Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
1
Securing dose quality of inhalable drug
TECHNICAL FIELD
The present invention relates to a method and a device applied in a single
dose dry powder inhaler for bringing a medicament dose into the dry powder
inhaler in preparation of an inhalation of the dose being enclosed in a sealed
dose container.
BACKGROUND
Within health care today administration of medicaments by inhalation for
distributing dry powder medicaments directly to the airways and lungs of a
user is becoming more and more popular, because it offers an efficient, fast,
and user friendly delivery of the specific medication substance.
Different types of inhalers are available on the market today, such as
metered dose inhalers (MDIs), nebulizers and dry powder inhalers (DPIs).
MDIs use medicaments in liquid form and may use a pressurized drive gas
to release a dose. Usually MDIs have a relatively low capacity for delivering
an effective dose of the active substance in a single inhalation and many
devices have problems with using a drive gas, which is environmentally
acceptable. Nebulizers are fairly big, non-portable devices. Dry powder
inhalers have become more and more accepted in the medical service,
because they deliver an effective dose in a single inhalation, they are
reliable,
often quite small in size and easy to operate for a user. Two types are
common, multi-dose dry powder inhalers and single dose dry powder
inhalers. Multi-dose devices have the advantage that a quantity of
medicament powder, enough for a large number of doses, is stored inside the
inhaler and a dose is metered from the store shortly before it is supposed to
be inhaled. Single dose inhalers either require reloading after each
administration or they may be loaded with a limited number of individually
packaged doses, where each package is opened shortly before inhalation of
the enclosed dose is supposed to take place.
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
2
Gelatin or plastic capsules and blisters made of aluminum or plastic, or
laminates comprising aluminum and plastic foil are common prior art
containers for metered single doses of dry powder medicaments. Typically,
the user has to open the inhaler, insert at least one container into the
inhaler, close it, push a button to force one or more sharp instrument(s) to
penetrate a selected container, such that the dose may be accessed by
streaming air when the user at leisure decides to inhale the dose. Besides a
method of breaking the container open inside the inhaler and pour out the
dose in a chamber first, the most common method of opening the container
is to punch one or more holes in the container itself or in a foil sealing the
container. In the first case the powder is poured onto a surface inside the
inhaler and made available for inhalation from there. In the second case the
dose is aerosolized by inhalation air being forced through the container or
the dose being shaken out of the container and immediately aerosolized' by
streaming air on the outside of the container.
There is a demand for an improved method and device, which will simplify
loading and opening of the dose container to make the powder dose enclosed
therein easily available to a user of the DPI.
SUMMARY
A method of making a metered dry powder medication dose, enclosed in a
dose container, accessible for inhalation with a minimum of exposure to
ambient atmosphere and a hand-operated device for carrying out the method
are disclosed.
The present invention relates to a single dose inhaler, which is provided with
a movable inhaler member, a so called slide, which has at least one
matching receptacle adapted for receiving a selected type of dose container.
The slide is movable between a first, protuding position, where the
receptacle(s) is accessible for loading of a sealed dose container by a user,
and a second, retracted position inside the inhaler.
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
3
In a particular embodiment the slide carrying an unopened, sealed dose
container is arranged to be pushed by a user's hand force from the first
position to the second position. During the motion the seal of the dose
container is opened by an opening member provided inside the inhaler. As
the container continues into the inhaler by the pushing action the dose
inside the container is accessed by an inhalation-induced flow element, e.g.
directed by a suction nozzle being in close proximity to the dose as it, too,
moves into the inhaler carried by the slide.
Optionally, the inhaler is provided with a breath-actuated latch mechanism
preventing the slide from being pushed from the first position, if the
receptacle is loaded with a dose container, unless a suction exceeding a
certain minimum magnitude is provided by the user sucking at a
mouthpiece of the inhaler. This breath-actuation helps the user, to
synchronize the pushing action with an act of inhalation.
DESCRIPTION OF THE DRAWINGS
The invention will be described in the form of a preferred and illustrative
embodiment and by means of the attached drawings, wherein like reference
numbers indicate like or corresponding elements and wherein:
FIG. 1 illustrates in a flow diagram a particular method of the present
invention;
FIG. 2 illustrates a slide applied in a single dose dry powder inhaler in a
top
view with slide in a protruding position (Fig. 2a) and slide in a
retracted position (Fig. 2b) and a side view (Fig. 2c) of slide and
inhaler;
FIG. 3 illustrates a sealed dose container carrying an enclosed dose adapted
for the slide in Fig. 2;
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
4
FIG. 4 illustrates in perspective (Fig. 4a), top (Fig. 4b) and front (Fig. 4c)
views a particular embodiment of a sealed dose container, adapted for
the present invention.
FIG. 5 illustrates two typical inhalation sequences (Fig 5a and Fig. 5b) when
applying the present invention to a dry powder inhaler.
DESCRIPTION OF AN ILLUSTRATIVE EMBODIMENT
The present invention relates to a method and a device for making a metered
dry powder medication dose in a dose container accessible inside a dry
powder inhaler (DPI) in direct connection with an inhalation of the enclosed
dose.
Advantages brought by the disclosure are
= Secures dose quality
= Simple inhaler design
= Few parts needed
= Low cost
= Small inhaler size
= Ease. of use
= Safety
= High level of user compliance
= Titratable dosing by user possible, i.e. different dose sizes may be
selected by user
= All types of inhalable dry powder drugs may be used
In a particular aspect of the present invention, the method comprises
bringing a sealed container carrying a single medicament dose into the
inhaler by means of a movable inhaler member, a so called slide. A user has
access to the slide in a first, protruding position. The slide comprises at
least
one matching receptacle designed for a particular type of dose container. An
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
advantage of the invention is that the user has access to the slide, but no
access to the internal parts of the inhaler, whereby unintentional damage or
contamination of sensitive parts is avoided. Pushing the slide inwards into
the inhaler body, the motion ends in a second, retracted position. Pushing
the slide is done preferably by hand or optionally by a motor. The dose
container is thus brought into the inhaler. The user pushes the slide into the
inhaler while at the same time inhaling through a mouthpiece of the inhaler.
As the dose container enters the inhaler, the container seal begins to be
opened up by an opener device integrated in the inhaler, thereby letting
ambient air into the container and into the dose powder. But at the same
time one or more flow elements of the inhaler gains access to the dose and a
concurrent release of the dose into an inhalation air-stream begins. The time
lapse between opening of the seal and dose release is extremely short, which
secures the quality of the dose when delivered to the inhaling user.
Typically,
the time the particles of the dose are exposed to the atmosphere before they
are entrained into inspiration air is only a split second. When the slide
reaches a second, fully retracted position, the dose has already been
delivered. Preferably, however, the slide cannot move from the first position
unless a sufficiently strong inhalation is already in progress. Thus, the
transport, container opening and dose delivery are carried out in a single
user-initiated action. In this way, the dose is exposed to the atmosphere for
a minimum time, in fact only for the duration of a complete dose delivery.
The exposure of the dose to the atmosphere is consistently short every time
and actually less than the inhalation time itself.
In a further aspect of the invention, illustrated in Figure 2a, 2b and 2c, the
slide 15 is provided with at least one matching receptacle 16 for a selected
type of single dose container 33 in a protective casing 41. Only the defined
selected type of dose container 33+41 can be inserted into the receptacle.
After the dose has been delivered the slide remains in the second, retracted
position until the user activates the slide, such that it comes out of the
inhaler 12 carrying the now spent empty container. The user removes the
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
6
spent container 33+41 and discards it. Pushing the slide without container
back into the inhaler closes the inhaler shut. The user may activate the slide
as needed when the time comes for administering a new dose. Thus, the user
needs never to have access to the inside of the inhaler. According to the
present invention, this novel method of protecting a dose and bringing it into
an inhaler 12, having a mouthpiece 11, makes it possible to arrange a very
efficient, high quality dose delivery with negligible risk of dose degradation
and of foreign matter being accidentally introduced into the inhaler by the
user.
By relying on the user to provide the energy to move the slide and the
inhalation effort for delivering the dose, a very simple, compact and robust
inhaler design is possible. As a result the cost is low and the uncomplicated
design makes the manufacturing simple and very little can go wrong with
the inhaler in the hands of a user.
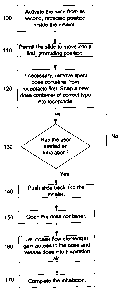
A particular method is described in a flow chart illustrated in Figure 1. A
movable inhaler member, constituting a slide, being in a normal, fully
retracted position inside the inhaler body, is activated in an optional step
100 by e.g. a pushbutton accessible on the inhaler. Preferably, the slide is
held in the fully retracted position by a latch mechanism, so that it cannot
move until the latch is released by any suitable means, e.g. a spring loaded
pushbutton. Preferably, when the slide is released it will come out of the
inhaler automatically, i.e. without further assistance from a user, into a
first,
fully protruding, dose loading position, step 110, where the slide is ready to
accept at least one dose container. If the inhaler has been used before, it is
possible that a spent container is still held in at least one receptacle in
the
slide and must be removed before a new one can be fitted. A new, selected
container of the correct type may now be snapped into the matching
receptacle, step 120. Preferably, the container snaps into place and remains
firmly held there, with no possibility of moving relative the slide. In a
particular embodiment of the present invention, on condition that a user has
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
7
started an inhalation, step 130, the slide is now pushed inwards into the
inhaler, step 140, bringing the container with it. As the container enters the
inhaler body it is opened, step 150, by suitable opening means and a stream
of air is then directed to the dose by an adapted flow element, e.g. a suction
nozzle, step 160. The particles of the dose are thereby released and
entrained into the inspiration airflow leaving the mouthpiece , step 170. In a
preferred embodiment of the present invention, the container is opened while
being transported into the inhaler. In an alternative embodiment the
container may be opened when the slide reaches its second, fully retracted
position, or proximate this position, inside the inhaler. See Figure 5 which
illustrates a typical opening of a container synchronized with an inhalation.
Diagram curve Y represents the suction power in kPa provided by the user
over time X and curve Z represents the container motion from 0 (starting
position) to 100 % (end position) in the DPI.
In a preferred embodiment according to the disclosure, however, it is
advantageous that the bringing of the slide with a dose container into the
inhaler is synchronized to a commenced inhalation, such that the time
during which the dose is exposed to the ambient atmosphere is minimized.
Obviously, the container must be opened before a stream of air can access
the dose. Preferably, the container starts to be opened at a predetermined
point along the stroke made by the slide. A stream of inspiration air is
directed into the container as it is being opened, whereby the air-stream
gains access to the enclosed dose.
The disclosed method must be adapted to the particular type of dose
container, which has been selected for insertion into a particular, adapted
dry powder inhaler. For instance, the receptacle in the slide, firmly holding
the dose container, must be adapted and matched to the container type.
Naturally, the piercing or opening members and the flow elements inside the
inhaler must also be adapted for the container type. Thus, the air flow
resulting from an inhalation is directed by proper channeling into the dose
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
8
container, preferably as soon as the container is being opened by the
piercing or opening members of the inhaler, such that the stream of air may
release the dose and bring the dose into the inspiration air of a user without
unnecessary delay. As already pointed out, different types of dose containers
may be selected and advantageously used in the present invention.
Examples of containers are aluminum or plastic single dose blisters of
varying size and design and also capsules of gelatin, cellulose or plastics. A
person of ordinary skill in the art will know how to adapt the receptacle in
the slide, the insertion of the dose container into the inhaler and the
inhaler
itself, including the piercing or cutting members and air flow channels, to a
particular type of dose container.
The disclosed method eliminates as far as possible any adverse influence
that e.g. humidity in the air may have on the fine particles in the dose, such
as creating particle aggregates and making aggregates more difficult to de-
aggregate when sucked up and delivered to a user of the inhaler. Minimizing
the dose exposure to the atmosphere may preferably be done by
implementing a breath actuation mechanism in the inhaler. The breath
actuation blocks the bringing of the slide and container into the inhaler
until
the user applies at least a minimum suction power to a mouthpiece of the
inhaler. For instance, a pressure sensitive flap may be arranged to open
when the applied suction is strong enough, thus letting air flow into the air
channels of the inhaler, which are in fluid connection with the mouthpiece.
When the flap opens, the blocking of the slide is removed and the slide. may
be pushed into the inhaler while the inhalation is in an early stage of
progress.
In a further aspect of the present invention a damper mechanism is attached
to the slide. In a particular embodiment the damper device provides a first
counterforce counteracting the slide motion out of the inhaler body after the
slide has been activated and released from its fully retracted position. The
slide motion out of the inhaler may optionally but preferably be governed
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
9
and powered by a spring mechanism that provides a spring force, which is
reasonably constant. The spring-driven motion is balanced by the damper
device along the full stroke, or part thereof, of the slide from a second,
fully
retracted position into a first, fully protruding position. The speed of the
slide coming out of the inhaler is thus kept constant. The same or a different
damper may provide a second counterforce, which may or may not be of the
same magnitude as the first counterforce, opposing an applied force pushing
the slide back into the inhaler body. The applied pushing force may be a
manual force provided by a user of the inhaler, or it may be provided by an
independent source of power, e.g. in a particular embodiment where the
slide is governed and powered by an electric motor device. The damper acts
to control the speed of the slide motion into the inhaler by providing a
suitable, second counterforce formed by the damper, such that the speed of
the slide into the inhaler is kept reasonably constant.
Disregarding the cost aspect, a motorized drive system may replace, the
spring mechanism and optionally the dampers and/or the manual pushing
force provided by a user. This version of moving the slide may e.g. be used
where a user has physical handicaps, which restricts or excludes manual
use of the device. As a person skilled in the art will realize, the driving
force,
necessary for driving the relative motion of the slide in and out of the
inhaler, may come from any type of power source, e.g. electric, hydraulic,
pneumatic, spring, mechanical or manual by a user. However, hand
operation by a user is normally preferred, because it offers a low cost,
simple
and safe administration of any type of inhalable dry powder drug.
In another embodiment, however, when a dose container is selected, which
is not suitable for concurrent transport, opening and dose delivery, the
disclosed method and device may be adjusted to comprise the bringing of the
container into a position in the inhaler where the container may be kept in
an unopened state prepared for later delivery. Preferably, a movable inhaler
member, e.g. a slide, is used for bringing the container into this position
and
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
keeping it there, until the container is opened by suitable means
incorporated in the inhaler, e.g. an opener or one or more sharp, piercing
instrument. Preferably, opening is triggered by an act of inhalation, such
that the enclosed dose may be entrained into inspiration air directly from the
container as soon as the container has been opened. In this case too, the
user has no access to the internals of the inhaler. However, such alternative
embodiments might prevent that a gradual and optionally prolonged release
of the dose is obtained. This is an important feature obtained in the
preferred embodiment. A corresponding function of another embodiment,
where the slide with the dose container is resting inside the device before
commencing an inhalation, will make this embodiment more complicated
leading to a more expensive design of the particular adapted inhaler.
In another aspect of the present invention, the time between opening of a
selected dose container and inhalation of the enclosed dose is on the order of
a split second, which is so short that it is negligible. Prior art inhalers
allow
much longer times between subjecting the selected dose to the ambient
atmosphere and an actual inhalation of the dose taking place. Some prior art
inhalers have no control over time lapse between breaking the dose
container open and a following inhalation. In any case, by the influence of
the ambient atmosphere and especially moisture, the dose may decompose
rapidly, such that when the user finally gets round to inhaling the dose, it
may have deteriorated seriously. The user will then unknowingly get a
smaller therapeutic effect than intended.
Generally, dry powder medicament doses need to be protected by an
enclosure not only during storage, but also when inserted in an inhaler
where the dose and its enclosure are kept in a ready state for delivery in an
inhalation at a point in time decided by the user. New types of dry powder
medicaments, not least for systemic treatment, have a rather short expiry
date and they are generally quite sensitive to ambient conditions, especially
moisture during storage and in use. Hence, the demands put on dose
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
11
protection and inhaler devices in handling sensitive doses are therefore
much higher than for prior art devices as used e.g. for administering
traditional medicaments against respiratory disorders. For instance, prior art
blister packages for dry powder medicaments, intended for inhaler use, often
use a fairly thin polymeric seal, which can be easily ripped or punched open
before the dose is supposed to be inhaled. Another common seal is a
peelable blister such that the blister is peeled open prior to inhalation of
the
enclosed dose. Yet another type of prior art dose container is the capsule.
Capsules are often made by gelatin, but polymers and cellulose and other
materials are also used. A common problem for prior art blisters and
capsules used for dry powder doses for inhalation is that the primary
package does not protect sensitive substances from moisture well enough
during storage and in use. Minimizing the time the primary package is
exposed to the atmosphere and minimizing the time during which the dose is
subjected to the ambient atmosphere after opening of the containerF are
therefore important aspects of inhaler and dose container design.
Naturally, using a new type of blister pack, a so-called pod, as a particular
embodiment of a sealed dose container, is to be preferred in an application
where the present invention is to be put to use. See Figure 3 illustrating a
sealed dose container 33 in a protective casing 41. The container encloses a
dose 23, illustrated for the benefit of the reader, although the dose is
located
under a seal 31. Containers providing high quality high barrier seals, such
as a pod, are particularly suited for use of the present invention. High
barrier seals require a pushing force of considerable strength to power
opening of the seal. The hand operated slide provides ample power to
overcome resistance from the sealing, as opposed to many prior art inhalers.
See Figure 4 illustrating a pod carrying a sealed container in a perspective
drawing. Figure 4a shows a sealed container 33 (seal 31) put into a
protective casing 41 adapted for insertion into a dry powder inhaler. Figure
4b shows a top view of the carrier/container and indicates depositions of dry
powder making up a metered dose inside the container 33 under a seal 31,
CA 02569343 2006-11-30
WO 2005/120616 PCT/SE2005/000843
12
for the benefit of the reader. Figure 4c illustrates a front view of the
carrier/ container in Figure 4b.
However, the present invention may also advantageously be applied to
conventional blister packs and capsules. Preferably, a person skilled in the
art may use the disclosure by adapting e.g. when the container seal is to be
opened during the course of moving the container from a starting position to
an end position and adapting how it is opened to the particular type of
container that is selected for use. Such adaptation is still within the scope
of
the present invention. An objective of the present invention is to make the
time between opening of the container and delivery of the dose inside as
short as possible and to make it impossible for the user to open the
container without commencing an inhalation. If an inhalation is broken off
prematurely for any reason, then the user will at least be aware that a,full
dosage may not have been delivered.
In cases where the medicament dosage is controlled by the user, a single
dose dry powder inhaler is preferred, because the user may then select a
dosage among pre-metered doses, which is well adjusted to the situation and
condition the user is in.
It will be understood by those skilled in the art that various modifications
and
changes may be made to the present invention without departing from the
scope thereof, which is defined by the appended claims.