Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
CONVERSION OF NITROGEN DIOXIDE (NO2) TO NITRIC OXIDE (NO)
TECHNICAL FIELD
This description relates to controllably generating nitric oxide.
BACKGROUND
Nitric oxide (NO), also known as nitrosyl radical, is a free radical that is
an
important signaling molecule in pulmonary vessels. Nitric oxide (NO) can
moderate
pulmonary hypertension caused by elevation of the pulmonary arterial pressure.
Inhaling
low concentrations of nitric oxide (NO), for example, in the range of 20-100
ppm can
rapidly and safely decrease pulmonary hypertension in a mammal by vasodilation
of
lo pulmonary vessels.
Some disorders or physiological conditions can be mediated by inhalation of
nitric
oxide (NO). The use of low concentrations of inhaled nitric oxide (NO) can
prevent,
reverse, or limit the progression of disorders which can include, but are not
limited to,
acute pulmonary vasoconstriction, traumatic injury, aspiration or inhalation
injury, fat
embolism in the lung, acidosis, inflammation of the lung, adult respiratory
distress
syndrome, acute pulmonary edema, acute mountain sickness, post cardiac surgery
acute
pulmonary hypertension, persistent pulmonary hypertension of a newborn,
perinatal
aspiration syndrome, haline membrane disease, acute pulmonary thromboembolism,
heparin-protamine reactions, sepsis, asthma and status asthmaticus or hypoxia.
Nitric
oxide (NO) can also be used to treat chronic pulmonary hypertension,
bronchopulmonary
dysplasia, chronic pulmonary thromboembolism and idiopathic or primary
pulmonary
hypertension or chronic hypoxia. Typically, the NO gas is supplied in a
bottled gaseous
form diluted in nitrogen gas (N2). Great care has to be taken to prevent the
presence of
even trace amounts of oxygen (02) in the tank of NO gas because the NO, in the
presence
of 02, is oxidized to nitrogen dioxide (NO2). Unlike NO, the part per million
levels of
NO2 gas is highly toxic if inhaled and can form nitric and nitrous acid in the
lungs.
SUMMARY
In one general aspect, an apparatus for converting nitrogen dioxide to nitric
oxide
includes a receptacle. The receptacle includes an inlet, an outlet, and a
surface-active
material coated with an aqueous solution of an antioxidant. The inlet is
configured to
1
CA 02576957 2012-04-10
72400-20
receive a gas flow and fluidly communicate the gas flow to the outlet through
the
surface-active material such that nitrogen dioxide in the gas flow is
converted to nitric
oxide.
According to another aspect of the present invention, there is provided
an apparatus for converting nitrogen dioxide to nitric oxide comprising a
receptacle
including an inlet, an outlet, and a surface-active material coated with an
aqueous
solution of an antioxidant, wherein the inlet is configured to receive a gas
flow and
fluidly communicate the gas flow including nitrogen dioxide to the outlet
through the
surface-active material such that nitrogen dioxide in the gas flow is
converted to nitric
oxide.
Implementations may include one or more of the following features. For
example, the antioxidant may be ascorbic acid, alpha tocopherol or gamma
tocopherol. The surface-active material may be saturated with the aqueous
solution
of the antioxidant. The surface-active material may include a substrate that
retains
water, such as a silica gel. The silica gel may be 35 to 70 sized mesh. The
receptacle may be a cartridge and may be at ambient temperature.
The surface-active material may be prepared using a solution of
ascorbic acid in water. The solution may be, for example, a 20% solution of
ascorbic
acid in water, a 25% solution of ascorbic acid in water, or a 30% solution of
ascorbic
acid in water. The surface-active material may be prepared by soaking the
surface-
active material in a solution of ascorbic acid in water, and air drying the
surface-active
material, or drying the surface-active material with a gas, before inserting
the surface-
active material into the receptacle.
The receptacle may include screen and glass wool adjacent to both the
inlet and the outlet. The screen and glass wool may be soaked in the aqueous
solution of antioxidant before being inserted in the receptacle.
In another general aspect, an apparatus for generating a therapeutic
gas including nitric oxide for use in delivering the therapeutic gas to a
mammal
includes a permeation cell and a receptacle. The permeation cell has liquid
nitrogen
2
CA 02576957 2012-04-10
72400-20
dioxide and is capable of diffusing gaseous nitrogen dioxide into an air flow.
The
receptacle includes an inlet, an outlet, and a surface-active material coated
with an
aqueous solution of an antioxidant. The inlet is configured to receive the air
flow from
the permeation cell and fluidly communicate the air flow to the outlet through
the
surface-active material coated with an aqueous solution of antioxidant to
convert the
gaseous nitrogen dioxide to nitric oxide at ambient temperature.
According to still another aspect of the present invention, there is
provided an apparatus for generating a therapeutic gas including nitric oxide
for use
in delivering the therapeutic gas to a mammal comprising: a permeation cell
having
liquid nitrogen dioxide and capable of diffusing gaseous nitrogen dioxide into
an air
flow; and a receptacle including an inlet, an outlet, and a surface-active
material
coated with an aqueous solution of an antioxidant, wherein the inlet is
configured to
receive the air flow including nitrogen dioxide from the permeation cell and
fluidly
communicate the air flow to the outlet through the surface-active material to
convert
the gaseous nitrogen dioxide to nitric oxide at ambient temperature.
Implementations may include one or more of the features noted above
and one or more of the following features. For example, an air pump capable of
generating an air flow may be included, and the permeation cell may be
configured to
receive the air flow generated by the air pump. The air pump may be portable.
An
oxygen-enrichment device capable of supplying oxygen in an air flow may be
included, and the permeation cell may be configured to received the air flow
enriched
by the oxygen-enrichment device.
2a
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
The receptacle may be a first receptacle and a second receptacle may be
included.
The second receptacle may include a second inlet, a second outlet, and a
second surface-
active material coated with an aqueous solution of an antioxidant. The second
inlet is
configured to receive the air flow from the first receptacle and fluidly
communicate the
air flow to the second outlet through the second surface-active material
coated with an
aqueous solution of antioxidant to convert the gaseous nitrogen dioxide to
nitric oxide at
ambient temperature. A flexible bag operable to inflate and deflate as the
mammal
breathes may be included, and the second receptacle may be positioned between
the
flexible bag and a point at which the air flow having the nitric oxide is
delivered to the
lo mammal.
Instead of the surface-active material and antioxidant, the second receptacle
may
include a second inlet, a second outlet, and activated alumina. The second
inlet may be
configured to receive the air flow from the first receptacle and fluidly
communicate the
air flow to the second outlet through the activated alumina to trap the
gaseous nitrogen
dioxide at ambient temperature.
Other features will be apparent from the following description, including the
drawings, and the claims.
DESCRIPTION OF DRAWING
Figs. 1 is a block diagram of a cartridge that converts NO2 to NO.
Figs. 2-10 are block diagrams of NO delivery systems using the cartridge of
Fig.
1.
Fig. 11 is a diagram of another cartridge that converts NO2 to NO.
Figs. 12-14 are diagrams of NO delivery systems using the cartridge of Fig.
11.
DETAILED DESCRIPTION
When delivering nitric oxide (NO) for therapeutic use to a mammal, it can be
important to avoid delivery of nitrogen dioxide (NO2) to the mammal. Nitrogen
dioxide
(NO2) can be formed by the oxidation of nitric oxide (NO) with oxygen (02).
The rate of
formation of nitrogen dioxide (NO2) is proportional to the oxygen (02)
concentration
multiplied by the square of the nitric oxide (NO) concentration - that is,
(02)
(NO)*(NO) = NO2.
A NO delivery system that converts nitrogen dioxide (NO2) to nitric oxide (NO)
is
provided. The system employs an surface-active material coated with an aqueous
solution of antioxidant as a simple and effective mechanism for making the
conversion.
3
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
More particularly, NO2 can be converted to NO by passing the dilute gaseous
NO2 over a
surface-active material coated with an aqueous solution of antioxidant. When
the
aqueous antioxidant is ascorbic acid (that is, vitamin C), the reaction is
quantitative at
ambient temperatures. The techniques employed by the system should be
contrasted for
other techniques for converting NO2 to NO. Two such techniques are to heat a
gas flow
containing NO2 to over 650 degrees Celsius over stainless steel, or 450
degrees Celsius
over Molybdenum. Both of these two techniques are used in air pollution
instruments
that convert NO2 in air to NO, and then measure the NO concentration by
chemiluminescence. Another method that has been described is to use silver as
a catalyst
at temperatures of 160 degrees Celsius to over 300 degrees Celsius.
One example of a surface-active material is silica gel. Another example of a
surface-active material that could be used is cotton. The surface-active
material may be
or may include a substrate capable of retaining water. Another type of surface-
active
material that has a large surface area that is capable of absorbing moisture
also may be
used.
Fig. 1 illustrates a cartridge 100 for generating NO by converting NO2 to NO.
The
cartridge 100, which may be referred to as a NO generation cartridge, a GENO
cartridge,
or a GENO cylinder, includes an inlet 105 and an outlet 110. Screen and glass
wool 115
are located at both the inlet 105 and the outlet 110, and the remainder of the
cartridge 100
is filled with a surface-active material 120 that is soaked with a saturated
solution of
antioxidant in water to coat the surface-active material. The screen and glass
wool 115
also is soaked with the saturated solution of antioxidant in water before
being inserted
into the cartridge 100. In the example of Fig. 1, the antioxidant is ascorbic
acid.
In a general process for converting NO2 to NO, an air flow having NO2 is
received
through the inlet 105 and the air flow is fluidly communicated to the outlet
110 through
the surface-active material 120 coated with the aquaeous antioxidant. As long
as the
surface-active material remains moist and the antioxidant has not been used up
in the
conversion, the general process is effective at converting NO2 to NO at
ambient
temperature.
The inlet 105 may receive the air flow having NO2 from an air pump that
fluidly
communicates an air flow over a permeation tube containing liquid NO2, such as
in the
system 200 of FIG 2. The inlet 105 also may receive the air flow having NO2,
for
example, from a pressurized bottle of NO2, which also may be referred to as a
tank of
NO2. The inlet 105 also may receive an air flow with NO2 in nitrogen (N2),
air, or oxygen
4
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
(02).The conversion occurs over a wide concentration range. Experiments have
been
carried out at concentrations in air of from about 2 ppm NO2 to 100 ppm NO2,
and even
to over 1000 ppm NO2. In one example, a cartridge that was approximately 6
inches long
and had a diameter of 1.5-inches was packed with silica gel that had first
been soaked in a
saturated aqueous solution of ascorbic acid. The moist silica gel was prepared
using
ascorbic acid (i.e., vitamin C) designated as A.C.S reagent grade 99.1% pure
from Aldrich
Chemical Company and silica gel from Fischer Scientific International, Inc.,
designated
as S8 32-1, 40 of Grade of 35 to 70 sized mesh. Other sizes of silica gel also
are
effective. For example, silica gel having an eighth-inch diameter also would
work.
The silica gel was moistened with a saturated solution of ascorbic acid that
had
been prepared by mixing 35% by weight ascorbic acid in water, stirring, and
straining the
water/ascorbic acid mixture through the silica gel, followed by draining. It
has been
found that the conversion of NO2 to NO proceeds well when the silica gel
coated with
ascorbic acid is moist. The conversion of NO2 to NO does not proceed well in
an
aqueous solution of ascorbic acid alone.
The cartridge filled with the wet silica gel/ascorbic acid was able to convert
1000
ppm of NO2 in air to NO at a flow rate of 150 ml per minute, quantitatively,
non-stop for
over 12 days. A wide variety of flow rates and NO2 concentrations have been
successfully tested, ranging from only a few ml per minute to flow rates of up
to 5,000 ml
per minute. The reaction also proceeds using other common antioxidants, such
as
variants of vitamin E (e.g., alpha tocopherol and gamma tocopherol).
The antioxidant/surface-active material GENO cartridge may be used for
inhalation therapy. In one such example, the GENO cartridge may be used as a
NO2
scrubber for NO inhalation therapy that delivers NO from a pressurized bottle
source.
The GENO cartridge may be used to remove any NO2 that chemically forms during
inhalation therapy. This GENO cartridge may be used to help ensure that no
harmful
levels of NO2 are inadvertently inhaled by the patient.
First, the GENO cartridge may be used to supplement or replace some or all of
the
safety devices used during inhalation therapy in conventional NO inhalation
therapy. For
3o example, one type of safety device warns of the presence of NO2 in air when
the
concentration of NO2 exceeds a preset or predetermined limit, usually 1 part
per million
or greater of NO2. Such a safety device may be unnecessary when a GENO
cartridge is
positioned in a NO delivery system just prior to the patient breathing the NO
laden air.
5
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
The GENO cartridge converts any NO2 to NO just prior to the patient breathing
the NO
laden air, making a device to warn of the presence of NO2 in air unnecessary.
Furthermore, a GENO cartridge placed near the exit of inhalation equipment and
gas plumbing lines (which also may be referred to as tubing) also reduces or
eliminates
problems associated with formation of NO2 that occur due to transit times in
the
ventilation, equipment. As such, use of the GENO cartridge reduces or
eliminates the
need to ensure the rapid transit of the gas through the gas plumbing lines
that is needed in
conventional applications.
Alternatively or additionally, a NO2 removal cartridge can be inserted just
before
lo the attachment of the delivery system to the patient to further enhance
safety and help
ensure that all traces of the toxic NO2 have been removed. The NO2 removal
cartridge
may be a GENO cartridge used to remove any trace amounts of NO2.
Alternatively, the
NO2 removal cartridge may include heat-activated alumina. A cartridge with
heat-
activated alumina, such as supplied by Fisher Scientific International, Inc.,
designated as
A505-212, of 8-14 sized mesh is effective at removing low levels of NO2 from
an air or
oxygen stream, and yet lets NO gas pass through without loss. Activated
alumina, and
other high surface area materials like it, can be used to scrub NO2 from a NO
inhalation
line.
In another example, the GENO cartridge may be used to generate NO for
therapeutic gas delivery. Because of the effectiveness of the NO generation
cartridge in
converting toxic NO2 to NO at ambient temperatures, liquid NO2 can be used as
the
source of the NO. When liquid NO2 is used as a source for generation of NO,
there is no
need for a pressurized gas bottle to provide NO gas to the delivery system. An
example
of such a delivery system is described in more detail with respect to Fig. 2.
By
eliminating the need for a pressurized gas bottle to provide NO, the delivery
system may
be simplified as compared with a conventional apparatus that is used to
deliver NO gas to
a patient from a pressurized gas bottle of NO gas. A NO delivery system that
does not
use pressurized gas bottles may be more portable than conventional systems
that rely on'
pressurized gas bottles.
Figs. 2-14 illustrate techniques using silica gel as the surface-active
material
employed in a GENO cartridge. As discussed previously, silica gel is only one
example
of a surface-active material that may be used in a NO generation system or
cartridge.
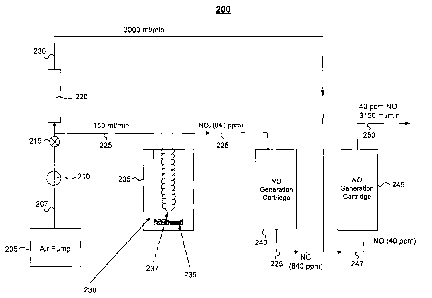
Fig. 2 illustrates a NO generation system 200 that converts liquid NO2 to NO
gas,
which then may be delivered to a patient for NO inhalation therapy. In
general, a flow of
6
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
air generated by an air pump 205 is passed through a gas permeation cell 235
having
liquid NO2 and its dimer N204 (collectively, 236). The air flow exiting the
gas permeation
cell 235 includes gaseous NO2, which is converted to NO gas by a NO generation
cartridge 240. The NO gas mixture may be delivered to a patient for inhalation
therapy,
for example, using a mask, a cannula, or a ventilator. The concentration of NO
in the NO
gas mixture delivered to the patent may be controlled by controlling the
temperature of
the gas permeation cell 235 or the air flow rate through the flow meter 220.
More particularly, the system 200 includes an air pump 205, a regulator 210, a
flow
diverter 215 and a flow meter 220. The system is configured such that air flow
207 from
the air pump 205 is divided into a first flow 225 of 150 ml/min and a second
flow 230 of
3000 ml/min. The air flow 207 may be dry or moist.
The flow 225 is passed through a gas permeation cell 235 containing liquid NO2
and
its dimer N2O4 (collectively, 236) and a gas permeation tube 237. The
permeation cell 235
also may be referred to as a permeation generator, a permeation device or a
permeation tube
holder. The NO2 diffuses through the gas porous membrane of the gas permeation
cell 235
into the flow 225. In one example, the flow 225 of 150 ml/min of air is
allowed to flow
through the permeation tube 237, such as a permeation tube supplied by KinTek
Corporation of Austin, Texas. The permeation tube 237 is designed to release
NO2 at a
steady rate such that the gas stream leaving the permeation tube in the flow
225 contains
about 840 ppm of NO2 when the permeation tube 237 is at a temperature of 40
degrees
Celsius. The region 238 is temperature controlled to maintain a temperature of
approximately 40 degrees Celsius. As discussed more fully below, maintaining
the
temperature of the permeation cell 235 helps to control the concentration of
NO delivered
to the patient.
The 150 ml of air containing 840 ppm of NO2 then flows through a NO generation
cartridge 240. In this example, the NO generation cartridge 240 is 6 inches
long with a
diameter of 1.5 inches and contains moist ascorbic acid on silica gel, which
serves as the
conversion reagent. The NO generation cartridge 240 may be an implementation
of
cartridge 100 of Fig. 1. The air stream 225 exiting from the NO generation
cartridge 240
contains 840 ppm of NO, with all or essentially all of the NO2 having been
converted to
NO.
The 225 flow of 150 ml/min with 840 ppm NO then mixes with the flow 230 of
3000 ml/min of air or oxygen to produce a flow 247 of 3150 ml/min containing
40 ppm of
NO. After mixing, the flow 247 passes through a second NO generation cartridge
245 to
7
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
remove any NO2 that may have been formed during the dilution of NO when the
flows 225
and 230 were mixed. The NO generation cartridges 240 and 245 may be sized the
same,
though this need not necessarily be so. For example, the NO generation
cartridge 245 may
be sized to have a smaller NO2 conversion capacity than the NO generation
cartridge 240.
The resulting flow 250 of air having NO is then ready for delivery to the
patient. The
system 200 may be designed to produce a steady flow of NO gas for a period as
short as a
few hours or as long as 14 days or more. In one test, the system 200 was shown
to deliver a
steady flow of 40 ppm NO gas in air, without NO2, for over 12 days, where the
NO and
NO2 concentrations were measured by a chemiluminescent gas analyzer.
As an alternative to the system 200, a NO generation system may include a
permeation tube that has a larger flow capacity than the permeation tube 237.
In such a
case, the larger permeation tube may be able to process all of the inhaled air
needed to be
delivered to the patient so that, for example, the flow 230 and the conversion
tube 245 are
not necessary.
The system 200 can be made portable, for example, if the air pump 205 used to
supply the -air is a portable air pump, such as a simple oil free pump. If
oxygen-enriched air
is needed by the patient, oxygen can be supplied in addition to, or in lieu
of, the air supplied
by the air pump 205. Oxygen can be supplied, for example, from an oxygen tank
or a
commercially available oxygen generator. Oxygen also can be supplied from a
tank that
has NO2 mixed with 02.
In some implementations, the permeation cell 238 and/or the two conversion
cartridges 240 and 245 may be disposable items.
The concentration of NO in the flow 250 exiting the system 200 is independent
of
the flow 225 through the permeation cell 235, as long as the flow 225 is
greater than a few
milliliters per minute. The concentration of NO in the flow 250 is a function
of the
temperature of the permeation cell 235 and to a lesser degree the air flow
rate 230. For
example, with a constant air flow rate 230, the system 200 is designed to
deliver 40 ppm
NO at a temperature of 40 degrees Celsius; however, the concentration of NO
can be
reduced to 20 ppm NO at 30 degrees Celsius and increased to 80 ppm NO at 50
degrees
Celsius. As such, a temperature controller can be used to adjust the
concentration of the
NO gas to be delivered. Once the desired NO concentration is selected and the
temperature
controller is set to maintain the particular temperature to deliver the
desired concentration,
the delivery rate of NO gas at the desired concentration remains constant. One
example of
a temperature controller is an oven, such as an oven available from KinTek
Corporation, in
8
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
which the permeation tube is placed. Another example of a temperature
controller is a
beaker of de-ionized water placed on a hot plate where the permeation tube is
placed in the
beaker. A thermometer may also be placed in the beaker to monitor the
temperature of the
water.
The NO generation system can be used to deliver a steady flow of NO gas
mixture
for use with a cannula, with the excess gas being vented to the environment.
The NO
generation system can be used with a ventilator, and, in such a case, the
delivery from the
NO generator must remain steady and cannot be shut off without endangering the
patient
receiving the NO. To handle the increased flow necessary during the air intake
to the
patient, the NO gas mixture may be used to inflate and then deflate a flexible
bag. If the air
flow to the patient is delayed in any way, a NO generation cartridge can be
inserted in the
NO generation system at the point immediately prior to inhalation to remove
any NO2 that
may form from NO reacting with 02 during such a delay. This helps to ensure
that even
very small amounts of NO2 that may be formed in the bag during the delay are
removed
prior to the therapeutic gas flow being inhaled by the patient.
A detector can be included in the therapeutic gas delivery system 200 to
detect the
concentration of NO in the therapeutic gas stream. The detector can also
detect the
concentration of NO2 in the therapeutic gas, if necessary, and may provide a
warning if
the NO concentration is outside a predetermined range or if the concentration
of NO2 is
above a threshold value. Examples of monitoring techniques include
chemiluminescence
and electrochemical techniques. The presence of nitric oxide can be detected
by, for
example, a chemiluminescence detector.
Fig. 3 depicts a NO generation system 300 that converts liquid NO2 to NO gas,
which then may be delivered to a patient for NO inhalation therapy. In
contrast to the
NO generation system 200 of Fig. 2, the NO generation system 300 includes an
activated
alumina cartridge 345. The activated alumina cartridge 345 removes any NO2
that forms
during a delay. In contrast to the NO generation cartridge 240, which removes
the NO2 by
converting the NO2 to NO, and thereby quantitatively recovering the NO2, the
activated
alumina cartridge 345 removes NO2 from the process gas stream without
generating NO.
Fig. 4 illustrates a therapeutic gas delivery system 400 that uses a NO
generation
cartridge 440, which maybe an implementation of NO generation cartridge 100 of
Fig. 1.
The system 400 uses a NO source 410 to provide gaseous NO in a flow 420
through
tubing. In one example, the NO source 410 may be a pressurized bottle of NO. A
flow of
air 430 through the tubing is generated by an air pump 435 and is mixed with
the flow
9
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
420. The air flow entering the NO generation cartridge 440 includes gaseous
NO. Any
NO2 gas that may have formed in flow 420 is removed by the NO generation
cartridge
440. The air flow 450 exiting the NO generation cartridge 440 includes
therapeutic NO
gas but is devoid of toxic levels of NO2. The air flow 450 then may be
delivered to a
patient for NO inhalation therapy.
Fig. 5 illustrates a therapeutic gas delivery system 500 that uses a NO
generation
cartridge 540, which may be an implementation of NO generation cartridge 100
of Fig. 1.
In contrast to therapeutic gas delivery system 400 of Fig. 4, the system 500
generates NO
from a NO2 source 510. The NO2 source 510 may use diffuse liquid NO2 in an air
flow
515 generated by an air pump 520 such that the flow 525 exiting the NO2 source
510
includes gaseous NO2. In some implementations, NO2 source 510 maybe a
pressurized
bottle of NO2,
In any case, the air flow 525 entering the NO generation cartridge 440
includes
gaseous NO2. The NO generation cartridge 440 converts the NO2 gas in flow 525
to NO.
The air flow 550 exiting the NO generation cartridge 540 includes therapeutic
NO gas but
is devoid or essentially devoid of NO2. The air flow 550 then may be delivered
to a
patient for NO inhalation therapy.
Fig. 6 illustrates a GENO pressure tank system 600 for delivering therapeutic
gas.
The system 600 includes a tank 620 having 40 ppm NO2 in air, which is
commercially
available, and a flow controller 622. In one example of tank 620, a 300 cu.
ft. tank lasts
1.2 days at an air flow of 5 L/min.
An air flow 625a of NO2 in air exits the flow controller 622 and enters a GENO
cartridge 640. The GENO cartridge 640 uses the NO2 as a precursor and converts
the
NO2 to NO. The air flow 625b exiting the GENO cartridge 640 includes
therapeutic NO
gas. The air flow 625b enters an activated alumina cartridge 660 to remove any
NO2 in
the air flow 625b. The air flow 625c that exits the activated alumina
cartridge 660 is
delivered to a patient for NO inhalation therapy.
The system 600 includes a NOx sample valve 665 and a NO-NO2 sensor 670
operable to detect NO2. A NO-NO2 sensor also may be referred to as a NO-NO2
detector.
3o The NOx sample valve 665 is operable to provide air samples from air flows
667a and
667b to the NO-NO2 sensor 670. Using the NO-NO2 detector 670 to detect the
presence
of any NOz in air flow 667a may provide an indication of a failure of the GENO
cartridge
640, and, as such, provides a prudent safeguard to ensure that no toxic NO2 is
delivered to
the patient.
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
In some implementations, the activated alumina cartridge 660 may be replaced
with a GENO cartridge.
Fig. 7 illustrates a GENO high-concentration NO2 pressure system 700 for
delivering therapeutic gas. In contrast to the system 600 of FIG. 6, the
system 700
includes two GENO cartridges 740 and 750 and a switching valve 745 to control
which of
the GENO cartridges 740 or 750 is used. When a NO-NO2 detector 770 detects the
presence of NO2 in the air flow 725d exiting the GENO cartridge being used,
the
switching valve 745 can be manipulated to switch the air flow 725c to pass
through the
other GENO cartridge 740 or 750. The ability to switch to a second GENO
cartridge in
lo the event of failure of a first GENO cartridge provides an additional layer
of safety for the
patient to whom the therapeutic gas is being delivered.
More particularly, the system 700 includes a tank 720 having 1000 ppm NO2 in
air
and a flow controller 722. In the example, the tank 720 is a 150 cu. ft. tank
at 2250 psi
and provides an air flow of 125 cc/min. At an air flow of 5 L/min of 40 ppm
delivered to
the patient, the tank 720 lasts approximately 23 days. The tank 720 is able to
provide an
air flow for a longer period than the expected life of each GENO cartridge 740
and 750,
which is, in the cartridge used in this example, less than two weeks. As such,
the ability
to switch from one GENO cartridge to another GENO cartridge helps to ensure
that the
contents of the tank are used or substantially used.
An'air flow 725a of NO2 in air exits the flow controller 722 and is mixed with
an
air flow 725b of 5 L/min that is generated by an air source 730, such as an
air pump. The
resulting air flow 725c enters the switching valve 745. The switching valve
745 controls
which of the GENO cartridges 740 or 750 receives the air flow 725c. As shown,
the
switching valve 745 is set such that the air flow 725c is provided to the GENO
cartridge
750. The GENO cartridge 750 converts the NO2 in the air flow 725c to NO. The
air flow
725d exiting the GENO cartridge 725d includes therapeutic NO gas. The air flow
725d
enters an activated alumina cartridge 760 to remove any NO2 in the air flow
725d. The
air flow 725e that exits the activated alumina cartridge 760 is delivered to a
patient for
NO inhalation therapy.
The system 700 includes a NO,, sample valve 765 and an NO-NO2 sensor 770
operable to detect NO2. The NO,, sample valve 765 is operable to provide air
samples
from air flows 767a and 767b to the NO-NO2 sensor 770. Using the NO-NO2 sensor
770
to detect the presence of any NO2 in air flow 767a may provide an indication
of a failure
of the GENO cartridge being used so that the second GENO cartridge may be
used. In
11
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
some implementations, the activated alumina cartridge 760 may be replaced with
a
GENO cartridge.
Fig. 8 illustrates a GENO high-concentration NO2 cartridge system 800 for
delivering therapeutic gas. In contrast to the systems 600 or 700 of FIGS. 6
and 7,
respectively, the system 800 includes a high-concentration NO2 cartridge as
the source of
the NO2 used to generate the NO. More particularly, the system 800 includes an
NO2
cartridge 800, such as a small butane tank or a cartridge conventionally used
to deliver
CO2. In one example of the system 800, a NO2 cartridge with dimensions of 1
inch by 6
inches and filled with 5% NO2 in CO2 was able to deliver NO2 for 14 days.
A NO2 shut-off valve 821 is adjacent to the cartridge 800 to shut-off delivery
of
NO2 from the cartridge 800. The system 800 also includes a flow controller 822
to ensure
a generally constant flow rate of the flow 825a exiting the flow controller
822. The flow
controller 822 is a glass tube with a small hole through which the gas flow
825a passes.
In various implementations of the system 800, the flow controller 822 may
ensure a
constant flow rate of 1 to 10 cc/min.
The gas flow 825a having NO2 exits the flow controller 822 and is mixed with
an
air flow 825b of approximately 5 L/min that is generated by an air source 830.
A gas
mixer 835 ensures that the air flows 825a and 825b are fully (or essentially
fully) mixed.
The resulting air flow 825c with NO2 enters a GENO cartridge 840 that
generates NO.
The system 800 also includes an activated alumina cartridge 860 to remove any
NO2 before the therapeutic gas including NO is delivered to the patient at the
rate of
approximately 5 L/min. The system 800 includes a NO,, sample valve 865 and a
NO-NO2
sensor 870 operable to detect NO2. In some implementations, the activated
alumina
cartridge 860 may be replaced with a GENO cartridge.
Fig. 9 illustrates a GENO permeation system 900 for delivering therapeutic
gas.
The system 900 includes an air flow 925a of approximately 5 L/min that flows
into a
GENO cartridge 940, which acts to humidify the air. After exiting the GENO
cartridge
940, the air flow 925a divides such that an air flow 925b passes through a
permeation
device 935 and an air flow 925c does not. The permeation device 935 includes
permeation tubing 937 and about 10 cc of liquid NO2 936 when the air flow 925a
begins.
The permeation device 935 may be an implementation of the permeation cell 235
of FIG.
2. The permeation device 935 is in a permeation oven 939 to maintain a
constant, or an
essentially constant, temperature to ensure the desired concentration of NO2
is diffused
into the air flow 925b. The air flow 925b and the air flow 925c mix to form
flow 925d
12
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
before entering the GENO cartridge 950. The GENO cartridge 950 converts the
NO2 to
NO.
The system 900 also includes an activated alumina cartridge 960 to receive air
flow 925e and remove any NO2 before the therapeutic gas including NO is
delivered to
the patient at the rate of approximately 5 L/min. The air flow 925f that exits
the activated
alumina cartridge is delivered to a patient for NO inhalation therapy. The
system 900
includes a NO, sample valve 965 and a NO-NO2 sensor 970 operable to detect N02-
Fig. 10 illustrates a GENO permeation system 1000 for delivering therapeutic
gas.
In contrast to the system 900 of Fig. 9, the system 1000 includes valves 1010
and 1015 to
lo control which of the GENO cartridges 1040 and 1050 first receives the air
flow. The
system 1000 uses liquid NO2 in a permeation device 1035 as a source of NO2 to
be
converted to NO. The system 1000 also includes an activated alumina cartridge
1060 to
remove any NO2 before the therapeutic gas including NO is delivered to the
patient at the
rate of approximately 5 L/min. The system 1000 also includes a NOx sample
valve 1065
and a NO-NO2 sensor 1070 operable to detect NO2.
The system 1000 receives an air flow 1025a of approximately 5 L/min into the
valve 1010, which, together with the valve 1015, controls which of GENO
cartridges
1040 or 1050 the air flow 1025a first passes through. More particularly, by
controlling
the position of the valves 1010 and 1015, the air flow 1025a can be made to
pass through
the GENO cartridge 1040, the permeation device 1025, the GENO cartridge 1050,
and
then the activated alumina cartridge 1060 before being delivered to the
patient. By
manipulating the position of the valves 1010 and 1015, the air flow 1025a also
can be
made to pass through the GENO cartridge 1050, the permeation device 1025, the
GENO
cartridge 1040, and then the activated alumina cartridge 1060 before being
delivered to
the patient.
For example, when the NO-NO2 sensor 1070 detects the presence of NO2 in the
air
flow 1025b, this may signal a need to manipulate the valves 1010 and 1015 to
cause the
order in which the GENO cartridges 1040 and 1050 are used to be switched --
that is, for
example, when the air flow 1025a flows through the GENO cartridge 1040 before
flowing through the GENO cartridge 1050, the values 1010 and 1015 are
manipulated to
cause the air flow 1025a to flow through GENO cartridge 1050 before flowing
through
the GENO cartridge 1040.
Fig. 11 illustrates a conceptual design of a GENO cartridge 1100 that converts
NO2 to NO. The GENO cartridge 1100 may be an implementation of the cartridge
100 of
13
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
Fig. 1. The GENO cartridge 1100 is approximately 6-inches long with a 1-inch
diameter.
The GENO cartridge 1100 includes silica gel saturated with an aqueous solution
of
ascorbic acid and receives an air flow from an air or oxygen gas bottle
containing NO2.
The air flow through the cartridge 1100 converts NO2 to NO, which exits the
cartridge
1100. The GENO cartridge 1100 works effectively at concentrations of NO2 from
5 ppm
to 5000 ppm. The conversion of NO2 to NO using the GENO cartridge 1100 does
not
require a heat source and may be used at ambient air temperature. The
conversion of
NO2 to NO using the GENO cartridge 1100 occurs substantially independently of
the flow
rate of the air flow through the GENO cartridge 1100.
Fig. 12 illustrates a therapeutic gas delivery system 1200 that includes a gas
bottle
1220 including NO2 and an GENO cartridge 1210, which may be an implementation
of
GENO cartridge 1100 of Fig. 11, for converting NO2 from the gas bottle 1220 to
NO for
delivery to a patient for NO inhalation therapy. The system 1200 is designed
to be
portable. In some implementations, the system 1200 may be designed to operate
without
the use of electronics or sensors. Depending on the capacity of the gas bottle
1220, the
system 1200 generally has capability to deliver therapeutic NO gas for one to
sixteen
hours.
The system 1200 may be employed to deliver therapeutic NO gas to a patient on
an emergency basis. Examples of such contexts include use by paramedics,
military
medics or field hospitals, and emergency rooms or a trauma center of a
hospital.
Fig. 13A depicts an exterior view 1300A of a therapeutic gas delivery system
with
a liquid NO2 source. Fig. 13B illustrates an interior view 1300B of the
therapeutic gas
delivery system shown in Fig. 13A. The therapeutic gas delivery system
includes a
permeation tube 1310 with a liquid NO2 source, which, for example, may be an
implementation of the permeation device 935 of Fig. 9. The therapeutic gas
delivery
system also includes GENO cartridges 1340 and 1350. The GENO cartridge 1340
receives an air flow 1325a from an air or oxygen source. After exiting the
GENO
cartridge 1340, the air flow is divided such that approximately 10% of the air
flow flows
through the permeation tube 1310 by which gaseous N02 is diffused into the air
now.
3o The air flow exiting the permeation tube 1310 and the other air flow that
did not flow
through the permeation tube 1310 flow through the GENO cartridge 1350, which
converts the NO2 to NO. The air flows 1325b and 1325c which exit the GENO
cartridge
1350 are delivered to the patient for NO inhalation therapy. The permeation
tube 1310
and the GENO cartridges 1340 and 1350 maybe disposable.
14
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
Depending on the capacity of the permeation tube 1310, the therapeutic gas
delivery system shown in Figs. 13A and 13B may have the capability to deliver
therapeutic NO gas for one to thirty days.
The therapeutic gas delivery system shown in Figs. 13A and 13B is able to
interface with a ventilator. The therapeutic gas delivery system shown in
Figs. 13A and
13B also may be employed to deliver therapeutic NO gas to a patient using a
cannula.
The use of the therapeutic gas delivery system with a cannula may enable NO
therapy to
occur outside of a hospital setting. One such example is the use of
therapeutic gas
delivery system for long-teen NO therapy that takes place at the patient's
home.
Fig. 13C depicts the exterior view 1300A of the therapeutic gas delivery
system
shown in Figs. 13A and 13B relative to a soda can 1350. As illustrated, the
implementation of the therapeutic gas delivery system shown in Figs. 13A-13C
is a small
device relative to conventional NO inhalation therapy systems and is slightly
larger than a
soda can.
Fig. 14 depicts an exterior view of a therapeutic gas delivery system 1400
that
uses GENO cartridges to convert NO2 to NO for use in NO inhalation therapy.
The
system 1400 includes GENO cartridge ports 1410 and 1415 through which a GENO
cartridge may be inserted or accessed. The system 1400 includes an inlet port
1420
through which air or oxygen flows into the system 1400 and an associated gauge
1425.
The system 1400 includes a flow value 1430 and display 1435 for controlling
the air flow.
The system 1400 includes GENO cartridge flow ports 1440.
The system 1400 also includes a temperature controller 1445 and a NOx detector
1450, which is accessible through a NOx detector access 1455. The system 1400
also
includes a GENO cartridge 1460 that is used to convert NO2 to NO essentially
just before
the air flow having NO exits the system 1400 through the outlet 1465. The GENO
cartridge 1460 may be referred to as a safety scrubber. The GENO cartridge
1460 may be
smaller than the GENO cartridges used elsewhere in the system 1400. The system
1400
also includes a backup input port 1470 and an exhaust fan 1475.
EXAMPLE 1
A cartridge six-inches in length with a diameter of 1.5-inches was used as the
NO
generation cartridge. Approximately 90 grams 35-70 sized mesh silica gel was
soaked in
a 25% ascorbic acid solution and air-dried at room temperature for two hours
before
being placed in the cartridge. A NO2 permeation tube was used as the source
gas for NO2.
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
Air from an air pump at a rate of 150 cc/min was flowed into the permeation
tube and
mixed, after it exited the cartridge, with 3 L/min of ambient air (which also
was from the
air pump). The permeation tube was placed in an oven with a temperature set at
32
degrees Celsius to provide a steady stream of 20 ppm NO2 for the cartridge.
The cartridge
lasted for 269 hours before ceasing to convert 100% of N02 to NO, achieving
breakthrough.
EXAMPLE 2
Two cartridges were each filled using 35-70 sized mesh silica gel and
approximately 40 grams of silica gel. The silica gel was prepared by being
soaked with a
25% solution of ascorbic acid until complete saturation, and then dried in an
oven for one
hour at 240 degrees Fahrenheit. The ascorbic acid solution was prepared by
mixing 25
grams of ascorbic acid in 100 ml of de-ionized water.
A 1000 ppm NO2 tank was used to flow NO2 through the two GENO cartridges at
a rate of 150 cc/min. The two cartridges were placed in series. Ambient air
from an air
tank was mixed in after the NO2 had passed through the first cartridge and
been converted
to NO. The air containing NO was then passed through the through the second
cartridge
in series. The air was passed through the cartridges at a rate of 3 L/min to
create a total
mixture of 40 ppm NO in air and free of any back reaction of NO2.
The two cartridges converted 100% of the NO2 for 104 hours. At the end of 104
hours, the experiment was stopped because the NO2 tank was empty. The two
cartridges
had not yet reached breakthrough after 104 hours.
Results may be improved by drying the silica gel with a gas, such as nitrogen
gas,
to remove dripping water/ascorbic acid solution from the silica gel.
EXAMPLE 3
A plastic PVC cartridge six-inches in length and having a diameter of 1.5-
inches
was used as the NO generator cartridge. The inside of the cartridge was filled
with an
ascorbic acid-silica mixture. To create the ascorbic acid silica mixture,
approximately
108 grams of 35-70 sized mesh was used. The silica gel was soaked in 25%
ascorbic acid
solution and then baked in an oven for one hour at 240 degrees Fahrenheit. The
ascorbic
acid solution was prepared by dissolving 25 grams of ascorbic acid in 100 ml
of de-
ionized water.
16
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
A 1000 ppm NO2 tank was attached to one end of the cartridge so that 1000 ppm
of NO2 flowed through the cartridge at a rate of 150 cc/min. The gas output of
the
cartridge was then mixed with air using an air pump that flowed at a rate of 3
L/min to
create a total mixture of 40 ppm NO in air. This cartridge lasted for a total
of 122 hours
before achieving breakthrough.
A NOx detector detected a slight concentration of NO2, varying from 0.15 ppm
to
0.25 ppm. The concentration of NO2 remained steady until breakthrough, making
it
likely that the detected NO2 concentration was not a failure in the 100%
efficiency of the
cartridge but rather was NO2 that was recreated in tubing after the cartridge.
A second,
lo smaller cartridge could be placed before the detector to eliminate the
small NO2 back
reaction.
EXAMPLE 4
A cartridge was prepared by using 35-70 sized mesh silica gel soaked in 25%
ascorbic acid solution and air dried for approximately one hour. A permeation
tube was
the source for the NO2 and a KinTek oven was used to raise the level of N02
required to
40ppm. To achieve this concentration, the oven was set at 45 degrees Celsius.
Air was
delivered to the permeation tube using an air pump at the rate of 200 cc/min.
Dilution air
was also provided by the air pump at the rate of 3 L/min. To add humidity to
the supply
of NO2, two jars filled with water were attached to the 200 cc/min air before
the air
entered the permeation tube. This helped to ensure that the air entering the
NO2 source
would be moisture rich and therefore that the NO2 entering the cartridge would
also be
moisture rich. Approximately every five days, the water in the first jar
receded to below
the end of the tubing and needed to be replenished so that the water level was
above the
bottom of the tube end. The second jar remained untouched for the entire
length of the
experiment. The cartridge lasted for 409 hours before ceasing to convert 100%
of NO2 to
NO, achieving breakthrough.
EXAMPLE 5
A cartridge six-inches long and having a diameter of 1.5-inches was prepared
by
using 108 grams of 35-70 sized mesh silica gel. The silica gel was soaked in a
25%
solution of ascorbic acid solution and dried at room temperature
(approximately 70
degrees Fahrenheit) for approximately two hours. The air-dried silica gel was
placed
inside the cartridge.
17
CA 02576957 2007-02-12
WO 2006/023616 PCT/US2005/029344
A flow of 40 ppm NO2 was sent through the silica-ascorbic acid cartridge at a
rate
of 3.2 L/min. The cartridge lasted for 299 hours before ceasing to convert
100% of NO2
to NO,_achieving breakthrough. The cartridge filled with air-dried silica gel
lasted longer
than a comparable cartridge filled with oven-dried silica gel. This
demonstrates oxidation
losses due to heating the ascorbic acid in the presence of air.
EXAMPLE 6
Approximately 40 grams of 35-70 sized mesh silica gel was soaked in a 33%
ascorbic acid solution and the dried in an oven at 240 degrees Fahrenheit
before being
placed in the cartridge. Ambient air at a flow rate of 3 L/min though an air
pump was
lo mixed with 1000 ppm of NO2 from a tank at a flow rate of 200 cc/min, which
created a
total flow rate of 3.2 L/min and a total N02/air mixture of 60 ppm NO2. The
cartridge
lasted for 25 hours before losing its 100% conversion ability. This
demonstrates that using
less silica gel/ascorbic acid in the cartridge results in a cartridge that
does not last as long.
The use of NO generation cartridge in which NO2 is quantitatively converted to
NO
is not limited to therapeutic gas delivery and may be applicable to many
fields. For
example, the NO generation cartridge maybe included in an air pollution
monitor. More
particularly, the NO generation cartridge can also be used to replace high
temperature
catalytic convertors that are widely used today in air pollution
instrumentation
measurement of the airborne concentration of NO2 gas. The current catalytic
convertors
expend significant electricity, and replacement of a catalytic convertor with
a device that
uses a NO generation cartridge may simplify the air pollution instruments, and
enable lower
cost, reduced weight, portable air pollution monitoring instruments.
Other implementations are within the scope of the following claims.
18