Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
IMPLANTABLE PRESSURE SENSOR WITH PACING CAPABILITY
Cross-Reference to Related Applications
[001] The present application claims the benefit of U.S. Provisional Patent
Application No. 60/608,077, filed September 8, 2004, the entire disclosure of
which is
incorporated herein by reference. The present application further relates to
U.S.
Patent Application Serial No. 10/077,566, filed February 15, 2002, entitled
Devices,
Systems and Methods for Endocardial Pressure Measurement, and U.S. Patent
Application No. 10/797,584, filed March 9, 2004, entitled Devices and Methods
for
Detecting and Treating Inadequate Tissue Perfusion, the entire disclosures of
which
are incorporated herein by reference.
Background of the Invention
[002] Congestive heart failure (CHF) is an end-stage chronic condition
resulting from the heart's inability to pump sufficient blood, and is a
significant factor
in morbidity, mortality and health care expenditure. There are a variety of
underlying
conditions that may lead to CHF, and a variety of therapeutic approaches
targeting
such conditions. The selection of the therapeutic approach, and the parameters
of the
particular therapeutic approach selected, is a function of the underlying
condition and
the degree to which it affects the heart's ability to pump blood. Endocardial
pressure,
particularly left ventricular (LV) pressure, is a good indicator of the
heart's ability to
pump blood and the effectiveness of any given therapy.
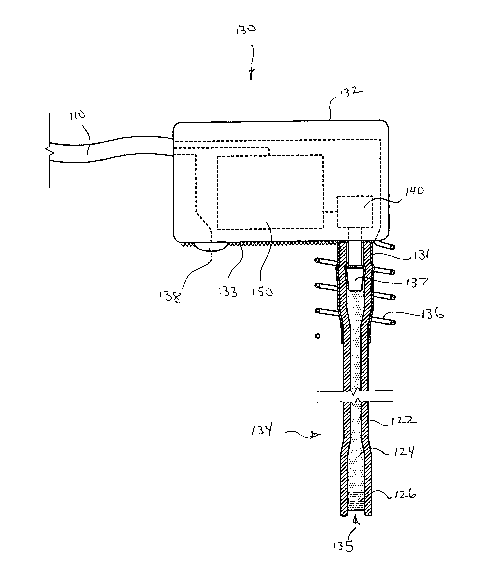
[003] Studies have shown that patients with moderate to severe CHF may
benefit from cardiac resynchronization therapy (CRT). CRT devices are similar
to
conventional pacemakers, except that in addition to a lead for pacing the
right
ventricle, a CRT device includes a lead for pacing the left ventricle. Left
ventricular
leads may be placed intravascularly using a coronary sinus lead, or surgically
using an
-1-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
epicardial lead. An example of a commercially available CRT device is the
InSync
system from Medtronic. However, such CRT systems do not have the ability to
measure LV pressure.
[004] U.S. Patent No. 5,353,800 to Pohnddrf et al. describes a pacing lead
that measures pressure using a hollow coiled needle. Pohndorf et al. describe
measuring LV pressure by placing the lead in the right ventricular chamber
with the
coiled needle extending through the septal wall into the left ventricular
chamber.
Although Pohndorf et al. describe a lead for measuring LV pressure, Pohndorf
et al.
do not describe a lead for pacing the left ventricle as would be needed for a
CRT
system. Consequently, there is a need for a device and system capable of both
LV
pacing and LV pressure measurement.
Summary of the Invention
[005] To address this need, the present invention provides devices and
methods for left ventricular or biventricular pacing plus left ventricular
pressure
measurement. By way of example, not limitation, the present invention provides
a
pacing lead having a combined electrode and pressure sensor assembly for left
ventricular pacing and pressure measurement. The assembly may include one or
more electrodes, a pressure sensor, and a pressure transmission catheter. The
assembly may be configured to be secured to the epicardial surface of the
heart, and
the pressure transmission catheter may be configured to extend through the
heart wall.
For example, the assembly may be positioned with respect to the heart such
that the
electrode is in a position to pace the LV, the pressure transmission catheter
passes
through a wall of the heart into the LV, and the pressure sensor resides
outside the
LV. Such a lead with a combined electrode and pressure sensor assembly for LV
pacing and pressure measurement is particularly suitable for biventricular
pacing and
-2-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
may be incorporated into a cardiac resynchronization therapy (CRT) system, for
example. The measured LV pressure may be used in an open loop system providing
LV pressure data to a physician, a closed loop system providing feedback
control to a
CRT system, or both, for example.
Brief Description of the Drawings
[006] Figure 1 is a schematic illustration of a pacing system including a
combined pacing and pressure sensing lead for the left ventricle;
[007] Figures 2A - 2C are schematic illustrations of various electrode
arrangements for the combined pacing and pressure sensing lead shown in Figure
1;
[008] Figure 3 is a more detailed schematic diagram illustrating a combined
pacing and pressure sensing lead;
[009] Figure 4 is a longitudinal cross-section of an alternative pressure
transmission catheter; and
[010] Figure 5 is a longitudinal cross-section o'f an alternative pressure
sensor arrangement.
[011] Figure 6 is a perspective view showing an illustrative assembly in
accordance with an exemplary embodiment of the present invention.
[012] Figure 7 is a perspective view showing a pressure transmission catheter
including a shaft.
[013] Figure 8 is an additional a perspective view showing the pressure
transmission catheter shown in the previous figure.
[014] Figure 9 is a schematic illustration showing a body and a cardiac
pacing system.
Detailed Description of the Invention
-3-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
[015] The following detailed description should be read with reference to the
drawings in which similar elements in different drawings are numbered the
same.
The drawings, which are not necessarily to scale, depict illustrative
embodiments and
are not intended to limit the scope of the invention.
[016] With reference to Figure 1, a system for left ventricular pacing and
pressure measurement is shown schematically. To facilitate a discussion of the
system, it is helpful to define and label some of the anatomical features of
the heart
200 shown in Figure 1. The heart 200 includes four chambers, including the
left
ventricle (LV) 202, the right ventricle (RV) 204, the left atrium (LA) 206,
and the
right atrium (RA) 208. The LV 202 is defined in part by LV free wall 230, and
the
RV 204 is defined in part by RV free wall 234. The LV 202 and the RV 204 are
separated by ventricular septal wall 232, and the LA 206 and the RA 208 are
separated by atrial septal wall 236.
[017] The right atrium 208 receives oxygen deprived blood returning from
the venous vasculature through the superior vena cava 216 and inferior vena
cava 218.
The right atrium 208 pumps blood into the right ventricle 204 through
tricuspid valve
242. The right ventricle 204 pumps blood through the pulmonary valve and into
the
pulmonary artery which carries the blood to the lungs. After receiving oxygen
in the
lungs, the blood is returned to the left atrium 206 through the pulmonary
veins. The
left atrium 206 pumps oxygenated blood through the mitral valve 244 and into
the left
ventricle 202. The oxygenated blood in the left ventricle 202 is then pumped
through
the aortic valve, into the aorta 217, and throughout the body via the arterial
vasculature.
[018] Returning to a discussion of the system illustrated in Figure 1, the
system generally includes a pulse generator 10 and a combined left ventricular
(LV)
-4-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
pacing and pressure sensing lead 100. The pulse generator 10 may comprise a
cardiac
resynchronization therapy (CRT) device for biventricular pacing, or a combined
CRT
and defibrillation (CRT-D) device for biventricular pacing and defibrillation.
Accordingly, the pulse generator 10 may accommodate three or four leads, for
example, including an atrial sensing lead 20, a right ventricular (RV) therapy
lead 30,
and a LV pacing lead 100.
[019] The LV lead 100 includes a lead body 110 having a proximal end
portion 112 connected to the pulse generator 10 and a distal end portion 114
connected to an electrode and pressure sensor assembly 130. The electrode and
pressure sensor assembly 130 may include a hermetically sealed housing 132
containing a pressure sensor and associated electronics as best seen in Figure
3. A
pressure transmission catheter (PTC) 134 may be connected to and extend from
the
housing 132, and may be configured to extend through a wall of the heart 200
and
into a chamber, such as through the LV free wal1230 and into the LV chamber
202 as
shown.
[020] A pacing electrode 136 may be mounted to a portion of the assembly
130, such as around the PTC 134 as shown. A reference electrode 138 may be
mounted to a portion of the assembly 130 and spaced from the pacing electrode
136,
such as on the bottom side of the housing 132. Alternatively, a single
electrode (e.g.,
136 or 138) may be implemented by using control circuitry to periodically
switch the
function (e.g., pace or reference) of the electrode. In this alternative
arrangement, the
control circuitry (e.g., electronics module 150 as described hereinafter)
would
communicate the timing, pacing stimulus and sensing parameters to and from the
electrode. As discussed in more detail with reference to Figures 2A - 2C, the
pacing
electrode 136 and the reference electrode 138 may be mounted in a variety of
places
-5-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
on the assembly 130 to effectively position the electrodes for pacing the LV
via the
myocardial and/or epicardial portions of the LV wall 230.
[021] In figure 1 pacing electrode 136 is shown having a length B and PTC
134 is show having a length A. In some useful embodiments of the present
invention,
PTC 134 is configured to extend through the LV free wall 230 of the heart 200.
In the
embodiment of figure 1, for example, length A of PTC 134 is greater than the
thickness of LV free wall 230. Also in the embodiment of figure 1, length B of
pacing electrode 136 is smaller than the thickness of LV free wall 230. Some
exemplary embodiments of the present invention include an electrode that is
dimensioned to contact the outer wall of a left ventricle without contacting
the blood
disposed within the left ventricle. With reference to figure 1, it will be
appreciated
that distance A is greater than distance B.
[022] With this arrangement, endocardial pressure (e.g., LV pressure) may
be measured via the PTC 134, which refers blood pressure from within the
chamber to
the pressure sensor contained in the housing 132. The pressure sensor (or
pressure
transducer), together with the associated electronics in the housing 132,
convert the
pressure signal into an electrical signal (analog or digital) which is
transmitted to the
pulse generator 10 via lead body 110. Accordingly, lead body 110 may contain
six
(or more) conductors; one each for power, ground, control in, data out, pacing
electrode 136, and reference electrode 138. Additional conductors may be
provided
in the lead body 110 to the extent that additional sensors (e.g., temperature
sensor) or
electrodes (e.g., ECG electrodes) are utilized. The electrical pressure signal
received
by the pulse generator 10 may be recorded, stored for later retrieval, or used
to control
pacing parameters or regimen. For example, the measured LV pressure may be
used
in an open loop system wherein telemetry is used to provide LV pressure data
to a
-6-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
physician who can monitor the effectiveness of the therapy and modify the
therapy as
needed. Alternatively, the measured LV pressure may be used in a closed loop
system wherein LV pressure data is used for feedback control of the pulse
generator
10.
[023] In some instances, it may be desirable to separate the combined
electrode and pressure sensor assembly 130 into two parts; a sensor assembly
portion
and an electrode assembly portion. In this alternative embodiment, the
electrodes
may be separated from the sensor assembly and take the form of a conventional
epicardial lead, and the sensor assembly may be essentially the same as before
(less
the electrodes). The sensor assembly portion and the epicardial lead portion
may
share a common lead connected to the pulse generator 10.
[024] A tissue in-growth promoting surface 133 such as polyester fabric may
be disposed on a bottom surface of the housing 132 to secure the assembly 130
to the
epicardial surface of the heart 200, such as the epicardial surface of the LV
free wall
230 as shown. In addition to the bottom surface of the housing 132, the tissue
in-
growth promoting surface 133 may also be disposed about the sides and top of
the
housing 132 to further enhance attachment to the outside of the heart wall.
Other
attachment means such as sutures, adhesive or the like may be used as in the
alternative or in addition to the tissue in-growth promoting surface 133.
[025] Reference may also be made to U.S. Patent No. 4,846,191 to
Brockway et al., U.S. Patent No. 6,033,366 to Brockway et al., U.S. Patent No.
6,296,615 to Brockway et al., and U.S. Published patent Application No.
2002/0120200 to Brockway et al. for examples of alternative embodiments of the
sensor assembly 130 onto which the electrodes 136, 138 may be disposed.
-7-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
[026] The assembly 130 may be mounted to the LV free wall 230 by a
conventional surgical technique, or a less invasive technique may be utilized,
such as
a transthoracic technique, where access to the cardiac space is gained via an
intercostal approach or a subxyphoid approach as known in the art. Examples of
suitable minimally invasive tools and methods are as described in U.S. Patent
No.
5,827,216 to Igo et al., assigned to Comedicus, Inc., and U.S. Patent No.
4,972,847 to
Dutcher et al., assigned to Enpath Medical, Inc., the entire disclosures of
which are
incorporated herein by reference. Examples of commercially available tools and
related components include the PerDUCER access device available from
Comedicus, Inc, Columbia Heights, Minnesota, the MyoPore sutureless unipolar
epicardial pacing lead and the FasTac myocardial lead implant tool
manufactured by
Enpath Medical, Minneapolis, Minnesota. Implantation of the system may take
place
during a contemporaneous open chest procedure (e.g., coronary artery bypass or
valve
repair/replacement), or the system may be implanted in a separate procedure.
[027] As an example of a surgical technique, a surgeon may perform a
median sternotomy, or mini-thoracotomy, cutting across the dermal layer, sub-
dermal
tissue layer, muscle layer, and sternum. The surgeon then cuts the pericardial
sac to
expose the heart 200, down to the LV apex. The PTC 134 is introduced through
the
LV free wall 230 and into the LV chamber 202 at the desired pacing location
using a
peelable-sheath introducer and a trocar. The trocar may be inserted into a
lumen of
the peelable sheath. The LV free wall 230 may be pierced with the trocar and
the
peelable sheath to form a hole in the LV free wall 2310. The trocar may be
removed
from the lumen of peelable sheath and the PTC 134 may be inserted into the
lumen of
the sheath. The peelable-sheath introducer facilitates insertion of the PTC
134 into
the heart wall and protects the PTC 134 from damage that may otherwise occur
during
-8-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
the insertion process. Following insertion of the PTC 134, the peelable-sheath
introducer is removed by peeling it off the PTC 134 and around the assembly
130. A
sheath retainer may be used to prevent splitting of the introducer inside the
heart wall
and to hold the assembly 130 in place while the introducer is removed.
[028] After a subcutaneous pocket is created for the pulse generator 10, the
lead body 110 may be tunneled from the cardiac space to the subcutaneous
pocket and
the proximal end 112 of the lead body 110 may be connected to the pulse
generator
10. The other sensing, RV pacing, and defibrillation electrodes may be placed
transvenously using conventional techniques, and subsequently connected to the
pulse
generator. The pocket and the chest are then closed.
[029] As seen in Figure 1, the combined electrode and sensor assembly 130
may be implanted on the heart 200 of a patient. In this exemplary embodiment,
the
PTC 134 is inserted directly into the left ventricle (LV) 202 across the left
ventricular
wall 230 for the purpose of measuring LV pressure. In particular, the housing
132
resides on the epicardial surface in the pericardial space, with the PTC 134
extending
across the epicardium, myocardium and endocardium, and into the LV chamber
202.
In this position, the pacing electrode 136 is in contact with the myocardium
and the
reference electrode 138 is*in contact with the epicardium. This allows for
pacing of
the LV and for monitoring of pressure in the LV chamber 202 of the heart 200.
[030] Although it is presently preferred to mount the assembly 130 on the
LV free wall 230 in order to pace and measure pressure in the LV for
biventricular
pacing applications, for example, other implant positions are also possible.
By way of
example, not limitation, the assembly 130 may be implanted such that the
distal end
of the PTC 134 resides in any chamber of the heart 200, such as the LV 202,
the RV
204, the LA 206, or the RA 208, to measure endocardial pressure in the
respective
-9-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
chamber. Also by way of example, not limitation, the assembly 130 may be
mounted
such that the pacing electrode 136 and the reference electrode 138 contact any
heart
wall, such as the LV free wa11230, the RV free wall 234, the ventricular
septum 232,
the atrial septum 236, the LA free wall 238, or the RA free wall, to pace the
respective
chamber. These alternative mounting positions permit the combined pacing and
pressure sensing lead 100 to be used to pace (or defibrillate) different
hearts walls and
measure pressure in different heart chambers.
[031] With reference to Figures 2A - 2C, various electrode arrangements are
schematically illustrated by way of example, not limitation. The illustrated
electrode
arrangements may be used in whole or in part, and may be combined in a variety
of
different ways to provide many permutations of possible arrangements.
[032] Figure 2A shows, in more detail, the embodiment illustrated in Figure
1, wherein the pacing electrode 136 comprises a metallic helical coil wound
around
the PTC 134, and the reference electrode 138 comprises a metallic button
extending
from the bottom of the housing 132. In this embodiment, the helical coil
electrode is
in contact with the myocardium, and the button electrode is in contact with
the
epicardium. The helical coil serves as both an electrode and as an anchor to
secure
the assembly 130 to the heart wall.
[033] Figure 2B shows the pacing electrode 136 and the reference electrode
138 comprising buttons extending from the bottom of the housing 132. In this
embodiment, both button electrodes 136, 138 are in contact with the
epicardium. A
plurality of button electrodes distributed about the bottom surface of the
housing 132
may be used (together with corresponding switching circuitry) to selectively
switch
between electrode pairs to obtain the desired pacing effect (e.g., to
establish or
maintain capture, to change thresholds, etc.).
-10-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
[034] Figure 2C shows the pacing electrode 136 and the reference electrode
138 comprising metallic rings disposed around and spaced apart on the PTC 134.
In
this embodiment, both ring electrodes 136, 138 are in contact with the
myocardium.
A plurality of ring electrodes distributed along the length of the PTC 143 may
be used
(together with corresponding switching circuitry) to selectively switch
between
electrode pairs to obtain the desired pacing effect (e.g., to establish or
maintaiii
capture, to change thresholds, etc.). In one embodiment, one ring electrode
may be
used for pacing (i.e., active) and the other ring electrode may serve as a
reference
electrode.
[035] With reference to Figure 3, additional details of an example
embodiment of the combined electrode and sensor assembly 130 are shown
schematically. The assembly 130 includes a sensor 140 comprising a pressure
transducer and an electronics module 150 contained within a housing 132. The
assembly 130 further includes a pressure transmission catheter (PTC) 134
extending
from the housing 132, a pacing electrode 136 extending around the PTC 134, and
a
reference electrode 138 disposed on the bottom of the housing 132.
[036] The housing 132 protects the pressure transducer 140 and the
electronics module 150 from the harsh environment of the human body. The
housing
132 may be fabricated of a suitable biocompatible material such as titanium or
ceramic and may be hermetically sealed. The proximal end of the housing 132
includes an electrical feedthrough to facilitate connection of the electronics
module
150, the pacing electrode 136, and the reference electrode 138 to the flexible
lead
body 110. The distal bottom side of the housing 132 includes a pressure
transducer
header to facilitate mounting of the pressure transducer 140 and to facilitate
connection to the PTC 134.
-11-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
[037] The pressure transducer 140 may be of the piezoresistive, optical,
resonant structure, or capacitive type. For example, the pressure transducer
may
comprise a piezoresistive wheatstone bridge type silicon strain gauge
available from
Sensonor of Horton, Norway. Examples of suitable pressure transducers are
disclosed
in U.S. Patent Application Serial No. 10/717,179, filed November 17, 2003,
entitled
Implantable Pressure Sensors, the entire disclosure of which is incorporated
herein by
reference.
[038] The electronics module 150 may provide excitation to the pressure
transducer 140, amplify the pressure and EGM signals, and digitally code the
pressure
and EGM information for communication to the pulse generator 10 via the
flexible
lead body 110. The electronics module 150 may also provide for temperature
compensation of the pressure transducer 140 and provide a calibrated pressure
signal.
A temperature measurement device may be included within the electronics module
150 to compensate the pressure signal from temperature variations. In an
alternative
embodiment, the electronics module 150 communicates or creates the stimulus to
drive the pacing electrode 136.
[039] The flexible lead body 110 connects the electronics module 150 of the
assembly 130 to the pulse generator. The lead body 110 may contain, for
example,
six conductors; one each for power, ground, control in, data out, pacing
electrode 136,
and reference electrode 138. The lead body 110 may incorporate conventional
lead
design aspects as used in the field of pacing and implantable defibrillator
leads. The
lead body 110 may optionally include one or more EGM electrodes, and the
number
of conductors may be modified to accommodate the EGM electrodes.
[040] The PTC 134, which is shown in longitudinal cross-section, may
comprise a tubular shaft 122 with a liquid-filled (or gel-filled) lumen 124
extending
-12-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
therethrough to a distal opening or port 135 containing a barrier 126. The
proximal
end of the PTC 134 is connected to the pressure transducer 140 via a nipple
tube 137
to establish a fluid path from the pressure transducer 140 to the distal end
of the PTC
134. The PTC 134 thus refers pressure from the pressure measurement site to
the
pressure transducer 140 located inside the housing 132. The PTC 134 may
optionally
include one or more EGM electrodes or other physiological sensors as described
in
U.S. Patent No. 6,296,615 to Brockway et al the disclosure of which is hereby
incorporated by reference herein.
[041] The barrier 126, which may comprise a gel plug and/or membrane,
may be disposed in or over the distal opening 135 to isolate the liquid-filled
lumen
124 of the PTC 134 from bodily fluids and to retain the fluid in the lumen,
without
impeding pressure transmission therethrough. In one embodiment, the fluid 124
is
chosen to be a fluorinated silicone oil and the gel 126 is chosen to be
dimethyl
silicone gel. Further aspects of suitable fluids 124 and gels 126 are
described in U.S.
Patent Application Serial No. 10/272,489, filed October 15, 2002, entitled
Improved
Barriers and Methods for Pressure Measurement Catheters, the entire disclosure
of
which is incorporated herein by reference.
[042] The PTC 134 may comprise a wide variety of materials, constructions
and dimensions depending on the particular clinical application and the bodily
tissue
in which the PTC 134 resides when implanted. For example, the PTC 134 may
comprise an extruded polycarbonate-polyurethane tube with a thermally formed
proximal flare to accommodate the nipple tube 137, and a thermally formed
distal
flare to increase the area of the sensing surface and thereby reduce pressure
measurement errors due to motion artifacts and thermal expansion artifacts.
The PTC
134 may also incorporate a polyester fabric tube 131 or other surface
modification.
-13-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
The PTC 134 may be annealed to improve its mechanical properties and may be
etched in solvent or solvent vapors to remove frayed edges.
[043] By way of example, not limitation, the PTC 134 may have an overall
length of approximately 26 mm, a proximal flare length of approximately 6.0
mm, a
distal flare length of approximately 5.5 mm, tapered transition lengths of
approximately 2.0 mm, a mid-shaft inside diameter of approximately 0.025
inches, a
proximal flare inside diameter of approximately 0.038 inches increasing to
0.059
inches to accommodate the nipple tube 137, a distal flare inside diameter of
approximately 0.042 inches, and a wall thickness of approximately 0.015
inches,
which are particularly suitable for LV pressure monitoring applications as
shown and
described with reference to Figure 1. Various different lengtlis, diameters,
tapers,
flares, wall thicknesses, coatings, coverings, surface treatments, etc. may be
incorporated into the PTC 134 depending on the application without departure
from
the present invention. Further details and alternative embodiments of the PTC
134 are
described in U.S. Patent Application No. 10/799,931, filed March 12, 2004,
entitled
Pressure Transmission Catheter for Implantable Pressure Sensors, the
disclosure of
which is incorporated herein by reference.
[044] In some instances, it may be desirable to provide one or more side
openings in the PTC 134 to increase the surface area for transfer of pressure
into the
fluid-filled lumen 124. An example of a side opening embodiment is illustrated
in
Figure 4. In the illustrated embodiment, the PTC 134 includes a tubular shaft
122
having a distal opening 135 in addition to one or more side openings 125. The
side
openings 125 may be provided in addition to or in place of the distal port
135. If the
distal port 135 is not used, it may be occluded with a suitable material such
as epoxy
-14-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
or a polymer, for example. The side openings 125 may be any desired shape,
such as
circular ports or rectangular windows, for example.
[045] The side ports 125 significantly increase surface area for pressure
transmission. For example, a 1.0 mm inside diameter tubular shaft 122 has a
distal
port 135 area of 0.78 mm2. The same sized tubular shaft 122 with two side
windows
each having a length of 3.00 mm and a height of 0.75 mm will add 4.50 mm2 in
opening area, an increase of 477%. Such side openings 125 provide several
advantages, including increased sampling area and increased pressure
transmission
efficiency, especially in the event that the tip of the catheter becomes
covered with
fibrous tissue.
[046] To retain the fluid 124 and the gel 126 inside the tubular shaft 122 of
the PTC 134, a membrane 123 may be disposed over the side openings 125. In
some
useful embodiments of the present invention, membrane 123 comprises a
resilient
and/or reversibly deformable material. For example, membrane 123 may comprise
an
elastomeric material. The term elastomeric generally refers to a rubber-like
material
(e.g., a material which can experience about a 5% deformation and return to
the
undeformed configuration). Examples of elastomeric materials include rubber
(e.g.,
natural rubber, silicone rubber, nitrile rubber, polysulfide rubber, etc.),
thermoplastic
elastomer (TPE), butyl, polyurethane, and neoprene. For example, the membrane
123
may comprise a thin walled (e.g., 0.002 inch thick wall) silicone rubber tube
slid over
the tubular shaft 122 adjacent the side openings 125. Silicone rubber that may
be
suitable in some applications is commercially available from Dow Coming
Corporation of Midland, Michigan which identifies this silicone rubber using
the
SILASTIC trademark. Alternatively, the membrane 123 may comprise a thin walled
polycarbonate-polyurethane that is bonded to the tubular shaft 122.
-15-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
[047] In addition to or in place of the thin membrane 123, a thin-walled
cover 127 may be placed over all or a portion of the tubular shaft 122 (and
membrane
123). The cover 127 may comprise a thin-walled tube or sock (closed-ended)
that
promotes tissue ingrowth (passivation) and that reduces the risk of
thromboemboli
formation. For example, the cover 127 may comprise a thin walled tube of ePTFE
or
a woven tube of Dacron.
[048] In addition to the use of cover 127 over the tubular shaft 122 of the
PTC 134, the use of a cover to reduce the risk of thromboemboli may also have
significant benefit for a wide variety of other blood pressure sensor
applications,
particularly when the underlying material tends to promote the thromboemboli.
For
example, a covering may be useful for a blood pressure sensor 160 as shown in
Figure
5. The pressure sensor 160 schematically shown in Figure 5 is similar to a
pressure
sensor described in U.S. Patent No. 6,221,024 to Miesel, the entire disclosure
of
which is incorporated herein by reference.
[049] The pressure sensor 160 includes a metallic housing 162 and a metallic
diaphragm 163 defining an oil-filled cavity 164. A capacitive pressure
transducer 166
and electronic integrated circuit 168 disposed in the cavity 164 detect
changes in
capacitance as a function of pressure impinging on the diaphragm 163. Because
metallic materials in contact with blood flow in a vessel or chamber may tend
to form
thromboemboli, a thin cover 167 composed of a material such as ePTFE may be
disposed about the housing 162 and/or the diaphragm 163 to reduce the
likelihood
therefor.
[050] The pressure sensor 160 includes a housing 162 and a diaphragm 163
defining an fluid-filled cavity 164. In the exemplary embodiment of figure 5,
housing
162 and diaphragm 163 both comprise metallic materials. In this exemplary
-16-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
embodiment, diaphragm 163 may be fixed to housing 162 by, for example,
welding,
brazing, and/or soldering. Examples of metallic materials that may be suitable
in
some applications include titanium, stainless steel, MP35N alloy, and
platinum. A
pressure transducer 166 and an electronic integrated circuit 168 disposed in
the cavity
164 provide a signal S that changes as a function of pressure impinging on the
diaphragm 163. In the embodiment of figure 5, a covering 167 is disposed over
the
housing 162 and diaphragm 163. Because metallic materials in contact with
blood
flow in a vessel or chamber may tend to form thromboemboli, a thin cover
composed
of a material such as, for example, ePTFE disposed about the housing 162
and/or the
diaphragm 163 may reduce the likelihood that thromboemboli will form.
[051] A number of materials may be suitable for use in covering 167.
Examples of such materials include fluoropolytetrafluoroethylene (PTFE),
ePTFE,
polyethylene(PE), polypropylene (PP), polyvinylchloride (PVC), polyurethane,
and
DACRON. A number of manufacturing processes may be used to create covering
167. For example, covering 167 may be woven from a plurality of fibers. By way
of a
second example, covering 167 may be formed from one or more sections of shrink
tubing. The shrink tubing sections may be positioned and then shrunk by the
application of heat. A spray process may also be used to apply covering 167.
For
example, spraying PTFE solids in a suitable solvent carrier is a process which
has
been found suitable for this application. Another material that may be used to
fabricate covering 167 is a thermoplastic generically known as parylene. There
are a
variety of polymers based on para-xylylene. These polymers are typically
placed onto
a substrate by vapor phase polymerization of the monomer. Parylene N coatings
are
produced by vaporization of a di(P-xylylene)dimer, pyrollization, and
condensation of
the vapor to produce a polymer that is maintained at comparatively lower
-17-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
temperature. In addition to parylene-N, parylene-C is derived from
di(monochloro-P-
xylylene) and parylene-D is derived from di(dichloro-P-xylylene). There are a
variety
of known ways to apply parylene to substrates. The use of paralene has been
disclosed
in U.S. Pat. Nos. 5,380,320 (to J. R. Morris), in 5,174,295 (to Christian et
al.), and in
6,067,491 (to Taylor et al.). The entire disclosure of these United States
Patents is
hereby incorporated herein.
[052] Figure 6 is a perspective view showing an illustrative assembly 300 in
accordance with an exemplary embodiment of the present invention. Assembly 300
comprises a shaft 302 having a wall 304 defining a lumen 306. In the
embodiment of
figure 6, wall 304 of shaft 302 defines a laterally oriented port 320 and an
axially
oriented port 322. With reference to figure 6 it will be appreciated that both
laterally
oriented port 320 and an axially oriented port 322 are disposed in fluid
communication with lumen 306.
[053] Figure 7 is a perspective view showing a pressure transmission catheter
301 including shaft 302 shown in the previous figure. In the embodiment of
figure
7, a gel plug 324 is disposed in lumen 306 proximate laterally oriented port
320 and
axially oriented port 322. Also in the embodiment of figure 7, a pressure
sensor 330
is disposed in fluid communication with lumen 306. A pressure transmitting
fluid 332
is disposed in lumen 306 for transferring pressure between gel plug 324 and
pressure
sensor 330.
[054] With reference to figure 7, it will be appreciated that a gel material
326
of gel plug 324 extends into laterally oriented port 320. In the embodiment of
figure
7, an exposed surface area of gel material 326 extending into laterally
oriented port
320 is generally equal to an outer surface area of laterally oriented port
320. Also in
the embodiment of figure 7, an exposed surface area of gel material proximate
axially
-18-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
oriented port 322 is generally equal to a lateral cross sectional area of
lumen 306. In
the exemplary embodiment of figure 7, the outer surface area of laterally
oriented port
320 is greater than the lateral cross-sectional area of lumen 306.
[055] Figure 8 is an additional a perspective view showing the pressure
transmission catheter 301 shown in the previous figure. In figure 8, a
membrane 334
is shown overlaying laterally oriented port 320. Also in figure 8, gel
material 326 of
gel plug 324 can be seen disposed in axially oriented port 322. Accordingly,
it will be
appreciated that membrane 334 covers laterally oriented port 320 and leaves
axially
oriented port 322 exposed in the exemplary embodiment of figure 8.
[056] Membrane 334 may comprise various materials without deviating from
the spirit and scope of the present invention. In some useful embodiments of
the
present invention, membrane 334 comprises a resilient and/or reversibly
deformable
material. For example, membrane 334 may comprise an elastomeric material. The
term elastomeric generally refers to a rubber-like material (e.g., a material
which can
experience about a 5% deformation and return to the undeformed configuration).
Examples of elastomeric materials include rubber (e.g., natural rubber,
silicone
rubber, nitrile rubber, polysulfide rubber, etc.), thermoplastic elastomer
(TPE), butyl,
polyurethane, and neoprene. Membrane 334 may comprise, for example, a thin
walled
(e.g., 0.002 inch thick wall) silicone rubber tube slid over shaft 302
adjacent laterally
oriented port 320. Silicone rubber that may be suitable in some applications
is
commercially available from Dow Corning Corporation of Midland, Michigan which
identifies this silicone rubber using the SILASTIC trademark. Alternatively,
membrane 334 may comprise a thin walled polycarbonate-polyurethane that is
bonded
to shaft 302.
-19-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
[057] The material(s) and ditnensions of membrane 334 may be selected
such that membrane 334 transfers a pressure being measured to gel plug 324. At
the
same time, the materials and dimensions of shaft 302 may be selected to
provide a
pressure transmission catheter with a desired level of structural integrity.
In some
exemplary embodiments, shaft 302 may comprise a first material and membrane
may
comprise a second material different from the first material. For example, the
second
material may comprise an elastomeric material and the first material may
comprise a
non-elastomeric material. By way of a second example, the first material may
have a
first modulus of elasticity and the second material may have a second modulus
of
elasticity that is greater than the first modulus of elasticity. Shaft 302 may
comprise
various materials without deviating from the spirit and scope of the present
invention.
Examples of materials that may be suitable in some applications include
polycarbonate, polyurethane (PU), polyethylene (PE), polypropylene (PP), and
polyvinylchloride (PVC) fluoropolytetrafluoroethylene (PTFE), and ePTFE.
[058] Figure 9 is a schematic illustration showing a body 550 and a cardiac
pacing system 500. Body 550 has a heart 552 that is disposed in a thoracic
cavity
560 of body 550. With reference to figure 9, it will be appreciated that a
pulse
generator 502 of cardiac pacing system 500 is disposed in a pocket 562 fonned
in
body 550. In the embodiment of figure 9, pocket 562 generally is disposed in a
pectoral region 564 of body 550.
[059] Heart 552 of body 550 includes a left ventricle 566 and a right
ventricle 568. A plurality of blood vessels are shown connecting with heart
552 in
figure 9. The blood vessels shown in figure 9 include a superior vena cava 570
and an
inferior vena cava 572. In the embodiment of figure 9, cardiac pacing system
500
comprises a pulse generator 502, a right atrial lead 503, a right ventricular
lead 504,
- 20 -
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
and a left ventricular lead 506. The right ventricular lead 504 is connected
to the
pulse generator 502 and configured to pace the right ventricle 554 of the
heart 552. In
figure 9, right ventricular lead is shown passing through the superior vena
cava 570 of
body 550. The left ventricular lead 506 of cardiac pacing system 500 is
connected to
the pulse generator 502 and configured to pace the left ventricle 566 of the
heart 552.
In some useful embodiments of the present invention, the left ventricular lead
506 is
also configured to measure left ventricular pressure. In the embodiment of
figure 9,
the left ventricular lead 506 comprises a pressure transmission catheter 520
and a
housing 522. Housing 522 may contain a pressure sensor and associated
electronics
as shown, for example, in Figure 3.
[060] With reference to figure 9, it will be appreciated that left ventricular
lead 506 extends between pulse generator 502 and the left ventricle 566 of the
heart
552. With continuing reference to figure 9, it will be appreciated that a
portion of left
ventricular lead 506 is disposed within thoracic cavity 560 and that left
ventricular
lead 506 is outside of any blood vessels. Some methods in accordance with the
present invention may comprise the step of positioning a conductor connected
to an
electrode andlor a pressure sensor so that it extends through a thoracic
cavity without
extending through any blood vessels.
[061] From the foregoing, it will be apparent to those skilled in the art that
the present invention provides, in exemplary non-limiting embodiments, devices
and
methods for left ventricular or biventricular pacing plus left ventricular
pressure
measurement, such as a pacing lead having a combined electrode and pressure
sensor
assembly for left ventricular pacing and pressure measurement. Further, those
skilled
in the art will recognize that the present invention may be manifested in a
variety of
forms other than the specific embodiments described and contemplated herein.
-21-
CA 02579571 2007-03-07
WO 2006/029186 PCT/US2005/031808
Accordingly, departures in form and detail may be made without departing from
the
scope and spirit of the present invention as described in the appended claims.
The
entire disclosure of all patents and patent applications mentioned in this
document are
hereby incorporated by reference herein.
-22-