Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-1=
"On-Line/In-Field Bayer Process Liquor Analysis"
Field of the Invention
This invention relates to on-line analysis of multi-component fluids, and in
particular on-line analysis of multi-component fluids containing impurities.
In
particular this invention relates to an apparatus and method for rapidly
performing
these determinations on-line and/or in-field, without having to convey batch
samples to a laboratory for analysis. In one embodiment this invention
provides
for determination of concentrations of alumina, total caustic and total alkali
in a
Bayer process liquor stream.
Background Art
Control of Bayer liquor composition is crucial to obtaining stable control of
the
Bayer process to maximise alumina yield and overall performance of the
process.
The components of a Bayer liquor include sodium hydroxide, sodium aluminate
and alumina, sodium carbonate, and a range of impurities. In general the
impurities maintain constant ratios to one another and can be treated in terms
of
physiochemical properties as a single impurity. The precision of existing on-
line
analysers is limited by the inability to resolve the effects produced by the
carbonate and impurities.
Devices developed in the past to analyse 'Bayer liquors in particular, have
utilised
some of the parameters comprising density, refractive index, sound velocity,
conductivity, maximum conductivity and viscosity in varying combinations to
determine liquor composition.
Conductivity is measured to determine the alumina/total caustic soda ratio.
The
maximum conductivity value observed during isothermal dilution is used to
determine the alumina / total caustic soda ratio.
Density is measured to determine total alkali (total OH- and C032-)
concentration.
A refractometer can be used to measure density in some slurry applications.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-2-
Refractive index and sound velocity are generally considered as alternatives
to
density measurement.
Sound velocity can be measured and used to determine concentration as total
caustic soda or total alkali. Conductivity and density measurements in
combination have been used to estimate values of aiumina and total caustic
soda
and sometimes total alkali. A refractometer can be substituted for the density
meter. All of these methods require temperature measurement for compensation.
Current methods are also typically used with laboratory feedback into the
equations to attempt to account for carbonate and impurity changes.
One example of an attempt at on-line liquor analysis was an apparatus
developed
in Hungary by Aluteru FKI. The Hungarian analyser measures Bayer liquor for
total caustic, total alkalinity and total alumina. The instrument utilises a
unique
relationship between the liquor components and liquor density, conductivity
and
maximum conductivity during isothermal dilution. This instrument relies on low
variability in liquor impurities for accuracy in readings. Thus a serious
disadvantage of the Hungarian analyser is that variability in the
concentration of
liquor impurities away from that for which the analyser is calibrated will
lead to
inaccuracies in parameters measured by the analyser.
Throughout the specification, unless the context requires otherwise, the word
"comprise" or variations such as "comprises" or "comprising", will be
understood to
imply the inclusion of a stated integer or group of integers but not the
exclusion of
any other integer or group of integers.
Disclosure of the Invention
Sound velocity and density have hitherto been considered to be directly
related
and not independent, and therefore not used in conjunction in hitherto known
devices and methods. Because of this belief, it wouldn't be contemplated to
measure both parameters of sound velocity and fluid density, in liquor
analysis.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-3-
Surprisingly, investigations into sound velocity conducted during the
development
of this invention have revealed that sound velocity has enough independence
from liquor density to provide another useful variable. The inventor's
laboratory
work conducted to date shows sufficient differences between density and sound
velocity characteristics to enable the two to be used together to provide
additional
data. Sound velocity is a function of density and bulk modulus (inverse of
compressibility). This test work has shown that changes in total caustic soda
and
alumina have quite different effects on sound velocity and density. That is,
the
bulk modulus plays an important part in the sound velocity values.
The test work also shows that the dilution technique can be used to provide
more
data than the simple alumina / total caustic soda ratio inferred by the
maximum
conductivity. It can be used to provide multiple equations for all
measurements
because the contributions to each measured variable by the different
components
during dilution change in different ways..
In accordance with the invention there is provided a process liquor analyser
having:
means to measure conductivity;
means to measure sound velocity;
means to measure density;
said analyser having a sample inlet to receive liquor sample and a water
inlet selectively fluidly connectable with said means to measure conductivity,
said
means to measure sound velocity, and said means to measure density, and
said means to measure conductivity, means to measure sound velocity and
means to measure density being contained within one or more vessels;
.25 said analyser operable to receive a process liquor sample from said
sample inlet and deliver said process liquor sample to said one or more
vessels to
take measurements of conductivity, sound velocity and density respectively of
said sample,
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-4-
and operable to drain said liquor sample, flush said one or more vessels
with water from said water inlet, and to drain water from said one or more
vessels
before a further said process liquor sample is received.
In the analysis of a Bayer process liquor, the concentration of alumina, total
caustic, and total alkali (and optionally total impurities) can then be
determined
from the measurements taken. This can be determined by regression (for
example linear analysis) to develop simultaneous equations to calculate the
liquor
components. Alternatively reference samples (controls) could be prepared, so
that in-field samples or on-line measurements can be assessed against the
controls. In an electronic device, the data pertaining to the controls could
be
incorporated in a look-up table in data storage memory (volatile or non-
volatile, for
example rom, ram, hard disk etc).
Thus, preferably said process liquor analyser is adapted for analysis of a
Bayer
process liquor, and includes processor means to determine the concentration of
alumina, total caustic, and total alkali (and optionally total impurities)
from the
measurements taken of conductivity, sound velocity and density.
Preferably said processor means is arranged to determine the concentration of
alumina, total caustic, and total alkali (and optionally total impurities)
using
simultaneous equations developed by regression of measurements taken of
conductivity, sound velocity and density.
Preferably the regression is in the form of a linear analysis.
Alternatively said processor means is arranged to determine the concentration
of
alumina, total caustic, and total alkali (and optionally total impurities)
through
comparison of measurements of conductivity, sound velocity and density with
measurements of conductivity, sound velocity and density for reference samples
acting as controls, the measurements for which are incorporated in a look-up
table
in memory or other data storage device.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-5-
Preferably said analyser is operable to controllably receive water from said
water
inlet to dilute said process liquor sample within said one or more vessels, to
allow
said means to measure conductivity to measure conductivity as said sample is
diluted, and optionally to measure maximum dilute liquor conductivity.
Preferably said analyser is adapted for analysis of a Bayer process liquor,
and
includes processor means to determine the concentration of alumina, total
caustic, and total alkali (and optionally total impurities) from the
measurements
taken of conductivity, sound velocity and density, and conductivity during
dilution.
Preferably said processor means is arranged'to determine the concentration of
alumina, total caustic, and total alkali (and optionally total impurities)
using
simultaneous equations developed by regression of measurements taken of
conductivity, sound velocity and density, and conductivity during dilution.
Preferably the regression is in the form of a linear analysis.
Alternatively said processor means is arranged to determine the concentration
of
alumina, total caustic, and total alkali (and optionally total impurities)
through
comparison of measurements of conductivity, sound velocity and density, and
conductivity during dilution, with measurements of conductivity, sound
velocity
and density, and conductivity during dilution for reference samples acting as
controls, the measurements for which are incorporated in a look-up table in
memory or other data storage device.
Preferably said reservoir includes an overflow to drain excess fluid as said
process liquor sample is diluted.
In further alternative arrangements, the parameters of sound velocity and
density
may also be measured as said process liquor sample is diluted, said
parameter(s)
measured being factored into the determination of the concentration of
alumina,
total caustic, and total alkali (and optionally total impurities).
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-6-
Preferably said analyser is operable to flush said sample inlet with water
from said
water inlet and drain water from said sample inlet before a further said
process
liquor sample is received. In this manner the previous sample can be flushed
before the next sample is taken.
Preferably said analyser is operable to flush said one or more vessels with
liquor
from said sample inlet and drain said one or more vessels before receiving a
said
process liquor sample for measurement. In this manner, the water used to flush
the previous sample can be purged so that it does not dilute the sample to be
measured.
Preferably said means to measure conductivity, means to measure sound
velocity, and means to measure density are contained within separate vessels
fluidly connected in series.
Preferably said means to rrieasure conductivity, means to measure sound
velocity, and means to measure density are connected in a circuit with a
reservoir
and a pump to recirculate said process liquor sample as measurements are
taken.
Preferably the means for measuring density is selected from a density meter
and
a refractometer. Density meters suitable for the apparatus include those that
work
on the Coriolis principle (eg those manufactured by Micromotion), those that
work
on the vibrating loop principle (eg those manufactured by Anton Paar), and
those
that work on the vibrating time principle (eg those manufactured by
Solartron).
The most suitable refractometers are those that measure total internal
reflection.
Other types are not as suitable for taking measurements in Bayer process
liquors,
but may be appropriate in other applications.
Preferably said means to measure conductivity utilises the toroidal
conductivity
measurement method. This method is most suitable for Bayer process liquors.
Contacting type conductivity measurement devices are not as suitable for Bayer
process liquors, but may be suitable in other applications. Suitable toroidal
types
may include units manufactured by Rosemont, Foxboro, and Yokogawa.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-7-
Preferably said analyser includes means to measure temperature associated with
said circuit.
Preferably said means to measure temperature is associated with one or more of
said one or more vessels.
Preferably said means to measure temperature is contained within a said vessel
with said means to measure conductivity.
Alternatively said means to measure temperature is contained within all three
vessels.
Preferably at least part of said circuit is associated with means to exercise
stabilising control over temperature of said process liquor sample.
Preferably said means to exercise stabilising control over temperature
includes a
water jacket which may be heated or cooled as required. A water jacket has the
advantage of possessing thermal momentum. Control over temperature to
counter effects of sample temperature change my be exerted by known means
such as Peltier effect devices, or known refrigerative devices operable in
reverse
cycle, or simple heaters. These may be in direct contact with the vessel(s) to
control the sample temperature, or in fluid contact with the water jacket
surrounding the vessel(s).
Also in accordance with the invention there is provided a method of performing
an
on-line/in-field analysis of a Bayer process liquor in a process stream, said
method including steps of taking measurements of conductivity, sound velocity
and density respectively of liquor directly in said process stream, and from
said
measurements, determining concentration of alumina, total caustic, total
alkali,
and optionally total impurities.
Further in accordance with the invention there is provided a method of
performing
an on-line/in-field analysis of a Bayer process liquor from a process stream,
said
method including steps of taking measurements of conductivity, sound velocity
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-8-
and density respectively of a sample taken from said process stream, and from
said measurements, determining concentration of alumina, total caustic, total
alkali, and optionally, total impurities.
Preferably regression is used to develop the equations necessary to calculate
the
liquor components. Measurements of density, conductivity, sound velocity and
temperature are taken on a number of fluids of varying composition to obtain
valid
regressions. The regressions provide a set of equations of the form:
TC = A1 + A2P + A3K + A4Vs
TA = B1 + B2p + B3K + B4Vs
AI = C, + C2p 'E' C3K '+' C4Vs
Where
TC = total caustic
TA = total alkali
Al = alumina
p = density
K = conductivity
VS = sound velocity
Temperature correction may be applied to the individual measurements in the
form:
Pcorrected = pmeasurejl + (treference - tmeasured)]
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-9-
Alternatively, temperature correction may be applied using the Arrhenius Law
method, where operation over a wide temperature range 'is required.
Alternatively, models describing the density, conductivity etc may be
developed
from the test work (or known models may be used) and tuned to the liquor of
interest yielding a set of equations where:
p = fw (TC, TA, Al, t)
x= fw (TC, TA, Al, t)
VS = f w(TC, TA, Al, t)
Commercially available models may be used or empirical models can be
developed from the test work. Where the range of variability is small, a
simple
linear empirical model of the following form may be used:
p D, + D2TC + D3TA + D4AI
x= El + E2TC + E3TA + E4AI
Vs = F, + F2TC + F3TA + F4AI
Temperature correction may be applied to the measurements or can be included
in the equations.
Having developed an appropriate model for the given fluid, any appropriate
mathematical method may be used to solve the simultaneous equations for the
unknown values of the liquor components.
Preferably said method includes taking measurements of conductivity during
dilution of said sample.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-10-
Preferably the temperature at which measurements are taken is maintained
within
a predetermined range. The actual temperature selected should be one which is
practical to operate at. Some density measurement devices require temperature
stability, however where a density measurement device is chosen that is not
temperature sensitive, temperature control is not so critical, as sound
velocity and
conductivity measurement can be readily temperature corrected.
Preferably said method includes stabilising the temperature of said sample
within
a narrow range of 2 C
Preferably said method includes stabilising the temperature of said sample
within
a narrow range of 1 C.
Preferably said method includes stabilising the temperature of said sample
within
a narrow range of 0.5 C.
The Liquor Analyser of the invention utilises four unique relationships which
exist
between each of the Bayer liquor component concentrations and the solution
density, characteristic sound velocity, conductivity and maximum conductivity
during isothermal dilution. With these four relationships, the individual
concentrations of the four components of the Bayer liquor can be determined,
the
four components being total caustic, total alkalinity, total alumina and
impurities.
There are two possible methods of analysis that may be performed according to
the invention. Both involve measuring conductivity, density (and/or refractive
index) and sound velocity, with the second method also including in addition,
measuring conductivity, density (or refractive index) and sound velocity
during
isothermal dilution.
The measurement of conductivity, density (and/or refractive index) and sound
velocity can be applied as a continuous on-line system not requiring sampling
or
physical processing. It has the advantage of being relatively simple to
measure
and provides improved precision over existing continuous on-line methods.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-11-
The measurement of these parameters during isothermal dilution requires
physical processing and may require sampling or a bypass stream.
The inclusion of refractive index measurement with density measurement
generally provides little extra benefit except to assist with diagnostics and
confirmation of results. However, it is theoretically possible to apply these
methods to solutions containing some solids and use the density and refractive
index measurements to resolve the solids content and also to correct the other
measurements for the effects of the solids. Refractive index measurements can
be made in ways that are not affected by the presence of suspended solids.
The method of the invention gathers sufficient parameters to derive an
adequate
number of equations to mathematically resolve the number of unknowns.
The invention provides an apparatus and a method capable of giving the
concentrations of alumina, total caustic and total alkali from a Bayer plant
process
stream on line and continuously without the need of laboratory analysis to
update
the calculation formulae. Sensors measure liquor temperature, density,
conductivity, sound velocity passage through the liquor and additionally
conductivity of variably diluted liquor and feed output signals to a computer
or
similar calculation device wherein they are used to solve a set of equations
and
output the liquor component parameters.
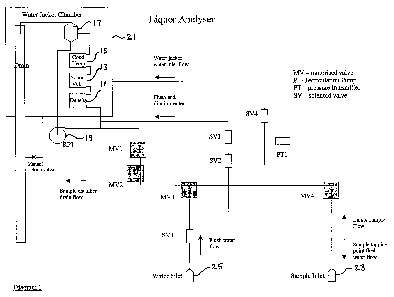
Brief Description of the Drawings
A preferred embodiment of the invention will now be described with reference
to
the attached schematic which is a diagram of the a process liquor analyser
according to the invention.
Best Mode(s) for Carrying Out the Invention
The liquor analyser apparatus and method of the embodiment is intended for use
in real-time or near real-time in-field/on-line analysis of. Bayer process
liquors to
determine the total alumina, total caustic, and total alkali concentrations in
the
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-12-
liquor. The embodiment obviates the need to have the analysis performed in a
laboratory (aside of any calibration analysis). In addition, the total
concentration
of impurities can also be determined.
The apparatus provides concentrations of alumina, total caustic and total
alkali by
relying on accurate determination of liquor temperature, density, sound
velocity,
conductivity and maximum conductivity of the liquor under isothermal dilution.
Automation of the apparatus is achieved using a programmable logic controller.
The apparatus is formed around a vessel 11 containing means to measure
density, a vessel 13 containing means to measure sound velocity, and a vessel
15
containing means to measure conductivity. The vessels 11, 13, and 15 are
fluidly
connected in series, with vessel 15 draining to a reservoir 17. A pump 19 is
provided to recirculate fluid from the reservoir 17 to the vessels 11, 13, 15
in a
closed circuit manner, when all valves are closed, as will be described later.
The
vessels 11, 13, 15 and reservoir 17 are contained within a water jacket
chamber
21 which is maintained at a suitable constant temperature. The temperature can
be any suitable temperature close to that of the sample, for example. In Bayer
process liquors the water temperature can be maintained at a temperature
between 50 and 60 C. Water jacket chamber 21 temperature control is via
electronic temperature controller and an electric heater.
The apparatus has a sample inlet 23 and a water inlet 25, and a drain 27. An
overflow 29 from the reservoir 17 connects to the drain to discharge fluid
during
dilution analysis of the fluid. Sample flows are controlled by automatic
operation
of motorised ball valves MV1, MV2, MV3, and MV4. Water flow control is
controlled via solenoid valves SV1, SV2, SV3, and SV4. The system has caustic
wash capability if an external supply of suitable wash is available.
Alternative
cleaning/descaling methods may be utilised if desired.
As will be described in more detail, under control of processor means in the
form
of a programmable logic controller, the apparatus automatically removes a
sample
of the liquor from the process stream and passes the sample into the vessels
11,
13, and 15 and reservoir 17. It should be noted that in alternative
embodiments
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-13-
the processor means may be a microprocessor, or may be incorporated in
computer software run on a stand-alone or networked computer.
The programmable logic controller records the magnitude of each of the four
measured variables of temperature, density, sound velocity, and conductivity,
once sample temperature stability at within 2 C is attained. Maximum
conductivity under isothermal dilution is determined immediately after.
Algorithms
to determine concentrations of alumina, total caustic, total alkali and
impurities are
performed in the controller.
The on-line liquor analyser operation will now be described. The programmable
logic controller is programmed to sample the process liquor stream at
predetermined intervals. The interval times are adjustable from the main
controller.
The programmable logic controller is programmed to operate the analyser by the
following sequential operations. Once the programmable logic controller
reaches
,
the end of an interval, at which time a sample is required, motorised valves
MV1
and MV2 are opened, draining flush water from the sample chambers. This water
has been present in the chambers since the last operation.
Filling of the vessels with sample liquor commences with motorised valve MV2
being closed and motorised valve MV4 opened, allowing sample liquor to flow
into
and fill the sample chambers. Motorised valves MV1 and MV4 are then closed.
The circulation pump 19 is started and circulates the sample through the
vessels
11, 13, and 15 and reservoir 17, scavenging any remaining water into the
sample.
The circulation pump 19 is stopped, motorised valves MV1 and MV2 are opened,
allowing the sample in the vessels 11, 13, and 15 and reservoir 17 to be
drained.
This sequence of filling, circulating and draining is repeated to remove any
remaining water from the flushing stage which would otherwise dilute the
sample.
Measurement of a sample of process liquor commences with motorised valve
MV2 being closed and motorised valve MV4 opened, allowing a sample of
process liquor to flow into and fill the vessels 11, 13,and 15 and reservoir
17.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-14-
Motorised valves MV1 and MV4 are then closed. The circulation pump 19 is
started and circulates the sample through the vessels 11, 13, and 15 and
reservoir 17. Valves MV3, MV4 and SV3 are opened for several seconds to
purge samples from the sample inlet 23 and fill it with water . The sample is
allowed to cool until the temperature is within 2 C of the selected constant
temperature and the rate of temperature change is less than 0.2 C per minute.
At this point the controller records measurements of temperature,
conductivity,
sound velocity and density.
Water is then slowly added to the sample, by intermittent operation of
solenoid
valves SV1, SV2 and SV3 to determine liquor conductivity during dilution and
maximum dilute liquor conductivity. As the sample is diluted, the conductivity
increases until it peaks and then begins to decline. , This peak measurement
is
recorded by the controller and used in algorithms with the other data to
determine
concentration of alumina, total caustic, total alkali and impurities in the
sample
liquor.
Most commonly, linear regression is used to develop the equations necessary to
calculate the liquor components. Measurements of density, conductivity, sound
velocity and temperature are taken on a number of fluids of varying
composition to
obtain valid regressions. The regressions provide a set of equations of the
form:
TC = A, + A2P + A3K + A4Vs + A5xmax
TA = B1 + B2P + B3K + B4Vs + B5Kmax
Al = C, + C2P + C3K + C4Vs + C5Kmax
Imp = H, + H2P + H3K + H4Vs + H5xmax
Where
TC = total caustic
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-15-
TA = total alkali
Al = alumina
p = density
K = conductivity
VS = sound velocity
Kmax = maximum conductivity during isothermal dilution
Imp = impurities
and AX, BX, Cx, and HX are constants.
Temperature correction may be applied to the individual measurements in the
form:
Pcorrected = pmeasured[1 + (treference - tmeasured)]
Alternatively, models describing the density, conductivity etc may be
developed
from the test work or known models may be used and tuned to the liquor of
interest yielding a set of equations where:
p = fw(TC, TA, Al, Imp, t)
x= fw(TC, TA, Al, Imp, t)
Vs = fw (TC, TA, Al, Imp, t)
Kmax = fvv (TC, TA, Al, Imp, t)
Where t = temperature
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-16-
Commercially available models may be used or empirical models can be
developed from the test work. Where the range of variability is small, a
simple
linear empirical model of the following form may be used:
p = D, + D2TC + D3TA + D4AI + D51mp
K= El + E2TC + E3TA + E4AI + E51mp
VS = F, + F2TC + F3TA + F4AI + F5Imp
Kmax = G, + G2TC + G3TA + G4AI + G51mp
where Dx, Ex, Fx, and Gx are constants.
In most cases, the last equation reduces to Kmax = J1 + J2=AI/TC, where Jx are
constants.
Temperature correction may be applied to the measurements or can be included
in the equations.
Prior to flushing, the pump 19 is stopped. The flushing of the liquor analyser
apparatus then commences with motorised valves MV1 and MV2 being opened
and the sample draining from the vessels 11, 13, and 15 and reservoir 17.
Motorised valve MV2 is closed and motorised valve MV3 and solenoid valve SV3
are both opened, allowing flush water to fill the vessels 11, 13, and 15 and
reservoir 17. Motorised valve MV1 is then closed, solenoid valve SV3 is closed
and circulation pump 19 is started to provide flushing water turbulence to
wash
the probes and vessels 11, 13, and 15 and reservoir 17. Washing of the vessels
11, 13, and 15 and reservoir 17 continues for 2 minutes (in practice, any time
sufficient to clean them), pump 19 is stopped, motorised valves MV1 and MV2
opened allowing the vessels 11, 13, and 15 to drain.
The above described flushing washing and draining procedure is repeated
sufficiently to adequately flush the equipment, after which the vessels 11,
13, and
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-17-
15 and reservoir 17 are filled with water, motorised valves MV1, MV3 and MV2
are closed.
In addition to this the programmable logic controller, at operator selectable
intervals, for example 20 minute intervals, briefly opens motorised valves
MV3,
MV4 and solenoid valve SV3 to flush the sample tapping point
While the programmable logic controller operates by solving simultaneous
equations to derive concentrations of alumina, total caustic, total alkali,
and total
impurities, in an alternative embodiment it may be possible to utilise a look-
up
table established from measured data of samples of known composition.
Generally the procedure will involve developing physiochemical property
models.
Normally it is best to develop the physiochemical property models by
laboratory
work using laboratory precision instruments. However, in some cases this can
be
done in the field with the field instruments. In each case it is necessary to
get
sufficient measurements across a variety of liquor compositions to adequately
describe in mathematical terms the relationships between the component values
and the measured physiochemical properties. This is more easily done in the
laboratory where experimental design can be applied and liquors can be
modified
to suit the design. In the field it is usually necessary to wait until
sufficient
variability has occurred in the liquor of interest. The model may be empirical
or
theoretical as long as it adequately describes the process over the normal
operating range. In some cases the physiochemical property models may already
be known in which case it is only necessary to test sufficiently to verify or
tune the
model for the application.
It should be appreciated that the scope of the invention is not limited to the
specific embodiment disclosed herein. The measurements may be taken in a
number of vessels to suit the particular instruments or a single vessel
containing
all of the measuring instruments.
Methods not involving dilution can be applied to full stream conditions or
simple
side streams but can be done also by sampling systems.
CA 02572319 2006-12-21
WO 2006/007631 PCT/AU2005/001029
-18-
Methods involving dilution (as described in the embodiment) will generally
require
a sampling system and some processing of the sample to maintain a constant or
relatively constant temperature. This can be performed with any conventional
temperature control method such as a heat exchanger, heat tracing or water
jacket. Methods involving dilution will most probably require the circulation
pump
to keep the fluid passing through the cells and to ensure a homogenous
solution
is achieved during dilution. A simple stirrer could be used if a single cell
is used.
Dilution can be achieved by a number of different means such as a dilution
pump,
solenoid valve or other valves. A pump may not be required in methods not
involving dilution.
Dilution methods may involve adding heated water to determine conductivity
during dilution and maximum conductivity during dilution. This technique may
be
applied when analysing spent liquor where scaling is not such an issue.
While the embodiment has been described in relation to Bayer process liquors,
it
may have application in analysis of multi-component fluids in other industries
where the particular measurements are suitable. Specifically contemplated is
analysis of liquors in the pulp and paper industry.