Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Dl'NAMIC MONITORING OF CELL ADHESION AND SPREADING USING THE RT-CES
SYSTEM
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims benefit of priority to the following patent
applications:
U.S. Patent Application Number 11/197,994, filed on August 4, 2005; U.S.
Patent
Application Number 11/198,831, filed on August 4, 2005; U.S. Patent
Application
Number 11/055,639 filed on February 9, 2005; U.S. Patent Application Number
10/987,732, filed on November 12, 2004; U.S. Provisional Patent Application
Number
60/630,131, filed on November 22, 2004; U.S. Provisional Patent Application
Number
60/630,071 filed on November 22, 2004; U.S. Provisional Paterit Application
Number
60/613,872 filed on September 27, 2004; U.S. Provisional Patent Application
Number
60/613,749, filed on September 27, 2004; U.S. Provisional Patent Application
Number
60/630,809 filed on November 24, 2004; U.S. Provisional Patent Application
Number
60/633,019 filed on December 3, 2004; U.S. Provisional Patent Application
Number
60/647,159 filed on January 26, 2005; U.S. Provisional Patent Application
Number
60/653,904 filed on February 27, 2005; U.S. Provisional Patent Application
Number
60/673,678 filed on Apri125, 2005; U.S. Provisional Patent Application Number
60/689,422 filed on June 10, 2005; PCT Patent Application Number
PCT/US05/27943
filed on August 4, 2005 and PCT Patent Application Number PCT/US05/27891 filed
on
August 4, 2005; U.S. Provisional Application Number 60/614,601, filed on
September
29, 2004; PCT Patent Application No. PCT/US05/04481, filed on February 9,
2005; PCT
Patent Application No. PCT/USO4/37696, filed on November 12, 2004; U.S.
Provisional
Patent Application Number 60/647,189, filed on January 26, 2005; U.S.
Provisional
Patent Application Number 60/647,075 filed on January 26, 2005; U.S.
Provisional
Patent Application Number 60/660,829 filed on March 10, 2005, and U.S.
Provisional
Patent Application Number 60/660,898 file on March 10, 2005. Each of the
patent
applications provided in this paragraph are incorporated by reference herein
in their
entirety.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
The present application incorporates by reference in their entirety the
following
patent applications: U. S. Provisional Application 60/519,567, filed on
November 12,
2003; U.S. Patent Application Number 10/705,447 filed on November 10, 2003; U.
S.
Provisional Application 60/397,749, filed on July 20, 2002; U. S. Provisional
Application
60/435,400, filed on December 20, 2002; U. S. Provisional Application
60/469,572, filed
on May 9, 2003; PCT application PCT/US03/22557, filed on July 18, 2003; U.S.
Patent
Application Number 10/705,615, filed on November 10, 2003; U. S. Provisional
Application 60/397,749 filed on July 20, 2002; U. S. Provisional Application
60/435,400,
filed on December 20, 2002; U. S. Provisional Application 60/469,572, filed on
May 9,
2003; and PCT application PCT/US03/22537, filed on July 18, 2003; U. S.
Provisional
Patent Application Number 60/542,927 filed on February 9, 2004; U. S.
Provisional
Application 60/548,713, filed on February 27, 2004; U.S. Provisional Patent
Application
Number 60/598,608, filed on August 4, 2004; U.S. Provisional Patent
Application
Number 60/598,609, filed on August 4, 2004;
TECHNICAL FIELD
The present application relates to microelectronic devices and methods of use
of
to detect changes in impedance of a cell, and more specifically to
microelectronic devices
coated with biological molecules or organic compounds and methods of
dynamically
monitoring cell adhesion and cell monitoring.
BACKGROUND
The cells malcing up the various tissues and organs systems are held together
by
specific molecules that essentially serve as "biological glue" conferring
shape, structure,
rigidity or plasticity. During embryogenesis, these biological molecules or
extracellular
matrix (ECM) proteins serve as "tracks" and direct cells to the appropriate
vicinity within
the embryo to give rise to tissues and organ systems. ECM proteins also play a
prominent
role during wound healing, and are involved in directing other cellular
processes such as
proliferation, survival, and differentiation. Failure of cells to interact
with the appropriate
biological surface or molecule can be detrimental to the faith of the cells
and can
contribute to cancer cell metastases.
2
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
There are several methods for assessing and quantifying cellular adhesion and
spreading on an ECM coated surface. The most widely used method is to apply
the cells
onto surfaces coated with appropriate ECM components, allow the cells to
attach and
adhere for a specified length of time and wash the unbound cells. The attached
cells are
then fixed, labeled with fluorescent reagent such as rhodamine phalloidin and
visualized
using an epi-fluorescent microscope or an epi-fluorescent confocal microscope.
Alternatively, the cells can be labeled with a dye such as crystal violet and
quantified by
either counting the stained cells using a light microscope or solubilizing the
stain and
obtaining absorbance reading using a spectrophotometer. Cells can also be pre-
labeled
with a fluorescent dye for live cells such as 6-carboxyfluorescein diacetate
(CFDA) and
then applied to appropriate ECM-coated surface. The unbound cells are washed
off and
the bound cells are quantified using a plate reader. An additional method for
assessing the
role of integrins and other adhesion proteins is to coat different surfaces
with antibodies
or peptides which are specific for the various receptors and then seed the
cells which are
expressing the appropriate integrin receptors. The interaction of integrin
receptor on the
cell surface with the antibody or peptide-coated surface will allow the cells
to adhere and
undergo specific morphological and biological changes which can then be
assessed by
using cell biological techniques discussed above.
While the assays just described for assessing and quantifying cell adhesion
have
been informative, there are certain caveats associated with each of these
assays. For
example, each of the assays described are end-point assays which provide a
"snapshot" of
the adhesion process. All the assays involve pre-labeling or post-labeling of
the cells and
also involve fixation and permeabilization leading to destruction of the cell.
In this
application we describe a label-free real-time assay using electronic cell
sensor
technology (RT-CES system) which addresses some of the major limitations of
the
current in vitro assays for assessing the interaction of bio-molecular coated
surfaces with
target cells. Furthermore, because the readout is non-invasive it precludes
the need for
fixation and lysis of the cells and allows for acquisition of information for
biological
events occurring after adhesion and spreading, such as proliferation and
differentiation.
~
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
SUMM:4Rl'
The present invention includes a microlectronic cell sensor array including a
non-
conductive substrate, a plurality of electrode arrays positioned on the
substrate, and a
biological molecule or an organic compound, and optionally a control molecule
or a
control compound positioned on a portion of the substrate. Each electrode
array includes
at least two electrodes and each electrode is separated from at least one
adjacent electrode
by an area of non-conductive material.
In another aspect of the present invention a method of coating a
microelectronic
cell sensor array with a biological molecule or organic compound is provided
including
providing a microelectronic cell sensor array and incubating a test solution
on a first
portion of the electrode array and optionally a control solution on a second
portion of the
electrode array. The microelectronic cell sensor array may include a non-
conductive
substrate and a plurality of electrode arrays positioned on the substrate.
Each electrode
array may include at least two electrodes and each electrode may be separated
from at
least one adjacent electrode by an area of non-conductive material. The test
solution may
include a biological molecule or organic compound and the control solution may
include
a vehicle control and optionally a control molecule or control compound. The
incubation occurs under conditions suitable for attaching the biological
molecule or
organic compound to the electrode array or to the nonconductive substrate.
In another aspect of the present invention, a method of monitoring cell
adhesion
or cell spreading is provided including-providing a microelectronic cell
sensor array
including a test portion and a control portion, coated at least in part with a
biological
molecule or organic compound and operably connected to an impedance analyzer.
A cell
or cell population is introduced to the test portion and the control portion.
A series of
impedaiice measurements of the test portion and the control portion are
performed. The
change in impedance and optionally a cell index (CI) of the test portion and
the change in
impedance and optionally a cell index (CI) of the control portion is
determined. The
change in impedance of the test portion is compared to the change in impedance
of the
control portion or alternatively the cell index (CI) of the test portion is
compared to the
4
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
cell index (Cl) of the control portion. Cell adhesion or cell spreading occurs
if the
comparison demonstrates.a_significant change in impedance.
In another aspect of the present invention, a method of identifi=ing a
compound or
biological molecule capable effecting cell adhesion or cell spreading is
provided
including providing a microelectronic cell sensor array that is at least in
part coated with
a biological molecule or organic compound and is operably connected to an
impedance
analyzer, introducing a cell or cell population to a test portion and to a
control portion of
the microelectronic cell sensor array, performing a series of impedance
measurements of
the test portion and the control portion, determining the change in impedatice
and
optionally a cell index (CI) of the test portion and the change in impedance
and
optionally a cell index (CI) of the control portion, comparing the change in
impedance of
the test portion to the change in impedance of the control portion or
comparing the cell
index (CI) of the test portion to the cell index (CI) of the control portion
and determirung
cell adhesion or cell spreading is effected if the comparison demonstrates a
significant
change in impedance. The substrate of the microelectronic cell sensor array is
coated
with biological molecule or organic compound capable of supporting cell
adhesion or
spreading on a test portion and a control biological molecule or control
organic
compound on a control portion.
In another aspect of the present invention, a method of identifying an
inhibitor of
cell adhesion or cell spreading is provided including providing a
microlectronic cell
sensor array operably connected to an impedance analyzer, the microelectronic
cell
sensor array including a non-conductive substrate, a plurality of electrode
arrays
positioned on the substrate, each electrode array including at least two
electrodes, and
each electrode is separated from at least one adjacent electrode by an area of
non-
conductive material and a biological molecule or organic compound positioned
on the
test portion of the substrate and on a control portion of the substrate. The
biological
molecule or organic compound may be known to be capable of increasing cellular
adhesion or cellular spreading. The method also includes preincubating a cell
or cell
population with a biological molecule or compound suspected of being an
effector or
inhibitor of cell spreading or cell adhesion defined as a test sample and
preincubating a
cell or cell population with a vehicle control defined as a control sample,
introducing the
5
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
test sample to the test portion and the control sample to the control portion,
perfoimiizg a
series of impedailce measurements of the test portion and the control portion,
determining
the change in impedance and optionally a cell index (CI) of the test portion
and the
change in impedance and optionally a cell index (CI) of the control portion,
comparing
the change in impedance of the test portion to the change in impedance of the
control
portion or comparing the cell index (CI) of the test portion to the cell index
(CI) of the
control portion, and determining cell adhesion or cell spreading is reduced or
inhibited if
the comparison demonstrates the change in impedance or cell index is greater
in the
control portion than the test portion.
BRIEF DESCRIPTION OF THE DRAWINGS
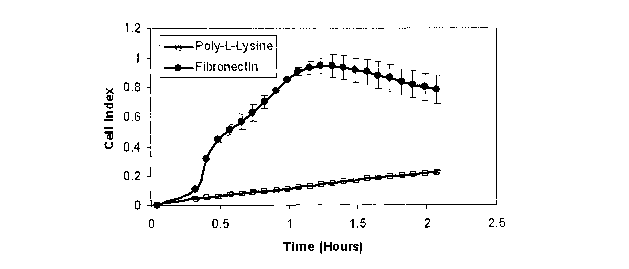
FIG. 1 depicts a graphical representation of attachment and spreading of
NIH3T3 cells
on ACEA E-plates coated with fibronectin (FN) and poly-L-Lysine (PLL) and
monitored
by the RT-CES system. The wells of ACEA E-plates were coated with 10 g/mL FN
or
with 50 g/mL PLL for 1 hour at 37 C. The wells were washed with PBS prior to
the
addition of media alone for background recording. NIH3T3 cells were added at a
density
of 10,000 cells per well and the adhesion and spreading of the cells were
monitored by
the RT-CES system.
FIG. 2 depicts images demonstrating attaclunent and spreading of NIH3T3 cells
on 16X
chamber slides coated with FN and PLL. 16X chamber slides were coated with PLL
and
FN as described in FIG. 1. NIH3T3 cells were added to the wells and at the
indicated
time points the cells were fixed and stained with rhodamine-phalloidin to
stain the actin
cytoskeleton. The cells were visualized and imaged with an epifluorescence
microscope.
FIG. 3 depicts a graphical representation of the effect of FN concentration
being coated
onto the ACEA E-plate on NIH3T3 cell attachment and spreading. ACEA E-plates
were
coated ~,ith increasinQ amounts of FN in the range of 0~Lg/mL to 20 g/mL.
5000
NIH3T3 cells were added to the wells and the attachment and spreading of the
cells were
6
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
monitol-ed by the RT-CES systeni. The inset shows the averaae cell index of
attachment
at 3 hours in response to increasing aniounts of FNT.
FIG. 4 depicts a graphical representation of inhibition of cell attachment and
spreading
using the RGD containing peptides. ACEA 16X E-plates were coated with FN as
described in FIG. 1. NIH3T3 cells were preincubated for 30 minutes with the
indicated
final concentration of the peptide GRGDS and also with the indicated
concentration of
the control peptide (GRADSP). The cells were added to E-plates coated with FN
and the
attaclunent and spreading of NIH3T3 cells were monitored by the RT-CES system.
FIG. 5 depicts a graphical representation of attachment and spreading of
Jurkat T cells
on ACEA E-plates coated with anti-CD-3 antibody. E-plates were coated with 10
g/mL
of OKT3 antibody (anti-CD-3) or a control antibody for 2 hours at room
temperature.
The wells were washed with PBS and then the background impedance determined
using
the RT-CES. 500,000 Jurkat T cells were added per well and the attachment and
spreading of the cells were monitored using the RT-CES system.
FIG. 6 depicts (A) Dynamic monitoring of cell attachment and spreading on PLL
and
FN-coated surfaces using the RT-CES system. (B) The RT-CES measurements
correlate
Z0 with the extent of cell attachment and spreading using conventional
phalloidin staining of
the actin cytoskeleton and immunofluorescence microscopy.
FIG 7 depicts (A) Quantitative and dynamic monitoring of cell attachment and
spreading
in response to increasing concentrations of FN using the RT-CES system. (B)
?5 Coniparison of ACEA units of Cell Index versus manual counting of the cells
for
different FN concentrations at 3 hours.
FIG. 8 depicts (A) Dose-dependent inhibition of cell attachment and spreading
in
response to cyclic-RGD peptides, using the RT-CES system. (B) Comparison of
cell
30 attachment and spreading in response to a control peptide and cyclic-RGD
peptides at
three hours.
7
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
FIG. 9 depicts (A) Dynamic monitoring of the dose-dependent effect of
Latriculin on
NIH3T33 cell attachment and spreading on FN-coated wells, using the RT-CES
system
(B) Analysis of the dose-dependent effect of Latriculin on NIH3T3 cell
attachment and
spreading at two hours.
FIG. 10 depicts (A) Dynainic monitoring of the effect of the Src inhibitor,
PP2 on
BxPC3 cell attaclunent and spreading on FN, using the RT-CES system. (B)
Comparison
of the extent of cell attachment and spreading on FN in response to PP2
compared to
DMSO control at two hours.
FIG. 11 depicts (A) Dynamic monitoring of cell attachment and spreading using
the RT-
CES system of BxPC3 cells transfected with an siRNA specific for c-Src or a
control
siRNA. (B) Comparison of the extent of cell attachment and spreading of BxPC3
cells
transfected with c-Src siRNA or a control siRNA at two hours.
DETAILED DESCRIPTION
A. Definitions
For clarity of disclosure, and not by way of limitation, the detailed
description of
the invention is divided irito the subsections that follow.
Unless defined otherwise, all technical and scientific terms used herein have
the
same meaiiing as is commonly understood by one of ordinary skill in the art to
which this
invention belongs. All patents, applications, published applications and other
publications referred to herein are incorporated by reference in their
entirety. If a
defmition set forth in this section is contrary to or otherwise inconsistent
with a definition
set forth in the patents, applications, published applications and other
publications that
are herein incorporated by reference, the definition set forth in this section
prevails over
the defmition that is incorporated herein by reference.
As used herein_ '-a7' or "an" rneans "at least one" or "one or more."
8
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
As used herein. "membrane' is a sheet of material.
As used herein. "biocompatible membrane" means a membrane that does not hare
deleterious effects on cells, including the viabilitv, attachment, spreadinQ,
motility,
growth, or cell division.
A"biomolecular coating" or a"bioloaical molecule coating" is a coating on a
surface that comprises a molecule that is a naturally occurriilg biological
molecule or
biochemical, or a biochemical derived from or based on one or more naturally
occurring
biomolecules or biochemicals. For example, a biological molecule coating can
include an
extracellular matrix component (e.g., fibronectin, collagens), or a derivative
thereof, or
can comprise a biochemical such as polylysine or polyornithine, which are
polymeric
molecules based on the naturally occurring biochemicals lysine and ornithine.
Polyineric
molecules based on naturally occurring biochemicals such as amino acids can
use
isomers or enantiomers of the naturally-occuring biochemicals.
An "organic compound coating" is a coating on a surface that includes an
organic
compound. For example an organic compound may include a natural ligand or an
agonist
or an antagonist for a cell surface receptor.
An "extracellular matrix component" is a molecule that occurs in the
extracellular
matrix of an animal. It can be a component of an extracellular matrix from any
species
and from any tissue type. Nonlimiting examples of extracellular matrix
components
include laminins, collagens fibronectins, other glycoproteins, peptides,
glycosaminoglycans, proteoglycans, etc. Extracellular matrix components can
also
include growth factors.
An "electrode" is a structure having a high electrical conductivity, that is,
an
electrical conductivity much higher than the electrical conductivity of the
surrounding
materials.
As used herein, an "electrode structure" refers to a single electrode,
particularly
one with a complex structure (as, for example, a spiral electrode structure),
or a collection
of at least two electrode elements that are electrically connected together.
All the
electrode elements within an "electrode structurC are electrically connected.
9
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
As used herein. "electrode element" refers to a single st1-uctural feature of
an
electrode structure, such as, for example, a fmRerlil:e projection of an
interdigitated
electrode structure.
As used herein, an "electrode array" or "electrode structure unit" is two or
more
electrode structures that are constructed to have dimensions and spacing such
that they
can, when connected to a signal source, operate as a unit to generate an
electrical field in
the region of spaces around the electrode structures. Preferred electrode
structure units of
the present invention can measure impedance changes due to cell attachment to
an
electrode surface. Non-limiting examples of electrode structure units are
interdigitated
electrode structure units and concentric electrode structure units.
An "electrode bus" is a portion of an electrode that connects individual
electrode
elements or substructures. An electrode bus provides a common conduction path
from
individual electrode elements or individual electrode substructures to another
electrical
connection. In the devices of the present invention, an electrode bus can
contact each
electrode element of an electrode structure and provide an electrical
connection path to
electrical traces that lead to a connection pad.
"Electrode traces" or "electrically conductive traces" or "electrical
traces:', are
electrically conductive paths that extend from electrodes or electrode
elements or
electrode structures toward one end or boundary of a device or apparatus for
connecting
the electrodes or electrode elements or electrode structures to an impedance
analyzer.
The end or boundary of a device may correspond to the coiuiection pads on the
device or
apparatus.
A "connection pad" is an area on an apparatus or a device of the present
invention
which is electrically connected to at least one electrode or all electrode
elements within at
least one electrode structure on an apparatus or a device and which can be
operatively
connected to external electrical circuits (e.g., an impedance measurement
circuit or a
signal source). The electrical connection between a connection pad and an
impedance
measurement circuit or a signal source can be direct or indirect, throuah any
appropriate
electrical conduction means such as leads or wires. Such electrical conduction
means
mav also go through electrode or electrical conduction paths located on other
regions of
the apparatus or device.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
"Interdiaitated" nzeans having projections comina one direction that interlace
with
projections coininQ from a different direction in the manner of the fingers of
folded hands
(witl-i the caveat that interdiaitated electrode elements preferably do not
contact one
another).
As used lierein, a"hiQh probability of contactinQ an electrode elenlent" means
tllat, if a cell is randomly positioned within the sensor area of a device or
apparatus of the
present invention, the probability of a cell (or particle) contacting on an
electrode
eleinent, calculated from the averagge diameter of a cell used on or in a
device or
apparatus of the present invention, the sizes of the electrode elements, and
the size of the
gaps between electrode elements, is greater than about 50%, more preferably
greater than
about 60%, yet more preferably greater than about 70%, and even more
preferably
b eater than about 80%, greater than about 90%, or greater than about 95%.
As used herein, "at least two electrodes fabricated on said substrate" means
that
the at least two electrodes are fabricated or made or produced on the
substrate. The at
least two electrodes can be on the same side of the substrate or on the
different side of the
substrate. The substrate may. have multiple layers, the at least two
electrodes can be
either on the same or on the different layers of the substrate.
As used herein, "at least two electrodes fabricated to a same side of said
substrate" means that.the at least two electrodes are fabricated on the sanie
side of the
substrate.
As used herein, "at least two electrodes fabricated to a same plane of said
substrate" meall.s that, if the nonconducting substrate has multiple layers,
the at least two
electrodes are fabricated to the same layer of the substrate.
As used herein, "said ... electrodes (or electrode structures) have
substantially
the same surface area" means that the surface areas of the electrodes referred
to are not
substantially different from each other, so'that the impedance change due to
cell
attachment or gTowth on any one of the electrodes (or electrode'structures)
referred to
will contribute to the overall detectable change in impedance to a same or
similar deo-ree
as the impedance change due to cell attachment or growth on any other of the
electrodes
(or electrode structures) referred to. In other words, where electrodes (or
electrode
structures) have substantially the same surface area, any one of the
electrodes can
11
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
contribute to overall change in impedance upon cell attaclmzent or growth on
the
electrode. In most cases, the ratio of surface. area between the larQest
electrode and the
smallest electrode that have "substantially the same surface area" is less
than 10.
Preferably, the ratio of.surfac.e area between the largest electrode and the
smallest
electrode of an electrode array is less than 5, 4, 3, 2, 1.5, 1.2 or 1.1. More
preferably, the
at least two electrodes of an electrode structure have nearly identical or
identical surface
area.
As used herein, "said device has a surface suitable for cell attachment or
growth"
means that the electrode and/or non-electrode area of the apparatus has
appropriate
physical, chemical or biological properties such that cells of interest can
viably attach on
the surface and new cells can continue to attach, while the cell culture
grows, on the
surface of the apparatus. However, it is not necessary that the device, or the
surface
thereof, contain substances necessary for cell viability or growth. These
necessary
substances, e.g., nutrients or growth factors, can be supplied in a medium.
Preferably,
when a suspension of viable, unimpaired, epithelial or endothelial cells is
added to the
"surface suitable for cell attachment" when at least 50% of the cells are
adhering to the
surface within twelve hours. More preferably, a surface that is suitable for
cell
attaclunent has surface properties so that at least 70% of the cells are
adhering to the
surface within twelve hours of plating (i.e., adding cells to the chamber or
well that.
comprises the said device). Even more preferably, the surface properties of a
surface that
is suitable for cell attachment results in at least 90% of the cells adhering
to the surface
within twelve hours of plating. Most preferably, the surface properties of a
surface that is
suitable for cell attachment results in at least 90% of the cells adhering to
the surface
within eight, six, four, two hours of plating.
?5 As used herein, "detectable change in impedance between or among said
electrodes" (or "detectable change in impedance between or among said
electrode
structures") means that the impedance between or among said electrodes (or
electrode
structures) would have a significant change that can be detected by an
impedance
analyzer or impedance measurement circuit when molecule binding reaction
occurs on
the electrode surfaces. The impedance change refers to the difference in
impedance
values when molecule bindina reaction occurs on the electrode surface of the
apparatus
12
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
and when no molecular reaction occurs on the electrode surface. Alternatively,
the
impedance chanQe refers to the difference in impedance values when cells are
attached to
the electrode surface and -,A~hen cells are not attached to the electrode
surface, or when the
number, type, activity, adhesiveness, or morphology of cells attached to the
electrode-
comprising surface of the apparatus changes. In most cases, the change in
impedance is
larger than 0.1 % to be detectable. Preferably, the detectable change in
impedance is
larger than 1%, 2%, 5%, or 8%. More preferably, the detectable change in
impedance is
larger than 10%. linpedance between or among electrodes is typically a
function of the
frequency of the applied electric field for measurement. "Detectable change in
impedance between or among said electrodes" does not require the impedance
change at
all frequencies being detectable. "Detectable change in impedance between or
among
said electrodes" only requires a detectable change in impedance at any single
frequency
(or multiple frequencies). In addition, impedance has two components,
resistance and
reactance (reactance can be divided into two categories, capacitive reactance
and
inductive reactance). "Detectable change in impedance between or among said
electrodes" requires only that either one of resistance and reactance has a
detectable
change at any single frequency or multiple frequencies. In the present
application,
impedance is the electrical or electronic impedance. The method for the
measurement of
such impedance is achieved by, (1) applying a voltage between or among said
electrodes
at a given frequency (or multiple frequencies, or having specific voltage
waveform) and
monitoring the electrical current through said electrodes at the frequency (or
multiple
frequencies, or having specific waveform), dividing the voltage amplitude
value by the
current amplitude value to derive the impedance value; (2) applying an
electric current of
a single frequency component (or multiple frequencies or having specific
current wave
form) through said electrodes and monitoring the voltage resulted between or
among said
electrodes at the frequency (or multiple frequencies, or having specific
waveform),
dividing the voltage amplitude value by the current amplitude value to derive
the
impedance value; (3) other methods that can measure or determine electric
impedance.
Note that in the description above of "dividing the voltage amplitude value by
the current
amplitude value to derive the impedance value", the "division~' is done for
the values of
current amplitude and voltage amplitude at same frequencies. Measurement of
such
13
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
electric impedance is an electronic or electrical process that does not
involve the use of
anv rea2ents.
As used herein. "said at least two electrodes have substantially different
surface
area" means that the surface areas of any electrodes are not similar to each
other so that
the impedance change due to cell attachment or growth on the larger electrode
will not
contribute to the overall detectable impedance to a saine or similar degree as
the
impedance change due to cell attachment or growth on the smaller electrodes.
Preferably, any impedance change due to cell attachment or growth on the
larger
electrode is significantly smaller than the impedance change due to cell
attachment or
growth on the smaller electrode. Ordinarily, the ratio of surface area between
the largest
electrode and the smallest electrode is more than 10. Preferably, the ratio of
surface area
between the largest electrode and the smallest electrode is more than 20, 30,
40, 50 or
100.
As used herein, "multiple pairs of electrodes or electrode structures
spatially
arranged according to wells of a multi-well microplate" means that the
multiple pairs of
electrodes or electrode structures of a device or apparatus are spatially
arranged to match
the spatial configuration of wells of a multi-well microplate so that, wlien
desirable, the
device can be inserted into, joined with, or attached to a multiwell plate
(for example, a
bottomless multiwell plate) such that multiple wells of the multi-well
microplate will
comprise electrodes or electrode structures.
As used herein, "arranged in a row-colunm configuration" means that, in terms
of
electric connection, the position of an electrode, an electrode array or a
switching circuit
is identified by both a row position number and a column position number.
As used herein, "each well contains substantially same number ... of cells"
means that the lowest number of cells in a well is at least 50% of the highest
number of
cells in a well. Preferably, the lowest number of cells in a well is at least
60%, 70%,
80%, 90%, 95% or 99% of the hiahest number of cells in a well. More
preferably, each
well contains an identical number of cells.
As used herein, "each well contains...same type of cells" means that, for the
intended purpose, each well contains same type of cells; it is not necessary
that each well
contains exactly identical type of cells. For example, if the intended purpose
is that each
14
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
well contains manuzialian cells, it is permissible if each well contains same
type of
manunalian cells, e.c., hunlan cells, or different mammalian cells, e.g.,
hunian cells as
well as other non-human manunalian cells such as mice, goat or monkey cells,
etc.
As used herein, "each well contains . . . serially different concentration of
a test
compound" means that each well contains a test compound with a serially
diluted
concentrations, e.g., an one-tenth serially diluted concentrations of 1 M, 0.1
M, 0.01 M,
etc.
As used herein, "dose-response curve" means the dependent relationship of
response of cells on the dose concentration of a test compound. The response
of cells can
be measured by many different parameters. For example, a test compound is
suspected to
have cytotoxicity and cause cell death. Then the response of cells can be
measured by
percentage of non-viable (or viable) cells after the cells are treated by the
test compound.
Plotting this percentage of non-viable (or viable) cells as a function of the
does
concentration of the test compound constructs a dose response curve. In the
present
application, the percentage of non-viable (or viable) cells can be expressed
in terms of
measured impedance values, or in terms of cell index derived from impedance
measurement, or in terms of cell change indexes. For exanlple, for a give cell
type and
under specific cellular physiological condition (e.g., a particular cell
culture medium),
cell index can be shown to have a linear correlation or positive correlation
with the
number of viable cells in a well from which cell index was derived from the
impedance
measurement. Thus, in the present application, one can plot cell index as a
function of
the dose concentration of the test compound to construct a "dose-response
curve". Note
that, generally, cell index not only correlate with the number of viable cells
in the wells
but also relate to the cell morphology and cell attachment. Thus plotting cell
index
versus doss concentration provides information not only about number of cells
but also
about their physiological status (e.g. cell morphology and cell adhesion).
Furthermore,
an important advantage offered by the system and devices of the present
i.nvention is that
in a sinale experiment, one can obtain "dose-response curves" at multiple time
points
since the system allows for the continuous monitoring of cells and provides
impedance
measurement at many time points over a time range as short as a few minutes to
as long as days or weeks.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
As used herein. "the electrodes have. alonR the lenath of the microchaimel. a
lenath that is substantially less than the largest single-dimension of a
particle to be
analyzed" means that the electrodes have, alona the len,th of the
microchannel, a length
that is at least less than 90% of the laraest sinale-dimension of a particle
to be analyzed.
Preferably, the electrodes have, along the length of the microchaimel, a
length that is at
least less than 80%, 70%, 60%, 50%, 40%, 30%, 20%, 10%, 5% of the largest
single-
dimension of a particle to be analyzed.
As used herein, "the microelectrodes span the entire height of the
microchannel"
means that the microelectrodes span at least 70% of the entire height of the
microchannel.
Preferably, microelectrodes span at least 80%, 90%, 95% of the entire height
of the
rnicrochamlel. More preferably, microelectrodes span at least 100% of the
entire height
of the microchannel.
As used herein, "an aperture having a pore size that equals to or is slightly
larger
than size of said particle" means that aperture has a pore size that at least
equals to the
particle size but less than 300% of the particle size. Here both pore size and
particle size
ar'e measured in terms of single dimension value.
As used herein, "microelectrode strip or electrode strip" means that a non-
conducting substrate strip on which electrodes or electrode structure units
are fabricated
or incorporated. The non-limiting examples of the non-conducting substrate.
strips
include polymer membrane, glass, plastic sheets, ceramics, insulator-on-
semiconductor,
fiber glass (like those for manufacturing printed-circuits-board). Electrode
structure units
having different geometries can be fabricated or made on the substrate strip
by any
suitable microfabrication, micromachining, or other methods. Non-limiting
examples of
electrode geometries include uzterdigitated electrodes, circle-on-line
electrodes, diamond-
on-line electrodes, castellated electrodes, or sinusoidal electrodes.
Characteristic
dimensions of these electrode geometries may vary from as small as less than 5
micron,
or less than 10 micron, to as large as over 200 micron, over 500 micron, over
1 mm. The
characteristic dimensions of the electrode geometries refer to the smallest
width of the
electrode elements, or smallest gaps between the adjacent electrode elements,
or size of a
repeatirig feature on the electrode geometries. The microelectrode strip can
be of any
geometry for the present invention. One exemplary aeometry for the
microelectrode
16
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
strips is rectanaular shape - having the width of the strip between less than
50 nucron to
over 10 mm. and having the length of the strip betu~een less than 60 micron to
over 15
mm. An exemplary geometryo of the microelectrode strips may have a geometry
having a
width of 200 micron and a length of 20 nun. A sinale microelectrode strip may
have two
electrodes serving as a measurement unit, or multiple such two-electrodes
serving as
multiple measurement units, or a sinQle electrode structure unit as a
measurement unit, or
multiple electrode structure units serving as multiple electrode structure
units. In one
exemplary embodiment, when multiple electrode structure units are fabricated
on a single
microelectrode strip, these electrode structure units are positioned along the
lengtll
direction of the strip. The electrode structure units may be of squared-shape,
or
rectangular-shape, or circle shapes: Each of electrode structure units may
occupy size
from less than 50 micron by 50 micron, to larger than 2 nun x 2mm.
As used herein, "sample" refers to anything which may contain a moiety to be
isolated, manipulated, measured, quantified, detected or analyzed using
apparatuses,
microplates or methods in the present application. The sample may be a
biological
sample, such as a biological fluid or a biological tissue. Examples of
biological fluids
include suspension of cells in a medium such as cell culture medium, urine,
blood,
plasma, serum, saliva, semen, stool, sputum, cerebral spinal fluid, tears,
mucus, amniotic
fluid or the like. Biological tissues are aggregates of cells, usually of a
particular kind
together with their intercellular substance that form one of the structural
materials of a
human, ai-dmal, plant, bacterial, fungal or viral structure, including
connective,
epithelium, muscle and nerve tissues. Examples of biological tissues also
include organs,
tumors, lymph nodes, arteries and individual cell(s). The biological samples
may further
include cell suspensions, solutions containing biological molecules (e.g.
proteins,
enzymes, nucleic acids, carbohydrates, chemical molecules binding to
biological
molecules).
As used herein, a "liquid (fluid) sample" refers to a sample that naturally
exists as
a liquid or fluid, e.g., a biological fluid. A "liquid sample" also refers to
a sample that
naturally exists in a non-liquid status, e.g.. solid or gas, but is prepared
as a liquid. fluid,
solution or suspension containing the solid or gas sample material. For
example, a liquid
17
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
sample can encompass a liquid. fluid_ solution or suspension contairuna a
biological
tissue.
A"compound" or "test compound" is any compound whose activity or direct or
indirect effect or effects on cells is investiQated in any assay. A test
compound can be
any compound, including, but not limited to, a small molecule, a large
molecule, a
molecular complex, an organic molecule, an inorganic molecule, a biomolecule
or
biological molecule such as but not limited to a lipid, a steroid, a
carbohydrate, a fatty
acid, an amino acid, a peptide, a protein, a nucleic acid, or any combination
thereof. A
test compound can be a synthetic compound, a naturally occurring compound, a
derivative of a naturally-occurring compound, etc. The structure of a test
compound can
be known or unknown. In one application of the present invention, a compound
is
capable of, or is suspected of, effecting cell adhesion or cell spreading. In
another
application of present invention, a compound is capable of, or is suspected
of, stimulating
or inhibiting cell adhesion or cell spreading. In still another application, a
compound is
capable of, or is suspected of, interacting witli cells (for example, binding
to cell surface
receptor, or inhibiting certain intracellular signal transduction pathway, or
activating
cells).
A "known compound" is a compound for which at least one activity is known. In
the present invention, a known compound preferably is a compound for which one
or
more direct or indirect effects on cells is known. Preferably, the structure
of a known
compound is known, but this need not be the case. Preferably, the mechanism of
action of
a lcnown compound on cells is luiown, for example, the effect or effects of a
known
compound on cells can be, as nonlimiting examples, effects- on cell viability,
cell
adhesion, apoptosis, cell differentiation, cell proliferation, cell
morphology, cell cycle,
IaE-mediated cell activation or stimulation, receptor-ligandbinding, cell
number, cell
quality, cell cycling, cell adhesion, cell spreading, etc.
An "impedance value" is the impedance measured for electrodes in a well with
or
without cell present. Impedance is generally a function of the frequency,
i.e., impedance
values depend on frequencies at ~~hich the measurement was conducted. For the
present
application, impedance value refers to impedance measured at either single
frequency or
multiple frequencies. Furthermore, impedance has two components, one
resistance
18
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
component and one i-eactance component. Impedance value in the present
application
refers to resistailce component, or reactance component, or both resistance
and reactance
component. Thus. when "impedance value" was measured or monitored, we are
referring
to that, resistance, or reactance, or both resistance and reactance were
measured or
monitored. In many embodiments of the methods of the present application,
impedance
values also refer to parameter values that are derived from raw, measured
impedance
data. For exaniple, cell index, or normalized cell index, or delta cell index
could be used
to repi-eseiit inipedance values.
A "Cell Index" or "CI" is a parameter that can derived from measured impedance
values and that ca.n be used to reflect the change in impedance values. There
are a
number of methods to derive or calculate Cell Index.
A"Normalized Cell Index" at a given time point is calculated by dividing the
Cell
Index at the time point by the Cell Index at a reference time point. Thus, the
Normalized
Cell Index is 1 at the reference time point.
A "delta cell index" at a given time point is calculated by subtracting the
cell
index at a standard time point frorim the cell index at the given time point.
Thus, the delta
cell index is the absolute change in the cell index from an initial time (the
standard time
point) to the measurement time.
A "Cell Change Index" or "CCI" is a parameter derived from Cell Index and
"CCI" at a time point is equal to the l st order derive of the Cell Index with
respect to
time, divided by the Cell Index at the time point. In other words, CCI is
calculated as
CCI (t) = dCl (t)
CI (t) = dt
As used herein, "target cell" or "target cells" refers to any cell that is to
be
monitored for adhesion or spreading. Non-limiting examples of target cells
include
eukaryotic or prokaryotic cells of interest. Eukaryotic cells of particular
interest may be
human cells, a human cell population or a human cell line. Immune cells may be
utilized
such as B-lymphocytes, T-lymphocytes Natural Killer (NK) cells, Cytotoxic T-
Lymphocytes (CTLs), neutrophils, easonophils, macrophages, Natural Killer
T(1'KT)
cells, PBMCs and the like.
19
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
As used herein. "primary cell" or "priniary cells" refers to any non-
inimortalized cell
that has been derived from various tissues and oraans of a patient or an
animal.
B. Devices and systems for monitoring cell-substrate impedance
Devices for Measurina Cell-Substrate Impedance
The present invention includes devices for measuring cell-substrate impedance
that comprise a nonconducting substrate; two or more electrode arrays
fabricated on the
substrate, where each.of the two or more electrode arrays comprises two
electrode
structures; and at least two connection pads, each of wliich is located on an
edge of the
substrate. Each electrode array of the device may have approximately u.iliform
electrode
resistance across the entire array. The substrate of the device has a surface
suitable for
attaching a biological molecule or organic coinpound (such as covalentl.y or
noncovelently bonding). The substrate may also be suitable for a attaching a
cell where
cell attachment or spreading on the substrate can result in a detectable
change in
impedance between or among the electrode structures within each electrode
array.
An electrode array is two or more electrode structures that are constructed to
have
dimensions and spacing such that they can, when connected to a signal source,
operate as
a unit to generate an electrical field in the region of spaces around the
electrode
structures. An electrode structure refers to a single electrode, particularly-
one with a
complex structure. (For example, an electrode structure can comprise two or
more
electrode elements that are electrically connected together.) In devices of
the preserit
invention, an electrode array comprises two electrode structures, each of
which comprises
multiple electrode elements, or substructures. In preferred embodiments of the
present
invention, the electrode structures of each of the two or more electrode
arrays of a device
have substantially the same surface area. In preferred embodiments of a device
of the
present invention, each of the two or more electrode arrays of a device
comprise two
electrode structures, and each electrode structure comprises multiple
electrode elements.
Each of the two electrode structures of an electrode array is connected to a
separate
connection pad that is located at the edge of the substrate.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Thus_ in devices of the present invention, for each of the two or more
electrode
arrays of the device. the first of the two electrode structures is connected
to one of the
two or more connection pads, and the second of the two elecuode structures is
connected
to another of the two or more connection pads. Preferably, each array of a
device is
individually addressed; meaning that the electrical traces and connection pads
of the
arrays are configured such that an array can be connected to an impedance
analyzer in
such a way that a measuring voltage can be applied across a single array at a
given time
by using switches (such as electronic switches).
Each electrode array of the device has an approximately uniform electrode
resistance distribution across the entire array. By "uniform resistance
distribution across
the array" is meant that when a measurement voltage is applied across the
electrode
sti-uctures of the array, the electrode resistance at any given location of
the array is
approximately equal to the.electrode resistance at ariy other location on
the.array.
Preferably, the electrode resistance at a first location on an array of the
device and the
electrode resistance at a second location on the same array does not differ by
more than
30%. More preferably, the electrode resistance at a first location on an array
of the device
and the electrode resistance at a second location on the same array does not
differ by
more than 15%. Even more preferably, the electrode resistance at a first
location on an
array of the device and a second location on the same array does not differ by
more than
5%. More preferably yet, the electrode resistance at a first location on an
array of the
device and a second location on the same array does not differ by more than
2%.
For a device of the present invention, preferred arrangements for the
electrode
elements, gaps between the electrodes and electrode buses in a given electrode
array are
used to allow all cells, no matter where they land and attach to the electrode
surfaces, to
contribute similarly to the total impedance change measured for the electrode
array. Thus,
it is desirable to have similar electric field strengths at any two locations
within any given
a.rray of the device when a measurement voltage is applied to the electrode
array. At any
aiven location of the array, the field streng-th is related to the potential
difference between
the nearest point on a first electrode structure of the array and the nearest
point on a
second electrode structure of the array. It is therefore desirable to have
similar electric
potential drops across the electrode elements and across the electrode buses
of a Qiven
21
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
arrav. Based on this requirement, it is preferred to have an approximately
uniform
electrode resistance distribution across the whole array where the electrode
resistance at a
location of interest is equal to the sum of the electrode resistance between
the nearest
point on a first electrode structure (that is the point on the first electrode
structure nearest
the locatiori of interest) and a first connection pad connected to the first
electrode
structure and the electrode resistance between the nearest point on a second
electrode
structure (that is the point on the first electrode structure nearest the
location of interest)
and a second connection pad connected to the second electrode structure.
Devices of the present invention are designed such that the arrays of the
device
have an approximately uniform distribution across the whole array. This can be
achieved,
for example, by having electrode structures and electrode buses of particular
spacing and
dimensions (lengths, widths, thiclcnesses and geometrical shapes) such that
the resistance
at any single location on the array is approximately equal to the resistance
at any single
other location on the array. In most embodiments, the electrode elements (or
electrode
structures) of a given array will have even spacing and be of similar
thicknesses and
widths, the electrode buses of a given array will be of similar thicknesses
and widths, and
the electrode traces leading from a given array to a connection pad will be of
closely
similar thicknesses and widths. Thus, in these preferred embodiments, an array
is
designed such that the lengths and geometrical shapes of electrode elements or
structures,
the lengths and geometrical shapes of electrode traces, and the lengths and
geometrical
shapes of buses allow for approximately uniform electrode resistance
distribution across
the array.
In some preferred embodiments of cell-substrate impedance measurement
devices, electrode structures comprise multiple electrode elements, and each
electrode
element connects directly to an electrode bus. Electrode elements of a first
electrode
structure connect to a first electrode bus, and electrode elements of a second
electrode
structure connect to a second electrode bus. In these embodiments, each of the
two
electrode buses connects to a separate connection pad via an electrical trace.
Although the
resistances of the traces contribute to the resistance at a location on the
array, for any two
locations on the array the trace connections from the first bus to a first
connection pad
and from the second bus to a second connection pad are identical. Thus, in
these
T)
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
preferred embodiments trace resistances do not need to be tahen into account
in designina
the geometry of the array to provide for uniform resistances across the array.
In preferred embodiments of the present invention, a device for monitoring
cell-
substrate impedance has two or more electrode arrays that share a connection
pad.
Preferably one of the electrode structures of at least one of the electrode
arrays of the
device is comlected to a connection pad that also connects to ati electrode
structure of at
least one other of the electrode arrays of the device. Preferably for at least
two arrays of
the device, each of the two or more arrays has a first electrode structure
connected to a
connection pad that connects with an electrode structure of at least one other
electrode
array, and each of the two or more arrays has a second electrode structure
that connects to
a connection pad that does not connect with any other electrode structures or
arrays of the
device. Thus, in preferred designs of a device there are at least two
electrode arrays each
of which has a first electrode structure that is connected to a common
connection pad and
a second electrode structure that is connected to an independent connection
pad.
In some preferred embodiments of the present invention, each of the electrode
structures of an array is connected to an electrode bus that is connected to
one of the two
or more connection pads of the device via an electrically conductive trace. In
preferred
embodiments, each of the two electrode structures is connected to a single
bus, such that
each array connects to two buses, one for each electrode structures. In this
arrangement,
each of the two buses connects to a separate connection pad of the substrate.
The electrically conductive traces that connect a bus with a connection can be
fabricated of any electrically conductive material. The traces can be
localized to the
surface of the substrate, and can be optionally covered with an insulating
layer.
Alternatively the traces can be disposed v.1 a second plane of the substrate.
Description of
arrangements and design of electrically conductive traces on impedance
measurement
devices can be found in parent U.S. Patent Application 10/705,447, herein
incorporated
by reference for all disclosure on fabrication and design of electrically
conductive trace
on substrates.
Appropriate electronic connection means such as metal clips engaged onto the
connection pads on the substrate and connected printed-circuit-boards can be
used for
leading the electronic connections from the connection pads on the devices to
ea-ternal
~
2
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
electronic circuitn? (e.g. an impedance anal}-zer). Description of the design
of cell-
subst rate impedance devices and their manufacture can be found in U.S. Patent
Application No. 10/705,447, herein incorporated by reference for all
description and
disclosure of the design, features, and manufacture of impedance device
comprising
electrode arrays.
Preferably the nonconducting substrate is planar, and is flat or
approxi.mately flat.
Exemplary substrates can comprise many materials, including, but not limited
to, silicon
dioxide on silicon, silicon-on-insulator (SOI) wafer, glass (e.g., quartz
glass, lead glass or
borosilicate glass), sapphire, ceramics, polymer, fiber glass, plastics, e.g.,
polyimide (e.g.
Kapton, polyimide film supplied by DuPont), polystyrene, polycarbonate,
polyvinyl
chloride, polyester, polypropylene and urea resin. Preferably, the substrate
and the
surface of the substrate are not going to interfere with molecular binding
reactions that
will occur at the substrate surface. For cell-substrate impedance monitoring,
any surface
of the nonconducting substrate that can be exposed to cells during the use of
a device of
the present invention is preferably biocompatible. Substrate materials that
are not
biocompatible can be made biocompatible by coating with another material, such
as
polymer or biomolecular coating.
All or a portion of the surface of a substrate can be chemically treated,
including
but not limited to, modifying the surface such as by addition of functional
groups, or
addition of charged or hydrophobic groups.
In some embodiments a portion of the surface of the substrate is modified to
display a biological molecule or organic compound of interest. Example of
biological
molecules or organic compounds that may be desired include those that are
involved or
may be involved in cell adhesion or cell spreading. The present invention
includes a
variety of biological molecules and organic compounds including a DNA
molecule, an
RNA molecule, a protein, a polypeptide and oligopeptide and the like.
Molecules of
particular interest may include an antibody, a ligand, a peptide, a receptor,
one or more
proteins or compounds present in the extracellular matrix (ECM), a molecule or
compound capable of binding an integrin, a cell surface receptor and the like.
In some
embodiments a peptide such as an argi.nine-glycine-aspartic acid (RGD) motif
or some
form thereof is the biological molecule. The present i_nvention also includes
organic
24
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
compounds that are agonists or antaRonists for a cell surface receptor
involved in cell
adhesion, includina integrins, _-rowth factor receptors. E-cadherins. N-
cadherins.
PECAMS and ICAMS.
The modification may ultimately result in a coated surface or a surface that
is
coated at least in part with a biological molecule or organic compound. The
coated
portion may represent a first portion, a second portion and the like. The
region may also
be referred to as a test portion or a control portion depending on the assay.
Wlien
utilizing wells with the present invention, an inner surface of the wells may
be coated at
least in part with a biological molecule or organic compound. The biological
molecule
or organic compound may interact with the substrate in any suitable fashion
that would
result in display of a biological molecule or organic compound. For example,
the
biological molecule or organic compound may be covalently bound, ionically
bound,
bound by Van der Waals forces and the like to the substrate or electrode.. The
biological
rriolecule or organic compound may be attached directly to the substrate or
electrode or
may be attached via an intermediate structure. As a nonlimiting example, a
biological
molecule or compound may be bound by incubating the molecule or compound in a
suitable medium such as phosphate buffered saline (PBS), borate buffered
saline (BBS)
and the like. Alternatively, an intermediate such as poly-L-lysine may be
applied to the
substrate then attached to the biological molecule or organic compound.
Descriptions of electrode arrays used for impedance measurement that apply to
the devices of the present invention are described in U.S. Patent Application
No.
10/705,447,.herein incorporated by reference for all disclosure relating to
electrode arrays
(or structural units), electrode structures, electrode materials, electrode
dimensions, and
methods of manufacturing electrodes on substrates.
Preferred electrode arrays for devices of the present invention include arrays
comprising two electrode structures, such as, for example, spiral electrode
arrays and
interdigitated arrays. In some preferred devices of the present invention,
electrode arrays
are fabricated on a substrate, in which the arrays comprises two electrode
structures, each
of which comprises multiple circle-on-line electrode elements, in which the
electrode
elements of one structure.altemate with the electrode elements of the opposite
electrode
s micture.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Preferablv. the electrode elements (or electrode structures) of an array of
the
present device of the present_invention are of approximately equal widths.
Preferably the
electrode elements (or electrode structures) of an array of the present device
of the
present invention are greater than 30 microns in width, more preferably from
about 50 to
about 300 inicrons in width, and nlore preferably yet about 90 microns in
width.
Preferably, the electrode elenients (or electrode structures) of an array of
the
present device of the present invention are approximately evenly spaced.
Preferably, the
gap between electrode elements (or electrode structures) of an array of the
present device
of the present invention is less than 50 microns in width, more preferably
from about 5 to
about 30 microns in width, and more preferably yet about 20 microns in width.
A device of the present invention can include one or more fluid-impermeable
receptacles which serve as fluid containers. Such receptacles may be
reversibly or
irreversibly attached to or formed within the substrate or portions thereof
(such as, for
example, wells formed as in a microtiter plate). In another exaniple, the
device of the
present invention includes microelectrode strips reversibly or irreversibly
attached to
plastic housings that have openings that correspond to electrode structure
units located on
the microelectrode strips. Suitable fluid container materials comprise
plastics, glass, or
plastic coated materials such as ceramics, glass, metal, etc. Descriptions and
disclosure of
devices that comprise fluid containers can be found in parent U.S. Patent
Application No.
10/705,447, herein incorporated by reference for all disclosure of fluid
containers and
fluid container structures that can engage a substrate comprising electrodes
for
impedance measurements, including their dimensions, design, composition, and
methods
of manufacture.
In preferred embodiments, each electrode array on the substrate of a device of
the
present invention is associated with a fluid-impermeable container or
receptacle, such as,
for example, a well. Preferably, the device of the present invention is
assembled to a
bottomless; multiwell plastic plate or strip with a fluid tight seal. The
device is
assembled such that a single array of the substrate is at the bottom of a
receptacle or well.
Preferably, each array of a device is associated with a well of a multiwell
plate. In some
preferred embodiments, a multiwell device for cell-substrate impedance
measurement has
"non-array" wells that are attached to the substrate but not associated with
arrays. Such
26
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
wells can optionally be used for performina non-impedance based assays, or for
vielAina
cells microscopically.
The design and assembly of multiwell impedance measurement devices is
described in U.S. Patent Application No. 10/705,447_ and also in U.S. Patent
Application
No. 10/987,732, both herein incorporated by reference for disclosure of
multiwell
impedance measurement devices, including their design, composition, and
manufacture.
A device of the present invention preferably has between 2 and 1,536 wells,
more
preferably between 4 and 384 wells, and even more preferably, between 16 and
96 wells,
all or less than all or which are associated with electrode arrays.
In some preferred embodiments, commercial tissue culture plates can be adapted
to fit a device of the present invention. Bottomless plates may also be custom-
made to
preferred dimensions. Preferably, well diameters are from about 1 millimeter
to about 20
millimeters, more preferably from about 2 millimeters to about 8 inillimeters
at the
bottom of the well (the end disposed on the substrate). The wells can have a
uniform
diameter or can taper toward the bottom so that the diameter of the container
at the end in
contact with the substrate is smaller than the diameter of the opposing end.
Methods of Use
The present invention also includes methods of using a device of the present
invention that comprises fluid containers situated over electrode arrays to
measure cell-
substrate impedance. Such methods include: providing a device of the present
invention
optionally including wells or fluid chambers situated over electrode arrays,
coating at
least in part the substrate or optionally the wells with a biological molecule
or organic
compound, attaching an impedance analyzer to a device of the present
invention, adding
cells to one or more fluid containers of the device, and measuring impedance
over one or
more arrays of the device. Methods of.performing cell assays using impedance
measurement devices can be found in parent U.S. Patent Application No.
10/987,732 and
U.S. Patent Application 10/705,447, both herein incorporated by reference for
all
disclosure of methods of using impedance measurement devices, as well as in
Sections D
and E of the present application.
27
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Cell-Substrate Impedance Measurement Systems
In another aspect, the present invention is directed to a cell-substrate
impedance
measurement svstem comprising a) at least one multiple-well cell-substrate
impedance
measuring device, in which at least two of the multiple wells comprise an
electrode array
at the bottom of the well and include a biolocrical molecule or or(yanic
compound coating;
b) an impedance analyzer electronically connected to the multiple-well cell-
substrate
impedance measuring device; c) a device station capable of engaging the one or
more
multiple-well devices and comprising electronic circuitry capable of selecting
and
connecting electrode arrays within any of the multiple wells to the impedance
analyzer;
and d) a software program connected to the device station and impedance
analyzer to
control the device station and perform data acquisition and data analysis from
the
iinpedance a.nalyzer.
In a cell-substrate impedance measurement system of the present invention, the
impedance analyzer engages connection pads of one or more multi-well devices
to
measure iinpedance. In one embodiment of the above system, the impedance
analyzer is
capable of measuring impedance between 0.1 ohm and 105 ohm in frequency range
of
1 Hz to 1 MHz. The impedance analyzer is preferably capable of measuring both
resistance and reactance (capacitive reactance and inductive reactance)
components of the
inipedance. In a preferred embodiment of the above system, the impedance
analyzer is
capable of measuring impedance between 0.1 ohm and 103 ohm in frequency range
of
100 Hz to 100 kHz.
A cell-substrate measurement system can be used to efficiently and
simultaneously
perform multiple assays by using circuitry of the device station to digitally
switch from
recording from measuring impedance over an array in one well to measuring
impedance
over an array in another well. In one embodiment of the above system, the
system under
software control is capable of completing an impedance measurement for an
individual
well at a single frequency within less than ten seconds. In another
embodiment, the
averaged time used by the system to complete an impedance measurement for an
individual well at a single frequency is less than one second.
A multiple-well cell-substrate impedance measuring device in a system of the
present
invention can be any multiple-well cell-subst.rate impedance measuring device
in which
28
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
at least two of the multiple wells comprise an electrode array at the bottom
of the well,
and in which at least two of the multiple wells comprise an electrode array
are
individually addressed. In one embodiment of the above system, the multi-well
device
takes the form of a specialized microtiter plate which has microelectronic
sensor arrays
integrated into the bottom of the wells and a biological molecule or organic
compound
covalently or noncovelently bound thereto.
A device used in a system of the present invention, when connected to an
impedance analyzer, can measure differences in impedance values that relate to
cell
behavior. For example, a cell-substrate impedance measuring device used in a
system of
the present uivention can measure differences in impedance values when cells
are
attached to the electrode array and when cells are not attached to the
electrode array, or
can detect differences in impedance values when the number, type, activity,
adhesiveness, or morphology of cells attached to the electrode-comprising
surface of the
apparatus changes. In particular the present invention can detect adhesion of
cells as well
as cell spreading.
Preferred devices that can be part of a cell-substrate impedance monitoring
system can be those described in U.S. Patent Application No. 10/705,447, and
in U.S.
Patent Application No. 10/987,732, both herein incorporated by reference for
disclosure
of cell-substrate impedance monitoring devices that comprise electrode arrays,
including
disclosure of their design, composition, and manufacture. Preferred devices
that can be
part of a cell-substrate impedance monitoring system can also be those
described in the
present application.
Preferably a multi-well device of a system of tlie present invention comprises
between 4 and 1,536 wells, some or all of which can comprise electrode arrays.
In some
embodiments of the present invention, a device station can comprise one or
more
platforms or one or more slots for positioning one or more multiwell devices.
The one or
more platforms or one or more slots can comprise sockets, pins or other
devices for
electrically comiecting the device to the device station. The device station
preferably can
be positioned in a tissue culture incubator during cell impedance measurement
assays. It
can be electrically connected to an impedance analyzer and computer that are
preferably
located outside the tissue culture incubator.
29
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
The device station comprises electronic circuitq that can cotinect to an
impedance monitorinR device and an impedance analvzer and electronic switches
that can
sWitch on and off connections to each of the two or more electrode arrays of
the
rnultiwell devices used in the system. The switches of the device station are
controlled by
a software program. The software program directs the device station to
coiuiect arrays of
the device to an impedance analyzer and monitor impedance from one or more of
the
electrode arrays. During impedance monitoring, the impedance analyzer can
monitor
impedance at one frequency or at more than one frequency. Preferably,
impedance
naonitoring is performed at more than one time point for a given assay, and
preferably,
impedance is monitored at at least three time points. The device station can
connect
individual arrays of a device to an impedance analyzer to monitor one, some,
or all of the
arrays of a device for a measurement time point. The switches of the device
station allow
the selected 'uldividual arrays to be monitored in rapid succession for each
desired
monitoring time point. Each monitoring time point is in fact a narrow time
frame (for
example from less than one second to minutes) of measurement in the assay
during which
impedance monitoring is performed. In some preferred embodiments of the
present
invention, the device station software is programmable to direct irripedance
monitoring of
any of the wells of the device that comprise arrays at chosen time intervals.
The software of the impedance monitoring system can also store and display
data.
Data can be displayed on a screen, as printed data, or both. Preferably the
software can
allow entry and display of experimental parameters, such as descriptive
information
including cells types, compound concentrations, time intervals monitored, etc.
Preferably, the software can also analyze impedance data. In preferred
embodiments, the software can calculate a cell index (CI) for one or more time
points for
one or more wells of the multiwell device. In some preferred embodiments, the
software
can calculate a cell change index (CCI) from impedance measurements of one or
more
wells of the multiwell device. The software can preferably generate plots of
impedance
data and impedance values, such as but not limited to CI or CCI, with respect
to time.
The softvvare may perform other analysis as well, such as calculate cell
number from CI,
generate dose-response curves based on impedance data, calculate IC values
based on
impedance values, and calculate kinetic parameters of cell orowth or behavior
based on
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
inipedance values and impedance value curves. The software of the impedance
nionitoring system can also store and display analyses of the data. such as
calculated
impedance values and kinetic parameters derived therefrom, Data can be
displayed on a
screen, as printed data, or both.
C. Methods for Calculating Cell Index (CI) and Cell Change Index (CCI)
Cell Index
Based on the dependent relationship between the measured impedance, cell
number (more accurately, the viable cell number, or attached cell number) and
cell
attachment status, it is possible to derive a so-called "cell number uidex" or
"cell index"
from the measured impedance frequency spectra that provides a useful index for
quantitating and comparing cell behavior in the impedance-based assays of the
present
invention. In some applications of the present invention, "cell index" in the
present
application is the same as "cell number index" in PCT Application No.
PCT/US03/22557,entitled "IMPEDANCE BASED DEVICES AND METHODS FOR
USE IN ASSAYS", filed on July 18, 2003 and in United States patent application
No.
10/705,447,entitled "IMPEDANCE BASED DEVICES AND METHODS FOR USE IN
ASSAYS," Attorney Docket No. ACE-00101.P.1.1-US, filed on November 10, 2003.,
U.S. Patent Application No. 10/987,732, filed November 12, 2004, U.S. Patent
application 10/705,447 and PCT Application No. PCT/US03/22557 are hereby
incorporated by reference for the discussions and disclosures of cell index
and cell
number index they contain.
Various methods for calculating such a cell number index can be used, some of
which are novel methods disclosed herein.
The present invention provides several methods of calculating cell index
numbers
for cells attached to two or more essentially identical arrays of a cell-
substrate impedance
device, where the cells are monitored for impeda.nce changes. In preferred
embodiments
of the present invention, the methods calculate cell index number with better
accuracy
than previous methods of calculating cell index for cells on two or more
arrays of a cell-
31
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
substrate monitorina device. In some preferred methods of the present
invention, methods
of calculatina a cell index rely on novel methods for calculatina the
resistances of
electrical traces leading to two or more essentially identical arrays. The
present invention
therefore also includes methods of calculating resistances of electrical
traces leading to
two or inore essentially identical arrays on a substrate.
By "essentially identical electrode arrays" or "essentially identical arrays'
is
meant that the dimensions and arrangement of electrodes, electrode structures,
and
electrode elements is the same for the referenced arrays. Thus, two
essentially identical
electrode arrays will have electrode structures of the same dimensions
(length, width,
thickness), where the electrode structures have the same number of electrode
elements,
and the arrangement of electrode structures and electrode elements in each
array are the
same. By arrangement is meant the distance between structures or elements (gap
widtli),
their physical position with respect to one another, and their geometry
(angles, degree of
curvature, circle-on-line or castellated geometries, etc.), including the same
features of
any electrode buses that may be connected to electrode structures or electrode
elements.
Electrodesof essentially identical arrays also comprise the same materials.
For the
purposes of calculating trace resistances and cell index number, a substrate
can have any
number of essentially identical arrays.
The following discussion provides novel methods of calculating cell index of
cells
adhered to arrays of a cell-substrate impedance monitoring device and novel
methods for
the calculation of the resistances of the electrical connection traces leading
to two or
more electrode arrays of a cell-substrate impedance monitoring device.
Impedance (Z) has two components, namely the resistance Rs and reactance
Xs. Mathematically, the impedance Z is expressed as follows,
Z=Rs+j Xs, (2)
where jdepicting that for the (serial) reactance component Xs, the
voltage applied over it is 90 degree phased-out from the current Qoing through
it. For the
(serial) resistance, the volta6e applied over it is in phase with the current
going through it.
32
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
As it is well-l:noArn in electronic and electrical engineering, the impedance
can also be
expressed in tenns of parallel resistance Rp and parallel reactance Xp, as
follows.
Z-RP*o Xp)/(Rp+j Xp), (3)
where jI. Nevertheless, these expressions (serial resistance and serial
reactance, or
parallel resistance and parallel reactance) are equivalent. Those who are
skilled in
electrical and electronic engineering can readily derive one form of
expression from the
parameter values in the other expression. For the sake of clarity and
consistency, the
description and discussion in the present invention utilizes the expression.
of serial
resistance and serial reactance. For simplicity, serial resistance and serial
reactance are
simply called resistance and reactance.
As described in US patent application no. 10/705,447, entitled "Impedance
based
devices and methods for use in assays", filed on November 10, 2003 and PCT
application
number PCT/US03/22557, entitled "Impedance based devices and methods for use
in
assays", filed on July 18, 2003, both of which are herein incorporated by
reference for
disclosures relating to cell-substrate impedance monitoring, monitoring cell-
substrate
impedance for detection or measurement of change in impeda nce can be done by
measuring impedance in any suitable range of frequencies. For, example, the
impedance
can be measured in a frequency range from about 1 Hz to about 100 MHz. In
another
example, the irnpedance can be_measured in a frequency range from about 100 Hz
to
.about 2 MHz. The iinpedance is typically a function of the frequency, i.e.,
the impedance
values change as frequency changes. Monitoring cell-substrate impedance can be
done
either in a single frequency or multiple frequencies. If the impedance
measurement is
performed at multiple frequencies, then a frequency-dependent impedance
spectrum is
obtained - i.e., there is an impedance value at each measured frequency. As
mentioned
above, the impedance has two components - a resistance component and a
reactance
component. A change in either resistance component or reactance component or
both
components can constitute a change in impedance.
As described in the US patent application no. 10i705.447,entitled --Impedance
based
devices and methods for use in assays", filed on November 10, 2003 and PCT
application
~,
~)
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
number PCT/LJ1S03/22557.entitled "Inipedance based devices and methods for use
in
assavs". filed on July 18. 2003, herein incorporated by reference for
disclosure of
methods of measuring electrical impedance, the method for the measurement of
electrical
(or electronic) impedance is achieved by , (1) applying a voltaae between or
among said
electrodes at a aiven frequency (or multiple frequencies, or having specific
voltage
waveform) and monitoring the electrical current through said electrodes at the
fi=equency
(or multiple frequencies, or having specific wavefonn), dividing the voltage
amplitude
value by the current amplitude value to derive the impedance value; (2)
applying an
electric current of a single frequency component (or multiple frequencies or
having
specific current wave form) tlirough said electrodes and monitoring the
voltage resulted
between or among said electrodes at the fiequency (or multiple frequencies, or
having
specific wavefonn), dividing the voltage amplitude value by the current
amplitude value
to derive the impedance value; (3) other methods that can measure or determine
electric
impedance. Note that in the description above of "dividing the voltage
amplitude value
by the current amplitude value to derive the impedaiice value", the "division"
is done for
the values of current amplitude and voltage amplitude at same frequencies. As
it is well-
known in electrical and electronic engineering, in such calculations (e.g.
divisions
mentioned above), the current amplitude and voltage amplitude are expressed in
the form
of complex numbers, which take into account of how big the current and the
voltage are
and what the phase difference between the sinusoidal waves of the current and
the
voltage is. Similarly, the impedance value is also expressed in a complex
foim, having
both resistance and reactance component, as shown in equations above.
As described in the US patent application no. 10/705,447, entitled "Impedance
based
devices and methods for use in assays", filed on November 10, 2003 and PCT
application
number PCT/US03/22557,entitled "Impedance based devices and methods for use in
assays", filed on July 18,. 2003, both incorporated herein by reference for
disclosure
relating to Cell Index or Cell Number Index, the measured cell-substrate
impedance can
be used to calculate a parameter termed Cell Index or Cell Number Index.
Various
methods for calculating such a cell number index can be used based on the
changes in
resistance. or reactance when cells are attached to the electrode structures
with respect to
th cases no cells are attached to the electrode structures. The impedance
(resistance and
34
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
reactance) of the electrode structures with no cell attached but with sanle
cell culture
medium over the electrode structures is sometimes referred as baseline
impedance. The
baseline unpedance may be obtained by one or more of the follouing ways: (1)
the
impedance measured for the electrode structures with a cell-free culture
medium
inti-oduced into the well containing the electrode structures, wherein the
culture medium
is the same as that used for the impedance measurements for the condition
where the cell
attachment or spreading is monitored; (2) the impedance measured shortly (e.g.
10
minutes) after the cell-containing medium was applied to the wells comprising
the
electrode structures on the well bottom (during the short period after cell-
containing
medium addition, cells do not have enough time to attach to the electrode
surfaces. The
length of this short-period may depend on cell type and/or surface treathnent
or
modification on the electrode surfaces); (3) the impedance measured for the
electrode
structures when all the cells in the well were killed by certain treatment
(e.g. high-
temperature treatment) and/or reagents (e.g. detergent) (for this method to be
used, the
treatment and/or reagents should not affect the dielectric property of the
medium wluch is
over the electrodes).
In one example (A), the cell index or cell number index can be calculated by:
(Al) at each measured frequency, calculating the resistance ratio by dividing
the resistance of the electrode arrays when cells are present and/or attached
to the
electrodes by the baseline resistance,
(A2) finding or determining tlie maximum value in the resistance ratio over
the
frequency spectrum,
(A3.) and subtracting one from the maximum value in the resistance ratio.
Using a mathematically formula, Cell Index is derived as
Cell Index = maa (4)
i=i,2-n'
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
'Where N is the number of the frequency points at which the impedance is
measured. For
example. if the frequencies used for the measurements are at 10 kHz. 25 kHz
and 50 l:Hz,
then N=' ). f~= 10 l:Hz. f= 25 kHz. f;= 50 kHz. R õ ( f,.) is the resistance
(cell-substrate
resistance) of the electrode arrays or electrode structures when the cells are
present on the
electrodes at the frequency f and Rh (f) is the baseline resistance of the
electrode array
or structures at the frequency f.
The cell index obtained for a given well reflects: 1) how many cells are
attached to the electrode surfaces in this well, 2) how well cells are
attached to the
electrode surfaces in the well. In this case, a zero or near-zero "cell index
or cell number
index" indicates that no cells or very small number of cells are present on or
attached to
the electrode surfaces. In other words, if no cells are present on the
electrodes, or if the
cells are not well-attached onto the electrodes, R,,õ ( f,. ) is about the
same as Rh ( f),
leading to Cell Index =0. A higher value of "cell nuinber index" indicates
that, for same
type of the cells and cells under similar physiological conditions, more cells
are attached
to the electrode surfaces. In otlier words, under same physiological
conditions, more
cells attached on the electrodes, the larger the values RCeõ ( f. ) is,
leading to a large value
for Cell Index. Thus Cell Index is a quantitative measure of cell number
present in a
well. A higher value of "cell index" may also indicate that, for same type of
the cells
and same number of the cells, cells are attached better (for example, cells
spread out
more, or cell adhesion to the electrode surfaces is stronger) on the electrode
surfaces.
Thus, for same number of the cells present in the well, change in a cell
status will lead to
a change in cell index. For example, an increase in cell ad.hesion or a cell
spread leading
to large cell/electrode contact area will result in an increase in R,,õ (f)
and a larger Cell
Index. On the other hand, a cell death or toxicity induced cell detachment,
cell rounding
up, will lead to smaller (f) and thus smaller Cell Index.
In another example (B), the cell number index can be calculated by:
36
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
(BI) at each measured frequency. calculating the reactance ratio by dividinR
the
reactance of the electrode arrays when cells are present on and/or attached to
the
electrodes by the baseline reactance,
(B2) findinQ or determining the maximum value in the reactance ratio over the
frequency spectrum,
(B3) and subtracting one from the maximum value in the resistance ratio.
In tl-iis case, a zero or near-zero "cell number index" indicates that no
cells or
very small number of cells are present on or attached to the electrode
surfaces. A higher
value of "cell number index" indicates that, for same type of the cells and
cells under
similar physiological conditions, more cells are attached to the electrode
surfaces.
In yet another example (C), the cell index can be calculated by:
(C1) at each measured frequency, subtracting the baseline resistance from the
resistance of the electrode arrays when cells are present or attached to the
electrodes
to determine the change in the resistance with the cells present relative to
the baseline
resistance;
(C2) then finding or determining the maximum value in the change of the
resistance.
In this case, "cell-number index" is derived based on the maximum change in
the resistance across the measured frequency range with the cells present
relative to the
baseline resistance. This cell index would have a dimension of olun.
In yet another example (D), the cell index can be calculated by:
(D1) at each measured frequency, calculating the magnitude of the impedance
(eualing to ~ R.' +~i'.,' , where R. and X, are the serial resistance and
reactance,
respectively).
(D2) subtracting the ma'grutude of the baseline impedance from the magnitude
of the
impedance of the electrode arrays when cells are present or attached to the
electrodes
37
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
to determine the change in the ma'cnitude of the impedance with the cells
present
relative to the baseline impedance;
(D3) then fmding or determining the maximum value in the change of the mao-
nitude
of the impedance.
In this case, "cell-nuniber index" is derived based on the maximum change in
the maQnitude of the impedance across the measured frequency range with the
cells
present relative to the baseline impedance. This cell index would have a
dimension of
ohm.
In yet another example (E), the index can be calculated by:
(El) at each measured frequency, calculating the resistance ratio by dividing
the
resistance of electrode arrays when cells are present or attached to the
electrodes by
the baseline resistance,
(E2) then calculating the relative change in resistance in each measured
frequency by
subtracting one from the resistance ratio,
(E3) then integrating all the relative-change value (i.e., summing together
all the
relative-change values at different frequencies).
In this case, "cell-number index" is derived based on multiple-frequency
points, instead of single peak-frequency like above examples. Again, a zero or
near-zero
"cell number index" indicates that on cells are present on the electrodes. A
higher value
of "cell number index" indicates that, for same type of the cells and
cellsunder similar
physiological conditions, more cells are attached to the electrodes.
In yet another example (F), the cell index can be calculated by:
(Fl) at each measured frequency, subtracting the baseline resistance from the
resistance of the electrode arrays when cells are attached to the electrodes
to
determine the change in the resistance with the cells present relative to the
baseline
impedance; (here the change in the resistance is given by
AR(f)- R,-,eu (.f )- Rs-ha.ccline (.fr ) for the frequency f. , R,-,,, and R,.-
ho,.el11e are the
serial resistances with the cells present on the electrode array and the
baseline serial
resistances; respectively);
38
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
(F3) analyzina the frequency dependency of the chanae of the resistance to
derive
certain parameters that can quantify such dependency. In one example. such
parameters can be calculated as PAR(,, )]2 . In another example, such
parameter
can be calculated as IDR( f,_ ). The parameter(s) are used as cell index or
cell
number index.
In this case, "cell-number u7dex" is derived based on the analysis of the
frequency spectrum of the change in the resistance. Depending how the
parameters are
calculated, the cell index may have a dimension of ohm.
In yet another example (G), the cell index can be calculated by:
(Gl) at each measured frequency, calculating the magnitude of the impedance
(equaling to JR~.'" + X t.2 , where R, and X, are the serial resistance and
reactance,
respectively).
(G2) subtracting the magnitude of the baseline impedance from the magnitude of
the
impedance of the electrode arrays when cells are attached to the electrodes to
determine the change in the magnitude of the impedance with the cells present
relative to the baseline impedance; (here, the change in the magnitude of the
impedance is given by AZ (f, )= ZCeõ (/ i)I -lZbaseline (.f; )I for the
fi=equency
Z,eõ ( f,~ )= R,-eeõ ( f. ) 2+ X.-~en ( f)~~ R,-cen and X,-eeõ being the
serial resistance
and reactance with the cells present on the electrode arrays, respectively,
ZeGõ ( f. )I is
the magnitude of the impedance of the electrode array with cells present on
the
electrode arrays, Zbaseline V) is the magnitude of the baseline impedance of
the
electrode array);
?5 (G3) analyzing the frequency dependency of the change of the magnitude of
the
impedance to derive certain parameters that can quantify such dependency. In
one
example, such parameters can be calculated as ~- In another example,
39
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
such parameter can be calculated as ~ hZ( f, )I . The parameter(s) are used as
cell
index or cell nun7ber index.
In this case, "cell-number index" is derived based on the analysis of the
frequency spectrum of the chanae in the magnitude of the impedance. Depending
how
the parameters are calculated, the cell index may have a dimension of ohn1.
As described in the US patent application no. 10/705,447, entitled "hnpedance
based devices and methods for use in assays", filed on November 10, 2003 and
PCT
application number PCTlUS03/22557,entitled "Inipedance based devices and
methods
for use in assays", filed on July 18,. 2003, and U.S. Patent Application No.
10/987,732,
all herein incorporated by reference for disclosure of Cell Index or Cell
Number Index
and its calculation, there are different methods for calculating the parameter
termed Cell
Index or Cell Number Index from the measured cell-substrate impedance
(resistance or
reactance). Cell Index or Cell Number Index is a quantitative measure of cells
in the
wells under cell-substrate impedance measurement.
It is worthwhile to point out that it is not necessary to derive such a "cell
number
index" for utilizing the impedance information for monitoring cell conditions
over the
electrodes. Actually, one may choose to directly use measured impedance (e.g.,
at a
single fixed frequency; or at a maximum relative-change frequency, or at
multiple
.20 frequencies) as an indicator of cell conditions. If measured impedance
values are directly
used for monitoring cell conditions, then resistance, or reactance or-both
resistance and
reactance can be used.
Still, deriving "cell index" or "cell number index" and using such index to
monitor cell conditions may have advantages. There are several advantages of
using
"cell number index" to monitor cell growth and/or attachment and/or viability
conditions.
First, one can compare the performance of different electrode geometries by
utilizing such cell number index.
Secondly, for a given electrode geometry; it is possible to construct
"calibration
curve" for depicting the relationship between the cell number and the cell
number index
bv performing impedance measurements for different number of cells added to
the
electrodes (in such an experiment, it is important to make sure that the
seeded cells have
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
well-attached to the electrode surfaces). With such a calibration curve, when
a new
impedance measurement is performed, it is then possible to estimate cell
number from the
newly-measured cell number index.
Thirdly, cell number index can also be used to compare different surface
conditions. For the same electrode geometry and same number of cells, a
surface
treatment given a larger cell number index indicates a better attachment for
the cells to
the electrode surface and/or better surface for cell attachment.
As shown above, for some methods of calculating cell index or cell number
index,
it is important to know the impedance (resistance and/or reactance) of the
electrode
structures with and without cells present on them. Based on the equation (1),
the
impedance of the electrode array (with or without cells present on the
electrodes) is given
by
Zelectrode-array= Ztotal - Ztrace - Z.rwrtch (5)
Where Z,s,,,;t,h is the impedance of electronic switch at its on stage,
Zt,.aCe is the
impedance of the electrical connection traces (or electrical conductive
traces) on the
substrate between the connection pads and the electrode buses, Ztotal is the
total
impedance measured at the impedance analyzer. By choosing electronic switches
with
good quality, it is possible to have all the electronic switches have a
consistent on-
impedance (mainly resistance). For example, the on=resistance of electronic
switches can
be about 3 ohni (+/- 10%) with the on reactance being negligible (for example,
less than
0.2 ohm in the frequency range of interest). Thus, if the trace impedance is
determined or
calculated, then formula (5) can be used to calculate the impedance of the
electrode
arrays with or without cells present.
A method is invented in the present application to determine the impedance of
electrical conductive (electrical connection) traces (mainly trace resistance,
trace
reactance is very small for the thin conductive film trace) based on the
relationships
among two or more essentially identical arrays on a cell-substrate impedance
monitorinQ
device. In the following, the four electrode arrays A, B. C and D as indicated
in Fiwre 1,
are used to illustrate this method. The electrical reactance (serial
reactance) of the
41
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
electronic switches and the electrical reactance (serial reactance) of the
electrical
connection traces are small as compared with_the corresponding electrical
resistances
(serial resistances). Thus, we focus on the analysis of the resistance of the
electrical
connection traces. The impedance determined from the impedance analyzer does
contain
both resistance (serial resistance, R,a,a, ) and reactance (serial reactance).
For the
electrode arrays A - D, the measured total resistance R,a,ar , the resistance
( R,,.QLe ) of
electrical conductive (connection) trace, the switch resistance and the
resistance
( Re_arra,, ) of the electrode array satisfy the following equations:
Re-arrar-A = R,a,al-A - R,race-A - R,,i,ch-A (6A)
Re-arra,,-B = R,a,al-B - R,race-B - R,fch-B (6B)
Re-arra,.-c = R,a,al-c - R,race-c - Rsw;,ch-c (6C)
Re-arrav-D = R,a,al-D - R(race-D - R..i,ch-D (6D)
With chosen electronic switches having consistent switch-on resistance,
R,,,,;,c,,_A,
R,,;,c,,_B , R,,,,;,c,,-c and R.,,;,ch-D have very similar values and can be
assumed to be the
same, R.,,,,;,c,, . Thus, in above equations, the known parameters are R,alal-
A , R,o,al-B ~
R,a,al-c: ) and R,o,al-D ~ and R,~,r,ch-A ~ R.,,eh-B ~ R.,,ch-c and R. õheh-D
. and there are eight
unknown parameters Re-array-A ~ Re-array-B 5 Re-array-c ~ and Re-array-D ) and
Rn=ac=e-A 5 R,race-B
R,race_c and R,,.ace_D . It is impossible to solve these equations for the
eight unknown
variables from these four equations directly. Additional relationships between
these
variables are needed to solve for them. Each trace resistance ( R,rac=e-A ,
R,raca-B )
R,race-c and R,rac=e-D ) depends on the metal film type used, and the geometry
of the trace
such as the how many rectangular segments the trace has, the film
thickness(es) of the
segments, the width(s) of the segments, the length(s) of the segment(s). For
example,
n A_; (7)
R, ~ L
?~ race-.4 - ,~ r *
d : -;
where N is the number of the se2nents of the trace-A, t,;-,, d4_; and L,q-; is
the thickness,
width and lenc-th of the i-th seament of the -traces for the electrode array
A, and p is the
42
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
resistivity of the thin fihii. The equation here applies to the film
comprisinQ a single type
of metal. The equation can be readily modified to be applicable to the film
comprisina
two or more metal nrpes (e.g. Qold film over chromium adhesion laver).
If the film thicl:ness is reasonably uniform (for eaanlple, less than 10% in
thickness variation) across the substrate, then the relationship among the
trace resistances
is simply determined by the pre-determined geometrical shapes (e.g. the
length, width of
the segments). For example, it would be straightforward to calculate the ratio
aA-,D
between the resistance of the electrically conductive traces for the electrode
array A to
the resistance of the electrically conductive traces for the electrode array D
as below,
where the film thickness is assumed to be the same everywhere on these traces
and the
resistivity is also the same everywhere on these traces,
~ P LA_; ~ LA-;
* ~,7 =1 A-!
a = R,ruce_A _ i=1 tA-i U~A-i _ . (8)
A-D R M L M L
n=ace-D ~P D-i D-i
#d
l=1 tD-; D-i ;-1 D-i
Similarly, one can determine the ratio aB-D and ac-D based on the pre-
determined
geometrical relationships for the traces of the electrode arrays B, C and D.
Note that
above equations can be similarly derived for the cases where the thin film in
these traces
comprises more than one metal type. Thus, based on the equalities
R.,,,.;,ch-A - R.,";lcl,-B = R.,,,;,a,-c = Rsõ-,Yd,-D = R"õl;leh ~ (9A)
Runc=e-A = aA-D o Rlrace-D ~ (9B)
R,race-B = aB-D R1race-D ~ (9C)
and Rlroce-C = ac-D * R,raee-D (9D)
equations (6A)-(6D) can be re-written in the followina format:
Re-arrar-.; = Rioaal-A - aA-D ' Rirnce-D - "av,ncn (10A)
Re-urrm,-B = RIaa!-B - aB-D' R,ruce-D - R.cxuch (l OB)
43
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Rc -urrm -( R,aml-(' - a('-D * Rvace-D - R,xaci, (1 OC)
Re-arra,.-n = R,~,a/-D - R,raee-D - R,,hch-D (l OD)
For equations (l0A) througll (10D), there are five unl:nown variables,
Re_arra,_A ,
Re-arrar-B 3 Re-arrav-(' and Re-arrav-D and R,ro,e-D = Mathematically, these
unk-iiown
variables cannot be determined from these equations. Additional iuiformation
is needed
to solve for these variables Re_arra,,_A , Re-array-B 3 Re-orrrn,-(: 3 and Re-
arrm,-D and Rlrac=e-D =
One approach is invented and described in the present invention. In this
approach, same biological or chemical solutions or suspensions are applied to
the
electrode-arrays A through D. Because the electrode arrays A tlirough D have
essentially
identical electrode structures, the electrode array resistances Re-or.an-A 3
Re-arratl-B 5
Re_ar,.o),_c and Re-arrav-D should be of same, or very similar value for such
a condition
when all the electrode arrays are exposed to the same biological or chemical
solutions or
suspensions, i.e.: Re-array-A "' Re-array-B ~ Re-array-C.C "" Re-array-D . If
we assume the averaged
electrode array resistance is Re_orray , then these approximate relationship
exists
Re-array-A ~ Re-array-B ~ Re-arrm,-C "-, Re-array-D "' Re-arra)- . Thus,
equations (10A - l OD) can
be changed to the following:
Re-arrav R7alal-A - aA-D ' R,race-D - R.cwilch (11A)
Re-arren, Ria,al-B - aB-D ' R,raee-D - R.s,,.;,eh (11B)
Re_array R,a,a/-C - aC-D = R,race-D - Rswi,ch (11 C)
Re-arrav R,a,al-D - R,race-D - R.,, ;,cn-D (11 D)
Thus, we would need to fmd Rlrac=e-D and Re_arra,, that satisfy the above
approximate equality as close as possible. One mathematical approach is to fmd
R,race-D
and R,_arra, that would result in the minimum value for the following
expression - an
expression that quantifies the differences between the two sides of the
approximate
equali-n. in (1 lA, 11B, 11 C and 11D),
44
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
-
F(R,rtrc=e-D' Re-arrtn' ) - LRe-orrrm (R,oltd-.-I - aA-DR,racr-D - R.~,,Ic'r,
)J 2
LRe-urrar -\R,am!-B - aB-DR,race-D - R.~ hch lJ ,
r l 2
LRe-arrar -\R,a,ar-C - aC-DR,ruc=e-D - R,'i,ch 1 T
- (RlalaI-D - R,ruce-D - R.,',,.;,Ch 2 (12)
The expression F(R,race-D ~ Re-arrm ) is the sum of the squared-differences
between the
two-sides of the approximate equality in (1 lA, 11B, 11 C and 11D). The
smaller
F(R race_D , Re_orro,, the closer the two sides of the approximate equality
(11 A, 11 B, 11 C
and 11D). Thus, values of R,ra,e_D and Re-arratthat result in the minimum
value of
F(R,,.ace_D , Re-arror ) should be determined. Mathematical approach involves
in the
calculation of the first order derivative of F(R,race-D, Re-arro, ) to R,roce-
D and to Re-arroland let such first order derivatives equal to zero. The
values of R,roce-D and Re-arra,- that
result in zero for these first-order-derivatives are those that result in the
minimum value
of F(R,rac=c-D ~ Re-arro,, )= The first order derivatives are as follows:
a'F(Rlrace-D,Re-aaror )J _ 2 o aA-D ' LRe-array - \Rlo,ar-A - aA-DR,race-D -
Rswllch !J+
a R,roce-D
2 o aB-D - [Re-arra>> - lRla,aI-B - aB-DRtrace-D - Rs~,ilch )J +
2 o aC-D 0 [Re-arrav - \Rla1a!-C - aC-D Rlrace-D - Rsu hch +
2 o LRe-arrar - \Rm,a!-D - Rlrace-D - R=,,,ch )J
=0; (13A)
~LF\Rlrace-D,Re-aarq~)J ( ll
_ ? = [Re-arrar - \Rtnlal-A - aA-DR,race-D - Rs~,i,ch !J+
a Re-arrav
2 .
LRr-arrqr - (Rla,al-B - aB-r>R,race-D - R.,rch / l +
~ I I
. = LRe-arrm' - (Rraral-(' - aC=_DR,raee-D - R.o+=aeh )J
2 * lRe-arrm- (Rmla!-D - Rrrace-D - R.M'i,dr )J
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
=0. (13B)
Equations (13A) and (13B) can be re-written as
Re-arrm' 0 La.-I-D -r IXB_D + aC_D + 1]+ Rlrace-D 0 la.-1-D2 + aB_!)7 + a(.-D2
+ 1,=
a.9-D o LRmlal-A - R., vilch J+ aB-D o LRlolal-B - Rs"=ilch J+
aC-D o LRralal-c - Rs~!ireh J+ LRlnral-D - Rsirilch J (14A)
4* Re-arrar + Rlrace-D * LaA-D + aB-D + aC_D + 1]=
[Rlnlal-A - Rswilch ~+ LRtolaf-B - Rswilch J+LR[olal-c - R.%wilch ~+ LRinlal-D
- R.cwilch ~
(14B)
Thus, we can solve for R,,.ace-D as follows:
_ 4 = S, - Aõ = S,
Rlrace-D (15) 49A,2 -Aõ =B12
where Aõ _IaA-D + aB-D + ac-D + 1] ;
A12 = [aA-D 2 + aB-D 2 + aC_D 2 + 1 ;
S1 = aA-D a LRlnlal-A - R.%N,ilch I+ aB-D ~ LRIala!-B - R.wilcb 1+
aC-D ~ LRlnlal-C.. - Rwilch J+ LRrolal-D - Rsvilch J~
B12 _ [aA_D + aB_D + aC_D +1];
S2 [Rrolal-A - 'Rilch J+ LRrntal-B - R.rx=ilclr )+ LRlnla!-C - R.M'ilch ]+
LRlnlal-D - Rswilch J'
Thus, With the determined R,ra,_,D . the trace resistances of R,race_A, Rlrace-
B ~ and Rlrace-C
can be calculated using equations (9B). (9C) and (9D). Furthermore, the
electrode array
resistance R, R R and Rc-crrcr-D can be calculated from the
.-arra: -., ' c-arra=,-B ' e-arrm-C'
46
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
measured resistance R,o,a,-n- R,,,,or-c and R,,,,respectivel y using equations
(l0A), (lOB). (I OC) and (I OD).
T11us_ one aspect of the present invention is directed to a method of
calculation of
the resistances of the electrical connection traces s from the measured_ total
resistances
for two or more essentially identical electrode arrays, comprising the
following steps:
(1) exposing the electrode arrays to the solutions havin-, same or similar
solutions or
suspensions;
(2) with an impedance analyzer or impedance measurement circuit, measuring
the resistance (serial resistance) for each electrode array, such resistance
being the sum of the resistance of electronic switches, the resistance of the
electrical connection traces between the connection pads and the electrode
structures (for example, between the connection pads and the electrode
buses), and the resistance of the electrode array with the solutions or
suspensions present;
(3) solving for the resistances of electrical connection traces using equation
(15)and equations (9B), (9C) and (9D), noting in the calculation with
equation (15), the geometrical relationships between the electrode arrays
are used to determine the factor aA_D , aB_D and ac-o
Another aspect of the present invention is directed to a method of calculating
the
resistance of the electrode arrays from the measured, total electrode
resistances for two ro
more essentially identical electrode arrays (such as, for example arrays A-D
in Figure 1)
if the same or similar solutions or suspensions are added to be in contact
with the
electrode assays, comprising the following steps:
(1) exposing the electrode arrays to the solutions having same or similar
solutions or suspensions;
(2) with an impedance analyzer or impedance measurement circuit, measuring
the resistance (serial resistance) for each electrode array, such resistance
being the sum of the resistance of electronic switches, the resistance of the
electrical connection traces between the connection pads and the electrode
47
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
structures (for example_ between the connection pads and the electrode
buses) and the resistance of the electrode arrays with the solutions or
suspensions present;
(3) solving for the resistances of electrical connection traces using equation
(15) and equations (9B), (9C) and (9D), noting in the calculation with
equation (15), the geometrical relationships between the electrode arrays
are used to determine the factor a.9_o , aB_o and aC_o ;
(4) calculating the resistances of the electrode arrays using equations (10A,
lOB, l OC and 10D) ).
In mairy applications, the solutions or suspensions (for example, cell
suspension)
applied to each electrode array may have different compositions. For exainple,
cell
suspensions of different cell numbers may be used so that the suspensions
applied to each
electrode array are quite different. Under such cases, the determination of
the resistance
of the electrode arrays with the cells present would require the determination
of the
resistance of the electrical connection traces by performing a "reference run"
or
"calibration run" in which the electrode arrays are exposed to a same,
reference solution.
From the "reference run", the resistances of the electrical connection traces
can be
determined. In a separate test, the electrode arrays are exposed to the
solutions or cell
suspensions of interest and the resistances for the electrode arrays under
such conditions
are measured with an impedance analyzer or impedance measuring circuit. The
resistance of the electrode arrays with such cell suspensions present can be
determined
(or continuously determined) from the measured resistance by subtracting the
sum of the
resistance of the electronic switches and the resistance of the electrical
connection traces
for corresponding electrode arrays from the measured resistances.
Thus, another aspect of the present invention is directed to a method of
calculatina the resistance of the electrode arrays from the total electrical
resistances
measured at an impedance anah,zer for essentialh~ identical electrode arrays
(such as
electrode arrays A-D in Figure 1 used as an example) if different solutions or
suspensions
of interest are applied to the electrode assays; comprising the folloNAring
steps:
48
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
(1) exposinQ ttie electrode arravs to the solutions having same or similar
solutions
or suspensions (reference solutions);
(2) with an impedance analyzer or impedance measurement circuit, measuanQ the
resistance (serial resistance) for each electrode array, such resistance
beinc, the
sum of the resistance of electronic switches. the resistance of the electrical
com7ection traces between the connection pads and the electrode structures
(for example, between the connection pads and the electrode buses) and the
resistance of the electrode arrays with the reference solutions present;
(3) solving for the resistances of electrical connection traces using equation
(15)
and equations (9B), (9C) and (9D), noting in the calculation with equation
(15), the geometrical relationships between the electrode arrays are used to
detennine the factor aA-o , aB-p and aC-o ;
(4) applying the solutions or suspensions of interest to each electrode array;
and
with an impedance analyzer or impedance measurement circuit, measuring the
resistance (serial resistance) of each electrode array, such resistance being
the
sum of the resistance of electronic switches, the resistance of the electrical
coiuiection traces between the connection pads and the electrode structures,
the resistance of the electrode arrays with the solutions or suspensions of
the
interest present,
(5) Calculating the resistance of the electrode arrays using equations (l0A),
(lOB), (10C) and (10D) by subtracting the electronic switch resistances and
the resistances of electrical connection traces from the measured resistances
in
the step (4).
Note that in above method, the steps of exposing the electrode arrays to
reference
solutions for the determination of the resistances of electrically conductive
traces (step
(1), (2) and (3)) may be performed before or after the steps of applying the
solutions or
suspensions of interest to the electrode arrays and measuring the total
electrical resistance
(step (4)). For example, step (4) may be performed first. After that, the
solutions or
suspensions of the interest may be removed from the electrode array. The
reference
solutions can then be added to the electrode arravs (step (1)). Step (2) and
step (3) can be
49
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
then performed to determine the resistances of electrical connection traces.
Finally, Step
(5) can be done.
in another approach, step (1) and (2) can be performed allead of step (4).
Another aspect of the present invention is directed to a method of detemlining
the
resistance of the electrode arrays with the cells present for a cell-based
assay based on the
total electrical resistance measured at an impedance analyzer for essentially
identical
electrode arrays. In this method, the electrode arrays are exposed to a same,
reference
solution (for exaniple, a same cell culture medium that does not contain any
cells) and
electrical measurement is conducted to determine the resistance of electrical
connection
traces. With the resistances of the electrical connection traces determined,
electrical
resistances of the electrode arrays with cell suspensions added to electrode
arrays can be
calculated from the total electrical resistances measured at an impedance
analyzer. Such
total electrical resistance would include the resistance of the electrode
arrays with.cells
present, the resistance of electronic switches and the resista.nce of
electrical connection
traces. The metllod comprises following steps
(1) exposing the electrode arrays to the solutions having same or similar
solutions
or suspensions (reference solutions);
(2) with an. impedance analyzer or impedance measurement circuit, measuring
the
resistance (serial resistance) for each electrode array, such resistance being
the
sum of the resistance of electronic switches, the resistance of the electrical
connection traces between the connection pads and the electrode structures
(for example, between the connection pads and the electrode buses) and the
resistance of the electrode arrays with the reference solutions present;
(3) solving for the resistances of electrical connection traces using equation
(15)
and equations (9B), (9C) and (9D), noting in the calculation with equation
(15), the geometrical relationships between the electrode arrays are used to
determine the factor aA_o , aB-D and ac-o ;
(4) applying the cell suspensions of interest to each electrode array; and
with an
impedance analyzer or impedance measurement circuit measuring the
resistance (serial resistance) of each electrode arrav, such resistance being
the
sum of the resistance of electronic s-witches_ the resistance of the
electrical
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
connection traces between the connection pads and the electrode structures.
the resistance of the electrode arrays with the cell suspensions of the
interest
present.
(5) Calculating the resistance of the electrode arrays using equations (10A),
(l OB), (I OC) and (l OD) by subtracting the electronic switch resistances and
the resistances of electrical connection traces from the measured resistances
in
step (4).
Note that in above method, the steps of exposing the electrode arrays to
reference
solution for the determination of the electrical resistance of electrically
conductive traces
(step (1), (2) and (3 ))) may be performed before or after the steps of
applying the solutions
of interest or cell suspensions of interest to the electrode arrays and
measuring the total
electrical resistance (step (4)). For example, step (4) may be performed
first, followed by
steps (1) and (2). In one approach, after step (4), the cell suspensions of
the interest may
be removed from the electrode array. Then reference solutions can be added to
the
electrode arrays. In another approach, after step (4), the cells are all lysed
with some cell
lysis solutions so that the electrodes are exposed to the same, reference
solutions for the
measurement and calculation of step (2) and (3). And then, step (5) is
performed to
determine the electrical resistance of electrode arrays with the cell
suspensions of interest
present.
The determination of the resistances of the electrical conductive traces for
the
electrode arrays that essentially identical electrode arrays may be, or may
not be, part of
the monitoring of cell-substrate impedance for cell-based assays. It depends
on how the
impedance data (measured at a single frequency or multiple frequencies,
measured at
multiple tune points) of the electrode arrays is analyzed.
In some assays, one is interested in the relative change in the resistance or
impedance of the electrode arrays with the cells present relative to the
baseline resistance
or impedance. For such cases, it is preferred to determine the resistance (or
impedance)
of the electrode arrays from the totaL measures resistance (or impedance) by
subtracting
the resistance of the electrical conductive traces and the resistance of
electronic svvitches.
51
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Thus. determination of the resistaiices or impedance of the electrically
conductive traces
may be required.
In some other assays, one is interested in the absolute changes in the
resistance (or
impedance) of the electrode arrays with cells present relative to the baseline
resistance (or
impedance). In these cases, one can directly subtract the measured resistance
or
impedance for the baseline condition from the measured resistance or impedance
for the
condition that the cells are present on the electrode arrays. The contribution
of the
resistance (or impedance) of the electronic switches and the resistance (or
impedance) of
the electrically conductive traces to the total measured resistance (or
impedance) values is
cancelled out in such subtractions. Thus, there is no need for determining the
resistances
of the electrically conductive traces.
In some assays, one is interested in calculating the Cell Index or Cell Number
Index based on the monitored impedance values. Depending on which method is
used
for calculating the Cell Index, it may, or may not, be necessary to determine
the
resistances of the electrically conductive traces. For example, for the Cell
Index
calculation method (A) described above, the resistances of the electrically
conductive
traces are needed, in order to remove the effect of the resistance of the
electrically
conductive traces on the analysis of the relative change of the resistance or
impedance.
In anotlier example, for the Cell Index calculation method (F) described
above, there is
no need to determine the resistances of the electrically conductive traces
since the effect
of the resistance of the electrically conductive traces is canceled out in the
calculations.
The monitoring of the cell-substrate impedance may be or may not be based on
the change with respect to the baseline impedance (or resistance). For
example, a cell-
based assay is performed to assess the effect of a test compound on the cells.
One
method in performing such an assay is by monitoring of the cell-substrate
impedance and
deterrriining the change in the cell-substrate impedance before and after the
addition of
the test compound to the cells. The monitoring of cell-substrate unpedance can
be
performed at a sinale frequency point or multiple frequency points, at a
single time point
or multiple time points after drug addition. For example, the impedan ce is
first measured
at a single frequency or multiple frequencies for the electrode arrays with
the cells
present jus-L before addition of test compound. The test compound is then
added to the
52
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
cells. The impedance is then measured asain at the sanie single frequency or
multiple
frequencies for the electrode arrays with the cells after the addition of test
compound.
Such post-compound addition measurement may be performed for many time points
continuously in a regular or uregular time intervals. The chanae in the cell-
substrate
impedances can be deterrnined or quantified by subtracting the impedance(s)
(resistance
and/or reactance) measured before addition of the test cotnpound from the
impedance(s)
(resistance and/or reactance) measured after addition of the test compound. If
the
measurement is done at multiple frequencies, a single parameter or multiple
parameters
may be further derived for each time point after compound addition based on
the
calculated change in the cell-substrate impedances. Such parameters are used
to quantify
the cell changes after compound addition. Such approaches can be used further
to
analyze the responses of the cells to a test compound at multiple
concentrations to derive
dose-dependent response curves.
Normalized Cell Index, Delta Cell Index
A "Normalized Cell Index" at a given time point is calculated by dividing the
Cell
Index at the time point by the Cell Index at a reference time point. Thus, the
Normalized
Cell Index is 1 at the reference time point. Normalized cell index is cell
index nomzalized
against cell index at a particular time point. In most cases in the present
applications,
normalized cell 'uzdex is derived as normalized relative to the time point
immediately
before a compound addition or treatment. Thus, normalized cell index at such
time point
(immediately before compound addition) is always unit one for all wells. One
possible
benefit for using such normalized cell index is to remove the effect from
difference in
cell number in different wells. A well having more cells may produce a larger
impedance
response following compound treatment. Using norinalized cell index, it helps
to remove
such variations caused by different cell numbers.
A "delta cell index" at a given time point is calculated by subtracting the
cell
index at a standard time point from the cell index at the given time point.
Thus, the delta
cell index is the absolute change in the cell index from an initial time (the
standard time
point) to the measurement time.
53
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Cell Chanae Index
The time-dependent cellular response (including cvtotoxicity response) may be
analyzed bv deriNIr:ng parameters that directly reflect the changes in cell
status. For
example, tirne dependent celluler response may be analyzed by calculating the
slope of
change in the measured impedance responses (that is equivalent to the first
order
derivative of the impedance response with respect to time, impedance response
here can
be measured impedance data or derived values such as cell index, normalized
cell index
or delta cell index). In another example, the time-dependent cellular
responses
(including cytotoxicresposnes) responses may be analyzed for their higher
order
derivatives with respect to time. Such high order derivatives may provide
additional
information as for how cells responding to different compounds and as for the
mechanisms of compound action.
As an example, we describe how one can to derive a parameter, called Cell
Change Index, based on the real time, quantitative information (i.e., cell
index, CI) about
biological status of cells in the wells provided from RT-CES system. This new
parameter, Cell Change Index (CCI), can effectively link time dependent cell
index I with
cell status, is calculated as,
CCI(t) = dCI(t) (5)
CI(t)=dt
Thus CCI is the normalized rate of change in cell index. CCI values can be
used to
quantify the cell status change. For cells in an exponential growth under
regular cell
culture condition, the cell index determined by a cell-substrate impedance
moiutoring
system described herein is expected to be a proportionate measure of the cell
number in
the well since the cell morphology and average extent of cell adhesion to the
electrode
surfaces among the whole cell population do not exhibit significant changes
over time.
Thus, the cell index (CI) increase with time following an exponential
function, such that
CI(t)=CI(0)*2D7' (6)
54
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
,here DT is the cell doublin, tinie. For such exponential growth culture.
CCI(t) is a
constant Rivino
CCI (~) = 0.69~ N~ 0_7 (7)
DT DT
Thus, several types of CCI(t) can be classified as:
(1) If CCI is about 0.7/DT, cell index increases in the same rate as that
expected
for an exponential growth of the cells.
(2) If CCI 0.7/DT, cell index increases faster than that expected for an
exponential growth (or log growth) of the cells. This indicates that cells may
grow faster than regular exponential growth, or cells may exhibit some
morphology change (e.g. cell spreading out or adhering better to the electrode
surfaces), leading to large impedance signal, or both of above effects, or
there
may be other cell behaviors occurring particular to the assay or culture
conditions.
(3) If CCI is more than zero but somewhat smaller than 0.7/DT, then cell index
increases in the rate slowed than that expected for an exponential growth.
This indicates that cell growth rate may be slowed down relative to
exponential growth, or cell growth may be somewhat inhibited by chemical
compounds added to the culture media or by other cell culture parameters, or
that certain populations of cells are dying off and detaching from the
electrode surfaces, or there nlay be other cell behaviors occurring particular
to
the assay or culture conditions.
(4) If CCI is about zero, then cell index shows a near constant value. This
may
indicate that the cell growth is nearly-completely inhibited. For example, all
the cells are arrested at certain points of cell cycle and are not progressing
further. Or, this may indicate that the number of cells dying off in the
culiure is nearly as the number of newly-divided cells. Alternatively this may
indicate that cells reach stationary phase of cell culture. A.lternativelv
this
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
mav- indicate that number of cells are above the detection upper limit of the
cell-substrate impedance monitoring system. There is also the possibility of
other cell behaviors occurring particular to the assay or culture conditions.
(5) If CCI is negative, then the cell index is decreasulg with time, showing
the
cells losing attachment to the electrode surface or changing
their morphology.
21
(6) If CCI is very negative, then the cell index decreases rapidly with time,
showing that either cells lose attachment to the electrode surfaces quickly or
cells change their morphology very quiclcly.
D. Methods for performing real=time cell-based.assays
The present invention provide cell-based assays that can be performed in real
time
to assess cell proliferation, cell growth, cell death, cell morphology, cell
membrane
propei-ties (for exainple, size, morphology, or composition of the cell
membrane) cell
adhesion, cell spreading and/or cell motility. Thus the assays can be
cytotoxicity assays,
proliferation assays, apoptosis assays, cell adhesion assays, cell activation
or stimulation
assays, anti-cancer compound efficacy assays, receptor-ligand binding or
signal
transduction analysis, assays of cytoskeletal changes, assays of cell
structural changes
(including but not limited to, changes in cell membrane size, morphology, or
composition), cell quantification, cell quality control, time-dependent
cytotoxicity
profiling, assays of cell differentiation or de-differentiation, detection or
quantitation of
neutralizing antibodies, specific T-cell mediated cytotoxic effect assays,
assays of cell
adhesivity or spreading, assays of cell-cell interactions, analysis of
microbial, viral, or
environmental toxins, etc.
The assays are real-time assays in the sense that cell behavior or cell status
being
assayed can be assessed continuously at regular or irregular intervals. Cell
behaviors, cell
responses, or cell status can be assayed and the results recorded or displayed
within
seconds to minutes of their occurrence. The cell response during an assay can
be
monitored essentially continuously over a selected time period. For example, a
culture
can be monitored every five to fifteen minutes for several hours to several
days after
addition of a reagent. The interval between impedance monitorins, whether
impedance
56
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
monitorinQ is perfomled at regular or irreaular intervals, and the duration of
the
impedance monitorinQ assay can be determined by the experimenter.
Thus, the cell-based impedance assays of the present invention avoid
inadvertently biased or misleadina evaluation of cell responses due to the
time point or
time points chosen for samplino, or assayinc, the cells. In addition, the
assays do not
require sampling of cell cultures or addition of reaQents and thus eliminate
the
inconvenience, delay in obtaining results, and error introduced by many
assays.
Descriptions of cell-substrate monitoring and associated devices, systems and
methods of use have been provided in United States provisional application
number
60/379,749, filed on July 20, 2002; United States provisional application
number
60/435,400, filed on December 20, 2002; United States Provisional application
60/469,572, filed on May 9, 2003, PCT application number
PCT/US03/22557,entitled
"Impedance based devices and methods for use in assays", filed on July 18,
2003; PCT
application number PCT/US03/22537,entitled "Impedaiice based apparatuses and
methods for analyzing cells and particles", filed on July 18, 2003; United
States patent
application number 10/705,447,entitled "Iinpedance based devices and methods
for use in
assays", filed on November 10, 2003; U.S. Patent Application No. 10/987,732
United
States patent application numberl0/705,615,entitled "Impedance based
apparatuses and
methods for analyzing cells and particles", filed on November 10, 2003, all
incorporated
herein by reference for their disclosure of cell-substrate impedance devices,
systems, and
methods of use. Additional details of cell-substrate impedance monitoring
technology is
furtlier disclosed in the present invention.
In brief, for measurement of cell-substrate or cell-electrode impedance using
the
technology of the present invention, cell-substrate impedance monitoring
devices are
used that have microelectrode arrays with appropriate geometries fabricated
onto the
bottom surfaces of wells such as microtiter plate wells, or have a similar
design of havinc,
multiple fluid containers (such as wells) having electrodes fabricated on
their bottom
surfaces facinc, into the fluid containers. Cells are introduced into the
fluid containers of
the devices, and make contact with and attach to the electrode surfaces. The
presence,
absence or chanae of properties of cells affects the electronic and ioni.c
passage on the
electrode sensor surfaces. Measuring the impedance bet ,e'n or amona
electrodes
57
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
provides important information about biological status of cells present on the
sensors.
vWhen there are changes to the bioloaical status of the cells analoaue
electronic readout
signals can be measured automatically and in real time, and can be converted
to digital
signals for processing and for analysis.
Preferably, cell-substrate impedance assays are performed using a system of
the
present invention that comprises a device of the present iuivention, an
impedance monitor,
a device station that comprises electronic circuitry and engages the device
and the
impedance analyzer, and a software program that controls the device station
and records
and analyzes impedance data.
Using a system of the present invention, a cell index can optionally be
automatically derived and provided based on measured electrode impedance
values. The
cell index obtained for a given well reflects: 1) how many cells are attached
to the
electrode surfaces in this well, and 2) how well (tightly or extensively)
cells are attached
to the electrode surfaces in this well. Thus, the more the cells of same type
in similar
physiological conditions attach the electrode surfaces, the larger the cell
index. And, the
better the cells attach to the electrode surfaces (e.g., the cells spread-out
more to have
larger contact areas, or the cells attach tighter to electrode surfaces), the
larger the cell
index.
In another aspect of the present invention, a method is provided for
performing
cell-based assays, comprising: a) providing a cell-substrate impedance
monitoring device
of the present invention that comprises two or more electrode arrays, each of
which is
associated with a fluid container of the device and coated at least in part
with a biological
rnolecule or organic compound; b) attaching the device to an impedance
monitor; c)
introducing cells into one or more fluid containers of the device; and d)
monitoring cell-
substrate impedance of at least one of the fluid containers that comprises an
electrode
array and cells. Preferably, impedance is monitored from the at least one
fluid container
to obtain impedance measurements at at least three time points. Preferably,
impedance
measurements or impedance values derived from impedance measurements from at
least
three time points are plotted versus time to generate one or more impedance
curves for
the one or more fluid containers.
58
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
In a related aspect of the present invention, a method is provided for
performing
cell-based assays in an impedance-monitoring system, comprising: a) providing
a cell-
substrate impedance monitoring system of the present invention that comprises
a device
having two or more electrode arrays, each of which is associated with a well
of the device
and coated at least in partwith a biological molecule or organic compound; b)
introducing cells into one or more wells of the device; and c) monitoring cell-
substrate
impedance of at least one of the wells that coniprises an electrode array and
cells.
Preferably, impedance is monitored from the one or more wells of the device to
obtain
impedance measurements at at least three time points. Preferably, impedance
measurements or impedance values derived from impedance measurements from at
least
three time points are plotted versus time to generate one or more impedance
curves for
the one or more wells.
The method can be used to assay cell status, where cell status includes, but
is not
limited to, cell attachment or adhesion status (e.g. the degree of cell
spread, the
. 15 attachment area of a cell, the degree of tightness of cell attachment,
cell morphology) on
the substrate including on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
nuinber of cells going through apoptosis andlor necrosis. The cell-based
assays that be
perfonned with above methods include, but are not limited to, cell adhesion,
cell
apoptosis, cell differentiation, cell proliferation, cell survival,
cytotoxicity, cell
morphology detection, cell quantification, cell quality control, time-
dependent
cytotoxicity profiling, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic changes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, and cell-based assays for screening and measuring ligand-receptor
binding.
In preferred embodiments of this aspect of the present invention, cells are
added
to at least two fluid containers of a device, each of which comprises an
electrode array, a
biological molecule coatincy or oraanic compound coating, and impedance is
monitored
from at least two wells that comprise cells and an electrode array.
The cells used in the assay can be primary cells isolated from any species or
cells
of cell lines. Primary cells can be from blood or tissue. The cells can be
engineered cells
59
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
into which nucleic acids or proteins have been introduced. In some
embodiments_
different cell types are added to different wells and the behavior of the cell
types is
compared.
An impedance monitoring assay can be from minutes to days or even weeks in
duration. Preferably, impedance is nlonitored at three or more time points,
although tlus
is not a requirement of the present invention. Impedance can be monitored at
regular or
irregular time intervals, or a combination of irregular and regular tnne
intervals. In one
embodiment of a cell-based impedance assay, the cell-substrate impedance is
monitored
at regular time intervals. In some embodiments of the present invention,
impedance is
monitored at irregular intervals and then at regular intervals during a
particular time
window of the assay. Impedance can be monitored at one frequency or at more
than one
frequency. For example, in some preferred embodiments, impedance is monitored
over a
range of frequencies for each time point at which impedance is monitored.
Preferably,
impedance is monitored at at least one frequency between about 1 Hz and about
100
MHz, more preferably at at least one frequency between about 100 Hz and about
21VIHz.
In yet another aspect, the present invention provides a method for performing
real-time cell-based assay investigating the effect of a compound on cells,
comprising: a)
providing an above described system; b) seeding the cells to the wells of
multiple-well
devices; c) adding a compound to the wells containing cells; d) monitoring
cell-substrate
impedance before and after adding the compound at a regular or irregular time
interval;
wherein the time dependent impedance change provides information about time
dependent cell status before addition of the compound and about time dependent
cell
status under the interaction of the compound. Information about cell status
includes, not
limited to, cell attachment or adhesion status (e.g. the degree of cell
spread, the
attachment area of a cell, the degree of tightness of cell attachment, cell
morphology) on
the substrate including on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
number of cells going through apoptosis and/or necrosis. Information about
cell status
may also include any compound-cell interaction leading to any change to one or
more of
above cell status indicators. For example, if the compound binds to a receptor
on the cell
surface and such binding leads to a change in cell morphology, then the
binding of
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
compound to the receptor can be assayed by the monitored cell-substrate
impedance. The
cell-based assays that be performed with above methods include, but not
limited to, cell
adhesion, cell apoptosis, cell differentiation, cell proliferation, cell
survival, cytotoxicity,
cell morphology detection, ce11 quantification, cell quality control, time-
dependent
cytotoxicity profiling, IQE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic chanQes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, cell-based assay for screening and measuring ligand-receptor binding.
In one embodiment of the above cell-based assay, the cell-substrate impedance
is
monitored at regular time intervals. In exemplary embodirnents, the impedance
is
measured at a regular 2 hour, 1 hour, 30 min or 15 min time interval before
and after
adding the compound. In the present application, a real-time assay means that
one can
perform the measurement on cell-substrate impedance with various time
resolutions, for
example, measurement taking place at a longer time interval such as everyhour
or every
two hours, or at a shorter time interval every minute or a few minutes. Real-
time assay
does not mean that the measurements are provided in a continuous,
uninterrupted fashion.
In another word, real-time assay does not mean that the measurements are
performed at
every single moment.
D.l. Cell proliferation assays
The present invention provides methods for performing cell proliferation
assays.
In these assays, an increase in monitored impedance is indicative of an
increases cell
number. The impedance measurements or impedance values derived from impedance
measurements can be plotted versus time to obtain growth curves for cells
growing in a
fluid container of a cell-substrate nlonitoring device of the present
invention.
The present invention provides a method of generating at least one cell growth
curve, comprising: providing a device of the present invention having two or
more
electrode arrays, each of which is associated with a fluid container of the
device;
attaching the device to an impedance analyzer; addin~ cells to one or more
fluid
containers of the device; monitoring impedance from the one or more fluid
containers to
obtain impedance measurements at three or more time points after adding the
cells to the
61
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
one or fluid containers; and plotting the impedance measurements or values for
the three
or more time points versus time to generate at least one growth curve for the
cells in the
one or more fluid containers.
The present invention also provides a method of generating at least one oTowth
cunJe using a system of the present invention, where the system includes a
multi-well
cell-substrate unpedance monitoring device, an impedance analyzer, a device
station, and
a software program. The method includes; providing a multi-well cell-substrate
impedance measuring system; adding cells to one or more wells of the system;
monitoring impedance from the one or more wells to obtain impedance
measurements at
tliree or more. time points after adding cells to the one or more wells; and
plotting
impedance measurements orimpedance values for the three or more time points
versus
time to generate a growth curve for the cells in the one or more wells.
Preferably, using a device or system of the present invention, impedance is
monitored at four or more time points, in which at least one of the four or
more time
points is measured from a fluid container prior to adding cells to the fluid
container.
Preferably, impedance is monitored at regular or irregular time intervals for
an assay
period of from minutes to days. In many cases, proliferation assays can be
performed by
monitoring impedance for a period of between several hours and several days.
It is preferable to perform replicate proliferation assays in which more than
one
fluid container is seeded with same number of cells of the same type. In this
case, a plot
can optionally be generated by plottuig averaged impedance measurements of
values at
assayed time points for replicate wells versus time. Preferably, a standard
deviation for
the averaged values is also calculated.
A growth curve can be generated by plotting impedance measurements versus
time, or by plotting cell index values that are calculated froni impedance
measurements,
such as normalized cell index values or delta cell index values versus time.
The
impedance measurement or cell index axis (typically the y-axis) can
optionally.use a log
scale.
An impedance value can be any indices of impedance derived from impedance
measurement, including, as nonlimiting examples, a cell index, a normalized
cell index or
a delta cell index. In certain embodiment, impedance value can also be a"ra'W'
measured
62
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
or monitored impedance value. Cell index (including normalized and delta cell
index)
can be a useful value for plottinc, srowth curves, as it relates impedance
measurements to
cell number. For cell Qrowth curves, a delta cell index for a given time point
can be
derived by subtractina the cell index at a baseline point, such as a time
poult after cell
attacliment and just before log phase growth, from the cell index measurement
at the
given time point. Preferably, determinations of impedance values and
generating growth
curves based on iinpedance measurements or values can be performed by
software, and
preferably by software that interfaces directly with the impedance analyzer.
For example,
where the growth assays are performed by a system of the present invention,
impedance
values (wllere used) can be measured or derived or calculated and growth
curves
generated by a software program that controls and receives data from the
impedance
analyzer.
A growth curve generated from impedance measurements or cell index values
(including nomialized cell index values and delta cell index values) can
optionally be
used to calculate one or more kinetic parameters of cell growth or behavior.
For example,
a growth curve can be used to calculate the length of a lag phase, cell
attachment time,
cell attachment rate, or a cell doubling time.
Conzparing Growth Curves of Two of More Cell Types
Two or more cell types can be seeded to separate wells in a proliferation
assay
using the methods of the present invention to generate growth curves of the
two or more
cell types. The growth curves or kinetic parameters derived from the growth
curves of the
cell types can be compared.
In this aspect, the invention includes a method of generating growth curves
for at
least two cell types, comprising: providing a device of the present invention
having two
or more electrode arrays, each of which is associated with a fluid container
of the device;
attaclung the device to an impedance analyzer; adding cells of two or more
cell types to
two or more fluid containers of the device, in which at least one of the two
or more fluid
containers receives one cell type and at least one other of the two or more
fluid containers
receives a different cell type, to provide two or more fluid containers
comprising two or
more different cell types; monitoring impedance from the two or more fluid
containers
63
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
comprisulg different cell types at three or more time points after adding the
two or more
cell t\pes to tlie two or more fluid containers; and plotting impedance
measurements or
impedance values for the three or more time points versus time to aenerate a
growth
curve for the two or more cell types.
The present invention also provides a method of generating at least one growth
curve using a system of the present invention, where the system includes a
multi-well
cell-substrate impedance monitoring device, an impedance analyzer, a device
station, and
a software program. The metliod includes; providing a multi-well cell-
substrate
impedance measuring system; adding cells of two or more cell types to two or
more wells
of the device, in which at least one of the two or more wells receives one
cell type and at
least one other of the two or more wells receives a different cell type, to
provide two or
more wells comprising two or more different cell types; monitoring impedance
from the
two or more wells comprising different cell types at three or more time points
after
adding the two or more cell types to the two or more wells; and plotting
impedance
measurements or impedance values for the three or more time points versus time
to
generate a growth curve for the two or more cell types.
As, described above for proliferation assays, impedance is preferably
monitored
using an impedance monitoring device or system at four or more time points,.
in which at
least one of the four or more time points is measured from fluid containers
prior to adding
cells to the fluid containers. Preferably, impedance is monitored at regular
or irregular
time intervals for an assay period of from minutes to days, for example, for a
period of
between several hours and several days.
It is preferable to perform replicate proliferation assays in which more than
one
fluid container is seeded with same number of cells of the same type. In this
case, a plot
can optionally be generated by plotting averaged impedance measurements of
values at
assayed time points for replicate wells versus time. Preferably, a standard
deviation for
the averaged values is also calculated.
Growth curves for different cell types can be generated as described above.
Impedance or impedance values, such as cell index, normalized cell index, or
delta cell
index can be plotted versus time. The impedance measurement or cell index axis
(typically the y-axis) can optionally use a log scale.
64
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
A growrth curve generated from impedance measurements or cell index values
(includina normalized cell index values and delta cell index values) can
optionally be
used to calculate one or more kinetic parameters of cell growth or behavior.
For example;
a g-ro-Mh curve can be used to calculate the duration of a lag phase, cell
attachment time,
cell attachment rate, or a cell doubling time.
Preferably, the growth curves of the two or more different cell types, or
kinetic
parameters derived from the growth curves of the two or more different cell
types, are
compared to determine differences among the cell types in proliferation
patterns or rates,
or in kinetic parameters that can be derived from growth curves. The different
cell types
used can be any cell types, including primary cells isolated from blood or
tissue of an
animal or human, or cells from cell lines. For example, proliferation rates of
two types of
primary cancer cell can be compared, or of primary cancer cells of tlie same
type but
different grades. In another example, primary cells of individuals of
different genotypes
can be compared. In another example, proliferation rates of primary or cell
line stem cells
can be compared. In yet another example, growth curves or parameters of
control and
genetically modified cells of a cell line can be compared. In yet another
example, growth
curves or parameters of cells infected with virus and control cells can be
compared.
D.2. Quantifying Cells Using Cell-Substrate lmpedance Devices
The present invention also includes a method of quantifying cells, compri
sing:
providing a device of the present invention having two or more electrode
arrays, each of
which is associated with a fluid container of the device; attaching the device
to an
impedance analyzer; adding cells to one or more fluid containers of the
device;
monitoring impedance from the one or more fluid containers to obtain impedance
measurements at one or more time points after adding the cells to the one or
more fluid
containers; deriving a cell index for the one or more time points; and using
the cell index
to determine the number of cells in the one or more fluid containers at least
one of the
one or more time points. The cell index is used to determine the number of
cells using a
formula that relates cell index to cell number, in which the formula is
obtained by:
providinQ a device for cell-substrate monitoring, attaching the device to an
impedance
mon.itor; addina, cells to one or more fluid containers of the device;
measuring impedance
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
of the one or more fluid containers comprising cells: calculating a cell index
from the
impedance measurements; determining the nunlber of cells of said at least one
fluid
container at the time of impedance monitoring by a means other than impedance
monitoring; and deriving a formula that relates the number of cells of the one
or more
fluid containers at the two or more time points with the impedance
measurements at the
two or mor-e time points.
In the embodiment of above method for obtaining the formula, sometime, the
number of cells introduced to the wells are pre-known or predetermined before
cells are
added in to the wells. Under such conditions, one assumes that there will be
no change in
cell number or little change in cell nuniber when the impedance measurement
for
obtaining the formula is performed.
The number of cells determined by a method other than impedance monitoring
can be determined by, for example, cell plating, hemacytometer counting, flow
cytometry, or Coulter counting.
The method can also be practiced using an impedance monitor ing system of the
present invention, where the system includes a multi-well cell-substrate
impedance
monitoring device, an impedance analyzer, a device station, and a software
program. The
method includes; providing a multi-well cell-substrate impedance measuring
system;
adding cells one or more wells of the system; monitoring impedance from the
one or
more wells comprising cells at one or more time points after adding the cells
to the one or
more wells; deriving a cell index for the one or more time points; and using
the cell index
to determine the number of cells in said at least well at at least one of said
one or more
time points.
The cell index is used to determine the number of cells using a formula that
relates cell index to cell number, in which the formula is obtained by:
providing a system
for cell-substrate monitoring, where the system comprises at least one multi-
well cell-
substrate impedance monitoring device, adding cells to one or more wells of a
device of
the system; measuring impedance of the one or more wells comprising cells at
two or
more time points; calculating a cell index from the impedance measurement at
the two or'
more time points; determining the number of cells of the one or more wells at
the two or
more time points by a means other than impedance monitorinQ: and deriving a
formula
66
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
that relates the number of cells of the one or more wells at the two or more
tinie points
with the impedance measurements at the two or more tinle points.
In the embodiment of above method for obtaining the formula, sometime, the
number of cells introduced to the wells are pre-known or predetermined before
cells are
added in to the wells. Under such conditions, one assumes that there will be
no change in
cell number or little change in cell number when the impedance measurement for
obtaining the fomlula is performed.
The number of cells detemlined by a method other than impedance monitoring
can be determined by, for example, cell plating, hemacytometer counting, flow
cytometry, or Coulter counting.
Formulas relating cell index (including normalized cell index and delta cell
index,
which can also be used) to cell number for a given cell type can be used lo
quantitate
cells of that type in assays using a cell-substrate impedance monitoring
device, such as a
device described herein. Generally, for a give cell type and for cells under
similar
physiological conditions, the derived formulas relating cell index to cell
number can be
used in subsequent assays. There is no need to obtain the formula each time
when an
assay is performed. However, it is worthwhile to point that .the formula can
only be valid
.as long as the cells are under same physiological conditions in the assays
where the
formula was derived and where the formula is used. If the cell condition is
different, for
example, the composition of culture medium changed, or the cell attachment
surface is
altered, then the formula will not hold. In another example, if cells are in
log-growth
phase in one assay and are in stationary phase in another assay, then the
formula may not
hold. Another point worth mentioni.ng here is that relates the fact the
derived cell index
or impedance also depends on cell attachment quality on the surface as well as
cell
morphology. If cell morphology or cell attachment changes during an assay,
then one
need to distinguish between the changes caused by change in cell number or in
cell
morphology or in cell attachment.
As an example, we can derive the correlation formula between cell index and
cell
number for NrII-i3T3 cells under the experimental conditions. The formula for
converting
cell index to cell number for this particular case is: Cell number = 2000*
Cell index -
67
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
145. To test this fonnula, we found the error in estimating cell number based
on the cell
index data shown in FiQure 8 as compared to the seeded cell number is less
than 20%
.5 D.3. Cell-based assays to test the effects of compounds on cells
In yet another aspect, the present invention provides a method for performuzg
a
cell-based assay investigating the effect of one or more test compounds on
cells,
comprising: providing a device of the present invention having two or more
electrode
arrays, each of which is associated with a fluid container or well that is at
least in part
coated with a biological compound or organic molecule; attaching the device to
an
impedance analyzer; introducing cells into two or more fluid containers of
tlie device that
comprise an electrode array; adding at least one test compound to at least one
of the one
or more of the fluid containers comprising cells and an electrode array to
provide at least
one test compound well; providing at least one control well to which cells are
added that
does not receive test compound; and monitoring cell-substrate impedance of the
one or
more test compound fluid containers and the one or more control fluid
containers at least
three time points after adding the one or more test compounds, and analyzing
impedance
measurements from the one or more test compound fluid containers and the one
or more
control fluid containers at at least three time points after adding the one or
more test
compounds, in which changes in impedance can provide information about cell
responses
to the one or more test compounds.
In a related aspect the present invention also provides a method for
performing a
cell-based assay investigating the effect of one or more test compounds on
cells, where
the system includes a multi-well cell-substrate impedance monitoring device,
an
impedance analyzer, a device station comprising electronic circuitry that
engages the
device and connects the two or more electrode arrays of the device to the
impedance
analyzer, and a software program that controls the device station and can
record and
analyze data from the impedance analyzer. The method includes; providing a
multi-well
cell-substrate impedance measuring system; introducing cells into two or more
wells of
the device coated with a biological molecule or orcyanic compound; adding at
least one
test compound to at least one of the one or more of the wells comprising cells
to provide
68
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
at least one test compound well; providing at least one control well to which
cells ai-e
added that does not receive test compound; monitoring cell-substrate impedance
of the
one or more test compound wells and the one or more control wells at at least
three time
points after adding the one or more test compounds; and analyzing impedance
measurements from the one or more test compound wells and the one or more
control
wells at at least tlv-ee time points after adding the one or more test
compounds, in which
changes in impedance can provide information about cell responses to the one
or more
test compounds.
A test compound can be any compound, including a small molecule, a large
molecule, a molecular complex, an organic molecule, an inorganic molecule, a
biomolecule such as but not limited to a lipid, a steroid, a carbohydrate, a
fatty acid, an
amino acid, a peptide, a protein, a nucleic acid, or any combination of these.
A test
coinpou.nd can be a syntlietic compound, a naturally occurring compound, a
derivative of
a naturally-occurring compound, etc. The structure of a test compound can be
known or
unlcnown.
Inforination about cell responses to the one or more test compounds includes,
but
is not limited to, information about cell attachment or adhesion status (e.g.
the degree of
cell spread, the attachnient area of a cell, the degree of tightness of cell
attachment, cell
morphology) on the substrate including on the electrodes, cell growth or
proliferation
status; number of viable cells and/or dead cells in the well; cytoskeleton
change and re-
organization and number of cells going through apoptosis and/or necrosis.
Information
about cell status may also include any compound-cell interaction leading to
any change to
one or more of above cell status indicators. For example, if the compound
binds to a
receptor on the cell surface and such binding leads to a change in cell
morphology, then
the binding of compound to the receptor can be assayed by the monitored cell-
substrate
impedance.
The cells used in the assay can be primary cells isolated from any species or
can
be cells of cell lines. The cells can be genetically engineered cells (For
example, cells
from a Qeneticall}~ modified organism, such as for example from a"Qene
knockout"
oraanism, or cells that have been enaineered to over-express an endogenous
gene or a
transQene. or cells whose normal Qene expression has been manipulated by use
of
69
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
antisense molecules or silencing Ri\TA.) In sonie embodiments. different cell
types are
added to different wells and the behavior-of the different cell types in
response to one or
more compounds is compared.
The cell-based assays that be performed with above methods include, but are
not
limited to, cell adhesion, cell spreading apoptosis, cell differentiation,
cell proliferation,
cell survival, cytotoxicity, cell morphology detection, cell quantification,
cell quality
control, time-dependent cytotoxicity profiling, IgE-mediated cell activation
or
stimulation, receptor-ligand binding, viral, bacterial, or environmental
toxiui mediated
cell pathologic changes or cell death, detection or quantification of
neutralizing
antibodies, specific T-cell mediated cytotoxic effect, and cell-based assay
for screening
or measuring ligand-receptor binding.
In the methods of the present invention that investigate test compound effects
on
cells, impedance is preferably monitored from at least one test compound well
at at least
one time point before adding said at least one test compound to said at least
one test
conZpound well. Preferably, impedance is monitored at four or more time
points, at least
one of which is prior to the addition of one or more test compounds.
Preferably,
impedance is monitored at regular or irregular time intervals for an assay
period of from
minutes to days, for example, for a period of between several hours and
several days. In
one embodiment of the above cell-based assay, the cell-substrate impedance is
monitored
at at least one time point prior to addition of the test compound, and at
regular time
intervals tllereafter. For example, impedance can be measured at one or more
intervals
before adding the compound and at a regular 2 hour, 1 hour, 30 min or 15 min
time
intervals after adding the compound. Preferably, impedance is measured at
three or more
time points spaced at regular intervals. In the present application, a real-
time assay
means allows one to perform the measurement on cell-substrate impedance with
various
time resolutions, for example, measurement taking place at a longer time
interval such as
every hour or every two hours, or at a shorter time interval every ininute or
a few
minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
example, in some preferred embodiments, impedance is monitored over a ranae of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
is monitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and about 2 MHz.
It is preferable to perform replicate test compound assays in which more than
one
fluid container of cetls receives the same compound at the same concentration.
hi tlus
case, impedance measurements or values can be averaged for the assayed time
points for
replicate wells. Preferably, a standard deviation for the averaged values is
also calculated.
In the methods of the present invention, analyzing impedance can comprise
plotting cell impedance versus time to obtain at least one test compound
impedance curve
and at least one control impedance curve. Preferably, at least one test
compound
impedance curve and said at least one control impedance curve are compared to
identify a
time frame, if any, in which a test compound curve differs significantly from
a control
curve, indicating a time frame of an . effect of a test compound on cells. For
example,
depending on the time frame at which a test compound curve differs
significantly from a
control curve, the test compound can be hypothesized to affect one or more of,
for
exaniple, cell attachment or adhesion, cell growth or proliferation,
cytoskeleton
organization or function, or apoptosis or cell death.
Preferably, data from impedance monitoring of.a well that comprises cells and
a
test compound is compared with data from impedance monitoring of a well.that
comprises cells in the absence of a test compound, however, this is not a
requirement of
the present invention. For example, it is also possible to compare impedance
measurements from one or more time points prior to the addition of compound to
compare impedance measurements from one or more time points after the addition
of
compound. Such comparisons can be used directly to assess the cells' response
to a
compound. It is also possible to calculate a cell index (or cell number index)
using the
impedance values obtained.
Methods of calculating a cell index (cell number index) are disclosed herein
as
well as in parent application U.S. Patent Application 10/705,447, U.S. Patent
Application
No. 10/987,732..both herein incorporated by reference for disclosures relating
to cell
number index and its calculation. The cell index calculated from impedance
measurements of wells receiving compound can be compared Arith the cell index
calculated from impedance measurements of control wells to assess the effect
of a
71
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
conlpound on cells. Altematively, cell index calculated from impedance
measurements of
wells from one or more time points after the addition of a compound can be
compared
with the cell index calculated from impedance measurements of wells from.one
or more
time points prior to the addition of a compound to assess the effect of a
compound on
cells. In some preferred embodiments, the cell index can be used as an
indicator of
cytotoxicity.
The derivation of cell index from iunpedance measurements is provided in
Section
C of the present application. Cell index values (including normalized cell
uidex values
and delta cell index values) from at least three time points from at least one
test
compound well and at least one control well can be plotted versus time to
obtain one or
more test compound cell index curve and one or more control cell index curves.
The one
or more test compound cell index curves and the one or more control cell index
curves
can be conipared to identify a time frame, if any, in which a test compound
curve differs
significantly from a control curve, uzdicating a time frame of an effect of a
test compound
on cells. For example, depending on the time frame at which a test compound
curve
differs significantly from a control curve, the test compound can be
hypothesized to
affect one or more of, for example, cell attachment or adhesion, cell growth
or
proliferation, cytoskeleton organization or function, or apoptosis or cell
death.
Cell index values at three or more assay time points for one or more test
.20 compound wells and one or more control wells can be used to derive cell
change index
(CCI) values or a second order derivatives of cell index at three or more
assay time
points. The calculation of cell change index is provided in Section C of the
present
application. The value of CCI at a give time point can be determined to be
either
approximately equal to 0.7, much greater than 0.7, greater than zero and less
than 0.7,
approximately equal to zero, less than zero, or much less than zero. These
values can
indicate cell behavior at an assay time point, as CCI approximately equal to
0.7 indicates
log rate growth, a CCI much greater than 0.7 indicates faster than log rate
growth, a CCI
greater than zero and less than 0.7 indicates slower than log rate growth, a
CCI
approximately equal to zero indicates no aTowth (a constant cell index), a CCI
less than
zero indicates cells are detaching from the substrate, and a CCI much less
than zero
indicates cell are detaching rapidly from the substrate.
72
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
For a Riven assay time point, differences in CCI value between control and
compound treated wells can indicate a time at which the compound has an_effect
on cells,
as well as providing information on the type of effect the compound has.
The CCI can further be used to obtain information on the effect of a test
compound by plotting CCI versus time for at least tlu-ee assay time points to
obtain a cell
change index cunle (CCI curve) for at least one control container or well and
at at least
one test compound container or well. One or more test compound CCI curves can
be
compared wit17 one or more control CCI curves to obtaul information on cell
statusor
behavior in response to said at least one test compound, wherein said cellular
status or
behavior is at least one of: cell attachment or adhesion status; cell growth
or proliferation
status; the number of viable cells or dead cells; cytoskeleton change or re-
organization; or
the number of cells going through apoptosis or necrosis.
Cell-based Assays with More Than One Cell Type
The present invention also provides metliods of comparing the effects of a
compound on two or more cell types. In one aspect, the method comprises:
providing a device of the present invention having two or more electrode
arrays, each of
which is associated with a fluid container of the device; attaching the device
to an
impedance analyzer; introducing cells into two or more fluid containers of the
device that
comprise an electrode array, wherein at least one of the two or more fluid
containers
receives one cell type and at least one other of the two or more fluid
containers receives a
different cell type; adding a test compound to the one or more fluid
containers receiving
one cell type and adding the test compound to the one or more fluid containers
receiving
a different cell type to provide at least two test compound fluid containers
that comprise
cells of different types; providing at least two control fluid containers that
do not receive
test compound, in which at least one of the control fluid containers receives
cells of the
one type and at least one of the control fluid containers receives cells of
the different
type; monitoring cell-substrate impedance of the two or more test compound
fluid
containers that comprise different cell types and the one or more control
fluid containers
at at least three time points after adding the one or more test compounds; and
analyzing
impedance measurements from the two or more test compound fluid containers
73
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
comprising diffei-ent cell types and from the one or more control fluid
containers at at
least three time points after adding the one or more test compounds, in -hich
changes in
impedance can provide information about cell responses to the one or more test
compounds.
In a related aspect the present invention also provides a method for
perfomiing a
cell-based assay investigating the effect of one or more test conipounds on
cells using a
cell-substrate impedance monitoring system of the present invention, where the
system
includes a multi-well cell-substrate impedance monitoring device, an impedance
analyzer, a device station comprising electronic circuitry that engages the
device and
com-iects the two or more electrode arrays of the device to the impedance
analyzer, and a
software program that controls the device station and can record and analyze
data from
the impedance analyzer. The method includes: providing a multi-well cell-
substrate
impedance measuring system; introducing cells into two or more wells of the
device that
conzprise an electrode array, wherein at least one of the two or more wells
receives one
cell type and at least one other of the two or more wells receives a different
cell type;
adding a test compound to the one or more wells receiving one cell type and
adding the
test compound to the one or more wells receiving a different cell type to
provide at least
two test compound wells that comprise cells of different types; providing at
least two
control wells that do not receive test compound, in which at least one of the
wells
receives cells of the one type and at least one of the control wells receives
cells of the
different type; monitoring cell-substrate impedance of the two or more test
compound
wells that coinprise different cell types and the one or more control wells at
at least three
time points after adding the one or more test compounds; and analyzing
impedance
measurements from the two or more test compound wells comprising different
cell types
and from the one or more control wells at at least three time points after
adding the one or
more test compounds, in which changes in impedance can provide information
about cell
responses to the one or more test compounds. -
In the methods of the present invention that investiaate test compound effects
on
cells, impedance is preferably monitored from at least two test compound wells
comprising different cell types at at least one time point before adding test
compound to
the at least one two compound wells. Preferably, impedance is monitored at
four or more
74
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
time points_ af least one of which is prior to the addition of one or more
test conlpounds.
Preferably. impedance is monitored at regular or irregular time intervals.for
an assay
period of from minutes to days, for example, for a period of between several
hours and
several days. In one embodiment of the above cell-based assay, the cell-
substrate
inlpedance is monitored at at least one time point prior to addition of the
test compound,
and at regular time intervals thereafter. For example, impedance can be
measured at one
or more intervals before adding the compound and at a regular 2 hour, 1 hour,
30 min or
niin time intervals after adding the compound. Preferably, impedance is
measured at
tliree or more time points spaced at regular intervals. In the present
application, a real-
10 time assay means allows one to perform the measurement on cell-substrate
impedance
with various time resolutions, for example, measurement taking place at a
longer time
interval such as every hour or every two hours, or at a shorter time interval
every minute
or a few minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
15 example, in some preferred embodiments, impedance is monitored over a range
of
frequencies for each time point at wh.ich impedance is monitored. Preferably,
impedance
is moilitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and= about 2 MHz.
As disclosed in an earlier section on compound assays, a test compound can be
any compound whose effect on cells can be investigated. A test compound used
in assays
comparing cell responses can be a compound whose effect on one or more of the
cell
types to be assayed is known, or can be a conlpound whose effects on any of
the cell
types to be assayed are unknown. In preferred methods of the present
invention, cells are
introduced into at least three wells of the device that each comprise an
electrode array,
and at least one well that comprises an electrode array and comprises cells
does not
receive a test compound. A control well that does not receive a test compound
can be
monitored, and its impedance data can be compared with that of wells that
receive a
compound to determine the effect of the test compounds on cells.
As disclosed in a previous section for compound assays, the cell types used in
the
3 '0 assay can be primary cells isolated from any species or can be cells of
cell lines. In some
preferred embodiments, the different cell t~,pes are the same t.Te of cell
from different
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
individuals, and thus have different genotypes. One or more of the cell types
can be
venetically engineered (For example, cells from a Qenetically modified
organism, such as
for example from a"gene knockout" organism, or cells that have been engineered
to
overexpress an endogenous gene or a transgene, or cells whose normal gene
expression
has been mauipulated by use of antisense molecules or silencing RNA.) In these
cases,
Qenetically modified cells can be compared with control cells. In another
example the
cells can be, for example, stem cells from different stages of differentiation
or of different
genotypes whose response to growth factors is being compared. In other
examples the
cells can be cancer cells where the test compound is tested for its cytotoxic
effects. The
cells can be primary cancer cells of the same type isolated from different
individuals, for
exanlple, or different cancer cell li.nes, or cancer cells of the same type
but of different
grades. In some embodiments, three or more different cell types are added to
different
wells and the behavior of the three or more different cell types in response
to one or more
compounds is compared. In preferred embodiments of the present invention, for
each cell
type tested there is a control performed in which the control does not receive
test
compound.
A variety of assays can be employed, where the effect of a test compound on
the
behavior of two or more cell types in the assay is under investigation. Such
assays
include, as noitlimiting examples, cell adhesion assays, apoptosis assays,
cell
differentiation assays, cell proliferation assays, cell survival assays,
cytotoxicity assays,
cell morphology detection assays, cell quantification assays, cell quality
control assays,
time-dependent cytotoxicity profiling assays, IgE-mediated cell activation or
stimulation
assays, receptor-ligand binding assays, viral, bacterial, or enviroiunental
toxin mediated
cell pathologic changes or cell death assays, detection or quantification of
neutralizing
antibodies, specific T-cell mediated cytotoxic effect assays, and cell-based
assays for
screeiiuig or measuring ligand-receptor binding.
In the assaysof the present invention is preferable to perform replicate test
compound assays in which more than one fluid container of cells of the same
type
receives the same compound at the same concentration. In this case, impedance
measurements or values can optionally be averaged for the assayed time points
for
replicate wells. Preferably, a standard deviation for the averaged values is
also calculated.
76
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Preferablv. time-dependent responses of the first and second types of cells
are
compared to see how similar or different the responses from the two types of
cells are.
In one method of the present invention, impedance from a first cell type well
is plotted
versus time to Qive a first cell type impedance curve and impedance from a
second cell
type well is plotted versus time to give a second cell type impedance curve.
Cell index
(including normalized cell index or delta cell index) from wells comprising
cells of
different types ca.n also be calculated from impedance data and plotted versus
time to
give cell index curves.
The impedance curves or cell index curves from the different cell types can be
compared to deterniine whether the time frame, magnitude, and duration of a
cells
response to a compound are similar or different. Preferably, impedance curves
or cell
index curves generated from control wells comprising each cell type in the
absence of
compound are compared with the test compound curves to assess the compound-
specific
effects on each cell type. The effects of the compounds on one or more of the
two or
more cell types can be effects on cell attachment or adhesion, cell growth or
proliferation;
the number of viable cells or dead cells; cytoskeleton organization or.
fimction; or the
number of cells going through apoptosis or necrosis in response to a test
compound.
Assays can be designed to investigate the compound's effects on particular
cellular
processes or activities.
The effect of a compound on at least one of the cell types used in the assay
may
be known. The mechanisin of action of a compound on at least one of the cell
types used
in the assay may be known. In such cases, comparison of the compound response
of one
or more different cell types with the compound response of a cell type whose
response to
the compound is characterized can give information as to the similarity or
difference in
response of a different cell type to the compound.
The CI derived from impedance data from wells comprising different cell types
and a test compound can be used to derive cell change index (CCI) values for
assay time
points. CCI values of particular cell types at assa), time points can be
compared. Such
comparisons can indicate wbether different cell types are responding similarly
to a
compound_ CCI can also be plotted versus time, and CCI curves of cells of
different types
77
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
assayed with one or more test compounds can be compared to determine the
similarities
or differences in cellular responses of different cell types to a test
compound.
Cell-based Assays iilith More Than One Con7pound
The present invention also provides metllods of comparing the effects of two
or
more different compounds on cells. In one aspect, the method comprises:
providing a device of the present invention having three or more electrode
arrays, each of
which is associated with a fluid container of the device; attaching the device
to an
impedance analyzer; introducing cells into three or more fl uid containers of
the device
that coinprise an electrode array; adding at least one test compound to at
least one of the
tliree or more fluid containers comprising cells and adding at least one
different test
compound to at least one other of the three or more fluid containers
comprising cells to
provide at least two different test compound fluid containers; providing as a
control fluid
container at least one of the three or more fluid containers, in which the
control fluid
container receives cells but does not receive compound; attaching an impedance
analyzer
to the device; monitoring cell=substrate impedance of the two or more
different test
compound fluid containers that comprise different compounds and the one or
more
control fluid containers at at least three time points after adding the one or
more test
compounds; and analyzing impedance measurements from the two or more different
test
conlpound fluid containers and from the one or more control fluid containers
at at least
three time points after adding the one or more test compounds, in which
changes in
impedance can provide information about cell responses to the one or more test
compounds.
In a related aspect, the present invention provides a method for performing a
cell-
based assay investigating the effect of two or more test compounds on cells
using a cell-
substrate impedance monitoring system. The method includes: a) providing a
cell-
substrate impedance monitoring system of the present invention; b) introducing
cells into
at least two wells of the device that each comprise an electrode array; c)
adding to at least
one well of the device comprising cells and an electrode array a first test
compound; d)
adding to at least one other well of the device comprising cells and an
electrode array a
second test compound, and e) monitoring cell-substrate impedance of at
l:zst.one well
78
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
comprisinR cells and a first compound and at least one well comprising cells
and a second
compound, in which changes in impedance can provide information about cell
responses
to the first and second compounds.
Preferably, time-dependent responses of cells to the first compound and the
second compound are compared to see how similar or different the responses
from the
two compounds are. In one preferred embodiment of this method, time-dependent
cytotoxic responses are compared.
The cells and test compound that can be used in the assay can be any as
described
above for assays testing effects of test compounds.
In the assays of the present invention is preferable to perform replicate test
compound assays in which more than one fluid container of cells of the same
type
receives the same compound at the same concentration. In this case, impedance
measurements or values can optionally be averaged for the assayed time points
for
replicate wells. Preferably, a standard deviation for the averaged values is
also calculated.
Impedance monitoring can be as described above for assays testing effects of
test
compounds. Preferably impedance is monitored from the at least two different
test
compound wells and at least one control well at at least one time point before
adding said
at least one test compound to said at least one test compound well.
Preferably, impedance
is monitored at four or more time points, at least one of which is prior to
the addition of
one or more test compounds. Preferably, impedance is monitored, at regular or
irregular
time intervals for an assay period of from minutes to days, for exainple, for
a period of
between several hours and several days. In one embodiment of the above cell-
based
assay, the cell-substrate impedance is monitored at at least one time point
prior to
addition of the test compound, and at regular time intervals thereafter. For
example,
impedance can be measured at one or more intervals before adding the compound
and at
a regular 2 hour, 1 hour, 30 min or 15 min time intervals after adding the
compound.
Preferably, impedance is measured at three or more time points spaced at
regular
intervals. In the present application, a real-time assay means allows one to
perform the
measurement on cell-substrate impedance with various time resolutions, for
example,
measurement taking place at a longer time interval such as every hour or every
two hours,
or at a shorter time interval every mi.nute or a few minutes.
79
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Impedance can be monitored at one frequency or at more than one frequency. For
exanlple_ in some preferred embodinlents, impedance is monitored over a range
of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
is monitored at at least one frequency between about 1 Hz and about 100 h411z,
more
preferably at at least one frequency between about 100 Hz and about 2 1\glz.
Preferably, data from impedance monitoring of wells that comprise different
test
compounds are compared.
In one embodiment, for at least two different compound wells, impedance at
three
or more assay time points can be plotted versus tinie. Preferably, for a
control well that
does not receive compound, impedance at the same three or more assay time
points is
also-plotted versus time. The impedance curves of different compound wells can
be
compared with the control impedance curve to determine whether the compounds
have a
similar or different effect on cells.
Cell uidex (including normalized cell index or delta cell index) from wells
comprising cells of different types can also be calculated from impedance data
and
plotted versus time to give cell index curves.
The impedance curves or cell index curves from the different cell types can be
compared to determine whether the time frame, magnitude, and duration the
response of
cells to different compounds are similar or different. Preferably, impedance
curves or cell
index curves generated from one or more control wells comprising cells in the
absence of
compound are coinpared with the test compound curves to assess the compound-
specific
effects of each compound. The effects of the compounds on cells can be for
example,
effects on cell attachment or adhesion, cell growth or proliferation; the
number of viable
cells or dead cells; cytoskeleton organization or function; or the number of
cells going
through apoptosis or necrosis in response to a test compound. Assays can be
designed to
investigate the compound's effects on particular cellular processes or
activities.
The effect on cells of one or more of the compounds used in the assay may be
knoNArn. The mechanism of action of one or more compounds used in the assay
may be
knoxvn. In such cases, comparison of the responses of cells to other test
compounds used
in the assay -;rith cellular responses to the one or more compounds whose
effects are
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
characterized can give information as to the similarity or difference in
response of
different compounds to a l:no-wn compound.
In.formation about cell responses to the compound includes, but is not limited
to,
information about cell attachn-tent or adhesion status (e.g. the degree of
cell spread, the
attachrnent area of a cell, the degree of tightness of cell attaclinient, cell
morphology) on
the substrate includina on the electrodes, cell growth or proliferation
status; number of
viable cells and/or dead cells in the well; cytoskeleton change and re-
organization and
number of cells going through apoptosis and/or necrosis. Information about
cell status
may also include any compound-cell interaction leading to any change to one or
more of
above cell status indicators. For example, if the compound binds to a receptor
on the cell
surface and such binding leads to a change in cell morphology, then the
binding of
compound to the receptor can be assayed by the monitored cell-substrate
impedance. The
cell-based assays that be performed with above methods include, but not
limited to, cell
adhesion, cell apoptosis, cell differentiation, cell proliferation, cell
survival, cytotoxicity,
] 5 cell morphology detection, cell quantification, cell quality control, time-
dependent
cytotoxicity profiling, IgE-mediated cell activation or stimulation, receptor-
ligand
binding, viral and bacterial toxin mediated cell pathologic changes and cell
death,
detection and quantification of neutralizing antibodies, specific T-cell
mediated cytotoxic
effect, cell-based assay for screening and measuring ligand-receptor binding.
A plurality of compounds can be assayed with multiple cell types. In one
prefen-ed embodiment of this method, time-dependent cytotoxic responses of
different
cell types to a set of compounds are compared. .
The CI derived from impedance data from wells comprising different cell types
and a test compound can be used to derive cell change index (CCI) values for
assay time
points. CCI values of particular cell types at assay time pouits can be
compared. Such
comparisons can indicate whether different cell types are responding similarly
to a
compound. CCI can also be plotted versus tinse, and CCI curves of cells of
different types
assayed with one or niore test compounds can be compared to determine the
similarities
or differences in cellular responses of different cell types to a test
compound.
For example, the time frame, magnitude; and duration of a difference in
response
as evidenced b~- the curves can indicate a difference in efficacy or mechanism
of
81
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
compounds. The inlpedance differences can.reflect differences in, for
exaniple, cell
attachment or adhesion, cell g-rowth or proliferation; the number of viable
cells or dead
cells: cytosl:eleton organization or function; or the number of cells goinc,
through
apoptosis or necrosis in response to a test compound.
A variety of assays can be employed, where the effect of two or more test
compound on the behavior cells is under investigation. Such assays include, as
nonliiniting examples, cell adhesion assays, apoptosis assays, cell
differentiation assays,
cell proliferation assays, cell survival assays, cytotoxicity assays, cell
morphology
detection assays, cell quantification assays, cell quality control assays,
time-dependent
cytotoxicity profiling assays, IgE-mediated cell activation or stimulation
assays, receptor-
ligand binding assays, viral, bacterial, or environmental toxin mediated cell
pathologic
changes or cell death assays, detection or quantification of neutralizing
antibodies,
specific T-cell mediated cytotoxic effect assays, and cell-based assays for
screeiung or
measuring ligand-receptor binding.
In one preferred embodiment of this method, time-dependent cytotoxic responses
of cells to a set of compounds are compared. "Cytotoxicity profiling" in which
the
impedance responses of cells in response to a plurality of potentially
cytotoxic
compounds are compared, can provide information on the efficacy and mechanism
of a
test compound. Cytotoxicity profiling can be performed by comparing any
combination
of impedance plots, kinetic parameters derived from impedance plots, CI plots,
CCI
values, and CCI plots.
In one embodiment of the method, analyzing the cytotoxicity response may
include derivation of the slope of change in the time dependent cytotoxicity
response at a
given compound concentration. In yet another embodiment of the method,
analyzing
real-time cytotoxicity response may include derivation of high-order
derivatives of the
time dependent cytotoxicity response with respect to time at a given compound
concentration.
Evaluatinc the Effeci of Different Concentrations qf a Compound os? Cells .
The present invention also includes methods of performing assays to test the
effect of different concentrations of one or more test compounds on cells.
82
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
In one aspecL a method for testing different concentrations of a test compound
on
cells comprises: providina a device of the present invention having three or
more
electrode arrays, each of which is associated with a fluid container of the
device;
attachinQ the device to an impedance analyzer; introducing cells into at least
two of the
three or more fluid containers of the device that comprise.an electrode array;
adding
diffei-ent concentrations of a test compound to the two or more fluid
containers of the
device that comprise cells; providing a control fluid container that comprises
cells but
does not receive compound; monitoring cell-substrate impedance of the two or
more
different test compound fluid containers that comprise different
concentrations of a test
compound and of the one or more control fluid containers at at least three
time points
after adding a test compound; and analyzing impedance measurements from the
two or
more different test compound fluid containers and one or more control fluid
containers at
at least three time points after adding a test compound, in which changes in
impedance
can provide information about cell responses to the test compounds.
In a related aspect, the present invention provides a method for performing a
cell-
based assay investigating the effect of two or more concentrations of a test
compound on
cells using a cell-substrate impedance monitoring system. The method includes:
providing a cell-substrate impedance monitoring system of the present
invention;
introducing cells into at least two of the three or more wells of the device
that comprise
an electrode array; adding different concentrations of a test compound to the
two or more
wells of the device that comprise cells; providing a control well that
comprises cells but
does not receive test compound; monitoring cell-substrate impedance of the two
or more
different test compound wells that comprise different concentrations of a test
compound
and the one or more control wells at at least three time points after adding a
test
compound; and analyzing inlpedance measurements from the two or more different
test
compound wells and the one or more control wells at at least three time points
after
adding a test compound, in which changes in impedance can provide information
about
cell responses to the test compounds.
The cells and test compound that can be used in the assay can be any as
described
above for assays testing effects of test compounds.
83
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Impedance monitoring can be as described above for assavs testinR effects of
test
compounds. Preferably impedance is monitored from the at least two different
test
compound wells and at least one control well at at least one time point before
adding said
at least one test compound to said at least one test compound well.
Preferably, impedance
is monitored at four or more time points, at least one of which is prior to
the addition of
one or more test compounds. Preferably, impedance is monitored at regular or
irregular
time intervals for an assay period of from minutes to days, for example, for a
period of
between several hours and several days. In one embodiment of the above cell-
based
assay, the cell-substrate impedance is monitored at at least one time point
prior to
addition of the test compound, and at regular time intervals thereafter. For
example,
impedance can be measured at one or more intervals before adding the compound
and at
a regular 2 hour, 1 hour, 30 min or 15 min tinze intervals after adding the
compound.
Preferably, impedance is measured at three or more time points spaced at
regular
intervals. In the present application, a real-time assay means allows one to
perform the
measurement on cell-substrate itnpedance with various time resolutions, for
example,
measurements taking place at a longer time interval such as every hour or
every two
hours, or at a shorter time interval every minute or a few minutes.
Impedance can be monitored at one frequency or at more than one frequency. For
example, in some preferred embodiments, impedance is monitored over a range of
frequencies for each time point at which impedance is monitored. Preferably,
impedance
is monitored at at least one frequency between about 1 Hz and about 100 MHz,
more
preferably at at least one frequency between about 100 Hz and about 2 MHz.
In one embodiment, for at least two different compound concentrations,
impedance or, preferably, cell index (including normalized cell index or delta
cell index),
at three or more assay time points is be plotted versus tune. Preferably, for
a control well
that does not receive compound, impedance at the same three or more assay time
points is
also plotted versus time. An impedance curve or cell index curve can give an
indication
of the time frame at which a compound affects cell response. In some preferred
embodiments, the cell index can be used as an indicator of cytotoxicity.
84
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
C>>totoxicitl= Profiling,
In another aspect_ the present invention provides a method for performinQ real-
time
cytotoxicity assay of a compound. comprising: a) providina an above described
system;
b) seeding cells to the wells of multiple-well devices; c) adding the compound
to the
wells contauun(y cells; d) monitoring cell-substrate impedance before and
after adding the
conipound at a regular or iuregular time interval; wherein the time dependent
impedance
change provides information about time dependent cytotoxicity of the compound.
In one
embodiment, the cell-substrate impedance is monitored at regular tiriie
intervals. In
exemplary embodiments, the impedance is measured at a regular 2 hour, 1 hour,
30 min
or 15 min time interval before and after adding the compound.
lil one embodiment of the above method, multiple wells with same cell types
are
used, wherein each well is added with the compound of different
concentrations. The
method provides the time-dependent and concentration-dependent cytotoxic
responses.
In yet auother aspect, the present invention provides a method for analyzing
and
comparing time-dependent cytotoxic effects of a first compound and a second
compound
on a cell type, comprising : a) performing a real-time cytotoxicity assay on a
cell type
with the first compound using the method described above; b) performing a real-
time
cytotoxicity assay on said cell type with the second compound using the method
described above; c) comparing the time-dependent cytotoxic responses of the
first
compound and the second compound to see how similar or different the responses
from
the two compounds are. In one embodiment of this method, time-dependent
cytotoxic
responses are determined for the first compound at multiple dose
concentrations. In
another embod"unent, time-dependent cytotoxic responses are determined for the
second
compound at multiple dose concentrations. In yet another embodiment, time-
dependent
cytotoxic responses are determined for both first compound and second compound
at
multiple dose concentrations.
In another embodiment of above methods, the first compound is a compound with
a known mechanism for its cytotoxic effect and the second compound is a
compound
with an unl:nown mechanism for its cytotoxic effect. If the time dependent
cytotoxic
responses from the second compound are similar to that of the first one, the
second
compound max, follow a similar mechanism for its cytotoxic effect to the first
compound.
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Various approaches may be used in comparing the cytotoxic responses of the
compounds. A cell index (or cell nunzber index) can optionally be calculated
using the
impedance values obtained. In one embodiment of the method described above,
time
dependent IC50 may be derived for the compounds and comparison between their
cytotoxic responses is done by comparing their time dependent IC50 curves
based on cell
index values. If the IC50 curves follow a similar time-dependent trend, the
two
coinpounds may follow a similar mechanism for inducing cytotoxicty effects. In
another
embodiment of the method described, direct comparison of time-dependent
cytotoxic
responses of two compounds are done where the concentrations for the two
compounds
may be the sanle or may be different. Direct comparison between time-dependent
cytotoxic responses may be done by analyzing the slope of change in the
measured
responses (that is equivalent to the first order derivative of the response
with respect to
tinie) and comparing the time-dependent slopes for the two compounds. In
another
approach, the time-dependent cytotoxic responses may be analyzed for their
higher order
derivatives witli respect to time. Comparing such high order derivatives may
provide
additional information as for the mechanisms of compound-induced cytotoxicity.
In one embodiment of the method, analyzing real-time cytotoxicity response may
include the derivation of time-dependent IC50 values for the compound on the
multiple
cell types. In another embodiment of the method, analyzing real-time
cytotoxicity
response may include derivation of the slope of change in the time dependent
cytotoxicity
response at a given compound concentration. In yet another embodiment of the
method,
analyzing real-time cytotoxicity response may include derivation of high-order
derivatives of the time dependent cytotoxicity response with respect to time
at a given
compound concentration.
In yet another embodiment, the above methods are applied to perform
cytotoxicity
profiling of multiple compounds on multiple cell types.
In another embodiment of the method, analyzing real-time cytotoxicity response
may include derivation of the slope of change in the time dependent
cytotoxicity response
at a given compound concentration. In yet another embodiment of the method,
analvzing
real-time cvtotoxiciry response may include derivation of high-order
derivatives of the
86
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
time dependent cvtotoxicin- response with respect to time at a aiven compound
concentration.
Some examples of compound assays that can be perfornied using a cell-substrate
impedance system of the present invention are provided by way of illustration
with
reference to the fi'ures. In these examples, cell index is calculated using
the same method
as the Cell Index calculation method (A) as described in Section C of the
present
application. In some of the figures of the present application, Normalized
Cell Index was
plotted. The Noiznalized Cell Index at a given time point is calculated by
dividing the
Cell Index at the time point by the Cell Index at a reference time point.
Thus, tlie
Normalized Cell Index is 1 at the reference time point.
As described in the present application, if the cell attachment conditions
remain
unchanged or exhibit little change over the course of an assay that uses
impedan ce
monitoring, then the larger the cell index, the larger the number of the cells
in the wells.
A decrease in cell index suggests that some cells are detaching from the
substrate surface
or dying under the influence of the coinpound. An increase in cell index
suggests that
more cells are attaching to the substrate surfaces, indicating an increase in
overall cell
number.
D.4. Dynamic Monitoring of Cell Adhesion and Spreading
The methods and devices of the present invention have a particular utility for
monitoring cell adhesion and spreading. More specifically, the present
invention
includes a method of monitoring cell adhesion or cell spreading including
providing a
inicroelectronic cell sensor array that displays a biological molecule or
organic molecule
on a test portion and a control portion, introducing a cell or cell population
to the test
portion and control portion, performing a series of impedance measurements of
the test
portion and the control portion, determining the change in impedance and
optionally a
cell index (CI) of the test portion and the control portion, comparing the
change in
impedance of the test portion to the change in impedance of the control
portion or
comparing the cell index (CI) of the test portion to the cell index. (CI) of
the control
87
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
portion and determining cell adhesion or cell spreading occurs if the
comparison
demonsiTates a sigmif cant change in impedance.
The device for use in monitoring cell adhesion and cell spreadinQ has many of
the
features previously discussed in the present document and those incorporated
by
reference. The device includes a non-conductive substrate, a plurality of
electrode arrays
positioned on the substrate, where each electrode array includes at least two
electrodes
and each electrode is separated from at least one electrode by an area of non-
conductive
material. The device also includes a biological molecule or organic compound
and
optionally a control molecule or a control compound positioned on a portion of
the
substrate.
As previously discussed, the non-conductive substrate may be substantially
flat
and may have two opposing ends along a longitudinal axis. The device may have
electrically conductive traces in electrical communication with at least one
of the
electrode arrays and extending substantially longitudinally to one of the two
opposing
ends. The substrate may be constructed at least in part from any suitable non-
conductive
material such as glass, sapphire, silicon dioxide on silicon or an appropriate
polymer.
The device may have electrodes that have a width at a widest point of more
than 1.5 and
less than 10 times the width of the area of non-conductive material. Each
electrode array
may have a plurality of evenly spaced electrodes and each electrode array may
be
provided in any of the previously described configurations such as but not
limited to
interdigitated, concentric, sinusoidal, castellated and the like.
The methods of the present invention utilize a biological molecule or organic
compound attached or closely associated with the substrate or electrode.
Molecules or
compounds of interest may be bound or associated with the substrate or
electrode to
evaluate the effect on cell adhesion or cell spreading such as inducing,
increasing,
decreasing or inhibition. The biological molecule or organic compound is
attached to the
substrate or electrode using techniques that utilize covalent bonds, ionic
bonds, Van der
Waals forces and the like. The biological molecule may be directly attached or
bound to
the device or may be bound via an intermediate compound such as by usinQ poly-
L-
lysine.
ss
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Examples of binding bioloo--ical molecules or organic compounds are provided
in
the examples. The general method includes providing a microelectronic cell
sensor array
and incubatuig the biological molecule or organic conipound along a portion of
the
substrate (such as but not limited to in a well) under conditions suitable to
form a bond or
to closely associate the molecule or compound with the substrate or electrode.
Although
tecluuques may vary, the incubation may occur utilizing a solution having an
appropriate
pH and salt concentration such as but not limited to phosphate buffered saline
(PBS),
borate buffered saline (BBS) and the like. The incubation may occur at a
temperature
appropriate for the molecule or compound such as 4 degrees Celsius, room
temperature,
37 degrees Celsius and the like. The device may be washed one or more times
using a
suitable solution or may be blocked.using a blocking solution such as a
solution including
a protein that does not noticeably affect the assay. In some embodiments
bovine serum
albumin (BSA) is provided in solution form as a blocking solution.
Biological molecules or organic compounds that may be of particular interest
include a DNA molecule, an RNA molecule, a protein, a polypeptide, an
oligopeptide,
individual amino acids and the like. Another example is an antibody such as a
polyclonal, monoclonal or humanized antibody or a fragnient thereof including
light
chain, heavy chain, Fc portion, Fab portion, Fab'2 portion and the like. Also,
the
biological molecule or organic compound may be a ligand, a receptor, may
target an
integrin or cell surface receptor or may be an agonist or an antagonist. In
one
embodiment, the biological molecule includes a molecule having an arginine-
glycine-
aspartic acid (RGD) motif. The biological molecule or organic compound may be
purified or may be provided as an extract. For example, when using
extracellular matrix
proteins, the proteins may be isolated then bound to the substrate or
electrode or
alternatively, a combination of proteins may be provided in the form of an
extract or
unpurified mixture.
Preferably, the display of the biological molecule or organic compound is such
that a corresponding binding member or cell having the appropriate cell
surface receptor
is capable of recognizina and binding the molecule or compound. For example,
when
utilizing an antibody as a biological molecule, the preferred display would
lil:ely be such
that the Fab pornion is positioned generally upwards relative to the substrate
and the Fc
89
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
portion positioned downwards toward the substrate. However, if the Fc portion
where to
be the desired bindinQ site for a cell or protein of interest (such as but not
limited to
protein A) it may be desired to have the Fc portion positioned generally
upwards.
Nonetheless, when proteins, DNA, RNA, polypeptides, oliQopeptides and the like
are
attached to the substrate of the device, it is likely that a variety of
orientations will result.
In some embodiments the device includes at least one well. The well acts as a
fluid container to perform the analysis. The well would include an electrode
array and
may include a biological molecule or organic conipound. The wells may be
defined by
the particular purpose such as a test well and a control well. The control
well provided as
a control for the experiment or test and the test well typically as an
experimental well.
Depending on the desired test, both the control well and test well may include
the same
biological molecule or organic compound or a different biological molecule or
organic
compound. The test well and the control well may provide a molecule or
compound in
the same concentration or they may be provided in different concentrations.
In one embodiment the test well and the control well contain different
biological
molecules or organic compounds. The test well may contain a biological
molecule that is
suspected of having an effect on cell adhesion or cell spreading and the
control well may
contain a biological molecule or organic compound with a known effect on cell
adhesion
or cell spreading (such as increasing, decreasing or no effect).
In another embodiment the same sample of biological molecule or organic
compound is aliquoted between the test well and the control well. In this
configuration,
different cells may be added to the test well versus the control well, a
compound such as
an inhibitor or inducer may be added to one of the wells (or preincubated with
the cells)
or the biological molecule or organic compound rriay be provided at different
concentrations.
Cells may be added directly to tlie devices such as in the test well or
control well
or may be preincubated with one or more compounds. Preincubation may be
desired
when assaying for an inhibitor of cell adhesion or cell spreading.
Alternatively; the
inhibitor or other compound of interest may be added to the device at the same
time as
the cell or cell population or may be added later. The types of cells that may
be used to
monitor cell adhesion or cell spreading may vary. Typically a cell type or
cell
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
capable of adhering to the device, the bioloRical molecule or the electrode
would be
desired. Cells may be from any suitable oraanism such as human. bovine_
hamster,
swine, and the like. The cells may be prokaryotic or eukaryotic. Cells may be
primarly
cells or cell lines. Human cells or cell lines derived from human cells have a
particular
utility. Cells may be those associated with the imnlune system such as B-
lymphocytes,
T-lymphocytes, macrophages, granulocytes, mast cells and PBMCs.
A series of impedance measurements are performed to detect changes in cell
adhesion or cell spreading. Impedance may be measured at regular time
intervals,
irregular time intervals, or a combination thereof. Time intervals of interest
may be 1)
after coating a device with a biological molecule or compound of interest and
before
adding cells, 2) shortly after adding cells and 3) one or more measurements
over time.
However alternative time points may be desired. The impedance of the test
portion such
as the test well and control well are preferably taken simultaneously or
nearly
simultaneously. The impedance values between the test portion and the control
portion
may be compared or corresponding cell indexes may be compared to one another
to
determine changes in cell adhesion or cell spreading.
Cell substrate impedance measurement is directly related to the number of
cells
added to the electrodes and the electrode area covered by the cells. During
cell adhesion
experiments the number of cells added to each well is fixed and therefore the
change in
impedance is primarily derived from the degree of cell attachment and
spreading on the
electrode surface. Therefore as the suspended cells settle onto the surface of
the
electrode, adhere and undergo morphological transfonnation, the cell substrate
impedance increases proportionately with the area of the electrode covered by
the cell. In
addition, the strengtli of attachment, which may depend on the cell attachment
receptors
expressed on the surface of the cell or the biological molecule coated onto
the surface of
the electrode can also affect cell electrode impedance measurements.
Cell Index
The methods of the present invention may include comparing one or more
impedance
measurements or comparing one or more cell indexes or cell index values. In
one
embodiment, a cell indea is deteruuned by calculating, for each measurement
frequency,
9i
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
the relative-change iu resistance (a component of impedance) of a well when
target cells
are present in the well ~3,ith respect to that of the well when no cell is
present and then
fmding the maximum relative-chanQe in resistance for all frequencies measured.
The
maximum relative-change in resistance is used as cell indea. (see equation (4)
in Section
C. Methods for Calculating Cell Index (CI) and Cell Change Index (CCI) of the
present invention). If impedance is measured_at a single frequency, then the
relative
change in resistance (a component of impedance) of a well when cells are
present in the
well with respect to that of the well when no cell is present. Other methods
for
calculating cell index have been disclosed in a previous Section C. Methods
for
Calculating Cell Index (CI) and Cell Change Index (CCI) of the present
invention).
92
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
Ex4h4PLES
Example 1: Coating a Microelectronic Plate with a_Biolobical Molecule or
Organic Compound including Extracellular Matrix (ECM) Protein
Manunalian cells express membrane -bound receptors called integrins which
interact with ECM proteins in a specific manner. Upon interaction of integrins
with their
cognate ECM proteins, a signali.ng cascade is initiated inside the cell
leading to
attachment, spreading, growth, differentiation and morphological dynamics
depending on
the integrin and the ECM substrate. Coating of ACEA E-plates with ECM proteins
will
allow label-free, quantitative and real-time measurements of cellular
interaction with the
ECM protein using the RT-CES system.
As a nonlimiting example, the steps involved in coating a microelectronic
plate,
such as the ACEA E-plates, and determining cellular response may include the
following
steps:
(1) Pipette 40-50 L of the appropriate ECM protein dissolved in
phosphate-buffered saline (PBS) at a pre-determined concentration into
the wells of the ACEA E-plate. As a control, add PBS alone to one of
the wells or alternatively, coat with poly-L-Lysine which coats the
surface but does not interact with integrins.
(2) Allow the E-plate to coat with the matrix solution for 1-2 hours at 37 C
or overnight at 4 C.
(3) Wash the wells once with PBS. Add 50 L of media to the wells and
obtain the background impedance using the RT-CES system.
(4) Add the cells at the appropriate density to the wells and monitor
attachment, spreading, uowth and morphological dynamics using the
RT-CES s;stem.
9-11
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
As an exanlple. we describe here the use of the ACEA RT-CESTM system to
measure and monitor the attachment and spreading of NIH-3 ) T3 cells on ACEA E-
plates
coated with fibronectin (FN) or with poly-L-lysine (PLL) as a control. 40 uL
of 10
JrnL FN solution in PBS and 40 L of 50 ghnL PLL were added to the wells of
ACEA 16X E-plate. The plate was placed at 37 C for lhour to allow coating to
take
place followed with PBS wash and then 50 L of media was added to E-plate to
measure
the background impedance. 5000 NIH3T3 cells in 100 L volume were then added
to the
wells and the plates were placed on the device station in the 37 C. The
attachment and
spreading of NIH3T3 cells on the two different surfaces were then monitored
every 5
minutes using the RT-CES system (FIG. 1). The kinetic trace of NIH3T3 cells on
FN
shows an immediate increase in cell index which correlates with the attachment
and
spreading of these cells wlule on PLL it is steady increase overtime. The
attachment and
spreading of NIH3T3 cells on FN is a rapid process and takes place within 5
minutes of
adding the cells to the coated wells (FIG. 2). The cells spread for
approximately an hour
at which time they contract due to stress fiber formation. On PLL the cells
attach non-
specifically due to charge interaction between PLL and the negatively charged
proteins
on the cell membrane. However, on PLL cell spreading does not take place for
at least the
first two hours after which the cells spread due to the fact that they secrete
their own
matrix. Also, to demonstrate that quantitative nature of the. Cell Index
readout, the wells
of ACEA E-plates were coated with increasing amounts of FN in the range of 0
g/mL to
20 g/mL and the attaclunent and spreading of NIH3T3 cells were monitored by
the RT-
CES (FIG. 3). T'he kinetic measurements for attachrnent and spreading clearly
indicate
that with increasing amounts of FN being coated on the wells, the index of
cell
attachnlent and spreading increases accordingly. In order to demonstrate the
specificity
of the interaction between integrins on the cell surface and the FN-coated
dish, arginine-
.glycine-aspartic acid (RGD) peptides were added to the wells in increasing
concentration
prior to adding the cells. As shown in FIG. 4, the RGD peptides effectively
block the
attachment and spreading of NU-I3T3 cells in a concentration-dependent manner,
whereas
a control peptide does not. In summary, these experiments demonstrate that the
wells in
ACEA E-plates containing the microelectronic cell sensor arrays can be coated
A-ith
matrix proteins which can affect cellular function in a specific way.
Furthermore, the
94
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
results obtained are very quantitative and can be performed in a high
throughput manner
and in real-time.
Example 2: Coating a Microelectronic Plate with a Biological Molecule or
Organic Compound Including an Antibody Against a Cell Surface
Receptor
In addition to coating the wells of ACEA E-plates with ECM proteins, the wells
can also be coated with other biological molecules such as antibodies specific
for a
particular receptor present on the cell surface. These antibodies can be
functional
antibodies (triggering a specific cellular response) or non-functional
antibodies. The main
requirement being that they recognize and bind to specific epitopes within the
receptor of
interest at the cell surface. As an example, we have coated the wells of ACEA
E-plate
with the OKT-3 antibody which is specific for the CD3 co-receptor of the T
cell receptor
complex. OKT-3 is a functional antibody and upon binding to the CD3 co-
receptor on T
lymphocytes triggers a signaling pathway leading to activation and adhesion of
the T cell.
As a control, the wells were also coated with an irrelevant mouse monoclonal
antibody.
As shown in FIG. 5, upon addition of Jurkat T cells, which express the CD-3
receptor, to
the wells coated witlz the OKT-3, the cells immediately attach and spread as
evidenced by
an immediate increase in cell index. However, in wells coated with the control
antibody
very little attachmerit and spreading is taking place.
The following steps provide a method of coating microelectronic plate (the
ACEA
E-plate) wells with an antibody specific. for cell surface receptors:
(1) Adjust the antibody to be coated to the appropriate concentration in
PBS and add 40-50 L per well of the ACEA E-plate. It may also
be required with some antibodies to covalently li.nk the antibody to
the surface of the sensors in the E-plate. Under those circumstances
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
the covalent cross-linking should be performed as directed by
antibody manufacturers.
(2) Allow the coating process to take place at 4 C overnight or at room
temperature for 1-2 hours.
(3) Wash the wells with PBS, add 50 L of the appropriate media and
perform background measurement using the RT-CES system.
(4) Add the appropriate density of cells in 100-150 RL volume to the
wells and monitor cellular status using the RT-CES system.
Example 3: Coating a Microelectronic Plate with a Biological Molecule or
Organic Compound Including a Ligand, a Peptide or a Compound
Directed Against a Specific Receptor on a Cell Surface That Effects
Cell Attachment, Growth, Differentiation or Morphological Changes
as Monitored by a Microelectronic System (RT-CES system)
The wells in ACEA E-plates can also be coated covalently or non-covalently
with
specific peptides, ligands or compounds wluch can illicit some specific
cellular response
such as adhesion and spreading. For example, RGD containing peptides when
coated
upon a surface, are sufficient to promote attachment and spreading of cells
via interaction
with specific integrin receptors on the cell surface.
The following steps need to be followed for coating the wells of ACEA E-plates
with peptides, ligands or compounds specific for a particular receptor or
other proteins on
the cell surface:
(1) Dissolve the peptide, ligand or compound to be used in PBS and
add to the wells of the ACEA E-plate. Allow coating to take place
at 4 C overnight or 1-2 hours at 4 C. The optimal time of coatin~
may need to be optimized. Alternatively, these reagents may need
96
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
to be covalently linked to the surface of the sensors and therefore
specialized linking procedures may need to be followed.
(2) 'A'ash the wells Arith PBS, add media and perform backg-round
impedance reading using the RT-CES system.
(3) Count and adjust the cells to be added to the appropriate density
and add to the wells which have been coated with the peptide,
ligand or compound.
(4) Monitor cellular response using the RT-CES.
Example 4: Dynamic Monitoring of Cell Adhesion and Spreading on Different
Surfaces Using the RT-CES system
Cells. All the cells used in this study were obtained from ATCC and maintained
at 37 C
incubator with 5 % CO2 saturation. NTH3T3 cells were maintained in DMEM media
containing 10 % FBS and 1% penicillin and streptomycin. Jurkat T cells and
BxPC3
cells were maintained in RPMI containing 10 % FBS and 1% penicillin and
strptomycin.
Cell Adhesion assays using the RT-CES system. ACEA e-plates were coated with
the
indicated ECM protein or PLL for 1 hour at 37 C. The plates were washed with
PBS and
coated with 0.5% BSA solution in PBS for 20 minutes at 37 C. The wells were
washed
with PBS prior to addition of the media and cells. The cells were trypsinized,
spun and
resuspended in serum-free media containing 0.25% BSA. The cells were adjusted
to
appropriate concentration and 100 L of the cell suspension was transferred to
the wells
of ACEA e-plates coated with the various ECM proteins. The adhesion and
spreading of
the cells were monito"red continuously every 3 minutes using the RT-CES system
for a
period of 1-3 hours dependinc, on the experiment. The electronic readout, cell
sensor
impedance is displayed as an arbitrary unit called the Cell Index (CI) where
the CI is
defined as Rn-Rb/Rp; Rn is defined as the cell-electrode impedance of the well
with the
cells at a particular time point and Rb is defined as the background impedance
of the
wells -with just the media alone.
97
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
lnhibitor Treannent and siRhjA Transfection. For assessment of the effect of
evclic RGD
peptides and chemical inhibitors on cell adhesion and spreading, cells were
pre-incubated
with the indicated concentration of the inhibitors for 15-30 minutes prior to
addition to
ECM-coated wells in e-plate. All other steps were exactly as described above.
BxPc3 cells were transfected with 20 nM of siSRC using siPORTatnine at a final
volume
of 60 L. Cells were assayed for adhesion function 48 hours after
transfection.
Im7nunofluorescence and Light Microscopy. The cells were seeded in 16X chamber
slides
coated with either PLL or FN. The cells were allowed to attach and were fixed
with 4%
parafannaldehyde at the indicated time points. The cells were permeabilized,
stained with
rhodamine-phalloidin (Molecular Probes), visualized and photographed using a
Nikon E-
400 epi-fluorescent niicroscope comiected to a digital camera.
In order to assess the extent of adhesion and spreading by the RT-CES system,
ACEA E-plates were coated with FN or PLL as a control. N1II3T3 cells were
applied
onto the coated wells and the extent of adhesion and spreading was monitored
by the. RT-
CES system. Simultaneously, chamber slides were also coated with FN and PLL
and the
same numbers of cells were.added to each well and its attachment and spreading
were
determined by staining with rhodamine-phalloidin and visualization by using
the
epifluorescent microscope. As shown in FIG. 6, application of the cells onto
the FN-
coated wells leads to a dramatic increase in cell index whereas on PLL it
leads to steady
increase overtime. Similarly, immunofluorescent images show that cell
attachnient on FN
is accompanied by immediate spreading which is maximal by 1 hour (FIG. 6A). On
PLL
coated wells, the cells tend to remain round even up two hours after initial
attachment
(FIG. 6A).
In order to determine the effect of FN concentration being coated on the
extent of
cell adhesion and spreadina, ACEA E-plates were coated with inereasing
concentration
of FNI ra.nging from 0 ug/mL to 20 g/mL. NIH3T3 cells were added to the wells
and the
ex-tent of attachment and spreading was monitored using the RT-CES system. As
shown
98
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
in FIG. 7A, the CI increases proportionately with increasinQ amounts of coated
FN. In
order to demonstrate that the CI is proportional to the number of cells
adhering to the
substrate, the cells were trvpsinized at 3 hours post-adhesion and manually
counted. As
shown in FIG. 7B, the ac.tual raw cell number at three hours for the different
FN
concentrations is proportional to the CI obtained at three hours. Taken
together, these
experiments demonstrate that the RT-CES system can be used to quantitatively
assess
cell attachn-ient and spreading under label free conditions and in real-time.
Example 5: Inhibition of Cell Attachment and Spreading Using RGD Containing
Peptides
Integi in heterodimers such as a5 (31 integrins which bind to FN recognize
specific
motif in FN, the arginine-glycine-aspartic acid (RGD) motif (1). It has been
shown
peptides containing the RGD motif can effectively compete for the binding of
cells
expressing the FN receptor to FN (2). In order to determine the extent of
inhibition of cell
attachment to FN by RGD containing peptides, NIH3T3 cells were detached and
incubated in the presence of increasing amounts of cyclic-RGD peptides (FIG.
8A) and
then plated onto FN-coated E-plates and monitored by the RT-CES system. As
seen in
FIG. 8, cyclic-RGD containing peptides blocked NIH3T3 cell adhesion and
spreading in
a concentration-dependent manner. A control peptide, laclcing the RGD motif
had no
effect on cell attachment and spreading (FIG. 8A and B). Comparison of the
relative
extent of cell attachment and spreading at two hours indicate that the 0.1 M
and 1 M
cyclic-RGD peptides block cells adhesion and spreading by 20 % and 40 %,
respectively.
In summary these experiments indicate that, perturbation with integrin
receptor function
can be assessed quantitatively and in real-time using the RT-CES system.
Example 6: Inhibition of Cell Attachment and Spreading Using Actin-Disrupting
Agents or Compounds and Specific Inhibitors of Signaling Proteins
Involved In Attachment and Spreading
Integ-in-mediated cell adhesion is knowm to orsanize the actin cytoskeleton in
a
specific manner. Vise versa. the actin cytoskeleton also participates in
organizina
99
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
integzins and other intracellular signalina proteins into signalina modules
which regulates
cell attachnlent and spreadinQ (1). To determine the.role of.the actin.cy-
tosheleton in cell
attachment and spreading using the RT-CES system, NIIH3T3 cells were detached
and
pre-i.ncubated with increasing concentrations of Latriculin, which is a potent
inhibitor of
actin polynlerization. The cells were then seeded onto FN-coated wells in ACEA
E-plates
and the extent of adhesion and spreading was monitored usiug the RT-CES
system. As
shown in FIG. 9A, Latriculin inhibits cell attachment and spreading in a
concentration-
dependent manner. Analysis of the extent of cell attachment and spreading at
two hours,
clearly demonstrates that Latriculin is a potent inhibitor of cell attachment
and spreading
(FIG. 9B).
One of the main signaling proteins which participates in integrin-mediated
cell
attachment and spreading is the Src family of non-receptor tyrosine kinases
(1). In order
to determine the contribution of Src family kinases to cell attachment and
spreading,
BxPC3 cells were pre-incubated with the Src kinase inhibitor PP2 and then
seeded onto
FN-coated wells in ACEA E-plate. The extent of cell attachment and spreading
was
rnonitored using the RT-CES system. As shown in FIG. 10, cell attachment and
spreading is significantly inhibited in the presence of the Src inhibitor. At
two hoursafter
seeding the cells treated with the PP2 compound displayed an approxinlately 60
%
inhibition of cell attachment and spreading relative to DMSO treated cells.
This fmding
confirms previous results using conventional methods to assess cell
attachnient and
spreading in the presence of the Src family inhibitor (3).
As an additional method for assessing the role of Src kinase in cell
attaclunent and
spreading, BxPC3 cells were transfected with a control siRNA or a siRNA
specific for
the c-Src mRNA. Forty eight hours after transfection, the cells were detached
and seeded
onto FN-coated wells in ACEA E-plate and the extent of cell adhesion and
spreading was
monitored using the RT-CES system. As shown in FIG. 11A and B, down regulation
of
the c-Src gene product leads to a 30 % decrease in cell attachment and
spreading at two
hours post-cell seeding. The disparity between the extent of cell attachment
and
spreading using the PP2 inhibitor and the c-Src siRNA can be explained by the
fact that
PP2 inhibits all Src family members and the siRNA is only specific for c-Src.
100
CA 02580548 2007-03-16
WO 2006/036952 PCT/US2005/034561
In sununan-. the RT-CES svstem can monitor and assess cell attachment and
spreadinR quantitativelv, under label-free conditions and in real-time. The
preclusion of
labelinQ saves on expensive reaQents and time. Moreover, the other major
advantaQe of
usinQ the RT-CES svstem is that since the readout is non-invasive, the user,
in addition to
nlonitorina the effect of matrix proteins on adhesion and spreading can
continue to
monitor its effect on other bioloaical events such as differentiation or
proliferation. Using
traditional methods, it would have been necessary to perform separate
experiments for
each of these events.
All headings used with the present invention are provided to assist the reader
and
are not intended as liuniting the scope of the invention.
20
101