-Fc, see Fig. 1) for immunization of mice
and rabbits can induce highly potent neutralizing antibody
responses in the immunized animals (geometric mean neutralizing
- 3 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
ti~'e~ s~:>~~"~ 61~ri4c~'~~ttjIantibodies can bind to RBD and block
RBD binding to ACE2. This suggests that RBD may be applied as a
subunit vaccine for prevention of SARS.
This invention discloses a recombinant fusion protein or
isolated polypeptides containing RBD linked to a human IgG-Fc
fragment (for facilitating RBD purification) as an antigen
(designated RBD-Fc, see Figure 1) that can induce highly potent
neutralizing antibody responses in immunized animals (mean
neutralizing titer 1:15,360 for rabbits and 1:12,553 for mice),
suggesting that RBD may be applied as a subunit vaccine for
prevention of SARS.
4
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
The spike (S) protein of severe acute respiratory syndrome
(SARS) coronavirus (CoV), a type I transmembrane envelope
glycoprotein, consists of Sl and S2 domains responsible for
virus binding and fusion, respectively. The Sl contains a
receptor-binding domain (RBD) that can specifically bind to
angiotensin-converting enzyme (ACE2), the receptor on target
cells.
This invention provides a vaccine comprising an effective amount
of the isolated polypeptide or recombinant protein containing
the sequence of RBD in the Severe Acute Respiratory Syndrome
(SARS) associated coronavirus spike protein or a functional
fragment thereof.
This invention also provides a vaccine comprising an effective
amount of a nucleic acid molecule comprising the sequence of a
fragment which encodes the sequence of RBD in the Severe Acute
Respiratory Syndrome associated coronavirus spike protein, or a
functional fragment thereof.
This invention also provides a recombinant fusion protein
containing sequence of RBD in the Severe Acute Respiratory
Syndrome associated coronavirus spike protein, or a functional
fragment thereof, and sequence of a human IgG Fc fragment
(designated RBD-Fc), or a functional fragment thereof.
RBD-Fc can induce highly potent antibody responses in the
immunized animals, including rabbits and mice. The antibodies
recognized the sequence of RBD on Sl domain in the Severe Acute
Respiratory Syndrome associated coronavirus spike protein, the
sequence of Si domain in the Severe Acute Respiratory Syndrome
associated coronavirus spike protein, and the sequence of the
- 5 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Se' spir'~ '~t I'Syndrome associated coronavirus spike
v~~e
protein.
The antibodies from animals (e.g., rabbits and mice) immunized
by RBD-Fc effectively blocked binding of RBD or S1 domain in the
Severe Acute Respiratory Syndrome associated coronavirus spike
protein to soluble ACE2 molecules or ACE2 expressed on cells.
The antibodies from animals (e.g., rabbits and mice)immunized by
RBD-Fc potently neutralized infection by SARS-CoV and by
HIV/SARS-CoV S pseudovirus with a neutralizing titer about 50-
300-fold higher than those of the mouse antisera induced by DNA
vaccines and vaccinia virus vectors encoding the full-length of
SARS-CoV S protein.
Depletion of anti-Fc antibodies from antisera did not affect
neutralizing activity.
This indicates that the sequence of RBD on S1 domain in the
Severe Acute Respiratory Syndrome associated coronavirus spike
protein can induce highly potent neutralizing antibody responses
and can be developed as an effective and safe subunit vaccine
for prevention of SARS.
The IgG Fc linked to RBD may significantly enhance the
immunogenicity of RBD to produce high levels of specific
antibodies against RBD. The method of linking IgG Fc to an
antigen may be used for inducing high levels of antibodies
against the corresponding antigen.
This invention provides a composition for increasing the
immunogenicity of an antigen comprising an effective amount of
an antigen and an IgG Fc domain, its functional fragment, or a
substance containing an IgG Fc domain or its functional
fragment. In an embodiment, the antigen and the IgG Fc are
- 6 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
~ ,t, ,p ~
liri~'k~d.'~ iI~~~Y l'!E,"~~::_giibodiment, they are linked to form a
fusion protein.
Finally, this invention also provides methods for using any of
the above compositions for immunization. In an embodiment, they
are used as vaccines.
- 7 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
i ,,,kt 'n :,~~E.:r 4i'.''' lt tF:.< .. 34 '
DE A 'fi;EI7'S~E~S~~~~P''~IDa1t~,F".,~!#E FIGURES
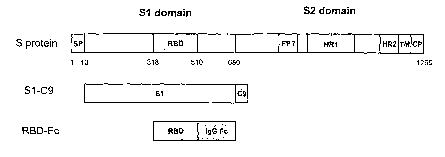
Figure 1. Schematic diagram of SARS-CoV S protein and the
recombinant fusion protein RBD-Fc. The S protein consists of S1
and S2 domains. There is a signal peptide (SP) located at the N-
terminus of the S protein. The Sl domain contains a receptor-
binding domain (RBD). The S2 domain contains a cytoplasm domain
(CP), a transmembrane domain (TM) and an ectodomain composed of
a putative internal fusion peptide (FP) and heptad repeat 1 and
2 (HR1 and HR2) regions. RBD-Fc consists of RBD and a human IgG-
Fc fragment. Sl-C9 contains S protein S1 domain and a C9
fragment.
Figure 2. Mouse antisera contained high titers of antibodies
binding to RBD on SARS-CoV S protein S1 domain. (A) Binding to
RBD-Fc by antisera (1:10,000) collected from mice before
immunization (pre-immune) and 4 days after each boost; (B)
Binding to RBD-Fc by mouse antisera collected 4 days after the
third boost at a series of 5-fold dilutions; and (C) Binding to
Sl-C9 protein by mouse antisera collected 4 days after the third
boost at a series of 5-fold dilutions. All samples were tested
in duplicate and data presented are mean values of two tests
(same for the following figures).
Figure 3. Rabbit antisera contained high titers of antibodies
binding to RBD. (A) Binding to RBD-Fc by antisera (1:10,000)
collected from rabbits before immunization (pre-immune) and 10
days after each boost; (B) Binding to RBD-Fc by rabbit antisera
collected 10 days after the first boost at a series of 5-fold
dilutions; and (C) Binding to Sl-C9 protein by rabbit antisera
collected 10 days after the first boost at a series of 5-fold
dilutions.
Figure 4. Neutralization of SARS-CoV infection by mouse antisera
directed against RBD-Fc. (A) Inhibition of CPE induced by SARS-
- 8 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
CoVFfn-'fV'dcell monolayer by mouse antisera in a
series of 2-fold dilutions was quantitated. The results obtained
from the experiment using antiserum from the mouse M8 was shown
here as an example. The CPE was recorded under a microscope and
the virus-neutralizing titers were calculated; and (B)
Neutralization of HIV/SARS-CoV S pseudovirus infection by mouse
antisera at a series of 2-fold dilutions. Inhibition of a
single-cycle infection of 293T cells expressing ACE2 by the
pseudovirus was determined in a luciferase assay.
Figure 5. Inhibition of CPE induced by SARS-CoV infection in
Vero E6 monolayer by rabbit antisera was detected as described
in Fig. 4A.
Figure 6. Neutralization of HIV/SARS-CoV S pseudovirus infection
by rabbit antisera. Inhibition of a single-cycle infection of
293T cells expressing ACE2 by the pseudovirus was determined in
a luciferase assay.
Figure 7. Effect of depletion of anti-Fc antibodies from the
rabbit antisera on binding to S1-C9 and virus-neutralizing
activity. The binding activity of anti-Fc-depleted arid untreated
rabbit antisera to human IgG (A) and S1-C9 (B) was measured by
ELISA. The neutralizing activity of the anti-Fc-depleted rabbit
antisera against HIV/SARS-CoV S pseudovirus was compared with
that of untreated rabbit antisera (C).
Figure 8. Mouse and rabbit antisera blocked binding of S1 which
contains RBD to ACE2. Inhibition of S1-C9 binding to soluble
ACE2 by mouse (A) and rabbit (B) antisera was measured by ELISA.
Inhibition of S1-C9 binding to cell-expressed ACE2 by rabbit
antisera was measured by flow cytometry (C) In the positive
control, no rabbit serum was added while in the negative
control, neither rabbit serum nor S1-C9 was added. Rabbit
- 9 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
~ ~~i~i'i~k~i=~~E '~~~~4'~=
anti a ~ binding to ACE2-expressing cells in a
dose-dependent manner (D).
- 10 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
DE12AIfi~AD 6-isMi2AI~ &4'iE INVENTION
This invention provides a vaccine comprising an effective amount
of the isolated polypeptide or recombinant protein containing
the receptor-binding domain (RBD) in the Severe Acute
Respiratory Syndrome associated coronavirus spike protein or a
functional fragment thereof. In an embodiment, appropriate
adjuvant(s) is/are used with the said vaccines which are
described in this invention. In a further embodiment, the
vaccines are conjugated.
As used herein, functional fragment is the part of the RBD which
carries out the function. In an embodiment, the function is to
bind receptors.
Peptide or Polypeptide or protein with RBD Sequence
This invention provides an isolated peptide or polypeptide or
protein comprising sequence of receptor-binding domain in the
Severe Acute Respiratory Syndrome associated coronavirus spike
protein or a functional fragment thereof, which can be used as a
vaccine for preventing infection by Severe Acute Respiratory
Syndrome associated coronavirus.
In an embodiment, the RBD is having the below sequence:
NITNLCPFGEVFNATKFPSVYAWERKKISNCVADYSVLYNSTFFS
TFKCYGVSATKLNDLCFSNVYADSFVVKGDDVRQIAPGQTGVIA
DYNYKLPDDFMGCVLAWNTRNIDATSTGNYNYKYRYLRHGKLRPFERDIS
NVPFSPDGKPCTPPALNCYWPLNDYGFYTTTGIGYQPYRVVVLSFELLNAP
ATV. (SEQ ID NO: 1)
This invention is intended to cover the below sequence or a
functional fragment of the below sequence:
- 11 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Nl'!TNL,C'P'V-,S-"E'VtNATKP,P'S'V~AWERKKISNCVADYSVLYNSTFFS
TFKCYGVSATKLNDLCFSNVYADSFVVKGDDVRQIAPGQTGVIA
DYNYKLPDDFMGCVLAWNTRNIDATSTGNYNYKYRYLRHGKLRPFERDIS
NVPFSPDGKPCTPPALNCYWPLNDYGFYTTTGIGYQPYRVVVLSFELLNAP
ATV. (SEQ ID NO: 1)
This invention is intended to cover the RBD sequences with
natural mutations in the SARS-CoV strains identified so far (6):
such as R344 , K; F360 --> S; L472 --> P; N479 -> K; D480 -> G; T487
S, and any unnatural mutations.
As it is known in this art, mutation, substitution, insertion
and deletion of the sequences are possible while the RBD
function or i.mmunogenicity remains unchanged. It is the
intention of this invention to include said mutation,
substitution, insertion and deletion.
Nucleic Acid Fragment Encoding RBD Sequence
Nucleic acid vaccines offer a new opportunity to immunize with
materials that are entirely gene-based, expressed by the
recipient's own cells. There is greater control over the
immunization process. The vaccine may be administered in skin
or muscle. Other molecules such as cytokines may be co-
expressed. In addition immunostimulatory DNA sequences may be
used to modulate the type of response (Th1 or Th2). The
duration of the response can be controlled by repeated exposure
to the genes, which are expressed transiently, by a variety of
delivery mechanisms such as: direct injection; electroporation;
mucosal delivery, etc. The ability to make DNA molecules
strictly by rational design makes it possible to bypass years of
development for the production of efficacious vaccines. These
vaccines are expressed and presented in the host, making an
ideal mimic of intracellular antigens. See U.S. Patent Nos.
6, 339, 068B1; 6, 821, 957B2 .
- 12 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
A DNA molecule comprising a nucleic acid fragment (e.g, DNA
vaccine) encoding sequence of receptor-binding domain in the
Severe Acute Respiratory Syndrome associated coronavirus spike
protein or a functional fragment thereof, can be used as a
vaccine for preventing infection by Severe Acute Respiratory
Syndrome associated coronavirus.
Live attenuated viruses (e.g., MVA) containing vectors
comprising the nucleic acid fragments or all molecules which
comprise the sequence of said fragments encoding sequence of
receptor-binding domain in the Severe Acute Respiratory Syndrome
associated coronavirus spike protein or a functional fragment
thereof, can be used as a vaccine for preventing infection by
Severe Acute Respiratory Syndrome associated coronavirus.
Fusion Protein or Polypeptide Containing RBD Sequence Linked with
IgG Fc Domain
A fusion protein or an isolated polypeptide that contain
sequence of receptor-binding domain in the Severe Acute
Respiratory Syndrome associated coronavirus spike protein or a
functional fragment thereof, linked to a substance comprising an
IgG Fc domain, its functional fragment or a substance containing
an IgG Fc domain or its functional fragment, can be used as a
vaccine for preventing infection by Severe Acute Respiratory
Syndrome associated coronavirus
An IgG molecule can be cleaved by the enzyme papain with the
hinge region at a site upstream of the inter-H chain disulfide
bonds to produce two Fab fragments and one Fc fragment (59). One
of the main functions of the Fc domain is responsible for
binding of IgG molecule to Fc receptors (FcyR) on cell surfaces
(60).
An example of the IgG sequence is illustrated below:
- 13 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
THTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPQVKFNWYVDGV
QVHNAKTKPREQQYNSTYRVVSVLTVLHQNWLDGKEYKCKVSNKALPAPIEKTISKAKGQ
PREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDG
SFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG. (SEQ ID NO: 2)
(61)
IgG Fc from different species should work the same.
This invention provides a pharmaceutical composition comprising
any of the above described isolated polypeptide or any of the
above-described the fusion protein and a pharmaceutically
acceptable carrier.
This invention provides a method for induction of antibodies
against Severe Acute Respiratory Syndrome-associated Coronavirus
in a subject comprising administering to the subject any of the
above vaccine or an effective amount of the isolated polypeptide
of any of the above described fusion protein or the composition.
In an embodiment, the induced antibodies are neutralizing.
As stated herein, subjects are organisms which have immune
response. The subject includes but is not limited to mammalians.
Said subject includes human but could be animals, such as dogs
and cats.
The above method may produce polyclonal br monoclonal antibody.
This invention provides the antibody generated by any of the
above methods. These antibodies may be used to treat or prevent
infection by Severe Acute Respiratory Syndrome-associated
Coronavirus.
Anti-idiotypic Antibody
- 14 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
This invention provides an anti-idiotypic antibody which should
mimic the RBD, or a functional portion thereof, against the
monoclonal antibody specific to RBD of SARS corona virus.
This invention also provides a vaccine comprising an effective
amount of the anti-idiotypic antibody, a functional portion
thereof or a single chain antibody which can function like the
anti-idiotypic antibody.
This invention provides a method for determining the
neutralizing epitope contained in S1 of the Severe Acute
Respiratory Syndrome Virus comprising steps of:
(a) generating peptide from the RBD sequence of SARS-CoV S
protein;
(b) immunizing animals (e.g., rabbits, mice etc) with the
peptides;
(c) collecting blood from the immunized animals; and
(d) testing the antisera collected from animals immunized
with the peptides derived from SARS-CoV s protein RBD
for neutralizing activity against SARS-CoV.
As an alternative, cells may be immunized in vitro. Spleen cells
of an appropriate host may be harvested and contact with the RBD
of SARS-CoV S protein. After the immunization, routine
procedure for production of monoclonal antibodies may be carried
out.
Epitope
This invention further provides the epitope of the RBD of SARS-
CoV S protein. In an embodiment, the epitope is determined by
the above-described method. In a further embodiment, the
epitope is a neutralization epitope.
- 15 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
An isolated polypeptide or recombinant protein containing the
sequence of the neutralizing epitope can be used as a vaccine.
The nucleic acid fragment, or the nucleic acid molecule which
contains the sequence of a fragment, encoding the sequence of
the neutralizing epitope or a vector comprising nucleic acid
fragment, or the nucleic acid molecule which contains the
sequence of a fragment, encoding the sequence of the
neutralizing epitope may be used as vaccine.
This invention also provides a compound containing the sequence
or conformation of the epitope. In an embodiment, the compound
is a peptide or polypeptide.
This invention further provides a composition comprising a
compound which contains the epitope, the isolated peptide or
polypeptide.
This invention also provides a vaccine comprising an effective
amount of any of the above composition.
This invention provides a method for induction of antibodies
against Severe Acute Respiratory Syndrome Virus in a subject
comprising administering to the subject the above vaccine.
Increase the Immunogenicity by Linking the IgG Fc Domain
This invention provides a method to increase the immunogenicity
of an antigen comprising linking of an IgG Fc domain, its
functional fragment or a substance containing the IgG Fc domain
or its functional fragment to said antigen.
This invention also provides a composition for increasing the
immunogenicity of an antigen comprising an effective amount of
- 16 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
an arrtigi~!n 11n'ked t o a=n 1gG Fc domain or its functional fragment
or a substance containing an IgG Fc domain or its functional
fragment.
In an embodiment of the above method or the composition, the
linkage results in a fusion protein.
This invention also provides a method to increase the
immunogenicity of an antigen in a subject comprising
administering to the subject the antigen linked to the IgG Fc
domain or its functional fragment, or a substance containing an
IgG Fc domain or its functional fragment.
This invention provides a composition for increasing the
immunogenicity of an antigen comprising an effective amount of
an antigen and a human IgG Fc domain, its functional fragment,
or a substance containing an IgG Fc domain or its functional
fragment. The IgG Fc domain can be from rabbit, mouse or any
other animals.
The antigen chosen can be of any origin so long as it can
illicit immune response. In an embodiment, the antigen
encompasses any antigen that can induce antibodies. In a
further embodiment, the antigen is derived from an infectious
agent. In a still further embodiment, it is of viral origin.
The increase in immunogenicity may result in high level of
neutralization antibodies, in high titer of antibodies against
the antigen or antibodies with high binding affinity.
Appropriate adjuvant(s) may also be used in the vaccine or the
composition to increase the immune response. Said usage of
adjuvants is well known in the art. The adjuvants include but
are not limited to saponin based adjuvants.
- 17 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Ths'It ihve~'4'c~ ~!M 1'fu'' th~'ltf"i'~~~rovides the above compositions and a
pharmaceutically acceptable carrier, thereby forming
pharmaceutical compositions.
This invention also provides a pharmaceutical composition
comprising a combination as described above and a
pharmaceutically acceptable carrier. For the purposes of this
invention, "pharmaceutically acceptable carriers" means any of
the standard pharmaceutical carriers. Examples of suitable
carriers are well known in the art and may include, but are not
limited to, any of the standard pharmaceutical carriers such as
a phosphate buffered saline solution and various wetting agents.
Other carriers may include additives used in tablets, granules
and capsules, etc. Typically such carriers contain excipients
such as starch, milk, sugar, certain types of clay, gelatin,
stearic acid or salts thereof, magnesium or calcium stearate,
talc, vegetable fats or oils, gum, glycols or other known
excipients. Such carriers may also include flavor and color
additives or other ingredients. Compositions comprising such
carriers are formulated by well-known conventional methods.
This invention demonstrates that a peptide derived from the HR2
region had inhibitory activity on SARS-CoV infection and it can
interact with a peptide derived from HR1 region to form six-
helix bundle, resembling the fusion-active core structure of
gp4l in HIV. These suggest a model of SARS-CoV entry into target
cell: upon binding of RBD on the S1 to ACE2, S2 changes
conformation by interaction between the HR1 and HR2 regions to
form fusogenic core and bring viral and target cell membrane
into close proximity, resulting in virus fusion and entry. This
indicates that the fragments containing the functional regions
in the S protein may be used as immunogens to induce antibodies
to block virus binding or fusion.
- 18 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
l..~
Thi~'~~iAvei~~yii~des a method for designing effective
and safe subunit vaccines for prevention of SARS using a
recombinant fusion protein (designated RBD-Fc, see Fig. 1)
containing RBD linked to human IgG Fc fragment (for facilitating
BRD purification) as an antigen to immunize rabbits and
evaluated the antibody titers of binding RBD and neutralizing
SARS-CoV was used in order to design effective and safe subunit
vaccines for prevention of SARS.
The invention will be better understood by reference to the
Experimental Details which follow, but those skilled in the art
will readily appreciate that the specific experiments detailed
are only illustrative, and are not meant to limit the invention
as described herein, which is defined by the claims which follow
thereafter.
- 19 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Ex~~~:9iitYezitdl=t~ DnetailLs~
Materials and Methods
Expression of recombinant RBD-Fc and S1-C9 proteins. Plasmid
encoding a 193-amino-acid fragment of SARS-CoV S protein,
corresponding to the receptor-binding domain, fused with the Fc
domain of human IgG1 (RBD-Fc) and plasmid encoding S1 protein
(residues 12-672) tagged with C9 at the C-terminus (S1-C9) has
been described previously (21, 23) . The RBD-Fc and S1-C9 proteins
were, respectively, expressed by transfecting 293T cells with
the plasmids using Fugene 6 reagents (Boehringer Mannheim,
Indianapolis, IN) according to the manufacturer's protocol.
Supernatants were harvested 72 h post-transfection. Recombinant
RBD-Fc fusion proteins were purified by Protein A Sepharose 4
Fast Flow (Amersham Biosciences, Piscataway, NJ), and S1-C9
proteins were purified by affinity chromatography with anti-C9
mouse monoclonal antibody (mAb) 1D4 (National Cell Culture
Center, Minneapolis, MN).
Immunization of mice with RBD-Fc. Ten Balb/c mice (4 wks old)
were immunized subcutaneously with 10 g purified RBD-Fc
resuspended in PBS (pH 7.2) in the presence of MLP+TDM Adjuvant
System (Sigma, Saint Louis, MI) and boosted with the same
antigen preparations at 3-wk intervals. Two Balb/c mice as
controls were treated in same way as the immunized mice except
that RBD-Fc was replaced by PBS. Pre-immune sera were collected
before starting the immunization and antisera were collected 4
days after each boost. Sera were kept at 4 C before use.
Production of rabbit antisera. Rabbit antisera directed against
RBD-Fc were produced at Covance Research Products Inc. (Denver,
PA) using their standard protocols. Briefly, NZW rabbits were
immunized intradermally with 150 g purified RBD-Fc resuspended
in phosphate-buffered solution (PBS, pH 7.2) in the presence of
Freund's complete adjuvant (FCA), and boosted with freshly
- 20 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
ii~l~ts~dnR~~ le immunogen and Freund' s incomplete
adjuvant (FIA) at 3-wk intervals. Pre-immune sera were collected
before starting the immunization and antisera were collected 10
days after each boost. Sera were kept at 4 C before use.
Enzyme-linked immunosorbent assay (ELISA). The reactivity of
mouse and rabbit sera with various antigens was determined by
ELISA. Briefly, 1pg/ml recombinant proteins (RBD-Fc or Sl-C9)
or purified human IgG (Zymed, South San Francisco, CA) were
used, respectively, to coat 96-well microtiter plates (Corning
Costar, Acton, MA) in 0.1 M carbonate buffer (pH 9.6) at 4 C
overnight. After blocking with 2% non-fat milk, serially diluted
mouse and rabbit sera, respectively, were added and incubated at
37 C for 1 h, followed by four washes with PBS containing 0.1%
Tween 20. Bound antibodies were detected by addition of HRP-
conjugated goat anti-mouse and rabbit IgG (Zymed) ,
respectively, and the substrate 3,3',5,5'-tetramethylbenzidine
(TMB) sequentially. Absorbance at 450 nm was measured by an
ELISA plate reader (Tecan US, Research Triangle Park, NC).
Neutralization of SARS-CoV infection. Neutralization of SARS-CoV
infection was assessed as previously described (29). Briefly,
Vero E6 cells were plated (5xl 04 cells/well) in 96-well tissue
culture plates and grown overnight. 100 TCID50 (50% tissue-
culture infectious dose) of SARS-CoV BJ01 strain (Accessi.on
number: AY278488) was mixed with an equal volume of diluted
mouse and rabbit sera, respectively, and incubated at 37 C for 1
h. The mixture was added to monolayers of Vero E6 cells.
Cytopathic effect (CPE) was recorded on days 3 post-infection as
previously described (29). The neutralizing titers represented
the dilutions of mouse and rabbit antisera that completely
prevented CPE in 50% of the wells (34) as calculated with Reed's
method (36).
- 21 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Netf 11 p~~tud~~5irus infection. HIV pseudotyped with
SARS-CoV S protein (HIV/SARS-CoV S) was prepared as previously
described (23, 24). In brief, 293T cells were co-transfected with
a plasmid encoding codon-optimized SARS-CoV S protein and a
plasmid encoding Env-defective, luciferase-expressing HIV-1
genome (pNL4-3.luc.RE)using Fugene 6 reagents (Boehringer
Mannheim). Supernatants containing HIV/SARS-CoV S protein were
harvested 48 h post-transfection and used for single-cycle
infection of ACE2-transfected 293T cells. Briefly, ACE2-
expressed 293T cells were plated (104 cells/well) in 96-well
tissue-culture plates and grown overnight. The pseudovirus was
preincubated with 2-fold serially diluted mouse and rabbit sera,
respectively, at 37 C for 1 h before addition to cells. The
culture was re-fed with fresh medium 24 h later and incubated
for an additional 48 h. Cells were washed with PBS and lysed
using lysis reagent included in a luciferase kit (Promega,
Madison, WI). Aliquots of cell lysates were transferred to 96-
well Costar flat-bottom luminometer plates (Corning Costar,
Corning, NY), followed by addition of luciferase substrate
(Promega). Relative light units were determined immediately on
the Ultra 384 luminometer (Tecan US).
Inhibition of Sl-protein binding to soluble ACE2. Recombinant
soluble ACE2 (R&D systems, Inc., Minneapolis, MN) at 2}ig/ml was
coated to 96-well ELISA plates (Corning Costar) in 0.1 M
carbonate buffer (pH 9.6) at 4 C overnight. After blocking with
2% non-fat milk, 2 ug/ml S1-C9 was added to the wells in the
presence of 2-fold serially diluted mouse and rabbit sera,
respectively. After incubation at 37 C for 1 h, the mAb 1D4
(National Cell Culture Center) was added and incubated at 37 C
for an additional 1 h. After washing, the HRP-conjugated goat
anti-mouse IgG (Zymed) and the substrate TMB were used for
detection.
- 22 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Me~~srlit inI~Y~~' of S1 binding to cell-expressed ACE2
by flow cytometry. 106 stable 293T/ACE2 cells were detached and
washed with Hank's balanced salt solution (HBSS) (Sigma, St.
Louis, MO) S1-C9 was added to the cells to a final
concentration of 1}ig/ml in the presence or absence of rabbit
sera at indicated dilutions, followed by incubation at room
temperature for 30 min. After thorough washes, the anti-C9 mAb
1D4 was added to the cells to a final concentration of 10 pg/ml
and incubated at room temperature for 30 min. Cells were washed
with HBSS and incubated with anti-mouse IgG-FITC conjugate
(Sigma) at 1:50 dilution at room temperature for an additional
30 min. After washing, cells were fixed with 1% formaldehyde in
PBS and analyzed in a Becton FACSCalibur flow cytometer
(Mountain View, CA) using Ce1lQuest software.
Experimental Results
Mouse and rabbit antisera directed against RBD-Fc contained high
titers of antibodies binding to RBD and S1 domains. Ten mice
(M1 to M10) were immunized with RBD-Fc and two control mice (N1
and N2) treated with PBS. Mouse antisera were collected before
immunization (pre-immune) and 4 days after each boost at
intervals of 3 wks. The serum samples at 1:10,000 dilutions were
tested for binding to the recombinant fusion protein RBD-Fc by
ELISA. As shown in Figure 2A, the antisera collected 4 days
after the first boost showed moderate binding activity. However,
this activity significantly increased after the second and third
boosts at 3 wks intervals. The antisera collected 4 days after
the third boost showed highest RBD-Fc binding activity.
Therefore, we used this batch of mouse antisera for the
subsequent antibody titration and neutralization experiments.
These antisera bound to RBD-Fc in dose-dependent manner with
geometric mean titer (GMT) at 1:312,500 (Figure 2B). Since RBD-
Fc also contains a human IgG-Fc fragment, the antibodies in the
mouse sera may also bind to Fc, in addition to RBD. Therefore,
- 23 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
{~ t~~~'S ~ G d':: ~Ã k' ti :i~ tt: t=' =.(x !" ~t :.:::t4 y~;'
we ~digtig ~~:3 , rE
~8tivity of mouse antisera against the
recombinant protein S1-C9, which contains RBD but not Fc. As
shown in Figure 2C, mouse antisera bound to S1-C9 in a pattern
similar to that shown in the experiments using RBD-Fc as an
antigen shown in Figure 2B, although the GMT (1:62,500) of the
antibodies against S1-C9 were lower than those to RBD-Fc. This
suggests that anti-RBD antibody is one of the major antibody
populations in mouse antisera.
Rabbit antisera collected before immunization (pre-immune) and
10 days after each boost at intervals of 3 wks were also tested
for their activity of binding to RBD-Fc and S1-C9. As shown in
Figure 3A, the antisera (1:10,000) collected 10 days after the
lst boost had the maximum reactivity with RBD-Fc and retained
the high levels after the 2nd and 3rd boosts. The GMT of the
antisera collected 10 days after the lst boost was 1:7,812,500
(Figure 3B). Therefore, we used these antiserum samples for the
subsequent studies These rabbit antisera also bound to S1-C9 in
a dose-dependent manner with GMT of 1:312,500 (Figure 3C), in
consistence with the results of using mouse antisera.
Mouse and rabbit antisera against RBD-Fc contained high titers
of SARS-CoV-neutralizing antibodies. The antisera were tested
for their neutralizing activity using two different assay
systems, i.e., infection of SARS-CoV in Vero E6 and of HIV
pseudotyped with SARS-CoV S protein (HIV/SARS-CoV S) in 293T
cells expressing ACE2. The antisera from 5 mice at 1:10,240 and
those from the remaining 5 mice at 1:5,120 fully protected Vero
E6 cells from SARS-CoV infection (i.e., no CPE was seen and the
cell monolayer remained intact). At higher serum dilutions, the
cell number decreased due to the CPE induced by SARS-CoV
replication in cells. Here we showed the results obtained from
the mouse M8 as an example (Figure 4A). Mean neutralizing
antibody titer calculated based on Reed's method (36) was
1:10,862. The pre-immune mouse sera at a 1:40 dilution had no
- 24 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
init~orf~ Ril3tl fi-
l~ ~~ c8RS-CoV infection. These mouse antisera
were also highly effective in inhibiting infection by HIV/SARS-
CoV S pseudovirus with 50% neutralizing titer (GMT) of 1:13,636
(Figure 4B), suggesting that the anti-RBD antibodies can inhibit
infection by SARS-CoV and pseudovirus containing SARS-CoV S
protein. Similarly, both rabbit antisera collected 10 days after
the first boost at dilution of 1:10,240 completely inhibited CPE
caused by SARS-CoV replication in Vero E6 cells with GMT of
neutralization of 1:14,482 (Figure 5). These rabbit antisera
also effectively inhibited infection by HIV/SARS-CoV S
pseudovirus (Figure 6). The antisera collected 10 days after the
second and third boosts possessed comparable neutralizing
activity against SARS-CoV and pseudovirus infection (data not
shown).
Depletion of anti-Fc antibodies from the antisera directed
against RBD-Fc did not affect the RBD-binding and neutralizing
activity. Since the recombinant fusion protein RBD-Fc also
contains a human IgG Fc fragment, it is expected that this
antigen will also induce anti-Fc antibodies. Indeed, the rabbit
antisera (1:100) reacted with human IgG-Fc coated in the wells
of plates (Figure 7A). However, the anti-Fc antibodies could be
depleted from the antisera by passing the antisera th-rough a
column conjugated with human IgG since the anti-Fc-depleted
antisera had no reaction with human IgG in ELISA (Figure 7A).
Anti-Fc-depleted antisera retained the RBD-binding activity
(Figure 7B) and neutralizing activity against infection by
HIV/SARS-CoV S pseudovirus (Figure 7C), comparable with the
untreated rabbit antisera. These results suggest that the anti-
Fc antibodies in the antisera induced by human IgG-Fc had no
contribution to the RBD-binding and virus-neutralizing activity
of the rabbit antisera.
Mouse and rabbit antisera against RBD-Fc effectively blocked RBD
binding to ACE2. We tested whether the anti-RBD antibodies in
- 25 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
N =_~ .~ ..... " 'E,~ : :[~,:~~, H;;l1 n lt~~w
th i,~.
~no s'e' ta~ ~1l1~a~ aj'-=~'~zsera block RBD binding to soluble and
cell-associated AEC2 using ELISA and flow cytometry,
respectively. Since RBD-Fc can also react with the anti-Fc
antibodies in the antisera directed against RBD-Fc, we used S1-
C9 which contains only RBD, but not Fc in all the experiments
for determining the binding of RBD. In ELISA assay, soluble ACE2
was coated on the wells of ELISA plates and Si-C9 significantly
bound to ACE2 (data not shown). Both mouse and rabbit anti-RBD
antisera effectively blocked S1 binding to ACE2 in a dose-
dependent manner while the pre-immune sera had no inhibitory
activity (Figure 8A and 8B). Interestingly, the sera from five
mice (M6 to M10) with better activity of inhibiting S1-ACE2
binding had more potent neutralizing activity against SARS-CoV
infection in Vero E6 cells than antisera from the mice M1 to M5
(data not shown) . Soluble ACE2 coated on plastics may lose the
native conformation, so we also used cell expressed ACE2, which
is expected to retain the native conformation, for detecting the
RBD-binding activity in a flow-cytometric assay. As shown in
Figure 8C, S1-C9 significantly bound to ACE2-expressed cells as
measured using anti-C9 mAb 1D4 (positive control) . If no S1-C9
was added (negative control), only background signals were
detected. Rabbit antisera at 1:100 effectively blocked S1
binding to ACE2-expressed cells while pre-immune rabbit sera at
the same dilution had no inhibitory activity. The inhibitory
activity of the rabbit antisera on S1 binding to ACE2-expressed
cells was dose-dependent. Depletion of anti-Fc antibodies from
the rabbit antisera did not affect the inhibitory activity of
the rabbit antisera on S1-ACE2 interaction (Figure 8D),
confirming that the anti-RBD activity is not mediated by anti-Fc
antibodies.
Discussion of the results
During the SARS pandemic of 2002/2003, despite the lack of
effective and specific therapy, most SARS patients recovered
- 26 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
fr te a,~'=u~e very few patents could be re-infected
by SARS-CoV (http://www.who.int/csr/sars/en). Neutralizing
antibodies were detectable in the convalescent sera of SARS
patients (37) . Passive transmission of the convalescent sera was
used for treatment of SARS-CoV infection
(http://www.crienglish.com/144/2003-5-7/11@12493.htm).
Inoculation of hyperimmune sera from mice infected by SARS-CoV
(38) or immunized with MVA/S (35) reduced the titers of SARS-CoV
in the respiratory tracts after challenge. Theses data suggest
that protective humoral immunity is achievable and vaccines can
be developed for prevention of SARS.
A number of vaccine candidates are in clinical trial and
preclinical study, including inactivated vaccines, DNA vaccines
and vaccinia virus-based vaccines encoding SARS-CoV S protein
(9, 10, 34, 35). These agents are effective in inducing
protective neutralizing antibody response in animals (34, 35). In
the present study, we used a recombinant fusion protein (RBD-Fc)
as an immunogen to immunize mice and rabbits since RBD is a key
functional domain in the S protein responsible for viral binding
to receptor on the target cell (24-26) and contains neutralizing
epitopes (39). Antibodies against RBD on the S proteins of other
coronaviruses, such as MHV, transmissible gastroenteritis virus
(TGEV) and human coronavirus (HCoV-229E)(40-42), and those
against receptors for the coronaviruses (43, 44) are highly
effective in blocking RBD-receptor interaction and neutralizing
infection by the corresponding coronaviruses. We found that the
RBD-Fc fusion protein elicited highly potent neutralizing
antibody responses in the immunized mice and rabbits and the
antisera could completely block SARS-CoV infection at the serum
dilutions of 1:5,120 to 1:10,240 (Figures 4 and 5) with
geometric mean neutralizing titers of 1:10,381 (mouse antisera)
and 1:14,451 (rabbit antisera), about 40-200 fold more potent
than those of antisera from mice immunized with DNA vaccines and
- 27 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
tr~~, ~~: ,,:~4,,: , Y ~t:,,:, +t~,: k tl,,~ 4~:;Ii '"=f tt tt::'.
vac~'~~lta~'~~v~~;~t~~a's -~' ~a'c~~~nes encoding the full-length S protein
(34, 35).
Since full-length S protein contains RBD and other viral
functional domains and multiple neutralizing epitopes, it is
expected to induce more potent neutralizing antibodies than RBD
alone. One possible reason why RBD actually elicited much higher
titers of neutralizing antibodies than full-length S protein is
that the latter contains non-neutralizing epitopes that may
elicit enhancing antibodies, like those induced by antigenic
sites on the envelope glycoproteins of HIV and Ebola virus (45-
48) . The S proteins from some coronaviruses could also induce
enhancing antibodies. For example, immunization of felines with
a vaccinia virus vector encoding the S protein of feline
infectious peritonitis virus (FIPV) resulted in enhancement of
virus replication after virus challenge (49, 50) and the
epitopes that elicit enhancing antibodies were localized in the
S protein (51). Although no enhanced virus replication was
observed in mice immunized with DNA vaccines and vaccinia virus-
based vaccines encoding SARS-CoV S protein (34, 35), this may not
exclude the possibility that the enhancing antibody titers are
lower than neutralizing antibody titers. In such case, enhancing
antibodies may suppress or "neutralize" the neutralizing-
antibody activity, resulting in reduced neutralizing-antibody
titers.
Another possibility of the recombinant fusion protein RBD-Fc
being able to induce highly potent neutralizing antibodies may
be because the antigen contains human IgG Fc fragment. Antigen-
presenting cells (APCs), such as dendritic cells and
monocytes/macrophages, can capture, process and present antigens
to T helper cells, which regulate antibody production. It has
been shown that APCs express the high-affinity receptor for IgG
Fc, FcgammaRI (also named CD64) and low-affinity receptor,
FcgammaRIII (CD16) (52, 53) . Through these receptors, APCs can
- 28 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
the immune complex containing antigen
and antibody IgG and enhance antibody response against the
immune complex, resulting in autoimmune diseases (53). However,
if the antigen is a viral protein, such as RBD in the SARS-CoV S
protein, conjugation of human IgG Fc to it may accelerate
presentation of RBD to immune cells for eliciting highly potent
anti-RBD antibody response and neutralizing SARS-CoV infection.
The mouse and rabbit antisera directed against RBD are effective
in binding to RBD on the S1 domain of SARS-CoV S protein
(Figures 2 and 3) and blocking RBD binding to soluble and cell-
expressed ACE2 (Figure 8). These confirm that the mouse and
rabbit antisera contain antibodies specifically targeted to RBD.
Although we have not tested the protective activity of the mouse
and rabbit anti-RBD antibodies in animal models against SARS-CoV
challenge, the high neutralizing titers of these antisera tested
in vitro suggest that RBD-Fc may induce strong protective
immunity in animals and humans, considering that the effective
protection against SARS-CoV infection can be achieved by the
convalescent sera from SARS patients with neutralizing antibody
titers ranging from 1:20 to 1:1,280 (37) and by antisera from
mice immunized by DNA vaccines and vaccinia virus-based vaccines
encoding S protein with low neutralizing antibody titers (1:50
to 1:284) (34, 35).
The sequence of S proteins, especially the S1 domains, of most
coronaviruses are highly variable (14), which is a major concern
in developing effective vaccines against virus strains with
distinct genotypes and phenotypes. However, recent studies have
shown that SARS-CoV strains are quite stable and do not change
as much as that was originally predicted (10). At the early
phase of SARS endemic in 2002/2003, 5 out of the 193 amino acid
residues in the RBD of SARS-CoV S protein are variable due to
the positive selection pressure in the process of transition
from animal (e.g., palm civet) SARS-like-CoV to human SARS-CoV.
- 29 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
d late phases (most virus strains were
isolated from SARS patients during these two phases), there is
no mutation in the RBD sequence.(6). Furthermore, the
conformation of RBD is relatively conserved to ensure the
binding of virus with different subtypes to a specific receptor
on the target cells, even though the linear sequence of RBD may
be variable. One example is B12 mAb which recognizes the
neutralizing epitopes on the CD4-binding domain on HIV-1 gp120
and neutralizes a broad range of HIV-1 primary isolates,
although the linear sequences of CD4-binding regions in gp120
from the corresponding strains are highly variable (54, 55). Our
data have shown that antibodies in the mouse and rabbit antisera
directed against RBD may primarily recognize conformational
epitopes on RBD since the antisera did not react with any of the
peptides overlapping the RBD sequence (He et al., unpublished
data). These suggest that anti-RBD antibodies may have
neutralizing activity with specificity against a broad spectrum
of SARS-CoV strains.
In summary, the recombinant fusion protein RBD-Fc is an ideal
vaccine candidate since it induces highly potent antibodies to
block S1-receptor interaction and to neutralize SARS-CoV
infection and has l'ow level of risk compared with inactivated
viruses or live attenuated virus vectors. Therefore RBD-Fc can
be further developed as an effective and safe subunit vaccine
for prevention of SARS.
- 30 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
Re:64t. ericr.4 a,
1. Ksiazek, T.G., D. Erdman, C.S. Goldsmith, S.R. Zaki, T.
Peret, S. Emery, S. Tong, C. Urbani, J.A. Comer, W. Lim, et
al. 2003. A novel coronavirus associated with severe acute
respiratory syndrome. N. Engl. J. Med. 348:1953-1966.
2. Drosten, C., S. Gunther, W. Preiser, W.S. Van Der, H.R.
Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova,
R.A. Fouchier, et al. 2003. Identification of a novel
coronavirus in patients with severe acute respiratory
syndrome. N. Engl. J. Med. 348:1967-1976.
3. Peiris, J.S., S.T. Lai, L.L. Poon, Y. Guan, L.Y. Yam, W.
Lim, J. Nicholls, W.K. Yee, W.W. Yan, M.T. Cheung, et al.
2003. Coronavirus as a possible cause of severe acute
respiratory syndrome. Lancet 361:1319-1325.
4. Marra, M.A., S.J.M. Jones, C.R. Astell, R.A. Holt, A.
Brooks-Wilson, Y.S.N. Butterfield, J. Khattra, J.K. Asano,
S.A. Barber, S.Y. Chan, et al. 2003. The genome sequence of
the SARS-associated coronavirus. Science 300:1399-1404.
5. Rota, P.A., M.S. Oberste, S.S. Monroe, W.A. Nix, R.
Campagnoli, J.P. Icenogle, S. Penaranda, B. Bankamp, K.
Maher, M.H. Chen, et al. 2003. Characterization of a Novel
Coronavirus Associated with Severe Acute Respiratory
Syndrome. Science 300:1394-1399.
6. Chinese SARS Molecular Epidemiology Consortium. 2004.
Molecular evolution of the SARS coronavirus during the
course of the SARS epidemic in China. Science 303:1666-
1669.
7. Holmes, K.V. and L. Enjuanes. 2003. VIROLOGY: The SARS
coronavirus: a postgenomic era. Science 300:1377-1378.
- 31 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
'~ ~.;' ~i~iY. Q. He, X. L. Liu,
Z.X. Zhuang, C.L.
8+F: Guan, , ~
Cheung, S.W. Luo, P.H. Li, L.J. Zhang, Y.J. Guan, et al.
2003. Isolation and characterization of viruses related to
the SARS coronavirus from animals in Southern China.
Science 302:276-278.
9. Fleck, F. 2004. SARS virus returns to China as scientists
race to find effective vaccine. Bull. World Health Organ
82:152-153.
10. Marshall, E. and M. Enserink.12004. Medicine. Caution urged
on SARS vaccines. Science 303:944-946.
11. Oba, Y. 2003. The use of corticosteroids in SARS. N. Engl.
J. Med. 348:2034-2035.
12. Wang, H., Y. Ding, X. Li, L. Yang, W. Zhang, and W. Kang.
2003. Fatal aspergillosis in a patient with SARS who was
treated with corticosteroids. N. Engl. J. Med. 349:507-508.
13. Enserink, M. 2004. One year after outbreak, SARS virus
yields some secrets. Science 304:1097.
14. Cavanagh,D. 1995. The coronavirus surface glycoprotein. In
The Coronaviridae. S.G.Siddell, editor. Plenum Press, New
York and London. 73-114.
15. Lai, M.M. and D. Cavanagh. 1997. The molecular biology of
coronaviruses. Adv. Virus Res. 48:1-100.
16. Gallagher, T.M. and M.J. Buchmeier. 2001. Coronavirus spike
proteins in viral entry and pathogenesis. Virology 279:371-
374.
17. Phillips, J. J. , M.M. Chua, G.F. Rall, and S.R. Weiss. 2002.
Murine coronavirus spike glycoprotein mediates degree of
viral spread, inflammation, and virus-induced
immunopathology in the central nervous system. Virology
301:109-120.
- 32 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
18IM" oIlmJ4; i~l-ARS-associated coronavirus. N. Engl. J
Med. 348:1948-1951.
19. Ho, T.Y., S.L. Wu, S.E. Cheng, Y.C. Wei, S.P. Huang, and
C.Y. Hsiang. 2004. Antigenicity and receptor-binding
ability of recombinant SARS coronavirus spike protein.
Biochem. Biophys. Res. Commun. 313:938-947.
20. Li, W.H., M.J. Moore, N.Y. Vasilieva, J.H. Sui, S.K. Wong,
A.M. Berne, M. Somasundaran, J.L. Sullivan, K. Luzuriaga,
T.C. Greenough, et al. 2003. Angiotensin-converting enzyme
2 is a functional receptor for the SARS coronavirus. Nature
426:450-454.
21. Prabakaran, P., X. Xiao, and D.S. Dimitrov. 2004. A model
of the ACE2 structure and function as a SARS-CoV receptor.
Biochem. Biophys. Res. Commun. 314:235-241.
22. Dimitrov, D.S. 2003. The Secret Life of ACE2 as a Receptor
for the SARS Virus. Cell 115:652-653.
23. Wang, P., J. Chen, A. Zheng, Y. Nie, X. Shi, W. Wang, G.
Wang, M. Luo, H. Liu, L. Tan, et al. 2004. Expression
cloning of functional receptor used by SARS coronavirus.
Biochem. Biophys. Res. Commun. 315:439-444.
24. Wong, S.K., W. Li, M.J. Moore, H. Choe, and M. Farzan.
2003. A 193-amino-acid fragment of the SARS coronavirus S
protein efficiently binds angiotensin-converting enzyme 2.
J. Biol. Chem. 279:3197-3201.
25. Xiao, X., S. Chakraborti, A.S. Dimitrov, K. Gramatikoff,
and D.S. Dimitrov. 2003. The SARS-CoV S glycoprotein:
expression and functional characterization. Biochem.
Biophys. Res. Commun. 312:1159-1164.
26. Babcock, G.J., D.J. Esshaki, W.D. Thomas, Jr., and D.M.
Ambrosino. 2004. Amino acids 270 to 510 of the severe acute
- 33 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
i~ad~~ ~~4'd~rft~'~' coronavirus spike protein are required
for interaction with receptor. J. Virol. 78:4552-4560.
27. Jiang, S., K. Lin, N. Strick, and A.R. Neurath. 1993. HIV-1
inhibition by a peptide. Nature 365:113.
28. Wild, C.T., D.C. Shugars, T.K. Greenwell, C.B. McDanal, and
T.J. Matthews. 1994. Peptides corresponding to a predictive
alpha-helical domain of human immunodeficiency virus type 1
gp4l are potent inhibitors of virus infection. Proc. Natl.
Acad. Sci. USA 91:9770-9774.
29. Liu, S., G. Xiao, Y. Chen, Y. He, J. Niu, C. Escalante, H.
Xiong, J. Farmar, A.K. Debnath, P. Tien, et al. 2004.
Interaction between the heptad repeat 1 and 2 regions in
spike protein of SARS-associated coronavirus: implication
for virus fusogenic mechanism and identification of fusion
inhibitors. Lancet 363:938-947.
30. Tripet, B., M.W. Howard, M. Jobling, R.K. Holmes, K.V.
Holmes, and R.S. Hodges. 2004. Structural characterization
of the SARS-coronavirus spike S fusion protein core. J.
Biol. Chem. 279:20836-20849.
31. Chan, D.C., D. Fass, J.M. Berger, and P.S. Kim. 1997. Core
structure of gp4l from the HIV envelope glycoprotein. Cell
89:263-273.
32. Bosch, B.J., Z.R. van der, C.A. de Haan, and P.J. Rottier.
2003. The coronavirus spike protein is a class I virus
fusion protein: structural and functional characterization
of the fusion core complex. J Virol 77:8801-8811.
33. Xu, Y., Y. Liu, Z. Lou, L. Qin, X. Li, Z. Bai, H. Pang, P.
Tien, G.F. Gao, and Z. Rao. 2004. Structural basis for
coronavirus-mediated membrane fusion: Crystal structure of
MHV spike protein fusion core. J. Biol. Chem. Apr. 27 [Epub
ahead of print].
- 34 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
3V:1'Y;-:Y. Huang, A. Roberts, B.R. MurPhY~
K. Subbarao, and G.J. Nabel. 2004. A, DNA vaccine induces
SARS coronavirus neutralization and protective immunity in
mice. Nature 428:561-564.
35. Bisht, H., A. Roberts, L. Vogel, A. Bukreyev, P.L. Collins,
B.R. Murphy, K. Subbarao, and B. Moss. 2004. Severe acute
respiratory syndrome coronavirus spike protein expressed by
attenuated vaccinia virus protectively immunizes mice.
Proc. Natl. Acad. Sci. U. S. A 101:6641-6646.
36. Reed, L.J. and H. Muench. 1938. A simple method of
estimating fifty per cent endpoints. Am. J. Hyg. 27:493-
497.
37. Zheng, B.J., K.H. Wong, J. Zhou, K.L. Wong, B.W. Young,
L.W. Lu, and S.S. Lee. 2004. SARS-related virus predating
SARS outbreak, Hong Kong. Emerg. Infect. Dis. 10:176-178.
38. Subbarao, K., J. McAuliffe, L. Vogel, G. Fahle, S. Fischer,
K. Tatti, M. Packard, W.J. Shieh, S. Zaki, and B. Murphy.
2004. Prior infection and passive transfer of neutralizing
antibody prevent replication of severe acute respiratory
syndrome coronavirus in the respiratory tract of mice. J
Virol 78:3572-3577.
39. Sui, J., W. Li, A. Murakami, A. Tamin, L.J. Matthews, S.K.
Wong, M.J. Moore, A.S. Tallarico, M. Olurinde, H. Choe, et
al. 2004. Potent neutralization of severe acute respiratory
syndrome (SARS) coronavirus by a. human mAb to Sl protein
that blocks receptor association. Proc. Natl. Acad. Sci. U.
S. A 101:2536-2541.
40. Kubo, H., Y.K. Yamada, and F. Taguchi. 1994. Localization
of neutralizing epitopes and the receptor-binding site
within the amino-terminal 330 amino acids of the murine
coronavirus spike protein. J. Virol. 68:5403-5410.
- 35 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
claude, B. Delmas, and H. Laude. 1994.
Major receptor-binding and neutralization determinants are
located within the same domain of the transmissible
gastroenteritis virus (coronavirus) spike protein. J.
Virol. 68:8008-8016.
42. Bonavia, A., B.D. Zelus, D.E. Wentworth, P.J. Talbot, and
K.V. Holmes. 2003. Identification of a receptor-binding
domain of the spike glycoprotein of human coronavirus HCoV-
229E. J Virol 77:2530-2538.
43. Williams, R.K., G.S. Jiang, and K.V. Holmes. 1991. Receptor
for mouse hepatitis virus is a member of the
carcinoembryonic antigen family of glycoproteins. Proc.
Natl. Acad. Sci. U. S. A 88:5533-5536.
44. Smith, A.L., C.B. Cardellichio, D.F. Winograd, M.S. de
Souza, S.W. Barthold, and K.V. Holmes. 1991. Monoclonal
antibody to the receptor for murine coronavirus MHV-A59
inhibits viral replication in vivo. J Infect. Dis. 163:879-
882.
45. Jiang, S., K. Lin, and A.R. Neurath. 1991. Enhancement of
human immunodeficiency virus type-1 (HIV-1) infection by
antisera to peptides from the envelope glycoproteins
gp120/gp41. J. Exp. Med. 174:1557-1563.
46. Geisbert, T.W., L.E. Hensley, J.B. Geisbert, and P.B.
Jahrling. 2002. Evidence against an important role for
infectivity-enhancing antibodies in Ebola virus infections.
Virology 293:15-19.
47. Takada, A. and Y. Kawaoka. 2003. Antibody-dependent
enhancement of viral infection: molecular mechanisms and in
vivo implications. Rev. Med. Virol 13:387-398.
- 36 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
~ F i{<: e= r,~E,
48I~""'ask' ~v;T~A ~tnabe K. Okazaki, H. Kida, and Y.
Kawaoka. 2001. Infectivity-enhancing antibodies to Ebola
virus glycoprotein. J. Virol. 75:2324-2330.
49. Vennema, H., R.J. de Groot, D.A. Harbour, M. Dalderup, T.
Gruffydd-Jones, M.C. Horzinek, and W.J. Spaan. 1990. Early
death after feline infectious peritonitis virus challenge
due to recombinant vaccinia virus immunization. J. Virol.
64:1407-1409.
50. Corapi, W.V., C.W. Olsen, and F.W. Scott. 1992. Monoclonal
antibody analysis of neutralization and antibody-dependent
enhancement of feline infectious peritonitis virus. J.
Virol. 66:6695-6705.
51. Olsen, C.W., W.V. Corapi, R.H. Jacobson, R.A. Simkins, L.J.
Saif, and F.W. Scott. 1993. Identification of antigenic
sites mediating antibody-dependent enhancement of feline
infectious peritonitis virus infectivity. J Gen Virol 74
Pt 4):745-749.
52. Grage-Griebenow, E., R. Zawatzky, H. Kahlert, L. Brade, H.
Flad, and M. Ernst. 2001. Identification of a novel
dendritic cell-like subset of CD64(+) / CD16(+) blood
monocytes. Eur. J Immunol. 31:48-56.
53. Yada, A., S. Ebihara, K. Matsumura, S. Endo, T. Maeda, A.
Nakamura, K. Akiyama, S. Aiba, and T. Takai. 2003.
Accelerated antigen presentation and elicitation of humoral
response in vivo by Fcgamma. Cell Immunol. 225:21-32.
54. Roben, P., J.P. Moore, M. Thali, J. Sodroski, C.F. Barbas,
III, and D.R. Burton. 1994. Recognition properties of a
panel of human recombinant Fab fragments to the CD4 binding
site of gp120 that show differing abilities to neutralize
human immunodeficiency virus type 1. J Virol 68:4821-4828.
- 37 -
CA 02569142 2006-11-29
WO 2005/120565 PCT/US2005/019266
55~~: ~4~NK~ss14 ':i~E~ E4i~t.A.~y'!~(~~ii,'~~If.{E; McKenna, E.A. Emini, C.
P. Chan, M.D.
Patel, S.K. Gupta, G.E. Mark, III, C.F. Barbas, III, D.R.
Burton, and A.J. Conley. 1997. Recombinant human monoclonal
antibody IgG1b12 neutralizes diverse human immunodeficiency
virus type 1 primary isolates. AIDS Res. Hum. Retroviruses
13:575-582.
56. Vennema, H., R.J. de Groot, D.A. Harbour, M. Dalderup, T.
Gruffydd-Jones, M.C. Horzinek, and W.J. Spaan. 1990. Early
death after feline infectious peritonitis virus challenge
due to recombinant vaccinia virus immunization. J. Virol.
64:1407-1409.
57. Corapi, W.V., C.W. Olsen, and F.W. Scott. 1992. Monoclonal
antibody analysis of neutralization and antibody-dependent
enhancement of feline infectious peritonitis virus. J.
Virol. 66:6695-6705.
58. Jiang, S., K. Lin, and A.R. Neurath. 1991. Enhancement of
human immunodeficiency virus type-1 (HIV-1) infection by
antisera to peptides from the envelope glycoproteins
gp120/gp41. J. Exp. Med. 174:1557-1563.
59. Chamow,S.M. and Ashkenazi.A. 1999. Antibody fusion
proteins. S.M.Chamow and Ashkenazi.A., editors. Wiley-Liss,
InC., New York.
60. Sondermann, P., R. Huber, V. Oosthuizen, and U. Jacob.
2000. The 3.2-A crystal structure of the human IgG1 Fc
fragment-Fc gammaRIII complex. Nature 406:267-273.
61. Huber, R., J. Deisenhofer, P.M. Colman, M. Matsushima, and
W. Palm. 1976. Crystallographic structure studies of an IgG
molecule and an Fc fragment. Nature 264:415-420.
- 38 -
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 38
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 38
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: