Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
PACIFIER WITH THIN-FILM RESERVOIR
AND METHOD FOR USE THEREOF
FIELD OF TAE INVENTION
The invention relates to a pacifier or nipple member for delivery of active
ingredients
into the oral cavity of an infant or patient. The pacifier has a porous nipple
member and a
reservoir or chamber therein for receiving a thin-film that contains an active
ingredient.
Active ingredients may include drugs and/or vitamins suitable for
administration to infants or
patients.
BACKGROUND OF THE RELATED TECHNOLOGY
It often is desirable to administer medications and/or vitamins to infants or
patients in
need thereof. Commonly used administration forms, however, experience certain
known
shortconiings. Due to an infant's natural propensity to suckle, pacifiers can
be a useful tool
for delivering such actives.
The prior art teaches pacifiers that have been modified to administer liquids
and liquid
medications to infants. Liquid medications may be difficult to insert into a
pacifier, which
can cause inaccuracy in the administration dosage. The present invention
provides a pacifier
that incorporates a thin-film dosage unit, thereby alleviating problems
associated with the
prior art.
SUMMARY OF THE INVENTION
In accordance with the present invention, there is provided a reusable
pacifier for
delivering an active ingredient to an infant or patient including: a porous
nipple member
having a nipple and a neck and having at least one reservoir therein for
receiving a thin-film
containing an active ingredient; and a base member removably attached to the
neck of the
nipple member and adapted to remain outside the oral cavity of the infant.
There also is provided a reusable pacifier for delivering an active ingredient
to an
infant or patient including: a porous nipple member having a nipple and a neck
and having at
least one reservoir therein; a thin-film housed in the reservoir, the film
containing an active
1
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
ingredient; ana a base memoer removably attached to the neck of the nipple
member and
adapted to remain outside the oral cavity of the infant.
Another embodiment of the present invention provides a single-use pacifier for
delivering an active ingredient to an infant or patient including: a base
member adapted to
remain outside the oral cavity of the infant, the base member including a
shield having a
concave side and a convex side; a porous nipple member disposed on the concave
side of the
base member, the nipple member having at least one reservoir therein; and a
thin-film housed
in the reservoir, the film containing an active ingredient.
In accordance with another embodiment of the present invention, there is
provided a
reusable pacifier for delivering an active ingredient to an infant or patient
including: a base
member adapted to remain outside the oral cavity of the infant, the base
member including a
shield having a concave side and a convex side; and a porous nipple member
disposed on the
concave side of the base member, the nipple member having at least one flap
defining at least
one chamber for receiving a thin-film containing an active ingredient.
Yet another embodiment of the present invention provides a baby bottle nipple
for
delivering an active ingredient to an infant including: a porous nipple member
having a
nipple and a neck and having at least one reservoir therein, wherein the neck
is adapted to
removably attach to a baby bottle; and a thin-film housed in the reservoir,
the film containing
an active ingredient.
The present invention also provides a method for delivering an active
ingredient to an
infant or patient including the steps of: providing a thin-film containing an
active ingredient;
providing a porous nipple member of a pacifier having a reservoir or chamber
for receiving
the thin-film containing an active; inserting the thin-film into the reservoir
or chamber of the
porous nipple member; and orally administering the pacifier to an infant or
patient thereby
allowing the thin-film to dissolve and release the active ingredient into the
oral cavity.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a side plan view of a reusable pacifier in accordance with an
embodiment
of the present invention;
2
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
Figure 2 is a side plan view or a single-use pacifier in accordance with an
embodiment of the present invention;
Figure 3 is a cross-sectional view of the reservoir in the nipple member taken
along
line 3-3 of Figure 1;
Figure 4 is a side plan view of a pacifier with insertion flaps for accessing
a reservoir
or chamber in accordance with an embodiment of the present invention;
Figure 5a is a side plan view of a reusable nipple member with a hollow
reservoir in
accordance with an embodiment of the present invention;
Figure 5b is a cross-sectional view of the reservoir in the nipple member
taken along
line 5-5 of Figure 5a;
Figure 6 is a side plan view of a nipple for a baby bottle in accordance with
an
embodiment of the present invention;
Figure 7 is a side plan view of a reusable pacifier including retaining
fingers on the
base portion in accordance with an embodiment of the present invention;
Figure 8 is a side plan view of a solid plug shaped to fit within the neck of
the nipple
member in accordance with an embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to delivery devices for drugs and other actives
which are
designed particularly for infants and patients such as the elderly, and those
who have had
surgery or other illnesses which prevent the ordinary dispensing of
medication. The devices
may be used for veterinary applications as well. The device of the present
invention may be
termed pacifiers or nipple members, since they are designed to administer the
active through
pores in the nipple member.
In particular, the present invention provides a pacifier or nipple unit for
bottles, both
of which are designed to deliver an active ingredient to a patient. The
pacifier includes a
porous nipple member and a base member. The pores in the nipple may be of any
suitable
shape and size. The nipple member includes a reservoir or other like chamber
therein for
receiving a thin-film that contains the active ingredient. The base member,
which is adapted
to remain outside the oral cavity of the infant or patient, may be removably
attached to the
nipple member. The pacifier may be a reusable or single-use device.
3
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
In one emboarment, the film containing active may be placed inside the hollow
portion of the nipple and which by means of the nipple's porosity is in
communication with
saliva in the patient's oral cavity such that the saliva dissolves the film
and releases the active
into the oral cavity.
In another embodiment, as discussed herein, the nipple has a reservoir or
chamber
adapted to receive a film containing an active, the reservoir or chamber
having pores which
allow passage of the film and its active contents for release into the oral
cavity.
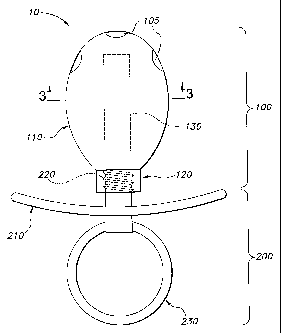
In accordance with some embodiments of the present invention, as shown in
Figs. 1
and 5a, the pacifier 10 includes a nipple member 100 and a base member 200.
The nipple
member 100 includes a nipple 110 wherein a rounded tip is contiguous with a
neck 120. The
nipple member 100 also has a reservoir therein 130 to receive a thin-film
containing the
active. Pores 105 permit the saliva to contact the film and the active to be
released in the oral
cavity. The pacifier 10 also may include a solid plug 300 shaped to fit within
the neck 120 of
the nipple member 100, as shown in Fig. 8.
The bo.se member 200 includes a shield 210, which may have a concave side and
a
convex side. The base member 200 may include a handle member 230 or 330 that
is
pivotally attached to the convex side of the shield 210.
In a reusable embodiment, the base member 200 includes an attachment member
220
disposed on the concave side of the shield 210. The neck 120 of the nipple
member 100
engages the attachment member 220 to removably attach the base member 200 to
the nipple
member 100. The attachment member 220 and neck 120 are attached via any
mechanical
connection known to those skilled in the art. For instance, the connection may
be a threaded
connection, as shown in Fig. 1. In this embodiment, the attachment member 220
and the
neck 120 of the nipple member 100 each include threaded-mating portions. When
the neck
120 engages the attachment member 220, a threaded connection is formed
therebetween
thereby removably attaching the nipple member 100 to the base member 200. The
nipple
member 100 may be removed from the base member 200 by unthreading the
connection.
This permits the user to reinsert a thin-film into the reservoir of the nipple
member as desired.
Other connections include, for example, but are not limited to, snap-fit or
bayonet-lock fit.
4
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
ln accordance with a single-use embodiment of the present invention, the
nipple
member 100 and base member 200 form a single molded device, as shown in Fig.
2. The
nipple member and base member are a one-piece construction. Alternatively, the
nipple
member and base member may be molded separately and then attached to one
another using a
suitable nontoxic adhesive, press fit or other similar attachment means. The
thin-film is
inserted into reservoir 130 formed in the nipple portion of the pacifier
during manufacture.
Single-use embodiments are constructed to be disposable devices. As such, it
may be
desirable to produce the pacifier in a hermetically sealed package for
sanitary reasons.
The nipple member of the pacifiers of the present invention may be constructed
from
any suitable resilient material that is acceptable for oral administration.
Such materials are
known to those skilled in the art. Suitable materials include, for example,
rubber or plastic
materials such as polyurethane, silicone, acrylics, polyethylene or polyvinyl
chloride. The
material is desirably porous or permeable to allow delivery of an active
ingredient into the
oral cavity of an infant or patient. For instance, the nipple member may
contain a series of
perforations or pores, such as pores 105 and slits 106, as shown in Figs. 1,
2, 4, 5a, 6 and 7.
The pores may be very small, as long as they are large enough to allow saliva
to pass through
the nipple to dissolve the film and release the active into the oral cavity.
The pores, however,
may be as large as desired to provide different release properties of the
active. For example,
it may be desirable to release some actives quickly, whereas other actives may
work more
effectively with a slower, controlled release. Moreover, the material may be
porous
throughout or selectively porous in certain regions to provide different
release properties.
The active ingredient desirably is released from a thin-film contained in the
reservoir
or chamber of the nipple member. More specifically, the infant's or patient's
sucking on the
nipple allows saliva to reach the thin-film via the porous material of the
nipple. The saliva
dissolves the film and the active ingredient contained therein is released
into the oral cavity.
The material may further be translucent so that a user can visually determine
when the film
has dissolved. The size and shape of the nipple member can vary to accommodate
different
age groups or various animals for use in veterinary medicine.
The reservoir 130 in the nipple member 100 is adapted to receive the thin-
film. The
reservoir may be such that the nipple member is hollow or a chamber sized to
fit the piece of
thin-film. In general, the films, when dried, have a thickness from about 3 m
to about 250
5
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
gm, or about u.1 mi1s to anoui 10 mils.""Desirably, the dried films will have
a thickness of
about 2 mils to about 8 mils, and more desirably, from about 3 mils to about 6
mils.
Accordingly, the reservoir may have a thickness of slightly larger than about
0.1 mils to
slightly larger than about 10 mils. The reservoir may have any shape suitable
to receive thin-
films as such. For example, as shown in Fig. 3, the reservoir 130 has a
generally flat shape.
As shown in Fig. 5b, the nipple member is generally hollow. The reservoir
could have square
or rounded edges.
In some embodiments, it may be desirable to include multiple reservoirs in the
nipple
member to house more than one film at a time. For example, it may be desirable
to deliver
more than one active ingredient to the infant or patient at the same time. In
such case,
different films each containing a different active may be inserted into
separate reservoirs in
the nipple meinber. Alternatively, it may be desirable to include a liquid,
such as water, in
one reservoir and a film in another reservoir. The liquid facilitates quick
dissolution of the
film as the infant or patient sucks on the nipple member. In other
embodiments, the infant's
or patient's saliva acts as the liquid source.
The film may be free-standing in the reservoir or it may be held in-place by a
retaining mechanism such as, but not limited to, clips or retaining fingers,
as depicted in Fig.
6. As shown in Fig. 6, the retaining fingers 125 are disposed on the internal
surface of the
nipple member 100. Alternatively, the retaining fingers 125 may be disposed on
the concave
side of the base member 200, as shown in Fig. 7. The film is thereby held in-
place insider the
reservoir once the nipple member is attached to the base member. Alternative
retaining
mechanisms are contemplated herein and would be known to those skilled in the
art.
In some embodiments, the film may have a preformed curled shape to fit inside
the
nipple member and mate therewith. Alternatively, the film may be manufactured
in a flat
shape and curled for insertion into the nipple member. The curled film may be
adapted to
abut against part or the entire inner surface of the nipple member.
In accordance with another embodiment of the present invention, the nipple
member
of the pacifier contains at least one chamber created by a flap 140 in the
external surface of
the nipple, as shown in Fig. 4. For instance, one or more flaps 140 may be
created by small
cuts in the surface of the nipple member. A thin-film may be inserted into
each of the flaps.
6
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
As in the embodiments described above, tne material of the nipple member is
porous, thereby
allowing release of the active from the film into the oral cavity.
Yet another embodiment of the present invention provides a nipple member for
attachment to a baby bottle as shown in Fig. 6. As described in the
embodiments above, the
nipple member 100 may include a reservoir 130 adapted to receive a thin-film
that contains
an active ingredient. More specifically, the nipple member 100 is hollow as
seen in Fig. 6.
The film is inserted into the hollow nipple member via the base 115 of the
nipple member
and held in-place against the internal surface of the nipple by retaining
fingers 125. Holding
the film against the internal surface of the nipple as such allows saliva or
liquid to reach the
film quickly and thus may assist in quickly releasing the active. The nipple
member similarly
is formed of a porous or permeable material, as shown by pores 105 in Fig. 6.
As the infant
sucks on the nipple to obtain the liquid contained in the bottle, the infant's
saliva and the
liquid together act to dissolve the film thereby releasing the active
ingredient into the oral
cavity. The nipple member for the baby bottle may be disposable or reusable by
reinserting
another film as desired.
The active ingredients used in embodiments of the present invention may be any
active suitable for administration to an infant or patient in need thereof.
The active ingredient
is desirably in a solid form, such as particulate, powder, dissolvable tablet,
or most desirably
a thin-film. The active ingredients include, without limitation,
pharmaceutical and cosmetic
actives, drugs, medicaments, antigens or allergens such as ragweed pollen,
spores,
microorganisms, seeds, mouthwash components, flavors, fragrances, enzymes,
preservatives,
sweetening agents, colorants, spices, vitamins and supplements and
combinations thereof.
Suitable active ingredients are more fully described in Applicants' co-pending
U.S.
Application Nos. 10,074,272, filed February 14, 2002, 10/768,809, filed
January 30, 2004,
and 10/856,176, filed May 28, 2004, which are incorporated herein by reference
in their
entirety. Particularly suitable actives for delivery to infants include
aspirin, benzocaine,
lidocaine, diphenhydramine, simethicone, vitamins and cold and cough
medications. Other
suitable actives include medicaments for the treatment of pain, such as, for
example, narcotic
analgesics including fentanyl. Some embodiments are adapted for emergency or
antidotal
use and therefore include, for example, poison antidotes as actives. In other
embodiments, it
may be desirable to include mucosally active vaccines, such as, but not
limited to, flu and
west nile virus vaccines.
7
CA 02579532 2007-03-06
WO 2006/031887 PCT/US2005/032746
The thin-films which contain the active ingredients may be any film disclosed
in U.S.
Application Nos. 10,074,272, 10/768,809 and 10/856,176, referred to above.
These
applications provide an extensive description of thin-films and methods for
producing such
films.
A variety of optional components also may be incorporated into the thin-films,
as
described in U.S. Application Nos. 10,074,272, 10/768,809 and 10/856,176,
referred to
above. These may include, without limitation, anti-foaming agents, pigments,
coloring
agents, sweetening agents and flavoring agents, among others.
The present invention also is directed to methods for delivering the active
ingredient
to the oral cavity of an infant or patient. In accordance therewith, at least
one thin-film
containing an active ingredient is provided. The thin-film may be inserted
into the reservoir
or chamber of the porous nipple member. The film may be inserted through the
neck of the
nipple member. The film may be free-standing or clipped or retained in-place
by a retaining
member. In the single-use, or disposable, embodiments, the final pacifier
device is
manufactured with a base member. In the reusable embodiments, the porous
nipple member
may be attached to the base member to form the pacifier apparatus. Once the
nipple member
and base member are attached, or manufactured as a disposable pacifier, the
pacifier may be
orally administered to an infant or patient. The infant's or patient's saliva
penetrates the
porous nipple member and dissolves the thin-film. In accordance with some
embodiments,
the film's dissolution is facilitated by wetting the reservoir or chamber. The
active ingredient
contained in the film is thereby released into the oral cavity of the infant
or patient.
Once the thin-film dissolves and the active is fully released, the pacifier
may be
removed from the oral cavity of the infant or patient. As discussed above, the
material
forming the nipple member may be translucent, thereby enabling the user to
determine when
the film is substantially consumed. In the reusable embodiments, the nipple
member may be
removed from the base member for administration of another thin-film as
desired.
8