Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02580541 2007-03-23
AN IMPROVED ELECTROCHROMIC MEDIUM
CAPABLE OF PRODUCING A PRE-SELECTED COLOR
BACKGROUND OF THE INVENTION
This invention relates to an improved electrochromic medium capable of
producing a pre-selected color and, more particularly, an improved
electrochromic
device having an electrochromic medium comprising at least three electroactive
materials whose concentrations may be chosen to produce a pre-selected
perceived
color, where the electrochromic medium generally maintains the pre-selected
perceived color throughout its normal range of voltages when used in an
electrochromic device.
Electrochromic devices have been proposed for commercial applications for
nearly seventy years (British Patent Specification No. 328,017 (1929) to F. H.
Smith).
However, the first commercially successful electrochromic device, a dimmable
rearview mirror for motor vehicles, was not introduced until 1987. Various
automatic
rearview mirrors for motor vehicles have been devised which automatically
change
from the full reflectance mode (day) to the partial reflectance mode(s)
(night) for glare
protection purposes from light emanating from the headlights of vehicles
approaching
from the rear. The electrochromic mirrors disclosed in U.S. Pat. No.
4,902,108, entitled
"Single-Compartment, Self-Erasing, Solution-Phase Electrochromic Devices,
Solutions
for Use Therein, and Uses Thereof', issued Feb. 20, 1990 to H. J. Byker;
Canadian
Patent No. 1,300,945, entitled "Automatic Rearview Mirror System for
Automotive
Vehicles", issued May 19, 1992 to J. H. Bechtel et al.; U.S. Pat. No.
5,128,799, entitled
"Variable Reflectance Motor Vehicle Mirror", issued Jul. 7, 1992 to H. J.
Byker; U.S.
Pat. No. 5,202,787, entitled "Electro-Optic Device", issued Apr. 13, 1993 to
H. J.
Byker et al.; U.S. Patent No. 5,204,778, entitled "Control System For
Automatic
Rearview Mirrors", issued Apr. 20, 1993 to J. H. Bechtel; U.S. Patent No.
5,278,693,
entitled "Tinted Solution-Phase Electrochromic Mirrors", issued Jan. 11, 1994
to D.A.
Theiste et al.; U.S. Patent No. 5,280,380, entitled "UV-Stabilized
Compositions and
Methods", issued Jan. 18, 1994 to H. J. Byker; U.S. Patent No. 5,282,077,
entitled
"Variable Reflectance Mirror", issued Jan. 25, 1994 to H. J. Byker; U.S.
Patent No.
5,294,376, entitled "Bipyridinium Salt Solutions", issued Mar. 15, 1994 to H.
J. Byker;
1
CA 02580541 2007-03-23
U.S. Patent No. 5,336,448, entitled "Electrochromic Devices with Bipyridinium
Salt
Solutions", issued August 9, 1994 to H. J. Byker; U.S. Patent No. 5,434,407,
entitled
"Automatic Rearview Mirror Incorporating Light Pipe", issued January 18, 1995
to F. T.
Bauer et al.; U.S. Patent No. 5,448,397, entitled "Outside Automatic Rearview
Mirror for
Automotive Vehicles", issued September 5, 1995 to W. L. Tonar; and U.S. Patent
No.
5,451,822, entitled "Electronic Control System", issued September 19, 1995 to
J. H.
Bechtel et al., each of which patents is assigned to the assignee of the
present invention,
are typical of modem day automatic rearview mirrors for motor vehicles. Such
electrochromic mirrors may be utilized in a fully integrated inside/outside
rearview
mirror system or as an inside or an outside rearview mirror system. In
general, in
automatic rearview mirrors of the types disclosed in the above referenced U.S.
Patents,
both the inside and the outside rearview mirrors are comprised of a relatively
thin
electrochromic medium sandwiched and sealed between two glass elements.
In most electrochromic mirrors, when the electrochromic medium which
functions as the media of variable transmittance is electrically energized, it
darkens and
begins to absorb light, and the more light the electrochromic medium absorbs
the darker
the mirror becomes. When the electrical voltage is decreased to zero, the
mirror returns
to its clear state. The electrochromic medium is contained in a sealed chamber
defined
by a transparent front glass element coated with a transparent conductor, a
peripheral
edge seal, and a rear mirror element having either a reflective layer or a
transparent
conductive layer in contact with the electrochromic medium depending on
whether the
mirror has a third or fourth surface reflector. The conductive layers on both
the front
glass element and the rear glass element are connected to electronic circuitry
which is
effective to electrically energize the electrochromic medium to switch the
mirror to
nighttime, decreased reflectance modes when glare is detected and thereafter
allow the
mirror to return to the daytime, high reflectance mode when the glare
subsides, as
described in detail in the aforementioned U.S. Patents. For clarity of
description of such
a structure, the front surface of the front glass element is referred to as
the first surface,
and the inside surface of the front glass element is referred to as the second
surface. The
inside surface of the rear glass element is referred to as the third
2
CA 02580541 2007-03-23
0
surface, and the back surface of the rear glass element is referred to as the
fourth
surface.
The electrochromic medium is typically comprised of solution-phase
electrochromic materials, electrodeposition type electrochromic materials,
surface
confined electrochromic materials or combinations thereof. The electrochromic
medium changes from a clear or high visible light transmission level, to a
lightly
colored state, to a moderately colored state, and to a dark or low visible
light
transmission colored state when various voltages are applied and
electrochemical
oxidation and reduction take place. An important factor in determining the
desirability
of an electrochromic device is its perceived color when in its clear state and
dark state
and any state therebetween. The perceived color of an electrochromic mirror
includes
the influences from the front glass element, the two transparent conductive
coatings, the
reflector and, most importantly, the electrochromic medium.
Generally speaking, there is a desire for a gray colored electrochromic medium
in interior mirrors and most exterior mirrors for motor vehicles because the
perceived
color of the reflected image will closely resemble the color of the object
before being
reflected. In addition, it is desirable that the electrochromic device
maintain this gray
color during its darkening and clearing transitions so that the perceived
colors of a
reflected image do not change during these transitions. However, arguments
have been
made for tinted or colored mirrors. For example, commonly assigned U.S. Patent
No.
5,278,693 to D. A. Theiste et al., discloses adding an electrochemically
inactive and
stable compound to a solution-phase electrochromic device to provide a blue
tint. This
electrochemically inactive compound is essentially a dye normally present at
low
levels, and will provide a perceived tint to the device only in the highest
reflectance or
transmittance states when little or no voltage is applied.
In other applications such as- architectural windows, sun roofs, displays and
specialty windows, various colors (e.g., blue, greens, purples, yellows) in
addition to
gray may be desirable for a number of reasons. For instance, it may be desired
that
certain electrochromic windows be tinted to match the decor of the room,
provide
contrast enhancement or gray scale dimming filters for displays emitting
particular
colors of light, or to give a building a particular color or appearance.
3
CA 02580541 2007-03-23
A problem in the art, then has been the inability to pre-select a color of an
electrochromic device while simultaneously ensuring that the device generally
maintains the desired color when in its clear state and dark state and any
state
therebetween. With such devices used as electrochromic rearview mirrors for
motor
vehicles and many window applications, a desired color is one that is
perceived as gray.
For other applications, colors that are perceived other than gray, (e.g., red,
yellow,
green, blue, purple) may be desirable.
Consequently, it is desirable to provide an impro ved electrochromic medium
having at least three electroactive materials whose concentrations may be
chosen to
produce a pre-selected perceived color, where the electrochromic medium
generally
maintains the pre-selected perceived color throughout its normal range of
voltages
when used in an electrochromic device.
OBJECTS OF THE INVENTION
Accordingly, a primary object of the present invention is to provide an
improved electrochromic medium having at least three electroactive materials
whose
relative concentrations may be chosen to produce a pre-selected perceived
color,
where the electrochromic medium generally maintains the pre-selected perceived
color throughout its normal range of voltages when used in an electrochromic
device.
Another object of the present invention is to provide an improved
electrochromic medium having at least three electroactive materials whose
concentrations may be chosen to produce a perceived gray color, where the
electrochromic medium generally maintains the gray color throughout its normal
range of voltages when used in an electrochromic device.
Yet another object of the present invention is to provide novel electroactive
materials.
SUMMARY OF THE INVENTION
The above and other objects, which will become apparent from the
specification as a whole, including the drawings, are accomplished in
accordance with
the present invention by providing an electrochromic device having an
electrochromic
medium that comprises at least three electroactive materials having absorption
spectra
4
CA 02580541 2007-03-23
that add together such that the color of the electrochemically activated
electrochromic
medium can be pre-selected by individually choosing the concentrations of the
at least
three electroactive materials. The electrochromic medium generally maintains
the
pre-selected perceived color throughout its normal range of voltages when used
in an
electrochromic device. The at least three electroactive materials include at
least one
electrochemically reducible material (cathodic material), at least one
electrochemically oxidizable material (anodic material) and at least one
additional
electroactive material which may be either an anodic or cathodic material.
Thus, there
are always at least three electroactive materials present in the medium, with
at least
two either being anodic or cathodic materials. The pre-selected color may be
chosen
from a wide variety of colors and may be, for example, gray, red, orange,
yellow,
green, blue, and purple. For electrochromic mirrors for motor vehicles and
many
window applications, a presently preferred color is gray.
In accordance with one aspect of the present invention, there is provided a
one-pot
method, comprising the steps of: mixing a heterocyclic azine compound; a
reducing
reagent; a base; an alkylating reagent; a member of the group consisting of. a
phase
transfer catalyst, water or both a phase transfer catalyst and water; and a
solvent in one
pot; stirring said mixture under an inert atmosphere; and separating a
compound from said
mixture, wherein said heterocyclic azine compound is reduced and alkylated.
In accordance with another aspect of the present invention, there is provided
a
two-phase one-pot method, comprising the steps of. mixing a heterocyclic azine
compound; a reducing reagent; a base; an alkylating reagent; a member of the
group
consisting of a phase transfer catalyst, water, or both a phase transfer
catalyst and water;
and a non-polar solvent in one pot; stirring said two-phase mixture under an
inert
atmosphere; and separating a compound from said two-phase mixture, wherein
said
heterocyclic azine compound is reduced and alkylated.
CA 02580541 2007-03-23
BRIEF DESCRIPTION OF THE DRAWINGS
The subject matter which is regarded as the invention is particularly pointed
out and distinctly claimed in the concluding portion of the specification. The
invention, together with further objects and advantages thereof, may best be
understood by reference to the following description taken in connection with
the
accompanying drawings, where like numerals represent like components, in
which:
FIG. 1 is an enlarged cross-sectional view of an electrochromic device;
FIG. 2 illustrates scaled absorption spectra for the electrochemically
activated states of the following individual electrochromic materials: 5,10-
dimethyl-5,10-dihydrophenazine (Al); 2,7-diphenoxy-5,1 0-dimethyl-5, 10-
dihydrophenazine (A6); 1,l'-dimethyl-4,4'-(1,3,5-triazine-2,4-diyl)
dipyridinium
diperchlorate (C4); 1,1'-dimethyl-2-(3-phenyl(n-propyl))-4,4'-dipyridinium
bis(hexafluorophosphate) (C5), where each absorption spectrum is scaled to the
relative concentrations of the activated states that would be present in an
activated
device; as well as the composite spectrum for the activated state of an
electrochromic medium originally comprising Al and 80% and A6 at 20% of the
total anodic materials, plus C4 at 50% and C5 at 50% of the total cathodic
materials;
5a
CA 02580541 2007-03-23
FIG. 3 illustrates scaled absorption spectra for the electrochemically
activated states of the following individual electrochromic materials: 5,10-
dimethyl-5,10-dihydrophenazine (A); 2,3-diphenyl-5, 1 0-dimethyl-5, 10-
dihydrophenazine (B); and tungsten trioxide (C), where each absorption
spectrum
is scaled to the relative concentrations of the activated states that would be
present
in an activated device; as well as the composite spectrum (D) which is the sum
of
the scaled spectra of the activated states of these three electrochromic
materials;
FIG. 4 shows several curves representing the color coordinates of various
electrochromic mirrors incorporating various electrochromic media as the
mirrors
are transitioned from their clear or high reflectance states to their dark or
low
reflectance states;
FIG. 5 shows several curves representing the color coordinates of various
electrochromic media incorporated in various electrochromic windows as the
windows are transitioned from their clear or high transmission states to their
dark
or low transmission states;
FIG. 6 shows several color coordinate curves indicating the staging
phenomenon in electrochromic windows incorporating various electrochromic
media as the windows are transitioned from their clear or high transmission
states
to their dark or low transmission states; and
FIG. 7 is a front elevational view schematically illustrating an
inside/outside electrochromic rearview mirror medium for motor vehicles where
the inside and outside mirrors incorporate the mirror assembly of the present
invention.
DETAILED DESCRIPTION
Figure 1 shows a cross-sectional view of an electrochromic device 110 which
may be a mirror, a window, a display device, and the like. Device 110 has a
front
transparent element 112 having a front surface 112a and a rear surface 112b,
and a
rear element 114 having a front surface 114a and a rear surface 114b. Since
some of
the layers of the mirror are very thin, the scale has been distorted for
pictorial clarity.
Also, for clarity of description of such a structure, the following
designations will be
6
CA 02580541 2009-07-22
used hereinafter. The front surface 112a of the front glass element will be
referred to
as the first surface and the back surface 112b of the front glass element as
the second
surface. The front surface 114a of the rear glass element will be referred to
as the
third -surface, and the back surface 114b of the rear glass element as the
fourth surface.
Front transparent element 112 may be any material which is transparent and
has sufficient strength to be able to operate in the conditions, e.g., varying
temperatures and pressures, commonly found in the automotive environment.
Front
element 112 may comprise any type of borosilicate glass, soda lime glass,
float glass
or any other material, such as, for example, a polymer or plastic, that is
transparent in
the visible region of the electromagnetic spectrum. Front element 112 is
preferably a
sheet of glass with a thickness ranging from 0.5 millimeters (mm) to about
12.7 mm.
Rear element 114 must meet the operational conditions outlined above, except
that if
the electrochromic device is a mirror, rear element 114 does not need to be
transparent, and therefore may comprise polymers, metals, glass, ceramics, and
preferably is a sheet of glass with a thickness ranging from 0.5 mm to about
12.7 mm.
A layer of a transparent electrically conductive material 116 is deposited on
the second surface 112b to act as an electrode. Transparent conductive
material 116
may be any material that: is substantially transparent to visible light; bonds
well to
front element 112 and maintains this bond when the epoxy seal 118 bonds
thereto; is
resistant to corrosion by any materials within the electrochromic device; is
resistant to
corrosion by the atmosphere; and has minimal diffuse or specular reflectance
and
good electrical conductance. Transparent conductive material 116 may include
two
sublayers 116a and 116b. Transparent conductive material 116 may be fluorine
doped tin
oxide (FTO), tin doped indium oxide (ITO), ITO/metal/ITO (IMI) as disclosed in
"Transparent Conductive Multilayer-Systems for FPD Applications", by J.
Stollenwerk, B.
Ocker, K.H. Kretschmer of LEYBOLD AG, Alzenau, Germany, and the materials
described
in above-referenced U.S. Patent No. 5,202,787, such as TECTM 20 or TECTM 15,
available
from Libbey Owens-Ford Co. (LOF) of Toledo, OH. Co-filed U.S. Patent No.
5,923,457
entitled "AN IMPROVED ELECTRO-OPTIC DEVICE INCLUDING A LOW SHEET
RESISTANCE, HIGH TRANSMISSION TRANSPARENT ELECTRODE" describes a low
sheet resistance, high transmission, scratch resistant transparent electrode
that forms strong
bonds with adhesives, is not
7
CA 02580541 2009-07-22
oxygen sensitive, and can be bent to form convex or aspheric electro-optic
mirror
elements or tempered in air without adverse side effects. Similar requirements
are
needed for the layer 120 deposited onto the third surface 114a, whether it is
a transparent
conductive material used in electrochromic windows and in mirrors having a
fourth
surface reflector, or a combined reflector/electrode (discussed below) used in
electrochromic mirrors having a third surface reflector.
The conductance of the layer(s) of transparent conductive material (116 and/or
TM
120) will depend on their thickness and composition. As a general rule TEC
coatings
from LOF are more color neutral than simple FTO and ITO coatings. This
difference in
color neutrality impacts the overall color of the reflected image when the
mirror is fully
darkened because almost all the reflection seen by the driver comes from the
first and
second surfaces. Thus, if there is transparent conductive material on the
second surface
of the mirror that is not color neutral it can impact the color of the
reflected image
TM
viewed by the driver. In certain automotive mirror systems it is beneficial to
use TEC
coatings as the transparent conductor on the interior mirror, but not larger
exterior
TM
mirrors. When thin glass is used for larger exterior mirrors TEC cannot be
used because
these coatings are applied on the float-line, and it is very difficult to make
thin glass on
large float-lines and even more difficult to apply coatings while making thin
glass on a
float-line. Therefore, thin glass coated on a float-line is not presently
commercially
available. If a simple ITO coating is used on the exterior mirror then the
reflected image
will not be color neutral when the mirror is in the fully darkened state and
one will notice
a difference in color between the reflected images of these exterior mirror
and interior
TM
mirrors made with TEC glass coatings. U.S. Patent No. 5,940,201 entitled "A
ELECTROCHROMIC MIRROR WITH TWO THIN GLASS ELEMENTS AND A
GELLED ELECTROCHROMIC MEDIUM" discloses a preferred color neutral
transparent conductive coating that can be used on the second surface of a
mirror or a
window and will eliminate the described problem. This color neutral
transparent
conductive coating provides a particularly advantageous combination with the
gray
electrochromic medium of the present invention. The combination of a bright
nearly
achromatic reflector, a gray electrochromic medium and a color neutral
transparent
conductive coating provides, for the first time, a rearview mirror which is
perceived as
8
CA 02580541 2009-07-22
neutral gray throughout all of its reflectance range, including intermediate
reflectances.
For electrochromic mirrors the reflector may be placed on the fourth surface,
in
which case a layer of a transparent conductive electrode is disposed on the
third surface
114a, or the reflector may be placed on the third surface 114a in accordance
with the
disclosure of U.S. Patent No. 5,818,625 entitled "ELECTROCHROMIC REARVIEW
MIRROR INCORPORATING A THIRD SURFACE METAL REFLECTOR" filed on or
about April 2, 1997. In this case the third surface reflector doubles as an
electrode and
the transparent conductive layer on the third surface is not necessary. A
heater (not
shown) may be placed directly on the fourth surface I 14b.
The coating 120 of the third surface 114a (whether a transparent conductor or
a
reflector/electrode) is sealably bonded to the coating 116 on the second
surface 112b near
the outer perimeter by a sealing member 118, thereby defining a chamber 122,
which may
be filled through fill hole 125. For electrochromic mirrors, sealing member
118 preferably
contains glass beads (not shown) to hold transparent elements 112 and 114 in a
parallel and
spaced-apart relationship. Sealing member 118 may be any material which is
capable of
adhesively bonding the coatings on the second surface 112b to the coatings on
the third surface
114a to seal the perimeter such that electrochromic medium 124 does not leak
from chamber
122. Optionally, the layer of transparent conductive coating 116 and the layer
on the third
surface 120 (transparent conductive material or reflector/electrode) may be
removed over a
portion where sealing member 118 is disposed (not the entire portion,
otherwise the drive
potential could not be applied to the two coatings). In such a case, sealing
member 118 must
bond well to glass.
The performance requirements for a perimeter seal member 118 used in an
electrochromic device are similar to those for a perimeter seal used in a
liquid crystal
device (LCD) which are well known in the art. The seal must have good adhesion
to
glass, metals and metal oxides, must have low permeabilities for oxygen,
moisture vapor
and other detrimental vapors and gases, and must not interact with or poison
the
electrochromic or liquid crystal material it is meant to contain and protect.
The perimeter
seal can be applied by means commonly used in the LCD industry such as
9
CA 02580541 2009-07-22
by silk-screening or dispensing. Totally hermetic seals such as those made
with glass
fit or solder glass can be used, but the high temperatures involved in
processing
(usually near 450-degrees Centigrade) this type of seal can cause numerous
problems
such as glass substrate warpage, changes in the properties of transparent
cbnductive
electrode and oxidation or degradation of the reflector. Because of their
lower
processing temperatures, thermoplastic, thermosetting or UV curing organic
sealing
resins are preferred. Such organic resin sealing systems for LCD's are
described in
U.S. Patent Numbers 4,297,401, 4,418,102, 4,695,490, 5,596,023 and 5,596,024.
Because of their excellent adhesion to glass, low oxygen permeability and good
solvent resistance, epoxy based organic sealing resins are preferred. These
epoxy
resin seals may be UV curing, such as described in U.S. Patent Number
4,297,401, or
thermally curing, such as with mixtures of liquid epoxy resin with liquid
polyamide
resin or dicyandiamide, or they can be homopolymerized. The epoxy resin may
contain fillers or thickeners to reduce flow and shrinkage such as fumed
silica, silica,
mica, clay, calcium carbonate, alumina, etc., and/or pigments to add color.
Fillers
pretreated with hydrophobic or silane surface treatments are preferred. Cured
resin
crosslink density can be controlled by use of mixtures of mono-functional, Bi-
functional and multi-functional epoxy resins and curing agents. Additives such
as
silanes or titanates can be used to improve the seal's hydrolytic stability,
and spacers
such as glass beads or rods can be used to control final seal thickness and
substrate
spacing. Suitable epoxy resins for use in a perimeter seal member 118 include
but are
TM
not limited to: "EPON RESIN" 813, 825, 826, 828, 830, 834, 862, 1001F, 1002F,
2012, DPS-155, 164, 1031, 1074, 58005, 58006, 58034, 58901, 871, 872 and DPL-
TM
862 available from Shell Chemical Co., Houston, Texas; "ARALITE" GY 6010, GY
6020, CY 9579, GT 7071, XU 248, EPN 1139, EPN 1138, PY 307, ECN 1235, ECN
1273, ECN 1280, MT 0163, MY 720, MY 0500, MY 0510 and PT 810 available from
TM
Ciba Geigy, Hawthorne, NY; "D.E.R." 331, 317, 361, 383, 661, 662, 667, 732,
736,
TM
"D.E.N." 431, 438, 439 and 444 available from Dow Chemical Co., Midland,
TM
Michigan. Suitable epoxy curing agents include V-15, V-25 and V-40 polyamides
TM T"
from Shell Chemical Co.; "AJICURE" PN-23, PN-34 and VDH available from
TM
Ajinomoto Co., Tokyo, Japan; "CUREZOL" AMZ, 2MZ, 2E4MZ, C11Z, CI7Z, 2PZ,,
TM
21Z and 2P4MZ available from Shikoku Fine Chemicals,- Tokyo, Japan; "ERISYS"
DDA or DDA accelerated with U-405, 24EMI, U-410 and U-415 available from CVC
CA 02580541 2009-07-22
TM
Specialty Chemicals, Maple Shade, NJ.; "AMICURE" PACM, 352, CG, CG-325 and
CG-1200 available from Air Products, Allentown, PA. Suitable fillers include
fumed
TM
silica such as "CAB-O-SIL" L-90, LM-130, LM-5, PTG, M-5, MS-7, MS-55, TS-720,
TM
HS-5, EH-5 available from Cabot Corporation, Tuscola, IL; "AEROSIL" R972,
R974,
R805, R812, R812 S, R202, US204 and US206 available from Degussa, Akron, OH.
TM i TM TM TM
Suitable clay fillers include BUCA, CATALPO, ASP NC, SATINTONE 5,
TM TM
SATINTONE SP-33, TRANSLINK 37, TRANSLINK 77, TRANSLINK 445,
TRANSLINK 555 available from Engelhard Corporation, Edison, NJ. Suitable
silica
TM
fillers are SILCRON G-130, G-300, G-100-T and G-100 available from SCM
Chemicals, Baltimore, MD. Suitable silane coupling agents to improve the
seal's
TM
hydrolytic stability are Z-6020, Z-6030, Z-6032, Z-6040, Z-6075 and Z-6076
available from Dow Corning Corporation, Midland, MI. Suitable precision glass
microbead spacers are available in an assortment of sizes from Duke
Scientific, Palo
Alto, CA.
In discussing colors it is useful to refer to the Commission Internationale de
I'Eclairage's (CIE) 1976 CIELAB Chromaticity Diagram (commonly referred to as
the L*a*b* chart). The technology of color is relatively complex, but a fairly
comprehensive discussion is given by F. W. Billmeyer and M. Saltzman in
Principles
of Color Technology, 2'd Edition, J. Wiley and Sons Inc. (1981), and the
present
disclosure, as it relates to color technology and terminology, generally
follows that
discussion. On the L*a*b* chart, L* defines lightness, a* denotes the
red/green value
and b* denotes the yellow/blue value. Each of the electrochromic media has an
absorption spectra at each particular voltage that may be converted to a three
number
designation, their L*a*b* values. To calculate a set of color coordinates,
such as
L*a*b* values, from the spectral transmission or reflectance, two additional
items are
required. One is the spectral power distribution of the source or illuminant.
The
present disclosure uses CIE Standard Illuminant A to simulate light from
automobile
headlamps and uses CIE Standard Illuminant D65 to simulate daylight. The
second
item needed is the spectral response of the observer. The present disclosure
uses the 2
degree CIE standard observer. The illuminant/observer combination generally
used
for mirrors is then represented as A/2 degree and the combination generally
used for
windows is represented as D65/2 degree.
11
CA 02580541 2007-03-23
In accordance with the present invention, the electrochromic device includes
an
electrochromic medium that comprises at least three electroactive materials
having
absorption spectra when electrochemically activated that add together such
that the color
of the electrochromic medium can be pre-selected by individually choosing the
concentrations of the at least three electroactive materials. The at least
three
electroactive materials include at least one reducible material (cathodic
material), at least
one oxidizable material (anodic material) and at least one additional
electroactive
material which may be either an anodic or cathodic material. Thus, there are
always
three electroactive materials present in the medium, with at least two either
being anodic
or cathodic materials. Generally, all three electroactive materials are
electrochromic such
that there is a change in the absorption coefficient at at least one
wavelength in the
visible spectrum when electrochemically activated. However, there are
instances where
it is desirable to have at least two electrochromic anodic materials combined
with at least
one generally colorless electroactive cathodic material or, alternatively, at
least two
electrochromic cathodic materials combined with at least one generally
colorless
electroactive anodic material. In any case, at least two of the electroactive
materials must
be electrochromic. Finally, if the at least three electroactive compounds in
their
non-activated, zero-potential, equilibrium states in the solution are not
ionic, the
electrochromic medium further includes an electrolyte, although it should be
understood
that an additional electrolyte may be included when one or more of the
electroactive
compounds is ionic.
The electrochromic medium includes electroactive cathodic and anodic materials
that may be independently chosen from at least the following three categories:
(i) Solution-Phase--a material contained in solution in the ionically
conducting electrolyte which remains in solution in the electrolyte when
electrochemically reduced or oxidized. Solution phase electroactive materials
may be contained in the continuous solution phase of a free-standing gel in
accordance with the teachings in U.S. Patent No. 5,928,572, entitled
"IMPROVED ELECTROCHROMIC LAYER AND DEVICES COMPRISING
SAME";
(ii) Surface-Confined--a material attached directly to an electronically
12
CA 02580541 2007-03-23
conducting electrode or confined in close proximity thereto which remains
attached or confined when electrochemically reduced or oxidized; and
(iii) Electrodeposition - a material contained in solution in the conically
-conducting electrolyte which forms a layer on the electronically, conducting
electrode when electrochemically reduced or oxidized.
In addition, the electrochromic medium may also include other materials like
solvents, light absorbers, light stabilizers, thermal stabilizers,
antioxidants, thickeners
or viscosity modifiers and a free standing gel (which includes a polymer
matrix).
The absorption spectra of the electrochromic materials when
electrochemically activated must add together such that the color of the
electrochromic medium can be pre-selected by individually choosing the
concentrations or layer thickness of the electrochromic materials. In a stable
device,
every electron that is removed through oxidation of an anodic material must be
balanced by one electron that is accepted through reduction of a cathodic
material.
Thus in an electrochromic medium containing three or more electroactive
materials,
the total number of anodic species that are oxidized must equal the total
number of
cathodic species that are reduced. This limitation is an important aspect in
ensuring
the ability to make a pre-selected color in accordance with the present
invention. To
illustrate this point, it is well known that one may add blue to yellow to
make green,
however, if an anodic material with a change from colorless to dark blue on
oxidation, and a cathodic material with a change from colorless to light
yellow on
reduction, are added together they will always produce an electrochromic
medium
with the same hue throughout it normal voltage range regardless of the ratios
of the
concentrations of the anodic and cathodic materials. This is because the total
amount
of anodic material oxidized must be equal to the total amount of cathodic
material
reduced. Thus, even if the amount of the cathodic material that turns yellow
on
reduction is doubled or even tripled the color will be the same because for
every
cathodic species that turns yellow, one anodic species will turn blue.
However, in
order for the concentration of both the cathodic electroactive materials and
the anodic
electroactive materials to be current limiting in solution-phase systems, the
total
concentration of one type may be different from the total concentration of the
other
type due to differences in diffusion coefficients in the electrochromic
medium. Often
13
CA 02580541 2007-03-23
the material(s) with smaller diffusion coefficients are present at slightly
higher
concentrations.
In order for an electrochromic medium containing multiple electroactive
anodic and cathodic materials to be able to make a pre-selected color, hand
generally
maintain the pre-selected perceived color during darkening and clearing
transitions
while simultaneously being desirable for commercial applications, the medium
should
be photochemically and thermally stable, and all of the anodic materials
present in the
electrochromic medium should have similar redox potentials to each other and
all of
the cathodic materials present in the electrochromic medium should have
similar
redox potentials to each other.
If the perceived color of the device is to be consistent throughout the
operation
of the electrochromie device (i.e., at various applied voltages and during
coloring and
clearing transitions) the redox potentials of all of the cathodic materials
electrochemically activated during normal operation must be similar to each
other,
preferably within 60 mV of each other, and the redox potentials of all the
anodic
materials electrochemically activated during operation must be similar to each
other,
preferably within 60 mV of each other. More preferably, the redox potentials
of all of
the cathodic materials are within 40 mV of each other and the redox potentials
of all
of the anodic materials are within 40 mV of each other.
Even if the redox potentials of the color-contributing cathodic materials are
not similar to one another, or the redox potentials of the color-contributing
anodic
materials are not similar to each other, a device containing such an
electrochromic
medium may still exhibit a single color due to a combination of all the colors
of the
cathodic materials or all the colors of the anodic materials at an applied
voltage high
enough to reduce all of the cathodic materials arriving at the cathode and
oxidize all of
the anodic materials arriving at the anode. However, at lower applied voltages
or
during coloring transitions or especially during clearing transitions, the
colors due to
the most easily reduced cathodic material, i.e., those with the highest redox
potentials,
and/or the most easily oxidized anodic materials, i.e., those with the lowest
redox
potentials will dominate the perceived color of the electrochromic medium.
This
phenomenon is commonly referred to as staging. If the redox potentials are
similar to
each other (and assuming the kinetics of the electrode reactions are at least
somewhat
similar and that the electrochromic materials have one color which only varies
in
14
CA 02580541 2007-03-23
perceived chroma throughout the voltage range of the device) then the color
due to the
electrochromic medium will be a consistent composite of all of the color
contributing
cathodic and anodic materials throughout the operation of the device at
various
applied voltages and during coloring and clearing transitions. Stated another
way, the
absorption spectra of the individual cathodic materials will add together and
the
absorption spectra of the individual anodic materials will add together, such
that the
resulting absorption spectra of the electrochromic medium will produce a
consistent
perceived color or hue throughout the operation of the device.
Electrochromic devices should preferably be photochemically stable. Devices
used in applications like rearview mirrors, especially on the exterior of
motor
vehicles, must have means that prevent harmful photons from reaching the
electrochromic medium or must have an electrochromic medium that is stable
with
respect to photochemical degradation, at least for sunlight exposure over the
useful
life of the device while the device is in the nominally clear state. For
electrochromic
devices used in applications like motor vehicle or architectural windows or
glazing,
the device must prevent harmful photons from reaching the electrochromic
medium or
must have an electrochromic medium that is stable with respect to
photochemical
degradation both in the nominally clear state and during electrochemical
activation.
For electrochromic devices and media which contain multiple cathodic
electrochromic
materials and/or multiple anodic electrochromic materials, photons harmful to
any one
of the electrochromic materials must be prevented from reaching that material,
or each
material and the medium as a whole must be stable with respect to
photochemical
degradation.
Finally, the electrochromic medium should preferably be thermally stable or
be such that the medium doesn't lose its ability to color or become
permanently
discolored due to thermal degradation. Many electrochromic media proposed in
the
art suffer from lack of thermal stability for one or more electrochromic
materials in
their nominally clear oxidation states or especially in their colored
oxidation states.
Lack of thermal stability results in poor cycle life for the electrochromic
device. In
electrochromic media that contain multiple cathodic and/or multiple anodic
materials,
every electrochromic material must be thermally stable enough in each of its
oxidation
states present in the device, with or without applied voltage, to provide the
device
with adequate thermal stability for its intended use and life, or the thermal
degradation
CA 02580541 2007-03-23
of these materials must not discolor the device or impede the proper operation
of the
device.
As stated above, the electrochromic media of the present invention comprises
at least three electroactive materials having absorption spectra in their
activated state
that add together such that a pre-selected color of the electrochromic medium
can be
made by individually choosing the concentrations, relative concentrations or
layer
thickness of the at least three electroactive materials contained in the
medium. This
pre-selected color may be a wide range of perceived colors, such as red,
orange,
yellow, green, blue, gray, etc.
Tables I through 9 list a number of cathodic electrochromic materials and a
number of anodic electrochromic materials that when dissolved in the proper
solvent
or solvent system, including enough dissolved electrolyte to provide ionic
conductivity to the solution, can be used as solution-phase electrochromic
materials.
The solvents used are generally the polar, aprotic organic solvents taught in
U.S.
Patent 4,902,108. In a number of these solvents, the materials in Tables I
through 9
exhibit two chemically reversible waves in a cyclic voltammogram run at an
inert
electrode at room temperature. The first cyclic voltammogram wave generally is
due
to a one electron per molecule reduction or one electron per molecule
oxidation which
is accompanied by a change from colorless or slightly colored to significantly
colored
(i.e. light absorbing at at least one wavelength in the visible spectrum). The
use of
these materials in electrochromic devices is normally restricted to the
electrochemical
activation of the materials to this one electron reduced state or one electron
oxidized
state. These reduced states for cathodic materials or oxidized states for
anodic
materials have a particular light absorption spectrum that generally follow
Beer's law
throughout their range of concentrations in activated electrochromic devices,
with the
exception of some materials which at higher concentrations of the reduced
state show
complication in the spectrum due to what is believed to be dimerization.
As long as the voltage applied to an electrochromic device containing these
materials is restricted to the normal range in which only the one electron
reduced state
or one electron oxidized state is produced at the electrodes, the materials
will make a
consistent color contribution varying only in the amount of absorption. If the
voltage
is too large, the color or visible light absorption spectrum of the twice
reduced state(s)
and/or twice oxidized state(s) will contribute to the overall spectrum of the
16
CA 02580541 2007-03-23
electrochromic medium and therefore the electrochromic device. Going outside
the
normal voltage range may and often will result in a perceived change in color
of the
medium. For several of the materials in Tables I and 2, the difference in
redox
potential for the first one electron reduction and the second one electron
reduction is
quite small and therefore the normal voltage range for a device containing
these
materials is quite limited. Generally if an electrochromic medium contains
both
anodic and cathodic electrochromic materials from Tables I through 9, then the
normal voltage across the medium is from about 0.3 volts less than the
difference in
redox potentials between the cathodic materials and the anodic materials to
about 0.2
to 0.4 volts more than the difference in these redox potentials.
The redox potentials in Tables 1 through 9 were determined by differential
pulse voltammetry at a platinum working electrode in an argon-purged propylene
carbonate solution containing 0.2 molar tetraethylammonium tetrafluoroborate
with
an internal reference compound of known redox potential. Ultimately, all of
the redox
potentials in Tables I through 9 are given relative to the redox potential of
5,10-
dimethyl-5,10-dihydrophenazine being set to 0.300 volts.
Tables 1 through 4 list four groups of cathodic electrochromic materials which
change from colorless or slightly colored to significantly colored when
electrochemically reduced. The tables also give the redox potentials for the
first one
electron reduction of each material and the wavelengths of maximum absorbance
and
the logarithms of the absorption coefficients at these wavelengths for the one
electron
reduced state of nearly all of the cathodic materials listed. Tables 5 and 6
list two
more groups of cathodic electrochromic materials and the redox potentials for
the first
one electron reduction for each material. The redox potential for
electrochemical
reduction is similar within each table or group.
All of the cathodic materials in Table 1 have their redox potentials between -
0.1 12 volts and -0.132 volts, however the one electron reduced materials have
different absorption spectra with different wavelengths of maximum absorbance,
which results in different perceived colors, when the materials are reduced.
For
example, in Table I materials 1 and 4 appear green in color when reduced and
materials 2 and 5 appear blue in color when reduced. By choosing various
relative
concentrations of, for instance, materials I and 2, the cathodic materials'
contribution
17
CA 02580541 2007-03-23
to the color of the electrochromic medium can range between blue, blue-green
and
green.
All of the cathodic materials in Table 2 have their redox potentials between -
0.192 volts and -0.216 volts. However, the spectral absorbances of the
materials in
their reduced states show that the materials appear different in color from
each other
and they can be combined in various relative concentrations to impart a
particular
color contribution, (different from any of the materials individually), to an
electrochromic medium containing this cathodic material combination.
All of the cathodic materials in Table 3 have their redox potentials between -
0.276 volts and -0.304 volts, however there are differences in their
absorption spectra
that lead to useful combinations of these cathodic materials in electrochromic
devices.
The cathodic materials in Table 4 have redox potentials similar to each other
and are between -0.340 volts and -0.376 volts. While materials 1, 3 and 5 have
at
least somewhat similar spectra and similar blue appearance in their reduced
states,
materials 2 and 4 have significantly different spectra and color appearance.
When
reduced, material 2 appears purple and material 4 appears green. This allows
for
particularly advantageous combinations for materials in Table 4 especially
with regard
to achieving gray color in an electrochromic mirror or window.
The cathodic materials in Table 5 have redox potentials between -0.424 and
-0.436. Although the absorption coefficients have not been measured, the
compounds
have different absorption spectra when electrochemically reduced and can be
combined with each other and/or anodic materials to give useful color
contributions to
the appearance of electrochromic devices.
The cathodic materials in Table 6 have redox potentials between -0.472 and
-0.492. Although the absorption coefficients have not been measured the
compounds
have different absorption spectra when electrochemically reduced and can be
combined with each other and/or anodic materials to give useful color
contributions to
the appearance of electrochromic devices.
18
CA 02580541 2007-03-23
O C
rZ
N ' 00 M V
N 0o O N 00
rt M ' ci M
N_ N t7 N
h h h
O C 00
M M N
CD 00
i- O N O O
to \J ~D
o
c' h -t O M
E v1 to
It CD 00 110 c:)
M O N M O
1~t d' T V
N O -- \0 1.0 -
~4 O C O O O O
cc N
CO
M CL .~
0 1 a
O 0 a O
o o 0 0
O O o
2 O O
cz
C C
X ca
u V X
i i a? n
o ..o .~ E .o
O E E o E
o
0 o G 'D . C
C o G s -
N O~ p b a
~ v ~r ~- v rr
v I - -~ v
v c c ,- a~
o. a a
7, 7,
v E E
.jr
c 140 't
N en ' N C
_a vii co i CO
o ' .o U
M lr1 V
19
CA 02580541 2007-03-23
^ ^ ^ ^ ^
vl v~ N ~' v1
ri ri ri ri ri
N 00 O N
N N N N
00 N N
00 rq
u^ O O O O
u Vr 4"1 to 'V'
N M v'1 V'1
O k M d d d
O ~r 00 00 00
N C O
N N N N
~ O O O O O
cc
c
N Q. 2 cn
cc
m
o
0
Q L 0 S O
o o_ ry
0 cc
cc a, o o x
0
CO cn
u
E
ate. ~ ~ ~ ~
'O
7~ ~T GL
x s v
x
I
CA 02580541 2007-03-23
O
M M
N 00
M M
^ ^ ^ ^
W G1 N
cti ~
a ,~ o M CD
_O O oo
O C\ rn
T7' 1 M
,o N V
N N C\ C\
Cl N N M
r O O O O
o
0 o 0
-~ L o
0 0
o
C ^
r ` = [ ~r
- L >l
~t ^ C
> C Lam`., 'C
C y) 0 ~
0 C. Lp
Q =L7 -
0 V'
aC) .C G .C
G 0 M ~
N --.
-- N M V
21
CA 02580541 2007-03-23
M M
-Y 00 In t0
r 1 M M M
00 00 v1
f'1 00 V N
00 N V'1 V1
~Q N 00 N
00 00 O
-
C y
m in 00 't 00
V' J N Vc
C~ Vl C\ M ON
M tI) M 'IT M
N O O
~_ M M M M M
O O O O
cs
a a
M o
is s
o a
er o 0
m o 2 o
o C
s
O cc
`~-. i i
cC O L. u
c3 0 A. v N
O cn
^C 0 c3
cn L-
2 . cu c
7 C_ x
=C = ~' N
4 L C cn - 0
a
_ cc
N
.õ
0 u
n 0
CS. N
`C C N C
C N
~y ~o C -C
,' N ~t N
N f V ~j O
>, 03
N M c1' V=1 *
22
CA 02580541 2007-03-23
N N M M
V O O O
1)
O
N O
W O
q a cj
E-~ o
0
0 E
cc =
C)
'- L
y
C
O GJ ~
N =.
co
O ~ O
O a
y c~ N
N
Ui N
N N
'p N N
^ N M
23
CA 02580541 2007-03-23
N N N ~7 co N
rq r- t- r- 00 00
.-, O O O O O O
C)
C,
N U)
C,
c U O
O p
L-a vii
r.~ O OC c
cr
Q O X
cQ C c3 U)
Q, ca
X -
o
c. n ~ c
o
c3
E O
C V n
X i
C)
C O a S] `~
cC % .C
Q'
O
to
cC CI a1 M
>, ..; C E
X 4) c3 a)
C 2 C. LL
N N N
'n 'C ~ N N N
N M V U1
24
CA 02580541 2007-03-23
Tables 7 through 9 list groups of anodic materials that are colorless or
slightly
colored which change to significantly colored when electrochemically oxidized.
The
tables also give the redox potentials for the first one electron oxidation of
each
material and the wavelengths of maximum absorbance and the logarithms 9f the
absorption coefficients at these wavelengths for the one electron oxidized
state of the
anodic materials listed.
All of the anodic materials in Table 7 have their redox potentials between
0.256 volts and 0.264 volts, however the one electron oxidized materials all
have
different absorption spectra. The oxidized materials appear blue, brown,
purple or
green and can be combined in selected relative concentrations in
electrochromic
devices to impart any of a number of particular or predetermined color
contributions.
All of the anodic materials in Table 8 have their redox potentials between
0.290 volts and 0.308 volts. The wavelength of maximum absorbance for the main
absorbance peak of the oxidized state of these materials varies from 460
nanometers
to 532 nanometers. Numerous useful combinations of these materials at selected
relative concentrations can be used in electrochromic devices to achieve a
particular
color appearance contribution.
Finally, all of the anodic materials in Table 9 have similar redox potential
to
each other and are between 0.344 volts and 0.352 volts. Even though the redox
potentials are similar the color appearance and absorption spectra are
different and
combinations at selected relative concentrations are useful for imparting a
particular
color appearance to electrochromic devices.
CA 02580541 2007-03-23
O r-
ri r
M N
N
M M
~O O
V' N 00
^ O M
~~ M M M
v W `~ `7 0
cc c
o ^ o o0
O (V
N N
G:: O O O O
N
a)
N Q)
Q)
a)
.3 => C
a) V Q
y "0 G. ON
C O O a~i
a) j, .fl
4, aci O ~'
' vl 1
y O
M >
_
E
L N
_1 O
Z L 1
'0
a) _O
N M
26
CA 02580541 2007-03-23
00
Vr I~ O O
c'1 (V N N
M M M M M M M
rV O - c1' 00 'ct ~t
-- ^ N ^ ^
c ^ O N O C\
UJ M N OM to N M
M M r l)* M M M M M
N ~t O N 00 \0 N O \O
yt 00 to 00 to O t~ O r- c-
c~
U~ = rn ~ c ~ v M v o
-~ rn o o, o -- ~ o
V Vr N N O N N ~' ~0
00 V' M M co p\
tI'1 V1 t^ '~ to to 'IT ~-
N O N N N O O O O 00
cl\ C\ C\ CN CD
N N N N OM O O O
W O O O O O O O O O
00
W o
W C
CC1
C
O
Q) a)
O C -
N N O C
O _ O
C N N
N O m cC
CL CO C ) C N
o a~ = -C N ca
O 0
C ..OC
0 .C - - - O
l O T T .C CL
_ ^ L
cC
'b vj N C O O >, C
N 'L7 = to
O C O 'a O
~, N C O O E
.. t
0 O A C T j, O
C C C '
0 E ^) Vi >
ca. E
~ LZ. l~ M ~. M ~ r= L1.
N N N N V') N N N N
N V V~ t- 00 G\
27
CA 02580541 2007-03-23
00 C:)
N N
t-
M 00
N p
`M.. rt
cv^ N 00
00
N
^ ^ ^ ^ ^
C4 V)
G1 O C O O
U o \0 '0 N \0
00 M C\ co
M
V1 ct
Tt
V
00 00 N
N - I' ct N
.-~ M M M M M
V O O O O O
N
ca
C
L, N
0 C
N
N C cc
N 4)
cz
a) o a
E
CL Q 0
'p C -
to
tn - N -
L1 .^.
.C `
0 N E
cn E
- O O
z p p vi V)
u vi ri ri
u W) kn Cj N
C - N M N
28
CA 02580541 2007-03-23
In accordance with an important aspect of the present invention, Table
shows the results of combining various concentrations of a number of the
materials
from Tables 1 through 9 in the electrochromic medium of an electrochrromic
device
and how the concentrations of at least three electroactive materials may be
chosen to
produce a device having a pre-selected perceived color. Because the anodic
materials
and the cathodic materials themselves are chosen such that they have similar
redox
potentials, the electrochromic medium maintains the predetermined perceived
color in
its electrochemically activated states throughout its normal range of
voltages.
29
CA 02580541 2007-03-23
O \7 O [- v1 10 ON M M
-- r a\ N r- \
00 N 00 00 -6 N N
u
a u
O L
(N N C14 C\ W) ~o 00 CD O 00 O N M 00 0O O 00
~t N M
N L N V O V1 M - 00 00
,.. Q N M i N N N N Q\
is
oo N %Z p O 0% O
L it l- %LJ 00 ~.,~
C h. M N (~ 'r
0
O O
oo M O
U 0
O
M 00 C\ C r C .... - v1
4c Q\ 00 ~ M M M M O~ (~ C' ~t tt M in
N O v1 O M 4 'el N M N r.: 6
.O
M M M M M M M M M M !t M er
C
'O
V1 .-. 'O
.i N v
a
Z r-.
`t T
O cl p
R
t0 O M
U O C n v1 T
,.. U N M i a
O u
C L. O
O M M
e
V U MM M M M M OM ,
U
~o 00
p o U
N
f0 C
N
ca
a r
6~ L t.. 0
O b
iG M 00 M M
u .~ Q M kn , C C
C
C v ,y
s... N p V'1 V1 v1 N'1 O , O , , i , i ~ _T
O a N 00 00
E
A C)
L. Q O - - - - kn CD N co h (N N ' ' N O~ 4 O
N
Q u v
p C
U "" N M 'cf ,n ,O r-~ 00 c~ O N M t7 v
CA 02580541 2007-03-23
~. o
~ o
o
O
O R
Ca o
o a)
_n n
a) 0
- p
E 2 C C
'fl _n 73
T 't7 t
.C r
Q T CG' N
a
a) N
E
C J Y v
C M 2
'D C n
N V
N ~ 7 N
N y
G C) N
T E E
7 7
II II II II
N M 'If vl
u U U U
a)
a)
C y N
N N C
s in.
-p N O T
O T ca -0
p q p ..
t7 - O
T .5 L
a T
s E -
u 'p vi 0. O
v -- r 31
E O
O
C G T O.
4. r'1 N to
N N vl N N
II II II It II
N M T vl %0
~ Q Q
31
CA 02580541 2009-07-22
The results of Table 10 are shown in terms of the L*a*b* color coordinates of
transmitted light when the electrochromic window devices were in their full
colored
state. This is the state in which L* is a minimum, the chroma is a maximum and
a*
and b* are furthest from the a* = 0 and b* = 0 origin (for the normal
operation of the
device). The electrochromic devices were fabricated using parallel, planar,
spaced
apart sheets of glass coated on the surfaces facing each other with fluorine-
doped tin
TM
oxide, (TEC 15 coated glass available from Libbey-Owens-Ford of Toledo, Ohio).
The spacing between the fluorine-doped tin oxide layers (cell spacing), was
137
microns. At least one electrochromic window device was filled with a propylene
carbonate solution containing each of the various millimolar (mM)
concentrations and
combinations of anodic material(s) and cathodic material(s) for each row of
Table 10.
The visible spectrum of the device in its clear state with no voltage applied
was
subtracted from the full colored state, normally with 0.6 to 1.0 volts
applied. This
difference spectra was converted to the color coordinates (Standard Illuminant
A/2-
degree), shown on the right hand side of the table by a standard method known
in the
art. Also shown is Y, the measure of brightness.
Referring specifically to Row 7, an electrochromic medium comprising an
anodic and a cathodic electrochromic material is shown whose relative
concentration
is found in commercially available elcctrochromic mirrors. In an
electrochromic
window with this electrochromic medium in the full colored state, the color
coordinates show a large negative a* or green appearance and a somewhat
smaller
negative b* or some blue appearance and the fully colored window appears green-
blue-green. Also of particular note are the electrochromic window devices with
the
concentrations/combinations given in rows 3 and 4 which have very low absolute
a*
and b* values and which appear nearly perfectly gray and the devices of rows
12 and
14 which also have relatively small values for a* and b* and give near neutral
gray
appearance with various applied voltages and transmission levels including the
lowest
transmission level or full colored state. In Table 10, all of the anodic
materials
combined in one device have redox potentials similar to each other and all of
the
cathodic materials combined in one device have redox potentials similar to
each other.
Therefore the devices have the same perceived color throughout their
coloration or
darkening range, which is to say the devices lack staging of colors both
during
coloration and clearing.
32
CA 02580541 2007-03-23
It should be understood that with the data in Tables 1 througn 10, not only
combinations of various anodic electrochromic materials and cathodic
electrochromic
materials can be chosen, but various relative concentrations of each anodic
material
and cathodic material can be chosen. All such combinations of electrochromic
materials that when combined give a gray device should be understood to be
within
the scope of the present invention.
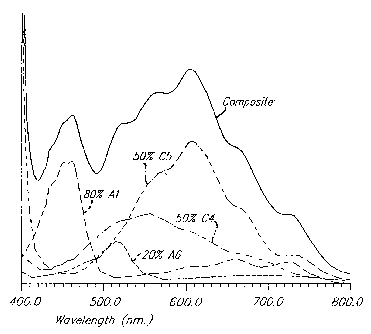
Figure 2 illustrates a method by which a predetermined color for an
electrochromic medium can be chosen. The visible light absorption spectrum for
the
colored state or in this case the cation radical of each of the compounds
listed below
for Figure 2 was determined. Each nominal spectrum was determined for the same
path length and concentration for the colored state of each material and was
scaled as
described below. As described earlier, in a stable electrochromic device the
number
of electrons added to the electrochromic medium equals the number of electrons
removed during electrochemical activation and, (as is this case for these
materials
listed below for Figure 2), if electrochemical activation involves one
electron
reduction for each cathodic compound and one electron oxidation for each
anodic
compound the total number or effective concentration of activated cathodic
species
will equal the total number or effective concentration of activated anodic
species.
Thus the percentages of the spectra for the cathodic species and the
percentages of the spectra for the anodic species will each add up to 100%.
Curve Al
shows 80% of the nominal spectrum of the cation radical of 5,1 0-dimethyl-5,
10-
dihydrophenazine, Curve A6 shows 20% of the nominal spectrum of the cation
radical of 2,7-diphenoxy-5,10-dimethyl-5,10-dihydrophenazine, Curve C4 shows
50%
of the nominal spectrum of the cation radical of 1,1'-dimethyl-4,4'-(1,3,5-
triazine-2,4-
diyl)dipyridinium diperchlorate, and Curve C5 shows 50% of the nominal
spectrum of
the cation radical of 1,1'-dimethyl-2-(3-phenyl(n-propyl))-4,4'-dipyridinium
bis(hexafluorophosphate). These scaled spectra were added together to give the
composite spectrum that would be essentially the same as that observed in an
electrochemically activated electrochromic medium containing these relative
concentrations of these electrochromic compounds.
The absorbances in Figure 2 are shown on a relative scale as the absorbance of
the electrochromic medium, once activated, will have the same shape (or
relative peak
heights and peak positions), shown but will increase as a whole as the voltage
is
33
CA 02580541 2007-03-23
increased. As described above, the absorbance spectrum shape will remain the
same
throughout the normal voltage range of the electrochromic medium which is
generally
from about 0.3 volts less than the difference in redox potentials between the
anodic
materials and the cathodic materials to about 0.2 to 0.4 volts more than the
difference
in these redox potentials. In this case, the normal operating voltage range
across the
medium for the materials in Figure 2 would be from about 0.35 volts to about
0.95
volts since the anodic materials have redox potentials around +0.300 volts and
the
cathodic materials have redox potentials around -0.350 volts for a difference
of 0.650
volts. Throughout this voltage range and different levels of darkening, an
electrochromic window containing an electrochromic medium comprised of the
electrochromic materials in Figure 2 in the given relative concentration
ratios will
maintain a constant blue-gray appearance. The device could be said to maintain
nearly constant hue as its magnitude of chroma is increased.
Figure 3 shows the scaled spectra of: the cation radical of 5,10-dimethyl-5,10-
dihydrophenazine in Curve A; the cation radical of 2,3-diphenyl-5,10-dimethyl-
5,10-
dihydrophenazine in Curve B; a tungsten trioxide film which has been
electrochemically reduced in the presence of lithium ion to form LixWO3 in
Curve C.
The sum or composite spectrum for the scaled spectra of these three
electrochromic
materials is shown in Curve D. An electrochromic device containing this
electrochromic medium has a surface confined W03 layer on one electrode
(either the
second or third surface) and a solution of propylene carbonate containing the
two
anodic materials and a lithium salt (e.g., LiC1Oõ to provide ionic
conductivity and a
lithium ion source), in contact with the other electrode and the W03 layer.
The
spectra are scaled such that 60% of the anodic material to be
electrochemically
activated is 5,10-dimethyl-5,10-dihydrophenazine and 40% is 2,3-diphenyl-5,10-
dimethyl-5,10-dihydrophenazine, and the tungsten trioxide film thickness is
chosen to
allow the absorbance in its reduced state to have the spectral contribution
relative to
the anodic materials shown in Figure 2.
The electrochromic device is still self-erasing, like an all solution-phase
device, since the oxidized anodic materials can diffuse to the reduced
tungsten
trioxide film and spontaneously exchange electrons to oxidize the reduced film
and
reduce the oxidized anodic materials. Thus the fully colored device would
spontaneously return to its clear condition even at open circuit.
34
CA 02580541 2007-03-23
The relative and total concentrations of these anodic materials which have
similar redox potentials and the thickness of tungsten trioxide layer can be
chosen to
give a gray appearing electrochromic device as is illustrated by the spectrum
in Curve
D. For this composite spectrum scaled to an L* value of 53.72, a moderate
amount of
coloration, the following color coordinates are obtained: a* = 2.18 and the b*
= -3.24
(for D65/2 degree). This is a remarkable achievement for tungsten trioxide
based
devices which usually suffer from being pure blue in appearance at moderate
coloration when the electrochromic medium includes tungsten trioxide as, an
electrochromic material.
Likewise, the anodic material can be in the form of a surface-confined layer,
such as a metal oxide (including M,VZOS, NiOXHy, MXCeO,, MXNb,OS, IrO, along
with Ce/Ti, Zr/Ce, and W/Ce mixed oxides). An electrochromic device containing
this electrochromic medium has the surface-confined layer on the second or
third
surface and a solution of the two or more cathodic materials, e.g., viologens,
in a
suitable solvent. The solution also contains a soluble ionic material
(typically a
lithium salt) in order to support ionic conductivity and to provide an ion
source for
intercalation of the surface-confined layer. The relative and total
concentrations of the
cathodic materials and the thickness of the surface-confined anodic layer can
be
chosen to give a pre-selected perceived color, including gray.
For an electrochromic medium containing an electrodeposition type
electrochromic material which is cathodic, two or more solution-phase anodic
materials of similar redox potential can be combined in the medium in relative
concentrations to produce a pre-selected perceived color appearance, including
gray.
The pre-selected relative concentrations of the anodic materials can be chosen
based
on the absorption spectra of the electrodeposited film, those of the anodic
materials
and the rate of the self-erasing reaction. For an anodic electrodeposition
type
electrochromic material, two or more cathodic materials with similar redox
potentials
can be combined in the electrochromic medium as described above to produce a
pre-
selected perceived color appearance, including gray.
In general, the absorption spectra of the electrochemically activated states
of
electrochromic materials can be scaled and summed in the fashion discussed
above to
choose materials and relative concentrations that will give an electrochromic
medium
with a particular (and pre-selected) perceived color throughout their normal
operating
CA 02580541 2007-03-23
voltage ranges. While the invention has been illustrated using several types
of
electroactive and electrochromic materials, being able to pre-select the
perceived color
is broad and applicable to electrochromic media comprised of organic,
inorganic,
organometallic, and polymeric materials, which may be solution-phase,
electrodeposition and surface confined electroactive and electrochromic
materials, as
well as combinations thereof.
In certain applications, such as architectural windows and motor vehicle
mirrors, the pre-selected color of the electrochromic medium may be one that
is
perceived as gray. In the broadest terms, a color that is perceived as gray is
an
achromatic color of lightness between black and white and, although achromatic
is
defined as a color perceived to have zero saturation and therefore no hue, it
should be
construed broader in the context of the present invention to mean a color
perceived to
have a little or moderate amount of chroma. Although the meaning of chroma
will be
understood to those skilled in the art, it may be helpful to refer to the
L*a*b* chart.
As stated above, on the L*a*b* chart, L* defines lightness, a* denotes the
red/green
value and b* denotes the yellow/blue value. According to the present invention
and
further described in the following paragraphs, a little or moderate amount of
saturation is defined as a color around (and including) a* = 0 and b* = 0 that
is
perceived as gray when viewed by human eyesight under particular conditions.
In the
narrowest sense, the gray color can be defined by a circle around a* = 0 and
b* = 0
having a radius C* where C* _ (a*z + b*Z)'a .
Figure 4 shows excursions in a*b* color coordinate space (A/2 degree) for a
number of electrochromic mirrors suitable for use as rearview mirrors in motor
vehicles. The excursions in color coordinate space for windows are generally
very
helpful for choosing electrochromic media for use in mirrors and visa versa,
however,
in contrast to the curves in Figure 5 (discussed below) which are nearly
linear, the
curves in Figure 4 have a definite semi-elliptical shape. The reason for this
is
believed to be as follows: the starting color coordinates for a mirror in its
high
reflectance state are determined largely by the color imparted to the light by
two
passes through the glass substrate(s), transparent electrode(s) and the non-
activated
electrochromic medium (each of which may have some slight absorption of light
at
some visible wavelengths), and. the slight non-uniform reflectance (with
respect to
light of visible wavelengths), due to the transparent electrode(s) and the
mirror
36
CA 02580541 2009-07-22
reflector layer(s). Thus electrochromic mirrors often appear slightly yellow
or
yellowish-green in their high reflectance state and the color coordinates for
all of the
mirrors shown in Figure 4 are in the green-yellow (-a*, +b*) quadrant in the
high
reflectance, zero-applied voltage state.
As the mirrors begin to dim by applying a voltage to, and thereby decreasing
the
transmission level of, the electrochromic medium, the color coordinates of the
reflected
light become largely determined by the color or visible light absorption
spectra of the
electrochromic medium. This is shown in Figure 4 by the excursion of the color
coordinates into the green-blue (-a*, -b*) quadrant as the applied voltage is
increased.
As the mirror continues to dim, the amount of light not absorbed by two passes
through
the electrochromic device, (including the electrochromic medium), starts to
become
comparable to the residual and secondary reflections due to the first surface
of the front
glass substrate, the interface between the front glass substrate and the
transparent
electrode layer and the interface between the transparent electrode layer and
the
electrochromic medium.
In general, these residual and secondary reflections are relatively colorless
if the
transparent electrode layer(s) provide for color suppression of the
transparent electrode
TM
structure, (as is the case for TEC 15 glass available from LOF of Toledo,
Ohio, or the
color neutral coatings disclosed in commonly assigned co-filed U.S. Patent No.
5,940,201
entitled "AN ELECTROCHROMIC MIRROR WITH TWO THIN GLASS ELEMENTS
AND A GELLED ELECTROCHROMIC MEDIUM. Therefore in this reflectance region
the color coordinates of the reflected light start to become less dominated by
the color or
visible light absorption spectra of the electrochromic medium and start to
become
dominated by the relatively colorless residual and secondary reflections and
the curves in
Figure 4 start to "turn around". As the reflectance continues to decrease, the
color
coordinates of the reflected light become largely dominated by the color of
the residual
and secondary reflections and often head toward relatively small absolute
values of a*
and b*. Thus, at the highest applied voltage or lowest reflectance levels, the
color
coordinates for the mirrors in Figure 4 (with the exception of Curve E) are
still in the
green-blue quadrant but are closer to the a*, b* equal 0, 0 than at
intermediate reflectance
levels.
37
CA 02580541 2009-07-22
The desirability of an electrochromic mirror for use as a motor vehicle
rearview
mirror, with regard to color, depends on the perceived color of the clear,
high reflectance
state; the perceived color of lowest reflectance state (often determined
mostly by residual
and secondary reflections); and the perceived color of the intermediate
reflectance states.
As stated earlier, commercial electrochromic rearview mirrors typically have a
slightly yellowish or yellowish-green tint in their high reflectance state
with L* typically
90 5, a* typically of -4 3 and b* typically 5 3. Most desirable for many
people
would be an L* as high as possible and a* and b* each as close to zero as
possible.
The actual electrochromic mirrors which were used to obtain the color
coordinate
curves in Figure 4 as a function of applied voltage are described below. The
mirrors with
tM
color coordinate Curves A, B and D were constructed of two flat sheets of TEC
15 glass
each 2.3 mm thick bonded together with an epoxy seal which provided a 137
micron
TM
spacing with the TEC 15 tin oxide coatings provided on surfaces 2 and 3. The
mirrors
had a fourth surface reflector made up of a conventional silver reflector over-
coated with
TM
copper and paint layers applied to the back surface of the sheet of TEC 15
glass that was
the rear glass element. The mirror with color coordinate Curve F was a large
outside
rearview mirror (about 12 centimeters high and 20 centimeters wide) which had
front and
rear glass elements that were 1.1 mm thick sheets of glass bonded together
with an epoxy
seal which provided a 180 micron spacing between surfaces 2 and 3. On surface
2 was a
color suppressed transparent electrode structure made up of about 300 A of
ITO, about
300 A of silicon dioxide, followed by about 1500 A of ITO and the coated glass
element
was essentially colorless when viewed both in transmission and reflection. On
Surface 3
was a reflector electrode structure made up of a first layer of chromium
metal, an
intermediate layer of rhodium metal and a top layer of silver-gold alloy which
contained
85% silver and 15% gold by weight. This reflector was essentially achromatic
in
appearance. In addition to the electrochromic materials described below, the
electrochromic medium of the mirror of Curve F also contained a polymer
matrix, which
with the electrochromic solution, formed a free-standing gel. The free-
standing gel
electrochromic medium was prepared according to the teachings of commonly
assigned
co-pending U.S. Patent 5,928,572 entitled, "IMPROVED ELECTROCHROMIC LAYER
AND DEVICES COMPRISING SAME" to W. L. Tonar, et al. This mirror had a high
38
CA 02580541 2007-03-23
end reflectance for CIE curve white light of 85%, a low end reflectance of 7%
and an
achromatic, "silver", or gray appearance at high, low and all intermediate
reflectance
levels.
Curve A shows the color coordinates (A/2 degree) for various reflectances
states
of an electrochromic mirror having an electrochromic medium comprising: 30 mM
1,1'-bis(3-phenyl(n-propyl))-4,4'-dipyridinium bis(tetrafluoroborate); 20 mM
5,10-dimethyl-5,10-dihydrophenazine; and 4 mM 2,3-diphenyl -5,10-dimethyl-5,10-
dihydrophenazine. Curve A has a maximum C* of 21.56 and a maximum a* of -
17.24.
Curve B shows the color coordinates (A/2 degree) for various reflectances
states of an
electrochromic mirror having an electrochromic medium comprising: 30 mM
1.1'-bis(3-phenyl(n-propyl))-4,4'-bipyridinium bis(tetrafluoroborate); 18 mM
5,10-dimethyl-5,10-dihydrophenazine; and 7.2 mM 2,3 -diphenyl-5,1 0-dimethyl-
5, 10-
dihydrophenazine. Curve B has a maximum C* of 20.24 and a maximum a* of -
13.15.
Curves C, E and D show color coordinates (A/2 degree) for the various
reflectance states of electrochromic mirrors commercially available in Europe,
the United
States, and throughout the world, respectively. Curve C has a maximum C* of
28.63 and
a maximum a* of -15.77, Curve D has a maximum C* of 23.53 and a maximum a* of
-20.48, and Curve E has a maximum C* of 31.13 and a maximum a* of -16.84. The
mirrors represented by Curves A and B (4 mM and 7.2 mM 2,3-diphenyl-5,10-
dimethyl-5,10-dihydrophenazine, respectively), when viewed at night in a motor
vehicle
have a neutral gray appearance, while the devices shown in Curves C and E have
blue
appearances, and the device shown in Curve D has a green or green-blue
appearance.
This seemingly small change in C* (the difference between 21.56 and 23.53 or
28.44)
represents a significant change in the perceived color of the device. Curve F
shows the
color coordinates (A/2 degree) for the various reflectances states of an
electrochromic
mirror having an electrochromic medium comprising: 12 mM 1,1'-dimethyl-2-
(3-phenyl(n-propyl))-4,4'-dipyridinium bis(hexafluorophosphate); 12 mM 1,1'-
dimethyl
4,4'-(1,3,5-triazine-2,4-diyl)dipyridinium diperchlorate; 16 mM 5,10-
39
CA 02580541 2007-03-23
dimethyI-5,10-dihydrophenazine (DMP); and 4 mM 2,7-diphenoxy-5,10-dimethyl-
5,10-dihydrophenazine. Curve F has a maximum C* of 13.51 and a maximum a* of -
7.48, and when viewed at night in a motor vehicle gives a neutral gray
appearance.
Thus, the difference in the perceived color of mirrors having a C* value bf
21.56 and
23.53 (a 8% change) is significant, whereas the difference in the perceived
color of
mirrors having a C* value of 13.51 and 21.56 (a 37% change) are both perceived
as
gray. It seems clear that, in the narrowest sense, a color is perceived as
gray for
reflected headlamps when viewed during night driving in a motor vehicle when
its
color coordinates (A/2-degree) have a maximum C* value below about 22,
especially
if the a* value is between -18 and zero.
Although it is not certain, it is generally believed, that for electrochromic
rearview mirrors for motor vehicles which are dimmed at night, when the
driver's eyes
are at least partially dark adapted, and which mirrors generally have their
perceived
color in the dimmed state determined by the color of reflected headlamp light,
that if
there is some perceived color, the most preferred or acceptable colors,
whether for
physiological or psychological reasons or not, are in the green-blue quadrant
of the a*,
b* color coordinate space. In fact, for mirror acceptance as relatively gray,
there tends
to be slightly more tolerance for excursions in the -b* or the blue direction
than in the
-a* or the green direction as long as the C* value stays below a maximum value
of
about 22. This is borne out in Figure 3 in that a mirror like that of Curve D
is
perceived as being somewhat green in the intermediate reflectance states,
(near
maximum C* values) during moderate glare night driving conditions. Mirrors
which
have color coordinate excursions during reflectance changes like Curves A and
B are
perceived as being much closer to gray, even at intermediate reflectance
levels, while
mirrors with Curves C and E are definitely perceived as blue in their
intermediate
reflectance states.
It has been determined that, for motor vehicle drivers at night, the
perception
will be that the mirror is essentially gray throughout its reflectance range
if it has a
maximum C* value of less than about 22, especially if the a* value between -18
and
zero. It has also been determined that Curve A is considered the limit of
acceptability
for a mirror perceived as gray in its intermediate reflectance states and
mirrors of
Curves B and F are considered to be essentially neutral or gray throughout
their entire
reflectance range.
CA 02580541 2007-03-23
Almost all commercial electrochromic rearview mirrors have most of their
color coordinate excursion in the green-blue quadrant. This may not be a total
coincidence since mirrors that have color coordinate excursions into the +a*
(red) and
-b* (blue) quadrant during their reflectance changes can appear purple which
gives an
eerie feeling to drivers using these mirrors during glare conditions at night.
Mirrors
that have color coordinate excursions into the -a* (green) and +b* (yellow)
quadrant
are considered undesirable by drivers and have difficulty being low enough in
reflectance to relieve strong glare. This is for the same reason that a dark
yellow
window still has significant light transmission. There is some thought that
mirrors
with a color coordinate excursion into the +a* (red) and +b* (yellow) quadrant
(especially with +a* values larger than the +b* values), would be desirable
for some
drivers who like red or orange display lighting in a motor vehicle but in
general
mirrors with this type of color coordinate excursion are controversial.
Therefore the -
a* (green) and -b* (blue) quadrant is preferred for the color coordinates of
rearview
mirrors in their intermediate reflectance states, especially if C* and a* are
limited as
described above.
Figure 5 shows color coordinate excursions (D65/2 degree) for four
electrochromic windows in Curves A through D (each made with TEC-15 glass with
a
cell spacing of 137 microns), and Curve E shows the color coordinate
excursions
(D65/2 degree) for the composite spectrum of Figure 2 multiplied by various
factors to
simulate various values of L* or levels of transmission. For each of the
experimental
electrochromic windows, the spectrum of the window at 0.0 volts is subtracted
from
the spectrum at each applied voltage so that the color coordinates are
calculated
essentially for the electrochromic medium alone.
Curve A is for an electrochromic window containing a propylene carbonate
solution of 28 mM 5,10-dimethyl-5,10-dihydrophenazine and 34 mM 1,1'-bis(3-
phenyl(n-propyl))-dipyridinium bis(tetrafluoroborate). As the voltage applied
to the
window is increased from 0.0 volts to 1Øvolts the color coordinates for
light
transmitted by the medium change from a L*, a*, b* of 100, 0, 0 to a fairly
green
slight blue appearance at a L*, a*, b* of 40.14, -36.47, -5.87. Simply using
straight
lines to connect the data points at various voltages results in a relatively
straight line
overall, and for this electrochromic medium containing only two materials the
color or
41
CA 02580541 2007-03-23
hue remains consistent throughout the normal voltage and transmission range of
the
device.
Curve B shows color coordinate data for an electrochromic medium for which
it was desired to make a window with a bright green appearance. The window was
filled with a propylene carbonate solution of 25 mM 5,10-dimethyl-5,10-
dihydrophenazine, 10 mM 1,1'-dibenzyl-2,2',6,6'-tetramethyl-4,4'-dipyridinium
bis(tetrafluoroborate) and 20 mM 1,1'-ethylene-4,4'-dimethyl-2,2'-dipyridinium
bis(hexafluorophosphate). As can be seen from Curve B this electrochromic
medium
changes from a L*, a*, b* equal 100, 0, 0 or colorless at 0.0 volts to a L*,
a*, b* equal
64.12, -40.58, 35.17 at 1.0 volts. Because the two cathodic materials have
similar
redox potential, even though they have significantly different absorption
spectra, the
medium has the same apparent bright green color or consistent hue throughout
its
normal voltage and transmission range.
Curve C is for an electrochromic window filled with a propylene carbonate
solution of 20 mM 5,10-dimethyl-5,10-dihydrophenazine, 4 mM 2,3-diphenyl-5,10-
dimethyl-5,10-dihydrophenazine and 30 mM 1,1'-dimethyl-4,4'-(1,3,5-triazine-
2,4-
diyl)-dipyridinium bis(tetrafluoroborate). This electrochromic medium had a
consistent hue with a perceived red/brown color throughout its normal voltage
and
transmission range which took the color coordinates for the medium from L*,
a*, b*
equal 100, 0, 0 at 0.0 volts to L*, a*, b* equal 53.70, 9.44, 9.70 at 1.0
volts.
Curve D shows what happens if the relative concentration of the anodic
materials in Curve C are reversed. The window for Curve D was filled with a
propylene carbonate solution of 4 mM 5,10-dimethyl-5,10-dihydrophenazine, 20
mM
2,3-diphenyl-5,10-dimethyl-5,10-dihydrophenazine and 30 mM 1,1'-dimethyl-4,4'-
(1,3,5-tri azine-2,4-diyl)-dipyridinium bis(tetrafluoroborate). This medium,
with its
reversed relative concentrations as compared to the window of Curve C; had a
consistent hue with a perceived red/magenta color throughout its normal
voltage and
transmission ranges which took the color coordinates from L*, a*, b* equal
100, 0, 0
at 0.0 volts to L*, a*, b* equal 43.70, 45.23, -27.19 at 1.0 volts.
Curve E shows color coordinates for the composite spectra of Figure 2
multiplied by various factors that made the L* value calculated for the
various scaled
spectra change through a range of L* values similar to the experimental
devices of
42
CA 02580541 2009-07-22
Curves A through D. At the highest absorbance, the color coordinates L*, a*,
b* were
equal to 26.91, -3.62,
-16.2. This medium has relatively small absolute values of a* and b* even
though the
value of L* is quite low. This small excursion in a*, b* for a large change,in
L* is
indicative of a relatively gray medium. An experimental window with an
electrochromic medium containing the electrochromic materials in the same
relative
concentrations shows a color coordinate excursion that is in excellent
agreement with
the excursion of the theoretical or calculated medium of Figure 2 and the
experimental
device, as expected from the teachings of this invention, had a gray with
slight blue-
gray appearance.
For color coordinate curves like those in Figure 5, it is interesting to note
that
a window containing the same electrochromic medium as a mirror will typically
have
a larger color coordinate excursion since the residual reflections that come
into play in
mirrors are not a significant factor in the apparent color of transmitted
light for
windows in their activated states. However, the color coordinate excursion for
windows are certainly valuable in designing electrochromic media for mirrors
and
vice versa. A general observation is that the grayer a window appears when
colored
the smaller its color coordinate excursion from a*, b* equal 0,0 for
coloration to a
given L* value. The curves in Figure 5 are nearly straight lines but do show
some
curvature. This is not unexpected as the Munsell loci of constant hue as a
function of
increasing chroma do show some curvature, see for example the figure of Page
63 and
the associated discussion in F. W. Billmeyer and M. Saltzman in Principles of
Color
Technology, 2"d Edition, J. Wiley and Sons Inc. (1981).
While some combinations of electrochromic materials maintain fairly
consistent perceived color or hue even when the redox potentials are not
similar, many
do not. Figure 6 shows the color coordinate curves (D65/2-degree) for three
windows
that show various amounts of staging. Curve A of Figure 6 is for an
electrochromic
medium in an electrochromic window filled with a propylene carbonate solution
of
30 mM 5,10-dimethyl-5,10-dihydrophenazine, 15 mM 1,1'-bis(3-phenyl(n-propyl))-
dipyridinium bis(tetrafluoroborate) and 15 mM 1,1'-ethylene-2,2'-dipyridinium
bis(hexafluorophosphate). This later compound has a redox potential of -0.252
on the
redox potential scale of the compounds of Table I through 9. Curve A starts at
a*, b*
equal 0, 0 at 0.0 volts and at higher voltages shows more curvature as
compared to the
curves in Figure 5. For the window of Curve A, there is very little perceived
change
43
CA 02580541 2007-03-23
in hue or color appearance as a function of voltage. This is because the
difference in
redox potential between the two cathodic materials is 44 millivolts so they
are still
similar within the definition of this invention.
The electrochromic medium for which the data of Curve B was measured was
contained in an electrochromic window filled with a propylene carbonate
solution of 8
mM 5-ethyl- l 0-methyl-5,10-dihydrophenazine, 20 mM 5, 1 0-dimethyl-5, 10-
dihydrodibenzo(A,C)phenazine and 34 mM 1,1'-ethylene-4,4'-dimethyl-2,2'-
dipyridinium
bis(hexafluorophosphate). This color coordinate curve shows significant
curvature and
the device shows readily distinguishable perceived colors, going from
greenish/yellow at
low voltage to reddish/brown at high voltages. The difference in redox
potential between
the two anodic materials is 80 millivolts and staging is readily apparent.
Curve C shows data for an electrochromic medium in an electrochromic window
filled with 8 mM N,N,N',N'-tetramethyl p-phenylenediamine, 20 mM 5,10-
diisopropyl
5, 1 0-dihydrophenazine and 34 mM 1, l'-bis(3-phenyl(n-propyl))-dipyridinium
bis(tetrafluoroborate). The redox potentials of the anodic materials differ by
88
millivolts and the color coordinate curve shows significant curvature. The
perceived
color of the device changes only slightly from blue to blue-purple through the
applied
voltage range. The slight variation in perceived color or hue variation may be
due to the
fact that, at the voltages where the absorption spectra changes shape, the
magnitude of
the chroma is already quite high and L* is quite small, thus obscuring the
change in hue.
The electrochromic medium comprises the electrochromic materials, and other
materials like solvents, light absorbers, light stabilizers, thermal
stabilizers, antioxidants,
and a free standing gel (which includes a polymer matrix). The polymer matrix
that may
optionally be used in the present invention is a part of a free-standing gel
that is disclosed
in commonly assigned co-pending U.S. Patent No. 5,928,572, entitled "IMPROVED
ELECTROCHROMIC LAYER AND DEVICES COMPRISING SAME" to W. L. Tonar
et al. For electrochromic mirrors, the free-standing gel cooperatively
interacts with glass
elements 112 and 114 to produce a mirror that acts as one thick unitary member
rather
than two glass elements held together only by a seal member. This allows one
to
construct a rearview mirror with thinner glass in order to decrease the
overall weight of
the mirror while
44
CA 02580541 2007-03-23
maintaining sufficient structural integrity so that the mirror will survive
the extreme
environments common to the automobile environment. For electrochromic windows
(especially larger windows), the polymer matrix cooperatively interacts with
glass
elements 112 and 114 such that the hydrostatic pressure that typically pccurs
from
gravity acting on the electrochromic medium (when the electrochromic medium
includes a solution) is reduced or eliminated.
During operation of an electrochromic mirror in the clear state and having a
third surface reflector, light rays enter through the front glass 112 and pass
through
the transparent conductive layer 116, the electrochromic medium in chamber
122,
before being reflected by the reflector/electrode disposed on the third
surface 114a
(unless the mirror has. a fourth surface reflector) of the mirror 110. Light
in the
reflected rays exit by'the same general path traversed in the reverse
direction. When a
sufficiently high voltage (in come cases of the proper polarity) is applied to
an
electrochromic device, electrochemical reduction takes place by electron
transfer to
the electrochromic medium from one of the electrodes (designated as the
cathode) and
electrochemical oxidation takes place by electron transfer from the
electrochromic
medium to the other electrode (designated as the anode). The electrochemical
reduction and/or the electrochemical oxidation give rise to a change in the
light
absorption properties of the material or materials reduced and/or oxidized. -
Operation,
or activation, of the device generally results in an increase in light
absorption at the
wavelengths of interest (although it is possible for operation of an already
colored
device to result in a decrease in light absorption at the wavelengths of
interest). When
the device is in its dark state or some state between its dark and clear
state, both the
entering rays and the.reflected rays are attenuated in proportion to the
degree to which
the electrochromic medium 124 is light absorbing.
Those skilled in the art will understand that the main difference between an
electrochromic motor vehicle mirror and an electrochromic window or some other
electrochromic device is the inclusion of a reflector for mirrors. By
following the
teachings outlined within the specification an electrochromic device may be
produced
having various pre-selected perceived colors, including gray, whether that
device is a
mirror, window, display, etc.
With respect to motor vehicle mirrors, Figure 7. shows a front elevational
view
schematically illustrating an inside mirror assembly 110 and two outside
rearview
CA 02580541 2007-03-23
mirror assemblies 11 la and 111 b for the driver-side and passenger-side,
respectively,
all of which are adapted to be installed on a motor vehicle in a conventional
manner
and where the mirrors face the rear of the vehicle and can be viewed by the
driver of
the-vehicle to provide a rearward view. Inside mirror assembly 110, and
outside
rearview mirror assemblies 11Ia and I i lb may incorporate light-sensing
electronic
circuitry of the type illustrated and described in the above-referenced
Canadian Patent
No. 1,300,945; U.S. patent No. 5,204,778; or U.S. Patent No. 5,451,822, and
other
circuits capable of sensing glare and ambient light and supplying a drive
voltage to the
electrochromic element. Mirror assemblies 110, 111a and 111 b are essentially
identical in that like numbers identify components of the inside and outside
mirrors.
These components may be slightly different in configuration but function in
substantially the same manner and obtain substantially the same results as
similarly
numbered components. For example, the shape of the front glass element of
inside
mirror 110 is generally longer and narrower than outside mirrors 111 a and 111
b.
There are also some different performance standards placed on inside mirror
110
compared with outside mirrors l l la and 11 lb. For example, inside mirror 110
generally, when fully cleared, should have a reflectance value of about 70
percent to
about 80 percent or higher whereas the outside mirrors often have a
reflectance of
about 50 percent to about 65 percent. Also, in the United States (as supplied
by the
automobile manufacturers), the passenger-side mirror l l lb typically has a
spherically
bent, or convex shape, whereas the driver-side mirror 111 a, and inside mirror
110
presently must be flat. In Europe the driver-side mirror l l la is commonly
flat or
aspheric, whereas the passenger-side mirror 11 lb has a convex shape. In Japan
both
mirrors have a convex shape. The following description is generally applicable
to all
mirror assemblies of the present invention.
The electrical circuit preferably incorporates an ambient light sensor (not
shown) and a glare light sensor 160, the glare light sensor being positioned
either
behind the mirror glass and looking through a section of the mirror with the
reflective
material completely or partially removed, or the glare light sensor can be
positioned
outside the reflective surfaces, e.g., in the bezel 144. Additionally, an area
or areas of
the electrode and reflector, such as 146 or the area aligned with sensor 160,
may be
completely removed, or partially removed in, for example, a dot or line
pattern, to
permit a vacuum fluorescent display, such as a compass, clock, or other
indicia, to
46
CA 02580541 2007-03-23
show through to the driver of the vehicle. Above-referenced co-pending U.S.
Patent
Application entitled "AN INFORMATION DISPLAY AREA ON
ELECTROCHROMIC MIRRORS HAVING A THIRD SURFACE REFLECTOR"
shows a presently preferred line pattern. The present invention is also
applicable to a
mirror which uses only one video chip light sensor to measure both glare and
ambient
light and which is further capable of determining the direction of glare. An
automatic
mirror on the inside of a vehicle, constructed according to this invention,
can also
control one or both outside mirrors as slaves in an automatic mirror system
Rearview mirrors embodying the present invention preferably include a bezel
144, which extends around the entire periphery of each individual assembly
110, 111 a
and/or 11 lb. The bezel 144 conceals and protects the spring clips (not shown)
and the
peripheral edge portions of sealing member and both the front and rear glass
elements
(described below). A wide variety of bezel designs are well known in the art,
such as,
for example the bezel taught and claimed in above-referenced U.S. Patent No.
5,448,397. There are also a wide variety of housings well known in the art for
attaching the mirror assembly 110 to the inside front windshield of an
automobile, or
for attaching the mirror assemblies l 11 a and l l lb to the outside of an
automobile. A
preferred housing for attaching an inside assembly is disclosed in above-
referenced
U.S. Patent No. 5,337,948.
The materials that are described in Examples 1-29 are believed to be novel
chemical substances except for the chemical substance of Examples 10 and 25.
Certain properties of some of these materials are shown in Tables 1-9. These
materials can be used as redox materials in such applications as redox
batteries, redox
indicators and mediated electron transfer in electro-organic synthesis.
Because they
significantly change their absorption spectra for visible light upon
electrochemical
reduction or electrochemical oxidation, they are also useful in electrochromic
media
for use in electrochromic windows, displays, mirrors, etc. In particular,
these
materials have colored state absorption spectra and redox potentials such that
they can
be placed in groups of materials with similar redox potentials. By selecting
two or
more materials with different colored state absorption spectra from a group
with
similar redox potentials, and by choosing relative concentrations of the two
or more
materials, one can design an electrochromic medium that has a pre-selected
perceived
color when incorporated in an electrochromic device and operated throughout
the
47
CA 02580541 2007-03-23
normal voltage range or transmission range of the device. These materials are
also
particularly useful in designing electrochromic media that result in
electrochromic
devices that have a perceived color of gray throughout their-normal ranges of
operation.
A number of the phenazine compounds listed in Tables 7 through 9 with the
following general structure:
R9 Rio Rt
Rg / N R2
R7 N R3
R6 R5 R4
[XXl
have advantageous characteristics as compared to phenazines previously studied
for
inclusion in electrochromic media. Most phenazines previously studied have
their
main visible light absorption peak with its wavelength for maximum absorbance
around 460 manometers for the electrochemically activated state. Combination
of a
phenazine compound as the anodic material with a typical 1,1'-substituted-4,4'-
dipyridinium salt as the cathodic material (with a wavelength for maximum
absorbance of visible light around 600 nanometers for the electrochemically
activated
state), gives rise to electrochromic media which are poorly absorbing in the
wavelength range from about 470 and to about 540 nanometers. These media and
devices containing them typically have green-blue-green appearance in daylight
and
somewhat greenish-blue appearance when used in a rearview mirror to relieve
glare
during night driving.
Phenazine compounds have been discovered that have substantial visible light
absorbance in the 470 to 540 nanometer range and, in fact, have their maximum
visible absorbance peak in this range. Of particular note are the phenazine
compounds with phenyl, phenoxy, vinyl, or substituted phenyl, e.g., tolyl, in
one or
more of the 2, 3, 7 and 8 positions. Remarkably, the normally electron
withdrawing
aryl groups listed above, have little if any effect on the redox potential for
the first one
electron oxidation of these compounds when substituted in these positions, and
yet
48
CA 02580541 2007-03-23
these groups red shift the absorption spectra of the oxidized or
electrochemically
activated state. Combining these novel phenazines in various relative
concentrations
with phenazines that absorb around 470 nanometers or less-results in
electrochromic
media that have a desirable color appearance, including gray, when activated.
In
addition, phenazine compounds with aryl group substitution, e.g., phenyl,
vinyl, tolyl,
etc., in one or more of the 2, 3, 7 and 8 positions can be combined in
electrochromic
media without impacting the photochemical or thermal stability of the media.
The
only potential drawback is that the non-activated, neutral state of these
compounds
can be slightly yellow due to tailing of the W absorbance of the non-activated
state.
This concern is largely overcome by placing methyl or alkyl group(s) adjacent
to or on the aryl substituent or on the aryl group in a position adjacent to
the
attachment between the phenazine and the aryl group. For example, 2,5,10-
trimethyl-
3-phenyl-5,10-dihydrophenazine; 1,5,10-trimethyl-2-phenyl-5,10-
dihydrophenazine;
2,7-di(o-tolyl)-5,10-dimethyl-5,10-dihydrophenazine and even 2,3-diphenyl-5,10-
dimethyl-5,10-dihydrophenazine, (two adjacent aryl groups), are either
colorless or
less yellow than 2-phenyl-5,10-dimethyl-5,10-dihydrophenazine, 2,7-diphenyl-
5,10-
dimethyl-5,10-dihydrophenazine and 2-vinyl-5, 1 0-dimethyl-5, 1 0-
dihydrophenazine.
Other phenazines with wavelengths of maximum absorption in the range of 470 to
540 nanometers for their electrochemically activated state, without being
substantially
yellow in their non-activated state are very useful as well for combinations
that
achieve a pre-selected color, especially gray, (e.g. 2,7-diphenoxy-5, I 0-
dimethyl-5, 10-
dihydrophenazine; 2-phenoxy-5,10-dimethyl-5,10-dihydrophenazine; and 5,10-
diisopropyl-5,10-dihydrophenazine).
In example 17, 5,1 0-dimethyl-5,1 0-dihydrophenazine was made from
phenazine in a novel one-pot synthesis. This same strategy can be applied to
alkylation of phenazines, triphenodithiazines, triphenodioxazines,
quinoxalinophenazines, phenazine-based dyes, phenoxazine-based dyes,
phenothiazine-based dyes and similar phenazine compounds. In the general
procedure, the azine starting material is both reduced and alkylated in the
same
reaction mixture. This procedure is novel because it teaches how to do both
reduction
and alkylation of the azine compound in a safe, rapid, cost-effective one-pot
reaction.
In prior literature there are references to reducing and alkylating phenazines
in
separate steps and usually one of the steps is hazardous and expensive. In the
49
CA 02580541 2007-03-23
reference, "The Direct Preparation of Some Dihydro and Other Phenazine
Derivatives," JACS (1957) pp. 6178-6179, phenazine was reduced with sodium or
potassium metal and was then alkylated with methyliodide. This method is
hazardous, tedious and expensive. In another reference, "Preparation and
Properties
of Electron Donor Acceptor Complexes of the Compounds Having Capto-dative
Substituents," J. Heterocyclic Chemistry (1989), Vol. 26, pp. 435-438,
phenazine was
reduced with sodium dithionite. The resulting dihydrophenazine was then
alkylated
by using butyl lithium for a lithium-proton exchange, and the dilithio adduct
was
alkylated with addition of methyl iodide. This process is a two-pot synthesis
that
involves a hazardous alkylation step.
In accordance with an embodiment of the present invention, the azine
compound, reducing reagent, base, alkylating reagent and phase transfer
catalyst are
added together in a polar aprotic solvent with a small amount of water
present. Upon
heating the azine is both reduced and alkylated. We have applied our process
to make
many alkylated: phenazines, e.g. 2,7-diphenyl.-5,10-dimethyl-5,10-
dihydrophenazine;
nitrogen heterocycles, e.g. N,N',N',N"-tetrabutylquino xalinophenazine; azine-
based
dyes, e.g. 3,7-dibutoxy-10-butylphenoxazine from 7-hydroxy-3H-phenoxazin-3-
one.
We have typically used sodium dithionite as the reducing reagent, however
other reducing reagents may work as well e.g. hypophosphorous acid. Our base
is
usually potassium carbonate or sodium carbonate powder. Alkylating reagents
can be
alkyl iodides, bromides, chlorides, triflates, mesylates, or tosylates. The
phase
transfer catalyst is essential and we have had good success with quaternary
ammonium halides or hydrogen sulfates. Crown ethers and quaternary phosphonium
catalysts may work as well. The best catalysts have proven to be "accessible"
quaternary ammonium salts, which is a term familiar to those skilled in the
art of
phase-transfer reactions. The best solvent is acetonitrile but other polar
aprotic
solvents may work. Also helpful to decrease reactions time is the addition of
a small
amount of water.
The procedure is as follows: For one mole of azine compound having two
azine nitrogen, the amounts of other reagents used are: 1.15 moles of sodium
dithionite (85%), 2.0 moles of sodium carbonate, 4.0 moles of alkyl halide,
0.115
moles of phase-transfer catalyst, 10 liters of acetonitrile, and 200
milliliters of water.
Combine all reagents in one pot and heat to reflux under an inert atmosphere
for a
CA 02580541 2007-03-23
minimum of 5 hours. Add 10 liters of water and filter off alkylated product.
These
are the presently preferred amounts of these reagents, however, it is our
intention to
teach that because the reaction is robust, these reagents will work to produce
alkylated
product, even when the amounts of reagents are not present in the preferred
amounts.
An alteration to these conditions is necessary when dialkylamino substituents
are present. In this case a 2-phase reaction consisting of a non-polar organic
solvent
and an aqueous hydroxide layer are substituted for the
acetonitrile/water/carbonate
combination in the above-mentioned process. This avoids quaternization of the
dialkylamino groups.
Also it is important to note that alkyl iodides are more reactive than alkyl
bromides and alkyl bromides are more reactive than alkyl chlorides. Sodium
iodide
can be added as a co-catalyst when using alkyl bromides or alkyl chlorides.
In conclusion, this one-pot reduction/alkylation process is widely applicable
in
alkylating phenazmes and related azine compounds, as is shown in Examples 1,
3, 4,
12, 13, 14 and 16.
The dipyridinium compounds listed in Tables 1 through 6 are commonly
referred to as viologens. In order to make viologens that are more difficult
to
electrochemically reduce, it is known to substitute the dipyridinium salts
with alkyl
groups at one or more of the 2, 2', 6 and 6' positions shown in the following
general
structure.
R, R,
Ri-N \ N-Rj'
X R R6' X
[m]
However, substitution with methyl groups in one or more of the 2, 2', 6 and 6'
position
leads to compounds with relatively acidic protons due to the strong electron
withdrawing power of the quaternarized nitrogen near the methyl group. In
addition,
1,1',2,2',6,6'-hexamethyl-4,4'-dipyridinium salts are only slightly soluble in
polar
organic solvents like cyclic esters and nitriles when the salt contains anions
like
tetrafluoroborate, hexafluorophosphate, perchlorate or halides. Example 29
describes
51
CA 02580541 2007-03-23
the synthesis of viologens that overcome these difficulties and provide
compounds
that have diffusion characteristics that are desirable. Compounds of structure
XXX
with one or more of the 2,2',6 and 6' positions substituted with aralkyl
group(s), e.g.,
2-phenylethyl and 3-phenyl(n-propyl), or long chain alkyl group(s), e.g.,
hexyl, which
have the other 2,2',6 and 6' positions substituted with methyl group(s) have
increased
solubility in polar organic solvents as compared to compounds of structure XXX
which have methyl groups in each of the 2,2',6 and 6' positions.
In general, substitution of one or more of the 2, 2', 6 and 6' positions with
2-
phenylethyl or 3-phenylpropyl results in a viologen which is more difficult to
electrochemically reduce, does not have proton(s) as acidic as if the
substitution were
a methyl group and because it is believed that the phenyl groups are well
solvated by
solvents like propylene carbonate these compounds are believed to have smaller
diffusion coefficients than similar viologens without these substitutions.
Certain aspects of the present invention are illustrated in more detail in the
following examples. Unless specified otherwise, all concentrations cited in
the
examples are at room temperature (20-27 degrees Celsius) and all temperatures
are in
degrees Celsius.
Example 1
Synthesis of 5-ethyl- l 0-methyl-5,10-dihydrophenazine
5-ethyl-l0-methyl-5,10-dihydrophenazine was made as follows:
5-methylphenazinium methosulfate salt was reduced and alkylated to 5-ethyl-10-
methyl-5,10-dihydrophenazine in a one-pot phase transfer reaction.
1.0 grams of the 5-methylphenazinium methosulfate salt was refluxed in a 2-
phase
slurry containing 50 milliliters of toluene, 10 milliliters of 4M aqueous
NaOH, 10
grams of sodium dithionite, 10 milliliters of iodethane, 0.1 grams of
tetrabutylammonium hydrogen sulfate and 50 milliliters of water.
This mixture was refluxed for 4 days after which the reaction was cooled and
the
lower aqueous layer separated and discarded. After two more water washes, the
toluene was removed and the crude product redissolved in 50 milliliters of hot
52
CA 02580541 2007-03-23
ethanol. The cooled solution produced 0.35 grams of 5-methyl-10-ethyl-5,10-
dihydrophenazine for a 48% yield.
Example 2
Synthesis of 2-vinyl-5,10-dimethyl-5,10-dihydrophenazine
A sample of 2-formyl-5,10-dimethyl-5,10-dihydrophenazine was prepared
according
to the procedure of Pokhodenko et.al., J. Chem. Soc., Chem. Commun, 1985, 72.
The
formyl group was converted to the vinyl group by the procedure of Ghosh and
Spiro,
J. Electrochem. Soc.. 128, 1281 (1981) for making 4-vinyl-1,10-phenanthroline.
Recrystallization from acetone/water gave a yellow solid with mass 236 and
electrochemistry consistent with an NN-dialkylated phenazine.
Example 3
Synthesis of 2,7-bis(o-tolyl)-5, I0-dimethyl-5,10-dihydrophenazine
2,7-bis(o-tolyl)-5,1 0-dimethyl-5,1 0-dihydrophenazine was prepared from 2,7-
dichlorophenazine. The 2,7-dichlorophenazine was prepared from 2-iodo-5-
chloronitrobenzene and 2-nitro-5-chloroaniline using an Ullmann type aryl
amination,
followed by reduction of the nitro groups and ferric chloride oxidation.
The o-tolyl groups were substituted for the chloro group at the 2,7-
dichlorophenazine
with a "Suzuki coupling" using o-tolyboronic acid. "Palladium Catalyzed Cross-
Coupling Reactions of Organoboron Compounds", N. Miyawra and A. Suzuki, Chem
Rev. 95 pp. 2457-2483 (1995). This cross-coupling reaction took about 3 weeks
to
go to completion.
The 2,7-bis(o-tolyl)phenazine (2.2 grams) was refluxed in acetonitrile
containing 2%
by volume water, 0.6 grams of methyltributyl ammonium chloride, 8.7 grams of
sodium dithionite, 1.6 grams of sodium carbonate, and 3.1 milliliters of
iodomethane.
After 40 hours, water was dripped into the refluxing reaction solution and
product
precipitated out. After cooling, the product was filtered off and
recrystallized from
acetonitrile.. 2.07 grams of product was isolated for an 87% yield for the
alkylation.
53
CA 02580541 2007-03-23
Example 4
Synthesis of 2,3-dimethyl-7-trifluoromethyl-5,10-diethyl-5,10-dihydrophenazine
2,3 dimethyl-7-trifluoromethyl-5,10-diethylphenazine was prepared from the 2,3-
dimethyl-7-trifluoromethylphenazine.
The 2,3-dimethyl-7-trifluoromethylphenazine was prepared in a 3-step process,
starting with 4,5-dimethyl.-l,2-phenylenediamine and 3-nitro-4-
bromobenzotrifluoride. The nucleophilic substitution product being the
biarylamine
was then reduced with stannous chloride in conc. HCI to the diamino
diphenylamine.
This compound was oxidized to the phenazine with ferric chloride, in a dilute
HCl
aqueous solution. Tomlinson: "The Preparation of 2:2'-diaminodiphenyl Amines",
J.
Chem. Soc., pp. 158-163 (1939).
24.0 grams of this phenazine was added to 500 milliliters of acetonitrile,
21.2 grams
of sodium carbonate, 69.6 grams of sodium dithionite, 3.4 grams of tetrabutyl
ammonium hydrogen sulfate and 78.0 grams of iodoethane. This mixture was
refluxed for 4 days before it went to ccmpletion. 400 milliliters of water was
slowly
added to the refluxing reaction slurry. The desired product precipitated out
and after
cooling was filtered off. Product was recrystallized from hot ethanol yielding
17.7
grams of 2,3-dimethyl-7trifluoromethyl-5,10-diethyl-5,10-dihydrophenazine.
This is
an overall yield of 30.1% starting from the 4,5-dimethyl-1,2-phenylenediamine.
Example 5
Synthesis of 2,3,5,10-tetramethyl-7-trifluoromethyl-5,10-dihydrophenazine
This material was prepared by the procedure of Synthesis Example 4 with the
exception that iodomethane was substituted for iodoethane in the alkylation
step.
Example 6
Synthesis of 2,3-diphenyl-5,I0-dimethyl-5,10-dihydrophenazine
54
CA 02580541 2007-03-23
2,3-diphenylphenazine was prepared according to the method of C.H.
Issidorides,
et.al., Tetrahedron 34, 217 (1978). The phenazine nitrogens were then
methylated by
the procedure of Synthesis Example 3.
Example 7
Synthesis of 2,5,10-trimethyl-3-phenyl-5,10-dihydrophenazine
2-methyl-3-phenylphenazine was prepared by the method of C.H. Issidorides,
et.al.,
Tetrahedron 34, 217 (1978), except for that 1-phenyl-1,2-propanedione was
substituted for benzil. The phenazine nitrogens were then methylated by the
procedure of Synthesis Example 3.
Example 8
Synthesis of 5,10-diisopropyl-5,10-dihydrophenazine
Phenazine, 9.0 grams, was stirred with 6.5 grams a finely divided metal alloy
of 10:1
potassium to sodium, in 150 milliliters of 1,2-dimethoxyethane, at 40 C, until
a brick
red slurry was formed: approximately 24 hours. 2-bromopropane, 14.1
milliliters,
was added and the reaction was allowed to stir for 2 hours at which time the
reaction
mixture was filtered, the filtrate was rotovaped to dryness and the product
loaded as a
solid onto a silica gel column. The column was prepared with and eluted with
8:2
hexane/ethylacetate. Removal of solvent from the target compound fractions
gave a
white solid which was recrystallized from methanol to give 2.1 grams of white
needles, m.p. 80-81 C. A mass of 306 was confirmed by mass spectrometry.
Example 9
Synthesis of 2,3,5,10-tetramethyl-5,10-dihydrobenzo(B)phenazine
The 2,3-dimethylbenzo(B)phenazine was prepared by the condensation of 2,3-
diaminonapthalene with 4,5-dimethyl-1,2-benzoquinone in 4:1 ethanol to acetic
acid
at reflux for 2 hours. The phenazine was alkylated by the procedure of
Synthesis
CA 02580541 2007-03-23
Example 8, using iodomethane. Electrochemical analysis was consistent with an
N,N'-dialkylated phenazine.
Example 10
Synthesis of 5,10-dimethyl-5,10-dihydrodibenzo(A,C)phenazine
Dibenzo (A,C) phenazine was prepared with 1,2-phenylenediamine and
phenanthrenequinone, using standard condensation conditions.
Dibenzo(A,C)phenazine, 4.2 grams, was alkylated by the procedure of Synthesis
Example 8, using methyl iodide to give 2.1 grams of yellow crystals.
Electrochemical analysis was consistent with an N,N'-dialkylated phenazine.
Example 11
Synthesis of 5,10-dimethyl-5,10-dihydrobenzo(A)phenazine
Benzo(A)phenazine was prepared with 1,2-phenylenediamine and 1,2-
naphthoquinone, using standard condensation conditions.
This phenazine was reduced with a 3:1 potassium/sodium metal alloy in
dimethoxyethane, to the brick red alkali metal adduct. Alkylation occurred
over 1
hour with addition of iodomethane. Residual K/Na alloy was quenched with
addition
of ethanol. The product was isolated with column chromatography and was
recrystallized from ethylacetate/hexane. 2.0 grams of product was isolated for
a 38%
overall yield.
Example 12
Synthesis of 2-phenoxy-5,10-dimethyl-5,10-dihydrophenazine
2-phenoxy-5,10-dimethyl-5,10-dihydrophenazine was prepared from the 2-chloro-
phenazine. 2-chlorophenazine was prepared using 4-chloro-1,2-phenylenediamine
and 1-iodo-2-nitrobenzene. This diphenylamine was reduced with stannous
chloride
to chloro-2,2'-diaminodiphenylamine and oxidized to the 2-chlorophenazine with
56
CA 02580541 2007-03-23
ferric chloride in dilute aqueous HCI. "Tomlinson: The Preparation of 2:2'-
Diaminodiphenylamines," J. Chem. Soc., pp. 158-163 (1939).
2-chlorophenazine was reacted with potassium phenolate in tetraglyme tq arrive
at the
2-phenoxyphenazine.
150 milligrams of 2-phenoxyphenazine was refluxed in 50 milliliters of
acetonitrile,
3 milliliters of iodomethane, 1.7 grams of sodium dithionite, 0.21 grams of
sodium
carbonate and 0.1 gram of tetrabutyl ammonium hydrogen sulfate. After 24
hours,
reaction was complete. 50 milliliters of water was added to the refluxing
reaction
mixture. An oil separated out which was isolated and dissolved in 20
milliliters of hot
ethanol. Upon cooling, 47 milligrams of crystalline 2-phenoxy-5,10-dimethyl-
5,10-
dihydrophenazine was isolated for a 31 % yield.
Example 13
Synthesis of 2,7-diphenoxy-5,10-dimethyl-5,10-dihydrophenazine
2,7-diphenoxy-5,10-dimethyl-5,10-dihydrophenazine was prepared from 2,7-
dichlorophenazine. 2,7-dicylrophenazine was made from the procedure described
in
Synthesis Example 3 for 2,7-bis(o-tolyl)phenazine.
The diphenoxyphenazine was produced by reaction of the dichlorophenazine with
potassium phenolate in tetraglyme. The resulting 2,7-diphenoxyphenazine 0.35
grams
was refluxed in 100 milliliters of acetonitrile, 1.7 grams of sodium
dithionite, 0.53
grams of sodium carbonate, 3 milliliters of iodomethane and 0.1 grams of
tetrabutyl
ammonium hydrogen sulfate. After refluxing for 3 days, 100 milliliters of
water was
added to the refluxing reaction shiny. The precipitated product was filtered
off and
recrystallized from ethanol. 210 milligrams of 2,7-diphenoxy-5,10-dimethyl-
5,10-
dihydrophenazine was isolated for a 55% yield.
Example 14
Synthesis of 1,5,10-trimethyl-2-phenyl-5,10-dihydrophenazine
57
CA 02580541 2007-03-23
1,5,10-trimethyl-2-phenyl-5,10-dihydrophenazine was made with a 5-step
process.
The first step involved a "Suzuki coupling" reaction with 2-nitro-6-bromo
toluene and
phenylboronic acid. The procedure used was from "Palladium Catal'yzed Cross-
Coupling Reactions of Arylboronic Acids with II-Deficient Heteroaryl
Chlorides,"
Tetrahedron, 48, pp. 8117-8126 (1992). This reaction was quantitative after 40
hours.
The 2-nitro-6-phenyltoluene was isolated as an oil from the "Suzuki coupling."
It was
then reduced to the 2-amino-6-phenyltoluene with stannous chloride in
concentrated
HC1 and methanol.
The next step is an Ullmann type aryl amination of the amine with 2-
iodonitrobenzene. This reaction was carried out in nitrobenzene with copper as
a
catalyst. Product was isolated by distillation of the solvent followed by
column
chromatography.
The resulting 2-nitrodiphenylamine was isolated as an impure oil and was
cyclized to
the 1-methyl-2-phenylphenazine with iron powder. "Direct Ring Closure Through
a
Nitro Group I. Certain Aromatic Compounds with the Formation of Nitrogen
Heterocycles: A New Reaction," by H.C. Waterman and D.L. Vivian, J. Org.
Chem.,
14, 289-297 (1949).
The 1-methyl-2-phenylphenazine was carried on to the final
reductionlalkylation step
as an oil. The oil was refluxed in 50 milliliters of acetonitrile, 1
milliliter of water, 0.9
grams of methyltributyl ammonium chloride, 2.1 grams of sodium carbonate, 8.7
grams of sodium dithionite and 2 milliliters of iodomethane. After 16 hours,
the
reaction was quenched by adding 50 milliliters of water to the refluxing
reaction
mixture. An oil separated which was isolated, then dissolved in ethyl acetate
and
washed with water. The ethyl acetate was removed and the oil cleaned up with
column chromatography. Recrystallization from ethanol yielded 88 milligrams of
1,5,10-trimethyl-2-phenyl-5,10-dihydrophenazine as a nearly white solid.
Example 15
58
CA 02580541 2007-03-23
Synthesis of 2-phenyl-5,10-dimethyl-5,10-dihydrophenazine
2-phenyl-5,10-dimethyl-5,10-dihydrophenazine was prepared in a 4-step process.
The first step involves aryl amination of 4-bromo-3-nitrobiphenyl with
aniline, in
dimethylformamide. The resulting 2-nitro-4-phenyldiphenylamine was ring closed
to
the 2-phenylphenazine using sodium ethoxide and sodium borohydride by the
procedure described in "A New Phenazine Synthesis, The Synthesis of
Griseolutein
Acid, Griseolutein A, and Methyl Diacetyl Griseolutein B", J. Chem. Soc..
Chem.
Commun.. 1423-1425 (1970).
The 2-phenylphenazine was reduced to the 2-phenyl-5,10-dihydrophenazine by
adding aqueous dithionite solution to a refluxing ethanol solution of the
phenazine.
This dihydro product was isolated and then alkylated in a refluxing solution
of
acetonitrile containing iodomethane and sodium carbonate. Product was
precipitated
out by addition of water and was isolated. It was carbon treated and
recrystallized
from a mixture of acetone and ethanol to yield a bright yellow crystalline
solid.
Example 16
Synthesis of 2,7-diphenyl-5,1 0-dimethyl-5,1 0-dihydrophenazine
2,7-diphenyl-5,10-dimethyl-5,10-dihydrophenazine was prepared from 2,7-
dichlorophenazine. 2,7-dichlorophenazine was made from the procedure described
in
Synthesis Example 3 for 2,7-bis(o-tolyl)phenazine.
The 2,7-diphenyiphenazine was made from a "Suzuki" cross-coupling reaction
with 2,7-dichlorophenazine and phenylboronic acid. Refer to the procedure
described
in "Palladium Catalyzed Cross-Coupling Reactions of Arylboronic Acids With
Deficient Heteroarylchlorides," Tetrahedron, 48, pp. 8117-8126.
660 milligrams of 2,7-diphenylphenazine was reduced and alkylated by
refluxing in 10 milliliters of acetonitrile, 0.2 milliliters of water, 1
milliliter
iodomethane, 3.5 grams of sodium dithionite, 0.21 grams of sodium carbonate
and 60
59
CA 02580541 2007-03-23
milligrams of methyl-tributyl ammonium chloride. After 40 hours the reaction
was
quenched by dripping in 20 milliliters of water to the refluxing reaction
slurry. 450
milligrams of 2,7-diphenyl-5,10-dimethyl-5,10-dihydrophenazine was isolated
for a
62.0% yield.
Example 17
Novel Method of Making 5,10-dimethyl-5,10-dihydrophenazine
5,10-dimethyl-5,10-dihydrophenazine can be easily made in a novel one-pot
synthesis beginning with phenazine. In this synthesis, both reduction and
alkylation
proceed rapidly under mild reaction conditions.
Under a nitrogen atmosphere, 650 grams of phenazine was refluxed in 3.5
liters of acetonitrile with 100 milliliters of water, 899 milliliters of
iodomethane
(alkylating reagent), 765 grams of sodium carbonate powder (base), 723 grams
of
sodium dithionite (reducing reagent) and 130 grams of methyltributyl ammonium
chloride (phase-transfer catalyst) present. Phenazine was completely reduced
and
methylated after 5 hours. At this time 4.5 liters of water was added to the
refluxing
reaction slurry over 25 minutes. Upon cooling to room temperature, nearly all
of the
5,10-dimethyl-5,10-dihydrophenazine had precipitated. This was filtered off
and
redissolved in 1.95 liters of hot toluene. This toluene solution was filtered
to remove
inorganic salts. After filtration, 0.95 liters of toluene was removed via
atmospheric
distillation under nitrogen. The reaction was cooled to 85 C and 1 liter of
ethanol was
added over 20 minutes.
The solution was cooled gradually to room temperature and kept at room
temperature for 4 hours before filtering. The resulting 5,10-dimethyl-5,10-
dihydrophenazine was washed with 1 liter of water followed by 1 liter of cold
ethanol.
This product was then dried to 650.2 grams of a white crystal for an 85.3%
yield.
Example 18
Synthesis of 1-methyl-1'-phenyl-4,4'-dipyridinium bis(hexafluorophosphate)
CA 02580541 2007-03-23
1-phenyl-1'-methyl-4,4'-dipyridinium bis(hexafluorophosphate) was made by
first
attaching the phenyl group and then the methyl group to 4,4'-dipyridyl. The
phenyl
group was attached using a procedure from the Canadian Patent #1031346
entitled,
"Preparation of Bipyridinium Compounds" by John G. Allen. The 4,4'-d~pyridyl
is
quaternized with 2,4-dinitrochlorobenzene at 35 C; and using only 1 equivalent
at this
temperature limits the quaternization to just one side of the 4,4'-dipyridyl.
The monoquaternarized intermediate is refluxed with 10 equivalents of
iodomethane,
in acetonitrile, to quaternize the remaining nitrogen. This reaction is
complete after
1 hour with a 97.6% yield. The mixed salt is dissolved in hot water, filtered
and
product precipated out with addition of a 1 molar ammonium hexafluorophosphate
solution.
Example 19
Synthesis of 1-(4-cyanophenyl)-1'-methyl-4,4'-bipyridinium
bis(hexafluorophosphate)
1-(4-cyanophenyl)-1'-methyl-4,4'-bipyridinium-bis(hexafluorophosphate) was
made similarly to 1-phenyl-1'-methyl-4,4'-dipyridinium-
bis(hexafluorophosphate) in
synthesis Example 18. The only difference is that 4-cyanoaniline was used to
displace
the 2,4-dinitrophenyl group instead of aniline. See Canadian Patent No.
1031346.
Example 20
Synthesis of 1-(4-methoxyphenyl)-1'-methyl-4,4'-dipyridinium
bis(hexafluorophosphate)
1-(4-methoxyphenyl)-1'-methyl-4,4'-dipyridinium bis(hexafluorophosphate) was
made similarly to 1-methyl-1'-phenyl-4,4'-dipyridinium
bis(hexafluorophosphate) in
Synthesis Example 18. The only difference is that para-anisidine was used to
displace
the 2,4-dinitrophenyl group instead of aniline. See Canadian Patent #1031346.
Example 21
61
CA 02580541 2007-03-23
Synthesis of 1-phenyl-1'-(4-dodecylphenyl)-4,4'-dipyridinium
bis(hexafluorophosphate)
This viologen was made with reference to Canadian Patent #1031345. First the
4,4'-
dipyridyl was quatemarized on one side at 35 C with I equivalent of 2,4-
dinitro-
chlorobenzene. After displacement with dodecylaniline, the second nitrogen was
quaternized with dinitrochlorobenzene. This quatemization was done with an
excess
of dinitrochlorobenzene at reflux temperature. This dinitrophenyl group was
then
displaced with aniline to give the dichloride salt of the desired product.
Metathesis to
the hexafluorophosphate was done in hot MeOH with an acetonitrile solution of
ammonium hexafluorophosphate.
Example 22
Synthesis of 1,2,6-trimethyl-1'-phenyl-4,4'-dipyridinium
bis(tetrafluoroborate)
2,6-dimethyl-4,4'-dipyridyl was quaternized with a 5-fold excess of
dinitrochlorobenzene at 50 C. The quaternization takes place at the unhindered
nitrogen to yield 2,6-dimethyl-1'-(2,4-dinitrophenyl)-4,4'-dipyridinium
chloride. This
is reacted with aniline (see Canadian Patent #1031346) to give -2,6-dimethyl-
1'-
phenyl-4,4'-dipyridinium chloride.
Finally the hindered nitrogen is quaternized with a 20-fold excess of
iodomethane in
refluxing acetonitrile. This quaternization is done after 1 hour and the
resultant di-
substituted dipyridinium salt is filtered off. This salt is dissolved in hot
water and
precipitated as the tetrafluoroborate salt with a 1 molar aqueous solution of
sodium
tetrafluoroborate.
Example 23
Synthesis of 1,1'-bis(2,6-dimethylphenyl)-4,4'-dipyridinium
bis(tetrafluoroborate)
7.0 ml of 2,6-dimethylaniline was added to 40 ml of a 3:2
dimethylformamide/H,O
solution and the mixture was heated to reflux under a nitrogen atmosphere; 5.0
g of
1,1'-bis(2,4-dinitrophenyl)-4,4'-dipyridinium (Cl" salt) in 50 ml water was
slowly
62
CA 02580541 2007-03-23
added (over 20 min) via a pressure-equalizing addition funnel. The black
solution
was refluxed for an additional 2.5 h, then cooled to produce a yellow-brown
oily
precipitate. The solid material was removed by filtration and discarded. The
volume
of the filtrate reduced to ca. 10 ml by rotary evaporation. Addition of
cppious
amounts of acetone produced a light brown solid which was redissolved in 10:1
methanol/water. This solution was treated with decolorizing charcoal and
filtered.
Aqueous sodium tetrafluoroborate was added and the solution was allowed to
stand at
room temperature overnight. The product was isolated as light brown needles by
vacuum filtration.
Example 24
Synthesis of 1,T"-bis(3,5-dimethylphenyl)-4,4'-dipyridinium
bis(tetrafluoroborate)
6.7 ml of 3,5-dimethylaniline was added to 30 ml of a 3:2
dimethylformamide/H,O
solution and the mixture was heated to reflux under a nitrogen atmosphere. 5.0
g of
1,1'-bis(2,4-dinitrophenyl)-4,4'-dipyridinium (Cl- salt) in 50 ml water was
slowly
added (over 20 min) via a pressure-equalizing addition funnel. The black
solution
was refluxed for an additional 5 h, then cooled to produce a yellow-brown
precipitate.
The solid material was removed by filtration and discarded. The volume of the
filtrate reduced to ca. 10 ml by rotary evaporation. Addition of copious
amounts of
acetone produced an orange-brown solid which was redissolved in water. Aqueous
sodium tetrafluoroborate was added, resulting in precipitation of the crude
product as
an orange solid. The product was purified first by digestion in ethanol, then
by
decolorizing charcoal treatment in methanol/acetonitrile. Following addition
of water
and removal of methanol and acetonitrile by rotary evaporation, the pure
product was
isolated as a chalky off-white solid by vacuum filtration.
Example 25
Synthesis of 1,1'-bis(2,4,6-trimethylphenyl)-4,4'-dipyridinium
bis(tetrafluoroborate)
7.5 ml of 2,4,6-trimethylaniline was added to 40 ml of a 3:2
dimethylformamide/H2O
solution and the mixture was heated to reflux under a nitrogen atmosphere. 5.0
g of
1,1'-bis(2,4-dinitrophenyl)-4,4'-dipyridinium (Cl" salt) in 50 ml water was
slowly
63
CA 02580541 2007-03-23
added (over 20 min) via a pressure-equalizing addition funnel. The black
solution
was refluxed for an additional 6 hours, then cooled to produce a yellow-brown
precipitate. The solid material was removed by filtration and discarded. The
volume
of the filtrate reduced to ca. 10 ml by rotary evaporation. Addition of
copious
amounts of acetone produced a yellow-brown solid which was redissolved in 10:1
methanol/water. Aqueous sodium tetrafluoroborate was added, causing formation
of a
bright yellow precipitate. The crude solid was isolated by vacuum filtration
and
washed with small portions of cold methanol and water. Purification was
achieved by
decolorizing charcoal treatment in methanol/acetonitrile. Following addition
of water
and removal of methanol and acetonitrile by rotary evaporation, the pure
product was
isolated as a bright yellow solid by vacuum filtration.
Example 26
Synthesis of 1-(3,5-dimethoxyphenyl)-I'-methyl-4,4'-dipyridinium
bis(hexafluorophosphate)
3.0 ml of 3,5-dimethoxyaniline was added to 25 ml of a 3:2
dimethylformamide/H,O
solution and the mixture was heated to reflux under a nitrogen atmosphere. 3.0
g of 1-
(2,4-dinitrophenyl)-4,4'-dipyridinium (Cl- salt) in 50 ml water was slowly
added (over
20 min) via a pressure-equalizing addition funnel. The orange-brown solution
was
refluxed for an additional 3 h, then cooled to produce a yellow precipitate.
The solid
material was removed by filtration and discarded. The volume of the filtrate
reduced
to ca. 10 ml by rotary evaporation. Addition of copious amounts of acetone
produced
a light yellow solid.
0.30 g of this solid was dissolved in 80 ml of acetonitrile, along with an
excess of
methyl iodide. The solution was refluxed under a nitrogen atmosphere for 4 h
and
then allowed to cool to room temperature. The resulting precipitate was
isolated as a
bright orange solid by vacuum filtration. This crude product (as a mixed Cl',
I- salt)
was redissolved in water. Aqueous sodium tetrafluoroborate was added and the
solution was refrigerated overnight. The product was isolated as a yellow-
orange
solid by vacuum filtration. Purification was achieved by first redissolving in
acetonitrile, then precipitating as the chloride salt by addition of a
solution of
64
CA 02580541 2007-03-23
tetraethylammonium chloride in acetone. The chloride salt was isolated by
filtration
and briefly air-dried. The product was then converted to the PF6 salt by
dissolving in
water, filtering, and adding aqueous ammonium hexafluorophosphate to the
filtrate.
The resulting precipitate was isolated as a chalky, off-white solid by vacuum
filtration. The color of this compound was less yellow than 1-(4-
methoxyphenyl)-1'-
methyl-4,4'-dipyridinium bis(hexafluorophosphate). This has advantages in
electrochromic devices when residual yellow color is undesirable.
Example 27
Synthesis of 1-methyl-l'-(2-methylphenyl)-4,4'-dipyridinium
bis(hexafluorophosphate)
3.0 ml of o-toluidine was added to 25 ml of a 3:2 dimethylformamide/H,O
solution
and the mixture was heated to reflux under a nitrogen atmosphere. 3.0 g of 1-
(2,4-
dinitrophenyl)-4,4'-dipyridinium (Cl- salt) in 50 ml water was slowly added
(over 20
min) via a pressure-equalizing addition funnel. The orange-brown solution was
refluxed for an additional 3.5 h, then cooled to produce a yellow precipitate.
The
solid material was removed by filtration and discarded. The volume of the
filtrate
reduced to ca. 10 ml by rotary evaporation. Addition of copious amounts of
acetone
produced a light yellow solid which was redissolved in water. Aqueous ammonium
hexafluorophosphate was added and the resulting white precipitate was isolated
by
vacuum filtration, washed with water, and dried in a vacuum oven.
030 g of this solid was dissolved in 50 ml of acetonitrile, along with ca. 1.0
g of
methyl iodide. The solution was refluxed under a nitrogen atmosphere for 6 h.
Addition of dilute aqueous ammonium hexafluorophosphate, followed by removal
of
acetonitrile by rotary evaporation, produced a light tan solid.
Example 28
Synthesis of 1-methyl-1'-(2,4,6-trimethylphenyl)-4,4'-dipyridinium
bis(hexafluorophosphate)
CA 02580541 2007-03-23
This compound was prepared from 1-(2,4-dinitrophenyl)-4,4'-dipyridinium
chloride
(see Synthesis Example 18) by reaction with excess methyl iodide in refluxing
acetonitrile. The 2,4-dinitrophenyl group was then displaced by reaction with
2,4,6-
trimethylaniline in 1:1 dimethylformamide/water (see Canadian Pateht
#1031346).
The crude product was isolated as a mixed halide salt by reducing the volume
of the
reaction mixture to only a few ml, adding 200 ml of acetone, and refrigerating
overnight. The resulting solid was isolated by filtration, redissolved in
water, and
precipitated as the hexafluorophosphate salt by addition of aqueous ammonium
hexafluorophosphate.
Example 29
Preparation Of Novel 2,2',6,6'-substituted-4,4'-dipyridinium salts
The procedure described here for preparing Compounds I-IV and VIII-XI are
based on
those outlined in:
1. (a) Minisci, Ton, Curr. Chem., Vol. 62, (1976), pp. 1-48
(b) Synthesis, (1973) pp. 1-24
2. Minisci, Mondelli, Gardini and Porta, Tetrahedron, Vol. 28, (1972), 2403
3. Citterio, Minisci and Franchi, J. Org. Chem., Vol. 45, (1980), 4752
4. Anderson and Kochi, J. Am. Chem. Soc., Vol. 92, (1970), 1651
5. Baltrop and Jackson, J. Chem. Soc., Perkin II, (1984), pp 367-371
Preparation of 2-(2-phenylethyl)-4,41-dipyridyl (I) and 2,2'-bis(2-
phenylethyl)-4,4'-
dipyridyl (II)
Procedure: To a stirring solution of 4,4'-dipyridyl (15.62 g; 0.1 mole) in a
mixture of
water (100 mL) and concentrated sulfuric acid (5.3 mL) were added
hydrocinnamic
acid (32.0 g; 0.213 mole) and silver nitrate (1.7 g; 0.01 mole) and the
mixture was
heated to about 80 C and while maintaining this temperature for 30 minutes
ammonium peroxy disulfate (22.82 g; 0.1 mole) was added in small portions.
After
the addition, the mixture was maintained at the same temperature for an
additional 2
hours. Then the reaction mixture was cooled to the room temperature and was
neutralized with aqueous sodium hydroxide (10%). The resulting dark brown-
colored
66
CA 02580541 2007-03-23
mixture was filtered and the filtrate was extracted several times with 25 mL
portions
of ethyl acetate. The organic layers were combined, dried over anhydrous
magnesium
sulfate, filtered and the filtrate was evaporated to remove the solvent
completely so
that_a dark-colored viscous oil was left behind. From this oily mixture, the
desired
compounds I and II were isolated as solids by silica gel column
chromatography. The
respective amounts of I and II so obtained were 4.7 g and 1.76 g.
Preparation of 2-(3-phenyl(n-propyl))-4,4'-dipyridyl (III) and 2,2'-bis(3-
phenyl(n-
propyl)-4,4'-dipyridyl (IV)
Procedure: Compounds III and IV were prepared by the same procedure as
described
for the preparation of Compounds I and II except for using 4-phenyl butyric
acid in
place of hydrocinnamic acid. The respective amounts of III and IV so obtained
were
3.3 g and 2.15 g.
Preparation of. 2,2',6-trimethyl-6'-(2-phenylethyl)-4,4'-dipyridyl (V); 2,2'-
dimethyl-
6,6'-bis(2-phenylethyl)-4,4'-dipyridyl (VI) and 2-methyl-2',6,6'-tris(2-
phenylethyl)-
4,4'-dipyridyl (VII)
Procedure: To a stirred suspension of sodium amide (29.2 g; 0.75 mole) in m-
xylene
(80 mL) was added 2,2',6,6'-tetramethyldipyridyl (5.3 g; 0.025 mole) under an
argon
atmosphere. After brief stirring, benzyl chloride (50 g; 0.39 mole) was added
slowly
over a period of 15-30 minutes and the mixture was refluxed for 15-20 hours.
After
this time the heating was stopped, the reaction mixture was cooled to room
temperature and cold water (5-10 mL) was added cautiously to destroy the
unreacted
sodium amide. The mixture was acidified with concentrated hydrochloric acid
and
was extracted with methylene chloride a few times with 25 mL portions. This
operation helped to remove the unreacted benzyl chloride and m-xylene solvent.
The
organic layer was separated and discarded. Now the aqueous solution was
basified
with sodium hydroxide (20% aqueous solution) and the mixture was extracted 2-
3 times with 25 mL portions of methylene chloride. The organic layers were
combined, dried over anhydrous magnesium sulfate and filtered. The filtrate on
complete evaporation of the solvent gave rise to a brown viscous oil (9.2 g).
The
67
CA 02580541 2007-03-23
desired Compounds V, VI and VII were isolated from the mixture by silica gel
column chromatography.
Preparation of 2,2',6-trimethyl-6'-n-hexyl-4,4'-dipyridyl (VIII) and 2,'2'-
dimethyl-6,6'-
bis(n-hexyl)-4,4'-dipyridyl (IX):
Procedure: To a magnetically stirred solution of 2,2'6,6'-tetramethyl-4,4'-
dipyridyl
(5.3 g; 0.025 mole) in pure tetrahydrofuran (80 mL) cooled to -78 C (dry ice
and 2-
propanol) was added under an argon atmosphere a cyclohexane solution (2.0 M)
of n-
butyl lithium (1.76 g; 0.0275 mole) from a dropping funnel over a period of 20
minutes. The solution turned deep blue immediately. The mixture was allowed to
warm up to -30 C for 5 minutes and was cooled back to -78 C. A solution of 1-
chloropentane (2.93 g; 0.0275 mole) in pure tetrahydrofuran (15 mL) was now
added
from a dropping funnel over a period of 10 minutes. After the addition, the
color of
the mixture became dark purple. After stirring at -78 C for a short period,
the mixture
was allowed to warm up to room temperature. Pure water (2-3 mL) was added
cautiously to destroy any unreacted butyl lithium still present and the
mixture was
diluted with more pure water (100 mL) and was extracted 2-3 times with 25 mL
portions of ethyl acetate. The organic layers were combined, dried over
anhydrous
magnesium sulfate, filtered, and the filtrate was evaporated to remove the
solvent
completely. This gave 7.2 g of a yellow-brown oil. Compounds VIII and IX were
isolated from the oil by silica gel column chromatography as solid products.
Preparation of 2,2',6-trimethyl-6'-(3-phenyl(n-propyl))-4,4'-dipyridyl (IX)
and 2,2'-
dimethyl-6,6'-bis(3-phenyl(n-propyl))-4,4'-dipyridyl (X)
Procedure: The Compounds X and XI were prepared in the same way as described
for the preparation of Compounds VIII and IX except for using 1-bromo-2-
phenylethane in place of 1-chloropentane as the alkylating agent.
Conversion of Compounds I through XI to Their Respective 1,1'-dimethyl-4,4'-
dipyridinium diiodide salts:
68
CA 02580541 2007-03-23
The diiodide salts of Compounds I through X were prepared by refluxing each of
these with an excess mole equivalent of iodomethane in pure acetonitrile for
24 to 48
hours, and the resulting diquaternary salts were filtered,-washed thoroughly
with fresh
acetonitrile, followed by rinsing with dry acetone.
Conversion of Compounds I - IV, VIII and IX to Their Respective 1,1'-dimethyl-
4,4'-
dipyridinium bis(hexafluorophosphate) salts:
Typical Procedure: The diiodide salt (5 mole) prepared as described above was
dissolved in pure water (100-150 mL) and the solution was stirred with
decolorizing
carbon (1.0 g) for 2-3 hours at room temperature. The suspension was filtered
and the
colorless filtrate was treated with an aqueous solution of one molar ammonium
hexafluorophosphate until the precipitation was complete. After standing for 1
hour,
the precipitate was filtered with suction, washed with pure water (20 mL) 2-3
times
and was recrystallized from water to obtain pure salt. The yields varied with
the
individual compounds anywhere from 20-80%.
Conversion of Compound VI to the 1,1'-dimethyl-4,4'-dipyridinium diperchlorate
salt:
The diiodide salt of Compound VI as prepared above was first dissolved in hot
pure
water (100 mL) and to the solution an aqueous solution (5%) of sodium
perchlorate
was added until the precipitation is complete. The precipitate was filtered,
washed 4-
tines with pure water (25 mL) and the wet precipitate was recrystallized and
purified by treatment with decolorizing carbon in a mixture (8:2 v/v) of
acetonitrile
and water. The yield of yellow-colored solid was 32%.
Conversion of Compound VII and VIII to the 1, 1 '-dimethyl-4,4'-dipyridinium
bis(tetrafluoroborate) salt:
The diiodide salt (2.0 g; 3.5 m mole) was dissolved in pure water (25 mL) at
room
temperature. The solution was treated with decolorizing carbon, filtered and
to the
colorless filtrate was added an aqueous solution of sodium tetrafluoroborate
(2 molar)
until the precipitation was complete. The resulting light yellow-colored
precipitate
69
CA 02580541 2009-07-22
was filtered, washed 4-5 times with 25 mL portions of pure water. The solid
precipitate was then recrystallized from hot water to obtain a colorless
solid.
Example 30
Green/Blue Electrochromic Device
Symmetrically substituted aryl viologens were prepared from reaction of the
appropriate aniline derivative with 1, l'-bis(2,4-dinitrophenyl)-4,4'-
dipvridinium as
has been previously described in Examples 23 and 24. Ferrocene was obtained
commercially (Aldrich) and purified by sublimation prior to use.
Two stock solutions, one containing 60 mM ferrocene in propylene carbonate
and the second containing 30 mM each 1,1'-bis(2,4,6-trimethylphenyl)-4,4'-
dipyridinium bis(tetrafluoroborate) and 1,1'-diphenyl-4,4'-dipyridinium
bis(tetrafluoroborate) in propylene carbonate were prepared in separate small
vials.
Both stock solutions were deoxygenated with dry nitrogen. Equal volumes of
each
stock solution were introduced into a clean vial to produce a mixture which
was
approximately 30 mM in ferrocene and 15 mM in each of the two viologen
derivatives. This multi-component mixture was then used to fill electrochromic
devices.
Electrochromic window devices were fabricated as is known in the art with
TM
TEC-20 glass from Libbey-Owens-Ford with a 137 micrometer cell spacing. The
devices were about 1' X 2" in area and were filled by introducing the solution
described above into the device through one of two holes drilled in the top
plate. Both
holes were then plugged using a hot glue gun.
Application of 1.2 V across this electrochromic device resulted in uniform
coloration to a green/blue state; however some staging (through a green
intermediate)
was observed on both coloring and clearing.
Example 31
Gray Electrochromic Device
CA 02580541 2009-07-22
Two stock solutions, one containing 60 mM ferrocene in propylene carbonate
and the second containing 30 mM each 1,1'-bis(2,4,6-timethylphenyl)-4,4'-
dipyridinium bis(hexafluorophosphate) and 1-(4-cyanophenyl)-I'-methyl-4,4'-
dipyridinium bis(tetrafluoroborate) in propylene carbonate were prepared in
separate
small vials. Both stock solutions were deoxygenated with dry nitrogen. Equal
volumes of each stock solution were introduced into a clean vial to produce a
mixture
which was approximately 30 mM in ferrocene and 15 mM in each of the two
viologen
derivatives. This multi-component mixture was then used to fill electrochromic
devices.
Electrochromic window devices were fabricated as is known in the art with
7M
TEC-20 glass from Libbey-Owens-Ford with a 137 micrometer cell spacing. The
devices were about 1' X 2" in area and were filled by introducing the solution
described above into the device through one of two holes drilled in the top
plate. Both
holes were then plugged using a hot glue gun.
Application of 1.2 V across this electrochromic device resulted in uniform
coloration to a dark blue/green (moderately gray) state. No staging was
observed
during either coloring or clearing.
Example 32
Electrochromic devices having colors ranging from green/gray and blue-green
gray
Three stock solutions, one containing 60 mMVl ferrocene in propylene
carbonate, one containing 60 mM 1,1'-bis(2,4,6-trimethylphenyl)-4,4'-
dipyridinium
bis(tetrafluoroborate)(V1) in propylene carbonate and one containing 60 mM
1,1'-
bis(3,5-dimethylphenyl)-4,4'-dipyridinium bis(tetrafluoroborate) (V2). in
propylene
carbonate were prepared. in separate small vials. All three stock solutions
were
deoxygenated with dry nitrogen. Aliquots from each of the stock solutions were
introduced into five clean vials in such a manner to produce the following
solutions:
A) 30 mM ferrocene/15 mM VI w/15 mM V2
B) 30 mM ferrocene/18 mM VI w/12 mM V2
C) 30 mM ferrocene/20 mM V 1 w/10 mM V2
D) 30 mM ferrocene/21 mM VI w/9 mM V2
71
CA 02580541 2009-07-22
E) 30 mM ferrocene/24 mM V 1 w/6 mM V2
These multi-component mixtures were then used to fill -electrochromic devices.
Electrochromic window devices were fabricated as is known in the art with
TM
TEC-20 glass from Libbey-Owens-Ford with a 137 micrometer cell spacing. The
devices were about 1' X 2" in area and were filled by introducing the
solutions
described above into the device through one of two holes drilled in the top
plate. Both
holes were then plugged using a hot glue gun.
Application of 1.2 V across each of these electrochromic devices resulted in
uniform coloration, with the exact color when full dark varying smoothly from
green-
gray for device A to blue-green gray for device E. Slight staging was observed
during
coloring of A, B, and C, while D and E exhibited no appreciable staging during
coloring or clearing.
Example 33
Gray Electrochromic Devices
A solution consisting of 25 mM 1,I'-dimethylferrocene, 100 mM 2-hydroxy-
4-methoxybenzophenone (as a UV stabilizer), 18 mM 1, 1'-bis(2,6-
dimethylphenyl)-
4,4'-dipyridinium bis(tetrafluoroborate), 12 mM 1, 1'-bis(3,5-dimethylphenyl)-
4,4'-
dipyridinium bis(tetrafluoroborate), and 3% (wt/wt) of polymethylmethacrylate
in
propylene carbonate was deoxygenated with dry nitrogen.
Electrochromic window devices were fabricated as is known in the art with
TM
TEC-20 glass from Libbey-Owens-Ford with a 137 micrometer cell spacing.
TM
Similarly, electrochromic mirrors were fabricated using a transparent TEC-20
front
TM
plate with either a TEC-20 back plate which had been previously silvered on
the side
opposite the conductive coating (fourth surface reflector) or coated with
another
reflective metal (third surface reflector). These devices measured about 2" X
5" and
were filled with the electrochromic solution described above via the vacuum
backfilling technique. The vacuum fill ports of the devices were plugged with
a UV
cure material.
After four months, representative L*a*b* (A/2-degree) values were as follows:
72
CA 02580541 2009-07-22
initial darkened
L* a* b* L* a* b*
fourth surface reflector 87.51 -1.42 +12.60 32.96 -0.3,7 -6.27
'third surface reflector 78.17 +0.07 +10.21 30.44 -5.12 -3.68
Example 34
Gray Electrochromic Device
TM
An electrochromic device was prepared from two pieces of TEC 15 glass
spaced apart by 137 microns by a perimeter epoxy seal. The device was filled
with a
nitrogen purged propylene carbonate solution of 14 mM 5,10-diisopropyl-5,10-
dihydrophenazine, 14 mM 5,10-dimethyl-5,10-dihydrobenzo(A,C)phenazine and 34
mM bis(3,5-dimethylphenyl)-4,4'-dipyridinium bis(tetrafluoroborate). In the
clear
state the device was slightly yellow and with 0.8 volts applied, the device
was very
dark gray. From the spectrum of the clear state, the color coordinates (A/2-
degree)
L*, a*, b* were found to be equal to 89.19, -0.27, 10.7 and at 0.8 volts L*,
a*, b*
were equal to 17.72, 9.03, 7.37. The CIE curve white light transmittances were
75%
clear and 2.5% darkened at 0.8 volts. Not only was the device gray when
activated, it
was remarkably low in transmission when fully darkened.
While the invention has been described in detail herein in accordance with
certain preferred embodiments thereof, many modifications and changes therein
may
be effected by those skilled in the art without departing from the spirit of
the
invention. Accordingly, it is our intent to be limited only by the scope of
the
appending claims and not by way of the details and instrumentalities
describing the
embodiments shown herein.
73